
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 21 August 2017
Sec. Neurocognitive Aging and Behavior
Volume 9 - 2017 | https://doi.org/10.3389/fnagi.2017.00263
Background: Transcranial magnetic stimulation (TMS) can be used to assess neurophysiology and the mechanisms of cortical brain plasticity in humans in vivo. As the use of these measures in specific populations (e.g., Alzheimer’s disease; AD) increases, it is critical to understand their reproducibility (i.e., test–retest reliability) in the populations of interest.
Objective: Reproducibility of TMS measures was evaluated in older adults, including healthy, AD, and Type-2 diabetes mellitus (T2DM) groups.
Methods: Participants received two identical neurophysiological assessments within a year including motor thresholds, baseline motor evoked potentials (MEPs), short- and long-interval intracortical inhibition (SICI, LICI) and intracortical facilitation (ICF), and MEP changes following intermittent theta-burst stimulation (iTBS). Cronbach’s α coefficients were calculated to assess reproducibility. Multiple linear regression analyses were used to investigate factors related to intraindividual variability.
Results: Reproducibility was highest for motor thresholds, followed by baseline MEPs, SICI and LICI, and was lowest for ICF and iTBS aftereffects. The AD group tended to show higher reproducibility than T2DM or controls. Intraindividual variability of baseline MEPs was related to age and variability of RMT, while the intraindividual variability in post-iTBS measures was related to baseline MEP variability, intervisit duration, and Brain-derived neurotrophic factor (BDNF) polymorphism.
Conclusion: Increased reproducibility in AD may reflect pathophysiological declines in the efficacy of neuroplastic mechanisms. Reproducibility of iTBS aftereffects can be improved by keeping baseline MEPs consistent, controlling for BDNF genotype, and waiting at least a week between visits.
Significance: These findings provide the first direct assessment of reproducibility of TMS measures in older clinical populations. Reproducibility coefficients may be used to adjust effect- and sample size calculations for future studies.
Transcranial magnetic stimulation (TMS) is a non-invasive means of electrically stimulating the brain through electromagnetic induction. TMS can be applied as single-pulse TMS to assess cortical reactivity, paired-pulse TMS to probe intracortical inhibition and facilitation, and patterned trains of pulses, termed rTMS, to induce changes in cortical excitability and metabolism that last beyond the stimulation itself. When directed to the primary motor cortex, the aftereffects of rTMS are typically measured as a change in cortical reactivity, i.e., the average amplitude of MEPs elicited by single-pulse TMS. In most instances, continuous low-frequency (∼1 Hz) rTMS tends to reduce MEP amplitude, while on-off patterns of higher frequency (5–20 Hz) rTMS tend to increase MEP amplitude. These neuromodulatory effects of rTMS are thought to rely on mechanisms of brain plasticity related to LTD and LTP, respectively (Pascual-Leone et al., 1994; Hallett, 2007).
Over the past decade, an ultra high-frequency patterned rTMS application termed TBS has emerged as a potential means to generate greater and longer-lasting neuromodulatory effects with a shorter duration of stimulation (Huang et al., 2005). TBS consists of 50 Hz pulse-triplets repeated every 200 ms in one of two protocols that parallel low- and high-frequency rTMS: 40 s (600 pulses) of cTBS reduces MEP amplitude by about 10–25% for about 50 min, while the same number of pulses delivered in a 2-s on, 8-s off iTBS pattern for 190 s increases MEP amplitude by about 15–35% for 60 min (Wischnewski and Schutter, 2015). These TBS protocols have been used to identify age-related changes in the mechanisms of plasticity across the lifespan in healthy individuals (Freitas et al., 2011b) and reveal altered neuroplastic mechanisms in autism spectrum disorders (Oberman et al., 2012), traumatic brain injury (Tremblay et al., 2015), schizophrenia (McClintock et al., 2011), Type-2 diabetes (Fried et al., 2016), and AD (Koch et al., 2012).
The growth in popularity of TMS techniques has led to an increased focus on the sources of inter- and intraindividual variability. For example, it has been demonstrated that activation of the target muscle prior to, during, or immediately after TBS can influence its effects on motor cortex excitability (Huang et al., 2008; Iezzi et al., 2008; Goldsworthy et al., 2014). In addition, carriers of the BDNF-Met allele may show altered response to neuromodulation paradigms including TBS (Cheeran et al., 2008; Lee et al., 2013; Di Lazzaro et al., 2015). Despite increased attention, only four studies (Hinder et al., 2014; Vernet et al., 2014; Vallence et al., 2015; Schilberg et al., 2017) have directly assessed the reproducibility of TBS aftereffects, and these largely focused on young healthy individuals. Similarly, while there have been more studies investigating the reproducibility of single and paired-pulse TMS measures, only two (Kimiskidis et al., 2004; Fleming et al., 2012) included subjects over the age of 50, and only one (Christie et al., 2007) exclusively recruited individuals over 65 years. As interest grows in using TMS and TBS to assess the intracortical and corticospinal excitability and the efficacy of neuroplastic mechanisms in older clinical populations (Freitas et al., 2011b; Di Lorenzo et al., 2016; Fried et al., 2016), it is critical to understand the reliability of these techniques in the populations of interest. The present study aims to fill this void through a direct assessment of the reproducibility of iTBS aftereffects and other common single- and paired-pulse TMS-based neurophysiological measurements in older adults, including those with impairments in cognition or glucose metabolism. The results from this study will serve as a guidepost for understanding how biomarkers of cortical reactivity and plasticity change with age or are affected by common diseases such as Type-2 diabetes and AD.
The study was carried out in accordance with the Declaration of Helsinki and the recommendations of the Institutional Review Board at Beth Israel Deaconess Medical Center with written informed consent from all subjects.
Retrospective data was obtained from 36 adults (17 females) of mean age 62.9 years (range: 50–79 years), who had participated in research at the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center between May 2012 and May 2015. The participants were drawn from different populations: nine participants (four males, mean ± SD age: 67.7 ± 6.9 years) had a probable diagnosis of mild-to-moderate AD (McKhann et al., 2011) with a CDR = 1.0 and an MMSE score between 18 and 23; 15 participants (nine males, mean ± SD age: 63.4 ± 7.3 years) had Type-2 diabetes (T2DM) but were otherwise cognitively intact (MMSE ≥ 27), and the remaining 12 healthy controls (six males, mean ± SD age: 58.6 ± 9.1 years) were both cognitively intact (MMSE ≥ 27) and non-diabetic (hemoglobin A1c < 6.2%). AD participants consisted of individuals who were randomized to a Sham-control group for a proof-of-principle study on the combined impact of daily rTMS and cognitive training (Brem et al., manuscript submitted for publication). T2DM and control participants were recruited for a study on cortical plasticity in T2DM (Fried et al., 2016). None of the participants had any unstable medical condition or comorbidity. Saliva was obtained from 24 participants (10 controls, 10 T2DM, 4 AD) to assess BDNF and APOE polymorphisms. All participants underwent anatomical MRI scan, structured neurological exam, medical history review, formal neuropsychological testing, and two identical TMS visits. Median time between TMS visits was 14 days (range: 2–344 days). Average (±SD) start time for the two TMS visits was 10:57 (±1:07) for Visit-A and 10:37 (±0:55) for Visit-B. Blood glucose levels were assessed in all T2DM subjects at the beginning of each TMS visit for the purpose of establishing that glucose levels were within a normative range defined a priori as 80–200 mg/dL.
A T1-weighted anatomical MRI scan was obtained in all participants and used for neuronavigation. Scans were completed on a 3T scanner (GE Healthcare, Ltd., United Kingdom) using a 3D spoiled gradient echo sequence: 162 axial-oriented slices for whole-brain coverage; 240-mm isotropic field-of-view; 0.937-mm × 0.937-mm × 1-mm native resolution; flip angle = 15°; TE/TR ≥ 2.9/6.9 ms; duration ≥ 432 s.
All parameters used in the study conformed to current recommended guidelines for the safe application of TMS endorsed by the IFCN (Rossi et al., 2009; Rossini et al., 2015). A Navigated Brain Stimulation system (Nexstim Plc, Finland) was used to identify the hand region of the left primary motor cortex and ensure consistent targeting throughout each TMS visit. At the first visit, the hotspot for the FDI muscle was marked on the participant’s MRI. The hotspot was defined as the site from which single-pulse TMS elicited MEPs that were more consistent and higher in amplitude in FDI than in either the abductor pollicis brevis or abductor digiti minimi muscles. The hotspot was reassessed at the second visit using the first visit as a reference. Following IFCN guidelines (Rossini et al., 2015), RMT (using both mono- and biphasic pulses) and AMT (using biphasic pulses) were measured. We defined RMT as the lowest intensity of stimulation that elicited MEPs ≥50 μV in at least five of ten pulses in the relaxed FDI, and AMT as the lowest intensity that elicited MEPs ≥200 μV in at least 5 of 10 pulses with the FDI slightly contracted. Single and paired-pulse TMS trials were separated by a randomized 5000–6000 ms interval to avoid applying patterned repetitive stimulation.
Neuronavigated paired-pulse TMS was applied using a handheld monophasic (posterior–anterior in the brain) figure-of-eight focal coil (Nexstim Plc, Finland). Three protocols were utilized: SICI, consisting of a subthreshold (80% RMT) conditioning pulse followed 3 ms by a suprathreshold (120% RMT) test pulse; ICF (subthreshold conditioning pulse followed 12 ms by a suprathreshold pulse); and LICI (suprathreshold conditioning pulse followed 100 ms by a suprathreshold test pulse) (Valls-Solé et al., 1992; Kujirai et al., 1993). A block of single monophasic-TMS pulses at 120% RMT provided a measure of unconditioned cortico-motor reactivity. Each block consisted of 50 trials and individual MEP amplitudes > 2.5 SD from the mean were excluded. Conditioned MEPs from SICI, LICI, and ICF blocks were averaged and expressed as the percent change from the unconditioned block. Paired-pulse measures could not be performed in two participants in whom RMT exceeded 83% of maximum stimulator output.
Neuronavigated iTBS was applied to participants using a handheld passive-cooling fluid-filled figure-of-eight coil (MCF-B65; 75 mm outer wing diameter) attached to a MagPro X100 stimulator (MagVenture A/S, Denmark). Intensity was 80% of AMT. The pattern was a 2-s train of biphasic bursts (three pulses at 50 Hz) repeated every 200 ms (30 pulses per train). Trains were repeated 20 times with an eight-second inter-train interval (600 pulses, 192 s). This protocol has been shown to potentiate cortico-motor reactivity for up to 60 min in healthy individuals (Huang et al., 2005; Wischnewski and Schutter, 2015).
Prior to iTBS, participants received three blocks of 30 single TMS pulses at 120% RMT using a hand-held biphasic (anterior–posterior, posterior–anterior in the brain) figure-of-eight coil (Nexstim Plc). Cortico-motor reactivity was reassessed in blocks of 30 TMS pulses at 5, 10, 20, 30, 40, and 50 min post-iTBS. The peak-to-peak amplitude of each recorded MEP was measured automatically. For each block, individual MEPs > 2.5 SD from the mean were excluded. All 90 pre-iTBS trials were averaged as a measure of baseline cortico-motor reactivity. MEP trials were averaged for each post-iTBS block and expressed as the percent change from baseline. MEPs were not obtained at 30-min post-iTBS in two participants and 50-min post-iTBS in one participant. In those participants, the corresponding time-point from the other visit was therefore excluded from subsequent analyses.
Neurophysiological data included three motor thresholds (mono- and biphasic RMT and biphasic AMT; expressed as percent of maximum stimulator output intensity), two measures of cortico-motor reactivity (unconditioned MEPs elicited with the monophasic coil that was used to assess the effects of the paired-pulse paradigms and baseline MEPs elicited with the biphasic coil that were used to assess the impact of iTBS), three paired-pulse measures (SICI, LICI, ICF; with the average amplitude of the conditioned MEPs expressed as the percent change the amplitude of unconditioned MEPs), and the six post-iTBS time-points (Post05, Post10, Post20, Post30, Post40, and Post50; with the average amplitude of MEPs from each time-point expressed as the percent change from the pre-iTBS baseline average). From the iTBS modulation, three further measures of plasticity were calculated: the Max+, or greatest change in MEP amplitude across all six time-points; the summed area under-the-curve for the first 20-min post-iTBS (AUC0-20), corresponding to the period of peak effect in neurotypical individuals (Wischnewski and Schutter, 2015); and the summed area under-the-curve across all post-iTBS time-points (AUC0-50). The area under-the-curve was calculated as the summed products of the average %Δ in MEP amplitude at two consecutive time-points and the time in minutes between them.
For all neurophysiological measures, Cronbach’s α coefficients (Cronbach and Warrington, 1951) were calculated between the two visits to assess test–retest reliability. The α coefficient provides a measure of internal consistency of a set of items (Tavakol and Dennick, 2011), in this case, the same subjects tested on two separate occasions. The α coefficient was calculated for all subjects together and for each group (AD, T2DM, controls) separately using the free online software program Cronbach alpha (v1.0.31) (Wessa, 2016).
Reliability coefficients, such as the α, can be used to adjust effect sizes (Baugh, 2002; Wright, 2014). Using this approach, it is possible to predict how the reproducibility of a given measure might affect a hypothetical effect size, which in turn could be used in a sample size calculation that takes into consideration the reproducibility (or lack thereof) of the measure of interest. To illustrate this point and provide a resource for future studies, adjustments for each measure were made to a hypothetical Cohen’s d effect size of 0.5, which corresponds to a within-subject change of half a standard deviation, and is considered a medium effect size (Cohen, 1992). First, a hypothetical, or idealized, Cohen’s d is converted into an r (Cohen, 1988, p. 23):
This idealized r is then adjusted for unreliability using the Cronbach’s α (Wright, 2014):
Finally, the corrected r is converted back into an adjusted d (Friedman, 1968, p. 246):
To investigate factors associated with intraindividual variability, additional analyses were performed in JMP Pro (v12.1.02) using a normal distribution and a two-tailed 95% confidence interval. Given the exploratory nature of these analyses, individual p-values were not adjusted for multiple comparisons and should be interpreted accordingly. For between-groups comparisons, the sample sizes in the present study provided 0.80 power to detect a medium effect size (Cohen’s d = 0.54). The first set of analyses concerned correlations between variables that were collected at each visit and thus were performed using the net difference between Visits A and B (ΔB-A) so that the direction of change between visits was taken into account. These analyses included: (1) how differences in baseline MEP amplitude relate to differences in RMT (for both mono- and biphasic pulses); (2) how differences in SICI, LICI, and ICF relate to differences in unconditioned monophasic MEP amplitudes and RMT; and (3) how differences in post-iTBS measures relate to differences in baseline biphasic MEP amplitudes, RMT, and AMT. The second set of analyses concerned factors, such as group, gender, age, inter-visit interval, and BDNF and APOE polymorphisms, that were assessed only once per participant. Multiple linear regression analyses were performed on the absolute value of the inter-visit difference (|ΔB-A|) to account for the amount of change between visits in either direction.
Data on motor thresholds, baseline cortico-motor reactivity measures, changes in MEP amplitude from the paired-pulse TMS and post-iTBS plasticity measures are shown in Table 1.
Figure 1 shows coefficients for all measures and all groups. Hypothetical effect- and sample sizes adjusted for these α coefficients are shown in Table 2. Following criteria for categorizing reproducibility in neurophysiological assessments (Portney and Watkins, 2009), we defined α ≥ 0.75 as high reproducibility, 0.50 ≤ α < 0.75 as moderate reproducibility, 0.25 ≤ α < 0.50 as low reproducibility, and α < 0.25 as very low to no reproducibility.
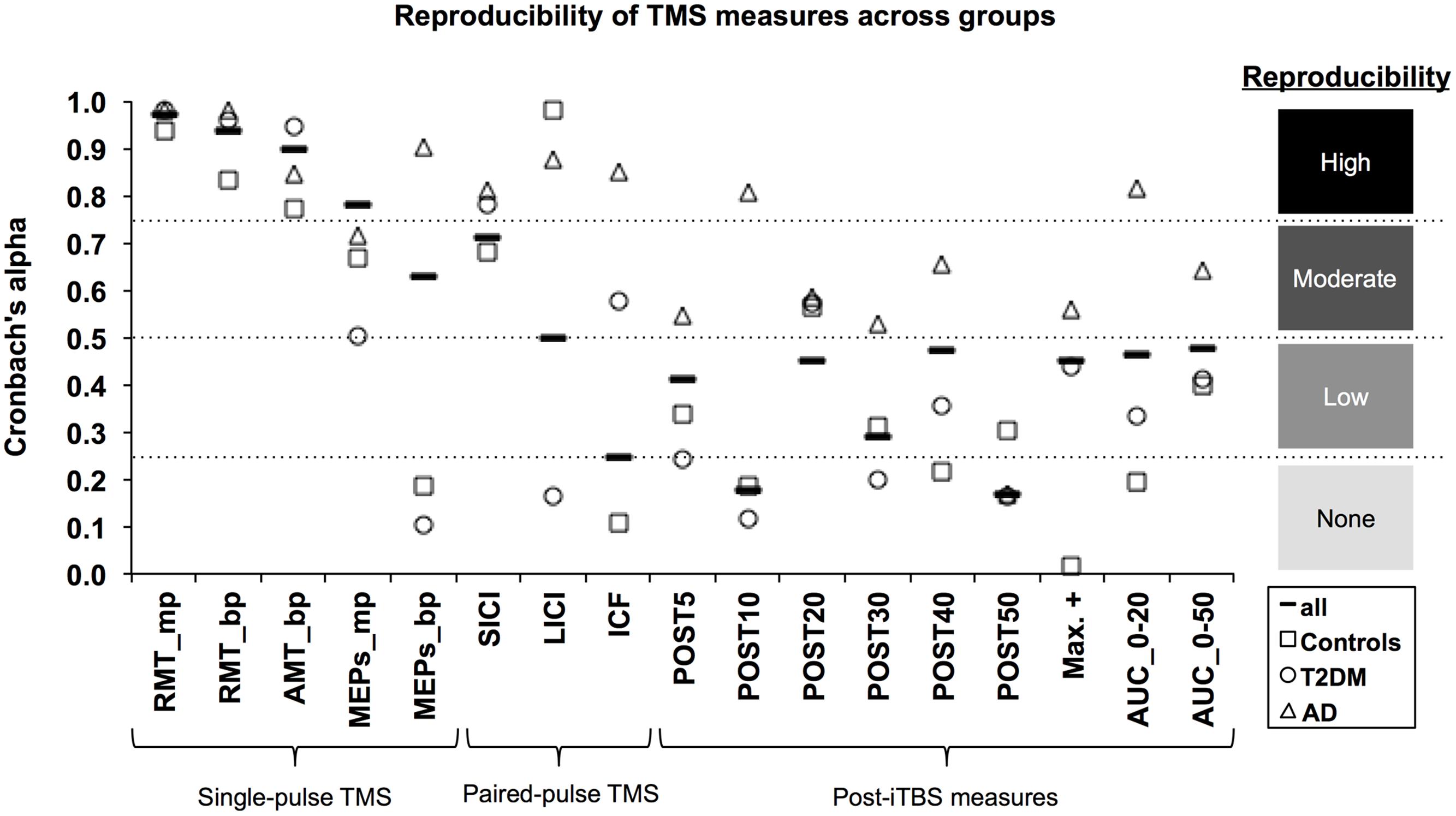
FIGURE 1. Reproducibility of TMS measures across groups. The Cronbach’s α coefficient (y-axis) was calculated as an index of reliability for each TMS-based measure (x-axis). α coefficients were calculated for all subjects (solid line marker) as well as for each group: Alzheimer’s disease (AD; triangle marker); Type-2 diabetes mellitus (T2DM; circle marker); and non-AD/non-T2DM controls (square marker). Following the approach of Portney and Watkins (2009), reproducibility was categorized as high (α ≥ 0.75), moderate (0.50 ≤ α < 0.75), low (0.25 ≤ α < 0.50), and very low to none (α < 0.25). mp, monophasic; bp, biphasic; RMT, resting motor threshold; AMT, active motor threshold; MEPs, motor evoked potentials; SICI, short-interval intracortical inhibition; LICI, long-interval intracortical inhibition; ICF, intracortical facilitation; iTBS, intermittent theta-burst stimulation; POST, minutes post-iTBS; Max+, maximum facilitation; AUC, area under-the-curve.
Considering all groups combined, the three motor thresholds had high reproducibility (α’s > 0.90). For baseline MEPs elicited at 120% of RMT, monophasic (α = 0.78) and biphasic pulses (α = 0.62) showed high and moderate reproducibility, respectively. Among the paired-pulse measures, reproducibility was moderate for SICI (α = 0.71) and LICI (α = 0.54), while ICF had very low to no reproducibility (α = 0.25). All post-iTBS measures demonstrated low reproducibility (α’s = 0.29–0.48); except for Post10 (α = 0.18) and Post50 (α = 0.17), which were not reproducible.
Considering each group separately, α coefficients tended to be higher for the AD group than for T2DM and controls. In particular, the AD group demonstrated high reproducibility for biphasic MEPs (α = 0.90), all three paired-pulse protocols (α’s > 0.81), Post10 (α = 0.81), and AUC0-20 (α = 0.82). Further, reproducibility in AD was moderate (α’s = 0.53–0.65) for all remaining post-iTBS measures, except Post50, which was not reproducible (α = 0.16). By comparison, both controls and T2DM individuals showed moderate reproducibility (α’s = 0.50–0.67) for baseline monophasic MEPs and no reproducibility (α’s = 0.11–0.19) for baseline biphasic MEPs. One positive outlier was LICI, which showed high reproducibility in controls (α = 0.98).
For measures assessed with a monophasic pulse, there was no significant relationship between the ΔB-A of baseline MEP amplitudes and the ΔB-A of RMT, R31 = 0.01, p = 0.934. Similarly, there were no significant relationships between the ΔB-A of any of the paired-pulses measurements with the ΔB-A of either RMT or baseline MEP amplitudes (|R|’s < 0.27, p’s > 0.13).
When using a biphasic coil, the ΔB-A of baseline MEP amplitudes was significantly correlated with the ΔB-A of RMT, R34 = -0.35, p = 0.035. Specifically, a 1% (maximum stimulator output) increase in the net difference of RMT was associated with a 70-μV decrease in the net difference of baseline MEP amplitude (Figure 2A). Further, the ΔB-A’s for all iTBS plasticity measures except Post30 and Post40 were significantly correlated with the ΔB-A of biphasic baseline MEPs amplitudes (R’s<-0.36, p < 0.04). In all cases, an increase in the net difference of baseline MEP amplitudes was associated with a decrease in the inter-visit difference of post-iTBS facilitation. This relationship was most apparent for AUC0-20, where a 1-mV increase in the inter-visit difference of baseline MEP amplitude was associated with a 390 (%Δ∗min) decrease in the net difference of the AUC (Figure 2B). By contrast, there were no significant relationships between the ΔB-A of any of the iTBS plasticity measures with the ΔB-A of either RMT or AMT (|R|’s < 0.32, p’s > 0.07). These results indicate that as much as 23% of the visit-to-visit variability in iTBS plasticity measures can be accounted for by the variability in the baseline MEP amplitude, which in turn is impacted by the variability in RMT.
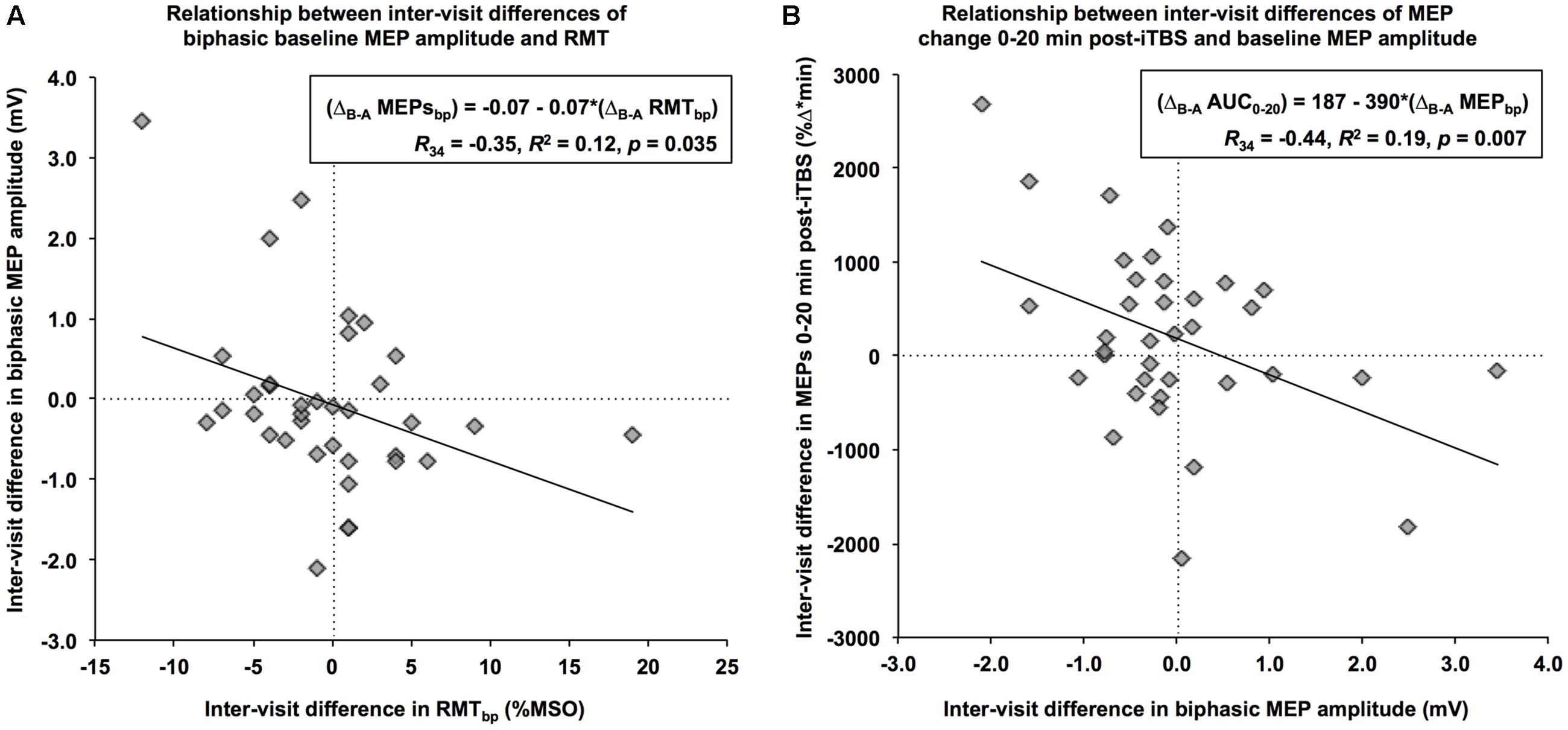
FIGURE 2. Relationships between the net differences of neurophysiological measures. (A) Using a biphasic pulse, an increase in RMT of 1% of maximum stimulator output from Visit-A to Visit-B (x-axis) was associated with a decrease of 0.07 mV in baseline MEP over the same period (y-axis). (B) An increase of 1-mV in baseline MEP amplitude from Visit-A to Visit-B (x-axis) was associated with an inter-visit decrease of 390 %Δ∗min in the MEP change during the first 20 min (y-axis).
In the T2DM subjects, blood glucose levels did not differ significantly between visits (p > 0.1) and no significant relationships were observed between changes in blood glucose levels and changes in any TMS measure between visits (p’s > 0.2).
Controlling for the interval between visits, as well as the age and gender of participants, the linear models yielded no difference between groups in the |ΔB-A| of any neurophysiological measure (F’s < 2.2, p’s > 0.13). These results indicate that the absolute amount of change between visits in motor thresholds as well as baseline reactivity, paired-pulse, and plasticity measures were equivalent across AD, T2DM and control participants at the 0.05 level.
The multiple regression analyses indicated a significant relationship between the |ΔB-A| of monophasic MEPs and age, controlling for group, gender, and inter-visit interval (F1,1 = 5.62, p = 0.025). Specifically, a 1-year increase in participant age was associated with a 0.03 mV increase in the absolute visit-to-visit difference in the amplitude of biphasic MEPs. Similarly, there was a significant relationship between the |ΔB-A| of Post05 facilitation and inter-visit interval, controlling for group, gender, and age (F1,1 = 6.42, p = 0.017). Specifically, a 1-day increase in the interval between visits was associated with a 0.03 mV decrease in the absolute inter-visit difference in the %Δ in MEP amplitudes at Post05. None of the other relationships were significant (F’s < 4.0, p’s > 0.05).
The multiple regression analyses demonstrated a significant effect of BDNF polymorphism on the |ΔB-A| of several post-iTBS measures after controlling for Group (Figure 3). Specifically, intraindividual variability was higher for BDNF-Met carriers than BDNF-Val homozygotes for Post05 (F1,1 = 6.76, p = 0.017) and Post50 (F1,1 = 6.79, p = 0.017), and AUC0-20 (F1,1 = 4.99, p = 0.037). By comparison, there was no significant effect of APOE status on the |ΔB-A| of any of TMS measures (F’s < 2.8, p’s > 0.1).
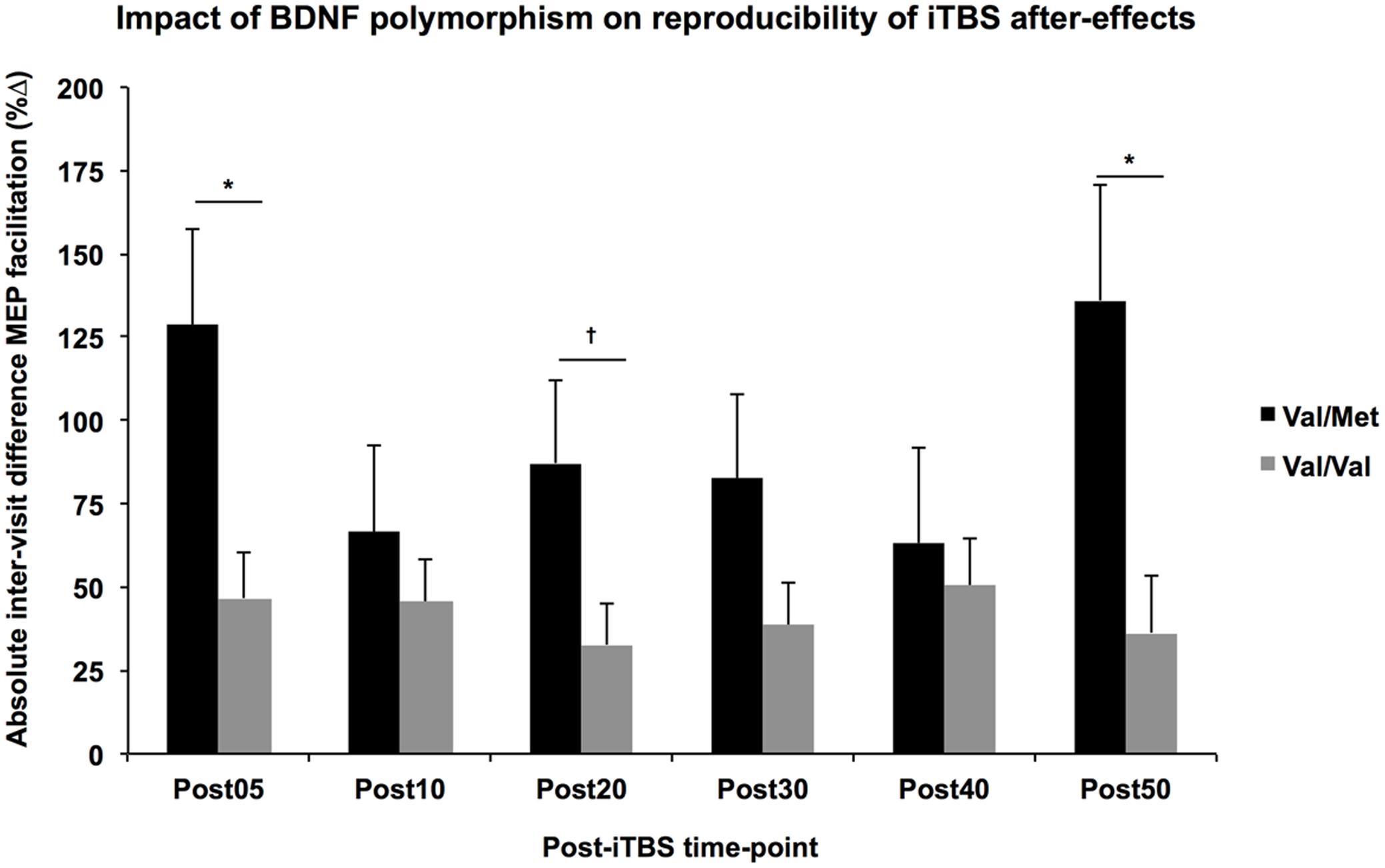
FIGURE 3. Impact of BDNF polymorphism on reproducibility of iTBS aftereffects. Controlling for Group, the absolute difference in MEP facilitation (y-axis) tended to be higher across all post-iTBS time-points (x-axis) in Val-Met carriers (black) than Val/Val homozygotes (gray). These differences were significant at 5- and 50-min post-iTBS, ∗p < 0.05, †p < 0.1.
The potential of TMS-based assessments to provide meaningful insights into human neurophysiology is constrained by its variability. In particular, the intraindividual variability of a measure reduces its sensitivity to detect meaningful changes over time or in responses to an intervention. As TMS is increasingly applied in different neuropsychiatric conditions, it is crucial to evaluate its reproducibility in the target populations. The current study offers the first direct analysis of reproducibility of single- and paired-pulse TMS, and patterned repetitive TMS in older healthy adults and those with impairments in cognition or glucose metabolism. The results show that reproducibility varies considerably across measures and populations. Motor thresholds remain the gold standard in test–retest reliability; SICI and LICI tended to be more reproducible than ICF, though variability in LICI and ICF differed considerably across groups. Lastly, measures of LTP-like plasticity from iTBS were among the least reproducible, especially for older healthy and diabetic individuals.
Two recent studies in younger healthy individuals have reported higher intraindividual variability in the response to iTBS (Hinder et al., 2014; Schilberg et al., 2017). The present results, based on data from healthy older adults and those with impairments in either cognition or glucose metabolism, are more-or-less consistent with those reports in younger adults and suggest that variability in the aftereffects of iTBS remains a significant challenge to its use as a biomarker for the efficacy of neuroplastic mechanisms across the lifespan. Previous studies have identified factors such as prior exercise (McDonnell et al., 2013), ongoing voluntary activity (Huang et al., 2008), and other state-dependent effects (Silvanto et al., 2007), that can influence the efficacy of TBS and thus increase intraindividual variability (for a review, see Ridding and Ziemann, 2010). Other factors such as BDNF polymorphisms and baseline cortico-motor reactivity are discussed below. Some factors could be disease-specific, such as fluctuations in blood glucose levels in T2DM, though, importantly, glucose levels (within the range of 80–200 mg/dL, specified a priori) were not found to influence variability in the present study. Interestingly, the AD group showed numerically higher reproducibility coefficients for nearly all measures, including RMT, SICI and iTBS aftereffects, which several studies in AD have shown to be abnormal and/or predictive of disease severity or response to treatment (Liepert et al., 2001; Di Lazzaro et al., 2004; Koch et al., 2012, 2015; Brem et al., 2013; Balla et al., 2014). It is possible, however unlikely, that some aspect of the Sham treatment (e.g., daily study visits or interaction with study staff) that the AD group underwent had some stabilizing effect on their neurophysiology. This possibility could be investigated further by conducting test–retest assessments in a similar AD cohort over a similar timeframe that did not include significant changes to their regular schedule. A more likely explanation is that the same pathological processes that cause certain measures to be abnormal in AD also exert a stabilizing effect on TMS measures. In particular, the reduction in LTP-like plasticity following iTBS (and in some cases conversion to a LTD-like response) (Koch et al., 2011, 2012; Di Lorenzo et al., 2016) could reflect pathological changes in the brains of AD patients that reduce state-dependent effect. In any case, the relatively high reproducibility of most TMS measures in AD appears to validate their use as surrogate biomarkers of AD cortical pathology (Freitas et al., 2011a).
Reliability coefficients such as Cronbach’s α can be used to adjust effect sizes to account for the fact that those calculations are made under the implicit assumption of perfect test–retest reliability (Baugh, 2002; Wright, 2014). In other words, detecting a significant change of any given size in a longitudinal design is more difficult for an unreliable measure than for a reliable one. In turn, an adjusted effect size can be used to provide a more realistic estimate of the sample size required to observe the desired effect given the reproducibility of the measure being tested. Table 2 shows adjustments to a hypothetical Cohen’s d of 0.5, which corresponds to a within-subject change of half a standard deviation, for each of the measures in the current analysis. Table 2 also shows the adjustments in the sample sizes required to detect the attenuated effects. The results of the present analysis can thus be used to more accurately plan for future studies using TMS-based neurophysiological measures as prognostic biomarkers in older healthy, diabetic, and AD populations.
Epidural recordings of cortico-spinal volleys in conscious humans receiving TMS have shown that depending on its shape, current direction, and intensity, a TMS pulse can result in direct depolarization of the layer-V pyramidal cell (D-waves) and/or indirect depolarization through local circuits of interneurons (I-waves) (Burke et al., 1993; Sakai et al., 1997). The use of posterior–anterior monophasic pulse waveforms, which primarily elicit early I-waves, yields higher reproducibility in measures of cortico-motor reactivity over biphasic stimulation, which elicits a more complex pattern of D-waves, and early and late I-waves depending on the intensity of stimulation (Di Lazzaro et al., 2003). Future studies should directly explore how the effect size and reproducibility of single-pulse, paired-pulse, and TBS-based TMS measures are influenced by physical TMS parameters such as pulse shape and duration, and induced current direction relative to the motor cortex.
The reproducibility of biphasic MEP amplitude (α = 0.62) was noticeably lower than that of biphasic RMT (α = 0.93). Given that MEPs were assessed using 120% of RMT, there appear to be factors that do not impact RMT but do add variability to batches of MEPs elicited at suprathreshold intensities. Moreover, the inter-visit change in biphasic baseline MEP amplitudes was inversely related to that of biphasic RMT, suggesting that stimulus-response curve (i.e., the relationship between TMS intensity and MEP size) itself varies across visits. It has been speculated that, at threshold intensities, the second half of the biphasic pulse (posterior–anterior in the present study) contributes primarily to the MEP, while at suprathreshold intensities, there is increasing influence of the first half of the pulse (anterior-posterior in the present study) (Di Lazzaro et al., 2003; Barker, 2017). There is at least some theoretical evidence that biphasic TMS pulses might be less effective than monophasic (posterior–anterior) at probing the neuromodulatory effects of TBS (Di Lazzaro and Rothwell, 2014). However, to our knowledge, this has never been directly investigated. Regardless, the relatively low reproducibility of baseline MEP amplitudes is an area of concern given that it is the basis on which post-iTBS measures are derived. Furthermore, a significant portion of the inter-visit variance in post-iTBS measures is accounted for by visit-to-visit difference in baseline MEP amplitudes. These results are consistent with a recent study showing variability in MEPs within a session is predictive of the response to cTBS (Hordacre et al., 2017). Furthermore, the present results imply that improving the consistency of baseline measures (within and across sessions) would decrease the variability of post-iTBS measures as well. Given that changes in stimulus-response curves might contribute to the changing relationship between RMT and suprathreshold MEP amplitudes, future studies should explore whether the reproducibility of these measures could be improved by choosing stimulation intensities based on individual stimulus-response curves rather than a fixed percent of RMT.
The use of neuronavigation has been shown to increase the consistency of MEPs (Julkunen et al., 2009). However, even with neuronavigation, handheld TMS remains prone to slight deviations in the position, orientation, and inclination of the TMS coil. Robot arms, such as the TMS Robot (Axilum® Robotics, Strasbourg) have been shown to improve the consistency of trial-to-trial MEPs over handheld TMS (Foucher et al., 2012; Ginhoux et al., 2013). Typically, MEP trials are elicited with individually spaced TMS pulses at a specific frequency range (e.g., 5000–6000 ms in the present study) with some random jitter incorporated to reduce the likelihood of train effects. Several recent studies combining TMS with concurrent EEG have highlighted to role of pre-stimulus oscillatory activity on cortico-motor excitability. Mäki and Ilmoniemi (2010) demonstrated that MEP amplitudes are inversely correlated with the amplitudes of pre-stimulus midrange-beta oscillations (15–18 Hz) over the stimulated motor cortex. Similarly, Iscan et al. (2016) showed that the variability of pre-stimulus power in the upper alpha band (10–12 Hz) was predictive of variability in ICF trials. Alternatively, Berger et al. (2014) suggest that the instantaneous phase of EEG oscillations across a range of frequencies is more predictive of MEP amplitude than spectral power. Together, these studies suggest that technological advances that allow for closed-loop systems to trigger TMS pulses timed to real-time EEG rhythms should result in more consistent MEPs. While these approaches offer the potential to improve the trial-to-trial consistency of MEPs, whether they would translate to greater reproducibility across visits remains to be explored.
While the multiple regression analyses did not show any significant difference between groups in terms of the absolute difference of the measures, participant age was significantly related to the absolute difference of monophasic baseline MEP amplitudes and inter-visit duration was significantly related to the absolute difference in Post05 facilitation, controlling for other factors such as group and gender. These results must be interpreted cautiously given the potential for Type-2 error in the present analysis. That the variability in baseline MEPs increases with age is not particularly surprising; however, its influence is not easily controlled, especially if the focus of the study is aging. It is more surprising that immediate iTBS aftereffects would be more consistent with greater time between visits. One possibility is that visits repeated under shorter intervals might be influenced by the iTBS from the previous visit, a phenomenon known as metaplasticity. It has been shown that the neuromodulatory effects of rTMS increase with consecutive daily application (Maeda et al., 2000; Valero-Cabré et al., 2008). Moreover, these metaplastic effects and state-dependent interactions may be altered by age (Opie et al., 2017) or neuropsychiatric disorders such as autism and Fragile X syndrome (Oberman et al., 2016). While the impact of multiple sessions separated by more than 24 h has not been well explored, a single application of iTBS was shown to alter the expression of GABA-precursor enzyme GAD67 for up to 7 days in the neocortex of rats (Trippe et al., 2009), suggesting the window for metaplastic effects might be longer than previously understood. Indeed, a follow-up analysis of the current data found that the absolute difference of Post05 facilitation was higher between visits conducted within 7 days than those separated by more than a week (Figure 4).
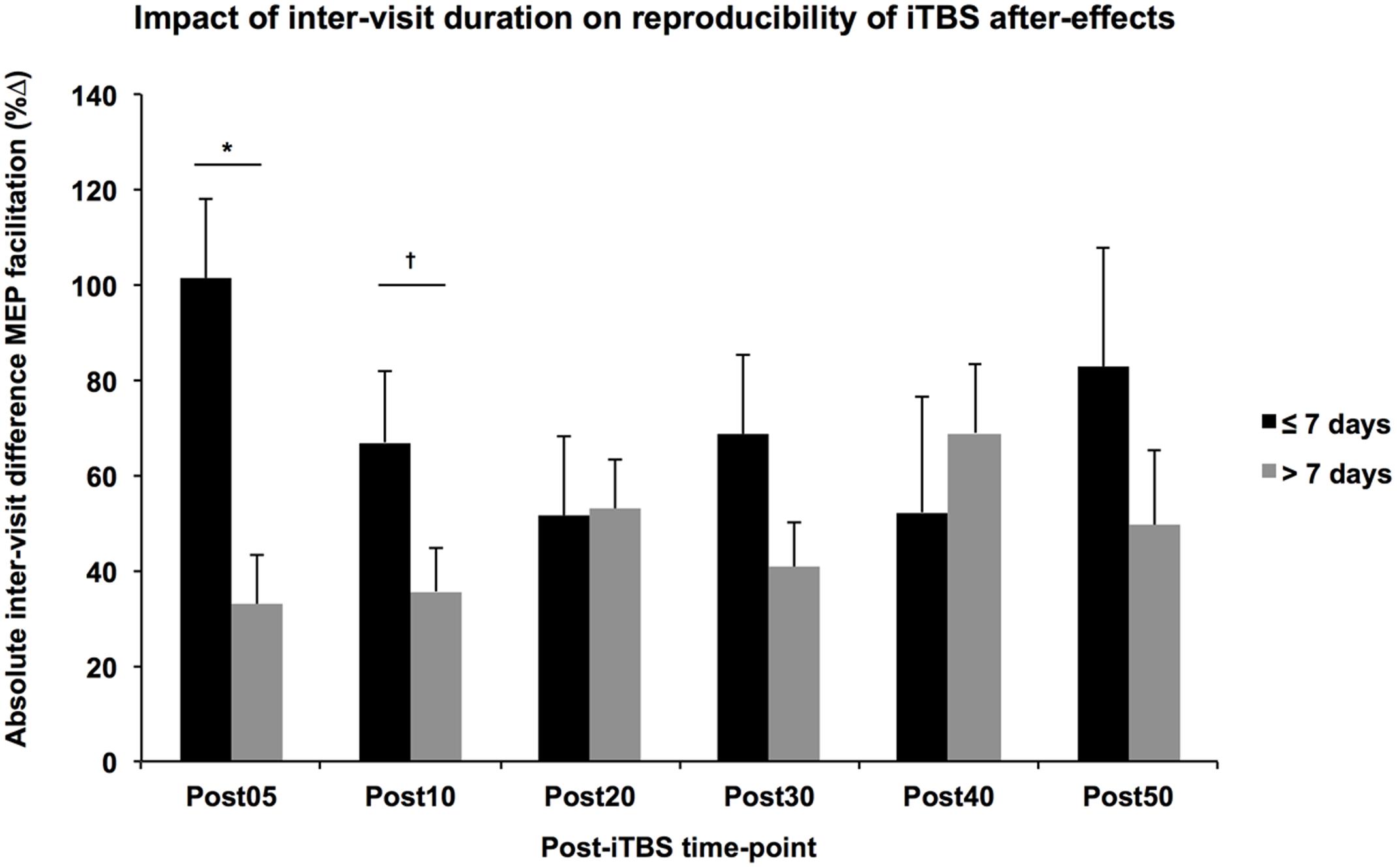
FIGURE 4. Impact of inter-visit duration on reproducibility of iTBS aftereffects. Controlling for Group, Age, and Gender, the absolute difference in MEP facilitation (y-axis) tended to be higher across all post-iTBS time-points (x-axis) in subjects that received their second visit within 7 days (black) than those whose second visit occurred greater than 7 days after the first (gray). These differences were significant at 5-min post-iTBS, ∗p < 0.05, †p < 0.1.
The multiple regression analyses showed that the absolute difference of several post-iTBS measures was higher in subjects with a BDNF-Met allele. While the generalizability of these findings is limited by the small sample size, they nonetheless provide insight into the debate over the role of BDNF polymorphisms in shaping the effects of neuromodulation. Several studies have reported a reduced impact of repetitive TMS in Met carriers (Cheeran et al., 2008; Cirillo et al., 2012; Lee et al., 2013; Chang et al., 2014; Di Lazzaro et al., 2015), still others have reported no difference (Li Voti et al., 2011; Mastroeni et al., 2013). The current results suggest that this divergence in the literature may be due to the fact that the BDNF Met allele leads to more variability in the response to neuromodulation rather than simply blunting its effects.
The present study was a retrospective analysis of data collected under different study protocols. As such there are a number of limitations, principally, the generally small and unequal sample sizes. While the Cronbach’s α is fairly robust to variations in sample size, the linear regression analyses are susceptible to limitations of sample size. As such, the analyses comparing absolute difference of visits between groups are likely underpowered to detect differences of the magnitude observed in the present study. Future studies with larger samples are needed to confirm the present findings. In addition, a number of the non-diabetic controls had hemoglobin A1c values indicating possible pre-diabetes, which may have contributed to the decreased reproducibility seen in this cohort. Further, HbA1c values were not available from the AD group, so the influence of impaired glucose metabolism could not be investigated in AD subjects, despite reports of high co-morbidity between AD and T2DM (Hölscher, 2011).
Motor thresholds remain the gold standard for reproducibility of any TMS measure as demonstrated by high Cronbach’s α coefficients. Post-iTBS measures of LTP-like plasticity demonstrate low reproducibility by comparison. Reproducibility was higher in the AD group, possibly reflecting pathological rigidity of neurophysiological systems. A number of factors may contribute to the intraindividual variability of iTBS aftereffects, including BDNF polymorphisms and variability in baseline MEP amplitudes, from which post-iTBS measures are calculated. Future studies can use the α coefficients to adjust expected effect size and required sample size calculations.
Based on these conclusions, we offer the following recommendations for future studies to potentially reduce the intraindividual variability in TMS measures, especially in the iTBS-induced modulation of cortico-motor reactivity. We note that these recommendations are based on exploratory analyses performed in a relatively small and heterogeneous group of subjects and further confirmatory studies are needed. (1) Waiting at least 7 days between repeated visits can reduce the probability of metaplastic effects, at least in healthy individuals. (2) Whenever possible, BDNF polymorphism should be taken into account, either by adding BDNF Met carrier status as a covariate, or by splitting the data into subgroups. (3) To reduce intraindividual variability in baseline MEP amplitudes and any resulting impact of this variability on post-iTBS measures, we recommend considering the use of a stimulation intensity derived from individual stimulus-response curves, rather than using a fixed percent of RMT.
Study concept and design: PF and AP-L. Data collection: PF. Data analysis: PF and AJ. Data interpretation: PF, AJ, PD-P, and AP-L. Drafting manuscript: PF. Revising manuscript: PF, AJ, PD-P, and AP-L. All authors approved the final version and agree to be accountable for the content of the work.
This study was primarily funded by the National Institutes of Health (NIH R21 NS082870). AJ was supported by a Postdoctoral Fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC PDF 454617) and a Fellowship from the Canadian Institutes of Health Research (CIHR 41791). AP-L was further supported in part by the Sidney R. Baer Jr. Foundation, the NIH (R01 MH100186, R01 HD069776, R01 NS073601, R21 MH099196, R21 NS085491, R21 HD07616), and Harvard Catalyst| The Harvard Clinical and Translational Science Center (NCRR and the NCATS NIH, UL1 RR025758). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr. Foundation.
AP-L serves on the scientific advisory boards for Magstim, Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Constant Therapy, and Neosync; and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank E. Seligson, N. Atkinson, and S. Saxena (Beth Israel Deaconess Medical Center) for their assistance in data collection and A. Connor (Beth Israel Deaconess Medical Center) for assistance with evaluation of participant health and medical history. A portion of the findings was presented at the 2nd International Brain Stimulation Conference, Barcelona, Spain, March 5-8, 2017.
AD, Alzheimer’s disease; AMT, active motor threshold; APOE, apolipoprotein-E; AUC0-20, area under-the-curve for 0–20 min post-iTBS; AUC0-50, area under-the-curve for 0–50 min post-iTBS; BDNF, brain-derived neurotrophic factor; CDR, clinical dementia rating; cTBS, continuous theta-burst stimulation; EEG, electroencephalography; FDI, first dorsal interosseous; ICF, intracortical facilitation; IFCN, International Federation of Clinical Neurophysiology; iTBS, intermittent theta-burst stimulation; LICI, long-interval intracortical inhibition; LTD, long-term depression; LTP, long-term potentiation; Max+, maximum facilitation; MEP, motor evoked potential; MMSE, mini-mental status exam; MRI, magnetic resonance imaging; Post05 … Post50, 5-min … 50-min post-iTBS; RMT, resting motor threshold; rTMS, repetitive transcranial magnetic stimulation; SICI, short-interval intracortical inhibition; T2DM, type-2 diabetes mellitus; TBS, theta-burst stimulation; TMS, transcranial magnetic stimulation; % Δ, percent change from baseline; ΔB-A, net difference between visits; |ΔB-A|, absolute difference between visits.
Balla, C., Maertens de Noordhout, A., and Pepin, J. L. (2014). Motor cortex excitability changes in mild Alzheimer’s disease are reversed by donepezil. Dement. Geriatr. Cogn. Disord. 38, 264–270. doi: 10.1159/000360617
Barker, A. T. (2017). Transcranial magnetic stimulation – past, present and future. Brain Stimul. Basic Transl. Clin. Res. Neuromodulation 10:540. doi: 10.1016/j.brs.2017.01.573
Baugh, F. (2002). Correcting effect sizes for score reliability: a reminder that measurement and substantive issues are linked inextricably. Educ. Psychol. Meas. 62, 254–263. doi: 10.1177/0013164402062002004
Berger, B., Minarik, T., Liuzzi, G., Hummel, F. C., and Sauseng, P. (2014). EEG oscillatory phase-dependent markers of corticospinal excitability in the resting brain. BioMed Res. Int. 2014:936096. doi: 10.1155/2014/936096
Brem, A.-K., Atkinson, N. J., Seligson, E. E., and Pascual-Leone, A. (2013). Differential pharmacological effects on brain reactivity and plasticity in Alzheimer’s disease. Front. Psychiatry 4:124. doi: 10.3389/fpsyt.2013.00124
Burke, D., Hicks, R., Gandevia, S. C., Stephen, J., Woodforth, I., and Crawford, M. (1993). Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J. Physiol. 470, 383–393. doi: 10.1113/jphysiol.1993.sp019864
Chang, W. H., Bang, O. Y., Shin, Y.-I., Lee, A., Pascual-Leone, A., and Kim, Y.-H. (2014). BDNF polymorphism and differential rTMS effects on motor recovery of stroke patients. Brain Stimulat. 7, 553–558. doi: 10.1016/j.brs.2014.03.008
Cheeran, B., Talelli, P., Mori, F., Koch, G., Suppa, A., Edwards, M., et al. (2008). A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. 586, 5717–5725. doi: 10.1113/jphysiol.2008.159905
Christie, A., Fling, B., Crews, R. T., Mulwitz, L. A., and Kamen, G. (2007). Reliability of motor-evoked potentials in the ADM muscle of older adults. J. Neurosci. Methods 164, 320–324. doi: 10.1016/j.jneumeth.2007.05.011
Cirillo, J., Hughes, J., Ridding, M., Thomas, P. Q., and Semmler, J. G. (2012). Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. Eur. J. Neurosci. 36, 2640–2649. doi: 10.1111/j.1460-9568.2012.08177.x
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates.
Cronbach, L. J., and Warrington, W. G. (1951). Time-limit tests: estimating their reliability and degree of speeding. Psychometrika 16, 167–188. doi: 10.1007/BF02289113
Di Lazzaro, V., Oliviero, A., Pilato, F., Mazzone, P., Insola, A., Ranieri, F., et al. (2003). Corticospinal volleys evoked by transcranial stimulation of the brain in conscious humans. Neurol. Res. 25, 143–150. doi: 10.1179/016164103101201292
Di Lazzaro, V., Oliviero, A., Pilato, F., Saturno, E., Dileone, M., Marra, C., et al. (2004). Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 75, 555–559. doi: 10.1136/jnnp.2003.018127
Di Lazzaro, V., Pellegrino, G., Di Pino, G., Corbetto, M., Ranieri, F., Brunelli, N., et al. (2015). Val66Met BDNF gene polymorphism influences human motor cortex plasticity in acute stroke. Brain Stimulat. 8, 92–96. doi: 10.1016/j.brs.2014.08.006
Di Lazzaro, V., and Rothwell, J. C. (2014). Corticospinal activity evoked and modulated by non-invasive stimulation of the intact human motor cortex. J. Physiol. 592, 4115–4128. doi: 10.1113/jphysiol.2014.274316
Di Lorenzo, F., Ponzo, V., Bonnì, S., Motta, C., Negrão Serra, P. C., Bozzali, M., et al. (2016). LTP-like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann. Neurol. 80, 202–210. doi: 10.1002/ana.24695
Fleming, M. K., Sorinola, I. O., Newham, D. J., Roberts-Lewis, S. F., and Bergmann, J. H. M. (2012). The effect of coil type and navigation on the reliability of transcranial magnetic stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 20, 617–625. doi: 10.1109/TNSRE.2012.2202692
Foucher, J., Lorgouilloux, K., Turek, J., Pham, B.-T., Elowe, J., Bayle, B., et al. (2012). “Robotic assistance in coil positioning improves reliability and comfort,” in Proceedings of the 3rd Annual Meeting of the German Society for Brain Stimulation – Modulating Emotions, Berlin.
Freitas, C., Mondragón-Llorca, H., and Pascual-Leone, A. (2011a). Noninvasive brain stimulation in Alzheimer’s disease: systematic review and perspectives for the future. Exp. Gerontol. 46, 611–627. doi: 10.1016/j.exger.2011.04.001
Freitas, C., Perez, J., Knobel, M., Tormos, J. M., Oberman, L., Eldaief, M., et al. (2011b). Changes in cortical plasticity across the lifespan. Front. Aging Neurosci. 3:5. doi: 10.3389/fnagi.2011.00005
Fried, P. J., Schilberg, L., Brem, A.-K., Saxena, S., Wong, B., Cypess, A. M., et al. (2016). Humans with Type-2 diabetes show abnormal long-term potentiation-like cortical plasticity associated with verbal learning deficits. J. Alzheimers Dis. 55, 89–100. doi: 10.3233/JAD-160505
Friedman, H. (1968). Magnitude of Experimental Effect and a Table for its Rapid Estimation. Available at: https://ntrs.nasa.gov/search.jsp?R=19690045051 [accessed October 20, 2016].
Ginhoux, R., Renaud, P., Zorn, L., Goffin, L., Bayle, B., Foucher, J., et al. (2013). “A custom robot for transcranial magnetic stimulation: first assessment on healthy subjects,” in Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC’13), Osaka. doi: 10.1109/EMBC.2013.6610758
Goldsworthy, M. R., Müller-Dahlhaus, F., Ridding, M. C., and Ziemann, U. (2014). Inter-subject variability of LTD-like plasticity in human motor cortex: a matter of preceding motor activation. Brain Stimulat. 7, 864–870. doi: 10.1016/j.brs.2014.08.004
Hallett, M. (2007). Transcranial magnetic stimulation: a primer. Neuron 55, 187–199. doi: 10.1016/j.neuron.2007.06.026
Hinder, M. R., Goss, E. L., Fujiyama, H., Canty, A. J., Garry, M. I., Rodger, J., et al. (2014). Inter- and intra-individual variability following intermittent theta burst stimulation: implications for rehabilitation and recovery. Brain Stimulat. 7, 365–371. doi: 10.1016/j.brs.2014.01.004
Hölscher, C. (2011). Diabetes as a risk factor for Alzheimer’s disease: insulin signalling impairment in the brain as an alternative model of Alzheimer’s disease. Biochem. Soc. Trans. 39, 891–897. doi: 10.1042/BST0390891
Hordacre, B., Goldsworthy, M. R., Vallence, A.-M., Darvishi, S., Moezzi, B., Hamada, M., et al. (2017). Variability in neural excitability and plasticity induction in the human cortex: a brain stimulation study. Brain Stimulat. 10, 588–595. doi: 10.1016/j.brs.2016.12.001
Huang, Y.-Z., Edwards, M. J., Rounis, E., Bhatia, K. P., and Rothwell, J. C. (2005). Theta burst stimulation of the human motor cortex. Neuron 45, 201–206. doi: 10.1016/j.neuron.2004.12.033
Huang, Y.-Z., Rothwell, J. C., Edwards, M. J., and Chen, R.-S. (2008). Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb. Cortex 18, 563–570. doi: 10.1093/cercor/bhm087
Iezzi, E., Conte, A., Suppa, A., Agostino, R., Dinapoli, L., Scontrini, A., et al. (2008). Phasic voluntary movements reverse the aftereffects of subsequent theta-burst stimulation in humans. J. Neurophysiol. 100, 2070–2076. doi: 10.1152/jn.90521.2008
Iscan, Z., Nazarova, M., Fedele, T., Blagovechtchenski, E., and Nikulin, V. V. (2016). Pre-stimulus alpha oscillations and inter-subject variability of motor evoked potentials in single- and paired-pulse TMS paradigms. Front. Hum. Neurosci. 10:504. doi: 10.3389/fnhum.2016.00504
Julkunen, P., Säisänen, L., Danner, N., Niskanen, E., Hukkanen, T., Mervaala, E., et al. (2009). Comparison of navigated and non-navigated transcranial magnetic stimulation for motor cortex mapping, motor threshold and motor evoked potentials. Neuroimage 44, 790–795. doi: 10.1016/j.neuroimage.2008.09.040
Kimiskidis, V. K., Papagiannopoulos, S., Sotirakoglou, K., Kazis, D. A., Dimopoulos, G., Kazis, A., et al. (2004). The repeatability of corticomotor threshold measurements. Neurophysiol. Clin. 34, 259–266. doi: 10.1016/j.neucli.2004.10.002
Koch, G., Di Lorenzo, F., Bonnì, S., Ponzo, V., Caltagirone, C., and Martorana, A. (2012). Impaired LTP- but not LTD-like cortical plasticity in Alzheimer’s disease patients. J. Alzheimers Dis. 31, 593–599. doi: 10.3233/JAD-2012-120532
Koch, G., Di Lorenzo, F., Del Olmo, M. F., Bonní, S., Ponzo, V., Caltagirone, C., et al. (2015). Reversal of LTP-like cortical plasticity in Alzheimer’s disease patients with Tau-related faster clinical progression. J. Alzheimers Dis. 50, 605–616. doi: 10.3233/JAD-150813
Koch, G., Esposito, Z., Codecà, C., Mori, F., Kusayanagi, H., Monteleone, F., et al. (2011). Altered dopamine modulation of LTD-like plasticity in Alzheimer’s disease patients. Clin. Neurophysiol. 122, 703–707. doi: 10.1016/j.clinph.2010.10.033
Kujirai, T., Caramia, M. D., Rothwell, J. C., Day, B. L., Thompson, P. D., Ferbert, A., et al. (1993). Corticocortical inhibition in human motor cortex. J. Physiol. 471, 501–519. doi: 10.1113/jphysiol.1993.sp019912
Lee, M., Kim, S. E., Kim, W. S., Lee, J., Yoo, H. K., Park, K.-D., et al. (2013). Interaction of motor training and intermittent theta burst stimulation in modulating motor cortical plasticity: influence of BDNF Val66Met polymorphism. PLoS ONE 8:e57690. doi: 10.1371/journal.pone.0057690
Li Voti, P., Conte, A., Suppa, A., Iezzi, E., Bologna, M., Aniello, M. S., et al. (2011). Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp. Brain Res. 212, 91–99. doi: 10.1007/s00221-011-2700-5
Liepert, J., Bär, K. J., Meske, U., and Weiller, C. (2001). Motor cortex disinhibition in Alzheimer’s disease. Clin. Neurophysiol. 112, 1436–1441. doi: 10.1016/S1388-2457(01)00554-5
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., and Pascual-Leone, A. (2000). Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 111, 800–805. doi: 10.1016/S1388-2457(99)00323-5
Mäki, H., and Ilmoniemi, R. J. (2010). EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clin. Neurophysiol. 121, 492–501. doi: 10.1016/j.clinph.2009.11.078
Mastroeni, C., Bergmann, T. O., Rizzo, V., Ritter, C., Klein, C., Pohlmann, I., et al. (2013). Brain-derived neurotrophic factor–a major player in stimulation-induced homeostatic metaplasticity of human motor cortex? PLoS ONE 8:e57957. doi: 10.1371/journal.pone.0057957
McClintock, S. M., Freitas, C., Oberman, L., Lisanby, S. H., and Pascual-Leone, A. (2011). Transcranial magnetic stimulation: a neuroscientific probe of cortical function in schizophrenia. Biol. Psychiatry 70, 19–27. doi: 10.1016/j.biopsych.2011.02.031
McDonnell, M. N., Buckley, J. D., Opie, G. M., Ridding, M. C., and Semmler, J. G. (2013). A single bout of aerobic exercise promotes motor cortical neuroplasticity. J. Appl. Physiol. 114, 1174–1182. doi: 10.1152/japplphysiol.01378.2012
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 263–269. doi: 10.1016/j.jalz.2011.03.005
Oberman, L., Eldaief, M., Fecteau, S., Ifert-Miller, F., Tormos, J. M., and Pascual-Leone, A. (2012). Abnormal modulation of corticospinal excitability in adults with Asperger’s syndrome. Eur. J. Neurosci. 36, 2782–2788. doi: 10.1111/j.1460-9568.2012.08172.x
Oberman, L. M., Ifert-Miller, F., Najib, U., Bashir, S., Heydrich, J. G., Picker, J., et al. (2016). Abnormal mechanisms of plasticity and metaplasticity in autism spectrum disorders and fragile X syndrome. J. Child Adolesc. Psychopharmacol. 26, 617–624. doi: 10.1089/cap.2015.0166
Opie, G. M., Vosnakis, E., Ridding, M. C., Ziemann, U., and Semmler, J. G. (2017). Priming theta burst stimulation enhances motor cortex plasticity in young but not old adults. Brain Stimulat. 10, 298–304. doi: 10.1016/j.brs.2017.01.003
Pascual-Leone, A., Valls-Solé, J., Wassermann, E. M., and Hallett, M. (1994). Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain J. Neurol. 117(Pt 4), 847–858. doi: 10.1093/brain/117.4.847
Portney, L. G., and Watkins, M. P. (2009). Foundations of Clinical Research: Applications to Practice, 3rd Edn. Upper Saddle River,NJ: Prentice Hall.
Ridding, M. C., and Ziemann, U. (2010). Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 588, 2291–2304. doi: 10.1113/jphysiol.2010.190314
Rossi, S., Hallett, M., Rossini, P. M., and Pascual-Leone, A. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039. doi: 10.1016/j.clinph.2009.08.016
Rossini, P. M., Burke, D., Chen, R., Cohen, L. G., Daskalakis, Z., Di Iorio, R., et al. (2015). Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin. Neurophysiol. 126, 1071–1107. doi: 10.1016/j.clinph.2015.02.001
Sakai, K., Ugawa, Y., Terao, Y., Hanajima, R., Furubayashi, T., and Kanazawa, I. (1997). Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of-eight-shaped coil. Exp. Brain Res. 113, 24–32. doi: 10.1007/BF02454139
Schilberg, L., Schuhmann, T., and Sack, A. T. (2017). Interindividual variability and intraindividual reliability of intermittent theta burst stimulation-induced neuroplasticity mechanisms in the healthy brain. J. Cogn. Neurosci. 29, 1022–1032. doi: 10.1162/jocn_a_01100
Silvanto, J., Muggleton, N. G., Cowey, A., and Walsh, V. (2007). Neural activation state determines behavioral susceptibility to modified theta burst transcranial magnetic stimulation. Eur. J. Neurosci. 26, 523–528. doi: 10.1111/j.1460-9568.2007.05682.x
Tavakol, M., and Dennick, R. (2011). Making sense of Cronbach’s alpha. Int. J. Med. Educ. 2, 53–55. doi: 10.5116/ijme.4dfb.8dfd
Tremblay, S., Vernet, M., Bashir, S., Pascual-Leone, A., and Théoret, H. (2015). Theta burst stimulation to characterize changes in brain plasticity following mild traumatic brain injury: a proof-of-principle study. Restor. Neurol. Neurosci. 33, 611–620. doi: 10.3233/RNN-140459
Trippe, J., Mix, A., Aydin-Abidin, S., Funke, K., and Benali, A. (2009). 𝜃 burst and conventional low-frequency rTMS differentially affect GABAergic neurotransmission in the rat cortex. Exp. Brain Res. 199, 411–421. doi: 10.1007/s00221-009-1961-8
Valero-Cabré, A., Pascual-Leone, A., and Rushmore, R. J. (2008). Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur. J. Neurosci. 27, 765–774. doi: 10.1111/j.1460-9568.2008.06045.x
Vallence, A.-M., Goldsworthy, M. R., Hodyl, N. A., Semmler, J. G., Pitcher, J. B., and Ridding, M. C. (2015). Inter- and intra-subject variability of motor cortex plasticity following continuous theta-burst stimulation. Neuroscience 304, 266–278. doi: 10.1016/j.neuroscience.2015.07.043
Valls-Solé, J., Pascual-Leone, A., Wassermann, E. M., and Hallett, M. (1992). Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr. Clin. Neurophysiol. 85, 355–364.
Vernet, M., Bashir, S., Yoo, W.-K., Oberman, L., Mizrahi, I., Ifert-Miller, F., et al. (2014). Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin. Neurophysiol. 125, 320–326. doi: 10.1016/j.clinph.2013.07.004
Wessa, P. (2016). Cronbach Alpha. Office for Research Development and Education. Available at: http://www.wessa.net/rwasp_cronbach.wasp/
Keywords: reproducibility of results, transcranial magnetic stimulation, cortical plasticity, aging, Type-2 diabetes, Alzheimer’s disease
Citation: Fried PJ, Jannati A, Davila-Pérez P and Pascual-Leone A (2017) Reproducibility of Single-Pulse, Paired-Pulse, and Intermittent Theta-Burst TMS Measures in Healthy Aging, Type-2 Diabetes, and Alzheimer’s Disease. Front. Aging Neurosci. 9:263. doi: 10.3389/fnagi.2017.00263
Received: 30 May 2017; Accepted: 24 July 2017;
Published: 21 August 2017.
Edited by:
Alessio Avenanti, Università di Bologna, ItalyReviewed by:
Vincenzo Di Lazzaro, Università Campus Bio-Medico, ItalyCopyright © 2017 Fried, Jannati, Davila-Pérez and Pascual-Leone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Fried, cGZyaWVkQGJpZG1jLmhhcnZhcmQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.