- 1Institute for Virology and Immunobiology, Julius-Maximilians-University of Würzburg, Würzburg, Germany
- 2HHV-6 Foundation, Santa Barbara, CA, United States
- 3Department of Cancer Biology and Genetics (CBG), Institute for Behavioral Medicine Research (IBMR), The Ohio State University, Columbus, OH, United States
First exposure to various human herpesviruses (HHVs) including HHV-6, HCMV and EBV does not cause a life-threatening disease. In fact, most individuals are frequently unaware of their first exposure to such pathogens. These herpesviruses acquire lifelong latency in the human body where they show minimal genomic activity required for their survival. We hypothesized that it is not the latency itself but a timely, regionally restricted viral reactivation in a sub-set of host cells that plays a key role in disease development. HHV-6 (HHV-6A and HHV-6B) and HHV-7 are unique HHVs that acquire latency by integration of the viral genome into sub-telomeric region of human chromosomes. HHV-6 reactivation has been linked to Alzheimer’s Disease, Chronic Fatigue Syndrome, and many other diseases. However, lack of viral activity in commonly tested biological materials including blood or serum strongly suggests tissue specific localization of active HHV-6 genome. Here in this paper, we attempted to analyze active HHV-6 transcripts in postmortem tissue biopsies from a small cohort of ME/CFS patients and matched controls by fluorescence in situ hybridization using a probe against HHV-6 microRNA (miRNA), miR-aU14. Our results show abundant viral miRNA in various regions of the human brain and associated neuronal tissues including the spinal cord that is only detected in ME/CFS patients and not in controls. Our findings provide evidence of tissue-specific active HHV-6 and EBV infection in ME/CFS, which along with recent work demonstrating a possible relationship between herpesvirus infection and ME/CFS, provide grounds for renewed discussion on the role of herpesviruses in ME/CFS.
Introduction
Human herpesvirus 6 (HHV-6) including both HHV-6A and HHV-6B are ubiquitous herpesviruses that remain latent in most of the population. In a neurological context, HHV-6 is commonly associated with post-hematopoietic stem cell transplant encephalitis (Schmidt-Hieber et al., 2011), but it has additionally been identified as a potential etiological pathogen in several chronic neurological disorders (Komaroff et al., 2020). It is difficult to establish a causal relationship between HHV-6 and the implicated neurological disorders as the virus lies latent in a high proportion of infected individuals (Lusso et al., 1991). The question of how HHV-6 can be an etiological pathogen in diseases that not all who are infected with HHV-6 experience is one that has been meticulously navigated by researchers investigating chronic neurological disorders of elusive etiology, particularly those investigating myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Recent pandemic has sparked interest in HHV-6A reactivation as a putative cause of ME/CFS-like symptoms observed in Long-COVID.
ME/CFS is a complex, multi-system disorder characterized by neurological, metabolic, and immune dysfunctions (Syndrome, 2015). Neurological disturbances that have been linked to ME/CFS include loss of concentration and memory, headache, unrefreshing sleep, and unexplained muscle and joint pain (Bjørklund et al., 2020). Hypoactivation of hypothalamic-pituitary-adrenal (HPA) axis (Morris et al., 2017; Vangeel et al., 2018) and the basal ganglia (Miller et al., 2014) have been associated with ME/CFS, along with autoantibodies against serotonin (Ortega-Hernandez et al., 2009) and a reduction in serotonin transporters in the hippocampus (Cleare et al., 2005) and anterior cingulate (Yamamoto et al., 2004). A recent meta-analysis found that abnormalities in the brain stem are often reported in ME/CFS neuroimaging studies (Shan et al., 2020). Muscle pain is common in ME/CFS (Wyller, 2019), though the cause of this pain is not well understood and there are a variety of proposed explanations (Gerwyn and Maes, 2017). One possible explanation models pain in ME/CFS as a result of microglial accumulation in the L4-L6 dorsal horn of the spine (Yasui et al., 2014), but this model has yet to be tested in humans. ME/CFS brains have been found to have reduced white matter volume and abnormal fractional anisotropy in the right arcuate fasciculus (Zeineh et al., 2015).
HHV-6 and Epstein-Barr virus (EBV) have been hypothesized as etiological pathogens in ME/CFS (Ariza, 2021; Cox et al., 2022). However misconceptions of earlier studies justifying detection of viremia as a requirement to demonstrate a causal relationship between these viruses and ME/CFS has led to the doubts regarding involvement of herpesviruses in ME/CFS (Soto and Straus, 2000). Recent findings have demonstrated that expression of HHV-6 miRNAs during virus reactivation, in the absence of viral DNA replication, is enough to cause decreased mitochondrial functioning (Schreiner et al., 2020; Hennig et al., 2022) potentially leading to alterations in mitochondrial metabolism, host innate immune response. We and others have reported that HHV-6 viral DNA and RNA are detected at a very low copy number in blood and serum of ME/CFS patients suggesting the possibility of localized virus reactivation. To understand the potential tissue specific viral reactivation in ME/CFS patients, we carried out fluorescence in situ hybridization (FISH) analysis in postmortem tissue biopsies of a small cohort of ME/CFS patients (n = 3) and controls (n = 24) and found abundant transcription of HHV-6 miR-U14 in frontal lobe, basal ganglia, and spinal cord of ME/CFS patents. Our results confirm tissue-specific HHV-6 reactivation in ME/CFS patients and provide a ground for reconsidering a causal role of HHV-6 in various clinical anomalies observed in ME/CFS.
Materials and methods
Sample collection
Deidentified formalin-fixed and paraffin-embedded (FFPE) postmortem tissue biopsies were acquired from Cambridge Brain Bank, Cambridge University Hospitals, United Kingdom under NHS Research Ethics Committee Approval number 10/H0308/56. Ethics Commission of University of Würzburg did not require the study to be reviewed or approved by an ethics committee because all the tissue samples were obtained under a written agreement that allows the use of samples for a specific project pre-approved by the Brain biobank. Donors gave informed written consent for the use of brain tissue for research. Biopsy samples included tissues from 3 ME/CFS patients and 3 patients with other clinical diagnosis (anorexia, non-Hodgkin’s lymphoma, and breast cancer) as controls. Patients’ age, sex, clinical diagnosis, and tissue sample details are listed in Supplementary Table S1. Additionally, control biopsies from 21 more cases without having ME/CFS were acquired from Cambridge Brain Bank and were also included in this study (Supplementary Table S1).
Fluorescence in situ hybridization (FISH)
FISH to detect HHV-6 miR-aU14 was carried out as described before (Descamps et al., 2021; Mysore et al., 2021).
Dual staining for HHV-6 miR-aU14 and viral proteins
Followed by FISH, slides were permeabilized with 0.2% Triton-X100 in PBS for 30 min at room temperature. After 3 washes with PBS, slides were blocked with 10% FCS for 30 min at room temperature. Slides were incubated with primary antibodies within a humidity chamber for 1 h at room temperature, followed by 3 PBS washes. Subsequently, slides were incubated with secondary antibodies within a humidity chamber for 1 h at room temperature, followed by PBS wash. Dehydration of the slides was performed with ethanol. Slides were then mounted using a mounting medium. All antibodies were diluted in 2% FCS (Sigma-Aldrich, United States). Antibodies used for immunofluorescence studies include anti-HHV-6B U94, anti-HHV-6B OHV3, anti-HHV-6 gB, anti-HHV-6 p41 and anti-EBV dUTPase. EBV dUTPase antibody is a rabbit polyclonal antibody against the full-length protein. Purkinje cells were stained using anti-GFAP (astrocyte specific staining, AB5804, Millipore) and anti-Iba1 (microglia specific staining, Ab178846, Abcam) monoclonal antibodies. Imaging for viral miR-aU14 and viral proteins were carried out using a LSM 780 (Carl Zeiss AG, Germany) confocal fluorescence microscope using ZEN software.
Results
Active HHV-6 infection in brain biopsies of ME/CFS patients
HHV-6 displays neurotropism and has long been proposed to be associated with ME/CFS. Reactivation of the virus in the neuronal system could be a cause for clinical symptoms related to the disease. However, no studies have been carried out so far to investigate potential tissue-specific HHV-6 reactivation in ME/CFS patients. We analyzed FFPE-post-mortem tissue biopsies from 3 ME/CFS patients and three patients with other clinical diagnosis (anorexia, non-Hodgkin’s lymphoma, and breast cancer) for potential signature of HHV-6 infection by immunofluorescence analysis using antibodies against HHV-6B U94 (potential marker for viral latency), HHV-6B late protein OHV-3 (marker for active infection) and HHV-6 glycoprotein gB and p41 (markers for active infection).
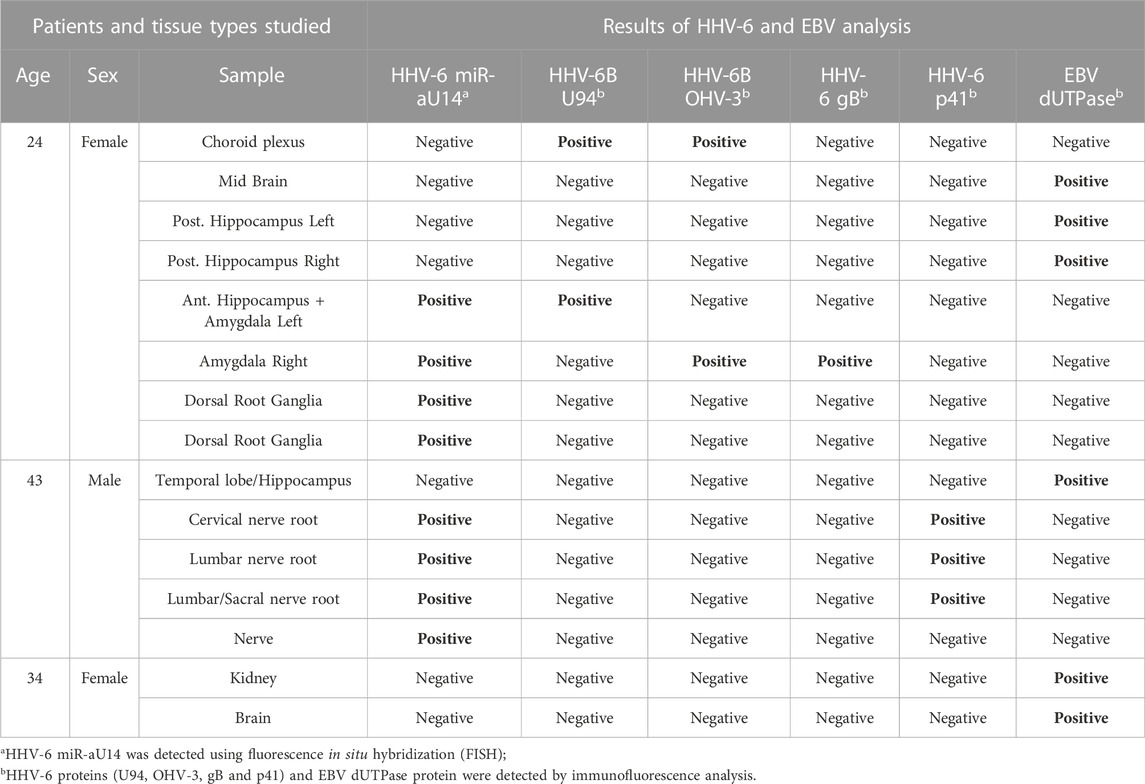
HHV-6B U94 and OHV-3 antigens were found in Choroid plexus tissues from one of the ME/CFS patients (Figure 1A). Additionally, U94 protein was detected in Ionized calcium binding adaptor molecule 1 (Iba1) positive neuroglial cells within the anterior Hippocampus and left Amygdala of the patient but not in the right amygdala (Figure 1B). Additionally, FISH analysis detected viral miR-aU14 within GFAP-positive astrocytes within the same tissues (Figure 1C). Another patient biopsies showed positive staining for HHV-6 p41 antigen in cervical, lumbar, and sacral nerve roots (Figures 2A,B) (Supplementary Table S1). In contrast to these findings, the third ME/CFS patient was negative for all HHV-6 proteins (Figure 2C) (Supplementary Table S1) like the control group. Taken together HHV-6 reactivation was observed in two of 3 ME/CFS patients in selected tissue sections (Table 1).
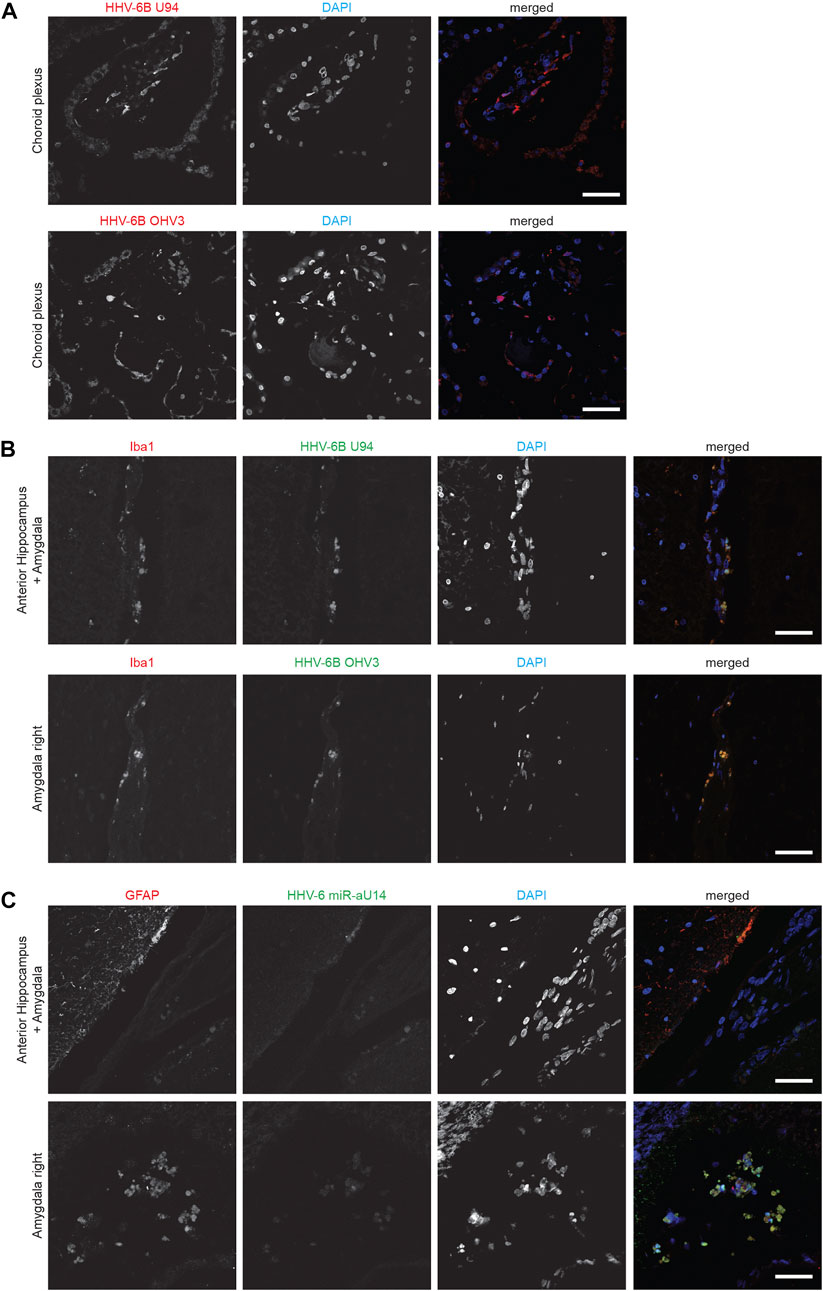
FIGURE 1. Representative fluorescence microscopy images of different human brain tissue types of ME/CFS patients. Tissues were co-stained with antibodies against HHV-6 specific proteins and/or neuronal tissue-specific marker and counter-stained for DAPI. (A) Human Choroid plexus tissues co-stained for HHV-6B U94 or OHV3, respectively. For each Panel images from the left to the right show markers for HHV-6 infection (red) and cell nuclei stained by DAPI (blue) and an overlay of the images. (B) Human anterior Hippocampus and Amygdala tissues co-stained for Iba1, HHV-6B U94 or OHV3. For each Panel images from the left to the right show astrocytes stained with Glial fibrillary acidic protein (GFAP) (red), markers for HHV-6 infection (green), cell nuclei stained by DAPI (blue) and an overlay of the images. (C) Human anterior Hippocampus and Amygdala tissues co-stained for Iba1 and HHV-6 miR-aU14. For each Panel images from the left to the right show astrocytes stained with GFAP (red), markers for HHV-6 infection (green), cell nuclei stained by DAPI (blue) and an overlay of the images. The scale bars represent 100 μm.
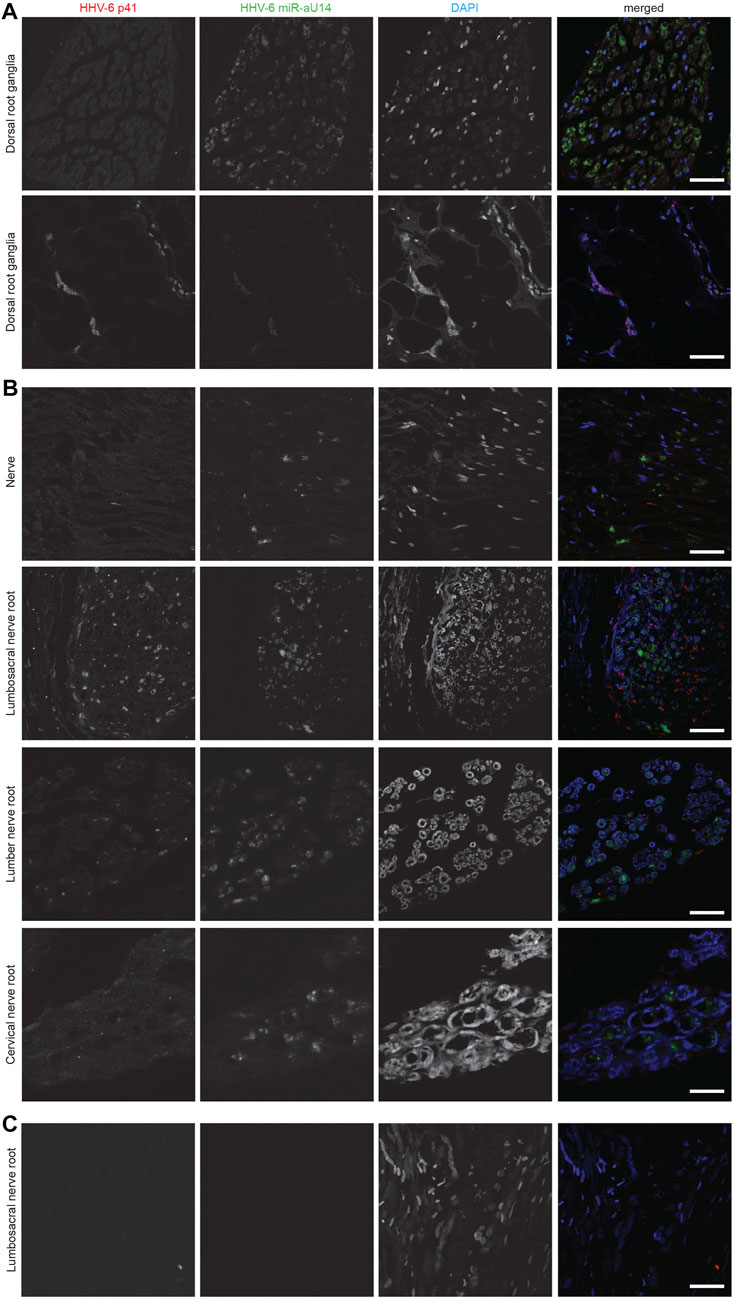
FIGURE 2. Representative fluorescence microscopy images of different human neuronal tissue types from two different ME/CFS patients and a control individual. For each panel images from the left to the right show HHV-6 p41 (red), a marker for active HHV-6A infection, miR-aU14 (green), cell nuclei stained with DAPI (blue) and an overlay of the images. (A) Human Dorsal root ganglia tissues of a ME/CFS patient. (B) Nerve, Lumbosacral nerve root, lumber nerve root and cervical nerve root tissues of a ME/CFS patient. (C) Lumbosacral nerve root tissue of a ME/CFS patient. The scale bars represent 100 μm.
Tissue specific localization of HHV-6 miRNA in ME/CFS
HHV-6 miRNA, miR-aU14 is expressed only during lytic HHV-6 infection and reactivation and is linked to disrupted mitochondrial function (Hennig et al., 2022). Because of their small size, miRNAs are often extremely stable and can serve as an ideal marker for virus reactivation studies (Descamps et al., 2021; Mysore et al., 2021). To study the presence of HHV-6 miR-aU14 in ME/CFS patient tissues, we carried out FISH analysis using FFPE tissue biopsies. HHV-6 p41 protein and miR-aU14 were observed within specific brain tissues of ME/CFS patients (Figure 2) (Table 1, Supplementary Table S1). Specifically, HHV-6 p41 was detected in the dorsal root ganglia of one of 3 ME/CFS patients (Figure 2A). Viral miR-aU14 was also found in the same patient in one of the two dorsal root ganglia samples analyzed (Figure 2A upper panel). Moreover, the same protein was detected in the Lumbosacral nerve root, lumbar nerve root, and cervical nerve root as well but not in the nerve tissue of another ME/CFS patient (Figure 2B). No viral miRNA was detected in the third ME/CFS patient. In summary, two out of the 3 ME/CFS patients showed the signature of ongoing HHV-6 infection/reactivation with viral miRNA production within localized neuronal cells of the brain.
Absence of HHV-6 infection within cerebellum of ME/CFS patients
We have previously shown association of active HHV-6 infection of cerebellum with mood disorders (Prusty et al., 2018). ME/CFS patients also suffer from psychological issues associated with depression. Therefore, we studied the cerebellum of these patients for potential HHV-6 infection. Interestingly, our study did not find any sign of HHV-6 infection within Purkinje neurons of ME/CFS patients (Figure 3). HHV6-U94 was not detected in cerebellum biopsies of all 3 ME/CFS patients. However, non-ME/CFS controls showed signs of HHV-6 U94 protein suggesting potential viral latency within this region (Figure 3). Analysis of HHV-6 miR-aU14, HHV-6B OHV-3, HHV-6 gB, and HHV6-p41 were all negative in the cerebellum biopsies of ME/CFS patients (Supplementary Table S1). These results suggest that HHV-6 infection within the cerebellum is not associated with any depressive physiology in ME/CFS patients.
FIGURE 3. Absence of HHV-6 infection in cerebellum of ME/CFS patients. Representative images showing Immuno fluorescence analysis for HHV-6B U94 in cerebellum samples. Cerebellum samples were stained using antibody against HHV-6B U94 together with GFAP (marker for astrocytes). DAPI was used to counterstain DNA. Each panel represents a different ME/CFS patient or a non-ME/CFS control. The scale bars represent 100 μm.
Co-infection of EBV in ME/CFS patients
Both HHV-6 and EBV infections are potential etiological pathogens for ME/CFS (Ariza, 2021; Cox et al., 2022). As active infection of HHV-6 was detected in multiple tissue biopsies from ME/CFS patients, we were interested in understanding potential co-EBV infection among these samples. EBV dUTPase has been detected in patients with chronic disorders. Hence, we analyzed post-mortem tissue biopsies for the presence of EBV dUTPase, which was abundantly detected in some tissue biopsies from ME/CFS patients, while all samples from non-ME/CFS control were negative for EBV dUTPase (Figure 4). Tissue samples from one of the ME/CFS patient showed positive staining for EBV dUTPase in the mid brain and right hippocampus (Figure 4) while the second patient showed positive EBV dUTPase staining only in hippocampus region. Interestingly, the third HHV-6 negative ME/CFS patient showed positive staining for EBV dUTPase in the left brain and kidney. None of the tissue biopsies were found to be dual positive for both HHV-6 and EBV. Overall, our results indicated that it is possible to have HHV-6 and EBV co-infection in ME/CFS patients.
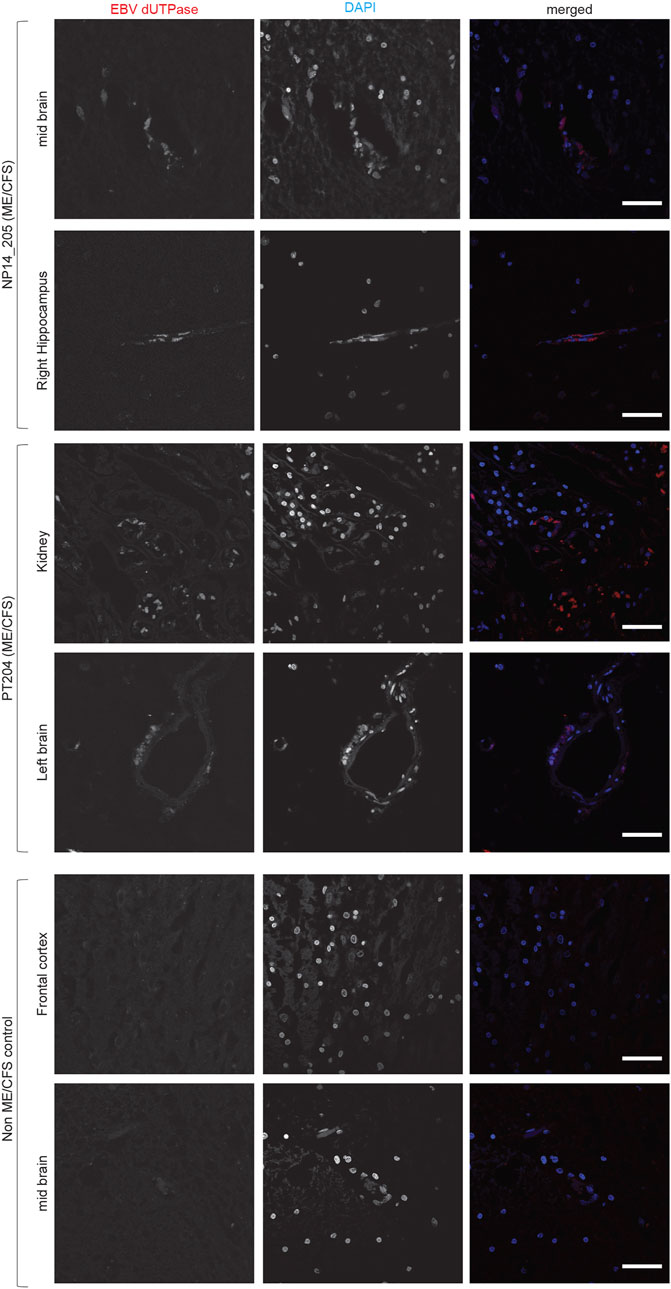
FIGURE 4. Co-infection of Epstein-Barr virus (EBV) in ME/CFS. Representative images showing Immuno fluorescence analysis for EBV dUTPase in multiple tissue samples from different ME/CFS patients and a non-ME/CFS control. Tissue samples were stained using a rabbit polyclonal antibody against EBV dUTPase. DAPI was used to counterstain DNA. The scale bars represent 100 μm.
Discussion
HHV-6 has previously been detected in the meninges (Chapenko et al., 2016), frontal lobe (Lin et al., 2002; Hemling et al., 2003; Chapenko et al., 2016), olfactory tract (Chapenko et al., 2016), optic tract (Chapenko et al., 2016), hippocampus (Esposito et al., 2015; Huang et al., 2015; Chapenko et al., 2016), and white matter (Cermelli et al., 2003; Opsahl and Kennedy, 2005) of the brain. Symptomatic HHV-6 is associated with neuroinflammation when identified in the brain (Komaroff et al., 2020). In addition to ME/CFS, active HHV-6 in the brain has been linked to mesial temporal lobe epilepsy (MTLE), multiple sclerosis (MS), and Alzheimer’s disease (AD).
MTLE is the most common form of epilepsy (Engel Jr, 2001). Active HHV-6A/B infection is more common in the mesial temporal lobe in MTLE patients than patients with other forms of epilepsy (Fotheringham et al., 2007) and HHV-6B positivity in astrocytes of the hippocampus has been associated with MTLE (Niehusmann et al., 2010; Li et al., 2011; Esposito et al., 2015; Huang et al., 2015). A potential mechanism of HHV-6 involvement in the MTLE could be a vicious cycle of infection inducing neuroinflammation which in turn increases viral reactivation and further inflammation (Tamai et al., 2017; Kwon et al., 2018; Bartolini et al., 2019). This explanation is also useful in hypothesizing HHV-6’s role in MS, with the addition of several other findings. HHV-6 is found in higher levels in MS plaques compared to other areas of the brain (Friedman et al., 1999; Cermelli et al., 2003; Opsahl and Kennedy, 2005) and HHV-6 positivity in oligodendrocytes and microglia of the white matter has been associated with MS (Friedman et al., 1999; Blumberg et al., 2000; Goodman et al., 2003). The ability to induce neuroinflammation by different mechanisms makes HHV-6 a potential etiological pathogen for AD (Reynaud et al., 2014; Eimer et al., 2018; Bortolotti et al., 2019), though findings supporting this association have been disputed (Jeong and Liu, 2019; Allnutt et al., 2020). HHV-6 positivity in the frontal cortex has been associated with AD (Lin et al., 2002; Hemling et al., 2003) in some cases.
Dysregulation in the frontal lobe, basal ganglia, and dorsal root ganglia (DRG) may be responsible for several characteristic symptoms of ME/CFS. The cognitive slowing observed in ME/CFS has been linked to hypoactivation of the frontal lobe (Zinn et al., 2018), the fatigue observed in ME/CFS has been linked to hypoactivation of the basal ganglia (Miller et al., 2014), and the unexplained muscle pain observed in ME/CFS has been linked to inflammation or pressure on or around the DRG (Hulens et al., 2021). Active HHV-6 has been found in the frontal lobe (Hemling et al., 2003; Chapenko et al., 2016), basal ganglia (Achim et al., 1994), and dorsal root ganglia (Hüfner et al., 2007). Active HHV-6 in these tissues has been associated with inflammation in the frontal lobe (Chapenko et al., 2016) and basal ganglia (Achim et al., 1994; Crawford et al., 2007). While active HHV-6 has been identified in the DRG and was found to increase the susceptibility of the sensory ganglia to alpha-herpesvirus infection (Hüfner et al., 2007), a clear link between symptomatic HHV-6 infection and dysregulation in the dorsal root ganglia has yet to be made. In context with one another and the results of the present study, these findings indicate that active HHV-6 infection in the frontal lobe, basal ganglia, and dorsal root ganglia may lead to several characteristic symptoms of ME/CFS, and future studies should elucidate these potential links.
Like many other herpesviruses, HHV-6 and HHV-7 display neurotropism. HHV-6 infection and reactivation has been shown in astrocytes (Donati et al., 2005), glial cells (Cassiani-Ingoni et al., 2005) and Purkinje neurons providing strong evidence that link HHV-6 infection to various neurological disorders. In this study, using FISH, we have found miR-aU14 in the axons of the spinal cord in ME/CFS patients (Figure 2) but not in controls, suggesting HHV-6 might also undergo retrograde transport within the axons like HSV-1 (Bearer et al., 2000).
The present finding of active HHV-6 infection in the cervical nerve root may propose a potential pathophysiological mechanism for a series of findings by Matsui et al., In 2012, Matsui et al. proposed a novel syndrome called “cervical neuro-muscular syndrome”, which established a link between treating cervical muscle lesions and the alleviation of autonomic dysfunction in ME/CFS (Matsui et al., 2012), which was followed in 2020 with a study establishing a relationship between cervical muscle lesions and parasympathetic nervous system dysfunction (Matsui et al., 2020). These findings were elucidated in a 2021 study in which Matsui et al. observed a relationship between treatment of cervical muscle lesions with low frequency electrical stimulation, reduction in severity across a range of ME/CFS symptoms, and reduction of pupil diameter in patients whose symptoms improved (Matsui et al., 2021). Taken together, these findings indicate a relationship between cervical muscle lesions, severity of ME/CFS symptoms, and autonomic dysfunction in ME/CFS, which provide intriguing context for the present identification of active HHV-6 infection in the cervical nerve roots of ME/CFS patients.
Additionally, several recent studies have identified higher copy numbers of HHV-6 and EBV in ME/CFS patients compared to healthy controls (Fevang et al., 2021; Lee et al., 2021; Gravelsina et al., 2022). Lee et al. observed an association between HHV-6B and HHV-7 viral load and severity of ME/CFS symptoms by analyzing salivary viral load. Gravelsina et al. found a higher viral load of HHV-6B in peripheral blood mononuclear cells (PBMC) corresponding to the severity of ME/CFS symptoms, with 84.2% of patients with severe ME/CFS having a load greater than 1,000 copies per million PBMC compared to 57.1% of patients with mild ME/CFS and 11.1% of health controls (Gravelsina et al., 2022). Fevang et al. found a higher average copy number of EBV in the peripheral blood of ME/CFS patients compared to healthy controls, however the difference was not statistically significant (Fevang et al., 2021).
EBV has also been associated with several neurological disorders ranging from inflammatory conditions such as encephalitis and encephalomyelitis to neurodegenerative diseases such as Parkinson’s Disease and Alzheimer’s Disease (Zhang et al., 2021). Furthermore, a recent study suggested that EBV was the leading cause of MS, a chronic inflammatory demyelinating disease of the central nervous system (Bjornevik et al., 2022). The results of the present study support the results of previous studies suggesting that the EBV dUTPase may contribute to the neurological symptomology observed in some patients with ME/CFS (Williams Ph et al., 2019).
Unlike many other neurological diseases, ME/CFS is not associated with high rate of mortality. Because of this reason, it is difficult to obtain tissue biopsies from patients to carry out these types of studies. Small sample size is one of the key limitations of our study. Despite the small sample size, we aim to present the findings of active HHV-6 and EBV in the tissues of ME/CFS patients, and its absence in healthy controls, to further reason renewed discussion and interest in the role of herpesviruses in ME/CFS.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by NHS Research Ethics Committee Approval number 10/H0308/56. The patients/participants provided their written informed consent to participate in this study.
Author contributions
BP designed and supervised the study; FK, DT, ZL, and AK carried out experimental work. BP, MA analyzed the data. All authors contributed to manuscript writing.
Acknowledgments
We thank Ms. Kristin Loomis and HHV-6 Foundation, United States for sponsoring and organizing the official documentation for FFPE tissue biopsy collection from Cambridge Bio Bank. We also thank Prof. David Hudnall for his help in sample procurement and initial experimental planning. MEA was funded by NIH-NIAID grant R01 AI084898. A word of thanks also goes to Prof. Marshall Williams for reading the manuscript and providing critical insights. The Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre. Late Prof. Gerhard Krüger will be missed for his thoughtful insights into tissue staining.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2022.1044964/full#supplementary-material
References
Achim, C. L., Wang, R., Miners, D. K., and Wiley, C. A. (1994). Brain viral burden in HIV infection. J. Neuropathol. Exp. Neurol. 53, 284–294. doi:10.1097/00005072-199405000-00010
Allnutt, M. A., Johnson, K., Bennett, D. A., Connor, S. M., Troncoso, J. C., Pletnikova, O., et al. (2020). Human herpesvirus 6 detection in Alzheimer’s disease cases and controls across multiple cohorts. Neuron 105, 1027. doi:10.1016/j.neuron.2019.12.031
Ariza, M. E. (2021). Myalgic encephalomyelitis/chronic fatigue syndrome: The human herpesviruses are back. Biomolecules 11, 185. doi:10.3390/biom11020185
Bartolini, L., Libbey, J. E., Ravizza, T., Fujinami, R. S., Jacobson, S., and Gaillard, W. D. (2019). Viral triggers and inflammatory mechanisms in pediatric epilepsy. Mol. Neurobiol. 56, 1897–1907. doi:10.1007/s12035-018-1215-5
Bearer, E. L., Breakefield, X. O., Schuback, D., Reese, T. S., and Lavail, J. H. (2000). Retrograde axonal transport of herpes simplex virus: Evidence for a single mechanism and a role for tegument. Proc. Natl. Acad. Sci. U. S. A. 97, 8146–8150. doi:10.1073/pnas.97.14.8146
Bjørklund, G., Dadar, M., Pivina, L., Doşa, M. D., Semenova, Y., and Maes, M. (2020). Environmental, neuro-immune, and neuro-oxidative stress interactions in chronic fatigue syndrome. Mol. Neurobiol. 57, 4598–4607. doi:10.1007/s12035-020-01939-w
Bjornevik, K., Cortese, M., Healy, B. C., Kuhle, J., Mina, M. J., Leng, Y., et al. (2022). Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 375, 296–301. doi:10.1126/science.abj8222
Blumberg, B. M., Mock, D. J., Powers, J. M., Ito, M., Assouline, J. G., Baker, J. V., et al. (2000). The HHV6 paradox: Ubiquitous commensal or insidious pathogen? A two-step in situ pcr approach. J. Clin. Virol. 16, 159–178. doi:10.1016/s1386-6532(99)00084-0
Bortolotti, D., Gentili, V., Rotola, A., Caselli, E., and Rizzo, R. (2019). HHV-6A infection induces amyloid-beta expression and activation of microglial cells. Alzheimers Res. Ther. 11, 104–111. doi:10.1186/s13195-019-0552-6
Cassiani-Ingoni, R., Greenstone, H. L., Donati, D., Fogdell-Hahn, A., Martinelli, E., Refai, D., et al. (2005). CD46 on glial cells can function as a receptor for viral glycoprotein-mediated cell-cell fusion. Glia 52, 252–258. doi:10.1002/glia.20219
Cermelli, C., Berti, R., Soldan, S. S., Mayne, M., D'Ambrosia, J. M., Ludwin, S. K., et al. (2003). High frequency of human herpesvirus 6 DNA in multiple sclerosis plaques isolated by laser microdissection. J. Infect. Dis. 187, 1377–1387. doi:10.1086/368166
Chapenko, S., Roga, S., Skuja, S., Rasa, S., Cistjakovs, M., Svirskis, S., et al. (2016). Detection frequency of human herpesviruses-6A, -6B, and-7 genomic sequences in central nervous system DNA samples from post-mortem individuals with unspecified encephalopathy. J. Neurovirol. 22, 488–497. doi:10.1007/s13365-015-0417-0
Cleare, A. J., Messa, C., Rabiner, E. A., and Grasby, P. M. (2005). Brain 5-HT1A receptor binding in chronic fatigue syndrome measured using positron emission tomography and [11C] WAY-100635. Biol. Psychiatry 57, 239–246. doi:10.1016/j.biopsych.2004.10.031
Cox, B. S., Alharshawi, K., Mena-Palomo, I., Lafuse, W. P., and Ariza, M. E. (2022). EBV/HHV-6A dUTPases contribute to myalgic encephalomyelitis/chronic fatigue syndrome pathophysiology by enhancing TFH cell differentiation and extrafollicular activities. JCI Insight 7 (11), e158193. doi:10.1172/jci.insight.158193
Crawford, J. R., Kadom, N., Santi, M. R., Mariani, B., and Lavenstein, B. L. (2007). Human herpesvirus 6 rhombencephalitis in immunocompetent children. J. Child. Neurol. 22, 1260–1268. doi:10.1177/0883073807307086
Descamps, V., Gautheret-Dejean, A., Pelletier, A. L., Bonnafous, P., Deschamps, L., and Prusty, B. K. (2021). Chronic persistent HHV-6B infection after sulfasalazine-induced DRESS with demonstration of HHV-6 encoded small noncoding RNAs (sncRNAs) in Crohn's-like colitis: Case report. Clin. Case Rep. 9, 841–844. doi:10.1002/ccr3.3680
Donati, D., Martinelli, E., Cassiani-Ingoni, R., Ahlqvist, J., Hou, J., Major, E. O., et al. (2005). Variant-specific tropism of human herpesvirus 6 in human astrocytes. J. Virol. 79, 9439–9448. doi:10.1128/JVI.79.15.9439-9448.2005
Eimer, W. A., Kumar, D. K. V., Shanmugam, N. K. N., Rodriguez, A. S., Mitchell, T., Washicosky, K. J., et al. (2018). Alzheimer’s disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 99, 1527–1532. e3. doi:10.1016/j.neuron.2018.11.043
Engel, J. R. (2001). Mesial temporal lobe epilepsy: What have we learned? Neuroscientist 7, 340–352. doi:10.1177/107385840100700410
Esposito, L., Drexler, J. F., Braganza, O., Doberentz, E., Grote, A., Widman, G., et al. (2015). Large-scale analysis of viral nucleic acid spectrum in temporal lobe epilepsy biopsies. Epilepsia 56, 234–243. doi:10.1111/epi.12890
Fevang, B., Wyller, V. B. B., Mollnes, T. E., Pedersen, M., Asprusten, T. T., Michelsen, A., et al. (2021). Lasting immunological imprint of primary epstein-barr virus infection with associations to chronic low-grade inflammation and fatigue. Front. Immunol. 12, 715102. doi:10.3389/fimmu.2021.715102
Fotheringham, J., Donati, D., Akhyani, N., Fogdell-Hahn, A., Vortmeyer, A., Heiss, J. D., et al. (2007). Association of human herpesvirus-6B with mesial temporal lobe epilepsy. PLoS Med. 4, e180. doi:10.1371/journal.pmed.0040180
Friedman, J., Lyons, M. J., Cu, G., Ablashl, D. V., Whitman, J. E., Edgar, M., et al. (1999). The association of the human herpesvirus-6 and MS. Mult. Scler. 5, 355–362. doi:10.1177/135245859900500509
Gerwyn, M., and Maes, M. (2017). Mechanisms explaining muscle fatigue and muscle pain in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A review of recent findings. Curr. Rheumatol. Rep. 19, 1. doi:10.1007/s11926-017-0628-x
Goodman, A. D., Mock, D. J., Powers, J. M., Baker, J. V., and Blumberg, B. M. (2003). Human herpesvirus 6 genome and antigen in acute multiple sclerosis lesions. J. Infect. Dis. 187, 1365–1376. doi:10.1086/368172
Gravelsina, S., Vilmane, A., Svirskis, S., Rasa-Dzelzkaleja, S., Nora-Krukle, Z., Vecvagare, K., et al. (2022). Biomarkers in the diagnostic algorithm of myalgic encephalomyelitis/chronic fatigue syndrome. Front. Immunol. 13, 928945. doi:10.3389/fimmu.2022.928945
Hemling, N., Röyttä, M., Rinne, J., Pöllänen, P., Broberg, E., Tapio, V., et al. (2003). Herpesviruses in brains in Alzheimer's and Parkinson's diseases. Ann. Neurol. 54, 267–271. doi:10.1002/ana.10662
Hennig, T., Prusty, A. B., Kaufer, B. B., Whisnant, A. W., Lodha, M., Enders, A., et al. (2022). Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA. Nature 605, 539–544. doi:10.1038/s41586-022-04667-4
Huang, C., Yan, B., Lei, D., Si, Y., Li, H., Chen, M.-W., et al. (2015). Apolipoprotein 4 may increase viral load and seizure frequency in mesial temporal lobe epilepsy patients with positive human herpes virus 6B. Neurosci. Lett. 593, 29–34. doi:10.1016/j.neulet.2014.12.063
Hüfner, K., Arbusow, V., Himmelein, S., Derfuss, T., Sinicina, I., Strupp, M., et al. (2007). The prevalence of human herpesvirus 6 in human sensory ganglia and its co-occurrence with alpha-herpesviruses. J. Neurovirol. 13, 462–467. doi:10.1080/13550280701447059
Hulens, M., Bruyninckx, F., Dankaerts, W., Rasschaert, R., De Mulder, P., Stalmans, I., et al. (2021). High prevalence of perineural cysts in patients with fibromyalgia and chronic fatigue syndrome. Pain Med. 22, 883–890. doi:10.1093/pm/pnaa410
Jeong, H.-H., and Liu, Z. (2019). Are HHV-6A and HHV-7 really more abundant in Alzheimer’s disease? Neuron 104, 1034–1035. doi:10.1016/j.neuron.2019.11.009
Komaroff, A. L., Pellett, P. E., and Jacobson, S. (2020). Human herpesviruses 6A and 6B in brain diseases: Association versus causation. Clin. Microbiol. Rev. 34, e00143. doi:10.1128/CMR.00143-20
Kwon, A., Kwak, B. O., Kim, K., Ha, J., Kim, S.-J., Bae, S. H., et al. (2018). Cytokine levels in febrile seizure patients: A systematic review and meta-analysis. Seizure 59, 5–10. doi:10.1016/j.seizure.2018.04.023
Lee, J. S., Lacerda, E. M., Nacul, L., Kingdon, C. C., Norris, J., O'Boyle, S., et al. (2021). Salivary DNA loads for human herpesviruses 6 and 7 are correlated with disease phenotype in myalgic encephalomyelitis/chronic fatigue syndrome. Front. Med. 8, 656692. doi:10.3389/fmed.2021.656692
Li, J.-M., Lei, D., Peng, F., Zeng, Y.-J., Li, L., Xia, Z.-L., et al. (2011). Detection of human herpes virus 6B in patients with mesial temporal lobe epilepsy in West China and the possible association with elevated NF-κB expression. Epilepsy Res. 94, 1–9. doi:10.1016/j.eplepsyres.2010.11.001
Lin, W. R., Wozniak, M. A., Cooper, R. J., Wilcock, G. K., and Itzhaki, R. F. (2002). Herpesviruses in brain and Alzheimer's disease. J. Pathol. 197, 395–402. doi:10.1002/path.1127
Lusso, P., Malnati, M., De Maria, A., Balotta, C., Derocco, S. E., Markham, P. D., et al. (1991). Productive infection of CD4+ and CD8+ mature human T cell populations and clones by human herpesvirus 6. Transcriptional down-regulation of CD3. J. Immunol. 147, 685–691.
Matsui, T., Hara, K., Iwata, M., Hojo, S., Shitara, N., Endo, Y., et al. (2021). Possible involvement of the autonomic nervous system in cervical muscles of patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). BMC Musculoskelet. Disord. 22, 419. doi:10.1186/s12891-021-04293-7
Matsui, T., Hara, K., Kayama, T., Iwata, M., Shitara, N., Hojo, S., et al. (2020). Cervical muscle diseases are associated with indefinite and various symptoms in the whole body. Eur. Spine J. 29, 1013–1021. doi:10.1007/s00586-019-06233-5
Matsui, T., Li, K., Hojo, S., and Sano, K. (2012). Cervical neuro-muscular syndrome: Discovery of a new disease group caused by abnormalities in the cervical muscles. Neurol. Med. Chir. 52, 75–80. doi:10.2176/nmc.52.75
Miller, A. H., Jones, J. F., Drake, D. F., Tian, H., Unger, E. R., and Pagnoni, G. (2014). Decreased basal ganglia activation in subjects with chronic fatigue syndrome: Association with symptoms of fatigue. PLoS One 9, e98156. doi:10.1371/journal.pone.0098156
Morris, G., Anderson, G., and Maes, M. (2017). Hypothalamic-pituitary-adrenal hypofunction in myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS) as a consequence of activated immune-inflammatory and oxidative and nitrosative pathways. Mol. Neurobiol. 54, 6806–6819. doi:10.1007/s12035-016-0170-2
Mysore, K. R., Phan, T. L., Himes, R. W., Schady, D., Eldin, K. W., Prusty, B. K., et al. (2021). Human herpesvirus 6 infection in pediatric liver transplantation: Single-center study of incidence, outcomes, and management. J. Pediatr. Infect. Dis. Soc. 10, 599–606. doi:10.1093/jpids/piaa166
Niehusmann, P., Mittelstaedt, T., Bien, C. G., Drexler, J. F., Grote, A., Schoch, S., et al. (2010). Presence of human herpes virus 6 DNA exclusively in temporal lobe epilepsy brain tissue of patients with history of encephalitis. Epilepsia 51, 2478–2483. doi:10.1111/j.1528-1167.2010.02741.x
Opsahl, M. L., and Kennedy, P. G. (2005). Early and late HHV-6 gene transcripts in multiple sclerosis lesions and normal appearing white matter. Brain 128, 516–527. doi:10.1093/brain/awh390
Ortega-Hernandez, O. D., Cuccia, M., Bozzini, S., Bassi, N., Moscavitch, S., Diaz-Gallo, L. M., et al. (2009). Autoantibodies, polymorphisms in the serotonin pathway, and human leukocyte antigen class II alleles in chronic fatigue syndrome: Are they associated with age at onset and specific symptoms? Ann. N. Y. Acad. Sci. 1173, 589–599. doi:10.1111/j.1749-6632.2009.04802.x
Prusty, B. K., Gulve, N., Govind, S., Krueger, G. R. F., Feichtinger, J., Larcombe, L., et al. (2018). Active HHV-6 infection of cerebellar Purkinje cells in mood disorders. Front. Microbiol. 9, 1955. doi:10.3389/fmicb.2018.01955
Reynaud, J. M., Jégou, J.-F., Welsch, J. C., and Horvat, B. (2014). Human herpesvirus 6A infection in CD46 transgenic mice: Viral persistence in the brain and increased production of proinflammatory chemokines via toll-like receptor 9. J. Virol. 88, 5421–5436. doi:10.1128/JVI.03763-13
Schmidt-Hieber, M., Schwender, J., Heinz, W. J., Zabelina, T., Kühl, J. S., Mousset, S., et al. (2011). Viral encephalitis after allogeneic stem cell transplantation: A rare complication with distinct characteristics of different causative agents. haematologica 96, 142–149. doi:10.3324/haematol.2010.029876
Schreiner, P., Harrer, T., Scheibenbogen, C., Lamer, S., Schlosser, A., Naviaux, R. K., et al. (2020). Human herpesvirus-6 reactivation, mitochondrial fragmentation, and the coordination of antiviral and metabolic phenotypes in myalgic encephalomyelitis/chronic fatigue syndrome. ImmunoHorizons 4, 201–215. doi:10.4049/immunohorizons.2000006
Shan, Z. Y., Barnden, L. R., Kwiatek, R. A., Bhuta, S., Hermens, D. F., and Lagopoulos, J. (2020). Neuroimaging characteristics of myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A systematic review. J. Transl. Med. 18, 335–346. doi:10.1186/s12967-020-02506-6
Soto, N. E., and Straus, S. E. (2000). Chronic fatigue syndrome and herpesviruses: The fading evidence. Herpes. 7, 46–50.
Syndrome, I. O. M. C. O. T. D. C. F. M. E. C. F. (2015). Beyond myalgic encephalomyelitis/chronic fatigue syndrome: Redefining an illness. NW Washington: National Academies Press.
Tamai, M., Kobayashi, N., Shimada, K., Oka, N., Takahashi, M., Tanuma, A., et al. (2017). Increased interleukin-1β and basic fibroblast growth factor levels in the cerebrospinal fluid during human herpesvirus-6B (HHV-6B) encephalitis. Biochem. Biophys. Res. Commun. 486, 706–711. doi:10.1016/j.bbrc.2017.03.102
Vangeel, E. B., Kempke, S., Bakusic, J., Godderis, L., Luyten, P., Van Heddegem, L., et al. (2018). Glucocorticoid receptor DNA methylation and childhood trauma in chronic fatigue syndrome patients. J. Psychosom. Res. 104, 55–60. doi:10.1016/j.jpsychores.2017.11.011
Williams, M., Cox, B., Lafuse, W., and Ariza, M. E. (2019). Epstein-barr virus dUTPase induces neuroinflammatory mediators: Implications for myalgic encephalomyelitis/chronic fatigue syndrome. Clin. Ther. 41, 848–863. doi:10.1016/j.clinthera.2019.04.009
Wyller, V. B. B. (2019). Pain is common in chronic fatigue syndrome–current knowledge and future perspectives. Scand. J. Pain 19, 5–8. doi:10.1515/sjpain-2018-2007
Yamamoto, S., Ouchi, Y., Onoe, H., Yoshikawa, E., Tsukada, H., Takahashi, H., et al. (2004). Reduction of serotonin transporters of patients with chronic fatigue syndrome. Neuroreport 15, 2571–2574. doi:10.1097/00001756-200412030-00002
Yasui, M., Yoshimura, T., Takeuchi, S., Tokizane, K., Tsuda, M., Inoue, K., et al. (2014). A chronic fatigue syndrome model demonstrates mechanical allodynia and muscular hyperalgesia via spinal microglial activation. Glia 62, 1407–1417. doi:10.1002/glia.22687
Zeineh, M. M., Kang, J., Atlas, S. W., Raman, M. M., Reiss, A. L., Norris, J. L., et al. (2015). Right arcuate fasciculus abnormality in chronic fatigue syndrome. Radiology 274, 517–526. doi:10.1148/radiol.14141079
Zhang, N., Zuo, Y., Jiang, L., Peng, Y., Huang, X., and Zuo, L. (2021). Epstein-barr virus and neurological diseases. Front. Mol. Biosci. 8, 816098. doi:10.3389/fmolb.2021.816098
Keywords: HHV-6, ME/CFS, EBV, epstein-barr virus, herpesvirus, viral pathology
Citation: Kasimir F, Toomey D, Liu Z, Kaiping AC, Ariza ME and Prusty BK (2022) Tissue specific signature of HHV-6 infection in ME/CFS. Front. Mol. Biosci. 9:1044964. doi: 10.3389/fmolb.2022.1044964
Received: 26 September 2022; Accepted: 05 December 2022;
Published: 14 December 2022.
Edited by:
Hem Chandra Jha, Indian Institute of Technology Indore, IndiaReviewed by:
Prafullakumar Tailor, National Institute of Immunology (NII), IndiaAna Afonso, Universidade NOVA de Lisboa, Portugal
Copyright © 2022 Kasimir, Toomey, Liu, Kaiping, Ariza and Prusty. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bhupesh K. Prusty, Ymh1cGVzaC5wcnVzdHlAdW5pLXd1ZXJ6YnVyZy5kZQ==
†These have authors contributed equally to this work
 Francesca Kasimir
Francesca Kasimir Danny Toomey2†
Danny Toomey2† Zheng Liu
Zheng Liu Maria Eugenia Ariza
Maria Eugenia Ariza Bhupesh K. Prusty
Bhupesh K. Prusty