- Division of Neurosurgery, Department of Surgery, University Malaya, Kuala Lumpur, Malaysia
The pedunculopontine nucleus (PPN) is situated in the upper pons in the dorsolateral portion of the ponto-mesencephalic tegmentum. Its main mass is positioned at the trochlear nucleus level, and is part of the mesenphalic locomotor region (MLR) in the upper brainstem. The human PPN is divided into two subnuclei, the pars compacta (PPNc) and pars dissipatus (PPNd), and constitutes both cholinergic and non-cholinergic neurons with afferent and efferent projections to the cerebral cortex, thalamus, basal ganglia (BG), cerebellum, and spinal cord. The BG controls locomotion and posture via GABAergic output of the substantia nigra pars reticulate (SNr). In PD patients, GABAergic BG output levels are abnormally increased, and gait disturbances are produced via abnormal increases in SNr-induced inhibition of the MLR. Since the PPN is vastly connected with the BG and the brainstem, dysfunction within these systems lead to advanced symptomatic progression in Parkinson's disease (PD), including sleep and cognitive issues. To date, the best treatment is to perform deep brain stimulation (DBS) on PD patients as outcomes have shown positive effects in ameliorating the debilitating symptoms of this disease by treating pathological circuitries within the parkinsonian brain. It is therefore important to address the challenges and develop this procedure to improve the quality of life of PD patients.
The Pedunculopontine Nucleus
The pedunculopontine nucleus (PPN) is situated in the upper pons in the dorsolateral part of the ponto-mesencephalic tegmentum. Its main mass is located at the level of trochlear nucleus, and is part of the mesenphalic locomotor region (MLR) in the upper brainstem (Olszewski and Baxter, 1954; Geula et al., 1993). Olszewski and Baxter (1954) divided the human PPN into two subnuclei, the pars compacta (PPNc) and pars dissipatus (PPNd). The PPNc is more prominent with a compact cluster of large neurons, whereas the PPNd is composed of small and medium-sized neurons scattered inside the superior cerebellar peduncle (SCP) and central tegmental tract (Olszewski and Baxter, 1954). The PPN comprises both cholinergic and non-cholinergic neurons, and possesses afferent and efferent projections to the cerebral cortex, thalamus, basal ganglia (BG), cerebellum, and spinal cord.
Eighty to ninety percentage of the PPNc contains cholinergic neurons amassed along the dorsolateral border of the SCP at trochlear nucleus levels with few dopaminergic neurons (Jones, 1991; Pahapill and Lozano, 2000; Winn, 2008). These thin unmyelinated axons diverge extensively over the brain supply nuclei in the BG, cerebellum, reticular formation in the lower brainstem, and also the spinal cord (Stein, 2009). PPNd neurons dispersed along the SCP from mid-encephalic to mid-pontine levels constitute mainly glutamatergic neurons (Rye et al., 1987; Lavoie and Parent, 1994a) while the rest are cholinergic (Mesulam et al., 1989).
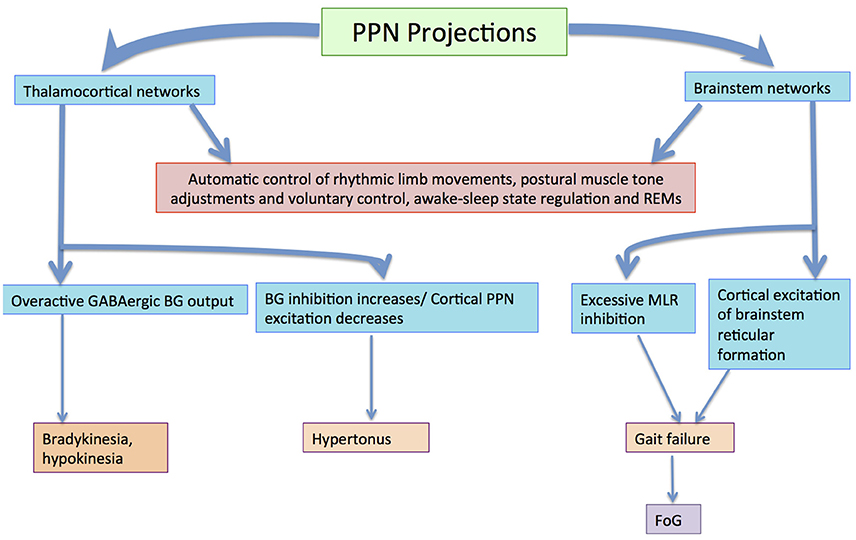
The PPNc and PPNd also possess GABAergic inhibitory neurons, whereas cholinergic neurons also contain neuropeptides and novel neuromodulators (Vincent et al., 1986; Vincent and Kimura, 1992; Lavoie and Parent, 1994a,b,c; Bevan and Bolam, 1995). The PPN possesses ascending and descending afferent and efferent (see Figure 1) projections, and PPN inputs approach from above and below its level. Descending networks from the cerebral cortex project via the BG and extrapyramidal system to the PPN, including the face, arm, trunk, and leg areas of the motor cortex (MCx), specifically Brodmann area 4 (von Monakow et al., 1979). The PPNc is also a primary constituent in a feedback loop to the thalamus from the spinal cord and limbic system (Pahapill and Lozano, 2000) and is a component of the ascending reticular activating system (ARAS), where cortical stimulation is modulated via ascending cholinergic connections to the thalamus (Steriade, 2004).
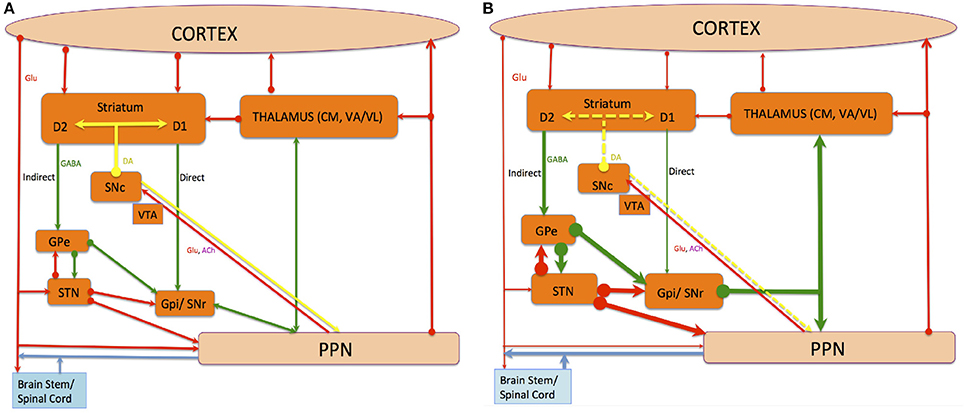
Figure 1. The connections of The PPN, and the direct and indirect pathway of BG-thalamocortical circuits under normal (A) and PD (B) conditions. Red, green, and yellow lines denote glutamatergic, GABAergic and dopaminergic projections respectively, while blue lines indicate chemically amalgamated projections. Thickening lines show increased activity whereas thinning lines show decreased activity when alterations occur in the average activity rate of specific projection pathways in PD compared with the normal state. Dotted yellow lines indicate loss. The striatum and STN deliver input from incoming cortical information to the BG. The GPi and SNr deliver output information from the BG to the rest of the brain and apply robust inhibitory control on targets in the thalamus and the brainstem. This tonic inhibitory input must be disinhibited to permit normal movements to occur. The striatum applies opposite influences on the GPi and SNr via two distinct classes of efferent neurons, namely the D1-receptor-rich “direct pathway” positively modulated by DA and the D2-receptor-rich “indirect pathway” negatively modulated by DA. The loss of DA in PD's causes disequilibrium in the activity of these two striatofugal pathways and their corresponding cortical inputs.
The PPN enhances the movement, motivational, and cognitive aspects of multifaceted behavioral responses (Garcia-Rill, 1986; Inglis and Winn, 1995; Reese et al., 1995; Takakusaki et al., 2004a). Stimulation of this area induces locomotion in animals, whereas damage leads to a number of neurological disorders included in Parkinson's disease (PD), Alzheimer's disease and schizophrenia due to its close ties with the BG and thalamus.
PPN Connectivity and Physiology
Motor Cortical Connections
The PPN possesses dense connections to the upper extremity regions of the MCx, followed by the lower extremity, trunk, and orofacial regions. Connections are more dense in the pre-MCx and frontal lobe compared to other regions (Muthusamy et al., 2007). Reciprocal connections also exist with the ipsilateral prefrontal MCx (Pahapill and Lozano, 2000). The PPN also obtains direct cortical afferent fibers from the primary and somatosensory motor area, pre-supplementary, dorsal, and ventral pre-MCx, as well as frontal eye fields (Kuypers and Lawrence, 1967; Moonedley and Graybiel, 1980; Matsumura et al., 2000). These connections implicate the PPN in cortical functions such as movement, cognition and sleep.
Striatal Connections
PPN efferent projections contacting the striatum are poorly arborized, excluding the ventral and peri-pallidal zone of the putamen (Lavoie and Parent, 1994c). These connections indicate that the PPN is also involved in limbic function. This is seen in the ventral striatum, also known as the nucleus accumbens (NAcc). Ascending PPN connections provide control over striatal input and output via connections with the thalamus and cerebral cortex, even in the absence of direct projections (Winn, 1998). PPN stimulation increases bursting activity in the NAcc (Floresco et al., 2003), where changes in release accompanying different firing patterns reveal two forceful conditions in dopamine (DA) levels in the striatum and NAcc. This is namely a tonic state with truncated but stable DA levels, and a phasic state correlated to behavioral actions and reactions to environmental stimuli. PPN cholinergic inputs therefore provide a functional duality ensuring the basal level of DA neurons response, whether stimulus-specific or anatomically diffuse. This also determines the response required in precise circumstances (Mena-Segovia et al., 2008b).
Thalamic Connections
Ascending PPN outputs project via the ventral and dorsal tegmental bundle pathways carry major cholinergic projections (Garcia-Rill, 1991) to all thalamic nuclei (Lavoie and Parent, 1994b). Strong cholinergic innervations to the intralaminar and reticular nuclei were also revealed (Mesulam et al., 1992a). These studies suggest that the thalamus obtains major cholinergic PPN input, especially toward “nonspecific” nuclei associated with the ARAS. The ARAS (Moruzzi and Magoun, 1949) stimulates the cortex using cholinergic input to the thalamus largely via PPN cholinergic cells (Pare et al., 1988; Steriade et al., 1988). This projection then travels to non-specific thalamic nuclei and produces rapid cortical oscillatory activity associated with arousal and rapid eye movement sleep (REMS) (Steriade, 2004). This stimulates reticular formation neurons in a positive-feedback procedure, whereas termination is induced through inhibitory activity of REMS-off aminergic neurons via REMS-on stimulated neuronal regulation positioned in the laterodorsal tegmental nucleus (LDT), and PPN regions (French and Muthusamy, 2016).
Pallidal Connections
The globus pallidus interna (GPi) of the globus pallidum (GP) sends inhibitory efferent fibers to the ipsilateral PPN. Anterograde tracer studies reveal that the PPN sends substantial efferent fibers to the GPi (Lavoie and Parent, 1994b) rather than the globus pallidus externa (GPe). In humans, the GP receives cholinergic innervations from the brainstem (Mesulam et al., 1983). Pallidal efferent pathways descend along the pallidotegmental tract to the Forel's field before dividing into the medial & lateral descending pathway into the PPN and midbrain tegmentum. The medial pathway then joins the medial longitudinal fasciculus in the pre-rubral field & terminates in the PPN, whereas the lateral pathway descends in the ventrolateral tegmentum before intermingling with the medial lemniscus and terminating in the PPN (Carpenter, 1976; DeVito et al., 1980).
Nigral Connections
Afferent gamma aminobutyric acid (GABA) endings from the substantia nigra (SN) profusely contact with synapses of PPN cell bodies and dendrites (Granata and Kitai, 1991). Reciprocally, the PPN sends efferent glutamatergic and cholinergic fibers to dopaminergic SN pars compacta (SNc) neurons (Charara et al., 1996) via multiple contacts with dendrites and cell bodies (Bolam et al., 1991; Mesulam et al., 1992b; Charara et al., 1996). These connections propose that strong excitatory influences on dopaminergic SNc neurons exerted from the PPN as pedunculonigral fibers branch more profusely in the SNc than SN pars reticulata (SNr).
The PPN also receives DA innervation over its anteroposterior extent (Rolland et al., 2009) from the SNc at posterior and mediodorsal levels, crossing through the medial lemniscus and reticular formation. These fibers tend to avoid cholinergic cell bodies but converge in neighboring non-cholinergic PPN parts through an anteroposterior and ventrodorsal gradient, particularly in the ventral cuneiform nucleus (CuN) located dorsally to the PPN. Furthermore, cell bodies analogous to the dopaminergic peri- and retrorubral cell clusters decrease rapidly posteriorly in the anterior PPN. Lavoie and Parent (1994b) also report that DA and cholinergic cells dominate adjoining but definite regions, with the dopaminergic population more anteriorly and laterodorsally located. Thus, the PPN along with the CuN receives dopaminergic innervation, endorsing that DA has a role in neural activity modulation of these structures. Intriguingly, DA fibers are heterogeneously dispersed, with central concentrations in the non-cholinergic PPN and ventral CuN border. This implies that functions such as postural muscle tone controlled by the PPN or locomotion via the CuN (Takakusaki et al., 2003) are directly influenced by DA. Furthermore, PPN and nigral dopaminergic neurons ascertain a direct loop moderating motor activity as both the cholinergic and especially non-cholinergic PPN project back to dopaminergic neurons of the SNc and ventral tegmental area (VTA) (Lavoie and Parent, 1994b; Mena-Segovia et al., 2008a).
PPN cholinergic projections have an expansive effect upon midbrain DA systems innervating both SNc and VTA neurons. Though less significant in controlling burst firing and population action of DA neurons, PPN neurons could be associated with sustaining the muscarinic-dependent tonic discharge of DA and specifying DA neuronal phasic signals to time sensory events. This suggests a responsibility for the PPN in associative learning. These phasic signals most likely work as a part of the ARAS in contribution of acetylcholine (ACh), to thalamocortical neuronal coherence in sensory stimuli integration.
PPN connections to the SNc and VTA alters DA release in different regions of the striatum, further affecting striatal inputs such as the cortex and thalamus. This modifies activity throughout the BG that eventually leads to behavioral changes. PPN afferents increase the number of neuronal burst firing in the VTA, though only in neurons that are already firing (Floresco et al., 2003). Extensive bilateral cholinergic innervation is also observed in the VTA, deriving primarily from the LDT and caudal PPN. An ipsilateral cholinergic projection originating from less dense regions of the cholinergic group projects to the SN. Cholinergic and glutamatergic PPN cells projecting to the SN and VTA (Beninato and Spencer, 1987; Bolam et al., 1991; Futami et al., 1995). This activates midbrain DA cells with short latencies (Scarnati et al., 1984; Lokwan et al., 1999) and evokes DA release in dopaminergic innervation areas (Forster and Blaha, 2003). Such topography indicates that cholinergic outflow from the PPN to functionally different systems vary depending on where afferent input is received. Input to dense cores of the group appears to affect cholinergic outflow to the mesolimbic DA system rather than the nigrostriatal system. However, this does not negate its influence on the nigrostriatal system as SN-projecting cells are also found throughout areas containing cholinergic cells. This implies that input received by an SN-projecting cell is more likely to affect the ipsilateral nigrostriatal system rather than the contralateral side. The VTA, however, appears to receive input from both sides of the cholinergic group. The identification of a distinct difference in cholinergic innervation of the SN and VTA relays important information on how cholinergic systems regulate CNS-controlling behavioral states including arousal and motor functions (Steriade and Buzsaki, 1990). Relative to this, the PPN also works with a parallel cholinergic input arising from the LDT. The PPN is thus part of two interrelated systems arising from cholinergic brainstem neurons modulating DA systems in the midbrain, another of which is the LDT.
Subthalamic Nuclei Connections
Glutamatergic afferents from the subthalamic nucleus (STN) to the PPN function through a positive feed-forward circuit arising from PPN cholinergic neurons. These projections converge with inputs from the cortex and GPe, affecting the activity of direct and indirect pathways (Bevan and Bolam, 1995).
A subpopulation of PPN neurons with ascending projections to the STN are distinct from neurons with descending projections to the gigantocellular reticular nucleus (GiN) (Mena-Segovia et al., 2008a; Ros et al., 2010). PPN projections are predominantly discrete to these two motor components, although cholinergic and non-cholinergic projections also surface from neurons within similar areas (Mena-Segovia et al., 2008a; Ros et al., 2010). This suggests that projection neurons in both pathways interact with each other, advocating an integrative role within PPN microcircuits. Similarly, the distribution of cholinergic, GABAergic, and glutamatergic neurons (Mena-Segovia et al., 2009; Wang and Morales, 2009; Martinez-Gonzalez et al., 2012) suggests that the rostral PPN is chiefly inhibitory being GABAergic, while the caudal PPN is chiefly excitatory being glutamatergic. Hence, motor projections to the STN and GiN are primarily glutamatergic with distinctive subtypes as they contain a diverse balance of calcium-binding proteins. Nonetheless, GABAergic constituents also exist (Bevan and Bolam, 1995). The quantity of cholinergic neurons in the caudal PPN is larger connecting to both targets, suggesting that cholinergic-mediated excitation of motor structures arise from the caudal PPN. Descending non-cholinergic neurons showed distinct electrophysiological properties compared to ascending non-cholinergic neurons, supporting the existence of functional differentiations concerning these two routes (Ros et al., 2010). Thus, descending PPN projections mediated via reticulospinal neurons of the GiN excites inhibitory interneurons in the spinal cord and modulate excitatory MLR output (Takakusaki et al., 2004a; Takakusaki, 2008). This concurs that the PPN is implicated in locomotion initiation (see Figure 1A).
Cerebellar and Spinal Cord Connections
Efferent fibers from deep cerebellar nuclei send collaterals to the PPN before reaching the thalamus (Hazrati and Parent, 1992), suggesting that the PPN acts as a well-designed consolidation epicenter between the BG and the cerebellum. Matsumura et al. (1997) also suggests that the PPN acts as a dispatch between the cerebral cortex and spinal cord, performing as a brainstem regulator center for interlimb movement synchronization and bimanual motor performance (Matsumura et al., 1997).
Parkinson's Disease
PD is a collection of neurodegenerative conditions affecting the brain, particularly pigmented nuclei in the extrapyramidal system of the midbrain and brainstem, the olfactory tubercle, cerebral cortex, and components of the peripheral nervous system (Braak et al., 2003). Ultimate physical debilities ensuing from these pathologies are motor deficiencies termed “parkinsonism.” These comprise dearth and movement slowness, known as akinesia and bradykinesia, muscle rigidity and resting tremor. Parkinsonism is produced primarily through BG functional impairments.
Principally, these problems result from dopaminergic neuronal degeneration in the midbrain leading to DA deficiency in areas receiving dopaminergic inputs, specifically from the post-commissural putamen and other BG areas (Braak et al., 2003). However, before dopaminergic degeneration occurs in the midbrain, Lewy neurites (LNs), and bodies (LBs), first form in the non-catecholaminergic dorsal glossopharyngeus-vagus complex and intermediate reticular zone projection neurons, and exclusive gain setting system nerve cell types, which are the coeruleus-subcoeruleus complex, caudal raphe nuclei, GiN, and olfactory bulb, tract, and/or anterior nucleus before nigral involvement (Del Tredici et al., 2002). This is possibly why PD patients develop anosmia during initial stages. This multisystem disorder first involves few susceptible nerve cell types in particular areas of the human nervous system, where the intracerebral development of abnormal proteinaceous LBs and LNs commences at definite locations and progress in a topographic order (Braak and Del Tredici, 2004). As the disease advances, constituents of the autonomic, limbic, and somatomotor systems become increasingly compromised. During pre-symptomatic stages 1–2, LB inclusion pathology is constricted to the medulla oblongata/pontine tegmentum and olfactory bulb/anterior nucleus. This means that SN involvement presumes an obvious prevailing pathology in the medulla oblongata. If it were possible to detect PD during this stage with an underlying therapy available, consequent neuronal loss in the SN could probably be prevented (Braak et al., 2003). In stages 3–4, the SN and other midbrain gray nuclei and forebrain undergo severe pathological changes as the process develops in an ascending manner traversing the upper border of the pontine tegmentum and enters midbrain and forebrain basal portions. More explicitly, the very first solitary LNs are observed in the SNc leading to granular aggregations, pale bodies, and LBs in melanized projection neurons, all of which are thin and sparsely myelinated axons (Braak et al., 2004). Classically, nigral pathology initiates in the SNc postero-lateral subnucleus (Braak and Braak, 1985; Gibb and Lees, 1991) and continue on in the postero-superior and posteromedial subnuclei, circumventing the SN magnocellular and anterior subnuclei while causing trivial lesions (Braak et al., 2003). Nuclear gray pathology from earlier stages is now severely exacerbated. Concurrently, the process impinges on the central amygdala subnucleus before extending into basolateral nuclei. LN complexes progressively fill the central subnucleus and characterize it off from contiguous structures (Sims and Williams, 1990; Amaral et al., 1992; Braak et al., 1994; Bohus et al., 1996). Other brain regions involved include the cholinergic PPN (Garcia-Rill, 1991; Inglis and Winn, 1995; Rye, 1997; Pahapill and Lozano, 2000), oral raphe nuclei, cholinergic magnocellular nuclei of the basal forebrain (Candy et al., 1983; Whitehouse et al., 1983; Mesulam et al., 1992a), and hypothalamic tuberomamillary nucleus (Del Tredici and Braak, 2004).
Excluding the SN and PPN, other striatal loop axes begin early myelination and oppose undergoing pathological changes (Braak et al., 2004). At stage 4, the poorly myelinated temporal mesocortex involving the transentorhinal region between the allocortex and neocortex is engaged in disease development for the first time (Braak et al., 2003). Most patients transcend into the symptomatic stages at this juncture. In the last stages 5–6, the disease reveals itself in all of its clinical dimensions as the process crosses the mature neocortex (Braak et al., 2004). During this stage, a plexus of LNs develop in the second sector of Ammon's horn (Dickson et al., 1994). This feature is typical of stages 4–6 that even when sections through the SN are unavailable, PD can be diagnosed based on Ammon's horn lesions alone (Del Tredici and Braak, 2004). During these final stages, the neurodegenerative progression reaches its supreme topographic degree. The SN appears practically stripped of melanoneurons, appearing colorless upon macroscopic inspection (Braak et al., 2004).
The BG and PD
The BG comprises the neostriatum containing the caudate nucleus (CN) and putamen, the GP containing the GPe and GPi, the STN, and the SN consisting of the SNr and SNc. These structures contribute to anatomically and functionally isolated loops involving certain thalamic and cortical regions. These parallel circuits differ based on the cortical function involved and are separated into “motor,” “associative,” and “limbic” loops (Alexander et al., 1986; Alexander and Crutcher, 1990; Middleton and Strick, 2000; Kelly and Strick, 2004). Reciprocal projections concerning the striatum and GPi/SNr are divided into two distinct pathways, namely a “direct” monosynaptic connection, and an “indirect” projection via the interpolated GPe/STN. GPi/SNr output projects mainly to ventral anterior (VA) and ventrolateral (VL) thalamic nuclei, which project to the cerebral cortex. Minor BG projections extend to the intralaminar centromedian and parafascicular thalamic nuclei, and brainstem structures such as the superior colliculus (SC), PPN, and reticular formation. The striatum also obtains prominent dopaminergic SNc input (Galvan and Wichmann, 2008). The BG are a major brain system modulated by dopaminergic input from the SN (Albin et al., 1989) with profound effects on behavior.
The striatum and STN obtains glutamatergic afferents from exclusive cerebral or thalamic regions and transfer this information to BG output nuclei, namely the GPi and SNr. These BG output nuclei fire tonically and rapidly (DeLong and Georgopoulos, 2011), thus brain areas receiving inputs from the BG are continuously under strong tonic inhibitions (Hikosaka, 2007). Decreases in SNr/ GPi neuronal activity is caused by direct input from the neostriatum, which are also GABAergic and inhibitory (Yoshida and Precht, 1971; Hikosaka et al., 1993b). An appealing theory states that the BG's chief purpose is apt behavior selection (Hikosaka et al., 1993a; Mink, 1996; Nambu et al., 2002), where unwanted behaviors are subdued by SNr/GPi-induced inhibition preservation or increment whilst required behaviors are liberated by SNr/GPi-induced inhibition decrement or elimination. Patients with BG dysfunctions portray involuntary movement disorders such as tremor, dyskinesia, dystonia, chorea, athetosis, and ballism (Denny-Brown, 1968). These involuntary movements are instigated by a disruption of the SNr/GPi-induced inhibition, consistent with parkinsonian symptoms displaying involuntary tremulous movements, diminished muscular power whether in activation or not, impaired posture with an inclination to bend the trunk forwards, festination from walking to running or poverty and slowness of movement without paralysis (DeLong and Wichmann, 2007), where the senses and intellect are uninjured initially. However, these patients also display difficulty in initiating purposeful movements known as akinesia, or slow and small movements known as bradykinesia and hypokinesia (see Figure 2). This movement disorder is elicited by an incomplete disinhibition of the SNr/GPi-induced inhibition on thalamocortical systems (Burbaud et al., 1998; Stein, 2009), ensuing in gait disturbances with difficulties initiating or terminating walking (Azulay et al., 2002). Additionally, dyskinesias induced by the DA pre-cursor levodopa (L-DOPA) or DA agonist apomorphine, are concomitant with the inadequate suppression of BG GABAergic output (Nevet et al., 2004). This leads to abnormal oscillatory firing of motor neurons in the aforementioned areas, inducing tremor or other involuntary movements.
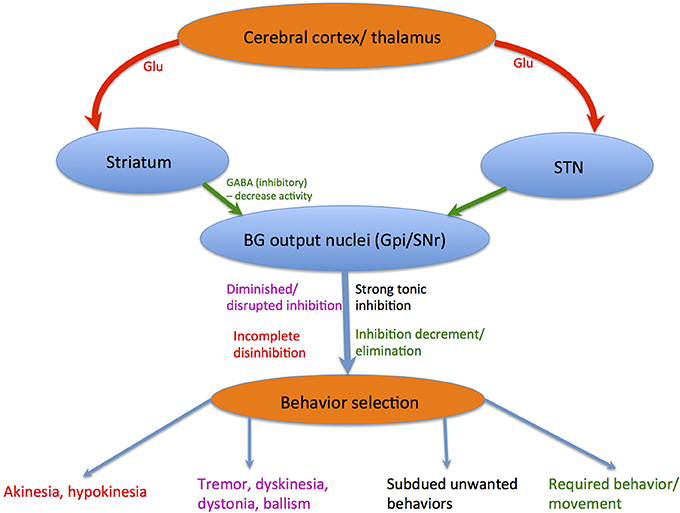
Figure 2. Behavior selection: Red lines depict glutamatergic pathways, whereas green lines depict GABAergic pathways. Blue lines depict chemically composite pathways. The corresponding colored notations show how each different movement disorder is elicited.
The BG is known for controlling locomotion and posture via SNr-GABAergic output (Takakusaki et al., 2004c). In PD patients, GABAergic BG output levels are abnormally increased (Miller and DeLong, 1987; DeLong, 1990; Filion, 1991). Takakusaki et al. (2004b) proposed that gait disturbances in PD are produced by abnormal increases in SNr-induced inhibition of the MLR. Furthermore, muscle rigidity might result from abnormally increased PPN inhibition that would otherwise produce muscle relaxation (see Figure 1B). Dystonia could be triggered by BG GABAergic output diminution to the PPN, depicted by focal and involuntary muscle tone, posture, or movement changes (Starr et al., 2005). Augmented GABAergic outputs would thus overwhelm target areas including the SC, MLR, PPN, thalamo-cortical circuits, and feasibly mouth movement and vocalization centers, ensuing in akinesia or hypokinesia (see Figure 2).
Another reason for a deranged BG-GABAergic output in the SNr/GPi would result from inputs coming from the GPe/neostriatum (Hikosaka, 2007). These motor features are often accompanied by non-motor issues such as depression, anxiety, autonomic dysfunction, sleep disorders, and cognitive impairment as a result of DA deficiency in non-motor portions of the striatum and more widespread progressive pathologic changes in the brainstem, thalamus, and eventually, the cerebral cortex (Braak et al., 2003).
The PPN and BG-Brainstem System
Ascending PPN projections provide substantial innervation to the SNc, STN, and GP (Mehler and Nauta, 1974; Graybiel, 1977; Nomura et al., 1980; Saper and Loewy, 1982; Edley and Graybiel, 1983; Jacobs and Azmitia, 1992; Spann and Grofova, 1992; Lavoie and Parent, 1994a,b,c). The inconsistency between the number of ascending and descending projections indicate that the PPN is not a major output component, but a modulating structure as it is part of the many auxiliary loops in BG circuitry and activity. This is because of its strategic position and network with the MCx, thalamus, SN, STN, and CuN. PPN neurons exert excitatory action upon various BG components facilitated mainly by Ach (Woolf and Butcher, 1986). However, the presence of glutamate and various neuropeptides within (Clements and Grant, 1990; Clements et al., 1991; Côté and Parent, 1992) suggest that the PPN also applies an expansive range of effects upon BG neurons through various chemo-specific neuronal systems (Parent and Hazrati, 1995). PPN neurons directly influence BG output nuclei, namely the SNr and GPi, and therefore directly affect information processed within the BG before approaching targets such as the thalamus. Since the PPN establishes highly reciprocal connections with the BG than any other brain region, both these structures exhibit complex physiological interdependence crucial for physiologic function (Mena-Segovia et al., 2004, 2008b). These structures are interconnected either directly or indirectly with every element, and the BG receives large converging input from the PPN (Garcia-Rill, 1991; Pahapill and Lozano, 2000; Mena-Segovia et al., 2004; Alderson and Winn, 2005).
The BG–brainstem (BG–BS) system functions throughout the mesopontine tegmentum in controlling diverse behavioral expressions. This includes automatic movement control comprising periodic limb movements and postural muscle tone adjustments throughout locomotion integrated with voluntary control. It is also involved in awake–sleep state regulation. The BG-BS system is thus accountable for the manifestation of volitionally-directed and emotionally-instigated motor behavior consolidation, and dysfunction of this system together with the cortico-BG loop triggers behavioral disorders (Takakusaki et al., 2004c). The BG performs planning and implementation of deliberate movements through parallel BG-thalamocortical loop sequences (Alexander and Crutcher, 1990; DeLong, 1990; Turner and Anderson, 1997), directing outflow to motor networks in the brainstem (Inglis and Winn, 1995; Hikosaka et al., 2000; Takakusaki et al., 2003) where central neuronal complexes for muscle tone and locomotor movement control are located (Garcia-Rill, 1991). Thus, BG outputs project through thalamocortical loops to the brainstem, and are involved in postural muscle tone and locomotion integrative assimilation (Takakusaki et al., 2004b). Hikosaka et al. (2000) postulates that the BG utilizes two types of output to regulate movements; one via thalamocortical systems, and another via brainstem motor networks (see Figure 3).
Figure 3. Volitional and automatic control of locomotor movements by the BG-BS system: GABAergic BG output to thalamocortical and brainstem neurons assimilates volitional and automatic movement control processes. Adapted from Villiger and Piersol (1912).
BG output to the cerebral cortex regulates voluntary movement control processes, whereas specific movement patterns such as saccades (Hikosaka and Wurtz, 1983a,b,c; Hikosaka, 1991; Hikosaka et al., 2000; Sparks, 2002), vocalization (Düsterhöft et al., 2000), and locomotion (Rossignol, 1996) are generated by exclusive neuronal systems in the brainstem and spinal cord. MCx projections are directed to the PPN (Matsumura et al., 2000) and pontomedullary reticular formation (Matsuyama and Drew, 1997), where muscle tone regulation and the locomotor system are coordinated simultaneously by dual feedback via net BG inhibition and MCx excitation to the brainstem. In PD, GABAergic BG output is overactive (Wichmann and DeLong, 1996, 2003), ensuing in sluggishness and movement decreases by thalamocortical neurons, known as bradykinesia and hypokinesia respectively. Contrarily, increases in BG inhibition together with PPN cortical excitation reductions would increase the level of muscle tone, known as hypertonus. Likewise, excessive MLR inhibitions and cortical excitation decreases in the brainstem reticular formation would educe gait failure. Furthermore, primary MCx inactivity would disrupt the locomotor programming necessary for defined gait control (Hanakawa et al., 1999; Pahapill and Lozano, 2000). Resultantly, this would constrain the degree of freedom for locomotion (Takakusaki et al., 2004c). Gait disturbances where delays are seen in freezing of gait (FoG), stance phase increases in movement sequences and movement speed decreases are also seen in PD invalids (Morris et al., 1994; Pahapill and Lozano, 2000). BG–BS system impairment would be the principal foundation for PD-induced gait deficiencies (Takakusaki et al., 2004c) as these gait failures resemble SNr-stimulated movement (Takakusaki et al., 2003).
In saccadic control, the direct and indirect pathways within the BG (Alexander and Crutcher, 1990; DeLong, 1990) cause GABAergic SNr tonic neuronal inhibition of SC output neurons, consequently preventing unnecessary saccades. The direct pathway from the CN to SNr results in SC neuronal disinhibition by eradicating this constant inhibition. Specifically, phasic GABAergic output neuronal activity in the CN permits saccade occurrence via tonic SNr-SC inhibition suspension (Hikosaka, 1989). The indirect pathway, involving the GPe and STN, further enhances the SNr-SC inhibition via excitatory cortical input (Nambu et al., 2002). Hence, direct CN-SNr and indirect CN-GPe-STN-SNr pathways induce contrasting SNr-SC system effects. Concurrent interactions within the two pathways generate additional discriminating information and heighten the target systems' neural signal spatial contrast. Inversely, behavior interchange from locomotor subdual when the indirect pathway dominates, to locomotor induction when the direct pathway dominates, is produced via consecutive communication of the pathways. This effect enhances temporal contrast. Thus, BG saccadic control can be summarized via two mechanisms. The first is by enhancement of tonic inhibition and disinhibition, while the second mechanism is through converging and sequencing. These two modules are elicited via direct and indirect pathway communication, and might influence brainstem networks besides thalamocortical networks (Hikosaka et al., 2000) (see Figure 4).
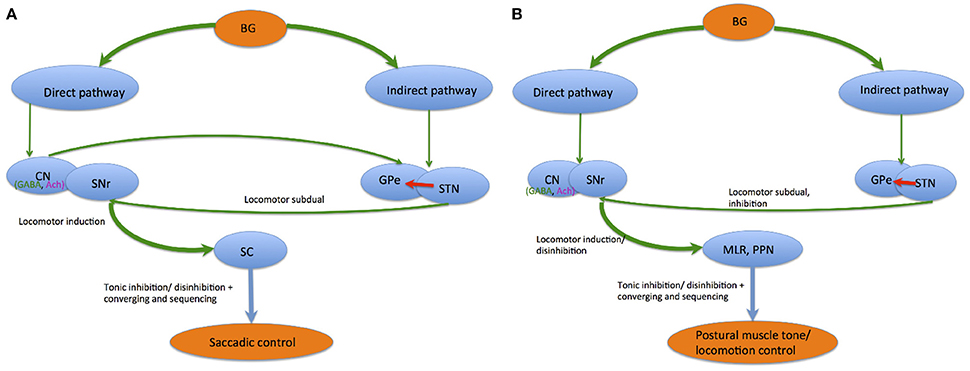
Figure 4. Saccadic control (A) and postural muscle tone/ locomotion control (B) by the direct and indirect pathways.
Similarly, disinhibition and inhibition regulations are key mechanisms for BG postural muscle tone and locomotion control. Locomotor and muscle tone control systems are normalized by the direct and indirect pathway balance via muscle tone inhibitory regions in the PPN, MLR, and SC receiving GABAergic input from the SNr. During locomotor movement preparation, tonic SNr neuronal tonic activity continuously inhibits both systems. When an activating signal occurs, the direct pathway releases activity in these systems, causing locomotion initiation followed by muscle tone level reduction. Parallel SNr organization to the MLR/PPN also regulates muscle tone level accompanying the initiation and termination of locomotion (Takakusaki et al., 2004c).
Cholinergic PPN neuronal loss in PD (Hirsch et al., 1987; Zweig et al., 1987, 1989; Jellinger, 1988) also attributes to attentive and cognitive damages and sleep defects (Scarnati and Florio, 1997). This validates that the BG-BS are also involved in non-motor function, specifically in REMS regulation, arousal and emotional motor behaviors (Takakusaki et al., 2004c).
Gait and Locomotion
As mentioned, the PPN is a central part of the MLR within the brainstem, where it generates and supports lower controlled locomotion (Skinner and Garcia-Rill, 1984; Skinner et al., 1990) via descending projections innervating foci in the lower brainstem and medulla, comprising the oral pontine reticular nucleus, the GiN, the medioventral medulla, and spinal cord regions (Mitani et al., 1988; Rye et al., 1988; Nakamura et al., 1989; Semba et al., 1990; Grofova and Keane, 1991; Scarnati et al., 2011). These projections are associated with gait control and posture primarily via locomotion inhibition, where increasing levels of high stimulation drives the frequency of stepping from walking to running (see Figure 5) (Garcia-Rill et al., 1987; Garcia-Rill, 1991).
The cholinergic PPNc induces locomotion (Garcia-Rill et al., 1987) together with other brainstem regions via prominent sensory nuclei stimulating locomotion through direct outputs to spinal cord regions of recognized locomotor generators (Pahapill and Lozano, 2000). PPN neuronal response to somatosensory excitation (Grunwerg et al., 1992; Reese et al., 1995) combined with cholinergic PPN neuronal thalamic projections and inputs from lamina 1 of the spinal cord, advocates that the PPN modulates sensory information to thalamic nuclei. Thus, the PPN plays a role as a dispatch amid the cerebral cortex and spinal cord, providing feedback information vital for posture and gait initiation modulation. This is enabled by ascending thalamic cholinergic projections and deep cerebellar nuclei networks (Pahapill and Lozano, 2000).
Non-cholinergic PPNd neurons receive input from the BG and limbic structures, propositioning that the PPN acts as an assimilator for BG motor choice output and incentive-motivated commands from the striatal-pallidal complex to deliver motivationally influenced activation of motor pattern generators in the pons, medulla and spinal cord (Inglis and Winn, 1995). Such factors affect motor function like kinesia paradoxica. Treatment via PPN activation would improve motor planning and permit increasing motivational ability in stimulating preserved motor programs for stereotyped movements (Pahapill and Lozano, 2000).
Reward, Motivation, and Compulsion
The PPN is accountable for the phasic activity bursts in SNc DA cells, which plays a key role in learning and preserving instrumental tasks (Scarnati et al., 1988; Futami et al., 1995). Primary PPN reward stimuli originate from the lateral hypothalamus, but excitatory reward-prediction stimuli spawns a condition stimulus-elicited DA surge traversing the ventral striatum–pallidum pathway, receiving predominantly limbic cortex input (Schultz et al., 1992). Striosomal cells regulate response to primary reward after conditioning via suppressing DA burst response through the striosomal-SNc pathway (Gerfen, 1992). Striosomal cells also modulate the adaptive scheduled reward expectation that annuls the predicted reward at the predicted interval (Schultz et al., 1997). DA cell activity is therefore an exclusive parallel act of PPN inactivation, compared to a secondary influence on motivation or abridged capability of task performance. The PPN thus serves as an integrative interface amidst innumerable stimuli necessary for executing intended behaviors (Kobayashi et al., 2004). This enables the fundamental activities for motor command initiation and external sensory dispensation via arousal regulation and attentive conditions through dopaminergic systems (Takakusaki et al., 2004c).
PPN lesions ensued in impaired attention (Inglis et al., 2001) and memory learning during a trained incitement and a prime reward (Inglis et al., 1994, 2000). This advocates that PPN inactivation has variable effects on non-dopaminergic cells in the VTA. Firstly, PPN neurons respond to the same task stimuli, whether visual- or auditory-activating DA cells. Secondly, PPN responses are governed by phasic inception patterns observed in DA cells. Thirdly, PPN cells respond before DA cells, permitting PPN-DA information transfer. Finally, PPN suppression subdues DA cell responses to stimuli without upsetting baseline firing frequency (Pan and Hyland, 2005). Therefore, auditory, visual, and somatosensory trained incitements stimulate DA cells (Romo and Schultz, 1990; Schultz and Romo, 1990), with bias toward auditory incitements at tremendously brief latencies, strictly programmed to the stimulus interval. The preference of PPN cells for tone and light increases the likelihood that homogenous afferent projections regulate DA cell action, with biasness for either constituent (Wallace and Fredens, 1988; Comoli et al., 2003). Thus, the PPN and SC supplement each other in dispatching sensory knowledge of different stimuli. PPN neuronal activity predisposed toward auditory responses has a functionally imperative role in reducing DA cell responses without substantial effects on baseline firing rate via a visual component inactivation. This establishes that the PPN selectively controls DA cell bursting rather than tonic resting activity (Floresco et al., 2003), and that PPN inputs are necessary for producing DA cell surge reactions to significant sensory stimuli (Schultz, 1998; Brown et al., 1999). The PPN is therefore imperative for arousal, attention, motivation, learning, and specifically stimulated–reward conditions (Steckler et al., 1994).
PPN lesions do not disrupt brain stimulation reward value however (Waraczynski and Perkins, 1998), suggesting that it performs as a primary sensory and motivational system interface toward delivering communication signals irrespective of reward value. DA cells typically react staggeringly when signals are reward-connected, while PPN cells react non-contingently, suggesting that separate, reward-information-bearing pathways gate PPN inputs. Hence PPN inputs have a dual role, to provide precise and brief latent information toward sensory stimuli intervals and advanced-level function information transmission concerning signals dispatched via sensory-attention regulating mechanisms (Pan and Hyland, 2005).
Research establishes that lateral hypothalamic brain stimulation not only rewards, but also drive-induces (Coons et al., 1965; Glickman and Schiff, 1967). Rewarding hypothalamic brain stimulation depends on trans-synaptic induced release of Ach in the VTA (Yeomans et al., 1993), where dominant portions of these fibers synapse in PPN cholinergic efferents relaying messages back to the VTA (Yeomans et al., 1993). These cholinergic PPN neurons provide non-specific facilitation for reward-connecting behaviors, and therefore act as a relay amid limbic-incentive organization and brainstem locomotor machinery (Steckler et al., 1994). Due to its position within the mesolimbic DA system encompassing the VTA and NAcc, it is entangled in brain mechanisms and neural circuitry formation involved in reward processing, which can lead to motivation and compulsion.
Rapid Eye Movement Sleep (REMS)
PPN and LDT cholinergic neurons are involved in arousal state maintenance and REMS generation (Rye, 1997). During sleep, PPN cholinergic activation of the cortex transpires via projections to the thalamocortical network to subdue slow delta waves and elicit cortical stimulation (Belaid et al., 2014). This leads to REMS, through REM-on and –off cellular activity together with the locus coeruleus and dorsal raphe nuclei (DRN) (McCarley and Chokroverty, 1994). Reduced inhibitory input from the SNr/GPi nuclei to the PPN results in higher intrinsic activity causing cortical activation and electroencephalography (EEG) desynchronization (Reiner et al., 1988) leading to REMS (Steriade, 1996). These neuronal mechanisms that induce REMS and muscular atonia together with PPN cholinergic projections are under SNr GABAergic inhibition regulation.
PD patients are known to experience several sleep disorders, including reduction of REMS sleep period and REMS behavior disorder (RBD) (Bliwise et al., 2000; Eisensehr et al., 2001). This is because decreases in BG dopaminergic activities is also involved in REMS reduction and RBD (Rye et al., 1999; Albin et al., 2000), hence providing a lucid explanation for the pathogenesis of sleep disturbances in PD (Takakusaki et al., 2004c; French and Muthusamy, 2016). A summary of the different maladies associated with the PPN in PD are listed in Table 1.
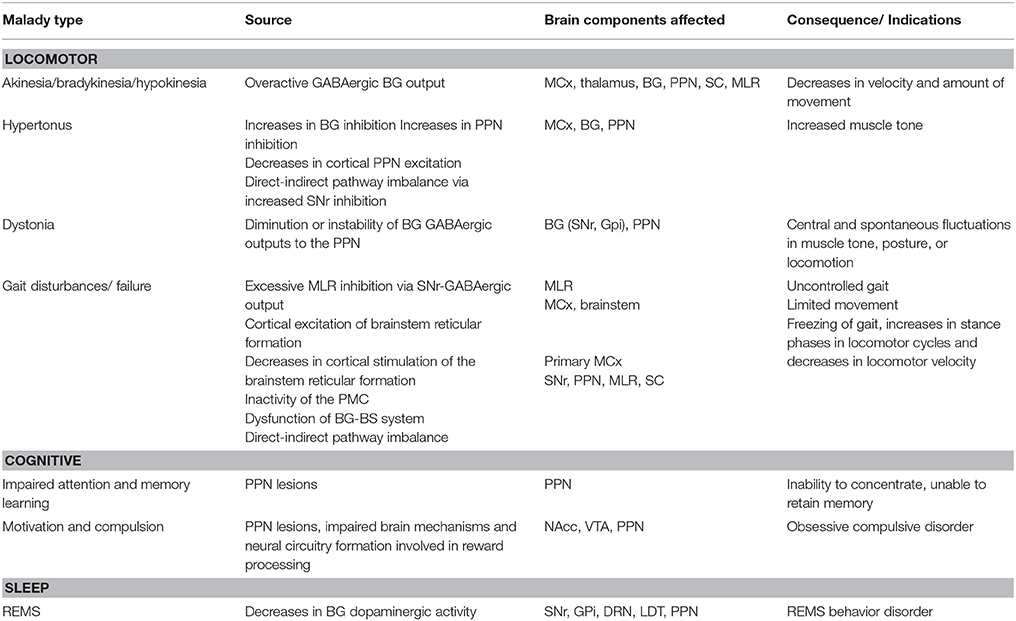
Table 1. Maladies associated with the PPN in PD, the source and affected brain components, as well as its consequence/ indications.
Treatment/Deep Brain Stimulation
Electrode recordings in deep brain stimulation (DBS) postulate that uncontrolled abnormal pathological oscillations throughout motor networks in the STN, GP, and thalamus are concomitant with motor symptoms in PD (Hammond et al., 2007). Similarly BG networks oscillating at a pathological beta (β) range of 20 Hz, driven by cerebral neurons firing in either “burst” or “tonic” modes is associated with akinesia (Stein, 2009). Successful alleviation of akinesia with L-DOPA enables the system to break away from this pathological beta repression (Kühn et al., 2008). High frequency stimulation, applied via DBS also subdues pathological synchronization (Brown and Eusebio, 2008). PD symptoms are lessened by DBS via preventing pathological neuronal network oscillations that destabilize them, and are successful as they abolish nodes responsible for oscillation generation itself. DBS is thus permanently effective by driving neurons tonically so that pathological oscillations causing the burst/silence mode are reversible.
Neurophysiologically, cortical bursts normally govern PPN input and orchestrate field potentials and neuronal discharges to the cortical rhythm so that PPN local field potentials and neuronal discharges are synchronized with those of MCx activity (Aravamuthan et al., 2008). However, these synchronies reverse after cell lesions and PPN neurons fire mostly throughout positive swings in the cortex when they should be silent. This indicates that inhibitory GP and SNr output is predominant input to the PPN, rather than excitatory MCx output. Stimulation of the GP and SNr also requires adequate glutamate conduction. This substitutes dominant PPN firing via inhibitory input for normal excitatory input from the MCx and STN to the PPN. The β-band is responsible for associated akinesia, where β suppression increases with the complexity of the intended movement while its latency predicts movement onset. This means that the earlier the suppression, the shorter the movement latency. Therapeutic interventions reducing akinetic symptoms reduce enhanced synchronization in the β band and facilitates regular gamma oscillations (Brown et al., 2001).
The PPN area might be a good prospective DBS objective concerning freezing and other gait disorders' treatment associated with PD, where data shows that cholinergic denervation due to PPN neuronal degeneration causes DA non-responsive gait and balance impairment (Karachi et al., 2010; Grabli et al., 2013). Imaging studies in PD patients propose that unilateral PPN DBS intensifies cerebral blood flow bilaterally into the central thalamus and cerebellum (Ballanger et al., 2009). However, recognized assessments support bilateral DBS (Khan et al., 2012) ascertained to be superior particularly for controlling FoG. Thevathasan et al. (2012) further supports this, concluding that bilateral stimulation was more successful in a specific subgroup of PD patients by ~200%. This study exhibited concrete unprejudiced, double-blinded proof that an explicit subcategory of Parkinsonian patients benefit from bilateral stimulation of a caudal PPN region just below the pontomesenphalic junction that discriminately improves FoG. This did not include inconsistencies in step length however, which could be furtive freezing interrupting the smooth execution of gait (Thevathasan et al., 2012). Long-term outcomes would unquestionably need further substantiation via supplementary studies or randomized trials with longer follow-up periods involving a higher number of patients and exclusive criteria.
Most PPN DBS studies denote alleviation in patients disturbed by freezing and falls although outcomes are variable. This possibly reflects patient choice, target option heterogeneity, surgical procedure differences and stimulation protocols (Hamani et al., 2016a). This leads to a number of challenges to be solved, including the prime target identification, surgical method choice that optimizes electrode placement, precision, and impact of surgical procedures, intraoperative target reliability, and procedural modifications in postoperative electrode position validation. Nonetheless, the procedure appears to provide benefit to selected patients and is comparatively safe. One important limitation in comparing studies from different centers and analyzing outcomes is great target variability and surgical techniques (Hamani et al., 2016b).
Chronic PPN stimulation is usually combined with stimulation of other targets, including the STN, GPi, and the caudal zona incerta due to its superiority compared to PPN DBS alone. However, combined stimulation poses challenges in the effectual assessment of DBS in each target, and also in enlightening the complex relationship between medication and stimulation. A particular problem is the use of low-frequency PPN stimulation and high-frequency stimulation in other targets, where this necessitates intricate programming or utilization of a supplementary pulse generator. Another issue is the concordance on the ideal target position within the PPN region, where it is ambiguous as to whether electrodes should be implanted in the rostral PPN at the level of the inferior colliculus or caudal PPN in a region about 4 mm below the inferior colliculus. A reasonable approach would be to insert contacts in both rostral and caudal PPN regions since available data is still vague, and also because the PPN is oriented along the long axis of the brainstem. Since the PPN is partially degenerated in PD, smaller-spaced electrodes might be preferable. It would also be vital to develop a specified set of resting and movement-related intraoperative local field potentials while conducting PPN DBS as frequency bands in the alpha, β, and theta ranges and movement-related potential were all recorded from the PPN region (Hamani et al., 2016b).
PPN DBS is still a relatively novel intervention in PD, and the numerous challenges mentioned before must be resolved. Despite these trials, the procedure provides benefit to selected patients and appears relatively safe. The future role of PPN DBS in the armamentarium of surgery for PD patients is still uncertain. Unquestionably, more studies are needed to provide more solid data on the advantages and limits of chronic stimulation (Hamani et al., 2016b).
Conclusion
Understanding the function of the PPN and its utility in the many neuronal circuitries of the brain is vital for neurophysiological knowledge. This would ensue in the understanding of how maladies such as Parkinson's disease occurs along amid its consequences, and subsequently help in producing the appropriate treatment needed to cure and control these disorders. A promising and long-term treatment would be DBS, which could vastly improve patients' quality of life. Further studies would definitely need to be conducted to elucidate further on such disorders especially in terms of genetics and biochemistry.
Author Contributions
IF was the main author of this paper who wrote this manuscript as part of a research project in order to understand better and summarize the physiology and pathophysiology of the pedunculopontine nucleus in associated with Parkinson's disease. KM provided the main oversight and general guidance in the completion of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This review was prepared in accordance with research conducted in conjunction with the High Impact Research Grant of University of Malaya (HIR -UM.C/625/1/HIR- MOHE/CHAN/12) and University Malaya Department of Surgery.
Abbreviations
Ach, Acetylcholine; ARAS, Ascending reticular activating system; BG, Basal ganglia; BG-BS, Basal ganglia-brainstem; CN, caudate nucleus; CuN, Cuneiform nucleus; DA, Dopamine; DBS, Deep brain stimulation; GABA, Gamma aminobutyric acid; GiN, Gigantocellular reticular nucleus; GP, Globus pallidus; GPe, Globus pallidus externa; GPi, globus pallidus interna; LN, Lewy neurites; LB, Lewy bodies; LDT, Laterodorsal tegmental nucleus; MCx, Motor cortex; MLR, mesenphalic locomotor region; NAcc, Nucleus accumbens; PPN, Pedunculopontine nucleus; PPNc, Pedunculopontine nucleus pars compacta; PPNd, Pedunculopontine nucleus pars dissipatus; PD, Parkinson's disease; REMS, Rapid eye movement sleep; VTA, Ventral tegmental area; VA, ventral anterior nucleus; VL, ventrolateral nucleus; SC, Superior colliculus; SN, Substantia nigra; SNc, Substantia nigra pars compacta; SNr, Substantia nigra pars reticulate; STN, Subthalamic nucleus.
References
Albin, R. L., Koeppe, R. A., Chervin, R. D., Consens, F. B., Wernette, K., Frey, K. A., et al. (2000). Decreased striatal dopaminergic innervation in REM sleep behavior disorder. Neurology 55, 1410–1412. doi: 10.1212/WNL.55.9.1410
Albin, R. L., Young, A. B., and Penney, J. B. (1989). The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375. doi: 10.1016/0166-2236(89)90074-X
Alderson, H. L., and Winn, P. (2005). “The pedunculopontine and reinforcement,” in The Basal Ganglia VIII, (Boston, MA: Springer), 523–532.
Alexander, G. E., and Crutcher, M. D. (1990). Functional architecture of basal ganglia circuits, neural substrates of parallel processing. Trends Neurosci. 13, 266–271. doi: 10.1016/0166-2236(90)90107-L
Alexander, G. E., DeLong, M. R., and Strick, P. L. (1986). Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381. doi: 10.1146/annurev.ne.09.030186.002041
Amaral, D. G., Price, J. L., Pitkanen, A., and Carmichael, S. T. (1992). “Anatomical organization of the primate amygdaloid complex,” in The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, ed J. P. Aggleton (New York, NY: Wiley-Liss), 1–66.
Aravamuthan, B. R., Bergstrom, D. A., French, R. A., Taylor, J. J., Parr-Brownlie, L. C., and Walters, Jr. (2008). Altered neuronal activity relationships between the pedunculopontine nucleus and motor cortex in a rodent model of Parkinson's disease. Exp. Neurol. 213, 268–280. doi: 10.1016/j.expneurol.2008.05.023
Azulay, J. P., Mesure, S., Amblard, B., and Pouget, J. (2002). Increased visual dependence in Parkinson's disease. Percep. Motor Skills 95(3 Suppl), 1106–1114. doi: 10.2466/pms.2002.95.3f.1106
Ballanger, B., Lozano, A. M., Moro, E., van Eimeren, T., Hamani, C., Chen, R., et al. (2009). Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson's disease: A [15O] H2O PET study. Hum. Brain Mapp. 30, 3901–3909. doi: 10.1002/hbm.20815
Belaid, H., Adrien, J., Laffrat, E., Tandé, D., Karachi, C., Grabli, D., et al. (2014). Sleep disorders in Parkinsonian macaques: effects of L-dopa treatment and pedunculopontine nucleus lesion. J. Neurosci. 34, 9124–9133. doi: 10.1523/JNEUROSCI.0181-14.2014
Beninato, M., and Spencer, R. F. (1987). A cholinergic projection to the rat substantia nigra from the pedunculopontine tegmental nucleus. Brain Res. 412, 169–174. doi: 10.1016/0006-8993(87)91455-7
Bevan, M. D., and Bolam, J. P. (1995). Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J. Neurosci. 15, 7105–7120.
Bliwise, D. L., Willians, M. L., Irbe, D., Ansari, F. P., and Rye, D. B. (2000). Inter-rater reliability for identification of REM sleep in Parkinson's disease. Sleep 23, 671–676.
Bohus, B., Koolhaas, J. M., Luiten, P. G., Korte, S. M., Roozendaal, B., and Wiersma, A. (1996). The neurobiology of the central nucleus of the amygdala in relation to neuroendocrine and autonomic outflow. Prog. Brain Res. 107, 447–460. doi: 10.1016/S0079-6123(08)61881-6
Bolam, J. P., Francis, C. M., and Henderson, Z. (1991). Cholinergic input to dopaminergic neurons in the substantia nigra: a double immunocytochemical study. Neuroscience 41, 483–494. doi: 10.1016/0306-4522(91)90343-M
Braak, H., and Braak, E. (1985). Nuclear configuration and neuronal types of the nucleus niger in the brain of the human adult. Hum. Neurobiol. 5, 71–82.
Braak, H., and Del Tredici, K. (2004). Poor and protracted myelination as a contributory factor to neurodegenerative disorders. Neurobiol. Aging 25, 19–23. doi: 10.1016/j.neurobiolaging.2003.04.001
Braak, H., Braak, E., Deniz, Y., de Vos, R. A., Jansen, E. N., Bohl, J., et al. (1994). Amygdala pathology in Parkinson's disease. Acta Neuropathol. 88, 493–500. doi: 10.1007/BF00296485
Braak, H., Del Tredici, K., Rüb, U., de Vos, R. A., Jansen, E. N., and Braak, E. (2003). Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging 24, 197–211.
Braak, H., Ghebremedhin, E., Rüb, U., Bratzke, H., and Del Tredici, K. (2004). Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 318, 121–134. doi: 10.1007/s00441-004-0956-9
Brown, J., Bullock, D., and Grossberg, S. (1999). How the basal ganglia use parallel excitatory and inhibitory learning pathways to selectively respond to unexpected rewarding cues. J. Neurosci. 19, 10502–10511.
Brown, P., and Eusebio, A. (2008). Paradoxes of functional neurosurgery: clues from basal ganglia recordings. Mov. Disord. 23, 12–20. doi: 10.1002/mds.21796
Brown, P., Oliviero, A., Mazzone, P., Insola, A., Tonali, P., and Di Lazzaro, V. (2001). Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson's disease. J. Neurosci. 21, 1033–1038.
Burbaud, P., Bonnet, B., Guehl, D., Lagueny, A., and Bioulac, B. (1998). Movement disorders induced by gamma-aminobutyric agonist and antagonist injections into the internal globus pallidus and substantia nigra pars reticulata of the monkey. Brain Res. 780, 102–107. doi: 10.1016/S0006-8993(97)01158-X
Candy, J. M., Perry, R. H., Perry, E. K., Irving, D., Blessed, G., Fairbairn, A. F., and Tomlinson, B. E. (1983). Pathological changes in the nucleus of Meynert in Alzheimer's and Parkinson's diseases. J. Neurol. Sci. 59, 277–289.
Carpenter, M. B. (1976). Anatomical organization of the corpus striatum and related nuclei. Res. Publ. Assoc. Res. Nerv. Ment. Dis. 55, 1.
Charara, A., Smith, Y., and Parent, A. (1996). Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. J. Comp. Neurol. 364, 254–266. doi: 10.1002/(SICI)1096-9861(19960108)364:2<254::AID-CNE5>3.0.CO;2-4
Clements, J. R., Toth, D. D., Highfield, D. A., and Grant, S. (1991). “Glutamate-like immunoreactivity is present within cholinergic neurons of the laterodorsal tegmental and pedunculopontine nuclei,” in The Basal Forebrain, (Boston, MA: Springer), 127–142.
Clements, J. R., and Grant, S. (1990). Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci. Lett. 120, 70–73.
Comoli, E., Coizet, V., Boyes, J., Bolam, J. P., Canteras, N. S., Quirk, R. H., et al. (2003). A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat. Neurosci. 6, 974–980. doi: 10.1038/nn1113
Coons, E. E., Levak, M., and Miller, N. E. (1965). Lateral hypothalamus: learning of food-seeking response motivated by electrical stimulation. Science 150, 1320–1321.
Côté, P. V., and Parent, A. (1992). Calbindin D-28k and choline acetyltransferase are expressed by different neuronal populations in pedunculopontine nucleus but not in nucleus basalis in squirrel monkeys. Brain Res. 593, 245–252. doi: 10.1016/0006-8993(92)91314-5
Del Tredici, K., and Braak, H. (2004). “Idiopathic Parkinson's Disease: Staging an α-Synucleinopathy with a Predictable Pathoanatomy,” in Madame Curie Bioscience Database [Internet] (Austin, TX: Landes Bioscience), 2000–2013.
Del Tredici, K., Rüb, U., De Vos, R. A., Bohl, J. R. E., and Braak, H. (2002). Where does Parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 61, 413–426. doi: 10.1093/jnen/61.5.413
DeLong, M. R. (1990). Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 13, 281–285. doi: 10.1016/0166-2236(90)90110-V
DeLong, M. R., and Georgopoulos, A. P. (2011). Motor functions of the basal ganglia. Compr. Physiol. doi: 10.1002/cphy.cp010221
DeLong, M. R., and Wichmann, T. (2007). Circuits and circuit disorders of the basal ganglia. Arch. Neurol. 64, 20–24. doi: 10.1001/archneur.64.1.20
Denny-Brown, D. (1968). Clinical symptomatology of diseases of the basal ganglia. Handb. Clin. Neurol. 6, 133–172.
DeVito, J. L., Anderson, M. E., and Walsh, K. E. (1980). A horseradish peroxidase study of afferent connections of the globus pallidus in Macaca mulatta. Exp. Brain Res. 38, 65–73. doi: 10.1007/BF00237932
Dickson, D. W., Schmidt, M. L., Lee, V. M., Zhao, M. L., Yen, S. H., and Trojanowski, J. Q. (1994). Immunoreactivity profile of hippocampal CA2/3 neurites in diffuse Lewy body disease. Acta Neuropathol. 87, 269–276. doi: 10.1007/BF00296742
Düsterhöft, F., Häusler, U., and Jürgens, U. (2000). On the search for the vocal pattern generator. A single-unit recording study. Neuroreport 11, 2031–2034.
Edley, S. M., and Graybiel, A. M. (1983). The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J. Comp. Neurol. 217, 187–215. doi: 10.1002/cne.902170207
Eisensehr, I., Lindeiner, H. V., Jäger, M., and Noachtar, S. (2001). REM sleep behavior disorder in sleep-disordered patients with versus without Parkinson's disease, is there a need for polysomnography? J. Neurol. Sci. 186, 7–11. doi: 10.1016/S0022-510X(01)00480-4
Filion, M. (1991). Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 547, 140–144. doi: 10.1016/0006-8993(91)90585-J
Floresco, S. B., West, A. R., Ash, B., Moore, H., and Grace, A. A. (2003). Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat. Neurosci. 6, 968–973. doi: 10.1038/nn1103
Forster, G. L., and Blaha, C. D. (2003). Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur. J. Neurosci. 17, 751–762. doi: 10.1046/j.1460-9568.2003.02511.x
French, I. T., and Muthusamy, K. A. (2016). A Review of sleep and its disorders in patients with Parkinson's Disease in relation to various brain structures. Front. Aging Neurosci. 8:114. doi: 10.3389/fnagi.2016.00114
Futami, T., Takakusaki, K., and Kitai, S. T. (1995). Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci. Res. 21, 331–342. doi: 10.1016/0168-0102(94)00869-H
Galvan, A., and Wichmann, T. (2008). Pathophysiology of parkinsonism. Clin. Neurophysiol. 119, 1459–1474. doi: 10.1016/j.clinph.2008.03.017
Garcia-Rill, E. (1986). The basal ganglia and the locomotor regions. Brain Res. Rev. 11, 47–63. doi: 10.1016/0165-0173(86)90009-3
Garcia-Rill, E. (1991). The pedunculopontine nucleus. Prog. Neurobiol. 36, 363–389. doi: 10.1016/0301-0082(91)90016-T
Garcia-Rill, E., Houser, C. R., Skinner, R. D., Smith, W., and Woodward, D. J. (1987). Locomotion-inducing sites in the vicinity of the pedunculopontine nucleus. Brain Res. Bull. 18, 731–738. doi: 10.1016/0361-9230(87)90208-5
Gerfen, C. R. (1992). The neostriatal mosaic: multiple levels of compartmental organization in the basal ganglia. Annu. Rev. Neurosci. 15, 285–320. doi: 10.1146/annurev.ne.15.030192.001441
Geula, C., Schatz, C. R., and Mesulam, M. M. (1993). Differential localization of NADPH-diaphorase and calbindin-D 28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience 54, 461–476.
Gibb, W. R., and Lees, A. J. (1991). Anatomy, pigmentation, ventral and dorsal subpopulations of the substantia nigra, and differential cell death in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 54, 388–396. doi: 10.1136/jnnp.54.5.388
Glickman, S. E., and Schiff, B. B. (1967). A biological theory of reinforcement. Psychol. Rev. 74, 81. doi: 10.1037/h0024290
Grabli, D., Karachi, C., Folgoas, E., Monfort, M., Tande, D., Clark, S., et al. (2013). Gait disorders in parkinsonian monkeys with pedunculopontine nucleus lesions: a tale of two systems. J. Neurosci. 33, 11986–11993. doi: 10.1523/JNEUROSCI.1568-13.2013
Granata, A. R., and Kitai, S. T. (1991). Inhibitory substantia nigra inputs to the pedunculopontine neurons. Exp. Brain Res. 86, 459–466. doi: 10.1007/BF00230520
Graybiel, A. M. (1977). Direct and indirect preoculomotor pathways of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J. Comp. Neurol. 175, 37–78. doi: 10.1002/cne.901750105
Grofova, I., and Keane, S. (1991). Descending brainstem projections of the pedunculopontine tegmental nucleus in the rat. Anat. Embryol. 184, 275–290. doi: 10.1007/BF01673262
Grunwerg, B. S., Krein, H., and Krauthamer, G. M. (1992). Somatosensory input and thalamic projection of pedunculopontine tegmental neurons. Neuroreport 3, 673–675. doi: 10.1097/00001756-199208000-00004
Hamani, C., Aziz, T., Bloem, B. R., Brown, P., Chabardes, S., Coyne, T., et al. (2016a). Pedunculopontine nucleus region deep brain stimulation in Parkinson disease: surgical anatomy and terminology. Stereotact. Funct. Neurosurg. 94, 298–306. doi: 10.1159/000449010
Hamani, C., Lozano, A. M., Mazzone, P. A., Moro, E., Hutchison, W., Silburn, P. A., et al. (2016b). Pedunculopontine nucleus region deep brain stimulation in Parkinson Disease: surgical techniques, side effects, and postoperative imaging. Stereotact. Funct. Neurosurg. 94, 307–319. doi: 10.1159/000449011
Hammond, C., Bergman, H., and Brown, P. (2007). Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 30, 357–364. doi: 10.1016/j.tins.2007.05.004
Hanakawa, T., Katsumi, Y., Fukuyama, H., Honda, M., Hayashi, T., Kimura, J., et al. (1999). Mechanisms underlying gait disturbance in Parkinson's disease. Brain 122, 1271–1282. doi: 10.1093/brain/122.7.1271
Hazrati, L. N., and Parent, A. (1992). Projection from the deep cerebellar nuclei to the pedunculopontine nucleus in the squirrel monkey. Brain Res. 585, 267–271. doi: 10.1016/0006-8993(92)91216-2
Hikosaka, O. (1989). “Role of basal ganglia in initiation of voluntary movements,” in Dynamic Interactions in Neural Networks: Models and Data, (New York, NY: Springer), 153–167. doi: 10.1007/978-1-4612-4536-0_9
Hikosaka, O. (1991). Role of the forebrain in oculomotor function. Prog. Brain Res. 87:101. doi: 10.1016/S0079-6123(08)63049-6
Hikosaka, O. (2007). GABAergic output of the basal ganglia. Prog. Brain Res. 160, 209–226. doi: 10.1016/S0079-6123(06)60012-5
Hikosaka, O., and Wurtz, R. H. (1983a). Visual and oculomotor functions of monkey substantia nigra pars reticulata. II. Visual responses related to fixation of gaze. J. Neurophysiol. 49, 1254–1267.
Hikosaka, O., and Wurtz, R. H. (1983b). Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J. Neurophysiol. 49, 1268–1284.
Hikosaka, O., and Wurtz, R. H. (1983c). Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J. Neurophysiol. 49, 1285–1301.
Hikosaka, O., Matsumura, M., Kojima, J., and Gardiner, T. W. (1993a). “Role of basal ganglia in initiation and suppression of saccadic eye movements,” in Role of the Cerebellum and Basal Ganglia in Voluntary Movement, eds N. Mano, I. Hamada, and M. R Delong (Okazaki: Elsevier Science Publishers), 213–219.
Hikosaka, O., Sakamoto, M., and Miyashita, N. (1993b). Effects of caudate nucleus stimulation on substantia nigra cell activity in monkey. Exp. Brain Res. 95, 457–472. doi: 10.1007/BF00227139
Hikosaka, O., Takikawa, Y., and Kawagoe, R. (2000). Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol. Rev. 80, 953–978. doi: 10.1152/physrev.2000.80.3.953
Hirsch, E. C., Graybiel, A. M., Duyckaerts, C., and Javoy-Agid, F. (1987). Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. U.S.A. 84, 5976–5980. doi: 10.1073/pnas.84.16.5976
Inglis, W. L., and Winn, P. (1995). The pedunculopontine tegmental nucleus, where the striatum meets the reticular formation. Prog. Neurobiol. 47, 1–29. doi: 10.1016/0301-0082(95)00013-L
Inglis, W. L., Dunbar, J. S., and Winn, P. (1994). Outflow from the nucleus accumbens to the pedunculopontine tegmental nucleus: a dissociation between locomotor activity and the acquisition of responding for conditioned reinforcement stimulated by d-amphetamine. Neuroscience 62, 51–64. doi: 10.1016/0306-4522(94)90314-X
Inglis, W. L., Dunbar, J. S., and Winn, P. (2000). Pedunculopontine tegmental nucleus lesions impair stimulus–reward learning in autoshaping and conditioned reinforcement paradigms. Behav. Neurosci. 114:285. doi: 10.1037/0735-7044.114.2.285
Inglis, W. L., Olmstead, M. C., and Robbins, T. W. (2001). Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions. Behav. Brain Res. 123, 117–131. doi: 10.1016/S0166-4328(01)00181-4
Jacobs, B. L., and Azmitia, E. C. (1992). Structure and function of the brain serotonin system. Physiol. Rev. 72, 165–229. doi: 10.1152/physrev.1992.72.1.165
Jellinger, K. (1988). The pedunculopontine nucleus in Parkinson's disease, progressive supranuclear palsy and Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 51, 540–543. doi: 10.1136/jnnp.51.4.540
Jones, B. E. (1991). Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience 40, 637–656. doi: 10.1016/0306-4522(91)90002-6
Karachi, C., Grabli, D., Bernard, F. A., Tandé, D., Wattiez, N., Belaid, H., et al. (2010). Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J. Clin. Invest. 120, 2745–2754. doi: 10.1172/JCI42642
Kelly, R. M., and Strick, P. L. (2004). Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog. Brain Res. 143, 447–459. doi: 10.1016/S0079-6123(03)43042-2
Khan, S. A., Javed, S., Mooney, L., White, P., Plaha, P., Whone, A., et al. (2012). Clinical outcomes from bilateral versus unilateral stimulation of the pedunculopontine nucleus with and without concomitant caudal zona incerta region stimulation in Parkinson's disease. Br. J. Neurosurg. 26, 722–725. doi: 10.3109/02688697.2012.659297
Kobayashi, Y., Inoue, Y., Yamamoto, M., Isa, T., and Aizawa, H. (2004). Pedunculopontine control of visually guided saccades. Prog. Brain Res. 143, 447–459. doi: 10.1016/S0079-6123(03)43041-0
Kühn, A. A., Kempf, F., Brücke, C., Doyle, L. G., Martinez-Torres, I., Pogosyan, A., et al. (2008). High-frequency stimulation of the subthalamic nucleus suppresses oscillatory β activity in patients with Parkinson's disease in parallel with improvement in motor performance. J. Neurosci. 28, 6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008
Kuypers, H. G., and Lawrence, D. G. (1967). Cortical projections to the red nucleus and the brain stem in the rhesus monkey. Brain Res. 4, 151–188. doi: 10.1016/0006-8993(67)90004-2
Lavoie, B., and Parent, A. (1994a). Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J. Comp. Neurol. 344, 232–241.
Lavoie, B., and Parent, A. (1994b). Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J. Comp. Neurol. 344, 190–209.
Lavoie, B., and Parent, A. (1994c). Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J. Comp. Neurol. 344, 210–231.
Lokwan, S. J., Overton, P. G., Berry, M. S., and Clark (1999). Stimulation of the pedunculopontine tegmental nucleus in the rat produces burst firing in A9 dopaminergic neurons. Neuroscience 92, 245–254. doi: 10.1016/S0306-4522(98)00748-9
Martinez-Gonzalez, C., Wang, H. L., Micklem, B. R., Bolam, J. P., and Mena-Segovia, J. (2012). Subpopulations of cholinergic, GABAergic and glutamatergic neurons in the pedunculopontine nucleus contain calcium-binding proteins and are heterogeneously distributed. Eur. J. Neurosci. 35, 723–734. doi: 10.1111/j.1460-9568.2012.08002.x
Matsumura, M., Nambu, A., Yamaji, Y., Watanabe, K., Imai, H., Inase, M., et al. (2000). Organization of somatic motor inputs from the frontal lobe to the pedunculopontine tegmental nucleus in the macaque monkey. Neuroscience 98, 97–110. doi: 10.1016/S0306-4522(00)00099-3
Matsumura, M., Watanabe, K., and Ohye, C. (1997). Single-unit activity in the primate nucleus tegmenti pedunculopontinus related to voluntary arm movement. Neurosci. Res. 28, 155–165. doi: 10.1016/S0168-0102(97)00039-4
Matsuyama, K., and Drew, T. (1997). Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: a study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 389, 617–641. doi: 10.1002/(SICI)1096-9861(19971229)389:4<617::AID-CNE6>3.0.CO;2-3
McCarley, R. W., and Chokroverty, S. (1994). Neurophysiology of sleep: basic mechanisms underlying control of wakefulness and sleep. Sleep Disord. Med. Basic Sci. Tech. Consider. Clin. Aspects 17–36. doi: 10.1016/B978-0-7506-9002-7.50008-2
Mehler, W. R., and Nauta, W. J. (1974). Connections of the basal ganglia and of the cerebellum. Stereotact. Funct. Neurosurg. 36, 205–222. doi: 10.1159/000102797
Mena-Segovia, J., Micklem, B. R., Nair, R. G., Ungless, M. A., and Bolam, J. P. (2009). GABAergic neuron distribution in the pedunculopontine nucleus defines functional subterritories. J. Comp. Neurol. 515, 397–408. doi: 10.1002/cne.22065
Mena-Segovia, J., Sims, H. M., Magill, P. J., and Bolam, J. P. (2008a). Cholinergic brainstem neurons modulate cortical gamma activity during slow oscillations. J. Physiol. 586, 2947–2960. doi: 10.1113/jphysiol.2008.153874
Mena-Segovia, J., Winn, P., and Bolam, J. P. (2008b). Cholinergic modulation of midbrain dopaminergic systems. Brain Res. Rev. 58, 265–271. doi: 10.1016/j.brainresrev.2008.02.003
Mena-Segovia, J., Bolam, J. P., and Magill, P. J. (2004). Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci. 27, 585–588. doi: 10.1016/j.tins.2004.07.009
Mesulam, M. M., Mufson, E. J., Wainer, B. H., and Levey, A. L. (1983). Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1–Ch6). Neuroscience 10, 1185–1201. doi: 10.1016/0306-4522(83)90108-2
Mesulam, M., Geula, C., Bothwell, M. A., and Hersh, L. B. (1989). Human reticular formation: cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J. Comp. Neurol. 283, 611–633. doi: 10.1002/cne.902830414
Mesulam, M., Hersh, L. B., Mash, D. C., and Geula, C. (1992a). Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J. Comp. Neurol. 318, 316–328. doi: 10.1002/cne.903180308
Mesulam, M. M., Mash, D., Hersh, L., Bothwell, M., and Geula, C. (1992b). Cholinergic innervation of the human striatum, globus pallidus, subthalamic nucleus, substantia nigra, and red nucleus. J. Comp. Neurol. 323, 252–268. doi: 10.1002/cne.903230209
Middleton, F. A., and Strick, P. L. (2000). Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res. Rev. 31, 236–250. doi: 10.1016/S0165-0173(99)00040-5
Miller, W. C., and DeLong, M. R. (1987). “Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate MPTP model of parkinsonism,” in The Basal Ganglia II, (Springer), 415–427.
Mink, J. W. (1996). The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425. doi: 10.1016/S0301-0082(96)00042-1
Mitani, A., Ito, K., Hallanger, A. E., Wainer, B. H., Kataoka, K., and McCarley, R. W. (1988). Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat. Brain Res. 451, 397–402. doi: 10.1016/0006-8993(88)90792-5
Moonedley, S., and Graybiel, A. M. (1980). “Connections of the nucleus tegmenti pedunculopontinis, pars compacta (TPc) in cat,” in Anatomical Record, Vol. 196 (New York, NY: John Wiley & Sons Inc., Wiley-Liss), A129–A129.
Morris, M. E., Iansek, R., Matyas, T. A., and Summers, J. J. (1994). The pathogenesis of gait hypokinesia in Parkinson's disease. Brain 117, 1169–1181. doi: 10.1093/brain/117.5.1169
Moruzzi, G., and Magoun, H. W. (1949). Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1, 455–473. doi: 10.1016/0013-4694(49)90219-9
Muthusamy, K. A., Aravamuthan, B. R., Kringelbach, M. L., Jenkinson, N., Voets, N. L., Johansen-Berg, H., et al. (2007). Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J. Neurosurg. 107, 814–8120. doi: 10.3171/JNS-07/10/0814
Nakamura, Y., Tokuno, H., Moriizumi, T., Kitao, Y., and Kudo, M. (1989). Monosynaptic nigral inputs to the pedunculopontine tegmental nucleus neurons which send their axons to the medial reticular formation in the medulla oblongata. An electron microscopic study in the cat. Neurosci. Lett. 103, 145–150.
Nambu, A., Tokuno, H., and Takada, M. (2002). Functional significance of the cortico–subthalamo–pallidal “hyperdirect” pathway. Neurosci. Res. 43, 111–117. doi: 10.1016/S0168-0102(02)00027-5
Nevet, A., Morris, G., Saban, G., Fainstein, N., and Bergman, H. (2004). Discharge rate of substantia nigra pars reticulata neurons is reduced in non-parkinsonian monkeys with apomorphine-induced orofacial dyskinesia. J. Neurophysiol. 92, 1973–1981. doi: 10.1152/jn.01036.2003
Nomura, S., Mizuno, N., and Sugimoto, T. (1980). Direct projections from the pedunculopontine tegmental nucleus to the subthalamic nucleus in the cat. Brain Res. 196, 223–227. doi: 10.1016/0006-8993(80)90728-3
Pahapill, P. A., and Lozano, A. M. (2000). The pedunculopontine nucleus and Parkinson's disease. Brain 123, 1767–1783. doi: 10.1093/brain/123.9.1767
Pan, W. X., and Hyland, B. I. (2005). Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J. Neurosci. 25, 4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005
Pare, D., Smith, Y., Parent, A., and Steriade, M. (1988). Projections of brainstem core cholinergic and non-cholinergic neurons of cat to intralaminar and reticular thalamic nuclei. Neuroscience 25, 69–86. doi: 10.1016/0306-4522(88)90007-3
Parent, A., and Hazrati, L. N. (1995). Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res. Rev. 20, 91–127.
Reese, N. B., Garcia-Rill, E., and Skinner, R. D. (1995). The pedunculopontine nucleus—auditory input, arousal and pathophysiology. Prog. Neurobiol. 47, 105–133. doi: 10.1016/0301-0082(95)00023-O
Reiner, A., Albin, R. L., Anderson, K. D., D'Amato, C. J., Penney, J. B., and Young, A. B. (1988). Differential loss of striatal projection neurons in Huntington disease. Proc. Natl. Acad. Sci. U.S.A. 85, 5733–5737. doi: 10.1073/pnas.85.15.5733
Ros, H., Magill, P. J., Moss, J., Bolam, J. P., and Mena-Segovia, J. (2010). Distinct types of non-cholinergic pedunculopontine neurons are differentially modulated during global brain states. Neuroscience 170, 78–91. doi: 10.1016/j.neuroscience.2010.06.068
Rolland, A. S., Tandé, D., Herrero, M. T., Luquin, M. R., Vazquez-Claverie, M., Karachi, C., et al. (2009). Evidence for a dopaminergic innervation of the pedunculopontine nucleus in monkeys, and its drastic reduction after MPTP intoxication. J. Neurochem. 110, 1321–1329. doi: 10.1111/j.1471-4159.2009.06220.x
Romo, R., and Schultz, W. (1990). Dopamine neurons of the monkey midbrain, contingencies of responses to active touch during self-initiated arm movements. J. Neurophysiol. 63, 592–606. doi: 10.1152/jn.1990.63.3.592
Rye, D. B. (1997). Contributions of the pedunculopontine region to normal and altered REM sleep. Sleep 20, 757–788. doi: 10.1093/sleep/20.9.757
Rye, D. B., Johnston, L. H., Watts, R. L., and Bliwise, D. L. (1999). Juvenile Parkinson's disease with REM sleep behavior disorder, sleepiness, and daytime REM onset. Neurology 53, 1868–1868.
Rye, D. B., Lee, H. J., Saper, C. B., and Wainer, B. H. (1988). Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmentum in the rat. J. Comp. Neurol. 269, 315–341. doi: 10.1002/cne.902690302
Rye, D. B., Saper, C. B., Lee, H. J., and Wainer, B. H. (1987). Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J. Comp. Neurol. 259, 483–528. doi: 10.1002/cne.902590403
Saper, C. B., and Loewy, A. D. (1982). Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry. Brain Res. 252, 367–372. doi: 10.1016/0006-8993(82)90404-8
Scarnati, E., and Florio, T. (1997). The pedunculopontine nucleus and related structures: functional organization. Adv. Neurol. 74, 97–110.
Scarnati, E., Campana, E., and Pacitti, C. (1984). Pedunculopontine-evoked excitation of substantia nigra neurons in the rat. Brain Res. 304, 351–361. doi: 10.1016/0006-8993(84)90339-1
Scarnati, E., Di Loreto, S., Proia, A., and Gallie, G. (1988). The functional role of the pedunculopontine nucleus in the regulation of the electrical activity of entopeduncular neurons in the rat. Arch. Ital. Biol. 126, 145–163.
Scarnati, E., Florio, T., Capozzo, A., Confalone, G., and Mazzone, P. (2011). The pedunculopontine tegmental nucleus: implications for a role in modulating spinal cord motoneuron excitability. J. Neural Transm. 118, 1409–1421. doi: 10.1007/s00702-010-0532-2
Schultz, W. (1998). Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27. doi: 10.1152/jn.1998.80.1.1
Schultz, W., and Romo, R. (1990). Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions. J. Neurophysiol. 63, 607–624. doi: 10.1152/jn.1990.63.3.607
Schultz, W., Apicella, P., Scarnati, E., and Ljungberg, T. (1992). Neuronal activity in monkey ventral striatum related to the expectation of reward. J. Neurosci. 12, 4595–4610.
Schultz, W., Dayan, P., and Montague, P. R. (1997). A neural substrate of prediction and reward. Science 275, 1593–1599.
Semba, K., Reiner, P. B., and Fibiger, H. C. (1990). Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38, 643–654. doi: 10.1016/0306-4522(90)90058-C
Sims, K. S., and Williams, R. S. (1990). The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience 36, 449–472. doi: 10.1016/0306-4522(90)90440-F
Skinner, R. D., and Garcia-Rill, E. (1984). The mesencephalic locomotor region (MLR) in the rat. Brain Res. 323, 385–389. doi: 10.1016/0006-8993(84)90319-6
Skinner, R. D., Kinjo, N., Henderson, V., and Garcia-Rill, E. (1990). Locomotor projections from the pedunculopontine nucleus to the spinai cord. Neuroreport 1, 183–186. doi: 10.1097/00001756-199011000-00001
Spann, B. M., and Grofova, I. (1992). Cholinergic and non-cholinergic neurons in the rat pedunculopontine tegmental nucleus. Anat. Embryol. 186, 215–227. doi: 10.1007/BF00174143
Sparks, D. L. (2002). The brainstem control of saccadic eye movements. Nat. Rev. Neurosci. 3, 952–964. doi: 10.1038/nrn986
Starr, P. A., Rau, G. M., Davis, V., Marks, W. J., Ostrem, J. L., Simmons, D., et al. (2005). Spontaneous pallidal neuronal activity in human dystonia: comparison with Parkinson's disease and normal macaque. J. Neurophysiol. 93, 3165–3176. doi: 10.1152/jn.00971.2004
Steckler, T., Keith, A. B., and Sahgal, A. (1994). Lesions of the pedunculopontine tegmental nucleus do not alter delayed non-matching to position accuracy. Behav. Brain Res. 61, 107–112.
Stein, J. F. (2009). Akinesia, motor oscillations and the pedunculopontine nucleus in rats and men. Exp. Neurol. 215, 1–4. doi: 10.1016/j.expneurol.2008.09.022
Steriade, M. (1996). Arousal: revisiting the reticular activating system. Science 272:225. doi: 10.1126/science.272.5259.225
Steriade, M. (2004). Acetylcholine systems and rhythmic activities during the waking–sleep cycle. Prog. Brain Res. 145, 179–196. doi: 10.1016/S0079-6123(03)45013-9
Steriade, M., and Buzsaki, G. (1990). Parallel activation of thalamic and cortical neurons by brainstem and basal forebrain cholinergic systems. Brain Cholinergic Syst. 3–62.
Steriade, M., Paré, D., Parent, A., and Smith, Y. (1988). Projections of cholinergic and non-cholinergic neurons of the brainstem core to relay and associational thalamic nuclei in the cat and macaque monkey. Neuroscience 25, 47–67. doi: 10.1016/0306-4522(88)90006-1
Takakusaki, K. (2008). Forebrain control of locomotor behaviors. Brain Res. Rev. 57, 192–198. doi: 10.1016/j.brainresrev.2007.06.024
Takakusaki, K., Habaguchi, T., Ohtinata-Sugimoto, J., Saitoh, K., and Sakamoto, T. (2003). Basal ganglia efferents to the brainstem centers controlling postural muscle tone and locomotion: a new concept for understanding motor disorders in basal ganglia dysfunction. Neuroscience 119, 293–308. doi: 10.1016/S0306-4522(03)00095-2
Takakusaki, K., Habaguchi, T., Saitoh, K., and Kohyama, J. (2004a). Changes in the excitability of hindlimb motoneurons during muscular atonia induced by stimulating the pedunculopontine tegmental nucleus in cats. Neuroscience 124, 467–480. doi: 10.1016/j.neuroscience.2003.12.016
Takakusaki, K., Oohinata-Sugimoto, J., Saitoh, K., and Habaguchi, T. (2004b). Role of basal ganglia–brainstem systems in the control of postural muscle tone and locomotion. Prog. Brain Res. 143, 231–237.
Takakusaki, K., Saitoh, K., Harada, H., and Kashiwayanagi, M. (2004c). Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neurosci. Res. 50, 137–151.
Thevathasan, W., Cole, M. H., Graepel, C. L., Cara, C. L., Jonathan, J. A., Jenkinson, J. S., et al. (2012). A spatiotemporal analysis of gait freezing and the impact of pedunculopontine nucleus stimulation. Brain. 135, 1446–1454. doi: 10.1093/brain/aws039
Turner, R. S., and Anderson, M. E. (1997). Pallidal discharge related to the kinematics of reaching movements in two dimensions. J. Neurophysiol. 77, 1051–1074. doi: 10.1152/jn.1997.77.3.1051
Villiger, E., and Piersol, G. A. (1912). Brain and Spinal Cord; A Manual for the Study of the Morphology and Fibre-Tracts of the Central Nervous System. Philadelphia, PA; London: J. B. Lippincott.
Vincent, S. R., and Kimura, H. (1992). Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46, 755–784. doi: 10.1016/0306-4522(92)90184-4
Vincent, S. R., Satoh, K., Armstrong, D. M., Panula, P., Vale, W., and Fibiger, H. C. (1986). Neuropeptides and NADPH-diaphorase activity in the ascending cholinergic reticular system of the rat. Neuroscience 17, 167–182. doi: 10.1016/0306-4522(86)90234-4
von Monakow, H. K., Akert, K., and Künzle, H. (1979). Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Experimental brain research 34, 91–105. doi: 10.1007/BF00238343
Wallace, M. N., and Fredens, K. (1988). Origin of high acetylcholinesterase activity in the mouse superior colliculus. Experimental brain research 72, 335–346. doi: 10.1007/BF00250255
Wang, H. L., and Morales, M. (2009). Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat. European Journal of Neuroscience 29, 340–358. doi: 10.1111/j.1460-9568.2008.06576.x
Waraczynski, M., and Perkins, M. (1998). Lesions of pontomesencephalic cholinergic nuclei do not substantially disrupt the reward value of medial forebrain bundle stimulation. Brain Res. 800, 154–169. doi: 10.1016/S0006-8993(98)00519-8
Whitehouse, P. J., Hedreen, J. C., White, C. L., and Price, D. L. (1983). Basal forebrain neurons in the dementia of Parkinson disease. Ann. Neurol. 13, 243–248. doi: 10.1002/ana.410130304
Wichmann, T., and DeLong, M. R. (1996). Functional and pathophysiological models of the basal ganglia. Curr. Opin. Neurobiol. 6, 751–758. doi: 10.1016/S0959-4388(96)80024-9
Wichmann, T., and DeLong, M. R. (2003). Pathophysiology of Parkinson's disease: the MPTP primate model of the human disorder. Ann. N. Y. Acad. Sci. 991, 199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x
Winn, P. (1998). Frontal syndrome as a consequence of lesions in the pedunculopontine tegmental nucleus: a short theoretical review. Brain Res. Bull. 47, 551–563. doi: 10.1016/S0361-9230(98)00136-1
Winn, P. (2008). Experimental studies of pedunculopontine functions: are they motor, sensory or integrative? Parkinsonism Relat. Disord. 14, S194–S198.
Woolf, N. J., and Butcher, L. L. (1986). Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain research bulletin 16, 603–637.
Yeomans, J. S., Mathur, A., and Tampakeras, M. (1993). Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav. Neurosci. 107, (1077).
Yoshida, M., and Precht, W. (1971). Monosynaptic inhibition of neurons of the substantia nigra by caudatonigral fibers. Brain Res. 32, 225–228. doi: 10.1016/0006-8993(71)90170-3
Zweig, R. M., Whitehouse, P. J., Casanova, M. F., Walker, L. C., Januarykel, W. R., and Price, D. L. (1987). Loss of pedunculopontine neurons in progressive supranuclear palsy. Annals of neurology 22, 18–25.
Keywords: Pedunculopontine nucleus, mesenphalic locomotor region, basal ganglia, substantia nigra, brainstem, Parkinson's disease, deep brain stimulation
Citation: French IT and Muthusamy KA (2018) A Review of the Pedunculopontine Nucleus in Parkinson's Disease. Front. Aging Neurosci. 10:99. doi: 10.3389/fnagi.2018.00099
Received: 31 May 2017; Accepted: 22 March 2018;
Published: 26 April 2018.
Edited by:
Elizabeth B. Torres, Rutgers University, The State University of New Jersey, United StatesReviewed by:
Jose Bargas, Universidad Nacional Autónoma de México, MexicoTipu Z. Aziz, John Radcliffe Hospital, United Kingdom
Copyright © 2018 French and Muthusamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isobel T. French, YmVsbGVpdGY4OEBnbWFpbC5jb20=
 Isobel T. French
Isobel T. French Kalai A. Muthusamy
Kalai A. Muthusamy