
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 07 March 2014
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 6 - 2014 | https://doi.org/10.3389/fnagi.2014.00036
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease in ageing individuals. It is now clear that genetic susceptibility and environmental factors play a role in disease etiology and progression. Because environmental factors are involved with the majority of the cases of PD, it is important to understand the role nutrition plays in both neuroprotection and neurodegeneration. Recent epidemiological studies have revealed the promise of some nutrients in reducing the risk of PD. In contrast, other nutrients may be involved with the etiology of neurodegeneration or exacerbate disease progression. This review summarizes the studies that have addressed these issues and describes in detail the nutrients and their putative mechanisms of action in PD.
Parkinson's Disease is a neurodegenerative disease that usually develops late in life and is characterized by the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Most cases of Parkinson's disease (PD) are idiopathic since their cause is unknown. Genetic susceptibility and environmental factors (Warner and Schapira, 2003) that mediate mitochondrial dysfunction, inflammation, abrogation of the autosomal-lysomal autophagy system (Beal, 2003), and endoplasmic reticulum stress (Ryu et al., 2002) play a role in disease development.
A growing body of evidence suggests that nutrition may play an important role in PD. Epidemiological and biochemical studies have recently identified promising components in certain food groups that may elicit neuroprotection in PD (Searles Nielsen et al., 2013; Shaltiel-Karyo et al., 2013). However, inclusion or exclusion of other food groups may trigger or exacerbate neurodegeneration. In this review, we focus on the role nutrition plays in promoting or slowing PD.
Dairy product consumption and drinking milk may increase one's risk of PD independently of calcium intake (Hellenbrand et al., 1996b; Chen et al., 2002; Park et al., 2005; Kyrozis et al., 2013), particularly in men (Chen et al., 2007a). Nonetheless, a positive association between milk consumption and PD risk was also observed in women in one study (Saaksjarvi et al., 2013). Preliminary research shows that individuals who consume large amounts of dairy products may often have low serum uric acid levels (Choi et al., 2005a). Serum urate and uric acid is inversely correlated with the risk of PD and disease duration (Weisskopf et al., 2007; Schlesinger and Schlesinger, 2008; Andreadou et al., 2009; Shen et al., 2013). The neuroprotective effects of serum urate may be limited to men (Gao et al., 2008; Shen et al., 2013) since the same is not observed in women (O'Reilly et al., 2010). In addition, the possible presence of dopaminergic neurotoxins, including pesticides and polychlorinated biphenyls in dairy products may increase the risk of PD (Chen et al., 2002). Accordingly, postmortem studies show higher levels of organochlorines, including dieldrin, an organochlorine pesticide, and polychlorinated biphenyls in the brains of PD patients compared to non-neurological controls (Fleming et al., 1994; Corrigan et al., 1998). Yet, the presence of dopaminergic neurotoxins may not be the only component responsible for the relationship between dairy products and PD. In fact, a strong positive association with the consumption of milk, but not cheese or yoghurt has been reported (Kyrozis et al., 2013). Therefore, other constituents in milk may be detrimental with regards to PD and additional studies are needed in order to identify them. The association between dairy products and PD should be interpreted with caution, however, as other studies have found conflicting results (Miyake et al., 2011c).
The health benefits associated with the intake of phytochemicals present in fruits and vegetables leads to decreased functional decline associated with aging and may slow the progression of PD (Liu, 2003). Epidemiological studies found that high intake of fruits, vegetables and fish was inversely associated with PD risk (Gao et al., 2007; Okubo et al., 2012). Dietary patterns, characteristic of a Mediterranean diet, are emerging as a potential neuroprotective alternative for PD (Alcalay et al., 2012).
Most fruits and vegetables are rich sources of antioxidants, including vitamins A, B (riboflavin), C, and E, which are present in low levels in some PD patients. Numerous studies have reported a decrease in peroxidase (Ambani et al., 1975), glutathion-peroxidase activities (Kish et al., 1985), and glutathione (Riederer et al., 1989) in the SN of PD patients postmortem; suggesting metabolic failure in antioxidant mechanisms and chemical processes can lead to lipid peroxidation and parkinsonian characteristics (Uttara et al., 2009).
Although the antioxidant capacity of some fruits and vegetables is evidenced in numerous studies, a recent investigation raised caution about the antioxidant properties of pomegranate. Contrary to the previously reported neuroprotective effects observed in Alzheimer's Disease (Hartman et al., 2006), pomegranate juice exacerbated oxidative stress and neurodegeneration in a rotenone model of PD (Tapias et al., 2013). However, the authors suggest that oxidative stress in a rotenone model may be substantially overwhelming and promegranate may act as a pro-oxidant.
Epidemiological studies have found a decrease in PD risk in individuals who consume foods containing carotenoids and β-carotene (Miyake et al., 2011a). Carotenoids possess antioxidant properties; they act as a reducing agent by protecting lipids through oxidation interference and free radical entrapment (Paiva and Russell, 1999). In mice, pretreatment with β-carotene partially protected against MPTP-induced neurotoxicity (Perry et al., 1985; Yong et al., 1986), but not in primates (Perry et al., 1987). Lycopene, another carotenoid compound, reduces oxidative stress and cognitive decline in a rotenone-induced rodent model of PD (Kaur et al., 2011). One should be cautious however about applying conclusions from animal models about the benefits of carotenoids to humans, since most animals do not absorb or metabolize carotenoids in a similar manner (Paiva and Russell, 1999).
Riboflavin is an integral component of the coenzymes flavin adenine dinucleotide and flavin mononucleotide. Flavin coenzymes participate in oxidation–reduction reactions where they are a major source of energy and are critical for carbohydrate, fat and protein metabolism (Massey, 2000). It has been suggested that riboflavin may be involved in glutathione depletion, cumulative mitochondrial DNA mutations, disturbed mitochondrial protein complexes, and abnormal iron metabolism (Coimbra and Junqueira, 2003). Despite these characteristics, some studies found that riboflavin is not associated with the risk of PD (Abbott et al., 2003; Murakami et al., 2010b), whereas another study observed improved motor skills in PD patients with daily supplementation of riboflavin for 6 months and elimination of red meat (Coimbra and Junqueira, 2003). However, several limitations of this study including omission of a placebo control group and the investigators not being blinded have lead others to question these findings (Ferraz et al., 2004). Another important consideration is that lower protein consumption may affect the absorption of levodopa (Pare et al., 1992; Crevoisier et al., 2003). Therefore, the apparent benefit in motor skills could have resulted from a better absorption of levodopa as opposed to riboflavin supplementation (Ferraz et al., 2004). In addition, intake of other related B vitamins including folate, vitamin B6 and B12 are not associated with a risk of PD (Chen et al., 2004). However, low intake of vitamin B6 is associated with an increased risk of PD (Murakami et al., 2010b). Larger placebo controlled blinded studies done over a longer period of time would be beneficial for determining if riboflavin or other related B vitamins are useful supplements for PD patients.
Recently, dietary intake of nicotine-containing vegetables from edible Solanaceae including tomatoes, potatoes, and peppers, was associated with a reduced risk of PD in men and woman who had never smoked cigarettes or tobacco (Searles Nielsen et al., 2013). It remains unclear as to whether the observed protective effect was due to the nicotine content or other components of this group of vegetables. Cruciferous vegetables such as cauliflower, cabbage, and broccoli, are another group of vegetables rich in antioxidants with neuroprotective capacity. For example, sulforaphane and erucin, are potent naturally occurring isothiocyanates found in cruciferous vegetables with antioxidant properties. Treatment with sulforaphane ameloriated motor deficits and protected dopaminergic neurons in a 6-OHDA mouse model of PD (Morroni et al., 2013). Similarly, erucin provided neuroprotective effects by preventing oxidative damage induced by 6-OHDA in an in vitro model (Tarozzi et al., 2012). Both, sulforaphane and erucin appear to be promising neuroprotective agents in chronic neurodegenerative diseases (Tarozzi et al., 2013). Taken together, these findings highlight the effects of some vegetables, fruits and constituents they contain as having neuroprotective potential.
Omega-3 polyunsaturated fatty acids (PUFAs) appear to be neuroprotective for several neurodegenerative diseases (Bousquet et al., 2011a). There have been no studies in PD patients that address whether omega-3s are neuroprotective, however, one study showed that supplementation with omega-3 PUFA reduced depression in PD patients (Da Silva et al., 2008). Current research focuses specifically on the omega-3 fatty acid docosahexaenoic acid (DHA). DHA is an essential factor in brain growth and development (Horrocks and Yeo, 1999) and has anti-inflammatory potential due to its ability to inhibit cyclooxygenase-2 (Massaro et al., 2006). DHA protects neurons against cytotoxicity, inhibition of nitrogen oxide (NO) production, and calcium (Ca2+) influx. DHA also increases the activities of antioxidant enzymes glutathione peroxidase and glutathione reductase (Wang et al., 2003). Furthermore, DHA supplementation reduced apoptosis in dopaminergic cells (Ozsoy et al., 2011) and replaced omega-6-PUFAs in the brains of mice post-MPTP treatment (Bousquet et al., 2008). Short-term administration of DHA reduced levodopa-induced dyskinesias in parkinsonian primates by up to 40% (Samadi et al., 2006). Long-term administration of uridine and DHA increased the amount of neural phosphatides in synaptic membranes (Wurtman et al., 2006) and dendritic spines in rodents (Sakamoto et al., 2007). In addition, a reduction in parkinsonian behaviors and elevated dopamine (DA) levels in 6-OHDA rodents was observed after treatment with these supplements (Cansev et al., 2008). Further research on DHA intake in PD patients is needed to assess whether it is beneficial in slowing disease progression.
The protective effects of DHA are mediated by a metabolic derivative known as neuroprotectin D1 (NPD1) (Bazan, 2009; Serhan and Petasis, 2011). NPD1 protects neurons against oxidative stress, inflammation, the disruption of the cytoskeleton, and from the activation of apoptotic signaling pathways. DHA may protect the brain by increasing glutathione reductase activity that results in decreased accumulation of oxidized proteins (Calon et al., 2004; Wu et al., 2004), lipid peroxide and reactive oxygen species (ROS) (Hashimoto et al., 2005). DHA also inactivates caspase activation signaling pathways (Calon et al., 2005), inhibits hyperphosphorylation of tau (Green et al., 2007) and regulates the PI3K/Akt cascade (Akbar and Kim, 2002). Other potential mechanisms of action of DHA include regulation of inflammation, transcription, and cell membrane properties (De Urquiza et al., 2000; Salem et al., 2000; Jump, 2002).
The precursor to DHA, eicosapentaenoic acid (EPA) is neuroprotective in experimental models of PD (Song et al., 2009; Meng et al., 2010; Taepavarapruk and Song, 2010; Luchtman et al., 2012). In in vitro models of PD, EPA attenuated an MPP+-induced reduction in cell viability and suppressed pro-inflammatory cytokines (Luchtman et al., 2013). A diet rich in EPA diminished hypokinesia induced by MPTP in mice and ameliorated procedural memory deficit (Luchtman et al., 2013).
Because DHA and EPA provide neuroprotection in animal models, more research is warranted to determine if they are beneficial for PD patients. This could be accomplished by following a large group of individuals at risk for PD, some of who are randomly chosen to receive a supplement and other who receive a placebo. The participants could be followed over several years to determine if they develop PD. Alternatively, a large intervention study testing supplements in patients at various stages of PD might reveal whether motor and/or cognitive symptoms are reduced.
The primary soybean isoflavone genistein is a source of protein that appears to be neuroprotective in ovariectomized rats following 6-OHDA injection, thus suggesting it may be useful for the prevention of PD in post-menopausal women (Kyuhou, 2008). In PD, genistein treatment resulted in dopaminergic neuron protection from lipopolysaccharide (LPS)-induced injury via inhibition of microglia activation (Wang et al., 2005). Genistein pretreatment improved spatial learning and memory in parkinsonian rats (Sarkaki et al., 2009) and restored tyrosine hydroxylase (TH), dopamine transporter (DAT) and Bcl-2 mRNA expression in the midbrain of MPTP-treated animals (Liu et al., 2008). Restored levels of DA and its metabolites, dihydroxyphenylacetic acid, and homovanillic acid, in the striatum were also observed after genistein administration. Additionally, genistein attenuated rotational behavior, protected SNpc neurons (Baluchnejadmojarad et al., 2009), and preserved motor function (Kyuhou, 2008) from 6-OHDA toxicities. Genistein's neuroprotective actions may regulate mitochondria-dependent apoptosis pathways and suppress ROS-induced NF-κ B activation (Qian et al., 2012). These studies suggest that it may be worthwhile to test the neuroprotective benefits of genistein in a clinical trial.
Caffeine is one of the most widely consumed substances. The health promoting benefits of caffeinated beverages is supported by numerous epidemiological studies (Prakash and Tan, 2011; Tanaka et al., 2011). An inverse association between PD and coffee, and caffeine from non-coffee sources, has been reported (Hellenbrand et al., 1997; Fall et al., 1999; Ascherio et al., 2001). In general, animal studies also indicate that caffeine is neuroprotective. The administration of caffeine to maneb- and paraquat-treated rodents reduced the number of degenerating dopaminergic neurons, microglial cells and nitrite content, while normalizing expression of IL-1β, p38 MAPK, NF-kB, and TK (Kachroo et al., 2010; Yadav et al., 2012). Acute and chronic administration of caffeine also reduced the effect of MPTP (Chen et al., 2001) and 6-OHDA treatment on striatal DA loss (Joghataie et al., 2004) and motor dysfunctions (Joghataie et al., 2004; Aguiar et al., 2006) in rats. Caffeine treatment partially restores DA metabolites in rats following 6-OHDA lesions (Aguiar et al., 2006), and provides neuroprotection in MPTP models of PD (Xu et al., 2010), thus extending its beneficial effects. It is important to note that a caffeine tolerance does not develop with long-term exposure in mice (Xu et al., 2002) and neuroprotection is still apparent with caffeine intake after the onset of neurodegeneration in rats (Sonsalla et al., 2012).
Genetic and pharmacological data from rodent studies indicate that caffeine reduces dopaminergic toxicity and slows disease progression through antagonism of adenosine A2A receptors (Morelli et al., 2010; Prediger, 2010; Xiao et al., 2011; Sonsalla et al., 2012). Inhibition of glutamate neurotransmission using A2A receptor antagonists, may relieve motor symptoms and provide neuroprotection in models of late-stage PD (reviewed in Popoli et al., 2004; Chen et al., 2007b). However, methylxanthine derivatives containing properties of monoamine oxidase B (MAO-B) inhibition, like 8-(3-chlorostyryl) caffeine, may cause oxidative stress via dysfunctional vesicular monoamine transporter 2 (VMAT2) and DA storage mechanisms early in PD (Golembiowska and Dziubina, 2012). Currently, clinical studies are underway to evaluate several A2A receptor antagonists for symptomatic relief and slowing of disease progression (reviewed in Hickey and Stacy, 2011). Caffeine has also shown cytoprotective effects through activation of the PI3K/Akt signaling pathway in SH-SY5Y cells (Nakaso et al., 2008). Therefore, caffeine's ability to down-regulate NO production, neuroinflammation, and microglial activation through these pathways may contribute to neuroprotection (Yadav et al., 2012). It is not fully established, however, that caffeine's neuroprotective role is the sole reason for reduced risk of PD. Nor is it known whether the association is causal rather than reverse causation; the protective effect of caffeine could also reflect an effect of symptoms of PD on caffeine consumption.
Estrogen has significant effects on caffeine's neuroprotective capabilities. Epidemiological studies have consistently demonstrated a greater improvement in male than female Parkinson's patients (Ascherio et al., 2001; Costa et al., 2010). Interestingly, post-menopausal women who are not taking hormone-replacement therapy receive the same neuroprotective benefits as men (Ascherio et al., 2001). However, high caffeine consumption was associated with an increased risk of PD among women using hormones (Ascherio et al., 2003). More recently, findings from a larger prospective study are consistent with a neuroprotective effect of caffeine intake in men and an attenuated effect in women due to hormone replacement therapy (Palacios et al., 2012a). With regards to animal models, estrogen and caffeine co-administration in MPTP-treated mice, prevented neuroprotection in males and females (Xu et al., 2006). Together these studies suggest that the beneficial effects of caffeine may be limited to men and post-menopausal women not receiving hormone-replacement therapy. However, an open-label study examining caffeine's symptomatic effects and tolerability in patients demonstrated improved non-motor aspects of PD with no gender differences (Altman et al., 2011). Currently adenosine A2A antagonists and caffeine are in phase II and III clinical trials for the symptomatic treatment of PD (Barkhoudarian and Schwarzschild, 2011).
Several epidemiological studies have addressed the influence of drinking tea (Camellia sinensis) on the risk of PD. A case-control study of Chinese PD patients showed that regular tea drinking protects against PD (Chan et al., 1998). Another study complimented the Chinese PD study showing a reduced risk for PD with tea consumption (two cups/day) (Checkoway et al., 2002). Similarly, a large prospective study showed a reduced risk of incident PD in subjects who habitually drank three or more cups of tea per day (Hu et al., 2007). A retrospective study associated drinking of more than three cups of tea per day with a delayed onset of motor symptoms in Israeli PD patients (Kandinov et al., 2009). Unfortunately, no distinction between green and black tea was made in these studies.
Several reports have revealed that both black and green tea exert neuroprotective effects in PD animal models (Bastianetto et al., 2006; Chaturvedi et al., 2006). Polyphenols in green and black tea extracts provide highly potent antioxidant-radical scavenging activities in brain mitochondrial membrane fractions (Zhao, 2009). In addition, polyphenols in tea reduce occurrence of disease and provide neuroprotection in cell culture and animal models (Nie et al., 2002; Pan et al., 2003b; Guo et al., 2007). In black tea, the polyphenol theaflavin (TF) possess a wide variety of pharmacological properties including antioxidative, antiapoptotic, and anti-inflammatory effects (Aneja et al., 2004; Gosslau et al., 2011). TF-mediated neuroprotection against MPTP-induced dopaminergic neurodegeneneration in rodents was evidenced by increased expression of nigral TH, DAT and reduced expression of apoptotic markers (Anandhan et al., 2012).
Similarly, the polyphenol (–)-epigallocatechin-3-gallate (EGCG) in green tea shows promise in neuroprotection, but one study showed that green tea drinking was unrelated to the risk of PD (Tan et al., 2008). EGCG inhibits nitric oxide and tumor necrosis factor-α secretion from LPS-activated microglia in dopaminergic mesencephalic cells (Li et al., 2004). Given that microglia play a key role in the generation of free radicals and inflammatory factors in the brain, EGCG was classified as neuroprotective in vivo (Li et al., 2004). Additionally, EGCG improved cell viability and attenuated MPP-induced intracellular ROS formation via the SIRT1/PGC-1α signaling pathway in MPP induced PC12 cells (Ye et al., 2012). EGCG reduced neuronal cell death and induced nitric oxide synthase (NOS) expression in an MPTP mouse model of PD, thus providing further evidence for its neuroprotection via NO reduction (Kim et al., 2010). Oral pretreatment with EGCG prevented dopaminergic neuron loss in MPTP-treated mice (Levites et al., 2001). In contrast, another study found subtle symptomatic relief but no neuroprotection with similar dose of EGCG in rats following a 6-OHDA lesion (Leaver et al., 2009). The differences between the results from these two studies may reflect the different mechanisms by which MPTP and 6-OHDA exert their neurotoxic effects. Also, the poor bioavailability of oral EGCG in rats may explain why similar doses led to different results in animal models (Kim et al., 2000).
Computational molecular modeling has shown that EGCG is a potent, non-competitive inhibitor that invokes various cellular neuroprotection/neurorescue mechanisms (Zhu et al., 2008). EGCG's mechanisms of action include iron-chelation, scavenging of oxygen and nitrogen radical species, activation of protein kinase C (PKC) signaling pathway and expression of pro-survival genes (Weinreb et al., 2009), and restoration of reduced PKC and extracellular signal-regulated kinases (ERK1/2) activities caused by 6-OHDA toxicity (Zhao, 2009). Tea and/or EGCG prevent neurotoxin-induced cell injury (Weinreb et al., 2004), MPTP-induced dopaminergic neurodegeneration and restore striatal levels of DA and its metabolites (Levites et al., 2001; Choi et al., 2002). Green tea polyphenols could also protect dopaminergic neurons against MPTP-induced injury by exerting inhibitory effects on DA-transporters, which block the uptake the metabolite MPP+ (Pan et al., 2003a).
In summary, tea consumption seems to be a promising lifestyle choice that may slow age-related deficits and neurodegenerative diseases. Given the evidence from preclinical studies, green tea polyphenols are currently being tested as a treatment for de novo PD patients (ClinicalTrials.gov identifier: NCT00461942).
Alcohol may exert neuroprotective effects in PD. One case-controlled study found an inverse association between total alcohol consumption and PD (Ragonese et al., 2003). A recent study suggests that low to moderate beer consumption may be associated with a lower PD risk, whereas greater liquor consumption may increase the risk of PD (Liu et al., 2013). Contrary to these findings, most of the epidemiological studies do not support an association between alcohol consumption and risk of PD (Benedetti et al., 2000; Checkoway et al., 2002; Hernan et al., 2003; Palacios et al., 2012b). Currently, the association between alcohol consumption and the risk of PD remains poorly understood.
Despite the conflicting results from epidemiological studies, specific components found in red wine including resveratrol and quercetin, may elicit neuroprotection against PD. Administration of resveratrol or quercetin before MPTP treatment reduced apoptotic cell death and modulated expression of Bax and Bcl-2 in PC12 cultures (Bournival et al., 2009, 2012). Resveratrol has elicited neuroprotective effects by preventing behavioral, biochemical, and histopathological changes that occur in PD animal models (Bureau et al., 2008; Khan et al., 2010). A diet containing resveratrol protects dopaminergic neurons and attenuates motor coordination in MPTP rodent models (Blanchet et al., 2008; Lu et al., 2008). Many studies suggest that the antioxidant actions of resveratrol are responsible for the neuroprotection from MPP+ toxicity (Alvira et al., 2007; Okawara et al., 2007).
Mechanistically, resveratrol reduces inflammation by trapping free radicals and preventing apoptosis of DA-producing neurons (Blanchet et al., 2008; Jin et al., 2008; Lu et al., 2008). In vitro studies showed that resveratrol protects DA neurons against LPS-induced neurotoxicity through the inhibition of microglial activation and subsequent pro-inflammatory factors (Zhang et al., 2010). Resveratrol-mediated neuroprotection has also been attributed to the inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and possibly activation of SIRT1 (Pallas et al., 2009; Zhang et al., 2010). However, one study suggested that SIRT1 activation does not play a major role in the protective effect of resveratrol against MPP+ cytotoxicity (Alvira et al., 2007). Although the evidence from in vitro and animal studies is promising, epidemiological studies do not support an association between red wine consumption and PD (Palacios et al., 2012b). Further research on the type and amount of dietary alcohol intake and the risk of PD would be very beneficial.
Dietary fat has shown inconsistent results in relation to PD. Rodent studies show diets high in fat exacerbate the progression of parkinsonism by exhibiting increased DA depletion in the SN, striatum, and nigrostriatal pathway (Choi et al., 2005b; Morris et al., 2010; Bousquet et al., 2011b). With regards to humans, epidemiological studies found a higher risk of PD among individuals with greater intake of total animal fat (Logroscino et al., 1996; Anderson et al., 1999; Johnson et al., 1999; Chen et al., 2003), whereas other studies show no significant relationship between PD and animal fat (Hellenbrand et al., 1996a; Chen et al., 2002, 2003; Powers et al., 2003). Moreover, the positive association between fat and PD risk reported earlier (Anderson et al., 1999) was not replicated in a larger study (Powers et al., 2003). Nonetheless, the conflicting results from these studies may be attributed to the specific type of fat in the diet, saturated or unsaturated, which is not always specified. Nor is the amount of animal protein consumed to supply the fat intake discussed.
In animal studies and clinical trials, a ketogenic diet, which is high in fat, provided symptomatic and beneficial disease-modifying activity in PD (Gasior et al., 2006). In fact in a small clinical trial, five PD patients on a hyperketonemia diet that substituted unsaturated for saturated fats showed improvement on the Unified Parkinson's Disease Rating Scale (Vanitallie et al., 2005). It should also be noted that the patients on the ketogenic diet ate only 8% protein. Low protein diets lead to better levodopa bioavailability (Pincus and Barry, 1987). It is therefore possible that the observed improvement may have been due to better absorption of synthetic dopamine in four of the patients since one patient was not taking anti-parkinson medication (Vanitallie et al., 2005). Because of the limited number of patients, the difficulty in adhering to a hyperketonemia diet, and the lack of healthy controls, the authors were not able to rule out a placebo effect. The promising results from this preliminary study suggest that another clinical trial of the ketogenic diet that includes a larger number of patients is warranted.
Dietary intake of PUFAs and monounsaturated fatty acids (MUFAs) might influence the risk of PD (Abbott et al., 2003; De Lau et al., 2005). It has been reported in other disease models that PUFA's have anti-inflammatory and neuroprotective properties (Blok et al., 1996; Simopoulos, 1999; Youdim et al., 2000; Kim et al., 2001) and MUFAs are thought to reduce oxidative stress (Colette et al., 2003; Moreno and Mitjavila, 2003). Unsaturated fatty acids are important constituents of neuronal cell membranes and the fatty acid composition of cell membranes is affected by diet. It has been demonstrated in other disease models that infants and young animals with dietary deficiencies in MUFAs and PUFAs have a decrease in brain function (Fernstrom, 1999; Simopoulos, 1999; Youdim et al., 2000; Moreno and Mitjavila, 2003). Moreover, it has been shown that PUFA intake is consistently associated with lower PD risk, and dietary fats modified the association of PD risk with pesticide exposure (Kamel, 2013; Kamel et al., 2013). Notably, PD was inversely associated with the N-3 precursor α-linolenic acid, an essential fatty acid, in a meta-analysis comprising nine studies (Kamel et al., 2013). The health benefit effects of α-linolenic acid may be due to its potential role in protecting against oxidative stress and inflammation (Hassan et al., 2010; Robinson and Mazurak, 2013; Zhang et al., 2013). These studies suggest that a diet high in PUFAs and low in saturated fats might reduce the risk of PD and protect from the toxic effects of neurotoxins, such as those possibly present in milk.
Alternatively, saturated fat could modify the risk of PD by affecting PUFA metabolism and inducing adverse changes in cell membrane lipid composition (Peers, 1997). Thus, fatty acids may contribute to an increased risk of PD via oxidative stress. PUFAs are concentrated in neuronal membranes and play a role in oxidative radical formation. Lipid peroxidation results in oxidative damage and can modify lipid composition of membranes, potentially leading to neuronal death (Farooqui and Horrocks, 1998). In addition, adverse essential fatty acid composition in the mitochondrial membrane may also induce phosphorylation uncoupling, causing energy failure (Peers, 1997). Thus, a high concentration of PUFAs may contribute to neural oxidative stress through lipid peroxidation. Additionally, PD patients have higher concentrations of PUFA peroxidation metabolites and lower concentrations of PUFA and glutathione in the SN compared to healthy controls, further supporting the hypothesis that energy failure may facilitate the onset and/or progression of PD (Chen et al., 2003). However, higher concentrations of PUFA peroxidation metabolites and lower PUFA may arise from several environmental factors in addition to nutrients.
The importance of fats in the pathogenesis of PD in some patients is suggested by genetic studies. Mutations in PARK2, which encodes the PD related factor Parkin, lead to an early onset familial form of PD (Kitada et al., 1998). Parkin is part of the E3 ubiquitin ligase complex that targets specific substrates for degradation via the ubiquitin−proteasome pathway (Shimura et al., 2000). Recently, it was shown that Parkin is a lipid-dependent regulator of fat uptake in mice and patient cells carrying mutations in PARK2 (Kim et al., 2011). These studies suggest that genetic mutations in the uptake or breakdown of fat may be associated with PD.
Lipid and cholesterol metabolism may also play a role in the pathogenesis of idiopathic PD, however the association between cholesterol and PD is highly debated (Hu, 2010). Lower plasma cholesterol concentrations (Lamperti, 1991) and decreased cholesterol biosynthesis is observed in cell lines from PD patients (Musanti et al., 1993), suggesting that low levels of cholesterol may play a role in PD development and/or progression. In contrast, higher total serum cholesterol may be associated with a modest slower progression of PD (Huang et al., 2011) and lower iron content in SN and globus pallidus in PD patients (Du et al., 2012). Interestingly, the association with increased cholesterol levels and decreased PD was seen primarily in women (De Lau et al., 2006). One possible explanation about the lack of an association between cholesterol levels and PD in men may be due to the gender differences of plasma concentration levels of the antioxidant coenzyme Q10 (De Lau et al., 2006), which are significantly higher in men than in women (Kaikkonen et al., 1999). In this regard, it should be noted that coenzyme Q10 has shown neuroprotective properties in numerous PD studies (Shults et al., 2004; Cleren et al., 2008). More recently, the total; HDL cholesterol ratio was found to be inversely associated with disease duration, thereby suggesting an effect of cardiometabolic protection in PD (Cassani et al., 2013). The results from this study must be interpreted with caution since no healthy controls were included in the analysis.
The studies cited above reflect our incomplete understanding regarding the association between fat intake and PD. The role that fat plays in PD is most likely related to the type of fat in the patient's diet (De Lau et al., 2005), the patient's HDL/LDL ratio, total cholesterol levels and genetic factors. Ideally, large prospective randomized controlled studies are needed to clarify the associations between fat intake and PD.
Meat is another source of animal fat and its consumption may be associated with the incidence of PD (Anderson et al., 1999) but the evidence from prospective studies is limited (Gaenslen et al., 2008). Interestingly, intake of processed meat and sausages was inversely associated with PD risk in women (Saaksjarvi et al., 2013). This finding is surprising given the higher incidence of mortality, cardiovascular diseases, and diabetes associated with processed meat consumption (Micha et al., 2010; Rohrmann et al., 2013). In the case of red meat, a positive association between red meat consumption and PD may be explained by the heme content that may act as a toxin when not digested properly. Heme is found in other meats also but not to the same extent. Hemin increases intracellular iron concentrations and hydroxyl radical production, contributing to iron deposits and mitochondrial damage (Schipper, 2000). In this context, iron intake from dietary nutrients may be related to risk for PD (Powers et al., 2003) but the evidence for this association is conflictive (Logroscino et al., 1998, 2008). Despite the inconsistent results, higher intake of iron is associated with neuroprotection in PD (Miyake et al., 2011b). Notwithstanding the positive results, the authors of this study noted that evaluation of dietary intake for 1 month prior to completing the questionnaire by the participants might not properly represent their typical diets.
It has been suggested that carbohydrates increase DA production in the brain by allowing easier passage of the DA precursor, tyrosine, through the blood-brain barrier into cerebrospinal fluid (Fernstrom et al., 1979; Wurtman et al., 2003). Carbohydrates with high glycemic index decrease the risk of PD by an insulin-induced increase in brain DA (Murakami et al., 2010a). A balanced diet of carbohydrate and protein mixture improved motor performance in PD patients (Berry et al., 1991). Yet, epidemiological studies about carbohydrate consumption and PD remain inconclusive. For example, the Nurses Health Study and Health Professionals Follow-up Study reported a non-significant direct association in women and inverse association in men for carbohydrate consumption and PD risk (Chen et al., 2003). In contrast, other studies have shown a positive association for total carbohydrate consumption and PD (Hellenbrand et al., 1996a; Abbott et al., 2003).
High carbohydrate diets are associated with an increased risk of type 2 diabetes (T2DM) (Salmeron et al., 1997a,b; Oba et al., 2013). Interestingly, numerous epidemiological studies indicate T2DM is associated with an increased risk of PD (Schernhammer et al., 2011; Xu et al., 2011; Sun et al., 2012; Cereda et al., 2013) but the evidence presented is conflictive (Simon et al., 2007; Palacios et al., 2011; Noyce et al., 2012). Nonetheless, T2DM is associated with more severe motor symptoms in PD (Kotagal et al., 2013). One possible explanation for the link between both chronic diseases is that alterations in common biological pathways may lead to neurodegeneration in patients with T2DM (Santiago and Potashkin, 2013b). In this regard, emerging research is beginning to elucidate the molecular networks and potential mechanisms implicated in both diseases (Santiago and Potashkin, 2013a; Mattson, 2014; Wang et al., 2014). Since carbohydrates are an important part of people's diets and its high consumption may increase risk for T2DM (Salmeron et al., 1997a,b; Oba et al., 2013) further research on the amount and type of dietary carbohydrates consumed in relationship to the risk of PD would be very beneficial.
Vitamin D deficiency is prevalent in PD patients (Sato et al., 1997); yet, it is unclear if a reduction in Vitamin D is a cause or consequence of PD. Vitamin D plays a role in regulating Ca2+ homeostasis (Garcion et al., 2002; Chan et al., 2009) and if disrupted, SNpc dopaminergic neuron loss is accelerated (Gleichmann and Mattson, 2011). This suggests that dietary regulation of vitamin D may be effective in protecting individuals from PD or slowing PD progression. In animal and cell culture models of PD, vitamin D supplementation was found to be beneficial in slowing disease progression (Wang et al., 2001; Smith et al., 2006; Holick, 2007). In human studies, however, high consumption of food containing vitamin D increased the risk of PD (Anderson et al., 1999). More recently, vitamin D3 supplementation stabilized PD patients' motor symptoms, preventing an increase in the Hoehn and Yahr stage, compared to a placebo-controlled group (Suzuki et al., 2013). It remains unknown if a reduction in vitamin D stemming from nutritional deficiencies causes an increase in PD and/or if an environmental factor such as UV radiation or exposure to sunlight plays a role. Therefore, more research needs to be done in order to link vitamin D supplementation and its effective in protecting individuals from PD or PD progression.
Vitamin C or ascorbate is highly concentrated in the central nervous system and its neuroprotective capabilities show promise in reducing lipid peroxidation levels and increasing catalase activity (Santos et al., 2008). Higher intake of vitamin C correlates with an increase risk of PD (Scheider et al., 1997). In contrast, in a case-controlled study, individuals consuming a diet rich in vitamin C showed a 40% reduction of PD risk (Hellenbrand et al., 1996a). Interestingly, in a pilot study in which high doses of vitamin C and E were given to early stage PD patients, a decrease in disease progression was observed (Fahn, 1992). Despite this progress, other studies have not found a significant association between intake of dietary vitamin C or vitamin C supplements and risk of PD (Zhang et al., 2002; Etminan et al., 2005). Collectively, the association of vitamin C and PD risk remains inconclusive and more studies are needed to clarify this association.
Vitamin E supplementation provides protective effects on DA neurons in the SNpc (Roghani and Behzadi, 2001), reduce DA loss (Lan and Jiang, 1997), and protect against paraquat toxicity (Storch et al., 2000; Osakada et al., 2004) in rodents and in vitro. Pretreatment with vitamin E reduces lipid peroxidation levels (Lan and Jiang, 1997), but depletion of striatal DA was not attenuated in animals (Gong et al., 1991; Chi et al., 1992). The potential benefits seen in vitamin E may be linked to its chain-breaking capabilities in biological membranes, preventing induced oxidative damage by trapping reactive oxyradicals. Yet, other studies have shown that vitamin E has no protective effects against DA-induced toxicity in PC12 cells (Offen et al., 1996) and only partial protection in MPTP-treated marmosets (Perry et al., 1987). A meta-analysis showed a protective effect against PD in humans with both moderate and high intake of vitamin E (Etminan et al., 2005), with a more significant effect observed in men than women (Zhang et al., 2002). In contrast, clinical trials show no neuroprotective benefits from vitamin E in PD patients (Fernandez-Calle et al., 1992; Lewitt, 1994).
Although researchers have started investigating the effect of individual nutrients through supplements the results of these studies remain inconclusive. Antioxidants are much more effective in combinations and therefore a combination of vitamins may be beneficial, perhaps acting synergistically. Thus, we suggest that choosing a diet that contains a variety of foods that are rich in multiple phytochemicals and other bioconstituents may provide a means of disease management. The total elimination of any one food group is not recommended. Additional prospective nutritional studies should help to resolve this issue.
A poor diet will have a negative impact on an individual's health. With regards to neurodegeneration, nutrition affects multiple aspects of neurodevelopment, neurogenesis and the functions of neurons and neural networks (Dauncey and Bicknell, 1999). Nutrition-gene interactions play a critical role in dysfunction and disease (Dauncey, 2012). Individual differences in genes such as single nucleotide polymorphisms, mutations and copy number variants significantly modify the effects of nutrition on gene expression (Dauncey, 2013).
A person's epigenome is just as important as their genome. An individual's epigenome reflects the interaction of the person's genome with their environment. Epigenomic modifications include DNA methylation, which may alter protein-DNA interaction and result in genes being expressed or turned off. Another type of modification is histone modification, which may lead to changes in DNA packaging. Histone modification may also lead to switching a gene on or off by making the DNA packaging more or less accessible to proteins. In addition, epigenetic regulation of gene expression through small non-coding RNAs is environmentally regulated. Epigenetic regulation of gene expression plays an important role in development and pathological processes (Dauncey et al., 2001; Babenko et al., 2012; Dauncey, 2012; Hackett et al., 2012; Park et al., 2012; Qureshi and Mehler, 2013). What a person eats and drinks will impact their epigenome (Dauncey, 1997, 2012; Langie et al., 2012). Currently the details about how individual nutrients affect the epigenome generally remain unknown. This area of nutrition research is still in its infancy. If we want to improve peoples' health it will be important to emphasize this area of research in the future because epigenetic changes also impact future generations since they may be inherited.
Currently, there is an abundance of preliminary evidence that indicates that some nutrients may increase an individual's risk for PD, while others may be neuroprotective (Figure 1, Supplementary Tables 1, 2). These results are not unexpected since nutrients affect mitochondrial energy function and provide vital antioxidant functions that ameliorate the free-radical byproducts of oxidative phosphorylation. A poor diet may lead to increased oxidative stress, which could impede the antioxidant defense system. In contrast, a well-balanced diet rich in a variety of foods, including numerous servings of vegetables and fruits (especially those containing nicotine) and moderate amounts of omega-3 fatty acids, tea, caffeine, and wine may provide neuroprotection.
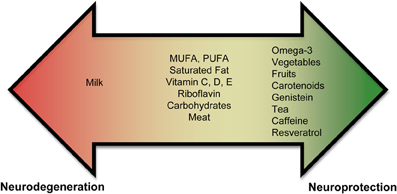
Figure 1. Role of nutrients in PD. Epidemiological and biochemical studies suggest that inclusion or exclusion of certain food groups may elicit neuroprotection or neurodegeneration. Foods are shown on a spectrum. Foods shown in the red promote neurodegeneration and foods in green promote neuroprotection. Foods shown in the middle (or yellow) part of the spectrum have conflicting results and need to be studied further to assess if they play a role in neurodegeneration or neuroprotection.
In spite of promising effectiveness of these nutrients in PD, we lack definitive evidence-based answers as a result of limited large prospective randomized controlled studies designed to address these issues. Indeed, there are several limitations in some epidemiological studies assessing dietary factors and PD that merit further attention. For example, the assumption that dietary patterns remain unchanged over time is a major limitation. Information on diet during development would be very helpful and may weaken or strength a result. In addition, patients with PD may experience non-motor symptoms at early stages such as constipation, dysphagia, depression, and hyposmia that may affect dietary choices and therefore may be responsible for the impairment of nutritional status observed in PD (Ponsen et al., 2004; Barichella et al., 2009). These factors may remain undetected and therefore not properly reported. Incorporation of these critical factors into clinical practice and epidemiological studies will greatly improve the reliability of studies assessing the role of nutrients in PD.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank an anonymous reviewer who made many useful suggestions to improve the manuscript. This work was supported by the US Army Medical Research and Materiel Command under awards number W81XWH-09-0708 and W81XWH-13-1-0025 to Judith A. Potashkin. Opinions, conclusions, interpretations and recommendations are those of the author and are not necessarily endorsed by the US Army.
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fnagi.2014.00036/abstract
Abbott, R. D., Ross, G. W., White, L. R., Sanderson, W. T., Burchfiel, C. M., Kashon, M., et al. (2003). Environmental, life-style, and physical precursors of clinical Parkinson's disease: recent findings from the Honolulu-Asia Aging Study. J. Neurol. 250(Suppl. 3), III30–III39. doi: 10.1007/s00415-003-1306-7
Aguiar, L. M., Nobre, H. V. Jr., Macedo, D. S., Oliveira, A. A., Freitas, R. M., Vasconcelos, S. M., et al. (2006). Neuroprotective effects of caffeine in the model of 6-hydroxydopamine lesion in rats. Pharmacol. Biochem. Behav. 84, 415–419. doi: 10.1016/j.pbb.2006.05.027
Akbar, M., and Kim, H. Y. (2002). Protective effects of docosahexaenoic acid in staurosporine-induced apoptosis: involvement of phosphatidylinositol-3 kinase pathway. J. Neurochem. 82, 655–665. doi: 10.1046/j.1471-4159.2002.01015.x
Alcalay, R. N., Gu, Y., Mejia-Santana, H., Cote, L., Marder, K. S., and Scarmeas, N. (2012). The association between Mediterranean diet adherence and Parkinson's disease. Mov. Disord. 27, 771–774. doi: 10.1002/mds.24918
Altman, R. D., Lang, A. E., and Postuma, R. B. (2011). Caffeine in Parkinson's disease: a pilot open-label, dose-escalation study. Mov. Disord. 26, 2427–2431. doi: 10.1002/mds.23873
Alvira, D., Yeste-Velasco, M., Folch, J., Verdaguer, E., Canudas, A. M., Pallas, M., et al. (2007). Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience 147, 746–756. doi: 10.1016/j.neuroscience.2007.04.029
Ambani, L. M., Van Woert, M. H., and Murphy, S. (1975). Brain peroxidase and catalase in Parkinson disease. Arch. Neurol. 32, 114–118. doi: 10.1001/archneur.1975.00490440064010
Anandhan, A., Tamilselvam, K., Radhiga, T., Rao, S., Essa, M. M., and Manivasagam, T. (2012). Theaflavin, a black tea polyphenol, protects nigral dopaminergic neurons against chronic MPTP/probenecid induced Parkinson's disease. Brain Res. 1433, 104–113. doi: 10.1016/j.brainres.2011.11.021
Anderson, C., Checkoway, H., Franklin, G. M., Beresford, S., Smith-Weller, T., and Swanson, P. D. (1999). Dietary factors in Parkinson's disease: the role of food groups and specific foods. Mov. Disord. 14, 21–27. doi: 10.1002/1531-8257(199901)14:1<21::AID-MDS1006>3.0.CO;2-Y
Andreadou, E., Nikolaou, C., Gournaras, F., Rentzos, M., Boufidou, F., Tsoutsou, A., et al. (2009). Serum uric acid levels in patients with Parkinson's disease: their relationship to treatment and disease duration. Clin. Neurol. Neurosurg. 111, 724–728. doi: 10.1016/j.clineuro.2009.06.012
Aneja, R., Odoms, K., Denenberg, A. G., and Wong, H. R. (2004). Theaflavin, a black tea extract, is a novel anti-inflammatory compound. Crit. Care Med. 32, 2097–2103. doi: 10.1097/01.CCM.0000142661.73633.15
Ascherio, A., Chen, H., Schwarzschild, M. A., Zhang, S. M., Colditz, G. A., and Speizer, F. E. (2003). Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology 60, 790–795. doi: 10.1212/01.WNL.0000046523.05125.87
Ascherio, A., Zhang, S. M., Hernan, M. A., Kawachi, I., Colditz, G. A., Speizer, F. E., et al. (2001). Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann. Neurol. 50, 56–63. doi: 10.1002/ana.1052
Babenko, O., Kovalchuk, I., and Metz, G. A. (2012). Epigenetic programming of neurodegenerative diseases by an adverse environment. Brain Res. 1444, 96–111. doi: 10.1016/j.brainres.2012.01.038
Baluchnejadmojarad, T., Roghani, M., Nadoushan, M. R., and Bagheri, M. (2009). Neuroprotective effect of genistein in 6-hydroxydopamine hemi-parkinsonian rat model. Phytother. Res. 23, 132–135. doi: 10.1002/ptr.2564
Barichella, M., Cereda, E., and Pezzoli, G. (2009). Major nutritional issues in the management of Parkinson's disease. Mov. Disord. 24, 1881–1892. doi: 10.1002/mds.22705
Barkhoudarian, M. T., and Schwarzschild, M. A. (2011). Preclinical jockeying on the translational track of adenosine A2A receptors. Exp. Neurol. 228, 160–164. doi: 10.1016/j.expneurol.2010.12.022
Bastianetto, S., Yao, Z. X., Papadopoulos, V., and Quirion, R. (2006). Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur. J. Neurosci. 23, 55–64. doi: 10.1111/j.1460-9568.2005.04532.x
Bazan, N. G. (2009). Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J. Lipid Res. 50(Suppl.), S400–S405. doi: 10.1194/jlr.R800068-JLR200
Beal, M. F. (2003). Mitochondria, oxidative damage, and inflammation in Parkinson's disease. Ann. N. Y. Acad. Sci. 991, 120–131. doi: 10.1111/j.1749-6632.2003.tb07470.x
Benedetti, M. D., Bower, J. H., Maraganore, D. M., McDonnell, S. K., Peterson, B. J., Ahlskog, J. E., et al. (2000). Smoking, alcohol, and coffee consumption preceding Parkinson's disease: a case-control study. Neurology 55, 1350–1358. doi: 10.1212/WNL.55.9.1350
Berry, E. M., Growdon, J. H., Wurtman, J. J., Caballero, B., and Wurtman, R. J. (1991). A balanced carbohydrate: protein diet in the management of Parkinson's disease. Neurology 41, 1295–1297. doi: 10.1212/WNL.41.8.1295
Blanchet, J., Longpre, F., Bureau, G., Morissette, M., Dipaolo, T., Bronchti, G., et al. (2008). Resveratrol, a red wine polyphenol, protects dopaminergic neurons in MPTP-treated mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 1243–1250. doi: 10.1016/j.pnpbp.2008.03.024
Blok, W. L., Katan, M. B., and Van Der Meer, J. W. (1996). Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J. Nutr. 126, 1515–1533.
Bournival, J., Plouffe, M., Renaud, J., Provencher, C., and Martinoli, M. G. (2012). Quercetin and sesamin protect dopaminergic cells from MPP+-induced neuroinflammation in a microglial (N9)-neuronal (PC12) coculture system. Oxid. Med. Cell. Longev. 2012, 921941. doi: 10.1155/2012/921941
Bournival, J., Quessy, P., and Martinoli, M. G. (2009). Protective effects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 29, 1169–1180. doi: 10.1007/s10571-009-9411-5
Bousquet, M., Calon, F., and Cicchetti, F. (2011a). Impact of omega-3 fatty acids in Parkinson's disease. Ageing Res. Rev. 10, 453–463. doi: 10.1016/j.arr.2011.03.001
Bousquet, M., Saint-Pierre, M., Julien, C., Salem, N. Jr., Cicchetti, F., and Calon, F. (2008). Beneficial effects of dietary omega-3 polyunsaturated fatty acid on toxin-induced neuronal degeneration in an animal model of Parkinson's disease. FASEB J. 22, 1213–1225. doi: 10.1096/fj.07-9677com
Bousquet, M., St-Amour, I., Vandal, M., Julien, P., Cicchetti, F., and Calon, F. (2011b). High-fat diet exacerbates MPTP-induced dopaminergic degeneration in mice. Neurobiol. Dis. 45, 529–538. doi: 10.1016/j.nbd.2011.09.009
Bureau, G., Longpre, F., and Martinoli, M. G. (2008). Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 86, 403–410. doi: 10.1002/jnr.21503
Calon, F., Lim, G. P., Morihara, T., Yang, F., Ubeda, O., Salem, N., et al. (2005). Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer's disease. Eur. J. Neurosci. 22, 617–626. doi: 10.1111/j.1460-9568.2005.04253.x
Calon, F., Lim, G. P., Yang, F., Morihara, T., Teter, B., Ubeda, O., et al. (2004). Docosahexaenoic acid protects from dendritic pathology in an Alzheimer's disease mouse model. Neuron 43, 633–645. doi: 10.1016/j.neuron.2004.08.013
Cansev, M., Ulus, I. H., Wang, L., Maher, T. J., and Wurtman, R. J. (2008). Restorative effects of uridine plus docosahexaenoic acid in a rat model of Parkinson's disease. Neurosci. Res. 62, 206–209. doi: 10.1016/j.neures.2008.07.005
Cassani, E., Cereda, E., Barichella, M., Madio, C., Cancello, R., Caccialanza, R., et al. (2013). Cardiometabolic factors and disease duration in patients with Parkinson's disease. Nutrition 29, 1331–1335. doi: 10.1016/j.nut.2013.04.013
Cereda, E., Barichella, M., Pedrolli, C., Klersy, C., Cassani, E., Caccialanza, R., et al. (2013). Diabetes and risk of Parkinson's disease. Mov. Disord. 28, 257. doi: 10.1002/mds.25211
Chan, C. S., Gertler, T. S., and Surmeier, D. J. (2009). Calcium homeostasis, selective vulnerability and Parkinson's disease. Trends Neurosci. 32, 249–256. doi: 10.1016/j.tins.2009.01.006
Chan, D. K., Woo, J., Ho, S. C., Pang, C. P., Law, L. K., Ng, P. W., et al. (1998). Genetic and environmental risk factors for Parkinson's disease in a Chinese population. J. Neurol. Neurosurg. Psychiatry 65, 781–784. doi: 10.1136/jnnp.65.5.781
Chaturvedi, R. K., Shukla, S., Seth, K., Chauhan, S., Sinha, C., Shukla, Y., et al. (2006). Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson's disease. Neurobiol. Dis. 22, 421–434. doi: 10.1016/j.nbd.2005.12.008
Checkoway, H., Powers, K., Smith-Weller, T., Franklin, G. M., Longstreth, W. T. Jr., and Swanson, P. D. (2002). Parkinson's disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 155, 732–738. doi: 10.1093/aje/155.8.732
Chen, H., O'Reilly, E., McCullough, M. L., Rodriguez, C., Schwarzschild, M. A., Calle, E. E., et al. (2007a). Consumption of dairy products and risk of Parkinson's disease. Am. J. Epidemiol. 165, 998–1006. doi: 10.1093/aje/kwk089
Chen, H., Zhang, S. M., Hernan, M. A., Willett, W. C., and Ascherio, A. (2002). Diet and Parkinson's disease: a potential role of dairy products in men. Ann. Neurol. 52, 793–801. doi: 10.1002/ana.10381
Chen, H., Zhang, S. M., Hernan, M. A., Willett, W. C., and Ascherio, A. (2003). Dietary intakes of fat and risk of Parkinson's disease. Am. J. Epidemiol. 157, 1007–1014. doi: 10.1093/aje/kwg073
Chen, H., Zhang, S. M., Schwarzschild, M. A., Hernan, M. A., Logroscino, G., Willett, W. C., et al. (2004). Folate intake and risk of Parkinson's disease. Am. J. Epidemiol. 160, 368–375. doi: 10.1093/aje/kwh213
Chen, J. F., Sonsalla, P. K., Pedata, F., Melani, A., Domenici, M. R., Popoli, P., et al. (2007b). Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog. Neurobiol. 83, 310–331. doi: 10.1016/j.pneurobio.2007.09.002
Chen, J. F., Xu, K., Petzer, J. P., Staal, R., Xu, Y. H., Beilstein, M., et al. (2001). Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J. Neurosci. 21, RC143.
Chi, D. S., Gong, L., Daigneault, E. A., and Kostrzewa, R. M. (1992). Effects of MPTP and vitamin E treatments on immune function in mice. Int. J. Immunopharmacol. 14, 739–746. doi: 10.1016/0192-0561(92)90070-2
Choi, H. K., Liu, S., and Curhan, G. (2005a). Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 52, 283–289. doi: 10.1002/art.20761
Choi, J. Y., Jang, E. H., Park, C. S., and Kang, J. H. (2005b). Enhanced susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in high-fat diet-induced obesity. Free Radic. Biol. Med. 38, 806–816. doi: 10.1016/j.freeradbiomed.2004.12.008
Choi, J. Y., Park, C. S., Kim, D. J., Cho, M. H., Jin, B. K., Pie, J. E., et al. (2002). Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology 23, 367–374. doi: 10.1016/S0161-813X(02)00079-7
Cleren, C., Yang, L., Lorenzo, B., Calingasan, N. Y., Schomer, A., Sireci, A., et al. (2008). Therapeutic effects of coenzyme Q10 (CoQ10) and reduced CoQ10 in the MPTP model of Parkinsonism. J. Neurochem. 104, 1613–1621. doi: 10.1111/j.1471-4159.2007.05097.x
Coimbra, C. G., and Junqueira, V. B. (2003). High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson's disease patients. Braz. J. Med. Biol. Res. 36, 1409–1417. doi: 10.1590/S0100-879X2003001000019
Colette, C., Percheron, C., Pares-Herbute, N., Michel, F., Pham, T. C., Brillant, L., et al. (2003). Exchanging carbohydrates for monounsaturated fats in energy-restricted diets: effects on metabolic profile and other cardiovascular risk factors. Int. J. Obes. Relat. Metab. Disord. 27, 648–656. doi: 10.1038/sj.ijo.0802299
Corrigan, F. M., Murray, L., Wyatt, C. L., and Shore, R. F. (1998). Diorthosubstituted polychlorinated biphenyls in caudate nucleus in Parkinson's disease. Exp. Neurol. 150, 339–342. doi: 10.1006/exnr.1998.6776
Costa, J., Lunet, N., Santos, C., Santos, J., and Vaz-Carneiro, A. (2010). Caffeine exposure and the risk of Parkinson's disease: a systematic review and meta-analysis of observational studies. J. Alzheimers Dis. 20(Suppl. 1), S221–S238. doi: 10.3233/JAD-2010-091525
Crevoisier, C., Zerr, P., Calvi-Gries, F., and Nilsen, T. (2003). Effects of food on the pharmacokinetics of levodopa in a dual-release formulation. Eur. J. Pharm. Biopharm. 55, 71–76. doi: 10.1016/S0939-6411(02)00124-8
Da Silva, T. M., Munhoz, R. P., Alvarez, C., Naliwaiko, K., Kiss, A., Andreatini, R., et al. (2008). Depression in Parkinson's disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. J. Affect. Disord. 111, 351–359. doi: 10.1016/j.jad.2008.03.008
Dauncey, M. J. (1997). From early nutrition and later development… to underlying mechanisms and optimal health. Br. J. Nutr. 78(Suppl. 2), S113–S123. doi: 10.1079/BJN19970226
Dauncey, M. J. (2012). Recent advances in nutrition, genes and brain health. Proc. Nutr. Soc. 71, 581–591. doi: 10.1017/S0029665112000237
Dauncey, M. J. (2013). Genomic and epigenomic insights into nutrition and brain disorders. Nutrients 5, 887–914. doi: 10.3390/nu5030887
Dauncey, M. J., and Bicknell, R. J. (1999). Nutrition and neurodevelopment: mechanisms of developmental dysfunction and disease in later life. Nutr. Res. Rev. 12, 231–253. doi: 10.1079/095442299108728947
Dauncey, M. J., White, P., Burton, K. A., and Katsumata, M. (2001). Nutrition-hormone receptor-gene interactions: implications for development and disease. Proc. Nutr. Soc. 60, 63–72. doi: 10.1079/PNS200071
De Lau, L. M., Bornebroek, M., Witteman, J. C., Hofman, A., Koudstaal, P. J., and Breteler, M. M. (2005). Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology 64, 2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F
De Lau, L. M., Koudstaal, P. J., Hofman, A., and Breteler, M. M. (2006). Serum cholesterol levels and the risk of Parkinson's disease. Am. J. Epidemiol. 164, 998–1002. doi: 10.1093/aje/kwj283
De Urquiza, A. M., Liu, S., Sjoberg, M., Zetterstrom, R. H., Griffiths, W., Sjovall, J., et al. (2000). Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science 290, 2140–2144. doi: 10.1126/science.290.5499.2140
Du, G., Lewis, M. M., Shaffer, M. L., Chen, H., Yang, Q. X., Mailman, R. B., et al. (2012). Serum cholesterol and nigrostriatal R2* values in Parkinson's disease. PLoS ONE 7:e35397. doi: 10.1371/journal.pone.0035397
Etminan, M., Gill, S. S., and Samii, A. (2005). Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson's disease: a meta-analysis. Lancet Neurol. 4, 362–365. doi: 10.1016/S1474-4422(05)70097-1
Fahn, S. (1992). A pilot trial of high-dose alpha-tocopherol and ascorbate in early Parkinson's disease. Ann. Neurol. 32(Suppl.), S128–S132. doi: 10.1002/ana.410320722
Fall, P. A., Fredrikson, M., Axelson, O., and Granerus, A. K. (1999). Nutritional and occupational factors influencing the risk of Parkinson's disease: a case-control study in southeastern Sweden. Mov. Disord. 14, 28–37. doi: 10.1002/1531-8257(199901)14:1<28::AID-MDS1007>3.0.CO;2-O
Farooqui, A. A., and Horrocks, L. A. (1998). Lipid peroxides in the free radical pathophysiology of brain diseases. Cell. Mol. Neurobiol. 18, 599–608. doi: 10.1023/A:1020261600498
Fernandez-Calle, P., Molina, J. A., Jimenez-Jimenez, F. J., Vazquez, A., Pondal, M., Garcia-Ruiz, P. J., et al. (1992). Serum levels of alpha-tocopherol (vitamin E) in Parkinson's disease. Neurology 42, 1064–1066. doi: 10.1212/WNL.42.5.1064
Fernstrom, J. D. (1999). Effects of dietary polyunsaturated fatty acids on neuronal function. Lipids 34, 161–169. doi: 10.1007/s11745-999-0350-3
Fernstrom, J. D., Wurtman, R. J., Hammarstrom-Wiklund, B., Rand, W. M., Munro, H. N., and Davidson, C. S. (1979). Diurnal variations in plasma concentrations of tryptophan, tryosine, and other neutral amino acids: effect of dietary protein intake. Am. J. Clin. Nutr. 32, 1912–1922.
Ferraz, H. B., Quagliato, E. A., Rieder, C. R., Silva, D. J., Teive, H. A., Barbosa, E. R., et al. (2004). Comments on the paper “High doses of riboflavin and the elimination of dietary red meat promote the recovery of some motor functions in Parkinson's disease patients. C.G. Coimbra and V.B.C. Junqueira. Brazilian Journal of Medical and Biological Research, 36: 1409-1417, 2003”. Braz. J. Med. Biol. Res. 37, 1297–1299. discussion: 1299–1302. doi: 10.1590/S0100-879X2004000900002
Fleming, L., Mann, J. B., Bean, J., Briggle, T., and Sanchez-Ramos, J. R. (1994). Parkinson's disease and brain levels of organochlorine pesticides. Ann. Neurol. 36, 100–103. doi: 10.1002/ana.410360119
Gaenslen, A., Gasser, T., and Berg, D. (2008). Nutrition and the risk for Parkinson's disease: review of the literature. J. Neural Transm. 115, 703–713. doi: 10.1007/s00702-007-0005-4
Gao, X., Chen, H., Choi, H. K., Curhan, G., Schwarzschild, M. A., and Ascherio, A. (2008). Diet, urate, and Parkinson's disease risk in men. Am. J. Epidemiol. 167, 831–838. doi: 10.1093/aje/kwm385
Gao, X., Chen, H., Fung, T. T., Logroscino, G., Schwarzschild, M. A., Hu, F. B., et al. (2007). Prospective study of dietary pattern and risk of Parkinson disease. Am. J. Clin. Nutr. 86, 1486–1494.
Garcion, E., Wion-Barbot, N., Montero-Menei, C. N., Berger, F., and Wion, D. (2002). New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 13, 100–105. doi: 10.1016/S1043-2760(01)00547-1
Gasior, M., Rogawski, M. A., and Hartman, A. L. (2006). Neuroprotective and disease-modifying effects of the ketogenic diet. Behav. Pharmacol. 17, 431–439. doi: 10.1097/00008877-200609000-00009
Gleichmann, M., and Mattson, M. P. (2011). Neuronal calcium homeostasis and dysregulation. Antioxid. Redox Signal. 14, 1261–1273. doi: 10.1089/ars.2010.3386
Golembiowska, K., and Dziubina, A. (2012). The effect of adenosine A(2A) receptor antagonists on hydroxyl radical, dopamine, and glutamate in the striatum of rats with altered function of VMAT2. Neurotox. Res. 22, 150–157. doi: 10.1007/s12640-012-9316-9
Gong, L., Daigneault, E. A., Acuff, R. V., and Kostrzewa, R. M. (1991). Vitamin E supplements fail to protect mice from acute MPTP neurotoxicity. Neuroreport 2, 544–546. doi: 10.1097/00001756-199109000-00012
Gosslau, A., En Jao, D. L., Huang, M. T., Ho, C. T., Evans, D., Rawson, N. E., et al. (2011). Effects of the black tea polyphenol theaflavin-2 on apoptotic and inflammatory pathways in vitro and in vivo. Mol. Nutr. Food Res. 55, 198–208. doi: 10.1002/mnfr.201000165
Green, K. N., Martinez-Coria, H., Khashwji, H., Hall, E. B., Yurko-Mauro, K. A., Ellis, L., et al. (2007). Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J. Neurosci. 27, 4385–4395. doi: 10.1523/JNEUROSCI.0055-07.2007
Guo, S., Yan, J., Yang, T., Yang, X., Bezard, E., and Zhao, B. (2007). Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson's disease through inhibition of ROS-NO pathway. Biol. Psychiatry 62, 1353–1362. doi: 10.1016/j.biopsych.2007.04.020
Hackett, J. A., Zylicz, J. J., and Surani, M. A. (2012). Parallel mechanisms of epigenetic reprogramming in the germline. Trends Genet. 28, 164–174. doi: 10.1016/j.tig.2012.01.005
Hartman, R. E., Shah, A., Fagan, A. M., Schwetye, K. E., Parsadanian, M., Schulman, R. N., et al. (2006). Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiol. Dis. 24, 506–515. doi: 10.1016/j.nbd.2006.08.006
Hashimoto, M., Tanabe, Y., Fujii, Y., Kikuta, T., Shibata, H., and Shido, O. (2005). Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr. 135, 549–555.
Hassan, A., Ibrahim, A., Mbodji, K., Coeffier, M., Ziegler, F., Bounoure, F., et al. (2010). An alpha-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-kappaB in rats with TNBS-induced colitis. J. Nutr. 140, 1714–1721. doi: 10.3945/jn.109.119768
Hellenbrand, W., Boeing, H., Robra, B. P., Seidler, A., Vieregge, P., Nischan, P., et al. (1996a). Diet and Parkinson's disease. II: a possible role for the past intake of specific nutrients. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 47, 644–650. doi: 10.1212/WNL.47.3.644
Hellenbrand, W., Seidler, A., Boeing, H., Robra, B. P., Vieregge, P., Nischan, P., et al. (1996b). Diet and Parkinson's disease. I: a possible role for the past intake of specific foods and food groups. Results from a self-administered food-frequency questionnaire in a case-control study. Neurology 47, 636–643. doi: 10.1212/WNL.47.3.636
Hellenbrand, W., Seidler, A., Robra, B. P., Vieregge, P., Oertel, W. H., Joerg, J., et al. (1997). Smoking and Parkinson's disease: a case-control study in Germany. Int. J. Epidemiol. 26, 328–339. doi: 10.1093/ije/26.2.328
Hernan, M. A., Chen, H., Schwarzschild, M. A., and Ascherio, A. (2003). Alcohol consumption and the incidence of Parkinson's disease. Ann. Neurol. 54, 170–175. doi: 10.1002/ana.10611
Hickey, P., and Stacy, M. (2011). Available and emerging treatments for Parkinson's disease: a review. Drug Des. Devel. Ther. 5, 241–254. doi: 10.2147/DDDT.S11836
Holick, M. F. (2007). Vitamin D deficiency. N. Engl. J. Med. 357, 266–281. doi: 10.1056/NEJMra070553
Horrocks, L. A., and Yeo, Y. K. (1999). Health benefits of docosahexaenoic acid (DHA). Pharmacol. Res. 40, 211–225. doi: 10.1006/phrs.1999.0495
Hu, G. (2010). Total cholesterol and the risk of Parkinson's disease: a review for some new findings. Parkinsons Dis. 2010, 836962. doi: 10.4061/2010/836962
Hu, G., Bidel, S., Jousilahti, P., Antikainen, R., and Tuomilehto, J. (2007). Coffee and tea consumption and the risk of Parkinson's disease. Mov. Disord. 22, 2242–2248. doi: 10.1002/mds.21706
Huang, X., Auinger, P., Eberly, S., Oakes, D., Schwarzschild, M., Ascherio, A., et al. (2011). Serum cholesterol and the progression of Parkinson's disease: results from DATATOP. PLoS ONE 6:e22854. doi: 10.1371/journal.pone.0022854
Jin, F., Wu, Q., Lu, Y. F., Gong, Q. H., and Shi, J. S. (2008). Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson's disease in rats. Eur. J. Pharmacol. 600, 78–82. doi: 10.1016/j.ejphar.2008.10.005
Joghataie, M. T., Roghani, M., Negahdar, F., and Hashemi, L. (2004). Protective effect of caffeine against neurodegeneration in a model of Parkinson's disease in rat: behavioral and histochemical evidence. Parkinsonism Relat. Disord. 10, 465–468. doi: 10.1016/j.parkreldis.2004.06.004
Johnson, C. C., Gorell, J. M., Rybicki, B. A., Sanders, K., and Peterson, E. L. (1999). Adult nutrient intake as a risk factor for Parkinson's disease. Int. J. Epidemiol. 28, 1102–1109. doi: 10.1093/ije/28.6.1102
Jump, D. B. (2002). Dietary polyunsaturated fatty acids and regulation of gene transcription. Curr. Opin. Lipidol. 13, 155–164. doi: 10.1097/00041433-200204000-00007
Kachroo, A., Irizarry, M. C., and Schwarzschild, M. A. (2010). Caffeine protects against combined paraquat and maneb-induced dopaminergic neuron degeneration. Exp. Neurol. 223, 657–661. doi: 10.1016/j.expneurol.2010.02.007
Kaikkonen, J., Nyyssonen, K., Tuomainen, T. P., Ristonmaa, U., and Salonen, J. T. (1999). Determinants of plasma coenzyme Q10 in humans. FEBS Lett. 443, 163–166. doi: 10.1016/S0014-5793(98)01712-8
Kamel, F. (2013). Epidemiology. Paths from pesticides to Parkinson's. Science 341, 722–723. doi: 10.1126/science.1243619
Kamel, F., Goldman, S. M., Umbach, D. M., Chen, H., Richardson, G., Barber, M. R., et al. (2013). Dietary fat intake, pesticide use, and Parkinson's disease. Parkinsonism Relat. Disord. 20, 82–87. doi: 10.1016/j.parkreldis.2013.09.023
Kandinov, B., Giladi, N., and Korczyn, A. D. (2009). Smoking and tea consumption delay onset of Parkinson's disease. Parkinsonism Relat. Disord. 15, 41–46. doi: 10.1016/j.parkreldis.2008.02.011
Kaur, H., Chauhan, S., and Sandhir, R. (2011). Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson's disease. Neurochem. Res. 36, 1435–1443. doi: 10.1007/s11064-011-0469-3
Khan, M. M., Ahmad, A., Ishrat, T., Khan, M. B., Hoda, M. N., Khuwaja, G., et al. (2010). Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson's disease. Brain Res. 1328, 139–151. doi: 10.1016/j.brainres.2010.02.031
Kim, H. Y., Akbar, M., and Kim, K. Y. (2001). Inhibition of neuronal apoptosis by polyunsaturated fatty acids. J. Mol. Neurosci. 16, 223–227. discussion: 279–284. doi: 10.1385/JMN:16:2-3:223
Kim, J. S., Kim, J. M., O, J. J., and Jeon, B. S. (2010). Inhibition of inducible nitric oxide synthase expression and cell death by (-)-epigallocatechin-3-gallate, a green tea catechin, in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson's disease. J.Clin. Neurosci. 17, 1165–1168. doi: 10.1016/j.jocn.2010.01.042
Kim, K. Y., Stevens, M. V., Akter, M. H., Rusk, S. E., Huang, R. J., Cohen, A., et al. (2011). Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J. Clin. Invest. 121, 3701–3712. doi: 10.1172/JCI44736
Kim, S., Lee, M. J., Hong, J., Li, C., Smith, T. J., Yang, G. Y., et al. (2000). Plasma and tissue levels of tea catechins in rats and mice during chronic consumption of green tea polyphenols. Nutr. Cancer 37, 41–48. doi: 10.1207/S15327914NC3701_5
Kish, S. J., Morito, C., and Hornykiewicz, O. (1985). Glutathione peroxidase activity in Parkinson's disease brain. Neurosci. Lett. 58, 343–346. doi: 10.1016/0304-3940(85)90078-3
Kitada, T., Asakawa, S., Hattori, N., Matsumine, H., Yamamura, Y., Minoshima, S., et al. (1998). Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608. doi: 10.1038/33416
Kotagal, V., Albin, R. L., Muller, M. L., Koeppe, R. A., Frey, K. A., and Bohnen, N. I. (2013). Diabetes is associated with postural instability and gait difficulty in Parkinson disease. Parkinsonism Relat. Disord. 19, 522–526. doi: 10.1016/j.parkreldis.2013.01.016
Kyrozis, A., Ghika, A., Stathopoulos, P., Vassilopoulos, D., Trichopoulos, D., and Trichopoulou, A. (2013). Dietary and lifestyle variables in relation to incidence of Parkinson's disease in Greece. Eur. J. Epidemiol. 28, 67–77. doi: 10.1007/s10654-012-9760-0
Kyuhou, S. (2008). Preventive effects of genistein on motor dysfunction following 6-hydroxydopamine injection in ovariectomized rats. Neurosci. Lett. 448, 10–14. doi: 10.1016/j.neulet.2008.10.045
Lamperti, E. (1991). “Decreased concentration of low density lipoprotein cholesterol in patients with parkinson's disease,” in Clinical Research, Vol. 39, eds R. Musanti, N. Rocca, G. Ghiselli, and E. Parat, 401A.
Lan, J., and Jiang, D. H. (1997). Desferrioxamine and vitamin E protect against iron and MPTP-induced neurodegeneration in mice. J. Neural Transm. 104, 469–481. doi: 10.1007/BF01277665
Langie, S. A., Lara, J., and Mathers, J. C. (2012). Early determinants of the ageing trajectory. Best Pract. Res. Clin. Endocrinol. Metab. 26, 613–626. doi: 10.1016/j.beem.2012.03.004
Leaver, K. R., Allbutt, H. N., Creber, N. J., Kassiou, M., and Henderson, J. M. (2009). Oral pre-treatment with epigallocatechin gallate in 6-OHDA lesioned rats produces subtle symptomatic relief but not neuroprotection. Brain Res. Bull. 80, 397–402. doi: 10.1016/j.brainresbull.2009.08.013
Levites, Y., Weinreb, O., Maor, G., Youdim, M. B., and Mandel, S. (2001). Green tea polyphenol (-)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 78, 1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x
Lewitt, P. A. (1994). Clinical trials of neuroprotection in Parkinson's disease: long-term selegiline and alpha-tocopherol treatment. J. Neural Transm. Suppl. 43, 171–181.
Li, R., Huang, Y. G., Fang, D., and Le, W. D. (2004). (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 78, 723–731. doi: 10.1002/jnr.20315
Liu, L. X., Chen, W. F., Xie, J. X., and Wong, M. S. (2008). Neuroprotective effects of genistein on dopaminergic neurons in the mice model of Parkinson's disease. Neurosci. Res. 60, 156–161. doi: 10.1016/j.neures.2007.10.005
Liu, R., Guo, X., Park, Y., Wang, J., Huang, X., Hollenbeck, A., et al. (2013). Alcohol consumption, types of alcohol, and Parkinson's disease. PLoS ONE 8:e66452. doi: 10.1371/journal.pone.0066452
Liu, R. H. (2003). Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 78, 517S-520S.
Logroscino, G., Gao, X., Chen, H., Wing, A., and Ascherio, A. (2008). Dietary iron intake and risk of Parkinson's disease. Am. J. Epidemiol. 168, 1381–1388. doi: 10.1093/aje/kwn273
Logroscino, G., Marder, K., Cote, L., Tang, M. X., Shea, S., and Mayeux, R. (1996). Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann. Neurol. 39, 89–94. doi: 10.1002/ana.410390113
Logroscino, G., Marder, K., Graziano, J., Freyer, G., Slavkovich, V., Lojacono, N., et al. (1998). Dietary iron, animal fats, and risk of Parkinson's disease. Mov. Disord. 13(Suppl. 1), 13–16.
Lu, K. T., Ko, M. C., Chen, B. Y., Huang, J. C., Hsieh, C. W., Lee, M. C., et al. (2008). Neuroprotective effects of resveratrol on MPTP-induced neuron loss mediated by free radical scavenging. J. Agric. Food Chem. 56, 6910–6913. doi: 10.1021/jf8007212
Luchtman, D. W., Meng, Q., and Song, C. (2012). Ethyl-eicosapentaenoate (E-EPA) attenuates motor impairments and inflammation in the MPTP-probenecid mouse model of Parkinson's disease. Behav. Brain Res. 226, 386–396. doi: 10.1016/j.bbr.2011.09.033
Luchtman, D. W., Meng, Q., Wang, X., Shao, D., and Song, C. (2013). omega-3 fatty acid eicosapentaenoic acid attenuates MPP+-induced neurodegeneration in fully differentiated human SH-SY5Y and primary mesencephalic cells. J. Neurochem. 124, 855–868. doi: 10.1111/jnc.12068
Massaro, M., Habib, A., Lubrano, L., Del Turco, S., Lazzerini, G., Bourcier, T., et al. (2006). The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc. Natl. Acad. Sci. U.S.A. 103, 15184–15189. doi: 10.1073/pnas.0510086103
Massey, V. (2000). The chemical and biological versatility of riboflavin. Biochem. Soc. Trans. 28, 283–296. doi: 10.1042/0300-5127:0280283
Mattson, M. P. (2014). Interventions that improve body and brain bioenergetics for Parkinson's disease risk reduction and therapy. J. Parkinsons Dis. 4, 1–13. doi: 10.3233/JPD-130335
Meng, Q., Luchtman, D. W., El Bahh, B., Zidichouski, J. A., Yang, J., and Song, C. (2010). Ethyl-eicosapentaenoate modulates changes in neurochemistry and brain lipids induced by parkinsonian neurotoxin 1-methyl-4-phenylpyridinium in mouse brain slices. Eur. J. Pharmacol. 649, 127–134. doi: 10.1016/j.ejphar.2010.09.046
Micha, R., Wallace, S. K., and Mozaffarian, D. (2010). Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 121, 2271–2283. doi: 10.1161/CIRCULATIONAHA.109.924977
Miyake, Y., Fukushima, W., Tanaka, K., Sasaki, S., Kiyohara, C., Tsuboi, Y., et al. (2011a). Dietary intake of antioxidant vitamins and risk of Parkinson's disease: a case-control study in Japan. Eur. J. Neurol. 18, 106–113. doi: 10.1111/j.1468-1331.2010.03088.x
Miyake, Y., Tanaka, K., Fukushima, W., Sasaki, S., Kiyohara, C., Tsuboi, Y., et al. (2011b). Dietary intake of metals and risk of Parkinson's disease: a case-control study in Japan. J. Neurol. Sci. 306, 98–102. doi: 10.1016/j.jns.2011.03.035
Miyake, Y., Tanaka, K., Fukushima, W., Sasaki, S., Kiyohara, C., Tsuboi, Y., et al. (2011c). Lack of association of dairy food, calcium, and vitamin D intake with the risk of Parkinson's disease: a case-control study in Japan. Parkinsonism Relat. Disord. 17, 112–116. doi: 10.1016/j.parkreldis.2010.11.018
Morelli, M., Carta, A. R., Kachroo, A., and Schwarzschild, M. A. (2010). Pathophysiological roles for purines: adenosine, caffeine and urate. Prog. Brain Res. 183, 183–208. doi: 10.1016/S0079-6123(10)83010-9
Moreno, J. J., and Mitjavila, M. T. (2003). The degree of unsaturation of dietary fatty acids and the development of atherosclerosis (review). J. Nutr. Biochem. 14, 182–195. doi: 10.1016/S0955-2863(02)00294-2
Morris, J. K., Bomhoff, G. L., Stanford, J. A., and Geiger, P. C. (2010). Neurodegeneration in an animal model of Parkinson's disease is exacerbated by a high-fat diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1082–R1090. doi: 10.1152/ajpregu.00449.2010
Morroni, F., Tarozzi, A., Sita, G., Bolondi, C., Zolezzi Moraga, J. M., Cantelli-Forti, G., et al. (2013). Neuroprotective effect of sulforaphane in 6-hydroxydopamine-lesioned mouse model of Parkinson's disease. Neurotoxicology 36, 63–71. doi: 10.1016/j.neuro.2013.03.004
Murakami, K., Miyake, Y., Sasaki, S., Tanaka, K., Fukushima, W., Kiyohara, C., et al. (2010a). Dietary glycemic index is inversely associated with the risk of Parkinson's disease: a case-control study in Japan. Nutrition 26, 515–521. doi: 10.1016/j.nut.2009.05.021
Murakami, K., Miyake, Y., Sasaki, S., Tanaka, K., Fukushima, W., Kiyohara, C., et al. (2010b). Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson's disease: a case-control study in Japan. Br. J. Nutr. 104, 757–764. doi: 10.1017/S0007114510001005
Musanti, R., Parati, E., Lamperti, E., and Ghiselli, G. (1993). Decreased cholesterol biosynthesis in fibroblasts from patients with Parkinson disease. Biochem. Med. Metab. Biol. 49, 133–142. doi: 10.1006/bmmb.1993.1016
Nakaso, K., Ito, S., and Nakashima, K. (2008). Caffeine activates the PI3K/Akt pathway and prevents apoptotic cell death in a Parkinson's disease model of SH-SY5Y cells. Neurosci. Lett. 432, 146–150. doi: 10.1016/j.neulet.2007.12.034
Nie, G., Cao, Y., and Zhao, B. (2002). Protective effects of green tea polyphenols and their major component, (-)-epigallocatechin-3-gallate (EGCG), on 6-hydroxydopamine-induced apoptosis in PC12 cells. Redox Rep. 7, 171–177. doi: 10.1179/135100002125000424
Noyce, A. J., Bestwick, J. P., Silveira-Moriyama, L., Hawkes, C. H., Giovannoni, G., Lees, A. J., et al. (2012). Meta-analysis of early nonmotor features and risk factors for Parkinson disease. Ann. Neurol. 72, 893–901. doi: 10.1002/ana.23687
Oba, S., Nanri, A., Kurotani, K., Goto, A., Kato, M., Mizoue, T., et al. (2013). Dietary glycemic index, glycemic load and incidence of type 2 diabetes in Japanese men and women: the Japan public health center-based prospective study. Nutr. J. 12, 165. doi: 10.1186/1475-2891-12-165
Offen, D., Ziv, I., Sternin, H., Melamed, E., and Hochman, A. (1996). Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson's disease. Exp. Neurol. 141, 32–39. doi: 10.1006/exnr.1996.0136
Okawara, M., Katsuki, H., Kurimoto, E., Shibata, H., Kume, T., and Akaike, A. (2007). Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem. Pharmacol. 73, 550–560. doi: 10.1016/j.bcp.2006.11.003
Okubo, H., Miyake, Y., Sasaki, S., Murakami, K., Tanaka, K., Fukushima, W., et al. (2012). Dietary patterns and risk of Parkinson's disease: a case-control study in Japan. Eur. J. Neurol. 19, 681–688. doi: 10.1111/j.1468-1331.2011.03600.x
O'Reilly, E. J., Gao, X., Weisskopf, M. G., Chen, H., Schwarzschild, M. A., Spiegelman, D., et al. (2010). Plasma urate and Parkinson's disease in women. Am. J. Epidemiol. 172, 666–670. doi: 10.1093/aje/kwq195
Osakada, F., Hashino, A., Kume, T., Katsuki, H., Kaneko, S., and Akaike, A. (2004). Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology 47, 904–915. doi: 10.1016/j.neuropharm.2004.06.029
Ozsoy, O., Seval-Celik, Y., Hacioglu, G., Yargicoglu, P., Demir, R., Agar, A., et al. (2011). The influence and the mechanism of docosahexaenoic acid on a mouse model of Parkinson's disease. Neurochem. Int. 59, 664–670. doi: 10.1016/j.neuint.2011.06.012
Paiva, S. A., and Russell, R. M. (1999). Beta-carotene and other carotenoids as antioxidants. J. Am. Coll. Nutr. 18, 426–433. doi: 10.1080/07315724.1999.10718880
Palacios, N., Gao, X., McCullough, M. L., Jacobs, E. J., Patel, A. V., Mayo, T., et al. (2011). Obesity, diabetes, and risk of Parkinson's disease. Mov. Disord. 26, 2253–2259. doi: 10.1002/mds.23855
Palacios, N., Gao, X., McCullough, M. L., Schwarzschild, M. A., Shah, R., Gapstur, S., et al. (2012a). Caffeine and risk of Parkinson's disease in a large cohort of men and women. Mov. Disord. 27, 1276–1282. doi: 10.1002/mds.25076
Palacios, N., Gao, X., O'Reilly, E., Schwarzschild, M., McCullough, M. L., Mayo, T., et al. (2012b). Alcohol and risk of Parkinson's disease in a large, prospective cohort of men and women. Mov. Disord. 27, 980–987. doi: 10.1002/mds.25050
Pallas, M., Casadesus, G., Smith, M. A., Coto-Montes, A., Pelegri, C., Vilaplana, J., et al. (2009). Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Curr. Neurovasc. Res. 6, 70–81. doi: 10.2174/156720209787466019
Pan, T., Fei, J., Zhou, X., Jankovic, J., and Le, W. (2003a). Effects of green tea polyphenols on dopamine uptake and on MPP+-induced dopamine neuron injury. Life Sci. 72, 1073–1083. doi: 10.1016/S0024-3205(02)02347-0
Pan, T., Jankovic, J., and Le, W. (2003b). Potential therapeutic properties of green tea polyphenols in Parkinson's disease. Drugs Aging 20, 711–721. doi: 10.2165/00002512-200320100-00001
Pare, S., Barr, S. I., and Ross, S. E. (1992). Effect of daytime protein restriction on nutrient intakes of free-living Parkinson's disease patients. Am. J. Clin. Nutr. 55, 701–707.
Park, L. K., Friso, S., and Choi, S. W. (2012). Nutritional influences on epigenetics and age-related disease. Proc. Nutr. Soc. 71, 75–83. doi: 10.1017/S0029665111003302
Park, M., Ross, G. W., Petrovitch, H., White, L. R., Masaki, K. H., Nelson, J. S., et al. (2005). Consumption of milk and calcium in midlife and the future risk of Parkinson disease. Neurology 64, 1047–1051. doi: 10.1212/01.WNL.0000154532.98495.BF
Perry, T. L., Yong, V. W., Clavier, R. M., Jones, K., Wright, J. M., Foulks, J. G., et al. (1985). Partial protection from the dopaminergic neurotoxin N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by four different antioxidants in the mouse. Neurosci. Lett. 60, 109–114. doi: 10.1016/0304-3940(85)90229-0
Perry, T. L., Yong, V. W., Hansen, S., Jones, K., Bergeron, C., Foulks, J. G., et al. (1987). Alpha-tocopherol and beta-carotene do not protect marmosets against the dopaminergic neurotoxicity of N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J. Neurol. Sci. 81, 321–331. doi: 10.1016/0022-510X(87)90106-7
Pincus, J. H., and Barry, K. M. (1987). Plasma levels of amino acids correlate with motor fluctuations in parkinsonism. Arch. Neurol. 44, 1006–1009. doi: 10.1001/archneur.1987.00520220012007
Ponsen, M. M., Stoffers, D., Booij, J., Van Eck-Smit, B. L., Wolters, E., and Berendse, H. W. (2004). Idiopathic hyposmia as a preclinical sign of Parkinson's disease. Ann. Neurol. 56, 173–181. doi: 10.1002/ana.20160
Popoli, P., Minghetti, L., Tebano, M. T., Pintor, A., Domenici, M. R., and Massotti, M. (2004). Adenosine A2A receptor antagonism and neuroprotection: mechanisms, lights, and shadows. Crit. Rev. Neurobiol. 16, 99–106. doi: 10.1615/CritRevNeurobiol.v16.i12.110
Powers, K. M., Smith-Weller, T., Franklin, G. M., Longstreth, W. T. Jr., Swanson, P. D., and Checkoway, H. (2003). Parkinson's disease risks associated with dietary iron, manganese, and other nutrient intakes. Neurology 60, 1761–1766. doi: 10.1212/01.WNL.0000068021.13945.7F
Prakash, K. M., and Tan, E. K. (2011). Clinical evidence linking coffee and tea intake with Parkinson's disease. Basal Ganglia 1, 127–130. doi: 10.1016/j.baga.2011.07.001
Prediger, R. D. (2010). Effects of caffeine in Parkinson's disease: from neuroprotection to the management of motor and non-motor symptoms. J. Alzheimers Dis 20(Suppl. 1), S205–S220. doi: 10.3233/JAD-2010-091459
Qian, Y., Guan, T., Huang, M., Cao, L., Li, Y., Cheng, H., et al. (2012). Neuroprotection by the soy isoflavone, genistein, via inhibition of mitochondria-dependent apoptosis pathways and reactive oxygen induced-NF-kappaB activation in a cerebral ischemia mouse model. Neurochem. Int. 60, 759–767. doi: 10.1016/j.neuint.2012.03.011
Qureshi, I. A., and Mehler, M. F. (2013). Epigenetic mechanisms governing the process of neurodegeneration. Mol. Aspects Med. 34, 875–882. doi: 10.1016/j.mam.2012.06.011
Ragonese, P., Salemi, G., Morgante, L., Aridon, P., Epifanio, A., Buffa, D., et al. (2003). A case-control study on cigarette, alcohol, and coffee consumption preceding Parkinson's disease. Neuroepidemiology 22, 297–304. doi: 10.1159/000071193
Riederer, P., Sofic, E., Rausch, W. D., Schmidt, B., Reynolds, G. P., Jellinger, K., et al. (1989). Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J. Neurochem. 52, 515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x
Robinson, L. E., and Mazurak, V. C. (2013). N-3 polyunsaturated fatty acids: relationship to inflammation in healthy adults and adults exhibiting features of metabolic syndrome. Lipids 48, 319–332. doi: 10.1007/s11745-013-3774-6
Roghani, M., and Behzadi, G. (2001). Neuroprotective effect of vitamin E on the early model of Parkinson's disease in rat: behavioral and histochemical evidence. Brain Res. 892, 211–217. doi: 10.1016/S0006-8993(00)03296-0
Rohrmann, S., Overvad, K., Bueno-De-Mesquita, H. B., Jakobsen, M. U., Egeberg, R., Tjonneland, A., et al. (2013). Meat consumption and mortality–results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 11:63. doi: 10.1186/1741-7015-11-63
Ryu, E. J., Harding, H. P., Angelastro, J. M., Vitolo, O. V., Ron, D., and Greene, L. A. (2002). Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson's disease. J. Neurosci. 22, 10690–10698.
Saaksjarvi, K., Knekt, P., Lundqvist, A., Mannisto, S., Heliovaara, M., Rissanen, H., et al. (2013). A cohort study on diet and the risk of Parkinson's disease: the role of food groups and diet quality. Br. J. Nutr. 109, 329–337. doi: 10.1017/S0007114512000955
Sakamoto, T., Cansev, M., and Wurtman, R. J. (2007). Oral supplementation with docosahexaenoic acid and uridine-5'-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res. 1182, 50–59. doi: 10.1016/j.brainres.2007.08.089
Salem, M. L., Kishihara, K., Abe, K., Matsuzaki, G., and Nomoto, K. (2000). N-3 polyunsaturated fatty acids accentuate B16 melanoma growth and metastasis through suppression of tumoricidal function of T cells and macrophages. Anticancer Res. 20, 3195–3203.
Salmeron, J., Ascherio, A., Rimm, E. B., Colditz, G. A., Spiegelman, D., Jenkins, D. J., et al. (1997a). Dietary fiber, glycemic load, and risk of NIDDM in men. Diabetes Care 20, 545–550. doi: 10.2337/diacare.20.4.545
Salmeron, J., Manson, J. E., Stampfer, M. J., Colditz, G. A., Wing, A. L., and Willett, W. C. (1997b). Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 277, 472–477. doi: 10.1001/jama.1997.03540300040031
Samadi, P., Gregoire, L., Rouillard, C., Bedard, P. J., Di Paolo, T., and Levesque, D. (2006). Docosahexaenoic acid reduces levodopa-induced dyskinesias in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkeys. Ann. Neurol. 59, 282–288. doi: 10.1002/ana.20738
Santiago, J. A., and Potashkin, J. A. (2013a). Integrative network analysis unveils convergent molecular pathways in Parkinson's disease and diabetes. PLoS ONE 8:e83940. doi: 10.1371/journal.pone.0083940
Santiago, J. A., and Potashkin, J. A. (2013b). Shared dysregulated pathways lead to Parkinson's disease and diabetes. Trends Mol. Med. 19, 176–186. doi: 10.1016/j.molmed.2013.01.002
Santos, L. F., Freitas, R. L., Xavier, S. M., Saldanha, G. B., and Freitas, R. M. (2008). Neuroprotective actions of vitamin C related to decreased lipid peroxidation and increased catalase activity in adult rats after pilocarpine-induced seizures. Pharmacol. Biochem. Behav. 89, 1–5. doi: 10.1016/j.pbb.2007.10.007
Sarkaki, A., Badavi, M., Aligholi, H., and Moghaddam, A. Z. (2009). Preventive effects of soy meal (+/- isoflavone) on spatial cognitive deficiency and body weight in an ovariectomized animal model of Parkinson's disease. Pak. J. Biol. Sci. 12, 1338–1345. doi: 10.3923/pjbs.2009.1338.1345
Sato, Y., Kikuyama, M., and Oizumi, K. (1997). High prevalence of vitamin D deficiency and reduced bone mass in Parkinson's disease. Neurology 49, 1273–1278. doi: 10.1212/WNL.49.5.1273
Scheider, W. L., Hershey, L. A., Vena, J. E., Holmlund, T., Marshall, J. R., and Freudenheim (1997). Dietary antioxidants and other dietary factors in the etiology of Parkinson's disease. Mov. Disord. 12, 190–196. doi: 10.1002/mds.870120209
Schernhammer, E., Hansen, J., Rugbjerg, K., Wermuth, L., and Ritz, B. (2011). Diabetes and the risk of developing Parkinson's disease in Denmark. Diabetes Care 34, 1102–1108. doi: 10.2337/dc10-1333
Schipper, H. M. (2000). Heme oxygenase-1: role in brain aging and neurodegeneration. Exp. Gerontol. 35, 821–830. doi: 10.1016/S0531-5565(00)00148-0
Schlesinger, I., and Schlesinger, N. (2008). Uric acid in Parkinson's disease. Mov. Disord. 23, 1653–1657. doi: 10.1002/mds.22139
Searles Nielsen, S., Franklin, G. M., Longstreth, W. T., Swanson, P. D., and Checkoway, H. (2013). Nicotine from edible Solanaceae and risk of Parkinson disease. Ann. Neurol. 74, 472–477. doi: 10.1002/ana.23884
Serhan, C. N., and Petasis, N. A. (2011). Resolvins and protectins in inflammation resolution. Chem. Rev. 111, 5922–5943. doi: 10.1021/cr100396c
Shaltiel-Karyo, R., Frenkel-Pinter, M., Rockenstein, E., Patrick, C., Levy-Sakin, M., Schiller, A., et al. (2013). A blood-brain barrier (BBB) disrupter is also a potent alpha-synuclein (alpha-syn) aggregation inhibitor: a novel dual mechanism of mannitol for the treatment of Parkinson disease (PD). J. Biol. Chem. 288, 17579–17588. doi: 10.1074/jbc.M112.434787
Shen, C., Guo, Y., Luo, W., Lin, C., and Ding, M. (2013). Serum urate and the risk of Parkinson's disease: results from a meta-analysis. Can. J. Neurol. Sci. 40, 73–79.
Shimura, H., Hattori, N., Kubo, S., Mizuno, Y., Asakawa, S., Minoshima, S., et al. (2000). Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 25, 302–305. doi: 10.1038/77060
Shults, C. W., Flint Beal, M., Song, D., and Fontaine, D. (2004). Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Exp. Neurol. 188, 491–494. doi: 10.1016/j.expneurol.2004.05.003
Simon, K. C., Chen, H., Schwarzschild, M., and Ascherio, A. (2007). Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 69, 1688–1695. doi: 10.1212/01.wnl.0000271883.45010.8a
Simopoulos, A. P. (1999). Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 70, 560S–569S.
Smith, M. P., Fletcher-Turner, A., Yurek, D. M., and Cass, W. A. (2006). Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem. Res. 31, 533–539. doi: 10.1007/s11064-006-9048-4
Song, C., Zhang, X. Y., and Manku, M. (2009). Increased phospholipase A2 activity and inflammatory response but decreased nerve growth factor expression in the olfactory bulbectomized rat model of depression: effects of chronic ethyl-eicosapentaenoate treatment. J. Neurosci. 29, 14–22. doi: 10.1523/JNEUROSCI.3569-08.2009
Sonsalla, P. K., Wong, L. Y., Harris, S. L., Richardson, J. R., Khobahy, I., Li, W., et al. (2012). Delayed caffeine treatment prevents nigral dopamine neuron loss in a progressive rat model of Parkinson's disease. Exp. Neurol. 234, 482–487. doi: 10.1016/j.expneurol.2012.01.022
Storch, A., Kaftan, A., Burkhardt, K., and Schwarz, J. (2000). 6-Hydroxydopamine toxicity towards human SH-SY5Y dopaminergic neuroblastoma cells: independent of mitochondrial energy metabolism. J. Neural Transm. 107, 281–293. doi: 10.1007/s007020050023
Sun, Y., Chang, Y. H., Chen, H. F., Su, Y. H., Su, H. F., and Li, C. Y. (2012). Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 35, 1047–1049. doi: 10.2337/dc11-1511
Suzuki, M., Yoshioka, M., Hashimoto, M., Murakami, M., Noya, M., Takahashi, D., et al. (2013). Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 97, 1004–1013. doi: 10.3945/ajcn.112.051664
Taepavarapruk, P., and Song, C. (2010). Reductions of acetylcholine release and nerve growth factor expression are correlated with memory impairment induced by interleukin-1beta administrations: effects of omega-3 fatty acid EPA treatment. J. Neurochem. 112, 1054–1064. doi: 10.1111/j.1471-4159.2009.06524.x
Tan, L. C., Koh, W. P., Yuan, J. M., Wang, R., Au, W. L., Tan, J. H., et al. (2008). Differential effects of black versus green tea on risk of Parkinson's disease in the Singapore Chinese Health Study. Am. J. Epidemiol. 167, 553–560. doi: 10.1093/aje/kwm338
Tanaka, K., Miyake, Y., Fukushima, W., Sasaki, S., Kiyohara, C., Tsuboi, Y., et al. (2011). Intake of Japanese and Chinese teas reduces risk of Parkinson's disease. Parkinsonism Relat. Disord. 17, 446–450. doi: 10.1016/j.parkreldis.2011.02.016
Tapias, V., Cannon, J. R., and Greenamyre, J. T. (2013). Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease. Neurobiol. Aging. 35, 1162–1176. doi: 10.1016/j.neurobiolaging.2013.10.077
Tarozzi, A., Angeloni, C., Malaguti, M., Morroni, F., Hrelia, S., and Hrelia, P. (2013). Sulforaphane as a potential protective phytochemical against neurodegenerative diseases. Oxid. Med. Cell. Longev. 2013:415078. doi: 10.1155/2013/415078
Tarozzi, A., Morroni, F., Bolondi, C., Sita, G., Hrelia, P., Djemil, A., et al. (2012). Neuroprotective effects of erucin against 6-hydroxydopamine-induced oxidative damage in a dopaminergic-like neuroblastoma cell line. Int. J. Mol. Sci. 13, 10899–10910. doi: 10.3390/ijms130910899
Uttara, B., Singh, A. V., Zamboni, P., and Mahajan, R. T. (2009). Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 7, 65–74. doi: 10.2174/157015909787602823
Vanitallie, T. B., Nonas, C., Di Rocco, A., Boyar, K., Hyams, K., and Heymsfield, S. B. (2005). Treatment of Parkinson disease with diet-induced hyperketonemia: a feasibility study. Neurology 64, 728–730. doi: 10.1212/01.WNL.0000152046.11390.45
Wang, J. Y., Sekine, S., and Saito, M. (2003). Effect of docosahexaenoic acid and ascorbate on peroxidation of retinal membranes of ODS rats. Free Radic. Res. 37, 419–424. doi: 10.1080/1071576031000070084
Wang, J. Y., Wu, J. N., Cherng, T. L., Hoffer, B. J., Chen, H. H., Borlongan, C. V., et al. (2001). Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 904, 67–75. doi: 10.1016/S0006-8993(01)02450-7
Wang, L., Zhai, Y. Q., Xu, L. L., Qiao, C., Sun, X. L., Ding, J. H., et al. (2014). Metabolic inflammation exacerbates dopaminergic neuronal degeneration in response to acute MPTP challenge in type 2 diabetes mice. Exp. Neurol. 251, 22–29. doi: 10.1016/j.expneurol.2013.11.001
Wang, X., Chen, S., Ma, G., Ye, M., and Lu, G. (2005). Genistein protects dopaminergic neurons by inhibiting microglial activation. Neuroreport 16, 267–270. doi: 10.1097/00001756-200502280-00013
Warner, T. T., and Schapira, A. H. (2003). Genetic and environmental factors in the cause of Parkinson's disease. Ann. Neurol. 53(Suppl. 3), S16–S23. discussion: S23-S15.
Weinreb, O., Amit, T., Mandel, S., and Youdim, M. B. (2009). Neuroprotective molecular mechanisms of (-)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 4, 283–296. doi: 10.1007/s12263-009-0143-4
Weinreb, O., Mandel, S., Amit, T., and Youdim, M. B. (2004). Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J. Nutr. Biochem. 15, 506–516. doi: 10.1016/j.jnutbio.2004.05.002
Weisskopf, M. G., O'Reilly, E., Chen, H., Schwarzschild, M. A., and Ascherio, A. (2007). Plasma urate and risk of Parkinson's disease. Am. J. Epidemiol. 166, 561–567. doi: 10.1093/aje/kwm127
Wu, A., Ying, Z., and Gomez-Pinilla, F. (2004). Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J. Neurotrauma 21, 1457–1467. doi: 10.1089/neu.2004.21.1457
Wurtman, R. J., Ulus, I. H., Cansev, M., Watkins, C. J., Wang, L., and Marzloff, G. (2006). Synaptic proteins and phospholipids are increased in gerbil brain by administering uridine plus docosahexaenoic acid orally. Brain Res. 1088, 83–92. doi: 10.1016/j.brainres.2006.03.019
Wurtman, R. J., Wurtman, J. J., Regan, M. M., McDermott, J. M., Tsay, R. H., and Breu, J. J. (2003). Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am. J. Clin. Nutr. 77, 128–132.
Xiao, D., Cassin, J. J., Healy, B., Burdett, T. C., Chen, J. F., Fredholm, B. B., et al. (2011). Deletion of adenosine A(1) or A((2)A) receptors reduces L-3,4-dihydroxyphenylalanine-induced dyskinesia in a model of Parkinson's disease. Brain Res. 1367, 310–318. doi: 10.1016/j.brainres.2010.08.099
Xu, K., Xu, Y., Brown-Jermyn, D., Chen, J. F., Ascherio, A., Dluzen, D. E., et al. (2006). Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. J. Neurosci. 26, 535–541. doi: 10.1523/JNEUROSCI.3008-05.2006
Xu, K., Xu, Y. H., Chen, J. F., and Schwarzschild, M. A. (2002). Caffeine's neuroprotection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity shows no tolerance to chronic caffeine administration in mice. Neurosci. Lett. 322, 13–16. doi: 10.1016/S0304-3940(02)00069-1
Xu, K., Xu, Y. H., Chen, J. F., and Schwarzschild, M. A. (2010). Neuroprotection by caffeine: time course and role of its metabolites in the MPTP model of Parkinson's disease. Neuroscience 167, 475–481. doi: 10.1016/j.neuroscience.2010.02.020
Xu, Q., Park, Y., Huang, X., Hollenbeck, A., Blair, A., Schatzkin, A., et al. (2011). Diabetes and risk of Parkinson's disease. Diabetes Care 34, 910–915. doi: 10.2337/dc10-1922
Yadav, S., Gupta, S. P., Srivastava, G., Srivastava, P. K., and Singh, M. P. (2012). Role of secondary mediators in caffeine-mediated neuroprotection in maneb- and paraquat-induced Parkinson's disease phenotype in the mouse. Neurochem. Res. 37, 875–884. doi: 10.1007/s11064-011-0682-0
Ye, Q., Ye, L., Xu, X., Huang, B., Zhang, X., Zhu, Y., et al. (2012). Epigallocatechin-3-gallate suppresses 1-methyl-4-phenyl-pyridine-induced oxidative stress in PC12 cells via the SIRT1/PGC-1alpha signaling pathway. BMC Complement. Altern. Med. 12:82. doi: 10.1186/1472-6882-12-82
Yong, V. W., Perry, T. L., and Krisman, A. A. (1986). Depletion of glutathione in brainstem of mice caused by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine is prevented by antioxidant pretreatment. Neurosci. Lett. 63, 56–60. doi: 10.1016/0304-3940(86)90012-1
Youdim, K. A., Martin, A., and Joseph, J. A. (2000). Essential fatty acids and the brain: possible health implications. Int. J. Dev. Neurosci. 18, 383–399. doi: 10.1016/S0736-5748(00)00013-7
Zhang, F., Shi, J. S., Zhou, H., Wilson, B., Hong, J. S., and Gao, H. M. (2010). Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 78, 466–477. doi: 10.1124/mol.110.064535
Zhang, S. M., Hernan, M. A., Chen, H., Spiegelman, D., Willett, W. C., and Ascherio, A. (2002). Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 59, 1161–1169. doi: 10.1212/01.WNL.0000028688.75881.12
Zhang, W., Li, R., Li, J., Wang, W., Tie, R., Tian, F., et al. (2013). Alpha-linolenic acid exerts an endothelial protective effect against high glucose injury via PI3K/Akt pathway. PLoS ONE 8:e68489. doi: 10.1371/journal.pone.0068489
Zhao, B. (2009). Natural antioxidants protect neurons in Alzheimer's disease and Parkinson's disease. Neurochem. Res. 34, 630–638. doi: 10.1007/s11064-008-9900-9
Keywords: Parkinson's disease, nutrition, neurodegeneration, neuroprotection, antioxidants
Citation: Seidl SE, Santiago JA, Bilyk H and Potashkin JA (2014) The emerging role of nutrition in Parkinson's disease. Front. Aging Neurosci. 6:36. doi: 10.3389/fnagi.2014.00036
Received: 05 November 2013; Accepted: 20 February 2014;
Published online: 07 March 2014.
Edited by:
Antonio Camins, University of Barcelona, SpainReviewed by:
Diego Ruano, University of Sevilla, SpainCopyright © 2014 Seidl, Santiago, Bilyk and Potashkin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Judith A. Potashkin, The Cellular and Molecular Pharmacology Department, The Chicago Medical School, Rosalind Franklin University of Medicine and Science, 3333 Green Bay Rd., North Chicago, IL 60064-3037, USA e-mail:anVkeS5wb3Rhc2hraW5Acm9zYWxpbmRmcmFua2xpbi5lZHU=
†These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.