
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Biosci., 07 February 2023
Sec. Protein Folding, Misfolding and Degradation
Volume 10 - 2023 | https://doi.org/10.3389/fmolb.2023.1142989
This article is part of the Research TopicGuardians of Protein Homeostasis (Proteostasis) in Health, Disease and AgingView all 6 articles
 Hemmo Meyer*
Hemmo Meyer* Johannes van den Boom
Johannes van den BoomThe AAA+ ATPase p97 (also called VCP or Cdc48) is a major protein unfolding machine with hundreds of clients in diverse cellular pathways that are critical for cell homeostasis, proliferation and signaling. In this review, we summarize recent advances in understanding how diverse client proteins are targeted to the p97 machine to facilitate client degradation or to strip clients from binding partners for regulation. We describe an elaborate system that is governed by at least two types of alternative adapters. The Ufd1-Npl4 adapter along with accessory adapters targets ubiquitylated clients in the majority of pathways and uses ubiquitin as a universal unfolding tag. In contrast, the family of SEP-domain adapters such as p37 can target clients directly to p97 in a ubiquitin-independent manner. Despite the different targeting strategies, both pathways converge by inserting the client into the p97 pore to initiate a peptide threading mechanism through the central channel of p97 that drives client protein unfolding, protein extraction from membranes and protein complex disassembly processes.
Proteins need to fold into a three-dimensional structure to become functional. Conversely a large fraction of these proteins have to be actively unfolded at least once in their lifetime for regulation or to assist their degradation. Protein unfolding is usually mediated by a family of ATPases Associated with diverse Activities (AAA) proteins (Erzberger and Berger, 2006; Khan et al., 2022). The most abundant and functionally versatile AAA protein is p97 (also called VCP or Cdc48). p97 has a key role in the ubiquitin-proteasome system and thus for protein homeostasis as it processes misfolded and ubiquitylated proteins to prepare them for degradation in the 26S proteasome in diverse pathways including ER-associated degradation (van den Boom and Meyer, 2018; Stach and Freemont, 2017; Ye et al., 2017). Moreover, p97 helps degradation of regulatory proteins for terminal inactivation in a number of signaling pathways. While the 26S proteasome has its own AAA protein ring, p97 is needed for clients that require extraction from membranes or partner proteins, but also for a subpopulation of monomeric proteins to provide unfolded peptide stretches that are essential for processing by the proteasome (Beskow et al., 2009; van den Boom and Meyer, 2018; Olszewski et al., 2019). In addition to its degradative function, p97 mediates regulatory unfolding and protein complex disassembly for example during protein phosphatase-1 (PP1) biogenesis (Weith et al., 2018). Missense mutations in p97 cause a multisystem proteinopathy (MSP-1) with features such as inclusion body myopathy, Paget’s disease of bone, frontotemporal dementia and amyotrophic lateral sclerosis (Al-Obeidi et al., 2018; Watts et al., 2004; Meyer and Weihl, 2014). Conversely, p97 is considered a promising cancer drug target (Anderson et al., 2015; Roux et al., 2021).
p97 has two AAA domains, D1 and D2 that form two stacked hexameric rings with a central channel (Figures 1A, B). The regulatory N-terminal domain is positioned at the periphery of the D1 ring and can be in a up or down position. Recent structural and biochemical work has revealed that client proteins are unfolded, or stripped from partners or membranes, by inserting them into the D1 pore and threading them through the central channel followed by ejection from the D2 pore (Bodnar and Rapoport, 2017; Weith et al., 2018; van den Boom et al., 2021). The active hexamer is in a stair-case configuration that allows a client threading mechanism that is common to many AAA unfoldases (Cooney et al., 2019; Twomey et al., 2019; Stach et al., 2020; Pan et al., 2021a; van den Boom et al., 2022; Xu et al., 2022). Hydrophobic residues in the pore loops engage in non-sequence-specific interactions with the client peptide backbone (Figure 1C). Threading is driven by a hand-over-hand mechanism in which ATP hydrolysis in the subunit at the bottom of the p97 spiral results in a discontinuity as it triggers the detachment of this subunit from the spiral and induces its reattachment to the client peptide at the top. Thus, the peptide is pulled through the pore with the progression of nucleotide binding and hydrolysis around the ring.

FIGURE 1. Structure and client threading mechanism of p97. (A) Domain structure of p97. Each p97 subunit comprises an N-terminal domain and two AAA ATPase domains, D1 and D2. (B) Cartoon representation of the p97 hexamer in staircase configuration around the central channel. (C) Hand-over-hand mechanism for client threading by p97. When the client protein (yellow) is inserted into the central channel of p97, hydrophobic pore loops of D1 and D2 interact with the client backbone. Each subunit binds two residues below the previous subunit (in clockwise direction) which sets the p97 hexamer in the right-handed staircase configuration. ATP hydrolysis in the lowermost subunit (orange) triggers the retraction of its pore loops and disengagement of the subunit (middle panel) creating a seam in the hexamer. Subsequent ADP release and re-binding of ATP induces re-engagement of this subunit as the topmost subunit (right panel). Propagation of ATP hydrolysis and up-movement of subunits around the hexamer in counterclockwise direction threads the client through the channel.
To achieve its versatility, p97 is assisted by a host of cofactor proteins that directly bind to p97 through dedicated interaction motifs and domains (Meyer and Weihl, 2014; Buchberger et al., 2015; Stach and Freemont, 2017). These cofactor proteins include client adapters, accessory adapters, membrane recruitment factors and client processing enzymes. A large body of cell biological and genetic data is available on the functions of these cofactors in the cell. Direct mechanistic analysis, however, has only become possible recently with the reconstitution of client unfolding in vitro. Key technical advances were the efficient in vitro ubiquitylation of a model client for ubiquitin-directed targeting and the discovery of a PP1 complex as a ubiquitin-independent client of p97 (Blythe et al., 2017; Bodnar and Rapoport, 2017; Weith et al., 2018; Mukherjee and Labib, 2019). This was combined with the use of GFP and later Eos fused to the clients, as well as the development of a FRET assay that monitor the unfolding or disassembly of clients, respectively, in real time (Blythe et al., 2017; Bodnar and Rapoport, 2017; Weith et al., 2018; Olszewski et al., 2019; van den Boom et al., 2021). Insertion and threading of clients in the p97 channel has been observed by cryo-EM, but can also be demonstrated by genetically encoded photocrosslinkers placed in the p97 pore loops and other critical positions (Bodnar and Rapoport, 2017; Weith et al., 2018). Thus, the field now profits from a powerful biochemical toolbox for dissecting and understanding the mechanism of client targeting to p97.
In this article, we will therefore focus on factors that have a biochemically proven function in the targeting of client proteins to the p97 channel and will only discuss a few additional candidates. For a more comprehensive discussion of p97 cofactors, we refer the reader to previous reviews (Meyer and Weihl, 2014; Buchberger et al., 2015; Stach and Freemont, 2017).
An important question has been how client proteins are recruited to p97 and, importantly, guided into the D1 pore to initiate client peptide threading. The molecular challenge is on the one hand to target a large diversity of clients for degradative unfolding, and on the other hand be selective for a direct target while sparing binding partners during regulatory protein complex disassembly. Early work had already suggested that p97 is directed by alternative, mutually exclusive client adapter proteins: the heterodimer Ufd1-Npl4 or members of a family of SEP-domain adapters (Figure 2) (Kondo et al., 1997; Meyer et al., 2000; Buchberger, 2022). Although structurally very different, both types of adapters bind p97 through a bipartite interaction mechanism. Ufd1-Npl4 appears to handle the majority of ubiquitin-modified clients, often to facilitate degradation in the proteasome. Ufd1-Npl4 is therefore crucial for pathways such as ER-associated degradation or ribosomal quality control as well as DNA-associated processes such as DNA repair and replication. In contrast, SEP-domain adapters such as p37 can mediate a selective, ubiquitin-independent targeting mechanism that discriminates a direct target in protein complex disassembly. With about 230,000 p97 hexamers and 70,000 copies of Ufd1-Npl4 in an exemplary human cancer cell (Beck et al., 2011), roughly one third of the p97 hexamers could be equipped with Ufd1-Npl4 at a time. In the same cell, more than 285,000 copies of SEP domain adapters exist (Figure 4A). However, not all p97 hexamers are thought to be stably occupied by adapters (Xue et al., 2016).
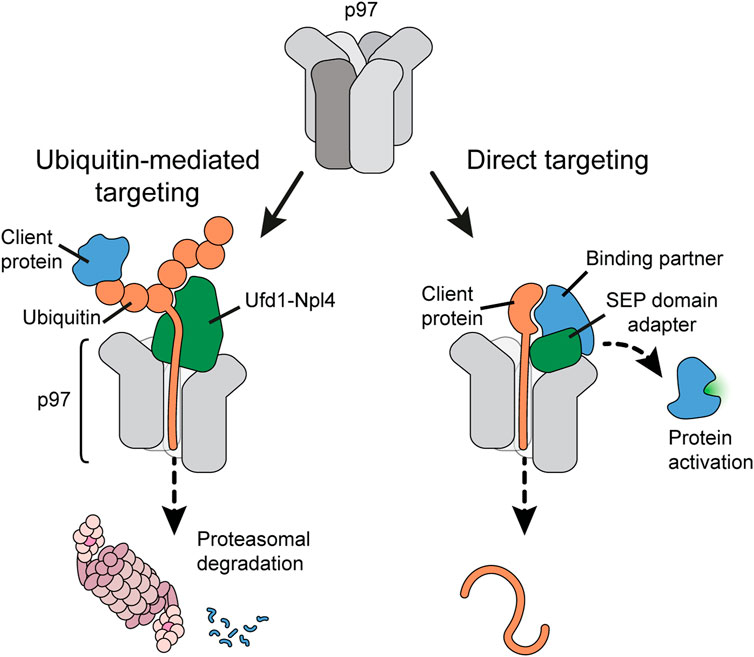
FIGURE 2. Client targeting pathways to p97. p97 uses (at least) two alternative strategies for client protein targeting. Ubiquitin-mediated targeting (left panel) recruits ubiquitylated client proteins to p97 using ubiquitin as a universal targeting tag. Ubiquitin chains attached to the client are bound by the Ufd1-Npl4 adapter. One ubiquitin is then melted and inserted by the N-terminus into the p97 pore. Continuous threading of the ubiquitin chain eventually leads to threading and unfolding of the client protein, often for subsequent degradation in the proteasome. In contrast, direct client targeting (right panel) uses adapter proteins of the SEP domain family to guide a p97 targeting region within the client into the p97 pore. Pulling the client protein through the p97 pore dislodges the protein from its binding partner, which can result in activation of the binding partner.
The Ufd1-Npl4 adapter is a heterodimer that cooperatively binds p97 through a SHP box in Ufd1 and a UBX-like domain in Npl4 (Figure 3A) (Bruderer et al., 2004). Npl4 does not bind p97 in the absence of Ufd1, and Ufd1 is destabilized upon Npl4 depletion suggesting that they always act together (Meyer et al., 2000; Wojcik et al., 2004). Recent cryo-EM structures of Ufd1-Npl4 with p97 and a ubiquitylated client protein revealed how Ufd1-Npl4 recruits clients and initiates their threading through the p97 channel (Twomey et al., 2019; Pan et al., 2021a; Pan et al., 2021b); (Figure 3B). Npl4 forms a tower above the D1 pore with two zinc binding domains resting on the D1 ring and the UBX-like domain interacting with one N-domain of p97 (Figures 3B, C). Ufd1 is poorly resolved in the published structures, but it binds to the N-domain of p97, opposite to the Npl4-bound N-domain, thereby positioning the Npl4 tower above the p97 pore.
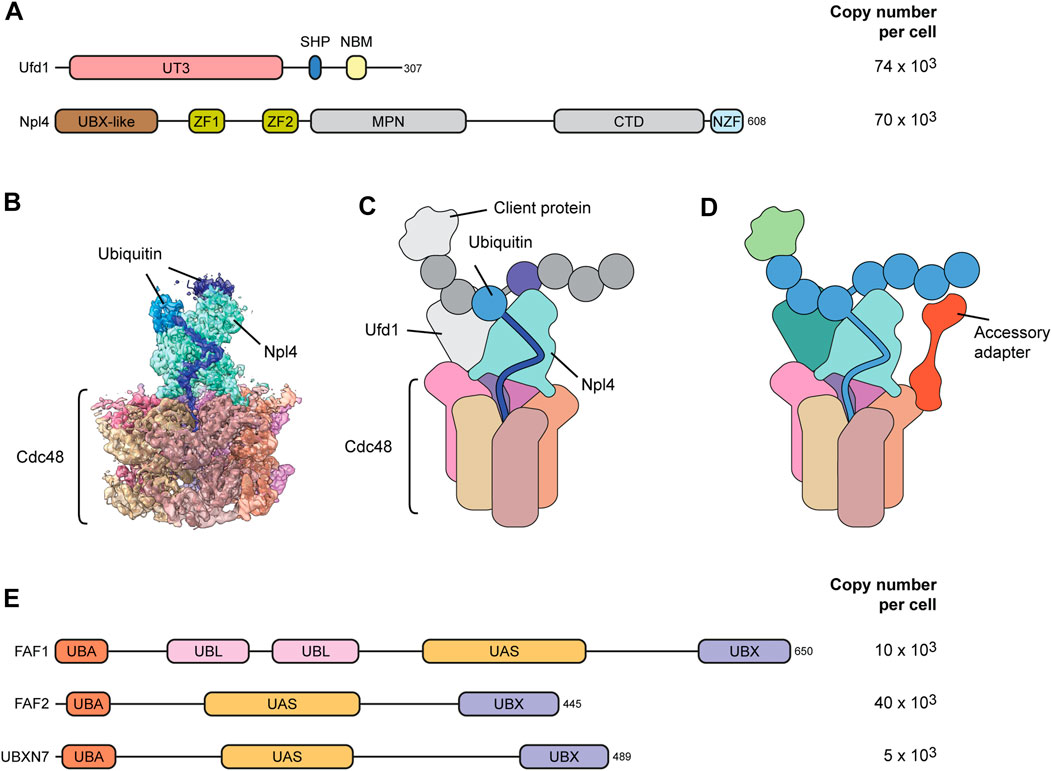
FIGURE 3. Adapter and accessory adapters for ubiqutin-mediated targeting. (A) Domain structure of the major Ufd1-Npl4 client adapter. Ufd1-Npl4 is a heterodimer that binds p97 cooperatively via interactions of a UBX-like (UBXL) domain in Npl4 and a SHP box in Ufd1 with p97 N-domains. The zinc fingers (ZF1 and ZF2) in Npl4 make contact with the top of the D1 ring. The UT3 domain in Ufd1 and the C-terminal domain (CTD) in Npl4 bind and position the ubiquitin chain. The ubiquitin-binding NZF is specific for metazoan Npl4. NBM, Npl4 binding motif. MPN, Mpr1, and Pad1 N-terminal domain. Copy numbers of indicated proteins compared to roughly 230 × 103 p97 hexamers in human U2OS osteosarcoma cells according to (Beck et al., 2011). (B) Cryo-EM structure (pdb 6OA9 and EMD-0665) of yeast Cdc48-Ufd1-Npl4 with a ubiquitinated model substrate in the central pore. Dark blue depicts one unfolded ubiquitin moiety bound to a groove in Npl4 (cyan). Additional ubiquitin moieties are bound on top. (C) Cartoon model of the structure in (B). Ufd1, the client and additional ubiquitin moieties are not resolved in the structure. (D) Speculative model for the function of accessory adapters such as FAF1, FAF2 or UBXN7. Accessory adapters enhance p97 affinity for the client by bridging p97 with the ubiquitin chain attached to the client, thereby assisting Ufd1-Npl4-mediated client targeting and unfolding. (E) Domain structure of p97 cofactor proteins that have been proven to act as accessory adapters cooperating with Ufd1-Npl4. They are characterized by UBX and UBA domains that bind p97 and ubiquitin, respectively, and possess a thioredoxin-like (UAS) domain of yet unknown function. UBL, ubiquitin-like domain.
Rather than binding the client directly, Npl4 interacts with the ubiquitin chain attached to the client (Twomey et al., 2019; Pan et al., 2021a; Sato et al., 2019; Pan et al., 2021b); (Figures 3B, C). The interaction is mediated by several moieties of the ubiquitin chain binding the top of the Npl4 tower. Importantly, one of the ubiquitin moieties is unfolded by extending into a groove in Npl4 that guides the elongated peptide stretch towards the p97 D1 pore and inserts it into the p97 channel (Twomey et al., 2019). p97 thereby first threads the ubiquitin chain and subsequently pulls in the attached client leading to unfolding of the client. This elegant strategy uses the ubiquitin modification as a universal unfolding tag that is independent of the client protein which it is conjugated to. This is very different from the regulatory particle of the proteasome which recruits the client through binding of the attached ubiquitin chain, but then requires an unfolded stretch of the client to insert the client directly into the AAA ring for unfolding while the ubiquitin chain is clipped off (Greene et al., 2020). This difference explains why some clients that do not have an unfolded peptide stretch require prior unfolding by p97 to facilitate processing and degradation by the proteasome (Beskow et al., 2009; Olszewski et al., 2019).
Starting the pulling on one of the ubiquitin moieties, however, also entails that the threading machine will soon encounter the branch point within ubiquitin chains and eventually the attachment site of the ubiquitin to the client (Twomey et al., 2019). Initial data indicated a requirement of a deubiquitinating enzyme that removes at least the distal parts of the ubiquitin chain (Bodnar and Rapoport, 2017). However, unfolding can occur in the absence of deubiquitinating enzymes (Olszewski et al., 2019) suggesting that p97 needs to be able to thread branch points and loops as shown by Ji and collegues (Ji et al., 2021). In support of that, independent data indicate that p97 can transport peptide loops (see below) and even peptides attached to oligonucleotides indicating a degree of plasticity regarding the structures that are threaded in the p97 channel (van den Boom et al., 2021; Kroning et al., 2022). An interesting question remains whether p97 can pull on only one strand when threading a loop, or on both strands as shown for the related AAA protein ClpB (Avellaneda et al., 2020). The function of Ufd1 in client targeting has not been uncovered yet. Intriguingly the globular UT3 domain of Ufd1 has the same fold as the p97 N-domain suggesting it could assist binding or unfolding the client.
In vitro, the Ufd1-Npl4 adapter is sufficient to trigger p97-driven unfolding of ubiquitylated clients. However, a growing body of evidence has been indicating that Ufd1-Npl4 cooperates with a family of accessory adapters comprising FAF1, FAF2 and UBXN7 during client targeting in the cell (Figures 3D, E). These accessory adapters are less abundant than Ufd1-Npl4 (Figure 3E) suggesting that they cooperate with Ufd1-Npl4 as needed. Like many p97 cofactors they contain a ubiquitin-binding UBA and a p97-interacting UBX domain at the N and C-terminus, respectively. In addition, the accessory adapters are characterized by a thioredoxin-like UAS domain of yet unknown function. Early data showed that yeast Ubx2 and its mammalian counterpart FAF2 (also called UBXD8) serves as targeting factor during p97-Ufd1-Npl4 mediated ER-associated degradation (Neuber et al., 2005; Schuberth and Buchberger, 2005). Of note, Ubx2 and FAF2 insert into the cytosolic leaflet of the ER membrane via a hydrophobic hairpin loop and can also partition into the mitochondrial membrane where it assists p97-Ufd1-Npl4 in mitochondria-associated degradation (Metzger et al., 2020). In contrast, FAF1 does not have a yeast orthologue. FAF1 (UBXN-3 in C. elegans) is distributed in both the cytosol and nucleoplasm but has functionally been mostly associated with various DNA-associated roles of p97-Ufd1-Npl4 such as degradation of the licensing factor CDT1 or extraction of topologically trapped DNA repair factor KU70/80 extraction (Franz et al., 2016; van den Boom et al., 2016). The third member, UBXN7 (Ubx5 in yeast), is localized exclusively in the nucleus and has been linked to various chromatin-associated functions of p97 (Alexandru et al., 2008; Verma et al., 2011; Puumalainen et al., 2014; Chauhan et al., 2021). Both FAF1 and UBXN7 have been connected to the extraction of the replicative helicase from DNA (Sonneville et al., 2017; Xia et al., 2021; Kochenova et al., 2022). The replicative helicase forms a ring consisting of the AAA proteins MCM2-7 and is assembled tightly around DNA during replication origin licensing. Consequently, the MCM2-7 ring needs to be actively dissociated at termination of replication or when the helicase encounters obstacles such at interstrand crosslinks. Disassembly is triggered by ubiquitylation of MCM7 which is then targeted and extracted by p97 and Ufd1-Npl4 leading to destabilization of the whole complex. Crucially, UBXN7 assists p97-Ufd1-Npl4-mediated extraction of the helicase (Sonneville et al., 2017; Xia et al., 2021; Kochenova et al., 2022). FAF1 can compensate for UBXN7, although this is controversial (Fujisawa et al., 2022; Tarcan et al., 2022).
Recent advances in reconstituting MCM2-7 disassembly from pure components have confirmed the involvement of the accessory adapters and brought more clarity in the molecular basis (Fujisawa et al., 2022). p97-Ufd1-Npl4 suffices to disassemble ubiquitylated MCM2-7 but only if the ubiquitin chains are very long. If ubiquitin chains are shorter with at least five ubiquitin moieties, p97-Ufd1-Npl4 is much less efficient but can be stimulated by any of the three factors. Surprisingly, truncation analysis revealed that the stimulatory effect does not depend on the UBA domain, at least for FAF1. In fact, apart from the UBX domain in FAF1, a helical domain located between the UAS and UBX domains was sufficient to stimulate disassembly of complexes modified with short ubiquitin chains. So far, it is unclear what this region binds to in the client. The authors conclude that a threshold for the ubiquitin chain length exists that can be overcome with the accessory adapters (Fujisawa et al., 2022). Since the analysis was done only with endpoint measurements it will be interesting to clarify whether the accessory adapters have a more general effect on the rate of client processing. Analysis of disassembly rates would also allow a more detailed dissection of the contribution of the different domains.
The alternative family of adapters is defined by the SEP (Shp1, eyes-closed, p47) domain combined with the p97-interaction module consisting of a SHP box and a UBX domain. Four SEP-domain proteins are encoded in the human genome (Figure 4A). p37 (also called UBXN2B), UBXN2A and UBXN11 do not contain ubiquitin-binding elements. p47 (also called NSFL1C) is an exception and harbors a ubiquitin-binding UBA domain. Whereas S. cerevisiae has only one SEP-domain protein, Shp1, with a UBA domain, the only orthologue in C. elegans lacks the UBA domain (Figure 4A).
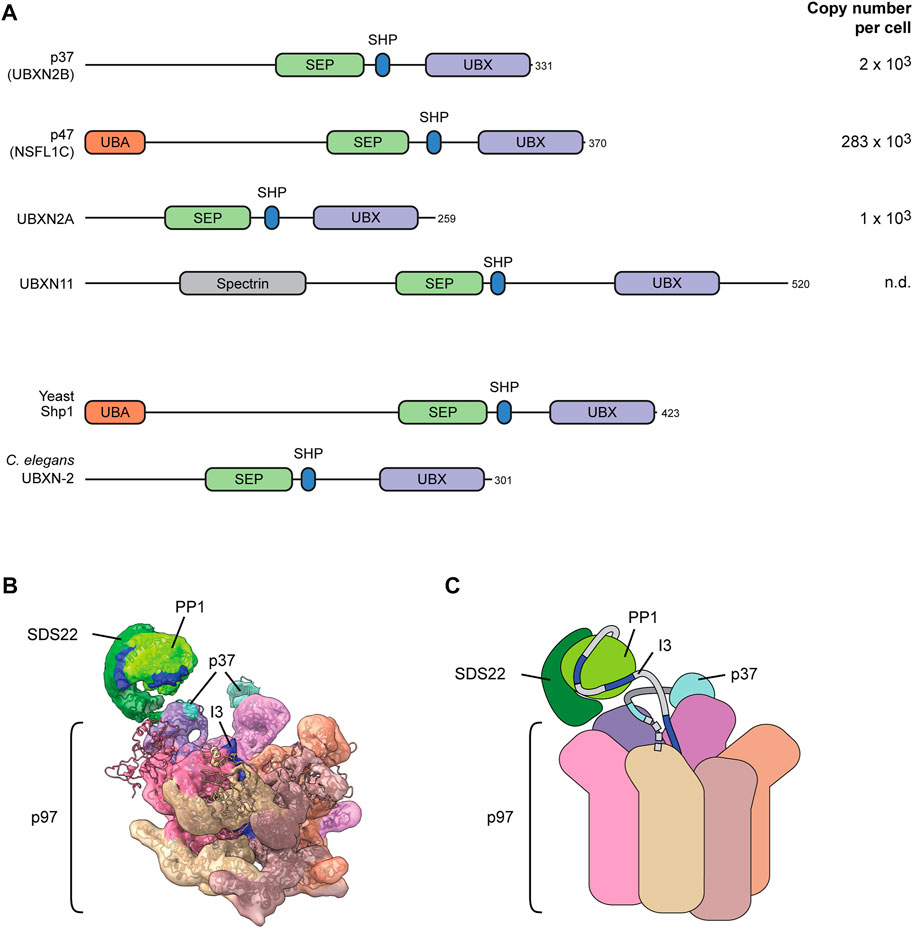
FIGURE 4. The SEP-domain adapters for direct client targeting. (A) Domain structure of the four human SEP domain proteins. Yeast and C. elegans code for only one ortholog as depicted. The UBX domain and the SHP box motif mediate binding to the p97 N-domain. The defining SEP domain contributes to a multivalent substrate binding mechanism. The UBA domain binds ubiquitin. Copy numbers of proteins in human U2OS osteosarcoma cells according to (Beck et al., 2011). n.d., not detected. (B) Cryo-EM structure of p97-p37-SDS22-PP1-I3. Dark blue indicates sections of I3 inserted into the p97 while still associated with PP1 (bright green). Note direct interaction of SDS22 (dark green) with an N-domain (purple) of p97. The SHP box and UBX domain of p37 (cyan) bridge two adjacent N-domains. (C) Cartoon model of the structure in (B). Dashed line indicates the N-terminal portion of p37 that is not resolved and includes the SEP domain.
Earlier work showed differential involvement of ubiquitin in the functions of p47 and p37 in SNARE-mediated mitotic membrane dynamics (Meyer, 2005; Totsukawa et al., 2011). The recently uncovered regulation of PP1 biogenesis by p97 and its SEP-domain adapters has brought clarity to the mechanism of client targeting and protein complex disassembly. Work in yeast already demonstrated that Shp1 (Suppressor of high copy PP1) is needed for activation of PP1 (rather than for its degradation) (Zhang et al., 1995). Further analysis in the mammalian system showed that newly synthesized PP1 is first held in an intermediate complex with its partners SDS22 (also called PPP1R7) and inhibitor-3 (I3, also called PPP1R11) to keep PP1 inactive (Weith et al., 2018; Cao et al., 2021). The SDS22-PP1-I3 complex is very stable and needs to be dissociated by p97 to allow assembly of PP1 holoenzymes with activating subunits (Weith et al., 2018; Cao et al., 2021). Conceptually, this process is reminiscent of ribosome biogenesis that involves the binding of a transient maturation factor, Rlp24, which needs to be extracted by the p97-related AAA protein Drg1 (Prattes et al., 2022).
SDS22-PP1-I3 disassembly can be reconstituted from purified components, which allowed detailed mechanistic dissection. Of note, p37 with p97 is sufficient to mediate SDS22-PP1-I3 disassembly without ubiquitylation involved (Weith et al., 2018). Cryo-EM revealed that p97-p37 is loaded with the client complex firmly on one of the p97 N-domains in a very different manner from Ufd1-Npl4 (van den Boom et al., 2022) (Figures 4B, C). The SDS22 subunit locks directly into the N-domain groove with a helix in SDS22 (van den Boom et al., 2022). This is surprising and reminiscent of AAA proteins such as VPS4 that, too, directly binds its client through an N-terminal domain (Monroe et al., 2017). The SEP-domain adapter is still essential for client targeting probably by positioning the substrate complex with direct contacts (Kracht et al., 2020). The p37 SHP box binds the same N-domain as SDS22 underneath PP1, while the p37 UBX domain interacts with the adjacent p97 N-domain (van den Boom et al., 2022). Site-specific crosslinks confirmed the position of the linker underneath PP1 and a multivalent interaction of p37 with the SDS22-PP1-I3 complex involving binding of the p37 SEP domain with I3 (Kracht et al., 2020). In the structure, I3 is already inserted in the channel of the spiral-shaped p97 hexamer while the bigger part of I3 is still associated with PP1 (van den Boom et al., 2022) (Figures 4B, C). Together with the firm attachment of the PP1 complex to one of the N-domains, this suggests a hold-and-extract mechanism for disassembly of the SDS22-PP1-I3 complex that may more widely be valid for other disassembly reactions.
A key question is how the direct client I3 is inserted into the p97 channel. Mapping of I3 revealed an internal recognition site in I3 that can also be dominantly crosslinked inside the p97 channel (van den Boom et al., 2021). Consistent with that, blocking both I3 termini by circularization of I3 did not prevent I3 unfolding suggesting that I3 is inserted as a loop (van den Boom et al., 2021). The SEP domain was not resolved in the structure, but also engages in I3 interaction and is essential for I3 unfolding (Kracht et al., 2020). It is therefore possible that the SEP domain has a function in I3 insertion, possibly even melting the I3-PP1 interaction equivalent to the melting of ubiquitin by Npl4 for insertion in the Ufd1-Npl4 pathway.
The other question is why different SEP domain adapters exist in metazoans. Like p37, UBXN2A can target I3 for unfolding in vitro raising the question what the functional or regulatory difference between the two adapters is in cells (Kracht et al., 2020). The more divergent UBNX11 does not target the PP1 complex in vitro. Surprisingly, despite the sequence similarity to p37, p47 is also not active. However, it can be changed to support PP1 complex disassembly by a transplant of the p37 SHP box-UBX linker to p47 (Kracht et al., 2020). It remains to be determined whether this reflects divergent client specificity of p47 compared to p37 and UBXN2A, or a possible lever for regulation.
It is important to note that, whereas p37 and UBXN2A are represented only by a few thousand copies in tissue culture cells, p47 is by far the most abundant p97 adapter (Beck et al., 2011) suggesting that more client proteins and functions of p47 are to be discovered. One such function in yeast is the ubiquitin-directed and Shp1-mediated processing of Def1 for regulation of RNA polymerase II degradation (Lehner et al., 2022). Another function of Cdc48 and Shp1 in yeast is a disassembly reaction associated with regulation of a cullin RING ubiquitin ligase (CRL) (Yen et al., 2012; Lauinger et al., 2020). In unchallenged cells, the transcription factor Met4 is constitutively degraded following ubiquitylation by the CRL Skp1-Cullin-F-box (SCF) with its substrate adapter Met30. In the response to Cd2+, however, SCF-Met30 is rapidly disassembled and, thus, Met4 is stabilized to help Cd2+ detoxification (Yen et al., 2012).
Importantly, SCF-Met30 disassembly is mediated by Cdc48 and Shp1, and requires autoubiquitylation of Met30 (Yen et al., 2012; Lauinger et al., 2020). Cdc48 can bind Met30 independently of Shp1, raising the possibility that Met30 binds Cdc48 directly similar to SDS22 binding to p97 in PP1 complex disassembly (Lauinger et al., 2020). Nevertheless, Shp1 is essential for SCF-Met30 disassembly, and this activity depends on its SEP domain, but is independent of the UBA domain in Shp1 (Lauinger et al., 2020). Likewise, Shp1 is essential for high temperature resistance of yeast but this does not require the UBA domain (Bohm and Buchberger, 2013). The role of Shp1 is therefore reminiscent of the role of p37 in PP1 complex disassembly, which forms multivalent interactions with the client complex in a ubiquitin-independent manner and binds the client directly through the SEP domain (Kracht et al., 2020). The activity of Cdc48 and Shp1 in SCF-Met30 regulation still needs to be validated and dissected in vitro. From the available information, however, and in contrast to Ufd1-Npl4, it seems unlikely that Shp1 uses ubiquitin attached to a client as a tag to initiate client unfolding. Rather, ubiquitylation may regulate the recruitment of the client complex similar to substrate recruitment to the proteasome. Shp1 then engages in direct interaction with the client protein to guide a protein stretch of the client into the pore for subsequent unfolding of the whole protein. This would reconcile the seemingly divergent observations regarding ubiquitin involvement for SEP domain adapters such as p37 and p47, and establish a common mechanism of direct insertion of elements of the client itself independently of whether the SEP domain adapter contains a UBA domain or not. Interestingly, Npl4 has evolutionarily gained the NZF as an additional ubiquitin-binding domain in metazoans (Meyer et al., 2002), which does not seem to be directly involved in the loading of ubiquitin into the D1 domain pore. It could therefore have a similar recruitment function as the UBA of p47 proposed here.
So far, no other p97 cofactor has been rigorously demonstrated to act as a client adapter independently of Ufd1-Npl4 or a SEP-domain adapter. However, cell biological work has identified interesting apparent candidates in specific cellular pathways in need of clarification. One such p97 cofactor complex forms with the cofactors UBXD1 and PLAA that have functionally been linked to sorting of caveolin-1 as well as to the endolysosomal damage response leading to lysophagy (Ritz et al., 2011; Papadopoulos et al., 2017). Of note, and consistent with that, mutations in PLAA cause neurodegeneration associated with endolysosomal sorting defects leading to epileptic encephalopathy in children (Hall et al., 2017). The endolysosome-associated function of PLAA appears to be conserved for the yeast orthologue Ufd3 (also called Doa1) (Ren et al., 2008). However, PLAA and UBXD1 (that only exists in metazoans) bind to the C-terminal tail of p97 close to the D2 domain exit pore. It is therefore difficult to rationalize how clients could be targeted to the D1 domain pore by UBXD1 and PLAA.
UBXN1 (also called SAKS1) is an interesting candidate that contains both a UBA and a UBX domains and has been linked to various protein quality control pathways (Ganji et al., 2018; Feng et al., 2021; Mukkavalli et al., 2021; Mengus et al., 2022). However, an adapter function has not been shown biochemically and UBXN1 apparently lacks a SHP box that seems typical for client adapters. Moreover, UBXN1 has been found associated with Ufd1-Npl4 suggesting that UBXN1 might act as an accessory adapter (Alexandru et al., 2008; Ganji et al., 2018). The DNA-dependent protease SPRTN has been linked to p97 and suggested to also act in recruiting clients (Fielden et al., 2020), but biochemical reconstitution showed the requirement of client ubiquitylation and the Ufd1-Npl4 adapter for p97-mediated unfolding of SPRTN substrates (Kroning et al., 2022). Yet another candidate, the UBXN10 cofactor, is critical for anterograde transport in cilia regulation (Raman et al., 2015). The fact that it lacks a ubiquitin-binding domain suggests a ubiquitin-independent targeting mechanism. Structure prediction, however, does not detect a dedicated domain that could help with client targeting, suggesting that UBXN10 may cooperate with another factor for targeting. Apart from further characterizing potential new client adapters, it will also be interesting to clarify whether cofactors such as DERL1/2 or SVIP may act not only in recruiting p97 to membrane but also directly as accessory adapters to stimulate client unfolding.
The dissection of p97 function in diverse pathways over the past 30 years has taught us how important controlled protein unfolding or protein complex disassembly is for cellular homeostasis and regulation. As we now know, the core threading machine is indiscriminate with regard to client proteins. It is therefore important to further understand how client proteins and protein complexes are targeted to p97 for unfolding and disassembly. This certainly includes understanding the role of p47 and determine what its clients are. New approaches to target specific adapter complexes may help (Jiang et al., 2022). Further work should also include examining the host of p97 interacting proteins for activities that assist client targeting or even serve as novel client adapters. Likewise, we need to understand how targeting is regulated, for example through posttranslational modifications other than ubiquitylation including phosphorylation or SUMOylation (Lee et al., 2023). With powerful biochemical unfolding and disassembly assays at hand, there is no excuse to not validate and dissect these activities mechanistically in vitro.
JvB Prepared the figures. HM wrote the manuscript with JvB.
This work was funded by DFG CRC1430 subproject A3 and Me1626/3-3 to HM.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Obeidi, E., Al-Tahan, S., Surampalli, A., Goyal, N., Wang, A. K., Hermann, A., et al. (2018). Genotype-phenotype study in patients with valosin-containing protein mutations associated with multisystem proteinopathy. Clin. Genet. 93, 119–125. doi:10.1111/cge.13095
Alexandru, G., Graumann, J., Smith, G. T., Kolawa, N. J., Fang, R., and Deshaies, R. J. (2008). UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell 134, 804–816. doi:10.1016/j.cell.2008.06.048
Anderson, D. J., Le Moigne, R., Djakovic, S., Kumar, B., Rice, J., Wong, S., et al. (2015). Targeting the AAA ATPase p97 as an approach to treat cancer through disruption of protein homeostasis. Cancer Cell 28, 653–665. doi:10.1016/j.ccell.2015.10.002
Avellaneda, M. J., Franke, K. B., Sunderlikova, V., Bukau, B., Mogk, A., and Tans, S. J. (2020). Processive extrusion of polypeptide loops by a Hsp100 disaggregase. Nature 578, 317–320. doi:10.1038/s41586-020-1964-y
Beck, M., Schmidt, A., Malmstroem, J., Claassen, M., Ori, A., Szymborska, A., et al. (2011). The quantitative proteome of a human cell line. Mol. Syst. Biol. 7, 549. doi:10.1038/msb.2011.82
Beskow, A., Grimberg, K. B., Bott, L. C., Salomons, F. A., Dantuma, N. P., and Young, P. (2009). A conserved unfoldase activity for the p97 AAA-ATPase in proteasomal degradation. J. Mol. Biol. 394, 732–746. doi:10.1016/j.jmb.2009.09.050
Blythe, E. E., Olson, K. C., Chau, V., and Deshaies, R. J. (2017). Ubiquitin- and ATP-dependent unfoldase activity of P97/VCP*NPLOC4*UFD1L is enhanced by a mutation that causes multisystem proteinopathy. Proc. Natl. Acad. Sci. U. S. A. 114, E4380–E4388. doi:10.1073/pnas.1706205114
Bodnar, N. O., and Rapoport, T. A. (2017). Molecular mechanism of substrate processing by the Cdc48 ATPase complex. Cell 169, 722–735. doi:10.1016/j.cell.2017.04.020
Bohm, S., and Buchberger, A. (2013). The budding yeast Cdc48(Shp1) complex promotes cell cycle progression by positive regulation of protein phosphatase 1 (Glc7). PLoS One 8, e56486. doi:10.1371/journal.pone.0056486
Bruderer, R. M., Brasseur, C., and Meyer, H. H. (2004). The AAA ATPase p97/VCP interacts with its alternative Co-factors, ufd1-npl4 and p47, through a common bipartite binding mechanism. J. Biol. Chem. 279, 49609–49616. doi:10.1074/jbc.M408695200
Buchberger, A., Schindelin, H., and Hanzelmann, P. (2015). Control of p97 function by cofactor binding. FEBS Lett. 589, 2578–2589. doi:10.1016/j.febslet.2015.08.028
Buchberger, A. (2022). Unfolding by cdc48/p97: Different strokes for different folks. Trends Cell Biol. 32, 278–280. doi:10.1016/j.tcb.2022.01.001
Cao, X., Lemaire, S., and Bollen, M. (2021). Protein phosphatase-1: Life-course regulation by SDS22 and inhibitor-3. FEBS J. 289, 3072–3085. doi:10.1111/febs.16029
Chauhan, A. K., Li, P., Sun, Y., Wani, G., Zhu, Q., and Wani, A. A. (2021). Spironolactone-induced XPB degradation requires TFIIH integrity and ubiquitin-selective segregase VCP/p97. Cell Cycle 20, 81–95. doi:10.1080/15384101.2020.1860559
Cooney, I., Han, H., Stewart, M. G., Carson, R. H., Hansen, D. T., Iwasa, J. H., et al. (2019). Structure of the Cdc48 segregase in the act of unfolding an authentic substrate. Science 365, 502–505. doi:10.1126/science.aax0486
Erzberger, J. P., and Berger, J. M. (2006). Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114. doi:10.1146/annurev.biophys.35.040405.101933
Feng, D., Wang, J., Yang, W., Li, J., Lin, X., Zha, F., et al. (2021). Regulation of Wnt/PCP signaling through p97/VCP-KBTBD7-mediated Vangl ubiquitination and endoplasmic reticulum-associated degradation. Sci. Adv. 7, eabg2099. doi:10.1126/sciadv.abg2099
Fielden, J., Wiseman, K., Torrecilla, I., Li, S., Hume, S., Chiang, S. C., et al. (2020). TEX264 coordinates p97-and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat. Commun. 11, 1274. doi:10.1038/s41467-020-15000-w
Franz, A., Pirson, P. A., Pilger, D., Halder, S., Achuthankutty, D., Kashkar, H., et al. (2016). Chromatin-associated degradation is defined by UBXN-3/FAF1 to safeguard DNA replication fork progression. Nat. Commun. 7, 10612. doi:10.1038/ncomms10612
Fujisawa, R., Polo Rivera, C., and Labib, K. P. M. (2022). Multiple UBX proteins reduce the ubiquitin threshold of the mammalian p97-UFD1-NPL4 unfoldase. Elife 11, e76763. doi:10.7554/eLife.76763
Ganji, R., Mukkavalli, S., Somanji, F., and Raman, M. (2018). The VCP-UBXN1 complex mediates triage of ubiquitylated cytosolic proteins bound to the BAG6 complex. Mol. Cell Biol. 38, e00154-18. doi:10.1128/MCB.00154-18
Greene, E. R., Dong, K. C., and Martin, A. (2020). Understanding the 26S proteasome molecular machine from a structural and conformational dynamics perspective. Curr. Opin. Struct. Biol. 61, 33–41. doi:10.1016/j.sbi.2019.10.004
Hall, E. A., Nahorski, M. S., Murray, L. M., Shaheen, R., Perkins, E., Dissanayake, K. N., et al. (2017). PLAA mutations cause a lethal infantile epileptic encephalopathy by disrupting ubiquitin-mediated endolysosomal degradation of synaptic proteins. Am. J. Hum. Genet. 100, 706–724. doi:10.1016/j.ajhg.2017.03.008
Ji, Z., Li, H., Peterle, D., Paulo, J. A., Ficarro, S. B., Wales, T. E., et al. (2021). Translocation of polyubiquitinated protein substrates by the hexameric Cdc48 ATPase. Mol. Cell 82, 570–584.e8. doi:10.1016/j.molcel.2021.11.033
Jiang, Z., Kuo, Y. H., Zhong, M., Zhang, J., Zhou, X. X., Xing, L., et al. (2022). Adaptor-specific antibody fragment inhibitors for the intracellular modulation of p97 (VCP) protein-protein interactions. J. Am. Chem. Soc. 144, 13218–13225. doi:10.1021/jacs.2c03665
Khan, Y. A., White, K. I., and Brunger, A. T. (2022). The AAA+ superfamily: A review of the structural and mechanistic principles of these molecular machines. Crit. Rev. Biochem. Mol. Biol. 57, 156–187. doi:10.1080/10409238.2021.1979460
Kochenova, O. V., Mukkavalli, S., Raman, M., and Walter, J. C. (2022). Cooperative assembly of p97 complexes involved in replication termination. Nat. Commun. 13, 6591. doi:10.1038/s41467-022-34210-y
Kondo, H., Rabouille, C., Newman, R., Levine, T. P., Pappin, D., Freemont, P., et al. (1997). p47 is a cofactor for p97-mediated membrane fusion. Nature 388, 75–78. doi:10.1038/40411
Kracht, M., Van Den Boom, J., Seiler, J., Kroning, A., Kaschani, F., Kaiser, M., et al. (2020). Protein phosphatase-1 complex disassembly by p97 is initiated through multivalent recognition of catalytic and regulatory subunits by the p97 SEP-domain adapters. J. Mol. Biol. 432, 6061–6074. doi:10.1016/j.jmb.2020.10.001
Kroning, A., Van Den Boom, J., Kracht, M., Kueck, A. F., and Meyer, H. (2022). Ubiquitin-directed AAA+ ATPase p97/VCP unfolds stable proteins crosslinked to DNA for proteolysis by SPRTN. J. Biol. Chem. 298, 101976. doi:10.1016/j.jbc.2022.101976
Lauinger, L., Flick, K., Yen, J. L., Mathur, R., and Kaiser, P. (2020). Cdc48 cofactor Shp1 regulates signal-induced SCF(Met30) disassembly. Proc. Natl. Acad. Sci. U. S. A. 117, 21319–21327. doi:10.1073/pnas.1922891117
Lee, H. G., Lemmon, A. A., and Lima, C. D. (2023). SUMO enhances unfolding of SUMO-polyubiquitin-modified substrates by the Ufd1/Npl4/Cdc48 complex. Proc. Natl. Acad. Sci. U. S. A. 120, e2213703120. doi:10.1073/pnas.2213703120
Lehner, M. H., Walker, J., Temcinaite, K., Herlihy, A., Taschner, M., Berger, A. C., et al. (2022). Yeast Smy2 and its human homologs GIGYF1 and -2 regulate Cdc48/VCP function during transcription stress. Cell Rep. 41, 111536. doi:10.1016/j.celrep.2022.111536
Mengus, C., Neutzner, M., Bento, A., Bippes, C. C., Kohler, C., Decembrini, S., et al. (2022). VCP/p97 cofactor UBXN1/SAKS1 regulates mitophagy by modulating MFN2 removal from mitochondria. Autophagy 18, 171–190. doi:10.1080/15548627.2021.1922982
Metzger, M. B., Scales, J. L., Dunklebarger, M. F., Loncarek, J., and Weissman, A. M. (2020). A protein quality control pathway at the mitochondrial outer membrane. Elife 9, e51065. doi:10.7554/eLife.51065
Meyer, H. H. (2005). Golgi reassembly after mitosis: The AAA family meets the ubiquitin family. Biochim. Biophys. Acta 1744, 108–119. doi:10.1016/j.bbamcr.2005.03.011
Meyer, H. H., Shorter, J. G., Seemann, J., Pappin, D., and Warren, G. (2000). A complex of mammalian Ufd1 and Npl4 links the AAA-ATPase, p97, to ubiquitin and nuclear transport pathways. Embo J. 19, 2181–2192. doi:10.1093/emboj/19.10.2181
Meyer, H. H., Wang, Y., and Warren, G. (2002). Direct binding of ubiquitin conjugates by the mammalian p97 adaptor complexes, p47 and Ufd1-Npl4. Embo J. 21, 5645–5652. doi:10.1093/emboj/cdf579
Meyer, H., and Weihl, C. C. (2014). The VCP/p97 system at a glance: Connecting cellular function to disease pathogenesis. J. Cell Sci. 127, 3877–3883. doi:10.1242/jcs.093831
Monroe, N., Han, H., Shen, P. S., Sundquist, W. I., and Hill, C. P. (2017). Structural basis of protein translocation by the Vps4-Vta1 AAA ATPase. Elife 6, e24487. doi:10.7554/eLife.24487
Mukherjee, P. P., and Labib, K. P. M. (2019). In vitro reconstitution defines the minimal requirements for cdc48-dependent disassembly of the CMG helicase in budding yeast. Cell Rep. 28, 2777–2783. doi:10.1016/j.celrep.2019.08.026
Mukkavalli, S., Klickstein, J. A., Ortiz, B., Juo, P., and Raman, M. (2021). The p97-UBXN1 complex regulates aggresome formation. J. Cell Sci. 134, jcs254201. doi:10.1242/jcs.254201
Neuber, O., Jarosch, E., Volkwein, C., Walter, J., and Sommer, T. (2005). Ubx2 links the Cdc48 complex to ER-associated protein degradation. Nat. Cell Biol. 7, 993–998. doi:10.1038/ncb1298
Olszewski, M. M., Williams, C., Dong, K. C., and Martin, A. (2019). The Cdc48 unfoldase prepares well-folded protein substrates for degradation by the 26S proteasome. Commun. Biol. 2, 29. doi:10.1038/s42003-019-0283-z
Pan, M., Yu, Y., Ai, H., Zheng, Q., Xie, Y., Liu, L., et al. (2021a). Mechanistic insight into substrate processing and allosteric inhibition of human p97. Nat. Struct. Mol. Biol. 28, 614–625. doi:10.1038/s41594-021-00617-2
Pan, M., Zheng, Q., Yu, Y., Ai, H., Xie, Y., Zeng, X., et al. (2021b). Seesaw conformations of Npl4 in the human p97 complex and the inhibitory mechanism of a disulfiram derivative. Nat. Commun. 12, 121. doi:10.1038/s41467-020-20359-x
Papadopoulos, C., Kirchner, P., Bug, M., Grum, D., Koerver, L., Schulze, N., et al. (2017). VCP/p97 cooperates with YOD1, UBXD1 and PLAA to drive clearance of ruptured lysosomes by autophagy. EMBO J. 36, 135–150. doi:10.15252/embj.201695148
Prattes, M., Grishkovskaya, I., Hodirnau, V. V., Hetzmannseder, C., Zisser, G., Sailer, C., et al. (2022). Visualizing maturation factor extraction from the nascent ribosome by the AAA-ATPase Drg1. Nat. Struct. Mol. Biol. 29, 942–953. doi:10.1038/s41594-022-00832-5
Puumalainen, M. R., Lessel, D., Ruthemann, P., Kaczmarek, N., Bachmann, K., Ramadan, K., et al. (2014). Chromatin retention of DNA damage sensors DDB2 and XPC through loss of p97 segregase causes genotoxicity. Nat. Commun. 5, 3695. doi:10.1038/ncomms4695
Raman, M., Sergeev, M., Garnaas, M., Lydeard, J. R., Huttlin, E. L., Goessling, W., et al. (2015). Systematic proteomics of the VCP-UBXD adaptor network identifies a role for UBXN10 in regulating ciliogenesis. Nat. Cell Biol. 17, 1356–1369. doi:10.1038/ncb3238
Ren, J., Pashkova, N., Winistorfer, S., and Piper, R. C. (2008). DOA1/UFD3 plays a role in sorting ubiquitinated membrane proteins into multivesicular bodies. J. Biol. Chem. 283, 21599–21611. doi:10.1074/jbc.M802982200
Ritz, D., Vuk, M., Kirchner, P., Bug, M., Schütz, S., Hayer, A., et al. (2011). Endolysosomal sorting of ubiquitylated caveolin-1 is regulated by VCP and UBXD1 and impaired by VCP disease mutations. Nat. Cell Biol. 13, 1116–1123. doi:10.1038/ncb2301
Roux, B., Vaganay, C., Vargas, J. D., Alexe, G., Benaksas, C., Pardieu, B., et al. (2021). Targeting acute myeloid leukemia dependency on VCP-mediated DNA repair through a selective second-generation small-molecule inhibitor. Sci. Transl. Med. 13, eabg1168. doi:10.1126/scitranslmed.abg1168
Sato, Y., Tsuchiya, H., Yamagata, A., Okatsu, K., Tanaka, K., Saeki, Y., et al. (2019). Structural insights into ubiquitin recognition and Ufd1 interaction of Npl4. Nat. Commun. 10, 5708. doi:10.1038/s41467-019-13697-y
Schuberth, C., and Buchberger, A. (2005). Membrane-bound Ubx2 recruits Cdc48 to ubiquitin ligases and their substrates to ensure efficient ER-associated protein degradation. Nat. Cell Biol. 7, 999–1006. doi:10.1038/ncb1299
Sonneville, R., Moreno, S. P., Knebel, A., Johnson, C., Hastie, C. J., Gartner, A., et al. (2017). CUL-2(LRR-1) and UBXN-3 drive replisome disassembly during DNA replication termination and mitosis. Nat. Cell Biol. 19, 468–479. doi:10.1038/ncb3500
Stach, L., and Freemont, P. S. (2017). The AAA+ ATPase p97, a cellular multitool. Biochem. J. 474, 2953–2976. doi:10.1042/BCJ20160783
Stach, L., Morgan, R. M., Makhlouf, L., Douangamath, A., Von Delft, F., Zhang, X., et al. (2020). Crystal structure of the catalytic D2 domain of the AAA+ ATPase p97 reveals a putative helical split-washer-type mechanism for substrate unfolding. FEBS Lett. 594, 933–943. doi:10.1002/1873-3468.13667
Tarcan, Z., Poovathumkadavil, D., Skagia, A., and Gambus, A. (2022). The p97 segregase cofactor Ubxn7 facilitates replisome disassembly during S-phase. J. Biol. Chem. 298, 102234. doi:10.1016/j.jbc.2022.102234
Totsukawa, G., Kaneko, Y., Uchiyama, K., Toh, H., Tamura, K., and Kondo, H. (2011). VCIP135 deubiquitinase and its binding protein, WAC, in p97ATPase-mediated membrane fusion. Embo J. 30, 3581–3593. doi:10.1038/emboj.2011.260
Twomey, E. C., Ji, Z., Wales, T. E., Bodnar, N. O., Ficarro, S. B., Marto, J. A., et al. (2019). Substrate processing by the Cdc48 ATPase complex is initiated by ubiquitin unfolding. Science 365, eaax1033. doi:10.1126/science.aax1033
Van Den Boom, J., Kueck, A. F., Kravic, B., Muschenborn, H., Giesing, M., Pan, D., et al. (2021). Targeted substrate loop insertion by VCP/p97 during PP1 complex disassembly. Nat. Struct. Mol. Biol. 28, 964–971. doi:10.1038/s41594-021-00684-5
Van Den Boom, J., Meyer, H., and Saibil, H. R. (2022). Structural basis of ubiquitin-independent PP1 complex disassembly by p97. bioRxiv.
Van Den Boom, J., and Meyer, H. (2018). VCP/p97-Mediated unfolding as a principle in protein homeostasis and signaling. Mol. Cell 69, 182–194. doi:10.1016/j.molcel.2017.10.028
Van Den Boom, J., Wolf, M., Weimann, L., Schulze, N., Li, F., Kaschani, F., et al. (2016). VCP/p97 extracts sterically trapped ku70/80 rings from DNA in double-strand break repair. Mol. Cell 64, 189–198. doi:10.1016/j.molcel.2016.08.037
Verma, R., Oania, R., Fang, R., Smith, G. T., and Deshaies, R. J. (2011). Cdc48/p97 mediates UV-dependent turnover of RNA Pol II. Mol. Cell 41, 82–92. doi:10.1016/j.molcel.2010.12.017
Watts, G. D., Wymer, J., Kovach, M. J., Mehta, S. G., Mumm, S., Darvish, D., et al. (2004). Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 36, 377–381. doi:10.1038/ng1332
Weith, M., Seiler, J., Van Den Boom, J., Kracht, M., Hulsmann, J., Primorac, I., et al. (2018). Ubiquitin-independent disassembly by a p97 AAA-ATPase complex drives PP1 holoenzyme formation. Mol. Cell 72, 766–777. doi:10.1016/j.molcel.2018.09.020
Wojcik, C., Yano, M., and Demartino, G. N. (2004). RNA interference of valosin-containing protein (VCP/p97) reveals multiple cellular roles linked to ubiquitin/proteasome-dependent proteolysis. J. Cell Sci. 117, 281–292. doi:10.1242/jcs.00841
Xia, Y., Fujisawa, R., Deegan, T. D., Sonneville, R., and Labib, K. P. M. (2021). TIMELESS-TIPIN and UBXN-3 promote replisome disassembly during DNA replication termination in Caenorhabditis elegans. EMBO J. 40, e108053. doi:10.15252/embj.2021108053
Xu, Y., Han, H., Cooney, I., Guo, Y., Moran, N. G., Zuniga, N. R., et al. (2022). Active conformation of the p97-p47 unfoldase complex. Nat. Commun. 13, 2640. doi:10.1038/s41467-022-30318-3
Xue, L., Blythe, E. E., Freiberger, E. C., Mamrosh, J. L., Hebert, A. S., Reitsma, J. M., et al. (2016). Valosin-containing protein (VCP)-Adaptor interactions are exceptionally dynamic and subject to differential modulation by a VCP inhibitor. Mol. Cell Proteomics 15, 2970–2986. doi:10.1074/mcp.M116.061036
Ye, Y., Tang, W. K., Zhang, T., and Xia, D. (2017). A mighty "protein extractor" of the cell: Structure and function of the p97/CDC48 ATPase. Front. Mol. Biosci. 4, 39. doi:10.3389/fmolb.2017.00039
Yen, J. L., Flick, K., Papagiannis, C. V., Mathur, R., Tyrrell, A., Ouni, I., et al. (2012). Signal-induced disassembly of the SCF ubiquitin ligase complex by Cdc48/p97. Mol. Cell 48, 288–297. doi:10.1016/j.molcel.2012.08.015
Keywords: protein homeostasis, ubiquitin, protein unfolding, protein phosphatase (PP) 1, protein quality control, DNA replication and damaged repair
Citation: Meyer H and van den Boom J (2023) Targeting of client proteins to the VCP/p97/Cdc48 unfolding machine. Front. Mol. Biosci. 10:1142989. doi: 10.3389/fmolb.2023.1142989
Received: 12 January 2023; Accepted: 30 January 2023;
Published: 07 February 2023.
Edited by:
David A. Dougan, La Trobe University, AustraliaReviewed by:
Alexander Buchberger, Julius Maximilian University of Würzburg, GermanyCopyright © 2023 Meyer and van den Boom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hemmo Meyer, aGVtbW8ubWV5ZXJAdW5pLWR1ZS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.