- 1Department of Psychiatry, Chungnam National University Hospital, Daejeon, South Korea
- 2Department of Neuropsychiatry, Seoul National University Bundang Hospital, Seongnam, South Korea
- 3Institute of Human Behavioral Medicine, Medical Research Center, Seoul National University, Seoul, South Korea
- 4Department of Psychiatry, National Center for Mental Health, Seoul, South Korea
- 5Department of Neuropsychiatry, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, South Korea
- 6Department of Neuropsychiatry, SMG-SNU Boramae Medical Center, Seoul, South Korea
- 7Department of Psychiatry, School of Medicine, Konkuk University Medical Center, Konkuk University, Seoul, South Korea
- 8Department of Nuclear Medicine, SMG-SNU Boramae Medical Center, Seoul, South Korea
- 9Department of Radiology, Seoul National University Hospital, Seoul, South Korea
- 10Department of Psychiatry, Seoul National University College of Medicine, Seoul, South Korea
This study aimed to investigate whether the midlife cognitive activity and physical activity moderate the relationship between apolipoprotein Eε4 (APOE4) and in vivo Alzheimer’s disease (AD) pathologies. In total, 287 non-demented older adults (mean age 72 years) from the Korean Brain Aging Study for the Early diagnosis and prediction of Alzheimer’s disease cohort were included. Participants underwent a comprehensive clinical assessment including the evaluation for midlife CA and physical activity, [11C]-Pittsburgh-Compound-B-positron emission tomography (PET), [18F]-fluorodeoxyglucose PET, structural magnetic resonance imaging (MRI), and APOE genotyping. We used linear regression and regression-based mediated-moderation models for statistical analyses. Neither midlife cognitive activity nor physical activity moderated the effect of APOE4 on β-amyloid (Aβ) retention itself. Midlife cognitive activity significantly moderated the effect of APOE4 on hippocampal volume [B (SE) = − 627.580 (252.327), t = −2.488, p = 0.014]: APOE4 carriers had smaller hippocampal volume than non-carriers at relatively high cognitive activity state (p = 0.004), but not at relatively low cognitive activity condition (p = 0.937). Midlife physical activity significantly moderated the effect of Aβ retention, which was closely related to APOE4, on AD-signature region cerebral glucose metabolism [AD-CM; B (SE) = 0.004 (0.002), t = 2.030, p = 0.043]: higher Aβ accumulation was associated with lower AD-CM in relatively low physical activity condition (p < 0.001), whereas no such association was observed in relatively high physical activity state (p = 0.791). The findings suggest that high midlife cognitive activity may accelerate hippocampal atrophy induced by APOE4, whereas high midlife physical activity may delay AD-related cerebral hypometabolism by weakening the influence of APOE4-associated Aβ retention.
Background
The apolipoprotein ε4 (APOE4) is the most well-evidenced risk gene for Alzheimer’s disease (AD; Corder et al., 1993) and is related to in vivo AD pathologies such as β-amyloid (Aβ) accumulation (Morris et al., 2010), reduced hippocampal volume (Hashimoto et al., 2001), and decreased AD-signature region cerebral glucose metabolism (AD-CM; Small et al., 1995; Lowe et al., 2014). APOE4 has complex effects on AD pathophysiology through both Aβ-mediated pathway (i.e., indirect effect of APOE4 on hippocampal volume or AD-CM reduction via Aβ accumulation) and Aβ-independent pathways (i.e., direct effect of APOE4 on hippocampal volume or AD-CM reduction not mediated by Aβ accumulation; Huang, 2010).
While APOE4 is a non-modifiable genetic risk factor, modifiable factors such as cognitive activity and physical activity have been associated with a decreased risk of cognitive decline (Ngandu et al., 2015) and AD dementia (Rovio et al., 2005; Kivipelto et al., 2008; Najar et al., 2019). However, studies on the in vivo neuropathological mechanisms underlying the association between cognitive activity or physical activity and AD-related cognitive decline have produced controversial findings (Valenzuela et al., 2008; Erickson et al., 2009; Liang et al., 2010; Bugg and Head, 2011; Head et al., 2012; Landau et al., 2012; Vemuri et al., 2012, 2016, 2017; Brown et al., 2013b; Wirth et al., 2014; Gidicsin et al., 2015; Ko et al., 2018). Such modifiable lifestyle activities may change the AD pathophysiological processes associated with APOE4. However, their moderation for the influence of APOE4 on AD pathologies remains poorly understood (Kivipelto et al., 2008; Head et al., 2012; Wirth et al., 2014).
Some previous studies have adopted current cognitive activity or physical activity to investigate the relationship between lifestyle activities and in vivo AD pathologies (Valenzuela et al., 2008; Erickson et al., 2009; Landau et al., 2012; Brown et al., 2013b; Wirth et al., 2014). However, as AD pathology, Aβ deposition, in particular, precedes the clinical symptom onset of dementia by 10–15 years (Villemagne et al., 2013), current activity itself could be affected by pre-existing AD pathology (i.e., reverse causation; de Bruijn et al., 2013; Jack et al., 2013b). In contrast, midlife cognitive and physical activities are less likely to be affected by AD pathology. Moreover, many previous studies indicated that such midlife activities are related with a decreased risk of late-life cognitive decline (Karp et al., 2009; Inzelberg et al., 2013; Najar et al., 2019) and AD dementia (Rovio et al., 2005; Andel et al., 2008; Kivipelto et al., 2008; Tolppanen et al., 2015).
Therefore, we aimed to investigate whether the midlife cognitive activity and physical activity can moderate the effect of APOE4 on in vivo AD pathologies measured by neuroimaging modalities.
Materials and Methods
Participants
The present study included 287 non-demented older adults [215 cognitively normal (CN), 72 mild cognitive impairment (MCI)] between 55 and 90 years of age who participated in the Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s disease (KBASE), an ongoing prospective cohort study initiated in 2014 (Byun et al., 2017). The CN group consisted of participants with a Clinical Dementia Rating (CDR; Morris, 1993) score of 0. All individuals with MCI met the core clinical criteria for MCI diagnosis recommended by the National Institute of Aging and Alzheimer’s Association guidelines (Albert et al., 2011), which are as follows: (1) memory complaints confirmed by an informant; (2) objective memory impairments; (3) preserved global cognitive function; (4) independance in functional activities; and (5) no dementia. Regarding Criterion 2, the age-, education-, and sex-adjusted z-scores for at least one of four episodic memory tests were < −1.0. The four memory tests were the Word List Memory, Word List Recall, Word List Recognition, and Constructional Recall tests, which are included in the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-K) neuropsychological battery (Lee et al., 2004). All MCI individuals had a CDR score of 0.5. The exclusion criteria were as follows: (1) presence of a major psychiatric illness, including alcohol-related disorders; (2) significant neurological or medical conditions or comorbidities that could affect mental function; (3) contraindications for an magnetic resonance imaging (MRI) scan (e.g., pacemaker or claustrophobia); (4) illiteracy; (5) the presence of significant visual/hearing difficulties and/or severe communication or behavioral problems that would make clinical examinations or brain scans difficult; (6) taking an investigational drug; and (7) pregnant or breastfeeding. All the participants received comprehensive neuropsychological and clinical evaluation including midlife cognitive activity and physical activity according to the KBASE assessment protocol (Byun et al., 2017). More detailed information on the KBASE study methodology including the enrollment and assessment of participants was described previously (Byun et al., 2017).
The Institutional Review Board of Seoul National University Hospital (C-1401-027-547) and Seoul Metropolitan Government-Seoul National University Boramae Medical center (26-2015-60) in South Korea approved the present study and all volunteers provided written informed consent prior to participation.
APOE Genotyping
Blood samples were obtained via venipuncture and DNA was extracted from whole blood. APOE genotyping was performed as described in a previous study (Park et al., 2017). If an individual has at least one APOE4 allele, we defined it as an APOE4 carrier.
Assessment of Midlife Cognitive and Physical Activities
Cognitive Activity
The cognitive activity of each subject was assessed using a 39 item expanded version (Wilson et al., 2005) of a previously reported 25-item autobiographical self-report questionnaire (Wilson et al., 2003; Landau et al., 2012). This questionnaire has sufficient internal consistency and temporal stability (Wilson et al., 2003, 2005). Participants were asked to report how often they engaged in common cognitively demanding activities with few barriers to participation, such as reading newspapers, magazines, or books; visiting a museum or library; attending a concert, play or musical and writing letters or a diary, at 5 age epochs: 6, 12, 18, and 40 years and the current age. Responses for each item were made using a 5-point frequency scale: 5, every day or almost every day; 4, several times a week; 3, several times a month; 2, several times a year; and 1, once a year or less. Among the 39 items, nine items were for current age (i.e., late-life) cognitive activity and nine items are for midlife (40 years of age) cognitive activity. The item scores for current age and midlife were averaged to yield current- and midlife cognitive activity value, respectively.
Physical Activity
Midlife physical activity (age 40–55 years) was assessed using the interviewer-administered Lifetime Total Physical Activity Questionnaire, a tool with demonstrated reliability (Friedenreich et al., 1998, 2004) and validity (Gill et al., 2015). This questionnaire assesses occupational, household, and leisure activities separately throughout a respondent’s lifetime. The frequency and duration of these activities were assessed by recording the number of years, months per year, weeks per month, days per week and hours per day that each activity was performed. The intensity of activity was estimated by the participant as sedentary, light, moderate or heavy. A metabolic equivalent (MET) value was assigned to each activity based on the Compendium of Physical Activities (Ainsworth et al., 2011). The index of midlife- and current physical activity was the average MET-hr./week spent on leisure activity at the ages of between 40–55 years old and over the past 3 years each. We selected leisure activities, but not occupational or household activities because we wanted to include only a modifiable factor that could be controlled. Most previous studies about the influence of physical activity on AD or dementia risk have focused only on leisure-time physical activity (Rovio et al., 2005; Tolppanen et al., 2015; Krell-Roesch et al., 2018).
Assessment of AD Neuroimaging Biomarkers
Measurement of Cerebral Aβ Accumulation
All subjects underwent simultaneous three-dimensional (3D) PiB-PET and T1-weighted MRI using a 3.0 T Biograph mMR (PET-MR) scanner (Siemens, Washington, DC, USA) according to the manufacturer’s approved guidelines. After 40 min from intravenous administration of 555 MBq of 11C-PiB (range, 450–610 MBq), the PiB-PET image data were collected in list mode (5 min × 6 frames). All PiB-PET images were processed with routine corrections for uniformity, UTE-based attenuation, and decay corrections, and reconstructed into a 256 × 256 image matrix using iterative methods (six iterations with 21 subsets). T1-weighted 3D MR images were acquired in the sagittal orientation with the following parameters; repetition time = 1,670 ms, echo time = 1.89 ms, field of view 250 mm, 256 × 256 matrix with 1.0 mm slice thickness. The image preprocessing was performed using Statistical Parametric Mapping 12 (Wellcome Department of Cognitive Neurology, London, UK1) and Individual Brain Atlases using Statistical Parametric Mapping software (IBASPM2). First, static PiB-PET images were co-registered to individual T1-weighted MR images and then transformation parameters for spatial normalization of individual T1-weighted MR images to a standard Montreal Neurological Institute (MNI) template were calculated. The inverse transformation parameters were used to transform coordinates from the automatic anatomic labeling (AAL) 116 atlas (Tzourio-Mazoyer et al., 2002) into an individual space for each subject (a resampling voxel size = 1 × 0.98 × 0.98 mm), and the non-gray matter portions of the atlas were individually masked using the cerebral gray matter segment image from each subject. Cerebellar gray matter was used as the reference region and mean [11C]-PiB uptake value was extracted from all the cerebellar lobular regions except for the vermis from a probabilistic cerebellar atlas (Institute of Cognitive Neuroscience, UCL; Cognitive Neuroscience Laboratory, Royal Holloway).
The AAL algorithm (Tzourio-Mazoyer et al., 2002) and a region combining method (Reiman et al., 2009) were applied to determine regions of interest (ROI) to characterize the [11C]-PiB level in the frontal, lateral parietal, posterior cingulate-precuneus, and lateral temporal regions. The standardized uptake value ratio (SUVR) values for each ROI were calculated by dividing the mean value for all voxels within each ROI by the mean cerebellar gray matter uptake value in the same image. A global cortical ROI consisting of the 4 ROIs was also defined, and a global Aβ retention value was calculated by dividing the mean PiB uptake value for all voxels of the global cortical ROIs by the mean cerebellar gray matter uptake value (Reiman et al., 2009). The global Aβ retention values had skewed distribution and were log-transformed in the analysis.
Hippocampal Volume Measurement
All T1-weighted images were acquired in the sagittal orientation using the abovementioned 3.0 T PET-MR machine. All MR images were automatically segmented using FreeSurfer version 5.33 with manual correction of minor segmentation errors. An adjusted hippocampal volume (HVa) was calculated as the unstandardized residual from the linear regression of total hippocampal volume (HVt) vs. the total intracranial volume (ICV) of the reference group (the young CN group of the study cohort; Lee et al., 2017). HVa indicates the volume deviated from the expected HVt according to the ICV in young CN subjects.
Measurement of AD-Signature Cerebral Glucose Metabolism
All subjects also underwent FDG-PET imaging using the same PET-MR machine as described previously. The participants fasted for at least 6 h and rested in a waiting room for 40 min prior to the scans after intravenous administration of 0.1 mCi/Kg of [18F]-FDG radioligands. The PET data collected in list mode (5 min × 4 frames) were processed for routine corrections such as uniformity, UTE-based attenuation, and decay corrections. After inspecting the data for any significant head movements, we reconstructed them into a 20-min summed image using iterative methods (6 iterations with 21 subsets). The following image processing steps were performed using SPM124 implemented in Matlab 2014a (Mathworks, Natick, MA, USA). First, static FDG-PET images were co-registered to individual T1 structural images, and transformation parameters for the spatial normalization of individual T1 images to a standard MNI template were calculated and used to spatially normalize the PET images to the MNI template. After smoothing the spatially normalized FDG-PET images with a 12-mm Gaussian filter, intensity normalization was performed using the pons as the reference region. AD-signature FDG ROIs, such as the angular gyri, posterior cingulate cortex, and inferior temporal gyri, which are sensitive to the changes associated with AD (Jack et al., 2014) were determined. AD-CM was defined as a voxel-weighted mean SUVR extracted from the AD-signature FDG ROIs.
Statistical Analysis
First, multiple linear regression analyses were conducted to examine the simple associations between midlife cognitive activity (or physical activity) and AD biomarkers using IBM SPSS Statistics software 23 (IBM Corp., Armonk, NY, USA). Then, we tested models including both Aβ-mediated- and Aβ-independent pathways of APOE4 effects using the Process Macro program (Hayes, 2017) to investigate systematically the effects of APOE4 on AD biomarkers and moderation by midlife cognitive activity or physical activity (Figure 1). The inference was determined by 95% bias-corrected bootstrap confidence intervals from 10,000 bootstrap samples. An effect was considered significant if the 95% confidence interval did not include zero. For all the analyses, age, sex, educational year, and clinical diagnosis (CN vs. MCI) were controlled as covariates, and p-value < 0.05 was considered significant.
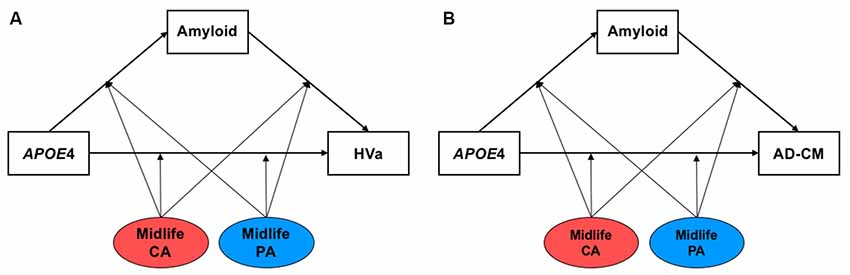
Figure 1. The hypothetical moderated mediation model to analyze the associations of apolipoprotein E ε4 (APOE4) with β-amyloid (Aβ) retention and (A) HVa or (B) AD-CM, and the moderation effect of cognitive and physical activity on the associations. The sequence of Alzheimer’s disease (AD) pathologies are based on hypothetical amyloid cascade model of AD pathophysiology (Jack et al., 2010; Jack et al., 2013a). APOE4, APOE ε4; CA, cognitive activity; PA, physical activity; HVa, adjusted hippocampal volume; AD-CM, AD-signature region cerebral glucose metabolism.
Data Availability
The datasets generated and analyzed during the present study are not publicly available, owing to ethical considerations and privacy restrictions. Data may be obtained from the corresponding author after approval by the Institutional Review Board of the Seoul National University Hospital, South Korea has been sought.
Results
Participant Characteristics
The characteristics of the subjects are shown in Table 1. Of the 287 study participants, 66 (23.0%) were APOE4 carriers. There were no differences between APOE4 carriers and non-carriers regarding age, sex, education, or midlife cognitive activity or physical activity. The proportion of MCI subjects was higher in the APOE4 carrier than in the non-carrier group. APOE4 carriers also had higher global Aβ accumulation, smaller HVa, and lower AD-CM than those of non-carriers.
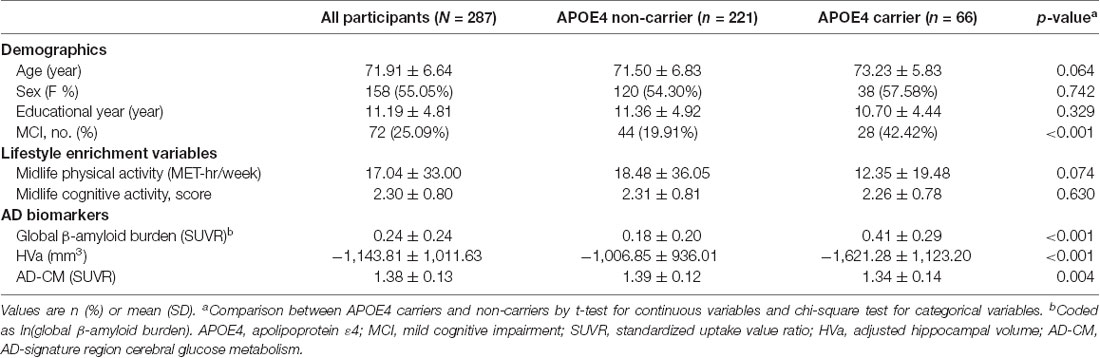
Table 1. Patient characteristics in the overall sample and in the strata by apolipoprotein E ε4 (APOE4) status (N = 287).
Simple Associations of APOE4, Cognitive Activity and Physical Activity With AD Biomarkers
Linear regression analyses showed that APOE4 positivity was significantly associated with increased global Aβ retention, decreased HVa, and decreased AD-CM (Table 2). In contrast, neither midlife cognitive activity nor physical activity was related to any of the AD neuroimaging biomarkers.
Moderation of Midlife Lifestyle Activities for the Association of APOE4 With Aβ Retention and HVa
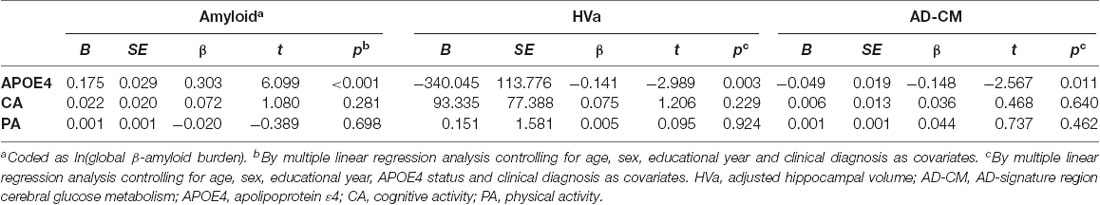
When a model including the moderating effect of midlife cognitive and physical activity for the association between APOE4, Aβ retention, and HVa (Figure 1A) was analyzed, midlife cognitive activity significantly moderated the Aβ-independent effect of APOE4 on HVa (Table 3 and Figure 2A). For the purpose of demonstration, the association between APOE4 carrier status and HVa was plotted for each of the high and low midlife cognitive activity state (Figure 3A). At relatively high cognitive activity (1SD above mean) condition, APOE4 carriers had significantly smaller HVa than non-carriers [B (SE) = −561.576 (193.159), t = −2.907, p = 0.004], whereas no such difference was found APOE4 carriers and non-carriers at relatively low cognitive activity (1 SD below mean) condition [B (SE) = 13.707 (173.037), t = 0.079, p = 0.937] (Figure 3A). In contrast, midlife cognitive activity moderated neither the APOE4 effect on Aβ retention itself nor the Aβ-mediated effect of APOE4 on HVa (Table 3 and Figure 2A). In contrast to midlife cognitive activity, midlife physical activity did not show any moderating effect on the influence of APOE4 on Aβ retention and HVa (Table 3 and Figure 2A). Even after current cognitive and physical activity were controlled in the model as additional covariates, the results were unchanged.
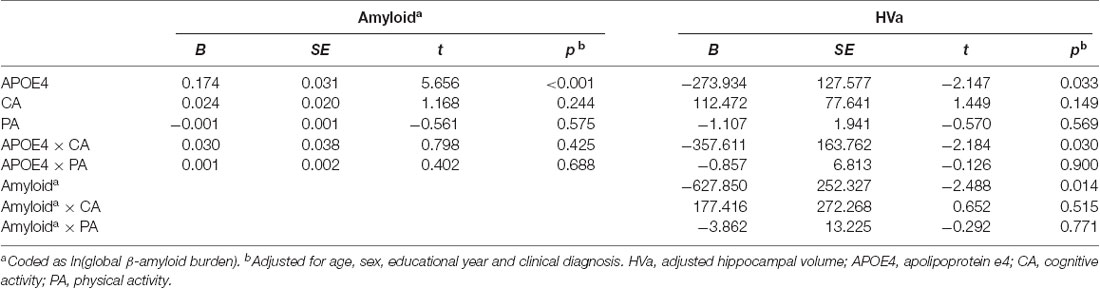
Table 3. Moderation of midlife activities for the association of APOE4 with Aβ retention and HVa: results from moderated mediation analysis based on PROCESS (N = 287).
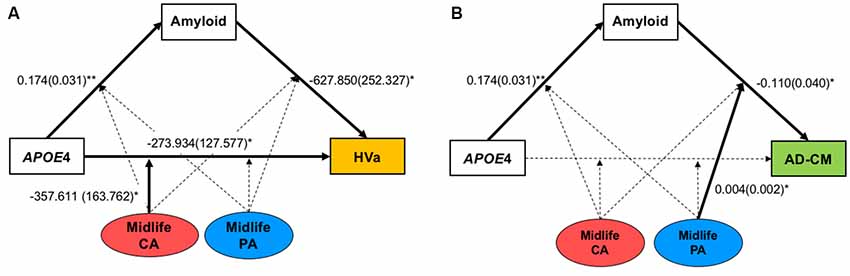
Figure 2. Results from moderated mediation model analyses for the associations of APOE4 with Aβ retention and (A) HVa or (B) AD-CM, and the moderation effect of midlife cognitive and physical activity for the associations. Values are standardized regression coefficients (standard error) for the associations or moderation effect with statistical significance. Bold lines also indicate significant association or moderation effect. *p < 0.05, **p < 0.005. APOE4, apolipoprotein ε4; CA, cognitive activity; PA, physical activity; HVa, adjusted hippocampal volume; AD-CM, AD-signature region cerebral glucose metabolism.
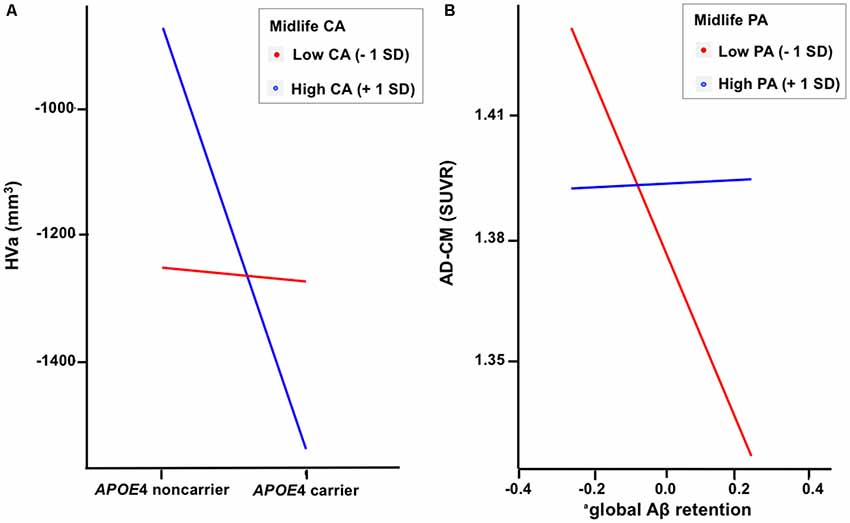
Figure 3. Plots to demonstrate the moderation effect of (A) midlife cognitive activity on the relationship between APOE4 and HVa and (B) midlife physical activity on the relationship between Aβ retention and AD-CM. aCoded as ln(global β-amyloid burden). APOE4, apolipoprotein ε4; CA, cognitive activity; PA, physical activity; HVa, adjusted hippocampal volume; AD-CM, AD-signature region cerebral glucose metabolism; <1 SD, 1 standard deviation below mean value; +1 SD,: 1 SD above mean value.
Moderation of Midlife Lifestyle Activities for the Association of APOE4 With Aβ Retention and AD-CM
While midlife cognitive activity did not have any moderation effect for the association between APOE4, Aβ retention, and AD-CM, midlife physical activity significantly moderated the effect of Aβ retention on AD-CM (Table 4 and Figure 2B), suggesting the effect of global Aβ retention, which is closely related to APOE4, on AD-CM can be changed by midlife physical activity level. For the purpose of demonstration, the association between global Aβ retention and AD-CM was plotted for each of the high and low midlife physical activity state (Figure 3B). At relatively low physical activity (1SD below mean) condition, higher global Aβ retention was significantly associated with lower AD-CM [B (SE) = −16.205 (0.179), t = −4.271, p < 0.001], whereas no such association was observed in relatively high physical activity (1 SD above mean) condition [B (SE) = 0.023(0.089), t = 0.264, p = 0.791] (Figure 3B). In contrast, midlife physical activity moderated neither the effect of APOE4 on Aβ retention nor the Aβ-independent effect of APOE4 on AD-CM (Table 4 and Figure 3B). Even after current cognitive and physical activity were controlled in the model as additional covariates, the results were similar.
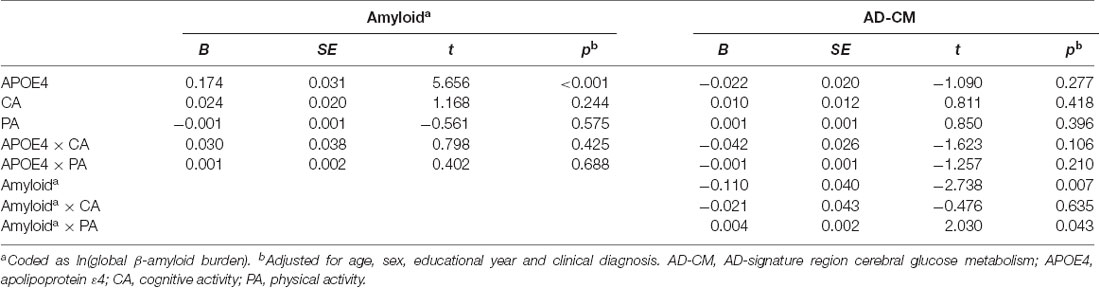
Table 4. Moderation of midlife activities for the association of APOE4 with Aβ retention and AD-CM: results from moderated mediation analysis based on PROCESS (N = 287).
Discussion
We observed that APOE4 was strongly associated with increased global Aβ accumulation and reduced HVa and AD-CM, whereas neither midlife cognitive activity nor physical activity was related to any of the AD biomarkers in bivariate association analysis. In terms of the moderating effects of midlife lifestyle activities, midlife cognitive activity moderated the Aβ-independent influence of APOE4 on HVa, and midlife physical activity moderated the Aβ-mediated influence of APOE4 on AD-CM, while neither activity moderated the APOE4 effects on Aβ accumulation.
Association Between APOE4, CA, and PA With AD Biomarkers
Consistent with previous reports (Small et al., 1995; Hashimoto et al., 2001; Morris et al., 2010; Lowe et al., 2014), APOE4 status was strongly associated with Aβ accumulation and the neurodegeneration biomarkers in our study.
In contrast, neither midlife cognitive activity nor physical activity was related to any of the AD neuroimaging biomarkers in bivariate analysis. Many studies investigating the association between cognitive activity or physical activity and AD biomarkers have reported inconsistent findings (Valenzuela et al., 2008; Erickson et al., 2009; Liang et al., 2010; Bugg and Head, 2011; Head et al., 2012; Landau et al., 2012; Vemuri et al., 2012, 2016, 2017; Brown et al., 2013b; Wirth et al., 2014; Gidicsin et al., 2015; Ko et al., 2018). Among them, only a few have focused on the effect of midlife activities (Vemuri et al., 2016, 2017; Ko et al., 2018) and have shown no direct association between midlife cognitive activity or physical activity and AD biomarkers, which was similar to our findings. Such a null association with Aβ accumulation and AD-related neurodegeneration biomarkers appears discordant with the finding that midlife lifestyle activities are associated with a decreased risk of late-life cognitive decline (Ngandu et al., 2015) or AD dementia (Rovio et al., 2005; Najar et al., 2019). Such a discrepancy was also observed in prior studies (Wilson et al., 2013; Gidicsin et al., 2015). A report based on the Harvard Aging Brain Study demonstrated that a history of greater cognitive activity is correlated with better cognitive performance, but not with Aβ accumulation, glucose metabolism, or hippocampal volume in CN older adults (Gidicsin et al., 2015). A neuropathological study also showed that greater past cognitive activity is related to slower late-life cognitive decline, independently of AD neuropathologies (Wilson et al., 2013). Taken together, a change in AD pathology itself is not likely to be the direct substrate underlying the effect of past lifestyle activities on cognitive benefit.
Moderation of Midlife Cognitive Activity or Physical Activity for APOE4 Effects on Aβ Deposition
In our study, neither midlife cognitive activity nor physical activity moderated the APOE4 effect on Aβ deposition itself. However, two previous studies reported a significant interaction effect between lifestyle activities and APOE4 on Aβ accumulation (Head et al., 2012; Wirth et al., 2014). They showed a beneficial effect of cognitive activity (Wirth et al., 2014) or physical activity (Head et al., 2012) on Aβ accumulation only in APOE4 carriers. This discrepancy might be attributed to different study methods and sample characteristics. We focused specifically on midlife activities to reduce the possibility of reverse causation (Jack et al., 2013b), whereas other studies adopted lifetime or recent 10-year lifestyle activities including current ones, which could be affected by underlying pathophysiological processes. In addition, the educational levels of their subjects (mean educational years: 16.86 (Wirth et al., 2014) and 16.23 (Head et al., 2012) were higher than those of our study (11.19 years). Another study (Vemuri et al., 2016) detected an inverse association between midlife cognitive activity and Aβ accumulation in APOE4 carriers only in the high education group (≥14 years), but not in the low education group (<14 years).
Moderation of Midlife Cognitive Activity for APOE4 Effects on HVa
Midlife cognitive activity moderated the Aβ-independent influence of APOE4 on HVa. More specifically, the direct negative effect of APOE4 on HVa was more evident in individuals with higher midlife cognitive activity than in those with a lower midlife cognitive activity. According to the APOE4 antagonistic pleiotropy hypothesis, APOE4 differentially impacts across different life stages. APOE4 offers cognitive benefits during early adulthood at the expense of a more rapid decline in cognitive function with aging (Tuminello and Han, 2011). Young CN individuals with APOE4 have elevated resting-state activity in the default mode network including the hippocampus compared to those without APOE4 (Filippini et al., 2009). APOE4 carriers in midlife have more strongly activated memory-related brain regions including the hippocampus to maintain the same level of performance than non-carriers, but this neural compensatory recruitment begins to decline by midlife (Bondi et al., 2005; Tuminello and Han, 2011). Such increased activity in the memory-related brain regions is also known to be related to atrophy of the medial temporal lobe including the hippocampus (O’Brien et al., 2010). Taken together, our results indicate that excessive midlife cognitive activity in APOE4 carriers may accelerate hippocampal atrophy by imposing hyperactivation of the related brain regions.
Moderation of Midlife Physical Activity for APOE4 Effects on AD-CM
While midlife physical activity did not moderate the Aβ-independent influence of APOE4 on AD-CM, it moderated the indirect pathway from APOE4 to AD-CM via Aβ accumulation. More specifically, Aβ accumulation, which is closely linked to APOE4, was associated with decreased AD-CM in individuals with a lower level of midlife physical activity, whereas such an inverse correlation between Aβ accumulation and AD-CM was not significant in those with a higher level of physical activity.
There are several possible pathways linking active physical activity and preserved AD-CM. First, physical activity has been suggested to increase cognitive or brain reserve through angiogenesis, increased cerebral blood flow and enhanced synaptic plasticity (van Praag, 2009; Brown et al., 2013a). Individuals with a greater reserve through active physical activity can tolerate a greater burden of cerebral Aβ accumulation and do not show reduced AD-CM as shown in the inactive physical activity group. Second, Aβ shares a consensus amino acid sequence with insulin and Aβ directly binds to the insulin receptor leading to increased insulin-resistance (Xie et al., 2002). Increased insulin resistance is associated with reduced AD-CM (Willette et al., 2015). As active physical activity decreases insulin-resistance (Balkau et al., 2008), it could prevent the reduction of AD-CM by Aβ. Finally, active physical activity also lowers chronic inflammation (Brown et al., 2013a). Increased cerebral Aβ accumulation induces neuroinflammation which can reduce AD-CM (Akiyama et al., 2000). Consistent with our result, a 21-year longitudinal follow-up study reported that midlife physical activity is inversely associated with a late-life risk of AD dementia only among APOE4 carriers (Rovio et al., 2005). Given the strong association between APOE4 and Aβ accumulation (Morris et al., 2010), our findings suggest that higher midlife physical activity decreases APOE4-related AD risk by weakening the influence of Aβ accumulation on further hypometabolism or neurodegeneration.
Strengths and Limitations
One of the key strengths of this study is the statistical approach using models including both the Aβ-mediated pathway and the Aβ-independent pathway of APOE4 influence. This approach made it possible to clarify the complex associations and interactions between APOE, midlife lifestyle factors, and AD biomarkers. Our findings, based on such complex models, may explain why studies about the effects of lifestyle activities on AD biomarkers have resulted in inconsistent findings. The relatively large sample size, particularly of the CN group, is another strong point of this study. Nevertheless, some limitations should also be mentioned. Because this was not a longitudinal study, we could not confirm causality for the observed associations. To overcome such a limitation in the study design, we used midlife cognitive activity and physical activity instead of current activities. Nevertheless, further long-term follow up studies are needed to clarify the causal aspects. Although the cognitive activity and physical activity questionnaires used in the present study were reliable and well-validated, they are based on self-reports and might be biased due to recall problems. To minimize such recall bias, we only included non-demented subjects. Although MCI individuals have some memory problems, their problems are confined to recent memory, not remote memory (Leyhe et al., 2009).
Conclusion
The current study was the first attempt to elucidate the moderating effect of modifiable midlife lifestyle factors on the influence of APOE4 on in vivo AD pathologies. Our findings suggest that high midlife cognitive activity may accelerate hippocampal atrophy induced by APOE4. In contrast, active midlife physical activity may delay AD-signature regional brain hypometabolism by weakening the influence of APOE4-associated Aβ accumulation. Overall, the information obtained in this study will be helpful to select preventive midlife lifestyle activities to reduce the negative influence of the genetic risk for AD.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Seoul National University Hospital, Seoul Metropolitan Government-Seoul National University Boramae Medical center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SJ and DYL is responsible for the study concept and design, acquisition, analysis and interpretation of data; and drafting and critically revising the manuscript for intellectual content. MB, DY, J-HL, KK, BS, and J-YL are responsible for the acquisition, analysis, and interpretation of data. S-HR, DWL, SS, YK, KMK, and C-HS participated in the analysis and interpretation of data. All authors read and approved the final manuscript.
Funding
This work was funded by a grant from Ministry of Science and ICT, South Korea (grant No: NRF-2014M3C7A1046042) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, South Korea (grant number: HI18C0630 and HI19C0149).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
APOE4, apolipoprotein ε4 allele; AD, Alzheimer’s disease; Aβ, β-amyloid; AD-CM, AD-signature region cerebral glucose metabolism; CA, cognitive activity; PA, physical activity; CN, cognitively normal; MCI, mild cognitive impairment; KBASE, the Korean Brain Aging Study for the Early Diagnosis and Prediction of AD; CDR, clinical dementia rating; CERAD-K, the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease Assessment Packet; PiB, [11C] Pittsburg compound B; PET, positron emission tomography; FDG, [18F]-fluorodeoxyglucose; MET, metabolic equivalent; MNI, Montreal Neurological Institute; AAL, automatic anatomic labeling; ROI, region of interest; SUVR, standardized uptake value ratio; HVa, adjusted hippocampal volume; HVt, total hippocampal volume; ICV, intracranial volume.
Footnotes
- ^ www.fil.ion.ucl.ac.uk/spm
- ^ www.thomaskoenig.ch/Lester/ibaspm.htm
- ^ http://surfer.nmr.mgh.harvard.edu/
- ^ www.fil.ion.ucl.ac.uk/spm
References
Ainsworth, B. E., Haskell, W. L., Herrmann, S. D., Meckes, N., Bassett, D. R. Jr., Tudor-Locke, C., et al. (2011). 2011 compendium of physical activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 43, 1575–1581. doi: 10.1249/MSS.0b013e31821ece12
Akiyama, H., Barger, S., Barnum, S., Bradt, B., Bauer, J., Cole, G. M., et al. (2000). Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421. doi: 10.1016/s0197-4580(00)00124-x
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2011). The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the national institute on aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 270–279. doi: 10.1016/j.jalz.2011.03.008
Andel, R., Crowe, M., Pedersen, N. L., Fratiglioni, L., Johansson, B., and Gatz, M. (2008). Physical exercise at midlife and risk of dementia three decades later: a population-based study of Swedish twins. J. Gerontol. A Biol. Sci. Med. Sci. 63, 62–66. doi: 10.1093/gerona/63.1.62
Balkau, B., Mhamdi, L., Oppert, J.-M., Nolan, J., Golay, A., Porcellati, F., et al. (2008). Physical activity and insulin sensitivity. The RISC study. Diabetes 57, 2613–2618. doi: 10.2337/db07-1605
Bondi, M. W., Houston, W. S., Eyler, L. T., and Brown, G. G. (2005). fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64, 501–508. doi: 10.1212/01.WNL.0000150885.00929.7E
Brown, B. M., Peiffer, J., and Martins, R. (2013a). Multiple effects of physical activity on molecular and cognitive signs of brain aging: can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol. Psychiatry 18, 864–874. doi: 10.1038/mp.2012.162
Brown, B. M., Peiffer, J., Taddei, K., Lui, J., Laws, S. M., Gupta, V. B., et al. (2013b). Physical activity and amyloid-β plasma and brain levels: results from the australian imaging, biomarkers and lifestyle study of ageing. Mol. Psychiatry 18, 875–881. doi: 10.1038/mp.2012.107
Bugg, J. M., and Head, D. (2011). Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 32, 506–514. doi: 10.1016/j.neurobiolaging.2009.03.008
Byun, M. S., Yi, D., Lee, J. H., Choe, Y. M., Sohn, B. K., Lee, J.-Y., et al. (2017). Korean brain aging study for the early Diagnosis and prediction of Alzheimer’s disease: methodology and baseline sample characteristics. Psychiatry Investig. 14, 851–863. doi: 10.4306/pi.2017.14.6.851
Corder, E. H., Saunders, A. M., Strittmatter, W. J., Schmechel, D. E., Gaskell, P. C., Small, G., et al. (1993). Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. doi: 10.1126/science.8346443
de Bruijn, R. F., Schrijvers, E. M., de Groot, K. A., Witteman, J. C., Hofman, A., Franco, O. H., et al. (2013). The association between physical activity and dementia in an elderly population: the Rotterdam study. Eur. J. Epidemiol. 28, 277–283. doi: 10.1007/s10654-013-9773-3
Erickson, K. I., Prakash, R. S., Voss, M. W., Chaddock, L., Hu, L., Morris, K. S., et al. (2009). Aerobic fitness is associated with hippocampal volume in elderly humans. Hippocampus 19, 1030–1039. doi: 10.1002/hipo.20547
Filippini, N., MacIntosh, B. J., Hough, M. G., Goodwin, G. M., Frisoni, G. B., Smith, S. M., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-ε4 allele. Proc. Natl. Acad. Sci. U S A 106, 7209–7214. doi: 10.1073/pnas.0811879106
Friedenreich, C. M., Courneya, K. S., and Bryant, H. E. (1998). The lifetime total physical activity questionnaire: development and reliability. Med. Sci. Sports Exerc. 30, 266–274. doi: 10.1097/00005768-199802000-00015
Friedenreich, C. M., McGregor, S., Courneya, K., Angyalfi, S., and Elliott, F. (2004). Case-control study of lifetime total physical activity and prostate cancer risk. Am. J. Epidemiol. 159, 740–749. doi: 10.1093/aje/kwh106
Gidicsin, C. M., Maye, J. E., Locascio, J. J., Pepin, L. C., Philiossaint, M., Becker, J. A., et al. (2015). Cognitive activity relates to cognitive performance but not to Alzheimer disease biomarkers. Neurology 85, 48–55. doi: 10.1212/WNL.0000000000001704
Gill, S. J., Friedenreich, C. M., Sajobi, T. T., Longman, R. S., Drogos, L. L., Davenport, M. H., et al. (2015). Association between lifetime physical activity and cognitive functioning in middle-aged and older community dwelling adults: results from the brain in motion study. J. Int. Neuropsychol. Soc. 21, 816–830. doi: 10.1017/s1355617715000880
Hashimoto, M., Yasuda, M., Tanimukai, S., Matsui, M., Hirono, N., Kazui, H., et al. (2001). Apolipoprotein E ε4 and the pattern of regional brain atrophy in Alzheimer’s disease. Neurology 57, 1461–1466. doi: 10.1212/wnl.57.8.1461
Hayes, A. F. (2017). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Guilford Publications.
Head, D., Bugg, J. M., Goate, A. M., Fagan, A. M., Mintun, M. A., Benzinger, T., et al. (2012). Exercise engagement as a moderator of the effects of APOE genotype on amyloid deposition. Arch. Neurol. 69, 636–643. doi: 10.1001/archneurol.2011.845
Huang, Y. (2010). Aβ-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol. Med. 16, 287–294. doi: 10.1016/j.molmed.2010.04.004
Inzelberg, R., Afgin, A. E., Massarwa, M., Schechtman, E., Israeli-Korn, S. S., Strugatsky, R., et al. (2013). Prayer at midlife is associated with reduced risk of cognitive decline in Arabic women. Curr. Alzheimer Res. 10, 340–346. doi: 10.2174/1567205011310030014
Jack, C. R. Jr., Knopman, D. S., Jagust, W. J., Petersen, R. C., Weiner, M. W., Aisen, P. S., et al. (2013a). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216. doi: 10.1016/s1474-4422(12)70291-0
Jack, C. R. Jr., Wiste, H. J., Lesnick, T. G., Weigand, S. D., Knopman, D. S., Vemuri, P., et al. (2013b). Brain β-amyloid load approaches a plateau. Neurology 80, 890–896. doi: 10.1212/WNL.0b013e3182840bbe
Jack, C. R. Jr., Knopman, D. S., Jagust, W. J., Shaw, L. M., Aisen, P. S., Weiner, M. W., et al. (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128. doi: 10.1016/s1474-4422(09)70299-6
Jack, C. R. Jr., Wiste, H. J., Weigand, S. D., Rocca, W. A., Knopman, D. S., Mielke, M. M., et al. (2014). Age-specific population frequencies of cerebral β-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 13, 997–1005. doi: 10.1016/S1474-4422(14)70194-2
Karp, A., Andel, R., Parker, M. G., Wang, H.-X., Winblad, B., and Fratiglioni, L. (2009). Mentally stimulating activities at work during midlife and dementia risk after age 75: follow-up study from the Kungsholmen Project. Am. J. Geriatr. Psychiatry 17, 227–236. doi: 10.1097/jgp.0b013e318190b691
Kivipelto, M., Rovio, S., Ngandu, T., Kåreholt, I., Eskelinen, M., Winblad, B., et al. (2008). Apolipoprotein E ε4 magnifies lifestyle risks for dementia: a population-based study. J. Cell. Mol. Med. 12, 2762–2771. doi: 10.1111/j.1582-4934.2008.00296.x
Ko, K., Byun, M. S., Yi, D., Lee, J. H., Kim, C. H., and Lee, D. Y. (2018). Early-life cognitive activity is related to reduced neurodegeneration in Alzheimer signature regions in late life. Front. Aging Neurosci. 10:70. doi: 10.3389/fnagi.2018.00070
Krell-Roesch, J., Feder, N. T., Roberts, R. O., Mielke, M. M., Christianson, T. J., Knopman, D. S., et al. (2018). Leisure-time physical activity and the risk of incident dementia: the mayo clinic study of aging. J. Alzheimers Dis. 63, 149–155. doi: 10.3233/jad-171141
Landau, S. M., Marks, S. M., Mormino, E. C., Rabinovici, G. D., Oh, H., O’Neil, J. P., et al. (2012). Association of lifetime cognitive engagement and low β-amyloid deposition. Arch. Neurol. 69, 623–629. doi: 10.1001/archneurol.2011.2748
Lee, J. H., Byun, M. S., Yi, D., Choe, Y. M., Choi, H. J., Baek, H., et al. (2017). Sex-specific association of sex hormones and gonadotropins, with brain amyloid and hippocampal neurodegeneration. Neurobiol. Aging 58, 34–40. doi: 10.1016/j.neurobiolaging.2017.06.005
Lee, D. Y., Lee, K. U., Lee, J. H., Kim, K. W., Jhoo, J. H., Kim, S. Y., et al. (2004). A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychol. Soc. 10, 72–81. doi: 10.1017/s1355617704101094
Leyhe, T., Müller, S., Milian, M., Eschweiler, G. W., and Saur, R. (2009). Impairment of episodic and semantic autobiographical memory in patients with mild cognitive impairment and early Alzheimer’s disease. Neuropsychologia 47, 2464–2469. doi: 10.1016/j.neuropsychologia.2009.04.018
Liang, K. Y., Mintun, M. A., Fagan, A. M., Goate, A. M., Bugg, J. M., Holtzman, D. M., et al. (2010). Exercise and Alzheimer’s disease biomarkers in cognitively normal older adults. Ann. Neurol. 68, 311–318. doi: 10.1002/ana.22096
Lowe, V. J., Weigand, S. D., Senjem, M. L., Vemuri, P., Jordan, L., Kantarci, K., et al. (2014). Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology 82, 1959–1967. doi: 10.1212/wnl.0000000000000467
Morris, J. C. (1993). The clinical dementia rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/wnl.43.11.2412-a
Morris, J. C., Roe, C. M., Xiong, C., Fagan, A. M., Goate, A. M., Holtzman, D. M., et al. (2010). APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann. Neurol. 67, 122–131. doi: 10.1002/ana.21843
Najar, J., Östling, S., Gudmundsson, P., Sundh, V., Johansson, L., Kern, S., et al. (2019). Cognitive and physical activity and dementia: a 44-year longitudinal population study of women. Neurology 92, e1322–e1330. doi: 10.1212/WNL.0000000000007021
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
O’Brien, J., O’Keefe, K., LaViolette, P., Deluca, A., Blacker, D., Dickerson, B., et al. (2010). Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74, 1969–1976. doi: 10.1212/wnl.0b013e3181e3966e
Park, J.-C., Han, S.-H., Cho, H. J., Byun, M. S., Yi, D., Choe, Y. M., et al. (2017). Chemically treated plasma Aβ is a potential blood-based biomarker for screening cerebral amyloid deposition. J. Alzheimers Res. Ther. 9:20. doi: 10.1186/s13195-017-0248-8
Reiman, E. M., Chen, K., Liu, X., Bandy, D., Yu, M., Lee, W., et al. (2009). Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. U S A 106, 6820–6825. doi: 10.1073/pnas.0900345106
Rovio, S., Kåreholt, I., Helkala, E.-L., Viitanen, M., Winblad, B., Tuomilehto, J., et al. (2005). Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurol. 4, 705–711. doi: 10.1016/S1474-4422(05)70198-8
Small, G. W., Mazziotta, J. C., Collins, M. T., Baxter, L. R., Phelps, M. E., Mandelkern, M. A., et al. (1995). Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA 273, 942–947. doi: 10.1001/jama.273.12.942
Tolppanen, A.-M., Solomon, A., Kulmala, J., Kåreholt, I., Ngandu, T., Rusanen, M., et al. (2015). Leisure-time physical activity from mid-to late life, body mass index and risk of dementia. Alzheimers Dement. 11, 434.e6–443.e6. doi: 10.1016/j.jalz.2014.01.008
Tuminello, E. R., and Han, S. D. (2011). The apolipoprotein e antagonistic pleiotropy hypothesis: review and recommendations. Int. J. Alzheimers Dis. 2011:726197. doi: 10.4061/2011/726197
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15, 273–289. doi: 10.1006/nimg.2001.0978
Valenzuela, M. J., Sachdev, P., Wen, W., Chen, X., and Brodaty, H. (2008). Lifespan mental activity predicts diminished rate of hippocampal atrophy. PLoS One 3:e2598. doi: 10.1371/journal.pone.0002598
van Praag, H. (2009). Exercise and the brain: something to chew on. Trends Neurosci. 32, 283–290. doi: 10.1016/j.tins.2008.12.007
Vemuri, P., Knopman, D. S., Lesnick, T. G., Przybelski, S. A., Mielke, M. M., Graff-Radford, J., et al. (2017). Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol. 74, 718–726. doi: 10.1001/jamaneurol.2017.0244
Vemuri, P., Lesnick, T. G., Przybelski, S. A., Knopman, D. S., Machulda, M., Lowe, V. J., et al. (2016). Effect of intellectual enrichment on AD biomarker trajectories Longitudinal imaging study. Neurology 86, 1128–1135. doi: 10.1212/wnl.0000000000002490
Vemuri, P., Lesnick, T. G., Przybelski, S. A., Knopman, D. S., Roberts, R. O., Lowe, V. J., et al. (2012). Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann. Neurol. 72, 730–738. doi: 10.1002/ana.23665
Villemagne, V. L., Burnham, S., Bourgeat, P., Brown, B., Ellis, K. A., Salvado, O., et al. (2013). Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort stud. Lancet. Neurol. 12, 357–367. doi: 10.1016/S1474-4422(13)70044-9
Willette, A. A., Bendlin, B. B., Starks, E. J., Birdsill, A. C., Johnson, S. C., Christian, B. T., et al. (2015). Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. 72, 1013–1020. doi: 10.1001/jamaneurol.2015.0613
Wilson, R. S., Barnes, L. L., Krueger, K. R., Hoganson, G., Bienias, J. L., and Bennett, D. A. (2005). Early and late life cognitive activity and cognitive systems in old age. J. Int. Neuropsychol. Soc. 11, 400–407. doi: 10.1017/s1355617705050459
Wilson, R. S., Bennett, D., Bienias, J., Mendes de Leon, C., Morris, M., and Evans, D. (2003). Cognitive activity and cognitive decline in a biracial community population. Neurology 61, 812–816. doi: 10.1212/01.wnl.0000083989.44027.05
Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Schneider, J. A., and Bennett, D. A. (2013). Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81, 314–321. doi: 10.1212/WNL.0b013e31829c5e8a
Wirth, M., Villeneuve, S., La Joie, R., Marks, S. M., and Jagust, W. J. (2014). Gene-environment interactions: lifetime cognitive activity, APOE genotype, and β-amyloid burden. J. Neurosci. 34, 8612–8617. doi: 10.1523/JNEUROSCI.4612-13.2014
Keywords: Alzheimer’s disease, APOE ε4, in vivo pathology, midlife, physical activity, cognitive activity
Citation: Jeon SY, Byun MS, Yi D, Lee J-H, Ko K, Sohn BK, Lee J-Y, Ryu S-H, Lee DW, Shin SA, Kim YK, Kang KM, Sohn C-H and Lee DY (2020) Midlife Lifestyle Activities Moderate APOE ε4 Effect on in vivo Alzheimer’s Disease Pathologies. Front. Aging Neurosci. 12:42. doi: 10.3389/fnagi.2020.00042
Received: 25 October 2019; Accepted: 07 February 2020;
Published: 27 February 2020.
Edited by:
Enrique Cadenas, University of Southern California, United StatesReviewed by:
Michael W. Jakowec, University of Southern California, United StatesBoon-Seng Wong, Singapore Institute of Technology, Singapore
Copyright © 2020 Jeon, Byun, Yi, Lee, Ko, Sohn, Lee, Ryu, Lee, Shin, Kim, Kang, Sohn and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Young Lee, c2VsZnBzeUBzbnUuYWMua3I=
† A complete list of the KBASE research group members can be found in http://kbase.kr/
 So Yeon Jeon
So Yeon Jeon Min Soo Byun
Min Soo Byun Dahyun Yi
Dahyun Yi Jun-Ho Lee
Jun-Ho Lee Kang Ko
Kang Ko Bo Kyung Sohn5
Bo Kyung Sohn5 Jun-Young Lee
Jun-Young Lee Chul-Ho Sohn
Chul-Ho Sohn Dong Young Lee
Dong Young Lee