
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mol. Biosci. , 15 March 2022
Sec. Molecular Diagnostics and Therapeutics
Volume 9 - 2022 | https://doi.org/10.3389/fmolb.2022.834651
This article is part of the Research Topic Cancer Treatment and Early Detection Targeting HER Receptors View all 5 articles
 Konstantinos Venetis1,2†
Konstantinos Venetis1,2† Edoardo Crimini2,3†
Edoardo Crimini2,3† Elham Sajjadi1,2
Elham Sajjadi1,2 Chiara Corti2,3
Chiara Corti2,3 Elena Guerini-Rocco1,2
Elena Guerini-Rocco1,2 Giuseppe Viale1,2
Giuseppe Viale1,2 Giuseppe Curigliano2,3
Giuseppe Curigliano2,3 Carmen Criscitiello2,3*
Carmen Criscitiello2,3* Nicola Fusco1,2*
Nicola Fusco1,2*HER2 status in breast cancer is assessed to select patients eligible for targeted therapy with anti-HER2 therapies. According to the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP), the HER2 test positivity is defined by protein overexpression (score 3+) at immunohistochemistry (IHC) and/or gene amplification at in situ hybridization (ISH). The introduction of novel anti-HER2 compounds, however, is changing this paradigm because some breast cancers with lower levels of protein expression (i.e. score 1+/2+ with no gene amplification) benefited from HER2 antibody-drug conjugates (ADC). Recently, a potential for HER2 targeting in HER2 “ultra-low” (i.e. score 0 with incomplete and faint staining in ≤10% of tumor cells) and MutL-deficient estrogen receptor (estrogen receptor)-positive/HER2-negative breast cancers has been highlighted. All these novel findings are transforming the traditional dichotomy of HER2 status and have dramatically raised the expectations in this field. Still, a more aware HER2 status assessment coupled with the comprehensive characterization of the clinical and molecular features of these tumors is required. Here, we seek to provide an overview of the current state of HER2 targeting in breast cancers beyond the canonical HER2 positivity and to discuss the practical implications for pathologists and oncologists.
Breast cancer is the most frequently diagnosed cancer in women and a leading cause of death worldwide (Sung et al., 2021). This malignancy is extremely heterogeneous in terms of clinicopathological and molecular characteristics, prognosis, and response to therapy (De Mattos-Arruda et al., 2018; Lopez et al., 2019; Filho et al., 2021). In 15–20% of cases, breast cancers show the overexpression of HER2, usually due to gene amplification (Seshadri et al., 1993). Given that HER2 is a potent (proto)oncogene, these tumors harbor a more aggressive behavior compared to HER2-negative breast cancers (Hamilton et al., 2021). The introduction of anti-HER2 monoclonal antibodies (e.g., trastuzumab, pertuzumab) back in the 90 s revolutionized the treatment landscape of HER2+ breast cancer, drastically improving the life expectations of these patients (Dieci and Miglietta 2021). Since then, several studies and harmonization efforts have been carried out to improve the sensitivity and specificity of HER2 pathological assessment, which is now considered highly reliable (Viale and Munzone 2019). However, the introduction of novel anti-HER2 antibody-drug conjugates (ADC) strategies is questioning the existing paradigm of HER2 testing (Banerji et al., 2019; Modi et al., 2020; Corti et al., 2021a).
According to the 2018 American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) guidelines, breast cancer is classified as HER2-positive when HER2 expression is scored as 3+ by immunohistochemistry (IHC) or 2+ IHC with gene amplification by reflex in-situ hybridization (ISH) (Wolff et al., 2018). For these tumors, there is a clinical recommendation for anti-HER2 targeted agents. On the contrary, tumors with IHC scores 0 and 1+, or 2+ with a negative ISH, are clinically HER2-negative because they lack a significant response to traditional anti-HER2 drugs (Pondé et al., 2018; Wolff et al., 2018). Lately, tumors with low levels of HER2 expression (i.e. IHC 1+ or 2+ with negative ISH), also referred to as HER2 “low” breast cancers, have been shown impressive response rates and progression-free survival (PFS) after ADC-based treatments (Iwata et al., 2018; Banerji et al., 2019; Schettini et al., 2021). Another rising layer of complexity is represented by the possible clinical relevance of the incomplete and faint staining in ≤10% of tumor cells displayed by a subset of IHC score 0 breast cancers. This HER2 “ultra-low” phenotype might explain some promising evidence on treatment response in HER2-negative breast cancer (Denkert et al., 2021). Recent preclinical studies on HER2 targeting in MutL-deficient estrogen receptor (ER)+/HER2-negative breast cancers have shown positive results (Punturi et al., 2021). These data might provide a further rationale for expanding our pathological armamentarium towards mismatch repair (MMR)-related biomarkers (Venetis et al., 2020b; Sajjadi et al., 2021b).
In this review, we present a thorough overview of the state of the art in breast cancer predictive pathology for HER2 targeting within and beyond the HER2 positivity spectrum. Practical technical hindrances for an accurate patient selection will be discussed in light of the most recent clinical trials.
The discovery of HER2 in the 1980s allowed the development of therapeutical strategies that have dramatically changed the natural history of HER2+ breast cancer, with significantly improved outcomes (Coussens et al., 1985; Slamon and Pegram 2001). HER2 testing is routinely recommended for all newly diagnosed breast cancers, and a re-characterization can be performed in some cases after neoadjuvant treatment and/or in case of tumor progression if a tissue sample is available (Viale and Fusco 2021; Wolff et al., 2018). This test relies on the combination of IHC and ISH (Schalper et al., 2014). In particular, IHC is an essay that identifies and describes the HER2 protein expression pattern and intensity on the cell membrane of breast cancer cells, while ISH detects the presence of gene amplification using HER2 and CEP17 probes (Pauletti et al., 1996; Slamon et al., 1989). The first step of the HER2 testing workflow requires the performance of IHC. Based on the completeness, intensity, and percentage of cells in which the staining is identified, HER2 IHC is scored using a three-tiered system, from 0 to 3+ (Figure 1) (Wolff et al., 2018). In the case of an equivocal result (score 2+), ISH is used as a reflex test (Press et al., 2002). Specifically, when ISH shows an average HER2 copy number ≥6.0 signals/cell, the case is HER2+ (Wolff et al., 2018). Taken together, HER2+ breast cancer is defined by a 3+ IHC score or 2+ IHC and positivity of ISH, while tumors with HER2 IHC scores of 1+ or 2+ without ISH amplification are defined as HER2-low (Tarantino et al., 2020). More recently, beyond the striking and established efficacy of HER2-directed therapies in HER2+ breast cancer, the possibility of targeting HER2 has been explored also in HER2-low breast cancer (Fusco et al., 2013; Fusco and Bosari 2016; Meric-Bernstam et al., 2019; Kreutzfeldt et al., 2020; Sartore-Bianchi et al., 2020; Shitara et al., 2020; Siena et al., 2021). Of note, even if the HER2 pathway activation is lower in HER2-low than in HER2+ breast cancer, the new anti-HER2 ADCs allow its targeting. New ADCs contain a strong chemotherapeutic payload that is guided against tumor cells thanks to their, even low, HER2 positivity.
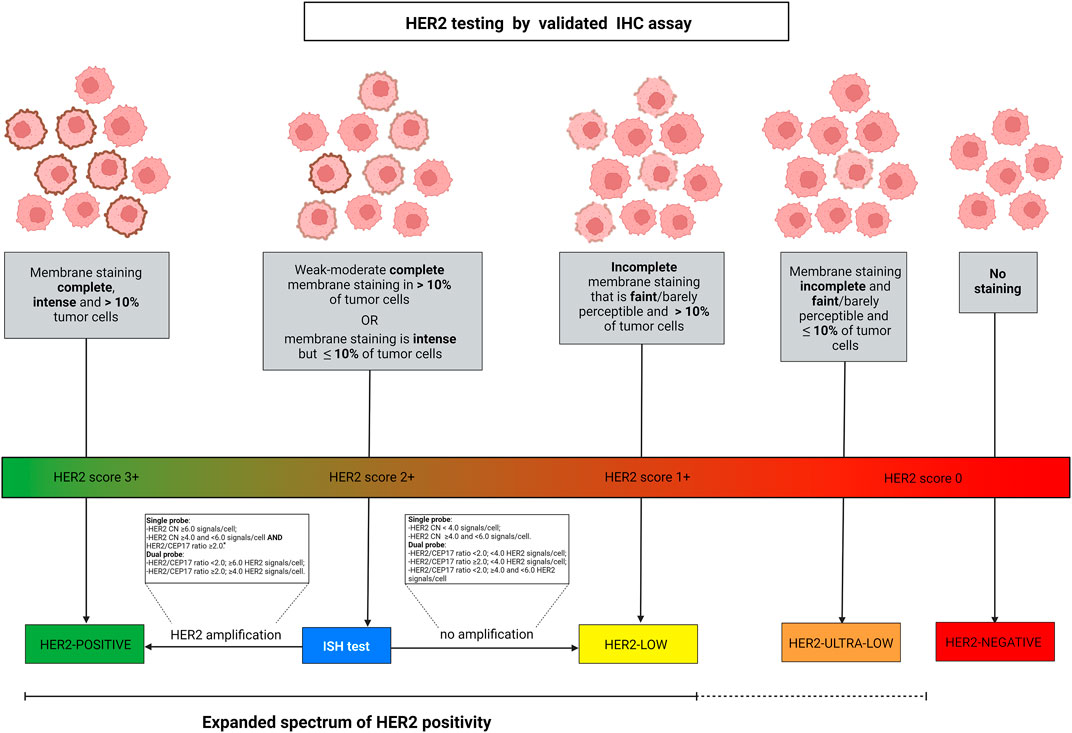
FIGURE 1. Algorithm for defining HER2 spectrum of expression according to ASCO/CAP guidelines. HER2-low diseases are identified by IHC score 2+ with negative ISH or IHC score 1+. Breast cancer is scored 2+ in case of weak-moderate complete membrane staining in >10% of tumor cells or if the membrane staining is intense but in ≤10% of tumor cells. Score 1+ is defined by faint or barely perceptible incomplete membrane staining in >10% of tumor cells. Gene amplification by ISH is assessed as detailed in the text boxes, according to the types of probes employed. Among the IHC score 0 category, two different types of expression are present, namely a complete lack of expression and the faint or barely perceptible incomplete membrane staining in ≤10% of tumor cells. Albeit HER2-negative, this latter type of tumor indeed shows the expression of the protein and can be described as HER2 “ultra-low”. The continuous versus the discontinued line depicts the different levels of evidence in the current clinical practice. Abbreviations, IHC, immunohistochemistry; ISH, in situ hybridization; HER2, human epidermal growth factor receptor 2. *, dual-probe ISH should be performed for final result. Source: Breast Biomarker Reporting, CAP Cancer Protocol Templates, v1.4.1.1, November 2021 update, available at: https://documents.cap.org/protocols/Breast.Bmk_1.4.1.1.REL_CAPCP.pdf. Created with biorender.com.
Although the ASCO/CAP guidelines have clearly defined the assessment criteria for HER2 status in breast cancer, HER2-low expression has not been formally defined (Tarantino et al., 2020). The precise identification of these patients is intrinsically dependent on the testing strategy and technique (Ercoli et al., 2017). The reproducibility of these tests might be affected by several pre-analytical and analytical issues (Fusco et al., 2021). Formalin fixation and artifacts, technical and biological heterogeneity represent factors that remarkably affect the analytical reliability of IHC, complicating the identification of HER2-low expression both in terms of both false positive and false negative results (Bussolati et al., 2015). These observations, along with the pooling of HER2 scores 0 and 1+ under the broader definition of HER2-negative breast cancer, may explain to a certain extent some of the discrepancies that have been observed in HER2 testing. In this context, several studies that assessed the reproducibility of HER2 testing between local and central laboratories revealed a remarkable inter-observer, intratumoral, and temporal heterogeneity of HER2 low status (Miglietta et al., 2021). Therefore, a precise assessment of HER2-low cases demands the harmonization of all methodologies coupled with straightforward and well-defined guidelines (Angerilli et al., 2021). In this regard, the role of pathologists is crucial, thus specific training and particular attention during HER2 testing is warranted. Alternative methodologies (e.g., RT-PCR, digital pathology) have already been proposed for the detection of this subset of patients (Jiang et al., 2016). Among these, machine learning-based predictors showed significant results in terms of speed, accuracy, and cost-effectiveness of predicting both HER2 status and anti-HER2 treatment response (Fusco et al., 2013; La Barbera et al., 2020; Yousif et al., 2021; Farahmand et al., 2022). However, the IHC-ISH combined test remains the gold standard.
The current evidence on HER2-targeting therapies in HER2-low breast cancer arises from several translational research studies employing various classes of monoclonal antibodies, ADC, and bispecific antibodies (Venetis et al., 2020a). Anti-HER2 vaccines and cellular immunotherapy have been also tested in HER2-low breast cancer with continuously gaining interest (Venetis et al., 2020a; Antonarelli et al., 2021a). The efficacy of trastuzumab, an anti-HER2 monoclonal antibody that binds the HER2 extracellular domain preventing receptor dimerization, is well-defined both in metastatic and early HER2+ breast cancer (Hudis 2007). The significant survival benefit obtained by the administration of this drug has been verified both in the adjuvant and neoadjuvant settings as well as in the first and subsequent lines of treatment (Slamon et al., 2001; Slamon and Pegram 2001; Piccart-Gebhart et al., 2005; Gianni et al., 2011; Slamon et al., 2011; Goldhirsch et al., 2013). Nevertheless, remarkable efficacy of trastuzumab was mainly observed in patients with HER2 3+ in IHC or 2+ and ISH amplification. In this context, the phase III study NSABP-47/NRG explored the role of adjuvant trastuzumab added to standard chemotherapy in HER2-low breast cancer (Fehrenbacher et al., 2020). However, the results of the study were negative since the invasive disease-free survival (DFS) and the overall survival (OS) were similar in the trastuzumab and the placebo arms (5-years IDFS: 89.8% with CHT plus trastuzumab versus 89.2% with CHT alone; hazard ratio [HR], 0.98; 95% CI, 0.76 to 1.25; p = 0.85; 5-years OS: 94.8% with CHT and 96.3% in CHT alone, HR, 1.33; 95% CI, 0.90 to 1.95; p = 0.15), thus highlighting the inefficacy of trastuzumab in HER2-low patients (Fehrenbacher et al., 2020). Pertuzumab, another anti-HER2 monoclonal antibody approved in combination with trastuzumab (dual blockade) for early and advanced breast cancer, was tested as monotherapy at two different dose levels in HER2-negative breast cancer in a phase II trial (Bachelot et al., 2019; Gianni et al., 2016; Swain et al., 2020; von Minckwitz et al., 2017). Regrettably, the results were not satisfactory in terms of efficacy, considering that only 3% (n = 2/78) of patients with IHC score 1+ and 2+ reached a partial response and 40% (n = 31/78) a stable disease (SD), with a short time to progression of 44 and 43 days in the two different dose arms (Gianni et al., 2010). The phase III SOPHIA trial demonstrated that margetuximab (a chimeric, Fc-engineered, immune-activating anti-ERBB2 immunoglobulin G1 (IgG1) monoclonal antibody sharing epitope specificity and Fc-independent antiproliferative effects with trastuzumab) in combination with chemotherapy prolongs PFS and objective response rate (ORR) if compared to trastuzumab plus chemotherapy, but the overall survival (OS) results are still awaited (Tarantino et al., 2021b; Tarantino et al., 2021c; Rugo et al., 2021). In a phase I trial of margetuximab, although HER2-low patients were included, only those with HER2+ disease experienced a response (Bang et al., 2017). Similarly, no responses were obtained in a phase II trial employing margetuximab in 22 patients with advanced breast cancer characterized by IHC score 2+ and absence of HER2 amplification, thus suggesting the efficacy of monoclonal antibodies strongly depends on high addiction to the HER2 pathway (Catenacci et al., 2020).
In HER2+ breast cancer, ado-trastuzumab-emtansine (T-DM1) is approved as adjuvant treatment for patients with residual disease after neoadjuvant therapy (von Minckwitz et al., 2019), and is still the standard of care as second-line treatment for HER2+ advanced breast cancer according to the results of TH3RESA and EMILIA trials (Diéras et al., 2017; Krop et al., 2017). Regarding T-DM1 efficacy in HER2-low breast cancer patients, the retrospective analysis of the 4,258 and 4,374 g trials showed that both PFS and ORR were significantly inferior in these patients compared to the canonical HER2+ (ORR 4.8 vs. 33.8% in the 4,258 g trial and 20 vs. 41.3% in the 4,374 g trial, while PFS 2.6 vs. 8.2 in the 4,258 g trial and 2.8 vs. 7.3 in the 4,374 g trial) (Burris et al., 2011; Krop et al., 2012). Moreover, T-DM1 was demonstrated to be less effective even in HER2 2+ and ISH-positive advanced breast cancer when compared to HER2 3+ (Yazaki et al., 2020). Trastuzumab-deruxtecan (T-DXd), another HER2-directed ADC containing a topoisomerase I inhibitor, demonstrated an impressive PFS benefit in HER2-positive pretreated breast cancer patients also in earlier lines, as emerged by the data of DESTINY-Breast 03 trial presented at ESMO 2021 congress (Cortes et al., 2021). T-DXd showed promising activity in a phase Ib trial including HER2-low patients (ORR 37%; PFS 11.1 months; mOS 29.4 months) (Modi et al., 2020). Similarly, trastuzumab-duocarmazine demonstrated interesting clinical activity in HER2-positive metastatic breast cancer patients in the phase I study (ORR 28% in HR-positive and 40% in HR-negative HER2-low metastatic breast cancer) (Banerji et al., 2019). The phase III YD985.002/TULIP trial comparing trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer terminated enrollment (Manich et al., 2021). The results presented at the ESMO 2021 provide evidence that treatment with SYD985 may represent a new therapeutic option for these patients. PF-06804103 is the other anti-HER2 ADC that was tested in HER2-low breast cancer patients in a phase I trial. To date, no results from this trial have been published, except for an abstract, that does not separately analyze the HER2-low cohort (Meric-Bernstam et al., 2019). Among bispecific antibodies, zenocutuzumab is an anti-HER2 and HER3 IgG1 more potent than pertuzumab in inhibiting HER2-HER3 heterodimerization which was initially tested in hormone receptor (HR)+/HER2-low breast cancer xenograft models, suggesting a potential synergistic effect with endocrine therapy (Geuijen et al., 2018; Antonarelli et al., 2021b). The drug was successively tested in clinical trials, among which a phase II, single-arm study enrolling endocrine-resistant metastatic breast cancer patients after progression to a CDK4/6 inhibitor (Pistilli et al., 2020; Eiger et al., 2021). Among the 50 patients enrolled, the clinical benefit rate at 24 weeks was 16.7%, with one patient that obtained a PR (Pistilli et al., 2020). Several clinical studies are currently ongoing with T-Dxd and other ADCs including HER2-low breast cancer patients (Table 1).
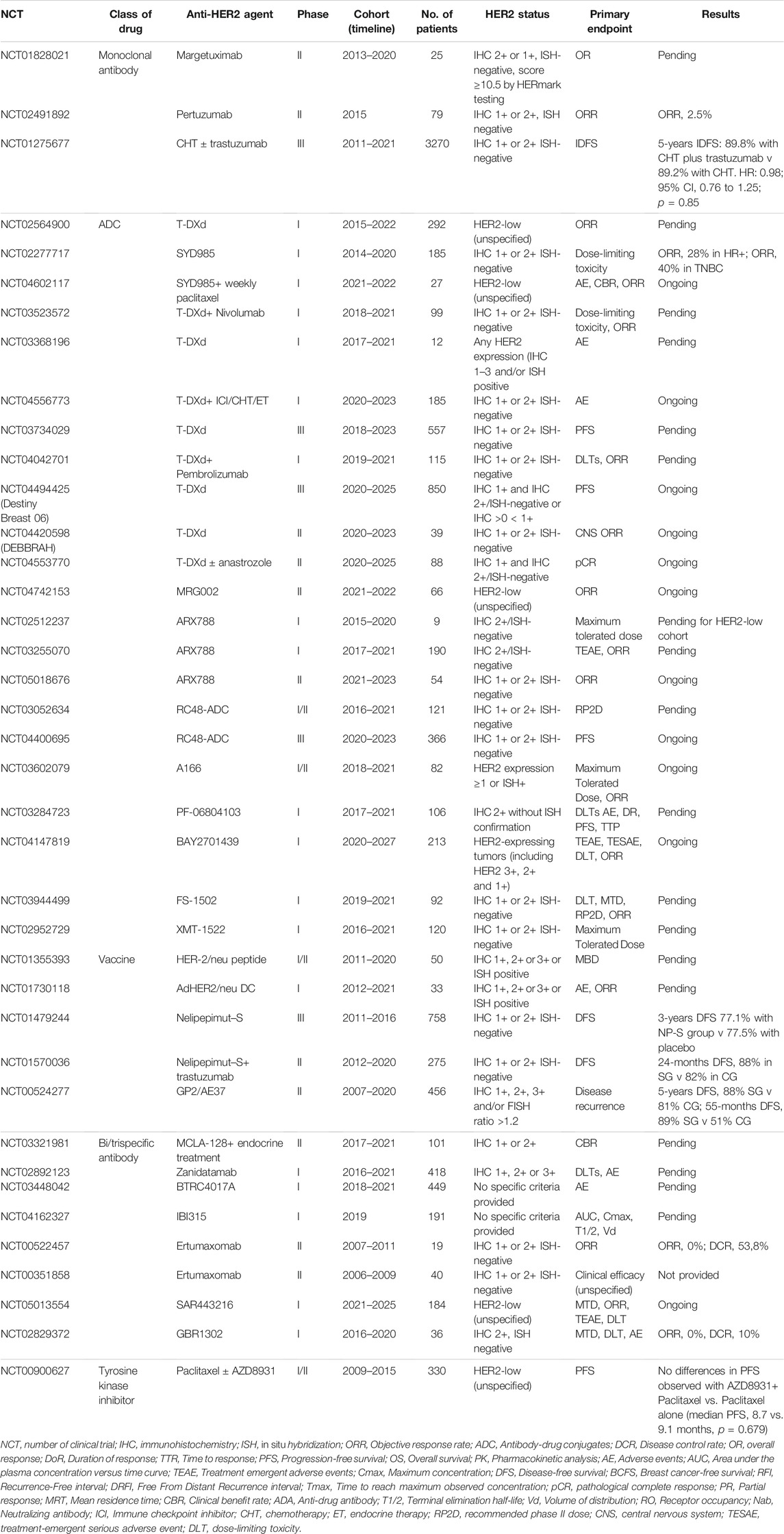
TABLE 1. Ongoing and recently completed clinical trials using HER2-targeting agents in HER2-low and/or “ultra-low” breast cancer patients.
Cancer vaccines have reached clinical trials based on the preclinical evidence of a synergistic effect with trastuzumab in HER2-low breast cancer (Tarantino et al., 2020; Corti et al., 2021a). Regrettably, the results of nelipepimut (an E75 HER2 peptide vaccine) plus trastuzumab versus trastuzumab plus placebo were negative in the adjuvant setting of HER2-low breast cancer (disease-free survival (DFS) was similar in the two groups of treatment: HR, 0.62; 95% CI 0.31–1.25; p = 0.18) (Clifton et al., 2020). Nevertheless, in patients with TNBC, DFS was improved in the experimental arm versus control (HR, 0.26; 95% CI, 0.08–0.81, p = 0.01) (Clifton et al., 2020). Similar results were obtained in two phase II clinical trials in the adjuvant setting with the other two HER2 peptide vaccines, GP2 and AE37 (Brown et al., 2020). With AE37, a DFS benefit was noted in an advanced stage, HER2 under-expression, and TNBC, while with GP2 there were no recurrences in patients with HER2-positive disease (Brown et al., 2020). A recent meta-analysis including 24 studies with E75 and GP2 vaccines showed a DFS benefit with the E75 vaccine (You et al., 2021).
According to the findings of retrospective analyses of the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-31 trial and North Central Cancer Treatment Group (NCCTG) N9831 trial, a subset of breast cancer patients resulted negative for HER2 biomarker assessment by ISH/IHC, benefited from anti-HER2 therapy (MacNeil et al., 2020). This postulates that the current HER2 assessment does not fully associate with HER2 signaling dysfunction. It should be noted, however, that a subset of HER2-negative tumors tested in core biopsy samples might respond to targeted therapy due to intra-tumor heterogeneity phenomena (Ercoli et al., 2017). Moreover, HER2 targeting may theoretically be possible even in score 0 tumors showing staining, albeit incomplete and faint, in ≤10% of tumor cells. To identify HER2-negative tumors susceptible to HER2 inhibition, several studies investigated the existence of a predictive marker that could detect patients benefiting from this therapy or the use of a functional signal profiling test to identify abnormal HER2-driven signaling activity.
Pathologists and oncologists are currently putting efforts into defining all possible HER2 entities for achieving the most accurate stratification of the patients and the selection of an appropriate therapeutic approach. In this regard, remarkable endeavors aimed to deepen into the clinical and molecular landscape of HER2-low breast cancer (Dieci and Miglietta 2021). The recent study published by Carsten Denkert et al. sought to determine this novel breast cancer subgroup by comparing its clinical and molecular characteristics with those of HER2-zero (i.e., complete HER2-negativity determined by IHC) breast cancer (Denkert et al., 2021). To achieve this, the authors performed IHC analysis in a cohort of 2,310 patients with HER2 non-amplified primary breast cancer treated with neoadjuvant combination chemotherapy in four prospective clinical trials. In terms of HR status, a significant difference was observed between the two groups since HER2-low breast cancer was enriched with HR+ tumors, while the HER2-zero cohort was enriched with HR- cases. Such correlation between HR signaling and HER2-low expression has also been reported in a retrospective, large-cohort, gene expression profiling study, where PAM50-based-subtypes accounted for approximately 80% of HER2-low-positive cases (Miglietta et al., 2021). Concerning the pathological complete response (pCR) data, in the HR+ cohort, the authors observed a lower pCR in patients with HER2-low breast cancer compared to those with HER2-zero disease with no effect on long-term survival (Denkert et al., 2021). On the contrary, even though such an association between HER2 status and pCR was not found in the TNBC group, longer disease-free and overall survival were reported in HER2-low patients in comparison with HER2-zero patients. Albeit this result was specifically seen in patients who did not achieve a pCR after neoadjuvant chemotherapy, to date, the prognostic role of HER2-low and potential correlation with sensitivity to chemotherapy still remain matters of controversy (Tarantino et al., 2020; Mutai et al., 2021; Schettini et al., 2021). In addition to these intriguing findings, the study published by Schettini et al. pointed out another important difference between these two entities which is related to gene expression data (Schettini et al., 2021). Indeed, it has been demonstrated that ERBB2 and luminal-related genes had higher levels of expression in HER2-low compared to HER2-cases in the HR+ group. Although the abovementioned studies highlighted major diversities between HER2-low and HER2-zero breast cancer in terms of biological and molecular characteristics, no robust evidence is available on whether HER2-low should be considered as a clinically separate entity (Dieci and Miglietta 2021; Omar and Arafat 2021). Considering that most of the available evidence derives from retrospective studies, results from the currently ongoing clinical trials are eagerly awaited.
Although targeting HER2 in HER2-negative breast cancer could sound like an oxymoron, it has been demonstrated to be at least theoretically possible. Hence, among IHC score 0 tumors, a substantial proportion of cases shows incomplete and faint staining in ≤10% of tumor cells. These HER2 “ultra-low” breast cancers might explain the positive results in some studies targeting HER2 in HER2-negative tumors. For example, a study by Bose et al. published in 2013 showed that HER2 pathogenic activating mutations occur irrespectively of HER2 IHC status as they do not necessarily lead to protein overexpression, representing an alternative mechanism for activating the HER2 pathway in breast cancer (Bose et al., 2013). Out of the eight HER2-mutated samples without gene amplification, for whom IHC data are available in the article, one was HER2-zero and harbored a V777L ERBB2 mutation, demonstrated to be an activating mutation as it strongly increased the phosphorylation of signaling proteins, indicating enhanced activity of the tyrosine kinase (Bose et al., 2013). Regrettably, the presence of HER2 positive cells within the score 0 category was not annotated in this study. Moreover, HER2 V777L-mutated breast cancer cell lines showed sensitivity to TKIs: lapatinib and neratinib, thus suggesting a possible role of HER2 targeting also in particular cases of HER2 “ultra-low” breast cancer (Bose et al., 2013). Regarding clinical studies, a phase II trial explored the efficacy of neratinib, a pan-HER inhibitor, in HER2-mutant advanced breast cancer, including 14 non-amplified breast cancers (Exman et al., 2019). Five of them (i.e., 36%, 90% CI 15–61%) obtained clinical benefit, including one complete response (CR), 1 PR, and 3 SD ≥ 6 months, while median PFS was 5 months (90% CI 2–8) (Exman et al., 2019). Nevertheless, this evidence is based on very rare findings given that HER2 activating mutations are described to occur in less than 2% of breast cancer, with a higher frequency in HR-positive compared to TNBC and in lobular than in ductal histology (Exman et al., 2019).
A recent study has proposed the application of anti-HER agents for ER+/HER2- breast cancers in the context of mismatch repair (MMR) status (Punturi et al., 2021). MMR is known as one of the fundamental DNA repair pathways (Piciotti et al., 2021) Defects in the MMR system are commonly due to molecular alterations involving the MutS and MutL dimeric homologs (Corti et al., 2019; Lopez et al., 2020). These complexes interact with each other to regulate the recognition and cleavage of incorrect base insertions (Sajjadi et al., 2021a). Almost 15–17% of ER+/HER2− breast cancer patients are correlated with endocrine treatment resistance due to MutL deficiency (Haricharan et al., 2017; Sajjadi et al., 2021). Punturi et al. have shown that in endocrine-treated, ER+/HER2− patients, the loss of MutL expression could activate HER2 by protecting it from lysosomal protein trafficking. Owing to this activation, MutL loss has been proposed as a marker to stratify ER+/HER2− breast cancer patients who would respond to anti-HER agents. This observation was based on multiple experimental model systems. Accordingly, based on gene expression microarray data from independent datasets, it has been shown that ER+/HER2− tumors with MutL loss have a relatively higher expression of ERBB2 compared to those with MutL proficient tumors, but this was not observed in the absence of treatment. These results were not only observed in cell line models but also in patient-derived xenograft models where ER+/HER2− tumors with MutL loss showed an increase in membrane HER2 levels after fulvestrant treatment. This finding was reflected by an increased sensitivity to HER inhibition. The data published in this study suggest that MutL loss inclines ER+/HER2− tumors to respond to a combination of anti-HER agents and endocrine treatment (Punturi et al., 2021). On the other hand, the currently available HER2 tests (IHC, ISH) provide information on the protein expression or gene amplification, which do not include the functional status of the HER2 biomarker. Therefore, the measurement of the signaling pathway activity of HER2 in addition to the common methods could stratify HER2-negative patients eligible for HER2 targeted therapies (Huang et al., 2017). In this approach, patients’ live tumor cells are applied on a biosensor that can identify dynamic HER2-driven signaling dysfunction. Among the HER2-negative samples, almost a quarter (27 out of 114 patients, 23.7%) have been related to abnormal HER2 signaling (MacNeil et al., 2020). This test has demonstrated the efficacy of various HER2 signal inhibitors in HER2-negative breast cancers with abnormal HER2 signaling (MacNeil et al., 2020). Further studies and clinical trials to evaluate the efficacy of HER2-targeted therapy in such patient populations are warranted.
Globally, HER2 expression is being increasingly perceived as a continuum spectrum, going beyond the classical dichotomous distinction between HER2-positive and HER2-negative cancer that led the treatment choice until today, especially in breast cancer. For this reason, it is becoming crucial to collect more solid evidence on targeting HER2 based on the whole spectrum of HER2 expression. Hence, a widely different efficacy of different classes of drugs and even of different drugs within the same class has been highlighted in the clinical trials according to the HER2 status (Figure 2). Monoclonal antibodies globally demonstrated to be ineffective in HER2-low breast cancer, because their activity relies mainly on the blockade of aberrant HER2 signaling via dimerization inhibition, HER2 internalization, and antibody-dependent cellular cytotoxicity (Hudis 2007; Eiger et al., 2021). They bind to the extracellular domain of the receptor thus they are more effective when the receptor is overexpressed, allowing more drugs to bind on the cell membrane inducing antibody-dependent cellular cytotoxicity. Moreover, monoclonal antibodies intrinsically act on the cell on which they bind, so in case of high intratumoral heterogeneity, their efficacy is impaired (Tarantino et al., 2020).
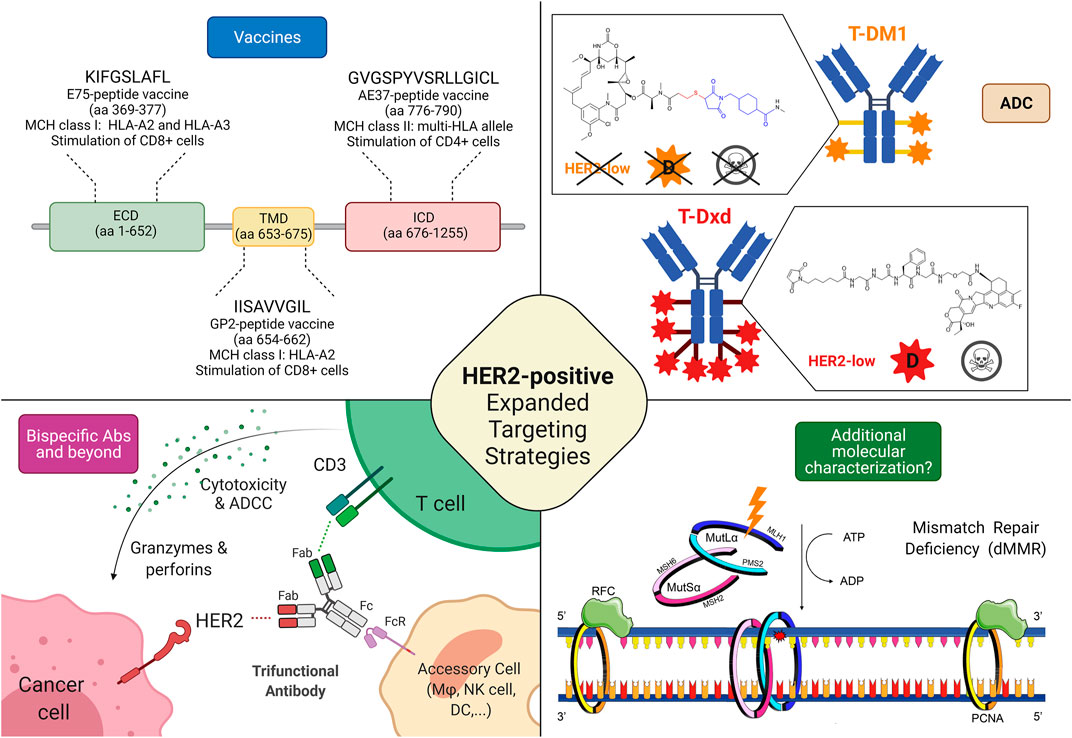
FIGURE 2. Possible targeting strategies in HER2-low and HER2-negative breast cancers. 1) Cancer vaccines: most of the research efforts related to cancer vaccines against HER2 regard targeting HER2-related peptides, such as E75 (ECD), GP2 (TMD), and AE37 (ICD); Interestingly, while a phase II trial testing AE37-based vaccine in the advanced stage (in the study defined as stage IIB or greater) did not demonstrate statistically significant survival benefit (5-years DFS) in the overall population, it showed a 5-years DFS of 83% in subjects with HER2-low disease, compared with 62.5% in the GM-CSF-only arm (HR, 0.375; CI: 0.142–0.988; p = 0.039). Similarly, in a subgroup with stage IIB or greater TNBC, a trend toward improved DFS with vaccination was found. The growing body of evidence coupled with subset analyses of this phase II trial led to another phase II clinical trial investigating AE37 in combination with pembrolizumab (NSABP FB-14) in TNBC patients with metastatic disease (NCT04024800). 2) Antibody-drug conjugates: several trials are currently testing ADC in HER2-low disease, with interesting results, particularly regarding those equipped with diffusable cytotoxic moiety as well as cleavable linkers, possibly accounting for bystander killing effect (skull symbol); 3) Bispecific antibodies (bsAbs) and trifunctional antibodies are characterized by the ability to target (at least) two different epitopes, enabling either inhibition of multiple oncogenic pathways or the forced connection between immune cells and cancer tissue. Several anti-HER2 bsAbs are under development, although only a minority is specifically investigated in HER2-low disease; 4) Additional molecular characterization is a possible new prospective, in the light of recent findings describing the predictive value of mismatch repair deficiency related to response to HER2 blockade in HER2-negative breast cancer. Abbreviations: T-DM1, trastuzumab emtansine; T-DXd, trastuzumab deruxtecan; HER2, human epidermal growth factor receptor 2; ECD, extracellular domain; TMD, transmembrane domain; ICD, intracellular domain; aa, amino acid; MHC, major histocompatibility complex; HLA, human leukocyte antigen; ADC, antibody-drug conjugate; D, diffusible cytotoxic moiety; MSH6, MutS Homolog 6; PMS2, PMS1 Homolog 2; MSH2, MutS Homolog 2; MLH1, MutL homolog 1; DFS, disease-free survival; bsAbs, bispecific antibody; CD, cluster of differentiation; ATP, Adenosine triphosphate; ADP, adenosine diphosphate; Fab, antigen-binding fragment; Fc, fragment crystallizable; FcR, Fc receptor; dMMR, mismatch repair deficient; ADCC, Antibody-dependent cellular cytotoxicity; RFC, replication factor C; PCNA, Proliferating cell nuclear antigen. Created with biorender.com.
On the other hand, ADCs can overcome some of the limitations encountered by monoclonal antibodies vehiculating and releasing a cytotoxic payload that can be internalized also by the surrounding cells that do not express HER2 (bystander effect) (Tarantino et al., 2021a; Corti et al., 2021b). In this perspective, the characteristic of the ADC, mainly the cytotoxic activity of the payload, the drug-antibody ratio (DAR), and the cleavability of the linker through which the payload is charged on the antibody, make the difference. T-DM1 is composed of trastuzumab and DM1 (emtansine), a tubulin polymerization inhibitor, linked through an uncleavable linker with a DAR of 3.5 (Krop et al., 2010). T-DM1 needs to be internalized and the antibody degraded before executing its cytotoxicity (Skeie et al., 2020). The mandatory internalization prevents the possibility of targeting HER2 non-expressing surrounding cells. In T-Dxd, on the contrary, trastuzumab is conjugated with deruxtecan, a topoisomerase I inhibitor with a DAR of eight via a cleavable but stable linker (Doi et al., 2017). The theoretical advantage given by the payload more potent, with higher DAR and by the cleavable linker thanks to the lysosomal enzymes present both in the endosomes and in the microenvironment translates in better activity in clinical trials enrolling HER2-low breast cancer patients, as previously reported. The presence of enzymes capable of cleaving the linker thus releasing the chemotherapeutic agent in the extracellular space and the higher payload confer to T-Dxd activity also on the cells that express HER2 at a lower level or even that do not express it at all (Tarantino et al., 2020). In Trastuzumab-duocarmazine the payload is a potent alkylating agent with a DAR of 2.8 and a cleavable linker (Banerji et al., 2019). On the whole, ADCs permit the target of HER2 independently from the cell and even the tumor addiction to HER2 pathway provided that a cleavable linker and a potent chemotherapeutic agent seem to be necessary to exert a sufficient bystander effect. Based on these findings, as T-Dxd and trastuzumab-duocarmazine demonstrated promising activity in HER2-low breast cancer but no data are currently available on survival endpoints, HER2-low expression in breast cancer can be classified as a tier IIb according to ESMO Scale for Clinical Actionability of molecular Targets (ESCAT), hoping that this evidence could reach a tier I in the future when more solid data on survival endpoints will be available (Mateo et al., 2018; Crimini et al., 2021). Focusing on HER2-negative breast cancer, the most promising therapeutical strategies are probably HER2 vaccines, as they assume that breast cancer cells express HER2 at a higher level than healthy tissues, even in cases of HER2-low and ultra-low breast cancers by IHC (You et al., 2021). In this perspective, the previously cited metanalysis supports further clinical trials on the topic to assess which subgroups of patients benefit from this approach (You et al., 2021). Moreover, the clinical validation of HER2 targeting in MutL deficient breast cancer is required to assess if this strategy could be implemented in the future.
In conclusion, targeting HER2 is revealing a fine and increasingly more complex work concerning the growing knowledge of the topic, that determines a deeper understanding of molecular mechanisms underlying the variable expression of HER2 in different tumors and even in different cells of the same tumor, a knowledge that is at the same time necessary to develop more effective therapies for breast cancer patients. For this, a tailored approach is warranted to assess HER2 status. Further prospective studies addressing the role of HER2 ultra-low expression along with additional complementary biomarkers would bring us a further step closer to the realization of the potentials of precision medicine for these patients.
KV and EC contributed to the literature search, conception and design of the article and wrote the first draft of the manuscript, which was initially reviewed by ES and CCo, who also contributed to the iconography together with NF. EG-R, GV, and GC provided critical revision. NF and CC supervised the preparation of the article and revised the manuscript. NF conceived the study. All the authors provided final approval to the submitted work.
The authors acknowledge support from the University of Milan through the APC initiative.
GC received honoraria for consulting/advisory role/speaker bureau and/or travel funding from Roche, Lilly, Bristol-Myers Squibb, Pfizer, Novartis, and Seagen; GV from MSD Oncology, Pfizer, Dako, Roche/Genetech, Astellas Pharma, Novartis, Bayer, Daiichi; Sankyo, Menarini, Ventana Medical Systems Dako/Agilent Technologies, Cepheid, and Celgene. CC received honoraria for consulting/advisory role/speaker bureau from Novartis, Eli-Lilly, Pfizer, MSD, Seagen and Roche; NF from Merck Sharp and Dohme (MSD), Boehringer Ingelheim, Novartis, AstraZeneca, and Daiichi Sankyo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Angerilli, V., Galuppini, F., Pagni, F., Fusco, N., Malapelle, U., and Fassan, M. (2021). The Role of the Pathologist in the Next-Generation Era of Tumor Molecular Characterization. Diagnostics 11, 339. doi:10.3390/diagnostics11020339
Antonarelli, G., Giugliano, F., Corti, C., Repetto, M., Tarantino, P., and Curigliano, G. (2021). Research and Clinical Landscape of Bispecific Antibodies for the Treatment of Solid Malignancies. Pharmaceuticals (Basel) 14, 884. doi:10.3390/ph14090884
Antonarelli, G., Corti, C., Tarantino, P., Ascione, L., Cortes, J., Romero, P., et al. (2021). Therapeutic Cancer Vaccines Revamping: Technology Advancements and Pitfalls. Ann. Oncol. 32, 1537–1551. doi:10.1016/j.annonc.2021.08.2153
Bachelot, T., Ciruelos, E., Schneeweiss, A., Puglisi, F., Peretz-Yablonski, T., Bondarenko, I., et al. (2019). Preliminary Safety and Efficacy of First-Line Pertuzumab Combined with Trastuzumab and Taxane Therapy for HER2-Positive Locally Recurrent or Metastatic Breast Cancer (PERUSE). Ann. Oncol. 30, 766–773. doi:10.1093/annonc/mdz061
Banerji, U., van Herpen, C. M. L., Saura, C., Thistlethwaite, F., Lord, S., Moreno, V., et al. (2019). Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: a Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 20, 1124–1135. doi:10.1016/s1470-2045(19)30328-6
Bang, Y. J., Giaccone, G., Im, S. A., Oh, D. Y., Bauer, T. M., Nordstrom, J. L., et al. (2017). First-in-human Phase 1 Study of Margetuximab (MGAH22), an Fc-Modified Chimeric Monoclonal Antibody, in Patients with HER2-Positive Advanced Solid Tumors. Ann. Oncol. 28, 855–861. doi:10.1093/annonc/mdx002
Bose, R., Kavuri, S. M., Searleman, A. C., Shen, W., Shen, D., Koboldt, D. C., et al. (2013). Activating HER2 Mutations in HER2 Gene Amplification Negative Breast Cancer. Cancer Discov. 3, 224–237. doi:10.1158/2159-8290.cd-12-0349
Brown, T. A., Mittendorf, E. A., Hale, D. F., Myers, J. W., Peace, K. M., Jackson, D. O., et al. (2020). Prospective, Randomized, Single-Blinded, Multi-center Phase II Trial of Two HER2 Peptide Vaccines, GP2 and AE37, in Breast Cancer Patients to Prevent Recurrence. Breast Cancer Res. Treat. 181, 391–401. doi:10.1007/s10549-020-05638-x
Burris, H. A., Rugo, H. S., Vukelja, S. J., Vogel, C. L., Borson, R. A., Limentani, S., et al. (2011). Phase II Study of the Antibody Drug Conjugate Trastuzumab-DM1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2) -Positive Breast Cancer after Prior HER2-Directed Therapy. Jco 129, 398–405. doi:10.1200/jco.2010.29.5865
Bussolati, G., Annaratone, L., and Maletta, F. (2015). The Pre-analytical Phase in Surgical Pathology. Recent Results Cancer Res. 199, 1–13. doi:10.1007/978-3-319-13957-9_1
Catenacci, D. V. T., Kang, Y. K., Park, H., Uronis, H. E., Lee, K. W., Ng, M. C. H., et al. (2020). Margetuximab Plus Pembrolizumab in Patients with Previously Treated, HER2-Positive Gastro-Oesophageal Adenocarcinoma (CP-MGAH22-05): a Single-Arm, Phase 1b-2 Trial. Lancet Oncol. 21, 1066–1076. doi:10.1016/s1470-2045(20)30326-0
Clifton, G. T., Hale, D., Vreeland, T. J., Hickerson, A. T., Litton, J. K., Alatrash, G., et al. (2020). Results of a Randomized Phase IIb Trial of Nelipepimut-S + Trastuzumab versus Trastuzumab to Prevent Recurrences in Patients with High-Risk HER2 Low-Expressing Breast Cancer. Clin. Cancer Res. 26, 2515–2523. doi:10.1158/1078-0432.ccr-19-2741
Cortes, J., Kim, S. B., Chung, W. P., Im, S. A., Park, Y. H., Hegg, R., et al. (2021). LBA1 - Trastuzumab Deruxtecan (T-DXd) vs Trastuzumab Emtansine (T-DM1) in Patients (Pts) with HER2+ Metastatic Breast Cancer (mBC): Results of the Randomized Phase III DESTINY-Breast03 Study. Ann. Oncol. 32, S1287–S1288. doi:10.1016/j.annonc.2021.08.2088
Corti, C., Giachetti, P., Eggermont, A. M. M., Delaloge, S., and Curigliano, G. (2021). Therapeutic Vaccines for Breast Cancer: Has the Time Finally Come? Eur. J. Cancer Nov 160, 150–174. doi:10.1016/j.ejca.2021.10.027
Corti, C., Giugliano, F., Nicolò, E., Ascione, L., and Curigliano, G. (2021). Antibody-Drug Conjugates for the Treatment of Breast Cancer. Cancers (Basel) 181, 126–142. doi:10.3390/cancers13122898
Corti, C., Sajjadi, E., and Fusco, N. (2019). Determination of Mismatch Repair Status in Human Cancer and its Clinical Significance: Does One Size Fit All? Adv. Anat. Pathol. Jul 26, 270–279. doi:10.1097/pap.0000000000000234
Coussens, L., Yang-Feng, T. L., Liao, Y.-C., Chen, E., Gray, A., McGrath, J., et al. (1985). Tyrosine Kinase Receptor with Extensive Homology to EGF Receptor Shares Chromosomal Location with Neu Oncogene. Science 230, 1132–1139. doi:10.1126/science.2999974
Crimini, E., Repetto, M., Aftimos, P., Botticelli, A., Marchetti, P., and Curigliano, G. (2021). Precision Medicine in Breast Cancer: From Clinical Trials to Clinical Practice. Cancer Treat. Rev. 98, 102223. doi:10.1016/j.ctrv.2021.102223
De Mattos-Arruda, L., Ng, C. K. Y., Piscuoglio, S., Gonzalez-Cao, M., Lim, R. S., De Filippo, M. R., et al. (2018). Genetic Heterogeneity and Actionable Mutations in HER2-Positive Primary Breast Cancers and Their Brain Metastases. Oncotarget 9 (9), 20617–20630. doi:10.18632/oncotarget.25041
Denkert, C., Seither, F., Schneeweiss, A., Link, T., Blohmer, J.-U., Just, M., et al. (2021). Clinical and Molecular Characteristics of HER2-Low-Positive Breast Cancer: Pooled Analysis of Individual Patient Data from Four Prospective, Neoadjuvant Clinical Trials. Lancet Oncol. 22, 1151–1161. doi:10.1016/s1470-2045(21)00301-6
Dieci, M. V., and Miglietta, F. (2021). HER2: a Never Ending story. Lancet Oncol. 22, 1051–1052. doi:10.1016/s1470-2045(21)00349-1
Diéras, V., Miles, D., Verma, S., Pegram, M., Welslau, M., Baselga, J., et al. (2017). Trastuzumab Emtansine versus Capecitabine Plus Lapatinib in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (EMILIA): a Descriptive Analysis of Final Overall Survival Results from a Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 18, 732–742. doi:10.1016/S1470-2045(17)30312-1
Doi, T., Shitara, K., Naito, Y., Shimomura, A., Fujiwara, Y., Yonemori, K., et al. (2017). Safety, Pharmacokinetics, and Antitumour Activity of Trastuzumab Deruxtecan (DS-8201), a HER2-Targeting Antibody-Drug Conjugate, in Patients with Advanced Breast and Gastric or Gastro-Oesophageal Tumours: a Phase 1 Dose-Escalation Study. Lancet Oncol. 18, 1512–1522. doi:10.1016/s1470-2045(17)30604-6
Eiger, D., Agostinetto, E., Saúde-Conde, R., and de Azambuja, E. (2021). The Exciting New Field of HER2-Low Breast Cancer Treatment. Cancers (Basel) 13. doi:10.3390/cancers13051015
Ercoli, G., Lopez, G., Ciapponi, C., Corti, C., Despini, L., Gambini, D., et al. (2017). Building up a High-Throughput Screening Platform to Assess the Heterogeneity of HER2 Gene Amplification in Breast Cancers. J. Vis. Exp. 13, 233–236. doi:10.3791/56686
Exman, P., Garrido-Castro, A. C., Hughes, M. E., Freedman, R. A., Li, T., Trippa, L., et al. (2019). Identifying ERBB2 Activating Mutations in HER2-Negative Breast Cancer: Clinical Impact of Institute-Wide Genomic Testing and Enrollment in Matched Therapy Trials. JCO Precis Oncol. 3. doi:10.1200/PO.19.00087
Farahmand, S., Fernandez, A. I., Ahmed, F. S., Rimm, D. L., Chuang, J. H., Reisenbichler, E., et al. (2022). Deep Learning Trained on Hematoxylin and Eosin Tumor Region of Interest Predicts HER2 Status and Trastuzumab Treatment Response in HER2+ Breast Cancer. Mod. Pathol. 35, 44–51. doi:10.1038/s41379-021-00911-w
Fehrenbacher, L., Cecchini, R. S., Geyer Jr, C. E., Rastogi, P., Costantino, J. P., Atkins, J. N., et al. (2020). NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy with or without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and with IHC 1+ or 2+. Jco 38, 444–453. doi:10.1200/jco.19.01455
Filho, O. M., Viale, G., Stein, S., Trippa, L., Yardley, D. A., Mayer, I. A., et al. (2021). Impact of HER2 Heterogeneity on Treatment Response of Early-Stage HER2-Positive Breast Cancer: Phase II Neoadjuvant Clinical Trial of T-DM1 Combined with Pertuzumab. Cancer Discov. 11, 2474–2487. doi:10.1158/2159-8290.cd-20-1557
Fusco, N., and Bosari, S. (2016). HER2 Aberrations and Heterogeneity in Cancers of the Digestive System: Implications for Pathologists and Gastroenterologists. World J. Gastroenterol. 22 (22), 7926–7937. doi:10.3748/wjg.v22.i35.7926
Fusco, N., Ragazzi, M., Sajjadi, E., Venetis, K., Piciotti, R., Morganti, S., et al. (2021). Assessment of Estrogen Receptor Low Positive Status in Breast Cancer: Implications for Pathologists and Oncologists. Histol. Histopathol 36, 1235–1245. doi:10.14670/HH-18-376
Fusco, N., Rocco, E. G., Del Conte, C., Pellegrini, C., Bulfamante, G., Di Nuovo, F., et al. (2013). HER2 in Gastric Cancer: a Digital Image Analysis in Pre-neoplastic, Primary and Metastatic Lesions. Mod. Pathol. 26, 816–824. doi:10.1038/modpathol.2012.228
Geuijen, C. A. W., De Nardis, C., Maussang, D., Rovers, E., Gallenne, T., Hendriks, L. J. A., et al. (2018). Unbiased Combinatorial Screening Identifies a Bispecific IgG1 that Potently Inhibits HER3 Signaling via HER2-Guided Ligand Blockade. Cancer Cell 33, 922–936. doi:10.1016/j.ccell.2018.04.003
Gianni, L., Lladó, A., Bianchi, G., Cortes, J., Kellokumpu-Lehtinen, P. L., Cameron, D. A., et al. (2010). Open-label, Phase II, Multicenter, Randomized Study of the Efficacy and Safety of Two Dose Levels of Pertuzumab, a Human Epidermal Growth Factor Receptor 2 Dimerization Inhibitor, in Patients with Human Epidermal Growth Factor Receptor 2-negative Metastatic Breast Cancer. J. Clin. Oncol. 28 (28), 1131–1137. doi:10.1200/JCO.2009.24.1661
Gianni, L., Dafni, U., Gelber, R. D., Azambuja, E., Muehlbauer, S., Goldhirsch, A., et al. (2011). Treatment with Trastuzumab for 1 Year after Adjuvant Chemotherapy in Patients with HER2-Positive Early Breast Cancer: a 4-year Follow-Up of a Randomised Controlled Trial. Lancet Oncol. 12, 236–244. doi:10.1016/s1470-2045(11)70033-x
Gianni, L., Pienkowski, T., Im, Y.-H., Tseng, L.-M., Liu, M.-C., Lluch, A., et al. (2016). 5-year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients with Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): a Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet Oncol. 17, 791–800. doi:10.1016/s1470-2045(16)00163-7
Goldhirsch, A., Gelber, R. D., Piccart-Gebhart, M. J., de Azambuja, E., Procter, M., Suter, T. M., et al. (2013). 2 Years versus 1 Year of Adjuvant Trastuzumab for HER2-Positive Breast Cancer (HERA): an Open-Label, Randomised Controlled Trial. Lancet 382, 1021–1028. doi:10.1016/S0140-6736(13)61094-6
Hamilton, E., Shastry, M., Shiller, S. M., and Ren, R. (2021). Targeting HER2 Heterogeneity in Breast Cancer. Cancer Treat. Rev. 100, 102286. doi:10.1016/j.ctrv.2021.102286
Haricharan, S., Punturi, N., Singh, P., Holloway, K. R., Anurag, M., Schmelz, J., et al. (2017). Loss of MutL Disrupts CHK2-dependent Cell-Cycle Control through CDK4/6 to Promote Intrinsic Endocrine Therapy Resistance in Primary Breast Cancer. Cancer Discov. 7, 1168–1183. doi:10.1158/2159-8290.cd-16-1179
Huang, Y., Burns, D. J., Rich, B. E., MacNeil, I. A., Dandapat, A., Soltani, S. M., et al. (2017). Development of a Test that Measures Real-Time HER2 Signaling Function in Live Breast Cancer Cell Lines and Primary Cells. BMC Cancer 17 (17), 199. doi:10.1186/s12885-017-3181-0
Hudis, C. A. (2007). Trastuzumab - Mechanism of Action and Use in Clinical Practice. N. Engl. J. Med. 357, 39–51. doi:10.1056/nejmra043186
Iwata, H., Tamura, K., Doi, T., Tsurutani, J., Modi, S., Park, H., et al. (2018). Trastuzumab Deruxtecan (DS-8201a) in Subjects with HER2-Expressing Solid Tumors: Long-Term Results of a Large Phase 1 Study with Multiple Expansion Cohorts. Jco 36, 2501. doi:10.1200/jco.2018.36.15_suppl.2501
Jiang, G., Zhang, S., Yazdanparast, A., Li, M., Pawar, A. V., Liu, Y., et al. (2016). Comprehensive Comparison of Molecular Portraits between Cell Lines and Tumors in Breast Cancer. BMC genomics 17, 525. doi:10.1186/s12864-016-2911-z
Kreutzfeldt, J., Rozeboom, B., Dey, N., and De, P. (2020). The Trastuzumab Era: Current and Upcoming Targeted HER2+ Breast Cancer Therapies. Am. J. Cancer Res. 10, 1045–1067.
Krop, I. E., LoRusso, P., Miller, K. D., Modi, S., Yardley, D., Rodriguez, G., et al. (2012). A Phase II Study of Trastuzumab Emtansine in Patients with Human Epidermal Growth Factor Receptor 2-positive Metastatic Breast Cancer Who Were Previously Treated with Trastuzumab, Lapatinib, an Anthracycline, a Taxane, and Capecitabine. J. Clin. Oncol. 30 (30), 3234–3241. doi:10.1200/JCO.2011.40.5902
Krop, I. E., Beeram, M., Modi, S., Jones, S. F., Holden, S. N., Yu, W., et al. (2010). Phase I Study of Trastuzumab-DM1, an HER2 Antibody-Drug Conjugate, Given Every 3 Weeks to Patients with HER2-Positive Metastatic Breast Cancer. Jco 28, 2698–2704. doi:10.1200/jco.2009.26.2071
Krop, I. E., Kim, S.-B., Martin, A. G., LoRusso, P. M., Ferrero, J.-M., Badovinac-Crnjevic, T., et al. (2017). Trastuzumab Emtansine versus Treatment of Physician's Choice in Patients with Previously Treated HER2-Positive Metastatic Breast Cancer (TH3RESA): Final Overall Survival Results from a Randomised Open-Label Phase 3 Trial. Lancet Oncol. 18, 743–754. doi:10.1016/s1470-2045(17)30313-3
La Barbera, D., Polónia, A., Roitero, K., Conde-Sousa, E., and Della Mea, V. (2020). Detection of HER2 from Haematoxylin-Eosin Slides through a Cascade of Deep Learning Classifiers via Multi-Instance Learning. J. Imaging 6, 82. doi:10.3390/jimaging6090082
Lopez, G., Costanza, J., Colleoni, M., Fontana, L., Ferrero, S., Miozzo, M., et al. (2019). Molecular Insights into the Classification of Luminal Breast Cancers: The Genomic Heterogeneity of Progesterone-Negative Tumors. Int. J. Mol. Sci. 20, 20. doi:10.3390/ijms20030510
Lopez, G., Venetis, K., Sajjadi, E., and Fusco, N. (2020). Mismatch Repair System Genomic Scars in Gastroesophageal Cancers: Biology and Clinical Testing. GastrointestDisord 2, 341–352. doi:10.3390/gidisord2040031
MacNeil, I. A., Burns, D. J., Rich, B. E., Soltani, S. M., Kharbush, S., Osterhaus, N. G., et al. (2020). New HER2-Negative Breast Cancer Subtype Responsive to Anti-HER2 Therapy Identified. J. Cancer Res. Clin. Oncol. 146, 605–619. doi:10.1007/s00432-020-03144-7
Mateo, J., Chakravarty, D., Dienstmann, R., Jezdic, S., Gonzalez-Perez, A., Lopez-Bigas, N., et al. (2018). A Framework to Rank Genomic Alterations as Targets for Cancer Precision Medicine: the ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). Ann. Oncol. 29, 1895–1902. doi:10.1093/annonc/mdy263
Meric-Bernstam, F., Hurwitz, H., Raghav, K. P. S., McWilliams, R. R., Fakih, M., VanderWalde, A., et al. (2019). Pertuzumab Plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer (MyPathway): an Updated Report from a Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol. 20, 518–530. doi:10.1016/s1470-2045(18)30904-5
Miglietta, F., Griguolo, G., Bottosso, M., Giarratano, T., Lo Mele, M., Fassan, M., et al. (2021). Evolution of HER2-Low Expression from Primary to Recurrent Breast Cancer. npj Breast Cancer 7, 137. doi:10.1038/s41523-021-00343-4
Modi, S., Park, H., Murthy, R. K., Iwata, H., Tamura, K., Tsurutani, J., et al. (2020). Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients with HER2-Low-Expressing Advanced Breast Cancer: Results from a Phase Ib Study. Jco 38, 1887–1896. doi:10.1200/jco.19.02318
Mutai, R., Barkan, T., Moore, A., Sarfaty, M., Shochat, T., Yerushalmi, R., et al. (2021). Prognostic Impact of HER2-Low Expression in Hormone Receptor Positive Early Breast Cancer. The Breast 60, 62–69. doi:10.1016/j.breast.2021.08.016
Omar, A., and Arafat, W. (2021). HER2-low-positive Breast Cancer from Four Neoadjuvant Clinical Trials. Lancet Oncol. 22, e426. doi:10.1016/s1470-2045(21)00456-3
Pauletti, G., Godolphin, W., Press, M. F., and Slamon, D. J. (1996). Detection and Quantitation of HER-2/neu Gene Amplification in Human Breast Cancer Archival Material Using Fluorescence In Situ Hybridization. Oncogene 13 (13), 63–72.
Piccart-Gebhart, M. J., Procter, M., Leyland-Jones, B., Goldhirsch, A., Untch, M., Smith, I., et al. (2005). Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 353, 1659–1672. doi:10.1056/nejmoa052306
Piciotti, R., Venetis, K., Sajjadi, E., and Fusco, N. (2021). Mismatch Repair Status Characterization in Oncologic Pathology: Taking Stock of the Real-World Possibilities. Jmp 2, 93–100. doi:10.3390/jmp2020009
Pistilli, B., Wildiers, H., Hamilton, E. P., Ferreira, A. A., Dalenc, F., Vidal, M., et al. (2020). Clinical Activity of MCLA-128 (Zenocutuzumab) in Combination with Endocrine Therapy (ET) in ER+/HER2-low, Non-amplified Metastatic Breast Cancer (MBC) Patients (Pts) with ET-Resistant Disease Who Had Progressed on a CDK4/6 Inhibitor (CDK4/6i). Jco 38, 1037. doi:10.1200/jco.2020.38.15_suppl.1037
Pondé, N., Brandão, M., El-Hachem, G., Werbrouck, E., and Piccart, M. (2018). Treatment of Advanced HER2-Positive Breast Cancer: 2018 and beyond. Cancer Treat. Rev. 67, 10–20. doi:10.1016/j.ctrv.2018.04.016
Press, M. F., Slamon, D. J., Flom, K. J., Park, J., Zhou, J. Y., and Bernstein, L. (2002). Evaluation of HER-2/neu Gene Amplification and Overexpression: Comparison of Frequently Used Assay Methods in a Molecularly Characterized Cohort of Breast Cancer Specimens. J. Clin. Oncol. 20 (20), 3095–3105. doi:10.1200/JCO.2002.09.094
Punturi, N. B., Seker, S., Devarakonda, V., Mazumder, A., Kalra, R., Chen, C. H., et al. (2021). Mismatch Repair Deficiency Predicts Response to HER2 Blockade in HER2-Negative Breast Cancer. Nat. Commun. 12, 2940. doi:10.1038/s41467-021-23271-0
Rugo, H. S., Im, S. A., Cardoso, F., Cortés, J., Curigliano, G., Musolino, A., et al. (2021). Efficacy of Margetuximab vs Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 7 (7), 573–584. doi:10.1001/jamaoncol.2020.7932
Sajjadi, E., Venetis, K., Piciotti, R., Gambini, D., Blundo, C., Runza, L., et al. (2021). Combined Analysis of PTEN, HER2, and Hormone Receptors Status: Remodeling Breast Cancer Risk Profiling. BMC cancer 21, 211152. doi:10.1186/s12885-021-08889-z
Sajjadi, E., Venetis, K., Piciotti, R., Invernizzi, M., Guerini-Rocco, E., Haricharan, S., et al. (2021b). Mismatch Repair-Deficient Hormone Receptor-Positive Breast Cancers: Biology and Pathological Characterization. Cancer Cell Int. May 17, 21266. doi:10.1186/s12935-021-01976-y
Sajjadi, E., Venetis, K., Piciotti, R., Invernizzi, M., Guerini-Rocco, E., Haricharan, S., et al. (2021a). Mismatch Repair-Deficient Hormone Receptor-Positive Breast Cancers: Biology and Pathological Characterization. Cancer Cell Int 21, 266. doi:10.1186/s12935-021-01976-y
Sartore-Bianchi, A., Lonardi, S., Martino, C., Fenocchio, E., Tosi, F., Ghezzi, S., et al. (2020). Pertuzumab and Trastuzumab Emtansine in Patients with HER2-Amplified Metastatic Colorectal Cancer: the Phase II HERACLES-B Trial. ESMO Open 5, e000911. doi:10.1136/esmoopen-2020-000911
Saura Manich, C., O'Shaughnessy, J., Aftimos, P. G., van den Tweel, E., Oesterholt, M., Escrivá-de-Romaní, S. I., et al. (2021). LBA15 Primary Outcome of the Phase III SYD985.002/TULIP Trial Comparing [vic-]trastuzumab Duocarmazine to Physician's Choice Treatment in Patients with Pre-treated HER2-Positive Locally Advanced or Metastatic Breast Cancer. Ann. Oncol. 32, S1288. doi:10.1016/j.annonc.2021.08.2088
Schalper, K. A., Kumar, S., Hui, P., Rimm, D. L., and Gershkovich, P. (2014). A Retrospective Population-Based Comparison of HER2 Immunohistochemistry and Fluorescence In Situ Hybridization in Breast Carcinomas: Impact of 2007 American Society of Clinical Oncology/College of American Pathologists Criteria. Arch. Pathol. Lab. Med. Feb 138, 213–219. doi:10.5858/arpa.2012-0617-oa
Schettini, F., Chic, N., Brasó-Maristany, F., Paré, L., Pascual, T., Conte, B., et al. (2021). Clinical, Pathological, and PAM50 Gene Expression Features of HER2-Low Breast Cancer. npj Breast Cancer 7, 1. doi:10.1038/s41523-020-00208-2
Seshadri, R., Firgaira, F. A., Horsfall, D. J., McCaul, K., Setlur, V., and Kitchen, P. (1993). Clinical Significance of HER-2/neu Oncogene Amplification in Primary Breast Cancer. The South Australian Breast Cancer Study Group. Jco 11, 1936–1942. doi:10.1200/jco.1993.11.10.1936
Shitara, K., Bang, Y.-J., Iwasa, S., Sugimoto, N., Ryu, M.-H., Sakai, D., et al. (2020). Trastuzumab Deruxtecan in Previously Treated HER2-Positive Gastric Cancer. N. Engl. J. Med. 382, 2419–2430. doi:10.1056/nejmoa2004413
Siena, S., Di Bartolomeo, M., Raghav, K., Masuishi, T., Loupakis, F., Kawakami, H., et al. (2021). Trastuzumab Deruxtecan (DS-8201) in Patients with HER2-Expressing Metastatic Colorectal Cancer (DESTINY-CRC01): a Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. Jun 22, 779–789. doi:10.1016/s1470-2045(21)00086-3
Skeie, M., Nikolaysen, F., Chitano, Y., and Stang, E. (2020). Hsp90 Inhibition and Co‐incubation with Pertuzumab Induce Internalization and Degradation of Trastuzumab: Implications for Use of T‐DM1. J. Cell Mol Med 24, 10258–10262. doi:10.1111/jcmm.15643
Slamon, D., Eiermann, W., Robert, N., Pienkowski, T., Martin, M., Press, M., et al. (2011). Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 365, 1273–1283. doi:10.1056/nejmoa0910383
Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., et al. (1989). Studies of the HER-2/Neu Proto-Oncogene in Human Breast and Ovarian Cancer. Science 244, 707–712. doi:10.1126/science.2470152
Slamon, D. J., Leyland-Jones, B., Shak, S., Fuchs, H., Paton, V., Bajamonde, A., et al. (2001). Use of Chemotherapy Plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer that Overexpresses HER2. N. Engl. J. Med. 344, 783–792. doi:10.1056/nejm200103153441101
Slamon, D., and Pegram, M. (2001). Rationale for Trastuzumab (Herceptin) in Adjuvant Breast Cancer Trials. Semin. Oncol. Feb 28, 13–19. doi:10.1053/sonc.2001.22812
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68, 394–424. doi:10.3322/caac.21660
Swain, S. M., Miles, D., Kim, S. B., Im, Y. H., Im, S. A., Semiglazov, V., et al. (2020). Pertuzumab, Trastuzumab, and Docetaxel for HER2-Positive Metastatic Breast Cancer (CLEOPATRA): End-Of-Study Results from a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Study. Lancet Oncol. 21, 519–530. doi:10.1016/S1470-2045(19)30863-0
Tarantino, P., Carmagnani Pestana, R., Corti, C., Modi, S., Bardia, A., Tolaney, S. M., et al. (2021). Antibody-drug Conjugates: Smart Chemotherapy Delivery across Tumor Histologies. CA Cancer J. Clin. Nov 12. doi:10.3322/caac.21705
Tarantino, P., Hamilton, E., Tolaney, S. M., Cortes, J., Morganti, S., Ferraro, E., et al. (2020). HER2-Low Breast Cancer: Pathological and Clinical Landscape. Jco 38, 1951–1962. doi:10.1200/jco.19.02488
Tarantino, P., Morganti, S., Uliano, J., Giugliano, F., Crimini, E., and Curigliano, G. (2021). Margetuximab for the Treatment of HER2-Positive Metastatic Breast Cancer. Expert Opin. Biol. Ther. 21, 127–133. doi:10.1080/14712598.2021.1856812
Tarantino, P., Uliano, J., Morganti, S., Giugliano, F., Crimini, E., and Curigliano, G. (2021). Clinical Development and Current Role of Margetuximab for the Treatment of Breast Cancer. Drugs Today 57, 551–558. doi:10.1358/dot.2021.57.9.3319148
Venetis, K., Invernizzi, M., Sajjadi, E., Curigliano, G., and Fusco, N. (2020a). Cellular Immunotherapy in Breast Cancer: The Quest for Consistent Biomarkers. Cancer Treat. Rev. 90, 102089. doi:10.1016/j.ctrv.2020.102089
Venetis, K., Sajjadi, E., Haricharan, S., and Fusco, N. (2020b). Mismatch Repair Testing in Breast Cancer: the Path to Tumor-specific Immuno-Oncology Biomarkers. Translational Cancer Res. 9, 4060–4064. doi:10.21037/tcr-20-1852
Viale, G., and Fusco, N. (2021). Pathology after Neoadjuvant Treatment - How to Assess Residual Disease. Breast Nov 16. doi:10.1016/j.breast.2021.11.009
Viale, G., and Munzone, E. (2019). Treatment Selection for Patients with Equivocal HER2 Status and in Luminal versus HER2-Enriched Disease. The Breast 48 (Suppl. 1), S49–s52. doi:10.1016/s0960-9776(19)31123-3
von Minckwitz, G., Huang, C. S., Mano, M. S., Loibl, S., Mamounas, E. P., Untch, M., et al. (2019). Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 380, 380617–380628. doi:10.1056/NEJMoa1814017
von Minckwitz, G., Procter, M., de Azambuja, E., Zardavas, D., Benyunes, M., Viale, G., et al. (2017). Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 377, 122–131. doi:10.1056/nejmoa1703643
Wolff, A. C., Hammond, M. E. H., Allison, K. H., Harvey, B. E., Mangu, P. B., Bartlett, J. M. S., et al. (2018). Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Jco 36, 2105–2122. doi:10.1200/jco.2018.77.8738
Yazaki, S., Hashimoto, J., Ogita, S., Nakano, E., Suzuki, K., and Yamauchi, T. (2020). Lower Response to Trastuzumab Emtansine in Metastatic Breast Cancer Patients with Human Epidermal Growth Factor Receptor 2 Immunohistochemistry Score of 2 and Fluorescence In Situ Hybridization Positive Compared with Immunohistochemistry Score of 3: a Retrospective Study. Anticancer Drugs 31, 973–978. doi:10.1097/cad.0000000000000939
You, Z., Zhou, W., Weng, J., Feng, H., Liang, P., Li, Y., et al. (2021). Application of HER2 Peptide Vaccines in Patients with Breast Cancer: a Systematic Review and Meta-Analysis. Cancer Cell Int 21, 489. doi:10.1186/s12935-021-02187-1
Keywords: breast cancer, biomarkers, HER2 low expression, HER2 ultra low, targeted therapy, antibody-drug conjugate, immunohistochemistry, fluorescence in situ hybrdization
Citation: Venetis K, Crimini E, Sajjadi E, Corti C, Guerini-Rocco E, Viale G, Curigliano G, Criscitiello C and Fusco N (2022) HER2 Low, Ultra-low, and Novel Complementary Biomarkers: Expanding the Spectrum of HER2 Positivity in Breast Cancer. Front. Mol. Biosci. 9:834651. doi: 10.3389/fmolb.2022.834651
Received: 13 December 2021; Accepted: 21 February 2022;
Published: 15 March 2022.
Edited by:
Libing Zhang, Tianjin University, ChinaReviewed by:
Manuel Salto-Tellez, Queen’s University Belfast, United KingdomCopyright © 2022 Venetis, Crimini, Sajjadi, Corti, Guerini-Rocco, Viale, Curigliano, Criscitiello and Fusco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Fusco, bmljb2xhLmZ1c2NvQHVuaW1pLml0; Carmen Criscitiello, Y2FybWVuLmNyaXNjaXRpZWxsb0B1bmltaS5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.