- Department of Molecular, Cell, and Developmental Biology, University of California, Santa Cruz, Santa Cruz, CA, United States
It has long been postulated that the inflammatory environment favors cell proliferation, and is conducive to diseases such as cancer. In the prostate gland, clinical data implicate important roles of prostatitis in the progression of both benign prostatic hyperplasia (BPH) and prostate cancer (PCa). However, their causal relationships have not been firmly established yet due to unresolved molecular and cellular mechanisms. By accurately mimicking human disease, vertebrate animals provide essential in vivo models to address this question. Here, we review the vertebrate prostatitis models that have been developed and discuss how they may reveal possible mechanisms by which prostate inflammation promotes BPH and PCa. Recent studies, particularly those involving genetically engineered mouse models (GEMMs), suggest that such mechanisms are multifaceted, which include epithelium barrier disruption, DNA damage and cell proliferation induced by paracrine signals, and expansion of potential cells of origin for cancer. Future research using rodent prostatitis models should aim to distinguish the etiologies of BPH and PCa, and facilitate the development of novel clinical approaches for prostatic disease prevention.
Introduction
Prostatitis is the inflammation of the prostate gland, and is characterized by immune cell (lymphocytes, neutrophils, macrophages, basophils, eosinophils) infiltration in the stromal compartment or localized regions surrounding the prostatic epithelial ducts. Often causing pelvic pain and sexual dysfunction, it is the most common urinary tract problem for men under the age of fifty (Collins et al., 1998). In the United States, prostatitis is estimated to account for two million visits to the clinics each year. Prostatitis is also gaining increasing attention because pathological and epidemiological evidence suggest that it is a significant etiologic factor in prostate cancer (PCa) (De Marzo et al., 2007; Sfanos et al., 2018). However, the mechanisms of prostatitis pathogenesis and its contribution to PCa development remain poorly understood. Vertebrate systems, particularly rodent models, provide invaluable tools to address these questions in the in vivo setting. By mimicking human prostatitis conditions and symptoms, those models allow for experimentation on various prostatic disease mechanisms and possible treatment options. In this review, we discuss commonly used vertebrate models of prostatitis in the field with a focus on their potential roles in elucidating the etiologic relationship of prostatitis, benign prostatic hyperplasia (BPH), and PCa.
Rodent Models for Different Types of Prostatitis
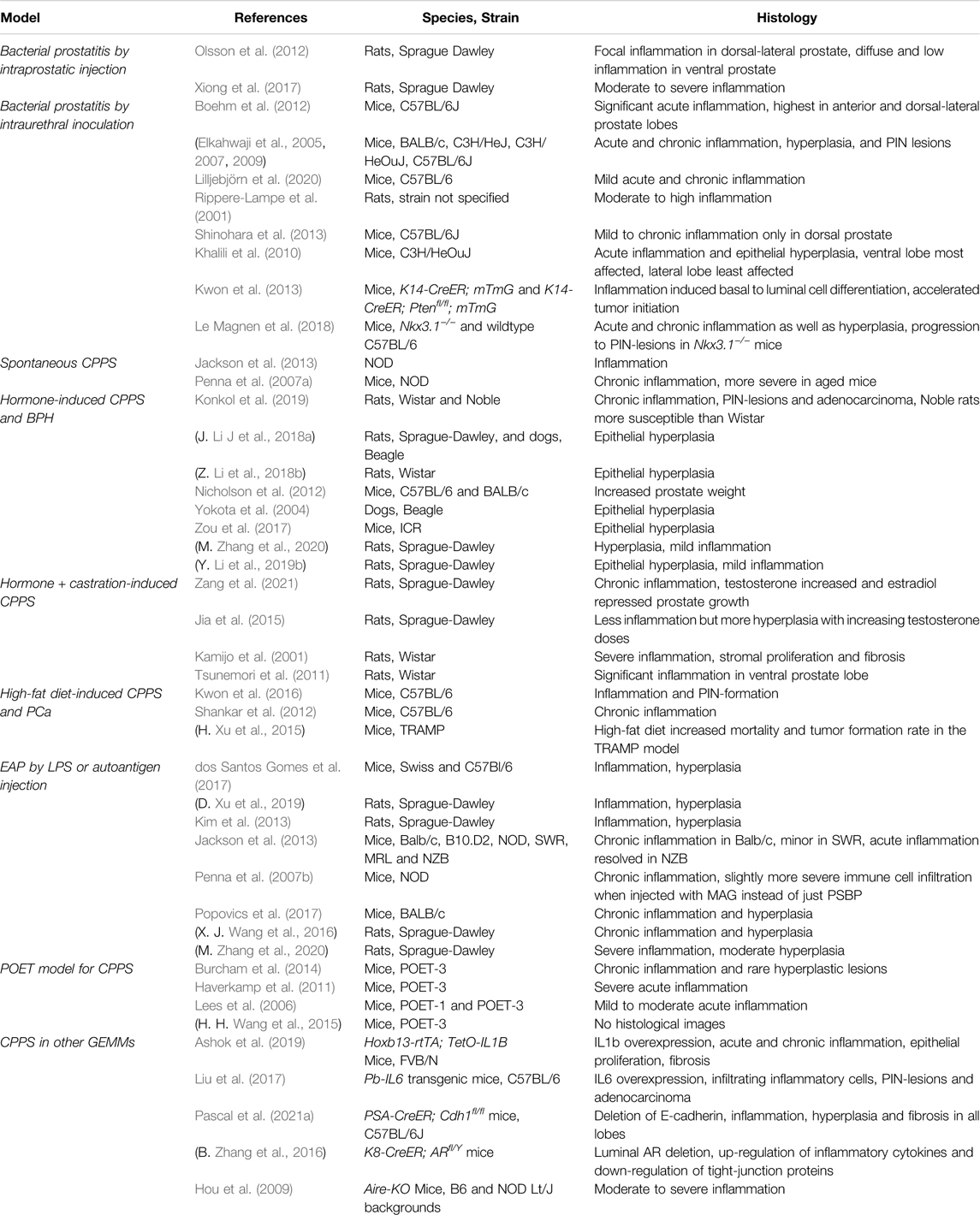
Clinically, prostatitis can be divided into four types: acute bacterial inflammation, chronic bacterial inflammation, abacterial prostatitis or chronic pelvic pain syndrome (CPPS), and asymptomatic prostatic chronic inflammation (Vykhovanets et al., 2007; Gill and Shoskes, 2016; Liu et al., 2020). Prostatitis pathology differs among the types of inflammation and may be distinguished by immune cell types and their localization in different regions of the prostate (Sfanos et al., 2018). For example, acute inflammation usually features neutrophil infiltration, whereas chronic inflammation is mostly characterized by lymphocytes and macrophages (Sfanos et al., 2018; Ashok et al., 2019). Type IV or asymptomatic inflammation, due to its lack of symptoms in patients, can only be diagnosed based on increased leukocytes in biopsy samples taken after a prostate-specific antigen (PSA) test in prostate cancer screens (Porcaro et al., 2015). As a result, animal models of asymptomatic prostatitis are rare and difficult to define. In contrast, various methods, including bacterial infection, hormone treatment, immunization, stress, and diet manipulation, have been used to study acute and chronic bacterial prostatitis as well as CPPS in rodent models (Vykhovanets et al., 2007) (summarized in Table 1).
Bacterial infection is frequently used to study acute and chronic bacterial inflammation and is induced either by direct injection of uropathogenic bacteria into the prostate lobes of rodents (Olsson et al., 2012; Xiong et al., 2017) or by inoculation via an intraurethral catheter (Rippere-Lampe et al., 2001; Elkahwaji et al., 2005; Elkahwaji et al., 2007; Elkahwaji et al., 2009; Khalili et al., 2010; Boehm et al., 2012; Shinohara et al., 2013; Le Magnen et al., 2018; Lilljebjörn et al., 2020). Different rodent species and strains have been used, including Wistar and Sprague-Dawley rats and C57BL/6 and C3H/HeJ mice. While some of the infected rodents recover spontaneously, many will develop chronic inflammation following initial acute inflammation response (Vykhovanets et al., 2007). Commonly used bacterial strains for infection include various uropathogenic Escherichia coli strains (Rippere-Lampe et al., 2001; Elkahwaji et al., 2005; Elkahwaji et al., 2007; Elkahwaji et al., 2009; Boehm et al., 2012; Lilljebjörn et al., 2020), as well as other species such as Propionibacterium acnes (Olsson et al., 2012; Shinohara et al., 2013). Although P. acnes infection might take longer to induce inflammation compared to E. coli, both can induce acute and chronic inflammation, with lesions featured by higher cell proliferation and diminished Nkx3.1 and androgen receptor (AR) expression (Shinohara et al., 2013). Clinically, chronic bacterial prostatitis is often developed from acute bacterial prostate inflammation. Therefore, these infection models are highly relevant as they mimic disease etiology.
In contrast to bacterial inflammation, the direct cause of abacterial prostatitis/CPPS remains unclear (Liu et al., 2020; Tsunemori and Sugimoto, 2021). Possible disease mechanisms include physical and chemical damage by urine reflux, sexually transmitted pathogens, diet, hormone imbalances, and autoimmunity (De Nunzio et al., 2011). Consequently, a wide range of animal models has been developed to explore the many potential causes of CPPS. Notably, certain rodents such as Wister, Lewis and Copenhagen rats, develop abacterial chronic prostatitis spontaneously as they age (Lundgren et al., 1984; Sharma et al., 1992; Keith et al., 2001). In men, aging is associated with increased prevalence of CPPS and a decline of the serum testosterone to estradiol ratio (T-to-E2 ratio) (Harman et al., 2001; Bernoulli et al., 2008). One proposed mechanism is that a decreased T-to-E2 ratio disrupts the balance between the immunosuppressive effect of testosterone and the pro-inflammatory effect mediated by estrogen (Cutolo et al., 2002). To mimic this, hormone-induced animal models of CPPS are often based on decreasing the T-to-E2 ratio, either by administration of estradiol or a combination of estradiol and testosterone (Kamijo et al., 2001; Tsunemori et al., 2011; Jia et al., 2015; Konkol et al., 2019; Zang et al., 2021). In other approaches, a high fat diet (HFD) has been shown to induce chronic inflammation in rodents (Shankar et al., 2012; Shankar et al., 2015; Xu et al., 2015; Kwon et al., 2016). HFD-induced oxidative stress and NF-κB and Stat3 signaling activation may play important roles in this process (Shankar et al., 2012; Shankar et al., 2015), but the mechanisms by which HFD promotes chronic inflammation remain to be fully elucidated.
One of the great advantages of using mouse models is the capability of genetically manipulating gene expression in vivo. Several genetically engineered mouse models (GEMMs) have been reported to be able to induce chronic inflammation. These include prostate-specific knockout of the gene encoding AR or E-cadherin (Zhang et al., 2016; Pascal et al., 2021b), which increases prostate epithelial barrier permeability. Genetically modified mice are particularly useful for modeling immune-related chronic prostate inflammation, whose phenotypes are commonly referred to as experimental autoimmune prostatitis (EAP). For example, an inherent lack of immunity can cause chronic prostatitis in aging NOD mice, a strain prone to developing organ-specific autoimmune disease (Kikutani and Makino, 1992). In these models, the autoimmune origin is evident by a T-cell response to prostate autoantigens and characterized by CD4+ T-cell intraprostatic infiltration (Penna et al., 2007a; Jackson et al., 2013). Other genetic models include overexpression of the pro-inflammatory cytokines IL-1β or IL-6 in transgenic mice (Liu et al., 2017; Ashok et al., 2019), and the Aire-deficient mouse model, in which knockout of the important immune regulator Aire led to development of chronic prostatitis (Hou et al., 2009).
Notably, EAP can also be triggered by injection of lipopolysaccharide (LPS), a component of the Gram-negative bacterial cell wall, which stimulates the release of pro-inflammatory cytokines to induce chronic inflammation (Kim et al., 2013; dos Santos Gomes et al., 2017; Xu et al., 2019). Other EAP models induce inflammation by injecting a combination of autoantigens with an adjuvant. These autoantigens are prostate specific, such as male accessory gland extract (Jackson et al., 2013) and prostate tissue homogenate (Wang et al., 2016; Popovics et al., 2017). However, as homogenized tissue contains multiple antigens, this makes some of these immunological models unfit to study T-cell/antigen specific interactions. Furthermore, many models use endogenous T-cell pools that have had previous antigen exposure, further limiting specificity of the T-cell response (Lees et al., 2006). Consequently, the prostate ovalbumin-expressing transgenic (POET) mouse model was developed as an antigen-specific autoimmune model of both acute and chronic prostate inflammation (Lees et al., 2006; Haverkamp et al., 2011; Burcham et al., 2014; Wang et al., 2015). Using the ARR2PB promoter, POET mice express high levels of membrane-bound ovalbumin in the different lobes of the prostate (Lees et al., 2006; Haverkamp et al., 2011). Using adoptive transfer of transgenic T-cells that recognize ovalbumin, the POET model circumvents general tolerance mechanisms and provides the opportunity to monitor a specific T-cell population during both chronic and acute prostate inflammation.
Vertebrate Models That Involve Inflammation and Benign Prostatic Hyperplasia
Benign prostatic hyperplasia (BPH) is a condition in which hyperplasia of the stromal and glandular prostatic cells causes prostate enlargement (Nickel, 2008). Clinically, BPH is characterized by lower urinary tract symptoms (LUTS) such as voiding, storage and post-micturition symptoms, and can be associated with bladder outlet obstruction (BOO) (Roehrborn, 2005; Chughtai et al., 2011). Some BOO animal models involve mechanical obstruction of the urethra by sutures or ligatures, thus directly affecting urine outflow (Austin et al., 2004; Kanno et al., 2016). However, the relevance of these models to BPH-induced BOO is unclear due to the invasiveness of the procedure and the fact that BPH is a disease that develops over a long period of time. Rather, animal models that recapitulate age-related spontaneous BPH development are desired. In contrast to spontaneous prostatitis models, only macaques, chimpanzees, and dogs are known to naturally develop BPH, with dogs being the most commonly used animal model for BPH (Sun et al., 2017; Zhang et al., 2021). Interestingly, since age-related change in hormone ratios is thought to contribute to BPH development, rodent models of BPH have been developed by castration and administration of testosterone and/or estrogen (Yokota et al., 2004; Nicholson et al., 2012; Zou et al., 2017; Li J. et al., 2018; Li Z. et al., 2018; Li Y. et al., 2019; Zhang et al., 2020), similar to the hormone-induced CPPS rodent models (Kamijo et al., 2001; Tsunemori et al., 2011; Jia et al., 2015; Konkol et al., 2019). Indeed, many aforementioned chronic prostatitis models also show BPH phenotypes. For example, high-fat diet as well as LPS and E. coli injection can induce both inflammation and BPH phenotypes in rodents (Elkahwaji et al., 2007; Escobar et al., 2009; Shankar et al., 2012; Kim et al., 2013; Kwon et al., 2016; dos Santos Gomes et al., 2017; Li Y. et al., 2019; Xu et al., 2019), and the EAP model used to induce chronic prostatitis can induce BPH in rats (Wang et al., 2016; Zhang et al., 2020). This overlap between animal models for prostatitis and BPH is reflected in clinical findings: biopsies taken from patients with BPH often show immune cell infiltration and markers of inflammation (Taoka et al., 2004; Penna et al., 2007b; Nickel, 2008; Robert et al., 2009; Taoka and Kakehi, 2017). Similarly, several studies found bacterial and viral strains in BPH specimens, suggesting that bacterial inflammation may play a role in BPH development (Nickel et al., 1999; Chughtai et al., 2011).
Mechanistically, it has been postulated that chronic inflammation can create a microenvironment that induces wound healing repair processes, leading to the activation of proliferative pathways and hence prostate hyperplasia (Taoka et al., 2004; Fibbi et al., 2010). For example, inflammation-induced leakage of the epithelial barrier could lead to an influx of luminal-secreted autoantigens into the stromal compartment and subsequently produce an autoimmune response (Chughtai et al., 2011; Li F. et al., 2019; Pascal et al., 2021b). Indeed, PSA has been detected in stroma surrounding BPH nodules from patients (O'Malley et al., 2014), and a large scale analysis of BPH patient tissues revealed that high serum PSA values were associated with inflammation (Gandaglia et al., 2013). One possible mechanism of epithelial barrier leakage may be through down-regulation of E-cadherin, an important regulator of the epithelial barrier and tissue homeostasis. E-cadherin expression is often found to be lower in BPH tissues (Kim et al., 2013; Li F. et al., 2019; Xu et al., 2019; Pascal et al., 2021a), and conditional knockout of E-cadherin in the mouse prostate causes loss of epithelial barrier function, inflammation and hyperplasia (Pascal et al., 2021b). Additionally, conditional overexpression of the pro-inflammatory cytokine interleukin-6 (IL-6) down-regulates E-cadherin (Liu et al., 2017), suggesting that a positive feedback loop between inflammation and epithelial barrier disruption may be present to promote BPH. Similarly, a recent study showed that attenuation of luminal epithelial AR signaling can induce prostate inflammation and impair epithelial cell tight junctions, while inflammation can suppress AR expression (Zhang et al., 2016). Such a positive feedback loop may also be involved in sustaining chronic inflammation during BPH progression. Despite these progresses, whether inflammation directly causes BPH or is an associated factor during BPH progression remains unclear. Further research is needed to clarify the relationship between chronic prostatitis and BPH.
Rodent Models for Studying the Relationship Between Prostatitis and Prostate Cancer
Prostate cancer (PCa) is the second leading cause of cancer-related morbidity and mortality in American men. The etiologic link between prostatitis and PCa has long been suggested (De Marzo et al., 2007; Sfanos et al., 2018). For example, in human prostatectomy specimens, lesions characterized by proliferating epithelial cells and activated inflammatory cells (named proliferative inflammatory atrophy, PIA) are often adjacent to areas of prostatic intraepithelial neoplasia (PIN) (De Marzo et al., 1999). Recently, inflammation in benign tissues identified in the Prostate Cancer Prevention Trial was positively associated with later development of PCa (Platz et al., 2017), strongly suggesting that chronic prostatitis is a precursor of PIN and PCa. To date, however, the mechanisms linking prostatitis and PCa development remain unclear. Uncovering these mechanisms should aid PCa prevention and early intervention. Below, we discuss three major possible avenues of how prostatitis may facilitate PCa progression (Figure 1) with a focus on applications of mouse models: 1) enhanced secretion of cytokines and growth factors to promote epithelial cell proliferation, 2) inflammation-induced epithelial cell DNA mutations, and 3) increasing basal-to-luminal differentiation to enlarge the pool of cells of origin for PCa.
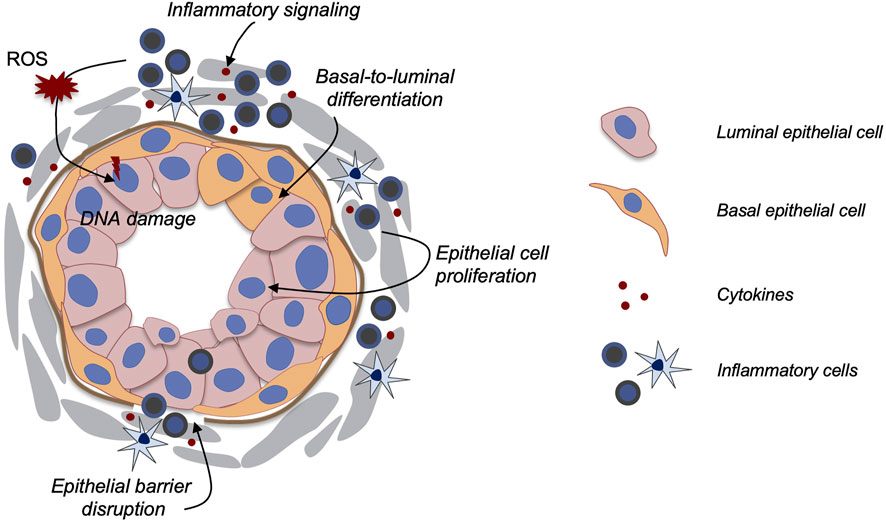
FIGURE 1. Model of how prostatitis promotes PCa. Multiple possible mechanisms have been proposed as illustrated in the diagram. Secreted cytokines may promote epithelial cell proliferation, basal-to-luminal cell differentiation, and epithelial barrier disruption. Inflammation-induced ROS production and Nkx3.1 down-regulation could also enhance DNA damage.
Enhanced Secretion of Cytokines and Growth Factors to Activate Epithelial Cell Proliferation
The mechanisms by which inflammation contributes to PCa development are multifaceted. One of the more direct ways may be through activating epithelial cell proliferation via paracrine signals from the inflammatory stroma. The normal prostate mostly contains quiescent cells, while cell proliferation is necessary for tissue wound healing. Interestingly, the reactive stroma observed in BPH and PCa undergoes changes resembling a wound healing response (Tuxhorn et al., 2001; Schauer and Rowley, 2011). Infiltration of inflammatory cells, increased growth factor availability, angiogenesis, and extracellular matrix remodeling are among the major features of such a pro-tumor microenvironment. The infiltrating inflammatory cells can produce a wide range of cytokines such as tumor necrosis factor (TNF) and interleukins (ILs), which can induce further secretion of growth factors to promote epithelial cell proliferation (Giri and Ittmann, 2001; Steiner et al., 2002; Sokol and Luster, 2015). For example, an in vitro study showed that in prostate epithelial cells, cytokines secreted by macrophages could activate ERK and Akt, two protein kinases that promote cell proliferation and survival (Dang and Liou, 2018). Furthermore, GEMMs offer great models to study the effects of inflammatory signaling on the prostate in vivo. In particular, overexpression of human IL-6 in the mouse prostate showed development of chronic inflammation and progressive neoplasia, with PIN lesions and prostate adenocarcinoma observed later (Liu et al., 2017). Moreover, in the genetic mouse prostatitis model where interleukin 1β (IL-1β) is overexpressed, increased expression of downstream cytokines were observed, along with formation of PIA-like lesions and high expression of the proliferation marker Ki67 (Ashok et al., 2019). As discussed previously regarding inflammation and BPH, these genetic mouse models suggest the involvement of a positive feedback loop between inflammation and epithelial barrier disruption to promote cell proliferation, as evidenced by the down-regulation of E-cadherin in the IL-6 overexpression model (Liu et al., 2017). However, it is important to note that cancer development requires more than just cell proliferation. Additional inflammation-induced mechanisms must be in play to explain the phenotypic differences between BPH and PCa.
Inflammation-Induced Oxidative Stress and Loss of Nkx3.1 can Induce DNA Damage
Studies across many organ types have suggested that inflammation can increase genomic instability (Colotta et al., 2009; Grivennikov et al., 2010). One proposed mechanism is the release of reactive oxygen species (ROS) by infiltrating inflammatory cells. ROS, such as superoxide, nitric oxide and hydrogen peroxide, are highly reactive oxygen-containing molecules that are produced during natural metabolic processes (Ihsan et al., 2018). Excess ROS production can result in an imbalance between ROS and antioxidants, leading to insufficient ROS degradation. This state of oxidative stress can cause oxidative damage in DNA, RNA, proteins, and lipids (Olinski et al., 2002; Lugrin et al., 2014; Ihsan et al., 2018). Notably, although much focus is placed on DNA damage, proteins and lipids are also important targets for oxidative attack, as modification of these molecules can increase the risk of mutagenesis (Reuter et al., 2010; Murata, 2018). Continuous exposure to inflammation and concurrent immune cell infiltration can lead to increased levels of ROS (Xia and Zweier, 1997; Eiserich et al., 1998), which can lead to genetic mutations and instability (Weitzman and Stossel, 1981; Weitzman and Gordon, 1990). It is hypothesized that in PIA lesions, where inflammatory injury stimulates epithelial cell proliferation, ROS released by infiltrating inflammatory cells can increase formation of PIN-lesions and carcinoma (Wiseman and Halliwell, 1996; Xia and Zweier, 1997; De Marzo et al., 1999). Using animal models, a mechanistic link between oxidative stress and inflammation has been established in mice susceptible to colon inflammation, in which knockout of Gpx1 and Gpx2, two genes that encode antioxidant enzymes, results in a high incidence of tumors in the intestinal epithelium (Chu et al., 2004). However, similar models in the PCa context are currently lacking. To functionally test the role of ROS in promoting inflammation-induced PCa, it will be very informative to genetically perturb the ROS production pathway in mice or combine ROS production perturbation with other oncogenic pathways to assess the effect on PCa development.
Genetic mouse model studies also suggested that another possible mechanism of inflammation-induced epithelial DNA damage could be related to Nkx3.1 down-regulation. Nkx3.1, besides serving as a transcription factor in prostate development, is also a tumor suppressor (Bhatia-Gaur et al., 1999; Kim et al., 2002). Its tumor suppressing functions can at least be partially attributed to its role in preventing DNA damage (Bowen and Gelmann, 2010; Bowen et al., 2013; Debelec-Butuner et al., 2015). PIN formation in Nkx3.1−/− mice was reported to be associated with deregulation of prooxidant and antioxidant enzymes, as well as oxidative damage in DNA (Ouyang et al., 2005). Notably, acute bacterial prostatitis in mice leads to down-regulation of Nkx3.1 (Khalili et al., 2010; Shinohara et al., 2013), and lower Nkx3.1 expression was also observed in the genetic prostatitis model of IL-1 overexpression (Ashok et al., 2019). Moreover, inducing prostate inflammation in Nkx3.1−/− mice accelerates PCa initiation (Le Magnen et al., 2018). These findings suggest that there may be a positive feedback or inflammatory storm mechanism at play. In such a model, inflammatory cytokines such as TNF-α and IL-1β could stimulate Nkx3.1 down-regulation (Markowski et al., 2008; Debelec-Butuner et al., 2014), which in turn would increase susceptibility to oxidative stress and further DNA damage (Ouyang et al., 2005). Such a combined environment of inflammatory signaling, oxidative stress, and high epithelial proliferation, could give rise to PIN and PCa (De Marzo et al., 1999).
Inflammation-Induced Basal-To-Luminal Differentiation Expands Cells of Origin for PCa
Cell of origin for PCa has been implicated as a link between prostatitis and PCa. A cell of origin is defined as a normal tissue cell that can give rise to a tumor after its oncogenic transformation (Blanpain, 2013; Lee and Shen, 2015). Tissue stem cells, due to their self-renewal and multipotent capabilities, can serve as potent cells of origin for cancer. In an earlier colon cancer study, inflammation induces tissue stem cell expansion, potentially enlarging the cellular pool for oncogenic transformation (Umar et al., 2009). In the prostate, lineage-tracing studies in mice have shown that epithelial basal cells are the stem cells that can generate luminal cells during prostate organogenesis (Ousset et al., 2012). However, basal stem cell activities become restricted in the mature prostate as basal and luminal cells are mostly two self-sustained lineages at adulthood and basal-to-luminal cell differentiation is rare (Choi et al., 2012; Wang et al., 2013). Importantly, basal-to-luminal differentiation appears to be an important step towards PCa initiation. In mouse lineage-tracing models, loss of the tumor suppressor gene Pten in basal cells promoted basal-to-luminal differentiation, and the resulting tumor had a luminal phenotype (Choi et al., 2012; Wang et al., 2013), resembling the predominant luminal feature in human PCa (Shen and Abate-Shen, 2010). In fact, loss of the basal cell layer is often considered a hallmark of PCa (Humphrey, 2007; Grisanzio and Signoretti, 2008). We previously showed that luminal cells are the favored cell type of origin for PCa (Wang et al., 2014). Therefore, by enhancing basal cell plasticity and basal-to-luminal differentiation, the cellular pool for oncogenic transformation is enlarged, potentially facilitating PCa development.
In light of this, it is particularly interesting to note that basal-to-luminal differentiation was reported to be enhanced in two prostatitis mouse models. When mice were either inoculated with uropathogenic E. coli (UPEC) or fed with HFD, basal cells rapidly proliferated and produced luminal cells (Kwon et al., 2013; Kwon et al., 2016). Both treatments also accelerated PCa initiation in the basal-specific Pten-knockout model (K14-Pten) (Kwon et al., 2013; Kwon et al., 2016). PCa developed relatively slowly in basal-specific Pten-knockout models since it takes time for Pten deletion to drive basal cells towards transformed luminal cells (Choi et al., 2012; Wang et al., 2013). K14-Pten mice treated with UPEC or HFD showed accelerated disease progression, indicating that faster basal-to-luminal differentiation due to inflammation-induced signals facilitated PCa development. In the future, identifying those signals that promotes basal-to-luminal differentiation should be beneficial for delaying PCa progression in patients with chronic prostatitis.
Conclusion and Discussion
Numerous rodent models have been developed to study the different types of clinically defined prostatitis. While bacterial prostatitis models have recapitulated many aspects of the acute and chronic bacterial inflammation observed in humans, it remains challenging to pinpoint the most relevant model for CPPS, since the molecular pathways responsible for abacterial chronic prostatitis are not yet fully understood. The etiology of chronic prostate inflammation can vary among individuals, and different CPPS models, including hormone, high fat, autoimmune, and GEMMs may capture different important aspects of CPPS development. These animal models have been playing crucial roles in our efforts to elucidate the relationship between prostatitis and other prostatic diseases such as BPH and PCa. The association of prostatitis to these diseases is well documented in clinical studies. In recent years, applications of GEMM prostatitis models have revealed possible mechanisms by which inflammation causes BPH and PCa. Among those mechanisms, disruption of the epithelial barrier and the ensuing auto feedback loop of enhanced inflammation appear to be a common theme. Nonetheless, inflammation may contribute to PCa development in many other ways, such as oxidative stress-induced DNA damage, down-regulation of the tumor suppressor Nkx3.1, and expansion of luminal epithelial cells as cells of origin. Future research utilizing rodent models will continue to shed light on the mechanistic, causal links between chronic prostate inflammation and progressive prostatic diseases, and should distinguish the etiology between BPH and PCa. Such insights will be invaluable for prostatic disease prevention and early intervention.
Author Contributions
JB and ZAW wrote the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
JB is supported by a Fulbright schoarship. This work is supported by NIH grant R01CA271452.
References
Ashok, A., Keener, R., Rubenstein, M., Stookey, S., Bajpai, S., Hicks, J., et al. (2019). Consequences of Interleukin 1β‐triggered Chronic Inflammation in the Mouse Prostate Gland: Altered Architecture Associated with Prolonged CD4 + Infiltration Mimics Human Proliferative Inflammatory Atrophy. Prostate 79, 732–745. doi:10.1002/pros.23784
Austin, J. C., Chacko, S. K., DiSanto, M., Canning, D. A., and Zderic, S. A. (2004). A Male Murine Model of Partial Bladder Outlet Obstruction Reveals Changes in Detrusor Morphology, Contractility and Myosin Isoform Expression. J. Urology 172, 1524–1528. doi:10.1097/01.ju.0000138045.61378.96
Bernoulli, J., Yatkin, E., Konkol, Y., Talvitie, E.-M., Santti, R., and Streng, T. (2008). Prostatic Inflammation and Obstructive Voiding in the Adult Noble Rat: Impact of the Testosterone to Estradiol Ratio in Serum. Prostate 68, 1296–1306. doi:10.1002/pros.20791
Bhatia-Gaur, R., Donjacour, A. A., Sciavolino, P. J., Kim, M., Desai, N., Young, P., et al. (1999). Roles for Nkx3.1 in Prostate Development and Cancer. Genes & Dev. 13, 966–977. doi:10.1101/gad.13.8.966
Blanpain, C. (2013). Tracing the Cellular Origin of Cancer. Nat. Cell Biol. 15, 126–134. doi:10.1038/ncb2657
Boehm, B. J., Colopy, S. A., Jerde, T. J., Loftus, C. J., and Bushman, W. (2012). Acute Bacterial Inflammation of the Mouse Prostate. Prostate 72, 307–317. doi:10.1002/pros.21433
Bowen, C., and Gelmann, E. P. (2010). NKX3.1 Activates Cellular Response to DNA Damage. Cancer Res. 70, 3089–3097. doi:10.1158/0008-5472.can-09-3138
Bowen, C., Ju, J.-H., Lee, J.-H., Paull, T. T., and Gelmann, E. P. (2013). Functional Activation of ATM by the Prostate Cancer Suppressor NKX3.1. Cell Rep. 4, 516–529. doi:10.1016/j.celrep.2013.06.039
Burcham, G. N., Cresswell, G. M., Snyder, P. W., Chen, L., Liu, X., Crist, S. A., et al. (2014). Impact of Prostate Inflammation on Lesion Development in the POET3+ Pten Mouse Model of Prostate Carcinogenesis. Am. J. Pathology 184, 3176–3191. doi:10.1016/j.ajpath.2014.08.021
Choi, N., Zhang, B., Zhang, L., Ittmann, M., and Xin, L. (2012). Adult Murine Prostate Basal and Luminal Cells Are Self-Sustained Lineages that Can Both Serve as Targets for Prostate Cancer Initiation. Cancer Cell 21, 253–265. doi:10.1016/j.ccr.2012.01.005
Chu, F.-F., Esworthy, R. S., Chu, P. G., Longmate, J. A., Huycke, M. M., Wilczynski, S., et al. (2004). Bacteria-Induced Intestinal Cancer in Mice with Disrupted Gpx1 and Gpx2 Genes. Cancer Res. 64, 962–968. doi:10.1158/0008-5472.can-03-2272
Chughtai, B., Lee, R., Te, A., and Kaplan, S. (2011). Role of Inflammation in Benign Prostatic Hyperplasia. Rev. Urol. 13, 147–150.
Collins, M. M., Stafford, R. S., O'Leary, M. P., and Barry, M. J. (1998). How Common Is Prostatitis? A National Survey of Physician Visits. J. Urology 159, 1224–1228. doi:10.1016/s0022-5347(01)63564-x
Colotta, F., Allavena, P., Sica, A., Garlanda, C., and Mantovani, A. (2009). Cancer-related Inflammation, the Seventh Hallmark of Cancer: Links to Genetic Instability. Carcinogenesis 30, 1073–1081. doi:10.1093/carcin/bgp127
Cutolo, M., Seriolo, B., Villaggio, B., Pizzorni, C., Craviotto, C., and Sulli, A. (2002). Androgens and Estrogens Modulate the Immune and Inflammatory Responses in Rheumatoid Arthritis. Ann. N. Y. Acad. Sci. 966, 131–142. doi:10.1111/j.1749-6632.2002.tb04210.x
Dang, T., and Liou, G.-Y. (2018). Macrophage Cytokines Enhance Cell Proliferation of Normal Prostate Epithelial Cells through Activation of ERK and Akt. Sci. Rep. 8, 7718. doi:10.1038/s41598-018-26143-8
De Marzo, A. M., Marchi, V. L., Epstein, J. I., and Nelson, W. G. (1999). Proliferative Inflammatory Atrophy of the Prostate. Am. J. pathology 155, 1985–1992. doi:10.1016/s0002-9440(10)65517-4
De Marzo, A. M., Platz, E. A., Sutcliffe, S., Xu, J., Grönberg, H., Drake, C. G., et al. (2007). Inflammation in Prostate Carcinogenesis. Nat. Rev. Cancer 7, 256–269. doi:10.1038/nrc2090
De Nunzio, C., Kramer, G., Marberger, M., Montironi, R., Nelson, W., Schröder, F., et al. (2011). The Controversial Relationship between Benign Prostatic Hyperplasia and Prostate Cancer: the Role of Inflammation. Eur. Urol. 60, 106–117. doi:10.1016/j.eururo.2011.03.055
Debelec-Butuner, B., Ertunc, N., and Korkmaz, K. S. (2015). Inflammation Contributes to NKX3.1 Loss and Augments DNA Damage but Does Not Alter the DNA Damage Response via Increased SIRT1 Expression. J. Inflamm. (Lond) 12, 12. doi:10.1186/s12950-015-0057-4
Debelec-Butuner, B., Alapinar, C., Varisli, L., Erbaykent-Tepedelen, B., Hamid, S. M., Gonen-Korkmaz, C., et al. (2014). Inflammation-mediated Abrogation of Androgen Signaling: An In Vitro Model of Prostate Cell Inflammation. Mol. Carcinog. 53, 85–97. doi:10.1002/mc.21948
dos Santos Gomes, F. O., Oliveira, A. C., Ribeiro, E. L., da Silva, B. S., dos Santos, L. A. M., de Lima, I. T., et al. (2017). Intraurethral Injection with LPS: an Effective Experimental Model of Prostatic Inflammation. Inflamm. Res. 67, 43–55. doi:10.1007/s00011-017-1094-7
Eiserich, J. P., Hristova, M., Cross, C. E., Jones, A. D., Freeman, B. A., Halliwell, B., et al. (1998). Formation of Nitric Oxide-Derived Inflammatory Oxidants by Myeloperoxidase in Neutrophils. Nature 391, 393–397. doi:10.1038/34923
Elkahwaji, J. E., Hauke, R. J., and Brawner, C. M. (2009). Chronic Bacterial Inflammation Induces Prostatic Intraepithelial Neoplasia in Mouse Prostate. Br. J. Cancer 101, 1740–1748. doi:10.1038/sj.bjc.6605370
Elkahwaji, J. E., Ott, C. J., Janda, L. M., and Hopkins, W. J. (2005). Mouse Model for Acute Bacterial Prostatitis in Genetically Distinct Inbred Strains. Urology 66, 883–887. doi:10.1016/j.urology.2005.04.013
Elkahwaji, J. E., Zhong, W., Hopkins, W. J., and Bushman, W. (2007). Chronic Bacterial Infection and Inflammation Incite Reactive Hyperplasia in a Mouse Model of Chronic Prostatitis. Prostate 67, 14–21. doi:10.1002/pros.20445
Escobar, E. L. O., Gomes-Marcondes, M. C. C., and Carvalho, H. F. (2009). Dietary Fatty Acid Quality Affects AR and PPARγ Levels and Prostate Growth. Prostate 69, 548–558. doi:10.1002/pros.20905
Fibbi, B., Penna, G., Morelli, A., Adorini, L., and Maggi, M. (2010). Chronic Inflammation in the Pathogenesis of Benign Prostatic Hyperplasia. Int. J. Androl. 33, 475–488. doi:10.1111/j.1365-2605.2009.00972.x
Gandaglia, G., Briganti, A., Gontero, P., Mondaini, N., Novara, G., Salonia, A., et al. (2013). The Role of Chronic Prostatic Inflammation in the Pathogenesis and Progression of Benign Prostatic Hyperplasia (BPH). BJU Int. 112, 432–441. doi:10.1111/bju.12118
Gill, B. C., and Shoskes, D. A. (2016). Bacterial Prostatitis. Curr. Opin. Infect. Dis. 29, 86–91. doi:10.1097/qco.0000000000000222
Giri, D., and Ittmann, M. (2001). Interleukin-8 Is a Paracrine Inducer of Fibroblast Growth Factor 2, a Stromal and Epithelial Growth Factor in Benign Prostatic Hyperplasia. Am. J. Pathology 159, 139–147. doi:10.1016/s0002-9440(10)61681-1
Grisanzio, C., and Signoretti, S. (2008). p63 in Prostate Biology and Pathology. J. Cell. Biochem. 103, 1354–1368. doi:10.1002/jcb.21555
Grivennikov, S. I., Greten, F. R., and Karin, M. (2010). Immunity, Inflammation, and Cancer. Cell 140, 883–899. doi:10.1016/j.cell.2010.01.025
Harman, S. M., Metter, E. J., Tobin, J. D., Pearson, J., Blackman, M. R., and Aging, B. L. S. o. (2001). Longitudinal Effects of Aging on Serum Total and Free Testosterone Levels in Healthy Men. J. Clin. Endocrinol. Metabolism 86, 724–731. doi:10.1210/jcem.86.2.7219
Haverkamp, J. M., Charbonneau, B., Crist, S. A., Meyerholz, D. K., Cohen, M. B., Snyder, P. W., et al. (2011). An Inducible Model of Abacterial Prostatitis Induces Antigen Specific Inflammatory and Proliferative Changes in the Murine Prostate. Prostate 71, 1139–1150. doi:10.1002/pros.21327
Hou, Y., DeVoss, J., Dao, V., Kwek, S., Simko, J. P., McNeel, D. G., et al. (2009). An Aberrant Prostate Antigen-specific Immune Response Causes Prostatitis in Mice and Is Associated with Chronic Prostatitis in Humans. J. Clin. Invest. 119, 2031–2041. doi:10.1172/JCI38332
Humphrey, P. A. (2007). Diagnosis of Adenocarcinoma in Prostate Needle Biopsy Tissue. J. Clin. pathology 60, 35–42. doi:10.1136/jcp.2005.036442
Ihsan, A. U., Khan, F. U., Khongorzul, P., Ahmad, K. A., Naveed, M., Yasmeen, S., et al. (2018). Role of Oxidative Stress in Pathology of Chronic Prostatitis/chronic Pelvic Pain Syndrome and Male Infertility and Antioxidants Function in Ameliorating Oxidative Stress. Biomed. Pharmacother. 106, 714–723. doi:10.1016/j.biopha.2018.06.139
Jackson, C. M., Flies, D. B., Mosse, C. A., Parwani, A., Hipkiss, E. L., and Drake, C. G. (2013). Strain-specific Induction of Experimental Autoimmune Prostatitis (EAP) in Mice. Prostate 73, 651–656. doi:10.1002/pros.22606
Jia, Y.-l., Liu, X., Yan, J.-y., Chong, L.-m., Li, L., Ma, A.-c., et al. (2015). The Alteration of Inflammatory Markers and Apoptosis on Chronic Prostatitis Induced by Estrogen and Androgen. Int. Urol. Nephrol. 47, 39–46. doi:10.1007/s11255-014-0845-4
Kamijo, T., Sato, S., and Kitamura, T. (2001). Effect of Cernitin Pollen-Extract on Experimental Nonbacterial Prostatitis in Rats. Prostate 49, 122–131. doi:10.1002/pros.1126
Kanno, Y., Mitsui, T., Kitta, T., Moriya, K., Tsukiyama, T., Hatakeyama, S., et al. (2016). The Inflammatory Cytokine IL-1β Is Involved in Bladder Remodeling after Bladder Outlet Obstruction in Mice. Neurourol. Urodynam. 35, 377–381. doi:10.1002/nau.22721
Keith, I. M., Jin, J., Neal, D., Teunissen, B. D., and Moon, T. D. (2001). Cell Relationship in a Wistar Rat Model of Spontaneous Prostatitis. J. Urology 166, 323–328. doi:10.1016/s0022-5347(05)66153-8
Khalili, M., Mutton, L. N., Gurel, B., Hicks, J. L., De Marzo, A. M., and Bieberich, C. J. (2010). Loss of Nkx3.1 Expression in Bacterial Prostatitis. Am. J. Pathology 176, 2259–2268. doi:10.2353/ajpath.2010.080747
Kikutani, H., and Makino, S. (1992). The Murine Autoimmune Diabetes Model: NOD and Related Strains. Adv. Immunol. 51, 285–322. doi:10.1016/s0065-2776(08)60490-3
Kim, H.-J., Park, J.-W., Cho, Y.-S., Cho, C.-H., Kim, J.-S., Shin, H.-W., et al. (2013). Pathogenic Role of HIF-1α in Prostate Hyperplasia in the Presence of Chronic Inflammation. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis. 1832, 183–194. doi:10.1016/j.bbadis.2012.09.002
Kim, M. J., Bhatia-Gaur, R., Banach-Petrosky, W. A., Desai, N., Wang, Y., Hayward, S. W., et al. (2002). Nkx3.1 Mutant Mice Recapitulate Early Stages of Prostate Carcinogenesis. Cancer Res. 62, 2999–3004.
Konkol, Y., Vuorikoski, H., Streng, T., Tuomela, J., and Bernoulli, J. (2019). Characterization a Model of Prostatic Diseases and Obstructive Voiding Induced by Sex Hormone Imbalance in the Wistar and Noble Rats. Transl. Androl. Urol. 8, S45–S57. doi:10.21037/tau.2019.02.03
Kwon, O.-J., Zhang, B., Zhang, L., and Xin, L. (2016). High Fat Diet Promotes Prostatic Basal-To-Luminal Differentiation and Accelerates Initiation of Prostate Epithelial Hyperplasia Originated from Basal Cells. Stem Cell Res. 16, 682–691. doi:10.1016/j.scr.2016.04.009
Kwon, O. J., Zhang, L., Ittmann, M. M., and Xin, L. (2013). Prostatic Inflammation Enhances Basal-To-Luminal Differentiation and Accelerates Initiation of Prostate Cancer with a Basal Cell Origin. Proc. Natl. Acad. Sci. USA. 111, E592–E600. doi:10.1073/pnas.1318157111
Le Magnen, C., Virk, R. K., Dutta, A., Kim, J. Y., Panja, S., Lopez-Bujanda, Z. A., et al. (2018). Cooperation of Loss of NKX3.1 and Inflammation in Prostate Cancer Initiation. Dis. Model Mech. 11. doi:10.1242/dmm.035139
Lee, S. H., and Shen, M. M. (2015). Cell Types of Origin for Prostate Cancer. Curr. Opin. Cell Biol. 37, 35–41. doi:10.1016/j.ceb.2015.10.002
Lees, J. R., Charbonneau, B., Hayball, J. D., Diener, K., Brown, M., Matusik, R., et al. (2006). T-Cell Recognition of a Prostate Specific Antigen Is Not Sufficient to Induce Prostate Tissue Destruction. Prostate 66, 578–590. doi:10.1002/pros.20307
Li, F., Pascal, L. E., Stolz, D. B., Wang, K., Zhou, Y., Chen, W., et al. (2019a). E‐cadherin Is Downregulated in Benign Prostatic Hyperplasia and Required for Tight Junction Formation and Permeability Barrier in the Prostatic Epithelial Cell Monolayer. Prostate 79, 1226–1237. doi:10.1002/pros.23806
Li, J., Tian, Y., Guo, S., Gu, H., Yuan, Q., and Xie, X. (2018a). Testosterone-induced Benign Prostatic Hyperplasia Rat and Dog as Facile Models to Assess Drugs Targeting Lower Urinary Tract Symptoms. PloS one 13, e0191469. doi:10.1371/journal.pone.0191469
Li, Y., Shi, B., Dong, F., Zhu, X., Liu, B., and Liu, Y. (2019b). Effects of Inflammatory Responses, Apoptosis, and STAT3/NF-Κb- and Nrf2-Mediated Oxidative Stress on Benign Prostatic Hyperplasia Induced by a High-Fat Diet. Aging 11, 5570–5578. doi:10.18632/aging.102138
Li, Z., Xiao, H., Wang, K., Zheng, Y., Chen, P., Wang, X., et al. (2018b). Upregulation of Oxytocin Receptor in the Hyperplastic Prostate. Front. Endocrinol. 9, 403. doi:10.3389/fendo.2018.00403
Lilljebjörn, L. V., Csizmadia, E., Hedblom, A., Canesin, G., Kalbasi, A., Li, M., et al. (2020). A Role of the Heme Degradation Pathway in Shaping Prostate Inflammatory Responses and Lipid Metabolism. Am. J. Pathology 190, 830–843. doi:10.1016/j.ajpath.2019.12.008
Liu, G., Zhang, J., Frey, L., Gang, X., Wu, K., Liu, Q., et al. (2017). Prostate-specific IL-6 Transgene Autonomously Induce Prostate Neoplasm through Amplifying Inflammation in the Prostate and Peri-Prostatic Adipose Tissue. J. Hematol. Oncol. 10, 14. doi:10.1186/s13045-016-0386-7
Liu, Y., Mikrani, R., Xie, D., Wazir, J., Shrestha, S., Ullah, R., et al. (2020). Chronic Prostatitis/chronic Pelvic Pain Syndrome and Prostate Cancer: Study of Immune Cells and Cytokines. Fundam. Clin. Pharmacol. 34, 160–172. doi:10.1111/fcp.12517
Lugrin, J., Rosenblatt-Velin, N., Parapanov, R., and Liaudet, L. (2014). The Role of Oxidative Stress during Inflammatory Processes. Biol. Chem. 395, 203–230. doi:10.1515/hsz-2013-0241
Lundgren, R., Holmquist, B., Hesselvik, M., and Müntzing, J. (1984). Treatment of Prostatitis in the Rat. Prostate 5, 277–284. doi:10.1002/pros.2990050305
Markowski, M. C., Bowen, C., and Gelmann, E. P. (2008). Inflammatory Cytokines Induce Phosphorylation and Ubiquitination of Prostate Suppressor Protein NKX3.1. Cancer Res. 68, 6896–6901. doi:10.1158/0008-5472.can-08-0578
Murata, M. (2018). Inflammation and Cancer. Environ. Health Prev. Med. 23, 50. doi:10.1186/s12199-018-0740-1
Nicholson, T. M., Ricke, E. A., Marker, P. C., Miano, J. M., Mayer, R. D., Timms, B. G., et al. (2012). Testosterone and 17β-Estradiol Induce Glandular Prostatic Growth, Bladder Outlet Obstruction, and Voiding Dysfunction in Male Mice. Endocrinology 153, 5556–5565. doi:10.1210/en.2012-1522
Nickel, J. C., Downey, J., Young, I., and Boag, S. (1999). Asymptomatic Inflammation And/or Infection in Benign Prostatic Hyperplasia. BJU Int. 84, 976–981. doi:10.1046/j.1464-410x.1999.00352.x
Nickel, J. C. (2008). Inflammation and Benign Prostatic Hyperplasia. Urologic Clin. N. Am. 35, 109–115. doi:10.1016/j.ucl.2007.09.012
O'Malley, K. J., Eisermann, K., Pascal, L. E., Parwani, A. V., Majima, T., Graham, L., et al. (2014). Proteomic Analysis of Patient Tissue Reveals PSA Protein in the Stroma of Benign Prostatic Hyperplasia. Prostate 74, 892–900. doi:10.1002/pros.22807
Olinski, R., Gackowski, D., Foksinski, M., Rozalski, R., Roszkowski, K., and Jaruga, P. (2002). Oxidative DNA Damage: Assessment of the Role in Carcinogenesis, Atherosclerosis, and Acquired Immunodeficiency Syndrome1 1This Article Is Part of a Series of Reviews on "Oxidative DNA Damage and Repair." the Full List of Papers May Be Found on the Homepage of the Journal. Free Radic. Biol. Med. 33, 192–200. doi:10.1016/s0891-5849(02)00878-x
Olsson, J., Drott, J. B., Laurantzon, L., Laurantzon, O., Bergh, A., and Elgh, F. (2012). Chronic Prostatic Infection and Inflammation by Propionibacterium Acnes in a Rat Prostate Infection Model. PloS one 7, e51434. doi:10.1371/journal.pone.0051434
Ousset, M., Van Keymeulen, A., Bouvencourt, G., Sharma, N., Achouri, Y., Simons, B. D., et al. (2012). Multipotent and Unipotent Progenitors Contribute to Prostate Postnatal Development. Nat. Cell Biol. 14, 1131–1138. doi:10.1038/ncb2600
Ouyang, X., DeWeese, T. L., Nelson, W. G., and Abate-Shen, C. (2005). Loss-of-function of Nkx3.1 Promotes Increased Oxidative Damage in Prostate Carcinogenesis. Cancer Res. 65, 6773–6779. doi:10.1158/0008-5472.can-05-1948
Pascal, L. E., Dhir, R., Balasubramani, G. K., Chen, W., Hudson, C. N., Srivastava, P., et al. (2021a). E-Cadherin Expression Is Inversely Correlated with Aging and Inflammation in the Prostate. Am. J. Clin. Exp. Urol. 9, 140–149.
Pascal, L. E., Mizoguchi, S., Chen, W., Rigatti, L. H., Igarashi, T., Dhir, R., et al. (2021b). Prostate-Specific Deletion of Cdh1 Induces Murine Prostatic Inflammation and Bladder Overactivity. Endocrinology 162. doi:10.1210/endocr/bqaa212
Penna, G., Amuchastegui, S., Cossetti, C., Aquilano, F., Mariani, R., Giarratana, N., et al. (2007a). Spontaneous and Prostatic Steroid Binding Protein Peptide-Induced Autoimmune Prostatitis in the Nonobese Diabetic Mouse. J. Immunol. 179, 1559–1567. doi:10.4049/jimmunol.179.3.1559
Penna, G., Mondaini, N., Amuchastegui, S., Degli Innocenti, S., Carini, M., Giubilei, G., et al. (2007b). Seminal Plasma Cytokines and Chemokines in Prostate Inflammation: Interleukin 8 as a Predictive Biomarker in Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Benign Prostatic Hyperplasia. Eur. Urol. 51, 524–533. doi:10.1016/j.eururo.2006.07.016
Platz, E. A., Kulac, I., Barber, J. R., Drake, C. G., Joshu, C. E., Nelson, W. G., et al. (2017). A Prospective Study of Chronic Inflammation in Benign Prostate Tissue and Risk of Prostate Cancer: Linked PCPT and SELECT Cohorts. Cancer Epidemiol. Biomarkers Prev. 26, 1549–1557. doi:10.1158/1055-9965.epi-17-0503
Popovics, P., Schally, A. V., Salgueiro, L., Kovacs, K., and Rick, F. G. (2017). Antagonists of Growth Hormone-Releasing Hormone Inhibit Proliferation Induced by Inflammation in Prostatic Epithelial Cells. Proc. Natl. Acad. Sci. U.S.A. 114, 1359–1364. doi:10.1073/pnas.1620884114
Porcaro, A. B., Novella, G., Molinari, A., Terrin, A., Minja, A., De Marco, V., et al. (2015). Prostate Volume Index and Chronic Inflammation of the Prostate Type IV with Respect to the Risk of Prostate Cancer. Urol. Int. 94, 270–285. doi:10.1159/000362176
Reuter, S., Gupta, S. C., Chaturvedi, M. M., and Aggarwal, B. B. (2010). Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 49, 1603–1616. doi:10.1016/j.freeradbiomed.2010.09.006
Rippere-Lampe, K. E., Lang, M., Ceri, H., Olson, M., Lockman, H. A., and O'Brien, A. D. (2001). Cytotoxic Necrotizing Factor Type 1-positive Escherichia coli Causes Increased Inflammation and Tissue Damage to the Prostate in a Rat Prostatitis Model. Infect. Immun. 69, 6515–6519. doi:10.1128/iai.69.10.6515-6519.2001
Robert, G., Descazeaud, A., Nicolaïew, N., Terry, S., Sirab, N., Vacherot, F., et al. (2009). Inflammation in Benign Prostatic Hyperplasia: A 282 Patients' Immunohistochemical Analysis. Prostate 69, 1774–1780. doi:10.1002/pros.21027
Roehrborn, C. G. (2005). Benign Prostatic Hyperplasia: an Overview. Rev. Urol. 7 Suppl 9 (Suppl. 9), S3–S14.
Schauer, I. G., and Rowley, D. R. (2011). The Functional Role of Reactive Stroma in Benign Prostatic Hyperplasia. Differentiation 82, 200–210. doi:10.1016/j.diff.2011.05.007
Sfanos, K. S., Yegnasubramanian, S., Nelson, W. G., and De Marzo, A. M. (2018). The Inflammatory Microenvironment and Microbiome in Prostate Cancer Development. Nat. Rev. Urol. 15, 11–24. doi:10.1038/nrurol.2017.167
Shankar, E., Bhaskaran, N., MacLennan, G. T., Liu, G., Daneshgari, F., and Gupta, S. (2015). Inflammatory Signaling Involved in High-Fat Diet Induced Prostate Diseases. J. Urol. Res. 2, 1018.
Shankar, E., Vykhovanets, E. V., Vykhovanets, O. V., MacLennan, G. T., Singh, R., Bhaskaran, N., et al. (2012). High-fat Diet Activates Pro-inflammatory Response in the Prostate through Association of Stat-3 and NF-Κb. Prostate 72, 233–243. doi:10.1002/pros.21425
Sharma, O. P., Adlercreutz, H., Stranberg, J. D., Zirkin, B. R., Coffey, D. S., and Ewing, L. L. (1992). Soy of Dietary Source Plays a Preventive Role against the Pathogenesis of Prostatitis in Rats. J. Steroid Biochem. Mol. Biol. 43, 557–564. doi:10.1016/0960-0760(92)90244-d
Shen, M. M., and Abate-Shen, C. (2010). Molecular Genetics of Prostate Cancer: New Prospects for Old Challenges. Genes Dev. 24, 1967–2000. doi:10.1101/gad.1965810
Shinohara, D. B., Vaghasia, A. M., Yu, S.-H., Mak, T. N., Brüggemann, H., Nelson, W. G., et al. (2013). A Mouse Model of Chronic Prostatic Inflammation Using a Human Prostate Cancer-Derived Isolate ofPropionibacterium Acnes. Prostate 73, 1007–1015. doi:10.1002/pros.22648
Sokol, C. L., and Luster, A. D. (2015). The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 7. doi:10.1101/cshperspect.a016303
Steiner, G. E., Djavan, B., Kramer, G., Handisurya, A., Newman, M., Lee, C., et al. (2002). The Picture of the Prostatic Lymphokine Network Is Becoming Increasingly Complex. Rev. Urol. 4, 171–177.
Sun, F., Báez-Díaz, C., and Sánchez-Margallo, F. M. (2017). Canine Prostate Models in Preclinical Studies of Minimally Invasive Interventions: Part II, Benign Prostatic Hyperplasia Models. Transl. Androl. Urol. 6, 547–555. doi:10.21037/tau.2017.03.62
Taoka, R., and Kakehi, Y. (2017). The Influence of Asymptomatic Inflammatory Prostatitis on the Onset and Progression of Lower Urinary Tract Symptoms in Men with Histologic Benign Prostatic Hyperplasia. Asian J. urology 4, 158–163. doi:10.1016/j.ajur.2017.02.004
Taoka, R., Tsukuda, F., Ishikawa, M., Haba, R., and Kakehi, Y. (2004). Association of Prostatic Inflammation with Down-Regulation of Macrophage Inhibitory Cytokine-1 Gene in Symptomatic Benign Prostatic Hyperplasia. J. Urology 171, 2330–2335. doi:10.1097/01.ju.0000127760.87421.e9
Tsunemori, H., Sugimoto, M., Xia, Z., Taoka, R., Oka, M., and Kakehi, Y. (2011). Effect of the Phytotherapeutic Agent Eviprostat on Inflammatory Changes and Cytokine Production in a Rat Model of Nonbacterial Prostatitis. Urology 77, 1507–1520. doi:10.1016/j.urology.2011.02.017
Tsunemori, H., and Sugimoto, M. (2021). Effects of Inflammatory Prostatitis on the Development and Progression of Benign Prostatic Hyperplasia: A Literature Review. Int J Urology 28, 1086–1092. doi:10.1111/iju.14644
Tuxhorn, J. A., Ayala, G. E., and Rowley, D. R. (2001). Reactive Stroma in Prostate Cancer Progression. J. Urology 166, 2472–2483. doi:10.1016/s0022-5347(05)65620-0
Umar, S., Sarkar, S., Wang, Y., and Singh, P. (2009). Functional Cross-Talk between β-Catenin and NFκB Signaling Pathways in Colonic Crypts of Mice in Response to Progastrin. J. Biol. Chem. 284, 22274–22284. doi:10.1074/jbc.m109.020941
Vykhovanets, E. V., Resnick, M. I., MacLennan, G. T., and Gupta, S. (2007). Experimental Rodent Models of Prostatitis: Limitations and Potential. Prostate Cancer Prostatic Dis. 10, 15–29. doi:10.1038/sj.pcan.4500930
Wang, H. H., Wang, L., Jerde, T. J., Chan, B. D., Savran, C. A., Burcham, G. N., et al. (2015). Characterization of Autoimmune Inflammation Induced Prostate Stem Cell Expansion. Prostate 75, 1620–1631. doi:10.1002/pros.23043
Wang, X.-J., Xia, L.-L., Xu, T.-Y., Zhang, X.-H., Zhu, Z.-W., Zhang, M.-G., et al. (2016). Changes in Erectile Organ Structure and Function in a Rat Model of Chronic Prostatitis/chronic Pelvic Pain Syndrome. Andrologia 48, 243–251. doi:10.1111/and.12437
Wang, Z. A., Mitrofanova, A., Bergren, S. K., Abate-Shen, C., Cardiff, R. D., Califano, A., et al. (2013). Lineage Analysis of Basal Epithelial Cells Reveals Their Unexpected Plasticity and Supports a Cell-Of-Origin Model for Prostate Cancer Heterogeneity. Nat. Cell Biol. 15, 274–283. doi:10.1038/ncb2697
Wang, Z. A., Toivanen, R., Bergren, S. K., Chambon, P., and Shen, M. M. (2014). Luminal Cells Are Favored as the Cell of Origin for Prostate Cancer. Cell reports.
Weitzman, S. A., and Stossel, T. P. (1981). Mutation Caused by Human Phagocytes. Science 212, 546–547. doi:10.1126/science.6259738
Weitzman, S., and Gordon, L. (1990). Inflammation and Cancer: Role of Phagocyte-Generated Oxidants in Carcinogenesis. Blood 76, 655–663. doi:10.1182/blood.v76.4.655.bloodjournal764655
Wiseman, H., and Halliwell, B. (1996). Damage to DNA by Reactive Oxygen and Nitrogen Species: Role in Inflammatory Disease and Progression to Cancer. Biochem. J. 313 ( Pt 1) (Pt 1), 17–29. doi:10.1042/bj3130017
Xia, Y., and Zweier, J. L. (1997). Superoxide and Peroxynitrite Generation from Inducible Nitric Oxide Synthase in Macrophages. Proc. Natl. Acad. Sci. U.S.A. 94, 6954–6958. doi:10.1073/pnas.94.13.6954
Xiong, Y., Qiu, X., Shi, W., Yu, H., and Zhang, X. (2017). Anti-inflammatory and Antioxidant Effect of Modified Bazhengsan in a Rat Model of Chronic Bacterial Prostatitis. J. Ethnopharmacol. 198, 73–80. doi:10.1016/j.jep.2016.12.039
Xu, D., Chen, P., Xiao, H., Wang, X., DiSanto, M. E., and Zhang, X. (2019). Upregulated Interleukin 21 Receptor Enhances Proliferation and Epithelial-Mesenchymal Transition Process in Benign Prostatic Hyperplasia. Front. Endocrinol. (Lausanne) 10, 4. doi:10.3389/fendo.2019.00004
Xu, H., Hu, M. B., Bai, P. D., Zhu, W. H., Liu, S. H., Hou, J. Y., et al. (2015). Proinflammatory Cytokines in Prostate Cancer Development and Progression Promoted by High-Fat Diet. Biomed. Res. Int. 2015, 249741. doi:10.1155/2015/249741
Yokota, T., Honda, K., Tsuruya, Y., Nomiya, M., Yamaguchi, O., Gotanda, K., et al. (2004). Functional and Anatomical Effects of Hormonally Induced Experimental Prostate Growth: A Urodynamic Model of Benign Prostatic Hyperplasia (BPH) in the Beagle. Prostate 58, 156–163. doi:10.1002/pros.10318
Zang, L., Tian, F., Yao, Y., Chen, Y., Shen, Y., Han, M., et al. (2021). Qianliexin Capsule Exerts Anti‐inflammatory Activity in Chronic Non‐bacterial Prostatitis and Benign Prostatic Hyperplasia via NF‐κB and Inflammasome. J. Cell Mol. Med. 25, 5753–5768. doi:10.1111/jcmm.16599
Zhang, B., Kwon, O.-J., Henry, G., Malewska, A., Wei, X., Zhang, L., et al. (2016). Non-Cell-Autonomous Regulation of Prostate Epithelial Homeostasis by Androgen Receptor. Mol. Cell 63, 976–989. doi:10.1016/j.molcel.2016.07.025
Zhang, J., Zhang, M., Tang, J., Yin, G., Long, Z., He, L., et al. (2021). Animal Models of Benign Prostatic Hyperplasia. Prostate Cancer Prostatic Dis. 24, 49–57. doi:10.1038/s41391-020-00277-1
Zhang, M., Luo, C., Cui, K., Xiong, T., and Chen, Z. (2020). Chronic Inflammation Promotes Proliferation in the Prostatic Stroma in Rats with Experimental Autoimmune Prostatitis: Study for a Novel Method of Inducing Benign Prostatic Hyperplasia in a Rat Model. World J. Urol. 38, 2933–2943. doi:10.1007/s00345-020-03090-6
Keywords: mouse model, prostatitis, prostate cancer, BPH, chronic inflammation
Citation: Bleeker J and Wang ZA (2022) Applications of Vertebrate Models in Studying Prostatitis and Inflammation-Associated Prostatic Diseases. Front. Mol. Biosci. 9:898871. doi: 10.3389/fmolb.2022.898871
Received: 18 March 2022; Accepted: 17 June 2022;
Published: 05 July 2022.
Edited by:
William C. Cho, QEH, Hong Kong SAR, ChinaReviewed by:
Ravi Sonkar, Boston University, United StatesCopyright © 2022 Bleeker and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhu A. Wang, endhbmczNkB1Y3NjLmVkdQ==
 Joosje Bleeker
Joosje Bleeker Zhu A. Wang
Zhu A. Wang