- 1Department of Research and Education, Centre of Expertise for Chronic Organ Failure (CIRO), Horn, Netherlands
- 2Department of Medical Psychology, Maastricht UMC+/School for Mental Health and Neurosciences (MHeNS), Maastricht, Netherlands
- 3Department of Respiratory Medicine, Maastricht University Medical Centre, NUTRIM School of Nutrition and Translational Research in Metabolism, Maastricht, Netherlands
- 4Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Alzheimer Centre Limburg, Maastricht University, Maastricht, Netherlands
- 5Department of Neurology, Maastricht University Medical Centre, Maastricht, Netherlands
- 6Department of Radiology, Maastricht University Medical Centre, Maastricht, Netherlands
- 7Department of Respiratory Medicine, Maastricht UMC+, Maastricht, Netherlands
The neural correlates of cognitive impairment in chronic obstructive pulmonary disease (COPD) are not yet understood. Structural brain abnormalities could possibly be associated with the presence of cognitive impairment through cigarette smoke, inflammation, vascular disease, or hypoxemia in these patients. This study aimed to investigate whether macrostructural brain magnetic resonance imaging (MRI) features of cerebral small vessel disease (SVD) and hippocampal volume (HCV) are related to cognitive performance in patients with COPD. A subgroup of cognitively high and low-performing COPD patients of the COgnitive-PD study, underwent a brain 3T MRI. SVD as a marker of vascular damage was assessed using qualitative visual rating scales. HCV as a marker of neurodegeneration was assessed using the learning embedding for atlas propagation (LEAP) method. Features of SVD and HCV were compared between cognitively high and low-performing individuals using Mann Whitney U tests and independent samples t-tests, respectively. No group differences were reported between 25 high-performing (mean age 60.3 (standard deviation [SD] 9.7) years; 40.0% men; forced expiratory volume in first second [FEV1] 50.1% predicted) and 30 low-performing patients with COPD (mean age 60.6 (SD 6.8) years; 53.3% men; FEV1 55.6% predicted) regarding demographics, clinical characteristics, comorbidities and the presence of the SVD features and HCV. To conclude, the current study does not provide evidence for a relationship between cerebral SVD and HCV and cognitive functioning in patients with COPD. Additional studies will be needed to determine other possible mechanisms of cognitive impairment in patients with COPD, including microstructural brain changes and inflammatory-, hormonal-, metabolic- and (epi)genetic factors.
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent chronic disease which is primarily characterized by progressive airflow limitation (Vestbo et al., 2013). Beyond respiratory impairment, patients with COPD often suffer from a variety of comorbid and biological conditions (Divo et al., 2012; Vanfleteren et al., 2013). These biological changes may have adverse effects on the brain leading to cognitive impairment. General cognitive impairment is four times more likely to occur in patients with COPD than in non-COPD controls and involves several cognitive domains, such as psychomotor speed, planning, memory and cognitive flexibility (Cleutjens et al., 2017). Impairments in working and verbal memory have been found in one out of three patients with COPD (Cleutjens et al., 2017). Although the pathogenesis of cognitive impairment in COPD has been searched in oxidative stress, hypoxemia, systemic inflammation and comorbidities, the exact pathways remain unknown (Dodd et al., 2010; Cleutjens et al., 2014a; Lahousse et al., 2015). Structural brain abnormalities are considered an important cause of cognitive impairment, especially decreased hippocampal volume (HCV), which has been associated with decreased memory (Soininen et al., 1994; Schuff et al., 2009; Wolz et al., 2010b), and features of cerebral small vessel disease (SVD; Cai et al., 2015). SVD refers to pathological processes affecting the small arteries, arterioles, venules and capillaries of the brain. For example, chronic hypoperfusion can occur, whereby the supply of oxygen and nutrients to the brain tissue is slowly cut (Cai et al., 2015). Hypoxemia is thought to act in an additive manner in the development of structural brain abnormalities (Miyamoto and Auer, 2000). Structural brain abnormalities associated with SVD can be seen on magnetic resonance imaging (MRI) as white matter hyperintensities (WMHs), lacunes, cerebral microbleeds and perivascular spaces (PVS). The main risk factors for SVD are increased age and hypertension.
In comparison to control participants, COPD patients showed smaller HCV (Li and Fei, 2013), reduced white matter integrity and regional gray matter density (Dodd et al., 2012; Zhang et al., 2012), periventricular white matter lesions (van Dijk et al., 2004), disturbed functional activation of gray matter (Dodd et al., 2012), and a higher prevalence of cerebral microbleeds (Lahousse et al., 2013). Possible mechanisms, common in COPD, which may contribute to brain abnormalities and cognitive impairment are cigarette smoke, inflammation, vascular disease and hypoxemia (Dodd et al., 2010). Yet, the relationship between structural brain abnormalities, especially HCV and features of SVD and cognitive functioning in COPD remains unexplored.
In order to verify whether SVD and HCV may explain the level of cognitive functioning in patients with COPD, this study aimed to assess whether and to what extent SVD and HCV differ between COPD patients with high and low cognitive performance. A priori, we hypothesized that COPD patients with low cognitive performances are characterized by a higher SVD load, and smaller HCV compared to COPD patients with high cognitive performances.
Materials and Methods
Design
This cross sectional study is part of a longitudinal study (COgnitive-PD study) aimed at mapping and understanding neuropsychological functioning in patients with COPD. Details of the methodology of this study and data concerning cognition have been described before (Cleutjens et al., 2014b, 2017).
Study Population
For this study, we selected the first 30 cognitively low-performing and the first 25 high-performing patients with COPD during inclusion of the COgnitive-PD study (n = 183) who were willing to undergo brain MRI. Six core tests of the detailed neuropsychological test battery from the Cognitive-PD study (Table 1) were used to distinguish cognitively high-performing individuals from low-performing individuals (Burgmans et al., 2009; for details about the tests, please see the Cognitive-PD study protocol (Cleutjens et al., 2014b)). In addition to the exclusion criteria of the COgnitive-PD study (Cleutjens et al., 2014b), patients were not eligible if they had contraindications to undergo a brain MRI (claustrophobia, a cardiac pacemaker, a cochlear implant, a neurostimulator, or other metal implants in the body). The study was approved by the Medical Ethics Committee of the Maastricht University Medical Centre (NL45127.068.13). All patients provided written informed consent to participate in the study.
Table 1. Cognitive performance per core subtest on the COgnitive-PD study neuropsychological test battery.
Brain MRI Acquisition
Patients had brain MRI on a research-dedicated 3-T MRI scanner (Siemens, Netherlands) operated by research-dedicated technical staff in the Maastricht University Medical Center. Sequences included T1-weighted sagittal sequence (TR = 8.14 ms, TE = 3.72 ms, FA = 8°, FOV = 256 × 256 × 155 mm, acquisition matrix = 256 × 256, number of slices = 155, voxel size = 1.00 mm isotropic), T2-weighted transversal sequence (TR = 3000 ms, TE = 80 ms, FA = 90°, FOV = 230 × 230 × 154 mm, acquisition matrix = 512 × 512, number of slices = 28, slice gap = 0.50 mm, voxel size = 0.45 × 0.45 × 5.5 mm), fluid-attenuated inversion recovery (FLAIR) sagittal sequence (TR = 4800 ms, TE = 275 ms, TI = 1650 ms, FOV = 250 × 250 × 154 mm, acquisition matrix = 240 × 240, number of slices = 275, slice gap = 0.56 mm, voxel size = 1.04 × 1.04 × 0.56 mm), and T2*-weighted transversal sequence (TR = 844 ms, TE = 16 ms, FA = 18°, FOV = 230 × 230 × 154 mm, acquisition matrix = 512 × 512, number of slices = 28, slice gap = 0.50 mm, voxel size = 0.45 × 0.45 × 5.5 mm).
MRI Analysis
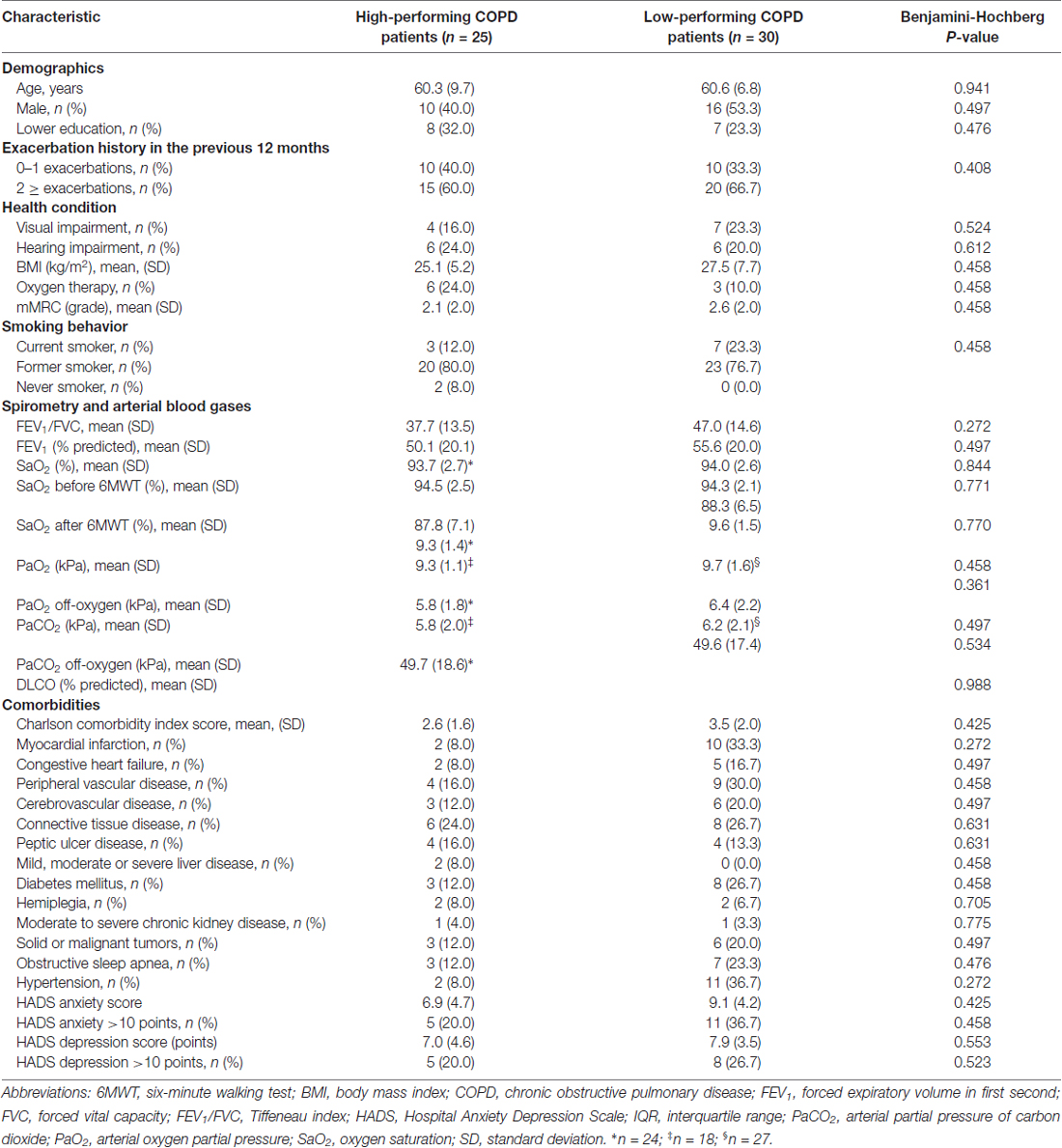
All MRIs were analyzed blinded to patient’s cognitive status. WMHs (Figure 1A) were classified as periventricular WMHs (PVWMHs; localized around the lateral ventricles) or deep WMHs (DWMHs; localized within the deep WM) on the Fazekas scale ranging from 0 to 3, using FLAIR and T2-weighted images (Fazekas et al., 1987). Additionally, the age-related white matter changes (ARWMC) scale was applied to rate WMHs more specifically per brain region (Wahlund et al., 2001). PVS (Figure 2) were defined as small, linear or pointy structures of cerebrospinal fluid intensity measuring <3 mm in size and following the course of the vessels, at the level of the basal ganglia and in the centrum semiovale. T2-weighted images were used in order to rate PVS on a qualitative scale (Potter et al., 2015). Microbleeds (Figure 1B) and lacunes (Figure 1C) were rated using T2*, T2 and FLAIR images. Microbleeds were defined as small (<5 mm), homogeneous, round foci of low signal intensity on T2* in the cerebellum, brainstem, basal ganglia, white matter, or cortico-subcortical junction, differentiated from vessel flow voids and mineral depositions in the globi pallidi (Wardlaw et al., 2013a). Lacunes were defined as rounded or ovoid lesions with diameters from 3 mm to 20 mm located in the basal ganglia, internal capsule, centrum semiovale, or brainstem and carefully distinguished from WMH and PVS (Wardlaw et al., 2013b). An SVD compound score, expressing the level of cerebral SVD load, was calculated according to the method of Staals et al. (2014) WMHs and PVS were rated by two trained raters. In a random sample of one third of the brain scans, the agreement between raters was high with intraclass correlation coefficient (ICC) greater than 0.80 for the Fazekas scale (both PVWMH and DWMH); ICC greater than 0.75 for the ARWMC except for the basal ganglia and the infratentorial area; ICC greater than 0.80 regarding PVS in the basal ganglia and greater than 0.65 in the centrum semiovale. Microbleeds and lacunes, were rated by an experienced neurologist (JS). Since one single MRI scanner performed all measurements no intervariability study was performed. HCV was measured on T1-weighted images using the Learning Embeddings Atlas Propagation (LEAP) method (Wolz et al., 2010a). HCV was normalized by multiplying the native space volume with the affine scaling factor (ASF), also derived with LEAP. An ASF value of >1 indicated expansion and a value <1 contraction required to register each individual’s brain to the atlas template (Buckner et al., 2004). LEAP data were provided by Wolz (Ixico Ltd. and Imperial College, London).
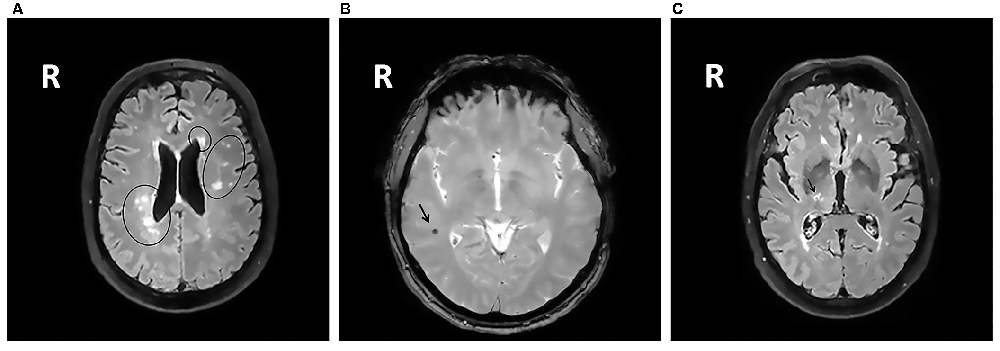
Figure 1. (A) Fluid-attenuated inversion recovery (FLAIR) brain magnetic resonance imaging (MRI) scan showing periventricular WMHs and deep WMHs (DWMHs) in both hemispheres. WMH, white matter hyperintensities; R, right. (B) T2*-weighted brain MRI scan showing a microbleed located within the right white matter. R, right. (C) FLAIR brain MRI scan showing a lacune located in the deep gray matter. R, right.
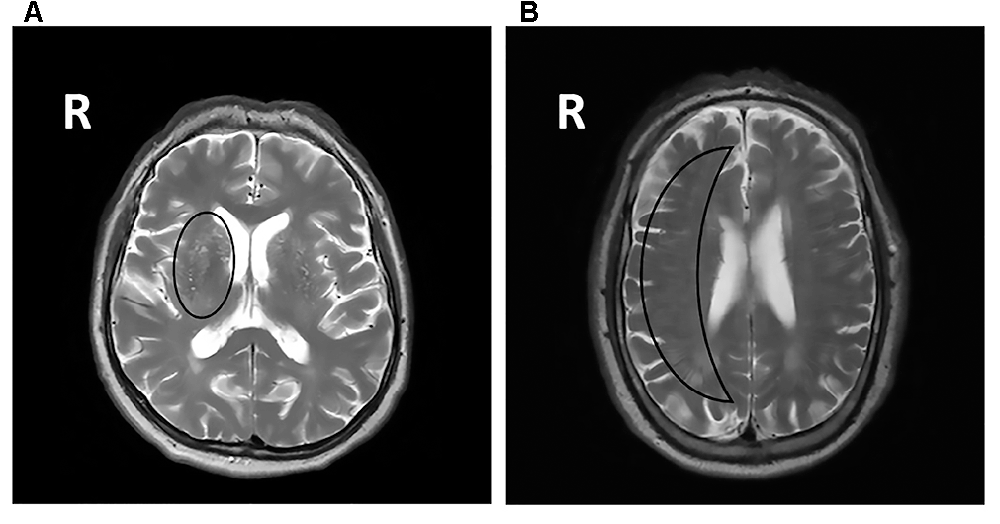
Figure 2. T2-weighted brain MRI scans showing symmetric extensive PVS at the level of the basal ganglia (A) and in the centrum semiovale (B). PVS, perivascular spaces; R, right.
Other Measures
Age, gender, educational level (according to The Dutch Standard Classification of Education (C.B.S., 2001)), marital status, visual and hearing impairment, handedness, smoking behavior and self-reported hypertension and comorbidities (Charlson Comorbidity Index (Charlson et al., 1987)) were recorded. Moreover, data on body mass index (BMI), long-term oxygen therapy, modified Medical Research Council (mMRC) dyspnea scale (American Thoracic Society, 1982), post-bronchodilator spirometry (forced vital capacity (FVC), FVC% predicted, forced expiratory volume in the first second (FEV1), FEV1% predicted and FEV1/FVC), resting arterial blood gases (arterial oxygen partial pressure [PaO2], arterial partial pressure of carbon dioxide [PaCO2] and oxygen saturation [SaO2]), and single breath carbon monoxide diffusing capacity (DLCO% predicted) were collected.
Statistical Analyses
All statistical analyses were done using SPSS 21.0 (SPSS Inc. Chicago, IL, USA). A p-value < 0.05 was considered as statistically significant. Categorical variables are described as frequencies, while continuous variables were tested for normality and are presented as mean and standard deviation (SD) or median and interquartile range (IQR).
Raw test scores on the neuropsychological test battery were transformed into age, gender and education corrected Z-scores based on normative data from the Maastricht Aging Study (MAAS). Cognitively low-performing individuals had a score less than 1.0 SD below the age-, gender- and education-specific mean of the MAAS study population for at least two core tests and high-performing individuals had a score more than 1.0 SD above the age-, gender- and education-specific mean of the MAAS study population for at least two core tests (Table 1; Singh et al., 2013).
Patient characteristics were compared between the groups using Chi-square tests for categorical variables and independent samples t-test or Mann-Whitney U test, as appropriate for continuous variables. Mann Whitney U tests were performed in order to compare high and low-performing individuals based on features of SVD. HCV were compared using independent samples t-tests. The p-values were adjusted using the Benjamini-Hochberg correction (Benjamini and Hochberg, 1995) for multiple tests, with a false discovery rate of 5%.
Results
Patient Characteristics
Cognitively low-performing patients had significantly worse mean scores on all core tests of the COgnitive-PD test battery compared to high-performing patients (Table 1). Demographic and clinical characteristics, including smoking behavior, FEV1, arterial blood gases and diffusion capacity were comparable between cognitively high and low-performing patients. The groups did not differ on reported comorbidities (Table 2).
Structural Brain Abnormalities in High and Low-Performing Patients with COPD
No differences were seen on the DWMH and PVWMH Fazekas subscales (Table 3). WMHs mapped specifically per brain region did not differ between low and high-performing patients. PVS in basal ganglia or centrum semiovale did not differ between the groups. There was no difference in presence of lacunes or microbleeds. The level of cereberal SVD load was comparable between the two groups. No significant difference was found between low and high-performing individuals regarding the left and right HCV (Table 3).
Discussion
Key Findings
The goal of the current brain MRI study was to assess whether differences in SVD features and/or HCV are related to the level of cognitive functioning in patients with COPD. In contrast to our hypothesis that COPD patients with low cognitive performances are characterized by a higher SVD load, and smaller HCV compared to COPD patients with high cognitive performances, we found that structural brain changes were comparable between COPD patients with low or high cognitively performance.
We did not find a difference in the compound score of SVD between high and low-performing COPD patients. Indeed, SVD may also be found in individuals with normal cognitive performance (Duning et al., 2005) and the exact pathophysiological link between SVD features and cognitive impairment remains not completely understood. It is suggested that individuals with normal cognitive performance have a greater cognitive reserve by which they are able to compensate certain brain pathologies better than others (Stern et al., 2005). However, the low and high-performing groups in our study were comparable for educational level, which is considered to be one on of the best indicators for cognitive reserve. Although not significant, we observed a trend towards more hypertension, one of the main risk factors for SVD (Pantoni and Garcia, 1997), in cognitively low-performing patients. 34.5% of patients without physician-diagnosed hypertension had an elevated systolic blood pressure (≥140) or elevated diastolic blood pressure of ≥90. Yet, the percentage of patients with elevated systolic or diastolic blood pressure did not differ between the low and high-performing group.
When we mapped WMHs specifically per brain region, these structural brain abnormalities were not able to differentiate both groups. A population-based study reported that COPD patients had more severe PVWMHs than persons without COPD (van Dijk et al., 2004). Characteristics of the control group were not reported. Our results show no difference in DWMHs between low and high-performing patients. Contradictory findings on the differential impact of PVWMHs and DWMHs on cognition exist, probably due to varying terminology and definitions for PVWMHs and DWMHs (Kim et al., 2008). Soriano-Raya et al. (2012) suggested that only DWMHs, not PVWMHs, are related to cognitive impairments in middle-aged individuals. De Groot et al. (2000) showed that PVWMHs are associated with cognition and DWMHs with depression. High and low cognitively performing COPD patients did not differ on symptoms of depression.
Van Dijk et al. (2004) did not find an association between COPD and lacunes. In addition, our study showed no difference in the incidence of lacunes between high and low-performing COPD patients. In a large population-based study, a higher prevalence of cerebral microbleeds was associated with COPD (Lahousse et al., 2013). Our study did not find differences in the presence of microbleeds between high and low-performing COPD patients. It might be that patients with COPD are at risk for microbleeds due to comorbid processes, such as systemic inflammation (Maclay and MacNee, 2013) without specific cognitive consequences.
Finally, no differences in the extent of PVS were found between high and low-performing patients. The exact causes of PVS are uncertain but abnormalities at the blood brain interface and inflammation have been associated with PVS (Wuerfel et al., 2008; Wardlaw et al., 2009). Studies suggest that smoking and reduced lung function cause inflammation and that both variables might act in an additive manner (Gan et al., 2005; Pelegrino et al., 2013). Our groups were comparable for lung function and smoking status which might explain the absence of differences in the extent of PVS.
MRI markers of SVD including WMHs, lacunes and microbleeds have been related to cognitive performance in population-based (Prins et al., 2005) and stroke cohorts (Gregoire et al., 2011; Patel et al., 2013). Yet, effect sizes have been small across studies (van der Vlies et al., 2012; Kloppenborg et al., 2014). The relatively weak or inconsistent correlations may be explained by the fact that it is not the individual lesions that determine the impact on cognitive performance, but the cumulative effect of these small, spatially distributed lesions. This is in line with findings of Schneider et al. (2007) who demonstrated multiple brain pathologies in cases with dementia using autopsy.
Rates of hippocampal atrophy are sensitive features of neurodegeneration. In normal aging (Ystad et al., 2009) and in several neurological disorders, including AD (Laakso et al., 1995), temporal lobe epilepsy (Kilpatrick et al., 1997), and depressed elderly (Steffens et al., 2011), measurements of volumes of the hippocampus already have been shown to be positively correlated with impaired performances on neuropsychological tests, especially in the memory domain. Also in COPD, HCV may be a proximity marker for cognitive (memory) impairment. Yet, no differences were observed in left and right HCV between cognitively low and high-performing COPD patients. Indeed, compared to cross-sectional studies, effect sizes are stronger across longitudinal studies (Raz et al., 2007).
Alternate mechanisms than structural brain abnormalities may explain cognitive performances are probably multifactorial. First, worse cognitive performance in the low-performing COPD group might be explained by damage caused by oxidative stress, like oxidized proteins, glycated products and lipid peroxidation which lead to degeneration of neurons and impairments in cognitive functioning (Popa-Wagner et al., 2013). Second, although not significant, the high-performing group more often received supplemental oxygen. Regular use of supplemental oxygen therapy has been shown to decrease the risk for cognitive impairment in patients with COPD (Thakur et al., 2010). Third, it is possible that the low-performing group had more comorbidities associated with cognition, other than mentioned in the Charlson comorbidity index (e.g., osteoporosis and Major Depressive Disorder) compared to the high-performing group. Fourth, mitochondrial dysfunction in COPD affects tissues with high energetic demands such as skeletal muscle, cardiac muscle and the central nervous system accompanied by cognitive deficits (Mancuso et al., 2009).
Limitations
Although we used a 3-T MRI scanner, the prevalence of detected SVD features was rather low. Furthermore, the number of patients investigated in this study was small and therefore we might have a low power to detect group differences in the outcome of interest. However, the group was relatively homogeneous with regard to demographical and clinical characteristics. Nevertheless, selection bias cannot be excluded, because, for instance, patients with personal interest are more willing to participate. We used visual rating scales, and although validated, these have some limitations, such as non-linearity of data, lack of sensitivity to small changes, and subjective assessment. By using non-parametric tests and calculating an ICC, we tried to compensate for these limitations. Moreover, Z scores below −1.0 SD and above +1 SD were considered as low and high cognitive performance, respectively. These score cutoff scores may not have been able to differentiate both groups sufficiently. Yet, Singh et al. (2013) considered a Z scores below −1.0 SD as being impaired in a COPD population. Though not the goal of this study, future inclusion of a control group could provide more insight into COPD specific brain-cognition correlations. Additionally, this cross-sectional study can only determine associations, no causal relationships nor the sequence of the development of cognitive impairment. Therefore, future studies should assess cognitive impairment at various stages of the disease, taking into account varying degrees of hypoxemia.
Conclusions
Using macrostructural MRI features of SVD and HCV, we found no differences between cognitively low and high-performing patients with COPD. This suggests that SVD and HCV are not related to cognitive performance in patients with COPD. Additional studies will be needed to get a better understanding of the mechanisms leading to cognitive impairment in COPD patients, including inflammatory factors, hormonal responses, metabolic disturbances, (epi)genetic or lifestyle factors, or microstructural brain changes using Diffusion Tensor Imaging.
Author Contributions
FAHMC, RWHMP, MAS, SB, HILJ, EHBMG, JS, FMEF, JBD, LEGWV, PAH, EFMW and DJAJ: substantial contributions to the conception or design of the work, and/or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published.
Funding
This work was supported by The Weijerhorst Foundation, Maastricht, Netherlands.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors like to thank Kiki Nap (KN) for her assistance in rating WMHs and PVS.
References
American Thoracic Society. (1982). Surveillance for respiratory hazards in the occupational setting [American Thoracic Society]. Am. Rev. Respir. Dis. 126, 952–956.
Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300.
Buckner, R. L., Head, D., Parker, J., Fotenos, A. F., Marcus, D., Morris, J. C., et al. (2004). A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage 23, 724–738. doi: 10.1016/j.neuroimage.2004.06.018
Burgmans, S., van Boxtel, M. P. J., Smeets, F., Vuurman, E. F. P. M., Gronenschild, E. H. B. M., Verhey, F. R. J., et al. (2009). Prefrontal cortex atrophy predicts dementia over a six-year period. Neurobiol. Aging 30, 1413–1419. doi: 10.1016/j.neurobiolaging.2007.11.028
Cai, Z., Wang, C., He, W., Tu, H., Tang, Z., Xiao, M., et al. (2015). Cerebral small vessel disease and Alzheimer’s disease. Clin. Interv. Aging 10, 1695–1704. doi: 10.2147/CIA.S90871
Charlson, M. E., Pompei, P., Ales, K. L., and Mackenzie, C. R. (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 40, 373–383. doi: 10.1016/0021-9681(87)90171-8
Cleutjens, F. A. H. M., Franssen, F. M. E., Spruit, M. A., Vanfleteren, L. E. G. W., Gijsen, C., Dijkstra, J. B., et al. (2017). Domain-specific cognitive impairment in patients with COPD and control subjects. Int. J. Chron. Obstruct. Pulmon. Dis. 12, 1–11. doi: 10.2147/COPD.S119633
Cleutjens, F. A. H. M., Janssen, D. J. A., Ponds, R. W. H. M., Dijkstra, J. B., and Wouters, E. F. M. (2014a). COgnitive-pulmonary disease. Biomed Res. Int. 2014:697825. doi: 10.1155/2014/697825
Cleutjens, F. A. H. M., Wouters, E. F. M., Dijkstra, J. B., Spruit, M. A., Franssen, F. M. E., Vanfleteren, L. E., et al. (2014b). The COgnitive-pulmonary disease (COgnitive-PD) study: protocol of a longitudinal observational comparative study on neuropsychological functioning of patients with COPD. BMJ Open 4:e004495. doi: 10.1136/bmjopen-2013-004495
De Groot, J. C., De Leeuw, F.-E., Oudkerk, M., Van Gijn, J., Hofman, A., Jolles, J., et al. (2000). Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann. Neurol. 47, 145–151. doi: 10.1002/1531-8249(200002)47:2<145::AID-ANA3>3.3.CO;2-G
Divo, M., Cote, C., de Torres, J. P., Casanova, C., Marin, J. M., Pinto-Plata, V., et al. (2012). Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 186, 155–161. doi: 10.1164/rccm.201201-0034OC
Dodd, J. W., Chung, A. W., van den Broek, M. D., Barrick, T. R., Charlton, R. A., and Jones, P. W. (2012). Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study. Am. J. Respir. Crit. Care Med. 186, 240–245. doi: 10.1164/rccm.201202-0355oc
Dodd, J. W., Getov, S. V., and Jones, P. W. (2010). Cognitive function in COPD. Eur. Respir. J. 35, 913–922. doi: 10.1183/09031936.00125109
Duning, T., Kugel, H., and Knecht, S. (2005). Excellent cognitive performance despite massive cerebral white matter changes. Neuroradiology 47, 749–752. doi: 10.1007/s00234-005-1420-6
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Gan, W. Q., Man, S. F. P., and Sin, D. D. (2005). The interactions between cigarette smoking and reduced lung function on systemic inflammation. Chest 127, 558–564. doi: 10.1378/chest.127.2.558
Gregoire, S. M., Charidimou, A., Gadapa, N., Dolan, E., Antoun, N., Peeters, A., et al. (2011). Acute ischaemic brain lesions in intracerebral haemorrhage: multicentre cross-sectional magnetic resonance imaging study. Brain 134, 2376–2386. doi: 10.1093/brain/awr172
Kilpatrick, C., Murrie, V., Cook, M., Andrewes, D., Desmond, P., and Hopper, J. (1997). Degree of left hippocampal atrophy correlates with severity of neuropsychological deficits. Seizure 6, 213–218. doi: 10.1016/s1059-1311(97)80008-8
Kim, K. W., Macfall, J. R., and Payne, M. E. (2008). Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol. Psychiatry 64, 273–280. doi: 10.1016/j.biopsych.2008.03.024
Kloppenborg, R. P., Nederkoorn, P. J., Geerlings, M. I., and van den Berg, E. (2014). Presence and progression of white matter hyperintensities and cognition: a meta-analysis. Neurology 82, 2127–2138. doi: 10.1212/WNL.0000000000000505
Laakso, M. P., Soininen, H., Partanen, K., Helkala, E. L., Hartikainen, P., Vainio, P., et al. (1995). Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: correlation with memory functions. J. Neural Transm. Park. Dis. Dement. Sect. 9, 73–86. doi: 10.1007/bf02252964
Lahousse, L., Tiemeier, H., Ikram, M. A., and Brusselle, G. G. (2015). Chronic obstructive pulmonary disease and cerebrovascular disease: a comprehensive review. Respir. Med. 109, 1371–1380. doi: 10.1016/j.rmed.2015.07.014
Lahousse, L., Vernooij, M. W., Darweesh, S. K., Akoudad, S., Loth, D. W., Joos, G. F., et al. (2013). Chronic obstructive pulmonary disease and cerebral microbleeds. The Rotterdam Study. Am. J. Respir. Crit. Care Med. 188, 783–788. doi: 10.1164/rccm.201303-0455OC
Li, J., and Fei, G. H. (2013). The unique alterations of hippocampus and cognitive impairment in chronic obstructive pulmonary disease. Respir. Res. 14:140. doi: 10.1186/1465-9921-14-140
Maclay, J. D., and MacNee, W. (2013). Cardiovascular disease in COPD: mechanisms. Chest 143, 798–807. doi: 10.1378/chest.12-0938
Mancuso, M., Calsolaro, V., Orsucci, D., Carlesi, C., Choub, A., Piazza, S., et al. (2009). Mitochondria, cognitive impairment, and Alzheimer’s disease. Int. J. Alzheimers Dis. 2009:951548. doi: 10.4061/2009/951548
Miyamoto, O., and Auer, R. N. (2000). Hypoxia, hyperoxia, ischemia, and brain necrosis. Neurology 54, 362–371. doi: 10.1212/WNL.54.2.362
Pantoni, L., and Garcia, J. H. (1997). Pathogenesis of leukoaraiosis: a review. Stroke 28, 652–659. doi: 10.1161/01.str.28.3.652
Patel, B., Lawrence, A. J., Chung, A. W., Rich, P., Mackinnon, A. D., Morris, R. G., et al. (2013). Cerebral microbleeds and cognition in patients with symptomatic small vessel disease. Stroke 44, 356–361. doi: 10.1161/STROKEAHA.112.670216
Pelegrino, N. R., Tanni, S. E., Amaral, R. A., Angeleli, A. Y., Correa, C., and Godoy, I. (2013). Effects of active smoking on airway and systemic inflammation profiles in patients with chronic obstructive pulmonary disease. Am. J. Med. Sci. 345, 440–445. doi: 10.1097/MAJ.0b013e31825f32a7
Popa-Wagner, A., Mitran, S., Sivanesan, S., Chang, E., and Buga, A. M. (2013). ROS and brain diseases: the good, the bad, and the ugly. Oxid. Med. Cell. Longev. 2013:963520. doi: 10.1155/2013/963520
Potter, G. M., Chappell, F. M., Morris, Z., and Wardlaw, J. M. (2015). Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovasc. Dis. 39, 224–231. doi: 10.1159/000375153
Prins, N. D., van Dijk, E. J., den Heijer, T., Vermeer, S. E., Jolles, J., Koudstaal, P. J., et al. (2005). Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain 128, 2034–2041. doi: 10.1093/brain/awh553
Raz, N., Rodrigue, K. M., Kennedy, K. M., and Acker, J. D. (2007). Vascular health and longitudinal changes in brain and cognition in middle-aged and older adults. Neuropsychology 21, 149–157. doi: 10.1037/0894-4105.21.2.149
Schneider, J. A., Arvanitakis, Z., Bang, W., and Bennett, D. A. (2007). Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. doi: 10.1212/01.WNL.0000271090.28148.24
Schuff, N., Woerner, N., Boreta, L., Kornfield, T., Shaw, L. M., Trojanowski, J. Q., et al. (2009). MRI of hippocampal volume loss in early Alzheimer’s disease in relation to ApoE genotype and biomarkers. Brain 132, 1067–1077. doi: 10.1093/brain/awp007
Singh, B., Parsaik, A. K., Mielke, M. M., Roberts, R. O., Scanlon, P. D., Geda, Y. E., et al. (2013). Chronic obstructive pulmonary disease and association with mild cognitive impairment: the Mayo Clinic Study of Aging. Mayo Clin. Proc. 88, 1222–1230. doi: 10.1016/j.mayocp.2013.08.012
Soininen, H. S., Partanen, K., Pitkänen, A., Vainio, P., Hänninen, T., Hallikainen, M., et al. (1994). Volumetric MRI analysis of the amygdala and the hippocampus in subjects with age-associated memory impairment: correlation to visual and verbal memory. Neurology 44, 1660–1668. doi: 10.1212/WNL.44.9.1660
Soriano-Raya, J. J., Miralbell, J., López-Cancio, E., Bargalló, N., Arenillas, J. F., Barrios, M., et al. (2012). Deep versus periventricular white matter lesions and cognitive function in a community sample of middle-aged participants. J. Int. Neuropsychol. Soc. 18, 874–885. doi: 10.1017/s1355617712000677
Staals, J., Makin, S. D. J., Doubal, F. N., Dennis, M. S., and Wardlaw, J. M. (2014). Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83, 1228–1234. doi: 10.1212/WNL.0000000000000837
Steffens, D. C., McQuoid, D. R., Payne, M. E., and Potter, G. G. (2011). Change in hippocampal volume on magnetic resonance imaging and cognitive decline among older depressed and nondepressed subjects in the neurocognitive outcomes of depression in the elderly study. Am. J. Geriatr. Psychiatry 19, 4–12. doi: 10.1097/JGP.0b013e3181d6c245
Stern, Y., Habeck, C., Moeller, J., Scarmeas, N., Anderson, K. E., Hilton, H. J., et al. (2005). Brain networks associated with cognitive reserve in healthy young and old adults. Cereb. Cortex 15, 394–402. doi: 10.1093/cercor/bhh142
Thakur, N., Blanc, P. D., Julian, L. J., Yelin, E. H., Katz, P. P., Sidney, S., et al. (2010). COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int. J. Chron. Obstruct. Pulmon. Dis. 5, 263–269. doi: 10.2147/copd.s10684
van der Vlies, A. E., Goos, J. D., Barkhof, F., Scheltens, P., and van der Flier, W. M. (2012). Microbleeds do not affect rate of cognitive decline in Alzheimer disease. Neurology 79, 763–769. doi: 10.1212/WNL.0b013e3182661f91
van Dijk, E. J., Vermeer, S. E., de Groot, J. C., van De Minkelis, J., Prins, N. D., Oudkerk, M., et al. (2004). Arterial oxygen saturation, COPD, and cerebral small vessel disease. J. Neurol. Neurosurg. Psychiatry 75, 733–736. doi: 10.1136/jnnp.2003.022012
Vanfleteren, L. E., Spruit, M. A., Groenen, M., Gaffron, S., van Empel, V. P., Bruijnzeel, P. L., et al. (2013). Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 728–735. doi: 10.1164/rccm.201209-1665oc
Vestbo, J., Hurd, S. S., Agusti, A. G., Jones, P. W., Vogelmeier, C., Anzueto, A., et al. (2013). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187, 347–365. doi: 10.1164/rccm.201204-0596PP
Wahlund, L. O., Barkhof, F., Fazekas, F., Bronge, L., Augustin, M., Sjögren, M., et al. (2001). A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32, 1318–1322. doi: 10.1161/01.str.32.6.1318
Wardlaw, J. M., Doubal, F., Armitage, P., Chappell, F., Carpenter, T., Muñoz Maniega, S., et al. (2009). Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann. Neurol. 65, 194–202. doi: 10.1002/ana.21549
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013a). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Wardlaw, J. M., Smith, C., and Dichgans, M. (2013b). Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–497. doi: 10.1016/s1474-4422(13)70060-7
Wolz, R., Aljabar, P., Hajnal, J. V., Hammers, A., Rueckert, D., and Alzheimer’s Disease Neuroimaging Initiative. (2010a). LEAP: learning embeddings for atlas propagation. Neuroimage 49, 1316–1325. doi: 10.1016/j.neuroimage.2009.09.069
Wolz, R., Heckemann, R. A., Aljabar, P., Hajnal, J. V., Hammers, A., Lötjönen, J., et al. (2010b). Measurement of hippocampal atrophy using 4D graph-cut segmentation: application to ADNI. Neuroimage 52, 109–118. doi: 10.1016/j.neuroimage.2010.04.006
Wuerfel, J., Haertle, M., Waiczies, H., Tysiak, E., Bechmann, I., Wernecke, K. D., et al. (2008). Perivascular spaces—MRI marker of inflammatory activity in the brain? Brain 131, 2332–2340. doi: 10.1093/brain/awn171
Ystad, M. A., Lundervold, A. J., Wehling, E., Espeseth, T., Rootwelt, H., Westlye, L. T., et al. (2009). Hippocampal volumes are important predictors for memory function in elderly women. BMC Med. Imaging 9:17. doi: 10.1186/1471-2342-9-17
Keywords: cerebral small vessel diseases, chronic obstructive pulmonary disease, cognition, hippocampus, magnetic resonance imaging
Citation: Cleutjens FAHM, Ponds RWHM, Spruit MA, Burgmans S, Jacobs HIL, Gronenschild EHBM, Staals J, Franssen FME, Dijkstra JB, Vanfleteren LEGW, Hofman PA, Wouters EFM and Janssen DJA (2017) The Relationship between Cerebral Small Vessel Disease, Hippocampal Volume and Cognitive Functioning in Patients with COPD: An MRI Study. Front. Aging Neurosci. 9:88. doi: 10.3389/fnagi.2017.00088
Received: 27 September 2016; Accepted: 20 March 2017;
Published: 30 March 2017.
Edited by:
Lia Fernandes, University of Porto, PortugalReviewed by:
Elizabeta Blagoja Mukaetova-Ladinska, Newcastle University, UKNeha Sehgal, Children’s Hospital of Philadelphia, USA
Copyright © 2017 Cleutjens, Ponds, Spruit, Burgmans, Jacobs, Gronenschild, Staals, Franssen, Dijkstra, Vanfleteren, Hofman, Wouters and Janssen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiona A. H. M. Cleutjens, ZmlvbmFjbGV1dGplbnNAY2lyby1ob3JuLm5s
 Fiona A. H. M. Cleutjens
Fiona A. H. M. Cleutjens Rudolf W. H. M. Ponds
Rudolf W. H. M. Ponds Martijn A. Spruit
Martijn A. Spruit Saartje Burgmans4
Saartje Burgmans4 Heidi I. L. Jacobs
Heidi I. L. Jacobs Julie Staals
Julie Staals Emiel F. M. Wouters
Emiel F. M. Wouters Daisy J. A. Janssen
Daisy J. A. Janssen