- 1German Center for Neurodegenerative Diseases (DZNE), Dresden, Germany
- 2Center for Regenerative Therapies (CRTD) TU Dresden, Dresden, Germany
To effectively promote life-long health and resilience against – for example – neurodegenerative diseases, evidence-based recommendations must acknowledge the complex multidimensionality not only of the diseases but also of personal lifestyle. In a straightforward descriptive and heuristic framework, more than 50 potential lifestyle factors cluster around diet (D), education (E), exercise (E), and purpose (P), unveiling their many relationships across domains and scales. The resulting systematics and its visualization might be a small but helpful step toward the development of more comprehensive, interdisciplinary models of lifestyle-dependent risk and resilience and a means to explain the opportunities and limitations of preventive measures to the public and other stakeholders. Most importantly, this perspective onto the subject implies that not all lifestyle factors are created equal but that there is a hierarchy of values and needs that influences the success of lifestyle-based interventions.
Introduction
Lifestyle-based health interventions promise solutions to pressing health problems by providing broad access to prevention and healthy aging. Preventing dementia is a prime target of these ambitions. After hundreds of failed clinical trials for treatments of neurodegenerative disease, especially of Alzheimer’s disease (AD) (Cummings et al., 2014), the insight has grown that neither conventional pharmacological targets nor immune-based strategies alone will be sufficient to conquer the problem at large. Some trials have thus reformulated their goal from curing manifest pathology to secondary prevention (Livingston et al., 2017).
This step is wise, because despite the failing therapeutic trials, within age-cohorts the risk for AD has been decreasing over the past decades (Derby et al., 2017). This “success” cannot be attributed to specific therapeutic interventions. Given that genetic factors did not change during this period, the decrease indicates that, in sum, modifiable factors must have exerted a measurable positive impact. As Fries et al. (2011) already wrote in 2011: “If we can accomplish morbidity compression without a strategy, as over the past thirty years, then we should be able to further improve if we have a plan.” How might such plan be developed?
Given the complexity of the subject, one critical prerequisite is to first systematize the available knowledge. This is necessary, because “lifestyle factors” are a category without sharp boundaries and a common definition but with many stakeholder-specific connotations.
The proposed DEEP framework is a simple way to support, yet not replace, this process across domains, disciplines, and stakeholders. It consists of common language descriptors that in the absence of a unifying theory and a comprehensive model of lifestyle-dependent risk and resilience offer a systematic summary accessible to stakeholders across the professional disciplines and the public alike.
Challenges of Lifestyle-Based Interventions
“Resilience” is here defined as the comprehensive ability to deal with adversity. Resilience is here not explicitly distinguished from “resistance,” which is often used to specifically identify the ability to ward off pathology. The term “lifestyle factors for risk and resilience” is a heuristic concept to structure the large group of potentially modifiable factors with proven or face value influence on health, that at least in theory can be influenced by the individual by his or her own actions. Actions based on these factors increase or decrease risk and reduce or promote resilience. Low levels of physical activity, for example, are associated with a shorter life expectancy and the increased incidence of cardiovascular disease, cancer, neurodegeneration, and other health issues (Lee et al., 2012); being physically active in turn reduces those risks and prolongs life (Schnohr et al., 2013). This duality might suggest the existence of an equilibrium that could or should be obtained and maintained.
The general usefulness of the term “lifestyle factors” notwithstanding, the heterogeneity of such factors and the conceptual and practical difficulty of capturing what people more broadly mean by “lifestyle” endanger the concept to become either too diffuse or too narrow. While in a scientific study context, lifestyle might have to be reduced to a manageable number of variables that can be measured and that show statistically significant correlations with outcome measures of interest (as well as relevant effect sizes), be it for example longevity or disease-free years, it is not trivial to answer the question of how these variables actually contribute to subjective “lifestyle.” The term “style” refers to this “how” and to rather personal ideas of “leading a good life.” “Lifestyle” is obviously much more than the sum of identifiable “lifestyle factors.”
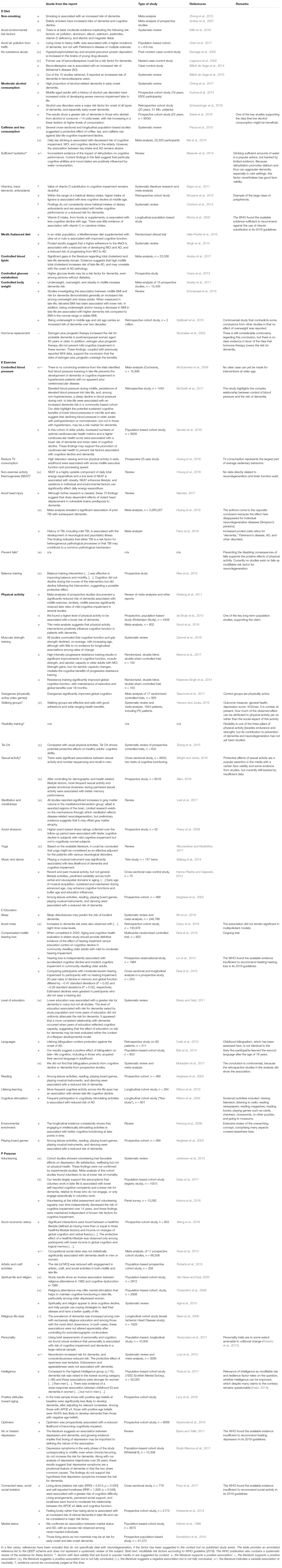
To understand the impact of lifestyle it is thus not advisable to restrict consideration to only those factors for which the “best” evidence (i.e., large effect sizes in population studies) exists, such as those in the SNAP scheme, which only covers smoking, nutrition, alcohol, and physical activity (Noble et al., 2015). A consensus publication in Lancet Neurology from 2017 highlighted nine factors (Livingston et al., 2017), which essentially match with other consensus lists (Deckers et al., 2015). They are also by and large identical to the set of factors that has been identified for longevity and the prevention of other, mostly age-related diseases, including cancer. The WHO lists eight such general factors plus four more specific to dementia (World Health Organization, 2017). In 2019, the WHO issued guidelines on these factors and supported its recommendations with detailed systematic reviews (World Health Organization, 2019). While this was an important step, the WHO has also been criticized for including recommendations with relatively weak evidence (management of hypertension and diabetes), while omitting other factors with potential benefit and low risks of side effects (treatment of hearing loss and depression) (Lancet, 2019). It was felt that the WHO had missed an opportunity to make even stronger statements for of public health and wake up governments to take the necessary actions. Moreover, in this domain and given the type of existing studies, it is problematic to base recommendations solely on best evidence as generated by clinical studies. The absence of evidence is also here no evidence of absence.
In addition, studies by necessity have to single out identifiable factors for the sake of design, feasibility, and statistical power. There are also first combinatorial and multi-domain studies such as the FINGERS trial (Ngandu et al., 2015) or the SMARTT trial (Yaffe et al., 2018). Even the most comprehensive multidomain study, however, will neither be able to capture the emerging qualities of actual lifestyle nor the one aspect that most people will intuitively value most: that this is their own chosen way of leading their life. The question of how to translate under such conditions from a study setting into everyday life remains extremely challenging.
The DEEP Framework
Dependent on stringency and definitions, at least 50 lifestyle risk and resilience factors can be identified (Table A1), that to a variable extent have been discussed in the literature. In this situation of an overabundance of potentially relevant aspects, a simple conceptual framework would facilitate knowledge management, promote sense-making in face of this complexity, and stimulate the development of more sophisticated and causal models across stakeholders, domains, and disciplines. A knowledge management framework can greatly support organization of lifestyle factors in their relations to each other and to larger-scale concepts such as “quality of life,” “well-being,” “happiness,” or, of course, “lifestyle” itself. The DEEP scheme visualizes key relationships between the large number of known (as well as perceived and hypothesized) lifestyle risk and resilience factors (Figures 1A,B).
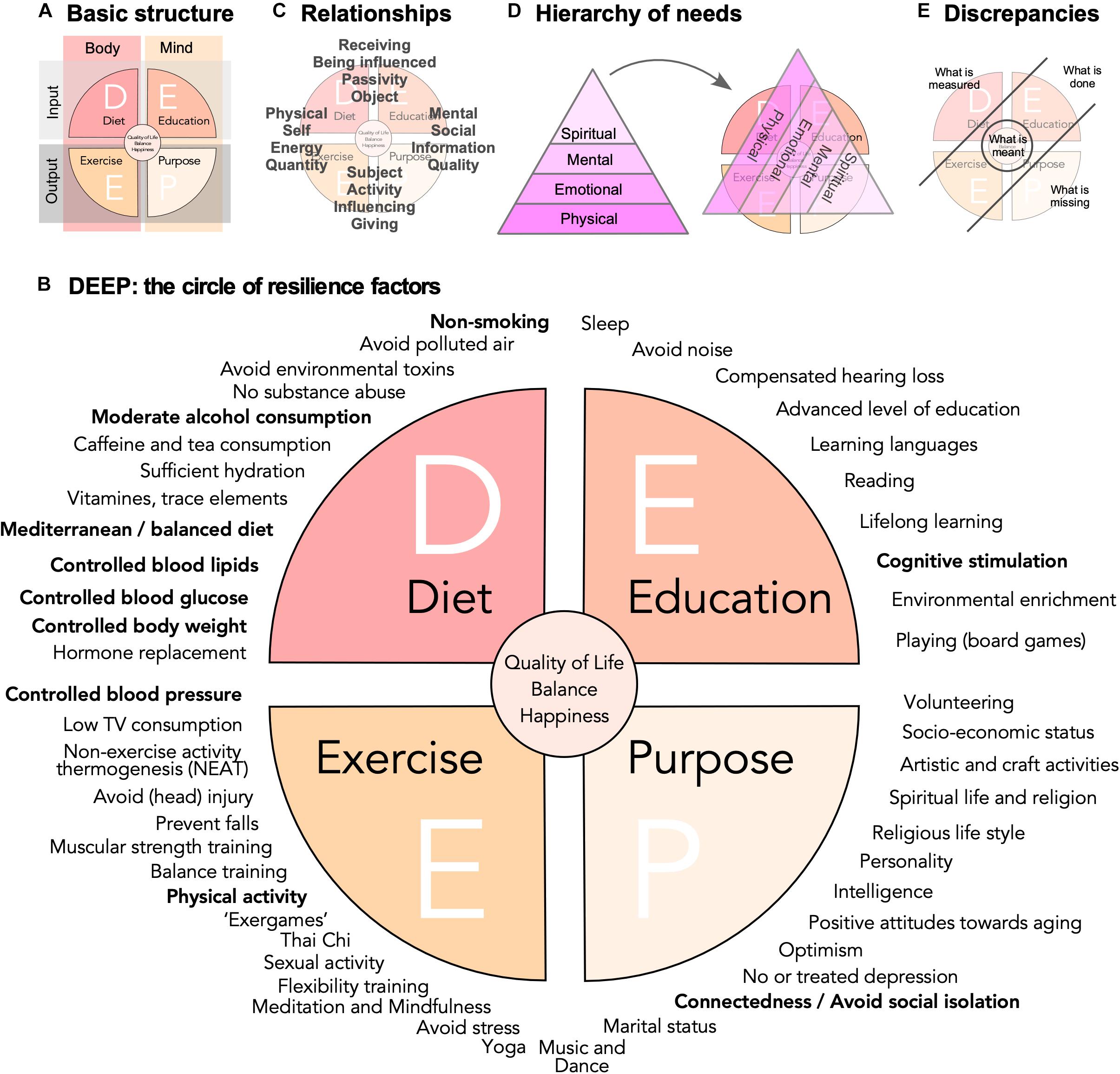
Figure 1. Risk and resilience factors for neurodegenerative disease. (A) The DEEP scheme is based on a four-field matrix, spanned out by body and mind as columns, and input and output as rows. (B) A non-exclusive list of potential lifestyle risk and resilience factors is represented as a circle with the four quadrants obtained in (A). The WHO factors from the 2019 guidelines are highlighted in bold. They cluster largely in the D quadrant. The WHO in addition states explicitly that vitamin substitution and polyunsaturated fatty acids should not be recommended. Quality of evidence varies greatly, but face validity and popularity might be very high for some factors, for which little data exist. (C) The depiction of the DEEP scheme highlights certain relation pairs and tension fields, which are here meant only as illustration of the complex network of relationships that exist and might influence the impact of lifestyle risk and resilience factors and interventions based on them. (D) As visualized by a hierarchy of needs that drive motivation, here depicted in a version according to Loehr and Schwartz (2005), people will assign different values to different factors and interventions. (E) Further depicts the discrepancy of what is measured (and recommended) and what is done on one side and what is perceived as missing and what is actually meant on the other.
Basis of the framework is a four-field matrix with Body and Mind as columns and Input and Output as rows (Figure 1A). “Diet” (D) would stand for bodily, physical intake and “Education” (E) for sensory, cognitive, and emotional input. “Exercise” (E) represents the domain of physical activity in the broadest sense, while the “Purpose” (P) quadrant is everything that relates between individual’s inner and outer world through his or her own actions. In other words, the four quadrants are related to (Line 1) what we take in (D) physically and (E) cognitively and (Line 2) what we spend (E) physically and (P) cognitively. This captures what essentially all more or less intuitive and research-based advice in this area tells us: Eat well (D), be physically and cognitively active (E+E), and engage (P). Labels for the quadrants and their explanation are emblematic and should not be taken too narrowly: “education,” for example, here stands for mental activity and function in a much broader sense than many scholarly definitions suggest. “Purpose” stands for socially and mentally (or even spiritually) goal-directed activities. The labels are associative anchors, permitting to bring structure into the widest possible scope. It is important not to over-define these categories.
The lifestyle factors, for whose effectiveness we have the best evidence, are some with the highest level of abstraction but with good measurability such as body weight or glucose levels. Education or physical activity cannot be measured directly but usually only be assessed via reductionistic proxies (e.g., VO2max for bodily fitness or self-reporting). For some factors in the P quadrant even qualitative proxies are a problem. But the P quadrant contains items that we would consider essentially human and which are determinants of well-being. To base our understanding of lifestyle solely on the relatively few quantifiable factors with “significant” effect sizes is as obviously incomplete as ascribing the genetic risk of complex disease only to the common polymorphisms with large effect sizes. Much like the “missing heritability” in the genetics of complex traits (Manolio et al., 2009), a large number of modifiable factors with small effect sizes and poor detectability will add up to explain a very large part of the total interindividual variance in lifestyle risk and resilience. As much as complex traits are “omnigenic” (Boyle et al., 2017), lifestyle-dependent risk and resilience will be “omni-factorial.”
Appreciating complexity is not per se an argument against pursuing lower hanging fruits, i.e., by implementing exercise programs, improving school food, and quit smoking (Norton et al., 2014). These are difficult enough. But while to reduce time spent sitting would be a valuable step forward, regularly standing up at work does not really amount to a different life style. Changing one’s life requires more than working down a check-list of good arguments.
The descriptive inclusiveness is also no argument against applying rigor and the full range of reductionistic instruments of evidence-based medicine to study the individual factors! Those approaches are mandatory for understanding effect sizes, weights of factors, and interaction effects.
The (P) Purpose Quadrant
Descriptions of lifestyle factors that are centered on diet, exercise, and cognitive activity/education might leave a blind spot exactly where many people would intuitively focus, when asked what matters most in life. Here, social and spiritual aspects rank very high: family and friends as well as mental and religious life, purpose, and autonomy. Certain “lifestyle” risk and resilience factors correspond to this preference: remaining in charge, the role of partnership and friendship, spirituality and religion, taking responsibility for others, etc. Such factors might exert a measurable impact: for example, positive beliefs about aging more or less compensated for the increased risk for AD associated with carrying the ApoEε4 polymorphism (Levy et al., 2018).
The dimension of Purpose-related factors is nevertheless often missing from the high-level aggregation in the discussion of “healthy lifestyle,” presumably because the evidence for these factors does not match the standards (of quantification) in the other domains. But for most people “health” in the sense of the medical professions is no end in itself. They intuitively have a broader, more implicit understanding, seamlessly integrating with “quality of life”: Leading a healthy life is more than the absence of disease. The concept of “successful aging,” otherwise not without its own problems (Bowling and Dieppe, 2005), can add this missing dimension.
Relationships
In the DEEP scheme, key relationships between domains are emphasized and the communality of lifestyle factors, to which many studies have pointed (Norton et al., 2014), are visible across the entire range of factors, independent of their nature. The scheme is thereby highly interdisciplinary and generous toward different scientific cultures. This is important, because no single discipline covers the full range of contributing factors.
The four quadrants can be paired in various, non-exclusive combinations (Figure 1C). The scheme thereby not only spans out between body and mind and input and output, but at the same time self and social, energy and information, reception and action, quantity and quality, etc. The challenge is to conceptualize, model, and measure such complex relationships and avoid pitfalls (Kempermann, 2017). The secret for livable strategies for successful (cognitive) aging based on lifestyle risk and resilience factors might foremost lie in the individual manifestation of these relationships. Neither represent these ranges dichotomies nor are the tension fields they establish orthogonal to each other. They are rather exemplary forces weaving a matrix of interdependencies.
Some of such interdependencies are known better than others and a few are widely acknowledged: the most obvious might be the relationship between energy intake and physical activity. Many studies calculate communalities, but what these interdependencies ultimately mean is rarely explored, especially for factors that are not linked in a way as obvious as in the case of caloric intake and expenditure. The classical triangle of (Mediterranean) diet, physical exercise, and cognitive activity/education also emphasizes potential links, but takes only three (albeit very important ones) out of an extensive network.
The step from the mere description in the DEEP scheme to a model of the network of factors requires different sets of data than presently available in most cases. Multivariate cohort studies, observational or interventional, can generate such data sets, if they collect information across all four DEEP quadrants.
The Question of Perspective
The discrepancy between lifestyle in the public-health-centered sense of recommendations and programs for prevention (mostly found in the DEE quadrants) and in what most people would see as integral parts of their personal lifestyle (represented by the P quadrant) might help to explain why the implementation of lifestyle interventions is so difficult. Recommendations based primarily on what can be measured (body weight, blood pressure, caloric intake, VO2max, etc.) see lifestyle largely through the filter of basic needs. While these are critical foundations of lifestyle they only partly coincide with our personal hierarchy of needs.
Maslow’s hierarchy of needs, usually displayed in form of a pyramid, remains a very popular means to visualize the essential observation that we differently value the driving forces behind our actions and, by extension, our lifestyle (Maslow, 1954). Various versions exist. Figure 1D depicts a variant from the coaching literature (Loehr and Schwartz, 2005), underscoring that such concepts are widely applied in professions that aim at empowering people to master change. People tend to favor a view that moves from the fulfillment of basic needs toward personal growth, self-actualization, and self-transcendence. While the depiction as pyramid somewhat blurs the fact that motivations arising from many different layers are effective concomitantly and synergistically, its suggestive strengths lie in the identification of an order and a hierarchical value we assign to them. A pyramid of needs, tilted by 120°, superimposed on the DEEP scheme, reveals that within the scope of lifestyle factors there is a hidden hierarchy of values.
Recommendations focusing on what can be measured might hence come across as superficial and trivial (Figure 1E). Emphasizing only the P quadrant is no valid solution either, because those strategies lack the concreteness of the physical dimension they have to build upon. But P might provide the drive to implement DEE.
DEEP Implementations
This implies that a holistic consideration of lifestyle risk and resilience factors along the DEEP scheme might help to develop causal models as basis of individualized strategies for healthy (cognitive) aging and the prevention of (neurodegenerative) disease. Interventions with large demonstrated effect sizes would obviously still be prioritized, but their practical realization and their contextual embedding would be improved by individualized multi-factorial approaches.
Physical activity, for example, remains the preventive “super factor” with massive effect sizes across many domains. Realizing its potential alone would profoundly change health and disease in any population. And most people know that they should (and actually would like to) be more physically active: there is no lack of insight and intentions. The implementation, however, of seemingly simple interventions based on reducing sedentary lifestyle and increasing physical activity is extremely difficult. Exercise-based programs often show remarkably little long-term effects on lifestyle. The same applies to nutritional recommendation, which are popular and often have high face validity. But dietary interventions are notoriously difficult to study, the underlying metabolic mechanisms to which these interventions refer show great inter-individual genetic differences, and eating food is much more than nutrition.
Measures centering only on single or few habits become isolated from the contexts of the individual’s life. New habits that were formed in the lab lack anchoring in everyday life.
Good and bad habits are context-sensitive and circumstances trigger habitual behaviors. Changing habits is difficult, if the world around us reinforces them. The question is, to which extent circumstances can be changed. While there is no general answer to this, there will be room for individual solutions that adapt lifestyle to conditions at hand and explore the available room for development. The DEEP scheme visualizes the scope of such co-factors.
What Lies at the Center?
The large cohort studies and meta-analyses indicate that the identifiable factors with large effect sizes are not independent of each other. On one side this suggests that an abstract “super factor” might be identifiable, comparable to the G factor in intelligence.
On the other hand, however, the communalities also indicate that there is more than one road to Rome. They might hide the range of options for the individual to achieve his or her goals.
The DEEP scheme might suggest that the greatest reduction in risk and the greatest level of resilience is found in individuals with a somewhat balanced nature within and between the four quadrants (Figure 1E). How would such balance relate to “quality of life”? While assessment of quality of life constitutes a major achievement in selecting appropriate, relevant “endpoints” of clinical studies, they are only surrogates for even more profound constructs such as happiness. Is a resilient individual also a happier individual? If so, what would be the direction of causality here? Positive psychology suggests that happiness can to some degree be induced. If “style” is an emerging property from the complexity of life, then so is happiness. An important question thus is, whether the assumed causality structure and interventional strategy can be put on its head. Is it possible to improve happiness and quality of life and see changes in measurable lifestyle factors as consequence or byproduct? Or, is the true center an attitude such as to “develop character in the face of the inevitable suffering,” as Peterson (2018) has put it, and thereby consider style of life in the light of giving meaning to life?
Working from the center and the intended result of “leading a good life,” including the full spectrum of our needs and values, is one way of altering contexts to our benefit. Ironically, despite being much more complex, multi-factorial changes might be more successful and ultimately easier: small steps at a time, but along different directions (as indicated in the many options of the DEEP scheme), might ultimately be more efficient and effective than focusing on one high-gain domain alone. They would lead to a diversification of the individual portfolio of lifestyle risk and resilience.
Data Availability
No datasets were generated or analyzed for this study.
Author Contributions
GK conceived the concept presented in this article, collected the relevant information, and wrote the manuscript.
Funding
This work was partly funded with support of the Helmholtz Network of Excellence for writing and publication of the article. The funder did not exert any influence on the content of this article.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Agli, O., Bailly, N., and Ferrand, C. (2015). Spirituality and religion in older adults with dementia: a systematic review. Int. Psychogeriatr. 27, 715–725. doi: 10.1017/S1041610214001665
Ahlskog, J. E., Geda, Y. E., Graff-Radford, N. R., and Petersen, R. C. (2011). Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin. Proc. 86, 876–884. doi: 10.4065/mcp.2011.0252
Allen, M. S. (2018). Sexual activity and cognitive decline in older adults. Arch. Sex Behav. 47, 1711–1719. doi: 10.1007/s10508-018-1193-8
Anstey, K. J., Ashby-Mitchell, K., and Peters, R. (2017). Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. J. Alzheimers Dis. 56, 215–228. doi: 10.3233/JAD-160826
Anstey, K. J., Cherbuin, N., Budge, M., and Young, J. (2011). Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes. Rev. 12, e426–e437. doi: 10.1111/j.1467-789X.2010.00825.x
Anstey, K. J., Sanden von, C., Salim, A., and O’Kearney, R. (2007). Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am. J. Epidemiol. 166, 367–378. doi: 10.1093/aje/kwm116
Bak, T. H., Nissan, J. J., Allerhand, M. M., and Deary, I. J. (2014). Does bilingualism influence cognitive aging? Ann. Neurol. 75, 959–963. doi: 10.1002/ana.24158
Balbag, M. A., Pedersen, N. L., and Gatz, M. (2014). Playing a musical instrument as a protective factor against dementia and cognitive impairment: a population-based twin study. Int. J. Alzheimer’s Dis. 2014, 1–6. doi: 10.1155/2014/836748
Beeri, M. S., Davidson, M., Silverman, J. M., Schmeidler, J., Springer, R. R., Noy, S., et al. (2008). Religious education and midlife observance are associated with dementia three decades later in Israeli men. J. Clin. Epidemiol. 61, 1161–1168. doi: 10.1016/j.jclinepi.2007.09.011
Billioti de Gage, S., Moride, Y., Ducruet, T., Kurth, T., Verdoux, H., Tournier, M., et al. (2014). Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 349, g5205. doi: 10.1136/bmj.g5205
Billioti de Gage, S., Pariente, A., and Bégaud, B. (2015). Is there really a link between benzodiazepine use and the risk of dementia? Expert. Opin. Drug Saf. 14, 733–747. doi: 10.1517/14740338.2015.1014796
Bowling, A., and Dieppe, P. (2005). What is successful ageing and who should define it? BMJ 331, 1548–1551. doi: 10.1136/bmj.331.7531.1548
Boyle, E. A., Li, Y. I., and Pritchard, J. K. (2017). An expanded view of complex traits: from polygenic to omnigenic. Cell 169, 1177–1186. doi: 10.1016/j.cell.2017.05.038
Byers, A. L., and Yaffe, K. (2011). Depression and risk of developing dementia. Nat. Rev. Neurol. 7, 323–331. doi: 10.1038/nrneurol.2011.60
Carey, I. M., Anderson, H. R., Atkinson, R. W., Beevers, S. D., Cook, D. G., Strachan, D. P., et al. (2018). Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8:e022404. doi: 10.1136/bmjopen-2018-022404
Chen, H., Kwong, J. C., Copes, R., Tu, K., Villeneuve, P. J., van Donkelaar, A., et al. (2017). Living near major roads and the incidence of dementia. Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 389, 718–726. doi: 10.1016/S0140-6736(16)32399-6
Cheng, C., Huang, C.-L., Tsai, C.-J., Chou, P.-H., Lin, C.-C., and Chang, C.-K. (2017). Alcohol-related dementia: a systemic review of epidemiological studies. Psychosomatics 58, 331–342. doi: 10.1016/j.psym.2017.02.012
Chung, N., Park, M.-Y., Kim, J., Park, H.-Y., Hwang, H., Lee, C.-H., et al. (2018). Non-exercise activity thermogenesis (NEAT): a component of total daily energy expenditure. JENB 22, 23–30. doi: 10.20463/jenb.2018.0013
Corsentino, E. A., Collins, N., Sachs-Ericsson, N., and Blazer, D. G. (2009). Religious attendance reduces cognitive decline among older women with high levels of depressive symptoms. J. Gerontol. A Biol. Sci. Med. Sci. 64, 1283–1289. doi: 10.1093/gerona/glp116
Craik, F. I. M., Bialystok, E., and Freedman, M. (2010). Delaying the onset of Alzheimer disease: bilingualism as a form of cognitive reserve. Neurology 75, 1726–1729. doi: 10.1212/WNL.0b013e3181fc2a1c
Crane, P. K., Walker, R., Hubbard, R. A., Li, G., Nathan, D. M., Zheng, H., et al. (2013). Glucose levels and risk of dementia. N. Engl. J. Med. 369, 540–548. doi: 10.1056/NEJMoa1215740
Crichton, G. E., Bryan, J., and Murphy, K. J. (2013). Dietary antioxidants, cognitive function and dementia–a systematic review. Plant Foods Hum. Nutr. 68, 279–292. doi: 10.1007/s11130-013-0370-0
Cummings, J. L., Morstorf, T., and Zhong, K. (2014). Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 6:37. doi: 10.1186/alzrt269
de Bruijn, R. F., Schrijvers, E. M. C., de Groot, K. A., Witteman, J. C. M., Hofman, A., Franco, O. H., et al. (2013). The association between physical activity and dementia in an elderly population: the Rotterdam Study. Eur. J. Epidemiol. 28, 277–283. doi: 10.1007/s10654-013-9773-3
Deal, J. A., Goman, A. M., Albert, M. S., Arnold, M. L., Burgard, S., Chisolm, T., et al. (2018). Hearing treatment for reducing cognitive decline: design and methods of the Aging and Cognitive Health Evaluation in Elders randomized controlled trial. Alzheimers Dement 4, 499–507. doi: 10.1016/j.trci.2018.08.007
Deal, J. A., Sharrett, A. R., Albert, M. S., Coresh, J., Mosley, T. H., Knopman, D., et al. (2015). Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am. J. Epidemiol. 181, 680–690. doi: 10.1093/aje/kwu333
Deckers, K., van Boxtel, M. P. J., Schiepers, O. J. G., de Vugt, M., Muñoz Sánchez, J. L., Anstey, K. J., et al. (2015). Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int. J. Geriatr. Psychiatry 30, 234–246. doi: 10.1002/gps.4245
Derby, C. A., Katz, M. J., Lipton, R. B., and Hall, C. B. (2017). Trends in dementia incidence in a birth cohort analysis of the Einstein aging study. JAMA Neurol. 74, 1345–1347. doi: 10.1001/jamaneurol.2017.1964
Emmerzaal, T. L., Kiliaan, A. J., and Gustafson, D. R. (2015). 2003-2013: a decade of body mass index, Alzheimer’s disease, and dementia. J. Alzheimers Dis. 43, 739–755. doi: 10.3233/JAD-141086
Etgen, T., Sander, D., Bickel, H., and Förstl, H. (2011). Mild cognitive impairment and dementia: the importance of modifiable risk factors. Dtsch. Arztebl. Int. 108, 743–750. doi: 10.3238/arztebl.2011.0743
Fiatarone Singh, M. A., Gates, N., Saigal, N., Wilson, G. C., Meiklejohn, J., Brodaty, H., et al. (2014). The Study of Mental and Resistance Training (SMART) study—resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 15, 873–880. doi: 10.1016/j.jamda.2014.09.010
Fries, J. F., Bruce, B., and Chakravarty, E. (2011). Compression of morbidity 1980–2011: a focused review of paradigms and progress. J. Aging Res. 2011, 1–10. doi: 10.4061/2011/261702
Gawronski, K. A. B., Kim, E. S., Langa, K. M., and Kubzansky, L. D. (2016). Dispositional optimism and incidence of cognitive impairment in older adults. Psychosom. Med. 78, 819–828. doi: 10.1097/PSY.0000000000000345
Griep, Y., Hanson, L. M., Vantilborgh, T., Janssens, L., Jones, S. K., and Hyde, M. (2017). Can volunteering in later life reduce the risk of dementia? A 5-year longitudinal study among volunteering and non-volunteering retired seniors. PLoS One 12:e0173885. doi: 10.1371/journal.pone.0173885
Groot, C., Hooghiemstra, A. M., Raijmakers, P. G., van Berckel, B. N. M., Scheltens, P., Scherder, E. J. A., et al. (2016). The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res. Rev. 25, 13–23. doi: 10.1016/j.arr.2015.11.005
Haier, R. J. (2014). Increased intelligence is a myth (so far). Front. Syst. Neurosci. 8:34. doi: 10.3389/fnsys.2014.00034
Hanna-Pladdy, B., and Gajewski, B. (2012). Recent and past musical activity predicts cognitive aging variability: direct comparison with general lifestyle activities. Front. Hum. Neurosci. 6:198. doi: 10.3389/fnhum.2012.00198
Hanson, S., and Jones, A. (2015). Is there evidence that walking groups have health benefits? A systematic review and meta-analysis. Br. J. Sports Med. 49, 710–715. doi: 10.1136/bjsports-2014-094157
Helmer, C., Damon, D., Letenneur, L., Fabrigoule, C., Barberger-Gateau, P., Lafont, S., et al. (1999). Marital status and risk of Alzheimer’s disease: a French population-based cohort study. Neurology 53, 1953–1958. doi: 10.1212/WNL.53.9.1953
Hertzog, C., Kramer, A. F., Wilson, R. S., and Lindenberger, U. (2008). Enrichment effects on adult cognitive development. Psychol. Sci. Public Interest 9:1.
Hoang, T. D., Reis, J., Zhu, N., Jacobs, D. R., Launer, L. J., Whitmer, R. A., et al. (2016). Effect of early adult patterns of physical activity and television viewing on midlife cognitive function. JAMA Psychiatry 73, 73–79. doi: 10.1001/jamapsychiatry.2015.2468
Holwerda, T. J., Deeg, D. J. H., Beekman, A. T. F., van Tilburg, T. G., Stek, M. L., Jonker, C., et al. (2014). Feelings of loneliness, but not social isolation, predict dementia onset: results from the Amsterdam Study of the Elderly (AMSTEL). J. Neurol. Neurosurg. Psychiatr. 85, 135–142. doi: 10.1136/jnnp-2012-302755
Huang, C.-H., Lin, C.-W., Lee, Y.-C., Huang, C.-Y., Huang, R.-Y., Tai, Y.-C., et al. (2018). Is traumatic brain injury a risk factor for neurodegeneration? A meta-analysis of population-based studies. BMC Neurol. 18:184. doi: 10.1186/s12883-018-1187-0
Infurna, F. J., Okun, M. A., and Grimm, K. J. (2016). Volunteering Is Associated with Lower Risk of Cognitive Impairment. J. Am. Geriatr. Soc. 64, 2263–2269. doi: 10.1111/jgs.14398
Jenkinson, C. E., Dickens, A. P., Jones, K., Thompson-Coon, J., Taylor, R. S., Rogers, M., et al. (2013). Is volunteering a public health intervention? A systematic review and meta-analysis of the health and survival of volunteers. BMC Public Health 13:773. doi: 10.1186/1471-2458-13-773
Kempermann, G. (2017). Cynefin as reference framework to facilitate insight and decision-making in complex contexts of biomedical research. Front. Neurosci. 11:634. doi: 10.3389/fnins.2017.00634
Killin, L. O. J., Starr, J. M., Shiue, I. J., and Russ, T. C. (2016). Environmental risk factors for dementia: a systematic review. BMC Geriatr. 16:175. doi: 10.1186/s12877-016-0342-y
Kuźma, E., Llewellyn, D. J., Langa, K. M., Wallace, R. B., and Lang, I. A. (2014). History of alcohol use disorders and risk of severe cognitive impairment: a 19-year prospective cohort study. Am. J. Geriatr. Psychiatry 22, 1047–1054. doi: 10.1016/j.jagp.2014.06.001
Lagnaoui, R., Bégaud, B., Moore, N., Chaslerie, A., Fourrier, A., Letenneur, L., et al. (2002). Benzodiazepine use and risk of dementia: a nested case-control study. J. Clin. Epidemiol. 55, 314–318.
Lancet, T. (2019). Editorial Reducing the risk of dementia. Lancet 393:2009. doi: 10.1016/S0140-6736(19)31085-2
Last, N., Tufts, E., and Auger, L. E. (2017). The effects of meditation on grey matter atrophy and neurodegeneration: a systematic review. J. Alzheimers Dis. 56, 275–286. doi: 10.3233/JAD-160899
Lee, I.-M., Shiroma, E. J., Lobelo, F., Puska, P., Blair, S. N., Katzmarzyk, P. T., et al. (2012). Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380, 219–229. doi: 10.1016/S0140-6736(12)61031-9
Levy, B. R., Slade, M. D., Pietrzak, R. H., and Ferrucci, L. (2018). Positive age beliefs protect against dementia even among elders with high-risk gene. PLoS One 13:e0191004. doi: 10.1371/journal.pone.0191004
Lin, F. R., Yaffe, K., Xia, J., Xue, Q.-L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern. Med. 173, 293–297. doi: 10.1001/jamainternmed.2013.1868
Livingston, G., Sommerlad, A., Orgeta, V., Costafreda, S. G., Huntley, J., Ames, D., et al. (2017). Dementia prevention, intervention, and care. Lancet 390, 2673–2734. doi: 10.1016/S0140-6736(17)31363-6
Loehr, J. E., and Schwartz, T. (2005). The Power of Full Engagement: Managing Energy, Not Time, Is the Key to High Performance and Personal Renewal. New York, NY: Simon & Schuster.
Low, L.-F., Harrison, F., and Lackersteen, S. M. (2013). Does personality affect risk for dementia? A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 21, 713–728. doi: 10.1016/j.jagp.2012.08.004
Ma, Q.-P., Huang, C., Cui, Q.-Y., Yang, D.-J., Sun, K., Chen, X., et al. (2016). Meta-analysis of the association between tea intake and the risk of cognitive disorders. PLoS One 11:e165861–e165818. doi: 10.1371/journal.pone.0165861
Manolio, T. A., Collins, F. S., Cox, N. J., Goldstein, D. B., Hindorff, L. A., Hunter, D. J., et al. (2009). Finding the missing heritability of complex diseases. Nature 461, 747–753. doi: 10.1038/nature08494
Masento, N. A., Golightly, M., Field, D. T., Butler, L. T., and van Reekum, C. M. (2014). Effects of hydration status on cognitive performance and mood. Br. J. Nutr. 111, 1841–1852. doi: 10.1017/S0007114513004455
Mavros, Y., Gates, N., Wilson, G. C., Jain, N., Meiklejohn, J., Brodaty, H., et al. (2017). Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: outcomes of the study of mental and resistance training. J. Am. Geriatr. Soc. 65, 550–559. doi: 10.1111/jgs.14542
McGrath, E. R., Beiser, A. S., DeCarli, C., Plourde, K. L., Vasan, R. S., Greenberg, S. M., et al. (2017). Blood pressure from mid- to late life and risk of incident dementia. Neurology 89, 2447–2454. doi: 10.1212/WNL.0000000000004741
McGuinness, B., Todd, S., Passmore, P., and Bullock, R. (2009). Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst. Rev. 18, CD004034. doi: 10.1002/14651858.CD004034.pub3
Mendez, M. F. (2017). What is the relationship of traumatic brain injury to dementia? JAD 57, 667–681. doi: 10.3233/JAD-161002
Mooventhan, A., and Nivethitha, L. (2017). Evidence based effects of yoga in neurological disorders. J. Clin. Neurosci. 43, 61–67. doi: 10.1016/j.jocn.2017.05.012
Morris, M. C., Evans, D. A., Bienias, J. L., Tangney, C. C., and Wilson, R. S. (2002). Vitamin E and cognitive decline in older persons. Arch. Neurol. 59, 1125–1132. doi: 10.1001/archneur.62.4.641
Mukadam, N., Sommerlad, A., and Livingston, G. (2017). The relationship of bilingualism compared to monolingualism to the risk of cognitive decline or dementia: a systematic review and meta-analysis. J. Alzheimers Dis. 58, 45–54. doi: 10.3233/JAD-170131
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
Noble, N., Paul, C., Turon, H., and Oldmeadow, C. (2015). Which modifiable health risk behaviours are related? A systematic review of the clustering of Smoking, Nutrition, Alcohol and Physical activity (“SNAP”) health risk factors. Prev. Med. 81, 16–41. doi: 10.1016/j.ypmed.2015.07.003
Nooyens, A. C. J., Milder, I. E. J., van Gelder, B. M., Bueno-de-Mesquita, H. B., van Boxtel, M. P. J., and Verschuren, W. M. M. (2015). Diet and cognitive decline at middle age: the role of antioxidants. Br. J. Nutr. 113, 1410–1417. doi: 10.1017/S0007114515000720
Norton, S., Matthews, F. E., Barnes, D. E., Yaffe, K., and Brayne, C. (2014). Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 13, 788–794. doi: 10.1016/S1474-4422(14)70136-X
Panza, F., Solfrizzi, V., Barulli, M. R., Bonfiglio, C., Guerra, V., Osella, A., et al. (2015). Coffee, tea, and caffeine consumption and prevention of late-life cognitive decline and dementia: a systematic review. J. Nutr. Health Aging 19, 313–328. doi: 10.1007/s12603-014-0563-8
Peavy, G. M., Salmon, D. P., Jacobson, M. W., Hervey, A., Gamst, A. C., Wolfson, T., et al. (2009). Effects of chronic stress on memory decline in cognitively normal and mildly impaired older adults. Am. J. Psychiatry 166, 1384–1391. doi: 10.1176/appi.ajp.2009.09040461
Perry, D. C., Sturm, V. E., Peterson, M. J., Pieper, C. F., Bullock, T., Boeve, B. F., et al. (2016). Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J. Neurosurg. 4, 511–526. doi: 10.3171/2015.2.JNS14503
Poey, J. L., Burr, J. A., and Roberts, J. S. (2017). Social connectedness, perceived isolation, and dementia: does the social environment moderate the relationship between genetic risk and cognitive well-being? Gerontologist 57, 1031–1040. doi: 10.1093/geront/gnw154
Qizilbash, N., Gregson, J., Johnson, M. E., Pearce, N., Douglas, I., Wing, K., et al. (2015). BMI and risk of dementia in two million people over two decades: a retrospective cohort study. Lancet Diabetes Endocrinol. 3, 431–436. doi: 10.1016/S2213-8587(15)00033-9
Ramage, S. N., Anthony, I. C., Carnie, F. W., Busuttil, A., Robertson, R., and Bell, J. E. (2005). Hyperphosphorylated tau and amyloid precursor protein deposition is increased in the brains of young drug abusers. Neuropathol. Appl. Neurobiol. 31, 439–448. doi: 10.1111/j.1365-2990.2005.00670.x
Ries, J. D., Hutson, J., Maralit, L. A., and Brown, M. B. (2015). Group balance training specifically designed for individuals with Alzheimer disease: impact on berg balance scale, timed up and go, gait speed, and mini-mental status examination. J. Geriatr. Phys. Ther. 38, 183–193. doi: 10.1519/JPT.0000000000000030
Roberts, B. W., Luo, J., Briley, D. A., Chow, P. I., Su, R., and Hill, P. L. (2017). A systematic review of personality trait change through intervention. Psychol. Bull. 143, 117–141. doi: 10.1037/bul0000088
Roberts, R. O., Cha, R. H., Mielke, M. M., Geda, Y. E., Boeve, B. F., Machulda, M. M., et al. (2015). Risk and protective factors for cognitive impairment in persons aged 85 years and older. Neurology 84, 1854–1861. doi: 10.1212/WNL.0000000000001537
Russ, T. C., Hannah, J., Batty, G. D., Booth, C. C., Deary, I. J., and Starr, J. M. (2017). Childhood cognitive ability and incident dementia: the 1932 Scottish mental survey cohort into their 10th decade. Epidemiology 28, 361–364. doi: 10.1097/EDE.0000000000000626
Russ, T. C., Stamatakis, E., Hamer, M., Starr, J. M., Kivimäki, M., and Batty, G. D. (2013). Socioeconomic status as a risk factor for dementia death: individual participant meta-analysis of 86 508 men and women from the UK. Br. J. Psychiatry 203, 10–17. doi: 10.1192/bjp.bp.112.119479
Sabia, S., Fayosse, A., Dumurgier, J., Dugravot, A., Akbaraly, T., Britton, A., et al. (2018). Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ 362, k2927. doi: 10.1136/bmj.k2927
Samieri, C., Perier, M.-C., Gaye, B., Proust-Lima, C., Helmer, C., Dartigues, J. F., et al. (2018). Association of cardiovascular health level in older age with cognitive decline and incident dementia. JAMA 320, 657–664. doi: 10.1001/jama.2018.11499
Schnohr, P., Marott, J. L., Lange, P., and Jensen, G. B. (2013). Longevity in male and female joggers: the Copenhagen city heart study. Am. J. Epidemiol. 177, 683–689. doi: 10.1093/aje/kws301
Schwarzinger, M., Pollock, B. G., Hasan, O. S. M., Dufouil, C., and Rehm, J., and QalyDays Study Group. (2018). Contribution of alcohol use disorders to the burden of dementia in France 2008-13: a nationwide retrospective cohort study. Lancet Public Health 3, e124–e132. doi: 10.1016/S2468-2667(18)30022-7
Sharp, E. S., and Gatz, M. (2011). Relationship between education and dementia. Alzheimer Dis. Assoc. Disord. 25, 289–304. doi: 10.1097/WAD.0b013e318211c83c
Shi, L., Chen, S.-J., Ma, M.-Y., Bao, Y.-P., Han, Y., Wang, Y.-M., et al. (2018). Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med. Rev. 40, 4–16. doi: 10.1016/j.smrv.2017.06.010
Shumaker, S. A., Legault, C., Rapp, S. R., Thal, L., Wallace, R. B., Ockene, J. K., et al. (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the women’s health initiative memory study: a randomized controlled trial. JAMA 289, 2651–2662. doi: 10.1001/jama.289.20.2651
Singh, B., Parsaik, A. K., Mielke, M. M., Erwin, P. J., Knopman, D. S., Petersen, R. C., et al. (2014). Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimers Dis. 39, 271–282. doi: 10.3233/JAD-130830
Singh-Manoux, A., Dugravot, A., Fournier, A., Abell, J., Ebmeier, K., Kivimäki, M., et al. (2017). Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA Psychiatry 74, 712–718. doi: 10.1001/jamapsychiatry.2017.0660
Stanmore, E., Stubbs, B., Vancampfort, D., de Bruin, E. D., and Firth, J. (2017). The effect of active video games on cognitive functioning in clinical and non-clinical populations: a meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 78, 34–43. doi: 10.1016/j.neubiorev.2017.04.011
Sundström, A., Westerlund, O., and Kotyrlo, E. (2016). Marital status and risk of dementia: a nationwide population-based prospective study from Sweden. BMJ Open 6, e8565–e8567. doi: 10.1136/bmjopen-2015-008565
Terracciano, A., Stephan, Y., Luchetti, M., Albanese, E., and Sutin, A. R. (2017). Personality traits and risk of cognitive impairment and dementia. J. Psychiatr. Res. 89, 22–27. doi: 10.1016/j.jpsychires.2017.01.011
Valls-Pedret, C., Sala-Vila, A., Serra-Mir, M., Corella, D., Torre de, R., and Martínez-González, M. Á, et al. (2015). Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Intern. Med. 175, 1094–1103. doi: 10.1001/jamainternmed.2015.1668
Van Ness, P. H., and Kasl, S. V. (2003). Religion and cognitive dysfunction in an elderly cohort. J. Gerontol. B Psychol. Sci. Soc. Sci. 58, S21–S29.
Verghese, J., Lipton, R. B., Katz, M. J., Hall, C. B., Derby, C. A., Kuslansky, G., et al. (2003). Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 348, 2508–2516. doi: 10.1056/NEJMoa022252
Weng, P.-H., Chen, J.-H., Chiou, J.-M., Tu, Y.-K., Chen, T.-F., Chiu, M.-J., et al. (2018). The effect of lifestyle on late-life cognitive change under different socioeconomic status. PLoS One 13:e0197676. doi: 10.1371/journal.pone.0197676
Wilson, R. S., Bennett, D. A., Bienias, J. L., Aggarwal, N. T., Mendes De Leon, C. F., Morris, M. C., et al. (2002). Cognitive activity and incident AD in a population-based sample of older persons. Neurology 59, 1910–1914. doi: 10.1212/01.wnl.0000036905.59156.a1
Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Schneider, J. A., and Bennett, D. A. (2013). Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology 81, 314–321. doi: 10.1212/WNL.0b013e31829c5e8a
World Health Organization (2017). Global Action Plan on the Public Health Response to Dementia. Geneva: World Health Organization, 2017–2025.
World Health Organization (2019). Risk Reduction of Cognitive Decline and Dementia. Geneva: World Health Organization, 1–96.
Wright, H., and Jenks, R. A. (2016). Sex on the brain! Associations between sexual activity and cognitive function in older age. Age Ageing 45, 313–317. doi: 10.1093/ageing/afv197
Yaffe, K., Barnes, D. E., Rosenberg, D., Dublin, S., Kaup, A. R., Ludman, E. J., et al. (2018). Systematic Multi-Domain Alzheimer’s Risk Reduction Trial (SMARRT): study protocol. JAD 13, 1–14. doi: 10.3233/JAD-180634
Zammit, A. R., Robitaille, A., Piccinin, A., Muniz-Terrera, G., and Hofer, S. M. (2018). Associations between aging-related changes in grip strength and cognitive function in older adults: a systematic review. J. Gerontol. A Biol. Sci. Med. Sci. 71:841. doi: 10.1093/gerona/gly046
Zheng, G., Liu, F., Li, S., Huang, M., Tao, J., and Chen, L. (2015). Tai chi and the protection of cognitive ability: a systematic review of prospective studies in healthy adults. Am. J. Prev. Med. 49, 89–97. doi: 10.1016/j.amepre.2015.01.002
Zhong, G., Wang, Y., Zhang, Y., Guo, J. J., and Zhao, Y. (2015). Smoking is associated with an increased risk of dementia: a meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS One 10:e0118333. doi: 10.1371/journal.pone.0118333
Appendix
Keywords: prevention, Alzheimer’s disease, neurodegeneration, public health, personal medicine
Citation: Kempermann G (2019) Making DEEP Sense of Lifestyle Risk and Resilience. Front. Aging Neurosci. 11:171. doi: 10.3389/fnagi.2019.00171
Received: 15 February 2019; Accepted: 19 June 2019;
Published: 17 July 2019.
Edited by:
Christian Gonzalez-Billault, Universidad de Chile, ChileReviewed by:
Ramesh Kandimalla, Texas Tech University Health Sciences Center, United StatesAlexei Verkhratsky, The University of Manchester, United Kingdom
Copyright © 2019 Kempermann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerd Kempermann, Z2VyZC5rZW1wZXJtYW5uQGR6bmUuZGU=; Z2VyZC5rZW1wZXJtYW5uQHR1LWRyZXNkZW4uZGU=
 Gerd Kempermann
Gerd Kempermann