
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mol. Biosci. , 17 March 2017
Sec. Nanobiotechnology
Volume 4 - 2017 | https://doi.org/10.3389/fmolb.2017.00014
 Yugal K. Mohanta1*
Yugal K. Mohanta1* Sujogya K. Panda2
Sujogya K. Panda2 Rasu Jayabalan3
Rasu Jayabalan3 Nanaocha Sharma4
Nanaocha Sharma4 Akshaya K. Bastia1*
Akshaya K. Bastia1* Tapan K. Mohanta5*
Tapan K. Mohanta5*In this experiment, biosynthesized silver nanoparticles (AgNPs) were synthesized using aqueous leaf extract of Erythrina suberosa (Roxb.). The biosynthesis of silver nanoparticle was continuously followed by UV-vis spectrophotometric analysis. The response of the phytoconstituents resides in E. suberusa during synthesis of stable AgNPs were analyzed by ATR- fourier-transform infrared spectroscopy. Further, the size, charge, and polydispersity nature of AgNPs were studied using dynamic light scattering spectroscopy. The morphology of the nanoparticles was determined by scanning electron microscopy. Current result shows core involvement of plant extracts containing glycosides, flavonoids, and phenolic compounds played a crucial role in the biosynthesis of AgNPs. The antimicrobial activities of silver nanoparticles were evaluated against different pathogenic bacterium and fungi. The antioxidant property was studied by radical scavenging (DPPH) assay and cytotoxic activity was evaluated against A-431 osteosarcoma cell line by MTT assay. The characteristics of the synthesized silver nanoparticles suggest their application as a potential antimicrobial and anticancer agent.
Silver nanoparticles are widely used in pharmaceutical industry in the fabrication of ointments and creams to inhibit burns and wounds related infections (Satyavani et al., 2011). The silver ion has strong inhibitory effect against a number of microorganisms (Mohanta and Behera, 2014). Biological synthesis or green synthesis of nanoparticles is an alternative and eco-friendly method for production of nanoparticles (Firdhouse and Lalitha, 2015; Chung et al., 2016; Nayak et al., 2016). The use of silver nanoparticles both as antimicrobial agent (Majeed et al., 2016) and as a potential drug carrier in treatment of cancer has recently gained considerable attention (Nayak et al., 2016).
Silver nanoparticles can be synthesized using a variety of chemicals and physical methods, involving chemical reduction (Vorobyova et al., 1999; Tan et al., 2002; Yu, 2007), photochemical reduction (Kéki et al., 2000; Pileni, 2000; Sun et al., 2001; Mallick et al., 2005), electrochemical reduction (Sandmann et al., 2000; Liu and Lin, 2004), and heat vaporization (Bae et al., 2002; Smetana et al., 2005). These processes involve several toxic chemicals as reducing agents. Because of using noble metal nanoparticles in areas of human contact (Song and Kim, 2008), there is an emergent need to develop eco-friendly biosynthesis processes that hinders the use of toxic chemicals.
To overcome the complication of toxicity in the synthesis and biological applications, plants or plant extracts have been established to have a leading role in the AgNPs bio synthesis process. Various chemical constituents/phyto-molecules have both protective and reductive activities which are mainly important for the reduction of silver ions adopting natural compounds and reductive enzyme complexes. In recent years, extracellular AgNPs were synthesized using different plant extracts as a potential reducing agent (Ahmed et al., 2016; Mohanta et al., 2016).
Erythrina suberosa (Roxb.) is a well-known medicinal plant and used as useful herbal drug in India (Panda, 2014). Its leaves are used for hypotensive, anti-spermatogenic, anti-androgen, anti-gonadotropin, anti-tumor activities, and the bark applied for urinary tract infection (Plants, 1968). E. suberosa belongs to the family Fabaceae (sub-family of Leguminasae) and known as the largest flowering plant family of Indian origin.
Till date, no report has present about biosynthesis of AgNPs utilizing an aqueous leaf extract of E. suberosa. In the current experiment, extract of E. suberosa leaf in a robust aqueous solution of silver nitrate resulted in the reduction of silver ions and the formation of silver nanoparticles. The resulted green-synthesized nanoparticles were examined by ultraviolet-visible spectroscopy (UV-Vis), Transmission Electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopy, and dynamic light scattering spectroscopy (DLS) to determine their size and charge. The antimicrobial and cytotoxic activities of silver nanoparticles were also evaluated.
Different chemicals and reagents used during this experiment include; silver nitrate (AgNO3), Mueller Hinton agar and Mueller Hinton broth and 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT), Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum (FBS), 1% penicillin-streptomycin solution, Bisbenzimide H 33342, and deionized water. All chemicals and reagents are purchased from Sigma Aldrich, India.
The plant E. suberosa was collected from the Similipal Biosphere Reserve (SBR), India during December, 2014. The SBR is well-known for its natural flora and fauna (Panda et al., 2011). The plant specimen with proper identification was deposited in the post-graduate department of botany, North Orissa University (NOU), Odisha, India. The healthy leaves with clean wash were shade dried and pulverized mechanically followed by percolating to get the homogenous size.
The shed dried healthy leaves were pulverized separately using mechanical grinder followed by sieving of 40 μm mesh size for further study. Exactly 10 g of leaf powder was added to 100 ml of sterile distilled water and sonicated for 15–20 min. The sonicated extracts were separated by centrifugation (~5,000 rpm) and supernatant was collected for further use. The purified extracts were filtered through Whatman® filter paper and the filtrate was stored at 4°C.
Qualitative phytochemical analysis of the E. suberosa extract was performed using the standard experimental procedures to observe the common phyto-constituents. Among the phyto-constituents, alkaloids are analyzed by adopting Mayer's, Wagner's, and Dragendorff's reagents whereas flavonoids by Shinoda alkaline reagent. Similarly, phenolic compounds were tested by lead acetate and alkaline reagent and triterpenes by Liberman-Burchard test. Further, the presence of saponins were determined by foam test and tannins by gelatin test (Parekh and Chanda, 2007; Subashini et al., 2011). The observations of these tests were indicated qualitatively as positive (+) or negative (−).
Folin-Ciocalteu method was adopted to determine the total amount of phenolics in the leaf extract with little modification (McDonald et al., 2001). All experiments set ups were made in triplicates. The total phenolics content (TPC) was revealed in terms of gallic acid equivalent (GAE) in mg/g sample.
The total amounts of flavonoids were determined by modified aluminum chloride method (Chang et al., 2002). All determinations were carried out in triplicates. The total flavonoids content (TFC) was expressed as GAE in mg/g sample.
To determine the antioxidant activity, 1, 1-diphenyl-2-picryl-hydrazil (DPPH) radical scavenging assay was followed (McDonald et al., 2001). The results were expressed in percentage of radical scavenging activity using butylated hydroxytoluene (BHT) as standard.
The reaction mixture was prepared in a clean glass test tube, by adding 0.5 ml of the aqueous extract and 4.5 ml aqueous solution of 1 mM AgNO3. On contrary, 0.5 ml of aqueous leaf extracts with 4.5 ml sterilized deionized water (as control) was kept under dark overnight at room temperature. The color change confirmed the synthesis nanoparticle and solutions with nanoparticle were centrifuged at 10,000 rpm for 45 min (C24-BL centrifuge, REMI, India) with successive washing with deionized water to evacuate any trace of un-utilized phyto-constituents. The remaining pellet was lyophilized and stored for further characterization. The sequence of experimental conditions was revamped for its reproducibility.
The bioreduction of silver ions (Ag+) into silver nanoparticles (Ag0) was monitored in aqueous solution by a UV-Vis spectrophotometer (Lambda 35® PerkinElmer, USA) at regular interval in wavelength ranges between 200 and 1,000 nm.
The Attenuated Total Reflection- FTIR spectroscopy analysis of AgNPs was conducted to confirm the promising role of a mixture of phytoconstituents of the plant extracts on the surface alternation and stabilization of biosynthesized AgNPs. The ATR-FTIR was performed using Bruker alpha spectrophotometer (Ettlinger, Germany) with a resolution of 4 cm−1. The samples were scanned in the spectral ranges of 4,000–500 cm−1 by an average of 25 scans per sample and the result obtained was analyzed through OPUS software.
The size and zeta potential (surface charge) of AgNPs were analyzed by Zetasizer (ZS 90, Malvern, UK). The dried samples were adequately diluted with phosphate buffer saline PBS (0.15 M, pH 7.2) prior to investigate in DLS instrument. A scattering angle of 90° was maintained during the assessing of the particle size distribution.
To further characterize the AgNPs, the Cryo-Transmission Electron microscopy (Cryo-TEM) (Technai™ F30 G2STWIN, FEI, USA) was used to observe the nanodimensional morphology. The synthesized AgNPs were drop coated in a copper grid with mesh size 300 and were observed at 300 kV.
The biosynthesized AgNPs obtained from the leaf extract of E. suberosa was tested for its antibacterial potential. The antibacterial activity was determined against Gram-positive bacteria including Bacillus subtilis (MTCC 736), Staphylococcus aureus (MTCC 737), and Gram-negative bacteria including Pseudomonas aeruginosa (MTCC 424), Escherichia coli (MTCC 443). The antibacterial activities of the AgNPs were determined by the agar cup and micro broth dilution method using Mueller Hinton medium. Approximate diameter of 6 and 2.5 mm depth wells were made on the exterior of the medium. Exactly 40 μl of synthesized AgNPs were filled in the wells and the same amount of AgNO3 solution without plant extracts was served as the control. The antibiotic Gentamycin was used as a positive control for the bacteria. The culture plates were incubated at 37°C for 24 h. After the growth period, the plates were removed and zones of inhibition were measured with Himedia antibiotic scale and the results were tabulated. The AgNPs with zones of inhibition greater or equal to 8 mm diameter were regarded as positive. This study was extended further with broth dilution test. Each experiment was carried out in triplicates. The mean ± SD of the zone of inhibitions were taken for calculating the antimicrobial activity of the extracts.
For micro broth dilution method, two-fold serial dilution of the AgNPs was prepared with the MilliQ water 96 well-microtriter plates. Each well of a microtriter plate was inoculated with 190 μL of the test inoculums (0.003 OD) and addition of 10 μL of the AgNPs was made. Only MH broth (190 μL) and AgNPs (10 μL) were taken in a well in order to rectify the absorption variation due to AgNP component present in the reaction mixture. After proper mixing, the plates were sealed with parafilms. The microtriter plates were kept in a shaker-incubator at 37°C for 24 h, and later reading was taken in a iMark™ Microplate Absorbance Reader (Biorad, USA) at 595 nm. The antibacterial activity in micro-broth dilution method was expressed in terms of percent (%) of inhibition.
The antifungal activity was tested against C. albicans (MTCC 227), C. kruseii (MTCC 9215), T. mentagrophytes (MTCC 8476), and C. viswanathii (MTCC 1929). Potato-dextrose (PD) medium was used to grow the test organism for inoculum. In order to perform the antifungal test, individual well of a microtriter plate was inoculated with the fungal inoculum (190 μL) and AgNPs (10 μL) as test solution. Mixture of 190 μL PD broth and 10 μL AgNPs were kept in a well as control to correct any absorption due to synthesized AgNPs. The standard antifungal drug Clotrimazole was used as a positive control.
The osteosarcoma cells (A-431, NCCS, Pune, India) were seeded in flask with Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and incubated at 37°C (5% CO2) for 24 h. Post-incubation, the attached cells were trypsinizated for 3–5 min to get the individual cells and centrifuged (800 rpm, 10 min). The cells were counted and distributed in 96-well Enzyme-linked immunosorbent assay (ELISA) plate with 5,000 cells in each well. The plate was incubated for 24 h at 37°C in a 5% CO2 atmosphere to allow the cells to form ~70–80% confluence as a monolayer (AshaRani et al., 2009).
Silver nanoparticles strongly reduce the Adenosine Triphosphate (ATP) content of the cell which ultimately cause mitochondrial damage and increases the generation of reactive oxygen species (ROS) in a dose-dependent manner (AshaRani et al., 2009). Hence the toxicity of AgNPs were determined at different concentrations (10, 50, 100, 150, 250 μg/ml) in triplicates and the population of cells were calculated by optical microscopy at 48 h.
To detect the cell viability, MTT solution was prepared in growth medium. MTT solution (200 μl) was added to each well of culture and kept for incubation (4–5 h). In the post-incubation period, the MTT solution was removed and DMSO (200 μl) was added to each well under dark condition followed by 15 min incubation. Later the optical density of the formazan product was taken at 595 nm in ELISA reader (Biorad, USA) (Nayak et al., 2016).
The wound healing activity of silver nanoparticles were performed by cell scratch assay (Chang et al., 2002; Yarrow et al., 2004; Chen et al., 2009). Normal fibroblast cell lines (BJ-5Ta) were used to assay the wound healing activity. For this assay, the cells (2 × 105 cells/mL) were seeded in normal cell culture medium DMEM supplemented with 10% phosphate buffer saline (PBS) and M199 medium. After seeding, the cells were incubated for 22–28 h in CO2 incubator. With conformation of ~70–80% confluence as a monolayer, the cells were scratched by sharp tips in the mid of the culture well. Thereafter, the ruptured cells were removed by repeated washing with medium. Subsequently the test samples (silver nanoparticles) were added to the scratched wells. During the experiments, Allantoin, a commercial wound healing drug at concentration 50 μg/ml (Sigma Aldrich) was used as +ve control and Hanks' Balanced Salt solution (HBSS) as –ve control. The culture plates were incubated again for 22–24 h until visible of appropriate growth. Later, the cells were fixed and stained to observe the wound healing activity and the photographs were taken in phase contrast microscope.
The phytochemical screening in both qualitatively and quantitatively of the aqueous extract of E. suberosa leaves has been summarized in Figure 1, Tables 1, 2. The investigation revealed the existence different phyto-constituents such as flavonoids, tannins, and phenolic compounds, glycoside and proteins in the leaf extract.
Being a valuable medicinal plant, the therapeutic uses and phytochemical investigations of E. suberosa are noteworthy. It is used as a potential herbal medicine against different types of body impairments. Our study does not confirm the presence of alkaloids, steroids, and sterols. As per the hypothetical mechanism of biosynthesis of AgNPs, there could be involvement of adequate complex antioxidant enzyme networks (Prasad, 2014). Current investigation result of antioxidant potential of E. suberosa offers an affirmative report toward hypothetical mechanism about involvement of antioxidant molecules from the leaf extract in the biogenic synthesis of silver nanoparticles. Previous research study revealed that, plant contains phenolic and flavonoids possess high antioxidant capabilities and hence biosynthesis of nanoparticles (Mohanta et al., 2016).
The significant antioxidant potential of AgNPs was evaluated by DPPH radical scavenging assay having IC50 30.04 μg/mL (Figure 2). The BHT was used as a standard. Kharat and Mendhulkar (2016) studied the antioxidant activity of synthesized nanoparticles using DPPH assay and observed the antioxidant potentials of photosynthesized nanoparticles (Kharat and Mendhulkar, 2016). They suggested that photosynthesized NPs can be used as a potential free radical scavenger. Priya et al. (2016) studied in vitro antioxidant activity of biosynthesized nanoparticles from P. pinnata extract and found significant free radical scavenging potential. Patra and Baek (2016) demonstrated presence of strong antioxidant activity in terms of DPPH radical scavenging (IC50 385.87 μg/mL) (Patra and Baek, 2016). The results strongly recommend the application of AgNPs as useful natural antioxidants for health preservation against different oxidative stress associated with degenerative diseases. In fact, antioxidant evaluation is essential for AgNPs before its use in vivo models and also human applications.
Bioreduction of silver ions to Ag nanoparticles was evaluated by UV–Vis spectroscopy which is the most simple and indirect method. For this experiment, 9 ml of 1 mM AgNO3 solution was taken as the initial amount to which 1 mL of aqueous leaf extract was added and incubated at room temperature (dark condition). After overnight incubation, a visible color change was observed from pale yellow to dark brown. The intensity of the color was increased with increasing in incubation time. In the present study, we observed the appearance of absorption peak at ~428 nm. Previous study reported that AgNPs give absorption peak at 420–450 nm as a result of its surface plasmon resonance (SPR) character (Mohanta et al., 2016; Nayak et al., 2016). In our study, the observed absorption peak at 428 nm further confirms the biosynthesis of Ag nanoparticles (Figure 3).
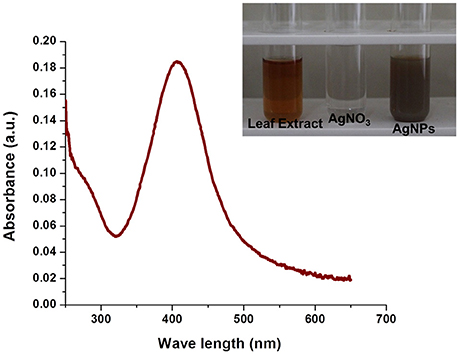
Figure 3. UV-Vis spectra of AgNPs synthesized by E. suberosa leaf extracts. Color change from pale yellow to brown upon synthesis of AgNPs (In set).
The FTIR spectra of the AgNPs confirmed the possible involvement of different functional groups in biosynthesis and stabilization of the nano-particles. It is comprehensible from the spectrum (Figure 4) which indicates the appearance of five prominent absorption peaks at ~3,740, 2,345, 1,680, 1,529, and 1,028 cm−1. The strong absorption was found at ~3,740 cm−1 indicating the presence of polyphenols due to the binding of silver ion with hydroxyl group which referred to the stretching of OH group or free hydroxyl group. Furthermore, the presence of −C = C− stretch at around 1,680 cm−1 confirms the presence of broad range of alkene group in the synthesized nanoparticles. The sharp band at ~1,529 cm−1 could possible due to N-O asymmetric stretching indicates the active involvement of nitro compounds. Another medium peak at ~1,028 cm−1 indicates the presence of aliphatic amines due to C-N stretching. The presence of characteristic functional groups such as alcohols, aldehydes, flavonoids, phenols, and nitro compounds as phyto-constituents were present in the leaves of E. suberosa which participates in the bioreduction process for synthesis of silver nanoparticles.
The DLS method revealed the hydrodynamic size and surface charge of AgNPs in aqueous colloidal milieu. With regard to size distributions, it has been found that the AgNPs show an average size of ~12 and 115 nm which confirm its bimodal distribution (Figure 5A). Moreover, the average size and charge of the AgNPs were confirmed to be ~73 nm and −15.8 mV respectively (Figure 5B). The degree of Zeta potential accord initial fluctuation of the particles in the media; but the value is quite adequate to avert further aggregation. Ultimately, the average size and Zeta potential of Ag nanoparticles gives a strong characteristic that can be utilized in biomedical sciences as a biosensor and active drug carrier (Ge et al., 2014).
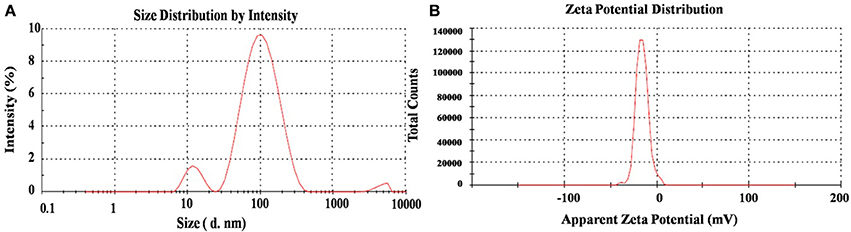
Figure 5. DLS spectra on (A) hydrodynamic size distribution and (B) Zeta potential (mV) of synthesized AgNPs.
Analysis of leaf mediated synthesis of silver nanoparticles by TEM proved the size of AgNPs in the range of nano scale, almost spherically shaped, and has a mean diameter of 15–34 nm (Figure 6). Most of the nanoparticles were approximately circular in shape with smooth edges. In the TEM image, the AgNPs were in physically close contact; but scattered by an adequately uniform distance between particles. Previous study also resulted the biosynthesized silver nanoparticles by Memecylon edule leaf extract with circular morphology (Arunachalam et al., 2013).
Pathogenic bacteria and fungi enlisted in Table 3 were taken for preliminary screening of antimicrobial activity using agar cup methods. In agar cup method, zone of inhibitions (ZI) was found maximum against P. aeruginosa, and S. aureus (Figure 7) whereas no zone of inhibition (ZI) was observed in E. coli and B. subtilis. Similarly, the inhibition was documented against C. kruseii and T. mentagrophytes (Figure 7) while no inhibition was observed against C. albicans and C. viswanathii. For confirmatory antimicrobial potential of AgNPs, the antimicrobial activity against pathogenic bacteria and fungi were carried out through micro broth dilution method and the results in terms of percentage (%) of inhibition were displayed in Table 4.
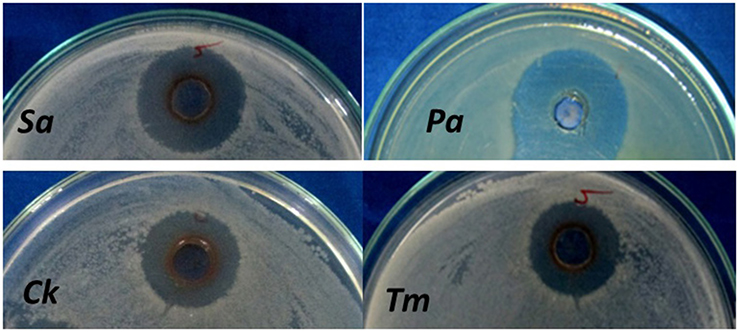
Figure 7. Antimicrobial activity of AgNPs. (Sa, Staphylococcus aureus; Pa, Pseudomonas aeruginosa; Ck, Candida kruseii; Tm, Trichophyton mentagrophytes).
Silver nanoparticles (AgNPs) have encouraging application in curative efficacy like wound care, skin cancer and breast cancer (Nayak et al., 2015). Moreover, these are being used as bone cementing and prosthetic materials for fast recovery. In order to support the development of anti-cancer therapy, the biosynthesized silver nanoparticles were taken for cytotoxic study against A-431 osteosarcoma cell line. The cell viability (%) of A-431 cell line post-treatment with biosynthesized silver nanoparticles was determined by studying the MTT assay. The viability of the cells (%) treated with various concentrations of AgNPs has shown in Figure 8. The IC50 values were determined to be 106.15 ± 2.6, 74.02 ± 2.4, and 136.73 ± 2.02 μg/mL for leaf extract, AgNPs, and AgNO3 respectively. The silver nanoparticles showed excellent anticancer activity against A-431 osteosarcoma cell line with similar IC50 values as reported earlier (Park et al., 2011; Gurunathan et al., 2013; Jeyaraj et al., 2013; Krishnaraj et al., 2014; Nayak et al., 2015). This study strongly revealed the significant antiproliferative activity of biosynthesized silver nanoparticles. However, such activity may be due to the synergetic effect of both nano sized silver and the bioactive phytocompounds attached on the surface of the nanoparticles. In depth study will be required to understand the real mechanism behind anticancer activity of the synthesized silver nanoparticles.
The wound healing activity of the silver nanoparticles was observed with positive effect (Figures 9A–C). This wound healing potential has strengthened the application of silver nanoparticles as potential medicine. The therapeutic applications of plant-extract-based scaffolds have been earlier reported to heal wounds and reconstitute the skin (Jin et al., 2013). Hence, the current research finding of potential wound healing capacity of plant extract based silver nanoparticles will be a positive additive for the biomedical applications.
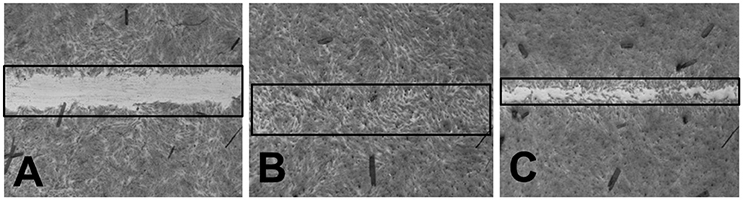
Figure 9. (A) BJ-5Ta cells treated with HBSS (−ve control) (B) BJ-5Ta cells treated with Allantoin (+ve control) (C) BJ-5Ta cells treated with silver nanoparticles.
Herein, a sustainable green approach was adopted to synthesize AgNPs using E. suberosa leaf extract which produce spherical-shaped AgNPs at ambient conditions without using any harmful reducing or capping agents. The involvement of plant extract in synthesis of AgNPs is confirmed by ATR-FTIR spectroscopy. Moreover, the functional size NPs was synthesized at room temperature. The protocol adopted here for the synthesis of AgNPs can be applied to other metal NPs due to highly oxidized in nature of E. suberosa leaf extract and is very likely to be able to reduce metals under different physiological conditions. Moreover, the abundancy and the biomedical applications such as antimicrobial, anti-cancer and wound healing nature of E. suberosa leaf extract mediated AgNPs potentially attracts for the up-scaling of metallic nanomaterials to explore various catalytic as well as biomedical applications.
YKM: conceived the idea and outlined the experimental procedure, performed the major experiments. SKP and RJ: performed the experiments. NS: drafted the manuscript. AB and TM: revised the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors are highly thankful to the authorities of North Orissa University, Baripada, National Institute of Technology, Rourkela for providing research facilities to carry out the complete experiments.
AgNPs, Silver Nanoparticles; SPR, Surface Plasmon Resonance; AgNO3, Silver Nitrate; nm, Nanometer; mm, Millimeter; IC50, Inhibitory concentrations; SBR, Similipal Biosphere Reserve; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DPPH, (1, 1-diphenyl-2-picrylhydrazyl); MTCC, Microbial Type Culture Collection; MH, Mueller-Hinton; PD, Potato Dextrose; DLS, Dynamic light scattering techniques; FE-SEM, Field emission scanning electron microscopy; FT-IR, Fourier transform infrared spectroscopy; UV-Vis, UV Visible spectrophotometer.
Ahmed, S., Ahmad, M., Swami, B. L., and Ikram, S. (2016). A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J. Adv. Res. 7, 17–28. doi: 10.1016/j.jare.2015.02.007
Arunachalam K. D., Annamalai S. K., and Hari, S. (2013). One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from Memecylon umbellatum. Int. J. Nanomedicine 8 1307–1315. doi: 10.2147/IJN.S36670
AshaRani, P. V., Low Kah Mun, G., Hande, M. P., and Valiyaveettil, S. (2009). Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3, 279–290. doi: 10.1021/nn800596w
Bae, C. H., Nam, S. H., and Park, S. M. (2002). Formation of silver nanoparticles by laser ablation of a silver target in NaCl solution. Appl. Surf. Sci. 197–198, 628–634. doi: 10.1016/S0169-4332(02)00430-0
Chen, Y., Lu, B., Yang, Q., Fearns, C., Yates, J. R., and Lee, J.-D. (2009). Combined integrin phosphoproteomic analyses and sirna-based functional screening identified key regulators for cancer cell adhesion and migration. Cancer Res. 69, 3713–3720. doi: 10.1158/0008-5472.CAN-08-2515
Chang, C.-C., Yang, M.-H., Wen, H.-M., and Chern, J.-C. (2002). Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10, 178–182.
Chung, I.-M., Park, I., Seung-Hyun, K., Thiruvengadam, M., and Rajakumar, G. (2016). Plant-mediated synthesis of silver nanoparticles: their characteristic properties and therapeutic applications. Nanoscale Res. Lett. 11, 40. doi: 10.1186/s11671-016-1257-4
Firdhouse, M. J., and Lalitha, P. (2015). Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015:829526. doi: 10.1155/2015/829526
Ge, L., Li, Q., Wang, M., Ouyang, J., Li, X., and Xing, M. M. Q. (2014). Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int. J. Nanomedicine 9, 2399–2407. doi: 10.2147/IJN.S55015
Gurunathan, S., Raman, J., Malek, S. N., John, P. A., and Vikineswary, S. (2013). Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells. Int. J. Nanomedicine 8, 4399–4413. doi: 10.2147/IJN.S51881
Jeyaraj, M., Sathishkumar, G., Sivanandhan, G., MubarakAli, D., Rajesh, M., Arun, R., et al. (2013). Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids Surf. B Biointerfaces 106, 86–92. doi: 10.1016/j.colsurfb.2013.01.027
Jin, G., Prabhakaran, M. P., Kai, D., Annamalai, S. K., Arunachalam, K. D., and Ramakrishna, S. (2013). Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 34, 724–734. doi: 10.1016/j.biomaterials.2012.10.026
Kéki, S., Török, J., Deák, G., Daróczi, L., and Zsuga, M. (2000). Silver Nanoparticles by PAMAM-Assisted Photochemical Reduction of Ag+. J. Colloid Interface Sci. 229, 550–553. doi: 10.1006/jcis.2000.7011
Kharat, S. N., and Mendhulkar, V. D. (2016). “Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract.” Mater. Sci. Eng. C 62, 719–724. doi: 10.1016/j.msec.2016.02.024
Krishnaraj, C., Muthukumaran, P., Ramachandran, R., Balakumaran, M. D., and Kalaichelvan, P. T. (2014). Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 4, 42–49. doi: 10.1016/j.btre.2014.08.002
Liu, Y.-C., and Lin, L.-H. (2004). New pathway for the synthesis of ultrafine silver nanoparticles from bulk silver substrates in aqueous solutions by sonoelectrochemical methods. Electrochem. Commun. 6, 1163–1168. doi: 10.1016/j.elecom.2004.09.010
Majeed, A., Ullah, W., Anwar, A. W., Shuaib, A., Ilyas, U., Khalid, P., et al. (2016). Cost-effective biosynthesis of silver nanoparticles using different organs of plants and their antimicrobial applications: a review. Mater. Technol. 7857, 1–8. doi: 10.1080/10667857.2015.1108065
Mallick, K., Witcomb, M. J., and Scurrell, M. S. (2005). Self-assembly of silver nanoparticles in a polymer solvent: formation of a nanochain through nanoscale soldering. Mater. Chem. Phys. 90, 221–224. doi: 10.1016/j.matchemphys.2004.10.030
McDonald, S., Prenzler, P. D., Antolovich, M., and Robards, K. (2001). Phenolic content and antioxidant activity of olive extracts. Food Chem. 73, 73–84. doi: 10.1016/S0308-8146(00)00288-0
Mohanta, Y. K., and Behera, S. K. (2014). Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by Streptomyces sp. SS2. Bioprocess Biosyst. Eng. 37, 2263–2269. doi: 10.1007/s00449-014-1205-6
Mohanta, Y. K., Panda, S. K., Biswas, K., Tamang, A., Bandyopadhyay, J., De, D., et al. (2016). Biogenic synthesis of silver nanoparticles from Cassia fistula (Linn.): in vitro assessment of their antioxidant, antimicrobial and cytotoxic activities. IET Nanobiotechnol. 10, 438–444. doi: 10.1049/iet-nbt.2015.0104
Nayak, D., Ashe, S., Rauta, P. R., Kumari, M., and Nayak, B. (2016). Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater. Sci. Eng. C 58, 44–52. doi: 10.1016/j.msec.2015.08.022
Nayak, D., Pradhan, S., Ashe, S., Rauta, P. R., and Nayak, B. (2015). Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J. Colloid Interface Sci. 457, 329–338. doi: 10.1016/j.jcis.2015.07.012
Panda, S. K. (2014). Ethno-medicinal uses and screening of plants for antibacterial activity from Similipal Biosphere Reserve, Odisha, India. J. Ethnopharmacol. 151, 158–175. doi: 10.1016/j.jep.2013.10.004
Panda, S. K., Dutta, S. K., and Bastia, A. K. (2011). Antidiarrheal activity of Terminalia arjuna Roxb. from India. J. Biologically Active Prod. Nature 1, 236–247. doi: 10.1080/22311866.2011.10719091
Parekh, J., and Chanda, S. V. (2007). In vitro antimicrobial activity and phytochemical analysis of some indian medicinal plants. Turk. J. Biol. 31, 53–58.
Park, M. V., Neigh, A. M., Vermeulen, J. P., de la Fonteyne, L. J., Verharen, H. W., Briedé, J. J., et al. (2011). The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32, 9810–9817. doi: 10.1016/j.biomaterials.2011.08.085
Patra, J. K., and Baek, K. H. (2016). Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential. IET Nanobiotechnol. 10, 326–333. doi: 10.1049/iet-nbt.2015.0102
Pileni, M. P. (2000). Fabrication and physical properties of self- organized silver nanocrystals. Pure Appl. Chem. 72, 53–65. doi: 10.1351/pac200072010053
Prasad, R. (2014). Synthesis of silver nanoparticles in photosynthetic plants. J. Nanoparticles 2014, 1–8. doi: 10.1155/2014/963961
Priya, R. S., Geetha, D., and Ramesh, P. S. (2016). Antioxidant activity of chemically synthesized AgNPs and biosynthesized Pongamia pinnata leaf extract mediated AgNPs – A comparative study. Ecotoxicol. Environ. Saf. 134, 308–318. doi: 10.1016/j.ecoenv.2015.07.037
Sandmann, G., Dietz, H., and Plieth, W. (2000). Preparation of silver nanoparticles on ITO surfaces by a double-pulse method. J. Electroanal. Chem. 491, 78–86. doi: 10.1016/S0022-0728(00)00301-6
Satyavani, K., Ramanathan, T., and Gurudeebam, S. (2011). Plant mediated synthesis of biomedical silver nanoparticles by using leaf extracts of Citrullus colosynthis. Res. J. Nanosci. Nanotechnol. 1, 95–101. doi: 10.3923/rjnn.2011.95.101
Smetana, A. B., Klabunde, K. J., and Sorensen, C. M. (2005). Synthesis of spherical silver nanoparticles by digestive ripening, stabilization with various agents, and their 3-D and 2-D superlattice formation. J. Colloid Interface Sci. 284, 521–526. doi: 10.1016/j.jcis.2004.10.038
Song, J. Y., and Kim, B. S. (2008). Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) leaf extract. Korean J. Chem. Eng. 25, 808–811. doi: 10.1007/s11814-008-0133-z
Subashini, S., Arunachalam, K. D., and Annamalai, S. K. (2011). Preclinical studies on the phytochemical, antimicrobial, and wound healing properties of indigofera aspalathoides leaves. J. Pharm. Res. 4, 3206–3211.
Sun, Y.-P., Atorngitjawat, P., and Meziani, M. J. (2001). Preparation of silver nanoparticles via rapid expansion of water in carbon dioxide microemulsion into reductant solution. Langmuir 17, 5707–5710. doi: 10.1021/la0103057
Tan, Y., Wang, Y., Jiang, L., and Zhu, D. (2002). Thiosalicylic acid-functionalized silver nanoparticles synthesized in one-phase system. J. Colloid Interface Sci. 249, 336–345. doi: 10.1006/jcis.2001.8166
Vorobyova, S. A., Lesnikovich, A. I., and Sobal, N. S. (1999). Preparation of silver nanoparticles by interphase reduction. Colloids Surf. A Physicochem. Eng. Asp. 152, 375–379. doi: 10.1016/S0927-7757(98)00861-9
Yarrow, J. C., Perlman, Z. E., Westwood, N. J., and Mitchison, T. J. (2004). A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 4:21. doi: 10.1186/1472-6750-4-21
Keywords: biosynthesis, silver nanoparticle, antimicrobial activity, antioxidant activity, cytotoxic activity
Citation: Mohanta YK, Panda SK, Jayabalan R, Sharma N, Bastia AK and Mohanta TK (2017) Antimicrobial, Antioxidant and Cytotoxic Activity of Silver Nanoparticles Synthesized by Leaf Extract of Erythrina suberosa (Roxb.). Front. Mol. Biosci. 4:14. doi: 10.3389/fmolb.2017.00014
Received: 28 December 2016; Accepted: 03 March 2017;
Published: 17 March 2017.
Edited by:
João Conde, Massachusetts Institute of Technology, USAReviewed by:
Vasco D. B. Bonifácio, Universidade Nova de Lisboa, PortugalCopyright © 2017 Mohanta, Panda, Jayabalan, Sharma, Bastia and Mohanta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yugal K. Mohanta, eWttb2hhbnRhQGdtYWlsLmNvbQ==
Akshaya K. Bastia, YmFzdGlhbm91QGdtYWlsLmNvbQ==
Tapan K. Mohanta, bm9zdG9jLnRhcGFuQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.