- 1Frank H. Netter MD School of Medicine, Quinnipiac University, North Haven, CT, United States
- 2Department of Psychiatry, Yale University School of Medicine, New Haven, CT, United States
- 3Department of Neuroscience, Yale University School of Medicine, New Haven, CT, United States
- 4Interdepartmental Neuroscience Program, Yale University School of Medicine, New Haven, CT, United States
Noradrenergic dysfunction contributes to cognitive impairment in Alzheimer's Disease (AD) and Parkinson's Disease (PD). Conventional therapeutic strategies seek to enhance cholinergic and dopaminergic neurotransmission in AD and PD, respectively, and few studies have examined noradrenergic dysfunction as a target for medication development. We review the literature of noradrenergic dysfunction in AD and PD with a focus on human imaging studies that implicate the locus coeruleus (LC) circuit. The LC sends noradrenergic projections diffusely throughout the cerebral cortex and plays a critical role in attention, learning, working memory, and cognitive control. The LC undergoes considerable degeneration in both AD and PD. Advances in magnetic resonance imaging have facilitated greater understanding of how structural and functional alteration of the LC may contribute to cognitive decline in AD and PD. We discuss the potential roles of the noradrenergic system in the pathogenesis of AD and PD with an emphasis on postmortem anatomical studies, structural MRI studies, and functional MRI studies, where we highlight changes in LC connectivity with the default mode network (DMN). LC degeneration may accompany deficient capacity in suppressing DMN activity and increasing saliency and task control network activities to meet behavioral challenges. We finish by proposing potential and new directions of research to address noradrenergic dysfunction in AD and PD.
Anatomical and Neurobiological Considerations
Alzheimer's Disease
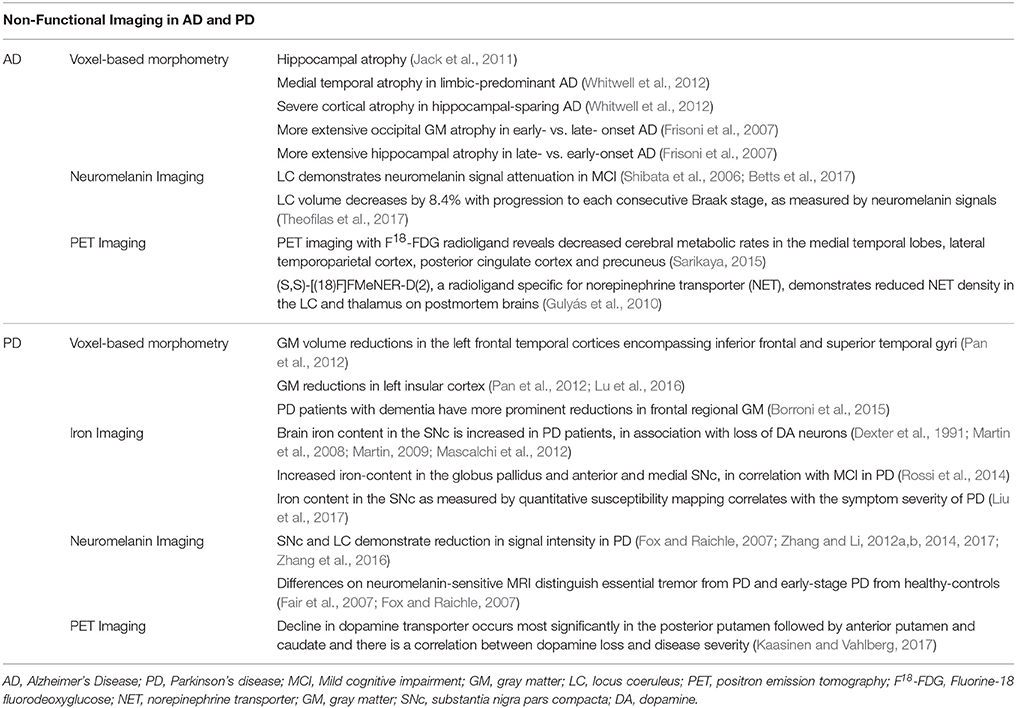
Alzheimer's disease (AD) is a well-known cause of dementia that is associated with the accumulation of intraneuronal neurofibrillary tangles (NFTs; Braak et al., 1994) and extraneuronal neuropil threads (Perry et al., 1991). NFTs are composed of abnormally phosphorylated tau, a protein supporting cytoskeletal structure in neurons (Delacourte and Defossez, 1986; Bancher et al., 1989; Braak et al., 1994). Neuropil threads (NTs) are composed of tau and ubiquitin and located typically at distal dendrites (Perry et al., 1991). The progression of AD, first detailed by Braak and Braak in 1995, was described in six stages that correlate with accumulation of NFTs and NTs. Stage I and II are regarded as the entorhinal stage and associated with accumulation of NFTs and NTs in the transentorhinal regions (Jellinger et al., 1991; Bancher et al., 1993; Braak et al., 1994). Stage III and IV are known as the limbic stage and progress to involve considerable portions of the entorhinal cortex. The limbic stage is also associated with minor hippocampal change. Clinically, stage III/IV are associated with impaired cognitive functioning and minor personality changes (Jellinger et al., 1991; Bancher et al., 1993; Braak et al., 1994). The last two stages, stage V and VI, represent the isocortical stages of AD. The feature that distinguishes the isocortical stages is significant involvement of the cerebral cortex and hippocampus. Stage V and VI manifest in clinically diagnosable AD.
It is important to note that AD does not follow a uniform pattern of neuroanatomical changes. A more recent study of AD pathology revealed three NFT distribution patterns described as typical AD, hippocampal-sparing AD and limbic predominant AD (Whitwell et al., 2012). Typical AD presents with diffuse NFT deposition in the hippocampus and cortex. Hippocampal-sparing AD demonstrates less NFT deposition in the hippocampus but more in the cortex, when compared to typical AD. Lastly, limbic predominant AD involves significantly more NFT deposition in the hippocampus than either of the other two groups. Thus, AD appears to be a relatively heterogeneous disease in terms of its etiological processes as characterized by chemical neuroanatomy.
Whereas clinical staging focuses on changes in the cerebral cortex and hippocampus, other studies have implicated changes in the locus coeruleus (LC) and nucleus basalis of Meynert (NbM). The loss of cholinergic neurons in the NbM was demonstrated as early as 1981 by Nissl-stained histological sections and cell counts in post-mortem studies of AD (Whitehouse et al., 1981). Patients with AD demonstrated degeneration of >75% of the neurons within the NbM (Whitehouse et al., 1982). The NbM cholinergic neurons project throughout the brain and loss of the cholinergic inputs may account for behavioral changes such as short-term memory loss, disorientation and problems with language in AD (Burns and Iliffe, 2009). Other studies demonstrated significant loss of LC neurons in AD (Matthews et al., 2002; Szot et al., 2006; Insua et al., 2010). In fact, LC degeneration has been documented in AD too as early as 1981 (Tomlinson et al., 1981). In comparative studies of disease burden in patients with advanced-stage AD the NbM demonstrated the most significant cell loss followed by the LC and transentorhinal cortex (Arendt et al., 2015). LC degeneration also occurs during healthy aging, which could amount to 50% of cell loss in the tenth decade of life (Manaye et al., 1995). In a longitudinal clinical-pathologic cohort study, 165 individuals completed a battery of cognitive tests and underwent brain autopsy upon death to examine neuronal density of the LC and other aminergic nuclei in the midbrain/brain stem (Wilson et al., 2013). Modeled together, only LC neuronal density was related to cognitive decline, suggesting that the LC may be a structural component of neural reserve.
Parkinson's Disease
Structural brain changes in Parkinson's Disease (PD) similarly follow a time course. PD is associated with the formation of intraneuronal aggregates of the protein alpha synuclein, known as Lewy bodies. The accumulation of Lewy bodies is closely associated with the degeneration of the ventrolateral substantia nigra including specifically the pars compacta (SNc; Fearnley and Lees, 1991; Damier et al., 1999). The loss of SNc neurons results in diminution of extranigral dopaminergic projections throughout the striatum, allocortex, and neocortex (Braak et al., 2003).
As with AD, post-mortem pathology has been described in six different stages which were determined using alpha-synuclein-immunopositive Lewy neurites and Lewy bodies to track disease progression in PD (Braak et al., 2003). Stage 1 is associated with lesions in the dorsal IX/X motor nucleus. Stage 2 includes the findings of stage 1 with additional involvement of the caudal raphe nuclei, gigantocellular reticular nucleus, and coeruleus-subcoeruleus complex. Stage 3 is characterized by significant lesions in the midbrain specifically in the area of the SNc. Progression to stage 4 represents first cortical involvement specifically in the transentorhinal region and allocortex. Stage 5 involves the neocortex with lesions present in sensory association areas and prefrontal regions. Finally, progression to stage six is characterized by additional lesions in sensory association areas and new lesions in the premotor, primary sensory, and primary motor areas. In summary, lesions in PD start at the dorsal motor nucleus and progress to involve the LC, SNc, transentorhinal and cortical regions, in that order. It is worth noting that Braak staging of PD implies an ascending propagation of pathology similar to that of prion diseases and offers to explain the neural processes underlying symptom progression. However, the exact mechanisms of stage progression remain unclear. In particular, studies have reported that only about half of postmortem brains in PD demonstrated Lewy body pathology consistent with the Braak staging (Kalaitzakis et al., 2008; Jellinger, 2009; Halliday et al., 2012), challenging the notion that PD progresses in a sequence similar to prion diseases (Surmeier et al., 2017).
Thus, whereas degeneration of the SNc is the most classic neuropathological finding in PD, postmortem studies also demonstrate Lewy body burden and associated loss of noradrenergic neurons in the LC (Chan-Palay and Asan, 1989; Braak et al., 2006; Szot, 2012). Interestingly, the loss of LC neurons in PD has been documented to occur earlier and in greater magnitude than that of the SNc (Rommelfanger and Weinshenker, 2007). For instance, in Braak staging of PD involvement of the LC starts in stage 2 while involvement of the SNc is not present until stage 3. Loss of LC neurons in PD is associated with depletion of noradrenergic inputs in the frontal cortex, cerebellum, striatum, thalamus, and hypothalamus (Kish et al., 1984; Shannak et al., 1994; Pavese et al., 2011), all of which receive projections from the LC. Together, although postmortem anatomical studies have focused on changes in the SNc, there is significant evidence in support of changes of the noradrenergic system as a critical etiological process of PD. Table 1 highlights the anatomical and neurobiological changes in AD and PD.
Structural Brain Imaging in AD and PD
Alzheimer's Disease
Many studies have correlated Braak staging with atrophic changes on magnetic resonance imaging (MRI) using voxel-based morphometry (VBM) (Ohnishi et al., 2001; Matsuda et al., 2002; Chetelat et al., 2003; Rémy et al., 2005; Matsuda, 2013), focusing largely on hippocampal atrophy as a biomarker of AD (Jack et al., 2011). The patterns of cerebral atrophy as detected on MRI vary across pathological subtypes of AD (Whitwell et al., 2012; Noh et al., 2014). Medial temporal cortical atrophy is seen in limbic-predominant while severe cortical atrophy is seen more in hippocampal-sparing AD (Whitwell et al., 2012). Distinct anatomical changes have also been identified on MRI when early and late onset AD are contrasted (Frisoni et al., 2007). Individuals with early onset AD tend to demonstrate more extensive occipital gray matter atrophy whereas those with late onset AD demonstrate more extensive hippocampal atrophy. The notion of prioritizing anatomical integrity of an isolated region as a biomarker of AD may be convenient; however, the anatomical heterogeneity brings to question the sensitivity of structural changes of individual brain regions as a diagnostic tool of AD.
Although the LC is implicated by postmortem anatomical studies, only recently have studies begun to employ high-resolution fast spin-echo T1-weighted imaging to reveal signal attenuation of the LC in individuals with mild cognitive impairment (MCI) and AD (Takahashi et al., 2015). This finding corresponds well to pathological findings of a decrease in neuromelanin contents as a result of LC neuronal loss in these patients. It is important to note that conventional MRI has not been successful in delineating the LC. However, fast spin-echo T1-weighted imaging, which relies on the paramagnetic properties of neuromelanin, has been demonstrated as a reliable method to quantify signal attenuation or volume loss in neuromelanin- containing tissues such as the LC and SNc (Tosk et al., 1992; Enochs et al., 1997). Neuromelanin is a pigment found in both SNc and LC (Lehéricy et al., 2012). Neuromelanin is identified in monoamines-containing neurons and formed by polymerization of 4,5-dihydroxyindole monomers (Charkoudian and Franz, 2006) via enzymatic processes that involve monoamine oxidase (Rabey and Hefti, 1990), and its concentrations increase with age. Neuromelanin can chelate metals and protect against oxidative stress (Tribl et al., 2009). On the other hand, excessive neuromelanin accumulation compromises neuronal integrity and causes dissolution of cell mass and decrease in neuromelanin signals, as may occur in neurodegenerative conditions. Neuromelanin has paramagnetic-T1 shortening effects and, when combined with metals like iron and copper, would make the SNc and LC appear hyper-intense on MRI. On neuromelanin-MRI there is signal attenuation in MCI participants who do or do not to progress to AD, suggesting that attenuated LC signal alone may not be diagnostic of AD. In fact, the LC exhibited signal changes during healthy aging (Shibata et al., 2006; Betts et al., 2017). Most recently, a stereological study of postmortem human brains reported that as the Braak staging increases by 1 unit, LC volume decreases by 8.4% (Theofilas et al., 2017). Together, although not necessarily pathognomonic of AD, LC volume measurements offer a promising biomarker to track the progression to AD from presymptomatic stages.
Parkinson's Disease
Findings from VBM studies of PD demonstrate varying results with the most common changes identified in the frontal and parieto-occipital regions (Biundo et al., 2011; Pan et al., 2012; Lee et al., 2013; Fioravanti et al., 2015). A meta-analysis of 498 patients with idiopathic PD revealed reductions in gray matter (GM) volume in the left frontal temporal cortices encompassing inferior frontal and superior temporal gyri (Pan et al., 2012). Other brain areas demonstrating GM reductions in PD were the left insular cortex, with insular GM density potentially related to memory scores (Pan et al., 2012; Lu et al., 2016), and the middle and superior frontal gyrus (Biundo et al., 2011). In a study where patients were scanned twice 2 years apart, PD but not controls demonstrated significant GM loss in the putamen and parietal cortex (Fioravanti et al., 2015). On the other hand, some studies did not reveal any significant differences in GM volume between PD and healthy controls (Menke et al., 2014; Borroni et al., 2015). In one of these studies PD patients were split into those with and without dementia and the PD group with dementia but not the group without demonstrated reductions in frontal regional GM volume (Borroni et al., 2015). However, compared to health controls, the two groups combined did not show frontal GM reduction. Together, these findings suggest that variability across studies may relate to the cognitive status of study participants. Other issues including sample size, duration of illness, medication status may all impact the imaging findings.
Neuromelanin Imaging has also been widely used to examine the integrity of SNc function in PD (Shibata et al., 2006; Ohtsuka et al., 2014; Castellanos et al., 2015; Reimão et al., 2015). In melanin-sensitive MR sequences both the SNc and the LC demonstrate reduction in signal intensity or contrast ratio (CR) in PD (Sasaki et al., 2006). The finding of decreased neuromelanin CR in the SNc has been reported in multiple studies of PD (Sasaki et al., 2006; Matsuura et al., 2013; Miyoshi et al., 2013; Mukai et al., 2013; Ohtsuka et al., 2014; Isaias et al., 2016). A recent work of neuromelanin-sensitive MRI employed an automated segmentation algorithm to quantify the volumes of the SNc and LC (Castellanos et al., 2015). Receiver operating characteristic analysis demonstrated better accuracy using the SNc than LC volumes in the diagnosis of PD. Neuromelanin-sensitive MRI has also been used to differentiate essential tremor from PD (Reimão et al., 2015) and early-stage PD from healthy controls (Ohtsuka et al., 2014). Thus, neuromelanin MRI offers a promising technique for characterizing structural and potentially functional changes of the SNc and LC in PD.
Summary
Neuromelanin-sensitive MRI has become a popular tool to investigate LC function and dysfunction. Neuromelanin CRs enable better visualization and assessment of the anatomical and functional integrity of the LC. In healthy adults neuromelanin CR of the LC increased until the sixth decade of life at which point it started to decrease (Shibata et al., 2006). This as well as another study (Clewett et al., 2016) suggests that LC neuromelanin accumulates in an inverted U pattern with age and peaks around 60 years. In older adults, greater LC signal intensity was associated with higher composite scores of cognitive reserve (Clewett et al., 2016). Neuromelanin CRs were greater in healthy adults as compared to patients with major depression, schizophrenia, MCI, PD, and AD (Liu et al., 2017). Together, these findings demonstrate the importance of LC imaging in investigating cognitive changes in neurodegenerative conditions and other psychiatric illnesses that implicate monoaminergic dysfunction.
Functional Brain Imaging in AD and PD
Functional MRI (fMRI) utilizes blood-oxygenation level dependent (BOLD) signals as a proxy for neural activity. The premise of using BOLD signals to reflect neural activities lies in the fact that oxyhemoglobin and deoxyhemoglobin exhibit different magnetic properties. As neurons are activated, more oxygen is consumed, resulting in a change in the ratio of oxyhemoglobin and deoxyhemoglobin, which can be picked up by BOLD imaging. Many studies have combined fMRI and a behavioral task to examine cerebral responses to cognitive and affective challenges. However, a decade of work has suggested that functional organization of the brain can be delineated by how BOLD signals of brain regions are correlated during a resting state. Low-frequency BOLD signal fluctuations reflect connectivity between functionally related brain regions (Biswal et al., 1995; Fair et al., 2007; Fox and Raichle, 2007). Studies of this “spontaneous” activity have provided insights into the intrinsic functional architecture of the brain and shown that coordinated spontaneous fluctuations are present in motor, visual, auditory, default mode, memory, language, dorsal attention, and ventral attention systems (Fox and Raichle, 2007). Other studies have suggested connectivity analysis of resting-state fMRI data as a useful alternative to characterizing functional subdivisions of a brain region (Zhang and Li, 2012a,b, 2014, 2017).
In a study of resting state functional connectivity (rsFC), the LC demonstrated positive connections to bilateral superior frontal gyrus, primary motor cortex, inferior parietal cortex, inferior temporal cortex, anterior parahippocampal gyrus, posterior insula, putamen, pallidum, ventrolateral thalamus, midbrain, and large areas of the cerebellum (Zhang et al., 2016). The LC showed negative connectivities too to a wide swath of brain regions, including bilateral visual cortex, middle/superior temporal cortex, precuneus, retrosplenial cortex, posterior parahippocampal gyrus, frontopolar cortex, caudate nucleus, and the dorsal and medial thalamus. Findings of positive connectivity support a role for norepinephrine (NE) in orienting and sensorimotor responses to external stimuli. The findings of negative connectivity to the precuneus support a role for the LC in regulating activity of the default-mode network (DMN). Together, through connectivity to the cerebral cortex the LC may suppress DMN activity in response to external stimuli and facilitate engagement of the saliency (“alert and orient”) and executive control systems (Li et al., 2007; Zhang and Li, 2010, 2012a,b). On the other hand, one should be cautioned against over-interpreting rsFC in terms of functional implications. A positive rsFC does not necessarily mean that the two brain regions are functionally congruent. Long range cortical-cortical/subcortical projections may target inhibitory interneurons within the recipient regions (Thomson and Lamy, 2007; Apicella et al., 2012; DeNardo et al., 2015). For instance, the amygdala and ventromedial prefrontal cortex (vmPFC) show positive rsFC (Veer et al., 2010; Schultz et al., 2012) but may respond in isolation or in opposite directions to behavioral challenges such as affective regulation (Urry et al., 2006) and extinction learning of fear (Phelps et al., 2004).
The proposition that the LC circuit responds to behavioral tasks is supported by task-based studies demonstrating LC activation to stimulus change. In an oddball task LC activity increased to the detection of novel, oddball stimuli (Krebs et al., 2017). In a picture-word interference task the LC responded to novel as compared to familiar stimuli (Krebs et al., 2013). In an attention task, exposure to the alerting condition was associated with increased activation of the LC (Neufang et al., 2015). The LC is also consistently activated in response to stimuli that trigger arousal responses via fear, pain, and anger (Liddell et al., 2005; Brooks et al., 2017; Gilam et al., 2017). The role of the LC in arousal is examined by a recent study using dexmedetomodine to elicit a state of reduced arousal (Song et al., 2017). Dexmedetomidine, a potent sedative, is an α2 agonist that reduces LC neuronal firing and NE release. Along with decreasing arousal, dexmedetomidine diminished connectivity between the LC and posterior cingulate cortex (PCC) as compared to the awake state. Further, dexmedetomidine disrupted PCC connectivity with subcortical and cortical structures central to executive control. These findings together support the role of the LC in facilitating attention shifts in response to stimulus change and in situations that demand a higher level of alertness (see Liu et al., 2017 for a review of task-related LC studies).
Finally, an overview of electrophysiological studies of LC neuronal functions would place human imaging findings in a clearer perspective (Aston-Jones and Waterhouse, 2016). First, cortical projections of the LC are predominantly ipsilateral whereas subcortical projections are more likely to be bilateral. Further, projections of individual LC neurons collateralize to cover functionally related brain regions. Neurons projecting to different circuits are segregated in the LC and may release NE asynchronously in response to task demands. Second, studies employing iontophoresis of NE in target regions and electrical stimulation of the LC showed that LC suppresses spontaneous neuronal activity in the cerebellum and cerebral cortex, establishing NE as an inhibitory neurotransmitter at central synapses. Other studies showed that the spontaneous activities are suppressed to a greater extent than stimulus-evoked activity, resulting in an increase of the signal noise ratio in response to stimulus. Third, LC neurons discharge both tonically and phasically. LC neurons discharge tonically at a moderate rate but respond to task-related stimuli phasically during focused attention. The Adaptive Gain Theory proposes that LC phasic responses facilitate behavioral adaptation to changing environment whereas tonic activities suppress behavior of low utility (Aston-Jones and Waterhouse, 2016).
Functional Imaging Studies of AD
The default mode network (DMN) comprises a set of brain regions, including parts of the precuneus and posterior cingulate cortex (PCC; Leech and Sharp, 2013), that are more active during mind wandering, retrieval of autobiographical memories, and monitoring of the arousal state (Leech and Sharp, 2013), than during exposure to environmental stimuli. A host of studies have noted changes in DMN activity and connectivity in AD during rest (Jacobs et al., 2013; Badhwar et al., 2016; Kim et al., 2016). Reduced DMN connectivities can be observed from early stages of MCI to late stage AD (Adriaanse et al., 2014; Gour et al., 2014; Badhwar et al., 2016), as well as in asymptomatic patients genetically at risk of AD (Chhatwal et al., 2013; Sala-Llonch et al., 2013; Quiroz et al., 2015). Reduced DMN connectivity in AD also correlates with disease severity, as measured by the Clinical Dementia Rating scale (Zhou et al., 2010; Petrella et al., 2011; Brier et al., 2012). Of all the brain networks studied, the DMN demonstrates the most consistent connectivity changes in AD.
The salience network (SAN) is a network of structures including most prominently the dACC and anterior insula that processes a constant stream of external stimuli to identify salient inputs for goal-directed behavior. In contrast to the DMN, which is an inwardly driven network, the SAN integrate sensory, visceral, and autonomic information to facilitate decision making (Seeley et al., 2007). Changes in the SAN activity and connectivity are also implicated in AD. Network based analyses frequently demonstrate hyperconnectivity within the SAN in patients with AD (Thomas et al., 2014; Wang et al., 2015; Badhwar et al., 2016). Abnormal SAN connectivity has been documented across the disease spectrum from early to late onset AD (Wang et al., 2015; Badhwar et al., 2016), in patients with autosomal dominant AD (Thomas et al., 2014), and in those who carry the APOEe4 gene, which is known to increase the risk for AD (Machulda et al., 2011; Goveas et al., 2013). One of the key hubs of the SAN is the anterior insular cortex (Seeley et al., 2007). In a large, voxel-based meta-analysis, the anterior insula demonstrated significant hyperconnectivity in AD (Badhwar et al., 2016). The SAN receives limbic and autonomic inputs and facilitates activity shifts between the DMN and frontoparietal network to help guide behavior (Seeley et al., 2007; He et al., 2014). Moreover, changes in SAN connectivity may perturb DMN function and vice versa (Bonnelle et al., 2012; Zhao Q. et al., 2017). Thus, the SAN plays a critical role in regulating DMN activity and failure to suppress DMN activity during task challenges correlates with worse cognitive performance. The implications of these changes for AD will be discussed in more depth later.
Other studies demonstrated hyperconnectivity of the limbic network (Gour et al., 2011, 2014; Badhwar et al., 2016), mostly in the hippocampus and entorhinal cortex, in AD (Badhwar et al., 2016). Intriguingly, anterior temporal network hyperconnectivity was correlated positively with memory performance in patients with early onset AD (Gour et al., 2014), perhaps reflecting a transient compensation before progression to late-stage dementia. Together with findings on the DMN, network connectivity changes appear to be a prominent feature of AD. The significance of these findings needs to be evaluated with longitudinal records of disease progression.
Functional Imaging Studies of PD
In patients with PD slower processing speeds were associated with decreased DMN connectivity at rest, specifically between the posterior cingulate, medial prefrontal and inferior parietal nodes (Disbrow et al., 2014). Off medication, PD patients were unable to suppress DMN activity during a facial emotion recognition task (Delaveau et al., 2010). In the Montreal card-sorting task, a task that involves manipulation of short-term memory, patients with PD showed less deactivation of the DMN as compared to healthy controls (van Eimeren et al., 2009). Another study reported worse performance on executive functioning, psychomotor speed and verbal memory in association with increased positive connectivity between the SAN and DMN (Putcha et al., 2016). In other words, reduced anti-correlation between these two networks was associated with impaired cognitive performance in PD. The latter study implicates dysregulation of both SAN and DMN in PD.
The right inferior parietal cortex (IPC), a region associated with the DMN (Mars et al., 2012; Davey et al., 2016), demonstrated aberrant connectivity in PD patients (Tahmasian et al., 2017) with and without depression (Wen et al., 2013), particularly in those who were cognitively impaired (Amboni et al., 2015; Gorges et al., 2015). Altered IPL activity was demonstrated consistently across different neural metrics, including the amplitude of low frequency fluctuations and regional homogeneity (Tahmasian et al., 2017). In a meta-analysis of rs-fMRI studies, connectivity changes in the right posterior IPC represent the most consistent finding in PD (Tahmasian et al., 2017) and may disrupt activity of the precuneus, PCC, mid-cingulate cortex, middle and superior frontal gyrus, and orbitofrontal gyrus. It is worth noting again that, the posterior IPC is part of the DMN (Davey et al., 2016). Sub-cluster analysis of functional connectivity identified four functionally dissociable subregions in the IPC (Zhang and Li, 2014; Bzdok et al., 2015) and the posterior subregion, in the area of angular gyrus and with the strongest connectivity to the DMN, is the exact region that demonstrated aberrant connectivity in PD (Tahmasian et al., 2017). PD patients demonstrated increased parietal cortical activation when off as compared to on medication (Herz et al., 2014). Mutation carriers of leucine-rich repeat kinase 2 (LRR2), a population with an elevated risk for PD, also demonstrated reduced connectivity between the IPL and posterior putamen, a task network region (Helmich et al., 2015). Altered DMN activity was associated with decreased SNc activity and with disease severity in PD (Wu et al., 2012). In summary, as with AD, dysconnectivity of the DMN appears a core neural feature and may contribute to cognitive decline in PD.
Other Imaging Studies
Conventional T1/T2 MRI has not been successful at identifying changes in the SNc or other structures in PD. More advanced techniques including magnetization transfer (Wolff and Balaban, 1989; Rademacher et al., 1999; van Waesberghe et al., 1999; Helms et al., 2009), adiabatic and MR microscopy (Lehéricy et al., 2012) and relaxometry have revealed changes in the SNc in individuals with PD. In addition to neuromelanin imaging, iron imaging has been studied in PD. Brain iron content is increased in association with dopamine loss in the SNc in PD patients (Dexter et al., 1991; Martin et al., 2008; Martin, 2009; Mascalchi et al., 2012). Thus, imaging iron with nonionizing MRI offers a strategy to detect neuroanatomical changes in PD. In a two-year follow-up study iron-related relaxation increased in the anterior globus pallidus, caudate nucleus and medial SNc and the changes in the globus pallidus and SNc were related to the development of MCI (Rossi et al., 2014). More recently an iron imaging study employing quantitative susceptibility mapping (QSM) reported increased QSM magnetic values in PD patients as compared to healthy controls. The difference between SNc iron deposition between healthy controls and patients with advanced PD was the most prominent, with SNc iron content correlated with symptom severity in PD (An et al., 2018).
Positron emission tomography (PET) imaging of PD focused primarily on changes in dopamine transporter (DAT) density. In a recent meta-analysis DAT and vesicular monoamine transporter in early to moderate PD is decreased most significantly in the posterior putamen followed by the anterior putamen and caudate. Moreover, disease severity was linearly correlated with dopamine loss (Kaasinen and Vahlberg, 2017). No studies to our knowledge investigated norepinephrine transporter (NET) density in PD. [(11)C]MENNET is a novel PET radiotracer with high affinity and selectivity for NET and may provide insights into noradrenergic dysfunction in PD (Adhikarla et al., 2016).
PET imaging has also demonstrated utility in tracking AD progression. Fluorine-18 fluorodeoxyglucose (FDG) PET imaging assessed regional cerebral glucose metabolism and showed decreased metabolic rates in the medial temporal lobes, lateral temporoparietal cortex, posterior cingulate cortex, and precuneus in patients with AD in comparison to normal aging (Sarikaya, 2015). In a post-mortem autoradiographic study of (S,S)-[(18)F]FMeNER-D(2), a selective ligand for NET, AD patients showed a reduction in NET density at the LC and thalamus in correlation with disease progression by Braak staging (Gulyás et al., 2010). The size of the LC is below the spatial resolution of PET imaging but in vivo imaging of NET density in the thalamus may be useful to highlight early noradrenergic dysfunction in AD (Gulyás et al., 2010).
Table 2 highlights VBM, neuromelanin and other imaging findings of AD and PD.
Noradrenergic Dysfunction in AD and PD
An Overview
The LC sends noradrenergic projections to the hippocampus (Loughlin et al., 1986), amygdala (Fallon et al., 1978), and prefrontal cortex (PFC; Loughlin et al., 1982). Phasic LC activation in response to target stimuli facilitates anticipation (Aston-Jones et al., 1985, 1994) and release of norepinephrine (NE) in the cortex (Mountcastle et al., 1972; Aston-Jones and Cohen, 2005) prior to a motivated action. NE signals in the PFC regulate attention, learning and working memory (Robbins, 2000). On the other hand, NE interacts with other catecholamines like dopamine (DA) to support these functions, with NE often playing a regulatory role in DA signaling. For example, chemical modulation or electrical stimulation of the LC increases the extracellular concentrations of both NE and DA (Smith and Greene, 2012). The ability of the LC to effect direct and indirect control of catecholamines has important implications on the arousal state as well as autonomic, motor, sensory, and cognitive functions.
Both AD and PD involve noradrenergic dysfunction. For example, orthostatic and postprandial hypotension in PD can be related to autonomic dysfunction and NE deficiency (Kaufmann and Goldstein, 2013). Disruption of the circadian rhythm and arousal/wakefulness cycles manifest in both diseases and are associated with noradrenergic dysfunction (Berridge and Waterhouse, 2003; Benarroch, 2009). In PD, decreased CSF concentration of NE is associated with freezing of gait and administration of a NE precursor improves gait (Tohgi et al., 1993a,b). Animal studies involving degeneration of the LC also support noradrenergic dysfunction in AD. In transgenic mice carrying homozygous forms of amyloid precursor protein/presenilin 1 genes, induction of LC degeneration with N-(2-chloroethyl)-N-ethyl-bromo-benzylamine resulted in an exacerbation of olfactory memory deficits and weakening of olfactory discrimination (Rey et al., 2012). As 90% of patients with early AD experience olfactory dysfunction (Hawkes, 2003), the latter study suggests a potential role of the LC in the etiological processes of early AD. In another study of transgenic mice with a low amyloid load, LC degeneration elicited with the same chemical triggered massive glial inflammation and augmented the deposition of amyloid plaques (Heneka et al., 2006). Together, these studies provide substantial evidence for noradrenergic dysfunction in AD and PD.
Noradrenergic Dysfunction and the Hippocampus in AD and PD
AD and PD are known to have distinct pathology, yet they share many cognitive and behavioral manifestations as the illness progresses, including dementia, motor dysfunction, and behavioral disinhibition. Both AD and PD are associated with significant degeneration of the LC (Bondareff et al., 1982; Marcyniuk et al., 1986; Rommelfanger and Weinshenker, 2007). In PD the degeneration of the LC occurs earlier and in greater magnitude in comparison to the SNc (Rommelfanger and Weinshenker, 2007). AD and PD also share extensive pathology in the hippocampus. In AD, memory deficits tend to manifest early and correlate with hippocampal pathology (Braak et al., 1994). In PD, loss of NE-containing neurons is associated with cognitive decline (Cash et al., 1987). As the LC projects heavily to the hippocampus and parahippocampal formation (Zhang et al., 2016), loss of noradrenergic signaling may lead to hippocampal dysfunction and memory deficits. In patients with MCI and a Clinical Dementia Rating score of 0.5, decreased LC connectivity to the left parahippocampal gyrus was associated with lower memory performance (Jacobs et al., 2015). These findings support the proposition that noradrenergic dysfunction in AD and PD contributes to memory impairment.
In rodent studies, the LC has extensive anatomical connectivity (likely stronger than the ventral tegmental area) to the hippocampus (Takeuchi et al., 2016). Optical activation of LC terminals in the dorsal hippocampus enhances object location memory (Kempadoo et al., 2016). Further, depletion of noradrenergic terminals in the hippocampus by neurotoxics or ablation of LC neurons impaired location memory in mice (Coradazzi et al., 2016; Moreno-Castilla et al., 2017). Numerous other studies have implicated noradrenergic control of other aspects of cognitive functioning (Caetano et al., 2013). Reduced cortical noradrenergic neurotransmission was associated with increased neophobia and impaired spatial memory in aged rats (Collier et al., 2004). Work on transgenetics associated reduced tissue levels of NE and LC degeneration with behavioral phenotypes of mouse models of AD (Francis et al., 2012; Rey et al., 2012). In transgenic mice that accumulated amyloid burden at early ages, treatment with a NE precursor increased CNS NE levels and improved learning in the Morris water maze (Kalinin et al., 2012). Noradrenergic innervations from the LC are needed to maintain beta amyloid clearance, and LC degeneration could contribute to the pathogenesis of AD (Kalinin et al., 2007).
The potential roles of NE in the etiological processes of AD and PD and memory impairment can also be examined with pharmacologic manipulations. Administration of methylphenidate, a stimulant medication known to increase NE and DA levels and commonly used in the treatment of ADHD (Weikop et al., 2007), improved word recall when taken before learning (Verster et al., 2010; Linssen et al., 2012). Administration of methylphenidate after learning also increased 1-week retention of intentionally and casually learned information (Izquierdo et al., 2008). In a fMRI study, methylphenidate administration increased connectivity between the LC and the hippocampus (Kline et al., 2016). The latter study implicates NE in producing a measurable change in connectivity between the LC and hippocampus, both of which suffer significant neuronal loss and functional dysconnectivity in AD and PD. Although methylphenidate increases the extracellular levels of DA in addition to NE, the finding of increased connectivity between the LC and hippocampus and its association with improved memory function helps establish a specific link to the noradrenergic system. Together, considered in the context of LC degeneration and the resulting denervation of the hippocampus, these findings support noradrenergic dysfunction as a neural mechanism of memory impairment in AD and PD.
Noradrenergic Connectivity Dysfunction in AD and PD
The LC undergoes early and significant deterioration and recent imaging studies suggest LC functional dysconnectivity that involves brain regions other than the hippocampus in AD. A recent work employed Granger causality mapping to create a model of directed connectivity and characterized regional functional coupling in AD and healthy controls (Zhao S. et al., 2017). With group differences determined with a generative model of pathology, the authors identified the LC and right orbitofrontal cortex as the two foci of disruption in AD. These findings provide further support of a critical role of the LC in functional impairment in AD.
The saliency network (SAN) facilitates network activity transition from the DMN to the frontoparietal network during task challenges (Seeley et al., 2007; He et al., 2014). For instance, the right anterior insula temporally precedes activation of the central executive network and de-activation of the DMN in task based paradigms (Sridharan et al., 2008). Temporal control over these network activities is central to an intact cognition, and impaired suppression of the DMN is associated with impaired performance. As discussed earlier, abnormal DMN connectivity represents one of the most consistent finding in AD. The SAN, specifically the anterior insula, also demonstrates abnormal connectivity in AD. DMN and SAN dysfunction may result in large part from noradrenergic dysfunction in AD. The insula responds strongly to deviances in a stream of continuous stimuli and does so across auditory, visual, and tactile modalities (Linden et al., 1999; Downar et al., 2000, 2001; Crottaz-Herbette and Menon, 2006). Regions of the SAN coactivate in response to faces of loved ones (Bartels and Zeki, 2004), pleasurable touch (Craig, 2002), emotional dimensions of pain (Peyron et al., 2000), “chills” to music (Blood and Zatorre, 2001) and other forms of saliency (Eisenberger et al., 2003; Singer et al., 2004), all supported by LC activity and an intact system of physiological arousal. As the LC shores up arousal and responses to salient stimuli, noradrenergic signaling is in a position to influence SAN and DMN network activities. It is highly likely that deficient noradrenergic signaling may compromise functional coupling between the SAN and DMN and, as a result, deficits in the suppression of DMN activity to meet task demands.
In support, atomoxetine, a selective NE reuptake inhibitor commonly used in the treatment of ADHD, increased connectivity between the right anterior insula and dorsolateral prefrontal cortex (Hernaus et al., 2017). Increased connectivity between these regions correlated with decreased reaction time variability in an N-back working memory test and predicted auditory digit span of the Wechsler Adult Intelligence Scale. In a predictive learning task atomoxetine facilitated extinction learning along with increased activity in the insula (Lissek et al., 2015). In a study of inhibitory control, atomoxetine improved performance in a stop-signal task in correlation with increased insula activity (Chamberlain et al., 2009). These findings suggest that NE influences insula activity and improves a broad spectrum of cognitive functions, providing a pathway whereby noradrenergic dysfunction may contribute to aberrant SAN and DMN activations in AD.
As discussed earlier, the IPL is part of the DMN (Bzdok et al., 2015) and consistently demonstrates abnormal connectivity in PD. Engagement in a cognitive task is associated with significant posterior IPL deactivation (Greicius et al., 2003; Buckner et al., 2008). Altered IPL and DMN connectivity in PD during task engagement (van Eimeren et al., 2009; Delaveau et al., 2010; Putcha et al., 2016) may represents a failure in activity transition between functional networks (Putcha et al., 2016). Successful task engagement requires suppression of internally driven processes such as mind-wondering, and the salience network monitors external stimuli and facilitates the suppression of DMN activity and these internally driven processes. One study discussed earlier demonstrated reduced anti-correlation between the SAN and DMN in link with diminished executive functioning, psychomotor speed and verbal memory (van Eimeren et al., 2009). The LC responds to saliency and functionally connects to key regions within the DMN including the IPL, PCC, and precuneus (Zhang et al., 2016). It is also one of the first brain regions affected in PD (Rommelfanger and Weinshenker, 2007). Thus, early deterioration and dysconnectivity of the LC is positioned to effect cognitive decline in PD.
Pharmacological investigations provide more evidence relating cognitive decline to LC dysfunction in PD. Enhancing noradrenergic signaling improves cognitive performance in PD (Bédard et al., 1998; Marsh et al., 2009; Weintraub et al., 2010; Obeso et al., 2011; Kehagia et al., 2014; Nombela et al., 2014; Ye et al., 2015). For example, naphtoxazine, a selective α1 agonist, as compared to placebo, ameliorated performance deficits in a reaction time task and the Stroop task in PD patients (Bédard et al., 1998). A recent study of healthy adults reported that LC functional connectivity increased in the Stroop task, implicating the noradrenergic system during interference control (Köhler et al., 2016). In other studies, atomoxetine improved performance on measures of global cognition (mini-mental status exam) and executive functions, as evaluated by the Frontal Systems Behavior Scale and Connors Adult ADHD Rating Scale (Marsh et al., 2009; Weintraub et al., 2010) in patients with PD. In a stop signal task, atomoxetine improved stop signal reaction times in a subset of PD patients (Obeso et al., 2011; Kehagia et al., 2014; Nombela et al., 2014; Ye et al., 2015). Atomoxetine also restored functional connectivity between the presupplementary motor area, inferior frontal gyrus, and subthalamic nucleus (Rae et al., 2016) of the cortical subcortical circuit to support response inhibition in PD patients. As discussed earlier, atomoxetine improved performance on stop signal tasks along with increased insular activity and engagement of the saliency network in healthy adults (Chamberlain et al., 2009). This is important because control of the DMN relies critically on SAN function (Bonnelle et al., 2012; Zhao Q. et al., 2017). In a study of medication-naïve adults with ADHD, atomoxetine facilitated suppression of the DMN in correlation with improved clinical symptoms (Lin and Gau, 2016). Together, these findings support noradrenergic dysfunction and the efficacy of noradrenergic agents in improving cognition in PD. Table 3 highlights key findings of DMN dysfunction in AD and PD.
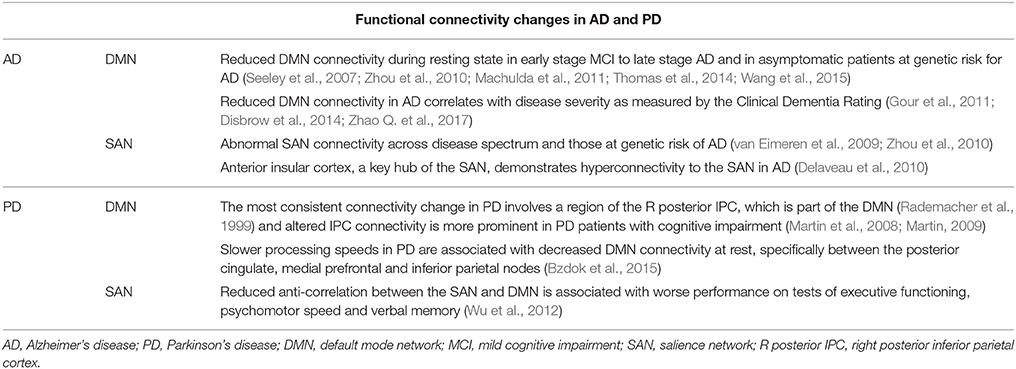
Table 3. Summary of functional connectivity changes of the default mode and saliency networks in Alzheimer's disease and Parkinson's disease.
Figure 1 illustrate a hypothetical model of noradrenergic circuit regulation of functional neural networks.
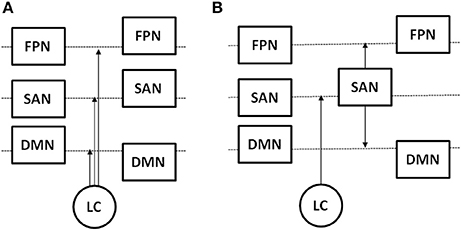
Figure 1. Hypothetical models of the influence of LC on the activity of the saliency network (SAN), frontoparietal task network (FPN), and default model network (DMN). Box position with respect to the dashed line represents changes in activity level from a resting to task state, and arrows indicate the location of LC action in response to a task challenge. (A) LC increases activity of the SAN and FPN and suppresses activity of the DMN; (B) LC increases activity of the SAN, which in turn increases activity of the FPN and suppresses activity of the DMN.
Conclusions and Future Research
Dysfunction of the noradrenergic system is a key feature of both AD and PD. The deficit is manifested in post-mortem studies which reveal loss of NE neurons in the LC in both diseases. It is also manifested in structural brain imaging as atrophic changes in structures connected with the LC and in functional imaging as changes in network activity and connectivity. Key networks implicated in noradrenergic dysfunction include the hippocampus, DMN and the SAN. Specifically, noradrenergic dysfunction may disrupt the ability to monitor external stimuli and shift attentional demands accordingly. These shifts in attentional demand rely on the SAN to regulate and suppress the DMN during task-oriented processes. Pharmacologic studies with NE reuptake inhibitors highlight the role of NE in remediating aberrations in functional connectivity and deficits in cognitive performance. Together, the studies may facilitate research of the etiology of AD and PD as well as development of pharmacotherapy for these debilitating illnesses (Calsolaro and Edison, 2015; Greig, 2015; Leissring, 2016; Schaeffer and Berg, 2017).
Despite the advances in methodology, there are limitations in current imaging techniques and pharmacological approaches to precisely localize the LC and quantify LC functions. For instance, neuromelanin imaging provides data with low spatial resolution with respect to the size of the LC and with signal non-uniformity in a given plane (Sasaki et al., 2008). These issues may compromise the utility of neuromelanin imaging in quantifying signal alteration in the LC. Pharmacological manipulations have been used to investigate noradrenergic dysfunction in AD and PD. However, many “noradrenergic” agents are not specific to the noradrenergic system. Further, because of co-localization of synaptic NE and DA, the findings from pharmacological studies would require careful interpretation.
A few potential research directions can be explored to further our knowledge of LC function in health and illness. First, despite electrophysiological studies characterizing the roles of LC neurons in cognitive performance in behaving primates, imaging work in humans has been hampered by the small size of LC. Thus, studies combining neuromelanin imaging to more accurately localize the LC and fMRI to explore task-related activations are needed to fully understand how LC and LC connectivity with SAN, DMN, and frontoparietal network partake in task challenges. As VTA/SNc and LC are both involved in saliency response, it would be of great interest to evaluate how the two systems are differentially involved in cognitive performance and whether reward plays a specific role in differentiating noradrenergic and dopaminergic circuit functions. Likewise, studies of pharmacological manipulations can take advantage of anatomical localization of the LC and more precisely pinpoint how noradrenergic agents influence regional activity and connectivity during cognitive performance. Second, there have been neuromelanin imaging studies of the VTA/SNc in PD but less work has focused on elucidating signal changes in the LC in either PD or AD. In particular, longitudinal studies to characterize how LC signals evolve during healthy aging and in individuals with MCI or at risk of developing PD and AD would be instrumental in determining the significance of neuromelanin imaging as a tool in predicting onset and progression of the illnesses. In individuals at risk for PD, it would also be of interest to contrast findings on VTA/SNc and LC to determine how the noradrenergic and dopaminergic circuits are differentially involved in the pathogenesis of PD. Likewise, longitudinal studies of PD and AD patients undergoing treatment may benefit from knowledge whether LC neuromelanin signal intensity may serve as a biomarker of treatment outcomes. Third, the LC projects to multiple brain regions and the interaction of LC with these neural networks are likely complex and defy simple correlation analyses. More sophisticated analytical tools such as Granger causality analysis (Duann et al., 2009; Ide and Chiang-shan, 2011; Ide and Li, 2011; Hu et al., 2015) and detrended partial cross correlation (Ide et al., 2017; Ide and Li, 2018) will be useful in delineating the direction of influence and distinguish direct functional interaction from influences via a common “third party.” These analyses would be tremendously useful in confirming the hypothesis that the LC response to saliency and its projection to the SAN facilitates activity transition from the DMN to frontoparietal network for goal-directed behavior. Together, these new studies will address many unanswered questions in cognitive and clinical neuroscience, and the findings would not only advance knowledge but also better patient care.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This review was supported by NIH grants MH113134, DA023248, and AA021449. We declare no conflicts of interest in the current work. The NIH was otherwise not involved in the preparation of or in the decision to publish this review.
References
Adhikarla, V., Zeng, F., Votaw, J. R., Goodman, M. M., and Nye, J. A. (2016). Compartmental modeling of [11C] MENET binding to the norepinephrine transporter in the healthy human brain. Nucl. Med. Biol. 43, 318–323. doi: 10.1016/j.nucmedbio.2016.02.008
Adriaanse, S. M., Binnewijzend, M. A., Ossenkoppele, R., Tijms, B. M., van der Flier, W. M., Koene, T., et al. (2014). Widespread disruption of functional brain organization in early-onset Alzheimer's disease. PLoS ONE 9:e102995. doi: 10.1371/journal.pone.0102995
Amboni, M., Tessitore, A., Esposito, F., Santangelo, G., Picillo, M., Vitale, C., et al. (2015). Resting-state functional connectivity associated with mild cognitive impairment in Parkinson's disease. J. Neurol. 262, 425–434. doi: 10.1007/s00415-014-7591-5
An, H., Zeng, X., Niu, T., Li, G., Yang, J., Zheng, L., et al. (2018). Quantifying iron deposition within the substantia nigra of Parkinson's disease by quantitative susceptibility mapping. J. Neurol. Sci. 386, 46–52. doi: 10.1016/j.jns.2018.01.008
Apicella, A. J., Wickersham, I. R., Seung, H. S., and Shepherd, G. M. (2012). Laminarly orthogonal excitation of fast-spiking and low-threshold-spiking interneurons in mouse motor cortex. J. Neurosci. 32, 7021–7033. doi: 10.1523/JNEUROSCI.0011-12.2012
Arendt, T., Brückner, M. K., Morawski, M., Jäger, C., and Gertz, H. (2015). Early neurone loss in Alzheimer's disease: cortical or subcortical? Acta Neuropathol. Commun. 3:10. doi: 10.1186/s40478-015-0187-1
Aston-Jones, G., and Cohen, J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28, 403–450. doi: 10.1146/annurev.neuro.28.061604.135709
Aston-Jones, G., Foote, S., and Segal, M. (1985). Impulse conduction properties of noradrenergic locus coeruleus axons projecting to monkey cerebrocortex. Neuroscience 15, 765–777. doi: 10.1016/0306-4522(85)90077-6
Aston-Jones, G., Rajkowski, J., Kubiak, P., and Alexinsky, T. (1994). Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J. Neurosci. 14, 4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994
Aston-Jones, G., and Waterhouse, B. (2016). Locus coeruleus: from global projection system to adaptive regulation of behavior. Brain Res. 1645, 75–78. doi: 10.1016/j.brainres.2016.03.001
Badhwar, A., Tam, A., Dansereau, C., Orban, P., Toro, R., and Bellec, P. (2016). Resting-state network dysfunction in alzheimer's disease: a systematic review and meta-analysis. Alzheimer's Dement. 8, 73–85. doi: 10.1016/j.dadm.2017.03.007
Bancher, C., Braak, H., Fischer, P., and Jellinger, K. A. (1993). Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer's and Parkinson's disease patients. Neurosci. Lett. 162, 179–182. doi: 10.1016/0304-3940(93)90590-H
Bancher, C., Brunner, C., Lassmann, H., Budka, H., Jellinger, K., Wiche, G., et al. (1989). Accumulation of abnormally phosphorylated τ precedes the formation of neurofibrillary tangles in Alzheimer's disease. Brain Res. 477, 90–99. doi: 10.1016/0006-8993(89)91396-6
Bartels, A., and Zeki, S. (2004). The neural correlates of maternal and romantic love. Neuroimage 21, 1155–1166. doi: 10.1016/j.neuroimage.2003.11.003
Bédard, M., El Massioui, F., Malapani, C., Dubois, B., Pillon, B., Renault, B., et al. (1998). Attentional deficits in Parkinson's disease: partial reversibility with naphtoxazine (SDZ NVI-085), a selective noradrenergic [alpha] 1 agonist. Clin. Neuropharmacol. 21, 108–117.
Benarroch, E. E. (2009). The locus ceruleus norepinephrine system: functional organization and potential clinical significance. Neurology 73, 1699–1704. doi: 10.1212/WNL.0b013e3181c2937c
Berridge, C. W., and Waterhouse, B. D. (2003). The locus coeruleus–noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res. Rev. 42, 33–84. doi: 10.1016/S0165-0173(03)00143-7
Betts, M. J., Cardenas-Blanco, A., Kanowski, M., Jessen, F., and Düzel, E. (2017). In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. Neuroimage 163, 150–159. doi: 10.1016/j.neuroimage.2017.09.042
Biswal, B., Zerrin Yetkin, F., Haughton, V. M., and Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn. Reson. Med. 34, 537–541. doi: 10.1002/mrm.1910340409
Biundo, R., Formento-Dojot, P., Facchini, S., Vallelunga, A., Ghezzo, L., Foscolo, L., et al. (2011). Brain volume changes in Parkinson's disease and their relationship with cognitive and behavioural abnormalities. J. Neurol. Sci. 310, 64–69. doi: 10.1016/j.jns.2011.08.001
Blood, A. J., and Zatorre, R. J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U.S.A. 98, 11818–11823. doi: 10.1073/pnas.191355898
Bondareff, W., Mountjoy, C. Q., and Roth, M. (1982). Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 32, 164–168. doi: 10.1212/WNL.32.2.164
Bonnelle, V., Ham, T. E., Leech, R., Kinnunen, K. M., Mehta, M. A., Greenwood, R. J., et al. (2012). Salience network integrity predicts default mode network function after traumatic brain injury. Proc. Natl. Acad. Sci. U.S.A. 109, 4690–4695. doi: 10.1073/pnas.1113455109
Borroni, B., Premi, E., Formenti, A., Turrone, R., Alberici, A., Cottini, E., et al. (2015). Structural and functional imaging study in dementia with Lewy bodies and Parkinson's disease dementia. Parkinsonism Relat. Disord. 21, 1049–1055. doi: 10.1016/j.parkreldis.2015.06.013
Braak, F., Braak, H., and Mandelkow, E. (1994). A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 87, 554–567. doi: 10.1007/BF00293315
Braak, H., Del Tredici, K., Rüb, U., De Vos, R. A., Steur, E. N. J., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211. doi: 10.1016/S0197-4580(02)00065-9
Braak, H., Rüb, U., and Del Tredici, K. (2006). Cognitive decline correlates with neuropathological stage in Parkinson's disease. J. Neurol. Sci. 248, 255–258. doi: 10.1016/j.jns.2006.05.011
Brier, M. R., Thomas, J. B., Snyder, A. Z., Benzinger, T. L., Zhang, D., Raichle, M. E., et al. (2012). Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. J. Neurosci. 32, 8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012
Brooks, J. C., Davies, W. E., and Pickering, A. E. (2017). Resolving the Brainstem Contributions to Attentional Analgesia. J. Neurosci. 37, 2279–2291. doi: 10.1523/JNEUROSCI.2193-16.2016
Buckner, R. L., Andrews-Hanna, J., and Schacter, D. (2008). The brain's default network: anatomy, function, and relevance to disease. Ann. N.Y. Acad. Sci. 1124, 1–38. doi: 10.1196/annals.1440.011
Bzdok, D., Heeger, A., Langner, R., Laird, A. R., Fox, P. T., Palomero-Gallagher, N., et al. (2015). Subspecialization in the human posterior medial cortex. Neuroimage 106, 55–71. doi: 10.1016/j.neuroimage.2014.11.009
Caetano, M. S., Jin, L. E., Harenberg, L., Stachenfeld, K. L., Arnsten, A. F., and Laubach, M. (2013). Noradrenergic control of error perseveration in medial prefrontal cortex. Front. Integr. Neurosci. 6:125. doi: 10.3389/fnint.2012.00125
Calsolaro, V., and Edison, P. (2015). Novel GLP-1 (glucagon-like peptide-1) analogues and insulin in the treatment for Alzheimer's disease and other neurodegenerative diseases. CNS Drugs 29, 1023–1039. doi: 10.1007/s40263-015-0301-8
Cash, R., Dennis, T., L'Heureux, R., Raisman, R., Javoy-Agid, F., and Scatton, B. (1987). Parkinson's disease and dementia: norepinephrine and dopamine in locus ceruleus. Neurology 37, 42–46. doi: 10.1212/WNL.37.1.42
Castellanos, G., Fernández-Seara, M. A., Lorenzo-Betancor, O., Ortega-Cubero, S., Puigvert, M., Uranga, J., et al. (2015). Automated neuromelanin imaging as a diagnostic biomarker for Parkinson's disease. Mov. Disord. 30, 945–952. doi: 10.1002/mds.26201
Chamberlain, S. R., Hampshire, A., Müller, U., Rubia, K., Del Campo, N., Craig, K., et al. (2009). Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol. Psychiatry 65, 550–555. doi: 10.1016/j.biopsych.2008.10.014
Chan-Palay, V., and Asan, E. (1989). Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson's disease with and without dementia and depression. J. Comp. Neurol. 287, 373–392. doi: 10.1002/cne.902870308
Charkoudian, L. K., and Franz, K. J. (2006). Fe (III)-coordination properties of neuromelanin components: 5, 6-dihydroxyindole and 5, 6-dihydroxyindole-2-carboxylic acid. Inorg. Chem. 45, 3657–3664. doi: 10.1021/ic060014r
Chetelat, G., Desgranges, B., de la Sayette, V., Viader, F., Berkouk, K., Landeau, B., et al. (2003). Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain 126, 1955–1967. doi: 10.1093/brain/awg196
Chhatwal, J. P., Schultz, A. P., Johnson, K., Benzinger, T. L., Jack, C. Jr, Ances, B. M., et al. (2013). Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81, 736–744. doi: 10.1212/WNL.0b013e3182a1aafe
Clewett, D. V., Lee, T., Greening, S., Ponzio, A., Margalit, E., and Mather, M. (2016). Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 37, 117–126. doi: 10.1016/j.neurobiolaging.2015.09.019
Collier, T. J., Greene, J. G., Felten, D. L., Stevens, S. Y., and Collier, K. S. (2004). Reduced cortical noradrenergic neurotransmission is associated with increased neophobia and impaired spatial memory in aged rats. Neurobiol. Aging 25, 209–221. doi: 10.1016/S0197-4580(03)00042-3
Coradazzi, M., Gulino, R., Fieramosca, F., Falzacappa, L. V., Riggi, M., and Leanza, G. (2016). Selective noradrenaline depletion impairs working memory and hippocampal neurogenesis. Neurobiol. Aging 48, 93–102. doi: 10.1016/j.neurobiolaging.2016.08.012
Craig, A. D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 3, 655–666. doi: 10.1038/nrn894
Crottaz-Herbette, S., and Menon, V. (2006). Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J. Cogn. Neurosci. 18, 766–780. doi: 10.1162/jocn.2006.18.5.766
Damier, P., Hirsch, E., Agid, Y., and Graybiel, A. (1999). The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122, 1437–1448. doi: 10.1093/brain/122.8.1437
Davey, C. G., Pujol, J., and Harrison, B. J. (2016). Mapping the self in the brain's default mode network. Neuroimage 132, 390–397. doi: 10.1016/j.neuroimage.2016.02.022
Delacourte, A., and Defossez, A. (1986). Alzheimer's disease: Tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J. Neurol. Sci. 76, 173–186. doi: 10.1016/0022-510X(86)90167-X
Delaveau, P., Salgado-Pineda, P., Fossati, P., Witjas, T., Azulay, J., and Blin, O. (2010). Dopaminergic modulation of the default mode network in Parkinson's disease. Eur. Neuropsychopharmacol. 20, 784–792. doi: 10.1016/j.euroneuro.2010.07.001
DeNardo, L. A., Berns, D. S., DeLoach, K., and Luo, L. (2015). Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat. Neurosci. 18, 1687–1697. doi: 10.1038/nn.4131
Dexter, D. T., Carayon, A., Javoy-Agid, F., Agid, Y., Wells, F., Daniel, S., et al. (1991). Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114, 1953–1975. doi: 10.1093/brain/114.4.1953
Disbrow, E. A., Carmichael, O., He, J., Lanni, K., Dressler, E., Zhang, L., et al. (2014). Resting state functional connectivity is associated with cognitive dysfunction in non-demented people with Parkinson's disease. J. Parkinson's Dis. 4, 453–465. doi: 10.3233/JPD-130341
Downar, J., Crawley, A. P., Mikulis, D. J., and Davis, K. D. (2000). A multimodal cortical network for the detection of changes in the sensory environment. Nat. Neurosci. 3, 277–283. doi: 10.1038/72991
Downar, J., Crawley, A. P., Mikulis, D. J., and Davis, K. D. (2001). The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. Neuroimage 14, 1256–1267. doi: 10.1006/nimg.2001.0946
Duann, J. R., Ide, J. S., Luo, X., and Li, C. S. (2009). Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. J. Neurosci. 29, 10171–10179. doi: 10.1523/JNEUROSCI.1300-09.2009
Eisenberger, N. I., Lieberman, M. D., and Williams, K. D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science 302, 290–292. doi: 10.1126/science.1089134
Enochs, W. S., Petherick, P., Bogdanova, A., Mohr, U., and Weissleder, R. (1997). Paramagnetic metal scavenging by melanin: MR imaging. Radiology 204, 417–423 doi: 10.1148/radiology.204.2.9240529
Fair, D. A., Schlaggar, B. L., Cohen, A. L., Miezin, F. M., Dosenbach, N. U., Wenger, K. K., et al. (2007). A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. Neuroimage 35, 396–405. doi: 10.1016/j.neuroimage.2006.11.051
Fallon, J. H., Koziell, D. A., and Moore, R. Y. (1978). Catecholamine innervation of the basal forebrain II. Amygdala, suprarhinal cortex and entorhinal cortex. J. Comp. Neurol. 180, 509–531. doi: 10.1002/cne.901800308
Fearnley, J. M., and Lees, A. J. (1991). Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain 114, 2283–2301. doi: 10.1093/brain/114.5.2283
Fioravanti, V., Benuzzi, F., Codeluppi, L., Contardi, S., Cavallieri, F., Nichelli, P., et al. (2015). MRI correlates of Parkinson's disease progression: a voxel based morphometry study. Parkinson's Dis. 2015:378032. doi: 10.1155/2015/378032
Fox, M. D., and Raichle, M. E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711. doi: 10.1038/nrn2201
Francis, B. M., Yang, J., Hajderi, E., Brown, M. E., Michalski, B., McLaurin, J., et al. (2012). Reduced tissue levels of noradrenaline are associated with behavioral phenotypes of the TgCRND8 mouse model of Alzheimer's disease. Neuropsychopharmacology 37, 1934–1944. doi: 10.1038/npp.2012.40
Frisoni, G. B., Pievani, M., Testa, C., Sabattoli, F., Bresciani, L., Bonetti, M., et al. (2007). The topography of grey matter involvement in early and late onset Alzheimer's disease. Brain 130, 720–730. doi: 10.1093/brain/awl377
Gilam, G., Lin, T., Fruchter, E., and Hendler, T. (2017). Neural indicators of interpersonal anger as cause and consequence of combat training stress symptoms. Psychol. Med. 47, 1561–1572. doi: 10.1017/S0033291716003354
Gorges, M., Müller, H., Lulé, D., Pinkhardt, E. H., Ludolph, A. C., Kassubek, J., et al. (2015). To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiol. Aging 36, 1727–1735. doi: 10.1016/j.neurobiolaging.2014.12.026
Gour, N., Felician, O., Didic, M., Koric, L., Gueriot, C., Chanoine, V., et al. (2014). Functional connectivity changes differ in early and late-onset alzheimer's disease. Hum. Brain Mapp. 35, 2978–2994. doi: 10.1002/hbm.22379
Gour, N., Ranjeva, J., Ceccaldi, M., Confort-Gouny, S., Barbeau, E., Soulier, E., et al. (2011). Basal functional connectivity within the anterior temporal network is associated with performance on declarative memory tasks. Neuroimage 58, 687–697. doi: 10.1016/j.neuroimage.2011.05.090
Goveas, J. S., Xie, C., Chen, G., Li, W., Ward, B. D., Franczak, M. B., et al. (2013). Functional network endophenotypes unravel the effects of apolipoprotein E epsilon 4 in middle-aged adults. PLoS ONE 8:e55902. doi: 10.1371/journal.pone.0055902
Greicius, M. D., Krasnow, B., Reiss, A. L., and Menon, V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100, 253–258. doi: 10.1073/pnas.0135058100
Greig, S. L. (2015). Memantine ER/donepezil: a review in Alzheimer's disease. CNS Drugs 29, 963–970. doi: 10.1007/s40263-015-0287-2
Gulyás, B., Brockschnieder, D., Nag, S., Pavlova, E., Kása, P., Beliczai, Z., et al. (2010). The norepinephrine transporter (NET) radioligand (S, S)-[18F] FMeNER-D2 shows significant decreases in NET density in the human brain in Alzheimer's disease: a post-mortem autoradiographic study. Neurochem. Int. 56, 789–798. doi: 10.1016/j.neuint.2010.03.001
Halliday, G., McCann, H., and Shepherd, C. (2012). Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson's disease? Expert Rev. Neurother. 12, 673–686. doi: 10.1586/ern.12.47
Hawkes, C. (2003). Olfaction in neurodegenerative disorder. Mov. Disord. 18, 364–372. doi: 10.1002/mds.10379
He, X., Qin, W., Liu, Y., Zhang, X., Duan, Y., Song, J., et al. (2014). Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer's disease. Hum. Brain Mapp. 35, 3446–3464. doi: 10.1002/hbm.22414
Helmich, R. C., Thaler, A., van Nuenen, B. F., Gurevich, T., Mirelman, A., Marder, K. S., et al. (2015). Reorganization of corticostriatal circuits in healthy G2019S LRRK2 carriers. Neurology 84, 399–406. doi: 10.1212/WNL.0000000000001189
Helms, G., Draganski, B., Frackowiak, R., Ashburner, J., and Weiskopf, N. (2009). Improved segmentation of deep brain grey matter structures using magnetization transfer (MT) parameter maps. Neuroimage 47, 194–198. doi: 10.1016/j.neuroimage.2009.03.053
Heneka, M. T., Ramanathan, M., Jacobs, A. H., Dumitrescu-Ozimek, L., Bilkei-Gorzo, A., Debeir, T., et al. (2006). Locus ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J. Neurosci. 26, 1343–1354. doi: 10.1523/JNEUROSCI.4236-05.2006
Hernaus, D., Santa, M. M. C., Offermann, J. S., and Van Amelsvoort, T. (2017). Noradrenaline transporter blockade increases fronto-parietal functional connectivity relevant for working memory. Eur. Neuropsychopharmacol. 27, 399–410. doi: 10.1016/j.euroneuro.2017.02.004
Herz, D. M., Eickhoff, S. B., Løkkegaard, A., and Siebner, H. R. (2014). Functional neuroimaging of motor control in parkinson's disease: a meta-analysis. Hum. Brain Mapp. 35, 3227–3237. doi: 10.1002/hbm.22397
Hu, S., Ide, J. S., Zhang, S., and Li, C. S. (2015). Anticipating conflict: neural correlates of a Bayesian belief and its motor consequence. Neuroimage 119, 286–295. doi: 10.1016/j.neuroimage.2015.06.032
Ide, J., Cappabianco, F., Faria, F., and Li, C. S. (2017). “Detrended partial cross correlation for brain connectivity analysis,” in Anonymous Advances in Neural Information Processing Systems, (Long Beach, CA) 889–897.
Ide, J. S., and Li, C. S. (2011). A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage 54, 455–464. doi: 10.1016/j.neuroimage.2010.07.042
Ide, J. S., and Li, C. S. (2011). Error-related functional connectivity of the habenula in humans. Front. Hum. Neurosci. 5:25. doi: 10.3389/fnhum.2011.00025
Ide, J. S., and Li, C. R. (2018) Time scale properties of task resting-state functional connectivity: Detrended partial cross-correlation analysis. Neuroimage 173, 240–248. doi: 10.1016/j.neuroimage.2018.02.029
Insua, D., Suárez, M., Santamarina, G., Sarasa, M., and Pesini, P. (2010). Dogs with canine counterpart of Alzheimer's disease lose noradrenergic neurons. Neurobiol. Aging 31, 625–635. doi: 10.1016/j.neurobiolaging.2008.05.014
Isaias, I. U., Trujillo, P., Summers, P., Marotta, G., Mainardi, L., Pezzoli, G., et al. (2016). Neuromelanin imaging and dopaminergic loss in Parkinson's disease. Front. Aging Neurosci. 8:196. doi: 10.3389/fnagi.2016.00196
Izquierdo, I., Bevilaqua, L. R., Rossato, J. I., Lima, R. H., Medina, J. H., and Cammarota, M. (2008). Age-dependent and age-independent human memory persistence is enhanced by delayed posttraining methylphenidate administration. Proc. Natl. Acad. Sci. U.S.A. 105, 19504–19507. doi: 10.1073/pnas.0810650105
Jack, C. R., Barkhof, F., Bernstein, M. A., Cantillon, M., Cole, P. E., DeCarli, C., et al. (2011). Steps to standardization and validation of hippocampal volumetry as a biomarker in clinical trials and diagnostic criterion for Alzheimer's disease. Alzheimer's Dement. 7, 474.e4–485.e4. doi: 10.1016/j.jalz.2011.04.007
Jacobs, H. I., Radua, J., Lückmann, H. C., and Sack, A. T. (2013). Meta-analysis of functional network alterations in Alzheimer's disease: toward a network biomarker. Neurosci. Biobehav. Rev. 37, 753–765. doi: 10.1016/j.neubiorev.2013.03.009
Jacobs, H. I., Wiese, S., van de Ven, V., Gronenschild, E. H., Verhey, F. R., and Matthews, P. M. (2015). Relevance of parahippocampal-locus coeruleus connectivity to memory in early dementia. Neurobiol. Aging 36, 618–626. doi: 10.1016/j.neurobiolaging.2014.10.041
Jellinger, K., Braak, H., Braak, E., and Fischer, P. (1991). Alzheimer lesions in the entorhinal region and isocortex in Parkinson's and Alzheimer's diseases. Ann. N.Y. Acad. Sci. 640, 203–209. doi: 10.1111/j.1749-6632.1991.tb00218.x
Jellinger, K. A. (2009). A critical evaluation of current staging of α-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta 1792, 730–740. doi: 10.1016/j.bbadis.2008.07.006
Kaasinen, V., and Vahlberg, T. (2017). Striatal dopamine in Parkinson's disease: a meta-analysis of imaging studies. Ann. Neurol. 82, 873–882. doi: 10.1002/ana.25103
Kalaitzakis, M., Graeber, M., Gentleman, S., and Pearce, R. (2008). The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of α-synuclein staging. Neuropathol. Appl. Neurobiol. 34, 284–295. doi: 10.1111/j.1365-2990.2007.00923.x
Kalinin, S., Gavrilyuk, V., Polak, P. E., Vasser, R., Zhao, J., Heneka, M. T., et al. (2007). Noradrenaline deficiency in brain increases β-amyloid plaque burden in an animal model of Alzheimer's disease. Neurobiol. Aging 28, 1206–1214. doi: 10.1016/j.neurobiolaging.2006.06.003
Kalinin, S., Polak, P. E., Lin, S. X., Sakharkar, A. J., Pandey, S. C., and Feinstein, D. L. (2012). The noradrenaline precursor L-DOPS reduces pathology in a mouse model of Alzheimer's disease. Neurobiol. Aging 33, 1651–1663. doi: 10.1016/j.neurobiolaging.2011.04.012
Kaufmann, H., and Goldstein, D. S. (2013). Autonomic dysfunction in Parkinson disease. Handb. Clin. Neurol. 117, 259–278. doi: 10.1016/B978-0-444-53491-0.00021-3
Kehagia, A. A., Housden, C. R., Regenthal, R., Barker, R. A., Müller, U., Rowe, J., et al. (2014). Targeting impulsivity in Parkinson's disease using atomoxetine. Brain 137, 1986–1997. doi: 10.1093/brain/awu117
Kempadoo, K. A., Mosharov, E. V., Choi, S. J., Sulzer, D., and Kandel, E. R. (2016). Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci. U.S.A. 113, 14835–14840. doi: 10.1073/pnas.1616515114
Kim, H. J., Cha, J., Lee, J., Shin, J. S., Jung, N., Kim, Y. J., et al. (2016). Distinctive resting state network disruptions among Alzheimer's disease, subcortical vascular dementia, and mixed dementia patients. J. Alzheimer's Dis. 50, 709–718. doi: 10.3233/JAD-150637
Kish, S. J., Shannak, K. S., Rajput, A. H., Gilbert, J. J., and Hornykiewicz, O. (1984). Cerebellar norepinephrine in patients with Parkinson's disease and control subjects. Arch. Neurol. 41, 612–614. doi: 10.1001/archneur.1984.04210080020007
Kline, R. L., Zhang, S., Farr, O. M., Hu, S., Zaborszky, L., Samanez-Larkin, G. R., et al. (2016). The effects of methylphenidate on resting-state functional connectivity of the basal nucleus of meynert, locus coeruleus, and ventral tegmental area in healthy adults. Front. Hum. Neurosci. 10:149. doi: 10.3389/fnhum.2016.00149
Köhler, S., Bär, K., and Wagner, G. (2016). Differential involvement of brainstem noradrenergic and midbrain dopaminergic nuclei in cognitive control. Hum. Brain Mapp. 37, 2305–2318. doi: 10.1002/hbm.23173
Krebs, R. M., Fias, W., Achten, E., and Boehler, C. N. (2013). Picture novelty attenuates semantic interference and modulates concomitant neural activity in the anterior cingulate cortex and the locus coeruleus. Neuroimage 74, 179–187. doi: 10.1016/j.neuroimage.2013.02.027
Krebs, R. M., Park, H. R., Bombeke, K., and Boehler, C. N. (2017). Modulation of locus coeruleus activity by novel oddball stimuli. Brain Imaging Behav. 12, 577–584. doi: 10.1007/s11682-017-9700-4
Lee, E. Y., Sen, S., Eslinger, P. J., Wagner, D., Shaffer, M. L., Kong, L., et al. (2013). Early cortical gray matter loss and cognitive correlates in non-demented Parkinson's patients. Parkinsonism Relat. Disord. 19, 1088–1093. doi: 10.1016/j.parkreldis.2013.07.018
Leech, R., and Sharp, D. J. (2013). The role of the posterior cingulate cortex in cognition and disease. Brain 137, 12–32. doi: 10.1093/brain/awt162
Lehéricy, S., Sharman, M. A., Santos, C. L. D., Paquin, R., and Gallea, C. (2012). Magnetic resonance imaging of the substantia nigra in Parkinson's disease. Mov. Disord. 27, 822–830. doi: 10.1002/mds.25015
Leissring, M. A. (2016). Aβ-degrading proteases: therapeutic potential in Alzheimer disease. CNS Drugs 30, 667–675. doi: 10.1007/s40263-016-0364-1
Li, C. S., Yan, P., Bergquist, K. L., and Sinha, R. (2007). Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage 38, 640–648. doi: 10.1016/j.neuroimage.2007.07.021
Liddell, B. J., Brown, K. J., Kemp, A. H., Barton, M. J., Das, P., Peduto, A., et al. (2005). A direct brainstem–amygdala–cortical ‘alarm’system for subliminal signals of fear. Neuroimage 24, 235–243. doi: 10.1016/j.neuroimage.2004.08.016
Lin, H., and Gau, S. S. (2016). Atomoxetine treatment strengthens an anti-correlated relationship between functional brain networks in medication-naïve adults with attention-deficit hyperactivity disorder: a randomized double-blind placebo-controlled clinical trial. Int. J. Neuropsychopharmacol. 19:pyv094. doi: 10.1093/ijnp/pyv094
Linden, D. E., Prvulovic, D., Formisano, E., Völlinger, M., Zanella, F. E., Goebel, R., et al. (1999). The functional neuroanatomy of target detection, an fMRI study of visual and auditory oddball tasks. Cereb. Cortex 9, 815–823. doi: 10.1093/cercor/9.8.815
Linssen, A. M., Vuurman, E., Sambeth, A., and Riedel, W. (2012). Methylphenidate produces selective enhancement of declarative memory consolidation in healthy volunteers. Psychopharmacology 221, 611–619. doi: 10.1007/s00213-011-2605-9
Lissek, S., Glaubitz, B., Gunturkun, O., and Tegenthoff, M. (2015). Noradrenergic stimulation modulates activation of extinction-related brain regions and enhances contextual extinction learning without affecting renewal. Front. Behav. Neurosci. 9:34. doi: 10.3389/fnbeh.2015.00034
Liu, K. Y., Marijatta, F., Hämmerer, D., Acosta-Cabronero, J., Düzel, E., and Howard, R. J. (2017). Magnetic resonance imaging of the human locus coeruleus: a systematic review. Neurosci. Biobehav. Rev. 83, 325–355. doi: 10.1016/j.neubiorev.2017.10.023
Loughlin, S. E., Foote, S., and Grzanna, R. (1986). Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience 18, 307–319. doi: 10.1016/0306-4522(86)90156-9
Loughlin, S. E., Foote, S. L., and Fallon, J. H. (1982). Locus coeruleus projections to cortex: topography, morphology and collateralization. Brain Res. Bull. 9, 287–294. doi: 10.1016/0361-9230(82)90142-3
Lu, Y. T., Chang, W., Chang, C., Lu, C., Chen, N., Huang, C., et al. (2016). Insula volume and salience network are associated with memory decline in parkinson disease: complementary analyses of voxel-based morphometry versus volume of interest. Parkinson's Dis. 2016:2939528. doi: 10.1155/2016/2939528
Machulda, M. M., Jones, D. T., Vemuri, P., McDade, E., Avula, R., Przybelski, S., et al. (2011). Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol. 68, 1131–1136. doi: 10.1001/archneurol.2011.108
Manaye, K. F., McIntire, D. D., Mann, D., and German, D. C. (1995). Locus coeruleus cell loss in the aging human brain: a non-random process. J. Comp. Neurol. 358, 79–87. doi: 10.1002/cne.903580105
Marcyniuk, B., Mann, D., and Yates, P. (1986). Loss of nerve cells from locus coeruleus in Alzheimer's disease is topographically arranged. Neurosci. Lett. 64, 247–252. doi: 10.1016/0304-3940(86)90336-8
Mars, R. B., Neubert, F. X., Noonan, M. P., Sallet, J., Toni, I., and Rushworth, M. F. (2012). On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 6:189. doi: 10.3389/fnhum.2012.00189
Marsh, L., Biglan, K., Gerstenhaber, M., and Williams, J. R. (2009). Atomoxetine for the treatment of executive dysfunction in Parkinson's disease: a pilot open-label study. Mov. Disord. 24, 277–282. doi: 10.1002/mds.22307
Martin, W. R. (2009). Quantitative estimation of regional brain iron with magnetic resonance imaging. Parkinsonism Relat. Disord. 15(Suppl. 3), S215–S218. doi: 10.1016/S1353-8020(09)70818-1
Martin, W. R., Wieler, M., and Gee, M. (2008). Midbrain iron content in early Parkinson disease: a potential biomarker of disease status. Neurology 70, 1411–1417. doi: 10.1212/01.wnl.0000286384.31050.b5
Mascalchi, M., Vella, A., and Ceravolo, R. (2012). Movement disorders: role of imaging in diagnosis. J. Magn. Reson. Imaging 35, 239–256. doi: 10.1002/jmri.22825
Matsuda, H. (2013). Voxel-based Morphometry of Brain MRI in Normal Aging and Alzheimer's Disease. Aging Dis. 4, 29–37.
Matsuda, H., Kitayama, N., Ohnishi, T., Asada, T., Nakano, S., Sakamoto, S., et al. (2002). Longitudinal evaluation of both morphologic and functional changes in the same individuals with Alzheimer's disease. J. Nucl. Med. 43, 304–311.
Matsuura, K., Maeda, M., Yata, K., Ichiba, Y., Yamaguchi, T., Kanamaru, K., et al. (2013). Neuromelanin magnetic resonance imaging in Parkinson's disease and multiple system atrophy. Eur. Neurol. 70, 70–77. doi: 10.1159/000350291
Matthews, K. L., Chen, C. P., Esiri, M. M., Keene, J., Minger, S. L., and Francis, P. T. (2002). Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol. Psychiatry 51, 407–416. doi: 10.1016/S0006-3223(01)01235-5
Menke, R. A., Szewczyk-Krolikowski, K., Jbabdi, S., Jenkinson, M., Talbot, K., Mackay, C. E., et al. (2014). Comprehensive morphometry of subcortical grey matter structures in early-stage Parkinson's disease. Hum. Brain Mapp. 35, 1681–1690. doi: 10.1002/hbm.22282
Miyoshi, F., Ogawa, T., Kitao, S. I., Kitayama, M., Shinohara, Y., Takasugi, M., et al. (2013). Evaluation of Parkinson disease and Alzheimer disease with the use of neuromelanin MR imaging and (123)I-metaiodobenzylguanidine scintigraphy. AJNR Am. J. Neuroradiol. 34, 2113–2118. doi: 10.3174/ajnr.A3567
Moreno-Castilla, P., Pérez-Ortega, R., Violante-Soria, V., Balderas, I., and Bermúdez-Rattoni, F. (2017). Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus 27, 547–557. doi: 10.1002/hipo.22711
Mountcastle, V. B., LaMotte, R. H., and Carli, G. (1972). Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J. Neurophysiol. 35, 122–136. doi: 10.1152/jn.1972.35.1.122
Mukai, M., Sugaya, K., Yabe, I., Goto, Y., Yokochi, F., Miyamoto, K., et al. (2013). Neuromelanin MRI in a family with mitochondrial parkinsonism harboring a Y955C mutation in POLG1. Parkinsonism Relat. Disord. 19, 821–824. doi: 10.1016/j.parkreldis.2013.04.011
Neufang, S., Geiger, M. J., Homola, G. A., Mahr, M., Akhrif, A., Nowak, J., et al. (2015). Modulation of prefrontal functioning in attention systems by NPSR1 gene variation. Neuroimage 114, 199–206. doi: 10.1016/j.neuroimage.2015.03.064
Noh, Y., Jeon, S., Lee, J. M., Seo, S. W., Kim, G. H., Cho, H., et al. (2014). Anatomical heterogeneity of Alzheimer disease: based on cortical thickness on MRIs. Neurology 83, 1936–1944. doi: 10.1212/WNL.0000000000001003
Nombela, C., Rittman, T., Robbins, T. W., and Rowe, J. B. (2014). Multiple modes of impulsivity in Parkinson's disease. PLoS ONE 9:e85747. doi: 10.1371/journal.pone.0085747
Obeso, I., Wilkinson, L., Casabona, E., Bringas, M. L., Alvarez, M., Alvarez, L., et al. (2011). Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson's disease. Exp. Brain Res. 212, 371–384. doi: 10.1007/s00221-011-2736-6
Ohnishi, T., Matsuda, H., Tabira, T., Asada, T., and Uno, M. (2001). Changes in brain morphology in Alzheimer disease and normal aging: is Alzheimer disease an exaggerated aging process? AJNR Am. J. Neuroradiol. 22, 1680–1685.
Ohtsuka, C., Sasaki, M., Konno, K., Kato, K., Takahashi, J., Yamashita, F., et al. (2014). Differentiation of early-stage parkinsonisms using neuromelanin-sensitive magnetic resonance imaging. Parkinsonism Relat. Disord. 20, 755–760. doi: 10.1016/j.parkreldis.2014.04.005
Pan, P. L., Song, W., and Shang, H. (2012). Voxel-wise meta-analysis of gray matter abnormalities in idiopathic Parkinson's disease. Eur. J. Neurol. 19, 199–206. doi: 10.1111/j.1468-1331.2011.03474.x
Pavese, N., Rivero-Bosch, M., Lewis, S. J., Whone, A. L., and Brooks, D. J. (2011). Progression of monoaminergic dysfunction in Parkinson's disease: a longitudinal 18 F-dopa PET study. Neuroimage 56, 1463–1468. doi: 10.1016/j.neuroimage.2011.03.012
Perry, G., Kawai, M., Tabaton, M., Onorato, M., Mulvihill, P., Richey, P., et al. (1991). Neuropil threads of Alzheimer's disease show a marked alteration of the normal cytoskeleton. J. Neurosci. 11, 1748–1755. doi: 10.1523/JNEUROSCI.11-06-01748.1991
Petrella, J. R., Sheldon, F. C., Prince, S. E., Calhoun, V. D., and Doraiswamy, P. M. (2011). Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 76, 511–517. doi: 10.1212/WNL.0b013e31820af94e
Peyron, R., Laurent, B., and García-Larrea, L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol. Clin. 30, 263–288. doi: 10.1016/S0987-7053(00)00227-6
Phelps, E. A., Delgado, M. R., Nearing, K. I., and LeDoux, J. E. (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43, 897–905. doi: 10.1016/j.neuron.2004.08.042
Putcha, D., Ross, R. S., Cronin-Golomb, A., Janes, A. C., and Stern, C. E. (2016). Salience and default mode network coupling predicts cognition in aging and Parkinson's disease. J. Int. Neuropsychol. Soc. 22, 205–215. doi: 10.1017/S1355617715000892
Quiroz, Y. T., Schultz, A. P., Chen, K., Protas, H. D., Brickhouse, M., Fleisher, A. S., et al. (2015). Brain imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: a cross-sectional study. JAMA Neurol. 72, 912–919. doi: 10.1001/jamaneurol.2015.1099
Rabey, J. M., and Hefti, F. (1990). Neuromelanin synthesis in rat and human substantia nigra. J. Neural Trans. Park. Dis. Dement. Sect. 2, 1–14. doi: 10.1007/BF02251241
Rademacher, J., Engelbrecht, V., Bürgel, U., Freund, H., and Zilles, K. (1999). Measuring in vivo myelination of human white matter fiber tracts with magnetization transfer, MR. Neuroimage 9, 393–406. doi: 10.1006/nimg.1998.0416
Rae, C. L., Nombela, C., Rodríguez, P. V., Ye, Z., Hughes, L. E., Jones, P. S., et al. (2016). Atomoxetine restores the response inhibition network in Parkinson's disease. Brain 139, 2235–2248. doi: 10.1093/brain/aww138
Reimão, S., Pita Lobo, P., Neutel, D., Guedes, L. C., Coelho, M., Rosa, M. M., et al. (2015). Substantia nigra neuromelanin-MR imaging differentiates essential tremor from Parkinson's disease. Mov. Disord. 30, 953–959. doi: 10.1002/mds.26182
Rémy, F., Mirrashed, F., Campbell, B., and Richter, W. (2005). Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study. Neuroimage 25, 253–266. doi: 10.1016/j.neuroimage.2004.10.045
Rey, N. L., Jardanhazi-Kurutz, D., Terwel, D., Kummer, M. P., Jourdan, F., Didier, A., et al. (2012). Locus coeruleus degeneration exacerbates olfactory deficits in APP/PS1 transgenic mice. Neurobiol. Aging 33, 426.e1–426.e11. doi: 10.1016/j.neurobiolaging.2010.10.009
Robbins, T. (2000). From arousal to cognition: the integrative position of the prefrontal cortex. Prog. Brain Res. 126, 469–483. doi: 10.1016/S0079-6123(00)26030-5
Rommelfanger, K. S., and Weinshenker, D. (2007). Norepinephrine: the redheaded stepchild of Parkinson's disease. Biochem. Pharmacol. 74, 177–190. doi: 10.1016/j.bcp.2007.01.036
Rossi, M. E., Ruottinen, H., Saunamaki, T., Elovaara, I., and Dastidar, P. (2014). Imaging brain iron and diffusion patterns: a follow-up study of Parkinson's disease in the initial stages. Acad. Radiol. 21, 64–71 doi: 10.1016/j.acra.2013.09.018
Sala-Llonch, R., Fortea, J., Bartres-Faz, D., Bosch, B., Lladó, A., Pena-Gomez, C., et al. (2013). Evolving brain functional abnormalities in PSEN1 mutation carriers: a resting and visual encoding fMRI study. J. Alzheimer's Dis. 36, 165–175. doi: 10.3233/JAD-130062
Sarikaya, I. (2015). PET imaging in neurology: Alzheimer's and Parkinson's diseases. Nucl. Med. Commun. 36, 775–781. doi: 10.1097/MNM.0000000000000320
Sasaki, M., Shibata, E., Kudo, K., and Tohyama, K. (2008). Neuromelanin-sensitive MRI. Clin. Neuroradiol. 18, 147–153. doi: 10.1007/s00062-008-8018-4
Sasaki, M., Shibata, E., Tohyama, K., Takahashi, J., Otsuka, K., Tsuchiya, K., et al. (2006). Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport 17, 1215–1218. doi: 10.1097/01.wnr.0000227984.84927.a7
Schaeffer, E., and Berg, D. (2017). Dopaminergic therapies for non-motor symptoms in Parkinson's disease. CNS Drugs 31, 551–570. doi: 10.1007/s40263-017-0450-z
Schultz, D. H., Balderston, N. L., and Helmstetter, F. J. (2012). Resting-state connectivity of the amygdala is altered following Pavlovian fear conditioning. Front. Hum. Neurosci. 6:242. doi: 10.3389/fnhum.2012.00242
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007
Shannak, K., Rajput, A., Rozdilsky, B., Kish, S., Gilbert, J., and Hornykiewicz, O. (1994). Noradrenaline, dopamine and serotonin levels and metabolism in the human hypothalamus: observations in Parkinson's disease and normal subjects. Brain Res. 639, 33–41. doi: 10.1016/0006-8993(94)91761-2
Shibata, E., Sasaki, M., Tohyama, K., Kanbara, Y., Otsuka, K., Ehara, S., et al. (2006). Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn. Reson. Med. Sci. 5, 197–200. doi: 10.2463/mrms.5.197
Singer, T., Seymour, B., O'Doherty, J., Kaube, H., Dolan, R. J., and Frith, C. D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162. doi: 10.1126/science.1093535
Smith, C. C., and Greene, R. W. (2012). CNS dopamine transmission mediated by noradrenergic innervation. J. Neurosci. 32, 6072–6080. doi: 10.1523/JNEUROSCI.6486-11.2012
Song, A. H., Kucyi, A., Napadow, V., Brown, E. N., Loggia, M. L., and Akeju, O. (2017). Pharmacological modulation of noradrenergic arousal circuitry disrupts functional connectivity of the locus coeruleus in humans. J. Neurosci. 37, 6938–6945. doi: 10.1523/JNEUROSCI.0446-17.2017
Sridharan, D., Levitin, D. J., and Menon, V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U.S.A. 105, 12569–12574. doi: 10.1073/pnas.0800005105
Surmeier, D. J., Obeso, J. A., and Halliday, G. M. (2017). Parkinson's Disease Is Not Simply a Prion Disorder. J. Neurosci. 37, 9799–9807. doi: 10.1523/JNEUROSCI.1787-16.2017
Szot, P. (2012). Common factors among Alzheimer's disease, Parkinson's disease, and epilepsy: possible role of the noradrenergic nervous system. Epilepsia 53, 61–66. doi: 10.1111/j.1528-1167.2012.03476.x
Szot, P., White, S. S., Greenup, J. L., Leverenz, J. B., Peskind, E. R., and Raskind, M. A. (2006). Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer's disease and dementia with Lewy bodies. J. Neurosci. 26, 467–478. doi: 10.1523/JNEUROSCI.4265-05.2006
Tahmasian, M., Eickhoff, S. B., Giehl, K., Schwartz, F., Herz, D. M., Drzezga, A., et al. (2017). Resting-state functional reorganization in Parkinson's disease: an activation likelihood estimation meta-analysis. Cortex 92, 119–138. doi: 10.1016/j.cortex.2017.03.016
Takahashi, J., Shibata, T., Sasaki, M., Kudo, M., Yanezawa, H., Obara, S., et al. (2015). Detection of changes in the locus coeruleus in patients with mild cognitive impairment and Alzheimer's disease: high-resolution fast spin-echo T1-weighted imaging. Geriatr. Gerontol. Int. 15, 334–340. doi: 10.1111/ggi.12280
Takeuchi, T., Duszkiewicz, A. J., Sonneborn, A., Spooner, P. A., Yamasaki, M., Watanabe, M., et al. (2016). Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362. doi: 10.1038/nature19325
Theofilas, P., Ehrenberg, A. J., Dunlop, S., Di Lorenzo Alho, A. T., Nguy, A., Leite, R. E. P., et al. (2017). Locus coeruleus volume and cell population changes during Alzheimer's disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimer's Dement. 13, 236–246. doi: 10.1016/j.jalz.2016.06.2362
Thomas, J. B., Brier, M. R., Bateman, R. J., Snyder, A. Z., Benzinger, T. L., Xiong, C., et al. (2014). Functional connectivity in autosomal dominant and late-onset Alzheimer disease. JAMA Neurol. 71, 1111–1122. doi: 10.1001/jamaneurol.2014.1654
Thomson, A. M., and Lamy, C. (2007). Functional maps of neocortical local circuitry. Front. Neurosci. 1, 19–42. doi: 10.3389/neuro.01.1.1.002.2007
Tohgi, H., Abe, T., and Takahashi, S. (1993a). The effects of L-threo-3, 4-dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J. Neural Trans. Parkinson's Dis. Dement. Sect. 5, 27–34. doi: 10.1007/BF02260912
Tohgi, H., Abe, T., Takahashi, S., Takahashi, J., Nozaki, Y., Ueno, M., et al. (1993b). Monoamine metabolism in the cerebrospinal fluid in Parkinson's disease: relationship to clinical symptoms and subsequent therapeutic outcomes. J. Neural Trans. Parkinson's Dis. Dement. Sect. 5, 17–26. doi: 10.1007/BF02260911
Tomlinson, B., Irving, D., and Blessed, G. (1981). Cell loss in the locus coeruleus in senile dementia of Alzheimer type. J. Neurol. Sci. 49, 419–428. doi: 10.1016/0022-510X(81)90031-9
Tosk, J. M., Holshouser, B. A., Aloia, R. C., Hinshaw, D. B., Hasso, A. N., MacMurray, J. P., et al. (1992). Effects of the interaction between ferric iron and L-dopa melanin on T1 and T2 relaxation times determined by magnetic resonance imaging. Magn. Reson. Med. 26, 40–45. doi: 10.1002/mrm.1910260105
Tribl, F., Asan, E., Arzberger, T., Tatschner, T., Langenfeld, E., Meyer, H. E., et al. (2009). Identification of L-ferritin in neuromelanin granules of the human substantia nigra: a targeted proteomics approach. Mol. Cell. Proteomics 8, 1832–1838. doi: 10.1074/mcp.M900006-MCP200
Urry, H. L., van Reekum, C. M., Johnstone, T., Kalin, N. H., Thurow, M. E., Schaefer, H. S., et al. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 26, 4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006
van Eimeren, T., Monchi, O., Ballanger, B., and Strafella, A. P. (2009). Dysfunction of the default mode network in Parkinson disease: a functional magnetic resonance imaging study. Arch. Neurol. 66, 877–883. doi: 10.1001/archneurol.2009.97
van Waesberghe, J., Kamphorst, W., De Groot, C. J., Van Walderveen, M. A., Castelijns, J. A., Ravid, R., et al. (1999). Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann. Neurol. 46, 747–54. doi: 10.1002/1531-8249(199911)46:5<747::AID-ANA10>3.0.CO;2-4
Veer, I. M., Beckmann, C. F., van Tol, M. J., Ferrarini, L., Milles, J., Veltman, D. J., et al. (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci. 4:41. doi: 10.3389/fnsys.2010.00041.
Verster, J. C., Bekker, E. M., Kooij, J. S., Buitelaar, J. K., Verbaten, M. N., Volkerts, E. R., et al. (2010). Methylphenidate significantly improves declarative memory functioning of adults with ADHD. Psychopharmacology 212, 277–281. doi: 10.1007/s00213-010-1952-2
Wang, Z., Xia, M., Dai, Z., Liang, X., Song, H., He, Y., et al. (2015). Differentially disrupted functional connectivity of the subregions of the inferior parietal lobule in Alzheimer's disease. Brain Struct. Funct. 220, 745–762. doi: 10.1007/s00429-013-0681-9
Weikop, P., Yoshitake, T., and Kehr, J. (2007). Differential effects of adjunctive methylphenidate and citalopram on extracellular levels of serotonin, noradrenaline and dopamine in the rat brain. Eur. Neuropsychopharmacol. 17, 658–671. doi: 10.1016/j.euroneuro.2007.02.014
Weintraub, D., Mavandadi, S., Mamikonyan, E., Siderowf, A. D., Duda, J. E., Hurtig, H. I., et al. (2010). Atomoxetine for depression and other neuropsychiatric symptoms in Parkinson disease. Neurology 75, 448–455. doi: 10.1212/WNL.0b013e3181ebdd79
Wen, X., Wu, X., Liu, J., Li, K., and Yao, L. (2013). Abnormal baseline brain activity in non-depressed Parkinson's disease and depressed Parkinson's disease: a resting-state functional magnetic resonance imaging study. PLoS ONE 8:e63691. doi: 10.1371/journal.pone.0063691
Whitehouse, P. J., Price, D. L., Clark, A. W., Coyle, J. T., and DeLong, M. R. (1981). Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126. doi: 10.1002/ana.410100203
Whitehouse, P. J., Price, D. L., Struble, R. G., Clark, A. W., Coyle, J. T., and Delon, M. R. (1982). Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239. doi: 10.1126/science.7058341
Whitwell, J. L., Dickson, D. W., Murray, M. E., Weigand, S. D., Tosakulwong, N., Senjem, M. L., et al. (2012). Neuroimaging correlates of pathologically defined subtypes of Alzheimer's disease: a case-control study. Lancet Neurol. 11, 868–877. doi: 10.1016/S1474-4422(12)70200-4
Wilson, R. S., Nag, S., Boyle, P. A., Hizel, L. P., Yu, L., Buchman, A. S., et al. (2013). Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 80, 1202–1208. doi: 10.1212/WNL.0b013e3182897103
Wolff, S. D., and Balaban, R. S. (1989). Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn. Reson. Med. 10, 135–144. doi: 10.1002/mrm.1910100113
Wu, T., Wang, J., Wang, C., Hallett, M., Zang, Y., Wu, X., et al. (2012). Basal ganglia circuits changes in Parkinson's disease patients. Neurosci. Lett. 524, 55–59. doi: 10.1016/j.neulet.2012.07.012
Ye, Z., Altena, E., Nombela, C., Housden, C. R., Maxwell, H., Rittman, T., et al. (2015). Improving response inhibition in Parkinson's disease with atomoxetine. Biol. Psychiatry 77, 740–748. doi: 10.1016/j.biopsych.2014.01.024
Zhang, S., Hu, S., Chao, H. H., and Li, C. R. (2016). Resting-state functional connectivity of the locus coeruleus in humans: in comparison with the ventral tegmental area/substantia nigra pars compacta and the effects of age. Cereb. Cortex 26, 3413–3427. doi: 10.1093/cercor/bhv172
Zhang, S., and Li, C. S. (2010). A neural measure of behavioral engagement: task-residual low-frequency blood oxygenation level-dependent activity in the precuneus. Neuroimage 49, 1911–1918. doi: 10.1016/j.neuroimage.2009.09.004
Zhang, S., and Li, C. S. (2014). Functional clustering of the human inferior parietal lobule by whole-brain connectivity mapping of resting-state functional magnetic resonance imaging signals. Brain Connect. 4, 53–69. doi: 10.1089/brain.2013.0191
Zhang, S., and Li, C. S. (2017). Functional connectivity parcellation of the human thalamus by independent component analysis. Brain Connect. 7, 602–616. doi: 10.1089/brain.2017.0500
Zhang, S., and Li, C. S. (2012a). Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage 59, 3548–3562. doi: 10.1016/j.neuroimage.2011.11.023
Zhang, S., and Li, C. S. (2012b). Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions. Front. Psychol. 3:172. doi: 10.3389/fpsyg.2012.00172
Zhao, Q., Li, H., Yu, X., Huang, F., Wang, Y., Liu, L., et al. (2017). Abnormal resting-state functional connectivity of insular subregions and disrupted correlation with working memory in adults with attention deficit/hyperactivity disorder. Front. Psychiatry 8:200. doi: 10.3389/fpsyt.2017.00200
Zhao, S., Rangaprakash, D., Venkataraman, A., Liang, P., and Deshpande, G. (2017). Investigating focal connectivity deficits in Alzheimer's disease using directional brain networks derived from resting-state fMRI. Front. Aging Neurosci. 9:211. doi: 10.3389/fnagi.2017.00211
Keywords: norepinephrine, dopamine, neurodegeneration, neurodegenerative, locus coeruleus, ventral tegmental area, midbrain, MRI
Citation: Peterson AC and Li CR (2018) Noradrenergic Dysfunction in Alzheimer's and Parkinson's Diseases—An Overview of Imaging Studies. Front. Aging Neurosci. 10:127. doi: 10.3389/fnagi.2018.00127
Received: 21 December 2017; Accepted: 16 April 2018;
Published: 01 May 2018.
Edited by:
Fernanda Laezza, University of Texas Medical Branch, United StatesReviewed by:
Richard Camicioli, University of Alberta, CanadaYoland Smith, Emory University, United States
Copyright © 2018 Peterson and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiang-shan R. Li, Y2hpYW5nLXNoYW4ubGlAeWFsZS5lZHU=
†ORCID 0000-0002-9393-1212
 Andrew C. Peterson
Andrew C. Peterson Chiang-shan R. Li
Chiang-shan R. Li