- 1Shaanxi Key Laboratory of Ischemic Cardiovascular Disease, Institute of Basic and Translational Medicine, Xi’an Medical University, Xi’an, China
- 2Shaanxi Key Laboratory of Chinese Herb and Natural Drug Development, Medicine Research Institute, Shaanxi Pharmaceutical Holding Group Co., Ltd., Xi’an, China
Cardiovascular diseases remain the leading cause of morbidity and mortality worldwide. Atherosclerosis is the main pathological basis of cardiovascular diseases and it is closely associated with hyperlipidemia, endothelial injury, macrophage-derived foam cells formation, proliferation and migration of vascular smooth muscle cells (VSMCs), platelet aggregation, and altered gut microbiota. Various symptomatic treatments, that are currently used to inhibit atherosclerosis, need to be administered in long term and their adverse effects cannot be ignored. Berberine (BBR) has beneficial effects on atherosclerosis through regulating multiple aspects of its progression. This review highlights the recent advances in understanding the anti-atherosclerosis mechanism of BBR. BBR alleviated atherosclerosis by attenuation of dyslipidemia, correction of endothelial dysfunction, inhibition of macrophage inflammation and foam cell formation, activation of macrophage autophagy, regulation of the proliferation and migration of VSMCs, attenuation of platelet aggregation, and modulation of gut microbiota. This review would provide a modern scientific perspective to further understanding the molecular mechanism of BBR attenuating atherosclerosis and supply new ideas for atherosclerosis management.
Highlights
1) Berberine attenuated atherosclerosis by regulating dyslipidemia.
2) Berberine alleviated atherosclerosis by affecting cellular targets, including ameliorating endothelial injury, inhibiting the formation of macrophage-derived foam cells, regulating the proliferation and migration of vascular smooth muscle cells, and suppressing platelet aggregation.
3) Berberine restrained atherosclerosis by modulating gut microbiota.
Introduction
According to the World Health Organization (WHO), an estimated 17.9 million people died of cardiovascular diseases, accounting for 30% of the total mortality worldwide (WHO, 2020). Atherosclerosis is the main pathological basis of cardiovascular diseases (Benjamin et al., 2019). The complex pathological mechanisms are developed by various factors, such as hyperlipidemia, endothelial injury, macrophage-derived foam cells formation, proliferation and migration of vascular smooth muscle cells (VSMCs), platelet aggregation, and altered gut microbiota (Tabas et al., 2015; Jonsson and Backhed, 2017; Fang et al., 2018; Qiao and Chen, 2018; Marchio et al., 2019). Atherosclerosis is initiated primarily by the accumulation of low-density lipoprotein cholesterol (LDL-C) in the vessel wall and subsequently intensified by oxidized low-density lipoprotein (oxLDL) (Marchio et al., 2019). Circulating oxLDL, increased chemokines together with the expression of adhesion proteins trigger the recruitment of immune cells, particularly monocytes (Buckley and Ramji, 2015). The monocytes then differentiate into macrophages, which engulf oxLDL and lead to foam cell formation—the hallmark of atherosclerosis (McLaren et al., 2011; Buckley and Ramji, 2015; Tabas and Bornfeldt, 2016). Subsequently, necrosis or apoptosis of foam cells, proliferation and migration of VSMCs coupled with chronic inflammatory response result in lesion development and atherosclerosis complications (McLaren et al., 2011; Buckley and Ramji, 2015; Basatemur et al., 2019).
Clinically, drugs used for symptomatic treatment mainly include lipid-lowering drugs (statins and niacins), antiplatelet and thrombolytic drugs (aspirin and urokinase), and anticoagulant drugs (warfarin). For atherosclerosis patients with ischemic symptoms, treatment of vasodilators and β-blockers such as phentolamine and propranolol can also be applied. Atherosclerosis can be effectively attenuated by these drugs, but the adverse effects of the drugs have been widely documented after long-term therapy. For example, statins can cause liver injury, myopathy, and rhabdomyolysis that cannot be ignored and there is an urgent need to develop new therapies (Björnsson, 2017; Liu et al., 2019).
The Nobel Prize in Physiology or Medicine in 2015 was awarded to Youyou Tu for the discovery of qinghaosu (artemisinin) and to William C. Campbell and Satoshi Omura for ivermectin’s discovery. This heralded a new golden age of natural product drug discovery (Li and Lou, 2018; Shen, 2015). Berberine (BBR, Figure 1) has beneficial effects on atherosclerosis through regulating multiple aspects of its progression (Neag et al., 2018; Feng et al., 2019). The guideline from the European Society of Cardiology and European Atherosclerosis Society suggested BBR as a dietary supplement and functional food for the treatment of dyslipidemia (Catapano et al., 2016). This review highlights the recent advances in understanding the anti-atherosclerosis mechanism of BBR, as shown in Figure 2. BBR alleviated atherosclerosis by attenuation of dyslipidemia, correction of endothelial dysfunction, inhibition of macrophage inflammation and foam cell formation, activation of macrophage autophagy, regulation of the proliferation and migration of VSMCs, attenuation of platelet aggregation, and modulation of gut microbiota. This review would provide a modern scientific perspective to further understanding the molecular mechanism of BBR attenuating atherosclerosis and supply new ideas for atherosclerosis management.
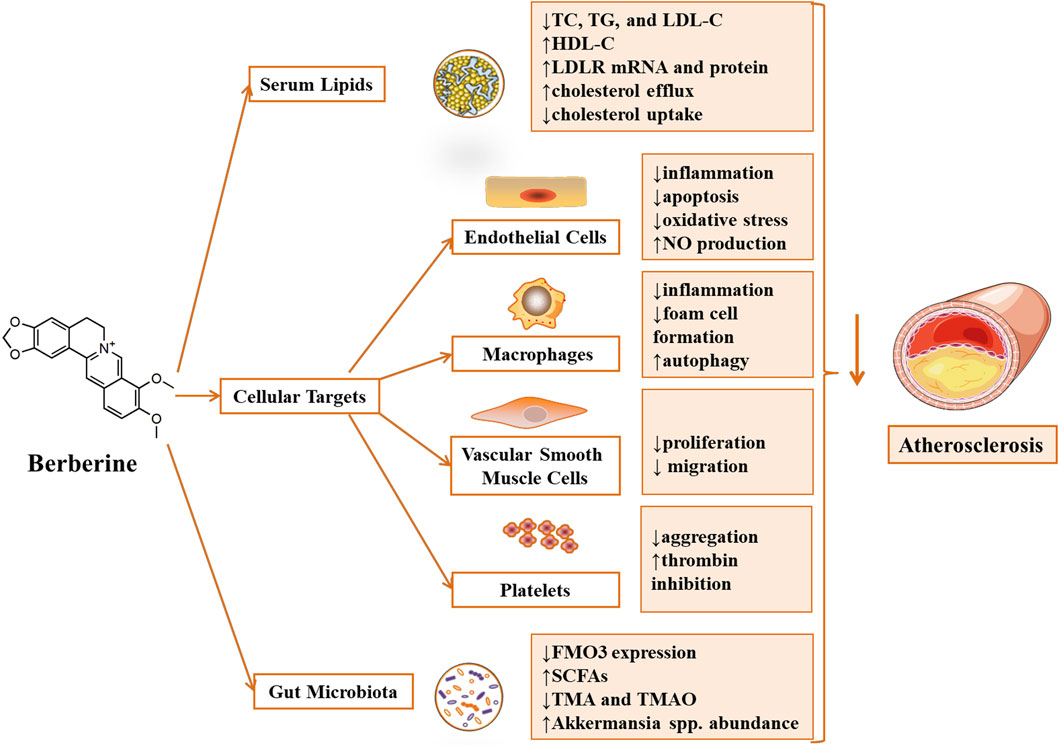
FIGURE 2. Atheroprotective effect and key molecular mechanism of Berberine (Fang et al., 2018). Berberine attenuated atherosclerosis by regulating dyslipidemia and gut microbiota. Meanwhile, Berberine alleviated atherosclerosis by affecting cellular targets, including ameliorating endothelial injury, inhibiting the formation of foam cells derived from macrophages, regulating the proliferation and migration of vascular smooth muscle cells and suppressing platelet aggregation. Annotations: ↓, reduction/down-regulation/inactivation; ↑, induction/up-regulation/activation.
Berberine Attenuated Atherosclerosis by Regulating Dyslipidemia
Hyperlipidemia, characterized by declined high-density lipoprotein (HDL) and increased total cholesterol (TC), triglyceride (TG), and LDL-C levels in serum, is a major risk factor of atherosclerosis. LDL-C plays a primary role in the formation of atherosclerosis plaque (Botham and Wheeler-Jones, 2013; Marchio et al., 2019). With the growing use of alternative herbal medicines for atherosclerosis management, BBR, as a bright new star, could alleviate atherosclerosis through regulating serum lipid profile.
According to the studies of Kong et al., orally administered BBR reduced the serum TC, TG, and LDL-C in hypercholesterolemic patients after a 3-months treatment. BBR activated extracellular signal-regulated kinase (ERK) and increased the mRNA stability of low-density lipoprotein receptor (LDLR), thus exhibited lipid-lowering effects in hyperlipidemic hamsters and HepG2 cells (Kong et al., 2004). This finding is consistent with a recent study conducted by Zhou et al., who suggested that BBR and its metabolites increased the LDLR mRNA and protein and had beneficial effects on inhibiting cellular lipid accumulation (Zhou et al., 2014). Clinical trials indicated that BBR increased plasma HDL-C and reduced TC, TG, and LDL-C after three months of administration (1.0 g daily) in subjects with low cardiovascular risk and patients with dyslipidemia and type 2 diabetes (Zhang et al., 2008; Derosa et al., 2013). The combination of BBR and simvastatin reduced serum LDL-C (46.2%) more effectively than that of BBR (26.8%) or simvastatin (28.3%) administered alone. This role might be attributed to the up-regulatory effects on LDLR expression of BBR, which is distinct from the inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase with statins (Kong et al., 2008). Another study by Brusq et al. demonstrated that BBR inhibited lipid synthesis in HepG2 cells through the activation of adenosine monophosphate-activated protein kinase (AMPK) in addition to upregulating the LDLR (Brusq et al., 2006). Recent studies showed that BBR could alleviate hyperlipidemia partly by promoting intracellular cholesterol efflux and decreasing cholesterol uptake by enterocytes (Wang et al., 2014; Li et al., 2015; Ma et al., 2020a).
Berberine Alleviated Atherosclerosis by Affecting Cellular Targets
Endothelial Cells
Vascular endothelium, the inner layer of the cardiovascular system, is a major regulator of vascular homeostasis in healthy individuals (Gimbrone and Garcia-Cardena, 2016). The healthy endothelium function mainly as a mechanical barrier between blood vessel walls and plasma molecules. Besides, it can respond to physical and chemical stimuli by producing numerous factors that regulate leukocyte attachment, vascular tone, thromboresistance, vessel wall inflammation, and VSMCs proliferation (Deanfield et al., 2007). Endothelial cell dysfunction plays a vital role in atherosclerosis lesion initiation and progression.
Berberine Suppressed Endothelial Proinflammation
A spectrum of factors lead to endothelial dysfunction, which results in the expression of endothelial-leukocyte adhesion molecules [e.g., vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and endothelial-leukocyte adhesion molecule-1], secreted chemokines [e.g., monocyte chemoattractant protein-1 (MCP-1), interleukin-8 (IL-8)] and other effector proteins (Gimbrone and Garcia-Cardena, 2016). These events bring about the recruitment of numerous inflammatory cells and trigger vascular inflammation.
BBR was reported to dramatically decrease oxLDL-stimulated adhesion of monocytes to human umbilical vein endothelial cells (HUVECs) by suppressing the expression of VCAM-1 and ICAM-1 (Huang et al., 2013). The results from Wang et al. showed that BBR attenuated the production of adhesion molecules and suppressed monocyte attachment to endothelial cells. Therefore, the hyperglycemia-induced endothelial injury was prevented partly by activating the AMPK signaling cascade (Wang et al., 2009b). Ko et al. revealed that BBR dose-dependently suppressed angiotensin II-induced U937 cells adhesion to HUVECs and mRNA expression of C-C chemokine receptor 2 (CCR-2) in U937 monocytes and MCP-1 in HUVECs, thus effectively alleviated angiotensin II-induced endothelial inflammation (Ko et al., 2007). HMC05, an extract containing BBR, inhibited attachment of monocytes to endothelial cells dose-dependently via decreasing the levels of VCAM-1, ICAM-1, MCP-1, and CCR-2 after tumor necrosis factor-α (TNF-α) induction, which was similar to that of BBR (Lee et al., 2011).
Berberine Inhibited Endothelial Cell Apoptosis
Apoptosis of vascular endothelial cells contributes to atherosclerosis development. The endothelial cells undergo apoptosis when exposed to various environmental changes, such as elevated oxLDL, blood glucose, and reactive oxygen species (ROS), decreased nitric oxide, and low shear stress (Paone et al., 2019).
BBR down-regulated the expression of proliferating cell nuclear antigen, nuclear factor κB (NF-κB), and lectin-like oxLDL receptor-1. Meanwhile, BBR inactivated phosphatidylinositol 3 kinase (PI3K)/AKT serine/threonine kinase (Akt), ERK1/2, and p38 mitogen-activated-protein kinase (MAPK) signaling pathways. Thus, BBR protected against oxLDL-caused endothelial dysfunction (Wang et al., 2009b; Caliceti et al., 2017; Xu et al., 2017). Pretreatment of BBR suppressed lipopolysaccharide (LPS)-induced apoptosis in HUVECs by blocking the c-Jun N-terminal kinase-mediated signaling pathway (Guo et al., 2016). BBR also alleviated high-glucose-mediated endothelial damage and enhanced vasodilatation via activating AMPK signaling cascade (Wang et al., 2009b).
Berberine Attenuated Oxidative Stress
Oxidative stress is the imbalance of excessive ROS generation and inactivated antioxidant defense systems. ROS generators in the vessel wall include nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, xanthine oxidase, mitochondrial enzymes, and uncoupled endothelial nitric oxide synthase (eNOS). The antioxidant enzymes in atherosclerosis contain superoxide dismutase, catalase, glutathione peroxidase, and paraoxonases (Förstermann et al., 2017).
BBR treatment ameliorated CD31+/CD42− microparticles-induced endothelial dysfunction through decreasing oxidative stress in HUVECs (Cheng et al., 2013). Studies conducted by Wang et al. (2009b), Zhang et al. (2013) demonstrated that BBR alleviated endothelial injury induced by high glucose and palmitate partly via activation of the AMPK signaling cascade and reduced generation of ROS. BBR could reduce intracellular ROS levels induced by TNF-α (Caliceti et al., 2017) and endothelial progenitor cells dysfunction caused by TNF-α could be improved by BBR via PI3K/Akt/eNOS signal pathway (Xiao et al., 2014). Furthermore, HMC05, an extract containing BBR, markedly inhibited the production of ROS and dose-dependently attenuated TNF-α-induced adhesion of monocytes to endothelial cells (Lee et al., 2011).
Berberine Activated Nitric Oxide Signaling Pathway
Nitric oxide (NO) produced by nitric oxide synthase (NOS) in endothelial cells is of great importance in regulating vascular tone. Neuronal NOS, eNOS, and inducible NOS are related to the production of NO. Neuronal NOS and eNOS function as anti-atherosclerosis factors, whereas inducible NOS is likely to play a pro-atherosclerosis role (Li et al., 2014). BBR showed atheroprotective effects by affecting the NO signaling pathway.
It was demonstrated that phosphorylation of eNOS at Ser1177 was enhanced by BBR dose-dependently, leading to an increased eNOS protein expression and NO production (Wang et al., 2009b). Zhang et al. (2013) reported that BBR considerably upregulated eNOS expression and NO levels in palmitate-treated HUVECs and ameliorated endothelial dysfunction. Bu-Shen-Ning-Xin Decoction, a Chinese herbal compound containing BBR, upregulated NO synthesis via estrogen receptor β pathway. Subsequently, NO suppressed apoptosis and NF-κB activity in endothelial cells and inhibited atherosclerosis progression (Wang et al., 2013). Elevated circulating endothelial microparticles (EMPs) are tightly linked to endothelial dysfunction. The diminished eNOS protein expression mediated by EMPs was markedly inhibited by BBR in HUVECs. Furthermore, BBR-induced decline in circulating CD31+/CD42− microparticles contributed to the improvement of endothelial function in healthy subjects (Wang et al., 2009a; Cheng et al., 2013).
Macrophages
Macrophages play critical roles in the initiation and progression of atherosclerosis. The inflammatory responses and macrophage-derived foam cell formation are the principal events in atherosclerosis (Moore et al., 2013; Tabas and Bornfeldt, 2016). BBR can achieve its atheroprotective functions by affecting the behavior of macrophages, such as inhibition of macrophage inflammation, foam cell formation, and activation of macrophage autophagy.
Anti-Inflammation
Macrophages constitute the most prominent inflammatory cells in atherosclerosis lesions. Activated macrophages produce a series of inflammation-related factors such as interleukin-1β (IL-1β), TNF-α, interleukin-6 (IL-6), IL-8, MCP-1, matrix metalloprotease-9 (MMP-9), and so on, which initiate inflammation to induce atherosclerosis (Kleemann et al., 2008).
BBR significantly downregulated the expression of proinflammatory genes such as IL-1β, IL-6, MCP-1, inducible NOS, cyclooxygenase-2, and MMP-9 through AMPK activation in macrophages (Jeong et al., 2009). In oxLDL-induced macrophages, BBR markedly upregulated miR150-5p level and decreased P2X7R-mediated extracellular matrix metalloproteinase inducer (EMMPRIN) and MMP-9 expression (Lu et al., 2021). In LPS-stimulated macrophages (RAW264.7), BBR treatment potently suppressed the expression of inflammatory cytokines such as TNF-α, IL-6, and MCP-1 through inhibition of NF-κB signaling via sirtuin 1-dependent mechanisms (Zhang et al., 2017). According to the study by Chen et al., BBR inhibited acetylated low-density lipoprotein-induced TNF-α, MCP-1, and IL-6 expression through peroxisome proliferator-activated receptor γ signaling pathway in macrophages (Chen et al., 2008). BBR tremendously inhibited TNF-α and IL-6 expression stimulated with an HIV protease inhibitor by modulating endoplasmic reticulum stress signaling pathways in murine macrophages (Zha et al., 2010). BBR reduced the expression of MMP-9 and EMMPRIN by suppressing the activation of p38 and NF-κB signaling pathways in human THP-1 macrophages (Huang et al., 2011; Huang et al., 2012b). BBR alleviated NLR Family Pyrin Domain Containing 3 inflammation activation by reducing IL-1β secretion via NF-κB inhibition in macrophages (Jiang et al., 2017). HMCO5 containing BBR suppressed the activation of NF-κB and subsequently inhibited the secretion of TNF-α and IL-1β in LPS stimulated RAW264.7 cells (Kim et al., 2007). In mouse RAW264.7 macrophages and primary hepatocytes, BBR significantly downregulated the proinflammatory cytokines (TNF-α, IL-6, IL-1β, and MCP-1) via suppressing the protein expression of endoplasmic reticulum stress genes (Wang et al., 2020b).
Berberine Inhibited Foam Cell Formation
Foam cell formation is a hallmark at the initial stage of atherosclerosis. The augmented ox-LDL influx and accumulation of cholesterol esters in intimal macrophages are responsible for this issue. Macrophages express a series of scavenger receptors (SR) with affinity to oxLDL, such as SR class A type I, CD36, and LOX-1. ATP-binding cassette transporters ABCA1 and ABCG1 and SR class B type I (SR-BI) in macrophages are involved in reverse cholesterol transport (Chistiakov et al., 2016; Chistiakov et al., 2017). These proteins protected macrophages from the formation of foam cells.
BBR can dose- and time-dependently downregulate oxLDL receptor-1 expression and facilitate SR-BI expression in macrophage-derived foam cells induced by oxLDL (Guan et al., 2010). Simultaneous administration of BBR and atorvastatin inhibited the expression of LOX-1 via the endothelin-1 receptor in monocyte/macrophages, which inhibited foam cell formation (Chi et al., 2014). BBR reduced foam cell formation by decreasing oxLDL internalization and increasing cholesterol efflux via the suppression of CD36, lectin-like oxLDL receptor-1, and adipocyte enhancer binding protein 1 in macrophages (Huang et al., 2012a). Macropinocytosis, excess free cholesterol-induced membrane ruffling, and hypercholesterolemic serum-induced cholesterol accumulation were inhibited by BBR in macrophages (Zimetti et al., 2015). BBR inhibited foam cell formation by increasing cholesterol efflux through enhancing liver X receptor α-ABCA1 expression in macrophages (Lee et al., 2010).
Berberine Promoted Macrophage Autophagy
Macrophage autophagy inhibited foam cell formation by the deficiency of oxLDL ingestion and the increase of efferocytosis and cholesterol efflux in macrophages. Therefore, promoting macrophage autophagy may alleviate atherosclerosis (Jia et al., 2006; Muller et al., 2011; Scherz-Shouval and Elazar, 2011; Shao et al., 2016).
BBR treatment alleviated inflammation in murine macrophages (J774A.1) by promoting autophagy, which was initiated by activation of the AMPK/mechanistic target of rapamycin (mTOR) signaling pathway (Fan et al., 2015). BBR-mediated sonodynamic therapy effectively induced cholesterol efflux by promoting ROS generation, and induced autophagy by regulating the PI3K/Akt/mTOR signaling pathway in THP-1 macrophages, peritoneal macrophages, and derived foam cells (Kou et al., 2017). BBR activated Sirt1 via the nicotinamide adenine dinucleotide synthesis pathway to promote transcription factor EB nuclear translocation and deacetylation, which in turn, triggered autophagy in peritoneal macrophages (Zheng et al., 2021). BBR reduced plaque area and alleviated inflammation in atherosclerosis rats with damp-heat syndrome via promoting LC3-II protein expression and inhibiting P62 protein expression. 3-methyladenine, an inhibitor of autophagy, significantly aggravated atherosclerosis progression (Ke et al., 2020).
Vascular Smooth Muscle Cells
VSMCs play a critical role in atherosclerosis progression. The aberrant proliferation and migration of VSMCs promote extracellular matrix formation in atherosclerosis plaque areas (Doran et al., 2008; Chistiakov et al., 2015). Studies confirmed that BBR could suppress the proliferation and migration of VSMCs to attenuate atherosclerosis.
Angiotensin II and heparin-binding epidermal growth factor were enormously inhibited by BBR via delaying or partially inactivating the Akt signaling pathway, which inhibited the proliferation and migration of VSMCs (Lee et al., 2006). Lysophosphatidylcholine induced VSMCs proliferation and migration, which triggered the intimal thickening in atherosclerosis lesions. BBR inhibited lysophosphatidylcholine-stimulated VSMCs proliferation and migration via suppression of ROS generation and ERK1/2 signaling pathway (Cho et al., 2005). BBR inhibited platelet-derived growth factor (PDGF)-induced VSMCs growth via activation of AMPK/p53/p21Cip1 signaling pathway and suppressed PDGF-stimulated migration via inhibition of Ras, Cell Division Cycle 42, and Rac Family Small GTPase 1 (Liang et al., 2008). Mechanical injury-induced VSMCs growth was prevented by BBR treatment through mitogen-activated protein kinase/ERK activation, early growth response gene, c-Fos, Cyclin D1, and PDGF subunit A expression, protein disulfide isomerase activation as well as phosphorylation of MAPKs (Liang et al., 2006; Wang et al., 2020a). BBR disrupted the binding of p27, p21 with S-phase kinase-associated protein-2, and induced G0/G1 phase arrest, which attenuated the proliferation of A7r5 induced by PDGF (Liu et al., 2011). Liu et al. found that BBR exerted anti-migratory properties in human VSMCs, possibly by downregulating MMP-2/9 and urokinase-type plasminogen activator and inhibiting AP-1 and NF-κB signaling pathways (Liu et al., 2014). BBR treatment dose-dependently inhibited VSMCs migration induced by upregulations of MMP-3 and MMP-9 via decreasing the phosphorylation of Akt at Ser473 with C. pneumoniae infection (Ma et al., 2015). HMC05, containing BBR and hesperidin in large quantities, protected VSMCs against oxidative stress by increasing NADPH: quinone oxidoreductase-1 gene expression via the regulation of Ras homolog family member A and/or Ras (Gum et al., 2014).
Platelets
Impaired regulation of platelet activation/aggregation is a prime cause of arterial thrombosis, this vital complication of atherosclerosis triggering myocardial infarction and stroke (Schafer and Bauersachs, 2008). The platelet activation and apoptosis would induce vascular occlusions and atherothrombotic events. BBR could inhibit these events by suppressing platelet aggregation and superoxide production via regulating NADPH oxidase, aldose reductase, and glutathione reductase in platelets with excess glucose. In addition, BBR inhibited platelet adhesive property and apoptosis induced by high glucose (Paul et al., 2019). BBR significantly inhibited rabbit platelet aggregation by suppressing the synthesis of thromboxane A2 (Huang et al., 2002). Molecular docking studies indicated that BBR interacted with thrombin by hydrogen bond and π-π interactions. Direct binding studies, competitive binding assay, and platelet aggregation assay demonstrated that BBR was a thrombin inhibitor showing direct activity in inhibiting platelet aggregation (Wang et al., 2017).
Berberine Reduced Atherosclerosis by Affecting Gut Microbiota
The gut microbiota and its metabolites play a critical role in atherosclerosis development (Mantziaris and Kolios, 2019). Trimethylamine (TMA), produced by gut microbiota, was converted to trimethylamine-N-oxide (TMAO) via flavin-containing monooxygenase form 3 (FMO3) in the liver (Schiattarella et al., 2017; Mantziaris and Kolios, 2019; Tang et al., 2019). It has been found that the BBR treatment reduced high-fat diet feeding-induced FMO3 expression and altered the composition of gut microbiota (Shi et al., 2018). The synthesis of TMA and TMAO were inhibited remarkably in choline-fed ApoE-/- and C57BL/6J mice by BBR via suppressing choline-to-TMA conversion. However, a slight increment was observed in chow-fed mice, indicating that BBR might decrease TMA production by gut microbiota only when the choline was overdosed (Li et al., 2021). There was a piece of evidence that BBR directly changed the bacterial community composition and function by reducing Clostridium spp. and subsequently activated farnesoid X receptor signaling (Tian et al., 2019). BBR treatment markedly increased Akkermansia spp. abundance in HFD-fed ApoE-/- mice, contributing to the anti-atherosclerotic properties of BBR (Zhu et al., 2018). In line with those findings, replenishment with Akkermansia significantly reduced atherosclerosis induced by a high-fat diet by attenuating the aortic and systemic metabolic inflammatory response (Li et al., 2016). A previous study revealed that BBR stimulated the gut bacteria-derived polyamines and enhanced mucin secretion in the colon of mice, exhibiting Akkermansia-promoting effects (Dong et al., 2021). According to the study of Wu et al., the abundance of Alistipes, Allobaculum, Blautia, Roseburia, and Turicibacter were significantly increased, and the abundance of Bilophila was altered after BBR treatment. Thus, the metabolism of lipid, glycan and the synthesis of short-chain fatty acids were promoted and the production of TMAO was reduced (Wu et al., 2020).
Concluding Remarks
Herbal medicines represent indispensable roles in new drug discovery, and they are relatively safe since herbs have been used for thousands of years in clinical practice. The atheroprotective effects of BBR have been explored during the past decades. We reviewed its anti-atherosclerotic effects from the perspective of molecular targets. Numerous evidences suggested that BBR had great therapeutic potential to attenuate atherosclerosis through lipid modification, anti-inflammatory, anti-oxidant, anti-apoptosis, anti-proliferative, anti-platelet aggregation, and gut microbiota modulatory activities. Among them, anti-inflammatory was the dominant factor. BBR significantly inhibited the expression of inflammatory factors and adhesion molecules, thus played anti-inflammatory role both in macrophages and endothelial cells.
Although a lot of knowledge has been gained in understanding the BBR-mediated atheroprotective potential, there are numerous questions ahead. The poor aqueous solubility and low dissolution of BBR lead to low oral bioavailability (< 1%) and have limited its clinical application (Liu et al., 2010). However, the poor bioavailability of BBR and its favorable atheroprotective effects are not contradictory. On the one hand, poorly absorbed BBR remained inside the gastrointestinal tract for a long time. It interacted comprehensively with the gut microbiota, which contributed to the anti-atherosclerosis effects of BBR by regulating the gut microbiota. On the other hand, BBR could convert into multiple metabolites. Many metabolites have anti-atherosclerotic effects, some metabolites showed even more potent anti-atherosclerotic effects than BBR (Cho, 2011; Cao et al., 2013; Wu et al., 2014; Zhou et al., 2014; Ning et al., 2015). In addition, various approaches have been explored to enhance its oral bioavailability (Mujtaba et al., 2021). BBR-trapped solid lipid nanoparticles and micelles had shown anti-hyperlipidemic and anti-atherosclerosis effects in animals (Ma et al., 2020b; Sailor et al., 2021). Some BBR analogs and derivatives also exhibited anti-atherosclerosis properties (Feng et al., 2017a; Feng et al., 2017b). Our understanding of BBR has been deepening by chemical, pharmacological, and system biological approaches (Liu et al., 2013). Especially, with the help of network pharmacology, computer-assisted molecular docking and genomic, and metabolomic profiling approaches, novel anti-atherosclerosis mechanisms/targets of BBR will be identified. In short, BBR could be a promising candidate for atherosclerosis management.
Author Contributions
LX and XZ summarized the literature and wrote this manuscript. A-HL, H-JL, C-XH, and WQ collected and analyzed references. DZ, P-QL, and LZ drew figures. H-LC planned the framework and polished the manuscript. All authors contributed significantly to the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (U1932130); the Key Program of Shaanxi Provincial Education Department (19JS058), the Program of Shaanxi Provincial Science and Technology Department (2021JQ-788), the Program of Shaanxi Administration of Traditional Chinese Medicine (2019-ZZ-ZY009); the Key Program of Weiyang District Bureau of Science, Technology and Industry Information Technology (201928) and the Talent Program of Xi’an Medical University (2015RCYJ01, 2018PT28, 2018DOC10, and 2020DOC28).
Conflict of Interest
Authors A-HI and H-LC were employed by the Shaanxi Pharmaceutical Holding Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Basatemur, G. L., Jørgensen, H. F., Clarke, M. C. H., Bennett, M. R., and Mallat, Z. (2019). Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 16 (12), 727–744. doi:10.1038/s41569-019-0227-9
Benjamin, E. J., Muntner, P., Alonso, A., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2019). Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 139 (10), e56–e528. doi:10.1161/CIR.0000000000000659
Björnsson, E. S. (2017). Hepatotoxicity of Statins and Other Lipid-Lowering Agents. Liver Int. 37 (2), 173–178. doi:10.1111/liv.13308
Botham, K. M., and Wheeler-Jones, C. P. D. (2013). Postprandial Lipoproteins and the Molecular Regulation of Vascular Homeostasis. Prog. lipid Res. 52 (4), 446–464. doi:10.1016/j.plipres.2013.06.001
Brusq, J.-M., Ancellin, N., Grondin, P., Guillard, R., Martin, S., Saintillan, Y., et al. (2006). Inhibition of Lipid Synthesis through Activation of AMP Kinase: an Additional Mechanism for the Hypolipidemic Effects of Berberine. J. lipid Res. 47 (6), 1281–1288. doi:10.1194/jlr.M600020-JLR200
Buckley, M. L., and Ramji, D. P. (2015). The Influence of Dysfunctional Signaling and Lipid Homeostasis in Mediating the Inflammatory Responses during Atherosclerosis. Biochim. Biophys. Acta (Bba) - Mol. Basis Dis. 1852 (7), 1498–1510. doi:10.1016/j.bbadis.2015.04.011
Caliceti, C., Rizzo, P., Ferrari, R., Fortini, F., Aquila, G., Leoncini, E., et al. (2017). Novel Role of the Nutraceutical Bioactive Compound Berberine in Lectin-like OxLDL Receptor 1-mediated Endothelial Dysfunction in Comparison to Lovastatin. Nutr. Metab. Cardiovasc. Dis. 27 (6), 552–563. doi:10.1016/j.numecd.2017.04.002
Cao, S., Zhou, Y., Xu, P., Wang, Y., Yan, J., Bin, W., et al. (2013). Berberine Metabolites Exhibit Triglyceride-Lowering Effects via Activation of AMP-Activated Protein Kinase in Hep G2 Cells. J. Ethnopharmacology 149 (2), 576–582. doi:10.1016/j.jep.2013.07.025
Catapano, A. L., Graham, I., De Backer, G., Wiklund, O., Chapman, M. J., Drexel, H., et al. (2016). 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: the Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the Special Contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR). Atherosclerosis 253, 281–344. doi:10.1016/j.atherosclerosis.2016.08.018
Chen, F. L., Yang, Z. H., Liu, Y., Li, L. X., Liang, W. C., Wang, X. C., et al. (2008). Berberine Inhibits the Expression of TNFα, MCP-1, and IL-6 in AcLDL-Stimulated Macrophages through PPARγ Pathway. Endocr 33 (3), 331–337. doi:10.1007/s12020-008-9089-3
Cheng, F., Wang, Y., Li, J., Su, C., Wu, F., Xia, W.-H., et al. (2013). Berberine Improves Endothelial Function by Reducing Endothelial Microparticles-Mediated Oxidative Stress in Humans. Int. J. Cardiol. 167 (3), 936–942. doi:10.1016/j.ijcard.2012.03.090
Chi, L., Peng, L., Hu, X., Pan, N., and Zhang, Y. (2014). Berberine Combined with Atorvastatin Downregulates LOX-1 Expression through the ET-1 Receptor in Monocyte/macrophages. Int. J. Mol. Med. 34 (1), 283–290. doi:10.3892/ijmm.2014.1748
Chistiakov, D. A., Bobryshev, Y. V., and Orekhov, A. N. (2016). Macrophage‐mediated Cholesterol Handling in Atherosclerosis. J. Cel. Mol. Med. 20 (1), 17–28. doi:10.1111/jcmm.12689
Chistiakov, D. A., Melnichenko, A. A., Myasoedova, V. A., Grechko, A. V., and Orekhov, A. N. (2017). Mechanisms of Foam Cell Formation in Atherosclerosis. J. Mol. Med. 95 (11), 1153–1165. doi:10.1007/s00109-017-1575-8
Chistiakov, D. A., Orekhov, A. N., and Bobryshev, Y. V. (2015). Vascular Smooth Muscle Cell in Atherosclerosis. Acta Physiol. 214 (1), 33–50. doi:10.1111/apha.12466
Cho, B. J., Im, E. K., Kwon, J. H., Lee, K. H., Shin, H. J., Oh, J., et al. (2005). Berberine Inhibits the Production of Lysophosphatidylcholine-Induced Reactive Oxygen Species and the ERK1/2 Pathway in Vascular Smooth Muscle Cells. Mol. Cell 20 (3), 429–434.
Cho, Y.-J. (2011). Anti-inflammatory Effect of Jatrorrhizine from Phellodendron Amurense in Lipopolysaccharide-Stimulated Raw264.7 Cells. J. Appl. Biol. Chem. 54 (2), 114–119. doi:10.3839/jabc.2011.020
Deanfield, J. E., Halcox, J. P., and Rabelink, T. J. (2007). Endothelial Function and Dysfunction. Circulation 115 (10), 1285–1295. doi:10.1161/CIRCULATIONAHA.106.652859
Derosa, G., D'Angelo, A., Bonaventura, A., Bianchi, L., Romano, D., and Maffioli, P. (2013). Effects of Berberine on Lipid Profile in Subjects with Low Cardiovascular Risk. Expert Opin. Biol. Ther. 13 (4), 475–482. doi:10.1517/14712598.2013.776037
Dong, C., Yu, J., Yang, Y., Zhang, F., Su, W., Fan, Q., et al. (2021). Berberine, a Potential Prebiotic to Indirectly Promote Akkermansia Growth through Stimulating Gut Mucin Secretion. Biomed. Pharmacother. 139, 111595. doi:10.1016/j.biopha.2021.111595
Doran, A. C., Meller, N., and McNamara, C. A. (2008). Role of Smooth Muscle Cells in the Initiation and Early Progression of Atherosclerosis. Atvb 28 (5), 812–819. doi:10.1161/ATVBAHA.107.159327
Fan, X., Wang, J., Hou, J., Lin, C., Bensoussan, A., Chang, D., et al. (2015). Berberine Alleviates Ox-LDL Induced Inflammatory Factors by Up-Regulation of Autophagy via AMPK/mTOR Signaling Pathway. J. Transl Med. 13, 92. doi:10.1186/s12967-015-0450-z
Fang, J., Little, P. J., and Xu, S. (2018). Atheroprotective Effects and Molecular Targets of Tanshinones Derived from Herbal Medicine Danshen. Med. Res. Rev. 38 (1), 201–228. doi:10.1002/med.21438
Feng, M., Kong, S.-Z., Wang, Z.-X., He, K., Zou, Z.-Y., Hu, Y.-R., et al. (2017a). The Protective Effect of Coptisine on Experimental Atherosclerosis ApoE−/− Mice Is Mediated by MAPK/NF-κB-dependent Pathway. Biomed. Pharmacother. 93, 721–729. doi:10.1016/j.biopha.2017.07.002
Feng, M., Zou, Z., Zhou, X., Hu, Y., Ma, H., Xiao, Y., et al. (2017b). Comparative Effect of Berberine and its Derivative 8-cetylberberine on Attenuating Atherosclerosis in ApoE−/− Mice. Int. Immunopharmacology 43, 195–202. doi:10.1016/j.intimp.2016.12.001
Feng, X., Sureda, A., Jafari, S., Memariani, Z., Tewari, D., Annunziata, G., et al. (2019). Berberine in Cardiovascular and Metabolic Diseases: from Mechanisms to Therapeutics. Theranostics 9 (7), 1923–1951. doi:10.7150/thno.30787
Förstermann, U., Xia, N., and Li, H. (2017). Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 120 (4), 713–735. doi:10.1161/CIRCRESAHA.116.309326
Gimbrone, M. A., and García-Cardeña, G. (2016). Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 118 (4), 620–636. doi:10.1161/CIRCRESAHA.115.306301
Guan, S., Wang, B., Li, W., Guan, J., and Fang, X. (2010). Effects of Berberine on Expression of LOX-1 and SR-BI in Human Macrophage-Derived Foam Cells Induced by Ox-LDL. Am. J. Chin. Med. 38 (6), 1161–1169. doi:10.1142/S0192415X10008548
Gum, S. I., Shin, H. M., and Cho, M. K. (2014). The Small GTPases Regulate HMC05-Induced NQO-1 Expression with an Antioxidant Effect in Smooth Muscle Cells. Biol. Pharm. Bull. 37 (10), 1626–1632. doi:10.1248/bpb.b14-00336
Guo, J., Wang, L., Wang, L., Qian, S., Zhang, D., Fang, J., et al. (2016). Berberine Protects Human Umbilical Vein Endothelial Cells against LPS-Induced Apoptosis by Blocking JNK-Mediated Signaling. Evidence-Based Complement. Altern. Med. 2016, 1–11. doi:10.1155/2016/6983956
Huang, C. G., Chu, Z. L., Wei, S. J., Jiang, H., and Jiao, B. H. (2002). Effect of Berberine on Arachidonic Acid Metabolism in Rabbit Platelets and Endothelial Cells. Thromb. Res. 106 (4-5), 223–227. doi:10.1016/s0049-3848(02)00133-0
Huang, Z., Cai, X., Li, S., Zhou, H., Chu, M., Shan, P., et al. (2013). Berberine-attenuated Monocyte Adhesion to Endothelial Cells Induced by Oxidized Low-Density Lipoprotein via Inhibition of Adhesion Molecule Expression. Mol. Med. Rep. 7 (2), 461–465. doi:10.3892/mmr.2012.1236
Huang, Z., Dong, F., Li, S., Chu, M., Zhou, H., Lu, Z., et al. (2012a). Berberine-induced Inhibition of Adipocyte Enhancer-Binding Protein 1 Attenuates Oxidized Low-Density Lipoprotein Accumulation and Foam Cell Formation in Phorbol 12-myristate 13-Acetate-Induced Macrophages. Eur. J. Pharmacol. 690 (1-3), 164–169. doi:10.1016/j.ejphar.2012.07.009
Huang, Z., Meng, S., Wang, L., Wang, Y., Chen, T., and Wang, C. (2012b). Suppression of oxLDL-Induced MMP-9 and EMMPRIN Expression by Berberine via Inhibition of NF-Κb Activation in Human THP-1 Macrophages. Anat. Rec. 295 (1), 78–86. doi:10.1002/ar.21489
Huang, Z., Wang, L., Meng, S., Wang, Y., Chen, T., and Wang, C. (2011). Berberine Reduces Both MMP-9 and EMMPRIN Expression through Prevention of P38 Pathway Activation in PMA-Induced Macrophages. Int. J. Cardiol. 146 (2), 153–158. doi:10.1016/j.ijcard.2009.06.023
Jeong, H. W., Hsu, K. C., Lee, J.-W., Ham, M., Huh, J. Y., Shin, H. J., et al. (2009). Berberine Suppresses Proinflammatory Responses through AMPK Activation in Macrophages. Am. J. Physiology-Endocrinology Metab. 296, E955–E964. doi:10.1152/ajpendo.90599.2008
Jia, G., Cheng, G., Gangahar, D. M., and Agrawal, D. K. (2006). Insulin‐like Growth Factor‐1 and TNF‐α Regulate Autophagy through C‐ Jun N‐terminal Kinase and Akt Pathways in Human Atherosclerotic Vascular Smooth Cells. Immunol. Cel Biol. 84 (5), 448–454. doi:10.1111/j.1440-1711.2006.01454.x
Jiang, Y., Huang, K., Lin, X., Chen, Q., Lin, S., Feng, X., et al. (2017). Berberine Attenuates NLRP3 Inflammasome Activation in Macrophages to Reduce the Secretion of Interleukin-1β. Ann. Clin. Lab. Sci. 47 (6), 720–728. https://pubmed.ncbi.nlm.nih.gov/29263046/.
Jonsson, A. L., and Bäckhed, F. (2017). Role of Gut Microbiota in Atherosclerosis. Nat. Rev. Cardiol. 14 (2), 79–87. doi:10.1038/nrcardio.2016.183
Ke, X., Huang, Y., Li, L., Xin, F., Xu, L., Zhang, Y., et al. (2020). Berberine Attenuates Arterial Plaque Formation in Atherosclerotic Rats with Damp-Heat Syndrome via Regulating Autophagy. Dddt 14, 2449–2460. doi:10.2147/DDDT.S250524
Kim, K. M., Choi, J. Y., Yoo, S.-E., Park, M. Y., Lee, B.-S., Ko, Y. H., et al. (2007). HMCO5, Herbal Extract, Inhibits NF-Κb Expression in Lipopolysaccharide Treated Macrophages and Reduces Atherosclerotic Lesions in Cholesterol Fed Mice. J. Ethnopharmacology 114 (3), 316–324. doi:10.1016/j.jep.2007.08.029
Kleemann, R., Zadelaar, S., and Kooistra, T. (2008). Cytokines and Atherosclerosis: a Comprehensive Review of Studies in Mice. Cardiovasc. Res. 79 (3), 360–376. doi:10.1093/cvr/cvn120
Ko, Y. J., Lee, J.-S., Park, B. C., Shin, H. M., and Kim, J.-A. (2007). Inhibitory Effects of Zoagumhwan Water Extract and Berberine on Angiotensin II-Induced Monocyte Chemoattractant Protein (MCP)-1 Expression and Monocyte Adhesion to Endothelial Cells. Vasc. Pharmacol. 47 (2-3), 189–196. doi:10.1016/j.vph.2007.06.004
Kong, W.-J., Wei, J., Zuo, Z.-Y., Wang, Y.-M., Song, D.-Q., You, X.-F., et al. (2008). Combination of Simvastatin with Berberine Improves the Lipid-Lowering Efficacy. Metabolism 57 (8), 1029–1037. doi:10.1016/j.metabol.2008.01.037
Kong, W., Wei, J., Abidi, P., Lin, M., Inaba, S., Li, C., et al. (2004). Berberine Is a Novel Cholesterol-Lowering Drug Working through a Unique Mechanism Distinct from Statins. Nat. Med. 10 (12), 1344–1351. doi:10.1038/nm1135
Kou, J. Y., Li, Y., Zhong, Z. Y., Jiang, Y. Q., Li, X. S., Han, X. B., et al. (2017). Berberine-sonodynamic Therapy Induces Autophagy and Lipid Unloading in Macrophage. Cell Death Dis. 8 (1), e2558. doi:10.1038/cddis.2016.354
Lee, J. S., Park, S.-Y., Thapa, D., Kim, A. R., Shin, H.-M., and Kim, J.-A. (2011). HMC05, Herbal Formula, Inhibits TNF-α-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. Evidence-Based Complement. Altern. Med. 2011, 1–11. doi:10.1093/ecam/nep126
Lee, S., Lim, H.-J., Park, H.-Y., Lee, K.-S., Park, J.-H., and Jang, Y. (2006). Berberine Inhibits Rat Vascular Smooth Muscle Cell Proliferation and Migration In Vitro and Improves Neointima Formation after Balloon Injury In Vivo. Atherosclerosis 186 (1), 29–37. doi:10.1016/j.atherosclerosis.2005.06.048
Lee, T.-S., Pan, C.-C., Peng, C.-C., Kou, Y. R., Chen, C.-Y., Ching, L.-C., et al. (2010). Anti-atherogenic Effect of Berberine on LXRα-ABCA1-dependent Cholesterol Efflux in Macrophages. J. Cel. Biochem. 111 (1), 104–110. doi:10.1002/jcb.22667
Li, G., and Lou, H.-X. (2018). Strategies to Diversify Natural Products for Drug Discovery. Med. Res. Rev. 38 (4), 1255–1294. doi:10.1002/med.21474
Li, H., Horke, S., and Förstermann, U. (2014). Vascular Oxidative Stress, Nitric Oxide and Atherosclerosis. Atherosclerosis 237 (1), 208–219. doi:10.1016/j.atherosclerosis.2014.09.001
Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W., and Xu, A. (2016). Akkermansia Muciniphila Protects against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe−/− Mice. Circulation 133 (24), 2434–2446. doi:10.1161/circulationaha.115.019645
Li, X.-Y., Zhao, Z.-X., Huang, M., Feng, R., He, C.-Y., Ma, C., et al. (2015). Effect of Berberine on Promoting the Excretion of Cholesterol in High-Fat Diet-Induced Hyperlipidemic Hamsters. J. Transl Med. 13, 278. doi:10.1186/s12967-015-0629-3
Li, X., Su, C., Jiang, Z., Yang, Y., Zhang, Y., Yang, M., et al. (2021). Berberine Attenuates Choline-Induced Atherosclerosis by Inhibiting Trimethylamine and Trimethylamine-N-Oxide Production via Manipulating the Gut Microbiome. NPJ Biofilms Microbiomes 7 (1), 36. doi:10.1038/s41522-021-00205-8
Liang, K.-W., Ting, C.-T., Yin, S.-C., Chen, Y.-T., Lin, S.-J., Liao, J. K., et al. (2006). Berberine Suppresses MEK/ERK-dependent Egr-1 Signaling Pathway and Inhibits Vascular Smooth Muscle Cell Regrowth after In Vitro Mechanical Injury. Biochem. Pharmacol. 71 (6), 806–817. doi:10.1016/j.bcp.2005.12.028
Liang, K.-W., Yin, S.-C., Ting, C.-T., Lin, S.-J., Hsueh, C.-M., Chen, C.-Y., et al. (2008). Berberine Inhibits Platelet-Derived Growth Factor-Induced Growth and Migration Partly through an AMPK-dependent Pathway in Vascular Smooth Muscle Cells. Eur. J. Pharmacol. 590 (1-3), 343–354. doi:10.1016/j.ejphar.2008.06.034
Liu, A., Wu, Q., Guo, J., Ares, I., Rodríguez, J.-L., Martínez-Larrañaga, M.-R., et al. (2019). Statins: Adverse Reactions, Oxidative Stress and Metabolic Interactions. Pharmacol. Ther. 195, 54–84. doi:10.1016/j.pharmthera.2018.10.004
Liu, J., Xiu, J., Cao, J., Gao, Q., Ma, D., and Fu, L. (2011). Berberine Cooperates with Adrenal Androgen Dehydroepiandrosterone Sulfate to Attenuate PDGF-Induced Proliferation of Vascular Smooth Muscle Cell A7r5 through Skp2 Signaling Pathway. Mol. Cel Biochem. 355 (1-2), 127–134. doi:10.1007/s11010-011-0846-x
Liu, S.-J., Yin, C.-X., Ding, M.-C., Xia, S.-Y., Shen, Q.-M., and Wu, J.-D. (2014). Berberine Suppresses In Vitro Migration of Human Aortic Smooth Muscle Cells through the Inhibitions of MMP-2/9, u-PA, AP-1, and NF-Κb. BMB Rep. 47 (7), 388–392. doi:10.5483/BMBRep.2014.47.7.186
Liu, X., Wu, W.-Y., Jiang, B.-H., Yang, M., and Guo, D.-A. (2013). Pharmacological Tools for the Development of Traditional Chinese Medicine. Trends Pharmacological Sciences 34 (11), 620–628. doi:10.1016/j.tips.2013.09.004
Liu, Y.-T., Hao, H.-P., Xie, H.-G., Lai, L., Wang, Q., Liu, C.-X., et al. (2010). Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain its Low Plasma Levels in Rats. Drug Metab. Dispos 38 (10), 1779–1784. doi:10.1124/dmd.110.033936
Lu, L., Huang, J., Xue, X., Wang, T., Huang, Z., and Li, J. (2021). Berberine Regulated miR150-5p to Inhibit P2X7 Receptor, EMMPRIN and MMP-9 Expression in oxLDL Induced Macrophages. Front. Pharmacol. 12, 639558. doi:10.3389/fphar.2021.639558
Ma, C.-Y., Shi, X.-Y., Wu, Y.-R., Zhang, Y., Qu, H.-L., Guo, Y.-L., et al. (2020a). Berberine Improves High-Fat Diet Induced Atherosclerosis and Hepatic Steatosis in Apoe-/-Mice by Down-Regulating PCSK9 via ERK1/2 Pathway. doi:10.21203/rs.3.rs-38344/v1
Ma, L., Zhang, L., Wang, B., Wei, J., Liu, J., and Zhang, L. (2015). Berberine Inhibits Chlamydia Pneumoniae Infection-Induced Vascular Smooth Muscle Cell Migration through Downregulating MMP3 and MMP9 via PI3K. Eur. J. Pharmacol. 755, 102–109. doi:10.1016/j.ejphar.2015.02.039
Ma, X., Zhang, T., Luo, Z., Li, X., Lin, M., Li, R., et al. (2020b). Functional Nano-Vector Boost Anti-atherosclerosis Efficacy of Berberine in Apoe() Mice. Acta Pharmaceutica Sinica B 10 (9), 1769–1783. doi:10.1016/j.apsb.2020.03.005
Mantziaris, V., and Kolios, G. (2019). Gut Microbiota, Atherosclerosis, and Therapeutic Targets. Crit. pathways Cardiol. 18 (3), 139–142. doi:10.1097/HPC.0000000000000187
Marchio, P., Guerra-Ojeda, S., Vila, J. M., Aldasoro, M., Victor, V. M., and Mauricio, M. D. (2019). Targeting Early Atherosclerosis: a Focus on Oxidative Stress and Inflammation. Oxidative Med. Cell Longevity 2019, 1–32. doi:10.1155/2019/8563845
McLaren, J. E., Michael, D. R., Ashlin, T. G., and Ramji, D. P. (2011). Cytokines, Macrophage Lipid Metabolism and Foam Cells: Implications for Cardiovascular Disease Therapy. Prog. lipid Res. 50 (4), 331–347. doi:10.1016/j.plipres.2011.04.002
Moore, K. J., Sheedy, F. J., and Fisher, E. A. (2013). Macrophages in Atherosclerosis: a Dynamic Balance. Nat. Rev. Immunol. 13 (10), 709–721. doi:10.1038/nri3520
Mujtaba, M. A., Akhter, M. H., Alam, M. S., Ali, M. D., and Hussain, A. (2021). An Updated Review on Therapeutic Potential and Recent Advances in Drug Delivery of Berberine: Current Status and Future prospect. Cpb 22. doi:10.2174/1389201022666210208152113 https://pubmed.ncbi.nlm.nih.gov/33557735/.
Muller, C., Salvayre, R., Nègre-Salvayre, A., and Vindis, C. (2011). Oxidized LDLs Trigger Endoplasmic Reticulum Stress and Autophagy: Prevention by HDLs. Autophagy 7 (5), 541–543. doi:10.4161/auto.7.5.15003
Neag, M. A., Mocan, A., Echeverría, J., Pop, R. M., Bocsan, C. I., Crişan, G., et al. (2018). Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 9, 557. doi:10.3389/fphar.2018.00557
Ning, N., He, K., Wang, Y., Zou, Z., Wu, H., Li, X., et al. (2015). Hypolipidemic Effect and Mechanism of Palmatine from Coptis Chinensis in Hamsters Fed High-Fat Diet. Phytother. Res. 29 (5), 668–673. doi:10.1002/ptr.5295
Paone, S., Baxter, A. A., Hulett, M. D., and Poon, I. K. H. (2019). Endothelial Cell Apoptosis and the Role of Endothelial Cell-Derived Extracellular Vesicles in the Progression of Atherosclerosis. Cell. Mol. Life Sci. 76 (6), 1093–1106. doi:10.1007/s00018-018-2983-9
Paul, M., Hemshekhar, M., Kemparaju, K., and Girish, K. S. (2019). Berberine Mitigates High Glucose-Potentiated Platelet Aggregation and Apoptosis by Modulating Aldose Reductase and NADPH Oxidase Activity. Free Radic. Biol. Med. 130, 196–205. doi:10.1016/j.freeradbiomed.2018.10.453
Qiao, L., and Chen, W. (2018). Atheroprotective Effects and Molecular Targets of Bioactive Compounds from Traditional Chinese Medicine. Pharmacol. Res. 135, 212–229. doi:10.1016/j.phrs.2018.07.012
Sailor, G. U., D. Ramani, V., Shah, N., Parmar, G. R., Gohil, D., Balaraman, R., et al. (2021). Design of experiment Approach Based Formulation Optimization of Berberine Loaded Solid Lipid Nanoparticle for Antihyperlipidemic Activity. ijps 83 (2), 204–218. doi:10.36468/pharmaceutical-sciences.766
Schafer, A., and Bauersachs, J. (2008). Endothelial Dysfunction, Impaired Endogenous Platelet Inhibition and Platelet Activation in Diabetes and Atherosclerosis. Cvp 6 (1), 52–60. doi:10.2174/157016108783331295
Scherz-Shouval, R., and Elazar, Z. (2011). Regulation of Autophagy by ROS: Physiology and Pathology. Trends Biochemical Sciences 36 (1), 30–38. doi:10.1016/j.tibs.2010.07.007
Schiattarella, G. G., Sannino, A., Toscano, E., Giugliano, G., Gargiulo, G., Franzone, A., et al. (2017). Gut Microbe-Generated Metabolite Trimethylamine-N-Oxide as Cardiovascular Risk Biomarker: a Systematic Review and Dose-Response Meta-Analysis. Eur. Heart J. 38 (39), 2948–2956. doi:10.1093/eurheartj/ehx342
Shao, B.-z., Han, B.-z., Zeng, Y.-x., Su, D.-f., and Liu, C. (2016). The Roles of Macrophage Autophagy in Atherosclerosis. Acta Pharmacol. Sin 37 (2), 150–156. doi:10.1038/aps.2015.87
Shen, B. (2015). A New golden Age of Natural Products Drug Discovery. Cell 163 (6), 1297–1300. doi:10.1016/j.cell.2015.11.031
Shi, Y., Hu, J., Geng, J., Hu, T., Wang, B., Yan, W., et al. (2018). Berberine Treatment Reduces Atherosclerosis by Mediating Gut Microbiota in apoE-/- Mice. Biomed. Pharmacother. 107, 1556–1563. doi:10.1016/j.biopha.2018.08.148
Tabas, I., and Bornfeldt, K. E. (2016). Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 118 (4), 653–667. doi:10.1161/CIRCRESAHA.115.306256
Tabas, I., García-Cardeña, G., and Owens, G. K. (2015). Recent Insights into the Cellular Biology of Atherosclerosis. J. Cel. Biol. 209 (1), 13–22. doi:10.1083/jcb.201412052
Tang, W. H. W., Bäckhed, F., Landmesser, U., and Hazen, S. L. (2019). Intestinal Microbiota in Cardiovascular Health and Disease. J. Am. Coll. Cardiol. 73 (16), 2089–2105. doi:10.1016/j.jacc.2019.03.024
Tian, Y., Cai, J., Gui, W., Nichols, R. G., Koo, I., Zhang, J., et al. (2019). Berberine Directly Affects the Gut Microbiota to Promote Intestinal Farnesoid X Receptor Activation. Drug Metab. Dispos 47 (2), 86–93. doi:10.1124/dmd.118.083691
Wang, J.-m., Yang, Z., Xu, M.-g., Chen, L., Wang, Y., Su, C., et al. (2009a). Berberine-induced Decline in Circulating CD31+/CD42− Microparticles Is Associated with Improvement of Endothelial Function in Humans. Eur. J. Pharmacol. 614 (1-3), 77–83. doi:10.1016/j.ejphar.2009.04.037
Wang, L., Deng, L., Lin, N., Shi, Y., Chen, J., Zhou, Y., et al. (2020a). Berberine Inhibits Proliferation and Apoptosis of Vascular Smooth Muscle Cells Induced by Mechanical Stretch via the PDI/ERS and MAPK Pathways. Life Sci. 259, 118253. doi:10.1016/j.lfs.2020.118253
Wang, L., Qiu, X.-M., Hao, Q., and Li, D.-J. (2013). Anti-inflammatory Effects of a Chinese Herbal Medicine in Atherosclerosis via Estrogen Receptor β Mediating Nitric Oxide Production and NF-Κb Suppression in Endothelial Cells. Cel Death Dis. 4, e551. doi:10.1038/cddis.2013.66
Wang, X., Zhang, Y., Yang, Y., Wu, X., Fan, H., and Qiao, Y. (2017). Identification of Berberine as a Direct Thrombin Inhibitor from Traditional Chinese Medicine through Structural, Functional and Binding Studies. Sci. Rep. 7, 44040. doi:10.1038/srep44040
Wang, Y., Huang, Y., Lam, K. S. L., Li, Y., Wong, W. T., Ye, H., et al. (2009b). Berberine Prevents Hyperglycemia-Induced Endothelial Injury and Enhances Vasodilatation via Adenosine Monophosphate-Activated Protein Kinase and Endothelial Nitric Oxide Synthase. Cardiovasc. Res. 82 (3), 484–492. doi:10.1093/cvr/cvp078
Wang, Y., Yi, X., Ghanam, K., Zhang, S., Zhao, T., and Zhu, X. (2014). Berberine Decreases Cholesterol Levels in Rats through Multiple Mechanisms, Including Inhibition of Cholesterol Absorption. Metabolism 63 (9), 1167–1177. doi:10.1016/j.metabol.2014.05.013
Wang, Y., Zhou, X., Zhao, D., Wang, X., Gurley, E. C., Liu, R., et al. (2020b). Berberine Inhibits Free Fatty Acid and LPS-Induced Inflammation via Modulating ER Stress Response in Macrophages and Hepatocytes. Plos One 15 (5), e0232630. doi:10.1371/journal.pone.0232630
WHO (2020). World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals. Retrieved August 20, 2021, from, Available at: https://apps.who.int/iris/handle/10665/332070.
Wu, H., He, K., Wang, Y., Xue, D., Ning, N., Zou, Z., et al. (2014). The Antihypercholesterolemic Effect of Jatrorrhizine Isolated from Rhizoma Coptidis. Phytomedicine 21 (11), 1373–1381. doi:10.1016/j.phymed.2014.05.002
Wu, M., Yang, S., Wang, S., Cao, Y., Zhao, R., Li, X., et al. (2020). Effect of Berberine on Atherosclerosis and Gut Microbiota Modulation and Their Correlation in High-Fat Diet-Fed ApoE−/− Mice. Front. Pharmacol. 11, 223. doi:10.3389/fphar.2020.00223
Xiao, M., Men, L. N., Xu, M. G., Wang, G. B., Lv, H. T., and Liu, C. (2014). Berberine Protects Endothelial Progenitor Cell from Damage of TNF-α via the PI3K/AKT/eNOS Signaling Pathway. Eur. J. Pharmacol. 743, 11–16. doi:10.1016/j.ejphar.2014.09.024
Xu, R. X., Sun, X. C., Ma, C. Y., Yao, Y. H., Li, X. L., Guo, Y. L., et al. (2017). Impacts of Berberine on Oxidized LDL-Induced Proliferation of Human Umbilical Vein Endothelial Cells. Am. J. Transl Res. 9 (10), 4375–4389. https://pubmed.ncbi.nlm.nih.gov/29118901/.
Zha, W., Liang, G., Xiao, J., Studer, E. J., Hylemon, P. B., Pandak, W. M., et al. (2010). Berberine Inhibits HIV Protease Inhibitor-Induced Inflammatory Response by Modulating ER Stress Signaling Pathways in Murine Macrophages. PLoS ONE 5 (2), e9069. doi:10.1371/journal.pone.0009069
Zhang, H., Shan, Y., Wu, Y., Xu, C., Yu, X., Zhao, J., et al. (2017). Berberine Suppresses LPS-Induced Inflammation through Modulating Sirt1/NF-Κb Signaling Pathway in RAW264.7 Cells. Int. Immunopharmacology 52, 93–100. doi:10.1016/j.intimp.2017.08.032
Zhang, M., Wang, C.-M., Li, J., Meng, Z.-J., Wei, S.-N., Li, J., et al. (20132013). Berberine Protects against Palmitate-Induced Endothelial Dysfunction: Involvements of Upregulation of AMPK and eNOS and Downregulation of NOX4. Mediators Inflamm. 2013, 1–8. doi:10.1155/2013/260464
Zhang, Y., Li, X., Zou, D., Liu, W., Yang, J., Zhu, N., et al. (2008). Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 93 (7), 2559–2565. doi:10.1210/jc.2007-2404
Zheng, Y., Kou, J., Wang, P., Ye, T., Wang, Z., Gao, Z., et al. (2021). Berberine-induced TFEB Deacetylation by SIRT1 Promotes Autophagy in Peritoneal Macrophages. Aging 13 (5), 7096–7119. doi:10.18632/aging.202566
Zhou, Y., Cao, S., Wang, Y., Xu, P., Yan, J., Bin, W., et al. (2014). Berberine Metabolites Could Induce Low Density Lipoprotein Receptor Up-Regulation to Exert Lipid-Lowering Effects in Human Hepatoma Cells. Fitoterapia 92, 230–237. doi:10.1016/j.fitote.2013.11.010
Zhu, L., Zhang, D., Zhu, H., Zhu, J., Weng, S., Dong, L., et al. (2018). Berberine Treatment Increases Akkermansia in the Gut and Improves High-Fat Diet-Induced Atherosclerosis in Apoe−/− Mice. Atherosclerosis 268, 117–126. doi:10.1016/j.atherosclerosis.2017.11.023
Zimetti, F., Adorni, M. P., Ronda, N., Gatti, R., Bernini, F., and Favari, E. (2015). The Natural Compound Berberine Positively Affects Macrophage Functions Involved in Atherogenesis. Nutr. Metab. Cardiovasc. Dis. 25 (2), 195–201. doi:10.1016/j.numecd.2014.08.004
Glossary
Akt AKT serine/threonine kinase
AMPK adenosine monophosphate-activated protein kinase
BBR berberine
CCR-2 C-C chemokine receptor 2
EMPs endothelial microparticles
eNOS endothelial nitric oxide synthase
ERK extracellular signal-regulated kinase
HDL high-density lipoprotein
HUVECs human umbilical vein endothelial cells
ICAM-1 intercellular adhesion molecule-1
IL-8 interleukin-8
IL-1β interleukin-1β
IL-6 interleukin-6
LDL-C low-density lipoprotein cholesterol
LDLR low-density lipoprotein receptor
LPS lipopolysaccharide
MAPK mitogen-activated-protein kinase
MCP-1 monocyte chemoattractant protein-1
MMP-9 matrix metalloprotease-9
NADPH nicotinamide adenine dinucleotide phosphate
NF-κB nuclear factor κB
NOS nitric oxide synthase
oxLDL oxidized low-density lipoprotein
PCNA proliferating cell nuclear antigen
PDGF platelet-derived growth factor
PI3K phosphatidylinositol 3 kinase
ROS reactive oxygen species
SR scavenger receptors
TC total cholesterol
TG triglyceride
TMA trimethylamine
TMAO trimethylamine-N-oxide
TNF-α tumor necrosis factor-alpha
VCAM-1 vascular cell adhesion molecule-1
VMSCs vascular smooth muscle cells
WHO World Health Organization
Keywords: atherosclerosis, berberine, molecular mechanism, cell targets, gut microbiota
Citation: Xing L, Zhou X, Li A-H, Li H-J, He C-X, Qin W, Zhao D, Li P-Q, Zhu L and Cao H-L (2021) Atheroprotective Effects and Molecular Mechanism of Berberine. Front. Mol. Biosci. 8:762673. doi: 10.3389/fmolb.2021.762673
Received: 22 August 2021; Accepted: 18 October 2021;
Published: 16 November 2021.
Edited by:
Zhe-Sheng Chen, St. John’s University, United StatesCopyright © 2021 Xing, Zhou, Li, Li, He, Qin, Zhao, Li, Zhu and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Ling Cao, Y2FvaHVpbGluZ19qenNAeGl5aS5lZHUuY24=
†These authors have contributed equally to this work
 Lu Xing
Lu Xing Xin Zhou
Xin Zhou Ai-Hong Li
Ai-Hong Li Hui-Jin Li
Hui-Jin Li Chun-Xia He
Chun-Xia He Wei Qin
Wei Qin Dong Zhao
Dong Zhao Peng-Quan Li
Peng-Quan Li Li Zhu
Li Zhu Hui-Ling Cao
Hui-Ling Cao