
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 04 February 2025
Sec. Infectious Agents and Disease
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1525051
This article is part of the Research Topic Research Advances and Challenges in Emerging and Re-Emerging Viral Diseases View all 17 articles
 Hongliang Chen1,2†
Hongliang Chen1,2† Yuan Li1†
Yuan Li1† Liping Yuan1†
Liping Yuan1† Fen Liu2
Fen Liu2 Qian Sun1,3
Qian Sun1,3 Qingkai Luo2
Qingkai Luo2 Yefei Lei2
Yefei Lei2 Yinglan Hou2
Yinglan Hou2 Jiayan Li2
Jiayan Li2 Liang Cai4
Liang Cai4 Shixing Tang1*
Shixing Tang1*Background: Elucidation of immune response differences is critical for uncovering underlying mechanisms and developing potential intervention measures among adults and children with COVID-19.
Methods: In this retrospective study, we analyzed serum biochemical markers and cytokine profiles among adults and children with COVID-19 in the First People’s Hospital of Chenzhou in Hunan, China from 1 December 2022 to 13 February 2023. A case–control study was conducted using propensity score matching (PSM) to mitigate possible confounding factors.
Results: The significant differences observed included lymphocyte exhaustion, an increased neutrophil-to-lymphocyte (NEU/LYM) ratio, high levels of C-reactive protein (CRP), and a cytokine storm, characterized by high levels of Th1 proinflammatory cytokines, including interleukin 1β (IL-1β), IL-6, IL-8, interferon type I (IFN-γ), and tumor necrosis factor (TNF-α) in the lung among severe adult COVID-19 patients. Additionally, systemic immune responses were observed in children with COVID-19.
Conclusion: Significant differences in immune responses between adults and children with COVID-19 highlight the different mechanisms and potential intervention measures of COVID-19.
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in late 2019 and the pandemic of coronavirus disease 2019 (COVID-19) highlighted the importance of global health preparedness and response system (Cucinotta and Vanelli, 2020) and the vulnerabilities of the existing systems particularly in low- and middle-income regions (Rosenthal and Waitzberg, 2023). The spectrum of COVID-19 clinical presentations is broad, ranging from asymptomatic infection, mild/moderate disease to severe cases (Contini et al., 2023). Severe COVID-19 patients may progress to acute respiratory distress syndrome (ARDS), and, in some cases, fatal outcomes, especially in elderly people and those with compromised immune systems (Chen et al., 2021; Hellman et al., 2024).
In general, SARS-CoV-2 infection is associated with abnormal immune responses (Mathew et al., 2020), including reduction of lymphocytes and increase of neutrophils in peripheral blood in adult COVID-19 patients (Jia et al., 2024) as well as heightened levels of proinflammatory cytokines with an initial decrease of interferon type I (IFN-I) (Ramasamy and Subbian, 2021). The overactive inflammation and inadequate early IFN response to SARS-CoV-2 infection can facilitate the prognosis of COVID-19 (Mehta et al., 2020), and cause serious complications (Lucas et al., 2020). However, different from adult COVID-19 patients, children usually rapidly activate innate immunity upon viral invasion and typically have milder symptoms (Dong et al., 2020), less reduction of peripheral blood lymphocytes (Ma et al., 2021), and weak cytokine storms (Yin et al., 2024). In addition, children often produce a focused antibody response primarily against the spike protein of SARS-CoV-2, with minimal IgG antibodies against viral nucleocapsid protein (Weisberg et al., 2021).
Previous studies have explored immune response differences between adults and children in various respiratory infections (Garofalo et al., 2005), such as infections with influenza viruses and respiratory syncytial virus (RSV). Children with RSV infection often demonstrate more robust IFN-I responses compared to adults, which contributes to distinct disease outcomes (Hijano et al., 2019). Similarly, adults with influenza virus infections usually display more pronounced inflammatory cytokine activity (Bahadoran et al., 2016). These findings highlight the age-related immune response variations in viral infections including SARS-CoV-2 infection in COVID-19 patients. Understanding the age-related immune disparities is critical for identifying tailored interventions and therapeutic approaches.
In December 2022, China adjusted its stringent control strategies, leading to outbreaks of COVID-19 in China (Lu et al., 2023), which provided us with the materials to investigate age-related immune response disparities between adult and pediatric severe COVID-19 patients in real-world settings. We collected bronchoalveolar lavage fluid (BALF) samples and blood in both the progressive and recovery stages of COVID-19. We analyzed cytokine profiles among adults and children with COVID-19 by using a case–control study design. Significant differences were identified in immune responses between adults and children with COVID-19, indicating the different mechanisms and potential intervention measures of COVID-19.
A total of 126 COVID-19 patients were enrolled in the study from the inpatients at the First People’s Hospital of Chenzhou in Hunan Province, China, from 28 December 2022 to 13 February 2023. Among them, 50 severe cases and 76 non-severe cases were diagnosed according to the guidelines for diagnosing and treating SARA-CoV-2 infection (version 10) issued by the National Health Commission of China (National Health Commission of the People’s Republic of China, 2023). Specifically, adults with COVID-19 were defined as non-severe if they met one of the following criteria: (1) absence of COVID-19 symptoms, such as shortness of breath, fever, cough, sore throat, fatigue, headache, muscle pain, nausea, vomiting, diarrhea, taste and smell loss; (2) presence of COVID-19-related symptoms without dyspnea; (3) respiratory rate (RR) < 30 breaths/min and oxygen saturation > 93% in the resting state; and (4) characteristic imaging manifestations of COVID-19 pneumonia. The severe adult COVID-19 patients are those with (1) shortness of breath, RR ≥ 30 breaths/min; (2) oxygen saturation ≤ 93% in the resting state; (3) arterial partial pressure of oxygen (PaO2)/inspired oxygen concentration (FiO2) ≤ 300 mmHg; (4) pulmonary infiltration >50%; and (5) presence of respiratory failure, septic shock, or multiple organ dysfunction. The criteria for diagnosis of non-severe children COVID-19 patients are (1) absence of COVID-19 symptoms, such as shortness of breath, fever, cough, sore throat, fatigue, headache, muscle pain, nausea, vomiting, diarrhea, taste, and smell loss; (2) presence of COVID-19-related symptoms without dyspnea; (3) persistent high fever for more than 3 days, cough or shortness of breath, but with the normal respiratory rate (RR), that is, RR < 60 breaths/min for children less than 2 months old, or RR < 50 breaths/min in the children of 2–12 months old, or RR < 40 breaths/min in those of 1–5 years old, or RR < 30 breaths/min for more than 5-year-old children, oxygen saturation > 93% in the resting state; and (4) characteristic imaging manifestations of COVID-19 pneumonia. The criteria for severe children COVID-19 patients included (1) high fever or persistent high fever for more than 3 days; (2) shortness of breath, that is, RR ≥ 60 breaths/min for less than 2 months old children, or RR ≥ 50 breaths/min for children of 2–12 months old, or RR ≥ 40 breaths/min for those of 1–5 years old, or RR ≥ 30 breaths/min for more than 5-year-old children except for cases with fever or crying; and (3) oxygen saturation ≤ 93% in the resting state. In addition, all the participants were hospitalized patients, and SARA-CoV-2 positive by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) on throat swabs. We collected social demographic information and medical data including clinical symptoms and signs, clinical laboratory results, such as blood tests, liver and kidney function analysis, and myocardial biomarkers from the inpatient medical record system upon admission. All the patients included in the study were evaluated for co-morbidities, including major chronic diseases, but not other pathogenic infections. However, none of the patients had life-threatening infections that were not related to COVID-19. Informed consent was obtained from the participants, and the ethics committee of Chenzhou First People’s Hospital approved the study (project number: cz2023015). The study design and the details of participant inclusion and exclusion are depicted in Figure 1.
Both BALF and serum samples that were used to analyze cytokine profiles were collected from patients in progressive stages of COVID-19 on the same day after admission. For each patient, approximately 1.5 mL of BALF samples were collected using fiber optic bronchoscopy. The lung segment was irrigated with saline solution at least 3 times, followed by negative pressure aspiration. At least 30% of the infused fluid was retrieved. The BALF samples were centrifuged, and the supernatant was stored at −80°C. In addition, venous blood was collected from patients in progressive and recovery stages of COVID-19, and the serum samples were stored at −80°C for analysis.
Cytokines including IFN-α, IFN-β, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-17, and TNF-α were detected by using multiplexed microsphere flow immunofluorescence luminescence method and a 12-item cytokine detection kit from Qingdao Realskil Biotechnology Co., Ltd. (Qingdao, China). The normal reference range is as follows: IL-1β ≤ 12.4 pg./mL, IL-2 ≤ 7.5 pg./mL, IL-4 ≤ 8.56 pg./mL, IL-5 ≤ 3.1 pg./mL, IL-6 ≤ 5.4 pg./mL, IL-8 ≤ 20.6 pg./mL, IL-10 ≤ 12.9 pg./mL, IL-12 ≤ 3.4 pg./mL, IL-17 ≤ 21.4 pg./mL, TNF-α ≤ 16.5 pg./mL, IFN-γ ≤ 23.1 pg./mL, and IFN-α ≤ 8.5 pg./mL (Liu et al., 2021).
Statistical analysis was conducted using R Software (Developed by the R Development Core Team, available under the GNU General Public License). GraphPad Prism 8 Software (Developed by GraphPad Software, Inc.). was used for data visualization. Demographic characteristics were presented for categorical variables whereas continuous data were described using average ± standard variation (SD) or median and interquartile range (IQR). The normal distribution for continuous variables was assessed using the Kolmogorov–Smirnov and Shapiro–Wilk tests. A chi-square test was performed to determine differences in the proportions of categorical variables. Propensity score matching (PSM) was conducted to reduce potential confounding effects. MATCHIT Package in R (Created and maintained by Kosuke Imai and collaborators, available on CRAN). was used to match severe and non-severe COVID-19 cases at a 1:1 ratio using the greedy nearest neighbor method. The overall quality of the matched samples was evaluated by comparing standardized mean differences (SMDs) for the covariates. In contrast, SMDs <0.1 indicate no significant difference in the demographic characteristics (such as age and sex) between the two groups (Lee and Little, 2017). Non-parametric Mann–Whitney U test or t-test was used to determine significant differences in blood biochemistry and inflammatory biomarkers between severe and non-severe patients (Mann and Whitney, 1947; Student, 1908). A p-value <0.05 was considered statistically significant.
The study recruited 126 COVID-19 patients including 86 adults and 40 children. There were 38 severe adult patients and 48 non-severe adult patients as well as 12 severe pediatric patients and 28 non-severe pediatric patients. The significant differences were a higher proportion of chronic diseases, mechanical ventilation, antiviral treatment, and mortality in adults than in children (Supplementary Table S1). To minimize the potential confounding factors, 36 severe adult patients and 36 non-severe adult patients, as well as 11 severe pediatric patients and 11 non-severe pediatric patients, were selected via PSM matching by age, sex, and chronic disease. For the matched adult severe and non-severe COVID-19 patients, the only differences were the proportion of antiviral treatment (41.7% vs. 5.6%) and mortality (25.0% vs. 5.6%). In contrast, no significant difference was found between matched severe and non-severe patients of children (Table 1). In addition, significant differences in the proportion of chronic diseases, mechanical ventilation, antiviral treatment, mortality, and vaccination with the COVID-19 vaccine were also observed between the matched adults and children with severe COVID-19 (Table 1).
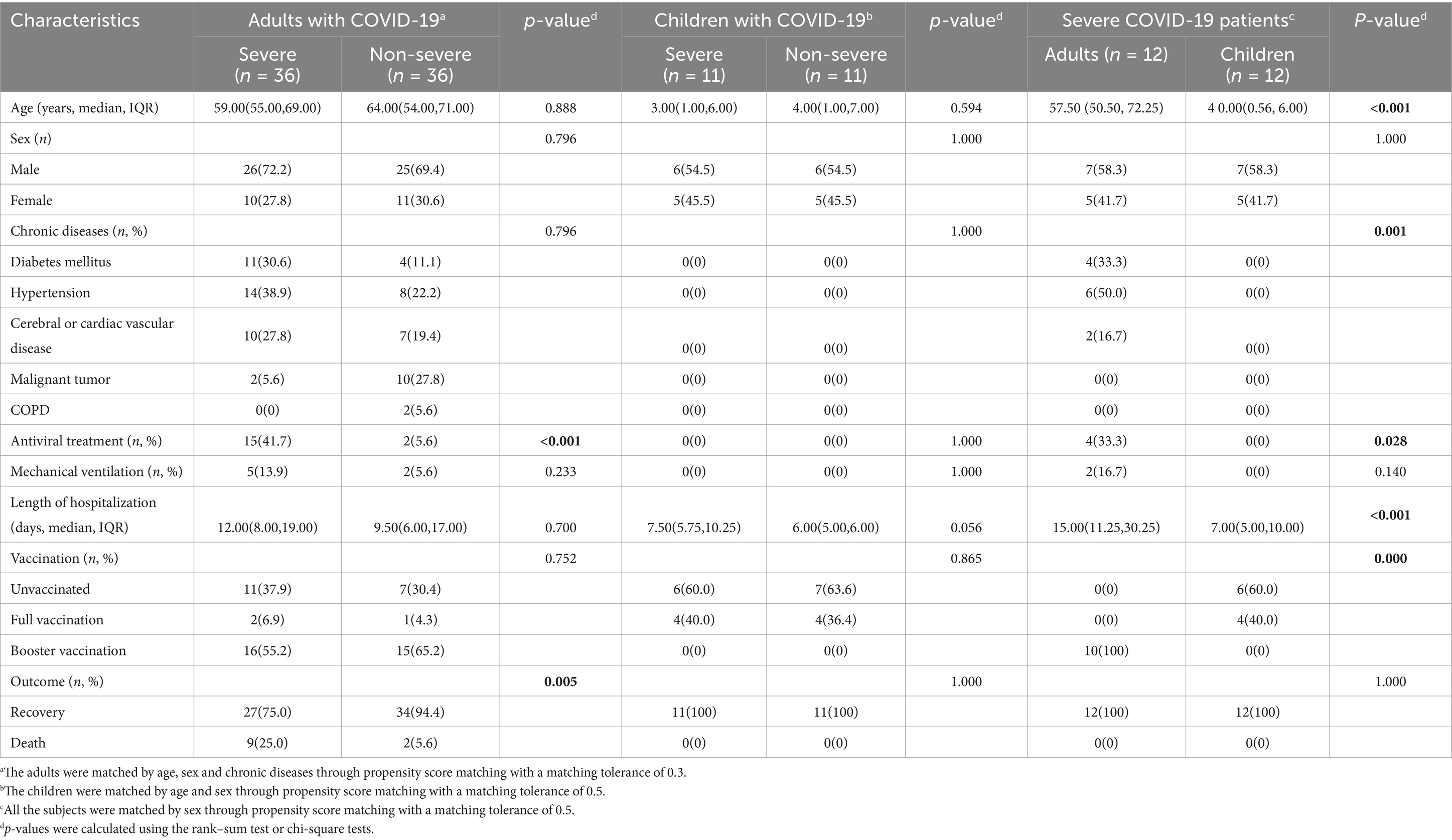
Table 1. Comparison of baseline information for the enrolled COVID-19 patients through propensity score matching.
Dramatic decrease of lymphocyte accounts and increased ratio of neutrophil/lymphocytes (NEU/LYM) were observed in adult patients. A significant difference was found between severe and non-severe adult patients in the lymphocyte accounts (0.85 × 109/L vs. 1.25 × 109/L, p = 0.001) and NEU/LYM ratio (10.40 vs. 5.70, p = 0.003, Supplementary Table S2; Figure 2). Furthermore, the age-related difference was also obtained between adults and children with severe COVID-19 in the lymphocyte accounts (0.85 × 109/L vs. 3.44 × 109/L, p = 0.003) and NEU/LYM ratio (8.40 vs. 1.80, p = 0.002, Supplementary Table S2; Figure 2). In addition, a higher level of C-reactive protein (CRP), an inflammation biomarker, was observed in adult severe patients than in child severe patients (35.80 mg/L vs. 2.89 mg/L, p = 0.005), and in the adult severe patients than in non-severe adult patients (42.60 vs. 28.10, p = 0.456). However, the difference was not statistically significant (Supplementary Table S2; Figure 2). Other blood biochemical results showed substantial differences between severe adult patients and pediatric patients, but the levels of these biomarkers were still within normal ranges (Supplementary Table S2).
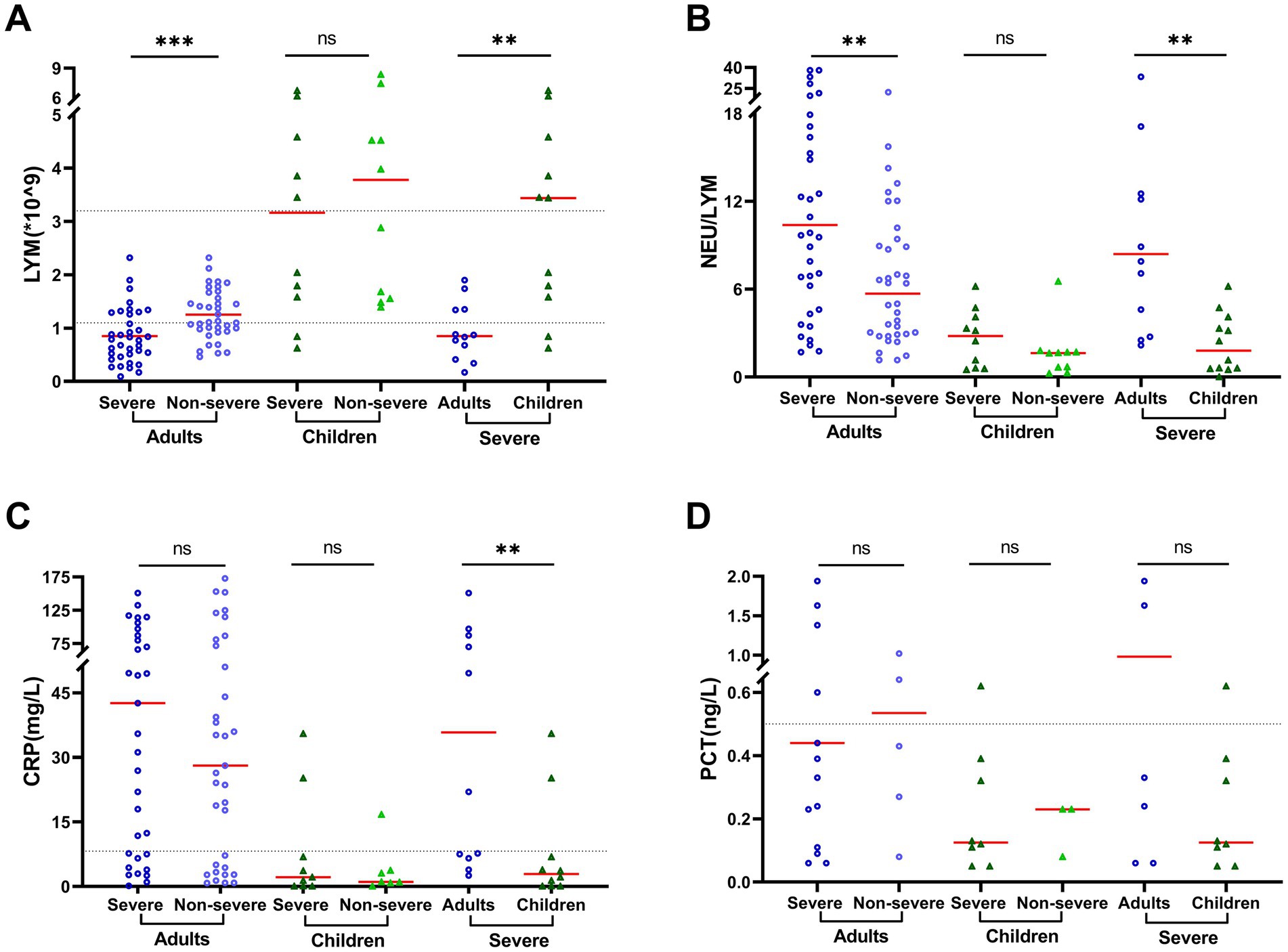
Figure 2. Clinical characteristics vary by age and patient outcome. Clinical measurements included (A) lymphocyte counts (LYM), (B) ratio of neutrophil vs. lymphocyte counts (NEU/LYM), (C) serum concentrations of C-reactive protein (CRP), and (D) serum concentrations of procalcitonin (PCT). The black dotted lines represent the reference range of 1.1–3.2 × 109/L for LYM, 0.068–8.2 mg/L for CRP, and 0–0.5 ng/L for PCT. Data are presented as median and interquartile range (IQR) when they are not normally distributed or mean ± SD when normally distributed. The comparison was conducted by rank–sum test or t-test. *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
Compared to non-severe adult COVID-19 patients, severe adult patients exhibited dramatically increased secretion of Th1 proinflammatory cytokines, in particular, IL-1β (40.79 vs. 207.84), IL-6 (112.90 vs. 304.15), and IL-8 (1454.66 vs. 2829.79, p = 0.001) in BALF (Supplementary Table S3). However, only IL-8 moderately increased in severe pediatric patients (451.62) and non-severe pediatric patients (355.87, Supplementary Table S3, Figure 3). Furthermore, higher levels of cytokines were observed in BALF in adults compared to children with severe COVID-19 (Supplementary Table S3; Figure 3). Specifically, a significant difference was observed for IL-6 (312.57 vs. 7.43, p = 0.05), IL-8 (4949.34 vs. 2054.95, p = 0.008), IL-5 (15.12 vs. 1.84, p = 0.013), IL-10 (3.56 vs. 1.57, p = 0.022), IL-17 (22.43 vs. 4.48, p = 0.007), IFN-γ (14.44 vs. 2.04, p = 0.004), and IFN-α (3.34 vs. 1.24, p = 0.005). However, no significant difference was observed for IL-1β (142.14 vs. 116.23, p = 0.371) between adults and children with severe COVID-19. In contrast, the level of TNF-α was higher in adults than in children (7.41 vs. 2.00) but did not reach statistical significance (p = 0.644).
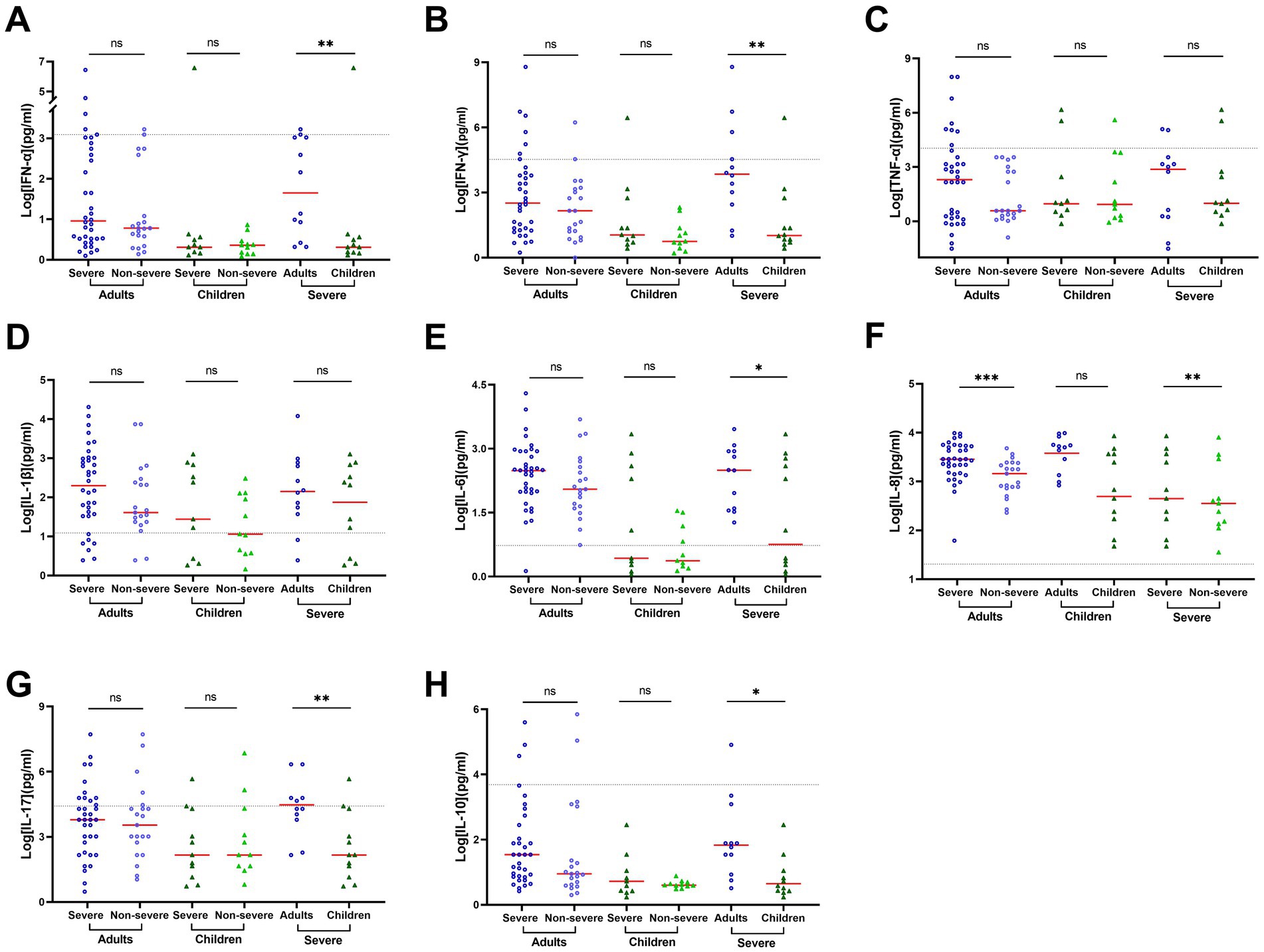
Figure 3. Cytokine concentrations vary by age and patient outcome in BALF of COVID-19 patients. Cytokine concentrations in BALF samples obtained during disease progression were determined using a 12-item cytokine detection kit for IFN-α (A), IFN-γ (B), TNF-α (C), IL-1β (D), IL-6 (E), IL-8 (F), IL-17 (G), and IL-10 (H). The black dotted lines represent the reference range, which is IFN-α ≤ 8.5 pg./mL, IFN-γ ≤ 23.1 pg./mL, TNF-α ≤ 16.5 pg./mL, IL-1β ≤ 12.4 pg./mL, IL-6 ≤ 5.4 pg./mL, IL-8 ≤ 20.6 pg./mL, IL-17 ≤ 21.4 pg./mL, and IL-10 ≤ 12.9 pg./mL, respectively. The concentrations in each group were compared by rank–sum test or t-test. Data are presented as expressed as median and interquartile range (IQR) when they are not normally distributed or mean ± SD when they were normally distributed. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
SARS-CoV-2 did not induce significant IFN-α response or secretion of proinflammatory cytokines in the serum in the adult COVID-19 patients though the levels of IL-6, IL-8, IL-17, and IL-10 moderately increased (Supplementary Table S4). In addition, the ratio of cytokine concentrations between adult patients with or without severe COVID-19 ranged from 0.66 to 1.68 with an average of 1.05 ± 0.26 (Supplementary Table S4), indicating no significant association of blood cytokines with the severity of COVID-19 in adult patients. In contrast, a relatively higher level of blood cytokines was observed in pediatric patients than in the adults, especially for IFN-α, IFN-γ, TNF- α, IL-1β, IL-6, IL-8, and IL-17 (Supplementary Table S4). Of note, a relatively lower level of cytokines was observed in severe pediatric patients than in non-severe pediatric patients. The corresponding ratios were 0.69 for IFN-α, 0.11 for TNF-α (p = 0.009), 0.32 for IL-1β (p = 0.012), 0.07 for IL-6 (p = 0.006), 0.18 for and IL-8 (p = 0.036, Supplementary Table S4; Figure 4). These results indicated stronger cytokine expression in serum in pediatric patients (especially non-severe pediatric patients) than in adults.
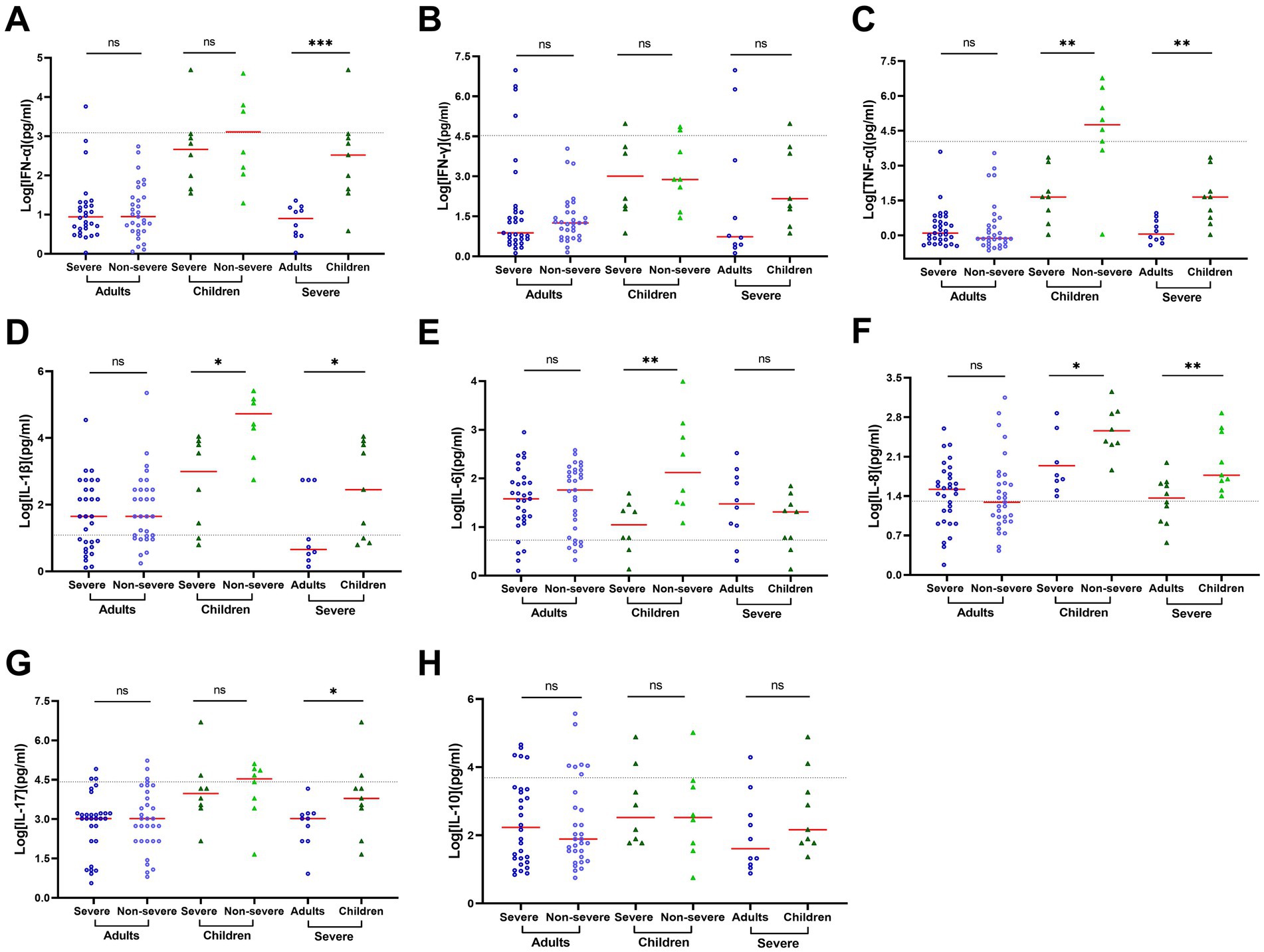
Figure 4. Cytokine concentrations vary by age and patient outcome in serum of progressive COVID-19 patients. Cytokine concentrations in serum samples obtained during disease progression were determined using a 12-item cytokine detection kit for IFN-α (A), IFN-γ (B), TNF-α (C), IL-1β (D), IL-6 (E), IL-8 (F), IL-17 (G) and IL-10 (H). The black dotted lines represent the reference range, which is IFN-α ≤ 8.5 pg./mL, IFN-γ ≤ 23.1 pg./mL, TNF-α ≤ 16.5 pg./mL, IL-1β ≤ 12.4 pg./mL, IL-6 ≤ 5.4 pg./mL, IL-8 ≤ 20.6 pg./mL, IL-17 ≤ 21.4 pg./mL, and IL-10 ≤ 12.9 pg./mL, respectively. The concentrations in each group were compared by rank–sum test or t-test. Data are presented as expressed as median and interquartile range (IQR) when they are not normally distributed or mean ± SD when they were normally distributed. *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001.
The levels of cytokines in BALF and serum were compared in COVID-19 patients whose BALF and serum samples were available. Significantly higher levels of IL-1β, IL-6, and IL-8 were observed in BALF than in serum in adult patients and pediatric severe patients, but not in non-severe pediatric patients (Table 2). For severe adult patients, the corresponding ratios of cytokines in BALF to serum were 70.94 for IL-1β (p < 0.001), 8.12 for IL-6 (p < 0.001), 93.58 for IL-8 (p < 0.001), 4.04 for TNF-α (p < 0.001), and 3.03 for IFN-γ (p = 0.010) while for non-severe adult patients, the corresponding ratios were 9.74 for IL-1β (p = 0.001), 1.47 for IL-6 (p = 0.010), and 130.19 for IL-8 (p = 0.001; Table 2). Interestingly, only a low level of IFN-α was detected, and there was no significant difference between BALF and serum in both severe patients (1.79 vs. 1.97, p = 0.421) and non-severe patients (1.75 vs. 1.78, p = 0.668). In contrast, a higher level of IL-10 was observed in serum than in BALF in both severe patients (4.10 vs. 2.91, p = 0.050) and non-severe patients (3.56 vs. 1.99, p = 0.006; Table 2). For severe pediatric patients, the corresponding ratios of cytokines in BALF to serum were 37.52 for IL-1β, 9.51 for IL-6, and 7.66 for IL-8 (p = 0.0015). In contrast, other cytokines, including IL-17 and IL-10, were relatively higher in serum than in BALF. Interestingly, except for IL-8, relatively higher levels of cytokines were observed in serum than in BALF in non-severe pediatric patients.
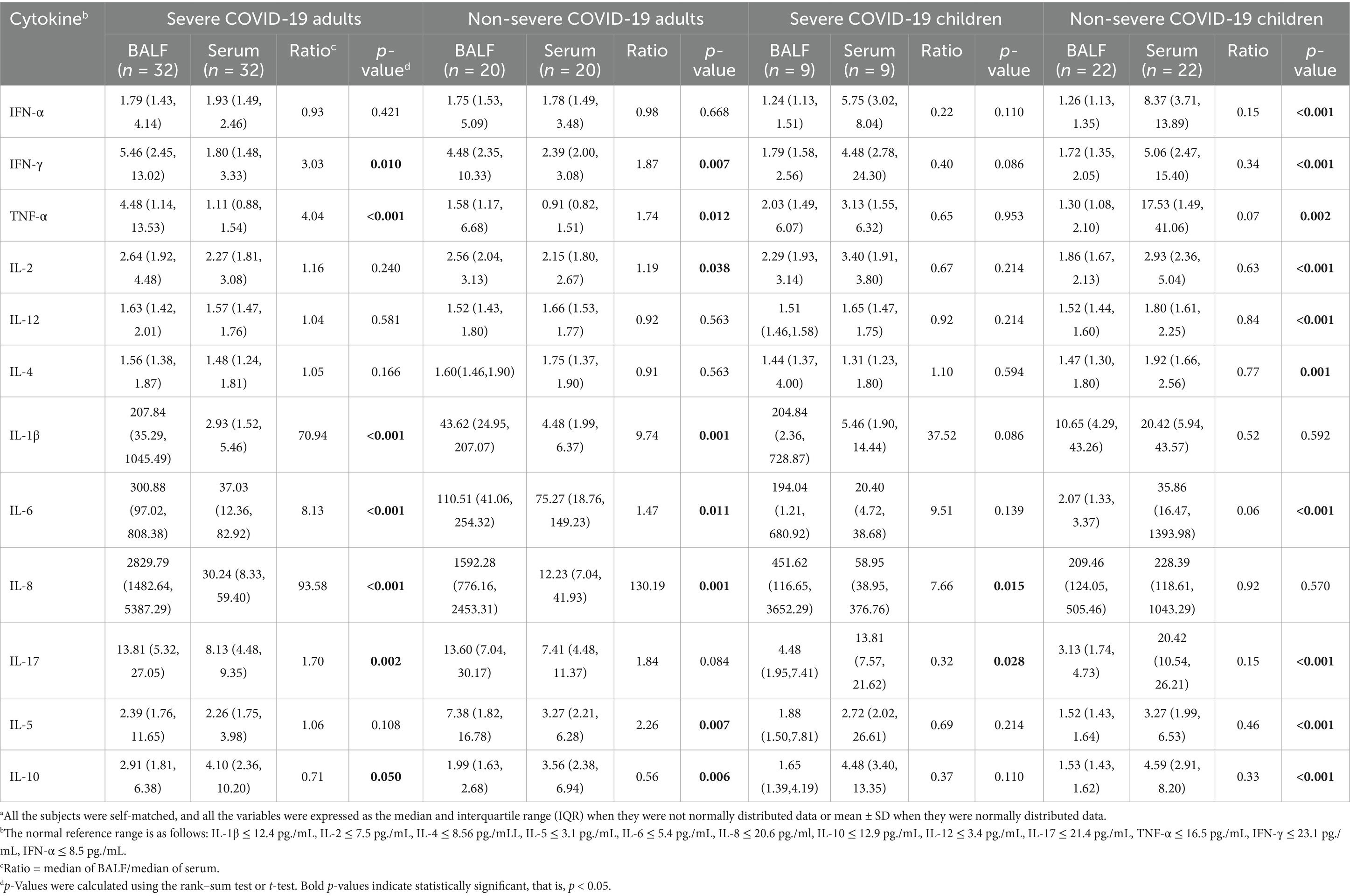
Table 2. Comparison of cytokine levels between the BALF and serum samples from COVID-19 patients.a
In this study, we aimed to simultaneously compare the inflammatory response profile in lung and blood among adults and children with severe and non-severe COVID-19 to identify the biomarkers that relate to the severity of COVID-19 and uncover the distinct mechanisms of pathogenesis caused by SARS-CoV-2 infection among adults and children. In our study, well-documented hallmarks of severe COVID-19, such as lymphopenia, a higher neutrophil-to-lymphocyte ratio (NEU/LYM), and the proinflammatory cytokine storm including IL-6, IL-1β, IL-8, and TNF-α were observed in adult COVID-19 patients rather than pediatric patients. These findings emphasize age-related distinct immune responses as major players in the pathogenesis of COVID-19 and key factors affecting the clinical outcome of SARS-CoV-2 infection.
Lymphocyte depletion, especially low CD3+ and CD3+CD8+ T cell counts is a common feature of adult COVID-19 patients, which may be the result of a large number of T cells migrating to the lungs and other inflammatory sites (Nasrollahi et al., 2023). Although the exact mechanism of lymphatic depletion in COVID-19 is not fully understood, it may involve the overexpression of the NKG2A receptor on CD8+ T cells since the NKG2A receptor is upregulated in COVID-19 patients, and decreases during recovery of COVID-19 (Sun et al., 2023). Furthermore, in COVID-19 patients, there is an observed upregulation in the expression of immune checkpoint molecules such as PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and VISTA. Interference with immune checkpoints may contribute to exacerbating lymphocyte depletion (Gurshaney et al., 2023). In addition, SARS-CoV-2 is known to impede antigen presentation by downregulating the expression of major histocompatibility complex (MHC) class I and II molecules, which hampers the activation of T cell-mediated immune responses, further complicating the immune system’s ability to combat the infection (Mohammed et al., 2022) effectively. Our results support the vital role and potential therapeutic targets of immune checkpoints in COVID-19. In contrast, pediatric patients displayed only moderate systemic inflammation without lymphopenia, which is consistent with the lack of proinflammatory cytokine storm in the lung. This indicates less susceptibility to hyperinflammation during SARS-CoV-2 infection in children, possibly due to differences in innate immune activation and cytokine regulation (Van De Garde et al., 2024).
Another significant difference between adults and pediatric COVID-19 patients is the level of CRP. During the course of COVID-19, cytokine storms lead to enhanced CRP production in adult patients. CRP is primarily regulated by IL-6 and IL-1β and can activate the complement system and potentially exacerbate inflammatory injury. Moreover, CRP-activated NLRP3 inflammasomes may precipitate complications, and lead to cell death (Maity et al., 2023). Although CRP is a non-specific biomarker of acute infections and elevates across diverse conditions, especially in bacterial infections (Giamarellos-Bourboulis et al., 2020), our results are consistent with previous studies to support it as a reliable biomarker to distinguish adults and children with COVID-19 (Rotulo and Palma, 2023). Augmented CRP is not only an early biomarker of SARS-CoV-2 infection but is also associated with the severity and prognosis of COVID-19.
Anti-viral infections are generally initiated by activating innate immune responses to produce interferons and proinflammatory cytokines to block infections (Mathew et al., 2020). IFN-α triggers the activation of interferon-stimulated genes (ISGs) in the epithelial cells, which is crucial for both innate and adaptive immune responses. Our results and previous studies indicated that during the early stage of SARS-CoV-2 infection, especially in severe adult COVID-19 patients, type I interferon response including IFN-α production and activity is markedly suppressed, which in turn facilitates persistent viral replication, excessive proinflammatory cytokine production, and systemic inflammation (Hadjadj et al., 2020), although excessive type I IFN response is a well-documented factor that can worsen COVID-19 by promoting hyperinflammation (Lee et al., 2020). Previous studies have shown that a delayed but enhanced IFN-I response during pathogenic coronavirus infection is critical for the development of ARDS and increases mortality (Kindler and Thiel, 2016; Channappanavar and Perlman, 2017). The timing and magnitude of IFN-I responses also play a crucial role in disease progression and outcome. However, pediatric COVID-19 patients usually exhibit higher IFN-α levels due to enhanced expression of RIG-I, MDA5, and toll-like receptors (TLRs 2/3/4/7/8) in the respiratory tract to trigger a robust and early antiviral response (Thorne et al., 2021). Furthermore, low levels of toll-like receptors (TLRs) and interferon regulatory factors (IRF) in children’s monocytes and dendritic cells result in relatively restrained activation of the IFN-I signaling pathway, which may reduce inflammatory responses and lower the severity of COVID-19. This restricted but effective IFN response in children may mitigate the cytokine storm and tissue damage, and decrease the likelihood of severe outcomes (Jia et al., 2024). Moreover, the combined elevation of IFN-γ and TNF-α can activate JAK/STAT1/IRF1 signaling pathway and promote nitric oxide production, which may lead to caspase-8/FADD-mediated inflammatory cell death (Dharra et al., 2023). Our results are consistent with previous studies and further confirm that in patients with severe COVID-19, decreased lung function is associated with elevated systemic levels of IFN-γ and TNF-α (Littlefield et al., 2022). Our results highlight the critical role of innate immune response during early SARS-CoV-2 infection and support the administration of antiviral therapy as soon as possible to eliminate or restrict viral replication.
Consistent with previous studies, we observed a dramatic increase of IL-6, IL-1β, IL-8, and TNF-α in BALF in adult COVID-19 patients, which is the main hyperinflammatory feature of severe COVID-19 patients while both IL-4 and IL-10 moderately increased to act as anti-inflammatory cytokines through a negative feedback mechanism (Xiong et al., 2020). Previous studies have found notable discrepancies in the intracellular gene expression between BALF and peripheral blood in COVID-19 patients (Manik and Singh, 2022). Our results indicated that SARS-CoV-2 infection results in enhanced secretion of Th1 proinflammatory cytokines, but a comparatively low secretion of Th2 anti-inflammatory cytokines in the BALF than in the serum of adult COVID-19 patients. The release of large amounts of cytokines and chemokines, particularly IL-6, IL-1β, IL-8, and TNF-α in the lung increases the expression of cellular adhesion molecules and VEGF, leading to an increase in pulmonary endothelial permeability, allowing viral dissemination and promoting the infiltration of neutrophils and inflammatory monocytes. Furthermore, the translocation of proinflammatory cytokines into the blood may lead to overactivation of Th1 cells and trigger systemic immune response (Manik and Singh, 2022). Conversely, children patients maintain cytokine levels within normal range and do not experience the cytokine storm typically seen in adult patients (Yuan et al., 2021). On the other hand, children are capable of sustaining appropriate but higher levels of inflammatory cytokines in the early stages of SARS-CoV-2 infection, which may aid in the more effective control of viral replication (Van De Garde et al., 2024). Of note, systemic immune responses are more often observed in children whose cytokine levels were observed to be higher in serum than in BALF. During acute SARS-CoV-2 infection, young children are relatively unaffected by the severe consequences reported in adults, but they are particularly vulnerable to multisystemic inflammatory syndrome in children (MIS-C). This may be due to immunodeficiency in children caused by Th2 polarization, which begins in utero and remains unchanged for the majority of the first decade of life (Hobbs et al., 2020). It should be noted that in children, early immune responses characterized by elevated levels of IL-17 can facilitate the quick clearance of viral infections (Rotulo and Palma, 2023), which is similar to our findings that serum IL-17 levels were higher in children than adults. The age-related immune polarization may explain why children are less susceptible to severe pulmonary complications but more prone to MIS-C (Wimmers et al., 2023). The significant difference in immune responses between adults and children could help to provide age-specific therapeutic strategies. For adults, interventions targeting cytokine storm are crucial to mitigate severe outcomes of COVID-19. Agents such as IL-6 inhibitors and anti-IL-1β antibodies could attenuate excessive inflammation while promoting viral clearance (Tasoudis et al., 2022).
Our study had several limitations. First, the participants were all hospitalized patients. We did not include people with mild COVID-19. Therefore, the results cannot be expanded directly to all SARS-CoV-2 infections. Second, we only analyzed cytokine profiles, but not lymphocyte subsets. Our results did not reflect the whole picture of abnormal immune responses in COVID-19 patients. Third, a single-center study with a small sample size of pediatric COVID-19 patients may limit the ability to identify the difference in cytokine levels between severe and non-severe pediatric patients. Additionally, our study did not include a control group of healthy subjects. A comparison with healthy controls could provide the baseline cytokine levels and strengthen the interpretation of the immune dysregulation in COVID-19 patients.
The severity of COVID-19 in adult patients is mainly caused by a deficiency in the innate immune response, which fails to control SARS-CoV-2 infection and replication, as well as overactivated immune response that results in cytokine storm in the lungs. In contrast, pediatric patients typically exhibit a moderate systemic immune response and a less cytokine storm in the lungs. The age-related differences uncover the different mechanisms related to the pathogenesis, prognosis, and treatment among adults and children with COVID-19. Cytokine profiles in COVID-19 patients may also offer new insights into the mechanism of long COVID-19 and facilitate the development of interventional measures to modulate immune responses and suppress inflammation in critically ill patients.
The original data presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the ethic committee of Chenzhou First People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
HC: Project administration, Resources, Writing – original draft, Writing – review & editing, Funding acquisition, Investigation, Methodology. YuL: Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Software, Validation, Visualization. LY: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. FL: Investigation, Validation, Writing – original draft. QS: Investigation, Writing – original draft, Data curation. QL: Data curation, Investigation, Writing – original draft. YeL: Data curation, Investigation, Writing – original draft. YH: Data curation, Investigation, Writing – original draft. JL: Data curation, Investigation, Writing – original draft. LC: Writing – original draft, Resources, Visualization. ST: Resources, Writing – original draft, Conceptualization, Project administration, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Bethune Charitable Foundation (20221206–03) and the Hunan Provincial Health High-Level Talent Scientific Research Project (R2023065).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1525051/full#supplementary-material
Bahadoran, A., Lee, S. H., Wang, S. M., Manikam, R., Rajarajeswaran, J., Raju, C. S., et al. (2016). Immune responses to influenza virus and its correlation to age and inherited factors. Front. Microbiol. 7:1841. doi: 10.3389/fmicb.2016.01841
Channappanavar, R., and Perlman, S. (2017). Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 39, 529–539. doi: 10.1007/s00281-017-0629-x
Chen, Y., Klein, S. L., Garibaldi, B. T., Li, H., Wu, C., Osevala, N. M., et al. (2021). Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res. Rev. 65:101205. doi: 10.1016/j.arr.2020.101205
Contini, C., Rotondo, J. C., Perna, B., Guarino, M., and De Giorgio, R. (2023). Special issue: advances in SARS-CoV-2 infection. Microorganisms 11:1048. doi: 10.3390/microorganisms11041048
Cucinotta, D., and Vanelli, M. (2020). WHO declares COVID-19 a pandemic. Acta Bio-Med. Atenei Parm. 91, 157–160. doi: 10.23750/abm.v91i1.9397
Dharra, R., Kumar Sharma, A., and Datta, S. (2023). Emerging aspects of cytokine storm in COVID-19: the role of proinflammatory cytokines and therapeutic prospects. Cytokine 169:156287. doi: 10.1016/j.cyto.2023.156287
Dong, Y., Mo, X., Hu, Y., Qi, X., Jiang, F., Jiang, Z., et al. (2020). Epidemiology of COVID-19 among children in China. Pediatrics 145:e20200702. doi: 10.1542/peds.2020-0702
Garofalo, R. P., Hintz, K. H., Hill, V., Patti, J., Ogra, P. L., and Welliver, R. C. (2005). A comparison of epidemiologic and immunologic features of bronchiolitis caused by influenza virus and respiratory syncytial virus. J. Med. Virol. 75, 282–289. doi: 10.1002/jmv.20268
Giamarellos-Bourboulis, E. J., Netea, M. G., Rovina, N., Akinosoglou, K., Antoniadou, A., Antonakos, N., et al. (2020). Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 27, 992–1000.e3. doi: 10.1016/j.chom.2020.04.009
Gurshaney, S., Morales-Alvarez, A., Ezhakunnel, K., Manalo, A., Huynh, T. H., Abe, J. I., et al. (2023). Metabolic dysregulation impairs lymphocyte function during severe SARS-CoV-2 infection. Commun. Biol. 6:374. doi: 10.1038/s42003-023-04730-4
Hadjadj, J., Yatim, N., Barnabei, L., Corneau, A., Boussier, J., Smith, N., et al. (2020). Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 369, 718–724. doi: 10.1126/science.abc6027
Hellman, U., Rosendal, E., Lehrstrand, J., Henriksson, J., Björsell, T., Wennemo, A., et al. (2024). SARS-CoV-2 infection induces hyaluronan production in vitro and hyaluronan levels in COVID-19 patients relate to morbidity and long-term lung impairment: a prospective cohort study. MBio 15:e0130324. doi: 10.1128/mbio.01303-24
Hijano, D. R., Vu, L. D., Kauvar, L. M., Tripp, R. A., Polack, F. P., and Cormier, S. A. (2019). Role of type I interferon (IFN) in the respiratory syncytial virus (RSV) immune response and disease severity. Front. Immunol. 10:566. doi: 10.3389/fimmu.2019.00566
Hobbs, C. V., Khaitan, A., Kirmse, B. M., and Borkowsky, W. (2020). COVID-19 in children: a review and parallels to other Hyperinflammatory syndromes. Front. Pediatr. 8:593455. doi: 10.3389/fped.2020.593455
Jia, R., Li, Z., Hu, S., Chang, H., Zeng, M., Liu, P., et al. (2024). Immunological characterization and comparison of children with COVID-19 from their adult counterparts at single-cell resolution. Front. Immunol. 15:1358725. doi: 10.3389/fimmu.2024.1358725
Kindler, E., and Thiel, V. (2016). SARS-CoV and IFN: too little, too late. Cell Host Microbe. 19, 139–141. doi: 10.1016/j.chom.2016.01.012
Lee, J., and Little, T. D. (2017). A practical guide to propensity score analysis for applied clinical research. Behav. Res. Ther. 98, 76–90. doi: 10.1016/j.brat.2017.01.005
Lee, J. S., Park, S., Jeong, H. W., Ahn, J. Y., Choi, S. J., Lee, H., et al. (2020). Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 5:eabd1554. doi: 10.1126/sciimmunol.abd1554
Littlefield, K. M., Watson, R. O., Schneider, J. M., Neff, C. P., Yamada, E., Zhang, M., et al. (2022). SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLoS Pathog. 18:e1010359. doi: 10.1371/journal.ppat.1010359
Liu, C., Chu, D., Kalantar-Zadeh, K., George, J., Young, H. A., and Liu, G. (2021). Cytokines: from clinical significance to quantification. Adv. Sci. Weinh Baden Wurtt Ger. 8:e2004433. doi: 10.1002/advs.202004433
Lu, G., Ling, Y., Jiang, M., Tan, Y., Wei, D., Jiang, L., et al. (2023). Primary assessment of the diversity of omicron sublineages and the epidemiologic features of autumn/winter 2022 COVID-19 wave in Chinese mainland. Front. Med. 17, 758–767. doi: 10.1007/s11684-022-0981-7
Lucas, C., Wong, P., Klein, J., Castro, T. B. R., Silva, J., Sundaram, M., et al. (2020). Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 584, 463–469. doi: 10.1038/s41586-020-2588-y
Ma, X., Liu, S., Chen, L., Zhuang, L., Zhang, J., and Xin, Y. (2021). The clinical characteristics of pediatric inpatients with SARS-CoV-2 infection: a meta-analysis and systematic review. J. Med. Virol. 93, 234–240. doi: 10.1002/jmv.26208
Maity, S., Santra, A., Hebbani, A. V., Pulakuntla, S., Chatterjee, A., Badri, K. R., et al. (2023). Targeting cytokine storm as the potential anti-viral therapy: implications in regulating SARS-CoV-2 pathogenicity. Gene 881:147612. doi: 10.1016/j.gene.2023.147612
Manik, M., and Singh, R. K. (2022). Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J. Med. Virol. 94, 869–877. doi: 10.1002/jmv.27405
Mann, H. B., and Whitney, D. R. (1947). On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 18, 50–60. doi: 10.1214/aoms/1177730491
Mathew, D., Giles, J. R., Baxter, A. E., Oldridge, D. A., Greenplate, A. R., Wu, J. E., et al. (2020). Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 369:eabc8511. doi: 10.1126/science.abc8511
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond. Engl. 395, 1033–1034. doi: 10.1016/S0140-6736(20)30628-0
Mohammed, R. N., Tamjidifar, R., Rahman, H. S., Adili, A., Ghoreishizadeh, S., Saeedi, H., et al. (2022). A comprehensive review about immune responses and exhaustion during coronavirus disease (COVID-19). Cell Commun. Signal CCS. 20:79. doi: 10.1186/s12964-022-00856-w
Nasrollahi, H., Talepoor, A. G., Saleh, Z., Eshkevar Vakili, M., Heydarinezhad, P., Karami, N., et al. (2023). Immune responses in mildly versus critically ill COVID-19 patients. Front. Immunol. 14:1077236. doi: 10.3389/fimmu.2023.1077236
National Health Commission of the People’s Republic of China. The guidelines for diagnosis and treatment of SARA-CoV-2 infection (version 10). (2023). Available at: https://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm (Accessed February 14, 2023).
Ramasamy, S., and Subbian, S. (2021). Critical determinants of cytokine storm and type I interferon response in COVID-19 pathogenesis. Clin. Microbiol. Rev. 34:e00299-20. doi: 10.1128/CMR.00299-20
Rosenthal, A., and Waitzberg, R. (2023). The challenges brought by the COVID-19 pandemic to health systems exposed pre-existing gaps. Health Policy Open. 4:100088. doi: 10.1016/j.hpopen.2022.100088
Rotulo, G. A., and Palma, P. (2023). Understanding COVID-19 in children: immune determinants and post-infection conditions. Pediatr. Res. 94, 434–442. doi: 10.1038/s41390-023-02549-7
Sun, Y., Luo, B., Liu, Y., Wu, Y., and Chen, Y. (2023). Immune damage mechanisms of COVID-19 and novel strategies in prevention and control of epidemic. Front. Immunol. 14:1130398. doi: 10.3389/fimmu.2023.1130398
Tasoudis, P. T., Arvaniti, C. K., Adamou, A. T., Belios, I., Stone, J. H., Horick, N., et al. (2022). Interleukin-6 inhibitors reduce mortality in coronavirus disease-2019: an individual patient data meta-analysis from randomized controlled trials. Eur. J. Intern. Med. 101, 41–48. doi: 10.1016/j.ejim.2022.04.004
Thorne, L. G., Reuschl, A. K., Zuliani-Alvarez, L., Whelan, M. V. X., Turner, J., Noursadeghi, M., et al. (2021). SARS-CoV-2 sensing by RIG-I and MDA5 links epithelial infection to macrophage inflammation. EMBO J. 40:e107826. doi: 10.15252/embj.2021107826
Van De Garde, M. D. B., Miranda-Bedate, A., Nanlohy, N. M., Jacobi, R. H. J., Meijer, A., Reukers, D. F. M., et al. (2024). Early immune profiling reveals distinct inflammatory responses between children and adults few days after primary SARS-CoV-2 infection. Front. Immunol. 15:1359993. doi: 10.3389/fimmu.2024.1359993
Weisberg, S. P., Connors, T. J., Zhu, Y., Baldwin, M. R., Lin, W. H., Wontakal, S., et al. (2021). Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 22, 25–31. doi: 10.1038/s41590-020-00826-9
Wimmers, F., Burrell, A. R., Feng, Y., Zheng, H., Arunachalam, P. S., Hu, M., et al. (2023). Multi-omics analysis of mucosal and systemic immunity to SARS-CoV-2 after birth. Cell 186, 4632–4651.e23. doi: 10.1016/j.cell.2023.08.044
Xiong, Y., Liu, Y., Cao, L., Wang, D., Guo, M., Jiang, A., et al. (2020). Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 9, 761–770. doi: 10.1080/22221751.2020.1747363
Yin, K., Peluso, M. J., Luo, X., Thomas, R., Shin, M. G., Neidleman, J., et al. (2024). Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2. Nat. Immunol. 25, 218–225. doi: 10.1038/s41590-023-01724-6
Keywords: COVID-19, cytokine, cytokine storm, inflammatory response, case–control study
Citation: Chen H, Li Y, Yuan L, Liu F, Sun Q, Luo Q, Lei Y, Hou Y, Li J, Cai L and Tang S (2025) Age-related immune response disparities between adults and children with severe COVID-19: a case–control study in China. Front. Microbiol. 16:1525051. doi: 10.3389/fmicb.2025.1525051
Received: 08 November 2024; Accepted: 13 January 2025;
Published: 04 February 2025.
Edited by:
Gianvito Lanave, University of Bari Aldo Moro, ItalyReviewed by:
Yuhang Wang, The University of Iowa, United StatesCopyright © 2025 Chen, Li, Yuan, Liu, Sun, Luo, Lei, Hou, Li, Cai and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shixing Tang, dGFtZ3NoaXhpbmdAc211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.