
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci. , 29 August 2022
Sec. Molecular Diagnostics and Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fmolb.2022.992044
This article is part of the Research Topic Oxidative Stress in Degenerative Bone and Joint Diseases: Novel Molecular Mechanisms and Therapeutic Potential View all 7 articles
 Lin Xia1
Lin Xia1 Ningji Gong2*
Ningji Gong2*Background: Osteoarthritis (OA) is a major factor causing pain and disability. Studies performed to date have suggested that synovitis is possibly a critical OA-related pathological change. Ferroptosis represents a novel type of lipid peroxidation-induced iron-dependent cell death. However, its effect on OA remains largely unclear.
Objective: This work focused on identifying and validating the possible ferroptosis-related genes (FRGs) involved in synovitis of OA through bioinformatics analysis.
Materials and Methods: The microarray dataset GSE55235 was downloaded in the database Gene Expression Omnibus (GEO). By the Venn diagram and GEO2R, differentially expressed genes (DEGs) and ferroptosis DEGs (FDEGs) were detected. DEGs were screened by GO and KEGG enrichment analysis, as well as protein-protein interaction (PPI) analysis. Besides, the software Cytoscape and database STRING were utilized to construct hub gene networks. Moreover, this study used the database NetworkAnalyst to predict the target miRNAs of the hub genes. Finally, the hub genes were confirmed by analysis of the receiver operating characteristic (ROC) curve on the GSE12021 and GSE1919 databases. Considering the relationship between ferroptosis and immunity, this study applied CIBERSORTx to analyze the immune infiltration in OA in addition.
Results: This work discovered seven genes, including ATF3, IL6, CDKN1A, IL1B, EGR1, JUN, and CD44, as the hub FDEGs. The ROC analysis demonstrated that almost all hub genes had good diagnostic properties in GSE12021 and GSE 1919.
Conclusion: This study discovered seven FDEGs to be the possible diagnostic biomarkers and therapeutic targets of synovitis during OA, which sheds more light on the pathogenesis of OA at the transcriptome level.
Osteoarthritis (OA) refers to the chronic joint degenerative disorder that is featured by synovial inflammation, cartilage degeneration, and reduced joint function (Kraus et al., 2015). As a result, patients experience symptoms such as swelling, pain, or stiffness, thereby negatively affecting their quality of life (Roseti et al., 2019). OA is associated with an increased morbidity rate in the last few decades because of the aging population and obesity (Nelson, 2018). According to a recent study, there will be about 400 million OA cases in China by 2030 (Zhang et al., 2020). OA does not exhibit any early symptoms, and none of the existing methods can sensitively and efficiently detect minor changes (Camacho-Encina et al., 2019). Therefore, the identification of biomarkers with high sensitivity and efficiency can contribute to the early diagnosis of OA and facilitate the study of the pathogenesis of OA in individualized treatments.
Ferroptosis, an iron-dependent oxidative type of cell death, is different from necrosis, autophagy, apoptosis, or other types of cell death (Dixon et al., 2012). Besides, it is characterized by the accumulation of lipid peroxidation in an iron-dependent manner, which is reflected by aberrant lipid oxide metabolism under the catalysis of excessive ferrous ions, generation of reactive oxygen species (ROS), and cell death mediated by the excessive oxidation of polyunsaturated fatty acid (PUFA). Recently, ferroptosis has become the hotspot for research on various types of disease, such as heart injury, brain injury, cancer, asthma, or acute kidney failure (Friedmann Angeli et al., 2014; Stockwell et al., 2017; Zhao et al., 2020). Ferroptosis and iron have been recently suggested to have important effects on the occurrence of OA (Yao et al., 2021; Miao et al., 2022), although their exact mechanism remains largely unclear. Since studies have been increasingly conducted to explore ferroptosis and its relation with some biological processes, targeting ferroptosis, either used alone or as adjuvant therapy, can be a feasible approach to treat OA (Qu et al., 2022). Therefore, it is necessary to further analyze ferroptosis-related genes (FRGs) that are related to synovial inflammation, which may provide potential therapeutic targets.
This study obtained data from OA cases and healthy subjects from the database Gene Expression Omnibus (GEO) for bioinformatics analysis with the purpose of screening the differentially expressed genes (DEGs). Then, the identified DEGs were intersected with the ferroptosis dataset with the purpose of obtaining the key FDEGs. By constructing the protein-protein interaction (PPI) network, the current work detected certain hub FDEGs as the key regulating factors for the occurrence of OA, which were the potential targets. These key FDEGs were then exposed to Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis. Thereafter, levels of these genes were analyzed in the GSE12021 and GSE1919 datasets. As demonstrated by the ROC curve, seven key genes had good diagnostic properties. Finally, given the potential relationship between ferroptosis and immunity, we used CIBERSORTx to analyze the immune infiltration in OA. This work may offer more insight into exploring the mechanism of ferroptosis in OA together with the diagnosis and treatment of OA.
The GSE55235, GSE12021 and GSE1919 datasets were acquired from the GEO database (https://www.ncbi.nlm.nih.gov/geo/). In addition, we downloaded the FRGs from the FerrDb online database (http://www.zhounan.org/ferrdb/).
The DEGs were screened and identified based on the program GEO2R (https://www.ncbi.nlm.nih.gov/geo/geo2r) in GSE55235. Besides, the DEGs were defined as |log FC|>1 (p < 0.05). The FDEGs were defined as DEGs from GSE55235 that overlapped with FRGs and were selected based on the Venn diagram.
The program DAVID (https://david.ncifcrf.gov/, version 6.8) was employed with the purpose of performing GO functional annotation and KEGG analysis, with the threshold set at p < 0.05. Furthermore, the GO terms were divided into three categories, including biological processes (BPs), molecular functions (MFs) as well as cellular components (CCs).
The STRING database (https://string-db.org/) was applied to construct the PPI network in order to obtain the diagram. In addition, the results were obtained in STRING online database and were then imported into the software Cytoscape v 3.9.1 to choose the critical nodes for the visualization of molecular interaction networks. Based on our constructed PPI network, the key genes were identified by the CytoHubba plugin.
With the aim of constructing the miRNA-gene interactions for the identified key genes, the online software NetworkAnalyst (version 3.0, https://www.networkanalyst.ca/) was applied.
The diagnostic accuracy of the FDEGs was analyzed by ROC curves in the dataset GSE12021 and GSE 1919.
To evaluate the function of immune microenvironment in OA formation, CIBERSORTx (https://cibersortx.stanford.edu/) was adopted for analyzing the immune infiltration differences between OA and normal groups. Then, the results were visualized by a bar-plot and box-plot.
The statistical software R version 4.2.0 was applied to perform the statistical analysis, with p-values of <0.05 suggesting statistical significance.
The GEO2R is a web-based tool that is specialized in analyzing DEGs in the OA group in relative to the healthy group. Besides, the two groups were compared using GEO2R in the GEO datasets GSE55235 and the results were downloaded for further analysis. Genes of |log FC| > 1 and p < 0.05 were identified as DEGs. Totally 1,443 DEGs, containing 920 upregulated and 523 downregulated DEGs were detected. A volcano plot was drawn to validate the results (Figure 1A). These DEGs were intersected with 486 FRGs downloaded from the FerrDb database (http://www.zhounan.org/ferrdb/). Finally, 53 FDEGs were selected on the basis of the Venn diagram (Figure 1B). A heatmap of the 53 FDEGs is presented in Figure 2.
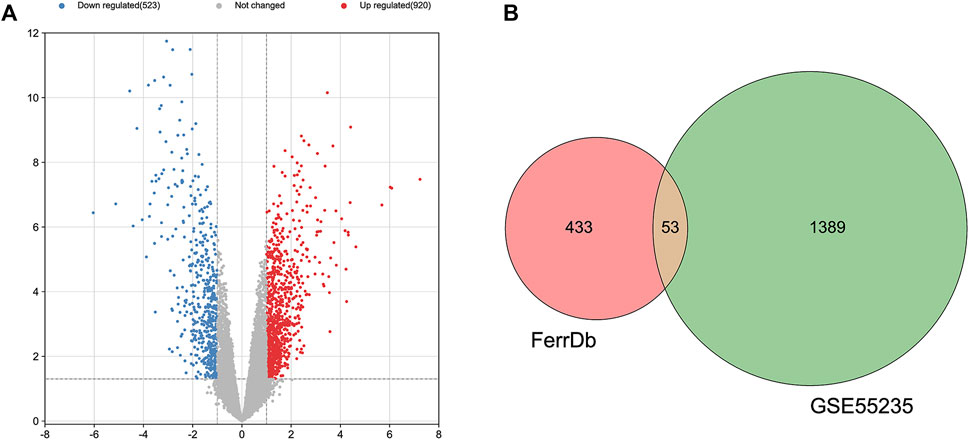
FIGURE 1. Identification of DEGs. (A) A volcano plot showing DEGs in the dataset GSE55235. (B) After the intersection, 53 FDEGs were identified based on the Venn diagram.
With the purpose of investigating the biological activities and pathways associated with FDEGs among the OA cases, GO and KEGG enrichment analyses (Yu et al., 2012; Luo and Brouwer, 2013) were performed on the 53 genes. Besides, the significantly enriched GO terms were cellular response to chemical stress, cellular response to oxidative stress (OS), response to OS (BP); apical part of the cell, apical plasma membrane, NADPH oxidase complex (CC); superoxide-generating NADA(P)H oxidase activity, oxidoreductase activity, acting on NAD(P)H, oxygen as acceptor, iron ion binding (MF) (Figure 3A). As revealed by the KEGG enrichment analysis, the 53 FDEGs were mostly associated with ferroptosis, FoxO pathway, AGE-RAGE pathway in diabetic complications, and HIF-1 signaling pathway (Figure 3B).
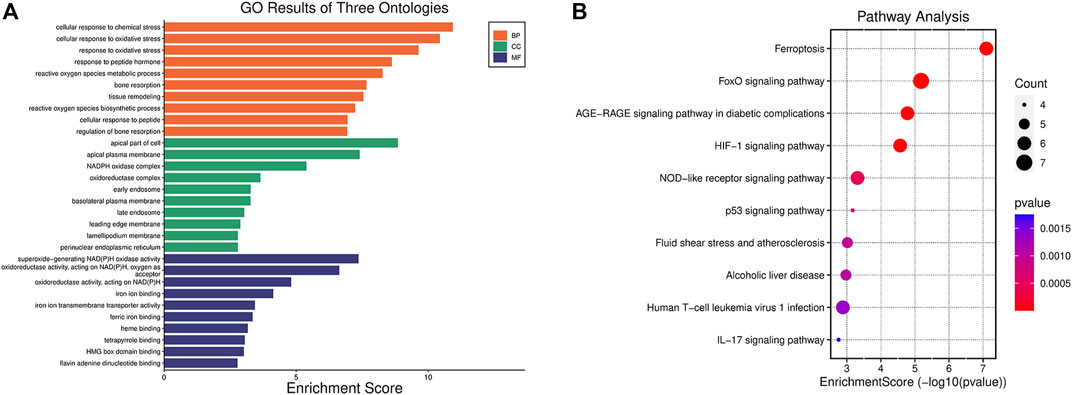
FIGURE 3. Functions and pathways explained by the 53 FDEGs. (A) GO enrichment analysis of the 53 FDEGs with bar-plot. (B) Results of the KEGG pathway analyses of the 53 FDEGs with dot-plot.
To analyze the PPI network of the 53 FDEGs, the database STRING (version 11.5, http://cn.string-db.org/) was applied. Cytoscape network visualization was obtained on the basis of the STRING database (Figure 4A). Using the MCC algorithm, 10 hub genes were obtained through the CytoHubba plugin of the software Cytoscape. Subsequently, according to the rank score, seven first-level hub genes, including ATF3, IL6, CDKN1A, IL1B, EGR1, JUN, and CD44 were identified with red nodes (Figure 4B; Table 1; Supplementary Table S2).

FIGURE 4. PPI analysis of the 53 FDEGs. (A) Cytoscape network visualization of the 53 FDEGs. (B) Network visualization of the 10 key genes by adopting the MCC algorithm of the CytoHubba plugin. Red and blue nodes represent the first-and second-level key genes, respectively.
To illustrate the possible pathways of the seven hub FDEGs, KEGG analyses were performed. On the basis of the KEGG analysis, these hub FDEGs were mostly related to the AGE-RAGE signaling pathway and Epstein-Barr virus infection (Figure 5).
The database NetworkAnalyst was adopted for predicting the target miRNA of the key genes. The possible miRNA-hub gene network was constructed to accurately investigate the molecular mechanism underlying the four ferroptosis-driver FDEGs. The miR-34a-5p and miR-155-5p interacted with all the four FDEMs, namely, ATF3, IL6, IL1B, and EGR1 (Figure 6).
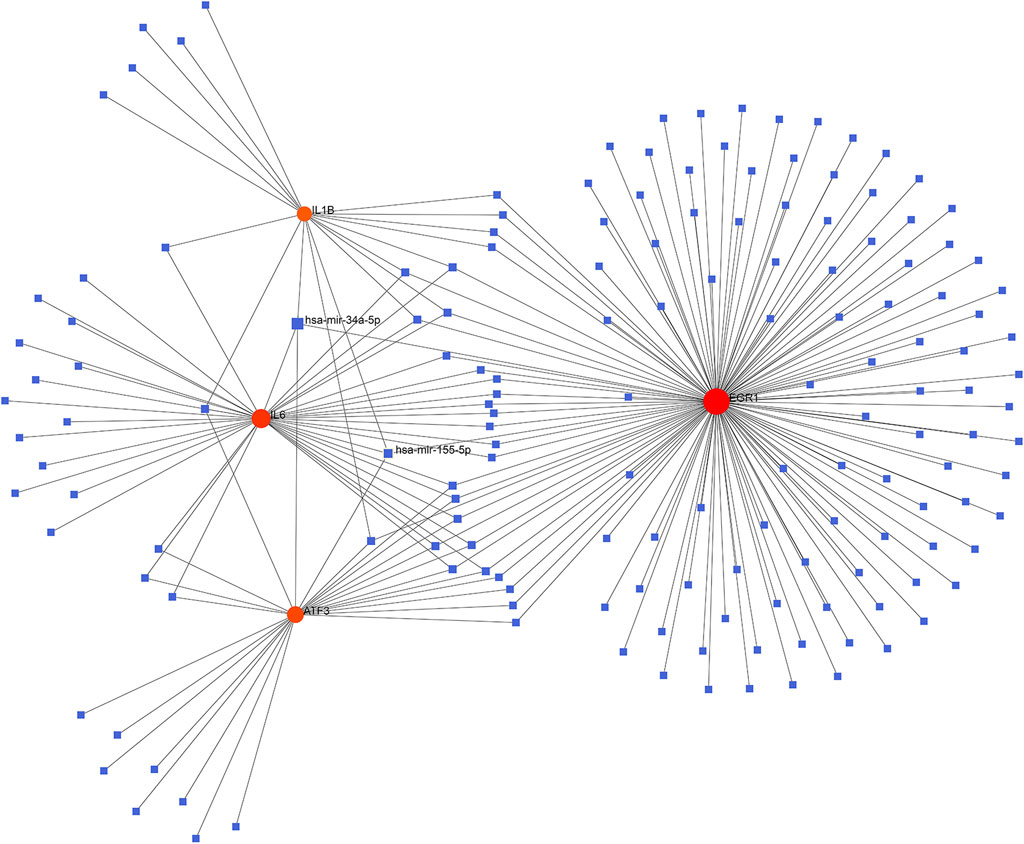
FIGURE 6. Establishment of the miRNA-gene interaction networks based on the four ferroptosis-driver key FDEMs. Red circles and blue squares represent key genes and miRNAs related to key FDEGs.
To verify whether the above seven key FDEGs had predictive significance, an analysis of the ROC curve was performed on the GSE12021 and GSE1919 datasets. It was observed that the AUCs of almost all hub genes were greater than 0.75 (Figure 7; Supplementary Table S1).

FIGURE 7. ROC analysis of seven key FDEGs. (A) on the GSE12021 dataset. (B) on the GSE1919 dataset.
CIBERSORTx was used to identify the types of immune cells involved in the formation of OA. We obtained the proprotions of 22 kinds of immune cell types between OA and normal synovium. Compared with normal synoviums, the proportions of T cells regulatory (Tregs), macrophages M0 and mast cells resting were relatively high, while T cells CD4 memory activated and mast cells activated were low (Figure 8; Supplementary Table S3).
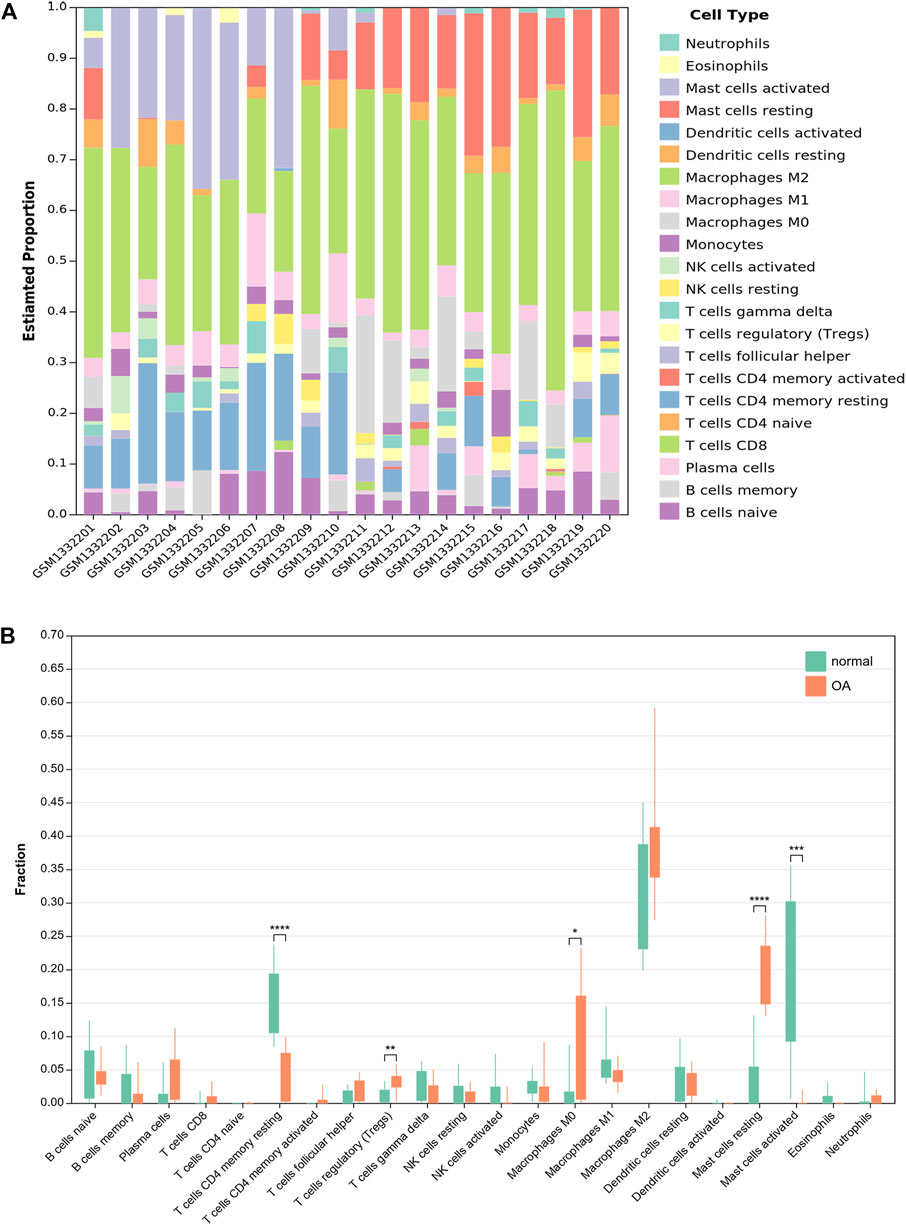
FIGURE 8. Results of immune infiltration by CIBERSORTx. (A) Bar-plot showed the composition of 22 kinds of immune cells in GSE55235. (B) Box-plot presented the difference of immune infiltration of 22 kinds of immune cells.
Previously, researchers focused mainly on articular cartilage while ignoring the function of soft tissues surrounding knee joints in the occurrence of OA (Trachana et al., 2019). Recently, increasing studies have suggested that synovitis may also occur during the early to late stage of OA (Wang et al., 2018). Therefore, studying the mechanism of synovitis is vital. However, little is known about the mechanism of synovitis in OA (Neumann et al., 2002). Moreover, while ferroptosis is extensively investigated in various tumor types (Wang et al., 2016; Zhang et al., 2018), its function in synovitis of OA is much less clear.
This work identified the molecular signatures of ferroptosis that were associated with synovitis of OA by analyzing the DEGs in OA cases compared to healthy subjects. Finally, 53 FDEGs were acquired by intersecting GSE55235 with FerrDb. Furthermore, GO and KEGG analyses were conducted in order to investigate the GO terms and pathways enriched by these 53 FDEGs. The oxidative stress and NADA(P)H oxidase were significantly enriched by these FDEGs, suggesting that these FDEGs were related to oxidation, which agreed with previous results (Shen et al., 2021). Through KEGG pathway enrichment, we observed that the genes were mostly enriched in the FoxO and HIF-1 signaling pathway. FoxO regulated the cell-aging mechanisms, such as defense against OS and autophagy (van der Horst and Burgering, 2007). Besides, the activation and level of FoxO are associated with OA and aging (Akasaki et al., 2014). HIF-1α is a major transcription factor regulating the developmental and cellular responses to hypoxia. The loss of HIF-1α has been shown to aggravate the level of MMP13 and degrade cartilage tissue (Bouaziz et al., 2016). Our results helped describe the aging and OS-related pathways, which might expand our understanding of the mechanisms of OA.
Furthermore, we examined the PPI results using the program cytoHubba in Cytoscape, which revealed seven key FDEGs, namely, ATF3, IL6, CDKN1A, IL1B, EGR1, JUN, and CD44. They have very high diagnostic efficiency in GSE12021 and GSE 1919. Furthermore, this work conducted KEGG analysis on the seven hub FDEGs. The results indicated that these hub FDEGs were mostly associated with AGE-RAGE pathway and the Epstein-Barr virus infection. Epstein-Barr virus was reported to show association with rheumatoid arthritis (Balandraud and Roudier, 2018). However, there are still few reports about Epstein-Barr virus infection and OA. According to our findings, this pathway is possibly related to ferroptosis-dependent synovial hyperplasia. However, further research is needed.
MiRNAs have been reported to be related to OA, and several miRNAs have been implicated in disease pathogenesis (Zhang et al., 2021). This work also established the miRNA-target gene network, based on which, miR-34a-5p and miR-155-5p interacted with all the four ferroptosis-driver hub FDEMs (ATF3, IL6, IL1B and EGR1) were selected. Mir-34a-5p expression is significantly increased in the synovium of patients with advanced OA, but the underlying mechanisms need to be further elucidated (Endisha et al., 2021). Recent research has found that mir-34a-5p targeting SIRT1 induces cell ferroptosis (Hao et al., 2022). But its role in OA is still unclear. Mir-155-5p was found to be related to OA (Soyocak et al., 2017), and it promoted polarization of macrophages M1 and reduced apoptosis of macrophages (Li et al., 2021). According to the results of our study, these miRNAs might participate in the ferroptosis of the synovium. However, their roles in regard to ferroptosis in OA synovial hyperplasia needs to be further clarified.
Ferroptosis has been reported to be associated with immune diseases such as rheumatoid arthritis (Zhao et al., 2022). Nevertheless, the exact mechanism between ferroptosis and immunity remains unclear. So, we used CIBERSORTx in order to investigate the infiltration of immune cells in OA synovium. The results showed that some immune cells in OA synovium were significantly different from normal synoviums. Compared with normal synoviums, the proportions of T cells regulatory (Tregs), macrophages M0 and mast cells resting were relatively high, while T cells CD4 memory activated and mast cells activated were low. However, the exact mechanism of their interaction remains to be further studied.
However, this study still has some limitations. At first, this work used a small sample size, which might induce bias. Second, more in-vivo and in-vitro studies were required to verify the diagnostic and prognostic significance of these factors. Finally, a comprehensive understanding of the hub-gene target of OA is necessary for the discovery and the progress of appropriate therapeutic strategies.
Our study identified seven hub FDEGs as possible diagnostic biomarkers and therapeutic targets for synovitis of OA. These seven hub genes (including ATF3, IL6, CDKN1A, IL1B, EGR1, JUN and CD44) were significantly downregulated. Among them, four were ferroptosis-driver genes, which were possibly associated with synovial hyperplasia. This study also predicted certain possible target miRNAs (miR-34a-5p and miR-155-5p), which might be related to the pathophysiological process of OA via ferroptosis. In addition, our analysis confirmed differences in immune infiltration between OA and normal specimens. Moreover, this study may offer more insight into the identification of markers for the diagnosis and treatment of OA.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
NG conceptualized the study design and analyze the data. LX wrote the manuscript.
This research was funded by the Foundation of Jinan Science and Technology Plan Project, grant number 202019167.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.992044/full#supplementary-material
Akasaki, Y., Hasegawa, A., Saito, M., Asahara, H., Iwamoto, Y., and Lotz, M. K. (2014). Dysregulated FOXO transcription factors in articular cartilage in aging and osteoarthritis. Osteoarthr. Cartil. 22, 162–170. doi:10.1016/j.joca.2013.11.004
Balandraud, N., and Roudier, J. (2018). Epstein-Barr virus and rheumatoid arthritis. Jt. Bone Spine 85, 165–170. doi:10.1016/j.jbspin.2017.04.011
Bouaziz, W., Sigaux, J., Modrowski, D., Devignes, C. S., Funck-Brentano, T., Richette, P., et al. (2016). Interaction of HIF1α and β-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc. Natl. Acad. Sci. U. S. A. 113, 5453–5458. doi:10.1073/pnas.1514854113
Camacho-Encina, M., Balboa-Barreiro, V., Rego-Perez, I., Picchi, F., Vanduin, J., Qiu, J., et al. (2019). Discovery of an autoantibody signature for the early diagnosis of knee osteoarthritis: Data from the osteoarthritis initiative. Ann. Rheum. Dis. 78, 1699–1705. doi:10.1136/annrheumdis-2019-215325
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 149, 1060–1072. doi:10.1016/j.cell.2012.03.042
Endisha, H., Datta, P., Sharma, A., Nakamura, S., Rossomacha, E., Younan, C., et al. (2021). MicroRNA-34a-5p promotes joint destruction during osteoarthritis. Arthritis Rheumatol. 73, 426–439. doi:10.1002/art.41552
Friedmann Angeli, J. P., Schneider, M., Proneth, B., Tyurina, Y. Y., Tyurin, V. A., Hammond, V. J., et al. (2014). Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell. Biol. 16, 1180–1191. doi:10.1038/ncb3064
Hao, R., Ge, J., Song, X., Li, F., Sun-Waterhouse, D., and Li, D. (2022). Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ. Toxicol. 37, 41–51. doi:10.1002/tox.23376
Kraus, V. B., Blanco, F. J., Englund, M., Karsdal, M. A., and Lohmander, L. S. (2015). Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr. Cartil. 23, 1233–1241. doi:10.1016/j.joca.2015.03.036
Li, G. S., Cui, L., and Wang, G. D. (2021). miR-155-5p regulates macrophage M1 polarization and apoptosis in the synovial fluid of patients with knee osteoarthritis. Exp. Ther. Med. 21, 68. doi:10.3892/etm.2020.9500
Luo, W., and Brouwer, C. (2013). Pathview: An R/bioconductor package for pathway-based data integration and visualization. Bioinformatics 29, 1830–1831. doi:10.1093/bioinformatics/btt285
Miao, Y., Chen, Y., Xue, F., Liu, K., Zhu, B., Gao, J., et al. (2022). Contribution of ferroptosis and GPX4's dual functions to osteoarthritis progression. EBioMedicine 76, 103847. doi:10.1016/j.ebiom.2022.103847
Nelson, A. E. (2018). Osteoarthritis year in review 2017: Clinical. Osteoarthr. Cartil. 26, 319–325. doi:10.1016/j.joca.2017.11.014
Neumann, E., Kullmann, F., Judex, M., JüSTEN, H. P., Wessinghage, D., Gay, S., et al. (2002). Identification of differentially expressed genes in rheumatoid arthritis by a combination of complementary DNA array and RNA arbitrarily primed-polymerase chain reaction. Arthritis Rheum. 46, 52–63. doi:10.1002/1529-0131(200201)46:1<52::AID-ART10048>3.0.CO;2-1
Qu, C., Peng, Y., and Liu, S. (2022). Ferroptosis biology and implication in cancers. Front. Mol. Biosci. 9, 892957. doi:10.3389/fmolb.2022.892957
Roseti, L., Desando, G., Cavallo, C., Petretta, M., and Grigolo, B. (2019). Articular cartilage regeneration in osteoarthritis. Cells 8, E1305. doi:10.3390/cells8111305
Shen, C., Gao, M., Chen, H., Zhan, Y., Lan, Q., Li, Z., et al. (2021). Reactive oxygen species (ROS)-responsive nanoprobe for bioimaging and targeting therapy of osteoarthritis. J. Nanobiotechnology 19, 395. doi:10.1186/s12951-021-01136-4
Soyocak, A., Kurt, H., Ozgen, M., Turgut Cosan, D., Colak, E., and Gunes, H. V. (2017). miRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene 627, 207–211. doi:10.1016/j.gene.2017.06.027
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., Dixon, S. J., et al. (2017). Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 171, 273–285. doi:10.1016/j.cell.2017.09.021
Trachana, V., Mourmoura, E., Papathanasiou, I., and Tsezou, A. (2019). Understanding the role of chondrocytes in osteoarthritis: Utilizing proteomics. Expert Rev. Proteomics 16, 201–213. doi:10.1080/14789450.2019.1571918
van der Horst, A., and Burgering, B. M. (2007). Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell. Biol. 8, 440–450. doi:10.1038/nrm2190
Wang, H., Wang, Q., Yang, M., Yang, L., Wang, W., Ding, H., et al. (2018). Histomorphology and innate immunity during the progression of osteoarthritis: Does synovitis affect cartilage degradation? J. Cell. Physiol. 233, 1342–1358. doi:10.1002/jcp.26011
Wang, S. J., Li, D., Ou, Y., Jiang, L., Chen, Y., Zhao, Y., et al. (2016). Acetylation is crucial for p53-mediated ferroptosis and tumor suppression. Cell. Rep. 17, 366–373. doi:10.1016/j.celrep.2016.09.022
Yao, X., Sun, K., Yu, S., Luo, J., Guo, J., Lin, J., et al. (2021). Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Transl. 27, 33–43. doi:10.1016/j.jot.2020.09.006
Yu, G., Wang, L. G., Han, Y., and He, Q. Y. (2012). clusterProfiler: an R package for comparing biological themes among gene clusters. Omics 16, 284–287. doi:10.1089/omi.2011.0118
Zhang, Q. C., Hu, S. Q., Hu, A. N., Zhang, T. W., Jiang, L. B., and Li, X. L. (2021). Autophagy-activated nucleus pulposus cells deliver exosomal miR-27a to prevent extracellular matrix degradation by targeting MMP-13. J. Orthop. Res. 39, 1921–1932. doi:10.1002/jor.24880
Zhang, Y., Shi, J., Liu, X., Feng, L., Gong, Z., Koppula, P., et al. (2018). BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat. Cell. Biol. 20, 1181–1192. doi:10.1038/s41556-018-0178-0
Zhang, Z., Huang, C., Jiang, Q., Zheng, Y., Liu, Y., Liu, S., et al. (2020). Guidelines for the diagnosis and treatment of osteoarthritis in China (2019 edition). Ann. Transl. Med. 8, 1213. doi:10.21037/atm-20-4665
Zhao, J., Dar, H. H., Deng, Y., St Croix, C. M., Li, Z., Minami, Y., et al. (2020). PEBP1 acts as a rheostat between prosurvival autophagy and ferroptotic death in asthmatic epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 117, 14376–14385. doi:10.1073/pnas.1921618117
Keywords: osteoarthritis, synovitis, ferroptosis, bioinformatics analysis, differentially expressed genes, ferroptosis DEGs
Citation: Xia L and Gong N (2022) Identification and verification of ferroptosis-related genes in the synovial tissue of osteoarthritis using bioinformatics analysis. Front. Mol. Biosci. 9:992044. doi: 10.3389/fmolb.2022.992044
Received: 15 July 2022; Accepted: 03 August 2022;
Published: 29 August 2022.
Edited by:
Li-Bo Jiang, Zhongshan Hospital, Fudan University, ChinaReviewed by:
Xingshan Wang, Beijing Jishuitan Hospital, ChinaCopyright © 2022 Xia and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningji Gong, Z29uZ25pbmdqaUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.