- 1Shanghai Key Laboratory of Psychotic Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Bio-X Institutes, Key Laboratory for the Genetics of Developmental and Neuropsychiatric Disorders, Ministry of Education, Shanghai Jiao Tong University, Shanghai, China
- 3Brain Science and Technology Research Center, Shanghai Jiao Tong University, Shanghai, China
- 4Shanghai Chang Ning Mental Health Center, Shanghai, China
- 5Neuropsychology and Applied Cognitive Neuroscience Laboratory, Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
The aim of this study was to investigate whether changes in cortical thickness correlated with cognitive function changes in healthy older adults after receiving cognitive training interventions. Moreover, it also aimed to examine the differential impacts of a multi-domain and a single-domain cognitive training interventions. Longitudinal magnetic resonance imaging (MRI) scanning was performed on participants 65–75 years of age using the Siemens 3.0 T Trio Tim with the Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence. The cortical thickness was determined using FreeSurfer Software. Cognitive functioning was evaluated using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). There were significant group × time interaction effects on the left supramarginal, the left frontal pole cortical regions; and a marginal significant group × time interaction effects on visuospatial/constructional and delayed memory scores. In a multi-domain cognitive training group, a number of cortical region changes were significantly positively correlated with changes in attention, delayed memory, and the total score, but significantly negatively correlated with changes in immediate memory and language scores. In the single-domain cognitive training group, some cortical region changes were significantly positively associated with changes in immediate memory, delayed memory, and the total score, while they were significantly negatively associated with changes in visuospatial/constructional, language, and attention scores. Overall, multi-domain cognitive training offered more advantages in visuospatial/constructional, attention, and delayed memory abilities, while single-domain cognitive training benefited immediate memory ability more effectively. These findings suggest that healthy older adults benefit more from the multi-domain cognitive training than single-domain cognitive training. Cognitive training has impacted on cortical thickness changes in healthy elderly.
Introduction
As the average human lifespan increases and the world’s aging population grows, issues surrounding the health and care of the aging are gaining increasing attention (Hu et al., 2014). Cognitive decline associated with aging, especially in episodic memory, attention, and executive functions, has been reported in both longitudinal (Meijer et al., 2009) and cross-sectional studies (Coubard et al., 2011; Kobayashi et al., 2015). As a means of prevention and treatment, cognitive training is designed to restore, increase, or optimize capacities in persons with cognitive decline or normal aging (Thompson and Foth, 2005; Belleville and Bherer, 2012; Rebok et al., 2014). Single-domain cognitive training focuses either on memory (Ball et al., 2002; Sisco et al., 2013), reasoning (Payne et al., 2012), strategy training (Kirchhoff et al., 2012) or processing speed (Cody et al., 2015). While multi-domain cognitive training combines several cognitive functions and demands their interplay (Corbett et al., 2015; Rahe et al., 2015). Beneficial changes at the behavioral level in cognitive function, as well as the structural level in the aging brain, have been proven possible as the result of cognitive training (Lustig et al., 2009; Reijnders et al., 2013; Rahe et al., 2015).
A number of randomized controlled trials (RCTs) have shown that cognitive training can improve cognitive performance in healthy older adults (Engvig et al., 2010; Mozolic et al., 2011; Kwok et al., 2013; Sisco et al., 2013; Corbett et al., 2015). For instance, Kwok et al. (2013) reported that the Active Mind cognitive-training program was effective in improving the cognitive function and quality of life for community-dwelling Chinese older adults in Hong Kong. Sisco et al. (2013) demonstrated that multifactorial memory training can improve the verbatim recall of prose in healthy older adults aged 65–91. Online cognitive training package (including memory, reasoning, attention training) in older adults has shown benefited to reasoning and verbal learning (Corbett et al., 2015).
Many studies have relied on structural brain imaging, such as whole-brain volume, regional gray matter volumes, and cortical thickness, to assess the effect of cognitive training in aging patients. The cortex, which is a tightly folded sheet of neurons, ranges in thickness between 1.5 and 4.5 mm (Parent and Carpenter, 1995). Measurements of cortical thickness can reflect the size, density, and arrangement of cells, which may be more closely linked with cognition ability than volumetric or intensity-based gray matter (GM) concentration measures, although these have also been shown to relate to other local measures of GM (Narr et al., 2007). Therefore, measurements of cortical thickness could provide important information about the regional integrity of the cerebral cortex and supply new insights into cognitive training (Liu et al., 2014).
The relationship between brain volumes and cognitive functions has been long-standing research interest. For example, some researchers have found positive correlations between GM volume and executive functions (Newman et al., 2007; Ruscheweyh et al., 2013) in cognitively healthy adults, and memory impairment is often associated with the atrophy of limbic structures (including the hippocampus and parahippocampal gyrus; Jack et al., 1992). However, fewer have shown the cortical thickness is correlated with cognitive functions (Ehrlich et al., 2012; Velayudhan et al., 2013; Kim et al., 2015b). For instance, Velayudhan et al. (2013) found that reduced thickness of the entorhinal cortex was related to reduced scores on perceptual and executive function tests, including perceptual speed and aspects of working memory in Alzheimer’s disease. In another study, right parahippocampal gyrus atrophy showed a significant correlation with the executive and visuospatial functions impairment in Parkinson’s disease (Kim et al., 2015b).
Taken together, we hypothesized that cortical thickness changes would significantly associated with changes in cognition, thereby revealing more brain mechanism of the cognitive training effect on healthy older adults. However, to our knowledge, no randomized, controlled study comparing multi-domain and single-domain cognitive training interventions in healthy older adults has been performed prior to our study. We also evaluated the effects of multi-domain and single-domain cognitive training interventions in healthy older adults.
Materials and Methods
Subjects
Participants were community-dwelling elders living in a neighborhood located in the Putuo District of Shanghai from March 2008 to April 2008. Inclusion criteria mandated that participants: (a) were 65–75 years of age; (b) had no severe physical or mental illnesses (such as brain tumor, cerebral infarction, cerebral hemorrhage, malnutrition, major depressive disorder and schizophrenia); (c) were able to live independently; (d) had no disability, and no difficulties in hearing, vision or communication; (e) had at least 1 year of formal education; and (f) had an education-adjusted normal score on the Chinese version of the Mini-Mental State Examination (MMSE; Li et al., 2006; >14 for those who had not finished primary school; >19 for those who completed primary school; or >24 for those who had graduated from middle school). Any participants exhibiting obvious cognitive decline, or who had received a diagnosis of Alzheimer’s disease or other major medical conditions, were excluded.
All participants underwent magnetic resonance imaging (MRI) scanning and cognitive assessments at baseline (Time 1) and a 12-month follow-up after the intervention (Time 2). Participants who were absent for one MRI scanning or one cognitive assessment were excluded from data analysis. All participants in this study provided a written informed consent form (LL(H)-09-04). This study was approved by the Human Research Ethics Committee of Tongji Hospital in Shanghai, China.
Cognitive Training Program
The cognitive training intervention was delivered by a trained expert in small groups (n ≤ 15). Each participant from the groups attended a total of 24 sessions, with each session being 60 min long, 2 days a week, for 12 weeks. The multi-domain cognitive training consisted of activities geared towards memory, reasoning, problem solving strategies, visuospatial map reading skills development, handcraft making, and health and physical exercise; the single-domain cognitive training specifically targeted reasoning training, including the Tower of Hanoi, numerical reasoning, Raven Progressive Matrices, and verbal reasoning. Booster training was provided to each group, which included one additional 60-min training session every month, from the 6-month follow-up to the 9-month follow-up after the intervention. A detailed description of the program was provided in the previous publication (Cheng et al., 2012).
Cognitive Assessment
The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS, Form A; Randolph et al., 1998) was administered to participants in order to assess cognitive functioning. It consists of 12 tasks that measure five domains of cognitive functioning: (a) immediate memory was measured using a list learning task (40 points) and a story memory task (24 points); (b) visuospatial/constructional ability was measured using a figure copy task (20 points) and a line orientation task (20 points); (c) language was measured using a picture naming task (10 points) and a semantic fluency task (40 points); (d) attention was measured using a digit span task (16 points) and a coding task (89 points); and (e) delayed memory was measured using a list recall task (10 points), a list recognition task (20 points), a story recall task (12 points), and a figure recall task (20 points). The RBANS includes six scores: total scale score and five index scores. Administration and scoring of the RBANS in this study were conducted by trained personnel according to standardized instructions (Randolph et al., 1998). RBANS (Form A) had good validity and reliability in a Chinese community-living elderly sample (Lim et al., 2010; Cheng et al., 2011).
Image Acquisition
Structural MRI scans were performed using a Siemens Tim Trio 3T scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12 channel head coil. The pulse sequence used for morphometric analyses was three-dimensional T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE), with the following parameters: repetition time (TR) = 1900 ms, echo time (TE) = 3.43 ms, flip angle = 9°, image matrix = 256 × 256 mm, field of view (FOV) = 24 cm. Each scan took 5 min. Each volume consisted of 160 sagittal slices with a 1.0 mm slice thickness, voxel size = 0.9 × 0.9 × 1.0 mm3. Images were reconstructed and visually checked for major artifacts (e.g., motion, ringing, wrap around and neurological abnormalities) before further processing.
Cortical Thickness Analysis
For the image analysis, we used the FreeSurfer software package 5.3.01 for Mac OS. To estimate cortical thickness, images were automatically processed with the longitudinal stream in FreeSurfer (Reuter et al., 2012). All scans from two time points (Time 1 and Time 2) were first preprocessed independently with the cross-sectional stream. Then, each subject’s base template was created from the scans of his or her two time points, which operates as an initial estimate for the segmentation and surface reconstruction. The two measurement time points were then registered to the base template in order to ensure non-biased analysis with regard to the two time points (Wenger et al., 2012). All reconstructed data were visually checked for segmentation accuracy at each time point. This work flow has been shown to significantly increase reliability and statistical power (Han et al., 2006). Based on gyral and sulcal anatomy, 34 distinct cortical regions were then segmented for each hemisphere, using the Desikan–Killiany Atlas (Desikan et al., 2006). Finally, the cortical thickness was measured as the shortest distance (in mm) between the white matter surface and the pial surface (Fischl and Dale, 2000).
Statistical Analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS; SPSSInc., Chicago, IL, USA) version 17.0 for Windows. The statistical significance threshold was set at p < 0.05. A repeated-measures analysis of variance (ANOVA) was performed to evaluate the training effect on RBANS scores and cortical thickness. Correlation between the RBANS change scores and cortical thickness changes in the participants using partial correlation after correcting for age, gender, years of education, and estimated total intracranial volume (eTIV). We computed RBANS change scores (Time 2 minus Time 1), as well as cortical thickness changes (Time 2 minus Time 1). We analyzed the multi-domain and single-domain cognitive training groups separately.
Results
Characteristics of Study Participants
The demographic and cognitive outcomes of healthy older adults at baseline and 12-month follow-up are shown in Table 1. At baseline, 24 subjects from the multi-domain training group and 24 subjects from the single-domain training group underwent cognitive assessment and the MRI scanning. Eighteen of the multi-domain training group and 18 of the single-domain training group finished the cognitive assessment and MRI scanning at 12-month follow-up after the intervention. A total of 12 participants withdrew, two due to left-handedness, one due to death, two due to intestinal cancer, one due to her husband’s death, two due to operation and four due to rejecting the scanning. There were no significant differences between groups in terms of age, gender, or any cognitive outcome measurements, except for the years of education (t = 2.63, p = 0.013) at the 12-month follow-up. Due to some participants missing one MRI scanning or one cognitive assessment at the 12-month follow-up, these results were excluded from data analysis, thus leading to the single-domain training group demonstrating a lower figure for years of education than the multi-domain training group at the 12-month follow-up.
The Effect of Training on Cortical Thickness and Cognition
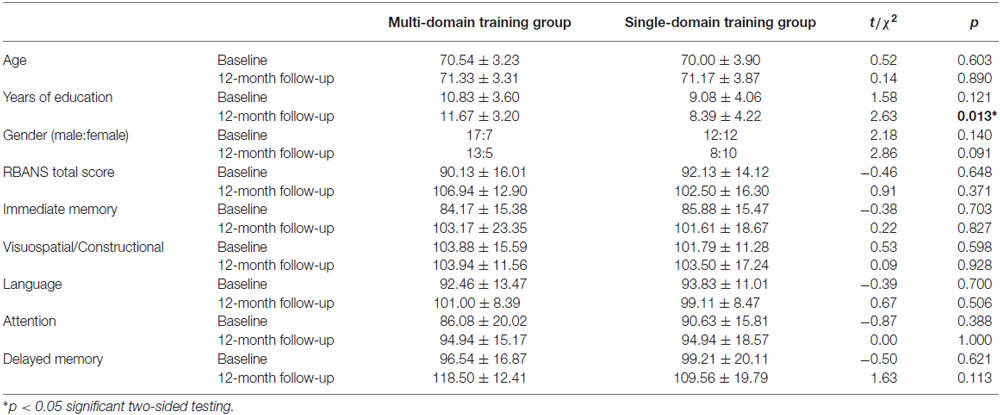
To investigate the impact of the cognitive training on the cortical thickness, the repeated-measures ANOVA showed a significant group × time interaction effects on the left supramarginal region (p = 0.025) and the left frontal pole region (p = 0.040; Figure 1). Tests of simple main effects revealed that the left supramarginal region (p = 0.020) cortical thickness was significantly reduced comparing to baseline in the single-domain training group, but not in the multi-domain training group. When the analyses were to investigate the impact of training on cognitive scores, a repeated-measures ANOVA revealed a marginal significant group × time interaction effects on visuospatial/constructional scores (p = 0.081) and delayed memory scores (p = 0.060). However, there were no group × time interaction effects on the other cortical regions and cognitive scores.
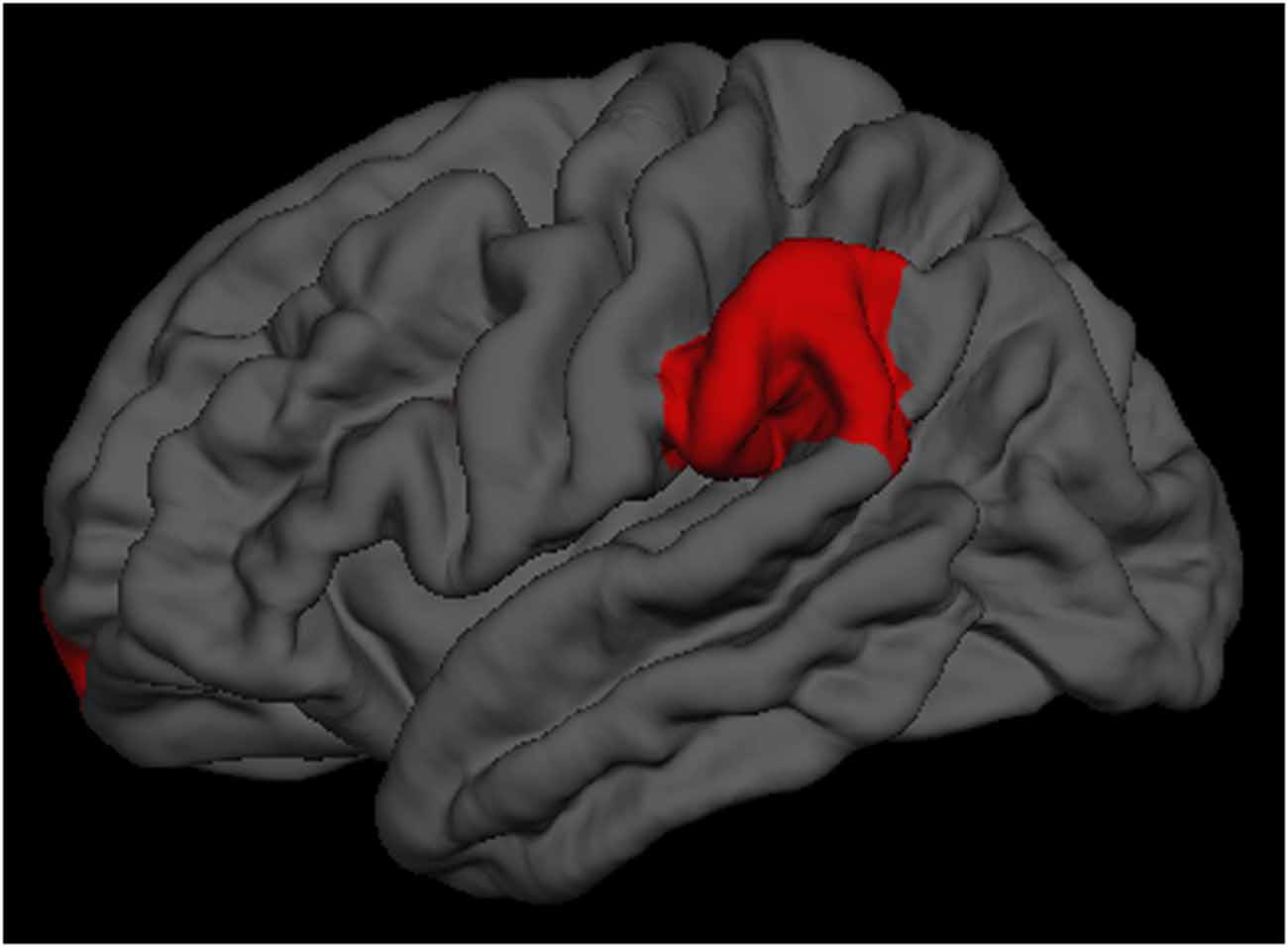
Figure 1. The left supramarginal and left frontal pole regions had significantly group × time interaction effects.
Associations of Cortical Thickness and Cognition
As shown in Tables 2, 3, the cortical thickness changes associated with specific cognitive functions change scores are somewhat different in the multi-domain and single-domain training groups. Table 2 presents the correlations between cognitive function change scores and cortical thickness changes in the multi-domain training group, after controlling for age, gender, education, and eTIV. It shows that the immediate memory change scores were significantly negatively associated with the changes in the left parahippocampal and right transverse temporal regions. In this group, visuospatial/constructional change scores were not significantly associated with any of the cortical thickness changes. However, significant negative correlations between language change scores and cortical thickness changes were observed in the change of the left parahippocampal region, and significant positive correlations between the attention change scores and cortical thickness changes were indicated in the change of the right frontal pole region. Furthermore, significant positive correlations between delayed memory change scores and cortical thickness changes were presented in the changes of the left inferior parietal, lateral occipital, lingual, superior parietal, and supramarginal, as well as the right cuneus, inferior parietal, lateral occipital, lateral orbitofrontal, pars orbitalis, posterior cingulate, rostral anterior cingulate, and insula regions. Finally, significant positive correlations between total change scores and cortical thickness changes were observed in changes to the left superior parietal and the right lateral occipital, temporal pole regions.
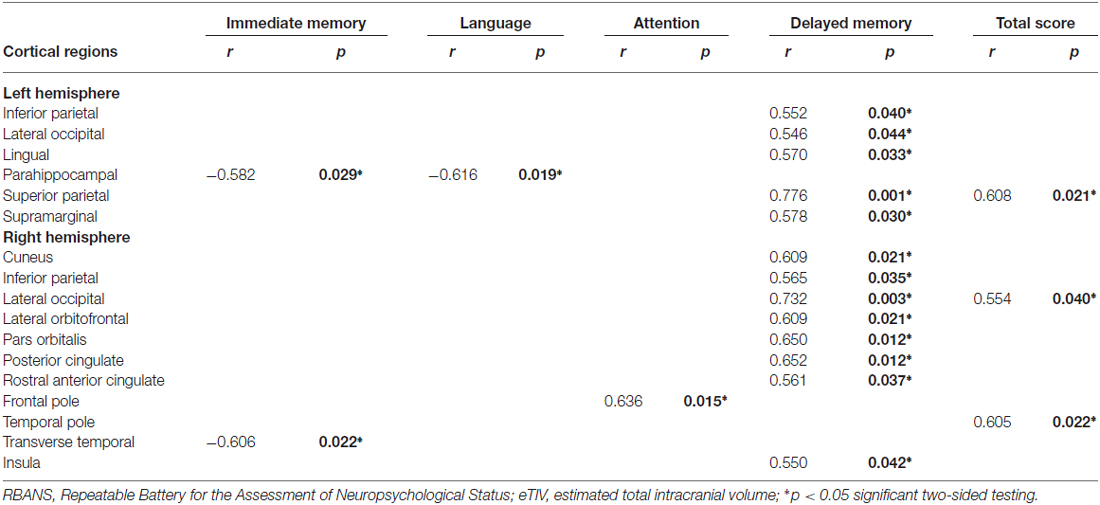
Table 2. Partial correlations between repeatable battery for the assessment of neuropsychological status (RBANS) change scores and cortical thickness changes in the multi-domain training group, by controlling for age, gender, education, and estimated total intracranial volume (eTIV).
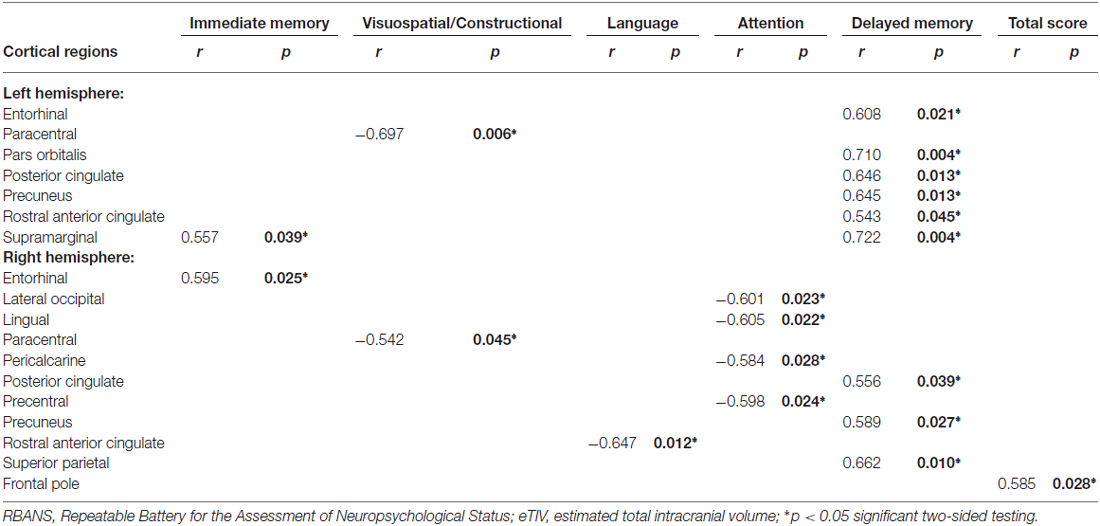
Table 3. Partial correlations between RBANS change scores and cortical thickness changes in the single-domain training group, by controlling for age, gender, education, and eTIV.
Table 3 presents the correlations between cognitive function change scores and cortical thickness changes in the single-domain training group, after controlling for age, gender, education, and eTIV. These results revealed that immediate memory change scores were significantly positively associated with the changes of the left supramarginal and the right entorhinal regions. Visuospatial/constructional change scores were significantly negatively associated with the changes of the left paracentral and the right paracentral regions and significant negative correlations between language change scores and cortical thickness changes were observed in the change of the right rostral anterior cingulate region. Attention change scores were significantly negatively correlated with the changes of the right lateral occipital, lingual, pericalcarine, and precentral regions. Significant positive correlations between delayed memory change scores and cortical thickness changes were presented in the changes of the left entorhinal, pars orbitalis, posterior cingulate, precuneus, rostral anterior cingulate, and supramarginal, as well as the right posterior cingulate, precuneus, and superior parietal regions. Lastly, significant positive correlations between total change scores and cortical thickness changes were observed in the change of the right frontal pole region.
Discussion
The main finding of this study was that the cortical thickness changes were significantly associated with cognitive function changes in healthy elderly after receiving cognitive training interventions, and healthy older adults benefited more from the multi-domain cognitive training than single-domain cognitive training. Single-domain training targets highly specific cognitive abilities, it may allow researchers to evaluate direct training-related effects (Cheng et al., 2012). However, multi-domain training may have more practical advantages for older adults because that will increase engagement and transfer (Binder et al., 2015; Kim et al., 2015a).
There were significantly group × time interaction effects on the left supramarginal and the left frontal pole cortical regions, which two regions were parts of attention network (Fan et al., 2005; Grant et al., 2013). These findings implicated that the multi-domain cognitive training would offer more advantages in attention in the healthy older adults. Due to the relative small sample size, the results from the repeated-measures ANOVA may lead to an underestimation of interaction effects, the visuospatial/constructional scores and delayed memory scores showed a marginal significant group × time interaction effects. As such, cognitive training could delay age-associated cortical change and cognitive decline, it was an efficacious behavioral strategy for improving or maintaining cognitive health in the healthy elderly (Kim et al., 2015a; Smith-Ray et al., 2015).
In our study, both the multi-domain and single-domain training groups demonstrated delayed memory change scores that were significantly positively correlated with many distinct cortical region changes. These distinct cortical regions exhibited normal functions that were directly involved in improving delayed memory. This result showed that cognitive training can improve delayed memory in older persons living in a community.
As indicated previously, changes in brain structure, as well as cognitive functions, are commonly seen in the aging population. The human brain is a complex network of functionally and structurally interconnected regions. For example, the attention network contains the integrated regions of the frontal lobe, superior parietal lobe, fusiform gyrus, anterior cingulated, thalamus, and left precentral gyrus (Fan et al., 2005). Similar to previous studies (Fan et al., 2005), we observed significantly positive correlations between changes in attention scores and right frontal poles in the multi-domain training group. However, in the single-domain training group, attention change scores were significantly negatively correlated with change in the right lateral occipital, lingual, pericalcarine, and precentral. Cognitive function in aging does not have a single etiology, as there is not one exclusive brain region or system that is affected. According to a compensation view (Grady, 2012; Walhovd et al., 2014; Morcom and Johnson, 2015), it may be that the right lateral occipital, lingual, pericalcarine, and precentral regions are indirectly involved in improving attention, and require other brain regions to be involved in the process. Our analysis suggests that multi-domain training was more beneficial to attention improvement than single-domain training in healthy older adult participants. In accordance with a compensation view, we also concluded that multi-domain cognitive training was more effective in improving visuospatial/constructional skills, and single-domain cognitive training was more effective in improving immediate memory.
There are several limitations to this study that should be kept in mind when interpreting our results. The first major limitation involves the relatively conservative number of subjects, as we only included a small proportion of all identified community members who met the criteria for the study. Larger sample size is needed to evaluate the effect of cognitive training on the aging brain. Second, the multi-domain training group had a slightly higher education level than the single-domain training group at the 12-month follow-up (as explained in “Results” Section). However, years of education was used as covariate in our analysis. Finally, our study only has two time points: MRI data at baseline and the 12-month follow-up. In other words, we did not collect data immediately following the completion of the intervention. These limitations may affect the external validity of the study, and deserve to be examined further.
Conclusion
In summary, our findings may contribute to an understanding of the mechanism of normal aging in the human brain and help to distinguish the different effects of multi-domain and single-domain cognitive training interventions in healthy older adults. Specifically, multi-domain cognitive training demonstrated more advantages in visuospatial/constructional, attention, and delayed memory abilities, while single-domain cognitive training primarily benefited immediate memory ability. In the end, multi-domain cognitive training may represent the best opportunity to improve the cognitive function of healthy older adults.
Author Contributions
Conceived the study: CL, JW. Designed the experiment: CL, JW, LJ, XC, TL. Performed the experiment: LJ, XC, TL, WL. Analyzed the data: LJ, YT, WL, RCC. Wrote the manuscript: LJ, XC, TL, RCC, CL, JW. All authors provided critical revisions to the manuscript and approved the final version of the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer KRB and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We thank other members of the research group: Yan Cheng, Wei Feng, Yikang Zhu, Huiru Cui, Hongmei Liu and Lanlan Zhang contributed to data collection. We thank Dr. Yi Jin from the Chinese Academy of Sciences for assistance in the data analysis. This work was supported by National Nature Science Foundation of China (81371505, 30770769), the Science and Technology Commission of Shanghai Municipality (134119a2501, 13dz2260500) and the Shanghai Mental Health Center (2013-YJ-09, 2014-YJ-05).
Footnotes
References
Ball, K., Berch, D. B., Helmers, K. F., Jobe, J. B., Leveck, M. D., Marsiske, M., et al. (2002). Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA 288, 2271–2281. doi: 10.1001/jama.288.18.2271
Belleville, S., and Bherer, L. (2012). Biomarkers of cognitive training effects in aging. Curr. Transl. Geriatr. Exp. Gerontol. Rep. 1, 104–110. doi: 10.1007/s13670-012-0014-5
Binder, J. C., Zöllig, J., Eschen, A., Mérillat, S., Röcke, C., Schoch, S. F., et al. (2015). Multi-domain training in healthy old age: hotel plastisse as an ipad-based serious game to systematically compare multi-domain and single-domain training. Front. Aging Neurosci. 7:137. doi: 10.3389/fnagi.2015.00137
Cheng, Y., Wu, W., Feng, W., Wang, J., Chen, Y., Shen, Y., et al. (2012). The effects of multi-domain versus single-domain cognitive training in non-demented older people: a randomized controlled trial. BMC Med. 10:30. doi: 10.1186/1741-7015-10-30
Cheng, Y., Wu, W., Wang, J., Feng, W., Wu, X., and Li, C. (2011). Reliability and validity of the repeatable battery for the assessment of neuropsychological status in community-dwelling elderly. Arch. Med. Sci. 7, 850–857. doi: 10.5114/aoms.2011.25561
Cody, S. L., Fazeli, P., and Vance, D. E. (2015). Feasibility of a home-based speed of processing training program in middle-aged and older adults with HIV. J. Neurosci. Nurs. 47, 247–254. doi: 10.1097/JNN.0000000000000147
Corbett, A., Owen, A., Hampshire, A., Grahn, J., Stenton, R., Dajani, S., et al. (2015). The effect of an online cognitive training package in healthy older adults: an online randomized controlled trial. J. Am. Med. Dir. Assoc. 16, 990–997. doi: 10.1016/j.jamda.2015.06.014
Coubard, O. A., Ferrufino, L., Boura, M., Gripon, A., Renaud, M., and Bherer, L. (2011). Attentional control in normal aging and Alzheimer’s disease. Neuropsychology 25, 353–367. doi: 10.1037/a002205
Desikan, R. S., Ségonne, F., Fischl, B., Quinn, B. T., Dickerson, B. C., Blacker, D., et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021
Ehrlich, S., Brauns, S., Yendiki, A., Ho, B. C., Calhoun, V., Schulz, S. C., et al. (2012). Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr. Bull. 38, 1050–1062. doi: 10.1093/schbul/sbr018
Engvig, A., Fjell, A. M., Westlye, L. T., Moberget, T., Sundseth, Ø., Larsen, V. A., et al. (2010). Effects of memory training on cortical thickness in the elderly. Neuroimage 52, 1667–1676. doi: 10.1016/j.neuroimage.2010.05.041
Fan, J., McCandliss, B. D., Fossella, J., Flombaum, J. I., and Posner, M. I. (2005). The activation of attentional networks. Neuroimage 26, 471–479. doi: 10.1016/j.neuroimage.2005.02.004
Fischl, B., and Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U S A 97, 11050–11055. doi: 10.1073/pnas.200033797
Grady, C. (2012). The cognitive neuroscience of aging. Nat. Rev. Neurosci. 13, 491–505. doi: 10.1038/nrn3256
Grant, J. A., Duerden, E. G., Courtemanche, J., Cherkasova, M., Duncan, G. H., and Rainville, P. (2013). Cortical thickness, mental absorption and meditative practice: possible implications for disorders of attention. Biol. Psychol. 92, 275–281. doi: 10.1016/j.biopsycho.2012.09.007
Han, X., Jovicich, J., Salat, D., van der Kouwe, A., Quinn, B., Czanner, S., et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32, 180–194. doi: 10.1016/j.neuroimage.2006.02.051
Hu, J. P., Guo, Y. H., Wang, F., Zhao, X. P., Zhang, Q. H., and Song, Q. H. (2014). Exercise improves cognitive function in aging patients. Int. J. Clin. Exp. Med. 7, 3144–3149.
Jack, C. R., Petersen, R. C., O’Brien, P. C., and Tangalos, E. G. (1992). MR-based hippocampal volumetry in the diagnosis of Alzheimer’ disease. Neurology 42, 183–188. doi: 10.1212/wnl.42.1.183
Kim, G. H., Jeon, S., Im, K., Kwon, H., Lee, B. H., Kim, G. Y., et al. (2015a). Structural brain changes after traditional and robot-assisted multi-domain cognitive training in community-dwelling healthy elderly. PLoS One 10:e0123251. doi: 10.1371/journal.pone.0123251
Kim, J. S., Yang, J. J., Lee, D. K., Lee, J. M., Youn, J., and Cho, J. W. (2015b). Cognitive impairment and its structural correlates in the parkinsonian subtype of multiple system atrophy. Neurodegener. Dis. 15, 294–300. doi: 10.1159/000430953
Kirchhoff, B. A., Anderson, B. A., Barch, D. M., and Jacoby, L. L. (2012). Cognitive and neural effects of semantic encoding strategy training in older adults. Cereb. Cortex 22, 788–799. doi: 10.1093/cercor/bhr129
Kobayashi, L. C., Smith, S. G., O’Conor, R., Curtis, L. M., Park, D., von Wagner, C., et al. (2015). The role of cognitive function in the relationship between age and health literacy: a cross-sectional analysis of older adults in Chicago, USA. BMJ Open 5:e007222. doi: 10.1136/bmjopen-2014-007222
Kwok, T., Wong, A., Chan, G., Shiu, Y. Y., Lam, K. C., Young, D., et al. (2013). Effectiveness of cognitive training for Chinese elderly in Hong Kong. Clin. Interv. Aging 8, 213–219. doi: 10.2147/CIA.s38070
Li, C., Wu, W., Jin, H., Zhang, X., Xue, H., He, Y., et al. (2006). Successful aging in Shanghai, China: definition, distribution and related factors. Int. Psychogeriatr. 18, 551–563. doi: 10.1017/s1041610205002966
Lim, M. L., Collinson, S. L., Feng, L., and Ng, T. P. (2010). Cross-cultural application of the repeatable battery for the assessment of neuropsychological status (RBANS): performances of elderly Chinese Singaporeans. Clin. Neuropsychol. 24, 811–826. doi: 10.1080/13854046.2010.490789
Liu, Y., Xie, T., He, Y., Duan, Y., Huang, J., Ren, Z., et al. (2014). Cortical thinning correlates with cognitive change in multiple sclerosis but not in neuromyelitis optica. Eur. Radiol. 24, 2334–2343. doi: 10.1007/s00330-014-3239-1
Lustig, C., Shah, P., Seidler, R., and Reuter-Lorenz, P. A. (2009). Aging, training and the brain: a review and future directions. Neuropsychol. Rev. 19, 504–522. doi: 10.1007/s11065-009-9119-9
Meijer, W. A., van Boxtel, M. P., Van Gerven, P. W., van Hooren, S. A., and Jolles, J. (2009). Interaction effects of education and health status on cognitive change: a 6-year follow-up of the Maastricht aging study. Aging Ment. Health 13, 521–529. doi: 10.1080/13607860902860821
Morcom, A. M., and Johnson, W. (2015). Neural reorganization and compensation in aging. J. Cogn. Neurosci. 27, 1275–1285. doi: 10.1162/jocn_a_00783
Mozolic, J. L., Long, A. B., Morgan, A. R., Rawley-Payne, M., and Laurienti, P. J. (2011). A cognitive training intervention improves modality-specific attention in a randomized controlled trial of healthy older adults. Neurobiol. Aging 32, 655–668. doi: 10.1016/j.neurobiolaging.2009.04.013
Narr, K. L., Woods, R. P., Thompson, P. M., Szeszko, P., Robinson, D., Dimtcheva, T., et al. (2007). Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cereb. Cortex 17, 2163–2171. doi: 10.1093/cercor/bhl125
Newman, L. M., Trivedi, M. A., Bendlin, B. B., Ries, M. L., and Johnson, S. C. (2007). The relationship between gray matter morphometry and neuropsychological performance in a large sample of cognitively healthy adults. Brain Imaging Behav. 1, 3–10. doi: 10.1007/s11682-007-9000-5
Payne, B. R., Jackson, J. J., Hill, P. L., Gao, X. F., Roberts, B. W., and Stine-Morrow, E. A. L. (2012). Memory self-efficacy predicts responsiveness to inductive reasoning training in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 67, 27–35. doi: 10.1093/geronb/gbr073
Rahe, J., Petrelli, A., Kaesberg, S., Fink, G. R., Kessler, J., and Kalbe, E. (2015). Effects of cognitive training with additional physical activity compared to pure cognitive training in healthy older adults. Clin. Interv. Aging 10, 297–310. doi: 10.2147/CIA.S74071
Randolph, C., Tierney, M. C., Mohr, E., and Chase, T. N. (1998). The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 20, 310–319. doi: 10.1076/jcen.20.3.310.823
Rebok, G. W., Ball, K., Guey, L. T., Jones, R. N., Kim, H. Y., King, J. W., et al. (2014). Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 62, 16–24. doi: 10.1111/jgs.12607
Reijnders, J., van Heugten, C., and van Boxtel, M. (2013). Cognitive interventions in healthy older adults and people with mild cognitive impairment: a systematic review. Aging Res. Rev. 12, 263–275. doi: 10.1016/j.arr.2012.07.003
Reuter, M., Schmansky, N. J., Rosas, H. D., and Fischl, B. (2012). Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 61, 1402–1418. doi: 10.1016/j.neuroimage.2012.02.084
Ruscheweyh, R., Deppe, M., Lohmann, H., Wersching, H., Korsukewitz, C., Duning, T., et al. (2013). Executive performance is related to regional gray matter volume in healthy older individuals. Hum. Brain Mapp. 34, 3333–3346. doi: 10.1002/hbm.22146
Sisco, S. M., Marsiske, M., Gross, A. L., and Rebok, G. W. (2013). The influence of cognitive training on older adults’ recall for short stories. J. Aging Health 25, 230S–248S. doi: 10.1177/0898264313501386
Smith-Ray, R. L., Hughes, S. L., Prohaska, T. R., Little, D. M., Jurivich, D. A., and Hedeker, D. (2015). Impact of cognitive training on balance and gait in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 70, 357–366. doi: 10.1093/geronb/gbt097
Thompson, G., and Foth, D. (2005). Cognitive-training programs for older adults: what are they and can they enhance mental fitness? Educ. Gerontol. 31, 603–626. doi: 10.1080/03601270591003364
Velayudhan, L., Proitsi, P., Westman, E., Muehlboeck, J. S., Mecocci, P., Vellas, B., et al. (2013). Entorhinal cortex thickness predicts cognitive decline in Alzheimer’s disease. J. Alzheimers Dis. 33, 755–766. doi: 10.3233/JAD-2012-121408
Walhovd, K. B., Fjell, A. M., and Espeseth, T. (2014). Cognitive decline and brain pathology in aging—need for a dimensional, lifespan and systems vulnerability view. Scand. J. Psychol. 55, 244–254. doi: 10.1111/sjop.12120
Keywords: aging, cognitive function, cortical thickness, multi-domain, single-domain
Citation: Jiang L, Cao X, Li T, Tang Y, Li W, Wang J, Chan RC and Li C (2016) Cortical Thickness Changes Correlate with Cognition Changes after Cognitive Training: Evidence from a Chinese Community Study. Front. Aging Neurosci. 8:118. doi: 10.3389/fnagi.2016.00118
Received: 24 December 2015; Accepted: 09 May 2016;
Published: 24 May 2016.
Edited by:
Junming Wang, University of Mississippi Medical Center, USAReviewed by:
Rubem C. A. Guedes, Universidade Federal de Pernambuco, BrazilMinghao Dong, Xidian University, China
Kenneth Ray Butler, University of Mississippi Medical Center, USA
Copyright © 2016 Jiang, Cao, Li, Tang, Li, Wang, Chan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jijun Wang, amlqdW53YW5nMjdAMTYzLmNvbQ==;
Chunbo Li, Y2h1bmJvX2xpQDE2My5jb20=
 Lijuan Jiang
Lijuan Jiang Xinyi Cao
Xinyi Cao Ting Li4
Ting Li4 Yingying Tang
Yingying Tang Jijun Wang
Jijun Wang Chunbo Li
Chunbo Li