- 1Key Laboratory of Pu-erh Tea Science, Ministry of Education, Yunnan Agricultural University, Kunming, China
- 2Tea Research Center of Yunnan, Yunnan Academy of Agricultural Sciences, Kunming, China
- 3College of Food Science and Technology, Yunnan Agricultural University, Kunming, China
- 4Pu’er Institute of Pu-erh Tea, Pu’er, China
- 5Department of Microbiology, Hyogo College of Medicine, Nishinomiya, Japan
- 6China-Japan Joint Center for Bioresource Research and Development, Yunnan Agricultural University, Kunming, China
- 7College of Veterinary Medicine, Yunnan Agricultural University, Kunming, China
- 8State Key Laboratory for Conservation and Utilization of Bio-Resources in Yunnan, Kunming, China
- 9College of Science, Yunnan Agricultural University, Kunming, China
Caffeine has been reported to delay aging and protect aging-associated disorders in Caenorhabditis elegans. However, the effects of low concentration of caffeine and its analogs on lifespan are currently missing. Herein, we report that at much lower concentrations (as low as 10 μg/ml), caffeine extended the lifespan of C. elegans without affecting food intake and reproduction. The effect of caffeine was dependent on IGF-1-like pathway, although the insulin receptor homolog, daf-2 allele, e1371, was dispensable. Four caffeine analogs, 1-methylxanthine, 7-methylxanthine, 1,3-dimethylxanthine, and 1,7-dimethylxanthine, also extended lifespan, whereas 3-methylxanthine and 3,7-dimethylxanthine did not exhibit lifespan-extending activity.
Introduction
Caffeine (1,3,7-trimethylxanthine) has been reported to act through daf-16 and cbp-1 to increase the lifespan of the nematode model organism Caenorhabditis elegans (Lublin et al., 2011). Subsequent studies found that caffeine induced longevity by mimicking dietary restriction and modulating the insulin/IGF-1-like signaling (IIS) pathway (Bridi et al., 2015). Moreover, as the most important molecular target of caffeine in vivo, the adenosine receptor might be involved in the lifespan-prolonging impact of caffeine (Chen et al., 1999).
Habitual human consumption of caffeine-containing foods and beverages is estimated to be in the range of 70–350 mg/person/day or 5–8 mg/kg/day (equivalent to three cups of coffee). In human, higher doses (400–500 mg/day) of caffeine may lead to side effects, including increased anxiety, increased blood pressure, headache, and confusion (Chen et al., 2010). The concentrations used in previous studies which reported the life-span extending effect of caffeine ranged from 1 to 100 mmol/L (18 mmol/L = 1 g/L), which is relatively higher than physiological doses (Lublin et al., 2011; Sutphin et al., 2012; Bridi et al., 2015). Thus, studies examining the effects of low caffeine concentrations on life span are currently missing.
Caffeine, which is widely consumed in beverages, possesses stimulant properties due to the blockage of adenosine receptors (Chen et al., 1999; Fredholm et al., 1999; Temple, 2009). Other methylxanthines interact with adenosine receptors as well, and their affinities vary according to different adenosine receptor subtypes and the chemical structures of the methylxanthines. Because the adenosine receptor might be involved in the lifespan-extending effect induced by caffeine (Chen et al., 1999), it is also possible that these caffeine analogs extend the lifespan of C. elegans.
The IIS pathway is an evolutionarily conserved pathway that regulates metabolism, development, stress resistance, and lifespan (El-Tanani and Green, 1996; Duanmu et al., 1999; Ackerman and Gems, 2012; Matilainen et al., 2013; Heimbucher and Hunter, 2015). In C. elegans, the insulin-like receptor, daf-2, signals through a phosphoinositide 3 (PI3)-kinase (age-1/aap-1) (Gottlieb and Ruvkun, 1994) signaling cascade that activates downstream serine/threonine kinases including pdk-1, akt-1, akt-2, and sgk-1. These kinases in turn function to negatively regulate the transcription factor, daf-16 (FOXO) to modulate the expression of downstream genes. High doses of caffeine (≥5 mmol/L) delayed larval development and increased lifespan, and these impacts were reversed when daf-2 or daf-16 was knocked-down by RNAi, which suggested that the effect of caffeine was dependent on the IIS pathway. In this study, we tested whether low concentrations of caffeine and its analogs could increase the lifespan of C. elegans.
Materials and Methods
Strains
All worm strains including daf-2 (e1370, e1371), age-1 (hx546), daf-16 (mu86), akt-1 (ok525), akt-2 (ok393), and daf-16 (mu86) mutants, as well as wild-type N2 (Bristol) and daf-16::GFP (zls356) strains, were obtained from the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN, United States).
C. elegans Assays
All strains were grown at 20°C on standard Nematode Growth Medium (NGM) seeded with Escherichia coli strain OP50 as the food source (Brenner, 1974).
Caffeine Treatments
Sterilized caffeine stock solution was added into NGM and OP50 to final concentrations as indicated. Plates were prepared the day before use. On the second day, 100 μl of bacterial solution was added to the control plate, and 100 μl of the bacterial solution containing 50-μg/ml caffeine was added to the treatment group.
Lifespan Analysis
Lifespan analysis was performed at 20°C as previously described (Kenyon et al., 1993). The strains grew for at least two generations at 20°C without starvation prior to lifespan analysis. The total numbers of animals in the experiments are listed in Tables 2, 3. In all experiments, lifespan analysis was performed using the time of egg laying as t = 0 for synchronization.
Body Length
During nematode development, body length can reflect the impact of drugs. In the nematode life cycle, drugs could play a role in promoting or delaying nematode spawning and development. In this study, we simultaneously cultured the treatment and control groups and examined their lengths. From the third day, 15 nematodes were randomly selected every 24 h from each group and anesthetized with 0.1% sodium azide delivered by a nematode gun. The nematode bodies were straightened and lengths determined under a microscope at 40× magnification and an eyepiece with a scale.
Brood Size Assays
Each worm was allowed to lay eggs and then transferred to a new NGM plate every 24 h until the egg laying period was complete. Hatched worms were counted after 48 h of incubation at 20°C (Li et al., 2008). C. elegans that crawled off the plate, exploded or bagged were not examined (Hsu et al., 2003). Twelve C. elegans were used for each treatment condition. The experiment was repeated at least three times.
Pharyngeal Pumping Rate Assay
In this study, the experimental and control group nematodes were simultaneously treated. Each group consisted of a certain number of synchronized nematodes. The time to swallow food was measured visually every 40 h. At each time, 15 nematodes were randomly selected from each group to determine the number of times they had swallowed (i.e., the number of times the pharyngeal muscles contracted) in 1 min at different time points.
DAF-16::GFP Nuclear Localization
At 20°C, transgenic worms expressing DAF-16::green fluorescent protein (GFP) were transferred to a plate containing 0 or 50 μg/ml caffeine at the L4 developmental stage. The worms were fixed by treatment with 25 nmol/L NaN3. Still images were captured using a mounted digital camera and a fluorescence channel of an Olympus Ckx41 microscope. The captured images were used to quantify visible GFP lesions at 20°C (Sutphin et al., 2012).
Quantitative RT-PCR Analysis
Fed worms were collected and RNA was prepared using TRIzol Reagent (Invitrogen, San Diego, CA, United States). After quantification, 1 μg of total RNA was used in a reverse-transcription reaction with SuperScript III (Invitrogen) to generate cDNA.
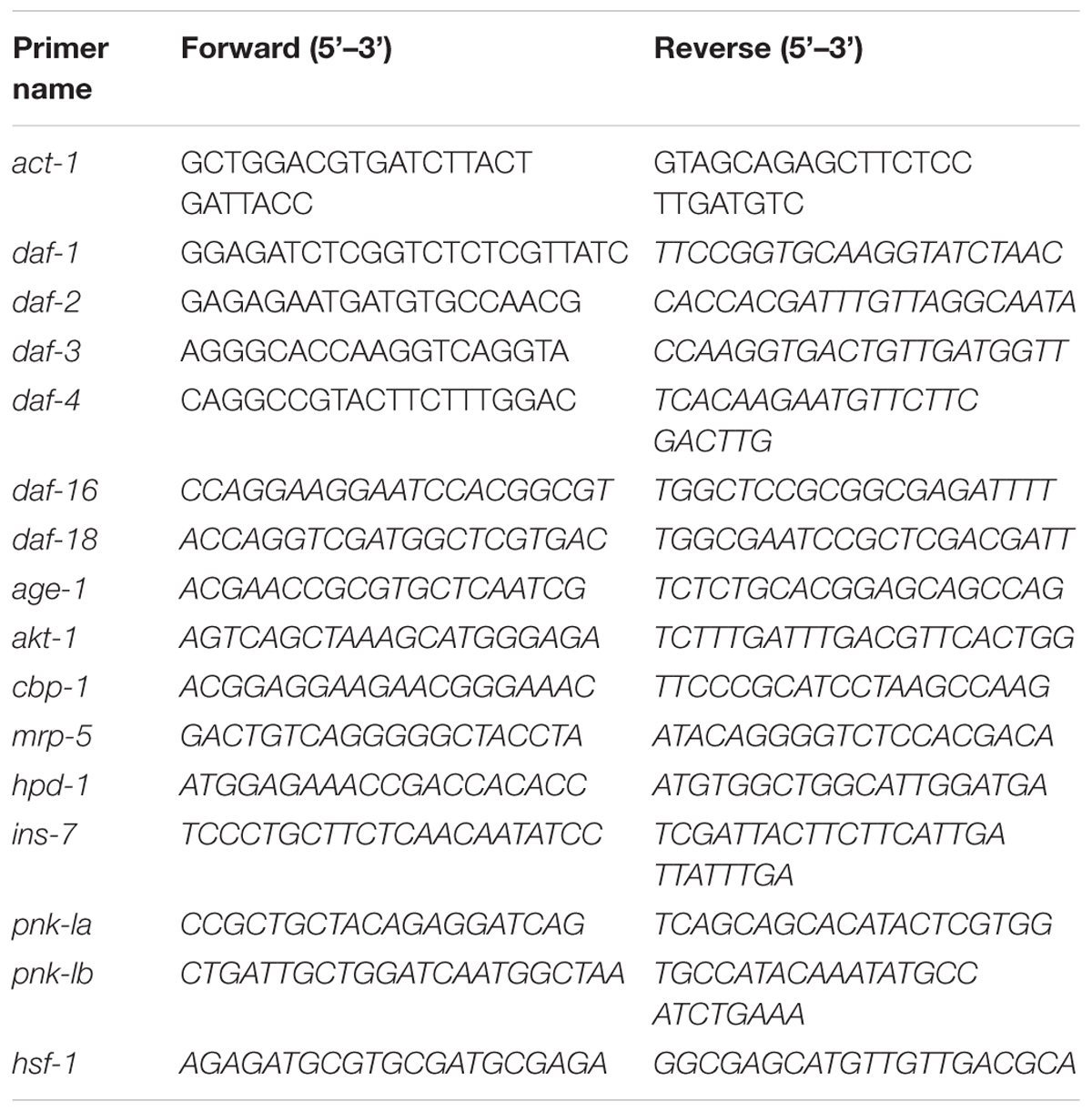
The PCR mixture consisted of 0.3 mM primers, cDNA, ROX, and 16 SYBR green mix (Invitrogen Platinum SYBR green qPCR Supermix UDG). The quantitative RT-PCR (qRT-PCR) was run with an ABI 7900. The level of each mRNA was analyzed using the ΔΔCt method and normalized to that of the corresponding act-1. Primer sequences were listed in Table 1.
Western Blotting Analysis
Extracts from day 1 adult N2 C. elegans grown on control or caffeine containing NGM plates were harvested and washed twice with cold M9 buffer. The animals were then washed with homogeneous buffer (HB buffer, 20 mmol/L Hepes at pH 7.6, 100 mmol/L NaCl, 10 mmol/L KCl, 1.5 mmol/L MgCl2, 0.1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.5 mmol/L ethyleneglycol-bis (2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA), 44 mmol/L sucrose, and 0.5% Triton X-100). The pellet was re-suspended in 3× volumes of HB buffer with 1.5 mmol/L NaF, 2 mmol/L Na2VO4 and protease inhibitor cocktail (Roche, Basel, Switzerland). The worms were then lysed by two freeze-thaw cycles. The lysate was transferred to a pyrolysis instrument at a frequency of 30 times/s for 2 min. The lysate was collected and centrifuged at 14,000 × g for 20 min. The supernatant was collected and protein concentration determined by Bradford assay (Pierce, Thermo Fisher Scientific, Rockford, IL, United States). Briefly, tissue lysates were prepared from C. elegans using radioimmunoprecipitation assay (RIPA) buffer (Solarbio, Beijing, China) according to manufacturer protocols. Cell and tissue extracts were normalized to determine protein concentrations by the bicinchoninic acid method. Proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, United States). After gentle washing, blocking, and incubation with the following primary antibodies, AKT 1/2/3 (SC-8312, Santa Cruz), and p-AKT 1/2/3 (Thr308; sc-16646-R, Santa Cruz), PVDF membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody. Protein bands were detected using a Pro-light Horseradish Peroxidase Chemiluminescence kit (Tiangen Biotech, Beijing, China). Images were acquired with a FluorChemTM E system (ProteinSimple, Santa Clara, CA, United States).
Statistical Analysis
Statistical analysis was performed using GraphPad (version 5.0). The Kaplan–Meier method was applied to calculate survival fractions and log-rank (Mantel-Cox) test was used to compare survival curves. The log-rank (Mantel-Cox) test or one-way analysis of variance (ANOVA) followed by Bonferroni post-test was used to check for significant differences between means for other comparisons. P-values lower than 0.05 were considered significant.
Results
Low Concentration of Caffeine Extends Lifespan of C. elegans Bristol N2 Strain
To determine whether caffeine extended the lifespan of worms, we exposed N2 wild-type C. elegans to caffeine and measured their lifespans. Low concentrations of caffeine increased their lifespans in a dose-dependent manner (Figures 1A,B). The time course of the effect of caffeine was also tested. Caffeine at 100 μg/ml was fed to worms on days 0, 3, 6, 9, and 12, and the results indicated that caffeine was effective at all these time-points (Figures 1C,D), and its effect was time-dependent. To exclude the possibility that caffeine decreased food intake by the worms, the pumping rate was measured. The result showed that caffeine did not reduce food intake (Figure 1E), which suggested that caffeine did not prolong lifespan by calorie restriction. Inconsistent with previous reports (Bridi et al., 2015), low concentrations of caffeine did not affect reproduction and body length of the worms (Figures 1F,G, Table 2, and Supplementary Table S1).
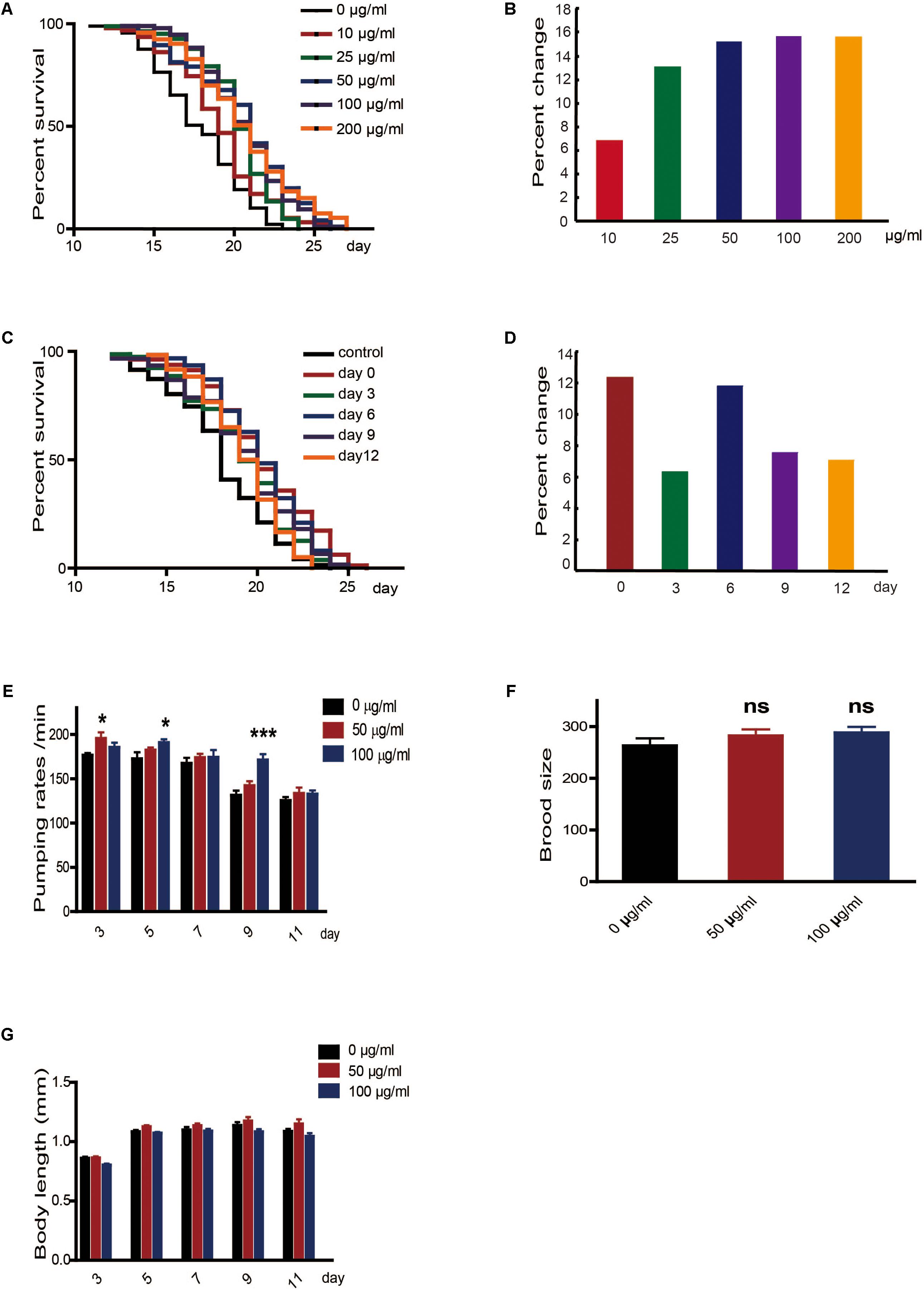
FIGURE 1. Low concentrations of caffeine increased C. elegans’ lifespan from the period of egg hatching. (A,B) Low concentration of caffeine increased C. elegans’ lifespan in a dose-dependent manner. (C,D) Caffeine treatment at various time points extended lifespan. (E) 50 μg/ml and 100 μg/ml caffeine did not reduce pumping rate, and 15 worms were used for each group. (F) 50 μg/ml and 100 μg/ml caffeine did not affect brood size, and 15 worms were used for each group. (G) 50 μg/ml and 100 μg/ml caffeine did not affect body length (head-to-tail). The log-rank (Mantel-Cox) test was used to analyze the differences. Error bars, values are expressed as mean ± SEM; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Effect of Caffeine Was Dependent on IGF-1 Pathway
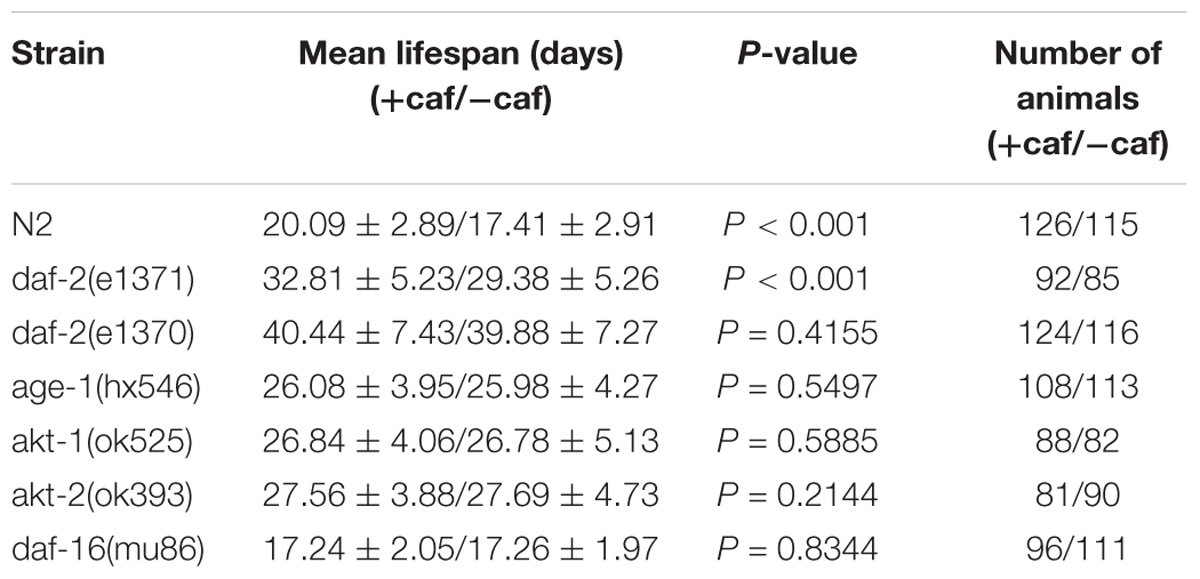
Several genetic pathways influence longevity and may mediate the lifespan-extending effects of caffeine. In worms, the insulin/IGF-1 signaling pathway is a major molecular pathway that influences lifespan (van Heemst, 2010). To investigate whether the insulin/IGF-1 pathway was involved in the lifespan extension effects of caffeine, daf-2, age-1, akt-1, akt-2, and daf-16 loss-of-function mutants were exposed to caffeine and their lifespans evaluated. Similar to wild-type worms, caffeine extended the lifespan of daf-2 (e1371) mutant worms (Figure 2A). However, in age-1 (hx546), akt-1 (ok525), akt-2 (ok393), and daf-16 (mu86) mutant strains, caffeine lost its effects on lifespan extension (Figures 2C–F). These results suggested that low concentrations of caffeine extended the lifespan of worms by modulating the IGF-1 pathway. The daf-2 (e1371) mutation did not affect caffeine’s effect, suggesting that the role of daf-2 (e1371) was not irreplaceable. However, caffeine did not further prolong life span in daf-2 (e1370) mutant strain (Figure 2B), which was consistent with previous study (Bridi et al., 2015). Since e1370 allele was a stronger mutation, these results suggested that caffeine’s effect was dependent on IGF-1 pathway.
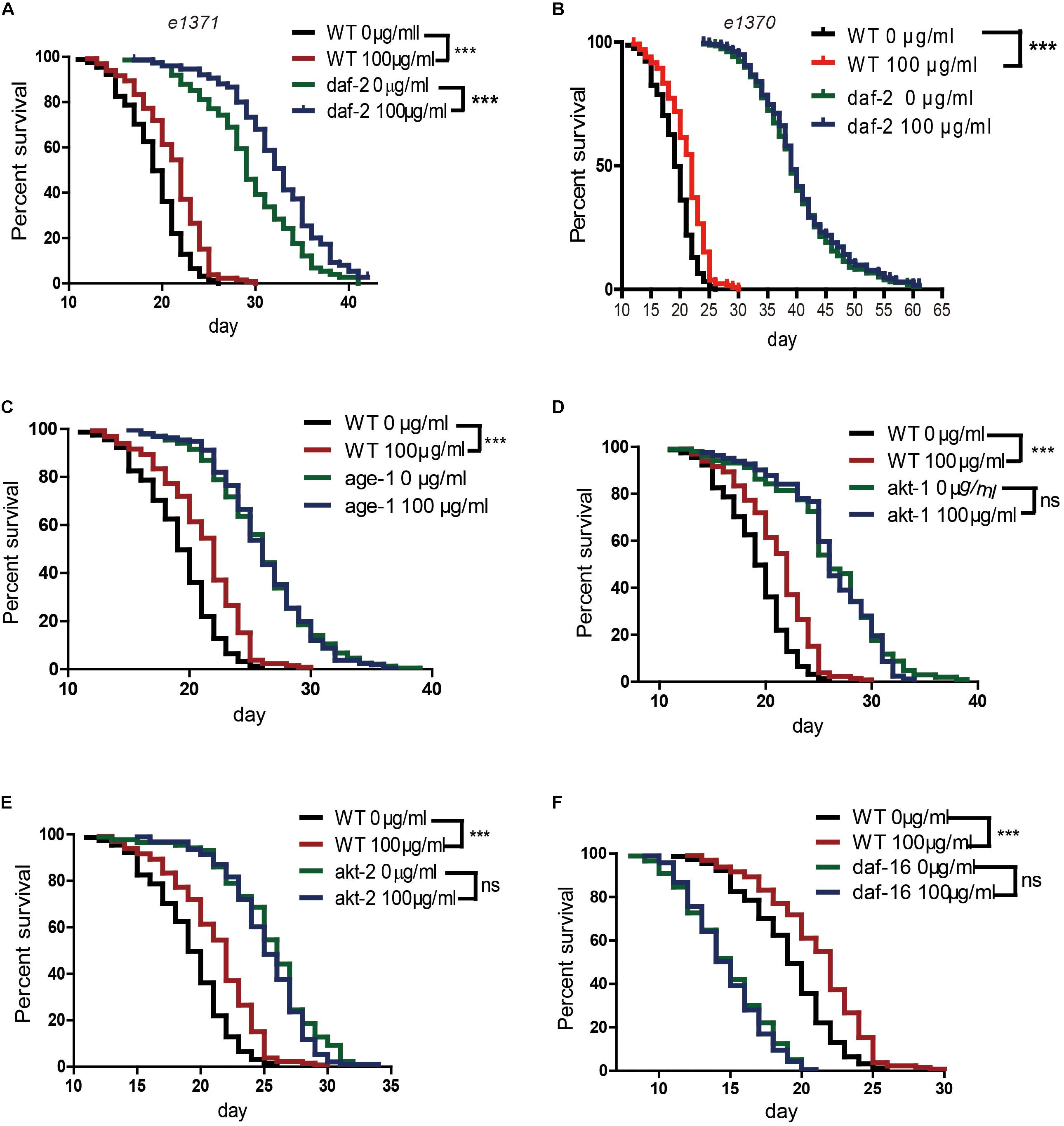
FIGURE 2. Effects of caffeine on lifespan from the time of egg hatching of N2 wild-type (WT) and IGF-1 pathway mutant strains. (A) N2 wild-type and daf-2 (e1371) mutant strain; (B) N2 wild-type and daf-2 (e1370) mutant strain; (C) N2 wild-type and age-1 (hx546) mutant strain; (D) N2 wild-type and akt1 (ok525) mutant strain; (E) N2 wild-type and akt-2 (ok393) mutant strain; (F) N2 wild-type and daf-16 (mu86) mutant strain. The log-rank (Mantel-Cox) test was used to analyze the differences. Error bars, values are expressed as mean ± SEM; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Acute Caffeine Treatment Induced DAF-16 Translocation to the Nucleus
The activity of daf-16 is regulated by subcellular localization. The reduction of IIS causes dephosphorylation and nuclear translocation of DAF-16 to activate transcription of target genes. To determine whether caffeine affects DAF-16 nuclear localization, we exposed GFP-labeled DAF-16 transgenic worm (zls356) to caffeine, and analyzed DAF-16 location. Animals exposed to caffeine showed higher DAF-16::GFP nuclear/cytoplasmic fluorescence ratios than vehicle-treated worms (Figure 3). These results confirmed that the effect of caffeine on lifespan was dependent on DAF-16, which is consistent with the results of mutant strains (Figure 2).
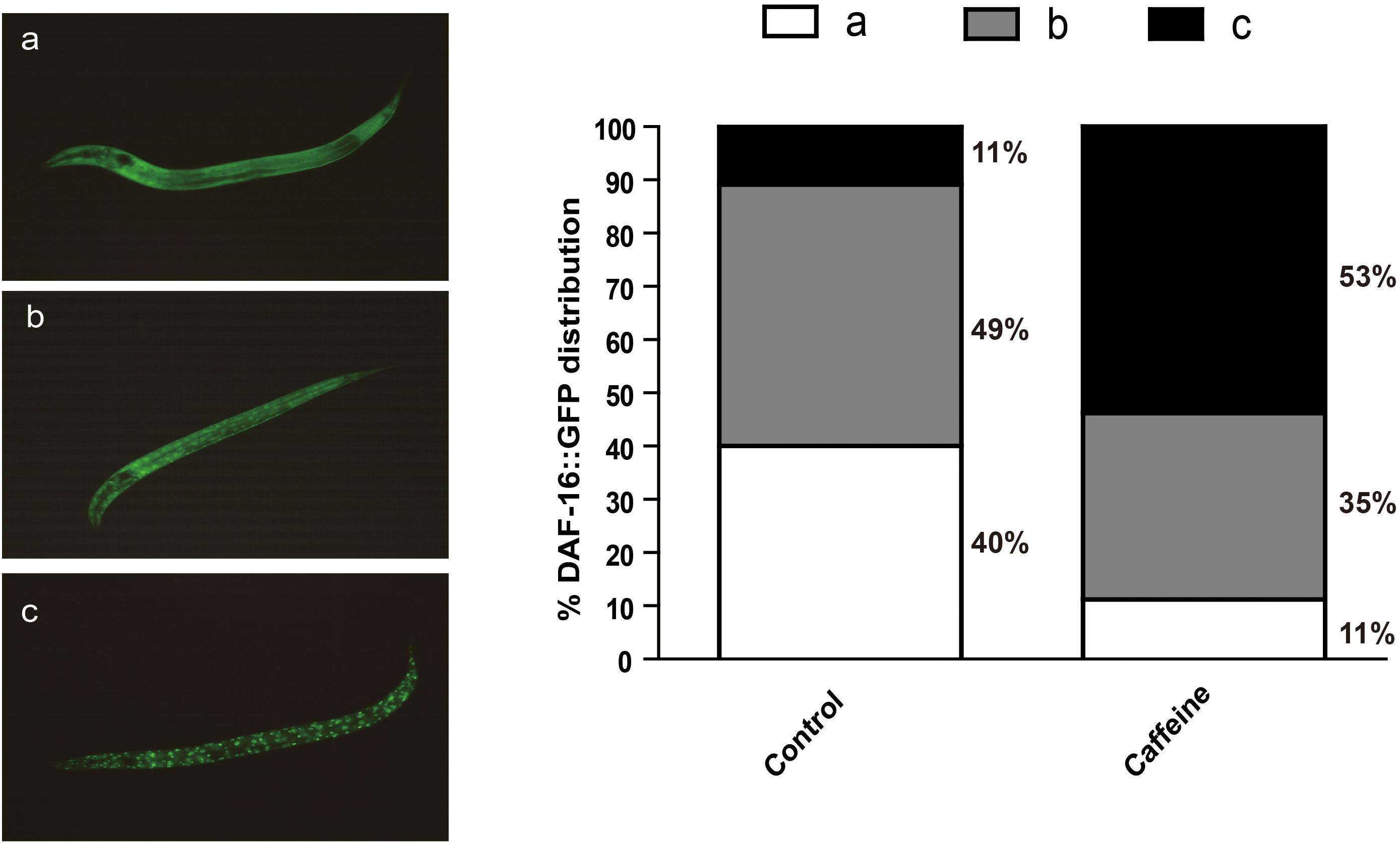
FIGURE 3. Acute caffeine (50 μg/ml) exposure translocates DAF-16 to the nucleus. The GFP localization status was characterized into three types defined as (a–c), as shown in the figure. The numbers of each type were counted and percentages were calculated. N = 176 and 197, respectively.
Caffeine Modulated Lifespan-Related Gene Expression in C. elegans
In order to further verify that caffeine extended lifespans of nematodes, we extracted nematode RNA and protein and examined expression of lifespan-related genes and akt phosphorylation. We found that caffeine significantly promoted daf-3, daf-4, and ins-7 mRNA expression in C. elegans (Figure 4A). Caffeine at 50 μg/mL inhibited AKT 1/2/3 expression and phosphorylation (Figures 4B–E), although the ratio of p-AKT/AKT was not significantly reduced. These results indicated that caffeine acted on AKT pathway and in turn promoted expression of downstream target genes.
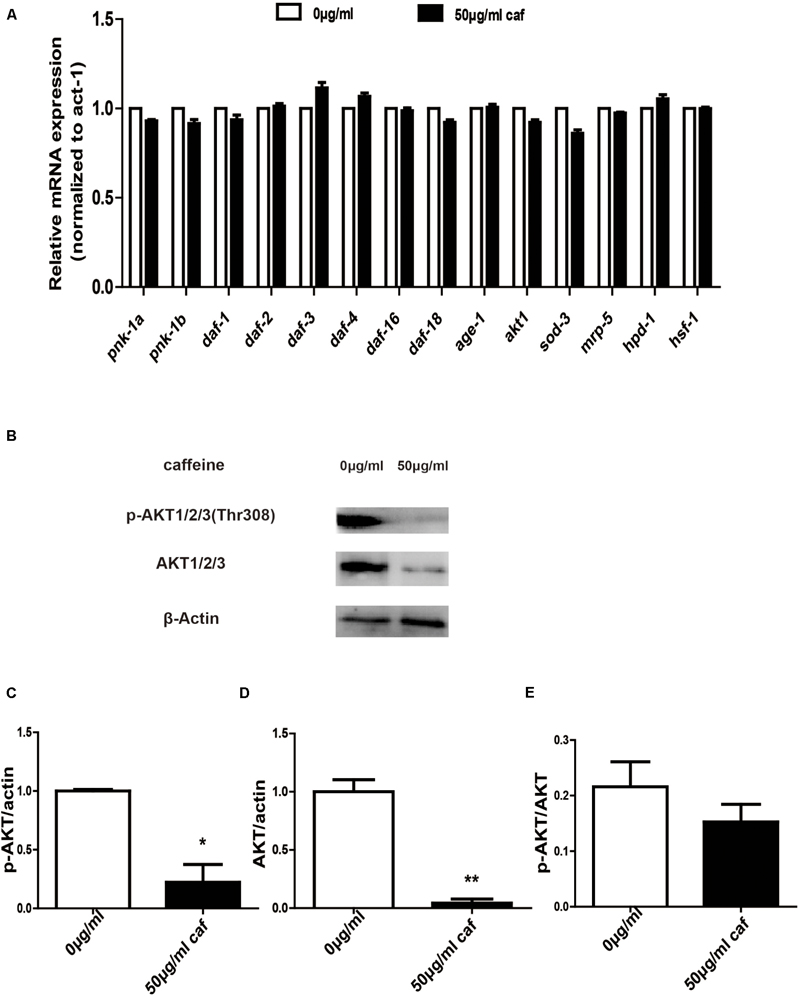
FIGURE 4. Caffeine modulated the AKT signaling pathway. (A) Caffeine increased longevity-related gene expression which acted downstream of AKT. (B) Caffeine prohibited expression and phosphorylation of AKT 1/2/3 (Thr308); (C–E) Quantification of expression and phosphorylation of AKT. One-way analysis of variance (ANOVA). Error bars, values are expressed as mean SEM; ∗p < 0.05; ∗∗p < 0.01.
The Effect of Methyl Substitution at Different Sites of Xanthine on Longevity of C. elegans
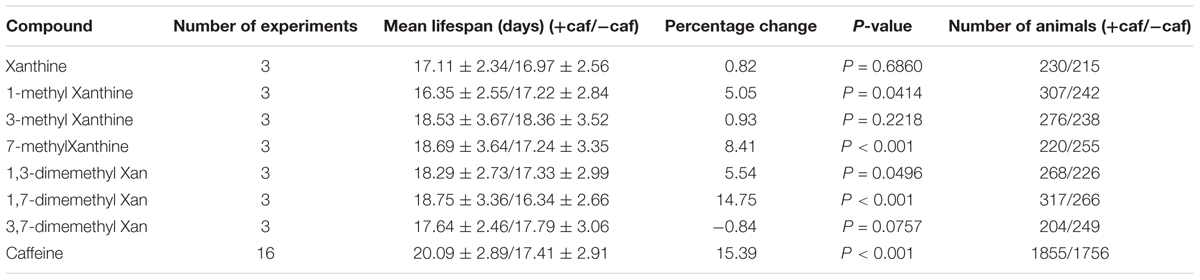
Caffeine (1,3,7-trimethylxanthine) is a xanthine with three methyl groups. Xanthine failed to prolong worm’s lifespan (Figure 5A). To identify the role of these methyl groups in the chemical structure of caffeine, we tested the effect of several caffeine analogs on longevity of C. elegans. Four analogs of caffeine (1-methylxanthine, 7-methylxanthine, 1,3-dimethylxanthine, and 1,7-dimethylxanthine) also extended worm lifespan, whereas 3-methylxanthine and 3,7-dimethylxanthine failed to exhibit lifespan-extending activity. These results indicated that the 1-methyl group was more important for the effect of caffeine, because all analogs containing 1-methyl extended lifespan (Figures 5B,E,F,H and Table 3). The role of 7-methyl was unclear because 7-methylxanthine, but not 3,7-dimethylxanthine, was effective (Figures 5D,G and Table 3). However, 3-methyl appeared unnecessary for the effects of caffeine, because 3-methylxanthine and 3,7-dimethylxanthine did not extend lifespan (Figures 5C,G and Table 3).
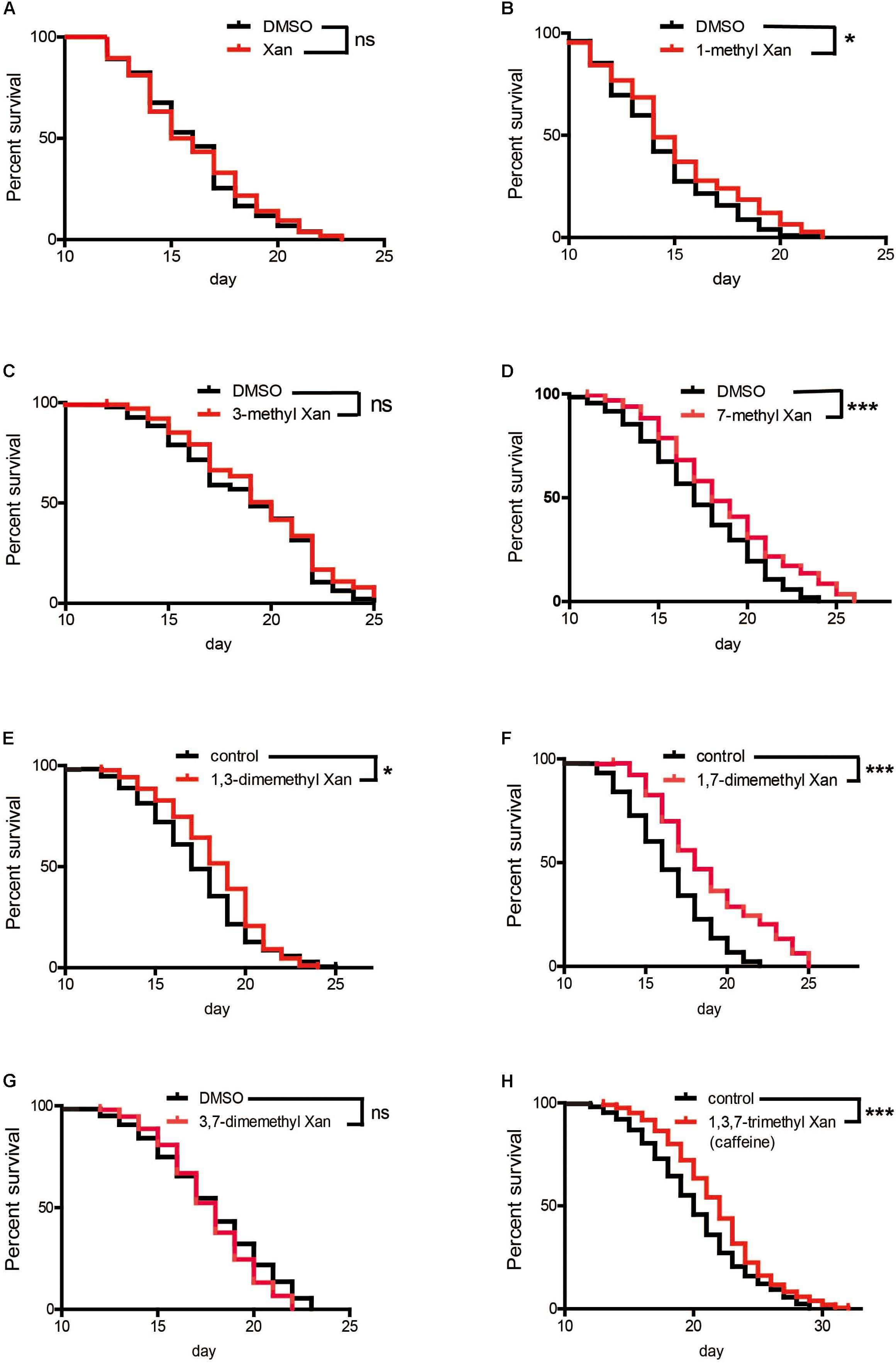
FIGURE 5. Effects of caffeine analogs on C. elegans’ lifespan from the period of egg hatching. (A–H) Four caffeine analogs, 1-methyl xanthine, 7-methyl xanthine, 1,3-dimethyl xanthine, and 1,7-dimethyl xanthine, also extended worm lifespan. Xanthine, 1-methyl xanthine, 3-methyl xanthine, 7-methyl xanthine, and 3,7-dimethyl xanthine were dissolved in DMSO to a final concentration of 0.1%, therefore 0.1% DMSO served as control. The log-rank (Mantel-Cox) test was used to analyze differences. Error bars, values are expressed as mean ± SEM; ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Discussion
In this study, we showed that low concentrations of caffeine extended the lifespan of C. elegans. Previous studies also reported that caffeine increased the lifespan of C. elegans, but the doses used ranged from 400 to 4000 μg/ml (Sutphin et al., 2012), which were all higher than physiological dose. The amounts we used in this study were much lower, and we showed that as low as 10 μg/ml caffeine still possessed the longevity-promoting effect. This low concentration was more close to the physiological dose. However, worms are quite different from humans, so the dose information acquired from these results was limited.
It seemed paradoxical about whether daf-2 was necessary for the effect of caffeine on longevity, considering the inconsistent results of e1371 and e1370 mutant strains. Results of previous daf-2 RNAi and mutant (e1370) experiments indicated that the presence of daf-2 was necessary for high concentration of caffeine’s effect (Bridi et al., 2015). Our results of e1370 suggested that low concentration of caffeine’s effect was dependent on daf-2 (e1370). However, low concentrations of caffeine further increased the lifespan of daf-2 (e1371) mutant worms, which implied daf-2 was not necessary. The difference was reasonable, since different daf-2 alleles can behave very differently (Patel et al., 2008). Therefore, whether daf-2 was crucial for the effect of low concentrations of caffeine on longevity needs further study.
Consistent with other reports, the presence of age-1, which acted downstream of daf-2, was irreplaceable in this study. There is a possibility that caffeine directly targeted age-1 to inhibit the AKT pathway and subsequently modulated downstream gene expression. Age-1 is a homolog of PI3K in mammals, and it has been reported that caffeine directly inhibited PI3K in vitro (half maximal inhibitory concentration-IC50 = 15 μg/ml for p110σ subunit) (Foukas et al., 2002). So it is possible that caffeine acted on age-1 independent of its receptor, daf-2.
Caffeine is one of the most widely consumed psychoactive substances in the world (Fredholm et al., 1999). Caffeine intake is associated with increased life expectancy in human and animal models (Lublin et al., 2011; Freedman et al., 2012). Nevertheless, the disadvantages of caffeine such as sleep deprivation and addiction have restricted its use for promoting human health. We aim to change the structure of caffeine by chemical derivation to obtain novel drugs that retain the effect of caffeine on human health but which are unable to cross the blood–brain barrier. According to results in this study, the 1-methyl moiety of caffeine was irreplaceable, but 3-methyl was unnecessary. These results were of importance because we could draw the conclusion that 1-methyl was more important than 3-methyl in lifespan extension. In future studies, the derivation of caffeine should focus on replacing the 3-methyl group, as it may lead to the discovery of a novel longevity-promoting compound.
Author Contributions
JS, XW, and CF conceived and designed the experiments. XD, YG, QH, ML, and XH performed the experiments. LY and SH conducted the daf-2 (e1371) mutant worms and analyzed the data. XD and YG analyzed the data. JS, XW, and CF contributed reagents, materials, and analysis tools. XD, YG, and CF wrote the manuscript.
Funding
This work was supported by Yunnan Provincial Department of Education Foundation (Project 2017ZZX033) and Yunnan Agricultural University Natural Foundation for Young Scientists (Project 2016ZR16).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR), for all nematode strains used in this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00211/full#supplementary-material
References
Ackerman, D., and Gems, D. (2012). Insulin/IGF-1 and hypoxia signaling act in concert to regulate iron homeostasis in Caenorhabditis elegans. PLoS Genet. 8:e1002498. doi: 10.1371/journal.pgen.1002498
Bridi, J. C., Barros, A. G., Sampaio, L. R., Ferreira, J. C., Antunes Soares, F. A., and Romano-Silva, M. A. (2015). Lifespan extension induced by caffeine in Caenorhabditis elegans is partially dependent on adenosine signaling. Front. Aging Neurosci. 7:220. doi: 10.3389/fnagi.2015.00220
Chen, J. F., Huang, Z., Ma, J., Zhu, J., Moratalla, R., Standaert, D., et al. (1999). A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J. Neurosci. 19, 9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999
Chen, J. F., Yu, L. Q., Shen, H. Y., He, J. C., Wang, X. T., Zheng, R. Y., et al. (2010). What knock-out animals tell us about the effects of caffeine. J. Alzheimers Dis. 20(Suppl. 1), S17–S24. doi: 10.3233/JAD-2010-1403
Duanmu, Z., Scislo, T., and Dunbar, J. C. (1999). Glycemic modulation of insulin/IGF-1 mediated skeletal muscle blood following sympathetic denervation in normal rats. Clin. Exp. Hypertens. 21, 1239–1255. doi: 10.3109/10641969909070847
El-Tanani, M. K., and Green, C. D. (1996). Insulin/IGF-1 modulation of the expression of two estrogen-induced genes in MCF-7 cells. Mol. Cell. Endocrinol. 121, 29–35. doi: 10.1016/0303-7207(96)03844-0
Fredholm, B. B., Bättig, K., Holmén, J., Nehlig, A., and Zvartau, E. E. (1999). Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133.
Freedman, N. D., Park, Y., Abnet, C. C., Hollenbeck, A. R., and Sinha, R. (2012). Association of coffee drinking with total and cause-specific mortality. N. Engl. J. Med. 366, 1891–1904. doi: 10.1056/NEJMoa1112010
Foukas, L. C., Daniele, N., Ktori, C., Anderson, K. E., Jensen, J., and Shepherd, P. R. (2002). Direct effects of caffeine and theophylline on p110 delta and other phosphoinositide 3-kinases. Differential effects on lipid kinase and protein kinase activities. J. Biol. Chem. 277, 37124–37130. doi: 10.1074/jbc.M202101200
Gottlieb, S., and Ruvkun, G. (1994). Daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics 137, 107–120.
Heimbucher, T., and Hunter, T. (2015). The C. elegans ortholog of USP7 controls DAF-16 stability in insulin/IGF-1-like signaling. Worm 4:e1103429. doi: 10.1080/21624054.2015.1103429
Hsu, A. L., Murphy, C. T., Kenyon, C. (2003). Regulation of aging and age-related disease by DAF-16 and heatshock factor. Science 300, 1142–1145. doi: 10.1126/science.1083701
Kenyon, C., Chang, J., Gensch, E., Rudner, A., and Tabtiang, R. (1993). A C. elegans mutant that lives twice as long as wild type. Nature 366, 461–464. doi: 10.1038/366461a0
Li, J., A. Ebata, A., Dong, Y., Rizki, G., Iwata, T., and Lee, S. S., (2008). Caenorhabditis elegans HCF-1 functions in longevity maintenance as a daf-16 regulator. PLoS Biol. 6:e233. doi: 10.1371/journal.pbio.0060233
Lublin, A., Isoda, F., Patel, H., Yen, K., Nguyen, L., Hajje, D., et al. (2011). FDA-approved drugs that protect mammalian neurons from glucose toxicity slow aging dependent on cbp and protect against proteotoxicity. PLoS One 6:e27762. doi: 10.1371/journal.pone.0027762
Matilainen, O., Arpalahti, L., Rantanen, V., Hautaniemi, S., and Holmberg, C. I. (2013). Insulin/IGF-1 signaling regulates proteasome activity through the deubiquitinating enzyme UBH-4. Cell Rep. 3, 1980–1995. doi: 10.1016/j.celrep.2013.05.012
Patel, D. S., Garzagarcia, A., Nanji, M., Mcelwee, J. J., Ackerman, D., Driscoll, P. C., et al. (2008). Clustering of genetically defined allele classes in the Caenorhabditis elegans DAF-2 insulin/IGF-1 receptor. Genetics 178, 931–946. doi: 10.1534/genetics.107.070813
Sutphin, G. L., Bishop, E., Yanos, M. E., Moller, R. M., and Kaeberlein, M. (2012). Caffeine extends life span, improves healthspan, and delays age-associated pathology in Caenorhabditis elegans. Longev. Healthspan 1:9. doi: 10.1186/2046-2395-1-9
Temple, J. L. (2009). Caffeine use in children: what we know, what we have left to learn, and why we should worry. Neurosci. Biobehav. Rev. 33, 793–806. doi: 10.1016/j.neubiorev.2009.01.001
Keywords: caffeine, C. elegans, lifespan, IGF-1 pathway, daf-2
Citation: Du X, Guan Y, Huang Q, Lv M, He X, Yan L, Hayashi S, Fang C, Wang X and Sheng J (2018) Low Concentrations of Caffeine and Its Analogs Extend the Lifespan of Caenorhabditis elegans by Modulating IGF-1-Like Pathway. Front. Aging Neurosci. 10:211. doi: 10.3389/fnagi.2018.00211
Received: 13 December 2017; Accepted: 22 June 2018;
Published: 16 July 2018.
Edited by:
Aurel Popa-Wagner, Department of Neurology, University Hospital Essen, GermanyReviewed by:
Eirini Lionaki, Foundation for Research and Technology Hellas, GreeceMaria Markaki, Foundation for Research and Technology Hellas, Greece
Copyright © 2018 Du, Guan, Huang, Lv, He, Yan, Hayashi, Fang, Wang and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chongye Fang, ZmFuZ2Nob25neWVAeW5hdS5lZHUuY24= Xuanjun Wang, d2FuZ3h1YW5qdW5AZ21haWwuY29t Jun Sheng, c2hlbmdqQHluYXUuZWR1LmNu
†These authors have contributed equally to this work.
 Xiaocui Du1,2,3†
Xiaocui Du1,2,3† Qin Huang
Qin Huang Ming Lv
Ming Lv Xiaofang He
Xiaofang He Xuanjun Wang
Xuanjun Wang Jun Sheng
Jun Sheng