
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 15 May 2018
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 10 - 2018 | https://doi.org/10.3389/fnagi.2018.00136
 Marina V. Shulskaya1*
Marina V. Shulskaya1* Anelya Kh. Alieva1
Anelya Kh. Alieva1 Ivan N. Vlasov1
Ivan N. Vlasov1 Vladimir V. Zyrin1
Vladimir V. Zyrin1 Ekaterina Yu. Fedotova2
Ekaterina Yu. Fedotova2 Natalia Yu. Abramycheva2
Natalia Yu. Abramycheva2 Tatiana S. Usenko3,4
Tatiana S. Usenko3,4 Andrei F. Yakimovsky4
Andrei F. Yakimovsky4 Anton K. Emelyanov3,4
Anton K. Emelyanov3,4 Sofya N. Pchelina3,4
Sofya N. Pchelina3,4 Sergei N. Illarioshkin2
Sergei N. Illarioshkin2 Petr A. Slominsky1
Petr A. Slominsky1 Maria I. Shadrina1
Maria I. Shadrina1Background: Parkinson’s disease (PD) is a complex disease with its monogenic forms accounting for less than 10% of all cases. Whole-exome sequencing (WES) technology has been used successfully to find mutations in large families. However, because of the late onset of the disease, only small families and unrelated patients are usually available. WES conducted in such cases yields in a large number of candidate variants. There are currently a number of imperfect software tools that allow the pathogenicity of variants to be evaluated.
Objectives: We analyzed 48 unrelated patients with an alleged autosomal dominant familial form of PD using WES and developed a strategy for selecting potential pathogenetically significant variants using almost all available bioinformatics resources for the analysis of exonic areas.
Methods: DNA sequencing of 48 patients with excluded frequent mutations was performed using an Illumina HiSeq 2500 platform. The possible pathogenetic significance of identified variants and their involvement in the pathogenesis of PD was assessed using SNP and Variation Suite (SVS), Combined Annotation Dependent Depletion (CADD) and Rare Exome Variant Ensemble Learner (REVEL) software. Functional evaluation was performed using the Pathway Studio database.
Results: A significant reduction in the search range from 7082 to 25 variants in 23 genes associated with PD or neuronal function was achieved. Eight (FXN, MFN2, MYOC, NPC1, PSEN1, RET, SCN3A and SPG7) were the most significant.
Conclusions: The multistep approach developed made it possible to conduct an effective search for potential pathogenetically significant variants, presumably involved in the pathogenesis of PD. The data obtained need to be further verified experimentally.
Parkinson’s disease (PD) is one of the most common human neurodegenerative disorders and belongs to the category of multifactorial diseases. In most cases, the disease is sporadic and is associated with a complex interaction of genetic and environmental factors. However, families with monogenic inheritance of PD have been identified, which has made it possible to reveal a number of genes, i.e., mutations that lead to the development of hereditary forms of the disease (Singleton and Hardy, 2016). Analysis of all available studies of the familial form of PD indicates that the fraction of monogenic forms does not account for more than 10% of all cases, whereas the expected number of cases attributable from a genetic viewpoint is 27%–40% (Hamza and Payami, 2010; Keller et al., 2012). In this regard, it is expedient to continue searching and identifying genes and mutations that lead to the development of monogenic forms of PD.
High throughput next-generation sequencing (NGS) has significantly accelerated the search process. The technology of whole-exome sequencing (WES) has allowed expansion of the spectrum of mutations in genes that were previously associated with PD pathogenesis (Gorostidi et al., 2016). However, the most interesting application of the technology is the search for new genes that are the genetic cause of various diseases. To do this, as a rule, large families with several generations in the pedigree are studied, and potential pathogenically significant variants are analyzed using the method of cosegregation within such a family. Over the past few years, the use of this approach in combination with NGS technologies has made it possible to identify a number of new genes involved in the pathogenesis of PD. These include VPS35, CHCHD2 and DNAJC13, associated with the development of the familial autosomal dominant form of the PD (Vilariño-Güell et al., 2011; Zimprich et al., 2011), DNAJC6, SYNJ1, VPS13C and PTRHD1 involved in the development of autosomal recessive form of PD (Edvardson et al., 2012; Lesage et al., 2016; Jansen et al., 2017; Khodadadi et al., 2017), and RAB39B with X-linked inheritance (Wilson et al., 2014).
However, PD is characterized by a late onset; therefore, large families consisting of several generations are extremely rare and, in most cases, one has to analyze small families and single unrelated patients. WES is known to identify up to 20,000 single-nucleotide variants (SNVs) in one analyzed sample (Bamshad et al., 2011), which leads to the emergence of a huge number of candidate variants and the need to develop a strategy for selecting a narrow range of potentially pathogenic variants. Currently, a number of imperfect tools allow assessment of the pathogenicity of identified variants using various parameters.
We analyzed 48 unrelated patients with an alleged autosomal dominant familial form of PD using WES technology and developed a comprehensive strategy for data analysis and selection of potential pathogenically meaningful variants using available bioinformatics resources.
Studied cohort included 48 unrelated patients with PD. By origin they were all Russians from Moscow and Saint Petersburg. Patients were selected and investigated according to the unified PD rating scale (UPDRS), and Hoehn & Yahr scores (Fahn and Elton, 1987; Goetz et al., 2004) at the Scientific Center of Neurology (Moscow) and Pavlov First Saint Petersburg State Medical University (Saint Petersburg). PD diagnosis was based on the UK PD Bank Criteria (Hughes et al., 1992). The average age at disease onset was 52.6 ± 17.0 (the mean ± standard deviation), sex ratio was 17/31 (M/F). Detailed statistical demographics of the participants is also provided (Supplementary Table S2). Written informed consent was obtained from all participating patients and families according to the Declaration of Helsinki. The study was approved by the Ethics Committee of the Scientific Center of Neurology and Pavlov First Saint Petersburg State Medical University. All patients were pre-typed using the P051-50 (lot C2-0911) and P052-50 (lot C1-0809) probe sets and EK1-FAM reagent kit for the SALSA MLPA MLPR (MRC-Holland b.v., Netherlands) according to the manufacturer’s recommendations (Filatova et al., 2014); all of them did not have any frequent mutations associated with pathogenesis of the PD.
Genomic DNA was obtained from leukocytes using AxyPrepBlood Genomic DNA Miniprep Kit (“Axygen”, USA) as recommended by the manufacturer. The concentration of isolated nucleic acids was measured using Qubit fluorometer (“Invitrogen”, USA) and Quant-iT RNA BR Assay Kit commercial kit as recommended by the manufacturer.
WES was performed using a TruSeq Exome Enrichment kit (Illumina, San Diego, CA, USA) on an Illumina HiSeq 2500 sequencer. Sequence consisted of 201121 exons in 20794 genes with an average coverage of 40×, providing genotype data for 90% of consensus coding exon bases. Resulting potentially pathogenically significant variants in genes listed in Supplementary Table S1 were validated by Sanger sequencing.
Bioinformatic analysis of obtained sequences was carried out using the software package “SNP and Variation Suite” (SVS) v.7.4 (Golden Helix, USA). The minor allele frequency (MAF) was estimated using the “1000 genomes” database (1KG1), the Exome sequencing project (ESP2), Exome Aggregation Consortium Database3 and Genome Aggregation Database (gnomAD4) using European population as a reference.
The possible pathogenicity of all detected new or rare non-synonymous heterozygous variants was assessed using the dbNSFP v2.9 database (Liu et al., 2013), consisting of SIFT, PROVEAN, Polyphen-2 HVAR and HDIV, FATHMM, MutationAssessor, MutationTaster, LRT, MetaSVM and MetaLR software (Dong et al., 2015), integrated into SVS. Additional annotation was carried out in Combined Annotation Dependent Depletion (CADD; Kircher et al., 2014) and Rare Exome Variant Ensemble Learner (REVEL; Ioannidis et al., 2016). A functional evaluation of selected annotated variants was carried out bioinformatically using the Pathway Studio database (Elsevier, New York City, NY, USA). Only connections based on reliable studies, in which statistically significant results were obtained, were taken into account as “reliable” bonds.
Variant call format (VCF) files obtained after the analysis of DNA samples from 48 patients with PD were analyzed using SVS: all heterozygous variants (in accordance with the assumed autosomal dominant nature of inheritance) were selected with a genotyping quality GQ > 99 and a coverage of at least 50 reads (Figure 1), because it is believed that variants with high DP are more reliable than those having a low read depth (Bansal, 2010). The total number of such variants for 48 samples was 201,442.
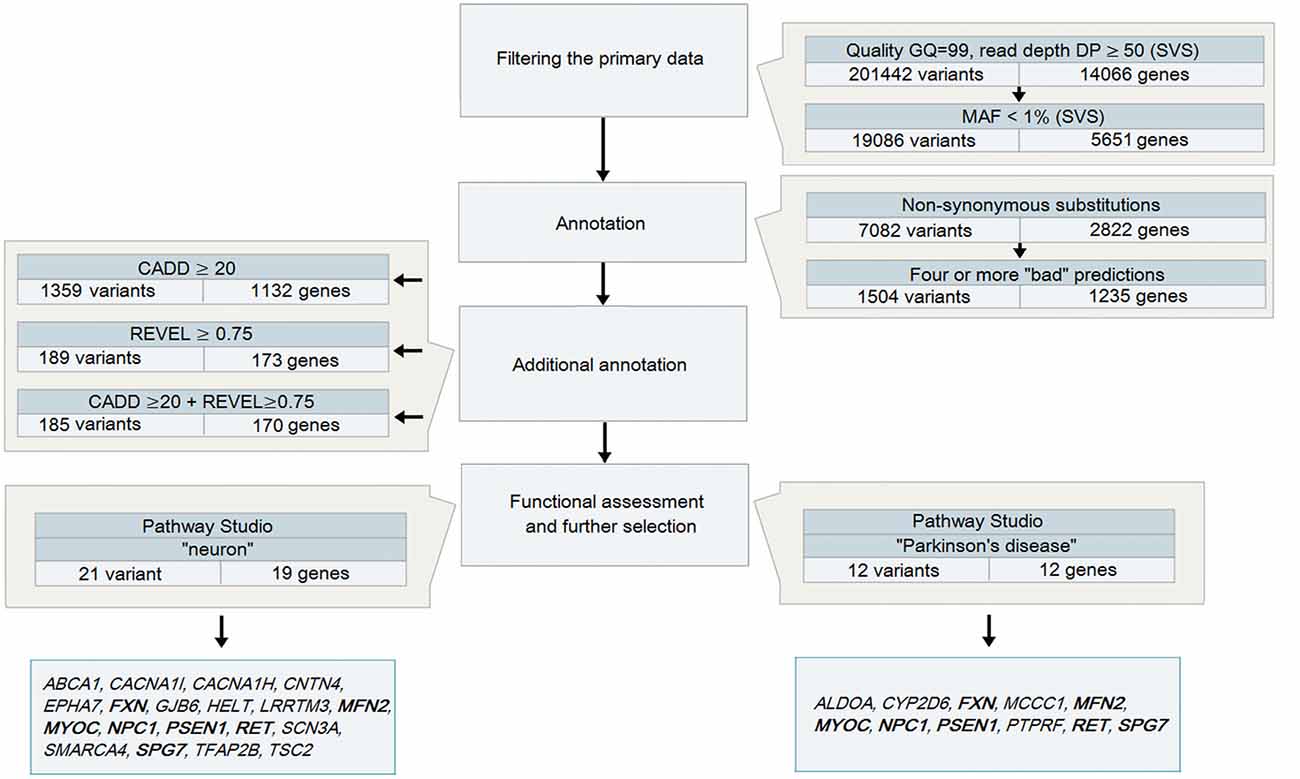
Figure 1. A strategy to analyze data obtained during whole-exome sequencing (WES) for patients with Parkinson’s disease (PD) Note. Genes highlighted in bold are those identified in both strategies.
The analysis of heterozygous variants revealed strong differences in the ratio between two allelic variants that were read during the sequencing. The assumption of the presence of false-positive heterozygotes was verified by the Sanger sequencing of individual exons of LRRK2. As a result, an approach for the elimination of false-positive heterozygotes was developed at the initial stage of the primary data analysis. The approach was based on the use of the following parameters: DP, i.e., approximated read depth (the number of high-quality mapped reads for the current position), and AD1 and AD2, which were the approximated read depth for the first and the second allele, respectively. Selection criteria were chosen empirically, i.e., heterozygous positions with different read depths and AD1/AD2 ratio were verified using Sanger sequencing (Table 1). As a result, the following criteria were chosen for selecting reliable heterozygous positions: “DP ≥ 50 and |AD1 – AD2|/DP <0.3”. Variants that did not meet the specified criteria were discarded as unreliable. The approach was implemented as a Python (version 2.7) script and is represented in Supplementary Materials.
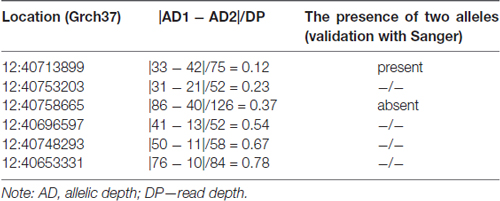
Table 1. Parameters that were used to develop an approach of screening for false-positive heterozygotes.
Further, all new or rare nonsynonymous variants with MAF that did not exceed 1% in 1KG and ESP were selected. As a result, 19,086 heterozygous nonsynonymous variants located in the coding regions of 5651 genes were identified. Two different mutations in one gene were not identified in any of the patients analyzed.
To narrow down the range of candidate variants, we used bioinformatics tools to predict the potential pathogenicity of variants by analyzing both the evolutionary conservation and the conservation in combination with changes in the structural and functional properties of the protein, as well as two tools with combined algorithms. Missense and nonsense variants, which were predicted to be pathogenic by at least four tools, were reserved for further analysis. This step reduced the number of potential variants by almost five times (up to 1504 variants in 1235 genes).
At this stage of the analysis, three pathogenically significant variants in the genes that have been known to be involved in the pathogenesis of PD were identified. All of them are described missense mutations.
To narrow the search even further, additional annotation was conducted using the prediction tools CADD and REVEL, which are neural networks integrating and combining a number of different annotation algorithms that were trained to identify potentially pathogenic variants. We selected the thresholds for the parameters used by the tools based on the objective requirements to the level of rigidity: a CADD score >20 allowed selection of variants belonging to 1% of the most pathogenic variants in the human genome (Kircher et al., 2014), while a REVEL score ≥0.75 provided high specificity for the annotation of rare variants (Ioannidis et al., 2016) and allowed us to narrow the search to 185 variants in 170 genes.
All variants that were identified on the previous stage were analyzed in Pathway Studio v.11.4 (Elsevier, New York City, NY, USA). Genes were selected using the keywords “PD” and “neuron” (Figure 2). As a result, 23 genes were identified (Figure 1, Supplementary Table S1); seven (FXN, MFN2, MYOC, NPC1, PSEN1, RET and SPG7) were associated with both keywords.
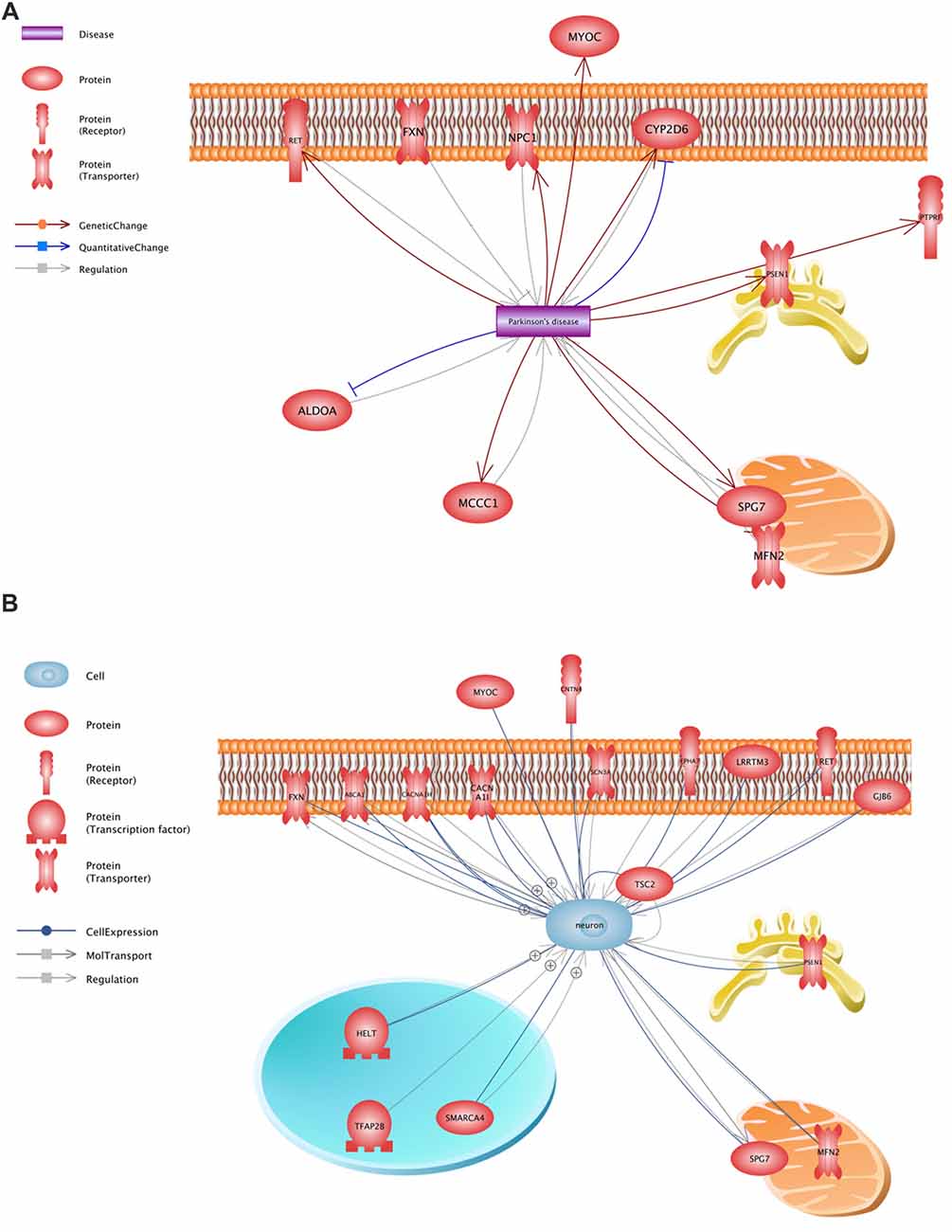
Figure 2. Functional assessment of the proteins encoded by the analyzed genes. (A)—a network constructed using the keyword “PD”, (B)—a network constructed using the keyword “neuron”.
The presence of potential pathogenically significant variants in all 23 genes was analyzed in 48 patients with PD. They were detected in 21 patients, which was 44% of the total number of PD patients analyzed. Four patients (8%) had two genes with potential pathogenically significant variants. It should be noted that practically only one variant in each gene was identified. Only SCN3A had three variants: each variant was found only once in three different patients.
In recent years, increasing numbers of medical genetics studies have been conducted using NGS technology, which is associated with increasing price accessibility and improved tools for in silico data analysis. The proportion of genetic factors involved in the pathogenesis of a disease and successfully identified using NGS ranges from 5.6% to 59% to date (Iglesias et al., 2014; Boillot et al., 2015; Izumi et al., 2015). At the same time, researchers found a large number of variants of unknown significance. It is believed that such variants should in no case be excluded from consideration (Wu and Jiang, 2013). As a result, the problem of correct evaluation of their pathogenicity arises. Currently available bioinformatics tools give conflicting predictions of pathogenicity, which greatly complicates the evaluation procedure, especially in the absence of a large family pedigree. The method of prediction is no less important because the decision on the potential pathogenicity is based on a different number of parameters. Tools with differing or combined algorithms in combination with the functional annotation are currently considered the closest to optimal (Wu and Jiang, 2013; Dong et al., 2015).
However, there is currently no optimal approach for selecting potentially pathogenic variants. Thus, an insufficient number of prediction tools is often used (Reis et al., 2013; Vona et al., 2014; Farlow et al., 2016) or no detailed description of the selection strategy is provided (Acke et al., 2014). The optimal approach for selecting and analyzing homozygous variants was proposed in a recent large-scale study devoted to the search for genetic risk factors in patients with early onset PD (Jansen et al., 2017). However, the proposed approach is not suitable for patients with late onset PD, in which heterozygous variants are analyzed. In this regard, we have developed our own multistep approach for the analysis and screening of candidate variants using almost all available bioinformatics resources for the analysis of exonic areas.
The developed approach for the elimination of false-positive heterozygous variants significantly reduces the number of analyzed data, thereby facilitating the work already at the first stages of the study. The tools used to assess the potential pathogenicity of the identified variants are presented in an amount sufficient to compile reliable data on the pathogenicity of analyzed variants because they are based on various predicting strategies, including combinations. CADD, which has been already proven in a similar study (Jansen et al., 2017) in combination with REVEL, which has more stringent evaluation criteria, allows researchers to obtain objective data in an amount sufficient for the subsequent functional analysis. In turn, the Pathway Studio database used for this purpose makes it possible to conduct an accurate and up-to-date functional evaluation.
The developed approach allows selection of only the most pathogenically significant substitutions, strictly excluding all other weakly pathogenic variants that do not fit at least one selection criteria. Thus, when analyzing the data obtained during the WES, none of the three variants identified in the known genes involved in the pathogenesis of PD reached the thresholds (Table 2). This can be explained by the fact that all three variants are most likely weakly pathogenic and considered only as risk factors for the development of PD.
Thus, the analysis of 48 unrelated patients with an alleged autosomal dominant form of PD allowed us to find a number of genetic factors presumably involved in the pathogenesis of the disease and form a list of 25 potential pathogenically significant variants located in the coding regions of 23 genes. In 22 genes, only one potential pathogenically significant variant was identified, while three variants were detected only in SCN3A. Each of these identified variants was found only once in the patients under study. Our findings confirm the recently advanced hypothesis that new genes associated with the pathogenesis of PD appear to include rare single mutations (Jansen et al., 2017). It should be noted that only 7 of 23 genes (FXN, MFN2, MYOC, NPC1, PSEN1, RET and SPG7) were selected using both keywords. Data of varying significance on the involvement of these genes in the development of the disease are available at present time.
The most convincing evidence of involvement is available for NPC1 and PSEN1 genes. For NPC1, a hypothesis on the contribution of heterozygous mutations to the risk of PD development was expressed earlier. The hypothesis was built primarily on the basis of information on the comorbidity of Niemann–Pick disease caused by mutations in NPC1 and parkinsonism (Kluenemann et al., 2013). The situation is the same for the variant p.L173F found in PSEN1, it was described as pathogenic in families with Alzheimer’s disease and parkinsonism (Kasuga et al., 2009; Jin et al., 2012). Because the whole symptom complex does not always produce an unambiguous diagnosis and distinguish the underlying disease from those associated with it, the variants we identified can be regarded at least as risk factors for PD development.
Three potential pathogenically significant variants were identified in SCN3A, therefore this gene is of importance to PD researchers. The protein encoded by SCN3A is a transmembrane sodium channel, which plays an important role in the neuronal function. Disruption of the functioning of such sodium channels can cause the emergence of a number of neurological diseases, including essential tremor (Bergareche et al., 2015), epilepsy (George, 2004; Helbig et al., 2008; Reid et al., 2009; Eijkelkamp et al., 2012; Oliva et al., 2012), multiple sclerosis (Waxman, 2006), painful neuropathy (Faber et al., 2012; Huang et al., 2014), myotonia (Jurkat-Rott et al., 2010; Stunnenberg et al., 2010) and paroxysmal myoplegia (Cannon, 2010). It is interesting to note that all three potentially pathogenetically significant variants have been identified by us for the first time and are located in the hydrophobic fifth segment of the SCHN3A protein that is directly responsible for ion transport. All these observations, although only indirectly thus far, support the possible involvement of SCN3A in the process of neurodegeneration.
Thus, the approach we developed to analyze the data obtained in the course of NGS for unrelated patients with an alleged autosomal dominant form of PD allowed effective searching for potential pathogenically meaningful variants, significantly reducing the search range from 7082 to 25 variants. A cohort of a large size would be optimal for the studies conducted with patients without a pronounced family history and without clear large pedigrees. Then one could speak of a clear association between a gene with multiple mutations in it and the development of the disease. In our limited cohort of patients, we observed three patients with different mutations in one gene, which may indicate its involvement in the pathogenesis of the disease. At the same time, these variants have not been identified in the EXAC, 1KG and ESP databases, which also may indicate their possible pathogenetic significance, however this significance has to be proved. The accumulation of the data from different groups of researchers will make it possible to clarify the role of individual genes in the development of the disease. The data obtained can be also verified further using experimental approaches, for example, by studying the mechanism of the influence of a nonsynonymous substitution, with such molecular genetic approaches as RNA interference or modeling using both animal models and cell culture. We realize that the results of the study can handle some false positives since there is the lack of ethnically matching sequenced Russian (or at least Slavonic) control population that can be used as the reference dataset for such type of analysis. Besides, the comparison of different datasets is also complicated by a well-known low concordance of differing sequencing platforms and pipelines used for variant calling (O’Rawe et al., 2013). Nevertheless, our results first of all provide some valuable data that will be further accumulated and estimated by the scientific community worldwide.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
MS performed next-generation sequencing data analysis, sanger sequencing studies and wrote the manuscript. EF, NA and AY contributed to the collection of the clinical data and its analysis. AA and TU carried out the genotyping of patients with PD. IV and AE performed the primary bioinformatics analysis of the next-generation sequencing data. MS and PS designed and coordinated the study and wrote the manuscript. SI and SP were involved in revising the manuscript critically for important intellectual content. VZ and AE performed the primary bioinformatics analysis of the next-generation sequencing data, IV created the final appropriate version of the Python script. All authors read and approved the final manuscript.
This work was supported by the Russian Science Foundation (grant no. 14-15-01047) and Federal Agency for Scientific Organizations (FASO) Russia (grant no. 01201355485).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2018.00136/full#supplementary-material
Acke, F. R., Malfait, F., Vanakker, O. M., Steyaert, W., De Leeneer, K., Mortier, G., et al. (2014). Novel pathogenic COL11A1/COL11A2 variants in Stickler syndrome detected by targeted NGS and exome sequencing. Mol. Genet. Metab. 113, 230–235. doi: 10.1016/j.ymgme.2014.09.001
Bamshad, M. J., Ng, S. B., Bigham, A. W., Tabor, H. K., Emond, M. J., Nickerson, D. A., et al. (2011). Exome sequencing as a tool for Mendelian disease gene discovery. Nat. Rev. Genet. 12, 745–755. doi: 10.1038/nrg3031
Bansal, V. (2010). A statistical method for the detection of variants from next-generation resequencing of DNA pools. Bioinformatics 26, i318–i324. doi: 10.1093/bioinformatics/btq214
Benitez, B. A., Davis, A. A., Jin, S. C., Ibanez, L., Ortega-Cubero, S., Pastor, P., et al. (2016). Resequencing analysis of five Mendelian genes and the top genes from genome-wide association studies in Parkinson’s disease. Mol. Neurodegener. 11:29. doi: 10.1186/s13024-016-0097-0
Bergareche, A., Bednarz, M., Sánchez, E., Krebs, C. E., Ruiz-Martinez, J., De La Riva, P., et al. (2015). SCN4A pore mutation pathogenetically contributes to autosomal dominant essential tremor and may increase susceptibility to epilepsy. Hum. Mol. Genet. 24, 7111–7120. doi: 10.1093/hmg/ddv410
Boillot, M., Morin-Brureau, M., Picard, F., Weckhuysen, S., Lambrecq, V., Minetti, C., et al. (2015). Novel GABRG2 mutations cause familial febrile seizures. Neurol. Genet. 1:e35. doi: 10.1212/NXG.0000000000000035
Cannon, S. C. (2010). Voltage-sensor mutations in channelopathies of skeletal muscle. J. Physiol. 588, 1887–1895. doi: 10.1113/jphysiol.2010.186874
Dong, C., Wei, P., Jian, X., Gibbs, R., Boerwinkle, E., Wang, K., et al. (2015). Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum. Mol. Genet. 24, 2125–2137. doi: 10.1093/hmg/ddu733
Edvardson, S., Cinnamon, Y., Ta-Shma, A., Shaag, A., Yim, Y. I., Zenvirt, S., et al. (2012). A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One 7:e36458. doi: 10.1371/journal.pone.0036458
Eijkelkamp, N., Linley, J. E., Baker, M. D., Minett, M. S., Cregg, R., Werdehausen, R., et al. (2012). Neurological perspectives on voltage-gated sodium channels. Brain 135, 2585–2612. doi: 10.1093/brain/aws225
Faber, C. G., Lauria, G., Merkies, I. S., Cheng, X., Han, C., Ahn, H. S., et al. (2012). Gain-of-function Nav1.8 mutations in painful neuropathy. Proc. Natl. Acad. Sci. U S A 109, 19444–19449. doi: 10.1073/pnas.1216080109
Fahn, B. S., Elton, R. L., and Committee MotUD. (1987). “Unified Parkinson’s disease rating scale,” in Recent Developments in Parkinson’s Disease, eds S. Fahn, C. D. Marsden, D. Calne and M. Goldstein (Florham Park, NY: Macmillan Health Care Information), 153–164.
Farlow, J. L., Robak, L. A., Hetrick, K., Bowling, K., Boerwinkle, E., Coban-Akdemir, Z. H., et al. (2016). Whole-exome sequencing in familial parkinson disease. JAMA Neurol. 73, 68–75. doi: 10.1001/jamaneurol.2015.3266
Filatova, E. V., Alieva, A. Kh., Shadrina, M. I., Shulskaya, M. V., Fedotova, E. Y. U., Illarioshkin, S. N., et al. (2014). Analysis of mutations in patients with suspected autosomal dominant form of the Parkinson disease. Mol. Genet. Microbiol. Virol. 29, 3–4. doi: 10.3103/S0891416814010029
George, A. L. Jr. (2004). Molecular basis of inherited epilepsy. Arch. Neurol. 61, 473–478. doi: 10.1001/archneur.61.4.473
Goetz, C. G., Poewe, W., Rascol, O., Sampaio, C., Stebbins, G. T., Counsell, C., et al. (2004). Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov. Disord. 19, 1020–1028. doi: 10.1002/mds.20213
Gorostidi, A., Marti-Masso, J. F., Bergareche, A., Rodriguez-Oroz, M. C., Lopez de Munain, A., and Ruiz-Martinez, J. (2016). Genetic mutation analysis of Parkinson’s disease patients using multigene next-generation sequencing Panels. Mol. Diagn. Ther. 20, 481–491. doi: 10.1007/s40291-016-0216-1
Hamza, T. H., and Payami, H. (2010). The heritability of risk and age at onset of Parkinson’s disease after accounting for known genetic risk factors. J. Hum. Genet. 55, 241–243. doi: 10.1038/jhg.2010.13
Helbig, I., Scheffer, I. E., Mulley, J. C., and Berkovic, S. F. (2008). Navigating the channels and beyond: unravelling the genetics of the epilepsies. Lancet Neurol. 7, 231–245. doi: 10.1016/s1474-4422(08)70039-5
Huang, J., Han, C., Estacion, M., Vasylyev, D., Hoeijmakers, J. G., Gerrits, M. M., et al. (2014). Gain-of-function mutations in sodium channel Nav1.9 in painful neuropathy. Brain 137, 1627–1642. doi: 10.1093/brain/awu079
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Iglesias, A., Anyane-Yeboa, K., Wynn, J., Wilson, A., Cho Truitt, M., Guzman, E., et al. (2014). The usefulness of whole-exome sequencing in routine clinical practice. Genet. Med. 16, 922–931. doi: 10.1038/gim.2014.58
Ioannidis, N. M., Rothstein, J. H., Pejaver, V., Middha, S., McDonnell, S. K., Baheti, S., et al. (2016). REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am. J. Hum. Genet. 99, 877–885. doi: 10.1016/j.ajhg.2016.08.016
Izumi, R., Niihori, T., Takahashi, T., Suzuki, N., Tateyama, M., Watanabe, C., et al. (2015). Genetic profile for suspected dysferlinopathy identified by targeted next-generation sequencing. Neurol. Genet. 1:e36. doi: 10.1212/NXG.0000000000000036
Jansen, I. E., Ye, H., Heetveld, S., Lechler, M. C., Michels, H., Seinstra, R. I., et al. (2017). Discovery and functional prioritization of Parkinson’s disease candidate genes from large-scale whole exome sequencing. Genome Biol. 18:22. doi: 10.1186/s13059-017-1147-9
Jin, S. C., Pastor, P., Cooper, B., Cervantes, S., Benitez, B. A., Razquin, C., et al. (2012). Pooled-DNA sequencing identifies novel causative variants in PSEN1, GRN and MAPT in a clinical early-onset and familial Alzheimer’s disease Ibero-American cohort. Alzheimers Res. Ther. 4:34. doi: 10.1186/alzrt137
Jurkat-Rott, K., Holzherr, B., Fauler, M., and Lehmann-Horn, F. (2010). Sodium channelopathies of skeletal muscle result from gain or loss of function. Cell Rep. 460, 239–248. doi: 10.1007/s00424-010-0814-4
Kasuga, K., Ohno, T., Ishihara, T., Miyashita, A., Kuwano, R., Onodera, O., et al. (2009). Depression and psychiatric symptoms preceding onset of dementia in a family with early-onset Alzheimer disease with a novel PSEN1 mutation. J. Neurol. 256, 1351–1353. doi: 10.1007/s00415-009-5096-4
Keller, M. F., Saad, M., Bras, J., Bettella, F., Nicolaou, N., Simón-Sánchez, J., et al. (2012). Using genome-wide complex trait analysis to quantify ‘missing heritability’ in Parkinson’s disease. Hum. Mol. Genet. 21, 4996–5009. doi: 10.1093/hmg/dds335
Khodadadi, H., Azcona, L. J., Aghamollaii, V., Omrani, M. D., Garshasbi, M., Taghavi, S., et al. (2017). PTRHD1 (C2orf79) mutations lead to autosomal-recessive intellectual disability and parkinsonism. Mov. Disord. 32, 287–291. doi: 10.1002/mds.26824
Kircher, M., Witten, D. M., Jain, P., O’Roak, B. J., Cooper, G. M., and Shendure, J. (2014). A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46, 310–315. doi: 10.1038/ng.2892
Kluenemann, H. H., Nutt, J. G., Davis, M. Y., and Bird, T. D. (2013). Parkinsonism syndrome in heterozygotes for Niemann-Pick C1. J. Neurol. Sci. 335, 219–220. doi: 10.1016/j.jns.2013.08.033
Lesage, S., Drouet, V., Majounie, E., Deramecourt, V., Jacoupy, M., Nicolas, A., et al. (2016). Loss of VPS13C function in autosomal-recessive parkinsonism causes mitochondrial dysfunction and increases PINK1/parkin-dependent mitophagy. Am. J. Hum. Genet. 98, 500–513. doi: 10.1016/j.ajhg.2016.01.014
Li, K., Tang, B. S., Liu, Z. H., Kang, J. F., Zhang, Y., Shen, L., et al. (2015). LRRK2 A419V variant is a risk factor for Parkinson’s disease in Asian population. Neurobiol. Aging 36, 2908.e11–2908.e15. doi: 10.1016/j.neurobiolaging.2015.07.012
Liu, X., Jian, X., and Boerwinkle, E. (2013). dbNSFP v2.0: a database of human non-synonymous SNVs and their functional predictions and annotations. Hum. Mutat. 34, E2393–E2402. doi: 10.1002/humu.22376
Oliva, M., Berkovic, S. F., and Petrou, S. (2012). Sodium channels and the neurobiology of epilepsy. Epilepsia 53, 1849–1859. doi: 10.1111/j.1528-1167.2012.03631.x
O’Rawe, J., Jiang, T., Sun, G., Wu, Y., Wang, W., Hu, J., et al. (2013). Low concordance of multiple variant-calling pipelines: practical implications for exome and genome sequencing. Genome Med. 5:28. doi: 10.1186/gm432
Reid, C. A., Berkovic, S. F., and Petrou, S. (2009). Mechanisms of human inherited epilepsies. Prog. Neurobiol. 87, 41–57. doi: 10.1016/j.pneurobio.2008.09.016
Reis, L. M., Tyler, R. C., Muheisen, S., Raggio, V., Salviati, L., Han, D. P., et al. (2013). Whole exome sequencing in dominant cataract identifies a new causative factor, CRYBA2, and a variety of novel alleles in known genes. Hum. Genet. 132, 761–770. doi: 10.1007/s00439-013-1289-0
Ross, O. A., Soto-Ortolaza, A. I., Heckman, M. G., Aasly, J. O., Abahuni, N., Annesi, G., et al. (2011). Association of LRRK2 exonic variants with susceptibility to Parkinson’s disease: a case-control study. Lancet Neurol. 10, 898–908. doi: 10.1016/S1474-4422(11)70175-2
Singleton, A., and Hardy, J. (2016). The evolution of genetics: Alzheimer’s and Parkinson’s diseases. Neuron 90, 1154–1163. doi: 10.1016/j.neuron.2016.05.040
Stunnenberg, B. C., Ginjaar, H. B., Trip, J., Faber, C. G., van Engelen, B. G., and Drost, G. (2010). Isolated eyelid closure myotonia in two families with sodium channel myotonia. Neurogenetics 11, 257–260. doi: 10.1007/s10048-009-0225-x
Vilariño-Güell, C., Wider, C., Ross, O. A., Dachsel, J. C., Kachergus, J. M., Lincoln, S. J., et al. (2011). VPS35 mutations in Parkinson disease. Am. J. Hum. Genet. 89, 162–167. doi: 10.1016/j.ajhg.2011.06.001
Vona, B., Müller, T., Nanda, I., Neuner, C., Hofrichter, M. A., Schroder, J., et al. (2014). Targeted next-generation sequencing of deafness genes in hearing-impaired individuals uncovers informative mutations. Genet. Med. 16, 945–953. doi: 10.1038/gim.2014.65
Waxman, S. G. (2006). Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat. Rev. Neurosci. 7, 932–941. doi: 10.1038/nrn2023
Wilson, G. R., Sim, J. C., McLean, C., Giannandrea, M., Galea, C. A., Riseley, J. R., et al. (2014). Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with α-synuclein pathology. Am. J. Hum. Genet. 95, 729–735. doi: 10.1016/j.ajhg.2014.10.015
Wu, J., and Jiang, R. (2013). Prediction of deleterious nonsynonymous single-nucleotide polymorphism for human diseases. ScientificWorldJournal 2013:675851. doi: 10.1155/2013/675851
Keywords: Parkinson’s disease, whole-exome sequencing, NGS, variant, mutation
Citation: Shulskaya MV, Alieva AKh, Vlasov IN, Zyrin VV, Fedotova EYu, Abramycheva NYu, Usenko TS, Yakimovsky AF, Emelyanov AK, Pchelina SN, Illarioshkin SN, Slominsky PA and Shadrina MI (2018) Whole-Exome Sequencing in Searching for New Variants Associated With the Development of Parkinson’s Disease. Front. Aging Neurosci. 10:136. doi: 10.3389/fnagi.2018.00136
Received: 13 February 2018; Accepted: 24 April 2018;
Published: 15 May 2018.
Edited by:
Catarina Oliveira, University of Coimbra, PortugalReviewed by:
Jorge L. Del-Aguila, Washington University in St. Louis, United StatesCopyright © 2018 Shulskaya, Alieva, Vlasov, Zyrin, Fedotova, Abramycheva, Usenko, Yakimovsky, Emelyanov, Pchelina, Illarioshkin, Slominsky and Shadrina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marina V. Shulskaya, bS5zaHVsc2theWFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.