- 1Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Psychiatry, Harvard Medical School, Belmont, MA, United States
- 3Division of Alcohol and Drug Abuse, Mailman Neuroscience Research Center, McLean Hospital, Belmont, MA, United States
Restless legs syndrome (RLS), a common neurological sensorimotor disorder in western countries, has gained more and more attention in Asian countries. The prevalence of RLS is higher in older people and females. RLS is most commonly related to iron deficiency, pregnancy and uremia. The RLS symptoms show a significant circadian rhythm and a close relationship to periodic limb movements (PLMs) in clinical observations, while the pathophysiological pathways are still unknown. The diagnostic criteria have been revised in 2012 to improve the validity of RLS diagnosis. Recent studies have suggested an important role of iron decrease of brain in RLS pathophysiology. Dopaminergic (DA) system dysfunction in A11 cell groups has been recognized long ago from clinical treatment and autopsy. Nowadays, it is believed that iron dysfunction can affect DA system from different pathways and opioids have a protective effect on DA system. Several susceptible single nucleotide polymorphisms such as BTBD9 and MEIS1, which are thought to be involved in embryonic neuronal development, have been reported to be associated with RLS. Several pharmacological and non-pharmacological treatment are discussed in this review. First-line treatments of RLS include DA agents and α2δ agonists. Augmentation is very common in long-term treatment of RLS which makes prevention and management of augmentation very important for RLS patients. A combination of different types of medication is effective in preventing and treating augmentation. The knowledge on RLS is still limited, the pathophysiology and better management of RLS remain to be discovered.
Background
The symptoms of the RLS were first described by Willis (1685) and then published by Ekbom (1960). Despite being introduced hundreds of years ago, it’s still a poorly recognized disorder because of the unclear pathophysiology and relatively low morbidity, resulting in limited recognition by primary care physicians and common misdiagnosis and under-diagnosis. RLS is considered as a common neurological sensorimotor disorder that manifests as an irresistible urge to move the body to relieve the uncomfortable sensations. There’s a significant circadian rhythm of the RLS, as it commonly worsens at night.
Epidemiology
It has been concluded that the prevalence rate ranged from 3.9 to 15% of the general population (Ohayon et al., 2012) from recent epidemiologic analyses of different countries. The estimated prevalence of the RLS is around 7–10% in Caucasians (Ohayon et al., 2012) while there is a much lower incidence ranging from 0.1 to 12% among Asian population (Cho et al., 2009; Tsuboi et al., 2009; Chen et al., 2010; Panda et al., 2012; Shi et al., 2015). Compared with other countries, RLS is much more common in western countries. As numerous studies in western countries indicated the prevalence of 10, 10–15, and 5.5% in United States, Canada, and Europe (United Kingdom, Spain, Germany, Italy), respectively (Phillips et al., 2000; Ohayon and Roth, 2002). On the other hand, studies from Asian countries showed a pretty low prevalence rate of RLS. The first Indian population study on RLS revealed a prevalence of 2.9% (Panda et al., 2012) while a prevalence of 0.96% among inhabitants of Ajimu in Japan who were older than 65 years old (Tsuboi et al., 2009). Another RLS study in Shanghai, China revealed a prevalence rate of 1.4% among 2941 eligible individuals older than 18 years old (Shi et al., 2015). The significant difference between different ethnic populations may be due to different genetic background, ethnicity, geography, and environmental influences including natural environment and diet habits. The different populations targeted, research methodologies and diagnosis criteria used may contribute to this result as well.
Most surveys have concluded that the prevalence and severity of the RLS both increase with age (Phillips et al., 2000; Ohayon and Roth, 2002), suggesting that the neurodegenerative process may play an important role in RLS. Life style of older people and the senile changes including cardiovascular changes and metabolism changes is also related to RLS (Koh et al., 2015; Cassel et al., 2016). In adults, the incidence of RLS is twice as high in women than in men (Berger et al., 2004), which may result from the hormones such as estrogen and progesterone and different social roles.
The age of onset varies widely from childhood to over 90 years of age. Though most patients in clinical practice are middle-aged or older, juvenile onset is not rare. 38–45% of adult patients complain about first symptoms before the age of 20 (Walters et al., 1996; Montplaisir et al., 1997). A study in 2007 found rates of 1.9 and 2.0% in 8–11 years old and 12–17 years old children and adolescence, respectively. Another study using a different diagnostic criteria for RLS in children that published in 2003 revealed a prevalence of 5.9% (Kotagal and Silber, 2004). A newest pediatric RLS diagnostic criteria that include consideration of typical words used by children, differential diagnosis and comorbidity was published in 2013 (Picchietti et al., 2013). But no gender difference is found in pediatric RLS (Per et al., 2017). This gender difference in adult but not in children might be due to pregnancy because nulliparous women have about the same prevalence of RLS as males (Berger et al., 2004). The different classifications of RLS including primary and secondary RLS may also contribute to these findings. Sleep, mood, cognition, and quality of life are significantly affected in pediatric RLS patients (Picchietti and Picchietti, 2010). And ADHD, depressive symptoms, and anxiety are very common comorbidities of pediatric RLS patients (Pullen et al., 2011).
Clinical Presentations and Diagnostic Criteria
Clinical Presentations
Restless legs syndrome manifests as an overwhelming urge to move the body to relieve the uncomfortable sensations, primarily when resting, sitting, or sleeping. The uncomfortable feelings are always described by the patients as “creeping, crawling tingling, tingling, pulling, or painful” deep inside the limbs (Trenkwalder et al., 2005), unilaterally or bilaterally occurring with the knees, the ankles or even the whole lower limbs (Trenkwalder et al., 2005). Sometimes even the phantom limbs can be involved (Skidmore et al., 2009). Commonly it affects the patient’s sleep. Insomnia is the most common reason for a patient with RLS to search for consult in clinical practice. The most common bedtime problems caused by RLS is difficulty initiating sleep (Mohri et al., 2008). It has a significant circadian pattern which presents as worsening symptoms in the evening and short remission in the morning after waking up (Kushida et al., 2007). The longer the course of the disease, the more likely the symptoms affect the arms or the other places of the body besides the legs. It’s not uncommon that patients developed arm restless syndrome with progressive disease (Freedom and Merchut, 2003). Movement such as walking, stretching, or bending the legs relieves the discomfort at least temporarily and partially (Trenkwalder et al., 2005).
Restless legs syndrome affects the patient’s HRQoL to different degrees according to various severities of the symptoms. HRQoL is measured by 36-Item Short Form Health Survey (SF-36), a 36 item survey used to construct eight scales among physical and mental health and health transition. Most patients have mild symptoms, only 11.9% of them seek for consult, while about 3.4% of all the patients need drug treatment (Hening, 2004). The main morbidities except for extreme discomfort were sleep loss and disruption of normal activities (Trenkwalder et al., 2005). Patients with mild or moderate symptoms manifested discomfort with less frequency, lower severity, and less influence of the symptoms on their sleep. Comparing with the general population, patients with severe or very severe RLS symptoms always reported apparent deficits (10–40 points on 100-points scales) in physical functioning, bodily pain, general health, vitality, social functioning, role-physical, and role-emotion (Trenkwalder et al., 2005). With deficient sleep at night, severely affected patients might complain about having difficulty in their daily life including their jobs and social activities (Kushida et al., 2007). A significant higher probability of 25% with ADHD were found in RLS patients compared to general population (Pullen et al., 2011). On the other hand, 20% of ADHD patients meet criteria for RLS as well (Zak et al., 2009; Yilmaz et al., 2011). Pathophysiological pathways of ADHD remains poorly understood, but the most popular theory is the dopamine deficit theory, which could be a shared pathway with RLS (Swanson et al., 2007). BTBD9, a RLS risk allele, may be related to certain subtypes of ADHD, which responds well to iron supplementation treatment (Schimmelmann et al., 2009). Disruption of sleep length and quality, daytime alertness can contribute to depression and anxiety. Increasing sympathetic tone due to PLMS may lead to cardiovascular disease and high blood pressure (Stevens, 2015).
Different from in adults, pain is a common presentation in pediatric RLS. 45% of children use the terms pain and hurts or hurting which makes growing pains a common misdiagnosis in pediatric RLS (Picchietti et al., 2013). And children always use their own words like “need to move, want to move and got to kick (Picchietti et al., 2011)” to describe “urge”. ADHD is also a very common comorbidity in pediatric RLS. Except for bad impact on sleep, mood and cognition, behavioral and educational changes are very common in pediatric RLS (Picchietti et al., 2013). It might be due to disruption of homework and ability to concentrate.
Associated Features
Circadian Rhythm
Both sensory and motor symptoms show significant circadian rhythm in RLS, which display as a peak at similar time at night. A study showed the increase in melatonin secretion to be the only changes preceding the sensory and motor symptoms in RLS patients, indicating melatonin might affect the symptoms by its inhibitory effect on dopamine secretion in central nervous system (Michaud et al., 2004). The intensity of RLS symptoms peaks on the falling phase of the core body temperature, another endogenous marker of circadian rhythm, while it decreases when core temperature increases (Hening et al., 1999; Barriere et al., 2005).
Previous studies have shown that plasma dopamine and its metabolites changes with circadian rhythm not only just in humans (Sowers and Vlachakis, 1984), but also in CSF in primate animals (Perlow et al., 1977) and in the striatum of rat (Schade et al., 1995; Castaneda et al., 2004). Another study indicated that the dopamine receptor responsiveness is modulated by the circadian rhythm at the level of spinal cord in decapitated Drosophila melanogaster (Andretic and Hirsh, 2000). Moreover, it has been revealed that sensitivity of dopamine receptors increased at night at the level of tubero-infundibular-dopaminergic system (Garcia-Borreguero et al., 2004). Additionally, a circadian variation of serum iron paralleled the CSF dopamine, as well as the severity of symptoms (Garcia-Borreguero et al., 2004). However, it is unclear whether the brain iron concentrations changes would follow this pattern.
Periodic Limb Movement Disorder
Periodic limb movement disorder, previously known as nocturnal myoclonus, is defined as involuntary movements of the patient’s limb or torso during awake or sleep which the patient is not aware of, different from the voluntary movement of the limb to relieve the discomfort in RLS patients (Hening et al., 1999; Trenkwalder et al., 2005). Nevertheless, it’s a very common phenomenon in RLS patients. A previous study indicated that PLMS was found in around 80% of RLS patients (Montplaisir et al., 1997). On the contrary, not a large percentage of patients having PLMS presented RLS. PLMS, which is not a specific feature for RLS patients, can be associated to many other conditions. The PSG is usually employed to measure the movements, while actigraphy is a helpful method for diagnosing and measuring PLMW or PLMS. PLMS is diagnosed on PSG by at least continuous four muscle contractions lasting 0.5–10 s and recurring during intervals of 5–90 s. The minimum amplitude of a leg movement event is an 8 μV increases in EMG voltage above resting EMG (Iber et al., 2007) in diagnostic criteria for PLMD.
A movement in PLMS starting in sleep can continue when waking up and vice versa. On the other hand, arousals happening before or during a movement event do not change the assessment of that event (Iber et al., 2007). Immobilization test is to measure PLMW in which the patient is asked to lie perfectly still. The PSG records the time the patient can stay still and the limb movements during an hour. It can be used to quantify the severity of RLS, to follow up the patient’s course of disease and to monitor treatment response (Trenkwalder et al., 2005).
Diagnostic Criteria
The diagnostic criteria have experienced a lot of improvements and revisions in the history, including the earliest informal Ekbom’s “criteria” for RLS in 1960, then DCSAD restless legs DIMS or DOES syndrome – essential features in 1979, ICSD diagnostic criteria for RLS in 1990, IRLSSG “minimal” criteria for diagnosis of RLS in 1995 and NIH/IRLSSG (NIH) “essential” criteria for diagnosis of RLS in 2003 (Allen et al., 2014). On the basis of previous diagnostic criteria, the four essential diagnostic criteria of RLS published by NIH/IRLSSG in 2003 emphasized the importance of the urge to move the legs in diagnosing RLS. The four essential criteria are shown below (Table 1).
Although, 2003 NIH/IRLSSG diagnostic criteria have defined RLS in a much more detailed way than those previous criteria. The same disadvantage still exist in this criteria, RLS “mimics” can’t be excluded according to this diagnostic criteria. Such as conditions like cramps, positional discomfort and local leg pathology. Then the diagnostic criteria were recently revised again by the IRLSSG in 2012 (International Restless Legs Syndrome Study Group, 2012). Comparing with 2003 NIH/IRLSSG diagnostic criteria, 2012 revised RLS diagnostic criteria have added an important essential criterion, noting that RLS should be differentiated with other conditions with similar symptoms such as myalgia, venous stasis, leg edema, arthritis, habitual foot tapping, and so on (International Restless Legs Syndrome Study Group, 2012). 2012 revised RLS diagnostic criteria also stated the stipulation of clinical course and clinical significance of RLS as presented (International Restless Legs Syndrome Study Group, 2012). The newest diagnostic criteria (Table 2) is much more rigorous than 2003 IRLSSG diagnostic criteria.
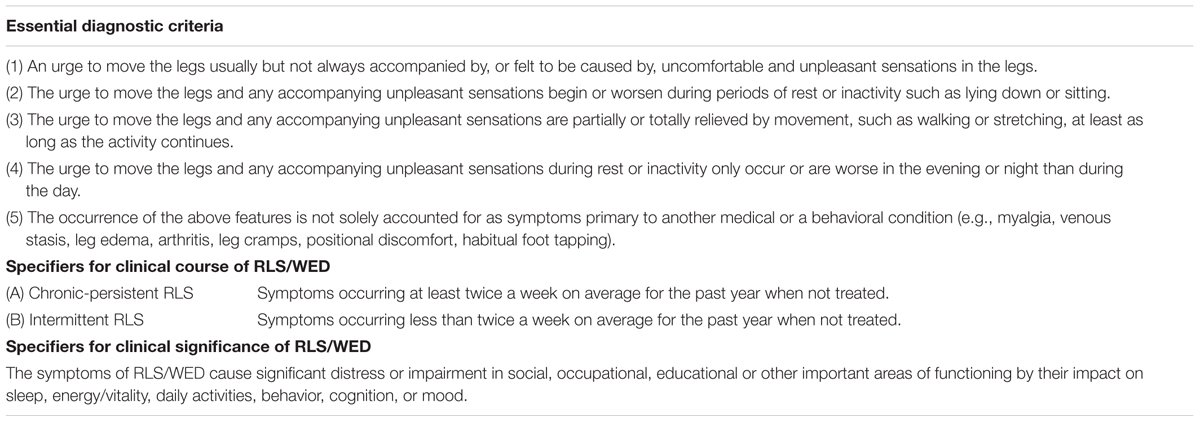
TABLE 2. 2012 revised IRLSSG diagnostic criteria (International Restless Legs Syndrome Study Group, 2012).
Specifier for clinical significance of RLS emphasizes that the influence of RLS on the patient’s function in social, occupational, educational, or other important areas should be evaluated. Different from in adults, functional consequences of RLS in children are mainly behavioral and educational domains (Arbuckle et al., 2010). The new diagnostic criteria set up a more rigorous method to ascertain a RLS case with more specific criteria and excluding standards. It has improved the validity of RLS diagnosis. On the other hand, it helps to classify the patient’s clinical course and clinical significance which helps the physician to better monitor the progression of RLS and support better cases for research samples (Allen et al., 2014). The specifier for clinical course does not apply for pediatric RLS or special cases of RLS secondary to pregnancy or medication (Picchietti et al., 2013). Generally, mild RLS severity with no family history, and young age at RLS onset are predictors of RLS remission. While most patients with severe RLS show a chronic clinical course (Lee et al., 2016).
Periodic limb movement during sleep is a very identified sign in RLS patients. It is found that 80–89% of RLS patients have excessive PLMS than same aged people (Montplaisir et al., 1997). PLMS is not very specific for RLS since it is a frequent condition among adults aged over 45 years (Hornyak et al., 2007). When PLMS present in a pattern different than expected for age excluding the states of other disease or medication, PLMS can support the diagnosis of RLS.
Considering that children might not understand the term “urge”, simple straightforward prompts should be asked, like “Do your legs bother you?” or “Do your legs bother you at night?” And common descriptions used by children about RLS sensations are “need to move, want to move and got to kick (Picchietti et al., 2011)”. RLS mimics like ADHD, sore leg muscles, growing pains and dermatitis should be carefully considered when diagnosing pediatric RLS (Picchietti et al., 2013).
Comparing with the previous diagnostic criteria, 2012 revised RLS diagnostic criteria is the only criteria that developed by a large international number of RLS clinical and research experts through an interdisciplinary, international and evidence-based approach which ensure it to be a world-wide consensus criteria which reduces the risk of cultural bias and avoids arbitrary and improve the validity. The specifiers for clinical course and significance are able to provide a method to define different target population which would help clinicians and researchers to offer better prevention and treatment strategies for specific groups of patients and to better elucidate etiopathogenesis (Allen et al., 2014).
On the other hand, some disadvantages still exist since the diagnostic criteria for RLS are subjective, some more objective and reliable diagnostic criteria need to be brought up for a further diagnosis and classification of RLS, such as biological markers, genetic features, PLMS measuring, polysomnography, and actigraphy changes. Some new tools are to be developed for standardize case ascertainment (Allen et al., 2014). A case-control study in Germany reveals that inositol metabolites increased specifically in RLS patients (Schulte et al., 2016). This might be a discovery approach using serum metabolite profiling in RLS.
Classification
The RLS includes two groups in general: primary RLS and secondary RLS.
Primary RLS is considered to be idiopathic when the cause is truly unknown. Among the idiopathic RLS, 40.9–92% of whom had a family history of RLS, indicating the important role of genetic factors in developing RLS (Winkelman et al., 1996; Winkelmann et al., 2002; Tison et al., 2005).
Most secondary RLS cases have an onset after 40 years old. Secondary RLS are those associated with a variety of neurological disorders, iron deficiency, pregnancy, or chronic renal failure (Winkelman et al., 1996; Curgunlu et al., 2012; Srivanitchapoom et al., 2014). A study in Turkey revealed that severity of RLS symptoms is very closely related to low ferritin level (Curgunlu et al., 2012). In the earliest studies of RLS, some revealed that 25% of RLS patients have iron deficiency condition. The other correlated factors were diabetic peripheral neuropathy (Zobeiri and Shokoohi, 2014), painful neuropathies (Rutkove et al., 1996), ADHD (Roy et al., 2015), migraine (Zanigni et al., 2014), AS (Tekatas and Pamuk, 2015), leprosy (Padhi and Pradhan, 2014), inflammatory chronic demyelinating neuropathies like multiple sclerosis (Deriu et al., 2009) and Guillain–Barré syndrome (Marin et al., 2010), thyroid disease (Rodriguez Martin et al., 2015), poliomyelitis (Kumru et al., 2014), chronic venous disorder (McDonagh et al., 2007), autoimmune disease including Sjögren’s syndrome (Theander et al., 2010), rheumatoid arthritis (Hening and Caivano, 2008), inflammatory bowel disease (Becker et al., 2015), and Crohn’s disease (Hoek et al., 2015). Epidemiology studies also showed that the prevalence of RLS significantly increased in post-stroke patients, mainly in patients whose stroke topography lied on pyramidal tract and the basal ganglia-brainstem axis which were primarily involved in motor functions (Sechi and Sechi, 2013). Some certain drugs can cause or worsen RLS symptoms, such as psycotropics like antidepressants and neuroleptics, dopaminergic drugs, and some other drugs (Giudice, 2010) such as cumulative dopaminergic agonists effects in Parkinson’s disease patients (McDonagh et al., 2007). A study revealed that the strongest evidence for drug-induced RLS are for the following: escitalopram, fluoxetine, L-dopa/carbidopa and pergolide, L-thyroxine, mianserin, mirtazapine, olanzapine, and tramadol (Hoque and Chesson, 2010).
According to the onset age of the symptoms, RLS is divided into early-onset RLS and late-onset RLS. Early-onset RLS refers to those who firstly have the symptoms before 45 years old. While late-onset RLS patients have the symptoms from or after 45 years old. A higher familial history rate was found in early-onset RLS comparing to late-onset RLS (Kotagal and Silber, 2004). Various clinical courses with periodic remissions are common in early-onset RLS. While a chronic progressive clinical course with more severe symptoms are seen in late-onset RLS. Pediatric RLS are always misdiagnosed as “growing pain”. Current researches demonstrate a relatively iron deficiency and renal failure to be exacerbating factors for pediatric RLS (Kotagal and Silber, 2004; Davis et al., 2005; Applebee et al., 2009; Sinha et al., 2009). Commonly pediatric RLS should be differentiated from ADHD while only RLS patients presents the need to move because of leg discomfort but not difficulty “sitting still” (Trenkwalder et al., 2005).
Differential Diagnosis
Very common conditions which should be differentiated with RLS include leg cramps, positional discomfort, local leg injury, arthritis, leg edema, venous stasis, peripheral neuropathy, radiculopathy, habitual foot tapping/leg rocking, anxiety, myalgia, and drug-induced akathisia (Allen et al., 2014). They can mimic RLS in different ways. Leg cramps are presented as knot of the muscle. Positional discomfort can be relieved by a positional shift. Arthritis patients have a limitation of the joints or joint erythema. Myalgias present as muscle soreness. Numbness happen to neuropathy patients as well as RLS patients, and both venous stasis and leg edema can manifest as swelling in the limbs (Davis et al., 2005; Applebee et al., 2009; Sinha et al., 2009; Karroum et al., 2012). Less common differential diagnostic conditions included myelopathy, myopathy, vascular or neurogenic claudication, hypotensive akathisia, orthostatic tremor, painful legs, and moving toes (Allen et al., 2014). Differential diagnosis is really important to RLS patients for their further treatment. Therefore clinical physicians have created additional diagnostic questionnaires and scales to improve the diagnosis of RLS in clinical practice (Popat et al., 2010). RLS-NIH questionnaire, developed in 2002 with three mandatory questions, showed sensitivity and specificity of 86 and 45% respectively (Popat et al., 2010). On the basis of this, an the RLS-EXP showed the sensitivity and specificity of 81 and 73% respectively (Popat et al., 2010). The CH-RLSq is more commonly used in clinical practice and by researchers.
Secondary RLS
Iron Deficiency
Early in 1953, Nordlander (1953) first proposed that iron deficiency might be an important part of the pathophysiological process in RLS which was supported by consistent prevalence studies and recent pharmacological researches. Low serum iron levels (normal range: 50–170 μg/dL for men; 65–176 μg/dL for women; 50–120 μg/dL for children) presented in 25% of patients with severe RLS (Ekbom, 1960). While 43% of patients complaining of “leg restless” were found to be in the condition of iron deficiency (Matthews, 1976). The severity of the symptoms was found to be correlated with serum ferritin levels (normal range: 15–200 ng/mL for men; 12–150 ng/mL for women; 7–140 ng/mL for children) (O’Keeffe et al., 1994; Mizuno et al., 2005). Numbers of pharmacological studies of iron supplement for RLS gained therapeutic effects (Nordlander, 1953). CSF biological studies showed lower iron and ferritin levels in RLS patients, along with higher transferrin levels (Mizuno et al., 2005). However, a current study has suggested that in normal circumstances, the brain does not respond to peripheral variations in iron status (Ward et al., 2014). This might explain why some RLS patients had a normal or over-loaded serum iron level while their CSF iron level was decreased. Various medical imaging studies including ultrasound studies and MRI studies revealed a decreased iron levels in the substantia nigra and putamen, especially in the very severe patients (Schmidauer et al., 2005; Moon et al., 2014). Other studies demonstrated iron decrease in the red nucleus, thalamus and the pallidum (Haba-Rubio et al., 2005; Rizzo et al., 2013). However, MRI was not capable of locating the iron deficiency condition to particular cells up to now. Autopsy studies also reported a decrease in iron concentration in the substantia nigra (Moon et al., 2014).
Pregnancy
15–25% of pregnant women have RLS in Western countries according to prevalence studies (Lee et al., 2001; Neau et al., 2010). A peak prevalence of RLS in pregnant women mainly occurred in the third trimester and gained a remission by 1 month after delivery (Ismailogullari et al., 2010). Surveys manifested that nulliparous women were at the same risk of RLS as same aged men, while the risk for women were increased after one pregnancy (OR 1.98) and two pregnancies (OR 3.04), even more after three or more pregnancies (OR 3.57) (Berger et al., 2004; Pantaleo et al., 2010). These findings may explain the gender difference in RLS prevalence. The reason why pregnant women have higher risk of having RLS remains unknown. One reason is that an increased demand of iron in pregnant women results in relative iron deficiency. The other established factors included hormonal status (prolactin, progesterone and estrogen), folate deficiency and stretch or compression of nerves due to fetal growth conflicting (Pantaleo et al., 2010; Pereira et al., 2013). Psychomotor behavioral change during the last weeks of pregnancy might also contribute to the symptoms. Anxiety, insomnia, and fatigue are always associated with pregnancy in the last trimester.
End-Stage Renal Disease Patients on Hemodialysis
Studies have revealed approximate prevalence of 20–30% RLS in hemodialysis than the prevalence of 3.9–15% among the general populations (Ohayon et al., 2012). A study including 166 patients in Serbian showed 22.7% of patients on hemodialysis were under the condition of RLS (Nikic et al., 2007). Other studies indicated a prevalence of 14.8% among 176 patients on hemodialysis in Brazil (Goffredo Filho et al., 2003) and a prevalence of 37.4% in 163 patients on hemodialysis in Iran (Rohani et al., 2015). Some of the studies revealed a predominance of female patients with RLS rather than male patients on hemodialysis (Al-Jahdali et al., 2009; La Manna et al., 2011; Haider et al., 2014), while a recent study in Saudi displayed no statistically difference between two genders (Wali and Alkhouli, 2015). Most of the RLS patients among end-stage renal disease indicated moderate to severe symptoms compared to most mild RLS symptoms among general populations (Wali and Alkhouli, 2015). Iron deficiency in end-stage renal disease patients may lead to anemia and affect the dopamine metabolism, contributing to RLS (Nikic et al., 2007), and uremia-related peripheral neuropathy and high serum calcium can be part of the physiology of RLS (Nikic et al., 2007). However, in a recent report, iron deficiency, anemia, and calcium were not found to be statistically related to RLS in hemodialysis patients (Wali and Alkhouli, 2015). Previous studies showed conflicts about the association between BMI and RLS in end-stage renal disease patients. A study with a large cohort found that the prevalence of RLS in end-stage renal disease patients was positively related to their BMI (Gao et al., 2009). The OR for RLS was 1.42 (95% CI: 1.3–1.6; p < 0.0001) for patients with BMI from 23 to 30 kg/m2 and 1.60 (95% CI: 1.5–1.8; p < 0.0001) for patients with highest BMI compared with patients with lowest BMI (Gao et al., 2009). Decreased number of dopamine receptors in obese people’s brain was considered to be the possible reason (Wang et al., 2001). However, another previous study revealed no such relationships (Kim et al., 2008). A recent cross-sectional study between control group, renal transplantation group and hemodialysis group found that prevalence in renal transplantation is significantly lower than in hemodialysis patients (Kahvecioglu et al., 2016). RLS is very prevalent in end-stage renal disease patients, and hemodialysis patients with RLS were found to have a higher risk of muscle atrophy (Giannaki et al., 2011), cardio/cerebrovascular events and mortality (Lin C.H. et al., 2015). To prevent more morbidity in end-stage renal disease patients, their RLS should be diagnosed in early stage and receive standard treatment of RLS or have a renal transplantation as soon as possible.
Pathophysiology
The pathophysiology of RLS is still partially understood. The most accepted pathways include genetics variants, abnormal iron metabolisms, dopaminergic dysfunction, and central opiate system.
Iron
It’s widely accepted that the local brain iron level plays an important role in RLS pathophysiology, however, the mechanism is still unclear. Recent studies demonstrated that the iron deficiency in brain was related to the function of blood-brain interface, as BBBs endothelial cells acted as an iron reservoir for the brain. A dysfunction of iron regulatory protein in the microvasculature incited dysregulation of iron transport across the BBB, resulting in a decrease of iron storage in endothelial cells (Lee et al., 2001). Biochemical studies on the effect of iron in brain indicated that several proteins containing iron were included in various processes like oxidative phosphorylation, oxygen transportation, myelin production and the synthesis and metabolism of neurotransmitters (Ward et al., 2014). Therefore, iron deficiency can lead to cellular damage by oxidation and modification of cellular compounds such as lipids, carbohydrates, protein, and DNA from hydroxyl radical production (Ward et al., 2014). The interactions between impaired neuronal iron uptake and the functions of the neuromelanin-containing and dopamine-producing cells play important roles in RLS pathophysiology (Michaud et al., 2004). Decreased extracellular dopamine, DAT, D1 and D2 receptors are found in iron deficiency, indicating that iron affect the brain dopaminergic transmission in different ways (Dauvilliers and Winkelmann, 2013).
Dopamine
A large number of pharmacological studies and clinical findings have provided evidence for the important role of dopaminergic system dysfunction in RLS (Winkelmann et al., 2001; Paulus and Trenkwalder, 2006; Galbiati et al., 2015). An improvement of RLS symptoms was found in patients receiving low-dose dopaminergic medications (Paulus and Trenkwalder, 2006; Galbiati et al., 2015) while a worsening of RLS symptoms in patients receiving dopamine antagonists (Winkelmann et al., 2001). The need for dopaminergic agonists to cross the BBB to be effective in RLS symptoms indicated that dopaminergic system in central nervous system was entailed in RLS pathophysiology rather than in peripheral nervous system (Garcia-Borreguero and Williams, 2014). Tyrosine hydroxylase is a rate-limiting step enzyme for the conversion of levodopa to dopamine. As iron is a cofactor of this enzyme, iron deficiency can alter the dopaminergic system in the brain (Dauvilliers and Winkelmann, 2013). Dopaminergic A11 cells, located in the midbrain and close to hypothalamus, have long axons and project diffusely throughout the spinal cord (Clemens and Hochman, 2004). They are the major source of dopamine in spinal cord. A11 cells arrive in the dorsal horn, then project to the motoneuronal site (Holstege et al., 1996). It’s found that stereotaxic bilateral 6-hydroxydopamine lesions into the A11 nucleus can result in an increased average number of standing episodes and total standing time comparing to the sham rats (Ondo et al., 2000), suggesting an important role of A11 dopaminergic cells in pathophysiological pathways in RLS. A significantly decreased N-acetylaspartate, creatinine ratio, and N-acetylaspartate concentrations were found in the medial thalamus of RLS patients in a recent study using proton magnetic resonance spectroscopy (Rizzo et al., 2012). Functional MRI and PET studies concluded an important role of medial thalamic nuclei in RLS pathophysiology. The medial thalamic nuclei are part of the limbic system, which is modulated by dopaminergic afferents. Another study found thalamic activity changes in the thalamocotrical circuit (Goulart et al., 2014). Therefore, it was hypothesized that the dopaminergic dysfunction might lead to an impairment of the medial pain system (Garcia-Borreguero and Williams, 2014; Goulart et al., 2014) and then caused the uncomfortable symptoms of RLS. Pharmacological studies also demonstrated that opioids had a protective effect in some RLS patients. An in vitro study in rats found that iron deficiency can cause cell death, mainly dopaminergic cells in substantia nigra and opioids could protect them from cell death under the condition of iron deprivation (Sun et al., 2011). The authors concluded from this result that an intact endogenous opioid system and opioid treatment could prevent the dopamine system from dysfunction in iron deficiency patients (Sun et al., 2011). A study of tsDCS in RLS patients showed supportive evidence of spinal cord hyperexcitability (Heide et al., 2014).
Genes
Studies demonstrate a strong relationship between genetic predisposition and early-onset RLS, of which more than 60% reveal a positive familial history. Moreover, inheritance was found to be related to late-onset RLS and secondary RLS (Winkelmann et al., 2000; Xiong et al., 2010; Yang et al., 2011). A study among 249 Canadian RLS patients found a family history existed in 77.1% of them while the rest 22.9% were sporadic (Yang et al., 2011). Variants in MEIS1, BTBD9 and MAP2K5/SKOR1 resulted in a much higher risk of RLS among a US population (Yang et al., 2011). So far, the GWASs have reported the following susceptible SNPs in association with RLS: MEIS1 (chromosome 2p14), BTBD9 (chromosome 6p21.2), PTPRD (chromosome 9p24.1-p23), MAP2K5/SCOR1 (chromosome 15q23) and chromosome 16q12.1 (Berger et al., 2002; Hogl et al., 2005; Stefansson et al., 2007; Schormair et al., 2008). Main function of these genes was related to embryonic neuronal development and limb development (Dauvilliers and Winkelmann, 2013; Garcia-Borreguero and Williams, 2014). In RLS autopsy cases, MEIS1 gene was found to be associated with an increase in H-ferritin, L-ferritin and divalent metal transporter-1 RNA expression in the thalamus (Catoire et al., 2011), suggesting MEIS1 gene mutants predisposed to lower iron condition. Another study used RNA interference techniques in a lymphoblastoid cell line in which the MEIS mRNA expression was blocked (Silver et al., 2010). Forty-eight hours later, an increase in transferring-2 receptor, ferroportin mRNA and BTBD9, and a decrease in hepcidin mRNA expression were observed (Silver et al., 2010), indicating that MEIS1 controlled cellular iron transfer to mitochondria, cellular export of iron and potentially affected BTBD9 expression and its downstream iron modulation. The effect of MEIS1 on iron homeostasis was also shown in Caenorhabditis elegans (Catoire et al., 2011). A study using dBTBD9 mutant flies showed significantly decreased brain dopamine and abnormal sleep phenotype which was completely retrieved by administering with pramipexole, a dopamine D2 receptor agonist (Freeman et al., 2012). Furthermore, RLS symptoms can be reconstructed by knocking-down dBTBD9 expression. While over expression of BTBD9 in HEK cells showed that the gene controlled iron homeostasis through the regulation of IRP2 (Freeman et al., 2012). The authors suggested that the BTBD9 protein belonged to a protein family which contained substrate adaptors for the Cul3 class (Freeman et al., 2012). Cul3 class of E3 ubiquitin ligases was shown to regulate sleep in flies (Stavropoulos and Young, 2011). A susceptible view point was that BTBD9 played a role in dopamine biosynthesis by unclear mechanisms (Shaw and Duntley, 2012). These SNPs had strong association with PLM in RLS patients (Moore et al., 2014). The exact pathophysiological pathways of these genes still remain unclear. An SNP study in the Spanish Caucasian population suggested a modest but significant association between vitamin D receptor rs731236 SNP and the risk for RLS. RLS patients carrying the allelic variant rs731236G had an earlier onset age while those carrying the allelic variant rs731236GG had more severe symptoms (Jimenez-Jimenez et al., 2015). A weak association between heme oxygenase genetic variants HMOX1 rs2071746 polymorphism and the risk of developing RLS was found in the Spanish population (Garcia-Martin et al., 2015). Linkage analyses in families identified several genetic loci for RLS: RLS1 (chromosome 12q12–q21), RLS2 (14q13–q21), RLS3 (9p24–p22), RLS4 (2q33), RLS5 (20p13), RLS6 (19p13), and RLS7 (16p12.1) (Desautels et al., 2001, 2005; Bonati et al., 2003; Kemlink et al., 2007). The authors suggested the inheritance mode of RLS could be a recessive mode or a dominant mode with variable of inheritance. The main SNP findings are summarized as below (Table 3).
Nervous System Structures
Previous theories about dopaminergic effect on RLS pathophysiology mainly focused on substantia nigra and dopaminergic A11 cell group (Ondo et al., 2000; Trenkwalder and Paulus, 2010), nevertheless, changes in dopaminergic neurons in basal ganglia in RLS patients were also found in autopsy studies. This result might be related to iron deficiency in brain (Connor et al., 2003). Dopaminergic projections to the spinal cord originate exclusively from A11 cell group (Noga et al., 2004). Dopamine acted as an excitatory and inhibitory neurotransmitter in spinal cord to regulate sensory, motor as well as autonomic functions (Dauvilliers and Winkelmann, 2013). Recent studies reported frequent PLMS in patients with spinal cord injury, indicating the center role of spinal cord in RLS pathophysiology process (Dauvilliers and Winkelmann, 2013). It might also result from the decreasing supraspinal inhibition to the spinal cord. Functional MRI studies showed changes of thalamic and cerebral activation in RLS (Bucher et al., 1997). However, postmortem studies in RLS patients showed no evidence of changes in the volume of tyrosine kinase (+) neurons or gliosis changes in A11 region in the posterior hypothalamus (Earley et al., 2009). This might result from the fact that only six cases are included in this study or the hypothesis that the manifestations of RLS may be secondary to dopamine metabolism or changes in the distal A11 synapses which is not as easily detected as structural or quantity changes in the cell bodies (Earley et al., 2009). Another recent study indicated that domperidone, a dopamine antagonist that cannot cross the BBB increased the frequency of RLS in patients with Parkinson’s Disease, proposing that peripheral dopaminergic neurons might play an important role in RLS (Rios Romenets et al., 2013). It’s widely believed that iron deficiency in brain causes the decrease in dopaminergic function which then motivates spinal hyperexcitability, leading to the spontaneous sensory and motor movements of RLS (Garcia-Borreguero and Williams, 2014).
Treatment
Treatment before RLS, three aspects should be considered: lifestyle change, medication effect and iron deficiency (defined as ferritin < 75 ng/mL or iron/TIBC ratio < 20%) (Mackie and Winkelman, 2015). Lifestyle like sleep deprivation, alcohol or tobacco use, decreased motility, or in medication (dopamine antagonists, antihistamines or serotonergic antidepressants, opioid discontinuation or blood loss) can result in earlier onset or increase severity in RLS symptoms. A detailed inquiry for the patient should be carried out. Treatment of RLS mainly include pharmacological and non-pharmacological treatment.
Pharmacological Treatment
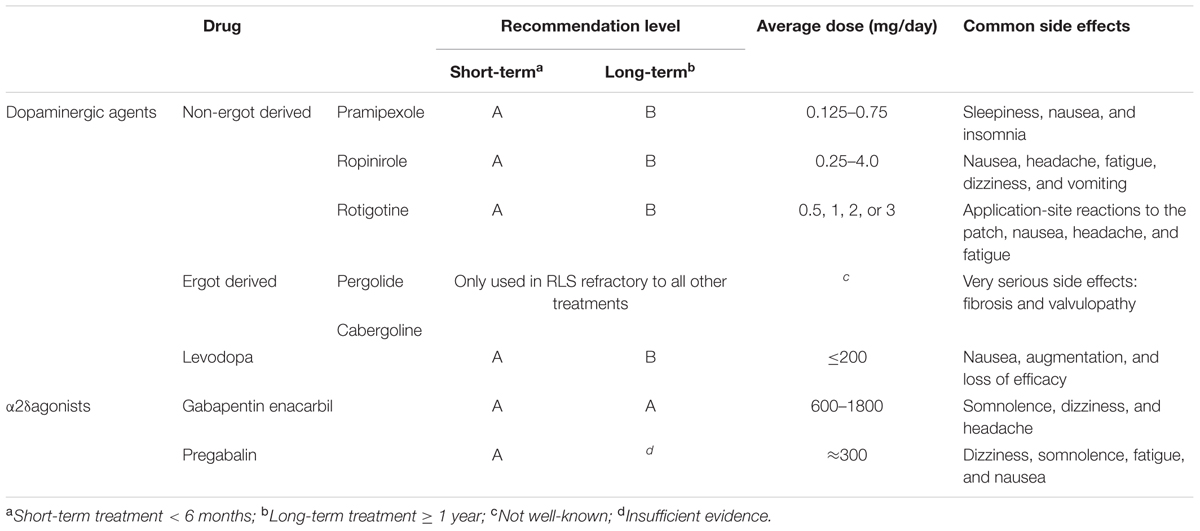
Dopaminergic agents are considered to be the first-line treatment for RLS, including pramipexole and ropinirole (Garcia-Borreguero et al., 2013; Silber et al., 2013). Pergolide and cabergoline are not recommended due to their association with increased risk of valvular heart disease (Zanettini et al., 2007). Ropinirole has a faster onset with shorter duration, while rotigotine is commonly used as a transdermal patch which continuously provides stable plasma drug concentrations, resulting in its particular therapeutic effect on patients with symptoms throughout the day (Mackie and Winkelman, 2015). α2δ agonists have become increasingly important in treating RLS, for being considered as possible first-line agents for RLS. A recent double-blind study over 12-week period compared the efficacy of pregabalin, pramipexole and placebo, demonstrating a better efficacy of pregabalin rather than dopamine agonist or placebo (Hubner et al., 2013). Moreover, the difference of the efficacy varied with drug doses (Hubner et al., 2013). Opioids have been found to be effective in treating RLS, but the potential drug abuse and side effects including respiratory depression and constipation limit its use in RLS, as they are not commonly advised as initial treatment of choice. A recent study on treating first-line agents refractory RLS with extended-release oxycodone–naloxone combination showed very impressive and persistent effect of this combination on RLS symptoms (Trenkwalder et al., 2013). Other pharmacological treatments include iron supplement, some other anticonvulsants and benzodiazepines. The choice of two major first-line agents should be considered with their side effects. Dopamine agonists can cause somnolence and ICDs like compulsive gambling or over-eating, while common side effects of α2δ agonists are weight gain, dizziness and gait instability. As a result, for initial treatment of RLS patients, dopaminergic agents are used as first-line agents in patients with very severe symptoms, over-weighted, comorbid depression, risk of falls, or cognitive impairment (Garcia-Borreguero et al., 2013), while α2δ agonists are advised as first-line agents in patients with severe sleep disturbance, comorbid anxiety, RLS-related pain, or previous history of ICDs (Garcia-Borreguero et al., 2013). Evidence-based guidelines on treating RLS were published by EFNS and IRLSSG in 2012 and 2013 respectively. Many other pharmacological treatment reviews and research papers of RLS have been published recently (Garcia-Borreguero and Williams, 2014; Hornyak et al., 2014). A conclusion of several medications is shown above (Table 4).
Other drugs such as dopaminergic agents (piribedil), anticonvulsants (gabapentin), opioids (tramadol, methodone), iron, hypnotic and sedative agents, folate, vitamin B12, magnesium, vitamin E, botulinum toxin, physiotherapy, phototherapy, and aerobic exercises are not recommended in clinical practice due to insufficient evidence. However, they can be used as auxiliary drugs concerning to the symptoms and comorbidities of the patient.
As depression is a very common comorbidity in RLS and many antidepressants such as selective serotonin reuptake inhibitors (SSRIs) and tricyclic antidepressants (TCAs) can worsen the symptoms of RLS, treatment of depression in RLS patients should be cautious. Since bupropion, a newer antidepressant, doesn’t show any evidence of exacerbation of RLS symptoms, it is used as an effective antidepressant in these patients (Mackie and Winkelman, 2015).
Restless legs syndrome is very common in women during pregnancy and lactation, with a prevalence of 15–25% (Hubner et al., 2013). Guidelines on treating RLS during pregnancy and lactation have been published by IRLSSG in 2014 (Picchietti et al., 2015) (Table 5). Once RLS is diagnosed in pregnancy, non-medicine treatment should be considered firstly. Patients should be educated about the natural course of RLS during pregnancy, in which RLS commonly remits or disappears after delivery. Moderate exercise and avoidance of aggravated factors such as iron deficiency, long-term immobility, serotonergic antidepressants should be suggested. Iron level should be measured to decide whether to treat the patient with iron. Iron therapy (oral or intravenous) should be given when serum ferritin < 75 ug/L. When serum ferritin > 75 ug/L with refractory RLS, drug treatment should be considered.
Loss of efficacy and augmentation are two main treatment failures after a long course of treatment of RLS. The probable reasons of them might be the natural worsening of RLS symptoms over time or compensatory response of CNS to chronic drug treatment (Mackie and Winkelman, 2015). Iron deficiency should be treated with iron supplement. Both of the intravenous and oral iron formulations are proved to be effective in some RLS patients (Trotti et al., 2012; Hornyak et al., 2014). Further study about effect of iron therapy in all RLS patients or only a certain type of RLS remains to be investigated.
Augmentation refers to a worsening of the symptoms that occurs very commonly among RLS patients after long-term treatment with some certain medications. An overall augmentation rate of 5.6% was reported (Liu et al., 2016). All the current dopaminergic drugs and another non-dopaminergic drug tramadol have been reported to show some degree of augmentation (Trenkwalder et al., 2007; Vetrugno et al., 2007; Hogl et al., 2010; Oertel et al., 2011), which are thought to be related to dose and duration of medication and individual factors such as iron deficiency (Garcia-Borreguero et al., 2013). The highest incidence rate of augmentation occurred with levodopa was up to 60–80% in RLS patients (Hogl et al., 2010). In addition, incidence rate was reported to be higher in patients treating with shorter-acting dopaminergic agents (pramipexole, ropinirole) than longer-acting dopaminergic agents (rotigotine, cabergoling). A possible explanation is the masking of earlier symptom onset by longer-acting dopaminergic agents (Garcia-Borreguero and Williams, 2014). To prevent augmentation, it is important to initiate the treatment with α2δ agonists for milder RLS patients (Silber et al., 2013), and a lowest effective dose of dopaminergic agents should be established to decrease the incidence and delay the occurrence of augmentation (Silber et al., 2013). Management of augmentation is not to increase the dose of dopaminergic agents, but to add a non-dopaminergic agent as a combination strategy (Silber et al., 2013). Moreover, loss of efficacy is the reduction of drug efficacy over time. In these cases, RLS symptoms are not worse than before initiating the treatment (Garcia-Borreguero and Williams, 2014), a combination therapy is recommended in loss of efficacy to decrease the side effects of certain medications, as well as to prevent augmentation.
Non-pharmacological Treatment
Sleep hygiene should be corrected before all the pharmacological treatment. Sleep deprivation, sleep disturbances and factors that can result in insomnia should all be avoided. Another common but easily neglected disorder is OSAS. Early treatment for OSAS is beneficial for improving sleep for RLS patients. Other non-pharmacological treatments have been proven to be effective in RLS. tsDCS showed a short-lasting clinical improvement in idiopathic RLS patients (Heide et al., 2014), while high-frequency rTMS resulted in an significant improvement in the motor symptoms and sleep disturbances in RLS patients (Lin Y.C. et al., 2015). But these non-pharmacological treatment for RLS studies are scarce. They show some advantages in symptomatic RLS patients who do not respond to or do not tolerate the classic pharmacological treatments. It may bring brand new solutions to these patients. On the other hand, these methods are non-invasive and safe, no significant side effects have been observed yet. Developing these new methods can be a great benefit for RLS patients.
Conclusion
There have been a lot of advances in the fields of RLS in recent years, as the diagnostic criteria have been revised in 2012. A deeper insight in pathophysiology of RLS has put iron metabolism dysfunction in an important position, as well as dopaminergic system dysfunction, has been demonstrated. Guidelines of long-term treatment of RLS are published by IRLSSG in 2013 to help with the treatment of RLS for clinicians. Prevention and management of augmentation for long-term treatment has been improved through clinical experience and researches. More researches should be done to discover the pathophysiology, and to find better treatment and management of augmentation of RLS.
Author Contributions
SG: drafting the manuscript and the tables; HJ, CH, JL, XX, GZ: giving advice on conception and design; JH, NX: revising the manuscript; ZL: giving advice on conception and design; TW: conception and design. Giving final approval of the version to be published.
Funding
This work was supported by grants 31171211 and 81471305 from the National Natural Science Foundation of China (to TW), grant 81200983 from the National Natural Science Foundation of China (to NX), grant 81301082 from the National Natural Science Foundation of China (to JH), grant 2012B09 from China Medical Foundation (to NX) and grant 0203201343 from Hubei Molecular Imaging Key Laboratory (to NX).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
ADHD, attention-deficit/hyperactivity disorder; AS, ankylosing spondylitis; BBBs, blood–brain barriers; CH-RLSq, Cambridge–Hopkins diagnostic questionnaire for RLS; CI, confidence interval; CSF, cerebrospinal fluid; Cul3, Cullin-3; DA, dopaminergic; DAT, dopamine transporter; DCSAD, Diagnostic Classification of Sleep and Arousal Disorders; DIMS, disorder of initiating and maintaining sleep; DOES, disorder of excessive somnolence; EFNS, European Federation of Neurological Societies; EXP, expanded screening questionnaire; GWASs, genome-wide association studies; HRQoL, health-related quality of life; ICDs, impulse control disorders; ICSD, International Classification of Sleep Disorders; IRLS, international restless legs scale; IRLSSG, International Restless Legs Syndrome Study Group; IRP2, iron regulatory protein-2; NIH, National Institutes of Health; OR, odd ratio; OSAS, obstructive sleep apnea syndrome; PLMs, periodic limb movements; PLMS, periodic limb movement during sleep; PLMW, periodic limb movement during wakefulness; PSG, polysomnogram; RLS, restless legs syndrome; rTMS, repetitive transcranial magnetic stimulation; SNPs, single nucleotide polymorphisms; tsDCS, transcutaneous spinal direct current stimulation.
References
Al-Jahdali, H. H., Al-Qadhi, W. A., Khogeer, H. A., Al-Hejaili, F. F., Al-Ghamdi, S. M., and Al Sayyari, A. A. (2009). Restless legs syndrome in patients on dialysis. Saudi J Kidney Dis. Transpl. 20, 378–385.
Allen, R. P., Picchietti, D. L., Garcia-Borreguero, D., Ondo, W. G., Walters, A. S., Winkelman, J. W., et al. (2014). Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 15, 860–873. doi: 10.1016/j.sleep.2014.03.025
Andretic, R., and Hirsh, J. (2000). Circadian modulation of dopamine receptor responsiveness in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 97, 1873–1878. doi: 10.1073/pnas.97.4.1873
Applebee, G. A., Guillot, A. P., Schuman, C. C., Teddy, S., and Attarian, H. P. (2009). Restless legs syndrome in pediatric patients with chronic kidney disease. Pediatr. Nephrol. 24, 545–548. doi: 10.1007/s00467-008-1057-x
Arbuckle, R., Abetz, L., Durmer, J. S., Ivanenko, A., Owens, J. A., Croenlein, J., et al. (2010). Development of the pediatric restless legs syndrome severity scale (P-RLS-SS): a patient-reported outcome measure of pediatric RLS symptoms and impact. Sleep Med. 11, 897–906. doi: 10.1016/j.sleep.2010.03.016
Barriere, G., Cazalets, J. R., Bioulac, B., Tison, F., and Ghorayeb, I. (2005). The restless legs syndrome. Prog. Neurobiol. 77, 139–165. doi: 10.1016/j.pneurobio.2005.10.007
Becker, J., Becker, F., Schindelbeck, K., Koch, P., Preig, J., Karge, T., et al. (2015). P344. Restless-legs-syndrome and iron deficiency in patients with inflammatory bowel disease. J. Crohns Colitis 9, 250–251. doi: 10.1093/ecco-jcc/jju027.463
Berger, K., Luedemann, J., Trenkwalder, C., John, U., and Kessler, C. (2004). Sex and the risk of restless legs syndrome in the general population. Arch. Intern. Med. 164, 196–202. doi: 10.1001/archinte.164.2.196
Berger, K., Von Eckardstein, A., Trenkwalder, C., Rothdach, A., Junker, R., and Weiland, S. K. (2002). Iron metabolism and the risk of restless legs syndrome in an elderly general population–the MEMO-Study. J. Neurol. 249, 1195–1199. doi: 10.1007/s00415-002-0805-2
Bonati, M. T., Ferini-Strambi, L., Aridon, P., Oldani, A., Zucconi, M., and Casari, G. (2003). Autosomal dominant restless legs syndrome maps on chromosome 14q. Brain 126, 1485–1492. doi: 10.1093/brain/awg137
Bucher, S. F., Seelos, K. C., Oertel, W. H., Reiser, M., and Trenkwalder, C. (1997). Cerebral generators involved in the pathogenesis of the restless legs syndrome. Ann. Neurol. 41, 639–645. doi: 10.1002/ana.410410513
Cassel, W., Kesper, K., Bauer, A., Grieger, F., Schollmayer, E., Joeres, L., et al. (2016). Significant association between systolic and diastolic blood pressure elevations and periodic limb movements in patients with idiopathic restless legs syndrome. Sleep Med. 17, 109–120. doi: 10.1016/j.sleep.2014.12.019
Castaneda, T. R., De Prado, B. M., Prieto, D., and Mora, F. (2004). Circadian rhythms of dopamine, glutamate and GABA in the striatum and nucleus accumbens of the awake rat: modulation by light. J. Pineal Res. 36, 177–185. doi: 10.1046/j.1600-079X.2003.00114.x
Catoire, H., Dion, P. A., Xiong, L., Amari, M., Gaudet, R., Girard, S. L., et al. (2011). Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann. Neurol. 70, 170–175. doi: 10.1002/ana.22435
Chen, N. H., Chuang, L. P., Yang, C. T., Kushida, C. A., Hsu, S. C., Wang, P. C., et al. (2010). The prevalence of restless legs syndrome in Taiwanese adults. Psychiatry Clin. Neurosci. 64, 170–178. doi: 10.1111/j.1440-1819.2010.02067.x
Cho, S. J., Hong, J. P., Hahm, B. J., Jeon, H. J., Chang, S. M., Cho, M. J., et al. (2009). Restless legs syndrome in a community sample of Korean adults: prevalence, impact on quality of life, and association with DSM-IV psychiatric disorders. Sleep 32, 1069–1076.
Clemens, S., and Hochman, S. (2004). Conversion of the modulatory actions of dopamine on spinal reflexes from depression to facilitation in D3 receptor knock-out mice. J. Neurosci. 24, 11337–11345. doi: 10.1523/JNEUROSCI.3698-04.2004
Connor, J. R., Boyer, P. J., Menzies, S. L., Dellinger, B., Allen, R. P., Ondo, W. G., et al. (2003). Neuropathological examination suggests impaired brain iron acquisition in restless legs syndrome. Neurology 61, 304–309. doi: 10.1212/01.WNL.0000078887.16593.12
Curgunlu, A., Doventas, A., Karadeniz, D., Erdincler, D. S., Ozturk, A. K., Karter, Y., et al. (2012). Prevalence and characteristics of restless legs syndrome (RLS) in the elderly and the relation of serum ferritin levels with disease severity: hospital-based study from Istanbul. Turkey. Arch. Gerontol. Geriatr. 55, 73–76. doi: 10.1016/j.archger.2011.06.002
Dauvilliers, Y., and Winkelmann, J. (2013). Restless legs syndrome: update on pathogenesis. Curr. Opin. Pulm. Med. 19, 594–600. doi: 10.1097/MCP.0b013e328365ab07
Davis, I. D., Baron, J., O’riordan, M. A., and Rosen, C. L. (2005). Sleep disturbances in pediatric dialysis patients. Pediatr. Nephrol. 20, 69–75. doi: 10.1007/s00467-004-1700-0
Deriu, M., Cossu, G., Molari, A., Murgia, D., Mereu, A., Ferrigno, P., et al. (2009). Restless legs syndrome in multiple sclerosis: a case-control study. Mov. Disord. 24, 697–701. doi: 10.1002/mds.22431
Desautels, A., Turecki, G., Montplaisir, J., Sequeira, A., Verner, A., and Rouleau, G. A. (2001). Identification of a major susceptibility locus for restless legs syndrome on chromosome 12q. Am. J. Hum. Genet. 69, 1266–1270. doi: 10.1086/324649
Desautels, A., Turecki, G., Montplaisir, J., Xiong, L., Walters, A. S., Ehrenberg, B. L., et al. (2005). Restless legs syndrome: confirmation of linkage to chromosome 12q, genetic heterogeneity, and evidence of complexity. Arch. Neurol. 62, 591–596. doi: 10.1001/archneur.62.4.591
Earley, C. J., Allen, R. P., Connor, J. R., Ferrucci, L., and Troncoso, J. (2009). The dopaminergic neurons of the A11 system in RLS autopsy brains appear normal. Sleep Med. 10, 1155–1157. doi: 10.1016/j.sleep.2009.01.006
Freedom, T., and Merchut, M. P. (2003). Arm restlessness as the initial symptom in restless legs syndrome. Arch. Neurol. 60, 1013–1015. doi: 10.1001/archneur.60.7.1013
Freeman, A., Pranski, E., Miller, R. D., Radmard, S., Bernhard, D., Jinnah, H. A., et al. (2012). Sleep fragmentation and motor restlessness in a Drosophila model of restless legs syndrome. Curr. Biol. 22, 1142–1148. doi: 10.1016/j.cub.2012.04.027
Galbiati, A., Marelli, S., Giora, E., Zucconi, M., Oldani, A., and Ferini-Strambi, L. (2015). Neurocognitive function in patients with idiopathic Restless Legs Syndrome before and after treatment with dopamine-agonist. Int. J. Psychophysiol. 95, 304–309. doi: 10.1016/j.ijpsycho.2014.12.005
Gao, X., Schwarzschild, M. A., Wang, H., and Ascherio, A. (2009). Obesity and restless legs syndrome in men and women. Neurology 72, 1255–1261. doi: 10.1212/01.wnl.0000345673.35676.1c
Garcia-Borreguero, D., Kohnen, R., Silber, M. H., Winkelman, J. W., Earley, C. J., Hogl, B., et al. (2013). The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 14, 675–684. doi: 10.1016/j.sleep.2013.05.016
Garcia-Borreguero, D., Larrosa, O., Granizo, J. J., De La Llave, Y., and Hening, W. A. (2004). Circadian variation in neuroendocrine response to L-dopa in patients with restless legs syndrome. Sleep 27, 669–673.
Garcia-Borreguero, D., and Williams, A. M. (2014). An update on restless legs syndrome (Willis-Ekbom disease): clinical features, pathogenesis and treatment. Curr. Opin. Neurol. 27, 493–501. doi: 10.1097/WCO.0000000000000117
Garcia-Martin, E., Jimenez-Jimenez, F. J., Alonso-Navarro, H., Martinez, C., Zurdo, M., Turpin-Fenoll, L., et al. (2015). Heme oxygenase-1 and 2 common genetic variants and risk for restless legs syndrome. Medicine 94, e1448. doi: 10.1097/MD.0000000000001448
Giannaki, C. D., Sakkas, G. K., Karatzaferi, C., Hadjigeorgiou, G. M., Lavdas, E., Liakopoulos, V., et al. (2011). Evidence of increased muscle atrophy and impaired quality of life parameters in patients with uremic restless legs syndrome. PLoS ONE 6:e25180. doi: 10.1371/journal.pone.0025180
Goffredo Filho, G. S., Gorini, C. C., Purysko, A. S., Silva, H. C., and Elias, I. E. (2003). Restless legs syndrome in patients on chronic hemodialysis in a Brazilian city: frequency, biochemical findings and comorbidities. Arq. Neuropsiquiatr. 61, 723–727. doi: 10.1590/S0004-282X2003000500004
Goulart, L. I., Delgado Rodrigues, R. N., and Prieto Peres, M. F. (2014). Restless legs syndrome and pain disorders: what’s in common? Curr. Pain Headache Rep. 18:461. doi: 10.1007/s11916-014-0461-0
Haba-Rubio, J., Staner, L., Petiau, C., Erb, G., Schunck, T., and Macher, J. P. (2005). Restless legs syndrome and low brain iron levels in patients with haemochromatosis. J. Neurol. Neurosurg. Psychiatry 76, 1009–1010. doi: 10.1136/jnnp.2003.030536
Haider, I., Anees, M., and Shahid, S. A. (2014). Restless legs syndrome in end stage renal disease patients on haemodialysis. Pak. J. Med. Sci. 30, 1209–1212. doi: 10.12669/pjms.306.5691
Heide, A. C., Winkler, T., Helms, H. J., Nitsche, M. A., Trenkwalder, C., Paulus, W., et al. (2014). Effects of transcutaneous spinal direct current stimulation in idiopathic restless legs syndrome patients. Brain Stimul. 7, 636–642. doi: 10.1016/j.brs.2014.06.008
Hening, W. (2004). The clinical neurophysiology of the restless legs syndrome and periodic limb movements. Part I: diagnosis, assessment, and characterization. Clin. Neurophysiol. 115, 1965–1974. doi: 10.1016/j.clinph.2004.03.032
Hening, W. A., and Caivano, C. K. (2008). Restless legs syndrome: a common disorder in patients with rheumatologic conditions. Semin. Arthritis Rheum. 38, 55–62. doi: 10.1016/j.semarthrit.2007.09.001
Hening, W. A., Walters, A. S., Wagner, M., Rosen, R., Chen, V., Kim, S., et al. (1999). Circadian rhythm of motor restlessness and sensory symptoms in the idiopathic restless legs syndrome. Sleep 22, 901–912. doi: 10.1093/sleep/22.7.901
Hoek, P. D., Smits, M. G., De Roos, N. M., Rijsman, R. M., and Witteman, B. J. (2015). Increased prevalence of restless legs syndrome in patients with Crohn’s disease. Eur. J. Gastroenterol. Hepatol. 27, 951–955. doi: 10.1097/MEG.0000000000000386
Hogl, B., Garcia-Borreguero, D., Kohnen, R., Ferini-Strambi, L., Hadjigeorgiou, G., Hornyak, M., et al. (2010). Progressive development of augmentation during long-term treatment with levodopa in restless legs syndrome: results of a prospective multi-center study. J. Neurol. 257, 230–237. doi: 10.1007/s00415-009-5299-8
Hogl, B., Kiechl, S., Willeit, J., Saletu, M., Frauscher, B., Seppi, K., et al. (2005). Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology 64, 1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3
Holstege, J. C., Van Dijken, H., Buijs, R. M., Goedknegt, H., Gosens, T., and Bongers, C. M. (1996). Distribution of dopamine immunoreactivity in the rat, cat and monkey spinal cord. J. Comp. Neurol. 376, 631–652. doi: 10.1002/(SICI)1096-9861(19961223)376:4<631::AID-CNE10>3.0.CO;2-P
Hoque, R., and Chesson, A. L. Jr. (2010). Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis. J. Clin. Sleep Med. 6, 79–83.
Hornyak, M., Feige, B., Voderholzer, U., Philipsen, A., and Riemann, D. (2007). Polysomnography findings in patients with restless legs syndrome and in healthy controls: a comparative observational study. Sleep 30, 861–865. doi: 10.1093/sleep/30.7.861
Hornyak, M., Scholz, H., Kohnen, R., Bengel, J., Kassubek, J., and Trenkwalder, C. (2014). What treatment works best for restless legs syndrome? Meta-analyses of dopaminergic and non-dopaminergic medications. Sleep Med. Rev. 18, 153–164. doi: 10.1016/j.smrv.2013.03.004
Hubner, A., Krafft, A., Gadient, S., Werth, E., Zimmermann, R., and Bassetti, C. L. (2013). Characteristics and determinants of restless legs syndrome in pregnancy: a prospective study. Neurology 80, 738–742. doi: 10.1212/WNL.0b013e318283baf3
Iber, C., Ancoli-Israel, S., Chesson, A., and Quan Sf for the American Academy of Sleep Medicine (2007). The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, 1st Edn. Westchester, IL: American Academy of Sleep Medicine.
International Restless Legs Syndrome Study Group (2012). IRLSSG Diagnostic Criteria for RLS. Available at: www.irlssg.org
Ismailogullari, S., Ozturk, A., Mazicioglu, M. M., Serin, S., Gultekin, M., and Aksu, M. (2010). Restless legs syndrome and pregnancy in Kayseri, Turkey: a hospital based survey. Sleep Biol. Rhythms 8, 137–143. doi: 10.1111/j.1479-8425.2010.00437.x
Jimenez-Jimenez, F. J., Garcia-Martin, E., Alonso-Navarro, H., Martinez, C., Zurdo, M., Turpin-Fenoll, L., et al. (2015). Association between vitamin D receptor rs731236 (Taq1) polymorphism and risk for restless legs syndrome in the Spanish Caucasian population. Medicine 94:e2125. doi: 10.1097/MD.0000000000002125
Kahvecioglu, S., Yildiz, D., Buyukkoyuncu, N., Celik, H., Tufan, F., Kilic, A. K., et al. (2016). Effect of renal transplantation in restless legs syndrome. Exp. Clin. Transplant 14, 45–49. doi: 10.6002/ect.2014.0163
Karroum, E. G., Leu-Semenescu, S., and Arnulf, I. (2012). Topography of the sensations in primary restless legs syndrome. J. Neurol. Sci. 320, 26–31. doi: 10.1016/j.jns.2012.05.051
Kemlink, D., Polo, O., Montagna, P., Provini, F., Stiasny-Kolster, K., Oertel, W., et al. (2007). Family-based association study of the restless legs syndrome loci 2 and 3 in a European population. Mov. Disord. 22, 207–212. doi: 10.1002/mds.21254
Kim, J. M., Kwon, H. M., Lim, C. S., Kim, Y. S., Lee, S. J., and Nam, H. (2008). Restless legs syndrome in patients on hemodialysis: symptom severity and risk factors. J. Clin. Neurol. 4, 153–157. doi: 10.3988/jcn.2008.4.4.153
Koh, S. Y., Kim, M. S., Lee, S. M., Hong, J. M., and Yoon, J. H. (2015). Impaired vascular endothelial function in patients with restless legs syndrome: a new aspect of the vascular pathophysiology. J. Neurol. Sci. 359, 207–210. doi: 10.1016/j.jns.2015.10.041
Kotagal, S., and Silber, M. H. (2004). Childhood-onset restless legs syndrome. Ann. Neurol. 56, 803–807. doi: 10.1002/ana.20292
Kumru, H., Portell, E., Barrio, M., and Santamaria, J. (2014). Restless legs syndrome in patients with sequelae of poliomyelitis. Parkinsonism Relat. Disord. 20, 1056–1058. doi: 10.1016/j.parkreldis.2014.06.014
Kushida, C., Martin, M., Nikam, P., Blaisdell, B., Wallenstein, G., Ferini-Strambi, L., et al. (2007). Burden of restless legs syndrome on health-related quality of life. Q. Life Res. 16, 617–624. doi: 10.1007/s11136-006-9142-8
La Manna, G., Pizza, F., Persici, E., Baraldi, O., Comai, G., Cappuccilli, M. L., et al. (2011). Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol. Dial. Transplant. 26, 1976–1983. doi: 10.1093/ndt/gfq681
Lee, C. S., Kim, T., Lee, S., Jeon, H. J., Bang, Y. R., and Yoon, I. Y. (2016). Symptom severity of restless legs syndrome predicts its clinical course. Am. J. Med. 129, 438–445. doi: 10.1016/j.amjmed.2015.12.020
Lee, K. A., Zaffke, M. E., and Baratte-Beebe, K. (2001). Restless legs syndrome and sleep disturbance during pregnancy: the role of folate and iron. J. Womens Health Gend. Based Med. 10, 335–341. doi: 10.1089/152460901750269652
Lin, C. H., Sy, H. N., Chang, H. W., Liou, H. H., Lin, C. Y., Wu, V. C., et al. (2015). Restless legs syndrome is associated with cardio/cerebrovascular events and mortality in end-stage renal disease. Eur. J. Neurol. 22, 142–149. doi: 10.1111/ene.12545
Lin, Y. C., Feng, Y., Zhan, S. Q., Li, N., Ding, Y., Hou, Y., et al. (2015). Repetitive transcranial magnetic stimulation for the treatment of restless legs syndrome. Chin. Med. J. 128, 1728–1731. doi: 10.4103/0366-6999.159344
Liu, G. J., Wu, L., Wang, S. L., Ding, L., Xu, L. L., Wang, Y. F., et al. (2016). Incidence of augmentation in primary restless legs syndrome patients may not be that high: evidence from a systematic review and meta-analysis. Medicine 95, e2504. doi: 10.1097/md.0000000000002504
Mackie, S., and Winkelman, J. W. (2015). Long-term treatment of restless legs syndrome (RLS): an approach to management of worsening symptoms, loss of efficacy, and augmentation. CNS Drugs 29, 351–357. doi: 10.1007/s40263-015-0250-2
Marin, L. F., Dos Santos, W. A., Pedroso, J. L., Ferraz, H. B., De Carvalho, L. B., and Do Prado, G. F. (2010). Restless legs syndrome associated with Guillain-Barre syndrome: a report of two cases. Parkinsonism Relat. Disord. 16, 418–419. doi: 10.1016/j.parkreldis.2010.03.004
Matthews, W. B. (1976). Letter: iron deficiency and restless legs. Br. Med. J. 1:898. doi: 10.1136/bmj.1.6014.898-a
McDonagh, B., King, T., and Guptan, R. C. (2007). Restless legs syndrome in patients with chronic venous disorders: an untold story. Phlebology 22, 156–163. doi: 10.1258/026835507781477145
Michaud, M., Dumont, M., Selmaoui, B., Paquet, J., Fantini, M. L., and Montplaisir, J. (2004). Circadian rhythm of restless legs syndrome: relationship with biological markers. Ann. Neurol. 55, 372–380. doi: 10.1002/ana.10843
Mizuno, S., Mihara, T., Miyaoka, T., Inagaki, T., and Horiguchi, J. (2005). CSF iron, ferritin and transferrin levels in restless legs syndrome. J. Sleep Res. 14, 43–47. doi: 10.1111/j.1365-2869.2004.00403.x
Mohri, I., Kato-Nishimura, K., Tachibana, N., Ozono, K., and Taniike, M. (2008). Restless legs syndrome (RLS): an unrecognized cause for bedtime problems and insomnia in children. Sleep Med. 9, 701–702. doi: 10.1016/j.sleep.2007.08.005
Montplaisir, J., Boucher, S., Poirier, G., Lavigne, G., Lapierre, O., and Lesperance, P. (1997). Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov. Disord. 12, 61–65. doi: 10.1002/mds.870120111
Moon, H. J., Chang, Y., Lee, Y. S., Song, H. J., Chang, H. W., Ku, J., et al. (2014). T2 relaxometry using 3.0-tesla magnetic resonance imaging of the brain in early- and late-onset restless legs syndrome. J. Clin. Neurol. 10, 197–202. doi: 10.3988/jcn.2014.10.3.197
Moore, H. T., Winkelmann, J., Lin, L., Finn, L., Peppard, P., and Mignot, E. (2014). Periodic leg movements during sleep are associated with polymorphisms in BTBD9, TOX3/BC034767, MEIS1, MAP2K5/SKOR1, and PTPRD. Sleep 37, 1535–1542. doi: 10.5665/sleep.4006
Neau, J. P., Porcheron, A., Mathis, S., Julian, A., Meurice, J. C., Paquereau, J., et al. (2010). Restless legs syndrome and pregnancy: a questionnaire study in the Poitiers District, France. Eur. Neurol. 64, 268–274. doi: 10.1159/000321413
Nikic, P. M., Andric, B. R., Stojanovic-Stanojevic, M., Dordevic, V., Petrovic, D., and Stojimirovic, B. B. (2007). [Restless legs syndrome prevalence in patients on chronic hemodialysis in central Serbia]. Vojnosanit. Pregl. 64, 129–134. doi: 10.2298/VSP0702129N
Noga, B. R., Pinzon, A., Mesigil, R. P., and Hentall, I. D. (2004). Steady-state levels of monoamines in the rat lumbar spinal cord: spatial mapping and the effect of acute spinal cord injury. J. Neurophysiol. 92, 567–577. doi: 10.1152/jn.01035.2003
Nordlander, N. B. (1953). Therapy in restless legs. Acta Med. Scand. 145, 453–457. doi: 10.1111/j.0954-6820.1953.tb07042.x
Oertel, W., Trenkwalder, C., Benes, H., Ferini-Strambi, L., Hogl, B., Poewe, W., et al. (2011). Long-term safety and efficacy of rotigotine transdermal patch for moderate-to-severe idiopathic restless legs syndrome: a 5-year open-label extension study. Lancet Neurol. 10, 710–720. doi: 10.1016/S1474-4422(11)70127-2
Ohayon, M. M., O’hara, R., and Vitiello, M. V. (2012). Epidemiology of restless legs syndrome: a synthesis of the literature. Sleep Med. Rev. 16, 283–295. doi: 10.1016/j.smrv.2011.05.002
Ohayon, M. M., and Roth, T. (2002). Prevalence of restless legs syndrome and periodic leg movement disorder in the general population. J. Psychosom. Res. 53, 547–554. doi: 10.1016/S0022-3999(02)00443-9
O’Keeffe, S. T., Gavin, K., and Lavan, J. N. (1994). Iron status and restless legs syndrome in the elderly. Age Ageing 23, 200–203. doi: 10.1093/ageing/23.3.200
Ondo, W. G., He, Y., Rajasekaran, S., and Le, W. D. (2000). Clinical correlates of 6-hydroxydopamine injections into A11 dopaminergic neurons in rats: a possible model for restless legs syndrome. Mov. Disord. 15, 154–158. doi: 10.1002/1531-8257(200001)15:1<154::AID-MDS1025>3.0.CO;2-Q
Padhi, T., and Pradhan, S. (2014). Prevalence of restless legs syndrome among leprosy patients: a hospital based study. Lepr. Rev. 85, 218–223.
Panda, S., Taly, A. B., Sinha, S., Gururaj, G., Girish, N., and Nagaraja, D. (2012). Sleep-related disorders among a healthy population in South India. Neurol. India 60, 68–74. doi: 10.4103/0028-3886.93601
Pantaleo, N. P., Hening, W. A., Allen, R. P., and Earley, C. J. (2010). Pregnancy accounts for most of the gender difference in prevalence of familial RLS. Sleep Med. 11, 310–313. doi: 10.1016/j.sleep.2009.04.005
Paulus, W., Dowling, P., Rijsman, R., Stiasny-Kolster, K., and Trenkwalder, C. (2007). Update of the pathophysiology of the restless-legs-syndrome. Mov. Disord. 22(Suppl. 18), S431–S439. doi: 10.1002/mds.21533
Paulus, W., and Trenkwalder, C. (2006). Less is more: pathophysiology of dopaminergic-therapy-related augmentation in restless legs syndrome. Lancet Neurol. 5, 878–886. doi: 10.1016/S1474-4422(06)70576-2
Per, H., Gunay, N., Ismailogullari, S., Oztop, D. B., and Gunay, O. (2017). Determination of restless legs syndrome prevalence in children aged 13-16years in the provincial center of Kayseri. Brain Dev. 39, 154–160. doi: 10.1016/j.braindev.2016.08.011
Pereira, J. C. Jr., Rocha, E., Silva, I. R., and Pradella-Hallinan, M. (2013). Transient Willis-Ekbom’s disease (restless legs syndrome) during pregnancy may be caused by estradiol-mediated dopamine overmodulation. Med. Hypotheses 80, 205–208. doi: 10.1016/j.mehy.2012.11.030
Perlow, M. J., Gordon, E. K., Ebert, M. E., Hoffman, H. J., and Chase, T. N. (1977). The circadian variation in dopamine metabolism in the subhuman primate. J. Neurochem. 28, 1381–1383. doi: 10.1111/j.1471-4159.1977.tb12336.x
Phillips, B., Young, T., Finn, L., Asher, K., Hening, W. A., Purvis, C., et al. (2000). Epidemiology of restless legs syndrome in adults. Arch. Intern. Med. 160, 2137–2141. doi: 10.1001/archinte.160.14.2137
Picchietti, D. L., Arbuckle, R. A., Abetz, L., Durmer, J. S., Ivanenko, A., Owens, J. A., et al. (2011). Pediatric restless legs syndrome: analysis of symptom descriptions and drawings. J. Child Neurol. 26, 1365–1376. doi: 10.1177/0883073811405852
Picchietti, D. L., Bruni, O., De Weerd, A., Durmer, J. S., Kotagal, S., Owens, J. A., et al. (2013). Pediatric restless legs syndrome diagnostic criteria: an update by the International Restless Legs Syndrome Study Group. Sleep Med. 14, 1253–1259. doi: 10.1016/j.sleep.2013.08.778
Picchietti, D. L., Hensley, J. G., Bainbridge, J. L., Lee, K. A., Manconi, M., Mcgregor, J. A., et al. (2015). Consensus clinical practice guidelines for the diagnosis and treatment of restless legs syndrome/Willis-Ekbom disease during pregnancy and lactation. Sleep Med. Rev. 22, 64–77. doi: 10.1016/j.smrv.2014.10.009
Picchietti, M. A., and Picchietti, D. L. (2010). Advances in pediatric restless legs syndrome: iron, genetics, diagnosis and treatment. Sleep Med. 11, 643–651. doi: 10.1016/j.sleep.2009.11.014
Popat, R. A., Van Den Eeden, S. K., Tanner, C. M., Kushida, C. A., Rama, A. N., Black, J. E., et al. (2010). Reliability and validity of two self-administered questionnaires for screening restless legs syndrome in population-based studies. Sleep Med. 11, 154–160. doi: 10.1016/j.sleep.2009.01.012
Pullen, S. J., Wall, C. A., Angstman, E. R., Munitz, G. E., and Kotagal, S. (2011). Psychiatric comorbidity in children and adolescents with restless legs syndrome: a retrospective study. J. Clin. Sleep Med. 7, 587–596. doi: 10.5664/jcsm.1456
Rios Romenets, S., Dauvilliers, Y., Cochen De Cock, V., Carlander, B., Bayard, S., Galatas, C., et al. (2013). Restless legs syndrome outside the blood-brain barrier–exacerbation by domperidone in Parkinson’s disease. Parkinsonism Relat. Disord. 19, 92–94. doi: 10.1016/j.parkreldis.2012.07.019
Rizzo, G., Manners, D., Testa, C., Tonon, C., Vetrugno, R., Marconi, S., et al. (2013). Low brain iron content in idiopathic restless legs syndrome patients detected by phase imaging. Mov. Disord. 28, 1886–1890. doi: 10.1002/mds.25576
Rizzo, G., Tonon, C., Testa, C., Manners, D., Vetrugno, R., Pizza, F., et al. (2012). Abnormal medial thalamic metabolism in patients with idiopathic restless legs syndrome. Brain 135, 3712–3720. doi: 10.1093/brain/aws266
Rodriguez Martin, C., Miranda Riano, S., Celorrio San Miguel, M., and Prieto De Paula, J. M. (2015). Restless legs syndrome and hypothyroidism. Rev. Clin. Esp. 215, 247–249.
Rohani, M., Aghaei, M., Jenabi, A., Yazdanfar, S., Mousavi, D., and Miri, S. (2015). Restless legs syndrome in hemodialysis patients in Iran. Neurol. Sci. 36, 723–727. doi: 10.1007/s10072-014-2026-8
Roy, M., De Zwaan, M., Tuin, I., Philipsen, A., Brahler, E., and Muller, A. (2015). Association between restless legs syndrome and adult ADHD in a German community-based sample. J. Atten. Disord. doi: 10.1177/1087054714561291 [Epub ahead of print].
Rutkove, S. B., Matheson, J. K., and Logigian, E. L. (1996). Restless legs syndrome in patients with polyneuropathy. Muscle Nerve 19, 670–672. doi: 10.1002/(SICI)1097-4598(199605)19:5<670::AID-MUS20>3.0.CO;2-Q
Schade, R., Vick, K., Ott, T., Sohr, R., Pfister, C., Bellach, J., et al. (1995). Circadian rhythms of dopamine and cholecystokinin in nucleus accumbens and striatum of rats–influence on dopaminergic stimulation. Chronobiol. Int. 12, 87–99. doi: 10.3109/07420529509064504
Schimmelmann, B. G., Friedel, S., Nguyen, T. T., Sauer, S., Ganz Vogel, C. I., Konrad, K., et al. (2009). Exploring the genetic link between RLS and ADHD. J. Psychiatr. Res. 43, 941–945. doi: 10.1016/j.jpsychires.2009.01.003
Schmidauer, C., Sojer, M., Seppi, M., Stockner, H., Hogl, B., Biedermann, B., et al. (2005). Transcranial ultrasound shows nigral hypoechogenicity in restless legs syndrome. Ann. Neurol. 58, 630–634. doi: 10.1002/ana.20572
Schormair, B., Kemlink, D., Roeske, D., Eckstein, G., Xiong, L., Lichtner, P., et al. (2008). PTPRD (protein tyrosine phosphatase receptor type delta) is associated with restless legs syndrome. Nat. Genet. 40, 946–948. doi: 10.1038/ng.190
Schulte, E. C., Altmaier, E., Berger, H. S., Do, K. T., Kastenmuller, G., Wahl, S., et al. (2016). Alterations in lipid and inositol metabolisms in two dopaminergic disorders. PLoS ONE 11:e0147129. doi: 10.1371/journal.pone.0147129
Sechi, G., and Sechi, E. (2013). Restless legs syndrome and cerebrovascular disease. Lancet Neurol. 12, 734. doi: 10.1016/S1474-4422(13)70106-6
Shaw, P. J., and Duntley, S. P. (2012). Neurological disorders: towards a mechanistic understanding of restless legs syndrome. Curr. Biol. 22, R485–R486. doi: 10.1016/j.cub.2012.05.004
Shi, Y., Yu, H., Ding, D., Yu, P., Wu, D., and Hong, Z. (2015). Prevalence and risk factors of restless legs syndrome among Chinese adults in a rural community of Shanghai in China. PLoS ONE 10:e0121215. doi: 10.1371/journal.pone.0121215
Silber, M. H., Becker, P. M., Earley, C., Garcia-Borreguero, D., Ondo, W. G., and Medical Advisory Board of the Willis-Ekbom Disease Foundation (2013). Willis-Ekbom disease foundation revised consensus statement on the management of restless legs syndrome. Mayo Clin. Proc. 88, 977–986. doi: 10.1016/j.mayocp.2013.06.016
Silver, N., Allen, R. P., and Earley, C. J. (2010). MEIS1 as a potential mediator of the RLS-iron pathology. Mov. Disord. 25, S513–S514.
Sinha, R., Davis, I. D., and Matsuda-Abedini, M. (2009). Sleep disturbances in children and adolescents with non-dialysis-dependent chronic kidney disease. Arch. Pediatr. Adolesc. Med. 163, 850–855. doi: 10.1001/archpediatrics.2009.149
Skidmore, F. M., Drago, V., Foster, P. S., and Heilman, K. M. (2009). Bilateral restless legs affecting a phantom limb, treated with dopamine agonists. J. Neurol. Neurosurg. Psychiatry 80, 569–570. doi: 10.1136/jnnp.2008.152652
Sowers, J. R., and Vlachakis, N. (1984). Circadian variation in plasma dopamine levels in man. J. Endocrinol. Invest. 7, 341–345. doi: 10.1007/BF03351014
Srivanitchapoom, P., Pandey, S., and Hallett, M. (2014). Restless legs syndrome and pregnancy: a review. Parkinsonism Relat. Disord. 20, 716–722. doi: 10.1016/j.parkreldis.2014.03.027
Stavropoulos, N., and Young, M. W. (2011). insomniac and Cullin-3 regulate sleep and wakefulness in Drosophila. Neuron 72, 964–976. doi: 10.1016/j.neuron.2011.12.003
Stefansson, H., Rye, D. B., Hicks, A., Petursson, H., Ingason, A., Thorgeirsson, T. E., et al. (2007). A genetic risk factor for periodic limb movements in sleep. N. Engl. J. Med. 357, 639–647. doi: 10.1056/NEJMoa072743
Stevens, M. S. (2015). Restless legs syndrome/Willis-Ekbom disease morbidity: burden, quality of life, cardiovascular aspects, and sleep. Sleep Med. Clin. 10, 369–373, xv–xvi. doi: 10.1016/j.jsmc.2015.05.017
Sun, Y. M., Hoang, T., Neubauer, J. A., and Walters, A. S. (2011). Opioids protect against substantia nigra cell degeneration under conditions of iron deprivation: a mechanism of possible relevance to the Restless Legs Syndrome (RLS) and Parkinson’s disease. J. Neurol. Sci. 304, 93–101. doi: 10.1016/j.jns.2011.02.003
Swanson, J. M., Kinsbourne, M., Nigg, J., Lanphear, B., Stefanatos, G. A., Volkow, N., et al. (2007). Etiologic subtypes of attention-deficit/hyperactivity disorder: brain imaging, molecular genetic and environmental factors and the dopamine hypothesis. Neuropsychol. Rev. 17, 39–59. doi: 10.1007/s11065-007-9019-9
Tekatas, A., and Pamuk, O. N. (2015). Increased frequency of restless leg syndrome in patients with ankylosing spondylitis. Int. J. Rheum. Dis. 18, 58–62. doi: 10.1111/1756-185X.12323
Theander, L., Strombeck, B., Mandl, T., and Theander, E. (2010). Sleepiness or fatigue? Can we detect treatable causes of tiredness in primary Sjogren’s syndrome? Rheumatology 49, 1177–1183. doi: 10.1093/rheumatology/keq023
Tison, F., Crochard, A., Leger, D., Bouee, S., Lainey, E., and El Hasnaoui, A. (2005). Epidemiology of restless legs syndrome in French adults: a nationwide survey: the INSTANT Study. Neurology 65, 239–246. doi: 10.1212/01.wnl.0000168910.48309.4a
Trenkwalder, C., Benes, H., Grote, L., Garcia-Borreguero, D., Hogl, B., Hopp, M., et al. (2013). Prolonged release oxycodone-naloxone for treatment of severe restless legs syndrome after failure of previous treatment: a double-blind, randomised, placebo-controlled trial with an open-label extension. Lancet Neurol. 12, 1141–1150. doi: 10.1016/S1474-4422(13)70239-4
Trenkwalder, C., Benes, H., Grote, L., Happe, S., Hogl, B., Mathis, J., et al. (2007). Cabergoline compared to levodopa in the treatment of patients with severe restless legs syndrome: results from a multi-center, randomized, active controlled trial. Mov. Disord. 22, 696–703. doi: 10.1002/mds.21401
Trenkwalder, C., and Paulus, W. (2010). Restless legs syndrome: pathophysiology, clinical presentation and management. Nat. Rev. Neurol. 6, 337–346. doi: 10.1038/nrneurol.2010.55
Trenkwalder, C., Paulus, W., and Walters, A. S. (2005). The restless legs syndrome. Lancet Neurol. 4, 465–475. doi: 10.1016/S1474-4422(05)70139-3
Trotti, L. M., Bhadriraju, S., and Becker, L. A. (2012). Iron for restless legs syndrome. Cochrane Database Syst. Rev. 5:CD007834. doi: 10.1002/14651858.CD007834.pub2
Tsuboi, Y., Imamura, A., Sugimura, M., Nakano, S., Shirakawa, S., and Yamada, T. (2009). Prevalence of restless legs syndrome in a Japanese elderly population. Parkinsonism Relat. Disord. 15, 598–601. doi: 10.1016/j.parkreldis.2009.02.014
Uetani, N., Chagnon, M. J., Kennedy, T. E., Iwakura, Y., and Tremblay, M. L. (2006). Mammalian motoneuron axon targeting requires receptor protein tyrosine phosphatases sigma and delta. J. Neurosci. 26, 5872–5880. doi: 10.1523/JNEUROSCI.0386-06.2006
Vetrugno, R., La Morgia, C., D’angelo, R., Loi, D., Provini, F., Plazzi, G., et al. (2007). Augmentation of restless legs syndrome with long-term tramadol treatment. Mov. Disord. 22, 424–427. doi: 10.1002/mds.21342
Wali, S. O., and Alkhouli, A. F. (2015). Restless legs syndrome among Saudi end-stage renal disease patients on hemodialysis. Saudi Med. J. 36, 204–210. doi: 10.15537/smj.2015.2.10036
Walters, A. S., Hickey, K., Maltzman, J., Verrico, T., Joseph, D., Hening, W., et al. (1996). A questionnaire study of 138 patients with restless legs syndrome: the ’Night-Walkers’ survey. Neurology 46, 92–95. doi: 10.1212/WNL.46.1.92
Wang, G. J., Volkow, N. D., Logan, J., Pappas, N. R., Wong, C. T., Zhu, W., et al. (2001). Brain dopamine and obesity. Lancet 357, 354–357. doi: 10.1016/S0140-6736(00)03643-6
Ward, R. J., Zucca, F. A., Duyn, J. H., Crichton, R. R., and Zecca, L. (2014). The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 13, 1045–1060. doi: 10.1016/S1474-4422(14)70117-6
Winkelman, J. W., Chertow, G. M., and Lazarus, J. M. (1996). Restless legs syndrome in end-stage renal disease. Am. J. Kidney Dis. 28, 372–378. doi: 10.1016/S0272-6386(96)90494-1
Winkelmann, J., Muller-Myhsok, B., Wittchen, H. U., Hock, B., Prager, M., Pfister, H., et al. (2002). Complex segregation analysis of restless legs syndrome provides evidence for an autosomal dominant mode of inheritance in early age at onset families. Ann. Neurol. 52, 297–302. doi: 10.1002/ana.10282
Winkelmann, J., Schadrack, J., Wetter, T. C., Zieglgansberger, W., and Trenkwalder, C. (2001). Opioid and dopamine antagonist drug challenges in untreated restless legs syndrome patients. Sleep Med. 2, 57–61. doi: 10.1016/S1389-9457(00)00025-3
Winkelmann, J., Schormair, B., Lichtner, P., Ripke, S., Xiong, L., Jalilzadeh, S., et al. (2007). Genome-wide association study of restless legs syndrome identifies common variants in three genomic regions. Nat. Genet. 39, 1000–1006. doi: 10.1038/ng2099
Winkelmann, J., Wetter, T. C., Collado-Seidel, V., Gasser, T., Dichgans, M., Yassouridis, A., et al. (2000). Clinical characteristics and frequency of the hereditary restless legs syndrome in a population of 300 patients. Sleep 23, 597–602. doi: 10.1093/sleep/23.5.1b
Xiong, L., Montplaisir, J., Desautels, A., Barhdadi, A., Turecki, G., Levchenko, A., et al. (2010). Family study of restless legs syndrome in Quebec, Canada: clinical characterization of 671 familial cases. Arch. Neurol. 67, 617–622. doi: 10.1001/archneurol.2010.67
Yang, Q., Li, L., Chen, Q., Foldvary-Schaefer, N., Ondo, W. G., and Wang, Q. K. (2011). Association studies of variants in MEIS1, BTBD9, and MAP2K5/SKOR1 with restless legs syndrome in a US population. Sleep Med. 12, 800–804. doi: 10.1016/j.sleep.2011.06.006
Yilmaz, K., Kilincaslan, A., Aydin, N., and Kor, D. (2011). Prevalence and correlates of restless legs syndrome in adolescents. Dev. Med. Child Neurol. 53, 40–47. doi: 10.1111/j.1469-8749.2010.03796.x
Zak, R., Fisher, B., Couvadelli, B. V., Moss, N. M., and Walters, A. S. (2009). Preliminary study of the prevalence of restless legs syndrome in adults with attention deficit hyperactivity disorder. Percept. Mot. Skills 108, 759–763. doi: 10.2466/pms.108.3.759-763
Zanettini, R., Antonini, A., Gatto, G., Gentile, R., Tesei, S., and Pezzoli, G. (2007). Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N. Engl. J. Med. 356, 39–46. doi: 10.1056/NEJMoa054830
Zanigni, S., Giannini, G., Melotti, R., Pattaro, C., Provini, F., Cevoli, S., et al. (2014). Association between restless legs syndrome and migraine: a population-based study. Eur. J. Neurol. 21, 1205–1210. doi: 10.1111/ene.12462
Keywords: clinical presentation, diagnostic criteria, iron deficiency, dopamine, A11 cell group, treatment, augmentation, loss of efficacy
Citation: Guo S, Huang J, Jiang H, Han C, Li J, Xu X, Zhang G, Lin Z, Xiong N and Wang T (2017) Restless Legs Syndrome: From Pathophysiology to Clinical Diagnosis and Management. Front. Aging Neurosci. 9:171. doi: 10.3389/fnagi.2017.00171
Received: 10 August 2016; Accepted: 16 May 2017;
Published: 02 June 2017.
Edited by:
Aurel Popa-Wagner, University of Rostock, GermanyReviewed by:
Antón Barreiro-Iglesias, Universidade de Santiago de Compostela, SpainJun Chang Su, Fourth Military Medical University, China
Shuqin Zhan, Xuan Wu Hospital of the Capital Medical University, China
Copyright © 2017 Guo, Huang, Jiang, Han, Li, Xu, Zhang, Lin, Xiong and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wang, d2FuZ3Rhb3doQGh1c3QuZWR1LmNu
†These authors have contributed equally to this work.
 Shiyi Guo
Shiyi Guo Jinsha Huang
Jinsha Huang Haiyang Jiang
Haiyang Jiang Chao Han
Chao Han Jie Li
Jie Li Xiaoyun Xu
Xiaoyun Xu Guoxin Zhang
Guoxin Zhang Zhicheng Lin2,3
Zhicheng Lin2,3 Nian Xiong
Nian Xiong Tao Wang
Tao Wang