- Department of Anesthesia, The Third Xiangya Hospital of Central South University, Changsha, China
Background: Members of the aging population who undergo surgery are at risk of postoperative cognitive dysfunction (POCD). Exploring an effective and reliable early predictor of POCD is essential to the identification of high-risk patients and to making prospective decisions. The purpose of this study was to examine whether preoperative insulin resistance is an independent predictor of POCD.
Methods: A total of 124 patients aged 60 years and older and who were scheduled for gastrointestinal surgery were enrolled in a prospective observational clinical study. All participants completed a battery of neuropsychological tests before surgery and 7 days later. POCD was defined as a decline of at least 1.5 SD on two or more of neuropsychological tests. Plasma concentration of the tumor necrosis factor α (TNF-α), C-reactive protein (CRP), and S-100β protein were measured. The status of insulin resistance was assessed by Homeostasis Model Assessment–Insulin Resistance (HOMA-IR). The relationship between HOMA-IR and POCD was assessed by Multivariable logistic regression models and the receiver operating characteristic (ROC) curve.
Results: Fifty one patients (41.1%) were diagnosed with POCD at 7 days after surgery. Preoperative HOMA-IR values of the POCD group were significantly higher than the non-POCD group. Furthermore, CRP and TNF-α levels of the POCD group were significantly higher at each postoperative time point (P < 0.05). The preoperative HOMA-IR value was an independent predictor of POCD (adjusted OR 1.88, 95% CI, 1.18–2.99) even after adjust for confounding variables, and when dichotomized, individuals above the HOMA-IR threshold (HOMA-IR > 2.6) had a three-times higher risk of POCD (OR 3.26; 95% CI, 1.07–9.91) compared to individuals below the threshold. The areas under the ROC curve of HOMA-IR was 0.804 (95% CI, 0.725–0.883; P < 0.001). The optimal cut-off value was found to be 0.583, with a sensitivity of 84.3% and specificity of 74%. The HOMA-IR value was positively associated with the TNF-α concentration at baseline (R2 = 0.43, P < 0.01) and 1 day after surgery (R2 = 0.3861, P < 0.01).
Conclusion: Preoperative insulin resistance is an effective predictor for the occurrence of POCD. Targeted prevention and treatment strategies of insulin resistance may be effective interventions of patients at risk for POCD.
Introduction
Postoperative cognitive dysfunction (POCD) is a common neuropsychological complication after surgery, with influences on various aspects of cognitive functioning, such as learning, memory, attention, and executive function, particularly in the elderly. Currently, the evaluation of POCD is based on a battery of neuropsychological test differences between pre- and postoperative performance and is often delayed. POCD has been confirmed to be associated with multiple poor outcomes, including increased surgical complications, prolonged hospitalization, impaired quality of life, increased risk of disability, and mortality (Lewis et al., 2004; Monk et al., 2008). Furthermore, POCD may accelerate the onset and progression of Alzheimer’s disease (Chen et al., 2014). However, to date, the underlying pathophysiology of POCD remains abstruse, being recognized as a result of interaction of multiple factors Thus, exploring the effective and reliable early predictor of POCD is essential to identifying high-risk patients and making prospective decisions.
Recently, there has been an increase in awareness on the contribution of metabolic risk factors such as obesity, diabetes, dyslipidemia to the occurrence of POCD (Feinkohl et al., 2016, 2017a,b, 2018). Indeed, Diabetes and obesity can affect the structure and function of the brain and show a much higher risk of cognitive impairment and even dementia (Bischof and Park, 2015; Schimming et al., 2017), especially in the elderly. Insulin resistance (IR) is not only at the core of obesity, diabetes, dyslipidemia, and metabolic syndrome (Sesti, 2006), but also represents a shared hallmark characteristic of neuropathological processes underlying cognition aging and Alzheimer’s disease (AD) (Frisardi et al., 2010; Heni et al., 2014; Akintola and van Heemst, 2015; Kullmann et al., 2016). There are numerous studies drawing a strong association between insulin resistance and poorer cognitive performance (Ekblad et al., 2017; Neergaard et al., 2017). In the central nervous system, insulin plays an important role in learning and formation of memory. Moreover, insulin resistance facilitates brain inflammatory responses and influences AD progression by increasing β-amyloid and reducing synaptic plasticity (Craft, 2005). Therefore, it may be that insulin resistance is a condition affecting both peripheral and central insulin signaling pathways, and insulin resistance constitutes a potential link between metabolic and cognitive dysfunction (Cholerton et al., 2013; Verdile et al., 2015). Regrettably, previous data only indicate that patients with metabolic risk factors may experience an increased risk of POCD; the influence of insulin resistance on POCD has not been proven in great detail. Therefore, exploring the relationship between insulin resistance and POCD is urgently needed through further research, especially in view of the modifiable nature that leaves room for risk reduction in high-risk individuals. These urged us to conduct the current research.
Thus, we aimed to evaluate the prospective association between insulin resistance and the subsequent risk of POCD, with a focus on whether insulin resistance is an independent predictor of POCD.
Materials and Methods
Participants and Study Design
This was a prospective observational clinical trial. All procedures of study were approved by the ethical committee of the Third Xiangya Hospital of Central South University, China (K18178). The study was registered on the Chinese Clinical Trial Registry (ChiCTR1800019768). All study participants provided written informed consent before enrollment.
Eligible patients were older than 60 years, classified under American Society of Anesthesiologists physical status I–III, and scheduled for elective gastrointestinal surgery lasting at least 2 h under general anesthesia. Additional inclusion criteria were that the participants must have the ability to communicate with the interviewer and complete a battery of neuropsychological testing. Exclusion criteria were any patients who had a preexisting psychiatric or neurological disease (e.g., intracranial tumors), had undergone surgery within the past 12 months, and registered a baseline Mini-Mental State Examination (MMSE) score < 23.
Clinical Measurements
On admission, all patients who were accepted provided detailed history records such as self-reports of a diagnosis of hypertension, diabetes mellitus, heart disease, and previous stroke, a detailed list of medication and were administered a clinical examination. Clinical examination includes the measurement of weight, height, waist circumference and blood pressure, using an average of 3 seated blood pressure measurements.
The metabolic syndrome was defined using a modified version of the definition recommended by the American Heart Association (Alberti et al., 2009). The presence of any 3 out of 5 of the following risk factors would qualify a person for the metabolic syndrome: (1) elevated waist circumference (man ≥ 85 cm, woman ≥80 cm), (2) elevated triglycerides ≥1.7 mmol/L or use of medication for dyslipidemia, (3) reduced HDL-C (males <1.0 mmol/L, females <1.3 mmol/L), (4) elevated blood pressure (systolic pressure above 130 mmHg or/and diastolic pressure higher than 85 mmHg or using an antihypertensive drug treatment), and (5) elevated fasting glucose (≥100 mg/dL or ≥5.56 mmol/L) or drug treatment of elevated glucose.
The homeostasis model assessment index (HOMA-IR) was used to assess the degree of insulin resistance (Ekblad et al., 2017). The HOMA-IR index was calculated using the following formula: Fasting Insulin × Fasting Glucose/22.5. A baseline HOMA-IR index greater than 2.6 was predefined arbitrarily as insulin resistance (Nagpal et al., 2016).
Plasma Biomarker Measurement
After admission, venous blood was obtained fasting in the morning for each patient and analyzed for biochemical parameters (triglycerides, HDL-C, fasting glucose and fasting Insulin levels) using standard laboratory techniques which is already a clinical routine in our hospital. At baseline and on postoperative days (PODs) 1, 3, and 7, blood samples were collected, processed by centrifugation, then were frozen at −70°C until assayed. Neuronal injury S100β protein and 2 biomarkers of inflammation CRP and TNF-α were measured in the central laboratory of our institution, using enzyme linked immunosorbent assay (ELISA) or turbidimetric inhibition immune assay.
Neuropsychological Test
Subjects were first screened with SDS, MMSE to exclude subjects with serious depression or cognitive impairment. Those enrolled participants completed a battery of neuropsychological tests conducted by a trained interviewer before surgery and 7 days after surgery. This cognitive test battery consisted of Verbal Learning and fluency Test, Visuospatial Memory and Delayed Recall Test, Benton Judgment of Line Orientation, Trail Making Test Parts A and B, Digit Span and Digit Symbol Substitution Test, which primarily focus on memory, attention, and executive function. Thedetailed content of these cognitive tests have been described previously (Lin et al., 2014).
A postoperative neuropsychological disorder in a test has been defined as a deterioration of one standard deviation (SD) compared to the preoperative test results (namely “the 1 SD criterion”) (Rasmussen et al., 2001). The standard deviation of each test is calculated from the corresponding preoperative assessments of all patients. For individuals, we subtracted the preoperative from the postoperative score and divided the results by the corresponding SD to obtain a value called Z score for each individual test, these Z scores were then used for the assessment of POCD. Patients were diagnosed as POCD if their Z score was less than at least 1.5 SD on two or more of eleven neuropsychological tests (Rasmussen et al., 2001).
In addition to the above neuropsychological tests, the following tests were also administered: (1) MMSE was used to exclude pre-existing cognitive impairment, (2) the Self-rating Depression scale (SDS) and the Self-rating Anxiety scale (SAS) were used to assess anxiety and depression at baseline, (3) the Visual analog scales 0–10 were used to assess postoperative pain and the presence of mood disorder.
Anesthesia and Surgery
During the entire perioperative period, all clinical management followed recognized clinical practice. No premedication was administered before surgery. All patients received general anesthesia. To ensure that all patients were under similar levels of anesthesia, the depth of anesthesia was monitored by the bispectral index, which is required to be maintained between 40 and 60. Except for this requirement, anesthetic agents and other aspects of management (blood pressure targets, use of vasoactive drugs) were at the attending anesthesiologist’s discretion. Generally, midazolam, sufentanil, neuromuscular blockade, and propofol were used for anesthesia induction, followed by volatile (sevoflurane) or/and intravenous (propofol) anesthesia. Endotracheal intubation was the most common form of airway maintenance. Postoperative pain therapy was most commonly treated with patient controlled analgesia device using sufentanil in the initial period. The surgery was carried out by skilled surgeons in a routine laparoscopic excision of gastrointestinal cancer. All aspects of the patient’s condition were documented in case report forms.
Statistical Analyses
Descriptive statistics of variables were examined in patients with and without POCD. Quantitative data were expressed as the mean ± standard deviation. Categorical data were expressed as the number and percentage. Statistical differences between two groups were investigated using t-test or Wilcoxon rank sum test, as appropriate for continuous data. The chi-square test or Fisher exact test was used for categorical data. Univariate logistic regression models were used; those variables which have statistical significance (p < 0.05) were then analyzed with the multivariate model. Multivariable logistic regression models were fitted to adjust for the odds ratio (OR) values and 95% confidence intervals (CI) of the independent predictors of POCD. The odds ratio estimates were adjusted for confounders age and postoperative pneumonia, and were then additionally mutually adjusted for all other metabolic components. The linear regression analysis was used to illustrate the relationship of HOMA-IR, CRP, and TNF-upalpha. Receiver operating characteristic (ROC) curves were configured to determine the best threshold of HOMA-IR for optimal predictive sensitivities and specificities of POCD. SPSS 22.0 was used for data analysis, where values of p < 0.05 were considered statistically significant. Graphics were created with GraphPad Prism Software version 8.0 for Windows.
Results
Enrolled Patients and Clinical Characteristics
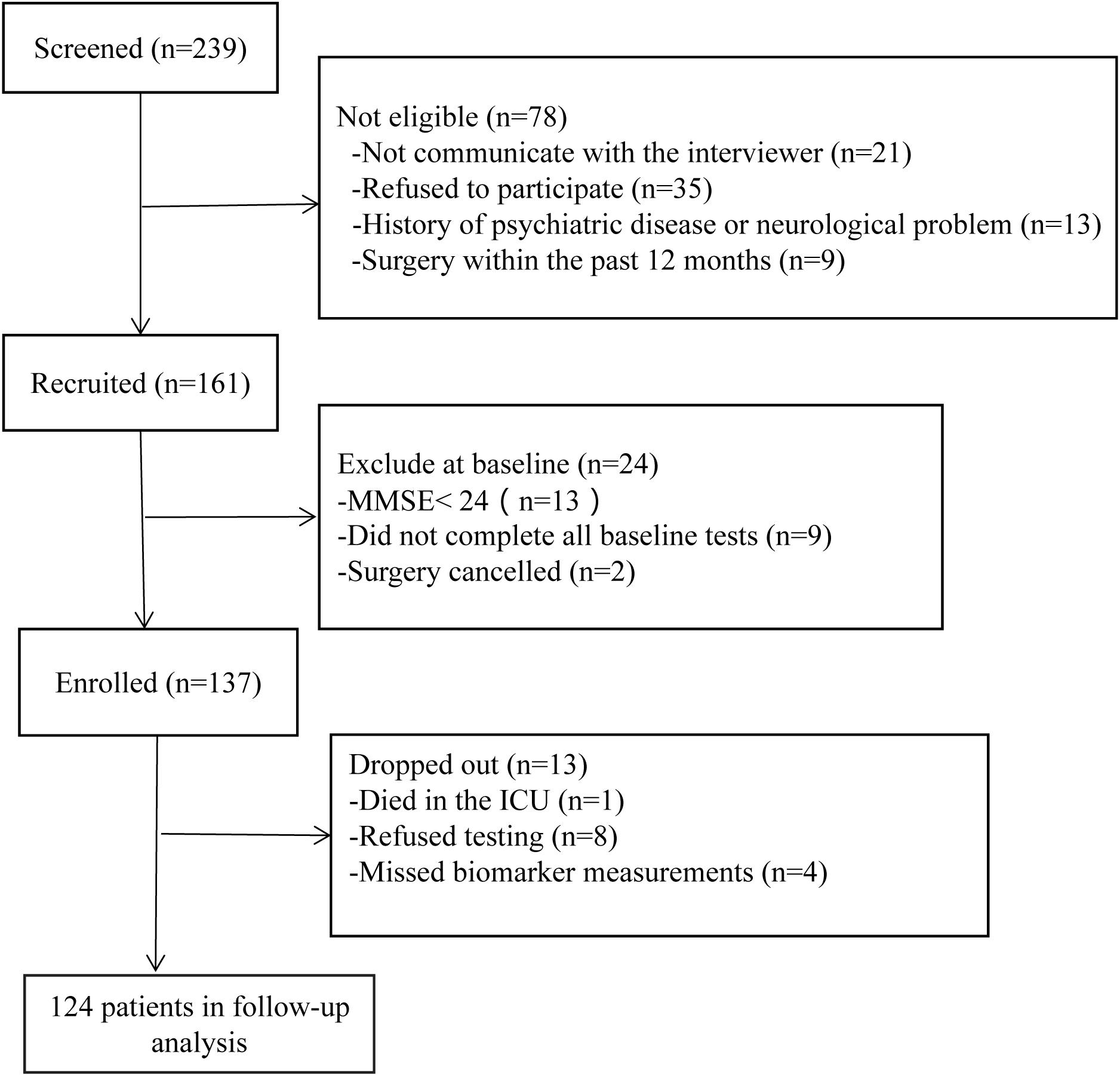
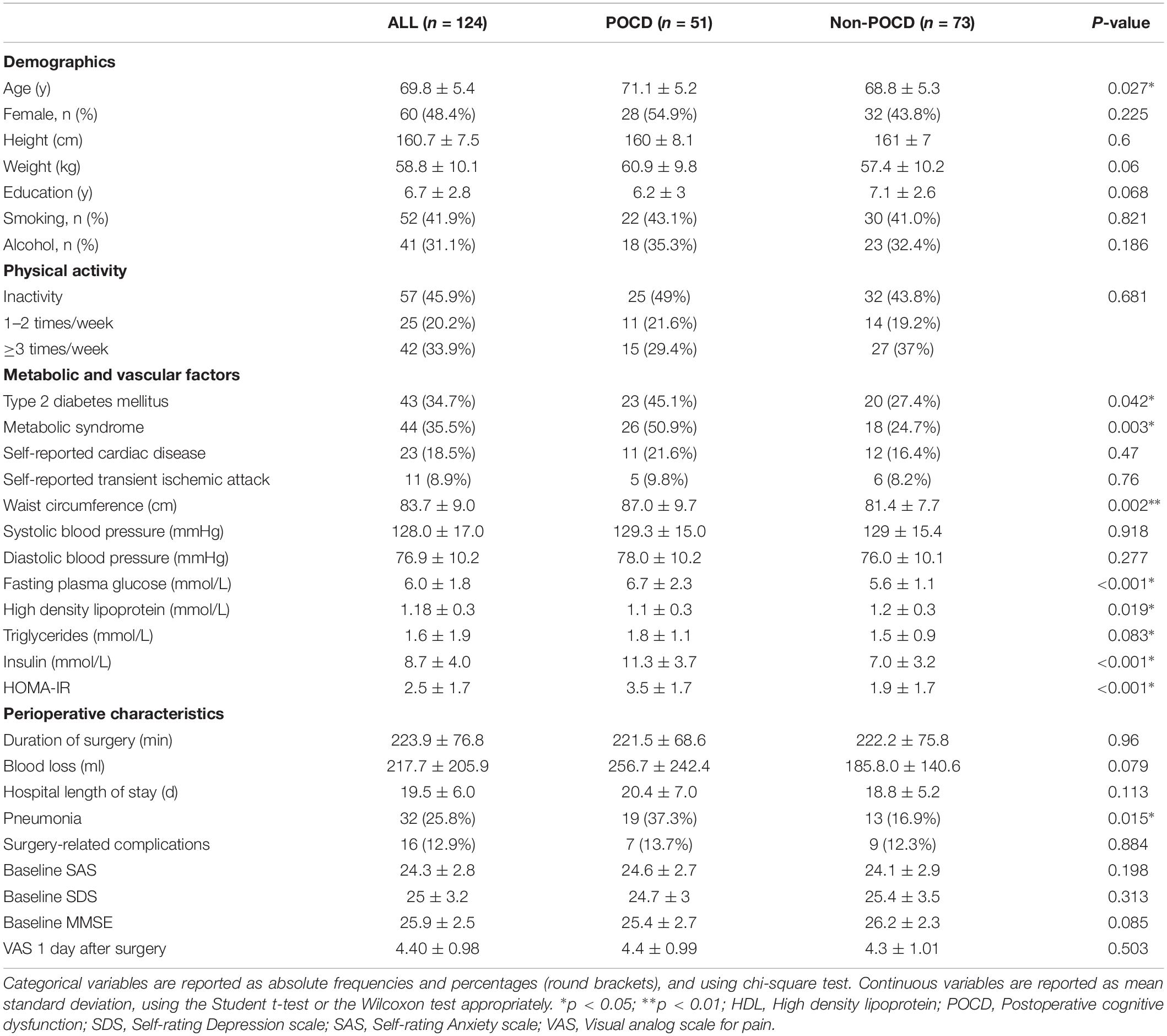
A total of 124 patients were included. The trial details are shown in Figure 1. The demographics and clinical characteristics of the participants are listed in Table 1. Fifty-one patients (41.1%) were diagnosed with POCD at 7 days after surgery. Compared to non-POCD, participants with POCD exhibited several abnormalities including elevated waist circumference (p = 0.002), elevated triglycerides (p = 0.083), and reported a higher incidence of pneumonia after surgery, but no surgery-related complications. Insulin and glucose level were marginally higher in the POCD group (p < 0.001). Furthermore, the prevalence of metabolic syndrome in patients with POCD (50.9%) was more than two times the prevalence in the non-POCD group (24.7%). Other cardiovascular risk factors such as hypertension, dyslipidemia, the level of physical activity, history of smoking, and alcoholism, were not statistically significant. Situations during perioperative period, such as ASA grade and the duration of time and blood loss, were observed with no significant difference (see Table 1).
Neuropsychological Test Results
Preoperative SAS and SDS showed no difference between the two groups (Table 1).
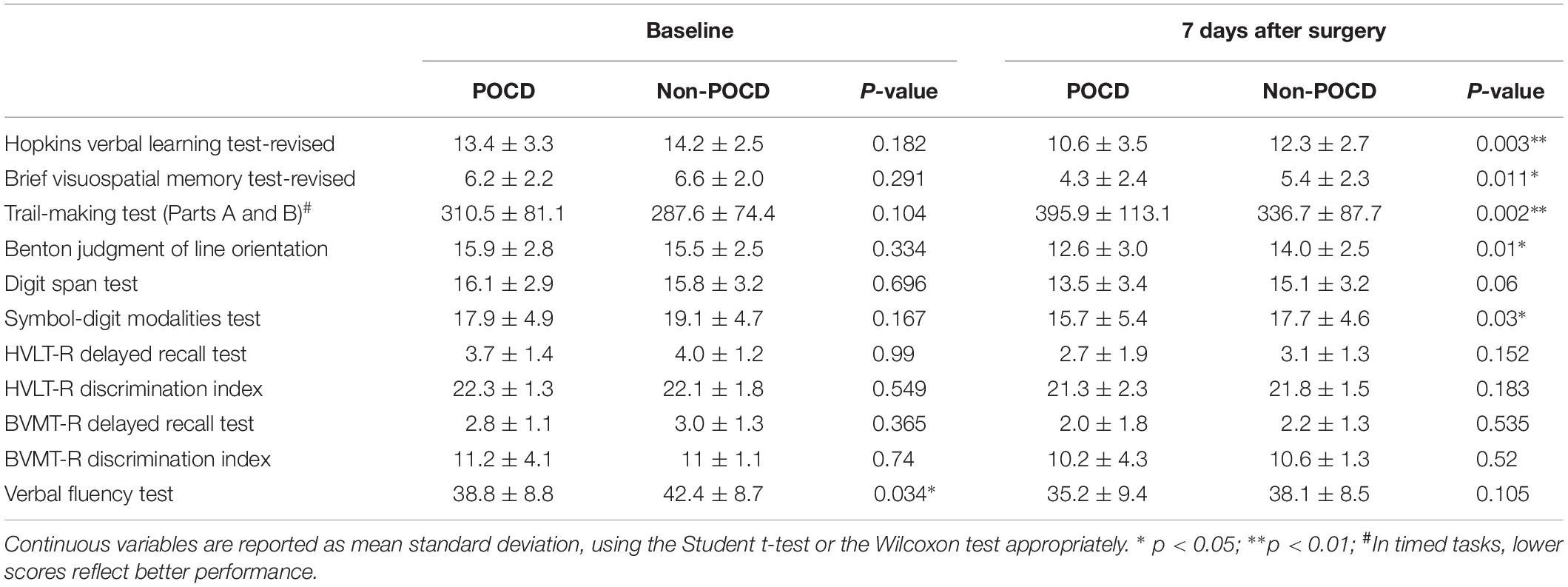
The results of neuropsychological testing of participants at baseline and at 7 days after surgery were listed in Table 2. Participants with POCD exhibited worse performance mainly in Visuospatial Memory, Trail Making Test, and Digit Span Test.
Primary Outcome: Relationship Between Metabolic Risk factors, IR and POCD
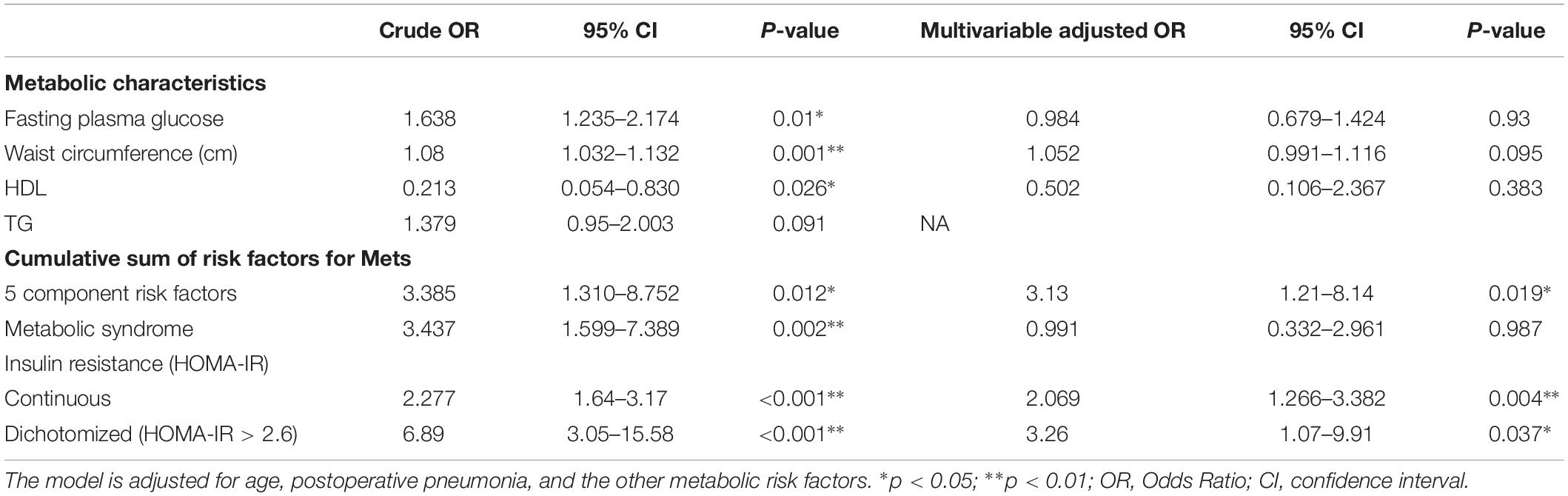
Table 3 presents the relations among metabolic risk factors, IR and incident POCD at follow-up. Insulin resistance was associated with an increased risk of POCD. The risk of POCD increased between 88% for every unit increase on the HOMA-IR index (OR 1.88, 95% CI, 1.18–2.99), and when dichotomized, individuals above the threshold of 2.6 had a three-times higher risk of POCD (OR 3.26; 95% CI, 1.07–9.91) compared to individuals below the HOMA-IR threshold. The relations among diabetes, metabolic syndrome, insulin resistance and POCD can be found in Supplementary Table S1. Furthermore, in our subgroup analyses by diabetics, we observed similar associations between HOMA-IR and the incidence of POCD in participants with diabetics (OR 2.38, 95% CI, 1.26–4.51) and participants who did not have diabetes (OR 2.48, 95% CI, 1.46–4.20) after adjustment for age and the other metabolic components (Table 4). At the same time, we also carried out a subgroup analysis based on obesity, dyslipidemia, hypertension, and metabolic syndrome. Subgroup analyses did not substantially change our findings (Table 4).
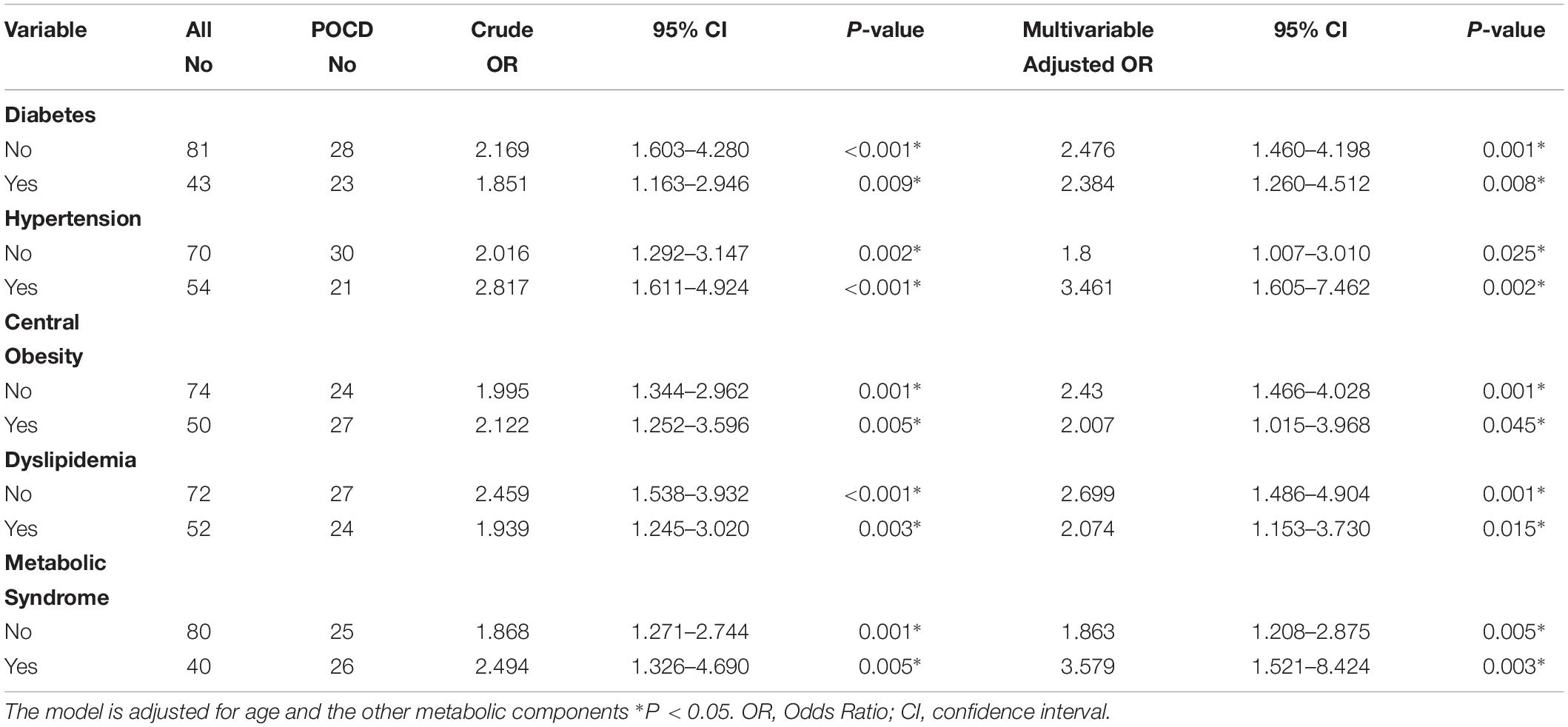
Table 4. Associations between Insulin Resistance and POCD in subgroup analysis based on metabolic members.
The ROC curves (Figure 2) shows that insulin resistance (assessed by the HOMA-IR) is the independent predictor of POCD. The areas under the curve of HOMA-IR was 0.804 (95% CI, 0.725–0.883; P < 0.001). The optimal cut-off value was found to be 0.583, with a sensitivity of 84.3% and specificity of 74%.
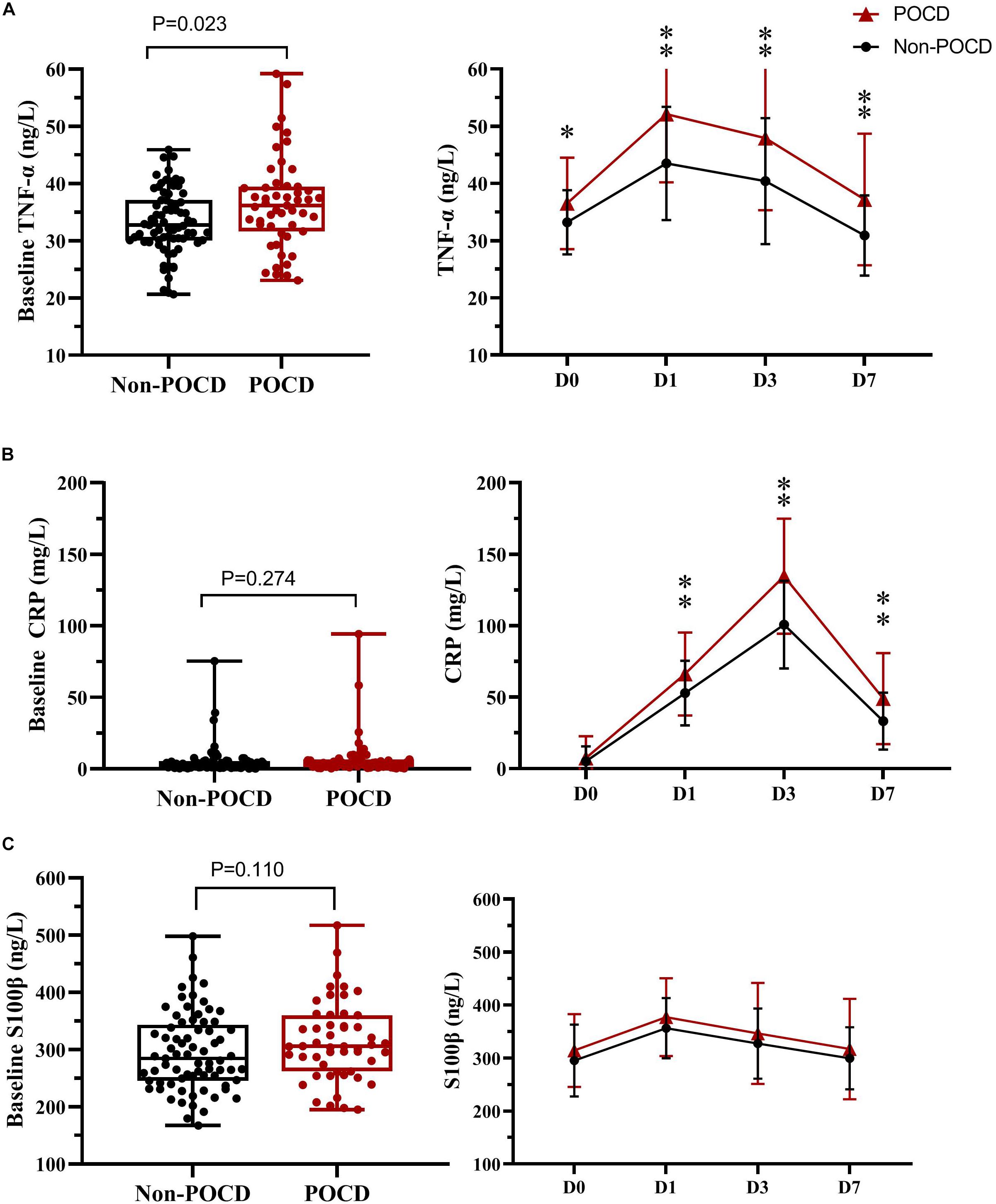
Figure 2. Receiver operating characteristic analysis of the preoperative HOMA-IR value. ROC, Receiver operating characteristic; HOMA-IR, Homeostasis Model Assessment–Insulin Resistance.
In addition, most metabolic risk factors showed a weak-to-moderate relationship with incident POCD after adjustment for age and postoperative pneumonia. However, after adjustment for all covariates and the other metabolic risk factors, associations were substantially meaningless. Only individuals with the worst metabolic condition, holding five risk factors for metabolic syndrome have a three-times larger odds (OR 3.13, 95% CI, 1.21–8.14) of developing POCD when compared to individuals with no metabolic risk factors. However, metabolic syndrome was not associated with an increased risk of incident POCD at follow-up.
Plasma Concentration of CRP, TNF-α, and S-100β Results
The levels of plasma biomarkers of brain injury and systemic inflammation are listed in Table 5. No significant differences were observed in preoperative concentrations of CRP and S100β; only the TNF-α concentrations at baseline were higher in the POCD group. Compared to the non-POCD group (Table 5 and Figure 3), patients with POCD had higher systemic inflammation (high CRP and TNF-α) and neuronal injury (high S-100β) at each postoperative time point. Most importantly, as shown in Figure 4, according to the linear regression equation, the HOMA-IR value was positively associated with a TNF-α concentration at baseline (R2 = 0.43, P < 0.01) and 1 days after surgery (R2 = 0.3861, P < 0.01). Furthermore, the HOMA-IR value was also positively associated with CRP concentration at baseline (R2 = 0.3288, P < 0.01) and 1 day after surgery (R2 = 0.2641, P < 0.01), indicating a possible cross-talk between insulin resistance and perioperative inflammation.
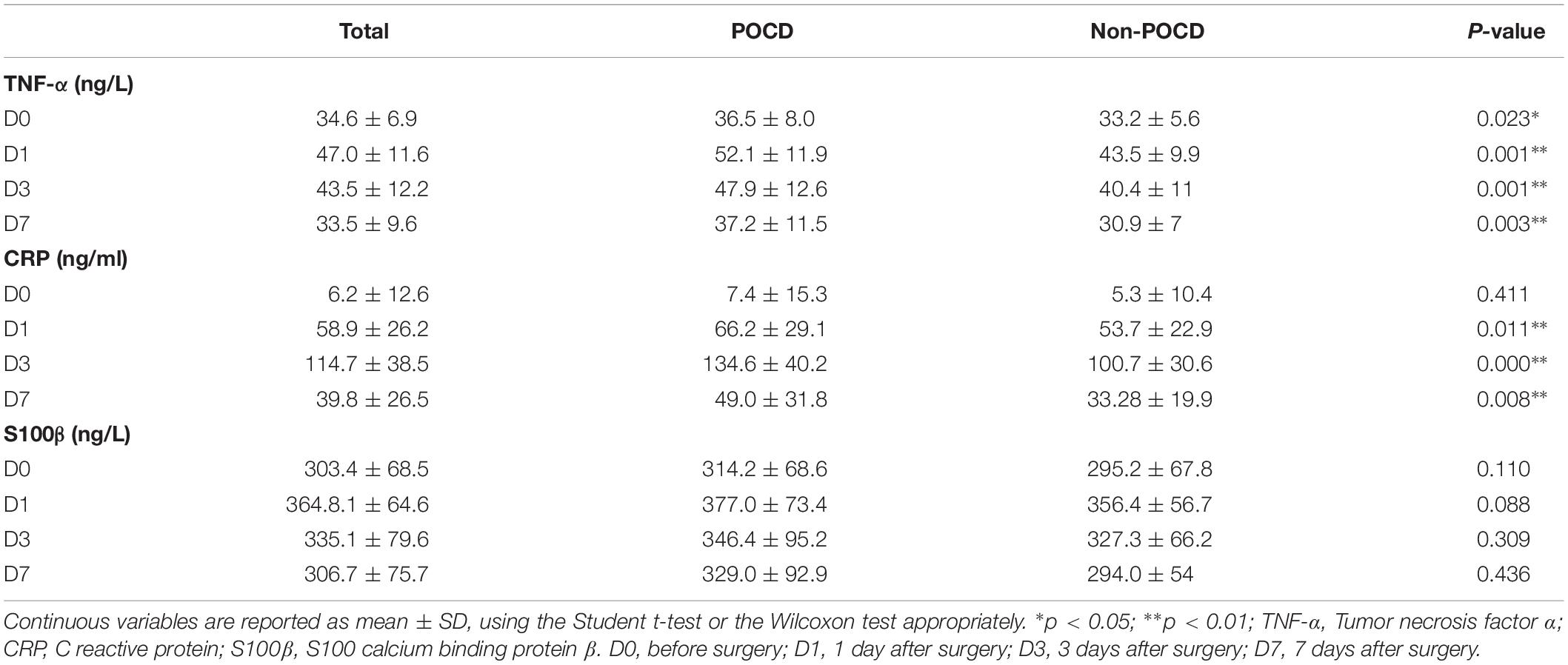
Table 5. Plasma Biomarker Levels in Patients with and without Postoperative Cognitive Dysfunction (POCD).
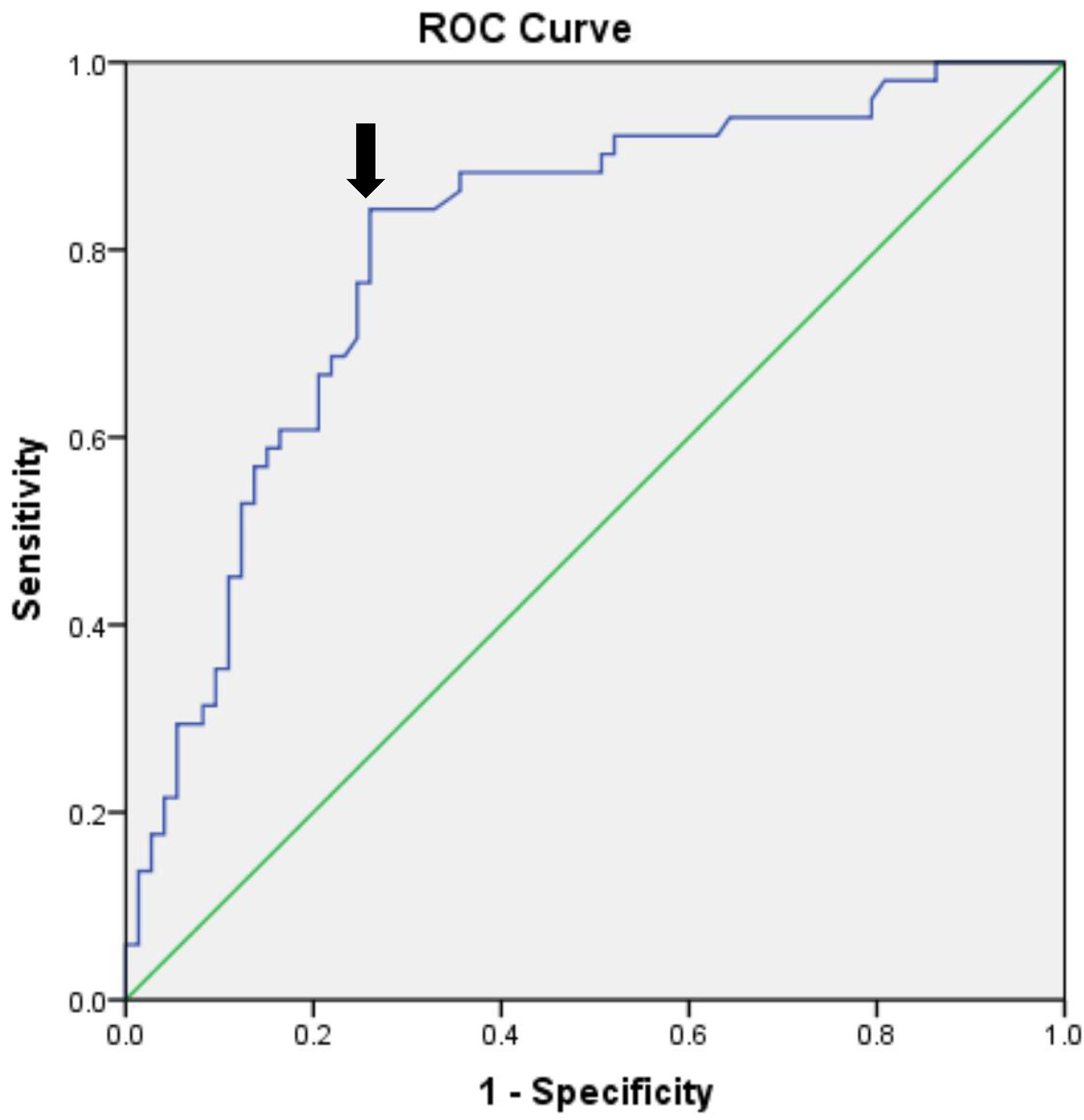
Figure 3. Plasma biomarker levels in two groups at different times. TNF-α (A), CRP (B), and S100β protein (C) were sampled at baseline and at 1, 3, and 7 days after surgery. ∗p < 0.05; ∗∗p < 0.01. POCD, postoperative cognitive dysfunction; Non-POCD, Non-postoperative cognitive dysfunction; TNF-α, Tumor necrosis factor α; CRP, C reactive protein; S100β, S100 calcium binding protein β. D0 at baseline; D1, 1 day after surgery; D3, 3 days after surgery; D7, 7 days after surgery.
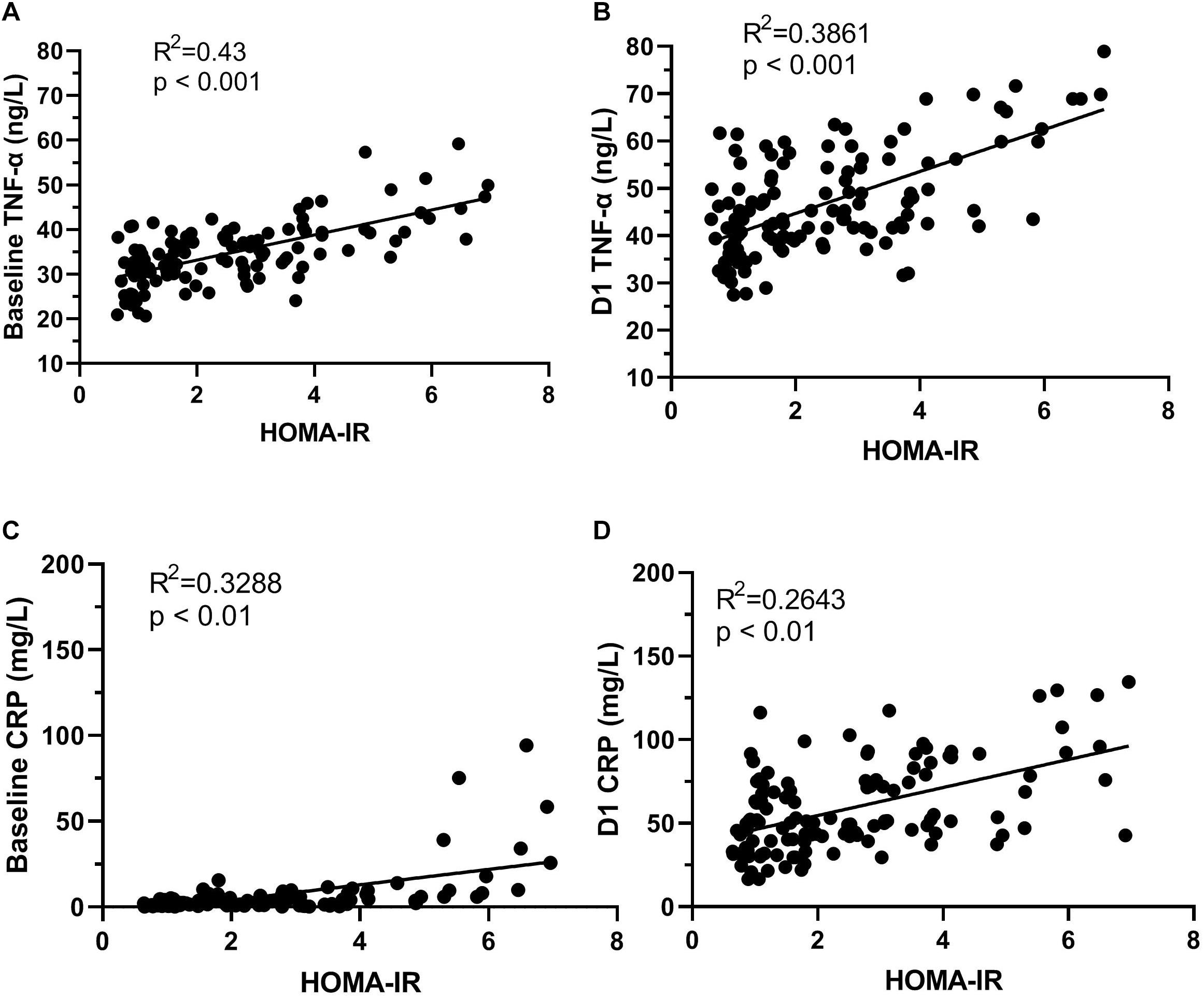
Figure 4. The correlation of HOMA-IR and systemic inflammation. HOMA-IR and TNF-α concentration at baseline (A); HOMA-IR and TNF-α concentration D1 (B). D1, HOMA-IR and CRP concentration at baseline (C); HOMA-IR and CRP concentration D1 (D). D1, 1 day after surgery.
Discussion
In this study, we found a high prevalence of preexisting insulin resistance in older patients undergoing gastrointestinal surgery, and most importantly, preexisting insulin resistance was associated with subsequent incident POCD. This association was independent of age, postoperative pneumonia, as well as all other metabolic derangement components. These findings suggest that preexisting insulin resistance associated with risk of POCD independently.
Insulin resistance is not only a shared hallmark characteristic of metabolic disease, but also a shared neuropathological process underlying cognition aging and AD.
Many studies have drawn a strong link between insulin resistance and cognitive decline (Akintola and van Heemst, 2015; Bischof and Park, 2015; Schimming et al., 2017), especially in the elderly. Indeed, peripheral hyperinsulinemia and insulin resistance can decrease insulin receptors expression at the blood-brain barrier, and reduce the insulin transport into the brain (Schwartz et al., 1990; Sartorius et al., 2015). These disturbances of insulin action can directly contribute to cognitive impairment and even AD. Currently, some researches described the potential association between AD and POCD (Arora et al., 2014; Evered et al., 2016). Postmortem studies of brain tissue on individuals with AD but not T2D have provided clear evidence showing that disturbance in insulin signaling, including decreased insulin levels and insulin sensitivity, as well as aberrant activation of the insulin receptor substrate (Biessels and Reagan, 2015), which also could be proved the relationship between insulin resistance and cognitive. In the present study, we find that the HOMA-IR value in individuals with POCD is higher than in those without POCD before operation, and individuals above the HOMA-IR threshold (HOMA-IR > 2.5) had three times larger odds of POCD. Although our study cannot draw causal conclusions, these results, combined with the previous pathological feature of AD, indicate the involvement of insulin resistance in POCD. Furthermore, evidence has accumulated that individuals with insulin resistance correspond to hippocampal atrophy, a sign of neurodegeneration (Ursache et al., 2012). Moreover, individuals with decreased peripheral insulin sensitivity have an impaired neuronal metabolism (Karczewska-Kupczewska et al., 2013) and brain glucose hypometabolism, evaluated by FDG-PET (Ohara et al., 2011; Thambisetty et al., 2013a,b). Therefore, the improvement of whole body insulin resistance could be a possible therapeutic option for neurodegeneration. Encouragingly, clinical research found that increasing CSF insulin by intranasal administration benefits the cognitive function in AD and mild cognitive impairment (MCI) patients (Craft et al., 2012; Claxton et al., 2013, 2015. In addition, animal experiments have shown that intranasal insulin administration prevent anesthesia-induced spatial learning and memory deficit in mice (Zhang et al., 2016), and this treatment in mice not only enhances object memory but also yields anxiolytic effects on behavior (Marks et al., 2009). All of these support insulin resistance as a key risk factor for the occurrence of POCD.
Currently, many animal studies have indicated that insulin and insulin resistance play a prominent role in amyloid beta metabolism and influence AD pathology (Cholerton et al., 2013; Verdile et al., 2015). However, the relationship between insulin resistance and amyloid load remains somewhat controversial in clinical research. In a longitudinal research, which included multiple OGTTs, PiB-PET data, as well as postmortem brain slices, amyloid accumulation was not confirmed to be relevant with either insulin resistance or glucose intolerance (Thambisetty et al., 2013b). Furthermore, AD-related regions with hypometabolism were observed; however, there were no increase in amyloid accumulation in T2D subjects (Roberts et al., 2014). This led us to draw a conclusion that there is no consistent pattern between amyloid accumulation and insulin resistance, which is also supported by research in patients with MCI or AD (Moran et al., 2015). Unfortunately, in our study, we did not evaluate amyloid accumulation in plasma or in CSF. Further studies with the evaluation of amyloid in blood or in CSF are necessary and useful to confirm (or not) literature data. In addition, this could represent a further investigation of the relationship between insulin resistance and POCD in terms of amyloid brain deposition.
Age has been shown to be an independent risk factor of POCD repeatedly (Monk et al., 2008). However, our study found no strong relation between age and POCD when the logistic regression model included HOMA-IR and age. In other words, if HOMA-IR was excluded from the logistic regression model, age was importantly associated with POCD, but inclusion of HOMA-IR weakened the relationship of age with POCD. This suggests that age could be acting as a substitute for insulin resistance status to a certain extent in analyses for risk of POCD. Previous research supports our results. Aging has been identified to be associated with insulin resistance, altered blood-brain barrier function for transport of insulin from the periphery to the brain decreased, and the direct effect on insulin receptor expression and activation; all of these might be responsible for insulin resistance in older individuals (Biessels and Reagan, 2015). Importantly, the elderly with insulin resistance are more susceptible to cognitive impairment and show an AD-like brain (Sheline and Raichle, 2013).
Many elderly individuals suffer from multiple metabolic diseases, such as diabetes, hypertension and abdominal obesity (Carracher et al., 2018; Bluher, 2019). These can directly result in the prevalence of insulin resistance which is probably over-represented in the surgical population and increases the risk of postoperative complications (Tzimas et al., 2015). Furthermore, the influence of metabolic derangement components on brain changes has been well proven and presents a higher risk of POCD (Feinkohl et al., 2016, 2017a,b, 2018). With metabolic syndrome considered to be a clustering of risk factors such as obesity, elevated blood glucose, elevated blood pressure, and dyslipidemia within a single individual, appear to confer an even greater risk for cognitive impairment than the sum of its individual components (Yaffe, 2007). The results of our study only support that individuals with a poor metabolic condition, holding five metabolic and vascular risk factors, have three-times larger odds of developing POCD than individuals with an ideal metabolic condition. However, the presence of a single metabolic member and metabolic syndrome itself does not provoke an increased risk of incident POCD at follow-up. All of these suggest that metabolic health is closely associated with brain health.
As noted in previous work, both clinical and preclinical studies indicate that inflammatory reactions could be responsible for incident POCD (Krenk et al., 2010; Skvarc et al., 2018). The results of our study support the potential role of increased systemic inflammation (high CRP and TNF-α) in the incident of POCD, but failed to show the relationship between elevated S100β and POCD. There are many possible mechanisms underlying this association; insulin resistance is supposed to play an important role. Indeed, peripheral hyperinsulinemia and insulin resistance facilitate brain inflammatory responses and influence AD pathology by increasing β-amyloid (Craft, 2005). In turn, these inhibit insulin receptors by activating TNF-α (Ferreira et al., 2014). How may inflammation account for the deleterious effect of insulin resistance on cognitive function? Most likely in theory, insulin resistance is associated with an inflammatory response and in turn, either the insulin resistant or inflammation or both, contribute to cognitive decline (Grundy, 2003; Ridker et al., 2003). Our findings are consistent with these results. We observed that the HOMA-IR value is positively associated with TNF-α and CRP concentrations at baseline and 1 day after surgery, respectively. Recently, Feng et al. (2013) found that surgery results in exaggerated neuroinflammation and persistent cognitive decline in a Rat Model of the Metabolic Syndrome. Moreover, blocking TNF-α in mice can effectively improve insulin sensitivity and prevent surgery-induced cognitive dysfunction (Terrando et al., 2010; Ferreira et al., 2014). These animal experiments corroborate our results that the negative impact of insulin resistance on inflammatory reactions could be accelerated because of surgical trauma, and some of the increased risk of POCD associated with insulin resistance is modified by inflammation.
Our study has limitations. One limitation is the lack of data on the ratio of CSF to serum insulin level which is closely associated with brain insulin resistance, because the influence of insulin resistance on cognitive function is mainly on the brain insulin signaling pathways. In addition, we did not evaluate amyloid beta accumulation in plasma or in CSF. This could represent a further investigation of the relationship between insulin resistance and cognitive impairment in terms of amyloid brain deposition. Additional studies with more complete data are necessary. Investigations should include POCD at 3 months and activities of daily living (ADLs). All of the above may be in favor to determine the exact contribution of insulin resistance to POCD.
Conclusion
In conclusion, insulin resistance is associated with an increased risk of POCD in the elderly. This observation of a link between insulin resistance and POCD suggests that insulin resistance is an effective predictor of incident POCD. Thus, consideration of insulin resistance status may help clinicians and patients to make prospective decisions. The findings warrant further direction of research, particularly with respect to the underlying mechanisms and possible treatment strategies of POCD.
DATA AVAILABILITY
The data for this manuscript will be made available by the authors to qualified researchers upon reasonable request. Requests to access the data should be directed to the corresponding author.
Ethics Statement
All participants provided a signed written informed consent before enrolment in the study. All procedures described in this study were carried out in accordance with the declaration of Chinese Clinical Trial Registry (ChiCTR1800019768).
Author Contributions
WO and XH designed the clinical experiment. WO directed the research group in all aspects. XH was the main investigator in this research. CQ contributed to sample selection and provision. BZ and JC were responsible for data acquisition. XH, GL, and WO carried out the data analysis and interpretation, and drafted the manuscript. All authors revised the manuscript.
Funding
This study was supported by the National Nature Science Foundation of China (Award Number: 8167051812 to WO) and Hunan Provincial Natural Science Foundation of China (Award Number: 2017JJ3424).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00197/full#supplementary-material
References
Akintola, A. A., and van Heemst, D. (2015). Insulin, aging, and the brain: mechanisms and implications. Front. Endocrinol. 6:13. doi: 10.3389/fendo.2015.00013
Alberti, K. G., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation 120, 1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644
Arora, S. S., Gooch, J. L., and Garcia, P. S. (2014). Postoperative cognitive dysfunction, Alzheimer’s disease, and anesthesia. Int. J. Neurosci. 124, 236–242. doi: 10.3109/00207454.2013.833919
Biessels, G. J., and Reagan, L. P. (2015). Hippocampal insulin resistance and cognitive dysfunction. Nat. Rev. Neurosci. 16, 660–671. doi: 10.1038/nrn4019
Bischof, G. N., and Park, D. C. (2015). Obesity and aging: consequences for cognition, Brain structure, and brain function. Psychosom. Med. 77, 697–709. doi: 10.1097/PSY.0000000000000212
Bluher, M. (2019). Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 15, 288–298. doi: 10.1038/s41574-019-0176-8
Carracher, A. M., Marathe, P. H., and Close, K. L. (2018). International diabetes federation 2017. J. Diabetes 10, 353–356. doi: 10.1111/1753-0407.12644
Chen, C. W., Lin, C. C., Chen, K. B., Kuo, Y. C., Li, C. Y., and Chung, C. J. (2014). Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheime’s Dement. 10, 196–204. doi: 10.1016/j.jalz.2013.05.1766
Cholerton, B., Baker, L. D., and Craft, S. (2013). Insulin, cognition, and dementia. Eur. J. Pharmacol, 719, 170–179. doi: 10.1016/j.ejphar.2013.08.008
Claxton, A., Baker, L. D., Hanson, A., Trittschuh, E. H., Cholerton, B., Morgan, A., et al. (2015). Long-Acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s Disease dementia. J. Alzheimers Dis. 44, 897–906. doi: 10.3233/Jad-141791
Claxton, A., Baker, L. D., Wilkinson, C. W., Trittschuh, E. H., Chapman, D., Watson, G. S., et al. (2013). Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer’s Disease. J. Alzheimers Dis. 35, 789–797. doi: 10.3233/Jad-122308
Craft, S. (2005). Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol. Aging 26, S65–S69. doi: 10.1016/j.neurobiolaging.2005.08.021
Craft, S., Baker, L. D., Montine, T. J., Minoshima, S., Watson, G. S., Claxton, A., et al. (2012). Intranasal insulin therapy for alzheimer disease and amnestic mild cognitive impairment a pilot clinical trial. Arch. Neurol. Chicago 69, 29–38. doi: 10.1001/archneurol.2011.233
Ekblad, L. L., Rinne, J. O., Puukka, P., Laine, H., Ahtiluoto, S., Sulkava, R., et al. (2017). Insulin resistance predicts cognitive decline: an 11-year follow-up of a nationally representative adult population sample. Diabetes Care 40, 751–758. doi: 10.2337/dc16-2001
Evered, L., Silbert, B., Scott, D. A., Ames, D., Maruff, P., and Blennow, K. (2016). Cerebrospinal fluid biomarker for alzheimer disease predicts postoperative cognitive dysfunction. Anesthesiology 124, 353–361. doi: 10.1097/ALN.0000000000000953
Feinkohl, I., Winterer, G., and Pischon, T. (2016). Obesity and post-operative cognitive dysfunction: a systematic review and meta-analysis. Diabetes Metab. Res. 32, 643–651. doi: 10.1002/dmrr.2786
Feinkohl, I., Winterer, G., and Pischon, T. (2017a). Diabetes is associated with risk of postoperative cognitive dysfunction: a meta-analysis. Diabetes Metab. Res. 33:e2884. doi: 10.1002/dmrr.2884
Feinkohl, I., Winterer, G., and Pischon, T. (2017b). hypertension and risk of post-operative cognitive dysfunction (POCD): a systematic review and meta-analysis. Clin. Pract. Epidemiol. Men. Health CP EMH 13, 27–42. doi: 10.2174/1745017901713010027
Feinkohl, I., Winterer, G., and Pischon, T. (2018). Associations of dyslipidaemia and lipid-lowering treatment with risk of postoperative cognitive dysfunction: a systematic review and meta-analysis. J. Epidemiol. Commun. H 72, 499–506. doi: 10.1136/jech-2017-210338
Feng, X., Degos, V., Koch, L. G., Britton, S. L., Zhu, Y., Vacas, S., et al. (2013). Surgery results in exaggerated and persistent cognitive decline in a rat model of the metabolic syndrome. Anesthesiology 118, 1098–1105. doi: 10.1097/ALN.0b013e318286d0c9
Ferreira, S. T., Clarke, J. R., Bomfim, T. R., and De Felice, F. G. (2014). Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 10, S76–S83. doi: 10.1016/j.jalz.2013.12.010
Frisardi, V., Solfrizzi, V., Seripa, D., Capurso, C., Santamato, A., Sancarlo, D., et al. (2010). Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer’s disease. Ageing Res. Rev. 9, 399–417. doi: 10.1016/j.arr.2010.04.007
Grundy, S. M. (2003). Inflammation, hypertension, and the metabolic syndrome. Jama 290, 3000–3002. doi: 10.1001/jama.290.22.3000
Heni, M., Schopfer, P., Peter, A., Sartorius, T., Fritsche, A., Synofzik, M., et al. (2014). Evidence for altered transport of insulin across the blood-brain barrier in insulin-resistant humans. Acta Diabetol. 51, 679–681. doi: 10.1007/s00592-013-0546-y
Karczewska-Kupczewska, M., Tarasow, E., Nikolajuk, A., Stefanowicz, M., Matulewicz, N., Otziomek, E., et al. (2013). The effect of insulin infusion on the metabolites in cerebral tissues assessed with proton magnetic resonance spectroscopy in young healthy subjects with high and low insulin sensitivity. Diabetes Care 36, 2787–2793. doi: 10.2337/dc12-1437
Krenk, L., Rasmussen, L. S., and Kehlet, H. (2010). New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesth. Scand. 54, 951–956. doi: 10.1111/j.1399-6576.2010.02268.x
Kullmann, S., Heni, M., Hallschmid, M., Fritsche, A., Preissl, H., and Haring, H. U. (2016). Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol. Rev. 96, 1169–1209. doi: 10.1152/physrev.00032.2015
Lewis, M., Maruff, P., and Silbert, B. (2004). Statistical and conceptual issues in defining post-operative cognitive dysfunction. Neurosci. Biobehav. Rev. 28, 433–440. doi: 10.1016/j.neubiorev.2004.05.002
Lin, G. X., Wang, T., Chen, M. H., Hu, Z. H., and Ouyang, W. (2014). Serum high-mobility group box 1 protein correlates with cognitive decline after gastrointestinal surgery. Acta Anaesthesiol, Scand. 58, 668–674. doi: 10.1111/aas.12320
Marks, D. R., Tucker, K., Cavallin, M. A., Mast, T. G., and Fadool, D. A. (2009). Awake Intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J. Neurosci. 29, 6734–6751. doi: 10.1523/Jneurosci.1350-09.2009
Monk, T. G., Weldon, B. C., Garvan, C. W., Dede, D. E., Aa, M. T., Heilman, K. M., et al. (2008). Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108, 18–30. doi: 10.1097/01.anes.0000296071.19434.1e
Moran, C., Beare, R., Phan, T. G., Bruce, D. G., Callisaya, M. L., Srikanth, V., et al. (2015). Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 85, 1123–1130. doi: 10.1212/Wnl.0000000000001982
Nagpal, M., De, D., Handa, S., Pal, A., and Sachdeva, N. (2016). Insulin Resistance and metabolic syndrome in young men with acne. JAMA Dermatol. 152, 399–404. doi: 10.1001/jamadermatol.2015.4499
Neergaard, J. S., Dragsbaek, K., Christiansen, C., Nielsen, H. B., Brix, S., Karsdal, M. A., et al. (2017). Metabolic syndrome. insulin resistance, and cognitive dysfunction: does your metabolic profile affect your brain? Diabetes 66, 1957–1963. doi: 10.2337/db16-1444
Ohara, T., Doi, Y., Ninomiya, T., Hirakawa, Y., Hata, J., Iwaki, T., et al. (2011). Glucose tolerance status and risk of dementia in the community: the Hisayama study. Neurology 77, 1126–1134. doi: 10.1212/WNL.0b013e31822f0435
Rasmussen, L. S., Larsen, K., Houx, P., Skovgaard, L. T., Hanning, C. D., Moller, J. T., et al. (2001). The assessment of postoperative cognitive function. Acta Anaesthesiol. Scand. 45, 275–289.
Ridker, P. M., Buring, J. E., Cook, N. R., and Rifai, N. (2003). C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14 719 initially healthy American women. Circulation 107, 391–397. doi: 10.1161/01.cir.0000055014.62083.05
Roberts, R. O., Knopman, D. S., Cha, R. H., Mielke, M. M., Pankratz, V. S., Boeve, B. F., et al. (2014). Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J. Nucl. Med. 55, 759–764. doi: 10.2967/jnumed.113.132647
Sartorius, T., Peter, A., Heni, M., Maetzler, W., Fritsche, A., Haring, H. U., et al. (2015). The brain response to peripheral insulin declines with age: a contribution of the blood-brain barrier? Plos One 10:e0126804. doi: 10.1371/journal.pone.0126804
Schimming, C., Luo, X., Zhang, C., and Sano, M. (2017). Cognitive performance of older adults in a specialized diabetes clinic. J. Diabetes 9, 929–935. doi: 10.1111/1753-0407.12503
Schwartz, M. W., Figlewicz, D. F., Kahn, S. E., Baskin, D. G., Greenwood, M. R., and Porte, D. (1990). Insulin binding to brain capillaries is reduced in genetically obese, hyperinsulinemic Zucker rats. Peptides 11, 467–472. doi: 10.1016/0196-9781(90)90044-6
Sesti, G. (2006). Pathophysiology of insulin resistance. Best Pract. Res. Clin. Endocrinol. Metab. 20, 665–679. doi: 10.1016/j.beem.2006.09.007
Sheline, Y. I., and Raichle, M. E. (2013). Resting state functional connectivity in preclinical Alzheimer’s Disease. Biol. Psychiat. 74, 340–347. doi: 10.1016/j.biopsych.2012.11.028
Skvarc, D. R., Berk, M., Byrne, L. K., Dean, O. M., Dodd, S., Lewis, M., et al. (2018). Post-operative cognitive dysfunction: an exploration of the inflammatory hypothesis and novel therapies. Neurosci. Biobehav. Rev. 84, 116–133. doi: 10.1016/j.neubiorev.2017.11.011
Terrando, N., Monaco, C., Ma, D. Q., Foxwell, B. M. J., Feldmann, M., and Maze, M. (2010). Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. U.S.A. 107, 20518–20522. doi: 10.1073/pnas.1014557107
Thambisetty, M., Beason-Held, L. L., An, Y., Kraut, M., Metter, J., Egan, J., et al. (2013a). Impaired glucose tolerance in midlife and longitudinal changes in brain function during aging. Neurobiol. Aging 34, 2271–2276. doi: 10.1016/j.neurobiolaging.2013.03.025
Thambisetty, M., Jeffrey Metter, E., Yang, A., Dolan, H., Marano, C., Zonderman, A. B., et al. (2013b). Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the baltimore longitudinal study of aging. JAMA Neurol. 70, 1167–1172. doi: 10.1001/jamaneurol.2013.284
Tzimas, P., Petrou, A., Laou, E., Milionis, H., Mikhailidis, D. P., and Papadopoulos, G. (2015). Impact of metabolic syndrome in surgical patients: should we bother? Br. J. Anaesth 115, 194–202. doi: 10.1093/bja/aev199
Ursache, A., Wedin, W., Tirsi, A., and Convit, A. (2012). Preliminary evidence for obesity and elevations in fasting insulin mediating associations between cortisol awakening response and hippocampal volumes and frontal atrophy. Psychoneuroendocrinology 37, 1270–1276. doi: 10.1016/j.psyneuen.2011.12.020
Verdile, G., Fuller, S. J., and Martins, R. N. (2015). The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis. 84, 22–38. doi: 10.1016/j.nbd.2015.04.008
Yaffe, K. (2007). Metabolic syndrome and cognitive disorders - Is the sum greater than its parts? Alz. Dis. Assoc. Dis. 21, 167–171. doi: 10.1097/Wad.0b013e318065bfd6
Keywords: insulin resistance, metabolic risk factors, postoperative cognitive dysfunction, elderly, tumor necrosis factor α
Citation: He X, Long G, Quan C, Zhang B, Chen J and Ouyang W (2019) Insulin Resistance Predicts Postoperative Cognitive Dysfunction in Elderly Gastrointestinal Patients. Front. Aging Neurosci. 11:197. doi: 10.3389/fnagi.2019.00197
Received: 24 April 2019; Accepted: 15 July 2019;
Published: 08 August 2019.
Edited by:
Ferdinando Franzoni, University of Pisa, ItalyCopyright © 2019 He, Long, Quan, Zhang, Chen and Ouyang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Ouyang, b3V5d3RnQDE2My5jb20=
 Xi He
Xi He Ge Long
Ge Long Chengxuan Quan
Chengxuan Quan