- 1Institute of Medical Biometry and Statistics (IMBI), Faculty of Medicine and Medical Center, University of Freiburg, Freiburg, Germany
- 2Freiburg Center for Data Analysis and Modelling (FDM), University of Freiburg, Freiburg, Germany
- 3Institute of Physics, University of Freiburg, Freiburg, Germany
- 4Centre for Integrative Biological Signalling Studies (CIBSS), University of Freiburg, Freiburg, Germany
Dynamic behavior of biological systems is commonly represented by non-linear models such as ordinary differential equations. A frequently encountered task in such systems is the estimation of model parameters based on measurement of biochemical compounds. Non-linear models require special techniques to estimate the uncertainty of the obtained model parameters and predictions, e.g. by exploiting the concept of the profile likelihood. Model parameters with significant uncertainty associated with their estimates hinder the interpretation of model results. Informing these model parameters by optimal experimental design minimizes the additional amount of data and therefore resources required in experiments. However, existing techniques of experimental design either require prior parameter distributions in Bayesian approaches or do not adequately deal with the non-linearity of the system in frequentist approaches. For identification of optimal experimental designs, we propose a two-dimensional profile likelihood approach, providing a design criterion which meaningfully represents the expected parameter uncertainty after measuring data for a specified experimental condition. The described approach is implemented into the open source toolbox Data2Dynamics in Matlab. The applicability of the method is demonstrated on an established systems biology model. For this demonstration, available data has been censored to simulate a setting in which parameters are not yet well determined. After determining the optimal experimental condition from the censored ones, a realistic evaluation was possible by re-introducing the censored data point corresponding to the optimal experimental condition. This provided a validation that our method is feasible in real-world applications. The approach applies to, but is not limited to, models in systems biology.
1 Introduction
With Fisher’s pioneering work on optimizing the design of agricultural experiments lying a century in the past, the design of informative experiments has long since become a foundation for most quantitative sciences. While there are undeniably practical aspects of conducting an experiment to generate the data used for analysis, planning a successful experiment requires consideration of statistical concepts even before any data is collected as this can help to develop the “logic of experimentation” (Bishop et al., 1982).
In systems biology, the underlying models used for analyses become increasingly complex. This is due to the fields aspiration to provide holistic descriptions of biological systems which are able to capture not only static properties of a system but the dynamic interactions of the system’s components (Kitano, 2002; Nurse and Hayles, 2011). For these systems, mathematical models are established to reduce the complexity of the biological components to their relevant features. The process of “building a model” is an intertwined process of finding a model which adequately describes the observed dynamics given the existing biological knowledge and providing the quantitative inputs for this model through experimentation (Kreutz and Timmer, 2009). The aim of systems biology is to construct “useful” models (Wieland et al., 2021), i.e. models that yield biological insights. Assessing whether a model is useful can be “notoriously difficult” (Liepe et al., 2013), even more so if the data obtained from experiments is insufficient to inform the model. Therefore, close cooperation of experimenters and theoreticians throughout the process increases the chance of generating data that is suitable for this task.
Biochemical processes can often be represented as ordinary differential equations (Nurse and Hayles, 2011; Liepe et al., 2013; Raue et al., 2013) which are often adequate representations of molecular dynamics. In general, this means that the observed biochemical compounds will be non-linearly related to the model parameters. Although such models are able to describe the system realistically, non-linearity proves to be a challenge in the analysis of the models properties. One consequence of non-linearity is the frequent absence of analytical solutions to the differential equations which determine the time-evolution of the biological states involved. Consequently, estimation of model parameters by optimization of the objective function, which measures the deviation of the model predictions to the measured data, is limited to numerical approaches (Raue et al., 2013). The difficulty of this “inverse problem” (Liepe et al., 2013) of determining the model parameters which describe the observed data the best is exacerbated in biological systems. Characterization of these systems can lead to a model with many parameters and biological states with the available data being noisy (Kreutz and Timmer, 2009). Additionally, the system is generally only partially observable, i.e. not all biochemical compounds in the model can be measured (Raue et al., 2009).
A major task in developing experiments in this defined setting is to propose practically feasible experiments which decrease the uncertainty about the value of parameters of interest. A well-known result from the classical theory of non-linear experimental design is that the optimal design depends on the “true model parameters,” i.e. the parameters that govern the true evolution of the system (Busetto et al., 2013), e.g. illustrated for the setting of Fisher’s dilution series experiments (Cochran, 1973). However, we are interested in inferring exactly these unknown parameters. A solid initial guess about the parameter values would solve the problem, but given the complex nature of the modeled systems, prior knowledge is usually sparse (Kreutz and Timmer, 2009; Bazil et al., 2012). A natural approach is then to design experiments sequentially (Cochran, 1973; Ford et al., 1989), i.e. measure the data in batches, updating the knowledge about the initial parameter values for each experimental design iteration.
Much of the classical literature on designing the optimal experiment is based on the Fisher information matrix (Ford et al., 1989; Atkinson and Donev, 1992; Fedorov, 2010). This is a natural approach in linear systems, as the inverted Fisher information matrix determines the covariance matrix of the estimated model parameters. Appropriate characteristics of this covariance matrix are then optimized by a suitable experimental design (Atkinson and Donev, 1992; Faller et al., 2003). However, application of the Fisher information matrix is known to be troublesome in non-linear systems if the amount of data is limited and statistical properties are far from asymptotic. The Wald confidence intervals implied by the Fisher information matrix might then only crudely reflect the existing uncertainty. Confidence intervals generated by the profile likelihood approach have more desirable properties in the finite sample case (Meeker and Escobar, 1995) and allow for the conceptional and operational definition of practical identifiability (Raue et al., 2009). Experimental planning in frequentist statistics should therefore make use of this powerful concept of quantifying parameter uncertainty and identifiability.
Approaches to the experimental design problem have also been developed in a Bayesian framework. The conceptual foundation of updating prior parameter knowledge given the newly measured data in Bayesian statistics provides natural solutions to the problem of experimental design. The information gain of an experiment can be reasonably quantified by means of the Shannon information (Lindley, 1956) and application of this theory to Bayesian experimental design provides a tool to plan optimal experiments for parameter inference (Huan and Marzouk, 2013; Liepe et al., 2013) and model discrimination (Busetto et al., 2013). However, we focus on a frequentist approach as it is usually not feasible to provide reasonable priors for all model parameter in the systems biology context.
There exist frequentist methods for experimental design if it is infeasible to provide prior information for all model parameters. If sets of parameters which are compatible with existing data about the system were known, the corresponding set of model trajectories would indicate for which observables and for which time points the model prediction is not yet reasonably constrained; such experimental conditions would then be “experimentally distinguishable” (Bazil et al., 2012). This set was previously constructed from efficient sampling of the parameter space (Bazil et al., 2012) or exploring the parameters along the likelihood profiles (Steiert et al., 2012). The latter method was applied in the DREAM6-Challenge (Dialogue for Reverse Engineering Assessments and Methods) and has been awarded as the best performing approach (Steiert et al., 2012). However, this approach assesses the impact of different sets of model parameters on the model predictions. In order to optimally design experiments which decrease parameter uncertainty, the logic of the design scheme has to be reversed: Instead of assessing the impact of different model parameters on the model prediction, the impact of different measurement outcomes on the parameter estimate of interest has to be assessed.
We propose a frequentist approach for optimal experimental design which realizes the full potential of the profile likelihood approach by extending the previously best-performing method (Steiert et al., 2012). For a specified experimental condition, we quantify the expected uncertainty of a targeted parameter of interest after a possible measurement. The parameter uncertainty after any specific measurement outcome is determined by the respective profile likelihood, effectively yielding a two-dimensional likelihood profile when accounting for different possible measurement outcomes. The range of reasonable measurement outcomes given the current data available before the measurement is quantified via the concept of validation profiles (Kreutz et al., 2012). The two-dimensional likelihood profile provides both the range of reasonable measurement outcomes of an intended experiment and their impact on the parameter likelihood profile. Hence, this allows for a definition of a design criterion which represents the expected average width of the confidence interval after measuring data for a certain experimental condition. The two-dimensional likelihood profiles therefore provide quantitative information usable for sequential experimental design and additionally serve as an intuitive tool to visualize the impact of an experiment on the uncertainty of the parameter of interest.
2 Materials and Methods
2.1 Mathematical Model
We introduce the concept of ordinary differential equation models, because they are frequently used for modeling of the dynamics of biological systems. However, we want to emphasize that the introduced method for experimental design is generic and only requires specification of a suitable likelihood function.
Biological quantities such as the concentration of a molecular compound are represented by mathematical states x(t) and are assumed to follow a set of ordinary differential equations
which generates the trajectories according to the unknown underlying true dynamic model parameters p0. The function f is typically defined by translating biochemical interactions, e.g. by the rate equation approach. The trajectories depend on the specific experiment conducted which is denoted by the experimental perturbations u, representing interventions such as external stimulation of the system or knockout of specific genes. The set of model parameters will usually include the initial values x0 of the model states.
Estimation of the true parameters typically requires measurement of time-resolved data on these states. However, some states in the considered system might not be observable at all or only indirectly accessible, e.g. if only a sum of different states can be observed (Raue et al., 2009). Additionally, measured data will usually be subject to random errors. Therefore, the set of observables
defines the types of data that can be measured. In this equation, ϵ describes the random error of the measurement which is usually assumed to be normally distributed, i.e.
The measured data of the system provides a set of scalar values yi which each corresponds to an experimental condition Di containing all information necessary to interpret the value yi. The experimental condition is uniquely defined as the measured observable, the time point of measurement and the corresponding experimental perturbation.
The objective function which indicates the agreement of experimental data with the model prediction given some parameters θ and measured data Y = {y1, …, yn} is the likelihood function
with ρ indicating the probability density for the considered data point. Maximizing this likelihood leads to the maximum likelihood estimate
2.2 Profile Likelihood
2.2.1 Parameter Profile Likelihood
The task of parameter inference is not completed with the identification of the maximum likelihood estimate. In general, other parameter estimates may provide other model trajectories which might fit similarly well to the given data. Additionally, replications of the same experiment will lead to different measurement results and therefore also different parameter estimates due to variance in the biological samples and the measurement process. From a frequentist standpoint, methods are required to construct confidence intervals for either individual parameters or multiple parameters jointly, which have a pre-defined coverage probability of containing the true parameter value if the experiment were to be replicated. Within the context of this paper, we focus exclusively on confidence intervals for individual parameters.
The commonly encountered Wald confidence intervals are based on a quadratic approximation of the likelihood and fail if the model features non-linear dynamics (Meeker and Escobar, 1995; Raue et al., 2009). The quadratic approximation of the likelihood depends on the parametrization of the model, may not respect boundaries of the parameter space and cannot capture global behavior such as the existence of local optima.
A more refined tool which reduces the high-dimensional likelihood onto the one-dimensional parameter of interest pi is the profile likelihood
with the parameter vector θ = {β, ω} being split into the parameter of interest pi = β and the nuisance parameters pi≠j = ω. The hats indicate maximum likelihood estimates, i.e.
are the nuisance parameters which maximize the likelihood if β is fixed to a specific value. The parameter profile likelihood is invariant under one-to-one parameter transformations and can accurately reduce complex shapes of the underlying likelihood function to an adequate one-dimensional representation. Confidence intervals can be constructed from the parameter profile by Wilks’ Theorem (Wilks, 1938) and take the form
with icdf representing the inverse cumulative distribution function. Note that high values of the profile likelihood defined in Eq. 4 correspond to lower values of the likelihood. This implies that parameter values β associated with a large profile likelihood value
Informally, Wilks’ theorem implies that asymptotically, these confidence intervals will attain the correct coverage probability α as they become equivalent to the Wald approximation. However, the finite sample properties of the profile likelihood intervals are superior. The notion of parameter profiles allows identifiability analyses on the parameters (Raue et al., 2009). Parameters can be: 1) Identifiable, in which case the width of the defined confidence interval is finite. 2) Structurally non-identifiable, in which case the profile likelihood is flat. This implies that any change of the parameter of interest can be compensated by changing other model parameters. 3) Practically non-identifiable, in which case the profile likelihood is not completely flat, but does not cross the confidence threshold to both sides such that the size of the confidence interval is infinite. While structural non-identifiability can only be resolved either by reparametrization of the model or qualitatively new experiments, practically non-identifiability can usually be resolved by providing higher quality data from similar experiments. Identifiability is distinct from the frequently encountered concept of sloppiness (Chis et al., 2016) which plays no role for the experimental design as discussed within this study. Due to the advantageous theoretical as well as practical properties of the profile likelihood, parameter uncertainties in this study are exclusively discussed in terms of their corresponding likelihood profile.
2.2.2 Validation Profile Likelihood
The parameter profile likelihood allows for the evaluation of the uncertainty of parameters given the current data. For some applications, assessing the “prediction uncertainty,” i.e. the uncertainty about the outcome of measuring at a certain experimental condition, might be more relevant. In a frequentist setting, one can readily extend the concept of the parameter profile likelihood to this setting in the form of the “validation profile likelihood” (Kreutz et al., 2012), also called “predictive profile likelihood” (Bjornstad, 1990), in which case the likelihood is reduced to the dimension of the measurement outcome of interest. Formally, this profile is defined by
with z defined as the outcome of measuring at experimental condition Dz and
2.3 Experimental Design Task
Understanding the task of designing an informative experiment requires clarification. We start by introducing the common terminology of the theory of optimal experimental design. The design region
The problem we are concerned with is the reduction of uncertainty for a single parameter by conducting an experiment at an informative design point. This means that given a set of admissible design points, we want to decide which of these experimental designs will best reduce the existing uncertainty about a pre-specified parameter. To put this into a more formal framework, we are looking for a design criterion
2.4 Measuring Parameter Uncertainty
2.4.1 Classical Theory
The optimal experimental design depends on the choice of a reasonable design criterion. Classical design theory solves this problem by applying the Fisher information matrix as the appropriate measure of information and establishing design criteria based on this matrix M(θ, ξ), i.e. the design criterion takes the form Φ(M(θ, ξ)) (Ford et al., 1989; Atkinson and Donev, 1992; Fedorov, 2010). The Fisher information matrix is concerned with the local behavior of the likelihood function around a specified parameter, which in application usually means in the neighborhood of the current maximum likelihood estimate of the parameters (Faller et al., 2003). For the same reasons discussed earlier, we propose that it is more adequate to utilize the profile likelihood of the parameter of interest pi to construct a measure of information which we can use to design an optimal experiment.
2.4.2 Confidence Distribution
There is no unique way to define the information available in the likelihood profile
This issue can be resolved by confidence distributions (Xie and Singh, 2013) which can be thought of simultaneously containing the information about the confidence intervals to all levels. This concept allows the construction of an object that has the form of a distribution estimator for the parameter of interest pi in the realm of frequentist statistics. The corresponding confidence density
2.4.3 Uncertainty as a Scalar Quantity
Ranking different experiments by their information content requires a way to order their corresponding design criterion values. A necessary step to achieve this is to reduce the confidence distribution of a parameter to a scalar value. We suggest utilizing the average confidence interval width
to summarize the information content of the confidence distribution. The function w assigns the width to the corresponding confidence interval. Different confidence interval widths are averaged by weighting with their respective confidence measure dα. The measure dα specifies the confidence that the true parameter value is covered by the interval CIα+dα(pi), but not by CIα(pi). Evaluation of this average confidence interval width does not require the explicit confidence distribution but only the individual confidence intervals. Thus, it can be directly calculated from the profile likelihood. In practice, we will only consider confidence intervals up to the 95%-level to ensure practical feasibility.
2.5 Two-Dimensional Profile Likelihood as a Design Criterion
In the previous sections, we proposed to quantify parameter uncertainty via the profile likelihood approach by definition of an average profile width in Eq. 8, which summarizes the existing uncertainty about the parameter of interest. Optimal experimental design aims at minimizing this measure of uncertainty by choosing an experimental condition for the next measurement which optimizes a suitable design criterion. However, for a given experimental condition D it is a priori unknown which value will result from a future measurement. This implies that the parameter profile likelihood
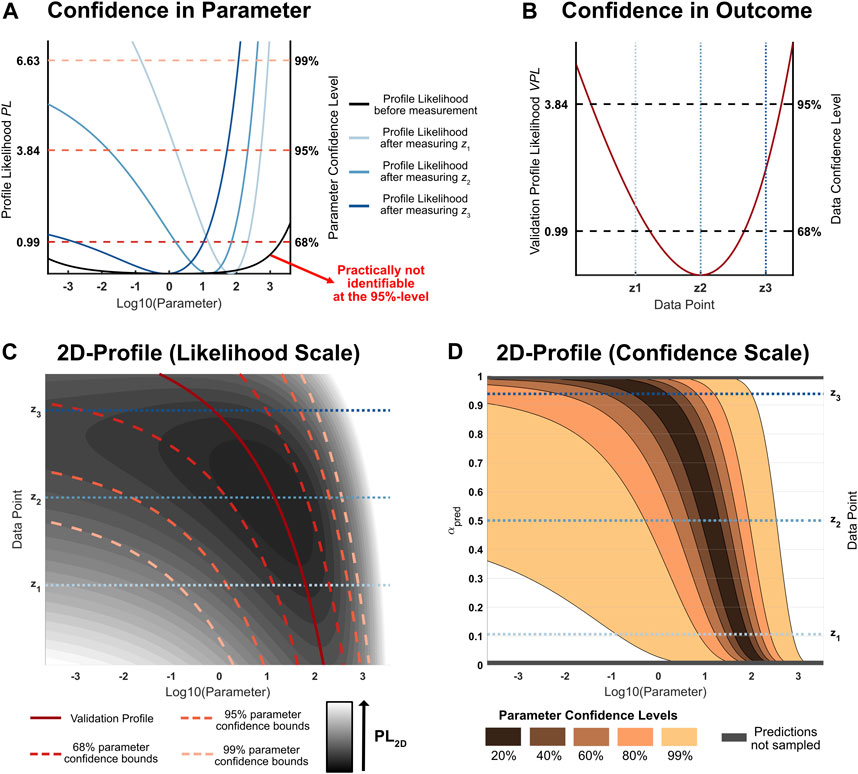
FIGURE 1. (A): Likelihood profiles of a hypothetical parameter of interest. Different measurement outcomes z1, z2, z3 for the same experimental condition lead to different updated parameter profiles which assess uncertainty about a parameter of interest. (B): Validation profile of the considered hypothetical experimental condition. This profile assesses the likelihood of a new measurement: The smaller the validation profile value, the more likely the respective outcome (C): 2D-Likelihood profile for the parameter of interest under some given experimental condition. The vertical axis corresponds to different possible measurement outcomes. If the outcome on the vertical axis would be observed, the profile likelihood after the measurement is given by the corresponding horizontal cross-section through the two-dimensional profile. In this example, lower values of the measurement outcome lead to narrow parameter confidence intervals after the measurement. (D): 2D-Likelihood profile on a confidence scale. Intervals of the same size on the y-axis hold equal confidence that a measurement will yield a data point in the corresponding interval. The prediction confidence levels on the vertical axis illustrate that the sampled two-dimensional likelihood profile covers most of the plausible measurement outcomes.
The plausibility of different possible measurement outcomes can be accounted for by weighting the average profile widths for different measurement outcomes by their likelihood of occurrence. As discussed in Section 2.2.2, this plausibility measure is implied by the validation profile likelihood. Figure 1B shows the validation profile for the specified design: z2 corresponds to the current maximum likelihood prediction for this experimental condition and is therefore the most likely measurement outcome given the current evidence, while z3 has a higher validation profile value than z1 and is therefore less likely. Therefore, the validation profile likelihood implies a predictive distribution which can be defined in analogy to the confidence distribution derived from the parameter profile likelihood. The corresponding predictive density ρpred(z|Y) associates different measurement outcomes with our confidence that the specific outcomes occur.
The concept of summarizing parameter uncertainty for a fixed measurement outcome and subsequent aggregation of different possible measurement outcomes based on the predictive density can be combined to construct a design criterion for an experimental condition of interest. To this end, each expected parameter profile width
W(pi|Y, D) exclusively depends on the given data Y and the experimental condition D of a subsequent experiment and thus by definition constitutes a design criterion. This quantity can be interpreted as the expected average profile width after measuring at the experimental condition D, where the average is taken over different parameter confidence levels and the expectation is taken over different possible measurement outcomes, weighted by their predicted plausibility. Given a set of experimental conditions {D1, …, Dn}, the optimal experiment D* to inform parameter pi is the one which minimizes W(pi|Y, D) given the current data Y, i.e.
The information necessary to evaluate the design criterion in Eq. 9 is summarized by a two-dimensional likelihood profile, defined as
For any fixed measurement outcome z, the resulting parameter profile likelihood can be extracted from this quantity. Simultaneously, the two-dimensional likelihood profile contains information about the plausibility of different measurement outcomes. Figure 1C illustrates this relationship: The profiles in Figure 1A are horizontal cross-sections from the two-dimensional likelihood profile (blue lines). The minimal profile value of each horizontal cross-section defines the path of the validation profile in Figure 1B (solid red line). The confidence intervals (dashed red lines) depend on the different possible measurement outcomes: Some measurement outcomes lead to more information about the parameter of interest than others as indicated by narrower confidence intervals.
The process of averaging confidence interval widths over the various parameter confidence levels and taking the expected value over the possible plausible data realizations is visualized in Figure 1D. The displayed two-dimensional profile is based on the same data as depicted in Figure 1C, but it has been transformed onto a different scale. The minimum of each horizontal cross section is shifted to the common null value, but still represents the trajectory of the validation profile. For the transformed two-dimensional likelihood profile, the vertical axis is proportional to the prediction confidence levels, i.e. intervals with the same length correspond to an equal confidence of yielding a measurement value in the given intervals. This transformation reveals that the interval [z1, z3] is a 83%-prediction interval for a future measurement outcome given the experimental condition. The horizontal gray patches at the top and the bottom of Figure 1D correspond to all the measurement outcomes for which the original two-dimensional profile likelihood was not sampled, because they are unlikely to occur. The trend of different parameter confidence intervals as a function of different data points is illustrated for five discrete confidence levels (shades of red). On this scale, the expected average profile width W(β|Y, D) is equal to the average of all the colored areas, where the smaller confidence intervals are included in the larger ones.
2.6 Experimental Design Workflow
Utilization of two-dimensional likelihood profiles as a tool for experimental design requires a ready-to-use workflow in applications. We provide an example for this workflow in a fully sequential experimental design scheme to put the previous definitions into a more practical context. Figure 2 shows a flowchart of the steps involved in this workflow. Starting from an initial data set, the model parameters are estimated and the profile likelihood is calculated for all model parameters to obtain information about existing parameter uncertainties. The likelihood profiles are calculated by numerical evaluation of Eq. 4 for a finite set of profile parameters. If there are non-identifiable parameters, the biologically most relevant parameter is targeted for improvement by the experimental design scheme.
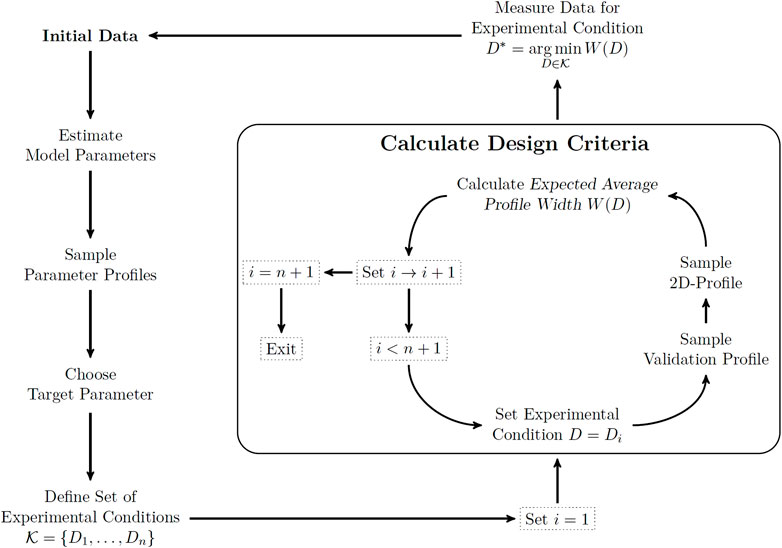
FIGURE 2. Workflow for the sequential experimental design scheme. Starting from the current data set (top left), the target parameter is chosen and relevant experimental conditions are specified. Calculating the two-dimensional profile likelihood and evaluating the expected average profile width for each experimental condition (box) reveals the optimal condition for the next measurement. Dotted rectangles specify the state of the for loop, while text without rectangles correspond to the experimental design steps involved.
After a representative set of experimental conditions has been defined, the design criterion in Eq. 9 needs to be evaluated for each of the experimental conditions by the following steps. First, a validation profile is calculated for the experimental condition. This validation profile provides the range of relevant measurement outcomes for the respective experimental condition. Therefore, the space on which the two-dimensional likelihood profile needs to be sampled is finite. This space is sampled by evaluating the parameter profile likelihood for a representative set of measurement outcomes. The expected average profile width is calculated from the two-dimensional likelihood profile by employing the discrete counterparts of all expressions appearing in Eq. 9. At this point the details are more of technical than conceptual relevance and we want to emphasize that an automated implementation of this algorithm is available and referred to at the end of this manuscript. The final step of the workflow is now to choose the experimental condition which provides the minimal value for the design criterion as the target for the next measurement. This workflow can be repeated after a new data point has been generated to determine a sequence of informative measurements.
3 Results
We illustrate the process of choosing the best experimental design for a parameter of interest by two examples. The first example is based on simulated data for a simple model with two consecutive reactions in which compound A is converted to compound B which is then converted to compound C and is therefore termed as ABC model in the following. This example will serve to illustrate the interpretation of the two-dimensional profile likelihood. The second example is based on the published experimental data for a model of erythropoietin (EPO) degradation (Becker et al., 2010) for which data has been censored in order to mimic a setting in which experimental design can be applied. This example serves to explain the full workflow of the sequential experimental design scheme in an application setting and illustrates the practical feasibility of our approach.
3.1 Experimental Design in the ABC-Model
The ABC model describes a simple case of a model in which the model predictions non-linearly depend on the model parameters. The reactions are illustrated in Figure 3A: State A is converted to state B with the rate p1 and B is subsequently converted into compound C with rate p2. In a biochemical setting, these three states might represent three conformations of three activation states in terms of different phosphorylations. The dynamics of this system are determined by the following differential equations:
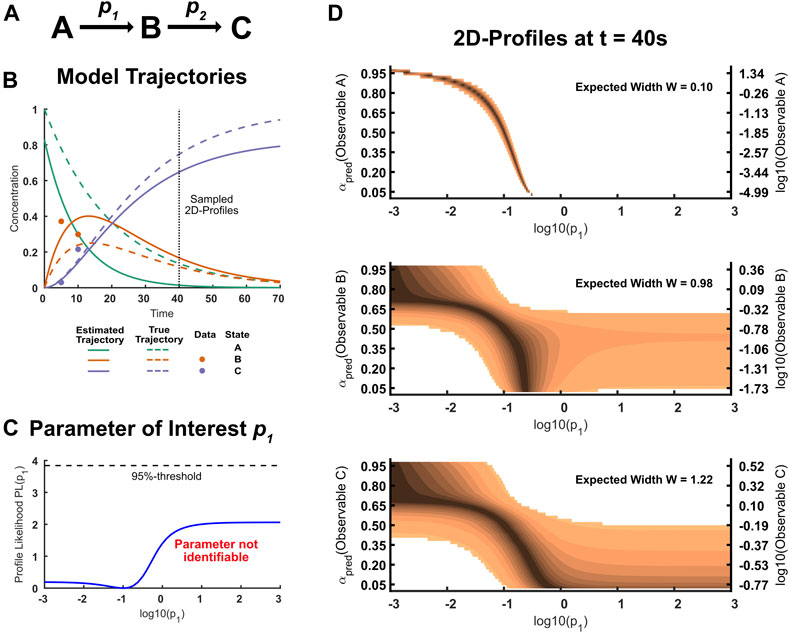
FIGURE 3. (A): States and parameters in the ABC-model. The model has three parameters: Two rate constants p1 and p2, and the initial concentration A0. The initial concentrations of B and C are assumed to be zero. (B): Trajectories of the ABC-Model. The dots correspond to the sparse data simulated from the true model. In this example, state B and C were assumed to be observable, but have only been observed at early time points. The true trajectory of state A yet differs considerably from the estimated trajectory. (C): Likelihood profile of the practically non-identifiable parameter p1. Because the initial concentration of state A is unknown, this parameter is difficult to estimate without information about state A. (D): 2D-Likelihood profiles for the three states A, B and C if measured at time point t = 40. The illustrated profiles are presented on a confidence scale according to Figure 1D. If state A was observable, the finite width of the 2D-profile to the 95% level indicates that any measurement outcome will make the parameter p1 identifiable. Note that possible values for A scatter across six orders of magnitude because the predictions for A are barely informed. Measuring state B or C will likely put an lower or upper limit on the parameter p1.
In order to illustrate the two-dimensional likelihood profile approach on a simple model for which we know the true underlying parameters, we defined the true model parameters and simulated data from this model. In this true model, the initial concentrations of state B and state C were set to zero which was assumed to be known for inference, such that the system is characterized by the three parameters {p1, p2, A0} which were assumed to be unknown and have to be estimated from data. The data set simulated from the true model parameters is sparse: state A has been assumed to not be observable and the two data points available for each state B and C have been generated with an initial concentration log(A0) = 0 from log-normal distributions with a standard deviation σlog = 0.2 After simulation of the data, the model parameters {p1, p2, A0} are optimized to estimate their values and the corresponding state trajectories.
The true as well as the estimated state trajectories are illustrated in Figure 3B: While the model predictions fit the data well, there is still considerable disagreement of the underlying true model and the best model fit. This is especially true for state A, considering that the differences between trajectories are analyzed on a log-scale, which measures relative differences. An analysis of parameter uncertainty reveals that parameter p2 is identifiable, as information for state B and C suffices to inform this rate. By similar reasoning, there is less information available for parameter p1 and the corresponding profile likelihood reveals that the parameter is practically non-identifiable over the whole considered parameter space, as illustrated in Figure 3C.
In our example, we want to inform this practically non-identifiable parameter p1 by choosing a measurement out of three possible experimental conditions. For demonstration purposes, the three experimental conditions of measuring either state A, B or C at the time point t = 40 are considered. The corresponding two-dimensional likelihood profiles are illustrated in Figure 3D. If it was possible to measure observable A, this would be highly informative and in fact guarantees that the parameter p1 is identifiable no matter the outcome of the measurement. This is intuitive, since the measurement of the yet unobserved quantity A highly constrains the possible dynamics. It should be noted that possible outcomes for the observable A vary across orders of magnitudes which can be attributed to the fact that the dynamics for A are poorly constrained given the current data set. The two-dimensional profiles associated with observable B and C reveal that the parameter p1 will likely not be identifiable even after the measurement. However, the magnitude of outcomes will yield at least an upper or a lower bound for the parameter of interest: Large values of B and C put an upper limit on p1, as this means that the reaction can not be arbitrarily fast, while low values of B and C put a lower limit of p1 because the reaction can not be arbitrarily slow. A not immediately obvious result from the two-dimensional profiles is that measuring observable B is more informative than measuring observable C as seen from the calculated design criterion. This example illustrates that two-dimensional likelihood profiles provide qualitative as well as quantitative information about how experiments impact parameter uncertainty.
3.2 Experimental Design in the Erythropoietin Degradation Model
The modeled system for the degradation of erythropoietin (EPO) (Becker et al., 2010) is an example of a non-linear model with intertwined reactions of biochemical states. EPO acts as a ligand by binding to the corresponding cell receptor to form a complex. This complex is internalized and then EPO is degraded. The mathematical model provided the insight that a combination of EPO receptor turnover and recycling guarantees that biochemical response to a broad range of ligand concentrations is possible (Becker et al., 2010).
A scheme of reactions in the biological system is illustrated in Figure 4A. The model features six dynamic states: EPO (Epo) and degraded EPO (dEpoe) outside of the cell, EPO receptors (EpoR) and EPO–EPO receptor complexes (EpoEpoR) on the cell membrane, and internalized EPO–EPO receptor complexes (EpoEpoRi) and degraded EPO (dEpoi) inside of the cell. The reactions illustrated in the figure can be translated into the following set of differential equations (Becker et al., 2010):
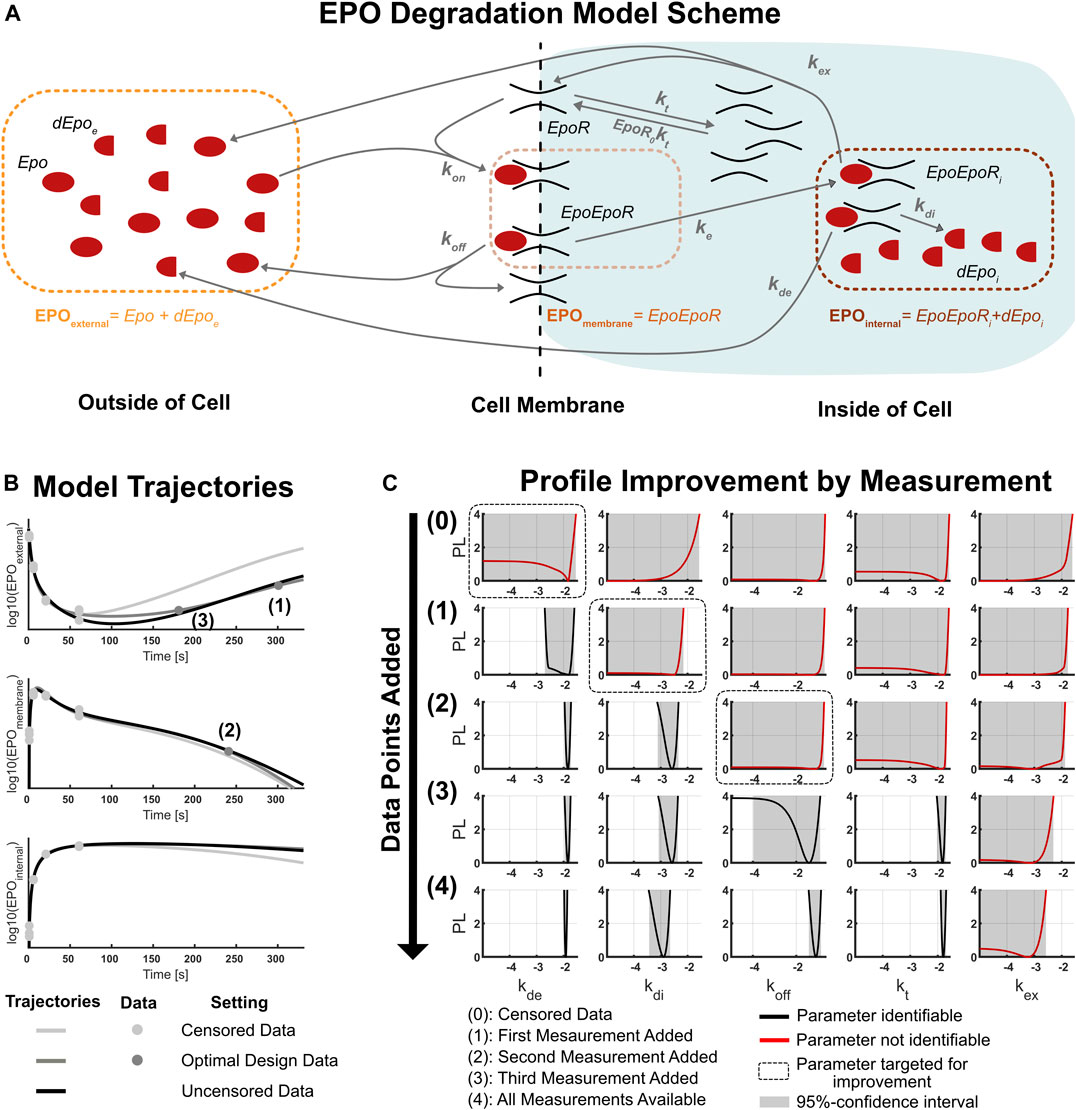
FIGURE 4. (A): Scheme of the biological dynamics in the EPO degradation model (Becker et al., 2010). There are six model states (black text) which interact through different biological reactions (gray arrows) and three observables (colored text). EPO is transported into the cell and degraded there. (B): Model trajectories for the observables of the EPO-model. The plotted curves are the best fit trajectories for three different data sets: the censored data set used at the start of the experimental design analysis, the data set after adding three sequentially proposed data points, and the uncensored published data set. The numbers indicate the order of the sequentially measured data points. (C): Change of parameter likelihood profiles during the sequential experimental design procedure. The targeted parameter always became identifiable after data for the optimal experimental condition proposed by the two-dimensional likelihood approach was added into the model. Incorporating the three optimal data points into the model already produces results of similar accuracy compared to the published data set with 36 additional data points.
There are seven dynamic parameters (kt, kon, koff, kex, ke, kde, kdi) and two unknown initial conditions (Epo0, EpoR0) in the model which are biologically interpretable as well as six further parameters which appear only in the observation function and not in the dynamic model. Because EPO can be traced with a radioactive marker, the concentration of EPO can be measured outside of the cell (EPOexternal), on the cell membrane (EPOmembrane) and inside of the cell (EPOinternal). This provides us with three different observables for the six dynamic states, i.e. the model is only partially observable.
The parameters of the model are identifiable except for one parameter given the complete data set of the study. In order to illustrate experimental design considerations, a model which is not yet well informed by data is required. Thus, we censored one half of the complete data set for all observables which respectively correspond to the later stages of the dynamics. This serves two purposes: First, we reduced the information content of the data, thus creating non-identifiabilities for some parameters. Second, we gain access to biological data for 3 (observables) x 4 (time points) = 12 experimental conditions which can be used to mimic real measurements.
The best fit model trajectories for the three observables are illustrated in Figure 4B for three sets of data (shades of gray). One set of trajectories corresponds to the censored data set, the next set corresponds to an optimal sequential experimental design with three additional measurements and the last set of trajectories corresponds to the original full data set with 3 (observables) x 4 (time points) x 3 (replications) = 36 additional data points. The predictions change significantly when adding the three optimal data points to the censored data set, while adding the rest of the data only changes the model trajectories slightly.
The three optimal data points were determined by applying the workflow for the sequential experimental design scheme shown in Figure 2. The iterative improvement of the likelihood profiles of the non-identifiable parameters by this workflow is illustrated in Figure 4C. Starting with the censored data set, five parameters are non-identifiable. This comprises the external and internal EPO degradation rate kde and kdi, the complex dissociation constant koff, the receptor turnover rate kt and the complex recycling rate kex.
The internal EPO degradation rate kde was targeted by the first experiment, and has been made identifiable after measuring EPOexternal at a late stage of the dynamics. Note that retrospectively, this choice was highly tailored to the identification of kde, as the profile likelihood for the other parameters only changed slightly. This underlines that experiments proposed by our approach aim specifically at improving the knowledge about the targeted parameter of interest.
In the second experiment, the internal EPO degradation rate kdi was targeted. The corresponding optimal experiment is a measurement of EPOmembrane at a late time point. Because this design is optimal at an earlier time point than the first measurement, this suggests that the first measurement of EPOexternal already carries information which could have been obtained from measuring EPOmembrane at the same time point, highlighting that model dynamics are highly intertwined. Imitating the measurement for the proposed experimental design again shows that the targeted parameter is identifiable after the experiment, while the others are still practically non-identifiable.
The third iteration of experimental design targeted the complex dissociation constant koff and revealed that measuring EPOexternal at an earlier time point is now more informative than measuring the observable EPOinternal, for which late time measurements are still not available. This highlights the fact that determination of the optimal experimental design is difficult by intuitive considerations and experimental design approaches provide non-trivial insights. This measurement removed the non-identifiability of both the targeted parameter koff and also the turnover rate kt which was not considered when planning the experiment.
A fourth iteration of the sequential experimental design was not conducted because the two-dimensional likelihood profiles for the last non-identifiable parameter kex indicate that a single additional data point for any of the remaining experimental conditions does not provide enough information to make the parameter identifiable. In fact, this is in line with the results of the final model with all data available, as the parameter is still practically non-identifiable given the complete data set. The two-dimensional likelihood profiles corresponding to the four experimental design iterations are illustrated in the Supplementary Figures S1–S4.
The comparison of parameter likelihood profiles for the design with three optimally chosen measurements with the full data set design of 36 new data points is shown in the last two rows of Figure 4C. The similarity of all profiles across all parameters indicates that three optimally chosen experimental conditions already yield much of the information contained in the set of all 36 data points. This underlines the ability of optimal experimental design to reduce the amount of data needed to remove non-identifiabilities for the parameters of interest. Therefore, application of the optimal sequential experimental design on a realistic biological model demonstrated the feasibility and merits of the two-dimensional likelihood profiles as an approach for experimental design.
4 Discussion
4.1 Experimental Design by Two-Dimensional Likelihood Profiles
A well-planned experiment can save time and resources. Therefore, optimal experimental design aimed at reducing the amount of data needed to inform the model is desirable in any context, but this task is often non-trivial for complex models such as those encountered in systems biology. We established a method for optimal experimental design aiming at reducing parameter uncertainty for a single parameter of interest in a frequentist setting. To this end, we define two-dimensional likelihood profiles which contain information about the likely parameter uncertainty after a measurement. Our approach for experimental design employs the theoretically appealing concept of likelihood profiles, which can serve as a measure for uncertainty in parameter estimates but also for a measure of uncertainty of measurement outcomes. These measures can be conceptually understood to imply confidence densities for parameters or predictive densities for measurement outcomes with strictly frequentist concepts. The presented approach allows for the evaluation of the impact of an experiment in a qualitative as well as in a quantitative manner.
The two-dimensional profile likelihood approach for experimental design was employed in two examples to illustrate its properties and establish feasibility of the method. The ABC reaction model features a non-linear relationship between model states and parameters and served to illustrate the features of two-dimensional likelihood profiles. In order to show practical feasibility of the approach in a realistic setting, an established erythropoietin degradation model (Becker et al., 2010) was investigated. To this end, half of the full data set has been censored to simulate a realistic setting for experimental design in which some model parameters were practically non-identifiable. A fully sequential experimental design procedure indicated that only 3 of the 36 censored data points were required to successfully remove all possible parameter non-identifiabilities.
4.2 Implementation and Limitations
The numerical implementation is provided as part of the Data2Dynamics (Steiert et al., 2019) modeling environment in MATLAB. The algorithm exploits the existing one dimensional profile likelihood calculation in order to construct the two-dimensional profile likelihood. Computationally, this amounts to about ∼1,000 local optimizations per two-dimensional likelihood profile, where local optimization is to be understood as deterministic optimization from a good initial guess for the parameters. Robustness of these fits is generally easier to obtain if the available data is appropriate for the size of the model, such that the model dynamics are constrained to some degree.
Problems associated with limited data availability go beyond numerical issues and are rooted in the structure of our approach. As a frequentist method, all information used in our experimental design scheme must stem from the data already measured. We have not assessed how much data needs to be initially available before a systematic experimental design procedure is practically feasible. However, the issue of lacking prior knowledge is not exclusive to our approach and a more general theme in non-linear experimental design. For the application in systems biology, initial data is often needed in proposing a suitable model, such that there will usually be data to start off with.
A practical limitation induced by insufficient data occurs if the range of reasonable measurement outcomes can not be predicted by the model, i.e. the validation profile reveals a practical non-identifiability of the model prediction. The existence of this non-identifiability complicates the estimation of the expected parameter uncertainty in the two-dimensional profile likelihood approach, because it relies on the prediction of the measurement outcomes given the model and current data. On the one hand, this fully utilizes the information available in the model, but on the other hand this constrains the applicability of the approach if the prediction for the measurement outcome is insufficiently constrained by the available model data. In case the model prediction of interest is not identifiable, a weak quadratic prior can be added to the validation profile in order to guarantee a finite sample space. This heuristic approach increases the scope of possible application settings. We emphasize that our experimental design procedure works best from a computational as well as methodical point of view if enough data is available such that model predictions are at least loosely constrained.
The usual assumption of the correctness of the model structure is especially important in our proposed method because it utilizes the model for predicting likely outcomes of the experiment and for calculating existing parameter uncertainties. This assumption is usually implicitly contained in any design strategy, but we emphasize that the full exploitation of the likelihood in our approach implies that the proposed experimental design will benefit greatly from solid prior knowledge about the model structure. This does not apply to prior knowledge about model parameters, because likelihood profiles account for parameter uncertainties.
The relationship between confidence intervals and likelihood profiles critically depends on the distributional assumption for the corresponding likelihood profile in Eq. 6. The implicit assumption that these likelihood ratios are
4.3 Comparison to Existing Methods
There are two conceptually different methods in the literature which we want to discuss, neglecting approaches based on the Fisher information matrix as reasoned before. One branch of methods deals with a Bayesian approach to experimental design which utilizes the Shannon information of the posterior distribution to plan optimal experiments. The other branch of methods discusses the concepts of frequentist approaches which find experimental designs by sampling relevant regions of the parameter space in order to assess the sensitivity of model predictions with respect to these parameters.
The Bayesian approach (Busetto et al., 2013; Huan and Marzouk, 2013; Liepe et al., 2013) is conceptually similar to our approach, but only applicable if suitable prior parameter distributions are available. The posterior parameter distribution after a possible measurement depends on unknown measurement outcomes which can be resolved by averaging the posterior distribution over the Bayesian predictive density. Similarly, our proposed frequentist method utilizes a predictive density for the measurement outcomes and a confidence density for the parameter estimates, eliminating the need for prior distributions. These “distributional estimators” (Xie and Singh, 2013) are implicitly derived from the likelihood profiles. This theoretical framework suggests the use of confidence and predictive densities in quantifying the confidence that an interval of parameter values or measurement outcomes contains the true parameter value or, respectively, a future measurement outcome.
Our method explicitly determines the impact of different plausible measurement outcomes of an experimental design on the parameter estimate of interest in order to derive a design criterion. This is different to existing frequentist approaches (Bazil et al., 2012; Steiert et al., 2012) which consider the sensitivity of model predictions to the different parameters which are consistent with the current data. Predictions which largely vary under these acceptable parameters indicate experimental conditions which are likely informative as they constrain the set of possible model dynamics. This approach has been awarded as best performing in the DREAM6 challenge (Steiert et al., 2012), although the feedback of the possible measurement results on the model parameter is not considered directly. This hinders intuitive interpretation of how a possible experiment feeds back into the parameter of interest and lacks a quantitative assessment of what constitutes a large variation of model predictions. Reversing the logic of this approach by considering the impact of likely model predictions on the parameter of interest leads to our refined approach, although this requires a higher computational cost.
4.4 Implications for Research
Our proposed approach can be used to select the most informative experimental design for a targeted parameter of interest. This is often relevant if there are certain biological parameters of interest which are not identifiable given the current data. We want to emphasize that although we discussed reduction of uncertainty for a single target parameter of interest, generalization to reducing the uncertainty for a model prediction, i.e. for a function of model parameters, is straightforward. The detailed quantitative and qualitative information gain by comparing two-dimensional profiles for the different experimental conditions comes with a higher computational cost compared to other approaches. As such, the detailed information provided by our method might be especially useful if experimental measurements require considerable time and resources and as such accuracy is favored over computational efficiency.
The experimental design approach only requires the existence of a suitable likelihood function and is therefore applicable in a broad spectrum of applications. We emphasize the novelty of our approach in employing confidence and predictive distributions as frequentist distributional measures for the confidence in parameter and measurement outcomes, which serve a similar function as Bayesian probabilities. Exploring the interaction of these concepts provides a point of interest for further research in frequentist experimental design.
4.5 Conclusion
To summarize, we established an experimental design procedure which aims at reducing the uncertainty for a parameter of interest. This design procedure reduces the likelihood function to a two-dimensional likelihood profile: One dimension informs our confidence of observing a certain measurement outcome for the given experimental condition, while the other dimension informs our confidence in the model parameter corresponding to the underlying true parameter. Testing our experimental design procedure on a simple model with simulated data and on a real model with experimental data revealed that our approach accurately predicted relevant experimental designs. Our method provides detailed information about possible experimental conditions on an easily interpretable quantitative as well as qualitative level.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: doi.org/10.6084/m9.figshare.16863559, https://github.com/Data2Dynamics/d2d.
Author Contributions
CK proposed the initial idea of exploiting two-dimensional likelihood profiles for experimental design. TL conceptualized the methods and formalized the presented approach with CK and JT providing input during the process. TL wrote the numerical implementation of the approach and created the results presented in this work. TL wrote a first manuscript draft and all authors revised the manuscript and provided contributions.
Funding
This work has been funded by the Federal Ministry of Education and Research of Germany (BMBF) (EA:Sys,FKZ031L0080), by the Federal Ministry of Education and Research of Germany (BMBF) within the Liver Systems Medicine network (LiSyM, Project number 031L0048), by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project number 272 983 813-TRR 179 and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germanys Excellence Strategy (CIBSS-EXC-2189-2100 249 960-390 939 984). The article processing charge was funded by the Baden-Württemberg Ministry of Science, Research and Art and the University of Freiburg in the funding programme Open Access Publishing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.800856/full#supplementary-material
References
Atkinson, A., and Donev, A. (1992). Optimum Experimental Designs. New York: Oxford University Press.
Bazil, J. N., Buzzard, G. T., and Rundell, A. E. (2012). A Global Parallel Model Based Design of Experiments Method to Minimize Model Output Uncertainty. Bull. Math. Biol. 74, 688–716. doi:10.1007/s11538-011-9686-9
Becker, V., Schilling, M., Bachmann, J., Baumann, U., Raue, A., Maiwald, T., et al. (2010). Covering a Broad Dynamic Range: Information Processing at the Erythropoietin Receptor. Science 328, 1404–1408. doi:10.1126/science.1184913
Bishop, T., Petersen, B., and Trayser, D. (1982). Another Look at the Statistician's Role in Experimental Planning and Design. Am. Stat. 36, 387–389. doi:10.1080/00031305.1982.10483058
Bjornstad, J. F. (1990). Predictive Likelihood: A Review. Stat. Sci. 5, 242–254. doi:10.1214/ss/1177012175
Busetto, A. G., Hauser, A., Krummenacher, G., Sunnåker, M., Dimopoulos, S., Ong, C. S., et al. (2013). Near-optimal Experimental Design for Model Selection in Systems Biology. Bioinformatics 29, 2625–2632. doi:10.1093/bioinformatics/btt436
Chis, O.-T., Villaverde, A. F., Banga, J. R., and Balsa-Canto, E. (2016). On the Relationship between Sloppiness and Identifiability. Math. Bioscie. 282, 147–161. doi:10.1016/j.mbs.2016.10.009
Cochran, W. G. (1973). Experiments for Nonlinear Functions (R.A. Fisher Memorial Lecture). J. Am. Stat. Assoc. 68, 771–781. doi:10.1080/01621459.1973.10481423
Faller, D., Klingmüller, U., and Timmer, J. (2003). Simulation Methods for Optimal Experimental Design in Systems Biology. Simulation 79, 717–725. doi:10.1177/0037549703040937
Ford, I., Titterington, D. M., and Kitsos, C. P. (1989). Recent Advances in Nonlinear Experimental Design. Technometrics 31, 49–60x. doi:10.1080/00401706.1989.10488475
Huan, X., and Marzouk, Y. M. (2013). Simulation-based Optimal Bayesian Experimental Design for Nonlinear Systems. J. Comput. Phys. 232, 288–317. doi:10.1016/j.jcp.2012.08.013
Kitano, H. (2002). Systems Biology: A Brief Overview. Science 295, 1662–1664. doi:10.1126/science.1069492
Kreutz, C., and Timmer, J. (2009). Systems Biology: Experimental Design. FEBS J. 276, 923–942. doi:10.1111/j.1742-4658.2008.06843.x
Kreutz, C., Raue, A., and Timmer, J. (2012). Likelihood Based Observability Analysis and Confidence Intervals for Predictions of Dynamic Models. BMC Syst. Biol. 6, 120. doi:10.1186/1752-0509-6-120
Liepe, J., Filippi, S., Komorowski, M., and Stumpf, M. P. H. (2013). Maximizing the Information Content of Experiments in Systems Biology. Plos Comput. Biol. 9, e1002888. doi:10.1371/journal.pcbi.1002888
Limpert, E., Stahel, W. A., and Abbt, M. (2001). Log-normal Distributions across the Sciences: Keys and Clues. BioScience 51, 341–352. doi:10.1641/0006-3568(2001)051[0341:LNDATS]2.0.CO;2
Lindley, D. V. (1956). On a Measure of the Information provided by an experiment. Ann. Math. Statist. 27, 986–1005. doi:10.1214/aoms/1177728069
Meeker, W. Q., and Escobar, L. A. (1995). Teaching about Approximate Confidence Regions Based on Maximum Likelihood Estimation. Am. Stat. 49, 48–53. doi:10.1080/00031305.1995.10476112
Nurse, P., and Hayles, J. (2011). The Cell in an Era of Systems Biology. Cell 144, 850–854. doi:10.1016/j.cell.2011.02.045
Raue, A., Kreutz, C., Maiwald, T., Bachmann, J., Schilling, M., Klingmüller, U., et al. (2009). Structural and Practical Identifiability Analysis of Partially Observed Dynamical Models by Exploiting the Profile Likelihood. Bioinformatics 25, 1923–1929. doi:10.1093/bioinformatics/btp358
Raue, A., Schilling, M., Bachmann, J., Matteson, A., Schelke, M., Kaschek, D., et al. (2013). Lessons Learned from Quantitative Dynamical Modeling in Systems Biology. PloS ONE 8, e74335. doi:10.1371/journal.pone.0074335
Steiert, B., Raue, A., Timmer, J., and Kreutz, C. (2012). Experimental Design for Parameter Estimation of Gene Regulatory Networks. PloS one 7, e40052. doi:10.1371/journal.pone.0040052
Steiert, B., Kreutz, C., Raue, A., and Timmer, J. (2019). “Recipes for Analysis of Molecular Networks Using the Data2dynamics Modeling Environment,” in Modeling Biomolecular Site Dynamics: Methods and Protocols. Editor W. S. Hlavacek (New York, NY: Springer New York), 341–362. doi:10.1007/978-1-4939-9102-0_16
Wasserstein, R. L., and Lazar, N. A. (2016). The ASA Statement on P-Values: Context, Process, and Purpose. Am. Stat. 70, 129–133. doi:10.1080/00031305.2016.1154108
Wieland, F.-G., Hauber, A. L., Rosenblatt, M., Tönsing, C., and Timmer, J. (2021). On Structural and Practical Identifiability. Curr. Opin. Syst. Biol. 25, 60–69. doi:10.1016/j.coisb.2021.03.005
Wilks, S. S. (1938). The Large-Sample Distribution of the Likelihood Ratio for Testing Composite Hypotheses. Ann. Math. Statist. 9, 60–62. doi:10.1214/aoms/1177732360
Keywords: experimental design, profile likelihood, systems biology, mathematical model, parameter uncertainty, prediction uncertainty, confidence distribution
Citation: Litwin T, Timmer J and Kreutz C (2022) Optimal Experimental Design Based on Two-Dimensional Likelihood Profiles. Front. Mol. Biosci. 9:800856. doi: 10.3389/fmolb.2022.800856
Received: 24 October 2021; Accepted: 07 January 2022;
Published: 23 February 2022.
Edited by:
Ernesto Perez-Rueda, Universidad Nacional Autónoma de México, MexicoReviewed by:
Juan Guillermo Diaz Ochoa, PERMEDIQ GmbH, GermanyLuis Diambra, National University of La Plata, Argentina
Copyright © 2022 Litwin, Timmer and Kreutz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tim Litwin, bGl0d2luQGltYmkudW5pLWZyZWlidXJnLmRl
 Tim Litwin
Tim Litwin Jens Timmer
Jens Timmer Clemens Kreutz
Clemens Kreutz