
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci. , 12 August 2021
Sec. Molecular Diagnostics and Therapeutics
Volume 8 - 2021 | https://doi.org/10.3389/fmolb.2021.621883
 Haifeng Li1,2†
Haifeng Li1,2† Xin An1,2†
Xin An1,2† Riqing Huang1,2†
Riqing Huang1,2† Lu Li1,2
Lu Li1,2 Chengbiao Chu1,3
Chengbiao Chu1,3 Wei Yang1,2
Wei Yang1,2 Zike Qin1,4
Zike Qin1,4 Zhuowei Liu1,4
Zhuowei Liu1,4 Fangjian Zhou1,4
Fangjian Zhou1,4 Cong Xue1,2*
Cong Xue1,2* Yanxia Shi1,2*
Yanxia Shi1,2*Introduction: The use of antibodies against programmed death receptor-1 (PD-1) and its ligand (PD-L1) has improved survival in metastatic urothelial carcinoma (mUC) patients. However, reliable and convenient biomarkers of early responses and outcomes are still lacking.
Materials and Methods: We retrospectively screened mUC patients who received anti–PD-1/PD-L1–based therapy at our institute. A modified urothelium immune prognostic index (mUIPI) based on the neutrophil-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH) was developed to characterize the three groups as good, intermediate, and poor mUIPI. Major observations were progression-free survival (PFS), overall survival (OS), and disease control rate (DCR).
Results: We identified 52 mUC patients with a median follow-up time of 29.8 months (95% CI, 26.3–53.2). Low NLR was with improved PFS and OS (hazard ratio [HR], 0.40, 95% CI, 0.18–0.92; HR, 0.27, 95% CI, 0.11–0.69, respectively). Normal LDH was associated with improved PFS but not OS (HR, 0.22, 95% CI, 0.10–0.52; HR, 0.86, 95% CI, 0.34–2.13, respectively). The median PFS for the poor, intermediate, and good mUIPI groups was 1.97 months (95% CI, 1.15 to NR), 3.48 months (95% CI, 1.58 to NR), and 14.52 months (95% CI, 5.75 to NR), respectively (p < 0.001). The median OS for the poor, intermediate, and good mUIPI was 12.82, 18.11, and 34.87 months, respectively (p = 0.28). A good mUIPI was associated with a higher DCR compared to intermediate and poor mUIPI (odds ratio [OR] 7.58, 95% CI, 1.73–43.69; OR, 6.49, 95% CI, 0.14–295.42, respectively). In the subgroup analysis, a good mUIPI was associated with improved PFS in the subgroups of male patients and patients with low urinary tract primary tumors, liver metastases, non–first-line treatment, and monotherapy.
Conclusions: mUIPI predicts early responses in mUC patients who received anti–PD-1/PD-L1–based therapy.
Urothelial cancer (UC) is one of the most common malignant tumors of the urinary system in the world (Siegel et al., 2019). In China, UC incidence is 6.61/100,000, ranking sixth of all malignant tumors with 32,900 deaths annually (Health Commission of Prc, 2019). For now, platinum-based chemotherapy remains the standard first-line treatment for metastatic UC (mUC). However, the prognosis of mUC remains poor, with a median overall survival (OS) of 9–15 months (Bukhari et al., 2018).
Antibodies that block programmed cell death-1 (PD-1) or its ligand 1 (PD-L1) have changed the therapeutic landscape of mUC and are recommended for patients who progress after platinum-based chemotherapy or who are platinum-ineligible (Bellmunt et al., 2017; Elias et al., 2018; Resch et al., 2018; Niglio et al., 2019). However, benefit populations are usually stratified by the positive PD-L1 expression, which has various thresholds. In some circumstances, even with the negative PD-L1 expression, patients still responded to anti–PD-1/PD-L1 treatment (Powles et al., 2018). Therefore, the role of PD-L1 expression in the entire population remains unclear. Furthermore, under the current guideline, the results of PD-L1 expression are not necessary when considering anti–PD-1/PD-L1 therapy (Bukhari et al., 2018), and tumor mutation burden (TMB) and microsatellite instability (MSI-H) are also potential predictors associated with immunotherapy outcomes in melanoma and non–small-cell lung cancer (NSCLC) (Arora et al., 2019). However, the predictive roles of these two biomarkers in mUC are not well established. Furthermore, the inconvenience of these two biomarkers has compromised their advantages for the sequencing process. Therefore, identifying effective and convenient biomarkers for patients who will likely benefit from anti–PD-1/PD-L1 therapy is critical in clinical practice.
The inflammatory process is associated with immunoresistance in patients with malignancies by promoting cancer growth and activating oncogenic signaling (Hanahan and Weinberg, 2011), and pretreatment inflammatory status is associated with clinical outcomes in cancer patients (Laird et al., 2016). Routine peripheral blood parameters, such as absolute neutrophil count, absolute platelet count, and lactate dehydrogenase (LDH) level, serve as potential inflammatory biomarkers that are correlated with poor outcomes in several cancer types (McMillan, 2013). In melanoma and NSCLC, elevated baseline LDH is associated with poor outcome of anti–PD-1 therapy (Ferrucci et al., 2015; Diem et al., 2016; Nakaya et al., 2018; Arora et al., 2019). The neutrophil-to-lymphocyte ratio (NLR), a novel inflammatory biomarker, reportedly has independent prognostic values in patients with melanoma and NSCLC treated with immunotherapy (Arora et al., 2019). In recent years, combining NLR and LDH as a comprehensive predictor has extended their utility in several cancers. The Lung Immune Prognostic Index, based on NLR and LDH, was excellent in predicting the prognosis of patients with advanced NSCLC who received anti–PD-1/PD-L1 therapy (Sorich et al., 2019). Likewise, another similar index, the urothelial immune prognostic index, UIPI, also provided evidence of the utility of combining inflammatory biomarkers in mUC patients receiving PD-1/PD-L1 inhibitors (Banna et al., 2020). However, the optional cut point and the stratification strategy of UIPI are still unclear.
Since accumulating evidence has shown the potential of NLR and LDH in patients with mUC receiving immunotherapy, exploiting the utility of UIPI is an urgent need for stratifying the potential benefited patients. Herein, we reinvestigated the role of UIPI in mUC patients who received anti–PD-1/PD-L1–based therapy and made a modification to test the utility of its capability as an effect and convenient predictor.
Patients who had a histologically confirmed diagnosis of mUC (bladder, renal pelvis, or ureter) and were treated with anti–PD-1/PD-L1–based therapy at the Sun Yat-sen University Cancer Center (SYSUCC) between April 2015 and October 2019 were retrospectively identified.
Laboratory results, such as complete blood cell counts, LDH, and albumin levels, at baseline before anti–PD-1/PD-L1–based treatment (within 30 days before the first treatment) were collected from the medical records. Demographic, clinical, and pathological data were also collected. Data for PD-L1 expression were analyzed on tumor cells by immunohistochemistry using 22C3 antibody, and PD-L1 expression >1% was considered PD-L1 positive (Arora et al., 2019). Routine treatment response assessments were performed every two cycles using RECIST (response evaluation criteria in solid tumors) v1.1 (Eisenhauer et al., 2009).
Modified UIPI (mUIPI) was developed based on pretreatment NLR and LDH. The cut point of NLR was in agreement with that in previous reports, which used five at the baseline setting (Ferrucci et al., 2015; Banna et al., 2020), while LDH levels greater than 220U/L were considered to be elevated baseline LDH according to the upper limit of the central laboratory (Tan et al., 2018). Patients were divided into three groups by mUIPI. Good mUIPI, with low NLR and normal LDH; intermediate mUIPI, with high NLR or elevated LDH; and poor mUIPI, with high NLR and elevated LDH.
The primary endpoint was progression-free survival (PFS), which was defined as the time from the initiation of anti–PD-1/PD-L1–based therapy to the date of disease progression, death, or last date of follow-up. Another endpoint was overall survival (OS), which was defined as the time from the initiation of anti–PD-1/PD-L1–based therapy to the date of mortality. The disease control rate (DCR) was observed as the third endpoint which was defined as complete plus partial response plus stable disease (CR + PR + SD), and progression disease (PD) as non-DCR (Eisenhauer et al., 2009) using the criteria of RECIST version 1.1.
A descriptive analysis was performed. Categorical variables are presented as numbers and percentiles, while medians and ranges are reported for continuous variables. Comparisons between patient characteristics were performed using χ2 or Fisher’s exact test for discrete variables, and the unpaired t-test, the Wilcoxon sign rank test, or analysis of variance for continuous variables. PFS and OS were estimated using the Kaplan–Meier method. Between-group differences in PFS and OS were calculated using the Cox regression model and the log-rank test. Hazard ratios (HRs) and associated 95% confidence intervals (CIs) were calculated using Efron’s method of handling ties. The odds ratio of the DCR was calculated by conditional maximum likelihood estimation (Fisher), and the 95% CI was calculated using exact methods. p-values for all two-sided tests are reported, and p-values less than 0.05 were considered statistically significant. All statistical analyses were performed using R software version 3.5.3.
Fifty-two patients treated with PD-1/PD-L1 inhibitors between April 2015 and October 2019 were included in this study. Baseline characteristics are summarized in Table 1 .With a median follow-up time of 15.5 months (95% CI, 9.8 to 36.6), median PFS was 6.2 months (95% CI, 4.2 to not reached [NR]) (Figure 1A), median OS was 20.53 months (95% CI, 15.3 to not reached [NR]) (Figure 2A). Median patient age was 62 years (range, 34–84). The majority of patients were male (N = 36, 69.2%). Twenty patients (61.54%) were former or current smokers, 32 (61.54%) exhibited liver metastasis, and 10 (19.23%) had an Eastern Cooperative Oncology Group (ECOG) performance status of two or greater. Thirty-one patients (59.61%) exhibited a lower urinary tract primary tumor. In the treatment strategy distribution, 24 patients (46.15%) were first-line setting, and 29 patients (55.76%) were combined with chemotherapy. With 16 available slices of the tumor sample, four patients were considered PD-L1 positive, and PD-L1 expression was not associated with immunotherapy outcomes in this cohort.
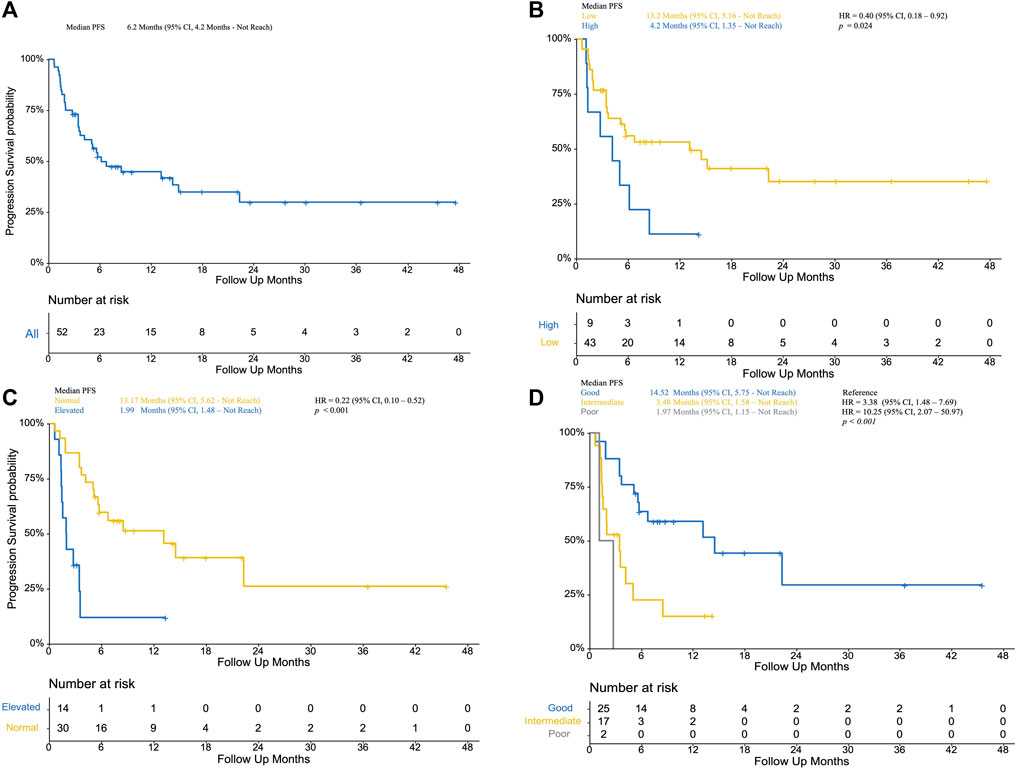
FIGURE 1. (A): Progression-free survival of included patients; (B) Kaplan–Meier curve of low NLR patients (yellow) and high NLR (blue, as reference) patients; (C) Kaplan–Meier curve of normal LDH (yellow) and elevated LDH (blue, as reference); (D) Kaplan–Meier curve of poor mUIPI (gray), intermediate mUIPI (yellow), and good mUIPI (blue, as reference).
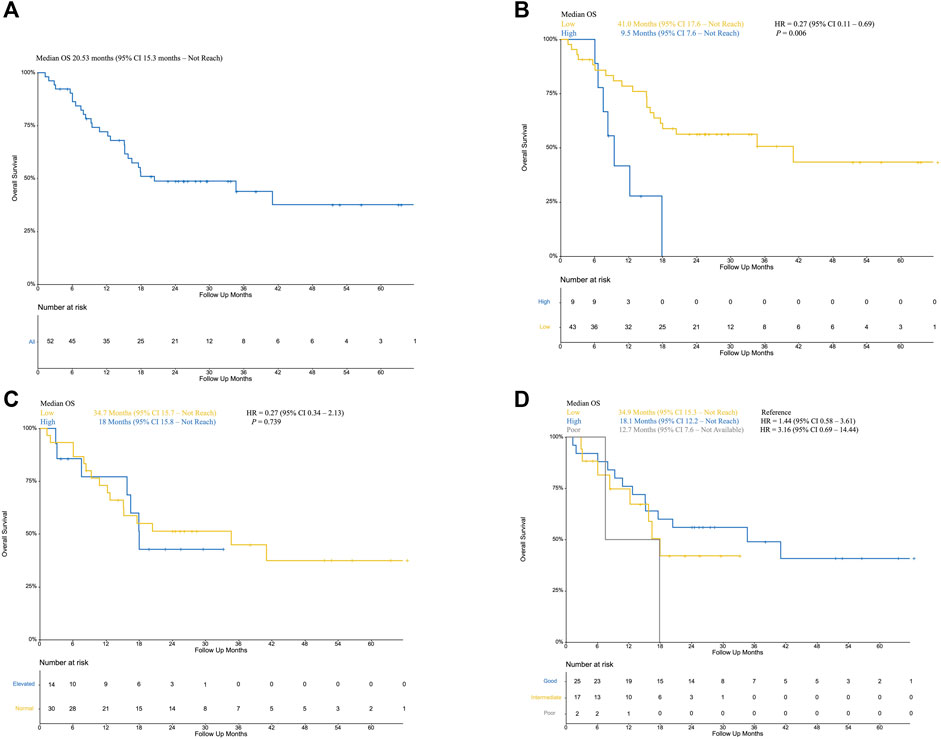
FIGURE 2. (A): Overall survival of included patients; (B) Kaplan–Meier curve of low NLR patients (yellow) and high NLR (blue, as reference) patients; (C) Kaplan–Meier curve of normal LDH (yellow) and elevated LDH (blue, as reference); (D) Kaplan–Meier curve of poor mUIPI (gray), intermediate mUIPI (yellow), and good mUIPI (blue, as reference).
The median level of NLR was 3.01, ranging from 1.03–7.66. In the predefined context above, forty-three patients were classified as low NLR, which was associated with improved PFS (13.2 VS 4.2 months, p = 0.024; HR, 0.40; 95% CI, 0.18–0.92) (Figure 1B) and improved OS (41.0 VS 9.5 months, p = 0.006; HR, 0.27; 95% CI, 0.11–0.69) (Figure 2B) but was not associated with higher DCR (odds ratio [OR], DCR VS non-DCR, 1.46, 95% CI, 0.26–6.89, p = 0.69) (Table 2). As for the LDH level, thirty patients had normal LDH, which was associated with improved PFS (13.17 VS 1.99 months, p < 0.001; HR, 0.22; 95% CI, 0.08–0.55, p < 0.001) (Figure 1C) and higher DCR (OR, DCR VS non-DCR, 10.84, 95% CI, 2.08–70.67, p < 0.001) (Table 2) but was not statistically associated with improved OS (34.7 VS 18 months, p = 0.739; HR, 0.27; 95% CI, 0.34–2.13) (Figure 2C).
In a multivariate analysis, among variables with p-values less than 0.1 or with clinical meaning, normal LDH (HR, 0.19; 95% CI, 0.07–0.48, p < 0.001) and first-line treatment (HR, 0.35; 95% CI, 0.14–0.91, p = 0.03) were independently associated with improved PFS, while low NLR (HR, 0.49; 95% CI, 0.18–1.36, p = 0.17) was not. However, the low NLR was an independent predictor in OS (HR, 0.30; 95% CI, 0.12–0.79, p = 0.014).
In the subgroup analysis, NLR and LDH had performed well in predicting clinical outcomes of patients treated with anti–PD-1/PD-L1 monotherapy in PFS and OS. (low NLR, HR, 0.343 for PFS, 0.128 for OS; normal LDH, HR, 0.042 for PFS, 0.194 for OS) (Supplementary Table1). And low NLR was also associated with improved survival outcomes in patients with liver metastases (Supplementary Table1).
Pretreatment NLR and LDH were combined with the calculation of mUIPI. Eight patients without baseline LDH were excluded from the UIPI analysis. Among 44 evaluable patients, 25 (56.81%) had good mUIPI, 17 (38.64%) had intermediate mUIPI, and 2 (4.55%) had poor mUIPI (Table 3).
The median PFS was 1.97 months (95% CI, 1.15 month to NR) vs. 3.48 months (95% CI, 1.58 months to NR) vs. 14.52 months (95% CI, 5.75 months to NR) for the poor, intermediate, and good mUIPI groups, respectively (p < 0.001) (Figure 1D). The median OS of each group was 12.7, 18.1, and 34.9 months, respectively. (Figure 2D). A good mUIPI was associated with higher DCR compared to intermediate and poor mUIPI (OR, non-DCR VS DCR, 0.13, 95% CI, 0.02–0.58; OR, 0.15, 95% CI, 0.003–7.04, respectively) (Table 2). In a multivariate analysis, intermediate and poor mUIPI groups were independently associated with shorter PFS (HR, 3.38, 95% CI, 1.48–7.69; HR, 10.25, 95% CI, 2.03–20.97, compared to good mUIPI, respectively). Therapy line was also associated with improved PFS (first-line VS ≥ second-line, HR, 0.39, 95% CI, 0.15–0.97) (Table 4). However, mUIPI failed to cross the statistical boundary in the cox regression analysis (Table 5).
In the subgroup analysis, good mUIPI was associated with improved PFS in male, current/former smoker, low-tract primary tumor, ECOG of 0–1 and two to three, liver and non-liver metastases, non–first-line treatment, and monotherapy subgroups (Figure 3). Meanwhile, good mUIPI was associated with higher DCR in male, low-tract primary tumor, liver metastases, non–first-line treatment, and monotherapy subgroups. As for the subgroup analysis of OS, good mUIPI was associated with prolonged OS in the subgroup of monotherapy (Figure 4).
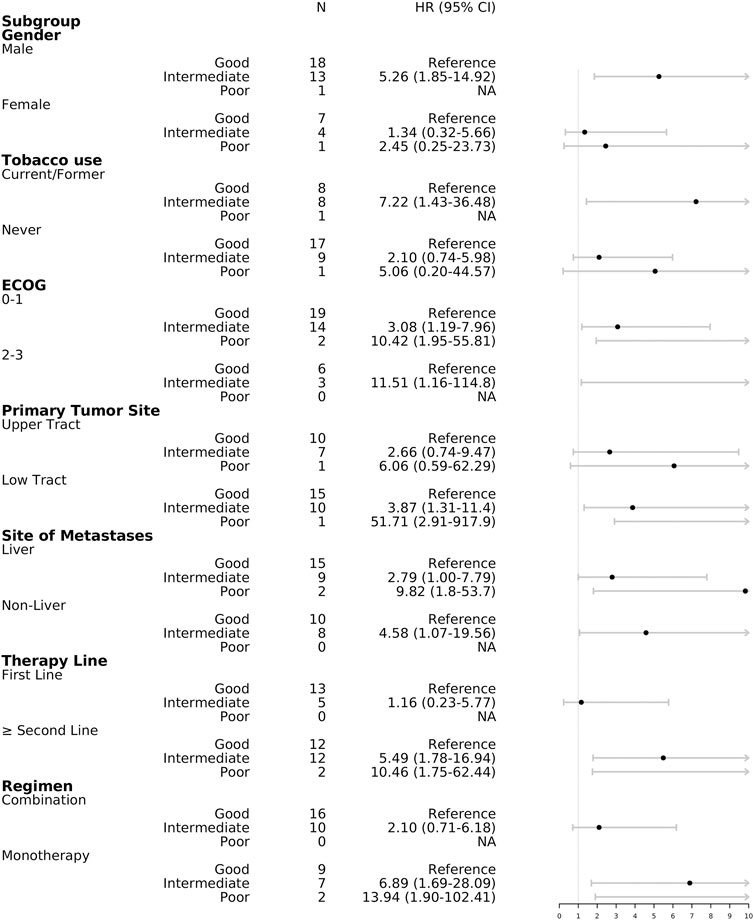
FIGURE 3. Forest plot of hazard ratios in the subgroup analysis comparing progression-free survival of mUIPI.

FIGURE 4. Forest plot of hazard ratios in the subgroup analysis comparing overall survival of mUIPI.
In the anti–PD-1/PD-L1 monotherapy subgroup, good mUIPI was associated with improved PFS, OS, as well as higher DCR. Median PFS was 1.97 months (95% CI, 1.15 month to NR) vs. 2.00 months (95% CI, 1.48 months to NR) vs. 22.34 months (95% CI, 5.94 months to NR) for poor, intermediate, and good mUIPI groups, respectively (p < 0.001), and median OS was 12.7 months (95% CI, 7.6 months to NR) vs. 15.8 months (95% CI, 8.4 months to NR) vs. NR (95% CI, 41.0 months to NR), respectively (p = 0.017). Furthermore, patients with good mUIPI had a higher DCR (good vs. intermediate, OR, 14.52, 95% CI, 1.34–523.98; good vs. poor, OR, 6.00, 95% CI, 0.10–342.93). In the multivariate analysis, good mUIPI was independently associated with improved PFS (intermediate vs. good, HR, 13.74, 95% CI, 1.46–129.08; poor vs. good, HR, 8.89, 95% CI, 1.09–72.57) and OS (intermediate vs. good, HR, 7.95, 95% CI, 1.14–55.61; poor vs. good, HR, 8.57, 95% CI, 1.11–66.26), whereas in the combined therapy subgroup, treatment outcomes were not statistically significantly correlated with mUIPI.
In the present study, we reinvestigated the utility of UIPI and developed the modified UIPI, based on a definite cut point of pretreatment NLR and LDH, which was significantly associated with PFS and short-term treatment response in patients with mUC treated with anti–PD-1/PD-L1–based therapy, exerting utility in several subgroups. Based on mUIPI, patients were stratified into three groups: good, intermediate, and poor mUIPI. Specifically, good mUIPI was significantly associated with prolonged PFS and higher DCR, indicating that it might represent a predictor for identifying populations that will benefit from anti–PD-1/PD-L1–based treatment.
In patients with malignancies, inflammatory status was closely correlated with poor clinical outcomes (Laird et al., 2016). With the advent of immune checkpoint inhibitors (ICIs), especially PD-1/PD-L1 inhibitors, inflammatory status is also associated with immunotherapy benefit (Arora et al., 2019). Several potential inflammatory biomarkers, including elevated neutrophils, lymphocytes, platelets, and LDH, are associated with poor outcomes in cancer. NLR, a novel parameter, is a well-known prognostic factor in cancer patients with late-stage melanoma or NSCLC treated with ICIs. In a retrospective cohort of 187 metastatic melanoma patients treated with ipilimumab, pretreatment NLR less than five was associated with improved survival (Ferrucci et al., 2015). In another retrospective cohort of 101 advanced NSCLC patients treated with nivolumab, high NLR 2 and 4 weeks after treatment was associated with poor treatment response and PFS (Nakaya et al., 2018). dNLR has also been investigated as a useful predictor of clinical outcomes in various cancer types, including melanoma, pancreas, and bladder cancer. In a retrospective study of 720 melanoma patients, dNLR of three or greater was independently associated with worse survival in patients treated with ipilimumab (Ferrucci et al., 2018). However, in a cohort of 123 muscle-invasive bladder cancer patients, elevated dNLR (>2.21) was correlated with nonresponse to preoperative chemotherapy (OR 2.70, 95% CI, 1.15–6.38) but was not statistically significantly associated with poor PFS or OS (van Kessel et al., 2016). In the present study, we found that NLR of five or greater was significantly correlated with inferior PFS and OS in mUC patients treated with anti–PD-1/PD-L1–based therapy. Furthermore, in the multivariate analysis, we reveal that high NLR was independently associated with inferior OS although it failed to cross the statistical boundary in PFS.
The serum LDH level is a standardized and convenient inflammatory biomarker in patients with cancer that is widely studied in the era of chemotherapy (Petrelli et al., 2015). Recently, elevated baseline LDH levels were been found to be associated with poor survival and a decreased response rate in patients treated with ICIs (Arora et al., 2019). In a cohort of 668 patients with upper tract urothelial carcinoma, preoperative elevated LDH was found to be correlated with worse OS in patients with localized disease (Tan et al., 2018). In the present study, we demonstrated that LDH represents an independent prognostic factor in patients with mUC treated with anti–PD-1/PD-L1–based therapy because normal LDH was associated with significantly improved PFS and treatment response. Therefore, LDH levels could be a useful and convenient predictor of response to immunotherapy.
Combining biomarkers, such as CII and LIPI, exploited the potential of NLR and LDH in colorectal and lung cancer (Casadei-Gardini et al., 2019; Sorich et al., 2019). In a similar previous study, UIPI was able to stratify benefited mUC patients treated with immunotherapy. However, the cut point and optional classification of UIPI remained inconclusive. In the present study, we modified the UIPI based on a definite cut point of pretreatment NLR and LDH. The mUIPI was able to identify three populations and showed that good mUIPI was significantly associated with superior immunotherapy outcomes. Furthermore, in patients treated with PD-1/PD-L1 inhibitors alone, mUIPI was also capable to identify a potential benefit subgroup, and it was useful for physicians to prescribe immunotherapy since patients with mUC usually suffer from renal function impairment, which is a contraindication for various cytotoxic drugs. Nevertheless, mUIPI is easy to extract from the routine blood parameters, while other prognostic factors, such as PD-L1 expression, are not definitively correlated with treatment outcomes in urothelial cancer and are not mandatory for prescribing the immunotherapy under the current guidelines. In addition, TMB, which was associated with immunotherapy outcomes in urothelial cancer, is limited by a complicated and expensive sequencing process. Therefore, UIPI, with its convenient generation and reliability, could be used to identify patients who might benefit from anti–PD-1/PD-L1–based therapy.
Combining PD-1/PD-L1 inhibitors with conventional chemotherapy is a recent and intriguing topic. IMvigor 130 was designed to investigate this modality. With the recent release of preliminary results, the study demonstrated that adding atezolizumab to platinum-based chemotherapy (PBC) improved PFS over PBC alone (Galsky et al., 2020). Regrettably, due to the PFS data of atezolizumab being absent, whether atezolizumab plus PBC provides a PFS advantage over atezolizumab alone in untreated mUC patients remained unknown. Meanwhile, in our study, combination therapy was not superior to monotherapy. However, the results might be comprised since the precisions of the combination therapy in our study consisted of various chemotherapy regimens rather than single PBC. In addition, some patients in our study were heavily treated, and some patients had a worse performance status and vulnerable renal function, which might have contributed to this dubious result. With ongoing treatment, cytotoxic drugs might further exacerbate renal malfunction, which might result in a worse prognosis for mUC patients. Therefore, more trials are needed to complete the picture and to identify populations who will benefit most from immunotherapy.
The nature of retrospective data is the primary limitation of our study, including missing clinical and laboratory data, as well as a high rate of patients with an unknown PD-L1 status. Another limitation was the small sample size of the cohort, which might lead to an underpower of this study, thus compromising the predicting strength of mUIPI. Also, the heterogeneity of chemotherapy regimens in the combination groups compromised the results of this study.
In summary, we demonstrated that mUIPI, based on pretreatment NLR and LDH levels, is correlated with treatment outcomes of anti–PD-1/PD-L1–based therapy in patients with mUC.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation. The key raw data has been deposited onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDA2021002042.
The studies involving human participants were reviewed and approved by Sun Yat-sen University Cancer Center Ethics Committees. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HL: writing—original draft, data curation, and statistical analysis; CX and FZ: conceptualization and analysis; RH and CC: writing; LL, ZQ, and WY: data curation; XA and ZL: writing—reviewing and editing; CX and YS: conceptualization, design, supervision, and writing—reviewing and editing.
This work was supported by the National Natural Science Foundation of China (81773279, 82073391 to YS), Science and Technology Planning Project of Guangdong Province (2016A050502015, 2013B021800062, and 2012B061700082 to YS), Young Teacher Foundation of Sun Yat-sen University (17ykzd33 to YS), and the Youth Program of National Natural Science Foundation of China (81702676 to CX).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmolb.2021.621883/full#supplementary-material
CII, colon inflammatory index; DCR, disease control rate; dNLR, derived NLR; ECOG PS, ECOG performance-status score; HR, hazard ratio; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; mUC, metastatic urothelial carcinoma; MSI-H, microsatellite instability; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non–small-cell lung cancer; OR, odds ratio; OS, overall survival; PFS, progression-free survival; PD-1, programmed cell death 1; PD-L1, programmed cell death 1 ligand 1; TMB, tumor mutation burden; UIPI, urothelium immune prognostic index.
Arora, S., Velichinskii, R., Lesh, R. W., Ali, U., Kubiak, M., Bansal, P., et al. (2019). Existing and Emerging Biomarkers for Immune Checkpoint Immunotherapy in Solid Tumors. Adv. Ther. 36 (10), 2638–2678. doi:10.1007/s12325-019-01051-z
Banna, G. L., Di Quattro, R., Malatino, L., Fornarini, G., Addeo, A., Maruzzo, M., et al. (2020). Neutrophil-to-lymphocyte Ratio and Lactate Dehydrogenase as Biomarkers for Urothelial Cancer Treated with Immunotherapy. Clin. Transl Oncol. 22 (11), 2130–2135. doi:10.1007/s12094-020-02337-3
Bellmunt, J., Powles, T., and Vogelzang, N. J. (2017). A Review on the Evolution of PD-1/pd-L1 Immunotherapy for Bladder Cancer: The Future Is Now. Cancer Treat. Rev. 54, 58–67. doi:10.1016/j.ctrv.2017.01.007
Bukhari, N., Al-Shamsi, H. O., and Azam, F. (2018). Update on the Treatment of Metastatic Urothelial Carcinoma. Scientific World J. 2018, 1–7. doi:10.1155/2018/5682078
Casadei-Gardini, A., Scarpi, E., Ulivi, P., Palladino, M. A., Accettura, C., Bernardini, I., et al. (2019). Prognostic Role of a New Inflammatory index with Neutrophil-To-Lymphocyte Ratio and Lactate Dehydrogenase (CII: Colon Inflammatory Index) in Patients with Metastatic Colorectal Cancer: Results from the Randomized Italian Trial in Advanced Colorectal Cancer (ITACa) Study. Cancer Manag. Res. 11, 4357–4369. doi:10.2147/CMAR.S198651
Diem, S., Kasenda, B., Spain, L., Martin-Liberal, J., Marconcini, R., Gore, M., et al. (2016). Serum Lactate Dehydrogenase as an Early Marker for Outcome in Patients Treated with Anti-PD-1 Therapy in Metastatic Melanoma. Br. J. Cancer 114 (3), 256–261. doi:10.1038/bjc.2015.467
Eisenhauer, E. A., Therasse, P., Bogaerts, J., Schwartz, L. H., Sargent, D., Ford, R., et al. (2009). New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 45 (2), 228–247. doi:10.1016/j.ejca.2008.10.026
Elias, R., Giobbie-Hurder, A., McCleary, N. J., Ott, P., Hodi, F. S., and Rahma, O. (2018). Efficacy of PD-1 & PD-L1 Inhibitors in Older Adults: a Meta-Analysis. J. Immunotherapy Cancer 6 (1), 26. doi:10.1186/s40425-018-0336-8
Ferrucci, P. F., Ascierto, P. A., Pigozzo, J., Del Vecchio, M., Maio, M., Antonini Cappellini, G. C., et al. (2018). Baseline Neutrophils and Derived Neutrophil-To-Lymphocyte Ratio: Prognostic Relevance in Metastatic Melanoma Patients Receiving Ipilimumab. Ann. Oncol. 29 (2), 524. doi:10.1093/annonc/mdx059
Ferrucci, P. F., Gandini, S., Battaglia, A., Alfieri, S., Di Giacomo, A. M., Giannarelli, D., et al. (2015). Baseline Neutrophil-To-Lymphocyte Ratio Is Associated with Outcome of Ipilimumab-Treated Metastatic Melanoma Patients. Br. J. Cancer 112 (12), 1904–1910. doi:10.1038/bjc.2015.180
Galsky, M. D., Arija, J. Á. A., Bamias, A., Davis, I. D., De Santis, M., Kikuchi, E., et al. (2020). Atezolizumab with or without Chemotherapy in Metastatic Urothelial Cancer (IMvigor130): a Multicentre, Randomised, Placebo-Controlled Phase 3 Trial. The Lancet 395, 1547–1557. doi:10.1016/s0140-6736(2030230-0)
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of Cancer: the Next Generation. Cell 144 (5), 646–674. doi:10.1016/j.cell.2011.02.013
Health Commission of Prc, N. (2019). Chinese Guidelines for Diagnosis and Treatment of Urothelial Carcinoma of Bladder 2018 (English Version). Chin. J. Cancer Res. 31 (1), 49–66. doi:10.21147/j.issn.1000-9604.2019.01.03
Laird, B. J. A., Fallon, M., Hjermstad, M. J., Tuck, S., Kaasa, S., Klepstad, P., et al. (2016). Quality of Life in Patients with Advanced Cancer: Differential Association with Performance Status and Systemic Inflammatory Response. Jco 34 (23), 2769–2775. doi:10.1200/JCO.2015.65.7742
McMillan, D. C. (2013). The Systemic Inflammation-Based Glasgow Prognostic Score: a Decade of Experience in Patients with Cancer. Cancer Treat. Rev. 39 (5), 534–540. doi:10.1016/j.ctrv.2012.08.003
Nakaya, A., Kurata, T., Yoshioka, H., Takeyasu, Y., Niki, M., Kibata, K., et al. (2018). Neutrophil-to-lymphocyte Ratio as an Early Marker of Outcomes in Patients with Advanced Non-small-cell Lung Cancer Treated with Nivolumab. Int. J. Clin. Oncol. 23 (4), 634–640. doi:10.1007/s10147-018-1250-2
Niglio, S. A., Jia, R., Ji, J., Ruder, S., Patel, V. G., Martini, A., et al. (2019). Programmed Death-1 or Programmed Death Ligand-1 Blockade in Patients with Platinum-Resistant Metastatic Urothelial Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. 76 (6), 782–789. doi:10.1016/j.eururo.2019.05.037
Petrelli, F., Cabiddu, M., Coinu, A., Borgonovo, K., Ghilardi, M., Lonati, V., et al. (2015). Prognostic Role of Lactate Dehydrogenase in Solid Tumors: a Systematic Review and Meta-Analysis of 76 Studies. Acta Oncologica 54 (7), 961–970. doi:10.3109/0284186X.2015.1043026
Powles, T., Durán, I., van der Heijden, M. S., Loriot, Y., Vogelzang, N. J., De Giorgi, U., et al. (2018). Atezolizumab versus Chemotherapy in Patients with Platinum-Treated Locally Advanced or Metastatic Urothelial Carcinoma (IMvigor211): a Multicentre, Open-Label, Phase 3 Randomised Controlled Trial. The Lancet 391 (10122), 748–757. doi:10.1016/s0140-6736(17)33297-x
Resch, I., Shariat, S. F., and Gust, K. M. (2018). PD-1 and PD-L1 Inhibitors after Platinum-Based Chemotherapy or in First-Line Therapy in Cisplatin-Ineligible Patients. Memo 11 (1), 43–46. doi:10.1007/s12254-018-0396-y
Siegel, R. L., Miller, K. D., and Jemal, A. (2019). Cancer Statistics, 2019. CA A. Cancer J. Clin. 69 (1), 7–34. doi:10.3322/caac.21551
Sorich, M. J., Rowland, A., Karapetis, C. S., and Hopkins, A. M. (2019). Evaluation of the Lung Immune Prognostic Index for Prediction of Survival and Response in Patients Treated with Atezolizumab for NSCLC: Pooled Analysis of Clinical Trials. J. Thorac. Oncol. 14 (8), 1440–1446. doi:10.1016/j.jtho.2019.04.006
Tan, P., Chen, J., Xie, N., Xu, H., Ai, J., Xu, H., et al. (2018). Is Preoperative Serum Lactate Dehydrogenase Useful in Predicting the Outcomes of Patients with Upper Tract Urothelial Carcinoma?. Cancer Med. 7 (10), 5096–5106. doi:10.1002/cam4.1751
van Kessel, K. E. M., de Haan, L. M., Fransen van de Putte, E. E., van Rhijn, B. W. G., de Wit, R., van der Heijden, M. S., et al. (2016). Elevated Derived Neutrophil-To-Lymphocyte Ratio Corresponds with Poor Outcome in Patients Undergoing Pre-operative Chemotherapy in Muscle-Invasive Bladder Cancer. Blc 2 (3), 351–360. doi:10.3233/BLC-160055
Keywords: PD-1 inhibitor, LDH, NLR, urothelial carcinoma, mUIPI
Citation: Li H, An X, Huang R, Li L, Chu C, Yang W, Qin Z, Liu Z, Zhou F, Xue C and Shi Y (2021) Role of a Modified Urothelium Immune Prognostic Index in Patients With Metastatic Urothelial Carcinoma Treated With Anti–PD-1/PD-L1–Based Therapy. Front. Mol. Biosci. 8:621883. doi: 10.3389/fmolb.2021.621883
Received: 16 November 2020; Accepted: 05 July 2021;
Published: 12 August 2021.
Edited by:
Kamran Ghaedi, University of Isfahan, IranReviewed by:
Giuseppe Luigi Banna, United Lincolnshire Hospitals NHS Trust, United KingdomCopyright © 2021 Li, An, Huang, Li, Chu, Yang, Qin, Liu, Zhou, Xue and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cong Xue, eHVlY29uZ0BzeXN1Y2Mub3JnLmNu; Yanxia Shi, c2hpeXhAc3lzdWNjLm9yZy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.