
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 19 May 2015
Sec. Neuroinflammation and Neuropathy
Volume 7 - 2015 | https://doi.org/10.3389/fnagi.2015.00062
This article is part of the Research TopicRole of Stem Cells in Skeletal Muscle Development, Regeneration, Repair, Aging and DiseaseView all 17 articles
 Julia Meireles Nogueira1,2
Julia Meireles Nogueira1,2 Katarzyna Hawrot1
Katarzyna Hawrot1 Colin Sharpe3
Colin Sharpe3 Anna Noble4
Anna Noble4 William M. Wood5
William M. Wood5 Erika C. Jorge2
Erika C. Jorge2 David J. Goldhamer5
David J. Goldhamer5 Gabrielle Kardon6
Gabrielle Kardon6 Susanne Dietrich1*
Susanne Dietrich1*Pax7 expressing muscle stem cells accompany all skeletal muscles in the body and in healthy individuals, efficiently repair muscle after injury. Currently, the in vitro manipulation and culture of these cells is still in its infancy, yet muscle stem cells may be the most promising route toward the therapy of muscle diseases such as muscular dystrophies. It is often overlooked that muscular dystrophies affect head and body skeletal muscle differently. Moreover, these muscles develop differently. Specifically, head muscle and its stem cells develop from the non-somitic head mesoderm which also has cardiac competence. To which extent head muscle stem cells retain properties of the early head mesoderm and might even be able to switch between a skeletal muscle and cardiac fate is not known. This is due to the fact that the timing and mechanisms underlying head muscle stem cell development are still obscure. Consequently, it is not clear at which time point one should compare the properties of head mesodermal cells and head muscle stem cells. To shed light on this, we traced the emergence of head muscle stem cells in the key vertebrate models for myogenesis, chicken, mouse, frog and zebrafish, using Pax7 as key marker. Our study reveals a common theme of head muscle stem cell development that is quite different from the trunk. Unlike trunk muscle stem cells, head muscle stem cells do not have a previous history of Pax7 expression, instead Pax7 expression emerges de-novo. The cells develop late, and well after the head mesoderm has committed to myogenesis. We propose that this unique mechanism of muscle stem cell development is a legacy of the evolutionary history of the chordate head mesoderm.
Adult skeletal muscle stem cells (satellite cells) accompany contractile muscle fibers and efficiently repair muscle after injury (reviewed in Relaix and Zammit, 2012). It is generally thought that one of the factors contributing to this efficient repair is that skeletal muscle stem cells are tissue-specific stem cells, solely committed to myogenesis. However, in a number of diseases including muscular dystrophies, cancer and HIV/Aids, the ability of muscle stem cells to repair muscle is compromised. Moreover, muscle regeneration declines when we age. This has been ascribed to inflammatory responses, changes to the stem cell niche and changes to the stem cells themselves. Current approaches investigate how these parameters could be targeted to reinstate the full regenerative capacity of muscle.
Overall, skeletal muscle function and repair is much the same in all areas of the body. Therefore, it is often overlooked that muscular dystrophies differentially target muscle groups in the head and in the trunk (reviewed in Emery, 2002). Moreover, head and trunk muscle and their accompanying muscle stem cells have a different developmental history (reviewed in Sambasivan et al., 2011, and see below). This tissue also contributes to the heart, an organ that in amniotes including humans cannot regenerate (reviewed in Garbern and Lee, 2013). Moreover, adult head and trunk muscle stem cells have divergent gene expression, proliferation and differentiation profiles (Sambasivan et al., 2009; Ono et al., 2010; Hebert et al., 2013). Thus, the head mesoderm and the muscle stem cells derived thereof are of great interest to develop both specialized skeletal muscle stem cells and cardiac cells for human therapy.
In the body, skeletal muscles and their accompanying stem cells develop from the segmented paraxial mesoderm, the somites (reviewed in Bryson-Richardson and Currie, 2008; Buckingham and Vincent, 2009; Relaix and Zammit, 2012). Muscles (myotomes) are laid down in waves, and while the first cells differentiate into contractile fibers, more cells are being added on from a dual muscle-dermis-competent precursor pool, stored in a specialized somitic compartment, the dermomyotome. This compartment also provides cells that emigrate into the periphery to provide the limb, hypobranchial/hypopharyngeal/hypoglossal, and in mammals, diaphragm muscles. Importantly, cells in the dermomyotome eventually shed their dermal competence, enter the myotome and become specialized muscle stem cells (Gros et al., 2005; Kassar-Duchossoy et al., 2005; Relaix et al., 2005; Schienda et al., 2006). These cells actively self-renew and provide differentiating cells during fetal and juvenile stages of development, thereby providing the bulk of the adult musculature. Eventually, the stem cells settle underneath the basal lamina of the differentiated muscle fibers and become adult muscle stem cells (satellite cells). In amniotes, these stem cells adopt a quiescent state, only to be activated when injuries occur; in anamniotes, the cells may remain mitotically active and continue to drive muscle growth (Bryson-Richardson and Currie, 2008; Buckingham and Vincent, 2009; Relaix and Zammit, 2012).
In amniotes, somites express the paralogous transcription factors Pax3 and Pax7 as soon as they form; in all jawed vertebrates, these genes continue to be expressed in the dermomyotome, with either Pax3 or both proteins also labeling the migratory muscle precursors (reviewed in Bryson-Richardson and Currie, 2008; Buckingham and Vincent, 2009; Relaix and Zammit, 2012). The genes keep cells in a proliferative state, but are also required to initiate myogenesis, and hence are referred to as premyogenic genes (Collins et al., 2009; Diao et al., 2012; Kawabe et al., 2012). Cells undertaking differentiation then switch on members of the MyoD family of transcription factors, which are crucial for myogenic differentiation (Weintraub et al., 1989). In the somites, Myf5 and MyoD are expressed first and commit cells to myogenesis. In a feed forward mechanism, they activate Myogenin which promotes cell cycle exit and entry into terminal differentiation (Penn et al., 2004). Mrf4 has an early expression phase in the mouse (Summerbell et al., 2002), but in most models, acts mainly during fetal myogenesis (Hinits et al., 2009; Della Gaspera et al., 2012, and Dietrich, unpublished observations).
The Pax3 and Pax7 genes arose as a result of the second of two rounds of whole genome duplications that occurred in the ancestors of jawed vertebrates 500 million years ago (Ohno et al., 1968; Holland et al., 1994). In jawless vertebrates, the single pax3/7 gene is also expressed in dermomyotomal muscle precursors (Kusakabe et al., 2011). Likewise, pax3/7 expression has also been found in the somites and muscle stem cell-like cells of the cephalochordate Amphioxus (Holland et al., 1999; Somorjai et al., 2012), indicating an ancient role as premyogenic genes. In jawed vertebrates, both genes were subject to subfunctionalisation: cells retaining muscle stem cells properties rely on the presence of Pax7 rather than Pax3, and in the absence of Pax7 function, the deposition and maintenance of the skeletal muscle stem cell pool is impaired (Seale et al., 2000; Kassar-Duchossoy et al., 2005; Relaix et al., 2006; Lepper et al., 2009; von Maltzahn et al., 2013). Moreover, in anamniote vertebrates such as the axolotl, in which fully differentiated, functional muscle can contribute to regeneration by returning to a stem cell state, or in experimental models where de-differentiation is induced in vitro, this occurs concomitant with a reactivation of pax7 (Kragl et al., 2009; Pajcini et al., 2010). Thus, the Pax7 gene is accepted as the universal skeletal muscle stem cell marker in jawed vertebrates.
In the head, the muscles that move the eye ball, move the gill arches and in jawed vertebrates, open and close the mouth, are derived from the non-somitic paraxial head mesoderm (Noden, 1983; Couly et al., 1992; Harel et al., 2009; Sambasivan et al., 2009; reviewed in Sambasivan et al., 2011). This tissue does not form segments, and in contrast to the trunk mesoderm, contributes to both, skeletal muscle and the heart. The early head mesoderm does not express the Pax3 gene and instead, harbors a complement of markers whose expression pattern is established in a step-wise fashion; eventually, the eye and jaw closure muscle anlagen express Pitx2, the most posterior eye muscle and muscle anlagen for the jaw and throat (branchiomeric muscles) express Tbx1, and all express Musculin (Msc/MyoR) (Mootoosamy and Dietrich, 2002; Bothe and Dietrich, 2006; Bothe et al., 2011). These transcription factors have overlapping roles. Notably, similar to Pax3 and Pax7 in the trunk, they keep cells in an immature state, control their survival and activate MyoD family members; once Mrf genes are expressed, myogenic differentiation is thought to occur in a similar fashion as in the body (Kitamura et al., 1999; Lu et al., 2002; Kelly et al., 2004; Diehl et al., 2006; Dong et al., 2006; Zacharias et al., 2011; Moncaut et al., 2012; Hebert et al., 2013; Castellanos et al., 2014).
In the adult, head muscle is equipped with muscle stem cells which express Pax7, underlining that Pax7 is the bona fide muscle stem cell marker (Harel et al., 2009; Sambasivan et al., 2009, reviewed in Sambasivan et al., 2011). These stem cells however are not immigrants from the somites. Rather, like the muscle they accompany, they are derived from the head mesoderm itself. In tune with this observation, head muscle stem cells continue to express the early head mesodermal markers. This implies that head muscle stem cells may have retained some of the properties of the early head mesoderm, and may therefore be suited to developing specialized muscle stem cells and cardiac cells for therapy.
To explore the developmental and therapeutic potential of head muscle stem cells, we need to understand when and how these cells are being generated. This is currently not known. The aim of this study therefore is to establish when and where head muscle stem cells emerge, using Pax7 as lead-marker. In order to understand the basic process common to all jawed vertebrates, we investigated the key models for vertebrate myogenesis, chicken, mouse, frog (sarcopterygians), and zebrafish (an actinopterygian). Our work shows that unequivocally, Pax7 expressing cells arise late in head muscle development, well after the onset of Myf5 and MyoD. Importantly, the cells arise from MyoD expressing precursor cells, and we propose that head mesodermal cells have to commit to myogenesis before being able to become a muscle stem cell.
Fertilised chicken eggs (Henry Stewart Ltd, Norfolk) were incubated in a humidified atmosphere at 38.5°C and staged according to Hamburger and Hamilton (1951). Embryos were harvested in 4% PFA.
Wildtype mice were provided by the Animals Resource Centre at the University of Portsmouth. Transgenic mouse driver lines carrying the improved Cre gene introduced into the Pax7 or the MyoD locus and reporter lines carrying a Cre-activateable LacZ, GFP, or YFP gene in the Rosa26 locus are described in Hutcheson et al. (2009), Kanisicak et al. (2009), and Wood et al. (2013) and were provided by the Kardon and Goldhamer laboratories. Mice were mated overnight; the appearance of a vaginal plug the next morning was taken as day 0.5 of development (E0.5). Pregnant females were sacrificed by cervical dislocation and the embryos were fixed in 4% PFA.
Adult J-strain and cardiac actin:GFP transgenic Xenopus laevis frogs were maintained in the European Xenopus Resource Centre (EXRC) at the University of Portsmouth at 18°C in a 14 h light 10 h dark cycle and fed 5 days each week using high protein trout pellets. Embryos were generated as described in Guille (1999), then dejellied in 2% cystein-HCl (pH 8.0), grown at 18–23°C in 0.1 × MBS (Gurdon, 1977), staged according to Nieuwkoop and Faber (1994) and harvested in MEMFA (Harland, 1991).
Zebrafish embryos were provided by the INCT de Medicina Molecular, Faculdade de Medicina, Universidade Federal de Minas Gerais. Breeding zebrafish (Danio rerio) were maintained at 28°C on a 14 h light/10 h dark cycle. Embryos were obtained by natural spawning, grown in egg water (0.3 gl/l Instant Ocean Salt, 1 mg l/l Methylene Blue) at 28°C and staged according to Kimmel et al. (1995). To prevent pigment formation, embryos post-24 hpf were raised in 0.2 mM 1-phenyl-2-thiourea (PTU, Sigma). Embryos were harvested in 4% PFA.
In chicken and mouse, whole mount in situ hybridisation, double in situ hybridisation, antibody staining, in situ hybridisation followed by antibody staining and vibratome sectioning was carried out as described by Dietrich et al. (1997, 1998, 1999), Mootoosamy and Dietrich (2002), Alvares et al. (2003), and Lours and Dietrich (2005). In situ hybridisation and antibody staining in Xenopus followed the protocols by Harland (1991) and Baker et al. (1995); for zebrafish the protocols by Thisse and Thisse (2008) were used. Beta galactosidase staining and antibody staining on cryosections was performed according to Hutcheson et al. (2009), a heat-induced epitope retrieval in 1.8 mM Citric Acid, 8.2 mM Sodium Citrate and signal amplification using the Streptavidin system was used for Pax7. Probes and antibodies are detailed in the table below (Tables 1A–E).
Whole embryos were cleared in 80% glycerol/PBS or, when fluorescent antibodies had been used, in 2.50 mg/ml 1,4-diazabicyclo[2.2.2]octane (DABCO) in 90% glycerol/ PBS. Vibratiome sections were mounted with glycerol, cryosections with Fluoromount (Sigma). Embryos and sections were photographed on a Zeiss Axioskop, using fluorescence or Nomarski optics. Sections in Figure 9 were photographed using a Zeiss LSM710 confocal miscroscope.
The work was has been approved by the University of Portsmouth Ethical Review Committee (AWERB No14005) and follows the jurisdiction of the Animals (Scientific Procedures) Act. The work involving Pax7-Cre, MyoDiCre, Rosa26-lacZ, and Rosa26-GFP mouse lines and the cardiac actin; GFP frog line is covered by personal licenses to G. Kardon, D. Goldhamer, and M. Guille.
Muscle stem cells have the ability to self renew and generate differentiating daughter cells, and this ability is linked to the expression and function of Pax7 (reviewed in Bryson-Richardson and Currie, 2008; Buckingham and Vincent, 2009; Relaix and Zammit, 2012). Adult head muscle stem cells express Pax7 (Harel et al., 2009; Sambasivan et al., 2009, reviewed in Sambasivan et al., 2011), and hence we used the emergence of Pax7 expression in the head mesoderm as a sign that head muscle stem cells are being laid down. We first analyzed the onset of Pax7 expression in the chicken head mesoderm, because chicken embryos are large and easy to obtain, and craniofacial muscle formation is well characterized in this model (reviewed in Noden and Francis-West, 2006). Using whole mount in situ hybridisation, we performed a time course for the expression of Pax7 mRNA from the stage the head mesoderm is being laid down by the primitive streak at HH4 to stage HH24 when craniofacial muscle anlagen are well established (Noden et al., 1999; Camp et al., 2012; Figures 1A–L). Moreover, we analyzed the onset of Pax7 protein expression (Figure 1M and not shown) and we confirmed the association of Pax7 expression domains with craniofacial skeletal muscle on serial frontal and cross sections (Figure 2).
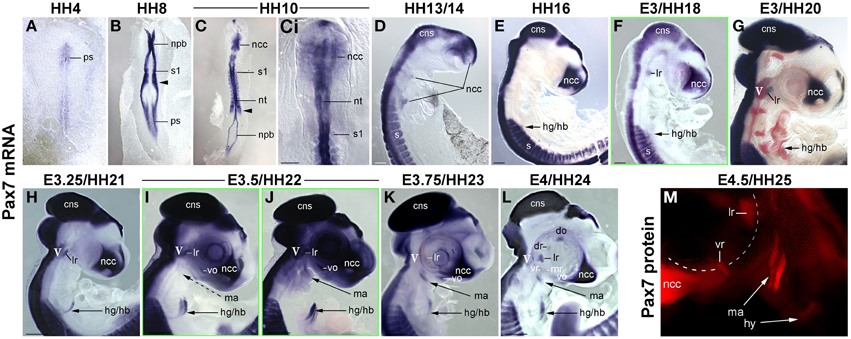
Figure 1. Time course of Pax7 expression in the chicken embryo; stages of development are indicated at the top of the panel. (A–L) Expression of Pax7 mRNA with (A-Ci) dorsal views, anterior to the top, (D–M) lateral views of the right side of embryos, anterior to the top, dorsal to the left. In (G), cranial ganglia are revealed by Isl1 expression (red staining). In (L), the eye was removed after staining. (M) Expression of Pax7 protein; lateral view of the left side of an embryo, anterior to the top, dorsal to the right, the eye has been removed before staining. The onset of Pax7 expression in craniofacial muscle anlagen is demarcated by green frames to the respective image. Throughout the first 5 days of development, Pax7 is well detectable in the central nervous system, cranial neural crest cells and the somites and somite-derived muscle precursors/embryonic muscle stem cells. In muscle anlagen derived from the paraxial head mesoderm, expression is first seen at stage HH18 in the lateral rectus eye muscle anlage, the only head muscle to express some trunk markers. In the other head mesoderm derived muscles, Pax7 mRNA can be detected in strongly stained specimen at stage 22; expression becomes more robust at stages 23–24 and is followed by protein expression at HH25. Abbreviations: cns, central nervous system; do, dorsal oblique eye muscle anlage; dr, dorsal rectus eye muscle anlage; hg/hb, hypoglossal/hypobranchial muscle anlage; lr, lateral rectus eye muscle anlage; ma, mandibular arch muscle anlage (jaw closure muscles); mr, medial rectus eye muscle anlage; ncc, neural crest cells; npb, neural plate border; nt, neural tube; ps, primitive streak; s/s1, somite/ somite 1; vo, ventral oblique eye muscle anlage; vr, ventral rectus eye muscle anlage; V, 5th cranial ganglion (trigeminal ganglion).
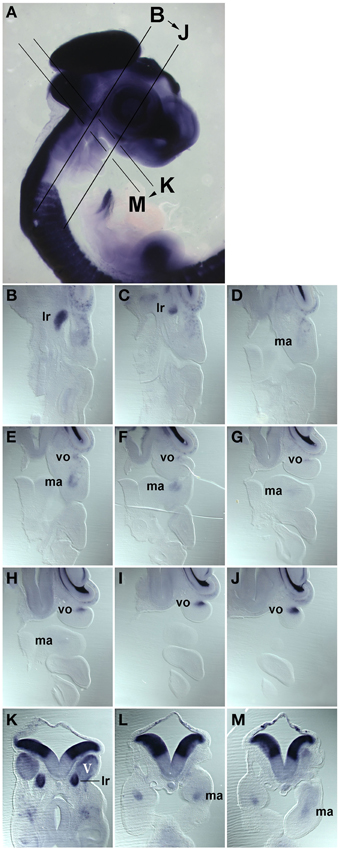
Figure 2. Series of frontal (B–J) and cross (K–M) sections of a HH22 chicken head, stained for the expression of Pax7 mRNA, the plane and order of sections in indicated in (A). (B–J) anterior to the top, lateral to the right; (K–M) dorsal to the top. Abbreviations as in Figure 1. The position of the Pax7 signals beneath the trigeminal ganglion, beneath the eye and in the core of the mandibular arch confirms that these are expression domains associated with developing head muscles.
We found that during early stages of development, Pax7 expression was associated with the epiblast bordering the primitive streak, the neural plate border/ dorsal neural tube and emerging neural crest cells (Figures 1A–C). Expression in the dorsal neural tube remained high at later stages of development while expression in neural crest cells only persisted in the trigeminal ganglion and the frontonasal neural crest (Figures 1D–M, cns, V, ncc). In the trunk, from HH7 onwards Pax7 was also expressed in the epithelialising somites (Figures 1B,C, arrowhead, and not shown), subsequently becoming confined to the muscle precursor/muscle stem cell lineage (Figures 1C–L). From HH16 onwards, Pax7 was also expressed in migratory muscle precursors that leave the somites to form the hypoglossal/hypopharyngeal (Figures 1E–L, hg/hb) and limb musculature (not shown). From HH20 onwards, expression was found in the embryonic muscle stem cells that populate the myotome, drive both fetal and perinatal muscle growth and give rise to the trunk adult muscle stem cells (Gros et al., 2005; Relaix et al., 2005; Ahmed et al., 2006; Schienda et al., 2006) and not shown. These findings are in agreement with published data and underline the robustness of our approach.
Expression associated with craniofacial muscles was first seen at HH18 in the developing lateral rectus eye muscle (Figure 1F, lr), located just beneath the also Pax7 positive trigeminal ganglion (Figures 1G–J, V, in G red staining for Isl1, Figure 2B, V). However, the lateral rectus is somewhat unusual as it is the only craniofacial muscle to express trunk markers such as Paraxis and Lbx1 (Mootoosamy and Dietrich, 2002, and see below). In the anlagen of the other craniofacial muscles, Pax7 staining did not emerge before day 3.5 of development. Expression was first seen in the ventral oblique eye muscle (Figures 1I,J, 2, vo), in strongly stained specimen followed by the anlagen of the jaw closure muscles (1st pharyngeal arch = mandibular arch muscles; Figures 1J, 2, ma). Pax7 mRNA expression became more robust at E3.75/HH23 and E4/HH24 and eventually began to encompass all muscle anlagen, with the staining in the ventral and medial rectus lagging behind that of the other eye muscle anlagen (Figures 1K,L). However, signals were always weak compared to the expression in the frontonasal neural crest cells, the central nervous system and the trunk musculature. Moreover, expression of Pax7 protein in craniofacial muscle anlagen was delayed compared to the expression of Pax7 mRNA and could only be detected from HH25 onwards (Figure 1M and not shown).
In the trunk, Pax7 expression precedes the expression of any marker for myogenic commitment and differentiation (Jostes et al., 1991). However, the late onset of Pax7 expression in the head musculature suggested that here, the sequence of marker gene expression and the set up of gene regulatory networks might be quite different. To explore this, we systematically analyzed the spatiotemporal distribution of early head mesoderm markers (Pitx2, Alx4, Musculin = Msc = MyoR, Tcf21 = Capsulin, Tbx1; Figure 3), of markers indicating the onset of myogenesis (Mrf family members; Figure 4), and of markers indicating cohesion and terminal differentiation of muscle anlagen (Cadherin4 = Cdh4 = R−Cadherin, Tnni1, sarcomeric Myosin; Figure 5).
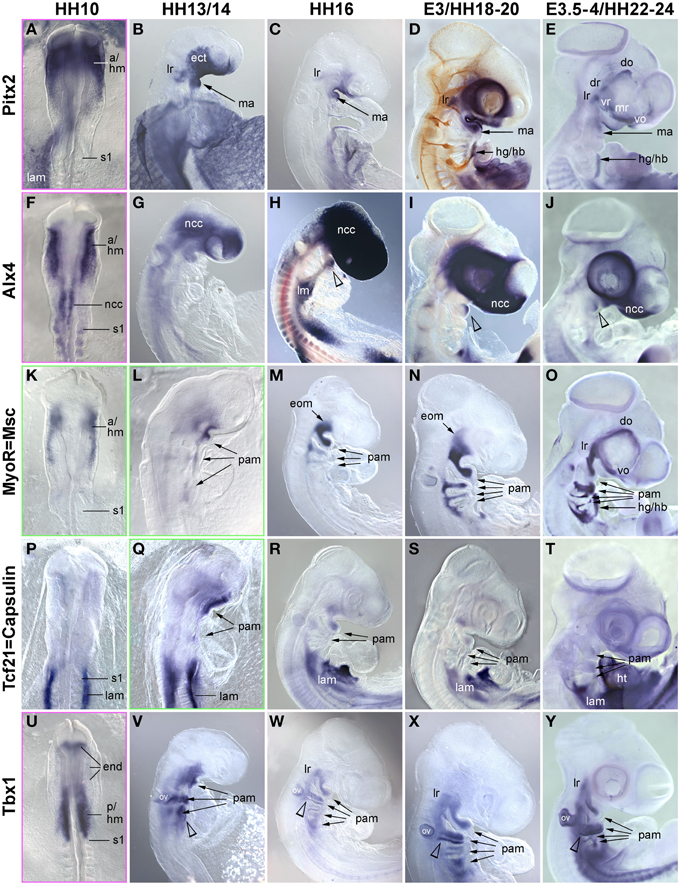
Figure 3. Time course for the mRNA expression of head mesoderm markers in chicken embryos at HH10 (dorsal views) and HH13/14-E4 (lateral views); in (D), cranial nerves are revealed with the RMO270 antibody (brown staining). Gene names are displayed on the left of the panel; developmental stages are indicated at the top. The onset of marker gene expression is demarcated by a green frame, for genes being expressed earlier than HH10, frames are displayed in magenta. Abbreviations as in Figure 1 and: a/hm, anterior head mesoderm; ect, surface ectoderm; eom, extraocular muscle anlagen; end, endoderm; lam, lateral mesoderm; ht, heart; ov, otic vesicle; pam, pharyngeal arch muscle anlagen; p/hm, posterior head mesoderm. The open arrowhead in (H,J) points at Alx4 expression in the mandibular arch ectoderm and in (V–Y) at Tbx1 expression in the posterior ectoderm of the hyoid (2nd pharyngeal) arch. Note that all head mesoderm markers begin their expression well before Pax7. With the exception of Alx4 which from HH13/14 onwards mainly labels cranial neural crest cells and Tcf21/Capsulin which throughout has lower expression levels than its paralog MSc/MyoR, all head mesoderm markers continue to strongly label the myogenic head mesoderm. Their expression domains are wider than that of Pax7, whose expression domain is nested in the expression domain of the head mesoderm genes (compare Figures 1, 4).
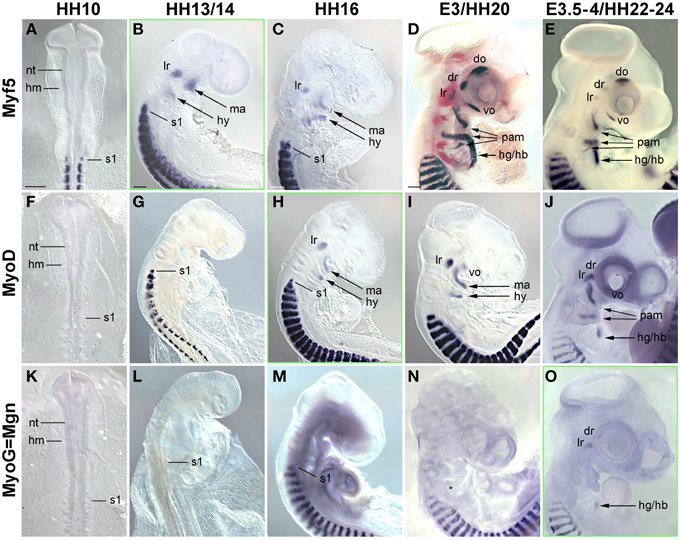
Figure 4. Time course for the mRNA expression of Mrf transcriptions factors in chicken embryos from HH10-E4; orientation of specimen and frames indicating the onset of expression as in Figure 3. Gene names and developmental stages are displayed as in Figure 3, Abbreviations as in Figures 1, 3 and: hy, hyoid arch. Myf5 and MyoD indicate the myogenic commitment of precursor cells and are expressed in developing craniofacial muscle anlagen from HH13/14 (Myf5) and HH16 (MyoD) onwards, i.e., significantly before the onset of Pax7. Expression of Myogenin (MyoG/Mgn) indicates the entry of cells into muscle differentiation and commences at E3.5-4, i.e., about the same time as Pax7 (compare with Figure 1).
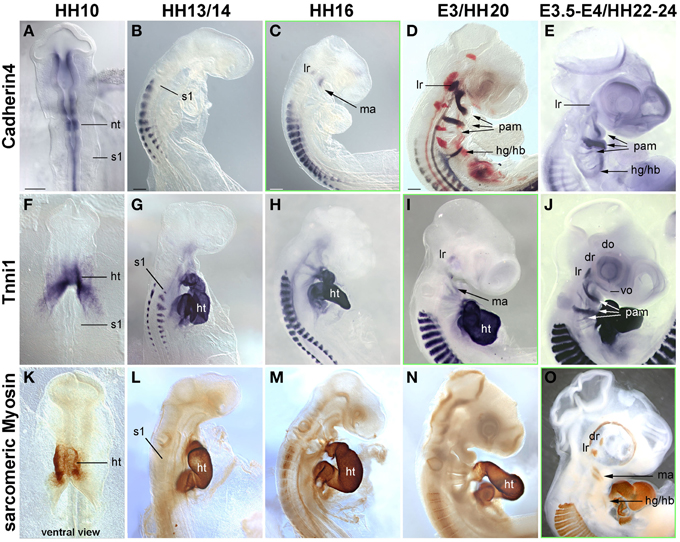
Figure 5. Time course in chicken embryos from HH10-E4 for markers indicating the cohesion of muscle anlagen (Cadherin 4–mRNA expression) and terminal differentiation [Troponin I 1 (Tnni1)–mRNA expression; sarcomeric Myosin–MF20 antibody staining]. Orientation of specimen and frames indicating the onset of expression as in Figures 3, 4; abbreviations as in Figures 1, 3, 4. The time course of Cadherin 4 expression resembles that of MyoD, and Tnni1 expression commences in many craniofacial muscle anlagen E3, i.e., both are expressed before or at the onset of Pax7 expression. Sarcomeric Myosins can be detected at E3.5-4, simultaneous to the onset of Pax7.
The early chicken head mesoderm is known to expresses the transcription factors Pitx2, Alx4, Msc, Capsulin, and Tbx1 (Bothe and Dietrich, 2006; von Scheven et al., 2006; Bothe et al., 2011), and in accord with this work, mRNA expression of Pitx2 and Tbx1 was first seen at HH6 in distinct rostro-caudal regions of the head mesoderm (not shown). At HH9 Alx4 expression emerged within the confines of the Pitx2 territory, followed by Msc expression at HH10. Between HH10 (Figures 3A,F,K,P,U) and HH13/14 (Figures 3B,G,L,Q,V), Tbx1 expression spread anteriorly and Msc expression spread posteriorly, co-labeling the branchiomeric mesoderm and the anlage of the caudal-most eye muscle, the lateral rectus. Also at HH13/14, Tcf21 expression commenced in the anlagen of the branchiomeric muscles, but remained weaker than that of its paralog Msc throughout (Figure 3Q). Alx4 on the other hand became strongly upregulated in craniofacial neural crest cells, thus, masking any residual mesodermal expression (Figures 3G–J, ncc). Owing to these changes, Pitx2, Msc, and Tbx1 became the most prominent markers for the myogenic head mesoderm, between HH16 to HH22-24 labeling the anlagen of the eye and mandibular arch muscles (Pitx2, Figures 3C–E), all eye and branchiomeric muscles (Msc; Figures 3M–O) or the branchiomeric muscles and the lateral rectus eye muscle (Tbx1, Figures 5W–Y). Respective expression domains for these markers were wider than those for Pax7 but included the Pax7 domains.
In the developing chicken somites, commitment to myogenesis and entry into differentiation is demarcated by the sequential expression of the MyoD family of Mrf genes, with Myf5 commencing first, followed by MyoD, MyoG and Mrf4 (Berti and Dietrich, unpublished observations). In the avian head mesoderm, expression of the Mrf family commenced at HH13/14, when Myf5 labeled the anlagen of the lateral rectus and the mandibular and hyoid arch muscles (Figure 4B, see also Noden et al., 1999). At HH16, expression of Myf5 was accompanied by that of MyoD (Figures 4C,H). Between HH20-HH24, all craniofacial muscle anlagen began to express these genes, with MyoD expression always following that of Myf5 (Figures 4D,E,I,J). MyoG expression was detected at HH22-24 (Figure 4O), yet Mrf4 was still silent at this stage (not shown). Thus, for all craniofacial muscles, including the peculiar lateral rectus eye muscle, expression of Myf and MyoD emerged well before that of Pax7; and MyoG expression began at approximately the same time as Pax7. This is different from the trunk where Pax7 is expressed before any of the Mrfs.
Cadherin 4 has been shown to act in the communication and differentiation of myogenic cells (Rosenberg et al., 1997) and to be expressed in the chicken lateral rectus eye muscle (Mootoosamy and Dietrich, 2002). Troponins and sarcomeric Myosins are components of the contractile proteins complexes in both cardiac and skeletal muscle, with Tnni1 specifically acting in the early developing slow-twitch muscle and (during embryogenesis) in the heart (see http://geisha.arizona.edu/geisha/). We therefore used these markers as indicators for the cohesion and terminal differentiation of muscle anlagen. Expression of these markers was first seen at HH16, when Cadherin4 labeled the lateral rectus and mandibular arch muscle anlagen (Figure 5C). Tnni1 expression commenced at HH20, at HH22-24 encompassing all craniofacial muscle anlagen (Figures 5I,J). At this stage, sarcomeric Myosins were also expressed, indicating the presence of functional skeletal muscle (Figure 5O and Noden et al., 1999). The onset of Cdh4 and Tnni1 expression before or concomitant with that of Pax7 suggests that in the head, the process of skeletal muscle development is well under way when the Pax7 cell lineage is being established.
In previous studies, we had shown that the early head mesoderm does not express the Pax7 paralog Pax3 (Mootoosamy and Dietrich, 2002; Bothe and Dietrich, 2006). However, stages at the onset of Pax7 expression have not been analyzed. Moreover, in the trunk Paraxis, Six1 and the Six1 co-activator Eya1 also act as premyogenic regulators (Wilson-Rawls et al., 1999; Grifone et al., 2005, 2007; Relaix et al., 2013). To explore if any of these genes might be in the position to serve as intermediaries between the head mesoderm genes, the Mrf genes and Pax7, we investigated the expression of these trunk premyogenic genes (Figure 6). Our analysis revealed that Pax3 expression overlapped with that of Pax7 in the neural tube, the trigeminal ganglion, the frontonasal neural crest and somites, but remained absent from craniofacial skeletal muscle anlagen (Figures 6A–E). Paraxis expression overlapped with the expression of Pax3 and Pax7 in the frontonasal neural crest cells and the somites, but, with the exception of the lateral rectus muscle, was also not expressed in craniofacial muscle anlagen (Mootoosamy and Dietrich, 2002; Figures 6F–J). Six1 showed a widespread expression, at HH5-10 encompassing the preplacodal ectoderm, both the mesoderm and the endoderm underneath the neural plate, and weakly, the developing somites (shown for HH10, Figure 6K). From HH13/14 onwards (Figures 6L–O), expression was strong in the otic vesicle and nasal pit, the trigeminal placodes, the posterior edge of the 2–4th pharyngeal arches and the pharyngeal pouches, the somites and the emerging migratory muscle precursors. Moreover, low-level, widespread Six1 expression was found throughout the head mesenchyme. However, expression was also found in the anlagen of branchiomeric muscles, with strongest expression in the hyoid arch. Expression of Eya1 (Figures 6P–T) was similar to that of Six1. Yet, while strong and lasting expression was detected in craniofacial neural crest cells, expression levels in craniofacial muscle anlagen declined. This suggests that in contrast to the other trunk pre-myogenic genes, Six1 and Eya1 may play an–albeit more subordinate than in trunk–role in head skeletal muscle development and may influence muscle development indirectly via the control of connective tissue development. Yet none of the trunk pre-myogenic markers seems to take over from the head mesoderm genes to prepare for myogenesis and/or muscle stem cell deployment.
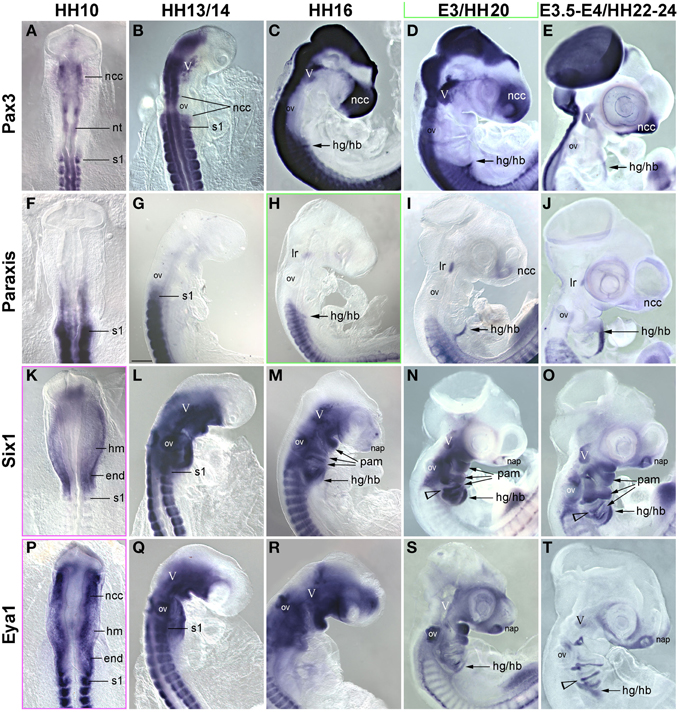
Figure 6. Time course for the mRNA expression of trunk pre-myogenic genes; embryos are displayed and annotated as in Figures 3–5. Abbreviations as before and: na, nasal pit. (A–E) Pax3 labels the central nervous system, the frontonasal neural crest, the trigeminal ganglion, the somites and the somite-derived hypobranchial and limb muscle precursors, but remains absent from genuine craniofacial muscle anlagen. (F–J) Paraxis expression overlaps with that of Pax3 and 7 in the somite-derived muscle precursors and in the frontonasal crest. Similar to Pax7, Paraxis is also expressed in the lateral rectus eye muscle, but is absent from all other craniofacial muscles. Six1 (K–O) and Eya1 (P–T) are expressed in the head mesoderm before and at HH10. From that stage onwards mesoderm expression becomes somewhat obscured by the overlying expression in neural crest cells. However, Six1 (but not Eya1) remains detectable in craniofacial muscle anlagen.
Our analysis in the chicken suggested that, in contrast to the trunk, Pax7 expressing cells associated with cranial skeletal muscle emerge late, well after the onset of markers for myogenic commitment and at the time that cells begin to enter terminal differentiation. To explore whether this unexpected timing is true also for other amniotes, we next investigated the mouse, establishing both the onset of mRNA (Figures 7A–D) and protein expression (Figures 7E–G; see also Figure 9). Moreover, we investigated the position of Pax7 protein expressing cells at birth (Figures 7H–J) and analyzed the fate of Pax7 positive cells from embryonic to late fetal stages of development (Figures 7K–Q).
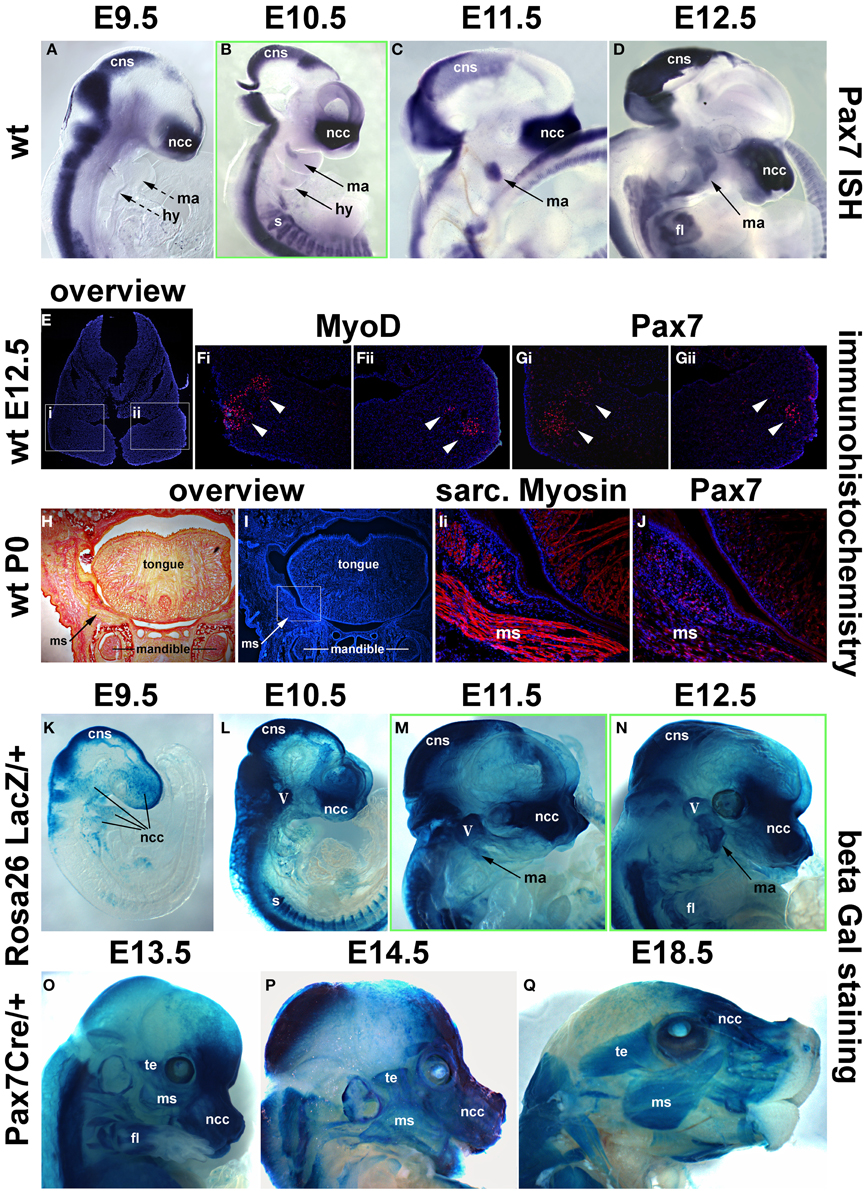
Figure 7. Time course of Pax7 expression in the mouse. (A–D) Pax7 mRNA expression from E9.5-E12.5 of development; lateral views of the right side of embryos, anterior to the top. Expression is readily detectable in the developing central nervous system, emigrating neural crest cells (prolonged expression in the frontonasal neural crest) and the somites. Head muscle anlagen show expression first at E10.5. (E) Serial cross sections of the mandibular arch at E12.5, dorsal to the top; (F,G) higher magnifications of the areas indicated by the boxes in (E) and stained for Dapi and MyoD protein (Fi,ii) or Dapi and Pax7 protein (Gi,ii). Note that MyoD and Pax7 domains overlap. (H,I) Serial frontal sections of the mandible at birth (P0), dorsal to the top, lateral to the left. (H) Sirius Red staining showing muscle fibers in yellow and bone and connective tissue in red. (I) Dapi staining of the same region, with (Ii) showing a magnification of the cheek and the floor of the mouth as indicated in (I). Skeletal muscle fibers are shown in red. (J) Subsequent section stained for Pax7 protein in red. Note the punctate, nuclear staining for Pax7, associated with the Myosin-positive muscle fibers. (K–Q) Lineage tracing of Pax7 expressing cells, revealed by beta galactosidase staining; lateral views of the right side of embryos, dorsal to the top. With a delay of 1 day, cells with a history of Pax7 expression can be detected in the central nervous system, the trigeminal ganglion, the frontonasal neural crest and the somites. In craniofacial muscle anlagen, cells with a history of Pax7 expression can be detected between E11.5 and E12.5, with a more robust staining appearing at E13.5. Eventually, all craniofacial muscles are stained and the staining is found in muscle fibers, indicating that, similar to the trunk, Pax7-positive cells contribute to fetal and perinatal muscle growth. Abbreviations as in Figures 1, 3, 4 and: fl, forelimb; ISH, in situ hybridisation; ms, masseter; te, temporalis muscle; wt, wildtype.
As in the chicken, mouse Pax7 expression was seen in neural crest cells, the central nervous system and in somites from early neurulation stages onwards (Jostes et al., 1991, and not shown). At E9.5, even though the first two pharyngeal arches were well developed, there was no expression in their myogenic mesodermal core (Figure 7A, ma, hy; dotted arrows). Expression in the pharyngeal arch muscle anlagen was first seen at E10.5, and became more robust between E11.5 -12.5 (Figures 7B–D, ma, hy; solid arrows). Also similar to the chicken, expression of Pax7 protein was delayed compared to the expression of mRNA and was first detected at E12.5, with the Pax7 domain overlapping with that of MyoD (Figures 7E–G). Both at E12.5 and in the newborn, Pax7 protein was located in the nuclei of cells (note the punctate staining in Figures 7Gi,Gii,J). In the newborn, the Pax7 staining was associated with muscle fibers revealed by antibodies detecting sarcomeric Myosins (compare Figures 7Ii,J), in line with studies that showed that at this stage, Pax7 expressing cells had assumed their mature satellite cell (adult muscle stem cell) position (Harel et al., 2009; Sambasivan et al., 2009).
Using the Pax7 Cre driver and the Rosa26RLacZ reporter, we traced cells that in their past expressed robust levels of Pax7 (Hutcheson et al., 2009). This approach allowed to visualize the contribution of Pax7 expressing neural crest cells to the trigeminal ganglion, the pharyngeal arches and the developing frontonasal skeleton with a delay of 1 day compared to the onset of mRNA expression; likewise, the Pax7 cell lineage in the central nervous system and in the somites could readily be traced with a delay of 1 day (Figures 7K–L, and not shown). LacZ positive cells contributing to the mandibular arch muscle anlagen were just about detectable at E11.5 (Figure 7M, ma, arrow), at E12.5, this contribution was more evident (Figure 7N, ma, arrow). By E13.5, virtually all developing craniofacial muscles had received a contribution of cells that once had expressed Pax7 (compare Figure 7O and Figure 9D, and see also Figures 9F,G,I), and at E14.5 (Figure 9P) and E18.5 (Figure 9Q), the cells had contributed to muscle fibers. This suggests that at fetal and perinatal stages of development Pax7 positive cells contribute to the growth of head skeletal muscle in a similar fashion as in the trunk, inferring a convergence of developmental pathways.
In the chicken, Pax7 expression in craniofacial muscle anlagen commenced after the onset of head mesoderm markers and after the onset of Myf5 and MyoD. To explore whether this is also true for mammals, we investigated the expression of Pitx2, Tbx1, Msc, Myf5, MyoD, and MyoG at E7.5-E10.5 of development (Figure 8 and not shown). Expression of Pitx2, Tbx1, and Msc commenced much earlier at E7.5-8 (not shown). At E9.5, Msc labeled the myogenic cells that will engage with the eye as well as the core of the first two pharyngeal arches; the same pattern was found at E10.5 (Figures 8A,B). Significantly, at both stages, both Myf5 as well as MyoD were well expressed in the anlagen of first and second arch muscles (Figures 8C–F), while MyoG was not yet active (Figures 8G,H). Thus, in both amniote models, the expression of the head mesoderm markers preceded the expression of Myf5 and MyoD, which in turn preceded the expression of Pax7.
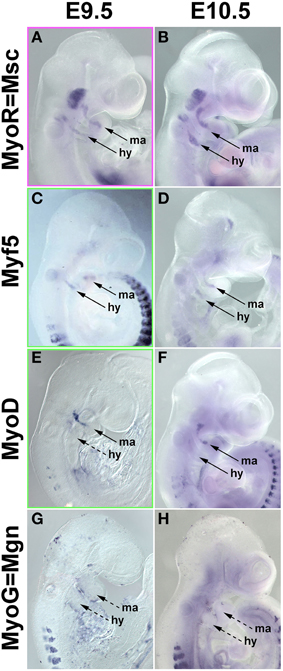
Figure 8. mRNA Expression of mouse head mesoderm markers and markers for myogenic commitment before and at the onset of Pax7 expression. Lateral views, dorsal to the top. Stages of development are indicated at the top of the panel, gene names on the left. (A,B) Musculin expression commences before E9.5 (not shown); at E9.5-10.5, the gene is widely expressed in the myogenic head mesoderm. Myf5 (C,D) and MyoD (E,F) expression commences at E9.5, i.e., before the onset of Pax7. (G,H) Myogenin expression is not yet detectable at these stages and commences slightly later at E11.5 (not shown).
Since MyoD expression in craniofacial muscle anlagen preceded the onset of Pax7, we began to explore whether MyoD might be upstream of Pax7, similar to what has been shown for P19 EC cells misexpressing MyoD (Gianakopoulos et al., 2011). For this we turned to the MyoDiCre mouse driver line (Kanisicak et al., 2009; Wood et al., 2013). We first established when the Rosa26 GFP reporter (R26NG; (Yamamoto et al., 2009) may reveal activity of the MyoDiCre driver in craniofacial muscle anlagen; we found that this was the case from E10.5 onwards (Figures 9A–D). At E12.5 and E13.5, the GFP expression pattern was highly similar to that of Pax7 mRNA and Pax7-driven LacZ (compare Figures 9C,D, 7D,N,O). To test whether the Pax7 mRNA we had detected earlier at E11.5 might colocalise with the GFP read-out of the MyoD locus, we simultaneously visualized the Pax7 mRNA and GFP driven by MyoDiCre (Figures 9Ei–iii). We found that indeed, cells with a history of MyoD expression engulfed the Pax7 domain located at the maxillary-mandibular junction. To directly test whether Pax7 expressing cells have a history of MyoD expression, we stained for Pax7 and GFP proteins on cryosections of MyoDiCre/+; R26NG/+ embryos at E12.5 (not shown) and E13.5 (Figures 9F–I). This revealed that not all cells with a history of MyoD expression also expressed Pax7. However, for head mesoderm-derived muscles, the majority of Pax7-positive nuclei were located in cells with MyoDiCre driven GFP expression (Figures 9G–I). In contrast, in the somite-derived tongue muscle most Pax7-positive nuclei were in GFP-negative cells (Figure 9H). Taken together, our data support the idea that head mesodermal cells express early Mrf and become myogenic before turning on Pax7.
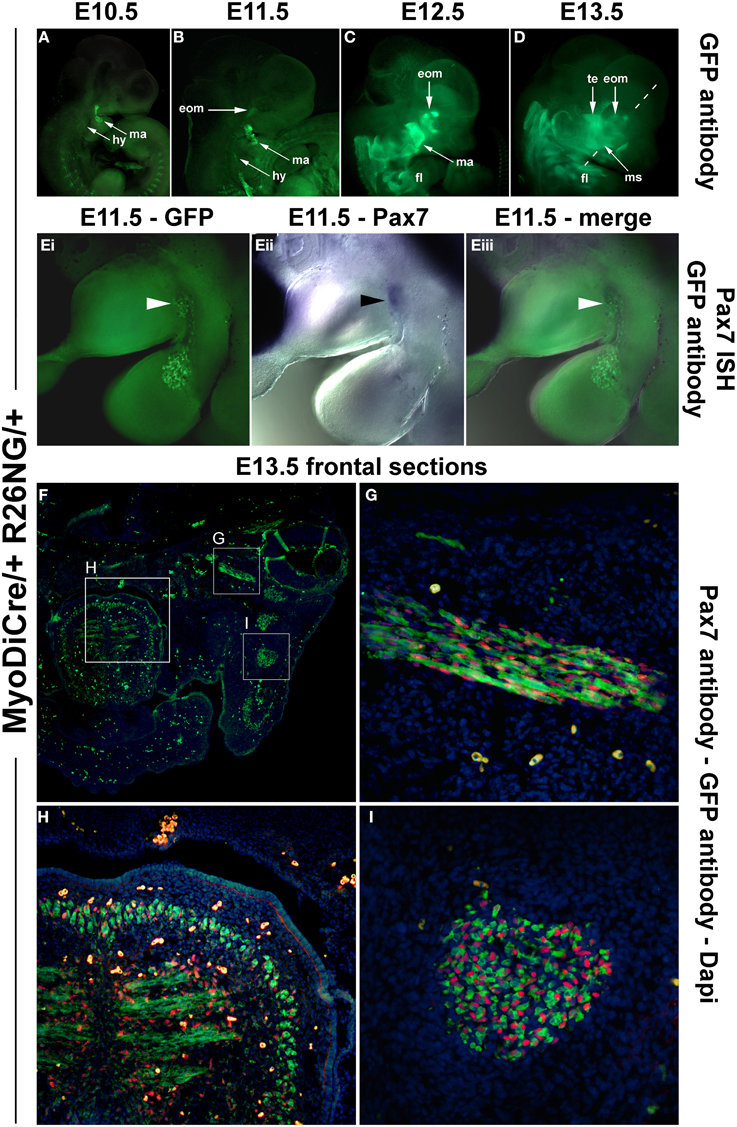
Figure 9. Lineage tracing of MyoD expressing cells in MyoDiCre/+ R26NG embryos, revealed by anti-GFP antibody (green) staining. (A–D) Lateral views of the right side of E10.5-E13.5 embryos; the dotted line indicates the sectional plane in (F–I). (Ei-iii) Lateral views of the left side of an E11.5 embryo, stained for Pax7 mRNA (blue) and GFP protein (green); dorsal to the top. (F) Frontal section of an E13.5 embryo, stained for Pax7 protein (red), GFP (green), and Dapi (blue). (G) Detail of the ventral rectus eye muscle, (H) detail of the tongue, (I) detail of the masseter as indicated in (D,F). The widely distributed bright green (F) or yellow cells (G–I) are autofluorescing blood cells. Cells with a history of MyoD expression can readily be detected at E10.5 and 11.5, first in the mandibular and hyoid arch, then in the developing extraocular muscles. In head-mesoderm-derived muscles, Pax7 mRNA and subsequent protein expression colocalises with that of MyoD-Cre driven GFP, and Pax7 containing nuclei reside in GFP expressing cells. In contrast, in the somite-derived tongue muscle, most Pax7-positive nuclei are not located in GFP expressing cells. Abbreviations as in Figures 1, 4, 5 and: eom, developing extraocular muscles.
Our analysis suggested that in amniotes, cells that eventually will populate the head muscle stem cell niche are being deployed after, possibly from, cells committed to skeletal muscle formation. However, in amniotes, overall skeletal myogenesis is delayed compared to anamniotes that rely on functional muscle during larval stages of development. Therefore, we investigate the emergence of Pax7 positive cells in craniofacial muscles of two anamniote models, the African clawed frog X. laevis (a sarcopterygian vertebrate like mouse and chicken) and the teleost fish D. rerio (zebrafish, an actinopterygian vertebrate).
In X. laevis, the genome was duplicated upon hybridisation between two ancestral species approximately 65 million years ago, and extant X. laevis is considered allotetraploid (Hughes and Hughes, 1993; Evans et al., 2004; reviewed in Evans, 2008). However, when we cloned partial cDNA sequences of the two duplicate pax7 genes, they had an identity of 87% (data not shown). Moreover, the pax7a probe had an identity of 85.5% and the pax7b probe of 78.7% with the corresponding sequence of the single Xenopus tropicalis pax7 gene, this however only shared 55% of nucleotides with its paralog pax3. Correspondingly, in situ hybridisation of X. laevis embryos with the pax7a and b probes alone, with a mix of both probes or with the X. tropicalis pax7 probe produced the same expression patterns, and hence only the data for the X. tropicalis probe are being shown (Figure 10). This analysis revealed expression in the central nervous system, in craniofacial neural crest cells, in the ventral diencephalon and the hypophysis as well as in the somitic dermomyotome, recapitulating the data by Maczkowiak et al. (2010), Daughters et al. (2011), Della Gaspera et al. (2012), Bandin et al. (2013) (Figures 10A,B and data not shown). Pax7 expression in areas of developing head muscle anlagen was detected from stage 39 onwards and became somewhat stronger at stages 40/41 [Figures 10C–Di; muscles were identified according to Ziermann and Olsson (2007) and Schmidt et al. (2013)]. However, expression levels remained low compared to other expression domains.
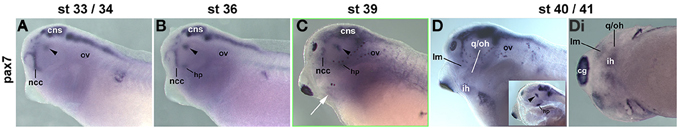
Figure 10. Time course of pax7 mRNA expression in Xenopus laevis. Lateral views, anterior to the left. Embryonic stages are indicated at the top. Inset in (D): pharyngeal arches and head mesenchyme were dissected away from the left side to reveal the brain. Up to stage 36, Pax7 expression is confined to the central nervous system including the ventral diencephalon (arrowhead), the hypophysis (hp), and the frontonasal neural crest cells. Weak expression is also seen in the somites. From stage 39 onwards, weak expression can be detected in craniofacial muscle anlagen. Abbreviations as before and: cg, cement gland; hp, hypophysis; ht, heart; first arch derived muscle: im, m. intermandibularis anlage; lm, m. levatores mandibulae anlage; second arch derived muscle: ih, m. interhyoideus anlage; oh, m. orbitohyoideus anlage; qh, m. quadrato-hyoangularis anlage; q/oh, common oh and qh precursor.
Previous studies have investigated the expression of some mesodermal and myogenic markers in the Xenopus embryos (Della Gaspera et al., 2012), but a systematic comparison with pax7 has not been carried out. We therefore cloned probes for the head mesodermal gene msc, for all mrf genes and for the muscle structural gene desmin. As expected, msc labeled the myogenic head mesoderm from early stages onwards (Figures 11A–Di and not shown); the exception is the mesoderm of the first arch which however is pitx2 and tbx1 positive (Della Gaspera et al., 2012). These markers are followed by the expression of myf5 and myod (Figures 11E–Hi,I–Li and not shown). myf5 and myod showed overlapping but non-identical expression patterns, with myf5 strongly labeling the 1st arch derived intermandibularis muscle anlage (Figures 11H,Hi, im) and myod the 1st arch derived levator mandibularis muscle (Figures 11L,Li, lm). Yet when myog and desmin expression commenced in developing head muscles at st36, the markers encompassed all craniofacial muscle anlagen, and they were followed by mrf4 expression at st39 (Figures 11M-Pi,Q-Ti,U-Xi). Thus, as in the two amniote models, frog pax7 expression in craniofacial muscle anlagen began late, after the commitment of cells to myogenesis and the onset of differentiation.
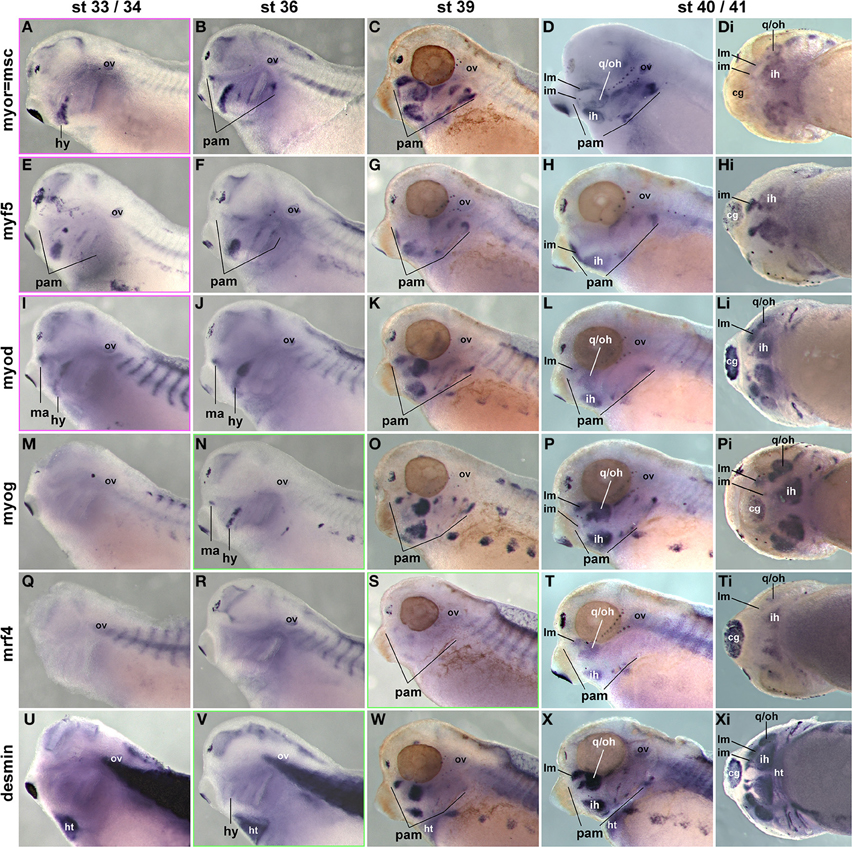
Figure 11. Time course of head mesoderm and muscle gene expression in Xenopus laevis. Same stages, views, and abbreviations as in Figure 11; markers are indicated on the left. Note that msc, myf5, myod, myog, and desmin are expressed before, mrf4 concomitant with the onset of pax7 expression.
In order to determine the onset of pax7 protein expression and to ascertain that expression domains are associated with skeletal muscle, we compared the expression of sarcomeric myosins (MF20 antibody staining, Figures 12A,C,E,G,I) and the read-out of the cardiac actin promoter (cardiac actin; GFP frogs; Figure 12K) with that of pax7 protein (Figures 12B,D,F,H,J,L,M). To associate expression with anatomical features, a diaminobenzidine staining was performed (Figures 12A–F); to better detect signals away from the surface, a fluorescent secondary antibody was used (Figures 12G–J,L,M). As a control, we performed an antibody staining at st26, focusing on the somitic expression (Figures 12A,Ai,B,Bi); this recapitulated the data by Daughters et al. (2011). Our stainings at st40 revealed pax7 protein expression in craniofacial muscle anlagen, with expression levels being significantly lower than those of sarcomeric myosins (Figures 12C–J).
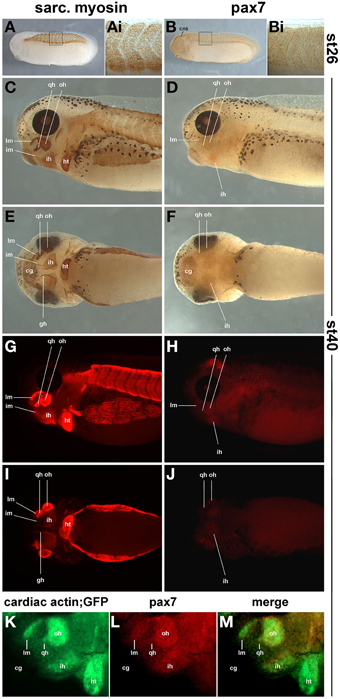
Figure 12. Sarcomeric myosin (A,C,E,G,I) and pax7 protein expression (B,D,F,H,J) in st26 Xenopus laevis control embryos and in craniofacial muscle anlagen embryos at st40. (K–M) GFP expression and pax7 protein expression in a st40 cardiac actin; GFP embryo. (A,B,C,D,G,H,K,L) lateral views, rostral to the left, dorsal; to the top; (E,F,I,J) ventral views, rostral to the left. In (A–F) a HRP-coupled secondary antibody was used, in (G–J) a secondary antibody coupled to Alexa fluor 594. In (K–M), Alexa fluor 488 and 594 coupled secondary antibodies were used to detect anti GFP and pax7 primaries, respectively. The staining at st26 recapitulates the known myosin and pax7 expression patterns. At st40, the strong expression of sarcomeric myosin and the activity of the cardiac actin promoter indicate that in particular in muscle anlagen associated with the first (mandibular) and second (hyoid) pharyngeal arch, muscle differentiation is well under way. In these muscle anlagen, a faint expression is visible for pax7. The pattern is punctuate, as expected for a nuclear localisation of the pax7 protein. Abbreviations as in Figures 1, 10, 11 and: somite derived muscle: gh, m. geniohyoideus anlage.
Chicken, mouse and frog all belong to the lobe-finned/limbed (sarcopterygian) class of osteichthyans, while zebrafish is a teleost that belongs to the ray-finned (actinopterygian) class (Clack, 2002). Thus, zebrafish is the model most distantly related to humans/ mammals. Teleosts have undertaken a 3rd genome duplication 350 million years ago (Postlethwait, 2007), and retained both pax7 copies (Seo et al., 1998). The coding sequences of these genes are 80.9% identical, and they share 83.6 and 82.6% (pax7b) identity with coding sequence of the single pax7 gene in the spotted gar, a holost fish (data not shown). Yet zebrafish pax7a sequences are 63.5/57.3% identical with pax3a/b sequences, and pax7b sequences are 61.3/56.2% identical with those from pax3a/b, respectively. This suggests that pax7a and b are likely to cross-hybridize with the mRNA of the duplicate gene but not with pax3a/b mRNAs. Here, we used a pax7a probe as it provides a more robust signal that the pax7b probe (Hughes, personal communication). This probe and the pax7 antibody (see below) had been used earlier (Seo et al., 1998; Hammond et al., 2007) and recapitulated pax7 expression in the nervous system and somites as displayed in these studies (not shown).
Craniofacial muscle anlagen begin to express myf5 and myod at 24 and 32 h post fertilization (hpf), with myod showing a more widespread expression (Lin et al., 2006; Hinits et al., 2009) and data not shown). myog and mrf4 are readily detectable at 48 hpf, and when at 72 hpf the animals rely on their head muscles to ventilate the gills and feed, structural proteins are well established (Schilling and Kimmel, 1997). In contrast, pax7a expression in craniofacial muscles was barely detectable at 72 hpf (Figures 13A,B, arrowheads) whereas expression of myod mRNA (single copy gene; Figures 13C,D) and of sarcomeric myosins (MF20 antibody staining, Figures 13E,F) was very strong at this stage. Thus, also in teleosts pax7 expressing future head muscle stem cells arise late and possibly after the cells committed to myogenesis.
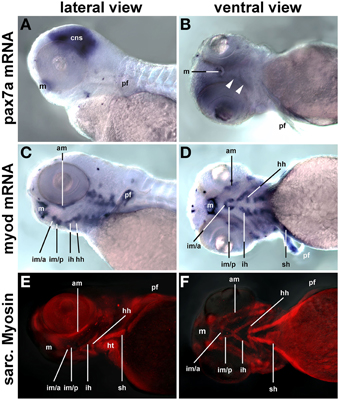
Figure 13. pax7a mRNA, myod mRNA, and sarcomeric myosin expression (MF20 antibody staining) in 72 hpf zebrafish larvae, lateral views, anterior to the left; markers are indicated on the left of the panel. While myogenic markers show robust expression in craniofacial muscle anlagen, pax7 mRNA is barely detectable (arrowheads). Abbreviations as before and: am, adductor mandibulae; hh, hyohyoideus; hpf, hours post fertilization; ih, interhyoideus; im/a, intermandibularis anterior muscle; im/p, intermandibularis posterior muscle; m, mouth; pf, pectoral fin; sh, sternohyoideus.
The vertebrate head mesoderm is a unique type of mesoderm as it forms both skeletal muscle and cardiac tissue. Specifically, the head mesoderm lining the lateral and ventral aspects of the pharynx retains the ability to contribute to skeletal muscle and the heart for a prolonged period of time, and it contributes the ventral muscles of the pharyngeal arches and the outflow tract of the heart (reviewed in Sambasivan et al., 2011). It is conversely debated whether in amniotes including humans, there are any proliferative cells in the mature heart; it is clear however that in contrast to for example the zebrafish (an anamniote) amniote heart regeneration is currently not possible (reviewed in Garbern and Lee, 2013). Yet, adult head skeletal muscle stem cells, besides expressing the muscle stem cell marker Pax7, retain the expression of the early head mesodermal markers (Harel et al., 2009; Sambasivan et al., 2009). These cells, when transplanted into trunk skeletal muscle lose their head-specific expression profile and contribute to trunk muscle regeneration. However, it is appealing to explore whether in the appropriate environment or niche, cells may be able to repair muscle in dystrophies predominantly affecting head muscles, or could be reprogrammed to regenerate the heart. Given that cardiovascular diseases are the predominant cause of death in the Western world (Garbern and Lee, 2013), the latter is of great medical importance.
Prerequisite to exploring the properties and therapeutic potential of head muscle stem cells is an understanding of their developmental biology. However, while some inroads into the unraveling of head skeletal muscle development have been made (reviewed in Sambasivan et al., 2011), timing and mechanisms controlling head muscle stem cell deployment are still largely unknown. Muscle stem cells rely on the expression and function of the Pax7 gene, and Pax7 is currently the most reliable marker for muscle stem cells (Seale et al., 2000; Kassar-Duchossoy et al., 2005; Relaix et al., 2006; Lepper et al., 2009; von Maltzahn et al., 2013). In this study, we used a comparative approach in the commonly used vertebrate models for myogenesis, chicken, mouse, Xenopus and zebrafish, and established, when and how Pax7 expressing head muscle stem cells emerge. These models represent both the lobe-finned/ limbed (sarcopterygian) and ray-finned (actinopterygian) class of “bony” (osteichthyan) vertebrates, and any shared characteristics point at evolutionarily conserved, basic mechanisms.
In vertebrates, the longitudinal body axis is laid down sequentially during gastrulation, proceeding from anterior to posterior (reviewed in Gilbert, 2000). The head therefore is always developmentally advanced, yet head skeletal muscles are known to develop late (Sambasivan et al., 2011). In tune with this delay, we found that Pax7 expressing head muscle stem cells also develop late. However, this delay is not proportionate: the avian embryo, for example, takes 21 days to develop, the head mesoderm is being laid down within the first day, the final pattern of head mesoderm markers is established within a further day, yet it takes about 1.5 days to Pax7 mRNA expression and another 12 h for readily detectable protein levels. This discrepancy is even more pronounced in anamniotes where Pax7 levels are low throughout and first detectable around the time of larval hatching. This suggests that the head mesoderm undertakes a series of so far ill-defined steps before head muscle stem cells can be deployed.
Using the aid of marker genes, we investigated the processes the head mesoderm is engaged in before the onset of Pax7. Notably, in all models examined here, the myogenic head mesoderm expresses Myf5 and/or MyoD (amniotes: co-expression, anamniotes: coexpression in most, but differential expression of myf5 or myod in selected muscle anlagen) before Pax7, indicating that the majority of cells have committed to a skeletal muscle fate. Moreover, several markers indicating the onset of myogenic differentiation are also expressed before the onset of Pax7. This is particularly evident in anamniotes where myog, the mrf that drives cell cycle exit and entry into terminal differentiation, is expressed before pax7; in amniotes, Pax7 expression begins at a similar time point as MyoG. With the exception of Six1, trunk pre-myogenic genes are not expressed (e.g., Pax3) or not expressed consistently (e.g., Eya1). Moreover, head mesoderm genes have been shown to act directly upstream of Mrf (Zacharias et al., 2011; Moncaut et al., 2012; Castellanos et al., 2014). This suggests that in the phase before the onset of Pax7, the myogenic head mesoderm proceeds from a precursor state to a state where skeletal muscle formation is initiated, without recruiting the upstream factors controlling trunk myogenesis. This also suggests that in contrast to the trunk, head muscle precursor cells do not go through a phase of Pax gene expression before becoming a muscle stem cell.
When Pax7 expression becomes detectable in the head mesoderm, the signal either occupies the same region as the Mrf signals or is nested within the MyoD expression domain. In turn, these markers overlap with the expression domains of the early head mesodermal genes. This indicates that in contrast to the early somite, the head mesoderm is not compartmentalized, and stem cells and differentiating cells emerge from within the same cell pool. This scenario is akin to the simultaneous renewal of stem cells and production of differentiating cells after -and from- Pax7 expressing cells that have populated the myotome; the same occurs in the muscle masses of the limbs, and in all muscles during fetal and perinatal stages of development (reviewed in Buckingham and Vincent, 2009). Elegant studies in the mouse showed that both in the head and in the trunk, differentiating muscle displays the membrane-bound ligand Delta which triggers Notch signaling in the neighboring cells. This in turn suppresses MyoD expression and maintains the muscle stem cell state of these cells (Mourikis et al., 2012; Czajkowski et al., 2014; reviewed in Mourikis and Tajbakhsh, 2014). However, in the trunk, the initial expression of Pax7 in the mouse dermomyotome is not controlled by a Notch-Delta lateral inhibition mechanism (Schuster-Gossler et al., 2007; Vasyutina et al., 2007). Similarly, the expression of Pax7 in the head mesoderm was not Delta-dependent (Czajkowski et al., 2014). Thus, additional parameters have to be considered for the establishment of the head muscle stem cell pool.
It is commonly held that at least in the amniote somite, all myogenic cells first express Pax3 and Pax7, the Pax genes are genetically and molecularly upstream of MyoD, and when MyoD expression commences, the pre-myogenic genes are downregulated (reviewed in Buckingham and Vincent, 2009). The same observation has been made in satellite cells where, upon asymmetric cell division, the cell set up to activate MyoD will switch off Pax7 and differentiate (Troy et al., 2012). Yet evidence is emerging that the linear progression from a Pax3/7+ state to a MyoD+ state is not obligatory: Lineage tracing and genetic cell ablations in the mouse have revealed that adult muscle stem cells have a history of Myf5, MyoD and Mrf4 expression, indicating that the expression of Mrfs that control the initial myogenic commitment does not prevent the maintenance of a stem cell state (Kanisicak et al., 2009; Biressi et al., 2013; Sambasivan et al., 2013; Wood et al., 2013). In anamniotes, the first cells to form contractile muscle do not express pax3/7 before undertaking myogenesis (reviewed in Bryson-Richardson and Currie, 2008), and in Xenopus, the pax7 lineage is established in a zone lateral to the somite that also expresses myoD (Daughters et al., 2011; Della Gaspera et al., 2012). In the mouse myoblast cell line C2C12, quiescent stem cells arise concomitant with contractile cells when the cells are cultured differentiation promoting medium (Yoshida et al., 1998), and when MyoD is misexpressed in P19 embryonic carcinoma cells, the gene activates pre-myogenic rather than myogenic genes, and does so directly (Gianakopoulos et al., 2011). Thus, evidence is accumulating that MyoD can act upstream of Pax7. Our data showed that in the mouse, cells with current and with a history of MyoD expression are situated in the same territory and arise well before the onset of Pax7. Importantly, the majority of Pax7 expressing cells develop from cells that previously expressed MyoD, and for the cells that do not display a history of MyoD expression, it cannot be excluded that thet expressed Myf5 before. Interestingly, the closest chordate relatives of vertebrates, the ascidians, develop cardiac and pharyngeal muscles from a bi-potential precursor in a similar fashion to vertebrates (Stolfi et al., 2010; Wang et al., 2013). In these animals, the pharyngeal muscle stem cells express the single mrf gene before some cells are set aside to become stem cells (Razy-Krajka et al., 2014). Thus, while more detailed lineage tracing will be required to fully elucidate this question; our data suggests that vertebrate head mesodermal cells similarly proceed through a phase of Mrf gene expression which sets the stage for the activation of Pax7 (a model is proposed in Figure 14).
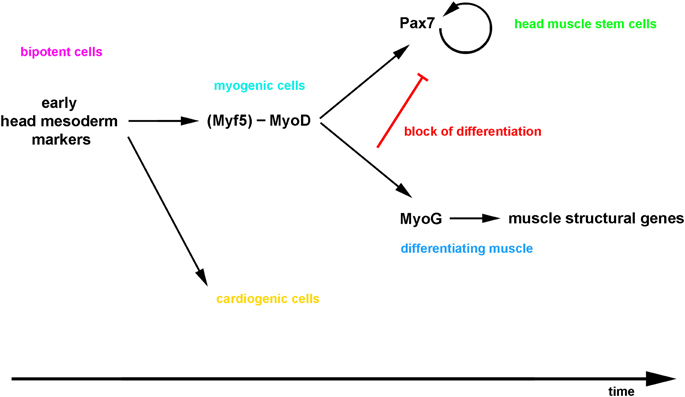
Figure 14. Proposed model for the emergence of craniofacial muscle stem cells: the bi-potential head mesodermal cells commit to myogenesis before adapting a muscle stem cell state, and then a lateral inhibition mechanism initiated by the differentiating cells controls the simultaneous production of functional muscle and the maintenance of the stem cell pool.
Circulatory pumps equipped with contractile cells—hearts—are widespread in the animal kingdom, and a conserved, core regulatory network involving Nk4/tinman-type transcription factors may already have been established in Cnidarians (Shimizu and Fujisawa, 2003). Likewise, skeletal muscle for locomotion, generated with the help of a MyoD-like basic-helix-loop-helix transcription factor, is widespread and may predate the evolution of bilaterians (Muller et al., 2003, but see also Steinmetz et al., 2012). Yet, typically cardiac and skeletal muscle lineages are exclusive. Vertebrates and their closest chordate relatives, the ascidians, have evolved a program that generates cells for the heart as well as skeletal muscle. However, this muscle is not used for locomotion but is associated with the function of the pharynx. The muscularisation of the pharynx has been seen as a key step during vertebrate evolution as it provided the basis for the active ventilation of gills and eventually, the evolution of jaws (Gans and Northcutt, 1983). A central component in this system is the Tbx1 gene (ascidians: single Tbx1/10 gene; (Stolfi et al., 2010). In the more distantly related cephalochordate Branchiostoma, the Tbx1/10 gene is expressed in the pharyngeal endoderm and mesoderm as well as the (ventral) somites (Mahadevan et al., 2004), while in the even further distant hemichordate Saccoglossus kowalevskii Tbx1/10 is only found in the pharyngeal endoderm and gill slits (Gillis et al., 2012). This suggests that during evolution of the ascidian-vertebrate ancestor, Tbx1/10 gene function has been linked both to the cardiac as well as the myogenic regulatory cascades. This implies that in contrast to the somite whose evolutionarily basic function is to generate skeletal muscle for locomotion, bi-potential pharyngeal cells have to commit to a myogenic fate before any muscle and muscle stem cells can be laid down. It is thus conceivable that, in the head, mesodermal cells have to express Myf5, MyoD, or Mrf4 before they can be set aside as a muscle stem cell.
Our work provides the basis for the–testable—hypothesis that head mesodermal cells have commit to myogenesis, and once this is achieved, cells can faithfully execute standard myogenic programs. In line with this, we have observed that Pax7 expressing head muscle stem cells provide the bulk of the head fetal muscles similar to muscle stem cells in the trunk (this study). Moreover, while the early head mesoderm is unable to provide muscle in a somitic environment (Mootoosamy and Dietrich, 2002), head muscle stem cells can shed their head mesodermal marker gene expression and regenerate trunk muscle (Sambasivan et al., 2009). Having established the emergence of head muscle stem cells, we can now explore the underlying molecular mechanisms and test, whether and in which environment head muscle stem cells can be redirected toward an earlier, bi-potential or a cardiogenic state.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are indebted to Roy Mootoosamy, Gudrun von Scheven, Ingo Bothe, Federica Berti, Samantha Maddison, Daniel Goodall, and Svenja Wöhrle for a helping hand with the in situ hybridisations and antibody stainings, and we thank Scott Rodaway, Kelsey Lewis, and Jennifer Lawson for setting up mouse matings, and Marco Aurélio Romano Silva, Rayan Silva de Paula and Erika Kelmer for the fish matings, and P. Gruss, S. Noji, T. Ogura, D. Srivastava, C. Redies, E. Olson, C. Tabin, A. Streit, R. Kelly, T. Braun, A. Fjose for providing in situ probes. We also would like to thank Frank Schubert, Lionel Chistiaen, Matt Guille, and Simon Hughes for most enjoyable and inspiring discussions, Matt Guille for critically reading the manuscript and Simon Hughes for sharing unpublished observations. The work was funded by a UoP start-up grant to SD, a travel grant for SD by the University of Utah, grant NICHD/NIH HS053728 to GK, grant NIH AR052777 to DJG, a SWB fellowship to JMN and a scholarship under the Silesian University of Technology program to KH.
Ahmed, M. U., Cheng, L., and Dietrich, S. (2006). Establishment of the epaxial-hypaxial boundary in the avian myotome. Dev. Dyn. 235, 1884–1894. doi: 10.1002/dvdy.20832
Alvares, L. E., Schubert, F. R., Thorpe, C., Mootoosamy, R. C., Cheng, L., Parkyn, G., et al. (2003). Intrinsic, Hox-dependent cues determine the fate of skeletal muscle precursors. Dev. Cell 5, 379–390. doi: 10.1016/S1534-5807(03)00263-6
Baker, C. V., Sharpe, C. R., Torpey, N. P., Heasman, J., and Wylie, C. C. (1995). A Xenopus c-kit-related receptor tyrosine kinase expressed in migrating stem cells of the lateral line system. Mech. Dev. 50, 217–228. doi: 10.1016/0925-4773(94)00338-N
Bandin, S., Morona, R., Moreno, N., and Gonzalez, A. (2013). Regional expression of Pax7 in the brain of Xenopus laevis during embryonic and larval development. Front. Neuroanat. 7:48. doi: 10.3389/fnana.2013.00048
Biressi, S., Bjornson, C. R., Carlig, P. M., Nishijo, K., Keller, C., and Rando, T. A. (2013). Myf5 expression during fetal myogenesis defines the developmental progenitors of adult satellite cells. Dev. Biol. 379, 195–207. doi: 10.1016/j.ydbio.2013.04.021
Bothe, I., and Dietrich, S. (2006). The molecular setup of the avian head mesoderm and its implication for craniofacial myogenesis. Dev. Dyn. 235, 2845–2860. doi: 10.1002/dvdy.20903
Bothe, I., Tenin, G., Oseni, A., and Dietrich, S. (2011). Dynamic control of head mesoderm patterning. Development 138, 2807–2821. doi: 10.1242/dev.062737
Braun, T., Bober, E., Buschhausen-Denker, G., Kohtz, S., Grzeschik, K. H., and Arnold, H. H. (1989). Differential expression of myogenic determination genes in muscle cells: possible autoactivation by the Myf gene products. EMBO J. 8, 3617–3625.
Bryson-Richardson, R. J., and Currie, P. D. (2008). The genetics of vertebrate myogenesis. Nat. Rev. Genet. 9, 632–646. doi: 10.1038/nrg2369
Buckingham, M., and Vincent, S. D. (2009). Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. 19, 444–453. doi: 10.1016/j.gde.2009.08.001
Camp, E., Dietrich, S., and Munsterberg, A. (2012). Fate mapping identifies the origin of SHF/AHF progenitors in the chick primitive streak. PLoS ONE 7:e51948. doi: 10.1371/journal.pone.0051948
Castellanos, R., Xie, Q., Zheng, D., Cvekl, A., and Morrow, B. E. (2014). Mammalian TBX1 preferentially binds and regulates downstream targets via a tandem T-site repeat. PLoS ONE 9:e95151. doi: 10.1371/journal.pone.0095151
Christophorou, N. A., Bailey, A. P., Hanson, S., and Streit, A. (2009). Activation of Six1 target genes is required for sensory placode formation. Dev. Biol. 336, 327–336. doi: 10.1016/j.ydbio.2009.09.025
Clack, J. A. (2002). Gaining Ground. The Origin and Evolution of Tetrapods. Bloomington, IN: Indiana University Press.
Collins, C. A., Gnocchi, V. F., White, R. B., Boldrin, L., Perez-Ruiz, A., Relaix, F., et al. (2009). Integrated functions of Pax3 and Pax7 in the regulation of proliferation, cell size and myogenic differentiation. PLoS ONE 4:e4475. doi: 10.1371/journal.pone.0004475
Couly, G. F., Coltey, P. M., and Le Douarin, N. M. (1992). The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development 114, 1–15.
Czajkowski, M. T., Rassek, C., Lenhard, D. C., Brohl, D., and Birchmeier, C. (2014). Divergent and conserved roles of Dll1 signaling in development of craniofacial and trunk muscle. Dev. Biol. 395, 307–316. doi: 10.1016/j.ydbio.2014.09.005
Daughters, R. S., Chen, Y., and Slack, J. M. (2011). Origin of muscle satellite cells in the Xenopus embryo. Development 138, 821–830. doi: 10.1242/dev.056481
Della Gaspera, B., Armand, A. S., Sequeira, I., Chesneau, A., Mazabraud, A., Lecolle, S., et al. (2012). Myogenic waves and myogenic programs during Xenopus embryonic myogenesis. Dev. Dyn. 241, 995–1007. doi: 10.1002/dvdy.23780
Diao, Y., Guo, X., Li, Y., Sun, K., Lu, L., Jiang, L., et al. (2012). Pax3/7BP is a Pax7- and Pax3-binding protein that regulates the proliferation of muscle precursor cells by an epigenetic mechanism. Cell Stem Cell 11, 231–241. doi: 10.1016/j.stem.2012.05.022
Diehl, A. G., Zareparsi, S., Qian, M., Khanna, R., Angeles, R., and Gage, P. J. (2006). Extraocular muscle morphogenesis and gene expression are regulated by Pitx2 gene dose. Invest. Ophthalmol. Vis. Sci. 47, 1785–1793. doi: 10.1167/iovs.05-1424
Dietrich, S., Abou-Rebyeh, F., Brohmann, H., Bladt, F., Sonnenberg-Riethmacher, E., Yamaai, T., et al. (1999). The role of SF/HGF and c-Met in the development of skeletal muscle. Development 126, 1621–1629.
Dietrich, S., Schubert, F. R., Healy, C., Sharpe, P. T., and Lumsden, A. (1998). Specification of the hypaxial musculature. Development 125, 2235–2249.
Dietrich, S., Schubert, F. R., and Lumsden, A. (1997). Control of dorsoventral pattern in the chick paraxial mesoderm. Development 124, 3895–3908.
Dong, F., Sun, X., Liu, W., Ai, D., Klysik, E., Lu, M. F., et al. (2006). Pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development 133, 4891–4899. doi: 10.1242/dev.02693
Emery, A. E. (2002). The muscular dystrophies. Lancet 359, 687–695. doi: 10.1016/S0140-6736(02)07815-7
Evans, B. J. (2008). Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana). Front. Biosci. 13, 4687–4706. doi: 10.2741/3033
Evans, B. J., Kelley, D. B., Tinsley, R. C., Melnick, D. J., and Cannatella, D. C. (2004). A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol. Phylogenet. Evol. 33, 197–213. doi: 10.1016/j.ympev.2004.04.018
Gans, C., and Northcutt, R. G. (1983). Neural crest and the origin of vertebrates: a new head. Science 220, 268–274. doi: 10.1126/science.220.4594.268
Garbern, J. C., and Lee, R. T. (2013). Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 12, 689–698. doi: 10.1016/j.stem.2013.05.008
Garg, V., Yamagishi, C., Hu, T., Kathiriya, I. S., Yamagishi, H., and Srivastava, D. (2001). Tbx1, a DiGeorge syndrome candidate gene, is regulated by sonic hedgehog during pharyngeal arch development. Dev. Biol. 235, 62–73. doi: 10.1006/dbio.2001.0283
Gianakopoulos, P. J., Mehta, V., Voronova, A., Cao, Y., Yao, Z., Coutu, J., et al. (2011). MyoD directly up-regulates premyogenic mesoderm factors during induction of skeletal myogenesis in stem cells. J. Biol. Chem. 286, 2517–2525. doi: 10.1074/jbc.M110.163709
Gillis, J. A., Fritzenwanker, J. H., and Lowe, C. J. (2012). A stem-deuterostome origin of the vertebrate pharyngeal transcriptional network. Proc. Biol. Sci. 279, 237–246. doi: 10.1098/rspb.2011.0599
Goulding, M. D., Lumsden, A., and Gruss, P. (1993). Signals from the notochord and floor plate regulate the region-specific expression of two Pax genes in the developing spinal cord. Development 117, 1001–1016. doi: 10.1016/0168-9525(93)90082-S
Grifone, R., Demignon, J., Giordani, J., Niro, C., Souil, E., Bertin, F., et al. (2007). Eya1 and Eya2 proteins are required for hypaxial somitic myogenesis in the mouse embryo. Dev. Biol. 302, 602–616. doi: 10.1016/j.ydbio.2006.08.059
Grifone, R., Demignon, J., Houbron, C., Souil, E., Niro, C., Seller, M. J., et al. (2005). Six1 and Six4 homeoproteins are required for Pax3 and Mrf expression during myogenesis in the mouse embryo. Development 132, 2235–2249. doi: 10.1242/dev.01773
Gros, J., Manceau, M., Thome, V., and Marcelle, C. (2005). A common somitic origin for embryonic muscle progenitors and satellite cells. Nature 435, 954–958. doi: 10.1038/nature03572
Guille, M. (1999). Microinjection into Xenopus oocytes and embryos. Methods Mol. Biol. 127, 111–123. doi: 10.1385/1-59259-678-9:111
Gurdon, J. B. (1977). Methods for nuclear transplantation in amphibia. Methods Cell Biol. 16, 125–139. doi: 10.1016/S0091-679X(08)60096-5
Hamburger, V., and Hamilton, H. L. (1951). A series of normal stages in the development of the chick embryo. J. Morph. 88, 49–92. doi: 10.1002/jmor.1050880104
Hammond, C. L., Hinits, Y., Osborn, D. P., Minchin, J. E., Tettamanti, G., and Hughes, S. M. (2007). Signals and myogenic regulatory factors restrict pax3 and pax7 expression to dermomyotome-like tissue in zebrafish. Dev. Biol. 302, 504–521. doi: 10.1016/j.ydbio.2006.10.009
Harel, I., Nathan, E., Tirosh-Finkel, L., Zigdon, H., Guimaraes-Camboa, N., Evans, S. M., et al. (2009). Distinct origins and genetic programs of head muscle satellite cells. Dev. Cell 16, 822–832. doi: 10.1016/j.devcel.2009.05.007
Harland, R. M. (1991). In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 36, 685–695. doi: 10.1016/S0091-679X(08)60307-6
Heanue, T. A., Reshef, R., Davis, R. J., Mardon, G., Oliver, G., Tomarev, S., et al. (1999). Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev. 13, 3231–3243. doi: 10.1101/gad.13.24.3231
Hebert, S. L., Daniel, M. L., and McLoon, L. K. (2013). The role of Pitx2 in maintaining the phenotype of myogenic precursor cells in the extraocular muscles. PLoS ONE 8:e58405. doi: 10.1371/journal.pone.0058405
Hinits, Y., Osborn, D. P., and Hughes, S. M. (2009). Differential requirements for myogenic regulatory factors distinguish medial and lateral somitic, cranial and fin muscle fibre populations. Development 136, 403–414. doi: 10.1242/dev.028019
Holland, L. Z., Schubert, M., Kozmik, Z., and Holland, N. D. (1999). AmphiPax3/7, an amphioxus paired box gene: insights into chordate myogenesis, neurogenesis, and the possible evolutionary precursor of definitive vertebrate neural crest. Evol. Dev. 1, 153–165. doi: 10.1046/j.1525-142x.1999.99019.x
Holland, P. W., Garcia-Fernandez, J., Williams, N. A., and Sidow, A. (1994). Gene duplications and the origins of vertebrate development. Dev. Suppl. 1994, 125–133.
Hughes, M. K., and Hughes, A. L. (1993). Evolution of duplicate genes in a tetraploid animal, Xenopus laevis. Mol. Biol. Evol. 10, 1360–1369.
Hutcheson, D. A., Zhao, J., Merrell, A., Haldar, M., and Kardon, G. (2009). Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for beta-catenin. Genes Dev. 23, 997–1013. doi: 10.1101/gad.1769009
Jostes, B., Walther, C., and Gruss, P. (1991). The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech. Dev. 33, 27–37. doi: 10.1016/0925-4773(90)90132-6
Kanisicak, O., Mendez, J. J., Yamamoto, S., Yamamoto, M., and Goldhamer, D. J. (2009). Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev. Biol. 332, 131–141. doi: 10.1016/j.ydbio.2009.05.554
Kassar-Duchossoy, L., Giacone, E., Gayraud-Morel, B., Jory, A., Gomes, D., and Tajbakhsh, S. (2005). Pax3/Pax7 mark a novel population of primitive myogenic cells during development. Genes Dev. 19, 1426–1431. doi: 10.1101/gad.345505
Kawabe, Y., Wang, Y. X., McKinnell, I. W., Bedford, M. T., and Rudnicki, M. A. (2012). Carm1 regulates Pax7 transcriptional activity through MLL1/2 recruitment during asymmetric satellite stem cell divisions. Cell Stem Cell 11, 333–345. doi: 10.1016/j.stem.2012.07.001
Kelly, R. G., Jerome-Majewska, L. A., and Papaioannou, V. E. (2004). The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum. Mol. Genet. 13, 2829–2840. doi: 10.1093/hmg/ddh304
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., and Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310. doi: 10.1002/aja.1002030302
Kitamura, K., Miura, H., Miyagawa-Tomita, S., Yanazawa, M., Katoh-Fukui, Y., Suzuki, R., et al. (1999). Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 126, 5749–5758.
Kragl, M., Knapp, D., Nacu, E., Khattak, S., Maden, M., Epperlein, H. H., et al. (2009). Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460, 60–65. doi: 10.1038/nature08152
Kusakabe, R., Kuraku, S., and Kuratani, S. (2011). Expression and interaction of muscle-related genes in the lamprey imply the evolutionary scenario for vertebrate skeletal muscle, in association with the acquisition of the neck and fins. Dev. Biol. 350, 217–227. doi: 10.1016/j.ydbio.2010.10.029
Lepper, C., Conway, S. J., and Fan, C. M. (2009). Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627–631. doi: 10.1038/nature08209
Lin, C. Y., Yung, R. F., Lee, H. C., Chen, W. T., Chen, Y. H., and Tsai, H. J. (2006). Myogenic regulatory factors Myf5 and Myod function distinctly during craniofacial myogenesis of zebrafish. Dev. Biol. 299, 594–608. doi: 10.1016/j.ydbio.2006.08.042
Lours, C., and Dietrich, S. (2005). The dissociation of the Fgf-feedback loop controls the limbless state of the neck. Development 132, 5553–5564. doi: 10.1242/dev.02164
Lu, J. R., Bassel-Duby, R., Hawkins, A., Chang, P., Valdez, R., Wu, H., et al. (2002). Control of facial muscle development by MyoR and capsulin. Science 298, 2378–2381. doi: 10.1126/science.1078273
Maczkowiak, F., Mateos, S., Wang, E., Roche, D., Harland, R., and Monsoro-Burq, A. H. (2010). The Pax3 and Pax7 paralogs cooperate in neural and neural crest patterning using distinct molecular mechanisms, in Xenopus laevis embryos. Dev. Biol. 340, 381–396. doi: 10.1016/j.ydbio.2010.01.022
Mahadevan, N. R., Horton, A. C., and Gibson-Brown, J. J. (2004). Developmental expression of the amphioxus Tbx1/ 10 gene illuminates the evolution of vertebrate branchial arches and sclerotome. Dev. Genes Evol. 214, 559–566. doi: 10.1007/s00427-004-0433-1
Moncaut, N., Cross, J. W., Siligan, C., Keith, A., Taylor, K., Rigby, P. W., et al. (2012). Musculin and TCF21 coordinate the maintenance of myogenic regulatory factor expression levels during mouse craniofacial development. Development 139, 958–967. doi: 10.1242/dev.068015
Mootoosamy, R. C., and Dietrich, S. (2002). Distinct regulatory cascades for head and trunk myogenesis. Development 129, 573–583.
Mourikis, P., Gopalakrishnan, S., Sambasivan, R., and Tajbakhsh, S. (2012). Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development 139, 4536–4548. doi: 10.1242/dev.084756
Mourikis, P., and Tajbakhsh, S. (2014). Distinct contextual roles for Notch signalling in skeletal muscle stem cells. BMC Dev. Biol. 14:2. doi: 10.1186/1471-213X-14-2
Muller, P., Seipel, K., Yanze, N., Reber-Muller, S., Streitwolf-Engel, R., Stierwald, M., et al. (2003). Evolutionary aspects of developmentally regulated helix-loop-helix transcription factors in striated muscle of jellyfish. Dev. Biol. 255, 216–229. doi: 10.1016/S0012-1606(02)00091-X
Nieuwkoop, P. D., and Faber, J. (1994). Normal Table of Xenopus laevis (Daudin). New York, NY: Garland Publishing Inc.
Noden, D. M. (1983). The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am. J. Anat. 168, 257–276. doi: 10.1002/aja.1001680302
Noden, D. M., and Francis-West, P. (2006). The differentiation and morphogenesis of craniofacial muscles. Dev. Dyn. 235, 1194–1218. doi: 10.1002/dvdy.20697
Noden, D. M., Marcucio, R., Borycki, A. G., and Emerson, C. P. Jr. (1999). Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev. Dyn. 216, 96–112.
Ohno, S., Wolf, U., and Atkin, N. B. (1968). Evolution from fish to mammals by gene duplication. Hereditas 59, 169–187. doi: 10.1111/j.1601-5223.1968.tb02169.x
Ono, Y., Boldrin, L., Knopp, P., Morgan, J. E., and Zammit, P. S. (2010). Muscle satellite cells are a functionally heterogeneous population in both somite-derived and branchiomeric muscles. Dev. Biol. 337, 29–41. doi: 10.1016/j.ydbio.2009.10.005
Pajcini, K. V., Corbel, S. Y., Sage, J., Pomerantz, J. H., and Blau, H. M. (2010). Transient inactivation of Rb and ARF yields regenerative cells from postmitotic mammalian muscle. Cell Stem Cell 7, 198–213. doi: 10.1016/j.stem.2010.05.022
Penn, B. H., Bergstrom, D. A., Dilworth, F. J., Bengal, E., and Tapscott, S. J. (2004). A MyoD-generated feed-forward circuit temporally patterns gene expression during skeletal muscle differentiation. Genes Dev. 18, 2348–2353. doi: 10.1101/gad.1234304
Postlethwait, J. H. (2007). The zebrafish genome in context: ohnologs gone missing. J. Exp. Zool. B Mol. Dev. Evol. 308, 563–577. doi: 10.1002/jez.b.21137
Razy-Krajka, F., Lam, K., Wang, W., Stolfi, A., Joly, M., Bonneau, R., et al. (2014). Collier/OLF/EBF-dependent transcriptional dynamics control pharyngeal muscle specification from primed cardiopharyngeal progenitors. Dev. Cell 29, 263–276. doi: 10.1016/j.devcel.2014.04.001
Relaix, F., Demignon, J., Laclef, C., Pujol, J., Santolini, M., Niro, C., et al. (2013). Six homeoproteins directly activate Myod expression in the gene regulatory networks that control early myogenesis. PLoS Genet. 9:e1003425. doi: 10.1371/journal.pgen.1003425
Relaix, F., Montarras, D., Zaffran, S., Gayraud-Morel, B., Rocancourt, D., Tajbakhsh, S., et al. (2006). Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 172, 91–102. doi: 10.1083/jcb.200508044
Relaix, F., Rocancourt, D., Mansouri, A., and Buckingham, M. (2005). A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature 435, 948–953. doi: 10.1038/nature03594
Relaix, F., and Zammit, P. S. (2012). Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139, 2845–2856. doi: 10.1242/dev.069088
Rosenberg, P., Esni, F., Sjodin, A., Larue, L., Carlsson, L., Gullberg, D., et al. (1997). A potential role of R-cadherin in striated muscle formation. Dev. Biol. 187, 55–70. doi: 10.1006/dbio.1997.8602
Sambasivan, R., Comai, G., Le Roux, I., Gomes, D., Konge, J., Dumas, G., et al. (2013). Embryonic founders of adult muscle stem cells are primed by the determination gene Mrf4. Dev. Biol. 381, 241–255. doi: 10.1016/j.ydbio.2013.04.018
Sambasivan, R., Gayraud-Morel, B., Dumas, G., Cimper, C., Paisant, S., Kelly, R., et al. (2009). Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev. Cell 16, 810–821. doi: 10.1016/j.devcel.2009.05.008
Sambasivan, R., Kuratani, S., and Tajbakhsh, S. (2011). An eye on the head: the development and evolution of craniofacial muscles. Development 138, 2401–2415. doi: 10.1242/dev.040972
Schienda, J., Engleka, K. A., Jun, S., Hansen, M. S., Epstein, J. A., Tabin, C. J., et al. (2006). Somitic origin of limb muscle satellite and side population cells. Proc. Natl. Acad. Sci. U.S.A. 103, 945–950. doi: 10.1073/pnas.0510164103
Schilling, T. F., and Kimmel, C. B. (1997). Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development 124, 2945–2960.
Schmidt, J., Piekarski, N., and Olsson, L. (2013). Cranial muscles in amphibians: development, novelties and the role of cranial neural crest cells. J. Anat. 222, 134–146. doi: 10.1111/j.1469-7580.2012.01541.x
Schuster-Gossler, K., Cordes, R., and Gossler, A. (2007). Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proc. Natl. Acad. Sci. U.S.A. 104, 537–542. doi: 10.1073/pnas.0608281104
Seale, P., Sabourin, L. A., Girgis-Gabardo, A., Mansouri, A., Gruss, P., and Rudnicki, M. A. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777–786. doi: 10.1016/S0092-8674(00)00066-0
Seo, H. C., Saetre, B. O., Havik, B., Ellingsen, S., and Fjose, A. (1998). The zebrafish Pax3 and Pax7 homologues are highly conserved, encode multiple isoforms and show dynamic segment-like expression in the developing brain. Mech. Dev. 70, 49–63. doi: 10.1016/S0925-4773(97)00175-5
Shimizu, H., and Fujisawa, T. (2003). Peduncle of Hydra and the heart of higher organisms share a common ancestral origin. Genesis 36, 182–186. doi: 10.1002/gene.10213
Somorjai, I. M., Somorjai, R. L., Garcia-Fernandez, J., and Escriva, H. (2012). Vertebrate-like regeneration in the invertebrate chordate amphioxus. Proc. Natl. Acad. Sci. U.S.A. 109, 517–522. doi: 10.1073/pnas.1100045109
Šošic, D., Brand-Saberi, B., Schmidt, C., Christ, B., and Olson, E. N. (1997). Regulation of paraxis expression and somite formation by ectoderm- and neural tube-derived signals. Dev. Biol. 185, 229–243. doi: 10.1006/dbio.1997.8561
Steinmetz, P. R., Kraus, J. E., Larroux, C., Hammel, J. U., Amon-Hassenzahl, A., Houliston, E., et al. (2012). Independent evolution of striated muscles in cnidarians and bilaterians. Nature 487, 231–234. doi: 10.1038/nature11180
Stolfi, A., Gainous, T. B., Young, J. J., Mori, A., Levine, M., and Christiaen, L. (2010). Early chordate origins of the vertebrate second heart field. Science 329, 565–568. doi: 10.1126/science.1190181
Summerbell, D., Halai, C., and Rigby, P. W. (2002). Expression of the myogenic regulatory factor Mrf4 precedes or is contemporaneous with that of Myf5 in the somitic bud. Mech. Dev. 117, 331–335. doi: 10.1016/S0925-4773(02)00208-3
Takahashi, M., Tamura, K., Buscher, D., Masuya, H., Yonei-Tamura, S., Matsumoto, K., et al. (1998). The role of Alx-4 in the establishment of anteroposterior polarity during vertebrate limb development. Development 125, 4417–4425.
Thisse, C., and Thisse, B. (2008). High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 3, 59–69. doi: 10.1038/nprot.2007.514
Troy, A., Cadwallader, A. B., Fedorov, Y., Tyner, K., Tanaka, K. K., and Olwin, B. B. (2012). Coordination of satellite cell activation and self-renewal by Par-complex-dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell 11, 541–553. doi: 10.1016/j.stem.2012.05.025
Vasyutina, E., Lenhard, D. C., Wende, H., Erdmann, B., Epstein, J. A., and Birchmeier, C. (2007). RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proc. Natl. Acad. Sci. U.S.A. 104, 4443–4448. doi: 10.1073/pnas.0610647104
von Maltzahn, J., Jones, A. E., Parks, R. J., and Rudnicki, M. A. (2013). Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 110, 16474–16479. doi: 10.1073/pnas.1307680110
von Scheven, G., Bothe, I., Ahmed, M. U., Alvares, L. E., and Dietrich, S. (2006). Protein and genomic organisation of vertebrate MyoR and Capsulin genes and their expression during avian development. Gene Expr. Patterns 6, 383–393. doi: 10.1016/j.modgep.2005.09.008
Wang, W., Razy-Krajka, F., Siu, E., Ketcham, A., and Christiaen, L. (2013). NK4 antagonizes Tbx1/10 to promote cardiac versus pharyngeal muscle fate in the ascidian second heart field. PLoS Biol. 11:e1001725. doi: 10.1371/journal.pbio.1001725
Weintraub, H., Tapscott, S. J., Davis, R. L., Thayer, M. J., Adam, M. A., Lassar, A. B., et al. (1989). Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc. Natl. Acad. Sci. U.S.A. 86, 5434–5438. doi: 10.1073/pnas.86.14.5434
Wilson-Rawls, J., Hurt, C. R., Parsons, S. M., and Rawls, A. (1999). Differential regulation of epaxial and hypaxial muscle development by paraxis. Development 126, 5217–5229.
Wood, W. M., Etemad, S., Yamamoto, M., and Goldhamer, D. J. (2013). MyoD-expressing progenitors are essential for skeletal myogenesis and satellite cell development. Dev. Biol. 384, 114–127. doi: 10.1016/j.ydbio.2013.09.012
Yamamoto, M., Shook, N. A., Kanisicak, O., Yamamoto, S., Wosczyna, M. N., Camp, J. R., et al. (2009). A multifunctional reporter mouse line for Cre- and FLP-dependent lineage analysis. Genesis 47, 107–114. doi: 10.1002/dvg.20474
Yoshida, N., Yoshida, S., Koishi, K., Masuda, K., and Nabeshima, Y. (1998). Cell heterogeneity upon myogenic differentiation: down-regulation of MyoD and Myf-5 generates ‘reserve cells’. J. Cell Sci. 111(Pt 6), 769–779.
Yoshioka, H., Meno, C., Koshiba, K., Sugihara, M., Itoh, H., Ishimaru, Y., et al. (1998). Pitx2, a bicoid-type homeobox gene, is involved in a lefty-signaling pathway in determination of left-right asymmetry. Cell 94, 299–305. doi: 10.1016/S0092-8674(00)81473-7
Zacharias, A. L., Lewandoski, M., Rudnicki, M. A., and Gage, P. J. (2011). Pitx2 is an upstream activator of extraocular myogenesis and survival. Dev. Biol. 349, 395–405. doi: 10.1016/j.ydbio.2010.10.028
Keywords: head muscle, muscle stem cells, Pax7, chicken, mouse, Xenopus, zebrafish, vertebrate embryo
Citation: Meireles Nogueira J, Hawrot K, Sharpe C, Noble A, Wood WM, Jorge EC, Goldhamer DJ, Kardon G and Dietrich S (2015) The emergence of Pax7-expressing muscle stem cells during vertebrate head muscle development. Front. Aging Neurosci. 7:62. doi: 10.3389/fnagi.2015.00062
Received: 12 January 2015; Accepted: 10 April 2015;
Published: 19 May 2015.
Edited by:
Ander Izeta, Instituto Investigación Sanitaria Biodonostia, SpainReviewed by:
Jaime J. Carvajal, Centro Andaluz de Biología del Desarrollo, SpainCopyright © 2015 Meireles Nogueira, Hawrot, Sharpe, Noble, Wood, Jorge, Goldhamer, Kardon and Dietrich. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susanne Dietrich, School of Pharmacy and Biomedical Sciences, Institute for Biomedical and Biomolecular Science, University of Portsmouth, St. Michael's Building, White Swan Road, Portsmouth PO1 2DT, UK,c3VzYW5uZS5kaWV0cmljaEBwb3J0LmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.