- Centro de Biologia Molecular “Severo Ochoa”, CSIC-UAM Cantoblanco, Madrid, Spain
The fatality rate of Covid-19 escalates with age and is larger in men than women. I show that these variations correlate strongly with the level of the viral receptor protein ACE2 in rat lungs, which is consistent with the still limited data on human ACE2. Surprisingly, lower receptor levels correlate with higher fatality. I propose two possible explanations of this negative correlation: First, a previous mathematical model predicts that the velocity of viral progression in the organism as a function of the receptor level has a maximum and declines for abundant receptor. Secondly, degradation of ACE2 by the virus may cause the runaway inflammatory response that characterizes severe CoViD-19. I present here a mathematical model that predicts the lethality as a function of ACE2 protein level based on the two above hypothesis. The model fits Covid-19 fatality rate across age and sex in three countries with high accuracy (
1 Introduction
The Covid-19 pandemics (Zhou et al., 2020) has caused millions fatalities worldwide (Dong et al., 2020), creating a tremendous threat to global health. It presents a strong gradient of fatalities across age and a sex bias with much higher severity in males than females. Analysis of seroprevalence studies (Pastor-Barriuso et al., 2020) and modelling studies that extrapolate the number of infections (Davies et al., 2020) indicate that, at young age, most SARS-CoV-2 infections are asymptomatic and the fatality rate is very low, whereas for the elderlies most infections are severe and a large fraction of them can be fatal. Understanding the biological reasons that underlie these striking differences is one of the most pressing problems of CoViD-19 research, which might lead to better prediction of the disease prognosis and possible treatments that approach the severity of the worst affected groups to that of the most protected ones.
Here I show that the case fatality rate of Covid-19 across age and sex correlates negatively with the level of the protein Angiotensin converting enzyme 2 (ACE2), the cellular receptor both of SARS and SARS-CoV-2 virus (Hamming et al., 2004; Zhou et al., 2020), which belongs to the anti-inflammatory axis of the Renin-Angiotensin-System (RAS) (Paz Ocaranza et al., 2020). The correlation is very strong with membrane-bound ACE2 protein in rat lungs, which decreases with age and is higher in old females than old males (Xie et al., 2006). The same qualitative pattern is observed for membrane-bound ACE2 in mice (Yoon et al., 2016), where all the anti-inflammatory axis of the RAS decreases with age. Data on ACE2 expression through age in humans were not available until recently, but the Covid-19 pandemics brought an explosion of studies. Despite apparently contradictory conclusions, all studies are consistent with a model in which ACE2 expression starts in late foetal life (Muus et al., 2020), it is lower in young children than in adults (Bunyavanich et al., 2020; Saheb Sharif-Askari et al., 2020), it reaches a maximum at young age and then it decreases during adulthood age both at the level of mRNA (Chen et al., 2020) and at the level of membrane-bound protein (Zhang et al., 2021). Thus these data support similar qualitative trajectories of ACE2 expression in rodents and humans, as hypothesized here and further discussed later. ACE2 is removed from the cell membrane by the metalloprotease ADAM17 (Lambert et al., 2005; Xu et al., 2017) whose expression increases with age (Dou et al., 2017; Liu et al., 2019), thereby suggesting that the rate of degradation increases with age.
The negative correlation between ACE2 and lethality is surprising: higher levels of the receptor decrease the lethality exponentially. The apparent paradox can be reconciled through a mathematical model of viral infection, developed before the COVID-19 pandemics, which predicted how viral propagation in the organism depends on the adsorption rate of viruses on cells (Fort and Méndez, 2002). Here I express this model in terms of receptor level and show that it predicts that the speed of viral propagation is a non-monotonic function of the receptor level, which reaches a maximum and decreases for high receptor expression.
The second mechanism that may underlie the negative correlation concerns the function of ACE2 not as viral receptor but as key enzyme for controlling the pro-inflammatory peptides of the RAS and the bradykinin system. Besides regulating blood pressure (BP) and electrolyte homeostasis in blood, the RAS (Paz Ocaranza et al., 2020) plays a central role in inflammatory processes (Agarwal et al., 2013), immune response (Satou et al., 2018) and coagulation (Lip, 2000; Remková and Remko, 2010), which characterize the most severe Covid-19 cases (Diamond, 2020; Ingraham et al., 2020). Its main player is the family of peptides derived from angiotensin I (Ang1-10), cleaved by the enzyme Renin from the protein angiotensinogen. Its pro-inflammatory arm is constituted by angiotensin II (Ang1-8), cleaved from Ang1-10 by the angiotensin converting enzyme (ACE) homologous to ACE2. Ang1-8 bound to the receptor ATR-1 triggers a cascade of reactions leading not only to vasoconstriction and increased BP but also to activation of the transcription factor NFkB that upregulates inflammatory cytokines (IL-1, TNF-α and IFN-γ among others), activates white blood cells and platelets, and favours thrombotic processes (Brasier, 2010). The enzyme ACE2 limits the level of Ang1-8 by converting its precursor Ang1-10 to Ang1-9 (Donoghue et al., 2020) that is subsequently cleaved by ACE to Ang1-7, and by directly converting Ang1-8 to Ang1-7, which belongs to the anti-inflammatory arm of the RAS since it favours vasodilation, reduces BP and attenuates inflammation (Chappell and Al Zayadneh, 2017).
The bradykinin system has vasodilatory effects that lower blood pressure and it is very strongly coupled with the RAS. It consists of two axis. The first axis is mediated by the pro-inflammatory peptide des-Arg9-bradykinin (DABK), which is downregulated by ACE2 and whose receptor BK1R is upregulated upon inflammation by Ang1-8 (in turn downregulated by ACE2) bound to the receptor ATR1. Stimulation of this axis leads to release of pro-inflammatory chemokines, lung inflammation and injury (Sodhi et al., 2018). The second axis is mediated by the peptide bradykinin (BK), which is downregulated by ACE and whose receptor BK2R is in turn activated by Ang1-7 and Ang1-9 produced by ACE2 and is stimulated by Ang1-8 bound to the receptor ATR2 (Kurisu et al., 2003). Therefore, ACE2 channels bradykinin from the first axis to the second one. A summary of the main inflammatory and anti-inflammatory components of the angiotensin and bradykinin system is represented in Figure 1.
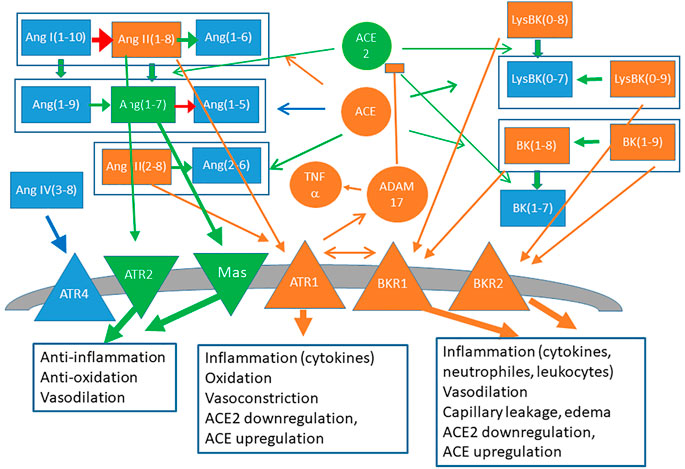
FIGURE 1. Schematic representation of the RAS (left) and bradykinin (right) systems. Signaling peptides are represented as rectangles, peptidases (ACE, ACE2), proteases (ADAM17) and cytokines (TNFα) are represented as circles and membrane receptors are represented as triangles. Components and links with mainly proinflammatory character are depicted in orange and anti-inflammatory components are depicted in green.
Upon viral entry the spike protein of SARS-CoV and probably also SARS-CoV-2 cause the internalization and degradation of ACE2 (Kuba et al., 2005) that critically contributes to lung damage (Imai et al., 2005; Imai et al., 2008; Jia, 2016). Decrease of ACE2 raises the severity of lung injury in other inflammatory diseases (Jia, 2016) and in aging rats (Schouten et al., 2016), which may be explained by the increase of Ang1-8 and its adverse effects.
Here I develop a set of mathematical models of SARS-CoV-2 lethality versus the pre-infection level of ACE2 based on two aspects: the influence of ACE2 on viral progression (Fort and Méndez, 2002) and the negative effect of its degradation. These models are fitted to the CFR of SARS-CoV-2 across six classes of age and sex in Italy, Spain and Germany, and support the hypothesis that the receptor level slows down the virus propagation in the infected organism, which fits the data better than the competing hypothesis that the viral progression is independent of the receptor level and ACE2 influences the lethality only through its negative effect on the inflammatory process. Furthermore, under the same hypothesis and by rescaling the parameters fitted to SARS-CoV-2 by the ratio between the binding rates of the spike proteins of SARS-CoV and SARS-CoV-2, the model predicts well the CFR of SARS-CoV, supporting again the negative relationship between receptor level and virus propagation.
2 Results
2.1 CoViD-19 Lethality Correlates Negatively With ACE2 Level
The level of the ACE2 protein in rat lungs were quantified across three adult age classes of the two sexes by Xie et al. (2006), who found that it strongly decays with age and it is higher in female than male rats, with largest difference in the oldest cohort where the expression is almost double for females. A similar pattern was observed in mice (Yoon et al., 2016). Observations on ACE2 protein in human lungs (Zhang et al., 2021) and ACE2 mRNA in the GTEx database (Chen et al., 2020) suggest that human ACE2 levels across age and sex are qualitatively similar to rodent data, apart for multiplicative factors that may depend on the organ: they decay with age and they are higher in females than in males. In children the situation is more complicated, since ACE2 protein in serum (Pavel et al., 2021) and ACE2 mRNA (Bunyavanich et al., 2020; Muus et al., 2020; Saheb Sharif-Askari et al., 2020) is lower in children than adults, indicating a non-monotonic trend with age. In fact, it has been observed that ACE2 starts being expressed in late foetal stage and reaches a cell-type dependent maximum at young age (Muus et al., 2020). ACE2 is shed from cell membranes to the serum through the protease ADAM17 (Xu et al., 2017), whose expression increases with age (Dou et al., 2017; Liu et al., 2019). As discussed later, this fact leads to predict that ACE2 levels in cell membranes attain their maximum at younger age than ACE2 mRNA and their decrease is faster. However, in comparison with adults, young children present lower ACE2 levels and reduced Covid-19 severity. We argue later that this reduced severity might be related with the higher expression in children of the alternative angiotensin receptor ATR2 that counteracts inflammation (Kaschina et al., 2017), which can be expected to alleviate the inflammatory consequences of low levels of ACE2.
Strikingly, the profile of ACE2 in adult rats in Ref. (Xie et al., 2006). is very strongly anti-correlated with the lethality of SARS-CoV-2. Figure 2 represents the level of the ACE2 protein in rats lung [horizontal axis, data from Figure 2 of (Xie et al., 2006)] versus the case fatality rate (CFR) of CoVid-19 registered in Italy (Istituto Superiore di Sanità, 2020), Spain (OPS, 2020) and Germany (Robert Koch Institute, 2020) in three uniform age classes (young,
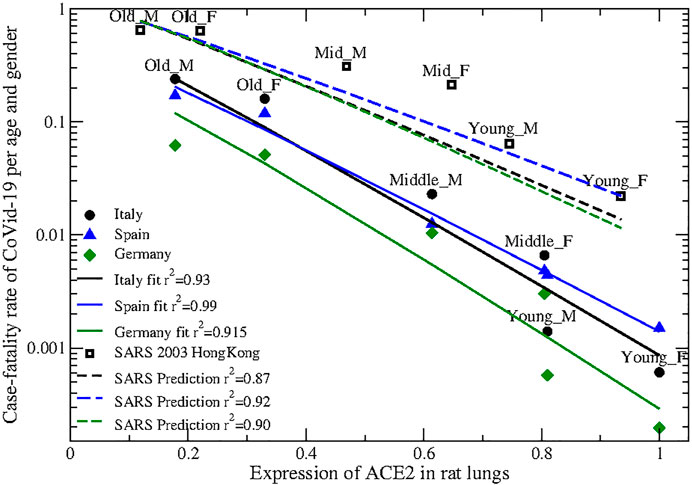
FIGURE 2. Expression of the ACE2 protein in rats lung (horizontal axis), normalized so that the highest expression is one, vs. case fatality rate (vertical axis) of SARS-CoV-2 (Circles: Italy; triangles: Spain; diamonds: Germany) and SARS 2003 (open squares) in three age classes (young 0–29, middle-age 30–59 and old
As for other two-parameter fits tested in this work, the fitted exponents for Italy and Germany coincide within the error and the CFR differ only by a multiplicative factor, supporting the robustness of the data. Data from Spain present higher mortality in the young ages, which might be attributed to more frequent undetected cases in young age with lower severity.
2.2 Mathematical Model of Covid-19 Lethality
Mathematical models of viral growth consider three processes: virus adsorption into susceptible cells, production of virus by infected cells after a delay time τ, and viral clearance by the immune system (Smith and Perelson, 2011). The simple mean-field model that does not consider explicit space predicts a minimal receptor density below which the virus does not grow and above which higher receptor levels accelerate the viral progression.
Considering spatial diffusion modifies this situation. Fort and Méndez proposed in 2002 a model of viral propagation through spatial diffusion on susceptible cells and showed that its solution can be expressed as a wave of reproducing viral particles that propagates in space with wave velocity v (Fort and Méndez, 2002), for which they were able to find an analytical approximation in terms of the parameters of the model: The adsorption rate
Here I express the mathematical solution to the wave velocity in terms of receptor density, assuming that the adsorption rate
Varying the receptor level A (adsorption rate in the original paper), three regimes appear: 1) When A is small the viral velocity v increases with A less than linearly. 2) For intermediate
The latter result is surprising: how can the virus progress more slowly for increasing receptor level? Since this is a mathematical model, the answer is readily found: in the model, viral particles are consumed when they enter a cell (this is embodied in the term
Next, I consider two models of Covid-19 death. The first model only considers variations of viral propagation with ACE2. Death occurs when the virus propagates through the upper respiratory tract or through endothelial cells, reaches the lungs and infects and destroys a critical fraction X of it. In the first model X is the same for all patients. The second model considers that ACE2 plays an essential role for reverting the inflammatory process propagated by the peptides of the angiotensin and bradykinin systems that ACE2 downregulates. I hypothesize that, if the ACE2 density in an infected organ is below a critical level
Combining these two assumptions with the three regimes of viral propagation described above gives six mathematical models. For each of them, I compute the time
I compute lethality as the probability that
The three models, combined with the exponential and the Gaussian distribution, yield six different functional forms of the CFR versus A that I fit to the data. Some fits have many free parameters, but simple approximations reduce their number to two (for the exponential distribution) or three (for the Gaussian distribution). Moreover, all fits are strongly regularized with rescaled ridge regression (Bastolla and Dehouck, 2019) to limit overfitting as much as possible (see Section 4).
Under both distributions, and for the CFR of the three countries, model 2 gives better fit than model 1 with the same number of free parameters. Under the exponential distribution (two free parameters), the relative quadratic error of the logarithm for models 1 and 2 is 0.37, 0.07, for Spain, 0.41, 0.14 for Italy and 0.53, 0.17 for Germany, respectively. Adopting the Gaussian distribution requires three parameters, with high risk of overfitting, but it maintains the same ranking: 0.04, 0.01 for Spain, 0.10, 0.04 for Italy and 0.14, 0.06 for Germany, see Supplementary Table S1. Model 3 does not improve the fit above Model 2 despite having one more parameter, which is due to the strong regularization that we apply, and combined with the Gaussian model it yields unrealistic negative parameters that suggest that it has too many parameters for the limited available observations. Model 2G with the Gaussian distribution is
In conclusion, for both distributions the hypothesis that the viral progression decreases with the receptor level (Model 2) fits the data much better than the competing hypothesis that the propagation is independent of the receptor level.
2.3 SARS 2003
An important prediction of Models 2 and 3 is that the fit parameters a and b depend on the adsorption constant per unit receptor k, with the fit parameters expressed as
2.4 NL63
It is natural to extend this analysis to the other human coronavirus that uses ACE2 as a receptor, NL63 that causes common cold and is not generally associated with pneumonia. Its spike protein contains a very stable receptor binding domain of 120 residues that showed high binding affinity for ACE2 (Wu et al., 2009). However, the complete S1 domain of the spike (717 residues) is much less stable and its affinity for ACE2 is so small that it could not be measured with binding assays (Hofmann et al., 2005; Mathewson et al., 2008; Glowacka et al., 2010), and it was conjectured that it is 10–100-fold smaller than that of SARS-CoV (Mathewson et al., 2008). Since the CFR of SARS peaks for old females, whose normalized ACE2 is equal to 22% of the maximum value, the model predicts that this is the level at which SARS-CoV propagates fastest. If the binding affinity of the NL63 spike is at least ten times smaller, the ACE2 level at which NL63 propagates fastest is more than double the highest ACE2 level, implying that NL63 is in the regime in which the ACE2 level enhances its propagation.
This analysis agrees with the apparently surprising data reported in Figure 3A of Ref. (Hofmann et al., 2005), which shows that ACE2 overexpression in 293T cells enhances NL63 infection three times more than SARS-CoV infection, indicating that higher receptor levels accelerate the propagation of NL63 more than that of SARS-CoV despite the latter has higher binding affinity.
3 Discussion
Since ACE2 is the SARS-CoV-2 receptor, we may expect that raising its level enhances the rate at which the virus propagates in the organism and worsens the outcome of the infection. However, a mathematical model of viral progression presents a regime in which the increase of the receptor level slows down the virus propagation in the organism. The observed relation between SARS-CoV-2 lethality and ACE2 levels suggests that this may be the relevant regime of SARS-CoV-2 infections, as further supported by the prediction of the age- and sex-dependent lethality of 2003 SARS-CoV.
3.1 Human ACE2
An important limitation of the present work is that it uses data of ACE2 protein levels in adult rat lungs (Xie et al., 2006) since similar data are not available for humans. Rat data are also consistent with observations in adult mice, which show decrease of ACE2 mRNA (Booeshaghi and Pachter, 2020) and membrane-bound ACE2 protein at old age and a general strengthening of the inflammatory arm of the RAS (Yoon et al., 2016).
Both for rodents and for humans ACE2 mRNA starts to be expressed in late foetal life (Muus et al., 2020), it is less expressed in young children than in adults (Bunyavanich et al., 2020; Saheb Sharif-Askari et al., 2020), as also found for ACE2 protein in serum (Pavel et al., 2021), it reaches a maximum at young age and then it decreases (Chen et al., 2020), see also Figure 3 of (Muus et al., 2020). In humans, membrane-bound ACE2, which is the relevant quantity for the present analysis, also decreases with age (Zhang et al., 2021) and it was found to be more abundant in children than in adult lungs (Ortiz et al., 2020), although this comparison may be debatable since it depends on the age examined and on some arbitrary thresholds.
ACE2 is removed from the cellular membrane and shed to the serum by the metalloprotease ADAM17 (Lambert et al., 2005; Xu et al., 2017) that is upregulated with age (Dou et al., 2017; Liu et al., 2019), consistent with the notion that ADAM17 is upregulated by the binding of Ang1-8 to the angiotensin II type 1 receptor (ATR1) which tend to increase with age (Yoon et al., 2016). The increase of ACE2 protein shedding with age implies that the age at which ACE2 protein expression is maximum is lower, and the rate at which it decreases with age is higher than ACE2 at the mRNA level. Since children express more than adults the receptor ATR2 that competes with ATR1 and counteracts its action (Kaschina et al., 2017), they are expected to present lower activation of ADAM17, which may partly explain why they suffer less severe Covid-19 despite having low level of ACE2. Note that ACE2 level of children does not prevent them from suffering of NL63 infections despite this virus is less efficient at binding ACE2.
Regarding sex differences, Ref. (Chen et al., 2020) found that ACE2 mRNA is lower in males than females, as in rats. Ref. (Muus et al., 2020). reached the opposite conclusion, but this seems an artefact of the fact that smoking enhances ACE2 expression (Saheb Sharif-Askari et al., 2020) and in their samples 50% of men were smokers compared to 25% of women. It has to be noted that the ACE2 gene is contained in the X chromosome, of which females have two copies. Although one of these copies is epigenetically silenced, about 15% of the X-linked genes escape this inactivation (Carrel and Willard, 2005) and heterochromatin is known to dysregulate with age. It is interesting that old female rats present almost exactly double ACE2 than males (Xie et al., 2006), as one would expect if the epigenetic silencing fades at old age. Consistently, some of the sex differences in human cardio-vascular diseases have been attributed to sex differences in the expression of ACE2, which acts as protective factor (Gupte et al., 2012).
The negative relation between ACE2 levels and severity of Covid-19 is supported by other risk and protective factors corrected for age, sex and other comorbidities in a large study in the United Kingdom (Williamson et al., 2020). Namely, being a current smoker constitute a curious protective factor (adjusted hazard ratio (AHR):
Finally, the GTEx database shows that, despite lungs are the organ that is more severely damaged by COVID-19, they do not present high expression of ACE2 mRNA (Gene Page, 2021), which is higher in tissues from reproductive organs, intestine, adipose tissue, kidney, hearth, thyroid, esophagus, breast, salivary glands and pancreas, among others. Some of these organs may be infected but they experience less damage, consistent with the negative correlation between ACE2 levels in lungs and lethality.
3.2 Role of ACE2 for Virus Propagation and Spike Mutant D614G
The above evidence strongly supports the negative correlation between ACE2 protein levels and severity of CoViD-19. This in turn supports the mathematical model presented here, based on the hypothesis that increased ACE2 may slow down viral propagation (Ortega-Cejas et al., 2004), which fits the CFR from Spain, Italy and Germany with
Our mathematical model predicts that SARS has higher relative mortality for young age with respect to old age (the observed ratio is 22% for SARS compared with 1.3% for SARS-CoV-2) due to the smaller binding affinity of the SARS spike for ACE2. It also predicts that mutations that decrease the binding of SARS-CoV-2 spike may generate a strain more severe for younger age.
The spike mutant D614G, which rapidly rose to almost fixation world-wide (Korber et al., 2020), presents an opportunity to assess this prediction since it presents lower affinity for ACE2 (Yurkovetskiy et al., 2020). It propagates faster in cell cultures than the original spike (Yurkovetskiy et al., 2020) and its detected cases tend to be younger (Wagner et al., 2020), in line with the above prediction. Nevertheless, the improved propagation of D614G was attribute to the higher population of the binding-competent open configuration (Yurkovetskiy et al., 2020), thus other possible interpretations exist. A direct relation between D614G and disease severity could not be proven, but there might be an indirect one since D641G is associated with increased viral load and viral load is associated with hospitalization (Wagner et al., 2020). This interesting mutant may deserve further study both computationally, testing whether it affects the age-mortality profile in countries where detailed data are available, and experimentally, comparing its kinetics with respect to the original virus as a function of ACE2 expression.
3.3 Implications of the Model
An important contribution of this work is that it contradicts the idea that the increase of ACE2 should always favour virus propagation and increase the risk. This idea had important practical consequences since it lead to propose that Angiotensin receptor blockers (ARB) and ACE inhibitors (ACE-I) commonly used to treat hypertension may favour viral propagation since they upregulate the viral receptor ACE2 (Ferrario et al., 2005), and should be discontinued (Diaz, 2020; Fang et al., 2020). Medical societies firmly opposed this suggestion due to lack of evidence (ACC, 2020; EMA, 2020; ESC, 2020), and it was proposed that withdrawal of anti-hypertensive drugs in patients that need them may be harmful (Vaduganathan et al., 2020).
SARS and probably also SARS-CoV-2 degrade ACE2 (Kuba et al., 2005), with detrimental effects on the lungs on which ACE2 has a protective effect (Imai et al., 2005; Nicholls and Peiris, 2005; Imai et al., 2008; Jia, 2016). Several papers proposed that the downregulation of ACE2 is a key factor for the severity of CoViD-19 and suggested that ACE-I and ARB that limit the effects of Ang1-8 may be beneficial for CoViD-19 patients (Annweiler et al., 2020; Ciaglia et al., 2020; Gurwitz, 2020; Offringa et al., 2020; Sun et al., 2020; Verdecchia et al., 2020). A similar idea was already proposed at the time of SARS, and a retrospective meta-analysis found that the use of ARB and ACE-I provides a consistent reduction in risk of pneumonia compared with controls (Caldeira et al., 2012). In the context of CoViD-19, a meta-analysis of several studies found that ARB and ACE-I mitigate the severity of COVID-19 for patients that already take them against hypertension (Guo et al., 2020).
The negative correlation between ACE2 levels and lethality of SARS-Cov-2 described here, and the mathematical prediction that the receptor level may slow down viral progression, contradict the fear that ARB and ACE-I may benefit the virus and suggests two complementary protective roles of high ACE2 levels. On one hand, they may slow down the propagation of the virus, an effect conceptually similar to that observed in recent experiments with soluble human ACE2 (Monteil et al., 2020). On the other hand, they reduce the accumulation of Ang1-8, whose proinflammatory and prothrombotic effects are thought to underlie the most severe complications of CoViD-19. This work thus supports the idea that ARB and ACE-I used to treat high blood pressure may limit the most adverse manifestations of CoViD-19.
A note of caution is required for the effect of these drugs on the bradykinin system. This system is strongly coupled with the RAS and causes vasodilation, reduces blood pressure and increases vascular permeability. Its over-activity can lead to increased inflammation, thrombosis and angioedema in the lungs, and it was proposed that it also mediates the severe manifestations of COVID-19 (Garvin et al., 2020; Nicolau et al., 2020; van de Veerdonk et al., 2020). The bradykinin system consists of two axes. The first one is downregulated by ACE2, which degrades the signalling peptide des-Arg9-bradykinin (DABK) whose receptor BK1R is upregulated by Ang1-8 (in turn downregulated by ACE2) bound to the receptor ATR1. Stimulation of this axis may lead to release of pro-inflammatory chemokines, lung inflammation and injury (Sodhi et al., 2018) and is expected to be reduced through ARB and ACE-I, which would exert a protective role. The other axis is downregulated by ACE, which degrades the signalling peptide BK, whose receptor BK2R is in turn activated by Ang1-7 and Ang1-9 produced by ACE2, and is stimulated by Ang1-8 bound to the receptor ATR2 (Kurisu et al., 2003). Thus, ACE2, ATR1 receptor blockers and even more ACE-I can upregulate the BK2R axis with pathological consequences, as observed in severe side-effects of ACE-I (Wood, 1995), and their use should be limited in the presence of hypotension. Nevertheless, studies of the effect of these drugs on COVID-19 patients found an overall positive balance.
Of course, clinical trials are necessary to establish whether ARB and ACE-I have a positive, negative or neutral effect on CoViD-19 severity. To this aim, the clinical trials NCT04312009 and NCT04311177 started in April 2020 at the University of Minnesota.
3.4 Positive Feedback Loop
It is noteworthy that degradation of ACE2 increases the level of Ang1-8, which in turn binds the ATR1 receptor and down-regulates ACE2 even further both at the mRNA and at the protein level (Deshotels et al., 2014). Thus, the SARS-CoV-2 infection may trigger a dangerous positive feedback loop that strongly raises Ang1-8, exacerbating inflammatory response (Agarwal et al., 2013; Satou et al., 2018) and coagulation problems (Lip, 2000; Remková and Remko, 2010), frequent complications of severe CoViD-19 patients (Diamond, 2020; Tseng et al., 2020). Positive correlation between Ang1-8 levels and viral load has been reported in CoViD-19 patients (Liu et al., 2020), supporting the link between severe CoViD-19 and dysregulation of the RAS.
Under this point of view, ARBs appear to be more favourable than ACE-I because they can interfere with the positive feedback loop of Ang1-8 and because Ang1-8 can be generated by other proteases if ACE is inhibited (Paz Ocaranza et al., 2020).
3.5 RAS Proteins as Prognostic Factors
The results presented here suggest a prognostic role for the measurements of key components of the RAS and the bradykinin system in bronchial aspirated lavage samples and in the serum, which may predict the severity of the disease already at an early stage and may allow detecting risk groups that need higher protection besides the elder, as supported by the association between ACE2 and known risk and protecting factors against CoViD-19 (Williamson et al., 2020). We are currently investigating this possibility through retrospective studies.
4 Methods
4.1 Case-Fatality-Rates and Expression Data
Case fatality rates (CFR) were taken from public sources (Istituto Superiore di Sanità, 2020; OPS, 2020; Robert Koch Institute, 2020) for CoViD-19 in Italy, Spain and Germany, respectively, and from Ref. (Karlberg et al., 2004). for the 2003 SARS outbreak in Hong-Kong. At the beginning of an outbreak, CFR underestimate the true fatality rate because their calculation assumes that all people currently infected will recover, which unfortunately is not true. This effect may not be uniform across age-sex classes if patients of some classes tend to die more rapidly, as assumed by the model. However, at a late epidemic stage this effect is expected to be small. On the other hand, CFR overestimate the true fatality rate because of undetected cases that tend to lower the denominator. Since age-sex classes with higher lethality also tend to have more severe cases and less undetected cases, the overestimation is larger for classes with smaller lethality, with the consequence of reducing the differences among classes for larger fraction of undetected cases. The data that we used do not allow correcting for this bias, which may account for some of the differences in the fit parameters.
Expression data presented in Ref. (Xie et al., 2006). were grouped in three age classes of 3 (young), 12 (middle) and 24 months (old). CFR were presented in bins of 10 years, and I grouped them in three equally spaced groups 0–29 (young), 30–59 (middle) and
For SARS CFR (Karlberg et al., 2004), ages were grouped differently: 0–44 (young), 45–74 (middle) and
4.2 Mathematical Model of Viral Propagation
The simplest mathematical model of viral propagation in an organism considers three populations: uninfected cells
The time that it takes for the virus to propagate through the upper respiratory tract (URT) can be estimated as
I consider three situations: 1) v is independent of A; 2) v decreases as
In each situation, the death time
In the model, death occurs if
a, b and c are positive fitting parameters. In Eq. 3G, there are five terms proportional to A,
4.3 Fit of the Models
The fitting parameters a and b are determined through regularized fits performed with rescaled ridge regression (Bastolla and Dehouck, 2019), which minimizes the quadratic error plus the term
Model (2G) coupled to the Gaussian distribution depends on three parameters but only the parameters a and b were fitted while c was fixed, fitting
4.4 Prediction for SARS
The models fitted to SARS-CoV-2 were rescaled in order to apply them to SARS-CoV, adopting the ratio between the binding rates of the spike proteins of both viruses to ACE2. The most precise measures available in the literature are
For predicting the CFR of the 2003 SARS outbreak, I used the parameters of SARS-CoV-2 and rescaled them with the ratio between the kinetic binding constant
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_2-aprile-2020.pdfhttps://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_76_COVID-19.pdfhttps://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/2020-04-28-en.pdf.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
I applied for funds for open access publishing from the CSIC library. This work is supported by the Spanish Research Council (CSIC) under the grant CSIC-COV19-108 (2020E165).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I would like to thank medical doctors, nurses and other public employees that fighted against CoViD-19 at the risk of their lives. This work is dedicated to my father, who passed away few days before the coronavirus outbreak became manifested in Italy, and my mother, who suffered cardiovascular diseases. I acknowledge useful discussions with several colleagues, in particular Patrick Chambers, Esther Lazaro, Manuel Fresno, Alberto Pascual-Garcia, David Abia, Alberto Rastrojo, Mario Mencia, Laura Garcia-Bermejo and Fatima Sanchez.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.706122/full#supplementary-material
References
ACC (2020). HFSA/ACC/AHA Statement Addresses Concerns Re: Using RAAS Antagonists in COVID-19. Medscape: American College of Cardiology (ACC).
Agarwal, D., Dange, R. B., Raizada, M. K., and Francis, J. (2013). Angiotensin II Causes Imbalance between Pro- and Anti-inflammatory Cytokines by Modulating GSK-3β in Neuronal Culture. Br. J. Pharmacol. 169, 860–874. doi:10.1111/bph.12177
Ajabshir, S., Asif, A., and Nayer, A. (2014). The Effects of Vitamin D on the Renin-Angiotensin System. J. Nephropathol. 3, 41–43. doi:10.12860/jnp.2014.09
Annweiler, C., Cao, Z., Wu, Y., Faucon, E., Mouhat, S., Kovacic, H., et al. (2020). Counter-regulatory 'Renin-Angiotensin' System-Based Candidate Drugs to Treat COVID-19 Diseases in SARS-CoV-2-Infected Patients. Iddt 20, 407–408. doi:10.2174/1871526520666200518073329
Bastolla, U., and Dehouck, Y. (2019). Can Conformational Changes of Proteins Be Represented in Torsion Angle Space? A Study with Rescaled ridge Regression. J. Chem. Inf. Model. 59, 4929–4941. doi:10.1021/acs.jcim.9b00627
Batlle, D., Jose Soler, M., and Ye, M. (2010). ACE2 and Diabetes: ACE of ACEs? Diabetes 59, 2994–2996. doi:10.2337/db10-1205
Ben Avraham, D., and Havlin, S. (2005). Diffusion and Reaction in Fractals and Disordered Systems. Cambridge University Press.
Biesalski, H. K. (2020). Vitamin D Deficiency and Co-morbidities in COVID-19 Patients - A Fatal Relationship? NFS J. 20, 10–21. doi:10.1016/j.nfs.2020.06.001
Booeshaghi, A. S., and Pachter, L. (2020). Decrease inACE2mRNA Expression in Aged Mouse Lung. biorxiv. doi:10.1101/2020.04.02.021451
Bour, S., Geleziunas, R., and Wainberg, M. A. (1995). The Human Immunodeficiency Virus Type 1 (HIV-1) CD4 Receptor and its central Role in Promotion of HIV-1 Infection. Microbiol. Rev. 59, 63–93. doi:10.1128/mr.59.1.63-93.1995
Brasier, A. R. (2010). The Nuclear Factor- B-Interleukin-6 Signalling Pathway Mediating Vascular Inflammation. Cardiovasc. Res. 86, 211–218. doi:10.1093/cvr/cvq076
Breiner, K. M., Urban, S., Glass, B., and Schaller, H. (2001). Envelope Protein-Mediated Down-Regulation of Hepatitis B Virus Receptor in Infected Hepatocytes. J. Virol. 75, 143–150. doi:10.1128/jvi.75.1.143-150.2001
Bunyavanich, S., Do, A., and Vicencio, A. (2020). Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA 323, 2427–2429. doi:10.1001/jama.2020.8707
Caldeira, D., Alarcao, J., Vaz-Carneiro, A., and Costa, J. (2012). Risk of Pneumonia Associated with Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: Systematic Review and Meta-Analysis. BMJ 345, e4260. doi:10.1136/bmj.e4260
Carrel, L., and Willard, H. F. (2005). X-inactivation Profile Reveals Extensive Variability in X-Linked Gene Expression in Females. Nature 434, 400–404. doi:10.1038/nature03479
Chappell, M. C., and Al Zayadneh, E. M. (2017). Angiotensin-(1-7) and the Regulation of Anti-fibrotic Signaling Pathways. J. Cell Signal 2, 134. doi:10.4172/2576-1471.1000134
Chen, J., Jiang, Q., Xia, X., Liu, K., Yu, Z., Tao, W., et al. (2020). Individual Variation of the SARS-CoV-2 Receptor ACE2 Gene Expression and Regulation. Aging Cell 19, e13168. doi:10.1111/acel.13168
Ciaglia, E., Vecchione, C., and Puca, A. A. (2020). COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 8, 206. doi:10.3389/fped.2020.00206
Davies, N. G., Klepac, P., Liu, Y., Prem, K., Jit, M., Eggo, R. M., et al. (2020). Age-dependent Effects in the Transmission and Control of COVID-19 Epidemics. Nat. Med. 26, 12051211. doi:10.1038/s41591-020-0962-9
Deshotels, M. R., Xia, H., Sriramula, S., Lazartigues, E., and Filipeanu, C. M. (2014). Angiotensin II Mediates Angiotensin Converting Enzyme Type 2 Internalization and Degradation through an Angiotensin II Type I Receptor-dependent Mechanism. Hypertension 64, 1368–1375. doi:10.1161/hypertensionaha.114.03743
Diamond, B. (2020). The Renin-Angiotensin System: An Integrated View of Lung Disease and Coagulopathy in COVID-19 and Therapeutic Implications. J. Exp. Med. 217, e20201000. doi:10.1084/jem.20201000
Diaz, J. H. (2020). Hypothesis: Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers May Increase the Risk of Severe COVID-19. J. Trav. Med. 27, taaa041. doi:10.1093/jtm/taaa041
Dong, E., Du, H., and Gardner, L. (2020). An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 20, 533–534. doi:10.1016/s1473-3099(20)30120-1
Donoghue, M., Hsieh, F., Baronas, E., Godbout, K., Gosselin, M., Stagliano, N., et al. (2020). A Novel Angiotensin-Converting Enzyme-Related Carboxypeptidase (ACE2) Converts Angiotensin I to Angiotensin 1-9. Circul. Res. 87, e1–e9. doi:10.1161/01.res.87.5.e1
Dou, H., Feher, A., Davila, A. C., Romero, M. J., Patel, V. S., Kamath, V. M., et al. (2017). Role of Adipose Tissue Endothelial ADAM17 in Age-Related Coronary Microvascular Dysfunction. Atvb 37, 1180–1193. doi:10.1161/atvbaha.117.309430
EMA (2020). EMA Advises Continued Use of Medicines for Hypertension, Heart or Kidney Disease during COVID-19 Pandemic. Medscape: European Medicines Agency (EMA).
Eriksen, R. S., Svenningsen, S. L., Sneppen, K., and Mitarai, N. (2018). A Growing Microcolony Can Survive and Support Persistent Propagation of Virulent Phages. Proc. Natl. Acad. Sci. USA 115, 337–342. doi:10.1073/pnas.1708954115
ESC (2020). Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. Medscape: European Society of Cardiology (ESC).
Fang, L., Karakiulakis, G., and Roth, M. (2020). Are Patients with Hypertension and Diabetes Mellitus at Increased Risk for COVID-19 Infection? Lancet Respir. Med. 8, e21. doi:10.1016/s2213-2600(20)30116-8
Ferrario, C. M., Jessup, J., Chappell, M. C., Averill, D. B., Brosnihan, K. B., Tallant, E. A., et al. (2005). Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2. Circulation 111, 2605–2610. doi:10.1161/circulationaha.104.510461
Fort, J., and Méndez, V. (2002). Time-delayed Spread of Viruses in Growing Plaques. Phys. Rev. Lett. 89, 178101. doi:10.1103/physrevlett.89.178101
Garvin, M. R., Alvarez, C., Miller, J. I., Prates, E. T., Walker, A. M., Amos, B. K., et al. (2020). A Mechanistic Model and Therapeutic Interventions for COVID-19 Involving a RAS-Mediated Bradykinin Storm. Elife 9, e59177. doi:10.7554/eLife.59177
Gene Page (2021). Gene Expression for ACE2 (ENSG00000130234.10). https://gtexportal.org/home/gene/ACE2.
Glowacka, I., Bertram, S., Herzog, P., Pfefferle, S., Steffen, I., Muench, M. O., et al. (2010). Differential Downregulation of ACE2 by the Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus and Human Coronavirus NL63. J. Virol. 84, 1198–1205. doi:10.1128/jvi.01248-09
Guo, X., Zhu, Y., and Hong, Y. (2020). Decreased Mortality of COVID-19 with Renin-Angiotensin-Aldosterone System Inhibitors Therapy in Patients with Hypertension: A Meta-Analysis. Hypertension 76, e13–e14. doi:10.1161/HYPERTENSIONAHA.120.15572
Gupte, M., Thatcher, S. E., Boustany-Kari, C. M., Shoemaker, R., Yiannikouris, F., Zhang, X., et al. (2012). Angiotensin Converting Enzyme 2 Contributes to Sex Differences in the Development of Obesity Hypertension in C57BL/6 Mice. Atvb 32, 1392–1399. doi:10.1161/atvbaha.112.248559
Gurwitz, D. (2020). Angiotensin Receptor Blockers as Tentative SARS‐CoV‐2 Therapeutics. Drug Dev. Res. 81, 537–540. doi:10.1002/ddr.21656
Hamming, I., Timens, W., Bulthuis, M., Lely, A., Navis, G., and van Goor, H. (2004). Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 203, 631–637. doi:10.1002/path.1570
Hofmann, H., Pyrc, K., van der Hoek, L., Geier, M., Berkhout, B., and Pohlmann, S. (2005). Human Coronavirus NL63 Employs the Severe Acute Respiratory Syndrome Coronavirus Receptor for Cellular Entry. Proc. Natl. Acad. Sci. 102, 7988–7993. doi:10.1073/pnas.0409465102
Imai, Y., Kuba, K., and Penninger, J. M. (2008). The Discovery of Angiotensin-Converting Enzyme 2 and its Role in Acute Lung Injury in Mice. Exper. Physiol. 93, 543–548. doi:10.1113/expphysiol.2007.040048
Imai, Y., Kuba, K., Rao, S., Huan, Y., Guo, F., Guan, B., et al. (2005). Angiotensin-converting Enzyme 2 Protects from Severe Acute Lung Failure. Nature 436, 112–116. doi:10.1038/nature03712
Ingraham, N. E., Barakat, A. G., Reilkoff, R., Bezdicek, T., Schacker, T., Chipman, J. G., et al. (2020). Understanding the Renin-Angiotensin-Aldosterone-SARS-CoV axis: a Comprehensive Review. Eur. Respir. J. 56, 2000912. doi:10.1183/13993003.00912-2020
Istituto Superiore di Sanità (2020). Epidemia COVID-19, Aggiornamento Nazionale: 30 Marzo 2020 (In Italian). https://www.epicentro.iss.it.
Jia, H. (2016). Pulmonary Angiotensin-Converting Enzyme 2 (ACE2) and Inflammatory Lung Disease. Shock 46, 239–248. doi:10.1097/shk.0000000000000633
Karlberg, J., Chong, D. S. Y., and Lai, W. Y. Y. (2004). Do men Have a Higher Case Fatality Rate of Severe Acute Respiratory Syndrome Than Women Do? Am. J. Epidemiol. 159, 229–231. doi:10.1093/aje/kwh056
Kaschina, E., Namsolleck, P., and Unger, T. (2017). AT2 Receptors in Cardiovascular and Renal Diseases. Pharmacol. Res. 125, 39–47. doi:10.1016/j.phrs.2017.07.008
Korber, B., Fischer, W. M., Gnanakaran, S., Yoon, H., Theiler, J., Abfalterer, W., et al. (2020). Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 182, 812–827. e19. doi:10.1016/j.cell.2020.06.043
Kuba, K., Imai, Y., Rao, S., Gao, H., Guo, F., Guan, B., et al. (2005). A Crucial Role of Angiotensin Converting Enzyme 2 (ACE2) in SARS Coronavirus-Induced Lung Injury. Nat. Med. 11, 875–879. doi:10.1038/nm1267
Kurisu, S., Ozono, R., Oshima, T., Kambe, M., Ishida, T., Sugino, H., et al. (2003). Cardiac Angiotensin II Type 2 Receptor Activates the Kinin/NO System and Inhibits Fibrosis. Hypertension 41, 99–107. doi:10.1161/01.hyp.0000050101.90932.14
Lambert, D. W., Yarski, M., Warner, F. J., Thornhill, P., Parkin, E. T., Smith, A. I., et al. (2005). Tumor Necrosis Factor-α Convertase (ADAM17) Mediates Regulated Ectodomain Shedding of the Severe-Acute Respiratory Syndrome-Coronavirus (SARS-CoV) Receptor, Angiotensin-Converting Enzyme-2 (ACE2). J. Biol. Chem. 280, 30113–30119. doi:10.1074/jbc.m505111200
Lip, G. (2000). Hypertension and the Prothrombotic State. J. Hum. Hypertens. 14, 687–690. doi:10.1038/sj.jhh.1001051
Liu, H., Wang, H., Cheng, D., Wang, Q., Pei, Z., Zhu, N., et al. (2019). Potential Role of a Disintegrin and Metalloproteinase-17 (ADAM17) in Age-Associated Ventricular Remodeling of Rats. RSC Adv. 9, 14321–14330. doi:10.1039/c9ra01190k
Liu, Y., Yang, Y., Zhang, C., Huang, F., Wang, F., Yuan, J., et al. (2020). Clinical and Biochemical Indexes from 2019-nCoV Infected Patients Linked to Viral Loads and Lung Injury. Sci. China Life Sci. 63, 364–374. doi:10.1007/s11427-020-1643-8
Marschall, M., Meier-Ewert, H., Herrler, G., Zimmer, G., and Maassab, H. F. (1997). The Cell Receptor Level Is Reduced during Persistent Infection with Influenza C Virus. Arch. Virol. 142, 1155–1164. doi:10.1007/s007050050149
Mathewson, A. C., Bishop, A., Yao, Y., Kemp, F., Ren, J., Chen, H., et al. (2008). Interaction of Severe Acute Respiratory Syndrome-Coronavirus and NL63 Coronavirus Spike Proteins with Angiotensin Converting Enzyme-2. J. Gen. Virol. 89, 2741–2745. doi:10.1099/vir.0.2008/003962-0
Michel, N., Allespach, I., Venzke, S., Fackler, O. T., and Keppler, O. T. (2005). The Nef Protein of Human Immunodeficiency Virus Establishes Superinfection Immunity by a Dual Strategy to Downregulate Cell-Surface CCR5 and CD4. Curr. Biol. 15, 714–723. doi:10.1016/j.cub.2005.02.058
Monteil, V., Kwon, H., Prado, P., Hagelkrüys, A., Wimmer, R. A., Stahl, M., et al. (2020). Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 181, 905–913. e7. doi:10.1016/j.cell.2020.04.004
Muus, C., Luecken, M. D., Eraslan, G., Waghray, A., Heimberg, G., Sikkema, L., et al. (2020). Integrated Analyses of Single-Cell Atlases Reveal Age, Sex, and Smoking Status Associations with Cell Type-specific Expression of Mediators of SARS-CoV-2 Viral Entry and Highlights Inflammatory Programs in Putative Target Cells. Preprint. doi:10.1101/2020.04.19.049254
Nicholls, J., and Peiris, M. (2005). Good ACE, Bad ACE Do Battle in Lung Injury, SARS. Nat. Med. 11, 821–822. doi:10.1038/nm0805-821
Nicolau, L. A. D., Magalhães, P. J. C., and Vale, M. L. (2020). What Would Sérgio Ferreira Say to Your Physician in This War against COVID-19: How about Kallikrein/kinin System? Med. Hypotheses 143, 109886. doi:10.1016/j.mehy.2020.109886
Offringa, A., Montijn, R., Singh, S., Paul, M., Pinto, Y. M., and Pinto-Sietsma*, S.-J. (2020). The Mechanistic Overview of SARS-CoV-2 Using Angiotensin-Converting Enzyme 2 to Enter the Cell for Replication: Possible Treatment Options Related to the Renin-Angiotensin System. Eur. Heart J. Cardiovasc. Pharmacother. 6, 317–325. doi:10.1093/ehjcvp/pvaa053
OPS (2020). Enfermedad por el coronavirus (COVID-19). https://www.mscbs.gob.es.
Ortega-Cejas, V., Fort, J., Méndez, V., and Campos, D. (2004). Approximate Solution to the Speed of Spreading Viruses. Phys. Rev. E 69, 031909. doi:10.1103/physreve.69.031909
Ortiz, M. E., Thurman, A., Pezzulo, A. A., Leidinger, M. R., Klesney-Tait, J. A., Karp, P. H., et al. (2020). Heterogeneous Expression of the SARS-Coronavirus-2 Receptor ACE2 in the Human Respiratory Tract. EBioMedicine 60, 102976. doi:10.1016/j.ebiom.2020.102976
Pastor-Barriuso, R., Pérez-Gómez, B., Hernán, M. A., Pérez-Olmeda, M., Yotti, R., Oteo-Iglesias, J., et al. (2020). Infection Fatality Risk for SARS-CoV-2 in Community Dwelling Population of Spain: Nationwide Seroepidemiological Study. BMJ 371, m4509. doi:10.1136/bmj.m4509
Pavel, A. B., Wu, J., Renert‐Yuval, Y., Del Duca, E., Glickman, J. W., Miller, R. L., et al. (2021). SARS‐CoV‐2 Receptor ACE2 Protein Expression in Serum Is Significantly Associated with Age. Allergy 76, 875–878. doi:10.1111/all.14522
Paz Ocaranza, M., Riquelme, J. A., García, L., Jalil, J. E., Chiong, M., Santos, R. A. S., et al. (2020). Counter-regulatory Renin-Angiotensin System in Cardiovascular Disease. Nat. Rev. Cardiol. 17, 116–129. doi:10.1038/s41569-019-0244-8
Pierce, C. A., Preston-Hurlburt, P., Dai, Y., Aschner, C. B., Cheshenko, N., Galen, B., et al. (2020). Immune Responses to SARS-CoV-2 Infection in Hospitalized Pediatric and Adult Patients. Sci. Transl. Med. 12, eabd5487. doi:10.1126/scitranslmed.abd5487
Remková, A., and Remko, M. (2010). The Role of Renin-Angiotensin System in Prothrombotic State in Essential Hypertension. Physiol. Res. 59, 13–23. doi:10.33549/physiolres.931525
Robert Koch Institute (2020). Coronavirus Disease 2019(COVID-19) Daily Situation Report of the Robert Koch Institute. https://www.rki.de.
Saheb Sharif-Askari, N., Saheb Sharif-Askari, F., Alabed, M., Temsah, M.-H., Al Heialy, S., Hamid, Q., et al. (2020). Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol. Ther. - Methods Clin. Dev. 18, 1–6. doi:10.1016/j.omtm.2020.05.013
Satou, R., Penrose, H., and Navar, L. G. (2018). Inflammation as a Regulator of the Renin-Angiotensin System and Blood Pressure. Curr. Hypertens. Rep. 20, 100. doi:10.1007/s11906-018-0900-0
Schneider-Schaulies, J., Schnorr, J. J., Brinckmann, U., Dunster, L. M., Baczko, K., Liebert, U. G., et al. (1995). Receptor Usage and Differential Downregulation of CD46 by Measles Virus Wild-type and Vaccine Strains. Proc. Natl. Acad. Sci. 92, 3943–3947. doi:10.1073/pnas.92.9.3943
Schouten, L. R. A., Helmerhorst, H. J. F., Wagenaar, G. T. M., Haltenhof, T., Lutter, R., Roelofs, J. J. T. H., et al. (2016). Age-dependent Changes in the Pulmonary Renin-Angiotensin System Are Associated with Severity of Lung Injury in a Model of Acute Lung Injury in Rats. Crit. Care Med. 44, e1226–e1235. doi:10.1097/ccm.0000000000002008
Smith, A. M., and Perelson, A. S. (2011). Influenza A Virus Infection Kinetics: Quantitative Data and Models. Wires Syst. Biol. Med. 3, 429–445. doi:10.1002/wsbm.129
Sodhi, C. P., Wohlford-Lenane, C., Yamaguchi, Y., Prindle, T., Fulton, W. B., Wang, S., et al. (2018). Attenuation of Pulmonary ACE2 Activity Impairs Inactivation of Des-Arg9 bradykinin/BKB1R axis and Facilitates LPS-Induced Neutrophil Infiltration. Am. J. Physiology-Lung Cell Mol. Physiol. 314, L17–L31. doi:10.1152/ajplung.00498.2016
Sun, M. L., Yang, J. M., Sun, Y. P., and Su, G. H. (2020). [Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 43, E014. doi:10.3760/cma.j.issn.1001-0939.2020.0014
Tseng, Y. H., Yang, R. C., and Lu, T. S. (2020). Two Hits to the Renin‐angiotensin System May Play a Key Role in severeCOVID‐19. Kaohsiung J. Med. Sci. 36, 389–392. doi:10.1002/kjm2.12237
Vaduganathan, M., Vardeny, O., Michel, T., McMurray, J. J. V., Pfeffer, M. A., and Solomon, S. D. (2020). Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 382, 1653–1659. doi:10.1056/nejmsr2005760
van de Veerdonk, F. L., Netea, M. G., van Deuren, M., van der Meer, J. W., de Mast, Q., Brüggemann, R. J., et al. (2020). Kallikrein-kinin Blockade in Patients with COVID-19 to Prevent Acute Respiratory Distress Syndrome. Elife 9, e57555. doi:10.7554/eLife.57555
Verdecchia, P., Cavallini, C., Spanevello, A., and Angeli, F. (2020). The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection. Eur. J. Intern. Med. 76, 14–20. doi:10.1016/j.ejim.2020.04.037
Wagner, C., Frazar, C. D., Roychoudhury, P., Lee, J., Moncla, L. H., Pelle, B., et al. (2020). Comparing Viral Load and Clinical Outcomes in Washington State across D614G Mutation in Spike Protein of SARS-CoV-2. https://github.com/blab/ncov-wa-d614g.
Walls, A. C., Park, Y.-J., Tortorici, M. A., Wall, A., McGuire, A. T., and Veesler, D. (2020). Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 181, 281–292. e6. doi:10.1016/j.cell.2020.02.058
Weibel, E. R. (1991). Fractal Geometry: A Design Principle for Living Organisms. Am. J. Physiology-Lung Cell Mol. Physiol. 261, L361–L369. doi:10.1152/ajplung.1991.261.6.l361
Williamson, E. J., Walker, A. J., Bhaskaran, K., Bacon, S., Bates, C., Morton, C. E., et al. (2020). Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 584, 430–436. doi:10.1038/s41586-020-2521-4
Wood, R. (1995). Bronchospasm and Cough as Adverse Reactions to the ACE Inhibitors Captopril, Enalapril and Lisinopril. A Controlled Retrospective Cohort Study. Br. J. Clin. Pharmacol. 39, 265–270. doi:10.1111/j.1365-2125.1995.tb04447.x
Wrapp, D., Wang, N., Corbett, K. S., Goldsmith, J. A., Hsieh, C.-L., Abiona, O., et al. (2020). Cryo-EM Structure of the 2019-nCoV Spike in the Prefusion Conformation. Science 367, 1260–1263. doi:10.1126/science.abb2507
Wu, K., Li, W., Peng, G., and Li, F. (2009). Crystal Structure of NL63 Respiratory Coronavirus Receptor-Binding Domain Complexed with its Human Receptor. Proc. Natl. Acad. Sci. 106, 19970–19974. doi:10.1073/pnas.0908837106
Xie, X., Xudong, X., Chen, J., Junzhu, C., Wang, X., Xingxiang, W., et al. (2006). Age- and Gender-Related Difference of ACE2 Expression in Rat Lung. Life Sci. 78, 2166–2171. doi:10.1016/j.lfs.2005.09.038
Xu, J., Sriramula, S., Xia, H., Moreno-Walton, L., Culicchia, F., Domenig, O., et al. (2017). Clinical Relevance and Role of Neuronal at 1 Receptors in ADAM17-Mediated ACE2 Shedding in Neurogenic Hypertension. Circ. Res. 121, 43–55. doi:10.1161/circresaha.116.310509
Yoon, H. E., Kim, E. N., Kim, M. Y., Lim, J. H., Jang, I. A., Ban, T. H., et al. (2016). Age-associated Changes in the Vascular Renin-Angiotensin System in Mice. Oxid. Med. Cel. Longev. 2016, 6731093. doi:10.1155/2016/6731093
Yurkovetskiy, L., Wang, X., Pascal, K. E., Tomkins-Tinch, C., Nyalile, T. P., Wang, Y., et al. (2020). Structural and Functional Analysis of the D614G SARS-CoV-2 Spike Protein Variant. Cell 183, 739–751. e8. doi:10.1016/j.cell.2020.09.032
Zhang, Z., Guo, L., Huang, L., Zhang, C., Luo, R., Zeng, L., et al. (2021). Distinct Disease Severity between Children and Older Adults with COVID-19: Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin. Infect. Dis. ciaa1911. doi:10.1093/cid/ciaa1911
Keywords: SARS-CoV-2, ACE2, viral propagation, mathematical model, inflammatory response, COVID-19
Citation: Bastolla U (2021) Mathematical Model of SARS-Cov-2 Propagation Versus ACE2 Fits COVID-19 Lethality Across Age and Sex and Predicts That of SARS. Front. Mol. Biosci. 8:706122. doi: 10.3389/fmolb.2021.706122
Received: 06 May 2021; Accepted: 30 June 2021;
Published: 12 July 2021.
Edited by:
Valentina Tozzini, Istituto Nanoscienze, ItalyReviewed by:
Guido Tiana, University of Milan, ItalyQian Wang, University of Science and Technology of China, China
Copyright © 2021 Bastolla. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ugo Bastolla, dWJhc3RvbGxhQGNibS5jc2ljLmVz
 Ugo Bastolla
Ugo Bastolla