
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Biosci. , 13 September 2022
Sec. RNA Networks and Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmolb.2022.986722
 Soudeh Ghafouri-Fard1
Soudeh Ghafouri-Fard1 Yadollah Poornajaf2
Yadollah Poornajaf2 Bashdar Mahmud Hussen3,4
Bashdar Mahmud Hussen3,4 Atefe Abak5
Atefe Abak5 Hamed Shoorei6,7
Hamed Shoorei6,7 Mohammad Taheri8,9*
Mohammad Taheri8,9* Guive Sharifi10*
Guive Sharifi10*Rho Associated Coiled-Coil Containing Protein Kinase 1 (ROCK1) is a protein serine/threonine kinase which is activated upon binding with the GTP-bound form of Rho. This protein can modulate actin-myosin contraction and stability. Moreover, it has a crucial role in the regulation of cell polarity. Therefore, it participates in modulation of cell morphology, regulation of expression of genes, cell proliferation and differentiation, apoptotic processes as well as oncogenic processes. Recent studies have highlighted interactions between ROCK1 and several non-coding RNAs, namely microRNAs, circular RNAs and long non-coding RNAs. Such interactions can be a target of medications. In fact, it seems that the interactions are implicated in therapeutic response to several medications. In the current review, we aimed to explain the impact of these interactions in the pathoetiology of cancers as well as non-malignant disorders.
Rho Associated Coiled-Coil Containing Protein Kinase 1 (ROCK1) human gene is located on 18q11.1 The protein serine/threonine kinase encoded by this gene is activated upon binding with the GTP-bound form of Rho. Functioning as a small GTPase, Rho can regulate construction of focal adhesion molecules and stress fibers in fibroblasts, establishment of adhesion molecules that induce platelet aggregation and lymphocyte adhesion. Activity of Rho is regulated through binding with GDP or GTP. ROCK1 is regarded as an important modulator of actin-myosin contraction and stability. Moreover, it has a crucial role in the regulation of cell polarity. Therefore, it participates in modulation of cell morphology, regulation of expression of genes, cell proliferation and differentiation, apoptotic processes as well as stemness and oncogenic processes (Rath and Olson, 2012).
In fact, members of the Rho family such as RhoA and RhoC can enhance production of actomyosin contractile force via ROCK1- and ROCK2-mediated phosphorylation of several downstream targets, such as LIMK1/2 and MLC (Riento and Ridley, 2003). ROCK proteins have catalytic kinase domain responsible for the substrate promiscuity, a coiled-coil region, and a split PH domain that is intersected by the protein kinase C conserved region 1 (Rath and Olson, 2012). A single Rho-binding domain (RBD) exists inside the coiled-coil region of both ROCK proteins (Fujisawa et al., 1996), in addition to several Rho GTPases-interacting regions which have been identified within the coiled-coil region of ROCK1, which contributes to its localization (Blumenstein and Ahmadian, 2004).
Recent studies have highlighted interactions between ROCK1 and several non-coding RNAs, namely microRNAs (miRNAs), circular RNAs (circRNAs) and long non-coding RNAs (lncRNAs). In the current review, we aimed to explain the impact of these interactions in the pathoetiology of cancers as well as non-malignant disorders. Figure 1 illustrates that aberrant expression of various ncRNAs could contribute in adversely modulating the ROCK1 pathway, with consequent triggering several kinds of cancers as well as a number of non-malignant conditions.
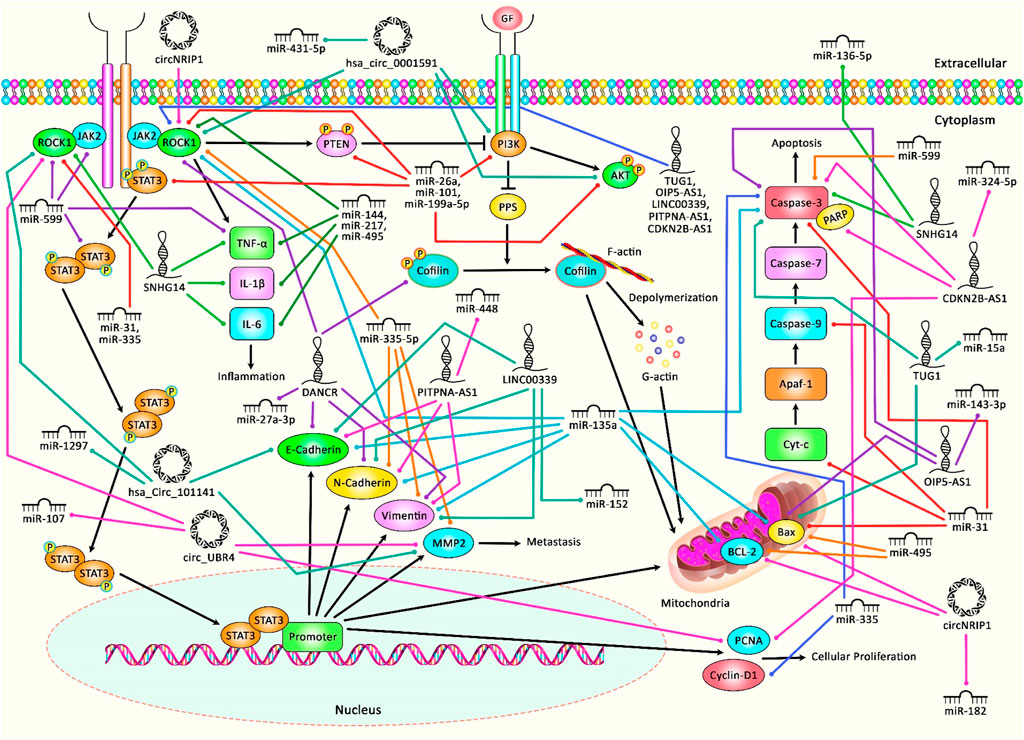
FIGURE 1. A schematic diagram of the role of several ncRNAs in triggering the ROCK1 signaling pathway in human disorders and malignancies. Overexpression of ROCK1 could result in triggering the activation of PTEN and PI3K, leading to PP1 and PP2A upregulation, and dephosphorylation of cofilin that could bind to G-actin and translocate to mitochondria, and eventually could cause cytochrome c release, caspases activation and apoptosis. ROCK1 could also play an effective role in activating and rapidly phosphorylating JAK2, which in turn could enhance downstream signaling cascades containing STAT3 and PI3K. Previous studies have authenticated that several ncRNAs (miRNAs, circRNAs, and lncRNAs) could have a crucial role in regulating the ROCK1 pathway in various human diseases as well as cancers. All the information regarding the role of these ncRNAs in the modulation of this cascade can be seen in Tables 1–7.
Interactions between miRNAs and ROCK1 have been assessed in different disorders, including metabolic syndrome, diabetes, acute lung injury, endometriosis, LPS-induced lung endothelial hyperpermeability and pneumonia. These miRNAs mainly bind to 3′ UTR of ROCK and suppress its expression. Thus, the underlying mechanisms of such interactions are shared between these disorders. For instance, Guo et al. showed up-regulation of levels of a ROCK1-targeting miRNA, namely miR-324-5p, in the circulation of patients with hyperglycemia or hyperlipidemia. Investigations in an animal model of diabetes type II and obesity also verified over-expression of miR-324-5p both in the peripheral blood and hepatic tissue. Up-regulation of this miRNA results in reduction of activity of the AKT/GSK pathway and enhancement of lipid buildup. Moreover, ROCK1 silencing has resulted in deterioration of lipid and glucose metabolism. Notably, ROCK1 silencing has overturned the effect of miR-324-5p inhibition on amelioration of glucose and lipid metabolism. Taken together, miR-324-5p was shown to regulate metabolism of glucose and lipid through influencing expression of ROCK1 (Guo et al., 2020). Another miRNA, miR-217, was shown to affect immune responses and proliferative and migratory potential of vascular smooth muscle cells (VSMCs) in high-glucose condition through modulation of ROCK1. Expression of miR-217 was increased in high glucose-exposed VSMCs as well as aorta VSMCs obtained from diabetic animals. Mechanistically, miR-217 can induce cell cycle arrest, inhibit of proliferation, reduce migration, and enhance apoptosis of VSMCs in high glucose conditions through regulation of expression of ROCK1 (Zhou et al., 2021).
Another experiment in an animal model of sepsis-induced acute lung injury demonstrated the effect of miR-539-5p in alleviation of lung injury through modulation of expression of ROCK1. miR-539-5p could also decrease apoptotic potential and inflammatory responses in LPS-treated pulmonary microvascular endothelial cells of mice. The effects of miR-539-5p in inhibition of caspase-3 activity and inhibition of release of inflammatory cytokines have been reversed by up-regulation of ROCK1 (Meng et al., 2019).
Another study revealed the down-regulation of miR-202-3p expression in primary endometrial stromal cells obtained from eutopic or ectopic endometriosis compared to endometrial stromal cells from normal endometrium. Functional studies have shown that up-regulation of miR-202-3p impairs viability, migratory potential, and invasion of these cells, while it is silencing has the opposite impact. miR-202-3p mimics could decrease expression of ROCK1 in endometrial stromal cells. Taken together, dysregulation of miR-202-3p can participate in the pathogenesis of endometriosis through influencing expression of ROCK1 (Zhang et al., 2020a). Table 1 indicates the role of ROCK1-interacting miRNAs in non-malignant disorders.
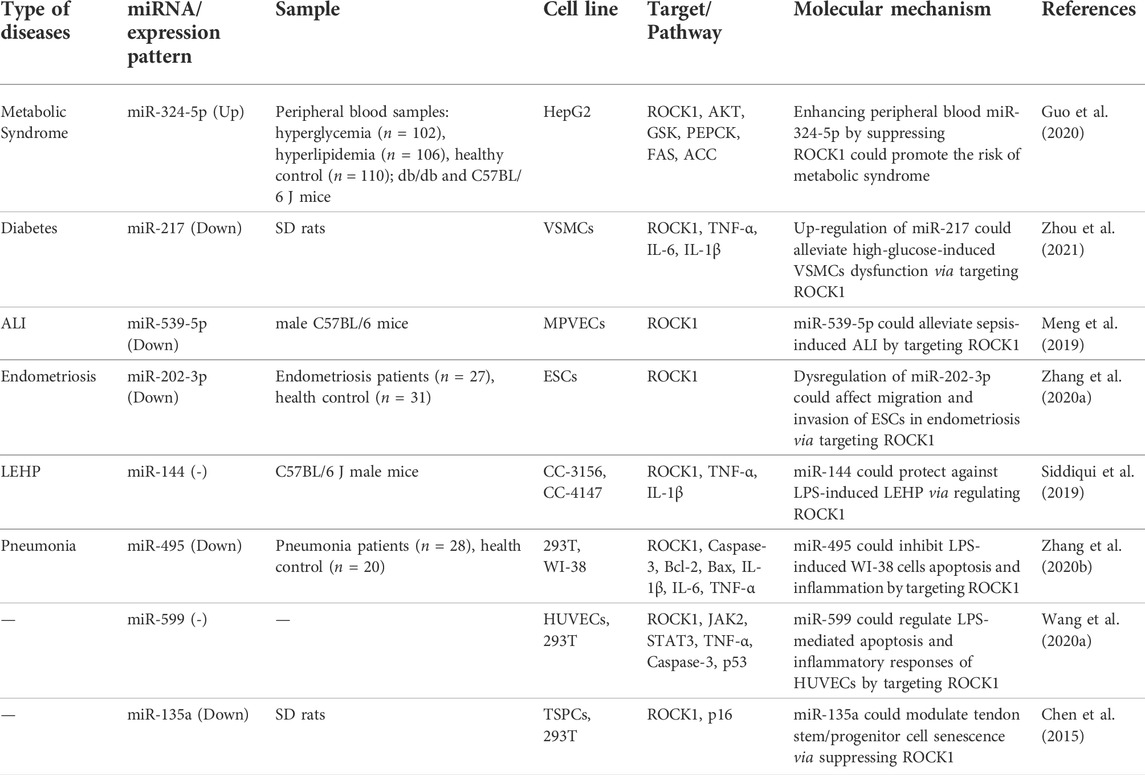
TABLE 1. ROCK1-interacting miRNAs in non-malignant conditions (ALI: acute lung injury, LEHP: LPS-induced lung endothelial hyperpermeability).
Similarly, cancer-related miRNAs can bind to 3′ UTR of ROCK1 to regulate its expression. A number of ROCK1-interacting miRNAs have been found to reduce tumor burden. For instance, experiments in non-small cell lung carcinoma cells showed that the tumor suppressor roles of miR-135a (Zhao et al., 2020), miR-148b (Luo and Liang, 2018) and miR-335-5p (Du et al., 2019) are exerted through modulation of expression of ROCK1. The interactions between miRNAs and ROCK1 have been mostly assessed in osteosarcoma cells among other cancers. miR-101 (Jiang et al., 2017), miR-139 (Fan et al., 2019), miR-144 (Liu et al., 2019), miR-202-5p (Li et al., 2018), miR-150 (Li et al., 2017a), miR-335 (Wang et al., 2017) and miR-214-5p (Zhang et al., 2017) are examples of down-regulated miRNAs in this type of cancer that were shown to directly regulate expression of ROCK1.
Roberto et al. measured expression of a number of ROCK1/ROCK2-targeting miRNAs, namely miR-124-3p, miR-138-5p, miR-139-5p, miR-335-5p and miR-584-5p in samples obtained from patients with Ewing sarcoma. They reported down-regulation of ROCK1 in these tissues; however its expression has not been associated with pathological factors. Expression levels of miR-124-3p, miR-139-5p and miR-335-3p were also shown to be reduced in these samples in correlation with ROCK1 levels. Down-regulation of miR-139-5p and miR-584-5p has been associated with disease progression. Moreover, down-regulation of miR-139-5p and miR-124-3p has been linked with poor clinical outcome. However, the results of in vitro studies on function of miR-139-5p were inconsistent. While its overexpression has led to a significant decrease in invasive abilities of cells, their clonogenic capability was enhanced (Roberto et al., 2020).
Expression levels of ROCK1-targeting miR-592 were reported to be decreased in clinical samples from patients with acute myeloid leukemia (AML) as well as AML cell lines. Down-regulation of miR-592 was associated with advanced French-American-British classification and adverse clinical outcomes. Functional studies also showed that up-regulation of miR-592 inhibits cell growth and metastatic capacity of cells, and enhances apoptosis (Xu et al., 2019). Table 2 shows the role of ROCK1-interacting miRNAs in cancers.
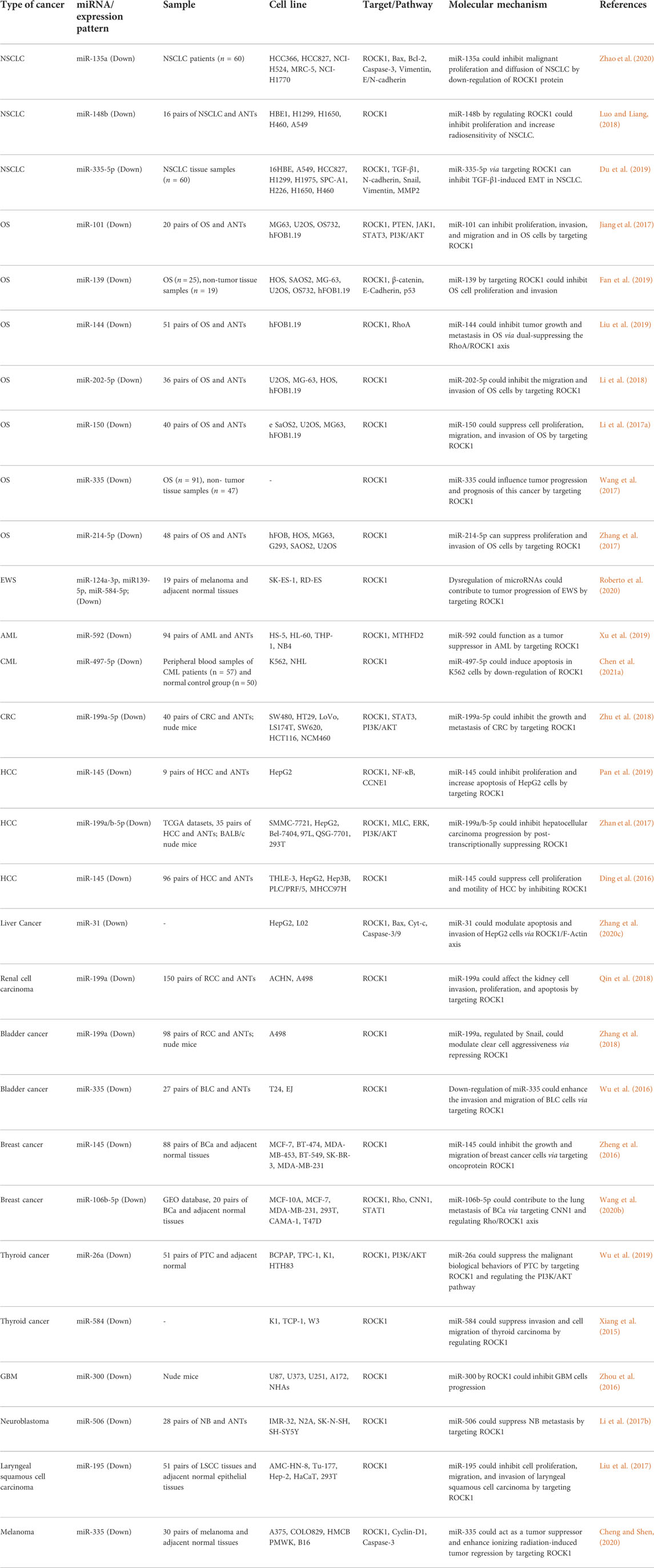
TABLE 2. ROCK1-interacting miRNAs in cancers (ANTs: adjacent non-cancerous tissues, NSCLC: non-small cell lung cancer, OS: osteosarcoma, EWS: Ewing sarcoma, AML/CML: acute/chronic myeloid leukemia, HCC: hepatocellular carcinoma, CRC: colorectal cancer).
CircRNAs mainly affect expression of ROCK1 through sponging ROCK1-interacting miRNAs. These interactions have been assessed in the context of non-alcoholic fatty liver disease and atherosclerosis. Expression of circ_0057558 was shown to be increased in nonalcoholic fatty liver disease, parallel with down-regulation of miR-206. Circ_0057558 silencing and up-regulation of miR-206 could decrease accumulation of lipids and secretion of triglycerides. Functionally, miR-206 could directly target ROCK1 and activate AMPK pathway through this route. In fact, circ_0057558 serves as a miR-206 sponge to suppress AMPK signals. Cumulatively, circ_0057558/miR-206/ROCK1/AMPK was found to be a functional axis in the etiology of nonalcoholic fatty liver disease (Chen et al., 2021b).
Another study reported the up-regulation of circ_UBR4 in an in vitro model of atherosclerosis. Moreover, expression levels of circ_UBR4 and ROCK1 have been found to be increased in sera of patients with atherosclerosis, parallel with down-regulation of miR-107. Circ_UBR4 silencing has led to induction of cell cycle arrest, suppression of cell viability, colony-forming capability, migration aptitude, and depression of expression of proliferating cell nuclear antigen and MMP2. miR-107 was found to act as a mediator of circ_UBR4 effects on ROCK1 expression. Taken together, circ_UBR4/miR-107/ROCK1 pathway has a possible role in the development of atherosclerosis through modulation of proliferative ability, migration, and cell cycle transition of human VSMCs (Zhang et al., 2021). Table 3 shows the role of ROCK1-interacting circRNAs in non-malignant conditions.
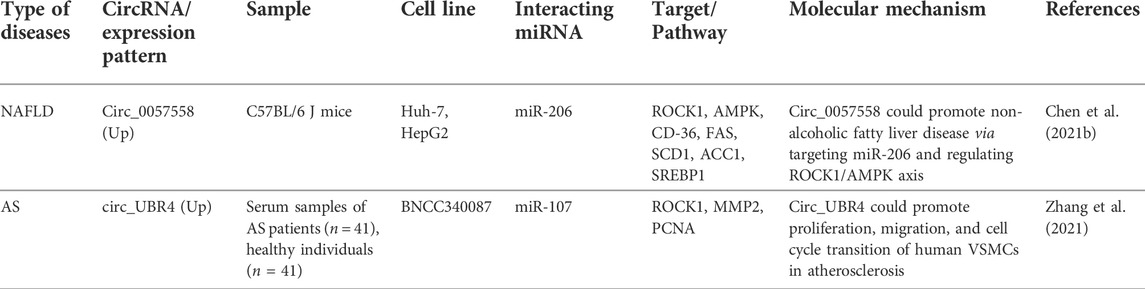
TABLE 3. ROCK1-interacting circRNAs in non-malignant conditions (NAFTD: Non-alcoholic fatty liver disease, AS: atherosclerosis).
A number of ROCK1-interacting circRNAs have been reported to be up-regulated in tissue or serum samples of patients with malignant conditions. For instance, circ-TIMELESS via the miR‐136‐5p/ROCK1 axis could regulate proliferation of lung squamous cell carcinoma cells (Zhang et al., 2020d). Moreover, hsa_circ_0001591 could promote metastasis and cell proliferation of human melanoma via modulation of ROCK1 through targeting miR-431-5p (Yin et al., 2021). hsa_circ_0043278 could promote cell proliferation and migration of NSCLC via sponging miR-520f and regulating ROCK1 expression (Cui et al., 2019). Finally, circ-ABCB10 could promote growth and metastasis of NPC by up-regulation of ROCK1 (Duan et al., 2020). Table 4 shows the role of ROCK1-interacting circRNAs in cancers.
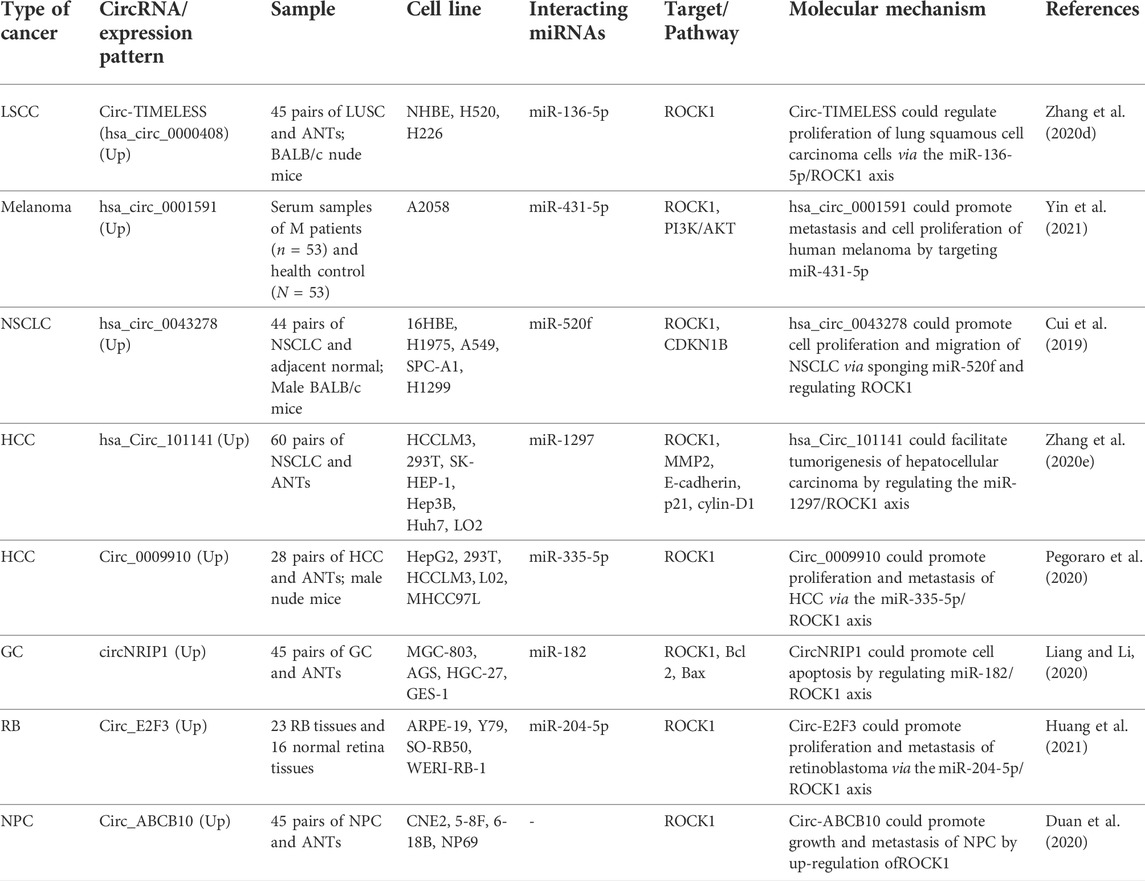
TABLE 4. ROCK1-interacting circRNAs in cancers (ANT: adjacent non-cancerous tissue, LSCC: Lung squamous cell carcinoma, NSCLC: Non-small cell lung cancer, HCC: Hepatocellular carcinoma, GC: gastric cancer, RB: retinoblastoma, NPC: Nasopharyngeal carcinoma).
Similar to circRNAs, lncRNAs can act as sponges for ROCK1-interacting miRNAs. Experiments in an animal model of Alzheimer’s disease confirmed reduction of spatial learning and memory abilities, noticeable pathological injuries, increase in apoptosis of hippocampal neurons and reduction of antioxidant ability. TUG1 silencing and miR-15a up-regulation could result in improvement of spatial learning and memory capacities, amelioration of pathological injuries, suppression of apoptosis of neurons, and enhancement of antioxidant capacity of hippocampal neurons in the animal model of Alzheimer’s disease. In vitro studies have also confirmed that TUG1 silencing and miR-15a up-regulation constrains apoptosis of hippocampal neurons. This miRNA directly targets ROCK1 (Li et al., 2020). Another study has shown that SNHG14 can assist in induction of inflammatory response by cerebral ischemia/reperfusion (I/R) injury via regulating miR-136-5p/ROCK1 axis (Zhong et al., 2019). SNHG7 is another ROCK1-interacting lncRNA which participates in the pathoetiology of cardiac fibrosis. Expression of this lncRNA was found to be up-regulated in the infarcted and peri-infarcted areas of animal models. SNHG7 silencing led to the reduction of expression levels of Col1 and α-SMA. Moreover, suppression of SNHG7 levels resulted in improvement of cardiac function after myocardial infarction. SNHG7 acts as a molecular sponge for miR-34-5p. Co-transfection of SNHG7 and miR-34-5p suppressed viability and proliferative ability of cardiac fibroblasts. Taken together, SNHG7 has a role in induction of cardiac fibrosis through modulation of miR-34-5p/ROCK1 axis (Wang et al., 2020c). Table 5 shows the role of ROCK1-interacting lncRNAs in non-malignant conditions.
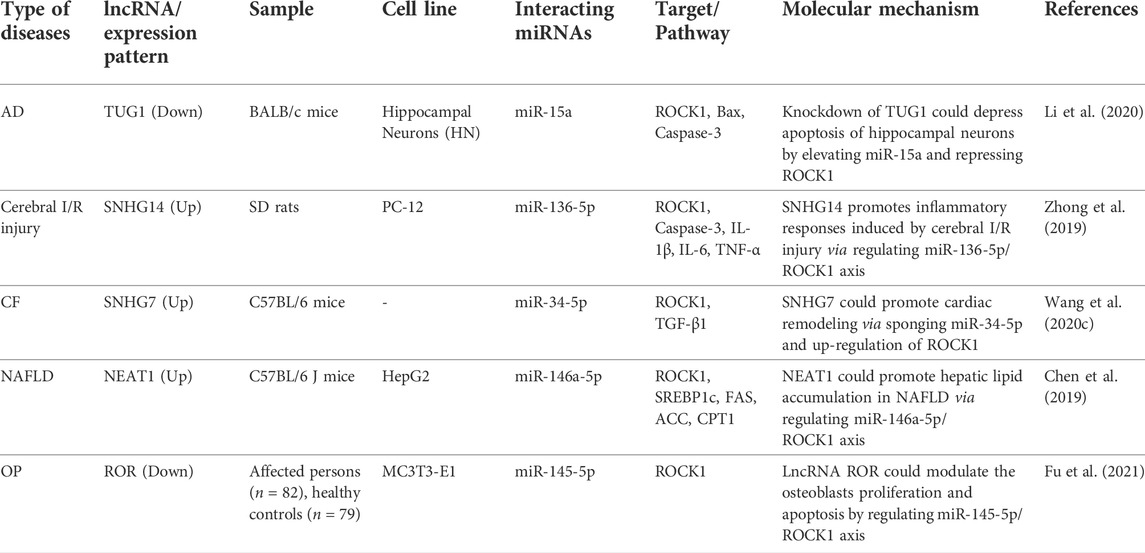
TABLE 5. ROCK1-interacting lncRNAs in non-malignant conditions (AD: Alzheimer’s disease, Cerebral I/R injury: Cerebral ischemia/reperfusion injury, CF: Cardiac fibrosis, NAFLD: Non-alcoholic fatty liver disorder, OP: Osteoporosis).
The impact of ROCK1-interacting lncRNAs on carcinogenesis has been evaluated in different cancers such as lung cancer, osteosarcoma, hepatocellular carcinoma and cervical cancer. For instance, PSMG3-AS1 via down-regulation of miR-340 and subsequent up-regulation of ROCK1 could promote cell migration and invasion of non-small cell lung carcinoma (Wang et al., 2021a). Moreover, KCNMB2-AS1 via sponging miR-374a-3p and regulating ROCK1 could assist in the progression of lung cancer (Yang et al., 2020).
In osteosarcoma, HAGLROS could promote cell invasion and metastasis via sponging miR-152 and up-regulation of ROCK1 (Zhou et al., 2020). Moreover, DANCR could promote proliferation and metastasis of these cells via sponging ROCK1-targeting miRNAs miR-335-5p and miR-1972 (Wang et al., 2018). Finally, HOXA11-AS could enhance the invasion and migration of osteosarcoma via sponging miR-124-3p and up-regulation of ROCK1 (Cui et al., 2017).
In cervical cancer, OIP5-AS1 (Song et al., 2020) and DANCR (Liang et al., 2019) were found to up-regulate ROCK1 via sponging miR-143-3p and miR‐335‐5p, respectively. Table 6 shows the role of ROCK1-interacting lncRNAs in cancers.
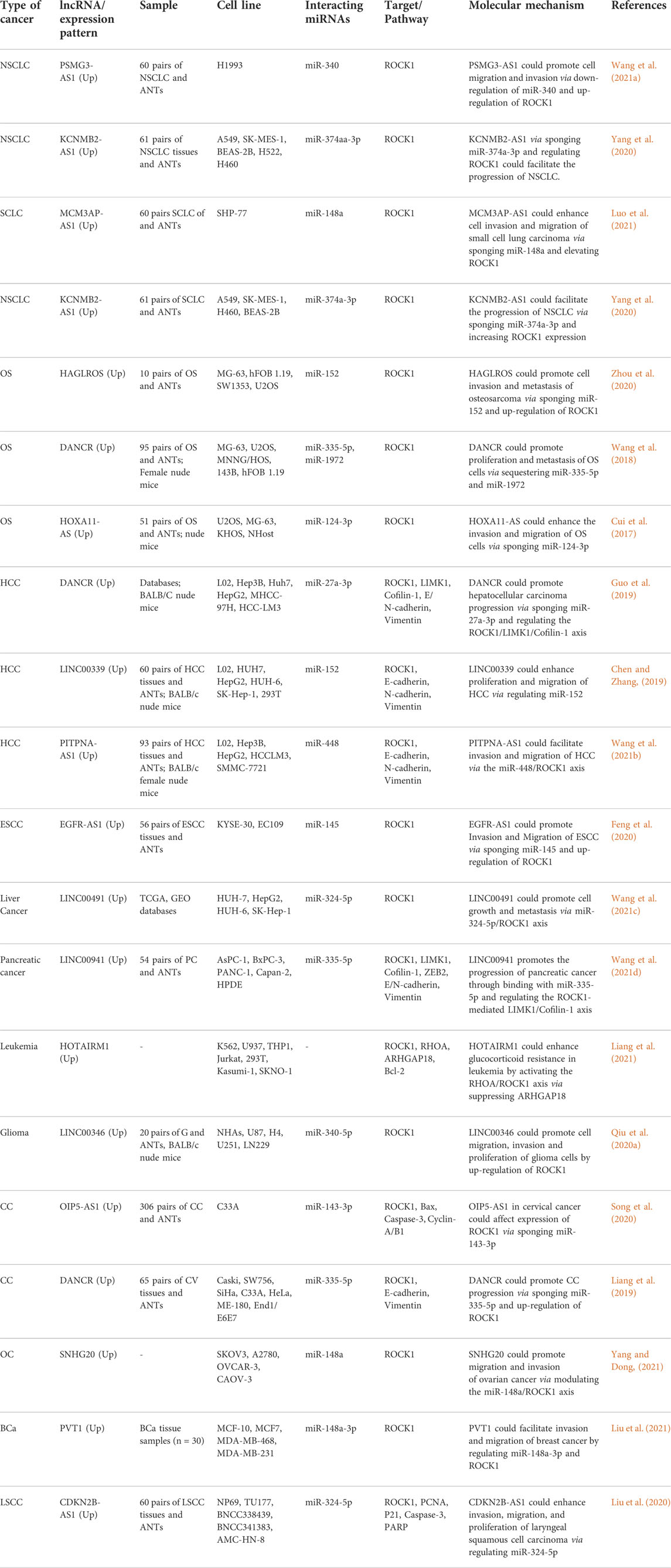
TABLE 6. ROCK1-interacting lncRNAs in cancers (ANT: adjacent non-cancerous tissue, NSCLC: non-small cell lung cancer, OS: osteosarcoma, HCC: hepatocellular carcinoma, ESCC: Esophageal squamous cell carcinoma, CC: cervical cancer, OC: ovarian cancer, BCa: breast cancer, LSCC: Laryngeal squamous cell carcinoma).
A number of therapeutic agents have been found to act through regulation of ROCK1-interacting non-coding RNAs. For instance, sevoflurane through regulation of circ_0079593/miR-633/ROCK1 axis could suppress tumorigenesis process in glioma (Cheng and Cheng, 2021). In addition, dexmedetomidine (DEX) could ameliorate cerebral I/R injury via the miR-214/ROCK1/NF-κB axis (Liu et al., 2021). Besides, the therapeutic effects of curcumin in osteoarthritis are possibly exerted via modulating the miR-143/ROCK1/TLR9 and miR-124/NF-kB pathways (Qiu et al., 2020b). Furthermore, some ROCK1-interacting non-coding RNAs can affect response to therapeutic agents. For example, circ_PIP5K1A via regulation of miR-493-5p/ROCK1 axis could regulate cisplatin resistance in lung cancer (Feng et al., 2021). Moreover, miR-136-5p could enhance cisplatin sensitivity and suppress invasion and migration in head and neck cancer cells via targeting the ROCK1 (Yang et al., 2021). Table 7 shows the mutual interactions between drug and ROCK1-interacting non-coding RNAs. Figure 2 represents the role of several miRNAs in various human disorders via regulating the ROCK1/NF-κB signaling pathway.
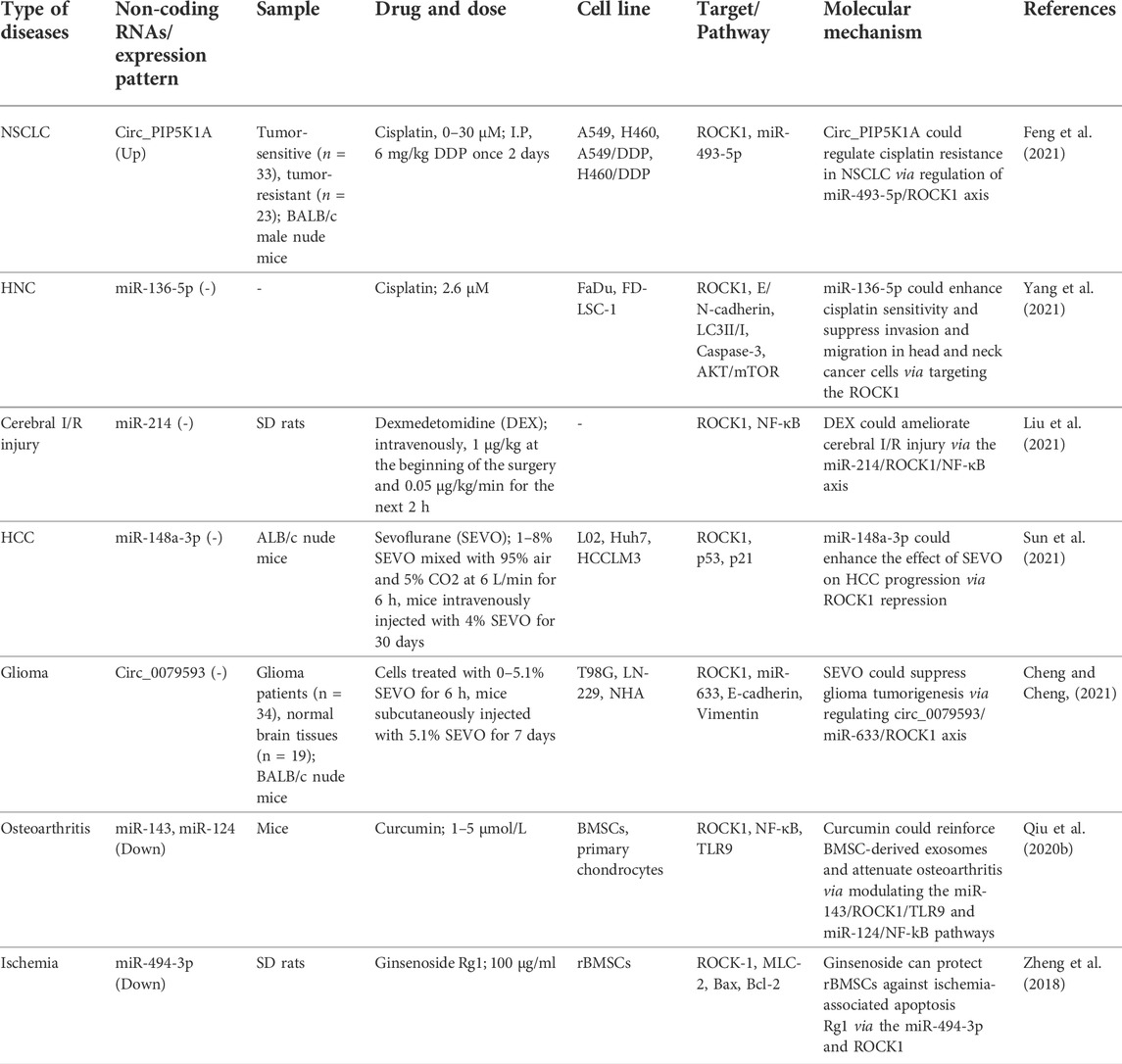
TABLE 7. Drug and ROCK1-interacting non-coding RNAs (NSCLC: Non-small cell lung cancer, HNC: Head and neck cancer, Cerebral I/R injury: cerebral ischemia/reperfusion injury, HCC: hepatocellular carcinoma).
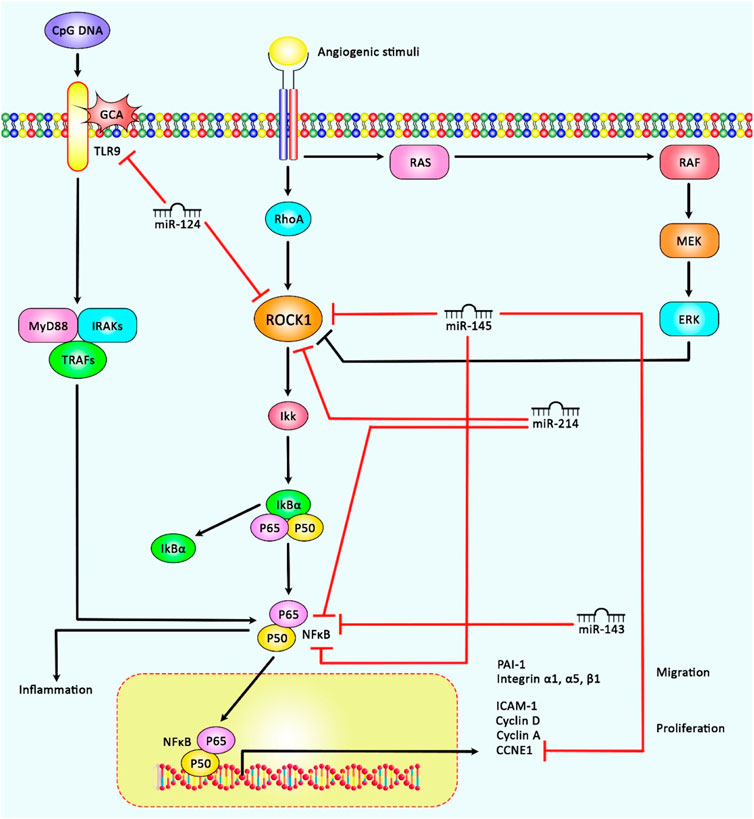
FIGURE 2. A schematic representation of the role of several miRNAs in regulating the ROCK1/NF-κB signaling cascade in cancers and non-malignant disorders. A recent study has detected that miR-145 could play a crucial role in inducing cell cycle suppression and activation of cell apoptosis, and thereby controlling hepatocellular carcinoma via down-regulation of the expression levels of ROCK1, NF-κB as well as CCNE1(27). Another research has demonstrated that up-regulation of miR-143 and miR-124 could down-regulate NF-kB and ROCK1 expression respectively, which could have a therapeutic role in Osteoarthritis (Qiu et al., 2020b). Moreover, accumulating evidence has represented that overexpression of ROCK1 could result in the activation of NF-κB that could in turn aggravate cerebral ischemia/reperfusion injury. Additionally, miR-214 via could target and negatively modulate ROCK1 and NF-κB expression, thereby could play a key role in the protection of DEX against cerebral ischemia/reperfusion injury (Liu et al., 2021)
Several non-coding RNAs have been shown to interact with ROCK1. The interaction between ROCK1 and these transcripts can affect development of different types of cancers as well as a number of non-malignant conditions such as metabolic syndrome, diabetes, acute lung injury, pneumonia, endometriosis, non-alcoholic fatty liver disease, cerebral ischemia/reperfusion injury, myocardial Infarction, osteoporosis and atherosclerosis.
CircRNAs and lncRNAs that influence expression of ROCK1 mainly act through sponging ROCK1-targeting miRNAs. Circ_0057558/miR-206, circ_UBR4/miR-107, circ-TIMELESS/miR‐136‐5p, has_circ_0001591/miR-431-5p, hsa_circ_0043278/miR-520f, hsa_Circ_101141/miR-1297, Circ_0009910/miR-335-5p, circNRIP1/miR-182, circ_E2F3/miR-204-5p, TUG1/miR-15a, SNHG14/miR-136-5p, SNHG7/miR-34-5p, NEAT1/miR-146a-5p, lnc-ROR/miR-145-5p, PSMG3-AS1/miR-340, KCNMB2-AS1/miR-374aa-3p, MCM3AP-AS1/miR-148a, HAGLROS/miR-152, DANCR/miR-335-5p, DANCR/miR-1972, DANCR/miR‐27a‐3p, HOXA11-AS/miR-124-3p, LINC00339/miR‐152, PITPNA-AS1/miR-448 and EGFR-AS1/miR-145 are examples of ROCK1-regulating axes which contribute in the development of human disorders.
In addition, interactions between non-coding RNAs and ROCK1 has important role in determination of response to a number of drugs such as cisplatin, dexmedetomidine, sevoflurane, curcumin and ginsenoside Rg1. In fact, alterations in the expression levels of ROCK1-interacting non-coding RNAs can affect expression of ROCK1 and induce sensitivity or resistance to these drugs through modulation of cell apoptosis or other fundamental aspects of cell biology. Thus, through modulation of expression of these non-coding RNAs, it is possible to enhance therapeutic effects of these substances.
Based on the above-mentioned evidence, it is clear that ROCK1 has direct or indirect interactions with numerous types of non-coding RNAs constructing a complex network. Identification of elements of this network is an important step for unraveling the molecular pathology of human disorders.
SG-F wrote the manuscript and revised it. MT and GS supervised and designed the study. YP, AA, HS, and BMH collected the data and designed the figures and tables. All authors read and approved the submitted version.
The authors would like to thank the clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study (Grant Number 43002285).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Blumenstein, L., and Ahmadian, M. R. (2004). Models of the cooperative mechanism for Rho effector recognition: implications for RhoA-mediated effector activation. J. Biol. Chem. 279 (51), 53419–53426. doi:10.1074/jbc.M409551200
Chen, K., and Zhang, L. (2019). LINC00339 regulates ROCK1 by miR-152 to promote cell proliferation and migration in hepatocellular carcinoma. J. Cell. Biochem. 120 (9), 14431–14443. doi:10.1002/jcb.28701
Chen, L., Wang, G-D., Liu, J-P., Wang, H-S., Liu, X-M., Wang, Q., et al. (2015). miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone 71, 210–216. doi:10.1016/j.bone.2014.11.001
Chen, X., Tan, X-R., Li, S-J., and Zhang, X-X. (2019). LncRNA NEAT1 promotes hepatic lipid accumulation via regulating miR-146a-5p/ROCK1 in nonalcoholic fatty liver disease. Life Sci. 235, 116829. doi:10.1016/j.lfs.2019.116829
Chen, N., Meng, Z., Song, J., Kong, L., Zhang, Y., Guo, S., et al. (2021). miR-497-5p induces apoptosis in K562 cells by downregulating ROCK1. Am. J. Transl. Res. 13 (8), 9278–9284.
Chen, X., Tan, Q-Q., Tan, X-R., Li, S-J., and Zhang, X-X. (2021). Circ_0057558 promotes nonalcoholic fatty liver disease by regulating ROCK1/AMPK signaling through targeting miR-206. Cell Death Dis. 12 (9), 809–812. doi:10.1038/s41419-021-04090-z
Cheng, S., and Cheng, J. (2021). Sevoflurane suppresses glioma tumorigenesis via regulating circ_0079593/miR-633/ROCK1 axis. Brain Res. 1767, 147543. doi:10.1016/j.brainres.2021.147543
Cheng, Y., and Shen, P. (2020). miR-335 acts as a tumor suppressor and enhances ionizing radiation-induced tumor regression by targeting ROCK1. Front. Oncol. 10, 278. doi:10.3389/fonc.2020.00278
Cui, M., Wang, J., Li, Q., Zhang, J., Jia, J., and Zhan, X. (2017). Long non-coding RNA HOXA11-AS functions as a competing endogenous RNA to regulate ROCK1 expression by sponging miR-124-3p in osteosarcoma. Biomed. Pharmacother. 92, 437–444. doi:10.1016/j.biopha.2017.05.081
Cui, J., Li, W., Liu, G., Chen, X., Gao, X., Lu, H., et al. (2019). A novel circular RNA, hsa_circ_0043278, acts as a potential biomarker and promotes non-small cell lung cancer cell proliferation and migration by regulating miR-520f. Artif. Cells Nanomed. Biotechnol. 47 (1), 810–821. doi:10.1080/21691401.2019.1575847
Ding, W., Tan, H., Zhao, C., Li, X., Li, Z., Jiang, C., et al. (2016). MiR-145 suppresses cell proliferation and motility by inhibiting ROCK1 in hepatocellular carcinoma. Tumour Biol. 37 (5), 6255–6260. doi:10.1007/s13277-015-4462-3
Du, W., Tang, H., Lei, Z., Zhu, J., Zeng, Y., Liu, Z., et al. (2019). miR-335-5p inhibits TGF-β1-induced epithelial–mesenchymal transition in non-small cell lung cancer via ROCK1. Respir. Res. 20 (1), 225–311. doi:10.1186/s12931-019-1184-x
Duan, Z., Dong, C., and Liu, J. (2020). Circ-ABCB10 promotes growth and metastasis of nasopharyngeal carcinoma by upregulating ROCK1. Eur. Rev. Med. Pharmacol. Sci. 24 (23), 12208–12215. doi:10.26355/eurrev_202012_24011
Fan, G., He, Z., Cao, L., Shi, X., Wu, S., and Zhou, G. (2019). miR-139 inhibits osteosarcoma cell proliferation and invasion by targeting ROCK1. Front. Biosci. 24, 1167–1177. doi:10.2741/4773
Feng, Z., Li, X., Qiu, M., Luo, R., Lin, J., and Liu, B. (2020). LncRNA EGFR-AS1 upregulates ROCK1 by sponging miR-145 to promote Esophageal squamous cell carcinoma cell invasion and migration. Cancer Biother. Radiopharm. 35 (1), 66–71. doi:10.1089/cbr.2019.2926
Feng, N., Guo, Z., Wu, X., Tian, Y., Li, Y., Geng, Y., et al. (2021). Circ_PIP5K1A regulates cisplatin resistance and malignant progression in non-small cell lung cancer cells and xenograft murine model via depending on miR-493-5p/ROCK1 axis. Respir. Res. 22 (1), 248–312. doi:10.1186/s12931-021-01840-7
Fu, Y., Hu, X., Gao, Y., Li, K., Fu, Q., Liu, Q., et al. (2021). LncRNA ROR/miR-145-5p axis modulates the osteoblasts proliferation and apoptosis in osteoporosis. Bioengineered 12 (1), 7714–7723. doi:10.1080/21655979.2021.1982323
Fujisawa, K., Fujita, A., Ishizaki, T., Saito, Y., and Narumiya, S. (1996). Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J. Biol. Chem. 271 (38), 23022–23028. doi:10.1074/jbc.271.38.23022
Guo, D., Li, Y., Chen, Y., Zhang, D., Wang, X., Lu, G., et al. (2019). DANCR promotes HCC progression and regulates EMT by sponging miR‐27a‐3p via ROCK1/LIMK1/COFILIN1 pathway. Cell Prolif. 52 (4), e12628. doi:10.1111/cpr.12628
Guo, J., Yang, C., Lin, Y., Hu, G., Wei, J., Zhang, X., et al. (2020). Enhanced peripheral blood miR-324-5p is associated with the risk of metabolic syndrome by suppressing ROCK1. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 1865 (8), 158727. doi:10.1016/j.bbalip.2020.158727
Huang, Y., Xue, B., Pan, J., and Shen, N. (2021). Circ-E2F3 acts as a ceRNA for miR-204-5p to promote proliferation, metastasis and apoptosis inhibition in retinoblastoma by regulating ROCK1 expression. Exp. Mol. Pathol. 120, 104637. doi:10.1016/j.yexmp.2021.104637
Jiang, R., Zhang, C., Liu, G., Gu, R., and Wu, H. (2017). MicroRNA-101 inhibits proliferation, migration and invasion in osteosarcoma cells by targeting ROCK1. Am. J. Cancer Res. 7 (1), 88–97.
Li, C. H., Yu, T. B., Qiu, H. W., Zhao, X., Zhou, C. L., and Qi, C. (2017). miR-150 is downregulated in osteosarcoma and suppresses cell proliferation, migration and invasion by targeting ROCK1. Oncol. Lett. 13 (4), 2191–2197. doi:10.3892/ol.2017.5709
Li, D., Cao, Y., Li, J., Xu, J., Liu, Q., and Sun, X. (2017). miR-506 suppresses neuroblastoma metastasis by targeting ROCK1. Oncol. Lett. 13 (1), 417–422. doi:10.3892/ol.2016.5442
Li, C., Ma, D., Yang, J., Lin, X., and Chen, B. (2018). miR-202-5p inhibits the migration and invasion of osteosarcoma cells by targeting ROCK1. Oncol. Lett. 16 (1), 829–834. doi:10.3892/ol.2018.8694
Li, X., Wang, S-W., Xi-Ling, L., Yu, F-Y., and Cong, H-M. (2020). Knockdown of long non-coding RNA TUG1 depresses apoptosis of hippocampal neurons in Alzheimer’s disease by elevating microRNA-15a and repressing ROCK1 expression. Inflamm. Res. 69 (9), 897–910. doi:10.1007/s00011-020-01364-8
Liang, L., and Li, L. (2020). Down-regulation of circNRIP1 promotes the apoptosis and inhibits the migration and invasion of gastric cancer cells by miR-182/ROCK1 axis. Onco. Targets. Ther. 13, 6279–6288. doi:10.2147/OTT.S221633
Liang, H., Zhang, C., Guan, H., Liu, J., and Cui, Y. (2019). LncRNA DANCR promotes cervical cancer progression by upregulating ROCK1 via sponging miR-335-5p. J. Cell. Physiol. 234 (5), 7266–7278. doi:10.1002/jcp.27484
Liang, L., Gu, W., Li, M., Gao, R., Zhang, X., Guo, C., et al. (2021). The long noncoding RNA HOTAIRM1 controlled by AML1 enhances glucocorticoid resistance by activating RHOA/ROCK1 pathway through suppressing ARHGAP18. Cell Death Dis. 12 (7), 702–714. doi:10.1038/s41419-021-03982-4
Liu, Y., Liu, J., Wang, L., Yang, X., and Liu, X. (2017). MicroRNA-195 inhibits cell proliferation, migration and invasion in laryngeal squamous cell carcinoma by targeting ROCK1. Mol. Med. Rep. 16 (5), 7154–7162. doi:10.3892/mmr.2017.7460
Liu, J. L., Li, J., Xu, J. J., Xiao, F., Cui, P. L., Qiao, Z. G., et al. (2019). MiR-144 inhibits tumor growth and metastasis in osteosarcoma via dual-suppressing RhoA/ROCK1 signaling pathway. Mol. Pharmacol. 95 (4), 451–461. doi:10.1124/mol.118.114207
Liu, F., Xiao, Y., Ma, L., and Wang, J. (2020). Regulating of cell cycle progression by the lncRNA CDKN2B-AS1/miR-324-5p/ROCK1 axis in laryngeal squamous cell cancer. Int. J. Biol. Markers 35 (1), 47–56. doi:10.1177/1724600819898489
Liu, W., Shao, C., Zang, C., Sun, J., Xu, M., and Wang, Y. (2021). Protective effects of dexmedetomidine on cerebral ischemia/reperfusion injury via the microRNA-214/ROCK1/NF-κB axis. BMC Anesthesiol. 21 (1), 203–210. doi:10.1186/s12871-021-01423-5
Luo, H., and Liang, C. (2018). MicroRNA-148b inhibits proliferation and the epithelial-mesenchymal transition and increases radiosensitivity in non-small cell lung carcinomas by regulating ROCK1. Exp. Ther. Med. 15 (4), 3609–3616. doi:10.3892/etm.2018.5845
Luo, H., Zhang, Y., Qin, G., Jiang, B., and Miao, L. (2021). LncRNA MCM3AP-AS1 sponges miR-148a to enhance cell invasion and migration in small cell lung cancer. BMC cancer 21 (1), 820–828. doi:10.1186/s12885-021-08365-8
Meng, L., Cao, H., Wan, C., and Jiang, L. (2019). MiR-539-5p alleviates sepsis-induced acute lung injury by targeting ROCK1. Folia Histochem. Cytobiol. 57 (4), 168–178. doi:10.5603/FHC.a2019.0019
Pan, J., Zhang, D., Zhang, J., Qin, P., and Wang, J. (2019). LncRNA RMRP silence curbs neonatal neuroblastoma progression by regulating microRNA-206/tachykinin-1 receptor axis via inactivating extracellular signal-regulated kinases. Cancer Biol. Ther. 20 (5), 653–665. doi:10.1080/15384047.2018.1550568
Pegoraro, V., Marozzo, R., and Angelini, C. (2020). MicroRNAs and HDAC4 protein expression in the skeletal muscle of ALS patients. Clin. Neuropathol. 39 (3), 105–114. PubMed PMID: 32000889. Epub 2020/02/01. eng. doi:10.5414/NP301233
Qin, Z., Wei, X., Jin, N., Wang, Y., Zhao, R., Hu, Y., et al. (2018). MiR-199a targeting ROCK1 to affect kidney cell proliferation, invasion and apoptosis. Artif. Cells Nanomed. Biotechnol. 46 (8), 1920–1925. doi:10.1080/21691401.2017.1396224
Qiu, H., Chen, Z., Lv, L., Tang, W., and Hu, R. (2020). Associations between microRNA polymorphisms and development of coronary artery disease: A case–control study. DNA Cell Biol. 39 (1), 25–36. doi:10.1089/dna.2019.4963
Qiu, B., Xu, X., Yi, P., and Hao, Y. (2020). Curcumin reinforces MSC-derived exosomes in attenuating osteoarthritis via modulating the miR-124/NF-kB and miR-143/ROCK1/TLR9 signalling pathways. J. Cell. Mol. Med. 24 (18), 10855–10865. doi:10.1111/jcmm.15714
Rath, N., and Olson, M. F. (2012). Rho-associated kinases in tumorigenesis: re-considering ROCK inhibition for cancer therapy. EMBO Rep. 13 (10), 900–908. PubMed PMID: 22964758. Epub 09/11. eng. doi:10.1038/embor.2012.127
Riento, K., and Ridley, A. J. (2003). Rocks: multifunctional kinases in cell behaviour. Nat. Rev. Mol. Cell Biol. 4 (6), 446–456. doi:10.1038/nrm1128
Roberto, G., Delsin, L., Vieira, G., Silva, M., Hakime, R., Gava, N., et al. (2020). ROCK1-predictedmicroRNAs dysregulation contributes to tumor progression in ewing sarcoma. Pathol. Oncol. Res. 26 (1), 133–139. doi:10.1007/s12253-017-0374-4
Siddiqui, M. R., Akhtar, S., Shahid, M., Tauseef, M., McDonough, K., and Shanley, T. P. (2019). miR-144–mediated inhibition of ROCK1 protects against LPS-induced lung endothelial hyperpermeability. Am. J. Respir. Cell Mol. Biol. 61 (2), 257–265. doi:10.1165/rcmb.2018-0235OC
Song, L., Wang, L., Pan, X., and Yang, C. (2020). lncRNA OIP5-AS1 targets ROCK1 to promote cell proliferation and inhibit cell apoptosis through a mechanism involving miR-143-3p in cervical cancer. Braz. J. Med. Biol. Res. 53, e8883. doi:10.1590/1414-431X20198883
Sun, Y., Liu, L., Xing, W., and Sun, H. (2021). microRNA-148a-3p enhances the effects of sevoflurane on hepatocellular carcinoma cell progression via ROCK1 repression. Cell. Signal. 83, 109982. doi:10.1016/j.cellsig.2021.109982
Wang, Y., Wang, N., Zeng, X., Sun, J., Wang, G., Xu, H., et al. (2017). MicroRNA-335 and its target Rock1 synergistically influence tumor progression and prognosis in osteosarcoma. Oncol. Lett. 13 (5), 3057–3065. doi:10.3892/ol.2017.5818
Wang, Y., Zeng, X., Wang, N., Zhao, W., Zhang, X., Teng, S., et al. (2018). Long noncoding RNA DANCR, working as a competitive endogenous RNA, promotes ROCK1-mediated proliferation and metastasis via decoying of miR-335-5p and miR-1972 in osteosarcoma. Mol. Cancer 17 (1), 89–14. doi:10.1186/s12943-018-0837-6
Wang, J., Du, A., Wang, H., and Li, Y. (2020). MiR-599 regulates LPS-mediated apoptosis and inflammatory responses through the JAK2/STAT3 signalling pathway via targeting ROCK1 in human umbilical vein endothelial cells. Clin. Exp. Pharmacol. Physiol. 47 (8), 1420–1428. doi:10.1111/1440-1681.13316
Wang, Z., Li, T-E., Chen, M., Pan, J-J., and Shen, K-W. (2020). miR-106b-5p contributes to the lung metastasis of breast cancer via targeting CNN1 and regulating Rho/ROCK1 pathway. Aging (Albany NY) 12 (2), 1867–1887. doi:10.18632/aging.102719
Wang, J., Zhang, S., Li, X., and Gong, M. (2020). LncRNA SNHG7 promotes cardiac remodeling by upregulating ROCK1 via sponging miR-34-5p. Aging (Albany NY) 12 (11), 10441–10456. doi:10.18632/aging.103269
Wang, X., Yang, Y., Dai, Y., Zhang, H., Xia, H., and Kan, Q. (2021). LncRNA PSMG3-AS1 downregulates miR-340 through methylation to upregulate ROCK1 and promote cell invasion and migration in non-small cell lung cancer. Research Square. doi:10.21203/rs.3.rs-153993/v1
Wang, Q-f., Wang, Q-l., and Cao, M-b. (2021). LncRNA PITPNA-AS1 as a potential diagnostic marker and therapeutic target promotes hepatocellular carcinoma progression via modulating miR-448/ROCK1 axis. Front. Med. 8, 668787. doi:10.3389/fmed.2021.668787
Wang, W., Yang, T., Li, D., Huang, Y., Bai, G., and Li, Q. (2021). LINC00491 promotes cell growth and metastasis through miR-324-5p/ROCK1 in liver cancer. J. Transl. Med. 19 (1), 504–514. doi:10.1186/s12967-021-03139-z
Wang, J., He, Z., Xu, J., Chen, P., and Jiang, J. (2021). Long noncoding RNA LINC00941 promotes pancreatic cancer progression by competitively binding miR-335-5p to regulate ROCK1-mediated LIMK1/Cofilin-1 signaling. Cell Death Dis. 12 (1), 36–15. doi:10.1038/s41419-020-03316-w
Wu, D., Niu, X., Pan, H., Zhou, Y., Qu, P., and Zhou, J. (2016). MicroRNA-335 is downregulated in bladder cancer and inhibits cell growth, migration and invasion via targeting ROCK1. Mol. Med. Rep. 13 (5), 4379–4385. doi:10.3892/mmr.2016.5055
Wu, Y., Li, S., and Jia, Y. (2019). MicroRNA-26a suppresses the malignant biological behaviors of papillary thyroid carcinoma by targeting ROCK1 and regulating PI3K/AKT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 23, 8940–8949. doi:10.26355/eurrev_201910_19292
Xiang, J., Wu, Y., Li, D-S., Wang, Z-Y., Shen, Q., Sun, T-Q., et al. (2015). miR-584 suppresses invasion and cell migration of thyroid carcinoma by regulating the target oncogene ROCK1. Oncol. Res. Treat. 38 (9), 436–440. doi:10.1159/000438967
Xu, Y., Li, K., Wang, S., and Yang, S. (2019). Mir-592 functions as a tumor suppressor in acute myeloid leukemia by targeting ROCK1 and predicts patients' prognosis. Eur. Rev. Med. Pharmacol. Sci. 23 (4), 1610–1619. doi:10.26355/eurrev_201902_17120
Yang, Q., and Dong, Y-J. (2021). LncRNA SNHG20 promotes migration and invasion of ovarian cancer via modulating the microRNA-148a/ROCK1 axis. J. Ovarian Res. 14 (1), 168–213. doi:10.1186/s13048-021-00889-8
Yang, H., Wang, Z., and Wang, Z. (2020). Long noncoding RNA KCNMB2-AS1 increases ROCK1 expression by sponging microRNA-374a-3p to facilitate the progression of non–small-cell lung cancer. Cancer Manag. Res. 12, 12679–12695. doi:10.2147/CMAR.S270646
Yang, B., Zang, J., Yuan, W., Jiang, X., and Zhang, F. (2021). The miR-136-5p/ROCK1 axis suppresses invasion and migration, and enhances cisplatin sensitivity in head and neck cancer cells. Exp. Ther. Med. 21 (4), 317. doi:10.3892/etm.2021.9748
Yin, D., Wei, G., Yang, F., and Sun, X. (2021). Circular RNA has circ 0001591 promoted cell proliferation and metastasis of human melanoma via ROCK1/PI3K/AKT by targeting miR-431-5p. Hum. Exp. Toxicol. 40 (2), 310–324. doi:10.1177/0960327120950014
Zhan, Y., Zheng, N., Teng, F., Bao, L., Liu, F., Zhang, M., et al. (2017). MiR-199a/b-5p inhibits hepatocellular carcinoma progression by post-transcriptionally suppressing ROCK1. Oncotarget 8 (40), 67169–67180. doi:10.18632/oncotarget.18052
Zhang, M., Wang, D., Zhu, T., and Yin, R. (2017). miR-214-5p targets ROCK1 and suppresses proliferation and invasion of human osteosarcoma cells. Oncol. Res. 25 (1), 75–81. doi:10.3727/096504016X14719078133401
Zhang, X., Li, P., Ding, Z., Wang, H., Wang, J., Han, L., et al. (2018). The putative tumor suppressor, miR-199a, regulated by Snail, modulates clear cell renal cell carcinoma aggressiveness by repressing ROCK1. Onco. Targets. Ther. 11, 103–112. doi:10.2147/OTT.S147184
Zhang, M., Zhang, Y., Li, L., Ma, L., and Zhou, C. (2020). Dysregulation of miR-202-3p affects migration and invasion of endometrial stromal cells in endometriosis via targeting ROCK1. Reprod. Sci. 27 (2), 731–742. doi:10.1007/s43032-019-00079-4
Zhang, J., Xiang, J., Liu, T., Wang, X., Tang, Y., and Liang, Y. (2020). miR-495 targets ROCK1 to inhibit lipopolysaccharides-induced WI-38 cells apoptosis and inflammation. Kaohsiung J. Med. Sci. 36 (8), 607–614. doi:10.1002/kjm2.12210
Zhang, X., Xu, L., and Yang, T. (2020). miR-31 modulates liver cancer HepG2 cell apoptosis and invasion via ROCK1/F-Actin Pathways. Onco. Targets. Ther. 13, 877–888. doi:10.2147/OTT.S227467
Zhang, W., Shi, J., Cheng, C., and Wang, H. (2020). CircTIMELESS regulates the proliferation and invasion of lung squamous cell carcinoma cells via the miR‐136‐5p/ROCK1 axis. J. Cell. Physiol. 235 (9), 5962–5971. doi:10.1002/jcp.29521
Zhang, T., Zhang, L., Han, D., Tursun, K., and Lu, X. (2020). Circular RNA hsa_Circ_101141 as a competing endogenous RNA facilitates tumorigenesis of hepatocellular carcinoma by regulating miR-1297/ROCK1 pathway. Cell Transpl. 29, 0963689720948016. doi:10.1177/0963689720948016
Zhang, Y., Zhang, C., Chen, Z., and Wang, M. (2021). Blocking circ_UBR4 suppressed proliferation, migration, and cell cycle progression of human vascular smooth muscle cells in atherosclerosis. Open Life Sci. 16 (1), 419–430. doi:10.1515/biol-2021-0044
Zhao, Y., Sun, X., Zhu, K., and Cheng, M. (2020). miR-135a inhibits malignant proliferation and diffusion of non-small cell lung cancer cells by down-regulating ROCK1 protein. Biosci. Rep. 40 (6), BSR20201276. doi:10.1042/BSR20201276
Zheng, M., Sun, X., Li, Y., and Zuo, W. (2016). MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol. 37 (6), 8189–8196. doi:10.1007/s13277-015-4722-2
Zheng, H-z., Fu, X-k., Shang, J-l., Lu, R-x., Ou, Y-f., and Chen, C-l. (2018). Ginsenoside Rg1 protects rat bone marrow mesenchymal stem cells against ischemia induced apoptosis through miR-494-3p and ROCK-1. Eur. J. Pharmacol. 822, 154–167. doi:10.1016/j.ejphar.2018.01.001
Zhong, Y., Yu, C., and Qin, W. (2019). LncRNA SNHG14 promotes inflammatory response induced by cerebral ischemia/reperfusion injury through regulating miR-136-5p/ROCK1. Cancer Gene Ther. 26 (7), 234–247. doi:10.1038/s41417-018-0067-5
Zhou, F., Li, Y., Hao, Z., Liu, X., Chen, L., Cao, Y., et al. (2016). MicroRNA-300 inhibited glioblastoma progression through ROCK1. Oncotarget 7 (24), 36529–36538. doi:10.18632/oncotarget.9068
Zhou, K., Xu, J., Yin, X., and Xia, J. (2020). Long noncoding RNA HAGLROS promotes cell invasion and metastasis by sponging miR-152 and upregulating ROCK1 expression in osteosarcoma. Comput. Math. Methods Med. 2020, 7236245. doi:10.1155/2020/7236245
Zhou, W., Ye, S., and Wang, W. (2021). miR-217 alleviates high-glucose-induced vascular smooth muscle cell dysfunction via regulating ROCK1. J. Biochem. Mol. Toxicol. 35 (3), e22668. doi:10.1002/jbt.22668
Keywords: miRNA, lncRNA, ROCK1, expression, biomarker
Citation: Ghafouri-Fard S, Poornajaf Y, Hussen BM, Abak A, Shoorei H, Taheri M and Sharifi G (2022) Implication of non-coding RNA-mediated ROCK1 regulation in various diseases. Front. Mol. Biosci. 9:986722. doi: 10.3389/fmolb.2022.986722
Received: 05 July 2022; Accepted: 18 August 2022;
Published: 13 September 2022.
Edited by:
Florence Pinet, INSERM U1167 Facteurs de risque et déterminants moléculaires des maladies liées au vieillissement, FranceReviewed by:
Alessio Colantoni, Italian Institute of Technology (IIT), ItalyCopyright © 2022 Ghafouri-Fard, Poornajaf, Hussen, Abak, Shoorei, Taheri and Sharifi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, TW9oYW1tYWQudGFoZXJpQHVuaS1qZW5hLmRl; Guive Sharifi, Z2libm93QHlhaG9vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.