Corrigendum: Identification of Cerebral Metal Ion Imbalance in the Brain of Ageing Octodon degus
- 1Centre for Healthy Brain Ageing, School of Psychiatry, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
- 2Mark Wainwright Analytical Centre, University of New South Wales, Sydney, NSW, Australia
- 3School of Medical Sciences, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
- 4School of Biotechnology and Biomolecular Sciences, Faculty of Science, University of New South Wales, Sydney, NSW, Australia
- 5Centre for Ageing and Regeneration, Faculty of Biological Sciences, Pontifical Catholic University of Chile, Santiago, Chile
- 6Neuropsychiatric Institute, Euroa Centre, Prince of Wales Hospital, Sydney, NSW, Australia
The accumulation of redox-active transition metals in the brain and metal dyshomeostasis are thought to be associated with the etiology and pathogenesis of several neurodegenerative diseases, and Alzheimer’s disease (AD) in particular. As well, distinct biometal imaging and role of metal uptake transporters are central to understanding AD pathogenesis and aging but remain elusive, due inappropriate detection methods. We therefore hypothesized that Octodon degus develop neuropathological abnormalities in the distribution of redox active biometals, and this effect may be due to alterations in the expression of lysosomal protein, major Fe/Cu transporters, and selected Zn transporters (ZnTs and ZIPs). Herein, we report the distribution profile of biometals in the aged brain of the endemic Chilean rodent O. degus—a natural model to investigate the role of metals on the onset and progression of AD. Using laser ablation inductively coupled plasma mass spectrometry, our quantitative images of biometals (Fe, Ca, Zn, Cu, and Al) appear significantly elevated in the aged O. degus and show an age-dependent rise. The metals Fe, Ca, Zn, and Cu were specifically enriched in the cortex and hippocampus, which are the regions where amyloid plaques, tau phosphorylation and glial alterations are most commonly reported, whilst Al was enriched in the hippocampus alone. Using whole brain extracts, age-related deregulation of metal trafficking pathways was also observed in O. degus. More specifically, we observed impaired lysosomal function, demonstrated by increased cathepsin D protein expression. An age-related reduction in the expression of subunit B2 of V-ATPase, and significant increases in amyloid beta peptide 42 (Aβ42), and the metal transporter ATP13a2 were also observed. Although the protein expression levels of the zinc transporters, ZnT (1,3,4,6, and 7), and ZIP7,8 and ZIP14 increased in the brain of aged O. degus, ZnT10, decreased. Although no significant age-related change was observed for the major iron/copper regulator IRP2, we did find a significant increase in the expression of DMT1, a major transporter of divalent metal species, 5′-aminolevulinate synthase 2 (ALAS2), and the proto-oncogene, FOS. Collectively, our data indicate that transition metals may be enriched with age in the brains of O. degus, and metal dyshomeostasis in specific brain regions is age-related.
Introduction
Alzheimer’s disease (AD) is the most common progressive age-related neurodegenerative disorder, characterized by debilitating effects on brain function, such as memory loss and decline in cognitive abilities ultimately resulting in loss of independent functioning (Teri et al., 1989; Baddeley et al., 1991; Terry et al., 1991). The two major pathological hallmarks of AD are extracellular amyloid plaques composed of insoluble amyloid beta (Aβ) protein, and intra-neuronal neurofibrillary tangles (NFTs) containing hyperphosphorylated tau (Khachaturian, 1985; Joachim et al., 1987; Selkoe et al., 1987; Mirra et al., 1991; Brun and Englund, 2002). While a substantial body of evidence has implicated neuroinflammation, oxidative stress, excitotoxicity, and oligomeric Aβ toxicity as AD contributing factors, the precise mechanism of AD etiopathology remains unclear (Halliwell, 1989). Several studies have shown that dysregulated transition metal metabolism, particularly Cu, Fe, and Zn may be casually linked to the neuropathology of AD, and may enhance Aβ aggregation and toxicity (Bush, 2003; Bush et al., 2003; Finefrock et al., 2003).
Over the last two decades, several studies using clinical diagnosis and post-mortem AD brain tissue have shown that the levels of Cu, Fe, and Zn accumulate in large concentrations within Aβ plaques (Lovell et al., 1998). Moreover, it has been demonstrated that Cu, Fe, and Zn are associated with the metabolism and functional roles of Aβ and amyloid precursor protein (APP; Smith et al., 2007). This has led to the hypothesis that abnormal biometal deposition may play an important role in the pathobiology of AD, and metal chelation may represent an important therapeutic strategy to prevent the onset or slow down the progression of AD (Bush, 2003; Faux et al., 2010; Bonda et al., 2011; Braidy et al., 2014). Levels of Cu and Fe are upregulated in the brain with age, suggesting that an age-dependent increase in bioactive transition metals may contribute to AD pathology (Maynard et al., 2002). However, the anatomical distribution of these metals within the brain remains unclear. The latter is important for further etiological exploration and the development of agents for the treatment and management of AD.
Apart from the involvement of Cu and Fe, Zn may also play a critical role in the pathogenesis of AD, although the exact mechanism remains unclear (Graham et al., 2014; Hancock et al., 2014; McCord and Aizenman, 2014; Rembach et al., 2014; Yuan et al., 2014). The transcription of APP is mediated by Zn-dependent transcription factors, Sp1 and NF-κB (Borchardt et al., 2000). Zn has also been shown to facilitate the oligomerization of Aβ (Taddeo et al., 2014). Furthermore, cleavage of APP by the protease α-secretase is inhibited by Zn (Parvathy et al., 2000). Therefore, it is highly likely that dysregulation of Zn may be involved in AD (Watt et al., 2014). The levels of Zn in brain tissue are modulated by two main families of Zn transporters; ZnTs (zinc transporters) and ZIPs (Zrt/Irt-like protein, SLC39). Briefly, the intracellular uptake of Zn by neurons is mediated by ZIP protein, and through activated Ca2+ voltage gated ion channels, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors, and N-methyl-D-aspartate (NMDA) receptors (Sensi et al., 2009). The export of zinc extracellularly, or from the cytosol to the lumen of intracellular organelles is mediated by ZnTs. Although several Zn transporters have previously been investigated in several AD models, a more comprehensive study is warranted (Sensi et al., 2009), especially in natural models which are likely more representative of late onset AD.
Several in vitro and in vivo models have been developed, the most commonly used being transgenic mice models (Braidy et al., 2012). However, due to the high complexity of AD pathology, transgenic mice models recapitulate many, but not all, of the major features of AD, and their validity for the sporadic form of AD is questionable (Braidy et al., 2012). In this context, the establishment and validation of a wild-type “natural” animal model of AD, which recapitulates the neuronal, neuropathological, and behavioral abnormalities of sporadic or late-onset AD, and is small and easy to handle, would be of great benefit.
Previous studies from our laboratory have identified the Octodon degus, a South American rodent endemic to Central Chile, as a “natural” rodent model for AD (Inestrosa et al., 2005). The O. degus is a diurnal, visual, and highly social rodent that “naturally” develops several symptoms that can be linked to the neuropathology of AD. This caviomorph rodent lives up to an average of 7 years in captivity, making it an interesting model for use in longitudinal studies, including those related to the neurobiology of AD (Colonnello et al., 2011; Uekita and Okanoya, 2011). The amino acid sequence of wild-type O. degus Aβ shares a high degree (97.5%) of genetic homology with humans (Inestrosa et al., 2005; Braidy et al., 2012). It was recently reported that age-relate changes in Aβ oligomers and tau phosphorylation in O. degus correlated with a decrease in both spatial and object recognition memory, and impaired postsynaptic and neural plasticity (Ardiles et al., 2012). However, changes in the levels and spatial distribution of Cu, Zn, and Fe, and other redox-active metals in the aged O. degus brain tissue are of particular interest.
In this study, we aim to further examine the homology between Alzheimer-type pathology in the South American rodent O. degus and sporadic AD in humans by studying the neuropathological abnormalities in the animal to determine if it is indeed a unique “natural” model for the study of the pathobiology of AD. We hypothesized that: (1) O. degus develop neuropathological abnormalities in the distribution of redox active biometals, such as Fe, Ca, Zn, Cu, and Al; (2) lysosomal markers are affected in the “natural” model, similar to human AD; (3) altered biometal trafficking pathways, including major Fe/Cu regulatory genes, selected ZnTs, and ZIPs may represent a potential mechanism for altered distribution of these biometals in brain sections across the entire life-cycle of the O. degus. To address these hypotheses, we quantitatively imaged the anatomical distribution of Cu, Fe, Zn, Ca, and Al, in the brains of aged O. degus by a laser ablation inductively coupled plasma (ICP) system using mass spectrometry (LA-ICPMS). We have delineated regions of interest (ROIs) and determined the biometal concentrations in the O. degus brain with advanced age. Using western blotting, we also investigated potential mechanisms for these changes by examining the expression of biometal trafficking pathways, including lysosomal function, major Fe/Cu regulatory genes, and selected ZnTs and ZIPs.
Materials and Methods
Animals
Octodon degus were obtained from a breeding colony at the animal facility of the University of Valparaiso and maintained in a controlled temperature room (23 ± 1°C), under a 12:12 h light/dark cycle, with water and food provided ad libitum. At the time of this study, 16 male O. degus were grouped by age, from 12 to 36 months of age (n = 8 per group). Ages were selected to represent the development of AD-like pathology (36 months). All efforts were made to minimize animal discomfort and stress while also limiting the number of animals used. Aged animals were anesthetized with Equitesin (2.5 ml/kg, i.p.) and injected with heparin (4 USP/kg, i.p.). Afterward, brains were surgically removed from their skulls and frozen in isopentane at -78.5°C. All procedures were conducted according to animal protocols approved by the Institutional Animal Care and Use Committee at the University of Valparaiso and Pontifical Catholic University of Chile.
Sample Preparation for LA-ICPMS
Cryosections of brains of 10 μm thickness were mounted on silanized slides. Neighboring sections were stained with hematoxylin and eosin staining.
LA-ICPMS Instrumentation
LA-ICPMS was performed using a NewWave NWR213 (New Wave UP 266, New Wave, Fremont, CA, USA) laser ablation pulsed laser sampling accessory connected to a PerkinElmer Nexion ICPMS. The material was ablated using a focused Nd:YAG laser and then transported by argon as carrier gas into the ICP plume. Following ion formation in the ICP ion source, the positively charged ions were extracted from the argon plasma (at ≈100 kPa) via the differentially pumped interface (at ≈130 Pa) between the sampler and skimmer cones into the high vacuum of the quadrupole mass analyzer, and were separated with respect to their mass-to-charge ratios and detected by the ion detector. The experimental parameters of LA-ICPMS were optimized with respect to the maximum ion intensity of 63Cu+ using a well-homogenized synthetic laboratory standard. Tissue was ablated using a laser setting of 25% of maximum power, with a repetition rate of 20 Hz, a laser spot size of 50 μm, and a scan speed of 25. The gas flows in the LA-sample chamber were 0.85 l/m for He, and 0.65 l/m for Ar. Elemental analysis on the ICPMS was performed using peak hopping scan mode, with a dwell time of 90.7 ms per amu, and an integration time of 24,048 ms. All mass spectrometric measurements were performed with established protocols in routine mode in Becker’s BrainMet Laboratory (BrainMet–Bioimaging of Metals in Brain and Metallomics) at Forschungszentrum Jülich. Optimized experimental parameters have been previously described (Matusch et al., 2010). Instrument response was calibrated using the NIST 614 and NIST 612 standards to produce images of the change in elemental distribution across the tissue. Image data was processed and calibrated using the IOLITE software, then rendered as color images using MATLAB.
Hematoxylin and Eosin Staining
Slides were over-stained with hematoxylin for 10 min and excess stain was removed by running under tap water for 2 min. Afterward, they were differentiated and destained by submerging in acid alcohol for a few seconds until sections appeared red then rinsed briefly under tap water to remove acid. Slides were blued in bicarbonate until nuclei stood out significantly then rinsed under tap water for 5 min. Afterward, the slides were submerged in 70% ethanol for 3 min, and then in eosin for 2 min. The slides were then taken through three changes of 95% ethanol, for 5 min each. Finally, slides were rinsed in 100% ethanol, coverslipped and mounted.
Western Blotting
Octodon degus brains were dissected on ice and immediately processed. Briefly, cortical tissue was homogenized in radioimmunoprecipitation assay buffer (RIPA buffer) [50 mM, Tris–Cl, pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, and 1% sodium dodecyl sulfate (SDS)], supplemented with a protease inhibitor cocktail (Sigma-Aldrich P8340) and phosphatase inhibitors (50 mM NaF, 1 mM Na3VO4, and 30 μM Na4P207), using a Potter homogenizer and then passed sequentially through different caliber syringes. Protein samples were centrifuged at 14,000 rpm at 4°C twice for 15 min. Total protein concentration was determined using the BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA). Hippocampal samples (20 μg) were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane The incubation with a primary antibody; then a secondary anti-goat peroxidase conjugated antibody (Pierce) was used and developed using an ECL kit (Western Lightning Plus ECL, PerkinElmer) following the manufacturer’s instructions.
Statistical Analysis
To validate the metal ion images (Figure 1) and quantify the levels of Cu, Fe, Zn, Ca, and Al in the hippocampus (Figure 2) and cortex (Figure 3) of O. degus with age, two-dimensional images were first acquired as previously described by da Silva and Arruda (2013). The raw data that was collected from the IOLITE software were exported as Microsoft Excel files, and each line that was formed by ablation was exported to a different worksheet. A total of 60 lines were required for mapping the entire slide surface. Therefore, 60 IOLITE files were generated, and each one was exported to a new worksheet, culminating in 60 Excel worksheets. Each worksheet contained the results of the analyte values on the entire slide surface. Blanks were added to account for the argon atmosphere where there was no ablation, and the blank signal was subtracted from the sample signal. The results were normalized using 13C as an internal standard. The average net 13C ion intensity was used as a surrogate of slice thickness, and calculated from histograms of pixel values (Becker et al., 2012). Afterward, excel files were converted into a text file to generate an image using MATLAB. The MATLAB image was generated using (x, y) coordinates. The x data set were collected from the LA-ICPMS, and the y data set was based on the number of lines. The z value representing the relative intensity at each point was correlated with the x and y matrix plane. For the x-axis, the ICP-MS acquisition time was considered, and LA system scan speed were used. For the y-axis, the spacing among the lines was used. Finally, data were converted into a matrix plane (x, y) in MATLAB. For data acquisition, a transpose function (w = z′) was used to adjust the image, and maintain relative intensity at each point. MATLAB images were saved as 8 bit grayscale TIFFs and were corrected for linear drift (factor of 1 was used at the end of the sample next to the standard, and the correction factor progressively increased or decreased line by line toward the other end) using IMAGENA (Osterholt et al., 2011). The hippocampus and cortex, including glass background and entire section trace metal concentrations were calculated from ion intensities averaged from freely drawn ROIs (representing the location of the hippocampus and cortex, respectively) within ion intensity images using PMOD version 3.01. This was repeated four times per sample to ensure greater reliability and accuracy of data. Microphotographs obtained prior to ablation and the RB4 Watson Paxinos Atlas2 were used to neuroanatomically define ROIs. Standard measurements were processed using Microsoft Excel algorithm calculating slope of the calibration curve. Data were analyzed based on concentration = (maximum counts - minimum counts) × (ion intensity-background)/(255 × slope). Results were expressed as mean ± standard error over the 5 vs 4 individual averages of regional element concentrations using Prism software (GraphPad Software Inc.). The classical Bonferroni threshold for 4 regions × 5 elements was p = 0.008. For the Holmes modified Bonferroni correction, a p-value of ≤0.05 was considered to be statistically significant for the first region, for the second 0.05/2, for the third 0.05/3 and so on.
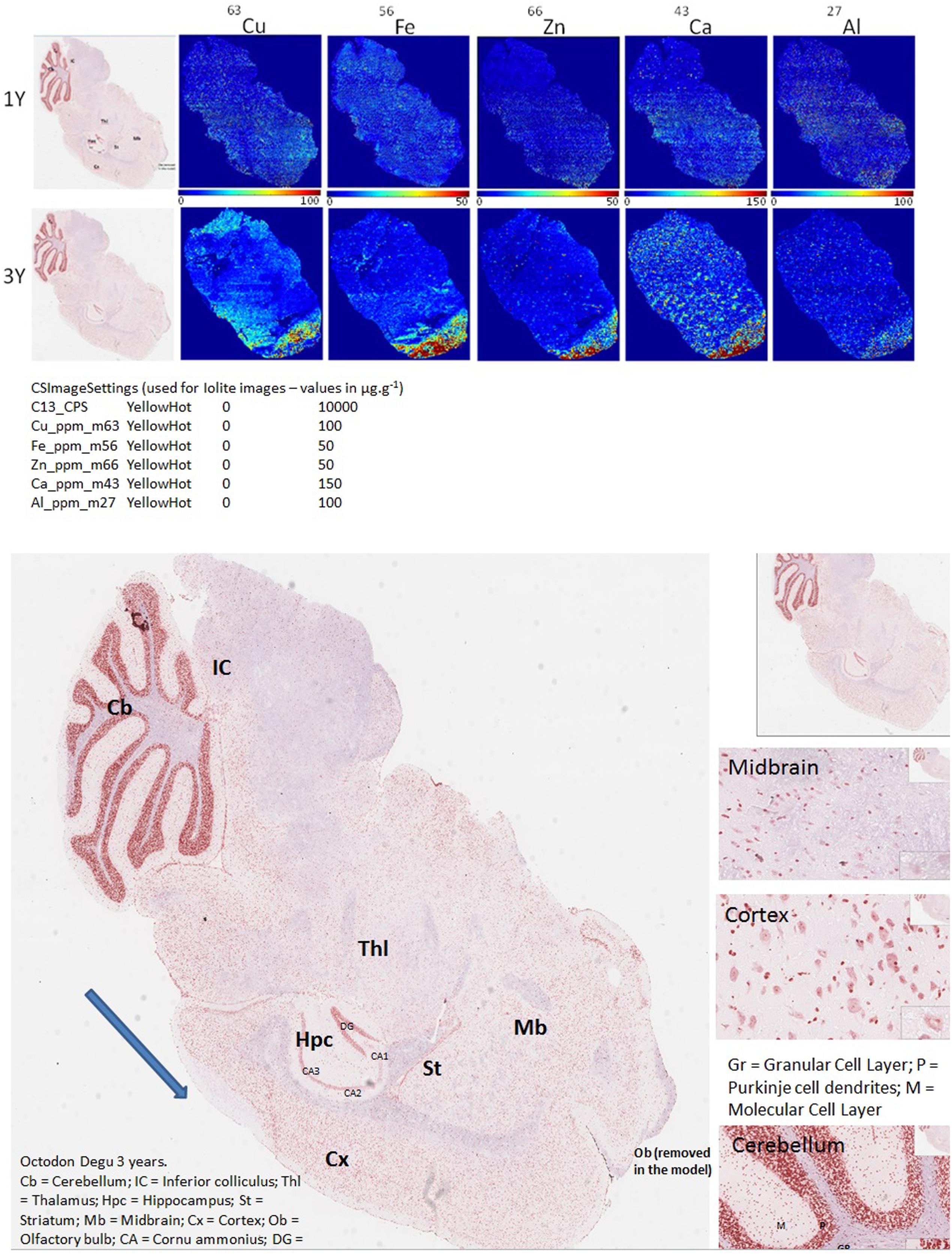
FIGURE 1. LA-ICPMS imaging of Cu, Fe, Zn, Ca, and Al, in whole brain, sagittal sections of O. degus with age. H&E stained histological sections are shown on the far left, and neighboring brain sections were used for LA-ICPMS of each of five metals.
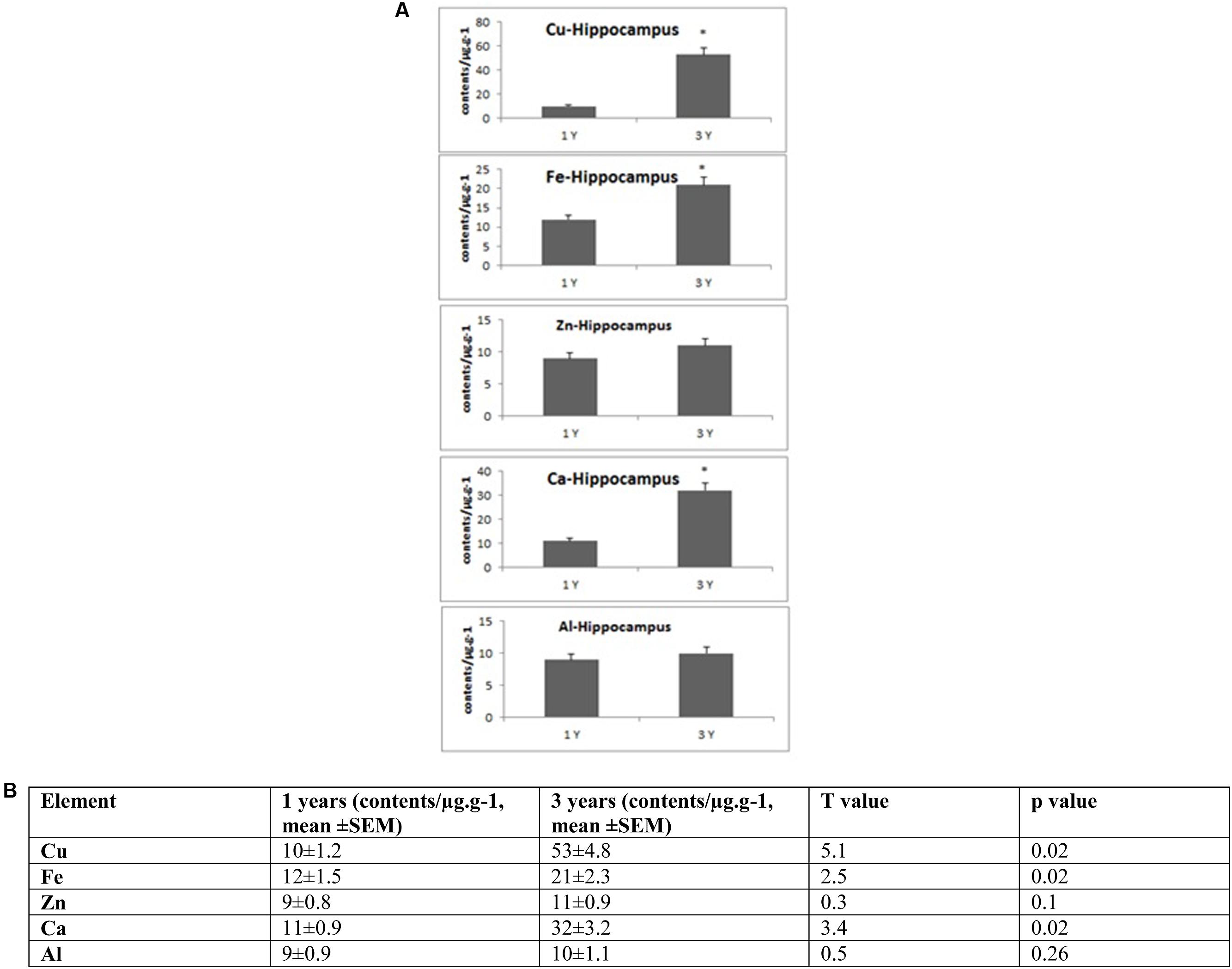
FIGURE 2. (A) Quantitative alteration of Cu, Fe, Zn, Ca, and Al in the hippocampus of O. degus with age. Significant difference between 1- and 3-year-old O. degus. ∗p < 0.05. (B) Table of absolute values mean ± SEM.
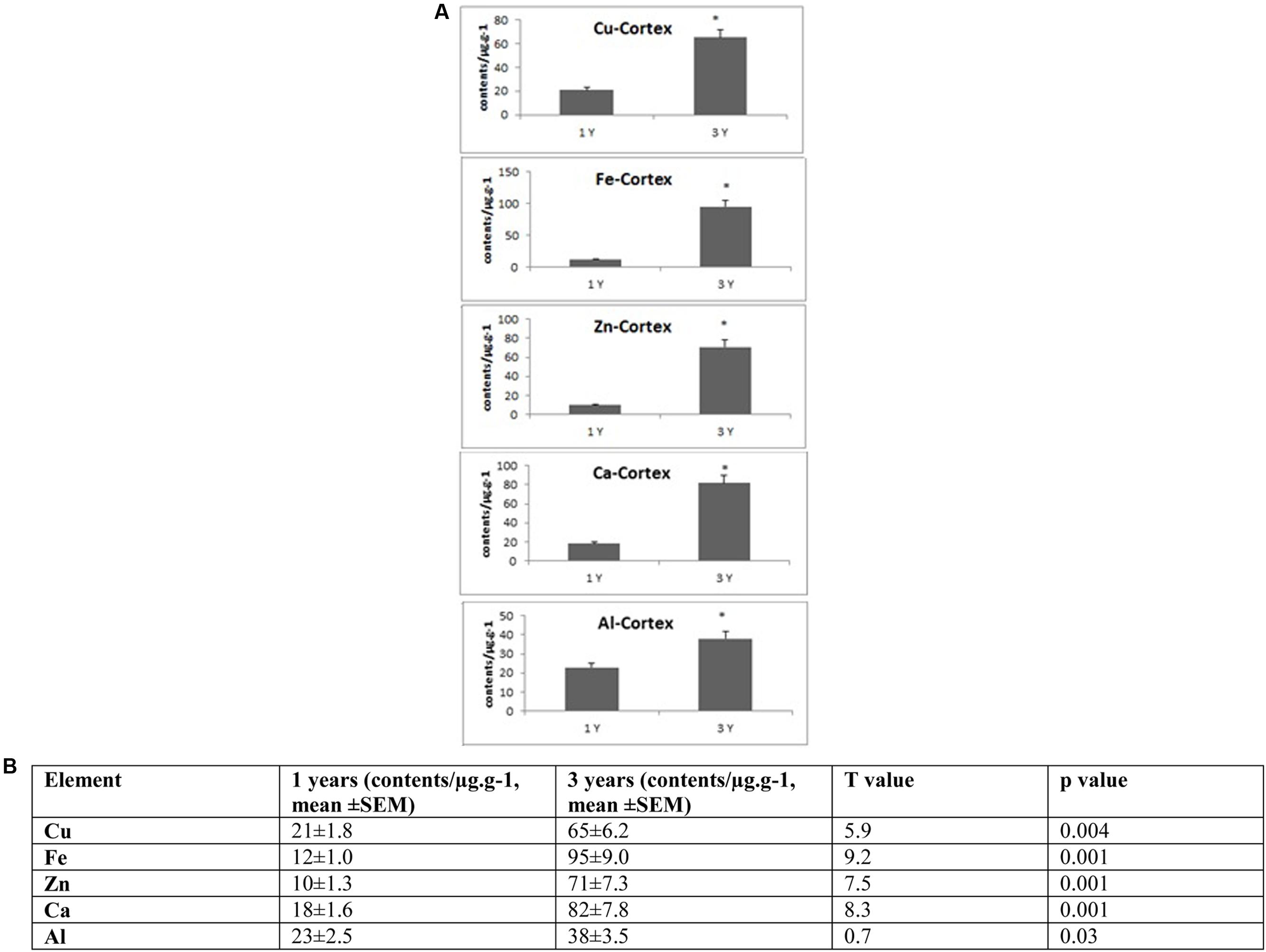
FIGURE 3. (A) Quantitative alteration of Cu, Fe, Zn, Ca, and Al in the frontal cortex of O. degus with age. Significant difference between 1- and 3-year-old O. degus. ∗p < 0.05. (B) Table of absolute values mean ± SEM.
Western blots (Figures 4–7), were quantified using ImageJ 1.47v (NIH; Rasband, W.S., ImageJ, U. S. National Institutes of Health, Bethesda, MD, USA3, 1997–2010). Statistical analysis was performed using Prism software (GraphPad Software Inc.). Data were analyzed using the Student’s t-test, and the post hoc Tukey’s multiple comparison test was applied. A p-value of ≤0.05 was considered to be statistically significant.
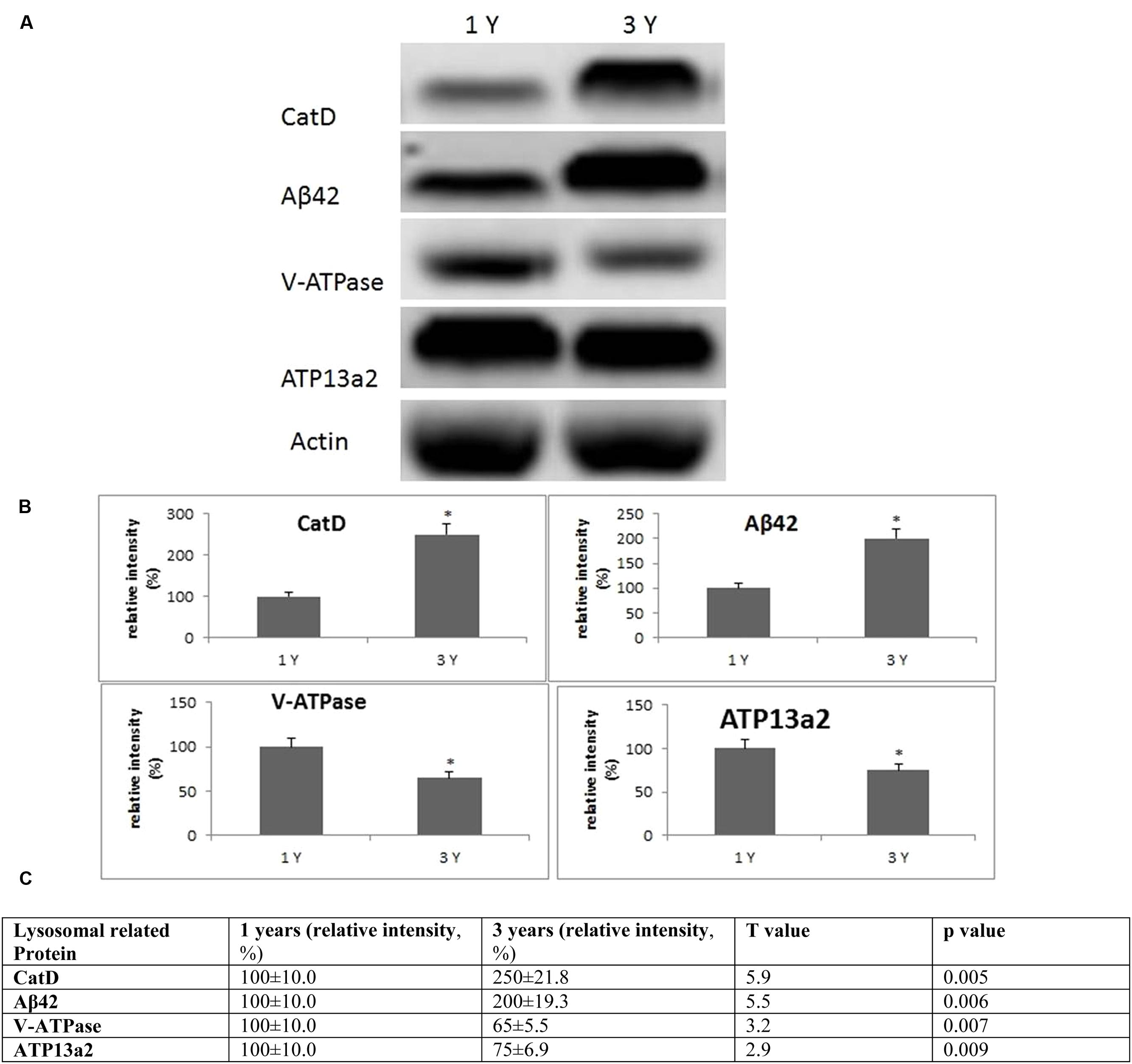
FIGURE 4. Evidence for lysosomal aberrations in the frontal cortex with age. (A) Altered levels of cathepsin D, V-ATPase, Aβ42, ATP13a2 were observed in the brain at 3 years of age compared to 1-year-old O. degus. The blots shown are representative data from an experiment repeated eight times. (B) Graphs are mean ± SEM brains from brains of eight O. degus for each age group. Significantly different ∗p < 0.01 compared to 1-year-old O. degus. (C) Table of absolute values mean ± SEM.
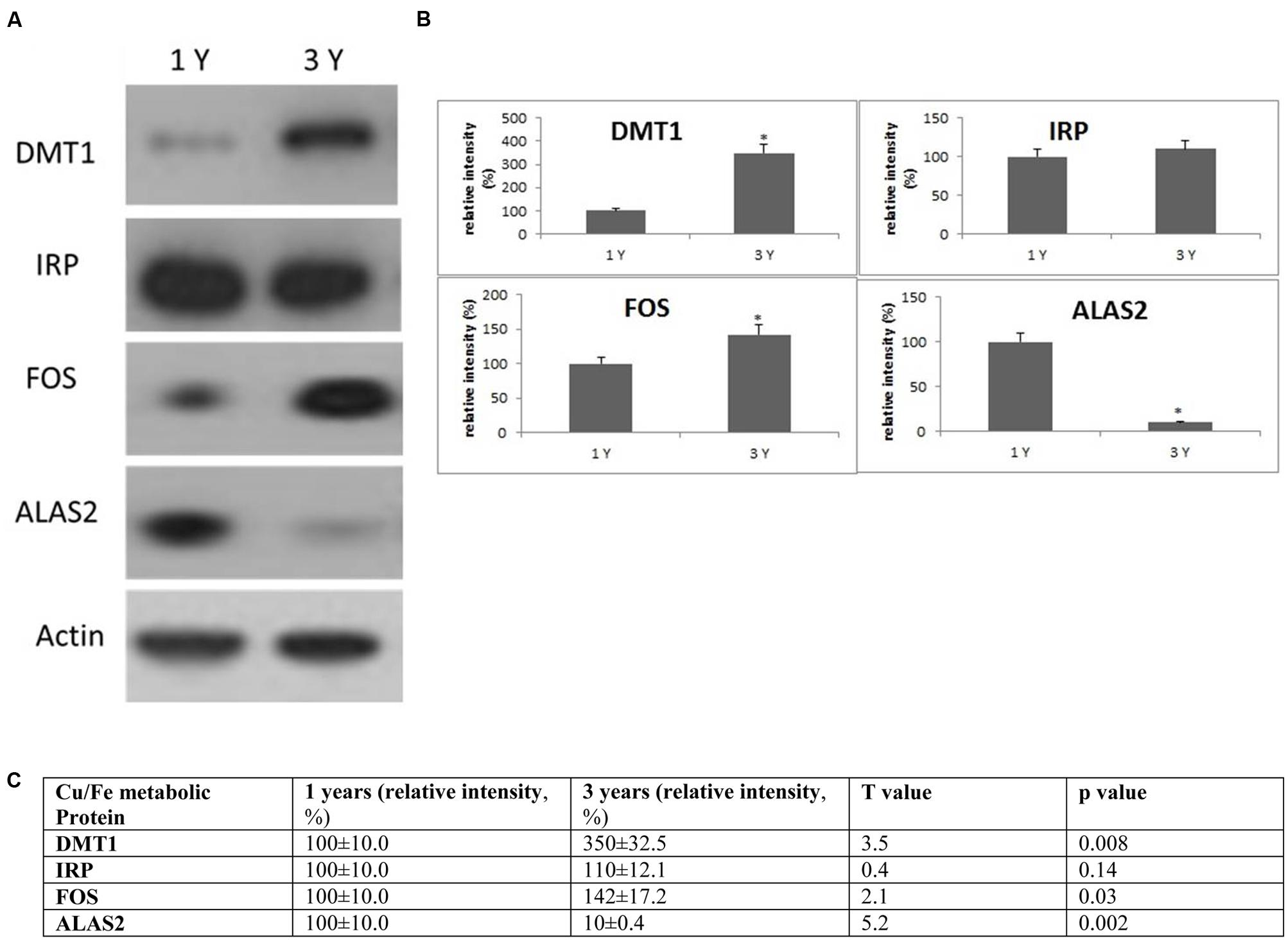
FIGURE 5. Alterations in the expression of Cu/Fe metabolic protein in the frontal cortex with age. (A) Altered levels of IRP2, DMT1, ALAS2, and FOS were reported in the brain at 3 years of age compared to 1-year-old O. degus. The blots shown are representative data from an experiment repeated eight times. (B) Graphs are mean ± SEM brains from brains of eight O. degus for each age group. Significantly different in 3-year-old ∗p < 0.01 compared to 1-year-old O. degus. (C) Table of absolute values mean ± SEM.
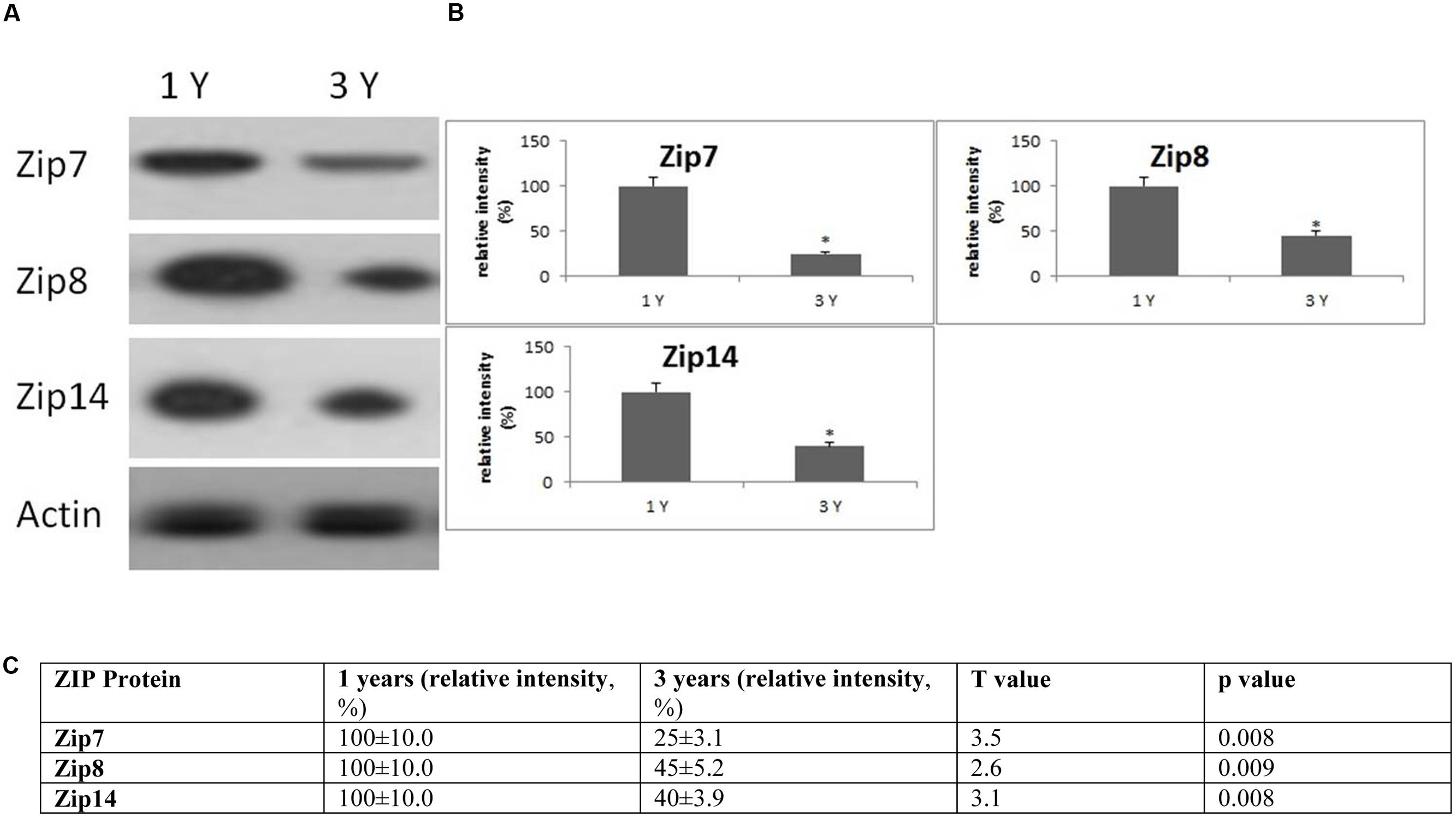
FIGURE 6. Changes in the expression of ZIP protein in the frontal cortex with age. (A) Altered levels of ZIP7,8 and ZIP14 were reported in the brain after 3 years of age compared to 1-year-old O. degus. The blots shown are representative data from an experiment repeated eight times. (B) Graphs are mean ± SEM brains from brains of eight O. degus for each age group. Significantly different in 3-year-old ∗p < 0.01 compared to 1-year-old O. degus. (C) Table of absolute values mean ± SEM.
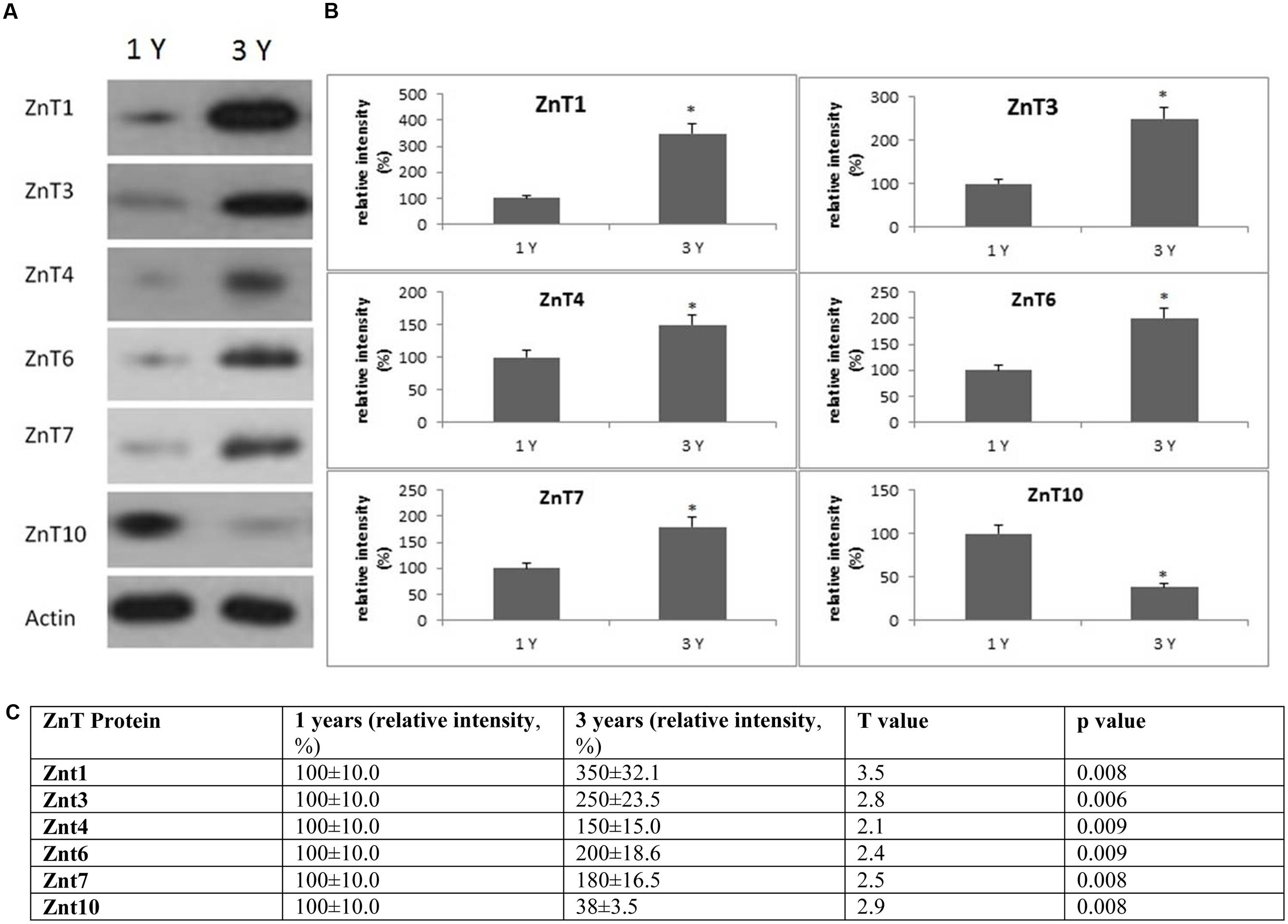
FIGURE 7. Changes in the expression of ZnT protein in the frontal cortex with age. (A) Altered levels of ZnT1,3,4,6,7, and 10 were reported in the brain after 3-years of age compared to 1-year-old O. degus. The blots shown are representative data from an experiment repeated eight times. (B) Graphs are mean ± SEM for brains of eight O. degus for each age group. Significance ∗p < 0.01 compared to 1-year-old O. degus. (C) Table of absolute values mean ± SEM.
Results
Microdistribution of Cu, Fe, Zn, Ca, and Al, in Whole Brain Sections of O. degus as a Natural Model of Aging and AD Progression
Biometal accumulation can only be examined in humans using post-mortem brain sections collected at the end stage of the disease, and the distribution of redox-active brain-metals during the pathobiology of AD remains unclear. By using the O. degus—a natural model for AD—dyshomeostasis in Cu, Fe, Zn, Ca, and Al distribution can be examined in various brain regions with age (from 1 to 3 years old) and AD progression (from early stage to late stage). Brain section elemental images are presented in Figure 1. These images were used for metals quantification in the brain regions of hippocampus and cortex (Figures 2, 3).
Several studies have shown that Cu is highly abundant in plaques but is present at lower than the limits of detection, in the whole brain of AD (Burdo and Connor, 2003; Bush, 2003; Feaga et al., 2011). Our Cu images in Figure 1 show that the amount of Cu in the brain of O. degus increases with age. These increases are focused in the hippocampal and cortical regions of O. degus. Figure 2 shows that Cu in the hippocampus of O. degus significantly increased with age, from 10.1 μg g-1 at 1 year of age to 52.1 μg g-1 at 3 years of age. Comparatively, an increase in the Cu level (21–65 μg g-1) was found in cortex from 1 to 3 years of age. Our imaging data suggests that Cu is enriched in the aging brain, and to a greater extent in the AD brain.
Similarly, Fe levels are increased with age in the brain of O. degus, indicating that, like Cu, Fe is an age-dependent enriched element in the brain (Figure 1). Fe was found to be increased in the hippocampus and cortex. The content of Fe in the hippocampus of 3 years old O. degus was in the range of 15–25 μg g-1, which was significantly higher than young O. degus (10–15 μg g-1). Figure 3 also shows an obvious Fe increase in the cortex of O. degus with age. The Fe content in the cortex of 3-year-old O. degus was about 90–100 μg g-1, which was considerably higher than that of the 1-year-old O. degus (10–15 μg g-1).
The dysregulation of Zn in AD pathology is also of major interest (Watt et al., 2014). Our quantitative images of Zn in this study show the cortex and hippocampus are Zn-rich regions in brain Figure 1. The average content of Zn in the hippocampus was 10–15 μg g-1 and did not significantly increase with age. However, in the cortex, the content of Zn increased significantly with age, from ∼10 μg g-1 in 1-year-old O. degus, to ∼80 μg g-1 in 3-year-old O. degus (Figures 1, 2).
Ca represents another important metal that plays a fundamental role in normal brain cell function (Chen and Nguyen, 2014). The level of Ca was found to increase with age throughout the brain (Figure 1), and in the hippocampus and cortex in particular, supporting the hypothesis of the imbalance of calcium homeostasis and disturbed Ca flux in brain components in the development of AD.
A link between Al and AD has also been the subject of scientific debate for several decades (Andrasi et al., 1995; De Sole et al., 2013). No significant increase in the levels of Al was observed in the hippocampus in our study. However, Al levels increased significantly in the cortex of aged O. degus, from ∼20 μg g-1 in 1-year-old O. degus, to ∼40 μg g-1 in 3-year-old O. degus.
Impaired Lysosomal Function in O. degus
Lysosomal dysfunction, has previously been reported in AD and several transgenic rodent models (Armstrong et al., 2014; Coffey et al., 2014; Xue et al., 2014). In frontal cortex, we found an age-dependent increase in cathepsin D activity from 1 to 3 years of age (Figure 4). An age-dependent reduction of subunit B2 of V-ATPase was also observed in the frontal cortex, the site of neuropathological changes in AD, suggestive of lysosomal impairment. We also examined the levels of Aβ peptide Aβ42, and the metal transporter, ATP13a2. Significantly increased concentrations of Aβ42, and decreased ATP13a2 levels were also observed after onset of neurological disease (Figure 4), providing further support for a relationship between AD and lysosomal aberrations.
IRP2, DMT1, ALAS2, and FOS expression in O. degus
To evaluate the biological significance of increased Cu and Fe with age, we measured the protein expression of IRP2 and DMT1 in the frontal cortex. DMT1 is a major transporter of divalent metal species and exists in two isoforms—one that contains an iron responsive element in the untranslated region and would be expected to be regulated by IRP2 (DMT1-IRE) and a second that lacks an IRE and should be regulated independently of IRP2 levels (DMT1-non-IRE) (Wilkinson and Pantopoulos, 2014). We found a significant increase in expression of DMT1-non-IRE in 3-year-old O. degus compared to 1-year-old O. degus (Figure 5). However, IRP2 protein expression remained unchanged (Figure 5).
We also assessed the expression of additional proteins related to Cu and Fe metabolism. The proteins of interest were proto-oncogene c-Fos (FOS), and 5-aminolevulinate synthase (ALAS2) (Figure 5). We found a significant increase in expression of FOS in 3-year-old O. degus compared to 1-year-old O. degus (Figure 5). However, the levels of ALAS2 significantly declined with age in 3-year-old O. degus compared to 1-year-old O. degus (Figure 5). This suggests that alterations in the ratio of Cu and Fe significantly alter intracellular Cu/Fe pathways.
Age-Specific Alterations to Zn Transport Pathways
Western blotting revealed age related changes in the protein expression of Zip and ZnT metal transporter proteins. The expression of the ER/Golgi-resident transporter, Zip7, significantly reduced in the frontal cortex in an age-dependent manner in 3-year-old O. degus. Similarly, significantly reduced expression of Zip8, and Zip14 were observed in 3-year-old O. degus (Figure 6). On the contrary, the protein expression of ZnT1, ZnT3, ZnT4, ZnT6, and ZnT7 were significantly increased with age in the frontal cortex of 3-year-old O. degus (Figure 7). However, the expression of ZnT10 was significantly reduced. Therefore, dysregulated expression of ZIP and ZnT family may be associated with the progression and neuropathology of AD.
Discussion
Natural animal models which closely resemble the etiopathogenesis of AD are needed for research on disease mechanisms and for drug development. No reliable “natural” model of AD is available, and current AD research largely uses transgenic models, which have a number of limitations, including the fact that they do not recapitulate the morphological and temporal patterns observed in clinical AD in humans (Braidy et al., 2012, 2015). Therefore, data on molecular mechanisms and drug efficacy testing using these genetically modified models should be interpreted with care, as many previous discoveries made in transgenic models have been lost in human translation (Braidy et al., 2012, 2015). Spontaneous age-related neurodegeneration reported in O. degus mimics many of the cellular and molecular events that have been observed extensively in experimental models and clinical AD (Inestrosa et al., 2005, 2015; Du et al., 2015). An interesting feature of the AD-like changes observed in O. degus brains is the fact that they occur in aged animals and so far, have never been detected in young animals (1-year-old; Inestrosa et al., 2015). In one study, 3-year-old wild-type O. degus have been shown to develop significant Aβ peptide deposition in blood vessel walls of the brain (van Groen et al., 2011). Capillary Aβ deposition in the cerebral cortex has been recently reported in a population-based study of 601 individuals aged greater than 85 years, and was associated with the severity of dementia and AD-type pathology (Makela et al., 2016). Moreover, hippocampal tau deposits were shown to occur parallel to loss of myelin, indicative of significant white matter degeneration in that same study (van Groen et al., 2011). Hippocampal tau deposits have also been detected in post-mortem AD brain tissue using THK5117, a radiotracer for positron emission tomography (PET) scan imaging of tau deposits (Lemoine et al., 2015). Moreover, white matter abnormalities have been shown to not only represent an early pathological event in AD, but may also be involved in Aβ aggregation and tau hyperphosphorylation (Sachdev et al., 2013). Taken together, we can postulate that amyloid and tau deposition are age-dependent processes in O. degus, as also occurs in clinical AD patients.
Abnormal metal deposition have also been reported in the APP/V717I transgenic mouse model and wild-type age-matched mice as controls using LA-ICPMS (Wang et al., 2012). However, very few studies using LA-ICPMS as a tool for biometal analysis, have been performed in the brain of “physiologically” aged rodent models. One study performed demonstrated a significant increase in Fe levels in the substantia nigra, the thalamus, and the CA1 region of the hippocampus of 14-month-old mice compared to 2-month-old mice (Becker et al., 2010). As iron is well known to form free radicals due to its high redox active properties, increased cerebral Fe levels may potentiate neurodegeneration. The same study showed that the levels of Zn remained unchanged with age. The latter supports the essential role of Zn as a major neurotransmitter that can be stored in vesicules. Another study showed that the global cerebral content of Cu was increased in 14-month-old mice compared to 2-month-old mice. However, reduced Cu uptake was noted in the striatum and ventral cortex in aged mice, which were associated with the highest decline in cerebral superoxide dismutase-1 (SOD-1). This further suggests that dysregulated Cu homeostasis may contribute to oxidative stress and neurodegeneration in the aging brain (Wang et al., 2010).
However, the current study is the first to identify specific age-related changes in biometal trafficking and transition metal distribution in O. degus, a potential “natural” model for AD. It is well established that deposition of the redox elements Fe and Cu contribute to Aβ-induced toxicity in the AD brain (Lovell et al., 1998; Bush, 2003; Bush et al., 2003; Matusch et al., 2010). Evidence shows the redox cycling of Cu contributes to the oxidative stress of AD (Burdo and Connor, 2003; Chen and Nguyen, 2014; Hancock et al., 2014; McCord and Aizenman, 2014). Although evidence for altered Cu levels in serum are conflicting, Cu has been found to be significantly dysregulated in patients with mild cognitive impairment (MCI) who subsequently progressed to dementia (Mueller et al., 2012). Furthermore, the levels of Cu have been shown to be increased with age in the hippocampus and cortex of APP/V717I transgenic mice (Wang et al., 2012). Moreover, the ratio of Cu/Fe as well as Cu levels may be a useful biomarker to distinguish between progressive and cognitively stable MCI patients (Mueller et al., 2012). This ratio is consistent with a previous study which examined the ratio of ceruloplasmin and transferrin (the major protein carriers of Cu and Fe, respectively) in serum from early AD cases: ceruloplasmin was increased while transferrin was decreased (Squitti et al., 2002). However, an earlier study supported an increase in serum ceruloplasmin, but challenged the decrease in serum transferrin in AD (Molaschi et al., 1996).
We also observed a significant increase in the levels of Fe in aged O. degus. This is in line with previous findings in the APP/V717I transgenic mouse model, and age-matched mice as controls using LA-ICPMS (Wang et al., 2012), suggesting that Fe is age-dependently enriched in the brain. The increased Fe level is likely due to the constant transportation of Fe to the brain mediated by capillary endothelial cells, and increased release of Fe into the blood via the blood–brain barrier (Burdo and Connor, 2003; Burdo et al., 2003). While Fe was found mainly enriched in the substantia nigra and corpus callosum of APP mice (Wang et al., 2012), Fe was primarily found in the substantia nigra and cortical area of age-matched controls. However, several studies have shown that Fe is enriched in plaques, and the hippocampus and frontal cortex are the main plaque deposition regions (Burdo and Connor, 2003; Leskovjan et al., 2009). Therefore, increased Fe content in the hippocampal and cortical areas of the O. degus is suggestive of defective metal transport in some micro-regions of the AD brain. Quantitative results from a previous study using LA-ICPMS show an increased tendency for Fe in the hippocampi of both APP and age-matched controls, suggesting regional variations in Fe reactions in the brain (Wang et al., 2012). The increased level of Fe in the brain can be attributed to the presence of iron-binding proteins. Ferritin and transferrin, represent the two-most important Fe carriers, and are commonly found in both the white and gray matter (Burdo et al., 1999; Thomas and Jankovic, 2004). Therefore, accumulation of Fe may be due to the functional role of ferritin in the brain of O. degus. Additionally, serum levels of Fe in AD patients are inconsistent, and no study has specifically evaluated the levels of non-heme (chelatable) iron (Mueller et al., 2012).
As well, Zn is an important redox-active metal that plays an important role in Aβ aggregation and extracellular plaque formation (Watt et al., 2014). Therefore, determining the mechanism(s) of Zn dysregulation in AD and aging is of particular interest. The microdistribution of zinc in aged O. degus is remarkably similar to a previous study in the APP/V717I transgenic mouse model (Wang et al., 2012). We observed a significant age-related increase in Zn levels in the cortex and hippocampus, which present as Zn-enriched regions.
Additionally, Ca represents another important metal that plays an important role in normal brain cell function (Lisek et al., 2015). It is well established that increased Aβ can reduce the membrane potential, thus enhancing Ca2+ influx, leading to an accumulation of Ca2+ in cells, leading to excitotoxicity and cell death via apoptotic processes (Greenamyre, 1991; Harkany et al., 2000; Barger, 2004; Hynd et al., 2004; Tsai et al., 2005; Koutsilieri and Riederer, 2007). Increased oxidative stress may further interfere with Ca immobilization, leading to cell death via additional cytotoxic pathways (Esposito et al., 2013; Ong et al., 2013). It has been previously reported that Ca2+ overload can modulate APP metabolism, and accelerate APP hydrolysis to generate more Aβ (Wang et al., 1994; Ye et al., 2010). Our study and others have shown that Ca increases with age in the brain, and provides further evidence supporting the hypothesis of the imbalance of calcium homeostasis and disturbed Ca flux in the brain can enhance AD progression (Peterson et al., 1985, 1989; Deary and Hendrickson, 1986; Martyn et al., 1989; Kelliher et al., 1999; O’Day and Myre, 2004; Attems et al., 2008).
Numerous studies have shown that Al may also contribute to the pathology of AD (Martyn, 1992; Copestake, 1993; Doll, 1993; Kruck, 1993; Rifat, 1993; Taylor et al., 1995). A collective body of published work indicates that: (1) Al accumulates in microtubules and induces cortical atrophy in vivo (Garruto and Brown, 1994), (2) Al may be involved in the formation of extracellular plaques and NFTs (Mera, 1991; McLachlan et al., 1992; Perl, 2006); (3) Increased incidence of AD has been observed in populations where there are high levels of Al in drinking water supplies (French et al., 1989; Martyn, 1992; Harrington et al., 1994; Polizzi et al., 2002; Gupta et al., 2005; Perl, 2006); (4) Al chelators have shown positive responses for the treatment of AD in transgenic rodent models (Guy et al., 1991; Justin Thenmozhi et al., 2015); and (5) Increased Al deposition is also associated with the pathogenesis of other neurodegenerative diseases including Parkinson’s disease and amyotrophic lateral sclerosis (Bharathi et al., 2008; Singla and Dhawan, 2014). While our study shows that Al is increased with age in the cortex, no other study has reported changes in Al distribution in the brain in other AD models, and it is still unclear whether Al levels are elevated in human AD patients. Thus, further studies are warranted to elucidate the association between Al levels and AD.
The expression of cellular trafficking systems plays a critical role in metal-induced toxicity. Abnormalities in the lysosomal pathway have been previously reported in AD pathology, prior to the development of NFTs or Aβ plaques (Cataldo and Nixon, 1990; Cataldo et al., 1994). Since proteolytic processing of APP is necessary for the formation of Aβ, lysosomal proteases have been linked with AD pathology (Vidoni et al., 2016). The increased expression of cathepsin D reported in this study may represent a compensatory mechanism to restore lysosomal function (Perez et al., 2015). This is supported by another study which showed that knockout of cathepsin D enhances abnormal tau phosphorylation, and oxidative stress in the brain (Khurana et al., 2010). Cathepsin D also has an important role of maintaining neuronal homeostasis, and optimal function of cathepsin D is therefore necessary for the clearance of unfolded/oxidized protein that are transported to lysosomes to facilitate their removal (Benes et al., 2008). Similarly, vesicular ATPases are required to acidify lysosomes and maintain an optimal acidic environment for lysosomes (Williamson et al., 2010). One study showed that degradation or reduced expression of V-ATPase can enhance AD pathology by making neurons more susceptible to insult following exposure to pathological concentrations of Aβ and tau (Williamson et al., 2010). Therefore, the observed changes in cathepsin D and V-ATPase may provide an additional mechanism for the failed protein degradation, abnormal accumulation of pathological hallmarks of AD, including Aβ (which was increased in this study), and age-related neurodegeneration.
Mutations, altered expression or aberrant functionality of several metal transporters belonging to the ATP7, ATP13, TRMPL, and ZnT families have also been implicated in neurodegenerative disorders (Lovell et al., 2005; Lyubartseva et al., 2010; Quadri et al., 2012; Yonova-Doing et al., 2012). Increased ATP13a2 expression has previously been shown to attenuate lysosomal dysfunction in fibroblasts derived from PD patients (Dehay et al., 2012a,b). Therefore, it is likely that downregulation of ATP13a2 in aged O. degus may impair lysosomal function. Neurodegenerative processes involving aberrant cellular metal trafficking may be intricately linked at the molecular level.
While Cu/Fe dysregulation may represent a useful biomarker for AD, on its own it is not conclusive. We analyzed the expression of several proteins, including: IRP2, a central regulator of intracellular iron and copper metabolism, and a downstream target of IRP2, the IRE-containing gene DMT1, FOS, and ALAS2 in the frontal cortex of O. degus. Alterations in the expression of regulatory proteins associated with the metabolism and transport of Cu and Fe suggest that homeostasis of these redox active metals is dysregulated in O. degus, and contributes to the age-related changes in the microdistribution of Cu and Fe (Mueller et al., 2012).
In the brain, Zn is transported by several members of the ZIP and ZnT family of transporters. A significant reduction in members of the ZIP family of transporters was observed in our study. In particular, our results are the first to show an age-related decline in the expression of ZIP7,8 and 14 in the brain of aged O. degus. It is likely that the loss of ZIP expression may stimulate perturbations in the metabolism of other redox-active metals (Dang et al., 2014; Grubman et al., 2014a,b). Excess unbound Zn may displace other redox-active transition metals from metalloproteins, and further promote formation of highly volatile free-radicals via Fenton chemistry (Wilson et al., 2012). Increased free Zn may also inhibit protein tyrosine phosphatases in the endoplasmic reticulum, inhibiting the propagation of “Zn wave” signals throughout cells, thus impairing pleiotropic cellular signaling processes important for cellular survival (Taylor et al., 2012).
Previous studies have reported upregulation of ZnT1, ZnT3, ZnT4, ZnT5, ZnT6, and ZnT7 protein isoforms in the hippocampus and neocortex in APP/PS1 mice (Lovell et al., 2005; Smith et al., 2006; Zhang et al., 2010). Similarly, we have shown that ZnT1, ZnT3, ZnT4, ZnT6, and ZnT7 are increased with age. On the other hand, a previous study showing that ZnT3 mRNA and protein levels are reduced in the human AD brain (Beyer et al., 2009). Two earlier studies have shown that ZnT1 is decreased significantly in the hippocampus of subjects with MCI (a stage from which 30% progress to develop dementia) and pre-clinical AD (PCAD; where subjects have no overt clinical manifestations of AD but pathology is revealed post-mortem) (Lovell et al., 2005), as well as in AD. Moreover, while ZnT4 and ZnT6 were elevated in PCAD and in the hippocampus of AD patients, no significant difference in levels was reported for either transporter between MCI and age-matched controls (Lyubartseva et al., 2010). ZnT3 is the main Zn transport protein and a major contributor to Zn2+ release from the synaptic cleft to Zn-enriched terminals, and may also be responsible for the abnormal distribution of Zn in the brain (Palmiter et al., 1996). Under normal physiological conditions, the levels of unbound Zn are regulated by metallothionein, and the endogenous antioxidant enzyme, SOD. However, under pathological conditions, Zn may remain unbound over a significant length of time, and may therefore interact with Aβ and enhance plaque deposition (Bosomworth et al., 2012).
Little information is known about the functional role of Zn10 in AD. A recent study has shown that ZnT10 expression is upregulated in post-mortem AD brain tissue (Bosomworth et al., 2013). By contrast, our results show that ZnT10 expression is reduced in the frontal cortex of aged O. degus. Under physiological conditions, ZnT10 is localized to the Golgi apparatus, and maybe translocated to the plasma membrane following an increase in Zn levels leading to a down-regulation of ZnT10 expression (Bosomworth et al., 2013). Therefore, it is possible that the increase in Zn may reduce ZnT10 expression early in disease course as reported in this study. Reduced expression of ZnT10 may exacerbate AD progression effluxing Zn into the extracellular space, and providing Zn ions to facilitate Aβ deposition and senile plaque formation.
Our current study has utilized LA-ICPMS and neuro-molecular biology techniques to examine the microdistribution of redox-active transition metals, and biometal trafficking pathways in the brain of aging O. degus. Since age is the greatest risk factor for AD, our present findings provide a rationale for further utilization of the metallomic approach in research and drug development to extend lifespan and maintain healthspan.
Author Contributions
NB, AP, TJ, CM, and PS wrote the draft, reviewed and interpreted the bioimages. HR, AR, and B-EJ processed the images. NI and PS provided the conceptual foundation of the research, writing of drafts, and interpretation of data. Animal tissue was obtained from NI’s laboratory.
Funding
This work was supported by a National Health and Medical Research Council of Australia Capacity Building Grant and a UNSW Faculty of Medicine Research Grant. NB is the recipient of an Alzheimer’s Australia Viertel Foundation Postdoctoral Research Fellowship at the University of New South Wales. TJ is a recipient of the University of New South Wales Postgraduate Award (UPA).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Rebecca Cooper Medical Research Foundation for their ongoing financial support and staff of the Bioanalytical Mass Spectrometry Facility at the University of New South Wales for assistance with GC-MS experiments. H&E sections were prepared by Ms Fei Shang.
Footnotes
- ^www.pmod.com
- ^http://www.callisto-science.org/NSI/Neuroscience_Image_Database/RUN_RBSC.PDF
- ^http://imagej.nih.gov/ij
References
Andrasi, E., Farkas, E., Scheibler, H., Reffy, A., and Bezur, L. (1995). Al, Zn, Cu, Mn and Fe levels in brain in Alzheimer’s disease. Arch. Gerontol. Geriatr. 21, 89–97. doi: 10.1016/0167-4943(95)00643-Y
Ardiles, A. O., Tapia-Rojas, C. C., Mandal, M., Alexandre, F., Kirkwood, A., Inestrosa, N. C., et al. (2012). Postsynaptic dysfunction is associated with spatial and object recognition memory loss in a natural model of Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 109, 13835–13840. doi: 10.1073/pnas.1201209109
Armstrong, A., Mattsson, N., Appelqvist, H., Janefjord, C., Sandin, L., Agholme, L., et al. (2014). Lysosomal network proteins as potential novel CSF biomarkers for Alzheimer’s disease. Neuromolecular Med. 16, 150–160. doi: 10.1007/s12017-013-8269-3
Attems, J., Preusser, M., Grosinger-Quass, M., Wagner, L., Lintner, F., and Jellinger, K. (2008). Calcium-binding protein secretagogin-expressing neurones in the human hippocampus are largely resistant to neurodegeneration in Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 34, 23–32.
Baddeley, A. D., Bressi, S., Della Sala, S., Logie, R., and Spinnler, H. (1991). The decline of working memory in Alzheimer’s disease. A longitudinal study. Brain 114(Pt 6), 2521–2542. doi: 10.1093/brain/114.6.2521
Barger, S. W. (2004). An unconventional hypothesis of oxidation in Alzheimer’s disease: intersections with excitotoxicity. Front. Biosci. 9:3286–3295. doi: 10.2741/1481
Becker, J. S., Kumtabtim, U., Wu, B., Steinacker, P., Otto, M., and Matusch, A. (2012). Mass spectrometry imaging (MSI) of metals in mouse spinal cord by laser ablation ICP-MS. Metallomics 4, 284–288. doi: 10.1039/c2mt00166g
Becker, J. S., Matusch, A., Palm, C., Salber, D., Morton, K. A., and Becker, J. S. (2010). Bioimaging of metals in brain tissue by laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) and metallomics. Metallomics 2, 104–111. doi: 10.1039/b916722f
Benes, P., Vetvicka, V., and Fusek, M. (2008). Cathepsin D-Many functions of one aspartic protease. Crit. Rev. Oncol. Hematol. 68, 12–28. doi: 10.1016/j.critrevonc.2008.02.008
Beyer, N., Coulson, D. T., Heggarty, S., Ravid, R., Irvine, G. B., Hellemans, J., et al. (2009). ZnT3 mRNA levels are reduced in Alzheimer’s disease post-mortem brain. Mol. Neurodegener. 4:53. doi: 10.1186/1750-1326-4-53
Bharathi, Vasudevaraju, P., Govindaraju, M., Palanisamy, A. P., Sambamurti, K., and Rao, K. S. (2008). Molecular toxicity of aluminium in relation to neurodegeneration. Indian J. Med. Res. 128, 545–556.
Bonda, D. J., Lee, H. G., Blair, J. A., Zhu, X., Perry, G., and Smith, M. A. (2011). Role of metal dyshomeostasis in Alzheimer’s disease. Metallomics 3, 267–270. doi: 10.1039/c0mt00074d
Borchardt, T., Schmidt, C., Camarkis, J., Cappai, R., Masters, C. L., Beyreuther, K., et al. (2000). Differential effects of zinc on amyloid precursor protein (APP) processing in copper-resistant variants of cultured Chinese hamster ovary cells. Cell Mol. Biol. 46, 785–795.
Bosomworth, H. J., Adlard, P. A., Ford, D., and Valentine, R. A. (2013). Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS ONE 8:e65475. doi: 10.1371/journal.pone.0065475
Bosomworth, H. J., Thornton, J. K., Coneyworth, L. J., Ford, D., and Valentine, R. A. (2012). Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 4, 771–779. doi: 10.1039/c2mt20088k
Braidy, N., Munoz, P., Palacios, A. G., Castellano-Gonzalez, G., Inestrosa, N. C., Chung, R. S., et al. (2012). Recent rodent models for Alzheimer’s disease: clinical implications and basic research. J. Neural Transm. 119, 173–195. doi: 10.1007/s00702-011-0731-5
Braidy, N., Poljak, A., Jayasena, T., Mansour, H., Inestrosa, N. C., and Sachdev, P. S. (2015). Accelerating Alzheimer’s research through ‘natural’ animal models. Curr. Opin. Psychiatry 28, 155–164. doi: 10.1097/YCO.0000000000000137
Braidy, N., Poljak, A., Marjo, C., Rutlidge, H., Rich, A., Jayasena, T., et al. (2014). Metal and complementary molecular bioimaging in Alzheimer’s disease. Front. Aging Neurosci. 6:138. doi: 10.3389/fnagi.2014.00138
Brun, A., and Englund, E. (2002). Regional pattern of degeneration in Alzheimer’s disease: neuronal loss and histopathological grading. Histopathology 41, 40–55.
Burdo, J. R., Antonetti, D. A., Wolpert, E. B., and Connor, J. R. (2003). Mechanisms and regulation of transferrin and iron transport in a model blood-brain barrier system. Neuroscience 121, 883–890. doi: 10.1016/S0306-4522(03)00590-6
Burdo, J. R., and Connor, J. R. (2003). Brain iron uptake and homeostatic mechanisms: an overview. Biometals 16, 63–75. doi: 10.1023/A:1020718718550
Burdo, J. R., Martin, J., Menzies, S. L., Dolan, K. G., Romano, M. A., Fletcher, R. J., et al. (1999). Cellular distribution of iron in the brain of the Belgrade rat. Neuroscience 93, 1189–1196. doi: 10.1016/S0306-4522(99)00207-9
Bush, A. I. (2003). The metallobiology of Alzheimer’s disease. Trends Neurosci. 26, 207–214. doi: 10.1016/S0166-2236(03)00067-5
Bush, A. I., Masters, C. L., and Tanzi, R. E. (2003). Copper, beta-amyloid, and Alzheimer’s disease: tapping a sensitive connection. Proc. Natl. Acad. Sci. U.S.A. 100, 11193–11194. doi: 10.1073/pnas.2135061100
Cataldo, A. M., Hamilton, D. J., and Nixon, R. A. (1994). Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer-disease. Brain Res. 640, 68–80. doi: 10.1016/0006-8993(94)91858-9
Cataldo, A. M., and Nixon, R. A. (1990). Enzymatically active lysosomal proteases are associated with amyloid deposits in Alzheimer brain. Proc. Natl. Acad. Sci. U.S.A. 87, 3861–3865. doi: 10.1073/pnas.87.10.3861
Chen, M., and Nguyen, H. T. (2014). Our “energy-Ca(2(+) signaling deficits” hypothesis and its explanatory potential for key features of Alzheimer’s disease. Front. Aging Neurosci. 6:329. doi: 10.3389/fnagi.2014.00329
Coffey, E. E., Beckel, J. M., Laties, A. M., and Mitchell, C. H. (2014). Lysosomal alkalization and dysfunction in human fibroblasts with the Alzheimer’s disease-linked presenilin 1 A246E mutation can be reversed with cAMP. Neuroscience 263, 111–124. doi: 10.1016/j.neuroscience.2014.01.001
Colonnello, V., Iacobucci, P., Fuchs, T., Newberry, R. C., and Panksepp, J. (2011). Octodon degus. A useful animal model for social-affective neuroscience research: basic description of separation distress, social attachments and play. Neurosci. Biobehav. Rev. 35, 1854–1863. doi: 10.1016/j.neubiorev.2011.03.014
Copestake, P. (1993). Aluminium and Alzheimer’s disease–an update. Food Chem. Toxicol. 31, 679–683. doi: 10.1016/0278-6915(93)90052-Z
da Silva, M. A., and Arruda, M. A. (2013). Laser ablation (imaging) for mapping and determining Se and S in sunflower leaves. Metallomics 5, 62–67. doi: 10.1039/c2mt20154b
Dang, T. N., Lim, N. K., Grubman, A., Li, Q. X., Volitakis, I., White, A. R., et al. (2014). Increased metal content in the TDP-43(A315T) transgenic mouse model of frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Front. Aging Neurosci. 6:15. doi: 10.3389/fnagi.2014.00015
De Sole, P., Rossi, C., Chiarpotto, M., Ciasca, G., Bocca, B., Alimonti, A., et al. (2013). Possible relationship between Al/ferritin complex and Alzheimer’s disease. Clin. Biochem. 46, 89–93. doi: 10.1016/j.clinbiochem.2012.10.023
Deary, I. J., and Hendrickson, A. E. (1986). Calcium and Alzheimer’s disease. Lancet 1, 1219. doi: 10.1016/s0140-6736(86)91205-5
Dehay, B., Martinez-Vicente, M., Ramirez, A., Perier, C., Klein, C., Vila, M., et al. (2012a). Lysosomal dysfunction in Parkinson disease: ATP13A2 gets into the groove. Autophagy 8, 1389–1391. doi: 10.4161/auto.21011
Dehay, B., Ramirez, A., Martinez-Vicente, M., Perier, C., Canron, M. H., Doudnikoff, E., et al. (2012b). Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 109, 9611–9616. doi: 10.1073/pnas.1112368109
Doll, R. (1993). Review: Alzheimer’s disease and environmental aluminium. Age Ageing 22, 138–153. doi: 10.1093/ageing/22.2.138
Du, L. Y., Chang, L. Y., Ardiles, A. O., Tapia-Rojas, C., Araya, J., Inestrosa, N. C., et al. (2015). Alzheimer’s disease-related protein expression in the retina of Octodon degus. PLoS ONE 10:e0135499. doi: 10.1371/journal.pone.0135499
Esposito, Z., Belli, L., Toniolo, S., Sancesario, G., Bianconi, C., and Martorana, A. (2013). Amyloid beta, glutamate, excitotoxicity in Alzheimer’s disease: are we on the right track? CNS Neurosci. Ther. 19, 549–555. doi: 10.1111/cns.12095
Faux, N. G., Ritchie, C. W., Gunn, A., Rembach, A., Tsatsanis, A., Bedo, J., et al. (2010). PBT2 rapidly improves cognition in Alzheimer’s disease: additional phase II analyses. J. Alzheimers Dis. 20, 509–516. doi: 10.3233/JAD-2010-1390
Feaga, H. A., Maduka, R. C., Foster, M. N., and Szalai, V. A. (2011). Affinity of Cu+ for the copper-binding domain of the amyloid-beta peptide of Alzheimer’s disease. Inorg. Chem. 50, 1614–1618. doi: 10.1021/ic100967s
Finefrock, A. E., Bush, A. I., and Doraiswamy, P. M. (2003). Current status of metals as therapeutic targets in Alzheimer’s disease. J. Am. Geriatr. Soc. 51, 1143–1148. doi: 10.1046/j.1532-5415.2003.51368.x
French, P., Gardner, M. J., and Gunn, A. M. (1989). Dietary aluminium and Alzheimer’s disease. Food Chem. Toxicol. 27, 495–498. doi: 10.1016/0278-6915(89)90040-9
Garruto, R. M., and Brown, P. (1994). Tau protein, aluminium, and Alzheimer’s disease. Lancet 343, 989. doi: 10.1016/S0140-6736(94)90119-8
Graham, S. F., Nasaruddin, M. B., Carey, M., Holscher, C., McGuinness, B., Kehoe, P. G., et al. (2014). Age-associated changes of brain copper, iron, and zinc in Alzheimer’s disease and dementia with Lewy bodies. J. Alzheimers Dis. 42, 1407–1413. doi: 10.3233/JAD-140684
Greenamyre, J. T. (1991). Neuronal bioenergetic defects, excitotoxicity and Alzheimer’s disease: use it and lose it. Neurobiol. Aging 12, 334–336; discussion 352–335. doi: 10.1016/0197-4580(91)90012-9
Grubman, A., Lidgerwood, G. E., Duncan, C., Bica, L., Tan, J. L., Parker, S. J., et al. (2014a). Deregulation of subcellular biometal homeostasis through loss of the metal transporter, Zip7, in a childhood neurodegenerative disorder. Acta Neuropathol. Commun. 2:25. doi: 10.1186/2051-5960-2-25
Grubman, A., White, A. R., and Liddell, J. R. (2014b). Mitochondrial metals as a potential therapeutic target in neurodegeneration. Br. J. Pharmacol. 171, 2159–2173. doi: 10.1111/bph.12513
Gupta, V. B., Anitha, S., Hegde, M. L., Zecca, L., Garruto, R. M., Ravid, R., et al. (2005). Aluminium in Alzheimer’s disease: are we still at a crossroad? Cell Mol. Life Sci. 62, 143–158.
Guy, S. P., Jones, D., Mann, D. M., and Itzhaki, R. F. (1991). Human neuroblastoma cells treated with aluminium express an epitope associated with Alzheimer’s disease neurofibrillary tangles. Neurosci. Lett. 121, 166–168. doi: 10.1016/0304-3940(91)90676-K
Halliwell, B. (1989). Oxidants and the central nervous system: some fundamental questions. Is oxidant damage relevant to Parkinson’s disease, Alzheimer’s disease, traumatic injury or stroke?. Acta Neurol Scand. Suppl. 126, 23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x
Hancock, S. M., Finkelstein, D. I., and Adlard, P. A. (2014). Glia and zinc in ageing and Alzheimer’s disease: a mechanism for cognitive decline? Front. Aging Neurosci. 6:137. doi: 10.3389/fnagi.2014.00137
Harkany, T., Penke, B., and Luiten, P. G. (2000). beta-Amyloid excitotoxicity in rat magnocellular nucleus basalis. Effect of cortical deafferentation on cerebral blood flow regulation and implications for Alzheimer’s disease. Ann. N. Y. Acad. Sci. 903, 374–386. doi: 10.1111/j.1749-6632.2000.tb06389.x
Harrington, C. R., Wischik, C. M., McArthur, F. K., Taylor, G. A., Edwardson, J. A., and Candy, J. M. (1994). Alzheimer’s-disease-like changes in tau protein processing: association with aluminium accumulation in brains of renal dialysis patients. Lancet 343, 993–997. doi: 10.1016/S0140-6736(94)90124-4
Hynd, M. R., Scott, H. L., and Dodd, P. R. (2004). Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 45, 583–595. doi: 10.1016/j.neuint.2004.03.007
Inestrosa, N. C., Reyes, A. E., Chacon, M. A., Cerpa, W., Villalon, A., Montiel, J., et al. (2005). Human-like rodent amyloid-beta-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol. Aging 26, 1023–1028. doi: 10.1016/j.neurobiolaging.2004.09.016
Inestrosa, N. C., Rios, J. A., Cisternas, P., Tapia-Rojas, C., Rivera, D. S., Braidy, N., et al. (2015). Age progression of neuropathological markers in the brain of the chilean rodent Octodon degus, a natural model of Alzheimer’s disease. Brain Pathol. 25, 679–691. doi: 10.1111/bpa.12226
Joachim, C. L., Morris, J. H., Selkoe, D. J., and Kosik, K. S. (1987). Tau epitopes are incorporated into a range of lesions in Alzheimer’s disease. J. Neuropathol. Exp. Neurol. 46, 611–622. doi: 10.1097/00005072-198711000-00001
Justin Thenmozhi, A., Raja, T. R., Janakiraman, U., and Manivasagam, T. (2015). Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 40, 767–776. doi: 10.1007/s11064-015-1525-1
Kelliher, M., Fastbom, J., Cowburn, R. F., Bonkale, W., Ohm, T. G., Ravid, R., et al. (1999). Alterations in the ryanodine receptor calcium release channel correlate with Alzheimer’s disease neurofibrillary and beta-amyloid pathologies. Neuroscience 92, 499–513. doi: 10.1016/S0306-4522(99)00042-1
Khachaturian, Z. S. (1985). Diagnosis of Alzheimer’s disease. Arch. Neurol. 42, 1097–1105. doi: 10.1001/archneur.1985.04060100083029
Khurana, V., Elson-Schwab, I., Fulga, T. A., Sharp, K. A., Loewen, C. A., Mulkearns, E., et al. (2010). Lysosomal dysfunction promotes cleavage and neurotoxicity of Tau In Vivo. PLoS Genet. 6:e1001026. doi: 10.1371/journal.pgen.1001026
Koutsilieri, E., and Riederer, P. (2007). Excitotoxicity and new antiglutamatergic strategies in Parkinson’s disease and Alzheimer’s disease. Parkinsonism Relat. Disord. 13(Suppl. 3), S329–S331. doi: 10.1016/S1353-8020(08)70025-7
Lemoine, L., Saint-Aubert, L., Marutle, A., Antoni, G., Eriksson, J. P., Ghetti, B., et al. (2015). Visualization of regional tau deposits using H-3-THK5117 in Alzheimer brain tissue. Acta Neuropathol. Commun. 3, 40. doi: 10.1186/s40478-015-0220-4
Leskovjan, A. C., Lanzirotti, A., and Miller, L. M. (2009). Amyloid plaques in PSAPP mice bind less metal than plaques in human Alzheimer’s disease. Neuroimage 47, 1215–1220. doi: 10.1016/j.neuroimage.2009.05.063
Lisek, M., Boczek, T., Ferenc, B., and Zylinska, L. (2015). Regional brain dysregulation of Ca-handling systems in ketamine-induced rat model of experimental psychosis. Cell Tissue Res. 363, 609–620. doi: 10.1007/s00441-015-2332-3
Lovell, M. A., Robertson, J. D., Teesdale, W. J., Campbell, J. L., and Markesbery, W. R. (1998). Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 158, 47–52. doi: 10.1016/S0022-510X(98)00092-6
Lovell, M. A., Smith, J. L., Xiong, S., and Markesbery, W. R. (2005). Alterations in zinc transporter protein-1 (ZnT-1) in the brain of subjects with mild cognitive impairment, early, and late-stage Alzheimer’s disease. Neurotox. Res. 7, 265–271. doi: 10.1007/BF03033884
Lyubartseva, G., Smith, J. L., Markesbery, W. R., and Lovell, M. A. (2010). Alterations of zinc transporter proteins ZnT-1, ZnT-4 and ZnT-6 in preclinical Alzheimer’s disease brain. Brain Pathol. 20, 343–350. doi: 10.1111/j.1750-3639.2009.00283.x
Makela, M., Paetau, A., Polvikoski, T., Myllykangas, L., and Tanskanen, M. (2016). Capillary amyloid-beta protein deposition in a population-based study (Vantaa 85+). J. Alzheimers Dis. 49, 149–157. doi: 10.3233/JAD-150241
Martyn, C. N. (1992). The epidemiology of Alzheimer’s disease in relation to aluminium. Ciba Found Symp. 169, 69–79; discussion 79–86.
Martyn, C. N., Singh, S., and Wood, P. J. (1989). Calcium metabolism in Alzheimer’s disease. A case-control study. Gerontology 35, 153–157.
Matusch, A., Depboylu, C., Palm, C., Wu, B., Hoglinger, G. U., Schafer, M. K., et al. (2010). Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson’s disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS). J. Am. Soc. Mass Spectrom. 21, 161–171. doi: 10.1016/j.jasms.2009.09.022
Maynard, C. J., Cappai, R., Volitakis, I., Cherny, R. A., White, A. R., Beyreuther, K., et al. (2002). Overexpression of Alzheimer’s disease amyloid-beta opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 277, 44670–44676. doi: 10.1074/jbc.M204379200
McCord, M. C., and Aizenman, E. (2014). The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 6:77. doi: 10.3389/fnagi.2014.00077
McLachlan, D. R., Fraser, P. E., and Dalton, A. J. (1992). Aluminium and the pathogenesis of Alzheimer’s disease: a summary of evidence. Ciba Found Symp. 169, 87–98; discussion 99–108.
Mirra, S. S., Heyman, A., McKeel, D., Sumi, S. M., Crain, B. J., Brownlee, L. M., et al. (1991). The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41, 479–486. doi: 10.1212/WNL.41.4.479
Molaschi, M., Ponzetto, M., Bertacna, B., Berrino, E., and Ferrario, E. (1996). Determination of selected trace elements in patients affected by dementia. Arch. Gerontol. Geriatr. 22(Suppl. 1), 39–42. doi: 10.1016/0167-4943(96)86910-X
Mueller, C., Schrag, M., Crofton, A., Stolte, J., Muckenthaler, M. U., Magaki, S., et al. (2012). Altered serum iron and copper homeostasis predicts cognitive decline in mild cognitive impairment. J. Alzheimers Dis. 29, 341–350. doi: 10.3233/JAD-2011-111841
O’Day, D. H., and Myre, M. A. (2004). Calmodulin-binding domains in Alzheimer’s disease proteins: extending the calcium hypothesis. Biochem. Biophys. Res. Commun. 320, 1051–1054. doi: 10.1016/j.bbrc.2004.06.070
Ong, W. Y., Tanaka, K., Dawe, G. S., Ittner, L. M., and Farooqui, A. A. (2013). Slow excitotoxicity in Alzheimer’s disease. J. Alzheimers Dis. 35, 643–668.
Osterholt, T., Salber, D., Matusch, A., Becker, J. S., and Palm, C. (2011). IMAGENA: image generation and analysis – an interactive software tool handling LA-ICPMS data. Int. J. Mass Spectrom. 307, 232–239. doi: 10.1016/j.ijms.2011.03.010
Palmiter, R. D., Cole, T. B., Quaife, C. J., and Findley, S. D. (1996). ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc. Natl. Acad. Sci. U.S.A. 93, 14934–14939. doi: 10.1073/pnas.93.25.14934
Parvathy, S., Turner, A. J., and Hooper, N. M. (2000). Inhibition of alpha-secretase by zinc metalloproteinase inhibitors. Methods Mol. Med. 32, 203–215. doi: 10.1385/1-59259-195-7:203
Perez, S. E., He, B., Nadeem, M., Wuu, J., Ginsberg, S. D., Ikonomovic, M. D., et al. (2015). Hippocampal endosomal, lysosomal, and autophagic dysregulation in mild cognitive impairment: correlation with a beta and tau pathology. J. Neuropathol. Exp. Neurol. 74, 345–358. doi: 10.1097/NEN.0000000000000179
Perl, D. P. (2006). Exposure to aluminium and the subsequent development of a disorder with features of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 77:811. doi: 10.1136/jnnp.2006.090613
Peterson, C., Gibson, G. E., and Blass, J. P. (1985). Altered calcium uptake in cultured skin fibroblasts from patients with Alzheimer’s disease. N. Engl. J. Med. 312, 1063–1065. doi: 10.1056/NEJM198504183121618
Peterson, C., Ratan, R., Shelanski, M., and Goldman, J. (1989). Changes in calcium homeostasis during aging and Alzheimer’s disease. Ann. N. Y. Acad. Sci. 568, 262–270. doi: 10.1111/j.1749-6632.1989.tb12515.x
Polizzi, S., Pira, E., Ferrara, M., Bugiani, M., Papaleo, A., Albera, R., et al. (2002). Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology 23, 761–774. doi: 10.1016/S0161-813X(02)00097-9
Quadri, M., Federico, A., Zhao, T., Breedveld, G. J., Battisti, C., Delnooz, C., et al. (2012). Mutations in SLC30A10 cause parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am. J. Hum. Genet. 90, 467–477. doi: 10.1016/j.ajhg.2012.01.017
Rembach, A., Hare, D. J., Doecke, J. D., Burnham, S. C., Volitakis, I., Fowler, C. J., et al. (2014). Decreased serum zinc is an effect of ageing and not Alzheimer’s disease. Metallomics 6, 1216–1219. doi: 10.1039/c4mt00060a
Rifat, S. L. (1993). Alzheimer’s disease and environmental aluminium. Age Ageing 22, 476–477. doi: 10.1093/ageing/22.6.476
Sachdev, P. S., Zhuang, L., Braidy, N., and Wen, W. (2013). Is Alzheimer’s a disease of the white matter? Curr. Opin. Psychiatry 26, 244–251. doi: 10.1097/YCO.0b013e32835ed6e8
Selkoe, D. J., Bell, D. S., Podlisny, M. B., Price, D. L., and Cork, L. C. (1987). Conservation of brain amyloid proteins in aged mammals and humans with Alzheimer’s disease. Science 235, 873–877. doi: 10.1126/science.3544219
Sensi, S. L., Paoletti, P., Bush, A. I., and Sekler, I. (2009). Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 10, 780–791. doi: 10.1038/nrn2734
Singla, N., and Dhawan, D. K. (2014). Influence of zinc on calcium-dependent signal transduction pathways during aluminium-induced neurodegeneration. Mol. Neurobiol. 50, 613–625. doi: 10.1007/s12035-014-8643-7
Smith, D. G., Cappai, R., and Barnham, K. J. (2007). The redox chemistry of the Alzheimer’s disease amyloid beta peptide. Biochim. Biophys. Acta 1768, 1976–1990. doi: 10.1016/j.bbamem.2007.02.002
Smith, J. L., Xiong, S., Markesbery, W. R., and Lovell, M. A. (2006). Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer’s disease brain. Neuroscience 140, 879–888. doi: 10.1016/j.neuroscience.2006.02.049
Squitti, R., Lupoi, D., Pasqualetti, P., Dal Forno, G., Vernieri, F., Chiovenda, P., et al. (2002). Elevation of serum copper levels in Alzheimer’s disease. Neurology 59, 1153–1161. doi: 10.1212/WNL.59.8.1153
Taddeo, M. A., Lee, S., and Shea, T. B. (2014). Synergistic inhibition of synaptic signaling in cortical cultures by subcytotoxic levels of oligomerized amyloid-beta and iron: alleviation by zinc. J. Alzheimers Dis. 41, 365–369. doi: 10.3233/JAD-132696
Taylor, G. A., Newens, A. J., Edwardson, J. A., Kay, D. W., and Forster, D. P. (1995). Alzheimer’s disease and the relationship between silicon and aluminium in water supplies in northern England. J. Epidemiol. Commun. Health 49, 323–324. doi: 10.1136/jech.49.3.323
Taylor, K. M., Hiscox, S., Nicholson, R. I., Hogstrand, C., and Kille, P. (2012). Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 5, ra11. doi: 10.1126/scisignal.2002585
Teri, L., Borson, S., Kiyak, H. A., and Yamagishi, M. (1989). Behavioral disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in Alzheimer’s disease. J. Am. Geriatr. Soc. 37, 109–116. doi: 10.1111/j.1532-5415.1989.tb05868.x
Terry, R. D., Masliah, E., Salmon, D. P., Butters, N., DeTeresa, R., Hill, R., et al. (1991). Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580. doi: 10.1002/ana.410300410
Thomas, M., and Jankovic, J. (2004). Neurodegenerative disease and iron storage in the brain. Curr. Opin. Neurol. 17, 437–442. doi: 10.1097/01.wco.0000137534.61244.d1
Tsai, V. W., Scott, H. L., Lewis, R. J., and Dodd, P. R. (2005). The role of group I metabotropic glutamate receptors in neuronal excitotoxicity in Alzheimer’s disease. Neurotox. Res. 7, 125–141. doi: 10.1007/BF03033782
Uekita, T., and Okanoya, K. (2011). Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav. Brain Res. 219, 302–309. doi: 10.1016/j.bbr.2011.01.042
van Groen, T., Kadish, I., Popovic, N., Popovic, M., Caballero-Bleda, M., Bano-Otalora, B., et al. (2011). Age-related brain pathology in Octodon degu: blood vessel, white matter and Alzheimer-like pathology. Neurobiol. Aging 32, 1651–1661. doi: 10.1016/j.neurobiolaging.2009.10.008
Vidoni, C., Follo, C., Savino, M., Melone, M. A. B., and Isidoro, C. (2016). The role of cathepsin D in the pathogenesis of human neurodegenerative disorders. Med. Res. Rev. 36, 845–870. doi: 10.1002/med.21394
Wang, G. T., Ladror, U. S., Holzman, T. F., Klein, W. L., and Krafft, G. A. (1994). Cleavage of fluorogenic substrates for APP-processing proteases by human brain extracts. Ca2(+)-substrate interaction is responsible for Ca2(+) stimulation of the neural protease activity. Mol. Chem. Neuropathol. 23, 191–199. doi: 10.1007/BF02815411
Wang, H. J., Wang, M., Wang, B., Li, M., Chen, H. Q., Yu, X. H., et al. (2012). The distribution profile and oxidation states of biometals in APP transgenic mouse brain: dyshomeostasis with age and as a function of the development of Alzheimer’s disease. Metallomics 4, 289–296. doi: 10.1039/c2mt00104g
Wang, L. M., Becker, J. S., Wu, Q., Oliveira, M. F., Bozza, F. A., Schwager, A. L., et al. (2010). Bioimaging of copper alterations in the aging mouse brain by autoradiography, laser ablation inductively coupled plasma mass spectrometry and immunohistochemistry. Metallomics 2, 348–353. doi: 10.1039/c003875j
Watt, N. T., Griffiths, H. H., and Hooper, N. M. (2014). Lipid rafts: linking prion protein to zinc transport and amyloid-beta toxicity in Alzheimer’s disease. Front. Cell Dev. Biol. 2:41. doi: 10.3389/fcell.2014.00041
Wilkinson, N., and Pantopoulos, K. (2014). The IRP/IRE system in vivo: insights from mouse models. Front. Pharmacol. 5:176. doi: 10.3389/fphar.2014.00176
Williamson, W. R., Wang, D., Haberman, A. S., and Hiesinger, P. R. (2010). A dual function of V0-ATPase a1 provides an endolysosomal degradation mechanism in Drosophila melanogaster photoreceptors. J. Cell Biol. 189, 885–899. doi: 10.1083/jcb.201003062
Wilson, M., Hogstrand, C., and Maret, W. (2012). Picomolar concentrations of free zinc(II) ions regulate receptor protein-tyrosine phosphatase beta activity. J. Biol. Chem. 287, 9322–9326. doi: 10.1074/jbc.C111.320796
Xue, X., Wang, L. R., Sato, Y., Jiang, Y., Berg, M., Yang, D. S., et al. (2014). Single-walled carbon nanotubes alleviate autophagic/lysosomal defects in primary glia from a mouse model of Alzheimer’s disease. Nano Lett. 14, 5110–5117. doi: 10.1021/nl501839q
Ye, H., Jalini, S., Mylvaganam, S., and Carlen, P. (2010). Activation of large-conductance Ca(2+)-activated K(+) channels depresses basal synaptic transmission in the hippocampal CA1 area in APP (swe/ind) TgCRND8 mice. Neurobiol. Aging 31, 591–604. doi: 10.1016/j.neurobiolaging.2008.05.012
Yonova-Doing, E., Atadzhanov, M., Quadri, M., Kelly, P., Shawa, N., Musonda, S. T., et al. (2012). Analysis of LRRK2, SNCA, Parkin, PINK1, and DJ-1 in Zambian patients with Parkinson’s disease. Parkinsonism Relat. Disord. 18, 567–571. doi: 10.1016/j.parkreldis.2012.02.018
Yuan, Y., Niu, F., Liu, Y., and Lu, N. (2014). Zinc and its effects on oxidative stress in Alzheimer’s disease. Neurol. Sci. 35, 923–928. doi: 10.1007/s10072-014-1668-x
Keywords: LA-ICPMS, metals, Alzheimer’s disease, bioimaging, Octodon degus
Citation: Braidy N, Poljak A, Marjo C, Rutlidge H, Rich A, Jugder B-E, Jayasena T, Inestrosa NC and Sachdev PS (2017) Identification of Cerebral Metal Ion Imbalance in the Brain of Aging Octodon degus. Front. Aging Neurosci. 9:66. doi: 10.3389/fnagi.2017.00066
Received: 02 May 2016; Accepted: 03 March 2017;
Published: 29 March 2017.
Edited by:
Agustin Ibanez, Institute of Cognitive and Translational Neuroscience, ArgentinaCopyright © 2017 Braidy, Poljak, Marjo, Rutlidge, Rich, Jugder, Jayasena, Inestrosa and Sachdev. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Perminder S. Sachdev, p.sachdev@unsw.edu.au
 Nady Braidy
Nady Braidy Anne Poljak
Anne Poljak Chris Marjo2
Chris Marjo2 Bat-Erdene Jugder
Bat-Erdene Jugder Nibaldo C. Inestrosa
Nibaldo C. Inestrosa Perminder S. Sachdev
Perminder S. Sachdev