
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 02 April 2015
Sec. Neurocognitive Aging and Behavior
Volume 7 - 2015 | https://doi.org/10.3389/fnagi.2015.00043
This article is part of the Research Topic The Metabolic-Inflammatory Axis in Brain Aging and Neurodegeneration View all 14 articles
A substantial number of studies on basal forebrain (BF) cholinergic neurons (BFCN) have provided compelling evidence for their role in the etiology of stress, cognitive aging, Alzheimer’s disease (AD), and other neurodegenerative diseases. BFCN project to a broad range of cortical sites and limbic structures, including the hippocampus, and are involved in stress and cognition. In particular, the hippocampus, the primary target tissue of the glucocorticoid stress hormones, is associated with cognitive function in tandem with hypothalamic-pituitary-adrenal (HPA) axis modulation. The present review summarizes glucocorticoid and HPA axis research to date in an effort to establish the manner in which stress affects the release of acetylcholine (ACh), glucocorticoids, and their receptor in the context of cognitive processes. We attempt to provide the molecular interactive link between the glucocorticoids and cholinergic system that contributes to BFCN degeneration in stress-induced acceleration of cognitive decline in aging and AD. We also discuss the importance of animal models in facilitating such studies for pharmacological use, to which could help decipher disease states and propose leads for pharmacological intervention.
Since its inception, the cholinergic hypothesis has generated considerable interest. It has been used to decipher the different orchestrating functions and dysfunctions in the nervous system associated with Alzheimer’s disease (AD) and other neurodegenerative diseases. This hypothesis has been used to further understand cognitive impairment and neurodegenerative diseases by evaluating and tracing brain functions in normal and aging brains (Gallagher and Colombo, 1995; Contestabile, 2011). The main components of the cholinergic pathway are: (1) the neurotransmitter acetylcholine (ACh); (2) acetylcholinesterase (AChE), which breaks down ACh; (3) choline acetyltransferase, an enzyme that synthesizes ACh; and (4) ACh receptors, specifically the nicotinic ACh receptor, and the muscarinic ACh receptor (mAChR). Evidence from previous research on normal aging (Drachman et al., 1982), AD (Whitehouse et al., 1982), and anti-cholinergic (Newhouse et al., 1988, 1994) and pro-cholinergic drug administration (Davis and Mohs, 1982) supports the major role of the cholinergic system in aged-related cognitive decline. Extensive research has established the relationship between cognitive impairment and the cholinergic system in the basal forebrain (BF; Baxter and Chiba, 1999). The involvement of the cholinergic system in regulating stress is also evident from studies that acute/inescapable stress enhanced release of ACh and induced expression of genes that regulate ACh availability in the hippocampus and prefrontal cortex (Mark et al., 1996; Kaufer et al., 1998). Cognitive processes are influenced by the acute and chronic stress-induced release of glucocorticoids, stress hormones that influence the function of the prefrontal cortex and hippocampus (Popoli et al., 2011). Stress and stress hormone plays a well-established role in mental health and impaired cognition. It is correlated with hippocampal volume and age-related cognitive decline (Lupien et al., 1994, 2009), suggesting that sustained stress, via glucocorticoid hypersecretion, leads to hippocampal damage (Uno et al., 1989). Prolonged overproduction of glucocorticoids can be detrimental to brain structure, whereas insufficient glucocorticoid signaling can lead to stress-related pathological conditions (Raison and Miller, 2003). This emphasizes the need for the careful regulation of glucocorticoid exposure. Impairment of the hypothalamic-pituitary-adrenal (HPA) axis in response to stress is also associated with cognitive dysfunction in aged animals (Issa et al., 1990; Bizon et al., 2001). In addition, HPA activity was blunt in elderly compared to young adult participants (Hatzinger et al., 2011). Therefore, new therapeutic approaches acting on the HPA axis and its receptor signaling should take into account.
Activation of the septo-hippocampal cholinergic system is considered as an important aspect in the adaptive response to stress and is influenced by neuronal and hormonal stimuli. This septo-hippocampal activation seems to initialize following activation of the pituitary-adrenocortical axis (Gilad et al., 1985; Gilad, 1987) and may then affect glucocorticoid secretion via the HPA axis (Herman et al., 1996). The HPA axis plays an important role in the adaptation to stress by modulating hippocampal activity. Thus, the hippocampus, along with cholinergic innervation from the BF, is involved in regulating the HPA axis stress response. Activation of the HPA axis mediates responses that enable an organism to maintain its homeostasis. Hence, neurodegeneration of cholinergic neurons, a pathological characteristic in AD and aging, makes the elderly vulnerable to stress, resulting in cognitive impairment.
The extent to which the cholinergic system is involved in stress, cognition, and neurological disorders has been reported in several significant research reports. Therefore, we attempt to develop a supporting background for our recent studies in rats (aged or with cholinergic lesions) on HPA axis dysfunction in response to stress and altered glucocorticoid receptor (GR) signaling in the hippocampus. We also describe the interactive link between the glucocorticoid and cholinergic systems in aging and stress.
An anatomical simplified overview of BF cholinergic neurons is summarized in Figure 1, including cells located in the medial septum (MS), the vertical limb of the diagonal band of Broca (VDB), and the nucleus basalis of Meynert–extending to the substantia innominata in the rodent brain. These structures send cholinergic projections to a broad range of neocortical sites as well as structures in the limbic system, including the hippocampus (Mesulam et al., 1983; Gallagher and Colombo, 1995). Thus, the septo-hippocampal pathway, which arises from the medial septal nucleus and nucleus of the diagonal band, is the main structure of the central cholinergic system and the main source of cholinergic innervation to the hippocampal formation. A topographical model of the septo-hippocampal pathway has been constructed based on various lesion, tracing, and immunocytochemical methods in the septal and hippocampal regions (Lewis et al., 1967; Dutar et al., 1995). The MS is connected to the hippocampus, via the fimbria and dorsal fornix, and to the medial cortex (Lewis and Shute, 1967; Teles-Grilo Ruivo and Mellor, 2013). The cornu ammonis (CA) 1 pyramidal and dentate granule (DG) cell layers in the dorsal hippocampus receive afferent inputs from the VDB, and these cell layers in the ventral hippocampus receive inputs from the both MS and VDB (McKinney et al., 1983; Nyakas et al., 1987).
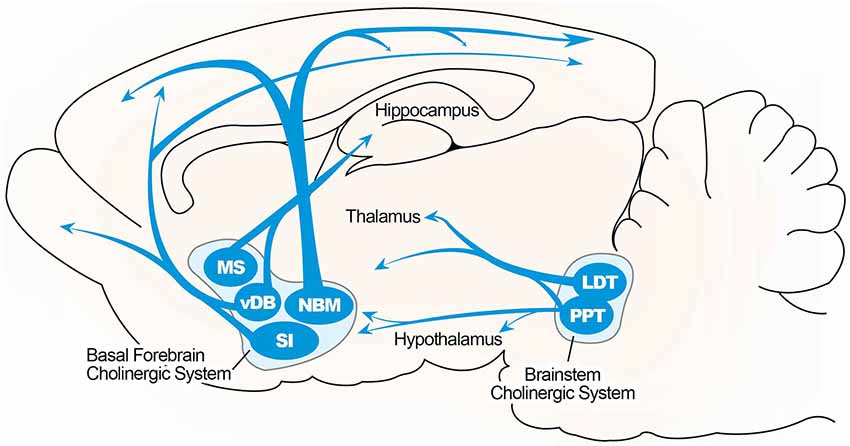
Figure 1. Illustrated overview of the basal forebrain cholinergic pathway. Cholinergic projections include the medial septum (MS), vertical limbs of the diagonal band of Broca (vDB), nucleus basalis of Meynert (NBM), and substantia innominate (SI) projecting to the hippocampus, thalamus, olfactory bulb, and cortical region. Cholinergic pontomesencephalon neurons include laterodorsal tegmental (LDT) and pedunculopontine tegmental nuclei (PPT) projecting to hindbrain, thalamus, hypothalamus, and basal forebrain.
The cholinergic hypothesis has been implicated in the etiology of AD, various types of dementia, and aging, and is rooted in degeneration of BF cholinergic neurons causing cognitive deficit (Bartus et al., 1982; Bartus, 2000; Sarter et al., 2003). This theory, however, remains controversial. Although enhancement of cholinergic function by cholinergic agents (e.g., AChE inhibitors) in AD and age-related cognitive deficit supported the hypothesis, other research subsequently pinpointed the involvement of other factors, such as dopamine projections to the frontal cortex, amyloid deposition, and increased glucocorticoid levels (Dumas and Newhouse, 2011). More specifically, hypersecretion of glucocorticoid, or A-beta-altered HPA axis function, have been implicated in hippocampal impairment in AD (Hibberd et al., 2000; Brureau et al., 2013) Additionally, elevated glucocorticoid levels and impaired GR signaling are associated with HPA dysfunction, resulting in cognitive decline in elderly subjects (Issa et al., 1990; Lupien et al., 1994; Bizon et al., 2001; Mizoguchi et al., 2009). Therefore, a possible pathophysiological link between glucocorticoids and the age-dependent decline in BF cholinergic function, especially in the CA1, CA3, and DG regions of hippocampus, has also been established (Hörtnagl et al., 1993). Craig et al. postulated a new cholinergic hypothesis version for AD, where loss of MS cholinergic input to the hippocampus induces hippocampal vulnerability, resulting in greater cognitive impairment in response to subsequent insults, such as stress or injury (Craig et al., 2011).
Application of the cholinergic hypothesis to animal model offers the ability to evaluate functional network and molecular pathway to understand neurocognitive diseases. Selection of animal (even among rodents) is important, as they differ in cholinergic tone, receptor activation, and relevance to human basal cholinergic activity (Van der Zee and Keijser, 2011). The adoption of the rodent as an animal model may compliment studies in primates due to effectiveness and cost-efficiency. Moreover, features such as short lifespans are an important factor to be considered in the study of late-onset or aging diseases (Gallagher et al., 2011). One major pathological hallmark of neurodegenerative disease that cause cognitive decline, is the dramatic loss of BF cholinergic projection neurons with reduced cholinergic innervation to the hippocampus and neocortex (Davies and Maloney, 1976; Whitehouse et al., 1981; Arendt et al., 1983; Mesulam, 2004). Additionally, the relative loss of cholinergic neurons and the decrease of the ACh synthesizing enzyme, choline acetyltransferase in the brain of AD patients is associated with cognitive impairment (Bartus, 2000). In animal studies, neurotoxin-induced BF lesions cause similar cognitive impairments (Olton, 1990). However, lesioning cholinergic BF neurons is challenging because they are intertwined with non-cholinergic neurons and there is a risk of damaging adjacent structures.
Although BF lesions using conventional lesion methods (e.g., electrolytic) produce varying manifestations of cognitive impairment, the development of methods that selectively interrupt the BF region has been attempted (Easton et al., 2012; Baxter and Bucci, 2013). An initial approach innovatively used 192IgG-saporin, a neurotoxin with a specific affinity for cholinergic neuron cell surface receptors (Wiley et al., 1991; Baxter and Bucci, 2013). A myriad of studies picked the hippocampal and septo-hippocampal regions of the BF as lesion sites to evaluate the cholinergic interventions observed in aging and cognition. Studies with selective BF neurotoxic lesion concluded this region was associated with cognitive function characterized by attention (Muir et al., 1993; Baxter et al., 1997, 1999; Bucci et al., 1998; Chiba et al., 1999; Chudasama et al., 2004), and learning and memory (Hepler et al., 1985; Hagan et al., 1988; Berger-Sweeney et al., 1994; Baxter et al., 1995; Janisiewicz et al., 2004). Furthermore, a significant correlation between cognitive impairment and decline in cholinergic markers for the septo-hippocampal projection in aged rats (Gallagher et al., 1990; Smith and Booze, 1995) supports the involvement of BF cholinergic neurons in cognition. More recently, animals with a saporin-induced partial loss of septo-hippocampal cholinergic neurons exhibited cognitive deficit (Brayda-Bruno et al., 2013). Other lesion studies extended the importance of the BF cholinergic system in the process of functional recovery from brain injury in young rats (Conner et al., 2005), which is intriguing in that animals with BF lesions may show deficits in cognitive function. Selective lesion of cholinergic inputs to the hippocampus has also been used to evaluate the effects of cholinergic receptors in regulating hippocampal ACh release (Thorne and Potter, 1995).
Though lesion studies have generated substantial controversy, the advent of this approach has generated new empirical tests and compelling information on the potential function of cholinergic and non-cholinergic neurons emanating from the BF. Additionally, this has unveiled the possible role played by loss of these neurons in stress, aging, and other neurocognitive diseases. More essentially, animal models are indispensable for recreating specific human pathogenic events, and invaluable for drug screening and therapeutic intervention assessment.
The HPA axis is an integral component in promoting resilience to stress. Most salient age-related changes in the stress response are thought to occur in the HPA axis. The HPA system is classically controlled by a range of afferent signals responsible for the coordinated release of various signaling markers. The hypothalamus is at the top of the hierarchy in the control of the central HPA axis. When the brain perceives a stressor, activation of the paraventricular nucleus of the hypothalamus triggers the release of corticotropin-releasing hormone. Corticotropin-releasing hormone stimulates pituitary adrenocorticotropic hormone (ACTH) release and activates the adrenal gland, which secretes glucocorticoids (cortisol in humans and corticosterone in rodents). Glucocorticoids released from the adrenal gland interact with the HPA axis by binding to specific GRs in the brain, forming a closed-loop feedback system and subsequently coping with stress (Sapolsky et al., 1986; Mizuno and Kimura, 1997; Srinivasan et al., 2013). Thus, glucocorticoids are the end product of the HPA axis and regulate a wide array of actions influencing neuronal function and metabolism (Figure 2).
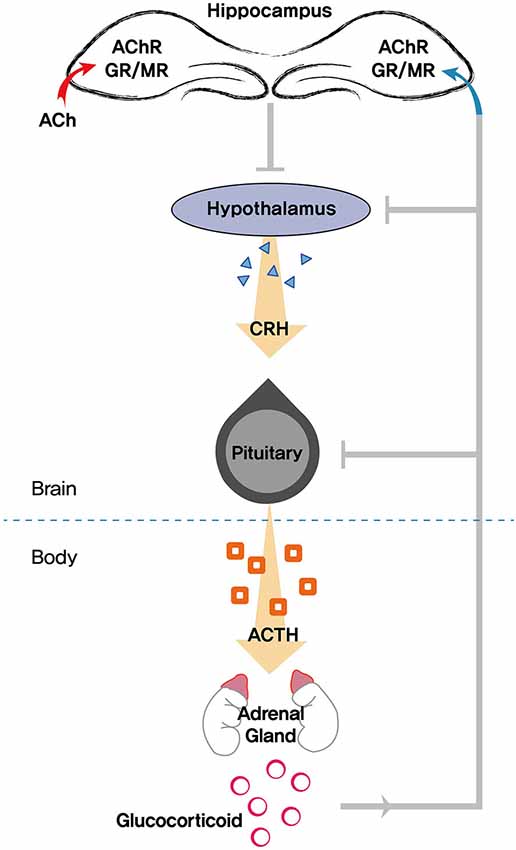
Figure 2. Schematic diagram showing the role of the hippocampus in modulating the HPA (hypothalamic pituitary adrenocortical) axis. Stress triggers the release of glucocorticoids, which exerts feedback to the hippocampus, hypothalamus, and pituitary. Acetylcholine (ACh) is also involved in mediating neuroendocrine, emotional, and physiological responses in tandem with the HPA axis. AChR, acetylcholine receptors; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone.
Two types of steroid hormone receptors have been identified in tandem in the brain: the mineralocorticoid receptor (MR) and the GR. Expression of MR is considerably more restricted in the brain compared to the ubiquitous expression of GR, and both mediate classical genomic and non-genomic glucocorticoid actions by acting as nuclear transcriptional activators and repressors (Reul and de Kloet, 1985; Joëls, 2008; van der Laan and Meijer, 2008; Groeneweg et al., 2011). Moreover, the secretion of glucocorticoids and their binding to receptors leads to auto-regulatory decreases in receptor availability and vice versa (Sapolsky et al., 1984; Reul et al., 1987a,b). Actions of GR described above extends the role of GR beyond mediating glucocorticoid feedback following stress, whereas MR participates in basal HPA tone (Reul et al., 1987a; De Kloet et al., 1998) and the genomic action in adaptation and homeostasis after stress exposure (de Kloet, 2008). After GR binding, these activated receptors are translocated to the nucleus where they bind to the glucocorticoid responsive elements (GRE) and affect the transcriptional activity of target genes (Funder, 1997). Stress causes a down-regulation of GR through the increase of circulating glucocorticoids, eventually decreasing sensitivity to nuclear transcriptional activities. Glucocorticoid-GR can also activate transcription by binding directly as a homodimer to the GRE DNA sequence present in the promotors of target genes (e.g., serum- and glucocorticoid-induced protein kinase, mitogen-activated protein kinase phosphatase-1, etc) that has been reported to regulate cognitive function (Lee et al., 2007; Vyas and Maatouk, 2013; Cestari et al., 2014).
HPA activity tends to increase with age due to inefficiencies of glucocorticoid negative-feedback inhibition, resulting in elevated plasma levels of ACTH and glucocorticoids (Mizoguchi et al., 2009; Aguilera, 2011). A decrease in glucocorticoid negative-feedback inhibition is associated with the loss of GR and altered GR signaling in the forebrain (Issa et al., 1990; Bizon et al., 2001; Lund et al., 2004; Lee et al., 2012). Increased levels of glucocorticoids appear to threaten hippocampal neurons and direct the loss of dendrite complexity in the hippocampus (Hibberd et al., 2000). Inappropriate HPA axis regulation in aging is reviewed by Aguilera (2011) who stresses its adverse effect in stress-related brain disorders.
A link between HPA deregulation and disruption of GR has been observed in AD and other neurodegenerative diseases (Brureau et al., 2013; Vyas and Maatouk, 2013). Interestingly, though extra-hypothalamic GR sites receive attention for this glucocorticoid-mediated HPA inhibitory feedback; the hippocampus receives the utmost attention with regards to these effects (McEwen et al., 1968, 1969; Sapolsky et al., 1984, 1986; Herman et al., 1989). The influence of glucocorticoids on the HPA axis markedly depends on the available GR in individual tissues (Simons, 2008). Abundant expression of GR in the forebrain (McEwen et al., 1969; McEwen and Wallach, 1973), and increased activity of the HPA axis in the absence of forebrain inhibition on HPA axis by damage of the forebrain (Jacobson and Sapolsky, 1991; van Haarst et al., 1996), contributes to the sequelae associated with GR malfunction. Taken together, forebrain GR expression is critical for HPA axis regulation in response to stress (Furay et al., 2008) and further adduces the role of GR in stress.
A further increase in ACh release was observed in the hippocampus after acute stress (Finkelstein et al., 1985; Gilad et al., 1985; Imperato et al., 1989). Corticosterone administration, mimicking the increase in the plasma corticosterone concentration produced by stress, induced hippocampal ACh (Imperato et al., 1989). These studies support the involvement of glucocorticoids in the cholinergic innervation of the hippocampus, and the activation of the HPA axis in the process. Stress-induced responses activated the septo-hippocampal cholinergic pathway within minutes (Gilad, 1987), which then induced ACh-mediated neuroendocrine, emotional, and physiological responses by stimulating the HPA axis (Newman et al., 2001). This HPA axis activation led to the release of corticosterone, a stress neurohormone (Nyakas et al., 1987; Calogero et al., 1988, 1990). Increased release of hippocampal ACh and glucocorticoids in response to stress was observed in young rats, but not in aged rats (Mizuno and Kimura, 1997). However, contradictory results have been reported. Proteomic analyses of the hippocampus of rats exposed to stress showed a decrease in a precursor protein of hippocampal cholinergic neurostimulating peptide (HCNP) leading to a loss of ACh production (Kim and Kim, 2007). HCNP stimulate the enzyme activity of choline acetyltransferase in neurons. It is also reported that expression levels of HCNP precursor protein mRNA were decreased in the hippocampus of AD patients (Maki et al., 2002). These reports indicate a possible intricate interplay between the level of glucocorticoids, ACh, and hippocampal cholinergic protein expression effecting septo-hippocampal cholinergic pathways.
Interestingly, a parallel increase in both plasma corticosterone and hippocampal ACh level has been validated as a consequence of elevated platform exposure, a relatively mild stress (Degroot et al., 2004). The interaction of glucocorticoid with ACh in the brain is well reviewed (Mora et al., 2012). Moreover, regulation of the HPA axis by glucocorticoid feedback and cholinergic brain function modulating stress responses depends on the intensity and predictability of stressful stimuli (Pitman et al., 1988; Martí and Armario, 1997; Morris and Rao, 2014).
Although ACh mediates its effects via both types of ACh receptors, mAChR are more involved in cognitive impairment and are densely present in the hippocampus (Dutar et al., 1995; Colgin et al., 2003; Drever et al., 2011). An excessive loss of mAChR in the hippocampus of Alzheimer’s patients, and severely impaired muscarinic signaling associated with age-related cognitive decline (Bartus et al., 1982; Zhang et al., 2007), reveal the connection between the muscarinic-dependent cholinergic system and cognitive impairment. Scopolamine-induced mAChR blockade resulted in cognitive deficit in healthy adult humans (Voss et al., 2010). Additionally, mAChR antagonists significantly elevated plasma corticosterone in stressed rats, suggesting an inhibitory effect of mAChR stimulation on pituitary-adrenal function (Kile and Turner, 1985). This result is indicative of a correlation between corticosterone levels, mAChR availability, and cognitive function.
The hippocampus is a brain structure crucially involved in memory, the neuroendocrine regulation of stress hormones, and termination of the stress response via HPA axis glucocorticoid-mediated inhibition (Mizuno and Kimura, 1997; Kim and Diamond, 2002). Studies showing hippocampal damage due to prolonged exposure to glucocorticoids or chronic stress in primates (Uno et al., 1989; Sapolsky et al., 1990) have been triggered an assessment of the cumulative impact of such exposures on the hippocampus using other animals. A number of studies reported that chronic stress or glucocorticoids contributed hippocampal cell death in adult rats (Sapolsky et al., 1985; Dachir et al., 1997). The hippocampus contains a high density of GR and is a target of glucocorticoid actions. Cognitive deficits are associated with a loss of hippocampal neurons, in particular pyramidal cells, due to increased glucocorticoid exposure (McEwen et al., 1968; McEwen, 1999; Hibberd et al., 2000). However, others failed to find hippocampal neuronal loss in rats (Bodnoff et al., 1995; Sousa et al., 1998; Coburn-Litvak et al., 2004), tree shrews (Vollmann-Honsdorf et al., 1997; Fuchs et al., 2001), primates (Leverenz et al., 1999), and humans (Müller et al., 2001). The inconsistencies in literatures on glucocorticoid-related cell death may arise from species-specific differences in expression levels of GR and MR (Conrad, 2008).
In any case, impairments of hippocampal dependent memory and synaptic plasticity, and structural alterations have been observed in the animals with chronic stress or corticosterone treatment (Bodnoff et al., 1995; Fuchs et al., 2001; Finsterwald and Alberini, 2014). Down-regulation of GR in the hippocampus follows the chronic corticosterone treatment. (Tornello et al., 1982). And GR signaling was altered in stress-related psychopathologies (Finsterwald and Alberini, 2014). Thus this indicates a connection between GR availability and cognitive function. As GR are responsible for negative feedback control of the adaptive stress response (De Kloet et al., 1998), a reduction in hippocampal GR is associated with post-stress glucocorticoid hypersecretion (Sapolsky, 1996). Additionally, glucocorticoid treatments exacerbated the cholinergic neurotoxin ethycholine aziridinium (AF64A)-induced cholinergic lesions in the hippocampus, suggesting a pathophysiological link between glucocorticoids and age-dependent declines in cholinergic function or cholinergic degeneration in AD (Hörtnagl et al., 1993). Moreover, the essential coordinating role of GR in regulating glucocorticoid secretion through the HPA axis in response to stress in aging is well documented (Issa et al., 1990; Herman et al., 1996; Bizon et al., 2001; Murphy et al., 2002; Furay et al., 2008; Mizoguchi et al., 2009).
Glucocorticoid receptor, when functioning as a ligand-dependent transcription factor, controls transcription by directly binding to positive and negative GRE, regulating transcriptional increases (anti-inflammatory) or decreases (HPA axis negative feedback) or inhibiting transcriptional activity of other factors (on pro-inflammatory molecules) (Silverman and Sternberg, 2012). The GR-DNA binding phenomenon is gradually receiving recognition, and research on the anti-inflammatory effects of GR-DNA binding has been reported in in vivo and in vitro studies (Reichardt et al., 1998, 2001; Schäcke et al., 2002; Clark, 2007). Target disruption of GR genes and impaired GR-DNA binding has been correlated with cognitive deficits in mice (Oitzl et al., 1997, 2001), while intra-hippocampal GR blockade with a GR antagonist produced memory impairments (Nikzad et al., 2011). Diminished GR signaling and GR mRNA in the aged hippocampus is related to memory impairment and HPA axis dysregulation (Bizon et al., 2001; Murphy et al., 2002; Lee et al., 2012). Furthermore, a decrease in the nuclear uptake of corticosterone, decreased nuclear translocation, and DNA binding deficits were observed in the hippocampus of the aged rat (Sapolsky et al., 1983; Murphy et al., 2002; Lee et al., 2012). This highlights the need to understand glucocorticoids-genomic interactions, as this may illuminate the role of GR in cognitive processes. Reduced expression of GR mRNA in the hippocampus and medial prefrontal cortex was also observed with memory-impaired aged rats relative to young controls and memory-unimpaired aged rats, with no change in the basal levels of circulating glucocorticoids (Bizon et al., 2001). Another source of GR signaling interference in hippocampal cognition may be mediated by the regulation of other intruding nuclear transcriptional factors, such as activator protein and nuclear factor κB (NF-κB; Yang-Yen et al., 1990; McKay and Cidlowski, 1998; Lund et al., 2004). Recently, reduced expression of FKBP5, a key GR modulator, and smaller hippocampal volumes were observed in posttraumatic stress disorder, which was reversed after cognitive behavioral therapy (Levy-Gigi et al., 2013). Supporting the above facts, decreased nuclear GR mRNA and protein was observed in aged rats with cognitive impairment, suggesting defective GR transport might affect the transcriptional properties of hippocampal neurons with HPA axis dysfunction and could have age-related impact on cognitive decline and the loss of stress regulation (Bizon et al., 2001; Lee et al., 2012).
Although high levels of glucocorticoids are not associated with the loss of hippocampal neurons (Leverenz et al., 1999), some studies suggest interplay between the accumulative factor of stress and aging in the process of cell loss (Hibberd et al., 2000). On the other hand, Notarianni recently proposed a role for GR signaling in the initiation and development of AD, implicating over-activation of GR with hypercortisolemia in promoting amyloid beta (Aβ) production that leads to Aβ deposition and associated neuroinflammation (Notarianni, 2013).
Chronic neuroinflammation in the BF is also linked to loss of cholinergic neurons and is responsible for cognitive impairment associated with aging and AD (Willard et al., 1999). Significant loss of cholinergic neurons in the MS/diagonal band was also observed in aged animals with memory impairment (Baskerville et al., 2006). A direct correlation between glucocorticoid regulation of GR via the HPA axis and impaired GR function as a mechanism for inflammation is well reviewed (Silverman and Sternberg, 2012), emphasizing its importance in the prevention and management of chronic stress. Further, upregulation of pro-inflammatory cytokines occurs in the cortex and hippocampus of rats with post-surgery stress, resulting in post-operative cognitive dysfunction. This surgery-induced inflammation can be reduced by acetylcholinesterase inhibitors (Kalb et al., 2013), pointing to the involvement of the cholinergic system in cognitive impairment associated with neuroinflammation.
Cognitive impairment was observed in healthy human subjects treated with scopolamine, a selective mAChR antagonist (Voss et al., 2010). Rats treated with scopolamine showed spatial working memory impairment in an 8-arm radial maze task and alterations in ventral hippocampi ACh release (Mishima et al., 2000). In addition, impairment of recognition memory in BF cholinergic lesioned animals was aggravated by scopolamine, emphasizing the importance of mAChR in cognitive function (Steckler et al., 1995). Deficit in the transduction of cholinergic mAChR signals has been detected in the hippocampus of aged rats (Smith and Booze, 1995), the cortex of aged monkeys (Vannucchi and Goldman-Rakic, 1991), and in AD patients (Flynn et al., 1991). Additionally, impaired mAChR binding was found in the striatum and hippocampus of aged rats (Anson et al., 1992; Yamagami et al., 1992; Nieves-Martinez et al., 2012).
Loss of mAChR exacerbates cognitive decline and AD pathology, such as increased plaques/tangles and cerebrovascular deposition of Aβ in AD mice (Medeiros et al., 2011). Treatment with selective muscarinic agonists resulted in reduced production of Aβ in AD patients (Hock et al., 2003). A speculative pathway, due to loss of mAChR function in rats, induced by selective hippocampal cholinergic lesions with AF64A is predicted to influence effects in stimulating nicotinic receptors that may modulate the release of ACh (Thorne and Potter, 1995). In context, it is predicted that loss of mAChR function might exert stimulatory effects on nicotinic receptors, which are well described in cognitive functions (Levin, 2013). This highlights the importance of mAChR in BF cognitive function. Decreased expression of mAChR in the hippocampal CA1 region of aged epileptic animals (Cavarsan et al., 2011), and severely impaired muscarinic signaling in the hippocampus of cognitively impaired rats (Zhang et al., 2007), illustrates the involvement of the cholinergic system with cognition. More recently, decreases in ACh and mAChR were observed in cognitively impaired mice (Park et al., 2013). Additionally, impaired hippocampal ACh release and cognitive deficits in mAChR knockout mice (Tzavara et al., 2003) coupled with mAChR antagonist impairment of memory in aged rats (Quirion et al., 1995; Klinkenberg and Blokland, 2010) implies a role for mAChR in the cholinergic hypothesis of cognition.
Activation of septo-hippocampal cholinergic neurons is manifested by increased release of ACh and choline uptake. This choline uptake is reduced below control levels in the presence of chronic stress, followed by an up-regulation of muscarinic binding sites (Finkelstein et al., 1985). Suppression of glucocorticoid secretion enhances hippocampal cholinergic transmission in rats (Mizoguchi et al., 2008). Furthermore, enhanced memory consolidation by striatal corticosterone injection was blocked by administration of scopolamine (Sánchez-Resendis et al., 2012), confirming the interactive relationship between glucocorticoids and cholinergic receptor. Glucocorticoid modulation of mAChR in lung (Scherrer et al., 1997), smooth muscle (Emala et al., 1997), chronic obstructive pulmonary disease (Johnson, 2005), and several brain nuclei in rats (Torres et al., 1991) suggests a similar pattern in hippocampal cholinergic neurons.
Rats with selective removal of hippocampal cholinergic input showed HPA axis dysfunction and decreased hippocampal GR levels (Han et al., 2002; Helm et al., 2002, 2004; Lim et al., 2012). Subsequent studies also revealed altered GR-protein kinase A (PKA)-NF-κB signaling in the hippocampus with loss of cholinergic input (Lim et al., 2011, 2012). The study regarding interactive effects of stress with loss of BFCN on cognitive function reported chronic stress induced impairment of working memory in rats with loss of hippocampal cholinergic input (Craig et al., 2008). Recently, we examined whether chronic stress aggravated cognitive deficit induced by selective BF cholinergic lesions leading to alterations in GR-PKA-NF-κB signaling. Lesioned rats receiving chronic stress showed a severe impairment in spatial memory and increased NF-κB signaling activation, which was substantiated by increased hippocampal pro-inflammatory gene expression, such as inducible nitric oxide synthase and cyclooxygenase-2 (Lee et al., 2013). These data indicate that the interaction between GR and ACh receptors is associated with stress-induced cognitive dysfunction.
The present review summarizes the current research and shows the importance of glucocorticoids and their receptor in modulating cognition in stress, aging, and AD via the cholinergic system. This may pave a new way in understanding the progression of the aforementioned diseases. Therefore, this review facilitates the understanding of the following: (1) involvement of both GR and ACh receptors in modulating cognition, thus providing a palliative approach for pharmaceutical interventions as these receptors are discussed in many research papers as a route to therapeutic intervention; (2) an interactive platform to mark the holistic consequences of cognitive impairment converging from varied neurological deficits; and finally; (3) an interactive role of glucocorticoids in the development of cognitive dysfunction and vulnerability of the hippocampus to such exposure. The main objective of this review was not only to highlight the possible underlying association between the various pathways and neural circuits involved in cognitive impairment, but also to enable the mitigation of such stress-induced cognitive morbidity by developing more effective pharmacotherapeutic strategies to ameliorate such diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This paper was written as part of Konkuk University’s research support program for its faculty on sabbatical leave in 2014 and supported by a grant (K15310) from the Korea Institute of Oriental Medicine (KIOM).
Aguilera, G. (2011). HPA axis responsiveness to stress: implications for healthy aging. Exp. Gerontol. 46, 90–95. doi: 10.1016/j.exger.2010.08.023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Anson, R. M., Cutler, R., Joseph, J. A., Yamagami, K., and Roth, G. S. (1992). The effects of aging on muscarinic receptor/G-protein coupling in the rat hippocampus and striatum. Brain Res. 598, 302–306. doi: 10.1016/0006-8993(92)90197-h
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arendt, T., Bigl, V., Arendt, A., and Tennstedt, A. (1983). Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s disease. Acta Neuropathol. 61, 101–108. doi: 10.1007/bf00697388
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bartus, R. T. (2000). On neurodegenerative diseases, models and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 163, 495–529. doi: 10.1006/exnr.2000.7397
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bartus, R. T., Dean, R. L., Beer, B., and Lippa, A. S. (1982). The cholinergic hypothesis of geriatric memory dysfunction. Science 217, 408–414. doi: 10.1126/science.7046051
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baskerville, K. A., Kent, C., Nicolle, M. M., Gallagher, M., and Mckinney, M. (2006). Aging causes partial loss of basal forebrain but no loss of pontine reticular cholinergic neurons. Neuroreport 17, 1819–1823. doi: 10.1097/wnr.0b013e32800fef5a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baxter, M. G., and Bucci, D. J. (2013). Selective immunotoxic lesions of basal forebrain cholinergic neurons: twenty years of research and new directions. Behav. Neurosci. 127, 611–618. doi: 10.1037/a0033781
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baxter, M. G., Bucci, D. J., Gorman, L. K., Wiley, R. G., and Gallagher, M. (1995). Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav. Neurosci. 109, 714–722. doi: 10.1037/0735-7044.109.4.714
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baxter, M. G., and Chiba, A. A. (1999). Cognitive functions of the basal forebrain. Curr. Opin. Neurobiol. 9, 178–183. doi: 10.1016/s0959-4388(99)80024-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baxter, M. G., Gallagher, M., and Holland, P. C. (1999). Blocking can occur without losses in attention in rats with selective removal of hippocampal cholinergic input. Behav. Neurosci. 113, 881–890. doi: 10.1037//0735-7044.113.5.881
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baxter, M. G., Holland, P. C., and Gallagher, M. (1997). Disruption of decrements in conditioned stimulus processing by selective removal of hippocampal cholinergic input. J. Neurosci. 17, 5230–5236.
Berger-Sweeney, J., Heckers, S., Mesulam, M. M., Wiley, R. G., Lappi, D. A., and Sharma, M. (1994). Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J. Neurosci. 14, 4507–4519.
Bizon, J. L., Helm, K. A., Han, J. S., Chun, H. J., Pucilowska, J., Lund, P. K., et al. (2001). Hypothalamic-pituitary-adrenal axis function and corticosterone receptor expression in behaviourally characterized young and aged long-evans rats. Eur. J. Neurosci. 14, 1739–1751. doi: 10.1046/j.0953-816x.2001.01781.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bodnoff, S. R., Humphreys, A. G., Lehman, J. C., Diamond, D. M., Rose, G. M., and Meaney, M. J. (1995). Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity and hippocampal neuropathology in young and mid-aged rats. J. Neurosci. 15, 61–69.
Brayda-Bruno, L., Mons, N., Yee, B. K., Micheau, J., Abrous, D. N., Nogues, X., et al. (2013). Partial loss in septo-hippocampal cholinergic neurons alters memory-dependent measures of brain connectivity without overt memory deficits. Neurobiol. Dis. 54, 372–381. doi: 10.1016/j.nbd.2013.01.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brureau, A., Zussy, C., Delair, B., Ogier, C., Ixart, G., Maurice, T., et al. (2013). Deregulation of hypothalamic-pituitary-adrenal axis functions in an Alzheimer’s disease rat model. Neurobiol. Aging 34, 1426–1439. doi: 10.1016/j.neurobiolaging.2012.11.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bucci, D. J., Holland, P. C., and Gallagher, M. (1998). Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J. Neurosci. 18, 8038–8046.
Calogero, A. E., Bernardini, R., Gold, P. W., and Chrousos, G. P. (1988). Regulation of rat hypothalamic corticotropin-releasing hormone secretion in vitro: potential clinical implications. Adv. Exp. Med. Biol. 245, 167–181. doi: 10.1007/978-1-4899-2064-5_13
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Calogero, A. E., Kamilaris, T. C., Johnson, E. O., Tartaglia, M. E., and Chrousos, G. (1990). Recovery of the rat hypothalamic-pituitary-adrenal axis after discontinuation of prolonged treatment with the synthetic glucocorticoid agonist dexamethasone. Endocrinology 127, 1574–1579. doi: 10.1210/endo-127-4-1574
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cavarsan, C. F., Avanzi, R. D., Queiroz, C. M., Xavier, G. F., Mello, L. E., and Covolan, L. (2011). m1 Acetylcholine receptor expression is decreased in Hippocampal CA1 region of aged epileptic animals. Aging Dis. 2, 301–307.
Cestari, V., Rossi-Arnaud, C., Saraulli, D., and Costanzi, M. (2014). The MAP(K) of fear: from memory consolidation to memory extinction. Brain Res. Bull. 105, 8–16. doi: 10.1016/j.brainresbull.2013.09.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chiba, A. A., Bushnell, P. J., Oshiro, W. M., and Gallagher, M. (1999). Selective removal of cholinergic neurons in the basal forebrain alters cued target detection. Neuroreport 10, 3119–3123. doi: 10.1097/00001756-199909290-00044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chudasama, Y., Dalley, J. W., Nathwani, F., Bouger, P., and Robbins, T. W. (2004). Cholinergic modulation of visual attention and working memory: dissociable effects of basal forebrain 192-IgG-saporin lesions and intraprefrontal infusions of scopolamine. Learn. Mem. 11, 78–86. doi: 10.1101/lm.70904
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Clark, A. R. (2007). Anti-inflammatory functions of glucocorticoid-induced genes. Mol. Cell. Endocrinol. 275, 79–97. doi: 10.1016/j.mce.2007.04.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Coburn-Litvak, P. S., Tata, D. A., Gorby, H. E., Mccloskey, D. P., Richardson, G., and Anderson, B. J. (2004). Chronic corticosterone affects brain weight and mitochondrial, but not glial volume fraction in hippocampal area CA3. Neuroscience 124, 429–438. doi: 10.1016/j.neuroscience.2003.11.031
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Colgin, L. L., Kubota, D., and Lynch, G. (2003). Cholinergic plasticity in the hippocampus. Proc. Natl. Acad. Sci. U S A 100, 2872–2877. doi: 10.1073/pnas.0530289100
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Conner, J. M., Chiba, A. A., and Tuszynski, M. H. (2005). The basal forebrain cholinergic system is essential for cortical plasticity and functional recovery following brain injury. Neuron 46, 173–179. doi: 10.1016/j.neuron.2005.03.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Conrad, C. D. (2008). Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neurosci. 19, 395–411. doi: 10.1515/revneuro.2008.19.6.395
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Contestabile, A. (2011). The history of the cholinergic hypothesis. Behav. Brain Res. 221, 334–340. doi: 10.1016/j.bbr.2009.12.044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Craig, L. A., Hong, N. S., Kopp, J., and McDonald, R. J. (2008). Emergence of spatial impairment in rats following specific cholinergic depletion of the medial septum combined with chronic stress. Eur. J. Neurosci. 27, 2262–2271. doi: 10.1111/j.1460-9568.2008.06179.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Craig, L. A., Hong, N. S., and McDonald, R. J. (2011). Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 35, 1397–1409. doi: 10.1016/j.neubiorev.2011.03.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dachir, S., Kadar, T., Robinzon, B., and Levy, A. (1997). Nimodipine’s protection against corticosterone-induced morphological changes in the hippocampus of young rats. Brain Res. 748, 175–183. doi: 10.1016/s0006-8993(96)01296-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Davies, P., and Maloney, A. J. (1976). Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 2:1403. doi: 10.1016/s0140-6736(76)91936-x
Davis, K. L., and Mohs, R. C. (1982). Enhancement of memory processes in Alzheimer’s disease with multiple-dose intravenous physostigmine. Am. J. Psychiatry 139, 1421–1424. doi: 10.1176/ajp.139.11.1421
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Degroot, A., Wade, M., Salhoff, C., Davis, R. J., Tzavara, E. T., and Nomikos, G. G. (2004). Exposure to an elevated platform increases plasma corticosterone and hippocampal acetylcholine in the rat: reversal by chlordiazepoxide. Eur. J. Pharmacol. 493, 103–109. doi: 10.1016/s0014-2999(04)00386-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
de Kloet, E. R. (2008). About stress hormones and resilience to psychopathology. J. Neuroendocrinol. 20, 885–892. doi: 10.1111/j.1365-2826.2008.01707.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
De Kloet, E. R., Vreugdenhil, E., Oitzl, M. S., and Joëls, M. (1998). Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301. doi: 10.1210/er.19.3.269
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drachman, D. A., Glosser, G., Fleming, P., and Longenecker, G. (1982). Memory decline in the aged: treatment with lecithin and physostigmine. Neurology 32, 944–950. doi: 10.1212/wnl.32.9.944
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Drever, B. D., Riedel, G., and Platt, B. (2011). The cholinergic system and hippocampal plasticity. Behav. Brain Res. 221, 505–514. doi: 10.1016/j.bbr.2010.11.037
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dumas, J. A., and Newhouse, P. A. (2011). The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol. Biochem. Behav. 99, 254–261. doi: 10.1016/j.pbb.2011.02.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dutar, P., Bassant, M. H., Senut, M. C., and Lamour, Y. (1995). The septohippocampal pathway: structure and function of a central cholinergic system. Physiol. Rev. 75, 393–427.
Easton, A., Douchamps, V., Eacott, M., and Lever, C. (2012). A specific role for septohippocampal acetylcholine in memory? Neuropsychologia 50, 3156–3168. doi: 10.1016/j.neuropsychologia.2012.07.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Emala, C. W., Clancy, J., and Hirshman, C. A. (1997). Glucocorticoid treatment decreases muscarinic receptor expression in canine airway smooth muscle. Am. J. Physiol. 272, L745–L751.
Finkelstein, Y., Koffler, B., Rabey, J. M., and Gilad, G. M. (1985). Dynamics of cholinergic synaptic mechanisms in rat hippocampus after stress. Brain Res. 343, 314–319. doi: 10.1016/0006-8993(85)90749-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Finsterwald, C., and Alberini, C. M. (2014). Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol. Learn. Mem. 112, 17–29. doi: 10.1016/j.nlm.2013.09.017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Flynn, D. D., Weinstein, D. A., and Mash, D. C. (1991). Loss of high-affinity agonist binding to M1 muscarinic receptors in Alzheimer’s disease: implications for the failure of cholinergic replacement therapies. Ann. Neurol. 29, 256–262. doi: 10.1002/ana.410290305
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fuchs, E., Flügge, G., Ohl, F., Lucassen, P., Vollmann-Honsdorf, G. K., and Michaelis, T. (2001). Psychosocial stress, glucocorticoids and structural alterations in the tree shrew hippocampus. Physiol. Behav. 73, 285–291. doi: 10.1016/s0031-9384(01)00497-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Funder, J. W. (1997). Glucocorticoid and mineralocorticoid receptors: biology and clinical relevance. Annu. Rev. Med. 48, 231–240. doi: 10.1146/annurev.med.48.1.231
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Furay, A. R., Bruestle, A. E., and Herman, J. P. (2008). The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology 149, 5482–5490. doi: 10.1210/en.2008-0642
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gallagher, M., Burwell, R. D., Kodsi, M. H., McKinney, M., Southerland, S., Vella-Rountree, L., et al. (1990). Markers for biogenic amines in the aged rat brain: relationship to decline in spatial learning ability. Neurobiol. Aging 11, 507–514. doi: 10.1016/0197-4580(90)90111-c
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gallagher, M., and Colombo, P. J. (1995). Ageing: the cholinergic hypothesis of cognitive decline. Curr. Opin. Neurobiol. 5, 161–168. doi: 10.1016/0959-4388(95)80022-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gallagher, M., Stocker, A. M., and Koh, M. T. (2011). Mindspan: lessons from rat models of neurocognitive aging. ILAR J. 52, 32–40. doi: 10.1093/ilar.52.1.32
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gilad, G. M. (1987). The stress-induced response of the septo-hippocampal cholinergic system. A vectorial outcome of psychoneuroendocrinological interactions. Psychoneuroendocrinology 12, 167–184. doi: 10.1016/0306-4530(87)90002-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gilad, G. M., Mahon, B. D., Finkelstein, Y., Koffler, B., and Gilad, V. H. (1985). Stress-induced activation of the hippocampal cholinergic system and the pituitary-adrenocortical axis. Brain Res. 347, 404–408. doi: 10.1016/0006-8993(85)90209-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Groeneweg, F. L., Karst, H., de Kloet, E. R., and Joëls, M. (2011). Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 209, 153–167. doi: 10.1530/joe-10-0472
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hagan, J. J., Salamone, J. D., Simpson, J., Iversen, S. D., and Morris, R. G. (1988). Place navigation in rats is impaired by lesions of medial septum and diagonal band but not nucleus basalis magnocellularis. Behav. Brain Res. 27, 9–20. doi: 10.1016/0166-4328(88)90105-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Han, J. S., Bizon, J. L., Chun, H. J., Maus, C. E., and Gallagher, M. (2002). Decreased glucocorticoid receptor mRNA and dysfunction of HPA axis in rats after removal of the cholinergic innervation to hippocampus. Eur. J. Neurosci. 16, 1399–1404. doi: 10.1046/j.1460-9568.2002.02191.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hatzinger, M., Brand, S., Herzig, N., and Holsboer-Trachsler, E. (2011). In healthy young and elderly adults, hypothalamic-pituitary-adrenocortical axis reactivity (HPA AR) varies with increasing pharmacological challenge and with age, but not with gender. J. Psychiatr. Res. 45, 1373–1380. doi: 10.1016/j.jpsychires.2011.05.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Helm, K. A., Han, J. S., and Gallagher, M. (2002). Effects of cholinergic lesions produced by infusions of 192 IgG-saporin on glucocorticoid receptor mRNA expression in hippocampus and medial prefrontal cortex of the rat. Neuroscience 115, 765–774. doi: 10.1016/s0306-4522(02)00487-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Helm, K. A., Ziegler, D. R., and Gallagher, M. (2004). Habituation to stress and dexamethasone suppression in rats with selective basal forebrain cholinergic lesions. Hippocampus 14, 628–635. doi: 10.1002/hipo.10203
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hepler, D. J., Olton, D. S., Wenk, G. L., and Coyle, J. T. (1985). Lesions in nucleus basalis magnocellularis and medial septal area of rats produce qualitatively similar memory impairments. J. Neurosci. 5, 866–873.
Herman, J. P., Prewitt, C. M., and Cullinan, W. E. (1996). Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit. Rev. Neurobiol. 10, 371–394. doi: 10.1615/critrevneurobiol.v10.i3-4.50
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Herman, J. P., Schäfer, M. K., Young, E. A., Thompson, R., Douglass, J., Akil, H., et al. (1989). Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J. Neurosci. 9, 3072–3082.
Hibberd, C., Yau, J. L., and Seckl, J. R. (2000). Glucocorticoids and the ageing hippocampus. J. Anat. 197(Pt. 4), 553–562. doi: 10.1046/j.1469-7580.2000.19740553.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hock, C., Maddalena, A., Raschig, A., Müller-Spahn, F., Eschweiler, G., Hager, K., et al. (2003). Treatment with the selective muscarinic m1 agonist talsaclidine decreases cerebrospinal fluid levels of A beta 42 in patients with Alzheimer’s disease. Amyloid 10, 1–6. doi: 10.3109/13506120308995249
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hörtnagl, H., Berger, M. L., Havelec, L., and Hornykiewicz, O. (1993). Role of glucocorticoids in the cholinergic degeneration in rat hippocampus induced by ethylcholine aziridinium (AF64A). J. Neurosci. 13, 2939–2945.
Imperato, A., Puglisi-Allegra, S., Casolini, P., Zocchi, A., and Angelucci, L. (1989). Stress-induced enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur. J. Pharmacol. 165, 337–338. doi: 10.1016/0014-2999(89)90735-8
Issa, A. M., Rowe, W., Gauthier, S., and Meaney, M. J. (1990). Hypothalamic-pituitary-adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J. Neurosci. 10, 3247–3254.
Jacobson, L., and Sapolsky, R. (1991). The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 12, 118–134. doi: 10.1210/edrv-12-2-118
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Janisiewicz, A. M., Jackson, O. 3rd, Firoz, E. F., and Baxter, M. G. (2004). Environment-spatial conditional learning in rats with selective lesions of medial septal cholinergic neurons. Hippocampus 14, 265–273. doi: 10.1002/hipo.10175
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Joëls, M. (2008). Functional actions of corticosteroids in the hippocampus. Eur. J. Pharmacol. 583, 312–321. doi: 10.1016/j.ejphar.2007.11.064
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Johnson, M. (2005). Corticosteroids: potential beta2-agonist and anticholinergic interactions in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2, 320–325; discussion 340–341. doi: 10.1513/pats.200504-040sr
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kalb, A., von Haefen, C., Sifringer, M., Tegethoff, A., Paeschke, N., Kostova, M., et al. (2013). Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS One 8:e62679. doi: 10.1371/journal.pone.0062679
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kaufer, D., Friedman, A., Seidman, S., and Soreq, H. (1998). Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature 393, 373–377. doi: 10.1038/30741
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kile, J. P., and Turner, B. B. (1985). Serotonergic and cholinergic interaction in the regulation of pituitary-adrenal function in rats. Experientia 41, 1123–1127. doi: 10.1007/bf01951690
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, J. J., and Diamond, D. M. (2002). The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci. 3, 453–462. doi: 10.1038/nrn849
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kim, H. G., and Kim, K. L. (2007). Decreased hippocampal cholinergic neurostimulating peptide precursor protein associated with stress exposure in rat brain by proteomic analysis. J. Neurosci. Res. 85, 2898–2908. doi: 10.1002/jnr.21407
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Klinkenberg, I., and Blokland, A. (2010). The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci. Biobehav. Rev. 34, 1307–1350. doi: 10.1016/j.neubiorev.2010.04.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, S. Y., Hwang, Y. K., Yun, H. S., and Han, J. S. (2012). Decreased levels of nuclear glucocorticoid receptor protein in the hippocampus of aged long-evans rats with cognitive impairment. Brain Res. 1478, 48–54. doi: 10.1016/j.brainres.2012.08.035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lee, S. Y., Kim, M. S., and Han, J. S. (2013). Activation of NF-kappa κB signaling in the hippocampus without cholinergic input was aggravated by chronic stress. Soc. Neurosci. Abstract.
Lee, C. T., Ma, Y. L., and Lee, E. H. (2007). Serum- and glucocorticoid-inducible kinase1 enhances contextual fear memory formation through down-regulation of the expression of Hes5. J. Neurochem. 100, 1531–1542. doi: 10.1111/j.1471-4159.2006.04284.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leverenz, J. B., Wilkinson, C. W., Wamble, M., Corbin, S., Grabber, J. E., Raskind, M. A., et al. (1999). Effect of chronic high-dose exogenous cortisol on hippocampal neuronal number in aged nonhuman primates. J. Neurosci. 19, 2356–2361.
Levin, E. D. (2013). Complex relationships of nicotinic receptor actions and cognitive functions. Biochem. Pharmacol. 86, 1145–1152. doi: 10.1016/j.bcp.2013.07.021
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Levy-Gigi, E., Szabó, C., Kelemen, O., and Kéri, S. (2013). Association among clinical response, hippocampal volume and FKBP5 gene expression in individuals with posttraumatic stress disorder receiving cognitive behavioral therapy. Biol. Psychiatry 74, 793–800. doi: 10.1016/j.biopsych.2013.05.017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lewis, P. R., and Shute, C. C. (1967). The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system and the subfornical organ and supra-optic crest. Brain 90, 521–540. doi: 10.1093/brain/90.3.521
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lewis, P. R., Shute, C. C., and Silver, A. (1967). Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J. Physiol. 191, 215–224. doi: 10.1113/jphysiol.1967.sp008246
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lim, C. S., Hwang, Y. K., Kim, D., Cho, S. H., Bañuelos, C., Bizon, J. L., et al. (2011). Increased interactions between PKA and NF-kappaB signaling in the hippocampus following loss of cholinergic input. Neuroscience 192, 485–493. doi: 10.1016/j.neuroscience.2011.05.074
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lim, C. S., Kim, Y. J., Hwang, Y. K., Bañuelos, C., Bizon, J. L., and Han, J. S. (2012). Decreased interactions in protein kinase A-Glucocorticoid receptor signaling in the hippocampus after selective removal of the basal forebrain cholinergic input. Hippocampus 22, 455–465. doi: 10.1002/hipo.20912
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lund, P. K., Hoyt, E. C., Bizon, J., Smith, D. R., Haberman, R., Helm, K., et al. (2004). Transcriptional mechanisms of hippocampal aging. Exp. Gerontol. 39, 1613–1622. doi: 10.1016/j.exger.2004.06.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lupien, S., Lecours, A. R., Lussier, I., Schwartz, G., Nair, N. P., and Meaney, M. J. (1994). Basal cortisol levels and cognitive deficits in human aging. J. Neurosci. 14, 2893–2903.
Lupien, S. J., Mcewen, B. S., Gunnar, M. R., and Heim, C. (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 10, 434–445. doi: 10.1038/nrn2639
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maki, M., Matsukawa, N., Yuasa, H., Otsuka, Y., Yamamoto, T., Akatsu, H., et al. (2002). Decreased expression of hippocampal cholinergic neurostimulating peptide precursor protein mRNA in the hippocampus in Alzheimer disease. J. Neuropathol. Exp. Neurol. 61, 176–185.
Mark, G. P., Rada, P. V., and Shors, T. J. (1996). Inescapable stress enhances extracellular acetylcholine in the rat hippocampus and prefrontal cortex but not the nucleus accumbens or amygdala. Neuroscience 74, 767–774. doi: 10.1016/0306-4522(96)00211-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martí, O., and Armario, A. (1997). Influence of regularity of exposure to chronic stress on the pattern of habituation of pituitary-adrenal hormones, prolactin and glucose. Stress 1, 179–189. doi: 10.3109/10253899709001107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McEwen, B. S. (1999). Stress and hippocampal plasticity. Annu. Rev. Neurosci. 22, 105–122. doi: 10.1146/annurev.neuro.22.1.105
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McEwen, B. S., and Wallach, G. (1973). Corticosterone binding to hippocampus: nuclear and cytosol binding in vitro. Brain Res. 57, 373–386. doi: 10.1016/0006-8993(73)90143-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McEwen, B. S., Weiss, J. M., and Schwartz, L. S. (1968). Selective retention of corticosterone by limbic structures in rat brain. Nature 220, 911–912. doi: 10.1038/220911a0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McEwen, B. S., Weiss, J. M., and Schwartz, L. S. (1969). Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res. 16, 227–241. doi: 10.1016/0006-8993(69)90096-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McKay, L. I., and Cidlowski, J. A. (1998). Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol. Endocrinol. 12, 45–56. doi: 10.1210/me.12.1.45
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McKinney, M., Coyle, J. T., and Hedreen, J. C. (1983). Topographic analysis of the innervation of the rat neocortex and hippocampus by the basal forebrain cholinergic system. J. Comp. Neurol. 217, 103–121. doi: 10.1002/cne.902170109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Medeiros, R., Kitazawa, M., Caccamo, A., Baglietto-Vargas, D., Estrada-Hernandez, T., Cribbs, D. H., et al. (2011). Loss of muscarinic M1 receptor exacerbates Alzheimer’s disease-like pathology and cognitive decline. Am. J. Pathol. 179, 980–991. doi: 10.1016/j.ajpath.2011.04.041
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mesulam, M. (2004). The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learn. Mem. 11, 43–49. doi: 10.1101/lm.69204
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mesulam, M. M., Mufson, E. J., Wainer, B. H., and Levey, A. I. (1983). Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10, 1185–1201. doi: 10.1016/0306-4522(83)90108-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mishima, K., Iwasaki, K., Tsukikawa, H., Matsumoto, Y., Egashira, N., Abe, K., et al. (2000). The scopolamine-induced impairment of spatial cognition parallels the acetylcholine release in the ventral hippocampus in rats. Jpn. J. Pharmacol. 84, 163–173. doi: 10.1254/jjp.84.163
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mizoguchi, K., Ikeda, R., Shoji, H., Tanaka, Y., Maruyama, W., and Tabira, T. (2009). Aging attenuates glucocorticoid negative feedback in rat brain. Neuroscience 159, 259–270. doi: 10.1016/j.neuroscience.2008.12.020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mizoguchi, K., Shoji, H., Ikeda, R., Tanaka, Y., Maruyama, W., and Tabira, T. (2008). Suppression of glucocorticoid secretion enhances cholinergic transmission in rat hippocampus. Brain Res. Bull. 76, 612–615. doi: 10.1016/j.brainresbull.2008.03.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mizuno, T., and Kimura, F. (1997). Attenuated stress response of hippocampal acetylcholine release and adrenocortical secretion in aged rats. Neurosci. Lett. 222, 49–52. doi: 10.1016/s0304-3940(97)13340-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mora, F., Segovia, G., Del Arco, A., de Blas, M., and Garrido, P. (2012). Stress, neurotransmitters, corticosterone and body-brain integration. Brain Res. 1476, 71–85. doi: 10.1016/j.brainres.2011.12.049
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Morris, M. C., and Rao, U. (2014). Cortisol response to psychosocial stress during a depressive episode and remission. Stress 17, 51–58. doi: 10.3109/10253890.2013.857398
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Muir, J. L., Page, K. J., Sirinathsinghji, D. J., Robbins, T. W., and Everitt, B. J. (1993). Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav. Brain Res. 57, 123–131. doi: 10.1016/0166-4328(93)90128-d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Müller, M. B., Lucassen, P. J., Yassouridis, A., Hoogendijk, W. J., Holsboer, F., and Swaab, D. F. (2001). Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur. J. Neurosci. 14, 1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Murphy, E. K., Spencer, R. L., Sipe, K. J., and Herman, J. P. (2002). Decrements in nuclear glucocorticoid receptor (GR) protein levels and DNA binding in aged rat hippocampus. Endocrinology 143, 1362–1370. doi: 10.1210/en.143.4.1362
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Newhouse, P. A., Potter, A., Corwin, J., and Lenox, R. (1994). Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology 10, 93–107. doi: 10.1038/npp.1994.11
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Newhouse, P. A., Sunderland, T., Tariot, P. N., Weingartner, H., Thompson, K., Mellow, A. M., et al. (1988). The effects of acute scopolamine in geriatric depression. Arch. Gen. Psychiatry 45, 906–912. doi: 10.1001/archpsyc.1988.01800340028004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Newman, M. B., Nazian, S. J., Sanberg, P. R., Diamond, D. M., and Shytle, R. D. (2001). Corticosterone-attenuating and anxiolytic properties of mecamylamine in the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 609–620. doi: 10.1016/s0278-5846(00)00178-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nieves-Martinez, E., Haynes, K., Childers, S. R., Sonntag, W. E., and Nicolle, M. M. (2012). Muscarinic receptor/G-protein coupling is reduced in the dorsomedial striatum of cognitively impaired aged rats. Behav. Brain Res. 227, 258–264. doi: 10.1016/j.bbr.2011.10.048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nikzad, S., Vafaei, A. A., Rashidy-Pour, A., and Haghighi, S. (2011). Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress 14, 459–464. doi: 10.3109/10253890.2010.548171
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Notarianni, E. (2013). Hypercortisolemia and glucocorticoid receptor-signaling insufficiency in Alzheimer’s disease initiation and development. Curr. Alzheimer Res. 10, 714–731. doi: 10.2174/15672050113109990137
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nyakas, C., Luiten, P. G., Spencer, D. G., and Traber, J. (1987). Detailed projection patterns of septal and diagonal band efferents to the hippocampus in the rat with emphasis on innervation of CA1 and dentate gyrus. Brain Res. Bull. 18, 533–545. doi: 10.1016/0361-9230(87)90117-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oitzl, M. S., de Kloet, E. R., Joëls, M., Schmid, W., and Cole, T. J. (1997). Spatial learning deficits in mice with a targeted glucocorticoid receptor gene disruption. Eur. J. Neurosci. 9, 2284–2296. doi: 10.1111/j.1460-9568.1997.tb01646.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oitzl, M. S., Reichardt, H. M., Joëls, M., and de Kloet, E. R. (2001). Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. U S A 98, 12790–12795. doi: 10.1073/pnas.231313998
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Olton, D. S. (1990). Dementia: animal models of the cognitive impairments following damage to the basal forebrain cholinergic system. Brain Res. Bull. 25, 499–502. doi: 10.1016/0361-9230(90)90242-r
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Park, S. J., Shin, E. J., Min, S. S., An, J., Li, Z., Hee Chung, Y., et al. (2013). Inactivation of JAK2/STAT3 signaling axis and downregulation of M1 mAChR cause cognitive impairment in klotho mutant mice, a genetic model of aging. Neuropsychopharmacology 38, 1426–1437. doi: 10.1038/npp.2013.39
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pitman, D. L., Ottenweller, J. E., and Natelson, B. H. (1988). Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol. Behav. 43, 47–55. doi: 10.1016/0031-9384(88)90097-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Popoli, M., Yan, Z., McEwen, B. S., and Sanacora, G. (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37. doi: 10.1038/nrn3138
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Quirion, R., Wilson, A., Rowe, W., Aubert, I., Richard, J., Doods, H., et al. (1995). Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired. J. Neurosci. 15, 1455–1462.
Raison, C. L., and Miller, A. H. (2003). When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry 160, 1554–1565. doi: 10.1176/appi.ajp.160.9.1554
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reichardt, H. M., Kaestner, K. H., Tuckermann, J., Kretz, O., Wessely, O., Bock, R., et al. (1998). DNA binding of the glucocorticoid receptor is not essential for survival. Cell 93, 531–541. doi: 10.1016/s0092-8674(00)81183-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reichardt, H. M., Tuckermann, J. P., Göttlicher, M., Vujic, M., Weih, F., Angel, P., et al. (2001). Repression of inflammatory responses in the absence of DNA binding by the glucocorticoid receptor. EMBO J. 20, 7168–7173. doi: 10.1093/emboj/20.24.7168
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reul, J. M., and de Kloet, E. R. (1985). Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511. doi: 10.1210/endo-117-6-2505
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reul, J. M., van den Bosch, F. R., and de Kloet, E. R. (1987a). Differential response of type I and type II corticosteroid receptors to changes in plasma steroid level and circadian rhythmicity. Neuroendocrinology 45, 407–412. doi: 10.1159/000124766
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Reul, J. M., van den Bosch, F. R., and de Kloet, E. R. (1987b). Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J. Endocrinol. 115, 459–467. doi: 10.1677/joe.0.1150459
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sánchez-Resendis, O., Medina, A. C., Serafin, N., Prado-Alcalá, R. A., Roozendaal, B., and Quirarte, G. L. (2012). Glucocorticoid-cholinergic interactions in the dorsal striatum in memory consolidation of inhibitory avoidance training. Front. Behav. Neurosci. 6:33. doi: 10.3389/fnbeh.2012.00033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sapolsky, R. M. (1996). Why stress is bad for your brain. Science 273, 749–750. doi: 10.1126/science.273.5276.749
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sapolsky, R. M., Krey, L. C., and McEwen, B. S. (1983). Corticosterone receptors decline in a site-specific manner in the aged rat brain. Brain Res. 289, 235–240. doi: 10.1016/0006-8993(83)90024-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sapolsky, R. M., Krey, L. C., and McEwen, B. S. (1984). Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc. Natl. Acad. Sci. U S A 81, 6174–6177. doi: 10.1073/pnas.81.19.6174
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sapolsky, R. M., Krey, L. C., and McEwen, B. S. (1985). Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 5, 1222–1227.
Sapolsky, R. M., Krey, L. C., and McEwen, B. S. (1986). The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr. Rev. 7, 284–301. doi: 10.1210/edrv-7-3-284
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sapolsky, R. M., Uno, H., Rebert, C. S., and Finch, C. E. (1990). Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J. Neurosci. 10, 2897–2902.
Sarter, M., Bruno, J. P., and Givens, B. (2003). Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol. Learn. Mem. 80, 245–256. doi: 10.1016/s1074-7427(03)00070-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schäcke, H., Döcke, W. D., and Asadullah, K. (2002). Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 96, 23–43. doi: 10.1016/s0163-7258(02)00297-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Scherrer, D., Lach, E., Landry, Y., and Gies, J. P. (1997). Glucocorticoid modulation of muscarinic and beta-adrenergic receptors in guinea pig lung. Fundam. Clin. Pharmacol. 11, 111–116. doi: 10.1111/j.1472-8206.1997.tb00176.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Silverman, M. N., and Sternberg, E. M. (2012). Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann. N Y Acad. Sci. 1261, 55–63. doi: 10.1111/j.1749-6632.2012.06633.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Simons, S. S. Jr. (2008). What goes on behind closed doors: physiological versus pharmacological steroid hormone actions. Bioessays 30, 744–756. doi: 10.1002/bies.20792
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Smith, M. L., and Booze, R. M. (1995). Cholinergic and GABAergic neurons in the nucleus basalis region of young and aged rats. Neuroscience 67, 679–688. doi: 10.1016/0306-4522(95)00076-u
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sousa, N., Madeira, M. D., and Paula-Barbosa, M. M. (1998). Effects of corticosterone treatment and rehabilitation on the hippocampal formation of neonatal and adult rats. An unbiased stereological study. Brain Res. 794, 199–210. doi: 10.1016/s0006-8993(98)00218-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Srinivasan, S., Shariff, M., and Bartlett, S. E. (2013). The role of the glucocorticoids in developing resilience to stress and addiction. Front. Psychiatry 4:68. doi: 10.3389/fpsyt.2013.00068
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Steckler, T., Keith, A. B., Wiley, R. G., and Sahgal, A. (1995). Cholinergic lesions by 192 IgG-saporin and short-term recognition memory: role of the septohippocampal projection. Neuroscience 66, 101–114. doi: 10.1016/0306-4522(94)00603-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Teles-Grilo Ruivo, L. M., and Mellor, J. R. (2013). Cholinergic modulation of hippocampal network function. Front. Synaptic Neurosci. 5:2. doi: 10.3389/fnsyn.2013.00002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thorne, B., and Potter, P. E. (1995). Lesion with the neurotoxin AF64A alters hippocampal cholinergic receptor function. Brain Res. Bull. 38, 121–127. doi: 10.1016/0361-9230(95)00076-q
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tornello, S., Orti, E., De Nicola, A. F., Rainbow, T. C., and McEwen, B. S. (1982). Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology 35, 411–417. doi: 10.1159/000123429
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Torres, N., Fanelli, M., Alvarez, A. L., Santajuliana, D., Finkielman, S., and Pirola, C. J. (1991). Glucocorticoid-induced hypertension in rats: role of the central muscarinic cholinergic system. J. Endocrinol. 129, 269–274. doi: 10.1677/joe.0.1290269
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tzavara, E. T., Bymaster, F. P., Felder, C. C., Wade, M., Gomeza, J., Wess, J., et al. (2003). Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol. Psychiatry 8, 673–679. doi: 10.1038/sj.mp.4001270
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Uno, H., Tarara, R., Else, J. G., Suleman, M. A., and Sapolsky, R. M. (1989). Hippocampal damage associated with prolonged and fatal stress in primates. J. Neurosci. 9, 1705–1711.
van der Laan, S., and Meijer, O. C. (2008). Pharmacology of glucocorticoids: beyond receptors. Eur. J. Pharmacol. 585, 483–491. doi: 10.1016/j.ejphar.2008.01.060
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van der Zee, E. A., and Keijser, J. N. (2011). Localization of pre- and postsynaptic cholinergic markers in rodent forebrain: a brief history and comparison of rat and mouse. Behav. Brain Res. 221, 356–366. doi: 10.1016/j.bbr.2010.11.051
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Haarst, A. D., Oitzl, M. S., Workel, J. O., and de Kloet, E. R. (1996). Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology 137, 4935–4943. doi: 10.1210/en.137.11.4935
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vannucchi, M. G., and Goldman-Rakic, P. S. (1991). Age-dependent decrease in the affinity of muscarinic M1 receptors in neocortex of rhesus monkeys. Proc. Natl. Acad. Sci. U S A 88, 11475–11479. doi: 10.1073/pnas.88.24.11475
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vollmann-Honsdorf, G. K., Flügge, G., and Fuchs, E. (1997). Chronic psychosocial stress does not affect the number of pyramidal neurons in tree shrew hippocampus. Neurosci. Lett. 233, 121–124. doi: 10.1016/s0304-3940(97)00647-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Voss, B., Thienel, R., Reske, M., Habel, U., and Kircher, T. (2010). Cognitive performance and cholinergic transmission: influence of muscarinic and nicotinic receptor blockade. Eur. Arch. Psychiatry Clin. Neurosci. 260(Suppl. 2), S106–S110. doi: 10.1007/s00406-010-0160-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vyas, S., and Maatouk, L. (2013). Contribution of glucocorticoids and glucocorticoid receptors to the regulation of neurodegenerative processes. CNS Neurol. Disord. Drug Targets 12, 1175–1193. doi: 10.2174/187152731131200125
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Whitehouse, P. J., Price, D. L., Clark, A. W., Coyle, J. T., and DeLong, M. R. (1981). Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann. Neurol. 10, 122–126. doi: 10.1002/ana.410100203
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Whitehouse, P. J., Price, D. L., Struble, R. G., Clark, A. W., Coyle, J. T., and Delon, M. R. (1982). Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science 215, 1237–1239. doi: 10.1126/science.7058341
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wiley, R. G., Oeltmann, T. N., and Lappi, D. A. (1991). Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 562, 149–153. doi: 10.1016/0006-8993(91)91199-b
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Willard, L. B., Hauss-Wegrzyniak, B., and Wenk, G. L. (1999). Pathological and biochemical consequences of acute and chronic neuroinflammation within the basal forebrain cholinergic system of rats. Neuroscience 88, 193–200. doi: 10.1016/s0306-4522(98)00216-4
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yamagami, K., Joseph, J. A., and Roth, G. S. (1992). Decrement of muscarinic receptor-stimulated low-KM GTPase in striatum and hippocampus from the aged rat. Brain Res. 576, 327–331. doi: 10.1016/0006-8993(92)90698-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yang-Yen, H. F., Chambard, J. C., Sun, Y. L., Smeal, T., Schmidt, T. J., Drouin, J., et al. (1990). Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 62, 1205–1215. doi: 10.1016/0092-8674(90)90396-v
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, H. Y., Watson, M. L., Gallagher, M., and Nicolle, M. M. (2007). Muscarinic receptor-mediated GTP-Eu binding in the hippocampus and prefrontal cortex is correlated with spatial memory impairment in aged rats. Neurobiol. Aging 28, 619–626. doi: 10.1016/j.neurobiolaging.2006.02.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: cholinergic neuron, stress, aging, basal forebrain, hippocampus, glucocorticoid, glucocorticoid receptor
Citation: Paul S, Jeon WK, Bizon JL and Han J-S (2015) Interaction of basal forebrain cholinergic neurons with the glucocorticoid system in stress regulation and cognitive impairment. Front. Aging Neurosci. 7:43. doi: 10.3389/fnagi.2015.00043
Received: 12 January 2015; Accepted: 12 March 2015;
Published online: 02 April 2015.
Edited by:
Enrique Cadenas, University of Southern California, USAReviewed by:
Junming Wang, University of Mississippi Medical Center, USACopyright © 2015 Paul, Jeon, Bizon and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jung-Soo Han, Department of Biological Sciences, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 143-701, South KoreaanNoYW4wNkBrb25rdWsuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.