
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 21 January 2020
Sec. Neurocognitive Aging and Behavior
Volume 11 - 2019 | https://doi.org/10.3389/fnagi.2019.00367
This article is part of the Research Topic Understanding Brain Aging View all 30 articles
 Cristina Udina1,2,3*
Cristina Udina1,2,3* Stella Avtzi4
Stella Avtzi4 Turgut Durduran4,5
Turgut Durduran4,5 Roee Holtzer6,7
Roee Holtzer6,7 Andrea L. Rosso8
Andrea L. Rosso8 Carmina Castellano-Tejedor1,2,3
Carmina Castellano-Tejedor1,2,3 Laura-Monica Perez1,2
Laura-Monica Perez1,2 Luis Soto-Bagaria1,2
Luis Soto-Bagaria1,2 Marco Inzitari1,2,3
Marco Inzitari1,2,3The integrity of the frontal areas of the brain, specifically the prefrontal cortex, are critical to preserve cognition and mobility in late life. Prefrontal cortex regions are involved in executive functions and gait control and have been related to the performance of dual-tasks. Dual-task performance assessment may help identify older adults at risk of negative health outcomes. As an alternative to neuroimaging techniques that do not allow assessment during actual motion, functional Near-Infrared Spectroscopy (fNIRS) is a non-invasive technique that can assess neural activation through the measurement of cortical oxygenated and deoxygenated hemoglobin levels, while the person is performing a motor task in a natural environment as well as during cognitive tasks. The aim of this review was to describe the use of fNIRS to study frontal lobe hemodynamics during cognitive, motor and dual-tasks in older adults. From the 46 included publications, 20 studies used only cognitive tasks, three studies used motor tasks and 23 used dual-tasks. Our findings suggest that fNIRS detects changes in frontal activation in older adults (cognitively healthy and mild cognitive impairment), especially while performing cognitive and dual-tasks. In both the comparison between older and younger adults, and in people with different neurological conditions, compared to healthier controls, the prefrontal cortex seems to experience a higher activation, which could be interpreted in the context of proposed neural inefficiency and limited capacity models. Further research is needed to establish standardized fNIRS protocols, study the cerebral hemodynamic in different neurological and systemic conditions that might influence cortical activation and explore its role in predicting incident health outcomes such as dementia.
The worldwide aging of the population makes tackling aging-associated disability an urgent priority. Cognitive impairment and mobility disability are key contributors to dementia and loss of independence in the activities of daily living and have a synergistic effect (Verghese et al., 2014). The integrity of the frontal areas of the brain, specifically the PFC, are critical to preserve cognition and mobility in late life (Beauchet et al., 2016). PFC regions carry out executive functions, i.e., higher order cognitive functions essential to plan and execute complex goal-directed actions, which are also key for motor control in older adults (Inzitari et al., 2007). The loss of integrity in frontal or prefrontal regions, either due to neurodegeneration, cerebrovascular disease or due to their interactions, contributes to the development of dementia (Burgmans et al., 2009; Kisler et al., 2017) and mobility impairments (De Laat et al., 2011). The PFC has also been implicated in performance of DT (Sala et al., 1995; Dux et al., 2006; Filmer et al., 2013), that are motor tasks performed simultaneously with a secondary, usually a cognitive task. DT increases the cognitive demand of walking and potentially results in a decrease in task performance in one or both tasks relative to when the tasks are performed separately as ST. DT performance assessment may help identify older adults at higher risk of incident cognitive decline (Ceïde et al., 2018; Rosso et al., 2019), disability, frailty and mortality (Verghese et al., 2012). One of the goals of the study of cognitive aging is to elucidate neural mechanisms that underlie the ability of the aging brain to cope with decline in cognitive functions and efficiency. Several hypothesis have been described and there is still no consensus regarding definitions of several concepts (Cabeza et al., 2018). Two of the previously described hyphotheses are: the “neural inefficiency hypothesis” (Rypma and D’Esposito, 2000; Holtzer et al., 2009) or “compensation by upregulation” (Cabeza et al., 2018), according to which older adults show increased activity of the same networks recruited by younger counterparts in order to meet behavioral demands, and the “capacity limitation hypothesis” (Cabeza, 2004; Holtzer et al., 2009) which postulates that older adults, while recruiting the same brain networks as young adults, would show a reduced activation compared to their younger counterparts (Holtzer et al., 2009; Stern, 2009).
Classic clinical and epidemiological studies have based their assessment of PFC on a static, structural basis, mainly through magnetic resonance imaging (MRI) techniques, which have shown a contribution of both cortical frontal and PFC volumes (Rosano et al., 2008; Weinstein et al., 2012) and subcortical alterations to executive dysfunction/dementia (Jokinen et al., 2009) and mobility limitations (Baezner et al., 2008). In addition, functional neuroimaging techniques, such as functional MRI (fMRI), allow the study of PFC by assessing the hemodynamic changes due to neurovascular coupling that are triggered by its neural activation (Buchbinder, 2016). fMRI studies assess whole brain function with a relatively high spatial resolution, are non-invasive and the most used technique to date to assess neural activity during specific task activation (Rosen and Savoy, 2012). Several fMRI studies have demonstrated the relevance of PFC for executive functions (Wager et al., 2004; Venkatraman et al., 2010; Yaple et al., 2019) and DT (Szameitat et al., 2002; Dux et al., 2006; Jurado and Rosselli, 2007). Limitations of both MRI and fMRI include their relatively high cost, unsuitability for many older adults due to metal implants in the body, claustrophobia or inability to lie still for long periods. Further, due to the nature of the scanner, the tasks are carried out in unnatural environments which may alter their relevance to the real-world and do not allow functional analysis of brain activity during locomotion. Imagined gait has been used as a way to study the neural correlates of locomotion with fMRI (Zwergal et al., 2012; Blumen et al., 2014); however, it is not entirely clear how well this mimics brain activation during actual walking. Other options, although they do not allow online assessment of gait either, include PET studies after walk trials with administration of fludeoxyglucose-18 tracer (la Fougère et al., 2010). We refer the reader to Holtzer et al. (2014) for a comprehensive review on neuroimaging of locomotion in aging.
Emerging alternatives to fMRI, based on near-infrared diffuse optical techniques, allow measurements in more realistic environments and during motion (Boas et al., 2014; Scholkmann et al., 2014). Accumulating evidence supports the use of these techniques for the study of frontal hemodynamic and metabolic changes (Agbangla et al., 2017; Gramigna et al., 2017). These diffuse optical techniques such as fNIRS (Durduran et al., 2010; Ferrari and Quaresima, 2012) allow the study of tissue composition by emitting near-infrared light (∼650–950 nm) into biological tissue and collecting the photons that undergo multiple scattering and absorption (i.e., diffuse) and emerge few centimeters away from the injection point (Delpy and Cope, 1997; Durduran et al., 2010). At these wavelengths the main absorbers in tissues, i.e., O2Hb and HHb, differentially absorb light in a wavelength dependent manner. Therefore, most common fNIRS methods can relate changes in the detected light intensity at different wavelengths to changes in oxygenated and deoxygenated hemoglobin concentrations by utilizing the modified Beer-Lambert law (Scholkmann et al., 2014). This is a signal similar to the blood oxygen level dependent (BOLD) signal from fMRI but can be obtained by portable (even wearable) instrumentation and flexible fiber-optic probes. The majority of the systems are using source and detector probes placed on the scalp of the head. The most common source-detector separations are of few centimeters. Able to detect signal coming from superficial cortical layers (Ferrari and Quaresima, 2012), fNIRS measurement is based on the neurovascular coupling (oxygen consumption to meet energy demands in activated cerebral areas cause an increase in blood flow resulting in an increase of O2Hb and decrease of HHb) and both the analysis and acquisition methods are still being developed with O2Hb changes appearing more reliable as a marker of brain activation since it has shown high reproducibility and stability over time (Plichta et al., 2006) and has the highest correlation to fMRI BOLD measures (Strangman et al., 2002). fNIRS studies usually consist of a combination of resting periods, to assess baseline brain activity, and different kinds of tasks. Brain activation is then calculated by comparing hemoglobin measurements at baseline and during the task, although there is a high heterogeneity in data processing and analysis methods. Regarding its advantages, fNIRS is a lower cost modality than fMRI, usable at point-of-care, and allows measurements during mobility tasks. These advantatges allow the potential use of the technique to assess cerebral blood flow and oxygenation with application in different pathologies (e.g., stroke, psychiatric disorders,…) resulting thus in continuous growth on relevant literature (Noda et al., 2017; Giacalone et al., 2019). However, the main limitations of most fNIRS devices include: (i) the limited penetration depth, allowing only the interrogation of superficial layers of the cortex in the adult brain, (ii) the assessment of a limited portion of the cortical surface with often a low spatial resolution with the probes that are attached to the scalp, not allowing complete whole-brain imaging, (iii) issues with extracerebral contamination from superficial tissues (i.e., cutaneous or skull perfusion) and (iv) motion artifacts.
Recent studies have expanded the use of fNIRS in the assessment of PFC of older adults during cognitive or motor tests (Verghese et al., 2017). These studies show changes in PFC hemodynamics during the execution of cognitive or motor tasks, and also report differences according to the person’s age and cognitive function. However, these findings are still preliminary and it is not yet clear if there is a specific pattern according to age or cognitive status, nor about how these differences should be interpreted. Recently published reviews have assessed the results of studies on fNIRS during cognitive tasks (Herold et al., 2018) or dual tasks (Gramigna et al., 2017; Herold et al., 2017; Leone et al., 2017; Vitorio et al., 2017; Stuart et al., 2018; Kahya et al., 2019) and some of them have chosen to focus on specific clinical profiles (Gramigna et al., 2017; Vitorio et al., 2017) or on methodological aspects such as fNIRS signal processing (Herold et al., 2017, 2018; Vitorio et al., 2017). To the best of our knowledge, our review is the first to focus specifically on older adults regardless of their clinical profile and to assess, from a clinical point of view, studies using only cognitive or motor tasks, as well as DTs.
The aim of this review is to describe, through an updated literature search, the use of optical techniques, specifically fNIRS, to study brain hemodynamics, with a focus on frontal regions, in relation to cognitive and physical function in normal and pathological older adult populations.
This is a narrative review. We performed, however, a search using pre-set criteria, to make sure that we considered all the relevant articles on the topic. We included manuscripts that have aimed to study frontal and prefrontal lobe hemodynamics (excluding those focusing on other brain regions) using fNIRS to measure oxygenated and deoxygenated hemoglobin levels during cognitive, motor and DTs in older adults. Articles were included if the mean age of the sample or a separately analyzed subgroup was 60 years or older. Review articles, studies assessing change in cerebral hemodynamics after an intervention, those not written in English and those that do not describe the age of the participants in the manuscript were excluded. In order to focus on most recent literature, we limited the publication date to the previous 5 years. The last search was performed on August 29th, 2018.
The article selection was performed in three phases (review of titles, abstracts, and full-texts). Two independent reviewers (CU and MI) reviewed the titles and abstracts resulting from the search, in order to assess potential inclusion. From the selected articles, we performed a full manuscript review to assess if the article met the eligibility criteria. Discrepancies were solved through consensus.
As depicted in the flow-chart (Figure 1), after removing duplicates, our search resulted in 134 items. After excluding records by title and abstract screening (n = 46), 89 full-text articles were assessed for eligibility. Studies not meeting the above described eligibility criteria such as sample/subgroup mean age (n = 19), the aim/topic focus of our review (n = 6) (i.e., use of NIRS to monitor cancer treatment), methodological aspects of the design of studies (n = 6) (i.e., different location of the probes or NIRS measures performed to assess the effect of an intervention) and review articles (n = 11), were excluded. We finally included 46 articles in our review.
Of the 46 included articles, 13 included a mix of younger and older participants (Heilbronner and Münte, 2013; Ohsugi et al., 2013; Beurskens et al., 2014; Müller et al., 2014; Oboshi et al., 2014; Hernandez et al., 2016; Bierre et al., 2017; Mirelman et al., 2017; Rosso et al., 2017; Hawkins et al., 2018) whereas 29 included only older adults (Doi et al., 2013; Heinzel et al., 2013, 2015; Niu et al., 2013; Clark et al., 2014; Vermeij et al., 2014; Dupuy et al., 2015; Holtzer et al., 2015, 2016, 2017a,b, 2018a,b; Laguë-Beauvais et al., 2015; Al-Yahya et al., 2016; Maidan et al., 2016, 2017; Mahoney et al., 2016; Nieuwhof et al., 2016; Osofundiya et al., 2016; Takeuchi et al., 2016; Uemura et al., 2016; Yeung et al., 2016a, b; Chen et al., 2017; Huppert et al., 2017; Verghese et al., 2017; Yap et al., 2017; Halliday et al., 2018; Katzorke et al., 2018; Lucas et al., 2018; Mori et al., 2018; Thumm et al., 2018). Moreover, 26 studies included only cognitively normal participants (Heilbronner and Münte, 2013; Heinzel et al., 2013, 2015; Ohsugi et al., 2013; Beurskens et al., 2014; Clark et al., 2014; Müller et al., 2014; Oboshi et al., 2014; Vermeij et al., 2014; Holtzer et al., 2015, 2016, 2017a,b, 2018a,b; Osofundiya et al., 2016; Bierre et al., 2017; Chen et al., 2017; Huppert et al., 2017; Mirelman et al., 2017; Rosso et al., 2017; Verghese et al., 2017; Halliday et al., 2018; Lucas et al., 2018), seven compared participants with different cognitive status [without cognitive impairment, with MCI or with mild AD] (Doi et al., 2013; Niu et al., 2013; Al-Yahya et al., 2016; Uemura et al., 2016; Yeung et al., 2016a, b; Yap et al., 2017; Katzorke et al., 2018), three studies focused on older adults with previous history of stroke (Al-Yahya et al., 2016; Hawkins et al., 2018; Mori et al., 2018), five assessed patients with parkinsonian syndromes (Mahoney et al., 2016; Maidan et al., 2016, 2017; Nieuwhof et al., 2016; Thumm et al., 2018) and two with Multiple Sclerosis (Hernandez et al., 2016; Chaparro et al., 2017).
Looking at the older adults subgroups that were included in the studies, there was a wide range of mean ages, from 61 ± 4 (Hernandez et al., 2016) to 88.1 ± 6 (Huppert et al., 2017). The largest sample size was 1052 participants (Heinzel et al., 2015) while a sample of 12 older adults was the smallest (Nieuwhof et al., 2016). Most source populations were community-dwelling but two studies included older adults living in nursing home (Osofundiya et al., 2016; Huppert et al., 2017). Ten studies did not describe the participant setting (Niu et al., 2013; Oboshi et al., 2014; Al-Yahya et al., 2016; Uemura et al., 2016; Maidan et al., 2017; Mirelman et al., 2017; Rosso et al., 2017; Katzorke et al., 2018; Mori et al., 2018; Thumm et al., 2018).
The majority, 29 studies, used O2Hb to assess brain activation while nine studies (Heilbronner and Münte, 2013; Beurskens et al., 2014; Müller et al., 2014; Al-Yahya et al., 2016; Hyodo et al., 2016; Nieuwhof et al., 2016; Rosso et al., 2017; Halliday et al., 2018; Katzorke et al., 2018) used both O2Hb and HHb and one used only Total Hb (Huppert et al., 2017). Two studies calculated the Total Oxygenation Index (O2Hb/Total Hb x 100) in order to assess brain hemodynamics (Clark et al., 2014; Bierre et al., 2017). In the following paragraphs, we will use the term activation to refer to changes in these hemoglobin indices.
Twenty-three studies measuring single cognitive or motor tasks performed intra-group comparisons of the cerebral activation during different tasks and the rest periods (see articles listed in Tables 1, 2A), whereas the other 23 studies compared cerebral hemodynamics between single and DT (see articles listed in Table 2B). Twenty-four studies performed comparisons of cerebral activation patterns between different groups (either young vs. old, MCI vs. cognitively normal, healthy vs. stroke etc.) (Heilbronner and Münte, 2013; Niu et al., 2013; Ohsugi et al., 2013; Beurskens et al., 2014; Müller et al., 2014; Oboshi et al., 2014; Laguë-Beauvais et al., 2015; Al-Yahya et al., 2016; Hernandez et al., 2016; Maidan et al., 2016, 2017; Mahoney et al., 2016; Osofundiya et al., 2016; Takeuchi et al., 2016; Uemura et al., 2016; Yeung et al., 2016a, b; Bierre et al., 2017; Mirelman et al., 2017; Rosso et al., 2017; Yap et al., 2017; Hawkins et al., 2018; Katzorke et al., 2018; Mori et al., 2018).
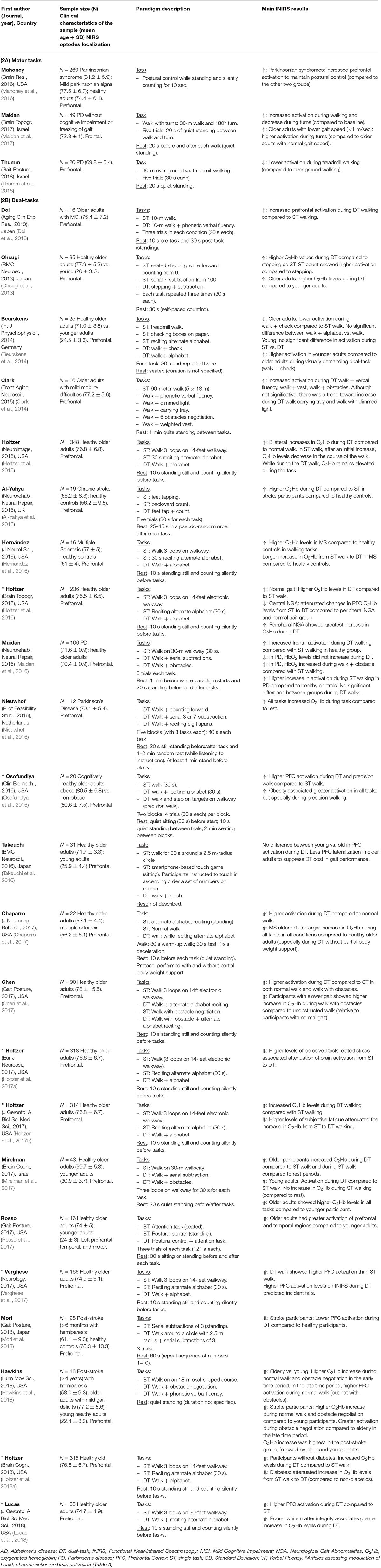
Table 2. Summary of the studies assessing fNIRS measures during motor and/or dual-tasks in older adults.
Some studies, beyond assessing frontal hemodynamics, investigated the influence of other clinical characteristics in the reported frontal activation findings (Albinet et al., 2014; Dupuy et al., 2015; Hyodo et al., 2016; Osofundiya et al., 2016; Holtzer et al., 2016, 2017a,b, 2018a; Verghese et al., 2017; Halliday et al., 2018; Lucas et al., 2018) (see Table 3).

Table 3. Studies assessing effect modification by different health characteristics on PFC activation.
We found 20 articles assessing cerebral activation during cognitive tasks (Table 1). The most frequent cognitive task was VF (Heinzel et al., 2013, 2015; Yeung et al., 2016a; Yap et al., 2017; Katzorke et al., 2018). Generally, VF tests ask the participants to produce the maximum number of words starting with a specific letter (phonemic) or belonging to a pre-specified semantic category (semantic). Three studies used N-back tests (Niu et al., 2013; Vermeij et al., 2014; Yeung et al., 2016b), which assess working memory function. N-back tasks are usually designed as conditions with increasing working-memory load: 0-back (subject has to detect if the presented stimulus is the one described as target), 1-back (the subject has to remember if the presented stimulus was presented on the previous position) and 2-back conditions (the participant must be able to remember if the stimulus is the same presented 2 positions before). From the twelve remaining studies, eleven used different tests of executive functions (i.e., Stroop, symbol digit coding and shifting attention test, Go/No go inhibition task, Trail Making Test part B, etc.) (Heilbronner and Münte, 2013; Albinet et al., 2014; Müller et al., 2014; Oboshi et al., 2014; Dupuy et al., 2015; Laguë-Beauvais et al., 2015; Hyodo et al., 2016; Bierre et al., 2017; Huppert et al., 2017; Halliday et al., 2017, 2018) and one used an episodic memory task (Uemura et al., 2016).
Regarding the studies that assessed frontal hemodynamics in cognitively healthy older adults, two studies by Heinzel et al. (2015) showed different activation patterns while performing VF tasks: one showed an increased activation and another found a decreased activation on bilateral inferior frontal junction in healthy older adults while middle frontal gyri and supramarginal gyri showed an increased activation (interpreted as compensatory mechanisms) (Heinzel et al., 2013). Cognitively healthy older adults showed an increased prefrontal activation while performing a working memory task with visual recognition (Oboshi et al., 2014) as well as with increasing working memory load during a N-back task (Vermeij et al., 2014). Studies using other executive function tests, found an increase in frontal lobe activation during executive function tasks (Heilbronner and Münte, 2013; Albinet et al., 2014; Müller et al., 2014; Bierre et al., 2017; Huppert et al., 2017). One study, instead of reporting only the mean values of O2Hb, addressed the association between O2Hb variability and behavioral results during an executive function task (Halliday et al., 2017). They reported that within-person O2Hb variability was associated with better accuracy and faster performance but between-person variability was associated with slower performance.
Healthy older adults showed higher frontal activation than younger persons while performing a visuomotor task with increasing executive function demand (Bierre et al., 2017). Moreover, a different activation pattern during executive function tests between age groups was observed. According to Heilbronner and Münte (2013), in older adults, activation shifted rostrally on the left hemisphere and dorsally on the right hemisphere during the inhibition task, while Müller et al. (2014) reported an additional activation in left medial and lateral PFC during the TMT-B (while more ventral activation was evidenced in younger counterparts). The effect of prioritization of a stimulus was assessed in one study (Laguë-Beauvais et al., 2015) where the participants were asked to prioritize one of two stimuli displayed (priority block) or to give the same priority to both stimuli (equal block). A change in the activation pattern between the priority and equal conditions was found only in the older adults group, with a less lateralized pattern (bilateral dorsolateral PFC activation) when not prioritizing either stimuli.
Regarding the studies assessing older adults with different cognitive status, three studies using VF tasks reported an increased activation during the task in MCI (Yeung et al., 2016a; Yap et al., 2017) and mild AD (Yap et al., 2017) while Katzorke et al. (2018) found a decreased activation during VF in MCI patients. Yap et al. (2017) compared the activation pattern in cognitively healthy older adults, MCI and mild AD and found the highest O2Hb increase in MCI older adults followed by healthy and AD participants, although the difference was not statistically significant. Increasing working memory load led to lower frontal lobe activation during a N-back task in MCI, compared to healthy controls (Niu et al., 2013; Yeung et al., 2016b). Only one study measured PFC activation during encoding and retrieval of episodic memory, and it found a decreased activation on bilateral dorsolateral cortex during memory retrieval in amnestic MCI (Uemura et al., 2016).
All the studies that used isolated motor tasks in order to assess PFC hemodynamics (n = 3) enrolled older adults with parkinsonian syndromes but were heterogeneous regarding the motor tasks paradigm (Table 2A). The reported results were also heterogeneous. According to Mahoney et al. (2016), older adults with parkinsonian syndromes showed higher PFC activation while performing a postural control task (compared to participants with mild parkinsonian signs or without these). Participants with PD walking on a straight walkway showed an increased PFC activation, compared to the baseline, and a decrease when performing 180° turns (Maidan et al., 2017). However, when comparing older adults with different gait speed, participants with gait speed lower than 1m/sec showed higher activation during turns, compared to those with normal gait speed. Thumm et al. reported lower O2Hb levels while walking on a treadmill vs. over-ground walking in PD participants (Thumm et al., 2018).
Twenty-three articles assessed PFC hemodynamics while performing DT (Table 2B). Studies included in this review used walking (Doi et al., 2013; Beurskens et al., 2014; Clark et al., 2014; Holtzer et al., 2015, 2016, 2017a,b, 2018a,b; Maidan et al., 2016; Nieuwhof et al., 2016; Takeuchi et al., 2016; Chaparro et al., 2017; Chen et al., 2017; Mirelman et al., 2017; Verghese et al., 2017; Hawkins et al., 2018; Lucas et al., 2018; Mori et al., 2018), feet tapping (Al-Yahya et al., 2016), stepping (Ohsugi et al., 2013) and postural control (Rosso et al., 2017) as the motor task and VF (Doi et al., 2013; Clark et al., 2014; Hawkins et al., 2018), calculation (Ohsugi et al., 2013; Al-Yahya et al., 2016; Maidan et al., 2016; Nieuwhof et al., 2016; Mirelman et al., 2017; Mori et al., 2018), alphabet (Beurskens et al., 2014; Holtzer et al., 2015, 2016, 2017a,b, 2018; Chaparro et al., 2017; Chen et al., 2017; Verghese et al., 2017; Lucas et al., 2018), digit span (Nieuwhof et al., 2016), visual (Beurskens et al., 2014) or attention (Takeuchi et al., 2016; Rosso et al., 2017) tasks as the added cognitive tasks. Other studies used challenging factors while walking such as obstacle negotiation or carrying a tray as the secondary task to assess DT performance (Clark et al., 2014; Maidan et al., 2016; Osofundiya et al., 2016; Chen et al., 2017; Mirelman et al., 2017; Hawkins et al., 2018).
The vast majority of studies reported an increase in PFC activation in cognitively healthy older adults while performing several types of DT compared to a ST (Ohsugi et al., 2013; Clark et al., 2014; Holtzer et al., 2015, 2017a,b; Maidan et al., 2016; Osofundiya et al., 2016; Chen et al., 2017; Mirelman et al., 2017; Verghese et al., 2017; Lucas et al., 2018). Only one article reported lower O2Hb levels during walking while performing a visual check task compared to ST walk in the older adults group (Beurskens et al., 2014).
Older older adults showed higher PFC activation during DT in most studies, compared to younger participants (Ohsugi et al., 2013; Mirelman et al., 2017; Rosso et al., 2017; Hawkins et al., 2018). Only one study reported lower activation in older adults, compared to younger older adults, during a walk and visual check DT (Beurskens et al., 2014) and Takeuchi et al. (2016) did not find significant differences between age groups.
The effect of dual tasking in older adults with MCI was assessed in one of the included studies, which found an increased activation during DT compared to ST walking (Doi et al., 2013). Frontal hemodynamics has also been studied in stroke patients, although these studies included participants with heterogeneous clinical characteristics (mainly the time after the stroke event) and DT paradigms (i.e., Task protocols). Compared to healthy controls, patients with stroke history showed higher activation during counting while feet tapping (Al-Yahya et al., 2016) but a lower activation during counting while walking in another study (Mori et al., 2018). Walking while negotiating obstacles caused a higher activation in stroke patients compared to younger adults (Hawkins et al., 2018). PD patients show an increase in frontal activation during DT that involve walking and counting or reciting digit spans compared to the resting baseline periods (Nieuwhof et al., 2016). Middle-aged Multiple Sclerosis older adults show increased PFC activation during ST and DT walking and larger increases in O2Hb levels from ST to DT when compared to healthy older adults (Hernandez et al., 2016; Chaparro et al., 2017). Multiple Sclerosis participants show an especially larger increase in activation (compared to healthy counterparts) when not provided with partial body weight support (Chaparro et al., 2017).
Other studies assessed how different variables modulate the PFC activation during cognitive, motor tasks and DT (Table 3). Publications from the “Central Control of Mobility in Aging” (CCMA) study, including community-dwelling older adults without dementia, found that activation of PFC during DT, compared to ST, was lower in participants with central NGA compared to peripheral NGA or with normal gait. In fact, the highest O2Hb increase during DT was showed by participants with peripheral NGA (Holtzer et al., 2016). Also in participants from the CCMA study, higher levels of self-perceived stress and fatigue were associated with attenuation of brain activation patterns (lower increase in O2Hb levels from ST to DT walking) (Holtzer et al., 2017a, b). Participants with diabetes from the same study showed lower PFC activation during DT, compared to non-diabetics (Holtzer et al., 2018a), while obese cognitively healthy older adults from a different study showed higher activation, especially during a precision walking task, compared to non-obese counterparts (Osofundiya et al., 2016). When combining fNIRS with cerebral microstructural white matter integrity assessment, using MRI with Diffusion Tensor Imaging (DTI), altered white matter integrity was associated to higher O2Hb levels during DT walk compared to normal walk in the CCMA study (Lucas et al., 2018). Using data from the same study, Verghese et al. (2017) revealed higher risk of incident falls in older adults with higher levels of PFC activation during DT. It is important to note that this is the only article included in our review that assessed the relationship between PFC hemodynamic and outcomes in a longitudinal manner. Furthermore, in a separate sample, fallers compared to non-fallers (history of falls in the previous 2 years) had higher activation while performing executive function tasks (Halliday et al., 2018). The effect of fitness level on frontal activation during executive functions tasks among cognitively healthy participants was addressed in three studies. Although they assessed the level of fitness with different instruments, it seems that higher levels of fitness might produce larger increases in prefrontal activation (Albinet et al., 2014). Two of these studies used two different versions of modified Stroop tasks and while one found a more left-lateralized activation in the high-fit participants (Hyodo et al., 2016), the other study found an increased activation in right inferior frontal gyrus in the high-fit group (Dupuy et al., 2015).
Our review identified 46 articles that reported the assessment of frontal and PFC hemodynamics in older adults using fNIRS during cognitive, motor and DTs.
This has revealed a quite homogeneous pattern of activation of the PFC in cognitively healthy older adults during cognitive and DTs compared to rest and to single-task conditions, respectively. This supports the use of fNIRS investigations to detect changes in frontal hemodynamics in older adults.
Cognitively healthy older adults, compared to younger ones, show a higher activation during executive function tasks and DTs (Ohsugi et al., 2013; Bierre et al., 2017; Mirelman et al., 2017; Rosso et al., 2017; Hawkins et al., 2018). However, one study reported lower activation during walking while performing a visual check task compared to ST walk in the older adults group and compared to the younger group (Beurskens et al., 2014). The results in older adults with various degrees of cognitive impairment are more heterogeneous. Overall, MCI older adults show increased PFC activation during VF tasks (Yeung et al., 2016a; Yap et al., 2017) and during DT compared to ST (Doi et al., 2013). However, gradually increasing working memory load causes a lower activation compared to healthy controls (Niu et al., 2013; Yeung et al., 2016b).
These findings are in line with previously proposed hypotheses, such as the “neural inefficiency theory” (Rypma and D’Esposito, 2000; Holtzer et al., 2009), according to which older adults show increased activity of the same networks recruited by younger counterparts in order to meet behavioral demands. On the other hand, the lower activation in the healthy old subgroup relative to younger adults could be interpreted as an inability to meet the increased cognitive demands during the more complex DT (Beurskens et al., 2014) and is supported by the “capacity limitation hypothesis” (Cabeza, 2004; Holtzer et al., 2009). This theory might also explain the decrease in activation in MCI older adults with increasing working memory load (Niu et al., 2013; Yeung et al., 2016b). Importantly, neural inefficiency and capacity limitation theories are not mutually exclusive and likely both play a role in determining activation levels.
Regarding the studies focusing on older adults with other specific diseases, the findings support an activation of PFC during gait as ST (Maidan et al., 2017) and DT (Nieuwhof et al., 2016) in adults with PD (compared to rest periods). The only study that assessed PFC during postural control found a higher activation in participants with parkinsonian syndromes relative to healthier controls (Mahoney et al., 2016). This could be interpreted in the context of the neural inefficiency theory, where adults with impaired postural mechanisms as seen in PD (Baltadjieva et al., 2006; Benítez-Rivero et al., 2013), need a higher PFC activation to maintain postural control. Similar results, of higher activation than healthy controls, were obtained in Multiple Sclerosis participants (Hernandez et al., 2016; Chaparro et al., 2017) whereas stroke patients reported more heterogeneous results. This might be due to different clinical characteristics of the samples and of the DT paradigms (Al-Yahya et al., 2016; Hawkins et al., 2018; Mori et al., 2018).
Studies that investigated the effect of several clinical variables on the PFC activation during DT found a higher activation in participants with peripheral NGA, lower stress and fatigue levels, obesity, non-diabetics and altered white matter integrity in MRI. The only study that assessed prediction of longitudinal outcomes of frontal hemodynamics, found a higher risk of falls associated with higher PFC activation. However, most of these findings come from a single sample. According to the results from three studies, higher levels of fitness might produce larger increases in prefrontal activation during executive functions tasks in healthy older adults (Albinet et al., 2014; Dupuy et al., 2015; Hyodo et al., 2016).
Overall, our findings suggest that fNIRS studies are able to detect changes in frontal and PFC activation in older adults (both cognitively healthy and MCI), especially while performing cognitive and DTs that are believed to engage the frontal areas of the brain. In particular, in both the comparison between older and younger adults, and in people with different neurological conditions, compared to healthier controls, the PFC seems to experience a higher activation, which could be interpreted in the context of proposed neural inefficiency and limited capacity models.
Main limitations of the fNIRS technique arise either due to physical or technological constraints of the setups, due to analysis methods, or due to the nature of the study itself. It is well known that the recorded signal contains information not only from brain activation due to a specific stimulus or task but is also affected by extra-cerebral (skull and scalp perfusion) as well as systemic parameters (heart and respiratory rate, blood pressure, Mayer waves). Nowadays, the fNIRS community has made not only technological improvements but also has developed an abundance of methods to attempt to overcome the abovementioned limitations (Tachtsidis and Scholkmann, 2016). Current instrumentation provides the ability of using multiple source detector pairs that cover a wide range of tissue penetration depth, giving the possibility to record short channel preparation and regress out signal coming from superficial tissue layers when using continuous wave light sources (Yücel et al., 2015). On the other hand, emerging methods that employ pulsed light sources [time-resolved NIRS (TRS)], allow for the possibility to discriminate between intra- and extra cerebral signals (Torricelli et al., 2014). These methods were prohibitively complex but have recently begun to become practical (Pifferi et al., 2016). In this context, to cover a large imaging area, multiple channels can be used in combination with MRI, thus overcoming the lack of anatomical information and allow for localization of the origin of NIRS signal (Okamoto et al., 2004). Another technical limitation, could originate from the differential path length factor (DPF), used in modified Beer-Lambert law (Cope and Delpy, 1988), that could lead in cross-talk between oxygenated and deoxygenated hemoglobin measurements and false calculations (Hoshi, 2007). Regarding the analysis methods of the acquired fNIRS signal, to date, there is no standard method established (Pfeifer et al., 2018). Some of the most common strategies include the use of low-pass filters to remove heart rate or instrumental noise and high pass filters to extract low frequency systemic noise. Signal analysis methods are also heterogeneous in the current literature (Kirilina et al., 2012; Zhang et al., 2016). Furthermore, in functional studies and especially in motor and DT, motion artifacts play an important role, therefore, motion correction processes are widely used, covering a wide range of proposed methods (Wavelet filtering, Kalman filtering, spline interpolation, etc.) (Cooper et al., 2012; Brigadoi et al., 2014). In general, for more accurate results when designing an fNIRS experimental protocol or analysis method, it is crucial to take into account, potential particularities that each studied population might have.
The heterogeneity in task protocols, methodology and small sample sizes in most of the included articles may limit the interpretation of the findings, although the studies with larger samples show promising results in similar directions. The great majority of the reviewed articles measured activation only over frontal areas, avoiding the assessment of possible compensatory activations in distant areas of the brain (Stern, 2005; Holtzer et al., 2009). This may be mainly due to the simplicity of the application over hairless areas and can be overcome with better probe designs.
Furthermore, differences in cerebral activation patterns detected by fNIRS could be actually related to structural alterations, as recently reported in an MRI-fNIRS study (Wagshul et al., 2019) where higher activation in healthy older adults during DT was related to reduced cortical volumes, especially in bilateral superior and rostral-middle frontal cortex. More evidence is needed supporting this concept.
Other gaps and limitations might limit the generalizability of the results produced by the studies published to date. Regarding studies reporting the results of the motor task alone (not as dual task), the studies are limited to older adults with Parkinson syndromes. The samples are also very heterogeneous regarding the mean age ranges and other clinical characteristics. In most of the included studies, inclusion criteria take into account age and cognitive function, but individuals of the samples or within the comparison groups might be heterogeneous regarding aspects which might affect the cerebral neurovascular coupling and metabolism, such as cardiovascular risk load, atherosclerosis, small vessels disease etc.
Our work is not exempt from limitations. In particular, the non-systematic search strategy might lead to possible missing relevant published literature on the topic. However, we consider our pre-defined search strategy sufficiently comprehensive to include the most if not all relevant ones.
Our findings support the potential role of fNIRS in research and clinical practice to study cognition and mobility in aging. As mentioned, fNIRS is a non-invasive technique, which can assess brain regions involved in executive functions, which are key to goal-oriented behaviors and preserved cognitive and motor functions. In particular, fNIRS allows to obtain relevant information regarding neural activation while the person is performing a real motor task in a natural environment, in a relatively inexpensive way. However, further research is needed to confirm those findings and to establish standardized protocols (for tasks protocols and fNIRS data acquisition and processing). Further research should also focus more on cerebral hemodynamic in different neurological diseases and on the influence of systemic conditions (e.g., vascular risk factors such as diabetes and hypertension) on brain activation patterns as assessed with fNIRS. Furthermore, fNIRS-derived brain activation patterns can be utilized as predictors of incident health outcomes including but not limited to dementia.
A recent study demonstrated that within session training resulted in improved DT walking that was coupled with reduced activation in the PFC among healthy older adults suggesting improved neural efficiency due to practice (Holtzer et al., 2018b). Moreover, the presence of fear of falling delayed practice-related improvements in PFC efficiency during DT walking (Holtzer et al., 2019). These findings suggest that fNIRS can be used to quantify neuroplasticity, monitor improvement in PFC efficiency due to practice and detect the effect of clinically relevant variables such as fear of falling on brain function and efficiency during active walking. Hence, it is appropriate to consider the inclusion of fNIRS at least as a secondary outcome measure in clinical trials designed to assess the effect of treatment on brain neuroplasticity and efficiency as well as for the development and monitoring of rehabilitation/training programs.
In conclusion, our review supports the use of fNIRS as a neuroimaging technique to study changes in the hemodynamic response in the frontal cortex during cognitively demanding tasks and during active walking under single and DT conditions in older adults. From a pathophysiological perspective this approach might help characterize the evolution of functional impairments in different neurological diseases in older adults as well as in healthy aging.
CU and MI designed the concept of this manuscript, reviewed abstracts and full-text publications to assess eligibility, and wrote the first draft of the manuscript. CU extracted the relevant data from the included publications to write the manuscript. SA, TD, RH, AR, CC-T, L-MP, and LS-B contributed in the redaction and revision of the manuscript.
This work was partially supported through the MEDPHOTAGE project (DTS16/00099) which was supported by the “Instituto de Salud Carlos III” (ISCIII) and “Fondo Europeo de Desarrollo Regional (FEDER)” through the “Desarrollo Tecnológico en Salud” (DTS) program (Spain).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
AD, Alzheimer’s disease; DT, dual-task; fNIRS, Functional Near-Infrared Spectroscopy; HHb, deoxygenated hemoglobin; MCI, mild cognitive impairment; NGA, Neurological Gait Abnormalities; O2Hb, oxygenated hemoglobin; PD, Parkinson’s disease; PFC, prefrontal cortex; ST, single task; VF, verbal fluency.
Agbangla, N. F., Audiffren, M., and Albinet, C. T. (2017). Use of near-infrared spectroscopy in the investigation of brain activation during cognitive aging: a systematic review of an emerging area of research. Ageing Res. Rev. 38, 52–66. doi: 10.1016/j.arr.2017.07.003
Albinet, C. T., Mandrick, K., Bernard, P. L., Perrey, S., and Blain, H. (2014). Improved cerebral oxygenation response and executive performance as a function of cardiorespiratory fitness in older women: a FNIRS study. Front. Aging Neurosci. 6:272. doi: 10.3389/fnagi.2014.00272
Al-Yahya, E., Johansen-Berg, H., Kischka, U., Zarei, M., Cockburn, J., and Dawes, H. (2016). Prefrontal cortex activation while walking under dual-task conditions in stroke. Neurorehabil. Neural Repair 30, 591–599. doi: 10.1177/1545968315613864
Baezner, H., Blahak, C., Poggesi, A., Pantoni, L., Inzitari, D., Chabriat, H., et al. (2008). Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology 70, 935–942. doi: 10.1212/01.wnl.0000305959.46197.e6
Baltadjieva, R., Giladi, N., Gruendlinger, L., Peretz, C., and Hausdorff, J. M. (2006). Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur. J. Neurosci. 24, 1815–1820. doi: 10.1111/j.1460-9568.2006.05033.x
Beauchet, O., Allali, G., Annweiler, C., and Verghese, J. (2016). Association of motoric cognitive risk syndrome with brain volumes: results from the GAIT study. J. Gerontol. A Biol. Sci. Med. Sci. 71, 1081–1088. doi: 10.1093/gerona/glw012
Benítez-Rivero, S., Marín-Oyaga, V. A., García-Solís, D., Huertas-Fernández, I., García-Gómez, F. J., Jesús, S., et al. (2013). Clinical features and 123 I-FP-CIT SPECT imaging in vascular parkinsonism and Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 84, 122–129. doi: 10.1136/jnnp-2012-302618
Beurskens, R., Helmich, I., Rein, R., and Bock, O. (2014). Age-related changes in prefrontal activity during walking in dual-task situations: a FNIRS study. Int. J. Psychophysiol. 92, 122–128. doi: 10.1016/j.ijpsycho.2014.03.005
Bierre, K. L., Lucas, S. J. E., Guiney, H., Cotter, J. D., and Machado, L. (2017). Cognitive difficulty intensifies age-related changes in anterior frontal hemodynamics: novel evidence from near-infrared spectroscopy. J. Gerontol. A Biol. Sci. Med. Sci. 72, 181–188. doi: 10.1093/gerona/glw061
Blumen, H. M., Holtzer, R., Brown, L. L., Gazes, Y., and Verghese, J. (2014). Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum. Brain Mapp. 35, 4090–4104. doi: 10.1002/hbm.22461
Boas, D. A., Elwell, C. E., Ferrari, M., and Taga, G. (2014). Twenty years of functional near-infrared spectroscopy: introduction for the special issue. Neuroimage 85, 1–5. doi: 10.1016/j.neuroimage.2013.11.033
Brigadoi, S., Ceccherini, L., Cutini, S., Scarpa, F., Scatturin, P., Selb, J., et al. (2014). Motion artifacts in functional near-infrared spectroscopy: a comparison of motion correction techniques applied to real cognitive data. Neuroimage 85, 181–191. doi: 10.1016/j.neuroimage.2013.04.082
Buchbinder, B. R. (2016). Functional Magnetic Resonance Imaging. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms, 1st Edn. Amsterdam: Elsevier.
Burgmans, S., van Boxtel, M. P. J., Smeets, F., Vuurman, E. F. P. M., Gronenschild, E. H. B. M., Verhey, F. R. J., et al. (2009). Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiol. Aging 30, 1413–1419. doi: 10.1016/j.neurobiolaging.2007.11.028
Cabeza, R. (2004). Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex 14, 364–375. doi: 10.1093/cercor/bhg133
Cabeza, R., Albert, M., Belleville, S., Craik, F. I. M., Duarte, A., Grady, C. L., et al. (2018). Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 19, 701–710. doi: 10.1038/s41583-018-0068-2
Ceïde, M. E., Ayers, E. I., Lipton, R., and Verghese, J. (2018). Walking while talking and risk of incident dementia. Am. J. Geriatr. Psychiatry 26, 580–588. doi: 10.1016/j.jagp.2017.12.009
Chaparro, G., Balto, J. M., Sandroff, B. M., Holtzer, R., Izzetoglu, M., Motl, R. W., et al. (2017). Frontal brain activation changes due to dual-tasking under partial body weight support conditions in older adults with multiple sclerosis. J. Neuroeng. Rehabil. 14, 1–10. doi: 10.1186/s12984-017-0280-8
Chen, M., Pillemer, S., England, S., Izzetoglu, M., Mahoney, J. R., and Holtzer, R. (2017). Neural correlates of obstacle negotiation in older adults: an FNIRS study. Gait Posture 58, 130–135. doi: 10.1016/j.gaitpost.2017.07.043
Clark, D. J., Rose, D. K., Ring, S. A., and Porges, E. C. (2014). Utilization of central nervous system resources for preparation and performance of complex walking tasks in older adults. Front. Aging Neurosci. 6:217. doi: 10.3389/fnagi.2014.00217
Cooper, R. J., Selb, J., Gagnon, L., Phillip, D., Schytz, H. W., Iversen, H. K., et al. (2012). A systematic comparison of motion artifact correction techniques for functional near-infrared spectroscopy. Front. Neurosci. 6:147. doi: 10.3389/fnins.2012.00147
Cope, M., and Delpy, D. T. (1988). System for long-term measurement of cerebral blood and tissue oxygenation on newborn infants by near infra-red transillumination. Med. Biol. Eng. Comput. 26, 289–294. doi: 10.1007/BF02447083
De Laat, K. F., Tuladhar, A. M., Van Norden, A. G. W., Norris, D. G., Zwiers, M. P., and De Leeuw, F. E. (2011). Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 134, 73–83. doi: 10.1093/brain/awq343
Delpy, D. T., and Cope, M. (1997). Quantification in tissue near–infrared spectroscopy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352, 649–659. doi: 10.1098/rstb.1997.0046
Doi, T., Makizako, H., Shimada, H., Park, H., Tsutsumimoto, K., Uemura, K., et al. (2013). Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a FNIRS study. Aging Clin. Exp. Res. 25, 539–544. doi: 10.1007/s40520-013-0119-5
Dupuy, O., Gauthier, C. J., Fraser, S. A., Desjardins-Crèpeau, L., Desjardins, M., Mekary, S., et al. (2015). Higher levels of cardiovascular fitness are associated with better executive function and prefrontal oxygenation in younger and older women. Front. Hum. Neurosci. 9:66. doi: 10.3389/fnhum.2015.00066
Durduran, T., Choe, R., Baker, W. B., and Yodh, A. G. (2010). Diffuse optics for tissue monitoring and tomography. Rep. Prog. Phys. 73:076701. doi: 10.1088/0034-4885/73/7/076701
Dux, P. E., Ivanoff, J., Asplund, C. L., and Marois, R. (2006). Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron 52, 1109–1120. doi: 10.1016/j.neuron.2006.11.009
Ferrari, M., and Quaresima, V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fnirs) development and fields of application. Neuroimage 63, 921–935. doi: 10.1016/j.neuroimage.2012.03.049
Filmer, H. L., Mattingley, J. B., and Dux, P. E. (2013). Improved multitasking following prefrontal TDCS. Cortex 49, 2845–2852. doi: 10.1016/j.cortex.2013.08.015
Giacalone, G., Zanoletti, M., Re, R., Germinario, B., Contini, D., Spinelli, L., et al. (2019). Time-Domain Near-Infrared Spectroscopy In Acute Ischemic Stroke Patients. Neurophotonics 6:015003. doi: 10.1117/1.NPh.6.1.015003
Gramigna, V., Pellegrino, G., Cerasa, A., Cutini, S., Vasta, R., Olivadese, G., et al. (2017). Near-infrared spectroscopy in gait disorders: is it time to begin? Neurorehabil. Neural Repair 31, 402–412. doi: 10.1177/1545968317693304
Halliday, D. W. R., Hundza, S. R., Garcia-Barrera, M. A., Klimstra, M., Commandeur, D., Lukyn, T. W., et al. (2018). Comparing executive function, evoked hemodynamic response, and gait as predictors of variations in mobility for older adults. J. Clin. Exp. Neuropsychol. 40, 151–160. doi: 10.1080/13803395.2017.1325453
Halliday, D. W. R., Mulligan, B. P., Garrett, D. D., Schmidt, S., Hundza, S. R., Garcia-Barrera, M. A., et al. (2017). Mean and variability in functional brain activations differentially predict executive function in older adults: an investigation employing functional near-infrared spectroscopy. Neurophotonics 5:011013. doi: 10.1117/1.nph.5.1.011013
Hawkins, K. A., Fox, E. J., Daly, J. J., Rose, D. K., Christou, E. A., and McGuirk, T. E. (2018). Prefrontal over-activation during walking in people with mobility deficits: interpretation and functional implications. Hum. Mov. Sci. 59, 46–55. doi: 10.1016/j.humov.2018.03.010
Heilbronner, U., and Münte, T. F. (2013). Rapid event-related near-infrared spectroscopy detects age-related qualitative changes in the neural correlates of response inhibition. Neuroimage 65, 408–415. doi: 10.1016/j.neuroimage.2012.09.066
Heinzel, S., Metzger, F. G., Ehlis, A. C., Korell, R., Alboji, A., Haeussinger, F. B., et al. (2013). Aging-related cortical reorganization of verbal fluency processing: a functional near-infrared spectroscopy study. Neurobiol. Aging 34, 439–450. doi: 10.1016/j.neurobiolaging.2012.05.021
Heinzel, S., Metzger, F. G., Ehlis, A. C., Korell, R., Alboji, A., Haeussinger, F. B., et al. (2015). Age and vascular burden determinants of cortical hemodynamics underlying verbal fluency. PLoS One 10:e0138863. doi: 10.1371/journal.pone.0138863
Hernandez, M. E., Holtzer, R., Chaparro, G., Jean, K., Balto, J. M., Sandroff, B. M., et al. (2016). Brain activation changes during locomotion in middle-aged to older adults with multiple sclerosis. J. Neurol. Sci. 370, 277–283. doi: 10.1016/j.jns.2016.10.002
Herold, F., Wiegel, P., Scholkmann, F., and Müller, N. (2018). Applications of functional near-infrared spectroscopy (FNIRS) neuroimaging in exercise–cognition science: a systematic, methodology-focused review. J. Clin. Med. 7:466. doi: 10.3390/jcm7120466
Herold, F., Wiegel, P., Scholkmann, F., Thiers, A., Hamacher, D., and Schega, L. (2017). Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics 4:041403. doi: 10.1117/1.nph.4.4.041403
Holtzer, R., Epstein, N., Mahoney, J. R., Izzetoglu, M., and Blumen, H. M. (2014). Neuroimaging of mobility in aging: a targeted review. J. Gerontol. A Biol. Sci. Med. Sci. 69, 1375–1388. doi: 10.1093/gerona/glu052
Holtzer, R., George, C. J., Izzetoglu, M., and Wang, C. (2018a). The effect of diabetes on prefrontal cortex activation patterns during active walking in older adults. Brain Cogn. 125, 14–22. doi: 10.1016/j.bandc.2018.03.002
Holtzer, R., Izzetoglu, M., Chen, M., and Wang, C. (2018b). Distinct FNIRS-Derived HbO2 trajectories during the course and over repeated walking trials under single- and dual-task conditions: implications for within session learning and prefrontal cortex efficiency in older adults. J. Gerontol. A 74, 1076–1083. doi: 10.1093/gerona/gly181
Holtzer, R., Kraut, R., Izzetoglu, M., and Ye, K. (2019). The effect of fear of falling on prefrontal cortex activation and efficiency during walking in older adults. Geroscience 41, 89–100. doi: 10.1007/s11357-019-00056-4
Holtzer, R., Mahoney, J. R., Izzetoglu, M., Wang, C., England, S., and Verghese, J. (2015). Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage 112, 152–159. doi: 10.1016/j.neuroimage.2015.03.002
Holtzer, R., Rakitin, B. C., Steffener, J., Flynn, J., Kumar, A., and Stern, Y. (2009). Age effects on load-dependent brain activations in working memory for novel material. Brain Res. 1249, 148–161. doi: 10.1016/j.brainres.2008.10.009
Holtzer, R., Schoen, C., Demetriou, E., Mahoney, J. R., Izzetoglu, M., Wang, C., et al. (2017a). Stress and gender effects on prefrontal cortex oxygenation levels assessed during single and dual-task walking conditions. Eur. J. Neurosci. 45, 660–670. doi: 10.1111/ejn.13518
Holtzer, R., Verghese, J., Allali, G., Izzetoglu, M., Wang, C., and Mahoney, J. R. (2016). Neurological gait abnormalities moderate the functional brain signature of the posture first hypothesis. Brain Topogr. 29, 334–343. doi: 10.1007/s10548-015-0465-z
Holtzer, R., Yuan, J., Verghese, J., Mahoney, J. R., Izzetoglu, M., and Wang, C. (2017b). Interactions of subjective and objective measures of fatigue defined in the context of brain control of locomotion. J. Gerontol. A Biol. Sci. Med. Sci. 72, 417–423. doi: 10.1093/gerona/glw167
Hoshi, Y. (2007). Functional near-infrared spectroscopy: current status and future prospects. J. Biomed. Optics 12:062106. doi: 10.1117/1.2804911
Huppert, T. J., Karim, H., Lin, C., Alqahtani, B. A., Greenspan, S. L., and Sparto, P. J. (2017). Functional imaging of cognition in an old-old population: a case for portable functional near-infrared spectroscopy. PLoS One 12:e0184918. doi: 10.1371/journal.pone.0184918
Hyodo, K., Dan, I., Kyutoku, Y., Suwabe, K., Byun, K., Ochi, G., et al. (2016). The association between aerobic fitness and cognitive function in older men mediated by frontal lateralization. Neuroimage 125, 291–300. doi: 10.1016/j.neuroimage.2015.09.062
Inzitari, M., Baldereschi, M., Di Carlo, A., Di Bari, M., Marchionni, N., Scafato, E., et al. (2007). Impaired attention predicts motor performance decline in older community-dwellers with normal baseline mobility: results from the italian longitudinal study on aging (ILSA). J. Gerontol. A Biol. Sci. Med. Sci. 62, 837–843. doi: 10.1093/gerona/62.8.837
Jokinen, H., Kalska, H., Ylikoski, R., Madureira, S., Verdelho, A., Van Der Flier, W. M., et al. (2009). Longitudinal cognitive decline in subcortical ischemic vascular disease -the ladis study. Cerebrovasc. Dis. 27, 384–391. doi: 10.1159/000207442
Jurado, M. B., and Rosselli, M. (2007). The elusive nature of executive functions: a review of our current understanding. Neuropsychol. Rev. 17, 213–233. doi: 10.1007/s11065-007-9040-z
Kahya, M., Moon, S., Ranchet, M., Vukas, R. R., Lyons, K. E., Pahwa, R., et al. (2019). Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: a systematic review. Exp. Gerontol. 128:110756. doi: 10.1016/j.exger.2019.110756
Katzorke, A., Zeller, J. B. M., Müller, L. D., Lauer, M., Polak, T., Deckert, J., et al. (2018). Decreased hemodynamic response in inferior frontotemporal regions in elderly with mild cognitive impairment. Psychiatry Res. Neuroimaging 274, 11–18. doi: 10.1016/j.pscychresns.2018.02.003
Kirilina, E., Jelzow, A., Heine, A., Niessing, M., Wabnitz, H., Brühl, R., et al. (2012). The physiological origin of task-evoked systemic artefacts in functional near infrared spectroscopy. Neuroimage 61, 70–81. doi: 10.1016/j.neuroimage.2012.02.074
Kisler, K., Nelson, A. R., Montagne, A., and Zlokovic, B. V. (2017). Cerebral blood flow regulation and neurovascular dysfunction in alzheimer disease. Nat. Rev. Neurosci. 18, 419–434. doi: 10.1038/nrn.2017.48
la Fougère, C., Zwergal, A., Rominger, A., Förster, S., Fesl, G., Dieterich, M., et al. (2010). Real versus imagined locomotion: a [18F]-FDG PET-FMRI comparison. Neuroimage 50, 1589–1598. doi: 10.1016/j.neuroimage.2009.12.060
Laguë-Beauvais, M., Fraser, S. A., Desjardins-Crépeau, L., Castonguay, N., Desjardins, M., Lesage, F., et al. (2015). Shedding light on the effect of priority instructions during dual-task performance in younger and older adults: a FNIRS study. Brain Cogn. 98, 1–14. doi: 10.1016/j.bandc.2015.05.001
Leone, C., Feys, P., Moumdjian, L., D’Amico, E., Zappia, M., and Patti, F. (2017). Cognitive-motor dual-task interference: a systematic review of neural correlates. Neurosci. Biobehav. Rev. 75, 348–360. doi: 10.1016/j.neubiorev.2017.01.010
Lucas, M., Wagshul, M. E., Izzetoglu, M., and Holtzer, R. (2018). Moderating effect of white matter integrity on brain activation during dual-task walking in older adults. J. Gerontol. A 74, 435–441. doi: 10.1093/gerona/gly131
Mahoney, J. R., Holtzer, R., Izzetoglu, M., Zemon, V., Verghese, J., and Allali, G. (2016). The role of prefrontal cortex during postural control in parkinsonian syndromes a functional near-infrared spectroscopy study. Brain Res. 1633, 126–138. doi: 10.1016/j.brainres.2015.10.053
Maidan, I., Bernad-Elazari, H., Giladi, N., Hausdorff, J. M., and Mirelman, A. (2017). When is higher level cognitive control needed for locomotor tasks among patients with Parkinson’s disease? Brain Topogr. 30, 531–538. doi: 10.1007/s10548-017-0564-0
Maidan, I., Nieuwhof, F., Bernad-Elazari, H., Reelick, M. F., Bloem, B. R., Giladi, N., et al. (2016). The role of the frontal lobe in complex walking among patients with Parkinson’s disease and healthy older adults: an FNIRS study”. Neurorehabil. Neural Repair 30, 963–971. doi: 10.1177/1545968316650426
Mirelman, A., Maidan, I., Bernad-Elazari, H., Shustack, S., Giladi, N., and Hausdorff, J. M. (2017). Effects of aging on prefrontal brain activation during challenging walking conditions. Brain Cogn. 115, 41–46. doi: 10.1016/j.bandc.2017.04.002
Mori, T., Takeuchi, N., and Izumi, S. (2018). Prefrontal cortex activation during a dual task in patients with stroke. Gait Posture 59, 193–198. doi: 10.1016/j.gaitpost.2017.09.032
Müller, L. D., Guhn, A., Zeller, J. B. M., Biehl, S. C., Dresler, T., Hahn, T., et al. (2014). Neural correlates of a standardized version of the trail making test in young and elderly adults: a functional near-infrared spectroscopy study. Neuropsychologia 56, 271–279. doi: 10.1016/j.neuropsychologia.2014.01.019
Nieuwhof, F., Reelick, M. F., Maidan, I., Mirelman, A., Hausdorff, J. M., and Olde Rikkert, M. G. M. (2016). Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: feasibility of using a new portable FNIRS device. Pilot Feasibility Stud. 2, 1–11. doi: 10.1186/s40814-016-0099-2
Niu, H., Li, X., Chen, Y., Ma, C., Zhang, J., and Zhang, Z. (2013). Reduced frontal activation during a working memory task in mild cognitive impairment: a non-invasive near-infrared spectroscopy study. CNS Neurosci. Ther. 19, 125–131. doi: 10.1111/cns.12046
Noda, T., Nakagome, K., Setoyama, S., and Matsushima, E. (2017). Working memory and prefrontal/temporal hemodynamic responses during post-task period in patients with schizophrenia: a multi-channel near-infrared spectroscopy study. J. Psychiatr. Res. 95, 288–298. doi: 10.1016/j.jpsychires.2017.09.001
Oboshi, Y., Kikuchi, M., Shimizu, Y., Yoshimura, Y., Hiraishi, H., Okada, H., et al. (2014). Pre-task prefrontal activation during cognitive processes in aging: a near-infrared spectroscopy study. PLoS One 9:e098779. doi: 10.1371/journal.pone.0098779
Ohsugi, H., Ohgi, S., Shigemori, K., and Schneider, E. B. (2013). Differences in dual-task performance and prefrontal cortex activation between younger and older adults. BMC Neurosci. 14:10. doi: 10.1186/1471-2202-14-10
Okamoto, M., Dan, H., Shimizu, K., Takeo, K., Amita, T., Oda, I., et al. (2004). Multimodal assessment of cortical activation during apple peeling by NIRS and FMRI. Neuroimage 21, 1275–1288. doi: 10.1016/j.neuroimage.2003.12.003
Osofundiya, O., Benden, M. E., Dowdy, D., and Mehta, R. K. (2016). Obesity-specific neural cost of maintaining gait performance under complex conditions in community-dwelling older adults. Clin. Biomech. 35, 42–48. doi: 10.1016/j.clinbiomech.2016.03.011
Pfeifer, M. D., Scholkmann, F., and Labruyère, R. (2018). Signal processing in functional near-infrared spectroscopy (fnirs): methodological differences lead to different statistical results. Front. Hum. Neurosci. 11:641. doi: 10.3389/fnhum.2017.00641
Pifferi, A., Contini, D., Dalla Mora, A., Farina, A., Spinelli, L., and Torricelli, A. (2016). New frontiers in time-domain diffuse optics, a review. J. Biomed. Optics 21:091310. doi: 10.1117/1.JBO.21.9.091310
Plichta, M. M., Herrmann, M. J., Baehne, C. G., Ehlis, A. C., Richter, M. M., Pauli, P., et al. (2006). Event-related functional near-infrared spectroscopy (FNIRS): are the measurements reliable? Neuroimage 31, 116–124. doi: 10.1016/j.neuroimage.2005.12.008
Rosano, C., Aizenstein, H., Brach, J., Longenberger, A., Studenski, S., and Newman, A. B. (2008). Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J. Gerontol. A Biol. Sci. Med. Sci. 63, 1380–1388. doi: 10.1093/gerona/63.12.1380
Rosen, B. R., and Savoy, R. L. (2012). FMRI at 20: has it changed the world? Neuroimage 62, 1316–1324. doi: 10.1016/j.neuroimage.2012.03.004
Rosso, A. L., Cenciarini, M., Sparto, P. J., Loughlin, P. J., Furman, J. M., and Huppert, T. J. (2017). Neuroimaging of an attention demanding dual-task during dynamic postural control. Gait Posture 57, 193–198. doi: 10.1016/j.gaitpost.2017.06.013
Rosso, A. L., Metti, A. L., Faulkner, K., Redfern, M., Yaffe, K., Launer, L., et al. (2019). Complex walking tasks and risk for cognitive decline in high functioning older adults. J. Alzheimers Dis. 71, S65–S73. doi: 10.3233/JAD-181140
Rypma, B., and D’Esposito, M. (2000). Isolating the neural mechanisms of age-related changes in human working memory. Nat. Neurosci. 3, 509–515. doi: 10.1038/74889
Sala, S., Baddeley, A., Papagno, C., and Spinnler, H. (1995). Dual-task paradigm: a means to examine the central executive. Ann. N. Y. Acad. Sci. 769, 161–172. doi: 10.1111/j.1749-6632.1995.tb38137.x
Scholkmann, F., Kleiser, S., Jaako Metz, A., Zimmermann, R., Mata Pavia, J., Wolf, U., et al. (2014). A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85, 6–27. doi: 10.1016/j.neuroimage.2013.05.004
Stern, Y. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 15, 394–402. doi: 10.1093/cercor/bhh142
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Strangman, G., Culver, J. P., Thompson, J. H., and Boas, D. A. (2002). A quantitative comparison of simultaneous bold FMRI and NIRS recordings during functional brain activation. Neuroimage 17, 719–731. doi: 10.1006/nimg.2002.1227
Stuart, S., Vitorio, R., Morris, R., Martini, D. N., Fino, P. C., and Mancini, M. (2018). Cortical activity during walking and balance tasks in older adults and in people with Parkinson’s disease: a structured review. Maturitas 113, 53–72. doi: 10.1016/j.maturitas.2018.04.011
Szameitat, A. J., Schubert, T., Müller, K., and Von Yves Cramon, D. (2002). Localization of executive functions in dual-task performance with FMRI. J. Cogn. Neurosci. 14, 1184–1199. doi: 10.1162/089892902760807195
Tachtsidis, I., and Scholkmann, F. (2016). False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward. Neurophotonics 3:031405. doi: 10.1117/1.NPh.3.3.031405
Takeuchi, N., Mori, T., Suzukamo, Y., Tanaka, N., and Izumi, S. (2016). Parallel processing of cognitive and physical demands in left and right prefrontal cortices during smartphone use while walking. BMC Neurosci. 17:9. doi: 10.1186/s12868-016-0244-0
Thumm, P. C., Maidan, I., Brozgol, M., Shustak, S., Gazit, E., Shema Shiratzki, S., et al. (2018). Treadmill walking reduces pre-frontal activation in patients with Parkinson’s disease. Gait Posture 62, 384–387. doi: 10.1016/j.gaitpost.2018.03.041
Torricelli, A., Contini, D., Pifferi, A., Caffini, M., Re, R., Zucchelli, L., et al. (2014). Time domain functional nirs imaging for human brain mapping. Neuroimage 85, 28–50. doi: 10.1016/j.neuroimage.2013.05.106
Uemura, K., Shimada, H., Doi, T., Hakizako, H., Tsutsumimoto, K., Park, H., et al. (2016). Reduced prefrontal oxygenation in mild cognitive impairment during memory retrieval. Int. J. Geriatr. Psychiatry 31, 583–591. doi: 10.1002/gps.4363
Venkatraman, V. K., Aizenstein, H., Guralnik, J., Newman, A. B., Glynn, N. W., Taylor, C., et al. (2010). Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage 49, 3436–3442. doi: 10.1016/j.neuroimage.2009.11.019
Verghese, J., Annweiler, C., Ayers, E., Barzilai, N., Beauchet, O., Bennett, D. A., et al. (2014). Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 83, 718–726. doi: 10.1212/WNL.0000000000000717
Verghese, J., Holtzer, R., Lipton, R. B., and Wang, C. (2012). Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J. Am. Geriatr. Soc. 60, 1901–1905. doi: 10.1111/j.1532-5415.2012.04145.x
Verghese, J., Wang, C., Ayers, E., Izzetoglu, M., and Holtzer, R. (2017). Brain activation in high-functioning older adults and falls: prospective cohort study. Neurology 88, 191–197. doi: 10.1212/WNL.0000000000003421
Vermeij, A., van Beek, A. H. E. A., Reijs, B. L. R., Claassen, J. A. H. R., and Kessels, R. P. C. (2014). An exploratory study of the effects of spatial working-memory load on prefrontal activation in low- and high-performing elderly. Front. Aging Neurosci. 6:303. doi: 10.3389/fnagi.2014.00303
Vitorio, R., Stuart, S., Rochester, L., Alcock, L., and Pantall, A. (2017). FNIRS response during walking — artefact or cortical activity? A systematic review. Neurosci. Biobehav. Rev. 83, 160–172. doi: 10.1016/j.neubiorev.2017.10.002
Wager, T. D., Jonides, J., and Reading, S. (2004). Neuroimaging studies of shifting attention: a meta-analysis. Neuroimage 22, 1679–1693. doi: 10.1016/j.neuroimage.2004.03.052
Wagshul, M. E., Lucas, M., Ye, K., Izzetoglu, M., and Holtzer, R. (2019). Multi-modal neuroimaging of dual-task walking: structural MRI and FNIRS analysis reveals prefrontal grey matter volume moderation of brain activation in older adults. Neuroimage 189, 745–754. doi: 10.1016/j.neuroimage.2019.01.045
Weinstein, A. M., Voss, M. W., Prakash, Chaddock, L., Szabo, A., White, S. M., et al. (2012). The association between aerobic fitness and executive function is mediated by prefrontal cortex volume. Brain Behav. Immun. 26, 811–819. doi: 10.1016/j.bbi.2011.11.008
Yap, K. H., Ung, W., Ebenezer, E. G. M., Nordin, N., Chin, P., Sugathan, S., et al. (2017). Visualizing hyperactivation in neurodegeneration based on prefrontal oxygenation: a comparative study of mild Alzheimer’s disease, mild cognitive impairment, and healthy controls. Front. Aging Neurosci. 9:287. doi: 10.3389/fnagi.2017.00287
Yaple, Z. A., Stevens, W. D., and Arsalidou, M. (2019). Meta-analyses of the n-back working memory task: FMRI evidence of age-related changes in prefrontal cortex involvement across the adult lifespan. Neuroimage 196, 16–31. doi: 10.1016/j.neuroimage.2019.03.074
Yeung, M. K., Sze, S. L., Woo, J., Kwok, T., Shum, D. H. K., Yu, R., et al. (2016a). Altered frontal lateralization underlies the category fluency deficits in older adults with mild cognitive impairment: a near-infrared spectroscopy study. Front. Aging Neurosci. 8:59. doi: 10.3389/fnagi.2016.00059
Yeung, M. K., Sze, S. L., Woo, J., Kwok, T., Shum, D. H., Yu, R., et al. (2016b). Reduced frontal activations at high working memory load in mild cognitive impairment: near-infrared spectroscopy. Dement. Geriatr. Cogn. Disord. 42, 278–296. doi: 10.1159/000450993
Yücel, M. A., Selb, J., Aasted, C. M., Petkov, M. P., Becerra, L., Borsook, D., et al. (2015). Short separation regression improves statistical significance and better localizes the hemodynamic response obtained by near-infrared spectroscopy for tasks with differing autonomic responses. Neurophotonics 2:035005. doi: 10.1117/1.NPh.2.3.035005
Zhang, X., Noah, J. A., and Hirsch, J. (2016). Separation of the global and local components in functional near-infrared spectroscopy signals using principal component spatial filtering. Neurophotonics 3:015004. doi: 10.1117/1.NPh.3.1.015004
Keywords: functional Near-Infrared Spectroscopy, gait, dual task, motor task, cognition, older adults, prefrontal cortex, cerebral hemodynamics
Citation: Udina C, Avtzi S, Durduran T, Holtzer R, Rosso AL, Castellano-Tejedor C, Perez L-M, Soto-Bagaria L and Inzitari M (2020) Functional Near-Infrared Spectroscopy to Study Cerebral Hemodynamics in Older Adults During Cognitive and Motor Tasks: A Review. Front. Aging Neurosci. 11:367. doi: 10.3389/fnagi.2019.00367
Received: 20 June 2019; Accepted: 16 December 2019;
Published: 21 January 2020.
Edited by:
Hans J. Grabe, University of Greifswald, GermanyReviewed by:
Dafin F. Muresanu, Iuliu Haţieganu University of Medicine and Pharmacy, RomaniaCopyright © 2020 Udina, Avtzi, Durduran, Holtzer, Rosso, Castellano-Tejedor, Perez, Soto-Bagaria and Inzitari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Udina, Y3VkaW5hQHBlcmV2aXJnaWxpLmNhdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.