- 1College of Basic Medical, Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Research Center of Integrative Medicine, School of Basic Medical Sciences, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3Department of Trauma and Foot-Ankle Surgery, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Lingnan Medical Research Center, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 5Department of Traumatology, The Third Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 6The Fifth Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
Copper is an indispensable trace metal element in human body, and copper deficiency is rare in clinic. However, diseases associated with serum copper deficiency, such as leukopenia, neutropenia, arthritis, osteoporosis, and bone defects, are well known. Copper ions can also achieve the effect of fighting pathogenic bacteria through the “contact killing” characteristic. Copper ion is also an important cofactor of bone matrix synthase, plays an important role in the pathophysiology of orthopedic diseases. The present review highlights the biological functions of copper in immunity, bone diseases and stem cells, as well as potential drug development targeting copper status for diagnostics and therapeutics of copper-associated bone diseases.
Introduction
Copper, an essential trace element as important co-factors of various chaperones and enzymes, is vital for maintenance of integrity and homeostasis of the human organism, such as the skeletal system (Pavelková et al., 2018; Maung et al., 2021). This current review summarizes advancement of copper involvement of immune response, and potential clinical implication for management of orthopedic diseases.
Copper regulation of the immune system
Trace element copper is necessary for a series of human physiological processes, including damage site repair and immune system function (Livingstone, 2017). Copper is an essential redox-active trace element for proper functioning of almost all organisms (Prohaska and Lukasewycz, 1990; Tapiero and Tew, 2003).
Generally, copper intake for infants of 0–6 months is 200, and 220 mg/d for 7–12-month-old infants. The intake of copper in children aged 1–3 years was 340 mg/day. Copper intake for children aged 4–8 was 440 mg/day; Copper intake for children aged 9–13 was 700 mg/day; Copper intake for adults 19–50 is 900 mg/day. 1,000 mg/day during pregnancy and 1,300 mg/day during lactation. Therefore, copper demand increases during pregnancy and lactation. Copper gluconate is the only copper supplement listed in the United States Pharmacopoeia Convention for oral use. Copper in food is mainly absorbed through the stomach and the upper part of the small intestine (Turnlund et al., 1997). Fifty years ago, Newberne et al. (1968) observed that copper-deficient mice infected with Salmonella typhimurium died faster and lived shorter than mice infected only with S. typhimurium in the control group.
Notably, copper is critical for immune functioning (Sullivan and Ochs, 1978; Prohaska and Lukasewycz, 1990; Tapiero and Tew, 2003). Copper metabolism changes during inflammation, and the level of serum copper metabolism increases. The lack of copper will lead to impaired energy production and affect the operation of the immune system (Hordyjewska et al., 2014). The immune system, mainly including innate and adaptive immunity, is a natural defensive response to stimulating factors such as infection, injury, and toxins (Medzhitov, 2008). Whilst excessive or improper immune response would cause numerous inflammatory diseases, such as cardiovascular disease (Ferrucci and Fabbri, 2018), and arthritis (Davidson and Diamond, 2001). Increasing evidence suggests the linking between copper deficiency and immune hypo-responsiveness (Immune tolerance). Reduced dietary copper after parturition causes immune suppression characterized by hypo-responsiveness to sheep red blood cells, B- and T-cell mitogens and alterations in splenic lymphocyte subpopulations (Prohaska and Lukasewycz, 1981; Lukasewycz and Prohaska, 1983; Vyas and Chandra, 1983; Lukasewycz et al., 1985; Blakley and Hamilton, 1987). Copper deficiency would cause the alteration of the acute-phase protein response to viral infection and may also affect lymphocyte responsiveness to mitogen stimulation (Arthington et al., 1996). During infection and inflammation, serum copper concentration increases, and ceruloplasmin activity also increases. These changes in copper metabolism result from interleukin-1-mediated increases in liver ceruloplasmin synthesis and release, an acute phase protein (Pekarek et al., 1972). Barber and Cousins showed that ceruloplasmin synthesis could be induced by intraperitoneal injection of interleukin-1 regardless of copper status (Barber and Cousins, 1988). Therefore, copper ion supplementation is necessary for ceruloplasmin synthesis in copper-deficient mice (Stabel and Spears, 1989). Thus, copper plays an important role in the mammalian immune system, and bacterial responses to copper could be a suitable target for future drug development.
The “contact killing” toxicity of copper alloy surface materials to clinically relevant pathogens can reduce the transmission of clinically relevant pathogens (Giachino and Waldron, 2020). In general, copper contributes to immunity through two pathways : 1) by participating in the development and differentiation of immune cells, and 2) by providing antifungal agents isolating machinery by metal in the host or as a bombarding property. Macrophages fight against invading pathogens by mobilizing copper ions in the body to create a copper-rich environment. The role of copper in the axis of infection has been further clarified by studies of copper excess or copper deficiency in resistance to pathogens such as mycobacterium Escherichia coli and Salmonella (Li et al., 2019). A large amount of evidence shows that copper regulation is key in innate and adaptive immune regulation. Disrupting key copper regulation often leads to a sharp increase in the pathogenicity of pathogens in the human body. In the future, copper containing complexes and body copper regulation will be the research hotspot of resistance to pathogens.
Application of copper in bone diseases
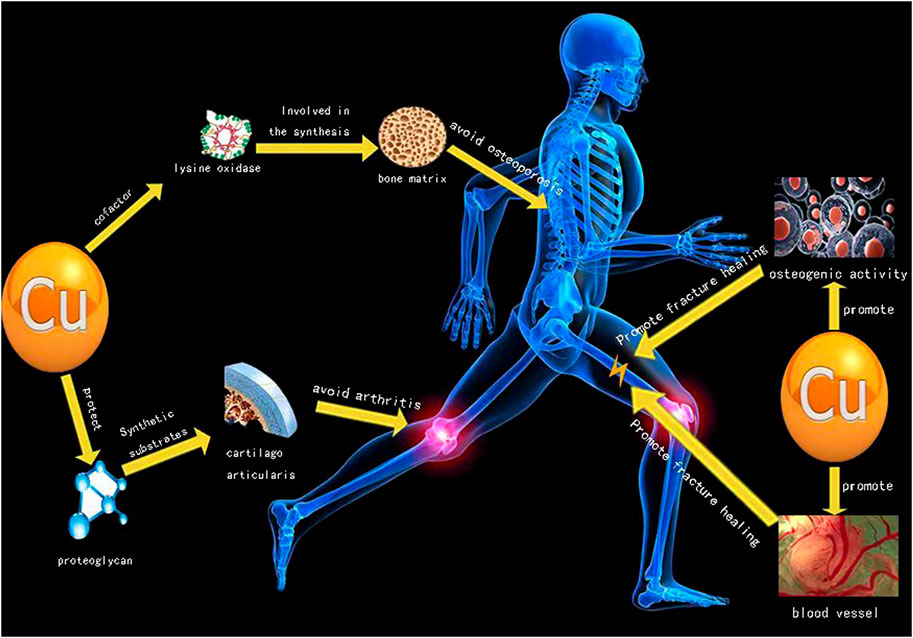
Arthritis, fracture and osteoporosis are among the three main diseases in orthopedics, and Cu plays an important role in treatment of these three main diseases (Figure 1). The Cu content in the human body is between 50 and 120 mg, and nearly two-thirds of Cu content is stored in muscles and bones. Liver is the key organ to maintain the content of copper in plasma (Olivares and Uauy, 1996; Turnlund et al., 1998). The content of copper in the body is related to bone deformity, increased osteoporosis, impaired melanin synthesis and immune response during development. When serum copper is deficient, it will increase the frequency of infection, cardiovascular diseases, changes in cholesterol metabolism and other trace element metabolic disorders (Olivares and Uauy, 1996; Turnlund et al., 1998).
Notably, scholars have compared healthy horses with horses suffered from developmental orthopedic disorders, indicating that horses with developmental orthopedic disorders are associated with reduced serum copper levels (Coskun et al., 2016). Furthermore, interference with trace elements, such as a lack of copper, iron, zinc and other metals, has long been regarded as a risk factor for osteoporosis (Hsieh and Navia, 1980; Davey, 1997; Wynchank and Saltman, 1997).
Copper is an essential cofactor of various enzymes involved in the synthesis of various bone matrix components (Lowe et al., 2002). Copper ion is essential for building strong bones via regulation of bone metabolism, and studies have shown that post-menopausal women with osteoporosis have significantly lower levels of copper than normal (Mahdavi-Roshan et al., 2015). As a key cofactor of lysine oxidase, copper participates in bone and cartilage metabolism, as a major enzyme involved in collagen cross-linking of cartilage-rich tissues. Animal studies have shown that copper deficiency can lead to bone strength reduction and bone loss, and eventually lead to osteoporosis. One study showed that bone mineral density in children under 3 years of age was positively correlated with age and serum copper levels (Wu et al., 2021b). Either copper deficiency or excessive copper accumulation would be detrimental to bones. Therefore, to maintain a moderate level of serum copper and healthy copper metabolism could facilitate the maintenance of healthy bone remodeling and reduction of the risk of hip fractures. But high levels of serum copper, especially in adult men, can increase the risk of fractures (Qu et al., 2018). Supplementation of trace elements (including copper) can prevent and reduce bone loss (Strause et al., 1994). Patients with thoracic hyperosteogeny (Cox et al., 1983), avascular necrosis of the femoral head (Milachowski, 1988), femoral neck fracture (Conlan et al., 1990), and lumbar osteoporosis had lower copper levels (Howard et al., 1992).
Involvement of copper in arthritis
Increasing evidence has suggested a disturbance in copper homeostasis and abnormal copper metabolism in rheumatoid disease, a chronic inflammatory disease of the whole joint (Scudder, 1976; Scudder et al., 1978; Rafter, 1987; Sarban et al., 2005). Copper plays an important role in immune response and anti-arthritis. A comprehensive meta-analysis suggests that increased serum level of copper is generally present in rheumatoid arthritis patients. The research history of association between copper and arthritis could be traced back to 40 years ago, Whitehouse et al. detected moderate anti-inflammatory and antiarthritic effects of various copper salts in rats (Whitehouse and Walker, 1978). Cartilage is composed of chondrocytes, water, and cartilage matrix. The extracellular matrix of articular cartilage contains proteoglycans, collagen, and non-collagenous proteins (Kleine and Singh, 1982; Chaminade et al., 1982; Fife and Brandt, 1984). Altered micro-environments in extracellular matrix composition and mechanical properties during arthritis progression are critically involved in arthritis pathology (Maldonado and Nam, 2013; Lin et al., 2020). Thus, targeting of extracellular matrix of cartilage is becoming increasingly attractive for arthritis therapy as modifications to the extracellular matrix (ECM) could be either causal or consequential of arthritis (Schultz, 2019).
Glycoprotein, a 550,000-Da non-collagenous cartilage matrix component, is present in hyaline cartilage and fibrocartilage (Fife et al., 1986). Pasqualicchio et al. (1996) have demonstrated copper supplementation is able to protect articular cartilage against synovial-induced proteoglycan depletion ex vivo, which reveals possible mechanisms by which copper exerts its anti-inflammatory and anti-arthritic actions. A series of studies have assessed serum copper levels in patients with rheumatoid arthritis (RA), with conflicting results. This is because inflammatory biomarkers such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor—α (TNF-α) up-regulate the synthesis and secretion of ceruloplasmin in liver cells during rheumatoid arthritis inflammation. When ceruloplasmin is transferred from the liver cell to the serum, the concentration of serum copper increases. Drugs containing copper ions also play an important role in the treatment of gout arthritis. Mubin et al. found that copper oxide nanoparticles could reduce serum levels in rats and treat gout arthritis caused by hyperuricemia (Kiyani et al., 2020). A copper-incorporated bioactive glass-ceramics scaffolds (Cu-BGC) can repair cartilage damage and reduce inflammatory response caused by osteoarthritis. However, a clinical trial suggests that magnetic wrist straps, and also copper bracelets, displayed neither statistically significant, nor clinically meaningful, therapeutic effects upon rheumatoid arthritis. Thus, to find a right therapeutic route on copper-based therapy is essential (Richmond et al., 2013).
To investigate role of copper on osteoclast function. Li and Yu (2007) isolated osteoclasts from the long limb bones of newborn rabbits and cultured them with cu-containing culture medium and deactivated human tooth slices. It was proved that extracellular copper ions could inhibit the absorption of osteoclasts. Previous studies have shown that Cu is an important cofactor of many enzymes. It is also an angiogenesis promoting and antibacterial agent. Milkovic et al. (2014) observed that low concentration of Cu could improve the viability and growth of human osteoblast like cells. In the treatment of bone defects by bone regeneration, new copper—containing composite materials can enhance osteogenic activity and promote angiogenesis at the early stage of the treatment process. At the same time, the composite can be combined with silicon to complete degradation (Wu et al., 2021a). Based on the research and development of metal manufacturing technology, there are three main methods to prepare cu-containing biomaterials to promote fracture healing via 1) supplementation of copper-containing biomaterials; 2) Add copper coating on metal surface; 3) Binding of copper nanoparticles with other functional bio-metals (Wang et al., 2021).
Copper involvement in cellular functions of mesenchymal stem cells
Mesenchymal stem cells are one of the most intensively studies adult stem cells. Bone marrow mesenchymal stem cells can reduce the proinflammatory potential of dendritic cells by inhibiting the production of tumor necrosis factor during innate immunity. In addition, incubation of plasmacytoid dendritic cells with mesenchymal stem cells up-regulated the production of the anti-inflammatory cytokine IL-10 (Aggarwal and Pittenger, 2005). Therefore, the combination therapy of mesenchymal stem cells on dendritic cells and plasmacytoid dendritic cells may be translated into effective anti-inflammatory and immunomodulation-associated therapeutics (Aggarwal and Pittenger, 2005; Uccelli et al., 2008). Mesenchymal stem cells have been proved to inhibit respiratory burst and delay the spontaneous apoptosis of dormant and activated neutrophils through IL-6-relevant mechanism (Raffaghello et al., 2007). Therefore, mesenchymal stem cells play an important role in the resting preservation of neutrophils and avoiding respiratory burst (Craddock et al., 1960; Uccelli et al., 2008).
Hypoxia inducible factors (HIFs) play a central role for cellular adaptation to low oxygen microenvironments through regulation of diverse downstream genes, which is also closely associated with the energy metabolism and immune alterations (Jaakkola et al., 2001). During inflammatory response, infected and inflamed tissues are usually hypoxic, and HIFs are conducive to the adaptation and normal functioning of various types of immune cells (Finlay et al., 2012). HIF-1 consists of two subunits: HIF-1α and HIF-1β. Under anoxic condition, HIF-1α abscond from the disassemble pathway, accumulate collect in the cytosol, and is translocated into the nucleus, where it dimerizes with HIF-1β, interacts with cofactors to assemble the HIF-1 transcriptional complex, and binds to the hypoxia-responsive element sites of its target genes, leading to transactivation of target genes expression. About 300 genes are regulated by HIF-1α (Fedele et al., 2002; Greijer et al., 2005). Hypoxia-inducible factor (HIF)-1-dependent signalling pathways regulating bone healing. Once bone injury or hypoxia happens, HIF-1a activation and stabilization occur. Vascular endothelial growth factor VEGF, stromal cell-derived factor-1, and CXC chemokine receptor CXCR 4 are directly positively regulated by HIF-1a. Increased expression of VEGF, SDF-1, and CXCR4 stimulates mesenchymal stem cell (MSC) homing (Lin et al., 2017). Remarkably, copper is essential for transactivation of HIF-1α for regulation of various of target gene expression (Martin et al., 2005). Additionally, Burghardt et al. found that copper in the concentration range of 0.1 mM could promote the proliferation of MSC and the osteogenic differentiation of MSCs. He also observed that copper could increase the activity of alkaline phosphatase in MSCs, and the expression of osteogenic markers such as type I collagen, o osteocalcin (OCN), and osteopontin (OPN) (Burghardt et al., 2015). The results of Li et al. (2019) suggest that sodium copper chlorophyllin can not only promote the proliferation and differentiation of bone marrow mesenchymal stem cells in mice with aplastic anemia, but also improve their immune regulation ability.
Conclusion
This review summarizes the effects of copper ion on immune response, arthritis, osteoporosis and fracture, and expounds the application progress of copper ion in biomedicine and copper-containing materials. As an important part of immune response, copper ions are released from ceruloplasmin at the stage of inflammatory reaction, resulting in increased serum copper. Copper ions can also achieve the effect of fighting pathogenic bacteria through the “contact killing” property.
The application of cu-containing nanomaterials and cu-containing coatings in promoting fracture healing and antibacterial research has attracted increasing attention of scholars. The application of cu-containing drugs and materials can avoid the issues of high cost and easy loss of biological activity, and can strongly stimulate the expression of related genes, with better clinical safety, thus inducing bone tissue repair and regeneration to complement each other. In conclusion, copper will provide a safe, efficient, and innovative activity platform for the biomedical field.
Author contributions
LX, JZ, and YaL: reference analyzation, original drafting, contributed equally; BW, WL, and YiL: study formulation, manuscript revision.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 81873326).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aggarwal, S., and Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105 (4), 1815–1822. doi:10.1182/blood-2004-04-1559
Arthington, J. D., Corah, L. R., and Blecha, F. (1996). The effect of molybdenum-induced copper deficiency on acute-phase protein concentrations, superoxide dismutase activity, leukocyte numbers, and lymphocyte proliferation in beef heifers inoculated with bovine herpesvirus-1. J. Anim. Sci. 74 (1), 211–217. doi:10.2527/1996.741211x
Barber, E. F., and Cousins, R. J. (1988). Interleukin-1-stimulated induction of ceruloplasmin synthesis in normal and copper-deficient rats. J. Nutr. 118 (3), 375–381. doi:10.1093/jn/118.3.375
Blakley, B. R., and Hamilton, D. L. (1987). The effect of copper deficiency on the immune response in mice. Drug. Nutr. Interact. 5 (2), 103–111.
Burghardt, I., Luthen, F., Prinz, C., Kreikemeyer, B., Zietz, C., Neumann, H. G., et al. (2015). A dual function of copper in designing regenerative implants. Biomaterials 44, 36–44. doi:10.1016/j.biomaterials.2014.12.022
Chaminade, F., Stanescu, V., Stanescu, R., Maroteaux, P., and Peyron, J. G. (1982). Noncollagenous proteins in cartilage of normal subjects and patients with degenerative joint disease. A gel electrophoretic study. Arthritis Rheum. 25 (9), 1078–1083. doi:10.1002/art.1780250908
Conlan, D., Korula, R., and Tallentire, D. (1990). Serum copper levels in elderly patients with femoral-neck fractures. Age Ageing 19 (3), 212–214. doi:10.1093/ageing/19.3.212
Coskun, A., Ozdemir, O., Erol, M., and Kirbiyik, H. (2016). The relationship of copper concentrations in feed and plasma to developmental orthopedic disease in foals. Veterinarski arh. 86 (3), 287–294. doi:10.1016/j.jhsa.2013.12.004
Cox, J. M., Gideon, D., and Rogers, F. J. (1983). Incidence of osteophytic lipping of the thoracic spine in coronary heart disease: Results of a pilot study. J. Am. Osteopath. Assoc. 82 (11), 837–838. doi:10.1515/jom-1983-830711
Craddock, C. G., Perry, S., Ventzke, L. E., Lawrence, J. S., Baker, M. H., and Paul, G. (1960). Evaluation of marrow granulocytic reserves in normal and disease states. Blood 15, 840–855. doi:10.1182/blood.v15.6.840.840
Davey, D. A. (1997). Osteoporosis, osteopenia and fracture risk: Widening the therapeutic horizons. S. Afr. Med. J. 87 (7), 285. doi:10.7196/samj.5400
Davidson, A., and Diamond, B. (2001). Autoimmune diseases. N. Engl. J. Med. 345 (5), 340–350. doi:10.1056/NEJM200108023450506
Fedele, A. O., Whitelaw, M. L., and Peet, D. J. (2002). Regulation of gene expression by the hypoxia-inducible factors. Mol. Interv. 2 (4), 229–243. doi:10.1124/mi.2.4.229
Ferrucci, L., and Fabbri, E. (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 15 (9), 505–522. doi:10.1038/s41569-018-0064-2
Fife, R. S., and Brandt, K. D. (1984). Identification of a high-molecular-weight (greater than 400 000) protein in hyaline cartilage. Biochim. Biophys. Acta 802 (3), 506–514. doi:10.1016/0304-4165(84)90370-2
Fife, R. S., Palmoski, M. J., and Brandt, K. D. (1986). Metabolism of a cartilage matrix glycoprotein in normal and osteoarthritic canine articular cartilage. Arthritis Rheum. 29 (10), 1256–1262. doi:10.1002/art.1780291011
Finlay, D. K., Rosenzweig, E., Sinclair, L. V., Feijoo-Carnero, C., Hukelmann, J. L., Rolf, J., et al. (2012). PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J. Exp. Med. 209 (13), 2441–2453. doi:10.1084/jem.20112607
Giachino, A., and Waldron, K. J. (2020). Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 114 (3), 377–390. doi:10.1111/mmi.14522
Greijer, A. E., P van der Groep, D. K., Shvarts, A., Semenza, G. L., Meijer, G. A., Belien, J. A. M., et al. (2005). Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). J. Pathol. 206 (3), 291–304. doi:10.1002/path.1778
Hordyjewska, A., Popiołek, Ł., and Kocot, J. (2014). The many “faces” of copper in medicine and treatment. Biometals 27 (4), 611–621. doi:10.1007/s10534-014-9736-5
Howard, G., Andon, M., Bracker, M., Saltman, P., and Strause, L. (1992). Low serum copper, a risk factor additional to low dietary calcium in postmenopausal bone loss. J. trace Elem. Exp. Med. 5, 23–31.
Hsieh, H. S., and Navia, J. M. (1980). Zinc deficiency and bone formation in Guinea pig alveolar implants. J. Nutr. 110 (8), 1581–1588. doi:10.1093/jn/110.8.1581
Jaakkola, P., Mole, D. R., Tian, Y. M., Wilson, M. I., Gielbert, J., Gaskell, S. J., et al. (2001). Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292 (5516), 468–472. doi:10.1126/science.1059796
Kiyani, M. M., Rehman, H., Hussain, M. A., Jahan, S., Afzal, M., Nawaz, I., et al. (2020). Inhibition of hyperuricemia and gouty arthritis in BALB/c mice using copper oxide nanoparticles. Biol. Trace Elem. Res. 193 (2), 494–501. doi:10.1007/s12011-019-01734-2
Kleine, T. O., and Singh, A. (1982). Isolation and characterization of glycoproteins in proteoglycan aggregates of calf rib cartilage. Connect. Tissue Res. 9 (3), 145–155. doi:10.3109/03008208209160255
Li, B. B., and Yu, S. F. (2007). In vitro study of the effects of copper ion on osteoclastic resorption in various dental mineralized tissues. Zhonghua Kou Qiang Yi Xue Za Zhi 42 (2), 110–113. doi:10.1631/jzus.2007.B0566
Li, C., Li, Y., and Ding, C. (2019). The role of copper homeostasis at the host-pathogen axis: From bacteria to fungi. Int. J. Mol. Sci. 20 (1), 175. doi:10.3390/ijms20010175
Lin, W., Xu, L., Zwingenberger, S., Gibon, E., Goodman, S. B., and Li, G. (2017). Mesenchymal stem cells homing to improve bone healing. J. Orthop. Transl. 9, 19–27. doi:10.1016/j.jot.2017.03.002
Lin, W., Xu, L., and Li, G. (2020). Molecular insights into lysyl oxidases in cartilage regeneration and rejuvenation. Front. Bioeng. Biotechnol. 8, 359. doi:10.3389/fbioe.2020.00359
Livingstone, C. (2017). Review of copper provision in the parenteral nutrition of adults [formula: See text]. Nutr. Clin. Pract. 32 (2), 153–165. doi:10.1177/0884533616673190
Lowe, N. M., Fraser, W. D., and Jackson, M. J. (2002). Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc. Nutr. Soc. 61 (2), 181–185. doi:10.1079/PNS2002154
Lukasewycz, O. A., Prohaska, J. R., Meyer, S. G., Schmidtke, J. R., Hatfield, S. M., and Marder, P. (1985). Alterations in lymphocyte subpopulations in copper-deficient mice. Infect. Immun. 48 (3), 644–647. doi:10.1128/IAI.48.3.644-647.1985
Lukasewycz, O. A., and Prohaska, J. R. (1983). Lymphocytes from copper-deficient mice exhibit decreased mitogen reactivity. Nutr. Res. 3 (3), 335–341. doi:10.1016/S0271-5317(83)80083-9
Mahdavi-Roshan, M., Ebrahimi, M., and Ebrahimi, A. (2015). Copper, magnesium, zinc and calcium status in osteopenic and osteoporotic post-menopausal women. Clin. Cases Min. Bone Metab. 12 (1), 18–21. doi:10.11138/ccmbm/2015.12.1.018
Maldonado, M., and Nam, J. (2013). The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 284873. doi:10.1155/2013/284873
Martin, F., Linden, T., Katschinski, D. M., Oehme, F., Flamme, I., Mukhopadhyay, C. K., et al. (2005). Copper-dependent activation of hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin regulation. Blood 105 (12), 4613–4619. doi:10.1182/blood-2004-10-3980
Maung, M. T., Carlson, A., Olea-Flores, M., Elkhadragy, L., Schachtschneider, K. M., Navarro-Tito, N., et al. (2021). The molecular and cellular basis of copper dysregulation and its relationship with human pathologies. FASEB J. 35 (9), e21810. doi:10.1096/fj.202100273RR
Medzhitov, R. (2008). Origin and physiological roles of inflammation. Nature 454 (7203), 428–435. doi:10.1038/nature07201
Milachowski, K. A. (1988). Investigation of ischaemic necrosis of the femoral head with trace elements. Int. Orthop. 12 (4), 323–330. doi:10.1007/BF00317832
Milkovic, L., Hoppe, A., Detsch, R., Boccaccini, A. R., and Zarkovic, N. (2014). Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J. Biomed. Mat. Res. A 102 (10), 3556–3561. doi:10.1002/jbm.a.35032
Newberne, P. M., Hunt, C. E., and Young, V. R. (1968). The role of diet and the reticuloendothelial system in the response of rats to Salmonella typhilmurium infection. Br. J. Exp. Pathol. 49 (5), 448–457. doi:10.3321/j.issn:0253-9829.2009.06.021
Olivares, M., and Uauy, R. (1996). Limits of metabolic tolerance to copper and biological basis for present recommendations and regulations. Am. J. Clin. Nutr. 63 (5), 846S–52S. doi:10.1093/ajcn/63.5.846
Pasqualicchio, M., Gasperini, R., Velo, G. P., and Davies, M. E. (1996). Effects of copper and zinc on proteoglycan metabolism in articular cartilage. Mediat. Inflamm. 5 (2), 95–99. doi:10.1155/S0962935196000154
Pavelková, M., Vysloužil, J., Kubová, K., and Vetchý, D. (2018). Biological role of copper as an essential trace element in the human organism. Ceska Slov. Farm. 67 (4), 143–153.
Pekarek, R. S., Powanda, M. C., and Wannemacher, R. W. (1972). The effect of leukocytic endogenous mediator (LEM) on serum copper and ceruloplasmin concentrations in the rat. Proc. Soc. Exp. Biol. Med. 141 (3), 1029–1031. doi:10.3181/00379727-141-36926
Prohaska, J. R., and Lukasewycz, O. A. (1981). Copper deficiency suppresses the immune response of mice. Science 213 (4507), 559–561. doi:10.1126/science.7244654
Prohaska, J. R., and Lukasewycz, O. A. (1990). Effects of copper deficiency on the immune system. Adv. Exp. Med. Biol. 262, 123–143. doi:10.1007/978-1-4613-0553-8_11
Qu, X., He, Z., Han, Q., Zhai, Z., Mao, Z., Yu, Z., et al. (2018). Serum copper levels are associated with bone mineral density and total fracture. J. Orthop. Transl. 14, 34–44. doi:10.1016/j.jot.2018.05.001
Raffaghello, L., Bianchi, G., Bertolotto, M., Montecucco, F., Busca, A., Franco, D., et al. (2007). Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 26 (1), 151–162. doi:10.1634/stemcells.2007-0416
Rafter, G. W. (1987). Rheumatoid arthritis: A disturbance in copper homeostasis. Med. Hypotheses 22 (3), 245–249. doi:10.1016/0306-9877(87)90190-3
Richmond, S. J., Gunadasa, S., Bland, M., and MacPherson, H. (2013). Copper bracelets and magnetic wrist straps for rheumatoid arthritis–analgesic and anti-inflammatory effects: A randomised double-blind placebo controlled crossover trial. PLoS One 8 (9), e71529. doi:10.1371/journal.pone.0071529
Sarban, S., Kocyigit, A., and Isikan, U. (2005). Synovial fluid and plasma selenium, copper, zinc, and iron concentrations in patients with rheumatoid arthritis and osteoarthritis. Biol. Trace Elem. Res. 106 (2), 123–132. doi:10.1385/BTER:106:2:123
Schultz, C. (2019). Targeting the extracellular matrix for delivery of bioactive molecules to sites of arthritis. Br. J. Pharmacol. 176 (1), 26–37. doi:10.1111/bph.14516
Scudder, P. R., Al-Timimi, D., McMurray, W., White, A. G., Zoob, B. C., and Dormandy, T. L. (1978). Serum copper and related variables in rheumatoid arthritis. Ann. Rheum. Dis. 37 (1), 67–70. doi:10.1136/ard.37.1.67
Scudder, P. R. (1976). Copper metabolism in rheumatoid arthritis and related disorders. London: University of London External.
Stabel, J. R., and Spears, J. W. (1989). Effect of copper on immune function and disease resistance. Adv. Exp. Med. Biol. 258, 243–252. doi:10.1007/978-1-4613-0537-8_22
Strause, L., Paul, S., Smith, K. T., Bracker, M., and Andon, M. B. (1994). Spinal bone loss in postmenopausal women supplemented with calcium and trace minerals. J. Nutr. 124 (7), 1060–1064. doi:10.1093/jn/124.7.1060
Sullivan, J. L., and Ochs, H. D. (1978). Copper deficiency and the immune system. Lancet 2 (8091), 686. doi:10.1016/s0140-6736(78)92806-4
Tapiero, H., and Tew, K. D. (2003). Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 57 (9), 399–411. doi:10.1016/s0753-3322(03)00081-7
Turnlund, J. R., Scott, K. C., Peiffer, G. L., M Jang, A., Keyes, W. R., Keen, C. L., et al. (1997). Copper status of young men consuming a low-copper diet. Am. J. Clin. Nutr. 65 (1), 72–78. doi:10.1093/ajcn/65.1.72
Turnlund, J. R., Keyes, W. R., Peiffer, G. L., and Scott, K. C. (1998). Copper absorption, excretion, and retention by young men consuming low dietary copper determined by using the stable isotope 65Cu. Am. J. Clin. Nutr. 67 (6), 1219–1225. doi:10.1093/ajcn/67.6.1219
Uccelli, A., Moretta, L., and Pistoia, V. (2008). Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 8 (9), 726–736. doi:10.1038/nri2395
Vyas, D., and Chandra, R. K. (1983). Thymic factor activity, lymphocyte stimulation response and antibody producing cells in copper deficiency. Nutr. Res. 3 (3), 343–349. doi:10.1016/s0271-5317(83)80084-0
Wang, P., Yuan, Y., Xu, K., Zhong, H., Yang, Y., Jin, S., et al. (2021). Biological applications of copper-containing materials. Bioact. Mat. 6 (4), 916–927. doi:10.1016/j.bioactmat.2020.09.017
Whitehouse, M. W., and Walker, W. R. (1978). Copper and inflammation. Agents Actions 8 (1), 85–90. doi:10.1007/BF01972407
Wu, Q., Xu, S., Wang, X., Jia, B., Han, Y., Zhuang, Y., et al. (2021a). Complementary and synergistic effects on osteogenic and angiogenic properties of copper-incorporated silicocarnotite bioceramic: In vitro and in vivo studies. Biomaterials 268, 120553. doi:10.1016/j.biomaterials.2020.120553
Wu, Z., Yuan, Y., Tian, J., Long, F., and Luo, W. (2021b). The associations between serum trace elements and bone mineral density in children under 3 years of age. Sci. Rep. 11 (1), 1890–1898. doi:10.1038/s41598-021-81501-3
Keywords: copper, immune response, osteoporosis, arthritis, fracture, mesenchymal stem cells, cu-containing biomaterials
Citation: Liu Y, Zhu J, Xu L, Wang B, Lin W and Luo Y (2022) Copper regulation of immune response and potential implications for treating orthopedic disorders. Front. Mol. Biosci. 9:1065265. doi: 10.3389/fmolb.2022.1065265
Received: 09 October 2022; Accepted: 17 November 2022;
Published: 05 December 2022.
Edited by:
Yiyi Xu, University of Gothenburg, SwedenReviewed by:
Meng Tian, Sichuan University, ChinaCopyright © 2022 Liu, Zhu, Xu, Wang, Lin and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Wang, Z3p0Y213YW5nYmluMTk3M0AxNjMuY29t; Weiping Lin, d2VpcGluZy5saW5AY3JtaC1jYXMub3JnLmhr; Yiwen Luo, Z3pobHl3QGd6dWNtLmVkdS5jbg==
†These authors have contributed equally to this work
 Yamei Liu
Yamei Liu Junlang Zhu3†
Junlang Zhu3† Liangliang Xu
Liangliang Xu Bin Wang
Bin Wang Weiping Lin
Weiping Lin