- 1Department of Biological Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI, United States
- 2Center for Cancer Immunotherapy, La Jolla Institute for Immunology, La Jolla, CA, United States
- 3Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee, Milwaukee, WI, United States
- 4Department of Biomedical Sciences, University of Wisconsin-Milwaukee, Milwaukee, WI, United States
The decades-long gap in antibiotic discovery has led to a significant health crisis due to antimicrobial resistance (AMR). The bacterial genus Xenorhabdus, which forms symbiotic relationships with the soil nematode Steinernema, are known to secrete a variety of antimicrobial compounds with potential effectiveness against AMR. These antimicrobial compounds are primarily bio-synthesized by non-ribosomal peptide synthetases (NRPS) and polyketide synthase (PKS) genes. In this study, we report that X. szentirmaii produces high levels of antibiotic activity during the stationary phase against diverse bacteria including known antibiotic resistant pathogens. It possesses 17 operons to encode predicted NRPS and PKS enzymes, designated as ste1 through ste17. The ste15-ste16 and ste17 operons are predicted to produce the known antibiotics Pax peptide and Fabclavine, respectively. Additionally, the newly identified operons ste3, ste4, ste5, ste8, ste9, and ste14 consist of single genes, each containing two or more NRPS genes. The ste13 operon harbors two NRPS genes, while the ste7 and ste12 operons contain three NRPS genes each. Further, RNA-seq analysis showed that lsrF that encodes a quorum sensing autoinducer-2 (AI-2) thiolase was expressed at high levels during stationary phase. These findings provide evidence that X. szentirmaii uses quorum sensing (QS) to synchronize the expression of multiple NRPS and PKS enzymes responsible for synthesizing various antimicrobial compounds. This study underscores the potential to leverage these regulatory insights for maximizing commercial applications of novel antibiotics combating AMR, as well as broader industrial uses.
Introduction
Antimicrobial resistance (AMR) is a major health crisis with a growing number of bacteria and fungi becoming resistant to current antibiotics (Brown and Wright, 2016). Approximately 4.95 million people die each year due to AMR infections (Antimicrobial Resistance, 2022). Resistance mechanisms in bacteria and fungi involve reducing drug uptake, altering the drug’s target, inactivating the drug, and actively expelling the drug (Reygaert, 2018). This rapid rise and prevalence of AMR underscores the urgent need to discover new antibiotics from natural sources with new modes of action and new chemistries. While significant efforts have been made to identify new antibiotics from natural resources, such as the well-studied bacterial genus Streptomyces (Chevrette et al., 2019), a promising new untapped natural resource of antibiotics lies within the Gram-negative Xenorhabdus bacteria, belonging to the order of Enterobacterales (Forst et al., 1997; Herbert and Goodrich-Blair, 2007; Booysen and Dicks, 2020).
Many of the antimicrobial compounds used in medicine today are produced by non-ribosomal peptide synthetase (NRPS) and polyketide synthase (PKS) genes (Snyder et al., 2007; Wang et al., 2014; Schwarzer et al., 2003; Sussmuth and Mainz, 2017; Felnagle et al., 2008). NRPSs also produce other secondary metabolites (SMs) such a siderophores, pigments, toxins, and immunosuppressive compounds (Snyder et al., 2007; Wang et al., 2014). Studies reveal that NRPSs are modular enzymes of three core domains consisting of an adenylation (A) domain, a peptidyl carrier protein transfer (PCP or P) domain and a condensation (C) domain (Drake et al., 2016; Tanovic et al., 2008). The A-domain performs the selection and activation of a specific amino acid. The PCP-domain shuttles the amino acid or peptide between A and C-domains facilitated by an enzyme phosphopantetheinyl transferase encoded by a conserved gene ngrA (Beld et al., 2014). Therefore, inactivation of ngrA abolishes the synthesis of all NRPS-derived antimicrobials (Ciezki et al., 2019). The C-domain accepts the amino acid and catalyzes the formation of a peptide bond. The number of peptide bonds in a non-ribosomal peptide (NRP) depends on the number and order of the NRPS modules (Bushley and Turgeon, 2010). The modular nature of NRPSs make them flexible to produce a wide range of structurally diverse antibiotics.
Bacterial species of the genus Xenorhabdus form species-specific mutualistic associations by colonizing the intestines of the soil-dwelling entomopathogenic Steinernema nematodes (EPNs) (Forst et al., 1997; Herbert and Goodrich-Blair, 2007; Booysen and Dicks, 2020). These EPNs with the Xenorhabdus bacteria invade insect larvae through natural openings (e.g., mouth and anus), migrate to the insect midgut, perforate the intestinal wall and enter the hemocoel where they release their Xenorhabdus symbionts (Snyder et al., 2007). The released Xenorhabdus bacteria multiply within the insect larvae and employ a dual strategy to utilize insects as a nutrient resource by (I) producing diverse toxins to kill the insects and (II) secreting numerous antibiotics to suppress growth of competing microbes. Both EPNs and Xenorhabdus utilize the nutrient resources of the insect cadaver to support their growth and reproduction.
Xenorhabdus nematophila is the most extensively studied species of Xenorhabdus. It contains multiple NRPS gene clusters that encode several small peptide compounds (Singh et al., 2015; Roumia et al., 2022). These compounds are composed of between 2 and 10 amino acids with a variety of modifications, including D-isomerization and cyclization (Wang et al., 2014). One of these antibiotics, called odilorhabdin, interacts with both a specific site on the 16S rRNA of bacterial ribosome and the anticodon loop of the A-site tRNA, thus inhibiting the ribosome-mediated cellular protein synthesis (Pantel et al., 2018). Additionally, odilorhabdin has been shown to reduce bacterial infections in animal models (Pantel et al., 2018). It is noteworthy that odilorhabdin binds to a novel site on the ribosomal 16S rRNA molecule that has not been targeted by any current antibiotics, suggesting that odilorhabdin is a new type of ribosome inhibitor. While X. nematophila has been studied extensively, the less well studied X. szentirmaii was found to produce higher levels of antimicrobial activity against a variety of microbial species including animal, plant, and fungal pathogens (Ciezki et al., 2019). Antibiotics of X. szentirmaii were also found to be active against other species of Xenorhabdus (Ciezki et al., 2019). This makes X. szentirmaii an excellent source for the discovery and isolation of novel antimicrobial compounds that have potential utility in medicine and agriculture.
Tobias et al. (2017) performed a comprehensive genomic and metabolomic data of Xenorhabdus strains, including the draft genome sequences of X. szentirmaii. Their study cataloged the NRPS-containing gene clusters in Xenorhabdus, including five such clusters in the X. szentirmaii genome (Tobias et al., 2017). Additionally, they fractionated cell-free liquid cultures of X. szentirmaii by HPLC followed by high-resolution electrospray ionization mass spectrometry (MS). This analysis led to the identification of five compounds (i.e., Xenematide, GameX peptide, Rhabdopeptide, Xenoamicin, Szentiamide) predicted to be synthesized by NRPS clusters. It should be noted that they did not identify the compound Fabclavine, although the related NRPS gene cluster was predicted. Conversely, the compound Xenematide was identified (Crawford et al., 2011), but not its corresponding gene clusters. Thus, the relationship between the biosynthetic gene clusters (BCGs) in the X. szentirmaii genome and its antibiotic production remains to be fully elucidated.
Antibiotics are a type of secondary metabolite typically produced by bacteria in planktonic culture during the stationary phase (Davies, 2013; Demain and Fang, 2000). These secondary metabolites confer competitive advantages by producing antimicrobial compounds (e.g., NRPs), influencing microbial survival through the synthesis of pigments and toxins (e.g., terpenes) and contributing to biofilm formation (e.g., phenazines) (Hibbing et al., 2010). They also play important roles in nutrient acquisition (Wang et al., 2011) and quorum sensing (Dietrich et al., 2006). Quorum sensing (QS), also known as density sensing, regulates a variety of cellular behavior, including bacterial luminescence, spore formation and biofilm formation, and antimicrobial drug resistance (Jiang et al., 2019; Zhao et al., 2020). Consequently, bacterial QS system is a promising therapeutic target for antibacterial strategies.
The QS system generally operates on three fundamental principles. First, members of the bacterial community produce signaling molecule known as autoinducers (AIs). AIs, at low cell densities, diffuse away, resulting in concentrations too low to be detected. However, at high cell densities, the cumulative production of AIs creates a localized high concentration, allowing detection and triggering a response (Kaplan and Greenberg, 1985). Second, AIs are recognized by receptors located in the cytoplasm or embedded in the cell membrane. Third, detection of AIs not only activates the expression of genes required for cooperative behavior but also stimulates additional AI production (Seed et al., 1995). This feed-forward autoinduction loop likely facilitates synchronized activity within the population and control a variety of bacterial behavior. However, the regulatory mechanism linking quorum sensing to microbial resistance and antimicrobial production is still unclear.
The antimicrobial compounds secreted by Xenorhabdus species have yet to achieve commercial utilization due to several technical challenges, such as insufficient knowledge of their molecular biochemistry, difficulties in large-scale purification, and the high cost (Dadgostar, 2019). To identify and characterize the antimicrobial compounds produced by X. szentirmaii, understanding the fundamental regulatory mechanisms governing antibiotic production by its NRPS and PKS genes is essential. In this study, we adopted a global bioinformatics approach to identify the potential biosynthetic gene clusters in the X. szentirmaii genome associated with antibiotic production. This involved performing NCBI BLAST searches using the PCP domain, followed by systematic analysis of the identified biosynthetic gene cluster. Through RNA-sequencing and subsequent mutational analyses, we identified novel operons with optimal expression in the stationary phase, which are associated with antimicrobial production and regulated by QS mechanisms.
Materials and methods
Bacterial strains
The following strains were used in this study: Xenorhabdus szentirmaii strain DSM16338 (nematode Steinernema Rerum), Xenorhabdus nematophila strain ATCC19061 (nematode Steinernema carpocapsae), Staphylococcus saprophyticus, Escherichia coli DH5α, a clinical ESBL-producing uropathogenic E. coli MWDL6 strain (Liedhegner et al., 2022), methicillin-resistant Staphylococcus aureus ATCC 43300, Enterococcus faecium ATCC 7171, Staphylococcus epidermidis, Klebsiella pneumoniae subsp. pneumoniae ATCC 33495, Neisseria flava, Streptococcus pneumoniae ATCC 6305, Acinetobacter baumannii ATCC 19606, and Candida albicans (ATCC 14053).
Overlay assay
Five μL culture of X. szentirmaii (OD600 = 1) was spotted on an LB agar plate. The plate was placed in a 30°C incubator overnight. The cells were then killed by chloroform vapor. One ml of tester strain (OD600 = 1) was mixed into 5 mL of soft agar medium (LB plus 0.7% agar) and overlaid onto the LB agar plate containing the killed bacteria. The plate was placed into a 37°C incubator overnight. For overlay assay with the cell-free supernatant: 5 μL of cell-free supernatant was spotted and dried on the LB-agar plate before overlaying with the tester strain.
Cross-streak analysis
Placed 30 μL of a diluted X. szentirmaii culture in the middle of a 150 mm Mueller Hinton (MH) plate and incubate for 48 h at 30°C. On the back of the plates, draw radiating lines 0.5 cm from the X. szentirmaii colony. Overnight cultures of each test strain were diluted to a 0.5 MacFarland and then streaked along its designated line. Growth inhibition was measured post-24 h and recorded. As a negative control to ensure neighboring bacteria did not inhibit each other, 30 μL of sterile phosphate buffered saline was added to a separate MH plate and test strains plated in the same orientation. No inhibition was evident between any of the strains (data not shown).
Plasmid construction
Two primers (forward primer with a Kpn1 site and reverse primer with a Bamh1 site) were used to amplify the upstream ~500 bases of our gene of interest (ste2, ngrA or lsrF) using the primers of interest (see Table 1). Both pKNOCK plasmid and PCR products were digested with the Kpn1-BamH1. The digested pKNOCK vector was then ligated with the PCR product to create various pKNOCK-yfg (your favorite gene) plasmids and transformed into E. coli S17 competent cells.
Bacterial gene disruption by conjugation
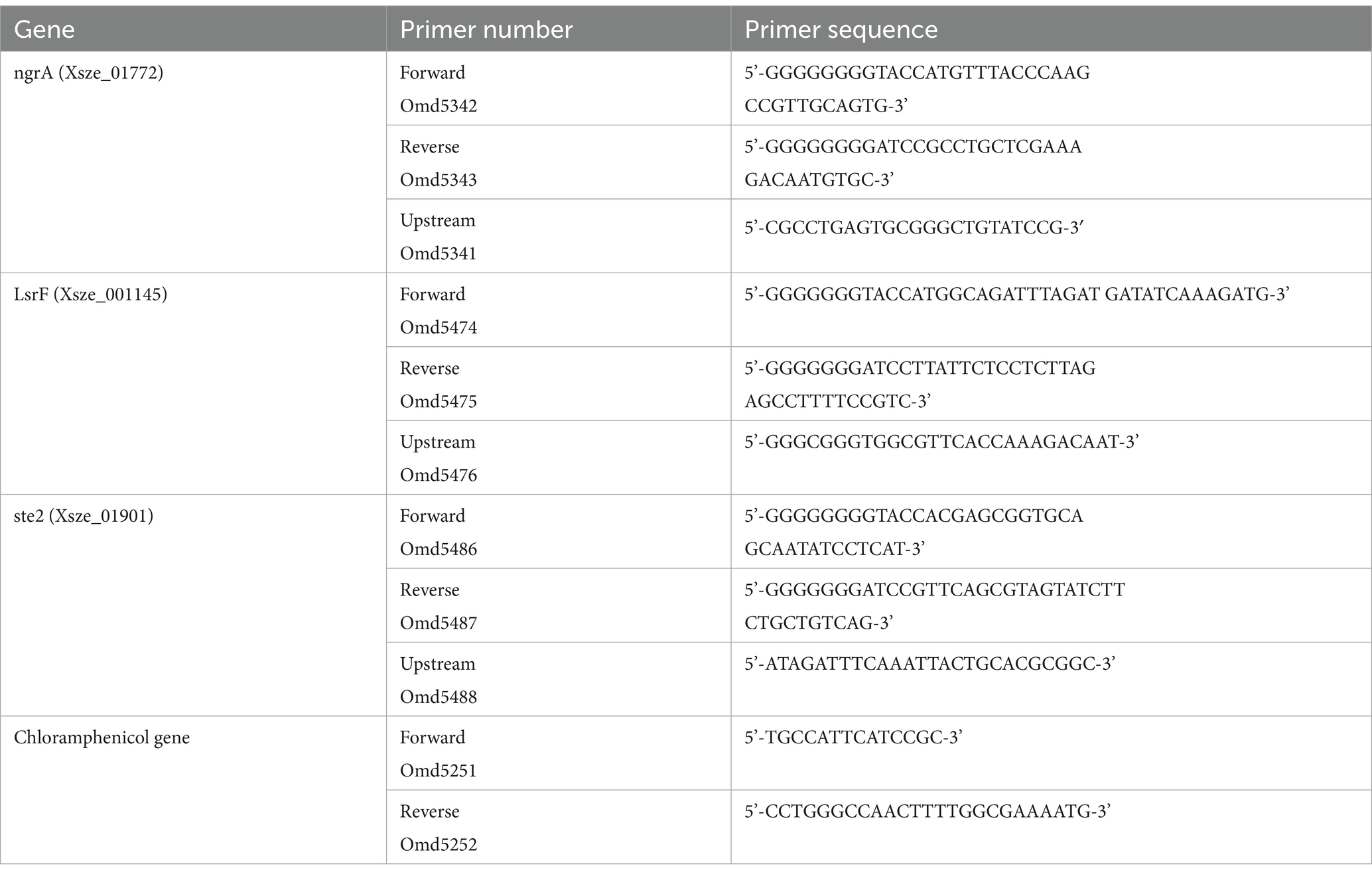
The S17 cells containing the plasmid pKNOCK-yfg was mixed with wild type X. szentirmaii cells in a 1:1 ratio (OD600 = 1). The cells were plated on an LB agar plate and placed in a 30°C incubator overnight. The cells were then streaked on an LB agar plate containing ampicillin (50 μg/mL) and chloramphenicol (20 μg/mL). The gene disruption was confirmed by PCR using an upstream primer (Table 2 for primers) and a reverse primer designed from the chloramphenicol gene.
RNA isolation and reverse-transcriptase PCR
Four single colonies of the bacterium X. szentirmaii were grown individually in a liquid medium in the presence of ampicillin (50 μg/mL). Cells were harvested at the log phase (OD600 = -0.5) and stationary phase (OD600 = 1.5). Total RNA was prepared by standard protocol using PureLink RNA isolation kit (Invitrogen, United States). Using 2 μg of total RNA, cDNA was prepared by random primers (NEB, USA, cat # S1330S) and NEB cDNA synthesis kit (NEB, USA, cat # E6300S). cDNA was then amplified by gene-specific primers (ngrA, ste2 or LsrF, see Table 1).
Library preparation, sequencing and RNA-seq analysis
RNA quality, determined using a NanoDrop 1,000 spectrophotometer (Thermo Fisher Scientific), Bioanalyzer 2,100 (Agilent), and Qubit fluorometer (Thermo Fisher Scientific), were: 260/280 ratio 2.0–2.1, 260/230 ratio 2.0–2.3, and RIN > 9. Sequencing libraries were prepared from 1 μg of total RNA from each sample using Illumina Stranded Total RNA Prep with Ribo-Zero Plus or Ribo-Zero Plus Microbiome (M-GL-02148 v1.0) and IDT for Illumina DNA/RNA UD INdexes (Illumina, 20,026,121). Prepared libraries were then sequenced using an Illumina MiSeq, with paired end reads of 150 bp.
The raw sequencing data were pre-processed using the FASTP program (NCBI GEO Submission = GSE289227) (Chen et al., 2018) to ensure quality control, trim adapters, and prune reads. The resulting FASTQ files were aligned with the X. szentirmaii genome (NIBV01000001.1 and NIBV01000002.1) using the STAR (Spliced Transcripts Alignment to a Reference) aligner tool (Dobin et al., 2013) with default parameters and the “alignIntronMax” parameter set to 1. Across all replicates, the uniquely mapped read fraction exceeded 70%, with absolute mapped reads ranging from 1.57 to 3.61 million. We used the gene-wise counts from the STAR to perform differential gene expression analysis via the DESeq2 program (v1.38.3) (Love et al., 2014). In this process, genes with a cumulative count of less than 10 across replicates were excluded. The remaining genes were analyzed using DESeq2 default parameters. A gene was deemed differentially expressed if its adjusted p-value was below 0.05 and it exhibited an absolute log2-fold change exceeding 1. To conduct principal component analysis (PCA), we utilized variance-stabilized gene expression values obtained via the “vst” function and derived the principal components using the “plotPCA” function of DESeq2 program.
Genome analysis
The X. szentirmaii genome sequences (accession numbers NIBV01000001 and NIBV01000002) were retrieved from NCBI database. The NCBI BLAST tool was used to BLAST the PCP domain sequences (RDPIEIELCT TFEQILSVKR VGIHDDFFEL GGHSLLAVKL VNHLKKAFGT ELSVALLAQY STVERLGEII RENKE) against the X. szentirmaii genome (Max target sequences 5,000, BLOSUM62, expected threshold 0.05) DNA sequence was analyzed by SnapGene software, protein sequences were analyzed and peptide sequences were predicted by using the website nrps.igs.umaryland.edu.
Results
The Xenorhabdus szentirmaii genome contains 17 operons that encode NRPS modules
NRPSs are modular enzymes of three core domains consisting of an adenylation (A) domain (~500 amino acids), a peptidyl carrier protein transfer (PCP or T) domain (~70–90 amino acids) and a condensation (C) domain (~350 amino acids) (Figure 1A) (Drake et al., 2016; Tanovic et al., 2008). To become functional, the PCP-domain of NRPS and PKS transitions from their inactive apo-forms to the functional halo-forms through the covalent attachment of the 4′-phosphopantetheine (P-pant) to a conserved serine residue. This reaction is catalyzed by the phosphopantetheinyl transferase (PPTase). In Xenorhabdus spp., the PPTase is encoded by the gene ngrA (Beld et al., 2014). The ngrA-PPTase is a conserved protein among Xenorhabdus species and is predicted to adopt a pseudo-2-fold architecture (Jumper et al., 2021; Figure 1B) like the Bacillus subtilis Sfp-type PPTase (Reuter et al., 1999). Disruption of the ngrA gene in Xenorhabdus spp. resulted in loss of antimicrobial secretion (Snyder et al., 2007; Wang et al., 2014; Schwarzer et al., 2003; Sussmuth and Mainz, 2017), suggesting that NRPS and PKS synthesized antimicrobials in Xenorhabdus. To further understand the role of ngrA-PPTase in biosynthesizing the antimicrobials, we disrupted the ngrA gene in the X. szentirmaii genome by insertional mutation using a pKNOCK based vector as described in the Materials and Methods. After an overlay assay with Staphylococcus saprophyticus, the wild type X. szentirmaii produced a zone of inhibition. However, the ΔngrA mutant strain did not show any anti-microbial properties (Figure 1C), further confirming that inactivation of ngrA abolishes the synthesis of all NRPS-mediated secondary metabolites (SMs) and bactericidal properties of X. szentirmaii.
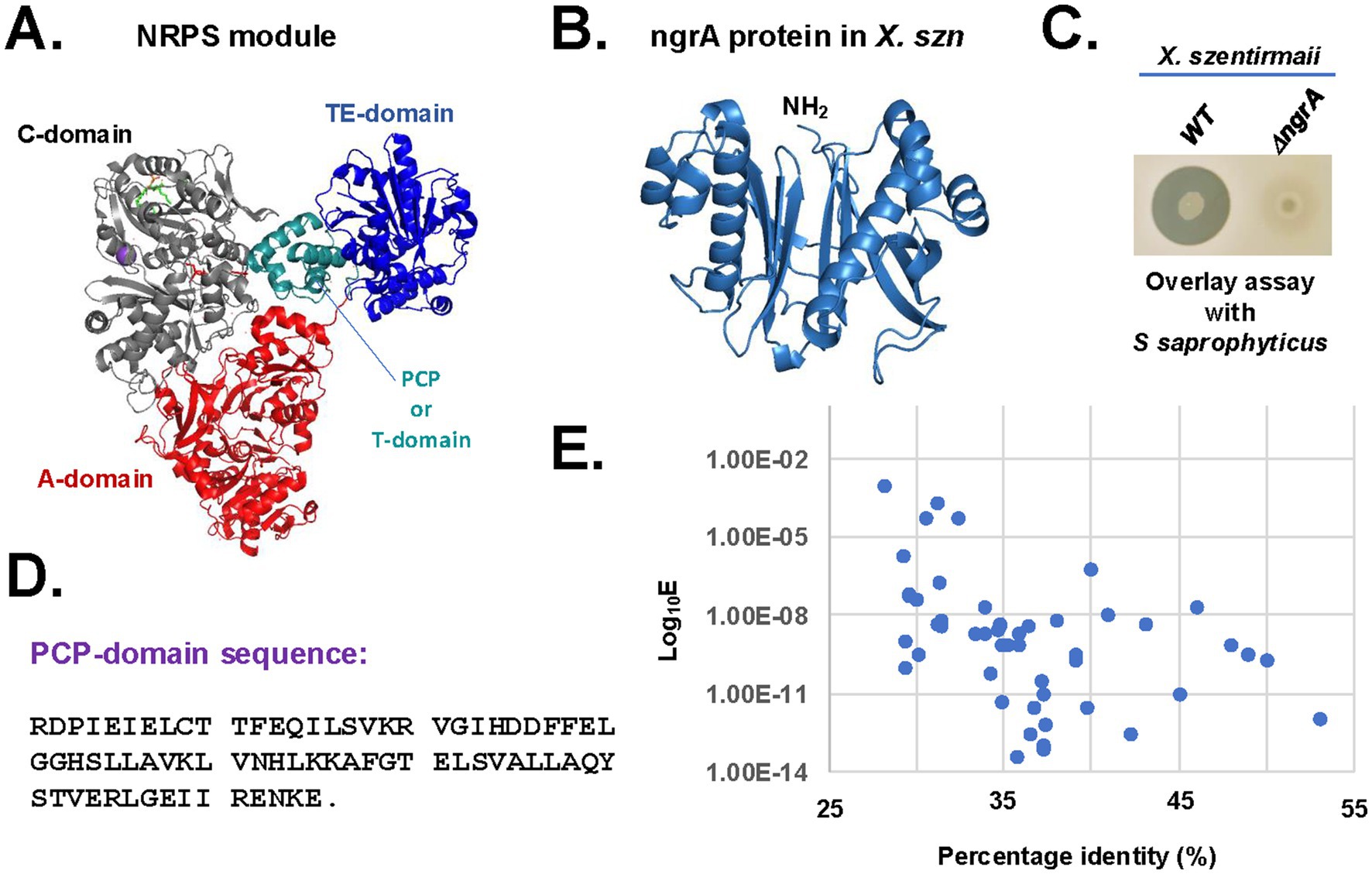
Figure 1. Inactivation of ngrA abolishes the synthesis of antimicrobials. (A) The crystal structure of Acinetobacter baumannii NRPS protein AB3403 (PDB ID = 4zxh). The condensation (C)-domain, adenylate (A)-domain, PCP-domain and thioesterase (TE)-domain are shown in gray, orange, red and blue colors, respectively. (B) The Alpha-Fold predicted structure of X. szentirmaii (X. szn) ngrA protein. (C) The bacterium X. szentirmaii (WT or ΔngrA mutant) was spotted on an LB medium and grown overnight. The indicated tester strain S. saprophyticus was overlaid and grown at 37°C for 24 h. The clearance zones indicate the antimicrobial activity. (D) Amino acid sequences of the PCP-domain. (E) The whole X. szentirmaii genome (GenBank accession numbers NIBV01000001 and NIBV01000002) was used to search for genes encoding homologs of the PCP-domain. After the BLAST search, the E-values and percentage identities were retrieved and graphed on a logarithmic scale.
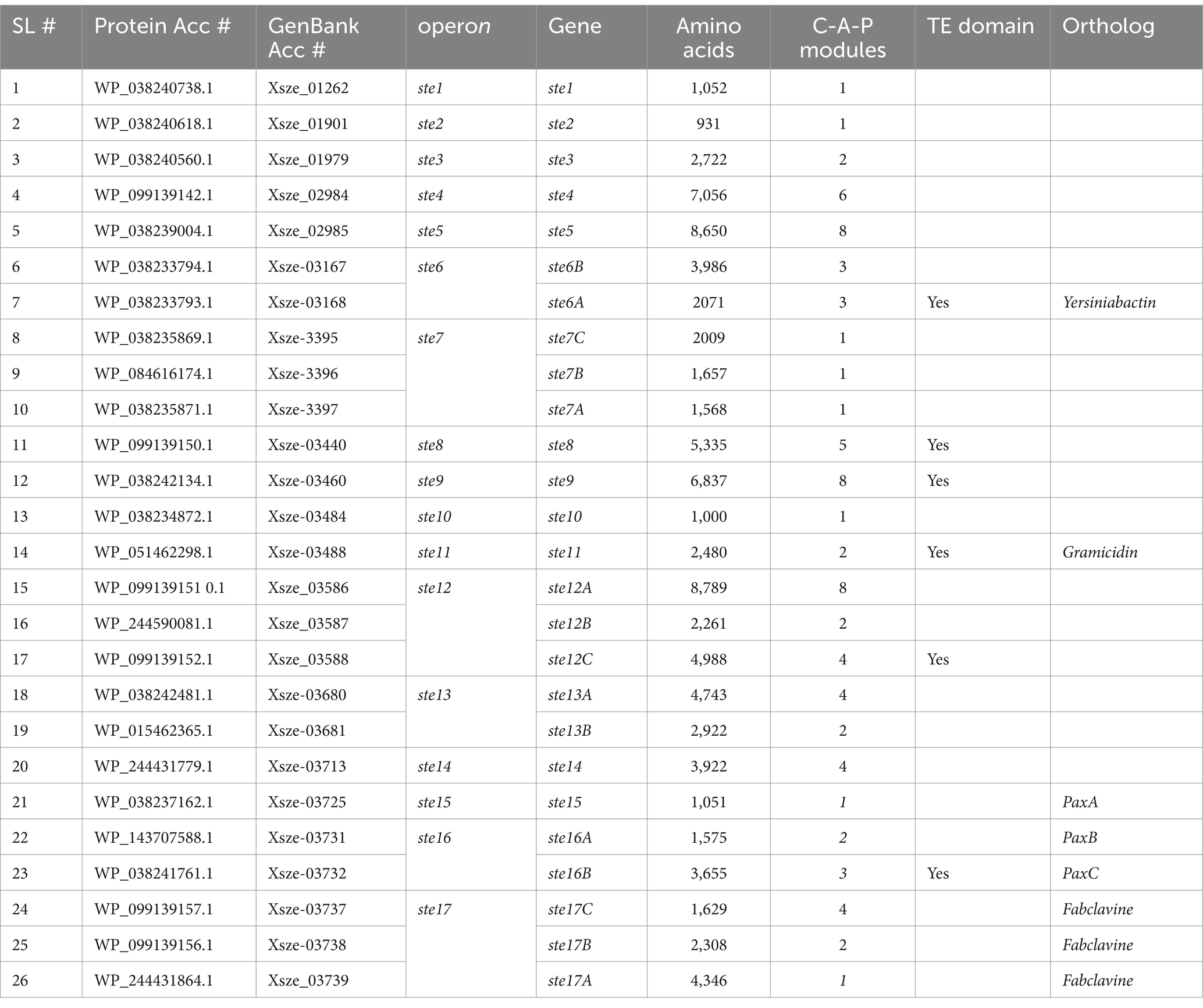
To identify genes encoding protein harboring the PCP domain sequence, the NCBI BLAST search tool was used on the genome of X. szentirmaii (Tobias et al., 2017) was analyzed (Figures 1D,E). A total of 64 BLAST-hits were obtained on NRPS, PKS and AMP-binding protein, with a percentage identity >30 and an E-value <10−4 (Figure 1E). These findings indicated that one or more PCP domains are present in a variety of metabolic enzymes, including ATP-hydrolyzing racemase, dehydrogenase, and non-ribosomal peptide synthetases. We focused on the proteins containing the NRPS-like modules (Table 2, protein accession numbers) and considered that two sequences are homologous if they are more than 30% identical over their entire lengths (Pearson, 2013). Then, we analyzed both upstream and downstream sequences of the respective genes to identify their neighboring genes using the SnapGene software (Table 2, see GenBank accession numbers). Additionally, the antiSMASH software (Blin et al., 2023) was used to search for the biosynthetic gene clusters and genes encoding NRPSs in the X. szentirmaii genome. Together, our analysis revealed that the PCP domain is distributed among 26 NRPS and PKS genes encoded by 17 operons (referred here to as ste1 - ste17, see Table 2 and Supplementary Figure 1).
Based on the sequence homology, five operons (ste6, ste11, ste15, ste16 and ste17) likely code for NRPS that catalyze the biosynthesis of orthologs of known compounds, whereas 12 operons (ste1, ste2, ste3, ste4, ste5, ste7, ste8, ste9, ste10, ste12, ste13 and ste14) are specific to X. szentirmaii with unknown functions. The ste6 operon is predicted to encode an NRPS that catalyzes the synthesis of a putative siderophore similar to Yersiniabactin (Perry and Fetherston, 2011), whereas the ste11 operon for the gramicidin-type compound (Dubos, 1939). The ste15 and ste16 operons are predicted to encode a composite NRPS that catalyzes the synthesis of antibiotic PAX-peptide (Fuchs et al., 2011). The ste17 operon appears to contain 11 open reading frames, which is predicted to encode a composite NRPS that catalyzes the synthesis of fabclavine-like compounds (Table 2; Supplementary Figure 1; Fuchs et al., 2014).
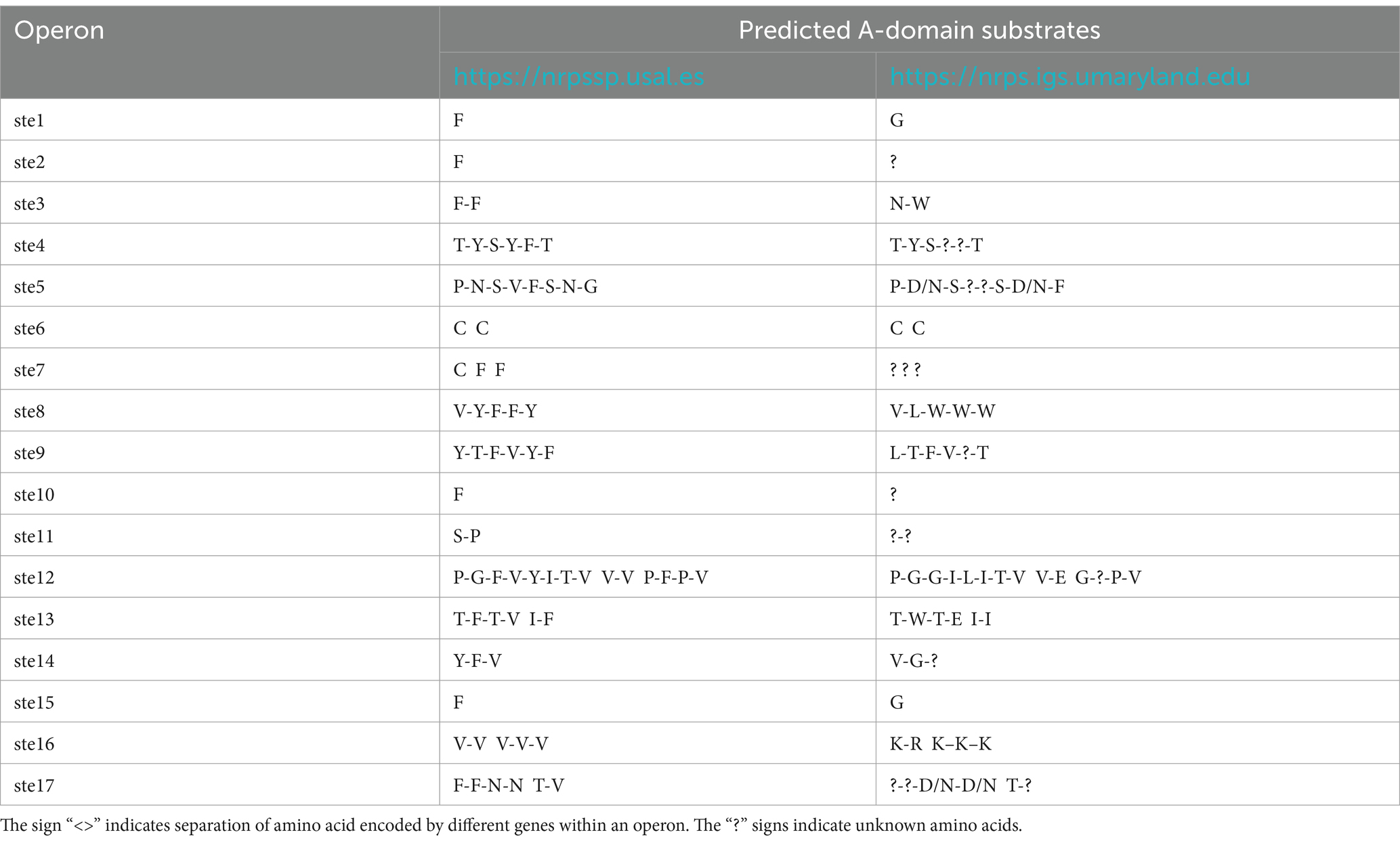
Both PKS/NRPS Analysis website1 and antiSMASH software predict that operons ste1, ste2, ste10 and 15 code for only one modular domain of the NRPS enzyme (Supplementary Figure 1). These observations suggest that these operons alone may not be capable of independently producing functional NRPs and they might work in conjunction with other operon (s) to code a functional NRP. The PKS/NRPS Analysis website predicts that operons ste3, ste4, ste5, ste8, and ste9, respectively, code for 2, 6, 8, 5, and 9C-A-P modular domains (Table 3). A terminal thioesterase (TE) domain at the end of these NRPSs suggests that they are likely stand-alone NRPSs. The operons ste13 and ste14 encode for 6 and 4 modular domains but lack a TE domain. The ste13 operon contains 2 NRPS genes (6 modular domains) and the ste7 and ste12 operons each contain 3 NRPS genes (3 and 14 modular domains, respectively).
The A-domain of NRPS modules plays a crucial role in NRP biosynthesis by selecting and activating a specific substrate (typically an amino acid). We utilized two online platforms to predict A-domain substrates (Table 3). Some predicted substrates varied between two platforms. For instance, the ste1-encoded A-domain was predicted to activate phenylalanine (Phe or F) on the nrpssp.usal.es platform, while the nrps.igs.umaryland.edu platform predicted glycine (Gly or G) as the substrate. These differences highlight the need for further refinement in A-domain substrate prediction. Despite these differences, our analysis revealed that X. szentirmaii contains a unique set of 17 NRPS-PKS operons, that are involved in synthesizing a unique repertoire of NRPs, whose functional significance remains to be further characterized.
Xenorhabdus szentirmaii and Xenorhabdus nematophila produce antimicrobials against pathogenic bacteria
Xenorhabdus nematophila has been studied extensively for its ability to secrete antimicrobial compounds. Here, we sought to compare the differences in antimicrobial secretion between X. nematophila and X. szentirmaii using an overlay assay with the tester strains S. saprophyticus and E. coli as described in the Materials and Methods. A large zone of inhibition was observed surrounding the X. szentirmaii when overlaid with S. saprophyticus (Figure 2A), and an even more prominent inhibition zone was seen when overlaid with E. coli when compared to X. nematophila (Figure 2A). These results further confirmed that X. szentirmaii produces a higher level of antimicrobials (Ciezki et al., 2019).
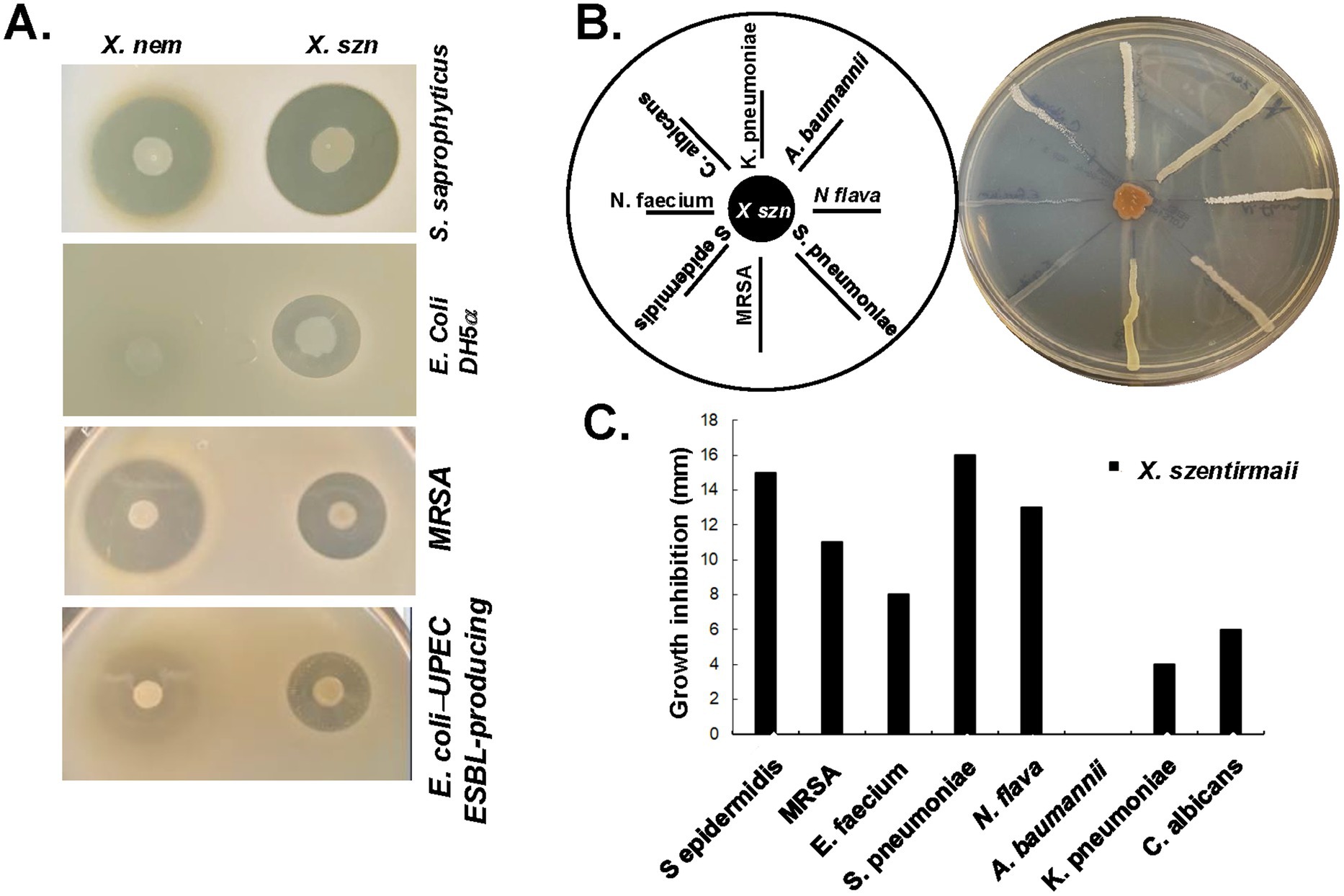
Figure 2. X. nematophila and X. szentirmaii are natural resources of antibiotics. (A) The bacteria X. nematophila and X. szentirmaii were subjected to overlay assays with four tester strains: S. saprophyticus, E. coli, MRSA and a clinical ESBL-producing UPEC strain. (B) The bacterium X. szentirmaii was grown for 48 h before cross-streaking with the indicated microorganisms. The distance of inhibition was measured and recorded in a bar diagram (C).
To assess the antimicrobial effects against antimicrobial resistant organisms recognized as “serious threats” by the CDC, the methicillin-resistant Staphylococcus aureus (MRSA) and an extended-spectrum beta-lactamase (ESBL)-producing uropathogenic E. coli (UPEC) (Figure 2A) were tested. Both X. szentirmaii and X. nematophila demonstrated strong anti-microbial effects against MRSA and ESBL-producing UPEC, with X. szentirmaii exhibiting stronger effects against the latter compared to X. nematophila (Figure 2A).
To further determine if X. szentirmaii secretes antimicrobial compounds capable of inhibiting growth of a variety of ESKAPE (Enterococcus, Staphylococcus, Klebsiella, Acinetobacter, Pseudomonas, Enterobacter) pathogens commonly associated with resistance, we conducted a cross-streak assay (Figure 2B). X. szentirmaii was grown for 48 h on an LB plate, followed by streaking seven pathogenic bacteria: (Klebsiella pneumoniae, Acinetobacter baumannii, Neisseria flava, Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecium) and a clinically relevant fungus (Candida albicans), as illustrated in Figure 2B. The plate was then grown at 37°C for 48 h. Except for the pathogen Acinetobacter baumannii, various zones of inhibition were evident for all tested bacteria and the yeast strain (Figures 2B,C), supporting potential application of Xenorhabdus bacteria against AMR pathogens.
Xenorhabdus szentirmaii produces antimicrobials during the stationary phase
To determine the specific growth stage of antibiotic production by X. szentirmaii, cell-free supernatants (CFSs) were collected from the log-and stationary-phases of bacterial cultures (Figure 3A) and subjected to overlay assays with the tester strain S. saprophyticus. Zones of inhibition were observed surrounding the CFSs collected at the stationary phase of cultures compared to log phases (Figure 3B). These data indicated two possibilities: (I) X. szentirmaii did not secrete antimicrobial compounds during the log phase and/or (II) number of cells at the log phase were too low to produce antimicrobials.
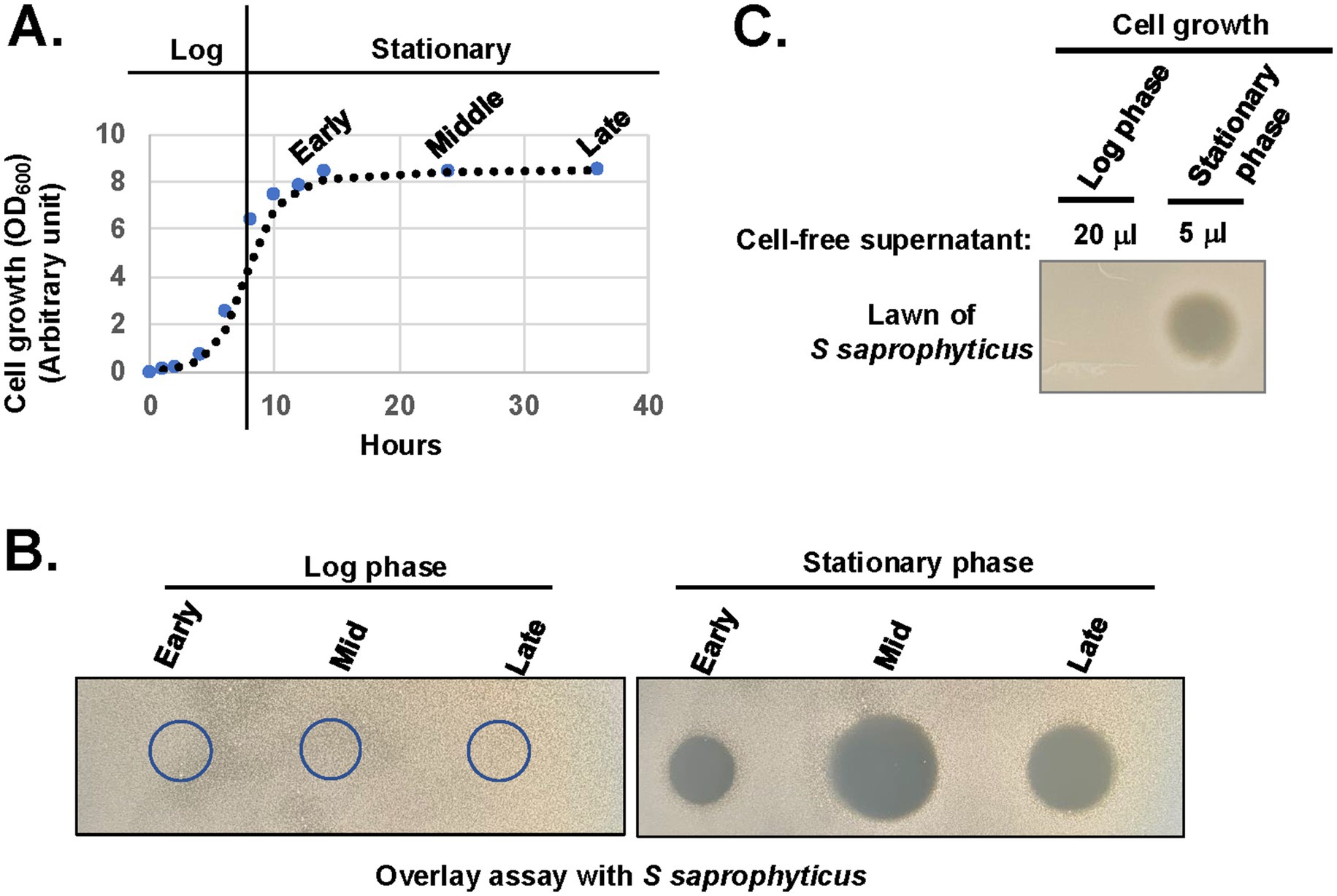
Figure 3. X. szentirmaii produces antimicrobials in the stationary phase. (A) The bacterium X. szentirmaii was grown for the indicated time and the optical density (OD) was measured at 600 mm. The experiments were repeated at least three times. One representative data are shown. (B) Cell-free supernatants were collected from both log and stationary phases, filter-sterilized, spotted (5 μL) on an agar plate, and subjected to overlay assay with the bacterium S. saprophyticus. The clearance zone indicates antibacterial activity. (C) The 20 μL or 5 μL of cell-free supernatants containing equal number of cells were collected, concentrated, and spotted on the LB medium and subjected to overlay assay.
To test the above possibilities, we collected CFSs containing an equal number of cells from both log and stationary phases, considering the lower cell density at the log phase (OD600 = ~0.5) compared to the stationary phase (OD600 < 1.5). CFS were then dried by speed vacuum, resuspended in equal volume of LB medium and spotted on the LB medium for the overlay assay with the tester strain S. saprophyticus. Zones of inhibition were observed surrounding the CFSs collected only at the stationary phase of cultures (Figure 3C). Taken together, these observations suggest that a certain cell density is required for the secretion of antimicrobial compounds and the secretion may be regulated by certain components of quorum sensing regulators.
Genome-wide analysis of transcripts expressed during log and stationary phases
In X. szentirmaii, the biosynthetic genes of secondary metabolites appear to be dominating during the late log and stationary phases (Figure 3). To understand the molecular details of the biosynthesis, secretion, and/or mode of action of antimicrobials, we performed a comparative analysis of transcripts expressed during log and stationary phases of growth to identify the deferentially expressed genes. Total RNA was isolated from X. szentirmaii grown at log and stationary phases (Figure 4A, four biological replicates referred here Dey1, Dey2, Dey3 and Dey4 for the log phase and Dey5, Dey6, Dey7 and Dey8 for the stationary phase, Supplementary Table 1). The equal amount of total RNA was subjected to RNA sequencing analysis. The RNA-seq data was then used to generate the PCA (principal component analysis) plot, which displayed distinct clustering (Figure 4B), indicating that there are discernable differences in gene expression between the log and stationary phases, and that the biological conditions associated with each phase lead to distinct gene expression pattern.
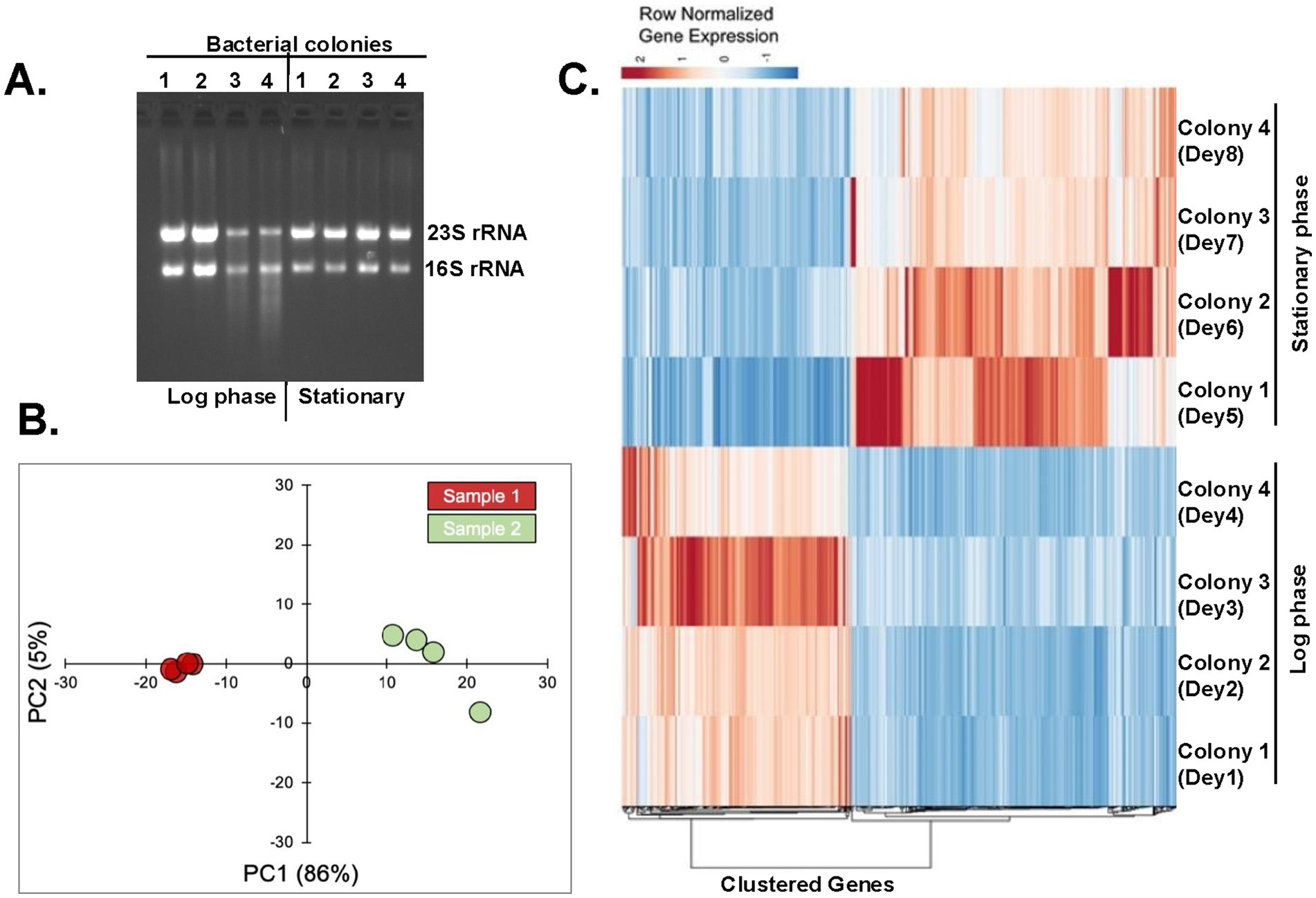
Figure 4. Analysis of transcripts expressed during log and stationary phases. (A) Four bacterial colonies were grown till the log and stationary phases. Total RNAs were isolated and run in an agarose gel. (B) The principal component analysis (PCA) plot shows two distinct clusters of samples from log-phase (green dots) and stationary-phase (red dots). (C) Heatmap of differentially expressed genes. The heatmap shows the row normalized TPM (Transcripts per million) values of 637 differential genes with FDR < 0.05 and absolute log2FC above >1.
The draft genome sequence featuring 3,810 genes of X. szentirmaii DSM 16338 was leveraged as a reference (Tobias et al., 2017). Our RNA-seq analyses identified 3,446 gene products. Approximately 637 genes were upregulated during the log or stationary phase (absolute log2 fold-change more than1; Figure 4C; Supplementary Table 2). Notably, as shown in Figure 5A, the functions of 118 genes remained uncharacterized. The remaining gene products include transcription factors (e.g., LysR), synthetases (e.g., dethiobiotin synthetase), transferases (e.g., GNAT family acetyltransferase), metabolic kinases (e.g., phosphoenolpyruvate carboxy-kinase), phosphatases (e.g., acid phosphatase and phosphotransferase), reductases (e.g., oxidoreductase), transporters (e.g., putative metal cation transporter P-type ATPase CtpV, permeases; e.g., ABC transporter permease), lyases (e.g., aspartate ammonia-lyase), chaperones (e.g., ClpB) and many membrane proteins (e.g., tricarboxylic transport membrane protein). Notably, no significant increase in transcripts encoding replication factors was observed. These findings suggest that these genes are involved in regulating various cellular processes, including transcription, transport, metabolisms, and biosynthesis of secondary metabolites and their modifiers. Together, these findings indicate that X. szentirmaii re-programs its physiological state upon reaching a certain cell density.
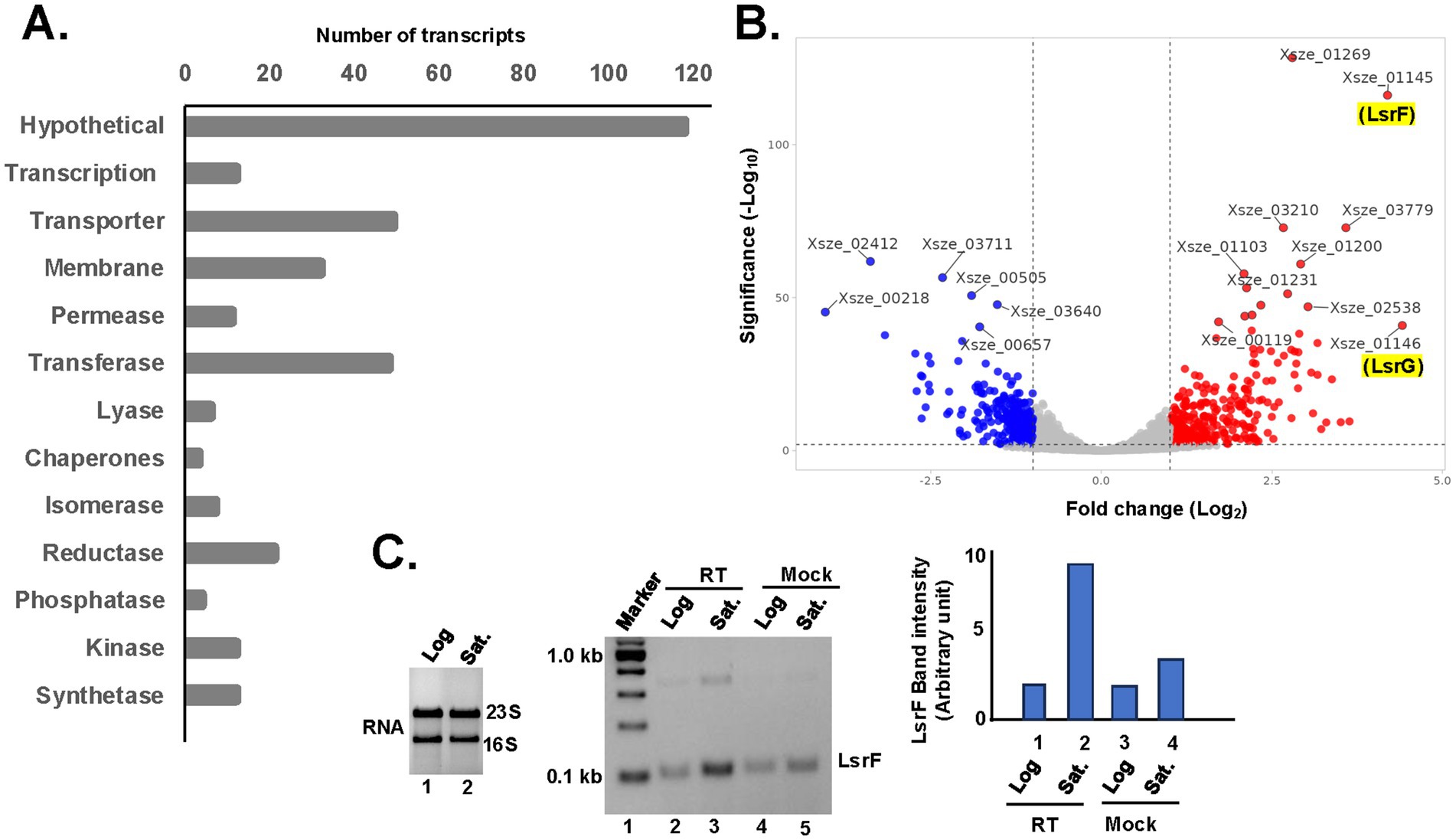
Figure 5. Upregulation of quorum sensing activities during the stationary phase. (A) The bar diagram shows the number of up-regulating genes during the stationary phase are classified based on their physiological functions. (B) Total RNA was isolated from the log and stationary phases of X. szentirmaii and subjected to RNA-seq analysis. Up and down regulated genes are shown in red and blue color, respectively, in the volcano plot. (C) (Left panel) Total RNA was isolated from the log and stationary phases of X. szentirmaii and run on an agarose gel. (Middle panel) cDNA was synthesized by reverse transcriptase (RT) from total RNA isolated from log and saturated (Sat.) phases and subjected to RT-PCR with the LsrF-specific primers. The PCR products were run in an agarose gel. A control reaction without RT (Mock) was also carried out. (Right panel) The band intensities of the PCR products were measured NIH-image J software and shown in a bar diagram.
The top 10 upregulated genes during the stationary phase (Figure 5B) are Xsze-01146 (encoding autoinducer-2 degrading protein LsrG), Xsze-01145 (autoinducer-2 thiolase, LsrF), Xsze-01251 (fumarate reductase subunit D), Xsze-03779 (urocanate hydratase), Xsze-03299 (dethiobiotin synthetase), Xsze-03232 (putative alcohol-acetaldehyde dehydrogenase), Xsze-01253 (fumarate reductase), Xsze-01252 (fumarate reductase subunit C), Xsze-03764 (tricarboxylic transport membrane protein) and Xsze-01254 (fumarate reductase flavoprotein subunit). Further genome analysis showed that lsrF and lsrG are two conserved structural genes of the lsr operon in X. szentirmaii, which is likely regulated by the transcription factor LsrR (Supplementary Figure 2). However, the functional significance of lsr operon in X. szentirmaii is yet to be determined.
To confirm that the lsrF gene expression was increased during the stationary phase, we conducted the reverse transcriptase (RT)-PCR analysis. Total RNA was prepared from both the log phase and the stationary phase of cell growth (Figure 5C) and subjected to RT-PCR analysis by two gene-specific primers of lsrF. The increased amount of PCR-amplified product (5-fold) in the presence of RT (Figure 5C, middle panel, lane 3, compared with the lane 5 and the right panel) confirmed that the expression of lsrF gene is induced during the stationary phase. These data indicate that LsrF and LsrG may have a distinct function in regulating antibiotic production by X. szentirmaii.
Consistent with the previous studies (Heinrich et al., 2017), our genome analysis further revealed that lsrF and lsrG are the only components of the lsr operon involved in regulating the QS system in X. szentirmaii. (Supplementary Figure 2). QS-mediated gene regulation arises from regulatory networks built upon the core mechanisms of AI production and detection. Thus, it appears that specific patterns may become apparent when investigating the role of QS network in antimicrobial production. It is plausible that certain regulatory systems within the QS network may have evolved to specifically control NPR biosynthesis. Future molecular studies will focus on the role of LsrF in NPR biosynthesis.
Increased expressions of ste2, ste10, ste14, ste17 and ngrA genes during the stationary phase
Xenorhabdus szentirmaii produced antimicrobials during the saturated phase when cells reached a certain cell density (Figure 3), and this production of antimicrobials depended on the function of NgrA-PPTase (Figure 1). In this study, we also identified that at least 17 NRPS operons regulated the expression of antimicrobial NRP (Table 2). However, the RNA-seq data showed that only the expression of ste10 (Xsze-03484) gene was increased (log2fold-change = 1.30 with a p-value 4.91E-08, Supplementary Table 2). These results suggested that the RNA-seq experiment may have failed to fully detect these NRPS transcripts likely due to their large size and/or short half-life. Additionally, it was possible that a 2-fold increase in transcripts might have not been captured by the high-throughput analysis. Therefore, we individually examined the raw FPKM (Fragments Per Kilobase of transcript per Million mapped reads) counts of each ste operon (Supplementary Figure 1) and analyzed their expression levels (Figure 6).
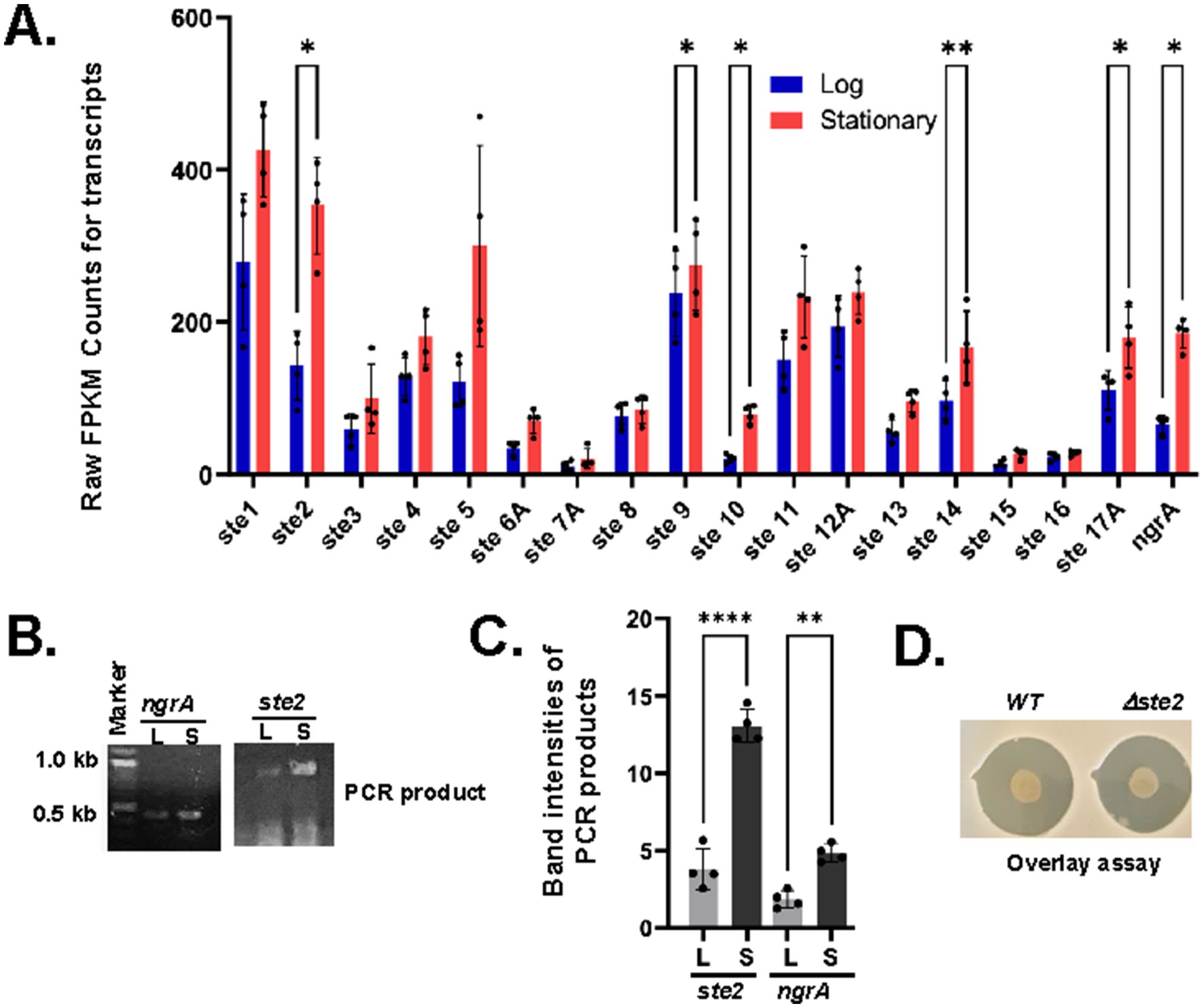
Figure 6. Upregulation of operons ste2, ste9, ste10, ste14A and ste17A during the stationary phase. (A) The average FPKM counts of the indicated ste genes are shown in a bar diagram with standard error (**p-value<0.001, paired t-test). (B) cDNA was synthesized by reverse transcriptase (RT) from total RNA isolated from log (L) and saturated (S) phases and subjected to RT-PCR with primers designed from the indicated ngrA and ste2 operons. (C) The PCR-amplified DNA bands were measured by ImageJ software and indicated in a bar diagram with standard error (*p-value<0.001, paired t-test). (D) Wild type (WT) and Δste2 strains of X. szentirmaii were subjected to overlay with the tester strain S. saprophyticus. The experiments were repeated at least three times. Results from one representative experiment data are shown.
Our analysis showed that there were significant variations in the FPKM counts for ste transcript levels. The average FPKM counts at log and stationary phases are 295 and 412 for ste1, 151 and 383 for ste2, 63 and 116 for ste3, 143 and 184 for ste4, 126 and 934 for ste5, 40 and 66 for ste6, 12 and 26 for ste7, 12 and 26 for ste8, 77 and 90 for ste8, 253 and 287 for ste9, 24 and 81 for ste10, 154 and 264 for ste11, 224 and 251 for ste12A, 64 and 107 for ste13, 107 and 189 for ste14, 15 and 30 for ste15, 24 and 26 for ste16A, and 124 and 198 for ste17A (Supplementary Table 1). These numbers show an overall increase in each NRPS transcript at the stationary phase. However, significant values were obtained for ste2 (~2.5-fold with a p-value = 0.029, paired t-test), ste9 (~0.85-fold with a p-value = 0.039, paired t-test), ste10 (3-fold with a p-value = 0.019, paired t-test), ste14A (~2-fold with a p-value =0.005, paired t-test) and ste17A (1.5-fold with a p-value = 0.037, paired t-test) (Figure 6A). Although the ste5 transcript level was increased by ~7-fold, a higher p-value (0.1962, paired t-test) indicated that this increased expression was not statistically significant (not analyzed). The increase in the NRPS transcript levels was consistent with the increase in the ngrA transcript level (Xsze_01772), which showed an average increase of ~3.5-fold with a p-value of 0.03 (paired t-test). Together, these findings suggest that the regulatory function and turn-over of the NRPSs might vary considerably based on their length and amino acid composition.
To validate the RNA-seq data, we performed reverse-transcript (RT)-PCR analysis targeting the operons ngrA and ste2. cDNA was synthesized from the total RNA isolated from both log and stationary phases and amplified using gene-specific primers. Consistent with the RNA-seq data, we found that transcription of ngrA and ste2 was significantly increased in bacteria grown at their stationary phase (Figures 6B,C). Taken together, our results suggest that the expressions of ngrA and ste2 increased during stationary phase, coinciding with the antibiotic production.
In this study, to further investigate the role of ste operons in antimicrobial secretion, we specifically disrupted the ste2 operon by insertional mutagenesis using a pKNOCK based vector (Materials and Methods), generating a Δste2 strain. An overlay assay with Staphylococcus saprophyticus was then conducted to compare the Δste2 strain and the wild type of strain. No difference in the zone of inhibition was observed between WT and Δste2 strains (Figure 6D). These results indicate that all ste operons likely function collectively to regulate antimicrobial secretion. Future studies will focus on disrupting each ste operon individually and in combination to evaluate their relative contribution to antimicrobial secretion.
Discussion
We focused our research on X. szentirmaii, a species that grows faster than its close relatives and produces more potent antimicrobial compounds against AMR (Figure 2). Genome analysis of the X. szentirmaii genome identified 17 operons (Table 3), encoding NRPS and PKS enzymes (ste1-ste17). RNA-seq analysis revealed that the expression of these operons increased during the stationary phase of its growth when X. szentirmaii secretes antibiotics. Furthermore, RNA-seq analysis identified that the quorum sensing (QS)-regulator LsrF is significantly elevated during the stationary phase, interring it may be a key factor in regulating the antibiotic production (Figure 5).
The X. szentirmaii genome contains at least 24 NRPS and/or PKS genes, which are distributed among 17 operons (Table 3). Based on sequence homology, some operons likely code for orthologs of known compounds. For example, ste6 operon is predicted to encode NRPSs to biosynthesize a siderophore similar to Yersiniabactin (Perry and Fetherston, 2011), while the operon ste11 to biosynthesize the gramicidin-type compound (Dubos, 1939). Both ste15 and ste16 operons are predicted to encode enzymes for synthesis the antibiotics PAX-peptide (Fuchs et al., 2011). The operon 17 is predicted to encode NRPSs to biosynthesize Fabclavine-like compound. The Fabclavine was first discovered in X. budapestensis and X. szentirmaii as a hexapeptide-polyketide, with a corresponding 50 kb biosynthesis gene cluster consisting of non-ribosomal peptide synthetases and one polyketide synthase (Fuchs et al., 2014). Later, several isoforms of Fabclavine and their corresponding biosynthesis gene clusters were reported, which have generated significant attention due to their broad-spectrum bioactivity against Gram-positive and-negative bacteria, fungi, and protozoa (Fuchs et al., 2014; Donmez Ozkan et al., 2019; Wenski et al., 2020). Recently it has been shown that Fabclavine from X. szentirmaii can kill the larva of the mosquito Aedes albopictus (Touray et al., 2024). Here, we report that the Fabclavine-like compound in X. szentirmaii is likely to be a heptapeptide, with a corresponding 28.6 kb biosynthesis gene cluster consisting of five genes (Supplementary Figure 1).
The products and functions of the rest of the operons are currently unknown. Some operons encode only one modular domain of the NRPS enzyme, indicating that these operons alone may not be capable of independently producing functional NRPs, but instead collaborate with other operon (s) to synthesize complete and functional NRPs. Additionally, some operons lack a terminal TE domain, indicating that they may work in conjunction with other genes that contains a TE domain to produce an NRP. It remains to be determined whether the five compounds reported in X. szentirmaii (i.e., Xenematide, GameX peptide, Rhabdopeptide, Xenoamicin, Szentiamide) (Tobias et al., 2017) are synthesized by a single operon or by NRPS encoded across multiple ste operons.
It appears that there are five stand-alone operons for NRPSs in X. szentirmaii. These operons are ste4 (encoding an NRP of six amino acids), ste6 (encoding an NRP of hexa-peptide), ste8 (a pentapeptide NRP), ste9 (another hexa-peptide NRP) and ste12 (encoding a fourteen-peptide NRP, Supplementary Figure 1). Each of these operons likely encodes a complete NRPS, making them attractive candidates for engineering and producing the natural bioactive NRP antibiotics, either within X. szentirmaii or in a heterologous E coli strain (Pfeifer et al., 2001), to combat the growing issue of antibiotic resistance.
Taken together, the X. szentirmaii genome contains a unique set of NRPSs that potentially synthesize a unique diversity of antimicrobial non-ribosomal peptides. However, the functional characterization of these operons at the molecular levels remains to be determined. Notably, the disruption of the ste2 operon did not impair the ability of X. szentirmaii to inhibit the S. saprophyticus growth (Figure 6D), suggesting that all ste operons might function collectively in mediating both intra-and interspecies competition. Ongoing research aims at disrupting each ste operon individually and in combination to evaluate their role in producing antimicrobial compounds. Additionally, future studies will aim to strategically express and engineer these NRPS operons in E coli.
Another key finding in our research is that antibiotic secretion in X. szentirmaii may be regulated by QS components. The LuxR/LuxI-type QS system, which facilitates cell–cell communication and bioluminescence in more than 100 Gram negative bacteria (Case et al., 2008), has been shown to regulate the biosynthesis of the non-ribosomal lipopeptide keanumycin D in Pseudomonas nunensis (Pflanze et al., 2023). Additionally, it is reported that the NRPS gene regulates the synthesis of some QS signal molecules in S. odorifera (Sun et al., 2020). In X. szentirmaii, the QS consists of only LsrF and LsrG (Supplementary Figure 2). Comparative analysis of transcripts expressed in cells at exponential and stationary growth phases showed a significant increase in the expression of lsrF and lsrG genes during the stationary phase, coinciding with the antibiotic production (Figure 5). These findings suggest that LsrF plays a crucial role in antibiotic secretion. In line with the previous reports (Pflanze et al., 2023; Sun et al., 2020), our results indicate that the QS system provide a substantial advantage to bacterial communities producing non-ribosomal peptides. This study provides new insights into microbiome research, contributing to the development of novel antibiotics.
The lsr operon in both E coli and Salmonella enterica contains multiple genes encoding the components for the uptake and manipulation of auto-inducers (AI)-2 (Wang et al., 2005). Specifically, within this operon, genes lsrA, lsrB, lsrC, and lsrD encode proteins involved in the transport of AI-2, while lsrF (Marques et al., 2014) and lsrG (Marques et al., 2011) encode proteins involved in the processing of the phosphorylated AI-2, thereby modulating quorum-sensing-regulated bacterial behaviors. The lsr operon is further regulated by its adjacent lsrR and lsrK genes, encoding a repressor and a metabolic kinase (Ha et al., 2018; Medarametla et al., 2021). Unlike E coli, the lsr operon in X. szentirmaii is distinct, it contains only lsrF and lsrG genes, lacking LsrB, a known receptor for AI-2(Miller et al., 2004). Consistently, it has been reported that AI-2 production is not involved in the global regulation of natural product biosynthesis in Xenorhabdus (Heinrich et al., 2017). However, we found that there is a 90% similarity between LsrF proteins in E. coli and X. szentirmaii, implying that these proteins have a common function in both bacteria (Supplementary Figure 2). The crystal structure shows that LsrF encodes a protein of ~32 kDa that folds into a homo-decameric enzyme and catalyzes the formation of key metabolites dihydroxy acetone phosphate and acetyl CoA (Marques et al., 2014; Supplementary Figure 2). However, the roles of these metabolites in the gene regulation or QS are not clear.
Our data indicate that the QS-regulators LsrF and LsrG may have a distinct function in regulating the antibiotics produced by X. szentirmaii. QS is an important mechanism to regulate the expression of secondary metabolites, ensuring that these compounds are produced when there is a sufficient population of microorganisms. Specifically, pathogenetic bacteria frequently rely on QS to synchronize the expression of virulence factors, leading to biofilm formation and invasion (Rutherford and Bassler, 2012). Thus, a deeper understanding of LsrF and LsrG is needed to further understand their roles in quorum sensing and antibiotic secretion, which can be applied to other pathogenic bacteria.
Conclusion
AMR is a major health crisis with growing numbers of bacteria and fungi becoming resistant to existing antibiotics (Brown and Wright, 2016). It is estimated that AMR costs up to $40 billion in direct costs alone in the United States (Zhen et al., 2019), which is primarily driven by some of the following WHO priority pathogens: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Escherichia coli (Nelson et al., 2021). Continuous efforts have been made to identify new antibiotics from natural resources, including the well-studied bacterial genus Streptomyces (Chevrette et al., 2019). However, a promising yet relatively untapped natural resource of antibiotics lies within the Gram-negative Xenorhabdus bacteria, which secretes numerous antimicrobial compounds (Forst et al., 1997). None of those compounds have been commercial utilized due to several technical challenges and a limited understanding of bacterial molecular biology. This highlights the need for fundamental molecular studies on these bacteria. In this study, we demonstrate that X. szentirmaii produces several NRPSs and PKSs through 17 operons and quorum sensing regulators. These NRPSs and PKSs biosynthesize potent antimicrobial NRPs, which are unique and substantiate the potential of Xenorhabdus szentirmaii as a promising natural source for future antimicrobial discoveries in a society that needs new antibiotics to combat AMR.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number GSE289227.
Author contributions
RD: Data curation, Investigation, Writing – review & editing. DV: Investigation, Writing – review & editing. AbC: Data curation, Formal analysis, Investigation, Writing – review & editing. KM: Investigation, Methodology, Writing – review & editing. JU: Data curation, Formal analysis, Investigation, Writing – review & editing. AnC: Data curation, Formal analysis, Methodology, Writing – review & editing. SM: Conceptualization, Investigation, Writing – review & editing. SF: Conceptualization, Writing – original draft, Writing – review & editing. TS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. MD: Conceptualization, Data curation, Investigation, Methodology, Resources, Software, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by departmental resources (Department of Biological Sciences, UW-Milwaukee, WI-53211, USA), with no external funding received. DOV was supported by a fellowship from the Office of Undergraduate Research (OUR), UW-Milwaukee. The RNA sequencing experiments were supported by the Great Lakes Genomics Center at the University of Wisconsin Milwaukee (RRID: SCR_017838) with Illumina library preparation and sequencing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1560663/full#supplementary-material
Footnotes
References
Antimicrobial Resistance, C. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Beld, J., Sonnenschein, E. C., Vickery, C. R., Noel, J. P., and Burkart, M. D. (2014). The phosphopantetheinyl transferases: catalysis of a post-translational modification crucial for life. Nat. Prod. Rep. 31, 61–108. doi: 10.1039/C3NP70054B
Blin, K., Shaw, S., Augustijn, H. E., Reitz, Z. L., Biermann, F., Alanjary, M., et al. (2023). antiSMASH 7.0: new and improved predictions for detection, regulation, chemical structures and visualisation. Nucleic Acids Res. 51, W46–W50. doi: 10.1093/nar/gkad344
Booysen, E., and Dicks, L. M. T. (2020). Does the future of antibiotics lie in secondary metabolites produced by Xenorhabdus spp.? A review. Probiotics Antimicrob. Proteins 12, 1310–1320. doi: 10.1007/s12602-020-09688-x
Brown, E. D., and Wright, G. D. (2016). Antibacterial drug discovery in the resistance era. Nature 529, 336–343. doi: 10.1038/nature17042
Bushley, K. E., and Turgeon, B. G. (2010). Phylogenomics reveals subfamilies of fungal nonribosomal peptide synthetases and their evolutionary relationships. BMC Evol. Biol. 10:26. doi: 10.1186/1471-2148-10-26
Case, R. J., Labbate, M., and Kjelleberg, S. (2008). AHL-driven quorum-sensing circuits: their frequency and function among the Proteobacteria. ISME J. 2, 345–349. doi: 10.1038/ismej.2008.13
Chen, S., Zhou, Y., Chen, Y., and Gu, J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Chevrette, M. G., Carlson, C. M., Ortega, H. E., Thomas, C., Ananiev, G. E., Barns, K. J., et al. (2019). The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 10:516. doi: 10.1038/s41467-019-08438-0
Ciezki, K., Wesener, S., Jaber, D., Mirza, S., and Forst, S. (2019). ngrA-dependent natural products are required for interspecies competition and virulence in the insect pathogenic bacterium Xenorhabdus szentirmaii. Microbiology (Reading) 165, 538–553. doi: 10.1099/mic.0.000793
Crawford, J. M., Portmann, C., Kontnik, R., Walsh, C. T., and Clardy, J. (2011). NRPS substrate promiscuity diversifies the xenematides. Org. Lett. 13, 5144–5147. doi: 10.1021/ol2020237
Dadgostar, P. (2019). Antimicrobial Resistance: implications and costs. Infect Drug Resist 12, 3903–3910. doi: 10.2147/IDR.S234610
Davies, J. (2013). Specialized microbial metabolites: functions and origins. J. Antibiot. (Tokyo) 66, 361–364. doi: 10.1038/ja.2013.61
Demain, A. L., and Fang, A. (2000). The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol. 69, 1–39. doi: 10.1007/3-540-44964-7_1
Dietrich, L. E., Price-Whelan, A., Petersen, A., Whiteley, M., and Newman, D. K. (2006). The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol. 61, 1308–1321. doi: 10.1111/j.1365-2958.2006.05306.x
Dobin, A., Davis, C. A., Schlesinger, F., Drenkow, J., Zaleski, C., Jha, S., et al. (2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21. doi: 10.1093/bioinformatics/bts635
Donmez Ozkan, H., Cimen, H., Ulug, D., Wenski, S., Yigit Ozer, S., Telli, M., et al. (2019). Nematode-associated Bacteria: production of Antimicrobial agent as a presumptive nominee for curing endodontic infections caused by Enterococcus faecalis. Front. Microbiol. 10:2672. doi: 10.3389/fmicb.2019.02672
Drake, E. J., Miller, B. R., Shi, C., Tarrasch, J. T., Sundlov, J. A., Leigh Allen, C., et al. (2016). Structures of two distinct conformations of holo-non-ribosomal peptide synthetases. Nature 529, 235–238. doi: 10.1038/nature16163
Dubos, R. J. (1939). Studies on a bactericidal agent extracted from a soil Bacillus: I. Preparation of the agent. Its activity in vitro. J. Exp. Med. 70, 1–10. doi: 10.1084/jem.70.1.1
Felnagle, E. A., Jackson, E. E., Chan, Y. A., Podevels, A. M., Berti, A. D., McMahon, M. D., et al. (2008). Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol. Pharm. 5, 191–211. doi: 10.1021/mp700137g
Forst, S., Dowds, B., Boemare, N., and Stackebrandt, E. (1997). Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Ann. Rev. Microbiol. 51, 47–72. doi: 10.1146/annurev.micro.51.1.47
Fuchs, S. W., Grundmann, F., Kurz, M., Kaiser, M., and Bode, H. B. (2014). Fabclavines: bioactive peptide-polyketide-polyamino hybrids from Xenorhabdus. Chembiochem 15, 512–516. doi: 10.1002/cbic.201300802
Fuchs, S. W., Proschak, A., Jaskolla, T. W., Karas, M., and Bode, H. B. (2011). Structure elucidation and biosynthesis of lysine-rich cyclic peptides in Xenorhabdus nematophila. Org. Biomol. Chem. 9, 3130–3132. doi: 10.1039/c1ob05097d
Ha, J. H., Hauk, P., Cho, K., Eo, Y., Ma, X., Stephens, K., et al. (2018). Evidence of link between quorum sensing and sugar metabolism in Escherichia coli revealed via cocrystal structures of LsrK and HPr. Sci. Adv. 4:p. eaar7063. doi: 10.1126/sciadv.aar7063
Heinrich, A. K., Hirschmann, M., Neubacher, N., and Bode, H. B. (2017). LuxS-dependent AI-2 production is not involved in global regulation of natural product biosynthesis in Photorhabdus and Xenorhabdus. PeerJ 5:e3471. doi: 10.7717/peerj.3471
Herbert, E. E., and Goodrich-Blair, H. (2007). Friend and foe: the two faces of Xenorhabdus nematophila. Nat. Rev. Microbiol. 5, 634–646. doi: 10.1038/nrmicro1706
Hibbing, M. E., Fuqua, C., Parsek, M. R., and Peterson, S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. doi: 10.1038/nrmicro2259
Jiang, Q., Chen, J., Yang, C., Yin, Y., and Yao, K. (2019). Quorum sensing: a prospective therapeutic target for bacterial diseases. Biomed. Res. Int. 2019:2015978. doi: 10.1155/2019/2015978
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., et al. (2021). Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589. doi: 10.1038/s41586-021-03819-2
Kaplan, H. B., and Greenberg, E. P. (1985). Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 163, 1210–1214. doi: 10.1128/jb.163.3.1210-1214.1985
Liedhegner, E., Bojar, B., Beattie, R. E., Cahak, C., Hristova, K. R., and Skwor, T. (2022). Similarities in virulence and extended Spectrum Beta-lactamase gene profiles among cefotaxime-resistant Escherichia coli wastewater and clinical isolates. Antibiotics (Basel) 11:260. doi: 10.3390/antibiotics11020260
Love, M. I., Huber, W., and Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15:550. doi: 10.1186/s13059-014-0550-8
Marques, J. C., Lamosa, P., Russell, C., Ventura, R., Maycock, C., Semmelhack, M. F., et al. (2011). Processing the interspecies quorum-sensing signal autoinducer-2 (AI-2): characterization of phospho-(S)-4,5-dihydroxy-2,3-pentanedione isomerization by LsrG protein. J. Biol. Chem. 286, 18331–18343. doi: 10.1074/jbc.M111.230227
Marques, J. C., Oh, I. K., Ly, D. C., Lamosa, P., Ventura, M. R., Miller, S. T., et al. (2014). LsrF, a coenzyme A-dependent thiolase, catalyzes the terminal step in processing the quorum sensing signal autoinducer-2. Proc. Natl. Acad. Sci. USA 111, 14235–14240. doi: 10.1073/pnas.1408691111
Medarametla, P., Kronenberger, T., Laitinen, T., and Poso, A. (2021). Structural characterization of LsrK as a quorum sensing target and a comparison between X-ray and homology models. J. Chem. Inf. Model. 61, 1346–1353. doi: 10.1021/acs.jcim.0c01233
Miller, S. T., Xavier, K. B., Campagna, S. R., Taga, M. E., Semmelhack, M. F., Bassler, B. L., et al. (2004). Salmonella Typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell 15, 677–687. doi: 10.1016/j.molcel.2004.07.020
Nelson, R. E., Hatfield, K. M., Wolford, H., Samore, M. H., Scott, R. D. II, Reddy, S. C., et al. (2021). National Estimates of healthcare costs associated with multidrug-resistant bacterial infections among hospitalized patients in the United States. Clin. Infect. Dis. 72, S17–S26. doi: 10.1093/cid/ciaa1581
Pantel, L., Florin, T., Dobosz-Bartoszek, M., Racine, E., Sarciaux, M., Serri, M., et al. (2018). Odilorhabdins, antibacterial agents that cause miscoding by binding at a new ribosomal site. Mol. Cell 70, 83–94.e7. doi: 10.1016/j.molcel.2018.03.001
Pearson, W. R. (2013). An introduction to sequence similarity ("homology") searching. Curr Protoc Bioinformatics 1, 3.1.1–3.1.8. doi: 10.1002/0471250953.bi0301s42
Perry, R. D., and Fetherston, J. D. (2011). Yersiniabactin iron uptake: mechanisms and role in Yersinia pestis pathogenesis. Microbes Infect. 13, 808–817. doi: 10.1016/j.micinf.2011.04.008
Pfeifer, B. A., Admiraal, S. J., Gramajo, H., Cane, D. E., and Khosla, C. (2001). Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291, 1790–1792. doi: 10.1126/science.1058092
Pflanze, S., Mukherji, R., Ibrahim, A., Günther, M., Götze, S., Chowdhury, S., et al. (2023). Nonribosomal peptides protect Pseudomonas nunensis 4A2e from amoebal and nematodal predation. Chem. Sci. 14, 11573–11581. doi: 10.1039/D3SC03335J
Reuter, K., Mofid, M. R., Marahiel, M. A., and Ficner, R. (1999). Crystal structure of the surfactin synthetase-activating enzyme sfp: a prototype of the 4′-phosphopantetheinyl transferase superfamily. EMBO J. 18, 6823–6831. doi: 10.1093/emboj/18.23.6823
Reygaert, W. C. (2018). An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol 4, 482–501. doi: 10.3934/microbiol.2018.3.482
Roumia, A. F., Hussein, W., EI-Sayed, G. M., Elmasry, A. M. A., and Fahim, S. F. (2022). In-silco and in-vitro characterization of a symbiotic association Bacteria isolated from Entomopathogenic nematodes and producers for biological control non-ribosomal peptides. Egypt. Acad. J. Biol. Sci. 14, 117–131. doi: 10.21608/eajbsf.2022.232303
Rutherford, S. T., and Bassler, B. L. (2012). Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2:2427. doi: 10.1101/cshperspect.a012427
Schwarzer, D., Finking, R., and Marahiel, M. A. (2003). Nonribosomal peptides: from genes to products. Nat. Prod. Rep. 20, 275–287. doi: 10.1039/b111145k
Seed, P. C., Passador, L., and Iglewski, B. H. (1995). Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177, 654–659. doi: 10.1128/jb.177.3.654-659.1995
Singh, S., Orr, D., Divinagracia, E., McGraw, J., Dorff, K., and Forst, S. (2015). Role of secondary metabolites in establishment of the mutualistic partnership between Xenorhabdus nematophila and the entomopathogenic nematode Steinernema carpocapsae. Appl. Environ. Microbiol. 81, 754–764. doi: 10.1128/AEM.02650-14
Snyder, H., Stock, S. P., Kim, S. K., Flores-Lara, Y., and Forst, S. (2007). New insights into the colonization and release processes of Xenorhabdus nematophila and the morphology and ultrastructure of the bacterial receptacle of its nematode host, Steinernema carpocapsae. Appl Environ Microbiol 73, 5338–5346. doi: 10.1128/AEM.02947-06
Sun, S. J., Liu, Y. C., Weng, C. H., Sun, S. W., Li, F., Li, H., et al. (2020). Cyclic dipeptides mediating quorum sensing and their biological effects in Hypsizygus marmoreus. Biomol. Ther. 10:298. doi: 10.3390/biom10020298
Sussmuth, R. D., and Mainz, A. (2017). Nonribosomal peptide synthesis-principles and prospects. Angew. Chem. Int. Ed. Engl. 56, 3770–3821. doi: 10.1002/anie.201609079
Tanovic, A., Samel, S. A., Essen, L. O., and Marahiel, M. A. (2008). Crystal structure of the termination module of a nonribosomal peptide synthetase. Science 321, 659–663. doi: 10.1126/science.1159850
Tobias, N. J., Wolff, H., Djahanschiri, B., Grundmann, F., Kronenwerth, M., Shi, Y. M., et al. (2017). Natural product diversity associated with the nematode symbionts Photorhabdus and Xenorhabdus. Nat. Microbiol. 2, 1676–1685. doi: 10.1038/s41564-017-0039-9
Touray, M., Ulug, D., Gulsen, S. H., Cimen, H., Hazir, C., Bode, H. B., et al. (2024). Natural products from Xenorhabdus and Photorhabdus show promise as biolarvicides against Aedes albopictus. Pest Manag. Sci. 80, 4231–4242. doi: 10.1002/ps.8127
Wang, H., Fewer, D. P., Holm, L., Rouhiainen, L., and Sivonen, K. (2014). Atlas of nonribosomal peptide and polyketide biosynthetic pathways reveals common occurrence of nonmodular enzymes. Proc. Natl. Acad. Sci. U. S. A. 111, 9259–9264. doi: 10.1073/pnas.1401734111
Wang, L., Li, J., March, J. C., Valdes, J. J., and Bentley, W. E. (2005). luxS-dependent gene regulation in Escherichia coli K-12 revealed by genomic expression profiling. J. Bacteriol. 187, 8350–8360. doi: 10.1128/JB.187.24.8350-8360.2005
Wang, Y., Wilks, J. C., Danhorn, T., Ramos, I., Croal, L., and Newman, D. K. (2011). Phenazine-1-carboxylic acid promotes bacterial biofilm development via ferrous iron acquisition. J. Bacteriol. 193, 3606–3617. doi: 10.1128/JB.00396-11
Wenski, S. L., Cimen, H., Berghaus, N., Fuchs, S. W., Hazir, S., and Bode, H. B. (2020). Fabclavine diversity in Xenorhabdus bacteria. Beilstein J. Org. Chem. 16, 956–965. doi: 10.3762/bjoc.16.84
Zhao, X., Yu, Z., and Ding, T. (2020). Quorum-sensing regulation of Antimicrobial Resistance in Bacteria. Microorganisms 8. doi: 10.3390/microorganisms8030425
Keywords: quorum sensing, NRPS, antibacterial, Xenorhabdus, AMR
Citation: Dey R, Valle DO, Chakraborty A, Mayer KA, Uppala JK, Chakraborty A, Mirza S, Skwor T, Forst S and Dey M (2025) Quorum sensing regulators and non-ribosomal peptide synthetases govern antibacterial secretions in Xenorhabdus szentirmaii. Front. Microbiol. 16:1560663. doi: 10.3389/fmicb.2025.1560663
Edited by:
John Osei Sekyere, University of Pretoria, South AfricaReviewed by:
Eugene A. Rogozhin, Institute of Bioorganic Chemistry (RAS), RussiaAhmed F. Roumia, Menoufia University, Egypt
Asem Sanjit Singh, Indian Institute of Science, India
Copyright © 2025 Dey, Valle, Chakraborty, Mayer, Uppala, Chakraborty, Mirza, Skwor, Forst and Dey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Madhusudan Dey, ZGV5bUB1d20uZWR1; Steven Forst, c2ZvcnN0QHV3bS5lZHU=
 Ritisha Dey1
Ritisha Dey1 Jagadeesh Kumar Uppala
Jagadeesh Kumar Uppala Troy Skwor
Troy Skwor Madhusudan Dey
Madhusudan Dey