
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 07 February 2025
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1533282
This article is part of the Research TopicGut Microbiota's Role in High-Altitude Animal AdaptationView all 17 articles
Introduction: Migratory birds exhibit unique annual cycles that complicate their gut microbiota. However, the annual dynamics of gut microbiota in migratory birds remain unclear, hindering our understanding of their environmental adaptation.
Methods: Here, we collected fecal samples from black-necked cranes (Grus nigricollis) across four seasons at their breeding grounds and used wintering ground data from databases to characterize their gut microbial compositions throughout the year.
Results and discussion: The results showed that the gut microbiota was clustered by season (Bray-Curtis: R2 = 0.348, p < 0.001; UniFrac: R2 = 0.352, p < 0.001). And the summer samples exhibited higher alpha (Simpson and Shannon), beta diversity (Bray-Curtis and UniFrac) and more diverse functions in gut microbiota compared to other seasons. Furthermore, in summer, the gut microbiota exhibited several balanced relative abundances at the family level, whereas Lactobacillaceae family dominated during the other seasons. Thirty-six ASVs were identified by random forest analysis to distinguish samples from distinct seasons. Despite having greater diversity, the summer gut microbiota had a simpler network structure than the other seasons (fewer edges and nodes). The dispersal limitation during random processes also significantly influenced gut microbial community assembly. Overall, the gut microbiota of the black-necked crane undergoes dynamic adjustments to adapt to seasonal environmental changes, which may be associated with the variations in diet across seasons. These results enhance our understanding of the gut microbiota of wild migratory birds and support further research on black-necked cranes.
Gut microbes form complex symbiotic relationships with their hosts (Nichols and Davenport, 2021). The gut microbiota is influenced by host conditions (Bajinka et al., 2020) and plays an important role in maintaining gut health and host metabolic pathways (Claus et al., 2011; Lee et al., 2022). Wild animals face more complex environmental changes (e.g., seasonal diet and elevation) than animals in captivity, and they undergo physiological and behavioral adjustments to adapt (Dallas and Warne, 2023). Research on wild animals has revealed that environmental changes influence the gut microbiota. For example, alpha and beta gut microbiota diversity increases with habitat elevation in pikas (Ochotona curzoniae) (Li H. et al., 2019). Furthermore, studies on Tibetan macaques (Macaca thibetana) (Xia et al., 2021) and ground squirrels (Spermophilus dauricus) (Yang et al., 2021) have revealed that their gut microbiota clusters by season. Additionally, during seasons of food abundance, the gut microbiota of animals exhibits higher diversity to meet the demands of digesting a diverse range of foods (Sun et al., 2016).
Migratory birds have a unique annual cycle (Schmiedová et al., 2023), and their periodic migrations between breeding and wintering grounds expose them to diverse challenges (Lu et al., 2022). Because of their exposure to complex and variable environments, wild migratory birds have emerged as pivotal models for investigating microbial-host interactions (Elzinga et al., 2019). During migration, birds must adapt to their different habitats and local food resources (Grond et al., 2018). The gut microbiota plays a crucial role in bird migration and habitat changes (Zhang F. et al., 2020). The gut microbiota assists birds in breaking down plant fibers and detoxifying harmful substances in their diet (Drovetski et al., 2019; Waite and Taylor, 2015; Zhang et al., 2021). During the cold season, bacteria such as Firmicutes become more abundant, facilitating energy intake (Liukkonen et al., 2024; Yao et al., 2023). Similarly, during migration, microbes associated with fat deposition, such as Corynebacterium, increase in abundance (Skeen et al., 2023; Thie et al., 2022; Zhang et al., 2021). However, the unique annual cycle of birds makes their gut microbiota complex and difficult to study (Wu et al., 2018).
Previous studies have identified seasonal variations in the diversity and functional composition of gut microbes in migratory birds. These include white-headed cranes (Antigone vipio) (Dong et al., 2021), black-winged stilts (Himantopus himantopus), black-tailed godwits (Limosa limosa), and redshanks (Tringa totanus) (Zhang et al., 2021). Some migratory birds have extended breeding or wintering periods, and a prolonged stay in one location can lead to changes in the gut microbiota. This has been observed in studies of wild relict gulls (Larus relictus) (Yao et al., 2023) and the great bustard (Otis tarda) (Lu et al., 2024).
Most studies on the microbiota of migratory birds have focused primarily on a single period of the annual cycle. However, understanding gut microbial changes throughout the annual cycle can provide valuable insights into the relationship between gut microbes and their hosts under varying environmental conditions, thereby aiding in the conservation of wild and rare avian species (Song et al., 2014).
The black-necked crane (Grus nigricollis) is a lifelong highland bird that is currently listed as threatened by the IUCN. High-altitude environments impose environmental pressures (e.g., hypoxia, low temperature, and high ultraviolet light) on animals (Liu et al., 2022), and consequently, birds inhabiting these environments adapt their physiological state and gut microbiota accordingly (Wang et al., 2020). For example, the Eurasian tree sparrow (Passer montanus) enlarges its digestive organs (Sun et al., 2023), whereas the Himalayan bluetail (Tarsiger rufilatus) enriches its gut with Lactobacillus and Pseudomonas to aid food metabolism (Zhang et al., 2024). Each year, black-necked cranes migrate from their wintering grounds (e.g., Yunnan-Kweichow Plateau, the southern slopes of the Himalayas) to their breeding grounds (Qinghai–Tibet Plateau, Xinjiang) in March and return in November (Gao et al., 2007; Wang et al., 2013). They have a relatively long breeding period (8 months) and a shorter wintering period (4 months) (Pu and Guo, 2023). Black-necked cranes primarily inhabit farmland areas during the winter and feed predominantly on wetlands during the breeding season in Zoige, China (Dong et al., 2016). As black-necked cranes transition from the growing season to the non-growing season in Zoige, they encounter different food resources. These seasonal differences, along with the differences between breeding and wintering periods, provide an excellent opportunity to study host-gut microbial interactions throughout the annual cycle of a migratory bird.
In this study, black-necked crane wintering data from previous studies were used in conjunction with fecal samples collected during the breeding period across the four seasons in Zoige. The resulting data were analyzed using 16S rRNA gene sequencing to determine the gut microbiota community structures of black-necked cranes throughout their annual cycle. We hypothesized that different seasons have different gut microbial community structures. Black-necked cranes’ gut microbiota would respond to the seasonal variation in food resources, showing higher diversity during seasons of food abundance.
Black-necked cranes are the only species known to inhabit and reproduce on high plateaus throughout their life. The Zoige Wetland National Nature Reserve in China is an important breeding site for black-necked cranes, whereas Caohai and Dashanbao in China are important wintering sites. Black-necked cranes primarily inhabit meadows or marsh meadows in Zoige (Bai et al., 2022), which is one of the hotspots for biodiversity, with plants from the Cyperaceae, Ranunculaceae, and Asteraceae families having the largest number of species. The Zoige area also supports a rich diversity of animal species, including amphibians, fish, and various arthropods such as Diptera and Coleoptera (Xiang et al., 2009). Furthermore, we found arthropods are main animal-deprived food of the black-necked crane’s diet in our previous study (Ma et al., 2024). However, in Caohai and Dashanbao during the wintering period, black-necked cranes primarily inhabit farmland areas (Wu et al., 2013), where they feed on grains, potatoes, and some invertebrates (Dong et al., 2016). We collected black-necked crane feces from 19 locations in Zoige in April and September 2022 as well as in July and November 2023 (spring: N = 30, summer: N = 30, autumn: N = 30, winter: N = 30). The spring and autumn samples are part of a dataset associated with a recently published paper (Ma et al., 2025). During sample collection, we observed black-necked cranes feeding for approximately 2–3 h and collected feces after the birds had left. Using sterile toothpicks, we extracted the internal portion of each fecal sample and placed it into a 15 mL centrifuge tube. The samples were stored in liquid nitrogen and sent to a laboratory in Chengdu, China. We also downloaded the gut microbiome data of black-necked cranes for the wintering period from the National Center of Biotechnology Information (NCBI; project numbers PRJNA681985) (Dashanbao; Zhao et al., 2021), PRJNA992803, and PRJNA995432 (Caohai; Wang et al., 2024). In total, 41 winter samples were obtained from the database (Supplementary Table S1; Figure 1).
Fecal DNA was extracted using an OMEGA Soil DNA Kit (M5635-02; Omega Bio-Tek, Norcross, GA, United States). Negative controls were used for extraction and amplification, and no detectable products were observed. The 16S rRNA region (V3–V4) of the gut microbiota was detected using the primers 338F/806R (Lee et al., 2012). The polymerase chain reaction (PCR; 25 μL) contained: 5 × reaction buffer 5 μL, 5 × GC buffer 5 μL, dNTP (2.5 mM) 2 μL, forward primer (10 uM) 1 μL, reverse primer (10 uM) 1 μL, DNA template 2 μL (20 ng/μL), ddH2O 8.75 μL, and Q5 DNA polymerase 0.25 μL. The amplification program was as follows: initial denaturation at 98°C for 2 min, denaturation at 98°C for 15 s, annealing at 55°C for 30 s, extension at 72°C for 30 s for 30 cycles, and final extension at 72 for °C 5 min. A DNA library was constructed using a TruSeq Nano DNA LT Library Prep Kit (Illumina). Paired-end sequencing of the 16S rRNA gene was conducted using an Illumina NovaSeq 6,000 platform at Personal Bio (Shanghai Personal Biotechnology Co., Ltd., Nanjing, China). The easyAmplicon pipeline1 was used to process the sequencing data. We used the “fastx_filter” function of VSEARCH (v2.14.1) to trim primers and perform quality filtering. The “derep_fulllength” function of VSEARCH was employed for the dereplication task, with a minimum unique size of 135. To denoise, we used the unoise3 function of USEARCH (v10.0.240), and the “usearch_global” function of VSEARCH was used to generate an amplicon sequence variant table. Rarefaction analysis was conducted using “alpha_div_rare” in USEARCH, and we did not observe obvious batch effects based on cluster dendrogram and PCA analysis (Supplementary Figure S1).
All statistical tests were conducted using R software (version 4.2.1, 2022). USEARCH was employed to calculate the alpha diversity measures, including the Shannon and Simpson indices, as well as the beta diversity metrics, which comprised the Bray-Curtis distance and the weighted UniFrac distance, for the microbiota analysis. Constrained ordination (partial canonical analysis of principal coordinates, CAP) and unconstrained ordination (nonmetric multidimensional scaling, NMDS) were performed to evaluate seasonal effects based on the Bray–Curtis distance and weighted UniFrac distance. For the CAP, we conducted permutational multivariate analysis of variance (PERMANOVA) and analysis of variance (ANOVA) to validate its significance using 999 permutations in the “vegan” v2.6.4 (Oksanen et al., 2007) package. CAP was performed using the “ordinate” function in the “phyloseq” v1.42.0 package (Hu L. et al., 2018). For NMDS, seasonal effects were detected using the analysis of similarities (ANOSIM) function in the “vegan” package with 999 permutations. NMDS was performed using the “metaMDS” function in the “vegan” package. To investigate the effect of season on the Shannon index, Simpson index, Bray–Curtis distance, and weighted UniFrac distance, we modeled season as a fixed factor, location and sample collection year as a random factor using “lme4” v 1.1.33 (Bates et al., 2014). We applied transformations using the “powerTransform” function from the “car” package (v3.1.2) (Fox et al., 2007) when the normality or constant variance of model residuals was not met. The indices that required transformation included the Simpson index, Bray–Curtis distance, and weighted UniFrac distance.
We used a random forest model to distinguish bacterial taxa between seasons, employing the machine learning algorithm in the “randomForest” v4.7.1.1 package (Breiman, 2001). The seasonal classification model was trained on 70% of the dataset. Error rates were estimated at the phylum, class, order, family, and genus levels, and the taxon level was selected to obtain the cross-validation error curve, as described in our previous study (Zhu et al., 2024).
A co-occurrence network was used to illustrate gut microbiota interactions at the family level. Spearman correlations among all samples were calculated and corrected for compositionality effects using 1,000 bootstrap iterations and permutations with the “ccrepe” package (v 1.38.1). p-values were adjusted for multiple testing using the default Benjamini–Hochberg–Yekutieli method, retaining values with an adjusted p < 0.05. To investigate the seasonal effects on topological properties, we extracted sub-networks of individual samples using the “subgraph” function in the “igraph” package by specifying individual vertices (Csardi and Nepusz, 2006). The number of edges, nodes, average degrees, and modularity were used to evaluate the complexity of the black-necked crane gut microbiota network. We used a generalized linear mixed model with a Poisson distribution for the number of edges and nodes, which are count data. For average and modularity, we employed a generalized linear mixed model with binomial error in the “lmer4” package. The sampling season was considered a fixed factor, and the sampling location, sample collection year was considered a random factor.
The Nearest Taxon Index (βNTI) was used to qualitatively evaluate the deterministic or stochastic processes of community assembly, using the “picante” package (v 1.8.2). If the βNTI is >2 or < −2 this indicates that the microbiota community is affected by the deterministic assembly process. However, if the βNTI is > −2 and < 2, this indicates that the gut microbiota community is impacted by a stochastic process. Phylogenetic-bin-based null model analysis (iCAMP) was also conducted using the “iCAMP” package (v 1.5.12) to examine the assembly mechanisms of different gut microbiota groups in black-necked cranes. The iCAMP results identified five assembly mechanisms: dispersal limitation, drift and others, heterogeneous selection, homogeneous selection, and homogenizing dispersal.
We used PICRUSt2 (Douglas et al., 2020) to predict the functional profiles of microbial communities across all samples on the basis of the 16S rRNA gene. The Shannon index, Simpson index of function were calculated using “vegan” package and we modeled season as a fixed factor, location and sample collection year as a random factor using “lme4” v 1.1.33 (Bates et al., 2014).
We obtained 10,949,408 high-quality reads from 161 samples (breeding: 8775868, wintering: 2173540), with an average of 68008.75 reads per sample. Rarefaction analysis revealed that the sequencing data captured most of the gut microbiota from each black-necked crane fecal sample (Supplementary Figure S2). In total, 18 phyla, 36 classes, 65 orders, 132 families, and 233 genera were identified.
The constrained ordination analysis (CAP) showed that gut microbiota exhibited a seasonal pattern (ANOVA and PERMANOVA, Bray-Curtis: R2 = 0.348, p < 0.001, UniFrac: R2 = 0.352, p < 0.001; Figures 2A,B), and the unconstrained ordination (NMDS) analysis revealed the same seasonal pattern (ANOVA, Bray-Curtis: R = 0.295 p < 0.001, UniFrac: R = 0.274, p < 0.001) based on Bray-Curtis and weighted UniFrac distances (Supplementary Table S2). They were relatively dispersed across different seasons (Figures 2A,B).
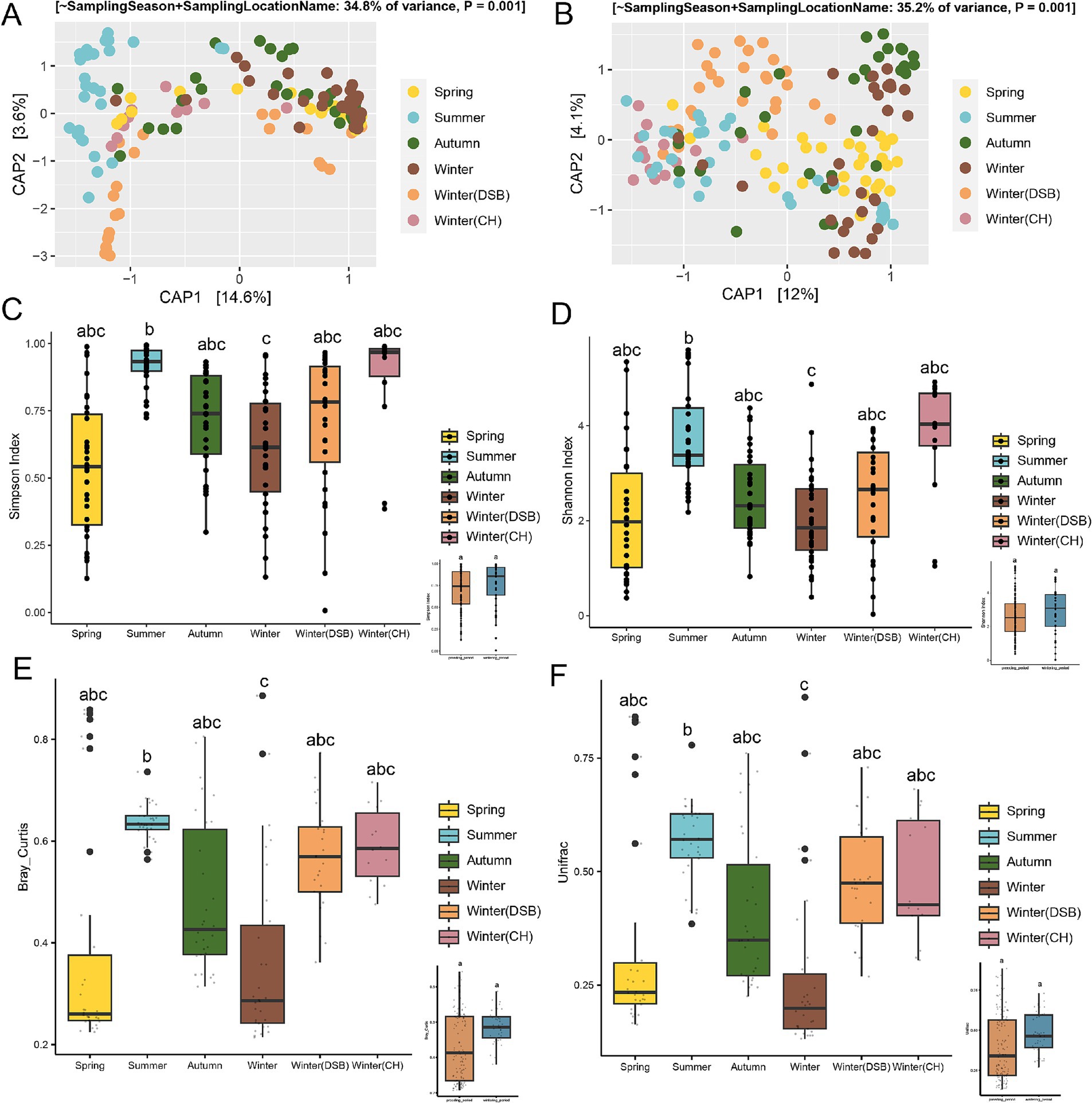
Figure 2. Seasonal variability in the gut microbiota of the black-necked crane. The constrained ordination (CAP) was based on (A) Bray-Curtis and (B) UniFrac distances. Alpha diversity of the gut microbiota was measured using (C) Simpson and (D) Shannon indices, with the inset plots showing alpha diversity between the breeding and wintering periods. Beta diversity of the gut microbiota was based on (E) Bray-Curtis and (F) UniFrac distances, with the inset plots illustrating beta diversity between the breeding and wintering periods. Different colors represent different seasons.
The alpha-diversity during the wintering period was higher than that during the breeding period. There were significant differences among the seasons (Simpson: χ2 = 57.719, p < 0.001; Shannon: χ2 = 24.494, p < 0.001; Figures 2C,D; Supplementary Table S3). The Simpson indices for the summer and winter Caohai samples were higher than those for the other seasons (Figure 2C; Supplementary Table S4). The Shannon index also indicated that the overwintering Caohai samples had higher diversity than the spring samples (Figure 2D; Supplementary Table S4).
Beta-diversity analysis showed that the wintering period had higher diversity than the breeding period, but the difference was not significant (Figures 2E,F). There were significant differences among the seasons (Bray-Curtis: χ2 = 30.907, p < 0.001; UniFrac: χ2 = 41.594, p < 0.001).
Summer, CH, and DSB had the highest beta diversity, whereas winter had the lowest diversity based on both Bray-Curtis and UniFrac distance (Figures 2E,F; Supplementary Table S4).
We observed variations in the relative abundances of the gut microbiota. Firmicutes were the dominant phylum in all groups, except for the winter (CH) group in which Proteobacteria were dominant (Supplementary Table S5).
At the family level, Lactobacillaceae were the dominant microbiota (spring: 68.2%; summer: 4.8%; autumn: 54.9%; winter: 76.4%; DSB: 42.2%; CH: 27.3%; Figure 3A; Supplementary Table S6), except for during the summer when Clostridiaceae_1 were dominant (8.7%; Supplementary Table S6). During the summer, certain other microorganisms exhibited relatively high abundances, such as Pseudomonadaceae (summer: 3.0%) and Enterobacteriaceae (summer: 7.2%; Figure 3A; Supplementary Table S6).
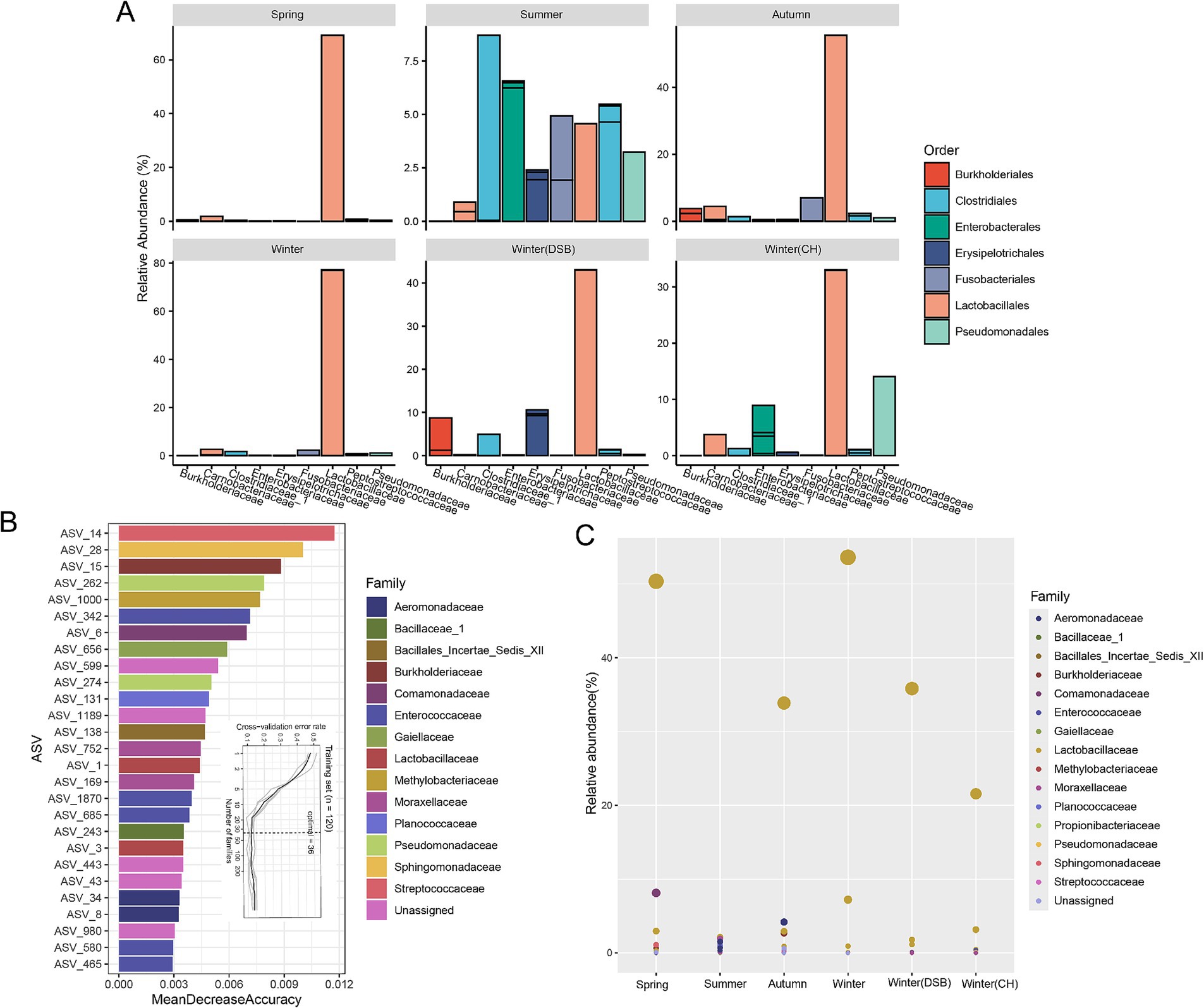
Figure 3. The dominant and distinct gut microbiota across seasons. (A) Normalized relative abundance of the most common genera in different seasons colored by order and separated at the top 10 family levels. Microbiota taxa were predicted using a random forest model for different seasons. (B) The top 36 microbiota ASVs were identified in the training set based on their relative abundance. (C) The abundance of microbiota across different seasons is shown, with bubble size representing abundance and color indicating family.
We further analyzed seasonal variations in gut microbiota biomarkers. The random forest-based model revealed that the ASV level provided the highest accuracy for classifying gut microbiota across different levels. The cross-validation error rate was 0.13 when using the 36 ASVs identified as having distinct microbiota (Figure 3B). The ASV of Lactobacillaceae was lower in abundance in the summer, whereas the ASVs of Moraxellaceae and Planococcaceae were higher (Figure 3C; Supplementary Figure S3).
In total, 40 nodes (families) and 42 connections (edges) were retained in the black-necked crane co-occurrence network. Only the module did not significantly differ between seasons (Supplementary Table S7). The summer network topology was simpler, whereas the spring and winter exhibited more nodes, edges, and degrees. The nodes, edges, and degrees in winter (CH) and summer were lower than those in other seasons (nodes: χ2 = 33.144, p < 0.001, edges: χ2 = 36.183, p < 0.001, Figure 4; Supplementary Tables S7, S8).
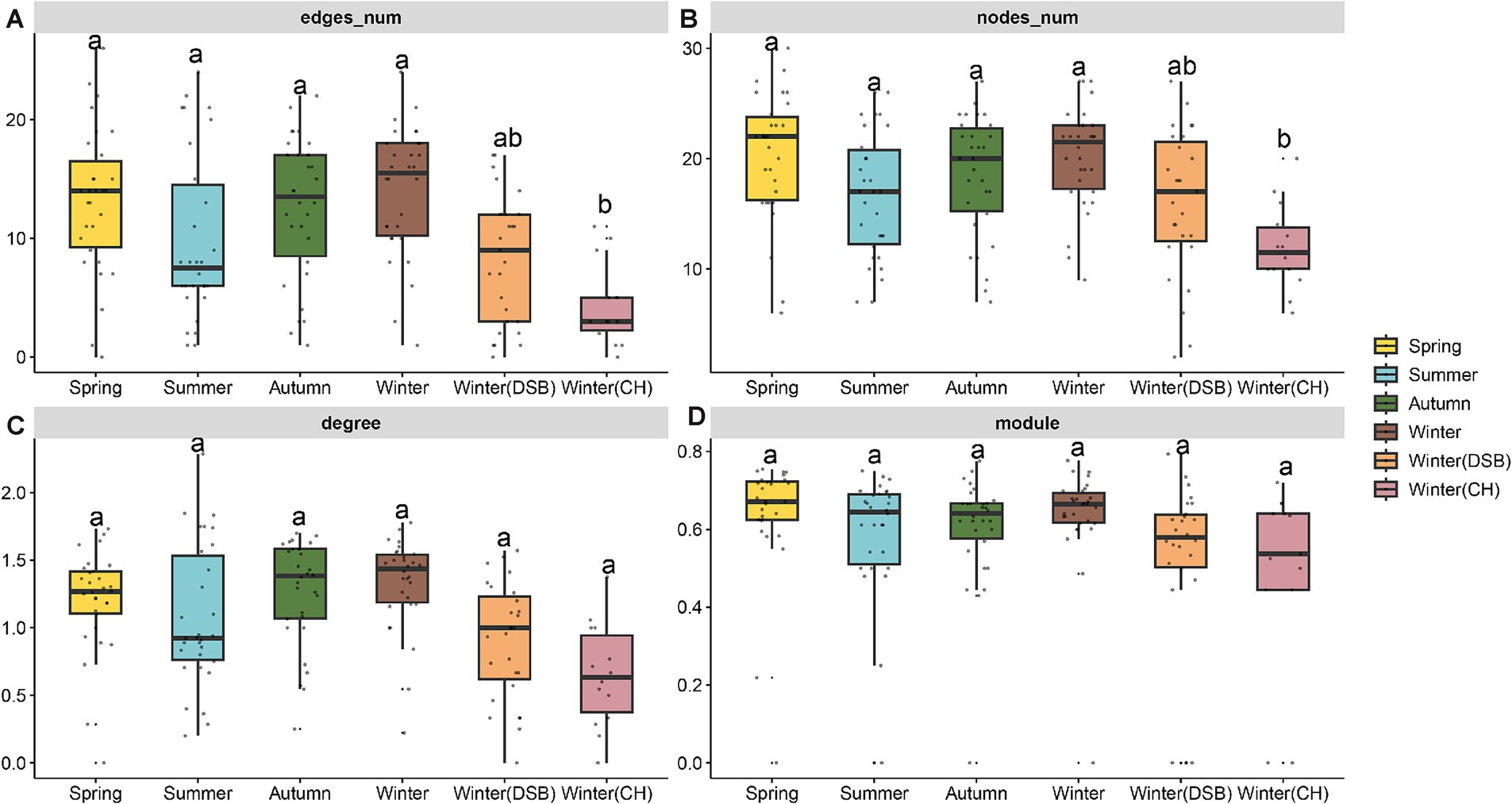
Figure 4. Topological properties of the co-occurrence network of black-necked crane gut microbiota in different seasons, (A) edges number, (B) nodes number, (C) average degree, and (D) modularity.
The βNTI results showed that −2 < βNTI > 2, indicating that the stochastic process is an important factor influencing gut microbiota assembly across all seasons (Figure 5A). ICAMP analysis was performed to evaluate the gut microbiota assembly processes in the different groups. The dispersal limitation (spring: 0.792; summer: 0.612; autumn: 0.728; winter: 0.445; DSB: 0.548; CH: 0.704) was the major driver of gut microbiota assembly in all seasons except winter (Figure 5B; Supplementary Table S9). However, the results for winter contradict the βNTI findings, as homogeneous selection (winter: 0.503), a deterministic process, was the major driver of gut microbiota assembly (Figures 5A,B).
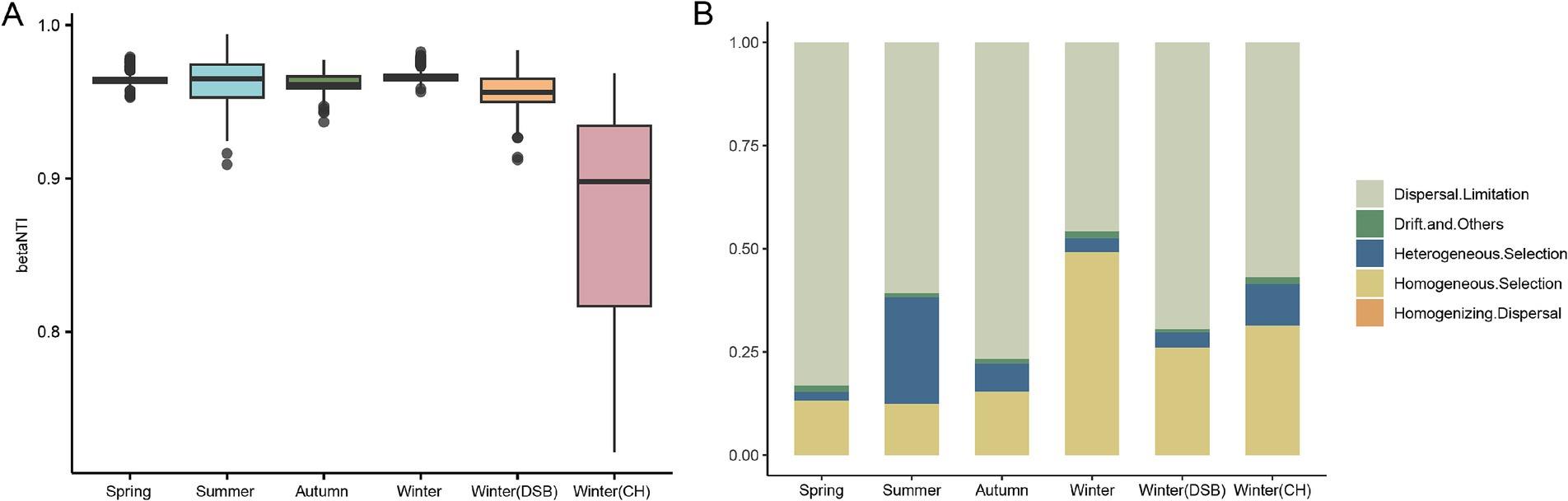
Figure 5. Importance of bacterial communities from different seasons in the gut microbiota of the black-necked crane. (A) The Nearest Taxon Index (βNTI) of the gut microbiota, −2 < βNTI <2 indicates that stochastic processes play a significant role in shaping the gut microbiota. (B) Relative importance of ecological processes for microbiota across different seasons.
The functional profiles inferred by PICRUSt2 indicated significant variations in the Simpson index (χ2 = 13.368, p < 0.05), while the Shannon index did not differ among seasons (χ2 = 7.296, p > 0.05). Additionally, both the Simpson and Shannon indices were higher in the summer samples relative to other seasons (Supplementary Figure S4).
Elucidating the changes in the gut microbiota of the migratory black-necked crane throughout its annual cycle is crucial for understanding its environmental adaptations. In this study, we examined the gut microbiota of the black-necked crane throughout its annual cycle and found differences in its composition, diversity, function, and co-occurrence networks. In most groups, stochastic processes were more important than deterministic processes in gut microbiota assembly.
Food resources in the environment are crucial factors that influence gut microbiota (diversity and composition) (Scott et al., 2013). In our previous study on the dietary of black-necked cranes in Zoige, we found that a greater diversity of arthropods dietary in black-necked cranes in autumn than in spring (Ma et al., 2024). The current study revealed that a similar trend in the gut microbiota diversity, with the black-necked crane exhibiting lower alpha diversity in spring than in autumn. This suggests that there may be an association between the richness of diet and diversity of gut microbiota. Our findings imply that as dietary diversity increases, so does the diversity of the gut microbiota. Additionally, the current study revealed highest alpha and beta diversity of gut microbiota in summer (Figure 2), which leads us to speculate that the diet of black-necked cranes is most diverse during this period. The abundant water and heat resources in Zoige during summer, which contribute to rich food availability (Zhang Z. et al., 2020), likely support the hypothesis by providing a more varied array of food resources for the cranes. Our findings suggest a link between diet and microbiota diversity, prompting the need for future research on the relationship between dietary intake and gut microbiota composition.
Significant differences in gut microbiota composition have also been reported between seasons of food abundance and scarcity (Orkin et al., 2019). Studies on Tibetan macaques (Macaca thibetana) have shown that during seasons of food abundance, gut microbiota exhibit a higher level of diversity (Sun et al., 2016). To adapt to the abundance of food, the gut microbiota shifts and exhibits higher intra- and inter-species diversity (Zhao et al., 2023). The gut microbiota of the Greater Horseshoe Bats (Rhinolophus ferrumequinum) (Xiao et al., 2019) and Forest Musk Deer (Moschus berezovskii) (Hu X. et al., 2018) were also found to show higher diversity in summer, which has an abundance of food compared with other seasons. Our findings are consistent with those of previous studies. We also observed that the gut microbiota exhibited higher alpha diversity in Caohai and its wintering grounds than at the other locations tested. This could be because human-maintained fields provide ample food, similar to the abundance observed under natural summer conditions (Bergmann et al., 2015).
We found that some gut microbiota families were enriched only in summer (e.g., Moraxellaceae, Planococcaceae, Bacillaceae_1). Moraxellaceae is associated with the benzoate degradation pathway (Torrecillas et al., 2023), Planococcaceae can modulate valine production (Li, 2018; Wu et al., 2024), and Bacillaceae_1 is linked to insect lipids (Li et al., 2022; Weththasinghe et al., 2022). Gut microbiota can rapidly respond to novel food components (Leeming et al., 2019). Previous studies have found that short-term dietary changes alter the gut microbiota of animals; however, these changes are difficult to observe after the return to a normal diet (Leeming et al., 2019). This enrichment likely reflects the animals’ need for diverse materials during digestion.
In summer, black-necked cranes had a more diverse microbiota and a lower relative abundance of dominant bacteria. However, during other seasons, the Lactobacillaceae family was dominant. An increase in a stable gut microbiota may represent an adaptation to cope with harsh environments (Jing et al., 2022; Santos et al., 2024). The Lactobacillaceae family’s strong adaptability allows for long-term colonization, maintenance of the intestinal barrier, and resistance to harmful bacteria, and helps hosts adapt to environmental changes (Santos et al., 2024). Extensive colonization by microorganisms ensures adequate energy intake (Ducarmon et al., 2019). The persistence of colonizing species in animals is likely due to their role in degrading storage carbohydrates, such as starch and fiber (Lee et al., 2024). Lactobacillaceae, known for their involvement in carbohydrate digestion, may colonize the gut for extended periods.
During summer, gut microbial samples revealed high microbial diversity but fewer nodes, edges, and degrees in the co-occurrence network, indicating a simpler network structure. This is likely due to the abundance of available food sources, which enables opportunistic bacteria to thrive and temporarily dominate (Stein et al., 2013). However, transient gut microbiota often have lower competitive adaptability in the gut than long-term colonizing species, which is why they do not persist (Lee et al., 2024). Once the season of food abundance has passed, these transient microbial changes are unlikely to persist. However, this situation is transient, and the complexity of the microbial network is expected to evolve.
We observed more complex microbial networks in other seasons, indicating that the microbiota networks had more nodes, edges, than those in summer. These complexities arise because of the harsh survival challenges that occur outside the summer. High environmental stress may cause the microbiota to establish more positive interactions within communities (Li G. et al., 2019) and support the stress-gradient hypothesis (Bertness and Callaway, 1994; Maestre et al., 2009). To overcome these difficulties, animals adjust their microbial networks to enhance their adaptability by increasing the complexity of their gut microbiota, which can be considered a strategy for biological adaptation to diverse environments (Faust and Raes, 2012). Adaptation has been found in many species, including wild ass (Equus kiang) (Gao et al., 2020), great tit (Parus major) (Bodawatta et al., 2021), Plateau Zokor (Eospalax baileyi) (Liu et al., 2024), and bharal (Pseudois nayaur) (Gao et al., 2024).
Dispersal limitation was the primary driver of the gut microbiota assembly in black-necked cranes during all seasons except winter. Dispersal limitations are important for microbiota assembly. This pattern has been observed in studies on honeybees (Apis cerana and Apis mellifera) (Ge et al., 2021) and birds such as the common nightingale (Luscinia megarhynchos) (Sottas et al., 2021), thrush nightingale (Luscinia luscinia) (Sottas et al., 2021), and green-winged teal (Anas crecca) (Wang et al., 2022). Dispersal limitations reduce the ease with which gut microbes spread between individuals. Previous studies on mammalian gut microbes have found that geographical proximity enhances microbial communication among animals, whereas increased physical distance is a key factor affecting the composition of gut microbes (Moeller et al., 2017). Birds, with higher mobility and broader activity ranges than other animals, experience reduced gut microbiota interactions among individuals (Weinhold, 2022). The reduced interaction of the gut microbiota could be a significant factor affecting the gut microbial composition of black-necked cranes.
This study investigated the annual cycle of gut microbiota in migratory black-necked cranes. We found that the diversity, composition, predicted dominant functions and co-occurrence networks of the gut microbiota varied across seasons. The summer samples exhibited greater alpha diversity and beta diversity, as well as more diverse functions compared to other seasons. In all seasons except summer, Lactobacillaceae dominated the gut microbiota. The network structure of the gut microbiota was simpler in summer than in other seasons. Dispersal limitations were identified as a key factor influencing the assembly of gut microbial communities. Overall, black-necked cranes exhibit dynamic adjustments in their gut microbiota to adapt to annual environmental changes, which might be related to the variation of their seasonal diet. Our research reports on the gut microbiota of black-necked cranes throughout their annual cycle, providing valuable insights for the study of migratory birds’ gut microbiota. Future research should focus on multi-year continuous sampling, particularly incorporating samples collected during migration and pay greater attention to the relationship between diet and the gut microbiota of animals.
The original contributions presented in the study are publicly available. This data can be found here: https://ngdc.cncb.ac.cn/gsa/s/qPJgx6YF.
The animal study was approved by College of Grassland Resources, Southwest Minzu University. The study was conducted in accordance with the local legislation and institutional requirements.
YuZ: Writing – original draft, Writing – review & editing. RM: Data curation, Investigation, Visualization, Writing – review & editing. Suolangduoerji: Investigation, Writing – review & editing. HL: Writing – review & editing. YiZ: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Writing – review & editing. SM: Investigation, Writing – review & editing. AN: Investigation, Writing – review & editing. KH: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The project has been funded by Sichuan and Technology Department of Sichuan Province (No. 2022YFS0487), the National Natural Science Foundation of China (No. 32101243), the Southwest Minzu University Research Startup Funds (Grant No. RQD2021049), and supported by “the Fundamental Research Funds for the Central Universities”, Southwest Minzu University (Grant No. ZYN2023086), and the project of Qinghai-Tietan Plateau Research in Southwest Minzu University (Grant No. 2024CXTD01).
We thank Anduo, Weimaduoji, Zerangzhaxi for helping sample collection. We obtained permission from the Zoige Wetland National Nature Reserve to collect all the samples and confirmed that we did not impact the animals during sampling. The project has been approved by the College of Grassland Resources, Southwest Minzu University (2024MDLS02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1533282/full#supplementary-material
Bai, J., Hou, P., Jin, D., Zhai, J., Ma, Y., and Zhao, J. (2022). Habitat suitability assessment of black-necked crane (Grus nigricollis) in the zoige grassland wetland ecological function zone on the eastern tibetan plateau. Diversity 14:579. doi: 10.3390/d14070579
Bajinka, O., Tan, Y., Abdelhalim, K. A., Özdemir, G., and Qiu, X. (2020). Extrinsic factors influencing gut microbes, the immediate consequences and restoring eubiosis. AMB Express 10, 1–11. doi: 10.1186/s13568-020-01066-8
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2014). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Bergmann, G. T., Craine, J. M., Robeson, M. S., and Fierer, N. (2015). Seasonal shifts in diet and gut microbiota of the American bison (Bison bison). PLoS One 10:e0142409. doi: 10.1371/journal.pone.0142409
Bertness, M. D., and Callaway, R. (1994). Positive interactions in communities. Trends Ecol. Evol. 9, 191–193. doi: 10.1016/0169-5347(94)90088-4
Bodawatta, K. H., Freiberga, I., Puzejova, K., Sam, K., Poulsen, M., and Jønsson, K. A. (2021). Flexibility and resilience of great tit (Parus major) gut microbiomes to changing diets. Anim. Microbiome 3, 1–14. doi: 10.1186/s42523-021-00076-6
Claus, S. P., Ellero, S. L., Berger, B., Krause, L., Bruttin, A., Molina, J., et al. (2011). Colonization-induced host-gut microbial metabolic interaction. MBio 2, e00271–e00210. doi: 10.1128/mBio.00271-10
Csardi, G., and Nepusz, T. (2006). The igraph software package for complex network research. InterJournal, Complex Systems 1695, 1–9. https://igraph.org
Dallas, J. W., and Warne, R. W. (2023). Captivity and animal microbiomes: potential roles of microbiota for influencing animal conservation. Microb. Ecol. 85, 820–838. doi: 10.1007/s00248-022-01991-0
Dong, H., Lu, G., Zhong, X., and Yang, X. (2016). Winter diet and food selection of the black-necked crane Grus nigricollis in Dashanbao, Yunnan, China. PeerJ 4:e1968. doi: 10.7717/peerj.1968
Dong, S., Xu, S., Zhang, J., Hussain, R., Lu, H., Ye, Y., et al. (2021). First report of fecal microflora of wild Bar-headed goose in Tibet plateau. Front. Vet. Sci. 8:791461. doi: 10.3389/fvets.2021.791461
Douglas, G. M., Maffei, V. J., Zaneveld, J. R., Yurgel, S. N., Brown, J. R., Taylor, C. M., et al. (2020). PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 38, 685–688. doi: 10.1038/s41587-020-0548-6
Drovetski, S. V., O’Mahoney, M. J., Matterson, K. O., Schmidt, B. K., and Graves, G. R. (2019). Distinct microbiotas of anatomical gut regions display idiosyncratic seasonal variation in an avian folivore. Anim. Microbiome 1, 1–11. doi: 10.1186/s42523-019-0002-6
Ducarmon, Q., Zwittink, R., Hornung, B., Van Schaik, W., Young, V., and Kuijper, E. (2019). Gut microbiota and colonization resistance against bacterial enteric infection. Microbiol. Mol. Biol. Rev. 83:e00007-19. doi: 10.1128/MMBR.00007-19
Elzinga, J., van der Oost, J., de Vos, W. M., and Smidt, H. (2019). The use of defined microbial communities to model host-microbe interactions in the human gut. Microbiol. Mol. Biol. Rev. 83:10-1128. doi: 10.1128/MMBR.00054-18
Faust, K., and Raes, J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550. doi: 10.1038/nrmicro2832
Fox, J., Friendly, G. G., Graves, S., Heiberger, R., Monette, G., Nilsson, H., et al., (2007). The car package. R Foundation for Statistical Computing 1109, 1431.
Gao, H., Chi, X., Li, G., Qin, W., Song, P., Jiang, F., et al. (2020). Gut microbial diversity and stabilizing functions enhance the plateau adaptability of Tibetan wild ass (Equus kiang). Microbiologyopen 9, 1150–1161. doi: 10.1002/mbo3.1025
Gao, H., Chi, X., Song, P., Gu, H., Xu, B., Cai, Z., et al. (2024). Maintaining the native gut microbiota of bharal (Pseudois nayaur) is crucial in ex situ conservation. Front. Microbiol. 15:1357415. doi: 10.3389/fmicb.2024.1357415
Gao, L., Qing, F., Yang, X., Wu, H., and Li, F. (2007). Satellite tracking on the migratory routes of wintering black-necked cranes at Dashanbao in Yunnan. Zool. Res. 28, 353–361. doi: 10.3321/j.issn:0254-5853.2007.04.003
Ge, Y., Jing, Z., Diao, Q., He, J.-Z., and Liu, Y.-J. (2021). Host species and geography differentiate honeybee gut bacterial communities by changing the relative contribution of community assembly processes. MBio 12:e0075121. doi: 10.1128/mBio.00751-21
Grond, K., Sandercock, B. K., Jumpponen, A., and Zeglin, L. H. (2018). The avian gut microbiota: community, physiology and function in wild birds. J. Avian Biol. 49:e01788. doi: 10.1111/jav.01788
Hu, X., Liu, G., Li, Y., Wei, Y., Lin, S., Liu, S., et al. (2018). High-throughput analysis reveals seasonal variation of the gut microbiota composition within forest musk deer (Moschus berezovskii). Front. Microbiol. 9:1674. doi: 10.3389/fmicb.2018.01674
Hu, L., Robert, C. A., Cadot, S., Zhang, X., Ye, M., Li, B., et al. (2018). Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 9:2738. doi: 10.1038/s41467-018-05122-7
Jing, X., Ding, L., Zhou, J., Huang, X., Degen, A., and Long, R. (2022). The adaptive strategies of yaks to live in the Asian highlands. Anim. Nutr. 9, 249–258. doi: 10.1016/j.aninu.2022.02.002
Lee, C. K., Barbier, B. A., Bottos, E. M., McDonald, I. R., and Cary, S. C. (2012). The inter-valley soil comparative survey: the ecology of Dry Valley edaphic microbial communities. ISME J. 6, 1046–1057. doi: 10.1038/ismej.2011.170
Lee, S., Meslier, V., Bidkhori, G., Garcia-Guevara, F., Etienne-Mesmin, L., Clasen, F., et al. (2024). Transient colonizing microbes promote gut dysbiosis and functional impairment. NPJ Biofilms Microbiomes 10:80. doi: 10.1038/s41522-024-00561-1
Lee, J. Y., Tsolis, R. M., and Bäumler, A. J. (2022). The microbiome and gut homeostasis. Science 377:eabp9960. doi: 10.1126/science.abp9960
Leeming, E. R., Johnson, A. J., Spector, T. D., and Le Roy, C. I. (2019). Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients 11:2862. doi: 10.3390/nu11122862
Li, J. (2018). Fecal metabolome and gut microbiome alterations in a mouse model of senescence accelerated mouse prone 8 (SAMP8). Chin. Tradit. Herb. Drug, 2265–2273. doi: 10.7501/j.issn.0253-2670.2018.10.006
Li, Y., Gajardo, K., Jaramillo Torres, A., Kortner, T. M., and Krogdahl, Å. (2022). Consistent changes in the intestinal microbiota of Atlantic salmon fed insect meal diets. Anim. Microbiome 4, 1–15. doi: 10.1186/s42523-021-00159-4
Li, G., Li, J., Kohl, K. D., Yin, B., Wei, W., Wan, X., et al. (2019). Dietary shifts influenced by livestock grazing shape the gut microbiota composition and co-occurrence networks in a local rodent species. J. Anim. Ecol. 88, 302–314. doi: 10.1111/1365-2656.12920
Li, H., Zhou, R., Zhu, J., Huang, X., and Qu, J. (2019). Environmental filtering increases with elevation for the assembly of gut microbiota in wild pikas. Microb. Biotechnol. 12, 976–992. doi: 10.1111/1751-7915.13450
Liu, G., Li, C., Liu, Y., Zheng, C., Ning, Y., Yang, H., et al. (2022). Highland adaptation of birds on the Qinghai-Tibet plateau via gut microbiota. Appl. Microbiol. Biotechnol. 106, 6701–6711. doi: 10.1007/s00253-022-12171-y
Liu, D., Li, B., Song, P., Jiang, F., and Zhang, T. (2024). Captivity shifts gut microbiota communities in plateau Zokor (Eospalax baileyi). Microorganisms 12:789. doi: 10.3390/microorganisms12040789
Liukkonen, M., Muriel, J., Martínez-Padilla, J., Nord, A., Pakanen, V. M., Rosivall, B., et al. (2024). Seasonal and environmental factors contribute to the variation in the gut microbiome: a large-scale study of a small bird. J. Anim. Ecol. 93, 1475–1492. doi: 10.1111/1365-2656.14153
Lu, Z., Guo, L., Meng, W., Meng, D., and Liu, J. (2024). Temporal changes in the gut microbiota of overwintering great bustard Otis tarda dybowskii. Endanger. Species Res. 53, 13–22. doi: 10.3354/esr01284
Lu, Z., Li, S., Wang, M., Wang, C., Meng, D., and Liu, J. (2022). Comparative analysis of the gut microbiota of three sympatric terrestrial wild bird species overwintering in farmland habitats. Front. Microbiol. 13:905668. doi: 10.3389/fmicb.2022.905668
Ma, R., Ma, S., Liu, H., Hu, L., Li, Y., He, K., et al. (2024). Seasonal changes in invertebrate diet of breeding black-necked cranes (Grus nigricollis). Ecol. Evol. 14:e70234. doi: 10.1002/ece3.70234
Ma, R., Ma, S., Zhang, Y., Hu, L., Tang, K., Liu, H., et al. (2025). Flexible host–microbe interaction aid adaptation of black-necked crane to seasonal shifts. Glob. Ecol. Conserv. e03458. doi: 10.1016/j.gecco.2025.e03458
Maestre, F. T., Callaway, R. M., Valladares, F., and Lortie, C. J. (2009). Refining the stress-gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 97, 199–205. doi: 10.1111/j.1365-2745.2008.01476.x
Moeller, A. H., Suzuki, T. A., Lin, D., Lacey, E. A., Wasser, S. K., and Nachman, M. W. (2017). Dispersal limitation promotes the diversification of the mammalian gut microbiota. Proc. Natl. Acad. Sci. 114, 13768–13773. doi: 10.1073/pnas.1700122114
Nichols, R. G., and Davenport, E. R. (2021). The relationship between the gut microbiome and host gene expression: a review. Hum. Genet. 140, 747–760. doi: 10.1007/s00439-020-02237-0
Oksanen, J., Kindt, R., Legendre, P., O’Hara, B., Stevens, M. H. H., Oksanen, M. J., et al. (2007). The vegan package. Community ecology package. 10, 719.
Orkin, J. D., Campos, F. A., Myers, M. S., Cheves Hernandez, S. E., Guadamuz, A., and Melin, A. D. (2019). Seasonality of the gut microbiota of free-ranging white-faced capuchins in a tropical dry forest. ISME J. 13, 183–196. doi: 10.1038/s41396-018-0256-0
Pu, Z., and Guo, Y. (2023). Autumn migration of black-necked crane (Grus nigricollis) on the Qinghai-Tibetan and Yunnan-Guizhou plateaus. Ecol. Evol. 13:e10492. doi: 10.1002/ece3.10492
Santos, A. A., Duarte, R., Duarte, M., Arella, F., Marques, V., Roos, S., et al. (2024). Impact of Lactobacillaceae supplementation on the multi-organ axis during MASLD. Life Sci. 354:122948. doi: 10.1016/j.lfs.2024.122948
Schmiedová, L., Kreisinger, J., Kubovčiak, J., Těšický, M., Martin, J. F., Tomášek, O., et al. (2023). Gut microbiota variation between climatic zones and due to migration strategy in passerine birds. Front. Microbiol. 14:1080017. doi: 10.3389/fmicb.2023.1080017
Scott, K. P., Gratz, S. W., Sheridan, P. O., Flint, H. J., and Duncan, S. H. (2013). The influence of diet on the gut microbiota. Pharmacol. Res. 69, 52–60. doi: 10.1016/j.phrs.2012.10.020
Skeen, H. R., Willard, D. E., Jones, A. W., Winger, B. M., Gyllenhaal, E. F., Tsuru, B. R., et al. (2023). Intestinal microbiota of Nearctic-Neotropical migratory birds vary more over seasons and years than between host species. Mol. Ecol. 32, 3290–3307. doi: 10.1111/mec.16915
Song, H., Zhang, Y., Gao, H., Guo, Y., and Li, S. (2014). Plateau wetlands, an Indispensible habitat for the black-necked crane (Grus nigricollis) – a review. Wetlands 34, 629–639. doi: 10.1007/s13157-014-0559-5
Sottas, C., Schmiedová, L., Kreisinger, J., Albrecht, T., Reif, J., Osiejuk, T. S., et al. (2021). Gut microbiota in two recently diverged passerine species: evaluating the effects of species identity, habitat use and geographic distance. BMC Ecol. Evol. 21, 1–14. doi: 10.1186/s12862-021-01773-1
Stein, R. R., Bucci, V., Toussaint, N. C., Buffie, C. G., Rätsch, G., Pamer, E. G., et al. (2013). Ecological modeling from time-series inference: insight into dynamics and stability of intestinal microbiota. PLoS Comput. Biol. 9:e1003388. doi: 10.1371/journal.pcbi.1003388
Sun, Y., Hao, Y., Zhang, Q., Liu, X., Wang, L., Li, J., et al. (2023). Coping with extremes: alternations in diet, gut microbiota, and hepatic metabolic functions in a highland passerine. Sci. Total Environ. 905:167079. doi: 10.1016/j.scitotenv.2023.167079
Sun, B., Wang, X., Bernstein, S., Huffman, M. A., Xia, D.-P., Gu, Z., et al. (2016). Marked variation between winter and spring gut microbiota in free-ranging Tibetan macaques (Macaca thibetana). Sci. Rep. 6:26035. doi: 10.1038/srep26035
Thie, N., Corl, A., Turjeman, S., Efrat, R., Kamath, P. L., Getz, W. M., et al. (2022). Linking migration and microbiota at a major stopover site in a long-distance avian migrant. Mov. Ecol. 10:46. doi: 10.1186/s40462-022-00347-0
Torrecillas, S., Rimoldi, S., Montero, D., Serradell, A., Acosta, F., Fontanillas, R., et al. (2023). Genotype x nutrition interactions in European sea bass (Dicentrarchus labrax): effects on gut health and intestinal microbiota. Aquaculture 574:739639. doi: 10.1016/j.aquaculture.2023.739639
Waite, D. W., and Taylor, M. W. (2015). Exploring the avian gut microbiota: current trends and future directions. Front. Microbiol. 6:673. doi: 10.3389/fmicb.2015.00673
Wang, W., Wang, F., Li, L., Wang, A., Sharshov, K., Druzyaka, A., et al. (2020). Characterization of the gut microbiome of black-necked cranes (Grus nigricollis) in six wintering areas in China. Arch. Microbiol. 202, 983–993. doi: 10.1007/s00203-019-01802-0
Wang, Y., Zhan, H., Saif, A., Zhang, X., and Su, H. (2024). Analysis of winter survival strategies of sympatric black-necked cranes, and common cranes from the perspective of diet and gut microbiota. Ecol. Indic. 160:111782. doi: 10.1016/j.ecolind.2024.111782
Wang, B., Zhong, H., Liu, Y., Ruan, L., Kong, Z., Mou, X., et al. (2022). Diet drives the gut microbiome composition and assembly processes in winter migratory birds in the Poyang Lake wetland, China. Front. Microbiol. 13:973469. doi: 10.3389/fmicb.2022.973469
Wang, N., Zhu, P., Wang, M., Ye, Y., and Qu, S. (2013). Size and distribution of the breeding population of black-necked crane in Haizishan, Sichuan Province. J. Ecol. Rural Environ. 29, 265–268. doi: 10.3969/j.issn.1673-4831.2013.02.022
Weinhold, A. (2022). Bowel movement: integrating host mobility and microbial transmission across host taxa. Front. Microbiol. 13:826364. doi: 10.3389/fmicb.2022.826364
Weththasinghe, P., Rocha, S. D., Øyås, O., Lagos, L., Hansen, J. Ø., Mydland, L. T., et al. (2022). Modulation of Atlantic salmon (Salmo salar) gut microbiota composition and predicted metabolic capacity by feeding diets with processed black soldier fly (Hermetia illucens) larvae meals and fractions. Anim. Microbiome 4:9. doi: 10.1186/s42523-021-00161-w
Wu, L., Niu, Y., Ren, B., Wang, S., Song, Y., Wang, X., et al. (2024). Naringenin promotes gastrointestinal motility in mice by impacting the SCF/c-kit pathway and gut microbiota. Food Secur. 13:2520. doi: 10.3390/foods13162520
Wu, Y., Yang, Y., Cao, L., Yin, H., Xu, M., Wang, Z., et al. (2018). Habitat environments impacted the gut microbiome of long-distance migratory swan geese but central species conserved. Sci. Rep. 8:13314. doi: 10.1038/s41598-018-31731-9
Wu, Z., Zhang, K., Li, W., and Jiang, P. (2013). Number, habitats, and roosting sites of wintering black-necked cranes in Huize nature reserve, Yunnan, China. Mt. Res. Dev. 33, 314–322. doi: 10.1659/MRD-JOURNAL-D-11-00066.1
Xia, T., Yao, Y., Wang, C., Dong, M., Wu, Y., Li, D., et al. (2021). Seasonal dynamics of gut microbiota in a cohort of wild Tibetan macaques (Macaca thibetana) in western China. Glob. Ecol. Conserv. 25:e01409. doi: 10.1016/j.gecco.2020.e01409
Xiang, S., Guo, R., Wu, N., and Sun, S. (2009). Current status and future prospects of Zoige marsh in eastern Qinghai-Tibet plateau. Ecol. Eng. 35, 553–562. doi: 10.1016/j.ecoleng.2008.02.016
Xiao, G., Liu, S., Xiao, Y., Zhu, Y., Zhao, H., Li, A., et al. (2019). Seasonal changes in gut microbiota diversity and composition in the greater horseshoe bat. Front. Microbiol. 10:2247. doi: 10.3389/fmicb.2019.02247
Yang, X., Yao, Y., Zhang, X., Zhong, J., Gao, F., Zhang, H., et al. (2021). Seasonal changes in the distinct taxonomy and function of the gut microbiota in the wild ground squirrel (Spermophilus dauricus). Animals 11:2685. doi: 10.3390/ani11092685
Yao, H., Zhang, Z., Wu, N., Wang, M., Wu, Q., Wu, H., et al. (2023). Comparative analysis of intestinal flora at different overwintering periods in wild relict gulls (Larus relictus): first evidence from northern China. Front. Microbiomes 2:1218281. doi: 10.3389/frmbi.2023.1218281
Zhang, Z., Qin, J., Sun, H., Yang, J., and Liu, Y. (2020). Spatiotemporal dynamics of dissolved organic carbon and freshwater browning in the Zoige alpine wetland, Northeastern Qinghai-Tibetan Plateau. Water 12:2453. doi: 10.3390/w12092453
Zhang, F., Xiang, X., Dong, Y., Yan, S., Song, Y., and Zhou, L. (2020). Significant differences in the gut bacterial communities of hooded crane (Grus monacha) in different seasons at a stopover site on the flyway. Animals 10:701. doi: 10.3390/ani10040701
Zhang, Z., Yang, Z., and Zhu, L. (2021). Gut microbiome of migratory shorebirds: current status and future perspectives. Ecol. Evol. 11, 3737–3745. doi: 10.1002/ece3.7390
Zhang, S., Zhou, C., Dong, Z., Feng, K., Peng, K., Wang, Z., et al. (2024). The diet–intestinal microbiota dynamics and adaptation in an elevational migration bird, the Himalayan bluetail (Tarsiger rufilatus). Ecol. Evol. 14:e11617. doi: 10.1002/ece3.11617
Zhao, Y., Sun, J., Ding, M., Hayat Khattak, R., Teng, L., and Liu, Z. (2023). Growth stages and inter-species gut microbiota composition and function in captive Red Deer (Cervus elaphus alxaicus) and blue sheep (Pseudois nayaur). Animals 13:553. doi: 10.3390/ani13040553
Zhao, J., Wang, Y., Zhang, M., Yao, Y., Tian, H., Sang, Z., et al. (2021). Structural changes in the gut microbiota community of the black-necked crane (Grus nigricollis) in the wintering period. Arch. Microbiol. 203, 6203–6214. doi: 10.1007/s00203-021-02587-x
Keywords: black-necked crane, annual cycle, gut microbiota, migratory birds, high-altitude
Citation: Zhang Y, Ma R, Suolangduoerji, Ma S, Nuertai A, He K, Liu H, and Zhu Y (2025) Annual cycle variations in the gut microbiota of migratory black-necked cranes. Front. Microbiol. 16:1533282. doi: 10.3389/fmicb.2025.1533282
Received: 23 November 2024; Accepted: 16 January 2025;
Published: 07 February 2025.
Edited by:
Wei Zhu, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Jian-Yu Jiao, Sun Yat-sen University, ChinaCopyright © 2025 Zhang, Ma, Suolangduoerji, Ma, Nuertai, He, Liu and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhu, c29fenkyMDAzQDEyNi5jb20=; eXpodUBzd3VuLmVkdS5jbg==
†These authors have contributed equally to this work
‡ORCID: Yujia Zhang, orcid.org/0009-0007-4844-3846
Ruifeng Ma, orcid.org/0009-0000-7716-3172
Suolangduoerji, orcid.org/0009-0000-0614-7407
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.