
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci. , 20 September 2022
Sec. RNA Networks and Biology
Volume 9 - 2022 | https://doi.org/10.3389/fmolb.2022.931525
This article is part of the Research Topic Insights in RNA Networks and Biology View all 11 articles
 Yan Zhang1
Yan Zhang1 Weihua Ma2
Weihua Ma2 Chao Ma1
Chao Ma1 Qinglu Zhang1
Qinglu Zhang1 Zhenya Tian1,3
Zhenya Tian1,3 Zhenqi Tian1
Zhenqi Tian1 Hongsong Chen1,3
Hongsong Chen1,3 Jianying Guo1
Jianying Guo1 Fanghao Wan1
Fanghao Wan1 Zhongshi Zhou1,4*
Zhongshi Zhou1,4*Heat shock proteins (Hsps) function as molecular chaperones that enable organisms to withstand stress and maintain normal life activities. In this study, we identified heat shock protein 70 (encoded by hsp70), which exhibits a higher expression in the mature male testis than in the unmature testis of Ophraella communa. Tissue expression profile revealed that Ochsp70 levels in males were highest in the testis, whereas those in females were highest in the head. Moreover, the expression of Ochsp70 was found to be significantly induced in female bursa copulatrix after mating. Double-stranded RNA dsOchsp70 was injected into males to performance RNA interference, which significantly decreased the male Ochsp70 expression levels within 20 d post-injection, whereas no effect was observed on the Ochsp70 expression level in the females after mating with dsOchsp70-injected males. However, significant downregulation of female fertility was marked simultaneously. Furthermore, knockdown of female Ochsp70 expression also led to a significant reduction in fertility. Finally, comparative transcriptomic analysis identified glucose dehydrogenase and insulin-like growth factor binding protein as putative downstream targets of Ochsp70. Overall, we deduced that Ochsp70 is an indispensable gene and a potential male mating factor in O. communa, which regulates reproduction.
When originating from their internal or external environments, the heat shock response of cells is activated to respond to the protein-damaging (proteotoxic) effects of stress (Sørensen et al., 2003). Heat shock genes are a subset of genes that encode for molecular chaperones called heat shock proteins, including a stress-related groups of proteins generated or synthesized by cells under the effect of high temperature (heat shock) or other stress stimuli. Hsps commonly exist in both prokaryotes and eukaryotes. Based on molecular weight (kDa), Hsps are divided into four types, Hsp90, Hsp70, Hsp60, and small Hsps, which are involved in the transport, folding, unfolding, assembly, and disassembly of multi-structured units, and in the degradation of misfolded or aggregated proteins (Lindquist, 1985; Feder and Hofmann, 1999; Pockley et al., 2007).
Hsp70 is a predominant Hsp family, and the previous studies were mainly focused on unraveling the important roles of this family in restoring the native conformation of proteins after experiencing stress (temperature, hypoxia, oxidative stress, pesticides, radiation, etc.) (Morimoto, 1993). In addition, members of the Hsp70 family are vital for the folding and intracellular trafficking of denovo synthesized proteins under normal conditions (Zatsepina et al., 2021). The highly dynamic nature of Hsp70 is a key factor responsible for its chaperone function (Clark and Peck, 2009; Mayer, 2013). Normally, Hsp70 is located in the cytoplasm, however, when cells are stimulated by heat stress, Hsp70 in the cytoplasm is rapidly transferred to the nucleus. Nuclear translocation of Hsp70 protects the cells from the damaging caused by hypoxia and high temperature (Velazquez and Lindquist, 1984). Interestingly, hsp70 gene expression has also been reported to determine the variation in fitness and geographical distribution of Nucella species (Sorte and Hofmann, 2005), and a similar phenomenon has been noted in marine organisms (Clark and Peck, 2009). During the evaluation of contaminated environments, the hsp70 gene may serve as a biomarker to detect adverse circumstances (Cristina et al., 2018). In mammals, certain Hsps have been identified in the seminal fluid, which play important roles in spermatogenesis, sperm-egg recognition, and the post-testicular maturation of mammalian spermatozoa (Walsh et al., 2008; Dun et al., 2012; Redgrove et al., 2012; Nixon et al., 2015). In boars, Hsp70 is associated with semen quality, which tends to decline significantly with Hsp70 levels (Huang et al., 2000). In insects, studies regarding the functional characterization of hsp70 are emergent. hsp70 gene is differentially regulated in response to diapause (Macrae, 2010), and a similar change is recorded for other influencing factors (King and MacRae, 2015). hsp70 is also involved in midgut metamorphosis in Spodoptera litura, wherein its expression is induced by hormones (Gu et al., 2012). In addition, hsp70 is associated with reproductive diapause (Baker and Russell, 2009) and aging, and has a positive effect in prolonging the lifespan of Drosophila melanogaster (Bourg et al., 2001).
Ophraella communa (Coleoptera: Chrysomelidae) is used worldwide as an important biological control agent of the ragweed Ambrosia artemisiifolia worldwide (Zhou et al., 2011). Ambrosia artemisiifolia invaded China in the 1930s (Li et al., 2015) and posed a serious threat to agriculture and ecosystem (Zhou et al., 2011; Smith et al., 2013). The O. communa feeds on foliage at both larval and adult stages, and either restricts the ragweed can not enter the vegetative genitals or die directly (Guo et al., 2011). Ragweed is spreading rapidly in China (Guo et al., 2011), and the new areas of A. artemisiifolia distribution lack a natural enemy population, making it particularly dangerous. Therefore, a prompt release of O. communa populations is required in these areas to prevent further propagation of this weed. In previous studies, we have investigated the biology and physiology of O. communa (Ma et al., 2019a; Ma et al. 2019b; Ma et al. 2020; Tian et al., 2021; Zhang et al., 2021), and found that these leaf beetles are bisexual reproductive insects that can mate multiple times per day after sexual maturity.
In the present study, we identified the hsp70 genes that were highly expressed from a cDNA library of male testes in O. communa. We noted that hsp70 is preferentially expressed in mature testes compared to unmature ones, and is also significantly upregulated in the bursa copulatrix (BC) of mated females. To further elucidate the potential functions of Ochsp70, we examined the tissue-specific transcript abundance patterns of Ochsp70 in males and females. Then, we used the RNA interference (RNAi) technique to further demonstrate its role in reproduction in males and females. Finally, a comparative transcriptome analysis of RNAi-treated females (dsgfp vs dshsp70) was carried out, and the potential mechanisms by which Ochsp70 regulates reproduction were discussed.
The A. artemisiifolia plants used in the present study were grown by following a previously reported method (Zhou et al., 2010). Ophraella communa population had been raised on ragweed plants for 1 year in the laboratory (Chinese Academy of Agricultural Sciences, Institute of Plant Protection, Beijing, China) at 27 ± 1°C, 70 ± 5% relative humidity, and a photoperiod of 14/10 h (light/dark).
Diverse tissues, including head, thorax, fat body, gut, male accessory glands (MAG), testis, bursa copulatrix (BC), were collected from eight male and female O. communa adults at day 5 post-eclosion. The post-mating bursa copulatrix (M-BC) tissue was obtained from 15 females immediately after mating, while the unmated bursa copulatrix (U-BC) tissue was obtained from 20 unmated females of the same age. All tissue samples collected for this study were immediately frozen in liquid nitrogen and stored at −80 C. Three biological replicates were used for quantitative real-time polymerase chain reaction (qPCR) analysis. Subsequently, total RNA from all samples was extracted following the manufacturer’s protocol using TRIzol™ reagent (Invitrogen, MA, United States). cDNA was synthesized using the TransScript® One-Step RT-PCR SuperMix (TransGen Biotech Co., Ltd, China) as per the recommended protocol.
The rapid amplification of cDNA ends (RACE) approach was used to amplify the full-length cDNA sequence according to the manufacturer’s guide (SMARTer® RACE 5ʹ/3ʹ Kit, Clontech, TaKaRa Bio Inc, United States) based on local transcriptome data. The primer sequences are listed in Supplementary Table S1. The complete Coding sequence region was analyzed according to the smart website (https://smart.embl.de/), and the conserved site was predicted using the Prosite tool (https://prosite.expasy.org/). The full-length cDNAs of hsp70 were used as query sequences to search for hsp70 homologs in other insect genomes available in GenBank using NCBI-BLASTn (http://www.ncbi.nlm.nih.gov/). Multiple sequence alignment was performed using DNAMAN 8.0, and phylogenetic trees were constructed by the maximum-likelihood method using MAGE 6.06 and phylogenetic relationships were determined by bootstrap analysis with values of 1,000 trials.
qPCR was performed to quantify the relative Ochsp70 expression levels in different tissues, including female and male, mating and unmating, and after double-stranded RNA (dsRNA) treatments. For this purpose, the ABI 7500 PCR detection system (Applied Biosystems, United States) was used. RPL19 was used as reference gene, as described by Zhang et al. (2020).
PCR was carried out using a gene-specific primer pair containing a T7 promoter sequence (5ʹ-TAATACGACTCACTATAGGG-3ʹ) at the 5′ end and a recombinant plasmid containing OcHsp70 as template. Thereafter, the PCR product was used as a template for dsRNA synthesis using Ambion™ MEGAscript® T7 Transcription Kit (Thermo-Fisher Scientific, CA, United States) according to the recommended protocol. The double-stranded green fluorescent protein (gfp) RNA, dsgfp, was used as blank (negative) control. Finally, the quality of dsOchsp70 was assessed using 1% agarose gel electrophoresis and quantified to 10 μg/ul. dsOcHsp70 and dsgfp were stored at −80 C for subsequent experiments (Jin et al., 2020).
For the RNAi experiment, newly emerged adults (males and females <12 h after eclosing) were injected with 500 ng of dsRNA in 100 nL water solution at the abdomen using the Nanoject III Programmable Nanoliter Injector (Drummond Scientific Co., Inc, PA, United States). At 5, 10, 15, and 20 d post injection (PI), the five injected adults of each biological replicate were collected for the evaluation of silencing efficiency using qPCR. The primers used in this study are listed in Supplementary Table S1.
Fecundity was assayed using single male-female mating pairs. The dsRNA (dshsp70 or dsgfp) injected adults (male or female) were mated with virgin (unmated) adults of the opposite sex and same age without dsRNA injection at 3 d PI. Each pair of adults was grouped in a Petri dish containing robust A. artemisiifolia leaves with wet cotton. The number of eggs laid from per pair per day was recorded every day until 20 d PI. The egg hatching rate was calculated as the percentage of hatched larvae among the total number of the eggs laid in the first 5 days.
To identify the potential interactors of hsp70 particularly related to reproduction, the global transcriptome profiles of dsOchsp70-treated and dsgfp-treated females were investigated and compared using high-throughput sequencing. To this end, RNA was extracted from all samples, and the Ochsp70-silencing efficiency of each sample was evaluated via qPCR before transcriptome sequencing.
Data from qPCR and bioassays were analyzed using SAS System for Windows V8. The qPCR data was analyzed using the 2−ΔΔCt method (Schmittgen, 2008). One-way ANOVA was performed to compare the variation between PCR data and bioassays, followed by a least significant difference (LSD) test for multiple comparisons. Differences among mean values were determined using a LSD test at p < 0.05.
The full-length cDNA of Ochsp70 was obtained by RACE-PCR and submitted to GenBank (GenBank number: OM162158), which consists of a 2,472 bp-long open reading frame encoding a polypeptide of 824 amino acids, and 186 bp long 5′ and 247 bp long 3′ untranslated regions. The molecular weight of OcHsp70 was predicted to be 91.98 kDa and the isoelectric point was 5.63, according to the ExPasy tools. The motif VEIVGGSSRIPAIKQ was found to be highly conserved in Ochsp70 and its homologs from other coleopteran species (Supplementary Figure S1), and Ochsp70 shares the HSPA4_like_NDB domain with these species. Homology analysis showed that the highest sequence similarity among OcHsp70 and other coleopteran Hsp70 proteins was 81.12% (Supplementary Figure S2). Meanwhile, phylogenetic analysis revealed that the Hsp70 clustered with strong bootstrapping support on the basis of the insect order of origin, whereas the amino acid sequences derived from insects of different orders were clustered in one clade, indicating that these Hsp70 are conserved within the same order of insects. The Ochsp70 sequence displayed the highest homology with that of Diabrotica virgifera (Supplementary Figure S1).
The relative expression of Ochsp70 in male testes was significantly higher in mature testes than in unmature testes (Figure 1A). We also observed that the expression level of OcHsp70 in BC (the female organ for storage of sperm and seminal fluid protein) increased significantly after mating (Figure 1B). Furthermore, the expression domain analysis of Ochsp70 revealed the highest expression in the testes in males and in the heads in females (Figure 1C,D).
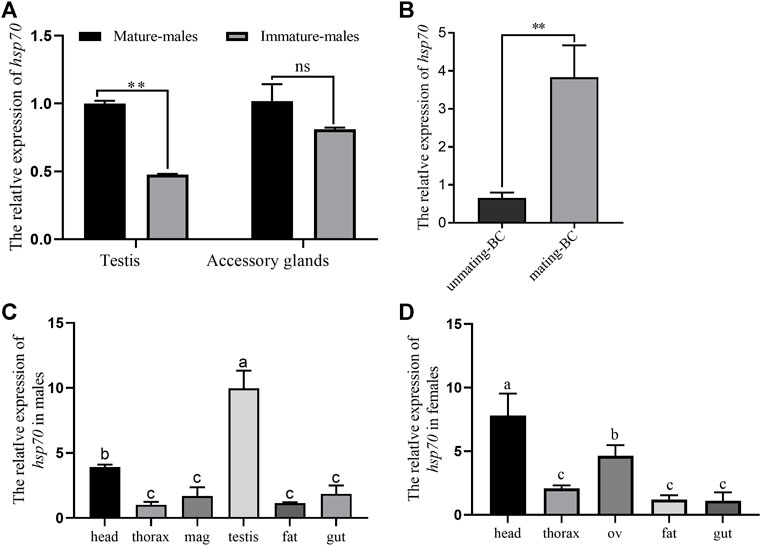
FIGURE 1. A) Expression levels of Ochsp70 in male testicular tissues; the expression patterns validation validate of the Ochsp70 transcriptome data in the male reproductive system. (B) Expression level of Ochsp70 in mating-bursa copulatrix (mating-BC) than unmating-bursa copulatrix (unmatingm-BC). (C) Expression profiles of Ochsp70 in different tissues of males beetles O. communa. (D) Expression profiles of Ochsp70 in different tissues of females beetles O. communa. Values are represent means ± SD. The data were analyzed usingby one-way ANOVA followed by the least significant difference (LSD) test. ∗*p < 0.05, ∗∗**p < 0.01.
The Ochsp70 expression was significantly reduced on the fifth day PI until 20th day PI in males (Figure 2A). In the meantime, we also tested the expression of Ochsp70 in the reproductive system of females that mated with males injected with dsOchsp70, and the results showed no significant changes (Figure 2B,C). However, the fecundity of these females was 26% lower than those administered dsgfp (Figure 2D). These results indicate that Ochsp70 is a putative male mating factor that plays an important role in reproduction.
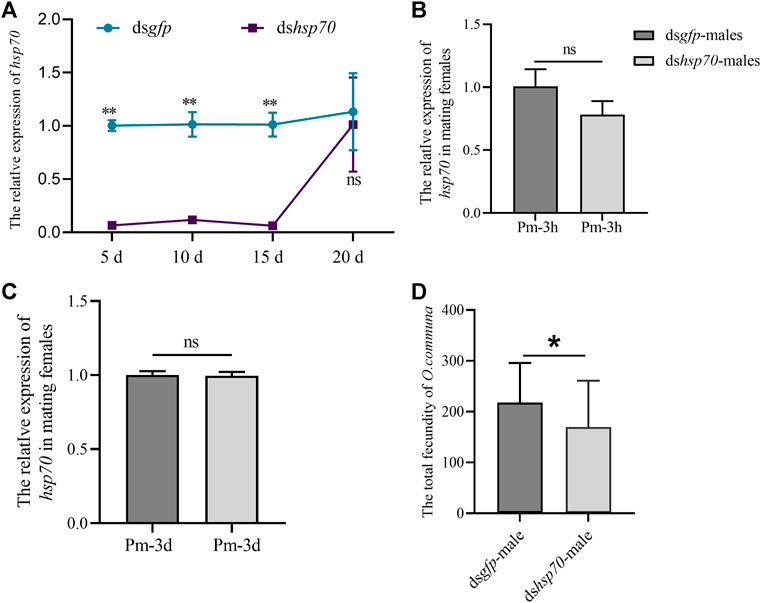
FIGURE 2. Functional characterization of Ochsp70 and evaluation of RNA silencing efficiency in males. (A) Expression levels of Ochsp70 (5, 10, 15, and 20 d) after dsRNA was injected into the males. (B) Expression levels of Ochsp70 in the reproductive system of females copulated with dsRNA-injected males, 3 h post-mating (Pm-3h). (C) Expression levels of Ochsp70 in the reproductive system of females copulated with dsRNA-injected males, 3 d post-mating (Pm-3d). (D) Effect of Ochsp70 on O. communa fecundity. Bars with the same letter are not significantly different from each other at p < 0.05, as per the LSD test.
To illustrate whether Ochsp70 was involved in regulating reproduction in females, dsOchsp70 was injected into freshly emerged females of O. communa. Similar to their male counterparts, the females displayed a significant reduction in Ochsp70 expression from the fifth to the 20th day PI (Figure 3A). Furthermore, the number of eggs laid by the dshsp70-treated females decreased by 56% compared to the control (Figure 3B). These results further suggest that Ochsp70 has a crucial role in the regulating the reproduction of O. communa females.
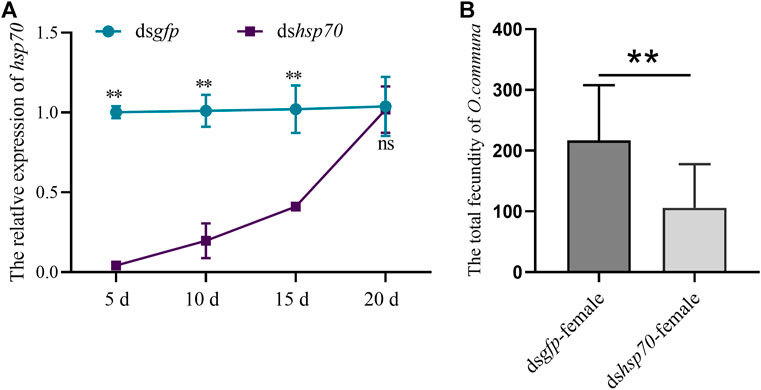
FIGURE 3. Functional characterization of Ochsp70 and evaluation of RNA silencing efficiency in females. (A) Expression levels of Ochsp70 (5, 10, 15, and 20 d) after dsRNA was injected dsRNA was injected into the females. (B) Effect of Ochsp70 on female fecundity. Bars with the same letter are not significantly different from each other at p < 0.05 level, as per the LSD test.
To elucidate the potential pathway of Ochsp70 mediated regulation of the reproduction in O. communa, total RNA was extracted from RNAi-treated mated female adults and was subjected to transcriptome sequencing (Supplementary Table S2). The raw data has been uploaded to NCBI (BioProject accession: PRJNA796368). Comparative transcriptomic analysis revealed significant alterations in the expression profiles of multiple genes associated with pathways involved in stress, reproductive development, and reproduction. Among the differentially expressed genes, we noticed that two fecundity-related genes, glucose dehydrogenase (evm.TU.chr5.700, GDH) and insulin-like growth factor binding protein (novel. 2003, IGF-BP) were downregulated 5.046 and 7.8136 times, respectively, after dsOchsp70 treatment. These results were subsequently validated by the relative expression levels quantified using qPCR (Figure 4).
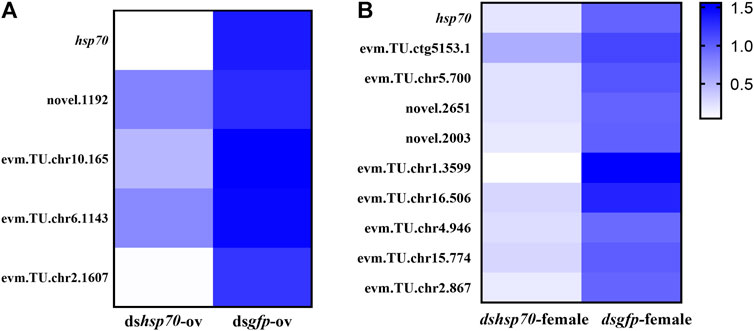
FIGURE 4. Verification of differential gene expression between two RNA interference-treated groups by quantitative real-time polymerase chain reaction represented as heat maps. (A) dshsp70-ov vs. dsgfp-ov. (B) dshsp70-female vs. dsgfp-female.
Insects produce Hsps in response to stress such as heat, cold, crowding, and anoxia. In concert with cochaperones and accessory proteins, Hsps mediate essential activities such as folding, assembly, intracellular localization, secretion, regulation, and degradation of other proteins (Hendrick and Hartl 1993). Previous studies have reported that Hsp exhibits characteristic and distinctive expression patterns during various stages of development, including gametogenesis (Dix, 1997) and embryogenesis (Heikkila, 1993; Krone et al., 2003), However, the role and significance of the high Hsp levels in the absence of stress stimuli remain unclear. In this study, the Ochsp70 gene was successfully isolated and was found to display a constitutive and preferential expres sion profile in male testes. Interestingly, knocking down male Ochsp70 resulted in diminished fertility in their female mates. A similar observation was made in Tribolium castaneum, wherein Hsp70 was found to be involved in reproductive regulation when Tchsp70 knock-down males were examined (Xu et al., 2013). In addition, Ochsp70 expression is highest in the female heads, which is similar with Nilaparvata lugens (Lu et al., 2018) and Cydia pomonellao (Yang et al., 2016), and some small hsp genes are also abundant in head (Sun et al., 2014; Li et al., 2019). As a chaperone, hsp might play an important role in maintaining the normal function of the insect brain, either olfaction or neuro/developmental processing (Yang et al., 2016). More importantly, Ochsp70 expression is highly in the female ovaries, which is consistent with the ovary-specific expression of Tchsp70 and Dmhsp70 (Marin and Tanguay, 1996; Xie et al., 2019), and constitutive expression of Hsp70 has been confirmed in mammalian oocytes (Dix, 1997), and hsp expression in female reproduction tissue and spermatogenesis was showed to correlate with HSP reproduction function (Neuer et al., 2000). Our results also revealed that the knockdown of female Ochsp70 expression led to reduced reproduction both males and females. This finding supports the previous prediction that the shsp and hsp70 genes may regulate reproduction in T. castaneum (Xie et al., 2019) and Agasicles hygrophila (Jin et al., 2020). However, expression level of hsp70 gene might be a balancer of benefits and costs. During the response of D. melanogaster against heat shock, the hsp70 expression increases, whereas the fecundity decreases (Krebs and Loeschcke, 1994; Huang et al., 2007), and growth and cell division are impeded (Feder, 1997).
In general, males offer male mating factors (e.g., seminal fluid proteins or other synthesized secretions) to females to ensure successful mating or to signify paternal investment (Thornhill, 1983; Avila et al., 2011). In the Hsp family, hsp60 was present in the upregulated gene cluster obtained from the mated females of D. melanogaster (Mack et al., 2006), and Hsp70 was identified as a seminal fluid protein in T. castaneum (Xu et al., 2013). In this study, female Ochsp70 expression was also induced through mating, which combined with the high expression levels of male Ochsp70 in the mature testis, suggests that Ochsp70 may functions as a male mating factor in O. communa. Similarly, 32 HSPs constitute a group of most abundant proteins in the adult testis proteomics of Bombyx mori, a 94.4 kDa Hsp70was also included (Zhang et al., 2014), which were considered to be associated with spermatogenesis, reproduction, mitosis, and fertilization. This phenomenon is even more comprehensible in mammals (Boelens et al., 2004; Jha et al., 2013; Zhang et al., 2014), wherein several hsp70 genes are expressed specifically in male germ cells (Dix, 1997; Neuer et al., 2000; Carreira and Santos, 2020). Testicular sperms are the most diverse of all cell types, so it is not surprising that spermatogenesis is accompanied by the expression of hsp gene different expression.
However, the knockdown of female Ochsp70 led to a reduction in egg production, suggesting that Ochsp70 may also be related to protein transport and nutrient supply in females, as observed previously (Marin and Tanguay, 1996). Hsp70 does not function independently and is associated with a team of cochaperones. In addition, hsp expression results from the activation of various intracellular signaling pathways (Feder and Hofmann, 1999). Liu et al. (2013) has been predicted that hsp90 is involved in regulating 20E and JH-inducible gene expression in Helicoverpa armigera, which may be another possible pathway for Hsp family-mediated reproductive regulation. In the present study, several pathways were revealed via RNA-sequencing analysis as potential downstream targets of Ochsp70 involved in the regulating the reproduction in O. communa, such as Foxo signaling pathway, MAPK signaling pathway and insect hormone biosynthesis. Particularly, we noticed that both GDH and IGF-BP were maximally down-regulated with decreasing expression of hsp70. Previous studies showed that GDH and IGF-BP are involved in reproduction-related pathways and homeostasis (Smykal and Raikhel, 2015), while GDH is also associated with lifespan regulation (Von Wyschetzki et al., 2015). Unfortunately, in our study, after we silenced Gdh and Igf-bp, respectively or combined, the female fertility was non-different (Supplementary materials). Meanwhile, when the expression of hsp70, Gdh and Igf-bp in female of O. communa was interfered simultaneously, the female fecundity decreased obviously, compared with the control (Supplementary materials). Hence, GDH and IGF-BP may be not directly regulate reproduction, and are not directly related to Hsp70. The process of Hsp70 involved in reproduction is multimodulated in males and females of O. communa, next we will contribute to explore and find out this mechanism.
Our study provides evidence that hsp70 is a regulator of O. communa reproduction. Our findings also supports the notion that Ochsp70 is a potential male mating factor. A high-throughput approach was used to analyze the potential regulatory mechanism of the function of Hsp family in reproduction. However, further studies are required to elucidate the gene regulatory network involved in Hsp-mediated regulation of reproduction.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
ZZ conceived, designed, coordinated the study, and helped with the drafting of the manuscript; YZ and QZ performed the experiments and participated in data analysis; WM participated in designing the study and drafted the manuscript; CM, HC, ZT, and JG were involved in data analysis; ZZ, YZ, WM, ZT, and FW composed the manuscript and performed statistical analyses. All authors have approved the manuscript for publication.
This study was supported by the National Natural Science Foundation of China (Nos. 31672089 and 31972340).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CZ declared a shared affiliation with the author(s). ZZ, YZ, CM, QZ, TZ, ZT, HC, JG, and FW to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.931525/full#supplementary-material
Avila, F. W., Sirot, L. K., LaFlamme, B. A., Rubinstein, C. D., and Wolfner, M. F. (2011). Insect seminal fluid proteins: Identification and function. Annu. Rev. Entomol. 56, 21–40. doi:10.1146/annurev-ento-120709-144823
Baker, D. A., and Russell, S. (2009). Gene expression during Drosophila melanogaster egg development before and after reproductive diapause. BMC Genomics 10, 242. doi:10.1186/1471-2164-10-242
Boelens, W. C., Wit, N. D., Verschuure, P., King, S. M., Kappe, G., Muijen, G. V., et al. (2004). Testis-specific human small heat shock protein HSPB9 is a cancer/testis antigen, and potentially interacts with the dynein subunit TCTEL1. Eur. J. Cell Biol. 83, 337–345. doi:10.1078/0171-9335-00396
Bourg, R. L., Valenti, P., Lucchetta, P., and Payre, F. (2001). Effects of mild heat shocks at young age on aging and longevity in Drosophila melanogaster. Biogerontology 2, 155–164. doi:10.1023/A:1011561107055
Carreira, R. P., and Santos, D. L. D. (2020). The role of HSP70 in sperm quality in HSP70s: Discovery, structure and functions. New York: Nova Science Publishers, Inc
Clark, M. S., and Peck, L. S. (2009). HSP70 heat shock proteins and environmental stress in antarctic marine organisms: A mini-review. Mar. Genomics 1, 11–18. doi:10.1016/j.margen.2009.03.003
Cristina, M., Bast, O. D., and Silvia, F. C. (2018). HSP70 as a biomarker: An excellent tool in environmental contamination analysis—a review. Water Air Soil Pollut. 229, 264. doi:10.1007/s11270-018-3920-0
Dix, D. J. (1997). HSP 70 expression and function during gametogenesis. Cell Stress Chaperones 2, 73–77. doi:10.1379/1466-1268(1997)002<0073:heafdg>2.3.co;2
Dun, M., Aitken, R. J., and Nixon, B. (2012). The role of molecular chaperones in spermatogenesis and the post-testicular maturation of mammalian spermatozoa. Hum. Reprod. Update 18, 420–435. doi:10.1093/humupd/dms009
Feder, K., and Feder, M. E. (1997). Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones 2, 60–71. doi:10.1379/1466-1268(1997)002<0060:dcohoi>2.3.co;2
Feder, M. E., and Hofmann, G. E. (1999). Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 61, 243–282. doi:10.1146/annurev.physiol.61.1.243
Gu, J., Huang, L. X., Shen, Y., Huang, L. H., and Feng, Q. L. (2012). Hsp70 and small Hsps are the major heat shock protein members involved in midgut metamorphosis in the common cutworm, Spodoptera litura. Insect Mol. Biol. 21, 535–543. doi:10.1111/j.1365-2583.2012.01158.x
Guo, J. Y., Zhou, Z. S., Zheng, X. W., Chen, H. S., Wan, F. H., and Luo, Y. H. (2011). Control efficiency of leaf beetle Ophraella communa, on the invasive common ragweed, Ambrosia artemisiifolia, at different growing stages. Biocontrol Sci. Technol. 21, 1049–1063. doi:10.1080/09583157.2011.603823
Heikkila, J. J. (1993). Heat shock gene expression and development. II. An overview of mammalian and avian developmental systems. Dev. Genet. 14, 87–91. doi:10.1002/dvg.1020140202
Hendrick, J. P., and Hartl, F.-U. (1993). Molecular chaperone functions of heat-shock proteins. Annu. Rev. Biochem. 62, 349–384. doi:10.1146/annurev.bi.62.070193.002025
Huang, L-H., Chen, B., and Kang, L. (2007). Impact of mild temperature hardening on thermotolerance, fecundity, and Hsp gene expression in Liriomyza huidobrensis. J. Insect Physiol. 53, 1199–1205. doi:10.1016/j.jinsphys.2007.06.011
Huang, S. Y., Kuo, Y. H., Lee, Y. P., Tsou, H. L., Lee, W. C., Ju, C. C., et al. (2000). Association of heat shock protein 70 with semen quality in boars. Anim. Reprod. Sci. 63, 231–240. doi:10.1016/s0378-4320(00)00175-5
Jha, K. N., Coleman, A. R., Wong, L., Salicioni, A. M., Howcroft, E., and Johnson, G. R. (2013). Heat shock protein 90 functions to stabilize and activate the testis-specific serine/threonine kinases, a family of kinases essential for male fertility. J. Biol. Chem. 288, 16308–16320. doi:10.1074/jbc.M112.400978
Jin, J., Zhao, M. T., Wang, Y., Zhou, Z. S., and Guo, J. Y. (2020). Induced thermotolerance and expression of three key hsp genes (Hsp70, Hsp21, and sHsp21) and their roles in the high temperature tolerance of Agasicles hygrophila. Front. Physiol. 10, 1593. doi:10.3389/fphys.2019.01593
King, A. M., and MacRae, T. H. (2015). Insect heat shock proteins during stress and diapause. Annu. Rev. Entomol. 60, 59–75. doi:10.1146/annurev-ento-011613-162107
Krebs, R., and Loeschcke, V. (1994). Costs and benefits of activation of the heat-shock response in Drosophila melanogaster. Funct. Ecol. 8, 730–737. doi:10.2307/2390232
Krone, P. H., Evans, T. G., and Blechinger, S. R. (2003). Heat shock gene expression and function during zebrafish embryogenesis. Semin. Cell Dev. Biol. 14, 267–274. doi:10.1016/j.semcdb.2003.09.018
Li, X. M., She, D. Y., Zhang, D. Y., and Liao, W. J. (2015). Life history trait differentiation and local adaptation in invasive populations of Ambrosia artemisiifolia in China. Oecologia 177, 669–677. doi:10.1007/s00442-014-3127-z
Li, Z. W., Li, X., Yu, Q. Y., Xiang, Z. H., Kishino, H., Zhang, Z., et al. (2009). The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol. Biol. 9, 215. doi:10.1186/1471-2148-9-215
Lindquist, S. L. (1985). The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191. doi:10.1146/annurev.bi.55.070186.005443
Liu, W., Zhang, F. X., Cai, M. J., Zhao, W. L., Li, X. R., Wang, J.-X., et al. (2013). The hormone-dependent function of Hsp90 in the crosstalk between 20-hydroxyecdysone and juvenile hormone signaling pathways in insects is determined by differential phosphorylation and protein interactions. Biochim. Biophys. Acta 1830, 5184–5192. doi:10.1016/j.bbagen.2013.06.037
Lu, K., Chen, X., Liu, W. T., Zhang, Z. C., Wang, Y., You, K., et al. (2018). Characterization of heat shock protein 70 transcript from Nilaparvata lugens (Stal): Its response to temperature and insecticide stresses. Pestic. Biochem. Physiol. 142, 102–110. doi:10.1016/j.pestbp.2017.01.011
Ma, C., Cui, S., Bai, Q., Tian, Z., Zhang, Y., Chen, G., et al. (2020). Olfactory co‐receptor is involved in host recognition and oviposition in Ophraella communa (Coleoptera: Chrysomelidae). Insect Mol. Biol. 29, 381–390. doi:10.1111/imb.12643
Ma, C., Cui, S., Tian, Z., Zhang, Y., Chen, G., Gao, X., et al. (2019a). OcomCSP12, a chemosensory protein expressed specifically by ovary, mediates reproduction in Ophraella communa (Coleoptera: Chrysomelidae). Front. Physiol. 10, 1290. doi:10.3389/fphys.2019.01290
Ma, C., Zhao, C., Cui, S., Zhang, Y., Zhou, Z., Chen, H., et al. (2019b). Identification of candidate chemosensory genes of Ophraella communa LeSage (Coleoptera: Chrysomelidae) based on antennal transcriptome analysis. Sci. Rep. 9, 15551. doi:10.1038/s41598-019-52149-x
Mack, P. D., Kapelnikov, A., Heifetz, Y., and Bender, M. (2006). Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 103, 10358–10363. doi:10.1073/pnas.0604046103
Macrae, T. H. (2010). Gene expression, metabolic regulation and stress tolerance during diapause. Cell. Mol. Life Sci. 67, 2405–2424. doi:10.1007/s00018-010-0311-0
Marin, R., and Tanguay, R. (1996). Stage-specific localization of the small heat shock protein Hsp27 during oogenesis in Drosophila melanogaster. Chromosoma 105, 142–149. doi:10.1007/BF02509495
Mayer, M. P. (2013). Hsp70 chaperone dynamics and molecular mechanism. Trends biochem. Sci. 38, 507–514. doi:10.1016/j.tibs.2013.08.001
Morimoto, & R. (1993). Cells in stress: Transcriptional activation of heat shock genes. Science 259, 1409–1410. doi:10.1126/science.8451637
Neuer, A., Spandorfer, S. D., Giraldo, P., Dieterle, S., Rosenwaks, Z., and Witkin, S. S. (2000). The role of heat shock proteins in reproduction. Hum. Reprod. Update 6, 149–159. doi:10.1093/humupd/6.2.149
Nixon, B., Bromfield, E. G., Dun, M., Redgrove, K. A., Mclaughlin, E. A., and Aitken, R. J. (2015). The role of the molecular chaperone heat shock protein A2 (HSPA2) in regulating human sperm-egg recognition. Asian J. Androl. 17, 568–573. doi:10.4103/1008-682X.151395
Pockley, A. G., Fairburn, B., Mirza, S., Slack, L. K., Hopkinson, K., and Muthana, M. (2007). A non-receptor-mediated mechanism for internalization of molecular chaperones. Methods, 43, 238–244. doi: doi: doi:10.1016/j.ymeth.2007.06.007
Redgrove, K. A., Brett, N., Baker, M. A., Louise, H., Gordon, B., Liu, D. Y., et al. (2012). The molecular chaperone HSPA2 plays a key role in regulating the expression of sperm surface receptors that mediate sperm-egg recognition. Plos One 7, e50851. doi:10.1371/journal.pone.0050851
Schmittgen, T. D., and Livak, K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. doi:10.1038/nprot.2008.73
Smith, M., Cecchi, L., Skjoth, C. A., Karrer, G., and Sikoparija, B. (2013). Common ragweed: A threat to environmental health in europe. Environ. Int. 61, 115–126. doi:10.1016/j.envint.2013.08.005
Smykal, V., and Raikhel, A. S. (2015). Nutritional control of insect reproduction. Curr. Opin. Insect Sci. 11, 31–38. doi:10.1016/j.cois.2015.08.003
Sørensen, J. G., Kristensen, T. N., and Loeschcke, V. (2003). The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 6, 1025–1037. doi:10.1046/j.1461-0248.2003.00528.x
Sorte, C., and Hofmann, G. (2005). Thermotolerance and heat-shock protein expression in Northeastern Pacific Nucella species with different biogeographical ranges. Mar. Biol. 146, 985–993. doi:10.1007/s00227-004-1508-2
Sun, M., Lu, M. X., Tang, X. T., and Du, Y. Z. (2014). Characterization and expression of genes encoding three small heat shock proteins in sesamia inferens (Lepidoptera: Noctuidae). Int. J. Mol. Sci. 15, 23196–23211. doi:10.3390/ijms151223196
Thornhill, R. (1983). Cryptic female choice and its implications in the scorpionfly Harpobittacus nigriceps. Am. Nat. 122, 765–788. doi:10.1086/284170
Tian, Z., Chen, G., Zhang, Y., Ma, C., Tian, Z., Gao, X., et al. (2021). Rapid adaptive evolution of Ophraella communa in new low temperature environment. J. Pest Sci. doi:10.21203/rs.3.rs-490874/v1
Velazquez, J. M., and Lindquist, S. (1984). hsp70: Nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell 36, 655–662. doi:10.1016/0092-8674(84)90345-3
Von Wyschetzki, K., Rueppell, O., Oettler, J., and Heinze, J. (2015). Transcriptomic signatures mirror the lack of the fecundity/longevity trade-off in ant queens. Mol. Biol. Evol. 32, 3173–3185. doi:10.1093/molbev/msv186
Walsh, A., Whelan, D., Bielanowicz, A., Skinner, B., Aitken, R. J., O., Bryan, M. K., et al. (2008). Identification of the molecular chaperone, heat shock protein 1 (chaperonin 10), in the reproductive tract and in capacitating spermatozoa in the male mouse. Biol. Reprod. 78, 983–993. doi:10.1095/biolreprod.107.066860
Wan, F., Jiang, M., and Zhan, A. (2017). “Biological invasions and its management in China,” in Common ragweed Ambrosia artemisiifolia L. Editors Z. S. Zhou, F. H. Wan, and J. Y. Guo (Springer Nature Singapore), 99–109.
Xie, J., Hu, X. X., Zhai, M. F., Yu, X. J., Song, X. W., Gao, S. S., et al. (2019). Characterization and functional analysis of hsp18.3 gene in the red flour beetle, Tribolium castaneum. Insect Sci. 026, 263–273. doi:10.1111/1744-7917.12543
Xu, J., Baulding, J., and Palli, S. R. (2013). Proteomics of Tribolium castaneum seminal fluid proteins: Identification of an angiotensin-converting enzyme as a key player in regulation of reproduction. J. Proteomics 78, 83–93. doi:10.1016/j.jprot.2012.11.011
Yang, X. Q., Zhang, Y. L., Wang, X. Q., Dong, H., Gao, P., and Jia, L. Y. (2016). Characterization of multiple heat-shock protein transcripts from Cydia pomonella: Their response to extreme temperature and insecticide exposure. J. Agric. Food Chem. 64, 4288–4298. doi:10.1021/acs.jafc.6b01914
Zatsepina, O. G., Evgen'ev, M. B., and Garbuz, D. G. (2021). Role of a heat shock transcription factor and the major heat shock protein Hsp70 in memory formation and neuroprotection. Cells 10, 1638. doi:10.3390/cells10071638
Zhang, Y., Chen, J., Chen, G., Ma, C., Chen, H., Gao, X., et al. (2020). Identification and validation of reference genes for quantitative gene expression analysis in Ophraella communa. Front. Physiol. 11, 355. doi:10.3389/fphys.2020.00355
Zhang, Y., Dong, Z., Gu, P., Zhang, W., Wang, D., Guo, X., et al. (2014). Proteomics analysis of adult testis from B ombyx mori. Proteomics 14, 2345–2349. doi:10.1002/pmic.201300507
Zhang, Y., Zhao, C., Ma, W., Cui, S., Chen, H., Ma, C., et al. (2021). Larger males facilitate population expansion in Ophraella communa. J. Anim. Ecol. 90, 2782–2792. doi:10.1111/1365-2656.13579
Zhou, Z. S., Guo, J. Y., Chen, H. S., and Wan, F. H. (2010). Effects of temperature on survival, development, longevity, and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a potential biological control agent against Ambrosia artemisiifolia (Asterales: Asteraceae). Environ. Entomol. 39, 1021–1027. doi:10.1603/EN09176
Keywords: hsp70, male mating factor, fertility, Ophraella communa, pathway
Citation: Zhang Y, Ma W, Ma C, Zhang Q, Tian Z, Tian Z, Chen H, Guo J, Wan F and Zhou Z (2022) The hsp70 new functions as a regulator of reproduction both female and male in Ophraella communa. Front. Mol. Biosci. 9:931525. doi: 10.3389/fmolb.2022.931525
Received: 29 April 2022; Accepted: 30 August 2022;
Published: 20 September 2022.
Edited by:
Gian Gaetano Tartaglia, Italian Institute of Technology (IIT), ItalyReviewed by:
Chenchen Zhao, Chinese Academy of Agricultural Sciences (CAAS), ChinaCopyright © 2022 Zhang, Ma, Ma, Zhang, Tian, Tian, Chen, Guo, Wan and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongshi Zhou, emhvdXpob25nc2hpQGNhYXMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.