- 1Department of Rehabilitation, Zhujiang Hospital, Southern Medical University, Guangzhou, China
- 2Department of Neurology, Zhengzhou People’s Hospital, Zhengzhou, China
Background: Stroke not only impacts patients physically but also economically. Post-stroke depression (PSD), as a common complication of stroke, always obstructs the process of stroke rehabilitation. Accordingly, defining the risk factors associated with PSD has extraordinary importance. Although there have been many studies investigating the risk factors for PSD, the results are inconsistent.
Objectives: The objectives of this study were to identify the risk factors for PSD by evidence-based medicine.
Data sources: A systematic and comprehensive database search was performed of PubMed, Medline, CENTRAL, EMBASE.com, the Cochrane library and Web of Science for Literature, covering publications from January 1, 1998 to November 19, 2016.
Study Selection: Studies on risk factors for PSD were identified, according to inclusion and exclusion criteria. The risk of bias tool, described in the Cochrane Handbook version 5.1.0, was used to assess the quality of each study. Meta-analysis was performed using RevMan 5.3 software.
Results: Thirty-six studies were included for review. A history of mental illness was the highest ranking modifiable risk factor; other risk factors for PSD were female gender, age (<70 years), neuroticism, family history, severity of stroke, and level of handicap. Social support was a protective factor for PSD.
Conclusion: There are many factors that have effects on PSD. The severity of stroke is an important factor in the occurrence of PSD. Mental history is a possible predictor of PSD. Prevention of PSD requires social and family participation.
Introduction
Stroke, which has been ranked the third most deadly disease, is prevalent worldwide (Mozaffarian et al., 2016). According to the report of World Health Organization (WHO), there are 15 million people suffering from stroke every year (European Stroke Initiative Executive EUSI Writing Committee Olsen et al., 2003).
Post-stroke depression (PSD) is considered the most frequent and important neuropsychiatric consequence of a stroke that negatively affects the outcome in stroke patients. A recent systematic review indicated that the frequency of PSD is 33% (95% confidence interval [CI], 29 to 36%) (Hackett and Pickles, 2014). The Diagnostic and Statistical Manual (DSM) IV categorizes PSD as a “mood disorder due to a general medical condition (i.e., stroke)” with the specifiers of depressive features, major depressive-like episodes, manic features, or mixed features (American Psychiatric Association, 2000). Persistent depression not only increases the disease deterioration, but also causes social function defects, and increases the risk of suicide. Moreover this condition can have an adverse effect on cognitive function, functional recovery and survival. Therefore, it is urgent to study the risk factors for PSD.
In recent decades, overwhelming evidence has indicated that PSD is associated with well-known risk factors such as medical history (i.e., predisposing illness and smoking) (Shi et al., 2015), a history of mental disorders (de Man-van Ginkel et al., 2013), and stroke characteristics (Jiang et al., 2014). PSD is highly prevalent among both men and women; however, it appears that PSD is more common in women than men. Although risk factors for PSD are increasingly reported, it still lacks of evidence based on medicine. Some studies have shown the risk factors for PSD are diverse at different time periods after the stroke. For example, Robinson et al. (1986) suggested that stroke patients with left hemisphere lesions are more likely to be depressed than those with right hemisphere and brainstem lesions, which is supported by most scholars. But Sun et al. (2014) suggested that there was a correlation between depression and right hemisphere lesion 6 months after stroke. In summary, the risk factors for PSD at different time points are complex and diverse, requiring further analysis to draw a convincing conclusion.
We undertook a systematic review of studies relevant to present practice to clearly identify important risk factors for PSD. If some of these risk factors can be avoided or prevented, it would be most helpful to take preventive measures in order to promote early diagnosis, implement early and adequate treatment, and improve quality of life. To obtain a more comprehensive estimate of the putative influence of PSD, we conducted a meta-analysis to examine the risk factors for PSD associated with different time periods after the stroke.
Materials and Methods
Search Strategy
A systematic and comprehensive database search was performed of PubMed, Medline, CENTRAL, EMBASE.com, Cochrane library and Web of Science for Literature published from January 1, 1998 to November 19, 2016. Only papers in English and human studies were considered. We used the following terms for the search strategy: two search themes were combined using the Boolean operator “and.” The first theme, depression, combined exploded versions of the Medical Subject Headings (MeSH) depression, depressive disorder, or depressive disorder, major. The second theme, stroke, combined exploded versions of the MeSH terms stroke, cerebrovascular disorders, or intracranial embolism and thrombosis.
Eligibility Criteria
Eligibility criteria accorded with the PICOS (participants, interventions, controls, outcomes, and studies) framework, as follows:
Participants
Participants were non-hospitalized adults with no history of stroke or transient ischemic attack (TIA) at the time of study initiation.
Interventions
The intervention variable was defined as depression, whereas the assessment of depression had to be prospectively performed at baseline and based upon an objective measure such as a neuropsychological mood scale or neuropsychiatric evaluation that complied with the Diagnostic Statistical Manual for Mental Disorders (DSM)-III/IV/V or the International Classification of Diseases (ICD)-7–10.
Controls
The comparison groups consisted of participants without depression at the time of study initiation.
Outcomes
The dichotomous outcome event of interest was a first-ever stroke during the follow-up period, including fatal and non-fatal ischemic stroke, TIA, and intracerebral hemorrhage (hereinafter referred as “all stroke”).
Studies
The study design was a community-, population-, or registry-based longitudinal cohort study reporting relative effect estimates, such as hazard ratios (HRs), relative risk (RRs), and odds ratios (ORs).
Study Selection
Two reviewers (Shi and Wu) independently evaluated potentially eligible studies that were identified by our search. Articles were screened for eligibility based on a review of the title and abstract only, and disagreements were resolved by consensus. Regarding the remaining papers, their full text was accessed and read independently by the initial two reviewers. When differences of opinion between reviewers occurred, these were resolved by discussion with a third member of the research team, and consensus was thereby reached.
Data Collection
We developed a specific data extraction sheet. One author (Shi) extracted data from the included studies and another (Wu) used statistical software to check the accuracy of inclusion. Any disagreement was resolved by discussion with the other authors. The data extracted from each study included (1) basic information: author, year of publication, published journals, the number of cases in each group, the proportion of men and women, average age, duration of follow-up time, study design; (2) statistical data (ORs and 95% Cis) for: demographic and social factors, medical history, history of mental disorders, stroke characteristics, impairments, neurocognitive outcome measures, biochemical factors, and other factors. The course of disease was defined as that of acute stage and subacute stage (≤3 months), recovery period (>3 months) (American Psychiatric Association, 2000). When there was any uncertainty about the data, we contacted the corresponding author for clarification. We also collected information suitable for a basic quality evaluation of the studies included, based on the comparability between stroke and non-stroke groups, the risk of selection bias, the evaluation of representativeness of the recruited samples, and the reliability of the depression assessment.
Statistical Analysis
For studies with data of sufficient quality, and similar in simulation learning and outcome measures, we combined data in a meta-analysis in order to provide a pooled effect estimate. All data were entered into RevMan 5.31, where standardized deviations and 95% CIs were calculated and pooled. The results were expressed as weighted OR with 95% CI for outcomes (Higgins and Green, 2011).
For each analysis, a heterogeneity test was performed using I2 statistics, which measure the extent of inconsistency among results and is interpreted approximately as the proportion of total variation across studies attributable to heterogeneity and not to chance. I2 = 25% was considered low, 50% moderate, and 75% high. I2 values higher than 50% were considered as having substantial heterogeneity, and the random-effects model was therefore applied for analysis of the data. In addition, we performed subgroup analysis according to prespecified variables, including study design and intervention characteristics (i.e., country, follow-up, stroke type). If there had been no statistical heterogeneity, we would have used a fixed-effect model. Subsequently, we performed subgroup analyses according to the study design which was chosen as a potential moderator because different designs were included in the meta-analysis and we considered it is important to be analyzed by subgroup.
To test for publication bias, a funnel plot, which graphs the effect size of each study according to its respective SE, was used. We assumed the existence of publication bias if there were no small studies with effect sizes favoring control groups. A two-tailed p-value of less than 0.05 was considered significant (Higgins and Green, 2011).
Results
Study Selection
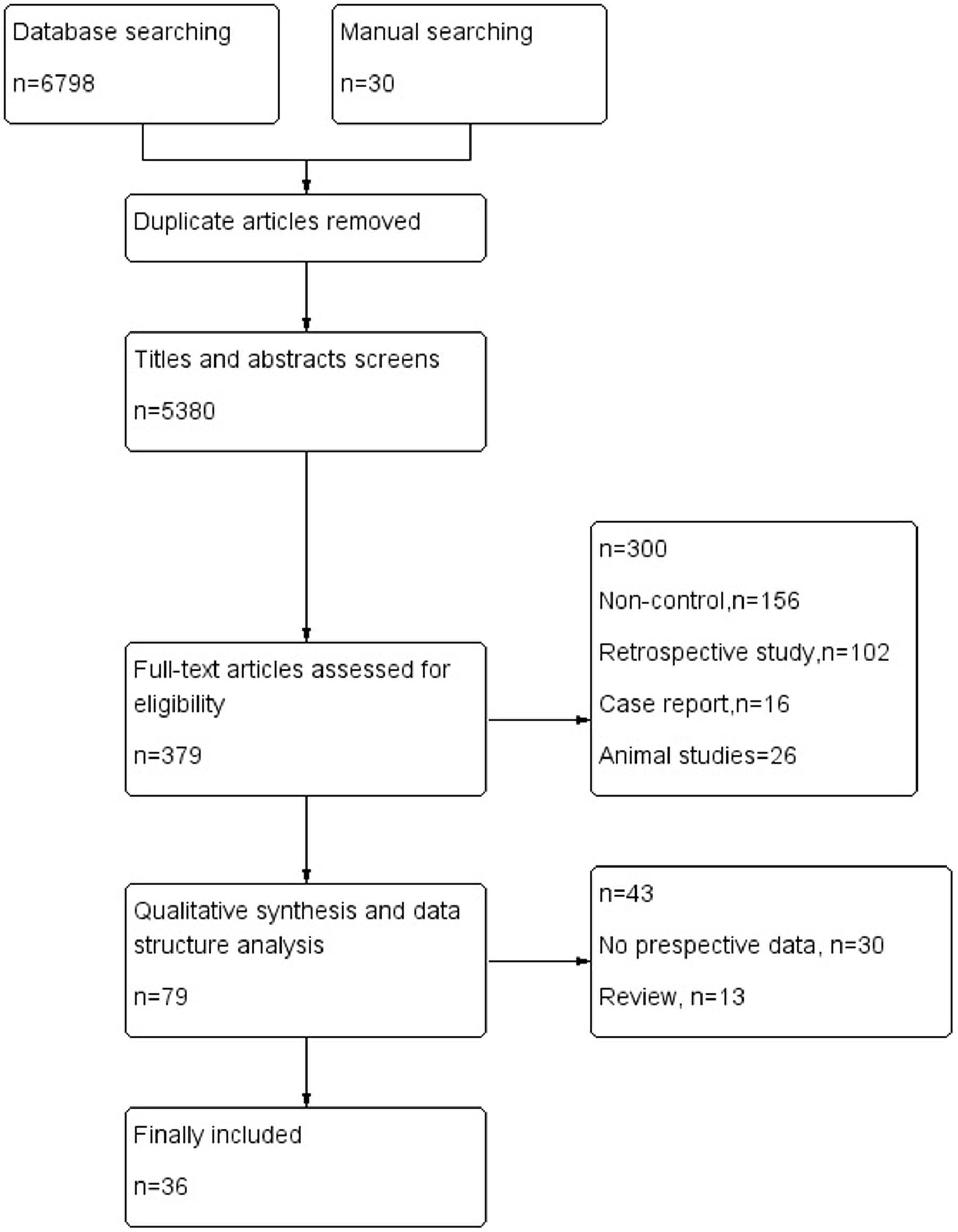
The electronic database search of PubMed, Medline, CENTRAL, EMBASE.com, the Cochrane library and Web of Science provideda total of 6798 citations, and 30 citations were found manually. After removing duplicate manuscripts, 5380 studies remained. Of these, 5000 were excluded based on the title and abstract review, leaving 379 for full text review. These 379 studies with their full text were retrieved and reviewed for eligibility, and 300 were excluded because of ineligible study design and outcome measures. The 79 studies met all the criteria, and these were selected for initial inclusion; after review, a total of 36 articles (Kotila et al., 1998; Pohjasvaara et al., 1998; Aben et al., 2002, 2006; Carota et al., 2005; Tang et al., 2005, 2011; Leentjens et al., 2006; Storor and Byrne, 2006; Brodaty et al., 2007; Lee et al., 2007; Lindén et al., 2007; van de Port et al., 2007; Fuentes et al., 2009; Jimenez et al., 2009; Schepers et al., 2009; Snaphaan et al., 2009; Farner et al., 2010; Ayerbe et al., 2011; Altieri et al., 2012; de Man-van Ginkel et al., 2013; Yang et al., 2013, 2015; Zhang et al., 2013; De Ryck et al., 2014a,b; Li et al., 2014; Ahn et al., 2015; Lewin-Richter et al., 2015; Shi et al., 2015; van Mierlo et al., 2015; Koh et al., 2016; Kootker et al., 2016; Malhotra et al., 2016; Metoki et al., 2016; Tsai et al., 2016) were included in the final analysis (Figure 1).
Study Characteristics
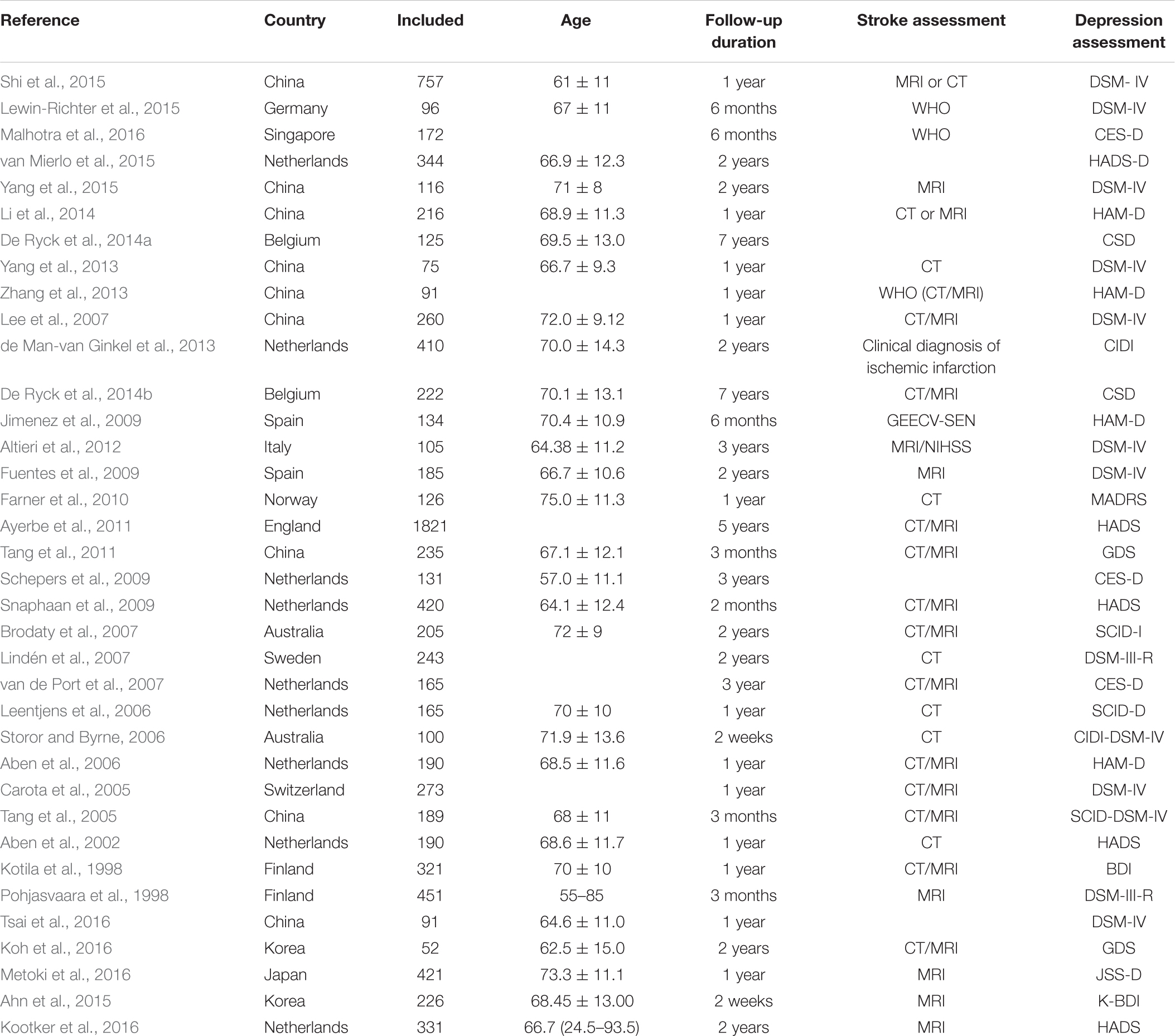
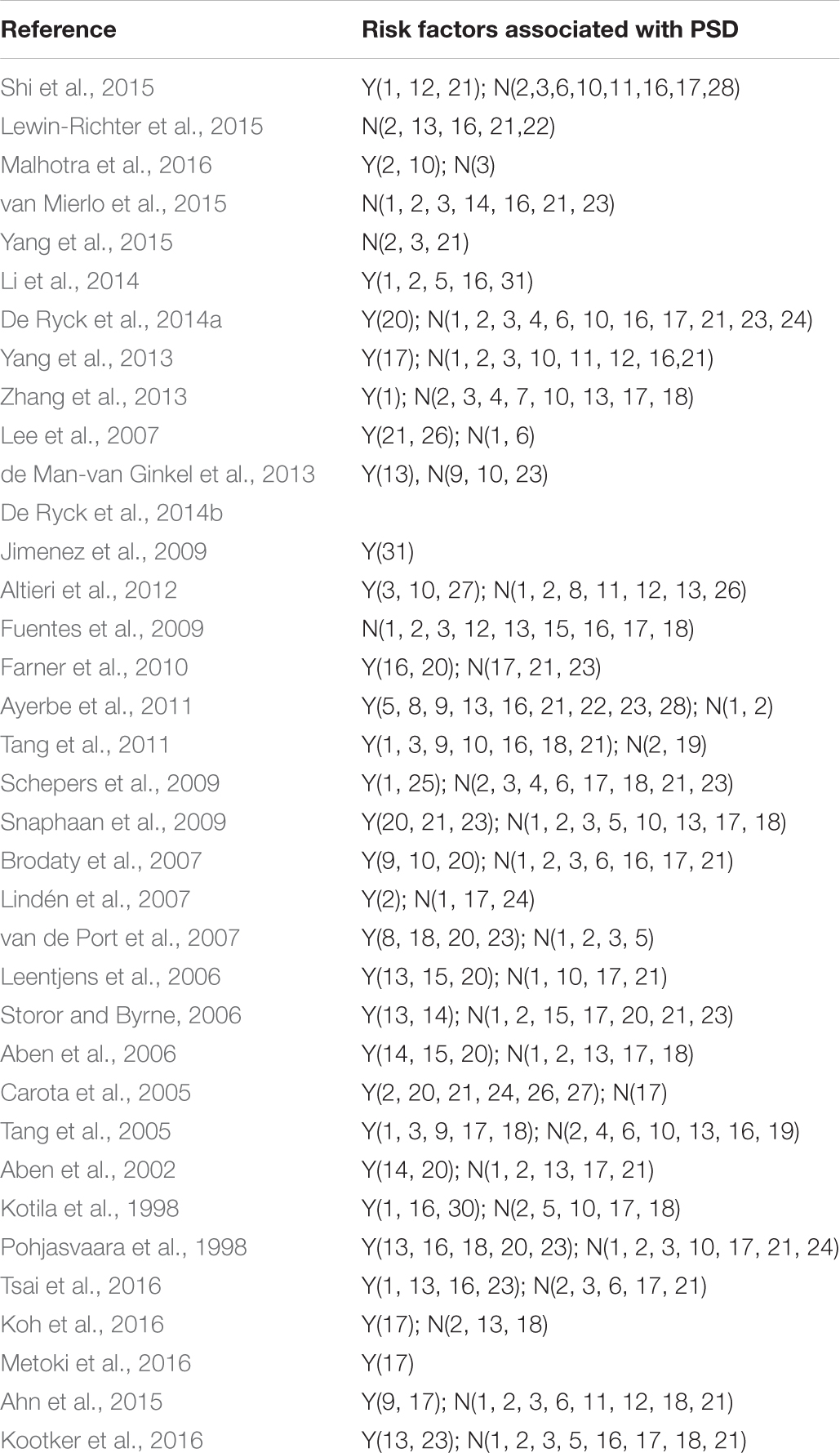
The selected studies included 14791 cases. Follow-up of studies was 2 weeks–15 years. Most of the included studies were combines with the ischemic stroke and hemorrhagic stroke. Tables 1, 2 shows the basic characteristics and risk factors for PSD of the included studies.
Meta-analysis Results
Sex
A total of eight articles reported sex (female) was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 2 shows that there is a high heterogeneity between the trials (χ2 = 27.03, I2= 70%). Therefore the random-effect model was used. Sex (female) was significant associated with PSD [OR = 1.77, 95% CI = 1.26–2.49], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
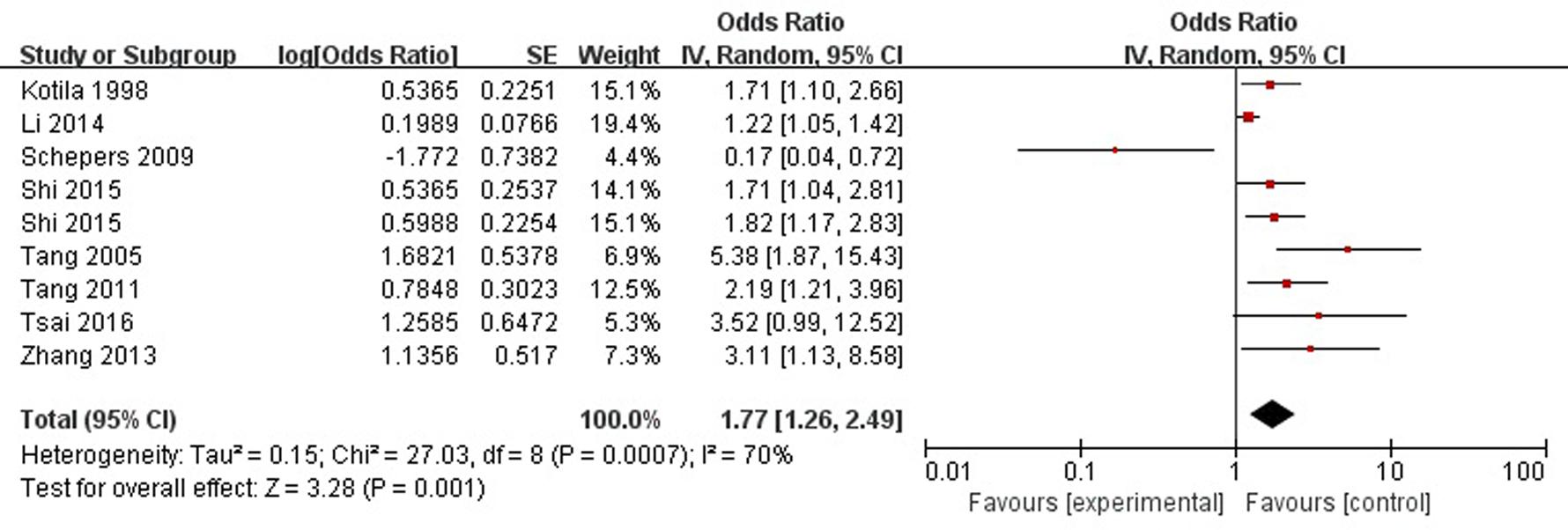
FIGURE 2. Meta-analysis of sex (female) and PSD risk in the acute stage and subacute stage (≤3 months).
Age
A total of two articles reported age (<70 years) was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 3 shows that there is a low heterogeneity between the trials (χ2 = 0.58, I2= 0%). Therefore the fixed-effect model was used. Age (<70 years) was significant associated with PSD [OR = 1.94, 95% CI = 1.36–2.79], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
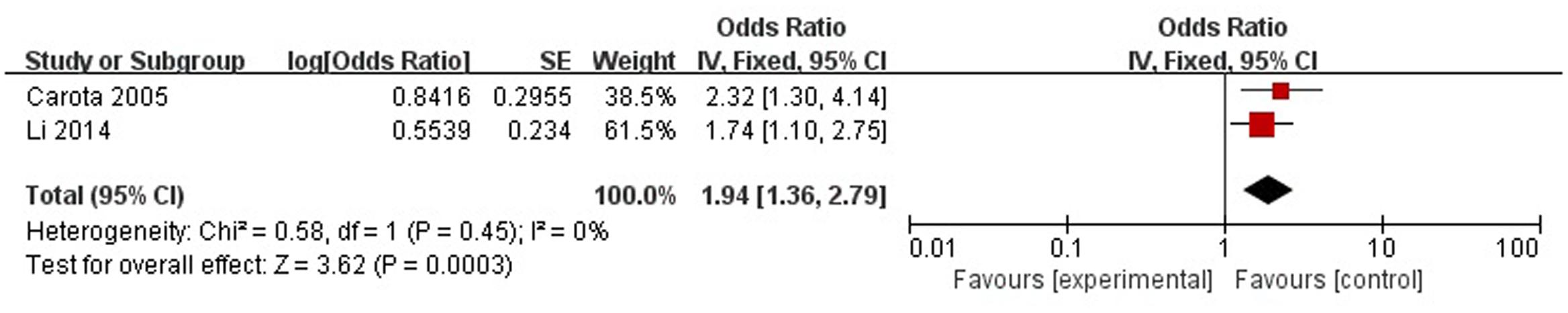
FIGURE 3. Meta-analysis of age (<70 years) and PSD risk in the acute stage and subacute stage (≤3 months).
Social Support
A total of three articles reported social support was a protective factor for PSD in the acute stage and subacute stage (≤3 months). Figure 4 shows that there is a high heterogeneity between the trials (χ2 = 3.84, I2= 48%). Therefore the random-effect model was used. Social support was associated with PSD [OR = 0.93, 95% CI = 0.87–0.99], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
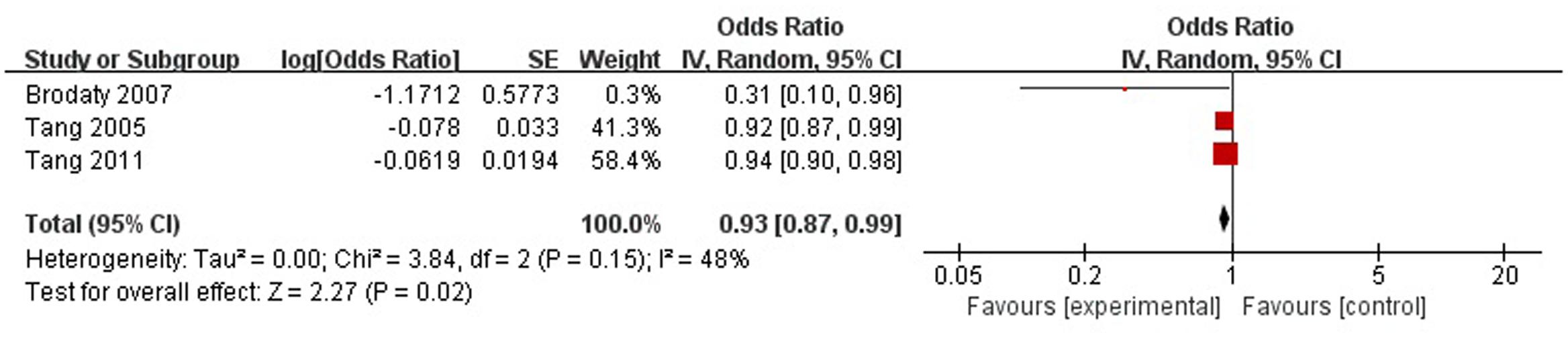
FIGURE 4. Meta-analysis of social support and PSD risk in the acute stage and subacute stage (≤3 months).
History of Mental Illness
A total of six articles reported history of mental illness (depression/anxiety/etc.) was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 5 shows that there is a high heterogeneity between the trials (χ2 = 48.55, I2= 90%). Therefore the random-effect model was used. Psychological history was significant associated with PSD [OR = 2.93, 95% CI = 1.42–6.05], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
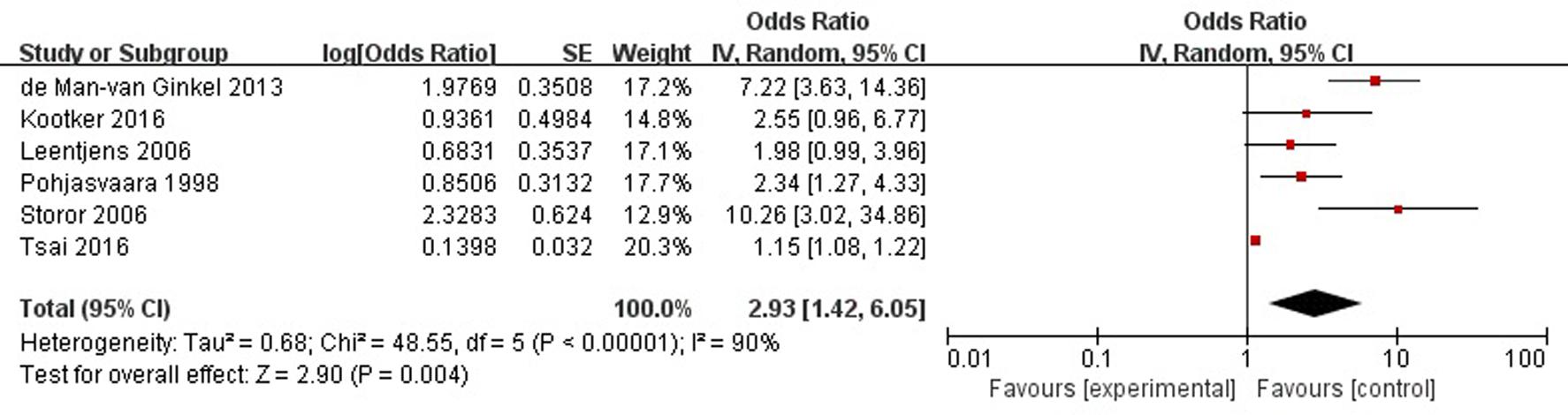
FIGURE 5. Meta-analysis of history of mental illness and PSD risk in the acute stage and subacute stage (≤3 months).
Neuroticism
A total of three articles reported neuroticism was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 6 shows that there is a high heterogeneity between the trials (χ2 = 4.93, I2= 59%). Therefore the random-effect model was used. Neuroticism was significant associated with PSD [OR = 1.08, 95% CI = 1.03–1.14], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
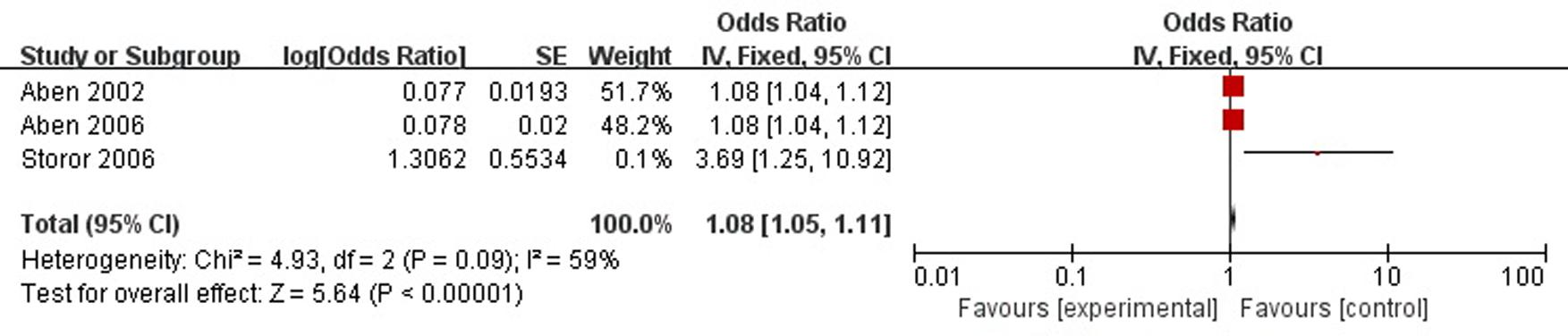
FIGURE 6. Meta-analysis of neuroticism and PSD risk in the acute stage and subacute stage (≤3 months).
Family History of Mental Illness
A total of two articles reported family history of mental illness was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 7 shows that there is a low heterogeneity between the trials (χ2 = 0.89, I2= 0%). Therefore the fixed-effect model was used. Family history was significant associated with PSD [OR = 1.95, 95% CI = 1.33–2.87], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
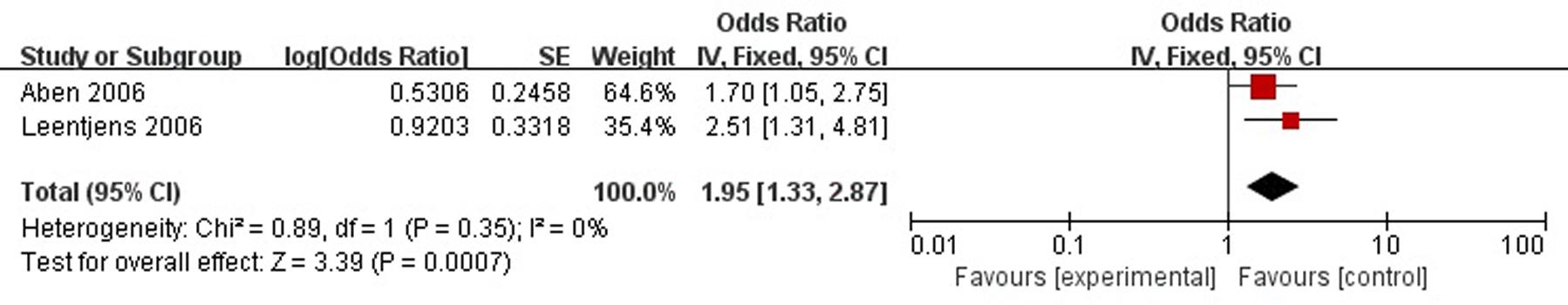
FIGURE 7. Meta-analysis of family history and PSD risk in the acute stage and subacute stage (≤3 months).
Severity of Stroke
A total of six articles reported severity of stroke was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 8 shows that there is a low heterogeneity between the trials (χ2 = 7.14, I2= 30%). Therefore the fixed-effect model was used. Severity of stroke was significant associated with PSD [OR = 1.12, 95% CI = 1.08–1.16], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
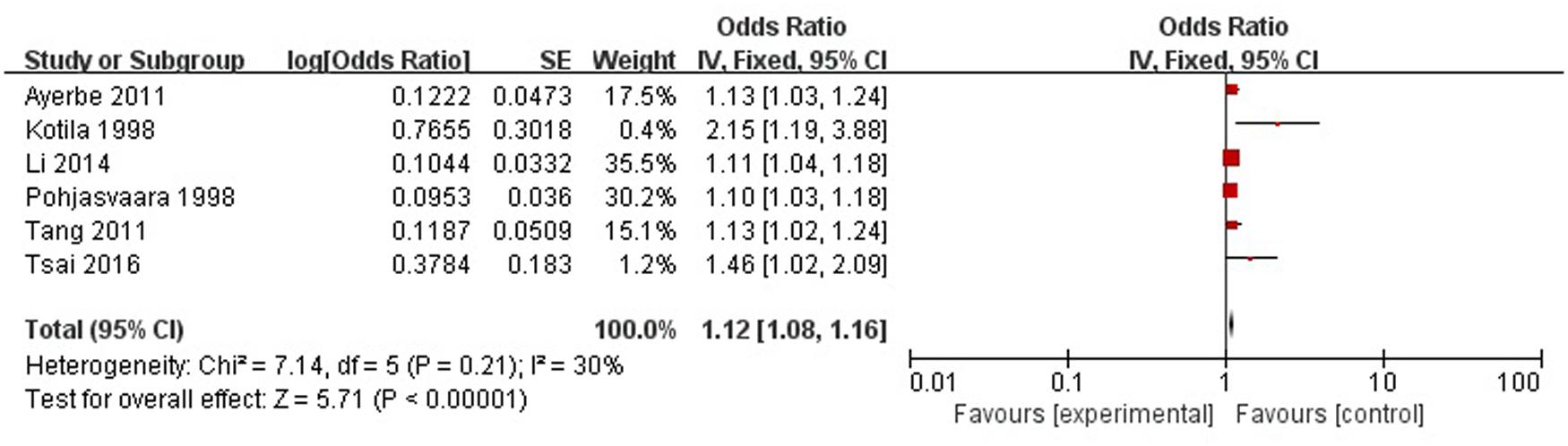
FIGURE 8. Meta-analysis of severity of stroke and PSD risk in the acute stage and subacute stage (≤3 months).
Level of Handicap
A total of four articles reported level of handicap was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 9 shows that there is a high heterogeneity between the trials (χ2 = 13.35, I2= 78%). Therefore the random-effect model was used. Level of handicap was significant associated with PSD [OR = 1.52, 95% CI = 1.32–1.75] in acute stage and subacute stage (≤3 months), but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
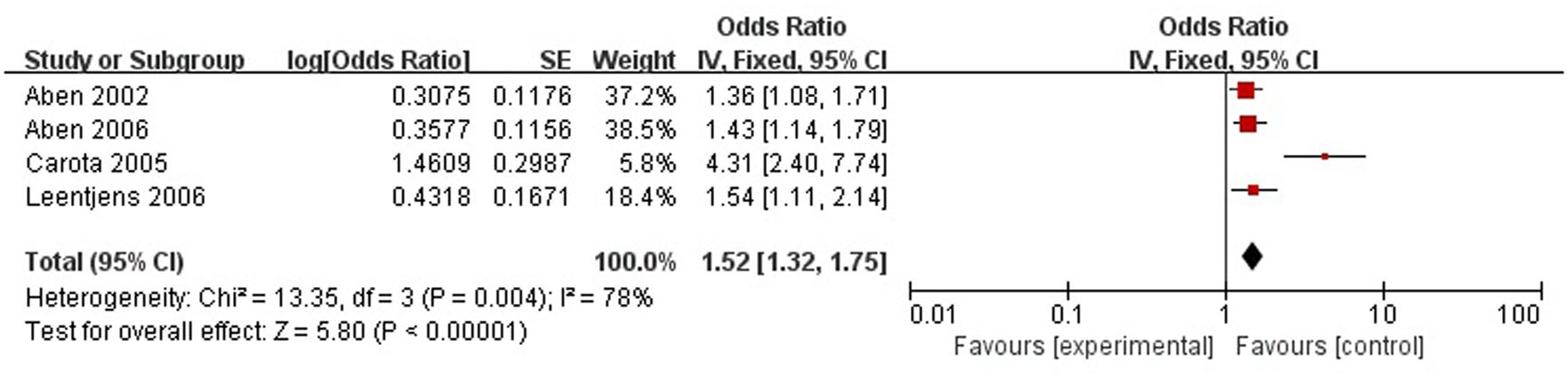
FIGURE 9. Meta-analysis of level of handicap and PSD risk in the acute stage and subacute stage (≤3 months).
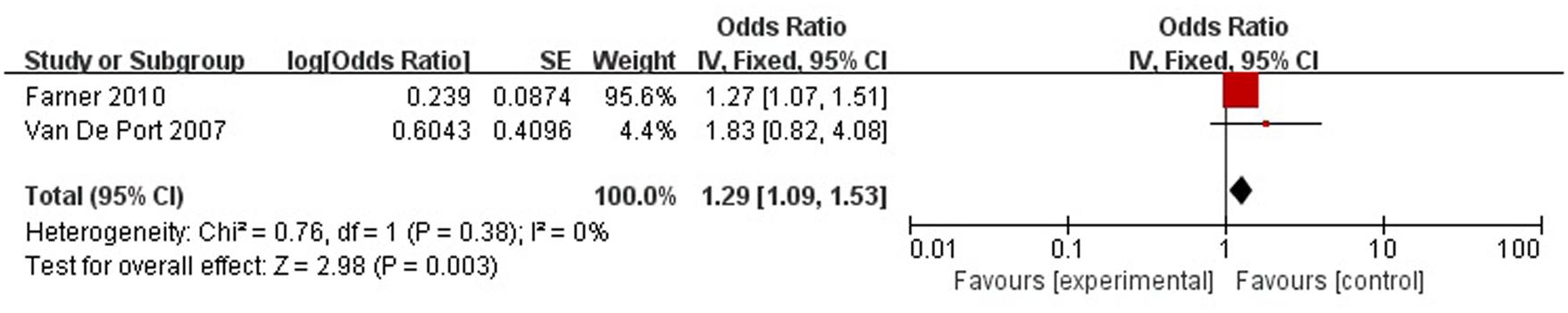
A total of two articles reported level of handicap was a risk factor for PSD in the recovery period (>3 months). Figure 10 shows that there is a low heterogeneity between the trials (χ2 = 0.76, I2= 0%). Therefore the fixed-effect model was used. Level of handicap was significant associated with PSD [OR = 1.29, 95% CI = 1.09–1.53] in recovery period, but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
Level of Independence
A total of five articles reported level of independence was a risk factor for PSD in the acute stage and subacute stage (≤3 months). Figure 11 shows that there is a high heterogeneity between the trials (χ2 = 19.02, I2= 79%). Therefore the random-effect model was used. Psychological history was significant associated with PSD [OR = 1.04, 95% CI = 0.87–1.24], but caution should be exercised while drawing conclusions. As a rule of thumb, tests for funnel plot asymmetry should only be used when there are at least 10 studies included in the meta-analysis, so we did not have a test for funnel plot asymmetry in this analysis.
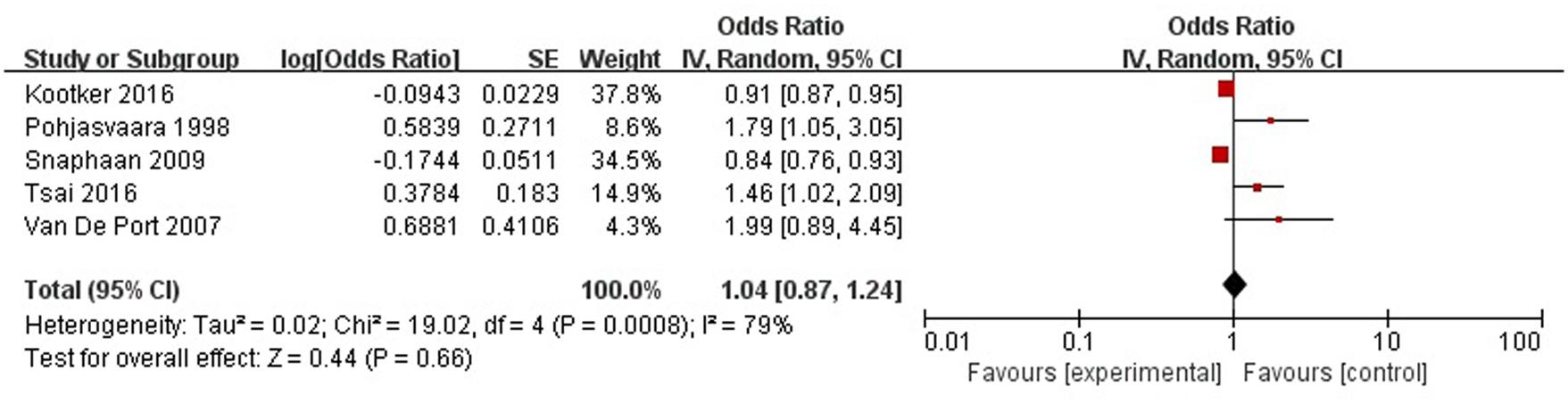
FIGURE 11. Meta-analysis of level of independence and PSD risk in the acute stage and subacute stage (≤3 months).
Other Data
Due to the limitations of some included studies, some data sets were insufficient to complete the meta-analysis (n < 2) and, in other studies, the original data could not be obtained. We show the unanalyzed data here, as follows:
Education
A total of four studies reported that education (>8 years) was a protective factor in the acute and subacute stages (≤3 months).
Predisposing Illness
Most of the included studies reported that predisposing illness, such as hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, and myocardial infarction, was not associated with PSD.
Location
In the included studies, the left hemisphere had an association with PSD in the acute and subacute stages. Brain damage in the left frontal lobe and left basal ganglia was associated with PSD.
Biochemical Factors
Most biochemical factors, such as interleukin-1β (IL-1β) and intercellular cell adhesion molecule-1 (ICAM-1) were not associated with PSD, but the levels of brain-derived neurotrophic factor (BDNF) and leptin were risk factors for PSD.
Discussion
Post-stroke depression is considered the most frequent and serious neuropsychiatric consequence of stroke (Cully et al., 2005). The reported prevalence of PSD varies widely, ranging from 25 to 79% (Gillen et al., 2001). Patients with PSD have more functional disability, poorer rehabilitation outcomes, and increased morbidity and mortality in the first year after stroke onset (Williams et al., 2004). Therefore, it is very important to find out the risk factors for PSD.
Views regarding the risk factors for PSD divide into two opposites: some propose a primary biological mechanism interpreting PSD (Li et al., 2014), whereas others claim that PSD is caused by social and psychological stressors owing to stroke (Farner et al., 2010). Besides some studies have come to different conclusions, for example, Tang et al. (2011) suggested that gender (female) was the important factor regarding PSD, while Ayerbe et al. (2011) failed to find this association. These controversies and other similar divergences all point toward the need for meta-analysis of this important topic.
This meta-analysis was conducted with 14,791 patients suffering from PSD. Many risk factors have been investigated over the last three decades; controversy exists concerning risk factors for the development of PSD. This meta-analysis has revealed that a history of mental disorders, stroke severity, gender and age are significantly associated with PSD.
History of Mental Disorders
Our meta-analysis showed that, in the acute and subacute stages (≤3 months), psychiatric history (i.e., depression, anxiety) was associated with PSD [OR = 3.95, 95% CI = 1.88–8.32], which suggests that a medical history of depression or other psychiatric disorders is one of the leading risk factors for PSD. Another study confirmed previous depressive episodes to be a predictor for PSD (Paolucci et al., 2005). Paolucci et al. (2006) found that, in the recovery period (>3 months), a psychiatric history was also associated with PSD (p < 0.001, χ2= 40.14) (Paolucci et al., 2006). In psychiatry, a mental illness may recur many times, and can lead to other mental illnesses (Burns et al., 2007). Accordingly, a history of psychiatric disorders could be a predisposing factor for PSD, simultaneously as a good indicator for PSD prevention.
Other major factors contributing to PSD are neuroticism and a family history of mental disorders. Our results suggest that neuroticism is strongly associated with PSD in the acute and subacute stages; this suggestion is supported by a study (Liu et al., 2015) indicating that high neuroticism conferred a greater risk of PSD (Andersen et al., 1995). Therefore, a personality assessment, as part of a screening test, could be useful for those at risk of developing depression. Moreover, both Aben et al. (2006) and Leentjens et al. (2006) showed that a family history of mental illness was associated with PSD. Mental illnesses are complex disorders resulting from the combined action of genetic and environmental factor. Thus, PSD may be explained by a genetic inheritance of family mental illness.
Stroke Characteristics
Brain damage caused by stroke is located mostly in the frontal lobe and basal ganglia where are highly correlated with emotional processing (Hornak et al., 1996; Pell and Leonard, 2003). Our meta-analysis revealed that stroke severity could be one of the most important risk factors for PSD, which conformed to the hypothesis that there may be an association between the extent of brain damage and depression (Vataja et al., 2004). Movement disorders, dysfunction, and life obstacles caused by brain damage could probably decline the self-confidence of patients and sudden stroke can also be regarded as a negative event for the sufferers, which might increase the incidence of depression. Consequently, stroke severity was identified as a vital factor for PSD because of its influence on the levels of handicap and independence that should remain as significant risk factors considered into the prevention of PSD.
There is a long-standing debate over the association between lesion locations and PSD. Robinson et al. (1984) and Starkstein and Robinson (1989) found that patients with left hemispheric lesions are more depressive than those with right hemispheric lesions and the severity of PSD is closely related to the extent of frontal lobe damage. But some other researches (Gainotti et al., 1999; Carson et al., 2000; Gainotti and Marra, 2002; Kutlubaev and Hackett, 2014; Wei et al., 2015) rejected this view, they suggested that lesion locations are not associated with PSD, and that the psychological model would play a role in the risk of PSD. In our study, we found that the damage of left hemisphere, especially in the left frontal lobe and left basal ganglia, had a close association with the extent of PSD in the acute and subacute stages, which suggested that the location of lesions do correlate with PSD. Our results are also supported by Vataja et al. (2001) and Rajashekaran et al. (2013). The possible reasons for this conjecture were that the left hemisphere is the dominant hemisphere responsible for positive emotions and language, and that the degree of neurological deficits in the left hemisphere is more serious in stroke patients according to the contrast of imaging data (Davidson and Irwin, 1999).
The frontal lobe and basal ganglia charge the heart of the emotional network (Jastorff et al., 2015), and these brain areas are more likely to change and then lead to depressive symptoms. At the same time, it is worth noting that using repetitive transcranial magnetic stimulation to stimulate focal brain is found more effective when it is administered to the left dorsolateral prefrontal cortex in patients with depression (Robinson and Jorge, 2016). This phenomenon also suggests that the prefrontal cortex plays an important role in depression. Of course, we cannot ignore the other phenomenon that in clinical observation, there are many patients with occipital lobe stroke or parietal lobe stroke having depressive symptoms but without obvious lesion locations in the emotional network. Accordingly, we should pay attention to the important role of psychological factors in the development of PSD (Gainotti et al., 1999, 2001). As we all know, stroke, as a serious stress event, is a heavy psychological blow to stroke patients. For instance, the neurologic impairment will cause a decline in or loss of the ability to work, which would make the stroke patients inevitably suffer from enormous social psychological pressure and finally lead to despair (Vallury et al., 2015; Eriksen et al., 2016). Therefore, psychological model is also play a role in the risk of PSD. Apart from stroke, serious diseases such as chronic heart disease and spinal cord injury, can also cause great physical and mental trauma leading to serious problems and a heavy family burden. However, there were statistics have shown that the incidence of depressive symptoms of the above diseases was lower than that of stroke (Fedoroff et al., 1991), which suggests that the psychological model just plays a part in the onset of PSD. Above all, we believe that stroke accompanying with the brain damage in the areas of the dominant emotional hemisphere or the emotional circuit, is more likely to occur depressive symptoms; and psychological factors also play an important role in the development of PSD. The differences found in previous findings are closely related to the design of this study. The different definitions of stroke, the difference in the imaging equipment, and the high heterogeneity of the subjects comprehensively lead to different results. A study with a large sample size, strict design, and unified diagnostic standards should be able to solve the above contradictions.
Personal and Social Factors
Our meta-analysis found that female gender was a significant risk factor for PSD in the acute and subacute stages, which was supported by previous studies. Paolucci et al. (2006) and Schepers et al. (2009) similarly suggested that gender was associated with PSD in the recovery period. Some studies considered that hormonal changes perhaps play a role in the occurrence of PSD because of their impacts on the moods of female patients (Beekman et al., 1999; Katona and Livingston, 2000). Stroke is a serious disease and a heavy blow to the patients as well. Women’s coping methods dealing with this situation are relatively inadequate and their psychological quality is also poor (Stewart et al., 2001; Berg et al., 2003; Verdelho et al., 2004). These help to explain why older women, especially those living alone, are more likely to get PSD. Moreover, possibly due to the decreased mental capacity and the slower recovery those reducing the quality of life and increasing mental pressure, the occurrence of depression may be more common in the middle-aged stroke patients than that in the elderly ones (>70 years), which has been shown in the included studies. It seems to be more difficult for the middle-aged (<70 years) to face their physical disorders and work capacity loss caused by the disease.
In the early stage of stroke, sudden behavioral disorders lead to a reduction in the number of patients engaging in social interactions (Boden-Albala et al., 2005). As social isolation has a negative impact on general health, it might play a role in the association with PSD (Tomaka et al., 2006). Limited activity gradually affects the mood and self-confidence of the patient; in their times of difficulty and stress, strong family and social support is extremely helpful and of importance for them, as it may contribute to improve their motivation adjusting to the disability and arouse their enthusiasm participating in social activities (Tsouna-Hadjis et al., 2000; Ayerbe et al., 2013; Northcott, 2013).
Patients with long-term education may develop better self-adjustment abilities (Ellen and Stéphan, 2013), which play a role in accommodating the change of PSD. Our meta-analysis revealed that living conditions at the time of stroke (alone/with family) were not associated with depression, so were housing conditions. In some studies (Robinson and Price, 1982), living alone did not predict depression, but in one study (Astrom et al., 1993) an association was found.
Other Factors
Our meta-analysis showed that the level of handicap was associated with PSD both in the acute and subacute stages and the recovery period. Burvill et al. (1997) and De Ryck et al. (2014a) and also suggested that the level of handicap was a risk factor for PSD in the recovery period. The level of handicap reflects the degree of disability, as well as the degree of brain damage. A high level of handicap would seriously impact the patients on their life and work, leading to great physiological and psychological trauma, and eventually results in PSD. Therefore, the level of handicap could also be a good indicator for PSD prevention.
It cannot be ignored that secondary neurodegeneration has a serious impact on PSD (Loubinoux et al., 2012). In secondary neurodegeneration, nerve damage after focal cerebral infarction not only affect the local lesions, but also affect the nerve fibers in remote brain areas (Liu et al., 2012). The axons connecting with the infarct neurons will have Wallerian degeneration and cause neuronal loss in remote brain areas (Dihne et al., 2002). Robinson and Bloom (1977) suggested that dysfunction of the (cortico)-striato-pallido-thalamic-cortical circuit would lead to depressive symptoms. Due to the influence of secondary neurodegeneration, even the stroke lesion is not included in the emotional circuit; this circuit could also be affected by the remote cerebral area. Though the thalamus and substantia nigra do not belong to the blood supply area of the middle cerebral artery (MCA), studies (Nakane et al., 1997; Zhao et al., 2001) have shown that MCA occlusion (MCAO) still affects excitability in the brain areas of the thalamus and substantia nigra, resulting in mood disorders. In addition, secondary neurodegeneration can also cause the release of inflammatory factors (Block et al., 2005) and aggravate the inflammatory response of remote cerebral area, which would play a role in the risk of PSD.
Robinson et al. (1986) proposed a theory about primary biological mechanism of PSD, following that more and more researchers focused on the biological principles of PSD. Li et al. suggested that inflammatory factors such as interleukin 1 (IL-1), interleukin 6 (IL-6), and interferon gamma (IFNγ), were important factors responsible for PSD (Li et al., 2014). In addition, other inflammatory factors, such as tumor necrosis factor alpha (TNF-α) (Spalletta et al., 2006), interleukin-8 (IL-8) (Spalletta et al., 2006), interleukin-18 (IL-18) (Kang et al., 2016), and high-sensitivity C-reactive protein (Hs-CRP) (Cheng et al., 2017) have also been found to play an important role in the onset of PSD. Under the stress of stroke, inflammatory factors increase dramatically. There are several plausible mechanisms for this association. First, IL-18 could induce the expression of indoleamine 2,3-dioxygenase (IDO), and overexpression of IDO can cause the depletion of 5-hydroxytryptamine (5-HT), leading to depressive symptoms (Maes et al., 2002; Spalletta et al., 2006). Second, Hs-CRP could increase the hyperintensity burden of white matter, which leads to depressive symptoms (Schmidt et al., 2011). At the same time, Cheng et al. (2017) suggested that homocysteine (HCY) also play a role in the risk of PSD, and overexpression HCY produced by stroke could affect the expression of BDNF (Folstein et al., 2007; Obeid et al., 2007). As an important nutrient for neurons, decreased release of BDNF can cause hippocampal atrophy and reduce synaptic plasticity (Failla et al., 2016), leading to the occurrence of depression. At present, the neurobiological mechanisms of PSD remain unclear, but the inflammatory factors and other biochemical factors [i.e., BDNF (Chen et al., 2005) and leptin (Söderberg et al., 1999)], provide new directions for the prediction of PSD.
Limitations
Although there were 36 studies included in our analysis, most primary data from these examined studies were not available, thus very little data could be effectively used in our analysis. For the same reason, we could not carry out subgroup analysis. Furthermore, the reliability of the selected studies was reduced due to the high heterogeneity of the data while most of the meta-analyses concerned the acute and subacute stages. Consequently, the data reflecting the outcomes in the recovery period were insufficient. In the included literature, there were some differences in the experimental designs and most studies included both ischemic stroke and hemorrhagic stroke, which could have affected the quality of the meta-analysis. To prove the significant advantages, large-scale, multiple-term, and high-quality studies are necessary.
Conclusion
There are many factors that have effects on PSD. The severity of stroke is an important factor in the occurrence of PSD. Mental history is a possible predictor of PSD. Prevention of PSD requires social and family participation.
Author Contributions
The literature was screened and methodological quality was assessed independently by YS and DY. WW provided overall expertise on post-stroke depression. YS and YZ performed the meta-analysis. YS drafted the manuscript. All authors approved the final version and agree to be accountable for this work.
Funding
This work was supported by National Natural Science Foundation of China (NNSFC), China; Contract grant number: 81473769; Natural Science Foundation of Guangdong Province, China; Contract grant number: 2014A030313335; Clinical Research Foundation of Southern Medical University, China; Contract grant number: LC2016PY037.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
A (personal and social factors): 1(sex), 2(age), 3(education), 4(employment), 5(alone), 6(marital status), 7(housing conditions), 8(social activities), 9(lack of family and social support); B (medical history): 10(predisposing illness), 11(alcohol abuse), 12(cigarette smoking); C (history of mental disorders): 13(mental disease), 14(neuroticism), 15(family history of disease); D (stroke characteristics): 16(severity), 17(location), 18(type); E (impairments): 19(physical condition before stroke), 20(level of handicap), 21(cognition), 22(Inability to work), 23(level of independence); F (neurocognitive outcome measures): 24(aphasia/dysphasia), 25(neglect); G (others): 26(overt sadness), 27(crying), 28(use of antidepressive at baseline), 29 (length of hospital stay), 30(active rehab program), 31(biochemical factors). Y, significant risk factors; N, no significant risk factors.
Footnotes
References
Aben, I., Denollet, J., Lousberg, R., Verhey, F., Wojciechowski, F., and Honig, A. (2002). Personality and vulnerability to depression in stroke patients A 1-year prospective follow-up study. Stroke 33, 2391–2395. doi: 10.1161/01.STR.0000029826.41672.2E
Aben, I., Lodder, J., Honig, A., Lousberg, R., Boreas, A., and Verhey, F. (2006). Focal or generalized vascular brain damage and vulnerability to depression after stroke: a 1-year prospective follow-up study. Int. Psychogeriatr. 18, 19–35. doi: 10.1017/S104161020500270X
Ahn, D. H., Lee, Y. J., Jeong, J. H., Kim, Y. R., and Park, J. B. (2015). The effect of post-stroke depression on rehabilitation outcome and the impact of caregiver type as a factor of post-stroke depression. Ann. Rehabil. Med. 39, 74–80. doi: 10.5535/arm.2015.39.1.74
Altieri, M., Maestrini, I., Mercurio, A., Troisi, P., Sgarlata, E., Rea, V., et al. (2012). Depression after minor stroke: prevalence and predictors. Eur. J. Neurol. 19, 517–521. doi: 10.1111/j.1468-1331.2011.03583.x
American Psychiatric Association (2000). Diagnostic and Statistical Manual-Text Revision (DSM-IV-TRim, 2000). Arlington, VA: American Psychiatric Association.
Andersen, G., Vestergaard, K., Ingemann-Nielsen, M., and Lauritzen, L. (1995). Risk factors for post-stroke depression. Acta Psychiatr. Scand. 92, 193–198. doi: 10.1111/j.1600-0447.1995.tb09567.x
Astrom, M., Olsson, T., and Asplund, K. (1993). Different linkage of depression to hypercortisolism early versus late after stroke. A 3-year longitudinal study. Stroke 24, 52–57. doi: 10.1161/01.STR.24.1.52
Ayerbe, L., Ayis, S., Rudd, A. G., Heuschmann, P. U., and Wolfe, C. D. (2011). Natural history, predictors, and associations of depression 5 years after stroke: the South London Stroke Register. Stroke 42, 1907–1911. doi: 10.1161/STROKEAHA.110.605808
Ayerbe, L., Ayis, S., Wolfe, C. D., and Rudd, A. G. (2013). Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br. J. Psychiatry 202, 14–21. doi: 10.1192/bjp.bp.111.107664
Beekman, A. T., Copeland, J. R., and Prince, M. J. (1999). Review of community prevalence of depression in later life. Br. J. Psychiatry 174, 307–311. doi: 10.1192/bjp.174.4.307
Berg, A., Palomäki, H., Lehtihalmes, M., Lönnqvist, J., and Kaste, M. (2003). Poststroke depression an 18-month follow-up. Stroke 34, 138–143. doi: 10.1161/01.STR.0000048149.84268.07
Block, F., Dihne, M., and Loos, M. (2005). Inflammation in areas of remote changes following focal brain lesion. Prog. Neurobiol. 75, 342–365. doi: 10.1016/j.pneurobio.2005.03.004
Boden-Albala, B., Litwak, E., Elkind, M., Rundek, T., and Sacco, R. (2005). Social isolation and outcomes post stroke. Neurology 64, 1888–1892. doi: 10.1212/01.WNL.0000163510.79351.AF
Brodaty, H., Withall, A., Altendorf, A., and Sachdev, P. S. (2007). Rates of depression at 3 and 15 months poststroke and their relationship with cognitive decline: the Sydney Stroke Study. Am. J. Geriatr. Psychiatry 15, 477–486. doi: 10.1097/JGP.0b013e3180590bca
Burns, T., Catty, J., Dash, M., Roberts, C., Lockwood, A., and Marshall, M. (2007). Use of intensive case management to reduce time in hospital in people with severe mental illness: systematic review and meta-regression. BMJ 335, 336. doi: 10.1136/bmj.39251.599259.55
Burvill, P., Johnson, G., Jamrozik, K., Anderson, C., and Stewart-Wynne, E. (1997). Risk factors for post-stroke depression. Int. J. Geriatr. Psychiatry 12, 219–226. doi: 10.1002/(SICI)1099-1166(199702)12:2<219::AID-GPS581>3.0.CO;2-E
Carota, A., Berney, A., Aybek, S., Iaria, G., Staub, F., Ghika-Schmid, F., et al. (2005). A prospective study of predictors of poststroke depression. Neurology 64, 428–433. doi: 10.1212/01.WNL.0000150935.05940.2D
Carson, A. J., MacHale, S., Allen, K., Lawrie, S. M., Dennis, M., House, A., et al. (2000). Depression after stroke and lesion location: a systematic review. Lancet 356, 122–126. doi: 10.1016/S0140-6736(00)02448-X
Chen, J., Zhang, C., Jiang, H., Li, Y., Zhang, L., Robin, A., et al. (2005). Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J. Cereb. Blood Flow Metab. 25, 281–290. doi: 10.1038/sj.jcbfm.9600034
Cheng, L. S., Tu, W. J., Shen, Y., Zhang, L. J., and Ji, K. (2017). Combination of high-sensitivity C-reactive protein and homocysteine predicts the post-stroke depression in patients with ischemic stroke. Mol. Neurobiol. doi: 10.1007/s12035-017-0549-8 [Epub ahead of print].
Cully, J. A., Gfeller, J. D., Heise, R. A., Ross, M. J., Teal, C. R., and Kunik, M. E. (2005). Geriatric depression, medical diagnosis, and functional recovery during acute rehabilitation. Arch. Phys. Med. Rehabil. 86, 2256–2260. doi: 10.1016/j.apmr.2005.07.292
Davidson, R. J., and Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3, 11–21. doi: 10.1016/S1364-6613(98)01265-0
de Man-van Ginkel, J. M., Hafsteinsdottir, T. B., Lindeman, E., Ettema, R. G., Grobbee, D. E., and Schuurmans, M. J. (2013). In-hospital risk prediction for post-stroke depression: development and validation of the Post-stroke Depression Prediction Scale. Stroke 44, 2441–2445. doi: 10.1161/STROKEAHA.111.000304
De Ryck, A., Fransen, E., Brouns, R., Geurden, M., Peij, D., Mariën, P., et al. (2014a). Psychosocial problems associated with depression at 18 months poststroke. Int. J. Geriatr. Psychiatry 29, 144–152. doi: 10.1002/gps.3974
De Ryck, A., Fransen, E., Brouns, R., Geurden, M., Peij, D., Marien, P., et al. (2014b). Poststroke depression and its multifactorial nature: results from a prospective longitudinal study. J. Neurol. Sci. 347, 159–166. doi: 10.1016/j.jns.2014.09.038
Dihne, M., Grommes, C., Lutzenburg, M., Witte, O. W., and Block, F. (2002). Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke 33, 3006–3011. doi: 10.1161/01.STR.0000039406.64644.CB
Ellen, W., and Stéphan, V.-L. (2013). Educational Research and Innovation Art for Art’s Sake? The Impact of Arts Education: The Impact of Arts Education. Paris: OECD Publishing.
Eriksen, S., Gay, C. L., and Lerdal, A. (2016). Acute phase factors associated with the course of depression during the first 18 months after first-ever stroke. Disabil. Rehabil. 38, 30–35. doi: 10.3109/09638288.2015.1009181
European Stroke Initiative Executive EUSI Writing Committee Olsen, T. S., Langhorne, P., Diener, H. C., Hennerici, M., et al. (2003). European stroke initiative recommendations for stroke management-update 2003. Cerebrovasc. Dis. 16, 311–337.
Failla, M. D., Juengst, S. B., Arenth, P. M., and Wagner, A. K. (2016). Preliminary associations between brain-derived neurotrophic factor, memory impairment, functional cognition, and depressive symptoms following severe TBI. Neurorehabil. Neural Repair 30, 419–430. doi: 10.1177/1545968315600525
Farner, L., Wagle, J., Engedal, K., Flekkoy, K. M., Wyller, T. B., and Fure, B. (2010). Depressive symptoms in stroke patients: a 13 month follow-up study of patients referred to a rehabilitation unit. J. Affect. Disord. 127, 211–218. doi: 10.1016/j.jad.2010.05.025
Fedoroff, J. P., Lipsey, J. R., Starkstein, S. E., Forrester, A., Price, T. R., and Robinson, R. G. (1991). Phenomenological comparisons of major depression following stroke, myocardial infarction or spinal cord lesions. J. Affect. Disord. 22, 83–89. doi: 10.1016/0165-0327(91)90088-A
Folstein, M., Liu, T., Peter, I., Buell, J., Arsenault, L., Scott, T., et al. (2007). The homocysteine hypothesis of depression. Am. J. Psychiatry 164, 861–867. doi: 10.1176/ajp.2007.164.6.861
Fuentes, B., Ortiz, X., Sanjose, B., Frank, A., and Diez-Tejedor, E. (2009). Post-stroke depression: can we predict its development from the acute stroke phase? Acta Neurol. Scand. 120, 150–156. doi: 10.1111/j.1600-0404.2008.01139.x
Gainotti, G., Antonucci, G., Marra, C., and Paolucci, S. (2001). Relation between depression after stroke, antidepressant therapy, and functional recovery. J. Neurol. Neurosurg. Psychiatry 71, 258–261. doi: 10.1136/jnnp.71.2.258
Gainotti, G., Azzoni, A., and Marra, C. (1999). Frequency, phenomenology and anatomical-clinical correlates of major post-stroke depression. Br. J. Psychiatry 175, 163–167. doi: 10.1192/bjp.175.2.163
Gainotti, G., and Marra, C. (2002). Determinants and consequences of post-stroke depression. Curr. Opin. Neurol. 15, 85–89. doi: 10.1097/00019052-200202000-00013
Gillen, R., Tennen, H., McKee, T. E., Gernert-Dott, P., and Affleck, G. (2001). Depressive symptoms and history of depression predict rehabilitation efficiency in stroke patients. Arch. Phys. Med. Rehabil. 82, 1645–1649. doi: 10.1053/apmr.2001.26249
Hackett, M. L., and Pickles, K. (2014). Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int. J. Stroke 9, 1017–1025. doi: 10.1111/ijs.12357
Higgins, J., and Green, S. (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0, The Cochrane Collaboration. Available at: training.cochrane.org/handbook [accessed November 14, 2011]. doi: 10.1002/14651858.CD008754.pub2
Hornak, J., Rolls, E., and Wade, D. (1996). Face and voice expression identification in patients with emotional and behavioural changes following ventral frontal lobe damage. Neuropsychologia 34, 247–261. doi: 10.1016/0028-3932(95)00106-9
Jastorff, J., Huang, Y. A., Giese, M. A., and Vandenbulcke, M. (2015). Common neural correlates of emotion perception in humans. Hum. Brain Mapp. 36, 4184–4201. doi: 10.1002/hbm.22910
Jiang, X., Lin, Y., and Li, Y. (2014). Correlative study on risk factors of depression among acute stroke patients. Eur. Rev. Med. Pharmacol. Sci. 18, 1315–1323.
Jimenez, I., Sobrino, T., Rodriguez-Yanez, M., Pouso, M., Cristobo, I., Sabucedo, M., et al. (2009). High serum levels of leptin are associated with post-stroke depression. Psychol. Med. 39, 1201–1209. doi: 10.1017/S0033291709005637
Kang, H. J., Bae, K. Y., Kim, S. W., Kim, J. T., Park, M. S., Cho, K. H., et al. (2016). Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology 72, 156–160. doi: 10.1016/j.psyneuen.2016.07.001
Katona, C., and Livingston, G. (2000). Impact of screening old people with physical illness for depression? Lancet 356, 91–92. doi: 10.1016/S0140-6736(00)02466-1
Koh, D. J., Kim, N. Y., and Kim, Y. W. (2016). Predictors of depressive mood in patients with isolated cerebellar stroke: a retrospective study. Ann. Rehabil. Med. 40, 412–419. doi: 10.5535/arm.2016.40.3.412
Kootker, J. A., van Mierlo, M. L., Hendriks, J. C., Sparidans, J., Rasquin, S. M., de Kort, P. L., et al. (2016). Risk factors for symptoms of depression and anxiety one year poststroke: a longitudinal study. Arch. Phys. Med. Rehabil. 97, 919–928. doi: 10.1016/j.apmr.2016.01.019
Kotila, M., Numminen, H., Waltimo, O., and Kaste, M. (1998). Depression after stroke: results of the FINNSTROKE Study. Stroke 29, 368–372. doi: 10.1161/01.STR.29.2.368
Kutlubaev, M. A., and Hackett, M. L. (2014). Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int. J. Stroke 9, 1026–1036. doi: 10.1111/ijs.12356
Lee, A. C., Tang, S. W., Yu, G. K., and Cheung, R. T. (2007). Incidence and predictors of depression after stroke (DAS). Int. J. Psychiatry Clin. Pract. 11, 200–206. doi: 10.1080/13651500601091212
Leentjens, A. F., Aben, I., Lodder, J., and Verhey, F. R. (2006). General and disease-specific risk factors for depression after ischemic stroke: a two-step Cox regression analysis. Int. Psychogeriatr. 18, 739–748. doi: 10.1017/S1041610206003486
Lewin-Richter, A., Volz, M., Jobges, M., and Werheid, K. (2015). Predictivity of early depressive symptoms for post-stroke depression. J. Nutr. Health Aging 19, 754–758. doi: 10.1007/s12603-015-0540-x
Li, J., Zhao, Y. D., Zeng, J. W., Chen, X. Y., Wang, R. D., and Cheng, S. Y. (2014). Serum Brain-derived neurotrophic factor levels in post-stroke depression. J. Affect. Disord. 168, 373–379. doi: 10.1016/j.jad.2014.07.011
Lindén, T., Blomstrand, C., and Skoog, I. (2007). Depressive disorders after 20 months in elderly stroke patients a case-control study. Stroke 38, 1860–1863. doi: 10.1161/STROKEAHA.106.471805
Liu, R., Yue, Y., Jiang, H., Lu, J., Yuan, Y., and Wang, Q. (2015). A risk prediction model of PSD in stroke survivors. Eur. Psychiatry 30, 712. doi: 10.1016/s0924-9338(15)30562-9
Liu, X., Tian, W., Li, L., Kolar, B., Qiu, X., Chen, F., et al. (2012). Hyperintensity on diffusion weighted image along ipsilateral cortical spinal tract after cerebral ischemic stroke: a diffusion tensor analysis. Eur. J. Radiol. 81, 292–297. doi: 10.1016/j.ejrad.2010.12.053
Loubinoux, I., Kronenberg, G., Endres, M., Schumann-Bard, P., Freret, T., Filipkowski, R. K., et al. (2012). Post-stroke depression: mechanisms, translation and therapy. J. Cell Mol. Med. 16, 1961–1969. doi: 10.1111/j.1582-4934.2012.01555.x
Maes, M., Verkerk, R., Bonaccorso, S., Ombelet, W., Bosmans, E., and Scharpe, S. (2002). Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 71, 1837–1848. doi: 10.1016/S0024-3205(02)01853-2
Malhotra, R., Chei, C.-L., Menon, E., Chow, W. L., Quah, S., Chan, A., et al. (2016). Short-term trajectories of depressive symptoms in stroke survivors and their family caregivers. J. Stroke Cerebrovasc. Dis. 25, 172–181. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.012
Metoki, N., Sugawara, N., Hagii, J., Saito, S., Shiroto, H., Tomita, T., et al. (2016). Relationship between the lesion location of acute ischemic stroke and early depressive symptoms in Japanese patients. Ann. Gen. Psychiatry 15, 12. doi: 10.1186/s12991-016-0099-x
Mozaffarian, D., Benjamin, E. J., Go, A. S., Arnett, D. K., Blaha, M. J., Cushman, M., et al. (2016). Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38–e360. doi: 10.1161/CIR.0000000000000350
Nakane, M., Tamura, A., Nagaoka, T., and Hirakawa, K. (1997). MR detection of secondary changes remote from ischemia: preliminary observations after occlusion of the middle cerebral artery in rats. Am. J. Neuroradiol. 18, 945–950.
Obeid, R., McCaddon, A., and Herrmann, W. (2007). The role of hyperhomocysteinemia and B-vitamin deficiency in neurological and psychiatric diseases. Clin. Chem. Lab. Med. 45, 1590–1606. doi: 10.1515/CCLM.2007.356
Paolucci, S., Gandolfo, C., Provinciali, L., Torta, R., Sommacal, S., Toso, V., et al. (2005). Quantification of the risk of post stroke depression: the Italian multicenter observational study DESTRO. Acta Psychiatr. Scand. 112, 272–278. doi: 10.1111/j.1600-0447.2005.00590.x
Paolucci, S., Gandolfo, C., Provinciali, L., Torta, R., Toso, V., and Group, D. S. (2006). The Italian multicenter observational study on post-stroke depression (DESTRO). J. Neurol. 253, 556–562. doi: 10.1007/s00415-006-0058-6
Pell, M. D., and Leonard, C. L. (2003). Processing emotional tone from speech in Parkinson’s disease: a role for the basal ganglia. Cogn. Affect. Behav. Neurosci. 3, 275–288. doi: 10.3758/CABN.3.4.275
Pohjasvaara, T., Leppavuori, A., Siira, I., Vataja, R., Kaste, M., and Erkinjuntti, T. (1998). Frequency and clinical determinants of poststroke depression. Stroke 29, 2311–2317. doi: 10.1161/01.STR.29.11.2311
Rajashekaran, P., Pai, K., Thunga, R., and Unnikrishnan, B. (2013). Post-stroke depression and lesion location: a hospital based cross-sectional study. Indian J. Psychiatry 55, 343–348. doi: 10.4103/0019-5545.120546
Robinson, R. G., and Bloom, F. E. (1977). Pharmacological treatment following experimental cerebral infarction: implications for understanding psychological symptoms of human stroke. Biol. Psychiatry 12, 669–680.
Robinson, R. G., and Jorge, R. E. (2016). Post-stroke depression: a review. Am. J. Psychiatry 173, 221–231. doi: 10.1176/appi.ajp.2015.15030363
Robinson, R. G., Kubos, K. L., Starr, L. B., Rao, K., and Price, T. R. (1984). Mood disorders in stroke patients. Importance of location of lesion. Brain 107(Pt 1), 81–93. doi: 10.1093/brain/107.1.81
Robinson, R. G., Lipsey, J. R., Rao, K., and Price, T. R. (1986). Two-year longitudinal study of post-stroke mood disorders: comparison of acute-onset with delayed-onset depression. Am. J. Psychiatry 143, 1238–1244. doi: 10.1176/ajp.143.10.1238
Robinson, R. G., and Price, T. R. (1982). Post-stroke depressive disorders: a follow-up study of 103 patients. Stroke 13, 635–641. doi: 10.1161/01.STR.13.5.635
Schepers, V., Post, M., Visser-Meily, A., van de Port, I., Akhmouch, M., and Lindeman, E. (2009). Prediction of depressive symptoms up to three years post-stroke. J. Rehabil. Med. 41, 930–935. doi: 10.2340/16501977-0446
Schmidt, H. D., Shelton, R. C., and Duman, R. S. (2011). Functional biomarkers of depression: diagnosis, treatment, and pathophysiology. Neuropsychopharmacology 36, 2375–2394. doi: 10.1038/npp.2011.151
Shi, Y., Xiang, Y., Yang, Y., Zhang, N., Wang, S., Ungvari, G. S., et al. (2015). Depression after minor stroke: prevalence and predictors. J. Psychosom. Res. 79, 143–147. doi: 10.1016/j.jpsychores.2015.03.012
Snaphaan, L., van der Werf, S., Kanselaar, K., and de Leeuw, F. E. (2009). Post-stroke depressive symptoms are associated with post-stroke characteristics. Cerebrovasc. Dis. 28, 551–557. doi: 10.1159/000247598
Söderberg, S., Ahrén, B., Stegmayr, B., Johnson, O., Wiklund, P.-G., Weinehall, L., et al. (1999). Leptin is a risk marker for first-ever hemorrhagic stroke in a population-based cohort. Stroke 30, 328–337. doi: 10.1161/01.STR.30.2.328
Spalletta, G., Bossu, P., Ciaramella, A., Bria, P., Caltagirone, C., and Robinson, R. G. (2006). The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol. Psychiatry 11, 984–991. doi: 10.1038/sj.mp.4001879
Starkstein, S. E., and Robinson, R. G. (1989). Affective disorders and cerebral vascular disease. Br. J. Psychiatry 154, 170–182. doi: 10.1192/bjp.154.2.170
Stewart, R., Prince, M., Mann, A., Richards, M., and Brayne, C. (2001). Stroke, vascular risk factors and depression: cross-sectional study in a UK Caribbean-born population. Br. J. Psychiatry 178, 23–28. doi: 10.1192/bjp.178.1.23
Storor, D. L., and Byrne, G. J. (2006). Pre-morbid personality and depression following stroke. Int. Psychogeriatr. 18, 457–469. doi: 10.1017/S1041610206003188
Sun, N., Li, Q. J., Lv, D. M., Man, J., Liu, X. S., and Sun, M. L. (2014). A survey on 465 patients with post-stroke depression in China. Arch. Psychiatr. Nurs. 28, 368–371. doi: 10.1016/j.apnu.2014.08.007
Tang, W. K., Chan, S. S., Chiu, H. F., Ungvari, G. S., Wong, K. S., Kwok, T. C., et al. (2005). Poststroke depression in Chinese patients: frequency, psychosocial, clinical, and radiological determinants. J. Geriatr. Psychiatry Neurol. 18, 45–51. doi: 10.1177/0891988704271764
Tang, W. K., Chen, Y. K., Lu, J. Y., Chu, W. C., Mok, V. C., Ungvari, G. S., et al. (2011). Cerebral microbleeds and depression in lacunar stroke. Stroke 42, 2443–2446. doi: 10.1161/STROKEAHA.111.614586
Tomaka, J., Thompson, S., and Palacios, R. (2006). The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. J. Aging Health 18, 359–384. doi: 10.1177/0898264305280993
Tsai, C. S., Wu, C. L., Hung, T. H., Chou, S. Y., and Su, J. A. (2016). Incidence and risk factors of poststroke depression in patients with acute ischemic stroke: a 1-year prospective study in Taiwan. Biomed. J. 39, 195–200. doi: 10.1016/j.bj.2015.10.004
Tsouna-Hadjis, E., Vemmos, K. N., Zakopoulos, N., and Stamatelopoulos, S. (2000). First-stroke recovery process: the role of family social support. Arch. Phys. Med. Rehabil. 81, 881–887. doi: 10.1053/apmr.2000.4435
Vallury, K. D., Jones, M., and Gray, R. (2015). Do family-oriented interventions reduce poststroke depression? A systematic review and recommendations for practice. Top. Stroke Rehabil. 22, 453–459. doi: 10.1179/1074935715Z.00000000061
van de Port, I. G., Kwakkel, G., Bruin, M., and Lindeman, E. (2007). Determinants of depression in chronic stroke: a prospective cohort study. Disabil. Rehabil. 29, 353–358. doi: 10.1080/09638280600787047
van Mierlo, M. L., van Heugten, C. M., Post, M. W., de Kort, P. L., and Visser-Meily, J. M. (2015). Psychological factors determine depressive symptomatology after stroke. Arch. Phys. Med. Rehabil. 96, 1064–1070. doi: 10.1016/j.apmr.2015.01.022
Vataja, R., Leppavuori, A., Pohjasvaara, T., Mantyla, R., Aronen, H. J., Salonen, O., et al. (2004). Poststroke depression and lesion location revisited. J. Neuropsychiatry Clin. Neurosci. 16, 156–162. doi: 10.1176/jnp.16.2.156
Vataja, R., Pohjasvaara, T., Leppavuori, A., Mantyla, R., Aronen, H. J., Salonen, O., et al. (2001). Magnetic resonance imaging correlates of depression after ischemic stroke. Arch. Gen. Psychiatry 58, 925–931. doi: 10.1001/archpsyc.58.10.925
Verdelho, A., Henon, H., Lebert, F., Pasquier, F., and Leys, D. (2004). Depressive symptoms after stroke and relationship with dementia: a three-year follow-up study. Neurology 62, 905–911. doi: 10.1212/01.WNL.0000115107.66957.8C
Wei, N., Yong, W., Li, X., Zhou, Y., Deng, M., Zhu, H., et al. (2015). Post-stroke depression and lesion location: a systematic review. J. Neurol. 262, 81–90. doi: 10.1007/s00415-014-7534-1
Williams, L. S., Ghose, S. S., and Swindle, R. W. (2004). Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am. J. Psychiatry 161, 1090–1095. doi: 10.1176/appi.ajp.161.6.1090
Yang, S., Hua, P., Shang, X., Cui, Z., Zhong, S., Gong, G., et al. (2015). A significant risk factor for poststroke depression: the depression-related subnetwork. J. Psychiatry Neurosci. 40, 259–268. doi: 10.1503/jpn.140086
Yang, S. R., Hua, P., Shang, X. Y., Hu, R., Mo, X. E., and Pan, X. P. (2013). Predictors of early post ischemic stroke apathy and depression: a cross-sectional study. BMC Psychiatry 13:164. doi: 10.1186/1471-244X-13-164
Zhang, W. N., Pan, Y. H., Wang, X. Y., and Zhao, Y. (2013). A prospective study of the incidence and correlated factors of post-stroke depression in China. PLoS ONE 8:e78981. doi: 10.1371/journal.pone.0078981
Zhao, F., Kuroiwa, T., Miyasaka, N., Nagaoka, T., Nakane, M., Tamura, A., et al. (2001). Characteristic changes in T(2)-value, apparent diffusion coefficient, and ultrastructure of substantia nigra evolving exofocal postischemic neuronal death in rats. Brain Res. 895, 238–244. doi: 10.1016/S0006-8993(00)03281-9
Keywords: post-stroke depression, PSD, risk factor, systematic review, meta-analysis
Citation: Shi Y, Yang D, Zeng Y and Wu W (2017) Risk Factors for Post-stroke Depression: A Meta-analysis. Front. Aging Neurosci. 9:218. doi: 10.3389/fnagi.2017.00218
Received: 20 April 2017; Accepted: 23 June 2017;
Published: 11 July 2017.
Edited by:
Aurel Popa-Wagner, University of Rostock, GermanyReviewed by:
Raluca Sandu Vintilescu, University of Medicine and Pharmacy of Craiova, RomaniaGuido Gainotti, Policlinico Universitario Agostino Gemelli, Italy
Copyright © 2017 Shi, Yang, Zeng and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Wu, d3V3ZW42NkAxNjMuY29t
†These authors have contributed equally to this work and are co-first authors.
 Yu Shi
Yu Shi Dongdong Yang
Dongdong Yang Yanyan Zeng1
Yanyan Zeng1 Wen Wu
Wen Wu