
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci. , 30 July 2021
Sec. Metabolomics
Volume 8 - 2021 | https://doi.org/10.3389/fmolb.2021.681955
This article is part of the Research Topic Metabolomics Perspectives for Clinical Medicine View all 14 articles
 Xiao-xue Tian1,2,3†
Xiao-xue Tian1,2,3† Shu-fen Zheng1,2,3†
Shu-fen Zheng1,2,3† Ju-e Liu2,3†
Ju-e Liu2,3† Yuan-yuan Wu2,3
Yuan-yuan Wu2,3 Lu Lin2,3
Lu Lin2,3 Hong-mei Chen2,3
Hong-mei Chen2,3 Li-wen Li2,3
Li-wen Li2,3 Min Qin2,3
Min Qin2,3 Zi-xian Wang2,3
Zi-xian Wang2,3 Qian Zhu2,3
Qian Zhu2,3 Wei-hua Lai2*
Wei-hua Lai2* Shilong Zhong1,2,3,4*
Shilong Zhong1,2,3,4*This study aims to evaluate the association between free triiodothyronine (FT3) and outcomes of coronary artery disease (CAD) patients, as well as to assess the predictive power of FT3 and related functional markers from the perspective of potential mechanism. A total of 5104 CAD patients with an average follow-up of three years were enrolled into our study. Multivariate Cox regression was used to evaluate the associations between FT3, FT4 (free thyroxin), FT3/FT4 and death, MACE. We developed and validated an age, biomarker, and clinical history (ABC) model based on FT3 indicators to predict the prognosis of patients with CAD. In the multivariable Cox proportional hazards model, FT3 and FT3/FT4 were independent predictors of mortality (Adjusted HR = 0.624, 95% CI = 0.486–0.801; adjusted HR = 0.011, 95% CI = 0.002–0.07, respectively). Meanwhile, emerging markers pre-brain natriuretic peptide, fibrinogen, and albumin levels are significantly associated with low FT3 (p < 0.001). The new risk death score based on biomarkers can be used to well predict the outcomes of CAD patients (C index of 0.764, 95% CI = 0.731–0.797). Overall, our findings suggest that low levels of FT3 and FT3/FT4 are independent predictors of death and MACE risk in CAD patients. Besides, the prognostic model based on FT3 provides a useful tool for the death risk stratification of CAD patients.
Coronary artery disease (CAD), also known as coronary atherosclerotic heart disease (CHD), is an inflammatory atherosclerotic disease that manifests as stable angina, unstable angina, myocardial infarction, and sudden cardiac death (álvarez-álvarez et al., 2017.; Pothineni et al., 2017; Musunuru and Kathiresan, 2019). Despite the prominent enrichment of treatments, patients with CAD have poor prognoses and high mortality (Li et al., 2015; Elmariah et al., 2018). The prognosis of CAD is affected by many factors, and the prognostic value of traditional risk factors for CAD is limited. A complex relationship exists between thyroid hormone (THs) levels and outcomes of CAD. THs play a central role in many cellular processes, including differentiation, growth, metabolism, and physiology (Boelaert; Gorman, 2006). Changes in TH concentrations in plasma, especially low triiodothyronine (T3) levels, represent hormonal imbalance, which is usual among patients suffering from an acute coronary event (Iervasi, 2008; Xu, 2012; Hao, 2014).
T3, the biologically active form of THs, is derived mainly from the peripheral transformation of precursor thyroxine (T4). Variations in the concentrations of T3 and T4 in plasma may exert a wide range of functions in several mechanisms, including heart dysfunction (Iervasi et al., 2003; Yazıcı et al., 2017). The levels of free triiodothyronine (FT3) and free thyroxin (FT4) are important indicators of the metabolic status of THs and have gained increasing attention as markers of many acute diseases (Iervasi et al., 2003; Zhang et al., 2016). The value of FT3 as an independent risk factor for the prognosis of patients with CAD remains controversial (Coceani et al., 2010; Wang et al., 2013; Lamprou et al., 2017; Wang et al., 2017). In addition, guidelines recommended for routine screening of low T3 syndrome in patients with CAD have not been pushed (Levine et al., 2016). Low T3 syndrome, known as nonthyroidal illness syndrome and euthyroid sick syndrome, is characterized by low serum levels of total T3 and FT3 with normal levels of thyroid stimulating hormone (TSH) and FT4; this condition is deemed as a strong prognostic determinant of chronic and systolic heart failure (HF) (Iervasi et al., 2003; Cooper and Biondi, 2012; Zhang et al., 2012; Jabbar et al., 2015; Yazıcı et al., 2017).
To the best of our knowledge, precise identification and discriminatory risk evaluation are important prerequisites to targeted treatment and prevention in high risk of all-cause death and major adverse cardiovascular (MACE) for CAD. Identifying novel and overlooked biomarkers, which not only guide the diagnosis and prognosis of patients with CAD but also detect new molecular mechanisms to elucidate the pathological progress of CAD, is important due to the limited predictive power of few predictors based on genetic factors and traditional clinical risk biomarkers available for risk stratification in patients with CAD. Hyperthyroidism is linked to an increased risk of thrombus (Kim et al., 2017). Data from epidemiological studies indicate that patients with low thyroid hormone levels are at higher risk of heart failure, and the prognosis of heart failure is also worse (Vale et al., 2019). A study suggested that a rise in thyroxine level is associated with the increase of FVIII, FIX, VWF and fibrinogen levels (Debeij et al., 2010). It has been reported that there is a positive correlation between FT3 level and cardiac ejection fraction, and a significant negative correlation with NT-proBNP. Although the imbalance of FT3 and FT4 levels is the most common manifestation of thyroid dysfunctions in patients with CAD, previous data on the prevalence of low FT3 and high FT4 levels in CAD patients are insufficient, and their prognostic roles are unclear. Thus, we evaluated the association of FT3 with other functional parameters and outcomes in 5104 CAD patients and assessed the predictive power of FT3 combined with other markers. The levels of thyroid hormone and related biomarkers are predictive of death in CAD, likely expressing different and synergistic pathogenic pathways, and their combined assay significantly improves the ability of risk stratification.
During the prospective study period, 5,104 Chinese CAD participants from Guangdong Provincial People’s Hospital, who underwent coronary angiography, were included. Patients were consecutively enrolled between January 2010 and December 2013 and followed up for all-cause death and MACE up to five years. The exclusion criteria for patients in single-center cohort study included the following: 1) age <18 years or >80 years, 2) renal insufficiency (defined as serum creatinine concentration > two times the upper limit of normal [230 μmol/L], history of renal transplantation or dialysis), 3) hepatic insufficiency (defined as serum transaminase concentration > two times the upper limit of normal [80 U/L], or a diagnosis of cirrhosis), 4) being pregnant or lactating, 5) advanced cancer or hemodialysis, 6) history of thyroid problems and use of antithyroid drugs or thyroid hormone medication, and 7) incomplete information about cardiovascular events during follow-up.
The primary endpoint of interest was all-cause death, followed by MACE. MACE is the occurrence of cardiac death, nonfatal myocardial infarctions, coronary revascularization, and cerebral infarction. All participants were followed up prospectively for the study endpoints by inpatient and outpatient hospital visits and telephone contacts with the patients or their families. At each follow-up assessment (every 6 months), the participants were questioned about new adverse cardiovascular events. From August 2010 to August 2018, all patients were followed up for the primary endpoint (all-cause death) and secondary endpoint (MACE), with a mean follow-up time of 3 years. Baseline information, including demographics, medical history, biochemical measurements, and medication, were obtained from the hospital information database. This study was approved by the Medical Ethical Review Committee of Guangdong Provincial People’s Hospital and conducted according to the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Eligible participants were evaluated for demographic factors (e.g., age and gender), clinical characteristics (e.g., The Synergy between PCI with TAXUS and Cardiac Surgery score, left ventricular ejection fraction [LVEF]), CAD risk factors (e.g., diabetes mellitus, smoking, hypertension, body mass index, and dyslipidemia), and current medication use. Baseline characteristics, including demographics, medical history, biochemical measurements, and medication, were obtained from the hospital information database. Follow-up data on mortality (time and cause of death) were used in the analysis as the primary outcome of interest. In some cases, these outcome data were unavailable. The serum concentrations of T3, FT3, T4, FT4, and of TSH have been determined and recorded. Normal range was as follows: T3, from 70 ng/dl to 170 ng/dl; FT3, from 3.28 to 6.47 pmol/L; T4, from 4.5 μg/dl to 12.0 μg/dl; FT4, from 7.64 to 16.03 pmol/L; TSH, from 0.49 to 4.91 μIU/ml, pro-brain-type natriuretic peptide (proBNP), from 0 to 125 pg/ml.
Continuous variables were expressed as mean ± SD, and categorical variables are described using number and percentage. Student’s t-test was used to compare continuously normal variables, the Wilcoxon rank-sum test was used for continuously abnormal variables, and the χ2-test was used for categorical variables. Cox proportional hazards analysis was performed to investigate the associations of clinical parameters, levels of FT3, FT4 or FT3/FT4 ratio with outcomes. In the multivariable analysis. We implemented three models to evaluate the impact of potential confounders. Model 1 included age, sex. Model 2 further adjusted for a history of diabetes, heart failure, aspartate aminotransferase, apolipoprotein a, creatine, creatine kinase MB, high-density lipoprotein cholesterol, glucose, lipoprotein (a), medication history of calcium channel blockers and proton pump inhibitors. Model 3 incorporated the components of model 2, heart rate, systolic blood pressure and diastolic blood pressure. Variables with p < 0.05 in the univariate Cox regression analysis were included in the multivariate Cox proportional hazard regression models by an improved backward stepwise procedure to select covariates based on the Akaike information criterion (AIC). The Spearman rank correlation coefficient (Spearman ρ) was used to determine the associations among FT3, FT4, FT3/FT4, and other risk factors. In addition, the Harrell–Lee C-index was used to quantify the additional predictive value of the new model over the traditional model containing common clinical variables. The area under the curve (AUC) of time-dependent receiver-operating characteristic (ROC) analysis was used to quantify the predictive performance. In addition, each selected biomarker was assigned a corresponding score based on its value on the nomogram. Thus, the total score of death and MACE risk was calculated by nomogram, and the cutoff points of the total score detected by x-tile analysis could be used to divide CAD patients into different categories.
The final model was represented as a nomogram. Calibration curves (2000 bootstrap resamples) were generated to verify the accuracy of the nomogram. Decision curve analyses were performed to assess the clinical utility of the nomogram. Finally, we used X-tile software (Version 3.6.2, calculated by the “rms package” of R software) to calculate the optimal cut-off points for linear prediction and established a prognostic risk stratification. Kaplan-Meier method was used to estimate the survival probabilities and obtain survival curves, which are used to illustrate the cumulative incidence of clinical endpoints based on the cut-off values, and the log-rank (Mantel–Cox) test was adopted to compare survival curves. A two-tailed p < 0.05 was statistically significant. All tests were performed using SAS (version 9.4) and R (version 3.6.2, http://www.R-project.org/).
A total of 5104 CAD patients were investigated, including 3,886 males (76.3%) and 1,218 females (23.7%), with an average age of 64.2 years at baseline, and a flowchart of the study population is shown in Figure 1. Table 1 provides the baseline characteristics of the participants. During an average of 3 years of follow-up, 274 (5.61%) died and 865 (16.9%) had MACE events. The mean ± standard deviations of FT3, FT4, and FT3/FT4 were 4.4 ± 0.93 pmol/L, 11.73 ± 2.86 pmol/L, and 0.39 ± 0.1 pmol/L, respectively. FT3 < 3.5 pmol/L and FT4 > 17.8 pmol/L are divided into low FT3 group (N = 326) and high FT4 group (N = 105). In addition, patients with FT3/FT4 <0.33 were classified as the low FT3/FT4 group (N = 1,194) in accordance with the lower quartile lower limit. The proportion of patients with HF was higher in the low FT3 group (14.11 vs 6.23%, p < 0.0001), and no significant difference was found in the proportion of patients with smoking history. The common clinical indicators in the low FT3 group were higher than those in the normal FT3 group. Myocardial damage indexes of proBNP, hydroxybutyrate dehydrogenase (HBDH), cardiac troponin (cTnl), high-sensitivity C-reactive protein (hs-CRP), and high-sensitivity cardiac troponin (hs-cTnl) in the low FT3 group were higher than those in the normal FT3 group (all p < 0.001). However, the level of albumin (ALB), triglyceride (TRIG), total protein (TP), hemoglobin (HGB), and lymphocyte in the low FT3 group was lower than that in the normal FT3 group (Supplementary Table S1).
A significant correlation exists between FT3/FT4 and ALB (r = 0.381, p < 0.001) and followed by proBNP (r = −0.285, p < 0.001). The results showed that ALB (r = 0.348), TP (r = 0.268), HCT (r = 0.279), and HGB (r = 0.276) were positively correlated with FT3 concentration (p < 0.001). Meanwhile, proBNP (r = −0.194), HBDH (r = −0.156), and DBIL (r = −0.161), and myocardial injury indicators Troponin I, high-sensitivity troponin T were negatively correlated with FT3 concentration (p < 0.001) (Table 2), Similar patterns of correlation strength were also found in FT3/FT4 ratio. In addition, statistically significant associations were also found between coagulation markers and thyroid hormone levels.
After an average of three years of follow-up, 116 (35.58%) of the 326 patients had MACE events and 63 (20.93%) died in the low FT3 group. Supplementary Tables S2–S3 provides univariable Cox modeling results for clinical indices and biomarkers in relation to mortality and MACE. As shown, age conferred increased risk for death (HR = 1.055, 95% CI = 1.041–1.069, and p = 3.33E-15). Biomarker data demonstrated a strong association between elevated FIB and risk for death (HR = 1.33, 95% CI = 1.23–1.43, and p = 8.06E-13). Low ALB levels may prompt increased death MACE risks (HR = 0.859, 95% CI = 0.837–0.881, and p = 3.98E-32; HR = 0.933, 95% CI = 0.918–0.947, and p = 6.98E-18, respectively). In the multivariable Cox regression analyses, FT3, FT4 and FT3/FT4 were all independent predictors of mortality after controlling for age and other clinical markers (p < 0.05) (Table 3). Notably, multivariate regression yielded similar results regarding relation between FT3, FT3/FT4 and MCACE risk, while after adjustment by clinical variables, no significant correlation was observed between FT4 and MACE risk (Adjusted HR = 1.008, 95% CI = 0.985–1.032). The cumulative survival curves of MACE and death in patients with different FT3, FT4, and FT3/FT4 levels are shown in Figure 2. Results demonstrate consistently the prognositic difference in different FT3, FT4 or FT3/FT4 ratio groups.
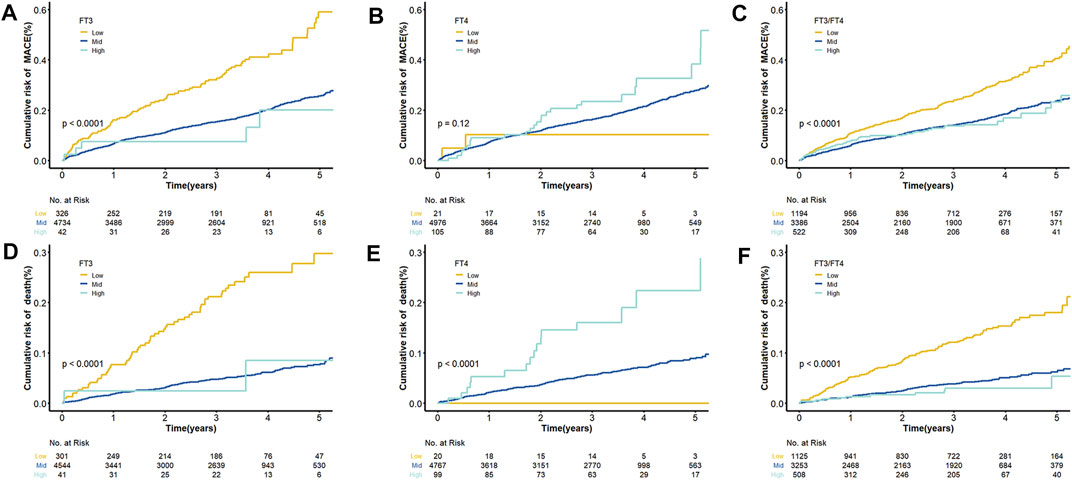
FIGURE 2. Cumulative Kaplan–Meier Curves for FT3, FT4 and the ratio of FT3 and FT4. (A) Cumulative Kaplan–Meier Curves for MACE according to FT3 quartiles; (B) Cumulative Kaplan–Meier Curves for MACE according to FT4 quartiles; (C) Cumulative Kaplan–Meier Curves for MACE according to FT3/FT4 quartiles; (D) Cumulative Kaplan–Meier Curves for death according to FT3 quartiles; (E) Cumulative Kaplan–Meier Curves for death according to FT4 quartiles; (F) Cumulative Kaplan–Meier Curves for death according to FT3/FT4 quartiles.
After univariate Cox regression analysis of 15 indicators significantly related to FT3 and FT4 (Supplementary Table S3), the indicators with p < 0.05 were screened out as the candidate variables for subsequent model constructions. A model including all candidate predictors were fitted. Then, the importance of each predictor variable in the model was measured by partial chi-square statistics minus degrees of freedom. Finally, the top three relevant candidate indicators were obtained. In the fitted predictive death model, proBNP (X2-df = 139.77), ALB (X2-df = 120.33), and HGB (X2-df = 88.67) were significantly correlated with death. However, proBNP (X2-df = 61.56) and ALB (X2-df = 60.97) were strongly independent predictors of MACE, and FIB (X2-df = 37.56) had greater prognostic value than other candidate variables involved in the outcome of MACE (Figure 3).
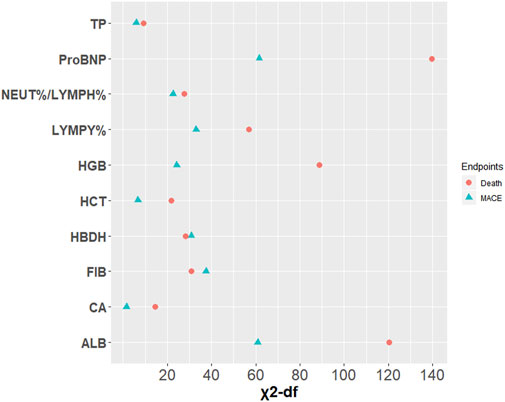
FIGURE 3. Relative importance of each variable in the full model. Measured by partial Wald χ2 minus the predictor degrees of freedom.
Our aim is to develop and validate a new predictive model based on FT3, including age, biomarker, and clinical history (ABC). HF, which was significantly correlated with endpoint events after multivariate Cox correction based on AIC stepwise regression, was selected as the clinical variable. The final predictive model established by age and candidate markers was represented via a nomogram (Figure 4).
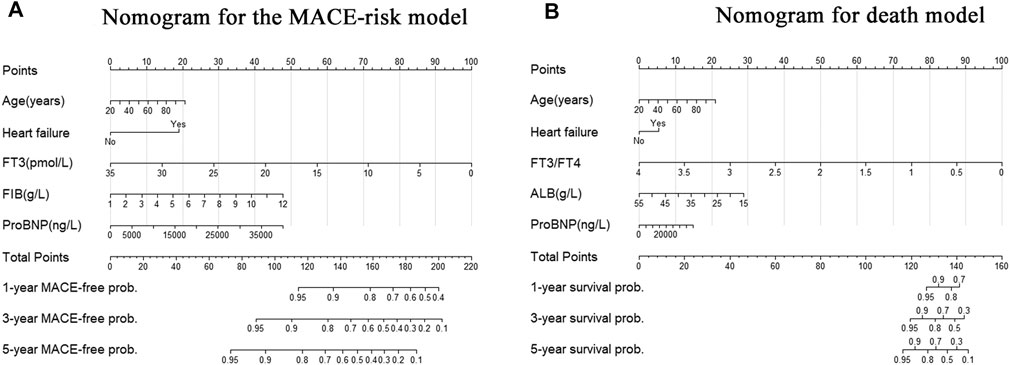
FIGURE 4. Nomogram for the new biomarker-based risk score. (A) Nomogram for the final biomarker-based ABC-MACE risk score; (B) Nomogram for the final biomarker-based ABC-death risk score. Note: The scores of each variable were added to obtain the total score, and a vertical line was drawn on the total score to obtain the corresponding probability of outcomes.
On the one hand, the final MACE predictive models including age, HF, FT3, FIB, and proBNP showed a C index of 0.621 (95% CI = 0.596–0.646), which was significantly higher than the traditional model consisting of common clinical variables (C index of 0.586, 95% CI = 0.554–0.618) (Table 4). On the other hand, the death predictive models of the single AUC of ALB, proBNP, and FT3/FT4 obtained 0.666, 0.708, and 0.634, respectively. The MACE predictive models of the single AUC of FIB, proBNP, and FT3 obtained 0.597, 0.594, and 0.55, respectively (Figure 5).
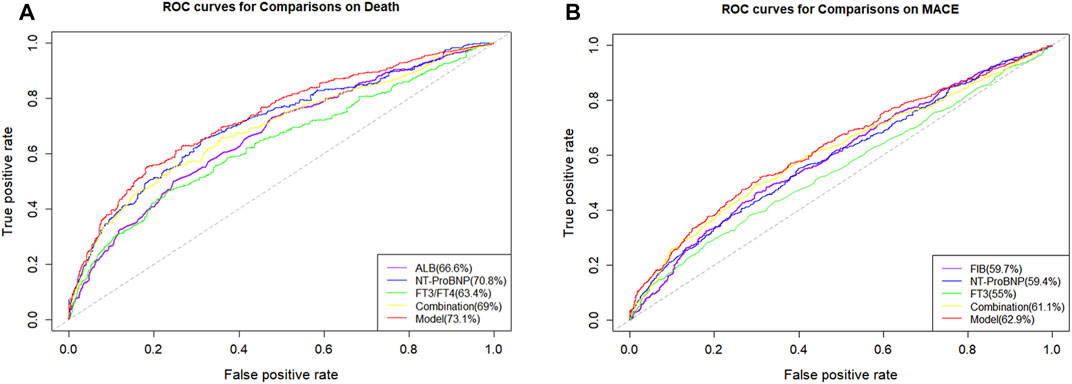
FIGURE 5. Receiver operating characteristics curves (ROC) of different parameters and models. (A) ROC curves of different parameters and models for predicting all-cause mortality; (B) ROC curves of different parameters and models for predicting MACE.
The final model was represented by a nomogram, which showed that low FT3/FT4 values; low ALB levels, high FIB levels, and high proBNP levels were associated with low survival rates. The calibration curves of the nomogram for the predicted five-year mortality and MACE risk demonstrated consistency with the prediction and observation of the primary cohort (Figure 6). Decision curve analysis showed that the use of nomogram to predict mortality adds more benefit than either the treat-all-patients scheme or the treat-none scheme (Figure 7). Finally, the prognostic model for death was divided into three groups according to the total score, as follows: low risk (<118), medium risk (118–124) and high risk (≥124). Similarly, the scores of low-, medium-, and high-risk of MACE groups were <113, 113–122, and ≥122, respectively. The cumulative survival curve of risk stratification is shown in Figure 8.
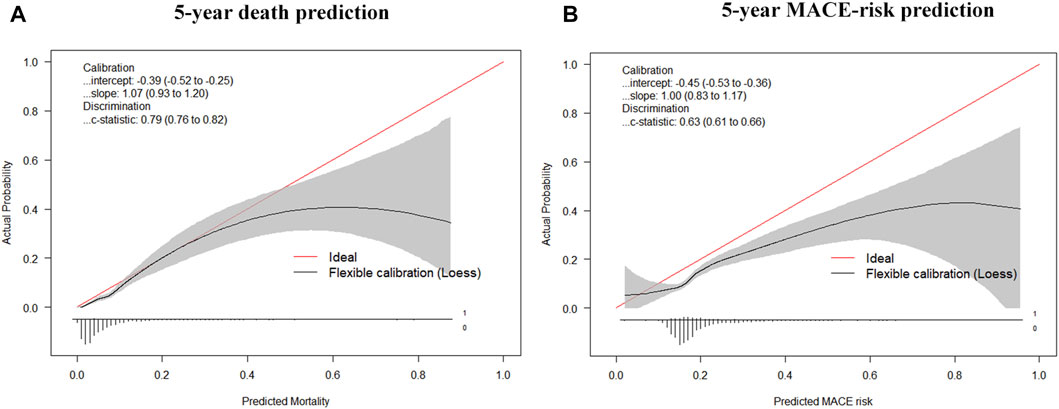
FIGURE 6. Calibration curves for the nomogram model. (A) Calibration curves of the prognostic nomogram for 5-years overall survival; (B) Calibration curves of the nomogram predicting risk of MACE.
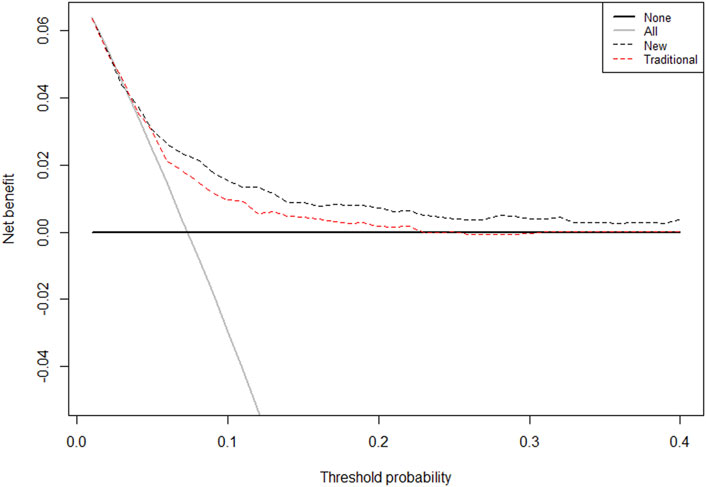
FIGURE 7. The Decision Curves Analysis (DCA) curve of the prognostic nomogram for predicting probability of overall survival at 5 years. Note: The traditional group-composed by parameters of age, sex, hypertension, diabetes, CHOL, HDLC, BMI, smoking, TRIG; Novel group: age, HF, FT3/FT4, ALB, and proBNP. The horizontal axis represents the threshold value, which is the reference probability of whether a patient receives treatment, and the vertical axis represents the net benefit rate after the advantages minus the disadvantages. Under the same threshold probability, the larger net benefit implies that patients can obtain the maximum benefit using the diagnosis of this model. The closer the curve in the DCA graph is to the top, the higher the value of the model diagnosis will be.
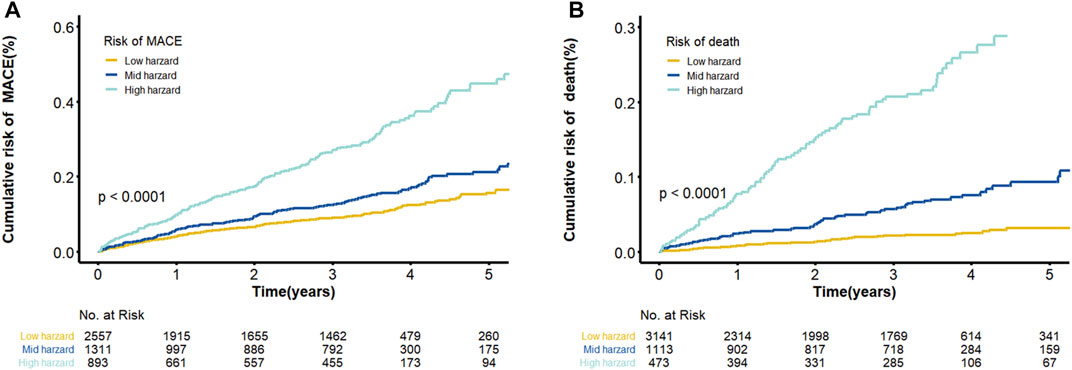
FIGURE 8. Cumulative Kaplan–Meier curves of the prediction model. (A) Cumulative risk of MACE by predicted 5-years ABC-MACE risk group; (B) Cumulative probability of mortality by predicted 5-years ABC-death risk group. Note: Survival curves stratified by the score calculated by the nomogram scoring system [low risk, moderate risk and high risk].
Our study demonstrated that the levels of FT3 and FT3/FT4 were independent predictors of death and MACE risk in patients with CAD on multivariate analyses including conventional CAD risk factors. We also proved that thyroid hormone indicators were significantly related to the emerging biomarkers proBNP, FIB, and ALB, which suggested that thyroid hormone levels may have an impact on adverse outcomes by the key biological pathways, such as heart function and injury, liver function, and coagulation. Finally, according to the predictive characteristics of FT3 and other cardiovascular biomarkers, we developed and validated a new, well-calibrated, biomarker-based risk score to assess the death risk of patients with CAD.
In our study, FT3 and FT3/FT4 are powerful prognostic markers. In recent years, the consequences on the cardiovascular system of milder forms of thyroid dysfunction have been increasingly recognized (Gomberg-Maitland and Frishman, 1998). The further clinical and experimental evidences suggest that a low FT3 level is a strong predictor of a poor prognosis in patients with chronic cardiovascular diseases. However, the relationship between low FT3 and adverse prognosis of CAD patients had a controversy in the last few years (Iervasi et al., 2003; Boelaert and Franklyn, 2005; Coceani et al., 2009; Iervasi, 2009; Ichiki, 2014). In this study, we found that more deaths and MACE occurred in the low FT3 group. Previous studies showed that FT3 promoted the progression of atherosclerosis probably due to the inhibition of lipoprotein enzyme activity and the decreasing clearance rate of total cholesterol (Chapidze et al., 2006; Asvold et al., 2007; Maria Moreno et al., 2019). Although FT3 is associated with lipid levels and linked with the increased mortality of CAD, the mechanism is worthy of further study due to the result of the interaction of many factors (Danese et al., 2000; Duntas, 2002; Erem, 2006).
On the basis of the relationship between FT3 and the prognosis of CAD, we also analyzed the correlation between FT3 and other clinical indicators of CAD patients to further understand whether or not the function was influenced by low FT3 with other common risk factors of CAD. Low T3 syndrome is associated with many traditional risk factors of CAD, such as proBNP, FIB, and ALB (Knezl et al., 2008; Chuang et al., 2014; Brozaitiene et al., 2016). Liang et al. reported that BNP was increased six-fold, and its promoter activity increased three to fivefold following T3 treatment (Liang et al., 2003). Afandi et al. reported that the decrease in thyroid hormone binding proteins is often a consequence of the acute phase response by impaired synthesis, rapid breakdown, and movement out of the plasma space (Afandi et al., 2000). However, there are no detailed data on thyroid parameters in patients with acute liver failure (ALF) so far. One study showed that more than 50% of ALF patients exhibit abnormal thyroid parameters, and their prognosis was worse than that of normal thyroid patients (Anastasiou et al., 2015). In addition, a study suggested that hyperthyroidism was associated with an increased risk of thrombosis, and patients with hyperthyroidism show higher levels of fibrinogen (Kim et al., 2017). Another study confirmed that VWF and fibrinogen mediate up to 10% of the association between FT4 and cardiovascular disease (Bano et al., 2019). In our study, the level of coagulation parameters we observed changes significantly with the increase of FT4 level, and seems to be less affected by FT3 level. It can be concluded from the existing literature that patients with hypothyroidism are at increased risk of bleeding complications due to impaired coagulation and fibrinolysis (Elbers et al., 2018). Notably, thyroid function with the pathophysiological mechanism of the heart and the prognosis of acute myocardial infarction is still being explored. A study demonstrated a significant relationship between the suppression of thyroid axis function, increased inflammation markers and increased NT-pro-BNP levels in CAD patients undergoing rehabilitation after ACS. They have also demonstrated that measures of FT4 and FT3/FT4 ratio together with NT-pro-BNP can be important prognostic markers of negative long-term outcomes (i.e., mortality) (Brozaitiene et al., 2016). A study aimed at explaining the relationship between T3 and cardiac function found that in the multiple regression analysis, FT3 levels were positively correlated with cardiac ejection fraction, and significantly negatively correlated with NT-proBNP(She et al., 2018). This is consistent with the correlation trend results of FT3 and proBNP obtained in this study. Overall, this study provides further insights into the relationship between thyroid hormones and liver function, thrombosis, and heart function. Whether the effect of thyroid hormones on cardiovascular events and cardiovascular death is mediated by coagulation factors or regulated by heart and liver functions requires further research in the future.
Identifying new biomarkers to effectively improve the prediction for prognosis and guide the individualized treatment of patients with CAD is critical (Malakar et al., 2019). As a quantitative tool for assessing risk and benefit, clinical predictive models provide risk estimates for the presence of the disease (diagnosis) or an event in the future course of disease (prognosis) for individual patients (Steyerberg and Vergouwe, 2014). We developed and validated a well-calibrated new risk score based on biomarkers to assess the risk of death in CAD patients. The novel ABC death score includes age, clinical history of HF, and biomarkers of proBNP, FT3/FT4, and ALB. The developed model including multiple and novel risk factors is useful for estimating prognosis in patients with CHD to inform treatment decisions or for use as a risk stratification tool in future research.
Although this novel ABC–CHD model has few variables, it provides reliable CV death prediction compared with models composed of traditional clinical factors. In our model, the most important prognostic variable is proBNP, which is a well-known marker of stress and dysfunction of cardiomyocytes (Beatty et al., 2015). Recent evidence suggests that proBNP also plays a role in metabolic pathways, including lipolysis and regulation of blood glucose levels, which are important in the pathophysiology of CAD (Zois et al., 2014). A study suggested that proBNP is independent of other prognostic markers, including systolic and diastolic dysfunction, left ventricular mass index, inducible ischemia, exercise capacity, C-reactive protein (CRP), cTnT, and New York Heart Association classification, which can predict cardiovascular morbidity and mortality (Bibbins-Domingo et al., 2007). Increasing concentration of proBNP may also be a signal of vascular dysfunction, in which the natriuretic peptides produce changes in vascular smooth muscle proliferation or contractility, in part via cyclic guanosine monophosphate cascades or nitric oxide synthesis (Ahluwalia et al., 2004).
In summary, these findings suggest that a simple test of biomarkers (i.e., proBNP and ALB) may stratify the risk of prognosis in patients with CAD and provide treatment strategies aimed at reducing future cardiovascular disease morbidity and mortality. The novel model can bring more incremental risk prediction (C index difference: 0.071) than the traditional predictive models of death. Furthermore, considering the powerful performance of the novel ABC-CHD model with a small number of variables provides a tool that may be easier to use in the clinical environment.
This study has several limitations. First, this study was based on a single center. Larger studies enrolling a more diverse population are needed to verify these findings and the external validity of prognostic models. Second, because measurements of FT3, FT4, and TSH were performed only once during initial hospitalization, we were unable to account for possible variations in thyroid function over time. Third, more patients with low FT3 have a history of HF compared with normal patients. Thus, the outcomes of patients may be biased. Further mechanism studies about low FT3 on the risk of death should be considered in the interaction with proBNP, FIB, and ALB. This complex relation merits further well-designed investigations.
This study demonstrated that low levels of FT3 and FT3/FT4 are independent predictors of death and MACE risk in CAD patients. The associations of thyroid hormone with other functional parameters (i.e. heart function and injury, liver function, and coagulation markers) warrant further study. A novel risk score for the prediction of death including age, HF, and three biomarkers was successfully developed and validated. It can be widely used to complement clinical assessment and guide management based on death and MACE risk stratification in patients with CAD because it is based on a small number of readily available biomarkers and clinical factors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Medical Ethical Review Committee of Guangdong Provincial People's Hospital (No. GDREC2010137 and GDREC2017071H) and conducted according to the Declaration of Helsinki. The patients/participants provided their written informed consent (No. 20100910, 20170211) to participate in this study.
X-XT, S-FZ, and J-EL wrote manuscript. X-XT, S-FZ, and Y-YW designed research. X-XT, S-FZ, MQ, QZ, Z-XW, and J-EL performed research. S-FZ, X-XT, MQ, QZ, and J-EL analyzed data.
This study was funded by the National Nature Science Foundation of China (No. 81872934 (Principle investigators: Shilong Zhong), 81673514 (Principle investigators: Shilong Zhong); http://www.nsfc.gov.cn/), the National key research and development program (No. 2017YFC0909301 (Principle investigators: Shilong Zhong); https://service.most.gov. cn/index/), the Key research and development program of Guangdong Province, China (2019B020229003 (Principle investigators: Shilong Zhong); http://pro.gdstc.gd.gov.cn/egrantweb/), and the Guangdong Provincial People's Hospital Clinical Research Fund (Y012018085). No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.681955/full#supplementary-material.
FT3, free triiodothyronine; FT4, free thyroxine; ALB, albumin; FIB, fibrinogen; proBNP, pro-B-type natriuretic peptide; HR, hazard ratio; 95%CI, 95% confidence interval.
Afandi, B., Schussler, G. C., Arafeh, A.-H., Boutros, A., and Yap, M. G. (2000). Selective Consumption of Thyroxine-Binding Blobulin during Cardiac Bypass Surgery. Metabolism-clinical Exp. 49 (2), 270–274. doi:10.1016/s0026-0495(00)91611-1
Ahluwalia, A., MacAllister, R. J., and Hobbs, A. J. (2004). Vascular Actions of Natriuretic Peptides. Cyclic GMP-dependent and -independent Mechanisms. Basic Res. Cardiol. 99 (2), 83–89. doi:10.1007/s00395-004-0459-6
álvarez-álvarez, M. M., Zanetti, D., Carreras-Torres, R., Moral, P., and Athanasiadis, G. (2017). A Survey of Sub-saharan Gene Flow into the Mediterranean at Risk Loci for Coronary Artery Disease. Eur. J. Hum. Genet. Ejhg 25 (4), 472–476. doi:10.1038/ejhg.2016.200
Anastasiou, O., Sydor, S., Sowa, J. P., Manka, P., and Canbay, A. (2015). Higher Thyroid-Stimulating Hormone, Triiodothyronine and Thyroxine Values Are Associated with Better Outcome in Acute Liver Failure. PLoS ONE 10 (7), e0132189. doi:10.1371/journal.pone.0132189
Asvold, B. O., Vatten, L. J., Nilsen, T. I. L., and Bjoro, T. (2007). The Association between TSH within the Reference Range and Serum Lipid Concentrations in a Population-Based Study. The HUNT Study. Eur. J. Endocrinol. 156 (2), 181–186. doi:10.1530/eje.1.02333
Bano, A., Chaker, L., de Maat, M., Atiq, F., Kavousi, M., Franco, O. H., et al. (2019). Thyroid Function and Cardiovascular Disease: The Mediating Role of Coagulation Factors. J. Clin. Endocrinol. Metab. 104 (8), 3203–3212. doi:10.1210/jc.2019-00072
Beatty, A. L., Ku, I. A., Bibbins-Domingo, K., Christenson, R. H., DeFilippi, C. R., Ganz, P., et al. (2015). Traditional Risk Factors versus Biomarkers for Prediction of Secondary Events in Patients with Stable Coronary Heart Disease: From the Heart and Soul Study. J. Am. Heart Assoc. 4 (7). doi:10.1161/jaha.114.001646
Bibbins-Domingo, K., Gupta, R., Na, B., Wu, A. H., Schiller, N. B., and Whooley, M. A. (2007). N-terminal Fragment of the Prohormone Brain-type Natriuretic Peptide (NT-proBNP), Cardiovascular Events, and Mortality in Patients with Stable Coronary Heart Disease. Jama 297 (2), 169–176. doi:10.1001/jama.297.2.169
Boelaert, K., and Franklyn, J. A. (2005). Boelaert Thyroid Hormone in Health and Disease. J. Endocrinol. 187 (1), 1–15. doi:10.1677/joe.1.06131
Brozaitiene, J., Mickuviene, N., Podlipskyte, A., Burkauskas, J., and Bunevicius, R. (2016). Relationship and Prognostic Importance of Thyroid Hormone and N-Terminal Pro-B-type Natriuretic Peptide for Patients after Acute Coronary Syndromes: a Longitudinal Observational Study. Bmc Cardiovasc. Disord. 16 (1), 45. doi:10.1186/s12872-016-0226-2
Chapidze, G., Dolidze, N., Williams, M., Sharadze, E., and Latsabidze, N. (2006). Peculiarities of Lipid Profile Parameters in Cardiac Patients with Hypo- and Hyperthyroidism. Georgian Med. News 4 (133), 44–46.
Chuang, C. P., Jong, Y. S., Wu, C. Y., and Lo, H. M. (2014). Impact of Triiodothyronine and N-Terminal Pro-B-type Natriuretic Peptide on the Long-Term Survival of Critically Ill Patients with Acute Heart Failure. Am. J. Cardiol. 113 (5), 845–850. doi:10.1016/j.amjcard.2013.11.039
Coceani, M., Iervasi, G., Pingitore, A., Carpeggiani, C., and L'Abbate, A. (2009). Thyroid Hormone and Coronary Artery Disease: from Clinical Correlations to Prognostic Implications. Clin. Cardiol. 32 (7), 380–385. doi:10.1002/clc.20574
Coceani, M., Iervasi, G., Pingitore, A., Carpeggiani, C., and L'Abbate, A. (2010). Thyroid Hormone and Coronary Artery Disease: From Clinical Correlations to Prognostic Implications. Clin. Cardiol. 32 (7), 380–385. doi:10.1002/clc.20574
Cooper, D. S., and Biondi, B. (2012). Subclinical Thyroid Disease. Lancet 379 (9821), 1142–1154. doi:10.1016/s0140-6736(11)60276-6
Danese, M. D., Ladenson, Paul, W., Meinert, C. L., and Powe, N. R. (2000). Clinical Review 115: Effect of Thyroxine Therapy on Serum Lipoproteins in Patients with Mild Thyroid Failure: A Quantitative Review of the Literature. J. Clin. Endocrinol. Metab. 85 (9), 2993–3001. doi:10.1210/jcem.85.9.6841
Debeij, J., Cannegieter, S. C., Zaane, B. V., Smit, J. W. A., Corssmit, E. P. M., Rosendaal, F. R., et al. (2010). The Effect of Changes in Thyroxine and Thyroid-Stimulating Hormone Levels on the Coagulation System. J. Thromb. Haemost. Jth 8 (12), 2823–2826. doi:10.1111/j.1538-7836.2010.04054.x
Duntas, L. H. (2002). Thyroid Disease and Lipids. Thyroid 12 (4), 287–293. doi:10.1089/10507250252949405
Elbers, L. P. B., Fliers, E., and Cannegieter, S. C. (2018). The Influence of Thyroid Function on the Coagulation System and its Clinical Consequences. J. Thromb. Haemost 16 (4), 634–645. doi:10.1111/jth.13970
Elmariah, S., Farrell, L. A., Furman, D., Lindman, B. R., Shi, X., Morningstar, J. E., et al. (2018). Association of Acylcarnitines with Left Ventricular Remodeling in Patients with Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Jama Cardiol. 3 (3), 242–246. doi:10.1001/jamacardio.2017.4873
Erem, C. (2006). Blood Coagulation, Fibrinolytic Activity and Lipid Profile in Subclinical Thyroid Disease: Subclinical Hyperthyroidism Increases Plasma Factor X Activity. Clin. Endocrinol. 64 (3), 323–329. doi:10.1111/j.1365-2265.2006.02464.x
Gomberg-Maitland, M., and Frishman, W. H. (1998). Frishman Thyroid Hormone and Cardiovascular Disease. Am. Heart J. 135–18796. doi:10.1016/s0002-8703(98)70081-x
Gorman, C. A.. (2006). Euthyroid Sick Syndrome: An Overview. Thyroid 7 (1), 125–132. doi:10.1089/thy.1997.7.125
Hao, P.. (2014). The Effect of Low FT3 Levels on Coronary Artery Calcification and MACE in Outpatients with Suspected Coronary Artery Disease. Coron. Artery Dis. 25 (5), 427–432. doi:10.1097/MCA.0000000000000095
Ichiki, T. (2014). Thyroid Hormone and Atherosclerosis. Vasc. Pharmacol. 52 (3-4), 151–156. doi:10.1016/j.vph.2009.09.004
Iervasi, G. (2008). Acute Effects of Triiodothyronine (T3) Replacement Therapy in Patients with Chronic Heart Failure and Low-T3 Syndrome: A Randomized, Placebo-Controlled Study. J. Clin. Endocrinol. Metab. 93 (4), 1351. doi:10.1210/jc.2007-2210
Iervasi, G., Pingitore, A., Landi, P., Raciti, M., Ripoli, A., Scarlattini, M., et al. (2003). Low-T3 Syndrome: a strong Prognostic Predictor of Death in Patients with Heart Disease. Circulation 107 (5), 708–713. doi:10.1161/01.cir.0000048124.64204.3f
Iervasi, G. (2009). Thyroid Hormones and the Cardiovascular System: Pathophysiology and Interventions. Biomed. Pharmacother. 63 (10), 0–753. doi:10.1016/j.biopha.2009.08.003
Jabbar, A., Ingoe, L., Pearce, S., Zaman, A., and Razvi, S. (2015). Thyroxine in Acute Myocardial Infarction (ThyrAMI) - Levothyroxine in Subclinical Hypothyroidism post-acute Myocardial Infarction: Study Protocol for a Randomised Controlled Trial. Trials 16 (1), 115. doi:10.1186/s13063-015-0621-5
Kim, N., Gu, J. Y., Yoo, H. J., Han, S. E., Kim, Y. I., Nam-Goong, I. S., et al. (2017). Contact System Activation and High Thrombin Generation in Hyperthyroidism. Eur. J. Endocrinol. 176, 583–589. doi:10.1530/EJE-16-0835
Knezl, V., Soukup, T., Okruhlicová, L., Slezák, J., and Tribulová, N. (2008). Thyroid Hormones Modulate Occurrence and Termination of Ventricular Fibrillation by Both Long-Term and Acute Actions. Physiol. Res. 57 (Suppl. 22), S91–S96. doi:10.33549/physiolres.931557
Lamprou, V., Varvarousis, D., Polytarchou, K., Varvarousi, G., and Xanthos, T. (2017). The Role of Thyroid Hormones in Acute Coronary Syndromes: Prognostic Value of Alterations in Thyroid Hormones. Clin. Cardiol. 40 (8), 528–533. doi:10.1002/clc.22689
Levine, G. N., Bates, E. R., Bittl, J. A., Brindis, R. G., Fihn, S. D., Fleisher, L. A., et al. (2016). ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients with Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 68 (10), 1082–1115. doi:10.1016/j.jacc.2016.03.513
Li, J., Li, X., Wang, Q., Hu, S., Wang, Y., Masoudi, F. A., et al. (2015). ST-segment Elevation Myocardial Infarction in China from 2001 to 2011 (The China PEACE-Retrospective Acute Myocardial Infarction Study): a Retrospective Analysis of Hospital Data. Lancet. 385 (9966), 441–451. doi:10.1016/S0140-6736(14)60921-1
Liang, F., Webb, P., Marimuthu, A., Zhang, S., and Gardner, D. G. (2003). Triiodothyronine Increases Brain Natriuretic Peptide (BNP) Gene Transcription and Amplifies Endothelin-dependent BNP Gene Transcription and Hypertrophy in Neonatal Rat Ventricular Myocytes. J. Biol. Chem. 278 (17), 15073–15083. doi:10.1074/jbc.m207593200
Malakar, A. K., Choudhury, D., Halder, B., Paul, P., Uddin, A., and Chakraborty, S. (2019). A Review on Coronary Artery Disease, its Risk Factors, and Therapeutics. J. Cell Physiol. 234 (10), 16812–16823. doi:10.1002/jcp.28350
Maria Moreno, P. d. L., Lombardi, A., Elena Silvestri, , , and Lanni, A. (2019). Fernando Goglia Metabolic Effects of Thyroid Hormone Derivatives. Thyroid 18 (2), 239–253. doi:10.1089/thy.2007.0248
Musunuru, K., and Kathiresan, S. (2019). Genetics of Common, Complex Coronary Artery Disease. Cell 177 (1), 132–145. doi:10.1016/j.cell.2019.02.015
Pothineni, N. V. K., Swathi, S., Kevin, K., Shirazi, L. F., Francesco, R., Shah, P. K., et al. (2017). Infections, Atherosclerosis, and Coronary Heart Disease. Eur. Heart J. 43, 43. doi:10.1093/eurheartj/ehx362
She, J., Feng, J., Deng, Y., Sun, L., Wu, Y., Guo, M., et al. (2018). Correlation of Triiodothyronine Level with In-Hospital Cardiac Function and Long-Term Prognosis in Patients with Acute Myocardial Infarction. Dis. markers 2018, 1–8. doi:10.1155/2018/5236267
Steyerberg, E. W., and Vergouwe, Y. (2014). Towards Better Clinical Prediction Models: Seven Steps for Development and an ABCD for Validation. Eur. Heart J. 35 (29), 1925–1931. doi:10.1093/eurheartj/ehu207
Vale, C., Neves, J. S., Von Hafe, M., Borges-Canha, M., and Leite-Moreira, A. (2019). The Role of Thyroid Hormones in Heart Failure. Cardiovasc. Drugs Ther. 33, 179–188. doi:10.1007/s10557-019-06870-4
Wang, B., Liu, S., Li, L., Yao, Q., Song, R., Shao, X., et al. (2017). Non-thyroidal Illness Syndrome in Patients with Cardiovascular Diseases: a Systematic Review and Meta-Analysis. Int. J. Cardiol. 226, 1–10. doi:10.1016/j.ijcard.2016.10.039
Wang, J., Ma, X., Qu, S., Li, Y., and Xu, J. (2013). High Prevalence of Subclinical Thyroid Dysfunction and the Relationship between Thyrotropin Levels and Cardiovascular Risk Factors in Residents of the Coastal Area of China. Exp. Clin. Cardiol. 18 (1), 16–20. doi:10.1161/CIRCOUTCOMES.111.000039
Xu, Y. (2012). A Low fT3 Level as a Prognostic Marker in Patients with Acute Myocardial Infarctions. Jpn. J. Med. 51 (21), 3009–3015. doi:10.2169/internalmedicine.51.7902
Yazıcı, S., Kırış, T., Ceylan, U. S., Terzi, S., Erdem, A., Atasoy, I., et al. (2017). Yeşilçimen Relation of Low T3 to One-Year Mortality in Non–ST-elevation Acute Coronary Syndrome Patients. J. Clin. Lab. Anal. 31, e22036. doi:10.1002/jcla.22036
Zhang, B., Peng, W., Wang, C., Li, W., and Xu, Y. (2012). A Low fT3 Level as a Prognostic Marker in Patients with Acute Myocardial Infarctions. Jpn. J. Med. 51 (21), 3009–3015. doi:10.2169/internalmedicine.51.7902
Zhang, M., Sara, J. D., Yasushi, M., Hossein, G., Bell, M. R., Rajiv, G., et al. (2016). Clinical Outcomes of Patients with Hypothyroidism Undergoing Percutaneous Coronary Intervention. Eur. Heart J. 37 (26), 2055–2065. doi:10.1093/eurheartj/ehv737
Keywords: free triiodothyronine, coronary artery disease, pre-brain natriuretic peptide, fibrinogen, metabolism, risk model, prognostic markers
Citation: Tian X-x, Zheng S-f, Liu J-e, Wu Y-y, Lin L, Chen H-m, Li L-w, Qin M, Wang Z-x, Zhu Q, Lai W-h and Zhong S (2021) Free Triiodothyronine Connected With Metabolic Changes in Patients With Coronary Artery Disease by Interacting With Other Functional Indicators. Front. Mol. Biosci. 8:681955. doi: 10.3389/fmolb.2021.681955
Received: 17 March 2021; Accepted: 12 July 2021;
Published: 30 July 2021.
Edited by:
Michal Jan Markuszewski, Medical University of Gdansk, PolandReviewed by:
Gaurav Sharma, University of Texas Southwestern Medical Center, United StatesCopyright © 2021 Tian, Zheng, Liu, Wu, Lin, Chen, Li, Qin, Wang, Zhu, Lai and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shilong Zhong, emhvbmdzbEBob3RtYWlsLmNvbQ==; Wei-hua Lai, bGFpd2VpaHVheEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.