- 1Department of Orthopaedics, Tianjin First Central Hospital, Tianjin, China
- 2Department of Orthopaedics, Baodi Peopele’s Hospital, Tianjin, China
Background/Aims: LncRNAs are a new modulator in the development of intervertebral disc degeneration. However, the functional role and mechanism of HOXC13-AS in intervertebral disc degeneration remain unclear.
Methods: qRT-PCR analysis was performed to measure the relative expression levels of HOXC13-AS and miR-497-5p, and the levels of IL-1β, IL-6, and TNF-α in the medium supernatant were analyzed by ELISA. The related mechanism between HOXC13-AS and miR-497-5p was detected by luciferase assays.
Results: The results revealed that TNF-α and IL-1β induced HOXC13-AS expression in NP cells. HOXC13-AS was overexpressed in IDD specimens compared to control specimens, and higher expression of HOXC13-AS was correlated with high Pfirrmann scores. Ectopic expression of HOXC13-AS promoted MMP-3 and ADAMTS4 and inhibited aggrecan and collagen II expression in NP cells. Furthermore, overexpression of HOXC13-AS increased the expression of inflammatory cytokines, including IL-1β, IL-6, and TNF-α. Our results demonstrated that TNF-α and IL-1β induced ADAMTS5 expression and suppressed miR-497-5p expression. miR-497-5p was downregulated in IDD specimens compared to control specimens, and the lower expression of miR-497-5p was correlated with high Pfirrmann scores. The miR-497-5p level was negatively proportional to HOXC13-AS expression in IDD specimens. Luciferase analysis data indicated that ADAMTS5 was a direct target gene of miR-497-5p. HOXC13-AS induced inflammatory cytokine expression and ECM degradation by modulating miR-497-5p/ADAMTS5.
Conclusion: HOXC13-AS may be a treatment target for IDD.
Introduction
Low back pain (LBP) is a common disorder that is experimentally and clinically concerning (Setton and Chen, 2004; Seguin et al., 2008; Inoue and Espinoza Orias, 2011). The etiology of LBP is still unclear, and the major cause of LBP is IDD (intervertebral disc degeneration) (Roughley, 2004; Raj, 2008; Loreto et al., 2011). IDD is usually considered a natural process in intervertebral disc aging, but several cases have indicated accelerated disc degeneration according to genetic and environmental factors (Johnson and Roberts, 2003; Le Maitre et al., 2005; Li et al., 2012). Disc cells secrete anomalous inflammatory cytokines due to smoking, excessive biomechanical loading, genetic predisposition, aging, and decreased nutrient transport, which can result in disc cell apoptosis, autophagy and senescence (Furukawa et al., 2009; Clouet et al., 2011; Wang et al., 2015; Li et al., 2017). However, the detailed mechanisms of these processes remain unknown.
LncRNAs are thought to be more than 200 nt long with limited or no protein-coding capacity and are essential modulators in aspects of cell biology through regulation at the posttranscriptional, transcriptional, and chromatin organization levels (Zhang et al., 2018; Zhao et al., 2018; Zou et al., 2018; Yu et al., 2018; Cao et al., 2019; Li et al., 2019). References have noted that lncRNAs play roles in cell molecular functions such as cell differentiation, metastasis, apoptosis, and proliferation (Pan et al., 2018; Xiao et al., 2018; Xu et al., 2018; Yang et al., 2018). The dysregulation of lncRNAs occurs in most types of diseases, including scoliosis, Parkinson’s disease, osteosarcoma, and IDD (Xi et al., 2017; Xu et al., 2018; Boros et al., 2020; Li et al., 2020). Recently, Gao et al. (2019) noted that HOXC13-AS was upregulated in HNSC samples and that HOXC13-AS knockdown suppressed cell invasion, proliferation and invasion by modulating HMGA2/miR-383-3p. Li et al. (2019) noted that HOXC13-AS was overexpressed in breast tumor samples and that HOXC13-AS overexpression induced cell growth by sponging PTEN/miR-497-5p. However, the functional role and mechanism of HOXC13-AS in IDD remain unclear.
We found that HOXC13-AS was overexpressed in IDD specimens compared to control specimens and that HOXC13-AS induced inflammatory cytokine expression and ECM degradation.
Materials and Methods
Sample Selection and Cell Transfection
Human IVD specimens from IDD patients and vertebral fracture cases were collected from our hospital. All patients underwent lumbar MRI, and the degree of disc degeneration was analyzed using modified Pfirrmann scoring. The NP cell line was obtained from ScienCell (San Diego, California, United States, No. Catalog #4800) and was cultured in F12/DMEM supplemented with streptomycin, penicillin, and serum. siRNA-NC and ADAMTS5 siRNA, pcDNA-HOXC13-AS and pcDNA-control, miR-497-5p scramble, and the mimics were synthetized by GenePharma. These vectors were transfected into NP cells using Lipofectamine 2000. The study was approved by the ethics committee of Tianjin First Central Hospital and followed the Declaration of Helsinki. Written consents were obtained from all cases.
Luciferase Assays
miR-497-5p was predicted to link with the ADAMTS5 3′-UTR using TargetScan software. Wild-type (WT) and mutant (Mut)-type 3′ UTR fragments of ADAMTS5 were cloned by PCR. NP cells were cultured in 96-well dishes and cotransfected with Mut ADAMTS5 3′ UTR and WT ADAMTS5 3′ UTR and miR-497-5p scramble and mimic. After 48 h, the luciferase value was analyzed using a luciferase analysis kit (Promega).
ELISA
The levels of IL-1β, IL-6, and TNF-α in the medium supernatant were analyzed by ELISA (IL-1β, IL-6, and TNF-α, R&D Systems) following the manufacturer’s instructions.
qRT-PCR
A TRIzol kit (Invitrogen) was used to extract RNA from NP cells and specimens. RT-qPCR analysis was applied to study HOXC13-AS, mRNA, and miR-497-5p expression levels using SYBR Reagent (TaKaRa, Beijing) on an ABI 7300 PCR system (Applied Biosystems, MA). The PCR primers were as follows: HOXC13-AS qF: TCCCACGGCTTTCTTAGGTCA, HOXC13-AS qR: GACTCAATTCCACGGAGATGC; ADAMTS5 qF: GAGGATTTATGTGGGCATCATTCATGTG, ADAMTS5 qR: CATATGGTCCCAACGTCTGC; miR-497-5p qF: CAGCAGCACACTGTGGTTTGT; U6 qF: CTCGCTTCGGCAGCACA, qR: AACGCTTCACGAATTTGCGT; GAPDH qF: GCTCTCTGCTCCTCCTGTTC, qR: ACGACCAAATCCGTTGACTC. U6 was used as a control for miR-497-5p, and GAPDH was applied for other genes.
Statistical Assay
The results are expressed as the means ± SD. Statistical assays were carried out using SPSS, and significant differences were determined with Student’s t test. Spearman’s two-tailed correlation analysis was used for HOXC13-AS and miR-497-5p expression. p < 0.05 was set to be statistically significant.
Results
TNF-α and IL-1β Induced HOXC13-AS and ADAMTS5 Expression and Suppressed miR-497-5p Expression
First, we noted that treatments with TNF-α and IL-1β induced HOXC13-AS expression in a dose-dependent manner in NP cells (Figures 1A,B). The miR-497-5p expression levels were decreased in NP cells treated with TNF-α and IL-1β (Figures 1C,D). Moreover, treatments with TNF-α and IL-1β increased ADAMTS5 expression in a dose-dependent manner in NP cells (Figures 1E,F).
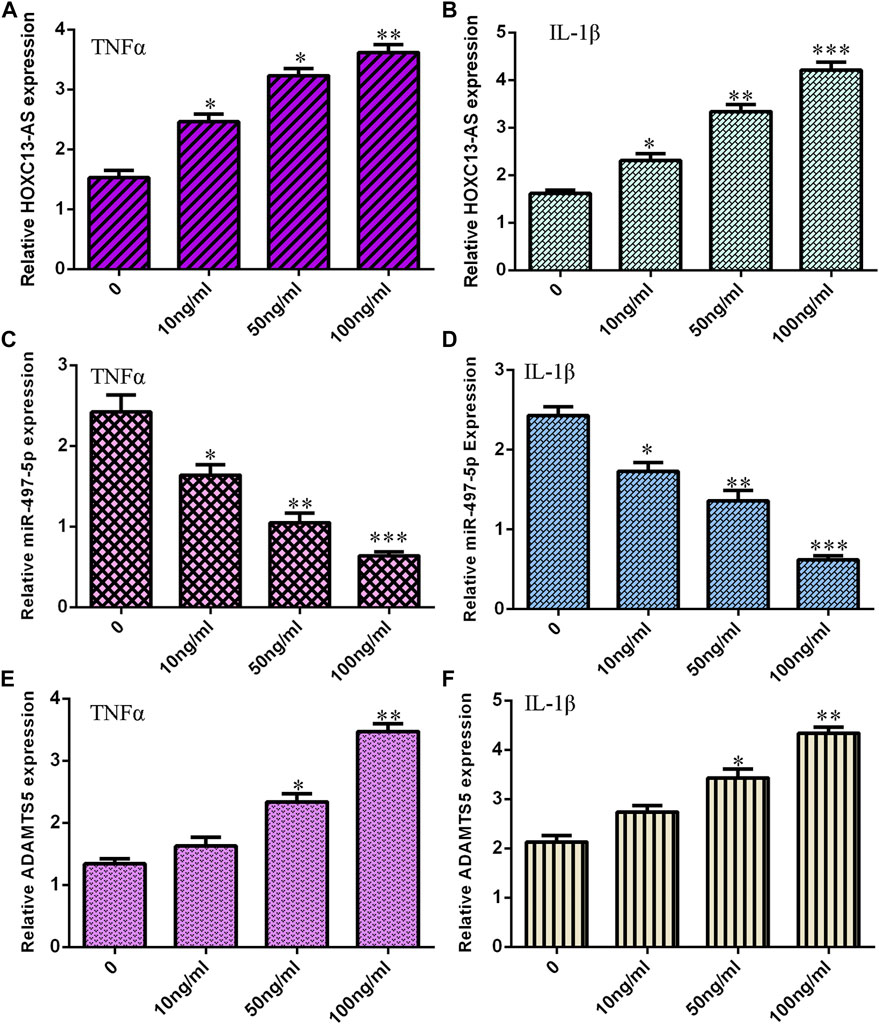
FIGURE 1. TNF-α and IL-1β induced HOXC13-AS and ADAMTS5 expression and suppressed miR-497-5p expression. (A) TNF-α induced HOXC13-AS expression in a dose-dependent manner in NP cells. (B) HOXC13-AS expression was measured by qRT-PCR. (C) miR-497-5p expression was decreased in NP cells treated with TNF-α. (D) miR-497-5p expression was detected by qRT-PCR. (E) TNF-α treatment increased ADAMTS5 expression in a dose-dependent manner in NP cells. (F) ADAMTS5 expression was determined by qRT-PCR. *p < 0.05, **p < 0.01, and ***p < 0.001.
HOXC13-AS was Upregulated in IDD Specimens
We then determined that HOXC13-AS was overexpressed in IDD specimens compared to control specimens by RT-qPCR (Figure 2A). Moreover, the higher expression of HOXC13-AS was correlated with high Pfirrmann scores (Figure 2B).
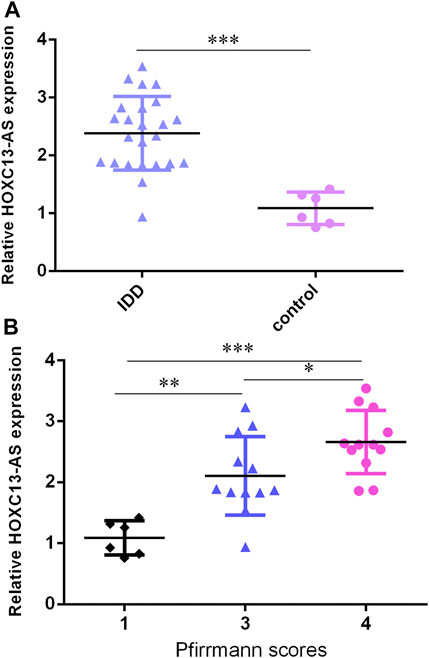
FIGURE 2. HOXC13-AS was upregulated in IDD specimens. (A) HOXC13-AS was overexpressed in IDD specimens compared to control specimens by RT-qPCR. (B) Higher expression of HOXC13-AS was correlated with high Pfirrmann scores. *p < 0.05, **p < 0.01, and ***p < 0.001.
miR-497-5p was Downregulated in IDD Specimens
Then, we found that miR-497-5p was downregulated in IDD specimens compared to control specimens by RT-qPCR (Figure 3A). Moreover, the lower expression of miR-497-5p was correlated with high Pfirrmann scores (Figure 3B). The miR-497-5p level was negatively proportional to HOXC13-AS expression in IDD specimens (Figure 3C).
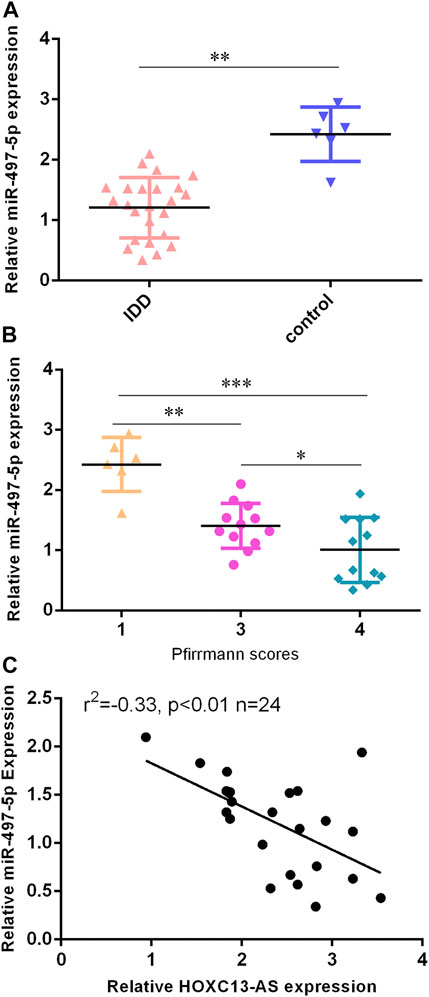
FIGURE 3. miR-497-5p was downregulated in IDD specimens. (A) miR-497-5p was downregulated in IDD specimens compared to control specimens by RT-qPCR. (B) Lower expression of miR-497-5p was correlated with high Pfirrmann scores. (C) miR-497-5p expression was negatively proportional to HOXC13-AS expression in IDD specimens. *p < 0.05, **p < 0.01, and ***p < 0.001.
miR-497-5p Targets ADAMTS5 Expression in NP Cells
We utilized TargetScan software to predict that miR-497-5p was linked to the ADAMTS5 3′-UTR (Figure 4A). miR-497-5p was obviously upregulated in NP cells after treatment with the miR-497-5p mimic (Figure 4B). Luciferase analysis data suggested that miR-497-5p overexpression inhibited the luciferase value of the wild-type reporter gene but not the mutated 3′UTR vector (Figure 4C). Ectopic miR-497-5p expression decreased ADAMTS5 levels in NP cells (Figure 4D). HOXC13-AS was obviously upregulated in NP cells after treatment with the pcDNA-HOXC13-AS vector (Figure 4E). Upregulation of HOXC13-AS expression inhibited miR-497-5p expression in NP cells (Figure 4F). Overexpression of HOXC13-AS suppressed ADAMTS5 expression in NP cells (Figure 4G).
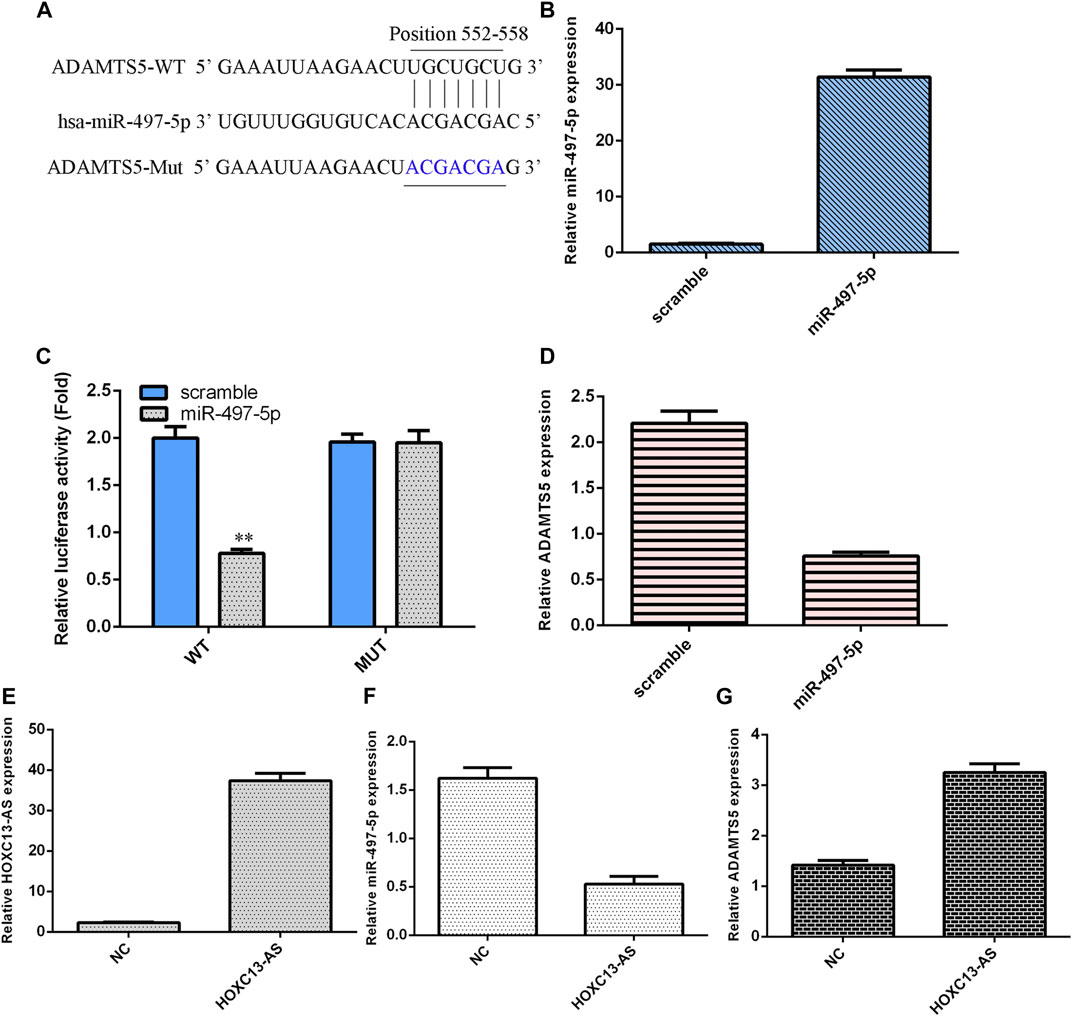
FIGURE 4. miR-497-5p targets ADAMTS5 expression in NP cells. (A) miR-497-5p was predicted to link with the ADAMTS5 3′-UTR using TargetScan software. (B) miR-497-5p was obviously upregulated in NP cells after treatment with the miR-497-5p mimic. (C) Luciferase analysis data showed that miR-497-5p overexpression inhibited the luciferase expression of the wild-type reporter gene but not the mutated 3′UTR vector. (D) Ectopic miR-497-5p expression decreased ADAMTS5 levels in NP cells. (E) HOXC13-AS expression was measured by qRT-PCR. (F) Upregulation of HOXC13-AS expression inhibited miR-497-5p expression in NP cells. (G) ADAMTS5 expression was measured by qRT-PCR. **p < 0.01.
HOXC13-AS Induced Inflammatory Cytokine Expression and ECM Degeneration
Ectopic expression of HOXC13-AS enhanced MMP-3 (Figure 5A) and ADAMTS4 (Figure 5B) expression in NP cells. Overexpression of HOXC13-AS decreased aggrecan (Figure 5C) and collagen II (Figure 5D) expression in NP cells. By ELISA, we determined that elevated expression of HOXC13-AS increased the expression levels of three inflammatory cytokines, IL-1β (Figure 5E), IL-6 (Figure 5F), and TNF-α (Figure 5G).
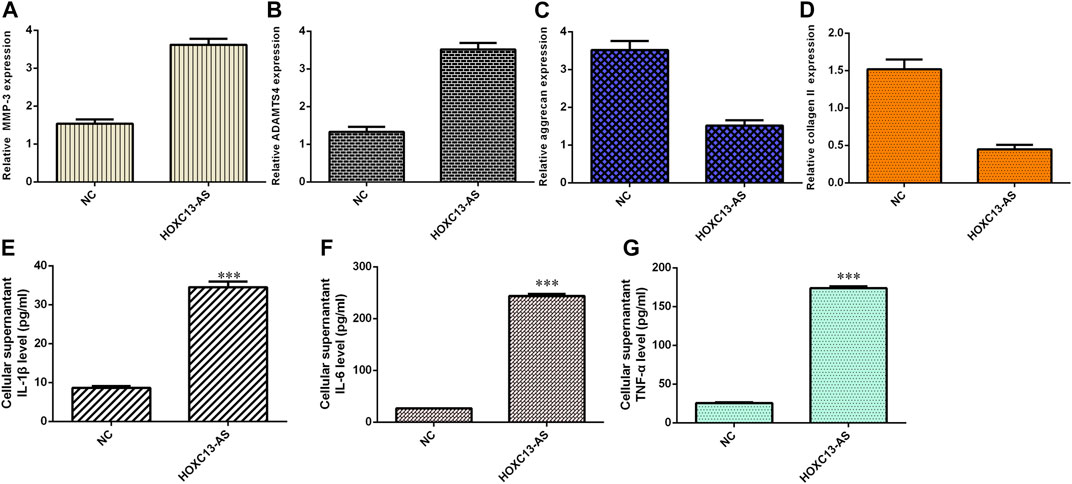
FIGURE 5. HOXC13-AS induced inflammatory cytokine expression and ECM degeneration. (A) MMP-3 expression was measured by qRT-PCR. (B) Ectopic expression of HOXC13-AS promoted ADAMTS4 expression in NP cells. (C) Aggrecan expression was determined by qRT-PCR. (D) Collagen II expression was measured by qRT-PCR. (E) IL-1β expression was determined by ELISA. (F) IL-6 expression was determined by ELISA. (G) TNF-α expression was determined by ELISA. ***p < 0.001.
miR-497-5p Suppressed Inflammatory Cytokine Expression and ECM Degeneration
Overexpression of miR-497-5p inhibited MMP-3 (Figure 6A) and ADAMTS4 (Figure 6B) expression in NP cells. Ectopic expression of miR-497-5p enhanced aggrecan (Figure 6C) and collagen II (Figure 6D) expression in NP cells. By ELISA, we showed that elevated expression of miR-497-5p increased the expression levels of three inflammatory cytokines, IL-1β (Figure 6E), IL-6 (Figure 6F), and TNF-α (Figure 6G).
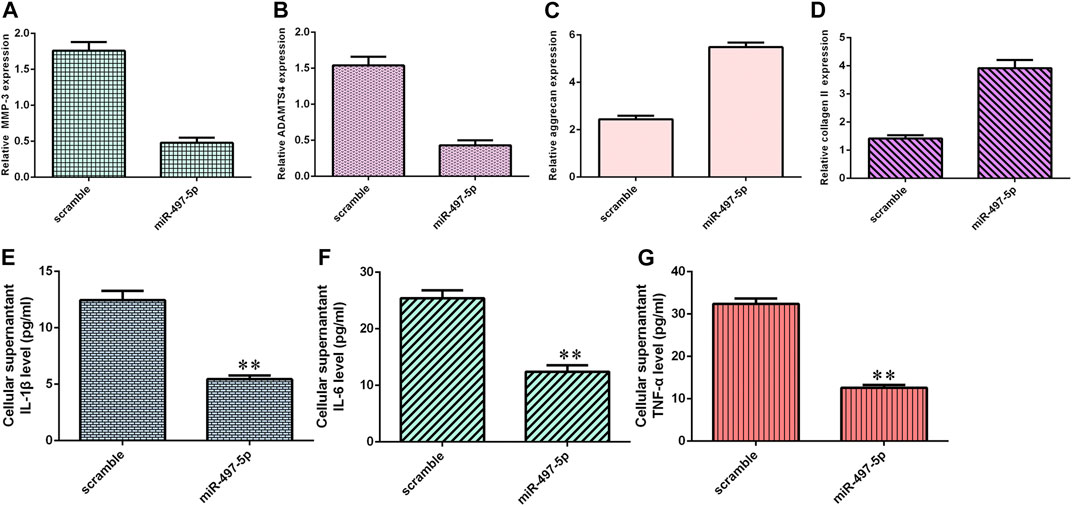
FIGURE 6. miR-497-5p suppressed inflammatory cytokine expression and ECM degeneration. (A) MMP-3 expression was detected by qRT-PCR. (B) Overexpression of miR-497-5p suppressed ADAMTS4 expression in NP cells. (C) Aggrecan expression was measured by qRT-PCR. (D) Collagen II expression was detected by qRT-PCR. (E) IL-1β expression was determined by ELISA. (F) IL-6 expression was determined by ELISA. (G) TNF-α expression was determined by ELISA. **p < 0.01.
HOXC13-AS Induced Inflammatory Cytokine Expression and ECM Degradation by Modulating miR-497-5p/ADAMTS5
We then explored the effects of three different treatment conditions on inflammatory cytokine expression and ECM degradation in NP cells. ADAMTS5 was obviously downregulated in NP cells after treatment with ADAMTS5 siRNA (Figure 7A). HOXC13-AS promoted ADAMTS4 and MMP-3 expression, while ADAMTS5 siRNA inhibited this function (Figures 7B,C). HOXC13-AS overexpression inhibited aggrecan and collagen II expression, while downregulation of ADAMTS5 expression enhanced this effect (Figures 7D,E). Elevated expression of HOXC13-AS promoted the expression of three inflammatory cytokines, IL-1β, IL-6, and TNF-α, while inhibition of ADAMTS5 expression decreased this effect (Figures 7F–H).
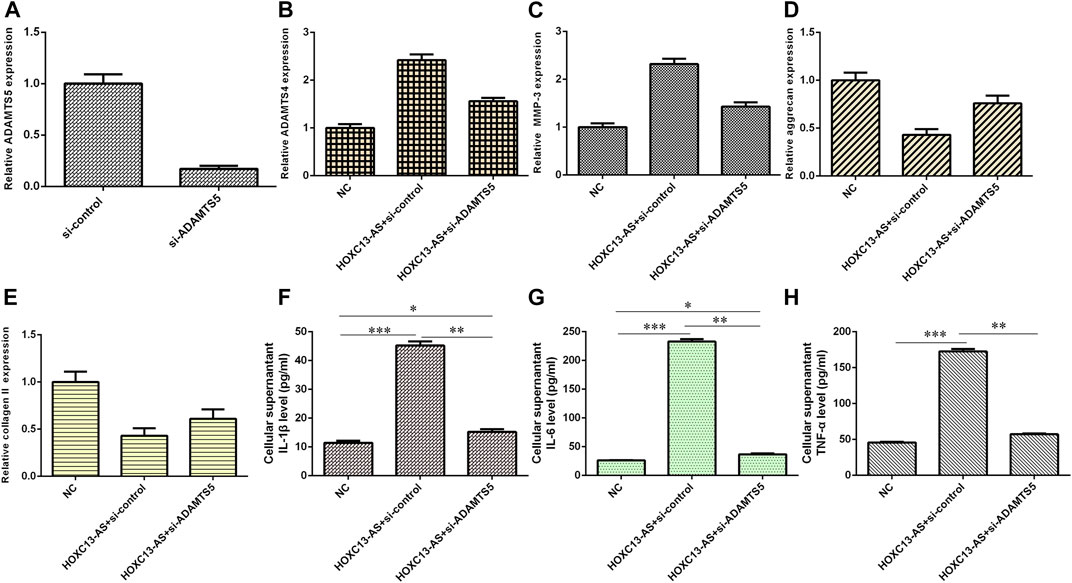
FIGURE 7. HOXC13-AS induced inflammatory cytokine expression and ECM degradation by modulating miR-497-5p/ADAMTS5. (A) ADAMTS5 was obviously downregulated in NP cells after treatment with ADAMTS5 siRNA. (B) ADAMTS4 expression was measured by qRT-PCR. (C) MMP-3 expression was measured by qRT-PCR. (D) Aggrecan expression was measured by qRT-PCR. (E) OXC13-AS overexpression inhibited collagen II expression, while downregulation of ADAMTS5 expression enhanced this effect. (F) IL-1β expression was determined by ELISA. (G) IL-6 expression was determined by ELISA. (H) TNF-α expression was determined by ELISA. *p < 0.05, **p < 0.01, and ***p < 0.001.
Discussion
Recently, abundant references have illustrated that lncRNA dysregulation is involved in the development of several diseases, including IDD (Li et al., 2018). In Ruan’s study, p53, p21, and NEAT1 were overexpressed in IDD samples, and ectopic expression of NEAT1 promoted ECM degradation by regulating MAPK/ERK1/2 pathway signaling expression (Ruan et al., 2018). Wang showed that linc-ADAMTS5 was negatively correlated with RREB1 to suppress ADAMTS5 and ECM degeneration in IDD (Wang K. et al., 2017). Moreover, Wang X. B. et al. (2017) found that RP11-296A18.3 was overexpressed in IDD samples and that RP11-296A18.3 knockdown decreased NP cell growth and ECM synthesis by modulating miR-138/HIF1A expression. Gao et al. (2019) noted that HOXC13-AS was upregulated in HNSC samples and that HOXC13-AS knockdown suppressed cell invasion, proliferation, and invasion by modulating HMGA2/miR-383-3p. Li X. W. et al. (2019) noted that HOXC13-AS was overexpressed in breast tumor samples and that HOXC13-AS overexpression induced cell growth by sponging PTEN/miR-497-5p. Our research revealed that TNF-α and IL-1β induced HOXC13-AS expression in NP cells. HOXC13-AS was overexpressed in IDD specimens compared to control specimens, and higher expression of HOXC13-AS was correlated with high Pfirrmann scores. Ectopic expression of HOXC13-AS promoted MMP-3 and ADAMTS4 and inhibited aggrecan and collagen II expression in NP cells. Overexpression of miR-497-5p suppressed inflammatory cytokine expression and ECM degeneration in NP cells. Furthermore, overexpression of HOXC13-AS increased the expression levels of three inflammatory cytokines, IL-1β, IL-6, and TNF-α.
LncRNAs act as posttranscriptional modulators of miRNA expression by functioning as “sponges” (Bian et al., 2017; Tian et al., 2018; Zhang et al., 2018; Cao et al., 2019). In line with previous data, we noted that upregulation of HOXC13-AS expression inhibited miR-497-5p expression in NP cells (Li et al., 2019). Furthermore, we utilized TargetScan software to predict that miR-497-5p was linked to the ADAMTS5 3′-UTR. Luciferase analysis data suggested that ADAMTS5 was a direct gene of miR-497-5p. Previous studies have suggested that ADAMTS5 plays critical roles in the progression of IDD (Ngo et al., 2017; Wang et al., 2018). In Seki’s study, they showed that ADAMTS5 siRNA injection inhibited NP sample degradation and ameliorated histologic and MRI grades (Seki et al., 2009). Zhao et al. (2011) indicated that IL-1β promoted ADAMTS-5 expression in NP cells. Our results illustrated that TNF-α and IL-1β induced ADAMTS5 expression and suppressed miR-497-5p expression. miR-497-5p was downregulated in IDD specimens compared to control specimens, and the lower expression of miR-497-5p was correlated with high Pfirrmann scores. The miR-497-5p expression level was negatively proportional to HOXC13-AS expression in IDD specimens. HOXC13-AS induced inflammatory cytokine expression and ECM degradation by modulating miR-497-5p/ADAMTS5.
To conclude, we found that HOXC13-AS was overexpressed in IDD specimens compared to control specimens and that HOXC13-AS induced inflammatory cytokine expression and ECM degradation by modulating miR-497-5p/ADAMTS5. These results suggest that HOXC13-AS may be a treatment target for IDD.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the ethics committee of Tianjin First Central Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WL and WJ have all contributed to the design and writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Bian, D., Shi, W., Shao, Y., Li, P., and Song, G. (2017). Long Non-coding RNA GAS5 Inhibits Tumorigenesis via miR-137 in Melanoma. Am. J. Transl Res. 9 (3), 1509–1520.
Boros, F. A., Maszlag-Török, R., Vécsei, R., and Klivényi, P. (2020). Increased Level of NEAT1 Long Non-coding RNA Is Detectable in Peripheral Blood Cells of Patients with Parkinson's Disease. Brain Res. 1730, 146672. doi:10.1016/j.brainres.2020.146672
Cao, C., Xu, Y., Du, K., Mi, C. Y., Yang, C. H., Xiang, L. L., et al. (2019). LINC01303 Functions as a Competing Endogenous RNA to Regulate EZH2 Expression by Sponging miR-101-3p in Gastric Cancer. J. Cell Mol. Med. 23 (11), 7342–7348. doi:10.1111/jcmm.14593
Clouet, J., Pot-Vaucel, M., Grimandi, G., Masson, M., Lesoeur, J., Fellah, B. H., et al. (2011). Characterization of the Age-dependent Intervertebral Disc Changes in Rabbit by Correlation between MRI, Histology and Gene Expression. BMC Musculoskelet. Disord. 12, 147. doi:10.1186/1471-2474-12-147
Furukawa, T., Ito, K., Nuka, S., Hashimoto, J., Takei, H., Takahara, M., et al. (2009). Absence of Biglycan Accelerates the Degenerative Process in Mouse Intervertebral Disc. Spine (Phila Pa 1976) 34 (25), E911–E917. doi:10.1097/brs.0b013e3181b7c7ec
Gao, C., Lu, W., Lou, W., Wang, L., and Xu, Q. (2019). Long Noncoding RNA HOXC13-AS Positively Affects Cell Proliferation and Invasion in Nasopharyngeal Carcinoma via Modulating miR-383-3p/HMGA2 axis. J. Cell Physiol. 234 (8), 12809–12820. doi:10.1002/jcp.27915
Inoue, N., and Espinoza Orias, A. A. (2011). Biomechanics of Intervertebral Disk Degeneration. Orthop. Clin. North. Am. 42(4): 487–499. doi:10.1016/j.ocl.2011.07.001
Johnson, W. E., and Roberts, S. (2003). Human Intervertebral Disc Cell Morphology and Cytoskeletal Composition: a Preliminary Study of Regional Variations in Health and Disease. J. Anat. 203 (6), 605–612. doi:10.1046/j.1469-7580.2003.00249.x
Le Maitre, C. L., Richardson, S. M., Baird, P., Freemont, A. J., and Hoyland, J. A. (2005). Expression of Receptors for Putative Anabolic Growth Factors in Human Intervertebral Disc: Implications for Repair and Regeneration of the Disc. J. Pathol. 207 (4), 445–452. doi:10.1002/path.1862
Li, X. W., Wang, Q., Rui, Y. Q., Zhang, C. Q., Wang, W. W., Gu, J. C., et al. (2019). HOXC13-AS Promotes Breast Cancer Cell Growth through Regulating miR-497-5p/PTEN axis. J. Cell Physiol. 234 (12), 22343–22351. doi:10.1002/jcp.28800
Li, Z., Li, X. Y., Chen, C., Chan, M. T. V., Wu, W. K. K., and Shen, J. X. (2017). Melatonin Inhibits Nucleus Pulposus (NP) Cell Proliferation and Extracellular Matrix (ECM) Remodeling via the Melatonin Membrane Receptors Mediated PI3K-Akt Pathway. J. Pineal Res. 63 (3). doi:10.1111/jpi.12435
Li, Z., Li, X. Y., Chen, C., Li, S. G., Shen, J. X., Tse, G., et al. (2018). Long Non-coding RNAs in Nucleus Pulposus Cell Function and Intervertebral Disc Degeneration. Cell Prolif 51 (5), e12483. doi:10.1111/cpr.12483
Li, Z., Li, X. Y., Chen, X., Li, S. G., Ho, I. H. T., Liu, X. D., et al. (2019). Emerging Roles of Long Non-coding RNAs in Neuropathic Pain. Cell Prolif 52 (1). doi:10.1111/cpr.12528
Li, Z., Shen, J., Wu, W. K. K., Yu, X., Liang, J., Qiu, G., et al. (2012). Leptin Induces Cyclin D1 Expression and Proliferation of Human Nucleus Pulposus Cells via JAK/STAT, PI3K/Akt and MEK/ERK Pathways. PLoS One 7 (12), e53176. doi:10.1371/journal.pone.0053176
Li, Z., Li, X., Shen, J., Zhang, L., Chan, M. T. V., and Wu, W. K. K. (2020). Emerging Roles of Non-coding RNAs in Scoliosis. Cel Prolif 53 (2), e12736. doi:10.1111/cpr.12736
Loreto, C., Musumeci, G., Castorina, A., and Martinez, G. (2011). Degenerative Disc Disease of Herniated Intervertebral Discs Is Associated with Extracellular Matrix Remodeling, Vimentin-Positive Cells and Cell Death. Ann. Anat. 193 (2), 156–162. doi:10.1016/j.aanat.2010.12.001
Ngo, K., Pohl, P., Wang, D., Leme, A. S., Lee, J., Di, P., et al. (2017). ADAMTS5 Deficiency Protects Mice from Chronic Tobacco Smoking-Induced Intervertebral Disc Degeneration. Spine (Phila Pa 1976) 42 (20), 1521–1528. doi:10.1097/brs.0000000000002258
Pan, Y., Wu, Y. J., Hu, J. L., Shan, Y. J., Ma, J., Ma, H. P., et al. (2018). Long Noncoding RNA HOTAIR Promotes Renal Cell Carcinoma Malignancy through Alpha-2, 8-sialyltransferase 4 by Sponging microRNA-124. Cell Prolif 51 (6), e12507. doi:10.1111/cpr.12507
Raj, P. P. (2008). Intervertebral Disc: Anatomy-Physiology-Pathophysiology-Treatment. Pain Pract. 8 (1), 18–44. doi:10.1111/j.1533-2500.2007.00171.x
Roughley, P. J. (2004). Biology of Intervertebral Disc Aging and Degeneration: Involvement of the Extracellular Matrix. Spine (Phila Pa 1976) 29 (23), 2691–2699. doi:10.1097/01.brs.0000146101.53784.b1
Ruan, Z., Ma, H., Li, J., Liu, H. Y., Jia, H. R., and Li, F. (2018). The Long Non-coding RNA NEAT1 Contributes to Extracellular Matrix Degradation in Degenerative Human Nucleus Pulposus Cells. Exp. Biol. Med. 243 (7), 595–600. doi:10.1177/1535370218760774
Seguin, C. A., Pilliar, R. M., Madri, J. A., and Kandel, R. A. (2008). TNF-alpha Induces MMP2 Gelatinase Activity and MT1-MMP Expression in an In Vitro Model of Nucleus Pulposus Tissue Degeneration. Spine (Phila Pa 1976) 33 (4), 356–365. doi:10.1097/brs.0b013e3181642a5e
Seki, S., Asanuma-Abe, Y., Masuda, K., Kawaguchi, Y., Asanuma, K., Muehleman, C., et al. (2009). Effect of Small Interference RNA (siRNA) for ADAMTS5 on Intervertebral Disc Degeneration in the Rabbit Anular Needle-Puncture Model. Arthritis Res. Ther. 11 (6), R166. doi:10.1186/ar2851
Setton, L. A., and Chen, J. (2004). Cell Mechanics and Mechanobiology in the Intervertebral Disc. Spine (Phila Pa 1976) 29 (23), 2710–2723. doi:10.1097/01.brs.0000146050.57722.2a
Tian, Chuang., Deng, Yuanyuan., Jin, Yong., Shi, Songshan., and Bi, Hai. (2018). Long Non-coding RNA RNCR3 Promotes Prostate Cancer Progression through Targeting miR-185-5p. Am. J. Transl Res. 10 (5), 1562–1570.
Wang, C., Wang, W. J., Yan, Y. G., Xiang, Y. X., Zhang, J., Tang, Z. H., et al. (2015). MicroRNAs: New Players in Intervertebral Disc Degeneration. Clin. Chim. Acta 450, 333–341. doi:10.1016/j.cca.2015.09.011
Wang, H., Hao, P., Zhang, H., Xu, C. P., and Zhao, J. Y. (2018). MicroRNA-223 Inhibits Lipopolysaccharide-Induced Inflammatory Response by Directly Targeting Irak1 in the Nucleus Pulposus Cells of Intervertebral Disc. IUBMB Life 70 (6), 479–490. doi:10.1002/iub.1747
Wang, K., Song, Y., Liu, W., Wu, X. H., Zhang, Y. K., Li, S. H., et al. (2017). The Noncoding RNA Linc-ADAMTS5 Cooperates with RREB1 to Protect from Intervertebral Disc Degeneration through Inhibiting ADAMTS5 Expression. Clin. Sci. 131 (10), 965–979. doi:10.1042/cs20160918
Wang, X. B., Lv, G. H., Li, J., Wang, B., Zhang, Q. S., and Lu, C. (2017). LncRNA-RP11-296A18.3/miR-138/HIF1A Pathway Regulates the Proliferation ECM Synthesis of Human Nucleus Pulposus Cells (HNPCs). J. Cell Biochem. 118 (12), 4862–4871. doi:10.1002/jcb.26166
Xi, Y. H., Jiang, T. W., Wang, W. H., Yu, J. M., Wang, Y., Wu, X. M., et al. (2017). Long Non-coding HCG18 Promotes Intervertebral Disc Degeneration by Sponging miR-146a-5p and Regulating TRAF6 Expression. Sci. Rep. 7 (1). doi:10.1038/s41598-017-13364-6
Xiao, M. Z., Feng, Y., Liu, C. D., and Zhang, Z. Y. (2018). Prognostic Values of Long Noncoding RNA PVT1 in Various Carcinomas: An Updated Systematic Review and Meta-Analysis. Cell Prolif 51 (6), e12519. doi:10.1111/cpr.12519
Xu, R. D., Feng, F., Yu, X. S., Liu, Z. D., and Lao, L. F. (2018). LncRNA SNHG4 Promotes Tumour Growth by Sponging miR-224-3p and Predicts Poor Survival and Recurrence in Human Osteosarcoma. Cell Prolif 51 (6), e12515. doi:10.1111/cpr.12515
Yang, C., Wu, K., Wang, S., and Wei, G. (2018). Long Non-coding RNA XIST Promotes Osteosarcoma Progression by Targeting YAP via miR-195-5p. J. Cell. Biochem. 119 (7), 5646–5656. doi:10.1002/jcb.26902
Yu, X., Zheng, H. Y., Tse, G., Zhang, L., and Wu, W. K. K. (2018). CASC2: An Emerging Tumour-Suppressing Long Noncoding RNA in Human Cancers and Melanoma. Cell Prolif 51 (6), e12506. doi:10.1111/cpr.12506
Zhang, J. M., Yin, M. N., Peng, G., and Zhao, Y. C. (2018). CRNDE: An Important Oncogenic Long Non-coding RNA in Human Cancers. Cell Prolif 51 (3), e12440. doi:10.1111/cpr.12440
Zhang, C., Su, C., Song, Q., Dong, F., Yu, S., and Huo, J. (2018). LncRNA PICART1 Suppressed Non-small Cell Lung Cancer Cells Proliferation and Invasion by Targeting AKT1 Signaling Pathway. Am. J. Transl Res. 10 (12), 4193–4201.
Zhao, C. Q., Zhang, Y. H., Jiang, S. D., Li, H., Jiang, L. S., and Dai, L. Y. (2011). ADAMTS-5 and Intervertebral Disc Degeneration: The Results of Tissue Immunohistochemistry and In Vitro Cell Culture. J. Orthopaedic Res. 29 (5), 718–725. doi:10.1002/jor.21285
Zhao, J., Gao, Z., Zhang, C., Wu, H., Gu, R., and Jiang, R. (2018). Long Non-coding RNA ASBEL Promotes Osteosarcoma Cell Proliferation, Migration and Invasion by Regulating microRNA-21. J. Cell. Biochem. 119 (8), 6461–6469. doi:10.1002/jcb.26671
Keywords: HOXC13-AS, intervertebral disc, miR-497-5p, ADAMTS5, lncRNA
Citation: Jing W and Liu W (2021) HOXC13-AS Induced Extracellular Matrix Loss via Targeting miR-497-5p/ADAMTS5 in Intervertebral Disc. Front. Mol. Biosci. 8:643997. doi: 10.3389/fmolb.2021.643997
Received: 19 March 2021; Accepted: 05 May 2021;
Published: 02 July 2021.
Edited by:
Yasuhito Ishigaki, Kanazawa Medical University, JapanReviewed by:
Kangcheng Zhao, Huazhong University of Science and Technology, ChinaZheng Li, Peking Union Medical College Hospital (CAMS), China
Copyright © 2021 Jing and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Liu, eXVndDEyQDE2My5jb20=
 Wanli Jing1
Wanli Jing1 Wei Liu
Wei Liu