- 1Department of Dermatology, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan
- 2International Center for Wound Repair and Regeneration, National Cheng Kung University, Tainan, Taiwan
Psoriasis, a chronic, multisystemic inflammatory disease affecting millions of people globally, manifests as erythematous, thick, scaly plaques on the skin. Clinical evaluation remains to be the benchmark for diagnosis and monitoring of this debilitating disease. With current advancements in targeted molecular therapy for psoriasis such as biologics, molecular detection methods may also help guide clinical decisions and therapeutic strategies through quantification of circulating biomarkers, which could reflect the underlying pathogenic events happening at a certain point of the disease course. In this review, we will discuss how biomarkers are detected in serum samples using enzyme-linked immunosorbent assay (ELISA). This review will feature candidate biomarkers supported by clinical data for psoriasis including, but not limited to, cytokines, chemokines, adipokines, and antimicrobial peptides. A better understanding of the common method used for biomarker detection would enable physicians to interpret and correlate laboratory results with the disease pathogenesis and clinical outcomes, e.g., severity assessment and/or therapeutic response. With better health outcomes as the main goal, the utility of such information to evaluate and even predict treatment response would be a major step closer towards patient-tailored management.
1 Introduction
Psoriasis is a chronic, immune-mediated condition manifesting as thick, raised, scaly plaques (World Health Organization, 2016). Psoriasis vulgaris or chronic plaque psoriasis constitutes 90% of all diagnosed psoriasis cases, some presenting with other systemic comorbidities, e.g., psoriatic arthritis, cardiovascular diseases, diabetes mellitus, cancer, and depression, thereby causing serious debilitation in one’s quality of life (Di Meglio et al., 2014; World Health Organization, 2016; Rendon and Schäkel, 2019).
Onset and exacerbation of psoriasis is multifactorial, including genetic and environmental factors (Armstrong and Read, 2020). Its complex inflammatory processes involve members of the adaptive immune system. Excessive activation of myeloid dendritic cells occurs due to the cytokines secreted by plasmacytoid dendritic cells (pDC), keratinocytes, macrophages, and T cells, resulting in the secretion of interleukin (IL)-12 and IL-23 (Alwan and Nestle, 2015). IL-12 drives naive T cells to differentiate into Th1 cells, which secretes interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNF-α). IL-23 plays a critical role for Th17- and Th22-mediated cytokine release, i.e., Th17 secretes IL-17, IL-22, and TNF-α, while Th22 produces IL-22 (Armstrong and Read, 2020). Overall, these inflammatory signals lead to the skin and systemic manifestations in psoriasis, e.g., keratinocyte proliferation, immune cells infiltrating skin lesions, vasodilation, and angiogenesis (Nestle et al., 2009; Armstrong and Read, 2020).
Skin biopsy is an invasive procedure seldom used in diagnosing psoriasis (Greaves and Weinstein, 1995). Less invasive diagnostics offer the advantage of clinching the diagnosis while allowing repetitive measurements when warranted. Circulating proteins, also referred to as biomarkers, can be quantified through less invasive procedures and can indicate disease activity and progression, i.e., significant deviation from normal levels likely reflects an abnormal process occurring within the body (Molteni and Reali, 2012; Aronson and Ferner, 2017).
Biomarker studies for psoriasis aim to seek for reliable yet minimally invasive indicators of disease status and/or treatment response. Although psoriasis is often clinically diagnosed, biomarkers may serve as an aid for clinicians in quantitatively monitoring disease activity and therapeutic response. In this review, we discussed serum protein biomarkers that have been clinically correlated with psoriasis, as summarized in Figure 1A and Table 1. Immunoassay methods were also briefly discussed in the context of serum biomarker detection. In doing so, we hope to provide clinicians with the fundamentals of immunoassays to facilitate better interpretation of immunoassay results and develop personalized and effective therapeutic strategies.
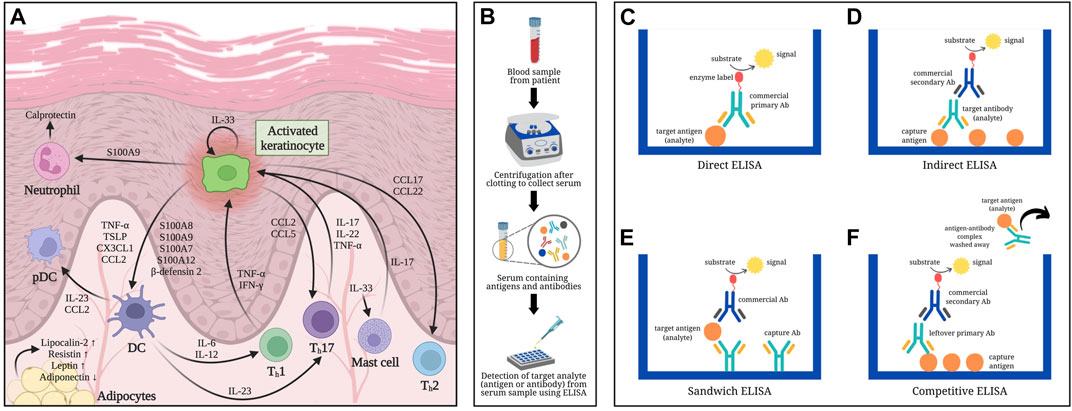
FIGURE 1. Overview of psoriasis-related serum biomarkers determined using ELISA-based methods: (A) Involvement of serum biomarkers in the pathogenesis of psoriasis. (B) Schematic diagram of serum collection from blood samples: Whole blood from a patient is allowed to clot first then centrifuged to separate the serum from clotted blood cells. (C) Direct ELISA: Antigens (analyte) from serum are immobilized onto a polystyrene microtiter plate followed by a blocking step (i.e., using bovine serum albumin) to cover any remaining plastic surface prior to adding the commercial enzyme-linked antibody (Ab) that would specifically bind to the antigens being measured. Signal emitted is directly proportional to the quantity of analytes. (D) Indirect ELISA: The microtiter plate is first coated with capture antigens, which would bind the antibodies (analyte) referred to as primary antibody from the serum sample. An enzyme-labeled secondary antibody (usually commercially obtained), which is host-specific to the primary antibody, is then added to detect the analytes captured onto the microtiter plate, hence emitting a signal proportional to the amount of analyte. (E) Sandwich ELISA: Capture antibodies specific to the analyte (antigen) are immobilized onto the wells of a microtiter plate, followed by the addition of the serum sample containing the antigens of interest. Once the antigens bind to the capture antibodies, a second antibody specific to a different epitope region of the analyte is added into the wells. The second antibody may or may not be labeled; in the latter case, an enzyme-conjugated third antibody, which is specific to the constant (Fc) region of the second antibody, would be included and act as the reporting antibody. The signal detected is directly correlated to the amount of antigens detected in the sample. (F) Competitive ELISA: Antigens are initially pre-coated onto the wells of a microtiter plate. The sample containing antigens to be measured are preincubated with primary antibodies to create mobile antibody-antigen complexes before adding the sample mixture onto the antigen-coated microtiter plates. Antigens, when present in high amounts, would result in fewer unbound primary antibodies. After washing off the sample mixture containing the mobile antibody-antigen complexes, any free primary antibodies would be captured by the immobilized antigens in the microtiter plate. A secondary antibody against the Fc region of the primary antibody is then added to report how much of it remained in the microtiter plate. Low signal detected in competitive ELISA implies that high amount of antigens were present in the sample, resulting in less unbound primary antibodies that could bind with the capture antigens on the microtiter plate.
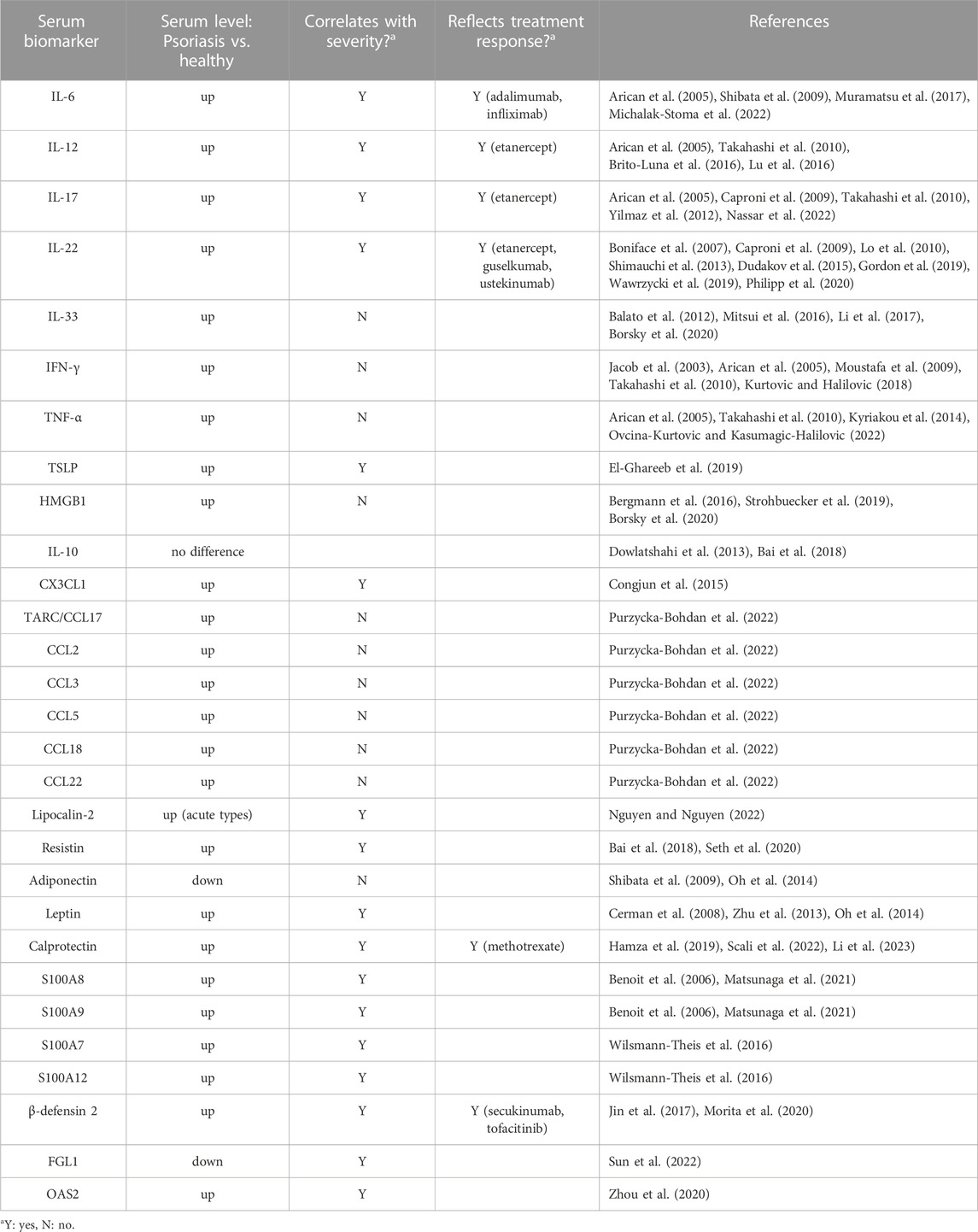
TABLE 1. Serum biomarkers for psoriasis in correlation with disease severity and/or treatment response.
2 Principles of protein-based immunoassay for serum biomarker detection
Biomarker detection may require a few drops of blood by finger prick method to a few milliliters by venipuncture (Paul and Veenstra, 2022). Serum can be separated from other blood components by centrifugation after allowing blood to coagulate (Figure 1B). Antibody-based techniques, e.g., enzyme-linked immunosorbent assay (ELISA), have high sensitivity and specificity in detecting proteins from serum. ELISA utilizes the specific binding between proteins and antibodies in producing a measurable signal (Alhajj and Farhana, 2023).
Regarded as the gold standard for immunoassays, ELISA was first described in 1972 as a simple yet sensitive analytical technique for antibody quantification, detecting less than 1 ng/mL of antibody (Engvall and Perlmann, 1972). The quantifiable signal results from an enzyme-substrate reaction indicating that the analyte is present and binds the reporting antibody conjugated with the enzyme (Figures 1C–F). Different types of ELISA (direct, indirect, sandwich, and competitive) mainly vary in the number of steps involved.
Direct ELISA is a straightforward manner of immunoassay (Figure 1C), wherein the antigens (analyte) from serum acts as capture molecules for its specific reporting enzyme-linked antibody (Aydin, 2015; Alhajj and Farhana, 2023). Indirect ELISA enables detection of antibody from serum when captured by immobilized antigens followed by the addition of an enzyme-labeled secondary antibody (Figure 1D) (Aydin, 2015; Alhajj and Farhana, 2023). Sandwich ELISA, as the name implies, involves two antibodies that capture the antigen of interest in between (Figure 1E). Commonly used for biomarker detection, sandwich ELISA has specific primary antibodies immobilized instead of directly coating the microtiter plate with analytes, thereby increasing its specificity while reducing nonspecific binding (Aydin, 2015; Alhajj and Farhana, 2023). Competitive ELISA is another platform that allows detection of antigens or biomarkers (Figure 1F). It is often useful when the analyte is small and difficult to be “sandwiched” in between antibodies (Gan and Patel, 2013; Aydin, 2015; Alhajj and Farhana, 2023).
Other techniques for biomarker detection include mass spectrometry, flow cytometry, and protein microarrays; however, these are not readily accessible in clinical settings hence were excluded in this review. ELISA is a well-established method that is feasible in most laboratories as commercial kits are often readily available. Given its high sensitivity and specificity, sandwich ELISA is most commonly used in biomarker assays (Del Campo et al., 2015). The subsequent section discusses psoriasis-related protein biomarkers, most of which were studied using sandwich-type ELISA.
3 Serum biomarkers for psoriasis
Candidate serum biomarkers for psoriasis discussed in this review include cytokines, chemokines, adipokines, and antimicrobial peptides, as summarized in Table 1.
3.1 Cytokines and chemokines
During inflammation, cytokines and chemokines are secreted by various immune cells to mediate signaling pathways and recruit effector cells towards the affected sites, hence may serve as candidate biomarkers for long-term inflammatory diseases such as psoriasis.
3.1.1 IL-6
With its implications on epidermal and dermal growth and differentiation, IL-6 is being explored as a dendritic cell (DC)-produced inflammatory marker as it induces T cell conversion to Th17, which in effect produces other inflammatory cytokines (Goodman et al., 2009; Pietrzak et al., 2020; Kutsuna et al., 2022). Baseline IL-6 levels in serum of psoriasis patients were found to be significantly elevated compared to healthy individuals (Arican et al., 2005; Shibata et al., 2009; Michalak-Stoma et al., 2022). Moreover, a significant correlation was noted between serum IL-6 and PASI scores in psoriasis vulgaris patients (Muramatsu et al., 2017). Therapeutic response was also reflected by significant decrease in serum IL-6 levels after treatment with biologics such as adalimumab and infliximab.
3.1.2 IL-12
IL-12 plays a role in T-cell-mediated immunity and is considered as one of the key cytokines responsible for formation and persistence of psoriatic plaques (Ergen and Yusuf, 2018). Patients with plaque psoriasis exhibited elevated serum IL-12, which also correlates with PASI scores (Arican et al., 2005; Takahashi et al., 2010; Brito-Luna et al., 2016). In addition, patients who were classified as non-responders to a 24-week treatment of etanercept were noted to have lower baseline IL-12 serum levels than the responders, hence may be suggestive of IL-12 as a potential biomarker or predictor for clinical response to etanercept, which is a TNF-inhibitor used to treat psoriasis (Lu et al., 2016). Moreover, serum IL-12 level could potentially serve as an indicator for clinical response to etanercept with 50% sensitivity and 96% specificity. In this study, clinical response was defined by a reduction of 75% in the PASI score after treatment.
3.1.3 IL-17
Produced by various immune cells such as Th17, neutrophils, and mast cells, IL-17 is a key cytokine responsible for keratinocyte proliferation and production of other cytokines and antimicrobial peptides in psoriasis (Veldhoen et al., 2006; Lin et al., 2011; Keijsers et al., 2014; Dyring-Andersen et al., 2017). Serum IL-17 concentration was higher in individuals with chronic plaque psoriasis than in healthy controls (Takahashi et al., 2010; Nassar et al., 2022). IL-17 in serum also correlated with severity in psoriasis patients (Arican et al., 2005; Takahashi et al., 2010; Yilmaz et al., 2012). It has been reported that IL-17 levels in serum decreased after treatment with etanercept, suggesting that IL-17 is a potential biomarker for both disease severity and therapeutic response monitoring (Caproni et al., 2009).
3.1.4 IL-22
Described as a proinflammatory cytokine produced by CD4 memory T cells (e.g., Th1, Th17, Th22), IL-22, a member of the IL-10 family, have been found to be significantly elevated in patients with psoriasis compared to healthy controls (Boniface et al., 2007; Lo et al., 2010; Shimauchi et al., 2013; Dudakov et al., 2015; Gordon et al., 2019; Wawrzycki et al., 2019). These studies also demonstrated that IL-22 levels exhibit a positive correlation with disease severity based on PASI scores, making it a potential cytokine suitable for monitoring psoriasis activity. IL-22 may also be a biomarker indicating treatment response due to the notable decrease in serum IL-22 levels after treatment with either etanercept, guselkumab (anti-IL-23) or ustekinumab (anti-IL-12/23) (Caproni et al., 2009; Gordon et al., 2019; Philipp et al., 2020).
3.1.5 IL-33
Regarded as both cytokine and damage-associated molecular pattern, IL-33 is released upon keratinocyte damage in psoriasis then activates inflammatory pathways in an autocrine manner (De la Fuente et al., 2015; Zeng et al., 2021). IL-33 secreted by endothelial cells could activate mast cells, which then initiate inflammation in unaffected psoriatic skin (Theoharides et al., 2010). Studies have reported that IL-33 levels are elevated in the serum of psoriasis patients than the healthy controls, although no correlation found between IL-33 levels and PASI scores (Mitsui et al., 2016; Li et al., 2017; Borsky et al., 2020). Despite having reported to be increased in other skin conditions such as vitiligo and atopic dermatitis (Tamagawa-Mineoka et al., 2014; Vaccaro et al., 2016), IL-33 elevation was significantly higher in psoriatic than in AD lesions, hence lesional IL-33 seem to correlate strongly with psoriasis in a localized manner (Balato et al., 2012).
3.1.6 IFN-γ
IFN-γ is a Th1-derived cytokine that mediates downstream processes favoring keratinocyte proliferation, a key occurrence in psoriasis pathogenesis (Nickoloff and Nestle, 2004). Although there was no overall significant correlation to disease type or severity in terms of PASI score, elevated IFN-γ serum concentration has been associated with active psoriasis in multiple studies (Jacob et al., 2003; Arican et al., 2005; Moustafa et al., 2009; Takahashi et al., 2010; Kurtovic and Halilovic, 2018). Altogether, increased IFN-γ likely indicates presence of psoriasis, though might not be suitable in terms of assessing disease severity due to conflicting reports regarding its correlation with PASI scores.
3.1.7 TNF-α
TNF-α is another key proinflammatory cytokine that promotes immune cell migration towards the skin resulting in keratinocyte proliferation, which is one of the hallmark manifestations of psoriasis (Conrad and Gilliet, 2018; Ogawa et al., 2018; Ovcina-Kurtovic and Kasumagic-Halilovic, 2022). In psoriasis, affected keratinocytes produce excessive TNF-α, hence activating dendritic cells to produce IL-12 and IL-23. These cytokines promote T cell differentiation into Th1 and Th17, which produce more TNF-α and other proinflammatory cytokines such as IL-17 and IFN-γ, leading to the pathologic manifestations of psoriasis, i.e., keratinocyte hyperproliferation, acanthosis, parakeratosis, and hypogranulosis (Conrad and Gilliet, 2018; Ogawa et al., 2018). Serum TNF-α was found to be significantly higher in those with psoriasis than those without (Arican et al., 2005; Takahashi et al., 2010; Kyriakou et al., 2014; Ovcina-Kurtovic and Kasumagic-Halilovic, 2022); however, majority of these studies report that its correlation with PASI scores was not significant.
3.1.8 Thymic stromal lymphopoietin (TSLP)
TSLP is a proallergic keratinocyte-derived cytokine, which has been described to induce dendritic cell maturation and IL-23 production in psoriasis pathogenesis (Volpe et al., 2014). Serum TSLP has been reported to be elevated in psoriasis patients with a significant correlation to disease severity (El-Ghareeb et al., 2019). TSLP may be a potential indicator for early detection and diagnosis of active psoriasis.
3.1.9 High-mobility group box 1 (HMGB1)
Previous studies found that HMGB1, a proinflammatory cytokine, was significantly increased in sera of psoriasis patients compared to healthy controls (Bergmann et al., 2016; Strohbuecker et al., 2019; Borsky et al., 2020); however, its relationship with disease severity is yet to be established.
3.1.10 IL-10
Despite being an anti-inflammatory cytokine expected to be downregulated in the presence of psoriasis, no significant difference has been noted in the baseline serum IL-10 levels between patients with psoriasis and healthy individuals (Dowlatshahi et al., 2013; Bai et al., 2018). In attempt to quantify serum IL-10 concentrations in psoriasis patients, multiple cytokine analysis approach was applied by Michalak-Stoma et al. (2022); however, signal intensities for IL-10 were insufficient for quantification, implying that this anti-inflammatory cytokine may not be an ideal serum biomarker for psoriasis.
3.1.11 Fractalkine (CX3CL1)
Fractalkine, a membrane-bound chemokine, was significantly elevated in the serum of psoriasis patients compared to healthy individuals (Congjun et al., 2015). In the same study, the positive correlation between CX3CL1 serum levels and PASI scores was also demonstrated, suggesting the potential utility of this biomarker to assess disease severity.
3.1.12 Thymus and activation-regulated chemokine (TARC/CCL17) and other chemokines
Thymus and activation-regulated chemokine or CCL17 serum levels were significantly higher in patients with chronic plaque psoriasis compared to that of the healthy controls (Purzycka-Bohdan et al., 2022). Moreover, serum TARC correlated positively with pruritus assessed using the visual analog scale, but no overall significant correlation was seen between serum TARC and PASI scores. Other chemokines, namely, CCL2/MCP-1, CCL3/MIP-1α, CCL5/RANTES, CCL18/PARC and CCL22/MDC, although not significantly correlated with disease severity, were also found to be elevated in patients with chronic plaque psoriasis.
3.2 Adipokines
Adipose-tissue derived mediators called adipokines have been linked with metabolic disturbances and chronic inflammation including psoriasis. Immune dysregulation in psoriasis leads to secretion of adipokines into the bloodstream, hence such molecules potentially reflect disease activity (Słuczanowska-Głabowska et al., 2023).
3.2.1 Lipocalin-2
Detected in blood samples through ELISA, lipocalin-2 is an adipokine that has been correlated with disease severity in psoriasis patients. In a study of 62 patients with psoriasis, the BSA and PASI scores in cases of psoriatic erythroderma and psoriasis vulgaris, as well as the severity score of generalized pustular psoriasis, were all noted to have positive correlation with lipocalin-2 levels (Nguyen and Nguyen, 2022). Furthermore, lipocalin-2 levels were significantly higher in patients with acute types of psoriasis than in chronic types, indicating the potential of this adipokine as a biomarker for psoriasis accompanied with acute inflammation.
3.2.2 Resistin
Resistin is a pro-inflammatory adipokine involved in TNF-α-related pathways in psoriasis (Nakajima et al., 2013; Müge et al., 2019). Serum resistin concentrations were significantly higher in psoriasis patients than that of healthy individuals (Bai et al., 2018). A correlation between serum resistin and PASI score was also reported, in which more severe cases of psoriasis had higher resistin levels than those with lower PASI scores (Seth et al., 2020).
3.2.3 Adiponectin
Psoriasis patients exhibited lower serum adiponectin levels relative to the healthy controls (Shibata et al., 2009; Oh et al., 2014), indicating that adiponectin’s supposedly anti-inflammatory role is downregulated in psoriasis. Furthermore, no significant correlation was noted between adiponectin levels in serum of patients and their PASI scores.
3.2.4 Leptin
Leptin, an energy-regulating secretory hormone from adipose tissue, has been associated with proinflammatory cytokine induction (Cerman et al., 2008). Serum leptin levels were found to be elevated in psoriasis patients compared to healthy controls and positively correlates with PASI scores (Cerman et al., 2008; Zhu et al., 2013; Oh et al., 2014).
3.3 Antimicrobial peptides
3.3.1 S100 proteins
Calprotectin is an antimicrobial peptide and calcium-binding soluble protein secreted by monocytes and neutrophils during inflammation (Voganatsi et al., 2001). Regarded as a potential novel biomarker for psoriasis, serum calprotectin was significantly higher and correlates positively with PASI scores in psoriatic patients than healthy controls (Scali et al., 2022; Li et al., 2023). Both PASI scores and serum calprotectin levels significantly decreased after 3-month methotrexate therapy of patients with psoriasis vulgaris, demonstrating its potential as a prognostic marker for treatment response (Hamza et al., 2019). Furthermore, calprotectin might indicate disease relapse as serum levels were noted to be significantly higher in relapsed cases compared to nonrelapsed cases of psoriasis.
Calcium-binding S100 proteins A8 (S100A8) and A9 (S100A9) levels were significantly increased in both serum and stratum corneum of psoriasis patients while also positively correlating with PASI scores (Benoit et al., 2006; Matsunaga et al., 2021), demonstrating the capability of these proteins in reflecting disease severity. Additionally, serum S100A7 (psoriasin) and S100A12 (calgranulin-c) were found to be elevated in psoriasis patients, with the latter being regarded as the most promising S100 protein biomarker thus far in terms of correlating with severity of psoriasis (Wilsmann-Theis et al., 2016).
3.3.2 β-defensin 2 (BD-2)
Inflammation drives the expression of BD-2 from keratinocytes (Cieślik et al., 2021). Baseline serum BD-2 levels were higher in patients with active psoriasis than healthy controls (Morita et al., 2020). Furthermore, serum BD-2 positively correlated with PASI score and was found to decrease after treatment with secukinumab, an anti-IL-17A drug. A similar trend was reported upon treatment with tofacitinib, a JAK-inhibitor (Jin et al., 2017). Serum BD-2 levels could potentially reflect psoriasis severity and treatment response to secukinumab or tofacitinib, although warranting further studies.
3.4 Other potential novel protein biomarkers
3.4.1 Fibrinogen-like protein 1 (FGL1)
FGL1, a 68-kD protein from the fibrinogen family, was found to be significantly lower in the serum of psoriasis patients than healthy controls (Sun et al., 2022). Despite this observation, PASI scores of patients with psoriasis remained positively correlated with serum FGL1 concentration.
3.4.2 2-5-Oligoadenylate synthase 2 (OAS2)
OAS2 is a potential novel biomarker identified from proteomic profiles of psoriasis patients, who exhibited significantly higher OAS2 serum levels than healthy controls (Zhou et al., 2020). The reported positive correlation between PASI score and OAS2 levels suggests that OAS2 potentially reflects disease severity. Moreover, OAS2 was shown to differentiate those with low PASI scores (PASI≤10) from healthy controls.
4 Discussion: future prospects and clinical application
Quantification of biomarkers from a small amount of blood or serum sample obtained via minimally invasive approaches offers plenty of advantages, including the willingness and increased compliance of patients, emphasizing the importance of biomarker identification and assays. One promising approach is by integrating high throughput technologies such as proteomics analysis as demonstrated in the discovery of OAS2 as a candidate biomarker for psoriasis (Zhou et al., 2020).
Establishing reliable biomarkers for psoriasis involves several stages. First is to identify detectable biomarkers that reflect key clinical outcomes (e.g., disease severity and treatment response). Validation studies, wherein biomarkers are assayed and correlated with the patients’ clinical manifestations, is preferably conducted in a larger cohort to better establish the correlation. Determining a biomarker’s correlation to the disease status (whether positive or negative), its cut-off values, sensitivity, and specificity provides useful information that can support a physician’s clinical decision-making and management of psoriasis patients.
Author contributions
Conceptualization: CC and C-CY; Writing—original draft: CC and C-CY; Writing—review and editing: CC and C-CY. All authors contributed to the article and approved the submitted version.
Funding
This research was supported in part by the National Science and Technology Council (NSTC), Taiwan (grant number NSTC 111-2314-B-006-101).
Acknowledgments
Figures were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alhajj, M., and Farhana, A. (2023). “Enzyme linked immunosorbent assay,” in StatPearls (Island: StatPearls Publishing).
Alwan, W., and Nestle, F. O. (2015). Pathogenesis and treatment of psoriasis: Exploiting pathophysiological pathways for precision medicine. Clin. Exp. Rheumatol. 33 (5), S2–S6.
Arican, O., Aral, M., Sasmaz, S., and Ciragil, P. (2005). Serum levels of TNF-alpha, IFN-gamma, IL-6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediat. Inflamm. 2005, 273–279. doi:10.1155/MI.2005.273
Armstrong, A. W., and Read, C. (2020). Pathophysiology, clinical presentation, and treatment of psoriasis: A review. Jama 323 (19), 1945–1960. doi:10.1001/jama.2020.4006
Aronson, J. K., and Ferner, R. E. (2017). Biomarkers-A general review. Curr. Protoc. Pharmacol. 76, 9.23.1–9.23.17. doi:10.1002/cpph.19
Aydin, S. (2015). A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides 72, 4–15. doi:10.1016/j.peptides.2015.04.012
Bai, F., Zheng, W., Dong, Y., Wang, J., Garstka, M. A., Li, R., et al. (2018). Serum levels of adipokines and cytokines in psoriasis patients: A systematic review and meta-analysis. Oncotarget 9 (1), 1266–1278. doi:10.18632/oncotarget.22260
Balato, A., Lembo, S., Mattii, M., Schiattarella, M., Marino, R., De Paulis, A., et al. (2012). IL-33 is secreted by psoriatic keratinocytes and induces pro-inflammatory cytokines via keratinocyte and mast cell activation. Exp. Dermatol 21 (11), 892–894. doi:10.1111/exd.12027
Benoit, S., Toksoy, A., Ahlmann, M., Schmidt, M., Sunderkötter, C., Foell, D., et al. (2006). Elevated serum levels of calcium-binding S100 proteins A8 and A9 reflect disease activity and abnormal differentiation of keratinocytes in psoriasis. Br. J. Dermatol 155 (1), 62–66. doi:10.1111/j.1365-2133.2006.07198.x
Bergmann, C., Strohbuecker, L., Lotfi, R., Sucker, A., Joosten, I., Koenen, H., et al. (2016). High mobility group box 1 is increased in the sera of psoriatic patients with disease progression. J. Eur. Acad. Dermatol Venereol. 30 (3), 435–441. doi:10.1111/jdv.13564
Boniface, K., Guignouard, E., Pedretti, N., Garcia, M., Delwail, A., Bernard, F. X., et al. (2007). A role for T cell-derived interleukin 22 in psoriatic skin inflammation. Clin. Exp. Immunol. 150 (3), 407–415. doi:10.1111/j.1365-2249.2007.03511.x
Borsky, P., Fiala, Z., Andrys, C., Beranek, M., Hamakova, K., Malkova, A., et al. (2020). Alarmins HMGB1, IL-33, S100A7, and S100A12 in psoriasis vulgaris. Mediat. Inflamm. 2020, 8465083. doi:10.1155/2020/8465083
Brito-Luna, M. J., Villanueva-Quintero, D. G., Sandoval-Talamantes, A. K., Fafutis-Morris, M., Graciano-Machuca, O., Sanchez-Hernandez, P. E., et al. (2016). Correlation of IL-12, IL-22, and IL-23 in patients with psoriasis and metabolic syndrome. Preliminary report. Prelim. Rep. Cytokine 85, 130–136. doi:10.1016/j.cyto.2016.06.020
Caproni, M., Antiga, E., Melani, L., Volpi, W., Del Bianco, E., and Fabbri, P. (2009). Serum levels of IL-17 and IL-22 are reduced by etanercept, but not by acitretin, in patients with psoriasis: A randomized-controlled trial. J. Clin. Immunol. 29 (2), 210–214. doi:10.1007/s10875-008-9233-0
Cerman, A. A., Bozkurt, S., Sav, A., Tulunay, A., Elbaşi, M. O., and Ergun, T. (2008). Serum leptin levels, skin leptin and leptin receptor expression in psoriasis. Br. J. Dermatol 159 (4), 820–826. doi:10.1111/j.1365-2133.2008.08742.x
Cieślik, M., Bagińska, N., Górski, A., and Jończyk-Matysiak, E. (2021). Human β-defensin 2 and its postulated role in modulation of the immune response. Cells 10 (11), 2991. doi:10.3390/cells10112991
Congjun, J., Yanmei, Z., Huiling, J., Zhen, Y., and Shuo, L. (2015). Elevated local and serum cx3cl1(fractalkine) expression and its association with disease severity in patients with psoriasis. Ann. Clin. Lab. Sci. 45 (5), 556–561.
Conrad, C., and Gilliet, M. (2018). Psoriasis: From pathogenesis to targeted therapies. Clin. Rev. Allergy & Immunol. 54 (1), 102–113. doi:10.1007/s12016-018-8668-1
De la Fuente, M., MacDonald, T. T., and Hermoso, M. A. (2015). The IL-33/ST2 axis: Role in health and disease. Cytokine & Growth Factor Rev. 26 (6), 615–623. doi:10.1016/j.cytogfr.2015.07.017
Del Campo, M., Jongbloed, W., Twaalfhoven, H. A., Veerhuis, R., Blankenstein, M. A., and Teunissen, C. E. (2015). Facilitating the validation of novel protein biomarkers for dementia: An optimal workflow for the development of sandwich immunoassays. Front. Neurol. 6, 202. doi:10.3389/fneur.2015.00202
Di Meglio, P., Villanova, F., and Nestle, F. O. (2014). Psoriasis. Cold Spring Harb. Perspect. Med. 4 (8), a015354. doi:10.1101/cshperspect.a015354
Dowlatshahi, E. A., van der Voort, E. A., Arends, L. R., and Nijsten, T. (2013). Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol 169 (2), 266–282. doi:10.1111/bjd.12355
Dudakov, J. A., Hanash, A. M., and van den Brink, M. R. (2015). Interleukin-22: Immunobiology and pathology. Annu. Rev. Immunol. 33, 747–785. doi:10.1146/annurev-immunol-032414-112123
Dyring-Andersen, B., Honoré, T. V., Madelung, A., Bzorek, M., Simonsen, S., Clemmensen, S. N., et al. (2017). Interleukin (IL)-17A and IL-22-producing neutrophils in psoriatic skin. Br. J. Dermatol 177 (6), e321–e322. doi:10.1111/bjd.15533
El-Ghareeb, M. I., Helmy, A., Al Kazzaz, S., and Samir, H. (2019). Serum TSLP is a potential biomarker of psoriasis vulgaris activity. Psoriasis (Auckl) 9, 59–63. doi:10.2147/ptt.S212774
Engvall, E., and Perlmann, P. (1972). Enzyme-linked immunosorbent assay, elisa. J. Immunol. 109 (1), 129–135. doi:10.4049/jimmunol.109.1.129
Ergen, E. N., and Yusuf, N. (2018). Inhibition of interleukin-12 and/or interleukin-23 for the treatment of psoriasis: What is the evidence for an effect on malignancy? Exp. Dermatol 27 (7), 737–747. doi:10.1111/exd.13676
Gan, S. D., and Patel, K. R. (2013). Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Invest. Dermatol 133 (9), e12. doi:10.1038/jid.2013.287
Goodman, W. A., Levine, A. D., Massari, J. V., Sugiyama, H., McCormick, T. S., and Cooper, K. D. (2009). IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J. Immunol. 183 (5), 3170–3176. doi:10.4049/jimmunol.0803721
Gordon, K. B., Armstrong, A. W., Foley, P., Song, M., Shen, Y. K., Li, S., et al. (2019). Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J. Invest. Dermatol 139 (12), 2437–2446.e1. doi:10.1016/j.jid.2019.05.016
Greaves, M. W., and Weinstein, G. D. (1995). Treatment of psoriasis. N. Engl. J. Med. 332 (9), 581–588. doi:10.1056/nejm199503023320907
Hamza, A., Hassan, E., Donia, H., and Maamon, Y. (2019). Serum calprotectin as a predictive biomarker in the treatment of psoriasis vulgaris with methotrexate. J. Egypt. Womenís Dermatologic Soc. 16 (2), 112–118. doi:10.4103/jewd.Jewd_12_19
Jacob, S. E., Nassiri, M., Kerdel, F. A., and Vincek, V. (2003). Simultaneous measurement of multiple Th1 and Th2 serum cytokines in psoriasis and correlation with disease severity. Mediat. Inflamm. 12 (5), 309–313. doi:10.1080/09629350310001619753
Jin, T., Sun, Z., Chen, X., Wang, Y., Li, R., Ji, S., et al. (2017). Serum human beta-defensin-2 is a possible biomarker for monitoring response to JAK inhibitor in psoriasis patients. Dermatology 233 (2-3), 164–169. doi:10.1159/000475809
Keijsers, R., Hendriks, A. G. M., van Erp, P. E. J., van Cranenbroek, B., van de Kerkhof, P. C. M., Koenen, H., et al. (2014). In vivo induction of cutaneous inflammation results in the accumulation of extracellular trap-forming neutrophils expressing RORγt and IL-17. J. Invest. Dermatol 134 (5), 1276–1284. doi:10.1038/jid.2013.526
Kurtovic, N. O., and Halilovic, E. K. (2018). Serum concentrations of interferon gamma (IFN-γ) in patients with psoriasis: Correlation with clinical type and severity of the disease. Med. Arch. 72 (6), 410–413. doi:10.5455/medarh.2018.72.410-413
Kutsuna, T., Hino, K., Hasegawa, H., Watamori, K., Kidani, T., Imai, H., et al. (2022). Psoriatic arthritis successfully treated with second-line anti-interleukin-6 treatment: A case report and review of the literature. J. Med. Case Rep. 16 (1), 402. doi:10.1186/s13256-022-03624-z
Kyriakou, A., Patsatsi, A., Vyzantiadis, T. A., and Sotiriadis, D. (2014). Serum levels of TNF-α, IL-12/23p40, and IL-17 in plaque psoriasis and their correlation with disease severity. J. Immunol. Res. 2014, 467541. doi:10.1155/2014/467541
Li, B., Li, G., Song, Z., and Zhang, Z. (2023). Serum calprotectin as a promising inflammatory biomarker in psoriatic arthritis: A 1-year longitudinal study. Rheumatol. Ther. 10 (1), 149–160. doi:10.1007/s40744-022-00501-5
Li, J., Liu, L., Rui, W., Li, X., Xuan, D., Zheng, S., et al. (2017). New interleukins in psoriasis and psoriatic arthritis patients: The possible roles of interleukin-33 to interleukin-38 in disease activities and bone erosions. Dermatology 233 (1), 37–46. doi:10.1159/000471798
Lin, A. M., Rubin, C. J., Khandpur, R., Wang, J. Y., Riblett, M., Yalavarthi, S., et al. (2011). Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J. Immunol. 187 (1), 490–500. doi:10.4049/jimmunol.1100123
Lo, Y. H., Torii, K., Saito, C., Furuhashi, T., Maeda, A., and Morita, A. (2010). Serum IL-22 correlates with psoriatic severity and serum IL-6 correlates with susceptibility to phototherapy. J. Dermatol Sci. 58 (3), 225–227. doi:10.1016/j.jdermsci.2010.03.018
Lu, J., Tang, S., Xie, S., Yi, X., Yu, N., Gao, Y., et al. (2016). The potential of IL-12 in predicting clinical response to etanercept treatment in patients with psoriasis. Int. J. Clin. Exp. Med. 9 (12), 23519–23524.
Matsunaga, Y., Hashimoto, Y., and Ishiko, A. (2021). Stratum corneum levels of calprotectin proteins S100A8/A9 correlate with disease activity in psoriasis patients. J. Dermatol 48 (10), 1518–1525. doi:10.1111/1346-8138.16032
Michalak-Stoma, A., Bartosińska, J., Raczkiewicz, D., Kowal, M., Kozak, J., Gujski, M., et al. (2022). Multiple cytokine analysis of Th1/Th2/Th9/Th17/Th22/treg cytokine pathway for individual immune profile assessment in patients with psoriasis. Med. Sci. Monit. 28, e938277. doi:10.12659/msm.938277
Mitsui, A., Tada, Y., Takahashi, T., Shibata, S., Kamata, M., Miyagaki, T., et al. (2016). Serum IL-33 levels are increased in patients with psoriasis. Clin. Exp. Dermatol 41 (2), 183–189. doi:10.1111/ced.12670
Molteni, S., and Reali, E. (2012). Biomarkers in the pathogenesis, diagnosis, and treatment of psoriasis. Psoriasis: Targets and Therapy, 55–66.
Morita, A., Tani, Y., Matsumoto, K., Yamaguchi, M., Teshima, R., and Ohtsuki, M. (2020). Assessment of serum biomarkers in patients with plaque psoriasis on secukinumab. J. Dermatol 47 (5), 452–457. doi:10.1111/1346-8138.15278
Moustafa, Y. M., Abdel Aal, I., Mohamed, E., Abdel Baky, A., and Taher, S. (2009). Assessment of serum interferon gamma and interleukin-4 in psoriasis vulgaris. Egypt. J. Med. Microbiol. 18 (3), 45–48.
Müge, A. E., Nilsel, İ., and Şehri, E. (2019). Association of leptin, resistin, and high-molecular-weight adiponectin levels with psoriasis area and severity index scores, obesity, and insulin resistance in psoriasis patients. Dermatol. Sin. 37 (1), 33–39. doi:10.4103/ds.ds_9_18
Muramatsu, S., Kubo, R., Nishida, E., and Morita, A. (2017). Serum interleukin-6 levels in response to biologic treatment in patients with psoriasis. Mod. Rheumatol. 27 (1), 137–141. doi:10.3109/14397595.2016.1174328
Nakajima, H., Nakajima, K., Tarutani, M., and Sano, S. (2013). Clear association between serum levels of adipokines and T-helper 17-related cytokines in patients with psoriasis. Clin. Exp. Dermatol 38 (1), 66–70. doi:10.1111/j.1365-2230.2012.04465.x
Nassar, A. A., Bakr, N. M., Elyousefi, E. H. I., Elkholy, B. M., and Fawzy, M. M. (2022). Serum immunoglobulin E and interleukin-17 levels in patients with chronic plaque psoriasis: A case-control study. J. Cosmet. Dermatol 21 (11), 6377–6384. doi:10.1111/jocd.15299
Nestle, F. O., Kaplan, D. H., and Barker, J. (2009). Psoriasis. N. Engl. J. Med. 361 (5), 496–509. doi:10.1056/NEJMra0804595
Nguyen, C. T. H., and Nguyen, O. P. T. (2022). Increased plasma lipocalin-2 levels correlate with disease severity and may be a marker of acute inflammatory response in patients with psoriasis. Dermatol Rep. 14 (4), 9469. doi:10.4081/dr.2022.9469
Nickoloff, B. J., and Nestle, F. O. (2004). Recent insights into the immunopathogenesis of psoriasis provide new therapeutic opportunities. J. Clin. Invest. 113 (12), 1664–1675. doi:10.1172/jci22147
Ogawa, E., Sato, Y., Minagawa, A., and Okuyama, R. (2018). Pathogenesis of psoriasis and development of treatment. J. Dermatology 45 (3), 264–272. doi:10.1111/1346-8138.14139
Oh, Y. J., Lim, H. K., Choi, J. H., Lee, J. W., and Kim, N. I. (2014). Serum leptin and adiponectin levels in Korean patients with psoriasis. J. Korean Med. Sci. 29 (5), 729–734. doi:10.3346/jkms.2014.29.5.729
Ovcina-Kurtovic, N., and Kasumagic-Halilovic, E. (2022). Serum levels of tumor necrosis factor - alpha in patients with psoriasis. Mater Sociomed. 34 (1), 40–43. doi:10.5455/msm.2022.33.40-43
Paul, J., and Veenstra, T. D. (2022). Separation of serum and plasma proteins for in-depth proteomic analysis. Separations 9 (4), 89. doi:10.3390/separations9040089
Philipp, S., Menter, A., Nikkels, A. F., Barber, K., Landells, I., Eichenfield, L. F., et al. (2020). Ustekinumab for the treatment of moderate-to-severe plaque psoriasis in paediatric patients (≥6 to < 12 years of age): Efficacy, safety, pharmacokinetic and biomarker results from the open-label CADMUS jr study. Br. J. Dermatol 183 (4), 664–672. doi:10.1111/bjd.19018
Pietrzak, A., Chabros, P., Grywalska, E., Pietrzak, D., Kandzierski, G., Wawrzycki, B. O., et al. (2020). Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postepy Dermatol Alergol. 37 (1), 41–45. doi:10.5114/ada.2018.78028
Purzycka-Bohdan, D., Nedoszytko, B., Zabłotna, M., Gleń, J., Szczerkowska-Dobosz, A., and Nowicki, R. J. (2022). Chemokine profile in psoriasis patients in correlation with disease severity and pruritus. Int. J. Mol. Sci. 23 (21), 13330. doi:10.3390/ijms232113330
Rendon, A., and Schäkel, K. (2019). Psoriasis pathogenesis and treatment. Int. J. Mol. Sci. 20 (6), 1475. doi:10.3390/ijms20061475
Scali, E., Dastoli, S., Procopio, A. C., Ricca, D., Mazzei, V., Cinaglia, P., et al. (2022). Evaluation of serum calprotectin as novel biomarker in psoriatic patients: A prospective pilot study. Minerva Med. 113 (5), 833–837. doi:10.23736/s0026-4806.22.08041-7
Seth, D., Ehlert, A. N., Golden, J. B., Damiani, G., McCormick, T. S., Cameron, M. J., et al. (2020). Interaction of resistin and systolic blood pressure in psoriasis severity. J. Invest. Dermatol 140 (6), 1279–1282.e1. doi:10.1016/j.jid.2019.07.727
Shibata, S., Saeki, H., Tada, Y., Karakawa, M., Komine, M., and Tamaki, K. (2009). Serum high molecular weight adiponectin levels are decreased in psoriasis patients. J. Dermatol Sci. 55 (1), 62–63. doi:10.1016/j.jdermsci.2009.02.009
Shimauchi, T., Hirakawa, S., Suzuki, T., Yasuma, A., Majima, Y., Tatsuno, K., et al. (2013). Serum interleukin-22 and vascular endothelial growth factor serve as sensitive biomarkers but not as predictors of therapeutic response to biologics in patients with psoriasis. J. Dermatol 40 (10), 805–812. doi:10.1111/1346-8138.12248
Słuczanowska-Głabowska, S., Staniszewska, M., Marchlewicz, M., Duchnik, E., Łuczkowska, K., Safranow, K., et al. (2023). Adiponectin, leptin and resistin in patients with psoriasis. J. Clin. Med. 12 (2), 663. doi:10.3390/jcm12020663
Strohbuecker, L., Koenen, H., van Rijssen, E., van Cranenbroek, B., Fasse, E., Joosten, I., et al. (2019). Increased dermal expression of chromatin-associated protein HMGB1 and concomitant T-cell expression of the DNA RAGE in patients with psoriasis vulgaris. Psoriasis (Auckl) 9, 7–17. doi:10.2147/ptt.S190507
Sun, X., Liu, L., Chen, S., Wang, J., Cai, X., Song, J., et al. (2022). Fibrinogen-like protein 1 as a novel biomarker of psoriasis severity. J. Inflamm. Res. 15, 4637–4647. doi:10.2147/jir.S378953
Takahashi, H., Tsuji, H., Hashimoto, Y., Ishida-Yamamoto, A., and Iizuka, H. (2010). Serum cytokines and growth factor levels in Japanese patients with psoriasis. Clin. Exp. Dermatology 35 (6), 645–649. doi:10.1111/j.1365-2230.2009.03704.x
Tamagawa-Mineoka, R., Okuzawa, Y., Masuda, K., and Katoh, N. (2014). Increased serum levels of interleukin 33 in patients with atopic dermatitis. J. Am. Acad. Dermatology 70 (5), 882–888. doi:10.1016/j.jaad.2014.01.867
Theoharides, T. C., Zhang, B., Kempuraj, D., Tagen, M., Vasiadi, M., Angelidou, A., et al. (2010). IL-33 augments substance P–induced VEGF secretion from human mast cells and is increased in psoriatic skin. Proc. Natl. Acad. Sci. 107 (9), 4448–4453. doi:10.1073/pnas.1000803107
Vaccaro, M., Cicero, F., Mannucci, C., Calapai, G., Spatari, G., Barbuzza, O., et al. (2016). IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch. Dermatol Res. 308 (7), 527–530. doi:10.1007/s00403-016-1675-2
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M., and Stockinger, B. (2006). TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24 (2), 179–189. doi:10.1016/j.immuni.2006.01.001
Voganatsi, A., Panyutich, A., Miyasaki, K. T., and Murthy, R. K. (2001). Mechanism of extracellular release of human neutrophil calprotectin complex. J. Leukoc. Biol. 70 (1), 130–134. doi:10.1189/jlb.70.1.130
Volpe, E., Pattarini, L., Martinez-Cingolani, C., Meller, S., Donnadieu, M. H., Bogiatzi, S. I., et al. (2014). Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J. Allergy Clin. Immunol. 134 (2), 373–381. doi:10.1016/j.jaci.2014.04.022
Wawrzycki, B., Pietrzak, A., Grywalska, E., Krasowska, D., Chodorowska, G., and Roliński, J. (2019). Interleukin-22 and its correlation with disease activity in plaque psoriasis. Arch. Immunol. Ther. Exp. Warsz. 67 (2), 103–108. doi:10.1007/s00005-018-0527-5
Wilsmann-Theis, D., Wagenpfeil, J., Holzinger, D., Roth, J., Koch, S., Schnautz, S., et al. (2016). Among the S100 proteins, S100A12 is the most significant marker for psoriasis disease activity. J. Eur. Acad. Dermatol Venereol. 30 (7), 1165–1170. doi:10.1111/jdv.13269
Yilmaz, S. B., Cicek, N., Coskun, M., Yegin, O., and Alpsoy, E. (2012). Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch. Dermatol Res. 304 (6), 465–469. doi:10.1007/s00403-012-1229-1
Zeng, F., Chen, H., Chen, L., Mao, J., Cai, S., Xiao, Y., et al. (2021). An autocrine circuit of IL-33 in keratinocytes is involved in the progression of psoriasis. J. Invest. Dermatol 141 (3), 596–606.e7. doi:10.1016/j.jid.2020.07.027
Zhou, Y., Wang, P., Yan, B. X., Chen, X. Y., Landeck, L., Wang, Z. Y., et al. (2020). Quantitative proteomic profile of psoriatic epidermis identifies OAS2 as a novel biomarker for disease activity. Front. Immunol. 11, 1432. doi:10.3389/fimmu.2020.01432
Keywords: psoriasis, ELISA, biomarkers, biologics, interleukins, skin inflammation
Citation: Cruz CJG and Yang C-C (2023) Clinical application of serum biomarkers for detecting and monitoring of chronic plaque psoriasis. Front. Mol. Biosci. 10:1196323. doi: 10.3389/fmolb.2023.1196323
Received: 29 March 2023; Accepted: 29 June 2023;
Published: 21 July 2023.
Edited by:
Aristidis M. Tsatsakis, University of Crete, GreeceReviewed by:
Rohit Saluja, All India Institute of Medical Sciences, IndiaCopyright © 2023 Cruz and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao-Chun Yang, eWFuZ2NjQG1haWwubmNrdS5lZHUudHc=
 Criselda Jean G. Cruz
Criselda Jean G. Cruz Chao-Chun Yang
Chao-Chun Yang