- 1Department of Antiviral Research, Institute of Advanced Virology, Thiruvananthapuram, Kerala, India
- 2Department of Microbiology and Immunology, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, IL, United States
- 3Clover Biopharmaceuticals, Boston, MA, United States
The emergence and re-emergence of viral diseases, which cause significant global mortality and morbidity, are the major concerns of this decade. Of these, current research is focused majorly on the etiological agent of the COVID-19 pandemic, SARS-CoV-2. Understanding the host response and metabolic changes during viral infection may provide better therapeutic targets for the proper management of pathophysiological conditions associated with SARS-CoV-2 infection. We have achieved control over most emerging viral diseases; however, a lack of understanding of the underlying molecular events prevents us from exploring novel therapeutic targets, leaving us forced to witness re-emerging viral infections. SARS-CoV-2 infection is usually accompanied by oxidative stress, which leads to an overactive immune response, the release of inflammatory cytokines, increasing lipid production, and also alterations in the endothelial and mitochondrial functions. PI3K/Akt signaling pathway confers protection against oxidative injury by various cell survival mechanisms including Nrf2-ARE mediated antioxidant transcriptional response. SARS-CoV-2 is also reported to hijack this pathway for its survival within host and few studies have suggested the role of antioxidants in modulating the Nrf2 pathway to manage disease severity. This review highlights the interrelated pathophysiological conditions associated with SARS-CoV-2 infection and the host survival mechanisms mediated by PI3K/Akt/Nrf2 signaling pathways that can help ameliorate the severity of the disease and provide effective antiviral targets against SARS-CoV-2.
Introduction
Coronaviruses (CoVs) are enveloped, non-segmented positive sense, single stranded RNA viruses belonging to the family Coronaviridae. They are amongst the largest group of viruses categorized into four genera: Alphacoronaviruses, Betacoronaviruses, Gammacoronaviruses, and Deltacoronaviruses, infecting different types of animals that can cause mild to severe respiratory and gastrointestinal complications in humans (Capron, 1987). Historically these viruses were linked to the human CoVs (229E and OC43) that caused mild upper respiratory tract diseases. The emergence and re-emergence of Coronavirus diseases in the twenty-first century has sparked public concern since 2002 with the severe acute respiratory syndrome Coronavirus (SARS-CoV) (Ksiazek et al., 2003) and the Middle East respiratory syndrome Coronavirus (MERS-CoV) outbreaks (Zaki et al., 2012; Cui et al., 2019). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible novel virus belonging to the family Coronaviridae (betacoronavirus 2B lineage), together with SARS-CoV and MERS-CoV viruses that caused previous outbreaks. SARS-CoV-2 is the etiological agent of the coronavirus disease (COVID-19) epidemic that emerged in late 2019 in Wuhan, China. On 11 March 2020, the World Health Organization (WHO) declared COVID-19 a pandemic with a high transmission rate, mortality, and morbidity (Team, 2020). Although a great deal of research has been conducted regarding viral emergence and transmission, there is very little available information explaining the pathophysiology and viable therapeutic options. In majority of cases, the infected patients do not require any special medical intervention, however in about 20% of the COVID-19 cases, patients do necessitate hospitalizations (Wu and McGoogan, 2020). Most of the hospitalizations are accounted to hyperinflammation damaging organs and endothelium of blood vessels, thrombosis and immunosuppression with a possible role in latent long COVID or post-COVID conditions (Ackermann et al., 2021; Zhu et al., 2022). Several reports in the past have already documented that oxidative stress and inflammation mutually reinforce each other and the same have also been observed in COVID-19 patients (Jensen et al., 2021). Although elevated levels of reactive oxygen species (ROS) have detrimental consequences on cell viability and many viruses have still evolved to induce oxidative stress for their own benefit of replication inside cells (Lee, 2018). Another important event associated with viral infection is the host lipid metabolism that plays vital role in oxidative stress, inflammatory response and thrombotic complications associated with SARS-CoV-2 infection (Casari et al., 2021). Lipids also form the structural foundations of viral and cellular membranes and thus during viral infection, viruses hijack host cellular signaling and lipid biogenesis to produce lipids and other metabolites in favour of the virus life cycle. Lipidomic approaches may provide valuable insights into the host response to COVID-19 and studies highlighting such roles may provide potential therapeutic targets (Abu-Farha et al., 2020). The virus-host harmonious balance is the key to the survival of these viruses. Since many of the antiviral signaling pathways are initiated due to infection induced oxidative stress, it is imperative to understand the mechanistic details of how host cells maintain the redox balance. A clear understanding will allow to effectively modulate the antiviral targets.
To date, there is no specific treatment strategy for curing COVID-19, however, new research and developments have paved pathways to select potent antiviral agents that can be used effectively as therapeutics against COVID-19. From the previous findings, it can be deduced that one of the best and most efficient cellular targets for the management of SARS-CoV-2 infection and pathogenicity could be the PI3K/Akt/Nrf2 signaling pathways. PI3K (phosphatidylinositol 3-kinase) is a family of enzymes that are involved in cell survival and intracellular trafficking. Nrf2 (nuclear factor erythroid 2–related factor 2) is a key transcription factor that acts as a sensor of oxidative stress and an important regulator of antioxidant defense mechanism via modulating the transcription of more than 200 cytoprotective genes (Tebay et al., 2015). Previous studies have shown that multiple viruses including Herpes simplex virus, Porcine circovirus, Influenza A viruses, vaccinia and cowpox viruses utilize the PI3K/Akt signalling pathways for replication and establising successful infection (Ehrhardt et al., 2007; Soares et al., 2009; Wei et al., 2012; Eaton et al., 2014). Studies have also reported that the PI3K pathway is activily involved in the endocytic uptake of influenza viruses (Ayllon et al., 2012) and ebola viruses (Saeed et al., 2008) thus demonstrating the extent of involvement of signalling pathways in viral infections. PI3K/Akt pathway has been shown to play a critical role in regulating SARS-CoV-2 entry (Shou et al., 2020) and evidence from other studies further suggest that the PI3K/Akt signaling pathway also inhibits NF-κB and subsequently reduces the expression of inflammatory cytokines (Li et al., 2021). Nrf2 and NF-κB has also been known to transcriptionally co-regulate the response of cells to oxidative stress and inflammation via the PI3K/Akt/Nrf2 signaling pathway (Lekshmi et al., 2019), thus making it a crucial target to develop host directed antiviral strategies against SARS-CoV2 and other related viruses.
A comprehensive analysis of all data suggests that PI3K/Akt/Nrf2 signaling could be a powerful tool to manage SARS-CoV-2 infection via antioxidant, anti-inflammatory, and lipid metabolism regulation. In this review, possible cellular targets and molecular mechanisms involved in SARS-CoV-2 infection, as well as therapeutic approaches to treat its pathophysiological complications, are discussed with special emphasis on PI3K/Akt/Nrf2 pathway.
Viruses hijack PI3K/Akt/Nrf2 pathway for survival
Several viruses exploit the host metabolic pathways in order to meet their needs of survival. The phosphatidylinositol 3-kinases (PI3K)-Akt pathway is one such signaling event that is central to metabolism and other cellular functions as well as a common target of many viruses (Cooray, 2004; Buchkovich et al., 2008; Diehl and Schaal, 2013). Although the PI3Ks belong to a large family of lipid kinases belonging to 3 classes: class 1 (1A and 1B), class II, and class III; the PI3K-Akt pathway falls within the class 1A PI3Ks. The class 1A PI3Ks get activated directly or indirectly via small GTPase RAS. The PI3K activation further phosphorylates and activates its most prominent effector Akt which then localizes to the plasma membrane. Viruses have evolved to utilize this pathway for successful entry into target cells or trafficking through the cytoplasm (Saeed et al., 2008; Feng et al., 2011; Fujioka et al., 2011; Izmailyan et al., 2012).
Nrf2, nuclear factor (erythroid-derived 2) -like 2, is a cytoprotective transcription factor belonging to the cap´n´collar basic leucine zipper family that binds to antioxidant response element (ARE) for regulating the transcription of genes encoding proteins which maintain cellular redox homeostasis and metabolic balance (Cuadrado et al., 2019). Under normal conditions, Nrf2 is maintained in an inactive state in the cytosol by binding with KEAP 1 (Kelch-like ECH-associated protein 1), an adaptor subunit of Cullin 3-based E3 ubiquitin ligase which acts as a sensor of oxidative stress. ROS, by modifying the specific cysteine residues, inactivates KEAP1 and thereby releases Nrf2 into the nucleus to induce transcription of Nrf2-responsive genes by binding with ARE. This in turn activates ARE-dependent gene expression of a series of cytoprotective and antioxidative proteins including heme oxygenase-1 (HO-1), glutathione peroxidase 1, glutathionine S-transferase (GST), glutathione reductase (GR), and superoxide dismutase (SOD), catalase (CAT), NAD(P) H dehydrogenase, quinone 1 (NQO1) and c-glutamylcysteine synthetase (Sun et al., 2021a).
The pathophysiology of respiratory viral infections generally involves a redox imbalance or oxidative stress that is associated with the release of cytokines, inflammation and cell death. Studies have shown crucial roles of overproduction of ROS in virus replication and virus-associated diseases (Gu and Korteweg, 2007). Since excessive oxidative stress can be detrimental to the host cells, several viruses maintain an optimal level of oxidative stress, enough to support its replication without killing cells, by manipulating the Nrf2 pathway. When ROS are released upon viral infection, the host cells activate an antioxidative defense mechanism, in which the Nrf2 pathway acts as a first line of defense for cytoprotection and detoxification. Virus-induced modulation of the host antioxidative response have been reported to be an important factor in the progression of several viral diseases. Recently several studies have also reported that groups of clinically relevant viruses can regulate the Nrf2 pathway in both positive and negative manner (Lee, 2018).
Human immunodeficiency virus type 1 (HIV-1), the etiological agent of acquired immunodeficiency syndrome (AIDS) is also linked with the development of neurocognitive disorders. The viral protein, gp120 is known for its causative role in the HIV-1-associated neurodegeneration through induction of oxidative stress. Based on studies conducted in HIV infected astrocytes, the use of Nrf2 activators was suggested as a promising approach to enhance lung innate immunity in HIV patients (Reddy et al., 2012). In 2012, Zhang et al., demonstrated that a major catechin from tea, Epigallocatechin-3-O-gallate (EGCG), was able to improve the cellular alterations induced by oxidative stress associated with Tat-induced HIV-1 transactivation by regulating nuclear levels of Nrf2 and NF-κB. The findings make the Nrf2 pathway the prime therapeautic target (Zhang et al., 2012). Hepatitis C Virus (HCV), responsible for chronic hepatitis, exerts differential effects on the Nrf2 pathway depending on the cellular context and level of oxidative stress. Numerous HCV proteins, including the core, NS3, and NS5A, cause hepatocellular damage as a result of oxidative stress. The production of ROS during HCV infection promotes the phosphorylation and nuclear translocation of Nrf2, which activates target genes such as HO-1 and glutamylcysteine synthetase heavy subunit (γGCSH) (Carvajal-Yepes et al., 2011; Ivanov et al., 2011). Numerous cellular kinases have been implicated in the phosphorylation and activation of Nrf2, including PI3K-Akt, JNK, ERK1/2, p38 MAPKs, and protein kinase C (PKC). In light of these findings, activation of Nrf2 pathway was suggested as one possible mechanism for HCV-infected cells to survive (Carvajal-Yepes et al., 2011; Ivanov et al., 2011).
Similarly, influenza viruses induce infection mainly through oxidative stress and respiratory inflammation. In addition, influenza viruses have also been shown to stimulate apoptosis and cytotoxicity in alveolar epithelial cells, as demonstrated by an increase in caspase 1, caspase 3, and the proinflammatory cytokine IL-8 via activation of Nrf2 pathway by facilitating nuclear translocation of Nrf2 and subsequent expression of Nrf2-target genes such as HO-1 (Kosmider et al., 2012). The suppression of Nrf2 gene was also found to enhance the replication of influenza virus which was reversed by the pharmacological induction of Nrf2 via EGCG supplementation (Kesic et al., 2011). In a proteomic analysis performed by Simon and colleagues, Nrf2 was found to be negatively affected by influenza virus infection. Thus, like HCV infection, influenza virus infection has also been found to induce differential antioxidative responses depending on cellular context (Simon et al., 2015). Likewise, positive or negative regulation of Nrf2 via PI3K/Akt or other signaling pathways through pharmacological modulators have shown to regulate the infection of various viruses including RSV, HCV, HBV, Herpes, DENV and Zika virus (Cho et al., 2009; Zhu et al., 2010; Schachtele et al., 2012; Huang et al., 2017).
The respiratory syndrome caused by SARS-CoV-2 continues to be a major healthcare concern around the globe because of no specific treatment availability for COVID-19. Since the treatments for COVID-19 are known to suppress the symptoms, modulating signaling pathways via therapeutic targets could be important for managing the disease severity. The PI3K/Akt signaling pathway has been identified as a novel therapeutic target against SARS-COV-2 infection due to its involvement in virus entry and host immune response. ACE2 and CD147 are known to be the prime entry receptors for SARS-COV-2 (Hoffmann et al., 2020; Wang et al., 2020). The reduced cell surface expression of ACE2 during infection results in angiotensin 2 accumulation, which upon binding to AT1R (angiotensin 2 receptor type 1) activates the inflammatory pathway via NF-κB signaling. Recent studies have demonstrated that CD147 and furin, as well as clatherin-mediated endocytosis, also induce P13K/Akt signaling (Khezri, 2021). A recent study has also shown that SARS-CoV-2 S protein can modulate inflammatory responses via the PI3K/Akt pathway to allow propagation of virus at early stages of infection (Al-Qahtani et al., 2022).
Apart from being exploited by the viruses during their life cycle, the PI3K/Akt pathway also serves to counteract viral invasion by inducing phosphorylation of IFN regulatory factor 3 (IRF3) and type I interferons (IFN-I) (Schabbauer et al., 2008; Joung et al., 2011). Infection with many double/single stranded viruses also activate the PI3K/Akt for TLR-mediated tyrosine phosphorylation and RIG-I dependent activation of the IRF3 (Sarkar et al., 2004; Yeon et al., 2015). A better understanding of how virus-induced lipid kinase pathways and oxidative stress communicates with the host’s antioxidative response, will provide insights into potential antiviral therapeutics that can be discovered and developed for efficient viral disease management.
Structure of SARS-CoV-2, its life cycle and host cell invasion
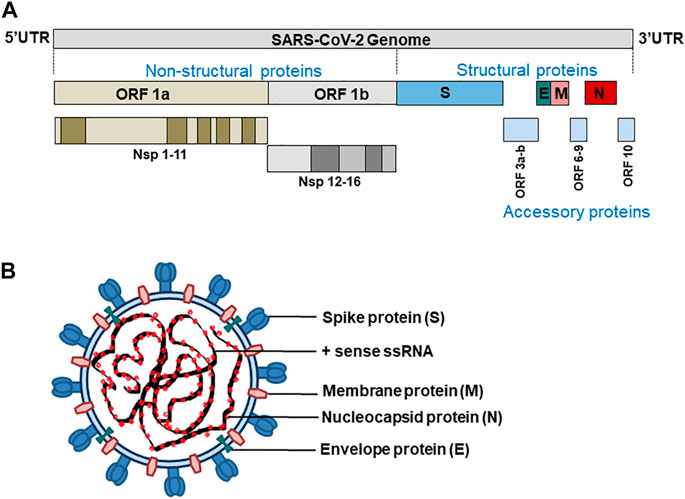
Coronaviruses have a genome size of 27–32 kb, which is generally larger than any other RNA viruses. SARS-CoV-2 has a genome size of approximately 29.9 kb and codes for 4 structural proteins S (spike glycoprotein), N (nucleocapsid protein), M (membrane protein), and E (Envelope protein), 16 non-structural (Nsp1-16), and nine accessory proteins (Orf3a, Orf3b, Orf6, Orf7a, Orf7b, Orf8, Orf9b, Orf9c, Orf10) (Gordon et al., 2020; Lu et al., 2020; Mariano et al., 2020) (Figure 1).
The proteins S, M, and E make up the viral envelope. The invasion of a host cell, the first step in SARS-CoV-2 infection, is mediated by the transmembrane spike protein (180–200 kDa). This allows SARS-CoV-2 virions to attach to the host cell membrane receptors (ACE2) and invade those cells by subsequent fusion of viral and host cell membranes or endocytosis.
The nucleocapsid protein N is recruited at the replication-transcription complex by Nsp3 where it plays a multifaceted role in the infection cycle of SARS-CoV-2. The N protein bind to and package the viral RNA to form ribonucleoprotein (RNP) complexes that locates in the internal face of the viral membrane as a separate layer from the envelope proteins S, M and E (Chang et al., 2014). N protein has two structured domains that allow it to carry out many functions during the viral life cycle, such as virion assembly, RNA replication/transcription, and immune system interference. Since the domains of the N protein are separated by a long flexible linker, it has a high degree of conformational freedom (McBride et al., 2014).
Similarly, the primary function of the membrane protein M, which is embedded by three transmembrane helices, is to drive the assembly of virions to the host cell and maintain other structural proteins at the budding site, and recruit the same by promoting membrane curvature (Neuman et al., 2011). Also, SARS-CoV-2-M proteins are reported to have high pro-apoptotic properties and induce apoptosis by disrupting the interaction of PDK1 (3-phosphoinositide-dependent protein kinase 1) with cell-survival protein PKB (protein kinase B)/Akt in cells expressing M-protein (Tsoi et al., 2014). Like M protein, SARS-CoV-2 envelope protein E also shows oligomerization properties. Coronavirus E protein has only one transmembrane domain that can self-interact to form ion channels and can also establish interactions with the nucleocapsid protein N (Stertz et al., 2007). The M protein oligomerizes at the membrane of the intermediary compartment of the endoplasmic reticulum and Golgi. The interaction of the C-terminus of E with M guides the recruitment of E and initiate virus budding into the host cells (Pervushin et al., 2009; Schoeman and Fielding, 2019).
In response to the viral infection, a “cytokine storm” (also known as hypercytokinemia) is triggered to induce further inflammatory changes in the pneumocytes. Excessive inflammation and apoptosis ultimately cause lung damage. The released viruses after cell apoptosis, further infects the adjacent type 2 alveolar epithelial cells in the same manner, resulting in acute respiratory distress syndrome (Jackson et al., 2022). Even though respiratory epithelial cells are the prime target for SARS-CoV-2, both direct and indirect cellular alterations due to virus replication, host response, and the triggered inflammatory and hypercoagulative consequences make the condition more lethal (Bezerra et al., 2022). COVID-19 patients mainly exhibit the viral nucleocapsid and spike proteins as main immunogens, and plasma or serum quantitative measurements in SARS-CoV-2 patients showed the N protein to be more sensitive to the adaptive immune response than the spike protein. This makes it an excellent indicator of early disease development (Burbelo et al., 2020).
SARS-CoV-2 infection and oxidative stress
Elevated ROS production and higher concentrations of oxidized biomolecules have been reported in the alveolar epithelium and endothelium of patients infected with viruses such as influenza (Buffinton et al., 1992), rhinovirus (Martinez et al., 2016), respiratory syncytial virus (RSV) (Biagioli et al., 1999), and many other viruses, however different viruses are known to employ diverse molecular mechanisms to exhibit these cellular effects. While maintaining a proper redox homeostasis is very important for the regulated balance of viral-induced ROS-activated immune cell signal transduction, its excessive production may further induce an impaired immune response, inflammatory reactions, mitochondrial dysfunction, and apoptosis impacting the disease pathogenesis (Chernyak et al., 2020). Mitochondria is the major producer of ROS (mtROS) in non-immune cells like endothelial cells while NADPH oxidase (NOX) and xanthine oxidase are the major sources of ROS in immune cells (Vorobjeva et al., 2017). Most viruses induce oxidative stress in order to facilitate viral replication in the host cell by activating innate immunity via NF-κB-dependent cytokine production. RSV is also reported to induce ROS production and cytokine burst in host cells. To control the ROS levels these viruses are known to acquire the ability to manipulate Nrf2 dependent antioxidant pathway in their favor. RSV ameliorates glutathione (GSH) levels and increases lipid peroxidation in type II epithelial cells of the airway and human alveoli resulting in the downregulation of the Nrf2 pathway. This in turn reduces the expression of Nrf2-dependent target genes; superoxide dismutase (SOD), catalase (CAT), hemoxigenase 1 (HO-1/HMOX1), glutathione S-transferase (GST), and glutathione peroxidase (GPx), and triggers interferon (IFN) and Toll-like receptor (TLR) pathway to combat the virus infection (Jamaluddin et al., 2009). The mechanism however, is different for the influenza virus. The influenza virus induces oxidative stress but it also favors translocation of Nrf2 and thereby activates the antioxidant defense mechanism for its survival in the host cells (Imai et al., 2008).
Oxidative stress is reported as the agent provocateur behind most viral infections and thus the host cell signaling activation accompanied by oxidative stress may have a profound impact on the pathogenesis of COVID-19 and related disorders. A recent study found that the activation of Nrf2/HMOX1 significantly suppressed SARS-CoV-2 replication through production of the metabolite biliverdin in different cell types. The same study also demonstrated that the virus impaired the Nrf2/HMOX1 axis through its NSP14 which interacted with the catalytic domain of the NAD-dependent deacetylase Sirtuin 1 (SIRT1) thereby inhibiting the Nfr2/HMOX1 pathway. While this finding revealed the crucial role of a viral protein in dysregulating the host antioxidant defense system, it further emphasized the important role of SIRT1/Nrf2 pathway in the host cell for the pathological management of SARS-CoV-2 infection via an antioxidant defense mechanism (Zhang et al., 2022).
Similarly, the Nrf2-dependent antioxidant pathway have been found to be suppressed in the biopsies of COVID-19 patients but interestingly the Nrf2 agonists like dimethyl fumarate (DMF) and 4-ocy-itaconate (4-OI) were reported to induce cellular antiviral effects that could inhibit the replication of SARS-CoV-2 by suppressing the pro-inflammatory response of the SARS-CoV-2 (Olagnier et al., 2020). Nrf2 has also been reported as an important transcriptional repressor of the inflammatory genes in macrophages by blocking the transcription of proinflammatory cytokines, most notably interleukin1β (IL-1β) (Hayes and Dinkova-Kostova, 2014).
The main protease (Mpro) in SARS-CoV-2 responsible for viral polyprotein processing is called 3C-like protease (3CLpro) or 3-chymotrypsin-like-proteases, a highly conserved protease among coronaviruses. It is a cysteine protease that corresponds to Nsp5 of coronavirus and acts as a potential drug target for antiviral therapy against the coronavirus. Several protease covalent inhibitors targeting 3CLpro like CLpro-1, GC376, rupintrivir (formerly AG7088), lufotrelvir (PF-07304814) have already been discovered by structural-based drug designs which are advantageous with low minimum side effects and maximum therapeutic efficacy. Of these, the prodrug PF-07304814 (lufotrelvir) entered clinical trials in September 2020 (Owen et al., 2021). As the Nrf2 pathway plays a significant role in the pathophysiology of both host cells and viruses, Nrf2 modulators have been recommended as promising supplements for the treatment of viral infections by reducing the effects of virus-induced oxidative stress. In 2021, Qi Sun and co-workers discovered oleanolic acid-derived semi-synthetic triterpenoids like bardoxolone and bardoxolone methyl compounds with electrophilic moieties as 3CL pro inhibitors that may covalently bind to the active site cysteine of SARS-CoV-2 3CLpro. These compounds were identified as Nrf2 activators that can inhibit the NF-κB pathway promoting resolution of inflammation, inhibiting viral replication, and thereby facilitating cytoprotection and tissue repair (Kobayashi et al., 2016). Using a murine model of infection and airway epithelial cells, Qu et al. demonstrated that SARS-CoV-2 can alter cellular redox balance and inhibit Nrf2-mediated antioxidant responses. Infection with SARS-CoV2 downregulated Nrf2 protein levels and Nrf2-dependent gene expression, resulting in increased inflammation and disease progression. In addition, mice lacking the Nrf2 gene exhibited worse clinical signs, had increased inflammation, and showed a tendency toward higher lung viral titers, demonstrating that Nrf2 has a protective role during SARS-CoV-2 infection. The results of this study provided a mechanistic explanation for the oxidative unbalance associated with SARS-CoV-2 infection, suggesting that activating Nrf2 by pharmacological agents could be a therapeutic strategy for COVID-19 (Qu et al., 2023). Similar observations have been reported for other viruses. In 2006, Jiang et al. discovered that α-Luminol (monosodium 5-amino-2-3-dihydro-1-4-phthalazine dione), an anti-inflammatory drug extensively used by Russian scientists, was able to suppress oxidative stress induced by the infection of temperature sensitive mutant virus Moloney murine leukemia virus (MoMuLV ts-1) (Jiang et al., 2006). In COVID-19 patients, in addition to viral propagation, the inflammatory response of host cells is also important in determining the disease outcome and fatality. In most viral infections the lethality is found to be associated with the inflammatory response orchestrated by the host immune system through cytokine storm rather than the cytolytic action of the pathogen (Fung et al., 2020; Guan et al., 2020). For the comprehensive management of SARS-CoV-2, it is always advised to introduce an anti-inflammatory and antioxidant therapy to complement an antiviral therapy to control inflammation without altering the host cell’s adaptive immunity against the infected virus.
Role of PI3K/Akt/Nrf2 pathway in SARS-CoV-2 infection induced inflammation
Cellular homeostasis and responses to stress and inflammation are regulated by Nrf2 through NF-ĸB-dependent pathways. There is compelling evidence that Nrf2 is capable of counteracting NF-ĸB-driven inflammation in many experimental models (Tu et al., 2019). The SARS-CoV-2 infection has similar pathophysiology to SARS-CoV and MERS-CoV infections, with aggressive inflammatory responses strongly implicated in the damage to the lungs. COVID-19 is a multifactorial and complex disease that primarily targets the airway epithelial cells of the respiratory tract, which is characterized by diffused alveolar edema in the lungs, infiltrations of inflammatory cells, epithelial dysfunction, and thrombosis (Song et al., 2020; Bridges et al., 2022).
Hepatocytes, monocytes, and other endothelial cells are also found to be susceptible to SARS-CoV-2 infection, and evidence suggests that virus-induced hyperinflammation can be triggered by virus-mediated intracellular sensing pathways in cellular targets (Figure 2). Similar to several other viral infections, the alveolar epithelial cells and macrophages recognize pathogen-associated molecular patterns (PAMPs), such as viral RNA, and damage-associated molecular patterns (DAMPs), such as ATP, DNA, and ASC oligomers, using a variety of pattern-recognition receptors (PRRs) during SARS-CoV-2 infection (Huang et al., 2020; Asha et al., 2021). These activated monocytes and polymorphonuclear cells infiltrate into the target cells and cause the release of proinflammatory cytokines and chemokines, like TNF-α, IL-1β, IL-6, CCl2, MCP1 and IP 10 upon interaction of viral particles with antigen-presenting cells (APCs). The release of these indicators of T helper 1 (TH1) cell-polarized response results in severe lung damage and multi-organ dysfunction. IL-1β is known to induce pyroptosis and is also found to be elevated during SARS-CoV-2 infection (Tang et al., 2021). These data strongly suggest that a COVID-19 patient’s disease severity is not only influenced by the virus but also by the host’s immune response.
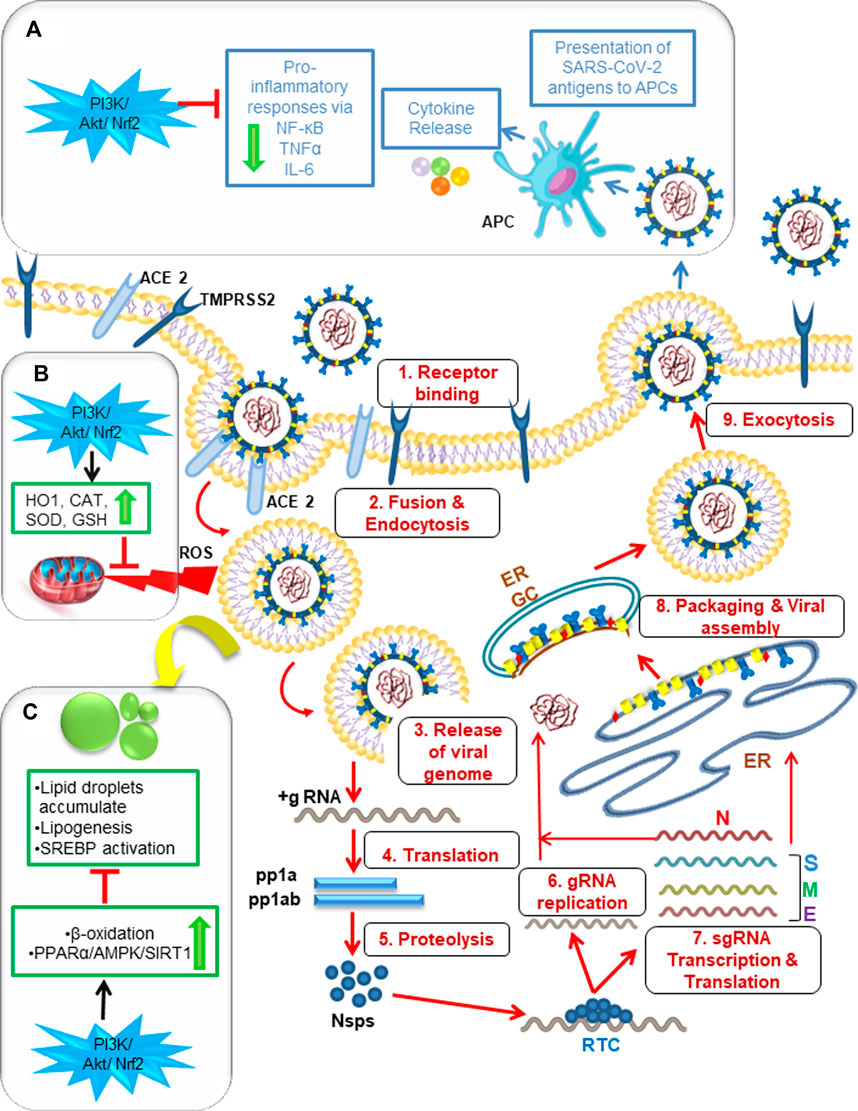
FIGURE 2. Graphical abstract showing the significance of P13K/Akt/Nrf2 signaling pathway for the management of SARS- CoV- 2 infection via modulating host cell inflammatory responses, antioxidant mechanism and lipid metabolism. 1–9: SARS-CoV-2 enters a target cell by either fusion or endocytosis followed by release of genetic material. Then subsequent events of translation and genome replication occurs leading to the final assembly and egress of virions to infect neighboring cells. (A) The released viral particles are recognized by APC of macrophages leading to host inflammatory response via “cytokine storm” that can be downregulated by PI3K/Akt/Nrf2 activators. (B) PI3K/Akt/Nrf2 activators can also downregulate ROS-induced oxidative stress produced by SARS-CoV-2 via antioxidant mechanism mediated by the enzymes HO1, SOD, CAT, GSH. (C) Lipid droplets can be downregulated via PI3K/Akt/Nrf2 pathway activators by beta oxidation and through the modification of PPARα/AMPK/SIRT1 signaling pathway. ACE2: angiotensin converting enzyme-2, TMPRSS2: Transmembrane serine protease 2, Nsps: non-structural proteins, ERGIC: ER-Golgi intermediate compartment, APC: antigen presenting cells, PI3K: phosphoinositide 3-kinase, Akt: serine/threonine-specific protein kinase B, Nrf2: nuclear factor erythroid 2–related factor 2, PPARα: Peroxisome proliferator-activated receptor, AMPK: AMP-activated protein kinase, SIRT1:Sirtuin, HO1: Heme Oxygenase-1, SOD: Superoxide dismutase, CAT: Catalase, GSH: Glutathione.
SARS-CoV-2 infection has been reported to initiate cell death by both apoptosis and necroptosis pathways. In a SARS-CoV-2-infected HFH4-hACE2 (Hepatocyte nuclear factor-3/forkhead homolog 4-human Angiotensin-converting enzyme 2) transgenic mouse model and in the postmortem lung sections of deceased COVID-19 patients, SARS-CoV-2 infection was found to activate caspase-8 to trigger cell apoptosis and inflammatory cytokine processing in the lung epithelial cells. The study further revealed massive inflammatory cell infiltration and pulmonary interstitial fibrosis, typical of immune pathogenesis as the reason of excessive lung damage in those diseased patients (Li et al., 2020). Such findings may assist in the development of specific therapeutic strategies to treat COVID-19.
ACE2-associated lung injury has also been suggested by both SARS-CoV infection and inflammatory cytokines such as IL-1β and TNF-α through enhancement of ACE2 shedding (Haga et al., 2008). In a study on SARS-CoV and human coronavirus NL63 infection, the spike protein was found to modulate ACE2 (Haga et al., 2008; Glowacka et al., 2010). The loss of pulmonary ACE2 function occurs as a result of the loss of catalytically active ACE2 ectodomains.
Ultimately, this resulted in acute lung injury by disrupting the renin-angiotensin system and enhancing inflammation and vascular permeability. The action of disintegrin and metalloprotease 17 (ADAM17, also known as TNF-α cleavage enzyme, TACE) constitutively sheds ACE2 to release enzymatically active soluble ACE2 (sACE2). Since SARS-CoV S protein-induced ACE2 shedding is tightly coupled with TNF-α production in cell culture conditions, it is possible that sACE2 plays a role in the inflammatory response to SARS-CoV and possibly SARS-CoV-2 as well (Haga et al., 2008; Fu et al., 2020). Angiotensin II (Ang II) is a vasoconstrictor that produces oxidative stress via ROS production and elevated blood pressure. In general ACE converts Ang I to Ang II which in turn is converted into Ang by ACE2. Ang remains bound to MAS receptor, a G protein-coupled receptor for Ang, in various tissues including the heart, brain, kidney, etc. To protect against aneurysms by activating PI3K/Akt/Nrf2 pathway (Shimada et al., 2015; Kamel et al., 2018). A recent study identified 34 compounds with anti-SARS-CoV-2 activity that targeted the mTOR/P13K/Akt pathway and DNA-damage response signaling pathways to block SARS-CoV, MERS-CoV and SARS-CoV-2 infection (Garcia et al., 2021). The kinase inhibitor berzosertib also blocked the SARS-CoV-2 at post entry levels in target epithelial cells (Garcia et al., 2021).
Previous studies have demonstrated the crucial role of ACE2/Ang/MAS axis in activating the Akt signaling to manage oxidative stress, inflammation, and hepatic steatosis (Cao et al., 2016) and that Akt inhibitors significantly reduce the ACE2 mediated lipid metabolism, thereby providing insights to manage the SARS-CoV-2 infection-induced metabolic changes in host cells (Cao et al., 2016).
Uncontrolled inflammatory responses known as the cytokine storms have been reported previously in case of SARS-CoV and MERS-CoV infections giving rise to heightened immune response leading to overproduction of proinflammatory cytokines such as the IL-6, TNF-α and IL-1β (Teijaro, 2017). SARS-CoV2 infection is also known to active immune response, more specifically in older adults or those with comorbidity, to a level that can give rise to uncontrolled inflammatory responses (Nile et al., 2020). It is very crucial to control excessive inflammation in COVID-19 patients with severe disease at right time, in absence of which, the condition quickly deteriorates leading to acute respiratory failure, cardiac damage or multi-organ failure (Costela-Ruiz et al., 2020). Naturally occurring phytochemicals, since decades, have been in use as therapeutics to manage diseases with minimal or no side effects. Flavonoids are secondary plant metabolites that have been shown to have anti-viral, anti-inflammatory and immunomodulatory activities (Chen et al., 2018; Hosseinzade et al., 2019; Liskova et al., 2020; Badshah et al., 2021). For example, Smilax campestris aqueous extract, containing the catechin and derivatives of quercetin, has been shown to reduce the production of proinflammatory cytokines such as the TNF-α, IL-1β, IL-6, IL-8, and MCP-1 in lipopolysaccharide-activated macrophages derived from THP-1 cells (Salaverry et al., 2020). Hesperetin and chrysin have been shown to have immunomodulatory potential in physiological and pathological conditions through the cellular as well as humoral responses (Sassi et al., 2017). Similarly, many flavonoids have also been demonstrated to exert immunomodulatory activities against human coronaviruses and in silico studies have further provided evidence that these flavonoids have potential to bind to ACE2 protein and ultimately inhibit the production of proinflammatory cytokines (Ngwa et al., 2020) thus making them an attractive therapeutic agent. Likewise, rhamnocitrin, a flavonoid extracted from Nervilia fordii, has shown its potential to inhibit the endothelial activation (via miR-185/STIM-1/SOCE/NFATc3) which is responsible for excessive cytokine production. Since a similar endothelial activation in case of SARS-CoV or COVID-19 have also been documented, rhamnocitrin may serve as potential modulator of the cytokine storm and effective management of COVID-19 (Lin et al., 2020).
Recent reports suggest the direct or indirect role of the PI3K/Akt signaling pathway in SARS-CoV, MERS-CoV and SARS-CoV-2 infection (Mizutani et al., 2005; Kindrachuk et al., 2015; Sun et al., 2021b). This signaling pathway has also shown to be the target of some flavonoids such as the quercetin, hesperidin, acacetin, geninstein, silibinin and delphinidin among many others (reviewed in (Zughaibi et al., 2021)) wherein they inhibit the deregulated signaling significantly in different types of cancer.
NLRP3 inflammasome has also been shown to be regulated by PI3K/Akt signaling in atherosclerosis and inhibitors of PI3K (GDC0941) and Akt (MK2206) significantly reduced the activation of NLRP3 and expression levels of p-p65/p65. The inhibitors further reduced the mitochondrial ROS in THP-1 cells and mice model (Liu et al., 2021). Since NLRP3 inflammasome is also reported to be activated by SARS-CoV-2, it’s imperative to understand the management of virus induced ROS and inflammatory response by modulating the PI3k/Akt signaling pathways using the anti-inflammatory and immunomodulatory properties of flavonoids. These natural products might be very helpful in minimizing the SARS-CoV-2 complications by regulating inflammatory mediators and endothelial activation by toll-like receptors (TLRs), NLRP3 inflammasome, Nrf2, bromodomain-containing protein 4 (BRD4), or 3CL pro (Liskova et al., 2021).
COVID-19 disease severity has also been correlated with TLR2 and MYD88 expressions, and it has been observed that TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. MyD88, the adaptor protein for TLRs, leads to the activation of NF-ĸB and MAPKs for the production of proinflammatory cytokines. As a result of TLR2 and Myd88 activation, during coronavirus infection, TLR2-dependent signaling leads to the production of proinflammatory cytokines independent of viral entry. In healthy tissues, the TLR-mediated signaling leads to the activation of the PI3K/Akt/Nrf2 pathway for the positive regulation of cell growth (Laird et al., 2009; Zheng et al., 2021). In light of these data, better therapeutic strategies to counter the ongoing COVID-19 pandemic could be developed and effectively manage disease burden.
SARS-CoV-2 infection and host lipid metabolism
Lipids are one of the fundamental components of a cell that make up the structural building blocks. As a signaling and energy storage molecule, it has a wide range of biological functions. Lipid plays a crucial role in the viral life cycle. The enveloped viruses, like SARS-CoV-2, are surrounded by a lipid bilayer and each step of the viral infection such as fusion of membrane to host cell, endocytosis, viral replication, maturation, and exocytosis utilizes host lipid metabolism (Abu-Farha et al., 2020). The coronaviruses create double-membrane vesicles (DMVs), a membranous structure consisting of viral proteins and some host factor, for viral genome amplification after seizing the intracellular membrane of host cells. Such a lipid micro-environment that contains specific phospholipid composition is ideal for viral replication. Recent studies have shown that an important lipid processing enzyme belonging to the phospholipase A2 superfamily, cytosolic phospholipase A2 enzyme (cPLA2) is crucial for DMV formation and viral replication (Muller et al., 2018).
Fatty acids and cholesterol are the inevitable components of viral replication as they constitute the major component of the viral membrane. Therefore, Acetyl-CoA carboxylase (ACC), fatty acid synthase (FASN), and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMG-CoA reductase, the major modulators of lipid metabolism, can act as possible antiviral targets against SARS-CoV2 infection (Heaton and Randall, 2011). The recent studies on HIV infection (Kulkarni et al., 2017), hepatitis C virus (HCV) infection (Yang et al., 2008), and Epstein–Barr virus (EBV) lytic and latent infection (Li et al., 2004) confirmed this hypothesis. An increase in the intracellular level of FAS was observed in all these conditions and it was also evident that FAS inhibition impaired the replication of the respiratory syncytial virus (RSV) and other respiratory viruses (Ohol et al., 2015). These findings make this enzyme a novel host-dependent antiviral target. The intracellular levels of fatty acids and cholesterol are regulated by a feedback mechanism mediated by SREBPs (Sterol regulatory element binding proteins), which are bound to the endoplasmic reticulum membrane as inactive precursors. When the cells are deprived of cholesterol, SREBPs are proteolytically cleaved and the active SREBP migrates to the nucleus for the transcriptional regulation of genes responsible for lipid metabolism. This can also be a potential candidate related to lipid metabolism-related antiviral approaches, that can also be modulated by the PI3K/Akt/Nrf2 pathway (Ye and DeBose-Boyd, 2011).
The major cellular receptors of SARS-CoV-2, ACE2 may be expressed in cholesterol-rich domains of lipid bilayer known as lipid rafts that serve as an entry port for certain viruses especially enveloped viruses. An experiment conducted in Vero E6 cells revealed that integrity of lipid rafts was required for productive infection of severe acute respiratory syndrome coronavirus (SARS-CoV) (Lu et al., 2008). The role of peroxisome proliferator-activated receptors (PPARs), belonging to the nuclear receptor superfamily, as an antiviral candidate is a recent matter of investigation during the COVID-19 pandemic. There are mainly 3 subtypes of PPAR receptors: PPARα, PPARγ, and PPARβ/δ that play well-established roles in cellular differentiation, proliferation, energetic homeostasis, glucose, and lipid metabolism. Several in vitro and in vivo studies revealed that the stimulation of PPAR by natural or synthetic agonists like curcumin, capsaicin, and eicosapentaenoic acid could prevent cytokine overproduction and the inflammatory cascade associated with virus infections (Ciavarella et al., 2020; Fantacuzzi et al., 2022). Pioglitazone, a PPAR agonist, is also proposed as an effective treatment in COVID-19 people affected by type 2 diabetes, cardiovascular complications, and hypertension by reducing inflammatory parameters and also by inhibiting 3CLpro thereby downregulating SARS-CoV-2 RNA synthesis and replication (Carboni et al., 2020). There is evidence that the PPARα/γ-adenosine 5′-monophosphate- (AMP-) activated protein kinase- (AMPK-) sirtuin-1 (SIRT1) pathway and fatty acid metabolism may be involved in influenza A virus (IAV) replication and pneumonia caused by IAV (Bei et al., 2021). These all synergistically work together to inhibit NF-κB signaling and suppress inflammation. Furthermore, Nrf2 and antioxidant response element (ARE) pathways also interact mutually with, PPARα/γ-AMPK to inhibit inflammation, constituting a positive feedback loop (Kauppinen et al., 2013). In adipose tissue, Gamma-oryzanol, the principal bioactive constituent of ice bran, reduced the levels of TNF-α, interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1) (Francisqueti-Ferron et al., 2021). Due to its potent and multi-PPAR activity, astaxanthin has been investigated as a therapeutic strategy to regulate inflammatory and immune responses, contrast cytokine storms, and prevent inflammatory effects following COVID-19 (Talukdar et al., 2020).
PI3K/Akt pathway is also known to play a significant role in host lipid biogenesis as revealed by the study on goose hepatocyte where researchers observed that inhibition of the PI3K-Akt-mTOR pathway drastically reduced the lipids accumulation in hepatocytes (Liu et al., 2016) s.
These alternative approaches that are under development, when backed up by clinical trials, can potentially be promising tools for reducing the pathophysiological complications of SARS-COV-2 infections.
Conclusion and future perspectives
Despite the widespread use of vaccines, the transmission of SARS-CoV-2 infection is on the rise, which enables new variants to emerge frequently. As a consequence of this unprecedented threat in the 21st century, to alleviate case fatality and patients’ symptoms, therapeutic interventions are urgently needed to compliment currently available vaccines. It is still required to conduct studies to develop a universal vaccine against COVID-19, which can neutralize all variants of SARS-CoV-2. Viruses and hosts have a strict interplay that can be destructed through metabolic disruption, which is an attractive novel strategy to combat viral infections. In this regard, broad spectrum antiviral compounds that target PI3K/Akt/Nrf2 signaling pathways to offer host-mediated antiviral responses in every manner, may be considered as the best drug candidates for the future management of COVID-19 and related post-COVID syndromes. Several FDA-approved inhibitors targetting PI3K and Akt are in use in clinical settings (Basile et al., 2022). While most of the inhibitors of PI3K are used to treat some forms of cancer, its utility in viral infections have not been reported adequately. Similarly the Akt inhibitor (Miltefosine) have been in use for Visceral and cutaneous leishmaniasis (Sundar and Olliaro, 2007), however the role in viral infections are still limited to research settings (Sharma et al., 2018). The processes of oxidative stress, inflammation, and changes in lipid metabolism are interconnected and all contribute to both SARS-CoV-2 infection and post-COVID-19 complications. According to the World Health Organization, many patients are experiencing short-to long-term post-COVID-19 sequelae such as cardiovascular, neurological, nephrological, gastro-intestinal, and even psychological effects. A major cause of mortality was reported to be thromboembolism, the cumulative risk product of all the above discussed pathophysiological conditions. PI3K/Akt pathway is known to be an important regulator of coagulation pathways and hence a key player in disease modulation (Shan et al., 2019). Another critical area of concern is the ‘postural orthostatic tachycardia syndrome’ (POTS) occurring after SARS-CoV-2 infection or COVID-19 vaccination. POTS is a condition in which there is an increase in heart rate of at least 30 beats per minute within 10 min of standing. SARS-CoV-2 infected people and those who have been vaccinated against COVID-19 have had an increased risk of cardiovascular diseases (CVDs), but it is unclear if this is due to the virus infection or the vaccination (Blitshteyn and Fedorowski, 2022). The situation is unfortunate as there is no complete cure. However, the overall immunity can be strengthened in order to compete with viral infections. Reports indicate that the best natural immunity boosters are functional foods that offer health benefits beyond their nutritional values. Incorporating functional food ingredients in diet can activate cell survival pathways like PI3K/Akt/Nrf2, that can reduce long-term health risks associated with COVID-19.
Overall, in this review article, we propose potential anti-inflammatory and antioxidant therapies that can also regulate lipid metabolism by targeting transcription factor Nrf2 via the PI3K/Akt signaling pathway. Combined, the research discussed in this article strongly suggests that activating Nrf2 could be a promising strategy for combating COVID-19. Further investigations along this line are needed to develop efficient counteracting strategies to ameliorate disease severity and improve treatment outcomes, especially for patients with underlying complications.
Author contributions
VSL, KA, MS, AA and BK, wrote the manuscript. VSL and UMA prepared the figures. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author MS is employed by Clover Biopharmaceuticals.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abu-Farha, M., Thanaraj, T. A., Qaddoumi, M. G., Hashem, A., Abubaker, J., and Al-Mulla, F. (2020). The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int. J. Mol. Sci. 21 (10), 3544. doi:10.3390/ijms21103544
Ackermann, M., Anders, H. J., Bilyy, R., Bowlin, G. L., Daniel, C., De Lorenzo, R., et al. (2021). Patients with COVID-19: In the dark-NETs of neutrophils. Cell Death Differ. 28 (11), 3125–3139. doi:10.1038/s41418-021-00805-z
Al-Qahtani, A. A., Pantazi, I., Alhamlan, F. S., Alothaid, H., Matou-Nasri, S., Sourvinos, G., et al. (2022). SARS-CoV-2 modulates inflammatory responses of alveolar epithelial type II cells via PI3K/AKT pathway. Front. Immunol. 13, 1020624. doi:10.3389/fimmu.2022.1020624
Asha, K., Khanna, M., and Kumar, B. (2021). Current insights into the host immune response to respiratory viral infections. Adv. Exp. Med. Biol. 1313, 59–83. doi:10.1007/978-3-030-67452-6_4
Ayllon, J., García-Sastre, A., and Hale, B. G. (2012). Influenza A viruses and PI3K: Are there time, place and manner restrictions? Virulence 3 (4), 411–414. doi:10.4161/viru.20932
Badshah, S. L., Faisal, S., Muhammad, A., Poulson, B. G., Emwas, A. H., and Jaremko, M. (2021). Antiviral activities of flavonoids. Biomed. Pharmacother. 140, 111596. doi:10.1016/j.biopha.2021.111596
Basile, M. S., Cavalli, E., McCubrey, J., Hernández-Bello, J., Muñoz-Valle, J. F., Fagone, P., et al. (2022). The PI3K/Akt/mTOR pathway: A potential pharmacological target in COVID-19. Drug Discov. Today 27 (3), 848–856. doi:10.1016/j.drudis.2021.11.002
Bei, Y., Tia, B., Li, Y., Guo, Y., Deng, S., Huang, R., et al. (2021). Anti-influenza A virus effects and mechanisms of emodin and its analogs via regulating pparα/γ-AMPK-SIRT1 pathway and fatty acid metabolism. Biomed. Res. Int. 2021, 9066938. doi:10.1155/2021/9066938
Bezerra, B. B., Silva, G. P. D. d., Coelho, S. V. A., Correa, I. A., Souza, M. R. M., Macedo, K. V. G., et al. (2022). Hydroxypropyl-beta-cyclodextrin (HP-BCD) inhibits SARS-CoV-2 replication and virus-induced inflammatory cytokines. Antivir. Res. 205, 105373. doi:10.1016/j.antiviral.2022.105373
Biagioli, M. C., Kaul, P., Singh, I., and Turner, R. B. (1999). The role of oxidative stress in rhinovirus induced elaboration of IL-8 by respiratory epithelial cells. Free Radic. Biol. Med. 26 (3-4), 454–462. doi:10.1016/s0891-5849(98)00233-0
Blitshteyn, S., and Fedorowski, A. (2022). The risks of POTS after COVID-19 vaccination and SARS-CoV-2 infection: it’s worth a shot. Nat. Cardiovasc. Res. 1 (12), 1119–1120. doi:10.1038/s44161-022-00180-z
Bridges, J. P., Vladar, E. K., Huang, H., and Mason, R. J. (2022). Respiratory epithelial cell responses to SARS-CoV-2 in COVID-19. Thorax 77 (2), 203–209. doi:10.1136/thoraxjnl-2021-217561
Buchkovich, N. J., Yu, Y., Zampieri, C. A., and Alwine, J. C. (2008). The TORrid affairs of viruses: Effects of mammalian DNA viruses on the PI3K-Akt-mTOR signalling pathway. Nat. Rev. Microbiol. 6 (4), 266–275. doi:10.1038/nrmicro1855
Buffinton, G. D., Christen, S., Peterhans, E., and Stocker, R. (1992). Oxidative stress in lungs of mice infected with influenza A virus. Free Radic. Res. Commun. 16 (2), 99–110. doi:10.3109/10715769209049163
Burbelo, P. D., Riedo, F. X., Morishima, C., Rawlings, S., Smith, D., Das, S., et al. (2020). Sensitivity in detection of antibodies to nucleocapsid and spike proteins of severe acute respiratory syndrome coronavirus 2 in patients with coronavirus disease 2019. J. Infect. Dis. 222 (2), 206–213. doi:10.1093/infdis/jiaa273
Cao, X., Yang, F., Shi, T., Yuan, M., Xin, Z., Xie, R., et al. (2016). Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis activates Akt signaling to ameliorate hepatic steatosis. Sci. Rep. 6, 21592. doi:10.1038/srep21592
Capron, L. (1987). Lipoproteins, diabetes and macroangiopathy. Journ. Annu. Diabetol. Hotel. Dieu 1987, 67–78.
Carboni, E., Carta, A. R., and Carboni, E. (2020). Can pioglitazone be potentially useful therapeutically in treating patients with COVID-19? Med. Hypotheses 140, 109776. doi:10.1016/j.mehy.2020.109776
Carvajal-Yepes, M., Himmelsbach, K., Schaedler, S., Ploen, D., Krause, J., Ludwig, L., et al. (2011). Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small Maf proteins. J. Biol. Chem. 286 (11), 8941–8951. doi:10.1074/jbc.M110.186684
Casari, I., Manfredi, M., Metharom, P., and Falasca, M. (2021). Dissecting lipid metabolism alterations in SARS-CoV-2. Prog. Lipid Res. 82, 101092. doi:10.1016/j.plipres.2021.101092
Chang, C. K., Hou, M. H., Hsiao, C. D., and Huang, T. h. (2014). The SARS coronavirus nucleocapsid protein-forms and functions. Antivir. Res. 103, 39–50. doi:10.1016/j.antiviral.2013.12.009
Chen, L., Wei, Y., Zhao, S., Zhang, M., Yan, X., Gao, X., et al. (2018). Antitumor and immunomodulatory activities of total flavonoids extract from persimmon leaves in H(22) liver tumor-bearing mice. Sci. Rep. 8 (1), 10523. doi:10.1038/s41598-018-28440-8
Chernyak, B. V., Popova, E. N., Prikhodko, A. S., Grebenchikov, O. A., Zinovkina, L. A., and Zinovkin, R. A. (2020). COVID-19 and oxidative stress. Biochem. (Mosc) 85 (12), 1543–1553. doi:10.1134/S0006297920120068
Cho, H. Y., Imani, F., Miller-DeGraff, L., Walters, D., Melendi, G. A., Yamamoto, M., et al. (2009). Antiviral activity of Nrf2 in a murine model of respiratory syncytial virus disease. Am. J. Respir. Crit. Care Med. 179 (2), 138–150. doi:10.1164/rccm.200804-535OC
Ciavarella, C., Motta, I., Valente, S., and Pasquinelli, G. (2020). Pharmacological (or synthetic) and nutritional agonists of PPAR-gamma as candidates for cytokine storm modulation in COVID-19 disease. Molecules 25 (9), 2076. doi:10.3390/molecules25092076
Cooray, S. (2004). The pivotal role of phosphatidylinositol 3-kinase–Akt signal transduction in virus survival. J. General Virology 85 (5), 1065–1076. doi:10.1099/vir.0.19771-0
Costela-Ruiz, V. J., Illescas-Montes, R., Puerta-Puerta, J. M., Ruiz, C., and Melguizo-Rodríguez, L. (2020). SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 54, 62–75. doi:10.1016/j.cytogfr.2020.06.001
Cuadrado, A., Rojo, A. I., Wells, G., Hayes, J. D., Cousin, S. P., Rumsey, W. L., et al. (2019). Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 18 (4), 295–317. doi:10.1038/s41573-018-0008-x
Cui, J., Li, F., and Shi, Z. L. (2019). Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17 (3), 181–192. doi:10.1038/s41579-018-0118-9
Diehl, N., and Schaal, H. (2013). Make yourself at home: Viral hijacking of the PI3K/Akt signaling pathway. Viruses 5 (12), 3192–3212. doi:10.3390/v5123192
Eaton, H. E., Saffran, H. A., Wu, F. W., Quach, K., and Smiley, J. R. (2014). Herpes simplex virus protein kinases US3 and UL13 modulate VP11/12 phosphorylation, virion packaging, and phosphatidylinositol 3-kinase/Akt signaling activity. J. Virol. 88 (13), 7379–7388. doi:10.1128/JVI.00712-14
Ehrhardt, C., Wolff, T., Pleschka, S., Planz, O., Beermann, W., Bode, J. G., et al. (2007). Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 81 (7), 3058–3067. doi:10.1128/JVI.02082-06
Fantacuzzi, M., Amoroso, R., and Ammazzalorso, A. (2022). PPAR ligands induce antiviral effects targeting perturbed lipid metabolism during SARS-CoV-2, HCV, and HCMV infection. Biol. (Basel) 11 (1), 114. doi:10.3390/biology11010114
Feng, S. Z., Cao, W. S., and Liao, M. (2011). The PI3K/Akt pathway is involved in early infection of some exogenous avian leukosis viruses. J. Gen. Virol. 92 (7), 1688–1697. doi:10.1099/vir.0.030866-0
Francisqueti-Ferron, F. V., Garcia, J. L., Ferron, A. J. T., Nakandakare-Maia, E. T., Gregolin, C. S., Silva, J. P. d. C., et al. (2021). Gamma-oryzanol as a potential modulator of oxidative stress and inflammation via PPAR-y in adipose tissue: A hypothetical therapeutic for cytokine storm in COVID-19? Mol. Cell Endocrinol. 520, 111095. doi:10.1016/j.mce.2020.111095
Fu, Y., Cheng, Y., and Wu, Y. (2020). Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virol. Sin. 35 (3), 266–271. doi:10.1007/s12250-020-00207-4
Fujioka, Y., Tsuda, M., Hattori, T., Sasaki, J., Sasaki, T., Miyazaki, T., et al. (2011). The Ras-PI3K signaling pathway is involved in clathrin-independent endocytosis and the internalization of influenza viruses. PLoS One 6 (1), e16324. doi:10.1371/journal.pone.0016324
Fung, S. Y., Yuen, K. S., Ye, Z. W., Chan, C. P., and Jin, D. Y. (2020). A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: Lessons from other pathogenic viruses. Emerg. Microbes Infect. 9 (1), 558–570. doi:10.1080/22221751.2020.1736644
Garcia, G., Sharma, A., Ramaiah, A., Sen, C., Purkayastha, A., Kohn, D. B., et al. (2021). Antiviral drug screen identifies DNA-damage response inhibitor as potent blocker of SARS-CoV-2 replication. Cell Rep. 35 (1), 108940. doi:10.1016/j.celrep.2021.108940
Glowacka, I., Bertram, S., Herzog, P., Pfefferle, S., Steffen, I., Muench, M. O., et al. (2010). Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 84 (2), 1198–1205. doi:10.1128/JVI.01248-09
Gordon, D. E., Jang, G. M., Bouhaddou, M., Xu, J., Obernier, K., White, K. M., et al. (2020). A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583 (7816), 459–468. doi:10.1038/s41586-020-2286-9
Gu, J., and Korteweg, C. (2007). Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 170 (4), 1136–1147. doi:10.2353/ajpath.2007.061088
Guan, W. J., Ni, Z. Y., Hu, Y., Liang, W. H., Ou, C. Q., He, J. X., et al. (2020). Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382 (18), 1708–1720. doi:10.1056/NEJMoa2002032
Haga, S., Yamamoto, N., Nakai-Murakami, C., Osawa, Y., Tokunaga, K., Sata, T., et al. (2008). Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U. S. A. 105 (22), 7809–7814. doi:10.1073/pnas.0711241105
Hayes, J. D., and Dinkova-Kostova, A. T. (2014). The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 39 (4), 199–218. doi:10.1016/j.tibs.2014.02.002
Heaton, N. S., and Randall, G. (2011). Multifaceted roles for lipids in viral infection. Trends Microbiol. 19 (7), 368–375. doi:10.1016/j.tim.2011.03.007
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181 (2), 271–280. doi:10.1016/j.cell.2020.02.052
Hosseinzade, A., Sadeghi, O., Naghdipour Biregani, A., Soukhtehzari, S., Brandt, G. S., and Esmaillzadeh, A. (2019). Immunomodulatory effects of flavonoids: Possible induction of T CD4+ regulatory cells through suppression of mTOR pathway signaling activity. Front. Immunol. 10, 51. doi:10.3389/fimmu.2019.00051
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (10223), 497–506. doi:10.1016/S0140-6736(20)30183-5
Huang, H., Falgout, B., Takeda, K., Yamada, K. M., and Dhawan, S. (2017). Nrf2-dependent induction of innate host defense via heme oxygenase-1 inhibits Zika virus replication. Virology 503, 1–5. doi:10.1016/j.virol.2016.12.019
Imai, Y., Kuba, K., Neely, G. G., Yaghubian-Malhami, R., Perkmann, T., van Loo, G., et al. (2008). Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133 (2), 235–249. doi:10.1016/j.cell.2008.02.043
Ivanov, A. V., Smirnova, O. A., Ivanova, O. N., Masalova, O. V., Kochetkov, S. N., and Isaguliants, M. G. (2011). Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS One 6 (9), e24957. doi:10.1371/journal.pone.0024957
Izmailyan, R., Hsao, J. C., Chung, C. S., Chen, C. H., Hsu, P. W. C., Liao, C. L., et al. (2012). Integrin β1 mediates vaccinia virus entry through activation of PI3K/Akt signaling. J. Virol. 86 (12), 6677–6687. doi:10.1128/JVI.06860-11
Jackson, C. B., Farzan, M., Chen, B., and Choe, H. (2022). Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 23 (1), 3–20. doi:10.1038/s41580-021-00418-x
Jamaluddin, M., Tian, B., Boldogh, I., Garofalo, R. P., and Brasier, A. R. (2009). Respiratory syncytial virus infection induces a reactive oxygen species-MSK1-phospho-Ser-276 RelA pathway required for cytokine expression. J. Virol. 83 (20), 10605–10615. doi:10.1128/JVI.01090-09
Jensen, I. J., McGonagill, P. W., Berton, R. R., Wagner, B. A., Silva, E. E., Buettner, G. R., et al. (2021). Prolonged reactive oxygen species production following septic insult. Immunohorizons 5 (6), 477–488. doi:10.4049/immunohorizons.2100027
Jiang, Y., Scofield, V. L., Yan, M., Qiang, W., Liu, N., Reid, A. J., et al. (2006). Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium alpha-luminol (Galavit). J. Virol. 80 (9), 4557–4569. doi:10.1128/JVI.80.9.4557-4569.2006
Joung, S. M., Park, Z. Y., Rani, S., Takeuchi, O., Akira, S., and Lee, J. Y. (2011). Akt contributes to activation of the TRIF-dependent signaling pathways of TLRs by interacting with TANK-binding kinase 1. J. Immunol. 186 (1), 499–507. doi:10.4049/jimmunol.0903534
Kamel, A. S., Abdelkader, N. F., Abd El-Rahman, S. S., Emara, M., Zaki, H. F., and Khattab, M. M. (2018). Stimulation of ACE2/ANG(1-7)/mas Axis by diminazene ameliorates alzheimer's disease in the D-galactose-ovariectomized rat model: Role of PI3K/Akt pathway. Mol. Neurobiol. 55 (10), 8188–8202. doi:10.1007/s12035-018-0966-3
Kauppinen, A., Suuronen, T., Ojala, J., Kaarniranta, K., and Salminen, A. (2013). Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25 (10), 1939–1948. doi:10.1016/j.cellsig.2013.06.007
Kesic, M. J., Simmons, S. O., Bauer, R., and Jaspers, I. (2011). Nrf2 expression modifies influenza A entry and replication in nasal epithelial cells. Free Radic. Biol. Med. 51 (2), 444–453. doi:10.1016/j.freeradbiomed.2011.04.027
Khezri, M. R. (2021). PI3K/AKT signaling pathway: A possible target for adjuvant therapy in COVID-19. Hum. Cell 34 (2), 700–701. doi:10.1007/s13577-021-00484-5
Kindrachuk, J., Ork, B., Hart, B. J., Mazur, S., Holbrook, M. R., Frieman, M. B., et al. (2015). Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob. Agents Chemother. 59 (2), 1088–1099. doi:10.1128/AAC.03659-14
Kobayashi, E. H., Suzuki, T., Funayama, R., Nagashima, T., Hayashi, M., Sekine, H., et al. (2016). Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624. doi:10.1038/ncomms11624
Kosmider, B., Messier, E. M., Janssen, W. J., Nahreini, P., Wang, J., Hartshorn, K. L., et al. (2012). Nrf2 protects human alveolar epithelial cells against injury induced by influenza A virus. Respir. Res. 13 (1), 43. doi:10.1186/1465-9921-13-43
Ksiazek, T. G., Erdman, D., Goldsmith, C. S., Zaki, S. R., Peret, T., Emery, S., et al. (2003). A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348 (20), 1953–1966. doi:10.1056/NEJMoa030781
Kulkarni, M. M., Ratcliff, A. N., Bhat, M., Alwarawrah, Y., Hughes, P., Arcos, J., et al. (2017). Cellular fatty acid synthase is required for late stages of HIV-1 replication. Retrovirology 14 (1), 45. doi:10.1186/s12977-017-0368-z
Laird, M. H., Rhee, S. H., Perkins, D. J., Medvedev, A. E., Piao, W., Fenton, M. J., et al. (2009). TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J. Leukoc. Biol. 85 (6), 966–977. doi:10.1189/jlb.1208763
Lee, C. (2018). Therapeutic modulation of virus-induced oxidative stress via the nrf2-dependent antioxidative pathway. Oxid. Med. Cell Longev. 2018, 6208067. doi:10.1155/2018/6208067
Lekshmi, V, S., Rauf, A. A., and Kurup, G. M. (2019). Sulfated polysaccharides from the edible marine algae Padina tetrastromatica attenuates isoproterenol-induced oxidative damage via activation of PI3K/Akt/Nrf2 signaling pathway - an in vitro and in vivo approach. Chem. Biol. Interact. 308, 258–268. doi:10.1016/j.cbi.2019.05.044
Li, F., Li, J., Wang, P. H., Yang, N., Huang, J., Ou, J., et al. (2021). SARS-CoV-2 spike promotes inflammation and apoptosis through autophagy by ROS-suppressed PI3K/AKT/mTOR signaling. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1867 (12), 166260. doi:10.1016/j.bbadis.2021.166260
Li, S., Zhang, Y., Guan, Z., Li, H., Ye, M., Chen, X., et al. (2020). SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct. Target Ther. 5 (1), 235. doi:10.1038/s41392-020-00334-0
Li, Y., Webster-Cyriaque, J., Tomlinson, C. C., Yohe, M., and Kenney, S. (2004). Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression. J. Virol. 78 (8), 4197–4206. doi:10.1128/jvi.78.8.4197-4206.2004
Lin, T., Luo, W., Li, Z., Zhang, L., Zheng, X., Mai, L., et al. (2020). Rhamnocitrin extracted from Nervilia fordii inhibited vascular endothelial activation via miR-185/STIM-1/SOCE/NFATc3. Phytomedicine 79, 153350. doi:10.1016/j.phymed.2020.153350
Liskova, A., Koklesova, L., Samec, M., Smejkal, K., Samuel, S. M., Varghese, E., et al. (2020). Flavonoids in cancer metastasis. Cancers (Basel) 12 (6), 1498. doi:10.3390/cancers12061498
Liskova, A., Samec, M., Koklesova, L., Samuel, S. M., Zhai, K., Al-Ishaq, R. K., et al. (2021). Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 138, 111430. doi:10.1016/j.biopha.2021.111430
Liu, D. D., Han, C. C., Wan, H. F., He, F., Xu, H. Y., Wei, S. H., et al. (2016). Effects of inhibiting PI3K-Akt-mTOR pathway on lipid metabolism homeostasis in goose primary hepatocytes. Animal 10 (8), 1319–1327. doi:10.1017/S1751731116000380
Liu, Z., Li, J., Lin, S., Wu, Y., He, D., and Qu, P. (2021). PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp. Anim. 70 (4), 488–497. doi:10.1538/expanim.21-0002
Lu, R., Zhao, X., Li, J., Niu, P., Yang, B., Wu, H., et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 395 (10224), 565–574. doi:10.1016/S0140-6736(20)30251-8
Lu, Y., Liu, D. X., and Tam, J. P. (2008). Lipid rafts are involved in SARS-CoV entry into Vero E6 cells. Biochem. Biophys. Res. Commun. 369 (2), 344–349. doi:10.1016/j.bbrc.2008.02.023
Mariano, G., Farthing, R. J., Lale-Farjat, S. L. M., and Bergeron, J. R. C. (2020). Structural characterization of SARS-CoV-2: Where we are, and where we need to Be. Front. Mol. Biosci. 7, 605236. doi:10.3389/fmolb.2020.605236
Martinez, I., García-Carpizo, V., Guijarro, T., García-Gomez, A., Navarro, D., Aranda, A., et al. (2016). Induction of DNA double-strand breaks and cellular senescence by human respiratory syncytial virus. Virulence 7 (4), 427–442. doi:10.1080/21505594.2016.1144001
McBride, R., van Zyl, M., and Fielding, B. C. (2014). The coronavirus nucleocapsid is a multifunctional protein. Viruses 6 (8), 2991–3018. doi:10.3390/v6082991
Mizutani, T., Fukushi, S., Saijo, M., Kurane, I., and Morikawa, S. (2005). JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1741 (1), 4–10. doi:10.1016/j.bbadis.2005.04.004
Muller, C., Hardt, M., Schwudke, D., Neuman, B. W., Pleschka, S., and Ziebuhr, J. (2018). Inhibition of cytosolic phospholipase A2α impairs an early step of coronavirus replication in cell culture. J. Virol. 92 (4), e01463. doi:10.1128/JVI.01463-17
Neuman, B. W., Kiss, G., Kunding, A. H., Bhella, D., Baksh, M. F., Connelly, S., et al. (2011). A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 174 (1), 11–22. doi:10.1016/j.jsb.2010.11.021
Ngwa, W., Kumar, R., Thompson, D., Lyerly, W., Moore, R., Reid, T. E., et al. (2020). Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules 25 (11), 2707. doi:10.3390/molecules25112707
Nile, S. H., Nile, A., Qiu, J., Li, L., Jia, X., and Kai, G. (2020). COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth Factor Rev. 53, 66–70. doi:10.1016/j.cytogfr.2020.05.002
Ohol, Y. M., Wang, Z., Kemble, G., and Duke, G. (2015). Direct inhibition of cellular fatty acid synthase impairs replication of respiratory syncytial virus and other respiratory viruses. PLoS One 10 (12), e0144648. doi:10.1371/journal.pone.0144648
Olagnier, D., Farahani, E., Thyrsted, J., Blay-Cadanet, J., Herengt, A., Idorn, M., et al. (2020). SARS-CoV2-mediated suppression of NRF2-signaling reveals potent antiviral and anti-inflammatory activity of 4-octyl-itaconate and dimethyl fumarate. Nat. Commun. 11 (1), 4938. doi:10.1038/s41467-020-18764-3
Owen, D. R., Allerton, C. M. N., Anderson, A. S., Aschenbrenner, L., Avery, M., Berritt, S., et al. (2021). An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 374 (6575), 1586–1593. doi:10.1126/science.abl4784
Pervushin, K., Tan, E., Parthasarathy, K., Lin, X., Jiang, F. L., Yu, D., et al. (2009). Structure and inhibition of the SARS coronavirus envelope protein ion channel. PLoS Pathog. 5 (7), e1000511. doi:10.1371/journal.ppat.1000511
Qu, Y., Haas de Mello, A., Morris, D. R., Jones-Hall, Y. L., Ivanciuc, T., Sattler, R. A., et al. (2023). SARS-CoV-2 inhibits NRF2-mediated antioxidant responses in airway epithelial cells and in the lung of a murine model of infection. Microbiol. Spectr. 2023, e0037823. doi:10.1128/spectrum.00378-23
Reddy, P. V., Gandhi, N., Samikkannu, T., Saiyed, Z., Agudelo, M., Yndart, A., et al. (2012). HIV-1 gp120 induces antioxidant response element-mediated expression in primary astrocytes: Role in HIV associated neurocognitive disorder. Neurochem. Int. 61 (5), 807–814. doi:10.1016/j.neuint.2011.06.011
Saeed, M. F., Kolokoltsov, A. A., Freiberg, A. N., Holbrook, M. R., and Davey, R. A. (2008). Phosphoinositide-3 kinase-akt pathway controls cellular entry of ebola virus. PLOS Pathog. 4 (8), e1000141. doi:10.1371/journal.ppat.1000141
Salaverry, L. S., Parrado, A. C., Mangone, F. M., Dobrecky, C. B., Flor, S. A., Lombardo, T., et al. (2020). In vitro anti-inflammatory properties of Smilax campestris aqueous extract in human macrophages, and characterization of its flavonoid profile. J. Ethnopharmacol. 247, 112282. doi:10.1016/j.jep.2019.112282
Sarkar, S. N., Peters, K. L., Elco, C. P., Sakamoto, S., Pal, S., and Sen, G. C. (2004). Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 11 (11), 1060–1067. doi:10.1038/nsmb847
Sassi, A., Mokdad Bzéouich, I., Mustapha, N., Maatouk, M., Ghedira, K., and Chekir-Ghedira, L. (2017). Immunomodulatory potential of hesperetin and chrysin through the cellular and humoral response. Eur. J. Pharmacol. 812, 91–96. doi:10.1016/j.ejphar.2017.07.017
Schabbauer, G., Luyendyk, J., Crozat, K., Jiang, Z., Mackman, N., Bahram, S., et al. (2008). TLR4/CD14-mediated PI3K activation is an essential component of interferon-dependent VSV resistance in macrophages. Mol. Immunol. 45 (10), 2790–2796. doi:10.1016/j.molimm.2008.02.001
Schachtele, S. J., Hu, S., and Lokensgard, J. R. (2012). Modulation of experimental Herpes encephalitis-associated neurotoxicity through sulforaphane treatment. PLOS ONE 7 (4), e36216. doi:10.1371/journal.pone.0036216
Schoeman, D., and Fielding, B. C. (2019). Coronavirus envelope protein: Current knowledge. Virol. J. 16 (1), 69. doi:10.1186/s12985-019-1182-0
Shan, X., Liu, Z., Wulasihan, M., and Ma, S. (2019). Edoxaban improves atrial fibrillation and thromboembolism through regulation of the Wnt-β-induced PI3K/ATK-activated protein C system. Exp. Ther. Med. 17 (5), 3509–3517. doi:10.3892/etm.2019.7379
Sharma, A., Bhomia, M., Yeh, T. J., Singh, J., and Maheshwari, R. K. (2018). Miltefosine inhibits Chikungunya virus replication in human primary dermal fibroblasts. F1000Res. 7, 9. doi:10.12688/f1000research.13242.1
Shimada, K., Furukawa, H., Wada, K., Wei, Y., Tada, Y., Kuwabara, A., et al. (2015). Angiotensin-(1-7) protects against the development of aneurysmal subarachnoid hemorrhage in mice. J. Cereb. Blood Flow. Metab. 35 (7), 1163–1168. doi:10.1038/jcbfm.2015.30
Shou, J., Wang, M., Cheng, X., Wang, X., Zhang, L., Liu, Y., et al. (2020). Tizoxanide induces autophagy by inhibiting PI3K/Akt/mTOR pathway in RAW264.7 macrophage cells. Archives Pharmacal Res. 43 (2), 257–270. doi:10.1007/s12272-019-01202-4
Simon, P. F., McCorrister, S., Hu, P., Chong, P., Silaghi, A., Westmacott, G., et al. (2015). Highly pathogenic H5N1 and novel H7N9 influenza A viruses induce more profound proteomic host responses than seasonal and pandemic H1N1 strains. J. Proteome Res. 14 (11), 4511–4523. doi:10.1021/acs.jproteome.5b00196
Soares, J. A., Leite, F. G. G., Andrade, L. G., Torres, A. A., De Sousa, L. P., Barcelos, L. S., et al. (2009). Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J. Virol. 83 (13), 6883–6899. doi:10.1128/JVI.00245-09
Song, J. W., Zhang, C., Fan, X., Meng, F. P., Xu, Z., Xia, P., et al. (2020). Immunological and inflammatory profiles in mild and severe cases of COVID-19. Nat. Commun. 11 (1), 3410. doi:10.1038/s41467-020-17240-2
Stertz, S., Reichelt, M., Spiegel, M., Kuri, T., Martínez-Sobrido, L., García-Sastre, A., et al. (2007). The intracellular sites of early replication and budding of SARS-coronavirus. Virology 361 (2), 304–315. doi:10.1016/j.virol.2006.11.027
Sun, F., Mu, C., Kwok, H. F., Xu, J., Wu, Y., Liu, W., et al. (2021). Capivasertib restricts SARS-CoV-2 cellular entry: A potential clinical application for COVID-19. Int. J. Biol. Sci. 17 (9), 2348–2355. doi:10.7150/ijbs.57810
Sun, Q., Ye, F., Liang, H., Liu, H., Lu, R., et al. (2021). Bardoxolone and bardoxolone methyl, two Nrf2 activators in clinical trials, inhibit SARS-CoV-2 replication and its 3C-like protease. Signal Transduct. Target Ther. 6 (1), 212. doi:10.1038/s41392-021-00628-x
Sundar, S., and Olliaro, P. L. (2007). Miltefosine in the treatment of leishmaniasis: Clinical evidence for informed clinical risk management. Ther. Clin. Risk Manag. 3 (5), 733–740.
Talukdar, J., Bhadra, B., Dattaroy, T., Nagle, V., and Dasgupta, S. (2020). Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed. Pharmacother. 132, 110886. doi:10.1016/j.biopha.2020.110886
Tang, Y., Sun, J., Pan, H., Yao, F., Yuan, Y., Zeng, M., et al. (2021). Aberrant cytokine expression in COVID-19 patients: Associations between cytokines and disease severity. Cytokine 143, 155523. doi:10.1016/j.cyto.2021.155523
Team, C. C.-R. (2020). Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, february 12-march 28, 2020. MMWR Morb. Mortal. Wkly. Rep. 69 (13), 382–386. doi:10.15585/mmwr.mm6913e2
Tebay, L. E., Robertson, H., Durant, S. T., Vitale, S. R., Penning, T. M., Dinkova-Kostova, A. T., et al. (2015). Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 88, 108–146. doi:10.1016/j.freeradbiomed.2015.06.021
Teijaro, J. R. (2017). Cytokine storms in infectious diseases. Semin. Immunopathol. 39 (5), 501–503. doi:10.1007/s00281-017-0640-2
Tsoi, H., Chen, Z. S., Lau, K. F., Tsui, S. K. W., and Chan, H. Y. E. (2014). The SARS-coronavirus membrane protein induces apoptosis via interfering with PDK1-PKB/Akt signalling. Biochem. J. 464 (3), 439–447. doi:10.1042/BJ20131461
Tu, W., Wang, H., Li, S., Liu, Q., and Sha, H. (2019). The anti-inflammatory and anti-oxidant mechanisms of the keap1/nrf2/ARE signaling pathway in chronic diseases. Aging Dis. 10 (3), 637–651. doi:10.14336/AD.2018.0513
Vorobjeva, N., Prikhodko, A., Galkin, I., Pletjushkina, O., Zinovkin, R., Sud'ina, G., et al. (2017). Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 96 (3), 254–265. doi:10.1016/j.ejcb.2017.03.003
Wang, K., Chen, W., Zhang, Z., Deng, Y., Lian, J. Q., Du, P., et al. (2020). CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 5 (1), 283. doi:10.1038/s41392-020-00426-x
Wei, L., Zhu, S., Wang, J., and Liu, J. (2012). Activation of the phosphatidylinositol 3-kinase/Akt signaling pathway during porcine circovirus type 2 infection facilitates cell survival and viral replication. J. Virol. 86 (24), 13589–13597. doi:10.1128/JVI.01697-12
Wu, Z., and McGoogan, J. M. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323 (13), 1239–1242. doi:10.1001/jama.2020.2648
Yang, W., Hood, B. L., Chadwick, S. L., Liu, S., Watkins, S. C., Luo, G., et al. (2008). Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology 48 (5), 1396–1403. doi:10.1002/hep.22508
Ye, J., and DeBose-Boyd, R. A. (2011). Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb. Perspect. Biol. 3 (7), a004754. doi:10.1101/cshperspect.a004754
Yeon, S. H., Song, M. J., Kang, H. R., and Lee, J. Y. (2015). Phosphatidylinositol-3-kinase and Akt are required for RIG-I-mediated anti-viral signalling through cross-talk with IPS-1. Immunology 144 (2), 312–320. doi:10.1111/imm.12373
Zaki, A. M., van Boheemen, S., Bestebroer, T. M., Osterhaus, A. D. M. E., and Fouchier, R. A. M. (2012). Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 367 (19), 1814–1820. doi:10.1056/NEJMoa1211721
Zhang, H. S., Wu, T. C., Sang, W. W., and Ruan, Z. (2012). EGCG inhibits tat-induced LTR transactivation: Role of Nrf2, AKT, AMPK signaling pathway. Life Sci. 90 (19-20), 747–754. doi:10.1016/j.lfs.2012.03.013
Zhang, S., Wang, J., Wang, L., Aliyari, S., and Cheng, G. (2022). SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol. Immunol. 19 (8), 872–882. doi:10.1038/s41423-022-00887-w
Zheng, M., Karki, R., Williams, E. P., Yang, D., Fitzpatrick, E., Vogel, P., et al. (2021). TLR2 senses the SARS-CoV-2 envelope protein to produce inflammatory cytokines. Nat. Immunol. 22 (7), 829–838. doi:10.1038/s41590-021-00937-x
Zhu, Y., Chen, X., and Liu, X. (2022). NETosis and neutrophil extracellular traps in COVID-19: Immunothrombosis and beyond. Front. Immunol. 13, 838011. doi:10.3389/fimmu.2022.838011
Zhu, Z., Wilson, A. T., Luxon, B. A., Brown, K. E., Mathahs, M. M., Bandyopadhyay, S., et al. (2010). Biliverdin inhibits hepatitis C virus nonstructural 3/4A protease activity: Mechanism for the antiviral effects of heme oxygenase? Hepatology 52 (6), 1897–1905. doi:10.1002/hep.23921
Keywords: Coronavirus, SARS-CoV-2, pandemic, COVID-19, Nrf2, oxidative stress, inflammation, PI3K/Akt pathway
Citation: Lekshmi VS, Asha K, Sanicas M, Asi A, Arya UM and Kumar B (2023) PI3K/Akt/Nrf2 mediated cellular signaling and virus-host interactions: latest updates on the potential therapeutic management of SARS-CoV-2 infection. Front. Mol. Biosci. 10:1158133. doi: 10.3389/fmolb.2023.1158133
Received: 03 February 2023; Accepted: 22 May 2023;
Published: 01 June 2023.
Edited by:
Rana Jahanban-Esfahlan, Tabriz University of Medical Sciences, IranReviewed by:
Shashikant Ray, Mahatma Gandhi Central University, IndiaShweta Jakhmola, University of California, San Diego, United States
Copyright © 2023 Lekshmi, Asha, Sanicas, Asi, Arya and Kumar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: V. S. Lekshmi, bHZzdmFuYWphQGdtYWlsLmNvbQ==; Binod Kumar, Ymlub2RfYmlvY2hlbUByZWRpZmZtYWlsLmNvbQ==
 V. S. Lekshmi
V. S. Lekshmi Kumari Asha
Kumari Asha Melvin Sanicas
Melvin Sanicas Abhila Asi
Abhila Asi U. M. Arya
U. M. Arya Binod Kumar
Binod Kumar