- 1Department of Aging and Geriatric Research, Center for Cognitive Aging and Memory, McKnight Brain Institute, University of Florida, Gainesville, FL, United States
- 2Department of Clinical and Health Psychology, University of Florida, Gainesville, FL, United States
- 3Department of Neuroscience, University of Florida, Gainesville, FL, United States
- 4Department of Psychology, Georgia State University, Atlanta, GA, United States
- 5Brain Rehabilitation Research Center, Malcom Randall Veterans Affairs Medical Center, Gainesville, FL, United States
Objective: Research has shown that depression is a risk factor for Alzheimer’s disease (AD) and subsequent cognitive decline. This is compounded by evidence showing an association between depression and reduced hippocampal volumes; a primary structure implicated in the pathogenesis of the disease. Less is known about the relationship between depression and other AD vulnerable regions such as the entorhinal cortex. Given the heterogeneity of depressive symptom presentation, we examined whether symptom dimensions were associated with hippocampal and entorhinal cortex volumes in community dwelling older adults.
Methods: Eighty-one community dwelling adults completed the Beck Depression Inventory – second edition and underwent structural neuroimaging. Measures of hippocampal and entorhinal cortex volumes were obtained using FreeSurfer software. Linear regression models included regions of interest as dependent variables, with depressive symptom dimensions, as independent variables, controlling for total intracranial volumes, age, education, and gender.
Results: Somatic symptoms were negatively associated with total, right, and left hippocampal volumes. Affective symptoms were negatively associated with total entorhinal cortex volumes, with a marginal main effect on left entorhinal cortex volumes.
Conclusion: Our findings provide support for examining depressive symptoms and their association with AD vulnerable regions along subdimensions of affective, cognitive, and somatic symptoms to better understand profiles of symptoms most associated with these regions. Conceptualizing depressive symptoms in this way may also better inform treatment approaches in terms of targeting types of symptoms that may be more closely linked to poorer brain and cognitive health outcomes.
Introduction
Understanding the neurobiological basis of depression in late life has received considerable attention (for review, see Disabato and Sheline, 2012), in part due to the evidence that depression may be a risk factor or prodrome for incident dementia (Green et al., 2003; Brommelhoff et al., 2009). Several brain structures have been shown to be associated with depression including the prefrontal cortex, cerebellum, and the cingulate gyrus (Ito, 1986; Dotson et al., 2009; Koenigs and Grafman, 2009). However, the hippocampus has perhaps been the most well-studied structure in the context of major depressive disorder (MDD) (for reviews, see Koolschijn et al., 2009; Lorenzetti et al., 2009; McKinnon et al., 2009). Several studies have shown that MDD in older adults is associated with decreased hippocampal volume (Hickie et al., 2005; Ballmaier et al., 2008; Zhao et al., 2008). These findings are consistent with some behavioral studies that have shown an association between depression and reduced performance on episodic and declarative memory tests (Bäckman and Forsell, 1994; Airaksinen et al., 2007; Herrmann et al., 2007); abilities that are largely dependent on hippocampal integrity (Tulving and Markowitsch, 1998). However, the findings on this topic are mixed. Some studies have found bilateral hippocampal volume reduction (Sheline et al., 1996; MacQueen et al., 2003), while others have noted a differential impact of depression on the right (Steffens et al., 2000; Bell-McGinty et al., 2002) and left hippocampi (Bremner et al., 2000), and yet others have not found any association with hippocampal volumes or differences between hemispheres (Pantel et al., 1997; Vakili et al., 2000; Posener et al., 2003).
Research on subclinical depressive symptoms and their association with memory and the hippocampus has shown a similar pattern of mixed findings (Dotson et al., 2009; Goveas et al., 2011; Enache et al., 2015; O’shea et al., 2015; Zimmerman et al., 2016), suggesting that there may be similar underlying mechanisms, although this is less clearly understood.
The role for depression in preclinical Alzheimer’s disease (AD) is compounded by emerging research which has shown that depression in late life may be a risk factor or prodromal manifestation of an underlying disease pathology (Jorm, 2000; Ownby et al., 2006; Sun et al., 2008). Although the directionality of the association between depression and AD is yet to be established, some longitudinal research does suggest that depressive symptoms precede cognitive decline (Cui et al., 2007; Zahodne et al., 2014). If depressive symptoms are a risk factor or are related to AD vulnerable regions such as the hippocampus, then examining other AD vulnerable or related regions may further identify those who may be at an increased risk-most notably; the entorhinal cortex, a region closely associated both structurally and functionally with the hippocampus. Layer II of the entorhinal cortex provides excitatory input to the hippocampus via the performant pathway and layer IV of the entorhinal cortex receives one of the major efferent projections from the hippocampus. The entorhinal cortex can be thought of as a bridge linking the hippocampus to the neocortex (Zola-Morgan et al., 1994). It is also one of the first regions to show neuronal loss in preclinical AD and is severely affected in later stages of the disease process (Gomez et al., 2014). However, few studies have examined the association of depression on both the hippocampus and the entorhinal cortex in a single study. One study found that early onset depression was associated with hippocampal volumes but not entorhinal cortex volumes and the converse with late onset depression (LoD) such that LoD was associated with smaller entorhinal cortex volumes but not hippocampal volumes (Gerritsen et al., 2011). These findings suggest that there may be differential mechanisms underlying presentation of depression. Research from the pediatric literature also suggests that there is a differential association with brain regions in unipolar versus bipolar depressive disorders (Serafini et al., 2014) highlighting the significance of how depressive symptoms present and the way this alters brain integrity. However, whether these findings would translate to subclinical levels of depressive symptoms is not clear. In a more recent study, Gatchel et al. (2017) found that depressive symptoms were marginally (partial r = 0.183, p = 0.055) associated with cerebral tau 18F T807 levels in the entorhinal cortex and significantly associated with inferior temporal lobes (partial r = 0.188, p = 0.050) in cognitive normal older adults, providing some preliminary support for an association between subclinical depression and the entorhinal cortex.
A consideration of not only severity of depression but also how we address differences in depressive symptom presentation warrants further consideration. The latter has received much less examination, which is surprising given that we understand depression to be a heterogeneous disorder. It may be possible that the presentation of depressive symptoms may differentially impact cognition and brain structure. Factor analytic studies of self-reported depression measures have typically identified a somatic, cognitive, and affective component, albeit in various combinations (Shafer, 2006; Elhai et al., 2012), supporting the consensus that depression is a highly heterogeneous disorder (Winokur, 1997). Examining subdimensions of depression scales may better inform our understanding of the mechanisms underlying the relationship between depression and the brain that may be missed when using only whole scale sum scores (Fried et al., 2014; Fried and Nesse, 2015; Dotson, 2017).
Little has been reported in terms of depression symptom dimensions and two primary AD vulnerable regions; the hippocampus and the entorhinal cortex, despite the accumulating evidence that total depressive symptom severity is associated with hippocampal volumes and that depression may be a risk factor for AD. The present study’s overarching aim is to explore this potential relationship and examine whether subdimensions of depressive symptoms are differentially related to hippocampal volumes. A second aim of the study is to examine whether subclinical depressive symptoms and subdimensions are associated with a closely related structure; the entorhinal cortex. As noted earlier, much less is known about the association between depression and the entorhinal cortex relative to the hippocampus. Thus, in the present study, we hypothesized that greater total depressive symptoms would be associated with smaller hippocampal and entorhinal cortex volumes. Furthermore, we hypothesized that subdimensions of depressive symptoms would be differentially associated with these two brain regions.
Materials and Methods
Participants
Data from 81 older adults (59% female, mean age: 71; range: 43–93) were included in the present study. The study procedures were approved by the University of Florida Institutional Review Board. Participants were recruited from the north-central Florida community via newspaper advertising and fliers. All participants provided written and verbal informed consent prior to taking part in the study. Participants were excluded if they reported a diagnosis of any neurological or psychiatric disorders, suicidal ideation and intention (participants were further screened to assess whether there was concern for this, no participants fulfilled this criteria), substance abuse, as well as neurodegenerative disease such as Parkinson’s disease or AD or self-reported difficulties or concern with thinking or memory. Further exclusionary criteria were based on whether the participants had any magnetic resonance imaging (MRI) [3.0 Tesla (3T)] contraindications, including metal or medical devices inside the body, pregnancy, or claustrophobia. Sample characteristics are displayed in Table 1.
Neuroimaging Procedure
Magnetic resonance imaging was performed using a 3T scanner (Achieva; Philips Electronics, Amsterdam, Netherlands) at the McKnight Brain Institute, University of Florida (Gainesville, FL, United States) with a 32-channel receive-only head coil. A high-resolution 3D T1 weighted MPRAGE scan was performed. Measures of hippocampal, entorhinal cortex, and intracranial volumes (ICV) were obtained using the following scanning parameters: voxel size = 1 mm isotropic; 1 mm slice thickness; TE = 3.2 ms; TR = 7.0 ms; FOV = 240 × 240; number of slices = 170; acquired in a sagittal orientation.
Magnetic resonance imaging T1-weighted scans were processed using FreeSurfer software (version 5.31). Hippocampal and entorhinal cortex volumes were obtained from the automatic subcortical segmentation stream. This method has shown to be reliable and produce accurate results of subcortical regions (Fischl et al., 2002; Jovicich et al., 2009). Each slice of all scans acquired was manually inspected by trained technicians and any errors in segmentation were manually corrected and re-processed through FreeSurfer. Total hippocampal and entorhinal cortex volumes were computed by summing the right and left volumes.
Depressive Symptoms
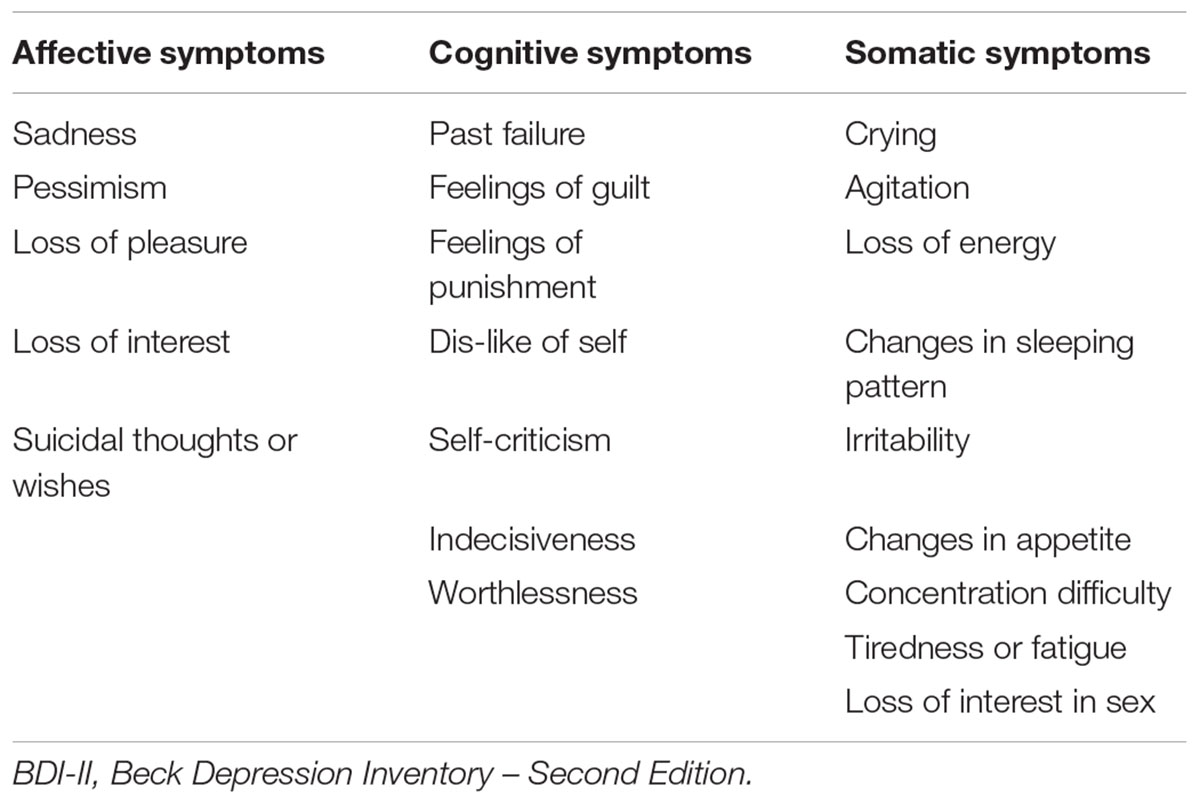
Depressive symptoms were measured using the Beck Depression Inventory – Second Edition (BDI-II; Beck et al., 1996). This is a widely used 21-item questionnaire that is used to assess for the presence of depressive symptoms over the past 2 weeks. Each item has a score between 0 and 3, thus total scores can range from 0 to 63. The range in the present sample was between 0 and 13, which indicates subclinical level of symptoms. The BDI-II has been previously shown to have strong psychometric properties in community dwelling older adults (Segal et al., 2008). The BDI-II has also been shown to have a three-factor structure reflecting a cognitive, somatic, and affective dimension that is present in both clinical and non-clinical samples (Vanheule et al., 2008). Table 2 shows each item corresponding to each of the three subscales used in the present study.
Statistical Analyses
All analyses were performed using SPSS version 22 (IBM Corp., Armonk, NY, United States). Data were screened for outliers by computing standardized z-scores. Outliers were identified as z > ±3 standard deviations above the mean and removed from analyses (n = 5). Several linear regression analyses were performed to examine the independent associations between total BDI-II scores on total hippocampal and entorhinal cortex volumes, controlling for covariates. BDI-II subdimensions were regressed onto the following outcome measures; total, right, and left hippocampal volumes and total right and left entorhinal cortex volumes. Age (continuous), sex, education (continuous), and ICV (continuous) were used as covariates in all the models. Covariates were entered in the first step of each model, followed by the cognitive, affective, and somatic symptom in steps 2, 3, and 4 (respectively) so that the effect of each of the scales could be estimated. All z-scored variables were used to facilitate interpretation of effects. Alpha was set at ≤0.05.
Results
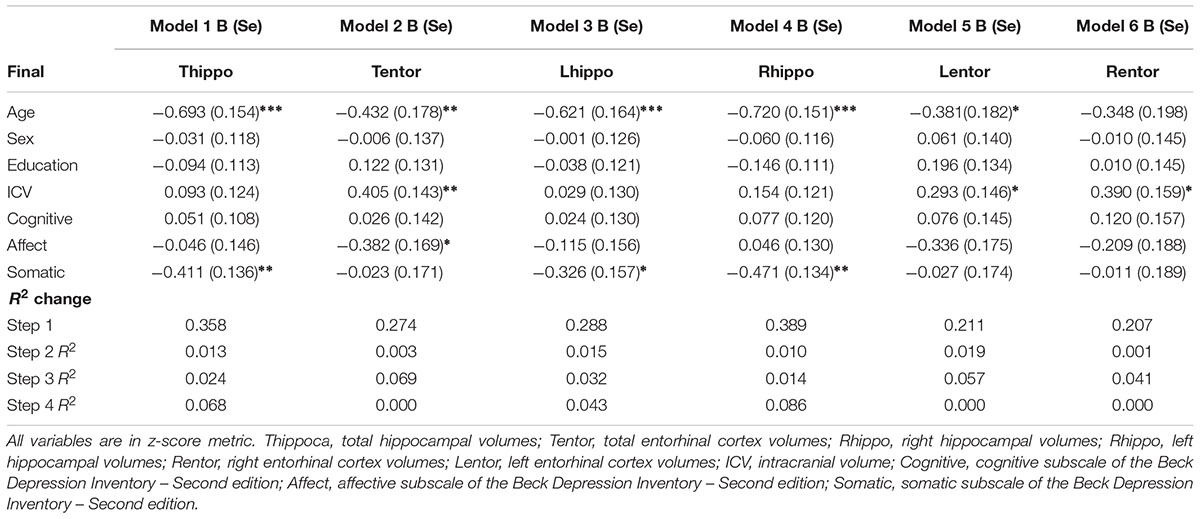
Results from the linear regression analyses revealed that older age was negatively associated with total, right, and left hippocampal volumes (Table 3). Increased age was also negatively associated with left entorhinal cortex volumes. Sex and education were not significant independent predictors in any of the models.
Total Symptoms
There was a significant main effect of total depressive symptoms on total hippocampal volumes but not total entorhinal cortex volumes. Greater depressive symptoms were associated with a decrease in total hippocampal volumes.
Symptom Dimensions
Cognitive
There was no main effect of cognitive symptoms on any of the outcome variables.
Affective
Higher scores on the affective symptom dimension were negatively associated with total entorhinal cortex volumes and marginally with left entorhinal cortex volumes (p = 0.056). Affective symptoms accounted for an additional 7% of the variance in total entorhinal cortex volumes, and almost 6% additional variance in left entorhinal cortex volume.
Somatic
Higher somatic depressive symptoms were negatively associated with total, right, and left hippocampal volumes but there was not a statistically significant association between this domain and entorhinal cortex volumes. The addition of somatic symptoms accounted for almost 7% of the variance in total hippocampal volumes, and 4% and almost 9% of the variance in left and right hippocampal volumes, respectively.
Discussion
We found that total depressive symptoms were negatively associated with total hippocampal volumes but not entorhinal cortex volumes. The association between total depressive symptoms and total hippocampal volumes was likely driven by somatic symptoms as this was the only subdimension significantly associated with total, left, and right hippocampal volumes. There was no association between somatic symptoms and entorhinal cortex volumes. Additionally, there was a significant negative association between affective symptoms and total entorhinal cortex volumes. There was also a marginally significant association between affective symptoms and left entorhinal cortex volumes. There were no other association between depressive symptom dimensions and any of the other brain regions. Our investigation of the subdimensions of depressive symptoms showed that somatic symptoms were the only symptom domain associated with hippocampal volumes. This is consistent with previous work which showed that somatic symptoms, but not affective or cognitive, were associated with thinner cortical precuneus volumes in older adults (Szymkowicz et al., 2017) as well as reduced overall gray and white matter volumes (Dotson et al., 2013; Kirton et al., 2014; Tang et al., 2014; Thames et al., 2015). Together, these findings highlight the significance of examining depressive symptoms in this way. Using only total scores may mask specific effects of groups of items or dimensions of depressive symptoms on brain volumes.
One explanation for the association between somatic symptoms and hippocampal volumes may be that some of the items underlying this dimension (“loss of energy,” “changes in sleeping patterns,” “irritability,” “tiredness or fatigue,” “concentration difficulties”) may reflect sleep quality. Previous research has shown a link between sleep quality/duration and brain volumes (Chao et al., 2014; Bernardi et al., 2016), including hippocampal volumes in older adults (Elcombe et al., 2015) and hippocampal glial integrity (Cross et al., 2013), although this topic in older adults is underexplored. The relationship between depression and sleep is well established (for reviews, see Tsuno et al., 2005; Bao et al., 2017), but the mechanisms through which these are related remain unclear. Disruptions in sleep quality and efficiency have been explained in terms of circadian rhythms disruptions due to abnormalities in plasma melatonin levels. Low plasma melatonin production at night is related to disruptions in circadian rhythms (Dawson and Encel, 1993).
Depression, as has largely been explained in terms of excess glucocorticoid production which proposes that depression is a type of stress response involving a chronic production of glucocorticoids which implicate the hypothalamic, pituitary, and limbic systems (Sheline, 2011). Several studies have shown that cumulative exposure to glucocorticoids has been related to hippocampal (i.e., a core limbic structure) volume reduction and impairments in hippocampal-dependent cognitive tasks in rodents (Woolley et al., 1990; Watanabe et al., 1992; Lee et al., 2017; Nguyen et al., 2018). Studies in humans have shown similar findings in healthy older adults who report high levels of stress concurrent with high levels of cumulative cortisol levels (Newcomer et al., 1999; Gold, 2015; Radley et al., 2015). There is also evidence that reductions in brain-derived neurotrophic factor (BDNF) following prolonged stress is associated with decreased hippocampal volumes (Mondelli et al., 2011). Excess glucocorticoid production is proposed to increase neuronal vulnerability to aging effects and/or pathological processes (Bishop et al., 2010).
Several studies have shown, for example, that about 50% of those with depression also have HPA hyperactivity (Arana et al., 1985; Checkley, 1996; Arborelius et al., 1999). Some research suggests that depression and sleep disorders may share some pathophysiological features (Thase, 2006; Srinivasan et al., 2009). This is supported by evidence which showed that the release of corticotropin-releasing hormone (CRH) in response to stress inhibits melatonin production in depressed patients (Kellner et al., 1997). However, further research is required to draw any firm conclusions.
Interpreting the relationship between affective symptoms of depression and entorhinal cortex volumes is more difficult to explain, largely due to the paucity of research on this topic. The differential impact of symptom subdimensions on the hippocampus versus entorhinal cortex may mirror findings which have shown that early-onset depression is association with hippocampal volumes reduction, whereas LoD is associated with entorhinal cortex volume reduction (Gerritsen et al., 2011). We may speculate that chronic stress due to life time or early experiences with depression impacts the hippocampus via pathophysiological processes outlined above and are more related to somatic symptoms. While the association between affective symptoms and entorhinal cortex (one of the first regions to be implicated in AD) may reflect an affective reaction to overt problems (such as memory lapses) due to an underlying preclinical AD process. However, we did not have information about time of onset of depressive symptoms to explore this further, which is a limitation to explaining our findings. Additionally, longitudinal studies would be better suited to explore this possibility and determine whether there is a differential relationship between subdimensions of depression and incidence of AD.
An alternative perspective might consider the role of neuroinflammation and its relation to depressive symptoms. Accumulating evidence suggest that chronic and low grade neuroinflammation may contribute to the pathogenesis of stroke, depression, and dementia as it alters molecular and cell function (for review, see Uzoni et al., 2015). Studies have shown that in MDD there are higher rates of neuroinflammation than in non-depressed participants (Tiemeier et al., 2003) and that elevated cytokines (inflammatory markers) can alter hippocampal neurogenesis (Maes et al., 2009; Caraci et al., 2010). Although less is known regarding the association between subclinical depressive symptoms and pro-inflammatory markers, some studies have shown that elevated cytokines IL-6 and Il-8 are associated with depressive symptoms (Penninx et al., 2003; Krishnadas and Cavanagh, 2012). Although the mechanisms underlying depression in the elderly are poorly understood, targeting and treating pro-inflammatory risk factors such diabetes and hypertension may optimize brain health by mitigating the effect of inflammation on molecular processes contributing to poor brain health and emergence of depressive symptoms. Other studies have also shown that exercise reduces levels of inflammation and depressive symptoms (Cotman et al., 2007; Trivedi et al., 2011), which may also be a viable treatment or protective lifestyle factor.
Additionally, with regard to brain health and protective mechanisms in the context of treatment, increasing brain or cognitive reserve may modulate responses to stress both at the molecular and psychological levels. Indeed, a more engaged and active lifestyle (a proxy of cognitive reserve) may protect against depressive symptoms (Van Gool et al., 2007). Additionally, other studies have shown that in healthy elderly and mild cognitive impairment samples with high cognitive reserve, there was greater network connectivity in the left frontal cortex (Franzmeier et al., 2017a,b), a region which supports memory functioning. Thus, the impact of depressive symptoms on brain integrity may be off-set by increasing cognitive reserve via engaging in a more stimulating and active lifestyle. Potentially, depressive symptoms could be treated from a multi-modal approach, one which focuses on enhancing brain health.
There are other limitations to our study that should be noted. The cross-sectional design is a limitation insofar as elucidating the causal mechanisms underlying the associations between symptom dimensions and hippocampal and entorhinal cortex volumes. For example, do somatic and affective symptoms cause hippocampal and entorhinal cortex volume reductions, respectively, or do smaller hippocampal and entorhinal cortex volumes cause one to be more susceptible to these symptoms? Follow-up studies will be required to better address this question. Another limitation includes the relatively small sample size and relatively homogenous Caucasian ethnicity of participants in the present study which may impact the generalizability of our findings. Future research should include a more ethnically diverse and larger sample size and/or examine whether these findings differ across race/ethnicity. Given that some previous work has shown that depressive symptom presentation in older adults vary as a function of ethnic status (Myers et al., 2002; Williams et al., 2015), this would seem important. Additionally, although we presented studies that support the hypothesis that depression may be a prodromal manifestation in AD, not all studies have provided consistent evidence (Rej et al., 2015; Almeida et al., 2016) and so caution should be exercised when considering that the presence of depressive symptoms may a prodrome of AD. Understanding the biological mechanisms underlying these two disorders will help bring clarity to this topic. Longitudinal studies will be necessary to fully understand this association. Another interesting avenue of research might examine whether depressive symptoms during a euthymic period also increases the risk for neurocognitive and brain dysfunction as this was not addressed in the present study.
Conclusion
Our findings provide support for examining depressive symptoms and their association with AD vulnerable regions along subdimensions of affective, cognitive, and somatic symptoms to better understand profiles of symptoms most associated with these regions. This approach may help to parse out those who may be at an increased risk of AD or region-dependent cognitive decline. Conceptualizing depressive symptoms in this way may also better inform treatment approaches in terms of targeting types of symptoms that may be more closely linked to poorer brain and cognitive health outcomes. For example, more prevalent self-reported somatic symptoms may signal the need to address lifestyle factors such as sleep, as well as physical and cognitive activity levels, and management of cardiovascular risk factors (i.e., diabetes and hypertension), whereas greater reports of affective symptoms may signal cognitive dysfunction or accelerated cognitive decline. Future research addressing the limitations discussed above would help to confirm and generalized these findings.
Ethics Statement
The study procedures and protocol were approved by the University of Florida Institutional Review Board. All participants provided written and verbal informed consent prior to taking part in the study in accordance with the Declaration of Helsinki.
Author Contributions
DO was involved in all aspects of the manuscript including hypothesis formation, statistical analyses, and writing of the manuscript. AO conducted volumetric analyses that were used in the manuscript. EP, JW, AW, and RC were involved in the conception of the study and RC was involved in the conception of the manuscript. All authors contributed to the intellectual content and approved the final version to be published.
Funding
This work was supported by an endowment from the McKnight Brain Research Foundation to the University of Florida, Center for Cognitive Aging and Memory.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnote
References
Airaksinen, E., Wahlin,Å., Forsell, Y., and Larsson, M. (2007). Low episodic memory performance as a premorbid marker of depression: evidence from a3-year follow-up. Acta Psychiatr. Scand. 115, 458–465. doi: 10.1111/j.1600-0447.2006.00932.x
Almeida, O. P., Hankey, G. J., Yeap, B. B., Golledge, J., and Flicker, L. (2016). Depression as a risk factor for cognitive impairment in later life: the health in men cohort study. Int. J. Geriatr. Psychiatry 31, 412–420. doi: 10.1002/gps.4347
Arana, G. W., Baldessarini, R. J., and Ornsteen, M. (1985). The dexamethasone suppression test for diagnosis and prognosis in psychiatry: commentary and review. Arch. Gen. Psychiatry 42, 1193–1204. doi: 10.1001/archpsyc.1985.01790350067012
Arborelius, L., Owens, M., Plotsky, P., and Nemeroff, C. (1999). The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 160, 1–12. doi: 10.1677/joe.0.1600001
Bäckman, L., and Forsell, Y. (1994). Episodic memory functioning in a community-based sample of old adults with major depression: utilization of cognitive support. J. Abnorm. Psychol. 103, 361–370. doi: 10.1037/0021-843X.103.2.361
Ballmaier, M., Narr, K. L., Toga, A. W., Elderkin-Thompson, V., Thompson, P. M., Hamilton, L., et al. (2008). Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. Am. J. Psychiatry 165, 229–237. doi: 10.1176/appi.ajp.2007.07030506
Bao, Y.-P., Han, Y., Ma, J., Wang, R.-J., Shi, L., Wang, T.-Y., et al. (2017). Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: meta-analysis and systematic review. Neurosci. Biobehav. Rev. 75, 257–273. doi: 10.1016/j.neubiorev.2017.01.032
Beck, A. T., Steer, R. A., and Brown, G. K. (1996). Beck Depression Inventory-II, Vol. 78, San Antonio, TX: Psychological Corporation, 490–498.
Bell-McGinty, S., Butters, M. A., Meltzer, C. C., Greer, P. J., Reynolds, C. F. III, and Becker, J. T. (2002). Brain morphometric abnormalities in geriatric depression: long-term neurobiological effects of illness duration. Am. J. Psychiatry 159, 1424–1427. doi: 10.1176/appi.ajp.159.8.1424
Bernardi, G., Cecchetti, L., Siclari, F., Buchmann, A., Yu, X., Handjaras, G., et al. (2016). Sleep reverts changes in human gray and white matter caused by wake-dependent training. Neuroimage 129, 367–377. doi: 10.1016/j.neuroimage.2016.01.020
Bishop, N. A., Lu, T., and Yankner, B. A. (2010). Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535. doi: 10.1038/nature08983
Bremner, J. D., Narayan, M., Anderson, E. R., Staib, L. H., Miller, H. L., and Charney, D. S. (2000). Hippocampal volume reduction in major depression. Am. J. Psychiatry 157, 115–118. doi: 10.1176/ajp.157.1.115
Brommelhoff, J. A., Gatz, M., Johansson, B., McArdle, J. J., Fratiglioni, L., and Pedersen, N. L. (2009). Depression as a risk factor or prodromal feature for dementia? Findings in a population-based sample of Swedish twins. Psychol Aging 24, 373–384. doi: 10.1037/a0015713
Caraci, F., Copani, A., Nicoletti, F., and Drago, F. (2010). Depression and Alzheimer’s disease: neurobiological links and common pharmacological targets. Eur. J. Pharmacol. 626, 64–71. doi: 10.1016/j.ejphar.2009.10.022
Chao, L. L., Mohlenhoff, B. S., Weiner, M. W., and Neylan, T. C. (2014). Associations between subjective sleep quality and brain volume in Gulf War veterans. Sleep 37, 445–452. doi: 10.5665/sleep.3472
Checkley, S. (1996). The neuroendocrinology of depression. Int. Rev. Psychiatry 8, 373–378. doi: 10.3109/09540269609051552
Cotman, C. W., Berchtold, N. C., and Christie, L. A. (2007). Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472. doi: 10.1016/j.tins.2007.06.011
Cross, N. E., Lagopoulos, J., Duffy, S. L., Cockayne, N. L., Hickie, I. B., Lewis, S. J., et al. (2013). Sleep quality in healthy older people: relationship with (1) H magnetic resonance spectroscopy markers of glial and neuronal integrity. Behav. Neurosci. 127, 803–810. doi: 10.1037/a0034154
Cui, X., Lyness, J. M., Tu, X., King, D. A., and Caine, E. D. (2007). Does depression precede or follow executive dysfunction? Outcomes in older primary care patients. Am. J. Psychiatry 164, 1221–1228. doi: 10.1176/appi.ajp.2007.06040690
Dawson, D., and Encel, N. (1993). Melatonin and sleep in humans. J. Pineal Res. 15, 1–12. doi: 10.1111/j.1600-079X.1993.tb00503.x
Disabato, B. M., and Sheline, Y. I. (2012). Biological basis of late life depression. Curr. Psychiatry Rep. 14, 273–279. doi: 10.1007/s11920-012-0279-6
Dotson, V. M. (2017). Variability in depression: what have we been missing? Am. J. Geriatr. Psychiatry 25, 23–24. doi: 10.1016/j.jagp.2016.10.005
Dotson, V. M., Davatzikos, C., Kraut, M. A., and Resnick, S. M. (2009). Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. J. Psychiatry Neurosci. 34, 367.
Dotson, V. M., Zonderman, A. B., Kraut, M. A., and Resnick, S. M. (2013). Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. Int. J. Geriatr. Psychiatry 28, 66–74. doi: 10.1002/gps.3791
Elcombe, E. L., Lagopoulos, J., Duffy, S. L., Lewis, S. J., Norrie, L., Hickie, I. B., et al. (2015). Hippocampal volume in older adults at risk of cognitive decline: the role of sleep, vascular risk, and depression. J. Alzheimers Dis. 44, 1279–1290. doi: 10.3233/JAD-142016
Elhai, J. D., Contractor, A. A., Tamburrino, M., Fine, T. H., Prescott, M. R., Shirley, E., et al. (2012). The factor structure of major depression symptoms: a test of four competing models using the Patient Health Questionnaire-9. Psychiatry Res. 199, 169–173. doi: 10.1016/j.psychres.2012.05.018
Enache, D., Cavallin, L., Lindberg, O., Farahmand, B., Kramberger, M. G., Westman, E., et al. (2015). Medial temporal lobe atrophy and depressive symptoms in elderly patients with and without Alzheimer disease. J. Geriatr. Psychiatry Neurol. 28, 40–48. doi: 10.1177/0891988714541873
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/S0896-6273(02)00569-X
Franzmeier, N., Buerger, K., Teipel, S., Stern, Y., Dichgans, M., Ewers, M., et al. (2017a). Cognitive reserve moderates the association between functional network anti-correlations and memory in MCI. Neurobiol. Aging 50, 152–162. doi: 10.1016/j.neurobiolaging.2016.11.013
Franzmeier, N., Göttler, J., Grimmer, T., Drzezga, A., Áraque-Caballero, M. A., Simon-Vermot, L., et al. (2017b). Resting-state connectivity of the left frontal cortex to the default mode and dorsal attention network supports reserve in mild cognitive impairment. Front. Aging Neurosci. 9:264. doi: 10.3389/fnagi.2017.00264
Fried, E. I., and Nesse, R. M. (2015). Depression sum-scores don’t add up: why analyzing specific depression symptoms is essential. BMC Med. 13:72. doi: 10.1186/s12916-015-0325-4
Fried, E. I., Nesse, R. M., Zivin, K., Guille, C., and Sen, S. (2014). Depression is more than the sum score of its parts: individual DSM symptoms have different risk factors. Psychol. Med. 44, 2067–2076. doi: 10.1017/S0033291713002900
Gatchel, J. R., Donovan, N. J., Locascio, J. J., Schultz, A. P., Becker, J. A., Chhatwal, J., et al. (2017). Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: a pilot study. J. Alzheimers Dis. 59, 975–985. doi: 10.3233/JAD-170001
Gerritsen, L., Comijs, H. C., van der Graaf, Y., Knoops, A. J., Penninx, B. W., and Geerlings, M. I. (2011). Depression, hypothalamic pituitary adrenal axis, and hippocampal and entorhinal cortex volumes—the SMART Medea study. Biol. Psychiatry 70, 373–380. doi: 10.1016/j.biopsych.2011.01.029
Gold, P. W. (2015). The organization of the stress system and its dysregulation in depressive illness. Mol. Psychiatry 20, 32–47. doi: 10.1038/mp.2014.163
Gomez, R., Summers, M., Summers, A., Wolf, A., and Summers, J. (2014). Depression Anxiety Stress Scales-21: measurement and structural invariance across ratings of men and women. Assessment 21, 418–426. doi: 10.1177/1073191113514106
Goveas, J. S., Espeland, M. A., Hogan, P., Dotson, V., Tarima, S., Coker, L. H., et al. (2011). Depressive symptoms, brain volumes and subclinical cerebrovascular disease in postmenopausal women: the Women’s Health Initiative MRI Study. J. Affect. Disord. 132, 275–284. doi: 10.1016/j.jad.2011.01.020
Green, R. C., Cupples, L. A., Kurz, A., Auerbach, S., Go, R., Sadovnick, D., et al. (2003). Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch. Neurol. 60, 753–759. doi: 10.1001/archneur.60.5.753
Herrmann, L. L., Goodwin, G. M., and Ebmeier, K. P. (2007). The cognitive neuropsychology of depression in the elderly. Psychol. Med. 37, 1693–1702. doi: 10.1017/S0033291707001134
Hickie, I., Naismith, S., Ward, P. B., Turner, K., Scott, E., Mitchell, P., et al. (2005). Reduced hippocampal volumes and memory loss in patients with early-and late-onset depression. Br. J. Psychiatry 186, 197–202. doi: 10.1192/bjp.186.3.197
Ito, M. (1986). Long-term depression as a memory process in the cerebellum. Neurosci. Res. 3, 531–539. doi: 10.1016/0168-0102(86)90052-0
Jorm, A. F. (2000). Is depression a risk factor for dementia or cognitive decline? Gerontology 46, 219–227.
Jovicich, J., Czanner, S., Han, X., Salat, D., van der Kouwe, A., Quinn, B., et al. (2009). MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage 46, 177–192. doi: 10.1016/j.neuroimage.2009.02.010
Kellner, M., Yassouridis, A., Manz, B., Steiger, A., Holsboer, F., and Wiedemann, K. (1997). Corticotropin-releasing hormone inhibits melatonin secretion in healthy volunteers–a potential link to low-melatonin syndrome in depression? Neuroendocrinology 65, 284–290.
Kirton, J. W., Resnick, S. M., Davatzikos, C., Kraut, M. A., and Dotson, V. M. (2014). Depressive symptoms, symptom dimensions, and white matter lesion volume in older adults: a longitudinal study. Am. J. Geriatr. Psychiatry 22, 1469–1477. doi: 10.1016/j.jagp.2013.10.005
Koenigs, M., and Grafman, J. (2009). The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 201, 239–243. doi: 10.1016/j.bbr.2009.03.004
Koolschijn, P., van Haren, N. E., Lensvelt-Mulders, G. J., Pol, H., Hilleke, E., and Kahn, R. S. (2009). Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 30, 3719–3735. doi: 10.1002/hbm.20801
Krishnadas, R., and Cavanagh, J. (2012). Depression: an inflammatory illness? J. Neurol. Neurosurg. Psychiatry 83, 495–502. doi: 10.1136/jnnp-2011-301779
Lee, S.-Y., Cho, W.-H., Lee, Y.-S., and Han, J.-S. (2017). Impact of chronic stress on the spatial learning and GR-PKAc-NF-κB signaling in the Hippocampus and cortex in rats following cholinergic depletion. Mol. Neurobiol. doi: 10.1007/s12035-017-0620-5 [Epub ahead of print].
Lorenzetti, V., Allen, N. B., Fornito, A., and Yücel, M. (2009). Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J. Affect. Disord. 117, 1–17. doi: 10.1016/j.jad.2008.11.021
MacQueen, G. M., Campbell, S., McEwen, B. S., Macdonald, K., Amano, S., Joffe, R. T., et al. (2003). Course of illness, hippocampal function, and hippocampal volume in major depression. Proc. Natl. Acad. Sci. U.S.A. 100, 1387–1392. doi: 10.1073/pnas.0337481100
Maes, M., Yirmyia, R., Noraberg, J., Brene, S., Hibbeln, J., Perini, G., et al. (2009). The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab. Brain Dis. 24, 27–53. doi: 10.1007/s11011-008-9118-1
McKinnon, M. C., Yucel, K., Nazarov, A., and MacQueen, G. M. (2009). A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J. Psychiatry Neurosci. 34, 41–54.
Mondelli, V., Cattaneo, A., Murri, M. B., Di Forti, M., Handley, R., Hepgul, N., et al. (2011). Stress and inflammation reduce BDNF expression in first-episode psychosis: a pathway to smaller hippocampal volume. J. Clin. Psychiatry 72, 1677–1684. doi: 10.4088/JCP.10m06745
Myers, H. F., Lesser, I., Rodriguez, N., Mira, C. B., Hwang, W. C., Camp, C., et al. (2002). Ethnic differences in clinical presentation of depression in adult women. Cultur. Divers. Ethnic Minor. Psychol. 8, 138–156. doi: 10.1037/1099-9809.8.2.138
Newcomer, J. W., Selke, G., Melson, A. K., Hershey, T., Craft, S., Richards, K., et al. (1999). Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Arch. Gen. Psychiatry 56, 527–533. doi: 10.1001/archpsyc.56.6.527
Nguyen, E. T., Caldwell, J. L., Streicher, J., Ghisays, V., Balmer, N. J., Estrada, C. M., et al. (2018). Differential effects of imipramine and CORT118335 (Glucocorticoid receptor modulator/mineralocorticoid receptor antagonist) on brain-endocrine stress responses and depression-like behavior in female rats. Behav. Brain Res. 336, 99–110. doi: 10.1016/j.bbr.2017.08.045
O’shea, D. M., Fieo, R. A., Hamilton, J. L., Zahodne, L. B., Manly, J. J., and Stern, Y. (2015). Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. Int. J. Geriatr. Psychiatry 30, 614–622. doi: 10.1002/gps.4192
Ownby, R. L., Crocco, E., Acevedo, A., John, V., and Loewenstein, D. (2006). Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 63, 530–538. doi: 10.1001/archpsyc.63.5.530
Pantel, J., Schröder, J., Essig, M., Popp, D., Dech, H., Knopp, M. V., et al. (1997). Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J. Affect. Disord. 42, 69–83. doi: 10.1016/S0165-0327(96)00105-X
Penninx, B. W., Kritchevsky, S. B., Yaffe, K., Newman, A. B., Simonsick, E. M., Rubin, S., et al. (2003). Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol. Psychiatry 54, 566–572. doi: 10.1016/S0006-3223(02)01811-5
Posener, J. A., Wang, L., Price, J. L., Gado, M. H., Province, M. A., Miller, M. I., et al. (2003). High-dimensional mapping of the hippocampus in depression. Am. J. Psychiatry 160, 83–89. doi: 10.1176/appi.ajp.160.1.83
Radley, J., Morilak, D., Viau, V., and Campeau, S. (2015). Chronic stress and brain plasticity: mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci. Biobehav. Rev. 58, 79–91. doi: 10.1016/j.neubiorev.2015.06.018
Rej, S., Begley, A., Gildengers, A., Dew, M. A., Reynolds, C. F. III, and Butters, M. A. (2015). Psychosocial risk factors for cognitive decline in late-life depression: findings from the MTLD-III Study. Can. Geriatr. J. 18, 43–50. doi: 10.5770/cgj.18.134
Segal, D. L., Coolidge, F. L., Cahill, B. S., and O’Riley, A. A. (2008). Psychometric properties of the Beck Depression Inventory—II (BDI-II) among community-dwelling older adults. Behav. Modif. 32, 3–20. doi: 10.1177/0145445507303833
Serafini, G., Pompili, M., Borgwardt, S., Houenou, J., Geoffroy, P. A., Jardri, R., et al. (2014). Brain changes in early-onset bipolar and unipolar depressive disorders: a systematic review in children and adolescents. Eur. Child Adolesc. Psychiatry 23, 1023–1041. doi: 10.1007/s00787-014-0614-z
Shafer, A. B. (2006). Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J. Clin. Psychol. 62, 123–146. doi: 10.1002/jclp.20213
Sheline, Y. I. (2011). Depression and the hippocampus: cause or effect? Biol. Psychiatry 70, 308–309. doi: 10.1016/j.biopsych.2011.06.006
Sheline, Y. I., Wang, P. W., Gado, M. H., Csernansky, J. G., and Vannier, M. W. (1996). Hippocampal atrophy in recurrent major depression. Proc. Natl. Acad. Sci. U.S.A. 93, 3908–3913. doi: 10.1073/pnas.93.9.3908
Srinivasan, V., Pandi-Perumal, S. R., Trakht, I., Spence, D. W., Hardeland, R., Poeggeler, B., et al. (2009). Pathophysiology of depression: role of sleep and the melatonergic system. Psychiatry Res. 165, 201–214. doi: 10.1016/j.psychres.2007.11.020
Steffens, D. C., Byrum, C. E., McQuoid, D. R., Greenberg, D. L., Payne, M. E., Blitchington, T. F., et al. (2000). Hippocampal volume in geriatric depression. Biol. Psychiatry 48, 301–309. doi: 10.1016/S0006-3223(00)00829-5
Sun, X., Steffens, D. C., Au, R., Folstein, M., Summergrad, P., Yee, J., et al. (2008). Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch. Gen. Psychiatry 65, 542–550. doi: 10.1001/archpsyc.65.5.542
Szymkowicz, S. M., Dotson, V. M., McLaren, M. E., De Wit, L., O’Shea, D. M., Talty, F. T., et al. (2017). Precuneus abnormalities in middle-aged to older adults with depressive symptoms: an analysis of BDI-II symptom dimensions. Psychiatry Res. 268, 9–14. doi: 10.1016/j.pscychresns.2017.08.002
Tang, W. K., Chen, Y. K., Liang, H. J., Chu, W. C. W., Mok, V. C. T., Ungvari, G. S., et al. (2014). Subcortical white matter infarcts predict 1-year outcome of fatigue in stroke. BMC Neurol. 14:234. doi: 10.1186/s12883-014-0234-8
Thames, A. D., Castellon, S. A., Singer, E. J., Nagarajan, R., Sarma, M. K., Smith, J., et al. (2015). Neuroimaging abnormalities, neurocognitive function, and fatigue in patients with hepatitis C. Neurol. Neuroimmunol. Neuroinflamm. 2:e59. doi: 10.1212/NXI.0000000000000059
Thase, M. E. (2006). Depression and sleep: pathophysiology and treatment. Dialogues Clin. Neurosci. 8, 217–226.
Tiemeier, H., Hofman, A., van Tuijl, H. R., Kiliaan, A. J., Meijer, J., and Breteler, M. M. (2003). Inflammatory proteins and depression in the elderly. Epidemiology 14, 103–107. doi: 10.1097/00001648-200301000-00025
Trivedi, M. H., Greer, T. L., Church, T. S., Carmody, T. J., Grannemann, B. D., Galper, D. I., et al. (2011). Exercise as an augmentation treatment for nonremitted major depressive disorder: a randomized, parallel dose comparison. J. Clin. Psychiatry 72, 677–684. doi: 10.4088/JCP.10m06743
Tsuno, N., Besset, A., and Ritchie, K. (2005). Sleep and depression. J. Clin. Psychiatry 66, 1254–1269. doi: 10.4088/JCP.v66n1008
Tulving, E., and Markowitsch, H. J. (1998). Episodic and declarative memory: role of the hippocampus. Hippocampus 8, 198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G
Uzoni, A., Ovidiu, C., Sandu, E. R., Buga, A. M., and Popa-Wagner, A. (2015). Aging, inflammation and depressive behavior: a review. Rev. Health Care 6, 67–80. doi: 10.7175/rhc.v6i2.1170
Vakili, K., Pillay, S. S., Lafer, B., Fava, M., Renshaw, P. F., Bonello-Cintron, C. M., et al. (2000). Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol. Psychiatry 47, 1087–1090. doi: 10.1016/S0006-3223(99)00296-6
Van Gool, C. H., Kempen, G. I., Bosma, H., van Boxtel, M. P., Jolles, J., and van Eijk, J. T. (2007). Associations between lifestyle and depressed mood: longitudinal results from the Maastricht Aging Study. Am. J. Public Health 97, 887–894. doi: 10.2105/AJPH.2004.053199
Vanheule, S., Desmet, M., Groenvynck, H., Rosseel, Y., and Fontaine, J. (2008). The factor structure of the beck depression inventory–II: an evaluation. Assessment 15, 177–187. doi: 10.1177/1073191107311261
Watanabe, Y., Gould, E., and McEwen, B. S. (1992). Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 588, 341–345. doi: 10.1016/0006-8993(92)91597-8
Williams, E. D., Tillin, T., Richards, M., Tuson, C., Chaturvedi, N., Hughes, A. D., et al. (2015). Depressive symptoms are doubled in older British South Asian and Black Caribbean people compared with Europeans: associations with excess co-morbidity and socioeconomic disadvantage. Psychol. Med. 45, 1861–1871. doi: 10.1017/S0033291714002967
Winokur, G. (1997). All roads lead to depression: clinically homogeneous, etiologically heterogeneous. J. Affect. Disord. 45, 97–108. doi: 10.1016/S0165-0327(97)00063-3
Woolley, C. S., Gould, E., and McEwen, B. S. (1990). Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 531, 225–231. doi: 10.1016/0006-8993(90)90778-A
Zahodne, L. B., Stern, Y., and Manly, J. J. (2014). Depressive symptoms precede memory decline, but not vice versa, in non-demented older adults. J. Am. Geriatr. Soc. 62, 130–134. doi: 10.1111/jgs.12600
Zhao, Z., Taylor, W. D., Styner, M., Steffens, D. C., Krishnan, K. R. R., and MacFall, J. R. (2008). Hippocampus shape analysis and late-life depression. PLOS ONE 3:e1837. doi: 10.1371/journal.pone.0001837
Zimmerman, M. E., Ezzati, A., Katz, M. J., Lipton, M. L., Brickman, A. M., Sliwinski, M. J., et al. (2016). Perceived stress is differentially related to hippocampal subfield volumes among older adults. PLOS ONE 11:e0154530. doi: 10.1371/journal.pone.0154530
Keywords: depressive symptom dimensions, hippocampal volumes, entorhinal cortex volumes, aging, older adults
Citation: O’Shea DM, Dotson VM, Woods AJ, Porges EC, Williamson JB, O’Shea A and Cohen R (2018) Depressive Symptom Dimensions and Their Association with Hippocampal and Entorhinal Cortex Volumes in Community Dwelling Older Adults. Front. Aging Neurosci. 10:40. doi: 10.3389/fnagi.2018.00040
Received: 03 December 2017; Accepted: 05 February 2018;
Published: 20 February 2018.
Edited by:
Aurel Popa-Wagner, University of Rostock, GermanyReviewed by:
Raluca Sandu Vintilescu, University of Medicine and Pharmacy of Craiova, RomaniaGianluca Serafini, University of Genoa, Italy
Copyright © 2018 O’Shea, Dotson, Woods, Porges, Williamson, O’Shea and Cohen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deirdre M. O’Shea, ZG1vMjEyM0B1ZmwuZWR1
 Deirdre M. O’Shea
Deirdre M. O’Shea Vonetta M. Dotson
Vonetta M. Dotson Adam J. Woods
Adam J. Woods Eric C. Porges1,2,3
Eric C. Porges1,2,3 Andrew O’Shea
Andrew O’Shea Ronald Cohen
Ronald Cohen