
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Aging Neurosci., 02 April 2019
Sec. Neurocognitive Aging and Behavior
Volume 11 - 2019 | https://doi.org/10.3389/fnagi.2019.00072
This article is part of the Research TopicLearning and MemoryView all 59 articles
Vitamin-C is a water soluble molecule that humans have lost the ability to produce. Vitamin-C plays a role in CNS functions such as neuronal differentiation, maturation, myelin formation and modulation of the catecholaminergic systems. A recent systematic review by our team indicated the need for further research into the relationship between plasma vitamin C and cognition in cognitively intact participants using plasma vitamin C concentrations instead of estimates derived from food-frequency-questionnaires (FFQ), and more sensitive cognitive assessments suitable for cognitive abilities vulnerable to aging. It was hypothesized that higher plasma vitamin C concentrations would be linked with higher cognitive performance. This cross-sectional trial was conducted on healthy adults (n = 80, Female = 52, Male = 28, 24–96 years) with a range of plasma Vitamin C concentrations. Cognitive assessments included The Swinburne-University-Computerized-Cognitive-Assessment-Battery (SUCCAB) and two pen and paper tests, the Symbol-Digits-Modalities-Test (SDMT) and Hopkins-Verbal-Learning-Test-Revised (HVLT-R). The pen and paper assessments were conducted to establish whether their scores would correlate with the computerized tasks. Plasma-Vitamin C concentrations were measured using two biochemical analyses. Participants were grouped into those with plasma vitamin-C concentrations of adequate level (≥28 μmol/L) and deficient level (<28 μmol/L). The SUCCAB identified a significantly higher performance ratio (accuracy/reaction-time) in the group with adequate vitamin-C levels vs. deficient vitamin-C on the choice reaction time (M = 188 ± 4 vs. 167 ± 9, p = 0.039), immediate recognition memory (M = 81 ± 3 vs. 68 ± 6, p = 0.03), congruent Stroop (M = 134 ± 3 vs. 116 ± 7, p = 0.024), and delayed recognition tasks (M = 72 ± 2 vs. 62 ± 4, p = 0.049), after adjusting for age (p < 0.05). Significantly higher scores in immediate recall on the HVLT-R (M = 10.64 ± 0.16 vs. 9.17 ± 0.37, p = 0.001), delayed recall (M = 9.74 ± 0.22 vs. 7.64 ± 0.51, p < 0.001), total recall (M = 27.93 ± 0.48 vs. 24.19 ± 1.11, p = 0.003) were shown in participants with adequate plasma Vitamin-C concentrations, after adjusting for vitamin-C supplementation dose (p < 0.05). Similarly, higher SDMT scores were observed in participants with adequate plasma Vitamin-C concentrations (M = 49.73 ± 10.34 vs. 41.38 ± 5.06, p = 0.039), after adjusting for age (p < 0.05). In conclusion there was a significant association between vitamin-C plasma concentrations and performance on tasks involving attention, focus, working memory, decision speed, delayed and total recall, and recognition. Plasma vitamin C concentrations obtained through vitamin C supplementation did not affect cognitive performance differently to adequate concentrations obtained through dietary intake.
Clinicaltrials.gov Unique Identifier: ACTRN 12615001140549, URL: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369440.
Because humans are unable to synthesize their own vitamin C they are particularly vulnerable to vitamin C deficiency (Hansen et al., 2014). Based on population averages, plasma concentrations <28 μmol/L are deemed to be deficient or marginally deficient, and ≥28 μmol/L is considered adequate (Hampl et al., 2004).
Although scurvy is considered to be relatively rare in Western populations, vitamin C deficiency is the fourth most prevalent nutrient deficiency reported in the United States (Schleicher et al., 2009). Reports from cross-sectional population surveys (Hampl et al., 2004; Schleicher et al., 2009) have consistently estimated that at least 10–15% of the adult population in the western world suffer from deficient vitamin C levels, with highest rates (30%) arising in the elderly (Hsiao et al., 2013), with some requiring hospitalization (Zhang et al., 1999; Harrison, 2012). The recent New Zealand CHALICE study (Pearson et al., 2017) revealed suboptimal levels in 62% of 400 healthy 50 year olds, and either a marginal or severe deficiency in 15.4% of the cohort.
Vitamin C blood concentrations are transient, with a number of factors affecting circulating concentrations (Lecomte et al., 1994; Brubacher et al., 2000; Padayatty et al., 2007; Fukushima and Yamazaki, 2010). Factors relating to plasma vitamin C depletion include malabsorption and certain acute and chronic diseases (Hoffman, 1985; Deicher and Hörl, 2003). Some medical conditions can reduce the absorption of vitamin C and/or increase the amount needed by the body. Low vitamin C concentrations have been associated with gastrointestinal disease, liver disease, cancer, asthma, and diabetes. Pregnancy also affects ascorbate levels, with lactation potentially leading to significant losses of maternal vitamin C.
One group particularly vulnerable to vitamin C deficiency are the elderly (>60 years) (Iqbal et al., 2004). Vitamin C levels in tissues, such as the brain and muscle are reduced with age to as little as 25% of those found in children (Lewin, 1976). This may be explained by increased reactive oxygen species [ROS and/or reduced ascorbate recycling in cells, as a result of reductions in the production of glutathione- a molecule required for recycling vitamin C (Rizvi et al., 2009)]. Lowered tissue levels with age have also been attributed to reduced fruit and vegetable consumption in the elderly (Newton et al., 1985; Iqbal et al., 2004).
A basic principle of nutrition is that most nutrients have a non-linear, inverted U-shaped association with optimum physiological function. In order to maintain optimal health, it is becoming increasingly acknowledged that vitamin C is required at concentrations (adequate/optimal) above those needed for the prevention of scurvy (severe depletion) (Carr and Frei, 1999). The category of marginal vitamin C deficiency can rarely be singled out from severe and marginal vitamin C deficiency. Some of the earliest signs of deficiency include fatigue, as deficiency progresses, individuals are known to present with severe fatigue, confusion, and depression (Basu and Donaldson, 2003).
Symptoms of vitamin C deficiency develop after weeks to months of vitamin C depletion (Basu and Donaldson, 2003). Depletion of vitamin C may be linked to a low average intake of <10 mg/d. Usually, ascorbate (reduced form of vitamin) has a half-life between 7 and 14 days in plasma, however, if ingestion stops completely, and levels drop below the sub-baseline, the half-life is significantly longer, approximately 35–40 days (Kallner et al., 1979; Noble et al., 2013). Organs such as the brain are particularly resilient to vitamin C depletion due to ascorbate being recycled by glutathione in astrocytes through the pentose phosphate pathway (Harrison and May, 2009). As plasma concentrations decline, more ascorbate is pumped into the cerebral spinal fluid in order to maintain homeostasis (Bowman et al., 2009). Duration of deficiency has been shown to influence brain ascorbate concentrations to a higher degree than the amount of depletion (Hornig, 1975). This is exemplified by observations in acute scurvy where brain concentrations of ascorbate are maintained through depletion of peripheral tissues, whereas marginal deficiency for longer periods of time has shown greater brain ascorbate depletions (27% depletion in 14 days in guinea pig brain tissue) (Spector, 1977).
The brain’s ability to recycle vitamin C and neurological symptoms that present from vitamin C deficiencies are reflections of the importance that the vitamin plays in central nervous functioning. The biological mechanisms/processes of vitamin C on brain and neuronal functioning have been well established (Travica et al., 2017). It is a vital co-factor in numerous processes such as the biosynthesis of collagen, carnitine, tyrosine, peptide hormones as well as myelin (Kocot et al., 2017). Vitamin C plays a crucial role in neurotransmission and neuronal maturation and functions (Eldridge et al., 1987). Ascorbic acid acts as a co-factor in the synthesis of neurotransmitters, particularly of catecholamines-dopamine and norepinephrine (Figueroa-Méndez and Rivas-Arancibia, 2015). Vitamin C is suggested to influence this process via modulating the binding of neurotransmitters to receptors and regulating their release (Rebec and Pierce, 1994). Vitamin C is also a cofactor for tryptophan-5-hydroxylase required for the conversion of tryptophan to 5-hydroxytryptophan in serotonin production (Gupta et al., 2014). Vitamin C deficiency has shown to decrease serotonin metabolites in both the cortex and striatum (Ward et al., 2013).
Moreover, ascorbic acid modulates the activity of excitatory receptors such as N-methyl-D-aspartate as well as inhibitory receptors such as aminobutyric acid (GABA) (Covarrubias-Pinto et al., 2015) and inhibits the binding of glutamate to the NMDA receptor, thus demonstrating a direct effect in preventing glutamate excitotoxicity which causes neuronal dysfunction and degeneration (Harrison and May, 2009).
Additionally, vitamin C has been shown to be involved in the biosynthesis of L-carnitine (Rebouche, 1991) playing a role in the transport of fatty acids into mitochondria for energy production. Enzymes can readily convert carnitine to acetyl-carnitine and vice versa, according to the metabolic needs of the cell (Mendelson, 2008). Acetyl-carnitine can cross the blood brain barrier and act as a precursor in the production of acetylcholine and supports healthy cerebral blood flow (White and Scates, 1990).
Vitamin C also plays a role in the modulation of neuronal metabolism by changing the preference for lactate over glucose as the primary energy source in neurons sustaining synaptic activity (Castro et al., 2008). Vitamin C is also involved in collagen synthesis in the brain (Harrison et al., 2009). It hydroxylases the amino acids proline and lysine and creates hydroxyproline which is found abundantly in collagen. Collagen is needed for blood vessel formation and integrity (Hansen et al., 2014). Ascorbate-dependent collagen synthesis has also been linked to the formation of myelin sheaths and regeneration of damaged sheaths that surround many nerve fibers (Guo et al., 2018). According to a study using mice deficient in an ascorbate transporter, the concentration of ascorbate in the brain was shown to be below a detectable level. The animals died due to capillary hemorrhage in the penetrating vessels of the brain (Sotiriou et al., 2002).
Due to a high level of poly-unsaturated fatty acids, combined with high rates of cellular metabolism of oxygen, glucose etc., the brain is particularly vulnerable to creating reactive oxygen species (Uttara et al., 2009). Ascorbic acid acts directly by scavenging reactive oxygen and nitrogen species produced during cell metabolism, and further recycles vitamin E involved in cell membrane integrity (Harrison and May, 2009). Additionally, vitamin C has been found to induce the expression of brain derived neurotrophic factor (BDNF) – a component of several survival pathways (Grant et al., 2005).
Alongside vitamin C, a number of nutrients have been shown to affect cognitive function (Gómez-Pinilla, 2008). Examples of these include magnesium (Slutsky et al., 2010), vitamin E (Kang et al., 2006), vitamin D (Garcion et al., 2002), selenium (Chen and Berry, 2003), and Vitamin B12. Amongst these nutrients, Vitamin B12 has been shown to be critical for cognitive function in several research trials, and will therefore be considered a covariate in the analysis of cognitive assessments (Kennedy, 2016). The effects of vitamin B12 (cobalamin), in particular on the central nervous system have been well documented. Vitamin B12 is a water-soluble vitamin required for red blood cell formation, neurological function, and DNA synthesis. More recent research has demonstrated a link between plasma vitamin B12 concentrations and cognitive function, and the potential to reduce the rate of brain atrophy with high dose B-vitamin supplementation that includes B12 (Riggs et al., 1996; Reay et al., 2013). Vitamin B12 deficiency has been linked to psychiatric disorders, including impaired memory, irritability, depression, and dementia (Lindenbaum et al., 1988). Neurological disorders due to vitamin B12 deficiency typically occur in both genders between the ages of 40 and 90 years, with a peak between 60 and 70 years (Healton et al., 1991; Savage and Lindenbaum, 1995; Kennedy, 2016).
A recent review demonstrated higher mean vitamin C concentrations in the cognitively intact groups of participants compared to cognitively impaired groups (Travica et al., 2017). One major limitation conducted in studies with healthy samples was the lack of sensitive cognitive assessments suitable for cognitively intact adults. Qualitative analysis of these studies that used a variety of cognitive assessments in the healthy population revealed a potential association between plasma vitamin C concentrations and cognition.
Additionally, a number of limitations have arisen in the measurement of vitamin C levels in a number of previous studies (Jama et al., 1996; Beydoun et al., 2015). The dietary assessments used have reliability and validity issues as a result of potential recall errors with self-report (Weinstein et al., 2001). Even when food consumption is recalled correctly, differences in storage and cooking can decrease the vitamin C level in the food and absorption can vary amongst participants (Weinstein et al., 2001). Given these issues, dietary self-reports do not necessarily reflect the vitamin C potential for biological action.
While blood samples are a more reliable measure of vitamin C status than self-reported vitamin C intakes, many factors can contribute to the instability of ascorbic acid in biological samples such as heat, light, and elevated pH (acidity). A number of previous studies failed to thoroughly explain blood sample handling and biochemical analysis, underestimation of vitamin C concentrations could occur if samples were not handled properly (Harrison, 2012).
Discriminating between the concentrations of those supplementing on vitamin C from those not supplementing has been an issue in previous studies. The cognitive significance of marginal and severe plasma vitamin C deficiency in comparison to adequate levels has not been thoroughly investigated. In most studies, upper and lower tertiles, quartiles, or quintiles are compared, making it difficult to compare groups between studies. Additionally, the use of dietary vitamin C supplementation has either been ignored in previous research or plasma vitamin C concentrations were not measured in those supplementing with vitamin C in randomized controlled and prospective trials. This may have compromised previous outcomes, as vitamin C supplementation may influence variances in plasma vitamin C concentration and may be complemented with the supplementation of other nutrients which are involved in brain function.
The present study was devised with the aim of exploring whether there is an association between plasma vitamin C concentrations and cognitive function in cognitively intact adults, using paper and pen and computerized cognitive assessments. We hypothesized that there would be a positive correlation between plasma vitamin C concentrations and cognitive performance and that those presenting with adequate plasma vitamin C concentrations would demonstrate higher cognitive performance than those displaying deficient (marginal/severe) plasma vitamin C concentrations. Furthermore, the relationship between vit C and cognition was investigated in participants who did not report vitamin C supplementation and in a larger group that included vitamin C supplementers.
This study was approved by the Human Research Ethics Committees at National Institute of Integrative Medicine and Swinburne University of Technology, and the trial was registered with the Australian New Zealand Clinical Trial Registry (ACTR12615001140549). Participants provided informed written consent.
Cognitively intact adults (>18 years) were sought to participate in this cross-sectional study investigating the association between plasma vitamin C concentrations and cognition. Asymptomatic participants were primarily recruited from The National Institute of Integrative Medicine (NIIM). We included participants displaying no major neurodegenerative condition, i.e., dementia (3MS score >79), and included those likely to be displaying a range of plasma vitamin C concentrations, i.e., varying diets, supplementation, age groups. Participants were excluded if they were pregnant or lactating, color blind, or taking antidepressants, antipsychotics, anxiolytics, illicit drugs or any cognitive enhancing drugs. Participants were also excluded if they were not able to give informed consent.
A number of cognitive assessments were performed. These included the Modified Mini Mental State Examination (3MS) (Tombaugh and McIntyre, 1992), Revised Hopkins Verbal Learning Test (HVLT-R) (Brandt, 1991), Symbol Digits Modalities Test (SDMT) (Sheridan et al., 2006) and the Swinburne University Computerized Cognitive Assessment Battery (SUCCAB) (Pipingas et al., 2010).
Participants were initially screened for cognitive impairment using the 3MS. This was implemented as a valid and reliable screening test for the purpose of evaluating major cognitive impairment (scale 0–100).
The HVLT-R is a validated paper and pen test designed to examine verbal learning, immediate and delayed recall, and delayed recognition. Participants were required to recognize and recall a list of 12 words immediately (across 3 trials) and after 40 min (4th trial). Delayed recognition was assessed by reading a longer list of words to participants (24 words) and having them respond with a ‘yes’ or ‘no’ if they recognized the word from the original list of 12 words. The 4th HVLT-R word recall trial and delayed recognition were completed after the SUCCAB. Trials 1–3 were scored out of 12 points, delayed recall (trial 4) was also scored out of 12 points and total recall was calculated from the sum of total correct responses for trials 1, 2, and 3 (out of 36 points). Recognition index was scored out of 12 for every correct word recognized.
The symbol digits modalities test (SDMT) (Sheridan et al., 2006) required participants to pair numbers with geometric figures, primarily assessing divided attention, tracking and visual screening. Participants were presented with a key consisting of numbers between 1 and 9 with a corresponding symbol under each number. The test consisted of rows of random symbols with blank squares below each symbol, and participants were given 90 s to fill in the blank squares with the corresponding number. Test scores ranged between 0 and 110.
The SUCCAB is a validated cognitive test battery consisting of eight computer-based cognitive tasks assessing various aspects of cognitive performance (Pipingas et al., 2010). Participants were asked to respond as quickly and accurately as possible in each task. A 4-button response box was used to complete the tasks, with buttons corresponding to task specific response dimensions: color (red, blue, green, or yellow), ‘yes’ or ‘no,’ or the spatial location of objects on the screen (top, bottom, left, or right). The eight tasks that comprised the SUCCAB battery are described in Table 1 below.
Participants initially completed a 10-min practice of the SUCCAB with the experimenter present, explaining and observing the participant during practice tasks. Following the practice run, and once participants were familiar and comfortable with the tasks, they completed the 30 min SUCCAB.
Fasting (8–12 h) blood tests were performed by the researcher/phlebotomist immediately after the completion of the SDMT. Blood was collected in 2, 6 ml lithium heparin vacutainer blood tubes. The blood tubes were immediately wrapped in foil and kept away from light. Each blood tube was spun in a centrifuge at 3,600 rpm. Plasma from the heparin tubes was aliquoted into separate 3 ml tubes. These were also wrapped in foil and kept away from light. Blood vitamin C concentrations were analyzed using two different biochemical analyses. An additional aim of the study was to compare and verify the effectiveness of an analysis conducted immediately at The National Institute of Integrative Medicine with one conducted by an external laboratory. This was done to ensure that the most reliable blood plasma vitamin C concentrations were used in analyses.
(a) Colorimetric analysis (Chung et al., 2001) is a method of determining the concentration of a chemical element or chemical compound in a solution with the aid of a color reagent. Ascorbic testing was conducted using the Ascorbic acid Assay Kit II (Sigma-Aldrich). In the assay, ascorbic acid concentration was determined using Ferric reducing/antioxidant and ascorbic acid assay. In the assay, Fe3+ is reduced to Fe2+ by antioxidants present in the sample which results in a colorimetric (593 nm) product. The color reaction is preceded by a reaction catalyzed by an enzyme. The addition of ascorbate oxidase to parallel samples oxidizes any ascorbic acid present allowing for the measurement of the ascorbic acid concentration. This analysis was performed in the NIIM lab using a Sigma-Aldrich kit and SoftMax® Pro Software on the EMax® Plus Microplate Reader. This analysis was run within 30 min of the blood being taken.
(b) A high performance liquid chromatographic (HPLC) (Chung et al., 2001) method was also used for the quantitation of vitamin C in plasma samples. The analysis was conducted 2–3 days following the blood test. The blood was spun in a centrifuge at 1,500 rpm, plasma separated and transported on dry ice (-80°C) and kept away from light. The HPLC testing was done by Sullivan Nicolaides Pathology (Queensland, Australia) using the Chromsystems® (Gräfelfing, Germany) Vitamin C Plasma/Serum HPLC kit. Level I and II Lyophilized quality vitamin C plasma controls were used. 100 μl of sample/calibrator/control was pipetted into a labeled light protected reaction vial. 100 μl of the internal Standard was added and mixed for 30 s (vortex). This was then centrifuged for 5 min at 15,000 × g and 20 μl of the supernatant was injected into the HPLC system. The HPLC analysis was carried out using a Waters 2695 Separations Module equipped with a dual wavelength UV detector set to 245 nm. The column temperature was maintained at 25°C. The generated chromatogram was used to determine vitamin C concentrations. Blood was also taken in a sodium citrate vacutainer tube for analysis of serum Vitamin B12 through Melbourne Pathology. A vitamin B12 immuno assay (Roche E602) was used to measure total vitamin B12 concentrations. This assay employs the competitive inhibition enzyme immunoassay technique (Lee and Griffiths, 1985). This was analyzed within 3 days of blood being taken.
Alongside assessing basic demographic information, a questionnaire assessed intake of prescribed medications, dietary supplementation (dose/frequency), smoking status, highest level of education, exercise (duration/type), family history of neurodegenerative disease, and any history of an incident possibly contributing to cognitive dysfunction. Exercise was defined as Moderate (50–65% of maximum heart rate) or vigorous (70–85% of maximum heart rate) (Swain and Franklin, 2006).
A long term (1 year) dietary intake of a number of additional nutrients was assessed using the computerized version of the dietary questionnaire for Epidemiological Studies, Version 2 (DQES v2) (Ireland et al., 1994). The Cancer Council DQES v2 covered five types of dietary intake based on the previous 12 months, incorporating 80 items: cereal foods, sweets and snacks, dairy products, meats and fish, fruit and vegetables and alcoholic beverages. It summarizes the intake of key nutrients all vital for CNS function such as magnesium, Vitamin E, selenium, folate, etc.
Secondly, an in-house developed short-term food frequency questionnaire (FFQ) was administered. This FFQ (based on the Cancer Council FFQ) assessed specifically what the consumption of vitamin C and vitamin B12 rich foods was within 2 weeks prior to the testing session.
Mood was assessed as a potential confounder to cognitive performance. The Bond Lader Mood questionnaire (Bond and Lader, 1974) is designed to measure four different concepts of mood: Mental Sedation or intellectual impairment, physical sedation or bodily impairment, tranquillization or calming effects, other types of feelings or attitudes. The scales comprise of 16, 10 cm lines anchored at each end by words descriptive of opposing statements (bipolar). On this linear scale, the participant indicated their mood by placing a mark between the 2 opposite words on either side. High reliability and validity have been demonstrated with this assessment. From the resultant scores, three derived measures can be isolated. These have been described as representing the following: alertness, calmness, and contentedness. Scores for each measure represent the unweighted average number of millimeters (maximum 100 mm) from the negative antonym for the individual scales contributing to the measure. We also added 2 scales from the cognitive demand battery that we implemented in our previous research (Massee et al., 2015). These scales assessed exhaustion/energy and stress/calmness and were measured the same way as the Bond Ladder scales.
Participants were asked to fast between 8–12 h prior to attending the testing session. They were also asked to refrain from any exercise on the morning of the testing session. Firstly, the demographic/history questionnaire and mood assessment was administered and followed by the 3MS assessment. The 3MS was followed by the FFQ, which assessed vitamin C and Vitamin B12 intake specifically. Following the FFQ, the HVLT-R cognitive assessment was administered. Participants then undertook the 10-min practice run of the SUCCAB before the commencement of the 30 min SUCCAB test battery. Following the SUCCAB, the SDMT and the delayed recognition and recall of the HVLT-R were then administered. The second FFQ (DQES v2) proceeded these cognitive tests and was followed by the blood testing and colorimetric analysis. Plasma was aliquoted and foiled for the biochemical analyses and specifically delivered on dry ice to the pathology company for HPLC analysis.
There had been no previous study exploring the effects of plasma vitamin C concentrations specifically using the SUCCAB and therefore no available data that could be used in a power analysis calculation. A recent study established aged-related mean SUCCAB values for each task in a sample of cognitively intact adults (n = 120, mean age = 53, SD = 16 years) (Pipingas et al., 2010). Results indicated significant differences for every 10 years of age of up to 100 ms (1 SD) on the spatial working memory task. Given the sensitivity of this task to cognitive aging, this is a useful tool in aging research to measure the degree of cognitive decline and to examine the efficacy of interventions. A sample size calculation indicated that 80 participants was sufficient to detect a significant mean difference of 100 ms (SD = 211) on the spatial working memory reaction time task in the SUCCAB, with 80% power and 95% confidence. This difference relates to 10 years of cognitive aging.
Analyses were performed using SPSS IBM statistics version 23 package. Statistical significance was set at p < 0.05. Values that were 2 standard deviations away from their means for the cognitive assessments were excluded from analysis. A Bonferroni correction adjustment was used in the ANOVA and ANCOVA analyses to avoid committing a type 1 error in the analyses. Descriptive analyses were conducted on all variables including mean dietary intakes, supplement doses, exercise etc.
A Bland–Altman analysis (Giavarina, 2015) was used to determine whether the two plasma vitamin C biochemical methods provided consistent results. This analysis involved correlating the difference (T-S) between the test (T = Colorimetric analysis) and comparative method (S = HPLC) against the average (T+S)/2 of the results obtained from the two methods. A Bland–Altman plot was also used to show the difference between the two methods against the average of both methods, the confidence intervals being displayed. Additionally, a Spearman’s correlation was conducted to determine the correlation between the two biochemical methods. As recommended by Bland and Altman, comparability was achieved if 95% of the data points were within ±2 SDs of the mean difference.
If consistency was demonstrated, the concentrations were averaged between the two biochemical analyses. Daily vitamin C consumption (mg/d) based on the food frequency questionnaire was also compared with the plasma vitamin C concentrations using a Spearman correlation.
Vitamin C blood plasma concentrations were correlated with cognitive performance using Spearman rank correlations. Scatterplots and Spearman correlations were used to assess the direction and strength of association between vitamin C concentrations and cognitive performance. This analysis was also used to visualize the impact of vitamin C supplementation on cognition relative to non-supplementers. In order to determine whether those who self-reported vitamin C supplementation would have an effect on cognitive performance, further Spearman correlations were conducted with the exclusion of those supplementing on vitamin C.
Primary analyses were conducted to compare cognitive performance between those in the adequate plasma vitamin C group with those in the deficient group. Average vitamin C concentrations were sub grouped into internationally established reference ranges representing adequate (≥28 μmol/L) or deficient (<28 μmol/L) concentrations. Descriptive statistics were compared between those with adequate versus deficient vitamin C concentrations using an analysis of variance (ANOVA) for continuous variables. A chi-square analysis was performed to assess the association between categorical variables such as gender, smoking status and prescribed medications and the adequate versus deficient status of participants (group effect) on plasma vitamin C concentrations.
For the SUCCAB, mean response time and percentage accuracy were calculated for each task. The accuracy percentage was divided by the mean response time (sec) to give an overall performance ratio score. This ratio also assisted in accounting for the issue of the speed versus accuracy trade-off that exists with increasing age (Starns and Ratcliff, 2010; Forstmann et al., 2011).
Lower reaction times are indicative of faster response times and with higher performance ratios indicating a combination of faster reaction times and greater trial accuracy. Finally, correlational analyses were performed between the scores on both of the paper and pen cognitive tasks and performance on each of the SUCCAB tasks. Cognitive performance was compared between those with adequate versus deficient vitamin C concentrations using an analysis of covariance (ANCOVA) for potential continuous confounder variables. Such potential confounding variables were assessed using the correlation regression analyses generated in the ANCOVA, with covariates included in the ANCOVA only if they had a significant effect (p < 0.05) on the cognitive performance variables. Additional ANCOVAs were conducted on cognitive performance between the deficient and adequate groups in which those supplementing on vitamin C were excluded. This was performed to investigate whether there would be a difference in cognitive performance between the groups with the exclusion of vitamin C supplementers.
A number of cognitive variables were derived from the HVLT-R assessment. Trials 1–3, delayed recall (trial 4), total recall, and recognition index were scored based on the number of correct responses. SDMT performance was scored out of 110 points.
The trial was conducted in Melbourne, Australia between November 2016 to January 2018. Participant demographic details are presented in Table 2.
There was a large age range (24–95) amongst the 80 recruited participants, the majority of participants were older (mean: 60.97 years ± 15.76). A majority of the sample was female (66.3%) with an average overweight BMI of 26.00 (SD = 3.16). 36.2% of the sample did not undertake any form of exercise 2 weeks prior to testing and a majority of those that did, reported a moderate intensity. Most participants reported no family history of neurodegenerative disease. On average, each of the assessed mood variables displayed mid-range values, indicating no extreme emotional display before the cognitive testing.
The majority (73.75%) of participants were on some form of prescribed medication, specifically anti-hypertensives (43.75%) and blood thinners (22.5%). Daily nutritional supplements are summarized in Table 3.
Supplements were only considered if they were taken within 1 week prior to the testing session. For those taking multivitamins, each individual vitamin was considered and contributed to intake. Most supplements included vitamin C, B12, D, magnesium and/or fish/krill oil. For those supplementing with vitamin C, concentrations and frequency of vitamin C supplementation did not vary immensely between participants. Frequent (almost daily) vitamin C supplementation was amongst those demonstrating plasma vitamin C concentrations >100 μmol/l. A majority reported multi-supplementation and not one single mineral/vitamin. This is exemplified by almost all of those supplementing on vitamin C also supplementing on some form of vitamin B12. 48 participants (60%) reported supplementation, with 42% (n = 20) of these reporting vitamin C supplementation in particular. Estimated FFQ nutritional intakes are displayed in Table 4.
On average, a majority of participants were within the RDI for the various B vitamins. However, close to one quarter met the RDI for vitamins C, A, E, D and iodine. Subgroup analyses (not shown) revealed no significant differences in nutritional intake between genders, age groups, BMI groups and those exercising.
The blood plasma vitamin C concentrations for each participant using each of the two biochemical techniques are displayed on Supplementary Figure S1.
Plasma concentrations ranged from 7 to 120 μmol/L. Between the two biochemical analyses, plasma vitamin C concentrations were similar for each participant. The Bland and Altman method (Giavarina, 2015) was used to compare the two methods. Firstly, the difference between the HPLC and colorimetric methods (HPLC - Colorimetric) was correlated with the average (HPLC + Colorimetric)/2 of the two methods. Spearman’s correlation indicated no correlation in this difference and average between the methods [rs(80) = 0.159, p = 0.16]. Furthermore, a Bland–Altman plot (Supplementary Figure S2) graphically demonstrated the difference of the two paired measurements against the mean of the two measurements. As recommended by Bland and Altman, comparability was achieved given 95% of the data points were within ±2 standard deviations of the mean difference (Giavarina, 2015).
Spearman’s correlation revealed a strong significant relationship between the two analyses (r = 0.96, p < 0.01). Given the high consistencies between the two biochemical analyses using the Bland and Altman method, their means were used for additional cognitive analyses such as correlations.
A majority (n = 67, 83.75%) of participants were within the adequate vitamin C reference range (≥28 μmol/L), displaying a mean plasma vitamin C concentration of 53.09 ± 19.77 μmol/L 16.25% (n = 13) were within the vitamin C deficiency range (<28 μmol/L), displaying a mean plasma concentration of 16.27 ± 6.45. Those deficient vitamin C were combined into one group and those displaying either adequate or optimal levels were combined into another group. As observed in Supplementary Figure S3, a Spearman correlation revealed a significant relationship between average daily dietary vitamin C intake and the plasma vitamin C concentrations [rs(80) = 0.438, p < 0.001].
Demographic, mood and nutritional differences between the adequate and deficient group using an ANOVA analysis are displayed in Table 5.
The mean age of the participants in the adequate vitamin C group was lower than in those in the deficient group (Table 5). A majority of participants in the adequate group were female compared to a majority of males in the deficient group. A Chi-square likelihood ratio test revealed that there was no significant association between gender and vitamin C group (adequate/deficient) [χ2(1) = 2.80, p = 0.094]. Using the present data set, an interaction between gender by plasma vitamin C concentrations on cognitive function will be analyzed more extensively in a subsequent trial. There was an association between smoking and vitamin C group, with smokers more likely to display deficient vitamin C concentrations [χ2(1) = 7.23, p = 0.007]. Three smokers were present in the deficient group as opposed to 1 in the adequate group. Additionally, there was an association between taking prescribed medications and vitamin C group, with those not taking any prescribed medications more likely to display adequate vitamin C concentrations [χ2(1) = 5.75, p = 0.016].
A higher percentage (92.3%) of participants were on prescribed medications in the deficient group than in the group with adequate vitamin C levels (61.2%). No statistically significant differences were observed in mood between the two groups.
Serum vitamin B12 levels were significantly higher in the adequate than deficient group (p = 0.016). Additionally, no participants in the deficient group reported supplementation of either vitamin C, vitamin B12, or magnesium and only 1 reported vitamin D supplementation. Vitamin C daily dietary intake was not significantly higher in the adequate group compared to the deficient group (p ≥ 0.05). There were no other statistically significant differences in dietary intake between the other measured nutrients. Additional analyses revealed no significant differences in daily vitamin C consumption between those supplementing on vitamin C (M = 81.68 ± 24.98) and those not (M = 83.75 ± 19.20, p > 0.05) in the adequate vitamin C group. Within the adequate group, those supplementing on vitamin C exhibited significantly higher mean vitamin C concentrations (M = 66.65 ± 25.91) than those not supplementing (M = 40.59 ± 17.2, p < 0.01). Additional descriptive comparisons between the deficient and adequate groups in which vitamin C supplementers were excluded revealed similar results to when vitamin C supplementers were included (not shown).
The spread of 3MS scores was restricted, with scores grouping between 80 and 100. Spearman’s correlation analysis revealed no relationship between vitamin C concentrations and 3MS performance [rs(80) = 0.025, p = 0.82]. Additionally, an analysis of covariance (adjusting for age, vitamin B12, mg, vitamin C dose supplementation) revealed no significant differences in 3MS scores between the adequate and deficient group [F(1,78) = 2.6, p = 0.11].
In order to assess the strength of association between plasma vitamin C and cognitive performance, mean plasma vitamin C concentrations were plotted against cognitive performance for each of the cognitive assessments in the HVLT-R and SDMT. Scatterplots were used to visualize cognitive performance between those supplementing on vitamin C relative to non-supplementers. Scatterplots are displayed in Figure 1.
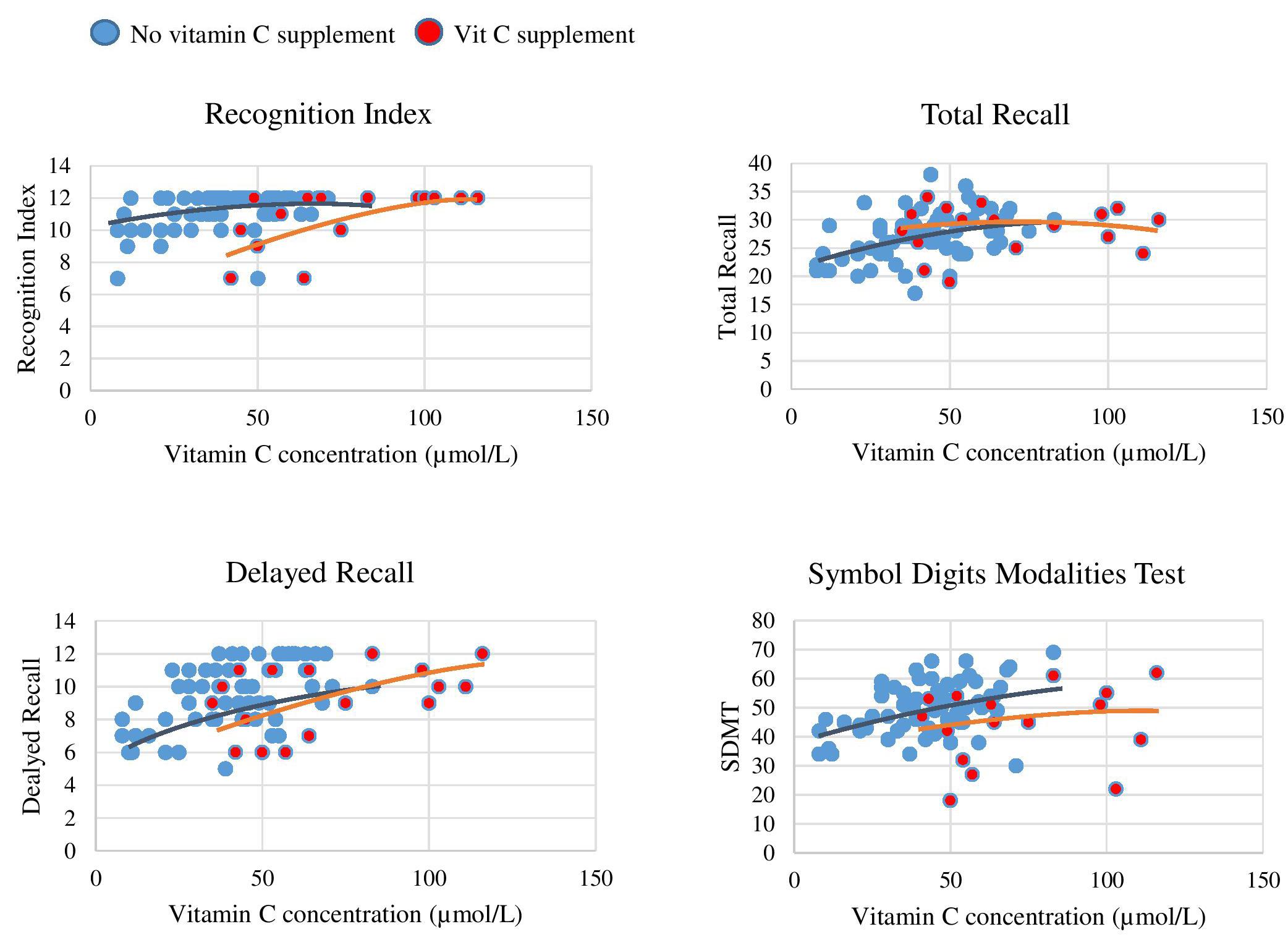
Figure 1. Plasma vitamin C concentrations and paper and pen test correlations. Red dots represent participants supplementing on vitamin C and blue dots those not supplementing on vitamin C. There is a plateau in performance on each of the cognitive measures once plasma concentration surpasses 70 μmol/L. In each graph there are two visual trend lines, one relating to data points of subgroup (a) participants not supplementing on vitamin C and subgroup (b) participants supplementing on vitamin C.
As displayed by the scatterplots, there was a plateau in cognitive performance with increasing vitamin C concentrations (>70 μmol/L), representing a non-linear relationship. The plots further demonstrate that the participants who supplemented on vitamin C were those exhibiting this plateau. Vitamin C Supplementation time frame and dose did not vary greatly in this group. Spearman correlation analysis was conducted to investigate the strength of these non-linear relationships for each cognitive measure, as presented in Table 6.
The Spearman correlations reveal a significant, positive relationship between vitamin C concentration and Trial 2, Trial 3, delayed recall, total recall and recognition index of the HVLT-R test (p < 0.01). There was a significant positive relationship between vitamin C concentrations and the SDMT test (p < 0.02).
When participants supplementing on vitamin C were not included in the correlational analysis, the relationship between vitamin C concentrations and each of the cognitive assessments appeared stronger (higher rs value). Spearman correlations for these relationships are displayed in Table 6. The Spearman correlations (with vitamin C supplementers excluded) displayed significant positive correlations between vitamin C concentrations and the cognitive assessments on both the HVLT-R and the SDMT.
The mean scores of the HVLT-R cognitive assessments and SDMT for the vitamin C adequate and deficient groups are displayed in Figure 2. ANCOVA analyses were performed to compare performance on the HVLT-R cognitive assessments and SDMT between adequate and deficient vitamin C concentrations while controlling for potential covariates (Table 7). Vitamin C supplementation dose was a significant covariate for four HVLT-R measures, number of prescribed medications for the recognition index measure and age for the SDMT test.
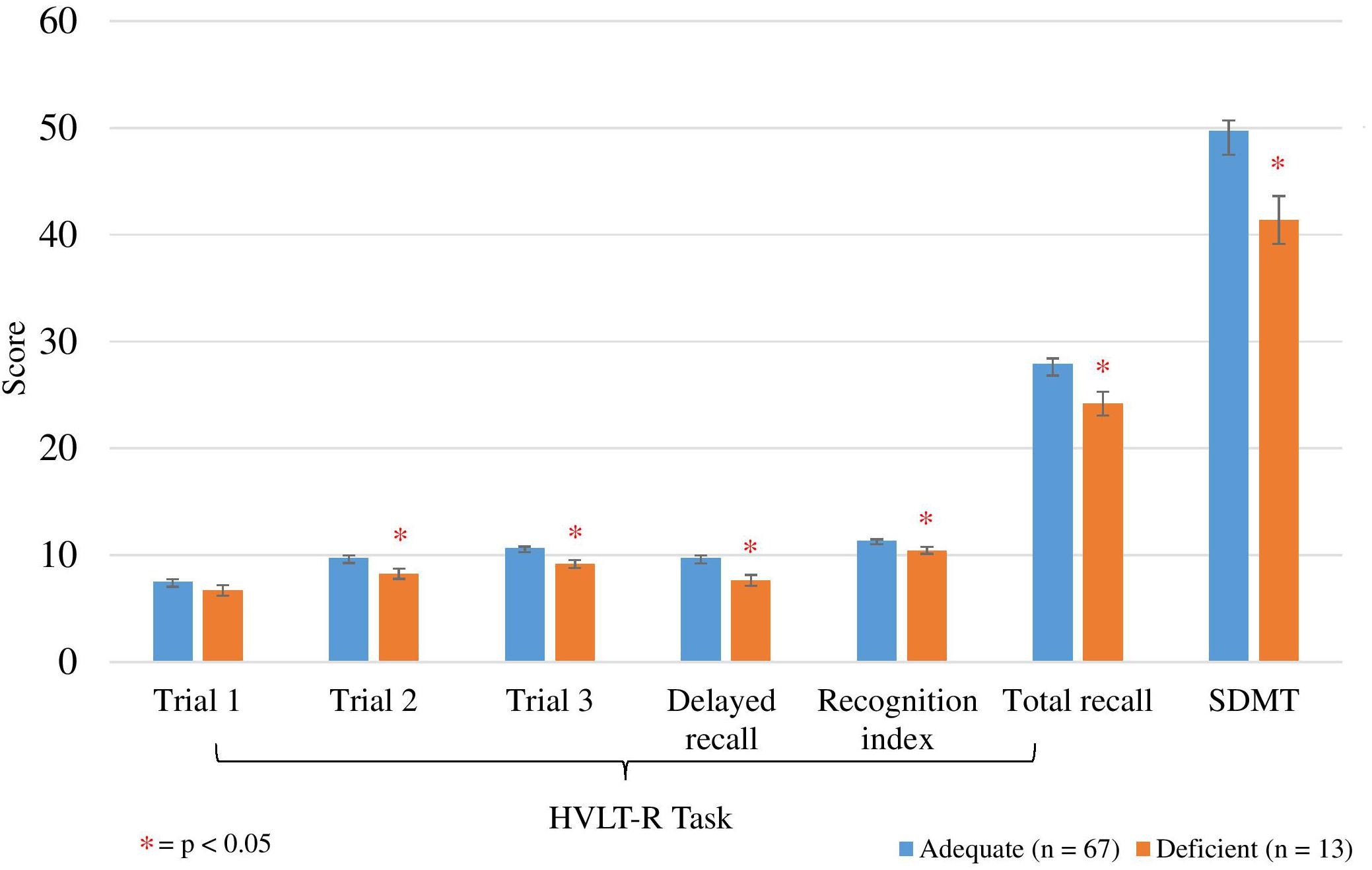
Figure 2. Comparison between adequate and deficient plasma vitamin C groups on HVLT-R cognitive measures and SDMT score. Participants grouped into adequate (n = 67) and deficient (n = 13) plasma vitamin C groups. Comparison assessed with an ANCOVA analysis, controlling for vitamin C supplement dose (Trials 1, 2, 3, Total recall), age (SDMT) and number of prescription medications (recognition index). Trials 1, 2, 3, delayed recall/recognition index scored out of 12 points; total recall scored out of 36 points. HVLT-R, Hopkins Verbal Learning Test Revised; SDMT, Symbol Digits Modalities Test; ∗ significant (p < 0.05). Delayed recall/recognition index scored out of 12 points; total recall scored out of 36 points.
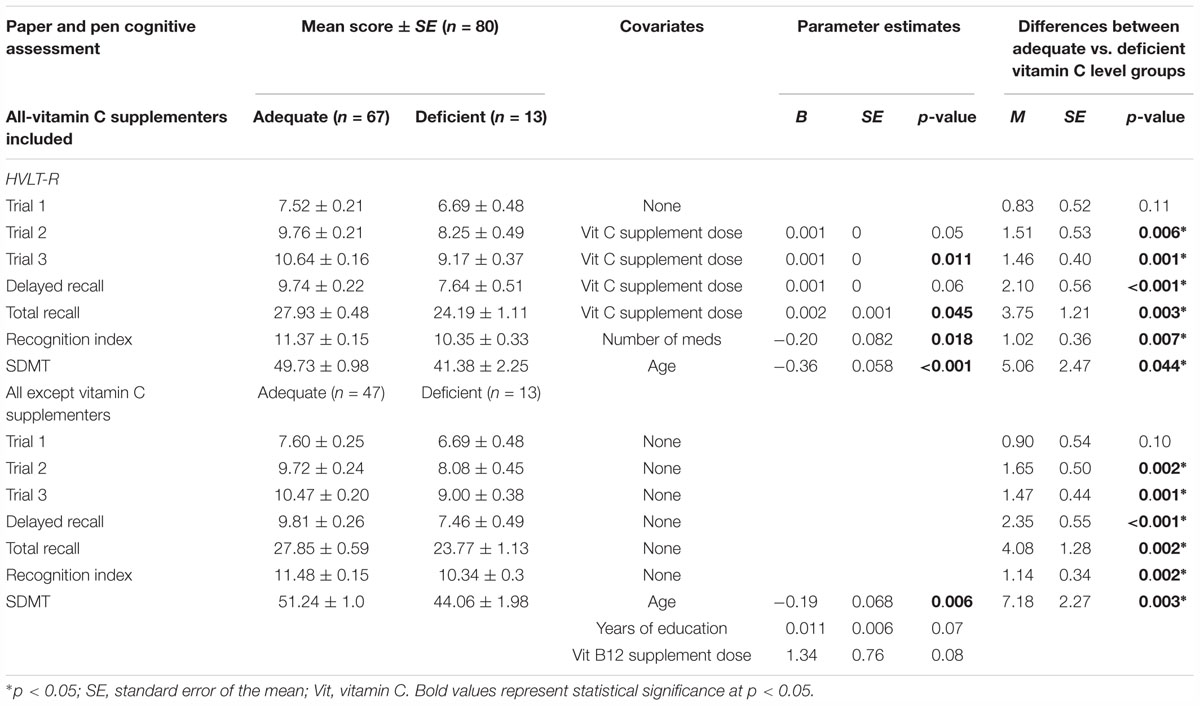
Table 7. Comparison between adequate and deficient plasma vitamin C groups on HVLT-R cognitive measures and SDMT score.
Significantly higher scores were observed on trials 2 and 3, delayed and total recall, and recognition on the HVLT-R in the adequate vitamin C group (Figure 2 and Table 7). On average, the adequate vitamin C group recalled close to 2 words more on both trials 2 and 3, delayed recall and recognized 1 more word based on the recognition index. Additionally, on average, those in the adequate vitamin C group recalled close to 4 words more on total recall. This group also had significantly higher SDMT scores then the deficient group (Table 7), with an average higher score of 7 points (1 SD).
An additional ANCOVA analysis was conducted (Table 7) comparing the adequate and deficient vitamin C groups with the exclusion of vitamin C supplementers (Table 7). As Table 7 demonstrates, when vitamin C supplementers were excluded, the cognitive differences between the adequate and deficient vitamin C groups on the HVLT-R task and SDMT did not vary greatly in comparison to when the supplementers were included. ANCOVA analyses were conducted in which each of the HVLT-R cognitive assessments and SDMT scores were compared between those supplementing on vitamin C (n = 20) and non-supplementers (n = 47) in the adequate group. Mean values on these cognitive tasks between those supplementing on vitamin C and non-vitamin C supplementers in the adequate group were not significantly different (Supplementary Table S1).
Mean vitamin C plasma concentrations were correlated with the reaction times for each of the SUCCAB tasks (Table 8). Lower reaction times were indicative of quicker response times.
The Spearman correlation (n = 80) revealed an inverse, significant, negative relationship between mean vitamin C plasma concentrations and reaction time on the congruent Stroop, spatial working memory and delayed recognition memory tasks. Similar to the paper and pen assessments, we conducted subgroup correlational analysis excluding participants supplementing on vitamin C, and found that the relationship between vitamin C concentrations and reaction times for each of the tasks appeared stronger (higher rs values) than when vitamin C supplementers were included.
The Spearman correlations (with vitamin C supplementers excluded) displayed statistically significant, inverse correlations between vitamin C concentrations and reaction time on choice reaction time, spatial working memory, the congruent and incongruent Stroop tasks and contextual memory tasks and a significant correlation on delayed recognition memory (Table 8, n = 60). The correlations between vitamin C and choice reaction time, incongruent Stroop and contextual memory were not significant when vitamin C supplementers were included (n = 80).
Spearman correlation ANCOVA analyses revealed age to be a significant covariate for the reaction times on the congruent and incongruent Stroop tasks, number of prescribed medications for reaction time on the contextual memory and delayed recognition tasks, and years of education for reaction time on the contextual memory task (Table 9). Analyses revealed lower (faster) mean reaction times in the adequate vitamin C group compared to the deficient for all of the SUCCAB tasks. Significant differences between the groups were observed on the congruent Stroop and contextual memory tasks (Figure 3). An additional ANCOVA analysis was conducted (Table 9) comparing the adequate and deficient vitamin C groups with the exclusion of vitamin C supplementers (Table 9).
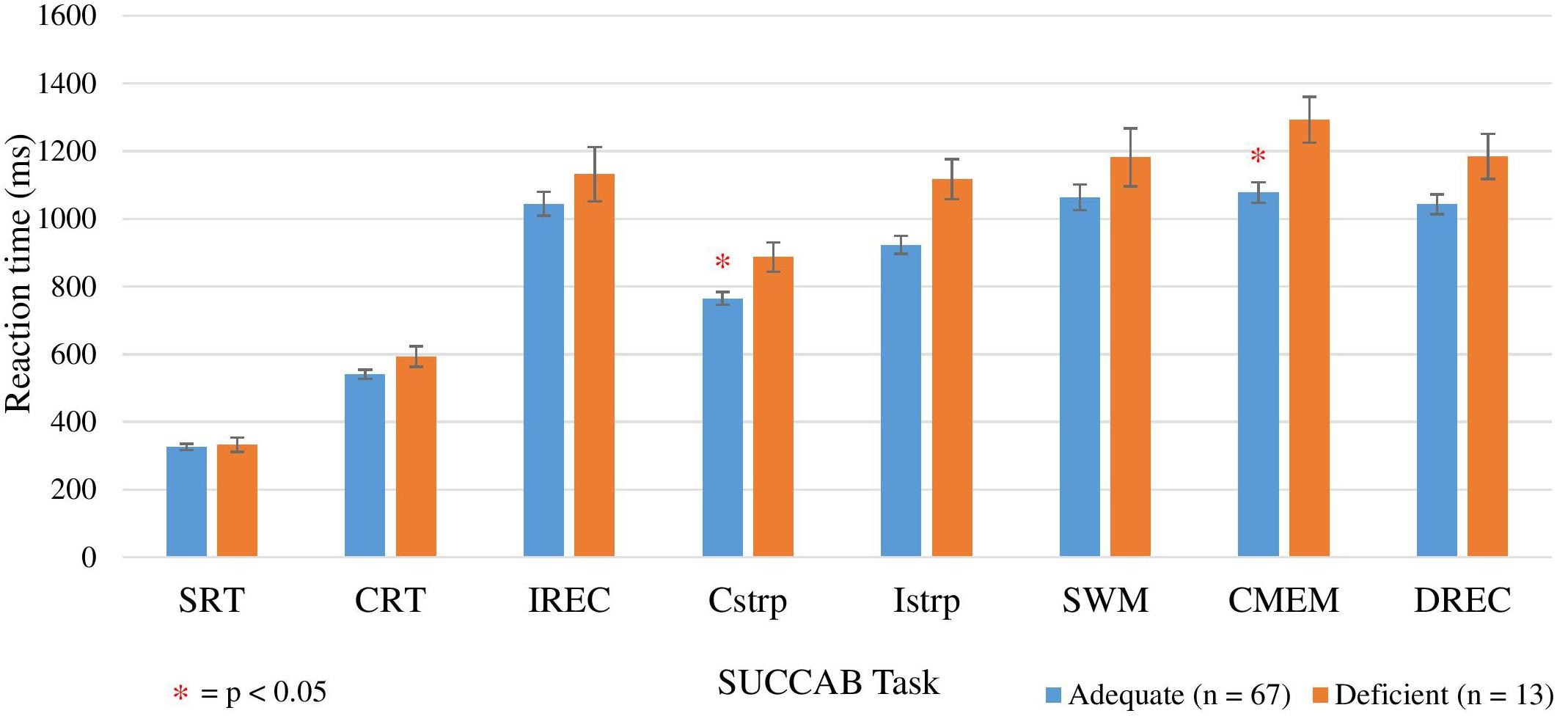
Figure 3. Comparison between adequate and deficient plasma vitamin C groups on SUCCAB reaction time. Participants grouped into adequate (n = 67) and deficient (n = 13) plasma vitamin C groups. Comparison assessed with an ANCOVA analysis, controlling for age on congruent and incongruent Stroop, numbers of medications and years of education on contextual memory and number of medications on delayed recognition memory. Reaction time assessed in milliseconds. SUCCAB, Swinburne University Computerized Cognitive Assessment Battery; SRT, simple reaction time; CRT, choice reaction time; IREC, immediate recognition memory; Cstrp, congruent Stroop; Istrp, incongruent Stroop; SWM, spatial working memory; CMEM, contextual memory; DREC, delayed recognition memory; ∗ significant (p < 0.05).
As Table 9 demonstrates, when vitamin C supplementers were excluded, significant differences between the groups were also observed on the congruent Stroop, contextual memory and additionally, the delayed recognition memory task. Mean reaction times on each SUCCAB task between those supplementing on vitamin C and non-vitamin C supplementers in the adequate group were not significantly different (Supplementary Table S2).
Additionally, analyses were conducted on the overall ratio performance (accuracy/reaction time) of each SUCCAB task. Mean vitamin C plasma concentrations were correlated with the mean ratio of each SUCCAB task (Table 10). The Spearman correlations revealed a positive, significant relationship between mean vitamin C plasma concentrations and ratios on the congruent Stroop and spatial working memory.
The Spearman correlation (with vitamin C supplementers excluded) displayed statistically significant, correlations between vitamin C concentrations and performance ratios on the congruent Stroop, spatial working memory and contextual memory, and additionally contextual memory and delayed recognition memory (Table 10, n = 60).
ANCOVA analyses revealed age to be a significant covariate for the ratios on the congruent and incongruent Stroop, spatial working memory and contextual memory. Analyses revealed higher ratios for each of the SUCCAB tasks in the adequate vitamin C group in comparison to the deficient group. Significant differences between the groups were observed on immediate recognition memory, congruent Stroop, choice reaction time and delayed recognition memory (Table 11 and Figure 4). As Table 11 demonstrates, when vitamin C supplementers were excluded, the mean scores on each of the cognitive measures on the HVLT-R task and SDMT did not vary greatly in comparison to when the supplementers were included.
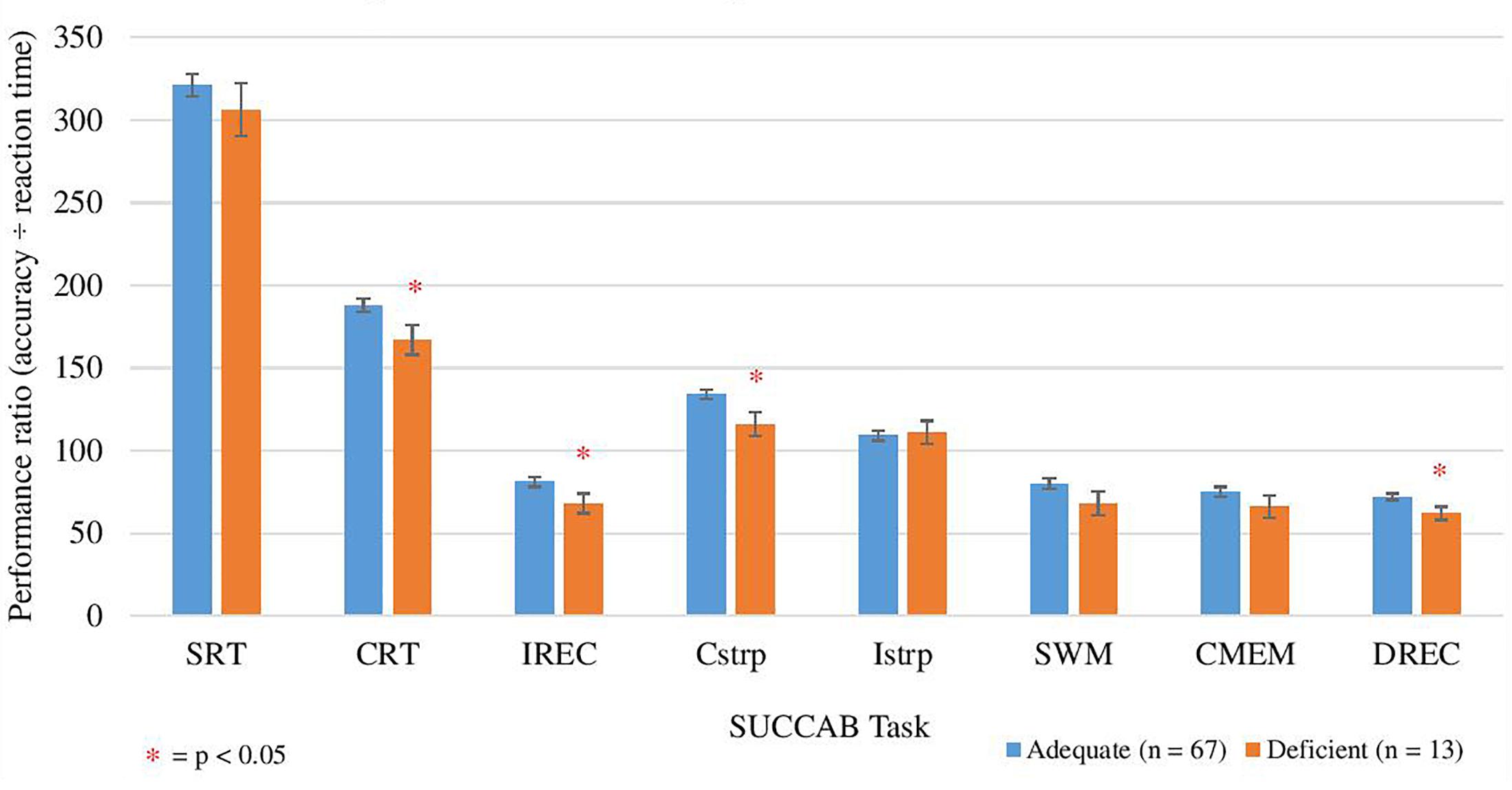
Figure 4. Comparison between adequate and deficient plasma vitamin C groups on SUCCAB ratio performance. Participants grouped into adequate (n = 67) and deficient (n = 13) plasma vitamin C groups. Comparison assessed with an ANCOVA analysis, controlling for age on congruent and incongruent Stroop, numbers of medications and years of education on contextual memory and number of medications on delayed recognition memory. Reaction time assessed in seconds. SUCCAB, Swinburne University Computerized Cognitive Assessment Battery; SRT, simple reaction time; CRT, choice reaction time; IREC, immediate recognition memory; Cstrp, congruent Stroop; Istrp, incongruent Stroop; SWM, spatial working memory; CMEM, contextual memory; DREC, delayed recognition memory; ∗ significant (p < 0.05).
An additional ANCOVA analysis was conducted in which each of the HVLT-R cognitive assessments and SDMT scores were compared between those supplementing on vitamin C (n = 20) and non-supplementers (n = 47) in the adequate group. Mean performance ratio on only the contextual memory task was significantly higher in the adequate vitamin C group not supplementing on vitamin C than in those self-reporting vitamin C supplementation (Supplementary Table S3).
Finally, results revealed no significant correlations between the HVLT-R scores and any of the reaction times and ratios on the SUCCAB tasks. Significant correlations were observed between the SDMT scores and ratios on immediate recognition memory, congruent and incongruent Stroop, spatial working memory and contextual memory.
Correlational analyses revealed a positive, significant correlation between vitamin C concentrations and performance ratio (accuracy ÷ reaction time) on the congruent Stroop, and spatial working memory tasks on the sensitive computerized SUCCAB. A significantly higher covariate adjusted performance ratio was only demonstrated on the choice reaction time, immediate recognition memory, congruent Stroop, and delayed recognition tasks in the group with adequate vitamin C levels.
Significantly higher covariate adjusted scores in immediate recall, delayed recall, total recall on the HVLT-R and SDMT scores were observed in those participants with adequate plasma vitamin C concentrations. A plateau in cognitive performance was observed on tasks in those supplementing with vitamin C and displaying vitamin C concentrations exceeding 70 μmol/L. ANCOVA analyses also indicated no additional cognitive benefits in those who self-reported vitamin C supplementation.
This is the first study to date to examine the link between vitamin C concentrations and cognition with the use of the highly sensitive computerized test SUCCAB, which can detect millisecond differences in reaction time (Pipingas et al., 2010), whereby 100 ms difference is related to 10 years of age on a the most difficult task (Pipingas et al., 2010). However, 8 studies (La Rue et al., 1997; Perrig et al., 1997; Lindeman et al., 2000; Grodstein et al., 2003; Péneau et al., 2011; Devore et al., 2013; Beydoun et al., 2015; Nooyens et al., 2015) have administered cognitive tests assessing cognitive domains that are similar to the SUCCAB tasks such as reaction time, visual perception, executive function, immediate and long term recognition/recall (tests include delayed word recall, trail making forward/backward test, Benton visual retention test, Halstead-Reitan categories test, etc.). These studies generally found a positive association between plasma vitamin C and cognition.
The positive relationship demonstrated between vitamin C concentrations and delayed recall, total recall and recognition on the HVLT-R assessment are consistent with one previous cross-sectional study using a computerized cognitive test (assessed working, implicit and explicit memory) (Perrig et al., 1997; Péneau et al., 2011) but contrary to a number of studies (Peacock et al., 2000; Grodstein et al., 2003) that used a lower quality design.
The positive relationship between vitamin C concentrations and SDMT scores found higher scores in those with adequate levels is consistent with a cross-sectional study (Sato et al., 2006) in which the highest fifth of plasma vitamin C concentrations were associated with better symbol digit substitution scores. No such association was discovered in a study which also used the letter substitution task but with a lower quality design by using a food frequency assessment instead of plasma to assess vitamin C concentration (Nooyens et al., 2015).
Vitamin C concentrations displayed an inverse relationship with reaction time on contextual memory with a trade-off on accuracy in the group in the adequate vitamin C level group. The congruent Stroop task performance was moderately related to plasma vitamin C concentrations, with a significantly higher performance and faster reaction time in the adequate vitamin C group. This finding is in contrast to a previous prospective study that used a FFQ to estimate vitamin C concentrations (Nooyens et al., 2015), but consistent with a higher quality randomized controlled trial using a multivitamin (80 mg vitamin C) (Chandra, 2001).
The choice reaction time performance ratio was positively associated with plasma vitamin C concentrations, with a significantly higher performance ratio in the adequate vitamin C group. These findings are in line with a previous cross-sectional (Perrig et al., 1997) and a 6-year prospective study (La Rue et al., 1997) which assessed visuo-spatial performance.
The significant correlations between the SDMT and a number of SUCCAB task ratios can be explained by reaction time and processing speed being the key components tested in both tasks. Both the SUCCAB and SDMT test fluid intelligence, a component of intelligence which relates to reasoning and solving novel problems, independent of any knowledge from the past. This intelligence peaks in young adulthood and then steadily declines. The cognitive decline can be attributed to lack of practice, along with age-related changes in the brain may contribute to the decline (Cunningham et al., 1975).
Lack of correlation between the HVLT-R measures and SUCCAB task performance may be explained by the HVLT-R not assessing reaction time and speed but rather focusing on accuracy of word recall and recognition, testing components of both fluid and crystalized intelligence. The SUCCAB tasks are based on visual processing whereas the HVLT-R is relying on verbal and auditory processing through spoken words. Previous research has demonstrated an asymmetry between auditory and visual processing (Cohen et al., 2009) and a stability of crystallized intelligence across most of adulthood which begins to decline after the age of 65 (Cunningham et al., 1975).
A number of explanations/mechanisms may attribute the observed results on both the paper and pen assessments and the computerized tests. It is established that vitamin C in higher concentrations stimulates the production of acetylcholine (Torda and Wolff, 1945) and an increase in brain acetylcholine receptor (AcChoR) numbers and distribution by influencing AcChoR expression through mRNA (Knaack and Podleski, 1985; Knaack et al., 1986). Vitamin C is involved as a co-factor in the conversion of dopamine into norepinephrine within the brain and adrenals (Harrison et al., 2009). Both norepinephrine and acetylcholine have been shown to play vital roles attention, focus and memory (Klinkenberg et al., 2011). Additionally, results may be explained by the involvement of vitamin C in serotonin production and neuronal absorption (Gupta et al., 2014), energy production through the synthesis of L-carnitine (Rebouche, 1991) and ROS scavenger action, enabling optimal mitochondrial function (Harrison and May, 2009).
The plateau in cognitive function observed in our study in those supplementing with vitamin C and displaying plasma concentrations >70 μmol/L can be explained by the uptake and maintenance of vitamin C in the CNS. Based on previous research, it has been established that in neuronal cells, the apparent Michaelis–Menten transport kinetics (Km) for ascorbate appears to be somewhat high (113 μmol/L); this affinity corresponds to plasma vitamin C concentrations of 30–60 μmol/L (May et al., 2006).
Although higher plasma ascorbic acid concentrations generally result in higher CSF concentrations (CSF: plasma ratio of about 3–4:1) (Quinn et al., 2003), these concentrations start to reach a steady state as plasma concentrations surpass 60 μmol/L. As plasma concentrations decline, relatively more ascorbate is pumped into the CSF in order to maintain homeostasis (May et al., 2006; Bowman et al., 2009). The variability in CSF starts to become apparent when the duration of vitamin C deficiency is extended. Thus, plasma vitamin C relates brain vitamin C status in a narrow window (<30 μmol/L) (Travica et al., 2017).
The cognitive plateau may be further explained by a possible over stimulation of norepinephrine in those displaying higher plasma vitamin C levels. Studies have indicated that norepinephrine does not display linear cognitive effects; instead, its modulation of cognitive and neuronal function maps on an Inverted-U curve (Arnsten and Li, 2005). During hyper noradrenergic states, noradrenergic α1 and β receptors are activated, leading to reduced neuronal signal efficiency and possible impairment of attentional selectivity and locomotor hyperactivity and distractibility (Arnsten and Li, 2005).
A strength of our study is its incorporation of two biochemical techniques in determining plasma vitamin C concentrations, due to the unstable nature of vitamin C in plasma. Furthermore, the present study is the first to assess vitamin C concentrations in a broad spectrum of cognitive abilities in a cognitively intact sample. The lack of spread in scores on the 3MS screening test is consistent with a number of previous cross-sectional studies (Jama et al., 1996; Ortega et al., 1997) and those discussed in a recent systematic review (Travica et al., 2017). Although the 3MS scores revealed minor variability, the other cognitive assessments were capable of detecting subtle, significant differences. Given the extensive link between vitamin B12 and cognition, a further strength of the present study is the use of serum vitamin B12 as a potential covariate.
The comparison between the food frequency questionnaire and biochemical analyses highlighted a moderate strength, significant correlation between the vitamin C measured FFQ intakes and the biochemical analyses, highlighting possible assessment inadequacies, with the lower quality study designs in previous studies. Limitations with the use of food frequency questionnaires have been well established, ranging from recall errors/bias (Pearson et al., 2017), nutrient content alterations as a result of storage and cooking (Weinstein et al., 2001), and no insight into nutrient absorption (Harrison, 2012).
Given the inadequacy of using a FFQ, our study was limited to only assessing the consumption of 20 nutrients. Other substances which have been shown to influence cognitive function such as choline (Poly et al., 2011), flavonoids (Letenneur et al., 2007) and plant based polyphenols (Vauzour, 2012), were not studied here. Although mean plasma vitamin C concentrations were higher in those supplementing on vitamin C in our study, dose and frequency were assessed using a self-report method, with no monitoring of when during the day and for how long (over 1 week prior to testing) vitamin C supplements were taken. Clinical research examining the pharmacokinetics of oral vitamin C intake has demonstrated that plasma vitamin C concentrations may exceed 100 μmol/l in healthy participants taking oral vitamin C doses over 200 mg, and that these levels can remain at these concentrations for as long as 24 h post-supplementation (depending on health/oxidative stress, etc.) (Padayatty et al., 2004). The participants displaying levels over 100 μmol/l within our sample were only those who reported vitamin C supplementation. All testing sessions were conducted in the morning with participants fasting. The individuals who self-reported vitamin C supplementation may have supplemented within 12–24 h prior to testing and reached plasma concentrations exceeding 100 μmol/l when blood was taken. Furthermore, those attaining adequate plasma vitamin C concentrations from diet performed similarly on the cognitive assessments to those supplementing on vitamin C.
There are a number of limitations arising in this study. The cross-sectional design is not suggestive of a causal relationship. Plasma vitamin C levels are dependent on recent dietary intake, therefore they are not very reproducible and also not representative of long-term consumption, which is possibly more relevant to neurologic outcome than short-term consumption (Bowman et al., 2009). The duration of depleted vitamin C concentrations were not known in our sample. This finding would be crucial, as previous studies (Spector and Lorenzo, 1973; Hornig, 1975) have demonstrated that the duration of deficiency seems to affect CNS concentrations to a greater extent than the amount of depletion.
Although our study used the Bond–Lader mood questionnaire before the cognitive assessments, we did not assess mood states post cognitive assessment. This final mood assessment would have provided an insight into the emotional affects that the cognitive tasks were having on participants by assessing subjective variables such as stress, fatigue, and energy.
An important area to be considered in future studies is cardiovascular health. Given the brain’s reliability on efficient cerebral blood flow for the delivery of vital biochemical, such as oxygen and glucose, previous studies have systematically demonstrated an effect of blood pressure and arterial stiffness on cognition (Fujishima et al., 1995; Tsao et al., 2013; Singer et al., 2014). Given the large percentage of participants in our study on blood pressure medications, cardiovascular health may have played a role in cognition over and above vitamin C concentrations.
Furthermore, additional biomarkers (other than Vitamin B12) which can influence both vitamin C concentrations and cognition could also be considered as potential covariates. These include corticosteroids such as cortisol (Sindi et al., 2012), inflammatory markers such as c-reactive protein alongside cytokines (McAfoose and Baune, 2009), white blood cells such as lymphocytes and neutrophils (Acid, 1977) and biomarkers reflecting oxidative stress such as glutathione peroxidase and glutathione (Revel et al., 2015). While our study measured plasma vitamin C and vitamin B12 levels biochemically, additional nutrients important for cognitive health such as folate, vitamin B6, vitamin D, and homocysteine should be assessed by blood tests. Alongside cardiovascular health markers, blood biomarkers which have consistently demonstrated links with both cognition and vitamin C concentrations such as cortisol, inflammatory markers and white blood cells should be considered. Although the present study did find a link between vitamin C levels and a variety of cognitive domains, future studies could confirm aspects of our results by focusing on specific cognitive domains, such as spatial working memory, and develop hypotheses and use sample sizes based on these specific cognitive domains.
In summary, using vitamin C plasma levels and validated pen and paper cognitive tests and age-sensitive computerized cognitive tests suitable for comparative analysis in cognitively intact participants, we found a significant association between vitamin C concentrations and performance on tasks involving attention, focus, working memory, decision speed, both delayed and total recall, and recognition.
In line with our hypothesis and biological roles of vitamin C on the central nervous system, significantly higher cognitive scores in adults with adequate plasma vitamin C levels (≥28 μmol/L) were predominantly observed on tasks involving decision speed and inhibition, focus, attention, recall (both immediate and delayed) and recognition compared to those with deficient vitamin C levels (<28 μmol/L). A plateau in cognitive performance was observed on tasks involving attention, focus, immediate and delayed recall in those supplementing with vitamin C and displaying vitamin C concentrations exceeding 70 μmol/L, a finding consistent with the homeostatic mechanism of vitamin C in the central nervous system. This is an indication of no additional cognitive benefits in those who self-reported vitamin C supplementation among those with non-deficient levels of plasma vitamin C. The findings from this cross-sectional study warrant future cohort or longitudinal, randomized controlled trials. This would not only enable repeated measures of vitamin C and cognition but also the assessment of a causal relationship between vitamin C and cognition.
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
AvS and NT conceptualized the study in discussion with KR and AP. IH provided software and statistical expertise. NT undertook data analysis and interpreted findings in discussion with KR and IH. NT prepared the manuscript with contributions from all co-authors (KR, AP, AvS, IH, and AnS). All authors approved the final version.
All testing was undertaken at the National Institute of Integrative Medicine (NIIM). Blood tests were paid for by NIIM.
AnS and AP have received research funding, consultancy, travel support, and speaking fees from the nutrition and supplement industry.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2019.00072/full#supplementary-material
Acid, A. (1977). The effect of ascorbic acid supplementation on some parameters of the human immunological defence system. Int. J. Vitam. Nutr. Res. 47, 248–257
Arnsten, A. F., and Li, B. -M. (2005). Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 57, 1377–1384. doi: 10.1016/j.biopsych.2004.08.019
Basu, T., and Donaldson, D. (2003). “Scurvy” in Encyclopedia of Food Sciences and Nutrition, eds B. Caballero, P. Finglas, F. Toldra (Cambridge, MA: Academic Press
Beydoun, M. A., Kuczmarski, M. F., Kitner-Triolo, M. H., Beydoun, H. A., Kaufman, J. S., Mason, M. A., et al. (2015). Dietary antioxidant intake and its association with cognitive function in an ethnically diverse sample of US adults. Psychosom. Med. 77, 68–82. doi: 10.1097/PSY.0000000000000129
Bond, A., and Lader, M. (1974). The use of analogue scales in rating subjective feelings. Br. J. Med. Psychol. 47, 211–218. doi: 10.1111/j.2044-8341.1974.tb02285.x
Bowman, G. L., Dodge, H., Frei, B., Calabrese, C., Oken, B. S., Kaye, J. A., et al. (2009). Ascorbic acid and rates of cognitive decline in Alzheimer’s disease. J. Alzheimers Dis. 16, 93–98. doi: 10.3233/JAD-2009-0923
Brandt, J. (1991). The hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin. Neuropsychol. 5, 125–142. doi: 10.1080/13854049108403297
Brubacher, D., Moser, U., and Jordan, P. (2000). Vitamin C concentrations in plasma as a function of intake: a meta-analysis. Int. J. Vitam. Nutr. Res. 70, 226–237. doi: 10.1024/0300-9831.70.5.226
Carr, A. C., and Frei, B. (1999). Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans–. Am. J. Clin. Nutr. 69, 1086–1107. doi: 10.1093/ajcn/69.6.1086
Castro, M. A., Angulo, C., Brauchi, S., Nualart, F., and Concha, I. I. (2008). Ascorbic acid participates in a general mechanism for concerted glucose transport inhibition and lactate transport stimulation. Pflügers Archi. 457, 519–528. doi: 10.1007/s00424-008-0526-1
Chandra, R. K. (2001). RETRACTED: effect of vitamin and trace-element supplementation on cognitive function in elderly subjects. Nutrition 17, 709–712. doi: 10.1016/S0899-9007(01)00610-4
Chen, J., and Berry, M. J. (2003). Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 86, 1–12. doi: 10.1046/j.1471-4159.2003.01854.x
Chung, W. Y., Chung, J. K. O., Szeto, Y. T., Tomlinson, B., and Benzie, I. F. (2001). Plasma ascorbic acid: measurement, stability and clinical utility revisited. Clin. Biochem. 34, 623–627. doi: 10.1016/S0009-9120(01)00270-3
Cohen, M. A., Horowitz, T. S., and Wolfe, J. M. (2009). Auditory recognition memory is inferior to visual recognition memory. Proc. Natl. Acad. Sci. U.S.A. 106, 6008–6010. doi: 10.1073/pnas.0811884106
Covarrubias-Pinto, A., Acuña, A. I., Beltrán, F. A., Torres-Díaz, L., and Castro, M. A. (2015). Old things new view: ascorbic acid protects the brain in neurodegenerative disorders. Int. J. Mol. Sci. 16, 28194–28217. doi: 10.3390/ijms161226095
Cunningham, W. R., Clayton, V., and Overton, W. (1975). Fluid and crystallized intelligence in young adulthood and old age. J. Gerontol. 30, 53–55. doi: 10.1093/geronj/30.1.53
De Benoist, B., McLean, E., Andersson, M., and Rogers, L. (2008). Iodine deficiency in 2007: global progress since 2003. Food Nutr. Bull. 29, 195–202. doi: 10.1177/156482650802900305
Deicher, R., and Hörl, W. H. (2003). Vitamin C in chronic kidney disease and hemodialysis patients. Kidney Blood Press. Res. 26, 100–106. doi: 10.1159/000070991
Devore, E. E., Kang, J. H., Stampfer, M. J., and Grodstein, F. (2013). The association of antioxidants and cognition in the Nurses’ health study. Am. J. Epidemiol. 177, 33–41. doi: 10.1289/EHP1691
Eldridge, C. F., Bunge, M. B., Bunge, R. P., and Wood, P. M. (1987). Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 105, 1023–1034. doi: 10.1083/jcb.105.2.1023
Figueroa-Méndez, R., and Rivas-Arancibia, S. (2015). Vitamin C in health and disease: its role in the metabolism of cells and redox state in the brain. Front. Physiol. 6:397. doi: 10.3389/fphys.2015.00397
Forstmann, B. U., Tittgemeyer, M., Wagenmakers, E. -J., Derrfuss, J., Imperati, D., and Brown, S. (2011). The speed-accuracy tradeoff in the elderly brain: a structural model-based approach. J. Neurosci. 31, 17242–17249. doi: 10.1523/JNEUROSCI.0309-11.2011
Fox, H. M., and Linkswiler, H. (1961). Pantothenic acid excretion on three levels of intake. J. Nutr. 75, 451–454. doi: 10.1093/jn/75.4.451
Frei, B., and Traber, M. G. (2001). The new US Dietary Reference Intakes for vitamins C and E. Redox Rep. 6, 5–9. doi: 10.1179/135100001101535978
Fujishima, M., Ibayashi, S., Fujii, K., and Mori, S. (1995). Cerebral blood flow and brain function in hypertension. Hypertens. Res. 18, 111–117. doi: 10.1291/hypres.18.111
Fukushima, R., and Yamazaki, E. (2010). Vitamin C requirement in surgical patients. Curr. Opin. Clin. Nutr. Metab. Care 13, 669–676. doi: 10.1097/MCO.0b013e32833e05bc
Garcion, E., Wion-Barbot, N., Montero-Menei, C. N., Berger, F., and Wion, D. (2002). New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 13, 100–105. doi: 10.1016/S1043-2760(01)00547-1
Giavarina, D. (2015). Understanding bland altman analysis. Biochem. Med. 25, 141–151. doi: 10.11613/BM.2015.015
Gómez-Pinilla, F. (2008). Brain foods: the effects of nutrients on brain function. Nat. Rev. Neurosci. 9, 568–578. doi: 10.1038/nrn2421
Grant, M. M., Barber, V. S., and Griffiths, H. R. (2005). The presence of ascorbate induces expression of brain derived neurotrophic factor in SH-SY5Y neuroblastoma cells after peroxide insult, which is associated with increased survival. Proteomics 5, 534–540. doi: 10.1002/pmic.200300924
Greger, J., Smith, S., and Snedeker, S. (1981). Effect of dietary calcium and phosphorus levels on the utilization of calcium, phosphorus, magnesium, manganese, and selenium by adult males. Nutr. Res. 1, 315–325. doi: 10.1016/S0271-5317(81)80033-4
Grodstein, F., Chen, J., and Willett, W. C. (2003). High-dose antioxidant supplements and cognitive function in community-dwelling elderly women. Am. J. Clin. Nutr. 77, 975–984. doi: 10.1093/ajcn/77.4.975
Guo, Y.-e, Suo, N., Cui, X., Yuan, Q., and Xie, X. (2018). Vitamin C promotes oligodendrocytes generation and remyelination. Glia 66, 1302–1316. doi: 10.1002/glia.23306
Gupta, P., Tiwari, S., and Haria, J. (2014). Relationship between depression and vitamin C status: a study on rural patients from western uttar pradesh in India. Int. J. Sci. Study 1, 37–39.
Hampl, J. S., Taylor, C. A., and Johnston, C. S. (2004). Vitamin C deficiency and depletion in the United States: the third national health and nutrition examination survey, 1988 to 1994. Am. J. Public Health 94, 870–875. doi: 10.2105/AJPH.94.5.870
Hansen, S. N., Tveden-Nyborg, P., and Lykkesfeldt, J. (2014). Does vitamin C deficiency affect cognitive development and function? Nutrients 6, 3818–3846. doi: 10.3390/nu6093818
Harrison, F., Allard, J., Bixler, R., Usoh, C., Li, L., May, J., et al. (2009). Antioxidants and cognitive training interact to affect oxidative stress and memory in APP/PSEN1 mice. Nutr. Neurosci. 12, 203–218. doi: 10.1179/147683009X423364
Harrison, F. E. (2012). A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer’s disease. J. Alzheimers Dis. 29, 711–726. doi: 10.3233/JAD-2012-111853
Harrison, F. E., and May, J. M. (2009). Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 46, 719–730. doi: 10.1016/j.freeradbiomed.2008.12.018
Healton, E. B., Savage, D. G., Brust, J. C., Garrett, T., and Lindenbaum, J. (1991). Neurologic aspects of cobalamin deficiency. Medicine 70, 229–245. doi: 10.1097/00005792-199107000-00001
Hoffman, F. A. (1985). Micronutrient requirements of cancer patients. Cancer 55, 295–300. doi: 10.1002/1097-0142(19850101)55:1+<295::AID-CNCR2820551315>3.0.CO;2-X
Holick, M. F. (1996). Vitamin D and bone health. J. Nutr. 126(Suppl. 4), 1159S–1164S. doi: 10.1093/jn/126.suppl_4.1159S
Hornig, D. (1975). Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann. N. Y. Acad. Sci. 258, 103–118. doi: 10.1111/j.1749-6632.1975.tb29271.x
Hsiao, P. Y., Mitchell, D., Coffman, D., Allman, R., Locher, J., Sawyer, P., et al. (2013). Dietary patterns and diet quality among diverse older adults: the university of alabama at birmingham study of aging. J. Nutr. Health Aging 17, 19–25. doi: 10.1007/s12603-012-0082-4
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and Its Panel on Folate, Other B Vitamins, and Choline (1998). Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington, DC: National Academies Press.
Iqbal, K., Khan, A., and Khattak, M. A. K. (2004). Biological significance of ascorbic acid (Vitamin C) in human health–a review. Pak. J. Nutr. 3, 5–13. doi: 10.3923/pjn.2004.5.13
Ireland, P., Jolley, D., Giles, G., O’Dea, K., Powles, J., Rutishauser, I., et al. (1994). Development of the Melbourne FFQ: a food frequency questionnaire for use in an Australian prospective study involving an ethnically diverse cohort. Asia Pac. J. Clin. Nutr. 3, 19–31.
Iuliano, S., Olden, A., & Woods, J. (2013). Meeting the nutritional needs of elderly residents in aged-care: are we doing enough? J. Nutr. Health Aging 17, 503–508. doi: 10.1007/s12603-013-0042-7
Jama, J. W., Launer, L. J., Witteman, J., Den Breeijen, J., Breteler, M., Grobbee, D., et al. (1996). Dietary antioxidants and cognitive function in a population-based sample of older persons the rotterdam study. Am. J. Epidemiol. 144, 275–280. doi: 10.1093/oxfordjournals.aje.a008922
Kallner, A., Hartmann, D., and Hornig, D. (1979). Steady-state turnover and body pool of ascorbic acid in man. Am. J. Clin. Nutr. 32, 530–539. doi: 10.1093/ajcn/32.3.530
Kang, J. H., Cook, N., Manson, J., Buring, J. E., and Grodstein, F. (2006). A randomized trial of vitamin E supplementation and cognitive function in women. Arch. Intern. Med. 166, 2462–2468. doi: 10.1001/archinte.166.22.2462
Kennedy, D. O. (2016). B vitamins and the brain: mechanisms, dose and efficacy—a review. Nutrients 8:68. doi: 10.3390/nu8020068
Klinkenberg, I., Sambeth, A., and Blokland, A. (2011). Acetylcholine and attention. Behav. Brain Res. 221, 430–442. doi: 10.1016/j.bbr.2010.11.033
Knaack, D., and Podleski, T. (1985). Ascorbic acid mediates acetylcholine receptor increase induced by brain extract on myogenic cells. Proc. Natl. Acad. Sci. U.S.A. 82, 575–579. doi: 10.1073/pnas.82.2.575
Knaack, D., Shen, I., Salpeter, M. M., and Podleski, T. R. (1986). Selective effects of ascorbic acid on acetylcholine receptor number and distribution. J. Cell Biol. 102, 795–802. doi: 10.1083/jcb.102.3.795
Kocot, J., Luchowska-Kocot, D., Kiełczykowska, M., Musik, I., and Kurzepa, J. (2017). Does vitamin c influence neurodegenerative diseases and psychiatric disorders? Nutrients 9:659. doi: 10.3390/nu9070659
La Rue, A., Koehler, K. M., Wayne, S. J., Chiulli, S. J., Haaland, K. Y., and Garry, P. J. (1997). Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am. J. Clin. Nutr. 65, 20–29. doi: 10.1093/ajcn/65.1.20
Lecomte, E., Herbeth, B., Pirollet, P., Chancerelle, Y., Arnaud, J., Musse, N., et al. (1994). Effect of alcohol consumption on blood antioxidant nutrients and oxidative stress indicators. Am. J. Clin. Nutr. 60, 255–261. doi: 10.1093/ajcn/60.2.255
Lee, D. S., and Griffiths, B. W. (1985). Human serum vitamin B12 assay methods—a review. Clin. Biochem. 18, 261–266. doi: 10.1016/S0009-9120(85)80028-X
Letenneur, L., Proust-Lima, C., Le Gouge, A., Dartigues, J. -F., and Barberger-Gateau, P. (2007). Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 165, 1364–1371. doi: 10.1093/aje/kwm036
Levine, M., Conry-Cantilena, C., Wang, Y., Welch, R. W., Washko, P. W., Dhariwal, K. R., et al. (1996). Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. U.S.A. 93, 3704–3709. doi: 10.1073/pnas.93.8.3704
Lewin, S. (1976). Vitamin C: Its Molecular Biology and Medical Potential. London: Academic Press Inc
Lindeman, R. D., Romero, L. J., Koehler, K. M., Liang, H. C., LaRue, A., Baumgartner, R. N., et al. (2000). Serum vitamin B12, C and folate concentrations in the New Mexico elder health survey: correlations with cognitive and affective functions. J. Am. Coll. Nutr. 19, 68–76. doi: 10.1080/07315724.2000.10718916
Lindenbaum, J., Healton, E. B., Savage, D. G., Brust, J. C., Garrett, T. J., Podell, E. R., et al. (1988). Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. New Engl. J. Med. 318, 1720–1728. doi: 10.1056/NEJM198806303182604
Madigan, S. M., Tracey, F., McNulty, H., Eaton-Evans, J., Coulter, J., McCartney, H., et al. (1998). Riboflavin and vitamin B-6 intakes and status and biochemical response to riboflavin supplementation in free-living elderly people. Am. J. Clin. Nutr. 68, 389–395. doi: 10.1093/ajcn/68.2.389
Martel, J. L., and Franklin, D. S. (2017). Vitamin, B1 (Thiamine). Available at: https://www.ncbi.nlm.nih.gov/books/NBK482360/ (accessed August 12, 2018).
Massee, L. A., Ried, K., Pase, M., Travica, N., Yoganathan, J., Scholey, A., et al. (2015). The acute and sub-chronic effects of cocoa flavanols on mood, cognitive and cardiovascular health in young healthy adults: a randomized, controlled trial. Front. Pharmacol. 6:93. doi: 10.3389/fphar.2015.00093
May, J. M., Li, L., Hayslett, K., and Qu, Z. -C. (2006). Ascorbate transport and recycling by SH-SY5Y neuroblastoma cells: response to glutamate toxicity. Neurochem. Res. 31, 785–794. doi: 10.1007/s11064-006-9077-z
McAfoose, J., and Baune, B. (2009). Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 33, 355–366. doi: 10.1016/j.neubiorev.2008.10.005
Mendelson, S. D. (2008). 10-Nutritional Supplements and Metabolic Syndrome. Metabolic Syndrome and Psychiatric Illness. San Diego, CA: Academic Press, 141–186.
Mollin, D., and Ross, G. (1952). The vitamin B12 concentrations of serum and urine of normals and of patients with megaloblastic anaemias and other diseases. J. Clin. Pathol. 5, 129–139. doi: 10.1136/jcp.5.2.129
National Health and Medical Research Council (2009). Australian Guidelines to Reduce Health Risks From Drinking Alcohol. Canberra: NHMRC.
Newton, H. M., Schorah, C., Habibzadeh, N., Morgan, D., and Hullin, R. (1985). The cause and correction of low blood vitamin C concentrations in the elderly. Am. J. Clin. Nutr. 42, 656–659. doi: 10.1093/ajcn/42.4.656
Noble, M., Healey, C. S., McDougal-Chukwumah, L. D., and Brown, T. M. (2013). Old disease, new look? A first report of parkinsonism due to scurvy, and of refeeding-induced worsening of scurvy. Psychosomatics 54, 277–283. doi: 10.1016/j.psym.2013.02.001
Nooyens, A. C., Milder, I. E., Van Gelder, B. M., Bueno-de-Mesquita, H. B., Van Boxtel, M. P., and Verschuren, W. M. (2015). Diet and cognitive decline at middle age: the role of antioxidants. Br. J. Nutr. 113, 1410–1417. doi: 10.1017/S0007114515000720
O’Keefe, C. A., Bailey, L. B., Thomas, E. A., Hofler, S. A., Davis, B. A., Cerda, J. J., et al. (1995). Controlled dietary folate affects folate status in nonpregnant women. J. Nutr. 125, 2717–2725.
Ortega, R. M., Requejo, A. M., Andrés, P., López-Sobaler, A. M., Quintas, M. E., Redondo, M. R., et al. (1997). Dietary intake and cognitive function in a group of elderly people. Am. J. Clin. Nutr. 66, 803–809. doi: 10.1093/ajcn/66.4.803
Padayatty, S. J., Doppman, J. L., Chang, R., Wang, Y., Gill, J., Papanicolaou, D. A., et al. (2007). Human adrenal glands secrete vitamin C in response to adrenocorticotrophic hormone.Am. J. Clin. Nutr. 86, 145–149. doi: 10.1093/ajcn/86.1.145
Padayatty, S. J., Sun, H., Wang, Y., Riordan, H. D., Hewitt, S. M., Katz, A., et al. (2004). Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 140, 533–537. doi: 10.7326/0003-4819-140-7-200404060-00010
Peacock, J. M., Folsom, A. R., Knopman, D. S., Mosley, T. H., Goff, D. C., and Szklo, M. (2000). Dietary antioxidant intake and cognitive performance in middle-aged adults. Public Health Nutr. 3, 337–343. doi: 10.1017/S1368980000000380
Pearson, J. F., Pullar, J. M., Wilson, R., Spittlehouse, J. K., Vissers, M., Skidmore, P. M., et al. (2017). Vitamin C status correlates with markers of metabolic and cognitive health in 50-year-olds: findings of the CHALICE cohort study. Nutrients 9:831. doi: 10.3390/nu9080831
Péneau, S., Galan, P., Jeandel, C., Ferry, M., Andreeva, V., Hercberg, S., et al. (2011). Fruit and vegetable intake and cognitive function in the SU. VI. MAX 2 prospective study. Am. J. Clin. Nutr. 94, 1295–1303. doi: 10.3945/ajcn.111.014712
Perrig, W. J., Perrig, P., and Stähelin, H. (1997). The relation between antioxidants and memory performance in the old and very old. J. Am. Geriatr. Soc. 45, 718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x
Pipingas, A., Harris, E., Tournier, E., King, R., Kras, M., and Stough, C. K. (2010). Assessing the efficacy of nutraceutical interventions on cognitive functioning in the elderly. Curr. Top. Nutraceutical Res. 8, 79–88.
Poly, C., Massaro, J. M., Seshadri, S., Wolf, P. A., Cho, E., Krall, E., et al. (2011). The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 94, 1584–1591. doi: 10.3945/ajcn.110.008938
Quinn, J., Suh, J., Moore, M. M., Kaye, J., and Frei, B. (2003). Antioxidants in Alzheimer’s disease-vitamin C delivery to a demanding brain. J. Alzheimers Dis. 5, 309–313. doi: 10.3233/JAD-2003-5406
Reay, J., Smith, M., and Riby, L. (2013). B vitamins and cognitive performance in older adults: review. ISRN Nutr. 2013:650983. doi: 10.5402/2013/650983
Rebec, G. V., and Pierce R. C. (1994). A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog. Neurobiol. 43, 537–565. doi: 10.1016/0301-0082(94)90052-3
Rebouche, C. J. (1991). Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 54, 1147S-1152S. doi: 10.1093/ajcn/54.6.1147s
Revel, F., Gilbert, T., Roche, S., Drai, J., Blond, E., Ecochard, R., et al. (2015). Influence of oxidative stress biomarkers on cognitive decline. J. Alzheimers Dis. 45, 553–560. doi: 10.3233/JAD-141797
Riggs, K. M., Spiro, A., Tucker, K., and Rush, D. (1996). Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am. J. Clin. Nutr. 63, 306–314. doi: 10.1093/ajcn/63.3.306
Rizvi, S. I., Pandey, K. B., Jha, R., and Maurya, P. K. (2009). Ascorbate recycling by erythrocytes during aging in humans. Rejuvenation Res. 12, 3–6. doi: 10.1089/rej.2008.0787
Sato, R., Helzlsouer, K., Comstock, G., and Hoffman, S. (2006). A cross-sectional study of vitamin C and cognitive function in older adults: the differential effects of gender. J. Nutr. Health Aging 10, 37–44.
Savage, D. G., and Lindenbaum, J. (1995). 11 Neurological complications of acquired cobalamin deficiency: clinical aspects. Baillières Clin. Haematol. 8, 657–678. doi: 10.1016/S0950-3536(05)80225-2
Schleicher, R. L., Carroll, M. D., Ford, E. S., and Lacher, D. A. (2009). Serum vitamin C and the prevalence of vitamin C deficiency in the united states: 2003–2004 national health and nutrition examination survey (NHANES). Am. J. Clin. Nutr. 90, 1252–1263. doi: 10.3945/ajcn.2008.27016
Sheridan, L. K., Fitzgerald, H. E., Adams, K. M., Nigg, J. T., Martel, M. M., Puttler, L. I., et al. (2006). Normative symbol digit modalities test performance in a community-based sample. Arch. Clin. Neuropsychol. 21, 23–28. doi: 10.1016/j.acn.2005.07.003
Sindi, S., Juster, R. -P., Wan, N., Nair, N., Ying Kin, N., and Lupien, S. (2012). Depressive symptoms, cortisol, and cognition during human aging: the role of negative aging perceptions. Stress 15, 130–137. doi: 10.3109/10253890.2011.599047
Singer, J., Trollor, J. N., Baune, B. T., Sachdev, P. S., and Smith, E. (2014). Arterial stiffness, the brain and cognition: a systematic review. Ageing Res. Rev. 15, 16–27. doi: 10.1016/j.arr.2014.02.002
Slutsky, I., Abumaria, N., Wu, L. -J., Huang, C., Zhang, L., Li, B., et al. (2010). Enhancement of learning and memory by elevating brain magnesium. Neuron 65, 165–177. doi: 10.1016/j.neuron.2009.12.026
Smith, P. F., Smith, A., Miners, J., McNeil, J., and Proudfoot, A. (2000). The safety aspects of dietary caffeine. Australia: Report from the Expert Working group, 202–223. Available at: https://www.foodstandards.gov.au/publications/Documents/safety%20aspects%20of%20dietary%20caffeine.pdf (accessed August 06, 2018).
Sotiriou, S., Gispert, S., Cheng, J., Wang, Y., Chen, A., Hoogstraten-Miller, S., et al. (2002). Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nat. Med. 8, 514–517. doi: 10.1038/0502-514
Spector, R. (1977). Vitamin homeostasis in the central nervous system. New Engl. J. Med. 296, 1393–1398. doi: 10.1056/NEJM197706162962409
Spector, R., and Lorenzo, A. (1973). Ascorbic acid homeostasis in the central nervous system. Am. J. Physiol. Leg. Content 225, 757–763. doi: 10.1152/ajplegacy.1973.225.4.757
Starns, J. J., and Ratcliff, R. (2010). The effects of aging on the speed–accuracy compromise: boundary optimality in the diffusion model. Psychol. Aging 25, 377–390. doi: 10.1037/a0018022
Swain, D. P., and Franklin, B. A. (2006). Comparison of cardioprotective benefits of vigorous versus moderate intensity aerobic exercise. Am. J. Cardiol. 97, 141–147. doi: 10.1016/j.amjcard.2005.07.130
Tombaugh, T. N., and McIntyre, N. J. (1992). The mini-mental state examination: a comprehensive review. J. Am. Geriatr. Soc. 40, 922–935. doi: 10.1111/j.1532-5415.1992.tb01992.x
Torda, C., and Wolff, H. G. (1945). Effect of vitamins on acetylcholine synthesis. The apparently specific action of Vitamin E. Proc. Soc. Exp. Biol. Med. 58, 163–165. doi: 10.3181/00379727-58-14882
Travica, N., Ried, K., Sali, A., Scholey, A., Hudson, I., and Pipingas, A. (2017). Vitamin C status and cognitive function: a systematic review. Nutrients 9:960. doi: 10.3390/nu9090960
Trumbo, P., Schlicker, S., Yates, A. A., and Poos, M. (2002). Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Dietetic Assoc. 102, 1621–1630. doi: 10.1016/S0002-8223(02)90346-9
Trumbo, P., Yates, A. A., Schlicker, S., and Poos, M. (2001). Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Am. Dietetic Assoc. 101, 294–301. doi: 10.1016/S0002-8223(01)00078-5
Tsao, C. W., Seshadri, S., Beiser, A. S., Westwood, A. J., DeCarli, C., Au, R., et al. (2013). Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81, 984–991. doi: 10.1212/WNL.0b013e3182a43e1c
Uttara, B., Singh, A. V., Zamboni, P., and Mahajan, R. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7, 65–74. doi: 10.2174/157015909787602823
Vauzour, D. (2012). Dietary polyphenols as modulators of brain functions: biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012:914273 doi: 10.1155/2012/914273
Ward, M. S., Lamb, J., May, J. M., and Harrison, F. E. (2013). Behavioral and monoamine changes following severe vitamin C deficiency. J. Neurochem. 124, 363–375. doi: 10.1111/jnc.12069
Weinstein, M., Babyn, P., and Zlotkin, S. (2001). An orange a day keeps the doctor away: scurvy in the year 2000. Pediatrics 108:e55. doi: 10.1542/peds.108.3.e55
White, H. L., and Scates, P. W. (1990). Acetyl-L-carnitine as a precursor of acetylcholine. Neurochem. Res. 15, 597–601. doi: 10.1007/BF00973749
Keywords: vitamin C, ascorbic acid, cognition, total recall, attention, central nervous system
Citation: Travica N, Ried K, Sali A, Hudson I, Scholey A and Pipingas A (2019) Plasma Vitamin C Concentrations and Cognitive Function: A Cross-Sectional Study. Front. Aging Neurosci. 11:72. doi: 10.3389/fnagi.2019.00072
Received: 13 November 2018; Accepted: 13 March 2019;
Published: 02 April 2019.
Edited by:
Enrique Cadenas, University of Southern California, United StatesReviewed by:
Kathy R. Magnusson, Oregon State University, United StatesCopyright © 2019 Travica, Ried, Sali, Hudson, Scholey and Pipingas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikolaj Travica, bnRyYXZpY2FAbmlpbS5jb20uYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.