
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 26 February 2025
Sec. Microbe and Virus Interactions with Plants
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1540651
This article is part of the Research Topic Soil Biodiversity and Regenerative Agriculture: The Path to Achieve SDGs View all 4 articles
Introduction: Endophytic fungi exhibit diverse interactions with plants, from pathogenic to mutualistic symbiosis, and the community composition is regulated by phytohormones. Yet, the composition and dynamics of endophytic fungi in Paris polyphylla var. chinensis (Franch.) Hara (PPC) during fresh seed (FD), sand-stored seed (SSD), and seedling (SS) stages remain unclear. Similarly, the overall impact of phytohormones on the management of endophytic fungal communities is yet to be elucidated.
Methods: We carried out a pot experiment to examine the effects of various stages of PPC seeds and the external addition of three phytohormones, namely, melatonin (MT), strigolactone (SL), and 24-epibrassinolide (BR) on the endophytic fungi of PPC seedlings. This was done through internal transcribed spacer (ITS) amplicon sequencing.
Results: The study of the endophytic fungal microbiome in FD, SSD, and SS stages of PPC revealed an increased richness and diversity of fungi during the SS stage, with significant changes in community composition observed. We found that Sordariomycetes played a crucial role in this process, potentially contributing to the establishment and growth of PPC seedlings. Additionally, this study investigated the influence of phytohormones on the phenotypic and physiological characteristics of PPC and its endophytic fungal community. Our results demonstrated that MT and SL significantly increased PPC biomass by 69.32 and 15.23%, respectively, while 2 mg/L of BR hindered the growth of PPC roots. MT, SL, and BR not only induced significant changes in the composition and diversity of the endophytic fungal community in PPC but also affected biomass potentially through specific regulation of potential biomarkers. Furthermore, phytohormones were shown to indirectly modify the endophytic fungal community by altering antioxidant system in plants.
Conclusion: This study provides novel insights into the dynamic changes of microbial communities in the FD, SSD, and SS stages. Furthermore, the differences among various phytohormones ultimately enhance our predictive understanding of how to directly or indirectly manipulate the plant microbiome to improve plant health.
Paris polyphylla var. chinensis (Franch.) Hara (PPC) is a perennial flowering herb that belongs to the Melanthiaceae family. Formerly, it was classified under the Liliaceae family and is endemic to China. This species is listed in the International Union for Conservation of Nature’s Red List (IUCN) (Wang et al., 2019). Additionally, the 2020 edition of Chinese Pharmacopeia includes PPC as one of the two original medicinal plants utilized in Paridis Rhizoma (State Pharmacopoeia Commission of the Prc, 2020). Modern pharmacological studies have demonstrated that the primary active component, steroid saponins, exhibits significant biological activities such as anti-tumor, anti-inflammatory, and hemostatic properties. Its efficacy and scope of application continue to expand (Guan et al., 2024). Research on the Chinese pharmaceutical market indicates that PPC is a key ingredient in over 80 traditional Chinese medicine formulations, with annual sales ranging from 800 to 1,050 tons. The annual consumption far exceeds its natural growth capacity, posing a threat of depletion to its wild medicinal resources (Cunningham et al., 2018).
Endophytic microorganisms in plants primarily originate from two sources: the external environment on the plant surface, and seeds (Liu et al., 2020). Endophytic microorganisms, including bacteria, fungi, and actinomycetes, are transmitted vertically between successive plant generations (Shade et al., 2017). The relationship between endophytic fungi and plants is extensive, ranging from being potential pathogens or saprophytes to mutualistic symbiosis (Zhang et al., 2024). Consequently, this intergenerational transmission significantly impacts plant health, quality, productivity, and microecology (Truyens et al., 2015; Nelson, 2018). In agricultural production, due to the lack of cost-effective tissue culture techniques, PPC can only be propagated via seeds. Fresh PPC seeds are immature, both morphologically and physiologically. Following 80 days of warm wet sand stratification before sowing, the PPC seeds complete their entire process of embryo morphological maturation, from nearly spherical to elliptical to cylindrical embryos. After an additional 60 days of low-temperature stratification, the seeds reach physiological maturation. During the sand stratification process, the seeds may be invaded and colonized by external fungi (Zhang et al., 2023). After reaching morphological and physiological maturity, changes in the internal nutritional and hormonal physiological levels of seeds alter the habitat and promote the growth of certain microorganisms within the seeds (Okunishi et al., 2005). Following seed germination, the seed coat gradually sheds, triggering the reassembly of microbial communities on the plant surface and within its tissues. These communities progressively converge toward a similar composition, with the presence of endophytic fungi serving as a key indicator of the completion of this assembly process (Shahzad et al., 2018). However, compared to the seed stage, this phase typically undergoes significant compositional changes. Studies have shown that the endophytic bacterial communities in seeds vary not only due to the seed development process and environmental conditions (Zhang et al., 2023), but also exhibit significant differences among different plant species (Links et al., 2014). Due to their positional advantage, endophytic fungi within the seeds may influence plant growth and adaptability, from seeds to seedling emergence, and continue to affect plant development over time. Therefore, understanding the dynamics of fungi during the processes of fresh seeds, seed storage, and early seedling germination is crucial for the selection and maintenance of endogenous microorganisms beneficial to plant growth and health, as well as monitoring changes in pathogenic bacteria.
The radicle begins to develop after seed germination, leading to seedling growth. PPC typically grows in valleys, beneath forests, or amid shrubbery, where plants compete for sunlight, air, and water. Relative to the fragility of seedlings during the early stages of growth, their swift and robust development influences subsequent growth and yield (Carrera-Castaño et al., 2020). Therefore, the early growth of seedlings is crucial because it enhances the plant’s ability to compete with other species in the field. Phytohormones have long been used to address sluggish early growth of plant seedlings. Research shows that phytohormones play a broad role in plant physiological processes, regulating plant growth and development, and they are critical in shaping the plant microbiome (Eichmann et al., 2021). These hormones can act as nutrients and signaling molecules to regulate the growth and metabolism of endophytic microorganisms (Xu et al., 2018), or selectively restrict the growth of certain members within the plant microbiome by modulating the reactive oxygen species (ROS) system (Stringlis and Pieterse, 2021). Brassinosteroids (BR) are a type of efficient, broad-spectrum steroidal plant hormone involved in processes like cell elongation, tissue differentiation, and responses to abiotic stress (Talaat and Shawky, 2012). Research confirms that BRs promote plant growth by enhancing antioxidant capacity and photosynthetic efficiency (Sytar et al., 2019). Moreover, BRs reportedly increase rhizosphere fungal diversity (Song et al., 2023), and positively impact arbuscular mycorrhizal (AM) symbiosis (Ren et al., 2021). Strigolactone (SL), a novel carotenoid-derived phytohormones is extensively involved in plant growth, including seed germination, seedling development, bud branching, stem elongation, and lateral root formation (Al-Babili and Bouwmeester, 2015). In terms of interactions with microorganisms, SL, similar to BR, has also been reported to participate in AM symbiosis. It reportedly influences significant differences in fungal community composition, but without significant differences in bacterial community composition (Carvalhais et al., 2019). SL can enhance plant resistance to pathogenic fungi (Marzec, 2016). Melatonin (N-acetyl-5-methoxytryptamine) is a natural indoleamine compound that aids in ROS scavenging, photosynthetic system regulation, leaf senescence delay, and tolerance promotion to some biotic stresses by reducing the abundance of several harmful fungi in the rhizosphere soil (Back, 2021; He et al., 2023). While previous studies successfully manipulated specific endophytic fungi in plants through the exogenous application of synthetic plant hormones, there have been no reports on manipulating endophytic fungi at the overall community level using phytohormones.
In this study, we delineated the endophytic fungal communities in PPC throughout three stages: fresh seeds (FD), sand-stored seed (SSD), and seedling (SS), and in response to the treatment of three phytohormones (MT, SL, and BR). The study objectives were as follows: (1) to examine the dynamic changes in endophytic fungi within PPC seeds from the fresh to the stored and seedling stages; (2) to scrutinize the specific impacts of the exogenous addition of three phytohormones on these endophytic fungi; and (3) to further analyze the correlation between the PPC endophytic fungal microbiota and certain physiological parameters, to understand the influence of these phytohormones on the assembly of PPC endophytic fungal microbiota.
The study was carried out at Biological Research Institute of Gansu Academy of Sciences and Heping testing base, Longnan (36.008 N, 103.970 E), Gansu, China. The experimental site is in the eastern monsoon region, and has a cold, semi-humid, and rainy climate, with an altitude of 1,700 m. The growing season of PPC seedlings is from December 2018 to October 2019. The soil is classified as Calcaric Cambisol according to the Food and Agricultural Organization (FAO) classification system. The initial physicochemical characteristics of the soil were: pH = 7.84, organic matter = 1.19 g/kg, TN = 0.63 g/kg and available K = 3.42 mg/kg. During the process of collecting the soil, large particles of impurities, including plant litter, plant roots, and gravel, were removed. The soil was thoroughly mixed before being filled into the flower pots.
In this study, PPC samples were collected at three stages: fresh seeds (FD), sand-stored seeds (SSD), and the seedling (SS) stage. The fresh fruits (Figure 1A) were collected from Guanshan, Huating City, Gansu Province, China, at an elevation of 2,000 m (35°200 N, 106°396 E). To obtain fresh seeds, the fruits were carefully processed by removing the pulp and peel (Figure 1B). Before sowing, fresh seeds require a two-step temperature-alternating sand storage protocol to break dormancy. Initially, seeds undergo a warm stratification phase of approximately 40 days at 18°C, during which PPC seeds complete the full process of embryonic morphological maturation, transitioning from nearly spherical to elliptical, and finally to a cylindrical embryo. After this, seeds are subjected to a cold stratification period of 60 days at 4°C, at which point the seeds attain physiological maturity. Seeds collected at this stage are used as the post-stratification sample (Figure 1C). The sand-stored seeds are then transplanted into plastic pots (17.6 cm × 26.5 cm × 23 cm). The pre-germination treatment of seeds is conducted under moist and cool conditions (with a 70% shade net) to ensure rapid and uniform germination following sowing. Post-germination, the seedlings are grown under natural temperature conditions, and seedling roots at the thirteenth week are collected as samples for the seedling stage (Figure 1D).
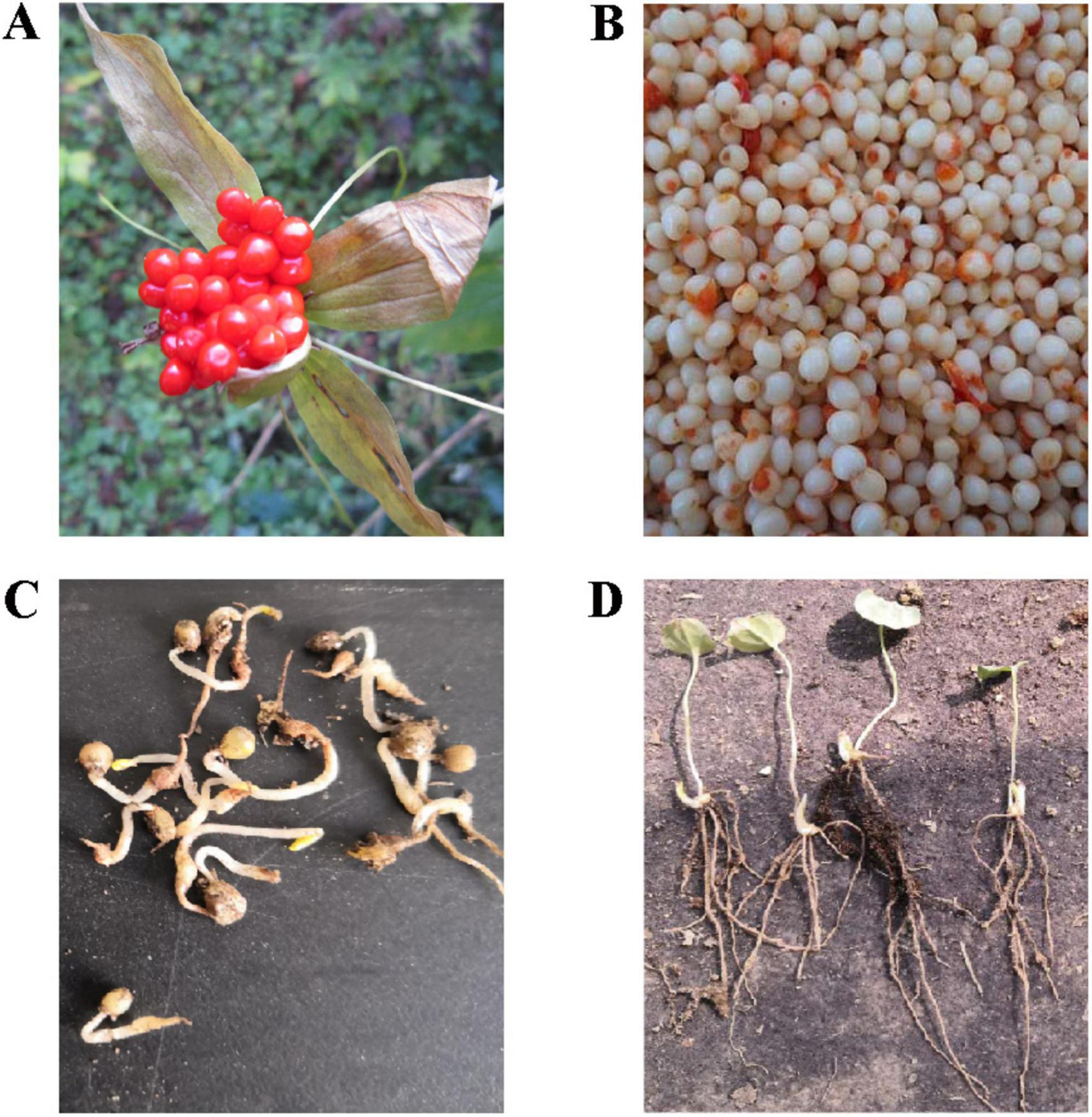
Figure 1. Experimental sampling. (A) Fresh fruit samples. (B) Fresh seed samples. (C) Post-stratification seed samples. (D) Seedling stage samples.
The present study implemented four treatments, which comprised individual inoculations with MT (MT), SL (SL), BR (BR), and an equivalent volume of sterile distilled water as the control (CK). The treatments were arranged in a randomized block design with three replicates per treatment, containing three seedlings per pot. The concentrations of MT, SL, and BR used in the experiment were in line with previous research by Ye et al. (2022), all at 2 mg/mL and 15 mL per pot. Starting from the 8th week of the seedling stage, applications were made once a week for five consecutive weeks. Seedling roots were collected in the 13th week to be used as samples for the seedling stage evaluation.
All collected samples underwent surface sterilization. Initially, the samples were rinsed with sterile distilled water at least three times, or until no turbidity was observed. Subsequently, the samples were immersed in a solution of 5% NaClO for 10 min, followed by three additional rinses with sterile distilled water. After this, 150 mL of the final rinse water was plated onto potato dextrose agar plates. The plates were then incubated at 28°C for 72 h to confirm the effectiveness of the seed surface sterilization. The samples were promptly stored at −80°C for subsequent utilization. The experiment included six treatments, named FD, SSD, SS (CK), MT, GR, and BR. Each treatment had three replicates, resulting in a total of 18 samples.
After phytohormone treatment, we randomly selected three pots per treatment group to measure root length, shoot length, and root fresh weight. Plant samples were placed in an oven at 105°C for 30 min to inactivate the enzymes. Next, they were dried at 70°C until they reached a constant weight; we then recorded the biomass. We calculated all indicators based on an average of three plants per pot. We transferred 1.0 g of mixed plant samples to a plastic centrifuge tube containing 18 mL of precooled 10 mM phosphate-buffered saline (130 mM NaCl, 7 mM Na2HPO4, and 3 mM NaH2PO4; pH 7.4). The plants were then homogenized using a homogenizer. Afterward, we centrifuged the homogenized sample at 3,000 rpm/min for 20 min at 4°C. We then collected and stored the supernatant at 4°C for physiological index measurement. In this study, we determined four physiological indices: malondialdehyde (MDA) content, hydrogen peroxide (H2O2), superoxide dismutase (SOD) activity, and catalase (CAT) activity. We measured all indices using plant Enzyme-Linked Immunosorbent Assay (ELISA) kits (Mibio, Shanghai, China) following the kit instructions. We purchased test kits for MDA (Lot. No.20200521), H2O2 (Lot. No.20200526), SOD (Lot. No.20200619), and CAT (Lot. No.20200603) from Suzhou Keming Biotechnology Co., Ltd. Net photosynthetic rate (Pn), stomatal conductance (Gs), inter-cellular CO2 (Ci), and transpiration rate (Tr) were determined by a portable infra-red gas analyzer—photosynthesis system, LI-6400XT (Lincoln, NE, United States) between 10 a.m. and 12 noon. The contents of Salicylic acid (SA), MT, SL, and BR in plant were measured using plant ELISA kits (Mibio, Shanghai, China) according to the provided instructions.
The total DNA of each sample was extracted using a DNeasy PowerSoil DNA Extraction Kit, following the manufacturer’s instructions (QIAGEN). The identification region for the analysis of fungal diversity, ITS I variable regions were amplified with universal primers (primers: ITS1F 5′-CTTGGTCATTTAGAGGAAGTAA-3′; ITS2 5′-GCTGCGTTCTTCATCGATGC-3′). Sequencing was conducted on an Illumina NovaSeq 6,000 instrument post PCR amplification and purification. Sequencing reads were processed to obtain effective sequences using barcodes for all samples. Then, alignment, filtration, and removal of chimeras from reads were accomplished using software applications such as Pandaseq, Prinseq, and Usearch. Sequences were clustered using Usearch at a similarity threshold of 0.97, chimeras were filtered from the resultant cluster sequences, and OTUs (operational taxonomic units) were collected for species classification. Then, sequences with a similarity threshold of 0.97 were aligned against the representative sequences of the OTU for additional analysis. Taxonomic data annotation was done for representative sequences of fungi using UNITE (version 8.0)1 databases, respectively. The microbial community structure was investigated using the Illumina NovaSeq 6,000 platform (Nanjing GENEPIONEER Biotechnology Co., Ltd., China) for high-throughput sequencing of fungal ITS rRNA.
Alpha diversity indices, including community richness (Chao1) and community diversity (Shannon), were utilized to indicate dissimilarity among samples. The beta diversities across the different stages were determined by principal coordinates analysis (PCoA), and the significant differences were determined by permutational multivariate analysis of variance (PERMANOVA). Non-metric multidimensional scaling (NMDS) and multivariate PERMANOVA were employed to reveal the succession of endophytic fungi, as well as to measure differences in community structure among different treatments. Co-occurrence network analysis and visualization were completed using Gephi software (v.0.9.2), and only significant relationships were included (R > 0.85, p < 0.05). Four node-level topological features were calculated per node: degree, betweenness, closeness, and eigenvector centrality. To investigate relationships between phytohormone contents, physiological properties, and endophytic fungal communities on PPC biomass, partial least squares path modeling (PLS-PM) was performed using the “plspm” package in R.3.1.3. Random forest analysis was performed using the “randomForest” package in R.3.1.3. Significant pearson correlation (p < 0.05) between phytohormone and microbial taxa were analyzed and visualized using the interactive networks in R.3.1.3, Cytoscape 3.3.0. Only significant relationships were included (R > 0.5, p < 0.05) in co-occurrence network analysis.
The data was analyzed using Analysis of Variance (ANOVA) in SPSS 17.0 for Windows (IBM SPSS Inc., Chicago, United States), and the results are expressed as the mean ± standard deviation. A p-value less than 0.05 suggested significant differences, while a p-value less than 0.01 indicated extremely significant differences.
In this study, the ITS gene was sequenced to identify endophytic fungi at three key growth stages of PPC: FS, SSD, and SS. A total of 831,954 high-quality sequences were generated from nine samples. These were classified into 209 fungal OTUs at a 97% clustering level, with 131, 141, and 185 OTUs in FS, SSD, and SS, respectively. Alpha diversity showed that the fungal richness (Chao1) and diversity (Shannon) during the SS stage were significantly higher than those of FS and SSD, with no significant difference between FS and SSD (Figure 2A). Furthermore, the PCoA, based on the Bray-Curtis metric (Figure 2B), revealed substantial differences in fungal composition between SS and the other two stages. Specifically, FS and SSD samples partially overlapped after clustering, while SS samples were separate from the other two stages on the first coordinate axis. The overall network of endophytic fungal communities at different stages portrayed a clear symbiotic pattern. The fungal communities of FS, SSD, and SS had meta-network node numbers of 130, 142, and 185, respectively, with significant edge numbers of 949, 951, and 1,700, respectively (p < 0.05) (Figure 2C). Generally, almost all links between OTUs exhibited positive correlations. Further comparison of the specific node topological characteristics showed that the betweenness centrality and closeness centrality values of SS were notably greater than those of FS and SSD (Figure 2D). Overall, FS and SSD endophytic fungi were significantly different from the SS stage. The dominant phyla of endophytic fungal communities under different treatments were Sordariomycetes (79.048%), Saccharomycetes (5.948%), Leotiomycetes (0.118%), Eurotiomycetes (0.116%), Mortierellomycetes (0.091%), Dothideomycetes (0.024%), Agaricomycetes (0.017%), Chytridiomycetes (0.002%), Glomeromycetes (0.002%), Spizellomycetes, and Ustilaginomycetes (Figure 2E).
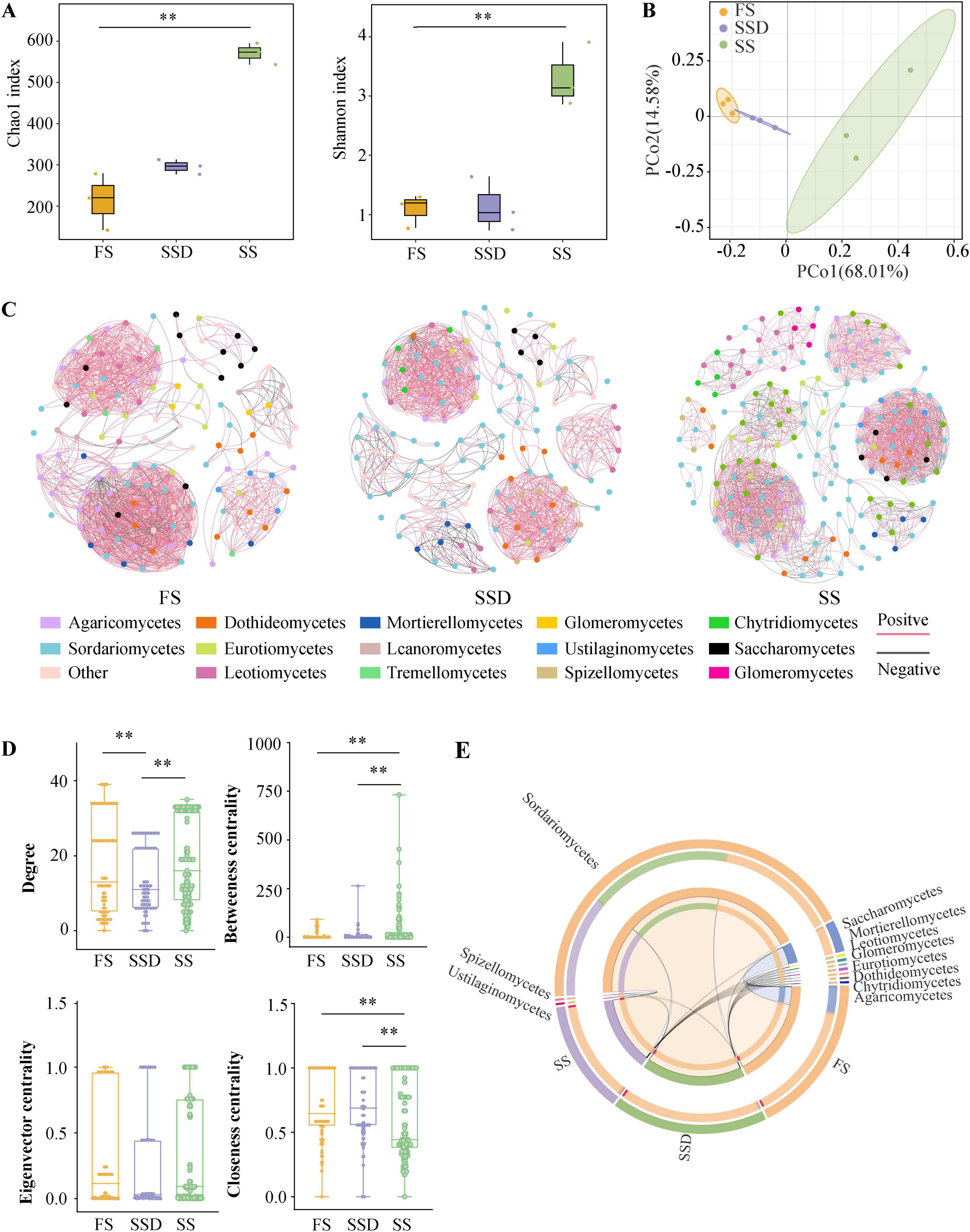
Figure 2. Endophytic fungal diversity and community composition in Paris polyphylla var. chinensis (Franch.) Hara (PPC) at fresh seed (FD), sand-stored seed (SSD), and seedling (SS) stages. (A) Alpha diversity data (Chao1 and Shannon index). (B) β-diversity data [Principal co-ordinates analysis (PCoA) based on Bray-Curtis dissimilarities]. (C) Co-occurrence networks at class level based on a correlation analysis. A link represents a significant correlation (Spearman’s |r| > 0.85 and FDR-corrected p < 0.05). Red and black links represent positive and negative relationships, respectively. (D) Comparison of node-level topological characteristics at class level. (E) Composition of the endophytic fungal communities at phylum level. **p < 0.01 (Dunn’s test).
We used random forest analysis to identify the endophytic fungal taxa distinguishing seeds at different stages (Figure 3A). Ranked by their importance value, the top 20 fungal OTUs mainly belonged to Ascomycota, specifically Stachybotryaceae, Claussenomyces, Nectriaceae, Sordariales, Fusarium, and Trichoderma. The relative abundance results showed that the four pathogenic fungi: Stachybotryaceae, Claussenomyces, Sordariales, and Fusarium, could be viewed as key taxa in the SS stage with a corresponding significant increase in the biocontrol fungus Trichoderma. The pathogen Nectriaceae could be considered a key taxon in the FS stage, showing a significantly higher abundance compared to the other two stages (Figure 3B).
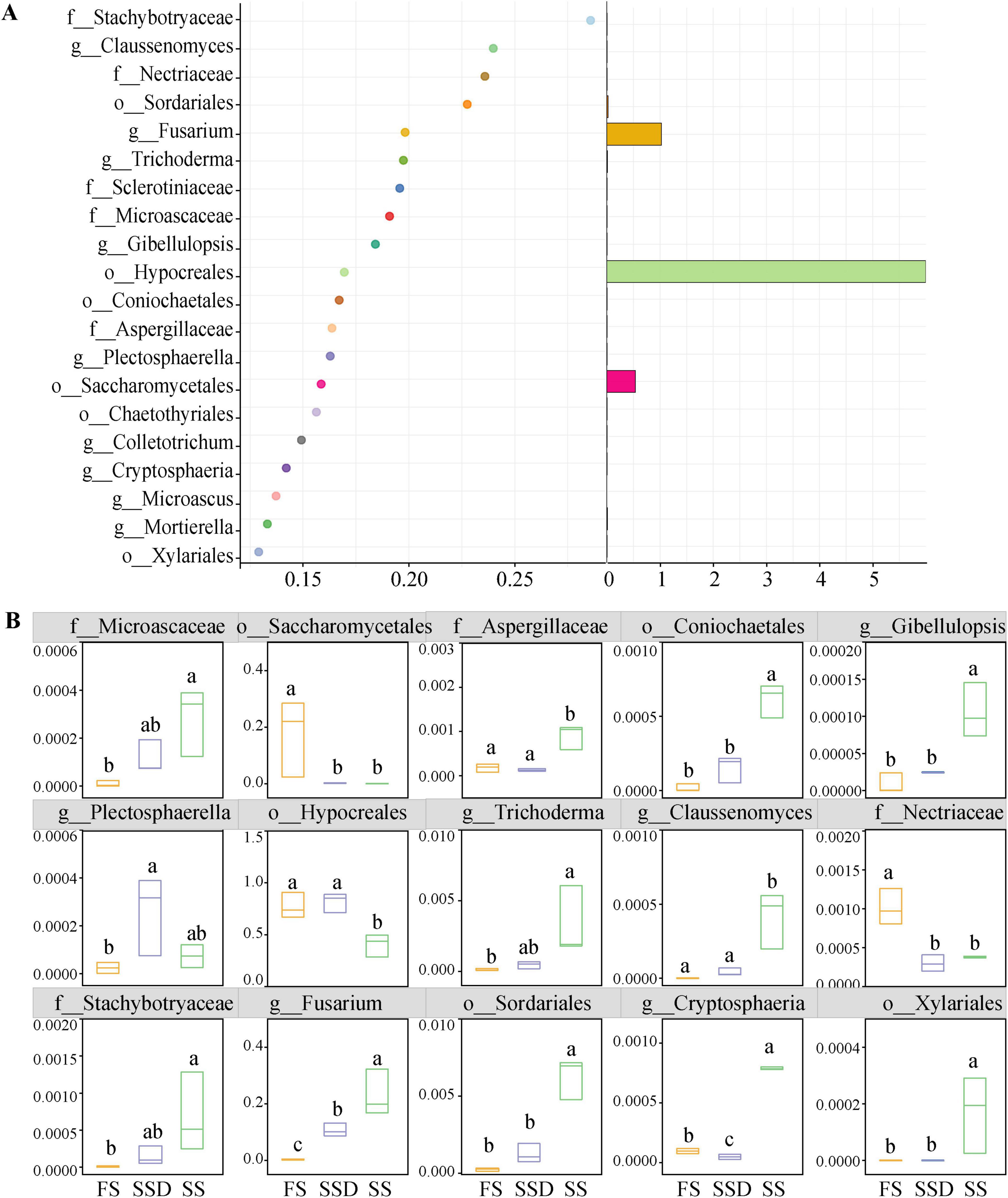
Figure 3. Predictor importance of the top 20 endophytic fungi taxonomic biomarkers (detected by random forest model) (A) and their relative levels of abundance at FD, SD, and SS stages (B). Different letters (a, b, and c) indicate significant differences among treatments according to Dunn’s test (p < 0.05), while the same letter signifies no significant difference.
We observed significant changes in the phenotype of PPC treated with sterile water, MT, SL, and BR (Table 1). In general, compared to CK, plants treated with MT depicted significantly enhanced robustness, with respective increases of 39.45, 10.34, 67.11, and 42.48% in root length, shoot length, fresh weight, and biomass (p < 0.05). SL did not affect the primary root growth significantly, but it led to a significant increase in shoot length, fresh weight, and biomass. BR notably improved shoot growth and exerted a distinct inhibitory effect on primary root growth, and did not significantly influence biomass accumulation. The activities of SOD and CAT were 32.81–108.48% and 20.63–42.86% higher in the three hormone-treated groups than the control, respectively (Table 1). Plants treated with MT exhibited low levels of MDA and H2O2. In contrast, SL and BR treatments significantly raised H2O2 levels, suggesting that the plants under these treatments might be experiencing oxidative stress. MT, SL and BR improved the Pn by10.74–40.00% than CK. Gs and Tr were 14.08–49.32% and 12.88–15.02% higher in MT and SL treatments, than CK, respectively, while Ci was significantly reduced. Treatment with BR significantly increased the gs and Ci transpiration rates compared to CK, while TR significantly decreased. With respect to the control, the phytohormone contents in the MT, SL, and BR treatments were 0.51, 0.69, and 0.34 mg/kg, respectively, marking an increase of 66.66, 56.25, and 66.52%, respectively. The content of SA significantly increased under the influence of all phytohormone treatments.
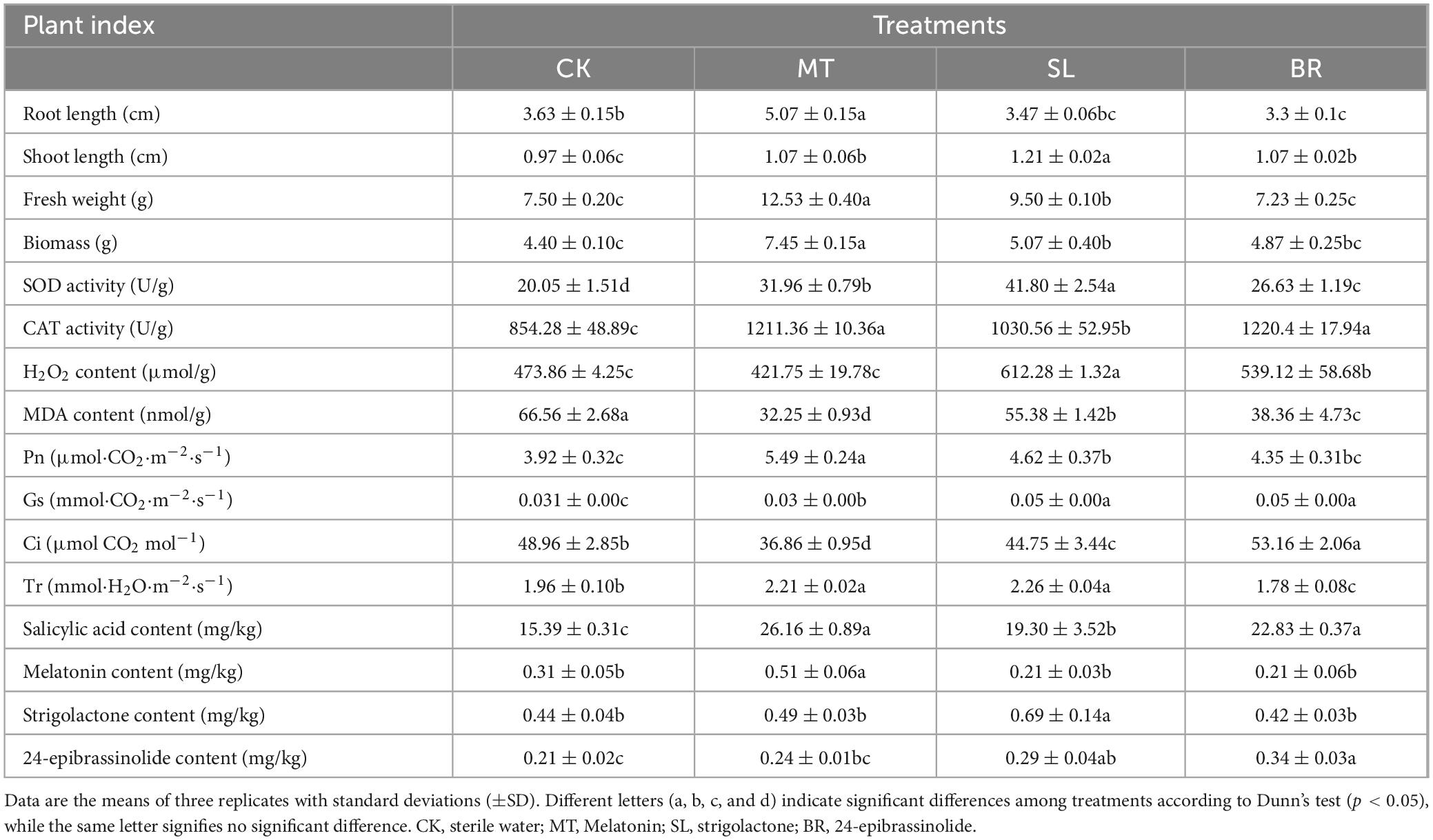
Table 1. Phenotype, physiological properties and phytohormone content of Paris polyphylla var. chinensis (Franch.) Hara (PPC) treated with different phytohormones.
We found that the Chao1 and Shannon indices of endophytic fungi in PPC under MT and SL treatments were significantly lower in comparison to CK (Figure 4A). We further analyzed the variations in soil microbial community structure under phytohormone treatments using NMDS analysis (Figure 4B). The NMDS plot revealed a statistically significant separation (p < 0.05) between the microbial communities of the CK treatment and those associated with MT, SL, and BR treatments, indicating that phytohormone application significantly altered microbial community composition.
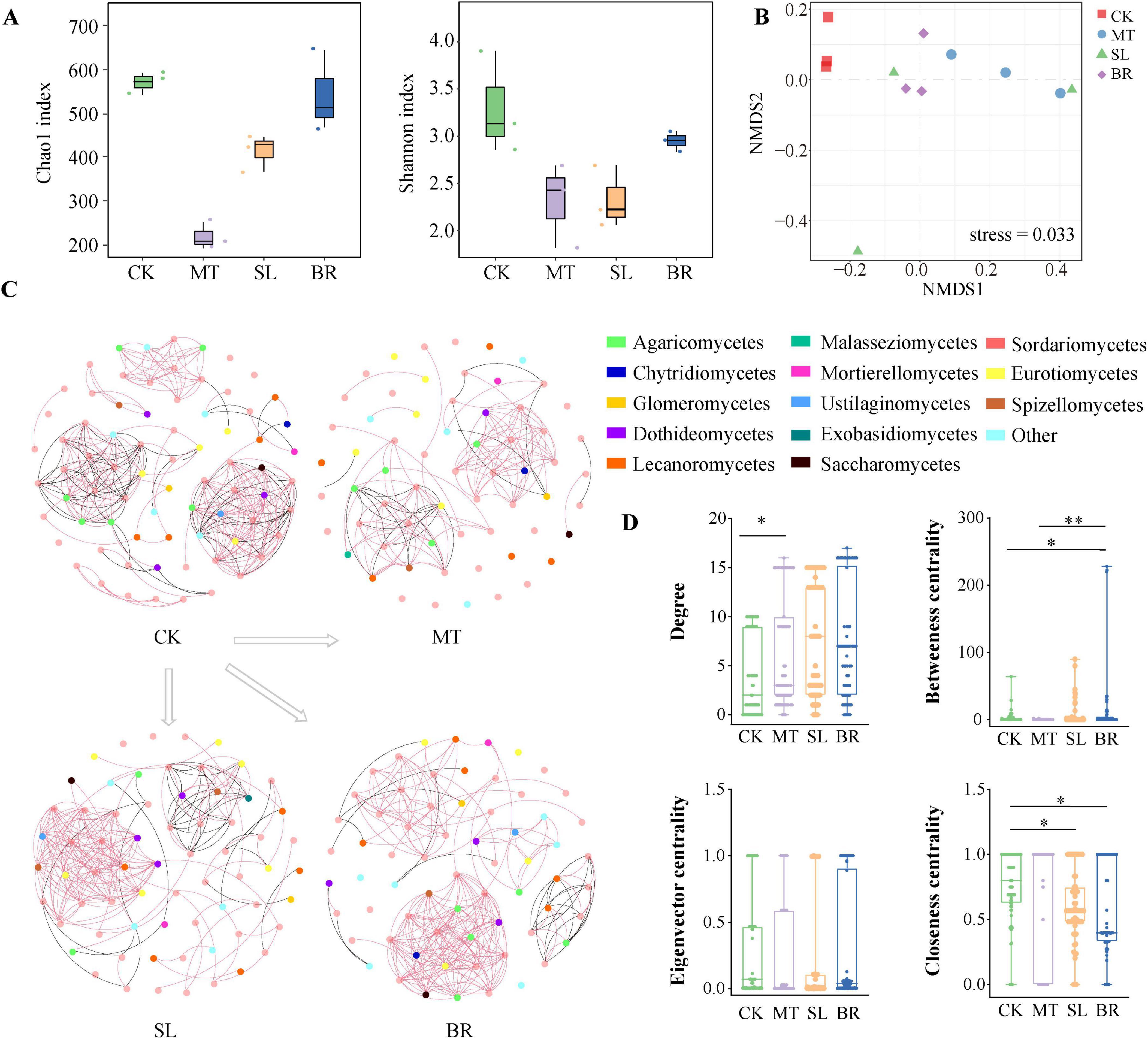
Figure 4. Endophytic fungal diversity and community composition of Paris polyphylla var. chinensis (Franch.) Hara (PPC) in different phytohormone treatments. (A) Alpha diversity data (Chao1 and Shannon index). (B) Non-metric multidimensional scaling (NMDS). (C) Co-occurrence networks at class level based on a correlation analysis. A link represents a significant correlation (Spearman’s |r| > 0.85 and FDR-corrected p < 0.05). Red and black links represent positive and negative relationships, respectively. (D) Comparison of node-level topological characteristics at class level. *p < 0.05, **p < 0.01 (Dunn’s test).
At the class level, Sordariomycetes, Eurotiomycetes, Mortierellomycetes, and Leotiomycetes were dominant in all samples (Figure 4C). Based on pertinent findings, we derived the co-occurrence network of the PPC endophytic fungal community across CK and various hormone treatments (Figure 4C). CK had 290 edges and 76 nodes, MT had 128 edges and 65 nodes, SL had 210 edges and 68 nodes, and BR had 240 edges and 67 nodes. MT demonstrated the fewest nodes and edges. We studied unique community-level topological traits at the node level, chiefly focusing on degree, betweenness centrality, closeness centrality, and eigenvector centrality (Figure 4D). The degree, betweenness centrality, and eigenvector centrality of BR were notably higher than those of CK, suggesting a more pronounced level of symbiotic connection.
To examine the potential direct and indirect effects of phytohormone, antioxidant systems, photosynthetic rate, and fungal communities on PPC biomass, we conducted a PLS-PM analysis based on the effects and relationships of known predictors (Figure 5). The final model fit our dataset well and suggested that the MT content could be the most significant factor directly influencing the PPC biomass. MT, SL, and BR appeared to directly influence the photosynthetic rate. Additionally, MT and BR might modulate the diversity of endophytic fungi indirectly through their influence on antioxidant enzyme activities. Furthermore, BR exerts a direct effect on the diversity of endophytic fungi. Interestingly, neither the α-diversity of endophytic fungi (including Chao1 and Shannon) nor the structure of their community (NMDS1) yielded a direct and significant influence on biomass.
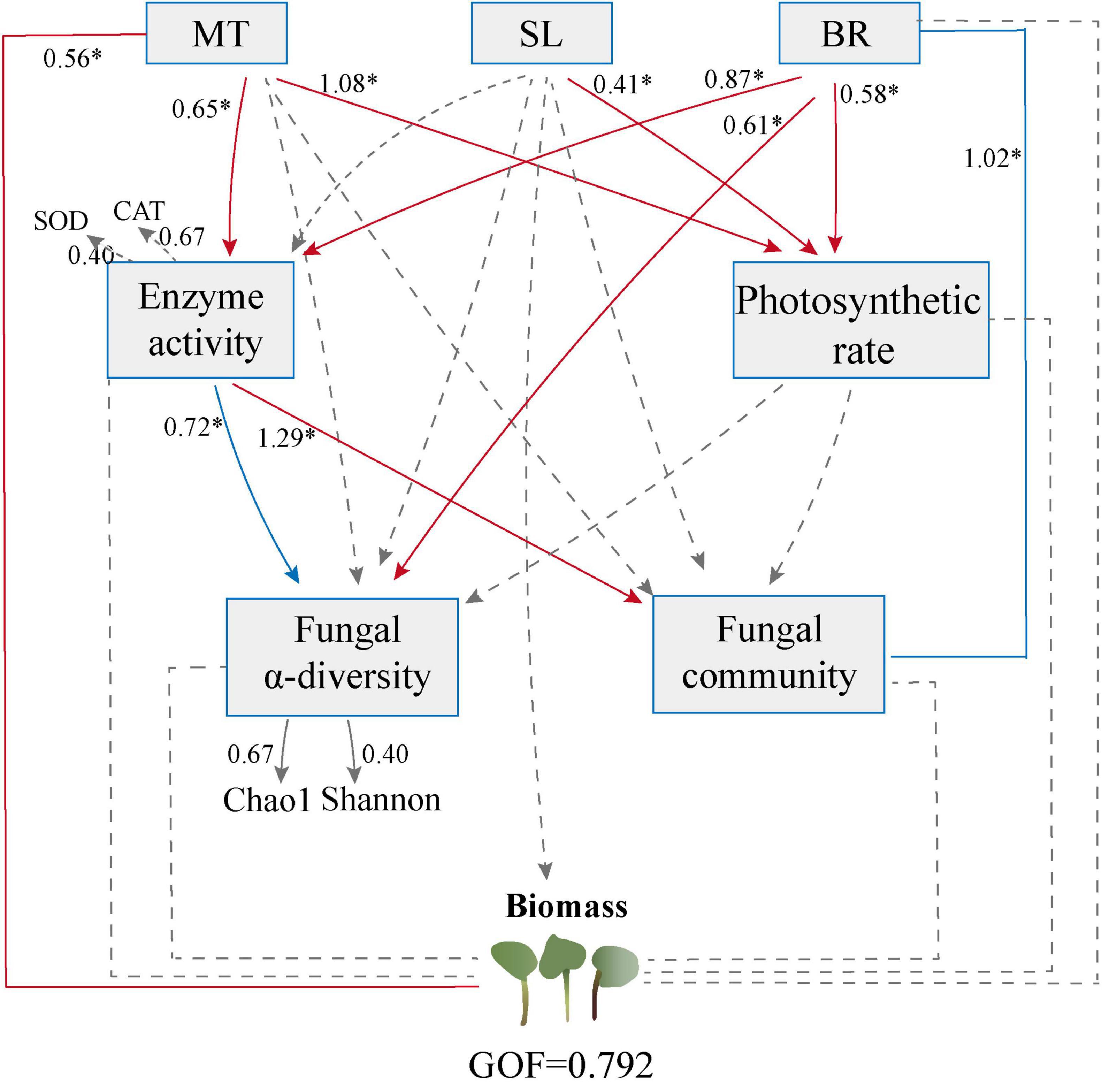
Figure 5. Partial least squares-path modeling (PLS-PM) analysis of direct and indirect influences of phytohormone contents, physiological properties and endophytic fungal communities on Paris polyphylla var. chinensis (Franch.) Hara (PPC) biomass. Positive and negative effects are presented by red and blue arrows, respectively. Path coefficients that were insignificantly different from zero are shown as dashed lines; *p < 0.05. The goodness-of-fit was used to assess the model.
We applied the random forest model to regress the relative abundance of root endophytic fungi at the genus level in PPC against different phytohormone treatments, determining which endophytic fungal taxa set apart from the different hormone treatments (Figure 6A). The model depicted the top 20 root microbiota with a variation index associated with different hormones. Figure 6B illustrates the specific shifts in significant fungal taxa under different hormone treatments, with MT augmenting the abundance of Funneliformis. SL notably increased the abundance of Funneliformis and significantly intensified the abundance of the pathogenic fungus Xylaria. BR markedly increased the abundance of Cryptosphaeria. A linear regression analysis revealed that among the key genera, Cryptosphaeria and Xylaria significantly negatively influenced biomass.
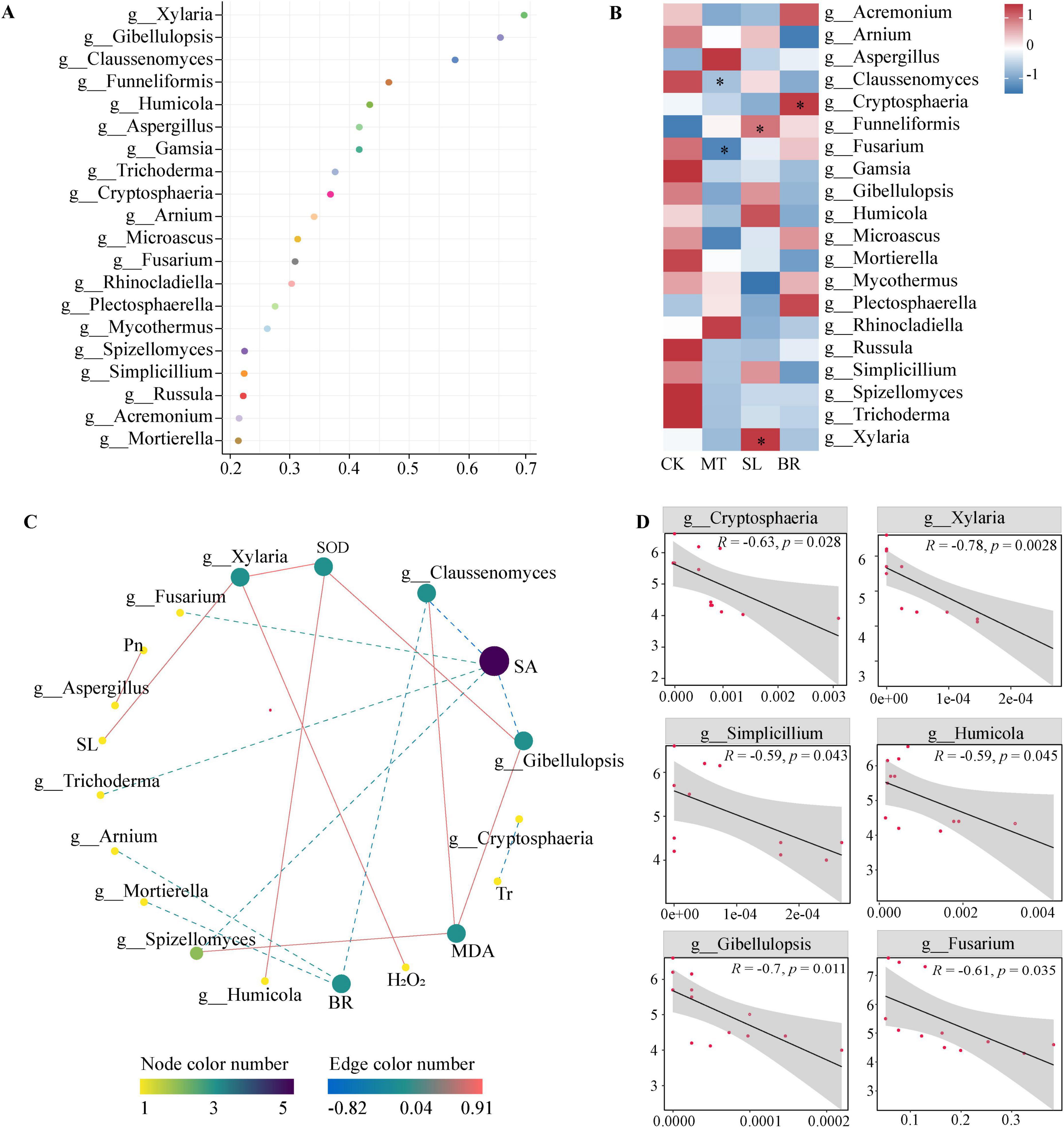
Figure 6. Potential biomarkers of endophytic fungi in response to different phytohormone treatments and their relative abundance in relation to plant physiology and biomass. (A) Genus annotation of the top 20 endophytic fungi in random forest model. (B) The heatmaps exhibit the changes in the relative abundance in response to different phytohormone treatments. *p < 0.05 indicate significant differences the treatments compared to CK (Dunn’s test). (C) Pearson analysis of phytohormone contents, physiological properties and biomarkers. A link represents a significant correlation (Pearson’s |r| > 0.5, and FDR-corrected p < 0.05). Red and blue links represent positive and negative relationships, respectively. (D) Linear regressions between the relative abundances of biomarkers and the Paris polyphylla var. chinensis (Franch.) Hara (PPC) biomass. The lines represent the least squares regression fit, and the shaded area are used to indicate the 95% confidence intervals. CK, sterile water; MT, Melatonin; SL, strigolactone; BR, 24-epibrassinolide.
Pearson correlation analysis showed that indicated that endophytic fungi had a stronger association with phytohormone and antioxidant systems than with photosynthetic systems (Figure 6C). The abundances of fungal genera Arnium, Claussenomyces, and Mortierella were found to negatively correlate with PPC BR content. The abundance of genera Spizellomyces, Claussenomyces, and Gibellulopsis showed a positive correlation with plant MDA content. SL content was found to have a significant negative correlation with Xylaria. Furthermore, there was a significant positive correlation between Pn and Aspergillus, as well as a significant negative correlation between Tr and Cryptosphaeria.
Owing to the notable changes within the endophytic fungal community in PPC spurred by phytohormones, we delved deeper into their potentially interlinked consequences on the increase in PPC biomass (Figure 6D). Our findings point out that among the key taxa significantly influencing alterations in the endophytic community under specified treatments with various phytohormones, species notably impacting biomass include Cryptosphaeria (R = −0.63, p = 0.028), Xylaria (R = −0.78, p = 0.002), Simplicillium (R = −0.59, p = 0.043), Humicola (R = −0.59, p = 0.045), Gibellulopsis (R = −0.7, p = 0.011), and Fusarium (R = −0.61, p = 0.035). Each of these revealed significant negative correlations with PPC biomass.
The study results indicate that there is no significant change in endophytic fungal diversity after PPC seeds are stored in sand. This can be attributed to the protective function of the seed coat, the outermost layer of PPC seeds, which shields the seeds from mechanical damage and prevents the invasion of diseases and pests (Zhang et al., 2023). Notably, Fusarium and Plectosphaerella are significantly enriched as potential biomarkers during this stage, with reports suggesting that these fungi are reputed for causing crop root rot and wilt through widespread colonization (Franke-Whittle et al., 2015; Yang et al., 2023).
Paris polyphylla var. chinensis (Franch.) Hara seedlings significantly alter the diversity and community structure of their endophytic fungi. It is posited that this shift is associated with the shedding of the seed coat, which initiates endophytic fungal interaction with the surrounding environmental microbiota. In this study, the increased relative abundance of symbiotic microorganisms such as Aspergillaceae, Coniochaetales, and Trichoderma during seed germination is ascribed to the efficient nutrient supply from the endosperm formed during germination, fostering the growth of the microbial communities within the seed. These microbial communities, possibly in a dormant state inside the seed, utilize the nutrients released during germination to break dormancy (Barret et al., 2015; Torres-Cortés et al., 2018).
The phylum Ascomycota was the most abundantly annotated group of endophytic fungi at various stages of PPC seed germination. In our study, the early seedling growth stage led to the enrichment of multiple Ascomycota biomarkers, including Gibellulopsis and Cryptosphaeria, two potential plant pathogens that may detriment growth (Trouillas and Gubler, 2016; Liao et al., 2024), as well as Sordariomycetes, a prevalent category with anti-pathogenic activities in plants (Wang et al., 2023). Furthermore, we found that the topological values of the Sordariomycetes taxa were significantly higher than those of other groups, indicating that Sordariomycetes often occupy central positions in the network. Therefore, we presuppose that Sordariomycetes plays a significant role during the PPC seedling growth stage.
The pot experiment demonstrated that both MT and SL significantly promoted PPC biomass accumulation. On the one hand, it has been shown that three phytohormones enhanced the Pn rate to varying degrees, providing plants with more carbon sources for synthesis and positively affecting plant growth (Yan D. et al., 2023). On the other hand, all three phytohormones can regulate the production and elimination of reactive oxygen, which in turn affects various aspects of plant growth and development (Devireddy et al., 2021). The three hormones notably increased the levels of SOD and CAT. MT exhibited lower levels of MDA and H2O2, implying that the oxidation-reduction state of the plant is relatively stable. Numerous reports have suggested that MT primarily functions to protect plants from various abiotic and biotic stresses by scavenging ROS and regulating antioxidant levels (Tan et al., 2012). Moreover, studies have found that MT does not significantly impact the membrane lipid peroxidation system (MDA and H2O2) of the plant under normal growth conditions but can decrease their MDA and H2O2 content under oxidative stress conditions (He et al., 2023; Darré et al., 2024). Based on this evidence, we posit that the natural slow growth of PPC seedlings may be due to oxidative stress in their environment. Therefore, additional supplementation of phytohormones for scavenging reactive oxygen species is deemed necessary. The inhibition of root growth in the BR treatment may be due to the relatively high concentration chosen for this study, which may not be optimal for PPC plants (Lv et al., 2018). Under SL treatment, a significant increase in H2O2 content and root length was observed, while shoot growth declined. The study revealed that the SL-induced accumulation of H2O2 initiates various reactions such as transcription and oxidative modification, ultimately stimulating root development (Tian et al., 2018). The restriction of shoot growth aligns with the conclusions drawn by Chen et al. (2009). Encouragingly, MT exerted a stronger effect than SL and BR on nearly all growth parameters. To some extent, this supports the role of melatonin as a primary regulator in PPC, in agreement with previous research findings (Carvalhais et al., 2019).
Manipulating the endophytic microbial communities in natural environments is critical for leveraging ecological interactions to enhance plant productivity. Therefore, it is essential to conduct pot experiments in uncontrolled, natural conditions, to examine the impact of phytohormones on the endophytic fungal community of PPC. In this study, all three phytohormones significantly altered the structure of the endophytic fungal community. We used PLS-PM to assess the relationships between hormone concentrations, physiological parameters (such as antioxidant enzyme activities and photosynthetic rates), endophytic fungal community composition (diversity and structure), and plant biomass. In the current study, MT and BR indirectly affected endophytic fungal diversity by enhancing the activity of antioxidant enzymes. Fungi like Gibellulopsis, Spizellomyces, and Claussenomyces showed significant positive correlations with MDA content, possibly due to the hypersensitive response triggered by plant infection and fungal recognition, which leads to increased lipid peroxidation of the cell membrane (Berrios and Rentsch, 2022). SOD and CAT are vital protective enzymes involved in scavenging ROS in plants. The coordination of ROS levels with the abundance and diversity of microbial populations is well-documented (Fuller et al., 2017). Xylaria and Humicola demonstrated significant positive correlations with SOD activity, which may be due to the tendency of commensal fungal genomes to enrich genes for ROS-scavenging enzymes (Berrios and Ely, 2020). MT content had a direct positive effect on biomass, but the diversity and overall structure of the endophytic fungal community did not vastly influence biomass. We conducted a subsequent random forest analysis to identify potential biomarkers of the endophytic fungal community responding to various phytohormone treatments. These key taxa are generally considered critical factors in differentiating microbial community structure and they exert varied effects on plant growth. In this study, MT exerted a significant positive impact on the biomass of PPC by markedly inhibiting the abundance of the pathogenic fungus Fusarium. The study showed the significant enrichment in two key taxa, Funneliformis and Xylaria, in that SL. Xylaria is identified as a pathogen that negatively influences crop root development (Garcia-Aroca et al., 2021). The results of linear analysis also indicate a negative correlation between Xylaria and biomass. In the BR treatment, Cryptosphaeria was significantly enriched, and reports have demonstrated that this pathogen can cause cankers in crops and forest plants (Yan C. et al., 2023). Consequently, Cryptosphaeria could be a key taxon responsible for the adverse effects of BR on PPC growth. In conclusion, the effects of various plant hormones on the endophytic fungi within PPC are specific and complex. MT, SL, and BR can regulate the antioxidant system, thereby influencing diversity and structural alterations within the endophytic fungal community. The findings of this study underline the view that phytohormones indirectly impact microbial communities by modifying plant physiological properties (Eichmann et al., 2021; Shao et al., 2024). The effects of MT, SL, and BR on the biomass of PPC are independent of changes in microbial community structure. Nevertheless, it is connected to the succession of specific key biomarker species affected by each hormone.
In summary, the study of the endophytic fungal microbiome in FS, SSD, and SS stages of PPC indicates that the seedling stage exhibits the greatest microbial richness and diversity, along with a significant shift in composition. We also identified Sordariomycetes as essential microorganisms in this process, potentially contributing to the establishment and growth of PPC seedlings. Moreover, this study explored the effects of phytohormones on the phenotypic, physiological characteristics, and endophytic fungal community of PPC. Our findings showed that MT and SL significantly boosted PPC biomass by 69.32 and 15.23%, respectively. However, 2 mg/L of BR hindered PPC root development. MT, SL, and BR notably induce changes in the endophytic fungal community composition and diversity within PPC, potentially affecting biomass through the specific regulation of potential biomarkers. Moreover, phytohormones can indirectly impact the endophytic fungal community by updating the plant antioxidant system. This research provides novel insights into the dynamics of microbial communities within FS, SSD, and SS. The variations among distinct phytohormones may ultimately enhance our predictive understanding regarding strategies to control plant microbiota, either directly or indirectly, for improving plant health.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1193989.
TP: Investigation, Methodology, Writing – original draft. TY: Funding acquisition, Resources, Supervision, Writing – review and editing. JS: Data curation, Project administration, Writing – review and editing. JZ: Formal Analysis, Investigation, Writing – review and editing. JS: Investigation, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Key research and development project of Gansu Academy of Sciences (2023ZDYF-01), Industrialization application Project of Gansu Academy of Sciences (2024YLCY-01).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Al-Babili, S., and Bouwmeester, H. J. (2015). Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 66, 161–186. doi: 10.1146/annurev-arplant-043014-114759
Back, K. (2021). Melatonin metabolism, signaling and possible roles in plants. Plant J. 105, 376–391. doi: 10.1111/tpj.14915
Barret, M., Briand, M., Bonneau, S., Préveaux, A., Valière, S., Bouchez, O., et al. (2015). Emergence shapes the structure of the seed microbiota. Appl. Environ. Microbiol. 81, 1257–1266. doi: 10.1128/aem.03722-14
Berrios, L., and Ely, B. (2020). Plant growth enhancement is not a conserved feature in the Caulobacter genus. Plant Soil 449, 81–95. doi: 10.1007/s11104-020-04472-w
Berrios, L., and Rentsch, J. D. (2022). Linking reactive oxygen species (ROS) to abiotic and biotic feedbacks in plant microbiomes: The dose makes the poison. Int. J. Mol. Sci. 23, 1422–1467. doi: 10.3390/ijms23084402
Carrera-Castaño, G., Calleja-Cabrera, J., Pernas, M., Gómez, L., and Oñate-Sánchez, L. (2020). An updated overview on the regulation of seed germination. Plants (Basel) 9, 2223–7747. doi: 10.3390/plants9060703
Carvalhais, L. C., Rincon-Florez, V. A., Brewer, P. B., Beveridge, C. A., Dennis, P. G., and Schenk, P. M. (2019). The ability of plants to produce strigolactones affects rhizosphere community composition of fungi but not bacteria. Rhizosphere 9, 18–26. doi: 10.1016/j.rhisph.2018.10.002
Chen, C., Zou, J., Zhang, S., Zaitlin, D., and Zhu, L. (2009). Strigolactones are a new-defined class of plant hormones which inhibit shoot branching and mediate the interaction of plant-AM fungi and plant-parasitic weeds. Sci. China C Life Sci. 52, 693–700. doi: 10.1007/s11427-009-0104-6
Cunningham, A. B., Brinckmann, J. A., Bi, Y. F., Pei, S. J., Schippmann, U., and Luo, P. (2018). Paris in the spring: A review of the trade, conservation and opportunities in the shift from wild harvest to cultivation of Paris polyphylla (Trilliaceae). J. Ethnopharmacol. 222, 208–216. doi: 10.1016/j.jep.2018.04.048
Darré, M., Zaro, M. J., Guijarro-Fuertes, M., Careri, L., and Concellón, A. (2024). Melatonin combined with wax treatment enhances tolerance to chilling injury in red bell pepper. Metabolites 14, 2218–1989. doi: 10.3390/metabo14060330
Devireddy, A. R., Zandalinas, S. I., Fichman, Y., and Mittler, R. (2021). Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 105, 459–476. doi: 10.1111/tpj.15010
Eichmann, R., Richards, L., and Schäfer, P. (2021). Hormones as go-betweens in plant microbiome assembly. Plant J. 105, 518–541. doi: 10.1111/tpj.15135
Franke-Whittle, I. H., Manici, L. M., Insam, H., and Stres, B. (2015). Rhizosphere bacteria and fungi associated with plant growth in soils of three replanted apple orchards. Plant Soil 395, 317–333. doi: 10.1007/s11104-015-2562-x
Fuller, A. W., Young, P., Pierce, B. D., Kitson-Finuff, J., Jain, P., Schneider, K., et al. (2017). Redox-mediated quorum sensing in plants. PLoS One 12:e0182655. doi: 10.1371/journal.pone.0182655
Garcia-Aroca, T., Price, P. P., Tomaso-Peterson, M., Allen, T. W., Wilkerson, T. H., Spurlock, T. N., et al. (2021). Xylaria necrophora, sp. nov., is an emerging root-associated pathogen responsible for taproot decline of soybean in the southern United States. Mycologia 113, 326–347. doi: 10.1080/00275514.2020.1846965
Guan, L., Zheng, Z., Guo, Z., Xiao, S., Liu, T., Chen, L., et al. (2024). Steroidal saponins from rhizome of Paris polyphylla var. chinensis and their anti-inflammatory, cytotoxic effects. Phytochemistry 219:113994. doi: 10.1016/j.phytochem.2024.113994
He, X., Yin, B., Zhang, J., Zhou, S., Li, Z., Zhang, X., et al. (2023). Exogenous melatonin alleviates apple replant disease by regulating rhizosphere soil microbial community structure and nitrogen metabolism. Sci. Total Environ. 884:163830. doi: 10.1016/j.scitotenv.2023.163830
Liao, K., Chu, T., Shi, Y., Xie, X., Li, L., Fan, T., et al. (2024). First report of gibellulopsis nigrescens causing yellow wilt on chinese cabbage in China. Plant Dis. 108:3197. doi: 10.1094/pdis-05-24-0989-pdn
Links, M. G., Demeke, T., Gräfenhan, T., Hill, J. E., Hemmingsen, S. M., and Dumonceaux, T. J. (2014). Simultaneous profiling of seed-associated bacteria and fungi reveals antagonistic interactions between microorganisms within a shared epiphytic microbiome on riticum and rassica seeds. New Phytol. 202, 542–553. doi: 10.1111/nph.12693
Liu, Y., Yan, H., Zhang, X., Zhang, R., Li, M., Xu, T., et al. (2020). Investigating the endophytic bacterial diversity and community structures in seeds of genetically related maize (Zea mays L.) genotypes. 3 Biotech 10:27. doi: 10.1007/s13205-019-2034-8
Lv, B., Tian, H., Zhang, F., Liu, J., Lu, S., Bai, M., et al. (2018). Brassinosteroids regulate root growth by controlling reactive oxygen species homeostasis and dual effect on ethylene synthesis in Arabidopsis. PLoS Genet. 14:e1007144. doi: 10.1371/journal.pgen.1007144
Marzec, M. (2016). Strigolactones as part of the plant defence system. Trends Plant Sci. 21, 900–903. doi: 10.1016/j.tplants.2016.08.010
Nelson, E. B. (2018). The seed microbiome: Origins, interactions, and impacts. Plant Soil 422, 7–34. doi: 10.1007/s11104-017-3289-7
Okunishi, S., Sako, K., Mano, H., Imamura, A., and Morisaki, H. (2005). Bacterial Flora of Endophytes in the Maturing Seed of Cultivated Rice (Oryza sativa). Microbes Environ. 20, 168–177. doi: 10.1264/jsme2.20.168
Ren, Y., Che, X., Liang, J., Wang, S., Han, L., Liu, Z., et al. (2021). Brassinosteroids benefit plants performance by augmenting arbuscular mycorrhizal symbiosis. Microbiol. Spectrum. 9:e0164521. doi: 10.1128/spectrum.01645-21
Shade, A., Jacques, M.-A., and Barret, M. (2017). Ecological patterns of seed microbiome diversity, transmission, and assembly. Curr. Opin. Microbiol. 37, 15–22. doi: 10.1016/j.mib.2017.03.010
Shahzad, R., Khan, A. L., Bilal, S., Asaf, S., and Lee, I. J. (2018). What is there in seeds? vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 9:24. doi: 10.3389/fpls.2018.00024
Shao, Q., Ran, Q., Li, X., Dong, C., Zhang, Y., and Han, Y. (2024). Differential responses of the phyllosphere abundant and rare microbes of Eucommia ulmoides to phytohormones. Microbiol. Res. 286:127798. doi: 10.1016/j.micres.2024.127798
Song, X. E., Cao, J., Guo, S., Wang, H., Dong, Q., Guo, P., et al. (2023). Effect of brassinolide on soil microorganisms in millet field polluted by tribenuron-methyl. Microorganisms 11, 2076–2607. doi: 10.3390/microorganisms11071829
State Pharmacopoeia Commission of the Prc. (2020). Pharmacopoeia of People’s Republic of China. China Med. Sci. Technol. Press 1, 274–275.
Stringlis, I. A., and Pieterse, C. M. J. (2021). Evolutionary “hide and seek” between bacterial flagellin and the plant immune system. Cell Host Microbe 29, 548–550. doi: 10.1016/j.chom.2021.03.010
Sytar, O., Kumari, P., Yadav, S., Brestic, M., and Rastogi, A. (2019). Phytohormone priming: Regulator for heavy metal stress in plants. J. Plant Growth Regul. 38, 739–752. doi: 10.1007/s00344-018-9886-8
Talaat, N. B., and Shawky, B. T. (2012). 24-Epibrassinolide ameliorates the saline stress and improves the productivity of wheat (Triticum aestivum L.). Environ. Exp. Bot. 82, 80–88. doi: 10.1016/j.envexpbot.2012.03.009
Tan, D. X., Hardeland, R., Manchester, L. C., Korkmaz, A., Ma, S., Rosales-Corral, S., et al. (2012). Functional roles of melatonin in plants, and perspectives in nutritional and agricultural science. J. Exp. Bot. 63, 577–597. doi: 10.1093/jxb/err256
Tian, Y., Fan, M., Qin, Z., Lv, H., Wang, M., Zhang, Z., et al. (2018). Hydrogen peroxide positively regulates brassinosteroid signaling through oxidation of the Brassinazole-Resistant1 transcription factor. Nat. Commun. 9:1063. doi: 10.1038/s41467-018-03463-x
Torres-Cortés, G., Bonneau, S., Bouchez, O., Genthon, C., Briand, M., Jacques, M. A., et al. (2018). Functional microbial features driving community assembly during seed germination and emergence. Front. Plant Sci. 9:902. doi: 10.3389/fpls.2018.00902
Trouillas, F. P., and Gubler, W. D. (2016). Cryptosphaeria dieback of fremont cottonwood caused by Cryptosphaeria pullmanensis and C. multicontinentalis in California. Plant Dis. 100, 777–783. doi: 10.1094/pdis-09-15-0972-re
Truyens, S., Weyens, N., Cuypers, A., and Vangronsveld, J. (2015). Bacterial seed endophytes: Genera, vertical transmission and interaction with plants. Environ. Microbiol. Rep. 7, 40–50. doi: 10.1111/1758-2229.12181
Wang, X., Peng, C., Liang, J., Liang, Q., Xu, C., and Guo, W. (2019). The complete chloroplast genome of Paris polyphylla var. chinensis, an endemic medicinal herb in China. Mitochondrial DNA B Resour. 4, 3888–3889. doi: 10.1080/23802359.2019.1687351
Wang, X., Wanasinghe, D. N., Zhang, J., Ma, J., Zhou, P., Zhang, L., et al. (2023). Insights from the Endophytic Fungi in Amphisphaeria (Sordariomycetes): A. orixae sp. nov. from Orixa japonica and its secondary metabolites. Microorganisms 11, 2076–2607. doi: 10.3390/microorganisms11051268
Xu, G., Yang, S., Meng, L., and Wang, B.-G. (2018). The plant hormone abscisic acid regulates the growth and metabolism of endophytic fungus Aspergillus nidulans. Sci. Rep. 8:6504. doi: 10.1038/s41598-018-24770-9
Yan, C., Hao, H., Wang, Z., Sha, S., Zhang, Y., Wang, Q., et al. (2023). Prediction of suitable habitat distribution of Cryptosphaeria pullmanensis in the world and China under climate change. J. Fungi (Basel) 9, 739. doi: 10.3390/jof9070739
Yan, D., Wang, J., Lu, Z., Liu, R., Hong, Y., Su, B., et al. (2023). Melatonin-mediated enhancement of photosynthetic capacity and photoprotection improves salt tolerance in wheat. Plants (Basel) 12, 2223–7747. doi: 10.3390/plants12233984
Yang, F., Jiang, H., Chang, G., Liang, S., Ma, K., Cai, Y., et al. (2023). Effects of rhizosphere microbial communities on cucumber fusarium wilt disease suppression. Microorganisms 11:1576. doi: 10.3390/microorganisms11061576
Ye, F., Jiang, M., Zhang, P., Liu, L., Liu, S., Zhao, C., et al. (2022). Exogenous melatonin reprograms the Rhizosphere microbial community to modulate the responses of barley to drought stress. Int. J. Mol. Sci. 23:9665. doi: 10.3390/ijms23179665
Zhang, J., Hu, H., Li, S., Shang, W., Jiang, J., Xu, X., et al. (2023). Diversity of fungal endophytes in american ginseng seeds. Plant Dis. 107, 2784–2791. doi: 10.1094/PDIS-10-22-2312-RE
Zhang, X., Huang, T., Liang, Y., Hussain, S., Peng, R., Wang, T., et al. (2024). Melatonin and 14-hydroxyed brassinosteroid combined promote kiwifruit seedling growth by improving soil microbial distribution, enzyme activity and nutrients uptake. Front. Plant Sci. 15:1336116. doi: 10.3389/fpls.2024.1336116
Keywords: Paris polyphylla var. chinensis (Franch.) Hara, endophytic fungi, phytohormones, phenotypic, antioxidant system
Citation: Peng T, Yang T, Sha J, Zhao J and Shi J (2025) Dynamics of endophytic fungi composition in paris polyphylla var. chinensis (franch.) hara seeds during storage and growth, and responses of seedlings to phytohormones. Front. Microbiol. 16:1540651. doi: 10.3389/fmicb.2025.1540651
Received: 06 December 2024; Accepted: 14 February 2025;
Published: 26 February 2025.
Edited by:
Anukool Vaishnav, GLA University, IndiaReviewed by:
Zeba I. Seraj, University of Dhaka, BangladeshCopyright © 2025 Peng, Yang, Sha, Zhao and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Yang, eWFuZ3RhbzgzODNAMTYzLmNvbQ==; Jianwu Shi, andzaGlAbnR1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.