
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 29 May 2017
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 9 - 2017 | https://doi.org/10.3389/fnagi.2017.00166
This article is part of the Research Topic Brain Networks for Studying Healthy and Pathological Aging Mechanisms and Intervention Efficacy View all 48 articles
Neurodegenerative diseases are rarely caused by a mutation in a single gene but rather influenced by a combination of genetic, epigenetic and environmental factors. Emerging high-throughput technologies such as RNA sequencing have been instrumental in deciphering the molecular landscape of neurodegenerative diseases, however, the interpretation of such large amounts of data remains a challenge. Network biology has become a powerful platform to integrate multiple omics data to comprehensively explore the molecular networks in the context of health and disease. In this review article, we highlight recent advances in network biology approaches with an emphasis in brain-networks that have provided insights into the molecular mechanisms leading to the most prevalent neurodegenerative diseases including Alzheimer’s (AD), Parkinson’s (PD) and Huntington’s diseases (HD). We discuss how integrative approaches using multi-omics data from different tissues have been valuable for identifying biomarkers and therapeutic targets. In addition, we discuss the challenges the field of network medicine faces toward the translation of network-based findings into clinically actionable tools for personalized medicine applications.
Neurodegenerative diseases are usually sporadic in nature and commonly influenced by a wide range of genetic, epigenetic and environmental factors. With the advent of new high-throughput technologies such as RNA sequencing, it has become essential to develop methods beyond the classical pathway analysis to systematically interpret large amounts of data in the context of health and disease. Despite the progress of high-throughput genomic studies the precise pathogenic mechanisms leading to the most prevalent neurodegenerative diseases remain elusive. To this end, the applications of network biology have been successful to provide biological insight and to decipher the molecular underpinnings of neurodegenerative diseases. Network biology is based on the premise that complex diseases, like neurodegenerative diseases, are frequently caused by alterations in many genes comprising multiple biological pathways. A network consists of nodes and edges that may represent genes, proteins, miRNAs, noncoding RNAs, drugs, or diseases connected through a wide range of interactions including, but not limited to physical, genetic, co-expression and colocalization. An example of network analysis of Alzheimer’s disease (AD) that identifies central hubs is shown in Figure 1. Integration of multi-omic information coupled with network-based approaches is becoming an essential step towards the advancement of personalized medicine (Figure 2). Some of the frequently used terms in network biology approaches are defined in Table 1.
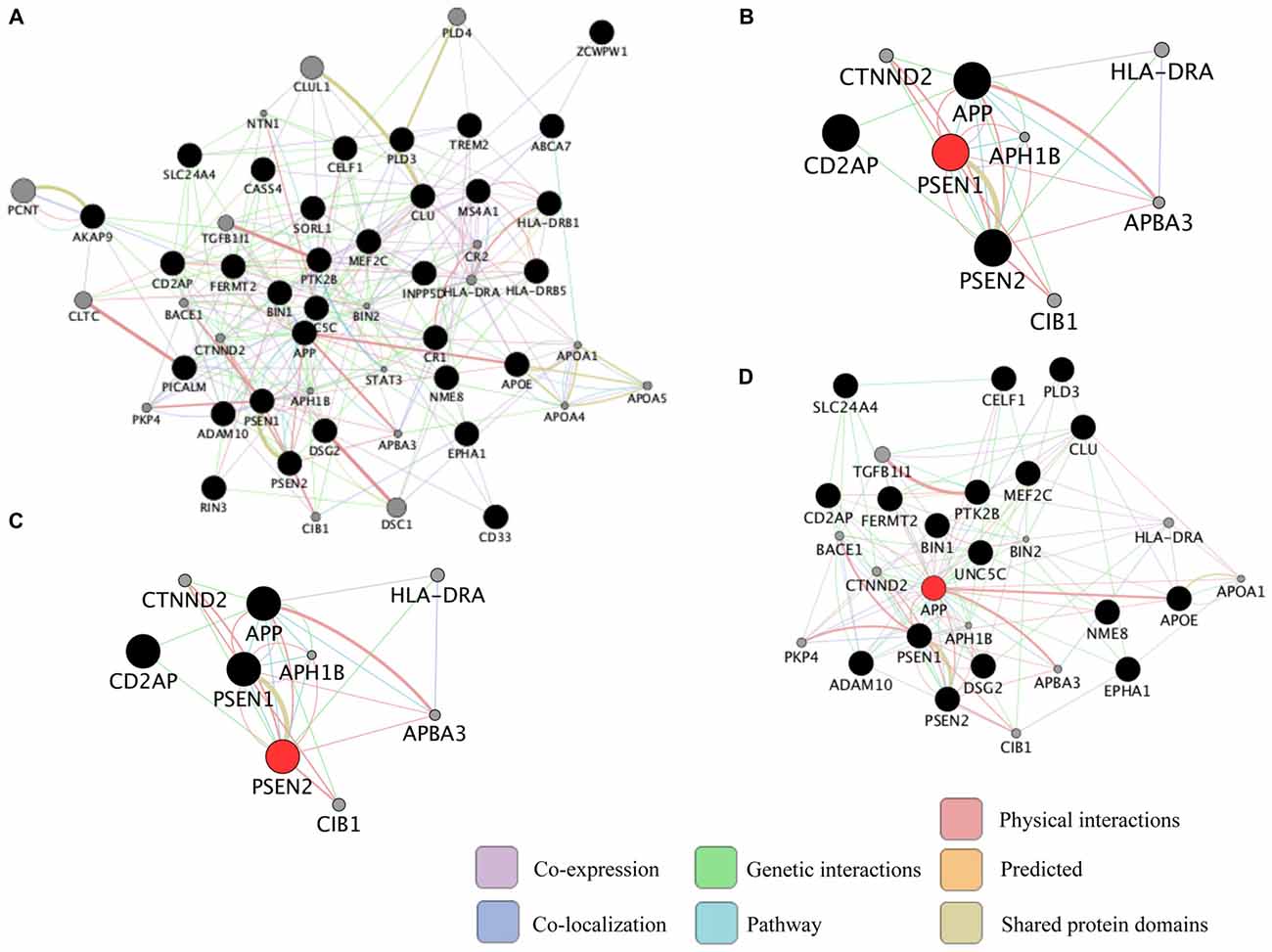
Representation of common biological networks. (A) Example of a network of interactions among genetic risk factors for Alzheimer’s disease (AD; black circles) and other related genes (gray circles). The color of the lines represents the type of interaction and the thickness is proportional to the strength of the association. (B–D) Presenilin 1 (PSEN1), PSEN2 and amyloid precursor protein (APP; red circles) are highly connected genes (hub genes) identified in the network. Hub genes usually play a central role in the disease. These networks were retrieved by GeneMANIA application in Cytoscape 3.1.1 as of September 2016 using the default settings to include the top 20 related genes and automatic weighting.
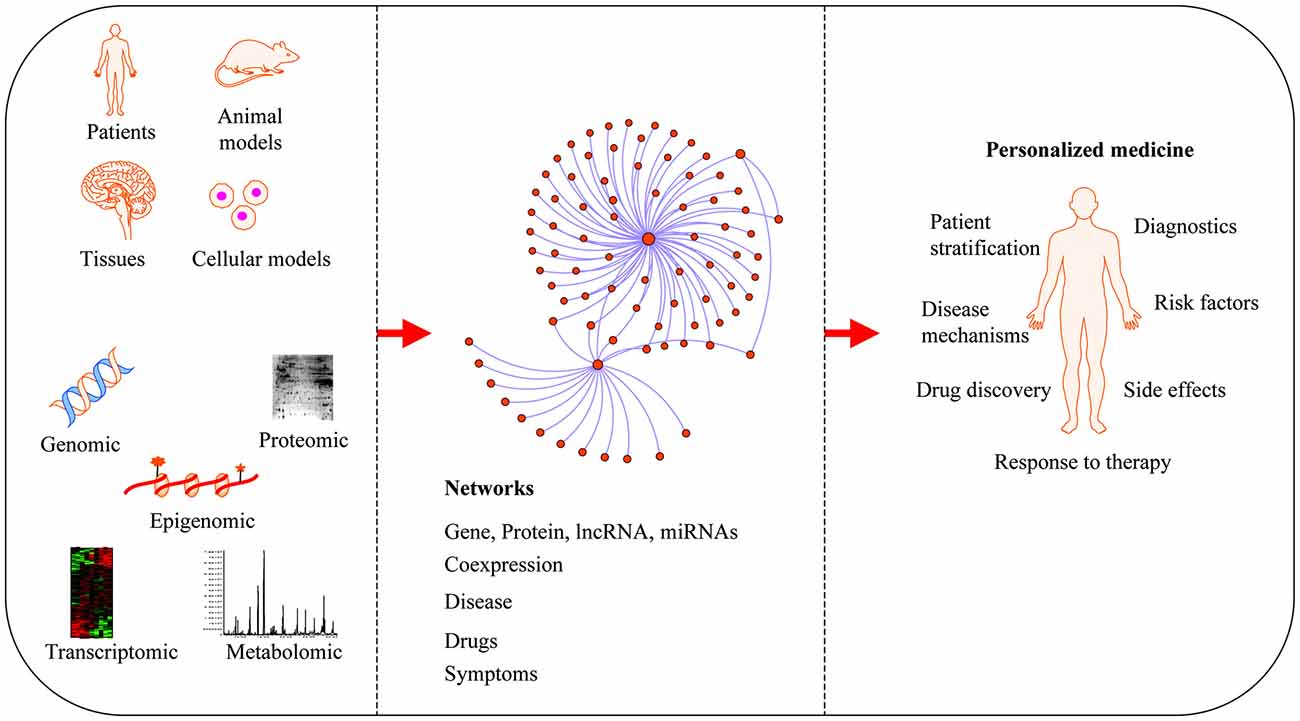
Applications of network medicine. Biological networks can be constructed from a wide range of different omic approaches including genomic, transcriptomic, epigenomic, metabolomic and proteomic datasets. In protein-protein interaction (PPI) networks, proteins are the nodes and their interactions are the edges. Network-based approaches have advanced the field of personalized medicine by providing novel mechanisms of disease, diagnostics and therapeutic targets.
Seminal work in network biology including the construction of the human disease network (Goh et al., 2007), the human functional linkage network (Linghu et al., 2009), the discovery of causal genes of obesity (Chen et al., 2008), and clinical biomarkers for cancer (Taylor et al., 2009), prompted efforts to study many different diseases using network-based approaches. In the last few years, there has been a steady growth in studies exploiting the concepts of network biology to understand neurodevelopmental and neurodegenerative diseases (Santiago and Potashkin, 2014a). For example, network approaches have successfully identified putative diagnostic biomarkers for Parkinson’s disease (PD; Santiago and Potashkin, 2013a, 2015; Santiago et al., 2014, 2016), and progressive supranuclear palsy (Santiago and Potashkin, 2014b) reviewed in Santiago and Potashkin (2013b, 2014a,c). In addition, network-based approaches have provided insights into the molecular mechanisms underlying co-morbid diseases associated with PD including diabetes (Santiago and Potashkin, 2013a) and cancer (Ibáñez et al., 2014). In this review article, we highlight the most recent advances in network biology applications to understand the most common neurodegenerative diseases with an emphasis on brain specific networks.
AD is the most prevalent neurodegenerative disease, responsible for the majority of the cases of dementia, affecting more than 44 million people worldwide with an estimated global cost of more than 600 billion dollars1. Although the exact mechanism of disease remains unclear, a complex combination of genetic, epigenetic, lifestyle, environmental factors and aging are believed to be responsible for most of the cases. Pathological features of AD include the accumulation of amyloid beta (Aβ) plaques and protein tau in neurofibrillary tangles (NFT). While most of the AD cases are late onset (LOAD) and sporadic, some genetic mutations in the amyloid precursor protein (APP), presenilin 1 (PSEN1) and presenilin 2 (PSEN2) are documented to cause early onset AD, which accounts for approximately 2% of the cases with symptoms appearing before the age of 65 (Goate et al., 1991; Levy-Lahad et al., 1995; Janssen et al., 2003). The apoliporotein E-ε4 (APOEε4) is the only genetic factor identified in more than 60% of the sporadic AD cases, however, it has also been found in healthy individuals thus suggesting that other genetic factors may be responsible for the disease (Coon et al., 2007). To date, emerging high-throughput genomic technologies have reported more than 2900 genetic variations associated with AD2.
Although these studies have been valuable to understand the genetic diversity associated with AD, the multi-factorial mechanisms leading to the disease are unclear. Network-based approaches have been successful to systematically interpret these results and to gain insight into the mechanisms of disease. In particular, integrative approaches combining multi-omic data in networks have been employed to identify susceptibility genes and pathways in AD. For example, combinatorial network analysis of proteomic and transcriptomic data revealed subnetworks enriched in pathways associated with the pathogenesis of AD including the downregulation of genes associated with the MAPK/ERK pathway and the upregulation of genes associated to the clathrin-mediated receptor endocytosis pathway (Hallock and Thomas, 2012; Table 2). In this regard, disruption of the clathrin-mediated receptor pathway can lead to increased levels of APP thereby contributing to disease progression (Schneider et al., 2008; Hallock and Thomas, 2012). Integrative approaches have led to the identification of potential additional genetic risk factors and biomarkers for AD. For instance, integration of genome wide association studies (GWAS), linkage analysis and expression profiling in a protein-protein interaction (PPI) network yielded a 108 potential risk factors for AD including EGFR, ACTB, CDC2, IRAK1, APOE, ABCA1 and AMPH. Among these genes, EGFR, APOE and ACTB were found to overlap with proteomic data from cerebrospinal fluid of AD patients (Talwar et al., 2014) thus providing potential biomarker candidates. Collectively, these studies reinforce the power of integrative network approaches to identify pathways, genetic risk factors and biomarkers for AD.
Weighted gene coexpression networks analysis (WGCNA) are increasingly being used to find highly co-expressed gene modules associated with a particular biological pathway or a clinical trait of interest (Langfelder and Horvath, 2008). For example, construction of gene co-expression networks from 1647 postmortem brain tissues from LOAD patients highlighted immune and microglia enriched modules, containing a key regulator of the immune system, known as TYROBP (Zhang et al., 2013). Likewise, WGCNA analysis uncovered astrocyte-specific and microglia-enriched modules in vulnerable brain regions that associated with early tau accumulation (Miller et al., 2013). Implementation of WGCNA in RNA-sequencing data using brain samples obtained from the temporal lobe of subjects with dementia with Lewy body (DLB), LOAD and cognitively normal patients, identified network modules specific to each disease. For example, two network modules enriched in myelination and innate immune response correlated with LOAD whereas network modules associated with synaptic transmission and the generation of precursor metabolites correlated with DLB and LOAD (Humphries et al., 2015). Further, genes previously implicated in LOAD including FRMD4B and ST18 (Miller et al., 2008; Zhang et al., 2013) were prominent hubs within the myelination network (Humphries et al., 2015). Together, these findings suggested the involvement of microglia and myelination in the pathogenesis of AD and established differences in biological pathways between LOAD and DLB. Besides innate immunity pathways, network analysis of transcriptomic data from the brain hippocampus of normal aged and AD subjects identified key transcriptional regulators related to insulin (INS1, INS2) and brain derived neurotrophic factor (BDNF) interacting with the retinoic acid receptor related orphan receptor (RORA, Acquaah-Mensah et al., 2015) previously implicated in autoimmunity and diabetes (Solt and Burris, 2012).
With the growing interest in personalized medicine, it has become essential to develop tools to stratify patients according to symptoms, prognosis, and disease stage. This is highly important due to the fact that some subgroups of patients within a specific disease may experience a faster disease progression or respond to therapy differently. There are several documented examples on how networks could accelerate individualized treatment. For instance, analysis of protein interaction networks identified unstable network modules in different brain regions, in particular, in the entorhinal cortex of AD patients. Specifically, several protein interactions were present or absent at different Braak stages thus providing network modules characteristic of disease progression in AD (Kikuchi et al., 2013). Interestingly, the network modules with the largest number of disappearing protein interactions at late stage were associated with the histone acetyltransferase and the proteasome complexes. These modules were interacting via UCHL5 thereby indicating the perturbation of the ubiquitin-proteosome system in AD. Likewise, network analysis of six relevant brain regions affected in AD uncovered 136 hub genes of which 72 correlated with the Mini Mental State Examination (MMSE) and NFT scores, both widely utilized indicators of disease severity in AD (Liang et al., 2012). Among these genes, there were important transcription factors and kinases associated with AD including LEF1, SOX9, YY1, TCF3, TFDP1, CDK5, CSK and MAP3K3. Among these genes, overactivation of CDK5 is a major trigger of tau hyperphosphorylation and NFT formation in AD suggesting it may be a target for therapeutic intervention (Wilkaniec et al., 2016).
Since some genetic risk factors have a stronger influence in the disease than others, patient categorization and stratification according to the genetic basis would be advantageous in personalized medicine. In the context of AD, APOEɛ4 is the strongest risk factor for LOAD accounting for more than 50% of the cases. APOEɛ4 carriers display different clinical and pathological features than those of non-carriers. For example, APOEɛ4 carriers perform worse on memory tasks (Marra et al., 2004) and have a higher amyloid beta deposition than non-carriers (Kandimalla et al., 2011; Jack et al., 2015). Moreover, APOEɛ4 carriers respond to treatment differently than non-carriers. For instance, a neuroprotective agent improved MMSE scores in APOEɛ4 carriers but not in non-carriers (Richard et al., 1997). WGCNA on a transcriptomic dataset from human cerebral cortex of LOAD identified distinct co-expression modules based on APOEɛ4 stratification (Jiang et al., 2016). Co-expression modules of APOEɛ4 carriers were enriched in hereditary disorders, neurological diseases, and nervous system development and function whereas modules of non-carriers were enriched in immunological and cardiovascular diseases thus suggesting that different biological processes could play a role in LOAD with different APOEε4 status (Jiang et al., 2016).
PD is the second most prevalent neurodegenerative disease after AD, affecting more than 10 million people worldwide. Pathological features include the accumulation of aggregated alpha synuclein (SNCA) in intraneuronal cytoplasmic inclusion known as Lewy bodies and the progressive loss of dopaminergic neurons in the substantia nigra pars compacta. Dopamine restorative drugs and deep brain stimulation are current therapies to treat patients, however, these treatments only alleviate motor symptoms but do not impact disease progression. Mutations in the LRRK2, PARK2, PARK7, PINK1 and SNCA genes are known to cause familial PD. Most of the PD cases, however, are sporadic resulting from a complex interplay between genetics and environmental factors. In fact, some of the same genetic variants including SNCA and LRRK2 implicated in familial PD have been also associated with sporadic PD (Satake et al., 2009; Simón-Sánchez et al., 2009; Lin and Farrer, 2014). To date, advances in genomics have identified 28 genes associated with PD (Lin and Farrer, 2014).
Several pathways have been linked to the pathogenesis of PD including mitochondrial dysfunction, endoplasmic reticulum stress, autophagy, inflammation and impaired insulin signaling (Mercado et al., 2013; Nolan et al., 2013; Santiago and Potashkin, 2013b; Lin and Farrer, 2014). Despite this progress, the precise disease-causing mechanisms of PD are not fully understood. Complementation of genomic and transcriptomic studies with system biology approaches have provided insights into some novel mechanisms of disease. For instance, differential co-expression network analysis (DCA) performed on transcriptomic data from PD substantia nigra at autopsy, uncovered a transcript isoform of SNCA with an extended 3′ untranslated region, termed aSynL, which influenced SNCA accumulation (Rhinn et al., 2012). Interestingly, the pattern of expression of the long aSynL isoform relative to the short isoforms was also observed in unaffected individuals harboring a PD risk variant in the SNCA locus (Rhinn et al., 2012).
Understanding the molecular events associated with the progression of PD could help delineate a timeline for effective therapeutic intervention. Gene co-expression network analysis showed differences in gene modules between PD and controls for different anatomic brain regions (Corradini et al., 2014). In PD, hub modules in the motor vagal nucleus, locus coeruleus, and substantia nigra were enriched in pathways related to stress response and neuron survival/degeneration mechanisms whereas in control samples gene modules were associated with neuroprotection and aging homeostasis. Interestingly, one of the main hubs in the substantia nigra of control samples was SIRT1, which has been widely implicated in neuroprotection in several neurodegenerative diseases (Donmez and Outeiro, 2013; Herskovits and Guarente, 2013). Analysis of PPI networks representing autophagy and mitochondrial dysfunction pathways identified key protein targets including p62, GABARAP, GBRL1 and GBRL2 that modulated 1-methyl-4-phenylpyridinium (MPP+) toxicity (Keane et al., 2015), a widely used toxin to mimic PD in animal and cellular models. Strikingly, overexpression of these proteins combined, but not each one alone, provided rescue of MPP+ toxicity. This result further strengthens the notion that targeting a cluster of genes rather than a single gene may be the route to an effective treatment.
Integrative system biology approaches incorporating network analyses have been valuable in identifying potential therapeutic targets in PD. For instance, construction of networks integrating genetic information from Gene expression Omnibus (GEO), the Parkinson’s disease database (ParkDB) and the Comparative Toxicogenomics Database (CTD), identified alvespimycin (17-DMAG) as a candidate neuroprotective agent for PD (Gao et al., 2014). Experimental validation showed that 17-DMAG attenuated rotenone-induced toxicity in vitro. Another approach combined human brain and blood transcriptomic data and identified RGS2 as a key regulator of LRRK2 function (Dusonchet et al., 2014), one of the most common genetic risk factor of PD. Of note, RGS2 protected against neuronal toxicity in a Caenorhabditis elegans model expressing wild type LRRK2. Combination of -omics data from different tissues, for example brain and blood, may be advantageous to understand neurodegeneration in light of the recent finding that demonstrated that cell types outside the brain contain genetic risk factors associated with PD (Coetzee et al., 2016) and thus may help uncover new putative therapeutic targets.
Huntington’s disease (HD) is one of the most common dominantly inherited neurodegenerative disorders. The symptoms include some motor symptoms, such as chorea and dystonia, as well as non-motor symptoms, including psychological changes, and cognitive decline leading to dementia (Ross et al., 2014). These symptoms are correlated with a selective degeneration of the striatal and cortical neurons (Ehrlich, 2012). Currently, there are no therapies to prevent the onset or slow the progression of HD.
This progressive and fatal disease is caused by abnormal extension of the CAG repeat coding for a polyglutamine (polyQ) tail in the huntingtin gene (HTT, MacDonald et al., 1993). Unaffected individuals have fewer than 36 repeats, whereas affected patients can have as many as 250 CAG repeats. It has been shown that the length of the polyQ extension is inversely proportional to the age of the disease onset (Orr and Zoghbi, 2007). Vesicle and mitochondrial transport, transcription regulation, neurogenesis and energy metabolism are among the cellular functions of the normal HTT protein (Borrell-Pagès et al., 2006). Both lost of function of the normal protein and gain of toxic properties of mutant HTT leads to HD pathology. In fact, it has been shown that whereas the normal HTT protein is neuroprotective, the mutant HTT is neurotoxic. Despite this progress, the molecular mechanisms involved in the complex phenotype of the disease are still largely unknown.
In order to understand the role of HTT in HD pathology, (Langfelder et al., 2016) expressed HTT with different CAG length in a mice model. They demonstrated that the length of the CAG repeats modified the transcriptome of the striatum, and to a lesser extent, the cortex. WGCNA allowed the identification of 13 striatal and five cortical gene coexpression modules that were strongly associated with Htt CAG length. Interestingly, cadherin and protocadherin (Pcdh) genes expression were dysregulated in four of the modules, indicating that regulatory factors of these genes, such as Rest, Ctcf and Rad21, could be involved in HTT toxicity in mice (Langfelder et al., 2016).
Similarly, WGCNA was performed on transcriptomic HD post mortem tissues including the frontal cortex, cerebellum and caudate nucleus regions. The authors found that genes involved in metalloprotein, stress response and angiogenesis were positively regulated in all the networks whereas genes involved in mitochondrion, glycolysis, intracellular protein transport, proteasome and synaptic vesicle were downregulated (Neueder and Bates, 2014). Analysis of the human transcriptome from HD patients compared to healthy samples confirmed that protein modification, vesicles transport, cell signaling and synaptic transmission are important pathways involved in HD (Mina et al., 2016). Interestingly, these modules were also found in a blood transcriptomic study (Mina et al., 2016). Despite the fact that dysregulation of similar pathways was observed in the blood and brain, there was no overlap in any of the individual genes common between the two tissues (Mina et al., 2016).
A system-based approach performed on human transcriptomic datasets from post mortem human cerebellum, frontal cortex and caudate nucleus from HD patients and controls showed that an astrocyte module is the network whose connectivity and expression is most altered in HD (Scarpa et al., 2016). This astrocyte module was located downstream of TGFβ -FOXO3 signaling. In this regard, the TGFβ pathway was upregulated in neural stem cell differentiated from HD patient induced pluripotent stem cells (iPSC; Ring et al., 2015). Analysis of corrected iPS cells expressing shorter polyQ tails showed a downregulation of TGFβ pathway target genes, including cyclin-dependent kinase inhibitor 2B (CDKN2B), inhibitor of DNA binding 2 (ID2), inhibitor of DNA binding 4 (ID4), paired-like homeodomain transcription factor 2 (PITX2), thrombospondin 1 (THBS1), and left-right determination factor 2 (LEFTY2, An et al., 2012). In addition, valproic acid and lithium, both affecting TGFβ signaling, have been shown to improve mood in HD patients (Grove et al., 2000; Liang et al., 2008; Watanabe et al., 2011; Scheuing et al., 2014; Raja et al., 2015).
HD non-motor symptoms such as stress-related psychiatric and sleep disturbances often precede the onset of motor symptoms (Duff et al., 2007) and system-based approaches have proposed that sleep and stress traits emerge from shared genetic and transcriptional networks (Jiang et al., 2015). Interestingly, the astrocyte network expression described by Scarpa et al also correlated with stress and sleep phenotype in a chronically stressed mouse model (Scarpa et al., 2016). Collectively, these results suggest that targeting components of the TGFβ signaling pathway may provide novel therapeutics for HD.
Widespread protein misfolding and aggregation is a hallmark of neurodegenerative diseases. Despite the fact that neurodegerative diseases are defined by a set of characteristic pathological and clinical features, there is some overlap in pathology, genetic risk factors, and mechanisms of disease. For example, accumulation of SNCA and Lewy body pathology, central in the pathogenesis of PD, are present in the brains of human AD and implicated in aberrant synapse formation (Hamilton, 2000; Kim et al., 2004). Several studies have identified Single nucleotide polymorphisms (SNPs) in the MAPT locus associated with PD and AD thus suggesting that a common genetic factor may put an individual at risk for both diseases (Desikan et al., 2015). In addition to MAPT, other genetic variants including PON1, GSTO, and NEDD9 have been associated with the risk of PD and AD thus strengthening the genetic overlap between both diseases (Xie et al., 2014). Not surprisingly, shared mechanisms related to oxidative stress, neuroinflammation, impaired insulin signaling, mitochondrial dysfunction, iron dyshomeostasis and nicotinic receptors have been implicated in the pathogenesis of AD and PD (Xie et al., 2014). Therefore, a system-level understanding of the disease-disease connections could accelerate the discovery of novel treatments for both neurodegenerative diseases.
A systems-based approach combining expression quantitative trait loci (eQTL) studies from cerebellum and frontal cortex of AD patients, GWAS from AD and PD and PPI networks indicated that some PD variants (cisSNPs, cis-acting SNPs) were associated with the expression of CRHR1, LRRC37A4 and MAPT located at 17q21 and suggestive of AD risk (Liu et al., 2015). Similarly, shortest path analysis on a network constructed from literature mining identified known genes that already have an association with AD and PD and seven previously unknown genes including ROS1, FMN1, ATP8A2, SNORD12C, ERVK10, PRS and C7ORF49 that may link both diseases (Kim et al., 2016). Besides finding shared genetic associations, network analysis employing the computation of a similarity matrix identified gene clusters related to DNA repair, RNA metabolism, and glucose metabolism shared in AD and PD (Calderone et al., 2016). Importantly, these pathways were not detected using the conventional gene ontology (GO) analysis thus highlighting the power of networks to uncover novel pathways.
In addition to the studies focused on AD and PD, recent network-based approaches have been applied to understand the molecular networks shared among other neurodegenerative diseases. One study focused on the dorsolateral prefrontal cortex (DLPFC) which is commonly affected in both AD and HD to construct coexpression networks using genome wide expression data from 600 postmorterm DLPFC tissues from AD, HD, and non-dementia controls. Differential coexpression analysis revealed a subnetwork of 242 genes enriched in pathways related to neuron differentiation, apoptosis, gap junction trafficking, and cellular metabolic processes (Narayanan et al., 2014). Interestingly, the 242 gene subnetwork overlapped with genes downregulated in postmortem brains of major depressive disorder, a condition that is associated with other neurodegenerative disorders including PD (Aarsland et al., 2012). Further inspection of this subnetwork identified a gained/lost gene coexpression patterns associated with chromatin organization and neural differentiation.
Aging is one of the most common risk factors associated with neurodegeneration. With an average age of onset of 60 for the most common neurodegenerative diseases, the risk of developing PD or AD significantly increases with age. Dopamine synthesis, a crucial neurotransmitter that becomes depleted in the brain of PD, declines with age (Ota et al., 2006) and amyloid deposits, characteristic pathology in AD, are found in the aging brain of non-demented individuals (Pike et al., 2007). Beyond the overlap in pathological features, aging and neurodegenerative disorders share several dysregulated pathways. A system-based approach that identifies molecular networks shared between aging and neurodegeneration should reveal shared mechanisms, some of which may be targets for slowing disease progression. Discovering unique dysregulataed pathways that are not aging-associated could pinpoint potential therapeutics targets unique for a particular neurodegenerative disease.
Several studies have employed system biology tools to better understand age-related neurodegeneration. For example, a comparative pathway and network analysis of the brain transcriptome revealed shared networks and pathways between aging and PD including inflammation, mitochondrial dysfunction and metal ion homeostasis (Glaab and Schneider, 2015). Interestingly, the expression of the most significant shared gene, NR4A2, gradually declined with aging and PD. They found that this aging-associated gene expression changes in NR4A2 might increase the risk of PD by mechanisms similar to gene mutations linked to PD (Glaab and Schneider, 2015).
Proteostasis functional decline is common in aging and neurodegenerative diseases. In fact, several studies have proposed a mechanistic link between aging and loss of protein homeostasis leading to protein aggregation and toxicity. In this context, chaperones play a pivotal role in protein assembly and folding and its dysregulation may lead to protein aggregation and proteotoxicity. A recent study identified a chaperone subnetwork that exhibited concordant repression and induction expression patterns in brain tissues from human aging, AD, HD and PD patients. Subsequent investigation led to the discovery of a subnetwork comprising HSC70, HSP90, the CCT/TRiC complex and HSP40 and TPR-domain related co-chaperones with aberrant expression that were required to prevent Aβ and polyQ-associated proteotoxicity in C. elegans (Brehme et al., 2014). This shared chaperome subnetwork in aging and neurodegeneration, which is critical to maintain protein homeostasis, provides new targets for therapeutic intervention in neurodegenerative diseases. Similarly, a recent meta-analysis of about 1600 microarrays from brain tissue of AD patients revealed a set of downregulated genes corresponding to metastable proteins prone to aggregation (Ciryam et al., 2016). Thus, targeting components of the proteome homeostasis network may enable novel therapeutic opportunities for neurodegenerative diseases.
Gene expression is temporally and spatially regulated by DNA methylation or histone modifications. These epigenetic changes could influence a global gene expression or target some specific genes. A role for epigenetic changes in gene expression has been proposed in aging and neurodegenerative disorders. Interestingly, many studies have reported a genome-wide tendency to DNA hypomethylation with age in different organs including the brain in aging animal models (Wilson et al., 1987; Brunet and Berger, 2014). These changes are proposed to play a role in the progression of aging (Benayoun et al., 2015; Zampieri et al., 2015). Interestingly, Humphries et al. (2015) has shown that hypomethylation was observed in a myelination network dysregulated in AD.
DNA methylation has been proposed as a biomarker for aging in cells, tissues and organs (Horvath, 2013). An acceleration of the epigenetic clock has been proposed in different neurodegenerative disorders. In this context, epigenetic age acceleration correlated with AD neuropathological markers such as neuritic plaques and amyloid load (Levine et al., 2015). In addition, an association between epigenetic age acceleration with episodic memory, working memory and cognitive decline was observed among individuals with AD (Levine et al., 2015). Histones modifications, such as acetylation and methylation, have been observed in AD models and patients (for review see Fischer, 2014). Interestingly, the epigenetic clock is also accelerated in brain regions from HD patients (Horvath et al., 2016).
Epigenetic modification is also proposed to contribute to neurodegeneration in PD. A genome wide DNA methylation and transcriptomic study in iPSC-derived dopaminergic neurons from LRRK2-associated PD patients identified common DNA methylation changes in LRRK2 and sporadic PD (Fernández-Santiago et al., 2015). DNA methylation changes in PD dopaminergic neurons correlated with the downregulation of RNA and protein expression of a network of transcription factors FOXA1, NR3C1, HNF4A and FOSL2, which have been implicated in PD. For instance, FOXA1 is a key determinant in the molecular and physiological properties of dopaminergic neurons (Pristerè et al., 2015) and HNF4A expression in blood has correlated with disease progression in PD (Santiago and Potashkin, 2015).
Several computational tools have been developed to facilitate the integration of epigenetic data in networks. For example, EpiRegNet is a publicly available web server that allows the construction of epigenetic regulatory networks from human transcriptomic data (Wang et al., 2011). Another model, the Artificial Epigenetic Regulatory Network (AERN) incorporated DNA methylation and chromatin modification in addition to genetic factors for the analysis of epigenetic networks (Turner et al., 2013). More recently, another computational model, the Biological Expression Language (BEL)3, enabled the analysis of functional consequences of epigenetic modifications in the context of disease mechanisms (Khanam Irin et al., 2015). Because BEL integrates literature-derived cause and effect relationships into networks, researchers can formulate novel hypotheses of disease mechanisms. Notably, BEL network modeling has been used to integrate epigenetic and genetic factors in a functional context in PD. Using this approach, SNCA, MAPT, DNMT1, CYP2E1, OLFR151, PRKAR2A and SEPW1, were found to be hypomethylated in PD and suggested to cause overexpression of genes that disrupt normal biological functions. Further, two SNPs, rs3756063 and rs7684318, were associated with hypomethylation of SNCA in PD patients (Khanam Irin et al., 2015). Collectively, these models demonstrate that the integration of epigenetic factors into networks can uncover novel mechanisms of disease.
The field of network medicine has undoubtedly accelerated the understanding of the molecular mechanisms leading to neurodegeneration. The most significant brain network-based studies of the most common neurodegenerative diseases are summarized in Table 2. While network-based methods provide an unbiased approach to decode complex diseases and generate novel hypothesis, experimental validation is essential for network findings to be translated into useful diagnostics and therapeutic applications. In this regard, a growing number of studies have successfully identified blood-based biomarkers with potential clinical applicability. For instance, network analysis identified SOD2, APP, HNF4A, PTBP1 and NAMPT as useful to distinguish PD patients from HC in blood samples obtained from two independent cohorts (Santiago and Potashkin, 2013a, 2015; Santiago et al., 2014, 2016). Among these biomarkers, HNF4A and PTBP1, showed a dynamic expression pattern in longitudinal samples thus showing potential to track the clinical course of PD patients. Likewise, network analysis identified PTPN1 as a useful blood biomarker to distinguish PD from progressive supranuclear palsy, an atypical parkinsonian disorder commonly misdiagnosed as PD (Santiago and Potashkin, 2014b). Despite the success in PD studies, experimental validation of network-based findings in AD and HTT studies in clinically relevant studies is mostly lacking. For example, a systems medicine approach identified TYROBP as a promising target for therapeutic intervention in AD but to the best of our knowledge there are no follow-up studies (Zhang et al., 2013). Similarly, the involvement of RORA (Acquaah-Mensah et al., 2015) and other potential targets in AD are yet to be validated.
Besides experimental validation, another aspect for consideration is the cell-type and tissue specific analysis. This is important since the analysis of gene expression studies from whole brain sections might lead to misleading results that are not relevant to the specific cell type affected in the disorder. To circumvent this problem, recent studies have successfully employed high-throughput technologies that enable a single-cell resolution. A notable example studied the changes in astrocyte and microglia reactivity in AD. They observed that genes within the immune response pathway were more pronounced in astrocytes than in microglia thus demonstrating that cell-type specific characterization of the molecular changes may be more informative (Orre et al., 2014). More details about limitations in system-biology approaches in the context of neurodegenerative diseases have been well described recently (De Strooper and Karran, 2016).
Another emerging area of research in the network biology field is the study of disease comorbidities. Several conditions including, diabetes, cancer, major depressive disorder and cardiovascular disease, for example, have been associated with neurodegenerative diseases. For example, insulin resistance and diabetes have been linked to AD and PD and drugs to treat diabetic patients have shown promising results in both disorders (Santiago and Potashkin, 2013b). In addition, analyses of shared networks between PD and diabetes have elucidated potential blood biomarkers for PD (Santiago and Potashkin, 2013a, 2014c; Santiago et al., 2014). More recently, an integrative transcriptomic meta-analysis of PD and major depression identified NAMPT as a potential blood biomarker for de novo PD patients (Santiago et al., 2016). Furthermore, treatment with an enzymatic product of NAMPT elicited neuroprotective effects via activation of SIRT1 in an in vitro model of PD (Zou et al., 2016). Therefore, understanding the molecular networks shared between comorbid diseases could reveal novel diagnostics and therapeutic targets. Network analysis of gene-drug interactions in PD and diabetes demonstrates that some drugs may be beneficial for treating both diseases (Figure 3). In this context, treatment with commonly prescribed drugs to treat diabetes including rosiglitazone, metformin, pioglitazone and exenatide have shown neuroprotective effects in PD models (Santiago and Potashkin, 2013b, 2014c; Aviles-Olmos et al., 2014; Carta and Simuni, 2015). In particular, treatment with exenatide improved motor and cognitive function in PD patients (Aviles-Olmos et al., 2013, 2014). Treatment with pioglitazone, however, did not result in disease-modifying benefits in PD patients (NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators, 2015; Simon et al., 2015). Nonetheless, it has been noted that a longer exposure to pioglitazone may have been required to observe an improvement in PD patients (Brundin and Wyse, 2015). As shown in Figure 3, interaction of these drugs with the peroxisome proliferator-activated receptor gamma (PPARG) may provide a mechanistic explanation to their neuroprotective effect. Some of these drugs are currently in clinical trials to determine if they are neuroprotective.
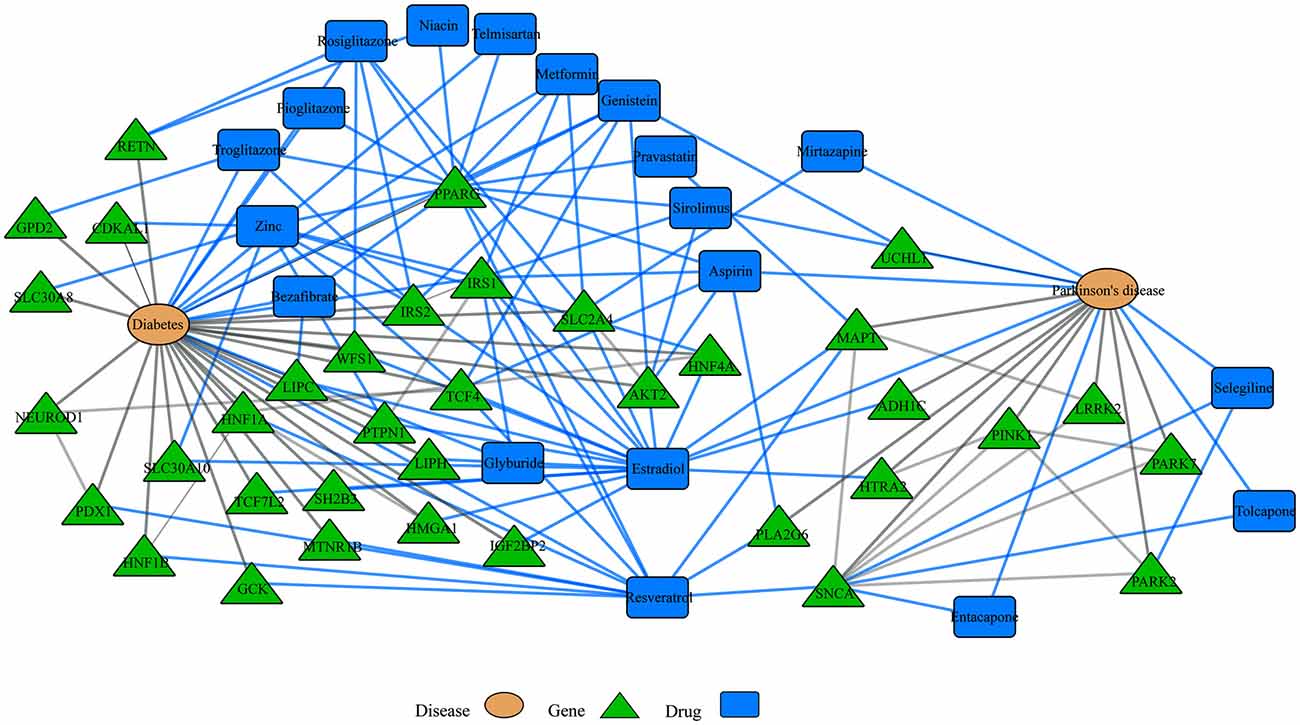
Disease-drugs networks. Interaction among different diseases, drugs and genes can be represented in a multi-level network model. For example, network-based approaches have been used to understand shared dysregulated pathways in Parkinson’s disease (PD) and diabetes. For instance, some drugs to treat diabetes patients have shown neuroprotective effects in PD and the observed neuroprotection may be mediated through their interaction with the peroxisome proliferator-activated receptor gamma (PPARG). Blue and gray lines represent drug interactions and disease interactions, respectively. This network was retrieved by iCTNet application in Cytoscape v3.1.1. using genetic associations from genome wide association studies (GWAS) and drug interactions from the Comparative Toxicogenomics Database (CTD) as of September 2016.
Nutrition is also recognized as an important component in the development and treatment of neurodegenerative diseases (Seidl et al., 2014). Given the promise of neuroprotective agents, the field of nutrigenomics is gaining interest among neuroscientists that are seeking to understand the complex nutrient-genetic interactions underlying neurodegeneration and neuroprotection. A recent example conducted a transcriptomic and epigenomic sequencing of the hypothalamus and hippocampus from a rodent model exposed to fructose consumption, which has been shown to contribute to the metabolic syndrome (Meng et al., 2016). Gene network analysis identified Bgn and Fmod as key genes involved in the observed metabolic alterations induced by fructose in mice. Strikingly, administration of docosahexaeonic acid (DHA) reversed the gene network changes elicited by fructose (Meng et al., 2016). This study provides evidence that integration of nutrigenomics coupled with network analysis can facilitate the identification of neuroprotective agents. Likewise, resveratrol, an antioxidant present in red wine, may also provide neuroprotection in PD patients and thus, could be tested in clinical trials (Figure 3).
In addition to a nutrient-rich diet, both physical exercise and cognitive training promote healthy aging (Kraft, 2012; Bamidis et al., 2014). It has been proposed that a combination of both together may be best to prevent cognitive decline and pathological aging (Kraft, 2012; Bamidis et al., 2014). In this regard, network analysis could be a useful tool to characterize the effects of physical exercise and cognitive training in the aging brain. Future studies directed at identifying gene expression changes associated with these lifestyle changes would be advantageous. Collectively, a multidimensional network approach that includes information about symptoms, drug treatments, comorbidities, nutrigenomics, physical exercise and cognitive training will be valuable to accelerate personalized treatment.
JAS and VB wrote the first draft of the manuscript. JAS, VB and JAP edited and reviewed the final draft of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by the US Army Medical Research and Materiel Command under awards number W81XWH-09-0708 and W81XWH13-1-0025 and the National Institute of Neurological Disorders and Stroke grant number U01NS097037 to JAP. The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Aarsland, D., Påhlhagen, S., Ballard, C. G., Ehrt, U., and Svenningsson, P. (2012). Depression in Parkinson disease—epidemiology, mechanisms and management. Nat. Rev. Neurol. 8, 35–47. doi: 10.1038/nrneurol.2011.189
Acquaah-Mensah, G. K., Agu, N., Khan, T., and Gardner, A. (2015). A regulatory role for the insulin- and BDNF-linked RORA in the hippocampus: implications for Alzheimer’s disease. J. Alzheimers Dis. 44, 827–838. doi: 10.3233/JAD-141731
An, M. C., Zhang, N., Scott, G., Montoro, D., Wittkop, T., Mooney, S., et al. (2012). Genetic correction of Huntington’s disease phenotypes in induced pluripotent stem cells. Cell Stem Cell 11, 253–263. doi: 10.1016/j.stem.2012.04.026
Aviles-Olmos, I., Dickson, J., Kefalopoulou, Z., Djamshidian, A., Ell, P., Soderlund, T., et al. (2013). Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Invest. 123, 2730–2736. doi: 10.1172/JCI68295
Aviles-Olmos, I., Dickson, J., Kefalopoulou, Z., Djamshidian, A., Kahan, J., Ell, P., et al. (2014). Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J. Parkinsons Dis. 4, 337–344. doi: 10.3233/JPD-140364
Bamidis, P. D., Vivas, A. B., Styliadis, C., Frantzidis, C., Klados, M., Schlee, W., et al. (2014). A review of physical and cognitive interventions in aging. Neurosci. Biobehav. Rev. 44, 206–220. doi: 10.1016/j.neubiorev.2014.03.019
Benayoun, B. A., Pollina, E. A., and Brunet, A. (2015). Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat. Rev. Mol. Cell Biol. 16, 593–610. doi: 10.1038/nrm4048
Borrell-Pagès, M., Zala, D., Humbert, S., and Saudou, F. (2006). Huntington’s disease: from huntingtin function and dysfunction to therapeutic strategies. Cell. Mol. Life Sci. 63, 2642–2660. doi: 10.1007/s00018-006-6242-0
Brehme, M., Voisine, C., Rolland, T., Wachi, S., Soper, J. H., Zhu, Y., et al. (2014). A chaperome subnetwork safeguards proteostasis in aging and neurodegenerative disease. Cell Rep. 9, 1135–1150. doi: 10.1016/j.celrep.2014.09.042
Brundin, P., and Wyse, R. (2015). Parkinson disease: laying the foundations for disease-modifying therapies in PD. Nat. Rev. Neurol. 11, 553–555. doi: 10.1038/nrneurol.2015.150
Brunet, A., and Berger, S. L. (2014). Epigenetics of aging and aging-related disease. J. Gerontol. A Biol. Sci. Med. Sci. 69, S17–S20. doi: 10.1093/gerona/glu042
Calderone, A., Formenti, M., Aprea, F., Papa, M., Alberghina, L., Colangelo, A. M., et al. (2016). Comparing Alzheimer’s and Parkinson’s diseases networks using graph communities structure. BMC Syst. Biol. 10:25. doi: 10.1186/s12918-016-0270-7
Carta, A. R., and Simuni, T. (2015). Thiazolidinediones under preclinical and early clinical development for the treatment of Parkinson’s disease. Expert Opin. Investig. Drugs 24, 219–227. doi: 10.1517/13543784.2015.963195
Chen, Y., Zhu, J., Lum, P. Y., Yang, X., Pinto, S., MacNeil, D. J., et al. (2008). Variations in DNA elucidate molecular networks that cause disease. Nature 452, 429–435. doi: 10.1038/nature06757
Ciryam, P., Kundra, R., Freer, R., Morimoto, R. I., Dobson, C. M., and Vendruscolo, M. (2016). A transcriptional signature of Alzheimer’s disease is associated with a metastable subproteome at risk for aggregation. Proc. Natl. Acad. Sci. U S A 113, 4753–4758. doi: 10.1073/pnas.1516604113
Coetzee, S. G., Pierce, S., Brundin, P., Brundin, L., Hazelett, D. J., and Coetzee, G. A. (2016). Enrichment of risk SNPs in regulatory regions implicate diverse tissues in Parkinson’s disease etiology. Sci. Rep. 6:30509. doi: 10.1038/srep30509
Coon, K. D., Myers, A. J., Craig, D. W., Webster, J. A., Pearson, J. V., Lince, D. H., et al. (2007). A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J. Clin. Psychiatry 68, 613–618. doi: 10.4088/jcp.v68n0419
Corradini, B. R., Iamashita, P., Tampellini, E., Farfel, J. M., Grinberg, L. T., and Moreira-Filho, C. A. (2014). Complex network-driven view of genomic mechanisms underlying Parkinson’s disease: analyses in dorsal motor vagal nucleus, locus coeruleus and substantia nigra. Biomed Res. Int. 2014:543673. doi: 10.1155/2014/543673
De Strooper, B., and Karran, E. (2016). The cellular phase of Alzheimer’s disease. Cell 164, 603–615. doi: 10.1016/j.cell.2015.12.056
Desikan, R. S., Schork, A. J., Wang, Y., Witoelar, A., Sharma, M., McEvoy, L. K., et al. (2015). Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol. Psychiatry 20, 1588–1595. doi: 10.1038/mp.2015.6
Donmez, G., and Outeiro, T. F. (2013). SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol. Med. 5, 344–352. doi: 10.1002/emmm.201302451
Duff, K., Paulsen, J. S., Beglinger, L. J., Langbehn, D. R., Stout, J. C., and Predict-HD Investigators of the Huntington Study Group. (2007). Psychiatric symptoms in Huntington’s disease before diagnosis: the predict-HD study. Biol. Psychiatry 62, 1341–1346. doi: 10.1016/j.biopsych.2006.11.034
Dusonchet, J., Li, H., Guillily, M., Liu, M., Stafa, K., Derada Troletti, C., et al. (2014). A Parkinson’s disease gene regulatory network identifies the signaling protein RGS2 as a modulator of LRRK2 activity and neuronal toxicity. Hum. Mol. Genet. 23, 4887–4905. doi: 10.1093/hmg/ddu202
Ehrlich, M. E. (2012). Huntington’s disease and the striatal medium spiny neuron: cell-autonomous and non-cell-autonomous mechanisms of disease. Neurotherapeutics 9, 270–284. doi: 10.1007/s13311-012-0112-2
Fernández-Santiago, R., Carballo-Carbajal, I., Castellano, G., Torrent, R., Richaud, Y., Sánchez-Danés, A., et al. (2015). Aberrant epigenome in iPSC-derived dopaminergic neurons from Parkinson’s disease patients. EMBO Mol. Med. 7, 1529–1546. doi: 10.15252/emmm.201505439
Fischer, A. (2014). Targeting histone-modifications in Alzheimer’s disease. What is the evidence that this is a promising therapeutic avenue? Neuropharmacology 80, 95–102. doi: 10.1016/j.neuropharm.2014.01.038
Gao, L., Zhao, G., Fang, J. S., Yuan, T. Y., Liu, A. L., and Du, G. H. (2014). Discovery of the neuroprotective effects of alvespimycin by computational prioritization of potential anti-Parkinson agents. FEBS J. 281, 1110–1122. doi: 10.1111/febs.12672
Glaab, E., and Schneider, R. (2015). Comparative pathway and network analysis of brain transcriptome changes during adult aging and in Parkinson’s disease. Neurobiol. Dis. 74, 1–13. doi: 10.1016/j.nbd.2014.11.002
Goate, A., Chartier-Harlin, M. C., Mullan, M., Brown, J., Crawford, F., Fidani, L., et al. (1991). Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 349, 704–706. doi: 10.1038/349704a0
Goh, K. I., Cusick, M. E., Valle, D., Childs, B., Vidal, M., and Barabási, A. L. (2007). The human disease network. Proc. Natl. Acad. Sci. U S A 104, 8685–8690. doi: 10.1073/pnas.0701361104
Grove, V. E. Jr., Quintanilla, J., and DeVaney, G. T. (2000). Improvement of Huntington’s disease with olanzapine and valproate. N. Engl. J. Med. 343, 973–974. doi: 10.1056/NEJM200009283431316
Hallock, P., and Thomas, M. A. (2012). Integrating the Alzheimer’s disease proteome and transcriptome: a comprehensive network model of a complex disease. OMICS 16, 37–49. doi: 10.1089/omi.2011.0054
Hamilton, R. L. (2000). Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using α-synuclein immunohistochemistry. Brain Pathol. 10, 378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x
Herskovits, A. Z., and Guarente, L. (2013). Sirtuin deacetylases in neurodegenerative diseases of aging. Cell Res. 23, 746–758. doi: 10.1038/cr.2013.70
Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biol. 14:R115. doi: 10.1186/gb-2013-14-10-r115
Horvath, S., Langfelder, P., Kwak, S., Aaronson, J., Rosinski, J., Vogt, T. F., et al. (2016). Huntington’s disease accelerates epigenetic aging of human brain and disrupts DNA methylation levels. Aging 8, 1485–1512. doi: 10.18632/aging.101005
Humphries, C. E., Kohli, M. A., Nathanson, L., Whitehead, P., Beecham, G., Martin, E., et al. (2015). Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. J. Alzheimers Dis. 44, 977–987. doi: 10.3233/JAD-141989
Ibáñez, K., Boullosa, C., Tabarés-Seisdedos, R., Baudot, A., and Valencia, A. (2014). Molecular evidence for the inverse comorbidity between central nervous system disorders and cancers detected by transcriptomic meta-analyses. PLoS Genet. 10:e1004173. doi: 10.1371/journal.pgen.1004173
Jack, C. R. Jr., Wiste, H. J., Weigand, S. D., Knopman, D. S., Vemuri, P., Mielke, M. M., et al. (2015). Age, sex, and APOE ε4 effects on memory, brain structure, and β-amyloid across the adult life span. JAMA Neurol. 72, 511–519. doi: 10.1001/jamaneurol.2014.4821
Janssen, J. C., Beck, J. A., Campbell, T. A., Dickinson, A., Fox, N. C., Harvey, R. J., et al. (2003). Early onset familial Alzheimer’s disease: mutation frequency in 31 families. Neurology 60, 235–239. doi: 10.1212/01.WNL.0000042088.22694.e3
Jiang, P., Scarpa, J. R., Fitzpatrick, K., Losic, B., Gao, V. D., Hao, K., et al. (2015). A systems approach identifies networks and genes linking sleep and stress: implications for neuropsychiatric disorders. Cell Rep. 11, 835–848. doi: 10.1016/j.celrep.2015.04.003
Jiang, S., Tang, L., Zhao, N., Yang, W., Qiu, Y., and Chen, H. Z. (2016). A systems view of the differences between APOE ε4 carriers and non-carriers in Alzheimer’s disease. Front. Aging Neurosci. 8:171. doi: 10.3389/fnagi.2016.00171
Kandimalla, R. J., Prabhakar, S., Binukumar, B. K., Wani, W. Y., Gupta, N., Sharma, D. R., et al. (2011). Apo-Eε4 allele in conjunction with Aβ42 and tau in CSF: biomarker for Alzheimer’s disease. Curr. Alzheimer Res. 8, 187–196. doi: 10.2174/156720511795256071
Keane, H., Ryan, B. J., Jackson, B., Whitmore, A., and Wade-Martins, R. (2015). Protein-protein interaction networks identify targets which rescue the MPP+ cellular model of Parkinson’s disease. Sci. Rep. 5:17004. doi: 10.1038/srep17004
Khanam Irin, A., Kodamullil, A. T., Gündel, M., and Hofmann-Apitius, M. (2015). Computational modelling approaches on epigenetic factors in neurodegenerative and autoimmune diseases and their mechanistic analysis. J. Immunol. Res. 2015:737168. doi: 10.1155/2015/737168
Kikuchi, M., Ogishima, S., Miyamoto, T., Miyashita, A., Kuwano, R., Nakaya, J., et al. (2013). Identification of unstable network modules reveals disease modules associated with the progression of Alzheimer’s disease. PLoS One 8:e76162. doi: 10.1371/journal.pone.0076162
Kim, Y. H., Beak, S. H., Charidimou, A., and Song, M. (2016). Discovering new genes in the pathways of common sporadic neurodegenerative diseases: a bioinformatics approach. J. Alzheimers Dis. 51, 293–312. doi: 10.3233/JAD-150769
Kim, S., Seo, J. H., and Suh, Y. H. (2004). α-synuclein, Parkinson’s disease, and Alzheimer’s disease. Parkinsonism Relat. Disord. 10, S9–S13. doi: 10.1016/j.parkreldis.2003.11.005
Kraft, E. (2012). Cognitive function, physical activity, and aging: possible biological links and implications for multimodal interventions. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 19, 248–263. doi: 10.1080/13825585.2011.645010
Langfelder, P., Cantle, J. P., Chatzopoulou, D., Wang, N., Gao, F., Al-Ramahi, I., et al. (2016). Integrated genomics and proteomics define huntingtin CAG length-dependent networks in mice. Nat. Neurosci. 19, 623–633. doi: 10.1038/nn.4256
Langfelder, P., and Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. doi: 10.1186/1471-2105-9-559
Levine, M. E., Lu, A. T., Bennett, D. A., and Horvath, S. (2015). Epigenetic age of the pre-frontal cortex is associated with neuritic plaques, amyloid load, and Alzheimer’s disease related cognitive functioning. Aging 7, 1198–1211. doi: 10.18632/aging.100864
Levy-Lahad, E., Wasco, W., Poorkaj, P., Romano, D. M., Oshima, J., Pettingell, W. H., et al. (1995). Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 269, 973–977. doi: 10.1126/science.7638622
Liang, D., Han, G., Feng, X., Sun, J., Duan, Y., and Lei, H. (2012). Concerted perturbation observed in a hub network in Alzheimer’s disease. PLoS One 7:e40498. doi: 10.1371/journal.pone.0040498
Liang, M. H., Wendland, J. R., and Chuang, D. M. (2008). Lithium inhibits Smad3/4 transactivation via increased CREB activity induced by enhanced PKA and AKT signaling. Mol. Cell. Neurosci. 37, 440–453. doi: 10.1016/j.mcn.2007.10.017
Lin, M. K., and Farrer, M. J. (2014). Genetics and genomics of Parkinson’s disease. Genome Med. 6:48. doi: 10.1186/gm566
Linghu, B., Snitkin, E. S., Hu, Z., Xia, Y., and Delisi, C. (2009). Genome-wide prioritization of disease genes and identification of disease-disease associations from an integrated human functional linkage network. Genome Biol. 10:R91. doi: 10.1186/gb-2009-10-9-r91
Liu, G., Bao, X., Jiang, Y., Liao, M., Jiang, Q., Feng, R., et al. (2015). Identifying the association between Alzheimer’s disease and Parkinson’s disease using genome-wide association studies and protein-protein interaction network. Mol. Neurobiol. 52, 1629–1636. doi: 10.1007/s12035-014-8946-8
MacDonald, M. E., Ambrose, C. M., Duyao, M. P., Myers, R. H., Lin, C., Srinidhi, L., et al. (1993). A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72, 971–983. doi: 10.1016/0092-8674(93)90585-e
Marra, C., Bizzarro, A., Daniele, A., De Luca, L., Ferraccioli, M., Valenza, A., et al. (2004). Apolipoprotein E ε4 allele differently affects the patterns of neuropsychological presentation in early- and late-onset Alzheimer’s disease patients. Dement. Geriatr. Cogn. Disord. 18, 125–131. doi: 10.1159/000079191
Meng, Q., Ying, Z., Noble, E., Zhao, Y., Agrawal, R., Mikhail, A., et al. (2016). Systems nutrigenomics reveals brain gene networks linking metabolic and brain disorders. EBioMedicine 7, 157–166. doi: 10.1016/j.ebiom.2016.04.008
Mercado, G., Valdés, P., and Hetz, C. (2013). An ERcentric view of Parkinson’s disease. Trends Mol. Med. 19, 165–175. doi: 10.1016/j.molmed.2012.12.005
Miller, J. A., Oldham, M. C., and Geschwind, D. H. (2008). A systems level analysis of transcriptional changes in Alzheimer’s disease and normal aging. J. Neurosci. 28, 1410–1420. doi: 10.1523/JNEUROSCI.4098-07.2008
Miller, J. A., Woltjer, R. L., Goodenbour, J. M., Horvath, S., and Geschwind, D. H. (2013). Genes and pathways underlying regional and cell type changes in Alzheimer’s disease. Genome Med. 5:48. doi: 10.1186/gm452
Mina, E., van Roon-Mom, W., Hettne, K., van Zwet, E., Goeman, J., Neri, C., et al. (2016). Common disease signatures from gene expression analysis in Huntington’s disease human blood and brain. Orphanet J. Rare Dis. 11:97. doi: 10.1186/s13023-016-0475-2
Narayanan, M., Huynh, J. L., Wang, K., Yang, X., Yoo, S., McElwee, J., et al. (2014). Common dysregulation network in the human prefrontal cortex underlies two neurodegenerative diseases. Mol. Syst. Biol. 10:743. doi: 10.15252/msb.20145304
Neueder, A., and Bates, G. P. (2014). A common gene expression signature in Huntington’s disease patient brain regions. BMC Med. Genomics 7:60. doi: 10.1186/s12920-014-0060-2
NINDS Exploratory Trials in Parkinson Disease (NET-PD) FS-ZONE Investigators. (2015). Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, couble-blind, randomised trial. Lancet Neurol. 14, 795–803. doi: 10.1016/s1474-4422(15)00144-1
Nolan, Y. M., Sullivan, A. M., and Toulouse, A. (2013). Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol. Med. 19, 187–196. doi: 10.1016/j.molmed.2012.12.003
Orre, M., Kamphuis, W., Osborn, L. M., Jansen, A. H., Kooijman, L., Bossers, K., et al. (2014). Isolation of glia from Alzheimer’s mice reveals inflammation and dysfunction. Neurobiol. Aging 35, 2746–2760. doi: 10.1016/j.neurobiolaging.2014.06.004
Orr, H. T., and Zoghbi, H. Y. (2007). Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30, 575–621. doi: 10.1146/annurev.neuro.29.051605.113042
Ota, M., Yasuno, F., Ito, H., Seki, C., Nozaki, S., Asada, T., et al. (2006). Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-[β-11C]DOPA. Life Sci. 79, 730–736. doi: 10.1016/j.lfs.2006.02.017
Pike, K. E., Savage, G., Villemagne, V. L., Ng, S., Moss, S. A., Maruff, P., et al. (2007). β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844. doi: 10.1093/brain/awm238
Pristerè, A., Lin, W., Kaufmann, A. K., Brimblecombe, K. R., Threlfell, S., Dodson, P. D., et al. (2015). Transcription factors FOXA1 and FOXA2 maintain dopaminergic neuronal properties and control feeding behavior in adult mice. Proc. Natl. Acad. Sci. U S A 112, E4929–E4938. doi: 10.1073/pnas.1503911112
Raja, M., Soleti, F., and Bentivoglio, A. R. (2015). Lithium treatment in patients With Huntington’s disease and suicidal behavior. Mov. Disord 30:1438. doi: 10.1002/mds.26260
Rhinn, H., Qiang, L., Yamashita, T., Rhee, D., Zolin, A., Vanti, W., et al. (2012). Alternative α-synuclein transcript usage as a convergent mechanism in Parkinson’s disease pathology. Nat. Commun. 3:1084. doi: 10.1038/ncomms2032
Richard, F., Helbecque, N., Neuman, E., Guez, D., Levy, R., and Amouyel, P. (1997). APOE genotyping and response to drug treatment in Alzheimer’s disease. Lancet 349:539. doi: 10.1016/s0140-6736(97)80089-x
Ring, K. L., An, M. C., Zhang, N., O’Brien, R. N., Ramos, E. M., Gao, F., et al. (2015). Genomic analysis reveals disruption of striatal neuronal development and therapeutic targets in human Huntington’s disease neural stem cells. Stem Cell Reports 5, 1023–1038. doi: 10.1016/j.stemcr.2015.11.005
Ross, C. A., Aylward, E. H., Wild, E. J., Langbehn, D. R., Long, J. D., Warner, J. H., et al. (2014). Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 10, 204–216. doi: 10.1038/nrneurol.2014.24
Santiago, J. A., Littlefield, A. M., and Potashkin, J. A. (2016). Integrative transcriptomic meta-analysis of Parkinson’s disease and depression identifies NAMPT as a potential blood biomarker for de novo Parkinson’s disease. Sci. Rep. 6:34579. doi: 10.1038/srep34579
Santiago, J. A., and Potashkin, J. A. (2013a). Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS One 8:e83940. doi: 10.1371/journal.pone.0083940
Santiago, J. A., and Potashkin, J. A. (2013b). Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol. Med. 19, 176–186. doi: 10.1016/j.molmed.2013.01.002
Santiago, J. A., and Potashkin, J. A. (2014a). A network approach to clinical intervention in neurodegenerative diseases. Trends Mol. Med. 20, 694–703. doi: 10.1016/j.molmed.2014.10.002
Santiago, J. A., and Potashkin, J. A. (2014b). A network approach to diagnostic biomarkers in progressive supranuclear palsy. Mov. Disord. 29, 550–555. doi: 10.1002/mds.25761
Santiago, J. A., and Potashkin, J. A. (2014c). System-based approaches to decode the molecular links in Parkinson’s disease and diabetes. Neurobiol. Dis. 72, 84–91. doi: 10.1016/j.nbd.2014.03.019
Santiago, J. A., and Potashkin, J. A. (2015). Network-based metaanalysis identifies HNF4A and PTBP1 as longitudinally dynamic biomarkers for Parkinson’s disease. Proc. Natl. Acad. Sci. U S A 112, 2257–2262. doi: 10.1073/pnas.1423573112
Santiago, J. A., Scherzer, C. R., and Potashkin, J. A. (2014). Network analysis identifies SOD2 mRNA as a potential biomarker for Parkinson’s disease. PLoS One 9:e109042. doi: 10.1371/journal.pone.0109042
Satake, W., Nakabayashi, Y., Mizuta, I., Hirota, Y., Ito, C., Kubo, M., et al. (2009). Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson’s disease. Nat. Genet. 41, 1303–1307. doi: 10.1038/ng.485
Scarpa, J. R., Jiang, P., Losic, B., Readhead, B., Gao, V. D., Dudley, J. T., et al. (2016). Systems genetic analyses highlight a TGFβ-FOXO3 dependent striatal astrocyte network conserved across species and associated with stress, sleep, and Huntington’s disease. PLoS Genet. 12:e1006137. doi: 10.1371/journal.pgen.1006137
Scheuing, L., Chiu, C. T., Liao, H. M., Linares, G. R., and Chuang, D. M. (2014). Preclinical and clinical investigations of mood stabilizers for Huntington’s disease: what have we learned? Int. J. Biol. Sci. 10, 1024–1038. doi: 10.7150/ijbs.9898
Schneider, A., Rajendran, L., Honsho, M., Gralle, M., Donnert, G., Wouters, F., et al. (2008). Flotillin-dependent clustering of the amyloid precursor protein regulates its endocytosis and amyloidogenic processing in neurons. J. Neurosci. 28, 2874–2882. doi: 10.1523/JNEUROSCI.5345-07.2008
Seidl, S. E., Santiago, J. A., Bilyk, H., and Potashkin, J. A. (2014). The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 6:36. doi: 10.3389/fnagi.2014.00036
Simon, D. K., Simuni, T., Elm, J., Clark-Matott, J., Graebner, A. K., Baker, L., et al. (2015). Peripheral biomarkers of Parkinson’s disease progression and pioglitazone effects. J. Parkinsons Dis. 5, 731–736. doi: 10.3233/JPD-150666
Simón-Sánchez, J., Schulte, C., Bras, J. M., Sharma, M., Gibbs, J. R., Berg, D., et al. (2009). Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat. Genet. 41, 1308–1312. doi: 10.1038/ng.487
Solt, L. A., and Burris, T. P. (2012). Action of RORs and their ligands in (patho)physiology. Trends Endocrinol. Metab. 23, 619–627. doi: 10.1016/j.tem.2012.05.012
Talwar, P., Silla, Y., Grover, S., Gupta, M., Agarwal, R., Kushwaha, S., et al. (2014). Genomic convergence and network analysis approach to identify candidate genes in Alzheimer’s disease. BMC Genomics 15:199. doi: 10.1186/1471-2164-15-199
Taylor, I. W., Linding, R., Warde-Farley, D., Liu, Y., Pesquita, C., Faria, D., et al. (2009). Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nat. Biotechnol. 27, 199–204. doi: 10.1038/nbt.1522
Turner, A. P., Lones, M. A., Fuente, L. A., Stepney, S., Caves, L. S., and Tyrrell, A. M. (2013). The incorporation of epigenetics in artificial gene regulatory networks. Biosystems 112, 56–62. doi: 10.1016/j.biosystems.2013.03.013
Wang, L. Y., Wang, P., Li, M. J., Qin, J., Wang, X., Zhang, M. Q., et al. (2011). EpiRegNet: constructing epigenetic regulatory network from high throughput gene expression data for humans. Epigenetics 6, 1505–1512. doi: 10.4161/epi.6.12.18176
Watanabe, T., Tajima, H., Hironori, H., Nakagawara, H., Ohnishi, I., Takamura, H., et al. (2011). Sodium valproate blocks the transforming growth factor (TGF)-β1 autocrine loop and attenuates the TGF-β1-induced collagen synthesis in a human hepatic stellate cell line. Int. J. Mol. Med. 28, 919–925. doi: 10.3892/ijmm.2011.768
Wilkaniec, A., Czapski, G. A., and Adamczyk, A. (2016). Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. J. Neurochem. 136, 222–233. doi: 10.1111/jnc.13365
Wilson, V. L., Smith, R. A., Ma, S., and Cutler, R. G. (1987). Genomic 5-methyldeoxycytidine decreases with age. J. Biol. Chem. 262, 9948–9951.
Xie, A., Gao, J., Xu, L., and Meng, D. (2014). Shared mechanisms of neurodegeneration in Alzheimer’s disease and Parkinson’s disease. Biomed Res. Int. 2014:648740. doi: 10.1155/2014/648740
Zampieri, M., Ciccarone, F., Calabrese, R., Franceschi, C., Bürkle, A., and Caiafa, P. (2015). Reconfiguration of DNA methylation in aging. Mech. Ageing Dev. 151, 60–70. doi: 10.1016/j.mad.2015.02.002
Zhang, B., Gaiteri, C., Bodea, L. G., Wang, Z., McElwee, J., Podtelezhnikov, A. A., et al. (2013). Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153, 707–720. doi: 10.1016/j.cell.2013.03.030
Keywords: Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, network biology, molecular mechanisms
Citation: Santiago JA, Bottero V and Potashkin JA (2017) Dissecting the Molecular Mechanisms of Neurodegenerative Diseases through Network Biology. Front. Aging Neurosci. 9:166. doi: 10.3389/fnagi.2017.00166
Received: 18 October 2016; Accepted: 12 May 2017;
Published: 29 May 2017.
Edited by:
Panagiotis D. Bamidis, Aristotle University of Thessaloniki, GreeceReviewed by:
Daniel Ortuño-Sahagún, Centro Universitario de Ciencias de la Salud, MexicoCopyright © 2017 Santiago, Bottero and Potashkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Judith A. Potashkin, anVkeS5wb3Rhc2hraW5Acm9zYWxpbmRmcmFua2xpbi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.