- 1Division of Translational Neuroscience, Department of Psychiatry, Duke University Medical Center, Durham, NC, USA
- 2Duke Institute for Brain Sciences, Durham, NC, USA
- 3Duke Center for the Study of Aging and Human Development, Durham, NC, USA
Personalized medicine aims to tailor diagnosis and treatment based on an individual's personal genetic make-up and other predictive biomarkers—rather than a one-size fits all approach. African-Americans comprise 13.2% of the U.S. population (U.S. Census Bureau, 2014) and minorities (consisting of all but non-Hispanic Caucasian), now 37% of the U.S. population, are projected to reach closer to 57% in 2060 (U.S. Census Bureau, 2012). Indeed in coming decades minority population, including African-Americans, may exceed the Caucasian population in the U.S.
Dementia, especially Alzheimer's disease (AD), has emerged as one of the biggest threats to public health and personal wellbeing among older adults. Epidemiological studies, by nature of their community sampling, have been able to study risk for AD in racially representative populations. In such studies, older African-Americans have been reported as being more likely than older Caucasians to develop AD and other dementias (Gurland et al., 1999; Dilworth-Anderson et al., 2008; Potter et al., 2009; Barnes and Bennett, 2014; Alzheimer's Association, 2015). Potter et al. (2009), Gurland et al. (1999), and Barnes and Bennett (2014) report that prevalence of cognitive impairment or AD among African-Americans may be two or three times higher than in Caucasians. By 2050, according to The Alzheimer's Association annual report from 2010, proportion of racial minorities with AD will increase from 20 to 42%, with African-Americans increasing from 9 to 12% (Alzheimer's Association, 2010). A more recent 2015 report suggests that 16% of African-Americans were diagnosed with AD or other dementias compared to 8% of Caucasians (Alzheimer's Association, 2015).
Current Status
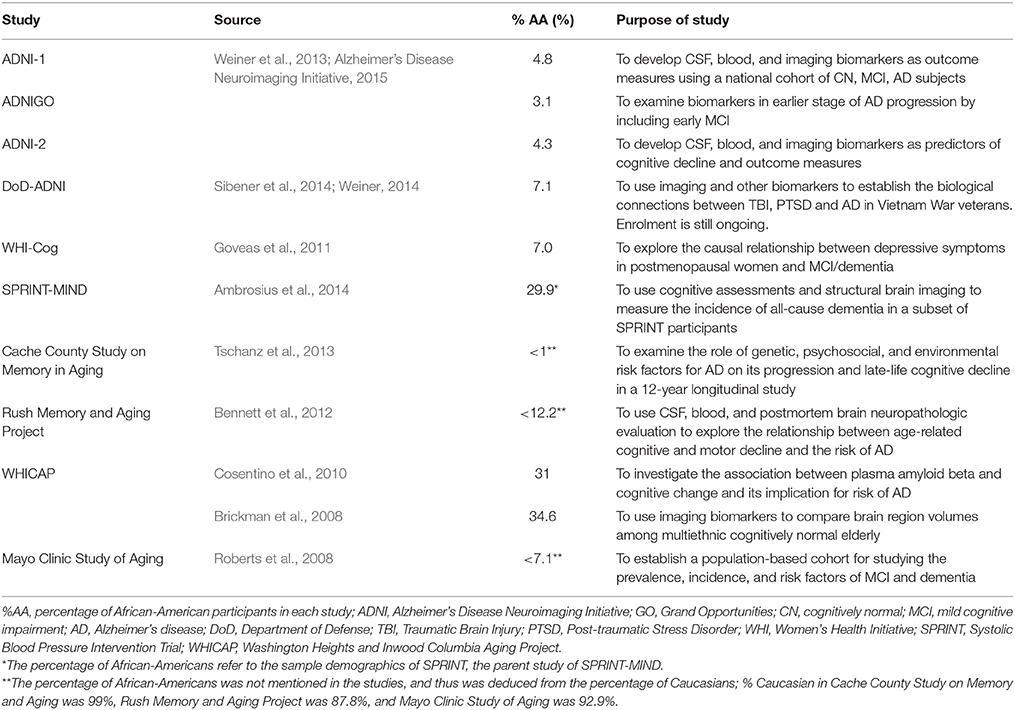
However, despite community studies suggesting they may be more susceptible to AD, African-Americans have been under-included in many prominent U.S. AD biomarker and clinical trials. In fact, barring a handful of studies in Asia and Africa, most of what we know about AD biomarkers and pathological changes comes almost exclusively from research studies of Caucasians (Brickman et al., 2008; Alzheimer's Association, 2010). Clinical trials and biomarker studies rely on convenience samples mostly recruited via advertisement. Further, we had great difficulty ascertaining the percentage of African-Americans in various trials since journals do not require trials to report a racial breakdown and many studies simply report percentage of Caucasians. Table 1 lists 10 major federally-funded biomarker studies conducted in the U.S. to illustrate that over half of these studies did not recruit adequate (approaching the USA national figure of 13%) numbers of African-American study subjects (for reasons specific to each study). For example, The Alzheimer's Disease Neuroimaging Initiative (ADNI) has made major contributions to our understanding of the pathological cascade and timeline of AD changes. Yet, ADNI-1 did not have sufficient number of African-Americans (< 5%) to reliably examine whether African-Americans differed from Caucasians. Likewise, with the exception of a multicenter trial of donepezil conducted exclusively in African-Americans (Griffith et al., 2006), almost all U.S. therapeutic information we have on what drugs work or do not work for AD (based on hundreds of industry sponsored AD clinical trials over four decades) is primarily derived from Caucasians.
Two major studies, SPRINT-MIND and the Washington Heights and Inwood Columbia Aging Project (WHICAP) did enroll sufficient numbers of African-Americans (Brickman et al., 2008; Cosentino et al., 2010; Ambrosius et al., 2014). Another study that had a substantive proportion of African-American participants was that conducted by Cosentino et al. (2010) as a part of WHICAP. Their goal was to associate plasma amyloid beta level with cognitive change indicative of AD progression, and in doing so, they recruited same number of Caucasian and African-American participants, each constituting 31% of the entire sample. They concluded that higher plasma amyloid beta level correlated with faster cognitive decline, noted in AD onset. As stated previously, to our knowledge, there has been only one US multicenter therapeutic drug trial done focused solely on African Americans with AD (Griffith et al., 2006). This 12-week multicenter trial of donepezil in 126 African Americans concluded it was safe and effective in mild-moderate AD (Griffith et al., 2006). However, the open label design (without placebo control) and the fact that ~51% of the subjects suffered adverse effects including diarrhea, hypertension and urinary tract infections, leaves open the question of whether the drug was truly effective.
Thus, while much progress has been made in AD research, it is clear that much of our knowledge about AD pathogenesis still comes from studies of Caucasians. One obstacle for recruiting African-American subjects is distrust of research studies led by hospitals, universities, and clinics due to historical mistreatments (Diaz et al., 2008; Ballard et al., 2010; Byrd et al., 2011; Lang et al., 2013). In this regards, it is encouraging to note that a recent survey of 5979 people in five US cities (Pease, 2013) found that 91% of African-Americans indicated interest in participating in research—suggesting that the onus is now on researchers to reach out to them and to not abuse their trust.
Recommendations for Future
While punitive approaches to enhance recruitment (e.g., tying grant payments to minority recruitment success or imposition of institutional penalties for failure to recruit sufficient minorities) may force change, we believe they are not ideal. We have some recommendations for enhancing African-American recruitment: (1) building long-standing partnerships with local African-American community organizations such as churches and initiations of forums, retreats, and other social events for greater approachability and getting to know the subjects on a more personal level (Ballard et al., 2010); (2) including a substantial budget for recruiting minorities in every major trial; (3) creating community based multiethnic registries (e.g., Brain Health Registry; Bryan ADRC Registry); (4) including a well powered hypothesis aimed at African Americans (or other major minority groups) in every major federally funded AD clinical trial; (5) explicit reporting in study reports and journal publications of percentage of African-Americans and other races, and whether race influenced any outcomes; (6) specific funding to conduct trials aimed at under-recruited minorities.
It is our hope that including a greater proportion of African-Americans in AD clinical trials will allow researchers to produce more generalizable results and a better understanding of race and ethnicity-specific differences in AD pathophysiology. This awareness and knowledge would help clinicians improve therapeutic target responses in patients and optimize the delivery of personalized care for more individuals with AD. Similar efforts aimed at Asians and Hispanics are already underway in other countries, and together these studies will help us paint a fuller picture of AD.
Author Contributions
JS was involved with topic selection, conducted necessary background literature research, and drafted the opinion article. PD oversaw the overall process and guided JS in selecting the topic and editing the draft.
Funding
JS was supported by the Wrenn Clinical Research Scholars program at Duke University.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer TR and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
PD has received grants from and/or served as an advisor/speaker to several companies. He owns shares in several companies whose products are not discussed here. JS's work in Dr. Doraiswamy's lab was made possible by the Wrenn Clinical Research Scholars program.
References
Alzheimer's Association (2010). 2010 Alzheimer's disease facts and figures, Alzheimers Dement. 6, 46–70. doi: 10.1016/j.jalz.2010.01.009
Alzheimer's Association (2015). 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 11, 332–384. doi: 10.1016/j.jalz.2015.02.003
Alzheimer's Disease Neuroimaging Initiative (2015). Key ADNI Tables Merged into One Table from Laboratory of Neuroimaging Image Data Archive. Available online at: https://ida.loni.usc.edu/pages/access/studyData.jsp (Accessed March 29, 2015).
Ambrosius, W. T., Sink, K. M., Foy, C. G., Berlowitz, D. R., Cheung, A. K., Cushman, W. C., et al. (2014). The design and rationale of a multi-center clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin. Trials 11, 532–546. doi: 10.1177/1740774514537404
Ballard, E. L., Gwyther, L. P., and Edmonds, H. L. (2010). Challenges and opportunities: recruitment and retention of African-Americans for Alzheimer's disease research: lessons learned. Alzheimer Dis. Assoc. Disord. 24, S19–S23. doi: 10.1097/WAD.0b013e3181f12432
Barnes, L. L., and Bennett, D. A. (2014). Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff. 33, 580–586. doi: 10.1377/hlthaff.2013.1353
Bennett, D. A., Schneider, J. A., Buchman, A. S., Barnes, L. L., Boyle, P. A., and Wilson, R. S. (2012). Overview and findings from the rush memory and aging project. Curr. Alzheimer Res. 9, 646–663. doi: 10.2174/156720512801322663
Brickman, A. M., Schupf, N., Manly, J. J., Luchsinger, J. A., Andrews, H., Tang, M. X., et al. (2008). Brain morphology in elderly African Americans, Caribbean Hispanics, and Caucasians from Northern Manhattan. Arch. Neurol. 65, 1053–1061. doi: 10.1001/archneur.65.8.1053
Byrd, G., Edwards, C. L., Kelkar, V. A., Phillips, R. G., Byrd, J. R., Pim-Pong, D. S., et al. (2011). Recruiting intergenerational African American males for biomedical research studies: a major research challenge. J. Natl. Med. Assoc. 103, 480–487. doi: 10.1016/S0027-9684(15)30361-8
Cosentino, S., Stern, Y., Sokolov, E., Scarmeas, N., Manly, J., Tang, M. X., et al. (2010). Plasma amyloid ß predicts cognitive decline. Arch. Neurol. 67, 1485–1490. doi: 10.1001/archneurol.2010.189
Diaz, V. A., Mainous, A. G., McCall, A. A., and Geesey, M. E. (2008). Factors affecting research participation in African-American college students. Fam. Med. 40, 46–51.
Dilworth-Anderson, P., Hendrie, H. C., Manly, J. J., Khachaturian, A. S., and Fazio, S. (2008). Diagnosis and assessment of Alzheimer's disease in diverse populations. Alzheimers Dement. 4, 305–309. doi: 10.1016/j.jalz.2008.03.001
Goveas, J. S., Espeland, M. A., Woods, N. F., Wassertheil-Smoller, S., and Kotchen, J. M. (2011). Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly Women: the Women's health initiative memory study. J. Am. Geriatr. Soc. 59, 57–66. doi: 10.1111/j.1532-5415.2010.03233.x
Griffith, P., Lichtenberg, P., Goldman, R., and Payne-Parrish, J. (2006). Safety and efficacy of Donepezil in African Americans with mild-to-moderate Alzheimer's disease. J. Natl. Med. Assoc. 98, 1590–1597.
Gurland, B. J., Wilder, D. E., Lantigua, R., Stern, Y., Chen, J., Killeffer, E. H., et al. (1999). Rates of dementia in three ethnoracial groups. Int. J. Geriatr. Psychiatry 14, 481–493.
Lang, R., Kelkar, V. A., Byrd, J. R., Edwards, C. L., Pericak-Vance, M., and Byrd, G. S. (2013). African American participation in health-related research studies: indicators for effective recruitment. J. Public Health Manag. Pract. 19, 110–118. doi: 10.1097/PHH.0b013e31825717ef
Pease, J. (2013). African-Americans Express Keen Interest in Medical Research Participation, UF Study Finds, University of Florida News. Available online at: http://news.ufl.edu/archive/2013/04/african-americans-express-keen-interest-in-medical-research-participation-uf-study-finds.html (Accessed April 2, 2013)
Potter, G. G., Plassman, B. L., Burke, J. R., Kabeto, M. U., Langa, K. M., Llewellyn, D. J., et al. (2009). Cognitive performance and informant reports in the diagnosis of cognitive impairment and dementia in African Americans and whites. Alzheimers Dement. 5, 445–453. doi: 10.1016/j.jalz.2009.04.1234
Roberts, R. O., Geda, Y. E., Knopman, D. S., Cha, R. H., Pankratz, V. S., Boeve, B. F., et al. (2008). The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30, 58–69. doi: 10.1159/000115751
Sibener, L., Zaganjor, I., Snyder, H. M., Bain, L. J., Egge, R., and Carrillo, M. C. (2014). Alzheimer's disease prevalence, costs, and prevention for military personnel and veterans. Alzheimers Dement. 10, S105–S110. doi: 10.1016/j.jalz.2014.04.011
Tschanz, J. T., Norton, M. C., Zandi, P. P., and Lyketsos, C. G. (2013). The Cache county study on memory in aging: factors affecting risk of Alzheimer's disease and its progress after onset. Int. Rev. Psychiatry 25, 673–685. doi: 10.3109/09540261.2013.849663
U.S. Census Bureau (2012). Projections Show a Slower Growing, Older, More Diverse Nation a Half Century from Now. Available online at: https://www.census.gov/newsroom/releases/archives/population/cb12-243.html
U.S. Census Bureau (2014). QuickFacts: United States. Available online at: https://www.census.gov/quickfacts/table/PST045215/00
Weiner, M. W. (2014). Effects of Traumatic Brain Injury (TBI) and Post-Traumatic Stress Disorder (PTSD) on Alzheimer's Disease (AD) in Veterans using ADNI (DoD ADNI). Lecture, A Study of Brain Aging in Vietnam War Veterans. Available online at: https://www.alz.org/research/funding/partnerships/2014_meeting/16c-Panel-ADNI-VeteransStudy-US.pdf.
Keywords: African-Americans, Alzheimer's disease, recruitment issues, biomarkers, personalized medicine
Citation: Shin J and Doraiswamy PM (2016) Underrepresentation of African-Americans in Alzheimer's Trials: A Call for Affirmative Action. Front. Aging Neurosci. 8:123. doi: 10.3389/fnagi.2016.00123
Received: 07 April 2016; Accepted: 13 May 2016;
Published: 03 June 2016.
Edited by:
P. Hemachandra Reddy, Texas Tech University, USAReviewed by:
Ramesh Kandimalla, Emory University, USATejaswini Parlapalle Reddy, Texas Tech University Health Science Center, USA
Copyright © 2016 Shin and Doraiswamy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaewook Shin, amFlLndvb2suc2hpbkBkdWtlLmVkdQ==
 Jaewook Shin
Jaewook Shin P. Murali Doraiswamy1,2
P. Murali Doraiswamy1,2