
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mol. Biosci., 22 January 2025
Sec. Molecular Diagnostics and Therapeutics
Volume 11 - 2024 | https://doi.org/10.3389/fmolb.2024.1522717
This article is part of the Research TopicApplications of High-Sensitivity Detection, Component Analysis, and Vector Construction for Extracellular Vesicles in Cancer Diagnosis and TreatmentView all articles
 Shankar Suman1*
Shankar Suman1* Wendy K. Nevala1
Wendy K. Nevala1 Alexey A. Leontovich2
Alexey A. Leontovich2 James W. Jakub3
James W. Jakub3 Liyi Geng1
Liyi Geng1 Sarah A. McLaughlin3
Sarah A. McLaughlin3 Svetomir N. Markovic1*
Svetomir N. Markovic1*Cytokines play a crucial role in mediating cell communication within the tumor microenvironment (TME). Tumor-associated macrophages are particularly influential in the regulation of immunosuppressive cytokines, thereby supporting tumor metastasis. The upregulation of Th2 cytokines in cancer cells is recognized for its involvement in suppressing anticancer immunity. However, the association between these cytokines and tumor-secreted extracellular vesicles (EVs) remains poorly understood. Therefore, our objective was to investigate the connection between tumor-promoting macrophages and melanoma-derived EVs. The analysis from altered cytokine profile data showed that melanoma-derived EVs upregulate Th2 cytokine expression in naïve macrophages, thereby contributing to the promotion of tumor-supporting functions. Notably, many of these cytokines were also found to be upregulated in metastatic melanoma patients (n = 30) compared to healthy controls (n = 33). Overall, our findings suggest a strong connection between melanoma secretory EVs and the induction of tumor-associated macrophages that facilitates the development of an immunosuppressive TME, supporting melanoma metastasis through regulation at both local and systemic levels.
The current understanding of the immune landscape in cutaneous melanoma depicts it as an immunogenic malignancy (Nirmal et al., 2022). Melanoma cells express a plethora of cytokines and growth factors crucial in primary tumor compared to distant metastases (Elias et al., 2010). These melanoma mediators are involved in autocrine and paracrine signaling to maintain the tumor microenvironment (TME) (Suman and Markovic, 2023; Buckels et al., 2019). These mediators which include cytokines and other growth factors as well as membrane bound vesicles, induce the development of immunosuppressive macrophages associated with tumor metastasis through the formation of premetastatic niches (Suman and Markovic, 2023; Salmi et al., 2019; Suman et al., 2024). Tumor-promoting macrophages (TPMs) also known as tumor associated macrophages in melanoma correlate with an unfavorable prognosis and are more prevalent in invasive melanomas than in benign nevi (Salmi et al., 2019). Within the domain of macrophage physiology, a portfolio of cytokines is considered to mediate the fate of a spectrum of functional forms of these cells (Arango Duque and Descoteaux, 2014). Tumor-secreted mediators including tumor-derived extracellular vesicles serve as a stimulus for macrophages in developing immunosuppressive phenotypes (Tian et al., 2023).
Extracellular vesicles (EVs) play significant roles in normal development and various physiological processes by facilitating communication between cells (De Toro et al., 2015). Furthermore, EVs exhibit a range of immunoregulatory functions as the proteins contained within these vesicles are likely involved in essential processes such as vesicle formation and trafficking, signal transduction, cytoskeletal organization, and antigen presentation (Robbins and Morelli, 2014). Tumor-derived EVs are important in conditioning the TME and promoting immune escape through transfer of carcinogenic lipids, nucleic acid, and proteins (Tan et al., 2022). Tumor-derived EVs promote immune evasion and interfere with immune responses in the TME. This process involves various mechanisms, which include induction of apoptosis in CD8+ T cells, generation of regulatory T cells (Tregs), suppression of natural killer (NK) cells, inhibition of the maturation and differentiation of monocytes, and enhancement of the suppressive functions of myeloid-derived suppressor cells (MDSCs) (Xie et al., 2022; Wang and Zhang, 2021). Tumor-derived EVs alter the myeloid differentiation pathway, directing toward the MDSC (CD11b+ Gr1+) lineage in a mouse model (Xiang et al., 2009). These tumor derived-EVs further involve in the metabolic reprogramming in macrophages to suppress their function and help developing premetastatic niche (Kersten et al., 2023). Melanoma-derived EVs also play a key role in developing an immune-tolerant microenvironment (Suman and Markovic, 2023) as it increases the expression of programmed cell death ligand 1 (PD-L1) on immature myeloid cells, which suppress T-cell activation (Fleming et al., 2019). Hood et al. showed that melanoma-derived EVs prepare microanatomic niches to facilitate lymphatic metastasis (Hood et al., 2011). Melanoma-derived EVs efficiently spread through the lymphatic system and preferentially bind to macrophages located in the subcapsular sinus (SCS) of tumor-draining lymph nodes (Pucci et al., 2016). When the SCS macrophage barrier is disrupted, tumor-derived EVs can enter the lymph node cortex, interact with B cells, and promote tumor-supporting humoral immunity (Pucci et al., 2016).
Macrophages are commonly classified as M1 and M2 based on their respective proinflammatory and tissue repair or anti-inflammatory functions. Nonetheless, TPMs demonstrate a diverse range of characteristics, encompassing both M1 and M2 phenotypes in the TME (Gao et al., 2022). TPMs are a crucial part of the TME, which play a significant role in various processes, including angiogenesis, extracellular matrix remodeling, immunosuppression, and resistance to therapy (Mantovani et al., 2022). However, the activated form of macrophages acts against tumors by engaging bidirectional interactions with both innate and adaptive immunity (Mantovani et al., 2022). Moreover, M2-like TPM is an essential component that contributes to lymphangiogenesis, immunosuppression, and drug resistance, making them important target for successful immunotherapy (Gao et al., 2022; Wang et al., 2024). The development of immunosuppressive macrophages represents a pivotal aspect of these processes. Soluble factors such as cytokines facilitate communication between tumors and macrophages to maintain the TME and establish a premetastatic niche (Suman and Markovic, 2023). However, their association with melanoma-derived EVs is not fully understood. This study investigates the link between melanoma-derived EVs and the development of TPMs through comprehensive cytokine profiling. Furthermore, it compares these associations in the blood plasma of patients with metastatic melanoma, elucidating the mechanisms by which melanoma cells regulate metastasis through cytokine-mediated pathways.
The human melanoma cell line (SKMEL-28) and monocyte cell line (THP1) were obtained from ATCC, United States, and cells were cultured in the recommended cell culture medium, following given instructions. EVs from SKMEL28 and human lymphatics from control (C-LEVs) and melanoma patients (P-LEVs) were collected by using previously described methods (Maus et al., 2019; Suman et al., 2024). In brief, we extracted LEVs from the lymphatic fluid of patients with melanoma downstream of primary cutaneous melanomas and compared it with those of control individuals (non-malignant post-operative fluid collected from the lymph node dissection field) (Supplementary Figure S1). We performed human cytokine antibody array analysis (RayBiotech, Norcross, GA) by following the manufacturer’s protocol to evaluate the expression levels of 80 human cytokines by the effect of melanoma-derived EVs on naive macrophages (M0) (Figure 1; Supplementary Figure S2). In brief, M0 macrophages were specifically generated from THP1 cells by treating them with 20 ng/mL phorbol 12-myristate-13-acetate (PMA) for 48 h. The adherent M0 cells were washed twice with 1× PBS to remove any residual PMA and non-adherent monocytes. These adherent M0 cells were then treated with EVs in complete Roswell Park Memorial Institute (RPMI) medium for 48 h. After the incubation, cells were washed again with cold 1× PBS to eliminate any remaining media and EVs before preparing the protein lysate for cytokine array analysis. An equal amount of protein from each sample set was applied to the cytokine array membrane according to the manufacturer’s instructions for cytokine analysis. The signals of the spots on the membrane were detected using chemiluminescence methods on the X-ray film. The intensity of protein signals was quantified by densitometric analysis using Quantity One software (Bio-Rad Laboratories, Hercules, CA). After background subtraction, results were expressed as the percentage of the mean of the relative positive controls.
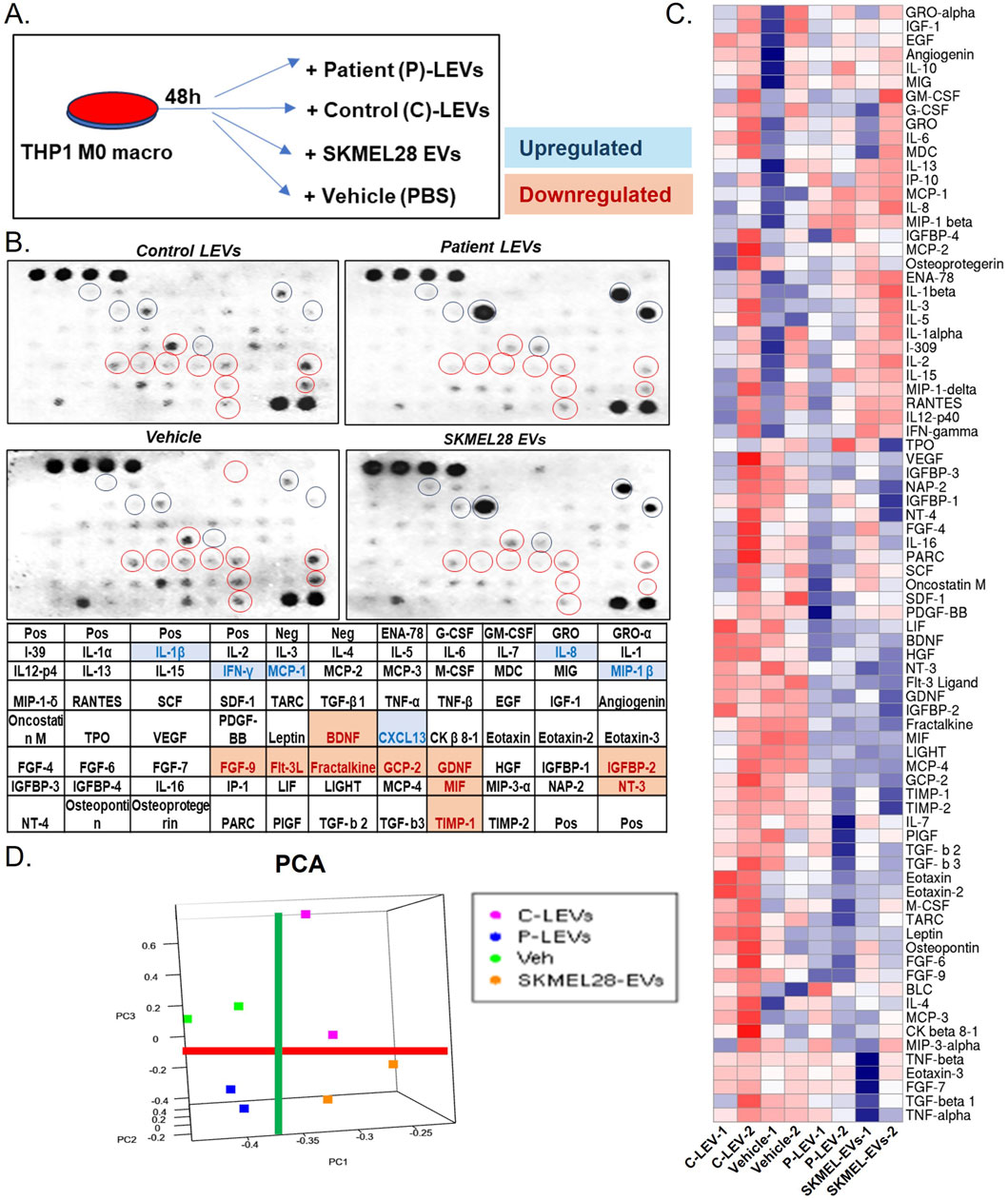
Figure 1. Melanoma-derived EVs transform naïve macrophages through an altered cytokine profile (A) Scheme of EV treatment (vehicle, SKMEL28 EVs, control LEVs, and melanoma patient LEVs) to the THP1 M0 macrophages; (B) human cytokine assay analysis of naïve macrophages treated with melanoma-derived EVs indicated several altered levels of cytokines, which appear similar in melanoma LEVs and SKMEL28 EVs compared to control LEVs and vehicle groups; (C) The heatmap shows all cytokines with altered levels induced by melanoma-derived EVs; and (D) principal component analysis (PCA) indicates the separation of melanoma EV-treated groups from control. The red line separates all control (C-LEVs and Veh) from melanoma-derived EVs (P-LEVs and SKMEL28-EVs), whereas the green line separates controls and melanoma-derived EVs.
Soluble proteins in plasma are measured using the 38-plex magnetic bead Luminex kit, following the manufacturer’s instructions (Millipore, Burlington, MA). In brief, 25 µL of varying-sized magnetic beads containing antibodies specific to the measured proteins was added to a 96-well plate, with 25 µL of the sample, assay buffer (background control), or standard curve dilutions (3.2–10,000 pg/mL). The beads and samples were incubated overnight at 4°C with gentle shaking. The next day, the magnetic beads were washed three times with wash buffer, and 25 µL of HRP-conjugated secondary antibodies was added and incubated at 4°C for 1 h with gentle shaking. After bead incubation with detection antibodies, 25 µL of streptavidin–PE was added for 20 min. After washing three times, the magnetic beads were enumerated using the Luminex 200 (Millipore, Burlington, MA). The concentrations of proteins were calculated from the standard curve using Milliplex Analyst software (Millipore, Burlington, MA).
We analyzed the effect of SKMEL28 EVs on human peripheral blood mononuclear cells (PBMCs) derived macrophages from 10 healthy human blood donors. In brief, PBMCs were cultured in RPMI medium for 24–48 h before treating with 5 ng/mL GM-CSF for 3 days. Adherent cells were further treated with control and melanoma-derived EVs for 48 h. Cell surface staining (CD206, CD163, and HLA-DR) and intracellular staining (MCP1, CXCL13, IL8, and MIP-1β) were performed following the manufacturer’s instructions (provided in the supplementary materials, Supplementary Table S1). Flow data were acquired by Bio-Rad ZE5 flow cytometry and analyzed using Flow Jo software v10.0 (Ashland, Oregon).
Normal, tumor, and metastatic tissue gene expressions were analyzed using the TNM plot data server (tnmplot.com) (Bartha and Győrffy, 2021). RNA-seq data were exported from the human skin cutaneous melanoma (SKCM) dataset for IP-10, GCSF, CXCL13, CCL2, CD163, and CD209 and analyzed using normal tissue from the non-cancer patients (n = 474), tumor tissue (n = 103), and metastatic melanoma tissue (n = 368). Furthermore, the gene chip database was used for the analysis of the genes that are absent in the RNA-seq data, which included CCL4, consisting of normal (n = 174), tumor (n = 253), and metastasis (n = 76) tissue genes. The correlation analysis of CD163 with macrophage-associated cytokine genes and other transcription factors was performed in the SKCM dataset using the TIMER2.0 webserver (http://timer.comp-genomics.org/timer/). Principal component analysis and maps as well as hierarchical clustering and heat maps were created using the R programming language (R4.2.2). All the other statistical analyses were conducted using GraphPad Prism V.10.3 software (La Jolla, California, United States).
To evaluate the cellular cytokine changes in the macrophages by the effect of melanoma-derived EVs, we treated THP1 monocytic cells with PMA to induce them to naïve macrophage. Furthermore, to check the cytokine modulation of naïve macrophages by the effect of melanoma-derived EVs, we performed cytokine array analyses of 80 key factors to determine macrophage phenotype state. A control set of EVs was collected from the lymphatic channel (control LEVs) from non-cancerous nodes to compare with the effect of EVs from melanoma-associated lymphatic channels (patient LEVs). We also analyzed SKMEL28 EVs and vehicle control to compare the direct effect of cancer cell EVs. Array data apparently show similar results on cytokine arrays for SKMEL28 and melanoma patient LEVs compared to respective controls (Figure 1). Among these, IL-1β, IL-8, IL-13, MIP-1β, MCP1, and CXCL13 were key upregulated cytokines, and MIF, IGFBP-2, FGF-9, FLT3L GCP-2, and NT3 are key downregulated cytokines (Figure 1B). Moreover, the patterns of cytokine expression in the macrophages are similar within melanoma and non-melanoma groups but distinct between them (Figures 1C, D). Most of these cytokines modulated by the melanoma-derived EVs on the naïve macrophages represent the induction of TPM-associated pathways (Supplementary Table S2). Interestingly, IL1β, IL8, and MCP1 are directly involved in the crosstalk with adipocytes in deregulated metabolism (Bing, 2015). CXCL13 is known to be secreted by M2 macrophages to promote cancer cell metastasis (Xie et al., 2021). In addition, MCP1 (CCL2) and MIP1β (CCL4) are considered TPM’s biomarkers (De la Fuente López et al., 2018).
The presence of systemic Th2 immune chronic inflammation in metastatic melanoma has been previously documented (Nevala et al., 2009). Our study was carried out to assess the levels of plasma cytokines in the metastatic stage of melanoma using the Luminex platform in newly diagnosed and previously untreated stage IV melanoma patients (n = 30) and healthy subjects (n = 33). The analysis identified 17 significantly altered cytokines, with 12 cytokines (IL4, IL5, IL6, IL8, IL9, IL10, IL13, MIP1, MCP1, IP-10, VEGF, and eotaxin) showing upregulation and five cytokines (IL2, IL7, IFN-γ, G-CSF, and GM-CSF) showing downregulation (Figure 2). Earlier research also revealed similar findings where melanoma patients associated with negative sentinel lymph nodes exhibit significantly elevated levels of IL-4, IL-6, and IL-10 compared to healthy controls, while IFN-gamma levels are notably lower (Porter et al., 2001). This suggests that a melanoma mediator including EVs may directly be involved in modulating these cytokines in systemic circulation. It is noteworthy that several cytokines with altered expressions in the melanoma patients’ blood (IL4, IL5, IL8, IL13, IP10, MCP1, MIP, and G-CSF) exhibited a similar expression level on melanoma EV-treated naïve macrophages (Figure 3A). Interestingly, all of these cytokines in macrophages are associated with the tumor-inflammatory environment and tumor-promoting functions (Chen et al., 2018). A previous study demonstrated that melanoma-secreted cytokines between primary and metastatic melanoma are significantly different (Elias et al., 2010). This finding highlights the role of melanoma cell behavior in promoting aggressiveness and mediating a tolerant immune microenvironment in the premetastatic niche. More importantly, melanoma cells directly target macrophages by releasing specific mediators in the form of EVs or cytokines to support the aggressive nature of metastatic melanoma cells (Wang et al., 2017a). This implies that melanoma-derived factors instigate systemic and local changes to establish an immunosuppressive environment through inducing tumor-promoting macrophages, which facilitate the metastasis of melanoma cells.
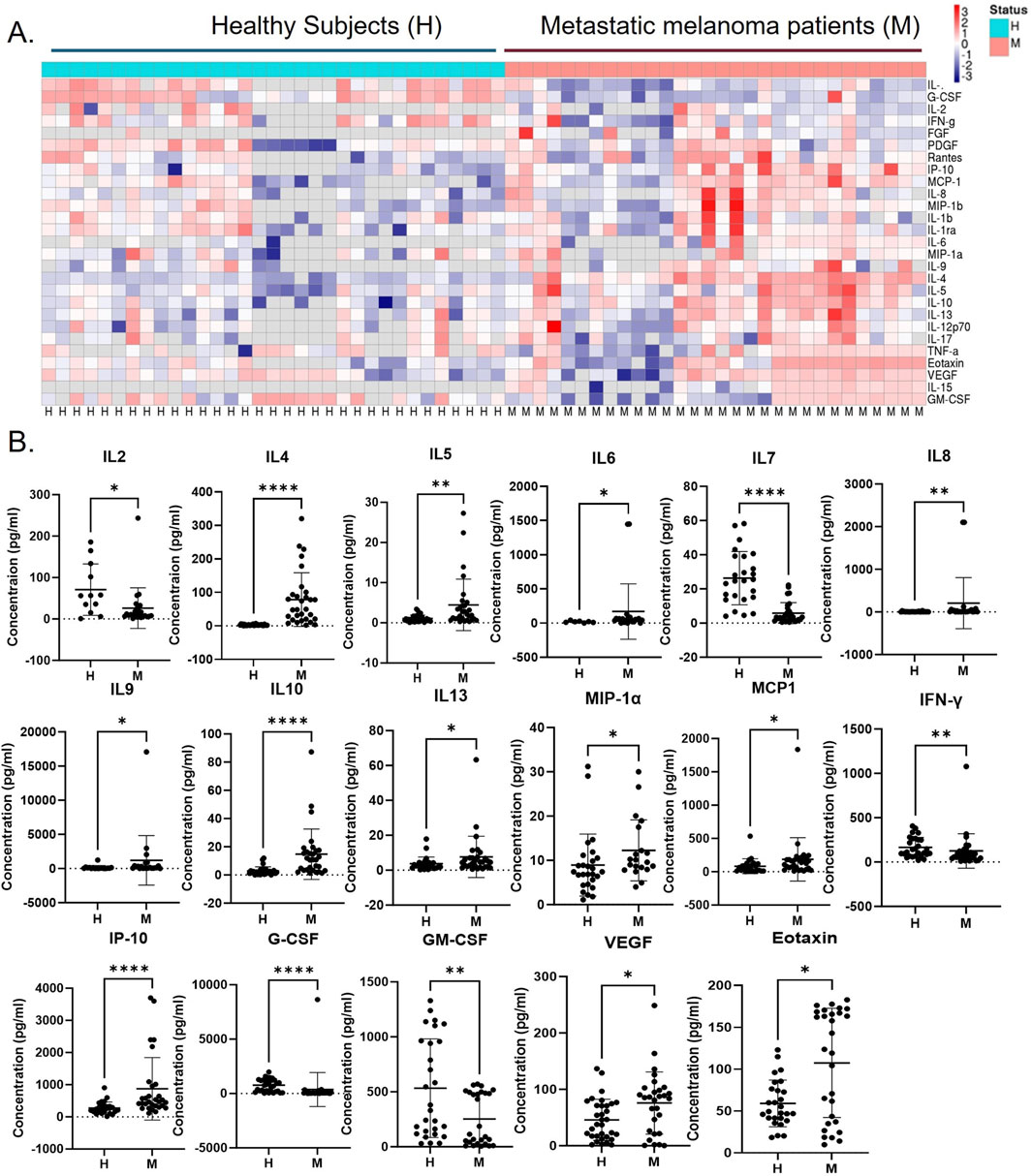
Figure 2. Immunosuppressive Th2 cytokine levels are upregulated in metastatic melanoma patients compared to healthy subjects. (A) The cytokine levels in the blood plasma of human stage IV metastatic melanoma (M; n = 30) and healthy subjects (H; n = 33) were analyzed using the Luminex kit, and the heatmap provided a summary of all analyzed cytokines, and (B) dot plots show cytokine levels between melanoma patients and healthy subjects with significant differences represented by *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, and ****p ≤ 0.0001.
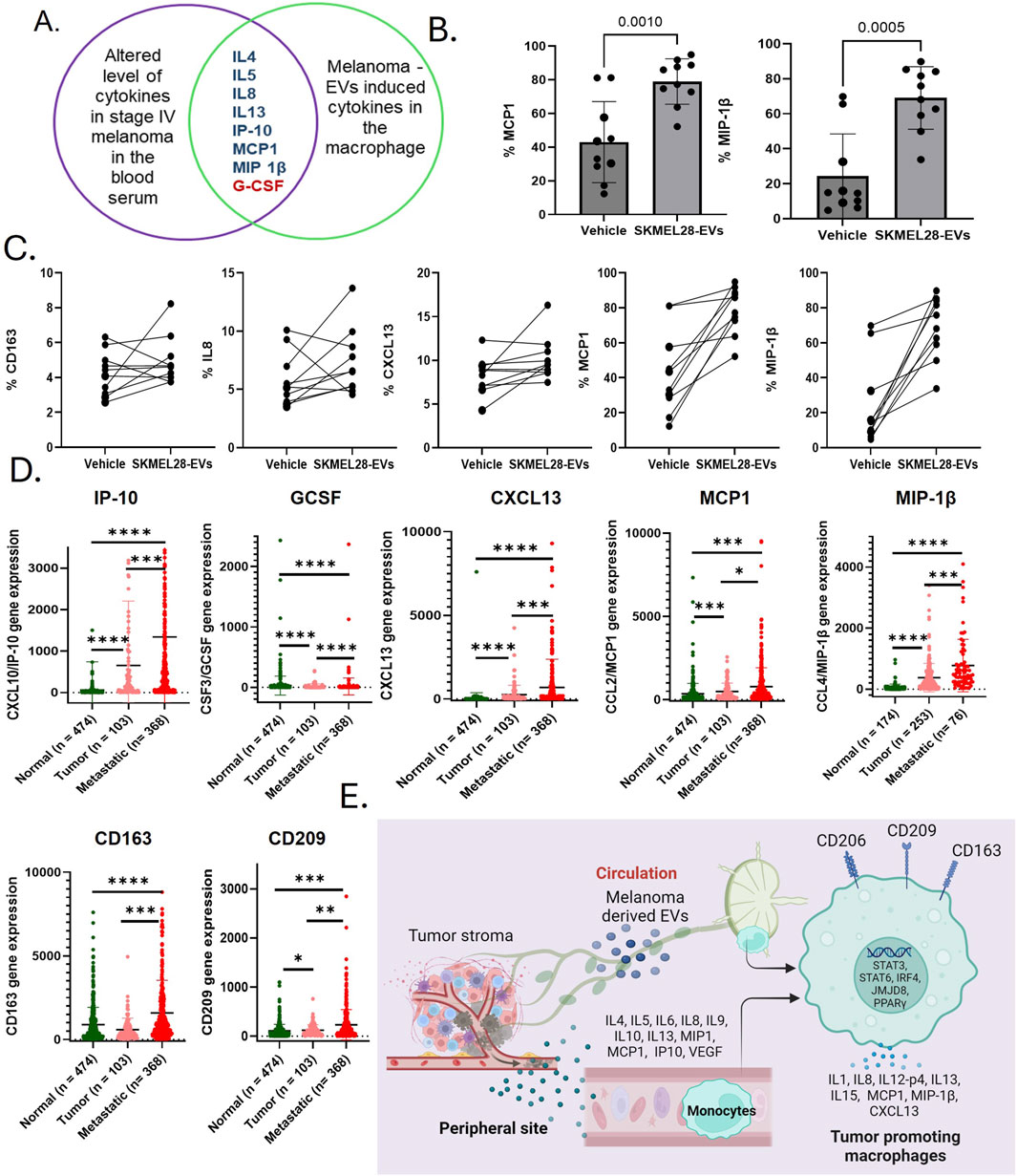
Figure 3. Immunosuppressive cytokines exhibiting tumor-promoting functions within macrophages have been implicated in dysfunction following exposure to melanoma-derived extracellular vesicles (EVs). (A) A Venn diagram shows the list of cytokines with similar regulation trends in the systemic and melanoma-derived EV-treated macrophages; (B) CCL2 and CCL4 are among the top upregulated cytokines by exposures of SKMEL28 EVs to human-derived macrophages (n = 10); (C) SKMEL28 EVs upregulate M2 macrophage markers CD163 and other tumor-promoting cytokines (IL-8, CXCL13, MCP1, and MIP1β) in naïve macrophages upon SKMEL28 EV treatment; (D) analysis of upregulated cytokines listed above in the Venn diagram in the SKCM dataset of normal tissue from non-cancer patients, tumor tissue, and metastatic melanoma tissue; and (E) data from this study summarize that melanoma-induced immunosuppression, driven by changes in the cytokine profile, plays a crucial role in the formation of the premetastatic niche and subsequent metastasis. This process is likely facilitated by the generation of tumor-promoting macrophages, which occurs through the modulation of various cytokines and transcriptional mechanisms after exposure to melanoma-secreted EVs.
TPMs have been implicated as a driver of tumor metastasis (Lin et al., 2019). Melanoma-derived EVs are important factors in the TME and contribute to the development of a niche that promotes tumor growth. The cytokine levels of IL4, IL5, IL8, IL13, IP10, MCP1, and MIP1β are commonly upregulated in the plasma of metastatic patients and melanoma EV-treated macrophages (Figure 3A). These cytokines are known for immunosuppressive macrophage activities that promote cancer progression (Supplementary Table S2). However, to know if melanoma-associated EVs transform macrophages, we further tested the key melanoma EV-induced macrophages cytokines and M2 macrophage surface markers in healthy PBMC-derived macrophages. For that, we treated M0 macrophages derived from healthy blood donors (n = 10) with SKMEL28 EVs. Interestingly, we found that MCP1 and MIP1β levels were upregulated in all the samples in the SKMEL28 EVs-treated group compared to the control group (Figures 3B, C). Moreover, IL-8 (8/10), CXCL-13 (9/10), and CD163 (8/10) levels were upregulated (Figure 3C), which validate that melanoma-derived EVs potentially transform macrophages to support tumor progression. Additionally, analysis of the TCGA-SKCM database revealed similar expression patterns of these gene sets in tumor and metastasis cases compared to the control, indicating the upregulation of tumor-promoting macrophages (CD163 and CD209) (Figure 3D). The heightened expression of cytokines in metastatic melanoma, either endogenously or resulting from induction in macrophages by melanoma-derived factors, signifies pivotal immunological mechanisms that have the potential to exacerbate the inflammatory microenvironment responses (Supplementary Figure S3). Several transcription regulators of TPMs also confirmed higher expression in metastatic melanoma patients in the TCGA-SKCM dataset (Supplementary Figure S4). Furthermore, a positive correlation between several immunosuppressive cytokines and TPM markers like CD163 was also observed in these datasets (Supplementary Figure S5). This evidence consistently supports the notion that factors secreted by tumors activate TPMs to modulate immune responses in melanoma as TPMs are pivotal in regulating T-lymphocytes and NK cells to facilitate metastasis (Yang et al., 2023). Notably, TPMs are recognized for their role in regulating a process akin to epithelial–mesenchymal transition, thereby promoting metastasis through the secretion of IL-8 (Fu et al., 2015). Cytokine array data show strong upregulation of IL-8, MCP1, CXCL13, and MIP1β among melanoma-derived EV-induced macrophage-associated cytokines, which signifies the role of these cytokines in inducing TPMs. Notably, MCP-1 is responsible for augmenting macrophage infiltration in the TME and acts as a potent macrophage-recruiting molecule, expressed in human malignant melanoma (Wang et al., 2017b). Moreover, MCP1 is positively correlated with the inflammatory activity of TPMs and enhances the tumor-promoting activation of the premetastatic systemic inflammatory T cell–IL17–neutrophil axis (Kersten et al., 2017). Strategies targeting TPMs and inhibiting MCP-1 have demonstrated efficacy in reducing angiogenesis and tumor growth in human melanoma xenografts (Gazzaniga et al., 2007). Previous studies also supported that MIP1β (Mukaida et al., 2020) and CXCL13 (Si and Hu, 2021; Chang et al., 2022) are involved in tumor promotion through their overexpression in macrophages.
In summary, our research findings demonstrate that melanoma-derived EVs can induce immunosuppressive activity in macrophages by upregulating levels of key cytokines, including IL-8, MCP-1, and CXCL13. Notably, our results revealed that these cytokine levels are significantly elevated in the blood plasma of patients diagnosed with metastatic melanoma, suggesting a systemic effect of the tumor on the immune environment. This also suggests that melanoma-associated cytokines and melanoma-derived EVs are interconnected with TPMs, highlighting their potential role in the progression of melanoma. The elevated levels of immunosuppressive cytokines (IL-4, IL-10, and IL-13) and decreased GM-CSF level in blood plasma of metastatic melanoma indicate their direct role in the transformation of macrophages into an immunosuppressive M2 phenotype (Hao et al., 2012). Our results strengthen that melanoma-derived EVs, which facilitate the communication between tumor cells and immune cells, ultimately create a favorable environment for tumor growth and metastasis by supporting the development of TPMs. Furthermore, the TCGA-SKCM dataset validates the transcriptional activation of immunosuppressive mechanisms through altered cytokine expression accountable for TPM generation within the TME, thereby bolstering metastasis.
Publicly available TCGA-SKCM dataset was analyzed in this study. The data can be found on online analysis platforms at TNMplot.com and timer.cistrome.org.
The studies involving humans were approved by the Institutional Review Board of Mayo Clinic. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from samples primarily isolated as part of a previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
SS: conceptualization, data curation, formal analysis, investigation, methodology, writing–original draft, and writing–review and editing. WKN: writing–review and editing, data curation, and formal analysis. AAL: writing–review and editing, software, and visualization. JWJ: formal analysis and writing–review and editing. LG: writing–review and editing. SAM: investigation and writing–review and editing. SNM: funding acquisition, investigation, project administration, resources, supervision, validation, and writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Institutes of Health (NIH).
The authors expressed their gratitude to the National Institutes of Health (NIH) R01 (CA 260259-1) for funding support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2024.1522717/full#supplementary-material
Arango Duque, G., and Descoteaux, A. (2014). Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol. 5, 491. doi:10.3389/fimmu.2014.00491
Bartha, Á., and Győrffy, B. (2021). TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int. J. Mol. Sci. 22 (5), 2622. doi:10.3390/ijms22052622
Bing, C. (2015). Is interleukin-1β a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte 4 (2), 149–152. doi:10.4161/21623945.2014.979661
Buckels, A., Zhang, Y., Jiang, J., Athar, M., Afaq, F., Shevde-Samant, L., et al. (2019). Autocrine/paracrine actions of growth hormone in human melanoma cell lines Biochem. Biophys. Rep. 21, 100716. doi:10.1016/j.bbrep.2019.100716
Chang, S.-J., Chao, C.-T., Kwan, A.-L., and Chai, C.-Y. (2022). The diagnostic significance of CXCL13 in M2 tumor immune microenvironment of human astrocytoma. Pathology Oncol. Res. 28, 1610230. doi:10.3389/pore.2022.1610230
Chen, Y., Tan, W., and Wang, C. (2018). Tumor-associated macrophage-derived cytokines enhance cancer stem-like characteristics through epithelial-mesenchymal transition. Onco Targets Ther. 11, 3817–3826. doi:10.2147/ott.S168317
De la Fuente López, M., Landskron, G., Parada, D., Dubois-Camacho, K., Simian, D., Martinez, M., et al. (2018). The relationship between chemokines CCL2, CCL3, and CCL4 with the tumor microenvironment and tumor-associated macrophage markers in colorectal cancer. Tumour Biol. 40 (11), 1010428318810059. doi:10.1177/1010428318810059
De Toro, J., Herschlik, L., Waldner, C., and Mongini, C. (2015). Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front. Immunol. 6, 203. doi:10.3389/fimmu.2015.00203
Elias, E. G., Hasskamp, J. H., and Sharma, B. K. (2010). Cytokines and growth factors expressed by human cutaneous melanoma. Cancers (Basel) 2 (2), 794–808. doi:10.3390/cancers2020794
Fleming, V., Hu, X., Weller, C., Weber, R., Groth, C., Riester, Z., et al. (2019). Melanoma extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res. 79 (18), 4715–4728. doi:10.1158/0008-5472.Can-19-0053
Fu, X. T., Dai, Z., Song, K., Zhang, Z. J., Zhou, Z. J., Zhou, S. L., et al. (2015). Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int. J. Oncol. 46 (2), 587–596. doi:10.3892/ijo.2014.2761
Gao, J., Liang, Y., and Wang, L. (2022). Shaping polarization of tumor-associated macrophages in cancer immunotherapy. Front. Immunol. 13, 888713. doi:10.3389/fimmu.2022.888713
Gazzaniga, S., Bravo, A. I., Guglielmotti, A., van Rooijen, N., Maschi, F., Vecchi, A., et al. (2007). Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J. Investig. Dermatol 127 (8), 2031–2041. doi:10.1038/sj.jid.5700827
Hao, N.-B., Lü, M.-H., Fan, Y.-H., Cao, Y.-L., Zhang, Z.-R., and Yang, S.-M. (2012). Macrophages in tumor microenvironments and the progression of tumors. J. Immunol. Res. 2012 (1), 948098. doi:10.1155/2012/948098
Hood, J. L., San, R. S., and Wickline, S. A. (2011). Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71 (11), 3792–3801. doi:10.1158/0008-5472.CAN-10-4455
Kersten, K., Coffelt, S. B., Hoogstraat, M., Verstegen, N. J. M., Vrijland, K., Ciampricotti, M., et al. (2017). Mammary tumor-derived CCL2 enhances pro-metastatic systemic inflammation through upregulation of IL1β in tumor-associated macrophages. Oncoimmunology 6 (8), e1334744. doi:10.1080/2162402x.2017.1334744
Kersten, K., You, R., Liang, S., Tharp, K. M., Pollack, J., Weaver, V. M., et al. (2023). Uptake of tumor-derived microparticles induces metabolic reprogramming of macrophages in the early metastatic lung. Cell. Rep. 42 (6), 112582. doi:10.1016/j.celrep.2023.112582
Lin, Y., Xu, J., and Lan, H. (2019). Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. and Oncol. 12 (1), 76. doi:10.1186/s13045-019-0760-3
Mantovani, A., Allavena, P., Marchesi, F., and Garlanda, C. (2022). Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21 (11), 799–820. doi:10.1038/s41573-022-00520-5
Maus, R. L. G., Jakub, J. W., Hieken, T. J., Nevala, W. K., Christensen, T. A., Sutor, S. L., et al. (2019). Identification of novel, immune-mediating extracellular vesicles in human lymphatic effluent draining primary cutaneous melanoma. Oncoimmunology 8 (12), e1667742. doi:10.1080/2162402X.2019.1667742
Mukaida, N., Sasaki, S. I., and Baba, T. (2020). CCL4 signaling in the tumor microenvironment. Adv. Exp. Med. Biol. 1231, 23–32. doi:10.1007/978-3-030-36667-4_3
Nevala, W. K., Vachon, C. M., Leontovich, A. A., Scott, C. G., Thompson, M. A., Markovic, S. N., et al. (2009). Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin. Cancer Res. 15 (6), 1931–1939. doi:10.1158/1078-0432.Ccr-08-1980
Nirmal, A. J., Maliga, Z., Vallius, T., Quattrochi, B., Chen, A. A., Jacobson, C. A., et al. (2022). The spatial landscape of progression and immunoediting in primary melanoma at single-cell resolution. Cancer Discov. 12 (6), 1518–1541. doi:10.1158/2159-8290.Cd-21-1357
Porter, G. A., Abdalla, J., Lu, M., Smith, S., Montgomery, D., Grimm, E., et al. (2001). Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann. Surg. Oncol. 8 (2), 116–122. doi:10.1007/s10434-001-0116-3
Pucci, F., Garris, C., Lai, C. P., Newton, A., Pfirschke, C., Engblom, C., et al. (2016). SCS macrophages suppress melanoma by restricting tumor-derived vesicle–B cell interactions. Science, 352(6282), 242–246. doi:10.1126/science.aaf1328
Robbins, P. D., and Morelli, A. E. (2014). Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 14 (3), 195–208. doi:10.1038/nri3622
Salmi, S., Siiskonen, H., Sironen, R., Tyynelä-Korhonen, K., Hirschovits-Gerz, B., Valkonen, M., et al. (2019). The number and localization of CD68+ and CD163+ macrophages in different stages of cutaneous melanoma. Melanoma Res. 29 (3), 237–247. doi:10.1097/cmr.0000000000000522
Si, Z., and Hu, H. (2021). Identification of CXCL13 as an immune-related biomarker associated with tumorigenesis and prognosis in cutaneous melanoma patients. Med. Sci. Monit. 27, e932052. doi:10.12659/msm.932052
Suman, S., and Markovic, S. N. (2023). Melanoma-derived mediators can foster the premetastatic niche: crossroad to lymphatic metastasis. Trends Immunol. 44 (9), 724–743. doi:10.1016/j.it.2023.07.002
Suman, S., Nevala, W. K., Leontovich, A. A., Ward, C., Jakub, J. W., Kim, Y., et al. (2024). Melanoma-derived extracellular vesicles induce CD36-mediated pre-metastatic niche. Biomolecules 14 (7), 837. doi:10.3390/biom14070837
Tan, Y., Tang, F., Li, J., Yu, H., Wu, M., Wu, Y., et al. (2022). Tumor-derived exosomes: the emerging orchestrators in melanoma. Biomed. and Pharmacother. 149, 112832. doi:10.1016/j.biopha.2022.112832
Tian, J. W., Zhang, H. J., Li, S. Y., Guo, Y. L., Chen, G., and Yu, Z. L. (2023). Tumor cell-derived extracellular vesicles in modulating phenotypes and immune functions of macrophages: mechanisms and therapeutic applications. J. Cancer 14 (8), 1321–1334. doi:10.7150/jca.84632
Wang, H., Yang, L., Wang, D., Zhang, Q., and Zhang, L. (2017b). Pro-tumor activities of macrophages in the progression of melanoma. Hum. Vaccin Immunother. 13 (7), 1556–1562. doi:10.1080/21645515.2017.1312043
Wang, H., Zhang, L., Yang, L., Liu, C., Zhang, Q., and Zhang, L. (2017a). Targeting macrophage anti-tumor activity to suppress melanoma progression. Oncotarget 8 (11), 18486–18496. doi:10.18632/oncotarget.14474
Wang, M., and Zhang, B. (2021). The immunomodulation potential of exosomes in tumor microenvironment. J. Immunol. Res. 2021 (1), 3710372. doi:10.1155/2021/3710372
Wang, S., Wang, J., Chen, Z., Luo, J., Guo, W., Sun, L., et al. (2024). Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. npj Precis. Oncol. 8 (1), 31. doi:10.1038/s41698-024-00522-z
Xiang, X., Poliakov, A., Liu, C., Liu, Y., Deng, Z. B., Wang, J., et al. (2009). Induction of myeloid-derived suppressor cells by tumor exosomes. Int. J. Cancer 124 (11), 2621–2633. doi:10.1002/ijc.24249
Xie, Q. H., Zheng, J. Q., Ding, J. Y., Wu, Y. F., Liu, L., Yu, Z. L., et al. (2022). Exosome-mediated immunosuppression in tumor microenvironments. Cells 11 (12), 1946. doi:10.3390/cells11121946
Xie, Y., Chen, Z., Zhong, Q., Zheng, Z., Chen, Y., Shangguan, W., et al. (2021). M2 macrophages secrete CXCL13 to promote renal cell carcinoma migration, invasion, and EMT. Cancer Cell. Int. 21 (1), 677. doi:10.1186/s12935-021-02381-1
Keywords: tumor-promoting macrophage, cytokines, extracellular vesicles, melanoma, immunosuppression, tumor microenvironment
Citation: Suman S, Nevala WK, Leontovich AA, Jakub JW, Geng L, McLaughlin SA and Markovic SN (2025) Melanoma-derived cytokines and extracellular vesicles are interlinked with macrophage immunosuppression. Front. Mol. Biosci. 11:1522717. doi: 10.3389/fmolb.2024.1522717
Received: 12 November 2024; Accepted: 13 December 2024;
Published: 22 January 2025.
Edited by:
Daniel X. Zhang, Hong Kong Metropolitan University, Hong Kong SAR, ChinaReviewed by:
Michael J. Wolyniak, Hampden–Sydney College, United StatesCopyright © 2025 Suman, Nevala, Leontovich, Jakub, Geng, McLaughlin and Markovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shankar Suman, c3VtYW4uc2hhbmthckBtYXlvLmVkdQ==; Svetomir N. Markovic, bWFya292aWMuc3ZldG9taXJAbWF5by5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.