- Anesthesiology and Peri-Operative Medicine (APOM), Oregon Health and Science University, Portland, OR, United States
The growth of the aging population, together with improved stroke care, has resulted in an increase in stroke survivors and a rise in recurrent events. Axonal injury and white matter (WM) dysfunction are responsible for much of the disability observed after stroke. The mechanisms of WM injury are distinct compared to gray matter and change with age. Therefore, an ideal stroke therapeutic must restore neuronal and axonal function when applied before or after a stroke, and it must also protect across age groups. Casein kinase 2 (CK2), is expressed in the brain, including WM, and is regulated during the development and numerous disease conditions such as cancer and ischemia. CK2 activation in WM mediates ischemic injury by activating the Cdk5 and AKT/GSK3β signaling pathways. Consequently, CK2 inhibition using the small molecule inhibitor CX-4945 (Silmitasertib) correlates with preservation of oligodendrocytes, conservation of axon structure, and axonal mitochondria, leading to improved functional recovery. Remarkably, CK2 inhibition promotes WM function when applied after ischemic injury by specifically regulating the AKT/GSK3β pathways. The blockade of the active conformation of AKT confers post-ischemic protection to young and old WM by preserving mitochondria, implying AKT as a common therapeutic target across age groups. Using a NanoString nCounter miRNA expression profiling, comparative analyses of ischemic WM with or without CX-4945 treatment reveal that miRNAs are expressed at high levels in WM after ischemia, and CX-4945 differentially regulates some of these miRNAs. Therefore, we propose that miRNA regulation may be one of the protective actions of CX-4945 against WM ischemic injury. Silmitasertib is FDA approved and currently in use for cancer and Covid patients; therefore, it is plausible to repurpose CK2 inhibitors for stroke patients.
Introduction
The Role of Casein Kinase 2 in Brain Ischemia
Casein Kinase 2 (CK2), is an unconventional protein kinase (PK) that is composed of two catalytic α-subunits (α and α’) and two β-subunits (Pinna and Allende, 2009). CK2 is shown to phosphorylate numerous substrates, including other PKs, thus acting as a “master regulator” (Meggio and Pinna, 2003; Poole et al., 2005). Unlike other PKs, the catalytic activity of CK2 is not regulated by second messengers or phosphorylation (Poole et al., 2005; Vilk et al., 2008) and it is constitutively active. In addition, CK2 is recently reported to be activated via a polymerization/depolymerization mechanisms (Kristensen et al., 2004; Lolli et al., 2012; Franchin et al., 2018) together with reactive oxygen species (ROS) (Leslie and Downes, 2002; Cosentino-Gomes et al., 2012; Srinivasan et al., 2013). CK2 regulates many cellular functions and its activity is vital for brain development (Lou et al., 2008) and cellular homeostasis (Blanquet, 2000; Brunet et al., 2015). For instance, direct interaction of CK2β with the transcription factor Olig2 is required for oligodendrocyte progenitor cell development and lineage (Huillard et al., 2010). On the other hand, upregulation of CK2 signaling is linked to many diseases, such as cancers (Sarno et al., 2002), cardiac hypertrophy (Hauck et al., 2008; Eom et al., 2011), and ischemic injury (Hu et al., 1993; Hu and Wieloch, 1993; Ka et al., 2015).
CK2 is constitutively expressed in the central nervous system (CNS) including glial cells such as adult oligodendrocytes (Huillard et al., 2010) and has a complex role in cellular injury (Ka et al., 2015). A previous report has shown that CK2 is a neuroprotectant that acts by directly modulating NADPH oxidase activity during cerebral ischemia (Kim et al., 2009). On the other hand, a brief and moderate AMPA receptor activation in rat oligodendrocyte cell cultures triggers CK2 activity to mediate excitotoxic injury (Canedo-Antelo et al., 2018). Accordingly, CK2 inhibition alleviates AMPA-mediated excitotoxic oligodendrocyte death by blocking AMPA receptor activation (Canedo-Antelo et al., 2019). Although these findings propose an intriguing role for CK2 signaling in brain ischemic injury mechanisms, the role of CK2 has remained unexplored in white matter (WM) function and ischemic injury mechanisms until recently (Baltan et al., 2018; Bastian et al., 2019b). WM is composed of astrocytes, oligodendrocytes, microglia, axons, and myelin that wraps them (Fields, 2008) therefore an ideal stroke therapeutic must be directed towards neurons, axons, and glial cells across all age groups. Currently, recombinant tissue plasminogen activator or endovascular thrombectomy are effective treatments for reperfusion after ischemic stroke. However, reperfusion alone is not sufficient to rescue dying cells due to the activation of injury-related pathways. Thus, there is an unmet need for the identification of post-ischemic injury mechanisms to develop effective stroke treatment. Axonal injury is an important independent risk factor and burden for adverse outcomes following a stroke, even in intravenous thrombolysis patients (Curtze et al., 2015). It is crucial, therefore, to search for therapeutic options that protect the entire brain by treating both gray and WM components against ischemia.
Mechanisms of Ischemic White Matter Injury Are Age-Dependent
Mechanisms underlying ischemic WM injury prove to be unexpectedly complex and distinct from gray matter (GM) injury (Wrathall et al., 1994; Agrawal and Fehlings, 1997; Fern and Ransom, 1997; McDonald et al., 1998; Sanchez-Gomez and Matute, 1999; Follett et al., 2000; Tekkök and Goldberg, 2001; Stys, 2004; Tekkök et al., 2007). WM injury mechanisms follow a spatiotemporal sequence of events; axons are injured directly by the loss of ionic homeostasis resulting in toxic accumulation of intracellular Na+ and Ca2+ (Stys et al., 1990; Fern et al., 1995; Wolf et al., 2001; Ouardouz et al., 2003; Underhill and Goldberg, 2007), while astrocytes due to reversal of Na+-dependent glutamate transporters release excessive glutamate (Tekkök et al., 2007) leading to injury of oligodendrocytes and the myelin they produce (Matute et al., 1997; McDonald et al., 1998; Li et al., 1999; Sanchez-Gomez and Matute, 1999; Tekkök and Goldberg, 2001; Alberdi et al., 2002; Micu et al., 2006; Tekkök et al., 2007). Consistent with this idea, removal of extracellular Ca2+, blockade of AMPA/kainate receptors, or blockade of reverse glutamate transport reduces ischemic WM injury. Moreover, glutamate accumulation triggers oxidative injury pathways by competing with cysteine. Together with mitochondrial dysfunction and nitric oxide synthetase (NOS) activation, reactive oxidative stress (ROS) production increases contributing to irreversible ischemic WM injury.
Aging is the most independent risk factor for stroke. Age-related changes in the molecular structure of WM dictate injury mechanisms by surpassing the ionic pathway and initiating injury by combined excitotoxic and oxidative injury pathways. Consequently, protective interventions in young WM become ineffective at promoting recovery of, or even injurious to, aging WM (Saab et al., 2016; Bastian et al., 2019a). For instance, in the aging axons, there is a significant increase in glutamate transporter-1 (GLT-1) levels leading to excessive extracellular glutamate accumulation presumably due to an increased need for glutamate signaling in aging WM to maintain its function (Baltan, 2009; Baltan et al., 2011). However, these adaptive changes act against the tissue by causing glutamate toxicity and mitochondrial energy depletion in aging axons during an ischemic episode (Stahon et al., 2016). To maintain proper axon function, axonal mitochondria exhibit unique and complex dynamics to proficiently buffer Ca2+, produce sufficient ATP, and effectively scavenge ROS. Ca2+ overload activates eNOS to produce nitric oxide (NO) and ROS, which are proposed as diffusible second messengers to link oligodendrocyte excitotoxicity to axon injury (Matute, 2010; Voccoli et al., 2014; Bastian et al., 2018). The fusion and fission processes of mitochondria are delicately regulated to coordinate the spatiotemporal properties of mitochondrial Ca2+ responses and the physiological and pathophysiological consequences of Ca2+ signals (Baltan, 2014b). By enhancing fusion or inhibiting fission, elongated mitochondria efficiently buffer Ca2+, thus preventing eNOS activation and subsequent ROS production (Lugus et al., 2011; Miller et al., 2013; Baltan, 2014b; Bastian et al., 2018). In aging axons, there is an increase in mitochondrial fusion, presumably to effectively buffer increased Ca2+ load and ROS production, which further alters the mitochondrial dynamics and function (Baltan, 2009; Stahon et al., 2016). Therefore, an age-dependent modification in mitochondrial bioenergetics may underlie the increased vulnerability of aging axons to ischemia. The intimate link between changes in aging WM structure and response to injury complicates the development of possible therapeutic options and warrants attention to identify beneficial interventions that act on shared molecular targets between young and aging WM.
Casein Kinase 2 Mediates Ischemic White Matter Injury
The optic nerve, a purely myelinated CNS WM tract, offers several advantages to study the mechanisms of WM injury. These advantages include minimal surgical injury due to isolation techniques, preservation of the three-dimensional structure of myelinated axons with their supporting glia, stable and quantifiable recording of action potentials for prolonged periods (Cavallotti et al., 2002; Cavallotti et al., 2003). Tissue collected at the end of the experiments can be further processed to quantify the proteins of interest. In addition, in fixed optic nerve tissue, the cellular and axonal structures can be immunolabeled with cell-specific antibodies or prepared for three-dimensional electron microscopy imaging for ultrastructural assessment. The corpus callosum (CC) is another WM tract and offers important advantages for the investigation of in vitro CC slices to quantify axon function and in vivo WM (selective WM ischemic by stereotaxic L-NIO injections) injury which can be assessed with behavioral tests (Nunez et al., 2016). These two WM tracts allow an excellent combined function-structure analysis of glial cells and axons after stroke.
We evaluated the expression and localization of CK2α in mouse optic nerves (MONs), using isoform-specific antibodies to support a biological basis for investigating CK2 signaling in WM (Figure 1). CK2α is expressed in axons and glial cells in MONs demonstrated by colocalization of CK2α subunit with GFAP (+) astrocyte nuclei and some processes, NF-200 (+) axons, Olig2 (+) oligodendrocytes, and PLP (+) myelin. The robust expression pattern of CK2α suggests an extensive kinase regulation of WM structure and function (Moreno et al., 1999; Brechet et al., 2008; Yoshimura and Rasband, 2014; Rosenberger et al., 2016). Indeed, CK2 signaling is different under physiological and ischemic stress conditions. Under normal physiological conditions, CK2 signaling is important for the clustering of Na+ channels at axon initial segments and nodes of Ranvier to enable and preserve axonal excitability (Brechet et al., 2008; Hien et al., 2014) and for oligodendrocyte development (Huillard et al., 2010). On the other hand, during ischemia, CK2 signaling mediates injury to glial cells and impairs axon function either directly and/or through Cdk5 and PTEN/AKT/GSK3β signaling regulation of downstream effectors (Figure 2). Subsequently, CK2 blockade preserves oligodendrocytes and axonal mitochondrial integrity, dynamics, and function (Baltan et al., 2018; Bastian et al., 2019b). Changes in signaling due to ischemia are achieved by either increasing CK2 activity (Charriaut-Marlangue et al., 1991; Hu and Wieloch, 1993; Hase et al., 2005; Chao et al., 2007) or by the movement of CK2 to a different subcellular compartment (Serrano et al., 1989; Charriaut-Marlangue et al., 1991; Diaz-Nido and Avila, 1992; Faust et al., 2001; Qaiser et al., 2014). CK2 can directly interfere with mitochondrial axonal transport (Pigino et al., 2009) to ultimately alter mitochondrial dynamics (Amiri and Hollenbeck, 2008; Liu et al., 2009; Twig et al., 2010) and function (Detmer and Chan, 2007). Another signaling pathway that CK2 regulates is Cdk5 (Lim et al., 2004) (Figure 2). Both CK2 and Cdk5 are expressed at nodes of Ranvier (Brechet et al., 2008; Cerda and Trimmer, 2011) and by oligodendrocytes (Huillard et al., 2010; Yang et al., 2013), where CK2 can inhibit Cdk5 by contact inhibition (Lim et al., 2004). Therefore, for Cdk5 to be activated, CK2 must move away from Cdk5. Cdk5 is tethered to the membrane by its association with p35, a protein with a membrane-anchoring domain. The Cdk5/p35 complex is then fully activated by Ca2+-dependent proteolytic cleavage of p35 to p25 by calpain, effectively removing its membrane-anchoring domain (Meyer et al., 2014). Local intracellular Ca2+ may be increased by the reversal of the Na+-Ca2+ exchanger or by activation of L-type Ca2+ channels (Brown, 2001; Baltan, 2009). The Cdk5 complex can then move from nodes of Ranvier to other cellular compartments to control AKT signaling (Lou et al., 2008) and axonal transport (Shea et al., 2004) to ultimately regulate mitochondrial dynamics (Amiri and Hollenbeck, 2008; Liu et al., 2009; Twig et al., 2010) and function (Detmer and Chan, 2007) (Figure 2). In addition, CK2 can regulate PTEN/AKT/GSK3β (Silva et al., 2008; Silva et al., 2010) signaling. CK2 inhibits PTEN by phosphorylation, which leads to the activation of AKT. AKT phosphorylates GSK3β to inhibit its activity (Cross et al., 1995), which could lead to changes in mitochondrial transport (Embi et al., 1980; Cohen and Frame, 2001), glycogen synthase (Embi et al., 1980; Cohen and Frame, 2001), and mitochondrial function (Detmer and Chan, 2007; Amiri and Hollenbeck, 2008; Liu et al., 2009; Twig et al., 2010).
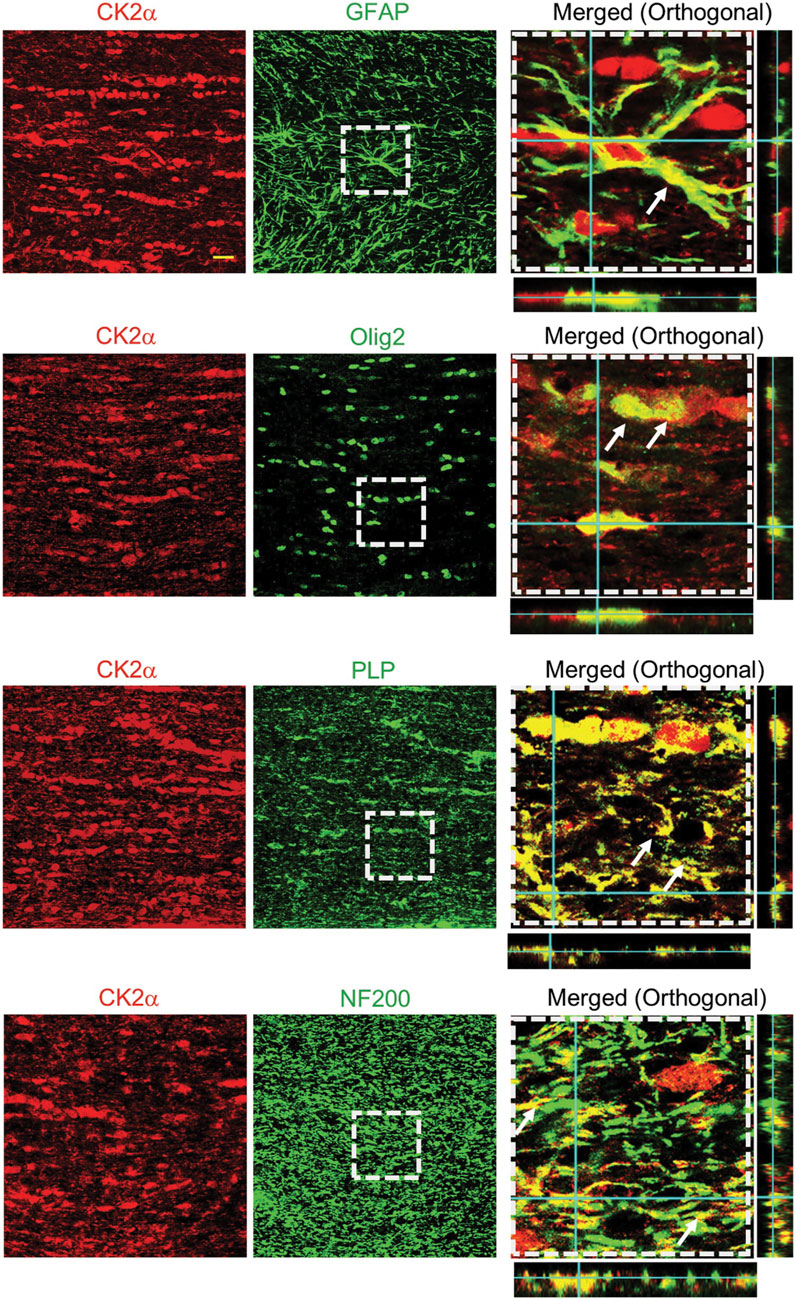
FIGURE 1. CK2α expression and localization in mouse optic nerves. CK2α subunits are expressed in optic nerve astrocytes, myelin sheath, and oligodendrocytes. To identify the cellular expression of CK2α subunits in the mouse optic nerve, CK2α was co-immunolabeled with glial fibrillary acidic protein (GFAP, astrocytes, top row), oligodendrocyte lineage transcription factor 2 (Olig2, oligodendrocytes, second row), myelin proteolipid protein (PLP, myelin, third row), and neurofilament protein (NF200, axons, bottom row). Note that the merged images (xy, xz and yz orthogonal view) in the right panels are enlarged areas (50 μm × 50 μm) indicated by the squares with dashed lines in the middle panels. Scale bar = 20 μm. (Figure from Bastian et al., 2019).
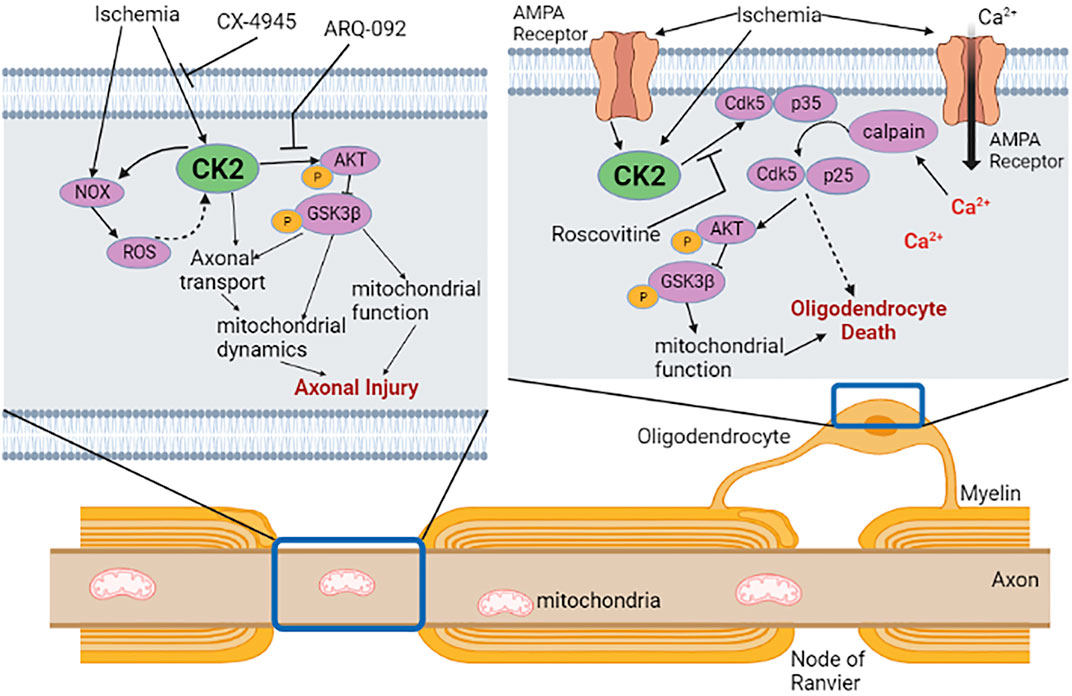
FIGURE 2. CK2 signaling in white matter ischemia. In axon, ischemia can directly activate CK2 or NADPH oxidase (NOX) to increase reactive oxygen species (ROS). CK2 then activates Akt/GSK3β signaling through phosphorylation to disrupt axonal mitochondrial dynamics and function. Specific CK2 inhibitor CX-4945 or phosphorylated Akt inhibitor ARQ-092 confers axonal protection when applied during or after ischemia. In oligodendrocytes, ischemia activates CK2 directly or via activation of AMPA receptors leading to an influx of Ca2+. Ca2+ activates calpain, which subsequently acts on Cdk5/p35 complex to untether Cdk5/p25 complex from the membrane that can then phosphorylate Akt/GSK3β in oligodendrocytes. Roscovitine, an inhibitor of Cdk5, improves axon function recovery following ischemia, presumably through protection of oligodendrocytes and/or axons. The effect of untethered Cdk5 on oligodendrocyte injury is yet to be investigated. Dotted arrow indicates potential interactions. Created with Biorender.com.
Our recent studies, for the first time, establish that a PK mediates ischemic injury in WM by CK2 signaling leading to activation of Cdk5 and PTEN/AKT/GSK3β pathways (Bastian et al., 2019b) (Figure 2). To identify the role of CK2 signaling in WM ischemic injury, we followed the preclinical recommendations of the Stroke Therapy Academic Industry Roundtable (STAIR) (Fisher et al., 2009). Based on this set of criteria, we use CX-4945 (Silmitasertib), a highly selective (Siddiqui-Jain et al., 2010; Son et al., 2013), specific (Battistutta et al., 2011), and potent CK2 inhibitor that can be administered orally (Ryan et al., 2005; Zhong et al., 2020) and crosses the blood-brain barrier (Vita et al., 2005; Sallam et al., 2008; Cheng et al., 2012; Zheng et al., 2013). CX-4945 is an FDA-approved anti-cancer drug (Martins et al., 2014). CX-4945 exerts dose-dependent protection to axon function (max protection at 5 µM) and the lack of any baseline effects of this drug on axon conduction allows comparison of recovery in in vitro experiments and behavioral assessments in in vivo experiments. Thus, we propose that CK2 inhibition protects the brain against ischemia by protecting axonal and glial compartments.
Equally important, CX-4945 confers similar protection to aging (12–14 months) and old (>20 months) WM when applied after ischemia promoting axon function recovery. The effects of aging on myelinated axons are more complicated and extensive than those in cortical GM. Despite larger and thicker aging axons, with longer and thicker mitochondria that correlate with lower ATP production, CX-4945 still provides post-ischemic protection to aging axon function by preserving mitochondrial integrity. We, therefore, suggest that CK2 signaling is a shared pathway underlying WM injury independent of age.
An important outcome measure emphasized by STAIR criteria is the consideration of the female sex. Stroke in females is associated with a decreased likelihood of excellent outcome after acute ischemic stroke, particularly in older age groups. There is a correlation between markers of WM integrity and functional outcomes in women, which implies a potential sex-specific WM injury mechanism (Etherton et al., 2017; Phan et al., 2018; Etherton et al., 2019). Therefore, evaluation of CK2 signaling in female WM injury and whether CK2 inhibition provides equal protection compared to male WM is a pending goal of our group.
Casein Kinase 2 Mediates Post-Ischemic WM Injury By Selectively Acting on the AKT Pathway
The finding that CK2 inhibition with CX-4945 when applied before or after the end of an ischemic episode promotes WM functional recovery raises the question of whether Cdk5 or AKT activation plays a distinct role in conferring post-ischemic WM protection (Figure 2). Inhibition of Cdk5 using Roscovitine protects axon function only when applied during ischemia, mainly acting on oligodendrocytes and axons (Figure 2). On the other hand, inhibition of the active conformation of AKT is beneficial when applied during or after ischemia suggesting that a window of opportunity exists in ameliorating ischemic injury in WM. AKT is involved in many neurological processes, and AKT isoforms are distinct regarding their tissue expression, pathway activation, and inhibitor sensitivity (Shaw and Kirshenbaum, 2006; Skeen et al., 2006). However, very few studies have examined AKT isoform expression at the cellular level, and cell- and age-specific AKT isoforms expression in WM remains unknown. Therefore, a systematic investigation of AKT isoforms and their contribution to axon and glia function is warranted.
Casein Kinase 2 Disrupts Axonal Mitochondria
We recently showed that the preservation of mitochondrial integrity is an essential component of post-ischemic protection of axon function in WM. (Baltan et al., 2011; Baltan, 2014a; Stahon et al., 2016). CX-4945 promotes young and aging axon function recovery following ischemia by preserving axonal mitochondria. Cdk5 directly impacts mitochondrial dynamics and function by increasing the production of ROS and phosphorylation of the mitochondrial fission protein Drp-1, leading to mitochondrial dysfunction (Sun et al., 2008; Morel et al., 2010; Cherubini et al., 2015; Jahani-Asl et al., 2015; Klinman and Holzbaur, 2015; Park et al., 2015). However, Cdk5 inhibition fails to exert post-ischemic protection to axon function, implying that Cdk5 signaling is important to alleviate oxidative injury specifically during ischemia. The finding that selective inhibition of phosphorylated AKT confers post-ischemic protection to axon function proposes a novel role for PTEN/AKT signaling in mediating mitochondrial disruption after an ischemic episode in WM. AKT activation contributes to increased glutamate release during OGD and ATP depletion, as well as enhanced excitotoxicity (Baltan et al., 2008) due to the upregulation of GLT-1 expression in astrocytes (Li et al., 2006; Ji et al., 2013; Zhang et al., 2013). As a result, the application of ARQ-092, which is a specific blocker for the active form of AKT (Yu et al., 2015; Lapierre et al., 2016), promotes axon function recovery suggesting that the active conformation of AKT is an important molecular target for post-ischemic protection of axon function (Figure 2). Moreover, the GSK3β isoform which is a part of the AKT/GSK3β signaling cascade has been reported to be a significant therapeutic target for cerebral ischemia (Koh S.-H. et al., 2008; Cowper-Smith et al., 2008). GSK3β inhibition decreases mitochondrial ROS production and prevents neuronal damage establishing an interesting relationship between GSK3β and mitochondria (Valerio et al., 2011). Because, we also observed that CK2 inhibition improved axon function recovery by decreasing the inactivation of GSK3β in WM, these findings suggest that GSK3β could be a common target to protect both GM and WM after ischemic stroke.
Casein Kinase 2 Mediates WM Injury By Regulating Micro RNAs (miRNAs)
The miRNAs emerge as important mediators of neuronal injury during an ischemia attack. However, the role and involvement of miRNAs remain unestablished in WM ischemic injury. Therefore, in our recent study, we characterized miRNA profiles in optic nerve following ischemia using the NanoString nCounter® miRNA Expression Panel. Together with in situ hybridization, and in silico KEGG pathway analysis, we show that the most abundant miRNAs in the optic nerve are expressed in astrocytes, and oxygen-glucose deprivation (OGD) differentially regulates miRNA expression in the optic nerve (Baltan et al., 2021).
Based on our analysis, it remains challenging to define whether miRNAs regulated by ischemia in WM are beneficial or detrimental to the recovery of axon function. Some miRNAs were reciprocally regulated by OGD or OGD and CX-4945 application. For instance, OGD and CX-4945 selectively modulated miR-1937a, miR-1937b, miR-1959, miR-200a, miR-501-3p, and miR-654-3p (Table 1) (Baltan et al., 2021). We propose that these miRNAs may be associated with the beneficial effects of CX-4945. In agreement, miR-501-3p is expressed in neurons which in optic nerve (pure WM tract without neuronal cell body) infers axonal expression (Baltan et al., 2021). Because CK2 inhibition promotes axon function by preserving axonal mitochondria (Bastian et al., 2019b), we hypothesize that CK2 inhibition exerts white matter protection by regulating miR-501-3p. Particularly, because miR-501-3p is shown to mediate the regulation of GluA1 subunit expression of AMPA receptors and the subsequent mitochondrial injury (Sripada et al., 2012). Furthermore, miR-501-3p is expressed in human and reported to be a novel serum biomarker, which relates to the severity of Alzheimer’s Disease (Hara et al., 2017). Hence, miR-501-3p appears as a promising target for further investigation. In addition, we determined that exosomal expression of miR-1959 and miR-1937b in astrocytes is also differentially regulated by OGD or OGD and CX-4945 (Jovičić and Gitler, 2017). Astrocytic exosomes are 50—100 ŋm membrane-bound vesicles, which contain and transfer a selected group of miRNAs to other cells. This suggests that astrocytes coordinate an efficient communication among glia cells in response to OGD or CK2 inhibition during ischemia in WM. It will be intriguing to identify the role and cellular target of miR-1959 and miR-1937b in WM. Another novel finding is that CX-4945 treatment affects some common KEGG signaling pathways. One of these signaling pathways is the ErbB signaling system (Li et al., 2020) which is important to maintain axon function as well as supporting glia and myelin. Expectedly, a disruption in ErbB signaling may impair axon function by causing myelin damage (Carroll et al., 1997). Additionally, ErbB signaling in neurons and macrophages/microglia determines neuroprotection and repair capacity after ischemia (Xu and Ford, 2005). Interestingly, CX-4945 regulates Wingless/int1 (Wnt) signaling, which is involved in neurogenesis after cerebral ischemia, implicating Wnt signaling as a therapeutic target for ischemic injury (Yu et al., 2018; Qiu et al., 2019). CX-4945 also regulates mTOR and axon guidance pathways that are important for neuroprotection after an ischemic stroke (Sofer et al., 2005; Koh P.-O. et al., 2008; Foster and Fingar, 2010; Koh, 2010; Hinman, 2014). However, further experiments are needed to validate whether the mechanisms of protection of CX-4945 are indeed mediated through these signaling pathways.
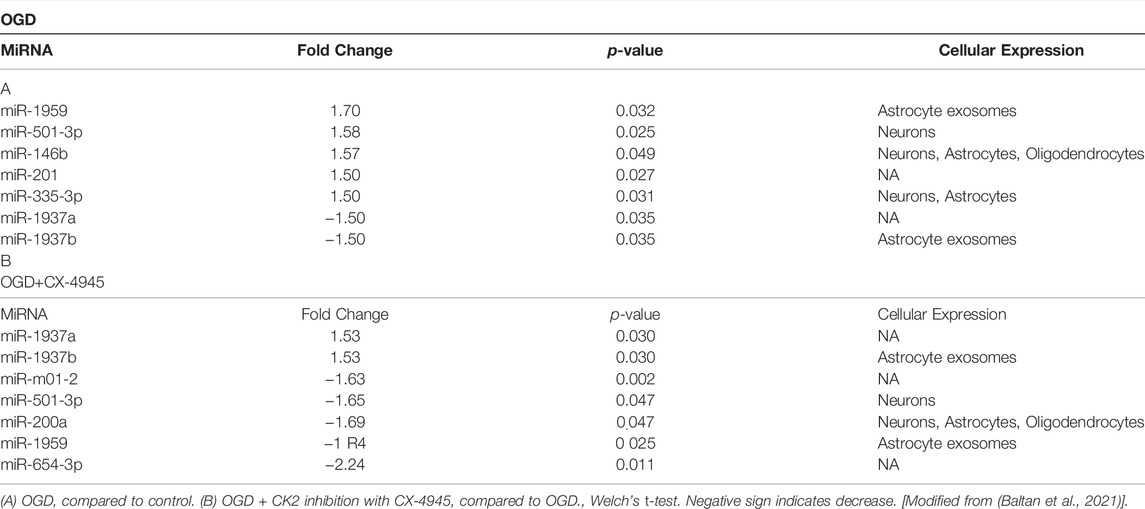
TABLE 1. List of miRNAs with fold changes after OGD and OGD with CK2 inhibition and their cellular expression.
Discussion
WM ischemic lesions are correlated to neurological deficits (Yamada et al., 2003; Sea Lee et al., 2005) and particularly the extent and localization of WM injury may dictate functional deficits and recovery in humans. Because the rodent brain has a relatively small WM (10–15%) (Zhang and Sejnowski, 2000) and most widely used stroke models spare corpus callosum, the injury mechanisms mostly provide information about neuronal populations. Neuroprotective approaches focused solely on neuronal survival may be one of the reasons for the failure in translating experimental findings successfully to clinical applications. It is crucial to consider WM integrity in experimental models to identify ideal therapeutic targets for stroke patients.
In summary, our recent studies provide evidence that CK2 signaling activates Cdk5 and AKT/GSK3ß signaling pathways to mediate WM ischemic injury. The downstream molecular pathways are activated in a spatiotemporal way such that Cdk5 signaling becomes significant during ischemia, while AKT signaling emerges as the key pathway during the post-ischemic period. Consistent with this, inhibition of CK2 or the activated form of AKT confers post-ischemic protection to axon function and promotes recovery in young and aging WM. The protective effects of CK2 inhibition correlate with the conservation of oligodendrocytes, axon structure, and axonal mitochondria. Several miRNAs are differentially regulated by CX-4945 compared to ischemia, and these miRNAs may participate in ischemic WM injury mechanisms. MiRNAs are promising candidates for biomarkers of injury and therapeutic interventions as they are readily detected in body fluids. We also show that CX-4945 regulates a group of murine-associated viral miRNAs (for example see Table 1) which may justify the use of CX-4945 in clinical trials for Covid19 patients (Baltan et al., 2021) (ClinicalTrials.gov Identifier: NCT04663737). Finally, our findings may have mechanistic and therapeutic implications for dementia, Alzheimer’s disease, multiple sclerosis, periventricular leukomalacia, and Parkinson’s disease that involve WM injury.
Author Contributions
All authors participated in writing the manuscript.
Funding
This work was supported by the National Institute of Aging (Grant Number AG033720) National Institute of Neurological Diseases and Stroke (NS094881).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Ngoc Wasson for editorial assistance.
References
Agrawal, S. K., and Fehlings, M. G. (1997). Role of NMDA and Non-NMDA Ionotropic Glutamate Receptors in Traumatic Spinal Cord Axonal Injury. J. Neurosci. 17 (3), 1055–1063. doi:10.1523/jneurosci.17-03-01055.1997
Alberdi, E., Sánchez-Gómez, M. V., Marino, A., and Matute, C. (2002). Ca2+ Influx through AMPA or Kainate Receptors Alone Is Sufficient to Initiate Excitotoxicity in Cultured Oligodendrocytes. Neurobiol. Dis. 9 (2), 234–243. doi:10.1006/nbdi.2001.0457
Amiri, M., and Hollenbeck, P. J. (2008). Mitochondrial Biogenesis in the Axons of Vertebrate Peripheral Neurons. Devel Neurobio 68 (11), 1348–1361. doi:10.1002/dneu.20668
Baltan, S. (2014a). “Age-Dependent Mechanisms of White Matter Injury after Stroke,” in White Matter Injury in Stroke and CNS Disease. Editors S. Baltan, S. T. Carmichael, C. Matute, G. Xi, and J. H. Zhang (New York, NY: Springer New York), 373–403. doi:10.1007/978-1-4614-9123-1_16
Baltan, S., Bastian, C., Quinn, J., Aquila, D., McCray, A., and Brunet, S. (2018). CK2 Inhibition Protects White Matter from Ischemic Injury. Neurosci. Lett. 687, 37–42. doi:10.1016/j.neulet.2018.08.021
Baltan, S., Besancon, E. F., Mbow, B., Ye, Z., Hamner, M. A., and Ransom, B. R. (2008). White Matter Vulnerability to Ischemic Injury Increases with Age Because of Enhanced Excitotoxicity. J. Neurosci. 28 (6), 1479–1489. doi:10.1523/jneurosci.5137-07.2008
Baltan, S. (2014b). Excitotoxicity and Mitochondrial Dysfunction Underlie Age-dependent Ischemic White Matter Injury. Adv. Neurobiol. 11, 151–170. doi:10.1007/978-3-319-08894-5_8
Baltan, S. (2009). Ischemic Injury to White Matter: an Age-dependent Process. Neuroscientist 15 (2), 126–133. doi:10.1177/1073858408324788
Baltan, S., Murphy, S. P., Danilov, C. A., Bachleda, A., and Morrison, R. S. (2011). Histone Deacetylase Inhibitors Preserve White Matter Structure and Function during Ischemia by Conserving ATP and Reducing Excitotoxicity. J. Neurosci. 31 (11), 3990–3999. doi:10.1523/JNEUROSCI.5379-10.2011
Baltan, S., Sandau, U. S., Brunet, S., Bastian, C., Tripathi, A., Nguyen, H., et al. (2021). Identification of miRNAs that Mediate Protective Functions of Anti-cancer Drugs during White Matter Ischemic Injury. ASN Neuro 13, 175909142110422. doi:10.1177/17590914211042220
Bastian, C., Quinn, J., Doherty, C., Franke, C., Faris, A., Brunet, S., et al. (2019a). “Role of Brain Glycogen during Ischemia, Aging and Cell-To-Cell Interactions,” in Brain Glycogen Metabolism. Editors M. DiNuzzo, and A. Schousboe (Cham: Springer International Publishing), 2019/11/02, 347–361. doi:10.1007/978-3-030-27480-1_12
Bastian, C., Quinn, J., Tripathi, A., Aquila, D., McCray, A., Dutta, R., et al. (2019b). CK2 Inhibition Confers Functional Protection to Young and Aging Axons against Ischemia by Differentially Regulating the CDK5 and AKT Signaling Pathways. Neurobiol. Dis. 126, 47–61. doi:10.1016/j.nbd.2018.05.011
Bastian, C., Zaleski, J., Stahon, K., Parr, B., McCray, A., Day, J., et al. (2018). NOS3 Inhibition Confers Post-Ischemic Protection to Young and Aging White Matter Integrity by Conserving Mitochondrial Dynamics and Miro-2 Levels. J. Neurosci. 38 (28), 6247–6266. doi:10.1523/JNEUROSCI.3017-17.2018
Battistutta, R., Cozza, G., Pierre, F., Papinutto, E., Lolli, G., Sarno, S., et al. (2011). Unprecedented Selectivity and Structural Determinants of a New Class of Protein Kinase CK2 Inhibitors in Clinical Trials for the Treatment of Cancer. Biochemistry 50 (39), 8478–8488. doi:10.1021/bi2008382
Blanquet, P. R. (2000). Casein Kinase 2 as a Potentially Important Enzyme in the Nervous System. Prog. Neurobiol. 60 (3), 211–246. doi:10.1016/s0301-0082(99)00026-x
Bréchet, A., Fache, M.-P., Brachet, A., Ferracci, G., Baude, A., Irondelle, M., et al. (2008). Protein Kinase CK2 Contributes to the Organization of Sodium Channels in Axonal Membranes by Regulating Their Interactions with Ankyrin G. J. Cell Biol. 183 (6), 1101–1114. doi:10.1083/jcb.200805169
Brown, G. C. (2001). Regulation of Mitochondrial Respiration by Nitric Oxide Inhibition of Cytochrome C Oxidase. Biochimica Biophysica Acta (BBA) - BioenergeticsProtein Struct. Mol. Enzym. 1504 (1), 46–57. doi:10.1016/s0005-2728(00)00238-3
Brunet, S., Emrick, M. A., Sadilek, M., Scheuer, T., and Catterall, W. A. (2015). Phosphorylation Sites in the Hook Domain of CaVβ Subunits Differentially Modulate CaV1.2 Channel Function. J. Mol. Cell. Cardiol. 87, 248–256. doi:10.1016/j.yjmcc.2015.08.006
Canedo-Antelo, M., Llavero, F., Zugaza, J., Matute, C., and Sánchez-Gómez, M. V. (2018) “Inhibition of Casein Kinase 2 Reduces aMPa-Induced Oligodendrocyte Death through Jnk Signaling and Er Stress Regulation,” in XII European Meeting on Glial Cells in Health and Disease (Spain: Bilbao).
Canedo-Antelo, M., Matute, C., and Sánchez-Gómez, M. V. (2019) “Protein Kinase CK2 and JNK Modulate Pro-apoptotic Effector Activation in AMPA-Induced Excitotoxicity in Oligodendrocytes,” in Neurogune 2nd Basque Neuroscience Meeting (Spain: San Sebastian).
Carroll, S. L., Miller, M. L., Frohnert, P. W., Kim, S. S., and Corbett, J. A. (1997). Expression of Neuregulins and Their Putative Receptors, ErbB2 and ErbB3, Is Induced during Wallerian Degeneration. J. Neurosci. 17 (5), 1642–1659. doi:10.1523/JNEUROSCI.17-05-01642.1997
Cavallotti, C., Cavallotti, D., Pescosolido, N., and Pacella, E. (2003). Age-related Changes in Rat Optic Nerve: Morphological Studies. Anatom Histol. Embryol. 32 (1), 12–16. doi:10.1046/j.1439-0264.2003.00431.x
Cavallotti, C., Pacella, E., Pescosolido, N., Tranquilli-Leali, F. M., and Feher, J. (2002). Age-related Changes in the Human Optic Nerve. Can. J. Ophthalmol. 37 (7), 389–394. doi:10.1016/s0008-4182(02)80040-0
Cerda, O., and Trimmer, J. S. (2011). Activity-dependent Phosphorylation of Neuronal Kv2.1 Potassium Channels by CDK5. J. Biol. Chem. 286 (33), 28738–28748. doi:10.1074/jbc.M111.251942
Chao, C. C., Ma, Y. L., and Lee, E. H. Y. (2007). Protein Kinase CK2 Impairs Spatial Memory Formation through Differential Cross Talk with PI-3 Kinase Signaling: Activation of Akt and Inactivation of SGK1. J. Neurosci. 27 (23), 6243–6248. doi:10.1523/JNEUROSCI.1531-07.2007
Charriaut-Marlangue, C., Otani, S., Creuzet, C., Ben-Ari, Y., and Loeb, J. (1991). Rapid Activation of Hippocampal Casein Kinase II during Long-Term Potentiation. Proc. Natl. Acad. Sci. U.S.A. 88 (22), 10232–10236. doi:10.1073/pnas.88.22.10232
Cheng, Y., Zhang, Y., Zhang, L., Ren, X., Huber-Keener, K. J., Liu, X., et al. (2012). MK-2206, a Novel Allosteric Inhibitor of Akt, Synergizes with Gefitinib against Malignant Glioma via Modulating Both Autophagy and Apoptosis. Mol. Cancer Ther. 11 (1), 154–164. doi:10.1158/1535-7163.MCT-11-0606
Cherubini, M., Puigdellívol, M., Alberch, J., and Ginés, S. (2015). Cdk5-mediated Mitochondrial Fission: A Key Player in Dopaminergic Toxicity in Huntington's Disease. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1852 (10 Pt A), 2145–2160. doi:10.1016/j.bbadis.2015.06.025
Cohen, P., and Frame, S. (2001). The Renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2 (10), 769–776. doi:10.1038/35096075
Cosentino-Gomes, D., Rocco-Machado, N., and Meyer-Fernandes, J. R. (2012). Cell Signaling through Protein Kinase C Oxidation and Activation. Ijms 13 (9), 10697–10721. doi:10.3390/ijms130910697
Cowper-Smith, C. D., Anger, G. J. A., Magal, E., Norman, M. H., and Robertson, G. S. (2008). Delayed Administration of a Potent Cyclin Dependent Kinase and Glycogen Synthase Kinase 3 β Inhibitor Produces Long-Term Neuroprotection in a Hypoxia-Ischemia Model of Brain Injury. Neuroscience 155 (3), 864–875. doi:10.1016/j.neuroscience.2008.05.051
Cross, D. A. E., Alessi, D. R., Cohen, P., Andjelkovich, M., and Hemmings, B. A. (1995). Inhibition of Glycogen Synthase Kinase-3 by Insulin Mediated by Protein Kinase B. Nature 378 (6559), 785–789. doi:10.1038/378785a0
Curtze, S., Melkas, S., Sibolt, G., Haapaniemi, E., Mustanoja, S., Putaala, J., et al. (2015). Cerebral Computed Tomography-Graded White Matter Lesions Are Associated with Worse Outcome after Thrombolysis in Patients with Stroke. Stroke 46 (6), 1554–1560. doi:10.1161/STROKEAHA.115.008941
Detmer, S. A., and Chan, D. C. (2007). Functions and Dysfunctions of Mitochondrial Dynamics. Nat. Rev. Mol. Cell Biol. 8 (11), 870–879. doi:10.1038/nrm2275
Díaz-Nido, J., and Avila, J. (1992). Protein Kinases Associated with Isolated Mitotic Spindles from Mammalian Cells: Identification of a Casein Kinase II-like Enzyme. Second Messengers Phosphoproteins 14 (1-2), 39–53.
Embi, N., Rylatt, D. B., and Cohen, P. (1980). Glycogen Synthase Kinase-3 from Rabbit Skeletal Muscle. Separation from Cyclic-AMP-dependent Protein Kinase and Phosphorylase Kinase. Eur. J. Biochem. 107 (2), 519–527.
Eom, G. H., Cho, Y. K., Ko, J.-H., Shin, S., Choe, N., Kim, Y., et al. (2011). Casein Kinase-2α1 Induces Hypertrophic Response by Phosphorylation of Histone Deacetylase 2 S394 and its Activation in the Heart. Circulation 123 (21), 2392–2403. doi:10.1161/CIRCULATIONAHA.110.003665
Etherton, M. R., Wu, O., Cougo, P., Giese, A.-K., Cloonan, L., Fitzpatrick, K. M., et al. (2017). Structural Integrity of Normal Appearing White Matter and Sex-specific Outcomes after Acute Ischemic Stroke. Stroke 48 (12), 3387–3389. doi:10.1161/STROKEAHA.117.019258
Etherton, M. R., Wu, O., Cougo, P., Lorenzano, S., Li, H., Cloonan, L., et al. (2019). Sex-specific Differences in White Matter Microvascular Integrity after Ischaemic Stroke. Stroke Vasc. Neurol. 4 (4), 198–205. doi:10.1136/svn-2019-000268
Faust, M., Jung, M., Günther, J., Zimmermann, R., and Montenarh, M. (2001). Localization of Individual Subunits of Protein Kinase CK2 to the Endoplasmic Reticulum and to the Golgi Apparatus. Mol. Cell Biochem. 227 (1-2), 73–80. doi:10.1007/978-1-4615-1723-8_9
Fern, R., and Ransom, B. R. (1997). Ischemic Injury of Optic Nerve Axons: the Nuts and Bolts. Clin. Neurosci. 4 (5), 246–250.
Fern, R., Ransom, B. R., and Waxman, S. G. (1995). Voltage-gated Calcium Channels in CNS White Matter: Role in Anoxic Injury. J. Neurophysiology 74 (1), 369–377. doi:10.1152/jn.1995.74.1.369
Fisher, M., Feuerstein, G., Howells, D. W., Hurn, P. D., Kent, T. A., Savitz, S. I., et al. (2009). Update of the Stroke Therapy Academic Industry Roundtable Preclinical Recommendations. Stroke 40 (6), 2244–2250. doi:10.1161/STROKEAHA.108.541128
Fields, R. D. (2008). White Matter in Learning, Cognition and Psychiatric Disorders. Trends Neurosci. 31 (7), 361–370. doi:10.1016/j.tins.2008.04.001
Follett, P. L., Rosenberg, P. A., Volpe, J. J., and Jensen, F. E. (2000). NBQX Attenuates Excitotoxic Injury in Developing White Matter. J. Neurosci. 20 (24), 9235–9241. doi:10.1523/jneurosci.20-24-09235.2000
Foster, K. G., and Fingar, D. C. (2010). Mammalian Target of Rapamycin (mTOR): Conducting the Cellular Signaling Symphony. J. Biol. Chem. 285 (19), 14071–14077. doi:10.1074/jbc.R109.094003
Franchin, C., Borgo, C., Cesaro, L., Zaramella, S., Vilardell, J., Salvi, M., et al. (2018). Re-evaluation of Protein Kinase CK2 Pleiotropy: New Insights provided by a Phosphoproteomics Analysis of CK2 Knockout Cells. Cell. Mol. Life Sci. 75 (11), 2011–2026. doi:10.1007/s00018-017-2705-8
Hara, N., Kikuchi, M., Miyashita, A., Hatsuta, H., Saito, Y., Kasuga, K., et al. (2017). Serum microRNA miR-501-3p as a Potential Biomarker Related to the Progression of Alzheimer's Disease. Acta Neuropathol. Commun. 5 (1), 10. doi:10.1186/s40478-017-0414-z
Hase, M., Depre, C., Vatner, S. F., and Sadoshima, J. (2005). H11 Has Dose-dependent and Dual Hypertrophic and Proapoptotic Functions in Cardiac Myocytes. Biochem. J. 388 (Pt 2), 475–483. doi:10.1042/BJ20041314
Hauck, L., Harms, C., An, J., Rohne, J., Gertz, K., Dietz, R., et al. (2008). Protein Kinase CK2 Links Extracellular Growth Factor Signaling with the Control of p27Kip1 Stability in the Heart. Nat. Med. 14 (3), 315–324. doi:10.1038/nm1729
Hien, Y. E., Montersino, A., Castets, F., Leterrier, C., Filhol, O., Vacher, H., et al. (2014). CK2 Accumulation at the Axon Initial Segment Depends on Sodium Channel Nav1. FEBS Lett. 588 (18), 3403–3408. doi:10.1016/j.febslet.2014.07.032
Hinman, J. D. (2014). The Back and Forth of Axonal Injury and Repair after Stroke. Curr. Opin. Neurol. 27 (6), 615–623. doi:10.1097/WCO.0000000000000149
Hu, B.-R., Ou Yang, Y.-B., and Wieloch, T. (1993). Heat-shock Inhibits Protein Synthesis and eIF-2 Activity in Cultured Cortical Neurons. Neurochem. Res. 18 (9), 1003–1007. doi:10.1007/BF00966760
Hu, B. R., and Wieloch, T. (1993). Casein Kinase II Activity in the Postischemic Rat Brain Increases in Brain Regions Resistant to Ischemia and Decreases in Vulnerable Areas. J. Neurochem. 60 (5), 1722–1728. doi:10.1111/j.1471-4159.1993.tb13396.x
Huillard, E., Ziercher, L., Blond, O., Wong, M., Deloulme, J.-C., Souchelnytskyi, S., et al. (2010). Disruption of CK2 β in Embryonic Neural Stem Cells Compromises Proliferation and Oligodendrogenesis in the Mouse Telencephalon. Mol. Cell Biol. 30 (11), 2737–2749. doi:10.1128/MCB.01566-09
Jahani-Asl, A., Huang, E., Irrcher, I., Rashidian, J., Ishihara, N., Lagace, D. C., et al. (2015). CDK5 Phosphorylates DRP1 and Drives Mitochondrial Defects in NMDA-Induced Neuronal Death. Hum. Mol. Genet. 24 (16), 4573–4583. doi:10.1093/hmg/ddv188
Ji, Y.-F., Zhou, L., Xie, Y.-J., Xu, S.-M., Zhu, J., Teng, P., et al. (2013). Upregulation of Glutamate Transporter GLT-1 by mTOR-Akt-NF-кB Cascade in Astrocytic Oxygen-Glucose Deprivation. Glia 61 (12), 1959–1975. doi:10.1002/glia.22566
Jovičić, A., and Gitler, A. D. (2017). Distinct Repertoires of microRNAs Present in Mouse Astrocytes Compared to Astrocytesecreted Exosomes. PLOS ONE 12 (2), e0171418. doi:10.1371/journal.pone.0171418
Ka, S.-O., Hwang, H. P., Jang, J.-H., Hyuk Bang, I., Bae, U.-J., Yu, H. C., et al. (2015). The Protein Kinase 2 Inhibitor Tetrabromobenzotriazole Protects against Renal Ischemia Reperfusion Injury. Sci. Rep. 5, 14816. doi:10.1038/srep14816
Kim, G. S., Jung, J. E., Niizuma, K., and Chan, P. H. (2009). CK2 Is a Novel Negative Regulator of NADPH Oxidase and a Neuroprotectant in Mice after Cerebral Ischemia. J. Neurosci. 29 (47), 14779–14789. doi:10.1523/JNEUROSCI.4161-09.2009
Klinman, E., and Holzbaur, E. L. F. (2015). Stress-Induced CDK5 Activation Disrupts Axonal Transport via Lis1/Ndel1/Dynein. Cell Rep. 12 (3), 462–473. doi:10.1016/j.celrep.2015.06.032
Koh, P.-O., Cho, J.-H., Won, C.-K., Lee, H.-J., Sung, J.-H., and Kim, M.-O. (2008a). Estradiol Attenuates the Focal Cerebral Ischemic Injury through mTOR/p70S6 Kinase Signaling Pathway. Neurosci. Lett. 436 (1), 62–66. doi:10.1016/j.neulet.2008.02.061
Koh, P.-O. (2010). Gingko Biloba Extract (EGb 761) Prevents Cerebral Ischemia-Induced p70S6 Kinase and S6 Phosphorylation. Am. J. Chin. Med. 38 (4), 727–734. doi:10.1142/S0192415X10008196
Koh, S.-H., Yoo, A. R., Chang, D.-I., Hwang, S. J., and Kim, S. H. (2008b). Inhibition of GSK-3 Reduces Infarct Volume and Improves Neurobehavioral Functions. Biochem. Biophysical Res. Commun. 371 (4), 894–899. doi:10.1016/j.bbrc.2008.05.006
Kristensen, L. P., Larsen, M. R., Højrup, P., Issinger, O.-G., and Guerra, B. (2004). Phosphorylation of the Regulatory β-subunit of Protein Kinase CK2 by Checkpoint Kinase Chk1: Identification of the In Vitro CK2β Phosphorylation Site. FEBS Lett. 569 (1-3), 217–223. doi:10.1016/j.febslet.2004.05.069
Lapierre, J.-M., Eathiraj, S., Vensel, D., Liu, Y., Bull, C. O., Cornell-Kennon, S., et al. (2016). Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-Phenyl-3h-Imidazo[4,5-B]pyridin-2-Yl)pyridin-2-Amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J. Med. Chem. 59 (13), 6455–6469. doi:10.1021/acs.jmedchem.6b00619
Leslie, N. R., and Downes, C. P. (2002). PTEN: The Down Side of PI 3-kinase Signalling. Cell. Signal. 14 (4), 285–295. doi:10.1016/s0898-6568(01)00234-0
Li, F., Liu, W.-C., Wang, Q., Sun, Y., Wang, H., and Jin, X. (2020). NG2-glia Cell Proliferation and Differentiation by Glial Growth Factor 2 (GGF2), a Strategy to Promote Functional Recovery after Ischemic Stroke. Biochem. Pharmacol. 171, 113720. doi:10.1016/j.bcp.2019.113720
Li, L.-B., Toan, S. V., Zelenaia, O., Watson, D. J., Wolfe, J. H., Rothstein, J. D., et al. (2006). Regulation of Astrocytic Glutamate Transporter Expression by Akt: Evidence for a Selective Transcriptional Effect on the GLT-1/EAAT2 Subtype. J. Neurochem. 97 (3), 759–771. doi:10.1111/j.1471-4159.2006.03743.x
Li, S., Mealing, G. A. R., Morley, P., and Stys, P. K. (1999). Novel Injury Mechanism in Anoxia and Trauma of Spinal Cord White Matter: Glutamate Release via Reverse Na+-dependent Glutamate Transport. J. Neurosci. 19 (14), RC16. doi:10.1523/jneurosci.19-14-j0002.1999
Lim, A. C. B., Hou, Z., Goh, C.-P., and Qi, R. Z. (2004). Protein Kinase CK2 Is an Inhibitor of the Neuronal Cdk5 Kinase. J. Biol. Chem. 279 (45), 46668–46673. doi:10.1074/jbc.M404760200
Liu, X., Weaver, D., Shirihai, O., and Hajnóczky, G. (2009). Mitochondrial 'kiss-And-Run': Interplay between Mitochondrial Motility and Fusion-Fission Dynamics. EMBO J. 28 (20), 3074–3089. doi:10.1038/emboj.2009.255
Lolli, G., Pinna, L. A., and Battistutta, R. (2012). Structural Determinants of Protein Kinase CK2 Regulation by Autoinhibitory Polymerization. ACS Chem. Biol. 7 (7), 1158–1163. doi:10.1021/cb300054n
Lou, D. Y., Dominguez, I., Toselli, P., Landesman-Bollag, E., O'Brien, C., and Seldin, D. C. (2008). The Alpha Catalytic Subunit of Protein Kinase CK2 Is Required for Mouse Embryonic Development. Mol. Cell Biol. 28 (1), 131–139. doi:10.1128/MCB.01119-07
Lugus, J. J., Ngoh, G. A., Bachschmid, M. M., and Walsh, K. (2011). Mitofusins Are Required for Angiogenic Function and Modulate Different Signaling Pathways in Cultured Endothelial Cells. J. Mol. Cell. Cardiol. 51 (6), 885–893. doi:10.1016/j.yjmcc.2011.07.023
Martins, L. R., Lúcio, P., Melão, A., Antunes, I., Cardoso, B. A., Stansfield, R., et al. (2014). Activity of the Clinical-Stage CK2-specific Inhibitor CX-4945 against Chronic Lymphocytic Leukemia. Leukemia 28 (1), 179–182. doi:10.1038/leu.2013.232
Matute, C. (2010). Calcium Dyshomeostasis in White Matter Pathology. Cell Calcium 47 (2), 150–157. doi:10.1016/j.ceca.2009.12.004
Matute, C., Sánchez-Gómez, M. V., Martínez-Millán, L., and Miledi, R. (1997). Glutamate Receptor-Mediated Toxicity in Optic Nerve Oligodendrocytes. Proc. Natl. Acad. Sci. U.S.A. 94 (16), 8830–8835. doi:10.1073/pnas.94.16.8830
McDonald, J. W., Levine, J. M., and Qu, Y. (1998). Multiple Classes of the Oligodendrocyte Lineage Are Highly Vulnerable to Excitotoxicity. Neuroreport 9 (12), 2757–2762. doi:10.1097/00001756-199808240-00014
Meggio, F., and Pinna, L. A. (2003). One‐thousand‐and‐one Substrates of Protein Kinase CK2? FASEB J. 17 (3), 349–368. doi:10.1096/fj.02-0473rev
Meyer, D. A., Torres-Altoro, M. I., Tan, Z., Tozzi, A., Di Filippo, M., DiNapoli, V., et al. (2014). Ischemic Stroke Injury Is Mediated by Aberrant Cdk5. J. Neurosci. 34 (24), 8259–8267. doi:10.1523/JNEUROSCI.4368-13.2014
Micu, I., Jiang, Q., Coderre, E., Ridsdale, A., Zhang, L., Woulfe, J., et al. (2006). NMDA Receptors Mediate Calcium Accumulation in Myelin during Chemical Ischaemia. Nature 439 (7079), 988–992. doi:10.1038/nature04474
Miller, M. W., Knaub, L. A., Olivera-Fragoso, L. F., Keller, A. C., Balasubramaniam, V., Watson, P. A., et al. (2013). Nitric Oxide Regulates Vascular Adaptive Mitochondrial Dynamics. Am. J. Physiology-Heart Circulatory Physiology 304 (12), H1624–H1633. doi:10.1152/ajpheart.00987.2012
Morel, M., Authelet, M., Dedecker, R., and Brion, J. P. (2010). Glycogen Synthase Kinase-3β and the P25 Activator of Cyclin Dependent Kinase 5 Increase Pausing of Mitochondria in Neurons. Neuroscience 167 (4), 1044–1056. doi:10.1016/j.neuroscience.2010.02.077
Moreno, F. J., Díaz-Nido, J., Jiménez, J. S., and Avila, J. (1999). Distribution of CK2, its Substrate MAP1B and Phosphatases in Neuronal Cells. Mol. Cell Biochem. 191 (1-2), 201–205. doi:10.1007/978-1-4419-8624-5_24
Nunez, S., Doroudchi, M. M., Gleichman, A. J., Ng, K. L., Llorente, I. L., Sozmen, E. G., et al. (2016). A Versatile Murine Model of Subcortical White Matter Stroke for the Study of Axonal Degeneration and White Matter Neurobiology. JoVE 109, 53404. doi:10.3791/53404
Ouardouz, M., Nikolaeva, M. A., Coderre, E., Zamponi, G. W., McRory, J. E., Trapp, B. D., et al. (2003). Depolarization-induced Ca2+ Release in Ischemic Spinal Cord White Matter Involves L-type Ca2+ Channel Activation of Ryanodine Receptors. Neuron 40 (1), 53–63. doi:10.1016/j.neuron.2003.08.016
Park, J., Choi, H., Min, J.-S., Kim, B., Lee, S.-R., Yun, J. W., et al. (2015). Loss of Mitofusin 2 Links Beta-Amyloid-Mediated Mitochondrial Fragmentation and Cdk5-Induced Oxidative Stress in Neuron Cells. J. Neurochem. 132 (6), 687–702. doi:10.1111/jnc.12984
Phan, H. T., Blizzard, C. L., Reeves, M. J., Thrift, A. G., Cadilhac, D. A., Sturm, J., et al. (2018). Factors Contributing to Sex Differences in Functional Outcomes and Participation after Stroke. Neurology 90 (22), e1945–e1953. doi:10.1212/WNL.0000000000005602
Pigino, G., Morfini, G., Atagi, Y., Deshpande, A., Yu, C., Jungbauer, L., et al. (2009). Disruption of Fast Axonal Transport Is a Pathogenic Mechanism for Intraneuronal Amyloid Beta. Proc. Natl. Acad. Sci. U.S.A. 106 (14), 5907–5912. doi:10.1073/pnas.0901229106
Pinna, L. A., and Allende, J. E. (2009). Protein Kinase CK2 in Health and Disease. Cell. Mol. Life Sci. 66 (11-12), 1795–1799. doi:10.1007/s00018-009-9148-9
Poole, A., Poore, T., Bandhakavi, S., McCann, R. O., Hanna, D. E., and Glover, C. V. C. (2005). A Global View of CK2 Function and Regulation. Mol. Cell Biochem. 274 (1-2), 163–170. doi:10.1007/s11010-005-2945-z
Qaiser, F., Trembley, J. H., Kren, B. T., Wu, J.-J., Naveed, A. K., and Ahmed, K. (2014). Protein Kinase CK2 Inhibition Induces Cell Death via Early Impact on Mitochondrial Function. J. Cell. Biochem. 115 (12), 2103–2115. doi:10.1002/jcb.24887
Qiu, C.-W., Liu, Z.-Y., Zhang, F.-L., Zhang, L., Li, F., Liu, S.-Y., et al. (2019). Post-Stroke Gastrodin Treatment Ameliorates Ischemic Injury and Increases Neurogenesis and Restores the Wnt/β-Catenin Signaling in Focal Cerebral Ischemia in Mice. Brain Res. 1712, 7–15. doi:10.1016/j.brainres.2019.01.043
Rosenberger, A. F. N., Morrema, T. H. J., Gerritsen, W. H., van Haastert, E. S., Snkhchyan, H., Hilhorst, R., et al. (2016). Increased Occurrence of Protein Kinase CK2 in Astrocytes in Alzheimer's Disease Pathology. J. Neuroinflammation 13, 4. doi:10.1186/s12974-015-0470-x
Ryan, Q. C., Headlee, D., Acharya, M., Sparreboom, A., Trepel, J. B., Ye, J., et al. (2005). Phase I and Pharmacokinetic Study of MS-275, a Histone Deacetylase Inhibitor, in Patients with Advanced and Refractory Solid Tumors or Lymphoma. Jco 23 (17), 3912–3922. doi:10.1200/JCO.2005.02.188
Saab, A. S., Tzvetavona, I. D., Trevisiol, A., Baltan, S., Dibaj, P., Kusch, K., et al. (2016). Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 91 (1), 119–132. doi:10.1016/j.neuron.2016.05.016
Sallam, H., Jimenez, P., Song, H., Vita, M., Cedazominguez, A., and Hassan, M. (2008). Age-dependent Pharmacokinetics and Effect of Roscovitine on Cdk5 and Erk1/2 in the Rat Brain. Pharmacol. Res. 58 (1), 32–37. doi:10.1016/j.phrs.2008.05.010
Sánchez-Gómez, M. V., and Matute, C. (1999). AMPA and Kainate Receptors Each Mediate Excitotoxicity in Oligodendroglial Cultures. Neurobiol. Dis. 6 (6), 475–485. doi:10.1006/nbdi.1999.0264
Sarno, S., Ghisellini, P., and Pinna, L. A. (2002). Unique Activation Mechanism of Protein Kinase CK2. J. Biol. Chem. 277 (25), 22509–22514. doi:10.1074/jbc.M200486200
Sea Lee, J., Han, M.-K., Hyun Kim, S., Kwon, O.-K., and Hyoung Kim, J. (2005). “Fiber Tracking by Diffusion Tensor Imaging in Corticospinal Tract Stroke: Topographical Correlation with Clinical Symptoms,” in NeuroImage. doi:10.1016/j.neuroimage.2005.02.036
Serrano, L., Hernández, M. A., Díaz-Nido, J., and Avila, J. (1989). Association of Casein Kinase II with Microtubules. Exp. Cell Res. 181 (1), 263–272. doi:10.1016/0014-4827(89)90200-0
Shaw, J., and Kirshenbaum, L. A. (2006). Prime Time for JNK-Mediated Akt Reactivation in Hypoxia-Reoxygenation. Circulation Res. 98 (1), 7–9. doi:10.1161/01.RES.0000200397.22663.b6
Shea, T. B., Zheng, Y.-L., Ortiz, D., and Pant, H. C. (2004). Cyclin-dependent Kinase 5 Increases Perikaryal Neurofilament Phosphorylation and Inhibits Neurofilament Axonal Transport in Response to Oxidative Stress. J. Neurosci. Res. 76 (6), 795–800. doi:10.1002/jnr.20099
Siddiqui-Jain, A., Drygin, D., Streiner, N., Chua, P., Pierre, F., O'Brien, S. E., et al. (2010). CX-4945, an Orally Bioavailable Selective Inhibitor of Protein Kinase CK2, Inhibits Prosurvival and Angiogenic Signaling and Exhibits Antitumor Efficacy. Cancer Res. 70 (24), 10288–10298. doi:10.1158/0008-5472.CAN-10-1893
Silva, A., Jotta, P. Y., Silveira, A. B., Ribeiro, D., Brandalise, S. R., Yunes, J. A., et al. (2010). Regulation of PTEN by CK2 and Notch1 in Primary T-Cell Acute Lymphoblastic Leukemia: Rationale for Combined Use of CK2- and -secretase Inhibitors. Haematologica 95 (4), 674–678. doi:10.3324/haematol.2009.011999
Silva, A., Yunes, J. A., Cardoso, B. A., Martins, L. R., Jotta, P. Y., Abecasis, M., et al. (2008). PTEN Posttranslational Inactivation and Hyperactivation of the PI3K/Akt Pathway Sustain Primary T Cell Leukemia Viability. J. Clin. Invest. 118 (11), 3762–3774. doi:10.1172/JCI34616
Skeen, J. E., Bhaskar, P. T., Chen, C.-C., Chen, W. S., Peng, X.-d., Nogueira, V., et al. (2006). Akt Deficiency Impairs Normal Cell Proliferation and Suppresses Oncogenesis in a P53-independent and mTORC1-dependent Manner. Cancer Cell 10 (4), 269–280. doi:10.1016/j.ccr.2006.08.022
Sofer, A., Lei, K., Johannessen, C. M., and Ellisen, L. W. (2005). Regulation of mTOR and Cell Growth in Response to Energy Stress by REDD1. Mol. Cell Biol. 25 (14), 5834–5845. doi:10.1128/MCB.25.14.5834-5845.2005
Son, Y. H., Song, J. S., Kim, S. H., and Kim, J. (2013). Pharmacokinetic Characterization of CK2 Inhibitor CX-4945. Arch. Pharm. Res. 36 (7), 840–845. doi:10.1007/s12272-013-0103-9
Srinivasan, S., Spear, J., Chandran, K., Joseph, J., Kalyanaraman, B., and Avadhani, N. G. (2013). Oxidative Stress Induced Mitochondrial Protein Kinase A Mediates Cytochrome C Oxidase Dysfunction. PLoS One 8 (10), e77129. doi:10.1371/journal.pone.0077129
Sripada, L., Tomar, D., and Singh, R. (2012). Mitochondria: One of the Destinations of miRNAs. Mitochondrion 12 (6), 593–599. doi:10.1016/j.mito.2012.10.009
Stahon, K. E., Bastian, C., Griffith, S., Kidd, G. J., Brunet, S., and Baltan, S. (2016). Age-Related Changes in Axonal and Mitochondrial Ultrastructure and Function in White Matter. J. Neurosci. 36 (39), 9990–10001. doi:10.1523/jneurosci.1316-16.2016
Stys, P. K., Ransom, B. R., Waxman, S. G., and Davis, P. K. (1990). Role of Extracellular Calcium in Anoxic Injury of Mammalian Central White Matter. Proc. Natl. Acad. Sci. U.S.A. 87 (11), 4212–4216. doi:10.1073/pnas.87.11.4212
Sun, K.-H., de Pablo, Y., Vincent, F., and Shah, K. (2008). Deregulated Cdk5 Promotes Oxidative Stress and Mitochondrial Dysfunction. J. Neurochem. 107 (1), 265–278. doi:10.1111/j.1471-4159.2008.05616.x
Tekkök, S. B., and Goldberg, M. P. (2001). AMPA/Kainate Receptor Activation Mediates Hypoxic Oligodendrocyte Death and Axonal Injury in Cerebral White Matter. J. Neurosci. 21 (12), 4237–4248. doi:10.1523/jneurosci.21-12-04237.2001
Tekkök, S. B., Ye, Z., and Ransom, B. R. (2007). Excitotoxic Mechanisms of Ischemic Injury in Myelinated White Matter. J. Cereb. Blood Flow. Metab. 27 (9), 1540–1552. doi:10.1038/sj.jcbfm.9600455
Twig, G., Liu, X., Liesa, M., Wikstrom, J. D., Molina, A. J. A., Las, G., et al. (2010). Biophysical Properties of Mitochondrial Fusion Events in Pancreatic β-cells and Cardiac Cells Unravel Potential Control Mechanisms of its Selectivity. Am. J. Physiology-Cell Physiology 299 (2), C477–C487. doi:10.1152/ajpcell.00427.2009
Underhill, S. M., and Goldberg, M. P. (2007). Hypoxic Injury of Isolated Axons Is Independent of Ionotropic Glutamate Receptors. Neurobiol. Dis. 25 (2), 284–290. doi:10.1016/j.nbd.2006.09.011
Valerio, A., Bertolotti, P., Delbarba, A., Perego, C., Dossena, M., Ragni, M., et al. (2011). Glycogen Synthase Kinase-3 Inhibition Reduces Ischemic Cerebral Damage, Restores Impaired Mitochondrial Biogenesis and Prevents ROS Production. J. Neurochem. 116 (6), 1148–1159. doi:10.1111/j.1471-4159.2011.07171.x
Vilk, G., Weber, J. E., Turowec, J. P., Duncan, J. S., Wu, C., Derksen, D. R., et al. (2008). Protein Kinase CK2 Catalyzes Tyrosine Phosphorylation in Mammalian Cells. Cell. Signal. 20 (11), 1942–1951. doi:10.1016/j.cellsig.2008.07.002
Vita, M., Abdel-Rehim, M., Olofsson, S., Hassan, Z., Meurling, L., Sidén, Å., et al. (2005). Tissue Distribution, Pharmacokinetics and Identification of Roscovitine Metabolites in Rat. Eur. J. Pharm. Sci. 25 (1), 91–103. doi:10.1016/j.ejps.2005.02.001
Voccoli, V., Tonazzini, I., Signore, G., Caleo, M., and Cecchini, M. (2014). Role of Extracellular Calcium and Mitochondrial Oxygen Species in Psychosine-Induced Oligodendrocyte Cell Death. Cell Death Dis. 5, e1529. doi:10.1038/cddis.2014.483
Wolf, J. A., Stys, P. K., Lusardi, T., Meaney, D., and Smith, D. H. (2001). Traumatic Axonal Injury Induces Calcium Influx Modulated by Tetrodotoxin-Sensitive Sodium Channels. J. Neurosci. 21 (6), 1923–1930. doi:10.1523/jneurosci.21-06-01923.2001
Wrathall, J. R., Choiniere, D., and Teng, Y. D. (1994). Dose-dependent Reduction of Tissue Loss and Functional Impairment after Spinal Cord Trauma with the AMPA/kainate Antagonist NBQX. J. Neurosci. 14 (11 Pt 1), 6598–6607. doi:10.1523/jneurosci.14-11-06598.1994
Xu, Z., and Ford, B. D. (2005). Upregulation of erbB Receptors in Rat Brain after Middle Cerebral Arterial Occlusion. Neurosci. Lett. 375 (3), 181–186. doi:10.1016/j.neulet.2004.11.039
Yamada, K., Mori, S., Nakamura, H., Ito, H., Kizu, O., Shiga, K., et al. (2003). Fiber-Tracking Method Reveals Sensorimotor Pathway Involvement in Stroke Patients. Stroke 34 (9), E159–E162. doi:10.1161/01.str.0000085827.54986.89
Yang, Y., Wang, H., Zhang, J., Luo, F., Herrup, K., Bibb, J. A., et al. (2013). Cyclin Dependent Kinase 5 Is Required for the Normal Development of Oligodendrocytes and Myelin Formation. Dev. Biol. 378 (2), 94–106. doi:10.1016/j.ydbio.2013.03.023
Yoshimura, T., and Rasband, M. N. (2014). Axon Initial Segments: Diverse and Dynamic Neuronal Compartments. Curr. Opin. Neurobiol. 27, 96–102. doi:10.1016/j.conb.2014.03.004
Yu, Y., Savage, R. E., Eathiraj, S., Meade, J., Wick, M. J., Hall, T., et al. (2015). Targeting AKT1-E17k and the PI3K/AKT Pathway with an Allosteric AKT Inhibitor, ARQ 092. PLoS One 10 (10), e0140479. doi:10.1371/journal.pone.0140479
Yu, Z., Cheng, C., Liu, Y., Liu, N., Lo, E. H., and Wang, X. (2018). Neuroglobin Promotes Neurogenesis through Wnt Signaling Pathway. Cell Death Dis. 9 (10), 945. doi:10.1038/s41419-018-1007-x
Zhang, K., and Sejnowski, T. J. (2000). A Universal Scaling Law between Gray Matter and White Matter of Cerebral Cortex. Proc. Natl. Acad. Sci. U.S.A. 97 (10), 5621–5626. doi:10.1073/pnas.090504197
Zhang, X., Shi, M., Bjoras, M., Wang, W., Zhang, G., Han, J., et al. (2013). Ginsenoside Rd Promotes Glutamate Clearance by Up-Regulating Glial Glutamate Transporter GLT-1 via PI3K/AKT and ERK1/2 Pathways. Front. Pharmacol. 4, 152. doi:10.3389/fphar.2013.00152
Zheng, Y., McFarland, B. C., Drygin, D., Yu, H., Bellis, S. L., Kim, H., et al. (2013). Targeting Protein Kinase CK2 Suppresses Prosurvival Signaling Pathways and Growth of Glioblastoma. Clin. Cancer Res. 19 (23), 6484–6494. doi:10.1158/1078-0432.CCR-13-0265
Zhong, B., Campagne, O., Salloum, R., Purzner, T., and Stewart, C. F. (2020). LC-MS/MS Method for Quantitation of the CK2 Inhibitor Silmitasertib (CX-4945) in Human Plasma, CSF, and Brain Tissue, and Application to a Clinical Pharmacokinetic Study in Children with Brain Tumors. J. Chromatogr. B 1152, 122254. doi:10.1016/j.jchromb.2020.122254
Keywords: mitochondria, micro RNA, CX-4945, silmitasertib, akt, post-ischemic protection, ischemia
Citation: Nguyen H, Zhu W and Baltan S (2022) Casein Kinase 2 Signaling in White Matter Stroke. Front. Mol. Biosci. 9:908521. doi: 10.3389/fmolb.2022.908521
Received: 30 March 2022; Accepted: 21 June 2022;
Published: 13 July 2022.
Edited by:
Andrea Venerando, University of Padua, ItalyReviewed by:
Jason D Hinman, University of California, Los Angeles, United StatesByung Gon Kim, Ajou University, South Korea
Copyright © 2022 Nguyen, Zhu and Baltan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Selva Baltan, YmFsdGFuQG9oc3UuZWR1
 Hung Nguyen
Hung Nguyen Wenbin Zhu
Wenbin Zhu Selva Baltan
Selva Baltan