- Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia
An important hallmark of the human immune system is to provide adaptive immunity against pathogens but tolerance toward self-antigens. The CC-chemokine receptor 7 (CCR7) provides a significant contribution in guiding cells to and within lymphoid organs and is important for acquiring immunity and tolerance. The CCR7 holds great importance in establishing thymic architecture and function and naïve and regulatory T-cell homing in the lymph nodes. Similarly, the receptor is a key regulator in cancer cell migration and the movement of dendritic cells. This makes the CCR7 an important receptor as a drug and prognostic marker. In this review, we discussed several biological roles of the CCR7 and its importance as a drug and prognostic marker.
Background
To ensure efficient functioning of the immune system, the interaction between immune and non-immune cells is imperative (Stockis et al., 2019). These cellular encounters greatly rely on the cells' ability to migrate to a defined site (Förster et al., 2008). The trafficking of immune cells is regulated by key regulators known as chemokines (Hughes and Nibbs, 2018). Some of these chemokines are produced during infection, while others such as CC-chemokine ligand 21 (CCL21) and CCL19 are expressed every time and function to control cell movement (Förster et al., 2008). Both CCL21 and CCL19 act as sole ligands for a CC-chemokine receptor 7 (CCR7) (Hauser and Legler, 2016). The CCR7 protein is the product of the CCR7 gene and is recently designated as a cluster of differentiation 197 (CD197) (Cuesta-Mateos et al., 2021). Different cells of the immunity system are responsible for CCR7 expression and along with its ligands play a key part in localizing antigen-presenting dendritic cells and T cell subpopulation to lymph nodes, where the cells establish close contacts to drive activation of antigen presentation (Lipscomb and Masten, 2002). The CCR7 is implicated in optimal induction of protective immunity and also for the stimulation of peripheral tolerance induction and immunity response regulation by CD4+CD25+ regulatory T cells (Cools et al., 2007) (Kondo et al., 2019).
CCR7 and Its Binders
There are two ligands for CCR7; CCL19 and CCL21. In order to have avid binding to glycosaminoglycans (Rot, 2010), CCL21 has a unique 12 basic amino acid patch in the long C-terminal tail of 32 residues (Proudfoot et al., 2017). The binding event is a prerequisite for effective presentation of CCL21 on endothelial cell surfaces (Miyasaka and Tanaka, 2004). The CCL21 presentation is specifically carried out by podoplanin, which is a proteoglycan expressed by different cell types and might regulate CCL21 availability (Johnson and Jackson, 2010). In mouse experimentation, due to gene duplication, two functional CCL21 variants have been noticed (Zlotnik et al., 2006). One is CCL21-Leu with leucine at position 65 and is expressed by the colon, lung, stomach, skin, and heart (Schumann, 2011). On the other hand, CCL21-Ser is expressed by lymph nodes, thymus, and spleen (Mori et al., 2001). It is interesting to know that the human genome only encodes CCL21-leu and not CCL21-Ser (Hauser and Legler, 2016). The CCL21 in humans and mice is yielded by fibroblastic reticular cells and endothelial venules (Link et al., 2011) (Al-Jokhadar et al., 2017) (Seth et al., 2011). The CCR7 is made of seven transmembrane domain containing proteins and facilitates its signaling pathways through heterotrimeric G proteins (Maghazachi, 2005). The expression of CCR7 is carried out by thymocytes, mature and semi-mature dendritic cells, regulatory T-cells, naïve T-and B-cells, and central memory T-cells (Schneider et al., 2007). In addition, CCR7 expression is carried out by different malignant cells. For CCR7, CCL19 and CCl21 had shown the same binding affinities though they initiate various singling pathways leading to different impacts (Müller et al., 2003). The CCL19 in contrast to CCL21 activates CCR7 internalization and phosphorylation, which shorten the time span of CCR7-mediated cell responses to CCL19 (Hauser and Legler, 2016). Similarly, the CCL19 can desensitize the CCR7 in its subsequent response to CCL21 ligation (Zidar et al., 2009). Together with CCL25, CCL19 and CCL21 have to potential to bind with high affinity to CC-X-chemokine receptors, which act as chemokine interceptors by internalizing ligands and transporting them (Förster et al., 2008).
Multifunctional Roles of CCR7 in Host Immunology
Significance of CCR7 in Immune Cell Regulation
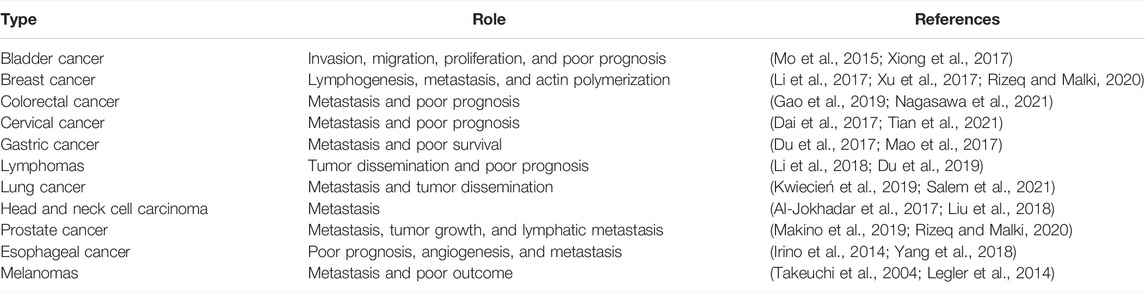
The localization of immune cells to defined functional compartments is controlled by CCR7-mediated signals (Worbs and Förster, 2007). The majority of the T-cells such as memory, naïve, and regulatory T-cells are allowed to penetrate lymph nodes involving a stepwise procedure of interaction of adhesion to endothelial cells (Nolz et al., 2011). In mice experimentation, CCR7 deficiency results in lack of T-cells in lymph nodes (Okada et al., 2002). It was also observed that T-cells are unable to home the lymph nodes but localize to the spleen in the absence of functional CCR7 (Sharma et al., 2015). The B cells in the CCR7-deficient case have the potential to migrate to splenic white pulp and lymph nodes (Katagiri et al., 2004). Though the CCR7 as a receptor of lymph node homing is well-established, evidence suggesting its role in lymphocyte recirculation is also very real (Link et al., 2007). The emigration of T-cells to peripheral tissues and entrance of T-cells to lymph nodes is also a CCR7-dependent step (Ebert et al., 2005). The dendritic cells are present as sentinels in the skin and alimentary, respiratory, and urogenital tracts (Hendry et al., 2017). The activation of dendritic cells by an infectious agent or inflammatory events drives the cells to undergo maturation, resulting in major changes in antigen uptake and presentation (Stockwin et al., 2000). The maturation of dendritic cells can be categorized by the higher expression of CCR7 and CD80, CH83, and CD86 (Chiesa et al., 2003). Very less is known about the exact mechanism of how trafficking of dendritic cells via different lymphatic events occurs (Alvarez et al., 2008). Furthermore, it is still under investigation how CCR7 and its ligands mobilize the dendritic cells (McKenna et al., 2005). Both the wild and CCR7-deficient mice were reported to have the same dendritic cell numbers in the peripheral organs (del Rio et al., 2007). This implies that CCR7 has no direct involvement in dendritic cell progenitor recruitment to mucosal and skin surfaces (Cutler and Jotwani, 2004). The migration ability of differentiated dendritic cells from bone marrow to lymph nodes is a major hinderance in CCR7-deicient mice (León et al., 2005). It is also analyzed that the turnover of dendritic cells from the lung, skin, and intestine depends on the CCR7 (Hintzen et al., 2006). In in vivo studies, it has been demonstrated that CCL19 and CCL21-Ser derived from lymph nodes take part in activating dendritic cell relocation into the lymph nodes (Denton et al., 2014). CCL19 and CCL21 are needed for dendritic cell guiding in the lymph nodes. Furthermore, research findings speculated that CCL19 and CCL21 are capable of priming T cells along with driving the dendritic cell migration. The uptake of antigens by mature dendritic cells is facilitated by CCR7 ligands (Seubert et al., 2008). A graphical illustration of the stepwise process of lymphocyte homing to the lymph nodes is provided in Figure 1.
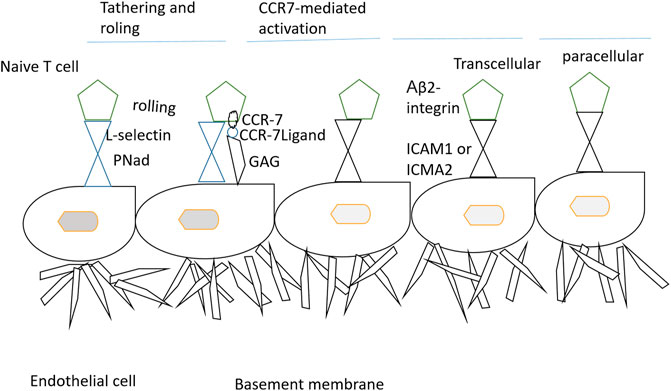
FIGURE 1. Stepwise mechanism of lymphocyte homing to the lymph nodes. The T-cells when emerging from the blood enter the peripheral lymph nodes through the tethering and rolling mechanism, activation of CCR7, firm arrest, and transendothelial migration. At first, L-selection of lymphocyte binds with peripheral node addressins (PNAd) and sialomucins on high endothelial venules (HEVs). The interaction results in T-cell attachment to HEVs and results in cell rolling. The rolling cells then interact with CCL21/CCL19 and thus are immobilized by glycosaminoglycans (GAGs). The signals from CCR7 and blood flow force induce conformational changes in αLβ2-integrins, thus allowing firm binding to intracellular addressin cell adhesion molecular 1 (ICAM1) and ICAM2. CCR7 also activates αLβ2-integrin mucosal addressin cell-adhesion molecule 1 (MAdCAM) (Förster et al., 2008).
The Role of CCR7 in Lymph-Node Homing
Upon entrance into the lymph node, naïve T-cells begin to migrate in a random walk pattern in the paracortical T-cell-rich area (Krummel et al., 2016) (Weninger et al., 2003). The CCR7-deficient T cells in popliteal lymph nodes have shown 30% reduced velocity as well as 50% reduced motility coefficient (Worbs et al., 2007). Furthermore, a notable dichotomy has been observed within the lymph nodes for chemokine receptor usage (Garcia et al., 2005). The CCR7 activates signals that allow the cell to migrate into the T-cell areas (Arnold et al., 2007). Upon activation, the follicular B-cells upregulate CCR7 and downregulate CXXR5. The differential chemokine receptor expression drives the movement of follicular B-cells to the T-cell zone to get help from CD4+ T cells (Eisenbarth et al., 2021). The expression of CCR7 on CD4+CXCR5+ follicular T cells permits the cells to enter into B cell follicles for providing help in antibody production and class switching (Hardtke et al., 2005). Overall, it can be concluded that CCR7 is a lymph-node receptor for dendritic cells and T-cells.
The Role of CCR7 in Immune Tolerance
The weak immunity in CCR7-deficient mice after administration of a model antigen further illustrates the multifaceted role of CCR7 and its ligand molecules on the immune system and their vital importance in paracortical area organization in the lymph node (Worbs and Förster, 2007). Studies have also shown that the CCR7-deficient mice impaired humoral immune responses in case of low antigen against replicating virus and high amount of virus glycoproteins (Scandella et al., 2007). These findings imply that when the antigen is sparse, CCR7 holds significant importance in interactions among immune cells (Qi et al., 2006). In some cases, the CCR7-mediated interactions are bypassed in providing adaptive immunity against a pathogen (Moretta et al., 2008). This was highlighted in CCR7-deficient mice where neutralizing immune responses were seen mounted against the choriomeningitis virus (Junt et al., 2008). It was also observed that for priming the naïve MHC-class-Ia-restricted CD8+ T cells, the presence of CCR7 is required, whereas MHC-class-II-restricted CD4+ T cells and naive MHC-class-Ib-restricted CD8+ T cells do not require chemokine receptor (Tzelepis et al., 2007). In addition, it was revealed that repeated administration of tetanus toxoid stimulated humoral and full-blown cellular immunity in CCR7-deficient mice (Macpherson et al., 2008). In auto-immune encephalitis, allergic asthma, and inflammatory bowel disease, substantial immune responses in mice were developed in the absence of CCR7 and its ligands (Griffith et al., 2014). The nonstop migration of dendritic cells from the periphery is a critical step in inducing immune tolerance in response to any food or environmental antigen (Zhang et al., 2021). The migration of tolerogenic or semi-mature dendritic cells into draining lymph nodes depends on CCR7 expression (Förster et al., 2012). This was tested in CCR7-deficient mice whether dendritic cell–mediated transportation of harmless antigens is required for peripheral tolerance (Worbs et al., 2006). The use of intravenous or subcutaneous injection of model antigen ovalbumin in wild-type mice results in systematic non-responsiveness toward model antigen ovalbumin (Steenblock et al., 2009). The mesenteric lymph node was identified as a site of antigen presentation to T cells (Buettner and Bode, 2012) (Jang et al., 2006). Further clarity on the point was obtained from studies where antigen delivery to the respiratory tract is carried out by intratracheal instillation or inhalation (Lombry et al., 2004). The antigen was labeled with fluorochrome to monitor its in vivo and ex vivo experimentations. The CCR7-deficient mice showed no effect of model antigen ovalbumin aerosol on reporter T-cells (Förster et al., 2008). Therefore, it can be summarized that under homeostatic conditions, the dendritic cells at mucosal sites can induce tolerance in the presence of CCR7 by sampling antigens and transporting them to draining lymph nodes to be efficiently presented to T-cells (Seth et al., 2011).
Suppression of the host immunity through forkhead box P3 (FOXP3) T-cells is considered an alternative method for efficient peripheral immune tolerance to foreign and self-antigens (Nishikawa and Sakaguchi, 2010). The regulatory T-cells can be naturally produced in the thymus when CCR7 is absent. In both wild and CCR7-deficient mice, the total number of FOXP3+ T-cells is the same (Schneider et al., 2007) (Smigiel et al., 2014). This can be rational that in vivo the cells are unable to reach the lymph nodes and incapable of placing themselves in the T-cell zone (Groom et al., 2012). In the lymph nodes, the exact mechanism behind the regulatory T-cell suppressive activity is still unknown (Wei et al., 2018). The regulatory T-cell homing T-cell zone of the lymph node is mediated by CCR7, proliferates, and expands when they encounter their cognate antigen (Schneider et al., 2007). Reduced number of activated T helper cells due to CCR7-dependent presence of regulatory T-cells is observed (Bayry et al., 2007). Schematically, the CCR7-mediated immune tolerance is presented in Figure 2.
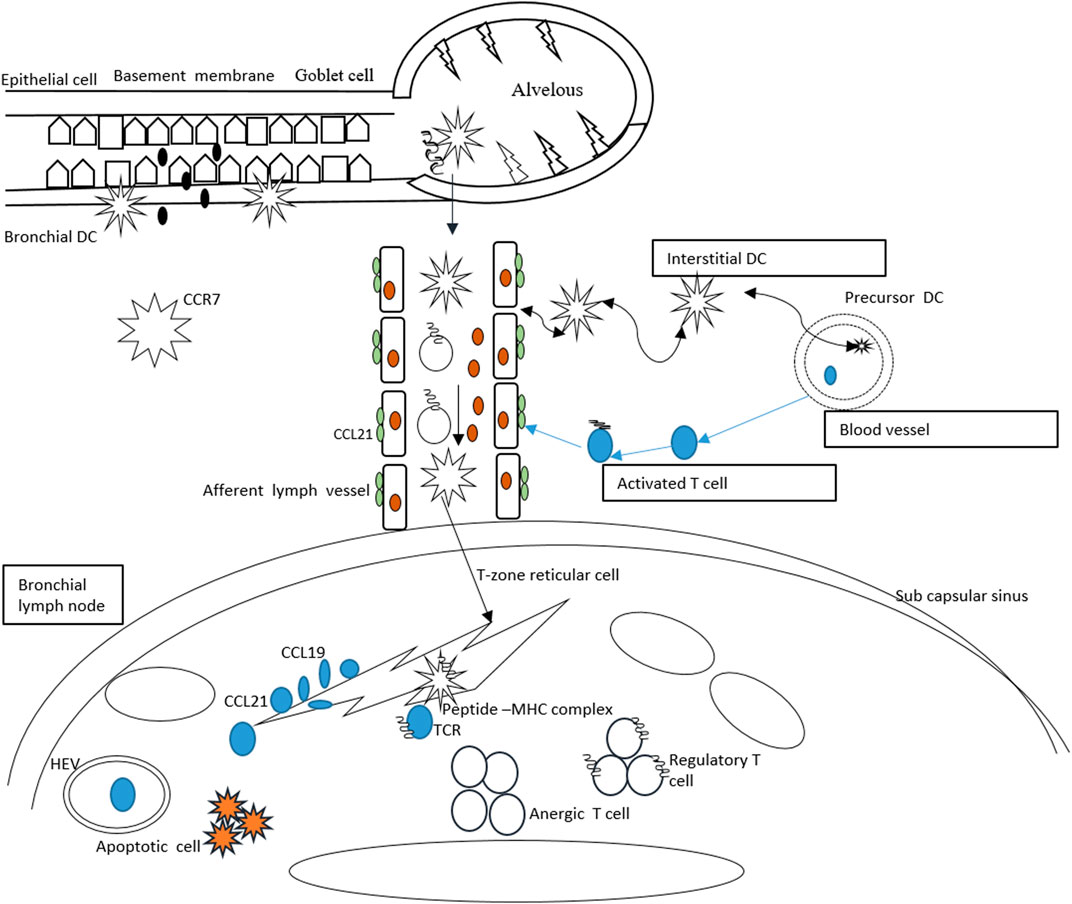
FIGURE 2. CCR7-mediated tolerance in response to inhaled antigens. The dendritic cells enter the lungs and produce interstitial and bronchial dendritic cells. The bronchial dendritic cells carry the antigens and upregulate the CCR7 and move toward lymphatic vessels to activate CCL21. The dendritic cells are passively transported into the draining lymph node. The dendritic cells then present antigens to the naïve T-cells and via endothelial venules, the T-cells penetrate into the lymph node. Migration of T-cells on reticular cells results in expression of CCL19/21.
The Role of CCR7 in Autoimmunity and Lymphoid Neogenesis
It has been observed that the absence of CCR7 is directly associated with the onset of spontaneous autoimmunity. This was evaluated in CCR7-deficient mice where lymphocyte infiltration was reported in different peripheral organs along with high auto-antibody titer resulting in IgG deposition in renal glomeruli. Further investigation reported that the emergence of autoimmunity is the product of ineffective negative selection of autoreactive T cells, defective regulatory T cell function, and lack of proper peripheral tolerance maintenance. It was also noticed that CCR7-deficient mice develop lymphoid at sites such as the stomach, lung, and colon; however, it is not exactly known about the extent of ectopic lymphoid structure contribution to autoimmunity establishment and maintenance. In the absence of CCR7, spontaneous lymphoid neogenesis is also witnessed emphasizing the fact that CCR7 is not needed for the process. Tertiary lymphoid structures are also formed due to transgenic expression of CCR7 in the pancreas and thyroid. Furthermore, tertiary lymphoid structure development in different organs is correlated with CCL21 ectopic expression in infection and autoimmunity. This process is hypothesized to be mediated by CCR7 as tertiary lymphoid structures are not formed in CCR7-deficient mice expressing CCL21. The functioning of CCR7 in regulatory T-cells is presented in Figure 3.
FIGURE 3. Functioning of CCR7 in regulatory T-cells. Almost all regulatory T-cells express CCR7 and use it for entering into lymph nodes. These cells homing to the lymph node allow their interaction with the antigens on the dendritic cells. As a result, the regulatory T-cells proliferate and expand upon presentation with the antigen. (A) Interfere with naïve T helper cell proliferation, (B) low number effector cells and inhibits differentiation, (C) regulatory T-cells exert suppressive activities by targeting dendritic cells, and (D) T-cells. (E) naive T cell conversion to regulatory T cells in the presence of low amount of antigens.
The Role of CCR7 in Thymus
The thymus is an important organ that maintains the pool of peripheral T cells. The CCR7 is revealed to be vital for organizing migratory events of cells in the thymus (Bunting et al., 2011). During embryogenesis, the CCL21 is reported to be involved in fetal hematopoietic progenitor recruitment in developing organs (Liu et al., 2005). The statement can be supported by the fact that CCR7-deficient mice are found to have a reduced number of thymocytes (Laan et al., 2009). Studies have also revealed that mouse overexpression of CCX-CKR possesses a low number of hematopoietic precursors in the thymic region (Bunting et al., 2013). The CCL19 and CCL21 in the adult thymus are not restricted to any compartment and are detectable in the medulla and cortex (Kwan and Killeen, 2004). As a result, CCR7 ligands are capable of guiding developed thymocyte migration through thymic compartments (Kwan and Killeen, 2004). The CD4 and CD8 expression in early progenitors is absent, and the cells are referred to as double negative cells (Ceredig and Rolink, 2002). The expression of CCR7 is prominent in the double-negative subpopulation cells (CD44hi CD25int) (Bulati et al., 2014). About fifty percent of these cells express CCR7 reflecting the role of CCR7 in cell migration from cortico–medullary junction (Braun et al., 2011). Recently, the CCR7 role in the translocation of double-positive thymocytes has been studied (Kwan and Killeen, 2004). The CCR7 expression is abundant in single-positive populations (Castro et al., 2014). These cells are found in high concentrations in the medulla. Interestingly, the immature CD4+ single-positive cells express very low CCR7 (Kurobe et al., 2006). On the other hand, immune cells that do not undergo negative selection and are mature produce a high amount of CCR7 (McDonald et al., 2015). Another important role of CCR7 expression is the mature thymocyte positioning near blood vessels prior to leaving the thymus (Kwan and Killeen, 2004). The thymus morphology disruption is the result of central tolerance breakdown and autoimmunity development (Lomada et al., 2007). During T cell production in the thymus, the absence of CCR7 signaling contributes to autoimmunity manifestation in CCR7-deficient mice. Along with this, it is also elucidated that CCR7-deficient mice reported defects in negative selection, which might be due to impaired T cell receptor stimulation, and this further signifies the contribution of CCR7 in central tolerance maintenance (Davalos-Misslitz et al., 2007). The role of CCR7 in the migration of thymocyte is given in Figure 4.
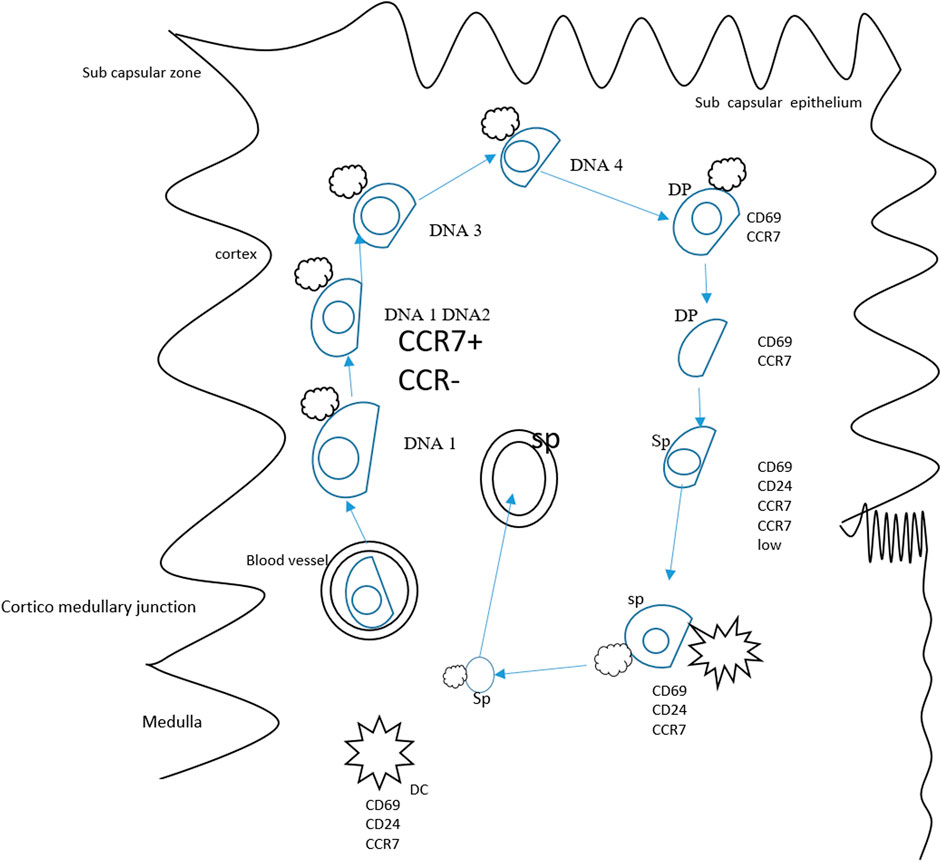
FIGURE 4. Role of CCR7 in the migration of thymocytes. Thymocyte progenitors derived from bone marrow travel to the thymus. As the CD4/8 lacks expression, the cells are known as double-negative cells. The DN1 cells differentiate at the thymic entry site, and transformation to DN2 occurs in the mid cortex. The DN3 thymocyte differentiation happens while cells migrate to the outer cortex and developed into DN4 cells in the sub-capsular zone. The double-negative dendritic cell transition to the double-positive phase is accomplished in reverse migration and the double-positive thymocytes enter the medulla. In the medulla, positively double-positive cells mature and result in the production of CD4+/CD8+. A small population of double-positive cells expresses CCR7 and might drive the migration of double-positive cells to the medulla from the cortex. The CCR7 also plays a key role in mature single positive CD62L cells and guides the maturation of these cells. In this period, thymocytes interact with dendritic cells and medullary and thymic epithelial cells, deleting auto-reactive thymocytes and guiding positive selection.
Role of CCR7 in Tumor Growth and Expansion
CCL19 and CCL21 are mostly expressed during the growth of lymphatic vessels and also in other lymphatic organs (Krishnamurty and Turley, 2020) (Wirsing et al., 2018). Disparate CCL19 and CCL21 bind to glycosaminoglycans (GAGs) and immobilize on endothelial cells (Jørgensen et al., 2021). Remarkably, literature reported that CCR7 stimulation with both CCL21 and CCL19 ligands enhances G-protein activation, migration of cells, signaling pathway of the ERK 1/2, and mobilization of calcium (Rizeq and Malki, 2020). Desensitization of the CCR7 and its activation of ERK are mainly facilitated by β-arrestin, suggesting that the effects of CCL19 may be more transitory than with CCL21 cytokines (van Gastel et al., 2018).
Moreover, semi-mature, CXCR4/CXCL12 expression is directly associated with the directing of cancer cells to the lungs, liver, and lymphatic nodes (Liu et al., 2020). The high-level expression of the CCR7/CCL21 axis has mostly related to metastasis lymph nodes regions, while it also plays a vital role in the progression of several different types of other malignancies, such as breast (Cabioglu et al., 2005), gastro (Mashino et al., 2002), melanoma (Takeuchi et al., 2004), neck (Tsuzuki et al., 2006), lung (Takanami, 2003), hepatocyte (Yang et al., 2018), cervical (Wang et al., 2021), thyroid (Wagner et al., 2008), tonsillar (Takeuchi et al., 2004), colon (Li et al., 2011), and prostate cancers (Berndt et al., 2013) as tabulated in Table 1. In many reported cases of these types of malignant cancers, increased size of tumor and invasions were due to CCR7 (Kodama et al., 2007).
The Role of CCR7 in Cancer Cell Migration
Cellular migration in situ and ex situ is dependent on the biochemical and physical properties of cells. For cells to come out from the blood veins and adhere to the endothelial layer, the chemokines must need to bind with GAGs located in the extracellular matrix (ECM) (Eble and Niland, 2009). There is an electrostatic type of interaction somewhere in the C-terminal region of the chemokine and is positively charged because of lysine and arginine, whereas GAGs possess a negative charge because of the presence of sulfate and carboxylate residues (Severin et al., 2010). Recent research works have reported that body cells can sense the physical and environmental stimuli and respond by altering cellular expression (Kraning-Rush et al., 2012). In addition, chemokines can enhance relocation toward an increasing meditation of a chemo-attractant (Yang et al., 2005). In mature dendritic cells, for example, immobilized CCL21 causes outgrowth of cell and integrin activation, while mobilized CCL19 and CCL21 increase the chemotaxis process (Haessler et al., 2011).
The migration of WBC and CCR7 (+) malignant cells spreading into secondary lymphatic organs is specifically regulated through the interaction of chemokine–chemokine receptors in the environment, and T-cell migration–mediated CCR7-proteins within SLT is very crucial for activation of T-cells in order to generate adaptive immunity (Castriconi et al., 2018). Exploring the migration response of CCR7 proteins coding T-cells within certain types of chemokine environment will facilitate a better understanding of the process of T-cell migration (van der Woude et al., 2017). A function examination of CCR7 in chemotaxis cells may also be helpful in understanding its function in cancer spreading (Wu et al., 2009).
CCR7 is of particular attention in understanding metastasis due to CD4 positive T-cells and dendritic cells needing expression of CCR7 to migrate with the lymphatic tissue (Roberts et al., 2016). The function of lymphatic organs as the extracellular fluids flow sink; it has been assumed that interstitial fluids flow and CCL21 role in conjunction to monitor the migrating of the cancer cells to lymphatic vessels in the development of metastases of cancer (Angeli and Randolph, 2006).
Several studies have revealed that CCL19 and CCL21 can vigorously drive the chemotaxis migration of CCR7-expressing tumor cells (Kabelitz and Wesch, 2003). Furthermore, CCL21 has also been observed to provoke the production of new lymphoid-like structures (Pitzalis et al., 2014). But, the function of CCL21 throughout tumor progression time remains slightly debatable. CCL21 is one of the effective chemo-attractant for tumor-penetrating white blood cells (Rizeq and Malki, 2020). The latest clinical research study described an increased outcome related to increased infiltration of CCR7 (+) T lymphocytes in advanced colon cell carcinoma (Banerje e et al., 2021). In stomach cancer expression of CCR7, early tumor cells were investigated as the most significant component in the determination of lymph node metastasis in cancers (Nagasawa et al., 2021).
CCR7 and Angiogenesis
CCR7 has also been linked to the formation of a new lymphoid vessel in breast carcinoma patient samples, but the actual mechanism is still unknown (Leong et al., 2021). This lymphoid angiogenesis is mainly facilitated by VEGF-C and the receptor of VEGFR-3 (Angeli and Randolph, 2006). Certainly, the high-level expression of this growth factor is well-reported in increased lymphoid node metastasis type of cancer (Ray and Cleary, 2017). Remarkably, there are several other types of reported studies signifying that each time cancer cells express CCL21 and increase the level of white blood cell recruitment in a specific subpopulation of T–cells CD8 positive and dendritic cells (Rizeq and Malki, 2020).
CCR7 as a Potential Drug Target
The transmembrane protein CCR7 is correlated with the spread of cancer to the lymph nodes in colon cancer and thus considered a beneficial therapeutic target (Salem et al., 2021). The structure of CCR7 attached to allosteric antagonist Cmp2105 was explained by Jaeger, Bruenle, and their colleagues (Jaeger et al., 2019). The CCR7 was fused with the 52.8 kDa; protein sialidase NanA to ensure its crystallization, and the crystals were distributed to a resolution of 2.1 Å. Cmp2105 was added to the CCR7 which made it more stabilized, and the IC50 of Cmp2105 in membrane-based competition assays was measured by radiolabeled CCL19, and its measured value was 35 NM. Surprisingly, the structure showed that Cmp2105 was found inside an intracellular space at the ends of transmembrane (TM) helices. As compared to CX3CL1 and CCR2, Cmp2105 stabilizes an inactive confirmation of CCR7. The similarity search of the 3-D model of 2.3 million compounds using Cmp2105 resulted in the finding that there were 293 compounds with similar pharmacophores to the Cmp2105. The thermal stability assays identified the top two best matches. One of these two was navarixin, which is also called SCH-527123, and MK-7123 antagonist, which shows larger efficacy and solubility. As navarixin has noticeable antimetastatic activity in colon cancer, soCZzmer, and some other cancers, therefore it is now in phase II clinical trials. Because of this observed antagonistic activity, there is the possibility of navarixin being utilized for preventing metastasis, which may likely contribute to the CCR7 antagonism mechanism. Furthermore, this study of CCR7 attached to an antagonist may provide a good platform for additional investigation of some available CCR7 antagonists.
CCR7 as a Prognostic Marker
Different research reported CCR7 as a cancer marker, but its effects on the OS of cancer patients are still unknown because different studies have shown distinguished results even in the same type of tumor in different patients, for example, rectal cancer and lung cancer. It is also reported that CCR7 has no notable effects on OS in other tumor types such as gastric cancer and breast cancer and SCCHN (Salem et al., 2021). This study found that the association between CCR7 and the diagnosis of several tumors has not been explained and reviewed yet. So, they conducted a meta-analysis to issue valid medical resources on the diagnostic value of CCR7. This meta-analysis included 30 studies in which there were 3,413 patients having 15 different types of tumors. The conducted meta-analysis showed that higher expression of CCR7 can independently be used as an indicator of poorer OS in patients having a tumor. Increased level of CCR7 was also correlated with the worst PFS; but there was no evidence to detect the association of CCR7 with DFS, RFS, and DSS. To investigate the prognostic value of CCR7 in other tumors, further investigation of the subgroup for overall survival (OS) values was performed and because of limited available data, the subgroup analysis for other values was not performed. The results showed that upregulation of CCR7 magnificently lowered the OS of esophageal and gastric tumors patients. Furthermore, the overexpression of CCR7 indicated poor OS in patients having breast cancer, but this prediction was not significant. Over CCR7 expression in patients with lung cancer predicted an association with the best diagnosis. The numbers of samples were not sufficient, which makes the results insignificant and that was of course one of the limitations of the study. Another factor was the negative prognostic factor in patients having tumors in the urogenital system and digestive system. Due to the limited sample size, the association between expressing CCR7 and tumor prognosis is considered not convincing, which can be improved by enlarging the sample size and some further analysis of the association of CCR7 with the clinical prognostic values.
Apart from CCR7 as a prognostic marker in cancer, there were some shortcomings of the work. First, the number of samples was not sufficient; second, CCR7 expression cutoff values were not the same in all studies, which can decrease the efficacy of the results; and third, the HR values were obtained from survival curves which can produce a statistical error. Significant heterogeneity was shown in this meta-analysis and that could be considered in different important factors, for example, type of tumor, method of analysis, the source of the sample, and cutoff value.
The results of this meta-analysis suggested that in some types of tumors, the overexpression of CCR7 is correlated to the worst prognosis of tumor patients (Zu et al., 2019). Though the predictions show that in lung cancer and colon cancer, the CCR7 expression is related to prognosis, but these results need to be improved (Günther et al., 2005). It is concluded that CCR7 is a good indicator in tumors, and these results should be considered carefully.
Conclusion
The CCR7 and its ligands have received great attention in recent times due to their versatile functioning in regulating leukocyte function during immunological responses. The chemokine ability to convey signals that are remarkably versatile and specific makes them powerful modulators of immunological responses against diverse antigens. Considering the importance of CCR7, in this review, we seek to address the importance of CCR7 in immune cell regulation, lymph node homing, immune tolerance, different types of cancer, and CCR7 as a therapeutic and prognostic marker. The literature reported herein might attract the readers for expanding their knowledge of chemokines and a better approach to novel therapeutics in the near future.
Author Contributions
FA planned this study, gathered the data, and prepared the whole review.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The researcher would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
References
Al-Jokhadar, M., Al-Mandily, A., Zaid, Kh., and Azar Maalouf, E. (2017). CCR7 and CXCR4 Expression in Primary Head and Neck Squamous Cell Carcinomas and Nodal Metastases – a Clinical and Immunohistochemical Study. Asian Pac J. Cancer Prev. 18, 1093–1104. doi:10.22034/APJCP.2017.18.4.1093
Alvarez, D., Vollmann, E. H., and von Andrian, U. H. (2008). Mechanisms and Consequences of Dendritic Cell Migration. Immunity 29, 325–342. doi:10.1016/j.immuni.2008.08.006
Angeli, V., and Randolph, G. J. (2006). Inflammation, Lymphatic Function, and Dendritic Cell Migration. Lymphatic Res. Biol. 4, 217–228. doi:10.1089/lrb.2006.4406
Arnold, C. N., Campbell, D. J., Lipp, M., and Butcher, E. C. (2007). The Germinal Center Response Is Impaired in the Absence of T Cell-Expressed CXCR5. Eur. J. Immunol. 37, 100–109. doi:10.1002/eji.200636486
Banerjee, A., Li, D., Guo, Y., Mahgoub, B., Paragas, L., Slobin, J., et al. (2021). Retargeting IL-2 Signaling to NKG2D-Expressing Tumor-Infiltrating Leukocytes Improves Adoptive Transfer Immunotherapy. J. Immunol. 207 (1), 333–343. doi:10.4049/jimmunol.2000926
Bayry, J., Triebel, F., Kaveri, S. V., and Tough, D. F. (2007). Human Dendritic Cells Acquire a Semimature Phenotype and Lymph Node Homing Potential through Interaction with CD4+CD25+ Regulatory T Cells. J. Immunol. 178, 4184–4193. doi:10.4049/jimmunol.178.7.4184
Berndt, B., Haverkampf, S., Reith, G., Keil, S., Niggemann, B., Zänker, K. S., et al. (2013). Fusion of CCL21 Non-migratory Active Breast Epithelial and Breast Cancer Cells Give Rise to CCL21 Migratory Active Tumor Hybrid Cell Lines. PLoS One 8, e63711. doi:10.1371/journal.pone.0063711
Braun, A., Worbs, T., Moschovakis, G. L., Halle, S., Hoffmann, K., Bölter, J., et al. (2011). Afferent Lymph-Derived T Cells and DCs Use Different Chemokine Receptor CCR7-dependent Routes for Entry into the Lymph Node and Intranodal Migration. Nat. Immunol. 12, 879–887. doi:10.1038/ni.2085
Buettner, M., and Bode, U. (2012). Lymph Node Dissection - Understanding the Immunological Function of Lymph Nodes. Clin.& Exp. Immunol. 169, 205–212. doi:10.1111/j.1365-2249.2012.04602.x
Bulati, M., Buffa, S., Martorana, A., Candore, G., Lio, D., Caruso, C., et al. (2014). Trafficking Phenotype and Production of Granzyme B by Double Negative B Cells (IgG+IgD−CD27−) in the Elderly. Exp. Gerontol. 54, 123–129. doi:10.1016/j.exger.2013.12.011
Bunting, M. D., Comerford, I., and McColl, S. R. (2011). Finding Their Niche: Chemokines Directing Cell Migration in the Thymus. Immunol. Cell. Biol. 89, 185–196. doi:10.1038/icb.2010.142
Bunting, M. D., Comerford, I., Seach, N., Hammett, M. V., Asquith, D. L., Körner, H., et al. (2013). CCX-CKR Deficiency Alters Thymic Stroma Impairing Thymocyte Development and Promoting Autoimmunity. Blood, J. Am. Soc. Hematol. 121, 118–128. doi:10.1182/blood-2012-06-434886
Cabioglu, N., Yazici, M. S., Arun, B., Broglio, K. R., Hortobagyi, G. N., Price, J. E., et al. (2005). CCR7 and CXCR4 as Novel Biomarkers Predicting Axillary Lymph Node Metastasis in T1Breast Cancer. Clin. Cancer Res. 11, 5686–5693. doi:10.1158/1078-0432.ccr-05-0014
Castriconi, R., Carrega, P., Dondero, A., Bellora, F., Casu, B., Regis, S., et al. (2018). Molecular Mechanisms Directing Migration and Retention of Natural Killer Cells in Human Tissues. Front. Immunol. 9, 2324. doi:10.3389/fimmu.2018.02324
Castro, R., Bromage, E., Abós, B., Pignatelli, J., González Granja, A., Luque, A., et al. (2014). CCR7 Is Mainly Expressed in Teleost Gills, where it Defines an IgD+IgM− B Lymphocyte Subset. J. I. 192, 1257–1266. doi:10.4049/jimmunol.1302471
Ceredig, R., and Rolink, T. (2002). A Positive Look at Double-Negative Thymocytes. Nat. Rev. Immunol. 2, 888–897. doi:10.1038/nri937
Chiesa, M. D., Vitale, M., Carlomagno, S., Ferlazzo, G., Moretta, L., and Moretta, A. (2003). The Natural Killer Cell-Mediated Killing of Autologous Dendritic Cells Is Confined to a Cell Subset Expressing CD94/NKG2A, but Lacking Inhibitory Killer Ig-like Receptors. Eur. J. Immunol. 33, 1657–1666. doi:10.1002/eji.200323986
Cools, N., Ponsaerts, P., Van Tendeloo, V. F. I., and Berneman, Z. N. (2007). Balancing between Immunity and Tolerance: an Interplay between Dendritic Cells, Regulatory T Cells, and Effector T Cells. J. Leukoc. Biol. 82, 1365–1374. doi:10.1189/jlb.0307166
Cuesta-Mateos, C., Brown, J. R., Terrón, F., and Muñoz-Calleja, C. (2021). Of Lymph Nodes and CLL Cells: Deciphering the Role of CCR7 in the Pathogenesis of CLL and Understanding its Potential as Therapeutic Target. Front. Immunol. 12, 927. doi:10.3389/fimmu.2021.662866
Cutler, C. W., and Jotwani, R. (2004). Antigen-presentation and the Role of Dendritic Cells in Periodontitis. Periodontol. 2000 35, 135–157. doi:10.1111/j.0906-6713.2004.003560.x
Dai, Y., Tong, R., Guo, H., Yu, T., and Wang, C. (2017). Association of CXCR4, CCR7, VEGF-C and VEGF-D Expression with Lymph Node Metastasis in Patients with Cervical Cancer. Eur. J. Obstetrics Gynecol. Reproductive Biol. 214, 178–183. doi:10.1016/j.ejogrb.2017.04.043
Davalos-Misslitz, A. C. M., Worbs, T., Willenzon, S., Bernhardt, G., and Förster, R. (2007). Impaired Responsiveness to T-Cell Receptor Stimulation and Defective Negative Selection of Thymocytes in CCR7-Deficient Mice. Blood, J. Am. Soc. Hematol. 110, 4351–4359. doi:10.1182/blood-2007-01-070284
del Rio, M.-L., Rodriguez-Barbosa, J.-I., Kremmer, E., and Förster, R. (2007). CD103− and CD103+ Bronchial Lymph Node Dendritic Cells Are Specialized in Presenting and Cross-Presenting Innocuous Antigen to CD4+ and CD8+ T Cells. J. Immunol. 178, 6861–6866. doi:10.4049/jimmunol.178.11.6861
Denton, A. E., Roberts, E. W., Linterman, M. A., and Fearon, D. T. (2014). Fibroblastic Reticular Cells of the Lymph Node Are Required for Retention of Resting but Not Activated CD8 + T Cells. Proc. Natl. Acad. Sci. U.S.A. 111, 12139–12144. doi:10.1073/pnas.1412910111
Du, H., Zhang, L., Li, G., Liu, W., Tang, W., Zhang, H., et al. (2019). CXCR4 and CCR7 Expression in Primary Nodal Diffuse Large B-Cell Lymphoma-A Clinical and Immunohistochemical Study. Am. J. Med. Sci. 357, 302–310. doi:10.1016/j.amjms.2019.01.008
Du, P., Liu, Y., Ren, H., Zhao, J., Zhang, X., Patel, R., et al. (2017). Expression of Chemokine Receptor CCR7 Is a Negative Prognostic Factor for Patients with Gastric Cancer: a Meta-Analysis. Gastric Cancer 20, 235–245. doi:10.1007/s10120-016-0602-8
Ebert, L. M., Schaerli, P., and Moser, B. (2005). Chemokine-mediated Control of T Cell Traffic in Lymphoid and Peripheral Tissues. Mol. Immunol. 42, 799–809. doi:10.1016/j.molimm.2004.06.040
Eble, J., and Niland, S. (2009). The Extracellular Matrix of Blood Vessels. Cpd 15, 1385–1400. doi:10.2174/138161209787846757
Eisenbarth, S. C., Baumjohann, D., Craft, J., Fazilleau, N., Ma, C. S., Tangye, S. G., et al. (2021). CD4+ T Cells that Help B Cells-Aa Proposal for Uniform Nomenclature. Trends Immunol. 42 (8), 658–669. doi:10.1016/j.it.2021.06.003
Förster, R., Braun, A., and Worbs, T. (2012). Lymph Node Homing of T Cells and Dendritic Cells via Afferent Lymphatics. Trends Immunol. 33, 271–280. doi:10.1016/j.it.2012.02.007
Förster, R., Davalos-Misslitz, A. C., and Rot, A. (2008). CCR7 and its Ligands: Balancing Immunity and Tolerance. Nat. Rev. Immunol. 8, 362–371. doi:10.1038/nri2297
Gao, L., Xu, J., He, G., Huang, J., Xu, W., Qin, J., et al. (2019). CCR7 High Expression Leads to Cetuximab Resistance by Cross-Talking with EGFR Pathway in PI3K/AKT Signals in Colorectal Cancer. Am. J. Cancer Res. 9, 2531–2543.
Garcia, G., Godot, V., and Humbert, M. (2005). New Chemokine Targets for Asthma Therapy. Curr. Allergy Asthma Rep. 5, 155–160. doi:10.1007/s11882-005-0090-0
Griffith, J. W., Sokol, C. L., and Luster, A. D. (2014). Chemokines and Chemokine Receptors: Positioning Cells for Host Defense and Immunity. Annu. Rev. Immunol. 32, 659–702. doi:10.1146/annurev-immunol-032713-120145
Groom, J. R., Richmond, J., Murooka, T. T., Sorensen, E. W., Sung, J. H., Bankert, K., et al. (2012). CXCR3 Chemokine Receptor-Ligand Interactions in the Lymph Node Optimize CD4+ T Helper 1 Cell Differentiation. Immunity 37, 1091–1103. doi:10.1016/j.immuni.2012.08.016
Günther, K., Leier, J., Henning, G., Dimmler, A., Weissbach, R., Hohenberger, W., et al. (2005). Prediction of Lymph Node Metastasis in Colorectal Carcinoma by Expressionof Chemokine Receptor CCR7. Int. J. cancer 116, 726–733. doi:10.1002/ijc.21123
Haessler, U., Pisano, M., Wu, M., and Swartz, M. A. (2011). Dendritic Cell Chemotaxis in 3D under Defined Chemokine Gradients Reveals Differential Response to Ligands CCL21 and CCL19. Proc. Natl. Acad. Sci. U.S.A. 108, 5614–5619. doi:10.1073/pnas.1014920108
Hardtke, S., Ohl, L., and Förster, R. (2005). Balanced Expression of CXCR5 and CCR7 on Follicular T Helper Cells Determines Their Transient Positioning to Lymph Node Follicles and Is Essential for Efficient B-Cell Help. Blood 106, 1924–1931. doi:10.1182/blood-2004-11-4494
Hauser, M. A., and Legler, D. F. (2016). Common and Biased Signaling Pathways of the Chemokine Receptor CCR7 Elicited by its Ligands CCL19 and CCL21 in Leukocytes. J. Leukoc. Biol. 99, 869–882. doi:10.1189/jlb.2mr0815-380r
Hendry, S., Salgado, R., Gevaert, T., Russell, P. A., John, T., Thapa, B., et al. (2017). Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors: A Practical Review for Pathologists and Proposal for a Standardized Method from the International Immuno-Oncology Biomarkers Working Group: Part 2: TILs in Melanoma, Gastrointestinal Tract Carcinomas, Non-small Cell Lung Carcinoma and Mesothelioma, Endometrial and Ovarian Carcinomas, Squamous Cell Carcinoma of the Head and Neck, Genitourinary Carcinomas, and Primary Brain Tumors. Adv. Anat. Pathol. 24, 311–335. doi:10.1097/pap.0000000000000161
Hintzen, G., Ohl, L., del Rio, M.-L., Pabst, O., Kocks, J. R., et al. (2006). Induction of Tolerance to Innocuous Inhaled Antigen Relies on a CCR7-dependent Dendritic Cell-Mediated Antigen Transport to the Bronchial Lymph Node. J. Immunol. 177, 7346–7354. doi:10.4049/jimmunol.177.10.7346
Hughes, C. E., and Nibbs, R. J. B. (2018). A Guide to Chemokines and Their Receptors. FEBS J. 285, 2944–2971. doi:10.1111/febs.14466
Irino, T., Takeuchi, H., Matsuda, S., Saikawa, Y., Kawakubo, H., Wada, N., et al. (2014). CC-chemokine Receptor CCR7: a Key Molecule for Lymph Node Metastasis in Esophageal Squamous Cell Carcinoma. BMC Cancer 14, 291–298. doi:10.1186/1471-2407-14-291
Jaeger, K., Bruenle, S., Weinert, T., Guba, W., Muehle, J., Miyazaki, T., et al. (2019). Structural Basis for Allosteric Ligand Recognition in the Human CC Chemokine Receptor 7. Cell. 178, 1222–1230. doi:10.1016/j.cell.2019.07.028
Jang, M. H., Sougawa, N., Tanaka, T., Hirata, T., Hiroi, T., Tohya, K., et al. (2006). CCR7 Is Critically Important for Migration of Dendritic Cells in Intestinal Lamina Propria to Mesenteric Lymph Nodes. J. Immunol. 176, 803–810. doi:10.4049/jimmunol.176.2.803
Johnson, L. A., and Jackson, D. G. (2010). Inflammation-induced Secretion of CCL21 in Lymphatic Endothelium Is a Key Regulator of Integrin-Mediated Dendritic Cell Transmigration. Int. Immunol. 22, 839–849. doi:10.1093/intimm/dxq435
Jørgensen, A. S., Brandum, E. P., Mikkelsen, J. M., Orfin, K. A., Boilesen, D. R., Egerod, K. L., et al. (2021). The C-Terminal Peptide of CCL21 Drastically Augments CCL21 Activity through the Dendritic Cell Lymph Node Homing Receptor CCR7 by Interaction with the Receptor N-Terminus. Cell. Mol. Life Sci. 78, 6963–6978. doi:10.1007/s00018-021-03930-7
Junt, T., Scandella, E., and Ludewig, B. (2008). Form Follows Function: Lymphoid Tissue Microarchitecture in Antimicrobial Immune Defence. Nat. Rev. Immunol. 8, 764–775. doi:10.1038/nri2414
Kabelitz, D., and Wesch, D. (2003). Features and Functions of Gamma Delta T Lymphocytes: Focus on Chemokines and Their Receptors. Crit. Rev. Immunol. 23, 339–370. doi:10.1615/critrevimmunol.v23.i56.10
Katagiri, K., Ohnishi, N., Kabashima, K., Iyoda, T., Takeda, N., Shinkai, Y., et al. (2004). Crucial Functions of the Rap1 Effector Molecule RAPL in Lymphocyte and Dendritic Cell Trafficking. Nat. Immunol. 5, 1045–1051. doi:10.1038/ni1111
Kodama, J., Hasengaowa, T., Kusumoto, T., Seki, N., Matsuo, T., Ojima, Y., et al. (2007). Association of CXCR4 and CCR7 Chemokine Receptor Expression and Lymph Node Metastasis in Human Cervical Cancer. Ann. Oncol. 18, 70–76. doi:10.1093/annonc/mdl342
Kondo, K., Ohigashi, I., and Takahama, Y. (2019). Thymus Machinery for T-Cell Selection. Int. Immunol. 31, 119–125. doi:10.1093/intimm/dxy081
Kraning-Rush, C. M., Califano, J. P., and Reinhart-King, C. A. (2012). Cellular Traction Stresses Increase with Increasing Metastatic Potential. PLoS One 7, e32572. doi:10.1371/journal.pone.0032572
Krishnamurty, A. T., and Turley, S. J. (2020). Lymph Node Stromal Cells: Cartographers of the Immune System. Nat. Immunol. 21, 369–380. doi:10.1038/s41590-020-0635-3
Krummel, M. F., Bartumeus, F., and Gérard, A. (2016). T Cell Migration, Search Strategies and Mechanisms. Nat. Rev. Immunol. 16, 193–201. doi:10.1038/nri.2015.16
Kurobe, H., Liu, C., Ueno, T., Saito, F., Ohigashi, I., Seach, N., et al. (2006). CCR7-dependent Cortex-To-Medulla Migration of Positively Selected Thymocytes Is Essential for Establishing Central Tolerance. Immunity 24, 165–177. doi:10.1016/j.immuni.2005.12.011
Kwan, J., and Killeen, N. (2004). CCR7 Directs the Migration of Thymocytes into the Thymic Medulla. J. Immunol. 172, 3999–4007. doi:10.4049/jimmunol.172.7.3999
Kwiecień, I., Polubiec-Kownacka, M., Dziedzic, D., Wołosz, D., Rzepecki, P., and Domagała-Kulawik, J. (2019). CD163 and CCR7 as Markers for Macrophage Polarization in Lung Cancer Microenvironment. Cent. J. Immunol. 44, 395.
Laan, M., Kisand, K., Kont, V., Möll, K., Tserel, L., Scott, H. S., et al. (2009). Autoimmune Regulator Deficiency Results in Decreased Expression of CCR4 and CCR7 Ligands and in Delayed Migration of CD4+Thymocytes. J. Immunol. 183, 7682–7691. doi:10.4049/jimmunol.0804133
Legler, D. F., Uetz-von Allmen, E., and Hauser, M. A. (2014). CCR7: Roles in Cancer Cell Dissemination, Migration and Metastasis Formation. Int. J. Biochem. Cell. Biol. 54, 78–82. doi:10.1016/j.biocel.2014.07.002
León, B., López-Bravo, M., and Ardavin, C. (2005). Monocyte-derived Dendritic Cells. Proc. Seminars Immunol. 17, 313–318. doi:10.1016/j.smim.2005.05.013
Leong, S. P., Naxerova, K., Keller, L., Pantel, K., and Witte, M. (2021). Molecular Mechanisms of Cancer Metastasis via the Lymphatic versus the Blood Vessels. Clin. \& Exp. Metastasis, 1–21. doi:10.1007/s10585-021-10120-z
Li, J., Sun, R., Tao, K., and Wang, G. (2011). The CCL21/CCR7 Pathway Plays a Key Role in Human Colon Cancer Metastasis through Regulation of Matrix Metalloproteinase-9. Dig. Liver Dis. 43, 40–47. doi:10.1016/j.dld.2010.05.013
Li, W., Xue, W., Wang, X., Fu, X., Sun, Z., Li, Z., et al. (2018). MiR-199a Mediated the Dissemination of Human Mantle Cell Lymphoma by Interacting with the CCR7/CCL21 Pair. Anticancer. Drugs 29, 861–870. doi:10.1097/cad.0000000000000656
Li, X., Sun, S., Li, N., Gao, J., Yu, J., Zhao, J., et al. (2017). High Expression of CCR7 Predicts Lymph Node Metastasis and Good Prognosis in Triple Negative Breast Cancer. Cell. Physiol. biochem. 43, 531–539. doi:10.1159/000480526
Link, A., Hardie, D. L., Favre, S., Britschgi, M. R., Adams, D. H., Sixt, M., et al. (2011). Association of T-Zone Reticular Networks and Conduits with Ectopic Lymphoid Tissues in Mice and Humans. Am. J. Pathology 178, 1662–1675. doi:10.1016/j.ajpath.2010.12.039
Link, A., Vogt, T. K., Favre, S., Britschgi, M. R., Acha-Orbea, H., Hinz, B., et al. (2007). Fibroblastic Reticular Cells in Lymph Nodes Regulate the Homeostasis of Naive T Cells. Nat. Immunol. 8, 1255–1265. doi:10.1038/ni1513
Lipscomb, M. F., and Masten, B. J. (2002). Dendritic Cells: Immune Regulators in Health and Disease. Physiol. Rev. 82 (1), 97–130. doi:10.1152/physrev.00023.2001
Liu, C., Ueno, T., Kuse, S., Saito, F., Nitta, T., Piali, L., et al. (2005). The Role of CCL21 in Recruitment of T-Precursor Cells to Fetal Thymi. Blood 105, 31–39. doi:10.1182/blood-2004-04-1369
Liu, H., Cheng, Q., Xu, D. S., Wang, W., Fang, Z., Xue, D. D., et al. (2020). Overexpression of CXCR7 Accelerates Tumor Growth and Metastasis of Lung Cancer Cells. Respir. Res. 21, 287–313. doi:10.1186/s12931-020-01518-6
Liu, M.-D., Wu, H., Wang, S., Pang, P., Jin, S., Sun, C.-F., et al. (2018). MiR-1275 Promotes Cell Migration, Invasion and Proliferation in Squamous Cell Carcinoma of Head and Neck via Up-Regulating IGF-1R and CCR7. Gene 646, 1–7. doi:10.1016/j.gene.2017.12.049
Lomada, D., Liu, B., Coghlan, L., Hu, Y., and Richie, E. R. (2007). Thymus Medulla Formation and Central Tolerance Are Restored in IKKα−/− Mice that Express an IKKα Transgene in Keratin 5+ Thymic Epithelial Cells. J. Immunol. 178, 829–837. doi:10.4049/jimmunol.178.2.829
Lombry, C., Marteleur, A., Arras, M., Lison, D., Louahed, J., Renauld, J.-C., et al. (2004). Local and Systemic Immune Responses to Intratracheal Instillation of Antigen and DNA Vaccines in Mice. Pharm. Res. 21, 127–135. doi:10.1023/b:pham.0000012160.00222.55
Macpherson, A. J., McCoy, K. D., Johansen, F.-E., and Brandtzaeg, P. (2008). The Immune Geography of IgA Induction and Function. Mucosal Immunol. 1, 11–22. doi:10.1038/mi.2007.6
Maghazachi, A. A. (2005). Insights into Seven and Single Transmembrane-Spanning Domain Receptors and Their Signaling Pathways in Human Natural Killer Cells. Pharmacol. Rev. 57, 339–357. doi:10.1124/pr.57.3.5
Makino, T., Izumi, K., Maolake, A., Natsagdorj, A., Iwamoto, H., Kadomoto, S., et al. (2019). Tumor Necrosis Factor-$α$ Upregulation of CCR7 Induces Prostate Cancer Cell Migration in Lymphatic Metastasis.
Mao, F.-y., Kong, H., Zhao, Y.-l., Peng, L.-s., Chen, W., Zhang, J.-y., et al. (2017). Increased Tumor-Infiltrating CD45RA−CCR7− Regulatory T-Cell Subset with Immunosuppressive Properties Foster Gastric Cancer Progress. Cell. Death Dis. 8, e3002. doi:10.1038/cddis.2017.388
Mashino, K., Sadanaga, N., Yamaguchi, H., Tanaka, F., Ohta, M., Shibuta, K., et al. (2002). Expression of Chemokine Receptor CCR7 Is Associated with Lymph Node Metastasis of Gastric Carcinoma. Cancer Res. 62, 2937–2941.
McDonald, B. D., Bunker, J. J., Erickson, S. A., Oh-Hora, M., and Bendelac, A. (2015). Crossreactive αβ T Cell Receptors Are the Predominant Targets of Thymocyte Negative Selection. Immunity 43, 859–869. doi:10.1016/j.immuni.2015.09.009
McKenna, K., Beignon, A.-S., and Bhardwaj, N. (2005). Plasmacytoid Dendritic Cells: Linking Innate and Adaptive Immunity. J. Virol. 79, 17–27. doi:10.1128/jvi.79.1.17-27.2005
Miyasaka, M., and Tanaka, T. (2004). Lymphocyte Trafficking across High Endothelial Venules: Dogmas and Enigmas. Nat. Rev. Immunol. 4, 360–370. doi:10.1038/nri1354
Mo, M., Zhou, M., Wang, L., Qi, L., Zhou, K., Liu, L.-F., et al. (2015). CCL21/CCR7 Enhances the Proliferation, Migration, and Invasion of Human Bladder Cancer T24 Cells. PLoS One 10, e0119506. doi:10.1371/journal.pone.0119506
Moretta, A., Marcenaro, E., Parolini, S., Ferlazzo, G., and Moretta, L. (2008). NK Cells at the Interface between Innate and Adaptive Immunity. Cell. Death Differ. 15, 226–233. doi:10.1038/sj.cdd.4402170
Mori, S., Nakano, H., Aritomi, K., Wang, C.-R., Gunn, M. D., and Kakiuchi, T. (2001). Mice Lacking Expression of the Chemokines CCL21-Ser and CCL19 (Plt Mice) Demonstrate Delayed but Enhanced T Cell Immune Responses. J. Exp. Med. 193, 207–218. doi:10.1084/jem.193.2.207
Müller, G., Höpken, U. E., and Lipp, M. (2003). The Impact of CCR7 and CXCR5 on Lymphoid Organ Development and Systemic Immunity. Immunol. Rev. 195, 117–135.
Nagasawa, S., Tsuchida, K., Shiozawa, M., Hiroshima, Y., Kimura, Y., Hashimoto, I., et al. (2021). Clinical Significance of Chemokine Receptor CXCR4 and CCR7 mRNA Expression in Patients with Colorectal Cancer. Anticancer Res. 41, 4489–4495. doi:10.21873/anticanres.15259
Nishikawa, H., and Sakaguchi, S. (2010). Regulatory T Cells in Tumor Immunity. Int. J. cancer 127, 759–767. doi:10.1002/ijc.25429
Nolz, J. C., Starbeck-Miller, G. R., and Harty, J. T. (2011). Naive, Effector and Memory CD8 T-Cell Trafficking: Parallels and Distinctions. Immunotherapy 3, 1223–1233. doi:10.2217/imt.11.100
Okada, T., Ngo, V. N., Ekland, E. H., Förster, R., Lipp, M., Littman, D. R., et al. (2002). Chemokine Requirements for B Cell Entry to Lymph Nodes and Peyer's Patches. J. Exp. Med. 196, 65–75. doi:10.1084/jem.20020201
Pitzalis, C., Jones, G. W., Bombardieri, M., and Jones, S. A. (2014). Ectopic Lymphoid-like Structures in Infection, Cancer and Autoimmunity. Nat. Rev. Immunol. 14, 447–462. doi:10.1038/nri3700
Proudfoot, A., Johnson, Z., Bonvin, P., and Handel, T. (2017). Glycosaminoglycan Interactions with Chemokines Add Complexity to a Complex System. Pharmaceuticals 10, 70. doi:10.3390/ph10030070
Qi, H., Egen, J. G., Huang, A. Y. C., and Germain, R. N. (2006). Extrafollicular Activation of Lymph Node B Cells by Antigen-Bearing Dendritic Cells. Science 312, 1672–1676. doi:10.1126/science.1125703
Ray, A., and Cleary, M. P. (2017). The Potential Role of Leptin in Tumor Invasion and Metastasis. Cytokine & Growth Factor Rev. 38, 80–97. doi:10.1016/j.cytogfr.2017.11.002
Rizeq, B., and Malki, M. I. (2020). The Role of CCL21/CCR7 Chemokine axis in Breast Cancer Progression. Cancers 12, 1036. doi:10.3390/cancers12041036
Roberts, E. W., Broz, M. L., Binnewies, M., Headley, M. B., Nelson, A. E., Wolf, D. M., et al. (2016). Critical Role for CD103+/CD141+ Dendritic Cells Bearing CCR7 for Tumor Antigen Trafficking and Priming of T Cell Immunity in Melanoma. Cancer Cell. 30, 324–336. doi:10.1016/j.ccell.2016.06.003
Rot, A. (2010). Chemokine Patterning by Glycosaminoglycans and Interceptors. Front. Biosci. 15, 645–660. doi:10.2741/3638
Salem, A., Alotaibi, M., Mroueh, R., Basheer, H. A., and Afarinkia, K. (2021). CCR7 as a Therapeutic Target in Cancer. Biochim. Biophys. Acta (BBA)-Reviews Cancer 1875, 188499.
Scandella, E., Fink, K., Junt, T., Senn, B. M., Lattmann, E., Förster, R., et al. (2007). Dendritic Cell-independent B Cell Activation during Acute Virus Infection: A Role for Early CCR7-Driven B-T Helper Cell Collaboration. J. Immunol. 178, 1468–1476. doi:10.4049/jimmunol.178.3.1468
Schneider, M. A., Meingassner, J. G., Lipp, M., Moore, H. D., and Rot, A. (2007). CCR7 Is Required for the In Vivo Function of CD4+ CD25+ Regulatory T Cells. J. Exp. Med. 204, 735–745. doi:10.1084/jem.20061405
Seth, S., Oberdörfer, L., Hyde, R., Hoff, K., Thies, V., Worbs, T., et al. (2011). CCR7 Essentially Contributes to the Homing of Plasmacytoid Dendritic Cells to Lymph Nodes under Steady-State and Inflammatory Conditions. J. I. 186, 3364–3372. doi:10.4049/jimmunol.1002598
Seubert, A., Monaci, E., Pizza, M., O’Hagan, D. T., and Wack, A. (2008). The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells. J. Immunol. 180, 5402–5412. doi:10.4049/jimmunol.180.8.5402
Severin, I. C., Gaudry, J.-P., Johnson, Z., Kungl, A., Jansma, A., Gesslbauer, B., et al. (2010). Characterization of the Chemokine CXCL11-Heparin Interaction Suggests Two Different Affinities for Glycosaminoglycans. J. Biol. Chem. 285, 17713–17724. doi:10.1074/jbc.m109.082552
Sharma, N., Benechet, A. P., Lefrancois, L., and Khanna, K. M. (2015). CD8 T Cells Enter the Splenic T Cell Zones Independently of CCR7, but the Subsequent Expansion and Trafficking Patterns of Effector T Cells after Infection Are Dysregulated in the Absence of CCR7 Migratory Cues. J. Immunol. 195, 5227–5236. doi:10.4049/jimmunol.1500993
Smigiel, K. S., Srivastava, S., Stolley, J. M., and Campbell, D. J. (2014). Regulatory T-Cell Homeostasis: Steady-State Maintenance and Modulation during Inflammation. Immunol. Rev. 259, 40–59. doi:10.1111/imr.12170
Steenblock, E. R., Wrzesinski, S. H., Flavell, R. A., and Fahmy, T. M. (2009). Antigen Presentation on Artificial Acellular Substrates: Modular Systems for Flexible, Adaptable Immunotherapy. Expert Opin. Biol. Ther. 9, 451–464. doi:10.1517/14712590902849216
Stockis, J., Roychoudhuri, R., and Halim, T. Y. F. (2019). Regulation of Regulatory T Cells in Cancer. Immunology 157, 219–231. doi:10.1111/imm.13064
Stockwin, L. H., McGONAGLE, D., Martin, I. G., and Blair, G. E. (2000). Dendritic Cells: Immunological Sentinels with a Central Role in Health and Disease. Immunol. Cell. Biol. 78, 91–102. doi:10.1046/j.1440-1711.2000.00888.x
Takanami, I. (2003). Overexpression of CCR7 mRNA in Nonsmall Cell Lung Cancer: Correlation with Lymph Node Metastasis. Int. J. cancer 105, 186–189. doi:10.1002/ijc.11063
Takeuchi, H., Fujimoto, A., Tanaka, M., Yamano, T., Hsueh, E., and Hoon, D. S. B. (2004). CCL21 Chemokine Regulates Chemokine Receptor CCR7 Bearing Malignant Melanoma Cells. Clin. cancer Res. 10, 2351–2358. doi:10.1158/1078-0432.ccr-03-0195
Tian, W. J., Feng, P. H., Wang, J., Yan, T., Qin, Q. F., Li, D. L., et al. (2021). CCR7 Has Potential to Be a Prognosis Marker for Cervical Squamous Cell Carcinoma and an Index for Tumor Microenvironment Change. Front. Mol. Biosci. 8, 583028. doi:10.3389/fmolb.2021.583028
Tsuzuki, H., Takahashi, N., Kojima, A., Narita, N., Sunaga, H., Takabayashi, T., et al. (2006). Oral and Oropharyngeal Squamous Cell Carcinomas Expressing CCR7 Have Poor Prognoses. Auris Nasus Larynx 33, 37–42. doi:10.1016/j.anl.2005.07.019
Tzelepis, F., Persechini, P. M., and Rodrigues, M. M. (2007). Modulation of CD4+ T Cell-dependent Specific Cytotoxic CD8+ T Cells Differentiation and Proliferation by the Timing of Increase in the Pathogen Load. PLoS One 2, e393. doi:10.1371/journal.pone.0000393
van der Woude, L. L., Gorris, M. A. J., Halilovic, A., Figdor, C. G., and de Vries, I. J. M. (2017). Migrating into the Tumor: a Roadmap for T Cells. Trends cancer 3, 797–808. doi:10.1016/j.trecan.2017.09.006
van Gastel, J., Hendrickx, J. O., Leysen, H., Santos-Otte, P., Luttrell, L. M., Martin, B., et al. (2018). β-Arrestin Based Receptor Signaling Paradigms: Potential Therapeutic Targets for Complex Age-Related Disorders. Front. Pharmacol. 9, 1369. doi:10.3389/fphar.2018.01369
Wagner, P. L., Moo, T.-A., Arora, N., Liu, Y.-F., Zarnegar, R., Scognamiglio, T., et al. (2008). The Chemokine Receptors CXCR4 and CCR7 Are Associated with Tumor Size and Pathologic Indicators of Tumor Aggressiveness in Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 15, 2833–2841. doi:10.1245/s10434-008-0064-2
Wang, Q., Zou, H., Wang, Y., Shang, J., Yang, L., and Shen, J. (2021). CCR7-CCL21 axis Promotes the Cervical Lymph Node Metastasis of Tongue Squamous Cell Carcinoma by Up-Regulating MUC1. J. Cranio-Maxillofacial Surg. 49, 562–569. doi:10.1016/j.jcms.2021.02.027
Wei, S. C., Duffy, C. R., and Allison, J. P. (2018). Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 8, 1069–1086. doi:10.1158/2159-8290.cd-18-0367
Weninger, W., Carlsen, H. S., Goodarzi, M., Moazed, F., Crowley, M. A., Baekkevold, E. S., et al. (2003). Naive T Cell Recruitment to Nonlymphoid Tissues: a Role for Endothelium-Expressed CC Chemokine Ligand 21 in Autoimmune Disease and Lymphoid Neogenesis. J. Immunol. 170, 4638–4648. doi:10.4049/jimmunol.170.9.4638
Wirsing, A. M., Ervik, I. K., Seppola, M., Uhlin-Hansen, L., Steigen, S. E., and Hadler-Olsen, E. (2018). Presence of High-Endothelial Venules Correlates with a Favorable Immune Microenvironment in Oral Squamous Cell Carcinoma. Mod. Pathol. 31, 910–922. doi:10.1038/s41379-018-0019-5
Worbs, T., Bode, U., Yan, S., Hoffmann, M. W., Hintzen, G., Bernhardt, G., et al. (2006). Oral Tolerance Originates in the Intestinal Immune System and Relies on Antigen Carriage by Dendritic Cells. J. Exp. Med. 203, 519–527. doi:10.1084/jem.20052016
Worbs, T., and Förster, R. (2007). A Key Role for CCR7 in Establishing Central and Peripheral Tolerance. Trends Immunol. 28, 274–280. doi:10.1016/j.it.2007.04.002
Worbs, T., Mempel, T. R., Bölter, J., von Andrian, U. H., and Förster, R. (2007). CCR7 Ligands Stimulate the Intranodal Motility of T Lymphocytes In Vivo. J. Exp. Med. 204, 489–495. doi:10.1084/jem.20061706
Wu, X., Lee, V., Chevalier, E., and Hwang, S. (2009). Chemokine Receptors as Targets for Cancer Therapy. Cpd 15, 742–757. doi:10.2174/138161209787582165
Xiong, Y., Huang, F., Li, X., Chen, Z., Feng, D., Jiang, H., et al. (2017). CCL21/CCR7 Interaction Promotes Cellular Migration and Invasion via Modulation of the MEK/ERK1/2 Signaling Pathway and Correlates with Lymphatic Metastatic Spread and Poor Prognosis in Urinary Bladder Cancer. Int. J. Oncol. 51, 75–90. doi:10.3892/ijo.2017.4003
Xu, B., Zhou, M., Qiu, W., Ye, J., and Feng, Q. (2017). CCR7 Mediates Human Breast Cancer Cell Invasion, Migration by Inducing Epithelial-Mesenchymal Transition and Suppressing Apoptosis through AKT Pathway. Cancer Med. 6, 1062–1071. doi:10.1002/cam4.1039
Yang, L., Chang, Y., and Cao, P. (2018). CCR7 Preservation via Histone Deacetylase Inhibition Promotes Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma Cells. Exp. Cell. Res. 371, 231–237. doi:10.1016/j.yexcr.2018.08.015
Yang, X., Coriolan, D., Murthy, V., Schultz, K., Golenbock, D. T., and Beasley, D. (2005). Proinflammatory Phenotype of Vascular Smooth Muscle Cells: Role of Efficient Toll-like Receptor 4 Signaling. Am. J. Physiology-Heart Circulatory Physiology 289, H1069–H1076. doi:10.1152/ajpheart.00143.2005
Zhang, Y., Li, M., Du, G., Chen, X., and Sun, X. (2021). Advancedoral Vaccine Delivery Strategies for Improving the Immunity. Adv. Drug Deliv. Rev. 177, 113928. doi:10.1016/j.addr.2021.113928
Zidar, D. A., Violin, J. D., Whalen, E. J., and Lefkowitz, R. J. (2009). Selective Engagement of G Protein Coupled Receptor Kinases (GRKs) Encodes Distinct Functions of Biased Ligands. Proc. Natl. Acad. Sci. U.S.A. 106, 9649–9654. doi:10.1073/pnas.0904361106
Zlotnik, A., Yoshie, O., and Nomiyama, H. (2006). The Chemokine and Chemokine Receptor Superfamilies and Their Molecular Evolution. Genome Biol. 7, 243. doi:10.1186/gb-2006-7-12-243
Keywords: CC-chemokine receptor 7, CCL19, CCL21, immune tolerance, drug target, prognostic marker
Citation: Alrumaihi F (2022) The Multi-Functional Roles of CCR7 in Human Immunology and as a Promising Therapeutic Target for Cancer Therapeutics. Front. Mol. Biosci. 9:834149. doi: 10.3389/fmolb.2022.834149
Received: 13 December 2021; Accepted: 26 May 2022;
Published: 06 July 2022.
Edited by:
Liaqat Ali, National University of Medical Sciences (NUMS), PakistanReviewed by:
Sudip Banerjee, Morehouse School of Medicine, United StatesJosé Luis Rodríguez-Fernández, Spanish National Research Council (CSIC), Spain
Youness El Bakri, South Ural State University, Russia
Copyright © 2022 Alrumaihi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faris Alrumaihi, Rl9hbHJ1bWFpaGlAcXUuZWR1LnNh
 Faris Alrumaihi
Faris Alrumaihi