- 1Department of Breast Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, China
- 2Wenzhou Medical University, Wenzhou, Zhejiang, China
Triple negative breast cancer is distinguished by its high malignancy, aggressive invasion, rapid progression, easy recurrence, and distant metastases. Additionally, it has a poor prognosis, a high mortality, and is unresponsive to conventional endocrine and targeted therapy, making it a challenging problem for breast cancer treatment and a hotspot for scientific research. Recent research has revealed that certain miRNA can directly or indirectly affect the occurrence, progress and recurrence of TNBC. Their expression levels have a significant impact on TNBC diagnosis, treatment and prognosis. Some miRNAs can serve as biomarkers for TNBC diagnosis and prognosis. This article summarizes the progress of miRNA research in TNBC, discusses their roles in the occurrence, invasion, metastasis, prognosis, and chemotherapy of TNBC, and proposes a treatment strategy for TNBC by interfering with miRNA expression levels.
1 Introduction
The International Agency for Research on Cancer (IARC) of the World Health Organization has released its latest global cancer data for 2020, revealing that breast cancer has surpassed lung cancer as the most common cancer in the world for the first time. The survival rate of breast cancer patients has significantly increased due to continuous advancements in early identification, individualized treatment, and chemotherapy approaches. However, it remains the leading cause of cancer-related deaths among women worldwide. Compared with other BC(Breast cancer) subtypes, TNBC(Triple negative breast cancer) has a worse prognosis and a higher early recurrence rate, typically with distant metastasis, owing to the absence of ER (Estrogen receptor),PR (Progesterone receptor) and Her-2(Human epidermal growth factor receptor 2) (Amorim et al., 2016; Volovat et al., 2020). TNBC makes for 10%–20% of all breast cancer cases, and the patients tend to be increasingly young (Shen et al., 2015; Araujo et al., 2022). Patients with metastatic TNBC have a median overall survival of about 18 months, and it is significantly less than patients with PR, ER positive, and HER2 enriched diseases, who may have a survival time of more than 5 years (Vagia et al., 2020). In order to improve the survival rate, it is necessary to identify its predictive biomarkers to assess metastatic rate, therapeutic effect, and even to create novel therapeutic approaches. MicroRNAs (miRNAs) are one of the promising molecular targets.
MiRNAs can be involved in regulating various pathophysiological processes, including proliferation, stress response, cell adhesion, inflammation, as well as cell survival, aging and apoptosis, all of which are closely related to the development of tumors (Xu et al., 2020; Rupaimoole and Slack, 2017). Multiple investigations have demonstrated that miRNA can target certain mRNAs to regulate a number of genes at the pre- and post-transcriptional levels (Du et al., 2020), hence promoting tumor growth, migration, invasion, angiogenesis, immune evasion and chemotherapy resistance (Miyoshi et al., 2017; Qu et al., 2017). (Figure 1) As a consequence, the abnormal expression of miRNA is closely associated with the occurrence and progression of BC. Some miRNAs have been identified to correlate with breast cancer subtypes and can be serve as the potential therapeutic application for BC. In perticular, miR-29a, miR-181a, miR-652 are related to Luminal A subtype (McDermott et al., 2014), miR-342 Luminal B subtype (Lowery et al., 2009), and miR-10b, miR-21 are related with HER-2 positive subtype (Anfossi et al., 2014). Furthermore, miRNA dysregulation contributes significantly to the activation or repression of TNBC-related gene expression. In recent years, numerous studies have discovered that the expression of miRNA in tumor tissue or blood of TNBC patients differs significantly from that of normal people, implying that miRNA may be closely relevant to the formation and progression of TNBC. High-throughput sequencing techniques have been established and developed, which has sped up and improved the accuracy of miRNA identification and expression detection (Thomas et al., 2019). In addition to traditional Northern blot method and fluorescent quantitative PCR technology, the second-generation sequencing (NGS) and microarray technology are being used to detect miRNA expression level (Hamam et al., 2017). Many oncology researchers use TCGA, GEO and other databases to analyze the relationship between miRNA imbalance and tumor occurrence and development (Urabe et al., 2019). This cutting-edge technique for bioinformatics analysis is crucial for the creation of miRNA biomarkers. In this paper, we reviewed recent research findings on miRNA in the diagnosis and prognosis of TNBC, and evaluated the prospects and viability of this field.
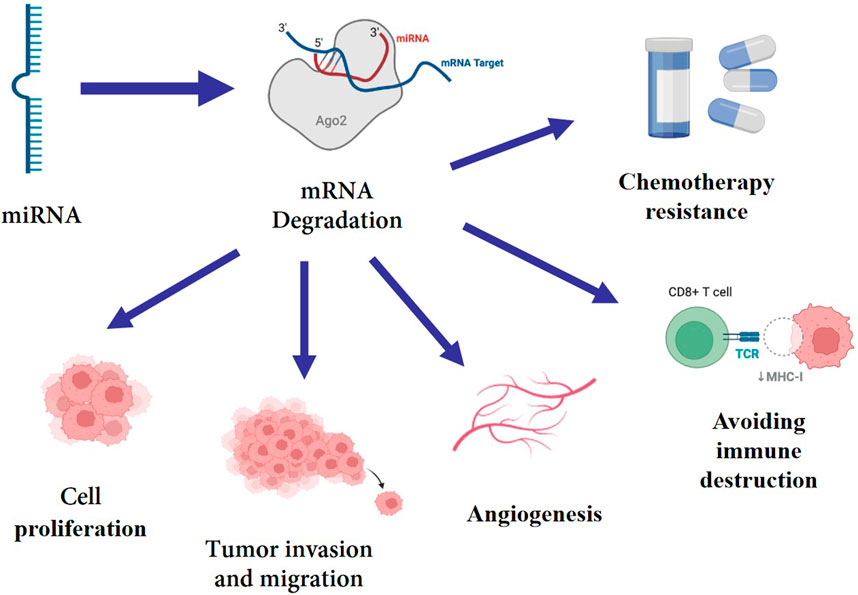
FIGURE 1. miRNA targets mRNA, mediating its degradation, which enhance cell proliferation, tumor invasion, migration, angiogenesis, immune evasion and chemotherapy resistance.
2 MiRNA with diagnostic and prognostic function
TNBC lacks specific diagnostic and prognostic markers due to tumor heterogeneity (Vargas and Harris, 2016). Currently, the diagnosis of TNBC is primarily based on the pathological detection and immunohistochemical detection, which is time-consuming and costly. It will greatly improve the diagnosis and treatment efficiency of TNBC, if one or several miRNAs can be identified to guide the clinical diagnosis and prognosis of TNBC. The prognosis can be early assessed using TNBC prognostic markers, and improved with early intervention.
2.1 miRNA assisting in diagnosis of TNBC
The existence of tumor markers in serum may result from the death of tumor cells, which are released into the blood after splitting, or it is believed to result from the spontaneous secretion of tumor cells. However, the mechanism underlying these two possibilities needs further investigation. Changes in the biological origin of microRNA, epigenetic regulation, transcription factors, and mutant proteins all contribute to altered microRNA expression patterns in breast cancer. It is reported that miRNA-9, miRNA-10b and miRNA-17-5p are abnormally upregulated in TNBC and have the potential to serve as diagnostic markers (Malla et al., 2019). Kahraman et al. used microarrays and Real Time Quantitative PCR(RT-qPCR)to assess the miRNA levels in the blood of healthy women and TNBC patients. They discovered that individuals with TNBC had considerably higher serum concentrations of miR-144-3p, miR-144-5p, miR-126-5p, and let-7d-5p than healthy women. Therefore, by identifying the presence of the aforementioned four miRNAs in patients’ blood, it may be possible to early diagnose TNBC (Kahraman et al., 2018). Using the rank aggregation method to conduct meta-analysis and integrating the miRNA expression profile data set of TNBC, Naorem et al. discovered six kinds of seriously dysregulated miRNAs (miR-135b-5p, miR-18a-5p, miR-9-5p, miR522-3p expression upregulated, miR-190b and miR-449a downregulated) with high prediction accuracy (Naorem et al., 2019). These six miRNAs may be a promising candidate for TNBC diagnostic biomarkers. Yang et al. demonstrated that the level of miR-195-5p in TNBC tissues is lower than that in healthy tissues using TCGA RNA sequence data analysis. Additionally, they measured the levels of miR-195-5p in 40 pairs of TNBC tissues and adjacent non-cancerous tissues, showing that it was considerably downregulated in TNBC tissues compared to paracancerous tissue (Yang et al., 2019). The above miRNAs are expressed differently in TNBC and normal tissues, and it is anticipated that they will function as biomarkers for the diagnosis of TNBC.
As a result of the differentiating expression of several miRNAs between TNBC and other types of BC, TNBC is predicted to be identifiable in many BC patients and these miRNAs will likely serve as biomarkers for differentiating between the various types of BC. Braicu et al. discovered that the miR-17-92 clusters (miR-17, miR-20a, miR-20b, and miR-93), miR-130, miR-22, and miR-29a/c can distinguish TNBC and DPBC (double positive breakthrough cancer, DPBC: ER+, PR+, Her-2) (Braicu et al., 2018). According to Pascull et al., TNBC subgroups had the highest levels of miR-155 expression relative to Luminal A and Luminal B, and HER2 amplification subgroups (Pasculli et al., 2020). Niedwiecki et al. examined the serum miR-200c levels in patients with two different BC subtypes and observed that TNBC patients had lower miRNA-200c levels than the ER/PR positive group as a whole (Niedzwiecki et al., 2018). In addition, there were statistically significant variations of miR-205 expression levels in the BC patients with different ER/PR states. Both the PR positive group and the ER positive group had higher levels of miR-205 than the PR negative group and the ER negative group, respectively. And the expression of miR-205 was found to be considerably higher in the ER+/PR + group compared to the ER -/PR - (i.e., TNBC) group. As a result, miR-205s low expression has an indication for TNBC (Petrovic et al., 2022). (Table 1)
Some biomedical companies and universities have developed specific miRNA detection kits for BC diagnosis in recent years, but there has been no report on targeted miRNA detection kits for TNBC diagnosis. We believe that it will be available soon.
2.2 miRNA assisting in determining prognosis
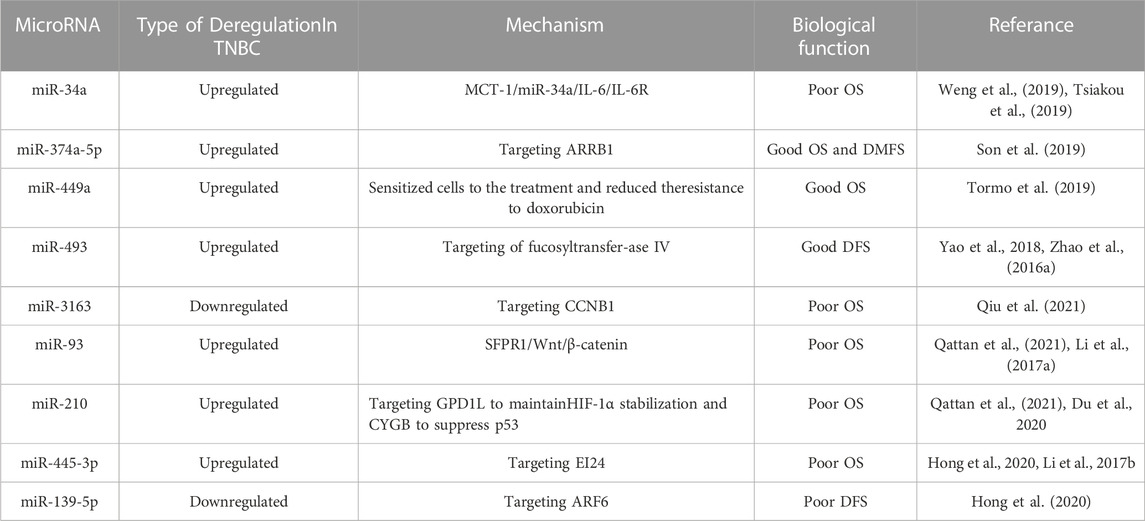
It will be absolutely crucial for the treatment of TNBC if we can predict the relationship between the up/downregulation of particular miRNAs and the prognosis of TNBC in order to determine the overall survival (OS), disease-free survival (DFS), and distant metastasis free survival (DMFS) of TNBC patients. We speculate that miRNA can be used as an effective therapeutic strategy and prognostic marker for TNBC. Weng et al. considered that the expression of the oncogene Multiple Copies in T-cell Malignancy 1 (MCT-1/MCTS1), which functions through MCT-1/miR-34a/IL-6/IL-6R, is a novel poor prognostic sign for patients with TNBC. By preventing the expression of IL-6R, which is supported by MCT-1, MiR-34a can enhance the prognosis of TNBC (Tsiakou et al., 2019; Weng et al., 2019). As a result, boosting miR-34a expression aids in improving TNBC’s prognosis. MiR-374a-5p can target arrestin beta 1 (ARRB1) and reduce its expression. Additionally, the expression of ARRB1 is positively connected with the survival rate of TNBC patients and negatively correlated with the histological grade of breast cancer (Son et al., 2019). Tormo et al. revealed that miR-449a high expression was noticeably associated with favourable prognosis, whereas miR-449b/c was unrelated to prognosis, based on their analysis of the GEO database (Tormo et al., 2019). Using tissue microarray (TMA), Yao et al. investigated the expression of miR-493 in breast cancer samples and found that patients with high miR-493 expression had improved DFS (Zhao et al., 2016a; Yao et al., 2018). Through bioinformatics analysis, Qiu et al. noticed that miR-3163 was connected to the poor OS of androgen receptor (AR) positive TNBCs. These results indicate that miR-3163 may be promising prognostic markers and therapeutic targets for AR positive TNBCs (Qiu et al., 2021). Through meta-analysis, Qattan et al. demonstrated an association of upregulated miR-93 and miR-210 with poor OS outcomes in TNBC patients (Li et al., 2017a; Du et al., 2020; Qattan et al., 2021). Hsiao Chin Hong et al. mined TCGA and GEO databases by logistic regression analysis and Gaussian mixture model, and then used the Kaplan-Meier method to conduct a comprehensive survival analysis, revealing that miR-455-3p was significantly related to OS, while miR-139-5p was significantly related to DFS, indicating that them were related to the recurrence of TNBC (Li et al., 2017b; Hong et al., 2020). All of the above investigations demonstrated that some particular miRNAs were associated with patient survival, prognosis, and recurrence and were therefore considered to be potential prognostic markers of TNBC (Table 2).
In conclusion, miRNA imbalance may become a potentially important tool for identifying key biomarkers in patients with TNBC. The above indicators can be detected by the patient’s blood or tumor tissue, with high sensitivity and specificity. In this case, miRNA not only can be employed as a potential marker to distinguish TNBC from other breast cancer, but also can be used as a biomarker, participating in canceration, predicting prognosis and evaluating treatment response. It is anticipated to be used in conjunction with conventional invasive biopsy to diagnose TNBC and predict its prognosis.
3 MiRNAs promoting tumor formation and progression
Tumor academia has come to terms with the notion that miRNA has a role in the initiation and progression of breast cancer, particularly TNBC, a heterogeneous subtype of the disease. Breast cancer progression is directly correlated with tumor growth, invasion, migration, and angiogenesis. Currently, it has been proven that a number of miRNAs participate in the aforementioned physiological processes of TNBC tumor cells, which can encourage the formation and progression of tumors. As a result, miRNA-based research is crucial for the early diagnosis and management of TNBC.
miR-20a-5p promotes TNBC cell proliferation by targeting Run related transcription factor 3 (RUNX3) and its immediate downstream targets Bim and p21 (Bai et al., 2018). miR-135b is a highly expressed miRNA in TNBC that targets the 3′-UTR of APC, which helps tumor cells proliferate and metastasize (Lv et al., 2019). A study in 2021 found that MDA-MB-231 cells (TNBC cells) expressed more miR-301a-3p than MCF-10A cells (human breast epithelial cells) did. Furthermore, miR-301a-3p overexpression can suppress mesenchymal homeobox 2 (MEOX2) expression, increasing the viability, migration and invasion of MDA - MB - 231 cells (Liu and Wang, 2021). In addition to targeting the appropriate genes to control the development of TNBC, some miRNAs can promote tumor progression by controlling the cell cycle and thwarting tumor cell apoptosis. And miR-502 could directly target H4K20 methyltransferase SET8, which is involved in cell proliferation and cycle, to encourage the transition of the cell cycle from the G phase to the M phase (Liu et al., 2016; Cantini et al., 2019). In TNBC tumor tissue, miR-301b was shown to be upregulated, and Song et al. discovered that it directly bound to the 3′-UTR of the CYLD lysine 63 diquinase mRNA to activate NF-kB p65 and prevent 5-FU from inducing tumor cell apoptosis (Song et al., 2018). Carcinogenic miRNA is linked to promoting the growth and invasion of TNBC tumor cells as well as tumor metastasis. Recently, Darbeheshti et al. discovered that miR-182-5p was considerably upregulated in TNBC tumor tissues compared to adjacent normal tissues, and that high miR-182-5p expression has a strong correlation with larger tumors, higher tumor grades, and positive lymph nodes. Moreover, miR-182-5p overexpression accelerates TNBC development and lymph node metastasis by downregulating the genes CHEK2 and RAD51 (Darbeheshti et al., 2022). In some way, the progress of TNBC is maintained by recruiting powerful tumor microenvironments (TMEs), which are mainly composed of cancer related fibroblasts (CAFs) that can recognize tumor markers. Scognamiglio et al. dicovered that the synergistic action of miR-185-5p, miR-652-5p and miR-1246 promoting fibroblast migration, and specific cancer-associated fibroblasts towards a pro-migratory functional state, finally boosting TNBC progression and migration (Scognamiglio et al., 2022). Wang et al. revealed that miR-1976 knockdown could enhance EMT and CSCs in vitro by targeting PIK3CG (Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit gamma) (Wang et al., 2020). (Figure 2)
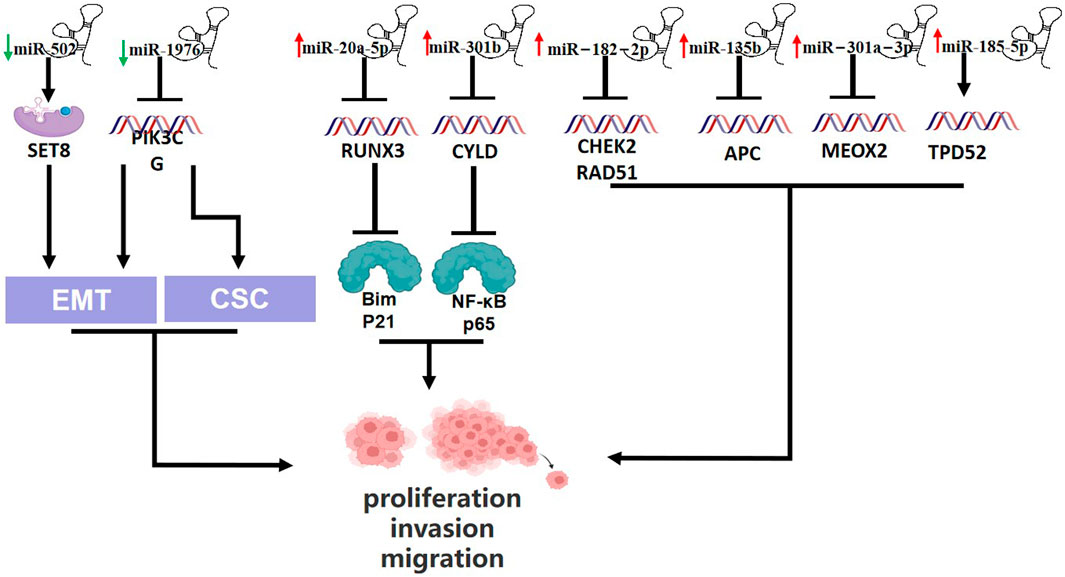
FIGURE 2. Schematic presentation of miRNAs involvement in promotion of triple-negative breast cancer and relationship between miRNAs and epithelial-to mesenchymal transition (EMT) and Cancer stem cells-like properties (CSC). Downregulated miR-502 enhance SET8 expression, which promotes EMT. miR-1976 knockdown could enhance EMT and CSCs by blocking PIK3CG. Upregulation of miR-20a-5p suppresses RUNX3, which suppresses Bim and p21. miR-301b is upregulated and represses CYLD, which block NF-KB p65. And high expression of miR-182-2p suppresses CHEK2 and RAD51. Upregulated miR-135b, miR-301a-3p and miR-185-5p block APC, MEOX2 and TPD52, respectively.
The abnormal expression of the above-mentioned miRNAs in TNBC promotes tumor cell formation, proliferation, invasion, and metastasis by targeting specific genes, regulating the cell cycle, inhibiting tumor cell apoptosis, and other mechanisms. These mechanisms also provide us with new therapeutic strategies. Whether we can use inhibitors of these molecules or block relevant signal pathways to control TNBC tumor cell proliferation and invasion speed, or even cause them to die due to a lack of relevant growth factors. A significant number of miRNAs that are abnormally expressed in TNBC are also listed in the TCGA and other tumor datasets in addition to the miRNAs mentioned above. These miRNAs can be employed as cancer-causing genes to take part in EMT, CSCs maintenance, epigenetic alterations, and other processes. Experiments are necessary to further our demonstration.
4 MiRNA inhibiting tumor formation and progression
Despite prior research on the role of miRNA in the initiation and progression of cancers, the role of miRNA in TNBC is not limited to this. It has been reported that some miRNAs can inhibit tumor formation, proliferation, invasion and migration, thereby preventing TNBC progression.
4.1 miRNA inhibiting tumor formation, proliferation and invasion
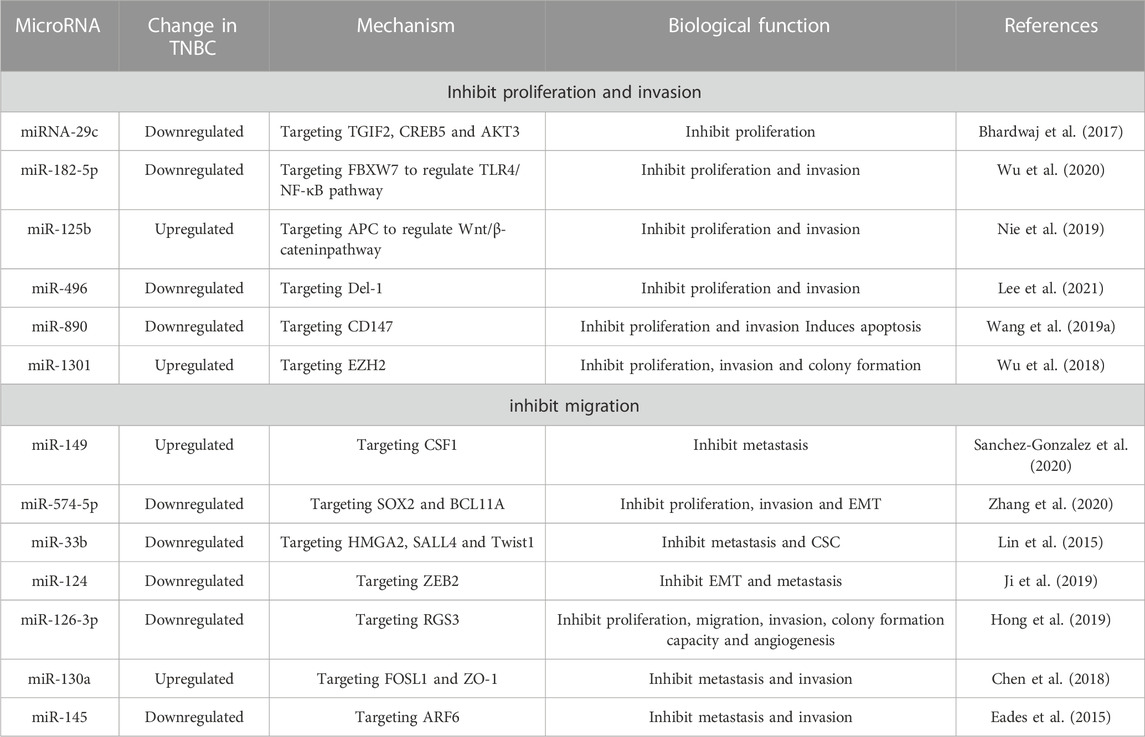
MiRNA-29c prevents preneoplastic TNBC cells from proliferating and populating the body by directly interacting with and regulating TGFB-induced factor homeobox 2 (TGIF2), CAMP-responsive element binding protein 5 (CREB5), and V-Akt murine thymoma viral oncogene homolog 3 (AKT3). As a result, miRNA-29c exerts significant influence during the early, preneoplastic phases of TNBC development (Bhardwaj et al., 2017). Wu et al. found that the downregulation of miR182-5p inhibits the production of inflammatory factors and the activation of inflammatory signals in TNBC cells by targeting FBXW7, thereby inhibiting the proliferation and invasion of TNBC and promoting apoptosis (Wu et al., 2020). Highly expressed in human TNBC tissues and cell lines, miR-125b inhibits proliferation and metastasis by binding 3′- UTR of APC, and preventing Wnt/β-catenin signaling pathway and EMT in cells (Nie et al., 2019). Lee et al. discovered that miR-496, which targets Del-1, prevents TNBC cancer cells from proliferating (Lee et al., 2021). MiR-890 negatively regulates the target gene CD147 in TNBC cells, which suppresses cell proliferation and invasion and induces apoptosis (Wang et al., 2019a). By interacting with the 3′-UTR of EZH2 mRNA, miR-1301 inhibits the proliferation, migration, and colony formation of TNBC cells after overexpression both in vivo and in vitro (Wu et al., 2018).
The above miRNAs inhibit the proliferation and invasion of TNBC by targeting specific genes. It is hoped that more mechanisms can be found in the future to inhibit the progress of TNBC.
4.2 miRNA inhibiting tumor metastasis
The capacity of primary breast cancers to metastasize is a essential aspect when grading and staging tumors and determining therapeutic approaches based on their clinical characteristics. Tumor suppressor miRNAs are vital for preventing the invasion, metastasis, and migration of tumor cells. Ismael et al. believed that overexpression of miR-149 in MDA-MB-231 cells inhibited THP-1 macrophage recruitment. By targeting CSF1, miR-149 inhibits CSF1 dependent communication between TNBC cells and THP-1 macrophages, thereby blocking the paracrine interactions between MDA-MB-231 cells and THP-1 cells and inhibiting breast cancer metastasis (Sanchez-Gonzalez et al., 2020). By MTT, colony formation, Transwell, xenotransplantation models in naked mice, Zhang et al. revealed that miR-574-5p levels were decreased in breast cancer tissues and cells, inhibited the proliferation, migration, and EMT of TNBC cells, and decreased the size and metastasis rate of tumors in vivo (Zhang et al., 2020). Additionally, some miRNAs target the corresponding targets to prevent local lymph node metastasis and/or distant metastasis of TNBC. Examples include miR-33b targeting HMGA2, SALL4, and Twist1 (Lin et al., 2015), miR-124 targeting ZEB2 (Ji et al., 2019), miR-126-3p targeting regulator of G protein signal 3 (RGS3) (Hong et al., 2019), miR-130a targeting FOSL1 and zona occludens 1 (zonula clusters 1, ZO-1 or called TJP1) (Chen et al., 2018), miR-145 targeting ARF6 (ADP-ribosylation factor 6) (Eades et al., 2015).
Some miRNAs can prevent TNBC from progressing by blocking the proliferation and peripheral infiltration of tumor cells as well as local and distant metastasis. The first discovered epigenetic regulated miRNA, miR-127, has been shown to target the gene BL6 (Saito et al., 2006) and suppress its expression in breast cancer (Chen et al., 2013; Wang et al., 2014). Garcia et al. confirmed that miR-127PD drastically decreased the activity of TNBC cells. And they observed a reduction in lung metastasis in mice treated with the miR-127PD system (Umeh-Garcia et al., 2020). miR-127PD also significantly reduced the spherulation capacity of four TNBC cells (MDA MB-231, MDA MB-157, MDA MB-468, HCC 1937), which was more effective than miR-34a (Raver-Shapira et al., 2007; Zhao et al., 2015; Adams et al., 2016; Zhao et al., 2016b). While miR-127PD is the precursor of miR-127-3p, which accumulates in cells after being processed and matured. Therefore, it makes sense to assume that miR-127-3p can prevent TNBC tumor cells from proliferating. They further used the tumorsphere assays, a widely accepted stem cell function test, and confirmed that miR-127 inhibited CSC, which has been of great significance in preventing the metastasis of TNBC and disease recurrence (Umeh-Garcia et al., 2020). (Table 3)
Metastasis is the most serious complication and leading cause of death in cancer patients. At present, breast cancer patients with distant metastasis essentially no longer have access to surgical treatment, and the median survival time is measured in months. TNBC has a greater capacity for invasion and metastasis than other BC types. If the key miRNAs that can promote the invasion and metastasis of TNBC are identified and blocked, the local metastasis of tumor cells, the ability of distant metastasis, and the mortality can be reduced, as well as the chance of radical surgery and the survival period can be increased. TNBC patients can benefit significantly from this.
5 Relationship between MicroRNA and chemotherapy resistance of tumor
Chemotherapy resistance is a major hindrance to neoadjuvant therapy. Unfortunately, treatment resistance is highly common, and this is one of the leading factors contributing to TNBC patients’ poor prognosis. MiRNA expression disorders, such as the upregulation of carcinogenic miRNA and the downregulation of tumor suppressor miRNA, are frequently seen in chemotherapy-resistant cancer cells. The uncontrolled expression of miRNA can be extrapolated into a direct connection to TNBC’s treatment resistance. It is now widely acknowledged that doxorubicin and platinum resistance are related to aberrant miRNA expression. Other chemoresistance research with miRNA are increasingly conducted as well.
5.1 miRNAs associated with doxorubicin and platinum chemoresistance
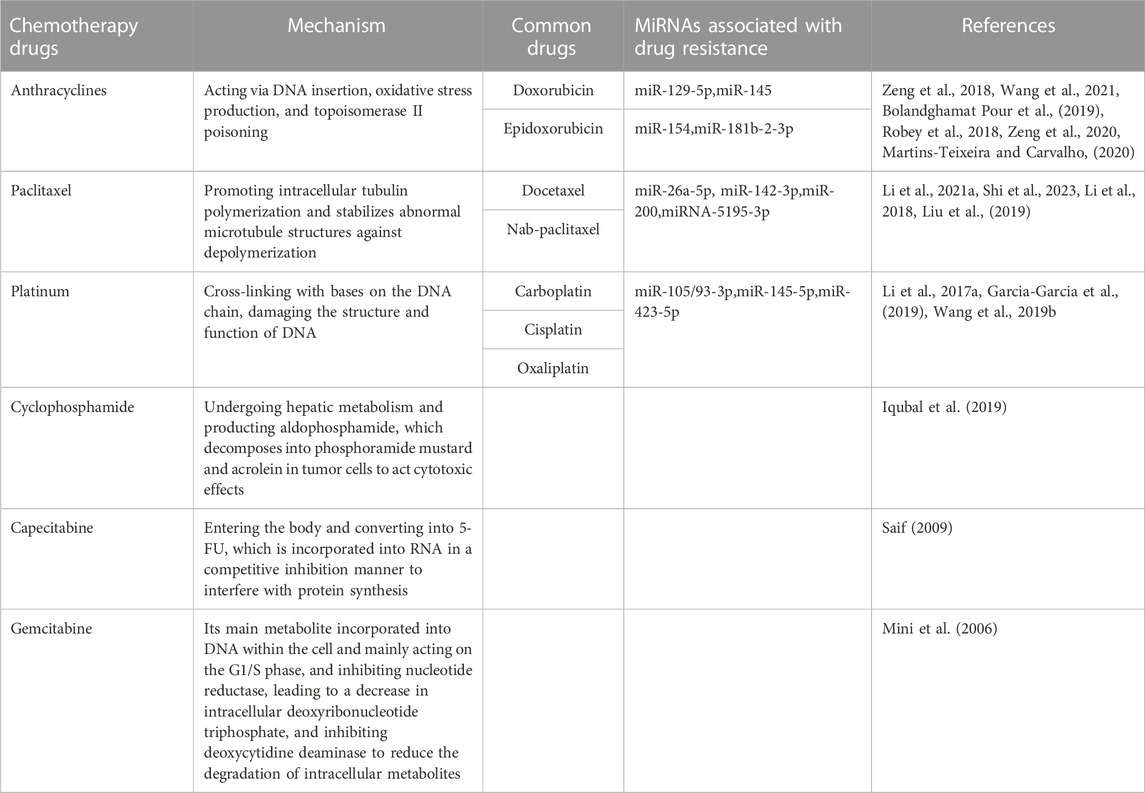
It is reported that the downregulation of miR-129-5p makes it resistant to doxorubicin (DOX or adriamycin) by promoting the apoptosis resistance induced by Sex-Determining Region Y-Box 2 (SOX2) (Zeng et al., 2018). By downregulating the multidrug resistance gene 1 (MDR1) and decreasing DOX efflux, increasing miR-145 expression can boost the sensitivity of MDA-MB-231 cells to DOX both in vitro and in vivo (Wang et al., 2021). While miR-154 promotes nicotinamide phosphoribosyltransferase (NAMPT), which further increases DOX chemical resistance, to prevent cell death (Bolandghamat Pour et al., 2019). Adenosine triphosphate binding cassette (ABC) transporter overexpression is the primary mechanism of acquired drug-resistance in multidrug-resistant cancer (Robey et al., 2018). While the increase of ABCC3(ATP binding cassette transporter family class C3) is related to the reduction of miR-181b-2-3p in MDA-MB-231/DOX (MDA-MB-231 cells resistant to DOX), decreasing the sensitivity of TNBC cells to DOX (Zeng et al., 2020). Furthermore, some miRNAs, such as miR-26a-5p, miR-142-3p, miR-200, and miRNA-5195-3p, are correlated with PTX resistance. Upregulation of miR-26a-5p promoted cellular cytotoxicity of PTX in vitro and in vivo (Li et al., 2021a). Higher miR-142-3p expression increased sensitivity to PTX treatment (Shi et al., 2023). MiRNA-200 inhibited PTX resistance (Li et al., 2018). PTX-resistant TNBC cells responded better to PTX therapy when miR-5195-3p was upregulated (Liu et al., 2019).
Platinum based first-line treatment are typically effective against breast cancer, and they can increase patients’ DFS, PFS, and ORR in both the early and late stages of TNBC. However, many patients with TNBC experience recurrence due to drug resistance, which lessens the therapeutic impact of cisplatin (DDP) on TNBC. MiR-105/93-3p is overexpressed in TBNC, which activate Wnt/β-catenin transmits signals by downregulation of SFRP1, thus endowing TNBC cells with cisplatin resistance (Li et al., 2017a). MiR-145-5p in MDA-MB-231 cells induces apoptosis and increases sensitivity to cisplatin therapy by downregulating transforming growth factor TGFβR2, albeit the precise regulatory mechanism is still unknown (Garcia-Garcia et al., 2019). And, the upregulation of miR-423-5p contributed to the drug resistance of MDA-MB-231 cells and had a substantial impact on the DDP resistance (Wang et al., 2019b).
Through pertinent pathways, these miRNAs contribute to doxorubicin and platinum resistance in TNBC. It is hoped that drug resistance can be overcome in the future to maximize the anti-tumor efficacy of doxorubicin and platinum.
5.2 MiRNAs that cause chemoresistance through other mechanisms
MiRNAs can directly target associated proteins or further regulate related signal pathways to regulate chemotherapy resistance. According to Cheng et al., chemotherapy-resistant cells have significantly higher levels of FSTL1, which was necessary for DDP and DOX chemoresistance in breast cancer cell lines. And there was a miR-137/FSTL1/integrinβ3/Wnt/β- Catenin signal axis maintaining stemness and enhancing chemoresistance in breast cancer cells (Cheng et al., 2019). Saatci et al. believed that inhibiting lysyl oxidase (LOX) can decrease collagen cross-linking and fibronectin assembly, boost drug absorption, and downregulate the expression of ITGA5/FN1, which inhibits FAK/Src signal transduction, induces apoptosis, and boosts chemotherapy sensitivity. Upregulation of miR-142-3p expression can target HIF-1α/The LOX/ITGA5/FN1 axis further inhibits the chemoresistance of TNBC (Saatci et al., 2020). As a tumor inhibitor, miR-17 inhibits the resistance of TNBC to DDP by promoting the expression of cell cycle inhibitor p27 and apoptosis. Therefore, miR-17, as a tumor chemotherapy sensitizer, may be an effective biological target to combat TNBC resistance (Wang et al., 2019c).
Additionally, a variety of mechanisms, including altered cell cycle and DNA damage regulation, decreased drug absorption, increased drug excretion, and others, might affect cancer drug resistance (Najminejad et al., 2019; Si et al., 2019). MiRNAs, as tissue specific regulators of the entire gene network related to drug resistance, have become research frontiers. Qattan et al. found that with had significantly aberrant expression of miR-19a/b-3p, miR-25-3p, miR-22-3p, miR-210-3p, miR-93-5p, and miR-199a-3p in TNBC patients is relavent to chemotherapy resistance. These miRNAs control the PAM (PI3K/Akt/mTOR), HIF-1, TNF, FoxO, Wnt and JAK/STAT, PD-1/PD-L1 pathway and EGFR tyrosine kinase inhibitor resistance (TKI) respectively (Qattan et al., 2021). In addition to miRNAs involved in chemotherapy resistance through the above mechanisms, some miRNAs can function as oncogenes. Their upregulation promotes chemotherapy resistance by inhibiting TNBC cell apoptosis, such as miR200a (Yu et al., 2018), miR-221/222 (Li et al., 2019), and miR1207-5p (Hou et al., 2019).
These indicators are especially well suited for addressing the treatment resistance issue of this clinically challenging subtype of breast cancer due to the mounting evidence of miRNA imbalance in TNBC. There is increasing number of studies on miRNA in chemotherapy resistance. We can consider using appropriate miRNA inhibitors or mimics to reverse the resistance to conventional chemotherapy drugs and enhance the effect of chemotherapy drugs. At present, this article focuses on the relationship between miRNA abnormal expression and DOX and platinum resistant TNBC. The connection between miRNA and chemoresistance to drugs like paclitaxel, cyclophosphamide, and capecitabine will then receive additional focus (Mini et al., 2006; Saif, 2009; Li et al., 2018; Zeng et al., 2018; Bolandghamat Pour et al., 2019; Iqubal et al., 2019; Liu et al., 2019; Martins-Teixeira and Carvalho, 2020; Zeng et al., 2020; Li et al., 2021a; Wang et al., 2021; Shi et al., 2023). In conclusion, many miRNAs’ drug resistance mechanisms have not yet been completely grasped, necessitating more research (Table 4).
6 miRNA and precision oncology
Precision oncology seeks to identify individual differences based on the personal genetic information of cancer patients, comprehend the phenotypes of disease, and direct personalized treatment (Yu and Snyder, 2016). Genomics provides valuable information on driving mutations and risk loci, while transcriptomics describes multiple expression patterns of mRNA and non-coding RNA (ncRNA), which can aid in deciphering genomic codes (Ma et al., 2018). The abnormal expression of miRNA in TNBC is also within the scope of precision oncology study because it is an indispensable component of ncRNA in the genome. By analyzing data from The Cancer Genome Atlas (TCGA), numerous scholars have currently examined the imbalance of miRNA in TNBC (Yang et al., 2019; Hong et al., 2020). Thanks to advancements in RNA sequencing, microarray technology, and high-throughput sequencing technology, more miRNA anomalies in TNBC have also been revealed.
In the previous section, we have described the abnormal expression of many miRNAs in TNBC. Upregulated oncogenic miRNAs may act as tumor promoters to increase the proliferation and/or invasion of TNBC cells, whereas downregulated tumor suppressor miRNAs may serve as tumor inhibitors to inhibit cancer cell growth, induce apoptosis, and enhance metastasis. Antisense oligonucleotides, miR mimics, and chemical modification of miRs are examples of current miRANA-based treatments (Nagini, 2017). Through the aforementioned techniques, it might be feasible to downregulate the expression of oncogenic miRNAs and upregulate the expression of tumor suppressor miRNAs, to inhibit the proliferation and invasion of TNBC. Many investigtions have demonstrated that miRNA can target related genes and obstruct their protein translation (Bai et al., 2018; Cantini et al., 2019; Lv et al., 2019; Son et al., 2019) (Wang et al., 2019a; Wu et al., 2020; Lee et al., 2021), which is connected to regulating tumor progression, chemotherapy resistance, and tumor immune surveillance. In the event that modifying the levels of miRNA prove to be challenging, we propose a hypothesis that corresponding antibodies can be created to attach to carcinogenic proteins and render them inactive, thereby slowing tumor progression. Furthermore, based on the abundance and pattern of miRNAs expression, BC subtypes could be reclassified, and the principal roles of related miRNAs were used to determine the precise biological functions of malignancies. Especially TNBC, the tumor is extremely heterogeneous, with ambiguous characteristics, and at this time there is no targeted medication. Therefore, precise target research orientig miRNA to provide individualized treatment for patients will be a blessing for all patients with TNBC.
7 Discussion
Breast cancer, the most frequent malignant tumor in women, has put women’s lives in danger worldwide. TNBC, lacking ER, PR and HER-2 expression, has a poor prognosis and is more prone to relapse due to the inability to use endocrine and monoclonal antibody targeted therapy. Epigenetic control, transcription factors, and/or mutant protein control alterations all contribute to altered miRNA expression patterns in breast cancer. Therefore, persistent aberrant miRNA expression may result in the development of tumors. This paper focuses on the role of particular miRNAs in the occurrence, development, and recurrence of TNBC. It also aims to identify some biomarkers that may reliably diagnose TNBC and assess the prognosis from a large pool of miRNAs in the future, so as to improve the prognosis of patients.
Despite numerous research on miRNA, there are currently a few precise, repeatable biological targets for the treatment of TNBC. Some studies have confirmed that miRNA is involved in regulating various metabolic pathways in cells. From the perspective of metabonomics, we can investigate whether miRNAs in TNBC modulate particular metabolic pathways of TNBC in the future, and then discover novel targeted biomarkers. The therapeutic scheme targeting miRNA may bring hope for the treatment of TNBC. A certain miRNA’s up- or downregulation, however, may affect the epigenetics of other tissues and cells, or potentially cause malignant changes, as many miRNAs do not exclusively target breast cells. As a result, there may be some application concerns with miRNA-targeted therapy for TNBC. It is worth considering whether changing the expression of miRNA will cause secondary tumors and damage other parts of the human body, and we must tread cautiously.
Along with miRNA, ohter RNAs, including LncRNA and CircRNA in ncRNA, have also been dicovered to be variably expressed in TNBC. And mounting evidence indicates that they may develop into potential biomarkers for diagnosis and prognosis, therapeutic targets, and improve the clinical outcomes for TNBC patients. For example, elevated expression of lncRNA H19 can be used to predict the efficacy of neoadjuvant chemotherapy (NAC) (Ozgur et al., 2020). NRAD1 is enriched in TNBC populations and is associated with a poor survival rate (Vidovic et al., 2020). And CircSEPT9 (Zheng et al., 2020), CircCD44 (Li et al., 2021b), etc., can be used as indicators of TNBC. It is interesting to note that lncRNA and circRNA can associate with miRNA, which affects how TNBC occurs and develops. The competitive endogenous RNA (ceRNA) theory (Salmena et al., 2011) postulates that lncRNA may function as a “sponge” for miRNA, competing with miRNA-targeted mRNA and influencing miRNA-mediated gene regulation (Poliseno et al., 2010; Cesana et al., 2011). CircRNAs can also regulate the proliferation, invasion and tumorigenesis of TNBC positively or negatively through the circRNA-miRNA-mRNA axis (Tian et al., 2021). This provides a theoretical foundation for our upcoming research on non-coding RNA medications used in combination to treat TNBC.
With a thorough study of TNBC, it is discovered that TNBC can be classified into various subtypes based on different traits and analytical techniques. In the future, we can further study the relationship between miRNAs and different TNBC subtypes, clarify that specific miRNAs play a role in promoting or inhibiting tumor progression in TNBC subtypes, and carry out precise individualized treatment to improve the efficacy. Although the impact of abnormally expressed miRNAs on the development of TNBC has been extensively investigated and the mechanism of some miRNAs has also been revealed. However, therapeutic application of exogenous miRNAs for the treatment of TNBC is rare due to their instability and low specificity in vivo. Consequently, it is vital to find solutions to the challenges of miRNA medication stability optimization and miRNA delivery improvement. The addition of miRNA degradation inhibitors can reduce miRNA decomposition and improve the drug stability of miRNA, thereby improving the efficacy. Secondly, medication delivery effects can be maximized and gene targets can be more precisely combined with the aid of nanotechnology or the employment of more than two carriers in a synergistic manner. This review’s key goals are to advance effective, less invasive treatment options, raise awareness of the role of miRNA in the emergence of TNBC, and enhance the prognosis for TNBC as much as feasible.
Author contributions
FYQ and ZXP contributed to the review topic. FYQ, YQH, YHJ, and ZXP collected the documents. FYQ wrote the first draft of the manuscript. YQH and YHJ wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version. YHJ and ZXP proposed the overall concept of this paper and revised the manuscript.
Funding
This work was supported by grants from Natural Science Foundation of Zhejiang Province: Study on the Mechanism of MALAT1 Regulating Lymph Node Metastasis of Three Negative Breast Cancer through CeRNA Mechanism and Its Correlation with Prognosis (No. LBY21H160001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2023.1162463/full#supplementary-material
References
Adams, B. D., Wali, V. B., Cheng, C. J., Inukai, S., Booth, C. J., Agarwal, S., et al. (2016). miR-34a silences c-SRC to attenuate tumor growth in triple-negative breast cancer. Cancer Res. 76 (4), 927–939. doi:10.1158/0008-5472.CAN-15-2321
Amorim, M., Salta, S., Henrique, R., and Jeronimo, C. (2016). Decoding the usefulness of non-coding RNAs as breast cancer markers. J. Transl. Med. 14, 265. doi:10.1186/s12967-016-1025-3
Anfossi, S., Giordano, A., Gao, H., Cohen, E. N., Tin, S., Wu, Q., et al. (2014). High serum miR-19a levels are associated with inflammatory breast cancer and are predictive of favorable clinical outcome in patients with metastatic HER2+ inflammatory breast cancer. PLoS One 9 (1), e83113. doi:10.1371/journal.pone.0083113
Araujo, J. M., De la Cruz-Ku, G., Cornejo, M., Doimi, F., Dyer, R., Gomez, H. L., et al. (2022). Prognostic capability of TNBC 3-gene score among triple-negative breast cancer subtypes. Cancers (Basel) 14 (17), 4286. doi:10.3390/cancers14174286
Bai, X., Han, G., Liu, Y., Jiang, H., and He, Q. (2018). MiRNA-20a-5p promotes the growth of triple-negative breast cancer cells through targeting RUNX3. Biomed. Pharmacother. 103, 1482–1489. doi:10.1016/j.biopha.2018.04.165
Bhardwaj, A., Singh, H., Rajapakshe, K., Tachibana, K., Ganesan, N., Pan, Y., et al. (2017). Regulation of miRNA-29c and its downstream pathways in preneoplastic progression of triple-negative breast cancer. Oncotarget 8 (12), 19645–19660. doi:10.18632/oncotarget.14902
Bolandghamat Pour, Z., Nourbakhsh, M., Mousavizadeh, K., Madjd, Z., Ghorbanhosseini, S. S., Abdolvahabi, Z., et al. (2019). Suppression of nicotinamide phosphoribosyltransferase expression by miR-154 reduces the viability of breast cancer cells and increases their susceptibility to doxorubicin. BMC Cancer 19 (1), 1027. doi:10.1186/s12885-019-6221-0
Braicu, C., Raduly, L., Morar-Bolba, G., Cojocneanu, R., Jurj, A., Pop, L. A., et al. (2018). Aberrant miRNAs expressed in HER-2 negative breast cancers patient. J. Exp. Clin. Cancer Res. 37 (1), 257. doi:10.1186/s13046-018-0920-2
Cantini, L., Bertoli, G., Cava, C., Dubois, T., Zinovyev, A., Caselle, M., et al. (2019). Identification of microRNA clusters cooperatively acting on epithelial to mesenchymal transition in triple negative breast cancer. Nucleic Acids Res. 47 (5), 2205–2215. doi:10.1093/nar/gkz016
Cesana, M., Cacchiarelli, D., Legnini, I., Santini, T., Sthandier, O., Chinappi, M., et al. (2011). A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 147 (2), 358–369. doi:10.1016/j.cell.2011.09.028
Chen, J., Wang, M., Guo, M., Xie, Y., and Cong, Y. S. (2013). miR-127 regulates cell proliferation and senescence by targeting BCL6. PLoS One 8 (11), e80266. doi:10.1371/journal.pone.0080266
Chen, X., Zhao, M., Huang, J., Li, Y., Wang, S., Harrington, C. A., et al. (2018). microRNA-130a suppresses breast cancer cell migration and invasion by targeting FOSL1 and upregulating ZO-1. J. Cell Biochem. 119 (6), 4945–4956. doi:10.1002/jcb.26739
Cheng, S., Huang, Y., Lou, C., He, Y., Zhang, Y., and Zhang, Q. (2019). FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin β3/Wnt signaling under miR-137 regulation. Cancer Biol. Ther. 20 (3), 328–337. doi:10.1080/15384047.2018.1529101
Darbeheshti, F., Kadkhoda, S., Keshavarz-Fathi, M., Razi, S., Bahramy, A., Mansoori, Y., et al. (2022). Investigation of BRCAness associated miRNA-gene axes in breast cancer: Cell-free miR-182-5p as a potential expression signature of BRCAness. BMC Cancer 22 (1), 668. doi:10.1186/s12885-022-09761-4
Du, Y., Wei, N., Ma, R., Jiang, S., and Song, D. (2020). A miR-210-3p regulon that controls the Warburg effect by modulating HIF-1α and p53 activity in triple-negative breast cancer. Cell Death Dis. 11 (9), 731. doi:10.1038/s41419-020-02952-6
Eades, G., Wolfson, B., Zhang, Y., Li, Q., Yao, Y., and Zhou, Q. (2015). lincRNA-RoR and miR-145 regulate invasion in triple-negative breast cancer via targeting ARF6. Mol. Cancer Res. 13 (2), 330–338. doi:10.1158/1541-7786.MCR-14-0251
Garcia-Garcia, F., Salinas-Vera, Y. M., Garcia-Vazquez, R., Marchat, L. A., Rodríguez-Cuevas, S., López-González, J. S., et al. (2019). miR‑145‑5p is associated with pathological complete response to neoadjuvant chemotherapy and impairs cell proliferation by targeting TGFβR2 in breast cancer. Oncol. Rep. 41 (6), 3527–3534. doi:10.3892/or.2019.7102
Hamam, R., Hamam, D., Alsaleh, K. A., Kassem, M., Zaher, W., Alfayez, M., et al. (2017). Circulating microRNAs in breast cancer: Novel diagnostic and prognostic biomarkers. Cell Death Dis. 8 (9), e3045. doi:10.1038/cddis.2017.440
Hong, H. C., Chuang, C. H., Huang, W. C., Weng, S. L., Chen, C. H., Chang, K. H., et al. (2020). A panel of eight microRNAs is a good predictive parameter for triple-negative breast cancer relapse. Theranostics 10 (19), 8771–8789. doi:10.7150/thno.46142
Hong, Z., Hong, C., Ma, B., Wang, Q., Zhang, X., Li, L., et al. (2019). MicroRNA-126-3p inhibits the proliferation, migration, invasion, and angiogenesis of triple-negative breast cancer cells by targeting RGS3. Oncol. Rep. 42 (4), 1569–1579. doi:10.3892/or.2019.7251
Hou, X., Niu, Z., Liu, L., Guo, Q., Li, H., Yang, X., et al. (2019). miR-1207-5p regulates the sensitivity of triple-negative breast cancer cells to Taxol treatment via the suppression of LZTS1 expression. Oncol. Lett. 17 (1), 990–998. doi:10.3892/ol.2018.9687
Iqubal, A., Iqubal, M. K., Sharma, S., Ansari, M. A., Najmi, A. K., Ali, S. M., et al. (2019). Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: Old drug with a new vision. Life Sci. 218, 112–131. doi:10.1016/j.lfs.2018.12.018
Ji, H., Sang, M., Liu, F., Ai, N., and Geng, C. (2019). miR-124 regulates EMT based on ZEB2 target to inhibit invasion and metastasis in triple-negative breast cancer. Pathol. Res. Pract. 215 (4), 697–704. doi:10.1016/j.prp.2018.12.039
Kahraman, M., Roske, A., Laufer, T., Fehlmann, T., Backes, C., Kern, F., et al. (2018). MicroRNA in diagnosis and therapy monitoring of early-stage triple-negative breast cancer. Sci. Rep. 8 (1), 11584. doi:10.1038/s41598-018-29917-2
Lee, S. J., Jeong, J. H., Lee, J., Park, H. Y., Jung, J. H., Kang, J., et al. (2021). MicroRNA-496 inhibits triple negative breast cancer cell proliferation by targeting Del-1. Med. Baltim. 100 (14), e25270. doi:10.1097/MD.0000000000025270
Li, C. Y., Miao, K. L., Chen, Y., Liu, L. Y., Zhao, G. B., Lin, M. H., et al. (2018). Jagged2 promotes cancer stem cell properties of triple negative breast cancer cells and paclitaxel resistance via regulating microRNA-200. Eur. Rev. Med. Pharmacol. Sci. 22 (18), 6008–6014. doi:10.26355/eurrev_201809_15936
Li, H. Y., Liang, J. L., Kuo, Y. L., Lee, H. H., Calkins, M. J., Chang, H. T., et al. (2017). miR-105/93-3p promotes chemoresistance and circulating miR-105/93-3p acts as a diagnostic biomarker for triple negative breast cancer. Breast Cancer Res. 19 (1), 133. doi:10.1186/s13058-017-0918-2
Li, J., Gao, X., Zhang, Z., Lai, Y., Lin, X., Lin, B., et al. (2021). CircCD44 plays oncogenic roles in triple-negative breast cancer by modulating the miR-502-5p/KRAS and IGF2BP2/Myc axes. Mol. Cancer 20 (1), 138. doi:10.1186/s12943-021-01444-1
Li, P. P., Li, R. G., Huang, Y. Q., Lu, J. P., Zhang, W. J., and Wang, Z. Y. (2021). LncRNA OTUD6B-AS1 promotes paclitaxel resistance in triple negative breast cancer by regulation of miR-26a-5p/MTDH pathway-mediated autophagy and genomic instability. Aging (Albany NY) 13 (21), 24171–24191. doi:10.18632/aging.203672
Li, S., Li, Q., Lu, J., Zhao, Q., Li, D., Shen, L., et al. (2019). Targeted inhibition of miR-221/222 promotes cell sensitivity to cisplatin in triple-negative breast cancer MDA-MB-231 cells. Front. Genet. 10, 1278. doi:10.3389/fgene.2019.01278
Li, Z., Meng, Q., Pan, A., Wu, X., Cui, J., Wang, Y., et al. (2017). MicroRNA-455-3p promotes invasion and migration in triple negative breast cancer by targeting tumor suppressor EI24. Oncotarget 8 (12), 19455–19466. doi:10.18632/oncotarget.14307
Lin, Y., Liu, A. Y., Fan, C., Zheng, H., Zhang, C., et al. (2015). MicroRNA-33b inhibits breast cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci. Rep. 5, 9995. doi:10.1038/srep09995
Liu, B., Zhang, X., Song, F., Zheng, H., Zhao, Y., Li, H., et al. (2016). MiR-502/SET8 regulatory circuit in pathobiology of breast cancer. Cancer Lett. 376 (2), 259–267. doi:10.1016/j.canlet.2016.04.008
Liu, H., and Wang, G. (2021). MicroRNA-301a-3p promotes triple-negative breast cancer progression through downregulating MEOX2. Exp. Ther. Med. 22 (3), 945. doi:10.3892/etm.2021.10377
Liu, M., Gong, C., Xu, R., Chen, Y., and Wang, X. (2019). MicroRNA-5195-3p enhances the chemosensitivity of triple-negative breast cancer to paclitaxel by downregulating EIF4A2. Cell Mol. Biol. Lett. 24, 47. doi:10.1186/s11658-019-0168-7
Lowery, A. J., Miller, N., Devaney, A., McNeill, R. E., Davoren, P. A., Lemetre, C., et al. (2009). MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 11 (3), R27. doi:10.1186/bcr2257
Lv, Z. D., Xin, H. N., Yang, Z. C., Wang, W. J., Dong, J. J., Jin, L. Y., et al. (2019). miR-135b promotes proliferation and metastasis by targeting APC in triple-negative breast cancer. J. Cell Physiol. 234 (7), 10819–10826. doi:10.1002/jcp.27906
Ma, L., Liang, Z., Zhou, H., and Qu, L. (2018). Applications of RNA indexes for precision oncology in breast cancer. Genomics Proteomics Bioinforma. 16 (2), 108–119. doi:10.1016/j.gpb.2018.03.002
Malla, R. R., Kumari, S., Gavara, M. M., Badana, A. K., Gugalavath, S., Kumar, D. K. G., et al. (2019). A perspective on the diagnostics, prognostics, and therapeutics of microRNAs of triple-negative breast cancer. Biophys. Rev. 11 (2), 227–234. doi:10.1007/s12551-019-00503-8
Martins-Teixeira, M. B., and Carvalho, I. (2020). Antitumour anthracyclines: Progress and perspectives. ChemMedChem 15 (11), 933–948. doi:10.1002/cmdc.202000131
McDermott, A. M., Miller, N., Wall, D., Martyn, L. M., Ball, G., Sweeney, K. J., et al. (2014). Identification and validation of oncologic miRNA biomarkers for luminal A-like breast cancer. PLoS One 9 (1), e87032. doi:10.1371/journal.pone.0087032
Mini, E., Nobili, S., Caciagli, B., Landini, I., and Mazzei, T. (2006). Cellular pharmacology of gemcitabine. Ann. Oncol. 17, v7–v12. doi:10.1093/annonc/mdj941
Miyoshi, J., Toden, S., Yoshida, K., Toiyama, Y., Alberts, S. R., Kusunoki, M., et al. (2017). MiR-139-5p as a novel serum biomarker for recurrence and metastasis in colorectal cancer. Sci. Rep. 7, 43393. doi:10.1038/srep43393
Nagini, S. (2017). Breast cancer: Current molecular therapeutic targets and new players. Anticancer Agents Med. Chem. 17 (2), 152–163. doi:10.2174/1871520616666160502122724
Najminejad, H., Kalantar, S. M., Abdollahpour-Alitappeh, M., Karimi, M. H., Seifalian, A. M., Gholipourmalekabadi, M., et al. (2019). Emerging roles of exosomal miRNAs in breast cancer drug resistance. IUBMB Life 71 (11), 1672–1684. doi:10.1002/iub.2116
Naorem, L. D., Muthaiyan, M., and Venkatesan, A. (2019). Identification of dysregulated miRNAs in triple negative breast cancer: A meta-analysis approach. J. Cell Physiol. 234 (7), 11768–11779. doi:10.1002/jcp.27839
Nie, J., Jiang, H. C., Zhou, Y. C., Jiang, B., Wang, Y. F., et al. (2019). MiR-125b regulates the proliferation and metastasis of triple negative breast cancer cells via the Wnt/β-catenin pathway and EMT. Biosci. Biotechnol. Biochem. 83 (6), 1062–1071. doi:10.1080/09168451.2019.1584521
Niedzwiecki, S., Piekarski, J., Szymanska, B., Pawlowska, Z., and Jeziorski, A. (2018). Serum levels of circulating miRNA-21, miRNA-10b and miRNA-200c in triple-negative breast cancer patients. Ginekol. Pol. 89 (8), 415–420. doi:10.5603/GP.a2018.0071
Ozgur, E., Ferhatoglu, F., Sen, F., Saip, P., and Gezer, U. (2020). Circulating lncRNA H19 may be a useful marker of response to neoadjuvant chemotherapy in breast cancer. Cancer Biomark. 27 (1), 11–17. doi:10.3233/CBM-190085
Pasculli, B., Barbano, R., Fontana, A., Biagini, T., Di Viesti, M. P., Rendina, M., et al. (2020). Hsa-miR-155-5p up-regulation in breast cancer and its relevance for treatment with poly[ADP-ribose] polymerase 1 (PARP-1) inhibitors. Front. Oncol. 10, 1415. doi:10.3389/fonc.2020.01415
Petrovic, N., Todorovic, L., Nedeljkovic, M., Božović, A., Bukumirić, Z., Tanić, N. D., et al. (2022). Dual function miR-205 is positively associated with ER and negatively with five-year survival in breast cancer patients. Pathol. Res. Pract. 238, 154080. doi:10.1016/j.prp.2022.154080
Poliseno, L., Salmena, L., Zhang, J., Carver, B., Haveman, W. J., and Pandolfi, P. P. (2010). A coding-independent function of gene and pseudogene mRNAs regulates tumour biology. Nature 465 (7301), 1033–1038. doi:10.1038/nature09144
Qattan, A., Al-Tweigeri, T., Alkhayal, W., Suleman, K., Tulbah, A., and Amer, S. (2021). Clinical identification of dysregulated circulating microRNAs and their implication in drug response in triple negative breast cancer (TNBC) by target gene network and meta-analysis. Genes (Basel) 12 (4), 549. doi:10.3390/genes12040549
Qiu, P., Guo, Q., Yao, Q., Chen, J., and Lin, J. (2021). Hsa-mir-3163 and CCNB1 may be potential biomarkers and therapeutic targets for androgen receptor positive triple-negative breast cancer. PLoS One 16 (11), e0254283. doi:10.1371/journal.pone.0254283
Qu, K., Zhang, X., Lin, T., Liu, T., Wang, Z., Liu, S., et al. (2017). Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: Evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci. Rep. 7 (1), 1692. doi:10.1038/s41598-017-01904-z
Raver-Shapira, N., Marciano, E., Meiri, E., Spector, Y., Rosenfeld, N., Moskovits, N., et al. (2007). Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 26 (5), 731–743. doi:10.1016/j.molcel.2007.05.017
Robey, R. W., Pluchino, K. M., Hall, M. D., Fojo, A. T., Bates, S. E., and Gottesman, M. M. (2018). Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 18 (7), 452–464. doi:10.1038/s41568-018-0005-8
Rupaimoole, R., and Slack, F. J. (2017). MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16 (3), 203–222. doi:10.1038/nrd.2016.246
Saatci, O., Kaymak, A., Raza, U., Ersan, P. G., Akbulut, O., Banister, C. E., et al. (2020). Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 11 (1), 2416. doi:10.1038/s41467-020-16199-4
Saif, M. W. (2009). Targeting cancers in the gastrointestinal tract: Role of capecitabine. Onco Targets Ther. 2, 29–41. doi:10.2147/ott.s3469
Saito, Y., Liang, G., Egger, G., Friedman, J. M., Chuang, J. C., Coetzee, G. A., et al. (2006). Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 9 (6), 435–443. doi:10.1016/j.ccr.2006.04.020
Salmena, L., Poliseno, L., Tay, Y., Kats, L., and Pandolfi, P. P. (2011). A ceRNA hypothesis: The rosetta stone of a hidden RNA language? Cell 146 (3), 353–358. doi:10.1016/j.cell.2011.07.014
Sanchez-Gonzalez, I., Bobien, A., Molnar, C., Schmid, S., Strotbek, M., Boerries, M., et al. (2020). miR-149 suppresses breast cancer metastasis by blocking paracrine interactions with macrophages. Cancer Res. 80 (6), 1330–1341. doi:10.1158/0008-5472.CAN-19-1934
Scognamiglio, I., Cocca, L., Puoti, I., Palma, F., Ingenito, F., Quintavalle, C., et al. (2022). Exosomal microRNAs synergistically trigger stromal fibroblasts in breast cancer. Mol. Ther. Nucleic Acids 28, 17–31. doi:10.1016/j.omtn.2022.02.013
Shen, X., Xie, B., Ma, Z., Yu, W., Wang, W., Xu, D., et al. (2015). Identification of novel long non-coding RNAs in triple-negative breast cancer. Oncotarget 6 (25), 21730–21739. doi:10.18632/oncotarget.4419
Shi, Y., Wang, J., Tao, S., Zhang, S., Mao, L., Shi, X., et al. (2023). miR-142-3p improves paclitaxel sensitivity in resistant breast cancer by inhibiting autophagy through the GNB2-AKT-mTOR Pathway. Cell Signal 103, 110566. doi:10.1016/j.cellsig.2022.110566
Si, W., Shen, J., Zheng, H., and Fan, W. (2019). The role and mechanisms of action of microRNAs in cancer drug resistance. Clin. Epigenetics 11 (1), 25. doi:10.1186/s13148-018-0587-8
Son, D., Kim, Y., Lim, S., Kang, H. G., Park, J. W., et al. (2019). miR-374a-5p promotes tumor progression by targeting ARRB1 in triple negative breast cancer. Cancer Lett. 454, 224–233. doi:10.1016/j.canlet.2019.04.006
Song, H., Li, D., Wu, T., Xie, D., Hua, K., Hu, J., et al. (2018). MicroRNA-301b promotes cell proliferation and apoptosis resistance in triple-negative breast cancer by targeting CYLD. BMB Rep. 51 (11), 602–607. doi:10.5483/BMBRep.2018.51.11.168
Thomas, A., Barriere, S., Broseus, L., Brooke, J., Lorenzi, C., Villemin, J. P., et al. (2019). GECKO is a genetic algorithm to classify and explore high throughput sequencing data. Commun. Biol. 2, 222. doi:10.1038/s42003-019-0456-9
Tian, T., Zhao, Y., Zheng, J., Jin, S., Liu, Z., and Wang, T. (2021). Circular RNA: A potential diagnostic, prognostic, and therapeutic biomarker for human triple-negative breast cancer. Mol. Ther. Nucleic Acids 26, 63–80. doi:10.1016/j.omtn.2021.06.017
Tormo, E., Ballester, S., Adam-Artigues, A., Burgués, O., Alonso, E., Bermejo, B., et al. (2019). The miRNA-449 family mediates doxorubicin resistance in triple-negative breast cancer by regulating cell cycle factors. Sci. Rep. 9 (1), 5316. doi:10.1038/s41598-019-41472-y
Tsiakou, A., Zagouri, F., Zografos, E., Samelis, G., Gazouli, M., Kalapanida, D., et al. (2019). Prognostic significance of miR-34 rs4938723 T > C polymorphism in triple negative breast cancer patients. Clin. Biochem. 68, 9–14. doi:10.1016/j.clinbiochem.2019.03.009
Umeh-Garcia, M., Simion, C., Ho, P. Y., Batra, N., Berg, A. L., Carraway, K. L., et al. (2020). A novel bioengineered miR-127 prodrug suppresses the growth and metastatic potential of triple-negative breast cancer cells. Cancer Res. 80 (3), 418–429. doi:10.1158/0008-5472.CAN-19-0656
Urabe, F., Matsuzaki, J., Yamamoto, Y., Kimura, T., Hara, T., Ichikawa, M., et al. (2019). Large-scale circulating microRNA profiling for the liquid biopsy of prostate cancer. Clin. Cancer Res. 25 (10), 3016–3025. doi:10.1158/1078-0432.CCR-18-2849
Vagia, E., Mahalingam, D., and Cristofanilli, M. (2020). The landscape of targeted therapies in TNBC. Cancers (Basel) 12, 916. doi:10.3390/cancers12040916
Vargas, A. J., and Harris, C. C. (2016). Biomarker development in the precision medicine era: Lung cancer as a case study. Nat. Rev. Cancer 16 (8), 525–537. doi:10.1038/nrc.2016.56
Vidovic, D., Huynh, T. T., Konda, P., Dean, C., Cruickshank, B. M., Sultan, M., et al. (2020). ALDH1A3-regulated long non-coding RNA NRAD1 is a potential novel target for triple-negative breast tumors and cancer stem cells. Cell Death Differ. 27 (1), 363–378. doi:10.1038/s41418-019-0362-1
Volovat, S. R., Volovat, C., Hordila, I., Hordila, D. A., Mirestean, C. C., Miron, O. T., et al. (2020). MiRNA and LncRNA as potential biomarkers in triple-negative breast cancer: A review. Front. Oncol. 10, 526850. doi:10.3389/fonc.2020.526850
Wang, B., Zhang, Y., Ye, M., Wu, J., and Chen, H. (2019). Cisplatin-resistant MDA-MB-231 cell-derived exosomes increase the resistance of recipient cells in an exosomal miR-423-5p-dependent manner. Curr. Drug Metab. 20 (10), 804–814. doi:10.2174/1389200220666190819151946
Wang, C., Xu, C., Niu, R., Hu, G., Gu, Z., and Zhuang, Z. (2019). MiR-890 inhibits proliferation and invasion and induces apoptosis in triple-negative breast cancer cells by targeting CD147. BMC Cancer 19 (1), 577. doi:10.1186/s12885-019-5796-9
Wang, J., Li, M., Han, X., Wang, H., Wang, X., Ma, G., et al. (2020). MiR-1976 knockdown promotes epithelial-mesenchymal transition and cancer stem cell properties inducing triple-negative breast cancer metastasis. Cell Death Dis. 11 (7), 500. doi:10.1038/s41419-020-2711-x
Wang, S., Li, H., Wang, J., Wang, D., Yao, A., and Li, Q. (2014). Prognostic and biological significance of microRNA-127 expression in human breast cancer. Dis. Markers 2014, 401986. doi:10.1155/2014/401986
Wang, S., Oh, D. Y., Leventaki, V., Drakos, E., Zhang, R., Sahin, A. A., et al. (2019). MicroRNA-17 acts as a tumor chemosensitizer by targeting JAB1/CSN5 in triple-negative breast cancer. Cancer Lett. 465, 12–23. doi:10.1016/j.canlet.2019.08.016
Wang, Y., Wang, Y., Qin, Z., Cai, S., Yu, L., Hu, H., et al. (2021). The role of non-coding RNAs in ABC transporters regulation and their clinical implications of multidrug resistance in cancer. Expert Opin. Drug Metab. Toxicol. 17 (3), 291–306. doi:10.1080/17425255.2021.1887139
Weng, Y. S., Tseng, H. Y., Chen, Y. A., Shen, P. C., Al Haq, A. T., Chen, L. M., et al. (2019). MCT-1/miR-34a/IL-6/IL-6R signaling axis promotes EMT progression, cancer stemness and M2 macrophage polarization in triple-negative breast cancer. Mol. Cancer 18 (1), 42. doi:10.1186/s12943-019-0988-0
Wu, Q., Chen, Z., Zhang, G., Zhou, W., Peng, Y., Liu, R., et al. (2018). EZH2 induces the expression of miR-1301 as a negative feedback control mechanism in triple negative breast cancer. Acta Biochim. Biophys. Sin. (Shanghai) 50 (7), 693–700. doi:10.1093/abbs/gmy050
Wu, X., Chen, H., Wu, M., Peng, S., and Zhang, L. (2020). Downregulation of miR-182-5p inhibits the proliferation and invasion of triple-negative breast cancer cells through regulating TLR4/NF-κB pathway activity by targeting FBXW7. Ann. Transl. Med. 8 (16), 995. doi:10.21037/atm-20-5192
Xu, J., Wu, K. J., Jia, Q. J., and Ding, X. F. (2020). Roles of miRNA and lncRNA in triple-negative breast cancer. J. Zhejiang Univ. Sci. B 21 (9), 673–689. doi:10.1631/jzus.B1900709
Yang, R., Xing, L., Zheng, X., Sun, Y., Wang, X., and Chen, J. (2019). The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol. Cancer 18 (1), 4. doi:10.1186/s12943-018-0933-7
Yao, L., Liu, Y., Cao, Z., Li, J., Huang, Y., Hu, X., et al. (2018). MicroRNA-493 is a prognostic factor in triple-negative breast cancer. Cancer Sci. 109 (7), 2294–2301. doi:10.1111/cas.13644
Yu, K. H., and Snyder, M. (2016). Omics profiling in precision oncology. Mol. Cell Proteomics 15 (8), 2525–2536. doi:10.1074/mcp.O116.059253
Yu, S. J., Yang, L., Hong, Q., Kuang, X. Y., Di, G. H., and Shao, Z. M. (2018). MicroRNA-200a confers chemoresistance by antagonizing TP53INP1 and YAP1 in human breast cancer. BMC Cancer 18 (1), 74. doi:10.1186/s12885-017-3930-0
Zeng, C., Fan, D., Xu, Y., Li, X., Yuan, J., Yang, Q., et al. (2020). Curcumol enhances the sensitivity of doxorubicin in triple-negative breast cancer via regulating the miR-181b-2-3p-ABCC3 axis. Biochem. Pharmacol. 174, 113795. doi:10.1016/j.bcp.2020.113795
Zeng, H., Wang, L., Wang, J., Chen, T., Li, H., Zhang, K., et al. (2018). microRNA-129-5p suppresses Adriamycin resistance in breast cancer by targeting SOX2. Arch. Biochem. Biophys. 651, 52–60. doi:10.1016/j.abb.2018.05.018
Zhang, K. J., Hu, Y., Luo, N., Li, X., Chen, F. Y., Yuan, J. Q., et al. (2020). miR-574-5p attenuates proliferation, migration and EMT in triple-negative breast cancer cells by targeting BCL11A and SOX2 to inhibit the SKIL/TAZ/CTGF axis. Int. J. Oncol. 56 (5), 1240–1251. doi:10.3892/ijo.2020.4995
Zhao, L., Feng, X., Song, X., Zhou, H., Zhao, Y., Cheng, L., et al. (2016). miR-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting FUT4. Oncol. Rep. 36 (2), 1007–1015. doi:10.3892/or.2016.4882
Zhao, Y., Tu, M. J., Wang, W. P., Qiu, J. X., Yu, A. X., and Yu, A. M. (2016). Genetically engineered pre-microRNA-34a prodrug suppresses orthotopic osteosarcoma xenograft tumor growth via the induction of apoptosis and cell cycle arrest. Sci. Rep. 6, 26611. doi:10.1038/srep26611
Zhao, Y., Tu, M. J., Yu, Y. F., Wang, W. P., Chen, Q. X., Qiu, J. X., et al. (2015). Combination therapy with bioengineered miR-34a prodrug and doxorubicin synergistically suppresses osteosarcoma growth. Biochem. Pharmacol. 98 (4), 602–613. doi:10.1016/j.bcp.2015.10.015
Keywords: triple negative breast cancer, miRNA, diagnosis, prognosis, drug resistance
Citation: Fu Y, Yang Q, Yang H and Zhang X (2023) New progress in the role of microRNAs in the diagnosis and prognosis of triple negative breast cancer. Front. Mol. Biosci. 10:1162463. doi: 10.3389/fmolb.2023.1162463
Received: 09 February 2023; Accepted: 30 March 2023;
Published: 13 April 2023.
Edited by:
Kamla Kant Shukla, All India Institute of Medical Sciences Jodhpur, IndiaReviewed by:
Prasant Yadav, The Ohio State University, United StatesSukhes Mukherjee, All India Institute of Medical Sciences, India
Copyright © 2023 Fu, Yang, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongjian Yang, eWhqemx5eUAxNjMuY29t; Xiping Zhang, enhwOTk2ODhAc2luYS5jb20=
 Yeqin Fu
Yeqin Fu Qiuhui Yang
Qiuhui Yang Hongjian Yang1*
Hongjian Yang1* Xiping Zhang
Xiping Zhang