
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 25 February 2025
Sec. Phage Biology
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1499566
 Chenyu Zheng1†
Chenyu Zheng1† Yaxin Di1†
Yaxin Di1† Yingchun Wang1
Yingchun Wang1 Huyang Zhao1
Huyang Zhao1 Ruichong Wang2
Ruichong Wang2 Guiwei Li3
Guiwei Li3 Xiaona Wang1
Xiaona Wang1 Zhifu San1
Zhifu San1 Yanping Jiang1
Yanping Jiang1 Wen Cui1
Wen Cui1 Jiaxuan Li1
Jiaxuan Li1 Li Wang1
Li Wang1 Xinyuan Qiao1*
Xinyuan Qiao1*The treatment of infections caused by drug-resistant Staphylococcus aureus has become increasingly difficult. In this study, the complete genome of phage 4086-1 against S. aureus was sequenced and shown to be 17,960 bp in size, with a GC content of 29.14%. Phylogenetic analysis demonstrated that phage 4086-1 exhibited a close relationship with the Staphylococcus phages SLPW, JPL-50, and LSA2366. BLAST analysis indicated that ORF12 of phage 4086-1 (termed Hol-4086) shares high identity with other reported phage-associated holins. Hol-4086 consists of 140 amino acids and exhibits high sequence identity with some members of the phage_holin_4_1 superfamily (18–125 amino acids). Hol-4086 was then expressed in Escherichia coli and detected using sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blotting. The results of a spot test demonstrated that Hol-4086 had substantial bacteriostatic effects on the host bacteria. S. aureus cells were exposed to Hol-4086 and observed using transmission electron microscopy and scanning electron microscopy. Bacterial cells treated with Hol-4086 showed ultrastructural and morphological changes. The detection of biofilm activity showed that Hol-4086 effectively inhibited and removed S. aureus biofilms. In vivo, treatment with Hol-4086 significantly reduced the number of bacteria, relieved inflammatory responses, and alleviated pathological changes in the organs of infected mice 48 h after treatment. These results demonstrate that Hol-4086 exhibits promising antibacterial potential as an alternative therapy for the treatment of infections caused by S. aureus.
Staphylococcus aureus is a pathogen with remarkable adaptive power that causes diverse severe infections, such as pneumonia, bacteremia, scalded skin syndrome, toxic shock syndrome, endocarditis, and sepsis (Cheung et al., 2021; Cong et al., 2020; Nwabuife et al., 2021). The treatment of infectious diseases caused by pathogenic bacteria is largely based on the use of antibiotics. The introduction of antibiotics into clinical medicine in the mid-20th century significantly reduced morbidity and mortality from bacterial infections; while pathogenic bacteria did not disappear, they did become more manageable (Loganathan et al., 2021). However, the emphasis on this treatment has led to an overreliance on antibiotics, leading to the emergence of antibiotic resistance. Treatment of infections caused by S. aureus has become more difficult because of the emergence of drug-resistant strains. These include methicillin-resistant S. aureus and vancomycin-resistant S. aureus (Cong et al., 2020; Lakhundi and Zhang, 2018; Turner et al., 2019). There is an urgent clinical need for non-antibiotic immune-based approaches to treat these infections because increasing antibiotic resistance is a serious threat to public health.
Phages are viruses that infect bacteria and were first discovered by Frederik Twort in 1915 and Felix d’Herelle in 1917 (Salmond and Fineran, 2015; Jariah and Hakim, 2019; Aranaga et al., 2022; Hatoum-Aslan, 2021). Phages are the most abundant biological entities and are estimated to amount to a total number of 1031 particles (Hesse and Adhya, 2019; Wei et al., 2020). Phages play a vital role in the treatment of antibiotic-resistant infections, one of the greatest threats to global health. Phage therapy has some strengths over traditional antibiotic therapy, including host specificity, and it does not affect other commensals (Anyaegbunam et al., 2022; Kakasis and Panitsa, 2019). Despite the great potential of phages, several constraints limit their broad application and acceptance in clinical practice. The emergence of phage-insensitive bacterial mutants, the stability of various types of phages, and the quality and safety requirements of phage preparations must be addressed in the coming years of phage research to advance the development of phage therapy (Pires et al., 2020; Bhargava et al., 2021). Thus, phage-encoded proteins with antibacterial properties have attracted considerable interest. Phage-encoded lysis proteins (endolysins and holins) can cause the breakdown of the bacterial membrane. Phages with single-stranded genomes encode cleavage effectors that inhibit the biosynthesis of bacterial peptidoglycan. In contrast, release of the phage progeny of double-stranded DNA (dsDNA) phages is mediated by two proteins (holin and endolysin) responsible for cell envelope disruption. Once the lytic life cycle is complete and the virion particles mature inside the bacterial cell, holin forms pores in the inner membrane, allowing endolysin to enter the cell wall. Subsequently, peptidoglycan is degraded by endolysin molecules, resulting in cell death and release of phage particles (Gutiérrez et al., 2018; Moghadam et al., 2020). However, some holins not only participate in phage-mediated cell lysis, but also participate in the process of phage DNA injection into host bacteria, destroying the cell wall and membrane of host bacteria from outside, and have strong lytic activity against bacteria (Qi et al., 2022; Nobrega et al., 2018).
Recently, phage proteins such as endolysins and holins have been explored as potential antimicrobial agents. Phage endolysin LysGH15 effectively protects against Staphylococcus epidermidis infection in mice. The number of bacteria decreased significantly in the organs of mice, and it also improved the pathological changes in the organs (Zhang et al., 2018). Phage endolysin LysSAP26, a gene potentially encoding the lysin of phage SAP-26, exhibits extended antibacterial activity. In animal experiments, mice infected with Acinetobacter baumannii were protected by LysSAP26, resulting in a survival rate of 40% (Kim et al., 2020). In the mouse infection model, Ply5218 repeated dosing (6, 24, and 48 h post-infection) not only alleviated the clinical symptoms caused by Streptococcus in piglets, but also significantly reduced the bacterial loads and the level of interleukin-6 (IL-6) (Wang et al., 2019). Moreover, research on the phage Lysin CF-301 has demonstrated that it can effectively remove biofilms and kill bacteria (Schuch et al., 2017). Meanwhile, many reports have indicated that holin proteins can cause cells to lose viability by forming holes in the cell membrane, which is also lethal to bacteria in vitro (Farkašovská et al., 2004). It was confirmed that the HolGH15 protein has an extracellular antibacterial function that can inhibit S. aureus and Pseudomonas aeruginosa. The combined application of LysGH15 enhanced antibacterial ability (Song et al., 2016).
In the present study, we isolated S. aureus phage 4086-1 from pig fecal samples (Tang, 2019) and analyzed its complete genome sequence. A putative holin protein from phage 4086-1 (named Hol-4086) was isolated and expressed in E. coli. The antibacterial effects of Hol-4086 were evaluated in vitro and in vivo, providing evidence for the feasibility of phage therapy for S. aureus infections.
Staphylococcus aureus CVCC 546 was purchased from the China Veterinary Culture Collection Center. S. aureus ATCC 43300 was purchased from the American Type Culture Collection Center. Phage 4086–1 was isolated from pig fecal samples and maintained in our laboratory. The phage 4086–1 was species-specific, attacking only S. aureus. E. coli pET-28a(+) and E. coli BL21 (DE3) were stored in our laboratory.
All animal experiments involved in this work were approved by the Animal Ethics Committee of Northeast Agricultural University, Harbin, China.
The purified lysate was added to the culture of exponentially grown propagation host strain (S. aureus CVCC 546), and the mixture was incubated at 37°C in LB broth until the liquid was clear. All the phage cultures were collected for DNA extraction. Chloroform was added to the phage cultures at a final concentration of 0.5% and the cultures were incubated at 37°C for 1 h to allow complete dissolution. The obtained lysate was centrifuged at 10,000 r/min for 10 min, and the supernatant was collected and filtered. The filtrate was treated with DNase I (final concentration 50 U/mL, Sigma, St. Louis, MO, United States) and RNase A (final concentration 50 U/mL, Sigma, St. Louis, MO, United States) for 1 h at 37°C. Crude phage particles were treated with 1 mol/L NaCl in an ice bath for 1 h and then concentrated using 10% (w/v) polyethylene glycol 8,000. After centrifugation at 10000 r/min for 15 min, the samples were collected and resuspended in 1 mL SM buffer (0.05 M Tris–HCl, pH 7.5, 0.1 M NaCl, 0.017 M MgSO4, 0.01% gelatin). Phage DNA was obtained using phenol-chloroform extraction as follows (Sambrook et al., 1989). Add DNase I and RNase A to the crude phage sample to a final concentration of 10 μg/mL and 5 μg/mL respectively, and incubate at 37°C for 1 h. EDTA (pH 8.0) was then added to a final concentration of 20 mM. Further, incorporate proteinase K and SDS to final concentrations of 50 μg/mL and 10% respectively, and incubate at 56°C for 1 h. Sequentially, add equal volumes of Tris-saturated phenol, phenol-chloroform-isoamyl alcohol (25:24:1), and chloroform. The mixture was stirred gently for 30 s, centrifuged at 5000 r/min for 5 min, and the supernatant was collected. Subsequently, add an equal volume of ice-cold ethanol and 1/10 volume of 3 M NaAc, and keep at −20°C overnight. Centrifuge at 12,000 r/min for 20 min at 4°C. Wash the precipitate with pre-cooled ethanol. Centrifuge and discard the supernatant. After drying at room temperature, dissolve in sterile ultrapure water and store at −20°C.
Purified phage genomic DNA was prepared for next-generation sequencing to obtain the phage 4086-1 sequence. The putative function of each open reading frame (ORF) was predicted and annotated using BLAST software. The amino acid sequences of DNA polymerase were used to construct neighbor-joining phylogenetic trees using MEGA X 1.0.2.0.
The DNA and protein sequences of the holin protein (Hol-4086) in phage 4086-1 were analyzed using BLAST. The TMHMM 2.0 Server1 was used to predict the transmembrane helices. Sequence alignment and Hol-4086 analysis were performed using BLAST2. The conserved domain was predicted using the NCBI CDD (Conserved Domains Database) search tool3.
The primers holF (5′-CGCGGATCCATGAATGAGGTAAAATTAAGATTTACG-3′) and holR (5′-CGAGCTCTTATCTATCTTCTCTTGGTCGTTCA-3′) were designed based on the complete sequence of the Hol-4086 gene. The reaction comprised of a 5 min denaturation at 94°C followed by 30 cycles of 30 s at 94°C, 30 s at 61°C, and 30 s at 72°C, and a final extension for 10 min at 72°C. The amplification products were inserted into the BamH I and Sac I sites of the pET-28a(+) vector. The recombinant plasmid, pET-Hol-4086, was transformed into competent E. coli BL21 (DE3) cells. A single positive colony was cultured in LB broth with 100 μg/mL ampicillin and grown to early mid-log phase (OD600 0.4–0.6). Next, the mixture was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 30°C for 8 h at 180 rpm. The precipitate was washed three times with phosphate buffered saline (PBS) (pH 7.4) and disrupted by sonication (4 s pulse, 4 s rest over 10 min) in an ice-cold water bath before centrifugation at 12,000 r/min. The supernatant was filtered using a 0.22 μm filter. All samples were stored for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting analysis.
The lytic activity of Hol-4086 was detected using spot testing. The culture of S. aureus (CVCC 546) was centrifuged to discard the supernatant. 2.5% glutaraldehyde fixative was added to fix at 4°C for more than 2 h. The cells were washed 3 times with 0.1 M PBS for 10 min each time, and the supernatant was discarded. After gradient dehydration with ethanol for 10 min and replacement with tert-butyl alcohol, the samples were freeze-dried for 4 h. Morphological and ultrastructural changes in S. aureus cells were visualized after exposure to Hol-4086 by transmission electron microscopy (TEM) (H-7650; Hitachi) and scanning electron microscopy (SEM) (SU8010; Hitachi).
To assess the efficacy of the holin in preventing adhesion of S. aureus to an abiotic surface, 50 μL S. aureus (mid-log phase, OD = 0.6) and 150 μL LB medium were added to the wells of a 96-well tissue culture plate. Wells containing only the LB medium were used as negative controls. Meanwhile, 100 μL PBS, antibiotics, or Hol-4086 lysate were added to the wells, respectively. After incubation at 37°C for 1, 4, 8, 12, 16, 20, and 24 h, the biofilms were stained with crystal violet. Finally, absorbance was recorded at 570 nm using a microplate reader.
To test the efficacy of the holin on removing S. aureus biofilm, after incubation at 37°C for 48 h to form a mature biofilm, the medium was discarded and the plate wells washed with PBS. Next, 200 μL PBS, antibiotics (ceftiofur sodium), or Hol-4086 lysate were added to the wells. After 1, 4, 8, 12, 16, 20, and 24 h incubation at 37°C, the microplate was stained with crystal violet, and the results were recorded as stated previously.
Ten healthy mice were randomly divided into two groups. The experimental group was injected with 250 μL lysate of Hol-4086. The mental state and death of the mice were observed and compared with the control group injected with the same dose of PBS to analyze whether lysate of Hol-4086 was toxic to mice.
To assess the effect of Hol-4086 treatment on septicemia caused by S. aureus infection, BALB/c mice aged 6–8 weeks were injected intraperitoneally with Hol-4086 lysate, ceftiofur, or PBS buffer 1 h after injection with 2× minimum lethal dose (2 × 108 CFU/mouse) of S. aureus bacterial suspension (n = 10 in each group). Mice uninfected or treated served as controls. Bacterial loads in the blood and vital organs were measured 48 h after treatment using serial dilution and plating techniques. The blood, hearts, lungs, livers, spleens and kidneys (100 mg) were selected and homogenized in 1 mL of PBS with TissueLyser II (QIAGEN, Hilden, Germany). The homogenate was serially diluted (1:10) with PBS and coated on the plate at 37°C for 12 h incubation, bacterial colonies (CFUs) were then counted.
The cytokines, including interferon alpha (IFN-α) and IL-6, were quantified using enzyme-linked immunosorbent assay (ELISA). Histopathological analyses were performed on the tissues of the main organs. The mice were euthanized 48 h after treatment, and their hearts, lungs, livers, spleens, and kidneys were removed and immediately placed in 4% formalin. The fixed tissues were stained with hematoxylin and eosin using conventional staining methods, and the samples were histologically examined under an optical microscope.
SPSS version 20.0 (SPSS Inc., Chicago, IL, United States) was used for all statistical analyses. Two-way analysis of variance was used to compare the quantities of bacteria and levels of cytokines in blood and organ samples. Error bars represent standard deviation.
The entire genome of phage 4086-1 was sequenced and annotated (Figure 1) (Accession number: PP541615). The phage genome consisted of circular double-stranded DNA with a genome size of 17,960 bp and a GC content of 29.14%. A total of 21 ORFs were predicted in the genome. Twelve ORFs were determined to have known functions and nine ORFs were classified as hypothetical proteins. ORF functions were predicted and classified into 4 categories: nucleotide metabolism-related, structure-related and packaging-related, lysis-related genes and hypothetical proteins genes. The results showed that ORF 12 probably encodes a holin protein (designated Hol-4086). Based on available databases, no functionally predicted genes related to virulence or lysogenicity were identified.
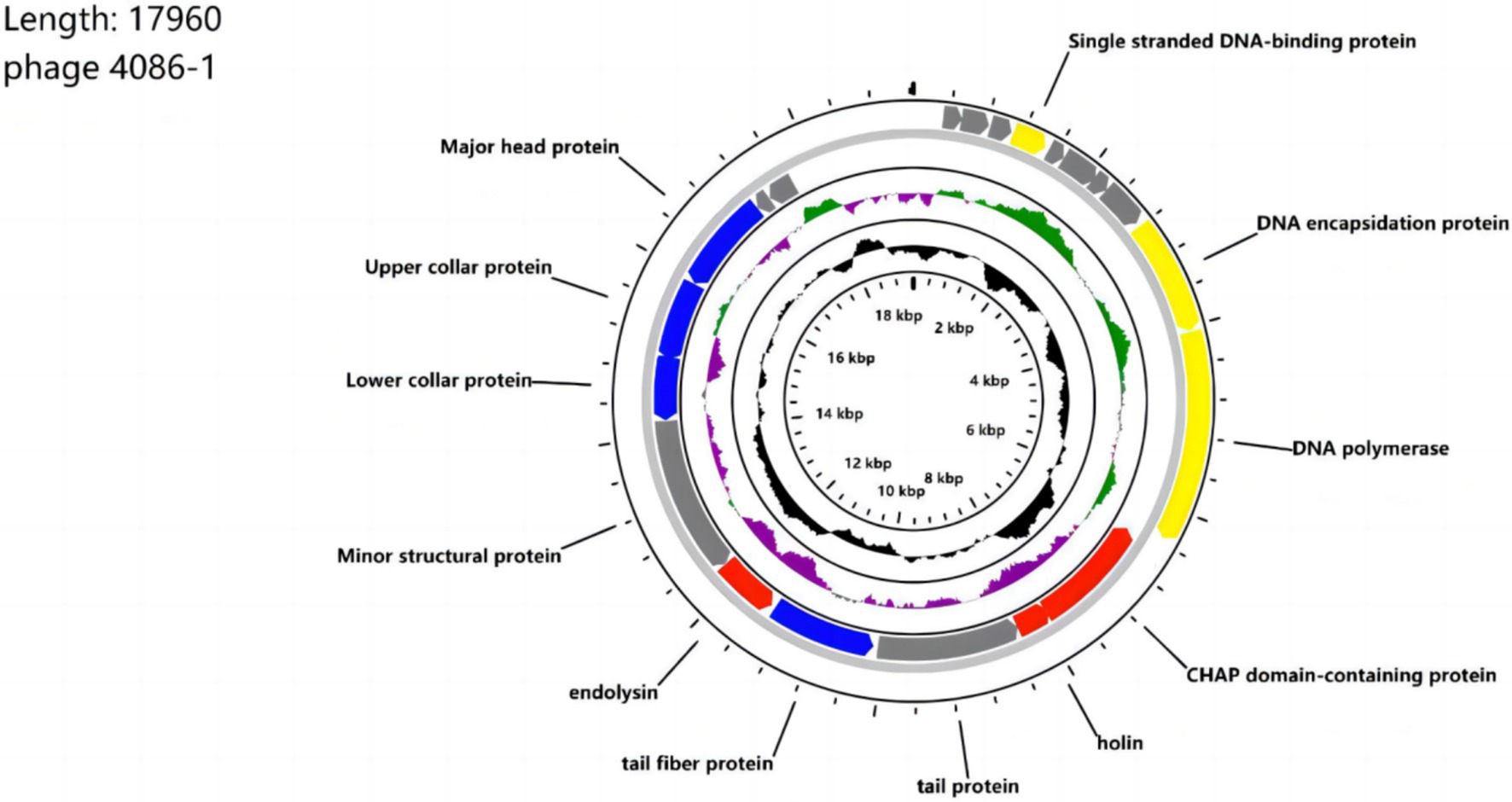
Figure 1. Genome map of phage 4086-1. The open reading frames (ORFs) are indicated by specific colors according to their functional categories. Consensus identities: red, lysis-related proteins; yellow, DNA and packaging-related proteins; blue; structural proteins; gray; hypothetical proteins. The GC skew is shown as inner circle holograms in green and purple. GC content is indicated by a black circular hologram.
Phylogenetic analysis demonstrated the genomic relationship of phage 4086-1 with other homologous phages. As shown in Figure 2, according to the amino acid sequence of the DNA polymerase (ORF10), phage 4086-1 is closely related to the Staphylococcus phages SLPW and JPL-50 (Figure 2A). Further analysis conducted based on the amino acid sequences of its major head protein (ORF 19), tail fiber protein (ORF 14) and endolysin (ORF 15). It was found that that the head protein of phage 4086-1 was closely related to Staphylococcus phages SLPW (Figure 2B). The tail protein of phage 4086-1 was closely related to Staphylococcus phages SCH1 (Figure 2C). And the endolysin of phage 4086-1 was closely related to Staphylococcus phages JPL-50 and LSA2366 (Figure 2D).
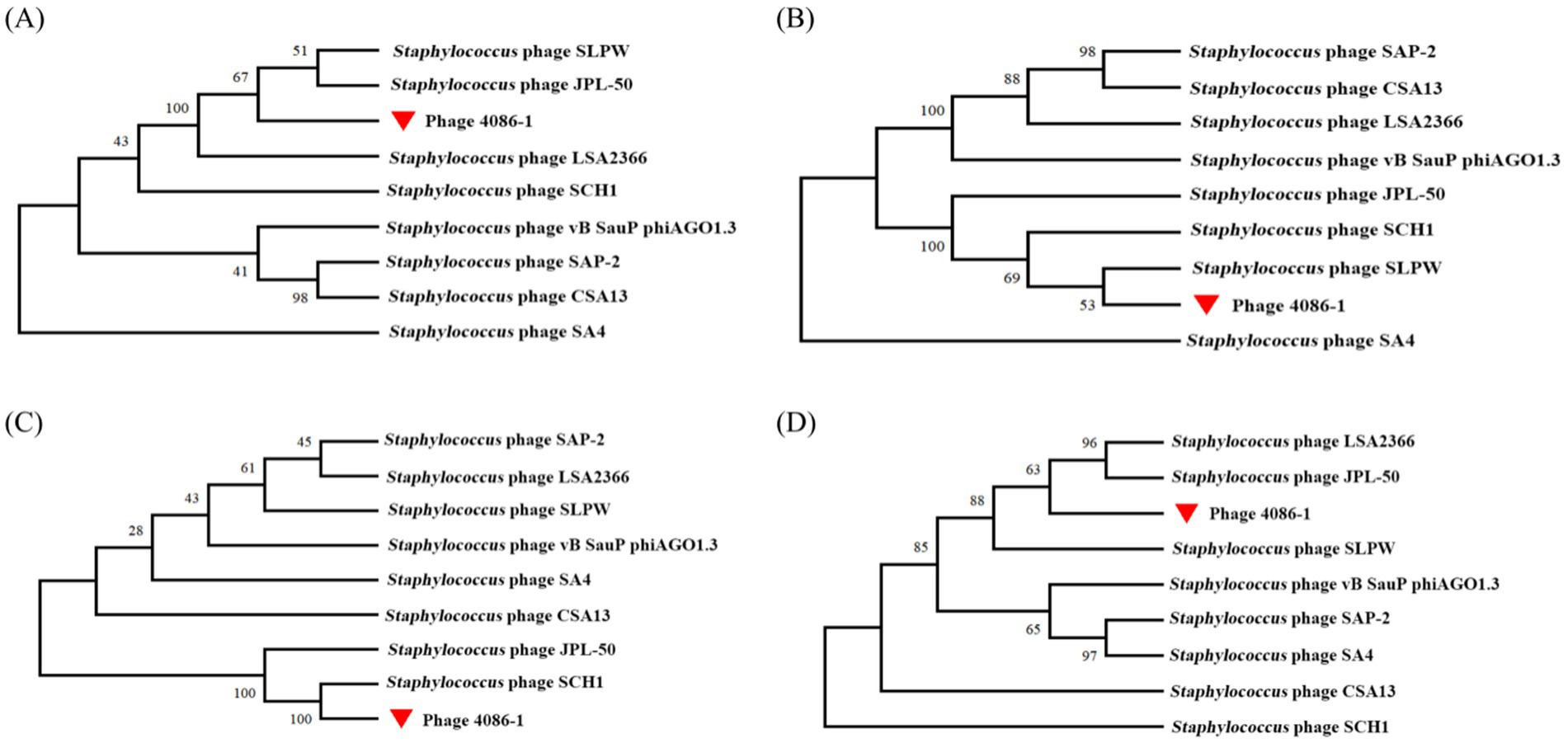
Figure 2. Phylogenetic analysis of the large terminase subunits from phage 4086-1 and other homologous phages. The phylogenetic analysis was constructed by comparing the sequence of the DNA polymerase (A), major head protein (B), tail fiber protein (C) and endolysin (D) to the corresponding sequences in homologous phages. The phage 4086-1 in the present study is highlighted using a red triangle.
The DNA sequence corresponding to or encoding Hol-4086 was compared to that of other published holins from Staphylococcus phages (Table 1). The results demonstrated that the holin region of phage 4086-1 (ORF 12) shared high sequence identity with different Staphylococcus phages (Accession number: PP477054). The results of TMHMM analysis suggested that Hol-4086 is a membrane protein with typical holin traits. This prediction indicated that Hol-4086 has two putative hydrophobic transmembrane domains (TMDs) with cytoplasmic N- and C-termini (Figure 3A). As shown in Figure 3B, Hol-4086 consists of 140 amino acids and exhibits high sequence identity with some members of the phage_holin_4_1 superfamily (18–125 amino acids).

Figure 3. Predicted Hol-4086 protein topology. (A) Topological model of Hol-4086. Red box, TMD1; blue box, TMD2. (B) Putative conserved domain model of Hol-4086. Yellow; conserved domain. TMD, transmembrane domain.
Sequences encoding Hol-4086 were successfully cloned into plasmid pET28a. Hol-4086 was then expressed in E. coli cells after induction by IPTG at 37°C. SDS-PAGE and western blotting revealed that the protein had the correct mass and a near homogeneity of 21 kDa, corresponding to the predicted size of the phage Hol-4086 protein (Figure 4).

Figure 4. Expression of Hol-4086. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed to detect Hol-4086 expression. (B) Western blot analysis of Hol-4086 expression. The lanes are as follows: M, molecular mass marker; 1, E. coli BL21-CodonPlus cells harboring pET-28a(+) cultures were collected after induction as a negative control; 2, supernatant from induced cell lysis of recombinants. The red arrow indicates the expressed Hol-4086.
After incubation for 12 h at 37°C, a bacteriostatic circle formed on the agar plate that had been treated with expressed Hol-4086 protein, which could not be observed in the negative controls (Figure 5A). These results demonstrate that the expressed Hol-4086 protein has substantial bacteriostatic effects on host bacteria.

Figure 5. Antibacterial activity of Hol-4086. (A) Effects of Hol-4086 on S. aureus as observed by spot testing. The holes are as follows: 1, 100 μL uninduced E. coli lysates; 2, 100 μL Hol-4086 lysate. (B) The effect of Hol-4086 on S. aureus observed by transmission electron microscopy (TEM) and scanning electron microscopy (SEM). (a) S. aureus cells without treatment with Hol-4086 observed by SEM. (b) Effects of Hol-4086 on S. aureus as observed by SEM. (c) S. aureus cells without treatment with Hol-4086 observed by TEM. (d) Effects of Hol-4086 on S. aureus as observed by TEM. Bars: SEM, 2.00 μm; TEM, 500 nm.
Staphylococcus aureus cells were exposed to Hol-4086 and observed using TEM and SEM. TEM and SEM images of the bacterial cells treated with Hol-4086 showed ultrastructural and morphological changes (Figure 5B). According to the SEM observations, Hol-4086 caused the surface shrinkage of S. aureus. In addition, TEM revealed that the cell wall of S. aureus was destroyed, resulting in the release of cellular contents.
Biofilm matrix disruption by Hol-4086 was measured by plate reader at OD570. The results of the tests performed for biofilm prevention and removal are shown in Figure 6. As shown in Figure 6A, after incubation with Hol-4086 or antibiotics, biofilm formation was significantly lower than that in the PBS group, and the amount of biofilm after incubation with Hol-4086 was slightly lower than that in the antibiotic group (p < 0.01). Hol-4086 also effectively removed S. aureus biofilms after incubation for 12 h (p < 0.01; Figure 6B).
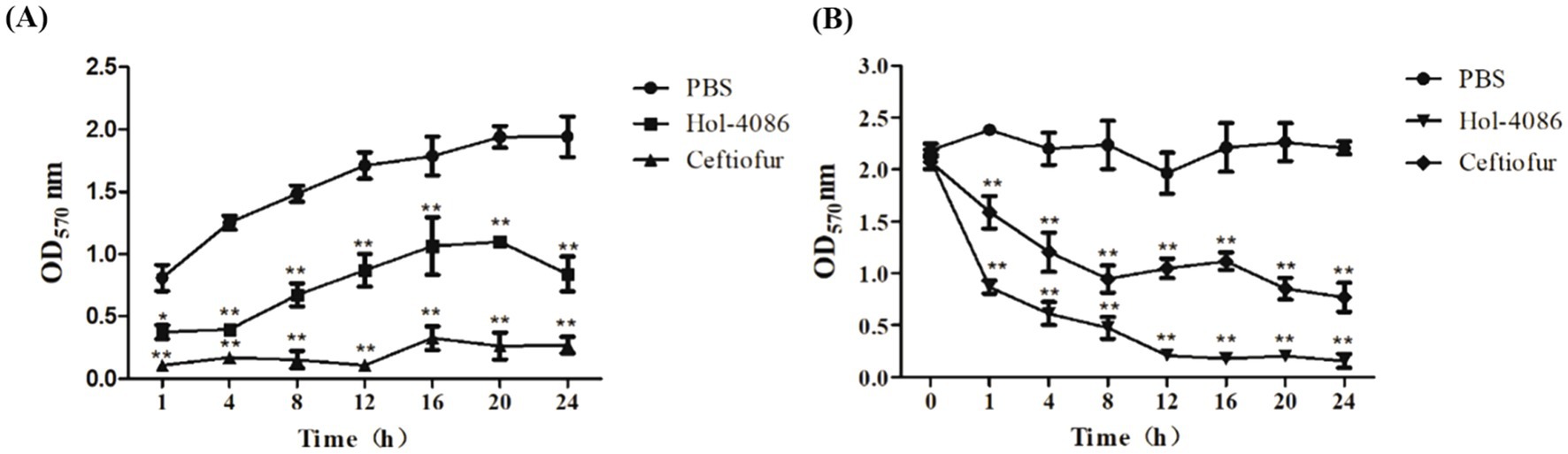
Figure 6. The effects of Hol-4086 on the removal of S. aureus biofilms. Biofilm prevention (A) and biofilm removal (B) by Hol-4086 against S. aureus in microtiter plates assays. The biomass amounts are presented as the OD570 values. *p < 0.05, **p < 0.01.
The endotoxin test results showed that the mice in the experimental group and the control group grew well without abnormalities, and the survival rate statistics were shown in Table 2, indicating that there was no toxic effect of Hol-4086 on mice.
Mice were infected with S. aureus to detect the effects of Hol-4086. The healthy mice that were not injected with bacteria or treated were as the control. S. aureus counts were determined using the plate-counting method. As shown in Figure 7, the bacterial loads in the blood, heart, liver, spleen, lung, and kidney of Hol-4086-treated mice decreased by 2.02, 0.16, 1.37, 0.05, 1.39, and 0.73 log units, respectively, 48 h after treatment, compared with the PBS group (p < 0.01).
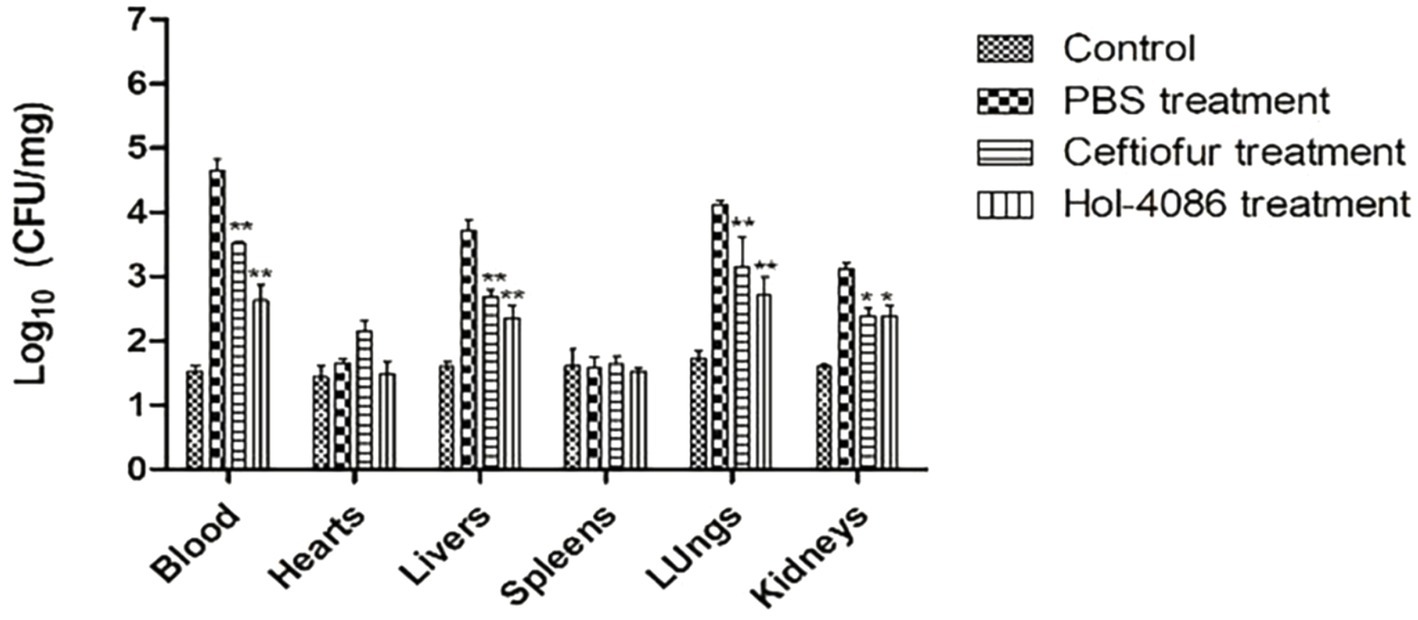
Figure 7. Bacterial loads in the blood and organs of mice. The bacterial loads in the blood, hearts, livers, spleens, lungs, and kidneys of Hol-4086-treated mice decreased by 2.02, 0.16, 1.37, 0.05, 1.39, and 0.73 log units, respectively, 48 h after treatment, compared with PBS group. Error bars represent the mean ± standard error of the mean from three independent replicates, *p < 0.05, **p < 0.01; two-way analysis of variance.
To assay the effect of Hol-4086 on S. aureus infection, the levels of IFN-ɑ and IL-6 were determined using ELISA kits. Compared with PBS group, the ceftiofur sodium and Hol-4086 groups both showed significant decreases in the levels of IFN-ɑ and IL-6. The concentration in the Hol-4086 group was slightly lower than that in the antibiotic-treated group (Figure 8). These results demonstrated that Hol-4086 effectively alleviated inflammatory responses to S. aureus infection.
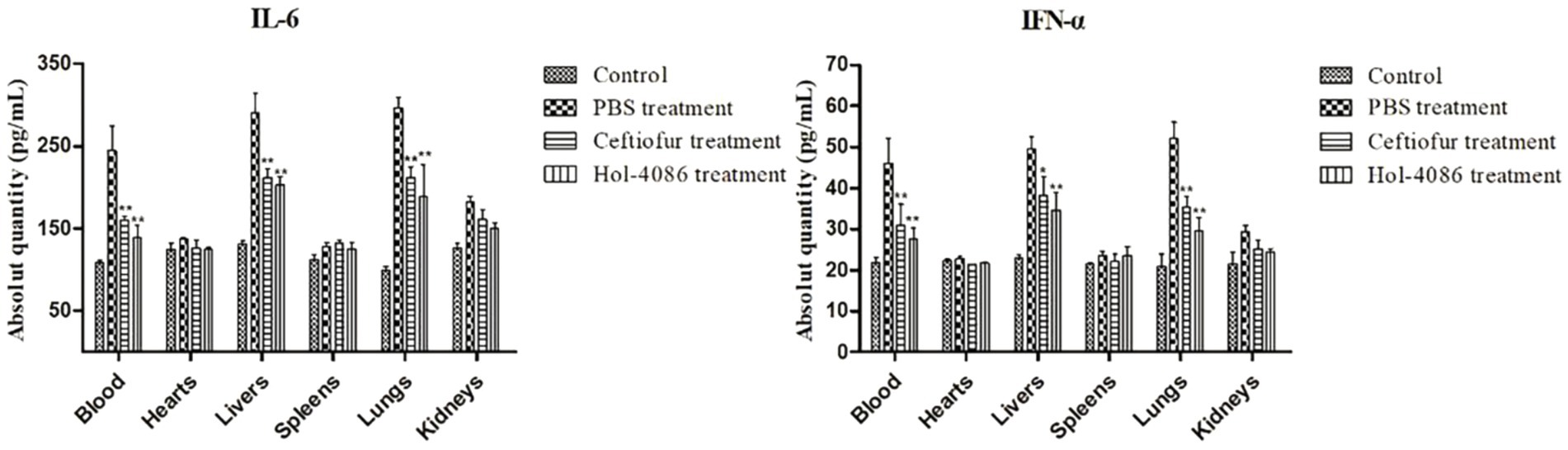
Figure 8. Levels of interleukin-6 (IL-6) and Interferon alpha (IFN-α) from the septicemia model mice after Hol-4086 treatment. Compared with PBS treatment, the ceftiofur and Hol-4086 groups both showed significant decrease in the levels of IFN-α and IL-6. Further, Hol-4086 treatment resulted in slightly lower levels than the antibiotic treatment. Error bars represent the mean ± standard error of the mean from three independent replicates. Compared with PBS treatment, *p < 0.05, **p < 0.01, two-way analysis of variance.
In the PBS group, congestion, extensive degeneration of hepatocytes, and inflammatory cell infiltration around small bile ducts presented in the livers. The lungs were congested, with alveolar epithelial cell hyperplasia, inflammatory cell infiltration, and local widening of alveolar septa. The kidneys were congested, with extensive degeneration and a small amount of necrosis of renal tubular epithelial cells. There was mild hyperplasia of the white pulp of the spleen (Figures 9F–J). After Hol-4086 treatment, pathological changes in the liver, lungs, and kidneys were alleviated (Figures 9P–T). The hearts of mice in the different groups did not show inflammation or other pathological changes (Figure 9).

Figure 9. Pathological changes and organ histopathology. Tissue sections were stained with hematoxylin and eosin and observed under a light microscope. Bars, 100 μm. Based on histopathological observations, the liver, lungs, and kidneys of the infected mice treated with phosphate buffered saline (PBS) showed different degrees of pathological changes. After treatment with Hol-4086, the pathological changes were alleviated. (A–E) The liver, lungs, kidneys, heart, and spleen of the mice without infection and treatment. (F–J) The liver, lungs, kidneys, heart, and spleen of infected mice after treatment with PBS for 48 h. (K–O) The liver, lungs, kidneys, heart, and spleen of infected mice after treatment with ceftiofur for 48 h. (P–T) The liver, lungs, kidneys, heart, and spleen of infected mice after treatment with Hol-4086 for 48 h.
The emergence and proliferation of antibiotic-resistant strains of S. aureus have stimulated the search for alternative strategies to manage bacterial infections (Pires et al., 2020; João et al., 2021). With rapid developments in molecular biology, several new antibacterial strategies have been proposed. Among these antimicrobial strategies, phage lysis-related proteins are considered the most promising biocontrol agents owing to their specific advantages, such as biosafety in vivo (Luo et al., 2020; Srinivasan et al., 2020).
In the present study, we sequenced the whole genome of a S. aureus phage with high lytic activity. Analysis of the phage 4086-1 genome indicated that it is a double-stranded DNA consisting of 17,960 bp, which showed high genomic similarity to S. aureus phage SLPW (Wang et al., 2016). Phage 4086-1 is structurally similar to the short-tailed phage CAS13, with an icosahedral head and a short, non-constricting tail (Tang, 2019; Cha et al., 2019). ORF12 was predicted to encode a protein composed of 140 amino acids that functioned as a holin (Hol-4086). After prokaryotic expression, the Hol-4086 lyase was shown to have the function of lysing S. aureus. In this study, purified protein was not obtained because of the low expression of Hol-4086. The toxic effect of holin on expressing cells may lead to a low exogenous expression output, which is an important reason for restricting the application of holin (Lu et al., 2020). Therefore, it is necessary to find ways to increase the expression output.
Phage lysis-related proteins are potential agents that inhibit bacterial growth (Zhang et al., 2019; Criel et al., 2021; Abdelkader et al., 2019). Endolysins can destabilize the biofilm structure by taking advantage of channels to reach the bacterial cells located inside the biofilm, thereby disrupting the biofilm (Sharma et al., 2018). LysGH15 efficiently prevented biofilm formation and showed notable disruptive activity against 24-h and 72-h biofilms formed by S. aureus and coagulase-negative species (Zhang et al., 2018). Many previous studies have shown that holin proteins can induce cracking in host bacteria (Song et al., 2016). The holin protein HolSSE1 and lyase LysSSE1 of Streptococcus dysenteriae phage have the efficacy of successfully removing bacterial biofilm. And they have a synergistic antibacterial effect when used together (Lu et al., 2021). In addition, it have shown that the phage holin protein pEF191 shows more efficient biofilm clearance of Enterococcus faecalis than the parent phage PEf771, which shows great potential in clinical bactericide (Xiang et al., 2024). In this study, Hol-4086 was characterized and demonstrated bacteriolytic activity against S. aureus. We determined the inhibition and removal effect of Hol-4086 on S. aureus biofilm. Hol-4086 had a better potential to prevent biofilm formation, most likely due to its rapid bacteriostatic activity prior to biofilm formation, which is similar to holin protein HolSSE1 (Lu et al., 2021). In addition, Hol-4086 was effective in removing S. aureus biofilms and could still be effective after 12 h of incubation. Taken together, all these results indicate that Hol-4086 has antibacterial activity against S. aureus and can either inhibit biofilm formation or effectively remove biofilms. It was previously reported that Lysin CF-301 was highly efficient for biofilm removal with a 90% minimum biofilm-eradicating concentration (MBEC90) value of 0.25 μg/mL (Schuch et al., 2017). Since we did not obtain the purified protein of Hol-4086 in this study, the dosage of Hol-4086 could not be determined. In the following study, we need to focus on the purification of Hol-4086 to determine its efficiency in removing biofilms and bacteriostatic efficiency.
Phage lysis-related proteins have great potential in clinical treatment. Singh et al. established bacteremia infection in mice by intraperitoneal injection of 109 CFU of S. aureus, followed by intraperitoneal or intravenous injection of phage lysis protein; the survival rate of mice was significantly increased, indicating that lysis protein can effectively control the infection caused by bacteremia (Singh et al., 2014). After treatment with the endolysin trxSA1 of phage IME-SA1, bacterial counts in cows were reduced to undetectable levels after 3 days, indicating that trxSA1 could effectively control mild clinical mastitis caused by S. aureus (Fan et al., 2016). Some studies have demonstrated that after infection with S. aureus, intraperitoneal injection of LysGH15 (50 μg) can provide 100% protection to mice and can significantly reduce the amount of bacteria in the blood and relieve inflammation (Xia et al., 2016; Zhang et al., 2016). In this study, we assayed the function of Hol-4086 and intraperitoneally injected mice with S. aureus to establish sepsis infection. We found that Hol-4086 significantly reduced bacterial loads in the blood, liver, and lungs, effectively reduced pathological damage, and alleviated inflammatory responses. These findings suggest that Hol-4086 is an active treatment in vivo and may be a potential therapeutic tool.
As a new antibacterial agent, Hol-4086 has similar functional activity and therapeutic effect to phage (Sharma et al., 2018; Zhang et al., 2018; Singh et al., 2014), which shows a great potential on S. aureus infection. Hol-4086 was still effective in removing S. aureus biofilms after 12 h of incubation, which is expected to be used as an environmental antimicrobial in the future. Endotoxin test showed that Hol-4086 did not cause damage to mice, which can provide a new idea and technical means for the treatment of clinical drug-resistant bacteria. More experiments still need to be carried out to explore the effect of Hol-4086 on eukaryotic cells before clinical practice. In addition, Hol-4086 expressed in E. coli has an inhibitory effect on the host bacteria itself, resulting in a low expression level and no purified protein can be obtained. Therefore, the influence of expression system and elements on the expression level should be further optimized in subsequent experiments for clinical treatment.
In summary, as a new type of antibacterial agent, Hol-4086 has a functional activity similar to that of phages, with no bacterial resistance or side effects. Hol-4086 has good therapeutic effects against S. aureus infections and can alleviate inflammation induced by infection. Thus, Hol-4086 may serve as a new therapeutic option for drug-resistant S. aureus in clinical settings.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found: https://www.ncbi.nlm.nih.gov/genbank/, PP541615; https://www.ncbi.nlm.nih.gov/genbank/, PP477054.
The animal study was approved by the Animal Ethics Committee of Northeast Agricultural University, Harbin, China. The study was conducted in accordance with the local legislation and institutional requirements.
CZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. YD: Investigation, Methodology, Writing – original draft. YW: Data curation, Writing – original draft. HZ: Investigation, Writing – original draft. RW: Supervision, Writing – original draft. GL: Project administration, Writing – original draft. XW: Writing – original draft. ZS: Project administration, Writing – original draft. YJ: Project administration, Writing – original draft. WC: Project administration, Writing – original draft. JL: Project administration, Writing – original draft. LW: Methodology, Writing – original draft. XQ: Funding acquisition, Resources, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Natural Science Foundation of China (grant number: 32072876) and Key Research and Development Program of Heilongjiang Province (grant number: GA21B004).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ELISA, Enzyme-linked immunosorbent assay; IPTG, Isopropyl β-d-1-thiogalactopyranoside; ORF, Open reading frame; PBS, Phosphate buffered saline; SDS-PAGE, Sodium dodecyl sulfate-polyacrylamide gel electrophoresis; SEM, Scanning electron microscopy; TEM, Transmission electron microscopy; TMD, Transmembrane domains.
Abdelkader, K., Gerstmans, H., Saafan, A., Dishisha, T., and Briers, Y. (2019). The preclinical and clinical Progress of bacteriophages and their lytic enzymes: the parts are easier than the whole. Viruses 11:96. doi: 10.3390/v11020096
Anyaegbunam, N. J., Anekpo, C. C., Anyaegbunam, Z. K. G., Doowuese, Y., Chinaka, C. B., Odo, O. J., et al. (2022). The resurgence of phage-based therapy in the era of increasing antibiotic resistance from research progress to challenges and prospects. Microbiol. Res. 264:127155. doi: 10.1016/j.micres.2022.127155
Aranaga, C., Pantoja, L. D., Martínez, E. A., and Falco, A. (2022). Phage therapy in the era of multidrug resistance in Bacteria: a systematic review. Int. J. Mol. Sci. 23:4577. doi: 10.3390/ijms23094577
Bhargava, K., Nath, G., Bhargava, A., Aseri, G. K., and Jain, N. (2021). Phage therapeutics: from promises to practices and prospectives. Appl. Microbiol. Biotechnol. 105, 9047–9067. doi: 10.1007/s00253-021-11695-z
Cha, Y., Chun, J., Son, B., and Ryu, S. (2019). Characterization and genome analysis of Staphylococcus aureus Podovirus CSA13 and its anti-biofilm capacity. Viruses 11:54. doi: 10.3390/v11010054
Cheung, G. Y. C., Bae, J. S., and Otto, M. (2021). 1--pathogenicity and virulence of Staphylococcus aureus. Virulence 12, 547–569. doi: 10.1080/21505594.2021.1878688
Cong, Y., Yang, S., and Rao, X. (2020). Vancomycin resistant Staphylococcus aureus infections: a review of case updating and clinical features. J. Adv. Res. 21, 169–176. doi: 10.1016/j.jare.2019.10.005
Criel, B., Taelman, S., Criekinge, W. V., Stock, M., and Briers, Y. (2021). PhaLP: a database for the study of phage lytic proteins and their evolution. Viruses 13:1240. doi: 10.3390/v13071240
Fan, J., Zeng, Z., Mai, K., Yang, Y., Feng, J., Bai, Y., et al. (2016). Preliminary treatment of bovine mastitis caused by Staphylococcus aureus, with trx-SA1, recombinant endolysin of S. aureus bacteriophage IME-SA1. Vet. Microbiol. 191, 65–71. doi: 10.1016/j.vetmic.2016.06.001
Farkašovská, J., Godány, A., and Vlček, C. (2004). Identification of a holin encoded by the Streptomyces aureofaciens phage μ1/6; functional analysis in Escherichia coli system. Springer 49, 679–684. doi: 10.1007/BF02931549
Gutiérrez, D., Fernández, L., Rodríguez, A., and García, P. (2018). Are phage lytic proteins the secret weapon to kill Staphylococcus aureus? mBio 9, e01923–e01917. doi: 10.1128/mBio.01923-17
Hatoum-Aslan, A. (2021). The phages of staphylococci: critical catalysts in health and disease. Trends Microbiol. 29, 1117–1129. doi: 10.1016/j.tim.2021.04.008
Hesse, S., and Adhya, S. (2019). Phage therapy in the twenty-first century: facing the decline of the antibiotic era; is it finally time for the age of the phage? Ann. Rev. Microbiol. 73, 155–174. doi: 10.1146/annurev-micro-090817-062535
Jariah, R. O. A., and Hakim, M. S. (2019). Interaction of phages, bacteria, and the human immune system: evolutionary changes in phage therapy. Rev. Med. Virol. 29:e2055. doi: 10.1002/rmv.2055
João, J., Lampreia, J., Prazeres, D. M. F., and Azevedo, A. M. (2021). Manufacturing of bacteriophages for therapeutic applications. Biotechnol. Adv. 49:107758. doi: 10.1016/j.biotechadv.2021.107758
Kakasis, A., and Panitsa, G. (2019). Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 53, 16–21. doi: 10.1016/j.ijantimicag.2018.09.004
Kim, S., Jin, J.-S., Choi, Y.-J., and Kim, J. (2020). LysSAP26, a new recombinant phage endolysin with a broad Spectrum antibacterial activity. Viruses 12:1340. doi: 10.3390/v12111340
Lakhundi, S., and Zhang, K. (2018). Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 31:e00020-18. doi: 10.1128/CMR.00020-18
Loganathan, A., Manohar, P., Eniyan, K., VinodKumar, C. S., Leptihn, S., and Nachimuthu, R. (2021). Phage therapy as a revolutionary medicine against gram-positive bacterial infections. Beni Suef Univ. J. Basic Appl. Sci. 10:49. doi: 10.1186/s43088-021-00141-8
Lu, N., Sun, Y., Wang, Q., Qiu, Y., Chen, Z., Wen, Y., et al. (2020). Cloning and characterization of endolysin and holin from Streptomyces avermitilis bacteriophage phiSASD1 as potential novel antibiotic candidates. Int. J. Biol. Macromol. 147, 980–989. doi: 10.1016/j.ijbiomac.2019.10.065
Lu, H., Xiong, W., Li, Z., Liu, B., Liu, R., and Liu, X. (2021). Activity of the lyases LysSSE1 and HolSSE1 against common pathogenic bacteria and their antimicrobial efficacy in biofilms. Bioorg. Chem. 116:105322. doi: 10.1016/j.bioorg.2021.105322
Luo, D., Huang, L., Gondil, V. S., Zhou, W., Yang, W., Jia, M., et al. (2020). A choline-recognizing monomeric Lysin, ClyJ-3m, shows elevated activity against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 64:e00311-20. doi: 10.1128/AAC.00311-20
Moghadam, M. T., Amirmozafari, N., Shariati, A., Hallajzadeh, M., Mirkalantari, S., Khoshbayan, A., et al. (2020). How phages overcome the challenges of drug resistant Bacteria in clinical infections. Infect Drug Resist 13, 45–61. doi: 10.2147/idr.s234353
Nobrega, F., Vlot, M., De Jonge, P., Dreesens, L., Beaumont, H., Lavigne, R., et al. (2018). Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 16, 760–773. doi: 10.1038/s41579-018-0070-8
Nwabuife, J. C., Pant, A. M., and Govender, T. (2021). Liposomal delivery systems and their applications against Staphylococcus aureus and methicillin-resistant Staphylococcus aureus. Adv. Drug Deliv. Rev. 178:113861. doi: 10.1016/j.addr.2021.113861
Pires, D. P., Costa, A. R., Pinto, G., Meneses, L., and Azeredo, J. (2020). Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 44, 684–700. doi: 10.1093/femsre/fuaa017
Qi, B., Xie, T., and Shi, H. (2022). Bioinformatics analysis and cloning and expression of endolysin and perforin from Salmonella bacteriophage SM-p2. J. Chin Instit Food 22, 32–39. doi: 10.16429/j.1009-7848.2022.08.004
Salmond, G. P. C., and Fineran, P. C. (2015). A century of the phage: past, present and future. Nat. Rev. Microbiol. 13, 777–786. doi: 10.1038/nrmicro3564
Schuch, R., Khan, B. K., Raz, A., Rotolo, J. A., and Wittekind, M. (2017). Bacteriophage Lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob. Agents Chemother. 61:e02666-16. doi: 10.1128/AAC.02666-16
Sharma, U., Vipra, A., and Channabasappa, S. (2018). Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov. Today 23, 848–856. doi: 10.1016/j.drudis.2018.01.026
Singh, P. K., Donovan, D. M., and Kumar, A. (2014). Intravitreal injection of the chimeric phage endolysin Ply187 protects mice from Staphylococcus aureus endophthalmitis. Antimicrob. Agents Chemother. 58, 4621–4629. doi: 10.1128/AAC.00126-14
Song, J., Xia, F., Jiang, H., Li, X., Hu, L., Gong, P., et al. (2016). Identification and characterization of HolGH15: the holin of Staphylococcus aureus bacteriophage GH15. J. Gen. Virol. 97, 1272–1281. doi: 10.1099/jgv.0.000428
Srinivasan, R., Chaitanyakumar, A., Subramanian, P., Mageswari, A., Gomathi, A., Aswini, V., et al. (2020). Recombinant engineered phage-derived enzybiotic in Pichia pastoris X-33 as whole cell biocatalyst for effective biocontrol of Vibrio parahaemolyticus in aquaculture. Int. J. Biol. Macromol. 154, 1576–1585. doi: 10.1016/j.ijbiomac.2019.11.042
Tang, T. (2019) Isolation and identification of Staphylococcus aureus phage and its preliminary application in the treatment of mastitis. [dissertation/master's thesis]. [Heilongjaing (Harbin)]: Northeast Agricultural University
Turner, N. A., Sharma-Kuinkel, B. K., Maskarinec, S. A., Eichenberger, E. M., Shah, P. P., Carugati, M., et al. (2019). Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat. Rev. Microbiol. 17, 203–218. doi: 10.1038/s41579-018-0147-4
Wang, Z., Ma, J., Wang, J., Yang, D., Kong, L., Fu, Q., et al. (2016). Application of the phage Lysin Ply5218 in the treatment of Streptococcus suis infection in piglets. Viruses 11:715. doi: 10.3390/v11080715
Wang, Z., Zheng, P., Ji, W., Fu, Q., Wang, H., Yan, Y., et al. (2019). SLPW: a virulent bacteriophage targeting methicillin-resistant Staphylococcus aureus in vitro and in vivo. Front. Microbiol. 7, 1–10. doi: 10.3389/fmicb.2016.00934
Wei, J., Peng, N., Liang, Y., Li, K., and Li, Y. (2020). Phage therapy: consider the past, embrace the future. Appl. Sci. 10:7654. doi: 10.3390/app10217654
Xia, F., Li, X., Wang, B., Gong, P., Xiao, F., Yang, M., et al. (2016). Combination therapy of LysGH15 and Apigenin as a new strategy for treating pneumonia caused by Staphylococcus aureus. Appl. Environ. Microbiol. 82, 87–94. doi: 10.1128/AEM.02581-15
Xiang, Y., Wang, S., Huang, H., Li, X., Li, H., Tu, Y., et al. (2024). A novel holin from an Enterococcus faecalis phage and application in vitro and in vivo. Microb. Pathog. 186:106471. doi: 10.1016/j.micpath.2023.106471
Zhang, H., Buttaro, B. A., Fouts, D. E., Sanjari, S., Evans, B. S., and Stevens, R. H. (2019). Bacteriophage φEf11 ORF28 endolysin, a multifunctional lytic enzyme with properties distinct from all other identified Enterococcus faecalis phage endolysins. Appl. Environ. Microbiol. 85:e00555-19. doi: 10.1128/AEM.00555-19
Zhang, Y., Cheng, M., Zhang, H., Dai, J., Guo, Z., Li, X., et al. (2018). Antibacterial effects of phage Lysin LysGH15 on planktonic cells and biofilms of diverse staphylococci. Appl. Environ. Microbiol. 84:e00886-18. doi: 10.1128/AEM.00886-18
Keywords: Staphylococcus aureus, phage, holin, phage perforin, phage therapy
Citation: Zheng C, Di Y, Wang Y, Zhao H, Wang R, Li G, Wang X, San Z, Jiang Y, Cui W, Li J, Wang L and Qiao X (2025) Biological function identification of phage holin Hol-4086 and treatment of Staphylococcus aureus infection. Front. Microbiol. 16:1499566. doi: 10.3389/fmicb.2025.1499566
Received: 21 September 2024; Accepted: 03 February 2025;
Published: 25 February 2025.
Edited by:
Felix Broecker, Idorsia Pharmaceuticals Ltd., SwitzerlandReviewed by:
António Machado, University of the Azores, PortugalCopyright © 2025 Zheng, Di, Wang, Zhao, Wang, Li, Wang, San, Jiang, Cui, Li, Wang and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinyuan Qiao, cWlhb3hpbnl1YW5AMTI2LmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.