
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Aging Neurosci. , 07 March 2018
Sec. Neurocognitive Aging and Behavior
Volume 10 - 2018 | https://doi.org/10.3389/fnagi.2018.00049
This article is part of the Research Topic Nutritional cognitive neuroscience: research at the crossroads of nutrition, psychology, and neuroscience View all 17 articles
Modifying nutritional intake through supplementation may be efficacious for altering the trajectory of cerebral structural decline evident with increasing age. To date, there have been a number of clinical trials in older adults whereby chronic supplementation with B vitamins, omega-3 fatty acids, or resveratrol, has been observed to either slow the rate of decline or repair cerebral tissue. There is also some evidence from animal studies indicating that supplementation with glycerophospholipids (GPL) may benefit cerebral structure, though these effects have not yet been investigated in adult humans. Despite this paucity of research, there are a number of factors predicting poorer cerebral structure in older humans, which GPL supplementation appears to beneficially modify or protect against. These include elevated concentrations of homocysteine, unbalanced activity of reactive oxygen species both increasing the risk of oxidative stress, increased concentrations of pro-inflammatory messengers, as well as poorer cardio- and cerebrovascular function. As such, it is hypothesized that GPL supplementation will support cerebral structure in older adults. These cerebral effects may influence cognitive function. The current review aims to provide a theoretical basis for future clinical trials investigating the effects of GPL supplementation on cerebral structural integrity in older adults.
Cerebral structure has been observed to decline with increasing age. Age-related reductions in cerebral structural integrity are evident at both the macro- and microstructural levels (e.g., reduced whole and regional volumes, elevated cortical thinning, increased severity of white matter lesions, and decreased integrity of microscopic white matter pathways), and may begin as early as young adulthood (Resnick et al., 2003; Allen et al., 2005; Fotenos et al., 2005; Walhovd et al., 2005; Bendlin et al., 2010; Hsu et al., 2010; Westlye et al., 2010; Sala et al., 2012; Taki et al., 2013a). There is also a growing literature purporting reduced cerebral structural integrity as a significant predictor of cognitive functioning apparent in older age (Davis et al., 2009; Bendlin et al., 2010; Lockhart et al., 2012; Arvanitakis et al., 2016).
Although all adults demonstrate at least some deterioration in cerebral structure (and cognitive function) with age, the trajectory of decline is not fixed. While some adults appear to demonstrate cerebral decline consistent with “normal aging,” others have conditions such as (in order of worsening dysfunction) “age-associated memory impairment” (AAMI), “mild cognitive impairment” (MCI), and Alzheimer's dementia (AD), all of which are characterized by more severe degradation of cerebral structure, as well as cognitive impairment (Anstey and Maller, 2003; Hänggi et al., 2011; Smith et al., 2011; Bosch et al., 2012; Maillard et al., 2012; Wang et al., 2014; Zheng et al., 2014). These conditions are not inevitable features of increasing age, and it may be possible to reduce their incidence within the population though administration of interventions capable of supporting cerebral structure in older adults.
One factor believed to influence cerebral structural integrity with age is nutritional intake (Scholey, 2018). Previous cross-sectional and longitudinal work has indicated that greater ingestion and bioavailability of B vitamins such as B6, B12, and folate (Erickson et al., 2008; Vogiatzoglou et al., 2008; Tangney et al., 2011; Hsu et al., 2015; Hooshmand et al., 2016; Köbe et al., 2016) or omega-3 polyunsaturated fatty acids (ω3-PUFA; Samieri et al., 2012; Tan et al., 2012; Titova et al., 2013; Pottala et al., 2014; Gu et al., 2016) predicts greater cerebral macro- and micro-structural integrity in older adults. Moreover, data from available clinical trials indicates that chronic supplementation with these nutrients, but also others such as resveratrol, may either enhance cerebral structural integrity, or improve the trajectory of cerebral decline over time (Smith et al., 2010; Douaud et al., 2013; Witte et al., 2013; Jerneren et al., 2015; Köbe et al., 2017; Zhang et al., 2017). It is possible that these structural effects, at least partly, underpin the benefits to cognitive performance in older adults following supplementation (Durga et al., 2007; Yurko-Mauro et al., 2010; de Jager et al., 2012; Witte et al., 2013).
The consumption of phospholipids (PL) and in particular the glycerophospholipids (GPL), may also benefit cerebral structure and subsequently cognitive function in older adults. The GPL species phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylserine (PS) are abundant in mammalian cell membranes, and there is growing evidence that provision of these GPL (particularly PC and PS) can improve cognitive function in animals via oral supplementation (Zanotti et al., 1989; Furushiro et al., 1997; Lim and Suzuki, 2000; Suzuki et al., 2001; Kataoka-kato et al., 2005; Yaguchi et al., 2009, 2010; Lee et al., 2010; Babenko and Semenova, 2011; Nagata et al., 2011; Park et al., 2013; Zhang et al., 2015; Qu et al., 2016; Wen et al., 2016a,b) or intraperitoneal/intracerebral injection (Drago et al., 1981; Zanotti et al., 1984; Corwin et al., 1985; Sakai et al., 1996; Blokland et al., 1999; Claro et al., 1999, 2006; Suzuki et al., 2000). Similar results are also evident following oral supplementation in older humans with varying levels of cognitive function (i.e., normal cognitive function with subjective memory complaints, age-related “cognitive dysfunction,” AAMI, MCI, or dementia). The details and findings of these trials are summarized in Tables 1–3.
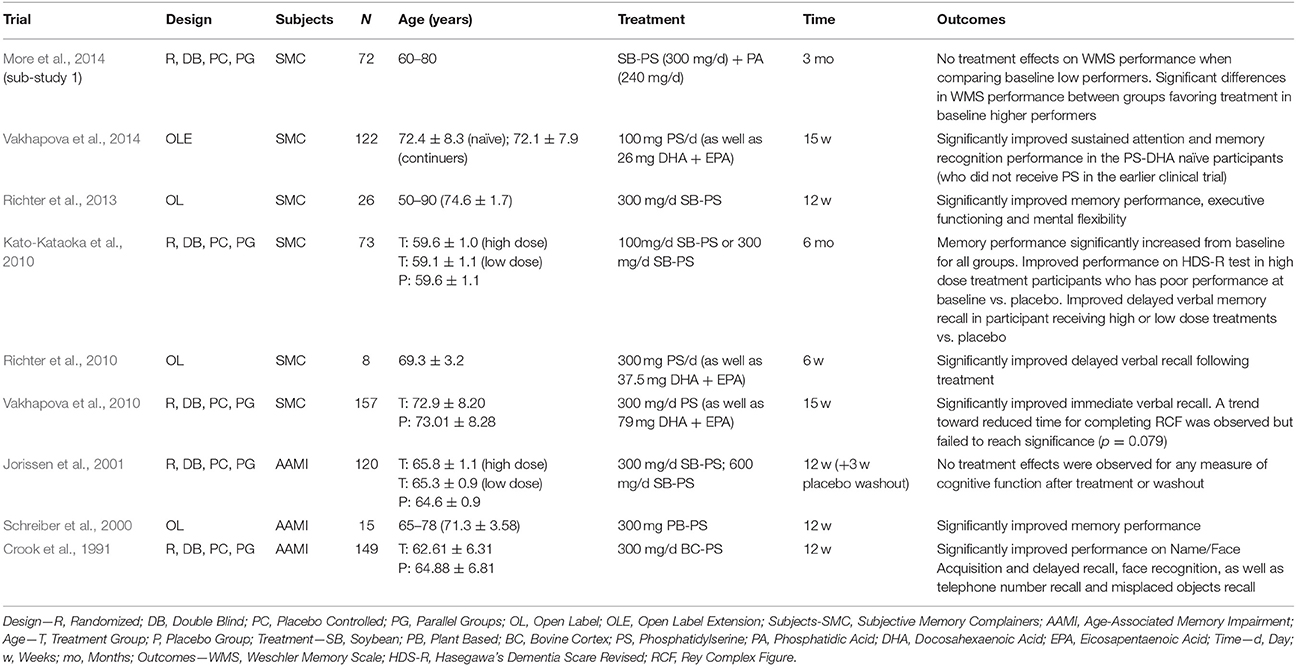
Table 1. GPL supplementation and cognitive function in older adults with subjective memory complaints (trials listed in reverse chronological order) and by subject type.
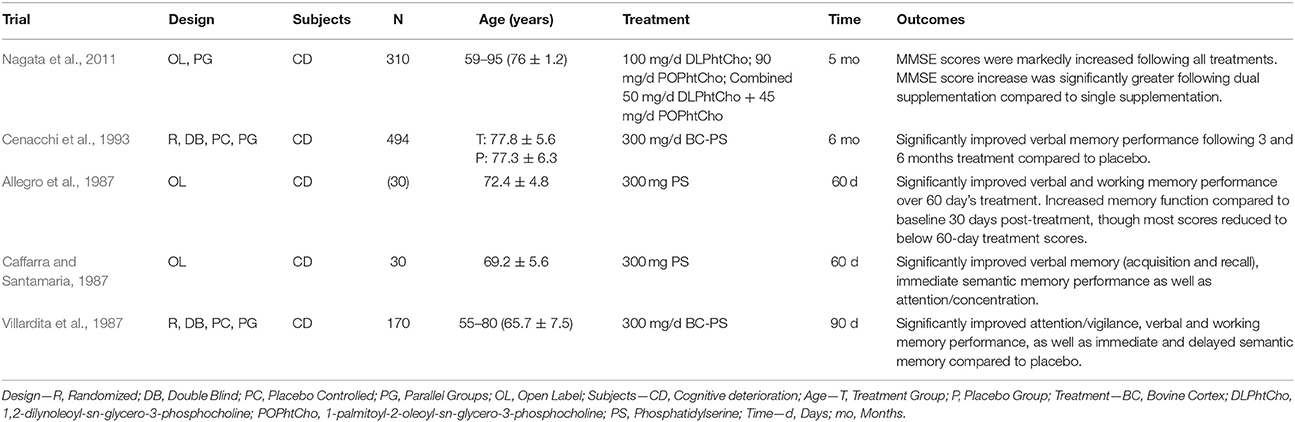
Table 2. GPL supplementation and cognitive function in older adults with age-related “cognitive deterioration” (trials listed in reverse chronological order).
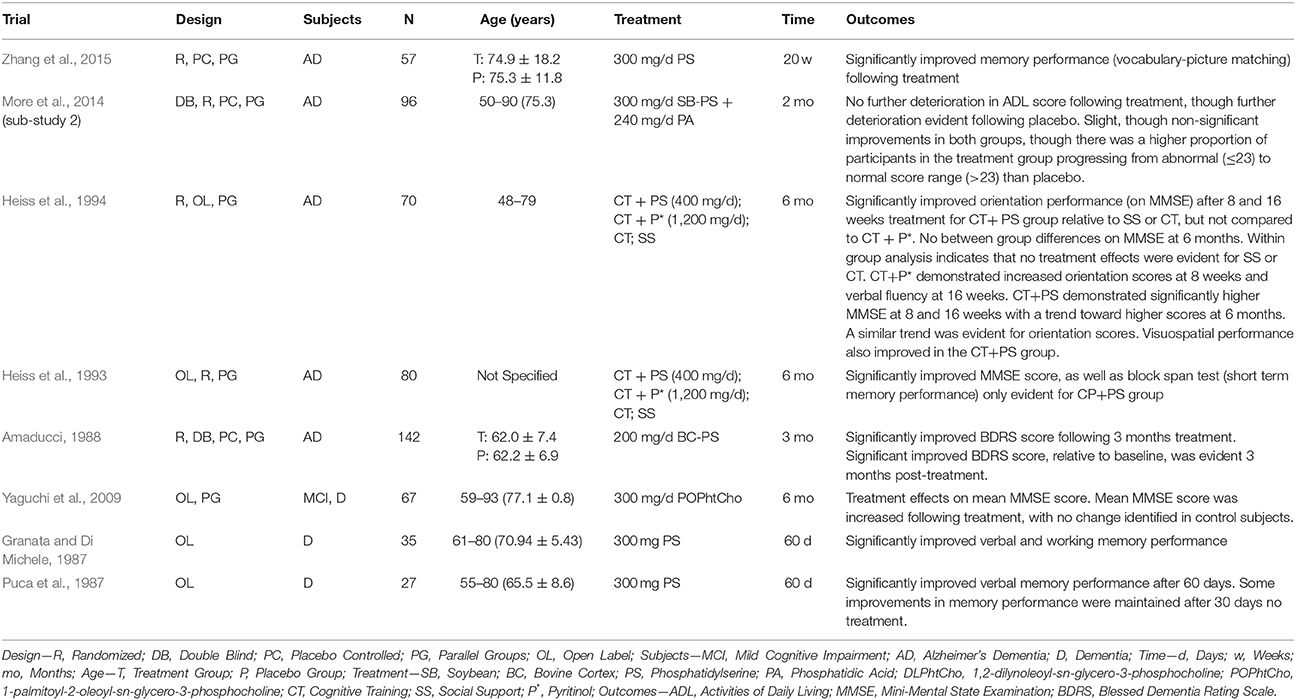
Table 3. GPL supplementation and cognitive function in older adults with dementia (trials listed in reverse chronological order) and by subject type.
While the evidence for GPL supplementation benefiting cognitive function is relatively robust, there is comparatively less work investigating their effects on cerebral structure. In a number studies with middle-aged and older rodents, GPL supplementation was found to improve hippocampal cell morphology (Nunzi et al., 1987; Crespo et al., 2004; Qu et al., 2016), as well as elevate cellular proliferation and survival within the dentate gyrus (Maragno et al., 2015). Likewise, 7 months oral supplementation with PE (1,2-dilinoleoyl-sn-glycero-3-phosphoethanolamine) has been observed to reduce age-related hippocampal neuron death in senescence accelerated SAMP8 rodents (Yaguchi et al., 2010). However, to date there have been no such studies with older humans.
Interestingly, GPL supplementation may potentially modify a number of factors predicting compromised cerebral structural in older humans. These include elevated homocysteine (HcY) concentrations, unbalanced activity of reactive oxygen species (ROS) activity and increased oxidative stress (OxS), higher levels of pro-inflammatory messengers, as well as poorer cardio- and cerebrovascular functioning. By modifying these factors, GPL supplementation may support cerebral structural integrity, and subsequently cognitive function, in older adults.
In this review, an introduction to GPL—their chemical structure, how they are synthesized in vivo and from what foods they are available from—will be presented. Following this, there will be an overview of each of the aforementioned factors, how they relate to cerebral structure (e.g., whole and regional cerebral volume, cortical thinning, severity of white matter lesions, and the integrity of microscopic white matter pathways) as well as cognitive function. This will be complemented with an overview of the available studies investigating the extent to which GPL supplementation modifies, or protects against, these risk factors. Overall, it is anticipated that GPL supplementation, particularly species containing choline and/or ω3-PUFA, may beneficially modify risk factors predicting cerebral structural decline, thereby supporting cerebral structure and subsequently cognitive function in older adults.
The term “phospholipid” (PL) may be used to describe any lipid (fatty acid) with a phosphoric acid residue (Hanahan, 1997). There are two major classes of PL—glycerophospholipids (GPL) and sphingolipids, both being essential components of cellular membranes, including those forming cerebral tissue. This review will focus on GPL, specifically PC, PE, and PS as these are the most common GPL species within mammalian cell membranes (Castro-Gómez et al., 2015), but also the most frequently examined GPL species in relation to neurocognitive health, or risk factors pertaining to neurocognitive health in older adults.
Glycerol forms the backbone of the GPL molecule and it contains three hydroxyl groups (sn-1, sn-2, and sn-3). A phosphate group distinguishing the overall species of GPL is attached to the glycerol backbone at sn-3 (Ridgway, 2016). The attached phosphate may be choline, ethanolamine, or serine, thereby forming PC, PE, or PS, respectively (Ridgway, 2016). Meanwhile, fatty acids are attached to glycerol at sn-1 and sn-2. Quite often sn-1 is occupied by a saturated fatty acid, while sn-2 is occupied by an unsaturated fatty acid, with polyunsaturated fatty acids being especially prevalent in GPL composing cerebral tissue membranes (Castro-Gómez et al., 2015). The combination of different phosphate groups and fatty acids gives rise to over 1,000 different subspecies of GPL (Vance, 2008). The structure of GPL species evident within mammalian cell membranes (PC, PE, PS as well as phosphatidylinositol) is presented in Figure 1.
The phosphate group at sn-3 forms the hydrophilic head of the GPL molecule, whereas the fatty acids attached at sn-1 and sn-2 form the hydrophobic tail (Castro-Gómez et al., 2015). The presence of a hydrophilic head and hydrophobic tail results in the formation bi-lipid layers when GPL are suspended together in aqueous solutions (Cooper and Hausman, 2007). This proclivity toward forming bi-lipid layers allows GPL (together with sphingolipids and proteins) to form the cell bi-lipid membrane, though different species of GPL appear to be differentially concentrated within membrane layers. PC is primarily located within the outer membrane layer (sometimes termed the outer leaflet) alongside sphingomyelin. Conversely, PE is mostly concentrated within the inner membrane layer (the inner leaflet), with PS being exclusively located within the inner leaflet (Devaux and Zachowski, 1994; Castro-Gómez et al., 2015).
GPL such as PC, PE, and PS may be synthesized in vivo via a number of different pathways. Some of these pathways involve de novo synthesis, whereas others involve remodeling of pre-existing GPL.
An essential process prior to the de novo synthesis of PC and PE (but also phosphatidylinositol) is the initial formation of phosphatidic acid (Kent, 1995). Once synthesized, phosphatidic acid is converted to either 1,2-diacylglycerol or cytidine diphosphate (CDP) diacylglycerol by two different enzymes—phosphatidic acid phosphatase and CDP-diacylglycerol synthase, respectively (Kent, 1995). 1,2-diacylglcerol is important for synthesis of PC and PE, whereas CDP-diacylglycerol is essential for the formation of phosphatidylinositol in mammalian cells (Kent, 1995). In mammalian cells, PS synthesis is not reliant upon phosphatidic acid synthesis. The major de novo and remodeling pathways associated with GPL biosynthesis in mammalian cells are outlined in Figure 2.
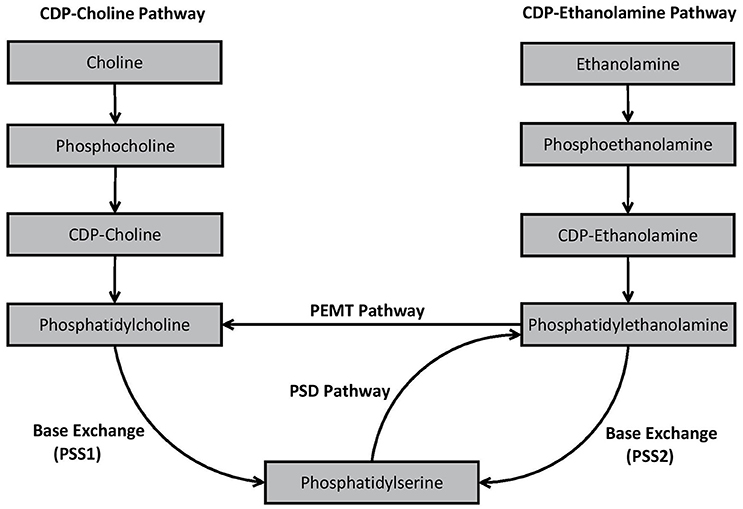
Figure 2. Biosynthesis pathways of phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine in mammalian cells.
As shown in Figure 2, PC is produced in vivo via two major pathways—the cytidine disphosphocholine (CDP-choline) pathway, and the phosphatidylethanolamine N-methyltransferase (PEMT) pathway. The CDP-choline pathway involves de novo synthesis of PC whereas the PEMT pathway involves remodeling of pre-existing PE. PE is synthesized analogously to PC via the CDP-ethanolamine pathway, but also by remodeling pre-existing PS via the PS decarboxylase (PSD) pathway. In mammalian cells, PS is primarily synthesized through base exchange reactions mediated by specialized synthases. PS synthase-1 (PSS1) causes free serine to be switched for choline from PC, while PSS2 switches ethanolamine in PE with free serine, thereby formulating PS. Detailed decriptions of the specific biochemical processes involved in each of these pathways have been provided elsewhere (Kent, 1995; Vance, 2008; Vance and Tasseva, 2013).
While different pathways may produce the same general species of GPL (e.g., CDP-choline and PEMT both produce PC), the pathway from which the GPL was synthesized appears to influence the fatty acid composition of the resultant GPL. For example, PC resulting from the CDP-choline pathway typically contains saturated, monounsaturated or diunsaturated fatty acid species, whereas PC synthesized via the PEMT pathway may also contain polyunsaturated fatty acids (PUFA), such as the ω3-PUFA species DHA (Delong et al., 1999; Pynn et al., 2011). Although this may be due to a substrate preference of the PEMT pathway for PE containing ω3-PUFA (Delong et al., 1999), others (e.g., Vance, 2014) suggest that the extent to which DHA enriched PC is produced is more likely dependent upon the availability of DHA within the overall reservoir of PE (Ridgway and Vance, 1988).
Differences in fatty acid composition depending on synthesis pathways are also apparent for PE. PE produced via the CDP-ethanolamine pathway predominately contains mono- or di-unsaturated fatty acid species at sn-2. Conversely, PE derived from the PSD pathway, predominately synthesizes PE containing PUFA such as the ω6-PUFA arachidonic acid (ARA) or ω3-PUFA's such as DHA or eicosapentaenoic acid (EPA) (Bleijerveld et al., 2007). Substrate specificity is apparent during PS synthesis in mammalian cells. Data from several studies indicate that PSS1 and PSS2 preference species of PC or PE containing ω3-PUFA, particularly DHA, compared to other subspecies (Kim et al., 2004; Kimura and Kim, 2013).
In addition to in vivo synthesis, GPL may be obtained from the diet. The estimated dietary intake of GPL is two to eight grams per day, representing one to ten percent of daily fat intake (Cohn et al., 2010). GPL are present in foods such as milk, eggs, skeletal, and organ (e.g., brain) meat from terrestrial animals (e.g., cow or pig), as well as fish (especially krill or squid). GPL may also be obtained by consuming certain seeds and legumes such as rapeseeds, sunflower seeds and soybeans (Weihrauch and Son, 1983).
The types of fatty acids contained within GPL appear to vary between food sources. The GPL derived from foods such as marine fish (e.g., krill or squid), mammalian brain (e.g., cow or pig) or eggs (particularly those from hens whose feed is fortified with ω3-PUFA) appear to contain ω3-PUFA such as DHA (Bourre et al., 1993; Bourre and Dumont, 2002; Favrelière et al., 2003; Chen and Li, 2008). In soybeans, ω3-PUFA is present in the form of α-linolenic acid (ALA) (Bourre and Dumont, 2002; Chen and Li, 2008), which may be later converted to longer chain ω3-PUFA species such as EPA and DHA (Burdge and Calder, 2005). Soybeans, but also fish, mammalian brain and eggs also provide varying levels of ω6-PUFA and other fatty acids (Bourre and Dumont, 2002; Favrelière et al., 2003; Chen and Li, 2008).
Aside from whole foods, GPL may be obtained from commercially available supplements. Importantly, the fatty acid composition of these supplemental GPL will vary depending on the food from which the GPL was extracted. GPL supplements may be consumed as capsules (e.g., krill oil), as well as powdered compounds (summarized in Küllenberg et al., 2012). Supplements in powder form are liquefiable thereby making them easier to consume. This may be particularly important for older adults especially those who experience difficulty swallowing (a common occurrence in the general population termed dysphagia; Aslam and Vaezi, 2013). These adults may be at an increased risk of developing nutritional insufficiency or deficiency (Sura et al., 2012).
Prior work has indicated that the bioavailability of ω3-PUFA may be increased following ingestion of whole foods or supplements containing this species of fatty acids (Popp-Snijders et al., 1986; Bourre et al., 1993; Yaqoob et al., 2000; Bourre and Dumont, 2002; Kew et al., 2004; Ulven et al., 2011; Browning et al., 2012). Other work demonstrates that the bioavailability of PUFA may be enhanced when they are consumed as GPL rather than triglycerides (Wijendran et al., 2002; Ramprasath et al., 2013). As such, GPL supplementation may be an effective method of increasing the bioavailability of nutrients such as ω3-PUFA. Given that other nutrients such as choline may also be attached to GPL, it is likely that GPL supplementation will also boost the bioavailability of these nutrients.
The concentrations of PL within cerebral tissue appears to decline with increasing age (Soderberg et al., 1990, 1991; Svennerholm et al., 1994, 1997). As such, increasing the dietary intake of PL, especially GPL, may benefit cerebral structure integrity due their incorporation into neuronal membranes. However, supplementation with GPL may also indirectly support cerebral structure by modifying, or protecting against, a number of factors that appear to predict poorer cerebral structure in older adults. It is these potential indirect effects that form the focus of this review. Specifically, this review will discuss biochemical (HcY, ROS activity and OxS, and inflammation) and biophysical (cardio- and cerebrovascular function) risk factors associated with poorer cerebral structural integrity, but also cognitive decline, and the extent to which GPL supplementation has been observed to modify, or protect against, these factors. There will also be a discussion of the potential pathways through which GPL supplementation may facilitate these effects. Overall, it is anticipated that, GPL supplementation will benefit cerebral structural integrity and subsequently cognitive function in older adults, via modification of, or protection against, the aforementioned factors.
HcY is a sulfur containing amino acid derived from s-adenosylhomocysteine, a by-product of methyl reactions involving s-adenosylmethionine (Selhub et al., 2000). A number of studies with older adults have determined that elevated HcY is a significant predictor of reduced total and regional cerebral tissue volumes (Williams et al., 2002; den Heijer et al., 2003b; Firbank et al., 2010; Narayan et al., 2011a; Rajagopalan et al., 2011; Tangney et al., 2011; Feng et al., 2013; Madsen et al., 2015), increased frequency and volume of white matter lesions or silent brain infarcts (Vermeer et al., 2002; Wright et al., 2005; Seshadri et al., 2008; Raz et al., 2012), as well as reduced cerebral microstructural integrity (Bettcher et al., 2014; Hsu et al., 2015). Given the link between measures of cerebral macro- and microstructural integrity and cognitive functioning, it follows that HcY may also be an important predictor of cognitive function. In fact multiple studies have observed an increased risk of developing MCI and dementia in adults with elevated HcY (Launtenschlager et al., 2005; Kim et al., 2007; Whalley et al., 2014), but also poorer performance across a number of cognitive domains (Narayan et al., 2011b; Allam et al., 2013; Jochemsen et al., 2013; Parizkova et al., 2017).
While HcY concentrations may be influenced by non-modifiable factors such as genetics and age, there are also a number of modifiable factors believed to impact HcY concentrations. One of the most important modifiable factors is nutritional intake, in particular B vitamin ingestion/absorption (Joosten et al., 1996; Jacques et al., 2001; Refsum et al., 2006). Vitamins B6, B12, and folate are essential for HcY metabolism, with lower intake or bioavailability of these vitamins predicting elevated HcY concentrations (Jacques et al., 2001; Refsum et al., 2006). Supplementation with these B vitamins has been shown to lower HcY concentrations (Eussen et al., 2006; McMahon et al., 2006; Smith et al., 2010; Clarke et al., 2014; Ford and Almeida, 2014). Moreover, data from the VITACOG study indicates that chronic supplementation with vitamins B6, B12 and folate is beneficial to cerebral structure, with reduced rates of total and regional cerebral atrophy due to significant reductions in HcY concentrations (Smith et al., 2010; Douaud et al., 2013; Jerneren et al., 2015). Lowering HcY through supplementation may also improve cognitive performance with several studies with older adults having observed improved memory performance and processing speed following HcY subsequent to B vitamin supplementation (Durga et al., 2007; de Jager et al., 2012). However, several systematic reviews and meta-analyses have indicated that lowering HcY through B vitamin supplementation does not improve cognitive function in older adults (Clarke et al., 2014; Ford and Almeida, 2014), though methodological weaknesses in these reviews has led to their results being criticized in more recent work (Smith and Refsum, 2016).
Overall, it would appear that lowering HcY through nutritional supplementation may benefit cerebral structural integrity in older adults. It may also be beneficial to cognitive function, though these latter effects remain controversial within the literature.
Choline is similar to B vitamins, in that its level of dietary intake appears to predict HcY concentrations in adults. A number of animal and plant based foods such as red meats, poultry, fish, eggs, and milk, but also broccoli, potatoes, green beans, legumes, and nuts, contain choline (Cho et al., 2006; Detopoulou et al., 2008). Following ingestion and absorption, choline may be oxidized into betaine, which functions as a methyl donor in the betaine-homocysteine-methyltransferase pathway, whereby HcY is converted to methionine in a reaction catalyzed by the enzyme betaine-homocysteine methyltransferase (Olthof et al., 2005). Importantly, increased ingestion or bioavailability of choline (or its metabolite betaine) has been observed to negatively predict HcY concentrations in adults (Schwab et al., 2002; Steenge et al., 2003; Cho et al., 2006; Chiuve et al., 2007; Atkinson et al., 2008; Detopoulou et al., 2008).
Choline is highlighted here as it forms the base attachment distinguishing PC (but also the sphingolipid known as sphingomyelin). In fact, choline represents 15% of PC molecular weight (Cheatham et al., 2012) thereby making PC an important carrier of choline that may be sourced from the diet. As such, greater ingestion of PC may be expected to negatively predict HcY concentrations. In several cross sectional studies utilizing FFQ data from the Framingham Heart Study (Cho et al., 2006) as well as the Nurses Health Studies (Chiuve et al., 2007), a number of dietary compounds containing choline were examined in relation to HcY. Cho et al. (2006), determined that estimated PC ingestion was inversely associated with HcY concentrations with similar associations being identified for other choline containing compounds including sphingomyelin, phosphocholine, and glycerophosphocholine. However, after controlling for additional factors related to HcY concentrations (e.g., B vitamin intake, caffeine, and alcohol consumption) PC was no longer associated with HcY concentrations, though other choline containing compounds remained as significant predictors. Interestingly, Chiuve et al. (2007) observed a negative association between choline from phosphocholine and glycerophosphocholine with HcY while there appeared to be a non-significant positive association with PC intake.
In perhaps the only study examining the direct effect of PC supplementation on HcY concentrations, Olthof et al. (2005) administered a daily oral dose of 34 g soybean lecithin (comprised of PC) delivering 2.6 g choline, to adult males aged 50–71 years over 2 weeks. Olthof et al. (2005) observed that HcY concentrations were 18% lower following treatment than placebo. However, it is important to note that the level of choline ingested in this study is well beyond the estimated mean daily intakes reported by both Cho et al. (2006) and Chiuve et al. (2007) (313 ± 61 and 323 mg/d, respectively). As such, it is possible that PC, due the provision of choline, will lower HcY at doses attainable through a combination of normal diet and supplementation than from diet alone.
Lowering HcY has been observed to benefit cerebral structural integrity in a number of clinical trials involving chronic B vitamin supplementation. As such, it is plausible that similar effects may be evident following chronic supplementation with PC due to the provision of choline. However, to date there has been minimal investigation of the use of PC as a means of lowering HcY. This is intriguing as choline, similar to select B vitamins, is an essential nutrient for the methylation of HcY. The relative lack of data determining the relationship between PC intake and HcY concentrations should be rectified by additional well-designed RCT's. The added utilization of advanced imaging methods in future studies will help determine how PC intake and subsequent HcY levels relate to cerebral structural integrity.
Despite there being a paucity of work determining how PC supplementation benefits cerebral structure in aging humans, it is plausible that PC supplementation may benefit cerebral structure by lowering HcY and alleviating the detrimental effects theorized to be associated with elevated concentrations of HcY. One effect of elevated HcY is an increased risk of hyperphosphorylated tau buildup as well as an increased presence of neurofibrillary tangles (NFT) within cerebral tissues (Luo et al., 2007; Zhang et al., 2008; Popp et al., 2009; Wei et al., 2011; Hooshmand et al., 2013; Li et al., 2014). Both of these factors have been linked to poorer cerebral macro- and microstructural integrity in adults across the spectrum of cognitive function (Whitwell et al., 2008; Polvikoski et al., 2010; Tosun et al., 2010; Glodzik et al., 2011, 2012; Marnane et al., 2016; de Souza et al., 2017; Hoy et al., 2017; Kantarci et al., 2017).
Increased HcY concentrations appears to exacerbate the production of hyperphosphorylated tau through inhibition of kinases, such as protein phosphatase 2A (PP2A), which are responsible for tau dephosphorylation (Luo et al., 2007; Zhang et al., 2008; Wei et al., 2011). However, lowering HcY concentration (through B vitamin supplementation) has been observed to alleviate PP2A inactivation thereby lowering tau hyperphosphorylation (Zhang et al., 2008; Wei et al., 2011). Further, while there have been no trials examining HcY lowering and changes to NFT load, given that NFT are largely composed of hyperphosphorylated tau proteins, lowering HcY should theoretically influence the extent to which NFT are formed and therefore evident within cerebral tissues. Although data is limited, PC supplementation, due to the provision of choline, has been observed to lower HcY concentrations. As such, it is possible that by lowering HcY, PC supplementation (administered in moderate to high doses) may facilitate reduced concentrations of hyperphosphorylated tau and subsequently NFT, thereby supporting cerebral structural integrity in the long term. These effects remain to be investigated in older adults.
In addition to increased hyperphosphorylated tau and NFT load, elevated HcY may facilitate more subtle effects potentially leading to reduced cerebral structural integrity. Elevated HcY concentrations inhibit cellular PEMT activity leading to reduced production of PC containing ω3-PUFA thereby facilitating distorted concentration ratios of PC and its precursor PE within cellular membranes (Innis et al., 2003; Miller et al., 2003; Devlin et al., 2007; Selley, 2007).
Lowered production of PC containing DHA, due to PEMT inhibition, may also partly explain the reduced concentrations of DHA observed within biological tissues when HcY levels are elevated (Innis et al., 2003; Miller et al., 2003; Li et al., 2006; Devlin et al., 2007; Selley, 2007; Rasmussen et al., 2010; Huang et al., 2013; Kume et al., 2013; Iglesia et al., 2017). The production of PC containing ω3-PUFA such as DHA by the PEMT pathway is a key process for transporting DHA from the liver into plasma and onwards to cerebral tissue (Smith and Refsum, 2016). Ultimately, lower concentrations of ω3-PUFA such as DHA within membranes (including those within cerebral tissue) may then facilitate an increased risk of OxS and elevated inflammation, subsequently leading to poorer cardio- and cerebrovascular function (elevated blood pressure, arterial stiffness, and permeability of the blood-brain barrier). These factors will be discussed in later sections of this review.
PC is a major dietary source of choline. As such increased ingestion of PC via supplementation, likely facilitates increased choline bioavailability thereby lowering HcY concentrations and subsequently alleviating the inhibitory effects of HcY upon PEMT activity. This would influence the extent to which ω3-PUFA are present in cellular membranes (including those in cerebral tissue) due to restored in vivo synthesis of PC comprised of ω3-PUFA such as DHA. However, depending on the source from which it as derived, the administered PC may also contain ω3-PUFA. Supplementing with PC containing ω3-PUFA such as DHA would likely facilitate increased concentrations of these PUFA within cell membranes. This would impact the extent to which factors such as elevated OxS and inflammation are apparent, thereby decreasing the likelihood of disturbed cardio- and cerebrovascular function, and subsequently the extent to which cerebral structure deteriorates over time.
Although a great deal more work is required examining how PC ingestion relates to HcY concentrations, the limited data available indicates that PC supplementation, in high enough doses, may lower HcY concentrations. By lowering HcY, PC supplementation may benefit cerebral structural integrity and potentially cognitive function in a similar way to B vitamins. It is hypothesized that chronic supplementation of GPL containing choline (i.e., PC) will significantly reduce HcY concentrations in older adults. Based upon earlier work whereby lowering HcY through nutritional supplementation benefited cerebral structural integrity, it is anticipated that HcY lowering via PC supplementation would likewise support cerebral structural integrity and subsequently cognitive functioning in older adults.
A ready supply of oxygen is essential for proper cellular functioning, although a consequence of oxygen metabolism is the formation of ROS (Chiurchiù et al., 2016). ROS are essential for many vital physiological functions including the destruction of pathogens during the inflammatory immune response and the regulation of smooth muscle tone (Popa-Wagner et al., 2013). Under normal conditions, ROS activity is balanced with that of endogenous antioxidants such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and glutathione (GSH), but also dietary antioxidants (Chiurchiù et al., 2016). However, unbalanced ROS activity may result in oxidative damage to lipids and proteins comprising cellular membranes thereby impacting cellular membrane structure and functioning (Popa-Wagner et al., 2013; Von Bernhardi et al., 2015).
Damage to biological tissues due to unbalanced ROS activity is termed oxidative stress (OxS). Cerebral tissue may be particularly susceptible to OxS, due to it requiring a considerable amount of oxygen so as to sustain disproportionate metabolic activity. Other factors facilitating an increased risk of OxS within cerebral tissues include a marked production of ROS through neurochemical reactions as well as deposits of iron that may promote oxidation (Popa-Wagner et al., 2013).
An additional factor contributing to cerebral tissues susceptibility to OxS, is that the PUFA within cellular membranes are highly susceptible to oxidative damage by ROS (Chan et al., 1982; Brett and Rumsby, 1994; Bongarzone et al., 1995), with increased ROS activity (without a corresponding increase in antioxidant activity) having been observed to facilitate destruction of myelin lipids and proteins (Chia et al., 1983a,b; Konat and Wiggins, 1985; Bongarzone et al., 1995). However, there appears to be an absence of data linking ROS activity or measures reflecting OxS with cerebral structural integrity, as determined using magnetic resonance imaging (MRI), in older humans. It is highly likely that oxidation of lipids and proteins within cerebral tissue membranes, at least partly contributes to those reductions in cerebral microstructural integrity identified in cognitively normal (Davis et al., 2009; Burzynska et al., 2010; Westlye et al., 2010) but also cognitively impaired older adults (i.e., MCI or AD) (Bosch et al., 2012; Wang et al., 2012; Zhang et al., 2013). Though they are not perfect measures, the diffusion tensor imaging (DTI) metrics “radial diffusivity” and “axonal diffusivity” have been suggested to reflect myelin and axonal integrity, respectively, within cerebral white matter (Song et al., 2002, 2003; Sun et al., 2006). Future work utilizing these measures may provide additional insight into how ROS activity, and subsequently OxS, contributes to declining cerebral structural integrity with age.
Due to a heightened susceptibility of cerebral tissues to OxS, it follows that measures indicative of OxS, or an elevated susceptibility to OxS due to unbalanced ROS activity (e.g., poorer concentrations of endogenous or dietary antioxidants), may be predictive of poorer cognitive function. In fact, compared to healthy adults, those with MCI or AD appear to demonstrate reduced concentrations of endogenous and diet derived antioxidants (Rinaldi et al., 2003; Padurariu et al., 2010; Mandal et al., 2015). In addition, adults with MCI or AD also demonstrate elevated OxS than cognitively normal adults, evidenced by increased concentrations of malondialdehyde (MDA), F2 and F4 isoprostanes (measures of ω6 and ω3-PUFA oxidation, respectively), and protein carbonyls (Mangialasche et al., 2009; Padurariu et al., 2010; Torres et al., 2011).
Conversely, greater intake of dietary antioxidants (e.g., carotenoids) are associated with greater cognitive performance in older adults (Jama et al., 1996; Berr et al., 1998; Akbaraly et al., 2007; Wengreen et al., 2007), though conflicting data is available (Crichton et al., 2013). Moreover, higher concentrations of endogenous antioxidants, such as GPx and GSH appear to predict better performance on the mini-mental state examination as well as the clinical dementia rating scale but also improved executive functioning in older adults with MCI, AD or “cognitive dysfunction” (Umur et al., 2011; Mandal et al., 2015; Revel et al., 2015).
Preclinical data appears to demonstrate that pre-treatment with GPL such as PS, may protect cells against the deleterious effects of elevated ROS, thereby reducing the risk of OxS. In one early study, Latorraca et al. (1993) suspended cultured human fibroblast cells in a solution containing acetaldehyde (37.5 mM) and 50 mU of xanthine-oxidase, which triggered a reaction resulting in high ROS production. Cell damage facilitated by increased ROS activity was quantified by measuring lactate dehydrogenase, with levels increasing by 40% when cells were exposed to both acetaldehyde and xanthine oxidase, compared to control (vehicle solution + acetaldehyde, but no xanthine oxidase). However, in cells pre-cultured with 13 μM PS for 4 days prior to suspension in the experimental solution, there was no significant increase in lactate dehydrogenase, suggesting that exposure to PS prevented tissue oxidative damage otherwise expected following increased ROS production.
Pre-culturing cells with GPL may facilitate decreased production of ROS, thereby lowering the risk of OxS occurring. Hashioka et al. (2007a) administered 400 ng/ml lipopolysaccharide (LPS) combined with 400 ng/ml of phorbol 12-myristate-12-acetate, to microglial cells sourced from rodents. This combination resulted in the production of the ROS and reactive nitrogen species—superoxide and nitric oxide, respectively, which together may form a powerful oxidant called peroxynitrite. However, when microglial cells were pre-cultured for 1 h with liposomes comprised of PS and PC or PC alone, both superoxide and nitric oxide production was markedly reduced. This would suggest that by lowering the production of superoxide and nitric oxide, treatment with liposomes comprised of PC and PS may facilitate reduced peroxynitrite production, lowering the likelihood of OxS. Similarly, Chaung et al. (2013) pre-cultured C6 cells (rodent glial cells modeling glioma) with 25 μM of DHA or 25 μM of PS, alone or in combination, for 24 h prior to these cells receiving electrical stimulation in order to stimulate ROS production. Across treatments, electrical stimulation of these glial cells successfully elevated ROS production, though for cells pre-cultured with either DHA or PS, ROS production was significantly lower. Moreover, the combination of DHA and PS appeared to facilitate even lower ROS production than when either treatment in isolation.
Prior work with animals indicates that supplementing with GPL containing ω3-PUFA may prevent OxS. Hiratsuka et al. (2008) fed rodents one of three experimental diets fortified with different lipid species. In the control diet lipids were issued as safflower oil (control), while experimental diets were fortified with the ω3-PUFA DHA delivered as either triglycerides or as GPL (PC, PE and SM) sourced from skipjack tuna ovaries. Rodents were fed their respective diets for a period of 5 weeks, though after the first 2 weeks' rodents received five daily injections of strepozotocin (40 mg/kg), so as to model diabetes, which subsequently caused elevated lipid peroxidation within cerebral tissue. Upon completion of the dietary intervention, no significant difference in cerebral lipid oxidation was identified between rodents within the control group or those receiving DHA as triglycerides. However, cerebral lipid oxidation was significantly lower in rodents fed DHA delivered as GPL, suggesting that supplementation with GPL containing DHA may lower the risk of OxS within cerebral tissue.
In addition to the above studies, others have determined that GPL supplementation may boost the concentrations of endogenous antioxidants. Liu et al. (2012) repeatedly administered pentylenetetrazol to induce epileptic-like seizures in rodents. This treatment facilitated a significant increase in the concentrations of reactive nitrogen species such as nitric oxide, while also reducing concentrations of endogenous antioxidants such as SOD within cerebral and liver tissues. Following seizure inducement, Liu et al. (2012) provided rodents with experimental diets fortified with 2.5 mg/kg DHA and/or 300 mg/kg PS for a period of 36 days. Dietary fortification with either DHA or PS was observed to increase SOD concentrations within cerebral tissue, whilst the combination of these nutrients boosted SOD concentrations within the liver. Nitric oxide was reduced in cerebral tissue following consumption of a diet fortified with DHA or PS, though liver concentrations were reduced following separate or combined treatment paradigms. Likewise, Zhang et al. (2015) observed that intracranial injection of 5.0 μL of amyloid-β1−42 facilitated a significant decline in SOD concentrations, while consumption of a diet enriched with PS sourced from bovine cortex (thereby containing DHA among other fatty acids) increased cerebral SOD expression in a dose dependent manner. More recently, Qu et al. (2016) modeled AD in rodents via intracerebral injection of amyloid-β25−35, subsequently reducing the concentrations of endogenous antioxidants such as SOD and GPx within the cortex and hippocampus. Compared to the AD control model, rodents modeling AD who received a medium (0.1 g/kg/d) or high (0.2 g/kg/d) dose of marine sourced PC (containing high levels of DHA and EPA) for 30 days demonstrated increased GPx within cortical tissue. Rodents that received a low (0.05 g/kg/d), medium or high dose of PC demonstrated elevated SOD activity within cortical tissue, although increased SOD activity within the hippocampus was only apparent following supplementation with the highest dose of PC. No treatment effects were observed within the hippocampus for GPx.
There is data from a range of preclinical and clinical trials indicating that supplementation with GPL may reduce the risk of OxS within biological tissues, including cerebral tissue. GPL from marine sources (e.g., krill or squid), mammalian (e.g., cow or pig) brain as well as soybeans may reduce the risk of OxS through the provision of fatty acids, especially ω3-PUFA such as DHA or EPA. Although maintaining redox balance is complex and facilitated via a wide range of biological processes, discussion in this review will be limited to two potential pathways through which supplementation with GPL, due to the provision of ω3-PUFA, may modulate the activity or concentrations of ROS and endogenous antioxidants thereby lowering the risk of OxS.
The first pathway through which GPL containing ω3-PUFA may reduce the risk of OxS involves upregulation of transcription factors associated with the synthesis of endogenous antioxidants. Prior work indicates that when cells are incubated with ω3-PUFA (DHA, EPA, or ALA) there appears to be an increase in the concentrations of endogenous antioxidants such as SOD, GSH, and GPx (Di Nunzio et al., 2011, 2016; Saw et al., 2013). Increased concentrations of these endogenous antioxidants has been linked to elevated activity of NF-E2-related factor 2 (NrF2)—a factor involved in the transcription of genes coding for those antioxidants (Di Nunzio et al., 2011, 2016; Saw et al., 2013). These effects may occur in response to oxidation of ω3-PUFA such as DHA and EPA, with the byproducts resulting from oxidation of these lipids (e.g., 4-hydroxy-2E-hexenal) subsequently inhibiting factors known to decrease NrF2 nuclear translocation, such as Keap1 (Gao et al., 2007). These putative effects were examined recently by Zhang et al. (2014), who identified that the administration of fish oil (rodent models) or pretreatment with the ω3-PUFA's DHA and EPA (rodent embryonic neuronal cells in vitro) significantly reduced the destruction of neuronal cells following oxygen-glucose deprivation. Zhang et al. (2014) also observed that cells pretreated with DHA, exhibited greater nuclear translocation of NrF2 following oxygen glucose deprivation. Moreover, they observed that 4-hydroxy-2E-hexenal, an end product of ω3-PUFA oxidation, was a far more potent inducer of NrF2 activity than 4-hydroxy-2E-nonenal—an end product of ω6-PUFA oxidation.
As indicated earlier, supplementation with ω3-PUFA is efficacious for elevating the bioavailability of these PUFA within biological tissues (Popp-Snijders et al., 1986; Bourre et al., 1993; Yaqoob et al., 2000; Bourre and Dumont, 2002; Favrelière et al., 2003; Kew et al., 2003, 2004; Browning et al., 2012). However, delivery of ω3-PUFA as PL may be a more effective for elevating tissue concentrations of these lipids than triglycerides (Ramprasath et al., 2013). Typically, increased concentrations of ω3-PUFA within membranes occurs at the expense of ω6-PUFA such as ARA (Calder, 2015). As such, increasing the bioavailability of ω3-PUFA within biological tissues, may increase the likelihood of these species of PUFA being oxidized thereby increasing the production of 4-hydroxy-2E-hexenal, rather than 4-hydroxy-2E-nonenal (from ω6-PUFA) leading to increased NrF2 activity and production of endogenous antioxidants. This process may account for the increased concentrations of endogenous antioxidants and therefore reduced risk of OxS, following GPL administration in earlier studies.
A second pathway through which GPL containing ω3-PUFA may reduce the risk of OxS is by downregulating the activity of select enzymes that produce ROS. One particular class of enzymes associated with ROS production are the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, abbreviated as Nox (Lambeth, 2004; Bedard and Krause, 2007). Nox are membrane bound proteins which facilitate electron transfer from NADPH onto molecular O2, thereby generating ROS, particularly superoxide. Dysregulated Nox activity may contribute to endothelial dysfunction, distorted smooth muscle growth as well as inflammation (Giordano and Visioli, 2014), and has been linked to an increased risk of MCI and AD, while also predicting Braak stage—a measure of AD pathology (Bruce-Keller et al., 2010; Ansari and Scheff, 2011). There are a number of different Nox enzymes (e.g., Nox1, Nox 2, Nox 3, Nox 4, Nox 5) which may be expressed in low to high concentrations within a wide variety of biological tissues (Lambeth, 2004; Bedard and Krause, 2007). Importantly, the activity of Nox proteins is regulated by a number of different proteins including p22phox, p47phox, p67phox, p40phox, and the GTPase Rac (Bedard and Krause, 2007).
Supplementation with GPL may downregulate Nox activity, subsequently lowering the risk of OxS, assuming the GPL contain ω3-PUFA. Richard et al. (2009) pre-cultured human aortic endothelial cells with DHA for 48 h, observing that DHA treatment facilitated a reduction in Nox4 expression and subsequently ROS production, which was otherwise upregulated due to exposure to angiotensin II and IL-1β. Additionally, the topical application of DHA to hairless mouse skin significantly attenuated the expression of Nox4 following exposure to ultraviolet radiation in a dose dependent manner compared to control (Rahman et al., 2011). In a more recent study by Depner et al. (2013), rodents were fed chow with a nutritional composition resembling that of the western diet regularly consumed by humans. Some of the rodents also received supplementation with ω3-PUFA's such as EPA and/or DHA, whereas controls only received olive oil. Although Depner et al. (2013) did not observe increased NrF2 expression following ingestion of any diet with added supplementation of ω3-PUFA, they did observe marked reduction in Nox regulating proteins including p22phox, p40phox, p47phox, p67phox, as well as RAC1, a member of the RAC subgroup of GTPase. Based upon the findings of these earlier studies, it is possible that supplementation of GPL (containing ω3-PUFA) may reduce the risk of OxS by down regulating the activity of enzymes known to produce ROS, as well as factors that stimulate the activity of those enzymes.
Overall, it would appear that supplementation with GPL, particularly those also containing ω3-PUFA, may modify the activity of several different protein messengers leading to reduced production of ROS, but increased synthesis of endogenous antioxidants, thereby lowering the risk of OxS. As such, it is anticipated that chronic supplementation with GPL (containing ω3-PUFA) is expected to facilitate a greater balance between the concentrations of ROS and endogenous antioxidants in older adults. This will likely manifest as lower concentrations of markers indicative of OxS. It is anticipated that in an aging sample, lower expression of OxS related markers or elevated expression of anti-oxidants (endogenous and dietary) will be associated with greater cerebral structural integrity, which in turn would be predictive of greater cognitive functioning.
Inflammation is an integral component of the immune response following injury to body tissues or infection. An important regulating influence of the inflammatory response is the production and release of cellular messengers specialized for either promoting or alleviating inflammation. Common pro-inflammatory messengers include interleukin (IL)-1β, IL-6, IL-8, IL-12, tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP), while examples of anti-inflammatory messengers include IL-4 and IL-10 (Baune et al., 2008; Marioni et al., 2010).
With increasing age, there appears to be a shift toward an immunologically primed state. This has been identified in cerebral tissue, such as the hippocampus, where the inflammatory response is typically of a greater magnitude and duration in older adults (for a review see Barrientos et al., 2015). This is an important observation in the context of neurocognitive health as data from multiple studies indicate that elevated concentrations of pro-inflammatory messengers, such as those listed earlier, are predictive of poorer cerebral structural integrity. These detrimental effects are evident as lower regional cerebral tissue volumes, increased volume of white matter lesions, as well as poorer cerebral microstructure (Baune et al., 2009; Wersching et al., 2010; Nagai et al., 2011; Bettcher et al., 2012, 2014, 2015; Miralbell et al., 2012; Satizabal et al., 2012; Arfanakis et al., 2013; Taki et al., 2013b; Sudheimer et al., 2014; Jiang et al., 2015).
Likewise, there appears to be a link between higher concentrations of pro-inflammatory messengers and poorer cognitive performance or increased rate of cognitive decline with age (Yaffe et al., 2003; Dimopoulos et al., 2006; Wright et al., 2006; Marioni et al., 2010; Economos et al., 2013; Mooijaart et al., 2013; Adriaensen et al., 2014; Tegeler et al., 2016). Greater concentration of pro-inflammatory messengers are evident in adults diagnosed with MCI or dementia compared to their cognitively normal counterparts (Dimopoulos et al., 2006; Magaki et al., 2007; Bermejo et al., 2008; Troller et al., 2010), with others having identified an increased risk of dementia in conjunction with elevated inflammation (Schmidt et al., 2002; Engelhart et al., 2004).
Interestingly, some studies appear to demonstrate a significant reduction in the strength of the relationship between inflammation and cognition after controlling for cerebral structural integrity (Arfanakis et al., 2013; Marsland et al., 2015). These results suggest that inflammation may detrimentally influence cognitive function, in part by promoting the deterioration of cerebral tissues. As such, the identification of interventions efficacious for modulating inflammatory messengers may be paramount for supporting cerebral structure, and subsequently cognitive function, in older adults.
Data from earlier pre-clinical studies demonstrate that GPL supplementation may modify the concentrations of pro-inflammatory messengers. In several studies, Treede et al. (2007, 2009) observed that administering TNF-α to caco-2 cells (human intestinal epithelial cells) triggered the activation of nuclear factor kappa-B (NF-KB), an important factor associated with the transcription of genes coding for pro-inflammatory messengers and the subsequent upregulation of these signaling messengers. However, simultaneous treatment with PC prevented NF-KB activation and subsequently lower concentrations of pro-inflammatory messengers (such as additional TNF-α) relative to control cultures. Similarly, cultures of human microglial cells exposed to with liposomes comprising PS and PC (sourced from pig brain or egg yolks, respectively), prior to the application of amyloid-β and interferon-γ, demonstrated lower concentrations of TNF-α (Hashioka et al., 2007b).
Building on the pre-clinical literature, Hartmann et al. (2009) use a rodent model of arthritis (involving injection of a solution containing carrageenan and kaolin into the knee joint). The injection of this solution triggered an inflammatory response consistent with arthritis—specifically increased heat, swelling and pressure sensitivity, though only within the joints that received the injection. Importantly, these effects were abated via oral administration of a non-steroidal anti-inflammatory drug or PC (dose of 150 mg/Kg)—though only the latter decreased the number of adherent leukocytes in the affected joints. In another study, increased concentrations of TNF-α and IL-6 were elicited in rodents following injection of LPS. However, rodents fed an experimental diet fortified with PC (1%) demonstrated significantly lower concentrations of TNF-α following LPS injection when compared to control rodents (Tokes et al., 2011). Similarly, consumption of a diet fortified with soybean derived PC (1%) facilitated anti-inflammatory effects in the gastrointestinal tract of dogs following small intestine ischemia (Ghyczy et al., 2008). In other work, the concentrations of both TNF-α and IL-6 were reduced in rodents which received PC supplementation for 3 days prior to, and 5 days after, an enema with trinitrobenzenesulfonic acid (Kovacs et al., 2012). Further, Jung et al. (2013) modeled multiple organ injury via LPS injection in otherwise healthy rodents. LPS injection triggered an increase in pro-inflammatory messengers such as TNF-α and IL-6, as well as the anti-inflammatory messenger IL-10. However, in rodents administered PC, a significant reduction in both TNF-α and IL-6 was apparent, while concentrations of IL-10 were unaffected.
Although the weight of research examining the concentration of inflammatory messengers in response to GPL focuses on PC, and to a lesser extent PS, several studies have also investigated PE. Despite observing anti-inflammatory effects in response to PC supplementation, Treede et al. (2007, 2009) were unable to replicate these effects when supplementing with PE. In a later study, Eros et al. (2009) modeled pleurisy (inflammation of lung tissue and tissue lining the chest cavity) in rodents via injection of 100 μL saline containing 2% carrageenan into the thoracic cavity at the 6th intercostal space. Carrageenan injection triggered an increase in total leukocyte count and as well as elevated leukocyte accumulation within lung tissue. Importantly, inflammation was reduced in rodents fed a special diet containing PC, PE and N-acylphosphatidylethanolamine or NAPE (diet composition-−1% PC, 0.4% PE, and 0.1% NAPE) for the 7 days prior to injection of carrageenan and 48 h thereafter. However, it is difficult to disentangle the anti-inflammatory effects associated with PC and those potentially from either PE or NAPE, as only combined supplementation was investigated.
The available literature suggests that PC supplementation may be capable of beneficially modifying inflammation. These effects are potentially due to PC being a significant source of choline (Cho et al., 2006; Chiuve et al., 2007) and therefore stimulating the cholinergic anti-inflammatory pathway. In this pathway, efferent fibers of the vagus nerve interact with acetylcholine receptors, notably α7 nicotinic acetylcholine (α7nACh) receptors on macrophages and other non-neural cytokine producing cells. The release of acetylcholine from vagus nerve activates these receptors which in turn facilitates inhibitory effects upon NF-KB, resulting in downregulated transcription of genes associated with pro-inflammatory messengers (Gallowitsch-Puerta and Pavlov, 2007).
In several studies, choline has been observed to function as an α7nACh receptor agonist. Parrish et al. (2008) cultured rodent macrophage cells with LPS in the presence or absence of choline for 4 h prior to measuring TNF-α concentrations. Choline treatment appeared to lower the concentrations of TNF-α following the presentation of LPS in a dose-dependent manner. This decline in TNF-α was found to have occurred because of reduced NF-KB activity. Parrish et al. then proceeded to demonstrate these effects in rodents, through intraperitoneal injection of choline (5 or 50 mg/kg) or saline at two time points (6 h as well as 30 min) prior to an injection of endotoxin (6 mg/kg). The higher choline dose reduced TNF-α concentrations in rodents previously injected with endotoxin, due to reduced NF-KB activity following activation of α7nACh receptors. Importantly, these results were then replicated in human whole blood and cultured macrophages. Similar observations were also made by Gurun et al. (2009) following administrations of CDP-choline (precursor to PC, but also containing choline) in rodents via intraplantar injection. These authors also observed that the anti-inflammatory effects of choline occurred in response to choline stimulating α7nACh receptors and subsequently suppressing NF-KB activity and therefore production of pro-inflammatory messengers. Though more work is needed to understand this potential mechanism, it is possible that PC, as a significant source of choline may influence the concentrations of pro-inflammatory messengers through the cholinergic anti-inflammatory pathway. This likely accounts for the empirical observations discussed earlier, whereby PC administration was associated with reduced concentrations of pro-inflammatory messengers such as TNF-α (Treede et al., 2007, 2009).
An additional pathway through which GPL supplementation may influence inflammation is through modifying the fatty acid composition of cell membranes, including inflammatory cells. Previous work has indicated that supplementation with ω3-PUFA primarily within PL facilitates increased bioavailability of ω3-PUFA within serum and membrane bound PL (Ramprasath et al., 2013). Increased ω3-PUFA within membrane GPL typically occurs at the expense of ω6-PUFA such as ARA (Calder, 2015). As such, there may be a proclivity toward the release of ω3-PUFA such as EPA or DHA, rather than the ω6-PUFA ARA, through the action of phospholipase A2, during the inflammatory response. Once released, these fatty acids may be metabolized by cyclooxygenase (COX) and/or lipoxygenase (LOX) enzymes resulting in the production of eicosanoids. Eicosanoids resulting from ARA metabolism are pro-inflammatory as they stimulate the production of inflammation inducing messengers such as TNF-α, IL-6, IL-8, and IL-1β (Wall et al., 2010; Serhan and Petasis, 2011). However, eicosanoids produced through metabolism of ω3-PUFA, particularly EPA, exert far less potent pro-inflammatory effects than those associated with ARA (Wall et al., 2010; Calder, 2015). Moreover, the metabolism of EPA or DHA by COX and LOX enzymes facilitates the synthesis of powerful anti-inflammatory and inflammation resolving agents such as resolvins, maresins and protectins (Bannenberg and Serhan, 2010; Serhan and Petasis, 2011; Calder, 2015). Furthermore, increased bioavailability of ω3-PUFA may help facilitate reduced nuclear translocation of NF-KB and therefore the transcription of genes associated with pro-inflammatory messengers (Wall et al., 2010; Calder, 2015).
Overall, it is anticipated that supplementation with GPL, especially those delivering choline and/or ω3-PUFA such as DHA, will beneficially modify the concentrations of inflammatory messengers. As chronic exposure to elevated concentrations of pro-inflammatory messengers appears to predict poorer cerebral structural integrity in older adults, it is predicted that lowering exposure to pro-inflammatory messengers will benefit cerebral structure. Subsequently, we would expect these effects to further benefit cognitive function in older adults.
Cardio- and cerebrovascular function are also pertinent factors predicting cerebral structure in older adults. One common measure of cardiovascular function is blood pressure (BP), specifically systolic and diastolic pressures (SBP or DBP, respectively). In middle aged and older adults, elevated BP (systolic and/or diastolic) or hypertension (clinically diagnosed high SBP and DBP) have been observed to predict poorer cerebral macrostructural integrity reflected as reduced total and regional cerebral volume (Den Heijer et al., 2003a, 2005; Raz et al., 2003; Beauchet et al., 2013), cortical thinning (Leritz et al., 2011; Alosco et al., 2014; Gonzalez et al., 2015), but also greater severity of cerebral white matter lesions or hyperintensities (Goldstein et al., 1998; Guo et al., 2009; Allan et al., 2015). Elevated BP also appears to predict poorer cerebral microstructural integrity (Kennedy and Raz, 2009; Leritz et al., 2010; Salat et al., 2012). An additional measure of cardiovascular function is arterial stiffness (AS). Increased AS has been observed to predict lower cerebral volume (Tsao et al., 2013; Lilamand et al., 2016) as well as increased severity of cerebral white matter lesions or hyperintensities (Henskens et al., 2008; Mitchell et al., 2011; Singer et al., 2014; van Sloten et al., 2015; Tsao et al., 2016). Both elevated BP and AS may contribute to poorer cerebral structural integrity, in part, due to chronically elevated pulsatile pressures damaging cerebral microvasculature. This may in turn facilitate reduced cerebral perfusion leading to ischemia and subsequent cerebral tissue decay (Gonzalez et al., 2015; van Sloten et al., 2015; Lilamand et al., 2016).
With an apparent association between poorer cardiovascular function and cerebral structural integrity, it follows that poorer cardiovascular function is predictive of reduced cognitive function. Elevated BP has been observed to predict poorer cognitive performance as well as an increased risk of developing MCI or dementia (Launer et al., 2000; Whitmer et al., 2005; Köhler et al., 2014; Chen et al., 2015; Alipour and Goldust, 2016) with similar effects having been observed in adults with elevated AS (Singer et al., 2014; Hajjar et al., 2016; Lim et al., 2016; Pase et al., 2016; Meyer et al., 2017). Given that there is a preponderance for increased BP (Neaton and Wentworth, 1992; Lloyd-Jones et al., 2005) and AS (Benetos et al., 2002; Mitchell et al., 2004) with age, targeting these outcomes with nutritional interventions may be benefit cerebral structural integrity, and therefore cognitive function in older adults.
Similarly, “blood-brain barrier” (BBB) integrity/permeability (an important measure of cerebrovascular function), may also influence cerebral structural integrity in older adults. The BBB is comprised of tightly compacted endothelial cells of capillaries perfusing cerebral tissue. Gases such as O2 and CO2 may freely diffuse across these capillary endothelial cells along their concentration gradients. However, the presence of “tight junctions,” composed of transmembrane proteins such as occluding and claudins, but also junction adhesion molecules, limits the paracellular flow of water, ions and other large molecules. Larger molecules may cross the BBB (either entering or leaving cerebral tissue), though movement of such molecules is normally dependent upon complex receptor and transporter systems (Popescu et al., 2009; Zeevi et al., 2010).
Maintenance of BBB integrity and therefore selective permeability is essential for ensuring optimal health of cerebral tissues. However, there appears to be a tendency toward reduced BBB integrity and increased permeability with older age. Pelegri et al. (2007) and Del Valle et al. (2009) have both observed increased BBB permeability with age in a rodent model of accelerated senescence (SAMP8). Likewise, reduced BBB permeability was identified in older relative to younger human adults in a systematic review and meta-analysis published around the same time (Farrall and Wardlaw, 2009).
Changes in BBB permeability with age appears to be predictive of the integrity of cerebral tissue, but also cognitive function, in older adults. In their systematic review, Farrall and Wardlaw (2009) identified a number of studies showing that elevated BBB permeability was correlated with increased severity of cerebral white matter lesions. Likewise, elevated BBB permeability is predictive of poorer memory (Wang et al., 2006) and performance on the mini-mental state examination (van de Haar et al., 2016). There also appears to be a preponderance for elevated BBB permeability in adults with either dementia (Alzheimer's and vascular subtypes) or MCI, relative to cognitively normal adults (Farrall and Wardlaw, 2009), particularly within the hippocampus, but also cerebral cortex and deep gray matter (Wang et al., 2006; Montagne et al., 2015; van de Haar et al., 2016). It is possible that increased BBB permeability detrimentally influences cognitive function through deleterious effects upon cerebral structure. Further work utilizing advanced structural neuroimaging methods, computerized tasks sensitive to subtle cognitive change, and importantly larger (and cognitively diverse) participant samples, will provide further insight into how changes to BBB permeability influence cerebral structural integrity and cognitive function with age.
As indicated earlier, GPL derived from marine sources (e.g., krill), but also mammalian brain or soybean may function as a source of ω3-PUFA. There is evidence suggesting that increased ω3-PUFA intake, or bioavailability, is beneficial to BP (Mori et al., 1999; Paschos et al., 2007; Takeuchi et al., 2007; Theobald et al., 2007; Witte et al., 2013) though such effects may be limited to adults with untreated (or poorly treated) hypertension (Campbell et al., 2012; Miller et al., 2014; Minihane et al., 2016). Similar benefits may also be observed for AS. In a systematic review and meta-analysis, Pase et al. (2011) identified that supplementation with ω3-PUFA was efficacious for improving pulse wave velocity and arterial compliance, indicating reduced AS. In a later trial, Pase et al. (2015) observed that daily supplementation of 6 g fish oil (480 mg DHA + 480 mg EPA) for 16 weeks significantly lowered central AS as measured by aortic augmentation pressure. Additional support for an association between ω3-PUFA intake/bioavailability and levels of arterial stiffening is provided by several studies with middle aged and older adults whereby greater tissue concentrations of ω3-PUFA predicted lower AS (Anderson et al., 2009; Sekikawa et al., 2013; Reinders et al., 2015; Lee et al., 2016). Given that prior work has indicated that GPL supplementation may effectively elevate the concentrations of ω3-PUFA within biological tissues (Ramprasath et al., 2013), it is anticipated that supplementation with GPL containing ω3-PUFA would also benefit measures of cardiovascular function such as BP or AS.
To date, very few studies have investigated whether GPL supplementation influences cardiovascular function in older humans. In one study, Vakhapova et al. (2011) administered a daily dose of PS (300 mg PS and 79 mg DHA and EPA; ratio of 3:1) to 157 older adults (mean age was ~72 years) daily for a period of 15 weeks. Following an initial 15-week double blind treatment phase, no differences were evident between treatment or control groups for either SBP or DBP. However, the trial was continued for an additional 15-weeks in an open label extension, though with a reduced dose of PS (100 mg PS and 26 mg DHA and EPA). The extension included 121 adults from the original trial phase, separated into two groups. Adults who previously received the PS supplement were designated as “continuers” whereas those originally receiving the placebo were termed “naïve.” Following an additional 15 weeks' intervention, Vakhapova et al. (2011) identified that in the adults designated as “continuers” DBP was significantly reduced from baseline, whereas no change was identified in the “naïve” group. However, it cannot be determined whether these effects are attributable to PS or the additional ω3-PUFA (or both), as simultaneous administration was the only experimental treatment. In another open label trial, Richter et al. (2013) administered a daily dose of 300 mg PS derived from soybeans (thereby containing ALA, which may be converted to longer chain ω3-PUFA such as DHA) to 30 adults aged between 50 and 90 years, for a period of 12 weeks. Following treatment, Richter et al. (2013) identified significant reductions to both SBP and DBP.
Although beneficial effects to cardiovascular function have been observed following GPL supplementation, contrary data are available. Jorissen et al. (2002) reported data relating to the safety of soybean derived PS when administered to older adults with AAMI in doses of either 300 or 600 mg, for a period of 12 weeks. The authors report that there was an absence of any significant group differences in any BP measure upon completion of the supplementation period. However, the absence of beneficial effects may be due to important methodological factors. In an earlier publication using data from the same study, Jorissen et al. (2001) suggested that a lack of cognitive benefits following GPL supplementation may be due to their PS having degraded by 50% by 15 months' post production. Although Jorissen et al. (2001) report that PS administration ended ~11 months after the treatments were manufactured, degradation may still have been advanced enough to minimize the likelihood of cardiovascular effects. Similar to Jorissen et al. (2002), Richter et al. (2013) administered PS in the form of gelatin capsules, though Richter et al. (2013) report that their capsules were specially engineered in order to GPL stability. Another pertinent difference between these studies is their use of parallel treatment groups. Jorissen et al. (2001, 2002) utilized a double blind, parallel groups design. Vakhapova et al. (2011) appears to have only performed within groups comparisons to determine whether adults designated “continuers” or “naïve” experienced cardiovascular benefits with no additional analyses having been performed so as to identify potential between groups effects. Likewise, Richter et al. (2013) performed an open label trial with no placebo group, so changes to cardiovascular function were only determined through within groups analyses. Lastly, there were marker differences in the samples sizes between these studies. Jorissen et al. (2002) and Vakhapova et al. (2011) included approximately 130 participants each, whereas Richter et al. (2013) only included 30 (26 by end of study). These differences in trial design, and size, may begin to account for the differences in results between these studies. As such, while GPL supplementation may benefit cardiovascular functioning, additional well designed clinical trials are required to confirm these effects in older adults.
While there has been some investigation of cardiovascular benefits following GPL supplementation, there appears to have been no clinical trials do date examining how GPL supplementation influences cerebrovascular function, particularly BBB permeability. Despite this, it is plausible that benefits to BBB permeability in older adults will be apparent following chronic ingestion of GPL containing choline and/or ω3-FA. Potential mechanisms through which GPL supplementation may benefit BBB permeability are highlighted in the following section.
Although multiple factors likely contribute to poorer cardio and cerebrovascular function, it has been suggested that both OxS and inflammation may be particularly important. Increased ROS activity, as well as OxS, are suggested to be key factors in the etiology of hypertension (Vaziri and Rodríguez-Iturbe, 2006; Briones and Touyz, 2010; Wu and Harrison, 2014). In addition, elevated inflammation is suggested to predict increased BP or the presence hypertension (Vaziri and Rodríguez-Iturbe, 2006; Dinh et al., 2014), though it may do so via reciprocal relationships with both OxS and endothelial dysfunction (Dinh et al., 2014). Furthermore, OxS has been positively linked with elevated AS (Patel et al., 2011; Kawamoto et al., 2016) potentially via remodeling of the vascular wall (Fleenor, 2013). Likewise, chronically elevated inflammation may initiate detrimental changes to the structural makeup of arterial walls, ultimately contributing to elevated AS (Jain et al., 2014).
Likewise, unbalanced ROS activity leading to OxS, but also elevated concentrations of certain pro-inflammatory messengers are suggested to facilitate increased BBB permeability (for reviews see Pun et al., 2009; Oakley and Tharakan, 2014; Rochfort and Cummins, 2015). Among other mechanisms, detrimental changes to tight junction proteins are often highlighted as a cause of increased BBB permeability following OxS or elevated concentrations of pro-inflammatory messengers such as TNF-α or IL-6 (Pun et al., 2009; Coisne and Engelhardt, 2011; Elahy et al., 2015; Varatharaj and Galea, 2017). Elevated HcY also predicts elevated BBB permeability in rodents (Kamath et al., 2006; Lominadze et al., 2006; Beard et al., 2011; Rhodehouse et al., 2013). Reduced BBB permeability has also been observed alongside elevated HcY in adults with MCI (Lehmann et al., 2003). Each of these risk mechanisms has been shown to be modifiable through supplementation with GPL containing choline and/or ω3-PUFA (see previous sections). Subsequently, it is anticipated that GPL supplementation, would further benefit BBB permeability in older adults. This remains to be investigated in well-designed clinical trials.
Although much more work is required, there is both direct and indirect evidence to suggest that GPL supplementation may benefit measures associated with cardio- and cerebro-vascular function in older adults. As such, it is hypothesized that chronic supplementation with GPL containing choline and/or ω3-PUFA will improve the aforementioned measures of cardio- and cerebro-vascular function in older adults, thereby supporting cerebral structural integrity and cognitive functioning in older adults.
Chronic supplementation with GPL has been observed to benefit cognitive function in animals and older adults (see introduction, as well as Tables 1–3). There is also data supporting the notion that GPL supplementation is beneficial to cerebral structure, though to date these effects have only been examined for in rodents. Despite a paucity of work in older humans, it is plausible that beneficial effects to cerebral structure are achievable, especially if the GPL administered contains choline and/or ω3-PUFA (DHA, EPA, or ALA). Long-term supplementation of GPL containing the aforementioned nutrients is expected to elevate the bioavailability of these nutrients, thereby resulting in modification of, or protection against, a number of factors associated with the destruction of cerebral tissues (see sections Homocysteine, Oxidative Stress, Inflammation, and Cardiovascular and Cerebrovascular Function). By minimizing or improving the factors outlined in this review, it is possible that GPL supplementation will lower the rate of cerebral structural decline over time. There is also the possibility that supplementation may facilitate at least mild repair to cerebral tissue given that GPL are major components of cellular membranes. Whether it be by reduced rate of decline, repair, or a combination of both, it is anticipated that by supporting cerebral structure, GPL supplementation may also influence the trajectory of cognitive decline with increasing age. Figure 3 outlines select pathways through which GPL supplementation may benefit cerebral structure, and subsequently cognitive function, in older adults. These effects should be examined in future well-designed clinical trials incorporating the use of advanced structural neuroimaging methods.
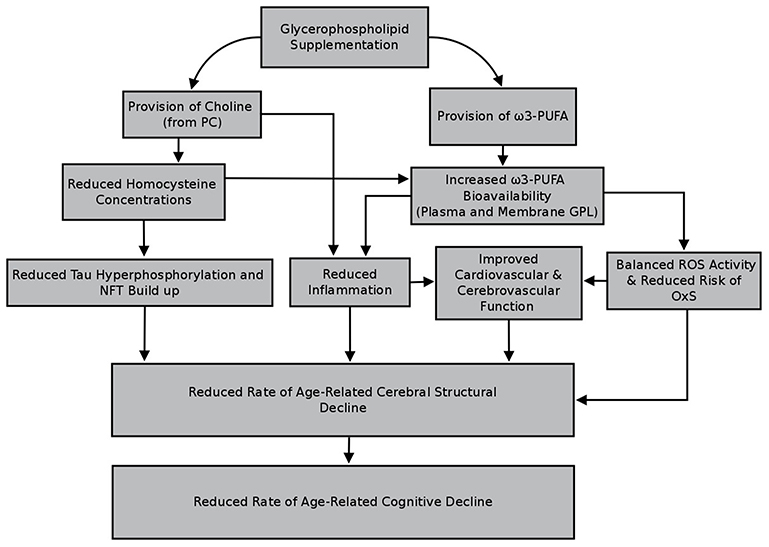
Figure 3. Select pathways through which glycerophospholipid supplementation may benefit cerebral structure and cognitive function in older adults.
There is substantial research indicating that cerebral structural integrity, at both the macro- and microstructural levels, is reduced with age. Modifying nutritional intake is quickly becoming recognized as a means of supporting cerebral structure with age, with a number of trials indicating that chronic supplementation with B vitamins, ω3-PUFA, or resveratrol, mediates reduced cerebral deterioration over time, perhaps even facilitating repair. This review discusses a number of different pathways through which benefits to cerebral structure may occur in response to GPL supplementation, thereby providing a theoretical basis for future human clinical trials.
Given that cerebral macro- and microstructural integrity is a pertinent predictor of cognitive function in older adults, it is plausible that through supporting cerebral structure, a reduced rate of cognitive deterioration may become apparent. Improving the trajectory of age-related cerebral deterioration and therefore cognitive decline through readily accessible interventions such as nutritional supplementation, may help lower the risk, and delay the onset, of age related conditions such as AAMI and MCI. Moreover, it may be possible to delay the onset of pathological conditions such as dementia, thereby contributing to a reduced incidence of this disease (Brookmeyer et al., 2007). Given the ease at which nutrition can be modified, and the relative absence of harmful side effects, nutritional supplementation, particularly with GPL, may well be a useful intervention for supporting neurocognitive health with increasing age.
JMR: Drafted the manuscript, with all additional authors (DJW, HM, AS, and AP) making significant contributions to the overall planning and development of the manuscript.
JMR is the recipient of an Australian Government Postgraduate research scholarship. DJW has received research funding and consultancy fees from Abbott Nutrition, Bayer Healthcare, and Neurobrands. HM has received research funding from Swisse-Wellness. AS has received research funding and consultancy fee from Abbott Nutrition, Australian Wine Research Institute, Barilla, Bayer Healthcare, Blackmores, Cognis, Cyvex, Dairy Health Innovation Consortium, Danone, Ginsana, GlaxoSmithKline Healthcare, Masterfoods, Martek, Naturex, Nestlé, Novartis, Red Bull, Sanofi, Unilever, Verdure Sciences, Wrigley. AP has received research funding and consultancy fees from Biostime, Blackmores, DSM, LifeVantage, Novasel Australia, Enzo Nutraceuticals and Swisse Wellness. AP was previously a member of the Scientific Advisory Panel for Swisse Wellness. Both DJW and AS have received grant funding from ARLA Foods to perform a clinical trial investigating the effects of a phospholipid supplement on neurocognitive health in older adults. AS has received research funding, consultancy and honoraria from the food industry.
AAMI, Age-associated memory impairment; MCI, Mild cognitive impairment; AD, Alzheimer's dementia; PL, Phospholipid; GPL, Glycerophospholipid; PC, Phosphatidylcholine; PE, Phosphatidylethanolamine; PS, Phosphatidylserine; CDP-choline, Cytidine disphosphocholine; PEMT, Phosphatidylethanolamine N-methyltransferase; PSS1, Phosphatidylserine synthase-1; PSS2, Phosphatidylserine synthase-2; PSD, Phosphatidylserine decarboxylase; PUFA, Polyunsaturated fatty acid; ω3/ω6, Omega 3 or 6; EPA, Eicosapentaenoic acid; DHA, Docosahexaenoic acid; ALA, α-Linolenic acid; AA, Arachidonic acid; HcY, Homocysteine; NFT, Neurofibrillary tangles; OxS, Oxidative stress; ROS, Reactive oxygen species; SOD, Superoxide dismutase; GSH, Glutathione; GPx, Glutathione peroxidase; MDA, Malondialdehyde; NrF2, NF-E2 related factor 2; NADPH, Nicotinamide adenine dinucleotide phosphate; Nox, NADPH oxidase; IL, Interleukin; TNF-α, Tumor necrosis factor alpha; NF-kB, Nuclear factor kappa B; COX, Cyclooxygenase; LOX, Lipoxygenase; BP, Blood pressure; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; AS, Arterial stiffness; BBB, Blood-brain barrier.
Adriaensen, W., Matheï, C., Vaes, B., van Pottelberg, G., Wallemacq, P., and Degryse, J. (2014). Interleukin-6 predicts short-term global functional decline in the oldest old: results from the BELFRAIL study. Age 36:9723. doi: 10.1007/s11357-014-9723-3
Akbaraly, N. T., Faure, H., Gourlet, V., Favier, A., and Berr, C. (2007). Plasma carotenoid levels and cognitive performance in an elderly population: results of the EVA study. J. Gerontol. Med. Sci. 63, 308–316. doi: 10.1093/gerona/62.3.308
Alipour, H., and Goldust, M. (2016). The association between blood pressure components and cognitive functions and cognitive reserve. Clin. Exp. Hypertens. 38, 95–99. doi: 10.3109/10641963.2015.1047946
Allam, M., Fahmy, E., Elatti, S. A., Amer, H., Abo-Krysha, N., and El-Sawy, E. (2013). Association between total plasma homocysteine level and cognitive functions in elderly Egyptian subjects. J. Neurol. Sci. 332, 86–91. doi: 10.1016/j.jns.2013.06.023
Allan, C. L., Zsoldos, E., Filippini, N., Sexton, C. E., Topiwala, A., Valkanova, V., et al. (2015). Lifetime hypertension as a predictor of brain structure in older adults: cohort study with a 28-year follow-up. Br. J. Psychiatry 206, 308–315. doi: 10.1192/bjp.bp.114.153536
Allegro, L., Favaretto, V., and Zilliotto, G. (1987). Oral Phosphatidylserine in elderly patients with cognitive deterioration: an open study. Clin. Trials J. 24, 104–108.
Allen, J. S., Bruss, J., Brown, C. K., and Damasio, H. (2005). Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol. Aging 26, 1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023
Alosco, M. L., Gunstad, J., Xu, X., Clark, U. S., Labbe, D. R., Riskin-jones, H. H., et al. (2014). The impact of hypertension on cerebral perfusion and cortical thickness in older adults. J. Am. Soc. Hypertens. 8, 561–570. doi: 10.1016/j.jash.2014.04.002
Amaducci, L. (1988). Phosphatidylserine in the treatment of Alzheimer's Disease: results of a multicenter study. Psychopharmacol. Bull. 24, 130–134.
Anderson, S. G., Sanders, T. A. B., and Cruickshank, J. K. (2009). Plasma fatty acid composition as a predictor of arterial stiffness and mortality. Hypertension 53, 839–845. doi: 10.1161/HYPERTENSIONAHA.108.123885
Ansari, M. A., and Scheff, S. W. (2011). Free radical biology and medicine NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic. Biol. Med. 51, 171–178. doi: 10.1016/j.freeradbiomed.2011.03.025
Anstey, K. J., and Maller, J. J. (2003). The role of volumetric MRI in understanding mild cognitive impairment and similar classifications. Aging Ment. Health 7, 238–250. doi: 10.1080/1360786031000120732
Arfanakis, K., Fleischman, D. A., Grisot, G., Barth, C. M., Varentsova, A., Morris, M. C., et al. (2013). Systemic inflammation in non-demented elderly human subjects: brain microstructure and cognition. PLoS ONE 8:e73107. doi: 10.1371/journal.pone.0073107
Arvanitakis, Z., Fleischman, D. A., Arfanakis, K., Leurgans, S. E., Barnes, L. L., and Bennett, D. A. (2016). Association of white matter hyperintensities and gray matter volume with cognition in older individuals without cognitive impairment. Brain Struct. Funct. 221, 2135–2146. doi: 10.1007/s00429-015-1034-7
Atkinson, W., Elmslie, J., Lever, M., Chambers, S. T., and George, P. M. (2008). Dietary and supplementary betaine: acute effects on plasma betaine and homocysteine concentrations under standard and postmethionine load conditions in healthy male subjects. Am. J. Clin. Nutr. 87, 577–585. doi: 10.1093/ajcn/87.3.577
Babenko, N. A., and Semenova, Y. A. (2011). Effects of exogenous phosphatidylserine on cognitive functions and phospholipid metabolism in the hippocampus of aged rats. Neurosci. Behav. Physiol. 41, 97–101. doi: 10.1007/s11055-010-9385-2
Bannenberg, G., and Serhan, C. N. (2010). Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim. Biophys. Acta 1801, 1260–1273. doi: 10.1016/j.bbalip.2010.08.002
Barrientos, R. M., Kitt, M. M., Watkins, L. R., and Maier, S. F. (2015). Neuroinflammation in the normal aging hippocampus. Neuroscience 309, 84–99. doi: 10.1016/j.neuroscience.2015.03.007
Baune, B. T., Ponath, G., Golledge, J., Varga, G., Arolt, V., Rothermundt, M., et al. (2008). Association between IL-8 cytokine and cognitive performance in an elderly general population the MEMO-study. Neurobiol. Aging 29, 937–944. doi: 10.1016/j.neurobiolaging.2006.12.003
Baune, B. T., Ponath, G., Rothermundt, M., Roesler, A., and Berger, K. (2009). Association between cytokines and cerebral MRI changes in the aging brain. J. Geriatr. Psychiatry Neurol. 22, 23–34. doi: 10.1177/0891988708328216
Beard, R. S. J., Reynolds, J. J., and Bearden, S. E. (2011). Hyperhomocysteinemia increases permeability of the blood-brain barrier by NMDA receptor-dependent regulation of adherens and tight junctions. Blood 118, 2007–2014. doi: 10.1182/blood-2011-02-338269
Beauchet, O., Celle, S., Roche, F., Bartha, R., Montero-Odasso, M., Allali, G., et al. (2013). Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J. Hypertens. 31, 1502–1516. doi: 10.1097/HJH.0b013e32836184b5
Bedard, K., and Krause, K. (2007). The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 87, 245–313. doi: 10.1152/physrev.00044.2005
Bendlin, B. B., Fitzgerald, M. E., Ries, M. L., Xu, G., Kastman, E. K., Thiel, B. W., et al. (2010). White matter in aging and cognition: a cross-sectional study of microstructure in adults aged eighteen to eighty-three. Dev. Neuropsychol. 35, 257–277. doi: 10.1080/87565641003696775
Benetos, A., Adamopoulos, C., Bureau, J., Temmar, M., Labat, C., Bean, K., et al. (2002). Determinants of accelerated progression of arterial stiffness in normotensive subjects and in treated hypertensive subjects over a 6-year period. Circulation 105, 1202–1207. doi: 10.1161/hc1002.105135
Bermejo, P., Martín-Aragón, S., Benedí, J., Susín, C., Felici, E., Gil, P., et al. (2008). Differences of peripheral inflammatory markers between mild cognitive impairment and Alzheimer's disease. Immunol. Lett. 117, 198–202. doi: 10.1016/j.imlet.2008.02.002
Berr, C., Richard, M. J., Roussel, A. M., and Bonithon-Kopp, C. (1998). Systemic oxidative stres and cognitive performance in the population-based EVA study. Free Radic. Biol. Med. 24, 1202–1208. doi: 10.1016/S0891-5849(97)00432-2
Bettcher, B. M., Watson, C. L., Walsh, C. M., Lobach, I. V., Neuhaus, J., Miller, J. W., et al. (2014). Interleukin-6, age, and corpus callosum integrity. PLoS ONE 9:e106521. doi: 10.1371/journal.pone.0106521
Bettcher, B. M., Wilheim, R., Rigby, T., Green, R., Miller, J. W., Racine, C. A., et al. (2012). C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain. Behav. Immun. 26, 103–108. doi: 10.1016/j.bbi.2011.07.240
Bettcher, B. M., Yaffe, K., Boudreau, R. M., Neuhaus, J., Aizenstein, H., Ding, J., et al. (2015). Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol. Aging 36, 948–954. doi: 10.1016/j.neurobiolaging.2014.11.004
Bleijerveld, O. B., Brouwers, J. F., Vaandrager, A. B., Helms, J. B., and Houweling, M. (2007). The CDP-ethanolamine pathway and phosphatidylserine decarboxylation generate different phosphatidylethanolamine molecular species. J. Biol. Chem. 282, 28362–28372. doi: 10.1074/jbc.M703786200
Blokland, A., Honig, W., Brouns, F., and Jolles, J. (1999). Cognition-enhancing properties of subchronic phosphatidylserine (ps) treatment in middle-aged rats: comparison of bovine cortex PS with egg PS and soybean PS. Nutrition 15, 778–783. doi: 10.1016/S0899-9007(99)00157-4
Bongarzone, E. R., Pasquini, J. M., and Soto, E. F. (1995). Oxidative damage to proteins and lipids of CNS myelin produced by in vitro generated reactive oxygen species. J. Neurosci. Res. 41, 213–221. doi: 10.1002/jnr.490410209
Bosch, B., Arenaza-Urquijo, E. M., Rami, L., Sala-Llonch, R., Junqué, C., Solé-Padullés, C., et al. (2012). Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol. Aging 33, 61–74. doi: 10.1016/j.neurobiolaging.2010.02.004
Bourre, J., Dumont, O., and Durand, G. (1993). Brain phospholipids as dietary source of (n-3) polyunsaturated fatty acids for nervous tissue in the rat. J. Neurochem. 60, 2018–2028. doi: 10.1111/j.1471-4159.1993.tb03486.x
Bourre, J. M., and Dumont, O. (2002). The administration of pig brain phospholipids versus soybean phospholipids in the diet during the period of brain development in the rat results in greater increments of brain docosahexaenoic acid. Neurosci. Lett. 335, 129–133. doi: 10.1016/S0304-3940(02)01172-2
Brett, R., and Rumsby, M. G. (1994). Susceptibility of myelin glycerophospholipids and sphingolipids to oxidative attack by hydroxyl free radicals as measured by the thiobarbituric acid test. Neurochem. Int. 24, 241–251. doi: 10.1016/0197-0186(94)90081-7
Briones, A. M., and Touyz, R. M. (2010). Oxidative stress and hypertension: current concepts. Curr. Hypertens. Rep. 12, 135–142. doi: 10.1007/s11906-010-0100-z
Brookmeyer, R., Johnson, E., Ziegler-Graham, K., and Arrighi, H. M. (2007). Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 3, 186–191. doi: 10.1016/j.jalz.2007.04.381
Browning, L. M., Walker, C. G., Mander, A. P., West, A. L., Madden, J., Gambell, J. M., et al. (2012). Incorporation of eicosapentaenoic and docosahexaenoic acids into lipid pools when given as supplements providing doses equivalent to typical intakes of oily fish. Am. J. Clin. Nutr. 96, 748–758. doi: 10.3945/ajcn.112.041343
Bruce-Keller, A. J., Gupta, S., Parrino, T. E., Knight, A. G., Ebenezer, P. J., Weidner, A. M., et al. (2010). NOX activity is increased in mild cognitive impairment. Antioxid. Redox Signal. 12, 1371–1380. doi: 10.1089/ars.2009.2823
Burdge, G. C., and Calder, P. C. (2005). Conversion of α-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reprod. Nutr. Dev. 45, 581–597. doi: 10.1051/rnd:2005047
Burzynska, A. Z., Preuschhof, C., Bäckman, L., Nyberg, L., Li, S.-C., Lindenberger, U., et al. (2010). Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage 49, 2104–2112. doi: 10.1016/j.neuroimage.2009.09.041
Caffarra, P., and Santamaria, V. (1987). The effects of phosphatidylserirne in patients with mild cognitive decline: an open trial. Clin. Trials J. 24, 109–114.
Calder, P. C. (2015). Marine omega-3 fatty acids and inflammatory processes: effects, mechanisms and clinical relevance. Biochim. Biophys. Acta 1851, 469–484. doi: 10.1016/j.bbalip.2014.08.010
Campbell, F., Dickinson, H. O., Critchley, J. A., Ford, G. A., and Bradburn, M. (2012). A systematic review of fish-oil supplements for the prevention and treatment of hypertension. Eur. J. Prev. Cardiol. 20, 107–120. doi: 10.1177/2047487312437056
Castro-Gómez, P., Garcia-serrano, A., Visioli, F., and Fontecha, J. (2015). Relevance of dietary glycerophospholipids and sphingolipids to human health. Prostaglandins Leukot. Essent. Fatty Acids 101, 41–51. doi: 10.1016/j.plefa.2015.07.004
Cenacchi, T., Bertoldin, T., Farina, C., Flori, M. G., and Crepaldi, G. (1993). Cognitive decline in the elderly: a double-blind, placebo controlled multicenter study on efficacy of phosphatidylserine administration. Aging 5, 123–133. doi: 10.1007/BF03324139
Chan, P. H., Yurko, M., and Fishman, R. A. (1982). Phospholipid degradation and cellular edema induced by free radicals in brain cortical slices. J. Neurochem. 38, 525–531. doi: 10.1111/j.1471-4159.1982.tb08659.x
Chaung, H., Chang, C., Chen, P., Chang, C., Liu, S., and Chen, C. (2013). Docosahexaenoic acid and phosphatidylserine improves the antioxidant activities in vitro and in vivo and cognitive functions of the developing brain. Food Chem. 138, 342–347. doi: 10.1016/j.foodchem.2012.10.082
Cheatham, C. L., Goldman, B. D., Fischer, L. M., Costa, K., Reznick, J. S., and Zeisel, S. H. (2012). Phosphatidylcholine supplementation in pregnant women consuming moderate-choline diets does not enhance infant cognitive function: a randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 96, 1465–1472. doi: 10.3945/ajcn.112.037184
Chen, K. H. M., Henderson, V. W., Stolwyk, R. J., Dennerstein, L., and Szoeke, C. (2015). Prehypertension in midlife is associated with worse cognition a decade later in middle-aged and older women. Age Ageing 44, 439–445. doi: 10.1093/ageing/afv026
Chen, S., and Li, K. W. (2008). Comparison of molecular species of various transphosphatidylated phosphatidylserine (PS) with bovine cortex PS by mass spectrometry. Chem. Phys. Lipids 152, 46–56. doi: 10.1016/j.chemphyslip.2008.01.001
Chia, L. S., Thompson, J. E., and Moscarello, M. A. (1983a). Changes in lipid phase behaviour in human myelin during maturation and aging: involvement of lipid peroxidation. Fed. Eur. Biochem. Sci. 157, 155–158. doi: 10.1016/0014-5793(83)81136-3
Chia, L. S., Thompson, J. E., and Moscarello, M. A. (1983b). Disorder in human myelin induced by superoxide radical: an in vitro investigation. Biochem. Biophys. Res. Commun. 117, 141–146. doi: 10.1016/0006-291X(83)91552-8
Chiurchiù, V., Orlacchio, A., and Maccarrone, M. (2016). Is modulation of oxidative stress an answer? the state of the art of redox therapeutic actions in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2016:7909380. doi: 10.1155/2016/7909380
Chiuve, S. E., Giovannucci, E. L., Hankinson, S. E., Zeisel, S. H., and Dougherty, L. W. (2007). The association between betaine and choline intakes and the plasma concentrations of homocysteine in women. Am. J. Clin. Nutr. 86, 1073–1081. doi: 10.1093/ajcn/86.4.1073
Cho, E., Zeisel, S. H., Jacques, P., Selhub, J., Dougherty, L., and Colditz, G. A. (2006). Dietary choline and betaine assessed by food-frequency questionnaire in relation to plasma total homocysteine concentration in the framingham offspring study. Am. J. Clin. Nutr. 83, 905–911. doi: 10.1093/ajcn/83.4.905
Clarke, R., Bennett, D., Parish, S., Lewington, S., Skeaff, M., Eussen, S. J., et al. (2014). Effects of homocysteine lowering with B vitamins on cognitive aging: meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 100, 657–666. doi: 10.3945/ajcn.113.076349
Claro, F. T., Patti, C. L., Abílio, V. C., Frussa-Filho, R., and Silva, R. H. (2006). Bovine brain phosphatidylserine attenuates scopolamine induced amnesia in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 881–886. doi: 10.1016/j.pnpbp.2006.01.013
Claro, F. T., Silva, R. H., and Frussa-Filho, R. (1999). Bovine brain phosphatidylserine attenuates scopolamine-induced amnesia. Physiol. Behav. 67, 551–554. doi: 10.1016/S0031-9384(99)00099-2
Cohn, J. S., Kamili, A., Wat, E., Chung, R. W. S., and Tandy, S. (2010). Dietary phospholipids and intestinal cholesterol absorption. Nutrients 2, 116–127. doi: 10.3390/nu2020116
Coisne, C., and Engelhardt, B. (2011). Tight junctions in brain barriers during central nervous system inflammation. Antioxid. Redox Signal. 15, 1285–1303. doi: 10.1089/ars.2011.3929
Cooper, G. M., and Hausman, R. E. (2007). “The composition of cells,” in The Cell: A Molecular Approach, eds G. M. Cooper and R. E. Hausman (Washington, DC: ASM Press), 43–72.
Corwin, J., Dean, R. L., Bartus, R. T., Rotrosen, J., and Watkins, D. L. (1985). Behavioral effects of phosphatidylserine in the aged fischer 344 rat: amelioration of passive avoidance deficits without changes in psychomotor task performance. Neurobiol. Aging 6, 11–15.
Crespo, D., Megias, M., Fernandez-Viadero, C., and Verduga, R. (2004). Chronic treatment with a precursor of cellular phosphatidylcholine ameliorates morphological and behavioral effects of aging in the rat hippocampus. Ann. N.Y. Acad. Sci. 1019, 41–43. doi: 10.1196/annals.1297.009
Crichton, G. E., Bryan, J., and Murphy, K. J. (2013). Dietary antioxidants, cognitive function and dementia-a systematic review. Plant foods Hum. Nutr. 68, 279–292. doi: 10.1007/s11130-013-0370-0
Crook, T. H., Tinklenberg, J., Yesavage, J., Petrie, W., Nunzi, M. G., and Massari, D. C. (1991). Effects of phosphatidylserine in age-associated memory impairment. Neurology 41, 644–649. doi: 10.1212/WNL.41.5.644
Davis, S. W., Dennis, N. A., Buchler, N. G., White, L. E., Madden, D. J., and Cabeza, R. (2009). Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage 46, 530–541. doi: 10.1016/j.neuroimage.2009.01.068
de Jager, C. A., Oulhaj, A., Jacoby, R., Refsum, H., and Smith, A. D. (2012). Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. Int. J. Geriatr. Psychiatry 27, 592–600. doi: 10.1002/gps.2758
de Souza, L. C., Chupin, M., Lamari, F., Jardel, C., Leclercq, D., Colliot, O., et al. (2017). CSF tau markers are correlated with hippocampal volume in Alzheimer's disease. Neurobiol. Aging 33, 1253–1257. doi: 10.1016/j.neurobiolaging.2011.02.022
Del Valle, J., Duran-Vilaregut, J., Manich, G., Camins, A., Pallas, M., Vilaplana, J., et al. (2009). Time-course of blood-brain barrier disruption in senescence-accelerated mouse prone 8 (SAMP8) mice. Int. J. Dev. Neurosci. 27, 47–52. doi: 10.1016/j.ijdevneu.2008.10.002
Delong, C. J., Shen, Y., Thomas, M. J., and Cui, Z. (1999). Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J. Biol. Chem. 274, 29683–29688. doi: 10.1074/jbc.274.42.29683
Den Heijer, T., Launer, L. J., Prins, N. D., van Dijk, E. J., Vermeer, S. E., Hofman, A., et al. (2005). Association between blood pressure, white matter lesions, and atrophy. Neurology 64, 263–267. doi: 10.1212/01.WNL.0000149641.55751.2E
Den Heijer, T., Skoog, I., Oudkerk, M., de Leeuw, F., de Groot, J., Hofman, A., et al. (2003a). Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol. Aging 24, 307–313. doi: 10.1016/S0197-4580(02)00088-X
den Heijer, T., Vermeer, S. E., Clarke, R., Oudkerk, M., Koudstaal, P. J., Hofman, A., et al. (2003b). Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126, 170–175. doi: 10.1093/brain/awg006
Depner, C. M., Philbrick, K. A., and Jump, D. B. (2013). Docosahexaenoic acid attenuates hepatic inflammation, oxidative stress, and fibrosis without decreasing hepatosteatosis in a ldlr -/- mouse model of western diet-induced nonalcoholic steatohepatitis. J. Nutr. 143, 315–323. doi: 10.3945/jn.112.171322
Detopoulou, P., Panagiotakos, D. B., Antonopoulou, S., and Pitsavos, C. (2008). Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am. J. Clin. Nutr. 87, 424–430. doi: 10.1093/ajcn/87.2.424
Devaux, P. F., and Zachowski, A. (1994). Maintenance and consequences of membrane phospholipid asymmetry. Chem. Phys. Lipids 73, 107–120. doi: 10.1016/0009-3084(94)90177-5
Devlin, A. M., Singh, R., Wade, R. E., Innis, S. M., Bottiglieri, T., and Lentz, S. R. (2007). Hypermethylation of Fads2 and altered hepatic fatty acid and phospholipid metabolism in mice with hyperhomocysteinemia. J. Biol. Chem. 282, 37082–37090. doi: 10.1074/jbc.M704256200
Di Nunzio, M., Valli, V., and Bordoni, A. (2011). Pro- and anti-oxidant effects of polyunsaturated fatty acid supplementation in HepG2 cells. Prostaglandins Leukot. Essent. Fat. Acids 85, 121–127. doi: 10.1016/j.plefa.2011.07.005
Di Nunzio, M., Valli, V., and Bordoni, A. (2016). PUFA and oxidative stress. Differential modulation of the cell response by DHA. Int. J. Food Sci. Nutr. 67, 834–843. doi: 10.1080/09637486.2016.1201790
Dimopoulos, N., Piperi, C., Salonicioti, A., Mitropoulos, P., Kallai, E., Liappas, I., et al. (2006). Indices of low-grade chronic inflammation correlate with early cognitive deterioration in an elderly Greek population. Neurosci. Lett. 398, 118–123. doi: 10.1016/j.neulet.2005.12.064
Dinh, Q. N., Drummond, G. R., Sobey, C. G., and Chrissobolis, S. (2014). Roles of inflammation , oxidative stress , and vascular dysfunction in hypertension. Biomed Res. Int. 2014:406960. doi: 10.1155/2014/406960
Douaud, G., Refsum, H., de Jager, C. A., Jacoby, R., Nichols, T. E., Smith, S. M., et al. (2013). Preventing Alzheimer's disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. U.S.A. 110, 9523–9528. doi: 10.1073/pnas.1301816110
Drago, F., Canonico, P. L., and Scapagnini, U. (1981). Behavioral effects of phosphatidylserine in aged rats. Neurobiol. Aging 2, 209–213. doi: 10.1016/0197-4580(81)90023-3
Durga, J., Boxtel, M. P. J., Van Schouten, E. G., Kok, F. J., Jolles, J., Katan, M. B., et al. (2007). Eff ect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet 369, 208–216. doi: 10.1016/S0140-6736(07)60109-3
Economos, A., Wright, C. B., Moon, Y. P., Rundek, T., Rabbani, L., Paik, M. C., et al. (2013). Interleukin 6 plasma concentration associates with cognitive decline: the northern Manhattan study. Neuroepidemiology 40, 253–259. doi: 10.1159/000343276
Elahy, M., Jackaman, C., Mamo, J. C. L., Lam, V., Dhaliwal, S. S., Giles, C., et al. (2015). Blood–brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun. Ageing 12, 1–9. doi: 10.1186/s12979-015-0029-9
Engelhart, M. J., Geerlings, M. I., Meijer, J., Kiliaan, A., Ruitenberg, A., van Swieten, J. C., et al. (2004). Inflammatory proteins in plasma and the risk of dementia. Arch. Neurol. 61, 668–672. doi: 10.1001/archneur.61.5.668
Erickson, K. I., Suever, B. L., Prakash, R. S., Colcombe, S. J., McAuley, E., and Kramer, A. F. (2008). Greater intake of vitamins B6 and B12 spares gray matter in healthy elderly: a voxel-based morphometry study. Brain Res. 1199, 20–26. doi: 10.1016/j.brainres.2008.01.030
Eros, G., Varga, G., Varadi, R., Czobel, M., Kaszaki, J., Ghyczy, M., et al. (2009). Anti-inflammatory action of a phosphatidylcholine, phosphatidylethanolamine and N-acylphosphatidylethanolamine-enriched diet in carrageenan-induced pleurisy. Eur. Surg. Res. 42, 40–48. doi: 10.1159/000167856
Eussen, S. J., Groot, L. C., De Joosten, L. W., Bloo, R. J., Clarke, R., Ueland, P. M., et al. (2006). Effect of oral vitamin B-12 with or without folic acid on cognitive function in older people with mild vitamin B-12 deficiency: a randomized, placebo-controlled trial. Am. J. Clin. Nutr. 84, 361–370. doi: 10.1093/ajcn/84.1.361
Farrall, A. J., and Wardlaw, J. M. (2009). Blood–brain barrier: ageing and microvascular disease – systematic review and meta-analysis. Neurobiol. Aging 30, 337–352. doi: 10.1016/j.neurobiolaging.2007.07.015
Favrelière, S., Perault, M. C., Huguet, F., Javel, D., De Bertrand, N., Piriou, A., et al. (2003). DHA-enriched phospholipid diets modulate age-related alterations in rat hippocampus. Neurobiol. Aging 24, 233–243. doi: 10.1016/S0197-4580(02)00064-7
Feng, L., Isaac, V., Sim, S., Ng, T. P., Krishnan, K. R., and Chee, M. W. (2013). Associations between elevated homocysteine, cognitive impairment, and reduced white matter volume in healthy old adults. Am. J. Geriatr. Psychiatry 21, 164–172. doi: 10.1016/j.jagp.2012.10.017
Firbank, M. J., Narayan, S. K., Saxby, B. K., Ford, G. A., and O'Brien, J. T. (2010). Homocysteine is associated with hippocampal and white matter atrophy in older subjects with mild hypertension. Int. Psychogeriatr. 22, 804–811. doi: 10.1017/S1041610210000499
Fleenor, B. S. (2013). Large elastic artery stiffness with aging: novel translational mechanisms and interventions. Aging Dis. 4, 76–83.
Ford, A. H., and Almeida, O. P. (2014). Effect of homocysteine lowering treatment on cognitive function: a systematic review and meta-analysis of randomized controlled trials. J. Alzheimer's Dis. 29, 133–149. doi: 10.3233/JAD-2012-111739
Fotenos, A. F., Snyder, A. Z., Girton, L. E., Morris, J. C., and Buckner, R. L. (2005). Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology 64, 1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11
Furushiro, M., Suzuki, S., Shishido, Y., Sakai, M., Yamatoya, H., Kudo, S., et al. (1997). Effects of oral administration phosphatidylserine avoidance of soybean in mice lecithin learning transphosphatidylated on impaired of passive avoidance in mice. Jpn. J. Pharmacol. 75, 447–450. doi: 10.1254/jjp.75.447
Gallowitsch-Puerta, M., and Pavlov, V. A. (2007). Neuro-immune interactions via the cholinergic anti-inflammatory pathway. Life Sci. 80, 2325–2329. doi: 10.1016/j.lfs.2007.01.002
Gao, L., Wang, J., Sekhar, K. R., Yin, H., Yared, N. F., Schneider, S. N., et al. (2007). Novel n-3 fatty acid oxidation products activate Nrf2 by destabilizing the association between Keap1 and Cullin3. J. Biol. Chem. 282, 2529–2537. doi: 10.1074/jbc.M607622200
Ghyczy, M., Torday, C., Kaszaki, J., Szabó, A., Czóbel, M., and Boros, M. (2008). Oral phosphatidylcholine pretreatment decreases ischemia-reperfusion induced methane generation and the inflammatory response in the small intestine. Shock 30, 596–602. doi: 10.1097/SHK.0b013e31816f204a
Giordano, E., and Visioli, F. (2014). Long-chain omega 3 fatty acids: molecular bases of potential antioxidant actions. Prostaglandins Leukot. Essent. Fat. Acids 90, 1–4. doi: 10.1016/j.plefa.2013.11.002
Glodzik, L., Mosconi, L., Tsui, W., Santi, S., De Zinkowski, R., Pirraglia, E., et al. (2012). Alzheimer's disease markers, hypertension, and gray matter damage in normal elderly. Neurobiol. Aging 33, 1215–1227. doi: 10.1016/j.neurobiolaging.2011.02.012
Glodzik, L., Santi, S., De Tsui, W. H., Mosconi, L., Zinkowski, R., Pirraglia, E., et al. (2011). Phosphorylated tau 231, memory decline and medial temporal atrophy in normal elders. Neurobiol. Aging 32, 2131–2141. doi: 10.1016/j.neurobiolaging.2009.12.026
Goldstein, I. B., Bartzokis, G., Hance, D. B., and Shapiro, D. (1998). Relationship between blood pressure and subcortical lesions in healthy elderly people. Stroke 29, 765–772. doi: 10.1161/01.STR.29.4.765
Gonzalez, C. E., Pacheco, J., Beason-Held, L. L., and Resnick, S. M. (2015). Longitudinal changes in cortical thinning associated with hypertension. J. Hypertens. 33, 1242–1248. doi: 10.1097/HJH.0000000000000531
Granata, Q., and Di Michele, J. (1987). Phosphatidylserine in elderly patients: an open trial. Clin. Trials J. 24, 99–103.
Gu, Y., Vorburger, R. S., Gazes, Y., Habeck, C. G., Stern, Y., Luchsinger, A., et al. (2016). White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Ann. Neurol. 79, 1014–1025. doi: 10.1002/ana.24674
Guo, X., Pantoni, L., Simoni, M., Bengtsson, C., Bjo, C., Lissner, L., et al. (2009). Blood pressure components and changes in relation to white matter lesions a 32-year prospective population study. Hypertension 54, 57–62. doi: 10.1161/HYPERTENSIONAHA.109.129700
Gurun, M. S., Parker, R., Eisenach, J. C., and Vincler, M. (2009). The effect of peripherally administered CDP-choline in an acute inflammatory pain model: the role of the α7 nicotinic acetylcholine receptor. Anesth. Analg. 108, 1680–1687. doi: 10.1213/ane.0b013e31819dcd08
Hajjar, I., Goldstein, F. C., Martin, G. S., and Quyyumi, A. A. (2016). Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension 67, 171–175. doi: 10.1161/HYPERTENSIONAHA.115.06277
Hanahan, D. J. (1997). “Introduction to lipids,” in A Guide to Phospholipid Chemistry (New York, NY: Oxford University Press), 3–24.
Hänggi, J., Streffer, J., Jäncke, L., and Hock, C. (2011). Volumes of lateral temporal and parietal structures distinguish between healthy aging, mild cognitive impairment, and Alzheimer's disease. J. Alzheimers. Dis. 26, 719–734. doi: 10.3233/JAD-2011-101260
Hartmann, P., Szabó, A., Eros, G., Gurabi, D., Horváth, G., Németh, I., et al. (2009). Anti-inflammatory effects of phosphatidylcholine in neutrophil leukocyte-dependent acute arthritis in rats. Eur. J. Pharmacol. 622, 58–64. doi: 10.1016/j.ejphar.2009.09.012
Hashioka, S., Han, Y., Fujii, S., Kato, T., Monji, A., Utsumi, H., et al. (2007a). Phospholipids modulate superoxide and nitric oxide production by lipopolysaccharide and phorbol 12-myristate-13-acetate-activated microglia. Neurochem. Int. 50, 499–506. doi: 10.1016/j.neuint.2006.10.006
Hashioka, S., Han, Y.-H., Fujii, S., Kato, T., Monji, A., Utsumi, H., et al. (2007b). Phosphatidylserine and phosphatidylcholine-containing liposomes inhibit amyloid beta and interferon-gamma-induced microglial activation. Free Radic. Biol. Med. 42, 945–954. doi: 10.1016/j.freeradbiomed.2006.12.003
Heiss, W. D., Kessler, J., Mielke, R., Szelies, B., and Herholz, K. (1994). Long term effects of phsopahtidylserine, pyritinol and cognitive training in Alzheimer's Disease: a Neuropsychological, EEG, and PET investigation. Dementia 5, 88–98.
Heiss, W. D., Kessler, J., Slansky, I., Mielke, R., Szelies, B., and Herholz, K. (1993). Activation PET as an instrument to determine therapeutic efficacy in Alzheimer's disease. Ann. N.Y. Acad. Sci. 695, 327–331.
Henskens, L. H., Kroon, A. A., van Oostenbrugge, R. J., Gronenschild, E. H., Fuss-Lejeune, M. M., Hofman, P. A., et al. (2008). increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 52, 1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024
Hiratsuka, S., Ishihara, K., Kitagawa, T., Wada, S., and Yokogoshi, H. (2008). Effect of dietary docosahexaenoic acid connecting phospholipids on the lipid peroxidation of the brain in mice. J. Nutr. Sci. Vitaminol. 54, 501–506. doi: 10.3177/jnsv.54.501
Hooshmand, B., Mangialasche, F., Kalpouzos, G., Solomon, A., Kåreholt, I., Smith, A. D., et al. (2016). Association of vitamin B12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: a longitudinal population-based study. JAMA Psychiatry 73, 606–613. doi: 10.1001/jamapsychiatry.2016.0274
Hooshmand, B., Polvikoski, T., Kivipelto, M., Tanskanen, M., Myllykangas, L., Erkinjuntti, T., et al. (2013). Plasma homocysteine, Alzheimer and cerebrovascular pathology: a population-based autopsy study. Brain 136, 2707–2716. doi: 10.1093/brain/awt206
Hoy, A. R., Ly, M., Carlsson, C. M., Okonkwo, O. C., Zetterberg, H., Blennow, K., et al. (2017). Microstructural white matter alterations in preclinical Alzheimer's disease detected using free water elimination diffusion tensor imaging. PLoS ONE 12, e0173982. doi: 10.1371/journal.pone.0173982
Hsu, J.-L., Chen, W.-H., Bai, C.-H., Leu, J.-G., Hsu, C.-Y., Viergever, M. A., et al. (2015). Microstructural white matter tissue characteristics are modulated by homocysteine: a diffusion tensor imaging study. PLoS ONE 10:e0116330. doi: 10.1371/journal.pone.0116330
Hsu, J.-L., Van Hecke, W., Bai, C.-H., Lee, C.-H., Tsai, Y.-F., Chiu, H.-C., et al. (2010). Microstructural white matter changes in normal aging: a diffusion tensor imaging study with higher-order polynomial regression models. Neuroimage 49, 32–43. doi: 10.1016/j.neuroimage.2009.08.031
Huang, T., Yu, X., Shou, T., Wahlqvist, M. L., and Li, D. (2013). Associations of plasma phospholipid fatty acids with plasma homocysteine in Chinese vegetarians. Br. J. Nutr. 109, 1688–1694. doi: 10.1017/S000711451200356X
Iglesia, I., Huybrechts, I., Mouratidou, T., Santabárbara, J., Chajès, V., Park, J. Y., et al. (2017). Folate and vitamin B 12 concentrations are associated with plasma DHA and EPA fatty acids in European adolescents: the Healthy Lifestyle in Europe by Nutrition in Adolescence (HELENA) study. Br. J. Nutr. 117, 124–133. doi: 10.1017/S0007114516004414
Innis, S. M., Davidson, A. G. F., Chen, A., Dyer, R., Melnyk, S., and James, S. J. (2003). Increased plasma homocysteine and S-adenosylhomocysteine and decreased methionine is associated with altered phosphatidylcholine and phosphatidylethanolamine in cystic fibrosis. J. Pediatr. 143, 351–356. doi: 10.1067/S0022-3476(03)00326-3
Jacques, P. F., Bostom, A. G., Wilson, P. W. F., Rich, S., Rosenberg, I. H., and Selhub, J. (2001). Determinants of plasma total homocysteine concentration in the Framingham Offspring cohort. Am. J. Clin. Nutr. 73, 613–621. doi: 10.1093/ajcn/73.3.613
Jain, S., Khera, R., Corrales, V. F., Townsend, R. R., and Chirinos, J. A. (2014). Inflammation and arterial stiffness in humans. Atherosclerosis 237, 381–390. doi: 10.1016/j.atherosclerosis.2014.09.011
Jama, J. W., Launer, J. L., Witteman, J. C. M., den Breeijen, J. H., Breteler, M. M. B., Grobbee, D. E., et al. (1996). Dietary antioxidants and cognitive function in a population-based sample of older persons. Am. J. Epidemiol. 144, 275–280. doi: 10.1093/oxfordjournals.aje.a008922
Jerneren, F., Elshorbagy, A. K., Oulhaj, A., Smith, S. M., Refsum, H., and Smith, A. D. (2015). Brain atrophy in cognitively impaired elderly: the importance of long-chain ω-3 fatty acids and B vitamin status in a randomized controlled trial. Am. J. Clin. Nutr. 102, 215–221. doi: 10.3945/ajcn.114.103283
Jiang, J., Wen, W., Brown, D. A., Crawford, J., Thalamuthu, A., Smith, E., et al. (2015). The relationship of serum macrophage inhibitory cytokine-1 levels with gray matter volumes in community-dwelling older individuals. PLoS ONE 10:e0123399. doi: 10.1371/journal.pone.0123399
Jochemsen, H. M., Kloppenborg, R. P., de Groot, L. C., Kampman, E., Mali, W. P., van der Graaf, Y., et al. (2013). Homocysteine, progression of ventricular enlargement, and cognitive decline: the Second Manifestations of ARTerial disease-Magnetic Resonance study. Alzheimers Dement. 9, 302–309. doi: 10.1016/j.jalz.2011.11.008
Joosten, E., Lesaffre, E., and Riezler, R. (1996). Are different reference intervals for methylmalonic acid and total homocysteine necessary in elderly people? Eur. J. Haematol. 57, 222–226. doi: 10.1111/j.1600-0609.1996.tb01367.x
Jorissen, B. L., Brouns, F., Van Boxtel, M. P., and Riedel, W. J. (2002). Safety of Soy-derived Phosphatidylserine in Elderly People Safety of Soy-derived Phosphatidylserine in Elderly People. Nutr. Neurosci. 5, 337–343. doi: 10.1080/1028415021000033802
Jorissen, B. L., Brouns, F., van Boxtel, M. P., Ponds, R. W., Verhey, F. R., Jolles, J., et al. (2001). The influence of soy-derived phosphatidylserine on cognition in age-associated memory impairment. Nutr. Neurosci. 4, 121–134. doi: 10.1080/1028415X.2001.11747356
Jung, Y. Y., Nam, Y., Park, Y. S., Lee, H. S., Hong, S. A., Keun, B., et al. (2013). Protective effect of phosphatidylcholine on lipopolysaccharide induced acute inflammation in multiple organ injury. Korean J. Physiol. Pharamcol. 17, 209–217. doi: 10.4196/kjpp.2013.17.3.209
Kamath, A. F., Chauhan, A. K., Kisucka, J., Dole, V. S., Loscalzo, J., Handy, D. E., et al. (2006). Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood 107, 591–593. doi: 10.1182/blood-2005-06-2506
Kantarci, K., Murray, M. E., Schwarz, C. G., Reid, R. I., Przybelski, S. A., Lesnick, T., et al. (2017). White-matter integrity on DTI and the pathologic staging of Alzheimer's disease. Neurobiol. Aging 56, 172–179. doi: 10.1016/j.neurobiolaging.2017.04.024
Kataoka-kato, A., Ukai, M., Sakai, M., Kudo, S., and Kameyama, T. (2005). Enhanced learning of normal adult rodents by repeated oral administration of soybean transphosphatidylated phosphatidylserine. J. Pharmacol. Sci. 98, 307–314. doi: 10.1254/jphs.FP0050366
Kato-Kataoka, A., Sakai, M., Ebina, R., Nonaka, C., Asano, T., and Miyamori, T. (2010). Soybean-derived phosphatidylserine improves memory function of the elderly japanese subjects with memory complaints. J. Clin. Biochem. Nutr. 47, 246–255. doi: 10.3164/jcbn.10-62
Kawamoto, R., Ninomiyax, D., and Kusunoki, T. (2016). Journal of Clinical Gerontology & Geriatrics Oxidative stress is associated with increased arterial stiffness in middle-aged and elderly community-dwelling persons. J. Clin. Gerontol. Geriatics 7, 136–140. doi: 10.1016/j.jcgg.2016.05.003
Kennedy, K. M., and Raz, N. (2009). Pattern of normal age-related regional differences in white matter microstructure is modified by vascular risk. Brain Res. 1297, 41–56. doi: 10.1016/j.brainres.2009.08.058
Kent, C. (1995). Eukaryotic phospholipid biosynthesis. Annu. Rev. Biochem. 64, 315–343. doi: 10.1146/annurev.bi.64.070195.001531
Kew, S., Banerjee, T., Minihane, A. M., Finnegan, Y. E., Muggli, R., Albers, R., et al. (2003). Lack of effect of foods enriched with plant-or marine-derived n-3 fatty acids on human immune function. Am. J. Clin. Nutr. 77, 1287–1295. doi: 10.1093/ajcn/77.5.1287
Kew, S., Mesa, M. D., Tricon, S., Buckley, R., Minihane, A. M., and Yaqoob, P. (2004). Effects of oils rich in eicosapentaenoic and docosahexaenoic acids on immune cell composition and function in healthy humans. Am. J. Clin. Nutr. 79, 674–681. doi: 10.1093/ajcn/79.4.674
Kim, H., Bigelow, J., and Kevala, J. H. (2004). Substrate preference in phosphatidylserine biosynthesis for docosahexaenoic acid containing species. Biochemistry 43, 1030–1036. doi: 10.1021/bi035197x
Kim, J., Park, M. H., Kim, E., Han, C., Jo, S. A., and Jo, I. (2007). Plasma homocysteine is associated with the risk of mild cognitive impairment in an elderly Korean population. J. Nutr. 137, 2093–2098. doi: 10.1093/jn/137.9.2093
Kimura, A. K., and Kim, H. (2013). Phosphatidylserine synthase 2: high efficiency for synthesizing phosphatidylserine containing docosahexaenoic acid. J. Lipid Res. 54, 214–222. doi: 10.1194/jlr.M031989
Köbe, T., Witte, A. V., Schnelle, A., Grittner, U., Tesky, V. A., Pantel, J., et al. (2016). Vitamin B-12 concentration, memory performance, and hippocampal structure in patients with mild cognitive impairment. Am. J. Clin. Nutr. 103, 1045–1054. doi: 10.3945/ajcn.115.116970
Köbe, T., Witte, A. V., Schnelle, A., Tesky, V. A., Pantel, J., Schuchardt, J., et al. (2017). Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 11:105. doi: 10.3389/fnins.2017.00105
Köhler, S., Baars, M. A., Spauwen, P., Schievink, S., Verhey, F. R. J., and van Boxtel, M. J. (2014). Temporal evolution of cognitive changes in incident hypertension: prospective cohort study across the adult age span. Hypertension 63, 245–251. doi: 10.1161/HYPERTENSIONAHA.113.02096
Konat, G. W., and Wiggins, R. C. (1985). Effect of reactive oxygen species on myelin membrane proteins. J. Neurochem. 45, 1113–1118. doi: 10.1111/j.1471-4159.1985.tb05530.x
Kovacs, T., Varga, G., Erces, D., Tokes, T., Tiszlavicz, L., Ghyczy, M., et al. (2012). Dietary phosphatidylcholine supplementation attentuates inflammatory mucosal damage in a rat model of experimental colitis. Shock 38, 177–185. doi: 10.1097/SHK.0b013e31825d1ed0
Küllenberg, D., Taylor, L. A., Schneider, M., and Massing, U. (2012). Health effects of dietary phospholipids. Lipids Health Dis. 11:3. doi: 10.1186/1476-511X-11-3
Kume, A., Kurotani, K., Sato, M., Ejima, Y., Pham, N. M., Nanri, A., et al. (2013). Polyunsaturated fatty acids in serum and homocysteine concentrations in Japanese men and women: a cross-sectional study. Nutr. Metab. 10:41. doi: 10.1186/1743-7075-10-41
Lambeth, J. D. (2004). Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 1–9. doi: 10.1038/nri1312
Latorraca, S., Piersanti, P., Tesco, G., Piacentini, S., Amaducci, L., and Sorbi, S. (1993). Effect of phosphaitydlserine on free radical susceptibility in human diploid fibroblasts. J. Neural Transm. 6, 73–77. doi: 10.1007/BF02252625
Launer, L. J., Ross, G. W., Petrovitch, H., Masaki, K., Foley, D., White, L. R., et al. (2000). Midlife blood pressure and dementia: the Honolulu – Asia aging study. Neurobiol. Aging 21, 49–55. doi: 10.1016/S0197-4580(00)00096-8
Launtenschlager, N. T., Flicker, L., Vasikaran, S., Leedman, P., and Almeida, O. P. (2005). Subjective memory complaints with and without objective memory impairment. Am. J. Geriatr. Psychiatry 13, 731–734. doi: 10.1097/00019442-200508000-00013
Lee, B., Sur, B., Han, J., Shim, I., Her, S., Lee, H., et al. (2010). Krill phosphatidylserine improves learning and memory in Morris water maze in aged rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1085–1093. doi: 10.1016/j.pnpbp.2010.05.031
Lee, M., Kwon, N., Yoon, S. R., and Kim, O. Y. (2016). Serum phospholipid docosahexaenoic acid is inversely associated with arterial stiffness in metabolically healthy men. Clin. Nutr. Res. 5, 190–203. doi: 10.7762/cnr.2016.5.3.190
Lehmann, M., Regland, B., Blennow, K., and Gottfries, C. G. (2003). Vitamin B12-B6-folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 16, 145–150. doi: 10.1159/000071002
Leritz, E. C., Salat, D. H., Milberg, W. P., Williams, V. J., Chapman, C. E., Grande, L. J., et al. (2010). Variation in blood pressure is associated with white matter microstructure but not cognition in African Americans. Neuropsychology 24, 199–208. doi: 10.1037/a0018108
Leritz, E. C., Salat, D. H., Williams, V. J., Schnyer, D. M., Rudolph, J. L., Lipsitz, L., et al. (2011). Thickness of the human cerebral cortex is associated with metrics of cerebrovascular health in a normative sample of community dwelling older adults. Neuroimage 54, 2659–2671. doi: 10.1016/j.neuroimage.2010.10.050
Li, D., Mann, N. J., and Sinclair, A. J. (2006). A significant inverse relationship between concentrations of plasma homocysteine and phospholipid docosahexaenoic acid in healthy male subjects. Lipids 41, 85–89. doi: 10.1007/s11745-006-5074-x
Li, J.-G., Chu, J., Barrero, C., Merali, S., and Praticò, D. (2014). Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann. Neurol. 75, 851–863. doi: 10.1002/ana.24145
Lilamand, M., Vidal, J.-S., Plichart, M., De Jong, L. W., Duron, E., and Hanon, O. (2016). Arterial stiffness and medial temporal lobe atrophy in elders with memory disorders. J. Hypertens. 34, 1331–1337. doi: 10.1097/HJH.0000000000000954
Lim, S., and Suzuki, H. (2000). Intakes of dietary docosahexaenoic acid ethyl ester and egg phosphatidylcholine improve maze-learning ability in young and old mice. J. Nutr. 130, 1629–1632. doi: 10.1093/jn/130.6.1629
Lim, S. L., Gao, Q., Nyunt, M. S., Gong, L., Lunaria, J. B., Lim, M. L., et al. (2016). Vascular health indices and cognitive domain function: Singapore longitudinal ageing studies. J. Alzheimer's Dis. 50, 27–40. doi: 10.3233/JAD-150516
Liu, S.-H., Chang, C.-D., Chen, P.-H., Su, J.-R., Chen, C.-C., and Chaung, H.-C. (2012). Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res. 1451, 19–26. doi: 10.1016/j.brainres.2012.02.060
Lloyd-Jones, D. M., Evans, J. C., and Levy, D. (2005). Hypertension in adults across rhe age spectrum: current outcomes and control in the community. JAMA 294, 466–472. doi: 10.1001/jama.294.4.466
Lockhart, S. N., Mayda, A. B., Roach, A. E., Fletcher, E., Carmichael, O., Maillard, P., et al. (2012). Episodic memory function is associated with multiple measures of white matter integrity in cognitive aging. Front. Hum. Neurosci. 6:56. doi: 10.3389/fnhum.2012.00056
Lominadze, D., Roberts, A. M., Tyagi, N., Moshal, K. S., and Tyagi, S. C. (2006). Homocysteine causes cerebrovascular leakage in mice. Am. J. Physiol. Hear. Circ. Physiol. 290, 1206–1213. doi: 10.1152/ajpheart.00376.2005
Luo, Y., Zhou, X., Yang, X., and Wang, J. (2007). Homocysteine induces t hyperphosphorylation in rats. Neuroreport 18, 18–21. doi: 10.1097/WNR.0b013e3282f29100
Madsen, S. K., Rajagopalan, P., Joshi, S. H., Toga, A. W., and Thompson, P. M. (2015). Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer's Disease Neuroimaging Initiative. Neurobiol. Aging 36, s203–s210. doi: 10.1016/j.neurobiolaging.2014.01.154
Magaki, S., Mueller, C., Dickson, C., and Kirsch, W. (2007). Increased production of inflammatory cytokines in mild cognitive impairment. Exp. Gerontol. 42, 233–240. doi: 10.1016/j.exger.2006.09.015
Maillard, P., Carmichael, O., Fletcher, E., Reed, B., Mungas, D., and DeCarli, C. (2012). Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 79, 442–448. doi: 10.1212/WNL.0b013e3182617136
Mandal, P. K., Saharan, S., Tripathi, M., and Murari, G. (2015). Archival report brain glutathione levels – a novel biomarker for mild cognitive impairment and Alzheimer's disease. Biol. Psychiatry 78, 702–710. doi: 10.1016/j.biopsych.2015.04.005
Mangialasche, F., Polidori, M. C., Monastero, R., Ercolani, S., Camarda, C., Cecchetti, R., et al. (2009). Biomarkers of oxidative and nitrosative damage in Alzheimer's disease and mild cognitive impairment. Ageing Res. Rev. 8, 285–305. doi: 10.1016/j.arr.2009.04.002
Maragno, H., Rodella, P., Freitas, S., Fernando, L., and Carlos, S. (2015). The effects of acute and chronic administration of phosphatidylserine on cell proliferation and survival in the dentate gyrus of adult and middle-aged rats. Brain Res. 1609, 72–81. doi: 10.1016/j.brainres.2015.03.017
Marioni, R. E., Strachan, M. W., Reynolds, R. M., Lowe, G. D. O., Mitchell, R. J., Fowkes, F. G., et al. (2010). Association between raised inflammatory markers and cognitive decline in elderly people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 59, 710–713. doi: 10.2337/db09-1163
Marnane, M., Al-jawadi, O. O., Mortazavi, S., Pogorzelec, K. J., Wang, B. W., Feldman, H. H., et al. (2016). Periventricular hyperintensities are associated with elevated cerebral amyloid. Neurology 86, 535–543. doi: 10.1212/WNL.0000000000002352
Marsland, A. L., Gianaros, P. J., Kuan, D. C., Sheu, L. K., Krajina, K., and Manuck, S. B. (2015). Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Beahv. Immun. 48, 195–204. doi: 10.1016/j.bbi.2015.03.015
McMahon, J. J., Green, T. J., Skeaff, C. M., Knight, R. G., Mann, J. I., and Williams, S. M. (2006). A controlled trial of homocysteine lowering and cognitive performance. N. Engl. J. Med. 34, 2764–2772. doi: 10.1056/NEJMoa054025
Meyer, M. L., Palta, P., Tanaka, H., Deal, J. A., and Wright, J. (2017). Association of central arterial stiffness and pressure pulsatility with mild cognitive impairment and dementia: the Atherosclerosis Risk in Communities Study-Neurocognitive Study (ARIC-NCS). J. Alzheimer's Dis. 57, 195–204. doi: 10.3233/JAD-161041
Miller, P. E., Van Elswyk, M., and Alexander, D. D. (2014). Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am. J. Hypertens. 27, 885–896. doi: 10.1093/ajh/hpu024
Miller, R. R., Leanza, C. M., Phillips, E. E., and Blacquire, K. D. (2003). Homocysteine-induced changes in brain membrane composition correlate with increased brain caspase-3 activities and reduced chick embryo viability. Comp. Biochem. Physiol. Part B 136, 521–532. doi: 10.1016/S1096-4959(03)00277-X
Minihane, A. M., Armah, C. K., Miles, E. A., Madden, J. M., Clark, A. B., Caslake, M. J., et al. (2016). Consumption of fish oil providing amounts of eicosapentaenoic acid and docosahexaenoic acid that can be obtained from the diet reduces blood pressure in adults with systolic hypertension: a retrospective analysis. J. Nutr. 146, 516–523. doi: 10.3945/jn.115.220475
Miralbell, J., Soriano, J. J., Spulber, G., López-Cancio, E., Arenillas, J. F., Bargalló, N., et al. (2012). Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiol. Aging 33, 1003.e9–17. doi: 10.1016/j.neurobiolaging.2011.09.020
Mitchell, G. F., Buchem, M. A., Van Sigurdsson, S., Gotal, J. D., Garcia, M., Aspelund, T., et al. (2011). Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility - Reykjavik Study. Brain 134, 3398–3407. doi: 10.1093/brain/awr253
Mitchell, G. F., Parise, H., Benjamin, E. J., Larson, M. G., Keyes, M. J., Vita, J. A., et al. (2004). Changes in arterial stiffness and wave reflection with advancing age in healthy men and women the framingham heart study. Hypertension 43, 1239–1245. doi: 10.1161/01.HYP.0000128420.01881.aa
Montagne, A., Barnes, S. R., Sweeney, M. D., Halliday, M. R., Sagare, A. P., Zhao, Z., et al. (2015). Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. doi: 10.1016/j.neuron.2014.12.032
Mooijaart, S. P., Sattar, N., Trompet, S., Lucke, J., Stott, D. J., Ford, I., et al. (2013). Circulating interleukin-6 concentration and cognitive decline in old age: the PROSPER study. J. Intern. Med. 274, 77–85. doi: 10.1111/joim.12052
More, M. I., Freitas, U., and Rutenberg, D. (2014). Positive effects of soy lecithin-derived phosphatidylserine plus phosphatidic acid on memory, cognition, daily functioning, and mood in elderly patients with Alzheimer's disease and dementia. Adv. Ther. 31, 1247–1262. doi: 10.1007/s12325-014-0165-1
Mori, T. A., Bao, D. Q., Burke, V., Puddey, I. B., and Beilin, L. J. (1999). Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension 34, 253–260. doi: 10.1161/01.HYP.34.2.253
Nagai, K., Kozaki, K., Sonohara, K., Akishita, M., and Toba, K. (2011). Relationship between interleukin-6 and cerebral deep white matter and periventricular hyperintensity in elderly women. Geriatr. Gerontol. Int. 11, 328–332. doi: 10.1111/j.1447-0594.2010.00686.x
Nagata, T., Yaguchi, T., and Nishizaki, T. (2011). DL- and PO-phosphatidylcholines as a promising learning and memory enhancer. Lipids Health Dis. 10:25. doi: 10.1186/1476-511X-10-25
Narayan, S. K., Firbank, M. J., Saxby, B. K., Stansby, G., Hansrani, M., O'Brien, J. T., et al. (2011a). Elevated plasma homocysteine is associated with increased brain atrophy rates in older subjects with mild hypertension. Dement. Geriatr. Cogn. Disord. 31, 341–348. doi: 10.1159/000328118
Narayan, S. K., Saxby, B. K., Firbank, M. J., O'Brien, J. T., Harrington, F., McKeith, I. G., et al. (2011b). Plasma homocysteine and cognitive decline in older hypertensive subjects. Int. Psychogeriatr. 23, 1607–1615. doi: 10.1017/S1041610211000779
Neaton, J. D., and Wentworth, D. (1992). Cholesterol, blood pressure, cigarette smoking, and death from coronary heart disease. Arch. Intern. Med. 152, 56–64. doi: 10.1001/archinte.1992.00400130082009
Nunzi, M. G., Milan, F., Guidolin, D., and Toffano, G. (1987). Dendritic spine loss in hippocampus of aged rats. Effect of brain phosphatidylserine administration. Neurobiol. Aging 8, 501–510. doi: 10.1016/0197-4580(87)90124-2
Oakley, R., and Tharakan, B. (2014). Vascular hyperpermeability and aging. Aging Dis. 5, 114–125. doi: 10.14336/AD.2014.0500114
Olthof, M. R., Brink, E. J., Katan, M. B., and Verhoef, P. (2005). Choline supplemented as phosphatidylcholine decreases fasting and postmethionine-loading plasma homocysteine concentrations in healthy men. Am. J. Clin. Nutr. 82, 111–117. doi: 10.1093/ajcn/82.1.111
Padurariu, M., Ciobica, A., Hritcu, L., Stoica, B., Bild, W., and Stefanescu, C. (2010). Neuroscience Letters Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and Alzheimer's disease. Neurosci. Lett. 469, 6–10. doi: 10.1016/j.neulet.2009.11.033
Parízková, M., Andel, R., Lerch, O., Marková, H., GaŽová, I., Vyhnálek, M., et al. (2017). Homocysteine and real-space navigation performance among non-demented older adults. J. Alzheimer's Dis. 55, 951–964. doi: 10.3233/JAD-160667
Park, H.-J., Shim, H. S., Kim, K. S., Han, J.-J., Kim, J. S., Ram, Y. A., et al. (2013). Enhanced learning and memory of normal young rats by repeated oral administration of Krill Phosphatidylserine. Nutr. Neurosci. 16, 47–53. doi: 10.1179/1476830512Y.0000000029
Parrish, W. R., Rosas-ballina, M., Gallowitsch-Puerta, M., Ochani, M., Ochani, K., Yang, L., et al. (2008). Modulation of TNF release by choline requires α7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 4, 567–574. doi: 10.2119/2008-00079
Paschos, G. K., Magkos, F., Panagiotakos, D. B., Votteas, V., and Zampelas, A. (2007). Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur. J. Clin. Nutr. 61, 1201–1206. doi: 10.1038/sj.ejcn.1602631
Pase, M. P., Beiser, A., Himali, J. J., Tsao, C., Satizabal, C. L., Vasan, R. S., et al. (2016). Aortic stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47, 2256–2262. doi: 10.1161/STROKEAHA.116.013508
Pase, M. P., Grima, N., Cockerell, R., Stough, C., Scholey, A., Sali, A., et al. (2015). The effects of long-chain omega-3 fish oils and multivitamins on cognitive and cardiovascular function: a randomized, controlled clinical trial the effects of long-chain omega-3 fish oils and multivitamins on. J. Am. Coll. Nutr. 34, 21–31. doi: 10.1080/07315724.2014.880660
Pase, M. P., Grima, N. A., and Sarris, J. (2011). Do long-chain n-3 fatty acids reduce arterial stiffness? A meta-analysis of randomised controlled trials. Br. J. Nutr. 106, 974–980. doi: 10.1017/S0007114511002819
Patel, R. S., Al, I., Morris, A. A., Ahmed, Y., Kavtaradze, N., Ali, S., et al. (2011). Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis 218, 90–95. doi: 10.1016/j.atherosclerosis.2011.04.033
Pelegri, C., Canudas, A. M., del Valle, J., Casadesus, G., Smith, M. A., Camins, A., et al. (2007). Increased permeability of blood–brain barrier on the hippocampus of a murine model of senescence. Mech. Ageing Dev. 128, 522–528. doi: 10.1016/j.mad.2007.07.002
Polvikoski, T. M., van Straaten, E. C. W., Barkhof, F., Sulkava, R., Aronen, H. J., Niinisto, L., et al. (2010). Frontal lobe white matter hyperintensities and neurofibrillary pathology in the oldest old. Neurology 75, 2071–2078. doi: 10.1212/WNL.0b013e318200d6f9
Popa-Wagner, A., Mitran, S., Sivanesan, S., Chang, E., and Buga, A. (2013). ROS and brain diseases: the good, the bad, and the ugly. Oxid. Med. Cell. Longev. 2013:963520. doi: 10.1155/2013/963520
Popescu, B. O., Toescu, E. C., Popescu, L. M., Bajenaru, O., Muresanu, D. F., Schultzberg, M., et al. (2009). Blood-brain barrier alterations in ageing and dementia. J. Neurol. Sci. 283, 99–106. doi: 10.1016/j.jns.2009.02.321
Popp, J., Lewczuk, P., Linnebank, M., Cvetanovska, G., Smulders, Y., Kölsch, H., et al. (2009). Homocysteine metabolism and cerebrospinal fluid markers for Alzheimer's disease. J. Alzheimer's Dis. 18, 819–828. doi: 10.3233/JAD-2009-1187
Popp-Snijders, C., Schouten, J. A., van Blitterswijk, W. J., and van der Veen, E. A. (1986). Changes in membrane lipid composition of human erythrocytes after dietary supplementation of (n-3) polyunsaturated fatty acids. Maintenance of membrane fluidity. Biochim. Biophys. Acta 854, 31–37. doi: 10.1016/0005-2736(86)90061-1
Pottala, J. V., Yaffe, K., Robinson, J. G., Espeland, M. A., Wallace, R., and Harris, W. S. (2014). Higher RBC EPA + DHA corresponds with larger total brain and hippocampal volumes: WHIMS-MRI study. Neurology 82, 435–442. doi: 10.1212/WNL.0000000000000080
Puca, F. M., Savarese, M. A., and Minervini, M. G. (1987). Exploratory trial of phosphatidylserine efficacy in mildly demented patients. Clin. Trials J. 24, 94–98.
Pun, P. B., Lu, J., and Moochhala, S. (2009). Involvement of ROS in BBB dysfunction. Free Radic. Res. 43, 348–364. doi: 10.1080/10715760902751902
Pynn, C. J., Henderson, N. G., Clark, H., Koster, G., Bernhard, W., and Postle, A. D. (2011). Specificity and rate of human and mouse liver and plasma phosphatidylcholine synthesis analyzed in vivo. J. Lipid Res. 52, 399–407. doi: 10.1194/jlr.D011916
Qu, M., Yang, X., Wang, Y., Tang, Q., Han, H., Wang, J., et al. (2016). Docosahexaenoic acid-phosphatidylcholine improves cognitive deficits in an Aβ23-35 Alzheimer's disease rat model. Curr. Top. Med. Chem. 16, 558–564. doi: 10.2174/1568026615666150813144437
Rahman, M., Kundu, J. K., Shin, J. W., Na, H., and Surh, Y. (2011). Docosahexaenoic acid inhibits UVB-induced activation of NF- k B and expression of COX-2 and NOX-4 in HR-1 hairless mouse skin by blocking MSK1 signaling. PLoS ONE 6:e28065. doi: 10.1371/journal.pone.0028065
Rajagopalan, P., Hua, X., Toga, A. W., Jack, C. R., Weiner, M. W., and Thompson, P. M. (2011). Homocysteine effects on brain volumes mapped in 732 elderly individuals. Neuroreport 22, 391–395. doi: 10.1097/WNR.0b013e328346bf85
Ramprasath, V. R., Eyal, I., Zchut, S., and Jones, P. J. H. (2013). Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Helath Dis. 12:178. doi: 10.1186/1476-511X-12-178
Rasmussen, L. E., Svensson, M., Jørgensen, K. A., Schmidt, E. B., and Christensen, J. H. (2010). The content of docosahexaenoic acid in serum phospholipid is inversely correlated with plasma homocysteine levels in patients with end-stage renal disease. Nutr. Res. 30, 535–540. doi: 10.1016/j.nutres.2010.07.004
Raz, N., Rodrigue, K. M., and Acker, J. D. (2003). Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav. Neurosci. 117, 1169–1180. doi: 10.1037/0735-7044.117.6.1169
Raz, N., Yang, Y., Dahle, C. L., and Land, S. (2012). Volume of white matter hyperintensities in healthy adults: contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim. Biophys. Acta 1822, 361–369. doi: 10.1016/j.bbadis.2011.08.007
Refsum, H., Nurk, E., Smith, D. A., Ueland, P. M., Gjesdal, C. G., Bjelland, I., et al. (2006). The hordaland homocysteine study: a community-based study of homocysteine, its determinants, and associations with disease. J. Nutr. 136, 1731s−1740s. doi: 10.1093/jn/136.6.1731S
Reinders, I., Murphy, R. A., Song, X., Mitchell, G. F., Visser, M., Cotch, M. F., et al. (2015). Higher plasma phospholipid n – 3 PUFAs , but lower n – 6 PUFAs , are associated with lower pulse wave velocity among older adults. J. Nutr. 145, 2317–2324. doi: 10.3945/jn.115.212282
Resnick, S. M., Pham, D. L., Kraut, M. A., Zonderman, A. B., and Davatzikos, C. (2003). Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J. Neurosci. 23, 3295–3301.
Revel, F., Gilbert, T., Roche, S., Drai, J., Blond, E., Ecochard, R., et al. (2015). Influence of oxidative stress biomarkers on cognitive decline. J. Alzheimers. Dis. 45, 553–560. doi: 10.3233/JAD-141797
Rhodehouse, B. C., Mayo, J. N., Beard, R. S., Chen, C.-H., and Bearden, S. E. (2013). Opening of the blood-brain barrier before cerebral pathology in mild hyperhomocysteinemia. PLoS ONE 8:e63951. doi: 10.1371/journal.pone.0063951
Richard, D., Wolf, C., Barbe, U., Kefi, K., Bausero, P., and Visioli, F. (2009). Biochemical and biophysical research communications docosahexaenoic acid down-regulates endothelial Nox 4 through a sPLA 2 signalling pathway. Biochem. Biophys. Res. Commun. 389, 516–522. doi: 10.1016/j.bbrc.2009.09.013
Richter, Y., Herzog, Y., Cohen, T., and Steinhart, Y. (2010). The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: a pilot study. Clin. Interv. Aging 5, 313–316. doi: 10.2147/CIA.S13432
Richter, Y., Herzog, Y., Lifshitz, Y., Hayun, R., and Zchut, S. (2013). The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clin. Interv. Aging 8, 557–563. doi: 10.2147/CIA.S40348
Ridgway, N. D. (2016). “Phospholipid synthesis in mammalian cells,” in Biochemistry of Lipids, Lipoproteins and Membranes, eds N. D. Ridgeway and R. S. McLeod (Amsterdam: Elsevier), 209–236.
Ridgway, N. D., and Vance, D. E. (1988). Specificity of rat hepatic phosphatidylethanolamine N-methyltransferase for molecular species of diacyl phosphatidylethanolamine. J. Biol. Chem. 263, 16856–16863.
Rinaldi, P., Polidori, M. C., Metastasio, A., Mariani, E., Mattioli, P., Cherubini, A., et al. (2003). Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer's disease. Neurobiol. Aging 24, 915–919. doi: 10.1016/S0197-4580(03)00031-9
Rochfort, K. D., and Cummins, P. M. (2015). The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem. Soc. Trans. 43, 702–706. doi: 10.1042/BST20140319
Sakai, M., Yamatoya, H., and Kudo, S. (1996). Pharmacological effects of phosphatidylserine enzymatically synthesized from soybean lecithin on brain functions in rodents. J. Nutr. Sci. Vitaminol. 42, 47–54. doi: 10.3177/jnsv.42.47
Sala, S., Agosta, F., Pagani, E., Copetti, M., Comi, G., and Filippi, M. (2012). Microstructural changes and atrophy in brain white matter tracts with aging. Neurobiol. Aging 33, 488–498. doi: 10.1016/j.neurobiolaging.2010.04.027
Salat, D. H., Williams, V. J., Leritz, E. C., Schnyer, D. M., Rudolph, J. L., Lipsitz, L. A., et al. (2012). Inter-individual variation in blood pressure is associated with regional white matter integrity in generally healthy older adults. Neuroimage 59, 181–192. doi: 10.1016/j.neuroimage.2011.07.033
Samieri, C., Maillard, P., Crivello, F., Proust-Lima, C., Peuchant, E., Helmer, C., et al. (2012). Plasma long-chain omega-3 fatty acids and atrophy of the medial temporal lobe. Neurology 79, 642–650. doi: 10.1212/WNL.0b013e318264e394
Satizabal, C. L., Zhu, Y. C., Mazoyer, B., Dufouil, C., and Tzourio, C. (2012). Circulating IL-6 and CRP are associated with MRI findings in the elderly The 3C-dijon study. Neurology 78, 720–727. doi: 10.1212/WNL.0b013e318248e50f
Saw, C. L., Yang, A. Y., Guo, Y., and Kong, A.-N. T. (2013). Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2 – ARE pathway. Food Chem. Toxicol. 62, 869–875. doi: 10.1016/j.fct.2013.10.023
Schmidt, R., Schmidt, H., Curb, J. D., Masaki, K., White, L. R., and Launer, L. J. (2002). Early inflammation and dementia : a 25-year follow-up of the honolulu-asia aging study. Ann. Neurol. 52, 168–174. doi: 10.1002/ana.10265
Scholey, A. (2018). Nutrients for neurocognition in health and disease: measures, methodologies and mechanisms. Proc. Nutr. Soc. 77, 73–83. doi: 10.1017/S0029665117004025
Schreiber, S., Kampf-sherf, O., Gorfine, M., Kelly, D., Oppenheim, Y., and Lerer, B. (2000). An open trial of plant-source derived phosphatydilserine for treatment of age- related cognitive decline. Isr. J. Psychiatry Relat. Sci. 37, 302–307.
Schwab, U., Toppinen, L., Alfthan, G., Saarinen, M., Aro, A., and Uusitupa, M. (2002). Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am. J. Clin. Nutr. 76, 961–967. doi: 10.1093/ajcn/76.5.961
Sekikawa, A., Shin, C., Masaki, K. H., Barinas-mitchell, E. J. M., Hirooka, N., Willcox, B. J., et al. (2013). Original article association of total marine fatty acids, eicosapentaenoic and docosahexaenoic acids, with aortic stiffness in Koreans whites, and Japanese Americans. Am. J. Hypertens. 26, 1321–1327. doi: 10.1093/ajh/hpt107
Selhub, J., Bagley, L. C., Miller, J., and Rosenberg, I. H. (2000). B vitamins, homocysteine, and neurocognitive function in the elderly. Am. J. Clin. Nutr. 71, 614s–620s. doi: 10.1093/ajcn/71.2.614s
Selley, M. L. (2007). A metabolic link between S -adenosylhomocysteine and polyunsaturated fatty acid metabolism in Alzheimer's disease. Neurobiol. Aging 28, 1834–1839. doi: 10.1016/j.neurobiolaging.2006.08.003
Serhan, C. N., and Petasis, N. A. (2011). Resolvins and protectins in inflammation-resolution. Chem. Rev. 111, 5922–5943. doi: 10.1021/cr100396c
Seshadri, S., Wolf, P. A., Beiser, A. S., Selhub, J., Au, R., Jacques, P. F., et al. (2008). Association of plasma total homocysteine levels with subclinical brain injury. Arch. Neurol. 65, 642–649. doi: 10.1001/archneur.65.5.642
Singer, J., Trollor, J. N., Baune, B. T., Sachdev, P. S., and Smith, E. (2014). Arterial stiffness, the brain and cognition: a systematic review. Ageing Res. Rev. 15, 16–27. doi: 10.1016/j.arr.2014.02.002
Smith, A. D., and Refsum, H. (2016). Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr. 36, 211–239. doi: 10.1146/annurev-nutr-071715-050947
Smith, A. D., Smith, S. M., de Jager, C. A., Whitbread, P., Johnston, C., Agacinski, G., et al. (2010). Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS ONE 5:e12244. doi: 10.1371/journal.pone.0012244
Smith, E. E., Salat, D. H., Jeng, J., McCreary, C. R., Fischl, B., Schmahmann, J. D., et al. (2011). Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology 76, 1492–1499. doi: 10.1212/WNL.0b013e318217e7c8
Soderberg, M., Edlund, C., Kristensson, K., and Dallner, G. (1990). Lipid compositions of different regions of the human brain during aging. J. Neurochem. 54, 415–423. doi: 10.1111/j.1471-4159.1990.tb01889.x
Soderberg, M., Edlund, C., Kristensson, K., and Dallner, G. (1991). Fatty acid composition of brain phospholipids in aging and in Alzheimer's Disease. Lipids 26, 421–425.
Song, S. K., Sun, S. W., Ju, W. K., Lin, S. J., Cross, A. H., and Neufeld, A. H. (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20, 1714–1722. doi: 10.1016/j.neuroimage.2003.07.005
Song, S.-K., Sun, S.-W., Ramsbottom, M. J., Chang, C., Russell, J., and Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17, 1429–1436. doi: 10.1006/nimg.2002.1267
Steenge, G. R., Verhoef, P., and Katan, M. B. (2003). Betaine supplementation lowers plasma homocysteine in healthy men and women. J. Nutr. 133, 1291–1295.
Sudheimer, K. D., O'Hara, R., Spiegel, D., Powers, B., Kraemer, H. C., Neri, E., et al. (2014). Cortisol, cytokines, and hippocampal volume interactions in the elderly. Front. Aging Neurosci. 6:153. doi: 10.3389/fnagi.2014.00153
Sun, S.-W., Liang, H.-F., Le, T. Q., Armstrong, R. C., Cross, A. H., and Song, S.-K. (2006). Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage 32, 1195–1204. doi: 10.1016/j.neuroimage.2006.04.212
Sura, L., Madhaven, A., Carnaby, G., and Crary, M. A. (2012). Dysphagia in the elderly: management and nutritional considerations. Clin. Interv. Aging 7, 287–298. doi: 10.2147/CIA.S23404
Suzuki, S., Kataoka, A., and Furushiro, M. (2000). Effect of intracerebroventricular administration of soybean lecithin transphosphorylated phosphatidylserine on scopolamine-induced amnesic mice. Jpn. J. Pharmacol. 84, 86–88. doi: 10.1254/jjp.84.86
Suzuki, S., Yamatoya, H., Sakai, M., and Kataoka, A. (2001). Oral administration of soybean lecithin transphosphatidylated phosphatidylserine improves memory impairment in aged rats. J. Nutr. 131, 2951–2956. doi: 10.1093/jn/131.11.2951
Svennerholm, L., Boström, K., and Jungbjer, B. (1997). Changes in weight and compositions of major membrane components of human brain during the span of adult human life of Swedes. Acta Neuropathol. 94, 345–352. doi: 10.1007/s004010050717
Svennerholm, L., Jungbjer, B., and Olsson, L. (1994). Membrane lipids of adult human brain: lipid composition of frontal and temporal lobe in subjects of age 20 to 100 years. J. Neurochem. 63, 1802–1811. doi: 10.1046/j.1471-4159.1994.63051802.x
Takeuchi, H., Sakurai, C., Noda, R., Sekine, S., Murano, Y., Wanaka, K., et al. (2007). Antihypertensive effect and safety of dietary a -linolenic acid in subjects with high-normal blood pressure and mild hypertension. J. Oleo Sci. 360, 347–360. doi: 10.5650/jos.56.347
Taki, Y., Thyreau, B., Kinomura, S., Sato, K., Goto, R., Wu, K., et al. (2013a). A longitudinal study of age- and gender-related annual rate of volume changes in regional gray matter in healthy adults. Hum. Brain Mapp. 34, 2292–2301. doi: 10.1002/hbm.22067
Taki, Y., Thyreau, B., Kinomura, S., Sato, K., Goto, R., Wu, K., et al. (2013b). Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum. Brain Mapp. 34, 2418–2424. doi: 10.1002/hbm.22073
Tan, Z. S., Harris, W. S., Beiser, A. S., Au, R., Himali, J. J., Debette, S., et al. (2012). Red blood cell ω-3 fatty acid levels and markers of accelerated brain aging. Neurology 78, 658–664. doi: 10.1212/WNL.0b013e318249f6a9
Tangney, C. C., Aggarwal, N. T., Li, H., Wilson, R. S., Decarli, C., Evans, D. A., et al. (2011). Vitamin B12, cognition, and brain MRI measures: a cross-sectional examination. Neurology 77, 1276–1282. doi: 10.1212/WNL.0b013e3182315a33
Tegeler, C., Lorraine, J., Sullivan, O., Bucholtz, N., Goldeck, D., Pawelec, G., et al. (2016). The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function - data from the Berlin Aging Study II. Neurobiol. Aging 38, 112–117. doi: 10.1016/j.neurobiolaging.2015.10.039
Theobald, H. E., Goodall, A. H., Sattar, N., Talbot, D. C., Chowienczyk, P. J., and Sanders, T. A. (2007). Low-dose docosahexaenoic acid lowers diastolic blood pressure in middle-aged men and women. J. Nutr. 137, 973–978. doi: 10.1093/jn/137.4.973
Titova, O. E., Sjögren, P., Brooks, S. J., Kullberg, J., Ax, E., Kilander, L., et al. (2013). Dietary intake of eicosapentaenoic and docosahexaenoic acids is linked to gray matter volume and cognitive function in elderly. Age 35, 1495–1505. doi: 10.1007/s11357-012-9453-3
Tokés, T., Eros, G., Bebes, A., Hartmann, P., Várszegi, S., Varga, G., et al. (2011). Protective effects of a phosphatidylcholine-enriched diet in lipopolysaccharide-induced experimental neuroinflammation in the rat. Shock 36, 458–465. doi: 10.1097/SHK.0b013e31822f36b0
Torres, L. L., Quaglio, N. B., de Souva, G. T., Garcia, R. T., Dati, L. M. M., Moreira, W. L., et al. (2011). Peripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer' s disease. J. Alzheimer's Dis. 26, 59–68. doi: 10.3233/JAD-2011-110284
Tosun, D., Schuff, N., Truran-Sacrey, D., Shaw, L. M., Trojanowski, J. Q., Aisen, P., et al. (2010). Relations between brain tissue loss, CSF biomarkers, and the ApoE genetic profile: a longitudinal MRI study. Neurobiol. Aging 31, 1340–1354. doi: 10.1016/j.neurobiolaging.2010.04.030
Treede, I., Braun, A., Jeliaskova, P., Giese, T., Füllekrug, J., Griffiths, G., et al. (2009). TNF-α- induced up-regulation of pro-inflammatory cytokines is reduced by phosphatidylcholine in intestinal epithelial cells. BMC Gastroenterol. 9:53. doi: 10.1186/1471-230X-9-53
Treede, I., Braun, A., Sparla, R., Ku, M., Giese, T., Turner, J. R., et al. (2007). Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 282, 27155–27164. doi: 10.1074/jbc.M704408200
Troller, J. N., Smith, E., Baune, B. T., Kochan, N. A., Campbell, L., Samaras, K., Crawford, J., et al. (2010). Systemic inflammation is associated with MCI and its subtypes: the sydney memory and aging study. Dement. Geriatr. Cogn. Disord. 30, 569–578. doi: 10.1159/000322092
Tsao, C. W., Beiser, A. S., Westwood, A. J., Decarli, C., Hamburg, N. M., Vita, J. A., et al. (2013). Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology 81, 984–991. doi: 10.1212/WNL.0b013e3182a43e1c
Tsao, C. W., Himali, J. J., Beiser, A. S., Larson, M. G., Decarli, C., and Mitchell, G. F. (2016). Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology 86, 619–626. doi: 10.1212/WNL.0000000000002368
Ulven, S. M., Kirkhus, B., and Lamglait, A. (2011). Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 46, 37–46. doi: 10.1007/s11745-010-3490-4
Umur, E. E., Oktenli, C., Celik, S., Tangi, F., Sayan, O., Sanisoglu, Y. S., et al. (2011). Increased iron and oxidative stress are separately related to cognitive decline in elderly. Geriatr. Gerontol. Int. 11, 504–509. doi: 10.1111/j.1447-0594.2011.00694.x
Vakhapova, V., Cohen, T., Kam, Y., Korczyn, A. D., Richter, Y., and Herzog, Y. (2014). Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in nondemented elderly individuals with memory complaints: results from an open-label extension study. Dement. Geriatr. Cogn. Disord. 38, 39–45. doi: 10.1159/000357793
Vakhapova, V., Cohen, T., Ritcher, Y., Herzog, Y., and Korczyn, A. D. (2010). Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo controlled trial. Dement. Geriatr. Cogn. Disord. 29, 467–474. doi: 10.1159/000310330
Vakhapova, V., Richter, Y., Cohen, T., Herzog, Y., and Korczyn, A. D. (2011). Safety of phosphatidylserine containing omega-3 fatty acids in non-demented elderly: a double-blind placebo-controlled trial followed by an open-label extension. BMC Neurol. 11:79. doi: 10.1186/1471-2377-11-79
van de Haar, H. J., Burgmans, S., Jansen, J. F., van Osch, M. J., van Buchem, M. A., Muller, M., et al. (2016). Blood-brain barrier leakage in patients with early Alzheimer disease. Radiology 281, 527–535. doi: 10.1148/radiol.2016152244
van Sloten, T. T., Protogerou, A. D., Henry, R. M. A., Schram, M. T., Launer, L. J., and Stehouwer, C. D. A. (2015). Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 53, 121–130. doi: 10.1016/j.neubiorev.2015.03.011
Vance, D. E. (2014). Phospholipid methylation in mammals: from biochemistry to physiological function. Biochim. Biophys. Acta 1838, 1477–1487. doi: 10.1016/j.bbamem.2013.10.018
Vance, J. E. (2008). Phosphatidylserine and phosphatidylethanolamine in mammalian cells: two metabolically related aminophospholipids. J. Lipid Res. 49, 1377–1387. doi: 10.1194/jlr.R700020-JLR200
Vance, J. E., and Tasseva, G. (2013). Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 1831, 543–554. doi: 10.1016/j.bbalip.2012.08.016
Varatharaj, A., and Galea, I. (2017). The blood-brain barrier in systemic inflammation. Brain. Behav. Immun. 60, 1–12. doi: 10.1016/j.bbi.2016.03.010
Vaziri, N. D., and Rodríguez-Iturbe, B. (2006). Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Neurol. 2, 582–593. doi: 10.1038/ncpneph0283
Vermeer, S. E., van Dijk, E. J., Koudstaal, P. J., Oudkerk, M., Hofman, A., Clarke, R., et al. (2002). Homocysteine, silent brain infarcts, and white matter lesions: the Rotterdam scan study. Ann. Neurol. 51, 285–289. doi: 10.1002/ana.10111
Villardita, C., Grioli, S., Salmeri, G., Nicoletti, F., and Pennisi, G. (1987). Multicentre clinical trial of brain phosphatidylserine in elderly patients with intellectual deterioration. Clin. Trials J. 24, 84–93.
Vogiatzoglou, A., Smith, S. M., and Bradley, K. M. (2008). Vitamin B 12 status and rate of brain volume loss in community-dwelling elderly. Neurology 71, 826–832. doi: 10.1212/01.wnl.0000325581.26991.f2
Von Bernhardi, R., Eugenín-von Bernhardi, L., and Eugenín, J. (2015). Microglial cell dysregulation in brain aging and neurodegeneration. Front. Aging Neurosci. 7:124. doi: 10.3389/fnagi.2015.00124
Walhovd, K. B., Fjell, A. M., Reinvang, I., Lundervold, A., Dale, A. M., Eilertsen, D. E., et al. (2005). Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol. Aging 26, 1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020
Wall, R., Ross, R. P., Fitzgerald, G. F., and Stanton, C. (2010). Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 68, 280–289. doi: 10.1111/j.1753-4887.2010.00287.x
Wang, H., Golob, E. J., and Su, M.-Y. (2006). Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J. Magn. Reson. Imaging 24, 695–700. doi: 10.1002/jmri.20669
Wang, P.-N., Chou, K.-H., Chang, N.-J., Lin, K.-N., Chen, W.-T., Lan, G.-Y., et al. (2014). Callosal degeneration topographically correlated with cognitive function in amnestic mild cognitive impairment and Alzheimer's disease dementia. Hum. Brain Mapp. 35, 1529–1543. doi: 10.1002/hbm.22271
Wang, Y., West, J. D., Flashman, L. A., Wishart, H. A., Santulli, R. B., Rabin, L. A., et al. (2012). Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim. Biophys. Acta 1822, 423–430. doi: 10.1016/j.bbadis.2011.08.002
Wei, W., Liu, Y.-H., Zhang, C.-E., Wang, Q., Wei, Z., Mousseau, D. D., et al. (2011). Folate/vitamin-B12 prevents chronic hyperhomocysteinemia-induced tau hyperphosphorylation and memory deficits in aged rats. J. Alzheimer's Dis. 27, 639–650. doi: 10.3233/JAD-2011-110770
Weihrauch, J. L., and Son, Y. (1983). The phospholipid content of foods. J. Am. Oil Chem. Soc. 60, 1971–1978. doi: 10.1007/BF02669968
Wen, M., Ding, L., Zhang, L., Zhou, M., Xu, J., Wang, J., et al. (2016a). DHA-PC and DHA-PS improved A β 1–40 induced cognitive deficiency uncoupled with an increase in brain DHA in rats. J. Funct. Foods 22, 417–430. doi: 10.1016/j.jff.2016.02.004
Wen, M., Xu, J., Ding, L., Zhang, L., Du, L., Wang, J., et al. (2016b). Eicosapentaenoic acid-enriched phospholipids improve A β 1 – 40-induced cognitive deficiency in a rat model of Alzheimer ' s disease. J. Funct. Foods 24, 537–548. doi: 10.1016/j.jff.2016.04.034
Wengreen, H. J., Munger, R. G., Corcoran, C. D., Zandi, P., Hayden, K. M., Fotuhi, M., et al. (2007). Antioxidant intake and cognitive function of elderly men and women: the Cache County study. J. Nutr. Health Aging 11, 230–237.
Wersching, H., Duning, T., Lohmann, H., Mohammadi, S., Stehling, C., Fobker, M., et al. (2010). Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 74, 1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b
Westlye, L. T., Walhovd, K. B., Dale, A. M., Bjørnerud, A., Due-Tønnessen, P., Engvig, A., et al. (2010). Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex 20, 2055–2068. doi: 10.1093/cercor/bhp280
Whalley, L. J., Duthie, S. J., Collins, A. R., Starr, J. M., Deary, I. J., Lemmon, H., et al. (2014). Homocysteine, antioxidant micronutrients and late onset dementia. Eur. J. Nutr. 53, 277–285. doi: 10.1007/s00394-013-0526-6
Whitmer, R. A., Sidney, S., Selby, J., Johnston, S. C., and Yaffe, K. (2005). Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 64, 277–281. doi: 10.1212/01.WNL.0000149519.47454.F2
Whitwell, J. L., Josephs, K. A., Murray, M. E., Kantarci, K., Pryzbelski, S. A., Weigand, S. D., et al. (2008). MRI correlates of neurofibrillary tangle pathology at autopsy A voxel-based morphometry study. Neurology 71, 743–749. doi: 10.1212/01.wnl.0000324924.91351.7d
Wijendran, V., Huang, M., Diau, G., Boehm, G., Nathanielsz, P. W., and Brenna, J. T. (2002). Efficacy of dietary arachidonic acid provided as triglyceride or phospholipid as substrates for brain arachidonic acid accretion in baboon neonates. Pediatr. Res. 51, 265–272. doi: 10.1203/00006450-200203000-00002
Williams, J. H., Periera, E. A. C., Budge, M. M., and Bradley, K. M. (2002). Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing 31, 440–444. doi: 10.1093/ageing/31.6.440
Witte, A. V., Kerti, L., Hermannstädter, H. M., Fiebach, J. B., Schreiber, S. J., Schuchardt, J. P., et al. (2013). Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb. Cortex 24, 3059–3068. doi: 10.1093/cercor/bht163
Wright, C. B., Paik, M. C., Brown, T. R., Stabler, S. P., Allen, R. H., Sacco, R. L., et al. (2005). Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke 36, 1207–1211. doi: 10.1161/01.STR.0000165923.02318.22
Wright, C. B., Sacco, R. L., Rundek, T. R., Delman, J. B., Rabbani, L. E., and Elkind, M. S. V. (2006). Interleukin-6 is associated with cognitive function: the Northern Manhattan Study. J. Stroke Cerebrovasc. Dis. 15, 34–38. doi: 10.1016/j.jstrokecerebrovasdis.2005.08.009
Wu, J., and Harrison, D. G. (2014). “Oxidative stress and hypertension,” in Blood Pressure and Artierla Wall Mechanics in Cardiovascular Diseases, eds M. E. Safar, M. F. O'Rourke, and E. D. Frohlich (London: Springer-Verlag), 175–191.
Yaffe, K., Lindquist, K., Penninx, B. W., Simonsick, E. M., Pahor, M., Kritchevsky, S., et al. (2003). Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61, 76–80. doi: 10.1212/01.WNL.0000073620.42047.D7
Yaguchi, T., Nagata, T., and Nishizaki, T. (2009). 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine improves cognitive decline by enhancing long-term depression. Behav. Brain Res. 204, 129–132. doi: 10.1016/j.bbr.2009.05.027
Yaguchi, T., Nagata, T., and Nishizaki, T. (2010). 1,2-Dilinoleoyl-sn-glycero-3-phosphoethanolamine ameliorates age-related spatial memory deterioration by preventing neuronal cell death. Behav. brain Funct. 6:52. doi: 10.1186/1744-9081-6-52
Yaqoob, P., Pala, H. S., and Newsholme, E. A. (2000). Encapsulated® sh oil enriched in a -tocopherol alters plasma phospholipid and mononuclear cell fatty acid compositions but not mononuclear cell functions. Eur. J. Clin. Invest. 30, 260–274. doi: 10.1046/j.1365-2362.2000.00623.x
Yurko-Mauro, K., McCarthy, D., Rom, D., Nelson, E. B., Ryan, A. S., Blackwell, A., et al. (2010). Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 6, 456–464. doi: 10.1016/j.jalz.2010.01.013
Zanotti, A., Aporti, F., Toffano, G., and Valzelli, L. (1984). Effects of phosphatidylserine on avoidance learning in rats (preliminary observations). Pharmacol. Res. Commun. 16, 485–493. doi: 10.1016/S0031-6989(84)80017-X
Zanotti, A., Valzelli, L., and Toffano, G. (1989). Chronic phosphatidylserine treatment improves spatial memory and passive avoidance in aged rats. Psychopharmacology 99, 316–321. doi: 10.1007/BF00445550
Zeevi, N., Pachter, J., McCullough, L. D., Wolfson, L., and Kuchel, G. A. (2010). The blood–brain barrier: geriatric relevance of a critical brain–body interface. J. Am. Geriatr. Soc. 58, 1749–1757. doi: 10.1111/j.1532-5415.2010.03011.x
Zhang, C.-E., Tian, Q., Wei, W., Peng, J.-H., Liu, G.-P., Zhou, X.-W., et al. (2008). Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol. Aging 29, 1654–1665. doi: 10.1016/j.neurobiolaging.2007.04.015
Zhang, M., Wang, S., Mao, L., Leak, R. K., Shi, Y., Zhang, W., et al. (2014). Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 34, 1903–1915. doi: 10.1523/JNEUROSCI.4043-13.2014
Zhang, Y., Schuff, N., Camacho, M., Chao, L. L., Fletcher, T. P., Yaffe, K., et al. (2013). MRI markers for mild cognitive impairment: comparisons between white matter integrity and gray matter volume measurements. PLoS ONE 8:e66367. doi: 10.1371/journal.pone.0066367
Zhang, Y. Y., Yang, L. Q., and Guo, L. M. (2015). Effect of phosphatidylserine on memory in patients and rats with Alzheimer's disease. Gent. Mol. Res. 14, 9325–9333. doi: 10.4238/2015
Zhang, Y.-P., Miao, R., Li, Q., Wu, T., and Ma, F. (2017). Effects of DHA supplementation on hippocampal volume and cognitive function in older adults with mild cognitive impairment: a 12-Month. J. Alzheimer's Dis. 55, 497–507. doi: 10.3233/JAD-160439
Keywords: glycerophospholipid, supplementation, intervention, cerebral structure, older adults
Citation: Reddan JM, White DJ, Macpherson H, Scholey A and Pipingas A (2018) Glycerophospholipid Supplementation as a Potential Intervention for Supporting Cerebral Structure in Older Adults. Front. Aging Neurosci. 10:49. doi: 10.3389/fnagi.2018.00049
Received: 23 October 2017; Accepted: 15 February 2018;
Published: 07 March 2018.
Edited by:
Aurel Popa-Wagner, Department Neurology, University of Medicine Essen, GermanyReviewed by:
Ryusuke Takechi, Curtin University, AustraliaCopyright © 2018 Reddan, White, Macpherson, Scholey and Pipingas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffery M. Reddan, anJlZGRhbkBzd2luLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.