- 1Department of Animal and Food Sciences, Texas Tech University, Lubbock, TX, United States
- 2USDA-ARS Livestock Issues Research Unit, Lubbock, TX, United States
- 3USDA-ARS, U.S. Meat Animal Research Center, Clay Center, NE, United States
- 4Department of Civil and Environmental Engineering, Rice University, Houston, TX, United States
- 5Sentinel Environmental Group, LLC, Houston, TX, United States
- 6College of Veterinary Medicine, Kansas State University, Manhattan, KS, United States
- 7Department of Veterinary Sciences, Texas Tech University, Lubbock, TX, United States
The objective was to longitudinally assess the prevalence of F. necrophorum subsp. necrophorum, F. necrophorum subsp. funduliforme, F. varium, and Salmonella enterica in the nasal cavity, ruminal fluid, and feces of finishing beef steers with and without LA. Crossbred steers (n = 225; 353 ± 39.6 kg) were transported to a feedlot and fed a high-concentrate diet. Nasal, ruminal fluid, and fecal samples were collected following feedlot arrival (d 5), 1 week after adaptation to a finishing diet (d 35), and the day before harvest (study end). Livers were collected at harvest and examined for LA, and cattle were subsequently assigned into either control or liver abscess groups. Overall LA prevalence was 18.7%. The concentration and prevalence of Salmonella decreased in ruminal fluid and increased in feces with days on feed (p < 0.01). Conversely, ruminal fluid prevalence of F. necrophorum subsp. necrophorum and F. varium increased with days on feed (p < 0.01). Fusobacterium abundance in ruminal fluid and feces was not indicative of LA development except for F. varium being more abundant in the ruminal fluid of steers with LA (p < 0.01). Abundance of F. necrophorum subsp. necrophorum was greater in abscessed liver tissue than healthy tissue (p = 0.03), although no other differences in bacterial abundance or prevalence were observed in livers. Overall, Fusobacterium and Salmonella prevalence in the nasal cavity, ruminal fluid, and feces were affected by days on feed, but their prevalence and abundance were not indicative of LA occurrence.
1 Introduction
Liver abscesses (LA) in finishing beef cattle are a significant economic concern for the feedlot industry because of decreased body weights and hot carcass weights (Brink et al., 1990; Brown and Lawrence, 2010), contributing to an estimated economic burden of almost $1 billion (Lawrence, 2024). On an individual-pen basis, LA prevalence ranges from 0 to 95.5%, with the overall prevalence increasing since 2012 (Grimes et al., 2024). Liver abscesses are complex, polymicrobial, and involve multiple organs (Broadway et al., 2024). The bacterial etiology of LA has been extensively studied, with Fusobacterium necrophorum subsp. necrophorum considered the primary causative agent (Amachawadi and Nagaraja, 2016; Pinnell and Morley, 2022; McDaniel et al., 2024a).
Historically considered a normal resident of the bovine gastrointestinal tract (GIT; Jang and Hirsh, 1994; Langworth, 1977), F. necrophorum subsp. necrophorum is an opportunistic pathogen commonly isolated in both necrotic respiratory infections (Seimiya et al., 2004; Tadepalli et al., 2009) and LA (Nagaraja and Chengappa, 1998). Recently, identification of F varium as the dominant species of Fusobacterium in the rumen of cattle has called into question the validity of previous culture-dependent methods (Schwarz et al., 2023; Deters et al., 2024a). Prevalence of F. necrophorum subsp. necrophorum in LA ranges from 71 to 100% (Herrick et al., 2022; Lechtenberg et al., 1988; Nagaraja and Chengappa, 1998). The common theory on etiology of LA suggests acidosis-induced rumenitis allows for bacterial invasion and colonization of the ruminal wall (Jensen et al., 1954; Nagaraja and Lechtenberg, 2007), thereby increasing bacterial translocation into portal vein circulation (Tadepalli et al., 2009). Once F. necrophorum subsp. necrophorum is translocated to the liver, leukotoxins and endotoxins protect it from phagocytosis (Emery et al., 1985; Tan et al., 1996) and induce hepatocyte-mediated apoptosis (Amachawadi and Nagaraja, 2016).
Recently, Salmonella enterica (denoted as Salmonella) has been isolated from LA (Amachawadi and Nagaraja, 2015; Amachawadi et al., 2017) at a prevalence of 27.5% nationally and 23.8% in association with F. necrophorum (Herrick et al., 2022). Nonetheless, in the High Plains cattle feeding region, the incidence of Salmonella alone or with F. necrophorum increases to 84.6 and 76.7%, respectively (Herrick et al., 2022). Hind-gut acidosis or stress-induced inflammation can increase the translocation of Salmonella across the intestinal epithelium, where Salmonella actively infects phagocytic and non-phagocytic cells (Ibarra and Steele-Mortimer, 2009; Sanz-Fernandez et al., 2020). Thus, the lymphatic system could provide another pathway for Salmonella to enter the liver besides portal vein circulation. Currently, little data substantiates the role of Salmonella in LA formation or the concentration and prevalence of Fusobacterium and Salmonella throughout the GIT in relation to LA occurrence. Therefore, we hypothesize that bacterial populations associated with LA will differ in the GIT of beef steers with and without LA. Our objective was to longitudinally assess the prevalence of F. necrophorum subsp. necrophorum, F. necrophorum subsp. funduliforme, F. varium, and Salmonella enterica in the nasal cavity, ruminal fluid, and feces of finishing beef steers with and without LA.
2 Materials and methods
All experimental procedures were approved by the Texas Tech University Institutional Animal Care and Use Committee (approval number 2022–1273) and conducted from May 2023 to February 2024.
2.1 Animal management
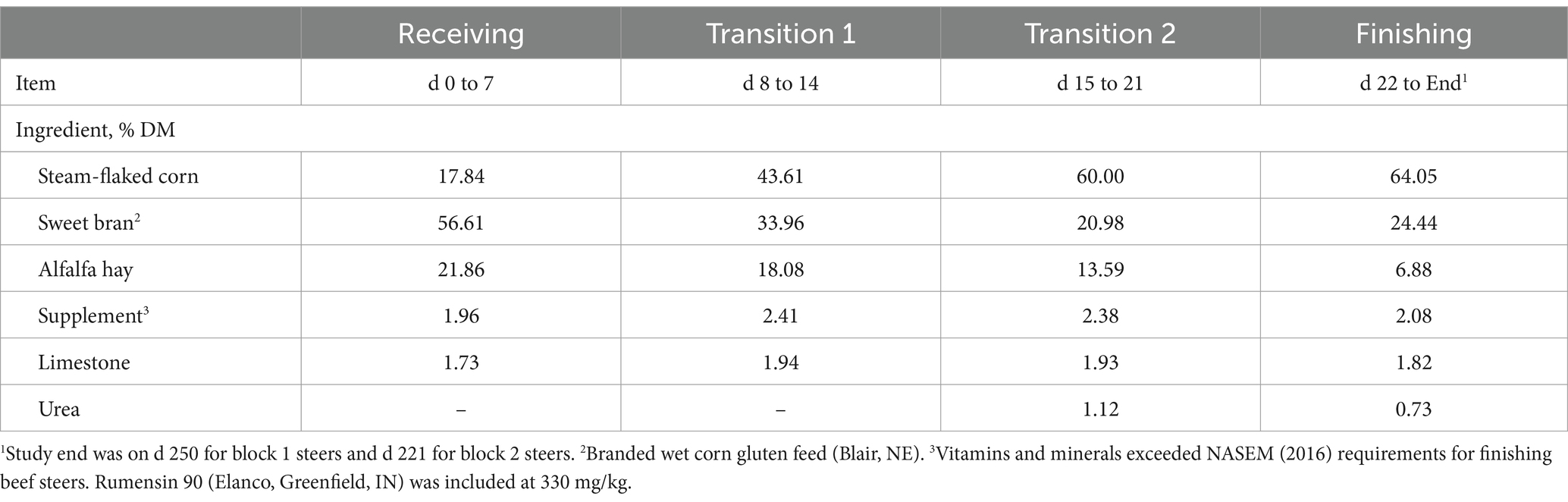
Crossbred steers (n = 225; 353 ± 39.6 kg) were sourced from the Texas Panhandle, transported to the Burnett Center for Research and Instruction, and blocked by arrival group into 2 source blocks. On d 0, steers were received in soil-surface, partially shaded outdoor pens (4.9 m × 30 m), administered vaccinations (Myco-B One Dose, American Animal Health, Fort Worth, TX; Bovilis Vista 5Q, Merck, Rahway, NJ; Bovilis Vision 7 with Spur, Merck; I-site XP, Huvepharma, Peachtree City, GA), anthelmintic (Cydectin; Elanco, Indianapolis, IN), and received a Revalor-XS implant (200 mg of trenbolone acetate +40 mg of estradiol 17β; Merck). On d 21, steers were moved into concrete, slatted-floor pens. From d 0 to study end (d 250 for block 1 and d 221 for block 2), steers were fed a standard grain-based finishing diet representative of those fed in the High Plains region (Table 1). At study end, steers were harvested at a commercial abattoir.
2.2 Sample collection
Nasal, ruminal fluid, and fecal samples were aseptically collected after feedlot arrival (d 5), 1 week after adaptation to the finishing diet (d 35), and the day before harvest (study end). Nasal samples (Salmonella only) were collected via a 5-in, rayon-tipped bacteriology swab (Fisher, Waltham, MA), whereas ruminal fluid was collected using a speculum and flexible tubing passed through the esophagus to the rumen, and feces were collected by rectal palpation. All samples were subsequently processed at the USDA-ARS Livestock Issues Research Unit (LIRU). At the commercial abattoir, LA prevalence was recorded by trained personnel from the Beef Carcass Research Center at West Texas A&M University. A 100-g sample of healthy and abscessed livers were collected before carcass chilling, placed in a sealable bag, and transported to LIRU. Liver samples were immediately emulsified using a blender for bacterial processing.
2.3 Sample processing for Salmonella enterica concentration and prevalence
Analysis of samples for Salmonella was conducted as described by Dornbach et al. (2023). To determine ruminal fluid, fecal, and liver Salmonella concentration and prevalence, 25 g of sample were diluted 1:10 in phosphate buffered saline (PBS) in a lateral filtered stomacher bag (Seward Ltd.; West Sussex, United Kingdom) and homogenized (Stomacher® 400 Circulator; Seward Ltd.) for 2 min at 230 rpm. For nasal Salmonella prevalence, swabs were suspended in 4.5 mL of PBS and vortexed. Salmonella concentrations were determined via spiral plating (Eddy Jet 2 W, Neutec Group Inc., Farmingdale, NY) 100 μL of homogenate onto xylose lysine deoxycholate (XLD; Becton, Dickinson and Co., Franklin Lakes, NJ) agar containing novobiocin (25 μg/mL). Additionally, 1 mL of homogenate was enriched in a 1:10 dilution of Tetrathionate Hajna (Remel, San Diego, CA) broth with iodine and incubated overnight at 37°C. Likewise, 1 mL of homogenate was enriched in Rappaport-Vassiliadis (Oxoid Ltd., Basingstoke, UK) broth and incubated at 42°C overnight. After incubation, enrichment broths were vortexed, and a 10-μL loop was used to streak enriched cultures onto XLD agar containing novobiocin (25 μg/mL). Following overnight incubation at 37°C and an additional 24 h at 25°C, phenotypic colonies were subjected to latex agglutination (Salmonella Test Kit; Oxoid Ltd) and confirmed by PCR using the invA gene (Rahn et al., 1992). Assay running conditions included an initial incubation at 95°C for 1 min, followed by 35 cycles of 95°C for 30 s, 64°C for 30 s, and 72°C for 30 s. Following the last cycle there was a 4 min incubation at 72°C. Assays were performed using a Bio-Rad C1000 Thermal Cycler (Hercules, CA).
2.4 Sample processing for Fusobacterium necrophorum and Fusobacterium varium abundance and prevalence
To determine the absolute abundance (i.e., copies/g) and prevalence (i.e., %) of ruminal fluid F. necrophorum and F. varium, dimethyl sulfoxide (DMSO) was added to a final concentration of 5% v/v, while fecal and liver samples were diluted 1:1 in PBS containing 10% DMSO. All samples were subsequently homogenized and frozen at-80°C. Frozen samples were then transported on dry ice to Sentinel Environmental in Houston, TX for analysis.
Ruminal fluid, fecal, and liver DNA were extracted using the ZymoBIOMICS™ 96 MagBead DNA kit (D4308-E, Zymo Research Corp. United States) and an OpenTrons OT-2 liquid-handling robot running a custom python script. Briefly, samples were partially thawed at room temperature to transfer 100 mg of sample into ZR BashingBead™ Lysis Tubes containing 375 μL ZymoBIOMICS™ Lysis Solution, 375 μL of DNA/RNA Shield™, and 1×106 copies of lambda phage genome (N3011S; New England Biolabs, Ipswich, MA). Following mechanical lysis with a Biospec Mini-Bead Beater-16 (BioSpec Products, Inc., Bartlesville, OK), samples were transferred to a 96-deep-well plate. Extraction of DNA was conducted with the OpenTrons OT-2. As described by Deters et al. (2024a), the qPCR primers and probes used were designed to target hgdA for F. necrophorum (hgdA-n) and F. varium (hgdA-v), as well as the leukotoxin promotor region, lktA-n for F. necrophorum subsp. necrophorum and lktA-f for F. necrophorum subsp. funduliforme (Table 2). Probe concentrations were optimized for each target gene. Assay running conditions were 95°C for 5 min followed by 45 cycles of 95°C for 15 s and 60°C for 40 s. Assays were performed using a Bio-Rad CFX96 Real-Time System. Lambda phage DNA was quantified to assess efficiency of extraction using primers and probes as described by Beller et al. (2002). Any sample with ≥102 copies/g of Fusobacterium were considered positive for prevalence.
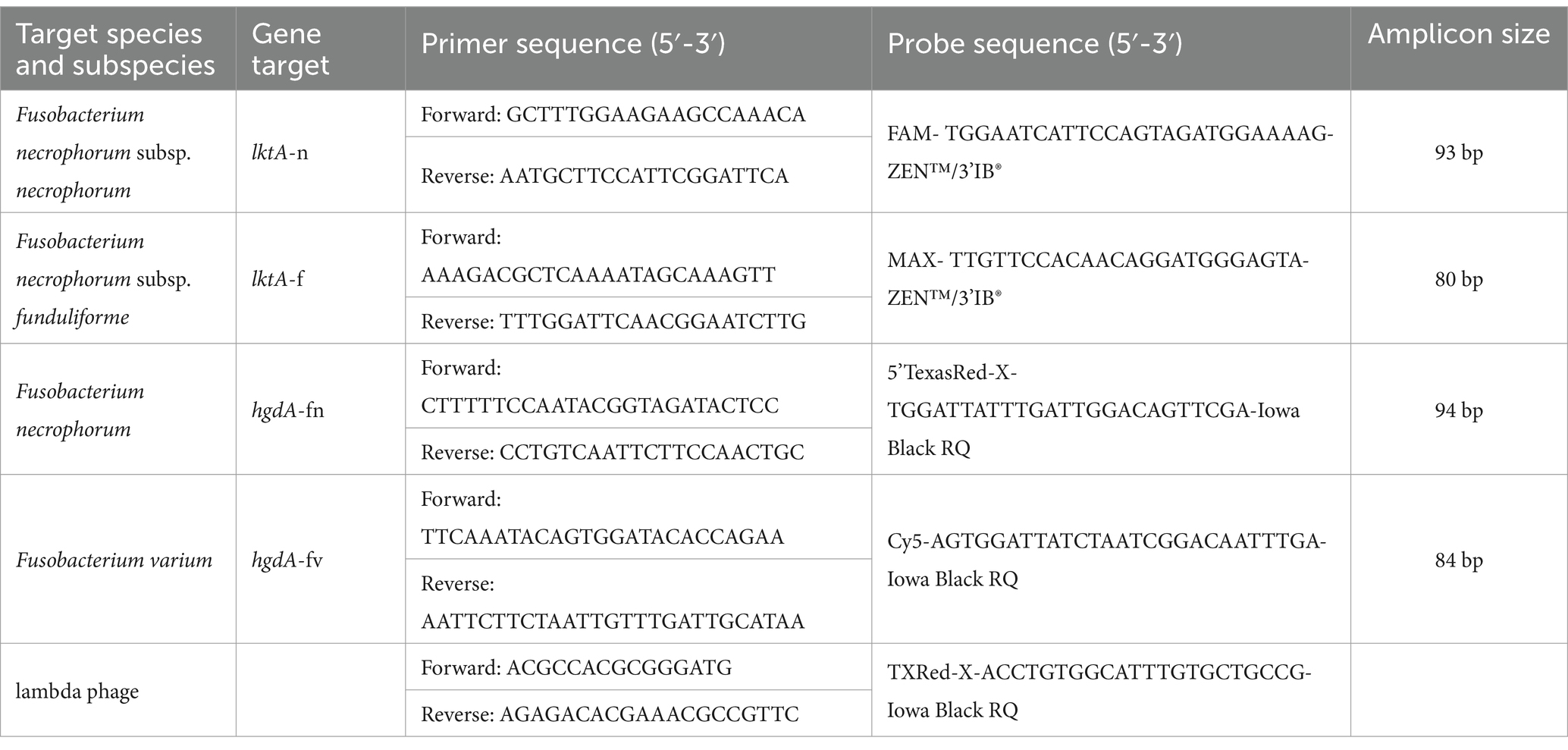
Table 2. Species and subspecies of Fusobacterium, genes targeted, and primer and probe sequences used in the qPCR assay.
2.5 Statistical analyses
The experimental design was a randomized complete block with individual steer as the experimental unit. The GLIMMIX and MIXED procedures of SAS 9.4 (SAS Inst., Cary, NC) were used to evaluate binomial and continuous data, respectively, with fixed effects of treatment, sampling time, and treatment × sampling time interaction. The interaction of block × treatment × sampling time was included as a random effect. Sampling time was the repeated measure and individual steer within block was the subject. The Kenward Roger adjustment was used to correct the degrees of freedom because of unequal treatment numbers. The covariance structure autoregressive (1) was used based on evaluation of the Akaike’s information criterion. Least squares means were separated using the Tukey option in the LSMEANS statement of SAS. Outliers were identified using the Cook’s D outlier test; ruminal fluid data for 1 steer at study end was omitted using these criteria. A p ≤ 0.05 was considered significant and tendencies were discussed at 0.05 < p ≤ 0.10.
3 Results
3.1 Salmonella enterica concentration and prevalence in the nasal cavity, ruminal fluid, feces, and liver
Overall LA prevalence was 18.7% (n = 42). No treatment × sampling time interactions were observed throughout the study (p ≥ 0.14). Nasal Salmonella concentration did not differ between steers with or without LA (p = 0.85; Table 3) or by collection day (p = 0.50). Moreover, no differences in ruminal fluid Salmonella concentration were observed between steers with or without LA (p = 0.37); however, ruminal fluid Salmonella concentration decreased from feedlot arrival to harvest (p < 0.01). Conversely, fecal Salmonella concentration was greatest before harvest (p < 0.01) and tended to be 5.9% greater in steers without LA (p = 0.07). Liver Salmonella concentrations were not affected by LA presence (p = 0.18; Table 4).
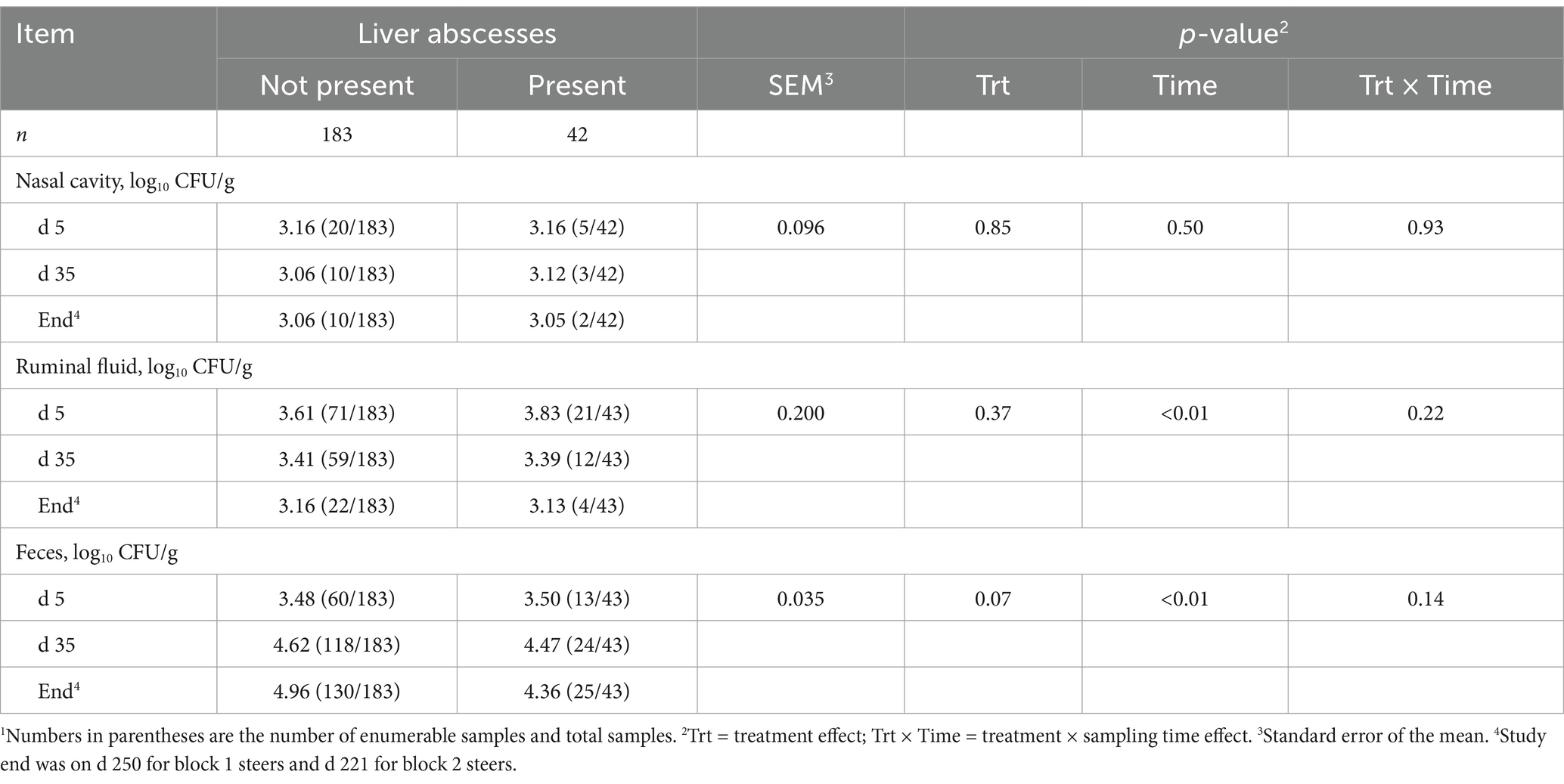
Table 3. Concentration of Salmonella enterica in the nasal cavity, ruminal fluid, and feces of finishing beef steers with and without liver abscesses.1
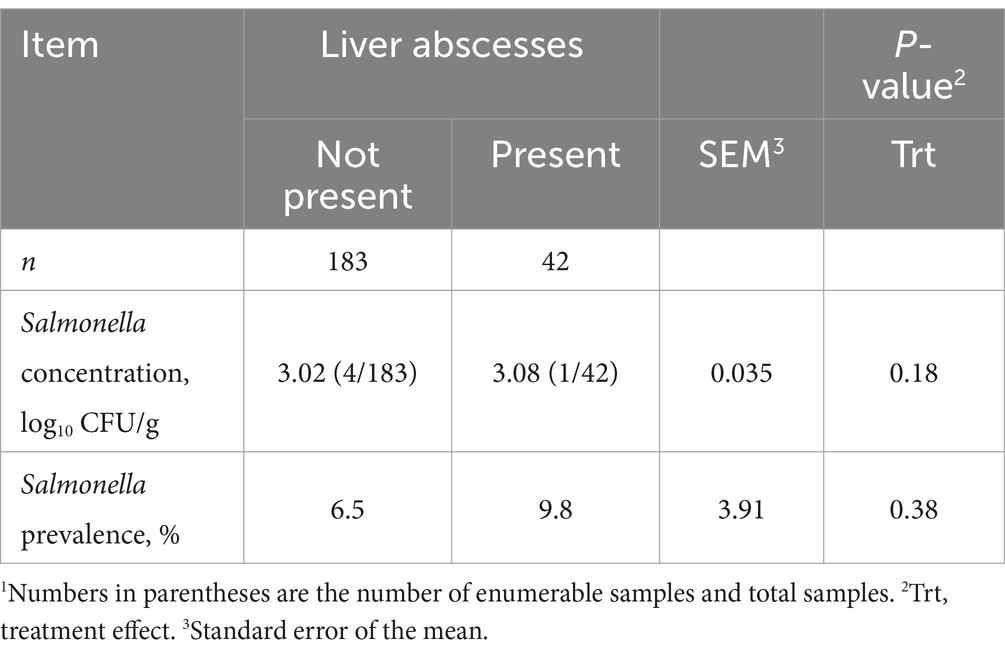
Table 4. Concentration and prevalence of Salmonella enterica in the livers of feedlot beef steers with and without liver abscesses.1
Mean nasal Salmonella prevalence was 34.5%, being greatest at feedlot arrival and least on d 35 (p < 0.01; Figure 1A), although prevalence was not indicative of the presence of LA (p = 0.73). Ruminal fluid Salmonella prevalence did not differ between steers with or without LA (p = 0.83; Figure 1B) whereby mean ruminal fluid Salmonella prevalence was 73.2%, with prevalence decreasing from 83.3% on d 35 to 55.3% at harvest (p < 0.01). Fecal Salmonella prevalence increased from 63.2% on d 5 to 96.6% by d 35 after transition to the finishing diet (p < 0.01; Figure 1C), with a mean fecal Salmonella prevalence of 81.7%. Fecal Salmonella prevalence tended to be 6.4% greater in steers with LA (p = 0.09), and liver Salmonella prevalence was 9.8 and 6.5% for steers with and without LA, respectively, but did not differ between groups (p = 0.47; Table 4).
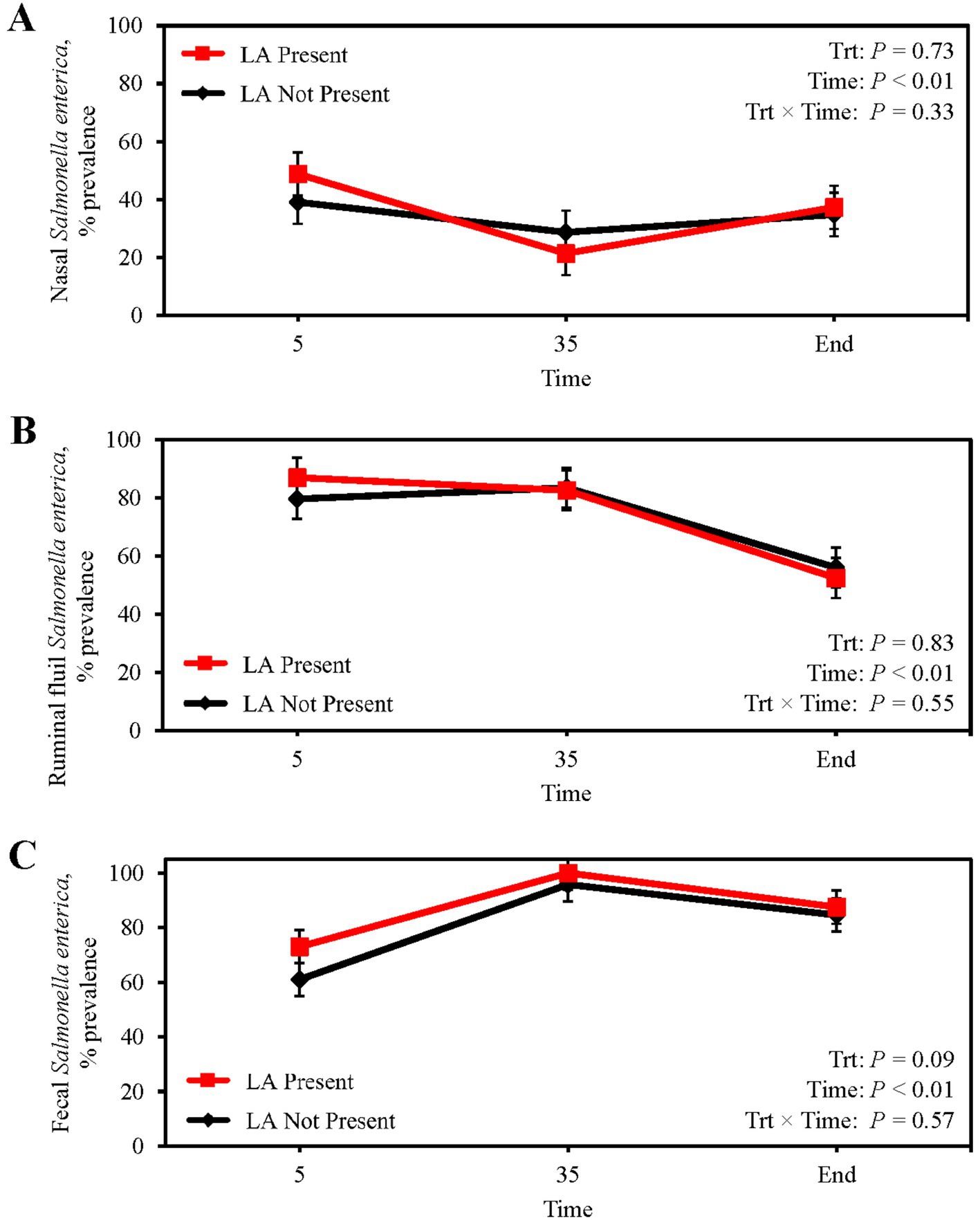
Figure 1. Prevalence of Salmonella enterica in the nasal cavity (A), ruminal fluid (B), and feces (C) of finishing beef steers with (Present; solid red line with square marker) and without (Not Present; solid black line with diamond marker) liver abscesses (LA). Samples were aseptically collected after feedlot arrival (d 5), 1 week after adaptation to the finishing diet (d 35), and the day before harvest (study end). Study end was on d 250 for block 1 steers and d 221 for block 2 steers. Error bars represent standard error of the mean. Trt = treatment effect; Trt × Time = treatment × sampling time effect.
3.2 Absolute abundance and prevalence of Fusobacterium necrophorum and Fusobacterium varium in ruminal fluid
Absolute abundance of F. necrophorum subsp. necrophorum (p = 0.38; Table 5) and subsp. funduliforme (p = 0.23) in ruminal fluid was not different between steers with or without a LA. Similarly, F. necrophorum subsp. necrophorum (p = 0.32) and subsp. funduliforme (p = 0.79) abundance in ruminal fluid did not change from feedlot arrival to harvest. Conversely, the abundance of F. varium in the ruminal fluid of steers with a LA was 192% greater than those without a LA (p < 0.01), although no differences across collection day were observed (p = 0.44). Ruminal fluid prevalence of F. necrophorum subsp. necrophorum, subsp. funduliforme, and F. varium were not suggestive of LA formation (p ≥ 0.16; Figures 2A–C). From feedlot arrival to harvest, F. necrophorum subsp. necrophorum and F. varium prevalence increased 54.4 and 300%, respectively (p < 0.01) regardless of LA prevalence. Mean F. necrophorum subsp. funduliforme prevalence in ruminal fluid was 99.2% and did not differ across collection day (p = 0.14).

Table 5. Absolute abundance (copies/g) of Fusobacterium necrophorum subsp. necrophorum, F. necrophorum subsp. funduliforme, and F. varium in the ruminal fluid of feedlot beef steers with and without liver abscesses.
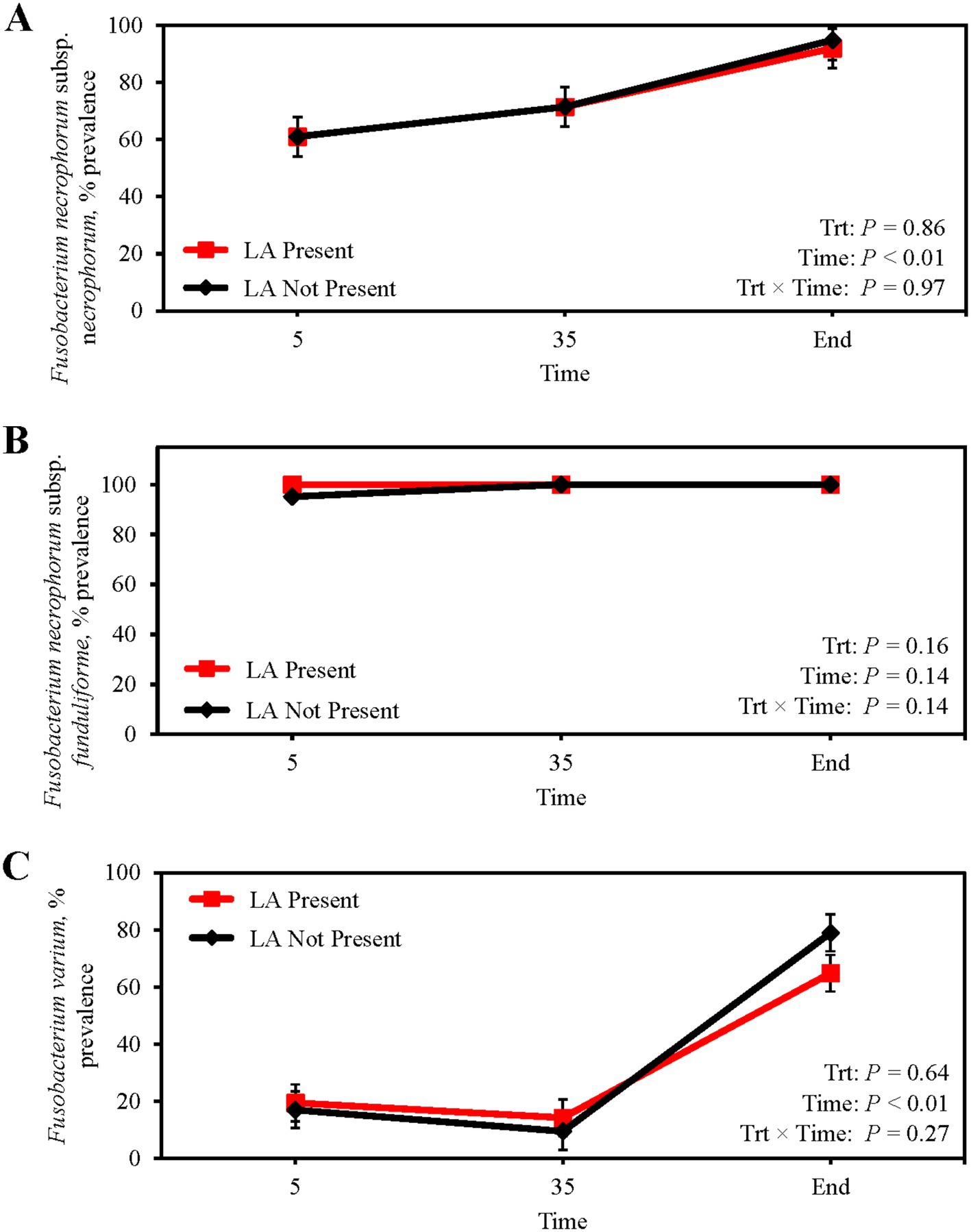
Figure 2. Prevalence of Fusobacterium necrophorum subsp. necrophorum (A) Fusobacterium necrophorum subsp. funduliforme (B) and Fusobacterium varium (C) in the ruminal fluid of finishing beef steers with (Present; solid red line with square marker) and without (Not Present; solid black line with diamond marker) liver abscesses (LA). Samples were aseptically collected after feedlot arrival (d 5), 1 week after adaptation to the finishing diet (d 35), and the day before harvest (study end). Study end was on d 250 for block 1 steers and d 221 for block 2 steers. Error bars represent standard error of the mean. Trt, treatment effect; Trt × Time = treatment × sampling time effect.
3.3 Absolute abundance and prevalence of Fusobacterium necrophorum and Fusobacterium varium in feces
Absolute abundance of F. necrophorum subsp. necrophorum in feces did not differ between steers with or without a LA (p = 0.28; Table 6) or from feedlot arrival to harvest (p = 0.32). Similarly, fecal abundance of F. necrophorum subsp. funduliforme did not differ with the presence of LA (p = 0.19) or longitudinally (p = 0.49). Fusobacterium varium tended to have a greater abundance in the feces of steers without a LA (p = 0.10), although no differences were observed across sampling days (p = 0.35). The presence of LA at harvest was not attributable to differences in the fecal prevalence of F. necrophorum subsp. necrophorum, subsp. funduliforme, and F. varium (p ≥ 0.26; Figures 3A–C). Mean fecal F. necrophorum subsp. necrophorum and subsp. funduliforme prevalence was 16.8 and 30.8%, respectively, and did not differ from feedlot arrival to harvest (p ≥ 0.61). Fecal F. varium prevalence increased from 10.8% at feedlot arrival to 36.3% at harvest (p < 0.01).
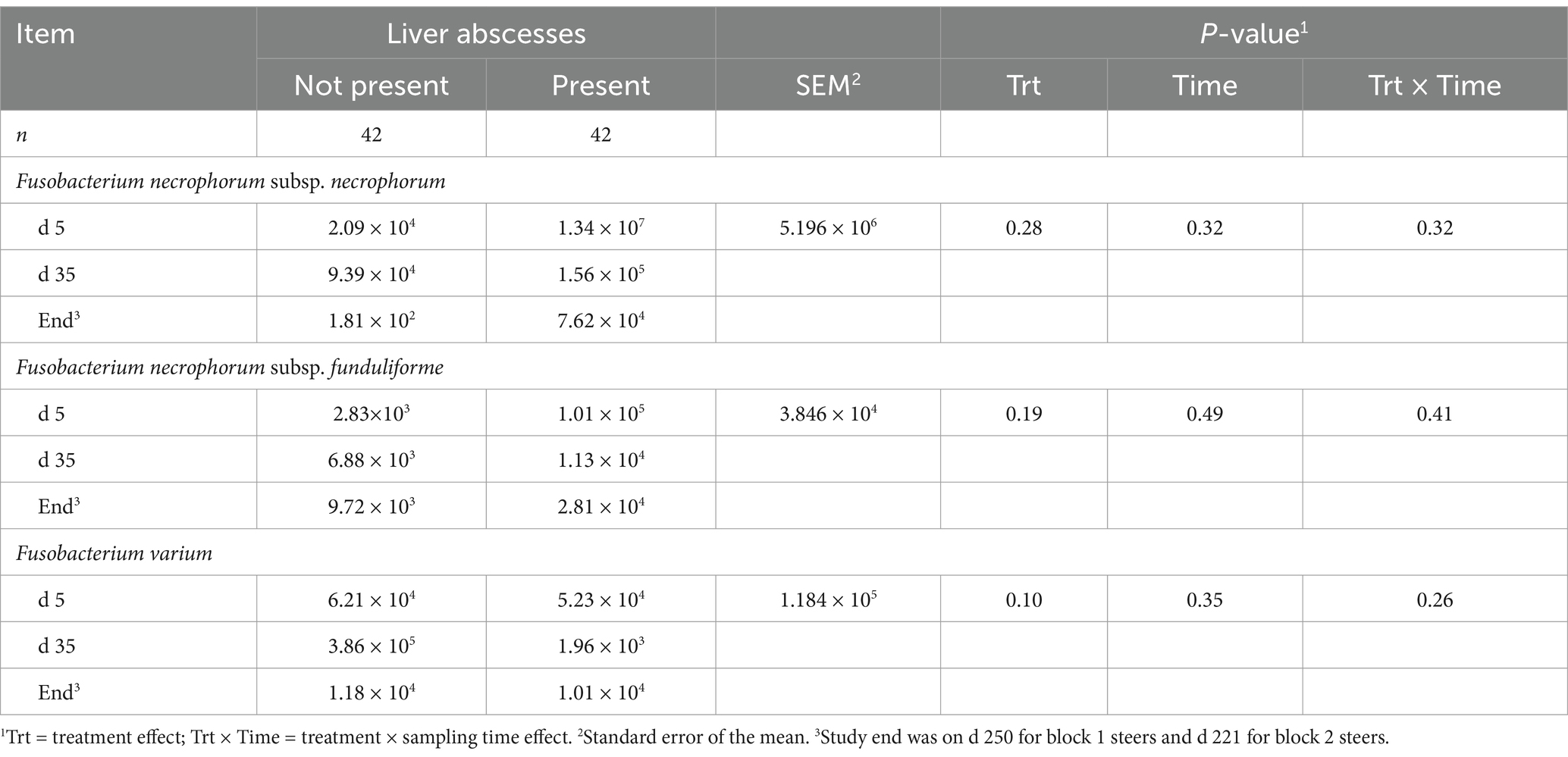
Table 6. Absolute abundance (copies/g) of Fusobacterium necrophorum subsp. necrophorum, F. necrophorum subsp. funduliforme, and F. varium in the feces of feedlot beef steers with and without liver abscesses.
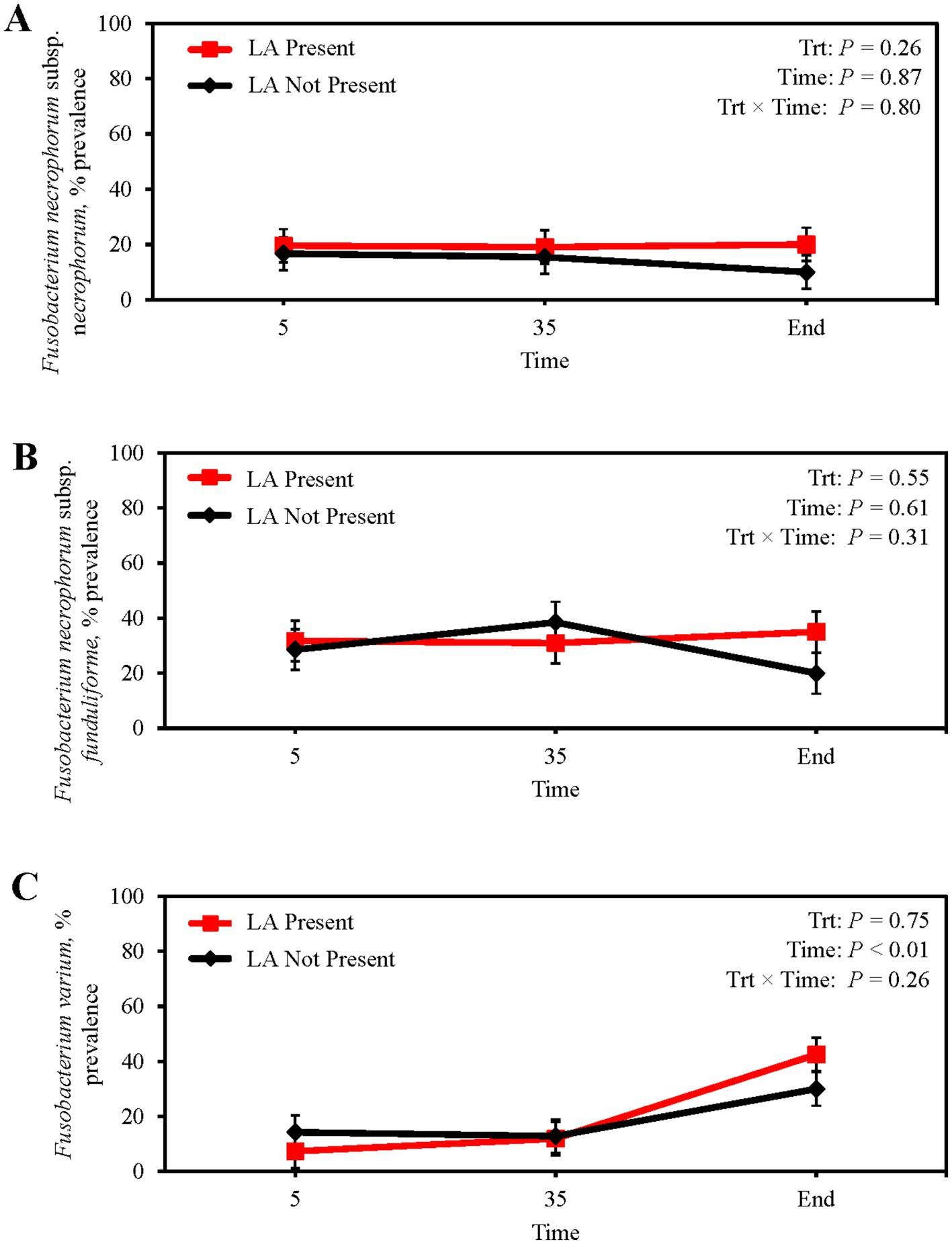
Figure 3. Prevalence of Fusobacterium necrophorum subsp. necrophorum (A) Fusobacterium necrophorum subsp. funduliforme (B) and Fusobacterium varium (C) in the feces of finishing beef steers with (Present; solid red line with square marker) and without (Not Present; solid black line with diamond marker) liver abscesses (LA). Samples were aseptically collected after feedlot arrival (d 5), 1 week after adaptation to the finishing diet (d 35), and the day before harvest (study end). Study end was on d 250 for block 1 steers and d 221 for block 2 steers. Error bars represent standard error of the mean. Trt = treatment effect; Trt × Time = treatment × sampling time effect.
3.4 Absolute abundance and prevalence of F. necrophorum and F. varium in livers.
Although liver F. necrophorum subsp. necrophorum prevalence was not affected by the presence of an abscess (p = 0.67; Table 7), F. necrophorum subsp. necrophorum abundance was 195% greater for steers with a LA compared with those without a LA (p = 0.03). The abundance (p ≥ 0.20) and prevalence (p ≥ 0.65) of F. necrophorum subsp. funduliforme and F. varium in liver were not affected by the presence of an abscess.

Table 7. Absolute abundance and prevalence of Fusobacterium necrophorum subsp. necrophorum, F. necrophorum subsp. funduliforme, and F. varium in the livers of feedlot beef steers with and without liver abscesses.
4 Discussion
Liver abscesses are frequently described as a polymicrobial infection, with most studies concluding that F. necrophorum is the primary causative agent (Broadway et al., 2024). Previous research has supported a causal link between acidosis-induced rumenitis and LA through increased bacterial translocation into portal vein circulation (Smith, 1944; Jensen et al., 1954; Nagaraja and Chengappa, 1998; Tadepalli et al., 2009). Nonetheless, venous drainage is not limited to the rumen, draining the entire GIT and associated visceral organs. Although a few studies have isolated Salmonella from LA (Amachawadi and Nagaraja, 2015; Amachawadi et al., 2017; Herrick et al., 2022), no literature has directly investigated a link between the presence of LA and Salmonella in the GIT.
4.1 Salmonella enterica in the nasal cavity, ruminal fluid, feces, and liver
Salmonella transmission primarily occurs through direct fecal-oral contamination (e.g., from other cattle, rodents, or birds) or indirectly through contaminated feed consumption (Cho et al., 2006; Stevens et al., 2009). Transdermal and intranasal Salmonella infection have also been recorded (Fedorka-Cray et al., 1995; Olafson et al., 2016). In dairy calves experiencing salmonellosis, 18.2% (4/22) of nasal secretions were positive for S. Typhimurium (Nolan et al., 1995). Moreover, dairy calves intranasally inoculated with S. Dublin (1.8 × 106 cells/calf) had positive nasal mucosal secretions for up to 9 d after inoculation and positive feces up to 14 d after inoculation (Nazer and Osborne, 1977). In the current study, nasal Salmonella concentrations remained low from feedlot arrival to harvest and were not indicative of LA presence. Shortly after steers were received in soil-surfaced pens, nasal Salmonella prevalence was 40.9% (Figure 1A); however, after placement onto clean, concrete slated-floor pens, prevalence decreased by 33.3%. Movement of cattle into concrete slated-floor pens likely lessened Salmonella reinfection. Miller et al. (2008) reported an increase in Salmonella enumeration on the hides of cattle exposed to dust. Nevertheless, from d 35 to study end, nasal Salmonella prevalence increased 29.3% despite the final collection occurring in winter. Allan et al. (2004) reported that Salmonella survival on biologically contaminated concrete surfaces was greater compared with non-contaminated concrete surfaces at either 4°C or 10°C. Therefore, accumulation of contaminated feces after 200 d in concrete pens and cross contamination with vectors, like birds and rodents, consuming feed from feed bunks during winter (Gwenzi et al., 2021) increased the likelihood of inhaling Salmonella.
Salmonella concentrations and prevalence in ruminal fluid were not associated with LA presence at harvest. Salmonella has previously been isolated from ruminal fluid at harvest, with prevalence ranging from 0.3 to 91% (Grau et al., 1968; Van Donkersgoed et al., 1999; McEvoy et al., 2003; Fegan et al., 2005). Previous literature attributed the variation in ruminal fluid Salmonella isolation to differences in ruminal pH and volatile fatty acid (VFA) concentrations, which are believed to exert bacteriostatic and bactericidal effects on Salmonella (Mattila et al., 1988; Corrier et al., 1990). To the authors’ knowledge, this study was the first to longitudinally assess Salmonella in the ruminal fluid of finishing beef cattle (Figure 1B). The decrease in both Salmonella concentration and prevalence with days on feed supports the previous notion that the rumen becomes an unfavorable environment for Salmonella persistence and multiplication as total VFA concentrations presumably increase with time on feed. From d 0 to 7, cattle received a high-forage receiving diet before being transitioned to a high-concentrate finishing diet by d 22. Although we did not measure ruminal VFA concentrations in the current study, Penner et al. (2009) reported total VFA concentrations increase, while ruminal pH concurrently decreases, in cattle that are consuming a high-concentrate diet.
In contrast to ruminal fluid, fecal Salmonella concentrations increased with days on feed, likely reflecting the colonization of Salmonella in the lower GIT where fermentative activity is more limited than in the rumen (Bolton et al., 2011). From feedlot arrival to harvest, fecal Salmonella concentrations increased 39.3% regardless of LA presence (Figure 1C). Jennings et al. (2021) previously reported that Salmonella incidence within ileal and colonic epithelial tissues increased with days on feed. From d 5 to 35, fecal Salmonella prevalence increased, corresponding to sample collections from late May to early August. Seasonality can partially explain this phenomenon as warmer seasons are favorable for Salmonella persistence in feedlots compared with colder seasons (Barkocy-Gallagher et al., 2003; Webb et al., 2017; Wottlin et al., 2022). Greater temperatures during summer can induce heat stress, resulting in feed intake disruptions and inflammation that increase Salmonella colonization of the GIT. Likavec et al. (2016) noted Salmonella incidence increased 54% for every 5°C increase in average temperature and 29% for every 5-unit increase in the temperature humidity index. Temperature stability is also important for long-term Salmonella survival in soil and manure (Holley et al., 2006; Semenov et al., 2007). Large fluctuations in manure or soil temperature, such as freeze–thaw cycles associated with colder months, can rapidly decrease environmental Salmonella concentrations (Semenov et al., 2007). This might also explain the decrease in fecal Salmonella prevalence from d 35 to harvest as cattle were harvested in winter when environmental temperature fluctuated between-14°C to 24°C.
From feedlot arrival to harvest, fecal Salmonella concentration tended to be greater in steers without LA, whereas Salmonella prevalence was greater in steers with LA. In the small intestine, Salmonella can either be endocytosed by M cells located in Peyer’s patches or induce cytoskeletal changes in epithelial cells, leading to membrane ruffling and bacterial internalization (Lostroh and Lee, 2001). Entry into cells can result in a pro-inflammatory response and uptake by macrophages and neutrophils (Clark et al., 1994; Johansson et al., 2006). Survival and replication within macrophages are essential for entry and persistence in lymph nodes and eventual liver colonization (Watson and Holden, 2010; Ilyas et al., 2017). Although not measured directly in the current study, the greater fecal Salmonella concentrations coupled with lesser fecal Salmonella prevalence could suggest cattle without LA had improved lower GIT epithelial integrity. In dairy steers ruminally inoculated with both F. necrophorum and S. Lubbock, 60% of ruminal and ileal tissue samples were positive for Salmonella (McDaniel et al., 2024b). While information regarding a synergistic relationship between Salmonella and F. necrophorum remains elusive, greater LA severity and prevalence has been noted when Salmonella and F. necrophorum are cultured in combination from LA (Herrick et al., 2022; McDaniel et al., 2024b). Further studies are warranted to validate these findings and determine whether Salmonella acts as a primary pathogen contributing to LA development or if its presence in the GIT facilitates Fusobacterium entry into the portal circulation.
Nationally, Salmonella has been isolated from 33.3% of LA from fed beef steers, with regional prevalence ranging from 0% in the North Plains and Pacific Northwest to 84.6% in the High Plains (Herrick et al., 2022). Seasonally, LA prevalence is reported to be less in January and greater in late spring/early summer (e.g., April to June; Grimes et al., 2024). In the current study, cattle were harvested from late January to early February. Mean liver Salmonella prevalence was not affected by the presence of LA. Likewise, Dockray (2022) did not find a difference in Salmonella prevalence or concentration between healthy and abscessed livers collected quarterly from commercial beef processing plants in the High Plains region. Although seasonality could potentially explain the decreased Salmonella prevalence observed in the current study compared with regional means, further studies are warranted to elucidate the effects of regionality and seasonality on Salmonella prevalence in healthy and abscessed livers.
4.2 Fusobacterium necrophorum subsp. necrophorum, subsp. funduliforme, and F. varium in ruminal fluid, feces, and liver
Until recently, F. necrophorum enumeration in ruminal contents relied on culture-dependent methods that incorporated selective growth medium containing lactate as the primary carbon source and indole as a growth indicator (Tan et al., 1994); however, this methodology to quantify F. necrophorum unintentionally inflated cell densities because of similar fermentative mechanisms shared with F. varium (Schwarz et al., 2023). Therefore, it is likely that for many years F. necrophorum subsp. necrophorum has been misidentified from culture methods (Schwarz et al., 2023; Deters et al., 2024a). The recent development of a qPCR assay (Deters et al., 2024a) to detect and quantify F. necrophorum subsp. necrophorum, subsp. funduliforme, and F. varium has greatly improved identification and enumeration of these bacterial species across different sample types.
We noted that F. necrophorum subsp. necrophorum and subsp. funduliforme abundance in ruminal fluid were not indicative of LA presence. Although in agreement with Deters et al. (2024a), samples in that study were collected once at harvest, not longitudinally before harvest as in the current study. Unlike Deters et al. (2024a), F. varium abundance in ruminal fluid in the current study was greater in cattle with LA. Nonetheless, the role of F. varium in LA development is still yet to be fully understood. Although F. varium is considered actively invasive (Manson McGuire et al., 2014), it lacks the leukotoxin gene found in F. necrophorum that induces abscess development (Narayanan et al., 2001). Unlike Schwarz et al. (2023), F. varium was not the dominant Fusobacterium species in ruminal fluid in the current study. Differences in sample collection day relative to harvest, feedlot regionality, and sample analysis (i.e., absolute vs. relative abundance) and processing (i.e., samples were not enriched prior to qPCR analysis in the current study) makes a direct comparison between studies difficult. Nevertheless, F. varium has been associated with human infections and diseases such as ulcerative colitis and acute kidney failure (Minami et al., 2009; Lee et al., 2022), justifying further research to understand its pathogenicity and risk as a potential zoonotic cattle pathogen.
From feedlot arrival to harvest, subsp. necrophorum and F. varium prevalence in ruminal fluid increased, a response that could be associated with long-term feeding of high-concentrate diets. Fusobacterium necrophorum can use lactate as a carbon and energy source, and increasing the proportion of grain in finishing cattle diets increases ruminal lactate production (Monteiro and Faciola, 2020) and F. necrophorum concentrations as well (Tan et al., 1996). Although it has been speculated whether F. varium uses lactate as an energy source, the presence of a lactate dehydrogenase gene in the F. varium genome highlights potential overlapping metabolic pathways and similar ecological niches with F. necrophorum (Schwarz et al., 2023).
At harvest, F. necrophorum subsp. necrophorum and F. varium prevalence in ruminal fluid was 93.3 and 71.2%, respectively, regardless of the LA status (Figures 2A,C). Deters et al. (2024a) reported F. necrophorum subsp. necrophorum was more prevalent in the ruminal contents of cattle with LA than without; however, mean subsp. necrophorum prevalence was less than 29% regardless of LA presence. This lead Deters et al. (2024a) to suggest that F. necrophorum subsp. necrophorum is not a normal inhabitant of the rumen. The data presented herein contradict that suggestion and agree with previous reports (Langworth, 1977; Wada, 1978; Smith and Thornton, 1993; Tadepalli et al., 2009) that infer subsp. necrophorum is a normal inhabitant of the rumen. Fusobacterium necrophorum subsp. funduliforme was present in 100% of ruminal fluid samples at harvest in the current study (Figure 2B). Deters et al. (2024a) reported F. necrophorum subsp. funduliforme prevalence was over 90% in ruminal content regardless of the LA status, therein validating subsp. funduliforme as a normal inhabitant of the rumen. Of note, ruminal fluid in the current study was collected from cattle longitudinally at 1 feedlot in the High Plains while Deters et al. (2024a) collected ruminal fluid from cattle originating from 12 feedlots at a Midwest commercial beef abattoir, and a better understanding of regional influences on ruminal F. necrophorum populations could prove worthwhile. Though ruminal fluid was not collected, Herrick et al. (2022) reported the incidence of F. necrophorum subsp. necrophorum and subsp. funduliforme in fed beef livers differed across regions of the U.S., suggesting a potential effect of region currently exists. Additionally, cross-sectional data from Deters et al. (2024a) was gathered from a single collection timepoint, thereby limiting the ability to assess temporal changes associated with dietary transition and disease progression. In contrast, the present study used a longitudinal approach to track shifts in Fusobacterium and Salmonella populations within individual animals over time in response to feedlot management.
Despite the belief that fecal excretion of F. necrophorum is the primary source of foot rot, the presence of F. necrophorum in feces is rare (Nagaraja et al., 2005). Smith and Thornton (1993) reported 2.5% (2/81) of calves sampled on a farm experiencing necrobacillosis were fecal positive for F. necrophorum biovar A (i.e., subsp. necrophorum). This led the authors to conclude that a surprisingly small proportion of cattle were excreting F. necrophorum despite high ruminal prevalence (83%). Moreover, neither fecal nor soil Fusobacterium were selected for model inclusion when estimating the LA occurrence within pens (Weinroth et al., 2019). In English sheep farms, Clifton et al. (2019) reported F. necrophorum was not ubiquitous in soil, and was only cultured from the surface of wet, highly-trafficked areas. This suggests F. necrophorum contamination of soil is transient. Kilama et al. (2024) reported that bull feces were negative for F. necrophorum subsp. necrophorum, subsp. funduliforme, and F. varium when assayed using qPCR; however, 12.5% of fecal samples were F. varium positive following enrichment. In the current study, fecal F. necrophorum subsp. necrophorum and subsp. funduliforme prevalence did not differ from feedlot arrival to harvest and were not associated with LA presence (Figures 3A,B). Jennings et al. (2021) reported colonic F. necrophorum subsp. necrophorum prevalence ranged from 0 to 19.6% over a 231-d feeding trial, with prevalence greatest on d 112. Mean fecal prevalence of F. necrophorum subsp. necrophorum in the current study was 16.8%. The increased prevalence of F. varium from d 35 to harvest is potentially associated with increased ruminal prevalence over the same timeframe (Figure 3C). Nonetheless, the lack of a similar response in F. necrophorum subsp. necrophorum warrants further investigation. Fecal Fusobacterium abundance in the current study was low and not altered by collection day or LA prevalence. Previously, Kim and Wells (2016) assigned 7 bovine fecal microbiome sequences out of 13,663 to Fusobacteria (0.0005%; Kim and Wells, 2016), further suggesting the lower GIT is an unfavorable environment for Fusobacterium survival and proliferation.
The abundance of F. necrophorum subsp. necrophorum in abscessed liver tissue was greater than in healthy liver tissue. This was expected and in agreement with previous reports (Stotz et al., 2021; McDaniel et al., 2024b). An unexpected response, however, was the lack of difference in F. necrophorum subsp. necrophorum prevalence between healthy and abscessed liver tissue. Even though earlier studies have documented F. necrophorum subsp. necrophorum in healthy liver tissue (Stotz et al., 2021; McDaniel et al., 2024a, 2024b), the prevalence of subsp. necrophorum in LA in the current study (54.8%) is less than the reported average of 79.3% for fed beef steers (Herrick et al., 2022). Potential reasons for this disparity include study scale (i.e., feedlot specific vs. national) and that half the liver samples received at LIRU from cattle recorded to have LA did not have a physical abscess in the processed sample. As a result, it is possible the abundance and prevalence reported in the current study is an underestimation.
When rumenitis or intestinal barrier dysfunction occurs, the translocation of gut bacteria and pathogens into portal circulation is not necessarily selective. Hence, it could be expected that greater ruminal fluid prevalence of F. necrophorum subsp. funduliforme will lead to greater liver prevalence of subsp. funduliforme than subsp. necrophorum. Nevertheless, prevalence of subsp. funduliforme in LA was 42.9% in the current study. Herrick et al. (2022) reported subsp. funduliforme prevalence to range from 15.4 to 44.0% in LA, with prevalence being greatest in the Pacific Northwest and lesser in the High Plains and Desert Southwest. Moreover, regardless of region, subsp. funduliforme prevalence in LA was not associated with subsp. necrophorum or Salmonella prevalence in LA (Herrick et al., 2022). Lesser subsp. funduliforme prevalence in LA is likely attributable to the weaker promoter associated with the lktA operon in F. necrophorum subsp. funduliforme, thereby inferring less virulence when compared with subsp. necrophorum. The leukotoxin operon in F. necrophorum subsp. necrophorum and subsp. funduliforme is tricistronic and comprised of lktB, lktA, and lktC genes. Work by Tan et al. (1992) noted F. necrophorum subsp. funduliforme leukotoxin specific mRNA expression was 18-fold less than subsp. necrophorum. Tadepalli et al. (2008) later validated this competitive disadvantage, reporting a 21-fold decrease in gene expression of lktA in F. necrophorum subsp. funduliforme.
The abundance of F. varium in livers was low, with prevalence ranging from 4.8 to 7.1% for healthy and abscessed livers, respectively. Deters et al. (2024b) reported qPCR prevalence of F. varium in LA to be 1% before enrichment and 10.4% after enrichment. As F. varium does not contain the leukotoxin gene found in F. necrophorum subsp. necrophorum and subsp. funduliforme to evade host defense mechanisms, it is not surprising F. varium abundance in the liver is the lowest of the 3 Fusobacterium quantified. Since F. varium is considered actively invasive, it is likely that a portion of F. varium will inevitably reach the liver (Schwarz et al., 2023); however, whether this pathogenesis aids in the ability for subsp. necrophorum to enter portal circulation has yet to be demonstrated.
5 Conclusion
The results of this study provide important insights into the dynamics of Fusobacterium and Salmonella populations within the GIT of feedlot cattle with and without LA. While direct correlations between bacterial populations and LA presence were not observed, the findings herein highlight the complexity of factors influencing pathogen persistence in the GIT. For instance, the observed differences in fecal Salmonella concentration and prevalence between steers with and without LA suggest that gut barrier function may influence the risk of LA development. Moreover, the transition to a high-concentrate diet appears to create an unfavorable environment in the rumen that limits Salmonella persistence but enhances the proliferation of F. necrophorum subsp. necrophorum and F. varium regardless of LA presence. Thus, high-concentrate feedlot diets potentiate the risk of a Fusobacterium infection in the rumen, while facilitating Salmonella persistence in the lower GIT with greater days on feed. Although current results suggest Fusobacterium species are normal inhabitants of the ruminal microbiome in feedlot cattle, fecal Fusobacterium abundance and prevalence is low. Nevertheless, Fusobacterium were prevalent in both healthy and abscessed livers, with subsp. necrophorum abundance being greater in abscessed liver tissue. In conclusion, entry of Fusobacteria and Salmonella into portal circulation is possible throughout the GIT though the abundance and prevalence of these bacterial populations are not directly suggestive of LA formation. These results underscore the need for further investigation into the complex interactions between host immunity, gut microbiome dynamics, and pathogen colonization. Future research should focus on how dietary transitions affect microbial communities in modulating Fusobacterium and Salmonella populations in feedlot cattle. Additionally, studies investigating the effects of feedlot health management practices on gut epithelial integrity and LA formation in feedlot cattle will aid in understanding the broader factors influencing LA susceptibility and progression.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Texas Tech University Institutional Animal Care and Use Committee (approval number 2022–1273). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
CD: Data curation, Formal Analysis, Investigation, Writing – original draft. PB: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing. JW: Investigation, Methodology, Resources, Writing – review & editing. KC: Investigation, Writing – review & editing. AT-S: Investigation, Writing – review & editing. LC: Investigation, Writing – review & editing. NB: Investigation, Writing – review & editing. JM: Formal Analysis, Investigation, Writing – review & editing. CS: Investigation, Writing – review & editing. JL: Investigation, Writing – review & editing. MT: Investigation, Writing – review & editing. TN: Conceptualization, Writing – review & editing. MG: Conceptualization, Writing – review & editing. KH: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Foundation for Food and Agriculture Research (grant no. #ICASALAWG-0000000061).
Acknowledgments
The authors thank the employees at the TTU Burnett Center, Dee Kucera at USDA-MARC, and Jessica Carroll for their hard work, support, and guidance during this project. Any opinions, findings, conclusions, or recommendations expressed in this presentation are those of the authors and do not necessarily reflect the view of the USDA. Mention of trade names or commercial products in this study is solely for the purpose of providing information and does not imply recommendation or endorsement by the USDA. The USDA is an equal opportunity provider and employer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allan, J. T., Yan, Z., Genzlinger, L. L., and Kornacki, J. L. (2004). Temperature and biological soil effects on the survival of selected foodborne pathogens on a mortar surface. J. Food Prot. 67, 2661–2665. doi: 10.4315/0362-028x-67.12.2661
Amachawadi, R. G., and Nagaraja, T. G. (2015). First report of an aerobic isolation of Salmonella enterica from liver abscesses of feedlot cattle. J. Clin. Microbiol. 53, 3100–3101. doi: 10.1128/JCM.01111-15
Amachawadi, R. G., and Nagaraja, T. G. (2016). Liver abscesses in cattle: a review of incidence in Holsteins and of bacteriology and vaccine approaches to control in feedlot cattle. J. Anim. Sci. 94, 1620–1632. doi: 10.2527/jas.2015-0261
Amachawadi, R. G., Purvis, T. J., Lubbers, B. V., Homm, J. W., Maxwell, C. L., and Nagaraja, T. G. (2017). Bacterial flora of liver abscesses in crossbred beef cattle and Holstein steers fed finishing diets with or without tylosin. J. Anim. Sci. 95, 3425–3434. doi: 10.2527/jas.2016.1198
Barkocy-Gallagher, G. A., Arthur, T. M., Rivera-Betancourt, M., Nou, X., Shackelford, S. D., Wheeler, T. L., et al. (2003). Seasonal prevalence of Shiga toxin-producing Escherichia coli including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J. Food Prot. 66, 1978–1986. doi: 10.4315/0362-028X-66.11.1978
Beller, H. R., Kane, S. R., Legler, T. C., and Alvarez, P. J. J. (2002). A real-time polymerase chain reaction method for monitoring anaerobic, hydrocarbon-degrading bacteria based on a catabolic gene. Environ. Sci. Technol. 36, 3977–3984. doi: 10.1021/es025556w
Bolton, D. J., Kelly, S., Lenahan, M., and Fanning, S. (2011). In vitro studies on the effect of pH and volatile fatty acid concentration, as influenced by diet, on the survival of inoculated nonacid- and acid-adapted Salmonella in bovine rumen fluid and feces. Foodborne Pathog. Dis. 8, 609–614. doi: 10.1089/fpd.2010.0713
Brink, D. R., Lowry, S. R., Stock, R. A., and Parrott, J. C. (1990). Severity of liver abscesses and efficiency of feed utilization of feedlot cattle. J. Anim. Sci. 68, 1201–1207. doi: 10.2527/1990.6851201x
Broadway, P. R., Nagaraja, T. G., Lawrence, T. E., Galyean, M. L., and Hales, K. E. (2024). Liver abscesses—new perspectives on a historic fed-cattle issue. Appl. Anim. Sci. 40, 237–243. doi: 10.15232/aas.2023-02498
Brown, T. R., and Lawrence, T. E. (2010). Association of liver abnormalities with carcass grading performance and value. J. Anim. Sci. 88, 4037–4043. doi: 10.2527/jas.2010-3219
Cho, S., Bender, J. B., Diez-Gonzalez, F., Fossler, C. P., Hedberg, C. W., Kaneene, J. B., et al. (2006). Prevalence and characterization of Escherichia coli O157 isolates from Minnesota dairy farms and county fairs. J. Food Prot. 69, 252–259. doi: 10.4315/0362-028x-69.2.252
Clark, M. A., Jepson, M. A., Simmons, N. L., and Hirst, B. H. (1994). Preferential interaction of Salmonella Typhimurium with mouse Peyer’s patch M cells. Res. Microbiol. 145, 543–552. doi: 10.1016/0923-2508(94)90031-0
Clifton, R., Giebel, K., Liu, N. L. B. H., Purdy, K. J., and Green, L. E. (2019). Sites of persistence of Fusobacterium necrophorum and Dichelobacter nodosus: a paradigm shift in understanding the epidemiology of footrot in sheep. Sci. Rep. 9:14429. doi: 10.1038/s41598-019-50822-9
Corrier, D. E., Hinton, A., Ziprin, R. L., Beier, R. C., and DeLoach, J. R. (1990). Effect of dietary lactose on cecal pH, bacteriostatic volatile fatty acids, and Salmonella Typhimurium colonization of broiler chicks. Avian Dis. 34, 617–625. doi: 10.2307/1591254
Deters, A., Shi, X., Bai, J., Kang, Q., Mathieu, J., and Nagaraja, T. G. (2024a). A real-time PCR assay for the detection and quantification of Fusobacterium necrophorum and Fusobacterium varium in ruminal contents of cattle. Appl. Anim. Sci. 40, 250–259. doi: 10.15232/aas.2023-02507
Deters, A., Shi, X., Lawrence, T. E., and Nagaraja, T. G. (2024b). First report of isolation of Fusobacterium varium from liver abscesses and ruminal and colonic epithelial tissues of feedlot cattle. Appl. Anim. Sci. 40, 244–249. doi: 10.15232/aas.2023-02512
Dockray, C. A. (2022). Association of liver abscess presence and epithelial integrity of the hindgut in feedlot cattle to Salmonella carriage in subiliac lymph nodes. M.S. Thesis. West Texas A&M. Available online at: https://hdl.handle.net/11310/5134.
Dornbach, C. W., Hales, K. E., Gubbels, E. R., Wells, J. E., Hoffman, A. A., Hanratty, A. N., et al. (2023). Longitudinal assessment of prevalence and incidence of Salmonella and Escherichia coli O157 resistance to antimicrobials in feedlot cattle sourced and finished in two different regions of the U.S. Foodborne Pathog. Dis. 20, 334–342. doi: 10.1089/fpd.2023.0009
Emery, D. L., Vaughan, J. A., Clark, B. L., Dufty, J. H., and Stewart, D. J. (1985). Cultural characteristics and virulence of strains of Fusobacterium necrophorum isolated from the feet of cattle and sheep. Aust. Vet. J. 62, 43–46. doi: 10.1111/j.1751-0813.1985.tb14231.x
Fedorka-Cray, P. J., Kelley, L. C., Stabel, T. J., Gray, J. T., and Laufer, J. A. (1995). Alternate routes of invasion may affect pathogenesis of Salmonella Typhimurium in swine. Infect. Immun. 63, 2658–2664. doi: 10.1128/IAI.63.7.2658-2664.1995
Fegan, N., Vanderlinde, P., Higgs, G., and Desmarchelier, P. (2005). A study of the prevalence and enumeration of Salmonella enterica in cattle and on carcasses during processing. J. Food Prot. 68, 1147–1153. doi: 10.4315/0362-028x-68.6.1147
Grau, F. H., Brownlie, L. E., and Roberts, E. A. (1968). Effect of some preslaughter treatments on the Salmonella population in the bovine rumen and faeces. J. Appl. Bacteriol. 31, 157–163. doi: 10.1111/j.1365-2672.1968.tb00353.x
Grimes, B. B., McEvers, T. J., Tennant, T. C., Johnson, J. W., and Lawrence, T. E. (2024). Relationship of liver abnormalities with carcass performance and value. Appl. Anim. Sci. 40, 358–375. doi: 10.15232/aas.2023-02482
Gwenzi, W., Chaukura, N., Muisa-Zikali, N., Teta, C., Musvuugwa, T., Rzymski, P., et al. (2021). Insects, rodents, and pets as reservoirs, vectors, and sentinels of antimicrobial resistance. Antibiotics (Basel) 10:68. doi: 10.3390/antibiotics10010068
Herrick, R. T., Rogers, C. L., McEvers, T. J., Amachawadi, R. G., Nagaraja, T. G., Maxwell, C. L., et al. (2022). Exploratory observational quantification of liver abscess incidence, specific to region and cattle type, and their associations to viscera value and bacterial flora. Appl. Anim. Sci. 38, 170–182. doi: 10.15232/aas.2021-02228
Holley, R. A., Arrus, K. M., Ominski, K. H., Tenuta, M., and Blank, G. (2006). Salmonella survival in manure-treated soils during simulated seasonal temperature exposure. J. Environ. Qual. 35, 1170–1180. doi: 10.2134/jeq2005.0449
Ibarra, J. A., and Steele-Mortimer, O. (2009). Salmonella--the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell. Microbiol. 11, 1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x
Ilyas, B., Tsai, C. N., and Coombes, B. K. (2017). Evolution of Salmonella-host cell interactions through a dynamic bacterial genome. Front. Cell. Infect. Microbiol. 7:428. doi: 10.3389/fcimb.2017.00428
Jang, S. S., and Hirsh, D. C. (1994). Characterization, distribution, and microbiological associations of Fusobacterium spp. in clinical specimens of animal origin. J. Clin. Microbiol. 32, 384–387. doi: 10.1128/jcm.32.2.384-387.1994
Jennings, J. S., Amachawadi, R. G., Narayanan, S. K., Nagaraja, T. G., Tedeschi, L. O., Smith, W. N., et al. (2021). Effects of corn stalk inclusion and tylosin on performance, rumination, ruminal papillae morphology, and gut pathogens associated with liver abscesses from finishing beef steers. Livest. Sci. 251:104623. doi: 10.1016/j.livsci.2021.104623
Jensen, R., Deane, H. M., Cooper, L. J., Miller, V. A., and Graham, W. R. (1954). The rumenitis-liver abscess complex in beef cattle. Am. J. Vet. Res. 15, 202–216.
Johansson, C., Ingman, M., and Wick, M. J. (2006). Elevated neutrophil, macrophage, and dendritic cell numbers characterize immune cell populations in mice chronically infected with Salmonella. Microb. Pathog. 41, 49–58. doi: 10.1016/j.micpath.2006.03.004
Kilama, J., Dahlen, C. R., Abbasi, M., Shi, X., Nagaraja, T. G., Crouse, M. S., et al. (2024). Fusobacterium necrophorum and Fusobacterium varium are commensal members of the bovine reproductive microbiota and may colonize calf prenatally. J. Anim. Sci. 2024:546. doi: 10.1101/2024.10.15.618546
Kim, M., and Wells, J. E. (2016). A meta-analysis of bacterial diversity in the feces of cattle. Curr. Microbiol. 72, 145–151. doi: 10.1007/s00284-015-0931-6
Langworth, B. F. (1977). Fusobacterium necrophorum: its characteristics and role as an animal pathogen. Bacteriol. Rev. 41, 373–390. doi: 10.1128/br.41.2.373-390.1977
Lawrence, T. (2024). Liver abscesses: Detrimental to offal and carcass value. Plains nutrition council 2024 spring conference proceedings, Amarillo, TX.
Lechtenberg, K. F., Nagaraja, T. G., Leipold, H. W., and Chengappa, M. M. (1988). Bacteriologic and histologic studies of hepatic abscesses in cattle. Am. J. Vet. Res. 49, 58–62. doi: 10.2460/ajvr.1988.49.01.58
Lee, S. J., Baek, Y. J., Kim, J. N., Lee, K. H., Lee, E. H., Yeom, J. S., et al. (2022). Increasing Fusobacterium infections with Fusobacterium varium, an emerging pathogen. PLoS One 17:e0266610. doi: 10.1371/journal.pone.0266610
Likavec, T., Pires, A. F., and Funk, J. A. (2016). Association between thermal environment and Salmonella in fecal samples from dairy cattle in midwestern United States. Can. J. Vet. Res. 80, 183–188.
Lostroh, C. P., and Lee, C. A. (2001). The Salmonella pathogenicity island-1 type III secretion system. Microbes Infect. 3, 1281–1291. doi: 10.1016/S1286-4579(01)01488-5
Manson McGuire, A., Cochrane, K., Griggs, A. D., Haas, B. J., Abeel, T., Zeng, Q., et al. (2014). Evolution of invasion in a diverse set of Fusobacterium species. MBio 5:e01864. doi: 10.1128/mBio.01864-14
Mattila, T., Frost, A. J., and O’Boyle, D. (1988). The growth of Salmonella in rumen fluid from cattle at slaughter. Epidemiol. Infect. 101, 337–345. doi: 10.1017/S0950268800054273
McDaniel, Z. S., Hales, K. E., Nagaraja, T. G., Lawrence, T. E., Tennant, T. C., Amachawadi, R. G., et al. (2024b). Validation of an experimental model to induce liver abscesses in Holstein steers using an acidotic diet challenge and intraruminal bacterial inoculation. Appl. Anim. Sci. 40, 398–413. doi: 10.15232/aas.2023-02485
McDaniel, Z. S., Hales, K. E., Salih, H., Deters, A., Shi, X., Nagaraja, T. G., et al. (2024a). Development of an experimental model for liver abscess induction in Holstein steers using an acidotic diet challenge and bacterial inoculation. J. Anim. Sci. 102:skae046. doi: 10.1093/jas/skae046
McEvoy, J. M., Doherty, A. M., Sheridan, J. J., Blair, I. S., and McDowell, D. A. (2003). The prevalence of Salmonella spp. in bovine fecal, rumen, and carcass samples at a commercial abattoir. J. Appl. Microbiol. 94, 693–700. doi: 10.1046/j.1365-2672.2003.01898.x
Miller, M. F., Loneragan, G. H., Harris, D. D., Adams, K. D., Brooks, J. C., and Brashears, M. M. (2008). Environmental dust exposure as a factor contributing to an increase in Escherichia coli O157 and Salmonella populations on cattle hides in feedyards. J. Food Prot. 71, 2078–2081. doi: 10.4315/0362-028x-71.10.2078
Minami, M., Ando, T., Okamoto, A., Sasaki, N., Ohkura, T., Torii, K., et al. (2009). Seroprevalence of Fusobacterium varium in ulcerative colitis patients in Japan. FEMS Immunol. Med. Microbiol. 56, 67–72. doi: 10.1111/j.1574-695X.2009.00550.x
Monteiro, H. F., and Faciola, A. P. (2020). Ruminal acidosis, bacterial changes, and lipopolysaccharides. J. Anim. Sci. 98:skaa248. doi: 10.1093/jas/skaa248
Nagaraja, T. G., and Chengappa, M. M. (1998). Liver abscesses in feedlot cattle: a review. J. Anim. Sci. 76, 287–298. doi: 10.2527/1998.761287x
Nagaraja, T. G., and Lechtenberg, K. F. (2007). Acidosis in feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 23, 333–350. doi: 10.1016/j.cvfa.2007.04.002
Nagaraja, T. G., Narayanan, S. K., Stewart, G. C., and Chengappa, M. M. (2005). Fusobacterium necrophorum infections in animals: pathogenesis and pathogenic mechanisms. Anaerobe 11, 239–246. doi: 10.1016/j.anaerobe.2005.01.007
Narayanan, S. K., Nagaraja, T. G., Chengappa, M. M., and Stewart, G. C. (2001). Cloning, sequencing, and expression of the leukotoxin gene from Fusobacterium necrophorum. Infect. Immun. 69, 5447–5455. doi: 10.1128/IAI.69.9.5447-5455.2001
Nazer, A. H., and Osborne, A. D. (1977). Experimental Salmonella Dublin infection in calves. Br. Vet. J. 133, 388–398. doi: 10.1016/S0007-1935(17)34040-X
Nolan, L. K., Giddings, C. W., Boland, E. W., Steffen, D. J., Brown, J., and Misek, A. (1995). Detection and characterization of Salmonella Typhimurium from a dairy herd in North Dakota. Vet. Res. Commun. 19, 3–8. doi: 10.1007/BF01839246
Olafson, P. U., Brown, T. R., Lohmeyer, K. H., Harvey, R. B., Nisbet, D. J., Loneragan, G. H., et al. (2016). Assessing transmission of Salmonella to bovine peripheral lymph nodes upon horn fly feeding. J. Food Prot. 79, 1135–1142. doi: 10.4315/0362-028X.JFP-15-414
Penner, G. B., Taniguchi, M., Guan, L. L., Beauchemin, K. A., and Oba, M. (2009). Effect of dietary forage-to-concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J. Dairy Sci. 92, 2767–2781. doi: 10.3168/jds.2008-1716
Pinnell, L. J., and Morley, P. S. (2022). The microbial ecology of liver abscesses in cattle. Vet. Clin. North Am. Food Anim. Pract. 38, 367–381. doi: 10.1016/j.cvfa.2022.08.004
Rahn, K., De Grandis, S. A., Clarke, R. C., McEwen, S. A., Galán, J. E., Ginocchio, C., et al. (1992). Amplification of an invA gene sequence of Salmonella Typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol. Cell. Probes 6, 271–279. doi: 10.1016/0890-8508(92)90002-F
Sanz-Fernandez, M. V., Daniel, J. B., Seymour, D. J., Kvidera, S. K., Bester, Z., Doelman, J., et al. (2020). Targeting the hindgut to improve health and performance in cattle. Animals (Basel) 10:1817. doi: 10.3390/ani10101817
Schwarz, C., Mathieu, J., Gomez, J. L., Miller, M. R., Tikhonova, M., Nagaraja, T. G., et al. (2023). Unexpected finding of Fusobacterium varium as the dominant Fusobacterium species in cattle rumen: potential implications for liver abscess etiology and interventions. J. Anim. Sci. 101:skad130. doi: 10.1093/jas/skad130
Seimiya, Y. M., Takahashi, M., Tamura, T., Murakami, R., Haritani, M., and Kimura, K. M. (2004). Fibrinonecrotic rhinitis caused by a concurrent infection of Fusobacterium necrophorum and Arcanobacterium pyogenes in a cow. J. Vet. Med. Sci. 66, 985–987. doi: 10.1292/jvms.66.985
Semenov, A. V., van Bruggen, A. H. C., van Overbeek, L., Termorshuizen, A. J., and Semenov, A. M. (2007). Influence of temperature fluctuations on Escherichia coli O157:H7 and Salmonella enterica server Typhimurium in cow manure. FEMS Microbiol. Ecol. 60, 419–428. doi: 10.1111/j.1574-6941.2007.00306.x
Smith, H. A. (1944). Ulcerative lesions of the bovine rumen and their possible relation to hepatic abscesses. Am. J. Vet. Res. 5, 234–242.
Smith, G. R., and Thornton, E. A. (1993). The prevalence of Fusobacterium necrophorum biovar a in animal feces. Epidemiol. Infect. 110, 327–331. doi: 10.1017/s0950268800068266
Stevens, M. P., Humphrey, T. J., and Maskell, D. J. (2009). Molecular insights into farm animal and zoonotic Salmonella infections. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 364, 2709–2723. doi: 10.1098/rstb.2009.0094
Stotz, M. K., Henry, D. D., and Crossland, W. L. (2021). Characterization of bacterial DNA identified in abscessed and non-abscessed bovine hepatic tissue at the time of harvest. J. Anim. Sci. 99:skab280. doi: 10.1093/jas/skab280
Tadepalli, S., Narayanan, S. K., Stewart, G. C., Chengappa, M. M., and Nagaraja, T. G. (2009). Fusobacterium necrophorum: a ruminal bacterium that invades the liver to cause abscesses in cattle. Anaerobe 15, 36–43. doi: 10.1016/j.anaerobe.2008.05.005
Tadepalli, S., Stewart, G. C., Nagaraja, T. G., and Narayanan, S. K. (2008). Leukotoxin operon and differential expressions of the leukotoxin gene in bovine Fusobacterium necrophorum subspecies. Anaerobe 14, 13–18. doi: 10.1016/j.anaerobe.2007.09.001
Tan, Z. L., Nagaraja, T. G., and Chengappa, M. M. (1992). Factors affecting the leukotoxin activity of Fusobacterium necrophorum. Vet. Microbiol. 32, 15–28. doi: 10.1016/0378-1135(92)90003-c
Tan, Z. L., Nagaraja, T. G., and Chengappa, M. M. (1994). Selective enumeration of Fusobacterium necrophorum from the bovine rumen. Appl. Environ. Microbiol. 60, 1387–1389. doi: 10.1128/aem.60.4.1387-1389.1994
Tan, Z. L., Nagaraja, T. G., and Chengappa, M. M. (1996). Fusobacterium necrophorum infections: virulence factors, pathogenic mechanism, and control measures. Vet. Res. Commun. 20, 113–140. doi: 10.1007/BF00385634
Van Donkersgoed, J., Graham, T., and Gannon, V. (1999). The prevalence of verotoxins, Escherichia coli O157:H7 and Salmonella in the faeces and rumen of cattle at processing. Can. Vet. J. 40, 332–338.
Wada, E. (1978). Studies on Fusobacterium species in the rumen of cattle. I. Isolation of genus Fusobacterium from rumen juice of cattle. Nippon Juigaku Zasshi 40, 435–439. doi: 10.1292/jvms1939.40.435
Watson, K. G., and Holden, D. W. (2010). Dynamics of growth and dissemination of Salmonella in vivo. Cell. Microbiol. 12, 1389–1397. doi: 10.1111/j.1462-5822.2010.01511.x
Webb, H. E., Brichta-Harhay, D. M., Brashears, M. M., Nightingale, K. K., Arthur, T. M., Bosilevac, J. M., et al. (2017). Salmonella in peripheral lymph nodes of healthy cattle at slaughter. Front. Microbiol. 8:2214. doi: 10.3389/fmicb.2017.02214
Weinroth, M. D., Martin, J. N., Doster, E., Geornaras, I., Parker, J. K., Carlson, C. R., et al. (2019). Investigation of tylosin in feed of feedlot cattle and effects on liver abscess prevalence, and fecal and soil microbiomes and resistomes1. J. Anim. Sci. 97, 4567–4578. doi: 10.1093/jas/skz306
Keywords: feedlot beef cattle, Fusobacterium necrophorum , Fusobacterium varium, Salmonella , liver abscess
Citation: Dornbach CW, Broadway PR, Wells JE, Childress KD, Thompson-Smith AC, Canterbury LG, Burdick Sanchez NC, Mathieu J, Schwarz C, Laverde Gomez J, Tikhonova M, Nagaraja TG, Galyean ML and Hales KE (2025) Longitudinal assessment of the prevalence of Fusobacterium necrophorum, Fusobacterium varium, and Salmonella enterica in the nasal cavity, ruminal fluid, and feces of finishing beef steers with and without liver abscesses. Front. Microbiol. 16:1565303. doi: 10.3389/fmicb.2025.1565303
Edited by:
Swayam Prakash, University of California, Irvine, United StatesReviewed by:
Bibek G. C., University of Texas Health Science Center at Houston, United StatesMoiz Ashraf Ansari, Texas A and M University, United States
Andrea Cunha, Cooperativa de Ensino Superior Politécnico e Universitário, Portugal
Copyright © 2025 Dornbach, Broadway, Wells, Childress, Thompson-Smith, Canterbury, Burdick Sanchez, Mathieu, Schwarz, Laverde Gomez, Tikhonova, Nagaraja, Galyean and Hales. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristin E. Hales, a3Jpc3Rpbi5oYWxlc0B0dHUuZWR1
 Colten W. Dornbach
Colten W. Dornbach Paul R. Broadway
Paul R. Broadway James E. Wells
James E. Wells Kallie D. Childress
Kallie D. Childress Aubrey C. Thompson-Smith1
Aubrey C. Thompson-Smith1 Nicole C. Burdick Sanchez
Nicole C. Burdick Sanchez T. G. Nagaraja
T. G. Nagaraja Kristin E. Hales
Kristin E. Hales