
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 15 January 2019
Sec. Alzheimer's Disease and Related Dementias
Volume 10 - 2018 | https://doi.org/10.3389/fnagi.2018.00437
This article is part of the Research Topic Frontiers in Aging Neuroscience Editor’s Pick 2021 View all 10 articles
 Angela Guarino1*
Angela Guarino1* Francesca Favieri1
Francesca Favieri1 Ilaria Boncompagni1
Ilaria Boncompagni1 Francesca Agostini1
Francesca Agostini1 Micaela Cantone1
Micaela Cantone1 Maria Casagrande2*
Maria Casagrande2*Alzheimer's disease is a severe irreversible syndrome, characterized by a slow and progressive cognitive decline that interferes with the standard instrumental and essential functions of daily life. Promptly identifying the impairment of particular cognitive functions could be a fundamental condition to limit, through preventive or therapeutic interventions, the functional damages found in this degenerative dementia. This study aims to analyse, through a systematic review of the studies, the sensitivity of four experimental paradigms (Wisconsin Card Sorting Test, Stroop Task, Go/No-Go Task, and Flanker Task) considered as golden standard instruments for executive functions assessment in elderly subjects affected by Alzheimer dementia. This review was carried out according to the PRISMA method. Forty-five studies comparing the executive performance of patients with Alzheimer's dementia (diagnosed according to different classification criteria for dementia) and healthy elderly patients both over the age of sixty, were selected. For the research, PubMed, PsycINFO, PsycArticles databases were used. The study highlighted the importance of using standard protocols to evaluate executive dysfunction in Alzheimer's disease. The Stroop task allows discriminating better between healthy and pathological aging.
The World Health Organization defines dementia as a “loss of intellectual capability of such severity as to interfere with the social or occupational functioning” (World Health Organization, 2010). It is a complex irreversible and chronic syndrome (Knapp et al., 2007) in which a slow and progressive cognitive decline affects the typical performance of the practical and essential functions of daily life (Boccardi, 2007).
The typical symptoms of dementia involve different cognitive domains, such as memory, spatial and temporal orienting, language and learning, comprehension, and communication skills (World Health Organization, 1992); moreover, alterations in emotional control, motivation and social behavior are often present (Kipps et al., 2009). This problem appears to be increasing in the general population (World Health Organization, 2012). It is estimated that about 46.8 million people worldwide, in 2015 had dementia; continually growing numbers that are expected to double every 20 years, reaching estimates of 74.7 million people in 2030 (Prince et al., 2016).
The calculation of the annual health costs related to dementias are similar to those of heart diseases, and they are much higher than those referred to cancer. These data would place dementia among the most expensive diseases in society (Prince et al., 2016). Most of these costs are attributable to home care and long-term institutionalization.
The most widespread form of dementia is Alzheimer's Dementia (AD), which is one of the main risk factors for death in the affected individuals (Alzheimer's Association, 2016). The average duration of the disease varies between 4 and 8 years, although some patients may survive up to 20 years after the onset of the AD (Xie et al., 2008). Alzheimer dementia is estimated to have increased by 35.4% in 2015, significantly raising the specific costs of this disease (Prince et al., 2016).
Alzheimer's disease includes a pre-dementia and a dementia phase (Taylor and Thomas, 2013). Pre-dementia represents the initial period of the disorder, in which the first symptoms associated with episodic memory loss begin (starting with the removal of the most recent memories and experiences), symptoms that however do not interfere with the management of the activities of the daily life (Förstl and Kurz, 1999). However, in this Mild Cognitive Impairment condition (MCI), not amnesic dysfunctions are also reported (Hodges et al., 2006). With the progression of the disease and the transition to the actual dementia phase, in addition to a worsening of the memory symptoms (which begin to affect even the most ancient memories and experiences), linguistic and spatial orienting deficits emerge that involves a severe functional difficulty (Hodges et al., 2006). High levels of anxiety and a general lack of motivation complete the clinical profile of AD (Steinberg et al., 2008; Dening and Sandilyan, 2015). On the other hand, in this first phase the procedural memory is still relatively preserved, but with the aggravation of the disease, there is a complete compromise of the entire memory domain (Pucci, 2004).
Balota and Faust (2002) have reported that individuals with AD present specific difficulties in selecting relevant information by separating them from irrelevant ones, highlighting their difficulty in dividing attention among multiple stimuli and in the attentional control. Likewise, lexical and semantic abilities would seem to be compromised, while phonological and syntax abilities would seem to be relatively conserved. As the disease progresses, the vocabulary tends to become impoverished and phonemic, and semantic paraphasias begin to appear, with a diminishment of expressive and understanding abilities (Pucci, 2004). In addition to language, the skills of spatial and temporal orienting, cognitive control of behavior, visuomotor integration, and executive functions are strongly compromised (Pucci, 2004).
Alzheimer's disease is characterized by a complex neuropsychological profile, associated with the gradual degeneration of the various cortical areas affected by this pathology. In the AD, the entorhinal cortex and the hippocampus seem to be compromised initially, that is, the structures involved in recording and consolidating information and in episodic memory (Du et al., 2001). Moreover, some studies have shown that patients with Alzheimer's disease showed severe lesions in the hippocampal and parahippocampal regions and the medial temporal lobe (Prvulovic et al., 2002; Machulda et al., 2003).
The AD, except the rare forms caused by genetic anomalies, derives from the presence and interaction of different conditions (Ngandu et al., 2015). Late age (Hebert et al., 2013), familiarity (Green et al., 2002) and the inheritance of the APOE-4 gene (Farrer et al., 1997) represent the risk factors most associated with the AD. Smoking, obesity (Beydoun et al., 2014), diabetes (Reitz et al., 2011), low levels of education and the inability to remain socially and mentally active, making it impossible to rely on their reserves cognitive (Wang et al., 2012) that, when are low, would be included among the risk factors indirectly associated with Alzheimer's disease. Regular physical activity (Sofi et al., 2011), a diet low in saturated fats (Loef and Walach, 2012), good cardiovascular health and the absence of brain lesions (McKee et al., 2013) represent protective factors for cognitive decline.
Executive functions represent a wide range of active cognitive processes, which allow responding in the appropriate way to environmental stimuli. This “umbrella term” includes verbal reasoning, problem-solving, planning, the ability to maintain sustained attention, resistance to interference, multitasking, cognitive flexibility, and the ability to cope with novelty (Stuss and Benson, 1986; Shallice, 1988; Damasio, 1995; Stuss et al., 1995; Grafman and Litvan, 1999; Burgess et al., 2000). To facilitate research in the field of Executive Functions, several authors (Miyake et al., 2000; Lehto et al., 2003; Diamond, 2013) have developed a tripartite classification that consists of:
- Inhibition, including inhibitory control, self-control (behavioral inhibition), and interference control (selective attention and cognitive inhibition). It includes the voluntary inhibition of dominant or automatic responses (Miyake et al., 2000) and would allow controlling behavior, thoughts and emotions, as well as attentional aspects, with the aim to respond appropriately to the needs of the task and specific objectives (Diamond, 2013);
- Updating, which allows keeping in mind and manipulating information. It involves the updating and the monitoring of the representations collected in the working memory (involvement of the Dorsolateral Prefrontal Cortex; Miyake et al., 2000), which allow responding appropriately to external tasks or stimuli, thanks to the processing of relevant information (Miyake et al., 2000);
- Cognitive flexibility (set-shifting), which allows modifying one's behavioral response to external stimuli (Baddeley and Hitch, 1994; Smith and Jonides, 1999; Diamond, 2013). It is characterized by the attentional shift between tasks or between different mental operations. This mechanism is commonly regarded as disengagement from an irrelevant task with subsequent anchorage on a relevant task to pursue a particular objective (Miyake et al., 2000). Diamond (2013) referring to this specific executive function uses the term Cognitive Flexibility, which allows underlining the ability to change the individual perspective not only from a spatial point of view but also by interpersonal and thoughtful perspectives.
Until the last 20 years, deficits in executive functions were rarely considered in the early stages of Alzheimer's disease (Allain et al., 2013). Some studies suggested that these were relatively preserved during the pre-clinical phase of the disorder (Broks et al., 1996; Razani et al., 2001). However, over the last years, this view has changed, and more recent studies have confirmed the presence in the AD of early impairment in a variety of tasks aimed at investigating executive functions (Binetti et al., 1996; Amieva et al., 2002; Bondi et al., 2002). These findings confirm that in the Alzheimer's disease executive functions are impaired from the early stages (Levy et al., 2002), primarily due to degeneration of the prefrontal cortex (Salat et al., 2001). In particular, the inhibitory abilities (Amieva et al., 2004), the attentional (Perry and Hodges, 1999) and the visuospatial functions (Cronin-Golomb and Amick, 2001) would be specifically compromised.
In patients with the AD, the attentional skills needed to resolve complex tasks would be impaired, such as divided attention, the ability to effectively disengage and shift attention (Perry and Hodges, 1999) and sustained attention (Berardi et al., 2005). Moreover, about the visuospatial functions the constructive praxia, visual-perceptive, and visual orienting abilities would seem to be damaged (Cronin-Golomb et al., 2007). When these cognitive deficits interfere with the performance of daily life activities, the patient can react to his/her cognitive impairment with mood swings, irritability and apathy. All these aspects outline the characteristic clinical profile associated with Alzheimer's disease.
Considering the relevance that the AD has on the life of patients affected by this disease, it is essential to understand the specific alterations involving the executive functioning thoroughly. With this purpose, recently, many researchers have focused on the use of experimental paradigms aimed at analyzing the deficits of executive functions in individuals affected by dementia (Sgaramella et al., 2001; Bullock and Lane, 2007; Cronin-Golomb et al., 2007; Ramanan et al., 2017). These studies highlighted how the various executive functions (Miyake et al., 2000) are differently affected by AD depending on the stage of the disease and by the personal characteristics of the patients.
Different experimental paradigms were used to evaluate executive functions in the AD. (Perry and Hodges, 1999) Given the heterogeneity of these paradigms and the vastness of studies aimed at investigating executive performance in patients with the AD, the objective of this review is to analyse the researches that address this issue through four specific behavioral tasks: Stroop Task, Wisconsin Card Sorting Test, Flanker Task, and Go/No-Go Task. These tasks were more commonly used to evaluate executive performance (Diamond, 2013).
Stroop Task (Stroop, 1935) is one of the most used paradigms for the study of executive functions. In particular, through the use of incongruent stimuli, it evaluates the management of the conflict and the inhibitory control of automatic responses. In the standard version of the Stroop Task, the stimuli are words written with colored inks. There are congruent (the word RED written in red ink) or incongruent (the word RED written in green ink) trials; the participant's goal is to respond by referring to the color of the ink ignoring the meaning of the word. In this way, two alternative and incompatible responses (color vs. word) are elicited, one of which is more spontaneous than the other (reading of the word vs. ink color denomination).
The Wisconsin Card Sorting Test (WCST) (Milner, 1963) is aimed to evaluate abstract reasoning, and cognitive flexibility understood as the ability to change one's strategies in response to environmental contingencies (Berg, 1948; Grant and Berg, 1948; Luria, 1973; Shallice, 1982). The WCST consists of four stimulus cards and two sets of 64 response cards. The cards vary in color, shape and number of elements represented. The test includes some ambiguous stimuli, and the pairing criteria vary according to a standardized order (Color, Form, Number). The task requires identifying the correct criterion with which to order the response cards to the stimulus cards; for each card placed by the participant, the experimenter provides feedback on the correctness of the performance. Based on the feedback from the experimenter, the participant can modify his/her behavior by identifying the appropriate strategy.
The Flanker Task (Eriksen and Eriksen, 1974) measures selective attention and the ability to control conflictual information. The task requires discriminating the central target stimulus between a series of lateral distractors (flanker). There are three types of conditions: the congruent trials, in which the target stimulus and the flankers have the same characteristics and required the same response; the incongruent trials, in which the target has different features with respect to the distractors, requiring an opposite response that generates conflict; finally, the neutral condition, in which the distractor is not confused with the targets presented in the task, and it does not cause conflict. The flanker effect (also called conflict or congruence effect) reveals the difficulty in ignoring the distractors due to the ambiguity of the stimuli used (Cohen and Shoup, 1997).
The Go/No-Go Task assesses sustained attention (vigilance) and impulsivity and allows obtaining information related to motor-type inhibitory control (Zahn et al., 1980, 1991; McGaughy and Sarter, 1995). The task consists in the presentation of a stimulus that requires a response from the participant (Go stimulus), and another stimulus for which the participant must, instead, inhibit any response (No-Go stimulus). A high percentage of errors indicates a difficulty in behavioral inhibition. Also, in this case, there are different versions of the task to investigate the inhibitory aspects and the influences of this ability from other variables, such as emotions (Schulz et al., 2007).
The central aim of this review is to analyse the sensitivity of four experimental paradigms (Stroop Task, Flanker Task, Wisconsin Card Sorting Test and Go/No-Go Task) in the study of executive functions in elderly subjects suffering from Alzheimer's dementia, in order to be able to consider and define the applicability and usefulness of these paradigms. Moreover, another objective of this work is to verify how the executive functioning in the AD is compromised concerning the normal operation of healthy elderly, with the aim of understanding how and where the cognitive impairment associated with dementia intervenes in a more evident way.
The systematic review was conducted using the PRISMA method (Moher et al., 2009), but without recording the protocol. This review considered all the works that investigated the executive functioning through the use of the cognitive tasks defined in the introduction.
Most of the considered studies refer to the diagnostic criteria of Alzheimer Disease of the National Institute of Neurological and Communications Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS-ADRDA) (McKhann et al., 1984) for the classification of the AD. However, we considered also the studies that used the diagnosis criteria of DSM (American Psychiatric Association, 1980, 1994), the Cambridge Diagnostic Examination of Elderly (CAMDEX) (Roth et al., 1986) or National Institutes of Health and the Alzheimer's Association published revised guidelines (NIA-AA) (McKhann et al., 2011).
The study was the result of systematic research in the PubMed, PsycArticles, and PsycINFO databases. The following keywords were used for the search: Stroop Task, Wisconsin Card Sorting Test, Go/No-Go Task, Flanker Task, and Alzheimer. Thespecific scripts are presented in Table 1.
All articles published on the topic up to the date of 1 July 2018 have been taken into account.
Two researchers performed the research independently, and the results were compared. The disagreements have been resolved with consensus methods. In case of lack of consensus among the researchers, a supervisor was used.
For the selection of the articles the following inclusion criteria were used: publications on “Peer Review-Journals”; use of the Stroop Task, the Flanker Task, the Wisconsin Card Sorting Test or, the Go/No-Go Task; the presence of a control group of healthy elderly.
The exclusion criteria were the following: (a) studies that did not present all the data useful for a critical analysis of the results; (b) the use of versions of the tasks that considered the emotional components of executive functions; (c) studies with methodological bias (for example with unspecified inclusion/exclusion criteria); (d) studies comparing the AD group with groups affected by other types of dementia or MCI without a healthy elderly group; single cases.
The initial results produced 858 articles. After the elimination of duplicates and irrelevant papers, by the title and abstract reading, 83 articles were read.
At the end of the revision work, 45 articles were included in the review. The flowchart presented in Figure 1 shows the selection of the studies.
The 45 selected articles have been categorized concerning the single paradigm used. Studies that used more experimental tasks were discussed in the different paragraphs concerning the results of each specific task. According to PICOS (Moher et al., 2009), information about participants, control groups, methods and results have been extracted. These data are presented in the different behavioral task tables.
The systematic research has allowed the identification of 30 studies (see Table 2) that used different versions of the Stroop Task to evaluate inhibitory control and selective attention in patients with Alzheimer's disease.
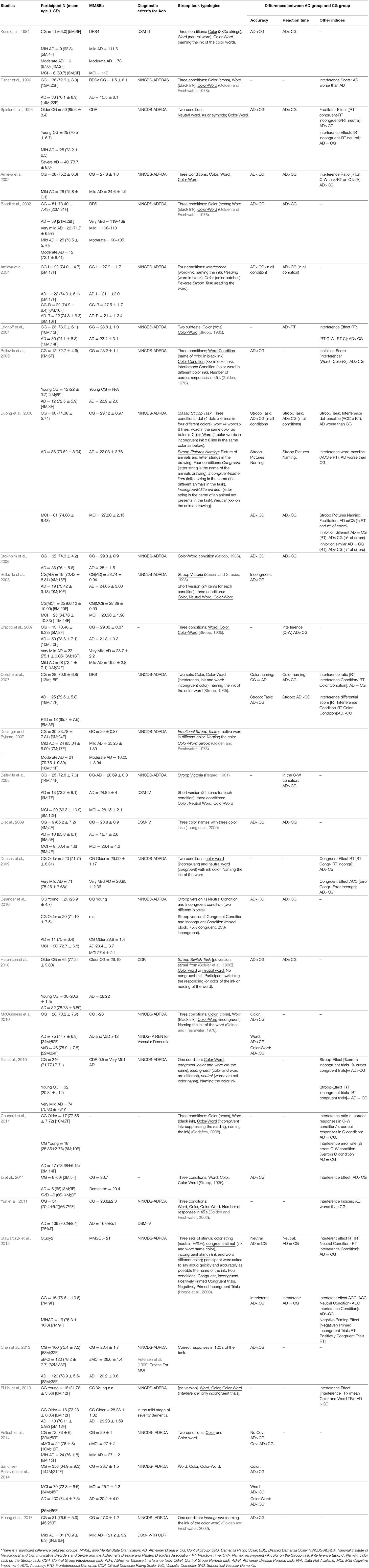
Table 2. Some characteristics of the studies that have used the Stroop Task to assess executive dysfunction in patients with Alzheimer's disease.
Within the 30 studies, only five of these did not use the diagnostic criteria of the National Institute of Neurological and Communicative Disorders and the Alzheimer Disease Association (McKhann et al., 1984), in particular, Koss et al. (1984) used the criteria for the diagnosis of AD diagnosis of DSM-III, medical observation and scores obtained at the Dementia Rating Scale (DRS) (Mattis, 1988); Li and collaborators (Li et al., 2009, 2011), have used the diagnostic criteria of the DSM-IV; (Fisher et al., 1990)used the Blessed Dementia Scale (Blessed et al., 1968) and Hutchison and colleagues (Hutchison et al., 2010) used the Clinical Dementia Rating Scale (CDR) (Morris, 1993).
The patients with the AD and their control groups included all participants over 65 years old, who differed from each other for the scores obtained at the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) or the DRS for the evaluation of the severity of dementia. Only in the study of Tse and colleagues (Tse et al., 2010), there was a significant difference between the age of patients with the AD and the healthy elderly group (patients with AD were older than people of the control group).
Compared to the Stroop Task analysis, all authors evaluated at least one of the following dependent variables: accuracy of responses, reaction times and interference effect (or Stroop effect).
In most of the studies, the analysis of accuracy showed worse performance in the patients with AD compared to healthy elderly (Koss et al., 1984; Spieler et al., 1996; Amieva et al., 2002, 2004; Bondi et al., 2002; Belleville et al., 2006, 2008; Duong et al., 2006; Stokholm et al., 2006; Collette et al., 2007; Doninger and Bylsma, 2007; Bélanger et al., 2010; Hutchison et al., 2010; McGuinness et al., 2010; Tse et al., 2010; Li et al., 2011; Stawarczyk et al., 2012; Chen et al., 2013; Peltsch et al., 2014; Sánchez-Benavides et al., 2014; Huang et al., 2017).
Furthermore, in all studies that also evaluated reaction times, it was found that patients with Alzheimer's disease are generally slower than healthy elderly people; (Koss et al., 1984; Spieler et al., 1996; Bondi et al., 2002; Amieva et al., 2004; Levinoff et al., 2004; Duong et al., 2006; Stokholm et al., 2006; Collette et al., 2007; Li et al., 2009; Bélanger et al., 2010; Hutchison et al., 2010; Tse et al., 2010) this result was not observed by Stawarczyk et al. (2012) that found similar reaction times in the two groups.
In the studies identified by systematic research, several indices were used to detect deficits in the inhibitory control through the Stroop Task. The most commonly used is the Interference Effect Index, generally calculated through the ratio or subtraction of the performance values (on reaction times or Accuracy) between the Neutral condition (Color or Word) and the interference condition (Color-Word). The results showed a higher interference effect in the patients with AD groups than in the healthy elderly people (Fisher et al., 1990; Levinoff et al., 2004; Duong et al., 2006; Collette et al., 2007; Li et al., 2009; Tse et al., 2010; Yun et al., 2011; Stawarczyk et al., 2012). The interference effect can also be considered as a measure of inhibition, and patients with AD would exhibit lower inhibitory ability than healthy participants (Amieva et al., 2004; Belleville et al., 2006). In addition, some studies have evaluated additional indices and effects: among these, Spieler and colleagues (Spieler et al., 1996) have identified a higher facilitator effect in patients with AD in the congruent trials of the Stroop Task; while Amieva and collaborators (Amieva et al., 2004) have shown a higher difficulty of patients with Alzheimer's disease in inhibiting previously imposed rules.
The systematic research allowed highlighting 14 studies (see Table 3) that used the WCST to investigate the executive function in patients with Alzheimer's disease. For this purpose, different versions of the test were used.
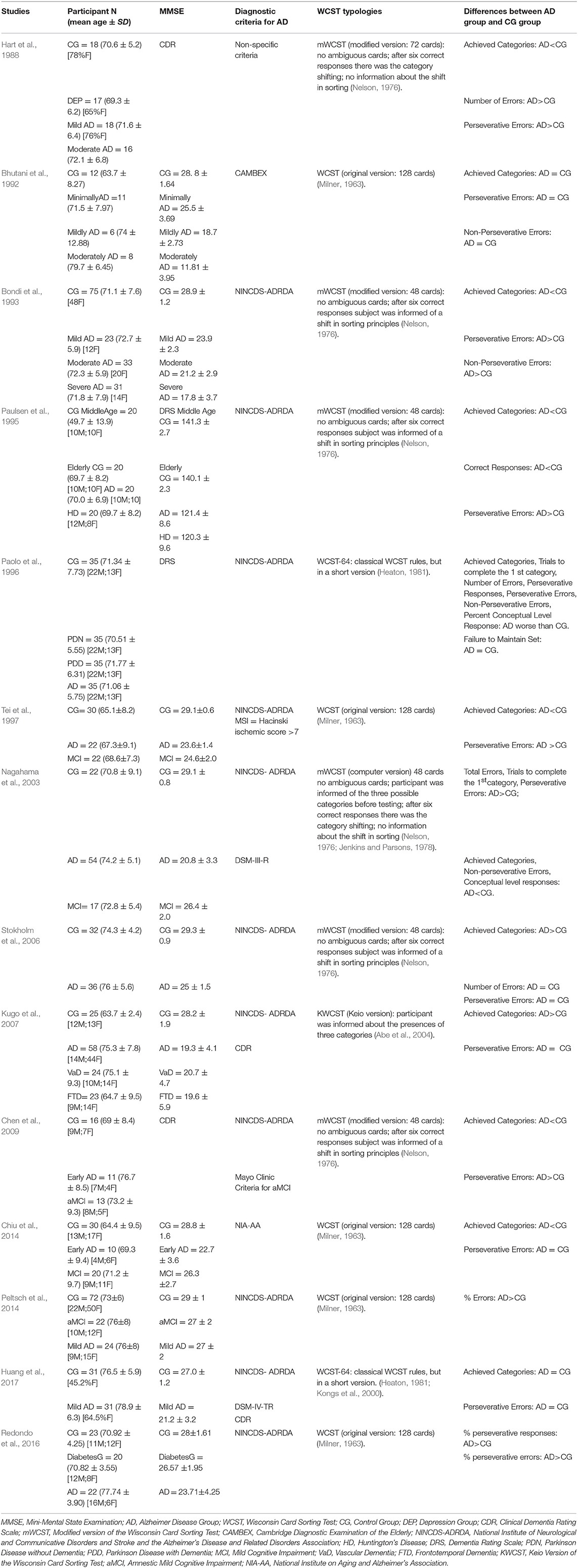
Table 3. Some characteristics of the studies that have used the Wisconsin Card Sorting Test to assess executive dysfunction in patients with Alzheimer's disease.
Almost all the studies used the diagnostic criteria NINCDS-ADRDA (McKhann et al., 1984) for the probable diagnosis of the AD. Hart et al. (1988) used non-specific criteria and the DRS score to define the AD; Bhutani et al. (1992) took into consideration the criteria of the Cambridge Diagnostic Examination of the Elderly (CAMDEX) (Roth et al., 1986); Chiu et al. (2014) used the criteria of the National Institutes of Health and the Alzheimer's Association, published revised guidelines (NIA-AA) (McKhann et al., 2011). Also, in this case, the participants were tested with the MMSE or DRS to verify the level of cognitive decline, except Chen et al. (2009) who took into account the IQ score to classify patients with the AD.
Concerning the characteristics of the groups, most of the studies matched healthy controls with patients with AD disease about age (considering an average age of over 65) and education. Redondo et al. (2016) and Peltsch et al. (2014) paired patients and healthy people only by considering years of education, groups of patients with AD were older than controls. In the Chiu study (2013), the AD group had lower education than healthy adults. Finally, the study by Kugo et al. (2007) considered patients with AD older and with lower levels of education compared to healthy controls.
Some of these studies compared the WCST performance of patients with the AD with that of patients with MCI, as well as with the healthy elderly group (Tei et al., 1997; Kugo et al., 2007; Chen et al., 2009; Chiu et al., 2014; Peltsch et al., 2014).
To evaluate performance in WCST, all studies used at least two of the following scores: number of completed categories, perseverative errors, total errors, and non-perseverative errors.
The results showed that patients with Alzheimer's disease complete fewer categories (Bondi et al., 1993; Paulsen et al., 1995; Paolo et al., 1996; Stokholm et al., 2006; Kugo et al., 2007; Chen et al., 2009; Chiu et al., 2014) and commit more perseverative errors than healthy people (Hart et al., 1988; Bondi et al., 1993; Paulsen et al., 1995; Nagahama et al., 2003; Kugo et al., 2007; Chen et al., 2009; Redondo et al., 2016). Nevertheless, five studies (Bhutani et al., 1992; Tei et al., 1997; Stokholm et al., 2006; Chiu et al., 2014; Peltsch et al., 2014; Huang et al., 2017) do not identify differences between the numbers of perseverative errors committed by patients compared to those of the control group. Some of these studies (Bhutani et al., 1992; Tei et al., 1997; Peltsch et al., 2014; Huang et al., 2017) did not identify any difference between patients with the AD and the control group even when they considered the number of completed categories.
Redondo et al. (2016) identified a higher percentage of both perseverative errors and perseverative responses in AD patients compared to healthy older; while Nagahama et al. (2003) and Bhutani et al. (1992) showed fewer non-perseverative errors in patients with the AD than in healthy patients. This result could indicate a poor set-shifting capacity in patients affected by Alzheimer's disease that is expressed through a general perseveration of the responses.
To analyse the motor inhibition in patients with Alzheimer's disease, four studies (see Table 4) used different versions of the Go/No-Go Task. All the studies used the NINCDS-ADRDA diagnostic criteria for diagnosis (McKhann et al., 1984). All participants (mean age over 65 years in both groups) underwent an assessment of cognitive decline through MMSE (Amieva et al., 2002; Stawarczyk et al., 2012; Rochat et al., 2013) or DRS, (Collette et al., 2007) which confirmed the presence of a higher decline in patients with AD compared to healthy controls. Further, all studies (Amieva et al., 2002; Collette et al., 2007; Stawarczyk et al., 2012; Rochat et al., 2013) used a group of healthy elderly people matched by age and education to the patient group with the AD.
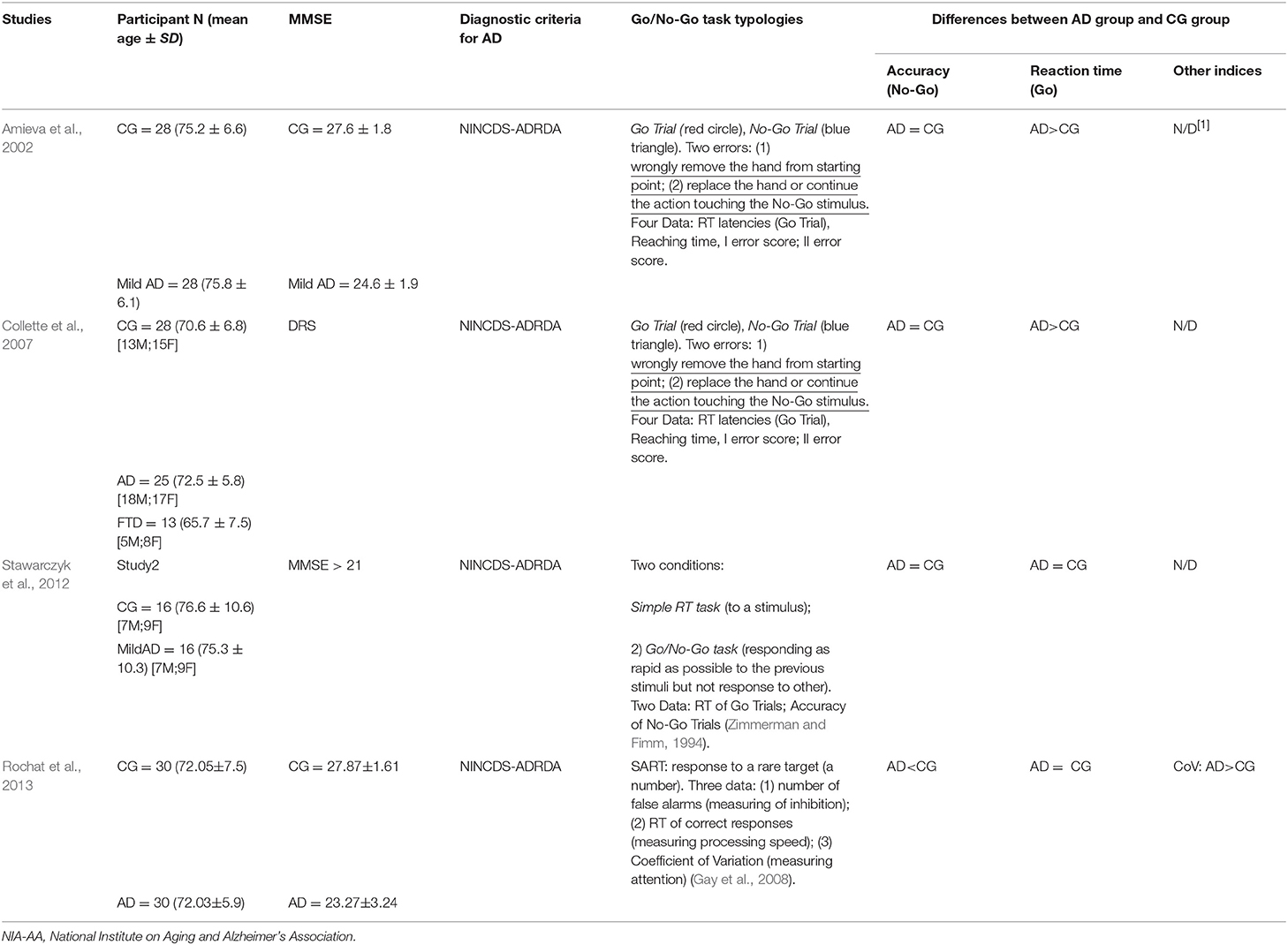
Table 4. Some characteristics of the studies that have used the Go/No-Go Task to assess executive dysfunction in patients with Alzheimer's disease.
The accuracy analysis was evaluated in all studies by considering the number of errors in the No-Go trials (false alarms). Responding to the No-Go stimulus, in fact, is viewed as an error due to impulsivity. Concerning this result, Rochat et al. (2013) shows a higher number of false alarms in the AD group compared to healthy older adults. The other studies (Amieva et al., 2002; Collette et al., 2007; Stawarczyk et al., 2012) did not reveal any difference between patients and controls in the motor inhibition.
Reaction times analysis, in the Go trials, was used to evaluate the global processing speed (Amieva et al., 2002; Collette et al., 2007; Stawarczyk et al., 2012; Rochat et al., 2013). In particular, Collette et al. (2007) and Amieva et al. (2002) confirm a slower performance of patients with AD compared to controls, while Stawarczyk et al. (2012) and Rochat et al. (2013) do not report significant differences between the groups.
The study by Stawarczyk et al. (2012) also analyzed the preservation of inhibitory control through the analysis of reaction times. However, they do not show any difference between controls and patients with Alzheimer's disease. Moreover, Rochat et al. (2013) also considered the Counting coefficient (Standard Deviation/Mean reaction times of the Go Trial) and observed a higher score in patients with AD compared to healthy people, indicating a worse performance to the task.
Overall, the studies that used the Go/No-Go task to assess the motor inhibitory control of patients with Alzheimer's dementia would seem to show a specific heterogeneity in the results. Two of the four studies analyzed (Amieva et al., 2002; Collette et al., 2007) tend to show a general slowdown in response times, which would not indicate a specific deficit in motor inhibition in this task. In contrast, only the study by Rochat and colleagues (Rochat et al., 2013) indicates the presence of an evident executive-motor deficit linked to inhibitory control in patients with Alzheimer's disease.
Four studies (Collette et al., 2009; Stawarczyk et al., 2012; Wang et al., 2013; Chen et al., 2017) used different types of flanker tasks inspired by the classic paradigm of Eriksen and Eriksen (Eriksen and Eriksen, 1974), to analyses cognitive inhibition and conflict control in patients with Alzheimer's disease (Table 2).
All the studies used for the probable diagnosis of the AD the diagnostic criteria NINCDS-ADRDA (McKhann et al., 1984).
In all studies, both groups (healthy elderly and elderly with Alzheimer's disease) have an average age over 65 years. For the assessment of the level of cognitive impairment, the scores at MMSE and those of the DRS were taken into consideration. Although the authors do not report in detail the scores obtained by the different groups, they still attest to higher cognitive impairment in patients with the AD. Two of the studies (Wang et al., 2013; Chen et al., 2017), compared patients with the AD with both a healthy control group and a group of subjects classified as MCI.
All the authors recorded reaction times and accuracy of responses in the Flanker Task. Chen et al. (2017) compared the performance between groups only through reaction times; this methodological choice is justified by the fact that the authors consider reaction times as the significant markers of cognitive functioning because it is more closely associated with neural functioning.
The analysis of the reaction times highlighted inconsistent results. Wang et al. (2013) and Chen et al. (2017) reported slower reaction times in patients with Alzheimer compared to healthy elderly, while Collette et al. (2009) and Stawarczyk et al. (2012) did not find significant differences between the two groups.
Analyzing accuracy, only the study by Wang et al. (2013) showed a higher percentage of errors in patients with the AD than in the control group. However, both general reaction times and accuracy give a measure of selective attention, and they do not inform about executive function.
Some of the authors (Collette et al., 2009; Wang et al., 2013) also evaluated the flanker effect index, given by the difference between incongruent and congruent trials in reaction times or accuracy. Specifically, Collette et al. (2009), considering accuracy, reported a higher flanker effect in the patients with the AD than in the control group; Wang et al. (2013) has instead recorded a higher flanker effect in patients the AD compared to the healthy people by considering both reaction times and accuracy. Moreover, Collette et al. (2009) also evaluated the facilitator effect (comparing the accuracy of the congruent trials of the same word and same category conditions to the neutral response condition of Word Flanker Task; see Table 5), this effect was higher in patients with the AD than in the two control groups (young adults and elderly healthy subjects).
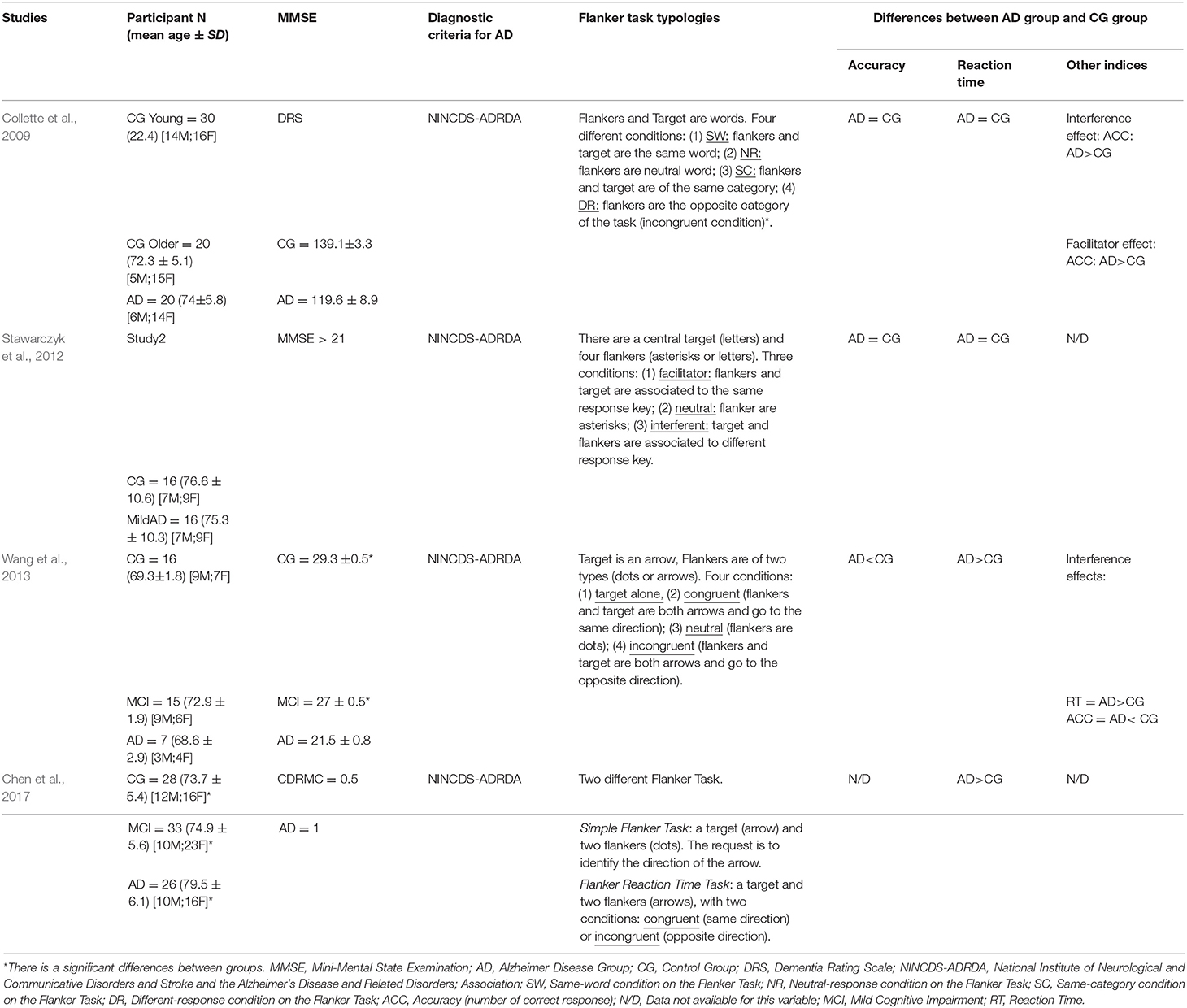
Table 5. Some characteristics of the studies that have used the Flanker Task to assess executive dysfunction in patients with Alzheimer's disease.
This systematic review was aimed to verify the sensitivity of four golden standard executive functions tasks in catching dysfunctions in these domains in the Alzheimer's disease. Because executive deficits interfere with the performance of daily life activities, by worsening the quality of life of individuals with the AD (Wecker et al., 2000; Collette et al., 2009), it is essential to take this cognitive dimension into account. It is important to note that the recognition of an impairment in the executive functions in the AD is the result of a route change, in fact until a few years ago it was believed that the executive functions were not affected in the pre-clinical stages of dementia (Broks et al., 1996; Razani et al., 2001). Today it is known that these cognitive functions are already damaged prematurely (Binetti et al., 1996; Bondi et al., 2002; Amieva et al., 2004).
A correct choice of the cognitive tasks to use for the assessment of cognitive impairment is a crucial element to take into account. Those considered in this review are the most used in the study of executive functions (Alvarez and Emory, 2006; Duchek et al., 2009; Diamond, 2013) and are widely utilized for cognitive assessment in patients with AD compared to healthy elderly or other pathologies (Collette et al., 2007; Stawarczyk et al., 2012; Peltsch et al., 2014).
They specifically evaluate the capacities of the motor (Go/No-Go Task) and cognitive inhibition (Stroop Task), the conflict control (Flanker Task), and the cognitive flexibility (WCST), the ability to suppress automatic responses and the ability to “resist” to interference (Stroop Task and Flanker task respectively); all skills affected by cognitive decline that are specially compromise in AD.
The Stroop Task seems to be the paradigm that best discriminates between healthy and pathological aging, and it is the most widely used in the research on the AD (Spieler et al., 1996; Belleville et al., 2008; Duchek et al., 2009; Bélanger et al., 2010; McGuinness et al., 2010; Stawarczyk et al., 2012; Peltsch et al., 2014). A neuropsychological assessment including this test can assess the executive system. It could highlight the individual's ability to move the patient's cognitive set, providing a measure of cognitive inhibition and attentional control, and it gives information about the ability to inhibit an overlearned response (i.e., a dominant response, such as the reading) in favor of an unusual stimulus (Spreen and Strauss, 1998). One aspect to consider about this task is that some authors (Hutchison et al., 2010) believe that memory is entailed in the resolution process involved in this task, the decline of which may compromise the overall performance and hinder the ability to focusing attention on the target.
The results obtained with the other tests were inconsistent, but it should be noted that few studies have used them. Concerning the ability of the Wisconsin Card Sorting Test to catch deficits in the cognitive flexibility of Alzheimer's disease, the results are inconsistent. The most critical aspect related to WCST is the complexity of the task. For this reason, some authors (Hart et al., 1988; Bondi et al., 1993; Paulsen et al., 1995; Stokholm et al., 2006) have introduced modified and simplified forms of this test. Contrary to the standard version, on these modified versions of the WSCT, there are fewer cards, and in some case, the subject was informed about a shift in sorting principles. Moreover, there were not ambiguous cards, and the sorting criteria changed after six correct responses. These aspects make the task easier and allow identifying, with higher sensitivity, the cognitive flexibility deficits in the AD (Bondi et al., 1993; Paulsen et al., 1995; Stokholm et al., 2006; Chen et al., 2009). If we considered the modified versions of the test, WCST seems to discriminate between AD patients and healthy subjects (Hart et al., 1988; Bondi et al., 1993; Paulsen et al., 1995; Nagahama et al., 2003; Stokholm et al., 2006; Chen et al., 2009). However, even using a simplified version of the WCST, some studies did not find clear differences between healthy elderly and patients with the AD. Bhutani et al. (1992) believe that the WCST is characterized by a “floor effect” that would not allow discriminating the normal cognitive decline and deterioration typical of dementia, an aspect also reaffirmed by Huang et al. (2017). Moreover, if we consider the studies that compared the performance of patients with the AD with those of elderly people suffering from other dementia diseases (temporal dementia, vascular dementia, Parkinson's with dementia), there are no differences, suggesting that this task not allow discriminating among different forms of dementia (Paulsen et al., 1995; Paolo et al., 1996; Kugo et al., 2007; Li et al., 2011).
The results about the Flanker Task would seem to indicate that this paradigm is not able to highlight a difference between healthy and pathological elderly, especially when reaction times are considered (Collette et al., 2009; Stawarczyk et al., 2012). The analysis of accuracy, instead, has a higher discriminating ability to indicate the actual deterioration in selective attention in patients with the AD (Collette et al., 2009; Wang et al., 2013; Chen et al., 2017). However, if we consider the only two studies that analyzed the flanker effect, both found an impaired conflict control in patients with AD compared to healthy people. The characteristics of the sample represent a weak point of these studies. Two of the four studies did not perform an exact pairing for age and education between patients with the AD and healthy controls. Moreover, the number of participants was very reduced (AD group: N = 26 and Control group: N = 28; AD group: N = 15 and Control group: N = 16) (Wang et al., 2013; Chen et al., 2017). For these reasons, and considering the few studies present, it is not possible to exclude that the Flanker Task is sensitive to catch impairment in selective attention and conflict control in Alzheimer's dementia, especially in light of the results obtained at the Stroop Task, which involves attentional aspects similar to those assessed by the Flanker Task (Baddeley and Hitch, 1994).
Also, the results relative to the Go/No-Go task did not indicate whether a deficit in the control of motor inhibition is present or not in patients with AD compared to healthy elderly. Only one study (Rochat et al., 2013) showed a higher number of false alarms in the AD group compared to healthy older adults, indicating an impaired motor inhibition in the AD. The other studies (Amieva et al., 2002; Collette et al., 2007; Stawarczyk et al., 2012) did not reveal any difference between patients and controls in the motor inhibition, suggesting that there not be in AD an executive deficit of this type. This difference between the Rochat's study and the other studies could be due to a different version of the Go/No-Go Task, that required to respond to a rare target (a number); this fact involves a harder inhibitory control ability, that could explain the difference between AD patients and elderly healthy Control Group. However, the comparison between patients with Alzheimer's dementia and patients with different types of dementia showed no difference (Collette et al., 2007; Kugo et al., 2007), suggesting a pattern of motor inhibition common to the different types of dementia.
This review highlighted several limitations in the examination of executive functions in Alzheimer's disease. In particular, many studies have used a numerically insufficient sample (Koss et al., 1984; Bhutani et al., 1992; Belleville et al., 2006; Chen et al., 2009; Li et al., 2009, 2011; Coubard et al., 2011; Stawarczyk et al., 2012; El Haj et al., 2013; Wang et al., 2013) others did not consider the level of education of the participants (Spieler et al., 1996; Amieva et al., 2002; Belleville et al., 2006) although this is a variable to be taken into account when analyzing executive functions (Contador et al., 2017). A further problem is related to the assessment of cognitive decline. The different studies have used different scales to evaluate the cognitive decline, and in some studies, the average scores obtained by the various groups are not reported (Paolo et al., 1996; Spieler et al., 1996; Collette et al., 2007; Coubard et al., 2011). Furthermore, the severity of the decline within the AD groups varies a lot as regards the scores at the MMSE (see Tables 2–5). This condition does not allow controlling the influence of these variables on the performance of the tasks used. Furthermore, some studies not matching groups by age, gender and education, and they have not always controlled other aspects (such as the severity of the AD or comorbidity diseases) that could influence task performance (Spieler et al., 1996; Amieva et al., 2002; Collette et al., 2007; Duchek et al., 2009; McGuinness et al., 2010). These dimensions indeed reduce the sensitivity of the instruments in the identification of differences between groups, especially in the case of an analysis of functions that are subject to a physiological decline with age, as also shown by some of the studies considered here that compare elderly control groups with younger control groups (Spieler et al., 1996; Bélanger et al., 2010; Hutchison et al., 2010).
Another critical point of the reviewed studies concerns the use of multiple forms of the same experimental paradigm. These miscellanea of test does not allow for an explicit comparison between the various researches. Therefore, it is difficult to arrive at definite conclusions. This aspect is particularly evident in the studies that have used the WCST. In this case, simplified versions of the test have shown a higher ability to identify differences associated with the AD (Paulsen et al., 1995; Nagahama et al., 2003; Chen et al., 2009), probably because they allow participants to overcome the floor effect.
Concerning the Go/No-Go and the Flanker task, the results were weak in light of the few studies found. Therefore, the systematic use of these tests would be useful, to verify their sensitivity to capture deficits in conflict control and motor inhibition in the AD.
However, regardless of these limitations, at the end of this review emerges an effective executive deficit in the inhibitory control (Chen et al., 2013; Peltsch et al., 2014; Sánchez-Benavides et al., 2014; Huang et al., 2017), and partly also in the cognitive flexibility (Paulsen et al., 1995; Stokholm et al., 2006; Chiu et al., 2014) in the AD. These results allow us to suggest a plausible identikit of executive functioning in Alzheimer's disease, characterized by an impairment in inhibition and cognitive flexibility. There are differences in performance in the various tasks, which could reflect, as previously underlying, differences in the levels of deterioration of the various executive functions analyzed during the AD progression.
In the light of the results of this review that showed a more or less marked discriminatory capacity of the examined tasks for the identification of the executive deficits in Alzheimer's disease, it would be advisable to insert these tasks within neuropsychological batteries. This could allow investigating more entirely and articulately the cognitive functioning of patients affected by the AD.
Overall, this review highlighted the importance of a comprehensive neuropsychological evaluation to allow a clear delineation of the aging profile associated with Alzheimer's disease.
An evaluation of this type could be inserted into pathological aging prevention programs, and it could be useful as a form of monitoring of executive functioning in aging. Furthermore, it could allow identifying the presence of even slight deficits, such as a Mild Cognitive Impairment, that could predict a degenerative disease like the AD. MCI and AD have different diagnostic criteria and different levels of cognitive impairment, for these reasons MCI was not considered in this work. However, it could be useful to conduct a systematic review taking into account the executive performance in MCI specifically.
The results of this review would be to decline it in a meta-analysis that could allow to better understanding the profile of executive functions in the Alzheimer's disease. Furthermore, it would be advisable to carry out a comparative analysis of the different experimental paradigms used to investigate the individual executive functions. This comparison could allow to a better understanding of the results obtained in this work, consenting to conclude whether the results are univocal regardless of the task used or if there is an effect of the type of the task that reinforces or weakens the conclusions to which this review has arrived.
AG, MaC and MiC: conception of review, wrote the manuscript; IB, FA and FF literature research, wrote the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Founding for Research from Ph.D. program in Psychology and Cognitivse Science. Department of Psychology, Sapienza Univeristy of Rome (Italy).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abe, M., Suzuki, K., Okada, K., Miura, R., Fujii, T., Etsurou, M., et al. (2004). Normative data on tests for frontal lobe functions: trail making test, verbal fluency, Wisconsin card sorting test (Keio version). No to shinkei 56, 567–574.
Allain, P., Etcharry-Bouyx, F., and Verny, C. (2013). Executive functions in clinical and preclinical Alzheimer's disease. Rev. Neurol. 169, 695–708. doi: 10.1016/j.neurol.2013.07.020
Alvarez, J. A., and Emory, E. (2006). Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16, 17–42. doi: 10.1007/s11065-006-9002-x
Alzheimer's Association (2016). 2016 Alzheimer's disease facts and figures. Alzheimer Dement. 12, 459–509. doi: 10.1016/j.jalz.2016.03.001
American Psychiatric Association (1980). Diagnostic and Statistical Manual of Mental Disorders DSM-III. Washington, DC: APA Diagnostic.
American Psychiatric Association (1994). Statistical Manual of Mental Disorders. Washington, DC: APA Diagnostic.
Amieva, H., Lafont, S., Auriacombe, S., Le Carret, N., Dartigues, J. F., Orgogozo, J. M., et al. (2002). Inhibitory breakdown and dementia of the Alzheimer type: a general phenomenon. J Clin Exp Neuropsychol. 24, 503–516. doi: 10.1076/jcen.24.4.503.1034
Amieva, H., Lafont, S., Rouch-Leroyer, I., Rainville, C., Dartigues, J. F., Orgogozo, J. M., et al. (2004). Evidencing inhibitory deficits in Alzheimer's disease through interference effects and shifting disabilities in the Stroop test. Arch. Clin. Neuropsychol. 19, 791–803. doi: 10.1016/j.acn.2003.09.006
Baddeley, A. D., and Hitch, G. J. (1994). Developments in the concept of working memory. Neuropsychology, 8, 485.
Balota, D. A., and Faust, M. (2002). “Attention in Alzheimer's disease,” in Handbook of Neuropsychology, Vol. 6, 2nd Edn, eds F. Boller and S. Cappa (New Brunswick, NJ: Elsevier Science), 51–80.
Bélanger, S., Belleville, S., and Gauthier, S. (2010). Inhibition impairments in Alzheimer's disease, mild cognitive impairment and healthy aging: effect of congruency proportion in a Stroop task. Neuropsychologia 48, 581–590. doi: 10.1016/j.neuropsychologia.2009.10.021
Belleville, S., Bherer, L., Lepage, É., Chertkow, H., and Gauthier, S. (2008). Task switching capacities in persons with Alzheimer's disease and mild cognitive impairment. Neuropsychologia 46, 2225–2233. doi: 10.1016/j.neuropsychologia.2008.02.012
Belleville, S., Rouleau, N., and Van der Linden, M. (2006). Use of the Hayling task to measure inhibition of prepotent responses in normal aging and Alzheimer's disease. Brain Cogn. 62, 113–119. doi: 10.1016/j.bandc.2006.04.006
Berardi, A. M., Parasuraman, R., and Haxby, J. V. (2005). Sustained attention in mild Alzheimer's disease. Dev. Neuropsychol. 28, 507–537. doi: 10.1207/s15326942dn2801_4
Berg, E. A. (1948). A simple objective technique for measuring flexibility in thinking. J. Gen. Psychol. 39, 15–22. doi: 10.1080/00221309.1948.9918159
Beydoun, M. A., Beydoun, H. A., Gamaldo, A. A., Teel, A., Zonderman, A. B., and Wang, Y. (2014). Epidemiologic studies of modifiable factors associated with cognition and dementia: systematic review and meta-analysis. BMC Public Health 14:643. doi: 10.1186/1471-2458-14-643
Bhutani, G. E., Montaldi, D., Brooks, D. N., and McCulloch, J. (1992). A neuropsychological investigation into frontal lobe involvement in dementia of the Alzheimer type. Neuropsychology 6:211. doi: 10.1037/0894-4105.6.3.211
Binetti, G., Magni, E., Padovani, A., Cappa, S. F., Bianchetti, A., and Trabucchi, M. (1996). Executive dysfunction in early Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr. 60, 91–93. doi: 10.1136/jnnp.60.1.91
Blessed, G., Tomlinson, B. E., and Roth, M. (1968). The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br. J. Psychiatry 114, 797–811. doi: 10.1192/bjp.114.512.797
Boccardi, M. (2007). La riabilitazione Nella Demenza Grave. Manuale pratico per operatori e Caregiver. Trento: Edizioni Erickson.
Bondi, M. W., Monsch, A. U., Butters, N., Salmon, D. P., and Paulsen, J. S. (1993). Utility of a modified version of the Wisconsin Card Sorting Test in the detection of dementia of the Alzheimer type. Clin. Neuropsychol. 7, 161–170. doi: 10.1080/13854049308401518
Bondi, M. W., Serody, A. B., Chan, A. S., Eberson-Shumate, S. C., Delis, D. C., Hansen, L. A., et al. (2002). Cognitive and neuropathologic correlates of Stroop Color-Word Test performance in Alzheimer's disease. Neuropsychology 16:335. doi: 10.1037//0894-4105.16.3.335
Bracco, L., Bessi, V., Piccini, C., Mosconi, L., Pupi, A., and Sorbi, S. (2007). Metabolic correlates of executive dysfunction. J. Neurol. 254:1052. doi: 10.1007/s00415-006-0488-1
Broks, P., Lines, C., Atchison, L., Challenor, J., Traub, M., Foster, C., et al. (1996). Neuropsychological investigation of anterior and posterior cortical function in early-stage probable Alzheimer's disease. Behav. Neurol. 9, 135–148. doi: 10.3233/BEN-1996-93-405
Bullock, R., and Lane, R. (2007). Executive dyscontrol in dementia, with emphasis on subcortical pathology and the role of butyrylcholinesterase. Curr. Alzheimer Res. 4, 277–293. doi: 10.2174/156720507781077313
Burgess, P. W., Veitch, E., de Lacy Costello, A., and Shallice, T. (2000). The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia 38, 848–863. doi: 10.1016/S0028-3932(99)00134-7
Chen, K. C., Weng, C. Y., Hsiao, S., Tsao, W. L., and Koo, M. (2017). Cognitive decline and slower reaction time in elderly individuals with mild cognitive impairment. Psychogeriatrics 17, 364–370. doi: 10.1111/psyg.12247
Chen, N. C., Chang, C. C., Lin, K. N., Huang, C. W., Chang, W. N., Chang, Y. T., et al. (2013). Patterns of executive dysfunction in amnestic mild cognitive impairment. Int. Psychogeriatr. 25, 1181–1189. doi: 10.1017/S1041610213000392
Chen, T. F., Chen, Y. F., Cheng, T. W., Hua, M. S., Liu, H. M., and Chiu, M. J. (2009). Executive dysfunction and periventricular diffusion tensor changes in amnesic mild cognitive impairment and early Alzheimer's disease. Hum. Brain Mapp. 30, 3826–3836. doi: 10.1002/hbm.20810
Chiu, M. J., Chen, Y. F., Chen, T. F., Yang, S. Y., Yang, F. P. G., Tseng, T. W., et al. (2014). Plasma tau as a window to the brain—negative associations with brain volume and memory function in mild cognitive impairment and early alzheimer's disease. Hum. Brain Mapp. 35, 3132–3142. doi: 10.1002/hbm.22390
Cohen, A., and Shoup, R. (1997). Perceptual dimensional constraints in response selection processes. Cogn. Psychol. 32, 128–181. doi: 10.1006/cogp.1997.0648
Collette, F., Amieva, H., Adam, S., Hogge, M., Van der Linden, M., Fabrigoule, C., et al. (2007). Comparison of inhibitory functioning in mild Alzheimer's disease and frontotemporal dementia. Cortex 43, 866–874. doi: 10.1016/S0010-9452(08)70686-5
Collette, F., Schmidt, C., Scherrer, C., Adam, S., and Salmon, E. (2009). Specificity of inhibitory deficits in normal aging and Alzheimer's disease. Neurobiol. Aging 30, 875–889. doi: 10.1016/j.neurobiolaging.2007.09.007
Contador, I., del Ser, T., Llamas, S., Villarejo, A., Benito-León, J., and Bermejo-Pareja, F. (2017). Impact of literacy and years of education on the diagnosis of dementia: A population-based study. J. Clin. Exp. Neuropsychol. 39, 112–119. doi: 10.1080/13803395.2016.1204992
Coubard, O. A., Ferrufino, L., Boura, M., Gripon, A., Renaud, M., and Bherer, L. (2011). Attentional control in normal aging and Alzheimer's disease. Neuropsychology 25:353. doi: 10.1037/a0022058
Cronin-Golomb, A., and Amick, M. (2001). Spatial abilities in aging, Alzheimer's disease and Parkinson's disease,” in Handbook of Neuropsychology, Vol. 6, 2nd Edn, eds F. Boller and S. Cappa (Amsterdam: Elsevier), 119–143.
Cronin-Golomb, A., Gilmore, G. C., Neargarder, S., Morrison, S. R., and Laudate, T. M. (2007). Enhanced stimulus strength improves visual cognition in aging and Alzheimer's disease. Cortex 43, 952–966. doi: 10.1016/S0010-9452(08)70693-2
Damasio, A. R. (1995). REVIEW: toward a neurobiology of emotion and feeling: operational concepts and hypotheses. Neuroscientist 1, 19–25. doi: 10.1177/107385849500100104
Dening, T., and Sandilyan, M. B. (2015). Dementia: definitions and types. Nurs. Stand. 29:37. doi: 10.7748/ns.29.37.37.e9405
Diamond, A. (2013). Executive functions. Annu. Rev. Psychol. 64, 135–168. doi: 10.1146/annurev-psych-113011-143750
Doninger, N. A., and Bylsma, F. W. (2007). Inhibitory control and affective valence processing in dementia of the Alzheimer type. J. Neuropsychol. 1, 65–83. doi: 10.1348/174866407X.180828
Du, A. T., Schuff, N., Amend, D., Laakso, M. P., Hsu, Y. Y., Jagust, W. J., et al. (2001). Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr. 71, 441–447. doi: 10.1136/jnnp.71.4.441
Duchek, J. M., Balota, D. A., Tse, C. S., Holtzman, D. M., Fagan, A. M., and Goate, A. M. (2009). The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer's disease. Neuropsychology 23:746. doi: 10.1037/a0016583
Duong, A., Whitehead, V., Hanratty, K., and Chertkow, H. (2006). The nature of lexico-semantic processing deficits in mild cognitive impairment. Neuropsychologia 44, 1928–1935. doi: 10.1016/j.neuropsychologia.2006.01.034
El Haj, M., Postal, V., and Allain, P. (2013). Destination memory in Alzheimer's disease: when i imagine telling ronald reagan about Paris. Cortex 49, 82–89. doi: 10.1016/j.cortex.2011.11.014
Eriksen, B. A., and Eriksen, C. W. (1974). Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 16, 143–149. doi: 10.3758/BF03203267
Farrer, L. A., Cupples, L. A., Haines, J. L., Hyman, B., Kukull, W. A., Mayeux, R., et al. (1997). Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA 278, 1349–1356. doi: 10.1001/jama.1997.03550160069041
Fisher, L. M., Freed, D. M., and Corkin, S. (1990). Stroop Color-Word Test performance in patients with Alzheimer's disease. J. Clin. Exp. Neuropsychol. 12, 745–758. doi: 10.1080/01688639008401016
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Förstl, H., and Kurz, A. (1999). Clinical features of Alzheimer's disease. Eur. Arch. Psychiatry Clin. Neurosci. Eur. 249, 288–290.
Gay, P., Rochat, L., Billieux, J., d'Acremont, M., and Van der Linden, M. (2008). Heterogeneous inhibition processes involved in different facets of self-reported impulsivity: Evidence from a community sample. Acta Psychol. 129, 332–339. doi: 10.1016/j.actpsy.2008.08.010
Godefroy, O. (2008). Fonctions Exécutives et Pathologies Neurologiques et Psychiatriques: Évaluation en Pratique Clinique. Groupe de Boeck.
Golden, C. J. (1976). Identification of brain disorders by the Stroop Color and Word Test. J. Clin. Psychol. 32, 654–658. doi: 10.1002/1097-4679(197607)32:3 < 654::AID-JCLP2270320336>3.0.CO;2-Z
Golden, C. J., and Freshwater, S. M. (1978). Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Wood Dale: Stoelting Company.
Golden, C. J., and Freshwater, S. M. (2002). The Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago: Stoelting.
Grafman, J., and Litvan, I. (1999). Importance of deficits in executive functions. Lancet 354, 1921–1923. doi: 10.1016/S0140-6736(99)90438-5
Grant, D. A., and Berg, E. (1948). A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 38:404. doi: 10.1037/h0059831
Green, R. C., Cupples, L. A., Go, R., Benke, K. S., Edeki, T., Griffith, P. A., et al. (2002). Risk of dementia among white and African American relatives of patients with Alzheimer disease. JAMA 287, 329–336. doi: 10.1001/jama.287.3.329
Hart, R. P., Kwentus, J. A., Wade, J. B., and Taylor, J. R. (1988). Modified Wisconsin Sorting Test in elderly normal, depressed and demented patients. Clin. Neuropsychol. 2, 49–56. doi: 10.1080/13854048808520085
Heaton, R. K. (1981). Wisconsin Card Sorting Test manual. Odessa, FL: Psychological Assessment Resources Inc.
Hebert, L. E., Weuve, J., Scherr, P. A., and Evans, D. A. (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. doi: 10.1212/WNL.0b013e31828726f5
Hodges, J. R., Erzinçliolu, S., and Patterson, K. (2006). Evolution of cognitive deficits and conversion to dementia in patients with mild cognitive impairment: a very-long-term follow-up study. Dement. Geriatr. Cogn. Disord. 21, 380–391. doi: 10.1159/000092534
Hogge, M., Salmon, E., and Collette, F. (2008). Interference and negative priming in normal aging and in mild Alzheimer's disease. Psychol. Belg. 48, 1–23. doi: 10.5334/pb-48-1-1
Huang, S. F., Liu, C. K., Chang, C. C., and Su, C. Y. (2017). Sensitivity and specificity of executive function tests for Alzheimer's disease. Appl. Neuropsychol. Adult 24, 493–504. doi: 10.1080/23279095.2016.1204301
Hutchison, K. A., Balota, D. A., and Ducheck, J. M. (2010). The utility of Stroop task switching as a marker for early-stage Alzheimer's disease. Psychol. Aging 25:545. doi: 10.1037/a0018498
Jenkins, R. L., and Parsons, O. A. (1978). Cognitive deficits in male alcoholics as measured by a modified Wisconsin Card Sorting Test. Alcohol Tech. Reports 7, 76–83.
Kipps, C. M., Mioshi, E., and Hodges, J. R. (2009). Emotion, social functioning and activities of daily living in frontotemporal dementia. Neurocase, 15, 182–189. doi: 10.1080/13554790802632892
Knapp, M., Prince, M., Albanese, E., Banerjee, S., Dhanasiri, S., Fernandez, J. L., et al. (2007). Dementia UK: a report into the prevalence and cost of dementia prepared by the Personal social Services Research Unit (PCSSRU) at the London School of Economics and Institute of Psychiatry at King's College London for the Alzheimer's Society. London: Alzheimer's Society.
Kongs, S. K., Thompson, L. L., Iverson, G. L., and Heaton, R. K. (2000). Wisconsin Card Sorting Test-64 Card Version (WCST-64). Odessa, FL: Psychological Assessment Resources.
Koss, E., Ober, B. A., Delis, D. C., and Friedland, R. P. (1984). The Stroop color-word test: indicator of dementia severity. Int. J. Neurosci. 24, 53–61. doi: 10.3109/00207458409079534
Kugo, A., Terada, S., Ata, T., Ido, Y., Kado, Y., Ishihara, T., et al. (2007). Japanese version of the frontal assessment battery for dementia. Psychiatry Res. 153, 69–75. doi: 10.1016/j.psychres.2006.04.004
Lehto, J. E., Juujärvi, P., Kooistra, L., and Pulkkinen, L. (2003). Dimensions of executive functioning: evidence from children. Br. J. Dev. Psychol. 21, 59–80. doi: 10.1348/026151003321164627
Leung, H. C., Skudlarski, P., Gatenby, J. C., Peterson, B. S., and Gore, J. C. (2000). An event-related functional MRI study of the Stroop color word interference task. Cereb. Cortex 10, 552–560. doi: 10.1093/cercor/10.6.552
Levinoff, E. J., Li, K. Z., Murtha, S., and Chertkow, H. (2004). Selective attention impairments in Alzheimer's disease: evidence for dissociable components. Neuropsychology 18:580. doi: 10.1037/0894-4105.18.3.580
Levy, G., Jacobs, D. M., Tang, M. X., Côté, L. J., Louis, E. D., Alfaro, B., et al. (2002). Memory and executive function impairment predict dementia in Parkinson's disease. Mov. Disord. 17, 1221–1226. doi: 10.1002/mds.10280
Li, C., Zheng, J., Wang, J., and Gui, L. (2011). Comparison between Alzheimer's disease and subcortical vascular dementia: attentional cortex study in functional magnetic resonance imaging. J. Int. Med. Res. 39, 1413–1419. doi: 10.1177/147323001103900428
Li, C., Zheng, J., Wang, J., Gui, L., and Li, C. (2009). An fMRI stroop task study of prefrontal cortical function in normal aging, mild cognitive impairment, and Alzheimer's disease. Curr. Alzheimer Res. 6, 525–530. doi: 10.2174/156720509790147142
Loef, M., and Walach, H. (2012). The combined effects of healthy lifestyle behaviors on all cause mortality: a systematic review and meta-analysis. Prev. Med. 55, 163–170. doi: 10.1016/j.ypmed.2012.06.017
Machulda, M. M., Ward, H. A., Borowski, B., Gunter, J. L., Cha, R. H., O'brien, P. C., et al. (2003). Comparison of memory fMRI response among normal, MCI, and Alzheimer's patients. Neurology 61, 500–506. doi: 10.1212/01.WNL.0000079052.01016.78
Mattis, S. (1988). Dementia Rating Scale DRS: Professional Manual. Odessa, FL: Psychological Assessment Resources.
McGaughy, J., and Sarter, M. (1995). Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology 117, 340–357. doi: 10.1007/BF02246109
McGuinness, B., Barrett, S. L., Craig, D., Lawson, J., and Passmore, A. P. (2010). Attention deficits in Alzheimer's disease and vascular dementia. J. Neurol. Neurosur. Psychiatry 81, 157–159. doi: 10.1136/jnnp.2008.164483
McKee, A. C., Stein, T. D., Nowinski, C. J., Stern, R. A., Daneshvar, D. H., Alvarez, V. E., et al. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64. doi: 10.1093/brain/aws307
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer's disease report of the NINCDS-ADRDA work group* under the auspices of department of health and human services task force on Alzheimer's disease. Neurology 34, 939–939. doi: 10.1212/WNL.34.7.939
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack Jr, C. R., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers. Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
Milner, B. (1963). Effects of different brain lesions on card sorting: the role of the frontal lobes. Arch. Neurol. 9, 90–100. doi: 10.1001/archneur.1963.00460070100010
Miyake, A., Friedman, N. P., Emerson, M. J., Witzki, A. H., Howerter, A., and Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn. Psychol. 41, 49–100. doi: 10.1006/cogp.1999.0734
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Morris, J. C. (1993). The clinical dementia rating (CDR): current version and scoring rules. Neurology. 43, 2412–2414.
Nagahama, Y., Okina, T., Suzuki, N., Matsuzaki, S., Yamauchi, H., Nabatame, H., et al. (2003). Factor structure of a modified version of the wisconsin card sorting test: an analysis of executive deficit in Alzheimer's disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 16, 103–112. doi: 10.1159/000070683
Nelson, H. E. (1976). A modified card sorting test sensitive to frontal lobe defects. Cortex 12, 313–324. doi: 10.1016/S0010-9452(76)80035-4
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
Paolo, A. M., Tröster, A. I., Blackwell, K. T., Koller, W. C., and Axelrod, B. N. (1996). Utility of a wisconsin card sorting test short form in persons with Alzheimer's and Parkinson's disease. J. Clin. Exp. Neuropsychol. 18, 892–897. doi: 10.1080/01688639608408310
Paulsen, J. S., Salmon, D. P., Monsch, A. U., Butters, N., Swenson, M. R., and Bondi, M. W. (1995). Discrimination of cortical from subcortical dementias on the basis of memory and problem-solving tests. JCLP 51, 48–58.
Peltsch, A., Hemraj, A., Garcia, A., and Munoz, D. P. (2014). Saccade deficits in amnestic mild cognitive impairment resemble mild Alzheimer's disease. Eur. J. Neurosci. 39, 2000–2013. doi: 10.1111/ejn.12617
Perry, R. J., and Hodges, J. R. (1999). Attention and executive deficits in Alzheimer's disease: a critical review. Brain 122, 383–404. doi: 10.1093/brain/122.3.383
Petersen, R. C., Smith, G. E., Waring, S. C., Ivnik, R. J., Tangalos, E. G., and Kokmen, E. (1999). Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 56, 303–308. doi: 10.1001/archneur.56.3.303
Prince, M., Ali, G. C., Guerchet, M., Prina, A. M., Albanese, E., and Wu, Y. T. (2016). Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers. Res. Ther. 8:23. doi: 10.1186/s13195-016-0188-8
Prvulovic, D., Hubl, D., Sack, A. T., Melillo, L., Maurer, K., Frölich, L., et al. (2002). Functional imaging of visuospatial processing in Alzheimer's disease. Neuroimage 17, 1403–1414. doi: 10.1006/nimg.2002.1271
Ramanan, S., Bertoux, M., Flanagan, E., Irish, M., Piguet, O., Hodges, J. R., et al. (2017). Longitudinal executive function and episodic memory profiles in behavioral-variant frontotemporal dementia and Alzheimer's disease. J. Int. Neuropsychol. Soc. 23, 34–43. doi: 10.1017/S1355617716000837
Razani, J., BOONE, K. B., Miller, B. L., Lee, A., and Sherman, D. (2001). Neuropsychological performance of right-and left-frontotemporal dementia compared to Alzheimer's disease. J. Int. Neuropsychol. Soc. 7, 468–480. doi: 10.1017/S1355617701744037
Redondo, M. T., Beltrán-Brotóns, J. L., Reales, J. M., and Ballesteros, S. (2016). Executive functions in patients with Alzheimer's disease, type 2 diabetes mellitus patients and cognitively healthy older adults. Exp. Gerontol. 83, 47–55. doi: 10.1016/j.exger.2016.07.013
Regard, M. (1981). Stroop Test–Victoria Version. Victoria, BC: Neuropsychological Laboratory, University of Victoria.
Reitz, C., Brayne, C., and Mayeux, R. (2011). Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 7:137. doi: 10.1038/nrneurol.2011.2
Rochat, L., Billieux, J., Van der Linden, A. C. J., Annoni, J. M., Zekry, D., Gold, G., et al. (2013). A multidimensional approach to impulsivity changes in mild Alzheimer's disease and control participants: cognitive correlates. Cortex 49, 90–100. doi: 10.1016/j.cortex.2011.08.004
Roth, M., Tym, E., Mountjoy, C. Q., Huppert, F. A., Hendrie, H., Verma, S., and Goddard, R. (1986). CAMDEX: a standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br J Psychiatry 149, 698–709. doi: 10.1192/bjp.149.6.698
Salat, D. H., Kaye, J. A., and Janowsky, J. S. (2001). Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Arch. Neurol. 58, 1403–1408. doi: 10.1001/archneur.58.9.1403
Sánchez-Benavides, G., Peña-Casanova, J., Casals-Coll, M., Gramunt, N., Molinuevo, J. L., Gómez-Ansón, B., et al. (2014). Cognitive and neuroimaging profiles in mild cognitive impairment and Alzheimer's disease: data from the spanish multicenter normative studies (NEURONORMA project). J. Alzheimers. Dis. 41, 887–901. doi: 10.3233/JAD-132186
Schulz, K. P., Fan, J., Magidina, O., Marks, D. J., Hahn, B., and Halperin, J. M. (2007). Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Arch. Clin. Neuropsychol. 22, 151–160. doi: 10.1016/j.acn.2006.12.001
Sgaramella, T. M., Borgo, F., Mondini, S., Pasini, M., Toso, V., and Semenza, C. (2001). Executive deficits appearing in the initial stage of Alzheimer's disease. Brain Cogn. 46, 264–268. doi: 10.1016/S0278-2626(01)80080-4
Shallice, T. (1982). Specific impairments of planning. Phil. Trans. R. Soc. Lond. B 298, 199–209. doi: 10.1098/rstb.1982.0082
Shallice, T. (1988). From Neuropsychology to Mental Structure. Cambridge: Cambridge University Press.
Smith, E. E., and Jonides, J. (1999). Storage and executive processes in the frontal lobes. Science 283, 1657–1661. doi: 10.1126/science.283.5408.1657
Sofi, F., Valecchi, D., Bacci, D., Abbate, R., Gensini, G. F., Casini, A., et al. (2011). Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J. Intern. Med. 269, 107–117. doi: 10.1111/j.1365-2796.2010.02281.x
Spieler, D. H., Balota, D. A., and Faust, M. E. (1996). Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer's type. J. Exp. Psychol. Hum. Percept. Perform. 22:461. doi: 10.1037/0096-1523.22.2.461
Spreen, O., and Strauss, E. A. (1998).Compendium of Neuropsychologial Tests. Administration, Norms, and Commentary. New York, NY: Oxford University Press.
Stawarczyk, D., Grandjean, J., Salmon, E., and Collette, F. (2012). Perceptual and motor inhibitory abilities in normal aging and Alzheimer disease (AD): A preliminary study. Arch. Gerontol. Geriatr. 54, e152–e161. doi: 10.1016/j.archger.2011.12.004
Steinberg, M., Shao, H., Zandi, P., Lyketsos, C. G., Welsh-Bohmer, K. A., Norton, M. C., et al. (2008). Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int. J. Geriatr. Psychiatry 23, 170–177. doi: 10.1002/gps.1858
Stokholm, J., Vogel, A., Gade, A., and Waldemar, G. (2006). Heterogeneity in executive impairment in patients with very mild Alzheimer's disease. Dement. Geriatr. Cogn. Disord. 22, 54–59.doi: 10.1159/000093262
Stroop, J.R. (1935). Studies of interference in serial verbal reactions. J. Exp. Psychol. 18:643. doi: 10.1037/0096-3445.121.1.15
Stuss, D. T., Shallice, T., Alexander, M. P., and Picton, T. W. (1995). A multidisciplinary approach to anterior attentional functions. Ann. N.Y. Acad. Sci. 769, 191–212. doi: 10.1111/j.1749-6632.1995.tb38140.x
Taylor, J. P., and Thomas, A. (2013). “Alzheimer's disease,” in Textbook of Old Age Psychiatry, eds. T. Dening and A. Thomas (Oxford: Oxoford University Press), 431–455.
Tei, H., Miyazaki, A., Iwata, M., Osawa, M., Nagata, Y., and Maruyama, S. (1997). Early-stage Alzheimer's disease and multiple subcortical infarction with mild cognitive impairment: neuropsychological comparison using an easily applicable test battery. Dement. Geriatr. Cogn. Disord. 8, 355–358. doi: 10.1159/000106655
Tse, C. S., Balota, D. A., Yap, M. J., Duchek, J. M., and McCabe, D. P. (2010). Effects of healthy aging and early stage dementia of the Alzheimer's type on components of response time distributions in three attention tasks. Neuropsychology 24:300. doi: 10.1037/a0018274
Wang, H. X., Xu, W., and Pei, J. J. (2012). Leisure activities, cognition and dementia. Biochim. Biophys. Acta 1822, 482–491. doi: 10.1016/j.bbadis.2011.09.002
Wang, P., Zhang, X., Liu, Y., Liu, S., Zhou, B., Zhang, Z., et al. (2013). Perceptual and response interference in Alzheimer's disease and mild cognitive impairment. Clin. Neurophysiol. 124, 2389–2396. doi: 10.1016/j.clinph.2013.05.014
Wecker, N. S., Kramer, J. H., Wisniewski, A., Delis, D. C., and Kaplan, E. (2000). Age effects on executive ability. Neuropsychology 14:409. doi: 10.1037/0894-4105.14.3.409
World Health Organization (1992). The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines (Vol. 1). Geneva: World Health Organization.
World Health Organization (2010). International Classification of Disease. Geneva: World Health Organization.
World Health Organization (2012). Dementia: A Public Health Priority. Geneva: World Health Organization.
Xie, J., Brayne, C., and Matthews, F. E. (2008). Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. BMJ 336, 258–262. doi: 10.1136/bmj.39433.616678.25
Yun, J. Y., Lee, D. Y., Seo, E. H., Choo, I. H., Park, S. Y., Kim, S. G., et al. (2011). Neural correlates of stroop performance in Alzheimer's disease: a FDG-PET study. Dement. Geriatr. Cogn. Disord. Extra 1, 190–201. doi: 10.1159/000329517
Zahn, T. P., Kruesi, M. J., and Rapoport, J. L. (1991). Reaction time indices of attention deficits in boys with disruptive behavior disorders. J. Abnorm. Cild Psychol. 19, 233–252. doi: 10.1007/BF00909980
Keywords: alzheimer's disease, executive functions, wisconsin card sorting test, stroop task, go/no-go task, flanker task
Citation: Guarino A, Favieri F, Boncompagni I, Agostini F, Cantone M and Casagrande M (2019) Executive Functions in Alzheimer Disease: A Systematic Review. Front. Aging Neurosci. 10:437. doi: 10.3389/fnagi.2018.00437
Received: 30 October 2018; Accepted: 20 December 2018;
Published: 15 January 2019.
Edited by:
Ashok Kumar, University of Florida, United StatesReviewed by:
Simone Migliore, Casa Sollievo della Sofferenza (IRCCS), ItalyCopyright © 2019 Guarino, Favieri, Boncompagni, Agostini, Cantone and Casagrande. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Angela Guarino, YW5nZWxhLmd1YXJpbm9AdW5pcm9tYTEuaXQ=
Maria Casagrande, bWFyaWEuY2FzYWdyYW5kZUB1bmlyb21hMS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.